Horizon Scanning Centre May Mepolizumab for severe refractory eosinophilic asthma first line SUMMARY NIHR HSC ID: 1643
|
|
|
- Rolf Henry
- 5 years ago
- Views:
Transcription
1 Horizon Scanning Centre May 2014 Mepolizumab for severe refractory eosinophilic asthma first line SUMMARY NIHR HSC ID: 1643 This briefing is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement on the safety, efficacy or effectiveness of the health technology covered and should not be used for commercial purposes or commissioning without additional information. Mepolizumab is intended to be used for the treatment of patients with severe refractory eosinophilic asthma that are on step 5 of the BTS/SIGN guidelines. If licensed, mepolizumab will offer an additional treatment option which targets the immune cells involved in airway inflammation. Mepolizumab is an anti-interleukin-5 humanised IgG 1 monoclonal antibody. Interleukin-5 stimulates the production, activation and maturation of eosinophils, a type of white blood cell involved in the body s defences. Mepolizumab causes a sustained reduction in the numbers of circulating eosinophils and may be effective in conditions characterised by eosinophilia, including eosinophilic asthma, where eosinophils accumulate in lung tissue producing airway inflammation, which is closely related to the risk of severe asthma exacerbations. Reduction in eosinophils may therefore lower the risk of severe asthma exacerbations and improve patient quality of life. Mepolizumab does not currently have marketing authorisation in the EU for any indication. 5.4 million people in the UK are receiving treatment for asthma; the equivalent of 1 in 12 adults or 1 in 11 children. The Health Survey for England (2010) estimated the lifetime prevalence of diagnosed asthma to be 17% in women and 16% in men. Approximately 5% of asthma sufferers are described as therapy resistant and are unable to get good control of their asthma despite using high levels of anti-asthma medicines. Mortality from asthma is rare in England and Wales, with 1,099 asthma-related deaths reported in The management of asthma aims to control the disease while minimising adverse reactions to treatment. The SIGN/British Thoracic Society (BTS) guideline recommends a stepwise approach where treatment is started at the step most appropriate to the initial severity of the asthma with the aim of achieving early control of symptoms and optimising respiratory function. Control is maintained by stepping up treatment as necessary and stepping down when control is good within the 5 steps. Mepolizumab in addition to standard care is currently in two phase III trials comparing its effect against placebo on asthma exacerbations, the need for oral corticosteroids, and the frequency of adverse events. These trials are expected to complete in the first half of This briefing presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health. NIHR Horizon Scanning Centre, University of Birmingham nihrhsc@contacts.bham.ac.uk Web:
2 TARGET GROUP Eosinophilic asthma: refractory; severe; first line; step 5 of the BTS/SIGN guidelines. TECHNOLOGY DESCRIPTION Mepolizumab (SB ) is an anti-interleukin-5 humanised IgG 1 monoclonal antibody. Interleukin-5 stimulates the production, activation and maturation of eosinophils, a type of white blood cell involved in the body s defence against parasites, allergic responses, tissue inflammation and immunity. Mepolizumab causes a sustained reduction in the numbers of circulating eosinophils and may be effective in conditions characterised by eosinophilia. In eosinophilic asthma, eosinophils accumulate in lung tissue producing airway inflammation, which is closely related to the risk of severe asthma exacerbations 1. A reduction in eosinophils may therefore lower the risk of severe asthma exacerbations and improve patient quality of life. In the phase III clinical trial mepolizumab is administered subcutaneously (SC) every 4 weeks at 100mg in addition to standard asthma care. Mepolizumab does not currently have Marketing Authorisation in the EU for any indication. Mepolizumab is also in phase III clinical trials for relapsing or refractory eosinophilic granulomatosis with polyangiitis (EGPA/Churg-Strauss syndrome) and for severe chronic obstructive pulmonary disease. Mepolizumab is also in phase II clinical trials for severe bilateral nasal polyposis. INNOVATION and/or ADVANTAGES If licensed, mepolizumab will offer an additional treatment option which targets the immune cells involved in airway inflammation. DEVELOPER GlaxoSmithKline. AVAILABILITY, LAUNCH OR MARKETING In phase III clinical trials. PATIENT GROUP BACKGROUND Asthma is a chronic disorder of the airways caused primarily by inflammation and constriction of the smooth muscle in airway walls (bronchoconstriction) 2. It is characterised by airflow obstruction and increased responsiveness of the airways to various stimuli 1. Symptoms include recurring episodes of wheezing, breathlessness, chest tightness, sputum production and coughing (particularly at night) 3, and vary in frequency and severity from 2
3 intermittent and mild, to frequent and severe 2. Allergic and non-allergic forms of asthma are described, although their inflammatory response to stimuli is the same a. In general, allergic asthma results from excess immunoglobulin E (IgE) produced in response to environmental allergens such as house dust mites, pollen and moulds. Non-allergic asthma can be triggered by factors such as anxiety, stress, exercise, cold air, smoke and infection 3. Eosinophilic asthma is characterised by high expression of Th2 cytokines, interleukin-5 and interleukin-13, and is generally responsive to inhaled corticosteroid (ICS) treatment in patients with milder asthma; however in some patients with severe asthma the eosinophilic inflammation persists despite high doses of ICS and systemic corticosteroids. Asthma usually develops in childhood but may start at any age. There is no cure for asthma, although patients may experience long periods of remission. Poorly controlled asthma can have a significant impact on the quality of life of the affected person and their family, with symptoms leading to fatigue and absence from school or work, though there may be variation in an individual s perception of the symptoms and how they adapt to the condition over time 2,4. Psychological problems, which can include stress, anxiety and depression, are up to 6 times more common in asthma sufferers than in the general population, and are particularly common in people with severe and difficult-to-control asthma 3. Clinical measures such as lung function may not correlate well with an individual s quality of life, but if asthma is well controlled, near maximal scores on quality of life instruments can be achieved 2. The diagnosis of asthma is a clinical one; there is no standardised definition of the type, severity or frequency of symptoms, nor of the findings on investigation 5. However, Asthma UK defines severe asthma as including those with therapy-resistant severe asthma and those who are not therapy-resistant but who have poorly managed difficult asthma 6. NHS or GOVERNMENT PRIORITY AREA This topic is relevant to: NHS England. 2013/14 NHS Standard Contract For Respiratory: Severe Asthma (Adult). A14/S/b. NHS England. 2013/14 NHS Standard Contract For Paediatric Medicine: Respiratory. E03/S/g. An outcome strategy for chronic obstructive pulmonary disease (COPD) and asthma in England (2011). CLINICAL NEED and BURDEN OF DISEASE 5.4 million people in the UK are receiving treatment for asthma; the equivalent of 1 in 12 adults or 1 in 11 children 7. Of these, it is estimated that 3.0 million are female and 2.4 million are male. The Health Survey for England (2010) estimated the lifetime prevalence of diagnosed asthma to be 17% in women and 16% in men 8. Approximately 5% of asthma sufferers are described as therapy resistant and are unable to get good control of their asthma despite using high levels of anti-asthma medicines 6. In , there were 64,240 hospital admissions for asthma (ICD-10 J45) in England, resulting in 148,987 bed days and 84,689 finished consultant episodes 9. Mortality from asthma is rare in England and Wales, with 1,099 asthma-related deaths (316 males, 783 females) reported in However, expert opinion suggests that it still accounts for nearly three potentially preventable deaths, sometimes in young persons, every day 11. a Expert personal opinion. 3
4 In 2013, there were 18,832,830 inhaled corticosteroid b prescription items dispensed in the community in England 12. Due to the lack of a standard definition for severe asthma the population likely to be eligible to receive mepolizumab could not be estimated from available routine published sources. PATIENT PATHWAY RELEVANT GUIDANCE NICE Guidance NICE technology appraisal. Omalizumab for treating severe persistent allergic asthma (review of technology appraisal guidance 133 and 201) (TA278). April NICE technology appraisal. Inhaled corticosteroids for the treatment of chronic asthma in children aged 12 years and over (TA138). March NICE technology appraisal. Omalizumab for severe persistent allergic asthma (TA133). November NICE quality standard. Quality standard for asthma (QS25). February NICE diagnostics guidance. Measuring fractional exhaled nitric oxide concentration in asthma: NIOX MINO, NIOX VERO and NObreath (DG12). April NICE interventional procedure guidance. Bronchial thermoplasty for severe asthma (IPG419). January NICE Clinical Knowledge Summaries: Asthma. June Other Guidance Scottish Intercollegiate Guidelines Network and British Thoracic Society. British guideline on the management of asthma: A national clinical guide. May 2008 (revised January 2012) 13. Scottish Intercollegiate Guidelines Network. Managing asthma in adults: a booklet for patients and their families and carers. December NHS quality improvement (Scotland) service guidance. Asthma services for children and young people: clinical standards. March CURRENT TREATMENT OPTIONS The management of asthma aims to control the disease while minimising adverse reactions to treatment; good control is characterised by no daytime symptoms, no night-time awakening due to asthma, normal lung function, no need for rescue medication, no exacerbations, and no limitations on activity including exercise 3,4. The SIGN/British Thoracic Society (BTS) guideline recommends a stepwise approach to treatment 13. Treatment is started at the step most appropriate to the initial severity of the asthma with the aim of achieving early control of symptoms and optimising respiratory function. Control is maintained by stepping up treatment as necessary and stepping down when control is good 3,4. b Based on BNF therapeutic class corticosteroids (respiratory). 4
5 The following stepwise and additive approach is recommended 13 : Step 1 - inhaled short-acting beta 2 -agonist. Step 2 - inhaled corticosteroid (ICS) µg/day (adults) or other preventer drug if inhaled steroid cannot be used. Step 3 - inhaled long-acting beta 2 -agonist (LABA). Benefit from LABA but inadequate control increase ICS dose to 800µg/day (adults). No response to LABA - stop use and increase ICS dose to 800µg/day (adults). Control still inadequate - consider leukotriene receptor agonist or modified release theophylline. Step 4 - increase ICS up to 2,000µg/day (adults) or addition of a fourth drug, e.g. leukotriene receptor antagonist, modified release theophylline, beta-2 agonist tablet (adults). Step 5 - daily oral, corticosteroids (lowest dose) and ICS at 2,000µg/day (adults). Consider other treatments to minimise the use of oral corticosteroids. NICE guidelines also recommend the use of omalizumab for severe persistent allergic IgEmediated asthma 3. Expert opinion 11 suggests that step 5 in the stepwise approach is the most difficult to manage, where the main aim of treatment is to control asthma using the lowest possible doses of medication. Other essential steps to treat asthma include: identification and elimination of disease triggers, identification and management of co-morbidities, regular scrutiny of inhaler technique and the provision of action plans and emergency passports c. EFFICACY and SAFETY A meta-analysis 16 of seven randomised placebo-controlled trials d which investigated the efficacy of mepolizumab in patients with eosinophilic asthma found that mepolizumab was associated with significantly decreased exacerbation risk compared to placebo (odds ratio 0.30, 95%CI 0.13 to 0.67, p=0.004) and significant improvement in scores on the Asthma Quality of Life Questionnaire (AQLQ) (mean difference from placebo 0.26, 95%CI 0.03 to 0.49, p=0.03). No significant differences in lung function measures between mepolizumab and placebo groups were observed, including FEV 1, PEF, or histamine PC 20. Trial Sponsor Status Source of information Location Design Participants Schedule NCT , MEA115661, EudraCT Number: ; mepolizumab in addition to standard care; phase III extension. GlaxoSmithKline. Ongoing. Trial registry 17. EU (incl UK), USA, Canada and other countries. Uncontrolled, single arm. n=660 (planned); aged 12 yrs; severe, refractory asthma being treated with a controller medication for 12 weeks; history of eosinophilic inflammation; completion of treatment during MEA (NCT ) or MEA (NCT ) studies; not current smoker; without hypersensitivity to study medication, significant change in health status, a study related SAE, significant laboratory/clinical abnormality, or significant new illness. Mepolizumab 100mg administered SC every 4 weeks for 12 mths, in addition to standard care. c Expert personal opinion d Including trials NCT and NCT
6 Follow-up Primary outcome/s Secondary outcome/s Expected reporting date Active treatment for 12 mths, follow-up 64 weeks. Adverse events (AEs), including local site reactions; withdrawals due to AEs; hospitalisations due to AEs (including asthma exacerbations); 12-lead ECG; vital signs; clinical laboratory measurements. Frequency of positive anti-mepolizumab binding antibodies and neutralising antibodies; annualised rate of exacerbations; asthma control questionnaire score (ACQ); Forced Expiratory Volume in 1 second (FEV1) measured by clinic spirometry; withdrawals due to lack of efficacy. Study completion date reported as April Trial NCT , MEA115666, EudraCT Number: ; mepolizumab in addition to standard care; phase III extension. DREAM, NCT , MEA112997, EudraCT Number: ; mepolizumab vs placebo; phase IIb/III. NCT , MEA115588, EudraCT Number: ; mepolizumab vs placebo, both in addition to standard care; phase III. Sponsor GlaxoSmithKline. GlaxoSmithKline. GlaxoSmithKline. Status Ongoing. Published. Completed but unpublished. Source of Trial registry 18. Publication 19, trial Trial registry 21. information registry 20. Location EU (incl UK), USA, Canada and other countries. EU (incl UK), USA, Canada and other countries. EU (incl UK), USA, Canada and other countries. Design Uncontrolled, single arm. Randomised, placebocontrolled. Randomised, placebocontrolled. Participants n=362 (screened) 347 (dosed) e ; aged 18 yrs; MEA (NCT ) study participation (receiving at least 2 doses); positive risk:benefit ratio if subject received mepolizumab in prior study; treated with a controller medication for 12 weeks; not received omalizumab in past 130 days; not current smoker; without hypersensitivity to study medication, significant change in health status, a study related SAE, significant laboratory/clinical abnormality, or significant new illness. n=621; aged yrs; severe refractory asthma; minimum weight 45kg; high dose ICS (i.e. 880mg/day fluticasone propionate or equivalent daily) for 12 mths; use of additional controller medication for 12 mths; persistent airflow obstruction (prebronchodilator FEV1<80% predicted at visit 1 or peak flow diurnal variability of >20% on 3 or more days during run-in); airway inflammation likely to be eosinophilic in nature (demonstrated by: peripheral blood eosinophils 300/mL, sputum eosinophils 3%, exhaled nitric oxide 50ppb or prompt deterioration of asthma control following a 25% reduction in regular maintenance dose of inhaled or oral corticosteroids); history of n=580 (planned); aged 12 yrs; severe, uncontrolled refractory asthma; minimum weight 45kg; requirement for high dose ICS 12 mths with or without maintenance oral corticosteroids; current treatment with an additional controller medication for 3 mths or a documented failure within 12 mths of an additional controller medication used for 3 mths; prior documentation of eosinophilic asthma or high likelihood of eosinophilic asthma (elevated peripheral blood eosinophil count 300 cell/µl in previous 12 mths or elevated peripheral blood eosinophil count of 150 cells/µl at visit 1 related to asthma); prebronchodilator FEV1<80% (in subjects 18 yrs), prebronchodilator FEV1<90% e Reported by the company. 6
7 Schedule Mepolizumab 100mg administered SC every 4 weeks in addition to standard care. 2 exacerbations in previous 12 mths; QTc<450msec or QTc<480msec for patients with bundle branch block; normal liver function tests. Patients excluded if current smoker, past smoking history >10 pack-year history, parasitic infection in 6 mths before study. Randomised to mepolizumab 750mg IV every 4 weeks; or mepolizumab 250mg IV every 4 weeks; or mepolizumab 75mg IV every 4 weeks; or placebo saline IV; all in addition to high doses of standard asthma medication. or FEV1:FVC ratio <0.8 (in subjects yrs); history of 2 exacerbations requiring treatment with systemic corticosteroids. Patients excluded if current smoker, past smoking history >10 pack-year history, presence of other clinically important lung condition, liver disease, severe or clinically significant cardiovascular disease, other eosinophilic disease, QTc(F) 450msec or QTc(F) 480msec for subjects with bundle branch block, immunodeficiency, received omalizumab within 130 days. Randomised to mepolizumab 75mg IV and placebo SC every 4 weeks for 28 weeks; or mepolizumab 100mg SC and placebo IV every 4 weeks for 28 weeks; or matching placebo IV and placebo SC every 4 weeks for 28 weeks. Follow-up Primary outcome/s Secondary outcome/s Active treatment until marketed (approximately mths) f, follow-up 12 weeks after final dose. AEs ECG parameters (QTcF and QT); clinical laboratory tests; vital signs; annualised rate of exacerbations; ACQ score; forced vital capacity (FVC); FEV1; immunogenicity (positive neutralising antibody); withdrawals due to lack of efficacy or AEs; hospitalisations due to AEs (including asthma exacerbations); frequency of immune reactions. Active treatment for 52 weeks (13 infusions), follow-up 8 and 24 weeks. Clinically significant asthma exacerbations. Time to first clinically significant asthma exacerbation requiring oral or systemic corticosteroids, hospitalisation and or emergency department (ED) visit; frequency of exacerbations requiring hospitalisation or ED visit; time to first exacerbation requiring hospitalisation or ED visit; frequency of investigator-defined exacerbations; time to first investigator-defined exacerbation; mean change in clinic pre-bronchodilator FEV1; mean change in post- bronchodilator FEV1; Active treatment for 32 weeks, follow-up 40 weeks. Clinically significant asthma exacerbations. Frequency of clinically significant exacerbations requiring hospitalisation or ED visits; lung function; quality of life (St George s respiratory questionnaire). f Reported by the company. 7
8 mean change in ACQ score. Key results - Compared with placebo, reduction in number of clinically significant exacerbations per patient per year, 48% (95% CI 31-61%) in the 75mg arm, 39% (95% CI 19-54) in the 250mg arm, and 52% (95% CI 36-64%) in the 750mg arm. Adverse effects (AEs) Expected reporting date - For the placebo, 75mg, 250mg, 750mg arms respectively, the most frequently reported AEs included: headache (17%, 21%, 21%, 21%); nasopharyngitis (15%, 22%, 22%, 19%); drugrelated adverse events included; infusion-related reaction (6%, 5%, 8%, 12%) and hypersensitivity (2%, 0%, <1%, 1%). Study completion date reported as June Compared with placebo, both treatment arms showed statistically significant reductions in the number of clinically significant exacerbations (75mg IV group, 47% reduction, p<0.001; 100mg SC group, 53% reduction, p<0.001) f. The most common AEs reported across the groups included: nasopharyngitis, headache, upper respiratory tract infection and asthma. For mepolizumab 75mg IV, mepolizumab 100mg and placebo respectively: frequency of AEs was, 84%, 78%, and 83%; frequency of serious AEs was 7%, 8%, and 14%. - Study completion date reported as January Trial NCT , MEA115575, EudraCT Number: ; mepolizumab vs placebo; phase III. NCT , RP#02/2115, SB /046, 9427-F C; mepolizumab vs placebo, both in addition to prednisolone; phase II. Sponsor GlaxoSmithKline. St.Joseph s Healthcare Hamilton. Status Completed but unpublished. Published. Source of Trial registry 22. Publication 23, trial registry 24. information Location EU (incl UK), USA, Canada, Australia Canada. and Mexico. Design Randomised, placebo-controlled. Randomised, placebo-controlled. f Reported by the company. 8
9 Participants Schedule Follow-up Primary outcome/s n=135 (planned); aged 12 yrs; severe, refractory asthma; requirement for regular treatment with maintenance systemic corticosteroids within 6 mths and stable oral corticosteroid dose for 4 weeks prior to visit 1; requirement for regular treatment with high dose ICS within 6 mths (in subjects 18 yrs, dose 880µg/day fluticasone propionate or equivalent; in subjects aged 12 to 17 ICS dose 440µg/day or equivalent or highest approved maintenance dose of ICS/LABA combination preparations); current treatment with an additional controller medication for 3 mths or a documented failure within 12 mths of an additional controller medication used for 3 mths; prior documentation of eosinophilic asthma or high likelihood of eosinophilic asthma (elevated peripheral blood eosinophil count 300 cell/µl in previous 12 mths or elevated peripheral blood eosinophil count of 150 cells/µl at visit 1 related to asthma); persistent airway obstruction (pre-bronchodilator FEV1<80% predicted); airway reversibility, hyperresponsiveness or airway variability. Patients excluded if current smoker, past smoking >10 pack-year history, presence of other clinically important lung condition, liver disease, severe or clinically significant cardiovascular disease, other concurrent medical conditions, any eosinophilic disease; QTc(F) 450msec or QTc(F) 480msec for subjects with bundle branch block, immunodeficiency, received omaluzumab within 130 days. Randomised to mepolizumab 100mg SC every 4 weeks for 20 weeks; or placebo SC administered every 4 weeks for 20 weeks. Active treatment for 20 weeks, follow-up 32 weeks. Reduction of oral corticosteroid (OCS) dose during weeks 20 to 24. n=20; aged yrs; asthma requiring treatment with oral prednisolone in addition to high dose ICS; sputum eosinophilia; abnormal FEV1 after withholding bronchodilators, before and after inhaled salbutamol (200mg) and methacholine PC20; same corticosteroid dose for 4 weeks. Patients excluded if exposure to a relevant seasonal environmental allergen known to worsen asthma during study period, respiratory tract infection within 4 weeks, or clinical exacerbation requiring increased prednisolone treatment within 4 weeks. Randomised to mepolizumab 750mg IV in weeks 2, 6,10,14 and 18; or matching IV placebo; both with prednisolone, tailing dose reduced by 5mg at weeks 6,10,14,18 and 22. Active treatment for 18 weeks, follow-up for 8 weeks up to week 26. Asthma exacerbations, mean reduction in the dose of prednisone as a percentage of maximum possible reduction (as measured by the Juniper ACQ). 9
10 Secondary outcome/s Key results Adverse effects (AEs) Expected reporting date 50% reduction in daily OCS dose; 5.0mg reduction in daily OCS dose; total reduction of OCS dose; median percentage reduction in OCS dose; safety (measured using AEs, clinical chemistry, haematological parameters, blood glucose, body weight, glycosylated haemoglobin and vital signs); AEs associated with chronic OCS use; mean and maximum change in QTc(F) and QTc(B). Compared to placebo, mepolizumab 100mg SC group had greater reductions in maintenance OCS (p=0.008) f. AEs were reportedly similar across treatment groups. The most common reported AEs in the two treatment groups were headache, nasopharyngitis, bronchitis, sinusitis, fatigue and asthma. Frequency of AEs was 92% in the placebo and 84% in the mepolizumab treatment group. Frequency of serious AEs was 18% in the placebo group and 1% in the mepolizumab group. Study completion date reported as December Study phase I - reduction in blood and sputum eosinophils. Study phase II - time to exacerbation; reduction in blood and sputum eosinophils; FEV1 change; symptom score. Study phase III reduction in blood and sputum eosinophils; FEV1 change; symptom score. A statistically significant difference was reported in the number of exacerbations (p=0.008), median time to exacerbation (p=0.003) and mean reduction in prednisolone dose between the mepolizumab group (±SD) (83.8±33.4%) and the placebo group (47.7±40.5%) (p=0.04). Few AEs were noted: in the mepolizumab group one patient reported progressive shortness of breath and another reported aches and tiredness when prednisolone dose was reduced to 2.5mg. - ESTIMATED COST and IMPACT COST The cost of mepolizumab is not yet known. The cost of selected inhaled corticosteroids and other treatments for asthma include: Drug Dose Unit cost 25, Beclometasone dipropionate µg twice daily adjusted to 800µg as necessary. 250µg/metered inhalation (100- dose unit): Budesonide µg twice daily. 100µg/metered inhalation (100- dose unit): Ciclesonide 160µg daily as a single dose. 160µg/metered inhalation (60- dose unit): Fluticasone propionate (Flixotide; Accuhaler) Mometasone fuorate (Asmanex; Twisthaler) µg twice daily. 400µg as a single dose in the evening or in 2 divided doses. 100µg/blister with Accuhaler: µg/metered inhalation (30- dose unit): f Reported by the company. 10
11 Omalizumab (Xolair) Subcutaneous injection. Dose determined by baseline IgE (IU/ml), measured before the start of treatment, and body weight (kg). The maximum recommended dose is 600mg every two weeks 26. A 0.5mL, 150mg/mL (75mg) prefilled syringe costs IMPACT - SPECULATIVE Impact on Patients and Carers Reduced mortality/increased length of survival Other: patients are likely to have to attend specialist centres to receive treatment f. Reduced symptoms or disability No impact identified Impact on Health and Social Care Services Increased use of existing services Re-organisation of existing services Other: patients are likely to have to attend specialist centres to receive treatment f but may also have less unplanned use of services, i.e. through A & E. Decreased use of existing services Need for new services None identified Impact on Costs and Other Resource Use Increased drug treatment costs Other increase in costs: patients are likely to have to attend specialist centres to receive treatment, necessitating day ward bed or chair facilities and appropriate expertise of staff f. Other: uncertain unit cost compared to existing treatments. Reduced drug treatment costs Other reduction in costs: None identified Other Issues Clinical uncertainty or other research question identified: Expert opinion suggests that it is unclear how mepolizumab reduces the acute exacerbations of asthma reported in trials. Studies showing its efficacy, which appears to be limited specifically to reduction of exacerbations perhaps in the face of reduced oral corticosteroid therapy with attendant improvement in quality of life, have been arbitrarily limited to patients above a threshold of blood and sputum eosinophilia. While this may theoretically be appropriate, the lack of comparison with low eosinophil groups inevitably casts doubt on whether this is a mandatory prerequisite for response to mepolizumab which should be incorporated into guidelines f. Expert opinion also notes that the most recent Lancet study suggests that even the lowest effective dosage used (75mg monthly) may be unnecessarily high f. None identified f Expert personal opinion. f Expert personal opinion 11
12 REFERENCES 1 Asthma and Allergy Foundation of America. Eosinophilic Asthma. Accessed 7 May National Institute for Health and Clinical Excellence. Inhaled corticosteroids for the treatment of chronic asthma in children aged 12 years and over. Technology appraisal TA138. London: NICE; March National Institute for Health and Care Excellence. Omalizumab for treating severe persistent allergic asthma (review of technology appraisal guidance 133 and 201). Technology appraisal TA278. London: NICE; April National Institute for Health and Clinical Excellence. Inhaled corticosteroids for the treatment of chronic asthma in children under the age of 12 years and over. Technology appraisal TA131. London: NICE; November National Institute for Health and Clinical Excellence. Guidance on the use of inhaler systems (devices) in children under the age of 5 years with chronic asthma. Technology appraisal TA10. London: NICE; August Asthma UK. Severe Asthma. Accessed 30 March NHS Choices. Asthma; Introduction. Accessed 30 March The Health Survey for England. Respiratory Health: Summary of key findings. Leeds: The NHS Information Centre Health and Social Care Information Centre. Hospital episode statistics, admitted patient care, England : Diagnosis Office for National Statistics. Mortality statistics: Deaths registered in England and Wales (Series DR), Mortality statistics: Deaths registered in 2012 (Series DR) Table Horizon Scanning Centre. Mastinib for severe asthma. September Health and Social Care Information Centre. Prescription cost analysis, England 2013 [NS]. Prescription cost analysis - England 2013: Data Scottish Intercollegiate Guidelines Network and British Thoracic Society. British guideline on the management of asthma: A national clinical guide. Edinburgh: SIGN; May 2008 (revised January 2012). 14 Scottish Intercollegiate Guidelines Network. Managing asthma in adults: a booklet for patients and their families and carers. Edinburgh: SIGN; December NHS Quality Improvement Scotland. Asthma services for children and young people: clinical standards March Liu Y, Zhang S, Li D et al. Efficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: A meta-analysis of randomized placebo-controlled trials. PLOS ONE 2013;8(3):e ClinicalTrials.gov. A study to determine long-term safety of mepolizumab in asthmatic subjects. Accessed 30 March ClinicalTrials.gov. MEA Open-label long term extension safety study of mepolizumab in asthmatic subjects. Accessed 1 May Pavord ID, Korn S, Howarth P et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380: ClinicalTrials.gov. Dose ranging efficacy and safety with mepolizumab in severe asthma (DREAM). Accessed 1 May ClinicalTrials.gov. Efficacy and safety study of mepolizumab adjunctive therapy in subjects with severe uncontrolled refractory asthma. Accessed 6 May ClinicalTrials.gov. Mepolizumab steroid-sparing study in subjects with severe refractory asthma. Accessed 6 May Nair P, Pizzichini MM, Kjarsgaard M et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. New England Journal of Medicine 2009;360: ClinicalTrials.gov. The Prednisone-sparing Effect of Anti-IL-5 Antibody (SB ). Accessed 7 May British National Formulary (BNF). Accessed 7 May
13 26 The electronic Medicines Compendium (emc). Summary of Product Characteristics, Xolair. Novartis Pharmaceuticals UK Ltd. Accessed 7 May
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
On completion of this chapter you should be able to: discuss the stepwise approach to the pharmacological management of asthma in children
 7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
7 Asthma Asthma is a common disease in children and its incidence has been increasing in recent years. Between 10-15% of children have been diagnosed with asthma. It is therefore a condition that pharmacists
(Asthma) Diagnosis, monitoring and chronic asthma management
 Dubai Standards of Care 2018 (Asthma) Diagnosis, monitoring and chronic asthma management Preface Asthma is one of the most common problem dealt with in daily practice. In Dubai, the management of chronic
Dubai Standards of Care 2018 (Asthma) Diagnosis, monitoring and chronic asthma management Preface Asthma is one of the most common problem dealt with in daily practice. In Dubai, the management of chronic
NG80. Asthma: diagnosis, monitoring and chronic asthma management (NG80)
 Asthma: diagnosis, monitoring and chronic asthma management (NG80) NG80 NICE has checked the use of its content in this product and the sponsor has had no influence on the content of this booklet. NICE
Asthma: diagnosis, monitoring and chronic asthma management (NG80) NG80 NICE has checked the use of its content in this product and the sponsor has had no influence on the content of this booklet. NICE
Technology appraisal guidance Published: 24 April 2013 nice.org.uk/guidance/ta278
 Omalizumab for treating severeere persistent allergic asthma Technology appraisal guidance Published: 24 April 2013 nice.org.uk/guidance/ta278 NICE 2018. All rights reserved. Subject to Notice of rights
Omalizumab for treating severeere persistent allergic asthma Technology appraisal guidance Published: 24 April 2013 nice.org.uk/guidance/ta278 NICE 2018. All rights reserved. Subject to Notice of rights
Horizon Scanning Centre November Secukinumab for active and progressive psoriatic arthritis. SUMMARY NIHR HSC ID: 5330
 Horizon Scanning Centre November 2012 Secukinumab for active and progressive psoriatic arthritis. SUMMARY NIHR HSC ID: 5330 Secukinumab is a high-affinity fully human monoclonal antibody that antagonises
Horizon Scanning Centre November 2012 Secukinumab for active and progressive psoriatic arthritis. SUMMARY NIHR HSC ID: 5330 Secukinumab is a high-affinity fully human monoclonal antibody that antagonises
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence
 Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Diagnostics Assessment Programme. Measurement of exhaled nitric oxide concentration in asthma; NIOX MINO and NObreath. Final scope
 Diagnostics Assessment Programme Measurement of exhaled nitric oxide concentration in asthma; NIOX MINO and NObreath Final scope February 2013 1. Introduction The Medical Technologies Advisory Committee
Diagnostics Assessment Programme Measurement of exhaled nitric oxide concentration in asthma; NIOX MINO and NObreath Final scope February 2013 1. Introduction The Medical Technologies Advisory Committee
Do We Need Biologics in Pediatric Asthma Management?
 Do We Need Biologics in Pediatric Asthma Management? Ting Fan LEUNG, MBChB, MD, FRCPCH, FAAAAI Professor and Chairman Department of Paediatrics The Chinese University of Hong Kong Asthma and Allergy by
Do We Need Biologics in Pediatric Asthma Management? Ting Fan LEUNG, MBChB, MD, FRCPCH, FAAAAI Professor and Chairman Department of Paediatrics The Chinese University of Hong Kong Asthma and Allergy by
fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK
 fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK 09 May 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
fluticasone furoate / vilanterol 92/22, 184/22 micrograms inhalation powder (Relvar Ellipta ) SMC No. (966/14) GlaxoSmithKline UK 09 May 2014 The Scottish Medicines Consortium (SMC) has completed its assessment
Horizon Scanning Centre March Denosumab for glucocorticoidinduced SUMMARY NIHR HSC ID: 6329
 Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Horizon Scanning Centre March 2014 Denosumab for glucocorticoidinduced osteoporosis SUMMARY NIHR HSC ID: 6329 This briefing is based on information available at the time of research and a limited literature
Asthma - Chronic. Presentations of asthma Cough Wheeze Breathlessness Chest tightness
 Asthma - Chronic Definition of asthma Chronic inflammatory disease of the airways 3 components: o Reversible and variable airflow obstruction o Airway hyper-responsiveness to stimuli o Inflammation of
Asthma - Chronic Definition of asthma Chronic inflammatory disease of the airways 3 components: o Reversible and variable airflow obstruction o Airway hyper-responsiveness to stimuli o Inflammation of
SEVERE ASTHMA NUCALA 100MG SUBCUTANEOUS INJECTION THE FIRST TARGETED ANTI IL-5 ADD-ON TREATMENT FOR ADULTS WITH SEVERE REFRACTORY EOSINOPHILIC ASTHMA
 SEVERE ASTHMA NUCALA 100MG SUBCUTANEOUS INJECTION THE FIRST TARGETED ANTI ADD-ON TREATMENT FOR ADULTS WITH SEVERE REFRACTORY EOSINOPHILIC ASTHMA FIND OUT MORE VISIT - WWW.NUCALA.CO.UK Vial not actual size
SEVERE ASTHMA NUCALA 100MG SUBCUTANEOUS INJECTION THE FIRST TARGETED ANTI ADD-ON TREATMENT FOR ADULTS WITH SEVERE REFRACTORY EOSINOPHILIC ASTHMA FIND OUT MORE VISIT - WWW.NUCALA.CO.UK Vial not actual size
Horizon Scanning Centre November Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line
 Horizon Scanning Centre November 2012 Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line SUMMARY NIHR HSC ID: 5206 This briefing is based on information available
Horizon Scanning Centre November 2012 Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line SUMMARY NIHR HSC ID: 5206 This briefing is based on information available
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Scottish Medicines Consortium
 Scottish Medicines Consortium montelukast 10mg tablets (Singulair ) No. (185/05) Merck, Sharp & Dohme Ltd (MSD) New indication: for asthmatic patients in whom montelukast is indicated in asthma, montelukast
Scottish Medicines Consortium montelukast 10mg tablets (Singulair ) No. (185/05) Merck, Sharp & Dohme Ltd (MSD) New indication: for asthmatic patients in whom montelukast is indicated in asthma, montelukast
Asthma: diagnosis and monitoring
 Asthma: diagnosis and monitoring NICE guideline: short version Draft for second consultation, July 01 This guideline covers assessing, diagnosing and monitoring suspected or confirmed asthma in adults,
Asthma: diagnosis and monitoring NICE guideline: short version Draft for second consultation, July 01 This guideline covers assessing, diagnosing and monitoring suspected or confirmed asthma in adults,
omalizumab 150mg powder and solvent for injection (Xolair ) No. (259/06) Novartis Pharmaceuticals UK Ltd.
 Scottish Medicines Consortium Re-Submission omalizumab 150mg powder and solvent for injection (Xolair ) No. (259/06) Novartis Pharmaceuticals UK Ltd. 8 December 2006 The Scottish Medicines Consortium (SMC)
Scottish Medicines Consortium Re-Submission omalizumab 150mg powder and solvent for injection (Xolair ) No. (259/06) Novartis Pharmaceuticals UK Ltd. 8 December 2006 The Scottish Medicines Consortium (SMC)
NICE guideline Published: 29 November 2017 nice.org.uk/guidance/ng80
 Asthma: diagnosis, monitoring and chronic asthma management NICE guideline Published: 29 November 2017 nice.org.uk/guidance/ng80 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Asthma: diagnosis, monitoring and chronic asthma management NICE guideline Published: 29 November 2017 nice.org.uk/guidance/ng80 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Diagnostics guidance Published: 2 April 2014 nice.org.uk/guidance/dg12
 Measuring fractional exhaled nitric oxide concentration in asthma: NIOX MINO, NIOX VERO and NObreath Diagnostics guidance Published: 2 April 2014 nice.org.uk/guidance/dg12 NICE 2017. All rights reserved.
Measuring fractional exhaled nitric oxide concentration in asthma: NIOX MINO, NIOX VERO and NObreath Diagnostics guidance Published: 2 April 2014 nice.org.uk/guidance/dg12 NICE 2017. All rights reserved.
Horizon Scanning Centre November Faldaprevir with BI for chronic hepatitis C infection, genotype 1 SUMMARY NIHR HSC ID: 7688
 Horizon Scanning Centre November 2012 Faldaprevir with BI 207127 for chronic hepatitis C infection, genotype 1 SUMMARY NIHR HSC ID: 7688 This briefing is based on information available at the time of research
Horizon Scanning Centre November 2012 Faldaprevir with BI 207127 for chronic hepatitis C infection, genotype 1 SUMMARY NIHR HSC ID: 7688 This briefing is based on information available at the time of research
RESPIRATORY CARE IN GENERAL PRACTICE
 RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
GINA. At-A-Glance Asthma Management Reference. for adults, adolescents and children 6 11 years. Updated 2017
 GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
GINA At-A-Glance Asthma Management Reference for adults, adolescents and children 6 11 years Updated 2017 This resource should be used in conjunction with the Global Strategy for Asthma Management and
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]
![Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]](/thumbs/85/91889043.jpg) Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
Global Initiative for Asthma (GINA) What s new in GINA 2016?
 Global Initiative for Asthma (GINA) What s new in GINA 2016? GINA Global Strategy for Asthma Management and Prevention GINA: A Brief History Established in 1993 Collaboration between NHLBI and WHO Multiple
Global Initiative for Asthma (GINA) What s new in GINA 2016? GINA Global Strategy for Asthma Management and Prevention GINA: A Brief History Established in 1993 Collaboration between NHLBI and WHO Multiple
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL
 DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
DR REBECCA THOMAS CONSULTANT RESPIRATORY PHYSICIAN YORK DISTRICT HOSPITAL Definition Guidelines contact complicated definitions Central to this is Presence of symptoms Variable airflow obstruction Diagnosis
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd
 roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
roflumilast 500 microgram tablets (Daxas ) SMC No. (635/10) Nycomed Ltd 06 August 2010 (Issued 10 September 2010) The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs)
 Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
Ustekinumab (Stelara) for psoriatic arthritis second line after disease modifying anti rheumatic drugs (DMARDs) January 2010 This technology summary is based on information available at the time of research
National Horizon Scanning Centre. Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis. April 2008
 Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Mannitol dry powder for inhalation (Bronchitol) for cystic fibrosis April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016
 Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Diagnosis and Management of Asthma in Children based on the British Thoracic Society and Scottish Intercollegiate Guidelines Network September 2016 Diagnosis: There is no lower limit to the age at which
Prof Neil Barnes. Respiratory and General Medicine London Chest Hospital and The Royal London Hospital
 Prof Neil Barnes Respiratory and General Medicine London Chest Hospital and The Royal London Hospital ASTHMA: WHEN EVERYTHING FAILS WHAT DO YOU DO? South GP CME 2013, Dunedin Saturday 17 th August 2013
Prof Neil Barnes Respiratory and General Medicine London Chest Hospital and The Royal London Hospital ASTHMA: WHEN EVERYTHING FAILS WHAT DO YOU DO? South GP CME 2013, Dunedin Saturday 17 th August 2013
Add on therapy in asthma. Local experience in context J Paul Dilworth, Nicola Marks, Lauren Geddes
 Add on therapy in asthma Local experience in context J Paul Dilworth, Nicola Marks, Lauren Geddes Programme Omalizumab Evidence and assessment Local Experience Dysfunctional breathing The other effective
Add on therapy in asthma Local experience in context J Paul Dilworth, Nicola Marks, Lauren Geddes Programme Omalizumab Evidence and assessment Local Experience Dysfunctional breathing The other effective
National Horizon Scanning Centre. Mepolizumab (Bosatria) for hypereosinophilic syndrome first line in combination with corticosteroids.
 Mepolizumab (Bosatria) for hypereosinophilic syndrome first line in combination with corticosteroids May 2008 This technology summary is based on information available at the time of research and a limited
Mepolizumab (Bosatria) for hypereosinophilic syndrome first line in combination with corticosteroids May 2008 This technology summary is based on information available at the time of research and a limited
Diagnosis, Treatment and Management of Asthma
 Diagnosis, Treatment and Management of Asthma Asthma is a complex disorder characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation.
Diagnosis, Treatment and Management of Asthma Asthma is a complex disorder characterized by variable and recurring symptoms, airflow obstruction, bronchial hyperresponsiveness, and an underlying inflammation.
Insulin degludec/insulin aspart (DegludecPlus) for type 1 diabetes
 Insulin degludec/insulin aspart (DegludecPlus) for type 1 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Insulin degludec/insulin aspart (DegludecPlus) for type 1 diabetes This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Pharmacy Management Drug Policy
 SUBJECT: : Nucala (mepolizumab), Cinqair (reslizumab), & Fasenra (benralizumab) POLICY NUMBER: Pharmacy-62 EFFECTIVE DATE: 12/15 LAST REVIEW DATE: 3/5/2018 If the member s subscriber contract excludes
SUBJECT: : Nucala (mepolizumab), Cinqair (reslizumab), & Fasenra (benralizumab) POLICY NUMBER: Pharmacy-62 EFFECTIVE DATE: 12/15 LAST REVIEW DATE: 3/5/2018 If the member s subscriber contract excludes
What s new in Asthma? Dr Alexandra Nanzer-Kelly Consultant Respiratory Physician Royal Brompton and Harefield Hospitals
 What s new in Asthma? Dr Alexandra Nanzer-Kelly Consultant Respiratory Physician Royal Brompton and Harefield Hospitals Asthma is an inflammatory disease Relaxed smooth muscles Air trapped in alveoli Tightened
What s new in Asthma? Dr Alexandra Nanzer-Kelly Consultant Respiratory Physician Royal Brompton and Harefield Hospitals Asthma is an inflammatory disease Relaxed smooth muscles Air trapped in alveoli Tightened
Study Investigators/Centers: GSK sponsored studies MEA112997, MEA115588, and MEA and a proof of concept investigator sponsored study CRT110184
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline
 umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Biologic Agents in the treatment of Severe Asthma
 Biologic Agents in the treatment of Severe Asthma Daniel L Maxwell, D.O., FACOI, FAASM Clinical Assistant Professor of Medicine Michigan State University College of Osteopathic Medicine College of Human
Biologic Agents in the treatment of Severe Asthma Daniel L Maxwell, D.O., FACOI, FAASM Clinical Assistant Professor of Medicine Michigan State University College of Osteopathic Medicine College of Human
Asthma COPD Overlap (ACO)
 Asthma COPD Overlap (ACO) Dr Thomas Brown Consultant Respiratory Physician Thomas.Brown@porthosp.nhs.uk Dr Hitasha Rupani Consultant Respiratory Physician Hitasha.rupani@porthosp.nhs.uk What is Asthma
Asthma COPD Overlap (ACO) Dr Thomas Brown Consultant Respiratory Physician Thomas.Brown@porthosp.nhs.uk Dr Hitasha Rupani Consultant Respiratory Physician Hitasha.rupani@porthosp.nhs.uk What is Asthma
Biologics in asthma Are we turning the corner? Roland Buhl Pulmonary Department Mainz University Hospital
 Biologics in asthma Are we turning the corner? Roland Buhl Pulmonary Department Mainz University Hospital Biologics in asthma - are we turning the corner? Allergic asthma anti - IgE Allergic airway inflammation
Biologics in asthma Are we turning the corner? Roland Buhl Pulmonary Department Mainz University Hospital Biologics in asthma - are we turning the corner? Allergic asthma anti - IgE Allergic airway inflammation
Clinical Implications of Asthma Phenotypes. Michael Schatz, MD, MS Department of Allergy
 Clinical Implications of Asthma Phenotypes Michael Schatz, MD, MS Department of Allergy Definition of Phenotype The observable properties of an organism that are produced by the interaction of the genotype
Clinical Implications of Asthma Phenotypes Michael Schatz, MD, MS Department of Allergy Definition of Phenotype The observable properties of an organism that are produced by the interaction of the genotype
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Interleukin (IL)-5 Antagonists: Mepolizumab and Reslizumab Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5
Cigna Drug and Biologic Coverage Policy Subject Interleukin (IL)-5 Antagonists: Mepolizumab and Reslizumab Table of Contents Coverage Policy... 1 General Background... 3 Coding/Billing Information... 5
Re-Submission. roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd. Published 11 September
 Re-Submission roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd 4 August 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Re-Submission roflumilast, 500 microgram, film-coated tablet (Daxas ) SMC No 635/10 AstraZeneca UK Ltd 4 August 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Putting NICE guidance into practice. Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80)
 Putting NICE guidance into practice Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80) Published: November 2017 Summary This report focuses on the recommendations
Putting NICE guidance into practice Resource impact report: Asthma: diagnosis, monitoring and chronic asthma management (NG80) Published: November 2017 Summary This report focuses on the recommendations
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes
 Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Liraglutide (Victoza) in combination with basal insulin for type 2 diabetes May 2011 This technology summary is based on information available at the time of research and a limited literature search. It
Omalizumab (Xolair ) ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September Indication
 ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
Treatment Options for Complicated/Severe Asthma. Henry J. Kanarek, MD Kanarek Allergy Asthma Immunology
 Treatment Options for Complicated/Severe Asthma Henry J. Kanarek, MD Kanarek Allergy Asthma Immunology www.kallergy.com 913-451-8555 Asthma Epidemiology World Health Organization, Asthma is one of the
Treatment Options for Complicated/Severe Asthma Henry J. Kanarek, MD Kanarek Allergy Asthma Immunology www.kallergy.com 913-451-8555 Asthma Epidemiology World Health Organization, Asthma is one of the
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
Not available 100/6mcg 2 BD formoterol (Fostair MDI) 100/6mcg 33
 COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
COMMISSIONING POLICY RECOMMENDATION TREATMENT ADVISORY GROUP FLUTICASONE FUROATE/VILANTEROL COMBINATION INHALER - ASTHMA Policy agreed by Vale of York CCG (date) Drug, Treatment, Device name Fluticasone
Searching for Targets to Control Asthma
 Searching for Targets to Control Asthma Timothy Craig Distinguished Educator Professor Medicine and Pediatrics Penn State University Hershey, PA, USA Inflammation and Remodeling in Asthma The most important
Searching for Targets to Control Asthma Timothy Craig Distinguished Educator Professor Medicine and Pediatrics Penn State University Hershey, PA, USA Inflammation and Remodeling in Asthma The most important
PCRS-UK briefing document Asthma guidelines. November 2017
 PCRS-UK briefing document Asthma guidelines November 2017 1 1. Background The Scottish Intercollegiate Guidelines Network (SIGN) and British Thoracic Society (BTS) have been collaborating on producing
PCRS-UK briefing document Asthma guidelines November 2017 1 1. Background The Scottish Intercollegiate Guidelines Network (SIGN) and British Thoracic Society (BTS) have been collaborating on producing
Scottish Medicines Consortium
 Scottish Medicines Consortium budesonide/formoterol 100/6, 200/6 turbohaler (Symbicort SMART ) No. (362/07) Astra Zeneca UK Limited 9 March 2007 (Issued May 2007) The Scottish Medicines Consortium (SMC)
Scottish Medicines Consortium budesonide/formoterol 100/6, 200/6 turbohaler (Symbicort SMART ) No. (362/07) Astra Zeneca UK Limited 9 March 2007 (Issued May 2007) The Scottish Medicines Consortium (SMC)
Local Omalizumab Treatment Protocol (For children 6 to <12 years of age)
 Local Omalizumab Treatment Protocol (For children 6 to
Local Omalizumab Treatment Protocol (For children 6 to
ERS Investor & Analyst Event. Munich Tuesday 9 th September 2014
 ERS Investor & Analyst Event Munich Tuesday 9 th September 2014 Darrell Baker SVP, Global Head of Respiratory Agenda GSK s Respiratory Portfolio Eosinophils Research in COPD Eosinophils Clinical Experience
ERS Investor & Analyst Event Munich Tuesday 9 th September 2014 Darrell Baker SVP, Global Head of Respiratory Agenda GSK s Respiratory Portfolio Eosinophils Research in COPD Eosinophils Clinical Experience
Asthma - An update BTS Asthma Guidelines 2016
 Asthma - An update BTS Asthma Guidelines 2016 Dr Ian Clifton Overview Diagnosis Supported self-management Non-pharmacological management Drugs / inhaled therapy Difficult asthma services Case discussions
Asthma - An update BTS Asthma Guidelines 2016 Dr Ian Clifton Overview Diagnosis Supported self-management Non-pharmacological management Drugs / inhaled therapy Difficult asthma services Case discussions
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy. December 2011
 Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Lacosamide (Vimpat) for partial-onset epilepsy monotherapy This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a
Difficult Asthma Assessment: A systematic approach
 Difficult Asthma Assessment: A systematic approach Dr Naghmeh Radhakrishna Respiratory, Sleep & Allergy Physician Allergy, Asthma & Clinical Immunology Service The Alfred Hospital Melbourne, Australia
Difficult Asthma Assessment: A systematic approach Dr Naghmeh Radhakrishna Respiratory, Sleep & Allergy Physician Allergy, Asthma & Clinical Immunology Service The Alfred Hospital Melbourne, Australia
Getting Asthma treatment right. Dr David Cremonesini Specialist Pediatrician American Hospital
 Getting Asthma treatment right Dr David Cremonesini Specialist Pediatrician American Hospital cdavid@ahdubai.com } Consultant Paediatrician from UK of 5.5 years } Speciality in Allergy / Asthma (PG Certificate)
Getting Asthma treatment right Dr David Cremonesini Specialist Pediatrician American Hospital cdavid@ahdubai.com } Consultant Paediatrician from UK of 5.5 years } Speciality in Allergy / Asthma (PG Certificate)
Amanda Hess, MMS, PA-C President-Elect, AAPA-AAI Arizona Asthma and Allergy Institute Scottsdale, AZ
 Amanda Hess, MMS, PA-C President-Elect, AAPA-AAI Arizona Asthma and Allergy Institute Scottsdale, AZ Financial Disclosures Advanced Practiced Advisory Board for Circassia Learning Objectives 1. Briefly
Amanda Hess, MMS, PA-C President-Elect, AAPA-AAI Arizona Asthma and Allergy Institute Scottsdale, AZ Financial Disclosures Advanced Practiced Advisory Board for Circassia Learning Objectives 1. Briefly
Asthma Management for the Athlete
 Asthma Management for the Athlete Khanh Lai, MD Assistant Professor Division of Pediatric Pulmonary and Sleep Medicine University of Utah School of Medicine 2 nd Annual Sports Medicine Symposium: The Pediatric
Asthma Management for the Athlete Khanh Lai, MD Assistant Professor Division of Pediatric Pulmonary and Sleep Medicine University of Utah School of Medicine 2 nd Annual Sports Medicine Symposium: The Pediatric
Technology appraisal guidance Published: 25 January 2017 nice.org.uk/guidance/ta431
 Mepolizumab for treating severeere refractory eosinophilic asthma Technology appraisal guidance Published: 25 January 2017 nice.org.uk/guidance/ta431 NICE 2017. All rights reserved. Subject to Notice of
Mepolizumab for treating severeere refractory eosinophilic asthma Technology appraisal guidance Published: 25 January 2017 nice.org.uk/guidance/ta431 NICE 2017. All rights reserved. Subject to Notice of
Relvar Ellipta (fluticasone furoate/vilanterol [as trifenatate]) for the treatment of patients with asthma
![Relvar Ellipta (fluticasone furoate/vilanterol [as trifenatate]) for the treatment of patients with asthma Relvar Ellipta (fluticasone furoate/vilanterol [as trifenatate]) for the treatment of patients with asthma](/thumbs/89/99956827.jpg) Relvar Ellipta (fluticasone furoate/vilanterol [as trifenatate]) for the treatment of patients with asthma Evidence Summary to support formulary decision making and guideline development Prescribing and
Relvar Ellipta (fluticasone furoate/vilanterol [as trifenatate]) for the treatment of patients with asthma Evidence Summary to support formulary decision making and guideline development Prescribing and
Horizon Scanning Centre March Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209
 Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
Horizon Scanning Centre March 2015 Ixekizumab for moderate to severe chronic plaque psoriasis SUMMARY NIHR HSC ID: 5209 This briefing is based on information available at the time of research and a limited
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
ADULT ASTHMA GUIDE SUMMARY. This summary provides busy health professionals with key guidance for assessing and treating adult asthma.
 ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
ADULT ASTHMA GUIDE SUMMARY This summary provides busy health professionals with key guidance for assessing and treating adult asthma. Its source document Asthma and Respiratory Foundation NZ Adult Asthma
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Include patients: with a confirmed diagnosis of asthma who have been free of asthma symptoms for 3 months or more.
 Corby Clinical Commissioning Group Kettering General Hospital NHS Trust Nene Clinical Commissioning Group rthampton General Hospital NHS Trust rthamptonshire Healthcare Foundation Trust Stepping down asthma
Corby Clinical Commissioning Group Kettering General Hospital NHS Trust Nene Clinical Commissioning Group rthampton General Hospital NHS Trust rthamptonshire Healthcare Foundation Trust Stepping down asthma
AR101 peanut allergy immunotherapy for adult and paediatric patients
 AR101 peanut allergy immunotherapy for adult and paediatric patients NIHRIO (HSRIC) ID: 11815 NIHR Innovation Observatory Evidence Briefing: May 2017 NICE ID: 8773 LAY SUMMARY Food allergy occurs when
AR101 peanut allergy immunotherapy for adult and paediatric patients NIHRIO (HSRIC) ID: 11815 NIHR Innovation Observatory Evidence Briefing: May 2017 NICE ID: 8773 LAY SUMMARY Food allergy occurs when
TRANSPARENCY COMMITTEE OPINION. 13 January 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 January 2010 XOLAIR 150 mg, powder and solvent for solution for injection Box containing 1 x 150 mg vial + 1 x
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 January 2010 XOLAIR 150 mg, powder and solvent for solution for injection Box containing 1 x 150 mg vial + 1 x
Position within the Organisation
 ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
ASTHMA TREATMENT GUIDELINES Document Description Document Type Service Application Guidelines All healthcare professionals(hcps) caring for patients with asthma Version 4.0 Ratification date September
B-cell lymphoma vaccine (BiovaxID) for follicular non-hodgkin s lymphoma
 B-cell lymphoma vaccine (BiovaxID) for follicular non-hodgkin s lymphoma May 2010 This technology summary is based on information available at the time of research and a limited literature search. It is
B-cell lymphoma vaccine (BiovaxID) for follicular non-hodgkin s lymphoma May 2010 This technology summary is based on information available at the time of research and a limited literature search. It is
Robert Kruklitis, MD, PhD Chief, Pulmonary Medicine Lehigh Valley Health Network
 Robert Kruklitis, MD, PhD Chief, Pulmonary Medicine Lehigh Valley Health Network Robert.kruklitis@lvh.com Correlation of a Asthma pathophyisology with basic science Asthma (Physiology) Bronchodilators
Robert Kruklitis, MD, PhD Chief, Pulmonary Medicine Lehigh Valley Health Network Robert.kruklitis@lvh.com Correlation of a Asthma pathophyisology with basic science Asthma (Physiology) Bronchodilators
Allergy and Immunology Review Corner: Chapter 75 of Middleton s Allergy Principles and Practice, 7 th Edition, edited by N. Franklin Adkinson, et al.
 Allergy and Immunology Review Corner: Chapter 75 of Middleton s Allergy Principles and Practice, 7 th Edition, edited by N. Franklin Adkinson, et al. Chapter 75: Approach to Infants and Children with Asthma
Allergy and Immunology Review Corner: Chapter 75 of Middleton s Allergy Principles and Practice, 7 th Edition, edited by N. Franklin Adkinson, et al. Chapter 75: Approach to Infants and Children with Asthma
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Is reslizumab effective in improving quality of life and asthma control in adolescent and adult patients with poorly controlled eosinophilic asthma?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2018 Is reslizumab effective in improving
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2018 Is reslizumab effective in improving
Drug Prior Authorization Guideline NUCALA (mepolizumab)
 Drug Prior Authorization Guideline MB9914 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below Restricted to Pulmonology, Allergy, and
Drug Prior Authorization Guideline MB9914 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below Restricted to Pulmonology, Allergy, and
Improving Outcomes in the Management & Treatment of Asthma. April 21, Spring Managed Care Forum
 Improving Outcomes in the Management & Treatment of Asthma April 21, 2016 2016 Spring Managed Care Forum David M. Mannino, M.D. Professor Department of Preventive Medicine and Environmental Health University
Improving Outcomes in the Management & Treatment of Asthma April 21, 2016 2016 Spring Managed Care Forum David M. Mannino, M.D. Professor Department of Preventive Medicine and Environmental Health University
Chronic obstructive pulmonary disease
 0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
Asthma. chapter 7. Overview
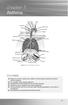 chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
The Asthma Guidelines: Diagnosis and Assessment of Asthma
 The Asthma Guidelines: Diagnosis and Assessment of Asthma Christopher H. Fanta, M.D. Partners Asthma Center Brigham and Women s Hospital Harvard Medical School Objectives Know how the diagnosis of asthma
The Asthma Guidelines: Diagnosis and Assessment of Asthma Christopher H. Fanta, M.D. Partners Asthma Center Brigham and Women s Hospital Harvard Medical School Objectives Know how the diagnosis of asthma
Bronchial asthma. E. Cserháti 1 st Department of Paediatrics. Lecture for english speaking students 5 February 2013
 Bronchial asthma E. Cserháti 1 st Department of Paediatrics Lecture for english speaking students 5 February 2013 Epidemiology of childhood bronchial asthma Worldwide prevalence of 7-8 and 13-14 years
Bronchial asthma E. Cserháti 1 st Department of Paediatrics Lecture for english speaking students 5 February 2013 Epidemiology of childhood bronchial asthma Worldwide prevalence of 7-8 and 13-14 years
Single Technology Appraisal (STA) Benralizumab for treating severe asthma
 Single Technology Appraisal (STA) Benralizumab for treating severe asthma Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please note: Comments received
Single Technology Appraisal (STA) Benralizumab for treating severe asthma Response to consultee and commentator comments on the draft remit and draft scope (pre-referral) Please note: Comments received
Allwin Mercer Dr Andrew Zurek
 Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Allwin Mercer Dr Andrew Zurek 1 in 11 people are currently receiving treatment for asthma (5.4 million people in the UK) Every 10 seconds, someone is having a potentially life-threatening asthma attack
Sponsor. Generic drug name. Trial indication(s) Protocol number. Protocol title. Clinical trial phase. Study Start/End Dates.
 Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
2017 Blue Cross and Blue Shield of Louisiana
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Policy Effective 4/1/2018
 Corporate Medical Policy Interleukin-5 Antagonists Notification File Name: Origination: Last CAP Review: Next CAP Review: Last Review: interleukin_5_antagonists 2/2016 11/2017 11/2018 2/2018 Policy Effective
Corporate Medical Policy Interleukin-5 Antagonists Notification File Name: Origination: Last CAP Review: Next CAP Review: Last Review: interleukin_5_antagonists 2/2016 11/2017 11/2018 2/2018 Policy Effective
Information for Parents and Young People on New and Emerging Treatments in Asthma
 Information for Parents and Young People on New and Emerging Treatments in Asthma Asthma continues to be a very common condition that causes a lot of distress to children and their families. For some it
Information for Parents and Young People on New and Emerging Treatments in Asthma Asthma continues to be a very common condition that causes a lot of distress to children and their families. For some it
New Therapies for Asthma
 New Therapies for Asthma Tracy Bridges, MD Speaker Disclosure: Dr. Bridges participates in speaker bureaus for Teva, Genetech & Astra Zeneca. Objectives: Discuss the use of LAMA s for Asthma Detail the
New Therapies for Asthma Tracy Bridges, MD Speaker Disclosure: Dr. Bridges participates in speaker bureaus for Teva, Genetech & Astra Zeneca. Objectives: Discuss the use of LAMA s for Asthma Detail the
Asthma 2015: Establishing and Maintaining Control
 Asthma 2015: Establishing and Maintaining Control Webinar for Michigan Center for Clinical Systems Improvement (Mi-CCSI) Karen Meyerson, MSN, APRN, NP-C, AE-C June 16, 2015 Asthma Prevalence Approx. 26
Asthma 2015: Establishing and Maintaining Control Webinar for Michigan Center for Clinical Systems Improvement (Mi-CCSI) Karen Meyerson, MSN, APRN, NP-C, AE-C June 16, 2015 Asthma Prevalence Approx. 26
Respiratory Pharmacology
 Allergy Targets of allergies Type I Histamine Leukotrienes Prostaglandins Bradykinin Hypersensitivity reactions Asthma Characterised by Triggered by Intrinsic Extrinsic (allergic) Mediators Result Early
Allergy Targets of allergies Type I Histamine Leukotrienes Prostaglandins Bradykinin Hypersensitivity reactions Asthma Characterised by Triggered by Intrinsic Extrinsic (allergic) Mediators Result Early
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line
 Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line August 2011 This technology summary is based on information available at the time of research and a limited
Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line August 2011 This technology summary is based on information available at the time of research and a limited
Nucala (mepolizumab injection for subcutaneous use)
 Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
AWMSG SECRETARIAT ASSESSMENT REPORT
 AWMSG SECRETARIAT ASSESSMENT REPORT Fluticasone furoate/vilanterol (as trifenatate) (Relvar Ellipta ) inhalation powder, 184/22 inhalation powder Reference number: 1216 FULL SUBMISSION This report has
AWMSG SECRETARIAT ASSESSMENT REPORT Fluticasone furoate/vilanterol (as trifenatate) (Relvar Ellipta ) inhalation powder, 184/22 inhalation powder Reference number: 1216 FULL SUBMISSION This report has
