Bone (Mineral) Density Studies
|
|
|
- Trevor Thompson
- 6 years ago
- Views:
Transcription
1 Bone (Mineral) Density Studies Policy Number: Original Effective Date: MM /23/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2014 Section: Radiology Place(s) of Service: Outpatient I. Description A bone mineral density (BMD) study is a non-invasive technique that is used to measure bone mineral content and bone mineral density. Its primary role is to detect osteoporosis and to predict the risk of s. Osteoporosis is a disease that results in the loss of bone mineral content or bone density. This leads to thinning and weakening of bones, increasing the risk of s. BMD can be measured with a variety of techniques in a variety of sites that are subdivided into central (hip or spine) and peripheral (wrist, finger or heel). Dual x-ray absorptiometry (DXA) is the most commonly used technique for BMD measurements. Quantitative computed tomography (QCT) may also be used, although it is not as readily available, and has the disadvantage of higher radiation exposure. Ultrasound (US) densitometry is as an office based technology for measuring BMD at peripheral sites, mainly the heel. Peripheral measurements can identify patients with low bone mass, but cannot predict response to pharmacologic therapy and is not a substitute for central BMD measurements. Therefore, central BMD measurements are recommended for the initial diagnosis and for repeat BMD measurements. II. Criteria/Guidelines A. An initial BMD study of the hip and/or spine is covered (subject to Limitations/Exclusions and Administrative Guidelines) to assess risk and the need for pharmacologic therapy in the following individuals considered to be at high-risk for osteoporosis: 1. Women 65 years of age or older and men 70 years of age or older 2. Postmenopausal women with any of the following risk factors: a. History of a low-impact * after age 45 in a first-degree relative b. Body weight of less than 127 lbs. or BMI of 20 or less c. Current smoker d. Surgical or natural menopause before age 40
2 Bone Mineral Density Studies 2 3. Premenopausal women with documented hypo-estrogenic amenorrhea for more than one year 4. Men or women with the following chronic conditions known to be associated with bone loss: a. Cushing's syndrome b. Male hypogonadism for more than five years c. Hyperthyroidism d. Hyperparathyroidism e. Ankylosing spondylitis f. Rheumatoid arthritis g. Systemic lupus erythematosus h. Leukemia i. Multiple myeloma j. Chronic kidney disease (stages III, IV and V) k. Celiac disease l. Prolonged severe loss of mobility (unable to ambulate outside of one's dwelling without a wheelchair for more than one year) 5. Patients taking any of the following medications associated with bone loss: a. Anticonvulsants b. Oral glucocorticoids for more than three months at a dose equivalent to 5 mg of prednisone or greater per day c. Aromatase inhibitors d. Chemotherapy agents (i.e., methotrexate or other antimetabolites) e. Depo-medroxy progesterone acetate (Depo-Provera) when used for more than two years f. Gonadotropin-releasing hormone (GnRH) agonists (buserelin, leuprolide, nafarelin) 6. Solid organ or allogeneic bone marrow transplant recipients 7. Patients with osseous abnormalities shown on imaging studies indicative of osteoporosis, osteopenia or a vertebral compression (defined as vertebral body height loss of 30% or more) 8. Adults who have had a documented low-impact (excluding fingers and toes) within the past two years 9. Children with a disease or condition associated with bone loss or on prolonged medications that are known to decrease bone mass B. Peripheral BMD studies are covered (subject to Limitations/Exclusions and Administrative Guidelines) if the hip/spine cannot be done or the patient is over the table limit for weight A low-impact is defined as a occurring spontaneously or from a fall from standing height, including s from activities such as a cough, sneeze or abrupt movement (e.g., opening a window)
3 Bone Mineral Density Studies 3 III. Limitations/Exclusions A. Routine BMD screening for osteoporosis is not recommended for women under the age of 65 and for men under the age of 70 B. Repeat central BMD studies are limited to one every two years (23 months must past since the previous BMD study) for patients whose last scan showed osteopenia/osteoporosis C. Repeat central BMD studies for individuals who previously tested normal may be retested no sooner than three years from the last BMD if they continue to meet the criteria listed above D. Repeat central BMD studies performed more frequently than once every two years are limited to individuals currently taking an aromatase inhibitor or on glucocorticoid therapy equivalent to 5.0 mg or more of prednisone per day for more than three months E. A follow up DXA is not covered when used to confirm a diagnosis obtained by an US or QCT F. Clinicians are encouraged to use the same facility and same scanner for initial and follow-up BMD studies whenever possible for greater consistency G. Dual Photon Absorptiometry (DPA) and images obtained during a BMD study (e.g., rapid vertebral assessment) are not covered H. 3D reconstruction is not covered for BMD studies IV. Administrative Guidelines A. Precertification is required for the following: 1. Patients under the age of 18. To precertify, please complete HMSA's Precertification Request and mail or fax the form as indicated. The request must include supporting documentation from the patient's medical records with the high-risk indications that would justify a BMD study as well as the proposed therapy for the patient 2. For follow-up studies performed more frequently than once every two years. Requests must include the previous BMD study results, other pertinent test findings and a list of medications the patient is currently taking B. Compliance with the provisions in this policy is subject to monitoring, post payment data analysis, and subsequent medical review. The following documentation should be kept in the patient's medical records and made available upon request: 1. Clinical indications to substantiate that the test is reasonable and necessary 2. Physician s order signed (not rubber-stamped) and dated (mm/dd/yy) 3. The diagnostic evaluation documenting symptoms, other diagnostic X-rays, and physical exam findings must have been dated at a time reasonably proximate to the scan 4. The name and medication regimen for patients undergoing drug therapy C. If a patient does not meet HMSA s guidelines for coverage but has indicated that he or she wants the services performed despite noncoverage, the patient should be asked to sign HMSA's Agreement of Financial Responsibility. A signed waiver indicates that the patient will be responsible for the denied charges D. When submitting a claim for a BMD study that does not meet HMSA guidelines, append modifier code GA to the CPT code for the service. The use of the GA modifier will alert HMSA
4 Bone Mineral Density Studies 4 that the claim should be processed to indicate that the patient will be financially responsible for the service, and that the noncovered charges should not be a provider adjustment E. The signed waiver should be kept in the patient's record. HMSA reserves the right to conduct periodic audits on claims submitted with the GA modifier and review medical records for signed waivers for this service F. The following codes are provided for informational purposes only. Inclusion/exclusion of a procedure or diagnosis does not constitute or imply coverage or provider reimbursement: ICD-9 codes Description Hyperparathyroidism (code range) Cushing's syndrome Post ablative ovarian failure Premature menopause Other ovarian failure Epilepsy (code range) Celiac disease Chronic kidney disease, Stage III (moderate) Stage IV (severe) Stage V Secondary hyperparathyroidism (of renal origin) Absence of menstruation Menopausal and postmenopausal disorders Systemic lupus erythematosus Rheumatoid arthritis Ankylosing Spondylitis Osteoporosis (Code range) Pathological, unspecified Pathological, humerus Pathologic of distal radius and ulna Disorders of bone and cartilage unspecified E932.0 Adrenal cortical steroids causing adverse effects in therapeutic use (cortisone derivatives, desoxycorticosterone derivatives, fluorinated corticosteroids). E932.2 Ovarian hormones and synthetic substitutes. To be used with ICD-9-CM V58.69 for reporting an individual receiving long term Depo Provera
5 Bone Mineral Density Studies 5 injections. V49.81 Asymptomatic postmenopausal status (age-related, natural) V58.65 Long term (current) use of steroids V58.69 Long-term (current) use of other medications (includes high-risk medications) V67.51 Following completed treatment with high-risk medication, not elsewhere classified. To be used for reporting an individual who has completed drug therapy for osteoporosis and is being monitored for response to therapy. V67.59 Following other treatment. To be used for reporting an individual who is receiving ongoing drug therapy for osteoporosis and is being monitored for effectiveness. V82.81 Special screening for other conditions, osteoporosis Applicable CPT codes: CPT Code Description Ultrasound bone density measurement and interpretation, peripheral site(s), any method Computed tomography, bone mineral density study, one or more sites; axial skeleton (e.g., hips, pelvis, spine) Dual energy X-ray absorptiometry (DXA), bone density study, one or more sites; axial skeleton (e.g., hips, pelvis, spine) appendicular skeleton (peripheral) (e.g., radius, wrist, heel) Codes that do not meet payment determination criteria: CPT code Description Vertebral assessment Bone density (bone mineral content) study, one or more sites; single photon absorptiometry Bone density (bone mineral content) study, one or more sites; dual photon absorptiometry, one or more sites HCPCS Code G0130 Description Single energy X-ray absorptiometry (SEXA) bone density study, one or more sites; appendicular skeleton (peripheral) (e.g., radius, wrist, heel)
6 Bone Mineral Density Studies 6 ICD-10 codes are provided for your information. These will not become effective until 10/1/2014: ICD-10 Description E21.0 Primary hyperparathyroidism E21.1 Secondary hyperparathyroidism, not elsewhere classified E21.2 Other hyperparathyroidism E21.3 Hyperparathyroidism unspecified E Symptomatic premature menopause E Asymptomatic premature menopause E28.39 Other primary ovarian failure E89.40 Asymptomatic postprocedure ovarian failure E89.41 Symptomatic postprocedural ovarian failure G Localization-related (focal) (partial) idiopathic epilepsy and epileptic syndromes with seizures of localized onset, not intractable, with status G Localization-related (focal) (partial) idiopathic epilepsy and epileptic syndromes with seizures of localized onset, not intractable, without status G Localization-related (focal) (partial) idiopathic epilepsy and epileptic syndromes with seizures of localized onset, intractable, with status G Localization-related (focal) (partial) idiopathic epilepsy and epileptic syndromes with seizures of localized onset, intractable, without status G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with simple partial seizures, not intractable, with status G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with simple partial seizures, not intractable, without status G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with simple partial seizures, intractable, with status G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with simple partial seizures, intractable, without status G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with complex partial seizures, not intractable, with status
7 Bone Mineral Density Studies 7 G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with complex partial seizures, not intractable, without status G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with complex partial seizures, intractable, with status G Localization-related (focal) (partial) symptomatic epilepsy and epileptic syndromes with complex partial seizures, intractable, without status G Generalized idiopathic epilepsy and epileptic syndromes, not intractable, with status G Generalized idiopathic epilepsy and epileptic syndromes, not intractable, without status G Generalized idiopathic epilepsy and epileptic syndromes, intractable, with status G Generalized idiopathic epilepsy and epileptic syndromes, intractable, without status G Other generalized epilepsy and epileptic syndromes, not intractable, with status G Other generalized epilepsy and epileptic syndromes, not intractable, without status G Other generalized epilepsy and epileptic syndromes, intractable, with status G Other generalized epilepsy and epileptic syndromes, intractable, without status G Special epileptic syndromes, not intractable, with status G Special epileptic syndromes, not intractable, without status G Other epilepsy, not intractable, with status G Other epilepsy, not intractable, without status G Other epilepsy, intractable, with status G Other epilepsy, intractable, without status G40.89 Other seizures G Epilepsy, unspecified, not intractable, with status G Epilepsy, unspecified, not intractable, without status G Epilepsy, unspecified, intractable, with status
8 Bone Mineral Density Studies 8 G Epilepsy, unspecified, intractable, without status K90.0 Celiac disease M05.70 Rheumatoid arthritis with rheumatoid factor of unspecified site without organ or M Rheumatoid arthritis with rheumatoid factor of right shoulder without organ or M Rheumatoid arthritis with rheumatoid factor of left shoulder without organ or M Rheumatoid arthritis with rheumatoid factor of unspecified shoulder without organ or M Rheumatoid arthritis with rheumatoid factor of right elbow without organ or M Rheumatoid arthritis with rheumatoid factor of left elbow without organ or M Rheumatoid arthritis with rheumatoid factor of unspecified elbow without organ or M Rheumatoid arthritis with rheumatoid factor of right wrist without organ or M Rheumatoid arthritis with rheumatoid factor of left wrist without organ or M Rheumatoid arthritis with rheumatoid factor of unspecified wrist without organ or M Rheumatoid arthritis with rheumatoid factor of right hand without organ or M Rheumatoid arthritis with rheumatoid factor of left hand without organ or M Rheumatoid arthritis with rheumatoid factor of unspecified hand without organ or M Rheumatoid arthritis with rheumatoid factor of right hip without organ or M Rheumatoid arthritis with rheumatoid factor of left hip without organ or M Rheumatoid arthritis with rheumatoid factor of unspecified hip without organ or M Rheumatoid arthritis with rheumatoid factor of right knee without organ or
9 Bone Mineral Density Studies 9 M Rheumatoid arthritis with rheumatoid factor of left knee without organ or M Rheumatoid arthritis with rheumatoid factor of unspecified knee without organ or M Rheumatoid arthritis with rheumatoid factor of right ankle and foot without organ or M Rheumatoid arthritis with rheumatoid factor of left ankle and foot without organ or M Rheumatoid arthritis with rheumatoid factor of unspecified ankle and foot without organ or M05.79 Rheumatoid arthritis with rheumatoid factor of multiple sites without organ or M Other rheumatoid arthritis with rheumatoid factor of right shoulder M Other rheumatoid arthritis with rheumatoid factor of left shoulder M Other rheumatoid arthritis with rheumatoid factor of right elbow M Other rheumatoid arthritis with rheumatoid factor of left elbow M Other rheumatoid arthritis with rheumatoid factor of right wrist M Other rheumatoid arthritis with rheumatoid factor of left wrist M Other rheumatoid arthritis with rheumatoid factor of right hand M Other rheumatoid arthritis with rheumatoid factor of left hand M Other rheumatoid arthritis with rheumatoid factor of right hip M Other rheumatoid arthritis with rheumatoid factor of left hip M Other rheumatoid arthritis with rheumatoid factor of right knee M Other rheumatoid arthritis with rheumatoid factor of left knee M Other rheumatoid arthritis with rheumatoid factor of right ankle and foot M Other rheumatoid arthritis with rheumatoid factor of left ankle and foot M05.89 Other rheumatoid arthritis with rheumatoid factor of multiple sites M05.9 Rheumatoid arthritis with rheumatoid factor, unspecified M Rheumatoid arthritis without rheumatoid factor, right shoulder M Rheumatoid arthritis without rheumatoid factor, left shoulder M Rheumatoid arthritis without rheumatoid factor, right elbow M Rheumatoid arthritis without rheumatoid factor, left elbow M Rheumatoid arthritis without rheumatoid factor, right wrist
10 Bone Mineral Density Studies 10 M Rheumatoid arthritis without rheumatoid factor, left wrist M Rheumatoid arthritis without rheumatoid factor, right hand M Rheumatoid arthritis without rheumatoid factor, left hand M Rheumatoid arthritis without rheumatoid factor, right hip M Rheumatoid arthritis without rheumatoid factor, left hip M Rheumatoid arthritis without rheumatoid factor, right knee M Rheumatoid arthritis without rheumatoid factor, left knee M Rheumatoid arthritis without rheumatoid factor, right ankle and foot M Rheumatoid arthritis without rheumatoid factor, left ankle and foot M06.08 Rheumatoid arthritis without rheumatoid factor, vertebrae M06.09 Rheumatoid arthritis without rheumatoid factor, multiple sites M06.9 Rheumatoid arthritis, unspecified M32.0 Drug-induced systemic lupus erythematosus M32.10 Systemic lupus erythematosus, organ or system involvement unspecified M32.11 Endocarditis in systemic lupus erythematosus M32.12 Pericarditis in systemic lupus erythematosus M32.13 Lung involvement in systemic lupus erythematosus M32.14 Glomerular disease in systemic lupus erythematosus M32.15 Tubulo-interstitial nephropathy in systemic lupus erythematosus M80.021A M80.022A M80.029A M80.031A M80.032A M80.039A M80.80xA Age-related osteoporosis with current pathological, right humerus, initial encounter for Age-related osteoporosis with current pathological, left humerus, initial encounter for Age-related osteoporosis with current pathological, unspecified humerus, initial encounter for Age-related osteoporosis with current pathological, right forearm, initial encounter for Age-related osteoporosis with current pathological, left forearm, initial encounter for Age-related osteoporosis with current pathological, unspecified forearm, initial encounter for Other osteoporosis with current pathological, unspecified site, initial encounter for
11 Bone Mineral Density Studies 11 M80.821A M80.822A M80.829A M80.831A M80.832A M80.839A Other osteoporosis with current pathological, right humerus, initial encounter for Other osteoporosis with current pathological, left humerus, initial encounter for Other osteoporosis with current pathological, unspecified humerus, initial encounter for Other osteoporosis with current pathological, right forearm, initial encounter for Other osteoporosis with current pathological, left forearm, initial encounter for Other osteoporosis with current pathological, unspecified forearm, initial encounter for M81.0 Age-related osteoporosis without current pathological M81.6 Localized osteoporosis M81.8 Other osteoporosis without current pathological M84.40xA M84.421A M84.422A M84.429A M84.431A M84.432A M84.433A M84.434A M84.439A M84.50xA M84.521A M84.522A M84.529A M84.531A Pathological, unspecified site, initial encounter for Pathological, right humerus, initial encounter for Pathological, left humerus, initial encounter for Pathological, unspecified humerus, initial encounter for Pathological, right ulna, initial encounter for Pathological, left ulna, initial encounter for Pathological, right radius, initial encounter for Pathological, left radius, initial encounter for Pathological, unspecified ulna and radius, initial encounter for Pathological in neoplastic disease, unspecified site, initial encounter for Pathological in neoplastic disease, right humerus, initial encounter for Pathological in neoplastic disease, left humerus, initial encounter for Pathological in neoplastic disease, unspecified humerus, initial encounter for Pathological in neoplastic disease, right ulna, initial encounter for
12 Bone Mineral Density Studies 12 M84.532A M84.533A M84.534A M84.539A M84.60xA M84.621A M84.622A M84.629A M84.631A M84.632A M84.633A M84.634A M84.639A Pathological in neoplastic disease, left ulna, initial encounter for Pathological in neoplastic disease, right radius, initial encounter for Pathological in neoplastic disease, left radius, initial encounter for Pathological in neoplastic disease, unspecified ulna and radius, initial encounter for Pathological in other disease, unspecified site, initial encounter for Pathological in other disease, right humerus, initial encounter for Pathological in other disease, left humerus, initial encounter for Pathological in other disease, unspecified humerus, initial encounter for Pathological in other disease, right ulna, initial encounter for Pathological in other disease, left ulna, initial encounter for Pathological in other disease, right radius, initial encounter for Pathological in other disease, left radius, initial encounter for Pathological in other disease, unspecified ulna and radius, initial encounter for N18.3 Chronic kidney disease, stage 3 (moderate) N18.4 Chronic kidney disease, stage 4 (severe) N18.5 Chronic kidney disease, stage 5 N25.81 Secondary hyperparathyroidism of renal origin N91.0 Primary amenorrhea N91.1 Secondary amenorrhea N91.2 Amenorrhea, unspecified N95.1 Menopausal and female climacteric states N91.2 Amenorrhea, unspecified Z78.0 Asymptomatic menopausal state
13 Bone Mineral Density Studies 13 Z79.3 Long term (current) use of hormonal contraceptives Z79.52 Long term (current) use of systemic steroids Z Other long term (current) drug therapy VI. Important Reminder The purpose of this Medical Policy is to provide a guide to coverage. This Medical Policy is not intended to dictate to providers how to practice medicine. Nothing in this Medical Policy is intended to discourage or prohibit providing other medical advice or treatment deemed appropriate by the treating physician. Benefit determinations are subject to applicable member contract language. To the extent there are any conflicts between these guidelines and the contract language, the contract language will control. This Medical Policy has been developed through consideration of the medical necessity criteria under Hawaii's Patients' Bill of Rights and Responsibilities Act (Hawaii Revised Statutes 432E-1.4), generally accepted standards of medical practice and review of medical literature and government approval status. HMSA has determined that services not covered under this Medical Policy will not be medically necessary under Hawaii law in most cases. If a treating physician disagrees with HMSA's determination as to medical necessity in a given case, the physician may request that HMSA reconsider the application of the medical necessity criteria to the case at issue in light of any supporting documentation. VI. References 1. Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM, McClung MR, Pollack RP, Petak SM, AACE Osteoporosis Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the Diagnosis and Treatment of Postmenopausal Osteoporosis. Endocr Pract Nov-Dec; 16(Suppl 3): BCBSA Medical Policy Reference Manual. Bone density studies. Policy # Last review March Diagnosis of Osteoporosis in Men, Premenopausal Women, and Children. J Clin Densitom 2004 Spring; 7(1): [82 references] 4. Hau Liu, Neil M. Paige, et.al: Screening for osteoporosis in men: A systematic review from the American College of Physicians Guideline. Ann Intern Med, May 2008; 148: Institute for Clinical Systems Improvement (ICSI). Health Care Guideline, Diagnosis and Treatment of Osteoporosis. Eighth Edition July National Osteoporosis Foundation: Clinicians Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; U.S. Preventive Services Task Force. Screening for Osteoporosis. Recommendation Statement. January Agency for Healthcare Research and Quality, Rockville, MD.
14 Bone Mineral Density Studies Ward L, et al; Bisphosphonate Therapy for Children and Adolescents with Secondary Osteoporosis. Premedline identifier Cochrane database sys rev Conference of Radiation Control Program Directors' Task Force on Bone Densitometry. Technical white paper: Bone Densitometry. October World Health Organization Collaborating Centre for Metabolic Bone Diseases. WHO Fracture Risk Assessment tool. Available online at
Bone (Mineral) Density Studies
 Bone (Mineral) Density Studies Policy Number: Original Effective Date: MM.05.002 03/23/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section: Radiology Place(s)
Bone (Mineral) Density Studies Policy Number: Original Effective Date: MM.05.002 03/23/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/28/2017 Section: Radiology Place(s)
Bone Mineral Density Studies in Adult Populations
 Bone Mineral Density Studies in Adult Populations Last Review Date: July 14, 2017 Number: MG.MM.RA10aC6 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician
Bone Mineral Density Studies in Adult Populations Last Review Date: July 14, 2017 Number: MG.MM.RA10aC6 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician
Contractor Number 03201
 Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Local Coverage Article for Bone Mass Measurements Coverage - 2012 CPT Updates (A51577) Contractor Information Contractor Name Noridian Administrative Services, LLC opens in new window Contractor Number
Bone Mass Measurement BONE MASS MEASUREMENT HS-042. Policy Number: HS-042. Original Effective Date: 8/25/2008
 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Clinical Appropriateness Guidelines: Advanced Imaging
 Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Quantitative CT (QCT) Bone Mineral Densitometry Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007
Clinical Appropriateness Guidelines: Advanced Imaging Appropriate Use Criteria: Quantitative CT (QCT) Bone Mineral Densitometry Effective Date: September 5, 2017 Proprietary Date of Origin: 05/21/2007
Cpt code for bone density of hips only
 Cpt code for bone density of hips only Enter a location: Find a13 This policy may apply to the following codes. Inclusion of a code in this section does not guarantee that it will be reimbursed. For further
Cpt code for bone density of hips only Enter a location: Find a13 This policy may apply to the following codes. Inclusion of a code in this section does not guarantee that it will be reimbursed. For further
Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Retirement Date N/A
 Local Coverage Determination (LCD): Bone Mass Measurement (L36460) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Bone Mass Measurement (L36460) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
MEDICAL POLICY SUBJECT: BONE DENSITOMETRY/ BONE DENSITY STUDIES. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: BONE DENSITOMETRY/ 07/20/06, 05/17/07, 06/19/08, 09/17/09, PAGE: 1 OF: 9 If the member's subscriber contract excludes coverage for a specific service it is not covered under that
MEDICAL POLICY SUBJECT: BONE DENSITOMETRY/ 07/20/06, 05/17/07, 06/19/08, 09/17/09, PAGE: 1 OF: 9 If the member's subscriber contract excludes coverage for a specific service it is not covered under that
Jurisdiction Nebraska. Retirement Date N/A
 If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): BONE Mass Measurement (L31620) Contractor Information Contractor Name
If you wish to save the PDF, please ensure that you change the file extension to.pdf (from.ashx). Local Coverage Determination (LCD): BONE Mass Measurement (L31620) Contractor Information Contractor Name
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bone_mineral_density_studies 12/1996 9/2017 9/2018 9/2017 Description of Procedure or Service Bone density
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: bone_mineral_density_studies 12/1996 9/2017 9/2018 9/2017 Description of Procedure or Service Bone density
Interpreting DEXA Scan and. the New Fracture Risk. Assessment. Algorithm
 Interpreting DEXA Scan and the New Fracture Risk Assessment Algorithm Prof. Samir Elbadawy *Osteoporosis affect 30%-40% of women in western countries and almost 15% of men after the age of 50 years. Osteoporosis
Interpreting DEXA Scan and the New Fracture Risk Assessment Algorithm Prof. Samir Elbadawy *Osteoporosis affect 30%-40% of women in western countries and almost 15% of men after the age of 50 years. Osteoporosis
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2016 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2016 Section: DME Place(s)
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017 Section: DME Place(s)
Continuous Glucose Monitoring System
 Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 4/1/2018 Section: DME Place(s)
Continuous Glucose Monitoring System Policy Number: Original Effective Date: MM.02.003 03/13/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 4/1/2018 Section: DME Place(s)
WCHQ Ambulatory Measure Specification Screening For Osteoporosis Measurement Period 07/01/16-06/30/17 Submission Period: 09/05/17-10/20/17
 MEASURE DESCRIPTION The percentage of women age 65 through 85 who had a minimum of one bone densitometry test at age 60 or above or have a diagnosis of osteoporosis or osteopenia. Disclaimer: Measures
MEASURE DESCRIPTION The percentage of women age 65 through 85 who had a minimum of one bone densitometry test at age 60 or above or have a diagnosis of osteoporosis or osteopenia. Disclaimer: Measures
Bone Densitometry. What is a Bone Density Scan (DXA)? What are some common uses of the procedure?
 Scan for mobile link. Bone Densitometry What is a Bone Density Scan (DXA)? Bone density scanning, also called dual-energy x-ray absorptiometry (DXA) or bone densitometry, is an enhanced form of x-ray technology
Scan for mobile link. Bone Densitometry What is a Bone Density Scan (DXA)? Bone density scanning, also called dual-energy x-ray absorptiometry (DXA) or bone densitometry, is an enhanced form of x-ray technology
sad EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Bone Mineral Density Studies sad EFFECTIVE DATE: 06 07 2011 POLICY LAST UPDATED: 11 06 2018 OVERVIEW Bone density studies can be used to identify individuals with osteoporosis and
Medical Coverage Policy Bone Mineral Density Studies sad EFFECTIVE DATE: 06 07 2011 POLICY LAST UPDATED: 11 06 2018 OVERVIEW Bone density studies can be used to identify individuals with osteoporosis and
DENOSUMAB (PROLIA & XGEVA )
 DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
DENOSUMAB (PROLIA & XGEVA ) UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 306.3 T2 Effective Date: July 2, 2018 Table of Contents Page INSTRUCTIONS FOR USE... 1 CONDITIONS OF COVERAGE...
Collagen Crosslinks, Any Method
 190.19 - Collagen Crosslinks, Any Method Collagen crosslinks, part of the matrix of bone upon which bone mineral is deposited, are biochemical markers the excretion of which provides a quantitative measurement
190.19 - Collagen Crosslinks, Any Method Collagen crosslinks, part of the matrix of bone upon which bone mineral is deposited, are biochemical markers the excretion of which provides a quantitative measurement
Growth Hormone Therapy
 Growth Hormone Therapy Policy Number: Original Effective Date: MM.04.011 05/21/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/23/2014 Section: Prescription Drugs Place(s)
Growth Hormone Therapy Policy Number: Original Effective Date: MM.04.011 05/21/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 05/23/2014 Section: Prescription Drugs Place(s)
Collagen Crosslinks, Any Method
 190.19 - Collagen Crosslinks, Any Method Collagen crosslinks, part of the matrix of bone upon which bone mineral is deposited, are biochemical markers the excretion of which provides a quantitative measurement
190.19 - Collagen Crosslinks, Any Method Collagen crosslinks, part of the matrix of bone upon which bone mineral is deposited, are biochemical markers the excretion of which provides a quantitative measurement
Bortezomib (Velcade)
 Bortezomib (Velcade) Policy Number: Original Effective Date: MM.04.003 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription Drugs Place(s)
Bortezomib (Velcade) Policy Number: Original Effective Date: MM.04.003 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription Drugs Place(s)
Lung-Volume Reduction Surgery ARCHIVED
 Lung-Volume Reduction Surgery ARCHIVED Policy Number: Original Effective Date: MM.06.008 04/15/2005 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST 03/22/2013 Section: Surgery Place(s) of
Lung-Volume Reduction Surgery ARCHIVED Policy Number: Original Effective Date: MM.06.008 04/15/2005 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST 03/22/2013 Section: Surgery Place(s) of
BMD: A Continuum of Risk WHO Bone Density Criteria
 Pathogenesis of Osteoporosis Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis AGING MENOPAUSE OTHER RISK FACTORS RESORPTION > FORMATION Bone Loss LOW PEAK BONE MASS Steven T Harris
Pathogenesis of Osteoporosis Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis AGING MENOPAUSE OTHER RISK FACTORS RESORPTION > FORMATION Bone Loss LOW PEAK BONE MASS Steven T Harris
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Remicade (Infliximab)
 Remicade (Infliximab) Policy Number: Original Effective Date: MM.04.016 11/18/2003 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/26/2013 Section: Prescription Drugs Place(s)
Remicade (Infliximab) Policy Number: Original Effective Date: MM.04.016 11/18/2003 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 07/26/2013 Section: Prescription Drugs Place(s)
Erythropoiesis Stimulating Agents (ESA)
 Erythropoiesis Stimulating Agents (ESA) Policy Number: Original Effective Date: MM.04.008 04/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription
Erythropoiesis Stimulating Agents (ESA) Policy Number: Original Effective Date: MM.04.008 04/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/01/2015 Section: Prescription
JOURNAL OF INTERNATIONAL ACADEMIC RESEARCH FOR MULTIDISCIPLINARY Impact Factor 1.393, ISSN: , Volume 2, Issue 7, August 2014
 HYPOVITAMINOSIS D IN INDIAN FEMALES WITH POSTMENOPAUSAL OSTEOPOROSIS DR. SHAH WALIULLAH 1 DR. VINEET SHARMA 2 DR. R N SRIVASTAVA 3 DR. YASHODHARA PRADEEP 4 DR. A A MAHDI 5 DR. SANTOSH KUMAR 6 1 Research
HYPOVITAMINOSIS D IN INDIAN FEMALES WITH POSTMENOPAUSAL OSTEOPOROSIS DR. SHAH WALIULLAH 1 DR. VINEET SHARMA 2 DR. R N SRIVASTAVA 3 DR. YASHODHARA PRADEEP 4 DR. A A MAHDI 5 DR. SANTOSH KUMAR 6 1 Research
Insulin Pumps - External
 Insulin Pumps - External Policy Number: Original Effective Date: MM.01.004 04/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/20174/1/2018 Section: DME Place(s) of
Insulin Pumps - External Policy Number: Original Effective Date: MM.01.004 04/01/2011 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/20174/1/2018 Section: DME Place(s) of
IEHP UM Subcommittee Approved Authorization Guidelines DEXA Scan
 Policy: IEHP UM Subcommittee Approved Authorization Guidelines IEHP considers bone mineral density testing using DEXA medically necessary for members who meet any of the following criteria: Women aged
Policy: IEHP UM Subcommittee Approved Authorization Guidelines IEHP considers bone mineral density testing using DEXA medically necessary for members who meet any of the following criteria: Women aged
Kyphoplasty and Vertebroplasty
 Kyphoplasty and Vertebroplasty Policy Number: Original Effective Date: MM.06.007 01/11/2005 Line(s) of Business: Current Effective Date: HMO; PPO 02/01/2012 Section: Surgery Place(s) of Service: Inpatient;
Kyphoplasty and Vertebroplasty Policy Number: Original Effective Date: MM.06.007 01/11/2005 Line(s) of Business: Current Effective Date: HMO; PPO 02/01/2012 Section: Surgery Place(s) of Service: Inpatient;
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 03/09/2004 Current Effective Date: 05/01/2017 POLICY A. INDICATIONS The indications below
DXA When to order? How to interpret? Dr Nikhil Tandon Department of Endocrinology and Metabolism All India Institute of Medical Sciences New Delhi
 DXA When to order? How to interpret? Dr Nikhil Tandon Department of Endocrinology and Metabolism All India Institute of Medical Sciences New Delhi Clinical Utility of Bone Densitometry Diagnosis (DXA)
DXA When to order? How to interpret? Dr Nikhil Tandon Department of Endocrinology and Metabolism All India Institute of Medical Sciences New Delhi Clinical Utility of Bone Densitometry Diagnosis (DXA)
Bevacizumab (Avastin)
 Bevacizumab (Avastin) Policy Number: Original Effective Date: MM.04.001 09/14/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2014 Section: Prescription Drugs Place(s) of Service:
Bevacizumab (Avastin) Policy Number: Original Effective Date: MM.04.001 09/14/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2014 Section: Prescription Drugs Place(s) of Service:
Posterior Tibial Nerve Stimulation
 Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
Posterior Tibial Nerve Stimulation Policy Number: Original Effective Date: MM.02.025 01/01/2015 Lines of Business: Current Effective Date: HMO; PPO; QUEST Integration 02/01/2015 Section: Medicine Place(s)
chapter Bone Density (Densitometry) RADIOPHARMACY INDICATIONS Radionuclide Localization Quality Control Adult Dose Range Method of Administration
 10766-04_CH04_redo.qxd 12/3/07 3:47 PM Page 17 chapter 4 Bone Density (Densitometry) RADIOPHARMACY Radionuclide Single radionuclide: 125 I t 1/2 : 60.1 days Energies: 23 31 kev Type: EC, x, γ, accelerator
10766-04_CH04_redo.qxd 12/3/07 3:47 PM Page 17 chapter 4 Bone Density (Densitometry) RADIOPHARMACY Radionuclide Single radionuclide: 125 I t 1/2 : 60.1 days Energies: 23 31 kev Type: EC, x, γ, accelerator
Velcade (bortezomib)
 Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
Velcade (bortezomib) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 03/09/2004 Current Effective Date: 03/01/2018 POLICY A. INDICATIONS The indications below
Bone Densitometry Pathway
 Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Bone Densitometry Pathway The goal of the Bone Densitometry pathway is to manage our diagnosed osteopenic and osteoporotic patients, educate and monitor the patient population at risk for bone density
Home Total Parenteral Nutrition for Adults
 Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Clinical Policy: (Forteo) Reference Number: CP.PHAR.188 Effective Date: 11.15.17 Last Review Date: 02.19 Line of Business: Commercial* (Exchange Plans), HIM, Medicaid Coding Implications Revision Log See
Disclosure and Conflicts of Interest Steven T Harris MD Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis
 Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis Steven T Harris MD FACP Clinical Professor of Medicine University of California, San Francisco Disclosure and Conflicts of Interest
Osteoporosis Diagnosis: BMD, FRAX and Assessment of Secondary Osteoporosis Steven T Harris MD FACP Clinical Professor of Medicine University of California, San Francisco Disclosure and Conflicts of Interest
Prophylactic Mastectomy
 Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Surgery Place(s) of Service: Inpatient I.
Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Surgery Place(s) of Service: Inpatient I.
FORTEO (teriparatide) INJECTION
 FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
FORTEO (teriparatide) INJECTION Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage
Objectives. Discuss bone health and the consequences of osteoporosis on patients medical and disability status.
 Objectives Discuss bone health and the consequences of osteoporosis on patients medical and disability status. Discuss the pathophysiology of osteoporosis and major risk factors. Assess the major diagnostic
Objectives Discuss bone health and the consequences of osteoporosis on patients medical and disability status. Discuss the pathophysiology of osteoporosis and major risk factors. Assess the major diagnostic
Bariatric Surgery MM /11/2001. HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient; Inpatient
 Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
Bariatric Surgery Policy Number: Original Effective Date: MM.06.003 09/11/2001 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 05/01/2012 Section: Surgery Place(s) of Service: Outpatient;
Use of DXA / Bone Density in the Care of Your Patients. Brenda Lee Holbert, M.D. Associate Professor Senior Staff Radiologist
 Use of DXA / Bone Density in the Care of Your Patients Brenda Lee Holbert, M.D. Associate Professor Senior Staff Radiologist Important Websites Resources for Clinicians and Patients www.nof.org www.iofbonehealth.org
Use of DXA / Bone Density in the Care of Your Patients Brenda Lee Holbert, M.D. Associate Professor Senior Staff Radiologist Important Websites Resources for Clinicians and Patients www.nof.org www.iofbonehealth.org
Applied Behavior Analysis Therapy for Treatment of Autism Spectrum Disorder
 Applied Behavior Analysis Therapy for Treatment of Autism Spectrum Disorder Policy Number: Original Effective Date: MM.12.022 01/01/2016 Line(s) of Business: Current Effective Date: HMO; PPO; Fed 87; FEP;
Applied Behavior Analysis Therapy for Treatment of Autism Spectrum Disorder Policy Number: Original Effective Date: MM.12.022 01/01/2016 Line(s) of Business: Current Effective Date: HMO; PPO; Fed 87; FEP;
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 : assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
: assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only.
 This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
This Coverage Policy applies to Individual Health Insurance Marketplace benefit plans only. INJECTABLE OSTEOPOSIS AGENTS SUBJECT Pharmacologic Agents: Bisphosphonates: Boniva IV (ibandronate) Reclast (zoledronic
Physical Therapy MM /15/2003
 Physical Therapy Policy Number: Original Effective Date: MM.09.005 07/15/2003 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/23/2017 Line(s) of Business Excluded: Federal Employee
Physical Therapy Policy Number: Original Effective Date: MM.09.005 07/15/2003 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/23/2017 Line(s) of Business Excluded: Federal Employee
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2013 Section:
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2013 Section:
DXA Best Practices. What is the problem? 9/29/2017. BMD Predicts Fracture Risk. Dual-energy X-ray Absorptiometry: DXA
 BMD Predicts Fracture Risk Ten Year Fracture Probability (%) 50 40 30 20 10 Age 80 70 60 50 E. Michael Lewiecki, MD Director, New Mexico Clinical Research & Osteoporosis Center Director, Bone TeleHealth
BMD Predicts Fracture Risk Ten Year Fracture Probability (%) 50 40 30 20 10 Age 80 70 60 50 E. Michael Lewiecki, MD Director, New Mexico Clinical Research & Osteoporosis Center Director, Bone TeleHealth
Section 4. Scans and tests. How do I know if I have osteoporosis? Investigations for spinal fractures. Investigations after you break a bone
 Section 4 Scans and tests How do I know if I have osteoporosis? Investigations for spinal fractures Investigations after you break a bone Investigations if you have risk factors Investigations for children
Section 4 Scans and tests How do I know if I have osteoporosis? Investigations for spinal fractures Investigations after you break a bone Investigations if you have risk factors Investigations for children
Somatuline Depot (lanreotide)
 Somatuline Depot (lanreotide) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 12/01/2017TBD POLICY A. INDICATIONS The indications
Somatuline Depot (lanreotide) Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 12/01/2017TBD POLICY A. INDICATIONS The indications
Torisel (temsirolimus)
 Torisel (temsirolimus) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 11/1/2017 POLICY A. INDICATIONS The indications below
Torisel (temsirolimus) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 11/1/2017 POLICY A. INDICATIONS The indications below
2013 ISCD Official Positions Adult
 2013 ISCD Official Positions Adult These are the Official Positions of the ISCD as updated in 2013. The Official Positions that are new or revised since 2007 are in bold type. Indications for Bone Mineral
2013 ISCD Official Positions Adult These are the Official Positions of the ISCD as updated in 2013. The Official Positions that are new or revised since 2007 are in bold type. Indications for Bone Mineral
Bone Mineral Densitometry with Dual Energy X-Ray Absorptiometry
 Bone Mineral Densitometry with Dual Energy X-Ray Absorptiometry R Gilles, Laurentius Ziekenhuis Roermond 1. Introduction Osteoporosis is characterised by low bone mass, disruption of the micro-architecture
Bone Mineral Densitometry with Dual Energy X-Ray Absorptiometry R Gilles, Laurentius Ziekenhuis Roermond 1. Introduction Osteoporosis is characterised by low bone mass, disruption of the micro-architecture
Applied Behavior Analysis Therapy for Treatment of Autism Spectrum Disorder
 Applied Behavior Analysis Therapy for Treatment of Autism Spectrum Disorder Policy Number: Original Effective Date: MM.12.022 01/01/2016 Line(s) of Business: Current Effective Date: HMO; PPO; Fed 87; FEP;
Applied Behavior Analysis Therapy for Treatment of Autism Spectrum Disorder Policy Number: Original Effective Date: MM.12.022 01/01/2016 Line(s) of Business: Current Effective Date: HMO; PPO; Fed 87; FEP;
Intensity Modulated Radiation Therapy (IMRT)
 Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 03/01/2015 Section: Radiology
Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 03/01/2015 Section: Radiology
Women s Imaging ICD-10-CM
 Women s Imaging ICD-10-CM Clinical Documentation Guides Brought to you by www.codingstrategies.com The Resource for Physician and Outpatient Coding, Compliance & ICD-10-CM OTHER CLINICAL DOCUMENTATION
Women s Imaging ICD-10-CM Clinical Documentation Guides Brought to you by www.codingstrategies.com The Resource for Physician and Outpatient Coding, Compliance & ICD-10-CM OTHER CLINICAL DOCUMENTATION
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment. William D. Leslie, MD MSc FRCPC
 Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Module 5 - Speaking of Bones Osteoporosis For Health Professionals: Fracture Risk Assessment William D. Leslie, MD MSc FRCPC Case #1 Age 53: 3 years post-menopause Has always enjoyed excellent health with
Case Finding and Risk Assessment for Osteoporosis
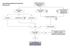 Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Extracorporeal Membrane Oxygenation (ECMO)
 Extracorporeal Membrane Oxygenation (ECMO) Policy Number: Original Effective Date: MM.12.006 05/16/2006 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 11/01/2014 Section: Other/Miscellaneous
Extracorporeal Membrane Oxygenation (ECMO) Policy Number: Original Effective Date: MM.12.006 05/16/2006 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 11/01/2014 Section: Other/Miscellaneous
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017
Intensity Modulated Radiation Therapy (IMRT)
 Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO 06/24/2011 Section: Radiology Place(s) of
Intensity Modulated Radiation Therapy (IMRT) Policy Number: Original Effective Date: MM.05.006 03/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO 06/24/2011 Section: Radiology Place(s) of
Residential Treatment (RTC)
 An Independent Licensee of the Blue Cross and Blue Shield Association Residential Treatment (RTC) BEACON HEALTH STRATEGIES, LLC ORIGINAL EFFECTIVE DATE HAWAII LEVEL OF CARE CRITERIA 2013 CURRENT EFFECTIVE
An Independent Licensee of the Blue Cross and Blue Shield Association Residential Treatment (RTC) BEACON HEALTH STRATEGIES, LLC ORIGINAL EFFECTIVE DATE HAWAII LEVEL OF CARE CRITERIA 2013 CURRENT EFFECTIVE
Medical Policy. MP Vertebral Fracture Assessment With Densitometry
 Medical Policy BCBSA Ref. Policy: 6.01.44 Last Review: 09/19/2018 Effective Date: 09/19/2018 Section: Radiology Related Policies 6.01.40 Whole Body Dual X-Ray Absorptiometry to Determine Body Composition
Medical Policy BCBSA Ref. Policy: 6.01.44 Last Review: 09/19/2018 Effective Date: 09/19/2018 Section: Radiology Related Policies 6.01.40 Whole Body Dual X-Ray Absorptiometry to Determine Body Composition
Low-Molecular-Weight Heparin
 Low-Molecular-Weight Heparin Policy Number: Original Effective Date: MM.04.019 10/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO 10/28/2011 Section: Prescription Drugs Place(s) of Service:
Low-Molecular-Weight Heparin Policy Number: Original Effective Date: MM.04.019 10/15/2007 Line(s) of Business: Current Effective Date: HMO; PPO 10/28/2011 Section: Prescription Drugs Place(s) of Service:
Using the FRAX Tool. Osteoporosis Definition
 How long will your bones remain standing? Using the FRAX Tool Gary Salzman M.D. Director Banner Good Samaritan/ Hayden VAMC Internal Medicine Geriatric Fellowship Program Phoenix, Arizona Using the FRAX
How long will your bones remain standing? Using the FRAX Tool Gary Salzman M.D. Director Banner Good Samaritan/ Hayden VAMC Internal Medicine Geriatric Fellowship Program Phoenix, Arizona Using the FRAX
denosumab (Prolia ) Policy # Original Effective Date: 07/21/2011 Current Effective Date: 04/19/2017
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Based on review of available data, the Company may consider the use of denosumab (Prolia) for the
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Kyphoplasty and Vertebroplasty
 Kyphoplasty and Vertebroplasty Policy Number: Original Effective Date: MM.06.007 01/11/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/27/2014 Section: Surgery Place(s)
Kyphoplasty and Vertebroplasty Policy Number: Original Effective Date: MM.06.007 01/11/2005 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 06/27/2014 Section: Surgery Place(s)
Bone Density Measurement in Women
 Bone Density Measurement in Women Revised 2005 Scope This guideline defines the medical necessity of bone mineral density (BMD) measurement using dualenergy x-ray absorptiometry (DXA or DEXA), and applies
Bone Density Measurement in Women Revised 2005 Scope This guideline defines the medical necessity of bone mineral density (BMD) measurement using dualenergy x-ray absorptiometry (DXA or DEXA), and applies
Osteoporosis: Risk Factors, Diagnostic Methods And Treatment Options
 ISPUB.COM The Internet Journal of Academic Physician Assistants Volume 1 Number 1 Osteoporosis: Risk Factors, Diagnostic Methods And Treatment Options K Ihrke Citation K Ihrke.. The Internet Journal of
ISPUB.COM The Internet Journal of Academic Physician Assistants Volume 1 Number 1 Osteoporosis: Risk Factors, Diagnostic Methods And Treatment Options K Ihrke Citation K Ihrke.. The Internet Journal of
Clinical Policy: Denosumab (Prolia, Xgeva) Reference Number: CP.PHAR.58
 Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Prolia, Xgeva) Reference Number: CP.PHAR.58 Effective Date: 03/11 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 04/01/2014 Section:
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 04/01/2014 Section:
Skeletal Manifestations
 Skeletal Manifestations of Metabolic Bone Disease Mishaela R. Rubin, MD February 21, 2008 The Three Ages of Women Gustav Klimt 1905 1 Lecture Outline Osteoporosis epidemiology diagnosis secondary causes
Skeletal Manifestations of Metabolic Bone Disease Mishaela R. Rubin, MD February 21, 2008 The Three Ages of Women Gustav Klimt 1905 1 Lecture Outline Osteoporosis epidemiology diagnosis secondary causes
Artificial Disc Replacement, Cervical
 Artificial Disc Replacement, Cervical Policy Number: Original Effective Date: MM.06.001 02/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO 11/01/2011 Section: Surgery Place(s) of Service:
Artificial Disc Replacement, Cervical Policy Number: Original Effective Date: MM.06.001 02/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO 11/01/2011 Section: Surgery Place(s) of Service:
Photochemotherapy MM /09/2004. HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service: Home; Office
 Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Parathyroid Hormone Analogs
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.30.36 Subject: Parathyroid Hormone Analogs Page: 1 of 6 Last Review Date: September 15, 2017 Parathyroid
SCHEDULE 2 THE SERVICES. A. Service Specifications
 SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No. 04/MSKT/0013 Service PAN DORSET FRACTURE LIAISON SERVICE Commissioner Lead CCP for Musculoskeletal & Trauma Provider Lead Deputy
SCHEDULE 2 THE SERVICES A. Service Specifications Service Specification No. 04/MSKT/0013 Service PAN DORSET FRACTURE LIAISON SERVICE Commissioner Lead CCP for Musculoskeletal & Trauma Provider Lead Deputy
Oxygen and Oxygen Equipment
 Oxygen and Oxygen Equipment Policy Number: Original Effective Date: MM.01.008 12/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 09/01/2013 Section: DME Place(s) of Service: Home I.
Oxygen and Oxygen Equipment Policy Number: Original Effective Date: MM.01.008 12/01/2010 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 09/01/2013 Section: DME Place(s) of Service: Home I.
Clinical Policy: Abaloparatide (Tymlos) Reference Number: CP.CPA.306 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Tymlos) Reference Number: CP.CPA.306 Effective Date: 06.13 Last Review Date: 08.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Osteoporosis: fragility fracture risk. Costing report. Implementing NICE guidance
 Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
Osteoporosis: fragility fracture risk Costing report Implementing NICE guidance August 2012 NICE clinical guideline 146 1 of 15 This costing report accompanies the clinical guideline: Osteoporosis: assessing
Pharmacy Management Drug Policy
 SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
SUBJECT: - Forteo (teriparatide), Prolia (denosumab), Tymlos (abaloparatide), Boniva injection (Ibandronate) POLICY NUMBER: Pharmacy-35 EFFECTIVE DATE: 9/07 LAST REVIEW DATE: 10/15/2018 If the member s
Gazyva (obinutuzumab)
 Gazyva (obinutuzumab) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/201807/01/2018 POLICY A. INDICATIONS The indications
Gazyva (obinutuzumab) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/201807/01/2018 POLICY A. INDICATIONS The indications
Osteoporosis. When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of.
 Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Osteoporosis When we talk about osteoporosis, we have to be familiar with the constituents of bone and what it is formed of. Osteoblasts by definition are those cells present in the bone and are involved
Sarena Ravi MD, MPH Endocrinologist. Franciscan Physicians Network Division of Endocrinology Chicago, IL
 Sarena Ravi MD, MPH Endocrinologist Franciscan Physicians Network Division of Endocrinology Chicago, IL Definition & Diagnosis of Osteoporosis Management of Osteoporosis in all Populations Long term Management
Sarena Ravi MD, MPH Endocrinologist Franciscan Physicians Network Division of Endocrinology Chicago, IL Definition & Diagnosis of Osteoporosis Management of Osteoporosis in all Populations Long term Management
Inpatient Mental Health
 Inpatient Mental Health BEACON HEALTH STRATEGIES, LLC ORIGINAL EFFECTIVE DATE HAWAII LEVEL OF CARE CRITERIA 2013 CURRENT EFFECTIVE DATE 2016 I. Description Acute Inpatient Psychiatric Services are the
Inpatient Mental Health BEACON HEALTH STRATEGIES, LLC ORIGINAL EFFECTIVE DATE HAWAII LEVEL OF CARE CRITERIA 2013 CURRENT EFFECTIVE DATE 2016 I. Description Acute Inpatient Psychiatric Services are the
Challenging the Current Osteoporosis Guidelines. Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA
 Challenging the Current Osteoporosis Guidelines Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Whom to screen Which test How to diagnose Whom to treat Benefits
Challenging the Current Osteoporosis Guidelines Carolyn J. Crandall, MD, MS Professor of Medicine David Geffen School of Medicine at UCLA Whom to screen Which test How to diagnose Whom to treat Benefits
Bone Mineral Density Studies
 Bone Mineral Density Studies Policy Number: 6.01.01 Last Review: 3/2019 Origination: 10/1988 Next Review: 3/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for bone
Bone Mineral Density Studies Policy Number: 6.01.01 Last Review: 3/2019 Origination: 10/1988 Next Review: 3/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for bone
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Actonel, Atelvia) Reference Number: CP.PMN.100 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the
Clinical Policy: (Actonel, Atelvia) Reference Number: CP.PMN.100 Effective Date: 03.01.18 Last Review Date: 02.19 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the
Understanding the Development of Osteoporosis and Preventing Fractures: WHO Do We Treat Now?
 Understanding the Development of Osteoporosis and Preventing Fractures: WHO Do We Treat Now? Steven M. Petak, MD, JD, FACE, FCLM Texas Institute for Reproductive Medicine And Endocrinology, Houston, Texas
Understanding the Development of Osteoporosis and Preventing Fractures: WHO Do We Treat Now? Steven M. Petak, MD, JD, FACE, FCLM Texas Institute for Reproductive Medicine And Endocrinology, Houston, Texas
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2015
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2015
Perjeta (pertuzumab)
 Perjeta (pertuzumab) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/201809/16/2018 POLICY A. INDICATIONS The indications
Perjeta (pertuzumab) Line(s) of Business: HMO; PPO; QUEST Integration Medicare Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/201809/16/2018 POLICY A. INDICATIONS The indications
PFIZER INC. What is the difference in incidence of fracture in women who ever or never used DMPA for contraception?
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
