Daily versus weekly azithromycin in cystic fibrosis patients
|
|
|
- Marcus Eaton
- 5 years ago
- Views:
Transcription
1 Eur Respir J 2007; 30: DOI: / CopyrightßERS Journals Ltd 2007 Daily versus weekly azithromycin in cystic fibrosis patients J. McCormack*, S. Bell #, S. Senini*, K. Walmsley*, K. Patel*, C. Wainwright ", D. Serisier +, M. Harris 1 and S. Bowler + ABSTRACT: Four randomised, placebo-controlled trials have previously documented the clinical benefits of azithromycin (AZM) in cystic fibrosis (CF) patients. The present study examined whether the beneficial effect of AZM is equivalent when administered daily or weekly. A double-blind, randomised study was carried out in 208 CF patients aged 6 58 yrs who were assigned to AZM either 250 mg daily (n5103) or 1,200 mg weekly (n5105) for 6 months, with assessments at baseline and at 1, 3, 6 and 7 months. Patients were taken from five adult and children CF centres in South-east Queensland, Australia. Equivalence was demonstrated between the two groups (daily versus weekly) with respect to improvements in lung function (forced expiratory volume in one second and forced vital capacity), C-reactive protein, days spent in hospital, admission rates and nutrition (body mass index, z- scores) using 95% confidence intervals with a tolerance interval of 10%. In patients aged,18 yrs the daily group had significantly better improvements in z-scores for height and weight after 6 months. In children, a nutritional advantage for daily administration was found. Gastro-intestinal adverse effects were more common with weekly therapy. Apart from these findings, daily and weekly administered azithromycin demonstrated similar outcomes for cystic fibrosis patients. KEYWORDS: Azithromycin, cystic fibrosis, macrolides, Pseudomonas aeruginosa The idea of using macrolides in cystic fibrosis (CF) originated from a Japanese study, which demonstrated beneficial effects from erythromycin in diffuse panbronchiolitis, a disease with many similarities to CF [1]. To date four clinical trials have been carried out to demonstrate the beneficial effects of azithromycin (AZM) in patients with CF [2 5]. Despite differences in study populations (adult, paediatric and mixed), duration (3 12 months) and dosage, all studies demonstrated clinical improvement, which included significant improvement in lung function in three out of the four studies [2 4]. Interestingly, the longest study (12 months) failed to demonstrate significant improvement in spirometry in patients receiving AZM when compared with placebo. Reductions in hospitalisation and requirements for antibiotic therapy, decreased systemic markers of inflammation, and improvements in quality of life have been reported. Respiratory exacerbations and requirements for i.v. antibiotics were reduced in three of the studies [2, 3, 5] Furthermore, AZM has been well tolerated in each of the four studies. None of the studies demonstrated an apparent change in airway flora. There is ongoing concern about the long-term effects of macrolide therapy and whether such therapy may predispose to increased antimicrobial resistance of bacteria including Staphylococcus aureus, Streptococcus pneumoniae and Haemophilus influenzae [6, 7]. AZM has been shown to accumulate within tissues and neutrophils and this has been related to its prolonged biological effects [8 10]. These properties are different to other commonly prescribed macrolides, such as erythromycin, roxithromycin and clarithromycin, and may be responsible for the proposed anti-inflammatory effects of AZM demonstrated in CF patients. In a recent study examining the pharmacokinetics of AZM in plasma, neutrophils and sputum in CF patients, WILMS et al. [11] demonstrated marked accumulation of AZM in neutrophils with a mean half-life of 12 days. The concentration of AZM in sputum was also very high and still detectable 10 days after the last dose was administered. The authors suggested that this long half-life and persistence in sputum may allow less frequent dosing (e.g. once a week) in patients with CF, which may encourage adherence. Many questions remain regarding the therapeutic use of AZM and other macrolides in patients with CF, including: dosing regimens (dose, AFFILIATIONS *Dept of Medicine and Infectious Diseases, and + Adult Cystic Fibrosis Unit, Mater Adult Hospital, # Adult Cystic Fibrosis Centre, The Prince Charles Hospital, " Dept of Paediatrics and Child Health, Royal Children s Hospital, and 1 Mater Children s Hospital, University of Queensland, South Brisbane, Queensland, Australia. CORRESPONDENCE J. McCormack Dept of Medicine and Infectious Diseases University of Queensland Mater Adult Hospital South Brisbane 4101 Queensland Australia Fax: jmccorma@mater.org.au Received: December Accepted after revision: May SUPPORT STATEMENT The study was supported by the Australian Cystic Fibrosis Research Trust (Sydney, Australia). STATEMENT OF INTEREST A statement of interest for this study can be found at statements.shtml European Respiratory Journal Print ISSN Online ISSN c EUROPEAN RESPIRATORY JOURNAL VOLUME 30 NUMBER 3 487
2 AZITHROMYCIN IN CF J. MCCORMACK ET AL. frequency and duration); who should be treated; whether other macrolides are effective; and the long-term benefits and risks of continued AZM therapy [12]. The major objective of the present study was to determine the clinical effects of AZM administered weekly in children and adults with CF using a parallel group, randomised double-blind study design of 6 months duration. The study was powered to determine whether weekly and daily AZM were equivalent in their effect on forced expiratory volume in one second (FEV1) % predicted. METHODS Design A double-blind randomised comparative study of 250 mg daily versus 1,200 mg weekly AZM in adults and children with CF in five Australian centres was carried out. Eligibility criteria for recruitment were: 1) age o6 yrs; 2) weight o25 kg; 3) clinical stability, defined as patients experiencing lung function (FEV1)ato90% of the mean outpatient FEV1 recorded over the previous 6 months, with o2 weeks since completion of last i.v./oral antibiotics for a pulmonary exacerbation; and 4) no concurrent change in medication. Patients were recruited irrespective of whether or not they were infected with Pseudomonas aeruginosa. Exclusion criteria included: 1) inability to provide informed consent; 2) known allergy to macrolides; 3) macrolide therapy in the previous 8 weeks; 4) severe liver disease (defined as proven cirrhosis and/or portal hypertension); 5) non-tuberculous mycobacteria isolated in sputum in the previous 2 yrs; 6) commencement of mucolytic or antiinflammatory therapy that had not been part of therapy prior to trial enrolment; 7) concomitant use of drugs contraindicated in patients taking macrolides (digoxin, cyclosporine, terfenadine, zidovidine or ergotamines); 8) transplant recipient patients; 9) patients who were pregnant, their partner was pregnant or they intended to get pregnant in the following 6 months; and 10) active involvement in another clinical trial. Usual concomitant therapy, including physiotherapy, maintenance antibiotics, inhaled medications and pancreatic and vitamin supplements, continued throughout the trial and was monitored by the clinic physicians. Ethical approval was granted at each of the participating hospitals. After written informed consent by patients, or parents/guardians for patients aged,18 yrs, patients were randomised to receive AZM daily or once a week for 6 months. Randomisation Randomisation was performed by the hospital pharmacy independently of trial staff prior to study commencement. Randomisation occurred in blocks of 10 patients and was stratified for sex and illness severity (FEV1,40, and.70% pred). There was further stratification by age in adults [13] and height in children [14] to ensure equal representation across treatments. Allocation generation A randomisation schedule was generated by the Mater Hospital Pharmacy (South Brisbane, Queensland, Australia) for use at all centres. A Microsoft Excel program was used to randomly generate numbers, and randomly assign dosing regimes to patient numbers in blocks of 10. Trial staff designated each patient the next available randomisation number in their stratification group from the randomisation schedule. Patients presented to the pharmacy with their allocated randomisation number and were provided with treatment from group A or B, according to the master randomisation schedule code held by the pharmacy. Allocation concealment The two treatment doses were concealed in identical capsules manufactured by Mater Hospital Pharmacy and presented in blister packs. Blister packs were labelled with code A or B to ensure pharmacy staff supplying the patients with their study treatment were also blinded to the treatment allocation. This label was removed prior to the pack being provided to the patient. All patients were required to take three capsules on one set day of the week, comprising either the entire weekly dose for the weekly treatment group, or one daily capsule complimented with two placebos (lactose) in the daily treatment group. All capsules looked and tasted the same. The daily group continued with one 250-mg capsule per day for the remaining 6 days, while intercurrent doses in the onceweekly regimen consisted of a placebo. Unblinding occurred subsequent to statistical analysis. Outcome measures Patients were assessed at baseline, after 1, 3 and 6 months of treatment and at 1 month post-treatment. Assessments at each time-point included the primary outcome of patient lung function (FEV1 as measured by spirometry). Spirometry was performed in specialised respiratory function laboratories according to the American Thoracic Society (ATS) criteria [15] and the percentage predicted was calculated using specific reference ranges for adults [13] and children [14]. A small number of outreach patients were assessed using a portable spirometer (Microlab 3500; Micro Medical Ltd, Kent, UK) provided by the Mater Children s Hospital Respiratory Laboratory, which was regularly calibrated using a 3-L calibration syringe (Hans Rudolph Inc., Kansas City, MI, USA). Secondary outcomes were also measured at each assessment and included forced vital capacity (FVC; measured by spirometry), percentage of patients admitted, mean days spent in hospital for respiratory indications, mean number of admissions for respiratory indications, nutritional status via body mass index (BMI) for adults (o18 yrs) and height, weight and BMI standardised z-scores for children and adolescent patients (f18 yrs old). Serum liver enzymes aspartate aminotransferase, alanine aminotransferase and the inflammatory mediator C-reactive protein (CRP) were measured by the Queensland Health Pathology Service (Brisbane, Queensland, Australia) using nephelometry. Serological evidence of infection with Legionella pneumophilia or Mycoplasma pneumoniae at baseline and after 6 months of treatment was sought to determine whether clinical improvement may be attributable to treatment of organisms susceptible to AZM. Acute respiratory exacerbations were treated as usual by attending physicians with admission to hospital for increased respiratory symptoms when appropriate. AZM treatment was continued throughout these periods. Formal criteria for the definition of respiratory exacerbations were not utilised in the present trial. 488 VOLUME 30 NUMBER 3 EUROPEAN RESPIRATORY JOURNAL
3 J. MCCORMACK ET AL. AZITHROMYCIN IN CF Where possible, an expectorated sputum sample was collected at each assessment and cultured by standard techniques for typical pathogens, including P. aeruginosa, H. influenzae, S. aureus and Burkholderia cepacia [16]. Throat swabs were collected to detect the presence of S. pneumoniae. AZM sensitivity testing of S. aureus isolates cultured before, during and after therapy was also performed [17]. Disc diffusion (Haemophilus spp.) and VITEK susceptibility panels (S. aureus) in an automated system (biomérieux, Marcy l Etoile, France) were used to determine antibiotic susceptibility. Clarithromycin was used to test macrolide susceptibility of Haemophilus spp. The National Committee for Clinical Laboratory Standards breakpoints were used to determine susceptibility of the isolates, as has been previously applied by PHAFF et al. [7]. According to these criteria, S. aureus with erythromycin minimal inhibitory concentration (MIC) of o8 mg?l -1 and Haemophilus spp. with clarithromycin disc zone o13 mm, corresponding to MIC o32 mg?l -1, are classified as resistant. Quality of life (QoL) was assessed using the disease-specific QoL questionnaires: Version 1 Cystic Fibrosis Questionnaire (CFQ) for either adults and adolescents.14 yrs old, or child and parent versions for younger children (researcher administered for patients 6 11 yrs old and self-administered for those aged yrs). Statistical analysis and sample size The primary study outcome was a change from baseline in FEV1 % pred. Additional analyses were conducted for secondary outcomes of change in FVC, CRP, nutritional status and time to first hospitalisation for exacerbation. Further secondary outcomes included change in number of admissions, length of stay and proportion of patients requiring hospital admissions for respiratory exacerbations relative to the 6-month period immediately prior to randomisation. Response heterogeneity was determined through sub-analyses of pulmonary level of illness severity (FEV1,40, and.70% pred), and distinct age group analyses for patients,14 yrs old and o14 yrs old to assess treatment responses in lung function, CRP, number of admissions and length of stay. In a previous study, involving 30 patients per arm, an SD of 22.5% was observed for a change in the primary outcome (FEV1 % pred) [2]. Based on this SD, the current authors originally calculated that a sample size of 92 patients per arm would be required to show equivalence in the primary outcome of change in FEV1 % pred with a tolerable interval of 10% with 85% power and a 5% significance level. However, the SD achieved in the current study was 20.04%; therefore, it was calculated that a sample size of 77 patients per arm was required to show equivalence. The present sample size calculations were based on the primary rather than secondary outcomes. These numbers were exceeded out of concerns regarding attrition and incomplete data. Analysis of the data was carried out using an intent-to-treat principle. To begin with, the demographic factors between the group that withdrew from the study and the remaining cohort were compared. No significant differences were found between any demographic factors (table 1), implying that bias was not introduced by the dropouts. Subsequently, generalised TABLE 1 Baseline characteristics of weekly and daily azithromycin (AZM) arms of the study Daily AZM Weekly AZM Participants n Age,14 yrs 24 (23) 30 (29) 14-,18 yrs 11 (11) 19 (18) o18 yrs 68 (66) 56 (53) Mean age yrs Sex female/male % 48/52 46/54 Height m Weight kg BMI # Z-score height " Z-score weight " FVC % pred CRP Pseudomonas aeruginosa 68 (66) 71 (68) Staphylococcus aureus 21 (20) 26 (25) Patients admitted to hospital + % Data are presented as n (%) or mean SD, unless otherwise stated. BMI: body mass index; FVC: forced vital capacity; % pred: % predicted; CRP: Creactive protein. # : patients aged o18 yrs; " : patients aged,18 yrs; + : i.v. antibiotics in 6 months prior to the study. estimating equations were used because of their ability to analyse correlated longitudinal data with missing values, assuming that the data was missing completely at random. All recruited and randomised participants were included in the analyses. All analyses reported significance at the p,0.05 level (two-tailed). At the bivariate level, analysis involved Fisher s exact test to examine the significance of the association between independent categorical variables. Assumption of normality was tested via the Shapiro and Francia Test, and where the assumption could not be met, data were transformed logarithmically or via square root transformation for analysis. When data could not be transformed to represent a normal distribution, the Wilcoxon Mann Whitney rank sum test was employed. Within-group mean changes from baseline and between-group comparisons were calculated using generalised estimating equation analyses with workable exchangeable correlation structure. Assessment of microbiological changes over the course of treatment employed the k coefficient to measure the agreement between baseline and 6- month treatment scores. With only two discrete levels of outcome, the Exact McNemar test was used to test significance of changes over the treatment period. RESULTS In total, 208 CF patients (47% female) with a mean (range) age of 21.2 (6 58) yrs were recruited from a total of 379 patients assessed for eligibility at five CF centres (two adult, two paediatric and one mixed; fig. 1). The baseline characteristics of the patients in each arm are described in table 1. There were no differences in any of the parameters examined. Out of the patients who completed the 6 month trial, 26 started in the c EUROPEAN RESPIRATORY JOURNAL VOLUME 30 NUMBER 3 489
4 AZITHROMYCIN IN CF J. MCCORMACK ET AL. Assessed for eligibility (n=379) Eligible (n=269) Recruited and randomised (n=208) Ineligible (n=110) Exclusion critiera Liver impairment (n=30), allergy (n=6), Mycobacterium avium (n=4), unstable/transplant (n=8), underweight (n=9), pregnancy (n=3) Taking AZM privately (n=7) Recruited to another study (n=2) Remoteness to centre making follow-up impractical (n=25) Deemed unsuitable by treating doctor (n=17) e.g. poor ability to perform spirometry Refusals (n=61) e.g. not interested, travel constraints, aversion to drug taking, needle phobia, no reason given Weekly treatment (n=105) Completed treatment (n=83) # Weekly treatment (n=103) Completed treatment (n=88) # Primary end-point analysis (with lung function data) Baseline (n=105) 1 month (n=98) 3 months (n=92) 6 months (n=81) 1 month post (n=49) Primary end-point analysis (with lung function data) Baseline (n=103) 1 month (n=96) 3 months (n=92) 6 months (n=81) 1 month post (n=54) FIGURE 1. Flow diagram showing the trial profile. AZM: azithromycin. # : during the trial a total of 37 (18%) subjects withdrew due to: choice to withdraw or inability to attend assessments (n514); loss to follow-up (n59); abdominal symptoms, nausea, pancreatitis, anorexia and weight loss, diarrhoea, vomiting (n59); ocular surface irritation (n51); lung transplant (n52), mood swings (n51); or photosensitivity (n51). summer, 51 in autumn, 37 in winter and 56 in the spring, but the starting season was equivalent for the weekly and daily arms (p50.42). Of the 208 enrolled patients, 26 were located at remote sites outside Brisbane (Australia) but their care was coordinated by one of the five centres. Tables 2 4 show the changes in FEV1 % pred from baseline to 1, 3, 6 and 7 months. Comparative values are shown for patients aged.14 yrs and,14 yrs old. There was no difference between the weekly and daily groups at any of the time-points. When all patients were included in the analysis of changes from baseline, the two treatment groups were equivalent to an interval of 3.2%. During the treatment period, adults and adolescents o14 yrs old and,14 yrs old proved equivalent to 2.4 and 8.7%, respectively. There was also no difference between the two groups when the relative change from baseline in FEV1 % pred was compared, as shown in figure 2. The FVC values also showed no differences between patients in the weekly or daily arms for adults or children (data not shown). The changes in CRP are shown in table 5. The decrease in CRP in the daily group was larger than the weekly group at 3 and 6 months (p50.04 and 0.02, respectively). There were no such differences at 1 or 7 months (p50.31 and 0.25, respectively). The percentage of patients admitted before and during the study period, the number of days spent in hospital, the number of admissions and the time to first exacerbation are shown in table 6. In the 6 months prior to the study, respiratory admission numbers were greater in the daily group but hospitalisation days were equivalent in both groups. No difference was demonstrated between the weekly and daily groups for hospital days, admission numbers or time to respiratory hospitalisation during the course of the study. When changes in nutritional status in the two groups were examined there were no differences in the change in BMI between the weekly and daily groups in the patients aged o18 yrs after 6 months of treatment (p50.88). However, in patients,18 yrs old, the daily group had significantly better improvements in z-scores for height and weight at 6 months (fig. 3) but this was not the case for BMI z-scores. Health-related QoL modalities were equivalent in the weekly and daily groups at baseline (data not shown). For patients who were o14 yrs of age, there were significant differences between groups in favour of weekly AZM in the physical domain at 6 months compared with baseline (+8.2 points; p50.02). There were no differences in any of the other domains between groups at the end of the study. For patients aged,14 yrs, the health domain of the CFQ for parents (parents responses) was significantly higher at 6 months for the daily 490 VOLUME 30 NUMBER 3 EUROPEAN RESPIRATORY JOURNAL
5 J. MCCORMACK ET AL. AZITHROMYCIN IN CF TABLE 2 Change from baseline in forced expiratory volume on one second (% pred) over 7 months and by treatment group in all patients # TABLE 4 Change from baseline in forced expiratory volume in one second (% pred) over 7 months and by treatment group in patients aged o14 yrs # Baseline 1 month " 3 months " 6 months " 7 months " Baseline 1 month " 3 months " 6 months " 7 months " Daily * * * Weekly * * * Mean difference e 95% CI p-value ## Daily * * * Weekly * * * * Mean difference e 95% CI p-value ## Data are presented as mean SD, unless otherwise stated. CI: confidence interval. # :n5208; " : change compared with baseline; + :n5103; 1 :n5105; e : mean difference between groups (daily versus weekly); ## : p-values relate to mean difference between groups (daily versus weekly); *: significant difference (p,0.05) from baseline. Data are presented as mean SD, unless otherwise stated. CI: confidence interval. # :n5154; " : change compared with baseline; + :n579; 1 :n575; e : mean difference between groups (daily versus weekly); ## : p-values relate to mean difference between groups (daily versus weekly); *: significant difference (p,0.05) from baseline. AZM (+15.6 points; p50.02) but there were no differences in any other domain. For children (children responses) no differences were found at 6 months compared with baseline in either group for any of the CFQ domains. P. aeruginosa was found in 66% of patients sputum on recruitment, with no difference between the weekly and daily treatment group. There was no change in this percentage over the study duration. S. aureus was identified in sputum from 47 out of 181 patients at baseline and from 22 out of 141 patients at 6 months. There was no significant difference in isolation rates between the weekly and daily groups. The number of patients in the weekly and daily groups who had isolates of S. aureus at baseline and 6 months were examined, and no difference was found between the two. In 24 patients with isolates at baseline and at least one other trial time-point, 14 S. aureus isolates were sensitive to AZM, five of which became resistant over the course of treatment. Of these five isolates, two were in the weekly group and three were in the daily group. There was no difference in the changes in FEV1 between these five patients and the other patients. There were no S. pneumoniae isolates in any patient at commencement or during the study. There was no difference between groups at baseline in isolation rate of H. influenzae, with two isolates in the weekly and one in the daily groups, and no isolates after 6 months of treatment. B. cepacia complex was isolated from five out of the 91 patients tested at baseline from the weekly treatment group, and one out of the 90 patients from the daily treatment group. At the end of 6 months, the isolation rates were three out of 69 and one out of 78 for the weekly and daily groups, respectively. After 6 months of treatment, no difference was found between the groups in the number of isolates of B. cepacia complex (p50.46) and no patients had acquired this infection during the course of treatment. TABLE 3 Change from baseline in forced expiratory volume in one second (% pred) over 7 months and by treatment group in patients aged,14 yrs # Baseline 1 month " 3 months " 6 months " 7 months " Daily * * Weekly Mean difference e % CI p-value ## Data are presented as mean SD, unless otherwise stated. CI: confidence interval. # :n554; " : change compared with baseline; + :n524; 1 :n530; e : mean difference between groups (daily versus weekly); ## : p-values relate to mean difference between groups (daily versus weekly); *: significant difference (p,0.05) from baseline. FEV1 % pred change # Daily Weekly Group FIGURE 2. Change in forced expiratory volume in one second (FEV1) between 6 months and baseline after 6 months of treatment. The error bars represent the median with the smallest and largest observations within 1.5 times the interquartile range (IQR). #: outliers between 1.5 and 3 times IQR from the quartiles. # : data point.3 times IQR from the quartiles. % pred: % predicted. c EUROPEAN RESPIRATORY JOURNAL VOLUME 30 NUMBER 3 491
6 AZITHROMYCIN IN CF J. MCCORMACK ET AL. TABLE 5 Change from baseline in C-reactive protein over 7 months and by treatment group 0.30 Baseline 1 month # 3 months # 6 months # 7 months # Daily * * * Weekly * Mean difference " % CI p-value Data are presented as mean SD, unless otherwise stated. CI: confidence interval. # : change compared with baseline; " : mean difference between groups (daily versus weekly); + : p-values relate to mean difference between groups (daily versus weekly); *: significant difference (p,0.05) from baseline. The adverse events of the study are listed in table 7. The most obvious difference between the two groups was the higher rate of gastro-intestinal effects in patients taking weekly AZM. It is likely that this was attributable to the larger dose. At 6 months five participants had dropped out of the daily treatment arm and 12 had dropped out of the weekly treatment arm (odds ratio 0.40, 95% confidence interval ). The most common adverse event involved nausea (daily n52, weekly n511), followed by diarrhoea (daily n53, weekly n58) and vomiting (daily n50, weekly n55). The total cost of AZM for 6 months was AU$407 and AU$622 for weekly and daily use, respectively; a factor of The cost of AZM was calculated based on the distribution charge from the Mater Hospital Pharmacy and did not include any ancillary charges. DISCUSSION The benefits of AZM in CF patients has been clearly demonstrated in four placebo-controlled, randomised controlled trials (RCTs) [2 5], as summarised in table 8. Further trials are needed to define optimal usage of AZM in CF. The previous studies have involved AZM administration daily or three times a week. The present study examined the potential for use of weekly dosing over a 6-month period in children and adults with CF with ages ranging 6 58 yrs. The weekly dose was 1,200 mg per Change in z-score l n BMI Height Weight FIGURE 3. Mean change in z-scores from baseline to 6 months in body mass index (BMI), height and weight in subjects aged,18 yrs. The error bars represent 95% confidence intervals. $: daily azithromycin; &: weekly azithromycin. week; this was selected because it is the regimen widely used in HIV/AIDS patients with low CD4 lymphocyte counts for prophylaxis against Mycobacterium avium infections and has been shown to be tolerable in that group [18]. At baseline, there were no significant differences between the characteristics of the two groups. The current study demonstrated equivalence between weekly and daily administered AZM for the primary end-point, FEV1 % pred. The magnitude of change in FEV1 for the daily therapy was similar to that seen at 3 months in an earlier study and in two out of the three previously published RCTs. Notably, the most recent and longest (12 months) RCT failed to demonstrate an improvement in spirometry [5]. Secondary end-points, including hospitalisation for respiratory treatments, i.v. antibiotic rates, time to hospitalisation and QoL, were equivalent between treatment arms in the present study. The current authors could not demonstrate any difference between those who received weekly or daily AZM. However, encouragingly, the effect on hospitalisation seen in earlier studies [2, 4] was also evident in the current study and was seen in both treatment arms. The number of days spent in hospital may be an important contributor to a patient s QoL and has important social and financial implications for both the patient and health systems. Whilst protocol definition for l n l n TABLE 6 Patients admitted, time spent in hospital, number of admissions and time to first exacerbation in study population Patients admitted % Mean days in hospital Mean number of admissions Mean days to first exacerbation Pre-trial # Trial p-value " Pre-trial # Trial p-value " Pre-trial # Trial p-value " Trial Daily 62 32, , , Weekly p-value # : 6 months immediately prior to study commencement; " : p-values relate to difference within group over time; + : p-values relate to differences between groups (daily versus weekly). 492 VOLUME 30 NUMBER 3 EUROPEAN RESPIRATORY JOURNAL
7 J. MCCORMACK ET AL. AZITHROMYCIN IN CF TABLE 7 Adverse events by treatment arm Daily Weekly p-value Total adverse events 24/103 34/ Gastro-intestinal adverse events # 9/103 27/ Frequency of four-fold increase from baseline in ALT 4/103 1/ Frequency of four-fold increase from baseline in AST 2/103 1/ Data are presented as n/total number of subjects, unless otherwise stated. ALT: alanine aminotransferase; AST: aspartate aminotransferase. # : gastro-intestinal adverse events of diarrhoea, nausea, abdominal symptoms, vomiting, constipation, pancreatitis, anorexia and weight loss; nongastro-intestinal adverse events of thrush, haemoptysis, headache, photosensitivity, rash, ocular surface irritation, dehydration, nightmares, difficulty concentrating, mood swings, hearing muffled, dry skin and hair, and pneumothorax. pulmonary exacerbations were not included in the present study, a surrogate marker, hospitalisation for pulmonary indications, was used and was not different between the two treatment arms. In an earlier study [2] and a study from the USA [4] there was a reduction in the number of days spent in hospital for patients who received AZM. No such reduction was observed in a study from the UK [3]. Therapeutic modalities that can reduce days spent in hospital are worthy of detailed evaluation, particularly in long-term studies. The mechanism of action of macrolides in CF is not yet established; however, anti-inflammatory and antimicrobial effects have been proposed. The evidence favouring each of these mechanisms has previously been summarised [12]. The study by WOLTER et al. [2] demonstrated that patients treated with AZM had a reduction in their mean CRP values in comparison with patients given placebo. The current study found differences between the weekly and daily treated patients at 3 and 6 months, but not at 1 and 7 months (table 5). These findings may suggest that the beneficial effects of AZM take several weeks to be established and are lost within a month of drug cessation whether the treatment is taken daily or weekly. Adverse events were similar overall between treatment groups but there were increased gastro-intestinal adverse events in the weekly group. In the current study there were no differences in BMI z-scores between daily and weekly treated patients in the adult population (fig. 3), but better improvements were noted in the height and weight z-scores in the daily group in patients,18 yrs old. The magnitude of the increase in growth parameters is relatively small, although this needs to be considered in light of a short duration study (6 months) and is likely to be an important clinical measure to determine benefit from AZM in younger patients with CF. The latter findings were unexpected; however, they may, in part, relate to the increased gastro-intestinal adverse effects in the patients receiving AZM weekly. If confirmed in other studies, this may have implications for future management. In the interim, weekly treatment should probably be avoided in younger patients. Assessment of changes in nutritional status has been TABLE 8 Study design and clinical outcomes in azithromycin in cystic fibrosis randomised controlled trials WOLTER [2] EQUI [3] SAIMAN [4] CLEMENT [5] Study design Country Australia UK USA France Patients n Population Adult Paediatric Adult/paediatric Adult/paediatric Duration months 3 15 # 6 12 Dosing regimen 250 mg?day mg?day -1" 500 mg MWF " 500 mg MWF " Clinical outcomes Baseline FEV1 % pred Relative % change in FEV1 %pred Hospital days Q «Q NR Intravenous antibiotic days Q «+ Q Q Inflammatory markers Q «Q NR QoL q «1 q e «FEV1: forced expiratory volume in one second; % pred: % predicted; QoL: quality of life; MWF: Monday, Wednesday, Friday (days of administration); NR: not reported; Q: decreased; «: unchanged; q: increased. # : 1-month run-in, 6 months each cycle (azithromycin/placebo), 2-month washout period; " : 250 mg for patients,40 kg; + : intravenous antibiotic courses (instead of days); 1 : quality of well being visual analogue score reported; e : physical functioning domain significantly increased (other domains unchanged). c EUROPEAN RESPIRATORY JOURNAL VOLUME 30 NUMBER 3 493
8 AZITHROMYCIN IN CF J. MCCORMACK ET AL. variable in the four placebo-controlled studies. In the Australian [2] and UK studies [3] there was no weight change between groups. In the USA study [4] there was a significant increase in weight in the AZM-treated patients compared with those receiving placebo. In the French study [5] there was no difference in BMI z-scores between the daily AZM and placebo groups. More studies on the effects of AZM are needed in younger patients with CF. The microbiological isolates from patients in either arm of the current trial were not extensively studied. The sputum samples were analysed according to established methods of the microbiological laboratories of each hospital. The rate of S. aureus isolates at baseline is consistent with that reported for Australian patients with CF [19]. In Australia, the rate of S. aureus was 40, 37 and 31% for children aged 0 10 yrs, adolescents aged yrs and adults with CF, respectively. The current analysis of S. aureus isolates demonstrated that five patients developed resistance to AZM during the course of the study. Patients in whom these resistant isolates were found did not have any different outcomes (primary or secondary). S. pneumoniae was not isolated and nor was any other upper respiratory tract bacterium acquired during the 6 months; however, it is recognised that such organisms may have been present in small numbers within the normal respiratory flora. Antibiotic resistance is a potential problem with long-term use of AZM [6, 7], and whilst numbers were small in the current study the potential for macrolide resistance of S. aureus to develop is has been noted [7]. The clinical impact of this phenomenon requires further study. There are several limitations of the present study. Detailed microbiological investigation of oropharyngeal bacteriology was not undertaken. The culture methods were those used in routine clinical practice at the five CF centres and had a predictably low yield. The fact that no S. pneumoniae isolates were found was, therefore, not surprising. It would be desirable in future studies for more detailed culture methods to examine for changes in macrolide susceptibility of bacteria, such as S. pneumoniae. Parameters of healthcare utilisation, including episodes of admission for treatment of respiratory symptoms and time to hospitalisation, were reported in the present study. Protocol definitions of respiratory exacerbations were not applied; however, patients were handled equally in both treatment arms. Future long-term studies of the impact of macrolides should include definitions of exacerbations. The clinical impact of AZM on patients with multi-resistant bacterial infections, such as methicillin-resistant S. aureus and B. cepacia complex, has not been formally studied. Patients with these organisms were included in the study but the numbers of patients were small and further studies are required to define the potential role of AZM in patients with multi-resistant bacterial infection. Similarly, the role of AZM in very young patients is uncertain, especially those without evidence of P. aeruginosa infection, and additional studies are anticipated [L. Saiman, Columbia University, New York, NY, USA; personal communication]. The current study and the recently published French study included younger children; however, numbers are small and study duration short (6 12 months). The present study demonstrated that whilst daily and weekly administered azithromycin provide similar outcomes over 6 months, treatment with 1,200 mg?week -1 was associated with increased gastro-intestinal adverse events and daily treatment with 250 mg had modest but more positive effects on growth in children with cystic fibrosis. These latter differences between the two groups are important; future studies involving 250 mg per week would help to determine if these observations are related to the large dose or to an inherent advantage of daily versus weekly administration. There are many other unanswered questions in this area, such as when azithromycin treatment should begin, is the benefit sustained, should administration be continuous or is there an optimal time limit, could other macrolides also be beneficial and could resistance and/or toxicity represent a significant problem? Further studies on these issues are needed to optimise the benefits of azithromycin therapy in cystic fibrosis patients. ACKNOWLEDGEMENTS The authors are grateful to S. Pain, K. Gilshenan and A. Chang (Director of the Mater Research Support Centre; all from Research Support Centre, Mater Health Services, South Brisbane, Australia) for statistical help, and M. Herwig (Dept of Medicine, University of Queensland, Mater Adult Hospital, Queensland, Australia) for clerical assistance. The authors would also like to thank all of the patients and their families, the pharmacy staff at all the hospitals and centre staff who assisted in so many ways with the execution of the study. REFERENCES 1 Kudoh S, Uetake T, Hagiwara K, et al. [Clinical effects of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis.] Nihon Kyobu Shikkan Gakkai Zasshi 1987; 25: Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002; 57: Equi A, Balfour-Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo controlled crossover trial. Lancet 2002; 360: Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003; 290: Clement A, Tamalet A, Le Roux E, Ravilly S, Fauroux B, Jais J-P. Long term effects of azithromycin in patients with cystic fibrosis: a double-blind, placebo-controlled trial. Thorax 2006; 61: Albrich WC, Monnet DL, Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis 2004; 10: Phaff SJ, Tiddens HA, Verbrugh HA, Ott A. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J Antimicrob Chemother 2006; 57: Amsden GW, Gray CL. Serum and WBC pharmacokinetics of 1500 mg of azithromycin when given either as a single 494 VOLUME 30 NUMBER 3 EUROPEAN RESPIRATORY JOURNAL
9 J. MCCORMACK ET AL. AZITHROMYCIN IN CF dose or over a 3 day period in healthy volunteers. J Antimicrob Chemother 2001; 47: Wildfeuer A, Laufen H, Zimmermann T. Distribution of orally administered azithromycin in various blood compartments. Int J Clin Pharmacol Ther 1994; 32: Scaglione F, Rossoni G. Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycin. J Antimicrob Chemother 1998; 41: Suppl. B, Wilms EB, Touw DJ, Heijerman HGM. Pharmacokinetics of azithromycin in plasma, blood, polymorphonuclear neutrophils and sputum during long-term therapy in patients with cystic fibrosis. Ther Drug Monit 2006; 28: Bell SC, Senini SL, McCormack JG. Macrolides in cystic fibrosis. Chron Respir Dis 2005; 2: Gore CJ, Crockett AJ, Pederson DG, Booth ML, Bauman A, Owen N. Spirometric standards for healthy adult lifetime nonsmokers in Australia. Eur Respir J 1995; 8: Spirxpert. Become an expert in spirometry. com/pivotal2.htm Date last updated: January Date last accessed: June 29, American Thoracic Society. ATS statement: Snowbird workshop on standardization of spirometry. Am Rev Respir Dis 1979; 119: Miller MB, Gilligan PH. Laboratory aspects of management of chronic pulmonary infections in patients with cystic fibrosis. J Clin Microbiol 2003; 41: Clinical Laboratory Standards Institute. Performance standards for antimicrobial sensitivity testing. 16th Informational Supplement 2006; 26: M100 S Havlir DV, Dube MP, Sattler FR, et al. Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N Engl J Med 1996; 335: Australian Cystic Fibrosis Data Registry cysticfibrosis.sitepoint.com.au/projects/dataregistry/ Date last updated: Date last accessed: June 10, EUROPEAN RESPIRATORY JOURNAL VOLUME 30 NUMBER 3 495
Long term azithromycin therapy in patients with cystic fibrosis
 The Turkish Journal of Pediatrics 2016; 58: 34-40 Original Long term azithromycin therapy in patients with cystic fibrosis Nagehan Emiralioğlu 1, Zeynelabidin Öztürk 2, Ebru Yalçın 1, Deniz Doğru 1, Uğur
The Turkish Journal of Pediatrics 2016; 58: 34-40 Original Long term azithromycin therapy in patients with cystic fibrosis Nagehan Emiralioğlu 1, Zeynelabidin Öztürk 2, Ebru Yalçın 1, Deniz Doğru 1, Uğur
Macrolide therapy in cystic fibrosis: new developments in clinical use
 Macrolide therapy in cystic fibrosis: new developments in clinical use Clin. Invest. (2013) 3(12), 1179 1186 Macrolide therapy, in particular azithromycin, has been shown to improve aspects of lung health
Macrolide therapy in cystic fibrosis: new developments in clinical use Clin. Invest. (2013) 3(12), 1179 1186 Macrolide therapy, in particular azithromycin, has been shown to improve aspects of lung health
Efficacy of Pseudomonas aeruginosa eradication regimens in bronchiectasis
 Efficacy of Pseudomonas aeruginosa eradication regimens in bronchiectasis Vallières, E., Tumelty, K., Tunney, M. M., Hannah, R., Hewitt, O., Elborn, J. S., & Downey, D. G. (2017). Efficacy of Pseudomonas
Efficacy of Pseudomonas aeruginosa eradication regimens in bronchiectasis Vallières, E., Tumelty, K., Tunney, M. M., Hannah, R., Hewitt, O., Elborn, J. S., & Downey, D. G. (2017). Efficacy of Pseudomonas
JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections
 Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
Journal of Antimicrobial Chemotherapy (1998) 41, Suppl. B, 69 73 JAC Efficacy and tolerance of roxithromycin versus clarithromycin in the treatment of lower respiratory tract infections G. Tatsis*, G.
Journal Club The ELITE Trial. Sandra Katalinic, Pharmacy Resident University Hospital of Northern British Columbia April 28, 2010
 Journal Club The ELITE Trial Sandra Katalinic, Pharmacy Resident University Hospital of Northern British Columbia April 28, 2010 Overview Journal article Title, journal, authors, funding Abstract Introduction
Journal Club The ELITE Trial Sandra Katalinic, Pharmacy Resident University Hospital of Northern British Columbia April 28, 2010 Overview Journal article Title, journal, authors, funding Abstract Introduction
TORCH: Salmeterol and Fluticasone Propionate and Survival in COPD
 TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
TORCH: and Propionate and Survival in COPD April 19, 2007 Justin Lee Pharmacy Resident University Health Network Outline Overview of COPD Pathophysiology Pharmacological Treatment Overview of the TORCH
Online Data Supplement. Impulse Oscillometry in Adults with Bronchiectasis
 Online Data Supplement Impulse Oscillometry in Adults with Bronchiectasis Wei-jie Guan *1, Ph. D.; Yong-hua Gao *2, Ph. D.; Gang Xu *3, Ph. D.; Zhi-ya Lin 1, Ph. D.; Yan Tang 1, M. D.; Hui-min Li 1, M.
Online Data Supplement Impulse Oscillometry in Adults with Bronchiectasis Wei-jie Guan *1, Ph. D.; Yong-hua Gao *2, Ph. D.; Gang Xu *3, Ph. D.; Zhi-ya Lin 1, Ph. D.; Yan Tang 1, M. D.; Hui-min Li 1, M.
P atients with cystic fibrosis (CF) experience repeated
 242 CYSTIC FIBROSIS Long term clinical outcome of home and hospital intravenous antibiotic treatment in adults with cystic fibrosis J Thornton, R Elliott, M P Tully, M Dodd, A K Webb... See end of article
242 CYSTIC FIBROSIS Long term clinical outcome of home and hospital intravenous antibiotic treatment in adults with cystic fibrosis J Thornton, R Elliott, M P Tully, M Dodd, A K Webb... See end of article
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
PFIZER INC. Study Initiation Date and Completion Dates: Information not available (Date of Statistical Report: 16 May 2004)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Goyal V, Grimwood K, Byrnes CA, et al. Amoxicillin
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Goyal V, Grimwood K, Byrnes CA, et al. Amoxicillin
LRI Children s Hospital
 Title: Prescribing in Cystic Fibrosis Page 1 of 10 LRI Children s Hospital Prescribing in Cystic Fibrosis Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team & AWP approval
Title: Prescribing in Cystic Fibrosis Page 1 of 10 LRI Children s Hospital Prescribing in Cystic Fibrosis Staff relevant to: Clinical staff working within the UHL Children s Hospital. Team & AWP approval
MEDICAL UPDATES. Chronic fatigue syndrome following infections in adolescents. azithromycin in pediatric patients with CF
 MEDICAL UPDATES Issue No.:14 July 2013 Chronic fatigue syndrome following infections in adolescents Antibacterial and immunomodulatory properties of azithromycin treatment implications for periodontitis.
MEDICAL UPDATES Issue No.:14 July 2013 Chronic fatigue syndrome following infections in adolescents Antibacterial and immunomodulatory properties of azithromycin treatment implications for periodontitis.
Pharmacokinetics and sputum penetration of azithromycin during once weekly dosing in cystic fibrosis patients
 Journal of Cystic Fibrosis 7 (2008) 79 84 www.elsevier.com/locate/jcf Pharmacokinetics and sputum penetration of azithromycin during once weekly dosing in cystic fibrosis patients E.B. Wilms a,, D.J. Touw
Journal of Cystic Fibrosis 7 (2008) 79 84 www.elsevier.com/locate/jcf Pharmacokinetics and sputum penetration of azithromycin during once weekly dosing in cystic fibrosis patients E.B. Wilms a,, D.J. Touw
C.S. HAWORTH 1, A. WANNER 2, J. FROEHLICH 3, T. O'NEAL 3, A. DAVIS 4, I. GONDA 3, A. O'DONNELL 5
 Inhaled Liposomal Ciprofloxacin in Patients With Non-Cystic Fibrosis Bronchiectasis and Chronic Pseudomonas aeruginosa: Results From Two Parallel Phase III Trials (ORBIT-3 and -4) C.S. HAWORTH 1, A. WANNER
Inhaled Liposomal Ciprofloxacin in Patients With Non-Cystic Fibrosis Bronchiectasis and Chronic Pseudomonas aeruginosa: Results From Two Parallel Phase III Trials (ORBIT-3 and -4) C.S. HAWORTH 1, A. WANNER
OPAT FOR INFECTION IN BRONCHIECTASIS
 OPAT FOR INFECTION IN BRONCHIECTASIS AN AUDIT EVALUATING THE USAGE OF OUTPATIENT ANTIBIOTIC THERAPY FOR INFECTIVE EXACERBATIONS OF BRONCHIECTASIS AGAINST CURRENT BRITISH THORACIC SOCIETY GUIDELINES Dr
OPAT FOR INFECTION IN BRONCHIECTASIS AN AUDIT EVALUATING THE USAGE OF OUTPATIENT ANTIBIOTIC THERAPY FOR INFECTIVE EXACERBATIONS OF BRONCHIECTASIS AGAINST CURRENT BRITISH THORACIC SOCIETY GUIDELINES Dr
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Blum CA, Nigro N, Briel M, et al. Adjunct prednisone
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd
 roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients
 Journal of Cystic Fibrosis 12 (2013) 482 486 www.elsevier.com/locate/jcf Original Article Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients
Journal of Cystic Fibrosis 12 (2013) 482 486 www.elsevier.com/locate/jcf Original Article Chronic Stenotrophomonas maltophilia infection and mortality or lung transplantation in cystic fibrosis patients
Cystic Fibrosis Panel Applications (Dornase Alfa) Contents
 Cystic Fibrosis Panel Applications (Dornase Alfa) Contents Page 2-4: Entry and Stopping Criteria for Treatment with Dornase Alfa Page 5-9: Application and consent forms for a one month trail and long term
Cystic Fibrosis Panel Applications (Dornase Alfa) Contents Page 2-4: Entry and Stopping Criteria for Treatment with Dornase Alfa Page 5-9: Application and consent forms for a one month trail and long term
Nebulised anti-pseudomonal antibiotics for cystic fibrosis (Review)
 Nebulised anti-pseudomonal antibiotics for cystic fibrosis (Review) Ryan G, Mukhopadhyay S, Singh M This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
Nebulised anti-pseudomonal antibiotics for cystic fibrosis (Review) Ryan G, Mukhopadhyay S, Singh M This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published
Bronchiectasis (non-cystic fibrosis), acute exacerbation: antimicrobial prescribing
 National Institute for Health and Care Excellence Bronchiectasis (non-cystic fibrosis), acute exacerbation: antimicrobial prescribing Evidence review NICE guideline NG117 December 2018 Disclaimer The
National Institute for Health and Care Excellence Bronchiectasis (non-cystic fibrosis), acute exacerbation: antimicrobial prescribing Evidence review NICE guideline NG117 December 2018 Disclaimer The
Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis
 Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Journal of Antimicrobial Chemotherapy (1991) 27, Suppl. A, 67-74 Clarithromycin versus penicillin in the treatment of streptococcal pharyngitis Joseph H. Levenstein* South Africa Academy of Family Practice,
Clinical Commissioning Policy Proposition: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (Adults)
 Clinical Commissioning Policy Proposition: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (Adults) Reference: NHS England 1621 First published: Month Year Prepared
Clinical Commissioning Policy Proposition: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (Adults) Reference: NHS England 1621 First published: Month Year Prepared
Early Pseudomonas Infection Control (EPIC) Clinical Study. Overview for Families
 Early Pseudomonas Infection Control (EPIC) Clinical Study Overview for Families What is the EPIC Clinical Study? The EPIC Clinical Study Compares different treatments for children with CF who have just
Early Pseudomonas Infection Control (EPIC) Clinical Study Overview for Families What is the EPIC Clinical Study? The EPIC Clinical Study Compares different treatments for children with CF who have just
Dr Conroy Wong. Professor Richard Beasley. Dr Sarah Mooney. Professor Innes Asher
 Professor Richard Beasley University of Otago Director Medical Research Institute of New Zealand Wellington Dr Sarah Mooney Physiotherapy Advanced Clinician Counties Manukau Health NZ Respiratory and Sleep
Professor Richard Beasley University of Otago Director Medical Research Institute of New Zealand Wellington Dr Sarah Mooney Physiotherapy Advanced Clinician Counties Manukau Health NZ Respiratory and Sleep
CONSORT 2010 checklist of information to include when reporting a randomised trial*
 Supplemental Figures for: Ramosetron Versus Ondansetron in Combination with Aprepitant and Dexamethasone for the Prevention of Highly Emetogenic Chemotherapy-induced Nausea and Vomiting: A Multicenter,
Supplemental Figures for: Ramosetron Versus Ondansetron in Combination with Aprepitant and Dexamethasone for the Prevention of Highly Emetogenic Chemotherapy-induced Nausea and Vomiting: A Multicenter,
Cystic Fibrosis Care Guidelines for Challenging Cystic Fibrosis
 Cystic Fibrosis Care Guidelines for Challenging Cystic Fibrosis APRIL 2018 Authors Steve Kent MD, CF Clinic Director, Victoria General Hospital (VGH), Victoria Mark Chilvers MD, CF Clinic Director, B.C.
Cystic Fibrosis Care Guidelines for Challenging Cystic Fibrosis APRIL 2018 Authors Steve Kent MD, CF Clinic Director, Victoria General Hospital (VGH), Victoria Mark Chilvers MD, CF Clinic Director, B.C.
Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages)
 Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages) NHS England Reference: 1732P NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages) NHS England Reference: 1732P NHS England INFORMATION READER BOX Directorate
Glossary. Acronyms used in cystic fibrosis peer review reports
 Glossary Acronyms used in cystic fibrosis peer review reports Acronym Definition Notes AC(T) Airway clearance (techniques) A term used in physiotherapy ACB Association of Clinical Biochemists Professional
Glossary Acronyms used in cystic fibrosis peer review reports Acronym Definition Notes AC(T) Airway clearance (techniques) A term used in physiotherapy ACB Association of Clinical Biochemists Professional
#8 - Respiratory System
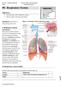 Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
Page1 #8 - Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Equipment: Remember to bring photographic atlas. Figure 1. Structures of the respiratory system.
The Bacteriology of Bronchiectasis in Australian Indigenous children
 The Bacteriology of Bronchiectasis in Australian Indigenous children Kim Hare, Amanda Leach, Peter Morris, Heidi Smith-Vaughan, Anne Chang Presentation outline What is bronchiectasis? Our research at Menzies
The Bacteriology of Bronchiectasis in Australian Indigenous children Kim Hare, Amanda Leach, Peter Morris, Heidi Smith-Vaughan, Anne Chang Presentation outline What is bronchiectasis? Our research at Menzies
Table S1. Data on IgG substitution for participants that were included in the per protocol analysis (n=62/arm).
 Bergman et al, Vitamin D 3 supplementation in patients with frequent respiratory tract infections - a randomised, double blind intervention study Supplementary tables Table S1. Data on IgG substitution
Bergman et al, Vitamin D 3 supplementation in patients with frequent respiratory tract infections - a randomised, double blind intervention study Supplementary tables Table S1. Data on IgG substitution
 PATCH Analysis Plan v1.2.doc Prophylactic Antibiotics for the Treatment of Cellulitis at Home: PATCH Analysis Plan for PATCH I and PATCH II Authors: Angela Crook, Andrew Nunn, James Mason and Kim Thomas,
PATCH Analysis Plan v1.2.doc Prophylactic Antibiotics for the Treatment of Cellulitis at Home: PATCH Analysis Plan for PATCH I and PATCH II Authors: Angela Crook, Andrew Nunn, James Mason and Kim Thomas,
ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS
 R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
TRANSPARENCY COMMITTEE OPINION. 15 October Date of Marketing Authorisation (national procedure): 16 April 1997, variation of 18 February 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 15 October 2008 MERONEM 1 g, powder for solution for IV Injection Box of 10 vials (CIP: 387 830-6) Applicant: ASTRAZENECA
Shared Care Guideline
 Shared Care Guideline Gentamicin for Nebulisation For the long term prophylaxis of chronic lung infections in non CF bronchiectasis Executive Summary Indication Nebulised gentamicin is indicated in patients
Shared Care Guideline Gentamicin for Nebulisation For the long term prophylaxis of chronic lung infections in non CF bronchiectasis Executive Summary Indication Nebulised gentamicin is indicated in patients
Dose-dependent effects of tobramycin in an animal model of Pseudomonas sinusitis Am J Rhino Jul-Aug; 21(4):423-7
 AMINOGLYCOSIDES Dose-dependent effects of tobramycin in an animal model of Pseudomonas sinusitis Am J Rhino. 2007 Jul-Aug; 21(4):423-7 http://www.ncbi.nlm.nih.gov/pubmed/17882910 Evaluation of the in-vivo
AMINOGLYCOSIDES Dose-dependent effects of tobramycin in an animal model of Pseudomonas sinusitis Am J Rhino. 2007 Jul-Aug; 21(4):423-7 http://www.ncbi.nlm.nih.gov/pubmed/17882910 Evaluation of the in-vivo
PFIZER INC. Study Center(s): A total of 6 centers took part in the study, including 2 in France and 4 in the United States.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Cystic Fibrosis Complications ANDRES ZIRLINGER, MD STANFORD UNIVERSITY MEDICAL CENTER MARCH 3, 2012
 Cystic Fibrosis Complications ANDRES ZIRLINGER, MD STANFORD UNIVERSITY MEDICAL CENTER MARCH 3, 2012 INTRODUCTION PNEUMOTHORAX HEMOPTYSIS RESPIRATORY FAILURE Cystic Fibrosis Autosomal Recessive Genetically
Cystic Fibrosis Complications ANDRES ZIRLINGER, MD STANFORD UNIVERSITY MEDICAL CENTER MARCH 3, 2012 INTRODUCTION PNEUMOTHORAX HEMOPTYSIS RESPIRATORY FAILURE Cystic Fibrosis Autosomal Recessive Genetically
Chapter 3 The Role of Nutrition in CF Care
 Chapter 3 The Role of Nutrition in CF Care S. King, N. Saxby & N. Sander Cystic fibrosis is the most common lethal autosomal recessive genetic condition affecting Caucasians 186,187. Over 3500 Australians
Chapter 3 The Role of Nutrition in CF Care S. King, N. Saxby & N. Sander Cystic fibrosis is the most common lethal autosomal recessive genetic condition affecting Caucasians 186,187. Over 3500 Australians
Experience the Powder of Non-nebulized Pa Treatment
 For people 6 years and older with cystic fibrosis (CF), Pseudomonas aeruginosa (Pa), and FEV 1 25% to 80% predicted and who do not have Burkholderia cepacia. Experience the Powder of Non-nebulized Pa Treatment
For people 6 years and older with cystic fibrosis (CF), Pseudomonas aeruginosa (Pa), and FEV 1 25% to 80% predicted and who do not have Burkholderia cepacia. Experience the Powder of Non-nebulized Pa Treatment
A NEW APPROACH TO THERAPEUTIC DRUG MONITORING OF TOBRAMYCIN IN CYSTIC FIBROSIS
 THE DOSING INSTITUTE CLINICAL REVIEW SERIES: TOBRAMYCIN IN CYSTIC FIBROSIS MAY 2017 A NEW APPROACH TO THERAPEUTIC DRUG MONITORING OF TOBRAMYCIN IN CYSTIC FIBROSIS THE DOSING INSTITUTE The Dosing Institute
THE DOSING INSTITUTE CLINICAL REVIEW SERIES: TOBRAMYCIN IN CYSTIC FIBROSIS MAY 2017 A NEW APPROACH TO THERAPEUTIC DRUG MONITORING OF TOBRAMYCIN IN CYSTIC FIBROSIS THE DOSING INSTITUTE The Dosing Institute
Dosage and Administration
 SIRTURO product information for healthcare providers 2 WARNINGS: An increased risk of death was seen in the SIRTURO (bedaquiline) treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81,
SIRTURO product information for healthcare providers 2 WARNINGS: An increased risk of death was seen in the SIRTURO (bedaquiline) treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81,
Drug Class Review on Macrolides
 Drug Class Review on Macrolides Preliminary Scan Report 5 July 2014 Last Report: Original August 2006 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Drug Class Review on Macrolides Preliminary Scan Report 5 July 2014 Last Report: Original August 2006 The purpose of reports is to make available information regarding the comparative clinical effectiveness
Briefing Document. FDA Pulmonary - Allergy Drugs Advisory Committee
 FDA Advisory Committee Briefing Materials Page 1 of 157 Briefing Document FDA Pulmonary - Allergy Drugs Advisory Committee Ivacaftor for the Treatment of Cystic Fibrosis in Patients Age 6 Years and Older
FDA Advisory Committee Briefing Materials Page 1 of 157 Briefing Document FDA Pulmonary - Allergy Drugs Advisory Committee Ivacaftor for the Treatment of Cystic Fibrosis in Patients Age 6 Years and Older
11/19/2012. The spectrum of pulmonary diseases in HIV-infected persons is broad.
 The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
PA Update: Oral Cystic Fibrosis Modulators
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Setting The setting was secondary care. The economic study was carried out in the USA.
 Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
Cost-effectiveness of IV-to-oral switch therapy: azithromycin vs cefuroxime with or without erythromycin for the treatment of community-acquired pneumonia Paladino J A, Gudgel L D, Forrest A, Niederman
Downloaded from:
 Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Bronchiectasis Domiciliary treatment. Prof. Adam Hill Royal Infirmary and University of Edinburgh
 Bronchiectasis Domiciliary treatment Prof. Adam Hill Royal Infirmary and University of Edinburgh Plan of talk Background of bronchiectasis Who requires IV antibiotics Domiciliary treatment Results to date.
Bronchiectasis Domiciliary treatment Prof. Adam Hill Royal Infirmary and University of Edinburgh Plan of talk Background of bronchiectasis Who requires IV antibiotics Domiciliary treatment Results to date.
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
JUERGEN FROEHLICH, JANICE DAHMS, DAVID CIPOLLA,
 Reduction in Frequency of Pulmonary Exacerbations With Inhaled ARD-315 in Non-Cystic Fibrosis Bronchiectasis (NCFB) Patients is Independent of Pseudomonas aeruginosa Susceptibility at Baseline JUERGEN
Reduction in Frequency of Pulmonary Exacerbations With Inhaled ARD-315 in Non-Cystic Fibrosis Bronchiectasis (NCFB) Patients is Independent of Pseudomonas aeruginosa Susceptibility at Baseline JUERGEN
Azithromycin and Chronic Obstructive Pulmonary Disease (COPD) Information for patients
 Azithromycin and Chronic Obstructive Pulmonary Disease (COPD) Information for patients page 2 What is azithromycin? Azithromycin is a type of antibiotic called a macrolide antibiotic. Antibiotics are used
Azithromycin and Chronic Obstructive Pulmonary Disease (COPD) Information for patients page 2 What is azithromycin? Azithromycin is a type of antibiotic called a macrolide antibiotic. Antibiotics are used
Supplementary Online Content
 Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Scottish Paediatric Cystic Fibrosis MCN. Protocols / Guidelines. Ivacaftor: A guideline for use in paediatric CF patients in Scotland
 Scottish Paediatric Cystic Fibrosis MCN Protocols / Guidelines Ivacaftor: A guideline for use in paediatric CF patients in Scotland Authors: Dr Carol Dryden Dr Jane Wilkinson Miss Julie Crocker, Registered
Scottish Paediatric Cystic Fibrosis MCN Protocols / Guidelines Ivacaftor: A guideline for use in paediatric CF patients in Scotland Authors: Dr Carol Dryden Dr Jane Wilkinson Miss Julie Crocker, Registered
Charles Feldman. Charlotte Maxeke Johannesburg Academic Hospital University of the Witwatersrand
 Opportunistic Infections Community Acquired Pneumonia Charles Feldman Professor of Pulmonology and Chief Physician Charlotte Maxeke Johannesburg Academic Hospital University of the Witwatersrand Introduction
Opportunistic Infections Community Acquired Pneumonia Charles Feldman Professor of Pulmonology and Chief Physician Charlotte Maxeke Johannesburg Academic Hospital University of the Witwatersrand Introduction
Indian Journal of Basic & Applied Medical Research; September 2013: Issue-8, Vol.-2, P
 Original article: Study of pulmonary function in different age groups Dr.Geeta J Jagia*,Dr.Lalita Chandan Department of Physiology, Seth GS Medical College, Mumbai, India *Author for correspondence: drgrhegde@gmail.com
Original article: Study of pulmonary function in different age groups Dr.Geeta J Jagia*,Dr.Lalita Chandan Department of Physiology, Seth GS Medical College, Mumbai, India *Author for correspondence: drgrhegde@gmail.com
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
pneumonia 2015;6:48 56
 pneumonia 2015 Aug 21;6:48 56 pneumonia Brief Report Anne B Chang a,b, Heidi Smith-Vaughan a,c, Theo P Sloots f, Patricia C Valery a, David Whiley f, Jemima Beissbarth a, Paul J Torzillo d,e a Menzies
pneumonia 2015 Aug 21;6:48 56 pneumonia Brief Report Anne B Chang a,b, Heidi Smith-Vaughan a,c, Theo P Sloots f, Patricia C Valery a, David Whiley f, Jemima Beissbarth a, Paul J Torzillo d,e a Menzies
Changes in the management of children with Cystic Fibrosis. Caroline Murphy & Deirdre O Donovan CF Nurses
 Changes in the management of children with Cystic Fibrosis Caroline Murphy & Deirdre O Donovan CF Nurses What Is Cystic Fibrosis? Cystic fibrosis (CF) is an inherited chronic disease that primarily affects
Changes in the management of children with Cystic Fibrosis Caroline Murphy & Deirdre O Donovan CF Nurses What Is Cystic Fibrosis? Cystic fibrosis (CF) is an inherited chronic disease that primarily affects
Eradication regimens for early or recurrent Pseudomonas aeruginosa infection
 Eradication regimens for early or recurrent Pseudomonas aeruginosa infection The Leeds Method of Management. April, 2008. Cystic fibrosis and eradication therapy for early or recurrent Pseudomonas aeruginosa
Eradication regimens for early or recurrent Pseudomonas aeruginosa infection The Leeds Method of Management. April, 2008. Cystic fibrosis and eradication therapy for early or recurrent Pseudomonas aeruginosa
Assessing response to treatment of exacerbations of bronchiectasis in adults
 Eur Respir J 2009; 33: 312 317 DOI: 10.1183/09031936.00122508 CopyrightßERS Journals Ltd 2009 Assessing response to treatment of exacerbations of bronchiectasis in adults M.P. Murray, K. Turnbull, S. MacQuarrie
Eur Respir J 2009; 33: 312 317 DOI: 10.1183/09031936.00122508 CopyrightßERS Journals Ltd 2009 Assessing response to treatment of exacerbations of bronchiectasis in adults M.P. Murray, K. Turnbull, S. MacQuarrie
Surveillance report Published: 6 April 2016 nice.org.uk. NICE All rights reserved.
 Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects
 Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects Karolyn S. Horn, Mark H. Gotfried, Judith N. Steenbergen,
Comparison of Omadacycline and Tigecycline Pharmacodynamics in the Plasma, Epithelial Lining Fluid, and Alveolar Macrophages in Healthy Subjects Karolyn S. Horn, Mark H. Gotfried, Judith N. Steenbergen,
INDIVIDUAL STUDY TABLE REFERRING TO PART OF THE DOSSIER Volume: Page:
 SYNOPSIS Protocol No.: TOPMAT-MIG-303 EudraCT No.: 2005-000321-29 Title of Study: A double-blind, randomised, placebo-controlled, multicentre study to investigate the efficacy and tolerability of in prolonged
SYNOPSIS Protocol No.: TOPMAT-MIG-303 EudraCT No.: 2005-000321-29 Title of Study: A double-blind, randomised, placebo-controlled, multicentre study to investigate the efficacy and tolerability of in prolonged
Microbiological surveillance in lung disease in ataxia telangiectasia
 Microbiological surveillance in lung disease in ataxia telangiectasia To the Editor: Ataxia telangiectasia is a progressive, neurodegenerative disease causing immunodeficiency, an increased risk of malignancy
Microbiological surveillance in lung disease in ataxia telangiectasia To the Editor: Ataxia telangiectasia is a progressive, neurodegenerative disease causing immunodeficiency, an increased risk of malignancy
C ystic fibrosis (CF) is an autosomal recessive disorder
 895 CYSTIC FIBROSIS Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial A Clement, A Tamalet, E Leroux, S Ravilly, B Fauroux, J-P Jais... See end
895 CYSTIC FIBROSIS Long term effects of azithromycin in patients with cystic fibrosis: a double blind, placebo controlled trial A Clement, A Tamalet, E Leroux, S Ravilly, B Fauroux, J-P Jais... See end
Clinical Trials Lecture 4: Data analysis
 Clinical Trials Lecture 4: Data analysis Dick Menzies, MD Respiratory Epidemiology and Clinical Research Unit Montreal Chest Institute TB Research methods course July 17, 2014 Lecture 4: Data analysis
Clinical Trials Lecture 4: Data analysis Dick Menzies, MD Respiratory Epidemiology and Clinical Research Unit Montreal Chest Institute TB Research methods course July 17, 2014 Lecture 4: Data analysis
Beta-lactamase production should have no effect on Azithromycin activity.
 AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
AZIMEX Composition Azimex 250 Capsules Each capsule contains Azithromycin (as dihydrate) 250 mg Capsules Azimex 500 mg Capsules Each capsule contains Azithromycin (as dihydrate) 500 mg Action Azithromycin
The role of serum Pseudomonas aeruginosa antibodies in the diagnosis and follow-up of cystic fibrosis
 The Turkish Journal of Pediatrics 2013; 55: 50-57 Original The role of serum Pseudomonas aeruginosa antibodies in the diagnosis and follow-up of cystic fibrosis Deniz Doğru 1, Sevgi Pekcan 1, Ebru Yalçın
The Turkish Journal of Pediatrics 2013; 55: 50-57 Original The role of serum Pseudomonas aeruginosa antibodies in the diagnosis and follow-up of cystic fibrosis Deniz Doğru 1, Sevgi Pekcan 1, Ebru Yalçın
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 18 October 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 18 October 2006 CUBICIN 350 mg (daptomycin), powder for perfusion solution Box of 1 bottle (CIP code: 567 219-3) CUBICIN
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
Antimicrobial Stewardship in Community Acquired Pneumonia
 Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
Antimicrobial Stewardship in Community Acquired Pneumonia Medicine Review Course 2018 Dr Lee Tau Hong Consultant Department of Infectious Diseases National Centre for Infectious Diseases Scope 1. Diagnosis
UMEC/VI vs. UMEC in subjects who responded to UMEC UMEC/VI vs. VI in subjects who responded to VI
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
You Can Observe a Lot By Just Watching. Wayne J. Morgan, MD, CM
 You Can Observe a Lot By Just Watching Wayne J. Morgan, MD, CM Disclosures Genentech Epidemiological Study of Cystic Fibrosis, Scientific Advisory Group CF Foundation Data Safety Monitoring Board Registry/Comparative
You Can Observe a Lot By Just Watching Wayne J. Morgan, MD, CM Disclosures Genentech Epidemiological Study of Cystic Fibrosis, Scientific Advisory Group CF Foundation Data Safety Monitoring Board Registry/Comparative
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Appendix D Clinical specialist statement template
 Appendix D Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of pseudomonas lung infection in cystic fibrosis Thank you for agreeing to give us a statement on your organisation
Appendix D Colistimethate sodium powder and tobramycin powder for inhalation for the treatment of pseudomonas lung infection in cystic fibrosis Thank you for agreeing to give us a statement on your organisation
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
SGRQ Questionnaire assessing respiratory disease-specific quality of life. Questionnaire assessing general quality of life
 SUPPLEMENTARY MATERIAL e-table 1: Outcomes studied in present analysis. Outcome Abbreviation Definition Nature of data, direction indicating adverse effect (continuous only) Clinical outcomes- subjective
SUPPLEMENTARY MATERIAL e-table 1: Outcomes studied in present analysis. Outcome Abbreviation Definition Nature of data, direction indicating adverse effect (continuous only) Clinical outcomes- subjective
CYSTIC FIBROSIS FOUNDATION INFO-POD Information You Need to Make Benefits Decisions
 CYSTIC FIBROSIS FOUNDATION INFO-POD Information You Need to Make Benefits Decisions Issue 1: Hypertonic Saline Summary: Preserving lung function is a crucial element in the care of the individual with
CYSTIC FIBROSIS FOUNDATION INFO-POD Information You Need to Make Benefits Decisions Issue 1: Hypertonic Saline Summary: Preserving lung function is a crucial element in the care of the individual with
Katherine Ronchetti*, Jo-Dee Tame*, Christopher Paisey, Lena P Thia, Iolo Doull, Robin Howe, Eshwar Mahenthiralingam, Julian T Forton
 The CF-Sputum Induction Trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial Katherine Ronchetti*,
The CF-Sputum Induction Trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial Katherine Ronchetti*,
CADTH CANADIAN DRUG EXPERT COMMITTEE FINAL RECOMMENDATION
 CADTH CANADIAN DRUG EXPERT COMMITTEE FINAL RECOMMENDATION LUMACAFTOR / IVACAFTOR (Orkambi Vertex Pharmaceuticals [Canada] Inc.) Indication: Cystic Fibrosis, F508del-CFTR mutation Recommendation: The CADTH
CADTH CANADIAN DRUG EXPERT COMMITTEE FINAL RECOMMENDATION LUMACAFTOR / IVACAFTOR (Orkambi Vertex Pharmaceuticals [Canada] Inc.) Indication: Cystic Fibrosis, F508del-CFTR mutation Recommendation: The CADTH
Jennifer R Bishop, Odette J Erskine and Peter G Middleton
 Timing of dornase alpha inhalation does not affect the efficacy of an airway clearance regimen in adults with cystic fibrosis: a randomised crossover trial Jennifer R Bishop, Odette J Erskine and Peter
Timing of dornase alpha inhalation does not affect the efficacy of an airway clearance regimen in adults with cystic fibrosis: a randomised crossover trial Jennifer R Bishop, Odette J Erskine and Peter
National Horizon Scanning Centre. Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis. April 2008
 Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
[No conflicts of interest]
![[No conflicts of interest] [No conflicts of interest]](/thumbs/86/94144068.jpg) [No conflicts of interest] Patients and staff at: Available evidence pre-calories Three meta-analyses: Gramlich L et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes
[No conflicts of interest] Patients and staff at: Available evidence pre-calories Three meta-analyses: Gramlich L et al. Does enteral nutrition compared to parenteral nutrition result in better outcomes
Lower airway microbiota for 'biomarker' measurements of cystic fibrosis disease progression?
 Lower airway microbiota for 'biomarker' measurements of cystic fibrosis disease progression? Sherrard, L. J., & Bell, S. C. (2018). Lower airway microbiota for 'biomarker' measurements of cystic fibrosis
Lower airway microbiota for 'biomarker' measurements of cystic fibrosis disease progression? Sherrard, L. J., & Bell, S. C. (2018). Lower airway microbiota for 'biomarker' measurements of cystic fibrosis
An Update in COPD John Hurst PhD FRCP
 An Update in COPD John Hurst PhD FRCP Reader in Respiratory Medicine / Honorary Consultant University College London / Royal Free London NHS Foundation Trust j.hurst@ucl.ac.uk What s new in COPD papers
An Update in COPD John Hurst PhD FRCP Reader in Respiratory Medicine / Honorary Consultant University College London / Royal Free London NHS Foundation Trust j.hurst@ucl.ac.uk What s new in COPD papers
Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function
 See Editorial, p 373 < Supplementary tables, a figure and additional methods are published online only. To view these files please visit the journal online http://thorax.bmj. com/content/vol65/issue5 1
See Editorial, p 373 < Supplementary tables, a figure and additional methods are published online only. To view these files please visit the journal online http://thorax.bmj. com/content/vol65/issue5 1
CYSTIC FIBROSIS IN AUSTRALIA 2008
 CYSTIC FIBROSIS IN AUSTRALIA 2008 11th Annual Report from the Australian Cystic Fibrosis Data Registry Cystic Fibrosis Australia 2010 This work is copyright. Apart from any use as permitted under the Copyright
CYSTIC FIBROSIS IN AUSTRALIA 2008 11th Annual Report from the Australian Cystic Fibrosis Data Registry Cystic Fibrosis Australia 2010 This work is copyright. Apart from any use as permitted under the Copyright
Dex single dose Pred- 5 day course Mean Difference Mean Difference Mean 3.5. Weight 100.0% IV, Fixed, 95% CI [-1.97, 0.37] 100.
![Dex single dose Pred- 5 day course Mean Difference Mean Difference Mean 3.5. Weight 100.0% IV, Fixed, 95% CI [-1.97, 0.37] 100. Dex single dose Pred- 5 day course Mean Difference Mean Difference Mean 3.5. Weight 100.0% IV, Fixed, 95% CI [-1.97, 0.37] 100.](/thumbs/90/101418001.jpg) Question : In the child with asthma exacerbation in the ED should prednisolone/prednisone vs. dexamethasone be used to prevent hospitalization, decrease time in the ED or improve pulmonary function? Forest
Question : In the child with asthma exacerbation in the ED should prednisolone/prednisone vs. dexamethasone be used to prevent hospitalization, decrease time in the ED or improve pulmonary function? Forest
Cystic fibrosis (CF) is a common fatal. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis
 Eur Respir J 2011; 37: 806 812 DOI: 10.1183/09031936.00072510 CopyrightßERS 2011 The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis R. Amin*,#, **, P. Subbarao*,#,",
Eur Respir J 2011; 37: 806 812 DOI: 10.1183/09031936.00072510 CopyrightßERS 2011 The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis R. Amin*,#, **, P. Subbarao*,#,",
Prophylactic antibiotic therapy for chronic obstructive pulmonary disease(copd)(review)
 Cochrane Database of Systematic Reviews Prophylactic antibiotic therapy for chronic obstructive pulmonary disease(copd)(review) HerathSC,PooleP Herath SC, Poole P. Prophylactic antibiotic therapy for chronic
Cochrane Database of Systematic Reviews Prophylactic antibiotic therapy for chronic obstructive pulmonary disease(copd)(review) HerathSC,PooleP Herath SC, Poole P. Prophylactic antibiotic therapy for chronic
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
What is Cystic Fibrosis? CYSTIC FIBROSIS. Genetics of CF
 What is Cystic Fibrosis? CYSTIC FIBROSIS Lynne M. Quittell, M.D. Director, CF Center Columbia University Chronic, progressive and life limiting autosomal recessive genetic disease characterized by chronic
What is Cystic Fibrosis? CYSTIC FIBROSIS Lynne M. Quittell, M.D. Director, CF Center Columbia University Chronic, progressive and life limiting autosomal recessive genetic disease characterized by chronic
