Ocular NSAIDs: A New Option
|
|
|
- Penelope Lawson
- 5 years ago
- Views:
Transcription
1 Insert to March 2006 Ocular NSAIDs: A New Option Evaluating performance parameters to optimize therapy. Potency and Penetration Efficacy Safety Clinical Experience to Date This continuing medical education activity is supported by an unrestricted educational grant from ISTA Pharmaceuticals, Inc. Jointly sponsored by The Dulaney Foundation and Cataract & Refractive Surgery Today.
2 Ocular NSAIDs: A New Option Evaluating performance parameters to optimize therapy. Jointly sponsored by The Dulaney Foundation and Cataract & Refractive Surgery Today. This continuing medical education activity is supported by an unrestricted educational grant from ISTA Pharmaceuticals, Inc. RELEASE DATE: MARCH 2006; EXPIRATION DATE: MARCH 31, 2007 STATEMENT OF NEED Topical nonsteroidal anti-inflammatory drugs (NSAIDs) offer several benefits after refractive and cataract surgery. These agents can reduce patients discomfort both during and after refractive procedures and reduce ocular inflammation, particularly in the cornea and conjunctiva. NSAIDs also lessen patients discomfort during cataract surgery, an important benefit when the ophthalmologist uses topical anesthesia. Furthermore, by helping to maintain pupillary dilation during the cataract procedure, these drugs can lower the rate of complications. NSAIDs control inflammation in the first few postoperative days, and they inhibit the development of cystoid macular edema (CME), which can occur 4 to 6 weeks after cataract surgery. The introduction of a new topical NSAID, bromfenac ophthalmic solution 0.09%, offers the ophthalmologist an additional clinical choice. This activity explores the utility of this agent and its unique properties, through available data and clinical experience, to facilitate therapeutic decision-making. TARGET AUDIENCE This activity is designed for ophthalmologists. LEARNING OBJECTIVES After the successful completion of this program, the participant should be able to: List the therapeutic applications of topical NSAIDs in ophthalmology and optimal performance characteristics in each of these applications Describe the relevance of IC 50 potency assays and in vitro tissue penetration data to NSAID performance and selection Compare NSAID clinical trial designs and study protocols Evaluate NSAIDs efficacy claims Describe available efficacy and safety data on bromfenac List barriers to patient compliance with NSAID therapy Incorporate the first b.i.d. NSAID into clinical protocols METHOD OF INSTRUCTION Participants should read the learning objectives and monograph in their entirety. After reviewing the material, they must complete the self-assessment test, which consists of a series of multiple-choice questions. Upon completing this activity as designed and achieving a passing score of 70% or higher on the self-assessment test, participants will receive a CME credit letter awarding AMA/PRA category 1 credit 4 weeks after the registration and evaluation materials are received. The estimated time to complete this activity as designed is 1 hour. ACCREDITATION This activity has been planned and implemented in accordance with essentials and standards of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of The Dulaney Foundation and Cataract & Refractive Surgery Today. The Dulaney Foundation is accredited by the ACCME to provide continuing medical education for physicians. 2 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I MARCH 2006
3 The Dulaney Foundation designates this educational activity for a maximum of one category 1 credit toward the AMA Physician s Recognition Award. Each physician should claim only those credits that he/she actually spent on the activity. This activity was reviewed for relevance, accuracy of content, balance of presentation, and time required for participation by William Trattler, MD; Terry Kim, MD; and Y. Ralph Chu, MD. DISCLOSURE In accordance with the disclosure policies of The Dulaney Foundation and to comply with ACCME guidelines, all program faculty are required to disclose to the activity participants: (1) the existence of any financial interest or other relationships with the manufacturers of any commercial products/devices, or providers of commercial services, that relate to the content of their presentation/material, or the commercial contributors of presentation/material, or the commercial contributors of this activity, that could be perceived as a real or apparent conflict of interest; and (2) the identification of a commercial product/device that is unlabeled for use or an investigational use of a product/device not yet approved. FACULTY DISCLOSURE DECLARATION The physician faculty whose material appears in this program has a financial interest, relationship, or affiliation in the following forms: Monte S. Dirks, MD, is a paid clinical researcher for ISTA Pharmaceuticals, Inc. He also receives research support and travel expenses from the company and is a member of the speaker s bureau for ISTA Pharmaceuticals, Inc. Eric D. Donnenfeld, MD, is a consultant for ISTA Pharmaceuticals, Inc., and was an investigator in the phase 3 bromfenac clinical trials. Dr. Donnenfeld is a paid consultant for Allergan, Inc., and Alcon Laboratories, Inc. Francis S. Mah, MD, is a paid consultant, and a member of the speaker s bureau for Allergan, Inc., Advanced Medical Optics, Inc., and ISTA Pharmaceuticals, Inc. Barry A. Schechter, MD, is a consultant for ISTA Pharmaceuticals, Inc. He also receives research support and travel expenses from the company and is a member of the speaker s bureau for ISTA Pharmaceuticals, Inc. Dr. Schechter has received unrestricted grant support from Allergan, Inc., for clinical research. MEET THE FACULTY Monte S. Dirks, MD, is in private practice at the Black Hills Regional Eye Institute in Rapid City, South Dakota, and is an Associate Clinical Professor of Ophthalmology at the University of South Dakota. He may be reached at (605) ; dirks1@aol.com. Eric D. Donnenfeld, MD, is a partner in Ophthalmic Consultants of Long Island and is Co-Chairman of Corneal and External Disease at the Manhattan Eye, Ear and Throat Hospital in New York. Dr. Donnenfeld may be reached at (516) ; eddoph@aol.com. Francis S. Mah, MD, is Assistant Professor in the Department of Ophthalmology and Co-Medical Director of the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh School of Medicine in Pennsylvania. Dr. Mah may be reached at (412) ; mahfs@upmc.edu. Barry A. Schechter, MD, is Director of the Department of Cornea and External Diseases at the Florida Eye Microsurgical Institute in Boynton Beach. Dr. Schechter may be reached at (561) ; barrys_@hotmail.com. A NEW NSAID The applications of ocular nonsteroidal anti-inflammatory drugs (NSAIDs) have expanded during the past few years to include the treatment of postoperative pain, ocular inflammation, and photophobia in patients undergoing intraocular and refractive surgery. Additionally, these agents are useful against intraoperative miosis. The recent introduction of bromfenac ophthalmic solution 0.09% offers clinicians another therapeutic option in the NSAID class. Although in the absence of well-controlled head-to-head clinical trials it is difficult to make direct efficacy comparisons between NSAIDs, published data on differences in penetration and potency, dosing schedule (b.i.d. vs. t.i.d. vs. q.i.d.), onset or duration of activity, and tolerability become useful in selecting among these agents. CONTENTS 4 Potency and Penetration Data available on the comparative potency and tissue penetration of available NSAIDs and the potential relevance of these differences for dosing and clinical performance is discussed. 7 Efficacy A review of the study design and results for the bromfenac phase 3 trials is presented. 10 Safety Clinical trial and postmarketing surveillance data on bromfenac is assessed. 11 Clinical Experience to Date An overview of clinical experience with bromfenac to date, focusing on evaluations of physician satisfaction and patient comfort. 13 CME Questionnaire MARCH 2006 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I 3
4 The Importance of Potency and Penetration Data available on the comparative potency and tissue penetration of available NSAIDs and the potential relevance of these differences for dosing and clinical performance is discussed. All nonsteroidal anti-inflammatory drugs (NSAIDs) act by inhibiting cyclooxygenases, key enzymes responsible for the production of prostaglandins in arachidonic acid metabolism. Cyclooxygenase-1 (COX-1) is expressed constitutively in almost all tissues, particularly the GI tract, platelets, endothelial cells, and kidneys. It appears to be responsible for the production of prostaglandins that maintain homeostatic functions, such as the integrity of the GI mucosa, platelet function, and the regulation of renal blood flow. Nonselective inhibition of COX-1 by systemic NSAIDs has been implicated in GI-toxicity. Cyclooxygenase-2 (COX-2) expression, on the other hand, is an inducible mediator of prostaglandin production, and increases dramatically during inflammation and carcinogenesis. Inhibition of COX-2 results in beneficial anti-inflammatory, pain, and analgesic affects. The degree to which the available NSAIDs inhibit either COX-1 or COX-2 differs. The relative potency of an agent against these mediators is measured by IC 50, the drug concentration required to inhibit enzyme activity by 50%. The lower the IC 50, the more potent is the molecule against the enzyme. Although in vitro data on IC 50 values are highly variable between laboratories and type of assay used, they provide some basis for evaluating relative potency. The focus is on the COX-2 enzyme, as this is the enzyme responsible for the mediation of pain and inflammation. According to available data, it took 3.7 times less bromfenac than diclofenac, and 6.5 times less bromfenac than amfenac to inhibit the COX-2 enzyme (Figure 1). 1-3 In summary, bromfenac is a more powerful inhibitor of COX-2 than either diclofenac or nepafenac, and therefore more potent than either diclofenac or nepafenac. Figure 1. In terms of ocular NSAID potency against the COX-2 enzyme, bromfenac is 3.7 and 6.5 times more potent than diclofenac and amfenac, respectively. 4 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I MARCH 2006
5 CHARACTERISTICS OF NSAIDS By Francis Mah, MD Ocular nonsteroidal anti-inflammatory drugs (NSAIDs) are used to manage the pain associated with refractive surgery, because this group of drugs has analgesic and sometimes mild anesthetic properties. NSAIDs are used after cataract surgery and other intraocular procedures to alleviate postoperative inflammation. For each application, it is essential that the NSAID reach target tissues efficiently and remain in effective concentrations over a sufficient period of time. Based on in vitro assays, it is clear that there are differences in these parameters between NSAIDs, and these differences may provide a guide for therapeutic decision making. Indeed, just as improvements in penetration and potency differentiated the fourth-generation fluoroquinolone antibiotics from their predecessors, the evolution of NSAIDs will be similar. It is apparent that it is the combination of potency and therapeutic penetration that will define clinical efficacy and lack of toxicity. Potency describes the strength of the activity and, therefore, affects the duration of activity in the NSAID class, which determines dosing. Without appropriate penetration of the drug, not all of the therapeutic benefits will be achieved. Although topical applications and surface activity is required (eg, in refractive surgery), NSAIDs also need to penetrate into the eye to protect and treat postoperative inflammation and possible cystoid macular edema (CME). Only with both qualities will the drug be useful to clinicians. In cataract surgery, an NSAID needs to penetrate the ocular tissues from the anterior chamber to the ciliary body and to the iris in order to strengthen the bloodaqueous barrier and prevent the release of proteins and pro-inflammatory mediators. Refractive surgeons use NSAIDs to manage the pain of ocular surface tissues, primarily after PRK. They also administer the agents in the eyes of post-lasik patients to make them more comfortable. In terms of NSAIDs penetration requirements for refractive surgery, they only need to infiltrate the cornea. It can be helpful to have some of the drug in the anterior chamber, but the drugs entering the cornea is sufficient for use after refractive surgery. Furthermore, the anti-inflammatory properties of NSAIDs provide protection against postoperative CME after cataract surgery. The vascular instability of CME lies within the retina and the choroid. Therefore, and most importantly, NSAIDs need to reach the back of the eye for the treatment and/or prophylaxis of CME. CME has been reported to be the most common cause of decreased vision following uncomplicated cataract surgery. In one study 1 utilizing the sensitive OCT technology to measure retinal thickness, 12% of routine, uncomplicated phacoemulsification resulted in CME. In that same study, the use of topical NSAIDs before and after the surgery resulted in zero cases of CME. As our surgical technology, skills, and outcomes improve, our patients are becoming more and more demanding. Patients are no longer happy with anything short of 20/20 vision, let alone other unmentionable complications, such as endophthalmitis. NSAIDs have been proven to treat CME, so it makes perfect sense that an NSAID that penetrates into the vitreous cavity and reaches the retina and choroid at high concentrations will prevent CME better than an NSAID that does not have the same properties. The optimal NSAID has a rapid delivery into targeted tissues but preferably all ocular tissues starting from the front and continuing to the back of the eye. Additionally, the longer the active levels of an NSAID are maintained in the target tissues, the more favorable the situation is for the patient in terms of his adherence to therapy because the frequency of dosing is lower. Bromfenac is a b.i.d. medication that controls pain to the same degree as the NSAIDs that are dosed q.i.d. and t.i.d. Due to bromfenac s potency as well as its ability to penetrate the anterior chamber and the vitreous cavity, twice-daily dosing is sufficient and should reduce the likelihood of cytotoxic effects while improving patients compliance. 1. McColgin AZ, Raizman MB. Efficacy of topical Voltaren in reducing the incidence of postoperative cystoid macular edema. Invest Ophthamol Vis Sci. 1999;40:S289. MARCH 2006 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I 5
6 Figure 2. The bromfenac molecule (left) is similar to the amfenac molecule (right). Figure 3. Ocular concentrations of bromfenac were detectable for the longest time period in comparison to nepafenac. TISSUE PENETRATION The chemical structure of bromfenac is identical to that of amfenac except for the addition of a bromine moiety at position 4 (Figure 2), which increases the lipophilicity of the molecule. 4 As a result, one could expect increased penetration and uptake of bromfenac into ocular tissues. This was demonstrated in a pharmacokinetic study in which a single drop of radiolabeled bromfenac (mean 0.29mg/drop) was administered to rabbits. Bromfenac was detectable in all ocular tissues over a 24-hour period. The highest concentrations detected were in the cornea, followed respectively by the iris and ciliary body, choroid, retina, and aqueous humor. 5 A similar study using a single drop of 0.3% amfenac demonstrated ocular penetration as well. 6 The results and relative ocular concentrations of these two trials are depicted in Figure 3. The clinical importance of enhanced penetration remains to be demonstrated. However, the enhanced potency of bromfenac allows for b.i.d. dosing and may account for the earlier efficacy (post-surgical day 2) seen in the reduction of pain and inflammation in clinical trials. 1.. Data on file at ISTA Pharmaceuticals, Inc. 2. Gamache DA, Graff G, Brady MT, et al. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. 3. Jett MF, Ramesha CS, Brown CD, et al. Characterization of the analgesic and anti-inflammatory activities of ketorolac and its enantiomers in the rat. J Pharmacol Exp Ther. 1999;288: Walsh DA, Moran HW, Shamblee DA, et al. Anti-inflammatory agents. 3. Synthesis and pharmacological evaluation of 2-amino-3benzoylphenylacetic acid and analogues. J Med Chem. 1984;11: Data on file at ISTA Pharmaceuticals, Inc. 6. Hellberg MR, Nixon JC. Use of non-steroidal anti-inflammatory agents in combination with compounds that have PF prostaglandin agonist activity to treat glaucoma and ocular hypertension. Alcon Laboratories, Inc., assignee. U.S. Patent 6,342,524 B1. Jan. 29, I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I MARCH 2006
7 Efficacy A review of the study design and results for the bromfenac phase 3 trials is presented. APPROVALS Bromfenac was approved in Japan in May 2000 under the trade name Bronuck for the treatment of blepharitis, conjunctivitis, scleritis, and postoperative inflammation. To date, more than 6 million patients have been treated with bromfenac in that country. The FDA approved the same formulation that was approved and used in Japan in March 2005 as the first nonsteroidal anti-inflammatory drug (NSAID) dosed b.i.d. for the treatment of ocular inflammation following cataract surgery. In January 2006, the FDA granted an expanded indication for bromfenac for the treatment of pain following cataract surgery. 1 PHASE 3 TRIALS Two US phase 3 clinical trials of bromfenac were completed, both of which demonstrated the drug s efficacy. A total of 527 patients were enrolled in the randomized, double-masked, placebo-controlled clinical trials, of which 356 received bromfenac and 171 received placebo. Patients enrolled in these trials had to have had undergone cataract surgery and had a Summed Ocular Inflammation Index Score (SOIS) of 3.7 out of 4 on day 1 postoperatively, with no presurgical dosing of an NSAID. Patients did not receive any anti-inflammatory agents until 1 day after surgery (after the inflammation had occurred). Patients then instilled bromfenac b.i.d. for 14 days. The endpoints measured included the reduction in summed cell and flare scores, a decrease in anterior chamber cell score, a reduction in anterior flare score, and a resolution of pain. Efficacy was measured by comparing the proportion of bromfenac versus placebo patients who achieved a SOIS of zero at days 3, 8, and 15, postoperatively (Figure 4). 2 At each post-treatment visit, for all efficacy measurements, the effect of bromfenac was statistically signifi- Figure 4. Bromfenac achieved statistical significance on the first treatment evaluation (2 treatment days) and achieved statistically significant reduction from baseline at all study visits for the treatment of inflammation. MARCH 2006 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I 7
8 EVALUATING NSAIDS CLINICAL EFFICACY By Barry A. Schechter, MD When reviewing nonsteroidal anti-inflammatory drugs (NSAIDs) efficacy data, one needs to consider such factors as predosing and the management of inflammation and pain postoperatively. The best way to compare the effectiveness of the different agents is to conduct head-to-head clinical studies. Unfortunately, none has been published thus far. Therefore, we have to compare the clinical trials of the available NSAIDs and extrapolate as to how efficacious the medications are by analyzing the different variables. One approach is to compare drugs affinities for the cyclooxygenase-2 (COX-2) enzyme, the major enzyme that produces prostaglandins that promote inflammation and pain. According to Fitzgerald et al 1 and Gamache et al, 2 bromfenac has a great affinity for COX-2 as far as the IC 50, at which the enzyme activity is inhibited by 50%, which is several log units more than diclofenac and amfenac. This stronger affinity allows for only b.i.d. dosing. The phase 3 clinical trials for bromfenac 0.09% were quite stringent. There was no predosing of medication in patients who were to undergo cataract surgery, and, in order for patients to be entered into the clinical trial, the postoperative eye had to be very inflamed (degree of cell and flare averaging 3.7 out of a possible 4). Results of the phase 3 clinical trials showed that after only 4 drops of bromfenac (2 days of therapy), there was already a statistically significant reduction in the amount of cell and flare in the anterior chamber. The similarly designed phase 3 trial for ketorolac showed that 7 days (28 drops) were needed to statistically reduce flare and required 14 days (56 drops) to show a statistically significant reduction in anterior chamber cells. 3 In the phase 3 clinical trial for nepafenac in which patients were predosed with the drug t.i.d., starting the day before surgery, statistical significance in reduction of cells and flare was noted on day 16 (48 drops). 4 The control of postoperative pain was assessed as a secondary endpoint. After 3 days of bromfenac treatment (4 drops), clinical significance versus placebo was achieved with 87.6% of bromfenac patients pain free. By 8 days (14-16 drops) of bromfenac treatment, clinical significance versus placebo was achieved with 93.3% of bromfenac patients pain free. On postoperative day 6 (24 drops), ketorolac first achieved statistical significance versus placebo. After 14 days of treatment with nepafenac (42 drops), approximately 95% of patients were pain free, however, nepafenac patients were predosed and treated immediately following surgery.. Only 3.1% of patients receiving bromfenac were dismissed from the phase 3 clinical trials due to lack of inflammation control, compared with 28% of ketorolac patients in the ketorolac phase 3 trial 5 and 10% for the nepafenac phase 3 trial. 6 Due to its analgesic properties, strong anti-inflammatory potential, and efficient tissue penetration, I have found bromfenac to be a versatile addition to my NSAID armamentarium. I have used bromfenac off label to treat nonsurgical iritis, peripheral inflammatory keratitis, Staphylococcus hypersensitivity reactions, and corneal abrasions. Furthermore, I have used it in patients with chemical keratoconjunctivitis and filamentary keratitis. When treating dry eye in conjunction with topical cyclosporine, my patients prefer bromfenac to ketorolac, which I had previously utilized, because there is less stinging. 7 NSAIDs allow patients with corneal abrasions to recuperate comfortably and return to work quickly. Previously, the standard treatment involved administering an antibiotic ointment, patching a patient s eye, and oftentimes oral opiates to control pain. Additionally, bromfenac is very helpful in patients who undergo refractive surgery; ie, LASIK, surface ablation, or limbal relaxing incisions. Bromfenac has been helpful after pterygium surgery with conjunctival grafting. Patients have not suffered significant pain, and there have been no problems with wound healing the NSAID is discontinued once the epithelium has healed. Bromfenac only needs to be used b.i.d., because it has potent inhibitory specificity for the COX-2 enzyme, which reduces the epithelial toxic effect of preservatives, which are present in nearly all commercially available topical NSAIDs. 1. FitzGerald GA, Patron C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345: Gamache DA, Graff G, Brady MT, et al. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of antiinflammatory efficacy. Inflammation. 2000;24: Heier J, Cheetham JK, Degryse R, et al. Ketorolac tromethamine 0.5% ophthalmic solution in the treatment of moderate to severe ocular inflammation after cataract surgery: a randomized, vehicle-controlled clinical trial. Am J Ophthalmol. 1999;127: Data on file at Alcon Laboratories, Inc. 5. Acular LS [package insert]. Irvine, CA: Allergan, Inc. 6. Personal communication with P. Cockrum, September 24, Schechter BA. The evaluation of ketorolac (Acular LS) to improve patient comfort during the induction phase of cyclosporine A (Restasis ophthalmic emulsion) therapy. J Ocular Pharmacol Ther. In press. 8 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I MARCH 2006
9 bromfenac placebo Figure 5. The resolution of pain after cataract surgery was rapid in patients that received bromfenac therapy. cant when compared with the effect of placebo. After 2 days of treatment with bromfenac, patients achieved a significant drop in their SOIS to less than 2 (P.0002), which dropped to less than 1 on day 7 of treatment (P.0001) and less than 0.5 on day 14 of treatment (P.0001). 2 On treatment day 3, 8% of the bromfenac patients compared with 1% of placebo patients achieved a SOIS of zero. Eighty-one percent of the bromfenac patients achieved improvements in inflammation on or before postoperative day 15 versus less than 52% of the patients who received placebo. 2 An additional efficacy endpoint was the time required for the resolution of ocular pain in patients who reported pain following cataract surgery (20% of subjects in the trials). The bromfenac group demonstrated a statistically significant difference in median time to resolution of ocular pain of 2 days, compared with 5 days for patients receiving the vehicle. Furthermore, 87.6% (P<.0001) of all patients treated with bromfenac were pain-free within 3 days of being treated with bromfenac b.i.d. (approximately 4 to 6 doses). By treatment day 8 (14 to 16 doses) 93.3% of these patients were pain-free versus 63.7% (P<.0001) of patients treated with vehicle during that period (Figure 5). 3 DOSING Bromfenac is the first NSAID to be approved for b.i.d. dosing. In contrast, approved dosing for nepafenac ophthalmic suspension and ketorolac tromethamine ophthalmic solution is one drop t.i.d. and one drop q.i.d., respectively. 4-6 According to a study by Ikeda et al, 7 which evaluated factors influencing patients use of ophthalmic solutions, patients who administered ophthalmic solutions once or twice daily were more compliant than those in other patient groups. Other factors influencing patients adherence to prescribed therapy were the number of ophthalmic solutions, patients age, taste of the drug, administration intervals, number of drops used, and hand washing before the application of ophthalmic solutions. 1. Data on file at ISTA Pharmaceuticals, Inc. 2. Donnenfeld ED, Holland EJ, Stewart R, Grillone LR, for the Bromfenac Study Group. Topical Xibrom 0.1%, an investigational NSAID for post-cataract surgery inflammation, markedly decreases inflammation. Paper presented at: The Annual ASCRS. April 15, 2005, Washington, DC. 3. Donnenfeld ED, Holland EJ, Stewart R, et al. Topical Xibrom 0.1%, an investigational NSAID, significantly and rapidly decreased post-cataract surgery inflammation and reduced ocular pain. Invest Ophthalmol Vis Sci. 2005;46:E-abstract Xibrom [package insert]. Irvine CA: ISTA Pharmaceuticals, Inc. 5. Data on file at Alcon Laboratories, Inc. 6. Acular LS [package insert]. Irvine, CA: Allergan, Inc. 7. Ikeda H, Sato M, Tsukamoto H, et al. Evaluation and multivariate statistical analysis of factors influencing patient adherence to ophthalmic solutions. Yakugaku Zasshi. 2001;121: MARCH 2006 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I 9
10 Safety Clinical trial and postmarketing surveillance data on bromfenac is assessed. In US phase 3 trials, the incidence rate of common treatment-emergent ocular adverse events associated with bromfenac was 2% of 356 patients. The incidence of burning and stinging in bromfenac patients was only 1.4%, and the incidence of cystoid macular edema was 1.4%. 1 In this same trial, four patients discontinued using bromfenac due to adverse ocular events, including iritis, lid edema, and cystoid macular edema, all of which were possibly related to bromfenac. Bromfenac is contraindicated in patients with known hypersensitivity to any ingredient in the formulation, and most commonly reported adverse experiences include abnormal sensation in the eye; conjunctival hyperemia; ocular irritation, pain, pruritus, and redness; headache, and iritis. These events were reported in 2% to 7% of patients. 2 As part of bromfenac s US New Drug Application, postmarketing information was submitted to the FDA from Senju Pharmaceuticals Inc. Ltd. (Osaka, Japan [bromfenac was developed by Senju Pharmaceuticals Inc. Ltd.]). The postmarketing surveillance survey included data from DATA CONFIRM BROMFENAC S SAFETY 2.7 million patients who had used bromfenac 0.09% in Japan between 2000 and 2004 for a wide variety of indications and treatment periods. 1 Since bromfenac s initial marketing in Japan beginning in July 2000 to date, the drug has been used in more than 6 million patients, and there has been no reported case of serious systemic adverse events and only 14 cases of serious ocular adverse events (including three corneal erosions, three corneal perforations, four corneal ulcers, three conjunctival disorders, and one epithelial defect), an event rate of %. 1-3 Three of the previously reported cases one corneal erosion in 2001, and two corneal erosions in 2002 were recently described in the literature. 4 Each of these cases resolved with conservative treatment. 1. Data on file at ISTA Pharmaceuticals, Inc. 2. Congdon NG, Schein OD, von Kulajta P, et al. Corneal complications associated with topical ophthalmic use of nonsteroidal anti-inflammatory drugs. J Cataract Refract Surg. 2001;27: Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108: Asai T, Nakagami T, Mochizuki M, et al. Three cases of corneal melting after instillation of a new nonsteroidal anti-inflammatory drug. Cornea. 2006;25: By Monte S. Dirks, MD Although bromfenac 0.09% is a new agent for US ophthalmologists, we have an unusually large body of information confirming its safety, including rigorous phase 3 clinical trials 1 and extensive postmarketing data from Japan. 2 Two US phase 3 clinical trials assessed the incidence of ocular effects with the use of bromfenac after cataract surgery. The results showed a minimal incidence of adverse events associated with bromfenac. The reported events were those expected following cataract surgery. Only 1.4% reported burning and stinging. Cystoid macular edema was reported in 1.4% of the bromfenac group compared with 4.7% of the placebo group. As an investigator in this study, I was impressed by the postoperative comfort reported by patients treated with bromfenac. 1 The most noteworthy safety data from the clinician s standpoint relate to the Japanese studies and the Japanese Ministry of Health s surveillance data. Despite genetic differences, the sample size was very large and the data span nearly 5 years. Certainly, we know more about bromfenac than we do about nepafenac, which has only been used in the US, and ketorolac, for which I was an investigator and witnessed its full development. To date, I believe there is sufficient data from which one can determine which ocular nonsteroidal anti-inflammatory drug (NSAID) is safe and comfortable. One of the major strengths of bromfenac is that it has one of the lowest incidence of burning and stinging upon instillation when compared with the other available ocular NSAIDs. 3-6 In refractive surgery, PRK patients in particular may benefit from bromfenac, because it is more comfortable in comparison with the sting/burn rate of ketorolac and nepafenac, which may add to these patients postoperative discomfort. Dry eye and pterygium patients may also prefer bromfenac over conventional topical anti-inflammatories, which can cause significant irritation on their own. Bromfenac works very well in patients who already have some ocular irritation. Last, bromfenac mitigates concerns about toxicity, because it is dosed b.i.d. as opposed to q.i.d. for ketorolac and t.i.d. for nepafenac. This less frequent-dosing also improves patient compliance. 1. Data on file, ISTA Pharmaceuticals, Inc. 2. Data on file, ISTA Pharmaceuticals, Inc. 3. Xibrom [package insert]. Irvine, CA: ISTA Pharmaceuticals, Inc. 4. Acular [package insert]. Irvine, CA: Allergan, Inc. 5. Nevanac [package insert]. Fort Worth, TX: Alcon Laboratories, Inc. 6. Voltaren [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. 10 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I MARCH 2006
11 Clinical Experience to Date An overview of clinical experience with bromfenac to date, focusing on evaluations of physician satisfaction and patient comfort. POSTMARKETING STUDIES US postmarking studies of bromfenac performed since its initial approval include examinations of the relative comfort of the drug upon instillation and its corneal anesthetic characteristics and physician satisfaction with a range of performance parameters. 1,2 ASSESSMENT OF COMFORT AND CORNEAL ANESTHESIA Twenty volunteers with normal, healthy eyes were enrolled in a US postmarketing study 1 that compared the comfort and corneal anesthetic properties of both bromfenac 0.09% and ketorolac 0.4%. Each subject was administered a single drop of drug in a random and masked fashion, and then they were asked to rate the severity of burning and stinging on a scale of 0 to 4. According to the study s results, 17 subjects (85%) reported no burning or stinging with bromfenac, compared with 7 subjects (35%) with ketorolac. None of the bromfenac subjects reported moderate or severe burning or stinging compared with 6 (30%) subjects who received ketorolac. 1 PHYSICIAN SATISFACTION An early experience study 2 performed in the US rated physicians initial experience with the drug. In the trial, participants treated a minimum of 10 patients with bromfenac. Results included data from 225 ophthalmologists and 2,604 patients who received bromfenac. The ophthalmologists rated the performance of bromfenac in control of inflammation, ease of use by patients, patient compliance, comfort on instil- Figure 6. In an early experience study 2 of bromfenac, physicians expectations of the drug s performance were either exceeded or met in 99% to 100% of reports. MARCH 2006 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I 11
12 lation, overall safety profile, and patient satisfaction, using four descriptors: (1) very satisfied (exceeded expectations); (2) satisfied (met expectations); (3) dissatisfied (met some expectations); and (4) very dissatisfied (did not meet expectations). According to the results of this study, ophthalmologists expectations were exceeded or met in 99% to 100% of reports (Figure 6). 1. Perry HD, Chou TY. A comparison of Xibrom and Acular LS in a test of comfort and corneal anesthesia. Abstract pending acceptance at ARVO as of February 15, Xibrom First Experience Trial Data on file, ISTA Pharmaceuticals, Inc. PERSONAL EXPERIENCE WITH BROMFENAC By Eric D. Donnenfeld, MD To date, I have had a positive experience with bromfenac. The initial clinical trials established that this b.i.d. drug could reduce inflammation following cataract surgery. The medication has been well tolerated, and patient acceptance has been exceptional. Notably, the medication induces little burning or stinging and is the most comfortable nonsteroidal anti-inflammatory drug (NSAID) I have used. However, it is difficult to make meaningful efficacy comparisons with other NSAIDs in the absence of head-tohead studies. One of the major concerns with any medication, and specifically with an NSAID, is the issue of corneal melts as was seen with generic diclofenac. 1 To date, I have not seen this problem with this medication, and bromfenac's track record in Japan, where it has been used for 5 years, suggests that it will be well tolerated. Continued experience in this market will confirm bromfenac's safety profile. In comparing available agents, it is possible to say that patient comfort and its b.i.d. dosing are major advantages of bromfenac. Numerous studies 2 involving dosing schedules have shown that a b.i.d. schedule is much better tolerated than a q.i.d. one. Simply stated, patients will take their medications twice per day but are not nearly as compliant with four-times-per-day dosing. Bromfenac's minimal burning and stinging is also a contributing feature for better patient compliance, because previous experience has shown that medications with side effects are not used as directed. NSAIDs in general are a potent addition to the antiinflammatory armamentarium following ophthalmic procedures. When they are used in conjunction with a topical corticosteroid, there is a synergy that dramatically increases onset of action and efficacy. Such a combination hastens the return of visual acuity and reduces the incidence of intraocular inflammation. Although the prevention of cystoid macula edema (CME) is one of the most important indications for a topical NSAID, the combination therapy more rapidly resolves CME. 3 In conclusion, NSAIDs have become an integral part of my surgical armamentarium. I use a topical NSAID for 3 days prior to any intraocular surgery and continue the agent for 3 weeks postoperatively. NSAIDs dramatically improve the patient's and surgeon's experience during cataract surgery by reducing pain, intraocular inflammation, and CME. Additionally, they maintain pupil size during cataract surgery and provide more rapid visual rehabilitation while not delaying wound healing like steroids can. I predict that NSAID use with cataract surgery will increase markedly as the efficacy of these medications is established and patient demand for painless, rapid visual rehabilitation postoperatively becomes the standard. The future for NSAIDs in ophthalmic surgery is bright with their safety and efficacy becoming established. Innovations include the possibility of intracameral usage in conjunction with topical therapy as well as even more potent and safer molecules in the future. 1. Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108: Kosoko O, Quigley HA, Vitale S, et al. Risk factors for noncompliance with glaucoma follow-up visits in a residents' eye clinic. Ophthalmology. 1998;105: Heier JS. Topping TM Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000;107: I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I MARCH 2006
13 CME QUESTIONS Circle the most appropriate answer in the ANSWER SECTION on the following page. 1. Which of the following are therapeutic applications for the topical NSAIDs described in the literature? a. treatment of postoperative pain b. treatment of postoperative inflammation c. prevention of cystoid macular edema d. all of the above 2. Inhibition of which cyclooxygenase enzyme reduces ocular inflammation and pain? a. COX-1 b. COX-2 3. Which characteristics of topical NSAIDs can be enhanced to improve their clinical performance? a. tissue penetration b. potency c. COX selectivity d. all of the above 4. In assessing relative potency of NSAIDs using in vitro assays, which statement is true? a. the higher the IC 50 value, the greater the relative potency b. the lower the IC 50 value, the greater the relative potency 5. A single drop of bromfenac produced measurable tissue levels in the cornea, iris, ciliary body, choroid, aqueous humor, and retina for 24 hours. a. true b. false 6. Which of the following parameters vary between NSAID clinical trials? a. whether NSAID dosing prior to surgery was allowed b. if and at what point concomitant steroid use was allowed c. the degree of inflammation or pain at treatment initiation d. at what time point, postoperatively, treatment was initiated e. all of the above 7. In clinical trials, at which time point was the reduction of inflammation produced by b.i.d. bromfenac statistically significant over placebo? a. day 3 b. day 8 c. day 15 d. all time points 8. More than 87% of patients reporting ocular pain who were treated with bromfenac in clinical trials were pain free within: a. 3 days b. 5 day c. 8 days d. 15 days 9. Research suggests that dosing of ophthalmic medications once or twice per day improves compliance. a. true b. false 10. Which statement about safety and tolerability is associated with bromfenac? a. the rate of burning and stinging upon instillation in US clinical trials was less than 1.4% b. the rate of serious ocular adverse events in Japanese postmarketing surveillance studies was % c. no serious systemic adverse events have been reported in Japan after more than 5 years and 6 million patient uses d. all of the above MARCH 2006 I INSERT TO CATARACT & REFRACTIVE SURGERY TODAY I 13
14 REGISTRATION/EVALUATION FORM To obtain AMA/PRA category 1 credit, you must: Read the learning objectives and the monograph and complete the self-assessment test. Complete this registration/evaluation form and record your test answers in the Answer Section below. Send the Registration/Evaluation form to The Dulaney Foundation, Post Office Box 25271, Tampa, FL , or fax to (813) Retain a copy of your test answers. Your answer sheet will be graded, and if you achieve a passing score of 70% or better, you will receive a CME credit letter awarding AMA/PRA category 1 credit by mail within 4 weeks. If you do not achieve a passing score, you will be notified and offered the opportunity to complete the activity again. ANSWER SECTION Circle the best answer for each question on page A B C D 2. A B 3. A B C D 4. A B 5. A B 6. A B C D E 7. A B C D 8. A B C D 9. A B 10. A B C D REGISTRATION FORM First name Last name Degree (MD, PhD) Position Specialty Institution or practice name Address City State Zip Code Country Telephone Fax address The processing fee has been underwritten by an unrestricted educational grant from ISTA Pharmaceuticals, Inc. I attest that I have completed this activity as designed and I am claiming (up to 1 credit) AMA/PRA category 1 credit. Signature Date Credit for this activity is available until March 31, The planning and execution of useful and educationally sound continuing education activities are guided in large part by input from participants. Please assist us in evaluating the effectiveness of this activity and make recommendations for future educational offerings by completing this evaluation form. Your response will help ensure that future programs are informative and meet the educational needs of all participants. Please note: CME credit letters and long-term credit retention information will only be issued upon receipt of this completed evaluation. Thank you for your cooperation! OBJECTIVES After successful completion of this program, you should be able to: List the therapeutic applications of topical NSAIDs in ophthalmology and optimal performance characteristics in each of these applications Describe the relevance of IC 50 potency assays and in vitro tissue penetration data to NSAID performance and selection List the goals of therapeutic intervention for dry eye disease Compare NSAID clinical trial designs and study protocols Evaluate NSAIDs efficacy claims List barriers to patient compliance with NSAID therapy Incorporate the first b.i.d. NSAID into clinical protocols OVERALL EVALUATION The information presented increased my awareness/understanding of the subject The information presented will influence how I practice The information presented will help me improve patient care The faculty demonstrated current knowledge of the subject The program was educationally sound and scientifically balanced The program avoided commercial bias or influence Overall, the program met my expectations I would recommend this program to my colleagues If you anticipate changing one or more aspects of your practice as a result of your participation in this activity, please provide a brief description of how you plan to do so: Please provide any additional comments pertaining to this activity (positive and negative) and suggestions for improvements: Please list any topics you would like to see addressed in future educational activities: Strongly Agree Strongly Disagree
15
16
A New Paradigm in NSAID Treatment
 V 5/6 pg Contents Common Uses of Ophthalmic NSAIDs A New Paradigm in NSAID History of (bromfenac ophthalmic solution).9% Efficacy of Phase III trials Pain data Post-Approval trials Pharmacokinetics, Penetration,
V 5/6 pg Contents Common Uses of Ophthalmic NSAIDs A New Paradigm in NSAID History of (bromfenac ophthalmic solution).9% Efficacy of Phase III trials Pain data Post-Approval trials Pharmacokinetics, Penetration,
WARNING LETTER. According to the Indications and Usage section of the FDA approved product labeling (PI):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
Better ophthalmic surgery outcomes in recent years have created more Demanding Patients.
 By Alaa EL Zawawi Prof. of Ophthalmology Alexandria - Egypt Launch Better ophthalmic surgery outcomes in recent years have created more Demanding Patients. 1 NSAIDs can play a pivotal role in facilitating
By Alaa EL Zawawi Prof. of Ophthalmology Alexandria - Egypt Launch Better ophthalmic surgery outcomes in recent years have created more Demanding Patients. 1 NSAIDs can play a pivotal role in facilitating
BARRY A. SCHECHTER ABSTRACT
 JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
INDICATIONS ACULAR 0,5 % is indicated for the relief of inflammation following ocular surgery.
 Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
BY BERNARD ZINMAN, MD
 Understanding the Relationship Between Hyperglycemia and Microvascular Complications Jointly sponsored by The Dulaney Foundation and DIABETIC MICROVASCULAR COMPLICATIONS TODAY. Release Date: August 2006.
Understanding the Relationship Between Hyperglycemia and Microvascular Complications Jointly sponsored by The Dulaney Foundation and DIABETIC MICROVASCULAR COMPLICATIONS TODAY. Release Date: August 2006.
See 17 for PATIENT COUNSELING INFORMATION.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
PHARMACOLOGY Class: Ketorolac trometamol is a member of the pyrrolo-pyrolle group of non-steroidal antiinflammatory
 ACULAR Eye Drops NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure: Chemical Name: ( )-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol
ACULAR Eye Drops NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure: Chemical Name: ( )-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol
Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST
 Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST Forward Looking Statements Certain statements contained
Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST Forward Looking Statements Certain statements contained
Innovation In Ophthalmology
 Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
13 NONCLINICAL TOXICOLOGY 5.1 Increased Bleeding Time Carcinogenesis, Mutagenesis, Impairment of 5.2 Delayed Healing
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
Case History. Slit lamp exam: Clinical Pearls in the Management of Iritis. 2- injection: Irregular SPK and staining AC: grade 3 cell & flare
 Clinical Pearls in the Management of Iritis Paul Karpecki, OD, FAAO Corneal Services and Ocular Disease Research Koffler Vision Group-Lexington, KY 68 y.o. Caucasian female Complains of photophobia and
Clinical Pearls in the Management of Iritis Paul Karpecki, OD, FAAO Corneal Services and Ocular Disease Research Koffler Vision Group-Lexington, KY 68 y.o. Caucasian female Complains of photophobia and
Anterior Segment Cataract Surgery
 Current Use of Non-steroidal Anti-inflammatory Drugs in the Treatment of Ocular Inflammation Related to Cataract Surgery Eric Donnenfeld Clinical Professor of Ophthalmology, New York University, New York,
Current Use of Non-steroidal Anti-inflammatory Drugs in the Treatment of Ocular Inflammation Related to Cataract Surgery Eric Donnenfeld Clinical Professor of Ophthalmology, New York University, New York,
Molecular Biology of Diabetic Retinopathy
 Opportunities for Therapeutic Intervention: Molecular Biology of Diabetic Retinopathy Jointly sponsored by The Dulaney Foundation and DIABETIC MICROVASCULAR COMPLICATIONS TODAY. Release Date: January 2006.
Opportunities for Therapeutic Intervention: Molecular Biology of Diabetic Retinopathy Jointly sponsored by The Dulaney Foundation and DIABETIC MICROVASCULAR COMPLICATIONS TODAY. Release Date: January 2006.
Rama Vadapalli * 1, Vankadara Naga Suresh 2. Online Access and Article Informtaion. Original Research Article
 Original Research Article An Interventional Follow up Study to Determine the Effect of Nepafenac 0.1% in Macular thickness in Patients who had Undergone Cataract Surgery as Determined By OCT Rama Vadapalli
Original Research Article An Interventional Follow up Study to Determine the Effect of Nepafenac 0.1% in Macular thickness in Patients who had Undergone Cataract Surgery as Determined By OCT Rama Vadapalli
84 Year Old with Rosacea
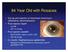 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution
 REVIEW Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution Hyung Cho 1 Kenneth J Wolf 1 Eric J Wolf 2 1 Department of Ophthalmology, Albert Einstein
REVIEW Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution Hyung Cho 1 Kenneth J Wolf 1 Eric J Wolf 2 1 Department of Ophthalmology, Albert Einstein
Case History. The SEVEN HABITS of Highly Effective Anterior Uveitis Management. SLEx findings: SLEx corneal findings: y.o.
 The SEVEN HABITS of Highly Effective Anterior Uveitis Management Case History! 68 y.o. Caucasian female of photophobia and blurred vision! As well as a headache over right eye for 2 days! Complains Paul
The SEVEN HABITS of Highly Effective Anterior Uveitis Management Case History! 68 y.o. Caucasian female of photophobia and blurred vision! As well as a headache over right eye for 2 days! Complains Paul
The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, Bromfenac 0.1% and Ketorolac 0.45%, on Cataract Surgery
 Original Article Yonsei Med J 215 Nov;56(6):1671-1677 pissn: 513-5796 eissn: 1976-2437 The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, and, on Cataract Surgery Ji Won Jung 1,2, Byung Hoon Chung
Original Article Yonsei Med J 215 Nov;56(6):1671-1677 pissn: 513-5796 eissn: 1976-2437 The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, and, on Cataract Surgery Ji Won Jung 1,2, Byung Hoon Chung
NSAIDs in Treatment of Retinal Disorders
 NSAIDs in Treatment of Retinal Disorders These drugs are playing new roles beyond their established use in cataract and refractive surgery. NON-STEROIDAL ANTI-INFLAMm a t o r y drugs are approved for the
NSAIDs in Treatment of Retinal Disorders These drugs are playing new roles beyond their established use in cataract and refractive surgery. NON-STEROIDAL ANTI-INFLAMm a t o r y drugs are approved for the
NSAIDs. The Evolving Role of. in Cataract Surgery
 Supplement to April 2011 The Evolving Role of NSAIDs in Cataract Surgery Strategies for ocular surface management with the newly approved q.d. formulation of bromfenac 0.09%. Featuring: Johnny Gayton,
Supplement to April 2011 The Evolving Role of NSAIDs in Cataract Surgery Strategies for ocular surface management with the newly approved q.d. formulation of bromfenac 0.09%. Featuring: Johnny Gayton,
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops
 AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Medical Science. Research Paper. 2nd yr post graduate, Dept. Of Ophthalmology, SVS Medical College
 Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
CME/CE POSTTEST CME/CE QUESTIONS
 CME/CE POSTTEST CME/CE QUESTIONS Controlling Asthma Severity: Identifying Unmet Needs and Optimizing Therapeutic Options There are no fees for participating in and receiving continuing medical education
CME/CE POSTTEST CME/CE QUESTIONS Controlling Asthma Severity: Identifying Unmet Needs and Optimizing Therapeutic Options There are no fees for participating in and receiving continuing medical education
REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE
 REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE Tina H Chiang 1, John G Walt 1, John P McMahon, Jr. 2, James E Mansfield, Jr. 2, Susan Simonyi 3 1 Allergan
REAL-WORLD UTILIZATION PATTERNS OF CYCLOSPORINE OPHTHALMIC EMULSION 0.05% WITHIN MANAGED CARE Tina H Chiang 1, John G Walt 1, John P McMahon, Jr. 2, James E Mansfield, Jr. 2, Susan Simonyi 3 1 Allergan
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project
 The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company AAO October 25, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye...
 Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Author's response to reviews
 Author's response to reviews Title:Efficacy of 1% carboxymethylcellulose sodium for treating dry eye after phacoemulsification: results from a multicenter, open-label, randomized, controlled study Authors:
Author's response to reviews Title:Efficacy of 1% carboxymethylcellulose sodium for treating dry eye after phacoemulsification: results from a multicenter, open-label, randomized, controlled study Authors:
Topical Bromfenac 0.09% vs Ketorolac 0.4% for the Control of Pain, Photophobia, and Discomfort Following PRK
 Topical Bromfenac 0.09% vs Ketorolac 0.4% for the Control of Pain, Photophobia, and Discomfort Following PRK Neal A. Sher, MD, FACS; Mikhail P. Golben, BS; William Bond, MD; William B. Trattler, MD; Shachar
Topical Bromfenac 0.09% vs Ketorolac 0.4% for the Control of Pain, Photophobia, and Discomfort Following PRK Neal A. Sher, MD, FACS; Mikhail P. Golben, BS; William Bond, MD; William B. Trattler, MD; Shachar
AWMSG SECRETARIAT ASSESSMENT REPORT. Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension. Reference number: 1509 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension Reference number: 1509 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology
AWMSG SECRETARIAT ASSESSMENT REPORT Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension Reference number: 1509 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology
Postoperative follow up and treatment after refractive surgery
 Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
Completed Clinical Research Trials Monte Dirks, M.D.
 Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
ASCRS completes fourth annual Clinical Survey
 ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
ASCRS completes fourth annual Clinical Survey More than 1,500 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education A note from the ASCRS Education
CORNEAL CONDITIONS CORNEAL TRANSPLANTATION
 GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
Vanderbilt Eye Institute Clinical Trials
 April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
April, 2010 Vanderbilt Eye Institute Clinical Trials Ophthalmology Actively Recruiting Studies For information on our clinical trials and other studies, please contact: Sandy Owings, COA, CCRP Clinic Director
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ENFLUAT 0.4 % Sterile Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Drug substance: Ketorolac Tromethamine 0,4 % Each bottle
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ENFLUAT 0.4 % Sterile Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Drug substance: Ketorolac Tromethamine 0,4 % Each bottle
Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice.
 Section Editor: Sumit Sam Garg, MD Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice. BY GARRICK CHAK, MD; AMANDA E. KIELY, MD; AND PRATAP CHALLA,
Section Editor: Sumit Sam Garg, MD Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice. BY GARRICK CHAK, MD; AMANDA E. KIELY, MD; AND PRATAP CHALLA,
The role of perioperative nonsteroidal anti-inflammatory drugs use in cataract surgery
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 18, Issue 1 Ser. 15 (January. 2019), PP 23-27 www.iosrjournals.org The role of perioperative nonsteroidal
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 18, Issue 1 Ser. 15 (January. 2019), PP 23-27 www.iosrjournals.org The role of perioperative nonsteroidal
CME. Clinical Uses of NSAIDs in Ophthalmic Surgery. Learning Objectives. September 1, Earn 1.0 hour CME credit
 September 1, 2005 Clinical Uses of NSAIDs in Ophthalmic Surgery CME Earn 1.0 hour CME credit Learning Objectives After reviewing the material, the participant should be able to: Discuss the use of NSAIDs
September 1, 2005 Clinical Uses of NSAIDs in Ophthalmic Surgery CME Earn 1.0 hour CME credit Learning Objectives After reviewing the material, the participant should be able to: Discuss the use of NSAIDs
Innovation In Ophthalmics
 Innovation In Ophthalmics Ophthalmic Innovation Summit @ AAO 2018 October 25, 2018 Mark Iwicki Chairman & CEO, Kala Pharmaceuticals Disclaimers and Notices This presentation contains forward-looking statements
Innovation In Ophthalmics Ophthalmic Innovation Summit @ AAO 2018 October 25, 2018 Mark Iwicki Chairman & CEO, Kala Pharmaceuticals Disclaimers and Notices This presentation contains forward-looking statements
Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac)
 EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
Surgery Post-operative
 Surgery Post-operative Redefining the Treatment Paradigm for Post-operative Inflammation Control The Role of Topical Non-steroidal Anti-inflammatory Drugs Oliver Findl Associate Professor of Ophthalmology
Surgery Post-operative Redefining the Treatment Paradigm for Post-operative Inflammation Control The Role of Topical Non-steroidal Anti-inflammatory Drugs Oliver Findl Associate Professor of Ophthalmology
OPHTHALMOLOGIC PEARLS FOR THE NON- OPHTHALMOLOGIST. David G. Gross D.O. Deen-Gross Eye Centers Merrillville-Hobart Deengrosseye.
 OPHTHALMOLOGIC PEARLS FOR THE NON- OPHTHALMOLOGIST David G. Gross D.O. Deen-Gross Eye Centers Merrillville-Hobart Deengrosseye.com A FEW OF THE AREAS WE WILL DISCUSS Red Eye Glaucoma Neuro ophthalmic tid
OPHTHALMOLOGIC PEARLS FOR THE NON- OPHTHALMOLOGIST David G. Gross D.O. Deen-Gross Eye Centers Merrillville-Hobart Deengrosseye.com A FEW OF THE AREAS WE WILL DISCUSS Red Eye Glaucoma Neuro ophthalmic tid
CONTRAINDICATIONS Hypersensitivity to any component of this product (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
ASCRS launches new Annual Clinical Survey
 ASCRS launches new Annual Clinical Survey Survey of more than 1,000 members measures current clinical opinions and practice patterns Survey overview The American Society of Cataract & Refractive Surgery
ASCRS launches new Annual Clinical Survey Survey of more than 1,000 members measures current clinical opinions and practice patterns Survey overview The American Society of Cataract & Refractive Surgery
Vankadara Naga Suresh * 1, Rama Vadapalli 2. Online Access and Article Informtaion. Original Research Article
 Original Research Article Effect of Prophylactic Bromfenac 0.07% on Cystoid Macular Edema Assessed using Optical Coherence Tomography Quantification of Total Macular Volume after Cataract Surgery Vankadara
Original Research Article Effect of Prophylactic Bromfenac 0.07% on Cystoid Macular Edema Assessed using Optical Coherence Tomography Quantification of Total Macular Volume after Cataract Surgery Vankadara
The Visian ICL Advantages
 The Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features found with the Visian ICL. These include:
The Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features found with the Visian ICL. These include:
Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery
 Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
Measure #191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery 2012 PHYSICIAN QUALITY REPORTING OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY DESCRIPTION: Percentage
Cataract Surgery Co-Management
 Cataract Surgery Co-Management Phacoemulsification, Clear-Lens Extraction, and LensX INCLUSION CRITERIA: Significant visual complaints (decreased VA, increased glare, decreased Activities of Daily Living
Cataract Surgery Co-Management Phacoemulsification, Clear-Lens Extraction, and LensX INCLUSION CRITERIA: Significant visual complaints (decreased VA, increased glare, decreased Activities of Daily Living
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
9 th Annual Residents Research Forum
 9 th Annual Residents Research Forum June 9, 2006 Rhode Island Country Club 150 Nayatt Road Barrington, Rhode Island 02806 2-7 PM Sponsored by: The Department of Ophthalmology at Rhode Island Hospital
9 th Annual Residents Research Forum June 9, 2006 Rhode Island Country Club 150 Nayatt Road Barrington, Rhode Island 02806 2-7 PM Sponsored by: The Department of Ophthalmology at Rhode Island Hospital
INDICATIONS For steroid responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye globe.
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Cataract and Refractive Surgery Co-Management Policy and Procedure Manual
 Cataract and Refractive Surgery Co-Management Policy and Procedure Manual Michael R. George, M.D. Chief Surgeon and Medical Director Tylock-George Eye Care Index of Cataract and Refractive Surgery Manual
Cataract and Refractive Surgery Co-Management Policy and Procedure Manual Michael R. George, M.D. Chief Surgeon and Medical Director Tylock-George Eye Care Index of Cataract and Refractive Surgery Manual
sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa
![sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa](/thumbs/78/77533900.jpg) PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only
 Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Assisting in Ophthalmology. Copyright 2011, 2007, 2003, 1999 by Saunders, an imprint of Elsevier Inc. All rights reserved.
 Assisting in Ophthalmology Learning Objectives Define, spell, and pronounce the terms listed in the vocabulary. Apply critical thinking skills in performing patient assessment and care. Explain the differences
Assisting in Ophthalmology Learning Objectives Define, spell, and pronounce the terms listed in the vocabulary. Apply critical thinking skills in performing patient assessment and care. Explain the differences
Cataract and Diabetic macular edema: What should I do?
 Cataract and Diabetic macular edema: What should I do? Irini Chatziralli Ophthalmic Surgeon, University Scholar 2 nd Department of Ophthalmology, University of Athens (Director: Prof P. Theodossiadis)
Cataract and Diabetic macular edema: What should I do? Irini Chatziralli Ophthalmic Surgeon, University Scholar 2 nd Department of Ophthalmology, University of Athens (Director: Prof P. Theodossiadis)
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
Post-LASIK infections
 Post-LASIK infections By Mohamed El-moddather Assiss. Prof. and head of department of ophthalmology AL-Azhar unizersity Assuit LASIK has become a common refractive procedure and is generally considered
Post-LASIK infections By Mohamed El-moddather Assiss. Prof. and head of department of ophthalmology AL-Azhar unizersity Assuit LASIK has become a common refractive procedure and is generally considered
ASCRS completes third annual Clinical Survey
 ASCRS completes third annual Clinical Survey More than 2,000 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education Survey Overview The third annual
ASCRS completes third annual Clinical Survey More than 2,000 members responded with clinical opinions and practice patterns to help drive the future of ASCRS education Survey Overview The third annual
INVELTYS (loteprednol etabonate ophthalmic suspension) 1%, for topical ophthalmic use Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
Internal document code 1 Nev061117iNZ
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NEVANAC TM (nepafenac) Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nevanac contains 1 mg in 1 ml of nepafenac. Excipient with known effect Benzalkonium
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NEVANAC TM (nepafenac) Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nevanac contains 1 mg in 1 ml of nepafenac. Excipient with known effect Benzalkonium
The Emergent Eye in the Acute Setting
 The Emergent Eye in the Acute Setting Todd P. Margolis MD, PhD Professor of Ophthalmology & Director of the F.I. Proctor Foundation UCSF Physical Exam-- Visual Acuity Essential Corrected visual acuity
The Emergent Eye in the Acute Setting Todd P. Margolis MD, PhD Professor of Ophthalmology & Director of the F.I. Proctor Foundation UCSF Physical Exam-- Visual Acuity Essential Corrected visual acuity
Condition: Pain management
 Condition: Pain management Description: In general, over-the-counter (OTC) pain medication or topical ophthalmic drugs (such as cyclopentolate) will be sufficient to ease discomfort in patients under the
Condition: Pain management Description: In general, over-the-counter (OTC) pain medication or topical ophthalmic drugs (such as cyclopentolate) will be sufficient to ease discomfort in patients under the
Corporate Presentation December Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG
 Corporate Presentation December 2018 Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking
Corporate Presentation December 2018 Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking
For last half century corticosteroids are used for ocular inflammation. They are used to suppress ocular inflammation in trauma, uveitis as well as
 The Professional Medical Journal DOI: 10.17957/TPMJ/17.3940 1. MBBS, DO, FCPS (Ophthalmology) Eye Specialist PAF Base Hospital Rafiqui Shorkot. 2. MBBS, DO, MCPS, FCPS (Ophthalmology) Classified Eye Specialist,
The Professional Medical Journal DOI: 10.17957/TPMJ/17.3940 1. MBBS, DO, FCPS (Ophthalmology) Eye Specialist PAF Base Hospital Rafiqui Shorkot. 2. MBBS, DO, MCPS, FCPS (Ophthalmology) Classified Eye Specialist,
InSite Vision DOUBle Phase 3 Clinical Program
 InSite Vision DOUBle Phase 3 Clinical Program AzaSite Plus (ISV 502) & DexaSite (ISV 305) For the Treatment of Blepharitis May 2011 Today s s Discussion Overview & Opportunity Phase 3 Trial Design/SPA
InSite Vision DOUBle Phase 3 Clinical Program AzaSite Plus (ISV 502) & DexaSite (ISV 305) For the Treatment of Blepharitis May 2011 Today s s Discussion Overview & Opportunity Phase 3 Trial Design/SPA
Ocular and periocular trauma
 Ocular and periocular trauma No financial disclosures. Tina Rutar M.D. Assistant Professor of Clinical Ophthalmology and Pediatrics Director, Visual Center for the Child University of California San Francisco
Ocular and periocular trauma No financial disclosures. Tina Rutar M.D. Assistant Professor of Clinical Ophthalmology and Pediatrics Director, Visual Center for the Child University of California San Francisco
Corporate Presentation NASDAQ: EYEG
 Corporate Presentation NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including
Corporate Presentation NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including
New Zealand Data Sheet
 New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
KNOW THE OPTIONS. Discover how the latest advances in vision correction can improve your sight.
 KNOW THE OPTIONS. LASIK VISIAN ICL PRK Discover how the latest advances in vision correction can improve your sight. Today, you can choose from several choices of permanent vision correction procedures
KNOW THE OPTIONS. LASIK VISIAN ICL PRK Discover how the latest advances in vision correction can improve your sight. Today, you can choose from several choices of permanent vision correction procedures
INFORMED CONSENT FOR AVASTIN TM (BEVACIZUMAB) INTRAVITREAL INJECTION
 INFORMED CONSENT FOR AVASTIN TM (BEVACIZUMAB) INTRAVITREAL INJECTION INDICATIONS: Age-related macular degeneration (AMD) is the leading cause of blindness in people over 50 years of age. It is caused by
INFORMED CONSENT FOR AVASTIN TM (BEVACIZUMAB) INTRAVITREAL INJECTION INDICATIONS: Age-related macular degeneration (AMD) is the leading cause of blindness in people over 50 years of age. It is caused by
 Traumatic Cataract Orbital Wall Fracture Vitreous Hemorrhage Optic Disc Hemorrhage a) Amblyopia b) Strabismus c) Trauma Playing with other children Sports Fire works BB gun Injecting needles .
Traumatic Cataract Orbital Wall Fracture Vitreous Hemorrhage Optic Disc Hemorrhage a) Amblyopia b) Strabismus c) Trauma Playing with other children Sports Fire works BB gun Injecting needles .
Although photocoagulation and photodynamic PROCEEDINGS PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION *
 PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
Ocular and Periocular Trauma. Tina Rutar, MD. Assistant Professor of Ophthalmology and Pediatrics. Director, Visual Center for the Child
 Ocular and Periocular Trauma Tina Rutar, MD Assistant Professor of Ophthalmology and Pediatrics Director, Visual Center for the Child University of California, San Francisco Phone: 415-353-2560 Fax: 415-353-2468
Ocular and Periocular Trauma Tina Rutar, MD Assistant Professor of Ophthalmology and Pediatrics Director, Visual Center for the Child University of California, San Francisco Phone: 415-353-2560 Fax: 415-353-2468
OPHTHALMOLOGY REFERRAL GUIDE FOR GPS
 OPHTHALMOLOGY REFERRAL GUIDE FOR GPS A guidebook to support general practitioners in the management and referral of a range of common eye problems. Contents 3 Introduction 4 Ophthalmic Workup 6 Acute Visual
OPHTHALMOLOGY REFERRAL GUIDE FOR GPS A guidebook to support general practitioners in the management and referral of a range of common eye problems. Contents 3 Introduction 4 Ophthalmic Workup 6 Acute Visual
Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery
 Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery David F. Chang, MD, 1 Ivan H. Garcia, MD, 2 John D. Hunkeler, MD, 3 Thomas Minas, MD 4 Objective: To evaluate the safety
Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery David F. Chang, MD, 1 Ivan H. Garcia, MD, 2 John D. Hunkeler, MD, 3 Thomas Minas, MD 4 Objective: To evaluate the safety
Building a Major Ophthalmic Pharmaceutical Company. Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015
 Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015 1 Important Information Any discussion of the potential use or expected success of our product
Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015 1 Important Information Any discussion of the potential use or expected success of our product
Aerie Pharmaceuticals, Inc. Additional Rhopressa Rocket 1 Analyses and Path Forward
 Aerie Pharmaceuticals, Inc. Additional Rhopressa Rocket 1 Analyses and Path Forward May 7, 2015 1 Important Information Any discussion of the potential use or expected success of our product candidates
Aerie Pharmaceuticals, Inc. Additional Rhopressa Rocket 1 Analyses and Path Forward May 7, 2015 1 Important Information Any discussion of the potential use or expected success of our product candidates
Dry-eye syndrome affects millions of
 CME EDUCATION EyeWorld Supplement July 2006 Making a Case for a Dry-Eye Treatment A CME Supplement from an EyeWorld Educational Symposium held at ASCRS ASOA 2006 in San Francisco I like tears as a first
CME EDUCATION EyeWorld Supplement July 2006 Making a Case for a Dry-Eye Treatment A CME Supplement from an EyeWorld Educational Symposium held at ASCRS ASOA 2006 in San Francisco I like tears as a first
Update on management of Anterior Uveitis
 Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Update on management of Anterior Uveitis Parthopratim Dutta Majumder Senior Consultant, Department of Uvea & Intraocular Inflammation Medical Research Foundation, Sankara Nethralaya ABCD of Treating a
Summary of the risk management plan (RMP) for Izba (travoprost)
 EMA/14138/2014 Summary of the risk management plan (RMP) for Izba (travoprost) This is a summary of the risk management plan (RMP) for Izba, which details the measures to be taken in order to ensure that
EMA/14138/2014 Summary of the risk management plan (RMP) for Izba (travoprost) This is a summary of the risk management plan (RMP) for Izba, which details the measures to be taken in order to ensure that
Evolution in Visual Freedom.
 Evolution in Visual Freedom. The EVO Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features
Evolution in Visual Freedom. The EVO Visian ICL Advantages Many vision correction procedures promise an improved level of vision, but few vision correction alternatives offer the quality and features
Investor Presentation December The vision to see past tomorrow
 Investor Presentation December 2011 The vision to see past tomorrow Safe Harbor Statement Certain statements contained herein are forward-looking statements (as such term is defined in the Private Securities
Investor Presentation December 2011 The vision to see past tomorrow Safe Harbor Statement Certain statements contained herein are forward-looking statements (as such term is defined in the Private Securities
Keratoconus Clinic. Optometric Co-management Opportunities
 Keratoconus Clinic Optometric Co-management Opportunities The Bochner Eye Institute established the first Keratoconus Clinic in Canada in 2008. The consultation and advanced imaging are OHIP covered. All
Keratoconus Clinic Optometric Co-management Opportunities The Bochner Eye Institute established the first Keratoconus Clinic in Canada in 2008. The consultation and advanced imaging are OHIP covered. All
A Multi-Disciplinary Approach to the Management of Cystoid MacularEdema
 Supplement to May/June 2008 Jointly sponsored by The Dulaney Foundation and Retina Today A Multi-Disciplinary Approach to the Management of Cystoid MacularEdema FEATURING: Jeffrey S. Heier, MD Eric D.
Supplement to May/June 2008 Jointly sponsored by The Dulaney Foundation and Retina Today A Multi-Disciplinary Approach to the Management of Cystoid MacularEdema FEATURING: Jeffrey S. Heier, MD Eric D.
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
ISSN: (Paper) eissn: (Online) JOURNAL OF ADVANCED ACADEMIC RESEARCH (JAAR) April 2017
 Topical proparacaine vs combined topical-intracameral lidocaine anesthesia in phacoemulsification surgery with preoperative counseling about intraoperative visual fear Kiran Shakya 1, Sangita Shakya 2,
Topical proparacaine vs combined topical-intracameral lidocaine anesthesia in phacoemulsification surgery with preoperative counseling about intraoperative visual fear Kiran Shakya 1, Sangita Shakya 2,
Cataract. What is a Cataract?
 Cataract What is a Cataract? We all have a lens in our eye. This is positioned just behind the iris, which is the coloured ring in the eye that gives your eye its colour. The lens function is to focus
Cataract What is a Cataract? We all have a lens in our eye. This is positioned just behind the iris, which is the coloured ring in the eye that gives your eye its colour. The lens function is to focus
Sponsor. Generic Drug Name. Trial Indications. Protocol Number. Protocol Title. Clinical Trial Phase. Study Start/End Dates. Reason for Termination
 Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
GENERAL INFORMATION CORNEAL TRANSPLANTATION
 GENERAL INFORMATION CORNEAL TRANSPLANTATION WHAT IS CORNEAL TRANSPLANTATION? A corneal transplant is an operation where a damaged or diseased cornea is replaced with donated, healthy tissue. Also called
GENERAL INFORMATION CORNEAL TRANSPLANTATION WHAT IS CORNEAL TRANSPLANTATION? A corneal transplant is an operation where a damaged or diseased cornea is replaced with donated, healthy tissue. Also called
