Cardiology Genetics Report Long QT Syndrome (LQTS) Panel
|
|
|
- Giles Sharp
- 5 years ago
- Views:
Transcription
1 Patient Name: Test(s) Requested: The ordered test and the genes analyzed are Genes Evaluated (Disease): Result: Only mutations detected appear here KCNQ1 (LQT1 and Jervell and LangeNielsen syndrome), KCNH2 (LQT2), SCN5A (LQT3), ANK2 (LQT4), KCNE1 (LQT5 and Jervell and LangeNielsen syndrome), KCNE2 (LQT6), KCNJ2 (LQT7 and Andersen Tawil syndrome), CACNA1C (LQT8 and Timothy syndrome), CAV3 (LQT9), SCN4B (LQT10), AKAP9 (LQT11), SNTA1 (LQT12) POSITIVE Gene cdna Variant Zygosity Classification KCNQ1 c.1663 C>T p.arg555cys (R555C) Heterozygous Diseasecausing mutation No other diseasecausing mutations associated with LQTS were detected by sequence analysis of the 12 genes of the LQTS panel in this individual. Interpretation: Expands on the genetics This individual is heterozygous for a published missense mutation in the KCNQ1 gene, consistent with a genetic form of LQTS. Familial Long QT syndrome is primarily an autosomal dominant disease caused by mutation(s) in cardiac ion channel genes. Mutations in these genes tend to prolong the duration of the ventricular action potential, thus lengthening the QT interval seen on an ECG (Goldenberg I et al., 2008; Priori S et al., 2004). LQTS is associated with increased risk for syncope, ventricular arrhythmia and sudden cardiac death in young adults with normal heart structure (Vincent G, 1998). Mutations in the KCNQ1 gene have been reported in approximately 39%62% of patients with autosomal dominant long QT syndrome, and are associated with increased risk of cardiac events triggered by emotion, exercise and vigorous activity, particularly swimming (Priori S et al., 2004; Vincent G, 2009). KCNQ1 R555C p.arg555cys (R555C) CGC>TGC: c.1663 C>T in exon 13 of the KCNQ1 gene (NM_ ) The R555C mutation in KCNQ1 has been reported multiple times in association with LQTS, including one study that found this mutation was associated with druginduced symptoms and often borderline or no QT prolongation in three families with LQTS (Donger C et al., 1997; Park K et al., 2005; Kapplinger J et al., 2009). Functional studies report that R555C results in abnormal potassium channel function (Peroz D et al., 2008; Coyan F et al., 2014). This mutation was absent from >2,600 published control alleles (Kapplinger J et al., 2009), and the R555C mutation was not observed in approximately 6,500 samples from individuals of European and African American ancestry in the NHLBI Exome Sequencing Project, indicating it is not a common benign variant in these populations. Located in the Cterminus of KCNQ1, R555C results in a nonconservative amino acid substitution, which is likely to impact secondary protein structure as these residues differ in polarity, charge, size and/or other properties (Park K et al., 2005). Other missense mutations affecting the same codon (R555H, R555S) as well as mutations in nearby codons (G548D, V554A, K557E, R562M) have also been observed in patients with LQTS, further supporting the functional importance of this residue and region of the protein (Stenson et al., 2014). In summary, R555C in the KCNQ1 gene is interpreted as a diseasecausing mutation. x 207 Perry Parkway Gaithersburg, MD Tel (301) Fax (301) Page 1 of 4
2 Patient Name: Outlines followup recommendations references to None management Providedguidelines, risks to family members Liz Butler or recommendations for genetic counseling. Recommendation: 1. It is recommended that this individual and any firstdegree relatives receive continued clinical evaluation and followup for LQTS. 2. Based on this genetic test result, targeted genetic testing is now available for this family, and is recommended for all of this patient's firstdegree relatives and other atrisk family members. Testing can identify family members at increased risk of developing LQTS based on the presence of the mutation. For this familial mutation, genetic testing of other family members only requires testing for this mutation, not the full LQTS panel. 3. Genetic counseling is recommended to discuss the implications of this test report, specifically the risk of recurrence for this family, and to provide testing for other atrisk family members. Methods: Describes the methodology used by the lab for that particular test. Using genomic DNA from the submitted specimen, the coding regions and splice junctions of the 12 genes (Only exons 144 for CACNA1C, only the KCNQ1binding domains including Ser1570 residue for AKAP9) were enriched by multiplex PCR and sequenced simultaneously by massively parallel (NextGen) sequencing on an Illumina HiSeq instrument with 1x50 pairedend reads. Bidirectional sequence was assembled, aligned to reference gene sequences based on human genome build GRCh37/UCSC hg19, and analyzed for sequence variants. Capillary sequencing was used to confirm all potentially pathogenic variants and to obtain sequence for regions where fewer than 15 reads were achieved by NextGen sequencing. Sequence alterations were reported according to the Human Genome Variation Society (HGVS) nomenclature guidelines. If present, apparently homozygous variants were confirmed using alternate primer pairs to significantly reduce the possibility of allele dropout. GeneDx 207 Perry Parkway Gaithersburg, MD Tel (301) Fax (301) Page 2 of 4
3 Patient Name: Supplemental Variant Information: Gene: cdna Variant (Protein) Classification Zygosity KCNQ1: c.1663 C>T p.arg555cys (R555C) Diseasecausing mutation Heterozygous Chr: Position 11: dbsnp 1000G 1000G_AMR 1000G_EAS 1000G_SAS 1000G_AFR 1000G_EUR 1000G_Highest 1000G_Highest_Sub ESP_EA ESP_AA ESP_Samples_Covered 6478 ESP_Avg_Rd_Depth 60.0 Sift PPH2 MutTaster_Score MutTaster_Pred rs (D) 1.0 (D) (D) D (D) Interpro_Domain Potassium channel, voltage dependent, KCNQ, Cterminal (1) ClinVar Pathogenic Provides evidence to support the variant in the attached result report This supplement provides evidence to support the classification of each reportable variant in the attached result report. This information is provided as a resource and may not be inclusive of all available information used by GeneDx for variant classification. The variant classification does not rely solely on any one of the individual criterion provided below. In addition, each criterion is weighted differently to derive the classification. This information is subject to change over time and may differ from what is currently available. This supplement is provided upon request to ordering providers. GeneDx has already conducted a thorough analysis of the data at the time testing was ordered and does not provide additional support to providers who have questions about the tools used in variant analysis and interpretation for a specific case. Results should always be interpreted in the context of the patient's clinical presentation. Note that the in silico scores used by GeneDx are precomputed and may change over time. In silico models use algorithms that predict the effect a variant may have on the protein, but they do not provide direct evidence regarding the actual impact on protein structure or function. The specificity of these models is typically low and they may produce discordant results (Flanagan 2010). In silico models should be interpreted with caution and only be used in combination with other available evidence to support the classification of any variant. Blank fields indicate that no data was available at time of analysis. For more information about the specific populations included in the 1000 Genomes Project, please see (McVean et al., 2012). For information about the populations included in the ESP data, please see The Exome Aggregation Consortium (ExAC) provides a betaversion of a set of exome sequencing data from a variety of largescale sequencing projects. A peerreviewed publication and final release of data are currently pending. The data includes low coverage and poor confidence calls which affect the data quality. The ExAC set includes data from individuals who were recruited for diseasespecific studies, including cancer and cardiovascular diseases. Therefore, GeneDx does not routinely consider data from the ExAC browser in variant interpretation at this time but will continue to evaluate the utility of this data set in a clinical setting and will include ExAC data once a peerreviewed publication is available and the full data set is released. REFERENCES: Genomes Project: McVean et al. (2012) Nature 491 (7422):5665 (PMID: ). 2. Exome Variant Server, NHLBI Exome Sequencing Project (ESP), Seattle, WA (URL: 3. SIFT: Kumar et al. (2009) Nature protocols 4 (7): (PMID: ) 4. Polyphen2: Adzhubei et al. (2010) Nature methods 7 (4):2489 (PMID: ) 5. Mutation Taster: Schwarz et al. (2014) Nature methods 11 (4):3612 (PMID: ) 6. SIFT and PolyPhen: Flanagan et al. (2010) Genet Test Mol Biomarkers Aug;14 (4):5337 (PMID ) 7. Interpro: Jones et al. (2014) Bioinformatics (Oxford, England) 30 (9): (PMID: ) 8. ClinVar: Landrum et al. (2014) Nucleic acids research 42 (Database issue):d9805 (PMID: ) GeneDx 207 Perry Parkway Gaithersburg, MD Tel (301) Fax (301) Page 3 of 4
4 Patient Name: Frequency/Catalog Information dbsnp NCBI repository for single base nucleotide substitutions and short deletion and insertion polymorphisms 1000G 1000 Genomes is a catalog of human genetic sequence variation obtained through genomic sequencing of 2500 individuals. Displayed valued indicates allelic frequency of the variant, with percent frequency displayed in parentheses. 1000G_AMR 1000G variant metafrequency percentage for individuals of American ancestry 1000G_EAS 1000G variant metafrequency percentage for individuals of East Asian ancestry 1000G_SAS 1000G variant metafrequency percentage for individuals of South Asian ancestry 1000G_AFR 1000G variant metafrequency percentage for individuals of African ancestry 1000G_EUR 1000G variant metafrequency percentage for individuals of European ancestry 1000G_Highest Highest reported percentage frequency of a variant in any 1000G subpopulation 1000G_Highest_Sub Subpopulation referenced in 1000G_Highest ESP_EA NHLBI Exome Sequencing Project (ESP) allelic frequency for individuals of European ancestry, including both healthy individuals and individuals from diseasespecific genetic studies ESP_AA NHLBI Exome Sequencing Project (ESP) allelic frequency for individuals of African ancestry, including both healthy individuals and individuals from diseasespecific genetic studies Information on Prediction Scores Sift Calculates the conservation of an amino acid and the protein tolerance to a proposed amino acid change. Scores range from 0 to 1 and are considered damaging if > PPH2 PolyPhen2 score predicts the probability that a variant is damaging using a machinelearning model and the HumDiv training dataset compiled from pathogenic variants known to cause human disorders. Scores range from 0 to 1: < 0.5 neutral, >= 0.5 possibly damaging, >= 0.9 damaging. MutTaster_Pred Predicts the effect of an amino acid variant. Predictions are: A Diseasecausing Automatic (known pathogenic), 'D' Diseasecausing, 'N' Polymorphism, P Polymorphism Automatic (known benign). MutTaster_Score Represents the strength of the calculated MutTaster_Pred matching a simple, automated prediction based on population statistics and ClinVar data (Example: A highscoring variant may be both calculated to be damaging and has a 'damaging' flag in ClinVar.) A high score does NOT indicate a high probability of accuracy. Interpro_Domain Domain in conserved site on which the variant is located. Domain annotations come from the Interpro database. The number in parentheses reflects how many times Interpro has assigned the variant position to that domain, typically derived from different predicting databases. ClinVar Classification of variant in ClinVar database, an NCBI archive of human variants with supporting evidence of phenotypic association. Report electronically signed by: Report electronically signed by: Sequence changes in the coding regions of KCNQ1, KCNH2, and SCN5A which are not known to definitively cause LQTS or a related arrhythmia: KCNH2, c.2690 A>C, p.lys897thr (K897T), Heterozygous; SCN5A, c.1673 A>G, p.his558arg (H558R), Homozygous References: Goldenberg et al. (2008) Current problems in cardiology 33 (11):62994 (PMID: ); Priori et al. (2004) Annals of the New York Academy of Sciences 1015 :96110 (PMID: ); Vincent et al. (1998) Annual review of medicine 49 :26374 (PMID: ); Vincent G. (Updated [4/2009]). RomanoWard Syndrome. In: GeneReviews at GeneTests Medical Genetics Information Resource (database online). Copyright, University of Washington, Seattle Available at Accessed [4/2012]; Donger C et al. KVLQT1 CTerminal Missense Mutation Causes a Forme Fruste LongQT Syndrome. Circulation. 96: , 1997; Park K et al. Impaired KCNQ1KCNE1 and phosphatidylinositol4,5bisphosphate interaction underlies the long QT syndrome. Circ Res. 96:730739, 2005; Kapplinger J et al., Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 6: , 2009; Peroz D et al. Kv7.1 (KCNQ1) properties and channelopathies. J Physiol 586.7: , 2008; Coyan et al. (2014) PloS one 9 (3):e93255; Exome Variant Server, NHLBI Exome Sequencing Project (ESP), Seattle, WA (URL: [12/2014]; Stenson et al. (2014) Human genetics 133 (1):19 (PMID: ) For additional information about this test result, please see attached brochure or visit the GeneDx website: cardiology/ Limitations: Genetic testing using the methods applied at GeneDx is expected to be highly accurate. Normal findings do not rule out the diagnosis of a genetic disorder since some genetic abnormalities may be undetectable with this test. This sequencing test cannot reliably detect mosaicism. Some genes have inherent sequence properties (for example: repeat, homology, or pseudogene regions, high GC content, rare polymorphisms) that may result in suboptimal data, and mutations in those regions may not be reliably identified. False negative results may also occur in the setting of bone marrow transplantation, recent blood transfusion, or suboptimal DNA quality. The chance of a false positive or false negative result due to laboratory errors incurred during any phase of testing cannot be completely excluded. Interpretations are made with the assumption that any information provided on family relationships is accurate. Consultation with a genetics professional is recommended for interpretation of results. Disclaimer: This test was developed and its performance characteristics determined by GeneDx. It has not been cleared or approved by the U.S. Food and Drug Administration. The laboratory is regulated under CLIA as qualified to perform highcomplexity testing. This test is used for clinical purposes. It should not be regarded as investigational or for research. GeneDx 207 Perry Parkway Gaithersburg, MD Tel (301) Fax (301) Page 4 of 4
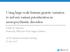
5 Patient Name: GeneDx Accession No: Date Specimen Obtained: Date Specimen Received: Date Test(s) Started: Date of Report: XXXXXXX 3/9/2015 3/10/2015 3/10/2015 4/3/2015 Image displays the location of the genetic alteration along the ion channel Heterozygous R555C in KCNQ1 Modified From Original Image: Inherited Arrhythmias Database This assay was developed and its performance determined by GeneDx for the sole purpose of identifying small sequence variants in the gene(s) tested. As with any genetic testing assay, this test does not detect large chromosomal aberrations, such as larger deletions and duplications (larger than 5bp) or rearrangements. Normal findings do not rule out the diagnosis of any disorder since some genetic abnormalities may be undetectable with this assay. Clinical implications of some variations may be unknown at the time of this report. This test should be used for clinical purposes only. It has not been cleared or approved by the FDA. The FDA has determined that such clearance or approval is not necessary. Pursuant to the requirements of CLIA '88, this laboratory has established and verified the test's accuracy and precision. CLIA ID#: 21D MD License: 953. GeneDx 207 Perry Parkway Gaithersburg, MD Tel (301) Fax (301)
Long QT. Long QT Syndrome. A Guide for Patients
 Long QT Long QT Syndrome A Guide for Patients Long QT Syndrome What is long QT syndrome? Long QT syndrome (LQTS) is a condition that affects the ability of the heart to beat (contract) regularly and efficiently.
Long QT Long QT Syndrome A Guide for Patients Long QT Syndrome What is long QT syndrome? Long QT syndrome (LQTS) is a condition that affects the ability of the heart to beat (contract) regularly and efficiently.
Reporting TP53 gene analysis results in CLL
 Reporting TP53 gene analysis results in CLL Mutations in TP53 - From discovery to clinical practice in CLL Discovery Validation Clinical practice Variant diversity *Leroy at al, Cancer Research Review
Reporting TP53 gene analysis results in CLL Mutations in TP53 - From discovery to clinical practice in CLL Discovery Validation Clinical practice Variant diversity *Leroy at al, Cancer Research Review
Prolonged QT Syndromes: Congenital and Acquired
 Prolonged QT Syndromes: Congenital and Acquired April 30, 2014 Elizabeth S. Kaufman, MD I have no financial disclosures. MetroHealth Campus, Case Western Reserve University Prolonged QT Syndromes Congenital
Prolonged QT Syndromes: Congenital and Acquired April 30, 2014 Elizabeth S. Kaufman, MD I have no financial disclosures. MetroHealth Campus, Case Western Reserve University Prolonged QT Syndromes Congenital
Long Q. Long QT Syndrome. A Guide for
 Long Q Long QT Syndrome A Guide for Introduction Long QT syndrome (LQTS) is a genetic heart disorder due to the malfunction of cardiac ion channels that results in 4,000 deaths annually in the United States
Long Q Long QT Syndrome A Guide for Introduction Long QT syndrome (LQTS) is a genetic heart disorder due to the malfunction of cardiac ion channels that results in 4,000 deaths annually in the United States
J. Peter van Tintelen MD PhD Clinical Geneticist Amsterdam, the Netherlands
 Inherited arrhythmia syndromes I: What you always wanted to know, but were afraid to ask: The basics of inherited arrhythmia syndromes and gene:cs J. Peter van Tintelen MD PhD Clinical Geneticist Amsterdam,
Inherited arrhythmia syndromes I: What you always wanted to know, but were afraid to ask: The basics of inherited arrhythmia syndromes and gene:cs J. Peter van Tintelen MD PhD Clinical Geneticist Amsterdam,
Concurrent Practical Session ACMG Classification
 Variant Effect Prediction Training Course 6-8 November 2017 Prague, Czech Republic Concurrent Practical Session ACMG Classification Andreas Laner / Anna Benet-Pagès 1 Content 1. Background... 3 2. Aim
Variant Effect Prediction Training Course 6-8 November 2017 Prague, Czech Republic Concurrent Practical Session ACMG Classification Andreas Laner / Anna Benet-Pagès 1 Content 1. Background... 3 2. Aim
Genetics of Sudden Cardiac Death. Geoffrey Pitt Ion Channel Research Unit Duke University. Disclosures: Grant funding from Medtronic.
 Genetics of Sudden Cardiac Death Geoffrey Pitt Ion Channel Research Unit Duke University Disclosures: Grant funding from Medtronic Duke U N I V E R S I T Y Sudden Cardiac Death High incidence 50-100 per
Genetics of Sudden Cardiac Death Geoffrey Pitt Ion Channel Research Unit Duke University Disclosures: Grant funding from Medtronic Duke U N I V E R S I T Y Sudden Cardiac Death High incidence 50-100 per
Basics of Structure/Function of Sodium and Potassium Channels Barry London, MD PhD
 Basics of Structure/Function of Sodium and Potassium Channels Barry London, MD PhD University of Pittsburgh Medical Center Pittsburgh, PA International Symposium of Inherited Arrhythmia Disorders and Hypertrophic
Basics of Structure/Function of Sodium and Potassium Channels Barry London, MD PhD University of Pittsburgh Medical Center Pittsburgh, PA International Symposium of Inherited Arrhythmia Disorders and Hypertrophic
No mutations were identified.
 Hereditary High Cholesterol Test ORDERING PHYSICIAN PRIMARY CONTACT SPECIMEN Report date: Aug 1, 2017 Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA Kelly Peters Sample Medical Group 123
Hereditary High Cholesterol Test ORDERING PHYSICIAN PRIMARY CONTACT SPECIMEN Report date: Aug 1, 2017 Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA Kelly Peters Sample Medical Group 123
Rhythm and Blues Drugs and QT Prolongation
 Rhythm and Blues Drugs and QT Prolongation Dr Martin Quinn St Vincents University Hospital Irish Medication Safety Network conference Farmleigh 18 Oct 2013 Drugs and QT Prolongation Anti-psychotic, antidepressant,
Rhythm and Blues Drugs and QT Prolongation Dr Martin Quinn St Vincents University Hospital Irish Medication Safety Network conference Farmleigh 18 Oct 2013 Drugs and QT Prolongation Anti-psychotic, antidepressant,
Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research
 Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research Application Note Authors John McGuigan, Megan Manion,
Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research Application Note Authors John McGuigan, Megan Manion,
Strength and weakness of genetic testing in clinical routine.
 Strength and weakness of genetic testing in clinical routine. Silvia G Priori MD PhD Molecular Cardiology, IRCCS Fondazione Maugeri Pavia, Italy AND Leon Charney Division of Cardiology, Cardiovascular
Strength and weakness of genetic testing in clinical routine. Silvia G Priori MD PhD Molecular Cardiology, IRCCS Fondazione Maugeri Pavia, Italy AND Leon Charney Division of Cardiology, Cardiovascular
Genetic Testing for Cardiac Ion Channelopathies
 Genetic Testing for Cardiac Ion Channelopathies Policy Number: 2.04.43 Last Review: 11/2018 Origination: 6/2007 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Genetic Testing for Cardiac Ion Channelopathies Policy Number: 2.04.43 Last Review: 11/2018 Origination: 6/2007 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Genetic Testing for Page 1 of 29 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Genetic Testing for Professional Institutional Original Effective Date: August 12,
Genetic Testing for Page 1 of 29 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Genetic Testing for Professional Institutional Original Effective Date: August 12,
Genetic Testing for Congenital Long QT Syndrome
 Genetic Testing for Congenital Long QT Syndrome Policy Number: 2.04.43 Last Review: 11/2013 Origination: 6/2007 Next Review: 11/2014 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Genetic Testing for Congenital Long QT Syndrome Policy Number: 2.04.43 Last Review: 11/2013 Origination: 6/2007 Next Review: 11/2014 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
MEDICAL GENOMICS LABORATORY. Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG)
 Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
TumorNext-HRD with OvaNext: Paired Germline and Tumor Analyses of Genes Involved in
 SAMPLE REPORT Ordered By Contact ID:1251298 Example, Doctor, MD MOCKORG44 (10829) 123 Somewhere LaneSuite 4 Heaven NV 78872 US Ph:123-123-1234 Fx:123-123-1223 Org ID:8141 Normal Specimen Accession #: 00-086947
SAMPLE REPORT Ordered By Contact ID:1251298 Example, Doctor, MD MOCKORG44 (10829) 123 Somewhere LaneSuite 4 Heaven NV 78872 US Ph:123-123-1234 Fx:123-123-1223 Org ID:8141 Normal Specimen Accession #: 00-086947
6/12/2018. Disclosures. Clinical Genomics The CLIA Lab Perspective. Outline. COH HopeSeq Heme Panels
 Clinical Genomics The CLIA Lab Perspective Disclosures Raju K. Pillai, M.D. Hematopathologist / Molecular Pathologist Director, Pathology Bioinformatics City of Hope National Medical Center, Duarte, CA
Clinical Genomics The CLIA Lab Perspective Disclosures Raju K. Pillai, M.D. Hematopathologist / Molecular Pathologist Director, Pathology Bioinformatics City of Hope National Medical Center, Duarte, CA
Merging single gene-level CNV with sequence variant interpretation following the ACMGG/AMP sequence variant guidelines
 Merging single gene-level CNV with sequence variant interpretation following the ACMGG/AMP sequence variant guidelines Tracy Brandt, Ph.D., FACMG Disclosure I am an employee of GeneDx, Inc., a wholly-owned
Merging single gene-level CNV with sequence variant interpretation following the ACMGG/AMP sequence variant guidelines Tracy Brandt, Ph.D., FACMG Disclosure I am an employee of GeneDx, Inc., a wholly-owned
Description. Page: 1 of 31. Genetic Testing for Cardiac Ion Channelopathies. Last Review Status/Date: December 2015
 Genetic Testing for Cardiac Ion Last Review Status/Date: December 2015 Genetic Testing for Cardiac Ion Description Page: 1 of 31 Genetic testing is available for patients suspected of having cardiac ion
Genetic Testing for Cardiac Ion Last Review Status/Date: December 2015 Genetic Testing for Cardiac Ion Description Page: 1 of 31 Genetic testing is available for patients suspected of having cardiac ion
Molecular Diagnostic Laboratory 18 Sequencing St, Gene Town, ZY Tel: Fax:
 Molecular Diagnostic Laboratory 18 Sequencing St, Gene Town, ZY 01234 Tel: 555-920-3333 Fax: 555-920-3334 www.moldxlaboratory.com Patient Name: Jane Doe Specimen type: Blood, peripheral DOB: 04/05/1990
Molecular Diagnostic Laboratory 18 Sequencing St, Gene Town, ZY 01234 Tel: 555-920-3333 Fax: 555-920-3334 www.moldxlaboratory.com Patient Name: Jane Doe Specimen type: Blood, peripheral DOB: 04/05/1990
Introduction to genetic variation. He Zhang Bioinformatics Core Facility 6/22/2016
 Introduction to genetic variation He Zhang Bioinformatics Core Facility 6/22/2016 Outline Basic concepts of genetic variation Genetic variation in human populations Variation and genetic disorders Databases
Introduction to genetic variation He Zhang Bioinformatics Core Facility 6/22/2016 Outline Basic concepts of genetic variation Genetic variation in human populations Variation and genetic disorders Databases
Code CPT Descriptor Test Purpose and Method Crosswalk Recommendation SEPT9 (Septin9) (e.g., colorectal cancer) methylation analysis
 ASSOCIATION FOR MOLECULAR PATHOLOGY Education. Innovation & Improved Patient Care. Advocacy. 9650 Rockville Pike. Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7995 amp@amp.org www.amp.org August
ASSOCIATION FOR MOLECULAR PATHOLOGY Education. Innovation & Improved Patient Care. Advocacy. 9650 Rockville Pike. Bethesda, Maryland 20814 Tel: 301-634-7939 Fax: 301-634-7995 amp@amp.org www.amp.org August
Pearls of the ESC/ERS Guidelines 2015 Channelopathies
 Pearls of the ESC/ERS Guidelines 2015 Channelopathies Carina Blomstrom Lundqvist Dept Cardiology, Uppsala, Sweden Content 2015 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias
Pearls of the ESC/ERS Guidelines 2015 Channelopathies Carina Blomstrom Lundqvist Dept Cardiology, Uppsala, Sweden Content 2015 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias
No mutations were identified.
 Hereditary Heart Health Test DOB: May 25, 1977 ID: 123456 Sex: Female Requisition #: 123456 ORDERING PHYSICIAN Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA SPECIMEN Type: Saliva Barcode:
Hereditary Heart Health Test DOB: May 25, 1977 ID: 123456 Sex: Female Requisition #: 123456 ORDERING PHYSICIAN Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA SPECIMEN Type: Saliva Barcode:
Protocol. Genetic Testing for Cardiac Ion Channelopathies
 Protocol Genetic Testing for Cardiac Ion Channelopathies (20443) Medical Benefit Effective Date: 04/0/8 Next Review Date: /8 Preauthorization Yes Review Dates: 05/09, 05/0, 03/, 03/2, 03/3, 03/4, 03/5,
Protocol Genetic Testing for Cardiac Ion Channelopathies (20443) Medical Benefit Effective Date: 04/0/8 Next Review Date: /8 Preauthorization Yes Review Dates: 05/09, 05/0, 03/, 03/2, 03/3, 03/4, 03/5,
Genetic Testing for Cardiac Ion Channelopathies. Description
 Genetic Testing for Cardiac Ion Page: 1 of 30 Last Review Status/Date: March 2017 Genetic Testing for Cardiac Ion Description Genetic testing is available for patients suspected of having cardiac ion channelopathies
Genetic Testing for Cardiac Ion Page: 1 of 30 Last Review Status/Date: March 2017 Genetic Testing for Cardiac Ion Description Genetic testing is available for patients suspected of having cardiac ion channelopathies
SNP Array NOTE: THIS IS A SAMPLE REPORT AND MAY NOT REFLECT ACTUAL PATIENT DATA. FORMAT AND/OR CONTENT MAY BE UPDATED PERIODICALLY.
 SAMPLE REPORT SNP Array NOTE: THIS IS A SAMPLE REPORT AND MAY NOT REFLECT ACTUAL PATIENT DATA. FORMAT AND/OR CONTENT MAY BE UPDATED PERIODICALLY. RESULTS SNP Array Copy Number Variations Result: LOSS,
SAMPLE REPORT SNP Array NOTE: THIS IS A SAMPLE REPORT AND MAY NOT REFLECT ACTUAL PATIENT DATA. FORMAT AND/OR CONTENT MAY BE UPDATED PERIODICALLY. RESULTS SNP Array Copy Number Variations Result: LOSS,
PALB2 c g>c is. VARIANT OF UNCERTAIN SIGNIFICANCE (VUS) CGI s summary of the available evidence is in Appendices A-C.
 Consultation sponsor (may not be the patient): First LastName [Patient identity withheld] Date received by CGI: 2 Sept 2017 Variant Fact Checker Report ID: 0000001.5 Date Variant Fact Checker issued: 12
Consultation sponsor (may not be the patient): First LastName [Patient identity withheld] Date received by CGI: 2 Sept 2017 Variant Fact Checker Report ID: 0000001.5 Date Variant Fact Checker issued: 12
Variant Annotation and Functional Prediction
 Variant Annotation and Functional Prediction Copyrighted 2018 Isabelle Schrauwen and Suzanne M. Leal This exercise touches on several functionalities of the program ANNOVAR to annotate and interpret candidate
Variant Annotation and Functional Prediction Copyrighted 2018 Isabelle Schrauwen and Suzanne M. Leal This exercise touches on several functionalities of the program ANNOVAR to annotate and interpret candidate
Key determinants of pathogenicity
 Key determinants of pathogenicity Session 6: Determining pathogenicity and genotype-phenotype correlation J. Peter van Tintelen MD PhD Clinical geneticist Academic Medical Center Amsterdam, the Netherlands
Key determinants of pathogenicity Session 6: Determining pathogenicity and genotype-phenotype correlation J. Peter van Tintelen MD PhD Clinical geneticist Academic Medical Center Amsterdam, the Netherlands
Genetic Testing for Cardiac Ion Channelopathies
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Genetic Testing and Analysis. (858) MRN: Specimen: Saliva Received: 07/26/2016 GENETIC ANALYSIS REPORT
 GBinsight Sample Name: GB4411 Race: Gender: Female Reason for Testing: Type 2 diabetes, early onset MRN: 0123456789 Specimen: Saliva Received: 07/26/2016 Test ID: 113-1487118782-4 Test: Type 2 Diabetes
GBinsight Sample Name: GB4411 Race: Gender: Female Reason for Testing: Type 2 diabetes, early onset MRN: 0123456789 Specimen: Saliva Received: 07/26/2016 Test ID: 113-1487118782-4 Test: Type 2 Diabetes
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 Genetic Testing for Page 1 of 23 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Genetic Testing for Professional Institutional Original Effective Date: August
Genetic Testing for Page 1 of 23 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Genetic Testing for Professional Institutional Original Effective Date: August
Ionchannels and channelopaties in the heart. Viktória Szőts
 Ionchannels and channelopaties in the heart Viktória Szőts Action of membrane transport protein ATP-powered pump Ion chanels Transporters 10 1-10 3 ions/s 10 7-10 8 ions/s 10 2-10 4 ions/s Cardiac K +
Ionchannels and channelopaties in the heart Viktória Szőts Action of membrane transport protein ATP-powered pump Ion chanels Transporters 10 1-10 3 ions/s 10 7-10 8 ions/s 10 2-10 4 ions/s Cardiac K +
Corporate Medical Policy
 Corporate Medical Policy Invasive Prenatal (Fetal) Diagnostic Testing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: invasive_prenatal_(fetal)_diagnostic_testing 12/2014 3/2018
Corporate Medical Policy Invasive Prenatal (Fetal) Diagnostic Testing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: invasive_prenatal_(fetal)_diagnostic_testing 12/2014 3/2018
SNP Array NOTE: THIS IS A SAMPLE REPORT AND MAY NOT REFLECT ACTUAL PATIENT DATA. FORMAT AND/OR CONTENT MAY BE UPDATED PERIODICALLY.
 SAMPLE REPORT SNP Array NOTE: THIS IS A SAMPLE REPORT AND MAY NOT REFLECT ACTUAL PATIENT DATA. FORMAT AND/OR CONTENT MAY BE UPDATED PERIODICALLY. RESULTS SNP Array Copy Number Variations Result: GAIN,
SAMPLE REPORT SNP Array NOTE: THIS IS A SAMPLE REPORT AND MAY NOT REFLECT ACTUAL PATIENT DATA. FORMAT AND/OR CONTENT MAY BE UPDATED PERIODICALLY. RESULTS SNP Array Copy Number Variations Result: GAIN,
Preventing Sudden Death in Young Athletes. Outline. Scope of the Problem. Causes of SCD in Young Athletes. Sudden death in the young athlete
 Preventing Sudden Death in Young Athletes Ronn E. Tanel, MD Director, Pediatric Arrhythmia Service UCSF Children s Hospital Associate Professor of Pediatrics UCSF School of Medicine Outline Sudden death
Preventing Sudden Death in Young Athletes Ronn E. Tanel, MD Director, Pediatric Arrhythmia Service UCSF Children s Hospital Associate Professor of Pediatrics UCSF School of Medicine Outline Sudden death
Is There a Genomic Basis to Acquired Channelopathic disease
 Is There a Genomic Basis to Acquired Channelopathic disease Yaniv Bar-Cohen, M.D. Associate Professor of Pediatrics Division of Cardiology / Electrophysiology Children s Hospital Los Angeles Keck School
Is There a Genomic Basis to Acquired Channelopathic disease Yaniv Bar-Cohen, M.D. Associate Professor of Pediatrics Division of Cardiology / Electrophysiology Children s Hospital Los Angeles Keck School
Ionchannels and channelopaties in the heart
 Ionchannels and channelopaties in the heart Csatorna müködés Több betegség Drugok kapcsolodása csat.hoz Sejtekbe ioncsat.expresszios módszerek, bemutatása Viktória Szőts Action of membrane transport protein
Ionchannels and channelopaties in the heart Csatorna müködés Több betegség Drugok kapcsolodása csat.hoz Sejtekbe ioncsat.expresszios módszerek, bemutatása Viktória Szőts Action of membrane transport protein
Clinical Policy Title: Genetic testing for long QT syndrome (LQTS)
 Clinical Policy Title: Genetic testing for long QT syndrome (LQTS) Clinical Policy Number: 04.01.02 Effective Date: Dec. 1, 2013 Initial Review Date: June 19, 2013 Most Recent Review Date: July 19, 2017
Clinical Policy Title: Genetic testing for long QT syndrome (LQTS) Clinical Policy Number: 04.01.02 Effective Date: Dec. 1, 2013 Initial Review Date: June 19, 2013 Most Recent Review Date: July 19, 2017
Name of Presenter: Marwan Refaat, MD
 NAAMA s 24 th International Medical Convention Medicine in the Next Decade: Challenges and Opportunities Beirut, Lebanon June 26 July 2, 2010 I have no actual or potential conflict of interest in relation
NAAMA s 24 th International Medical Convention Medicine in the Next Decade: Challenges and Opportunities Beirut, Lebanon June 26 July 2, 2010 I have no actual or potential conflict of interest in relation
Variant Classification: ACMG recommendations. Andreas Laner MGZ München
 Variant Classification: ACMG recommendations Andreas Laner MGZ München laner@mgz-muenchen.de Overview Introduction ACMG-AMP Classification System Evaluation of inter-laboratory concordance in variant classification
Variant Classification: ACMG recommendations Andreas Laner MGZ München laner@mgz-muenchen.de Overview Introduction ACMG-AMP Classification System Evaluation of inter-laboratory concordance in variant classification
Section: Effective Date: Subsection: Original Policy Date: Subject: Page: Last Review Status/Date: Background
 Genetic Testing for Cardiac Ion Last Review Status/Date: March 2014 Genetic Testing for Cardiac Ion Description Page: 1 of 22 Genetic testing is available for patients suspected of having cardiac ion channelopathies
Genetic Testing for Cardiac Ion Last Review Status/Date: March 2014 Genetic Testing for Cardiac Ion Description Page: 1 of 22 Genetic testing is available for patients suspected of having cardiac ion channelopathies
Clinical Policy Title: Genetic testing for long QT syndrome (LQTS)
 Clinical Policy Title: Genetic testing for long QT syndrome (LQTS) Clinical Policy Number: 04.01.02 Effective Date: Dec. 1, 2013 Initial Review Date: June 19, 2013 Most Recent Review Date: July 20, 2016
Clinical Policy Title: Genetic testing for long QT syndrome (LQTS) Clinical Policy Number: 04.01.02 Effective Date: Dec. 1, 2013 Initial Review Date: June 19, 2013 Most Recent Review Date: July 20, 2016
Assessing Laboratory Performance for Next Generation Sequencing Based Detection of Germline Variants through Proficiency Testing
 Assessing Laboratory Performance for Next Generation Sequencing Based Detection of Germline Variants through Proficiency Testing Karl V. Voelkerding, MD Professor of Pathology University of Utah Medical
Assessing Laboratory Performance for Next Generation Sequencing Based Detection of Germline Variants through Proficiency Testing Karl V. Voelkerding, MD Professor of Pathology University of Utah Medical
CHAPTER IV RESULTS Microcephaly General description
 47 CHAPTER IV RESULTS 4.1. Microcephaly 4.1.1. General description This study found that from a previous study of 527 individuals with MR, 48 (23 female and 25 male) unrelated individuals were identified
47 CHAPTER IV RESULTS 4.1. Microcephaly 4.1.1. General description This study found that from a previous study of 527 individuals with MR, 48 (23 female and 25 male) unrelated individuals were identified
MRC-Holland MLPA. Description version 07; 26 November 2015
 SALSA MLPA probemix P266-B1 CLCNKB Lot B1-0415, B1-0911. As compared to version A1 (lot A1-0908), one target probe for CLCNKB (exon 11) has been replaced. In addition, one reference probe has been replaced
SALSA MLPA probemix P266-B1 CLCNKB Lot B1-0415, B1-0911. As compared to version A1 (lot A1-0908), one target probe for CLCNKB (exon 11) has been replaced. In addition, one reference probe has been replaced
Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals
 Bansal et al. BMC Medicine (2017) 15:213 DOI 10.1186/s12916-017-0977-3 RESEARCH ARTICLE Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals
Bansal et al. BMC Medicine (2017) 15:213 DOI 10.1186/s12916-017-0977-3 RESEARCH ARTICLE Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals
Variant interpretation exercise. ACGS Somatic Variant Interpretation Workshop Joanne Mason 21/09/18
 Variant interpretation exercise ACGS Somatic Variant Interpretation Workshop Joanne Mason 21/09/18 Format of exercise Compile a list of tricky variants across solid cancer and haematological malignancy.
Variant interpretation exercise ACGS Somatic Variant Interpretation Workshop Joanne Mason 21/09/18 Format of exercise Compile a list of tricky variants across solid cancer and haematological malignancy.
Genetic testing in Cardiomyopathies
 Genetic testing in Cardiomyopathies Silvia Giuliana Priori Cardiovascular Genetics, Langone Medical Center, New York University School of Medicine, New York, USA and Molecular Cardiology, IRCCS Fondazione
Genetic testing in Cardiomyopathies Silvia Giuliana Priori Cardiovascular Genetics, Langone Medical Center, New York University School of Medicine, New York, USA and Molecular Cardiology, IRCCS Fondazione
Using large-scale human genetic variation to inform variant prioritization in neuropsychiatric disorders
 Using large-scale human genetic variation to inform variant prioritization in neuropsychiatric disorders Kaitlin E. Samocha Hurles lab, Wellcome Trust Sanger Institute ACGS Summer Scientific Meeting 27
Using large-scale human genetic variation to inform variant prioritization in neuropsychiatric disorders Kaitlin E. Samocha Hurles lab, Wellcome Trust Sanger Institute ACGS Summer Scientific Meeting 27
A guide to understanding variant classification
 White paper A guide to understanding variant classification In a diagnostic setting, variant classification forms the basis for clinical judgment, making proper classification of variants critical to your
White paper A guide to understanding variant classification In a diagnostic setting, variant classification forms the basis for clinical judgment, making proper classification of variants critical to your
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Exeter RGC Approved: Sept 2013 1. Disorder/condition
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Exeter RGC Approved: Sept 2013 1. Disorder/condition
MutationTaster & RegulationSpotter
 MutationTaster & RegulationSpotter Pathogenicity Prediction of Sequence Variants: Past, Present and Future Dr. rer. nat. Jana Marie Schwarz Klinik für Pädiatrie m. S. Neurologie Exzellenzcluster NeuroCure
MutationTaster & RegulationSpotter Pathogenicity Prediction of Sequence Variants: Past, Present and Future Dr. rer. nat. Jana Marie Schwarz Klinik für Pädiatrie m. S. Neurologie Exzellenzcluster NeuroCure
Hereditary Cardiovascular Conditions. genetic testing for undiagnosed diseases
 Hereditary Cardiovascular Conditions genetic testing for undiagnosed diseases What is Hypertrophic Cardiomyopathy (HCM)? normal heart heart with hcm Extra or thick heart muscle Typically in the left ventricle
Hereditary Cardiovascular Conditions genetic testing for undiagnosed diseases What is Hypertrophic Cardiomyopathy (HCM)? normal heart heart with hcm Extra or thick heart muscle Typically in the left ventricle
Shashikant Kulkarni, M.S (Medicine)., Ph.D., FACMG Associate Professor of Pathology & Immunology Associate Professor of Pediatrics and Genetics
 Shashikant Kulkarni, M.S (Medicine)., Ph.D., FACMG Associate Professor of Pathology & Immunology Associate Professor of Pediatrics and Genetics Director of Cytogenomics and Molecular Pathology Evidence-based
Shashikant Kulkarni, M.S (Medicine)., Ph.D., FACMG Associate Professor of Pathology & Immunology Associate Professor of Pediatrics and Genetics Director of Cytogenomics and Molecular Pathology Evidence-based
Case presentations from diagnostic exome sequencing results in ion channels M. Koko, U. Hedrich, H. Lerche DASNE meeting 2017, Eisenach
 Case presentations from diagnostic exome sequencing results in ion channels M. Koko, U. Hedrich, H. Lerche DASNE meeting 2017, Eisenach CASE 1: Clinical picture Patient s presentation: 38 year old jewish
Case presentations from diagnostic exome sequencing results in ion channels M. Koko, U. Hedrich, H. Lerche DASNE meeting 2017, Eisenach CASE 1: Clinical picture Patient s presentation: 38 year old jewish
ICD in a young patient with syncope
 ICD in a young patient with syncope Konstantinos P. Letsas, MD, FESC Second Department of Cardiology Evangelismos General Hospital of Athens Athens, Greece Case presentation A 17-year-old apparently healthy
ICD in a young patient with syncope Konstantinos P. Letsas, MD, FESC Second Department of Cardiology Evangelismos General Hospital of Athens Athens, Greece Case presentation A 17-year-old apparently healthy
Using SuSPect to Predict the Phenotypic Effects of Missense Variants. Chris Yates UCL Cancer Institute
 Using SuSPect to Predict the Phenotypic Effects of Missense Variants Chris Yates UCL Cancer Institute c.yates@ucl.ac.uk Outline SAVs and Disease Development of SuSPect Features included Feature selection
Using SuSPect to Predict the Phenotypic Effects of Missense Variants Chris Yates UCL Cancer Institute c.yates@ucl.ac.uk Outline SAVs and Disease Development of SuSPect Features included Feature selection
CITATION FILE CONTENT/FORMAT
 CITATION For any resultant publications using please cite: Matthew A. Field, Vicky Cho, T. Daniel Andrews, and Chris C. Goodnow (2015). "Reliably detecting clinically important variants requires both combined
CITATION For any resultant publications using please cite: Matthew A. Field, Vicky Cho, T. Daniel Andrews, and Chris C. Goodnow (2015). "Reliably detecting clinically important variants requires both combined
The State of the Molecular Autopsy for Sudden Death in the Young
 The State of the Molecular Autopsy for Sudden Death in the Young Michael J. Ackerman, MD, PhD Windland Smith Rice Cardiovascular Genomics Research Professor Professor of Medicine, Pediatrics, and Pharmacology
The State of the Molecular Autopsy for Sudden Death in the Young Michael J. Ackerman, MD, PhD Windland Smith Rice Cardiovascular Genomics Research Professor Professor of Medicine, Pediatrics, and Pharmacology
State of the Molecular Autopsy
 State of the Molecular Autopsy Michael J. Ackerman, MD, PhD Windland Smith Rice Cardiovascular Genomics Research Professor Professor of Medicine, Pediatrics, and Pharmacology Director, Long QT Syndrome/Genetic
State of the Molecular Autopsy Michael J. Ackerman, MD, PhD Windland Smith Rice Cardiovascular Genomics Research Professor Professor of Medicine, Pediatrics, and Pharmacology Director, Long QT Syndrome/Genetic
Variant Classification. Author: Mike Thiesen, Golden Helix, Inc.
 Variant Classification Author: Mike Thiesen, Golden Helix, Inc. Overview Sequencing pipelines are able to identify rare variants not found in catalogs such as dbsnp. As a result, variants in these datasets
Variant Classification Author: Mike Thiesen, Golden Helix, Inc. Overview Sequencing pipelines are able to identify rare variants not found in catalogs such as dbsnp. As a result, variants in these datasets
MRC-Holland MLPA. Description version 08; 30 March 2015
 SALSA MLPA probemix P351-C1 / P352-D1 PKD1-PKD2 P351-C1 lot C1-0914: as compared to the previous version B2 lot B2-0511 one target probe has been removed and three reference probes have been replaced.
SALSA MLPA probemix P351-C1 / P352-D1 PKD1-PKD2 P351-C1 lot C1-0914: as compared to the previous version B2 lot B2-0511 one target probe has been removed and three reference probes have been replaced.
MEDICAL GENOMICS LABORATORY. Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG)
 Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with receipt) Saliva (OGR-575 DNA Genotek;
Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with receipt) Saliva (OGR-575 DNA Genotek;
MEDICAL GENOMICS LABORATORY. Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG)
 Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with
Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with
CHR POS REF OBS ALLELE BUILD CLINICAL_SIGNIFICANCE
 CHR POS REF OBS ALLELE BUILD CLINICAL_SIGNIFICANCE is_clinical dbsnp MITO GENE chr1 13273 G C heterozygous - - -. - DDX11L1 chr1 949654 A G Homozygous 52 - - rs8997 - ISG15 chr1 1021346 A G heterozygous
CHR POS REF OBS ALLELE BUILD CLINICAL_SIGNIFICANCE is_clinical dbsnp MITO GENE chr1 13273 G C heterozygous - - -. - DDX11L1 chr1 949654 A G Homozygous 52 - - rs8997 - ISG15 chr1 1021346 A G heterozygous
CONGENITAL LONG QT SYNDROME(CLQTS) ASSOCIATED WITH COMPLETE ATRIOVENTRICULAR BLOCK. A CASE REPORT.
 CONGENITAL LONG QT SYNDROME(CLQTS) ASSOCIATED WITH COMPLETE ATRIOVENTRICULAR BLOCK. A CASE REPORT. SAHA Annual Congress 2017. Samkelo Jiyana, Adele Greyling, Andile Nxele, ZM,Makrexeni,L.Pepeta. BACKGROUND
CONGENITAL LONG QT SYNDROME(CLQTS) ASSOCIATED WITH COMPLETE ATRIOVENTRICULAR BLOCK. A CASE REPORT. SAHA Annual Congress 2017. Samkelo Jiyana, Adele Greyling, Andile Nxele, ZM,Makrexeni,L.Pepeta. BACKGROUND
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT MARCH 13, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201208 MARCH 13, 2012 Updates to the 2012 Healthcare Common Coding System This bulletin updates information published by the Indiana Health Coverage Programs
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201208 MARCH 13, 2012 Updates to the 2012 Healthcare Common Coding System This bulletin updates information published by the Indiana Health Coverage Programs
Abstract. Optimization strategy of Copy Number Variant calling using Multiplicom solutions APPLICATION NOTE. Introduction
 Optimization strategy of Copy Number Variant calling using Multiplicom solutions Michael Vyverman, PhD; Laura Standaert, PhD and Wouter Bossuyt, PhD Abstract Copy number variations (CNVs) represent a significant
Optimization strategy of Copy Number Variant calling using Multiplicom solutions Michael Vyverman, PhD; Laura Standaert, PhD and Wouter Bossuyt, PhD Abstract Copy number variations (CNVs) represent a significant
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
JULY 21, Genetics 101: SCN1A. Katie Angione, MS CGC Certified Genetic Counselor CHCO Neurology
 JULY 21, 2018 Genetics 101: SCN1A Katie Angione, MS CGC Certified Genetic Counselor CHCO Neurology Disclosures: I have no financial interests or relationships to disclose. Objectives 1. Review genetic
JULY 21, 2018 Genetics 101: SCN1A Katie Angione, MS CGC Certified Genetic Counselor CHCO Neurology Disclosures: I have no financial interests or relationships to disclose. Objectives 1. Review genetic
MRC-Holland MLPA. Description version 18; 09 September 2015
 SALSA MLPA probemix P090-A4 BRCA2 Lot A4-0715, A4-0714, A4-0314, A4-0813, A4-0712: Compared to lot A3-0710, the 88 and 96 nt control fragments have been replaced (QDX2). This product is identical to the
SALSA MLPA probemix P090-A4 BRCA2 Lot A4-0715, A4-0714, A4-0314, A4-0813, A4-0712: Compared to lot A3-0710, the 88 and 96 nt control fragments have been replaced (QDX2). This product is identical to the
Case Demonstrations in Congenital and Acquired Long QT Syndrome
 Case Demonstrations in Congenital and Acquired Long QT Syndrome Can You Make A Correct ECG Interpretation? Li Zhang, MD; 1-2 G. Michael Vincent, MD 1 1. LQTS Studies, Department t of Medicine i LDS Hospital,
Case Demonstrations in Congenital and Acquired Long QT Syndrome Can You Make A Correct ECG Interpretation? Li Zhang, MD; 1-2 G. Michael Vincent, MD 1 1. LQTS Studies, Department t of Medicine i LDS Hospital,
TEST INFORMATION Test: CarrierMap GEN (Genotyping) Panel: CarrierMap Expanded Diseases Tested: 311 Genes Tested: 299 Mutations Tested: 2647
 Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
Welcome to the Genetic Code: An Overview of Basic Genetics. October 24, :00pm 3:00pm
 Welcome to the Genetic Code: An Overview of Basic Genetics October 24, 2016 12:00pm 3:00pm Course Schedule 12:00 pm 2:00 pm Principles of Mendelian Genetics Introduction to Genetics of Complex Disease
Welcome to the Genetic Code: An Overview of Basic Genetics October 24, 2016 12:00pm 3:00pm Course Schedule 12:00 pm 2:00 pm Principles of Mendelian Genetics Introduction to Genetics of Complex Disease
MRC-Holland MLPA. Description version 14; 28 September 2016
 SALSA MLPA probemix P279-B3 CACNA1A Lot B3-0816. As compared to version B2 (lot B2-1012), one reference probe has been replaced and the length of several probes has been adjusted. Voltage-dependent calcium
SALSA MLPA probemix P279-B3 CACNA1A Lot B3-0816. As compared to version B2 (lot B2-1012), one reference probe has been replaced and the length of several probes has been adjusted. Voltage-dependent calcium
CRISPR/Cas9 Enrichment and Long-read WGS for Structural Variant Discovery
 CRISPR/Cas9 Enrichment and Long-read WGS for Structural Variant Discovery PacBio CoLab Session October 20, 2017 For Research Use Only. Not for use in diagnostics procedures. Copyright 2017 by Pacific Biosciences
CRISPR/Cas9 Enrichment and Long-read WGS for Structural Variant Discovery PacBio CoLab Session October 20, 2017 For Research Use Only. Not for use in diagnostics procedures. Copyright 2017 by Pacific Biosciences
SALSA MLPA probemix P241-D2 MODY mix 1 Lot D As compared to version D1 (lot D1-0911), one reference probe has been replaced.
 mix P241-D2 MODY mix 1 Lot D2-0413. As compared to version D1 (lot D1-0911), one reference has been replaced. Maturity-Onset Diabetes of the Young (MODY) is a distinct form of non insulin-dependent diabetes
mix P241-D2 MODY mix 1 Lot D2-0413. As compared to version D1 (lot D1-0911), one reference has been replaced. Maturity-Onset Diabetes of the Young (MODY) is a distinct form of non insulin-dependent diabetes
MRC-Holland MLPA. Description version 19;
 SALSA MLPA probemix P6-B2 SMA Lot B2-712, B2-312, B2-111, B2-511: As compared to the previous version B1 (lot B1-11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). SPINAL
SALSA MLPA probemix P6-B2 SMA Lot B2-712, B2-312, B2-111, B2-511: As compared to the previous version B1 (lot B1-11), the 88 and 96 nt DNA Denaturation control fragments have been replaced (QDX2). SPINAL
PERSONALIZED GENETIC REPORT CLIENT-REPORTED DATA PURPOSE OF THE X-SCREEN TEST
 INCLUDED IN THIS REPORT: REVIEW OF YOUR GENETIC INFORMATION RELEVANT TO ENDOMETRIOSIS PERSONAL EDUCATIONAL INFORMATION RELEVANT TO YOUR GENES INFORMATION FOR OBTAINING YOUR ENTIRE X-SCREEN DATA FILE PERSONALIZED
INCLUDED IN THIS REPORT: REVIEW OF YOUR GENETIC INFORMATION RELEVANT TO ENDOMETRIOSIS PERSONAL EDUCATIONAL INFORMATION RELEVANT TO YOUR GENES INFORMATION FOR OBTAINING YOUR ENTIRE X-SCREEN DATA FILE PERSONALIZED
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: None Genetic Testing for PTEN Hamartoma Tumor Syndrome Description The PTEN hamartoma tumor syndrome (PHTS) includes several syndromes
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: None Genetic Testing for PTEN Hamartoma Tumor Syndrome Description The PTEN hamartoma tumor syndrome (PHTS) includes several syndromes
The Role of Defibrillator Therapy in Genetic Arrhythmia Syndromes
 The Role of Defibrillator Therapy in Genetic Arrhythmia Syndromes RHEA C. PIMENTEL, MD, FACC, FHRS UNIVERSITY OF KANSAS HOSPITAL MID AMERICA CARDIOLOGY AUGUST 19, 2012 Monogenic Arrhythmia Syndromes Mendelian
The Role of Defibrillator Therapy in Genetic Arrhythmia Syndromes RHEA C. PIMENTEL, MD, FACC, FHRS UNIVERSITY OF KANSAS HOSPITAL MID AMERICA CARDIOLOGY AUGUST 19, 2012 Monogenic Arrhythmia Syndromes Mendelian
NGS for Cancer Predisposition
 NGS for Cancer Predisposition Colin Pritchard MD, PhD University of Washington Dept. of Lab Medicine AMP Companion Society Meeting USCAP Boston March 22, 2015 Disclosures I am an employee of the University
NGS for Cancer Predisposition Colin Pritchard MD, PhD University of Washington Dept. of Lab Medicine AMP Companion Society Meeting USCAP Boston March 22, 2015 Disclosures I am an employee of the University
The Genetics and Prevention of Sudden Cardiac Death
 The Genetics and Prevention of Sudden Cardiac Death Sudden cardiac death (SCD), a serious public health problem Every day, between 1,600 and 2,000 people die worldwide from genetically caused SCD. 1 SCD
The Genetics and Prevention of Sudden Cardiac Death Sudden cardiac death (SCD), a serious public health problem Every day, between 1,600 and 2,000 people die worldwide from genetically caused SCD. 1 SCD
Congenital long QT syndrome of particularly malignant course connected with so far unknown mutation in the sodium channel SCN5A gene
 CASE REPORT Cardiology Journal 2013, Vol. 20, No. 1, pp. 78 82 10.5603/CJ.2013.0012 Copyright 2013 Via Medica ISSN 1897 5593 Congenital long QT syndrome of particularly malignant course connected with
CASE REPORT Cardiology Journal 2013, Vol. 20, No. 1, pp. 78 82 10.5603/CJ.2013.0012 Copyright 2013 Via Medica ISSN 1897 5593 Congenital long QT syndrome of particularly malignant course connected with
Advances in genetic diagnosis of neurological disorders
 Acta Neurol Scand 2014: 129 (Suppl. 198): 20 25 DOI: 10.1111/ane.12232 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd ACTA NEUROLOGICA SCANDINAVICA Review Article Advances in genetic diagnosis
Acta Neurol Scand 2014: 129 (Suppl. 198): 20 25 DOI: 10.1111/ane.12232 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd ACTA NEUROLOGICA SCANDINAVICA Review Article Advances in genetic diagnosis
Silvia G Priori MD PhD
 Novel therapies for the long QT syndrome. Silvia G Priori MD PhD Molecular Cardiology, IRCCS Fondazione Salvatore Maugeri Pavia, Italy and Leon Charney Division of Cardiology, Cardiovascular Genetics Program,
Novel therapies for the long QT syndrome. Silvia G Priori MD PhD Molecular Cardiology, IRCCS Fondazione Salvatore Maugeri Pavia, Italy and Leon Charney Division of Cardiology, Cardiovascular Genetics Program,
Using the Bravo Liquid-Handling System for Next Generation Sequencing Sample Prep
 Using the Bravo Liquid-Handling System for Next Generation Sequencing Sample Prep Tom Walsh, PhD Division of Medical Genetics University of Washington Next generation sequencing Sanger sequencing gold
Using the Bravo Liquid-Handling System for Next Generation Sequencing Sample Prep Tom Walsh, PhD Division of Medical Genetics University of Washington Next generation sequencing Sanger sequencing gold
Functional analysis of DNA variants
 Functional analysis of DNA variants GS011143, Introduction to Bioinformatics The University of Texas GSBS program, Fall 2012 Ken Chen, Ph.D. Department of Bioinformatics and Computational Biology UT MD
Functional analysis of DNA variants GS011143, Introduction to Bioinformatics The University of Texas GSBS program, Fall 2012 Ken Chen, Ph.D. Department of Bioinformatics and Computational Biology UT MD
Title:Exome sequencing helped the fine diagnosis of two siblings afflicted with atypical Timothy syndrome (TS2)
 Author's response to reviews Title:Exome sequencing helped the fine diagnosis of two siblings afflicted with atypical Timothy syndrome (TS2) Authors: Sebastian Fröhler (Sebastian.Froehler@mdc-berlin.de)
Author's response to reviews Title:Exome sequencing helped the fine diagnosis of two siblings afflicted with atypical Timothy syndrome (TS2) Authors: Sebastian Fröhler (Sebastian.Froehler@mdc-berlin.de)
AD (Leave blank) TITLE: Genomic Characterization of Brain Metastasis in Non-Small Cell Lung Cancer Patients
 AD (Leave blank) Award Number: W81XWH-12-1-0444 TITLE: Genomic Characterization of Brain Metastasis in Non-Small Cell Lung Cancer Patients PRINCIPAL INVESTIGATOR: Mark A. Watson, MD PhD CONTRACTING ORGANIZATION:
AD (Leave blank) Award Number: W81XWH-12-1-0444 TITLE: Genomic Characterization of Brain Metastasis in Non-Small Cell Lung Cancer Patients PRINCIPAL INVESTIGATOR: Mark A. Watson, MD PhD CONTRACTING ORGANIZATION:
Variant Classification: ACMG recommendations. Andreas Laner MGZ München
 Variant Classification: ACMG recommendations Andreas Laner MGZ München laner@mgz-muenchen.de OVERVIEW Introduction ACMG-AMP Classification System Evaluation of inter-laboratory concordance in variant classification
Variant Classification: ACMG recommendations Andreas Laner MGZ München laner@mgz-muenchen.de OVERVIEW Introduction ACMG-AMP Classification System Evaluation of inter-laboratory concordance in variant classification
P NK. Breast Cancer Genetic Risk Test. Test Report
 Test Report CONTENTS SECTION 1 1-1. Patient Information 1-2. Test Report Summary 1-3. Test Report Details 1-4. List of Genes Tested SECTION 2 2-1. About the Test 2-2. References 1-1. Patient Information
Test Report CONTENTS SECTION 1 1-1. Patient Information 1-2. Test Report Summary 1-3. Test Report Details 1-4. List of Genes Tested SECTION 2 2-1. About the Test 2-2. References 1-1. Patient Information
NGS in neurodegenerative disorders - our experience
 Neurology Clinic, Clinical Center of Serbia Faculty of Medicine, University of Belgrade Belgrade, Serbia NGS in neurodegenerative disorders - our experience Marija Branković, MSc Belgrade, 2018 Next Generation
Neurology Clinic, Clinical Center of Serbia Faculty of Medicine, University of Belgrade Belgrade, Serbia NGS in neurodegenerative disorders - our experience Marija Branković, MSc Belgrade, 2018 Next Generation
Family stories: to illustrate inheritance and the impact on families. Dr Claire Turner Consultant clinical geneticist
 Family stories: to illustrate inheritance and the impact on families Dr Claire Turner Consultant clinical geneticist Overview With respect to inherited cardiac conditions Simple modes of inheritance (Mendelian)
Family stories: to illustrate inheritance and the impact on families Dr Claire Turner Consultant clinical geneticist Overview With respect to inherited cardiac conditions Simple modes of inheritance (Mendelian)
SALSA MLPA probemix P315-B1 EGFR
 SALSA MLPA probemix P315-B1 EGFR Lot B1-0215 and B1-0112. As compared to the previous A1 version (lot 0208), two mutation-specific probes for the EGFR mutations L858R and T709M as well as one additional
SALSA MLPA probemix P315-B1 EGFR Lot B1-0215 and B1-0112. As compared to the previous A1 version (lot 0208), two mutation-specific probes for the EGFR mutations L858R and T709M as well as one additional
