Supplementary Online Content
|
|
|
- Julius Barber
- 5 years ago
- Views:
Transcription
1 Supplementary Online Content de la Poza Abad M, Mas Dalmau G, Moreno Bakedano M, et al; Delayed Antibiotic Prescription (DAP) Group. Prescription strategies in acute uncomplicated respiratory infections: a randomized clinical trial. Published online December 21, JAMA Oncol. doi: /jamainternmed eappendix 1. General exclusion criteria and specific inclusion and exclusion criteria eappendix 2. Primary outcomes and secondary outcomes efigure 1. CONSORT 2010 checklist of information to include when reporting a randomised trial efigure 2. The TIDieR (Template for Intervention Description and Replication) Checklist This supplementary material has been provided by the authors to give readers additional information about their work American Medical Association. All rights reserved.
2 SUPPLEMENTAL MATERIAL eappendix 1 General exclusion criteria - Previous participation in the Delayed antibiotic prescription trial. - Patient is very affected or they have been very affected for a week (constantly) - Patient with signs and symptoms suggestive of disease o serious affectation and/or of complications (particularly pneumonia, mastoiditis, peritonsillar abscess, peritonsillar cellulitis, intraorbital or intracranial complications). - Patient at a risk high of serious complications due to a previous comorbidity. This includes serious conditions involving the heart, lungs, kidney, liver, or neuromuscular disorders, immunosuppression and cystic fibrosis. - If the patient is older than 65 with acute cough and two or more of the following criteria or older than 80 with acute cough and one or more of the following criteria: admission in the previous year, type I or II Diabetes, history of cardiac failure or current use of oral corticosteroids. Specific inclusion and exclusion criteria Acute pharyngitis - Inclusion criteria: adults presenting to the family doctor with sore throat as the main symptom and at least 2 of the Centor criteria: (1) pharyngo-tonsillar exudate, (2) history of fever or feeling of dysthermia, (3) tender anterior cervical adenopathy, and (4) absence of cough. - Exclusion criteria: other causes of sore throat (ulcers, aphthas or thrush), presence of all four Centor criteria, presence of only one or none of the Centor criteria, poor general condition, immunosuppression, antibiotic use in the previous two weeks, history of rheumatic fever, repeated pharyngotonsillitis (more than five episodes in the previous year), pregnant women, and/or use of rapid antigen techniques in the visit. Rhinosinusitis 2015 American Medical Association. All rights reserved.
3 - Inclusion criteria: acute inflammation of the mucous membrane in the nose and pharynx for more than one week, with rhinitis as the main sign and at least one symptom or sign of the sinuses: purulent rhinorrhea or sinus pain. - Exclusion criteria: symptoms for less than a week, poor general condition*, suspected pneumonia, antibiotic use in the previous two weeks and/or use of rapid C-reactive protein test in the visit.. (*) Poor general condition: Patient is very affected or they have been very affected for a week. Acute bronchitis - Inclusion criteria: adults with uncomplicated acute disease presenting with cough as the main symptom and at least one symptom or sign of lower respiratory tract involvement: expectoration, chest pain, shortness of breath or wheezing. - Exclusion criteria: suspected pneumonia (crepitant rales, bronchial murmur, asymmetrical auscultation, tachypnea, vomiting and/or severe diarrhea), bronchial asthma, other acute respiratory or chronic diseases except for mild-to-moderate chronic obstructive pulmonary disease (cystic fibrosis, tuberculosis), active cardiovascular disease, psychiatric diagnoses, dementia, poor general condition, institutionalized in care centers, prior antibiotic use in the previous two weeks, history of admission in the previous year due to respiratory infections and/or use of rapid Creactive protein test in the visit. Exacerbations of mild to moderate chronic obstructive pulmonary disease - Inclusion criteria: > 40 years old, smokers or ex-smokers of over 10 packages-year, with one of the two following diagnoses: chronic bronchitis (cough for more than 3 months, for 3 or more consecutive years), or chronic obstructive pulmonary disease diagnosed with spirometry in the last 2 years (FEV1/FVC <0.7% and FEV1 50%), with an infectious exacerbation and one or both of the following Anthonisen criteria: increased expectoration volume and/or increased shortness of breath. - Exclusion criteria: purulent expectoration, no spirometry in the previous 2 years, severe chronic obstructive pulmonary disease (FEV1 <50%), neoplasm, pregnancy, tracheotomy, poor general condition, antibiotic use in the previous two weeks, suspected pneumonia (crepitant rales, bronchial murmur, asymmetrical auscultation, tachypnea, vomiting and/or severe diarrhea) and/or use of rapid C-reactive protein test in the visit American Medical Association. All rights reserved.
4 eappendix 2 Primary outcome - Duration and severity of symptoms. Patients filled out a daily questionnaire for a maximum of 30 days. Each symptom was scored using a six-point Likert scale (0=no problem, 1=very little problem, 2=slight problem, 3=moderately bad, 4=bad, 5=very bad, 6=as bad as it could be). Symptom scores of 5 or 6 were considered severe and the symptoms scores of 3 or 4 were considered moderate. We included common symptoms such as fever, discomfort or general pain, cough, difficulty sleeping, and changes in everyday life, and specific symptoms according to the condition. Specific symptoms collected for patients with pharyngitis were: swallowing difficulties, headache, nasal mucosity and sore throat. The specific symptoms collected for patients with rhinosinusitis were: spontaneous facial pain, facial pain on touch, headache, nasal mucosity and sore throat. The specific symptoms collected for patients with bronchitis and mild-moderate chronic obstructive pulmonary disease were: expectoration or phlegm, breathlessness, chest pain on breathing and chest noises on breathing. Secondary outcomes - Antibiotic use. Patients were asked about antibiotic use during the last 30 days. - Satisfaction with health care. Patients completed a questionnaire with the Likert scale when they no longer had symptoms. - Belief in the effectiveness of antibiotics. Patients completed a questionnaire with a Likert scale when they no longer had symptoms. - Absenteeism. Patients were asked about the number of days of absence from work or doing their daily activities when they no longer had symptoms. - Risk of complications (pneumonia, abscesses or cellulitis). At 30 days, physicians assessed the complications from clinical records and patient interviews. - Risk of need for unscheduled health care. At 30 days, physicians assessed this need from clinical records and patient interviews American Medical Association. All rights reserved.
5 efigure 1 Section/Topic Title and abstract Introduction Background and objectives CONSORT 2010 checklist of information to include when reporting a randomised trial* Item No 1a 1b Checklist item Identification as a randomised trial in the title Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) 1 Reported on page No 3,4 2a Scientific background and explanation of 5,6 rationale 2b Specific objectives or hypotheses 6 Methods Trial design 3a Description of trial design (such as parallel, factorial) including allocation ratio 3b Important changes to methods after trial commencement (such as eligibility 6,7 Not criteria), with reasons Participants 4a Eligibility criteria for participants 6 and Supplement 1 4b Settings and locations where the data were collected Interventions 5 The interventions for each group with sufficient details to allow replication, including how and when they were actually administered Outcomes 6a Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed 6b Any changes to trial outcomes after the trial commenced, with reasons Sample size 7a How sample size was determined 8 7b When, explanation of any interim analyses and stopping guidelines Randomisation: Sequence 8a Method used to generate the random ,7 7 and Supplement 2 Not Not
6 generatio allocation sequence n 8b Type of randomisation; details of any restriction (such as blocking and block size) Allocation 9 Mechanism used to implement the random concealm allocation sequence (such as sequentially ent numbered containers), describing any mechanis steps taken to conceal the sequence until m interventions were assigned 10 Who generated the random allocation Implementation sequence, who enrolled participants, and who assigned participants to interventions Blinding 11a If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how 11b If relevant, description of the similarity of Statistical methods Results Participant flow (a diagram is strongly recommended) 12a 12b 13a 13b interventions Statistical methods used to compare groups for primary and secondary outcomes Methods for additional analyses, such as subgroup analyses and adjusted analyses For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome For each group, losses and exclusions after randomisation, together with reasons Not 8,9 8,9 Figure 1 Figure 1 Recruitment 14a Dates defining the periods of recruitment 6 and follow-up 14b Why the trial ended or was stopped 12 Baseline data 15 A table showing baseline demographic and clinical characteristics for each group 9 and Table 1 Numbers analysed Table 1 Outcomes and estimation 16 For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups 17a 17b For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) For binary outcomes, presentation of both absolute and relative effect sizes is recommended 9-11 and Table 2-5 Table 5
7 Ancillary analyses 18 Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory Harms 19 All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) Discussion Limitations 20 Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses Generalisability 21 Generalisability (external validity, applicability) of the trial findings Interpretation 22 Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence Other information Registration 23 Registration number and name of trial registry Protocol 24 Where the full trial protocol can be accessed, if available Funding 25 Sources of funding and other support (such as supply of drugs), role of funders 9-11 and Table 2-5 9,10 12, , (ref.27) 4,9,14 *We strongly recommend reading this statement in conjunction with the CONSORT 2010 Explanation and Elaboration for important clarifications on all the items. If relevant, we also recommend reading CONSORT extensions for cluster randomised trials, non-inferiority and equivalence trials, nonpharmacological treatments, herbal interventions, and pragmatic trials. Additional extensions are forthcoming: for those and for up to date references relevant to this checklist, see
8 efigure 2 Item number The TIDieR (Template for Intervention Description and Replication) Checklist*: Information to include when describing an intervention and the location of the information Item Where located ** Primary pap (page or appe number) Other (details) BRIEF NAME 1. Provide the name or a phrase that describes the intervention. WHY 2. Describe any rationale, theory, or goal of the elements essential to the intervention. WHAT 3. Materials: Describe any physical or informational materials used in the intervention, including those provided to participants or used in intervention delivery or in training of intervention providers. Provide information on where the materials can be accessed (e.g. online appendix, URL). 4. Procedures: Describe each of the procedures, activities, and/or processes used in the intervention, including any enabling or support activities. WHO PROVIDED 5. For each category of intervention provider (e.g. psychologist, nursing assistant), describe their expertise, 1 3 and
9 background and any specific training given. HOW 6. Describe the modes of delivery (e.g. face-to-face or by some other mechanism, such as internet or telephone) of the intervention and whether it was provided individually or in a group. WHERE 7. Describe the type(s) of location(s) where the intervention occurred, including any necessary infrastructure or relevant features. 8 8 WHEN and HOW MUCH 8. Describe the number of times the 8 intervention was delivered and over what period of time including the number of sessions, their schedule, and their duration, intensity or dose. TAILORING 9. If the intervention was planned to be Not personalised, titrated or adapted, then describe what, why, when, and how. MODIFICATIONS 10. ǂ If the intervention was modified during Not the course of the study, describe the changes (what, why, when, and how). HOW WELL 11. Planned: If intervention adherence or Not
10 fidelity was assessed, describe how and by whom, and if any strategies were used to maintain or improve fidelity, describe them. 12. ǂ Actual: If intervention adherence or fidelity was assessed, describe the extent to which the intervention was delivered as planned. Not ** Authors - use N/A if an item is not for the intervention being described. Reviewers use? if information about the element is not reported/not sufficiently reported. If the information is not provided in the primary paper, give details of where this information is available. This may include locations such as a published protocol or other published papers (provide citation details) or a website (provide the URL). ǂ If completing the TIDieR checklist for a protocol, these items are not relevant to the protocol and cannot be described until the study is complete. * We strongly recommend using this checklist in conjunction with the TIDieR guide (see BMJ 2014;348:g1687) which contains an explanation and elaboration for each item. * The focus of TIDieR is on reporting details of the intervention elements (and where relevant, comparison elements) of a study. Other elements and methodological features of studies are covered by other reporting statements and checklists and have not been duplicated as part of the TIDieR checklist. When a randomised trial is being reported, the TIDieR checklist should be used in conjunction with the CONSORT statement (see as an extension of Item 5 of the CONSORT 2010 Statement. When a clinical trial protocol is being reported, the TIDieR checklist should be used in conjunction with the SPIRIT statement as an extension of Item 11 of the SPIRIT 2013 Statement (see For alternate study designs, TIDieR can be used in conjunction with the appropriate checklist for that study design (see
CONSORT 2010 checklist of information to include when reporting a randomised trial*
 CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
CONSORT 2010 checklist of information to include when reporting a randomised trial* Section/Topic Title and abstract Introduction Background and objectives Item No Checklist item 1a Identification as a
CONSORT 2010 checklist of information to include when reporting a randomised trial*
 Supplemental Figures for: Ramosetron Versus Ondansetron in Combination with Aprepitant and Dexamethasone for the Prevention of Highly Emetogenic Chemotherapy-induced Nausea and Vomiting: A Multicenter,
Supplemental Figures for: Ramosetron Versus Ondansetron in Combination with Aprepitant and Dexamethasone for the Prevention of Highly Emetogenic Chemotherapy-induced Nausea and Vomiting: A Multicenter,
Details on the procedure and devices used for assessment and calculation of
 SUPPLEMENTAL METHODS Details on the procedure and devices used for assessment and calculation of cardiovascular parameters The peripheral psychophysiological activation was registered via impedance cardiography
SUPPLEMENTAL METHODS Details on the procedure and devices used for assessment and calculation of cardiovascular parameters The peripheral psychophysiological activation was registered via impedance cardiography
Title 1 Descriptive title identifying the study design, population, interventions, and, if applicable, trial acronym
 SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents* Section/item Item No Description Addressed on page number Administrative information Title 1 Descriptive
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents* Section/item Item No Description Addressed on page number Administrative information Title 1 Descriptive
a) Subjects must be willing and able to give signed and dated written informed consent.
 Detailed inclusion and exclusion criteria Inclusion Criteria 1) Signed Written Informed Consent a) Subjects must be willing and able to give signed and dated written informed consent. 2) Target Population
Detailed inclusion and exclusion criteria Inclusion Criteria 1) Signed Written Informed Consent a) Subjects must be willing and able to give signed and dated written informed consent. 2) Target Population
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients
R eview. Cough: Controversies and Consensus Brian s Case. Acute Cough
 R eview Cough: Controversies and Consensus 2011 Copyright Not for Sale or Commercial Distribution Irvin Mayers, MD, FRCPC Unauthorised use prohibited. Authorised users can download, display, view and print
R eview Cough: Controversies and Consensus 2011 Copyright Not for Sale or Commercial Distribution Irvin Mayers, MD, FRCPC Unauthorised use prohibited. Authorised users can download, display, view and print
Respiratory System Virology
 Respiratory System Virology Common Cold: Rhinitis. A benign self limited syndrome caused by several families of viruses. The most frequent acute illness in industrialized world. Mild URT illness involving:
Respiratory System Virology Common Cold: Rhinitis. A benign self limited syndrome caused by several families of viruses. The most frequent acute illness in industrialized world. Mild URT illness involving:
CONSORT 2010 Statement Annals Internal Medicine, 24 March History of CONSORT. CONSORT-Statement. Ji-Qian Fang. Inadequate reporting damages RCT
 CONSORT-Statement Guideline for Reporting Clinical Trial Ji-Qian Fang School of Public Health Sun Yat-Sen University Inadequate reporting damages RCT The whole of medicine depends on the transparent reporting
CONSORT-Statement Guideline for Reporting Clinical Trial Ji-Qian Fang School of Public Health Sun Yat-Sen University Inadequate reporting damages RCT The whole of medicine depends on the transparent reporting
Randomized Controlled Trial
 Randomized Controlled Trial Training Course in Sexual and Reproductive Health Research Geneva 2016 Dr Khalifa Elmusharaf MBBS, PgDip, FRSPH, PHD Senior Lecturer in Public Health Graduate Entry Medical
Randomized Controlled Trial Training Course in Sexual and Reproductive Health Research Geneva 2016 Dr Khalifa Elmusharaf MBBS, PgDip, FRSPH, PHD Senior Lecturer in Public Health Graduate Entry Medical
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Stewart CP, Kariger P, Fernald L, et al. Effects
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Stewart CP, Kariger P, Fernald L, et al. Effects
USAID Health Care Improvement Project. pneumonia) respiratory infections through improved case management (amb/hosp)
 Improvement objective: : decrease morbidity and mortality due to acute upper (rhinitis, sinusitis, pharyngitis) and lower (bronchitis, pneumonia) respiratory infections through improved case management
Improvement objective: : decrease morbidity and mortality due to acute upper (rhinitis, sinusitis, pharyngitis) and lower (bronchitis, pneumonia) respiratory infections through improved case management
Respiratory System. Respiratory System Overview. Component 3/Unit 11. Health IT Workforce Curriculum Version 2.0/Spring 2011
 Component 3-Terminology in Healthcare and Public Health Settings Unit 11-Respiratory System This material was developed by The University of Alabama at Birmingham, funded by the Department of Health and
Component 3-Terminology in Healthcare and Public Health Settings Unit 11-Respiratory System This material was developed by The University of Alabama at Birmingham, funded by the Department of Health and
Upper Respiratory Tract Infections / 42
 Upper Respiratory Tract Infections 1 Upper Respiratory Tract Infections Acute tonsillitispharyngitis Acute otitis media Acute sinusitis Common cold Acute laryngitis Otitis externa Mastoiditis Acute apiglottis
Upper Respiratory Tract Infections 1 Upper Respiratory Tract Infections Acute tonsillitispharyngitis Acute otitis media Acute sinusitis Common cold Acute laryngitis Otitis externa Mastoiditis Acute apiglottis
Guidelines for Reporting Non-Randomised Studies
 Revised and edited by Renatus Ziegler B.C. Reeves a W. Gaus b a Department of Public Health and Policy, London School of Hygiene and Tropical Medicine, Great Britain b Biometrie und Medizinische Dokumentation,
Revised and edited by Renatus Ziegler B.C. Reeves a W. Gaus b a Department of Public Health and Policy, London School of Hygiene and Tropical Medicine, Great Britain b Biometrie und Medizinische Dokumentation,
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
From protocol to publication: ensuring quality in the reporting of continence research Workshop 20 Monday, August 23rd 2010, 14:00 17:00
 From protocol to publication: ensuring quality in the reporting of continence research Workshop 20 Monday, August 23rd 2010, 14:00 17:00 Time Time Topic Speaker 14:00 14:15 Introduction Rufus Cartwright
From protocol to publication: ensuring quality in the reporting of continence research Workshop 20 Monday, August 23rd 2010, 14:00 17:00 Time Time Topic Speaker 14:00 14:15 Introduction Rufus Cartwright
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
CHECK-LISTS AND Tools DR F. R E Z A E I DR E. G H A D E R I K U R D I S TA N U N I V E R S I T Y O F M E D I C A L S C I E N C E S
 CHECK-LISTS AND Tools DR F. R E Z A E I DR E. G H A D E R I K U R D I S TA N U N I V E R S I T Y O F M E D I C A L S C I E N C E S What is critical appraisal? Critical appraisal is the assessment of evidence
CHECK-LISTS AND Tools DR F. R E Z A E I DR E. G H A D E R I K U R D I S TA N U N I V E R S I T Y O F M E D I C A L S C I E N C E S What is critical appraisal? Critical appraisal is the assessment of evidence
Reducing unnecessary antibiotic use in respiratory tract infections in children
 Reducing unnecessary antibiotic use in respiratory tract infections in children -a secondary care perspective Dr Conor Doherty (Consultant in paediatric infectious diseases and immunology GGC) Current
Reducing unnecessary antibiotic use in respiratory tract infections in children -a secondary care perspective Dr Conor Doherty (Consultant in paediatric infectious diseases and immunology GGC) Current
ACHA Clinical Benchmarking Program
 Acute Care Measure: Avoidance of Antibiotic Treatment for Adults Aged 18-64 with Acute Bronchitis Background The American College of Chest Physicians defines acute bronchitis as "an acute respiratory infection
Acute Care Measure: Avoidance of Antibiotic Treatment for Adults Aged 18-64 with Acute Bronchitis Background The American College of Chest Physicians defines acute bronchitis as "an acute respiratory infection
I have no disclosures
 Disclosures Streptococcal Pharyngitis: Update and Current Guidelines Richard A. Jacobs, MD, PhD Emeritus Professor of Medicine Division of Infectious Diseases I have no disclosures CID 2012:55;e 86-102
Disclosures Streptococcal Pharyngitis: Update and Current Guidelines Richard A. Jacobs, MD, PhD Emeritus Professor of Medicine Division of Infectious Diseases I have no disclosures CID 2012:55;e 86-102
ASTHMA-COPD OVERLAP SYNDROME 2018: What s All the Fuss?
 ASTHMA-COPD OVERLAP SYNDROME 2018: What s All the Fuss? Randall W. Brown, MD MPH AE-C Association of Asthma Educators Annual Conference July 20, 2018 Phoenix, Arizona FACULTY/DISCLOSURES Randall Brown,
ASTHMA-COPD OVERLAP SYNDROME 2018: What s All the Fuss? Randall W. Brown, MD MPH AE-C Association of Asthma Educators Annual Conference July 20, 2018 Phoenix, Arizona FACULTY/DISCLOSURES Randall Brown,
Respiratory Diseases and Disorders
 Chapter 9 Respiratory Diseases and Disorders Anatomy and Physiology Chest, lungs, and conducting airways Two parts: Upper respiratory system consists of nose, mouth, sinuses, pharynx, and larynx Lower
Chapter 9 Respiratory Diseases and Disorders Anatomy and Physiology Chest, lungs, and conducting airways Two parts: Upper respiratory system consists of nose, mouth, sinuses, pharynx, and larynx Lower
PIDS AND RESPIRATORY DISORDERS
 PRIMARY IMMUNODEFICIENCIES PIDS AND RESPIRATORY DISORDERS PIDS AND RESPIRATORY DISORDERS 1 PRIMARY IMMUNODEFICIENCIES ABBREVIATIONS COPD CT MRI IG PID Chronic obstructive pulmonary disease Computed tomography
PRIMARY IMMUNODEFICIENCIES PIDS AND RESPIRATORY DISORDERS PIDS AND RESPIRATORY DISORDERS 1 PRIMARY IMMUNODEFICIENCIES ABBREVIATIONS COPD CT MRI IG PID Chronic obstructive pulmonary disease Computed tomography
The RESPIRATORY System. Unit 3 Transportation Systems
 The RESPIRATORY System Unit 3 Transportation Systems The Respiratory System Functions of the Respiratory System Warms, moistens, and filters incoming air Nasal cavity Resonating chambers for speech and
The RESPIRATORY System Unit 3 Transportation Systems The Respiratory System Functions of the Respiratory System Warms, moistens, and filters incoming air Nasal cavity Resonating chambers for speech and
Appropriate Use of Antibiotics for the Treatment of Acute Upper Respiratory Tract Infections in Adults
 Appropriate Use of Antibiotics for the Treatment of Acute Upper Respiratory Tract Infections in Adults Kyong Ran Peck, M.D. Division of Infectious Diseases Sungkyunkwan University School of Medicine, Samsung
Appropriate Use of Antibiotics for the Treatment of Acute Upper Respiratory Tract Infections in Adults Kyong Ran Peck, M.D. Division of Infectious Diseases Sungkyunkwan University School of Medicine, Samsung
Statement on the use of delayed prescriptions of antibiotics for infants and children
 Statement on the use of delayed prescriptions of antibiotics for infants and children Endorsed by the Royal College of General Practitioners Background Delayed prescribing (also known as back up prescribing)
Statement on the use of delayed prescriptions of antibiotics for infants and children Endorsed by the Royal College of General Practitioners Background Delayed prescribing (also known as back up prescribing)
an inflammation of the bronchial tubes
 BRONCHITIS DEFINITION Bronchitis is an inflammation of the bronchial tubes (or bronchi), which are the air passages that extend from the trachea into the small airways and alveoli. Triggers may be infectious
BRONCHITIS DEFINITION Bronchitis is an inflammation of the bronchial tubes (or bronchi), which are the air passages that extend from the trachea into the small airways and alveoli. Triggers may be infectious
Online appendices. Table of Contents TABLE OF CONTENTS 1 APPENDIX 1. SEARCH STRATEGIES 2 APPENDIX 2. PRISMA FLOW DIAGRAM 4
 Online appendices Table of Contents TABLE OF CONTENTS 1 APPENDIX 1. SEARCH STRATEGIES 2 APPENDIX 2. PRISMA FLOW DIAGRAM 4 APPENDIX 3. CHARACTERISTICS OF THE INCLUDED STUDIES 5 APPENDIX 4. RISK OF BIAS
Online appendices Table of Contents TABLE OF CONTENTS 1 APPENDIX 1. SEARCH STRATEGIES 2 APPENDIX 2. PRISMA FLOW DIAGRAM 4 APPENDIX 3. CHARACTERISTICS OF THE INCLUDED STUDIES 5 APPENDIX 4. RISK OF BIAS
Appendices. Appendix A Search terms
 Appendices Appendix A Search terms Database Search terms Medline 1. Ipilimumab; 2. MDX-010; 3. MDX-101; 4. Yervoy; 5. BMS-734016; 6. Nivolumab; 7. ONO-4538; 8. BMS-936558; 9. MDX-1106; 10. Opdivo; 11.
Appendices Appendix A Search terms Database Search terms Medline 1. Ipilimumab; 2. MDX-010; 3. MDX-101; 4. Yervoy; 5. BMS-734016; 6. Nivolumab; 7. ONO-4538; 8. BMS-936558; 9. MDX-1106; 10. Opdivo; 11.
Web appendix: Supplementary data
 Web appendix: Supplementary data Azad MA, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, Friesen C, Abou-Setta AM, Zarychanski R. Probiotic supplementation during pregnancy or infancy for the
Web appendix: Supplementary data Azad MA, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, Friesen C, Abou-Setta AM, Zarychanski R. Probiotic supplementation during pregnancy or infancy for the
TENNCARE Bundled Payment Initiative: Description of Bundle Risk Adjustment for Wave 5 Episodes
 TENNCARE Bundled Payment Initiative: Description of Bundle Risk Adjustment for Wave 5 Episodes Mastectomy, Breast Cancer Medical Oncology, Breast Biopsy, Tonsillectomy, Otitis media, Anxiety, Non-emergent
TENNCARE Bundled Payment Initiative: Description of Bundle Risk Adjustment for Wave 5 Episodes Mastectomy, Breast Cancer Medical Oncology, Breast Biopsy, Tonsillectomy, Otitis media, Anxiety, Non-emergent
Comorbidome, Pattern, and Impact of Asthma-COPD Overlap Syndrome in Real Life
 Comorbidome, Pattern, and Impact of Asthma-COPD Overlap Syndrome in Real Life Job F. M. van Boven, PharmD, PhD; Miguel Román-Rodríguez, MD; Josep F. Palmer, MD; Núria Toledo-Pons, MD; Borja G. Cosío, MD,
Comorbidome, Pattern, and Impact of Asthma-COPD Overlap Syndrome in Real Life Job F. M. van Boven, PharmD, PhD; Miguel Román-Rodríguez, MD; Josep F. Palmer, MD; Núria Toledo-Pons, MD; Borja G. Cosío, MD,
Supplementary Online Content
 1 Supplementary Online Content 2 3 4 5 Hay AD, Little P, Harnden A, et al. Effect of oral prednisolone on symptom duration in nonasthmatic adults with acute lower respiratory tract infection: a randomized
1 Supplementary Online Content 2 3 4 5 Hay AD, Little P, Harnden A, et al. Effect of oral prednisolone on symptom duration in nonasthmatic adults with acute lower respiratory tract infection: a randomized
lozenge/spray) within the previous 8 hours; a longer acting or slow-release analgesic during the previous 24 hours (e.g. piroxicam, naproxen); any
 Benzocaine: Primary: Chrubasik S, Beime B, Maora F. Efficacy of a benzocaine lozenge in the treatment of uncomplicated sore throat. Eur Arch Otorhinolaryngol 2012; 269:571-77. Extended Abstract: Study
Benzocaine: Primary: Chrubasik S, Beime B, Maora F. Efficacy of a benzocaine lozenge in the treatment of uncomplicated sore throat. Eur Arch Otorhinolaryngol 2012; 269:571-77. Extended Abstract: Study
UPPER RESPIRATORY TRACT INFECTIONS. IAP UG Teaching slides
 UPPER RESPIRATORY TRACT INFECTIONS 1 INTRODUCTION Most common problem in children below 5 years. In this age group they get about 6 8 episodes per year. It includes infections of nasal cavity, throat,
UPPER RESPIRATORY TRACT INFECTIONS 1 INTRODUCTION Most common problem in children below 5 years. In this age group they get about 6 8 episodes per year. It includes infections of nasal cavity, throat,
Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List
 Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List (1) Have all important studies that you are aware of been included? No additional
Reviewer No. 1 checklist for application of: inclusion of Nifurtimox + eflornithine in the WHO Essential Medicines List (1) Have all important studies that you are aware of been included? No additional
SCREENING AND PREVENTION
 These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
These protocols are designed to implement standard guidelines, based on the best evidence, that provide a consistent clinical experience for AHC II Integrated Clinical Delivery Network patients and allow
The Respiratory System
 130 20 The Respiratory System 1. Define important words in this chapter 2. Explain the structure and function of the respiratory system 3. Discuss changes in the respiratory system due to aging 4. Discuss
130 20 The Respiratory System 1. Define important words in this chapter 2. Explain the structure and function of the respiratory system 3. Discuss changes in the respiratory system due to aging 4. Discuss
The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews
 The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews David Moher 1, Alessandro Liberati 2, Douglas G Altman 3, Jennifer Tetzlaff 1 for the QUOROM Group
The QUOROM Statement: revised recommendations for improving the quality of reports of systematic reviews David Moher 1, Alessandro Liberati 2, Douglas G Altman 3, Jennifer Tetzlaff 1 for the QUOROM Group
Asthma Assessment & Review
 ASTHMA RESOURCE PACK Section 5B Asthma Assessment & Review In this section: 1. Primary Care initial assessment and review Asthma Resource Pack Section 5B: Asthma Assessment & Review Version 3.0 Last Updated:
ASTHMA RESOURCE PACK Section 5B Asthma Assessment & Review In this section: 1. Primary Care initial assessment and review Asthma Resource Pack Section 5B: Asthma Assessment & Review Version 3.0 Last Updated:
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Efficiency
 Quality ID #333: Adult Sinusitis: Computerized Tomography (CT) for Acute Sinusitis (Overuse) National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Quality ID #333: Adult Sinusitis: Computerized Tomography (CT) for Acute Sinusitis (Overuse) National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Reports of efficacy and safety studies of primary immunodeficiency
 2. SYNOPSIS TITLE OF STUDY: Clinical Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of IGIV3I GRIFOLS [Immune Globulin Intravenous (Human)] for Replacement Therapy in Primary Immunodeficiency
2. SYNOPSIS TITLE OF STUDY: Clinical Study to Evaluate the Safety, Efficacy, and Pharmacokinetics of IGIV3I GRIFOLS [Immune Globulin Intravenous (Human)] for Replacement Therapy in Primary Immunodeficiency
RESPIRATORY TRACT INFECTIONS. CLS 212: Medical Microbiology Zeina Alkudmani
 RESPIRATORY TRACT INFECTIONS CLS 212: Medical Microbiology Zeina Alkudmani Lower Respiratory Tract Upper Respiratory Tract Anatomy of the Respiratory System Nasopharynx Oropharynx Respiratory Tract Infections
RESPIRATORY TRACT INFECTIONS CLS 212: Medical Microbiology Zeina Alkudmani Lower Respiratory Tract Upper Respiratory Tract Anatomy of the Respiratory System Nasopharynx Oropharynx Respiratory Tract Infections
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection](/thumbs/93/111890101.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
SYNOPSIS. Clinical Study Report IM Double-blind Period
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #331: Adult Sinusitis: Antibiotic Prescribed for Acute Viral Sinusitis (Overuse) National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Quality ID #331: Adult Sinusitis: Antibiotic Prescribed for Acute Viral Sinusitis (Overuse) National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY
Bronchiectasis in Adults - Suspected
 Bronchiectasis in Adults - Suspected Clinical symptoms which may indicate bronchiectasis for patients Take full respiratory history including presenting symptoms, past medical & family history Factors
Bronchiectasis in Adults - Suspected Clinical symptoms which may indicate bronchiectasis for patients Take full respiratory history including presenting symptoms, past medical & family history Factors
The Throat. Image source:
 The Throat Anatomy Image source: http://anatomyforlayla.blogspot.co.za/2007/04/blog-post.html The Throat consists of three parts: 1. The Nasopharynx is the upper part of the throat and it is situated behind
The Throat Anatomy Image source: http://anatomyforlayla.blogspot.co.za/2007/04/blog-post.html The Throat consists of three parts: 1. The Nasopharynx is the upper part of the throat and it is situated behind
Unconscious exchange of air between lungs and the external environment Breathing
 Respiration Unconscious exchange of air between lungs and the external environment Breathing Two types External Exchange of carbon dioxide and oxygen between the environment and the organism Internal Exchange
Respiration Unconscious exchange of air between lungs and the external environment Breathing Two types External Exchange of carbon dioxide and oxygen between the environment and the organism Internal Exchange
5/5/2013. The Respiratory System. Chapter 16 Notes. The Respiratory System. Nasal Cavity. Sinuses
 The Respiratory System Chapter 16 Notes The Respiratory System Objectives List the general functions of the respiratory system. Identify the organs of the respiratory system. Describe the functions of
The Respiratory System Chapter 16 Notes The Respiratory System Objectives List the general functions of the respiratory system. Identify the organs of the respiratory system. Describe the functions of
New Patient Questionnaire Pediatric Orthopaedic Surgery
 Page 1 of 5 New Patient Questionnaire Pediatric Orthopaedic Surgery First Name: Last Name: Middle: DOB: Height: Weight: Primary Care Physician/Pediatrician Name: Address: Phone Number: Chief Compliant
Page 1 of 5 New Patient Questionnaire Pediatric Orthopaedic Surgery First Name: Last Name: Middle: DOB: Height: Weight: Primary Care Physician/Pediatrician Name: Address: Phone Number: Chief Compliant
EXACERBATION ASSESSMENT FORM
 EXACERBATION ASSESSMENT FORM ID NUMBER: 0a) Form Completion Date... 0b) Staff Code... Administrative Information 1) Date of clinic visit: 2) What type of Event is this?... Participant/HCU-triggered...
EXACERBATION ASSESSMENT FORM ID NUMBER: 0a) Form Completion Date... 0b) Staff Code... Administrative Information 1) Date of clinic visit: 2) What type of Event is this?... Participant/HCU-triggered...
Pathway diagrams Annex F
 Pathway diagrams Annex F Fig 1 Asthma: The patient journey Asthma is diagnosed Making the diagnosis of asthma Confirming the diagnosis may depend on history, response to treatment, measurement of airflow
Pathway diagrams Annex F Fig 1 Asthma: The patient journey Asthma is diagnosed Making the diagnosis of asthma Confirming the diagnosis may depend on history, response to treatment, measurement of airflow
UMEC/VI vs. UMEC in subjects who responded to UMEC UMEC/VI vs. VI in subjects who responded to VI
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
EXACERBATION ASSESSMENT FORM
 EXACERBATION ASSESSMENT FORM ID NUMBER: VERSION: 1.0 05/27/14 0a) Form Completion Date... 0b) Staff Code... Instructions: This form should be completed when a participant comes to the clinical center for
EXACERBATION ASSESSMENT FORM ID NUMBER: VERSION: 1.0 05/27/14 0a) Form Completion Date... 0b) Staff Code... Instructions: This form should be completed when a participant comes to the clinical center for
Clinical Implications of Asthma Phenotypes. Michael Schatz, MD, MS Department of Allergy
 Clinical Implications of Asthma Phenotypes Michael Schatz, MD, MS Department of Allergy Definition of Phenotype The observable properties of an organism that are produced by the interaction of the genotype
Clinical Implications of Asthma Phenotypes Michael Schatz, MD, MS Department of Allergy Definition of Phenotype The observable properties of an organism that are produced by the interaction of the genotype
Bronchitis. Anatomy of the Lungs The lungs allow us to fill our blood with oxygen. The oxygen we breathe is absorbed into our blood in the lungs.
 Bronchitis Introduction Bronchitis is an inflammation of the bronchial tubes, the airways that carry air to the lungs. It causes shortness of breath, wheezing and chest tightness as well as a cough that
Bronchitis Introduction Bronchitis is an inflammation of the bronchial tubes, the airways that carry air to the lungs. It causes shortness of breath, wheezing and chest tightness as well as a cough that
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS
 TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
TARGET POPULATION Eligibility Inclusion Criterion Exclusion Criterion RECOMMENDATIONS Recommendation PULMONARY FUNCTION TESTING (SPIROMETRY) Conditional: The Expert Panel that spirometry measurements FEV1,
RESPIRATORY CARE IN GENERAL PRACTICE
 RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
RESPIRATORY CARE IN GENERAL PRACTICE Definitions of Asthma and COPD Asthma is due to inflammation of the air passages in the lungs and affects the sensitivity of the nerve endings in the airways so they
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Persistent Obstructive Sleep Apnea After Tonsillectomy. Learning Objectives. Mary Frances Musso, DO Pediatric Otolaryngology
 Persistent Obstructive Sleep Apnea After Tonsillectomy Mary Frances Musso, DO Pediatric Otolaryngology Learning Objectives Recognize indications for tonsillectomy List patients at risk for persistent OSA
Persistent Obstructive Sleep Apnea After Tonsillectomy Mary Frances Musso, DO Pediatric Otolaryngology Learning Objectives Recognize indications for tonsillectomy List patients at risk for persistent OSA
Respiratory system. Applied Anatomy &Physiology
 Respiratory system Applied Anatomy &Physiology Anatomy The respiratory system consists of 1)The Upper airway : Nose, mouth and larynx 2)The Lower airways Trachea and the two lungs. Within the lungs,
Respiratory system Applied Anatomy &Physiology Anatomy The respiratory system consists of 1)The Upper airway : Nose, mouth and larynx 2)The Lower airways Trachea and the two lungs. Within the lungs,
SPECIFIED PHYSICAL CONDITIONS MATRIX
 SPECIFIED PHYSICAL CONDITIONS MATRIX I. Compensation for ACUTE CONDITIONS A1 Proof Lump Sum Enhancer Declaration under penalty of perjury (1) asserting the manifestation of one or more conditions (or the
SPECIFIED PHYSICAL CONDITIONS MATRIX I. Compensation for ACUTE CONDITIONS A1 Proof Lump Sum Enhancer Declaration under penalty of perjury (1) asserting the manifestation of one or more conditions (or the
Rhinosinusitis. John Ramey, MD Joseph Russell, MD
 Rhinosinusitis John Ramey, MD Joseph Russell, MD Disclosure Statement RSFH as a continuing medical education provider, accredited by the South Carolina Medical Association, it is the policy of RSFH to
Rhinosinusitis John Ramey, MD Joseph Russell, MD Disclosure Statement RSFH as a continuing medical education provider, accredited by the South Carolina Medical Association, it is the policy of RSFH to
PLEASE COMPLETE ALL SECTIONS OF THIS FORM
 PLEASE COMPLETE ALL SECTIONS OF THIS FORM Patient Name: Date of Birth: Referring Doctor? (Name, telephone number and address) Chief Complaint: Why have you come here? How did it start? What are the symptoms?
PLEASE COMPLETE ALL SECTIONS OF THIS FORM Patient Name: Date of Birth: Referring Doctor? (Name, telephone number and address) Chief Complaint: Why have you come here? How did it start? What are the symptoms?
Azithromycin for sore throat and chest congestion
 Azithromycin for sore throat and chest congestion "I've nearly finished a course of this for a chest infection... "When I start with sore throat in winter it always ends up being bronchitis and I'm off
Azithromycin for sore throat and chest congestion "I've nearly finished a course of this for a chest infection... "When I start with sore throat in winter it always ends up being bronchitis and I'm off
How many tonsillectomies are necessary?
 How many tonsillectomies are necessary? An eleven year retrospective study of indications and eligibility for childhood tonsillectomy in UK Dana Šumilo, Ronan Ryan, Tom Marshall Preventing Overdiagnosis,
How many tonsillectomies are necessary? An eleven year retrospective study of indications and eligibility for childhood tonsillectomy in UK Dana Šumilo, Ronan Ryan, Tom Marshall Preventing Overdiagnosis,
Appropriate Antibiotic Prescribing. Frank Romanelli, Pharm.D., MPH, AAHIVP Professor & Associate Dean Paul F. Parker Endowed Professor of Pharmacy
 Appropriate Antibiotic Prescribing Frank Romanelli, Pharm.D., MPH, AAHIVP Professor & Associate Dean Paul F. Parker Endowed Professor of Pharmacy Objectives Discuss CDCs Core Elements of abx stewardship.
Appropriate Antibiotic Prescribing Frank Romanelli, Pharm.D., MPH, AAHIVP Professor & Associate Dean Paul F. Parker Endowed Professor of Pharmacy Objectives Discuss CDCs Core Elements of abx stewardship.
TENNCARE Bundled Payment Initiative: Description of Bundle Risk Adjustment for Wave 3 Episodes
 TENNCARE Bundled Payment Initiative: Description of Bundle Risk Adjustment for Wave 3 Episodes Respiratory Infection (RI); Pneumonia (PNA); Inpatient Urinary Tract Infection (UTI-I; Outpatient Urinary
TENNCARE Bundled Payment Initiative: Description of Bundle Risk Adjustment for Wave 3 Episodes Respiratory Infection (RI); Pneumonia (PNA); Inpatient Urinary Tract Infection (UTI-I; Outpatient Urinary
INDEPENDENT MEDICAL EXAMINATION
 INDEPENDENT MEDICAL EXAMINATION I have had the opportunity to examine for the purpose of independent medical examination, Mr. John Doe. He was seen in my office at 38 East 32nd Street in New York on February
INDEPENDENT MEDICAL EXAMINATION I have had the opportunity to examine for the purpose of independent medical examination, Mr. John Doe. He was seen in my office at 38 East 32nd Street in New York on February
Supplementary Online Content
 Supplementary Online Content Lee C-C, Lee M-tG, Chen Y-S. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Internal Medicine. Published online October 5, 2015.
Supplementary Online Content Lee C-C, Lee M-tG, Chen Y-S. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Internal Medicine. Published online October 5, 2015.
Clinical Study Design: From Pilot to Randomized Controlled Trials
 Clinical Study Design: From Pilot to Randomized Controlled Trials Thomas C. Hulsey, MSPH, Sc.D. Professor and Chair, Department of Epidemiology Director, Clinical Research Education, CTSI What is a clinical
Clinical Study Design: From Pilot to Randomized Controlled Trials Thomas C. Hulsey, MSPH, Sc.D. Professor and Chair, Department of Epidemiology Director, Clinical Research Education, CTSI What is a clinical
Re-Screening Medical History Questionnaire
 Building Trades National Medical Screening Program Re-Screening Medical History Questionnaire Name: Address: _ City: _State: Zip Phone Number (include Area Code): Social Security # Date of Birth If female,
Building Trades National Medical Screening Program Re-Screening Medical History Questionnaire Name: Address: _ City: _State: Zip Phone Number (include Area Code): Social Security # Date of Birth If female,
Instructions: Please bring these forms to your Physical Examination & TB Test and have the Doctor fill them out. (Where applicable)
 Instructions: Please bring these forms to your Physical Examination & TB Test and have the Doctor fill them out. (Where applicable) 1. The physician s examination certification form. Ask your doctor to
Instructions: Please bring these forms to your Physical Examination & TB Test and have the Doctor fill them out. (Where applicable) 1. The physician s examination certification form. Ask your doctor to
Pre-Admission Testing Questionnaire
 Pre-Admission Testing Questionnaire Approximately 2 weeks prior to your surgery date you will receive a telephone call from our Pre-Admission Testing department. During this conversation, a Registered
Pre-Admission Testing Questionnaire Approximately 2 weeks prior to your surgery date you will receive a telephone call from our Pre-Admission Testing department. During this conversation, a Registered
Burden of major Respiratory Diseases
 Burden of major Respiratory Diseases WHO Survey Ryazan region of Russia, Ryazan region of Russia, health care system: 104 hospitals district hospitals 32 rural hospitals 44 65 out-patient departments
Burden of major Respiratory Diseases WHO Survey Ryazan region of Russia, Ryazan region of Russia, health care system: 104 hospitals district hospitals 32 rural hospitals 44 65 out-patient departments
National Asthma Educator Certification Board Detailed Content Outline
 I. THE ASTHMA CONDITION 9 20 1 30 A. Pathophysiology 4 6 0 10 1. Teach an individual with asthma and their family using simple language by illustrating the following with appropriate educational aids a.
I. THE ASTHMA CONDITION 9 20 1 30 A. Pathophysiology 4 6 0 10 1. Teach an individual with asthma and their family using simple language by illustrating the following with appropriate educational aids a.
Chronic Sinusitis. Acute Sinusitis. Sinusitis. Anatomy of the Paranasal Sinuses. Sinusitis. Medical Topics - Sinusitis
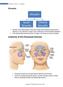 1 Acute Chronic is the inflammation of the inner lining of the parnasal sinuses due to infection or non-infectious causes such as allergies or environmental pullutants. If the inflammation lasts more than
1 Acute Chronic is the inflammation of the inner lining of the parnasal sinuses due to infection or non-infectious causes such as allergies or environmental pullutants. If the inflammation lasts more than
ARIKAYCE IMPORTANT SAFETY INFORMATION
 ARIKAYCE The first and only FDA-approved medication for the treatment of refractory (difficult to treat) MAC lung disease as part of a combination antibacterial drug treatment plan MAC=Mycobacterium avium
ARIKAYCE The first and only FDA-approved medication for the treatment of refractory (difficult to treat) MAC lung disease as part of a combination antibacterial drug treatment plan MAC=Mycobacterium avium
Clinical Practice Guideline: Tonsillectomy in Children, Baugh et al Otolaryngology Head and Neck Surgery, 2011 J and: 144 (1 supplement) S1 30.
 Pediatric ENT Guidelines Jane Cooper, FNP, CORLN References: Clinical Practice Guideline: Tympanostomy tubes in children, Rosenfeld et al., American Academy of Otolaryngology Head and Neck Surgery Foundation
Pediatric ENT Guidelines Jane Cooper, FNP, CORLN References: Clinical Practice Guideline: Tympanostomy tubes in children, Rosenfeld et al., American Academy of Otolaryngology Head and Neck Surgery Foundation
Downloaded from:
 Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
MEDICATION GUIDE XELJANZ (ZEL JANS ) (tofacitinib)
 MEDICATION GUIDE XELJANZ (ZEL JANS ) (tofacitinib) Read this Medication Guide before you start taking XELJANZ and each time you get a refill. There may be new information. This Medication Guide does not
MEDICATION GUIDE XELJANZ (ZEL JANS ) (tofacitinib) Read this Medication Guide before you start taking XELJANZ and each time you get a refill. There may be new information. This Medication Guide does not
2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Process
 Quality ID #52 (NQF 0102): Chronic Obstructive Pulmonary Disease (COPD): Long-Acting Inhaled Bronchodilator Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
Quality ID #52 (NQF 0102): Chronic Obstructive Pulmonary Disease (COPD): Long-Acting Inhaled Bronchodilator Therapy National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL
SYNOPSIS. Date 15 June 2004
 Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Drug product Drug substance(s) Document No. Edition No. Study code SYMBICORT pmdi 160/4.5 mg per actuation Budesonide/formoterol SD-039-0719 Date 15 June 2004 SYNOPSIS A Six-Month, Randomized, Open-Label
Chapter 10 Respiratory System J00-J99. Presented by: Jesicca Andrews
 Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
Chapter 10 Respiratory System J00-J99 Presented by: Jesicca Andrews 1 Respiratory System 2 Respiratory Infections A respiratory infection cannot be assumed from a laboratory report alone; physician concurrence
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
ASTHMA RESOURCE PACK Section 3. Chronic Cough Guidelines
 ASTHMA RESOURCE PACK Section 3 Chronic Cough Guidelines NHS Fife Guidelines for the Management of Chronic Cough in Adults In this section: 1. Introduction 2. Scope Guidelines for Management of Chronic
ASTHMA RESOURCE PACK Section 3 Chronic Cough Guidelines NHS Fife Guidelines for the Management of Chronic Cough in Adults In this section: 1. Introduction 2. Scope Guidelines for Management of Chronic
BELLWORK page 343. Apnea Dyspnea Hypoxia pneumo pulmonary Remember the structures of the respiratory system 1
 BELLWORK page 343 Apnea Dyspnea Hypoxia pneumo pulmonary respiratory system 1 STANDARDS 42) Review case studies that involve persons with respiratory disorders, diseases, or syndromes. Citing information
BELLWORK page 343 Apnea Dyspnea Hypoxia pneumo pulmonary respiratory system 1 STANDARDS 42) Review case studies that involve persons with respiratory disorders, diseases, or syndromes. Citing information
PATHOLOGY & PATHOPHYSIOLOGY
 PATHOLOGY & PATHOPHYSIOLOGY DISORDERS OF THE RESPIRATORY SYSTEM DISORDERS OF THE RESPIRATORY SYSTEM Disorders of the Respiratory System Infections Degenerative Tumours Immune Trauma Congenital Upper respiratory
PATHOLOGY & PATHOPHYSIOLOGY DISORDERS OF THE RESPIRATORY SYSTEM DISORDERS OF THE RESPIRATORY SYSTEM Disorders of the Respiratory System Infections Degenerative Tumours Immune Trauma Congenital Upper respiratory
Supplementary Online Content
 Supplementary Online Content Dharmarajan K, Wang Y, Lin Z, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. doi:10.1001/jama.2017.8444 etable
Supplementary Online Content Dharmarajan K, Wang Y, Lin Z, et al. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. doi:10.1001/jama.2017.8444 etable
Is reslizumab effective in improving quality of life and asthma control in adolescent and adult patients with poorly controlled eosinophilic asthma?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2018 Is reslizumab effective in improving
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2018 Is reslizumab effective in improving
I understand that as a patient, I have both rights and responsibilities. I have received a copy of this document for my reference.
 1. Patient Rights and Responsibilities Acknowledgement I understand that as a patient, I have both rights and responsibilities. I have received a copy of this document for my reference. 2. Notice of Privacy
1. Patient Rights and Responsibilities Acknowledgement I understand that as a patient, I have both rights and responsibilities. I have received a copy of this document for my reference. 2. Notice of Privacy
Medical History. Participant Id#: Acrostic: Tech ID#: Date: / / 1 How would you say your health currently compares with other persons of your age?
 Multi-Ethnic Study of Atherosclerosis Exam 5 Participant Id#: Acrostic: Tech ID#: Medical History Interviewer Administered Date: / / Month Day Year The following are some questions about your medical history.
Multi-Ethnic Study of Atherosclerosis Exam 5 Participant Id#: Acrostic: Tech ID#: Medical History Interviewer Administered Date: / / Month Day Year The following are some questions about your medical history.
BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012
 BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012 Evidence base for Home Oxygen therapy in COPD, non-copd respiratory disease and nonrespiratory
BTS Guideline for Home Oxygen use in adults Appendix 9 (online only) Key Questions - PICO 10 December 2012 Evidence base for Home Oxygen therapy in COPD, non-copd respiratory disease and nonrespiratory
PULMONARY CARE OF CENTRAL FLORIDA, P.A. Date: / /
 PULMONARY CARE OF CENTRAL FLORIDA, P.A. Date: / / Patient Name Age DOB: / / Family Physician Referring Physician Telephone Number Telephone Number Pharmacy: Phone: Fax: MEDICAL HISTORY 1. What is your
PULMONARY CARE OF CENTRAL FLORIDA, P.A. Date: / / Patient Name Age DOB: / / Family Physician Referring Physician Telephone Number Telephone Number Pharmacy: Phone: Fax: MEDICAL HISTORY 1. What is your
Study No.: Title: Rationale: Phase: Study Period Study Design: Centres: Indication: Treatment: Objectives : Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Problem Based Learning Session. Mr Robinson is a 67 year old man. He visits the GP as he has had a cough and fever for 5 days.
 Problem Based Learning Session Mr Robinson is a 67 year old man. He visits the GP as he has had a cough and fever for 5 days. The GP takes a history from him and examines his chest. Over the left base
Problem Based Learning Session Mr Robinson is a 67 year old man. He visits the GP as he has had a cough and fever for 5 days. The GP takes a history from him and examines his chest. Over the left base
