A New Paradigm in NSAID Treatment
|
|
|
- Shannon Carpenter
- 6 years ago
- Views:
Transcription
1 V 5/6 pg Contents Common Uses of Ophthalmic NSAIDs A New Paradigm in NSAID History of (bromfenac ophthalmic solution).9% Efficacy of Phase III trials Pain data Post-Approval trials Pharmacokinetics, Penetration, and Potency Safety of Oral NSAIDs vs. Ophthalmic NSAIDs Japanese experience and post-approval surveillance data US Pivotal trials V 5/6 pg Current Uses of Ophthalmic NSAIDs Intraoperative miosis Relief of: Pain Inflammation,,4 photophobia Ocular allergy 5 Xibrom dosed BID is indicated only for the treatment of postoperative inflammation and the reduction of ocular pain in patients who have undergone cataract surgery Reduction of post-cataract cystoid macular edema (CME) Current NSAIDs Common Issues Dosing Schedule: Compliance & Convenience BID Patient Tolerability : Burning & Stinging: Acular 4%; Acular LS % Voltaren 5-5%.4% TID Nevanac QID Acular ; Acular LS; Voltaren Sticky Sensation: Nevanac 5-% Conditions for which bromfenac ophthalmic solution has been studied or for which data are available. Note: All topical nonsteroidal anti-inflammatory drugs may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Acular onset of effect Statistical Significance vs placebo st demonstrated: Day 4-6 for reduction in anterior chamber cells Day 6-8 for reduction in anterior chamber flare Day 6-8 for ocular pain. Ohara K, et al. Jpn J Clin Ophthalmol. 4;58:5-8.. Data on File, ISTA Pharmaceuticals; Xibrom US Phase III Trials. Kawaguchi T, et al. Folia Ophthalmol Jpn. ;54:6. 4. Ohara K, et al. Jpn J Cataract & Refractive Surgery 4;8: Miyake-Kashima M, et al. Jpn J Ophthalmol. 4;48:58. V 5/6 pg. Physicians Desk Reference. 4 & 6.. Heier, J. Ketorolac Tromethamine.5% Ophthalmic Solution in the treatment of Moderate to Severe Ocular Inflammation after cataract Surgery: A Randomized, Vehicle-Controlled Clinical Trial: AJO, Mar 999; : 5-59 V 5/6 pg4 History of Published Comparative Papers From Trials in Japan Japan Approval, May : blepharitis, conjunctivitis, scleritis and post-operative inflammation 6 Years & approximately. million patient uses established track record of efficacy & safety US Approval, March 5: same formulation as used in Japan Inflammation Post-Surgery Significantly less flare in first 4 days for bromfenac (BID) vs. diclofenac (QID) Significantly less AC cells & protein in first days for bromfenac (BID) than diclofenac (TID) Relief of Seasonal Allergy Bromfenac equal in efficacy to pemirolast Prevention of Miosis in Cataract Surgery Bromfenac equal in efficacy to diclofenac 4. Data on File. ISTA Pharmaceuticals V 5/6 pg5. Kawaguchi T, et al. Folia Ophthalmol Jpn. ;54:6.. Ohara K, et al. Jpn J Cataract Refract Surgery 4;8:-.. Miyake-Kashima M, et al. Jpn J Ophthalmology 4; 48; Ohara K et al. Jpn J Clin Ophthalmol 4; 58; 5-8 V 5/6 pg6
2 V 5/6 pg (bromfenac ophthalmic solution).9% Indicated as: the first and only BID dosed NSAID for the treatment of postoperative inflammation and the reduction of ocular pain in patients who have undergone cataract extraction In The US: Contraindicated: in patients with known hypersensitivity to any ingredient in the formulation Most commonly reported adverse experiences: Abnormal sensation in eye, conjunctival hyperemia, eye irritation, eye pain, eye pruritus, eye redness, headache and iritis. These events were reported in %-% of patients Effect of BID on Compliance Evaluation and Multivariate Statistical Analysis of Factors Influencing Patient Adherence to Ophthalmic Solutions Proper Use M isuse ophthalmic patients Ophthalmology Department at Hiroshima University Drugs Applied - x daily, 6% Drugs Applied - 5 x daily 44% Drugs Applied - 5 x daily, 56% Drugs Applied - x daily, 94% % % % % 4% 5% 6% % 8% 9% % % of Patients. Published: Yakugaku Zasshi ;():99-86 V 5/6 pg8 Phase III Trials: Key Points US Phase III Protocol Review No pre-surgical dosing of an NSAID No anti-inflammatory agent until ONE DAY after surgery Summed Ocular Inflammation score prior to treatment =. High level of inflammation for routine cataract procedure : Dosed BID Data presented in following slides represent patients treated with Xibrom as their only anti-inflammatory (no steroid used) V 5/6 pg9 Number of cataract surgery patients Number of US sites Baseline summed cells and flare: (prior to treatment) Pre-Surgery Dosing w/nsaid or Steroid Xibrom (or placebo) Dose Efficacy Endpoint Measures SOIS = Summed Ocular Inflammation Score: Anterior chamber cell grade added to anterior chamber flare grade 5 (56 Xibrom, placebo) 9. NO BID for 4 days Reduction in Summed Cell & Flare Score Reduction in AC Cell Score Reduction in AC Flare Score Resolution of Pain Randomized, double masked, placebo-controlled studies Similar protocol to ketorolac.5% Phase III ONLY NSAID pivotal trial requiring an end-point of zero V 5/6 pg Visit & Schedule US Phase III Trials: Inflammation Reduction: Summed Ocular Inflammation Score Evaluation Days Post-Surgery Day = Day of Surgery Days Post- Initiation Approximate Doses of Xibrom High Inflammation.5 Days After Cataract Surgery 8 5 P =. P <. vs. placebo st nd rd 4th 5th Day : Return to office for Entry Criteria Evaluation (still no anti-inflammatory used) Day Day 8 Day 5 Day Day No Inflammation.5 SOIS Score.5.5 Days of Xibrom achieved statistical significance on st treatment evaluation ( treatment days) achieved statistically significant reduction from baseline at all study visits Xibrom TM Note: Day represents Return to office for entry Criteria Evaluation (no antiinflammatory used) Day represents the nd day of therapy V 5/6 pg Data represent results from patients on Xibrom or placebo, only. No rescue medications V 5/6 pg
3 V 5/6 pg US Phase III Trials: Individual Mean Cell and Flare Scores by Visit : Summary Of Phase III Efficacy Results Statistical Significance Achieved in Every Time-Point Measure High.5 Inflammation Cells Score.5.5 No Inflammation Days of treatment P<. P<. Flare Score Days After Cataract Surgery Days After Cataract Surgery Note: Day represents Return to office for entry Criteria Evaluation (no antiinflammatory used) Day represents the nd day of therapy.5 Days of treatment Xibrom statistical significance achieved on st treatment visit for both cell and flare reduction Statistical significance achieved through each treatment visit: Days 8 to 5 Reduction in Summed Ocular Inflammation Score Reduction in Cells Reduction in Flare Patients Achieving Summed Cells & Flare Score of Zero Use of NSAIDs pre-operatively was disallowed Achievement of Statistical Significance vs placebo Day Day Day 4 p <. p =. p <. p<. Data represent results from patients on Xibrom or placebo, only. No rescue medications V 5/6 pg4 Acular.5% Pivotal Trial : Statistical Significance Achieved within 6 4 Day Measures In this specific study, use of NSAIDs pre-operatively was disallowed Reduction in Cells Reduction in Flare Patients Achieving Summed Cells & Flare Score of Zero Reduction in Ocular Pain Achievement of Statistical Significance vs placebo Day - 4 Day 6-8 Day 4-6 p <.5 p <. ISTA Pharmaceuticals, Inc US Phase III Trials: ndary End. Point Time to Resolution of Ocular Pain Percent Unresolved Time to Resolution of Ocular Pain (days) log rank p<.. Heier, J, et al. Am J Ophthalmol 999; : 5-59 V 5/6 pg5 Estimated median for Time to Resolution of Ocular Pain: Xibrom =. days : = 5. days (p =.) V 5/6 pg6 Overall Pain Relief: Percentage of All Subjects Pain Free Percentage of Patients Pain Free % 9% 8% % 6% 5% 4% % % % % 5.% 6.8% 8.6% 9.% 9.5% 59.% 6.% 8 4 Study day Xibrom (n = 56) 69.6% (n=) No patients in Xibrom group reported pain at day 4. Subjects with missing data points on day were treated as treatment failures. Missing data were treated as LOCF. p=.6 p<. At Day 8 = 9.% of all Xibrom subjects were pain free vs. 6.% placebo Post-Approval US Trials Comfort vs. Acular LS H.D. Perry, MD, T.Y. Chou, MD Physician Satisfaction 589 Ophthalmologists/, Patient Experiences CME Post-Cataract Surgery vs. Acular and Voltaren D.S. Rho, MD, S.M. Soll, MD, B.J. Markovitz, MD Macular Edema Post-Glaucoma Surgery vs. Acular A.M. Solish, MS, MD V 5/6 pg V 5/6 pg8
4 V 5/6 pg9 Post-Approval Trials: Comfort A Comparison of and Acular LS in a Test of Comfort and Corneal Anesthesia Henry D. Perry, MD Timothy Y. Chou, MD Purpose: Compare comfort of Xibrom and Acular LS normal healthy volunteers Methods: subjects administered single drop of each test agent to their eyes in a random and masked fashion. Burning and stinging on a 4 scale. Results: Subjective Assessment of Comfort Upon Instillation Level of Burning or Stinging Xibrom Acular LS Number of Patients Reporting None to Severe Burning & Stinging None Mild Moderate 5 Severe Xibrom: of (85%) reported no burning or stinging Acular LS: of (5%) reported no burning or stinging Xibrom: of (%) reported moderate or severe burning or stinging Acular LS: 6 or (%) reported moderate or severe burning or stinging V 5/6 pg Post Marketing Trials: Physician Satisfaction Xibrom First Experience (XFE) Trial MDs placed a minimum of patients on Xibrom May through June, 5 Jan through Mar, 6 MDs rated Xibrom performance: Very Satisfied: Exceeded My Expectations Satisfied: Met All My Expectations Dissatisfied: Met Some of My Expectations Very Dissatisfied: Did Not Meet My Expectations Robust MDs experiences captured: 589 MDs reported to date, patients experiences reported V 5/6 pg ISTA Pharmaceuticals, Inc :. Early Experience Program Exceeded All Expectations Met All Expectations 589 ophthalmologists:, patient experiences MD Expectations: Exceeded or Met in 99% to % of Reports Met Some Expectations Did Not Meet Expectations % 5% % 58% 6% 6% 4% 9% 8% Over all Patients Satisfaction Safety Comfort Upon Instillation % 9% Patient Compliance 8% 9% Ease of Use by Patients % % % % % % % % % % % % 46% 5% Control of Inflammation V 5/6 pg Phase 4 Trials: Cystoid Macular Edema (CME) Phase 4 Trials: Cystoid Macular Edema (CME) A Comparison of Bromfenac, Ketorolac, and Diclofenac for of Acute Pseudophakic Cystoid Macular Edema (Preliminary Data) David S. Rho, MD, Stephen M. Soll, MD, Bruce J. Markovitz, MD Purpose: Comparison of Xibrom, Acular, and Voltaren for treatment of acute CME post-cataract surgery Methods: 64 post-cataract patients with acute CME randomized to one of three regimens: Xibrom BID, Acular QID, or Voltaren QID, in the affected eye Visual acuities measured; Snellen VA charts and ETDRS charts Initial VA Initial VA (ETDRS letters) Final VA Final VA (ETDRS letters) ETDRS Letters gained Bromfenac /5 ± ±. /58.4 ± ±.6 5. ±. P-values for ETDRS letters gained within treatment groups: Bromfenac p <.5 Diclofenac p <.5 Ketorolac p <.5 Diclofenac /5 ± ± 5. / ± 4..6 ± ±.5 Ketorolac /6 ± ±. / ± 4.6. ±.9.6 ± 8.6 P-values for ETDRS letters gained between treatment groups: Bromfenac vs Diclofenac p =.8 Bromfenac vs Ketorolac p =.5 Ketorolac vs Diclofenac p =.96. Rho DS. ARVO 6; A5 V 5/6 pg. Rho DS. ARVO 6; A5 V 5/6 pg4
5 V 5/6 pg5 Phase 4 Trials: Macular Edema Post-Glaucoma Surgery A Prospective, Randomized Comparison of Bromfenac with Ketorolac for the of Patients with Reduced Visual Acuity Following Glaucoma Surgery Alfred M. Solish, MS, MD Purpose: Comparison of Xibrom and Acular for treatment of macular edema post-glaucoma surgery Methods: post-glaucoma surgery patients with decreased corrected visual acuity (VA), whose vision had not improved with topical steroids, were randomized to either Xibrom BID or Acular QID Corrected visual acuities were compared following 4-6 weeks of treatment Phase 4 Trials: Macular Edema Post-Glaucoma Surgery Initial IOP (mmhg) Follow-up IOP (mmhg) Follow-up (days) Change in VA (lines corrected) Patients gaining VA (> lines) Patients losing VA (> line) Bromfenac /6 (.5%) /6 (.5%) Ketorolac /9 (%) 5/9 (6.%) P (t-test) More patients had VA improvement with Xibrom than with Acular, though the difference was not statistically significant. Solish AM. AGS 6; A95. Solish AM. AGS 6; A95 V 5/6 pg6 Chemical Structures and Related Activities Bromfenac Amfenac O O Penetration & Potency Data Br C C H N CH CO Na. ½H O H N CH CO Na. H O Helps answer the question: How and why is Xibrom BID? Chemical structure of bromfenac is similar to amfenac, shown above Substitution of the benzoyl ring has pronounced effects on in-vivo and in-vitro potency and absorption (B >x more potent than A in this study) In general, compounds that contain a halogen, are more potent (I-~ Br- > Cl > F > H).. Walsh D. J Medicinal Chem. 984;:9-88 V 5/6 pg V 5/6 pg8 Octanol/Water (O/W) Partition Coefficients Amfenac Penetration: Data From Amfenac Patent 6,646, B The octanol/water partition coefficient for bromfenac compared to other NSAIDs and Steroids at physiological ph.4 Drug Bromfenac Amfenac O/W Partition Coefficients.. Ketorolac.88 The higher the coefficient, the higher the penetration across membranes.. unit difference = fold difference. Ruiz J et.al Journal of Computer-Aided Molecular Des QSAR tables V 5/6 pg9 No level detected: Aqueous humor Choroid at hrs Retina at 6 hrs V 5/6 pg
6 V 5/6 pg Concentrations of Radioactivity in Ocular Tissues Following a Single Topical Ocular Dose of 4 C-Bromfenac Comparison of Ocular Concentrations of 4C- Bromfenac and 4C-Nepafenac 4C-Bromfenac 4C-Nepafenac 8 4 Retina and choroid detectable through 4 hour time-point Choroid not detectable at hours Detectable levels thru 4 hours and beyond for the above ocular tissues. Baklayan GA. ASCRS 6;P. Retina not detectable at 6 hours V 5/6 pg Concentrations of Radioactivity in Ocular Tissues Following a Single.9%Topical Ocular Dose of 4 C-Bromfenac Concentration (ug equiv./g) Iris-Cliary Body Aqueous Humor Choroid Retina hr hr 4 hr 8 hr hr 4 hr Time Detectable levels thru 4 hours and beyond for the above ocular tissues. McNamara TR. ARVO 6;A586. V 5/6 pg PK Profile of a Single.9%Topical Ocular Dose of Bromfenac in Subjects Undergoing Cataract Surgery BF conc. in Time of instillation aqueous humor No. of cases before surgery (min) (ng/ml) (mean + SD) t < 9.8 < t < < t < < t < < t < < t < < t < < t < < t < < t < < t <. +.5 t > 5 Bromfenac concentrations in aqueous humor at grouped -min intervals. Ogawa T. ARVO 6;A68. V 5/6 pg4 PK Profile of a Single.9%Topical Ocular Dose of Bromfenac in Subjects Undergoing Cataract Surgery PK Profile of a Single.9%Topical Ocular Dose of Bromfenac in Subjects Undergoing Cataract Surgery Human recombinant prostaglandin G/H synthase Rabbit alveolar macrophages IC5 4. nm,.5 ng/ml nm, 8.8 ng/ml Human Rabbit Tmax (hr).. Cmax (ng/ml) T / (hr).4. IC5 value of bromfenac in human and rabbit COX- inhibitory activity PK parameters of bromfenac in aqueous humor of human and rabbit. Ogawa T. ARVO 6;A68. V 5/6 pg5. Ogawa T. ARVO 6;A68. V 5/6 pg6
7 V 5/6 pg PK Profile of a Single.9%Topical Ocular Dose of Bromfenac in Subjects Undergoing Cataract Surgery COX- Hypothesis, BF Conc. in AH (ng/ml)... Human data Human COX- IC5 Rabbit data Rabbit COX- IC Time after instillation (hrs) Estimation of duration for retaining the effective concentration of bromfenac after instillation over the IC5 value of COX-. Ogawa T. ARVO 6;A68. COX- constitutive PGs GI cytoprotection Platelet aggregation Renal function (blood flow) Arachidonic acid. Oka T. Curr Eye Res 4;9():-4. Guex-Crosier Y. Klin Monatsbl Augenheilkd ;8(5):5-8 COX- inducible PGs Inflammation Fever Pain Headache Carcinogenesis V 5/6 pg8 Potency Measurement: IC 5 Relative Potency of NSAIDs In Vitro: IC 5 vs COX- and COX- enzymes Drug concentration required to inhibit enzyme activity by 5% The smaller the number, the more potent the molecule Lower IC 5 = Greater Potency IC5, COX - IC5, COX - Bromfenac.5 um. um Diclofenac.95 um.85 um Amfenac.5 um.5 um.x V 5/6 pg9 6.5X COX II Enzyme most responsible for pain and inflammation IC5 of bromfenac for COX- is 8 fold greater than ketorolac The clinical significance of these data is unknown. The Cox- and Cox- in vitro assay is highly variable between laboratories and types of assays used.. Data on file BR54. ISTA Pharmaceuticals:. Gamache et al Inflammation LS:. Waterbury et al. Curr Med Res Opin 6;(6):-4 V 5/6 pg4 Safety US Phase III Trials: Common - Emergent Ocular Adverse Events (> % incidence rate) Extensive testing from Phase I Phase III 6 years of ophthalmic use Over. million ophthalmic uses,45 patients officially tracked by institutions A very safe drug: No reported drug related serious systemic side effects Clinical studies demonstrated a minimal incidence of adverse events n (% of Subjects) Adverse Event (n = 56) (n = ) Iritis 5 (.%) (8.%) Ocular pain 5 (4.%) (.%) Abnormal sensation in eye (6.5%) 4 (8.%) Eye pruritus 4 (.9%) 5 (.9%) Eye irritation 9 (.5%) 8 (4.%) Eye redness 8 (.%) (.6%) Photophobia (.%) 9 (.%) Other Reported Adverse Events: ONLY.4% incidence of burning and stinging in Xibrom patients CME reported: Xibrom.4% : 4.% (p <.5) V 5/6 pg4 Note: Xibrom is not indicated for the treatment of CME nor Photophobia V 5/6 pg4
8 V 5/6 pg4 Experience with Bromfenac Sodium Ophthalmic Solution in Japan (-6) Well established 6 Year safety profile (Bronuck) Post Marketing Safety Report (Japan) Filed with Minister of Health, Labour and Welfare /5 Marketed since /: There have been no drug related serious systemic adverse events reported with use of bromfenac sodium ophthalmic solution. million uses & in over.8 million patients in Japan Zero drug related serious systemic events reported Serious ocular adverse events Reported in.% of patients (6) 4 corneal events reported in the Japanese post-marketing surveillance program involving,45 patients observed from May to January 4 (.4%). Dosing schedules, length of therapy, and indications may vary between Xibrom and bromfenac ophthalmic solution used in Japan Products in Japan and U.S. are identical Patients or Usage of Xibrom (Bronuck) Total Patients Surveyed (/ /6) Patient Age: Month to 5 Years Patient Age: 65 Years or Older Patients with known pre-existing liver disorders Patients with known pre-existing kidney disorders More than times per day More than 4 times per day Between 5-99 drops of drug Between -49 drops of drug Between 5-99 drops of drug Number of patients,45 49, , Over drops of drug 45. Data on file. ISTA Pharmaceuticals. Kitao N, Shimoji H, Fukuda M. Atarashii Ganka 5;:99-8. Xibrom, in the US, is indicated for BID dosing for 4 days of treatment V 5/6 pg44 Summary of HIDDEN/BACK-UP SLIDES A Very Potent NSAID IC 5 data for bromfenac & diclofenac & amfenac in similar models Rapidly Treats Significant Inflammation Excellent penetration BID dose Compliance & Convenience Comfortable.4% burning/stinging Safe 6 years experience &. million uses V 5/6 pg45 V 5/6 pg46 US Phase III Trials: Grading Scale for Cells & Flare : US Phase III Trials Grade 4 Cells (Count) -5 (trace) > 5 Grade 4 Flare Complete Absence Very Slight Moderate Marked Intense Inclusion Criteria: Patients were required to have a Summed Ocular Inflammation Score (cell score + flare score) at 6 to hours, after surgery PRIOR to Patients with EXISTING significant inflammation were treated with Xibrom, or placebo, BID started AFTER inflammation had developed Cells & flare graded each visit st treatment visit: days of Xibrom treatment SOIS = Summed Ocular Inflammation Score: Anterior chamber cell grade added to anterior chamber flare grade V 5/6 pg4 V 5/6 pg48
9 V 5/6 pg49 Safety Issues have occurred with all ORAL NSAIDs Diclofenac (Voltaren ) Gastrointestinal perforations, ulcerations, and bleeding Ketorolac (Toradol ) Gastrointestinal perforations, ulcerations, and bleeding Black box warning in U.S., withdrawn from market in France and Germany 4 deaths from Bromfenac (Duract ) Hepatotoxicity, acute hepatic failures, when used outside of labeled dosage Manufacturer voluntarily withdrew from US market 4 deaths in US Phase III Trials: Liver Function Tests Show No Effect on Liver Liver Function Test Aspartate aminotransferase (AST) Alanine transaminase (ALT) γ-glutamyl transpeptidase (GGT) Total bilirubin Direct bilirubin Alkaline phosphatase No significant differences between treatment groups No subjects with results > CTC grade n (% of Patients) With Toxicity Grade = at Study Termination (n = 4) (.9%) 4 (.%) 8 (.%) (.6%) (n = 5) (.%) (.%) (.9%). Macario A, et.al Pain Medicine Vol.,. Physicians Desk Reference. 4. CTC = common toxicity criteria V 5/6 pg5 ISTA JapanPost-MarketingSurveilanceProgram programto document saf et y a n d ef f i c ac y V5/6pg5
Ocular NSAIDs: A New Option
 Insert to March 2006 Ocular NSAIDs: A New Option Evaluating performance parameters to optimize therapy. Potency and Penetration Efficacy Safety Clinical Experience to Date This continuing medical education
Insert to March 2006 Ocular NSAIDs: A New Option Evaluating performance parameters to optimize therapy. Potency and Penetration Efficacy Safety Clinical Experience to Date This continuing medical education
WARNING LETTER. According to the Indications and Usage section of the FDA approved product labeling (PI):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE David E.I. Pyott President and Chief Executive Officer PO Box 19534
Better ophthalmic surgery outcomes in recent years have created more Demanding Patients.
 By Alaa EL Zawawi Prof. of Ophthalmology Alexandria - Egypt Launch Better ophthalmic surgery outcomes in recent years have created more Demanding Patients. 1 NSAIDs can play a pivotal role in facilitating
By Alaa EL Zawawi Prof. of Ophthalmology Alexandria - Egypt Launch Better ophthalmic surgery outcomes in recent years have created more Demanding Patients. 1 NSAIDs can play a pivotal role in facilitating
PHARMACOLOGY Class: Ketorolac trometamol is a member of the pyrrolo-pyrolle group of non-steroidal antiinflammatory
 ACULAR Eye Drops NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure: Chemical Name: ( )-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol
ACULAR Eye Drops NAME OF THE DRUG Non-proprietary name: ketorolac trometamol. Chemical Structure: Chemical Name: ( )-5-Benzoyl-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol
INDICATIONS ACULAR 0,5 % is indicated for the relief of inflammation following ocular surgery.
 Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
Page 1 of 5 SCHEDULING STATUS Schedule 3 PROPRIETARY NAME (AND DOSAGE FORM) ACULAR 0,5 % COMPOSITION ACULAR 0,5 % contains: Preservatives: Benzalkonium chloride 0,01 % m/v Disodium edetate 0,1 % m/v PHARMACOLOGICAL
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops
 AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
AUSTRALIAN PRODUCT INFORMATION ACULAR (ketorolac trometamol) Eye Drops 1 NAME OF THE MEDICINE Ketorolac trometamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION ACULAR contains ketorolac trometamol 5 mg/ml.
See 17 for PATIENT COUNSELING INFORMATION.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEVANAC* safely and effectively. See full prescribing information for NEVANAC. NEVANAC (nepafenac
13 NONCLINICAL TOXICOLOGY 5.1 Increased Bleeding Time Carcinogenesis, Mutagenesis, Impairment of 5.2 Delayed Healing
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ILEVRO* (nepafenac ophthalmic suspension), 0.3% safely and effectively. See full prescribing information
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ENFLUAT 0.4 % Sterile Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Drug substance: Ketorolac Tromethamine 0,4 % Each bottle
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ENFLUAT 0.4 % Sterile Eye Drops 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Drug substance: Ketorolac Tromethamine 0,4 % Each bottle
Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST
 Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST Forward Looking Statements Certain statements contained
Lauren Silvernail, CFO & VP Corporate Development to present at 2005 Thomas Weisel Partners Healthcare Conference on September 7, 2005 at 3:50 pm EST Forward Looking Statements Certain statements contained
Surgery Post-operative
 Surgery Post-operative Redefining the Treatment Paradigm for Post-operative Inflammation Control The Role of Topical Non-steroidal Anti-inflammatory Drugs Oliver Findl Associate Professor of Ophthalmology
Surgery Post-operative Redefining the Treatment Paradigm for Post-operative Inflammation Control The Role of Topical Non-steroidal Anti-inflammatory Drugs Oliver Findl Associate Professor of Ophthalmology
NSAIDs in Treatment of Retinal Disorders
 NSAIDs in Treatment of Retinal Disorders These drugs are playing new roles beyond their established use in cataract and refractive surgery. NON-STEROIDAL ANTI-INFLAMm a t o r y drugs are approved for the
NSAIDs in Treatment of Retinal Disorders These drugs are playing new roles beyond their established use in cataract and refractive surgery. NON-STEROIDAL ANTI-INFLAMm a t o r y drugs are approved for the
Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution
 REVIEW Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution Hyung Cho 1 Kenneth J Wolf 1 Eric J Wolf 2 1 Department of Ophthalmology, Albert Einstein
REVIEW Management of ocular infl ammation and pain following cataract surgery: focus on bromfenac ophthalmic solution Hyung Cho 1 Kenneth J Wolf 1 Eric J Wolf 2 1 Department of Ophthalmology, Albert Einstein
Case History. Slit lamp exam: Clinical Pearls in the Management of Iritis. 2- injection: Irregular SPK and staining AC: grade 3 cell & flare
 Clinical Pearls in the Management of Iritis Paul Karpecki, OD, FAAO Corneal Services and Ocular Disease Research Koffler Vision Group-Lexington, KY 68 y.o. Caucasian female Complains of photophobia and
Clinical Pearls in the Management of Iritis Paul Karpecki, OD, FAAO Corneal Services and Ocular Disease Research Koffler Vision Group-Lexington, KY 68 y.o. Caucasian female Complains of photophobia and
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd
 nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
nepafenac 1mg/mL eye drops, suspension (Nevanac ) SMC No. (813/12) Alcon Laboratories (UK) Ltd 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Anterior Segment Cataract Surgery
 Current Use of Non-steroidal Anti-inflammatory Drugs in the Treatment of Ocular Inflammation Related to Cataract Surgery Eric Donnenfeld Clinical Professor of Ophthalmology, New York University, New York,
Current Use of Non-steroidal Anti-inflammatory Drugs in the Treatment of Ocular Inflammation Related to Cataract Surgery Eric Donnenfeld Clinical Professor of Ophthalmology, New York University, New York,
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
BARRY A. SCHECHTER ABSTRACT
 JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS Volume 22, Number 2, 2006 Mary Ann Liebert, Inc. The Evaluation of Ketorolac (Acular LS ) to Improve Patient Comfort During the Induction Phase of Cyclosporin-A
CONTRAINDICATIONS Hypersensitivity to any component of this product (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OMIDRIA safely and effectively. See full prescribing information for OMIDRIA. OMIDRIA (phenylephrine
sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa
![sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa sodium [2-(2,6-dichloroanilino)phenyl] acetate, a phenylacetic acid derivative CH 2 COONa](/thumbs/78/77533900.jpg) PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
PRODUCT INFORMATION VOLTAREN * OPHTHA Eye Drop (diclofenac sodium) NAME OF THE MEDICINE Active ingredient: Chemical name: Molecular formula: C 14 H 10 Cl 2 NNaO 2 CAS number: 15307-79-6 Molecular weight:
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye...
 Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
Overview & pathophysiology of Dry Eye and the use of cyclosporine eye drops in dry eye... This Allergan sponsored session was held on July 24, 2005, Hotel Satya Ashoka, Jabalpur. The session was followed
! Somatic! Visceral! Neuropathic! Psychogenic. ! Analgesic! Relief without sedation! Works on peripheral pain receptors
 PAIN, PAIN, GO AWAY. TYPES OF PAIN! Somatic! Visceral! Neuropathic! Psychogenic Jill Autry, OD, RPh Eye Center of Texas, Houston drjillautry@tropicalce.com ANALGESIA! Mild! Aspirin! Paracetamol! Moderate!
PAIN, PAIN, GO AWAY. TYPES OF PAIN! Somatic! Visceral! Neuropathic! Psychogenic Jill Autry, OD, RPh Eye Center of Texas, Houston drjillautry@tropicalce.com ANALGESIA! Mild! Aspirin! Paracetamol! Moderate!
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES TRANSMITTED BY FACSIMILE
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Cary Rayment President and Chief Executive Officer Alcon, Inc. C/O
Investor Presentation December The vision to see past tomorrow
 Investor Presentation December 2011 The vision to see past tomorrow Safe Harbor Statement Certain statements contained herein are forward-looking statements (as such term is defined in the Private Securities
Investor Presentation December 2011 The vision to see past tomorrow Safe Harbor Statement Certain statements contained herein are forward-looking statements (as such term is defined in the Private Securities
LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%)
 Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
Published on: 14 Apr 2017 LOWPROST Eye Drops (Bimatoprost Ophthalmic Solution 0.01%) For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only Composition Each ml contains: Bimatoprost...
Medical Science. Research Paper. 2nd yr post graduate, Dept. Of Ophthalmology, SVS Medical College
 Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Dr. A.Narasimha Rao Dr. N.Deepika Dr. D.Chandrakanth Reddy Dr. M. Vijaya Ramaraju ABSTRACT KEYWORDS Research Paper Medical Science Comparative Evaluation of the Anti- Inflammatory Effect of Topical 1 %
Case History. The SEVEN HABITS of Highly Effective Anterior Uveitis Management. SLEx findings: SLEx corneal findings: y.o.
 The SEVEN HABITS of Highly Effective Anterior Uveitis Management Case History! 68 y.o. Caucasian female of photophobia and blurred vision! As well as a headache over right eye for 2 days! Complains Paul
The SEVEN HABITS of Highly Effective Anterior Uveitis Management Case History! 68 y.o. Caucasian female of photophobia and blurred vision! As well as a headache over right eye for 2 days! Complains Paul
CONTRAINDICATIONS None (4).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXYCU safely and effectively. See full prescribing information for DEXYCU. DEXYCU (dexamethasone
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXYCU safely and effectively. See full prescribing information for DEXYCU. DEXYCU (dexamethasone
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company AAO October 25, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ AAO October 25, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, Bromfenac 0.1% and Ketorolac 0.45%, on Cataract Surgery
 Original Article Yonsei Med J 215 Nov;56(6):1671-1677 pissn: 513-5796 eissn: 1976-2437 The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, and, on Cataract Surgery Ji Won Jung 1,2, Byung Hoon Chung
Original Article Yonsei Med J 215 Nov;56(6):1671-1677 pissn: 513-5796 eissn: 1976-2437 The Effects of Two Non-Steroidal Anti-Inflammatory Drugs, and, on Cataract Surgery Ji Won Jung 1,2, Byung Hoon Chung
84 Year Old with Rosacea
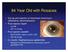 84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
84 Year Old with Rosacea S/p tap and injection of intravitreal vancomycin, ceftazidime, dexamethasone Post-injection day#1 Va HM IOP 14 mmhg Post-injection week#3 BCVA 20/20-3 (plano +0.50 x 180) IOP 23
Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice.
 Section Editor: Sumit Sam Garg, MD Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice. BY GARRICK CHAK, MD; AMANDA E. KIELY, MD; AND PRATAP CHALLA,
Section Editor: Sumit Sam Garg, MD Topical Corticosteroid and NSAID Therapies for Ocular Inflammation A concise overview for clinical practice. BY GARRICK CHAK, MD; AMANDA E. KIELY, MD; AND PRATAP CHALLA,
Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery.
 Poster #33516 Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery. JEFFREY H. LEVENSON, MD 1 (PRESENTING AUTHOR);
Poster #33516 Iontophoresis Platform Safely and Effectively Delivers Dexamethasone to Manage Post Operative Inflammation and Pain Following Cataract Surgery. JEFFREY H. LEVENSON, MD 1 (PRESENTING AUTHOR);
INDICATIONS For steroid responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye globe.
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Condition: Pain management
 Condition: Pain management Description: In general, over-the-counter (OTC) pain medication or topical ophthalmic drugs (such as cyclopentolate) will be sufficient to ease discomfort in patients under the
Condition: Pain management Description: In general, over-the-counter (OTC) pain medication or topical ophthalmic drugs (such as cyclopentolate) will be sufficient to ease discomfort in patients under the
Rama Vadapalli * 1, Vankadara Naga Suresh 2. Online Access and Article Informtaion. Original Research Article
 Original Research Article An Interventional Follow up Study to Determine the Effect of Nepafenac 0.1% in Macular thickness in Patients who had Undergone Cataract Surgery as Determined By OCT Rama Vadapalli
Original Research Article An Interventional Follow up Study to Determine the Effect of Nepafenac 0.1% in Macular thickness in Patients who had Undergone Cataract Surgery as Determined By OCT Rama Vadapalli
INVELTYS (loteprednol etabonate ophthalmic suspension) 1%, for topical ophthalmic use Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project
 The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
The Biomedical Ocular Coil Drug delivery Comfort (OCDC) project Prof.dr. R.M.M.A. Nuijts (MUMC+) Dr. A. Dias (Eyegle BV) Prof.dr. R. Tuinier (TU/e) I. Schefman, BA (Eyegle BV) Dr. H. Theunissen (MVC, Part.
The role of perioperative nonsteroidal anti-inflammatory drugs use in cataract surgery
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 18, Issue 1 Ser. 15 (January. 2019), PP 23-27 www.iosrjournals.org The role of perioperative nonsteroidal
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 18, Issue 1 Ser. 15 (January. 2019), PP 23-27 www.iosrjournals.org The role of perioperative nonsteroidal
Efficacy of Bromfenac Sodium Ophthalmic Solution for Treatment of Dry Eye Disease
 ORIGINAL CLINICAL STUDY Efficacy of Bromfenac Sodium Ophthalmic Solution for Treatment of Dry Eye Disease Hiroshi Fujishima, MD,* Miki Fuseya, MD,Þ Masarou Ogata, MD,Þ and Dogru Murat, MD, PhDþ Purpose:
ORIGINAL CLINICAL STUDY Efficacy of Bromfenac Sodium Ophthalmic Solution for Treatment of Dry Eye Disease Hiroshi Fujishima, MD,* Miki Fuseya, MD,Þ Masarou Ogata, MD,Þ and Dogru Murat, MD, PhDþ Purpose:
Internal document code 1 Nev061117iNZ
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME NEVANAC TM (nepafenac) Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nevanac contains 1 mg in 1 ml of nepafenac. Excipient with known effect Benzalkonium
NEW ZEALAND DATA SHEET 1. PRODUCT NAME NEVANAC TM (nepafenac) Eye Drops 0.1% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Nevanac contains 1 mg in 1 ml of nepafenac. Excipient with known effect Benzalkonium
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
SUPPLEMENTARY INFORMATION Contents METHODS... 2 Inclusion and exclusion criteria... 2 Supplementary table S1... 2 Assessment of abnormal ocular signs and symptoms... 3 Supplementary table S2... 3 Ocular
PRODUCT MONOGRAPH. Pr ACULAR. ketorolac tromethamine ophthalmic solution 0.5% w/v. with benzalkonium chloride 0.01% w/v as preservative.
 PRODUCT MONOGRAPH Pr ACULAR ketorolac tromethamine ophthalmic solution 0.5% w/v with benzalkonium chloride 0.01% w/v as preservative Pr ACULAR LS ketorolac tromethamine ophthalmic solution 0.4% w/v with
PRODUCT MONOGRAPH Pr ACULAR ketorolac tromethamine ophthalmic solution 0.5% w/v with benzalkonium chloride 0.01% w/v as preservative Pr ACULAR LS ketorolac tromethamine ophthalmic solution 0.4% w/v with
NSAIDs. The Evolving Role of. in Cataract Surgery
 Supplement to April 2011 The Evolving Role of NSAIDs in Cataract Surgery Strategies for ocular surface management with the newly approved q.d. formulation of bromfenac 0.09%. Featuring: Johnny Gayton,
Supplement to April 2011 The Evolving Role of NSAIDs in Cataract Surgery Strategies for ocular surface management with the newly approved q.d. formulation of bromfenac 0.09%. Featuring: Johnny Gayton,
Completed Clinical Research Trials Monte Dirks, M.D.
 Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
Completed Clinical Research Trials Monte Dirks, M.D. Monte Dirks, M.D. - Primary Investigator Norfloxacin -Merck (1986) Safety and efficacy of norfloxacin vs. tobramycin in the treatment of external ocular
KEY MESSAGES. Details of the evidence supporting these recommendations can be found in the above CPG, available on the following websites:
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
QUICK REFERENCE FOR HEALTHCARE PROVIDERS KEY MESSAGES 1. Glaucoma is a chronic eye disease that damages the optic nerve, & can result in serious vision loss and irreversible blindness. 2. Glaucoma diagnosis
New Zealand Data Sheet
 New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
Optometric Postoperative Cataract Surgery Management
 Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
Financial Disclosures Optometric Postoperative Cataract Surgery Management David Dinh, OD Oak Cliff Eye Clinic Dallas Eye Consultants March 10, 2015 Comanagement Joint cooperation between two or more specialists
TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%)
 Published on: 23 Sep 2014 TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%) Composition Loteprednol Etabonate 5 mg (0.5% w/v) Tobramycin...3 mg (0.3% w/v) Benzalkonium Chloride.. 0.01%
Published on: 23 Sep 2014 TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%) Composition Loteprednol Etabonate 5 mg (0.5% w/v) Tobramycin...3 mg (0.3% w/v) Benzalkonium Chloride.. 0.01%
PRODUCT MONOGRAPH. (ketorolac tromethamine ophthalmic solution 0.45% w/v) Topical Non-steroidal Anti-inflammatory Agent
 PRODUCT MONOGRAPH Pr ACUVAIL (ketorolac tromethamine ophthalmic solution 0.45% w/v) Topical Non-steroidal Anti-inflammatory Agent Allergan Inc. Markham, Ontario L6G 0B5 Date of Preparation: June 13, 2011
PRODUCT MONOGRAPH Pr ACUVAIL (ketorolac tromethamine ophthalmic solution 0.45% w/v) Topical Non-steroidal Anti-inflammatory Agent Allergan Inc. Markham, Ontario L6G 0B5 Date of Preparation: June 13, 2011
Postoperative follow up and treatment after refractive surgery
 Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
Postoperative follow up and treatment after refractive surgery George Kontadakis, MD, MSc, PhD Institute of Vision and Optics and Department of Ophthalmology University of Crete Target of postoperative
AWMSG SECRETARIAT ASSESSMENT REPORT. Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension. Reference number: 1509 FULL SUBMISSION
 AWMSG SECRETARIAT ASSESSMENT REPORT Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension Reference number: 1509 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology
AWMSG SECRETARIAT ASSESSMENT REPORT Nepafenac (Nevanac ) 1 mg/ml eye drops, suspension Reference number: 1509 FULL SUBMISSION This report has been prepared by the All Wales Therapeutics and Toxicology
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NEVANAC 1 mg/ml eye drops, suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of suspension contains 1 mg nepafenac.
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NEVANAC 1 mg/ml eye drops, suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml of suspension contains 1 mg nepafenac.
Citation for published version (APA): van Sorge, A. A. (2002). Reflections on flurbiprofen eyedrops Groningen: s.n.
 University of Groningen Reflections on flurbiprofen eyedrops van Sorge, Adriaan Alastair IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
University of Groningen Reflections on flurbiprofen eyedrops van Sorge, Adriaan Alastair IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
Update on Rhopressa TM QD (netarsudil ophthalmic solution) 0.02% and Roclatan TM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.
 Update on Rhopressa TM QD (netarsudil ophthalmic solution) 0.02% and Roclatan TM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% 1 Important Information Any discussion of the potential use or
Update on Rhopressa TM QD (netarsudil ophthalmic solution) 0.02% and Roclatan TM (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% 1 Important Information Any discussion of the potential use or
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
Innovation In Ophthalmology
 Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
BROMFENAC- bromfenac solution/ drops Hi-Tech Pharmacal Co., Inc
 BROMFENAC- bromfenac solution/ drops Hi-Tech Pharmacal Co., Inc. ---------- HIGHLIGHT S OF PRESCRIBING INFORMAT ION These highlights do not include all the information needed to use Bromfenac Ophthalmic
BROMFENAC- bromfenac solution/ drops Hi-Tech Pharmacal Co., Inc. ---------- HIGHLIGHT S OF PRESCRIBING INFORMAT ION These highlights do not include all the information needed to use Bromfenac Ophthalmic
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
Charles C. Wykoff MD PhD Rahul N. Khurana MD
 HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
HDWallpapers Suprachoroidal Triamcinolone Acetonide with & without Intravitreal Aflibercept for DME: Results of the 6 Month Prospective Phase 1/2 Hulk trial Blanton Eye Institute Charles C. Wykoff MD PhD
Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac)
 EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
EMA/368070/2015 Summary of the risk management plan (RMP) for Omidria (phenylephrine / ketorolac) This is a summary of the risk management plan (RMP) for Omidria, which details the measures to be taken
CONTRAINDICATIONS Active ocular infections (4).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXTENZA safely and effectively. See full prescribing information for DEXTENZA. DEXTENZA (dexamethasone
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXTENZA safely and effectively. See full prescribing information for DEXTENZA. DEXTENZA (dexamethasone
Vankadara Naga Suresh * 1, Rama Vadapalli 2. Online Access and Article Informtaion. Original Research Article
 Original Research Article Effect of Prophylactic Bromfenac 0.07% on Cystoid Macular Edema Assessed using Optical Coherence Tomography Quantification of Total Macular Volume after Cataract Surgery Vankadara
Original Research Article Effect of Prophylactic Bromfenac 0.07% on Cystoid Macular Edema Assessed using Optical Coherence Tomography Quantification of Total Macular Volume after Cataract Surgery Vankadara
Ophthalmic Antihistamine Step Therapy Program Summary
 Ophthalmic Antihistamine Step Therapy Program Summary FDA APPROVED INDICATIONS AND DOSAGE 1-8 Drug FDA Indication(s) Administration and Dosing Bepreve Treatment of itching associated Instill one drop into
Ophthalmic Antihistamine Step Therapy Program Summary FDA APPROVED INDICATIONS AND DOSAGE 1-8 Drug FDA Indication(s) Administration and Dosing Bepreve Treatment of itching associated Instill one drop into
Chemical Names: Prednisolone acetate: 11ß,17,21-Trihydroxypregna-1,4-diene-3,20-dione 21-acetate.
 PRED-G (gentamicin and prednisolone acetate ophthalmic suspension, USP) 0.3%/1% sterile DESCRIPTION PRED-G sterile ophthalmic suspension is a topical anti-inflammatory/anti-infective combination product
PRED-G (gentamicin and prednisolone acetate ophthalmic suspension, USP) 0.3%/1% sterile DESCRIPTION PRED-G sterile ophthalmic suspension is a topical anti-inflammatory/anti-infective combination product
Cataract Surgery: The Importance of NSAIDS AFTER. Considerations on penetration, efficacy, increasing pharmacy fills and decreasing callbacks
 Supplement to SEPTEMBER 25, 2015 The Importance of NSAIDS AFTER Cataract Surgery: Considerations on penetration, efficacy, increasing pharmacy fills and decreasing callbacks 2015 0000 Shutterstcok Copyright
Supplement to SEPTEMBER 25, 2015 The Importance of NSAIDS AFTER Cataract Surgery: Considerations on penetration, efficacy, increasing pharmacy fills and decreasing callbacks 2015 0000 Shutterstcok Copyright
Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults
 Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults First Lower Dose NSAID Using SoluMatrix Fine Particle Technology to be Reviewed
Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults First Lower Dose NSAID Using SoluMatrix Fine Particle Technology to be Reviewed
PRECISION PROGRAM. Injection Technique Quick-Reference Guide. Companion booklet for the Video Guide to Injection Technique
 Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
Injection Technique Quick-Reference Guide PRECISION PROGRAM Companion booklet for the Video Guide to Injection Technique Available at www.ozurdexprecisionprogram.com Provides step-by-step directions with
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain
 TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
Study Number CAIN457C2302 (core study) and CAIN457C2302E1 (extension study)
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Secukinumab Therapeutic Area of Trial Uveitis Approved Indication Investigational Study Number CC2302 (core study) and CC2302E1
PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile
 PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile PRED-G sterile ophthalmic ointment is a topical anti-inflammatory/anti-infective combination product for ophthalmic
PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile PRED-G sterile ophthalmic ointment is a topical anti-inflammatory/anti-infective combination product for ophthalmic
Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use
 Int Ophthalmol (2012) 32:507 517 DOI 10.1007/s10792-012-9589-2 REVIEW Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use Michael Amon Massimo
Int Ophthalmol (2012) 32:507 517 DOI 10.1007/s10792-012-9589-2 REVIEW Loteprednol etabonate ophthalmic suspension 0.5 %: efficacy and safety for postoperative anti-inflammatory use Michael Amon Massimo
NEVANAC (Nepafenac) Ophthalmic Suspension 0.1% w/v
 PRODUCT MONOGRAPH Pr NEVANAC (Nepafenac) Ophthalmic Suspension 0.1% w/v Nonsteroidal Anti-Inflammatory Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date
PRODUCT MONOGRAPH Pr NEVANAC (Nepafenac) Ophthalmic Suspension 0.1% w/v Nonsteroidal Anti-Inflammatory Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date
Elements for a public summary. Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Glaucoma is an eye disease that can result in damage to the optic nerve and loss of vision (blindness). It is the major cause
VI.2 VI.2.1 Elements for a public summary Overview of disease epidemiology Glaucoma is an eye disease that can result in damage to the optic nerve and loss of vision (blindness). It is the major cause
For last half century corticosteroids are used for ocular inflammation. They are used to suppress ocular inflammation in trauma, uveitis as well as
 The Professional Medical Journal DOI: 10.17957/TPMJ/17.3940 1. MBBS, DO, FCPS (Ophthalmology) Eye Specialist PAF Base Hospital Rafiqui Shorkot. 2. MBBS, DO, MCPS, FCPS (Ophthalmology) Classified Eye Specialist,
The Professional Medical Journal DOI: 10.17957/TPMJ/17.3940 1. MBBS, DO, FCPS (Ophthalmology) Eye Specialist PAF Base Hospital Rafiqui Shorkot. 2. MBBS, DO, MCPS, FCPS (Ophthalmology) Classified Eye Specialist,
ILEVRO (Nepafenac) Ophthalmic Suspension 0.3% w/v
 PRODUCT MONOGRAPH Pr ILEVRO (Nepafenac) Ophthalmic Suspension 0.3% w/v Nonsteroidal Anti-Inflammatory Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date
PRODUCT MONOGRAPH Pr ILEVRO (Nepafenac) Ophthalmic Suspension 0.3% w/v Nonsteroidal Anti-Inflammatory Novartis Pharmaceuticals Canada Inc. 385 Bouchard Blvd. Dorval, Quebec H9S 1A9 www.novartis.ca Date
ROCKLATAN (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005%, for topical ophthalmic use Initial U.S. Approval: 2019
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ROCKLATAN safely and effectively. See full prescribing information for ROCKLATAN. ROCKLATAN (netarsudil
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ROCKLATAN safely and effectively. See full prescribing information for ROCKLATAN. ROCKLATAN (netarsudil
William F. Mieler, MD University of Illinois at Chicago Chicago, IL
 William F. Mieler, MD University of Illinois at Chicago Chicago, IL Advisory Board Genentech Data and Safety Monitoring Committee ThromboGenics (in the past) Acucela Corticosteroids and NSAIDs are the
William F. Mieler, MD University of Illinois at Chicago Chicago, IL Advisory Board Genentech Data and Safety Monitoring Committee ThromboGenics (in the past) Acucela Corticosteroids and NSAIDs are the
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
Aerie Pharmaceuticals, Inc. Additional Rhopressa Rocket 1 Analyses and Path Forward
 Aerie Pharmaceuticals, Inc. Additional Rhopressa Rocket 1 Analyses and Path Forward May 7, 2015 1 Important Information Any discussion of the potential use or expected success of our product candidates
Aerie Pharmaceuticals, Inc. Additional Rhopressa Rocket 1 Analyses and Path Forward May 7, 2015 1 Important Information Any discussion of the potential use or expected success of our product candidates
Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery
 Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery David F. Chang, MD, 1 Ivan H. Garcia, MD, 2 John D. Hunkeler, MD, 3 Thomas Minas, MD 4 Objective: To evaluate the safety
Phase II Results of an Intraocular Steroid Delivery System for Cataract Surgery David F. Chang, MD, 1 Ivan H. Garcia, MD, 2 John D. Hunkeler, MD, 3 Thomas Minas, MD 4 Objective: To evaluate the safety
Combining strategies
 Supplement to OCULAR SURGERY US EDITION NEWS JULY 25, 2016 Combining strategies in the counseling and care of patients with cataracts This OCULAR SURGERY NEWS supplement is produced by SLACK Incorporated
Supplement to OCULAR SURGERY US EDITION NEWS JULY 25, 2016 Combining strategies in the counseling and care of patients with cataracts This OCULAR SURGERY NEWS supplement is produced by SLACK Incorporated
New Treatments Following Cataract Surgery October 23, 2018
 Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders New Treatments Following Cataract Surgery October 23, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE
Delivering Innovative Ophthalmic Products to Patients with Serious Eye Disorders New Treatments Following Cataract Surgery October 23, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
Balanced Analgesia With NSAIDS and Coxibs. Raymond S. Sinatra MD, Ph.D
 Balanced Analgesia With NSAIDS and Coxibs Raymond S. Sinatra MD, Ph.D Prostaglandins and Pain The primary noxious mediator released from damaged tissue is prostaglandin (PG) PG is responsible for nociceptor
Balanced Analgesia With NSAIDS and Coxibs Raymond S. Sinatra MD, Ph.D Prostaglandins and Pain The primary noxious mediator released from damaged tissue is prostaglandin (PG) PG is responsible for nociceptor
1165 Creditstone Road, Unit #1 June 16, 2015 Vaughan, Ontario L4K 4N7
 PRODUCT MONOGRAPH KETOROLAC Ketorolac Tromethamine Ophthalmic Solution 0.5% w/v with benzalkonium chloride 0.01% w/v as preservative Topical Non-Steroidal Anti-Inflammatory Agent AA PHARMA INC. DATE OF
PRODUCT MONOGRAPH KETOROLAC Ketorolac Tromethamine Ophthalmic Solution 0.5% w/v with benzalkonium chloride 0.01% w/v as preservative Topical Non-Steroidal Anti-Inflammatory Agent AA PHARMA INC. DATE OF
D90 (27/10/2005) Final SmPC NL/H/653/01
 1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
Roclatan TM Mercury 1 Phase 3 12-month Topline Results. For Investor Use
 Roclatan TM Mercury 1 Phase 3 12-month Topline Results For Investor Use 1 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product
Roclatan TM Mercury 1 Phase 3 12-month Topline Results For Investor Use 1 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
CHARTING THE NEW COURSE FOR MIGS
 CHARTING THE NEW COURSE FOR MIGS SEE WHAT S ON THE HORIZON CyPass Micro-Stent the next wave in micro-invasive glaucoma surgery. MICRO-INVASIVE GLAUCOMA SURGERY (MIGS) OFFERS A REVOLUTIONARY APPROACH TO
CHARTING THE NEW COURSE FOR MIGS SEE WHAT S ON THE HORIZON CyPass Micro-Stent the next wave in micro-invasive glaucoma surgery. MICRO-INVASIVE GLAUCOMA SURGERY (MIGS) OFFERS A REVOLUTIONARY APPROACH TO
Building a Major Ophthalmic Pharmaceutical Company. Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015
 Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015 1 Important Information Any discussion of the potential use or expected success of our product
Building a Major Ophthalmic Pharmaceutical Company Aerie Pharmaceuticals, Inc. Company Overview June 2-3, 2015 1 Important Information Any discussion of the potential use or expected success of our product
Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1
 Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1 Fast onset of pain relief with 7% reduction in visual analog scale (VAS) scores
Effective pain management begins with OFIRMEV (acetaminophen) injection FIRST Proven efficacy with rapid reduction in pain 1 Fast onset of pain relief with 7% reduction in visual analog scale (VAS) scores
Levocetirizine dihydrochloride
 INSERT TEXT UAP Levocetirizine dihydrochloride Allerzet 5 mg Tablet Antihistamine FORMULATION Each film-coated tablet contains: Levocetirizine dihydrochloride.. 5 mg PRODUCT DESCRIPTION Levocetirine 5
INSERT TEXT UAP Levocetirizine dihydrochloride Allerzet 5 mg Tablet Antihistamine FORMULATION Each film-coated tablet contains: Levocetirizine dihydrochloride.. 5 mg PRODUCT DESCRIPTION Levocetirine 5
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only
 Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
POST-CATARACT SURGERY TREATMENT PULL-THROUGH PROTOCOLS AND OUTCOMES WHERE DOES PROLENSA FIT WITHIN THE TREATMENT PARADIGM?
 POST-CATARACT SURGERY TREATMENT PULL-THROUGH PROTOCOLS AND OUTCOMES WHERE DOES PROLENSA FIT WITHIN THE TREATMENT PARADIGM? SPONSORED BY BRINGING THE DISCUSSION DIRECTLY TO YOU This supplement captures
POST-CATARACT SURGERY TREATMENT PULL-THROUGH PROTOCOLS AND OUTCOMES WHERE DOES PROLENSA FIT WITHIN THE TREATMENT PARADIGM? SPONSORED BY BRINGING THE DISCUSSION DIRECTLY TO YOU This supplement captures
Page 1 RED EYES. conjunctivitis keratitis episcleritis / scleritis. Frank Larkin Moorfields Eye Hospital. acute glaucoma anterior uveitis
 The RED EYE and ALLERGIC EYE DISEASE DIAGNOSIS & MANAGEMENT Frank Larkin Moorfields Eye Hospital RED EYES conjunctivitis keratitis episcleritis / scleritis acute glaucoma anterior uveitis post-op. / trauma
The RED EYE and ALLERGIC EYE DISEASE DIAGNOSIS & MANAGEMENT Frank Larkin Moorfields Eye Hospital RED EYES conjunctivitis keratitis episcleritis / scleritis acute glaucoma anterior uveitis post-op. / trauma
Abela-Formanek, C., M. Amon, et al. (2011). "Biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes wit
 Abela-Formanek, C., M. Amon, et al. (2011). "Biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with uveitis having cataract surgery: Long-term follow-up."
Abela-Formanek, C., M. Amon, et al. (2011). "Biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in eyes with uveitis having cataract surgery: Long-term follow-up."
Cataract surgery is a hugely important means of preventing poor-quality vision or vision loss, especially in the elderly and people
 Nepafenac in the Treatment of Ocular Inflammation Following Cataract Surgery (Pseudophakic Macular Oedema) an Update Hadi Kjærbo Scandinavian Eye Center, Hellerup, Denmark; Department of Ophthalmology,
Nepafenac in the Treatment of Ocular Inflammation Following Cataract Surgery (Pseudophakic Macular Oedema) an Update Hadi Kjærbo Scandinavian Eye Center, Hellerup, Denmark; Department of Ophthalmology,
You can C-ME after Uveitis
 You can C-ME after Uveitis Abstract: Approximately 50% of uveitis patients will present with vision loss secondary to cystoid macular edema[1]. Two patients with uveitis present with a constant decrease
You can C-ME after Uveitis Abstract: Approximately 50% of uveitis patients will present with vision loss secondary to cystoid macular edema[1]. Two patients with uveitis present with a constant decrease
