n BASIC-TRANSLATIONAL REVIEW
|
|
|
- Sharon Booker
- 5 years ago
- Views:
Transcription
1 n BASIC-TRANSLATIONAL REVIEW At the Bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases April Barnado,*,1 Leslie J. Crofford,* and Jim C. Oates *Department of Medicine, Vanderbilt University, Nashville, Tennessee, USA; and Department of Medicine, Medical University of South Carolina, Charleston, South Carolina, USA RECEIVED JUNE 1, 2015; REVISED OCTOBER 15, 2015; ACCEPTED NOVEMBER 5, DOI: /jlb.5BT R < SEE CORRESPONDING ARTICLE ON PAGE 253 ABSTRACT Neutrophil extracellular traps are associated with a unique form of cell death distinct from apoptosis or necrosis, whereby invading microbes are trapped and killed. Neutrophil extracellular traps can contribute to autoimmunity by exposing autoantigens, inducing IFN-a production, and activating the complement system. The association of neutrophil extracellular traps with autoimmune diseases, particularly systemic lupus erythematosus, will be reviewed. Increased neutrophil extracellular trap formation is seen in psoriasis, antineutrophil cytoplasmic antibody-associated vasculitis, antiphospholipid antibody syndrome rheumatoid arthritis, and systemic lupus erythematosus. Neutrophil extracellular traps may promote thrombus formation in antineutrophil cytoplasmic antibody-associated vasculitis and antiphospholipid antibody syndrome. In systemic lupus erythematosus, increased neutrophil extracellular trap formation is associated with increased disease activity and renal disease, suggesting that neutrophil extracellular traps couldbeadisease activity marker. Neutrophil extracellular traps can damage and kill endothelial cells and promote inflammation in atherosclerotic plaques, which may contribute to accelerated atherosclerosis in systemic lupus erythematosus. As neutrophil extracellular traps induce IFN-a production, measuring neutrophil extracellular traps may estimate IFN-a levels and identify which systemic lupus erythematosus patients have elevated levels and may be more likely to respond to emerging anti-ifn-a therapies. In addition to anti-ifn-a therapies, other novel agents, such as N-acetyl-cysteine, DNase I, and peptidylarginine deiminase inhibitor 4, target neutrophil extracellular traps. Neutrophil extracellular traps offer insight into the pathogenesis of autoimmune diseases and provide promise in developing disease markers and novel therapeutic agents in systemic lupus erythematosus. Priority areas for basic research Abbreviations: b 2 GP1 = b-2-glycoprotein 1, ACPA = autoantibodies to citrullinated protein antigen, ANCA = antineutrophil cytoplasmic antibody, APS = antiphospholipid antibody syndrome, C5 = complement component 5, cfdna = cell-free DNA, CRP = C-reactive protein, CVD = cardiovascular disease, EC = endothelial cell, ENA = extractable nuclear antigen, ESR = erythrocyte sedimentation rate, GN = glomerulonephritis, HCQ = hydroxychloroquine, (continued on next page) based on clinical research insights will be identified, specifically the potential role of neutrophil extracellular traps as a biomarker and therapeutic target in systemic lupus erythematosus. J. Leukoc. Biol. 99: ; Introduction Neutrophils play a key role in innate immunity with multiple strategies for defending the body against pathogens. Neutrophils initially migrate to the site of infection and phagocytose and kill bacteria with the aid of proteolytic enzymes, antimicrobial proteins, and ROS [1]. Neutrophils can also degranulate to release antimicrobial factors in the extracellular space to kill bacteria [1]. More recently, neutrophils were found to kill bacteria by forming extracellular structures called NETs, which are composed mainly of histones, DNA, and proteases, such as NE. NETs were first discovered in 1996 as a unique form of cell death, distinct from necrosis or apoptosis [2]. This process was further described and termed NETosis in 2004 [1]. In NETosis, the neutrophils extrude large amounts of chromatin and granule proteins, such as NE and MPO, which trap and kill microorganisms. NETs contain the invading microorganism to prevent the spread of infection and use their highly localized concentration of antimicrobial peptides to degrade virulence factors and kill the microorganism [1]. Whereas NETs have a role in pathogen defense, NETs can also lead to toxic effects in the host. Importantly, NETs expose autoantigens, such as nucleic acids and proteins, in an inflammatory milieu that can stimulate an autoimmune response in a susceptible individual. See the related review that highlights the involvement of NETs in the innate immune system and autoimmune diseases [3]. NETosis is a novel form of cell death where neutrophils extrude extracellular fibers composed of chromatin and granule proteins that trap and kill microorganisms. The association of increased NET formation and autoimmunity was first described in ANCA-associated vasculitis and 1. Correspondence: Dept. of Medicine, Vanderbilt University, T3113 Medical Center North, st Ave., South, Nashville, TN 37232, USA. april.barnado@vanderbilt.edu /16/ Society for Leukocyte Biology Volume 99, February 2016 Journal of Leukocyte Biology 265
2 subsequently in other autoimmune diseases, including psoriasis,sle,aps,andra[4 9]. Proteins found in NETs may be the source of key autoantigens, including MPO and PR3, in ANCA-associated vasculitis, dsdna antibodies in SLE, and ACPAs in RA. Not only do the protein contents of NETs serve as the targets for autoantibody and immune complex formation, but also, they induce further NETosis, resulting in a positive-feedback loop. The exact molecular mechanisms that trigger NETosis are not fully understood. Several pathways have been suggested, such as induction by ROS generated from NOX. MPO and NE then promote chromatin dencondensation with PAD-4 citrullinating histones [10]. There are conflicting reports as to whether these processes are both required and sufficient for NETosis [10]. NETs were originally described as being activated upon stimulation with IL-8, PMA, and LPS, along with gram-positive and -negative bacteria, fungi, and parasites [1, 11]. However, in autoimmune diseases, proinflammatory cytokines, such as TNF-a and IL-17, and autoantibodies not only stimulate NETosis but also affect the protein contents of NETs [7]. NETs can also promote autoimmunity through production of type I IFNs and activation of the classic and alternative pathways of the complement system. In psoriasis and SLE, the contents of NETs, namely the antimicrobial peptides and the self-dna, are able to induce IFN-a production from the pdc [4]. pdcs are the major producers of IFN-a and normally do so in response to invading viruses. NETs with their nuclear contents of dsdna similarly induce IFN-a from pdcs. IFN-a can then activate both the innate and adaptive immune systems, specifically inducing a Th type 1 pathway, inhibiting T cell apoptosis, and activating B cells and antibody production [12]. In addition to increased NET formation in patients with autoimmune diseases compared with healthy controls, there is enhanced NET stability with decreased clearance of NETs, particularly in SLE and ANCA-associated vasculitis [13 15]. There are various mechanisms for the decreased clearance of NETs. One degrader of NETs is an endonuclease called DNase I. In a subset of SLE and ANCA-associated vasculitis patients, there is low DNase I activity and DNase I inhibitors, resulting in decreased clearance of NETs [13 16]. There are also NETprotecting antibodies that prevent access of DNase I to the NETs to prevent NET degradation [13 16]. Complement also plays a role in NET degradation. Complement interacts with NETs, with C1q and C3b depositing on the NETs [15]. Deposited C1q inhibits NET degradation through inhibition of DNase I. Furthermore, nondegraded NETs activate complement in vitro [15]. Overall, a positive-feedback loop exists where NETs activate complement, which then further increases NETs by preventing (continued from previous page) LDG = low-density granulocyte, MMP = metalloproteinase, MPO = myeloperoxidase, mtor = mammalian target of rapamycin, NAC = N-acetyl-cysteine, NE = neutrophil elastase, NET = neutrophil extracellular trap, NOX = NADPH oxidase, PAD-4 = peptidylarginine deiminase 4, pdc = plasmacytoid dendritic cell, PNH = paroxysmal nocturnal hemoglobinuria, PR3 = proteinase 3, RA = rheumatoid arthritis, rh = recombinant human, rm = recombinant mouse, ROS = reactive oxygen species, SLE = systemic lupus erythematosus, SLEDAI = systemic lupus erythematosus disease activity index, SRI = systemic lupus erythematosus responder index their degradation. In addition to the role of NETs in autoimmunity, NETs may be a potential disease activity biomarker and a therapeutic target. ASSOCIATION OF NETS WITH DISEASE ACTIVITY IN AUTOIMMUNE DISEASES The ability of NETs to induce autoimmunity has been suggested in various autoimmune diseases (Table 1 and Fig. 1). NETs were first implicated in the pathogenesis of ANCAassociated vasculitis, which is a group of diseases characterized by a systemic necrotizing vasculitis that affects small vessels, predominantly of the lungs and kidneys, and includes granulomatosis with polyangiitis (Wegener s) and microscopic polyangiitis. Patients have ANCAs antibodies directed against neutrophil granule proteins PR3 or MPO. These autoantibodies are pathogenic in animal models of ANCAassociated vasculitis [19, 20]. The granule protein contents of NETs may be the antigen source for these pathogenic autoantibodies. ANCAs can then directly stimulate NETosis in vitro [5, 14, 21]. In a study with 38 patients with ANCAassociated vasculitis, patients sera had a high ability to induce NET formation compared with healthy controls [14]. Increased NET formation was associated with increased disease activity [14]. Furthermore, DNase I activity levels were significantly lower, leading to impaired NET degradation in the serum of patients with ANCA-associated vasculitis vs. healthy controls [14]. Anti-NET antibodies that can inhibit the DNase I activity were found in some patients and led to a further reduction in the degradation of NETs [14]. Overall, there seems to be a NET-ANCA vicious cycle, where there is excessive formation and decreased clearance of NETs that could lead to ANCA production, which then accelerates further NET formation [14]. Better understanding of the mechanisms of the anti-net antibodies and the NET-ANCA cycle couldhelpidentifypotential therapeutic targets for ANCA-associated vasculitis. ANCA-associated vasculitis is a group of diseases characterized by a systemic necrotizing vasculitis that affects small vessels, predominantly of the lungs and kidneys, and includes granulomatosis with polyangiitis (Wegener s) and microscopic polyangiitis. Patients frequently have antibodies directed against neutrophil granule proteins PR3 or MPO. There is also in vivo evidence of NET formation in the affected glomeruli and interstitium of renal biopsies from patients with ANCA-associated vasculitis [5]. A single case study found abundant NETs in a thrombus from a patient with ANCAassociated vasculitis [17]. Increased NET formation may cause thrombus formation. Specifically, histones within the NETs can bind platelets and blood coagulants [22, 23]. The role of NETs in thrombosis in ANCA-associated vasculitis has clinical relevance, as patients with ANCA-associated vasculitis have an increased risk of venous thrombosis during periods of active disease [24, 25]. More studies are needed to determine whether NETs are a pathogenic mechanism in thrombosis formation in patients with ANCA-associated vasculitis. 266 Journal of Leukocyte Biology Volume 99, February
3 BASIC-TRANSLATIONAL REVIEW Barnado et al. NETs in autoimmune diseases TABLE 1. Association of NETs with autoimmune diseases Study Disease NETs measurement Results Proposed role of NETs [4] Psoriasis In vitro Endogenous antimicrobial peptide (LL-37) converts self-dna into a potent trigger of pdcs to release IFN-a in psoriatic skin. [5] ANCA-associated vasculitis [14] ANCA-associated vasculitis [17] ANCA-associated vasculitis NET protein content LL-37 drives production of IFN-a that contributes to autoimmunity. In vivo NET formation in 15 renal biopsies The granule protein contents of NET may be the antigen source for pathogenic autoantibodies, ANCAs. In vivo In vivo Association of increased NET formation with increased disease activity, as measured by the Birmingham Vasculitis Activity Score NETs were found in glomerular crescents and in thrombi of patients with ANCA-associated vasculitis. [8] APS In vitro Decreased degradation of NETs in sera of patients with APS was associated with increased levels of antibodies against NETs. [9] APS In vitro Increased circulating levels of NETs were associated with the presence of APS autoantibodies; an APS autoantibody, b 2 GP1, interacted with neutrophils to stimulate NETosis. [7] RA In vivo Increased NET formation was seen in synovial tissue, rheumatoid nodules, and skin; increased percentage of netting neutrophils was associated with increased serum CRP, ESR, ACPA, and IL-17 [18] RA In vitro Increased NET formation compared with controls Increased NET formation may contribute to thrombus formation in ANCA-associated vasculitis. NETs may promote thrombin formation in APS. NETs may be the source of citrullinated autoantigens that are pathogenic in RA. Research Question: Does increased NET formation lead to thrombus formation in patients with ANCA-associated vasculitis? NETs may play a pathogenic role in thrombus formation in another autoimmune disease called APS. In APS, patients are at an increased risk of recurrent arterial and venous thrombosis and pregnancy morbidity. APS can occur in a primary form, where there is no underlying autoimmune disease, or a secondary form, where there is an underlying autoimmune disease, such as SLE. Patients with both primary and secondary APS have autoantibodies to phospholipids and surface proteins, such as lupus anticoagulant, anticardiolipin, and b 2 GP1, which can promote thrombus formation by activating ECs, monocytes, and platelets [26 28]. b 2 GP1 can interact with neutrophils to stimulate NETosis in vitro in a process that is dependent on ROS and TLR4 signaling [9]. Furthermore, NETs promoted thrombin generation in vitro [9]. A positive correlation was seen with the presence of b 2 GP1 and other APS antibodies lupus anticoagulant and anticardiolipin with circulating levels of NETs [9]. These increased levels of circulating NETs may be a result of the presence of antiphospholipid autoantibodies but also could result from decreased degradation of NETs, as has been suggested by another in vitro study in APS [8]. Future in vivo studies are needed to confirm the findings of increased NET formation and decreased NET degradation in patients with APS and to characterize the pathways in which autoantibodies in APS may stimulate NETosis and promote thrombus formation. APS is an autoimmune disease where patients are at increased risk of recurrent arterial and venous thrombosis and pregnancy morbidity, such as miscarriages, fetal loss, and preeclampsia. Patients can have persistently elevated levels of autoantibodies to phospholipids and surface proteins, such as anticardiolipin and b 2 GP1 and the presence of a circulating lupus anticoagulant. Research Questions: What are the mechanisms by which NETs promote thrombus formation in patients with APS? Do levels of NETs increase during clinical events associated with APS, such as acute thrombotic episodes, or pregnancy complications, such as miscarriages or preeclampsia? NETs have also been implicated in the pathogenesis of RA, which is a chronic autoimmune disease characterized by inflammation and subsequent damage of the synovial joints. Volume 99, February 2016 Journal of Leukocyte Biology 267
4 ASSOCIATION OF NETS WITH DISEASE ACTIVITY IN SLE Figure 1. Overview of potential pathogenic roles of NETs in autoimmune diseases. NETs externalize citrullinated histones that act as autoantigens in RA. The contents of NETs, LL-37 and DNA, induce IFN-a from pdcs, which contribute to autoimmunity in SLE and psoriasis. The MPO content of NETs is the antigenic source for pathogenic ANCA in ANCA-associated vasculitis. NETs activate platelets and coagulation factors, such as tissue factor, to promote thrombosis in APS and ANCAassociated vasculitis. MMP-9, externalized on NETs, damages ECs to promote vasculopathy and potentially accelerated atherosclerosis in SLE. Electron micrograph of polymorphonuclear neutrophil (PMN) undergoing NETosis courtesy of Drs. Elizabeth Johnson and Leslie Crofford. Patients with RA have increased levels of inflammatory cytokines, such as TNF-a and IL-17, and pathogenic ACPA, years before clinical diagnosis [29]. Citrullination is a post-translation modification that can generate neoantigens, rendering proteins and immune complexes immunogenic and arthrogenic [30, 31]. Whereas histone citrullation is important in NETosis, it is not sufficient [10]. In an autocrine feed-forward loop, NETs present these citrullinated histones [32] that serve as proinflammatory and immunostimulatory autoantigens to promote joint inflammation and destruction [6, 33, 34]. Neutrophils from RA cases have increased NET formation in vitro compared with controls [18], with NETs detected in synovial tissue, rheumatoid nodules, and skin [7]. Furthermore, there were significant correlations between the percentage of peripheral blood netting neutrophils and serum levels of CRP, ESR, ACPA, and IL-17 [7]. Autoantibodies, such as ACPA and rheumatoid factor, and inflammatory cytokines, such as TNF-a and IL-17, induced NETosis in RA cases [7]. In addition, NETs externalized citrullinated autoantigens that are key RA autoantigens [7]. This study suggests a cycle where NETs are exposing citrullinated antigens to the immune system with these same citrullinated antigens and inflammatory cytokines, further perpetuating NETosis [7]. In addition, ACPA and inflammatory cytokines, such as TNF-a, induced distinct protein contents in the NETs. Future studies are needed to confirm these findings but also to investigate how different autoantibodies and inflammatory cytokines stimulate NETs and affect their protein contents. RA is a chronic autoimmune disease characterized by inflammation and subsequent damage of the synovial joints. Patients with RA can have pathogenic ACPA. Similar to ANCA-associated vasculitis and RA, there is in vivo evidence for NETs in SLE (Table 2), which is a chronic autoimmune disease with multisystem organ involvement that can range from a mild disease course, where the skin and joints are affected, to a more severe disease course, involving the kidneys and CNS. The disease is characterized by fluctuating periods of increased disease activity called flares. In SLE, there is a break in self-tolerance that results in the production of autoantibodies to nucleic acids and associated proteins, such as dsdna. These autoantibodies can form immune complexes that deposit in the skin and kidneys causing tissue damage. SLE is a chronic autoimmune disease characterized by multisystem organ involvement ranging from skin and joint and renal and CNS involvement with fluctuating episodes of increased disease activity. Patients can have autoantibodies to nucleic acids and associated proteins, including antinuclear antibodies, anti-smith, anti-dsdna, and antiphospholipid antibodies. Netting neutrophils were present in 6 of 9 renal biopsies from SLE patients with class III or IV SLE nephritis [6]. Patients with class IV SLE nephritis, a diffuse-proliferative form, typically with active inflammatory lesions, had a higher percentage of netting neutrophils infiltrating the glomeruli compared with patients with class III SLE nephritis, a focal-proliferative form, typically with active inflammatory lesions. Patients with higher activity biopsy indices and anti-dsdna levels had more netting neutrophils infiltrating the glomeruli compared with patients with lower activity biopsy indices and anti-dsdna levels. NETs were also found in skin lesions in SLE patients with various forms of cutaneous lupus [6]. In addition, there are multiple studies investigating the association of NET degradation with the presence of SLE nephritis and disease activity. One study with 94 SLE patients confirmed that sera from a subset of patients failed to degrade NETs [15]. In a retrospective analysis of 61 patients, the inability to degrade NETs correlated with the presence of SLE nephritis and increased disease activity [13], as measured by the SLEDAI [38]. Renal involvement and pleurisy, as measured by SLEDAI, were also associated with the inability to degrade NETs [13]. SLEDAI is a validated and commonly used disease activity measure, where the physician assigns points to clinical and lab parameters that represent active SLE based on their presence or absence in the prior 10 d. SLEDAI is a validated disease activity measure, where a physician assigns points to a list of 24 items, 16 of which are based on clinical symptoms, and 8 are laboratory measures based on their presence or absence within the prior 10 d. More severe organ involvement, such as renal involvement, is weighted with more points. The points are then summed with a possible score ranging from 0 to 105, with higher scores indicating higher disease activity. A score of 6 or more is consistent with active disease that requires therapy. Research Question: How are the protein contents of NETs influenced by exposure to different autoantibodies and inflammatory cytokines? With the use of a different measure to assess NET formation, one cross-sectional study assessed levels of cfdna, products of NETs [35]. Compared with controls, SLE patients had significantly higher mean concentrations of cfdna [35]. SLE patients 268 Journal of Leukocyte Biology Volume 99, February
5 BASIC-TRANSLATIONAL REVIEW Barnado et al. NETs in autoimmune diseases TABLE 2. Association of NETs with SLE Study NETs measurements Disease measurements Results Proposed role of NETs [13] In vivo, inability to degrade NETs [15] In vivo, inability to degrade NETs [35] In vivo, NET products (cfdna) [16] In vivo, inability to degrade NETs [14] In vivo, ability of sera IgG from SLE patients to induce NET formation [6] In vivo, percentage of netting neutrophils, LDGs Presence of SLE nephritis Disease activity, as measured by SLEDAI Active nephritis (active lesions on renal biopsy, active urinary sediment, and proteinuria), serum albumin, creatinine clearance rates Disease activity, as measured by SLEDAI, and additional clinical and lab parameters Disease activity, as measured by SLEDAI Activity indices on renal biopsies, dsdna levels Inability to degrade NETs correlated with the presence of SLE nephritis. Inability to degrade NETs correlated with higher SLEDAI scores and the presence of renal involvement and pleurisy. Higher concentrations of cfdna correlated with the presence of active nephritis and with increased levels of 24 h urinary protein and decreased levels of albumin and creatinine clearance rates. Decreased ability to degrade NETs was associated with a higher SLEDAI score, nephritis, elevated dsdna, and low C3. No significant association between the ability to induce NETs and SLEDAI Class IV SLE nephritis patients had higher percentage of netting neutrophils than class III SLE nephritis patients. Higher percentage of netting neutrophils was associated with higher activity indices on renal biopsies and higher dsdna levels. LDGs have increased ability to form NETs; LDGs have a propensity to damage and kill ECs and stimulate IFN-a synthesis by pdcs. [36] In vitro EC damage by use of HUVECs MMP-9 is up-regulated and externalized on NETs and damages ECs. [37] In vitro HDL oxidation, cholesterol efflux capacity NETs promote HDL oxidation and decreased cholesterol efflux capacity. Decreased ability to degrade NETs may be associated with increased disease activity and renal disease. NETs externalize dsdna, an autoantigen that is immunostimulatory and pathogenic in SLE. NETs promote IFN-a, production that contributes to autoimmunity in SLE. NETs damage and kill ECs; MMP-9, contained in NETs, contributes to EC dysfunction in SLE. NETs oxidize HDL, rendering it dysfunctional and proatherogenic, possibly contributing to accelerated atherosclerosis in SLE. with active nephritis, defined as active urinary sediment, proteinuria, or active lesions on a renal biopsy, had higher concentrations of cfdna than SLE patients with inactive nephritis and no history of nephritis. cfdna positively correlated with 24 h urinary protein and negatively correlated with albumin levels and the creatinine clearance rate. In summary, with the use of 3 different measures to assess NETs, there are multiple studies supporting the association between increased NET formation and increased disease activity and renal involvement in patients with SLE. To show more convincingly the association of NETs and SLE disease activity, one prospective longitudinal study conducted an analysis of 69 SLE patients. The authors measured the degradation of NETs, along with other serologic and clinical parameters, every 2 mo for a mean 2.5 y [16]. To define normal vs. decreased degradation of NETs, the ability of healthy controls to degrade NETs was measured. If a SLE patient had NET degradation at a cut-off set as 3 SD or lower below the mean NET degradation of controls, then the SLE patient was defined as Volume 99, February 2016 Journal of Leukocyte Biology 269
6 having a decreased ability to degrade NETs. Among the 69 SLE patients, 28 (41%) displayed, at least once, a decreased ability to degrade NETs, with 2 SLE patients having a reduced ability to degrade NETs at all time points measured. SLE patients who had decreased NET degradation at least once were more likely to be dsdna positive compared with SLE patients with normal NET degradation. In an attempt to describe temporal associations of clinical and laboratory parameters with NET degradation, this study analyzed items predominantly from the SLEDAI to assess disease activity that occurred 2 mo before, concomitantly, and 2 mo after NET degradation measurement. An increase in SLEDAI was significantly associated with decreased NET degradation and seen before, at, and after sampling of NET degradation. Furthermore, manifestations of active GN, such as cellular casts, hematuria, and proteinuria, were all significantly associated with decreased NET degradation and seen at the time of sampling. Proteinuria was associated with decreased NET degradation and seen before, during, and after NET sampling. In addition, lab parameters, such as elevated dsdna, CRP, low levels of C3, and leukopenia, were all associated with decreased NET degradation and seen at the time of sampling. This prospective longitudinal study showed that a subset of SLE patients had a decreased ability to degrade NETs, which was associated with a higher SLEDAI score, GN, and known disease activity markers, such as elevated dsdna and low C3 levels. Decreased NET degradation even preceded an increase in SLEDAI and proteinuria. This study suggests that NET degradation may have value as a biomarker of both predicting and detecting disease activity and renal involvement in SLE. However, larger prospective longitudinal studies that assess NET degradation and disease activity are needed. Research Questions: Do all patients with SLE have increased NETosis, or does only a subset of patients with specific autoantibodies have increased NETosis? In which subsets of patients with SLE does increased NETosis correlate with increased disease activity? NETS AS AUTOIMMUNE DISEASE BIOMARKERS Multiple cross-sectional studies and 1 longitudinal prospective study suggest that the measurement of NET degradation may be useful as a biomarker to predict and assess disease activity and renal disease. However, there is a recent, small study with 23 patients with SLE that did not find an association between increased NETs and disease activity, as measured by SLEDAI [14]. Of note, this study assessed for the presence of NETs differently by measuring the ability of sera IgG from SLE patients to induce NET formation instead of measuring NET degradation, as done in the previously discussed studies. These contrasting results highlight the importance of standardizing and validating the way NETs are measured in SLE patients. Whereas more studies in the literature, including a prospective one, tend to use NET degradation as the measure of increased NET formation or NETosis, it is not clear if this method is more accurate to capture NETosis compared with measuring the ability for sera to induce NETs or measuring NET products, such as cfdna. Another consideration is how to define normal versus increased NET formation. Some studies do not define specific cutoffs, whereas others define decreased NET degradation, for example, as 3 SD or lower than the mean for healthy controls [16]. The standardization of what is defined as decreased NET degradation or increased NET formation is needed. In addition, autoantibodies to NETs could interfere with serum NETs measurements using an ELISA. Any measurement technique proposed as a biomarker should also be relatively easy to perform in a clinical lab setting. Prospective longitudinal studies with larger sample sizes are needed to measure the test characteristics of any measure of NETosis to predict and correlate with disease activity and renal involvement. As illustrated by current disease activity markers, such as complement and dsdna levels, the clinical heterogeneity of SLE may limit which biomarkers are useful in assessing for disease activity and renal involvement. For example, not all SLE patients have anti-dsdna antibodies. Furthermore, SLE patients with anti-dsdna antibodies may not have increased levels associated with increased disease activity. This limitation can also be seen with complement levels with the lack of association between low complements and increased disease activity. Potentially, not all SLE patients will have increased NETosis or NETosis that correlates with increased disease activity. Future studies would need to clearly define in which SLE patients it may be beneficial to use NET measurement to help clinicians with predicting and assessing disease activity and renal involvement. Research Questions: What is the best method (ability of sera to induce NETs, NET degradation, NET products, such as cfdna) to measure increased NET formation in SLE patients? How will we define normal NET formation versus increased NET formation? Which measurement of NETosis could be most easily performed in clinical labs? In addition to SLE, the understanding of NETs in ANCAassociated vasculitis has led to the identification of potential biomarkers for disease activity and treatment response. In a recent study, increased expression of a granulocyte geneexpression signature was associated with increased disease activity and decreased treatment response in patients with ANCAassociated vasculitis [39]. The source of this signature was a subset of neutrophils called LDGs, which are proinflammatory [6, 36, 40] and able to undergo spontaneous NETosis without stimulation [40]. This study was the first to show the presence of LDGs in patients with ANCA-associated vasculitis, as LDGs had previously been shown in patients with SLE [40]. Furthermore, the LDGs in the patients with ANCA-associated vasculitis, produced NETs containing MPO and PR3, major autoantigens in ANCA-associated vasculitis. Future studies are needed to assess the potential pathogenic role of LDGs and NETs in ANCAassociated vasculitis. Research Questions: What is the role of LDGs in the pathogenesis of ANCA-associated vasculitis? Could LDGs be potential biomarkers for ANCA-associated vasculitis and SLE? In SLE, measures of molecular signatures or mrna expression of type I IFN are used as pharmacodynamic markers in 270 Journal of Leukocyte Biology Volume 99, February
7 BASIC-TRANSLATIONAL REVIEW Barnado et al. NETs in autoimmune diseases developing new SLE treatments. Type I IFNs are a cytokine family of IFN-a, IFN-b, IFN-Τ, IFN-k, and IFN-v [41]. Whereas individual type I IFNs, such as IFN-a, are difficult to measure, as a result of the small quantities in peripheral blood, the type I IFNs as a family are easier to measure in terms of mrna expression. In SLE, there is an overexpression of type I IFNinducible mrnas in blood and in involved tissues in ;60% of patients [41]. Increased expression of mrnas induced by type I IFN is associated with increased disease activity [41]. The measurement of the type I IFN signature is a potential pharmacodynamic marker to evaluate the inhibition of anti-ifn therapies, as well as select SLE patients with an increased type I IFN signature that may benefit from these therapies. Research Question: Does NETosis correlate with an increased type I IFN signature and increased IFN-a levels in patients with SLE? Similar to the IFN signature, NETs are increased in the blood and involved tissues of SLE patients. Increased NET formation and decreased NET degradation are associated with increased disease activity. NETs are able to induce IFN-a production from pdcs. Therefore, the measurement of NETs may allow for an indirect measure of IFN-a. The measurement of the IFN signature captures multiple type I IFNs, not just IFN-a. The measurement of NETs may be a more specific way to estimate IFN-a levels and identify which SLE patients at baseline in clinical trials have elevated IFN-a levels and may be more likely to respond to anti-ifn-a therapies. ASSOCIATION OF NETOSIS WITH EC DYSFUNCTION AND ATHEROSCLEROSIS IN AUTOIMMUNE DISEASES NETs may play a role in the pathogenesis of premature CVD in SLE. SLE patients have substantially increased morbidity and mortality from CVD, with the incidence of myocardial infarction 5 times higher in patients with SLE compared with the general population [42]. CVD occurs at earlier ages in SLE patients than in the general population, with traditional and diseaserelated risk factors not fully accounting for this increased risk [43, 44]. One proposed mechanism is an altered innate immune response that contributes to EC dysfunction and damage leading to plaque formation [37]. SLE patients have EC dysfunction with evidence for accelerated EC apoptosis [45], which results in loss of NO release, leading to impaired vasodilation, which may potentiate atherosclerosis [45]. Increased EC apoptosis in SLE patients correlated with reduced endothelial function, as measured by brachial artery flow-mediated dilation [45]. In addition, increased EC apoptosis in SLE patients correlated with elevated tissue factor, which is associated with a prothrombogenic phenotype [45]. Neutrophils and NETs may play a central role in increased EC apoptosis in SLE. There is evidence that neutrophils and NETs enhance EC killing and death in vitro [6]. In summary, NETs accelerate EC death that may contribute to both atherosclerotic and thrombotic events in SLE patients. Other mechanisms have also been implicated in the role of NETs in EC damage and the pathogenesis of accelerated atherosclerosis in SLE. MMP-9 is up-regulated and externalized on the surface of the NETs and able to damage ECs [36]. Furthermore, the MMPs from the NETs cause secretion of MMP- 2 from the ECs, resulting in further EC dysfunction. NETs may also mediate HDL oxidation in SLE patients [37]. Compared with controls, SLE patients have increased levels of oxidized HDL, which is a proinflammatory lipoprotein with impaired cholesterol efflux capacity. NET-derived MPO, NOX, and NOS promote HDL oxidation and decrease cholesterol efflux capacity in vitro [37]. In summary, NETs lead to the generation of a dysfunctional HDL that impairs cholesterol efflux capacity, further promoting proinflammatory responses in the vascular system that may contribute to accelerated atherosclerosis in SLE. Recently, an inflammatory role of neutrophils and macrophages in the pathogenesis of atherosclerosis has been described. Specifically, NETs were found in atherosclerotic plaques in apolipoprotein E-deficient mice [46]. Cholesterol crystals induced neutrophils to undergo NETosis, which primed macrophages to release pro-il-1b [46]. In addition, the cholesterol crystals, bound to the cell surface of the macrophages, activated the inflammasome, which further propagated inflammation [46]. In vitro human studies showed that cholesterol crystals triggered NETosis [46]. Whether neutrophils, NETs, and macrophages interact in human plaques to generate an inflammatory state needs further human in vivo studies. Research Questions: Do NETs propagate inflammation in atherosclerotic lesions in humans? Is the NET-mediated inflammatory process in atherosclerosis more pronounced in patients with autoimmune diseases, such as SLE, compared with the general population? NETS AS POTENTIAL THERAPEUTIC TARGETS NETs represent a novel target for therapy in SLE and other autoimmune diseases. The literature currently focuses on targeting NETs in developing SLE therapies, but potential therapies may be applicable to other autoimmune diseases. There are both conventional medications already used in SLE, as well as novel agents that target NETs (Table 3 and Fig. 2). As a defense against invading pathogens, NETs release ROS, with ROS important in NET formation. Treatment of neutrophils with NAC blocks ROS and NET formation in vitro [47]. NAC also blocks the mtor in SLE T cells in vitro and in vivo [49, 65]. In 2 small studies, NAC improved SLE disease activity [48, 49]. Future randomized, controlled clinical trials are needed to assess fully the efficacy of NAC in reducing disease activity in SLE. Another possible target is treatment with DNase I, which is an endonuclease that is secreted into the extracellular space and found in the blood and gastrointestinal tract with a presumed function of digesting extracellular DNA [66]. With their DNA contents, DNase I is an important degrader of NETs. In SLE patients, sera levels of DNase I are decreased [13, 15, 16]. With these decreased levels, it was hypothesized that administration of Volume 99, February 2016 Journal of Leukocyte Biology 271
8 TABLE 3. Therapies that target NETs in SLE Drug Mechanism of action Study Results Outstanding questions NAC DNase I PAD-4 inhibitor Eculizumab Vitamin D (calcitriol) Antimalarials Antioxidant reduces ROS and NET formation. Endonuclease to digest extracellular DNA and degrade NETs Deiminates histones (NET contents) rhmab to C5 may block NET formation. Unknown, reduces NET formation Blocks processing of NETs through TLR9 in pdcs [47] NAC blocks NET formation in vitro. What dose and frequency of NAC should be used? [48] NAC reduces SLE disease activity and attention deficit hyperactivity disorder scores. Will NAC be effective in reducing disease activity in SLE in controlled trials? [49] NAC reduces disease activity and blocks mtor in T lymphocytes. [50] In mice, rmdnase I prolonged What are the in vivo effects of survival and improved kidney DNase I in SLE patients? histology. [51] In mice, DNase I had no effect on survival and kidney disease. [52] In mice, DNase I reduced reactive B cells, production of type I IFN, and immune complex deposition in the kidneys. [53] In a phase Ib study, there were no significant differences in adverse reactions in the treatment versus placebo groups. Serum markers of disease activity were unchanged in the treatment group. [54] In mice, PAD-4 decreased What are the in vitro and in vivo autoimmune responses and effects of PAD-4 inhibitors in protected against vascular SLE patients? damage. [55] In mice, PAD-4 decreased proteinuria, improved skin involvement, and improved EC dysfunction. [56] In PNH patients, eculizumab inhibited markers of NET formation in vivo. [57] In a phase I study, there were no significant differences in adverse reactions in the treatment versus placebo groups, with complement inhibition persisting for 10 d; there were no significant changes in SLEDAI scores. [58] In vitro, vitamin D reduced NET formation and decreased rates of EC apoptosis in SLE patients with low vitamin D. [59] In vivo, vitamin D improved endothelial function in SLE patients with low vitamin D. [37] In vitro, chloroquine inhibited NET formation in SLE neutrophils. Sifalimumab Human mab to IFN-a [60] In a phase Ia placebo-controlled study, there was no safety signal seen with dose-dependent inhibition of the type I IFN signature with sifalimumab; there were trends toward improvement in SLEDAI with sifalimumab. [61] In a phase Ib, placebo-controlled study, adverse events were Does eculizumab block NET formation in SLE patients? In larger SLE studies, will eculizumab be shown to be safe and effective in reducing disease activity? Will vitamin D affect NET formation and improve EC dysfunction in larger studies? Will vitamin D reduce CVD in SLE patients? Will antimalarials inhibit NET formation in SLE in vivo? Is sifalimumab generally safe in SLE patients? Is sifalimumab only effective for SLE patients with increased (continued on next page) 272 Journal of Leukocyte Biology Volume 99, February
9 BASIC-TRANSLATIONAL REVIEW Barnado et al. NETs in autoimmune diseases TABLE 3. (continued) Drug Mechanism of action Study Results Outstanding questions similar in both groups; however, more discontinuations and deaths were seen with sifalimumab; there was dosedependent neutralization of the type I IFN signature with no difference in SLEDAI scores. [62] In a phase IIb, placebo-controlled study, adverse events were similar in both groups, except with higher rates of herpes zoster seen with sifalimumab. There was a significantly higher percentage of patients with an SRI response at d 365 with sifalimumab. Rontalizumab Human mab to IFN-a [63] In a phase I, placebo-controlled study, adverse events were similar, except a higher infection rate with rontalizumab; rontalizumab reduced levels of the type I IFN signature but did not reduce levels of IFN-inducible proteins or anti-dsdna levels. BILAG, British Isles Lupus Assessment Group. [64] In a phase IIb, placebo-controlled study, response rates to BILAG and SRI were similar; prespecified subgroup analyses showed a trend toward improvement in flare rates and a decrease in corticosteroid use. type I IFN signatures, or can it be used in all SLE patients? Will sifalimumab reduce disease activity in larger phase II and III studies? Will infection risk, such as from viral reactivation, limit rontalizumab and sifalimumab s use? Are other subsets of type I IFN, which are not blocked by sifalimumab or rontalizumab, important drivers of clinical disease in SLE? DNase I would reduce the extracellular DNA found in SLE that triggers multiple autoimmune pathways leading to inflammation and tissue damage. DNase I was administered to multiple models of lupus-prone mice with mixed results [66]. One group that uses rmdnase I showed improvement in histologic changes in kidneys and survival in the NZB/NZW F1 mouse model [50]. However, with the use of the same model, another group showed that DNase I had no effect in improving disease in the mice [51]. With the use of an estrogen-induced mouse model of lupus, DNase I treatment did improve disease in the mice [52]. rhdnase I was given to 17 SLE nephritis patients in a phase Ib, randomized, double-blinded, placebo-controlled trial to determine the drug s safety and pharmacokinetics [53]. rhdnase I was well tolerated without significant adverse events. However, serum markers of disease activity were unchanged. Future studies with more SLE patients are needed to assess the safety and efficacy of rhdnase I. Research Question: What are the in vivo effects of DNase I in SLE patients? Another potential drug target also involves inhibiting a major component of NETs. PAD-4 is a hydrolase that deiminates histones, which are prominent in NETs. Without PAD-4, NETs cannot form, as illustrated by PAD-4-deficient mice [32, 67]. In one lupus-prone mouse model the New Zealand Mixed 2328 which is prone to proliferative GN and a prominent type I IFN signature, PAD inhibitors decreased autoimmune responses and protected against NET-mediated vascular damage [54]. In lupus-prone MRL/Mp-lpr/lpr mice with proliferative GN and a much less prominent type I IFN signature, PAD inhibitors blocked NET formation, decreased immune complex deposition in the kidneys and proteinuria, and improved skin involvement [55]. Treatment with the PAD inhibitors also improved EC dysfunction [55]. PAD inhibitors represent an anti-net target that may be an effective, novel therapy in SLE to treat not only disease manifestations but also to improve endothelial dysfunction. To date, trials have not been conducted in humans. Research Question: What are the in vitro and in vivo effects of PAD-4 inhibitors in SLE patients? Another potential target of NETs is the complement system. Complement deposits on NETs and is important in regulating NETs by preventing their degradation. Eculizumab is a rhmab that is a terminal complement inhibitor and approved for the Volume 99, February 2016 Journal of Leukocyte Biology 273
10 determine if improved endothelial function translates to improved CVD outcomes. Research Question: Does vitamin D block NET formation and improve EC dysfunction in vivo in SLE patients? Figure 2. Overview of potential therapies that target NETs. Neutrophils release NETs, which contain histones, complexes of DNA and LL-37, an antimicrobial peptide, elastase, and MPO. NAC is an antioxidant that can reduce ROS, which promotes NETosis. DNase I digests extracellular DNA and degrades NETs. PAD-4 inhibitor deiminates histones, an important component of NETs. Eculizumab, a mab to C5, can block complement activation that stimulates NETosis. The target by which vitamin D reduces NET formation is unknown. Antimalarials block processing of NETs through TLR9 in pdcs. Sifalimumab and rontalizumab are mab to IFN-a, which is a product of NETosis that can stimulate further NETosis. Electron micrograph of polymorphonuclear neutrophil (PMN) undergoing NETosis courtesy of Drs. Elizabeth Johnson and Leslie Crofford. treatment of PNH, a rare, acquired hemolytic anemia, where the complement system attacks RBCs, and atypical hemolytic uremic syndrome a hemolyticanemiathatcanresultinacute kidney failure and a low platelet count. Specifically, eculizumab binds to the terminal C5 and inhibits the cleavage of C5 to C5a and C5b by the C5 convertasae, which prevents the formation of the terminal complement complex. Eculizumab inhibited NET formation in PNH patients [56]. Eculizumab has been studied in 24 SLE patients in a phase I, randomized, placebocontrolled, double-blind trial to evaluate its safety, pharmacodynamic, and pharmacokinetic properties [57]. There were no significant differences in adverse events in the treatment vs. placebo groups. Complement inhibition of.80% was observed in the treatment group and persisted for 10 d but then returned to baseline at 14 d. There were no significant changes in disease activity scores, as measured by SLEDAI, in exploratory analyses. However, this initial, small controlled study shows promising safety data to justify future, larger phase I and II studies. Research Question: Does eculizumab block NET formation in vitro and in vivo in SLE patients? In addition to the above novel agents, known agents used in SLE also represent NET-targeted therapy. In a small study of 5 SLE patients, vitamin D (calcitriol) reduced NET formationinneutrophilsfromslepatientswithlowvitamin D [58]. In addition, vitamin D decreased rates of apoptosis in ECs [58]. This study suggests that vitamin D reduces the cytotoxic effects of NETs on ECs. A small in vivo study of 22 stable SLE patients with vitamin D deficiency (,20 ng/ml) showed that vitamin D improved endothelial function [59]. These studies would need to be expanded in a larger SLE population with vitamin D tested in controlled trials to Antimalarials, such as HCQ, are the cornerstone of drug therapy in SLE. Antimalarials have the potential to block the processing of NETs through TLR9 in pdcs. Chloroquine, an antimalarial, significantly inhibited NET formation in control and SLE neutrophils in vitro [37]. However, this potential mechanism has not been investigated in vivo. Commonly used medications in SLE, such as azathioprine, mycophenolate mofetil, and cyclophosphamide, have effects on neutrophils. However, currently, there are no studies investigating the effects of these medications on NET formation. Furthermore, it is not clear if corticosteroids affect NETs. Both in vitro and in vivo studies show that corticosteroids do not affect the activation of pdcs and thus, do not reduce IFN-a levels [68]. As NETs promote autoimmunity through IFN-a production, it is not surprising that 1 study suggests that corticosteroids do not block NET formation in vitro [34]. These findings have not been replicated or studied in vivo in SLE patients. Research Question: Do antimalarials and other commonly used medications in SLE, such as azathioprine, mycophenolate mofetil, and cyclophosphamide, block NET formation in vivo in SLE patients? With NETs being potent inducers of IFN-a, drugs that target IFN, specifically IFN-a, have been studied. In SLE, there are multiple studies that establish the presence of an IFN signature, an overexpression of type I IFN-induced genes [69]. These increased gene-expression levels correlate with increased SLE disease activity [69]. As it is difficult to measure the low quantities of IFN-a, the gene-expression levels are typically measured. pdcs are the major producers of IFN-a and normally do so in response to invading viruses. NETs with their nuclear contents of dsdna similarly induce IFN-a from pdcs. IFN-a can then activate the innate and adaptive immune systems, specifically inducing a Th type 1 pathway, inhibiting T cell apoptosis, and activating B cells and antibody production [12]. Several drugs have been developed that block IFN-a, including sifalimumab (MEDI-545) and rontalizumab (RG7415), as well as other similar neutralizing antibodies to IFN-a and IFN-g [70]. Sifalimumab and rontalizumab are both fully human mab that bind to IFN-a and prevent IFN-a from signaling to its receptor. However, sifalimumab and rontalizumab target slightly different IFN-a subtypes. In a phase Ia, randomized, double-blind, placebo-controlled study, the safety profile, tolerability, and pharmacokinetics of sifalimumab were examined in 69 patients with SLE [60]. The pharmacodynamics of the type I IFN signature were assessed by measuring the expression level of type I IFN-inducible mrnas in whole blood, along with the expression of 2 IFN-a/b-inducible protein products in affected skin biopsies. Adverse event rates were similar between the sifalimumab and placebo groups. The SLE patients, with an elevated type I IFN signature at baseline, had a dose-dependent inhibition of the type I IFN signature with sifalimumab. In 274 Journal of Leukocyte Biology Volume 99, February
4. KIDNEYS AND AUTOIMMUNE DISEASE
 How to Cite this article: Kidneys and Autoimmune Disease - ejifcc 20/01 2009 http://www.ifcc.org 4. KIDNEYS AND AUTOIMMUNE DISEASE Maksimiljan Gorenjak 4.1 Autoimmune diseases The human immune system limits
How to Cite this article: Kidneys and Autoimmune Disease - ejifcc 20/01 2009 http://www.ifcc.org 4. KIDNEYS AND AUTOIMMUNE DISEASE Maksimiljan Gorenjak 4.1 Autoimmune diseases The human immune system limits
ANCA-associated vasculitis and organ-on-chips disease model
 Vaskulitis-zentrum Süd Tübingen-Kirchheim ANCA-associated vasculitis and organ-on-chips disease model Elena Csernok Klinik für Innere Medizin, Rheumatologie und Immunologie Kreiskliniken Esslingen Klinik
Vaskulitis-zentrum Süd Tübingen-Kirchheim ANCA-associated vasculitis and organ-on-chips disease model Elena Csernok Klinik für Innere Medizin, Rheumatologie und Immunologie Kreiskliniken Esslingen Klinik
DC were seeded into tissue culture dishes in IMDM 2% FCS, and added with PMN. (1:1; PMN: DC) for 16h also in the presence of DNAse (100 U/ml); DC were
 Supplementary methods Flow cytometric analysis of DCs. DC were seeded into tissue culture dishes in IMDM 2% FCS, and added with PMN (1:1; PMN: DC) for 16h also in the presence of DNAse (100 U/ml); DC were
Supplementary methods Flow cytometric analysis of DCs. DC were seeded into tissue culture dishes in IMDM 2% FCS, and added with PMN (1:1; PMN: DC) for 16h also in the presence of DNAse (100 U/ml); DC were
Supplementary Figure Legends
 Supplementary Figure Legends Supplementary Figure 1. Comparison of RNP IC-mediated NET formation. Quantification of DNA release induced by ICs consisting of SmRNP combined with SLE IgG 961 (n = 10), 1032
Supplementary Figure Legends Supplementary Figure 1. Comparison of RNP IC-mediated NET formation. Quantification of DNA release induced by ICs consisting of SmRNP combined with SLE IgG 961 (n = 10), 1032
How the Innate Immune System Profiles Pathogens
 How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
How the Innate Immune System Profiles Pathogens Receptors on macrophages, neutrophils, dendritic cells for bacteria and viruses Broad specificity - Two main groups of bacteria: gram positive, gram-negative
Autoimmune diagnostics. A comprehensive product line for the detection of autoantibodies
 Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
Autoimmune diagnostics A comprehensive product line for the detection of autoantibodies Autoimmune diagnostics Autoimmune diseases are chronic inflammatory processes with an indeterminate etiology. They
Year 2004 Paper one: Questions supplied by Megan
 QUESTION 53 Endothelial cell pathology on renal biopsy is most characteristic of which one of the following diagnoses? A. Pre-eclampsia B. Haemolytic uraemic syndrome C. Lupus nephritis D. Immunoglobulin
QUESTION 53 Endothelial cell pathology on renal biopsy is most characteristic of which one of the following diagnoses? A. Pre-eclampsia B. Haemolytic uraemic syndrome C. Lupus nephritis D. Immunoglobulin
Histopathology: Glomerulonephritis and other renal pathology
 Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Autoimmune Disease. Autoimmunity. Epidemiology. ACR Criteria for Diagnosis. Signs and Symptoms. Autoreactivity: Reactivity to self antigens:
 Autoimmunity Reactivity to self antigens: Autoreactivity: Autoimmune Disease T cells B cells Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection Epidemiology SLE Pathogenesis
Autoimmunity Reactivity to self antigens: Autoreactivity: Autoimmune Disease T cells B cells Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection Epidemiology SLE Pathogenesis
Autoimmunity. Autoimmune Disease
 Autoimmunity Reactivity to self antigens: T cells B cells Autoimmune Disease Autoreactivity: Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection 1 SLE Pathogenesis Immune
Autoimmunity Reactivity to self antigens: T cells B cells Autoimmune Disease Autoreactivity: Leading to tissue damage or dysfunction Occurring in the absence of ongoing infection 1 SLE Pathogenesis Immune
The Immune System: The Mind Body Connection. Presented by Margaret Kemeny, Ph.D. Department of Psychiatry, University of California, San Francisco
 The Immune System: The Mind Body Connection Presented by Margaret Kemeny, Ph.D. Department of Psychiatry, University of California, San Francisco Psychoneuroimmunology Investigation of the bidirectional
The Immune System: The Mind Body Connection Presented by Margaret Kemeny, Ph.D. Department of Psychiatry, University of California, San Francisco Psychoneuroimmunology Investigation of the bidirectional
Significance of Anti-C1q Antibodies in Patients with Systemic Lupus Erythematosus as A Marker of Disease Activity and Lupus Nephritis
 THE EGYPTIAN JOURNAL OF IMMUNOLOGY Vol. 23 (1), 2016 Page: 00-00 Significance of Anti-C1q Antibodies in Patients with Systemic Lupus Erythematosus as A Marker of Disease Activity and Lupus Nephritis 1
THE EGYPTIAN JOURNAL OF IMMUNOLOGY Vol. 23 (1), 2016 Page: 00-00 Significance of Anti-C1q Antibodies in Patients with Systemic Lupus Erythematosus as A Marker of Disease Activity and Lupus Nephritis 1
MOLECULAR IMMUNOLOGY Manipulation of immune response Autoimmune diseases & the pathogenic mechanism
 MOLECULAR IMMUNOLOGY Manipulation of immune response Autoimmune diseases & the pathogenic mechanism SCHMAIEL SHIRDEL CONTENT 2 Introduction Autoimmune diseases Classification Involved components Autoimmune
MOLECULAR IMMUNOLOGY Manipulation of immune response Autoimmune diseases & the pathogenic mechanism SCHMAIEL SHIRDEL CONTENT 2 Introduction Autoimmune diseases Classification Involved components Autoimmune
Neutrophils and NETs: A double edged sword
 Neutrophils and NETs: A double edged sword Paola Migliorini Immunologia Clinica e Allergologia, Università di Pisa NET: neutrophil extracellular traps In neutrophils (also in eosinophils and mast cells)
Neutrophils and NETs: A double edged sword Paola Migliorini Immunologia Clinica e Allergologia, Università di Pisa NET: neutrophil extracellular traps In neutrophils (also in eosinophils and mast cells)
Lupus as a risk factor for cardiovascular disease
 Lupus as a risk factor for cardiovascular disease SØREN JACOBSEN Department Rheumatology, Rigshospitalet Søren Jacobsen Main sponsors: Gigtforeningen Novo Nordisk Fonden Rigshospitalet Disclaimer: Novo
Lupus as a risk factor for cardiovascular disease SØREN JACOBSEN Department Rheumatology, Rigshospitalet Søren Jacobsen Main sponsors: Gigtforeningen Novo Nordisk Fonden Rigshospitalet Disclaimer: Novo
A clinical syndrome, composed mainly of:
 Nephritic syndrome We will discuss: 1)Nephritic syndrome: -Acute postinfectious (poststreptococcal) GN -IgA nephropathy -Hereditary nephritis 2)Rapidly progressive GN (RPGN) A clinical syndrome, composed
Nephritic syndrome We will discuss: 1)Nephritic syndrome: -Acute postinfectious (poststreptococcal) GN -IgA nephropathy -Hereditary nephritis 2)Rapidly progressive GN (RPGN) A clinical syndrome, composed
1. The scavenger receptor, CD36, functions as a coreceptor for which TLR? a. TLR ½ b. TLR 3 c. TLR 4 d. TLR 2/6
 Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Innate Immunity: Nonspecific Defenses of the Host
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 16 Innate Immunity: Nonspecific Defenses of the Host Host Response to Disease Resistance- ability
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 16 Innate Immunity: Nonspecific Defenses of the Host Host Response to Disease Resistance- ability
Glomerular pathology in systemic disease
 Glomerular pathology in systemic disease Lecture outline Lupus nephritis Diabetic nephropathy Glomerulonephritis Associated with Bacterial Endocarditis and Other Systemic Infections Henoch-Schonlein Purpura
Glomerular pathology in systemic disease Lecture outline Lupus nephritis Diabetic nephropathy Glomerulonephritis Associated with Bacterial Endocarditis and Other Systemic Infections Henoch-Schonlein Purpura
Overview of the immune system
 Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Overview of the immune system Immune system Innate (nonspecific) 1 st line of defense Adaptive (specific) 2 nd line of defense Cellular components Humoral components Cellular components Humoral components
Innate Immunity: (I) Molecules & (II) Cells
 Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Approach to Glomerular Diseases: Clinical Presentation Nephrotic Syndrome Nephritis
 GLOMERULONEPHRITIDES Vivette D Agati Jai Radhakrishnan Approach to Glomerular Diseases: Clinical Presentation Nephrotic Syndrome Nephritis Heavy Proteinuria Renal failure Low serum Albumin Hypertension
GLOMERULONEPHRITIDES Vivette D Agati Jai Radhakrishnan Approach to Glomerular Diseases: Clinical Presentation Nephrotic Syndrome Nephritis Heavy Proteinuria Renal failure Low serum Albumin Hypertension
Scleritis LEN V KOH OD
 Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
Scleritis LEN V KOH OD 2014 PUCO 1 Introduction A painful, destructive, and potentially blinding disorder Highly symptomatic High association with systemic disease Immunosuppresssive agents 2014 PUCO 2
Cutaneous Immunology: Innate Immune Responses. Skin Biology Lecture Series
 Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
Cutaneous Immunology: Innate Immune Responses Skin Biology Lecture Series The Immune Response: Innate and Adaptive Components Source: Wolff, Goldsmith, Katz, Gilchrest, Paller, Leffell. Fitzpatrick s Dermatology
Test Name Results Units Bio. Ref. Interval
 135091662 Age 45 Years Gender Male 29/8/2017 120000AM 29/8/2017 100215AM 29/8/2017 110825AM Ref By Final RHEUMATOID AUTOIMMUNE COMREHENSIVE ANEL ANTI NUCLEAR ANTIBODY / FACTOR (ANA/ANF), SERUM ----- 20-60
135091662 Age 45 Years Gender Male 29/8/2017 120000AM 29/8/2017 100215AM 29/8/2017 110825AM Ref By Final RHEUMATOID AUTOIMMUNE COMREHENSIVE ANEL ANTI NUCLEAR ANTIBODY / FACTOR (ANA/ANF), SERUM ----- 20-60
Editing file. Color code: Important in red Extra in blue. Autoimmune Diseases
 Editing file Color code: Important in red Extra in blue Autoimmune Diseases Objectives To know that the inflammatory processes in autoimmune diseases are mediated by hypersensitivity reactions (type II,
Editing file Color code: Important in red Extra in blue Autoimmune Diseases Objectives To know that the inflammatory processes in autoimmune diseases are mediated by hypersensitivity reactions (type II,
Anaphylactic response in rabbit Part II
 Anaphylactic response in rabbit Part II Introduction Four types of hypersensitivity reactions: Type I: allergy Type II: antibodies Type III: immune complex Type IV: T-cells Type I Hypersensitivity ALLERGY
Anaphylactic response in rabbit Part II Introduction Four types of hypersensitivity reactions: Type I: allergy Type II: antibodies Type III: immune complex Type IV: T-cells Type I Hypersensitivity ALLERGY
Case Presentation VASCULITIS. Case Presentation. Case Presentation. Vasculitis
 Case Presentation VASCULITIS The patient is a 24 year old woman who presented to the emergency room with left-sided weakness. She was confused and complained of a severe headache. She was noted to have
Case Presentation VASCULITIS The patient is a 24 year old woman who presented to the emergency room with left-sided weakness. She was confused and complained of a severe headache. She was noted to have
Committee Approval Date: May 9, 2014 Next Review Date: May 2015
 Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Medication Policy Manual Policy No: dru248 Topic: Benlysta, belimumab Date of Origin: May 13, 2011 Committee Approval Date: May 9, 2014 Next Review Date: May 2015 Effective Date: June 1, 2014 IMPORTANT
Understanding Autoimmune Diseases: Evolving Issues. Alvina D. Chu, M.D. April 23, 2009
 Understanding Autoimmune Diseases: Evolving Issues Alvina D. Chu, M.D. April 23, 2009 Objectives Define the key pathogenic characteristics of: Type I diabetes mellitus Multiple sclerosis Rheumatoid arthritis
Understanding Autoimmune Diseases: Evolving Issues Alvina D. Chu, M.D. April 23, 2009 Objectives Define the key pathogenic characteristics of: Type I diabetes mellitus Multiple sclerosis Rheumatoid arthritis
HYPERSENSITIVITY REACTIONS D R S H O AI B R AZ A
 HYPERSENSITIVITY REACTIONS D R S H O AI B R AZ A HYPERSENSITIVITY REACTIONS Are exaggerated immune response upon antigenic stimulation Individuals who have been previously exposed to an antigen are said
HYPERSENSITIVITY REACTIONS D R S H O AI B R AZ A HYPERSENSITIVITY REACTIONS Are exaggerated immune response upon antigenic stimulation Individuals who have been previously exposed to an antigen are said
Self-tolerance. Lack of immune responsiveness to an individual s own tissue antigens. Central Tolerance. Peripheral tolerance
 Autoimmunity Self-tolerance Lack of immune responsiveness to an individual s own tissue antigens Central Tolerance Peripheral tolerance Factors Regulating Immune Response Antigen availability Properties
Autoimmunity Self-tolerance Lack of immune responsiveness to an individual s own tissue antigens Central Tolerance Peripheral tolerance Factors Regulating Immune Response Antigen availability Properties
Immune System. Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka
 Immune System Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka Content Standards 35.1 In innate immunity, recognition and response rely on traits common to groups of pathogens 35.2
Immune System Presented by Kazzandra Anton, Rhea Chung, Lea Sado, and Raymond Tanaka Content Standards 35.1 In innate immunity, recognition and response rely on traits common to groups of pathogens 35.2
VASCULITIS. Case Presentation. Case Presentation
 VASCULITIS Case Presentation The patient is a 24 year old woman who presented to the emergency room with left-sided weakness. She was confused and complained of a severe headache. She was noted to have
VASCULITIS Case Presentation The patient is a 24 year old woman who presented to the emergency room with left-sided weakness. She was confused and complained of a severe headache. She was noted to have
Is it Autoimmune or NOT! Presented to AONP! October 2015!
 Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
.,Dr Ali Alkazzaz Babylon collage of medicine 2016
 .,Dr Ali Alkazzaz Babylon collage of medicine 2016 Lupus history Lupus is the Latin word for wolf 1 st used medically in the 10 th century Described clinically in the 19 th century Butterfly rash in 1845
.,Dr Ali Alkazzaz Babylon collage of medicine 2016 Lupus history Lupus is the Latin word for wolf 1 st used medically in the 10 th century Described clinically in the 19 th century Butterfly rash in 1845
Fc receptors, phagocytosis role 128
 Subject Index Adaptive immunity dependence on innate immunity 9, 10 evolution 10 Aging anti-inflammatory agents in counteraction 202 beneficial polymorphisms 199 201 definition 18, 189 innate immunity
Subject Index Adaptive immunity dependence on innate immunity 9, 10 evolution 10 Aging anti-inflammatory agents in counteraction 202 beneficial polymorphisms 199 201 definition 18, 189 innate immunity
Dr Rodney Itaki Lecturer Anatomical Pathology Discipline. University of Papua New Guinea School of Medicine & Health Sciences Division of Pathology
 Vasculitis Dr Rodney Itaki Lecturer Anatomical Pathology Discipline University of Papua New Guinea School of Medicine & Health Sciences Division of Pathology Disease Spectrum Hypersensitivity vasculitis/microscopic
Vasculitis Dr Rodney Itaki Lecturer Anatomical Pathology Discipline University of Papua New Guinea School of Medicine & Health Sciences Division of Pathology Disease Spectrum Hypersensitivity vasculitis/microscopic
All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity
 1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
The Innate Immune Response
 The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
The Innate Immune Response FUNCTIONS OF THE IMMUNE SYSTEM: Recognize, destroy and clear a diversity of pathogens. Initiate tissue and wound healing processes. Recognize and clear damaged self components.
Introduce the important components of the immune system Show how they interact & protect the body
 Immunology in Rheumatic Diseases Knowledge of immunology forms the basis of understanding many of the Rheumatologic diseases and has become the focus of many exciting new treatment strategies. AIMS OF
Immunology in Rheumatic Diseases Knowledge of immunology forms the basis of understanding many of the Rheumatologic diseases and has become the focus of many exciting new treatment strategies. AIMS OF
Diseases of Immunity 2017 CL Davis General Pathology. Paul W. Snyder, DVM, PhD Experimental Pathology Laboratories, Inc.
 Diseases of Immunity 2017 CL Davis General Pathology Paul W. Snyder, DVM, PhD Experimental Pathology Laboratories, Inc. Autoimmunity Reflects a loss of immunologic tolerance Mechanisms Auto-antibodies
Diseases of Immunity 2017 CL Davis General Pathology Paul W. Snyder, DVM, PhD Experimental Pathology Laboratories, Inc. Autoimmunity Reflects a loss of immunologic tolerance Mechanisms Auto-antibodies
LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS LUPUS 101 SLE SUBSETS AUTOIMMUNE DISEASE 11/4/2013 HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS
 LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
Vasculitis. Edward Dwyer, M.D. Division of Rheumatology. Vasculitis
 Edward Dwyer, M.D. Division of Rheumatology VASCULITIS is a primary inflammatory disease process of the vasculature Determinants of the Clinical Manifestations of : Target organ involved Size of vessel
Edward Dwyer, M.D. Division of Rheumatology VASCULITIS is a primary inflammatory disease process of the vasculature Determinants of the Clinical Manifestations of : Target organ involved Size of vessel
EDITORIAL. Issue Seventeen, October Editorial Team. Issue Seventeen. Info link
 EDITORIAL, October 2004 Welcome to the Spring 2004 Edition of InfoLink. The feature article in this edition has been written by Dr Rodger Laurent, Head of Department, PaLMS Rheumatology Laboratory. The
EDITORIAL, October 2004 Welcome to the Spring 2004 Edition of InfoLink. The feature article in this edition has been written by Dr Rodger Laurent, Head of Department, PaLMS Rheumatology Laboratory. The
Innate Immunity: (I) Molecules & (II) Cells. Part II: Cells (aka the Sentinels)
 Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Innate Immunity: (I) Molecules & (II) Cells Stephanie Eisenbarth, M.D., Ph.D. FOCIS Advanced Course 2/19/18 Department of Laboratory Medicine Yale School of Medicine Department of Immunobiology Yale School
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Medical Immunology Practice Questions-2016 Autoimmunity + Case Studies
 Medical Immunology Practice Questions-2016 Autoimmunity + Case Studies Directions: Each of the numbered items or incomplete statements in this section is followed by answers or by completions of the statement.
Medical Immunology Practice Questions-2016 Autoimmunity + Case Studies Directions: Each of the numbered items or incomplete statements in this section is followed by answers or by completions of the statement.
Disease causing organisms Resistance Immunity
 Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Part 1 Disease causing organisms Resistance Immunity Bacteria Most common pathogens Anthrax Cholera Staphylococcus epidermidis bacteria Bacterial diseases Tuberculosis Cholera Bubonic Plague Tetanus Effects
Elevated Expression of Pentraxin 3 in Anti-neutrophil Cytoplasmic Antibody-associated Glomerulonephritis with Normal Serum C-reactive Protein
 CASE REPORT Elevated Expression of Pentraxin 3 in Anti-neutrophil Cytoplasmic Antibody-associated Glomerulonephritis with Normal Serum C-reactive Protein Risa Ishida 1,KentaroNakai 1, Hideki Fujii 1, Shunsuke
CASE REPORT Elevated Expression of Pentraxin 3 in Anti-neutrophil Cytoplasmic Antibody-associated Glomerulonephritis with Normal Serum C-reactive Protein Risa Ishida 1,KentaroNakai 1, Hideki Fujii 1, Shunsuke
Willcocks et al.,
 ONLINE SUPPLEMENTAL MATERIAL Willcocks et al., http://www.jem.org/cgi/content/full/jem.20072413/dc1 Supplemental materials and methods SLE and AASV cohorts The UK SLE cohort (n = 171) was obtained from
ONLINE SUPPLEMENTAL MATERIAL Willcocks et al., http://www.jem.org/cgi/content/full/jem.20072413/dc1 Supplemental materials and methods SLE and AASV cohorts The UK SLE cohort (n = 171) was obtained from
Autoimmune diseases, their pathogenic mechanisms and treatment of unwanted immune responses (Janeway s Immunobiology)
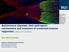 Autoimmune diseases, their pathogenic mechanisms and treatment of unwanted immune responses (Janeway s Immunobiology) Picture source: https://www.klini kum.uniheidelberg.de/fil eadmin/pressest elle/pm_neu/20
Autoimmune diseases, their pathogenic mechanisms and treatment of unwanted immune responses (Janeway s Immunobiology) Picture source: https://www.klini kum.uniheidelberg.de/fil eadmin/pressest elle/pm_neu/20
Innate Immunity. Chapter 3. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples. Antimicrobial peptide psoriasin
 Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
Chapter Know Differences and Provide Examples Innate Immunity kin and Epithelial Barriers Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive Immunity
History. Chapter 13. Complement Components. Complement Pathways
 History Chapter 13 Complement Jules Border in 1890 s discovered complement Paul Ehrlich coined the term complement The activity of blood serum that completes the action of antibody Now: Set of serum proteins
History Chapter 13 Complement Jules Border in 1890 s discovered complement Paul Ehrlich coined the term complement The activity of blood serum that completes the action of antibody Now: Set of serum proteins
Innate Immunity. Natural or native immunity
 Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
Immunity and Infection. Chapter 17
 Immunity and Infection Chapter 17 The Chain of Infection Transmitted through a chain of infection (six links) Pathogen: Disease causing microorganism Reservoir: Natural environment of the pathogen Portal
Immunity and Infection Chapter 17 The Chain of Infection Transmitted through a chain of infection (six links) Pathogen: Disease causing microorganism Reservoir: Natural environment of the pathogen Portal
Complement. History. Chapter 7. Complement Components. Complement Pathways. Pathways of complement activation
 History Chapter 7 Complement Jules Border in 1890 s discovered complement Paul Ehrlich coined the term complement The activity of blood serum that completes the action of antibody Now: Set of serum proteins
History Chapter 7 Complement Jules Border in 1890 s discovered complement Paul Ehrlich coined the term complement The activity of blood serum that completes the action of antibody Now: Set of serum proteins
Vasculitis local: systemic
 Vasculitis Inflammation of the vessel wall. Signs and symptoms: 1- local: according to the involved tissue 2- systemic:(fever, myalgia, arthralgias, and malaise) Pathogenesis 1- immune-mediated 2- infectious
Vasculitis Inflammation of the vessel wall. Signs and symptoms: 1- local: according to the involved tissue 2- systemic:(fever, myalgia, arthralgias, and malaise) Pathogenesis 1- immune-mediated 2- infectious
VASCULITIS PRODUCT HIGHLIGHTS
 VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
VASCULITIS PRODUCT HIGHLIGHTS AESKU.DIAGNOSTICS offers a comprehensive and complete diagnostic portfolio in the field of vasculitis diagnostics. Not only are screening and profiling s available but also
Chapter 22: The Lymphatic System and Immunity
 Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Autoimmunity. Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens
 Autoimmunity Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens Autoimmune disease can be caused to primary defects in B cells, T cells and possibly
Autoimmunity Autoimmunity arises because of defects in central or peripheral tolerance of lymphocytes to selfantigens Autoimmune disease can be caused to primary defects in B cells, T cells and possibly
Mechanisms of Autontibodies
 Mechanisms of Autontibodies Production in Rheumatic Diseases Eisa Salehi PhD Tehran University of Medical Sciences Immunology Department Introduction Rheumatic diseases: Cause inflammation, swelling, and
Mechanisms of Autontibodies Production in Rheumatic Diseases Eisa Salehi PhD Tehran University of Medical Sciences Immunology Department Introduction Rheumatic diseases: Cause inflammation, swelling, and
Physiology Unit 3. ADAPTIVE IMMUNITY The Specific Immune Response
 Physiology Unit 3 ADAPTIVE IMMUNITY The Specific Immune Response In Physiology Today The Adaptive Arm of the Immune System Specific Immune Response Internal defense against a specific pathogen Acquired
Physiology Unit 3 ADAPTIVE IMMUNITY The Specific Immune Response In Physiology Today The Adaptive Arm of the Immune System Specific Immune Response Internal defense against a specific pathogen Acquired
Innate Immunity. Natural or native immunity
 Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
Innate Immunity 1 Innate Immunity Natural or native immunity 2 When microbes enter in the body 3 Secondly, it also stimulates the adaptive immune system 4 Immunologic memory 5 Components of Innate Immunity
Autoimmune Diseases. Betsy Kirchner CNP The Cleveland Clinic
 Autoimmune Diseases Betsy Kirchner CNP The Cleveland Clinic Disclosures (financial) No relevant disclosures Learning Objectives Explain the pathophysiology of autoimmune disease Discuss safe administration
Autoimmune Diseases Betsy Kirchner CNP The Cleveland Clinic Disclosures (financial) No relevant disclosures Learning Objectives Explain the pathophysiology of autoimmune disease Discuss safe administration
Innate immunity. Abul K. Abbas University of California San Francisco. FOCiS
 1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
1 Innate immunity Abul K. Abbas University of California San Francisco FOCiS 2 Lecture outline Components of innate immunity Recognition of microbes and dead cells Toll Like Receptors NOD Like Receptors/Inflammasome
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
PNH Glossary of Terms
 AA Absolute neutrophil count Alendronate Allergen ALT Anemia Antibodies Anticoagulant Anticoagulation Antigen Antithymocyte globulin (ATG) Aplastic Aplastic anemia Band Bilirubin Blast cells Bone marrow
AA Absolute neutrophil count Alendronate Allergen ALT Anemia Antibodies Anticoagulant Anticoagulation Antigen Antithymocyte globulin (ATG) Aplastic Aplastic anemia Band Bilirubin Blast cells Bone marrow
Rheumatologic Lab Tests
 Rheumatologic Lab Tests What the Practitioner Needs to Know Mary Nakamura M.D. 2008 Rheumatologic Lab Tests Are rarely diagnostic of any specific disease If you do not have in mind a rheumatologic disease
Rheumatologic Lab Tests What the Practitioner Needs to Know Mary Nakamura M.D. 2008 Rheumatologic Lab Tests Are rarely diagnostic of any specific disease If you do not have in mind a rheumatologic disease
2. Innate immunity 2013
 1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
1 Innate Immune Responses 3 Innate immunity Abul K. Abbas University of California San Francisco The initial responses to: 1. Microbes: essential early mechanisms to prevent, control, or eliminate infection;
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia
 Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
Alida R Harahap & Farida Oesman Department of Clinical Pathology Faculty of Medicine, University of Indonesia Foreign molecules = antigens Immune response Immune system non-specific specific cellular humoral
Cell-Derived Inflammatory Mediators
 Cell-Derived Inflammatory Mediators Introduction about chemical mediators in inflammation Mediators may be Cellular mediators cell-produced or cell-secreted derived from circulating inactive precursors,
Cell-Derived Inflammatory Mediators Introduction about chemical mediators in inflammation Mediators may be Cellular mediators cell-produced or cell-secreted derived from circulating inactive precursors,
9/25/2013 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
11/25/2017. THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS BARRIER DEFENSES INNATE IMMUNITY OF VERTEBRATES
 THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS Exoskeleton made of chitin forms the first barrier to pathogens Digestive system is protected by a chitin-based barrier and lysozyme,
THE IMMUNE SYSTEM Chapter 43 IMMUNITY INNATE IMMUNITY EXAMPLE IN INSECTS Exoskeleton made of chitin forms the first barrier to pathogens Digestive system is protected by a chitin-based barrier and lysozyme,
Natural Defense Mechanisms
 Color code: Important in red Extra in blue For team error adjustments, click here Natural Defense Mechanisms Objectives To know First (non-specific immunity) and second (adaptive immunity) lines of defense
Color code: Important in red Extra in blue For team error adjustments, click here Natural Defense Mechanisms Objectives To know First (non-specific immunity) and second (adaptive immunity) lines of defense
Glomerular diseases mostly presenting with Nephritic syndrome
 Glomerular diseases mostly presenting with Nephritic syndrome 1 The Nephritic Syndrome Pathogenesis: proliferation of the cells in glomeruli & leukocytic infiltrate Injured capillary walls escape of RBCs
Glomerular diseases mostly presenting with Nephritic syndrome 1 The Nephritic Syndrome Pathogenesis: proliferation of the cells in glomeruli & leukocytic infiltrate Injured capillary walls escape of RBCs
GOODPASTURE'S SYNDROME WITH CONCOMITANT IMMUNE COMPLEX MIXED MEMBRANOUS AND PROLIFERATIVE GLOMERULONEFRITIS
 GOODPASTURE'S SYNDROME WITH CONCOMITANT IMMUNE COMPLEX MIXED MEMBRANOUS AND PROLIFERATIVE GLOMERULONEFRITIS VESNA JURČIĆ 1, ANDREJA ALEŠ RIGLER 2, INSTITUTE OF PATHOLOGY, FACULTY OF MEDICINE, UNIVERSITY
GOODPASTURE'S SYNDROME WITH CONCOMITANT IMMUNE COMPLEX MIXED MEMBRANOUS AND PROLIFERATIVE GLOMERULONEFRITIS VESNA JURČIĆ 1, ANDREJA ALEŠ RIGLER 2, INSTITUTE OF PATHOLOGY, FACULTY OF MEDICINE, UNIVERSITY
MAF Shalaby Prof. Rheumatology Al Azhar University, Cairo, Egypt.
 MAF Shalaby Prof. Rheumatology Al Azhar University, Cairo, Egypt. AUTOIMMUNE DISEASE RA SLE VASCULITIS RELAPSING POLYCHONDRITIS SS DM/PM SJOGREN S SYNDROME RHEUMATOID ARTHRITIS Classically immune mediated
MAF Shalaby Prof. Rheumatology Al Azhar University, Cairo, Egypt. AUTOIMMUNE DISEASE RA SLE VASCULITIS RELAPSING POLYCHONDRITIS SS DM/PM SJOGREN S SYNDROME RHEUMATOID ARTHRITIS Classically immune mediated
Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals. Taniawati Supali. Department of Parasitology
 Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals Taniawati Supali Department of Parasitology 1 Defense mechanism in human Th17 (? ) Acute Chronic Th1 Th 2 Intracellular Treg
Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals Taniawati Supali Department of Parasitology 1 Defense mechanism in human Th17 (? ) Acute Chronic Th1 Th 2 Intracellular Treg
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases. Abul K. Abbas UCSF
 Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Tolerance, autoimmunity and the pathogenesis of immunemediated inflammatory diseases Abul K. Abbas UCSF Balancing lymphocyte activation and control Activation Effector T cells Tolerance Regulatory T cells
Dr Ian Roberts Oxford
 Dr Ian Roberts Oxford Oxford Pathology Course 2010 for FRCPath Present the basic diagnostic features of the commonest conditions causing renal failure Highlight diagnostic pitfalls. Crescentic GN: renal
Dr Ian Roberts Oxford Oxford Pathology Course 2010 for FRCPath Present the basic diagnostic features of the commonest conditions causing renal failure Highlight diagnostic pitfalls. Crescentic GN: renal
Immune System AP SBI4UP
 Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
Immune System AP SBI4UP TYPES OF IMMUNITY INNATE IMMUNITY ACQUIRED IMMUNITY EXTERNAL DEFENCES INTERNAL DEFENCES HUMORAL RESPONSE Skin Phagocytic Cells CELL- MEDIATED RESPONSE Mucus layer Antimicrobial
ANCA associated vasculitis in China
 ANCA associated vasculitis in China Min Chen Renal Division, Peking University First Hospital, Beijing 100034, P. R. China 1 General introduction of AAV in China Disease spectrum and ANCA type Clinical
ANCA associated vasculitis in China Min Chen Renal Division, Peking University First Hospital, Beijing 100034, P. R. China 1 General introduction of AAV in China Disease spectrum and ANCA type Clinical
Innate Immunity. Connection Between Innate and Adaptive Immunity. Know Differences and Provide Examples Chapter 3. Antimicrobial peptide psoriasin
 Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Know Differences and Provide Examples Chapter * Innate Immunity * kin and Epithelial Barriers * Antimicrobial peptide psoriasin -Activity against Gram (-) E. coli Connection Between Innate and Adaptive
Lupus Related Kidney Diseases. Jason Cobb MD Assistant Professor Renal Division Emory University School of Medicine October 14, 2017
 Lupus Related Kidney Diseases Jason Cobb MD Assistant Professor Renal Division Emory University School of Medicine October 14, 2017 Financial Disclosures MedImmune Lupus Nephritis Kidney Biopsy Biomarkers
Lupus Related Kidney Diseases Jason Cobb MD Assistant Professor Renal Division Emory University School of Medicine October 14, 2017 Financial Disclosures MedImmune Lupus Nephritis Kidney Biopsy Biomarkers
Hematopoiesis. Hematopoiesis. Hematopoiesis
 Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Chapter. Cells and Organs of the Immune System Hematopoiesis Hematopoiesis- formation and development of WBC and RBC bone marrow. Hematopoietic stem cell- give rise to any blood cells (constant number,
Disorders of the kidney. Urine analysis. Nephrotic and nephritic syndrome.
 Disorders of the kidney. Urine analysis. Nephrotic and nephritic syndrome. Azotemia and Urinary Abnormalities Disturbances in urine volume oliguria, anuria, polyuria Abnormalities of urine sediment red
Disorders of the kidney. Urine analysis. Nephrotic and nephritic syndrome. Azotemia and Urinary Abnormalities Disturbances in urine volume oliguria, anuria, polyuria Abnormalities of urine sediment red
LABORATORY STANDARDS IN THE DIAGNOSIS AND THERAPY MONITORING OF SYSTEMIC LUPUS ERYTHEMATOSUS
 LABORATORY STANDARDS IN THE DIAGNOSIS AND THERAPY MONITORING OF SYSTEMIC LUPUS ERYTHEMATOSUS Prof. Sandor Sipka, M.D., Ph.D. 3rd Department of Medicine, Institute for Internal Medicine, Medical and Health
LABORATORY STANDARDS IN THE DIAGNOSIS AND THERAPY MONITORING OF SYSTEMIC LUPUS ERYTHEMATOSUS Prof. Sandor Sipka, M.D., Ph.D. 3rd Department of Medicine, Institute for Internal Medicine, Medical and Health
Components of the innate immune system
 Components of the innate immune system Before our discussion about innate immunity Differences between innate and adaptive systems: Innate immune system = natural = native -Germline: prepared before exposure
Components of the innate immune system Before our discussion about innate immunity Differences between innate and adaptive systems: Innate immune system = natural = native -Germline: prepared before exposure
Application of genetic signatures to clinical care of lupus
 Application of genetic signatures to clinical care of lupus Dr Durga Prasanna Misra MD, MRCP(UK), DM Assistant Professor Department of Clinical Immunology, JIPMER, Puducherry. LAYOUT Genetics of lupus
Application of genetic signatures to clinical care of lupus Dr Durga Prasanna Misra MD, MRCP(UK), DM Assistant Professor Department of Clinical Immunology, JIPMER, Puducherry. LAYOUT Genetics of lupus
Subject Index. Bcl-2, apoptosis regulation Bone marrow, polymorphonuclear neutrophil release 24, 26
 Subject Index A1, apoptosis regulation 217, 218 Adaptive immunity, polymorphonuclear neutrophil role 31 33 Angiogenesis cancer 178 endometrium remodeling 172 HIV Tat induction mechanism 176 inflammatory
Subject Index A1, apoptosis regulation 217, 218 Adaptive immunity, polymorphonuclear neutrophil role 31 33 Angiogenesis cancer 178 endometrium remodeling 172 HIV Tat induction mechanism 176 inflammatory
Lecture on Innate Immunity and Inflammation. Innate Immunity: An Evolutionary View
 Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Lecture on Innate Immunity and Inflammation Evolutionary View Epithelial barriers to infection Four main types of innate recognition molecules:tlrs, CLRs, NLRs, RLRs NF-κB, the master transcriptional regulator
Immunity to Viruses. Patricia Fitzgerald-Bocarsly September 25, 2008
 Immunity to Viruses Patricia Fitzgerald-Bocarsly September 25, 2008 The Immune System Deals with a Huge Range of Pathogens Roitt, 2003 Immune Responses to Viruses Viruses are dependent on the host cell
Immunity to Viruses Patricia Fitzgerald-Bocarsly September 25, 2008 The Immune System Deals with a Huge Range of Pathogens Roitt, 2003 Immune Responses to Viruses Viruses are dependent on the host cell
Chapter 17. The Lymphatic System and Immunity. Copyright 2010, John Wiley & Sons, Inc.
 Chapter 17 The Lymphatic System and Immunity Immunity Innate Immunity Fast, non-specific and no memory Barriers, ph extremes, Phagocytes & NK cells, fever, inflammation, complement, interferon Adaptive
Chapter 17 The Lymphatic System and Immunity Immunity Innate Immunity Fast, non-specific and no memory Barriers, ph extremes, Phagocytes & NK cells, fever, inflammation, complement, interferon Adaptive
THE KIDNEY AND SLE LUPUS NEPHRITIS
 THE KIDNEY AND SLE LUPUS NEPHRITIS JACK WATERMAN DO FACOI 2013 NEPHROLOGY SIR RICHARD BRIGHT TERMINOLOGY RENAL INSUFFICIENCY CKD (CHRONIC KIDNEY DISEASE) ESRD (ENDSTAGE RENAL DISEASE) GLOMERULONEPHRITIS
THE KIDNEY AND SLE LUPUS NEPHRITIS JACK WATERMAN DO FACOI 2013 NEPHROLOGY SIR RICHARD BRIGHT TERMINOLOGY RENAL INSUFFICIENCY CKD (CHRONIC KIDNEY DISEASE) ESRD (ENDSTAGE RENAL DISEASE) GLOMERULONEPHRITIS
AN OVERVIEW OF ANCA-ASSOCIATED VASCULITIS
 GRANULOMATOSIS WITH POLYANGIITIS (GPA), MICROSCOPIC POLYANGIITIS (MPA), and EOSINOPHILIC GRANULOMATOSIS WITH POLYANGIITIS (EGPA) AN OVERVIEW OF ANCA-ASSOCIATED VASCULITIS What is ANCA-associated Vasculitis?
GRANULOMATOSIS WITH POLYANGIITIS (GPA), MICROSCOPIC POLYANGIITIS (MPA), and EOSINOPHILIC GRANULOMATOSIS WITH POLYANGIITIS (EGPA) AN OVERVIEW OF ANCA-ASSOCIATED VASCULITIS What is ANCA-associated Vasculitis?
Arteriosclerosis & Atherosclerosis
 Arteriosclerosis & Atherosclerosis Arteriosclerosis = hardening of arteries = arterial wall thickening + loss of elasticity 3 types: -Arteriolosclerosis -Monckeberg medial sclerosis -Atherosclerosis Arteriosclerosis,
Arteriosclerosis & Atherosclerosis Arteriosclerosis = hardening of arteries = arterial wall thickening + loss of elasticity 3 types: -Arteriolosclerosis -Monckeberg medial sclerosis -Atherosclerosis Arteriosclerosis,
Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel:
 Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel: 4677363 aalshamsan@ksu.edu.sa Learning Objectives By the end of this lecture you will be able to: 1 Understand the physiological
Cytokines (II) Dr. Aws Alshamsan Department of Pharmaceu5cs Office: AA87 Tel: 4677363 aalshamsan@ksu.edu.sa Learning Objectives By the end of this lecture you will be able to: 1 Understand the physiological
Unit 5 The Human Immune Response to Infection
 Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
Unit 5 The Human Immune Response to Infection Unit 5-page 1 FOM Chapter 21 Resistance and the Immune System: Innate Immunity Preview: In Chapter 21, we will learn about the branch of the immune system
Potential Rebalancing of the Immune System by Anti-CD52 Therapy
 Potential Rebalancing of the Immune System by Anti-CD52 Therapy Johanne Kaplan, PhD VP Neuroimmunology Research Genzyme March 26, 2013 RESTRICTED USE SEE TRAINING MEMO 2011 DO Genzyme NOT 1COPY Corporation
Potential Rebalancing of the Immune System by Anti-CD52 Therapy Johanne Kaplan, PhD VP Neuroimmunology Research Genzyme March 26, 2013 RESTRICTED USE SEE TRAINING MEMO 2011 DO Genzyme NOT 1COPY Corporation
