Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice.
|
|
|
- Jessie Jordan
- 6 years ago
- Views:
Transcription
1 Section Editor: Sumit Sam Garg, MD Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice. BY GARRICK CHAK, MD; AMANDA E. KIELY, MD; AND PRATAP CHALLA, MD When beginning to prescribe anti-inflammatory medications, it is common to be confused with respect to the various preparations that are commercially available. What is the difference in potency between prednisolone, loteprednol, and difluprednate? How do I choose which nonsteroidal drop to use for my patient? In this second installment from Dr. Garrick Chak and colleagues, they review the basics and intricacies of ophthalmic steroidal and nonsteroidal formulations. Knowledge of the specifics detailed here will allow you to better tailor your medical management of patients. As always, if you have any recommendation for Residents and Fellows, please let me know. Sumit Sam Garg, MD, section editor Topical corticosteroids and nonsteroidal antiinflammatory drugs (NSAIDs) are often used to treat ocular inflammation. An appreciation of the subtle differences may help physicians determine which medication to prescribe as they strive to offer patient-centered care. PEARLS FOR TOPICAL CORTICOSTEROIDS Topical ophthalmic corticosteroid agents can be classified as ketone steroids (prednisolone, difluprednate, dexamethasone, fluorometholone, and rimexolone) or ester steroid (loteprednol) based on pharmacologic design. Loteprednol is formulated with an ester instead of a ketone group at the C-20 position. 1 Thought to be cataractogenic, the C-20 ketone group forms a covalent bond with lens proteins that are found only in steroid-induced cataracts. 1 Although this is a widely accepted hypothesis for steroid-induced cataracts, other mechanisms may exist. In clinical practice, corticosteroids are often grouped broadly by anti-inflammatory potency, defined as the binding affinity of the drug to the glucocorticoid receptor. As a brief review, the corticosteroid binds to a glucocorticoid receptor that is located in the cytosol. Once bound, the glucocorticoid receptor is activated and migrates into the nucleus, where it modulates signaling pathways and protein expression (more than 5,000 genes are targeted via corticosteroids). 2 Corticosteroid therapies disrupt the inflammatory cascade by inhibiting the release of arachidonic acid from cell membrane phospholipids, thus preventing the formation of prostaglandins (cyclooxygenase [COX] pathway) as well as leukotrienes and other inflammatory mediators (lipoxygenase pathways). EFFICACY VERSUS POTENCY The anti-inflammatory potency of a drug is a pharmacologically relative term in some instances relative to hydrocortisone 3 and in other instances relative to dexamethasone 4 and is not necessarily tantamount to its clinical efficacy topically. For instance, topical dexamethasone alcohol 0.1% is known to have a sixfold higher potency and double the half-life of topical prednisolone 1% (Table 1). Even so, the latter attained a peak aqueous concentration that was more than 21 to 36 times higher than the former and also persisted with a detectable drug aqueous concentration after 24 hours (whereas the former was undetectable) because of superior penetration of the drug. 5 Thus, when selecting the appropriate corticosteroid for the patient, the eye care provider should note that the efficacy of a topical corticosteroid comprises a combination of variables such as potency, vehicle, drug concentration, duration of action, and ocular penetration. NOVEMBER/DECEMBER 2014 CATARACT & REFRACTIVE SURGERY TODAY 15
2 Topical Potency (Clinical Efficacy) Name TABLE 1. TOPICAL CORTICOSTEROIDS IN THE UNITED STATES Anti-inflammatory Potency (Relative to Hydrocortisone) Average IOP Rise, mm Hg Half-life of Drug, hrs Highest Difluprednate x as long as betamethasone Highest Higher Higher High Prednisolone Dexamethasone Prednisolone phosphate Loteprednol etabonate % with spike > 10 mm Hg (901 patients) Moderate Rimexolone 25 2% with spike > 10 mm Hg (98 patients) Moderate Moderate Dexamethasone phosphate Fluorometholone Mild Medrysone Mild Fluorometholone alcohol Low Hydrocortisone Low Cortisone SOLUBILITY, PENETRATION, AND CONVENIENCE Within a class of corticosteroids, the, the phosphate, or the alcohol form somewhere in between and phosphate in the solubility spectrum gives physicians an idea of the drug s propensity for corneal penetration and may change the relative anti-inflammatory efficacy of the drug. Aqueous humor samples have shown that prednisolone achieves higher drug concentrations than prednisolone sodium phosphate in the presence of an intact corneal epithelium. 6 The form is more lipophilic and available as a suspension, which leads to longer contact time and better penetration. The phosphate form is more hydrophilic, however, and is available as a solution. Topical prednisolone sodium phosphate 1% is less effective than topical prednisolone 1% due to bioavailability and penetration (lower ability to achieve high aqueous humor concentration of the drug through an intact corneal epithelium). 7 Generally, a suspension (for instance, prednisolone ) must be shaken vigorously for the medication to be homogenous upon application, 8 whereas a solution (eg, prednisolone sodium phosphate), an emulsion (eg, difluprednate), or a gel (eg, loteprednol etabonate) removes this responsibility from those who find shaking agitating. Even among prednisolone suspensions, a generic version has been shown to have poorer dose uniformity and may require more shaking in order to achieve the same dose uniformity as a brand-name version. 9 Besides the convenience of not requiring shaking, difluprednate 0.05% does not contain benzalkonium chloride (BAK); instead, it uses sorbic acid as a preservative. Alternative topical corticosteroids without BAK include preservative-free dexamethasone 0.1%, preservative-free loteprednol 0.5%, and compounded preservative-free methylprednisolone 1%. Table 1 provides a quick reference list of topical corticosteroids that are frequently prescribed in the United States RISK AND REWARDS, IOP ELEVATION With corticosteroid therapy, higher anti-inflammatory rewards do not come without the potential for higher risk, such as IOP elevation, cataractogenesis, epithelial breakdown into a geographic ulcer if administered in the presence of a herpetic dendritic ulcer, and fungal infection with long-term corticosteroid use. Generally, the risk 16 CATARACT & REFRACTIVE SURGERY TODAY NOVEMBER/DECEMBER 2014
3 TABLE 2. NSAIDs IN THE UNITED STATES Drug Brand Name Manufacturer Dosing COX-2 IC50, µm Bromfenac Xibrom 0.09% Formerly Ista Pharmaceuticals; now generic Diclofenac Voltaren 0.1% Ophtha CD Preservative b.i.d Benzalkonium chloride (BAK) 0.005% Bromday 0.09% Bausch + Lomb Daily BAK 0.005% Prolensa 0.07% Bausch + Lomb Daily BAK 0.005% Voltaren 0.1% Ophtha Dicloftil 0.1% Alcon q.i.d BAK 0.005% Alcon q.i.d. Thimerosal 0.004% Farmigea (not available in the United States) q.i.d. Preservative free Flurbiprofen Ocufen 0.03% Allergan q.i.d Thimerosal 0.005% Ketorolac Acular 0.5% Allergan q.i.d BAK 0.01% Acular LS 0.4% Allergan q.i.d. BAK 0.006% Acuvail 0.45% Allergan b.i.d. Preservative free Nepafanac Nevanac Alcon t.i.d BAK 0.005% Ilevro Alcon Daily BAK 0.005% for a steroid-related IOP spike is correlated to the potency of the topical steroid; other influencing factors include the duration and frequency of the drug s administration as well as the susceptibility of the individual. About one-third of the general population are potential moderate steroid responders (IOP increase of 6-15 mm Hg). About 5% to 6% of the general population, in addition to the 33% mentioned previously, are severe responders (IOP increase > 15 mm Hg, with many having a marked IOP increase of > 31 mm Hg after 4-6 weeks of topical steroid use). 15,16 Despite proper tapering of topical corticosteroid therapy, IOP may not necessarily decrease in steroidresponsive patients who are at increased risk of developing open-angle glaucoma. Also, topical corticosteroids may yield a crossover effect with IOP elevation in the fellow eye from systemic absorption. 17 The provider should be aware that corticosteroid use may lead to a dose-dependent IOP spike that occurs more frequently, more severely, and more rapidly in children than in adults. 18 Due to the potential IOP elevation with the stronger corticosteroids, softer corticosteroids have been strategically designed to reduce the risk of IOP elevation. Loteprednol and rimexolone are rapidly hydrolyzed into their respective inactive metabolite, and fluorometholone despite a surprisingly high pharmacologic potency is considered a soft steroid because of its limited corneal penetration. 5 Placebo-controlled trials have been conducted, but there has not been a randomized headto-head comparison of the softer steroids. By knowing the profile of each corticosteroid, an ophthalmic provider can select the most appropriate antiinflammatory medication for the patient. NSAID PEARLS NSAIDs produce a variety of ocular effects. The growing body of scientific evidence suggests they may be beneficial in diabetic retinopathy, diabetic macular edema, age-related macular degeneration, and even ocular tumors. The longest-standing and most widespread uses of NSAIDs, however, are for reducing postoperative inflammation and preventing and treating cystoid macular edema (CME) associated with intraocular surgery. This article focuses on those applications. NSAIDs reduce inflammation by inhibiting COX enzymes (COX-1 and COX-2), thereby limiting the production of prostaglandins via the arachidonic acid cascade. Prostaglandins mediate multiple inflammatory changes, increasing vasodilation and vascular permeability. 19,20 In the eye, the drugs also disrupt the blood-aqueous barrier, leading not only to iritis but also increasing the risk of CME as inflammatory mediators leak into the eye. Topical NSAIDs have been shown to be more effective than corticosteroids in re-establishing the blood-aqueous barrier and can thus play a critical role in the management of postoperative 18 CATARACT & REFRACTIVE SURGERY TODAY NOVEMBER/DECEMBER 2014
4 and other ophthalmic inflammation. 21 When choosing an NSAID, several factors are worth considering. EFFICACY As a rule, the inhibition of COX-2 inducible in inflammatory conditions determines the clinical efficacy of an ophthalmic NSAID (Table 2). 22,23 Interestingly, however, evidence does not support a direct correlation between in vitro potency, measured by the IC50 (the concentration required to reduce enzyme activity to half), and either bioavailability or medication effectiveness. 24 Flach et al compared the anti-inflammatory effects of diclofenac (IC50 = µm) and ketorolac (IC50 = 0.12 µm) in a double-masked study of 120 postoperative patients using both a laser cell and flare meter and clinical observation. The investigators found the two treatments to be equivalent. 25 Bromfenac has the lowest IC50 of the group (0.023 µm), indicating greatest potency. A 2007 study, however, compared the in vivo concentration and in vitro PGE2 inhibition of amfenac, its prodrug nepafenac (nepafanac is converted to bioactive amfenac primarily by ocular tissue hydrolases 26 ), ketorolac, and bromfenac. Nepafenac proved to be most bioavailable with the shortest time to peak concentration and the highest peak aqueous humor concentration. Amfenac was more potent at COX-2 inhibition than either bromfenac or ketorolac (the most potent COX-1 inhibitor). 27 On the other hand, another study conducted that same year suggested ketorolac was as effective as nepafenac clinically (assessed using BCVA, anterior chamber inflammation on examination, and pain control) and perhaps better tolerated, with greater reported satisfaction and compliance among patients. 28,29 Although flurbiprofen reduces intraoperative miosis and inflammation after cataract surgery, the weight of the scientific evidence suggests it is less effective than other available NSAIDs. 30 DOSING SCHEDULE While maximizing drug effect may be necessary in some patients (eg, those with persistent macular edema), in many routine cases, it is just as important that ease of drop use facilitates patients adherence to therapy. Several studies have examined reduced dosing schedules for bromfenac, ketorolac, and nepafenac. Among dosing schedules for nepafenac, dosing three times a day resulted in better pain control on day 1 after cataract surgery. By postoperative day 3, patients using nepafenac only once daily were equally comfortable, however, and by day 14, there was no measureable difference in inflammation. 28 A more recent small study suggested that bromfenac administered just once daily was equivalent to nepafenac dosed three times daily after cataract surgery, based on measures of anterior chamber inflammation, BCVA, macular volume/retinal thickness, and IOP. 31 Twice-daily dosing of ketorolac has been evaluated versus placebo but not head-to-head with other agents. SIDE EFFECTS In the 1990s, reports of corneal melting associated with topical NSAID use caused significant concern in the ophthalmology community. Most cases were associated with a now-discontinued diclofenac product (DSOS) and felt to be related to the vitamin E-based solubilizer tocophersolan it contained. 32,33 However, a few cases of corneal melt have since been associated with other formulations of ophthalmic diclofenac. One proposed mechanism is depletion of the neuropeptide substance P within the corneal epithelium, which is associated with delayed wound healing and a risk of neurotrophic keratopathy. 34 It is also speculated that diclofenac increases the production of lipoxygenase-derived LTB4, a polymorphonuclear chemotactic, leading to corneal inflammation and melting. 33 CONCLUSION In general, the ophthalmic practitioner should consider the patient s profile when prescribing topical corticosteroids or NSAIDs. With corticosteroids, matching the penetration and potency of the drug with consideration of clinical context, contraindications, monitoring of the potential development of open-angle glaucoma, and the patient s physical limitations guides selection of the topical corticosteroid that is most appropriate for the patient. Before prescribing an NSAID, it behooves practitioners to determine whether a patient is predisposed to delayed wound healing (as in diabetes, rheumatoid arthritis, or other autoimmune inflammatory conditions) or has likely corneal denervation (as in severe ocular surface disease, a history of herpetic keratitis, or after multiple complex ocular surgeries). Certainly, as with topical steroids, patients should not follow a prolonged, unsupervised course of topical NSAIDs. n Section Editor Sumit Sam Garg, MD, is the medical director, vice chair of clinical ophthalmology, and an assistant professor of ophthalmology at the Gavin Herbert Eye Institute at the University of California, Irvine, School of Medicine. He also serves on the ASCRS Young Physicians and Residents Clinical Committee and is involved in residents and fellows education. Dr. Garg may be reached at gargs@uci.edu. Garrick Chak, MD, and Amanda E. Kiely, MD, are glaucoma fellows at the Duke Eye Center in Durham, North Carolina. They both acknowledged no financial interest in the 20 CATARACT & REFRACTIVE SURGERY TODAY NOVEMBER/DECEMBER 2014
5 products or companies mentioned herein. Dr. Chak may be reached at and Dr. Kiely may be reached at Pratap Challa, MD, is the director of the ophthalmology residency program and an associate professor of ophthalmology at the Duke Eye Center in Durham, North Carolina. He acknowledged no financial interest in the products or companies mentioned herein. Dr. Challa may be reached at 1. Comstock TL, Decory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam. 2012;2012: Cidlowski JA. Glucocorticoids and their actions in cells. Retina. 2009;29(6):S21-S MedCalc. Steroid equivalence calculator. Accessed October 15, Kelly HW. Comparison of inhaled corticosteroids. Ann Pharmacother. 1998;32: Awan MA, Agarwal PK, Watson DG, et al. Penetration of topical and subconjunctival corticosteroids into human aqueous humor and its therapeutic significance. Br J Ophthalmol. 2009;93: Kuplerman A, Leibowitz HM. Biological equivalence of ophthalmic prednisolone suspensions. Am J Ophthalmol. 1976;82: McGhee CNJ, Noble MJ, Watson DG, et al. Penetration of topically applied prednisolone sodium phosphate into human aqueous humour. Eye. 1989;3: Fiscella RG, Jensen M, Van Dyck G. Generic prednisolone suspension substitution. Arch Ophthalmol. 1998;116(5): Stringer W, Bryant R. Dose uniformity of topical corticosteroid preparations: difluprednate ophthalmic emulsion 0.05% versus branded and generic prednisolone ophthalmic suspension 1%. Clin Ophthalmol. 2010;4: Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Hardman JG, Limbird LE, Gilman AG, eds. Goodman & Gilman s The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill; 2001: Rhee DJ, Colby KA, Sobrin L, Rapuano CJ. Ophthalmologic Drug Guide. 2nd ed. New York, NY: Springer Science & Business Media; 2010: Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20: Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther. 2013;2: Tajika T, Waki M, Tsuzuki M, et al. Pharmacokinetic features of difluprednate ophthalmic emulsion in rabbits as determined by glucocorticoid receptor-binding bioassay. J Ocul Pharmacol Ther. 2011;27(1): Jones III R, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17: Cohen A. Steroid induced glaucoma. In: Rumelt S, ed. Glaucoma-Basic and Clinical Concepts. com/books/glaucoma-basic-and-clinical-concepts/steroid-induced-glaucoma. Accessed October 13, Palmberg PF, Mandell A, Wilensky JT, et al. The reproductivity of the intraocular pressure response to dexamethasone. Am J Ophthalmol. 1975;80: Lam DSC, Fan DSP, Ng JSK, et al. Ocular hypertensive and anti-inflammatory responses to different dosages of topical dexamethasone in children: a randomized trial. Clin Experiment Ophthalmol. 2005;33(3): Ophthalmic NSAIDs Review (2008). Provider Synergies, LLC. NVRx_DCR_ _Ophthalmic_NSAIDs.pdf. Accessed September 24, Rowen S. Preoperative and postoperative medications used for cataract surgery. Curr Opin Ophthalmol. 1999;10: Jampol LM, Jain S, Pudzisz B, Weinreb RN. Nonsteroidal anti-inflammatory drugs and cataract surgery. Arch Ophthalmol. 1994;112(7): Gallemore RP. NSAIDs in treatment of retinal disorders. Review of Ophthalmology. 2006;6(45): Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55(2): Warner TD, Vojnovic I, Bishop-Bailey D, Mitchell JA. Influence of plasma proteins on the potencies of inhibitors of cyclooxygenase-1 and -2. FASEB J. 2006;20(3): Flach AJ, Dolan BJ, Donahue ME, et al. Comparative effects of ketorolac 0.5% or diclofenac 0.1% ophthalmic solutions on inflammation after cataract surgery. Ophthalmology. 1998;105(9): Gaynes BI, Onyekwuluje A. Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension. Clin Ophthalmol. 2008; 2(2): Walters T, Raizman M, Ernest P, et al. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007;33(9): Maxwell WA, Reiser HJ, Stewart RH, et al. Nepafenac dosing frequency for ocular pain and inflammation associated with cataract surgery. J Ocul Pharmacol Ther. 2008;24(6): Duong HV, Westfield KC, Chalkley TH. Ketorolac tromethamine LS 0.4% versus nepafenac 0.1% in patients having cataract surgery. Prospective randomized double-masked clinical trial. J Cataract Refract Surg. 2007;33(11): Blaydes JEJ, Kelley EP, Walt JG, et al. Flurbiprofen 0.03% for the control of inflammation following cataract extraction by phacoemulsification. J Cataract Refract Surg. 1993;19(4): Cable M. Comparison of bromfenac 0.09% QD to nepafanac 0.1% TID after cataract surgery: pilot evaluation of visual acuity, macular volume, and retinal thickness at a single site. Clin Ophthalmol. 2012;6: Gaynes BI, Fiscella R. Topical nonsteroidal anti-inflammatory drugs for ophthalmic use. Drug Safety. 2002;25: Congdon NG, Schein OD, von Kulajta P, et al. Corneal complications associated with topical ophthalmic use of nonsteroidal antiinflammatory drugs. J Cataract Refract Surg. 2001;27(4): Yamada M, Ogata M, Kawai M, et al. Topical diclofenac sodium decreases substance P content in tears. Arch Ophthalmol. 2002;120:51-54.
Better ophthalmic surgery outcomes in recent years have created more Demanding Patients.
 By Alaa EL Zawawi Prof. of Ophthalmology Alexandria - Egypt Launch Better ophthalmic surgery outcomes in recent years have created more Demanding Patients. 1 NSAIDs can play a pivotal role in facilitating
By Alaa EL Zawawi Prof. of Ophthalmology Alexandria - Egypt Launch Better ophthalmic surgery outcomes in recent years have created more Demanding Patients. 1 NSAIDs can play a pivotal role in facilitating
Anterior Segment Cataract Surgery
 Current Use of Non-steroidal Anti-inflammatory Drugs in the Treatment of Ocular Inflammation Related to Cataract Surgery Eric Donnenfeld Clinical Professor of Ophthalmology, New York University, New York,
Current Use of Non-steroidal Anti-inflammatory Drugs in the Treatment of Ocular Inflammation Related to Cataract Surgery Eric Donnenfeld Clinical Professor of Ophthalmology, New York University, New York,
A New Paradigm in NSAID Treatment
 V 5/6 pg Contents Common Uses of Ophthalmic NSAIDs A New Paradigm in NSAID History of (bromfenac ophthalmic solution).9% Efficacy of Phase III trials Pain data Post-Approval trials Pharmacokinetics, Penetration,
V 5/6 pg Contents Common Uses of Ophthalmic NSAIDs A New Paradigm in NSAID History of (bromfenac ophthalmic solution).9% Efficacy of Phase III trials Pain data Post-Approval trials Pharmacokinetics, Penetration,
WARNING LETTER. According to the Indications and Usage section of the FDA approved product labeling (PI):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
Update on management of Anterior Uveitis
 Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
BARRY A. SCHECHTER ABSTRACT
 JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
Case History. The SEVEN HABITS of Highly Effective Anterior Uveitis Management. SLEx findings: SLEx corneal findings: y.o.
 The SEVEN HABITS of Highly Effective Anterior Uveitis Management Case History! 68 y.o. Caucasian female of photophobia and blurred vision! As well as a headache over right eye for 2 days! Complains Paul
The SEVEN HABITS of Highly Effective Anterior Uveitis Management Case History! 68 y.o. Caucasian female of photophobia and blurred vision! As well as a headache over right eye for 2 days! Complains Paul
Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use
 Int Ophthalmol (2012) 32:507 517 DOI 10.1007/s10792-012-9589-2 REVIEW Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use Michael Amon Massimo
Int Ophthalmol (2012) 32:507 517 DOI 10.1007/s10792-012-9589-2 REVIEW Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use Michael Amon Massimo
INDICATIONS ACULAR 0,5 % is indicated for the relief of inflammation following ocular surgery.
 Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
84 Year Old with Rosacea
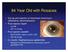 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
Ocular NSAIDs: A New Option
 Insert to March 2006 Ocular NSAIDs: A New Option Evaluating performance parameters to optimize therapy. Potency and Penetration Efficacy Safety Clinical Experience to Date This continuing medical education
Insert to March 2006 Ocular NSAIDs: A New Option Evaluating performance parameters to optimize therapy. Potency and Penetration Efficacy Safety Clinical Experience to Date This continuing medical education
INVELTYS (loteprednol etabonate ophthalmic suspension) 1%, for topical ophthalmic use Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
See 17 for PATIENT COUNSELING INFORMATION.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
Postoperative follow up and treatment after refractive surgery
 Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Case History. Slit lamp exam: Clinical Pearls in the Management of Iritis. 2- injection: Irregular SPK and staining AC: grade 3 cell & flare
 Clinical Pearls in the Management of Iritis Paul Karpecki, OD, FAAO Corneal Services and Ocular Disease Research Koffler Vision Group-Lexington, KY 68 y.o. Caucasian female Complains of photophobia and
Clinical Pearls in the Management of Iritis Paul Karpecki, OD, FAAO Corneal Services and Ocular Disease Research Koffler Vision Group-Lexington, KY 68 y.o. Caucasian female Complains of photophobia and
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Medical Science. Research Paper. 2nd yr post graduate, Dept. Of Ophthalmology, SVS Medical College
 Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Topical Corticosteroids: Making Sense of the Options
 DICAL EDU G ME CA UIN TIO TIN CON CME N A CONTINUING MEDICAL EDUCATION PUBLICATION ISSUE 3 Topical Corticosteroids: Making Sense of the Options ERIC D. DONNENFELD, MD Topical corticosteroid prescribing
DICAL EDU G ME CA UIN TIO TIN CON CME N A CONTINUING MEDICAL EDUCATION PUBLICATION ISSUE 3 Topical Corticosteroids: Making Sense of the Options ERIC D. DONNENFELD, MD Topical corticosteroid prescribing
13 NONCLINICAL TOXICOLOGY 5.1 Increased Bleeding Time Carcinogenesis, Mutagenesis, Impairment of 5.2 Delayed Healing
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
Surgery Post-operative
 Surgery Post-operative Redefining the Treatment Paradigm for Post-operative Inflammation Control The Role of Topical Non-steroidal Anti-inflammatory Drugs Oliver Findl Associate Professor of Ophthalmology
Surgery Post-operative Redefining the Treatment Paradigm for Post-operative Inflammation Control The Role of Topical Non-steroidal Anti-inflammatory Drugs Oliver Findl Associate Professor of Ophthalmology
The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, Bromfenac 0.1% and Ketorolac 0.45%, on Cataract Surgery
 Original Article Yonsei Med J 215 Nov;56(6):1671-1677 pissn: 513-5796 eissn: 1976-2437 The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, and, on Cataract Surgery Ji Won Jung 1,2, Byung Hoon Chung
Original Article Yonsei Med J 215 Nov;56(6):1671-1677 pissn: 513-5796 eissn: 1976-2437 The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, and, on Cataract Surgery Ji Won Jung 1,2, Byung Hoon Chung
Rama Vadapalli * 1, Vankadara Naga Suresh 2. Online Access and Article Informtaion. Original Research Article
 Original Research Article An Interventional Follow up Study to Determine the Effect of Nepafenac 0.1% in Macular thickness in Patients who had Undergone Cataract Surgery as Determined By OCT Rama Vadapalli
Original Research Article An Interventional Follow up Study to Determine the Effect of Nepafenac 0.1% in Macular thickness in Patients who had Undergone Cataract Surgery as Determined By OCT Rama Vadapalli
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
Completed Clinical Research Trials Monte Dirks, M.D.
 Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
NSAIDs in Treatment of Retinal Disorders
 NSAIDs in Treatment of Retinal Disorders These drugs are playing new roles beyond their established use in cataract and refractive surgery. NON-STEROIDAL ANTI-INFLAMm a t o r y drugs are approved for the
NSAIDs in Treatment of Retinal Disorders These drugs are playing new roles beyond their established use in cataract and refractive surgery. NON-STEROIDAL ANTI-INFLAMm a t o r y drugs are approved for the
PHARMACOLOGY Class: Ketorolac trometamol is a member of the pyrrolo-pyrolle group of non-steroidal antiinflammatory
 ACULAR Eye Drops NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure: Chemical Name: ( )-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol
ACULAR Eye Drops NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure: Chemical Name: ( )-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol
Impact of the Topical Ophthalmic Corticosteroid Loteprednol Etabonate on Intraocular Pressure
 Adv Ther (2016) 33:532 552 DOI 10.1007/s12325-016-0315-8 REVIEW Impact of the Topical Ophthalmic Corticosteroid Loteprednol Etabonate on Intraocular Pressure John D. Sheppard. Timothy L. Comstock. Megan
Adv Ther (2016) 33:532 552 DOI 10.1007/s12325-016-0315-8 REVIEW Impact of the Topical Ophthalmic Corticosteroid Loteprednol Etabonate on Intraocular Pressure John D. Sheppard. Timothy L. Comstock. Megan
TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%)
 Published on: 23 Sep 2014 TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%) Composition Loteprednol Etabonate 5 mg (0.5% w/v) Tobramycin...3 mg (0.3% w/v) Benzalkonium Chloride.. 0.01%
Published on: 23 Sep 2014 TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%) Composition Loteprednol Etabonate 5 mg (0.5% w/v) Tobramycin...3 mg (0.3% w/v) Benzalkonium Chloride.. 0.01%
NSAIDs. The Evolving Role of. in Cataract Surgery
 Supplement to April 2011 The Evolving Role of NSAIDs in Cataract Surgery Strategies for ocular surface management with the newly approved q.d. formulation of bromfenac 0.09%. Featuring: Johnny Gayton,
Supplement to April 2011 The Evolving Role of NSAIDs in Cataract Surgery Strategies for ocular surface management with the newly approved q.d. formulation of bromfenac 0.09%. Featuring: Johnny Gayton,
2665 Meadowpine Blvd June 2, 1987 Mississauga, Ontario L5N 8C7
 PRODUCT MONOGRAPH Pr FLAREX7 Fluorometholone Acetate Ophthalmic Suspension 0.1% Corticosteroid : Alcon Canada Inc Date of Preparation: 2665 Meadowpine Blvd June 2, 1987 Mississauga, Ontario L5N 8C7 9HN842974
PRODUCT MONOGRAPH Pr FLAREX7 Fluorometholone Acetate Ophthalmic Suspension 0.1% Corticosteroid : Alcon Canada Inc Date of Preparation: 2665 Meadowpine Blvd June 2, 1987 Mississauga, Ontario L5N 8C7 9HN842974
For last half century corticosteroids are used for ocular inflammation. They are used to suppress ocular inflammation in trauma, uveitis as well as
 The Professional Medical Journal DOI: 10.17957/TPMJ/17.3940 1. MBBS, DO, FCPS (Ophthalmology) Eye Specialist PAF Base Hospital Rafiqui Shorkot. 2. MBBS, DO, MCPS, FCPS (Ophthalmology) Classified Eye Specialist,
The Professional Medical Journal DOI: 10.17957/TPMJ/17.3940 1. MBBS, DO, FCPS (Ophthalmology) Eye Specialist PAF Base Hospital Rafiqui Shorkot. 2. MBBS, DO, MCPS, FCPS (Ophthalmology) Classified Eye Specialist,
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
Steroids and Controlled Substances
 Steroids and Controlled Substances Marc R. Bloomenstein OD, FAAO Scottsdale, Arizona Disclosure Presenter is on speakers panel of Alcon, Allergan, Abbott, Bausch + Lomb, Inspire, STAAR Surgical, Odyssey
Steroids and Controlled Substances Marc R. Bloomenstein OD, FAAO Scottsdale, Arizona Disclosure Presenter is on speakers panel of Alcon, Allergan, Abbott, Bausch + Lomb, Inspire, STAAR Surgical, Odyssey
sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa
![sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa](/thumbs/78/77533900.jpg) PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
Anti-inflammatory effectiveness in the cornea of topically administered prednisolone
 Anti-inflammatory effectiveness in the cornea of topically administered prednisolone Howard M. Leibowitz and Allan Kupferman The relative ability of two of the most widely used ophthalmic prednisolone
Anti-inflammatory effectiveness in the cornea of topically administered prednisolone Howard M. Leibowitz and Allan Kupferman The relative ability of two of the most widely used ophthalmic prednisolone
Generics are Cheaper and Just as Effective
 Disclosures Generics are Cheaper and Just as Effective Robert L. Stamper, MD UCSF Department of Ophthalmology No current pharmaceutical industry connections Data & Safety Monitor for Cypass - Chair - Transcend
Disclosures Generics are Cheaper and Just as Effective Robert L. Stamper, MD UCSF Department of Ophthalmology No current pharmaceutical industry connections Data & Safety Monitor for Cypass - Chair - Transcend
Vankadara Naga Suresh * 1, Rama Vadapalli 2. Online Access and Article Informtaion. Original Research Article
 Original Research Article Effect of Prophylactic Bromfenac 0.07% on Cystoid Macular Edema Assessed using Optical Coherence Tomography Quantification of Total Macular Volume after Cataract Surgery Vankadara
Original Research Article Effect of Prophylactic Bromfenac 0.07% on Cystoid Macular Edema Assessed using Optical Coherence Tomography Quantification of Total Macular Volume after Cataract Surgery Vankadara
INDICATIONS For steroid responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye globe.
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
RETINA TODAY. for Therapeutic Use in the Posterior Segment CME ACTIVITY. Jointly sponsored by the Dulaney Foundation and Retina Today.
 Supplement to CME ACTIVITY RETINA TODAY November/December 2010 Jointly sponsored by the Dulaney Foundation and Retina Today. for Therapeutic Use in the Posterior Segment Jointly sponsored by the Dulaney
Supplement to CME ACTIVITY RETINA TODAY November/December 2010 Jointly sponsored by the Dulaney Foundation and Retina Today. for Therapeutic Use in the Posterior Segment Jointly sponsored by the Dulaney
Cataract and Diabetic macular edema: What should I do?
 Cataract and Diabetic macular edema: What should I do? Irini Chatziralli Ophthalmic Surgeon, University Scholar 2 nd Department of Ophthalmology, University of Athens (Director: Prof P. Theodossiadis)
Cataract and Diabetic macular edema: What should I do? Irini Chatziralli Ophthalmic Surgeon, University Scholar 2 nd Department of Ophthalmology, University of Athens (Director: Prof P. Theodossiadis)
AWMSG SECRETARIAT ASSESSMENT REPORT. Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension. Reference number: 1509 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension Reference number: 1509 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology
AWMSG SECRETARIAT ASSESSMENT REPORT Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension Reference number: 1509 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology
Combining strategies
 Supplement to OCULAR SURGERY US EDITION NEWS JULY 25, 2016 Combining strategies in the counseling and care of patients with cataracts This OCULAR SURGERY NEWS supplement is produced by SLACK Incorporated
Supplement to OCULAR SURGERY US EDITION NEWS JULY 25, 2016 Combining strategies in the counseling and care of patients with cataracts This OCULAR SURGERY NEWS supplement is produced by SLACK Incorporated
Innovation In Ophthalmology
 Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
D90 (27/10/2005) Final SmPC NL/H/653/01
 1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
Managing the Patient with POAG
 Managing the Patient with POAG Vision Institute Annual Fall Conference Mitchell W. Dul, OD, MS, FAAO mdul@sunyopt.edu Richard J. Madonna, MA, OD, FAAO rmadonna@sunyopt.edu Ocular Hypertension (OHT) Most
Managing the Patient with POAG Vision Institute Annual Fall Conference Mitchell W. Dul, OD, MS, FAAO mdul@sunyopt.edu Richard J. Madonna, MA, OD, FAAO rmadonna@sunyopt.edu Ocular Hypertension (OHT) Most
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
OPHTHALMIC GENERIC FORMULATIONS BY ADREA R. BENKOFF, M.D.
 OPHTHALMIC GENERIC FORMULATIONS BY ADREA R. BENKOFF, M.D. WHAT YOU NEED TO KNOW ABOUT OPHTHALMIC GENERICS GENERIC REQUIREMENTS WHO MAKES GENERIC OPHTHALMIC PRODUCTS DO THEY WORK AS WELL AS THE BRAND PRODUCT
OPHTHALMIC GENERIC FORMULATIONS BY ADREA R. BENKOFF, M.D. WHAT YOU NEED TO KNOW ABOUT OPHTHALMIC GENERICS GENERIC REQUIREMENTS WHO MAKES GENERIC OPHTHALMIC PRODUCTS DO THEY WORK AS WELL AS THE BRAND PRODUCT
The role of perioperative nonsteroidal anti-inflammatory drugs use in cataract surgery
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 18, Issue 1 Ser. 15 (January. 2019), PP 23-27 www.iosrjournals.org The role of perioperative nonsteroidal
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 18, Issue 1 Ser. 15 (January. 2019), PP 23-27 www.iosrjournals.org The role of perioperative nonsteroidal
PRODUCT MONOGRAPH. (loteprednol etabonate ophthalmic gel 0.5 % w/w) Professed Standard. Corticosteroid
 PRODUCT MONOGRAPH Pr LOTEMAX GEL (loteprednol etabonate ophthalmic gel 0.5 % w/w) Professed Standard Corticosteroid Bausch & Lomb Incorporated Rochester, NY, USA 14609 www.bausch.com Date of Preparation:
PRODUCT MONOGRAPH Pr LOTEMAX GEL (loteprednol etabonate ophthalmic gel 0.5 % w/w) Professed Standard Corticosteroid Bausch & Lomb Incorporated Rochester, NY, USA 14609 www.bausch.com Date of Preparation:
Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution
 REVIEW Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution Hyung Cho 1 Kenneth J Wolf 1 Eric J Wolf 2 1 Department of Ophthalmology, Albert Einstein
REVIEW Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution Hyung Cho 1 Kenneth J Wolf 1 Eric J Wolf 2 1 Department of Ophthalmology, Albert Einstein
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
ASCRS launches new Annual Clinical Survey
 ASCRS launches new Annual Clinical Survey Survey of more than 1,000 members measures current clinical opinions and practice patterns Survey overview The American Society of Cataract & Refractive Surgery
ASCRS launches new Annual Clinical Survey Survey of more than 1,000 members measures current clinical opinions and practice patterns Survey overview The American Society of Cataract & Refractive Surgery
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NEVANAC 1 mg/ml eye drops, suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of suspension contains 1 mg nepafenac.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NEVANAC 1 mg/ml eye drops, suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of suspension contains 1 mg nepafenac.
Anterior Segment Disease and the Systemic Link Mile Brujic, OD, FAAO
 Anterior Segment Disease and the Systemic Link Mile Brujic, OD, FAAO brujic@prodigy.net Summary As optometry s role in health care increases, so does our responsibility to appropriately diagnose and appropriately
Anterior Segment Disease and the Systemic Link Mile Brujic, OD, FAAO brujic@prodigy.net Summary As optometry s role in health care increases, so does our responsibility to appropriately diagnose and appropriately
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops
 AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
Abbreviated Drug Evaluation: Fluocinolone acetonide intravitreal implant (Retisert )
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Methylprednisolone iv to po dose
 Methylprednisolone iv to po dose The Borg System i Methylprednisolone iv to po dose PRESTON T. PHILLIPS A R C H I T E C T ford residence southampton, ny Methylprednisolone iv to po dose Chemotherapy. Cytarabine
Methylprednisolone iv to po dose The Borg System i Methylprednisolone iv to po dose PRESTON T. PHILLIPS A R C H I T E C T ford residence southampton, ny Methylprednisolone iv to po dose Chemotherapy. Cytarabine
William F. Mieler, MD University of Illinois at Chicago Chicago, IL
 William F. Mieler, MD University of Illinois at Chicago Chicago, IL Advisory Board Genentech Data and Safety Monitoring Committee ThromboGenics (in the past) Acucela Corticosteroids and NSAIDs are the
William F. Mieler, MD University of Illinois at Chicago Chicago, IL Advisory Board Genentech Data and Safety Monitoring Committee ThromboGenics (in the past) Acucela Corticosteroids and NSAIDs are the
How Do Ocular Corticosteroids and NSAIDs Work?
 DICAL EDU G ME CA UIN TIO TIN CON CME N A CONTINUING MEDICAL EDUCATION PUBLICATION ISSUE 6 How Do Ocular Corticosteroids and NSAIDs Work? Membrane Phospholipids Corticosteroids Daniel r. saban, PHD Corticosteroids
DICAL EDU G ME CA UIN TIO TIN CON CME N A CONTINUING MEDICAL EDUCATION PUBLICATION ISSUE 6 How Do Ocular Corticosteroids and NSAIDs Work? Membrane Phospholipids Corticosteroids Daniel r. saban, PHD Corticosteroids
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
Cataract Surgery Co-Management
 Cataract Surgery Co-Management Phacoemulsification, Clear-Lens Extraction, and LensX INCLUSION CRITERIA: Significant visual complaints (decreased VA, increased glare, decreased Activities of Daily Living
Cataract Surgery Co-Management Phacoemulsification, Clear-Lens Extraction, and LensX INCLUSION CRITERIA: Significant visual complaints (decreased VA, increased glare, decreased Activities of Daily Living
Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension
 REVIEW Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension Bruce I Gaynes 1,2 Anne nyekwuluje 1 1 Departments of phthalmology and 2 Pharmacology, Rush College of Medicine,
REVIEW Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension Bruce I Gaynes 1,2 Anne nyekwuluje 1 1 Departments of phthalmology and 2 Pharmacology, Rush College of Medicine,
CME. Clinical Uses of NSAIDs in Ophthalmic Surgery. Learning Objectives. September 1, Earn 1.0 hour CME credit
 September 1, 2005 Clinical Uses of NSAIDs in Ophthalmic Surgery CME Earn 1.0 hour CME credit Learning Objectives After reviewing the material, the participant should be able to: Discuss the use of NSAIDs
September 1, 2005 Clinical Uses of NSAIDs in Ophthalmic Surgery CME Earn 1.0 hour CME credit Learning Objectives After reviewing the material, the participant should be able to: Discuss the use of NSAIDs
Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery
 Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery David F. Chang, MD, 1 Ivan H. Garcia, MD, 2 John D. Hunkeler, MD, 3 Thomas Minas, MD 4 Objective: To evaluate the safety
Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery David F. Chang, MD, 1 Ivan H. Garcia, MD, 2 John D. Hunkeler, MD, 3 Thomas Minas, MD 4 Objective: To evaluate the safety
! Somatic! Visceral! Neuropathic! Psychogenic. ! Analgesic! Relief without sedation! Works on peripheral pain receptors
 PAIN, PAIN, GO AWAY. TYPES OF PAIN! Somatic! Visceral! Neuropathic! Psychogenic Jill Autry, OD, RPh Eye Center of Texas, Houston drjillautry@tropicalce.com ANALGESIA! Mild! Aspirin! Paracetamol! Moderate!
PAIN, PAIN, GO AWAY. TYPES OF PAIN! Somatic! Visceral! Neuropathic! Psychogenic Jill Autry, OD, RPh Eye Center of Texas, Houston drjillautry@tropicalce.com ANALGESIA! Mild! Aspirin! Paracetamol! Moderate!
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
ASCRS completes fourth annual Clinical Survey
 ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
Chemical Names: Prednisolone acetate: 11ß,17,21-Trihydroxypregna-1,4-diene-3,20-dione 21-acetate.
 PRED-G (gentamicin and prednisolone acetate ophthalmic suspension, USP) 0.3%/1% sterile DESCRIPTION PRED-G sterile ophthalmic suspension is a topical anti-inflammatory/anti-infective combination product
PRED-G (gentamicin and prednisolone acetate ophthalmic suspension, USP) 0.3%/1% sterile DESCRIPTION PRED-G sterile ophthalmic suspension is a topical anti-inflammatory/anti-infective combination product
FLAREX PRODUCT MONOGRAPH. Fluorometholone Acetate Ophthalmic Suspension. 0.1% w/v. Corticosteroid. Date of Preparation: June 2, 1987
 PRODUCT MONOGRAPH Pr FLAREX Fluorometholone Acetate Ophthalmic Suspension 0.1% w/v Corticosteroid Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date of
PRODUCT MONOGRAPH Pr FLAREX Fluorometholone Acetate Ophthalmic Suspension 0.1% w/v Corticosteroid Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date of
AUSTRALIAN PRODUCT INFORMATION FML (FLUOROMETHOLONE) EYE DROPS
 AUSTRALIAN PRODUCT INFORMATION FML (FLUOROMETHOLONE) EYE DROPS 1. NAME OF THE MEDICINE FML fluorometholone 1 mg/ml eye drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION FML eye drops contain fluorometholone
AUSTRALIAN PRODUCT INFORMATION FML (FLUOROMETHOLONE) EYE DROPS 1. NAME OF THE MEDICINE FML fluorometholone 1 mg/ml eye drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION FML eye drops contain fluorometholone
Slide 1. Slide 2. Slide 3
 Slide 1 Use of Steroids After Cataract Surgery March 16, 2016 Erica Person, MD MS FAAO Slide 2 Outline Results from prednisolone vs. difluprednate Evaluation of new agent for cataract surgery Laser alternative
Slide 1 Use of Steroids After Cataract Surgery March 16, 2016 Erica Person, MD MS FAAO Slide 2 Outline Results from prednisolone vs. difluprednate Evaluation of new agent for cataract surgery Laser alternative
FLAREX * PRODUCT MONOGRAPH. Fluorometholone Acetate Ophthalmic Suspension. 0.1% w/v. Corticosteroid. Date of Preparation: June 2, 1987
 PRODUCT MONOGRAPH Pr FLAREX * Fluorometholone Acetate Ophthalmic Suspension 0.1% w/v Corticosteroid Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date of
PRODUCT MONOGRAPH Pr FLAREX * Fluorometholone Acetate Ophthalmic Suspension 0.1% w/v Corticosteroid Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date of
POST-CATARACT SURGERY TREATMENT PULL-THROUGH PROTOCOLS AND OUTCOMES WHERE DOES PROLENSA FIT WITHIN THE TREATMENT PARADIGM?
 POST-CATARACT SURGERY TREATMENT PULL-THROUGH PROTOCOLS AND OUTCOMES WHERE DOES PROLENSA FIT WITHIN THE TREATMENT PARADIGM? SPONSORED BY BRINGING THE DISCUSSION DIRECTLY TO YOU This supplement captures
POST-CATARACT SURGERY TREATMENT PULL-THROUGH PROTOCOLS AND OUTCOMES WHERE DOES PROLENSA FIT WITHIN THE TREATMENT PARADIGM? SPONSORED BY BRINGING THE DISCUSSION DIRECTLY TO YOU This supplement captures
In this supplement, we will
 Supplement to EyeWorld September 2015 The X Factors: Three areas that will improve refractive cataract surgery outcomes Click to read and claim CME credit Experts take aim at threats to optimal results
Supplement to EyeWorld September 2015 The X Factors: Three areas that will improve refractive cataract surgery outcomes Click to read and claim CME credit Experts take aim at threats to optimal results
CONTRAINDICATIONS Hypersensitivity to any component of this product (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only
 Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Applications of Sustained Release Delivery Systems in Ocular Disease
 Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
Applications of Sustained Release Delivery Systems in Ocular Disease Formulation and Delivery Systems For Peptide and Protein 2012 December 5, 2012 Christopher A. Rhodes, Ph.D. Principal, Christopher A
New Zealand Data Sheet
 New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
Investor Presentation December The vision to see past tomorrow
 Investor Presentation December 2011 The vision to see past tomorrow Safe Harbor Statement Certain statements contained herein are forward-looking statements (as such term is defined in the Private Securities
Investor Presentation December 2011 The vision to see past tomorrow Safe Harbor Statement Certain statements contained herein are forward-looking statements (as such term is defined in the Private Securities
8/7/12. Anterior Seg Grand Rounds Case III. New Advances in the Management of Viral Eye Disease. Slit lamp exam: Sign: 68 y.o.
 New Advances in the Management of Viral Eye Disease Anterior Seg Grand Rounds Case III 68 y.o. Caucasian female!! Dr.Paul Karpecki!! Corneal Services and Ocular Disease Research!! Koffler Vision Group
New Advances in the Management of Viral Eye Disease Anterior Seg Grand Rounds Case III 68 y.o. Caucasian female!! Dr.Paul Karpecki!! Corneal Services and Ocular Disease Research!! Koffler Vision Group
BETAGAN Allergan Levobunolol HCl Glaucoma Therapy
 BETAGAN Allergan Levobunolol HCl Glaucoma Therapy Action And Clinical Pharmacology: Levobunolol is a noncardioselective beta- adrenoceptor antagonist, equipotent at both beta1 and beta2 receptors. Levobunolol
BETAGAN Allergan Levobunolol HCl Glaucoma Therapy Action And Clinical Pharmacology: Levobunolol is a noncardioselective beta- adrenoceptor antagonist, equipotent at both beta1 and beta2 receptors. Levobunolol
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME FML 0.1% w/v eye drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Fluorometholone 1 mg/ml (0.1% w/v) For the full list of excipients, see Section 6.1. 3. PHARMACEUTICAL
NEW ZEALAND DATA SHEET 1. PRODUCT NAME FML 0.1% w/v eye drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Fluorometholone 1 mg/ml (0.1% w/v) For the full list of excipients, see Section 6.1. 3. PHARMACEUTICAL
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution)
 VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
New Treatments Following Cataract Surgery October 23, 2018
 Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders New Treatments Following Cataract Surgery October 23, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE
Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders New Treatments Following Cataract Surgery October 23, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE
CASES JAMBALAYA. EXPERTS RECIPES for PREVENTING COMPLICATIONS CATARACT AND REFRACTIVE. Highlights From a CME Symposium.
 CME Monograph CATARACT AND REFRACTIVE CASES JAMBALAYA EXPERTS RECIPES for PREVENTING and MANAGING COMPLICATIONS Highlights From a CME Symposium Original Release: March 15, 2014 Last Review: February 27,
CME Monograph CATARACT AND REFRACTIVE CASES JAMBALAYA EXPERTS RECIPES for PREVENTING and MANAGING COMPLICATIONS Highlights From a CME Symposium Original Release: March 15, 2014 Last Review: February 27,
Internal document code 1 Nev061117iNZ
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NEVANAC TM (nepafenac) Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nevanac contains 1 mg in 1 ml of nepafenac. Excipient with known effect Benzalkonium
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NEVANAC TM (nepafenac) Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nevanac contains 1 mg in 1 ml of nepafenac. Excipient with known effect Benzalkonium
Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study
 Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study James H. Peace, M.D. 1, Casey K. Kopczynski, Ph.D. 2, and Theresa Heah, M.D. 2 1 Inglewood, CA 2
Ocular Hypotensive Efficacy of Netarsudil Ophthalmic Solution 0.02% Over a 24-Hour Period: A Pilot Study James H. Peace, M.D. 1, Casey K. Kopczynski, Ph.D. 2, and Theresa Heah, M.D. 2 1 Inglewood, CA 2
Herpetic Eye Disease Jason Duncan, OD, FAAO Diplomate, American Board of Optometry Associate Professor, Southern College of Optometry
 Herpetic Eye Disease Jason Duncan, OD, FAAO Diplomate, American Board of Optometry Associate Professor, Southern College of Optometry I have what?! How to break the news Meet the Herpes Quick virology
Herpetic Eye Disease Jason Duncan, OD, FAAO Diplomate, American Board of Optometry Associate Professor, Southern College of Optometry I have what?! How to break the news Meet the Herpes Quick virology
Switch from BAK-preserved to preservative-free latanoprost decreases anterior chamber flare in POAG patients
 https://doi.org/10.1007/s10792-017-0792-z ORIGINAL PAPER Switch from BAK-preserved to preservative-free latanoprost decreases anterior chamber flare in POAG patients Ph. A. Kestelyn. Ph. G. Kestelyn. D.
https://doi.org/10.1007/s10792-017-0792-z ORIGINAL PAPER Switch from BAK-preserved to preservative-free latanoprost decreases anterior chamber flare in POAG patients Ph. A. Kestelyn. Ph. G. Kestelyn. D.
ILUVIEN IN DIABETIC MACULAR ODEMA
 1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
1 ILUVIEN IN DIABETIC MACULAR ODEMA Marie Tsaloumas Consultant Ophthalmic Surgeon Queen Elizabeth Hospital, Birmingham bars conference 2104 1 2 Declaration of interest I have sat on Advisory boards for
PRODUCT MONOGRAPH. Pr Lotemax. (loteprednol etabonate ophthalmic suspension 0.5% w/v) Corticosteroid. Professed Standard
 PRODUCT MONOGRAPH Pr Lotemax (loteprednol etabonate ophthalmic suspension 0.5% w/v) Corticosteroid Professed Standard Bausch and Lomb, Inc. Rochester, NY 14609 www.bausch.com Date of Preparation: January
PRODUCT MONOGRAPH Pr Lotemax (loteprednol etabonate ophthalmic suspension 0.5% w/v) Corticosteroid Professed Standard Bausch and Lomb, Inc. Rochester, NY 14609 www.bausch.com Date of Preparation: January
Do You Want Steroids with THAT?! Bruce Onofrey, OD, RPh, FAAO
 Do You Want Steroids with THAT?! Bruce Onofrey, OD, RPh, FAAO Disclosure Statement: Nothing to disclose Please remember to complete your session evaluations on the Academy.18 meeting app Tweet about this
Do You Want Steroids with THAT?! Bruce Onofrey, OD, RPh, FAAO Disclosure Statement: Nothing to disclose Please remember to complete your session evaluations on the Academy.18 meeting app Tweet about this
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project
 The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
Bromfenac eyedrops in the treatment of diabetic macular edema: a pilot study
 EJO ISSN 1120-6721 Eur J Ophthalmol 2017; 27 (3): 326-330 DOI: 10.5301/ejo.5000888 Original Research Article Bromfenac eyedrops in the treatment of diabetic macular edema: a pilot study Antonio Pinna,
EJO ISSN 1120-6721 Eur J Ophthalmol 2017; 27 (3): 326-330 DOI: 10.5301/ejo.5000888 Original Research Article Bromfenac eyedrops in the treatment of diabetic macular edema: a pilot study Antonio Pinna,
IOP measurements: 8.00 am (trough drug levels) and am (peak drug levels)(2 hours post dose)
 This overview of 2% eye drops was presented by Dr. Ravin N. Das, at Hotel Satya Ashoka, on 19-6-2004 in place of Dr. H. S. Ray due to unforseen circumstances. This dinner meeting was sponsored by Cipla
This overview of 2% eye drops was presented by Dr. Ravin N. Das, at Hotel Satya Ashoka, on 19-6-2004 in place of Dr. H. S. Ray due to unforseen circumstances. This dinner meeting was sponsored by Cipla
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML-NEO Liquifilm Ophthalmic Suspension COMPOSITION FML-NEO Liquifilm Ophthalmic Suspension contains per ml: Fluorometholone 1,0
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML-NEO Liquifilm Ophthalmic Suspension COMPOSITION FML-NEO Liquifilm Ophthalmic Suspension contains per ml: Fluorometholone 1,0
Trifluridine Ophthalmic Solution, 1% Sterile
 Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
