American College of Rheumatology
|
|
|
- Carmel Chandler
- 5 years ago
- Views:
Transcription
1 SPECIAL REPORT Highlights from the American College of Rheumatology Meeting Chicago, IL 2011 > Highlights of the American College of Rheumatology 2011 Annual Scientific Meeting > First Lupus Nephritis Diagnosis and Management Guidelines Released > The Latest Treatment Advances for Lupus > Update on Biomarkers > Women with Lupus Can Have a Safe Pregnancy and Healthy Baby > Vitamin D: Intriguing Data > Systemic Lupus Erythematosus Registries: Providing Critical Information > Cognitive Function in Systemic Lupus Erythematosus
2 ACR SPECIAL REPORT Highlights of the American College of Rheumatology 2011 Annual Scientific Meeting While research continues on new therapies for lupus, the 2011 American College of Rheumatology (ACR) was particularly notable for its insights into how to design such trials, according to ALR Scientific Advisory Board Chair Mary (Peggy) K. Crow, MD. One of the most important and, yes, even exciting presentations came from David Wofsy, MD, professor of rheumatology at the University California-San Francisco, she said. Dr. Wofsy s presentation, highlighted here, provided a what if perspective on the abatacept clinical trial for lupus nephritis, demonstrating that the trial would have had far better outcomes if its primary endpoints had been less restrictive. The exercise demonstrates the importance of choosing the most appropriate outcome measures for clinical trials in lupus, Dr. Crow said, and reiterates the learning curve that still exists in designing clinical trials for what is a very complex and heterogenous disease. It s a message to keep an open mind and understand that we still don t know the best way to design the trials yet, but that we re learning all the time, she said. She also found hope in the results of a large pregnancy study presented at the meeting by ALR grantees Jane Salmon, MD, of the Hospital for Special Surgery in New York, and Jill Buyon, MD, of New York University School of Medicine. The study showed that pregnancy in women with lupus was far safer than previously thought. The message is that pregnancy is certainly an option for lupus patients, Dr. Crow said. The study also demonstrated the growing importance of lupus registries of real-life patients in improving our understanding of the disease, its complications, and its progression. On the basic science side, Dr. Crow highlighted the numerous studies focused on the role of the innate immune system the part of the immune system designed to respond immediately to threats, versus the adaptive immune system, which takes longer to mount a response in lupus. The role of the innate immune system, which was not really recognized as being so significant in lupus pathogenesis not so long ago, continues to be stronger and stronger, she said. Of particular interest, she said, is the role of neutrophils and immune system nucleic acid-containing complexes that activate the innate immune system, contributing to the production of pro-inflammatory chemicals such as interferon. More advanced, however, is work on biomarkers in lupus. Dr. Crow highlighted a study she and her colleagues presented, which linked clinical features to gene and protein expression patterns. Once validated in additional studies, she said, it could eventually help clinicians predict the course of the disease and select therapies based on the molecular characteristics of the disease and the patient, just as we re starting to do today in cancer. More information about lupus and treatment advances can be found by visiting Alliance for Lupus Research. All Rights Reserved. 2
3 First Lupus Nephritis Diagnosis and Management Guidelines Released About 60% of people with lupus will eventually develop lupus-related kidney disease, or lupus nephritis. Yet until now, there was no consensus on the best way to diagnose and manage this common complication. That changed at ACR, when ALR grantee and ALR Scientific Advisory Board (SAB) member Bevra H. Hahn, MD, of the University of California-Los Angeles School of Medicine, presented new guidelines from the American College of Rheumatology. The guidelines, which will be published in an upcoming issue of Arthritis & Rheumatism, were developed by a 24-member committee that Dr. Hahn headed, and vetted by separate task force. The guidelines recommend that physicians perform kidney biopsies on anyone with clinical signs of the disease; classify the disease stage based on the International Society of Nephrology /Renal Pathology Society s classification; and treat the disease based on that classification. Other key recommendations include: All patients should receive hydroxychloroquine, which can reduce the risk of long-term kidney damage Patients may receive azathioprine during the induction phase of therapy, but it should no longer be used as maintenance therapy Patients with a proteinuria of 0.5 g/24 hours or greater, or an equivalent protein/creatinine ratio, should receive an angiotensin converting enzyme inhibitor (ACEi) or angiotensin receptor blocker Patients should maintain a blood pressure of 130/80 mmhg or less Patients with low-density lipoprotein (LDL) cholesterol levels greater than 100 mg/dl should receive a statin Women of reproductive age should be counseled about pregnancy For induction therapy, the panel recommended either mycophenolate mofetil (MMF) or cyclophosphamide (CYC) in patients with Class III/IV lupus nephritis, but MMF (2-3 g/day for 6 months) for African Americans and Hispanics. All patients should also receive a glucocorticoid IV pulse for 3 days, then prednisone 0.5-1mg/kg per day with eventual tapering to the lowest effective dose. The guidelines also offer a choice of induction method for CYC: either the low-dose, Euro-Lupus regimen (500 mg IV every 2 weeks for 6 weeks) for those of Caucasian or European background; or the high-dose, National Institutes of Health regimen ( mg/m 2 body surface area monthly for 6 months). Both provide similar improvement according to several studies. If patients fail the first induction drug, they should be switched to the other. If patients fail both MMF and CYC, the guidelines suggest starting rituximab (Rituxan ) or calcineurin inhibitors. Several openlabel trials suggest that rituximab may benefit patients with lupus nephritis. Once the acute phase of the disease improves, doctors can maintain patients on MMF (1-2 g/day) or azathioprine (2 mg/kg/d ± low dose). Patients with Class V membranous lupus nephritis should start on MMF (2-3 g daily for 6 months) plus prednisone (0.5 mg/kg/day for 6 months) and, if they improve, receive maintenance therapy 3
4 with MMF or azathioprine. If they do not improve, they should be started on CYC ( mg/m 2 monthly for 6 months), plus a glucocorticoid pulse followed by daily prednisone ( mg/kg/day). The guidelines also address management in pregnant women. Women with a history of Class III or higher disease do not require treatment if there is no evidence of disease activity. Those with mild disease activity should receive hydroxychloroquine (200mg-400mg daily), while those with clinically active disease should receive prednisone at doses required to suppress activity and, if necessary, azathioprine not to exceed 2/mg/kg/day). These are only guidelines designed to remind clinicians of what is good clinical practice, said Dr. Hahn. Clinicians can still treat individual patients outside of the recommendations, she said. Key point: These guidelines represent the best evidence to date about available treatments for lupus nephritis. If your doctor is not treating your disease according to these recommendations, share them and discuss possible treatment changes. 4
5 The Latest Treatment Advances for Lupus IFNa-Kinoid: A Vaccine for Lupus? What if there was a vaccine that could harness the strength of your own immune system to block the activity of a key protein involved in the underlying disease processes of lupus? It s not as far-fetched as it sounds. Researchers from the Université Catholique de Louvain, in Brussels, Belgium, presented intriguing data from a small study in 28 patients with mild-to-moderate lupus, 21 of whom received a novel immunotherapy treatment called IFN-a-kinoid, 7 of whom received a placebo. i The injectable treatment, from Paris-based pharmaceutical company NeoVacs, blocks production of interferon alpha (IFNa) inflammatory cytokines. These cytokines stimulate the autoantibody response that forms the hallmark of lupus pathology. Participants received increasingly higher doses of the study drug, with the initial, lowest dose injected at the trial s start; a slightly higher dose a week later; another, higher dose at 1 month; and the final, highest dose, given to half the participants at day 84. All participants were followed for 3 to 15 months. Other than some mild irritation at the injection site, there were no significant side effects. All patients produced antibodies against IFNa, demonstrating that the vaccine had the desired immunological effect. Participants whose blood was positive for the IFNa signature demonstrated a significant reduction in IFNa activity compared to those receiving a placebo (about two-thirds of those with lupus have the IFNa signature). In addition, there was a statistically significant improvement in complement C3, a biomarker for disease activity; a reduction of the IFNa gene signature; and lower levels of dsdna antibodies, all of which suggest a reduction in disease activity. The results were so encouraging, researchers said, that they plan to begin Phase II trials in 2012 to assess the ability of the vaccine to prevent lupus flares. Key point: Another possible treatment for lupus is moving forward to the next phase of clinical trials. Belimumab (Benlysta) for Renal Nephritis In 2011, belimumab (Benlysta) became the first new drug approved to treat lupus in more than 50 years. Now researchers are investigating its potential for lupus nephritis. In a study presented at ACR, researchers evaluated various kidney-related biomarkers from patients who participated in the large, phase III trials that led to the drug s approval. None had severe active lupus nephritis, nor were the studies designed to evaluate the effect of belimumab on kidney function. Nonetheless, in this secondary analysis researchers found that individuals who received belimumab for 1 year had lower rates of renal flares, higher rates of lupus nephritis remission, and shorter time to first renal remission than those who received standard therapy (the control group). They also showed greater improvement in proteinuria (a marker of kidney dysfunction) and improvement on 2 assessments used to measure renal function, the SELENA-SLEDAI and BILAG. Key point: Belimumab may have some benefits for people with lupus nephritis. Human Genome Sciences, which developed belimumab, is considering whether to begin clinical trials focused on patients with lupus nephritis. 5
6 Update on Belimumab (Benlysta) Several posters presented during the ACR meeting highlighted long-term safety and efficacy outcomes from the pivotal clinical trials that led to belimumab s (Benlysta) approval. In one study, researchers combined data from 3 clinical trials involving 2,133 patients who received belimumab for 76 weeks. Researchers found similar rates of adverse events overall between the group that received a placebo and the group that received belimumab; as well as similar numbers of participants in each group who dropped out of the trial because of adverse events. Participants receiving belimumab did have higher rates of depression, infection, and reactions at the infusion site than the placebo group. ii Key point: The safety data seen in individual belimumab trials remained relevant even when all the trials were combined. A second study assessed safety and efficacy data in individuals who had been receiving belimumab for 6 years. iii Researchers reported on 296 patients with lupus who participated in the original clinical trials for the drug. After 6 years, 208 remained on the drug. Rates of adverse events remained stable or actually declined over the time period. Participant scores on the SLE Responder Index (SRI), which measures disease activity, continued to improve, while the number of patients experiencing new flares fell. After the first year on the drug, 38% of patients receiving belimumab experienced at least one BILAG A or 2 new B flares compared to 44% of those receiving a placebo; but by year 6, that figure had dropped to 11%. In addition, while after 1 year on belimumab 84% of participants had experienced an SRI flare (17% severe) compared to 85% (19% severe) on placebo, after 6 years that had dropped to 42% (5% severe). Long-term participants also demonstrated increases in complement C3 or C4 and declining autoantibody levels over the time period. In addition, patients required about a third fewer corticosteroids after 6 years than when they first joined the trial. Key point: Belimumab appears safe over time, with continued increases in efficacy. In a third study, researchers evaluated various biomarkers in clinical trial participants who received belimumab for 1 year. iv They found that individuals with higher baseline disease activity (a SELENA- SLEDAI score of 10 or higher); positive anti-dsdna levels; and low complement (C3/C4) all responded better to belimumab than those with lower disease activity. In other words, the sicker the patients, the greater their response. Patients with positive anti-dsdna and low complement had response rates of 51.5% with the 10 mg/kg dose compared to response rates of 31.7% with placebo. That compares to overall response rates of all participants receiving belimumab of 50.6% and 38.8% for placebo. Key point: Patients with greater disease activity are more likely to respond to belimumab therapy than those with mild-to-moderate disease activity. In a fourth study, researchers evaluated the effects of lupus on health-related quality of life, a measure that addresses numerous areas in which the disease impacts the quality of life. v Other studies find that the impact of lupus is as significant as that of AIDS, rheumatoid arthritis, diabetes, and congestive heart failure. In the clinical trials that led to the approval of belimumab, participants quality of life was assessed at the beginning of the trial and again at the end. When researchers evaluated that data, they found that those who benefitted the most from the drug also saw their quality of life improve the greatest. For instance, in response to the question: Compared with 1 year ago, how are you today? 76% of responders said they were somewhat/much better and 34% responded that they were much better compared to just 33.5% and 14.6% of nonresponders, respectively. Responders also experienced significant improvements in fatigue. Key point: People who respond to belimumab experience not just disease improvement, but improvement in their overall quality of life, including less fatigue. 6
7 Abatacept (Orencia) for Lupus Nephritis One reason it has been so difficult to find effective treatments for lupus and lupus nephritis is related not to the potential efficacy of the medication itself, but to the design of clinical trials developed to assess these investigational compounds. That became quite clear during two presentations this year. Efficacy and Safety of Abatacept Over 12 Months in Patients with Lupus Nephritis: Results From a Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase II/III Study In the first, researcher Richard Furie, MD, of the North Shore-Long Island Jewish Health System in Lake Success, NY, presented the result of a 12-month study of abatacept in 228 patients with lupus nephritis who also received mycophenolate mofetil (MMF). vi The study found no significant improvement compared to participants who did not receive the study drug. One bright spot is that the 122 patients with nephrotic lupus nephritis, a more serious form of the disease, experienced a greater improvement in their urinary protein-to-creatinine ratio in the second 6 months than those in the placebo group, suggesting that more study should be done in this subset of patients. There were no significant safety issues related to abatacept. Abatacept for Lupus Nephritis: Alternative Outcome Measures Support Opposing Interpretations of Data From a Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase II/III Study In the second presentation, researcher David Wofsy of the VA Medical Center in San Francisco showed that the results would have been far different if different endpoints, or outcomes, were used. vii In the original study, patients had to achieve a complete response on two successive visits, defined as an egfr (a test used to measure kidney status) within 10% of the pre-treatment value; a urine protein-tocreatinine ratio less than 30 mg/mmol; and normal urine sediment. This requirement, said Dr. Wofsy, is a very high bar to meet, with few, if any, other studies in lupus nephritis requiring such stringent values or requiring that they be met on two successive visits. Dr. Wofsy s analysis used a less stringent but still clinically relevant definition of complete response (which is currently being used in another lupus nephritis trial). It required a blood creatinine level that was either normal or less than or equal to 125% of the baseline ratio; a urine protein/creatinine ratio less than 0.5 mg/mg; and a prednisone dose less than or equal to 10 mg/day at the end of the study. When this criteria was used, he reported, there were significant improvements between those patients who received the study drug and those who did not, particularly among patients with the most severe nephritis when the study began. We are locked into a language that is not ours, Dr. Wofsy said after presenting the data. Phrases like complete and partial response come from the oncology world, he noted. We need to get away from this language that doesn t describe our disease. Key point: Abatacept may have some benefit for patients with lupus nephritis; but, more importantly, clinical trial endpoints significantly impact trial results. Looking Down the Road: New Therapies Under Investigation for Lupus What s next in lupus therapies? Here s a look at one compound still in the very early stages of investigation as a lupus treatment. The results of this trial were presented at the 2011 ACR meeting. Sirukumab This human monoclonal IgG1 kappa antibody is being developed by Centocor Research & Development, a division of Johnson & Johnson Pharmaceutical. It targets and inhibits interleukin-6, 7
8 which plays a role in autoimmune diseases like lupus. The study presented at ACR was designed to evaluate the safety and pharmacokinetics of various dosages of sirukumab in 33 patients with cutaneous lupus or 15 patients with systemic lupus erythematosus (SLE). The study showed that the drug was generally safe in both groups, with all patients exhibiting decreases in complement C3 and C4. None had any flares, nor did any participants develop antibodies to the drug itself. viii Sirukumab is also being investigated in a Phase II trial for lupus nephritis. Key point: This study highlights the potential of another drug to treat lupus. 8
9 Update on Biomarkers A holy grail of lupus is to identify a panel of blood tests that can tell physicians how a patient s disease will progress and what treatments would be most beneficial. This is particularly difficult given that the disease presents so differently in so many patients. But a poster presented at the ACR meeting by researchers at the Hospital for Special Surgery in New York City, including ALR Scientific Advisory Board Chair Mary Peggy Crow, MD, reports on a recent study that may eventually provide that information. ix The researchers conducted genetic analyses and measured levels of 44 autoantibodies and proinflammatory cytokines on 169 blood samples from 23 patients with lupus and 5 healthy donors that had been collected over 3 years. The two most common gene signatures were type 1 interferon (IFN-1) inducible genes and neutrophil granule-related genes. Patients with neither gene signature had only mild disease; while those with both the IFN-1 and neutrophil signatures were more likely to experience vascular complications such as cardiovascular and kidney damage, and much higher levels of anti-ssa/ro autoantibodies. Patients who expressed the IFN-1 signature only were more likely to have mucocutaneous manifestations, such as those affecting the skin, scalp, and nails, and much higher levels of tumor necrosis factor. Key point: This study provides promising data on the potential of a biomarker panel to identify subsets of lupus disease that could guide treatment decisions. Additional clinical trials are necessary, however. 9
10 Women with Lupus Can Have a Safe Pregnancy and Healthy Baby One of the most devastating effects of lupus is that it often affects women during their childbearing years. For years, doctors warned women with lupus to avoid pregnancy, citing high rates of complications during pregnancy and delivery, as well as risks to the baby. But the results of a major study presented at the ACR found that women whose disease is stable even those with lupus nephritis can have a healthy pregnancy, delivery, and baby. The Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus (PROMISS) is designed to find ways to predict the likelihood of problems in women with lupus who become pregnant. Researchers, including ALR grantees Jill Buyon, MD of New York University Medical Center and Jane Salmon, MD, of the Hospital for Special Surgery in New York City, are following 4 groups of women: those with lupus, those with lupus and antiphospholipid syndrome, those with antiphospholipid syndrome only, and healthy women who have already had at least 1 healthy pregnancy and delivery. The study presented at ACR was based on 333 pregnancies. Most of the women with lupus had markers of serious disease, including a history of lupus nephritis, the presence of anti-dna autoantibodies, antiphospholipid antibodies, and/or low complement levels. Overall, researchers reported, about 19% of the women with lupus experienced some type of complication during their pregnancy or delivery compared to 5% of healthy women. However, the complication rate in the general population is about 10%, and other studies have reported complication rates in women with lupus as high as 30%. Those studies, however, had a broader definition of complications than this one, Dr. Buyon noted. In this study, complications were defined as miscarriage, stillborn birth, early delivery (before 36 weeks), newborns who were small for their gestational age, and preeclampsia in the mother. The extraordinary news, said Dr. Buyon during a press conference about the study, is that just 15 patients (4%) had a severe flare during their pregnancy, while just 18% experienced a mild-tomoderate flare. Few of the women required steroids during their flares. Women who had slightly higher disease activity when they got pregnant; anticoagulant antibodies; slightly higher uric acid in the second and third trimesters; and a smaller complement increase as the pregnancy progressed were most likely to develop complications, as were women with a past history of kidney disease. What the study says is that even patients with a history of lupus nephritis can do well during pregnancy, Dr. Buyon said, if they wait to become pregnant until their disease is stable and are closely managed by obstetrical specialists. x Key point: It is possible to have a healthy pregnancy and a healthy baby if you work closely with your doctor on the timing of pregnancy and are closely monitored throughout pregnancy. 10
11 Vitamin D: Intriguing Data They ve been calling vitamin D the miracle vitamin for years, implicating low levels of the so-called sunshine vitamin in everything from heart disease to autoimmune conditions like multiple sclerosis and, yes, lupus. What is less clear, however, is if supplementing with vitamin D in people with low blood levels has any effect on lupus disease progression or its symptoms. Although the research is still in its infancy, it is providing some intriguing clues. Here s what we learned at this year s meeting: Twenty people with low-to-moderate lupus disease activity and vitamin D deficiency received 100,000 IU of vitamin D a week for 4 weeks, then 100,000 a month for 6 months. xi All demonstrated dramatic improvements in their blood levels of vitamin D levels with no safety issues or negative side effects. Perhaps most important is that levels of aberrant T cells declined after 2 months of supplementation, as did levels of 2 inflammatory cytokines and anti-dna autoantibodies. In addition, the participants demonstrated reduced B-cell activation. These represent very preliminary results, said lead researcher Benjamin Terrier, MD, of the Internal Medicine Department of the Pitié-Salpétrière Hospital in Paris, France, during a press conference. Given the absence of a control group, he said, he couldn t even say for sure that the immunologic effects were related to the vitamin D. However, he also called the results encouraging because they demonstrated the safety of such high doses of vitamin D in people with lupus. Next step: Randomized clinical trials ALR grantee Michelle Petri, MD, of Johns Hopkins University School of Medicine in Baltimore, presented results from another study of vitamin D in lupus patients. She and her colleagues evaluated vitamin D levels in 922 patients between July 2009 and December They found that younger age, higher cholesterol levels, higher systolic (the top number) blood pressure, a higher urine protein/creatinine ratio (a sign of renal problems), and obesity were associated with low vitamin D levels. In addition, patients with low vitamin D levels were more likely to have lupusrelated kidney and cardiovascular damage. However, supplementing with vitamin D had no effect on patients symptoms, although it did lead to a significant reduction in their urine protein/ creatinine ratio and an increase in complement C4 and C4, both signs of disease improvement. There was no effect on anti-dna autoantibodies, however. Yet, Dr. Petri noted, there was a trend towards a reduction in the amount of prednisone patient required to control their symptoms. While the reduction was not statistically significant, it is suggestive that increasing vitamin D levels can impact disease severity. Key point: Supplementing with vitamin D may provide some benefits for people with lupus who have low blood levels of the vitamin, but large, randomized clinical trials are necessary. 11
12 Systemic Lupus Erythematosus Registries: Providing Critical Information Much of what we re learning about lupus these days comes from patient registries, essentially, large databases that contain numerous types of information, including patient symptoms, treatments, side effects, biomarkers, and genetic analyses. They are invaluable for helping to identify possible problems with treatments, discover new treatment possibilities, or highlight certain clues regarding the progression or regression of the disease the deserve additional investigation. You don t have to sign up to be in a registry; all information is anonymous and doctors provide the data. In addition to the pregnancy study highlighted here, other registry-related studies included: Biologics use in SLE in 23 Centers Data from the International Registry for Biologics In SLE (IRBIS) Although belimumab (Benlysta) is the only approved biologic drug for lupus, physicians use numerous such agents off label. This study analyzed the use of biologics among 359 patients in 23 centers participating in the International Registry for Biologics in SLE (IRBIS). The study found that rituximab (Rituxan) was the main biologic used. Other patients were treated with belimumab, epratuzumab, abatacept (Orencia), etanercept (Enbrel), and adalimumab (Humira), some after participating in clinical trials for the drug. The researchers focused on the outcomes in those patients taking rituximab, finding that about 40% were also receiving cyclophosphamide. Most patients were started on rituximab because they had developed lupus nephritis or blood-related complications. After one year, patients on rituximab had improved SLEDAI scores and taking lower doses of glucocorticosteroids, even without adding any other immunosuppressants. xii Key point: Rituximab can provide important benefits for patients with lupus that is not responding to traditional immunosuppressant drugs. Large Scale Analysis of Serum Tumor Necrosis Factor Alpha Levels in Systemic Lupus Erythematosus Blood levels of tumor necrosis alpha (TNFa) are high in some patients with lupus but not in others. Researchers studied 653 patients from the Lupus Family Registry and Repository at the Oklahoma Medical Research Foundation to evaluate any correlation between TNF-a levels and disease severity. They found that higher levels of TNF-a were associated with higher levels of the inflammatory protein interferon alfa (IFN-a) regardless of the patient s ethnic background. However, African Americans were most likely to have the high TNF-a/IFN-a levels, with European-Americans least likely, while European Americans had the greatest proportion of low TNF/low IFN. While IFN-a was associated with the characteristic autoantibodies of lupus, only TNF-a was associated with IFN-a, suggesting some relationship between TNF-a and the characteristics of the disease. xiii Key point: TNF-a levels may provide an important biomarker for tracking lupus disease progression. More studies are needed, however. The Georgia Lupus Registry: Differences in Age-Specific Incidence Rates Between Black and White Females with SLE The Georgia Lupus Registry is a population-based registry designed to estimate the incidence and prevalence of lupus in Atlanta, GA. Researchers reviewed 8,000 medical records and found
13 cases of lupus. Based on census data for the region, they determined that African-American women developed lupus earlier than white women, with the rate increasing more dramatically at puberty and peaking between ages 30 and 39. Rates among white women, however, increased more gradually and peaked between ages 60 and 69. Key point: Although lupus is often considered a disease that affects young women, hormones may have a greater affect on its development in black women than white women. Status and Missing Days At Work in a Large Population of Patients with Systemic Lupus Erythematosus (SLE) Researchers from Toronto Western Hospital in Canada used a large lupus registry to evaluate how the disease affected patients ability to work. Of the 1,242 individuals evaluated, with an average age of 40.5, about half were not working, although 80% had previously worked. Among those working, 30% had missed work since their last doctor visit, and half said that their illness make it difficult for them to do their job. Researchers found that the longer patients had the disease, the greater their disease activity, and the more troublesome symptoms they had, such as fatigue, weakness, and difficulty thinking, the more likely they were to miss work. xiv Key point: The researchers note that improving work-related interventions could reduce disability among individuals who want to work, but whose lupus interferes in their ability to work. 13
14 Cognitive Function in Systemic Lupus Erythematosus It has been known for years that lupus affects the central nervous system (CNS) as well as other organ systems, resulting in problems with thinking, remembering, and learning. These cognitive issues, along with other neurologic manifestations such as seizures and psychosis, affect about 80% of patients with lupus, said Robin L. Brey, MD, of the University of Texas Health Science Center in San Antonio, while about 70% of those with lupus experience mood disorders. Her comments came during a talk on cognitive issues in patients with lupus. Neuropsychiatric lupus (NPSLE) can even occur without any other evidence of the disease, making it difficult to know if the neurological manifestation is due to lupus or some other condition, she said. In fact, a host of other factors can influence cognitive function in normal people and in patients with autoimmune diseases, including sleep, social and cultural issues, psychiatric disorders such as depression and anxiety, preexisting or lupus-related central-nervous system (CNS) disorders, brain damage, medications, metabolic abnormalities and infection. The challenge for us is determining whether or not the neurological symptoms are due to the lupus, are primary central-nervous system (CNS) mediated, lupus-mediated CNS pathology, or whether they are secondary to other conditions, she said. It may also be that the autoimmune dysfunction itself triggers the cognitive dysfunction, with some studies finding similar levels of cognitive dysfunction in patients with rheumatoid arthritis, multiple xv, xvi sclerosis, and lupus. Does this mean that cognitive dysfunction in these patient groups is not related to the underlying disease? Dr. Brey asked. I say no, it doesn t mean that at all. Instead, she suggested that the underlying inflammation of all three diseases plays a role. Thus, she said, the quest for looking for a specific cognitive dysfunction pattern in lupus that is not seen in other disease entities will stop us in our tracks in terms of progress. Instead, what these studies show is that the result of inflammatory damage to the brain can be cognitive dysfunction. It is a process that you can come to by several different mechanisms. Brain involvement in lupus may also be related to the damage that occurs in tiny blood vessels throughout the body, including those in the brain. In addition, she said, there also appears to be dysfunction in the blood-brain-barrier, which may allow autoantibodies access to the brain, leading to the production pro-inflammatory proteins further damaging the brain. In the San Antonio Lupus Study of Neuropsychiatric Disease (SALUD) study she and her colleagues conducted, in which 65% of the patients were Hispanic, depression, disease activity, prednisone use, persistently positive antibodies, higher SLEDAI scores, were all risk factors for cognitive dysfunction, as was Hispanic ethnicity. xvii One reason Hispanics may have poorer cognitive function, she said, is that these patients are sicker overall and the effects of lupus in the brain are more pronounced. 14
15 References i Houssiau FA, et al. Active Immunization Against IFN with IFN-Kinoid in SLE Patients Is Safe, Immunogenic and Induces Down-Regulation of IFN-Mediated Genes. Abstract ii iii Wallace DJ, et al. Safety Profile of Belimumab, a B-Lymphocyte Stimulator Specific Inhibitor, in Phase 2 and 3 Clinical Trials of Patients with Active Systemic Lupus Erythematosus. Abstract 578 Merrill JT, et al. Sustained Disease Improvement and Safety Profile Over the 1500 Patient-Year Experience (6 years) with Belimumab in Patients with Systemic Lupus Erythematosus. Abstract 584. iv Van Vollenhoven RF, et al. Factors Associated with Belimumab Treatment Benefit: Results From Phase 3 Studies in Patients with Systemic Lupus Erythematosus. Abstract v vi vii Strand V. Responders in the Phase 3 Belimumab Clinical Trials in Patients with Systemic Lupus Erythematosus Reported Improvements in Fatigue and Health-Related Quality of Life At Week 52. Abstract Furie R, et al. Efficacy and Safety of Abatacept Over 12 Months in Patients with Lupus Nephritis: Results From a Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase II/III Study. Abstract Wofsy D, et al. Abatacept for Lupus Nephritis: Alternative Outcome Measures Support Opposing Interpretations of Data From a Multicenter, Randomized, Double-Blind, Placebo-Controlled Phase II/III Study. Abstract viii Szepietowski JC, et al. A Phase 1, Randomized, Double-Blind, Placebo-Controlled, Multiple, Intravenous, Ascending-Dose Study of Sirukumab in Patients with Cutaneous or Systemic Lupus Erythematosus. Abstract ix Olferiev M. Analysis of Longitudinal Gene and Protein Expression Data Classifies SLE Patients Based on Molecular Profiles Associated with Disease Activity, Serology and Specific Organ Manifestations. Abstract x Buyon JP, et al. Favorable Prognosis in a Large, Prospective Multicenter Study of Lupus Pregnancies. Abstract xi Terrier et al. Restoration of Regulatory T Cells-Th17 Cells Balance in Systemic Lupus Erythematosus Through Vitamin D Supplementation. Abstract 577. xii Van Vollenhoven RF, et al. Biologics use in SLE in 23 Centers - Data from the International Registry for Biologics In SLE (IRBIS). Abstract 609. xiii Weckerle CE. Large Scale Analysis of Serum Tumor Necrosis Factor Alpha Levels in Systemic Lupus Erythematosus. Abstract xiv Morrison S, et al. Status and Missing Days At Work in a Large Population of Patients with Systemic Lupus Erythematosus (SLE). Abstract xv Hanly JG, Omisade A, Su L, Farewell V, Fisk JD. Assessment of cognitive function in systemic lupus erythematosus, rheumatoid arthritis, and multiple sclerosis by computerized neuropsychological tests. Arthritis Rheum. 2010;62(5): xvi Antonchak MA, Saoudian M, Khan AR, Brunner HI, Luggen ME. J Rheumatol. 2011;38(6): xvii McLaurin EY, Holliday SL, Williams P, Brey RL. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology Jan 25;64(2): More information about lupus and treatment advances can be found by visiting The Alliance for Lupus Research Special Report on the 2011 American College of Rheumatology Meeting was made possible in part by generous support from Genentech Alliance for Lupus Research. All Rights Reserved. Contents herein may not be reproduced, republished or distributed without the prior written permission of the Alliance for Lupus Research. To request permission to reproduce, republish or distribute any part of this report, contact us at or info@lupusresearch.org. 15
Committee Approval Date: May 9, 2014 Next Review Date: May 2015
 Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Lupus Related Kidney Diseases. Jason Cobb MD Assistant Professor Renal Division Emory University School of Medicine October 14, 2017
 Lupus Related Kidney Diseases Jason Cobb MD Assistant Professor Renal Division Emory University School of Medicine October 14, 2017 Financial Disclosures MedImmune Lupus Nephritis Kidney Biopsy Biomarkers
Lupus Related Kidney Diseases Jason Cobb MD Assistant Professor Renal Division Emory University School of Medicine October 14, 2017 Financial Disclosures MedImmune Lupus Nephritis Kidney Biopsy Biomarkers
Lupus. Fast facts. What is lupus? What causes lupus? Who gets lupus?
 Lupus Systemic lupus erythematosus, referred to as SLE or lupus, is sometimes called the "great imitator." Why? Because of its wide range of symptoms, people often confuse lupus with other health problems.
Lupus Systemic lupus erythematosus, referred to as SLE or lupus, is sometimes called the "great imitator." Why? Because of its wide range of symptoms, people often confuse lupus with other health problems.
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS
 Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
Arthritis & Rheumatology Clinics of Kansas PATIENT EDUCATION SYSTEMIC LUPUS ERYTHEMATOSUS Introduction: There is perhaps no rheumatic disease that evokes so much fear and confusion among both patients
Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study.
 1. Title Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study. 2. Background Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune and
1. Title Efficacy and Safety of Belimumab in the treatment of Systemic Lupus Erythematosus: a Prospective Multicenter Study. 2. Background Systemic Lupus Erythematosus (SLE) is a chronic, autoimmune and
Aurinia Pharmaceuticals Announces Voclosporin Meets Primary Endpoint in Phase IIB AURA-LV Study in Lupus Nephritis
 August 15, 2016 Aurinia Pharmaceuticals Announces Voclosporin Meets Primary Endpoint in Phase IIB AURA-LV Study in Lupus Nephritis Conference call and webcast at 8am ET First therapeutic agent to meet
August 15, 2016 Aurinia Pharmaceuticals Announces Voclosporin Meets Primary Endpoint in Phase IIB AURA-LV Study in Lupus Nephritis Conference call and webcast at 8am ET First therapeutic agent to meet
Taming the wolf: Treating to target, treating to remission new strategies for SLE
 Taming the wolf: Treating to target, treating to remission new strategies for SLE Ronald van Vollenhoven Seattle, April 28, 2017 Disclosures Research support, consultancy: Abbott (AbbVie), Biotest, BMS,
Taming the wolf: Treating to target, treating to remission new strategies for SLE Ronald van Vollenhoven Seattle, April 28, 2017 Disclosures Research support, consultancy: Abbott (AbbVie), Biotest, BMS,
Corporate Presentation January 2013
 NASDAQ: ANTH Corporate Presentation January 2013 1 Forward Looking Statements This presentation contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as
NASDAQ: ANTH Corporate Presentation January 2013 1 Forward Looking Statements This presentation contains "forward-looking" statements within the meaning of Section 27A of the Securities Act of 1933, as
Lupus and Your Kidneys. Michael P. Madaio, MD Professor of Medicine Chairman of Medicine Medical College of Georgia Augusta, Georgia
 Lupus and Your Kidneys Michael P. Madaio, MD Professor of Medicine Chairman of Medicine Medical College of Georgia Augusta, Georgia Kidney Inflammation and Abnormal Function as a Result of Lupus (Lupus
Lupus and Your Kidneys Michael P. Madaio, MD Professor of Medicine Chairman of Medicine Medical College of Georgia Augusta, Georgia Kidney Inflammation and Abnormal Function as a Result of Lupus (Lupus
* Autoantibody positive (i.e. antinuclear antibody or ANA titre 1:80 or anti-double stranded (ds) DNA antibody 30 IU/mL)
 Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
Benlysta (belimumab) Policy Number: 5.02.509 Last Review: 05/2018 Origination: 06/2011 Next Review: 05/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Benlysta
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Benlysta (belimumab) Prior Authorization Criteria Program Summary
 Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Identification of clinical and serological factors during induction treatment of lupus nephritis that are associated with renal outcome
 To cite: Dall Era M, Levesque V, Solomons N, et al. Identification of clinical and serological factors during induction treatment of lupus nephritis that are associated with renal outcome. Lupus Science
To cite: Dall Era M, Levesque V, Solomons N, et al. Identification of clinical and serological factors during induction treatment of lupus nephritis that are associated with renal outcome. Lupus Science
Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab
 (2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
(2016) 25, 346 354 http://lup.sagepub.com PAPER Elevated BLyS levels in patients with systemic lupus erythematosus: Associated factors and responses to belimumab DA Roth 1, A Thompson 2, Y Tang 2*, AE
ONE of the following:
 Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Learning about Lupus. Learning About Lupus. Lupus Society of Illinois
 Learning About Lupus Learning about Lupus Lupus Society of Illinois 525 W. Monroe Street, Suite 900 Chicago, Illinois 60661 Robert S. Katz, M.D. Professor of Medicine Rush University Medical Center Northwestern
Learning About Lupus Learning about Lupus Lupus Society of Illinois 525 W. Monroe Street, Suite 900 Chicago, Illinois 60661 Robert S. Katz, M.D. Professor of Medicine Rush University Medical Center Northwestern
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December Reference : NHSCB/A3C/1b
 Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Rheumatoid Arthritis: Counseling Women Who Are Trying to Conceive
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience
 Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience References Sanna G, Bertolaccini ML. Neuropsychiatric manifestations
Neuropsychiatric Systemic Lupus Erythematosus (NPSLE) Case presentations and topic discussion The Rheumatology Unit UMMC experience References Sanna G, Bertolaccini ML. Neuropsychiatric manifestations
SYSTEMIC LUPUS ERYTHEMATOSUS: CURRENT CONCEPTS AND CLINICAL PEARLS. Dr Sheila Vasoo Consultant Division of Rheumatology NUHS
 SYSTEMIC LUPUS ERYTHEMATOSUS: CURRENT CONCEPTS AND CLINICAL PEARLS Dr Sheila Vasoo Consultant Division of Rheumatology NUHS Listen to the Patient Concepts Diagnosis Immunopathogenesis Clinical Pearls Disease
SYSTEMIC LUPUS ERYTHEMATOSUS: CURRENT CONCEPTS AND CLINICAL PEARLS Dr Sheila Vasoo Consultant Division of Rheumatology NUHS Listen to the Patient Concepts Diagnosis Immunopathogenesis Clinical Pearls Disease
ENFERMEDADES AUTOINMUNES SISTÉMICAS. Dr. J. María Pego Reigosa
 ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. J. María Pego Reigosa ABSTRACT NUMBER: 888 PHASE 3 TRIAL RESULTS WITH BLISIBIMOD, A SELECTIVE INHIBITOR OF B-CELL ACTIVATING FACTOR, IN SUBJECTS WITH MODERATE-TO-SEVERE
ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. J. María Pego Reigosa ABSTRACT NUMBER: 888 PHASE 3 TRIAL RESULTS WITH BLISIBIMOD, A SELECTIVE INHIBITOR OF B-CELL ACTIVATING FACTOR, IN SUBJECTS WITH MODERATE-TO-SEVERE
Lupus nephritis. Vladimir Tesar Department of Nephrology, General University Hospital, Prague, Czech Republic
 Lupus nephritis Vladimir Tesar Department of Nephrology, General University Hospital, Prague, Czech Republic Disclosure of Interests Abbvie, Amgen, Baxter, Bayer, Boehringer-Ingelheim, Calliditas, Chemocentryx,
Lupus nephritis Vladimir Tesar Department of Nephrology, General University Hospital, Prague, Czech Republic Disclosure of Interests Abbvie, Amgen, Baxter, Bayer, Boehringer-Ingelheim, Calliditas, Chemocentryx,
REMISSION OF ACTIVE LUPUS NEPHRITIS WITH VOCLOSPORIN: RESULTS OF THE AURA-LV STUDY
 REMISSION OF ACTIVE LUPUS NEPHRITIS WITH VOCLOSPORIN: RESULTS OF THE AURA-LV STUDY V. Dobronravov* 1, M. A. Dooley 2, S. A. Haq 3, I. Adzerikho 4, O. Bugrova 5, D. Isenberg 6, F. Houssiau 7, N. Solomons
REMISSION OF ACTIVE LUPUS NEPHRITIS WITH VOCLOSPORIN: RESULTS OF THE AURA-LV STUDY V. Dobronravov* 1, M. A. Dooley 2, S. A. Haq 3, I. Adzerikho 4, O. Bugrova 5, D. Isenberg 6, F. Houssiau 7, N. Solomons
Ronald F. van Vollenhoven Unit for Clinical Therapy Research, Inflammatory Diseases (ClinTRID) The Karolinska Institute Stockholm, Sweden
 Ronald F. van Vollenhoven Unit for Clinical Therapy Research, Inflammatory Diseases (ClinTRID) The Karolinska Institute Stockholm, Sweden Disclosures Research Grants: AbbVie, Amgen, BMS, GSK, Pfizer, Roche,
Ronald F. van Vollenhoven Unit for Clinical Therapy Research, Inflammatory Diseases (ClinTRID) The Karolinska Institute Stockholm, Sweden Disclosures Research Grants: AbbVie, Amgen, BMS, GSK, Pfizer, Roche,
UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS
 UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. October 20, 2012 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement Diagnosis Treatment Pregnancy
UNDERSTANDING SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. October 20, 2012 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement Diagnosis Treatment Pregnancy
Additional file 2: Details of cohort studies and randomised trials
 Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
Reference Randomised trials Ye et al. 2001 Abstract 274 R=1 WD=0 Design, numbers, treatments, duration Randomised open comparison of: (45 patients) 1.5 g for 3, 1 g for 3, then 0.5 to 0.75 g IV cyclophosphamide
SELECTED ABSTRACTS. All (n) % 3-year GS 88% 82% 86% 85% 88% 80% % 3-year DC-GS 95% 87% 94% 89% 96% 80%
 SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
SELECTED ABSTRACTS The following are summaries of selected posters presented at the American Transplant Congress on May 5 9, 2007, in San Humar A, Gillingham KJ, Payne WD, et al. Review of >1000 kidney
IRACON Management of Lupus Nephritis: Old is gold, New is Trendy
 IRACON 2016 Management of Lupus Nephritis: Old is gold, New is Trendy First episode of LN (III/IV): Induction Low dose is equally considerable as high dose CYC Switch to the other agent if no improvement
IRACON 2016 Management of Lupus Nephritis: Old is gold, New is Trendy First episode of LN (III/IV): Induction Low dose is equally considerable as high dose CYC Switch to the other agent if no improvement
Definition Chronic autoimmune disease The body s immune system starts attacking itself Can affect most organs and tissues in the body Brain, lungs, he
 LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
Erythrocyte-bound C4d in combination with complement and autoantibody status for the monitoring of SLE
 To cite: Merrill JT, Petri MA, Buyon J, et al. Erythrocytebound C4d in combination with complement and autoantibody status for the monitoring of SLE. Lupus Science & Medicine 2018;5:e000263. doi:10.1136/
To cite: Merrill JT, Petri MA, Buyon J, et al. Erythrocytebound C4d in combination with complement and autoantibody status for the monitoring of SLE. Lupus Science & Medicine 2018;5:e000263. doi:10.1136/
LONG-TERM OUTCOME OF PATIENTS WITH LUPUS NEPHRITIS: A SINGLE CENTER EXPERIENCE
 & LONG-TERM OUTCOME OF PATIENTS WITH LUPUS NEPHRITIS: A SINGLE CENTER EXPERIENCE Senija Rašić 1 *, Amira Srna 1, Snežana Unčanin 1, Jasminka Džemidžić 1, Damir Rebić 1, Alma Muslimović 1, Maida Rakanović-Todić
& LONG-TERM OUTCOME OF PATIENTS WITH LUPUS NEPHRITIS: A SINGLE CENTER EXPERIENCE Senija Rašić 1 *, Amira Srna 1, Snežana Unčanin 1, Jasminka Džemidžić 1, Damir Rebić 1, Alma Muslimović 1, Maida Rakanović-Todić
Lupus Nephritis New (?) Treatments. Aurélie HUMMEL Service de Néphrologie Hôpital Necker Enfants-Malades Paris
 Lupus Nephritis New (?) Treatments Aurélie HUMMEL Service de Néphrologie Hôpital Necker Enfants-Malades Paris Introduction Lupus nephritis : 30-50% of patients with lupus = mortality risk factor Mok Series
Lupus Nephritis New (?) Treatments Aurélie HUMMEL Service de Néphrologie Hôpital Necker Enfants-Malades Paris Introduction Lupus nephritis : 30-50% of patients with lupus = mortality risk factor Mok Series
Market Access CTR Summary
 Market Access CTR Summary Study No.: BEL114246 Title: Efficacy of Belimumab Treatment in a Subpopulation of Systemic Lupus Erythematosus (SLE) Patients: A Pooled Analysis of the HGS1006-C1056 (BLISS-52)
Market Access CTR Summary Study No.: BEL114246 Title: Efficacy of Belimumab Treatment in a Subpopulation of Systemic Lupus Erythematosus (SLE) Patients: A Pooled Analysis of the HGS1006-C1056 (BLISS-52)
LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS LUPUS 101 SLE SUBSETS AUTOIMMUNE DISEASE 11/4/2013 HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS
 LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
9/25/2013 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397
 Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
10/25/2018. Autoimmunity and how to treat it. Disclosure. Why do we get autoimmunity? James Verbsky MD/PhD Pediatric Rheumatology/Immunology
 Autoimmunity and how to treat it James Verbsky MD/PhD Pediatric Rheumatology/Immunology Disclosure None I will mention drug names and some brand names but I have no financial interest or any other ties
Autoimmunity and how to treat it James Verbsky MD/PhD Pediatric Rheumatology/Immunology Disclosure None I will mention drug names and some brand names but I have no financial interest or any other ties
Development of SLE among Possible SLE Patients Seen in Consultation: Long-Term Follow-Up. Disclosures. Background. Evidence-Based Medicine.
 Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
Belimumab: Present and future
 Mini Review imedpub Journals http://www.imedpub.com Journal of Autoimmune Disorders Belimumab: Present and future CD Tripathi and Preeta Kaur Chugh Abstract Belimumab, a B lymphocyte stimulator (BLyS)
Mini Review imedpub Journals http://www.imedpub.com Journal of Autoimmune Disorders Belimumab: Present and future CD Tripathi and Preeta Kaur Chugh Abstract Belimumab, a B lymphocyte stimulator (BLyS)
New Drug Evaluation: Belimumab Injection, Intravenous and Subcutaneous
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Understanding Myositis Medications
 Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
AURION STUDY: 48-WEEK DATA OF MULTI-TARGET THERAPY WITH VOCLOSPORIN, MMF AND STEROIDS FOR ACTIVE LUPUS NEPHRITIS
 AURION STUDY: 48-WEEK DATA OF MULTI-TARGET THERAPY WITH VOCLOSPORIN, MMF AND STEROIDS FOR ACTIVE LUPUS NEPHRITIS The 12th International Congress on Systemic Lupus Erythematosus (LUPUS 2017) & the 7th Asian
AURION STUDY: 48-WEEK DATA OF MULTI-TARGET THERAPY WITH VOCLOSPORIN, MMF AND STEROIDS FOR ACTIVE LUPUS NEPHRITIS The 12th International Congress on Systemic Lupus Erythematosus (LUPUS 2017) & the 7th Asian
Cohen: Well, hi to my listeners, this is Dr. Marc Cohen, and I am happy again to discuss with you advances in the efficacy and safety of TNF
 Cohen: Well, hi to my listeners, this is Dr. Marc Cohen, and I am happy again to discuss with you advances in the efficacy and safety of TNF inhibitors. This is a subject of great interest to me and I
Cohen: Well, hi to my listeners, this is Dr. Marc Cohen, and I am happy again to discuss with you advances in the efficacy and safety of TNF inhibitors. This is a subject of great interest to me and I
Uncontrolled Moderate-to-Severe-Asthma: Latest Data from the Floor of CHEST 2018
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
NEWS RELEASE Genentech Contacts: Media: Joe St. Martin (650) Investor: Karl Mahler Thomas Kudsk Larsen (973)
 NEWS RELEASE Genentech Contacts: Media: Joe St. Martin (650) 467-6800 Investor: Karl Mahler 011 41 61 687 8503 Thomas Kudsk Larsen (973) 235-3655 Biogen Idec Contacts: Media: Christina Chan (781) 464-3260
NEWS RELEASE Genentech Contacts: Media: Joe St. Martin (650) 467-6800 Investor: Karl Mahler 011 41 61 687 8503 Thomas Kudsk Larsen (973) 235-3655 Biogen Idec Contacts: Media: Christina Chan (781) 464-3260
ENFERMEDADES AUTOINMUNES SISTÉMICAS. Dr. José María Pego Reigosa
 ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. José María Pego Reigosa ABSTRACT NUMBER: 2754 Comparison of Individually Tailored vs Systematic Rituximab Regimens to Maintain ANCA-Associated Vasculitis Remissions:
ENFERMEDADES AUTOINMUNES SISTÉMICAS Dr. José María Pego Reigosa ABSTRACT NUMBER: 2754 Comparison of Individually Tailored vs Systematic Rituximab Regimens to Maintain ANCA-Associated Vasculitis Remissions:
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC
 Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Update on the Treatment of Rheumatoid Arthritis Sabrina Fallavollita MDCM McGill University Canadian Society of Internal Medicine
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Update on the Treatment of Rheumatoid Arthritis Sabrina Fallavollita MDCM McGill University Canadian Society of Internal Medicine
29/10. Treatment (brand name, manufacturer): For the treatment of: Background: Rituximab (MabThera, Roche) SLE in adults (unlicensed)
 GENERAL POLICY Policy Ref: 29/10 Treatment (brand name, manufacturer): For the treatment of: Background: Commissioning position: Rituximab (MabThera, Roche) SLE in adults (unlicensed) There are frequent
GENERAL POLICY Policy Ref: 29/10 Treatment (brand name, manufacturer): For the treatment of: Background: Commissioning position: Rituximab (MabThera, Roche) SLE in adults (unlicensed) There are frequent
New Drug Evaluation: Belimumab Injection, Intravenous and Subcutaneous
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Recent advances in management of Pulmonary Vasculitis. Dr Nita MB
 Recent advances in management of Pulmonary Vasculitis Dr Nita MB 23-01-2015 Overview of the seminar Recent classification of Vasculitis What is new in present classification? Trials on remission induction
Recent advances in management of Pulmonary Vasculitis Dr Nita MB 23-01-2015 Overview of the seminar Recent classification of Vasculitis What is new in present classification? Trials on remission induction
Actemra (tocilizumab) CG-DRUG-81
 Market DC Actemra (tocilizumab) CG-DRUG-81 Override(s) Prior Authorization Approval Duration 1 year Medications Line of Business Quantity Limit Actemra (tocilizumab) vials VA MCD and All L-AGP May be subject
Market DC Actemra (tocilizumab) CG-DRUG-81 Override(s) Prior Authorization Approval Duration 1 year Medications Line of Business Quantity Limit Actemra (tocilizumab) vials VA MCD and All L-AGP May be subject
Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z
 Topic Page: Systemic Lupus Erythematosus Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z What Is It? Lupus is thought to develop when the immune system
Topic Page: Systemic Lupus Erythematosus Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z What Is It? Lupus is thought to develop when the immune system
Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies
 Original Article Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies Col K Narayanan *, Col V Marwaha +, Col K Shanmuganandan #, Gp Capt S Shankar
Original Article Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies Col K Narayanan *, Col V Marwaha +, Col K Shanmuganandan #, Gp Capt S Shankar
Corporate Medical Policy
 Corporate Medical Policy Rituximab for the Treatment of Rheumatoid Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: rituximab_for_the_treatment_of_rheumatoid_arthritis 4/2008
Corporate Medical Policy Rituximab for the Treatment of Rheumatoid Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: rituximab_for_the_treatment_of_rheumatoid_arthritis 4/2008
ANCA associated vasculitis in China
 ANCA associated vasculitis in China Min Chen Renal Division, Peking University First Hospital, Beijing 100034, P. R. China 1 General introduction of AAV in China Disease spectrum and ANCA type Clinical
ANCA associated vasculitis in China Min Chen Renal Division, Peking University First Hospital, Beijing 100034, P. R. China 1 General introduction of AAV in China Disease spectrum and ANCA type Clinical
EXTENDED REPORT. Clinical and epidemiological research
 1 Allegheny Singer Research Institute, West Penn Allegheny Health System, Temple University School of Medicine, Pittsburgh, Pennsylvania, USA 2 Instituto Nacional de Ciencias Medicas y Nutricion Salvador
1 Allegheny Singer Research Institute, West Penn Allegheny Health System, Temple University School of Medicine, Pittsburgh, Pennsylvania, USA 2 Instituto Nacional de Ciencias Medicas y Nutricion Salvador
Lupus. and the Kidneys
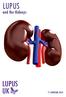 Lupus and the Kidneys LupusuK 2015 This information booklet has been produced by LUPUS UK 2015 LUPUS UK LUPUS UK is the national charity caring for those with systemic lupus erythematosus (SLE) and discoid
Lupus and the Kidneys LupusuK 2015 This information booklet has been produced by LUPUS UK 2015 LUPUS UK LUPUS UK is the national charity caring for those with systemic lupus erythematosus (SLE) and discoid
Importance of Awareness and Research for Lupus
 Importance of Awareness and Research for Lupus Dec 9, 2017 LFA Indiana Chapter Symposium Susan Manzi MD MPH Chair, Department of Medicine Director Lupus Center of Excellence Allegheny Health Network Pittsburgh,
Importance of Awareness and Research for Lupus Dec 9, 2017 LFA Indiana Chapter Symposium Susan Manzi MD MPH Chair, Department of Medicine Director Lupus Center of Excellence Allegheny Health Network Pittsburgh,
Which outcome measures in SLE clinical trials best reflect medical judgment?
 To cite: Thanou A, Chakravarty E, James JA, et al. Which outcome measures in SLE clinical trials best reflect medical judgment? Lupus Science & Medicine 2014;1:e000005. doi:10.1136/lupus-2013-000005 Received
To cite: Thanou A, Chakravarty E, James JA, et al. Which outcome measures in SLE clinical trials best reflect medical judgment? Lupus Science & Medicine 2014;1:e000005. doi:10.1136/lupus-2013-000005 Received
The only biologic approved to treat SLE: now with multiple delivery options
 The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
Lupus The Clinical Perspective
 Lupus The Clinical Perspective Anca D. Askanase, M.D., M.P.H. Associate Professor of Medicine Director Lupus Center Columbia University Medical Center New York-Presbyterian Hospital Systemic Lupus Erythematosus
Lupus The Clinical Perspective Anca D. Askanase, M.D., M.P.H. Associate Professor of Medicine Director Lupus Center Columbia University Medical Center New York-Presbyterian Hospital Systemic Lupus Erythematosus
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Belimumab Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 11/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Belimumab Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 4 References... 4 Effective Date... 11/15/2017 Next
THE KIDNEY AND SLE LUPUS NEPHRITIS
 THE KIDNEY AND SLE LUPUS NEPHRITIS JACK WATERMAN DO FACOI 2013 NEPHROLOGY SIR RICHARD BRIGHT TERMINOLOGY RENAL INSUFFICIENCY CKD (CHRONIC KIDNEY DISEASE) ESRD (ENDSTAGE RENAL DISEASE) GLOMERULONEPHRITIS
THE KIDNEY AND SLE LUPUS NEPHRITIS JACK WATERMAN DO FACOI 2013 NEPHROLOGY SIR RICHARD BRIGHT TERMINOLOGY RENAL INSUFFICIENCY CKD (CHRONIC KIDNEY DISEASE) ESRD (ENDSTAGE RENAL DISEASE) GLOMERULONEPHRITIS
Contents. Welcome to EULAR 2012 Berlin... iii AbstractsReviewers... iv. Speakers Abstracts
 vii Welcome to EULAR 2012 Berlin... iii AbstractsReviewers... iv Speaker Presentations Speakers Abstracts Wednesday, 6 June 2012 SP0001 Pre-conference outlook... 2 SP0002 WIN Session 1... 2 SP0003 SP0005
vii Welcome to EULAR 2012 Berlin... iii AbstractsReviewers... iv Speaker Presentations Speakers Abstracts Wednesday, 6 June 2012 SP0001 Pre-conference outlook... 2 SP0002 WIN Session 1... 2 SP0003 SP0005
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 08/19/14 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Diagnosis and Treatment of Neurosarcoidosis
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/focus-on-neurology-and-psychiatry/diagnosis-and-treatment-ofneurosarcoidosis/3892/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/focus-on-neurology-and-psychiatry/diagnosis-and-treatment-ofneurosarcoidosis/3892/
To live with lupus, we need to know about lupus.
 To live with lupus, we need to know about lupus. Zineb Aouhab, MD Assistant Professor of Medicine Loyola University Medical Center Division of Rheumatology 1 Where did the word lupus come from? The word
To live with lupus, we need to know about lupus. Zineb Aouhab, MD Assistant Professor of Medicine Loyola University Medical Center Division of Rheumatology 1 Where did the word lupus come from? The word
ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION
 UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
Evidence Based Treatment of SLE with Treatment Algorithm. Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College
 Evidence Based Treatment of SLE with Treatment Algorithm Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College Natural Histoty Inflammatory multisystem disease Onset usually between
Evidence Based Treatment of SLE with Treatment Algorithm Dr. Md. Mujibur Rahman Professor of Medicine Shaheed Suhrawardy Medical College Natural Histoty Inflammatory multisystem disease Onset usually between
Living with Lupus: An Insider s Perspective
 Living with Lupus: An Insider s Perspective Pamela Thorpe, MD, FACP Lupus Foundation of America, Inc. Philadelphia Tri-State Chapter Volunteer May 2014 My Own Story Is it Lupus Yet? The What What is this?
Living with Lupus: An Insider s Perspective Pamela Thorpe, MD, FACP Lupus Foundation of America, Inc. Philadelphia Tri-State Chapter Volunteer May 2014 My Own Story Is it Lupus Yet? The What What is this?
LUPUS. and Medication LUPUSUK 2015
 9 LUPUS and Medication LUPUSUK 2015 LUPUS and Medication The types of drugs used in lupus can be broadly divided into those that treat the disease itself (e.g. hydroxychloroquine and prednisolone) and
9 LUPUS and Medication LUPUSUK 2015 LUPUS and Medication The types of drugs used in lupus can be broadly divided into those that treat the disease itself (e.g. hydroxychloroquine and prednisolone) and
Corporate Presentation. April 2017
 Corporate Presentation April 2017 Forward-Looking Statements Certain of the statements made in this presentation may constitute forward-looking information within the meaning of applicable Canadian securities
Corporate Presentation April 2017 Forward-Looking Statements Certain of the statements made in this presentation may constitute forward-looking information within the meaning of applicable Canadian securities
Summary of Risk Minimization Measures
 Table 6.1.4-1: Summary of Risk Minimization Measures Safety Concern Vaccination Hepatic and renal impairment Combination therapy Elderly Routine Risk Minimization Measures Specific subsection on vaccination
Table 6.1.4-1: Summary of Risk Minimization Measures Safety Concern Vaccination Hepatic and renal impairment Combination therapy Elderly Routine Risk Minimization Measures Specific subsection on vaccination
Switching Treatment Between Mycophenolate Mofetil and Azathioprine in Lupus Patients: Indications and Outcomes
 Arthritis Care & Research Vol. 66, No. 12, December 2014, pp 1905 1909 DOI 10.1002/acr.22364 2014, American College of Rheumatology ORIGINAL ARTICLE Switching Treatment Between Mycophenolate Mofetil and
Arthritis Care & Research Vol. 66, No. 12, December 2014, pp 1905 1909 DOI 10.1002/acr.22364 2014, American College of Rheumatology ORIGINAL ARTICLE Switching Treatment Between Mycophenolate Mofetil and
Antibody-Drug Conjugates in Glioblastoma Multiforme: Finding Ways Forward
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
New Evidence reports on presentations given at ACR Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab
 New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
New Evidence reports on presentations given at EULAR Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis
 New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
Contents. Welcome Address... iii EULAR 2017 Abstracts Reviewers... iv. Speakers Abstracts
 vi Welcome Address... iii EULAR 2017 Abstracts Reviewers... iv Speaker Presentations Speakers Abstracts Wednesday, 14 June 2017 SP0001 SP0004 Joint EULAR - APLAR session: novel animal models - where no
vi Welcome Address... iii EULAR 2017 Abstracts Reviewers... iv Speaker Presentations Speakers Abstracts Wednesday, 14 June 2017 SP0001 SP0004 Joint EULAR - APLAR session: novel animal models - where no
Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response
 ARD Online First, published on April 5, 2012 as 10.1136/annrheumdis-2011-200937 1 Unit for Clinical Therapy Research, Infl ammatory Diseases (ClinTRID), The Karolinska Institute, Stockholm, Sweden 2 Division
ARD Online First, published on April 5, 2012 as 10.1136/annrheumdis-2011-200937 1 Unit for Clinical Therapy Research, Infl ammatory Diseases (ClinTRID), The Karolinska Institute, Stockholm, Sweden 2 Division
CENTRAL NERVOUS SYSTEM VASCULITIS
 What is central nervous system (CNS) vasculitis? Central nervous system (CNS) vasculitis is among a family of rare disorders characterized by inflammation of the blood vessels, which restricts blood flow
What is central nervous system (CNS) vasculitis? Central nervous system (CNS) vasculitis is among a family of rare disorders characterized by inflammation of the blood vessels, which restricts blood flow
Immuno-Oncology: Perspectives on Current Therapies & Future Developments
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/medical-industry-feature/immuno-oncology-perspectives-currenttherapies-future-developments/9502/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/medical-industry-feature/immuno-oncology-perspectives-currenttherapies-future-developments/9502/
Effect of mycophenolate mofetil on the white blood cell count and the frequency of infection in systemic lupus erythematosus.
 Thomas Jefferson University Jefferson Digital Commons Department of Medicine Faculty Papers Department of Medicine 10-22-2015 Effect of mycophenolate mofetil on the white blood cell count and the frequency
Thomas Jefferson University Jefferson Digital Commons Department of Medicine Faculty Papers Department of Medicine 10-22-2015 Effect of mycophenolate mofetil on the white blood cell count and the frequency
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
Moderately to severely active ulcerative colitis
 Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients
Clinical Trials in Rheumatology
 Clinical Trials in Rheumatology Rüdiger Müller Johannes von Kempis Clinical Trials in Rheumatology Authors Rüdiger Müller Division of Rheumatology and Rehabilitation Department of Internal Medicine Kantonsspital
Clinical Trials in Rheumatology Rüdiger Müller Johannes von Kempis Clinical Trials in Rheumatology Authors Rüdiger Müller Division of Rheumatology and Rehabilitation Department of Internal Medicine Kantonsspital
LUPUZOR (SYSTEMIC LUPUS ERYTHEMATOSUS AND LUPUS NEPHRITIS) - FORECAST AND MARKET ANALYSIS TO 2022
 REFERENCE CODE GDHC279DFR PUBLICATION DATE OCTOBER 2013 LUPUZOR (SYSTEMIC LUPUS ERYTHEMATOSUS AND Executive Summary Table below presents the key metrics of Lupuzor for Systemic Lupus Erythematosus (SLE)
REFERENCE CODE GDHC279DFR PUBLICATION DATE OCTOBER 2013 LUPUZOR (SYSTEMIC LUPUS ERYTHEMATOSUS AND Executive Summary Table below presents the key metrics of Lupuzor for Systemic Lupus Erythematosus (SLE)
Treat-to-target in lupus: what does the future hold?
 International Journal of Clinical Rheumatology Treat-to-target in lupus: what does the future hold? Implementing a treat-to-target (T2T) strategy that aims to improve disease outcomes through achievement
International Journal of Clinical Rheumatology Treat-to-target in lupus: what does the future hold? Implementing a treat-to-target (T2T) strategy that aims to improve disease outcomes through achievement
Guideline on clinical investigation of medicinal products for the treatment of systemic lupus erythematosus and lupus nephritis
 26 February 2015 EMA/CHMP/51230/2013 corr 1 Committee for Medicinal Products for Human use (CHMP) Guideline on clinical investigation of medicinal products for the treatment of systemic lupus erythematosus
26 February 2015 EMA/CHMP/51230/2013 corr 1 Committee for Medicinal Products for Human use (CHMP) Guideline on clinical investigation of medicinal products for the treatment of systemic lupus erythematosus
IACFS/ME Newsletter April 2014 Attachment 9a
 Second Study on Blood test for FM. A second study on a blood test for fibromyalgia was reported at the 2013 American College of Rheumatology meeting. On July, 8, 2012, information about a diagnostic blood
Second Study on Blood test for FM. A second study on a blood test for fibromyalgia was reported at the 2013 American College of Rheumatology meeting. On July, 8, 2012, information about a diagnostic blood
Persistent disease activity may have significant implications for your SLE patients life journey. 1,2
 Persistent disease activity may have significant implications for your SLE patients life journey. 1,2 Is it time to increase the focus on disease activity reduction? BENLYSTA (belimumab) is indicated as
Persistent disease activity may have significant implications for your SLE patients life journey. 1,2 Is it time to increase the focus on disease activity reduction? BENLYSTA (belimumab) is indicated as
Chapter 12: Lupus nephritis Kidney International Supplements (2012) 2, ; doi: /kisup
 http://www.kidney-international.org chapter 12 & 2012 KDIGO Chapter 12: Lupus nephritis Kidney International Supplements (2012) 2, 221 232; doi:10.1038/kisup.2012.25 INTRODUCTION This chapter makes treatment
http://www.kidney-international.org chapter 12 & 2012 KDIGO Chapter 12: Lupus nephritis Kidney International Supplements (2012) 2, 221 232; doi:10.1038/kisup.2012.25 INTRODUCTION This chapter makes treatment
New Evidence reports on presentations given at EULAR Tocilizumab for the Treatment of Rheumatoid Arthritis
 New Evidence reports on presentations given at EULAR 2012 Tocilizumab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Tocilizumab monotherapy is superior to adalimumab monotherapy
New Evidence reports on presentations given at EULAR 2012 Tocilizumab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Tocilizumab monotherapy is superior to adalimumab monotherapy
High Impact Rheumatology
 High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
A Clinical Context Report
 Rheumatoid Arthritis in Practice An Expert Commentary with Diane Horowitz, MD A Clinical Context Report Rheumatoid Arthritis in Practice Jointly Sponsored by: and Rheumatoid Arthritis in Practice Expert
Rheumatoid Arthritis in Practice An Expert Commentary with Diane Horowitz, MD A Clinical Context Report Rheumatoid Arthritis in Practice Jointly Sponsored by: and Rheumatoid Arthritis in Practice Expert
Benlysta. Benlysta (belimumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.99.01 Subject: Benlysta Page: 1 of 5 Last Review Date: June 22, 2018 Benlysta Description Benlysta (belimumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.99.01 Subject: Benlysta Page: 1 of 5 Last Review Date: June 22, 2018 Benlysta Description Benlysta (belimumab)
Highlights on the American College of Rheumatology Meeting 2006
 Highlights on the American College of Rheumatology Meeting 2006 The ACR 2006 meeting was held in Washington DC, the capital of United States between November 11 and November 15. The weather was fine and
Highlights on the American College of Rheumatology Meeting 2006 The ACR 2006 meeting was held in Washington DC, the capital of United States between November 11 and November 15. The weather was fine and
