Notch signaling in pancreatic cancer: oncogene or tumor suppressor?
|
|
|
- Sabrina Parker
- 6 years ago
- Views:
Transcription
1 Review Notch signaling in pancreatic cancer: oncogene or tumor suppressor? Jacqueline L. Avila 1 and Joseph L. Kissil 2 1 Molecular and Cellular Oncogenesis Program, The Wistar Institute, Philadelphia, PA 19104, USA 2 Department of Cancer Biology, The Scripps Research Institute, Jupiter, FL 33458, USA The Notch signaling pathways are known to play critical roles during pancreatic development, but it remains unclear what functions are important in the adult organ. One area of debate is the role of Notch signaling in the development of pancreatic ductal adenocarcinoma (PDAC) and proposed precursor lesions, pancreatic intraepithelial neoplasia (PanIN). Initial studies revealed that Notch signaling is reactivated during PDAC initiation and development, suggesting that Notch promotes PDAC and may therefore represent a target for drug development. However, more recent work reveals a tumor suppressive role for Notch receptors in the context of PanIN development. Here, we summarize the current literature describing Notch signaling in the development of PDAC, and discuss the potential of the Notch pathway as a therapeutic target. The development of pancreatic ductal adenocarcinoma PDAC is one of the most lethal types of cancer the number of patients diagnosed with the disease annually is nearly equal to the number of mortalities [1]. PDAC is believed to evolve through the progression of noninvasive precursor lesions, including PanIN, which are classified into four stages of increasing nuclear and architectural abnormalities: PanIN-1A, -1B, -2, and -3 [2]. Similar to other epithelial cancers, the progression of PanIN lesions coincides with specific genetic events [3]. The most commonly mutated oncogene in PDAC is KRAS; activating mutations are found in >95% of PDAC cases [4]. KRAS mutations are also found in PanIN lesions, with approximately 36% of PanIN-1A lesions and nearly 87% of PanIN-2 and -3 lesions harboring a KRAS mutation [2]. The near universal presence of KRAS mutations in PDAC and their high prevalence in PanIN lesions suggest that activation of KRAS is both an initiating event and necessary for the development of PDAC, making this an attractive candidate for a targeted therapy. However, efforts to develop inhibitors directly targeting Kras to date have failed to result in clinically effective treatment options [5]. Thus, focus has shifted from directly targeting KRAS to identifying and targeting pathways that KRAS relies upon to effect transformation. Corresponding author: Kissil, J.L. (jkissil@scripps.edu) Keywords: pancreatic ductal adenocarcinoma; Notch; PanIN; gamma-secretase inhibitorkras /$ see front matter ß 2013 Elsevier Ltd. All rights reserved. j.molmed One such candidate is the Notch signaling pathway. Notch signaling has previously been shown to be required for Ras-induced transformation of fibroblasts [6] and Hras driven tumorigenesis in a mouse mammary tumor model [7]. Furthermore, the Notch signaling pathway appears to be activated in human pancreatic cancer, given that the expression of receptors, ligands, and downstream targets are induced compared with normal epithelial tissue [8]. However, recent studies utilizing mouse models have revealed both oncogenic and tumor suppressive roles for Notch signaling in PDAC development. These conflicting results necessitate further investigation into the role of Notch and strategies for Notch inhibition to treat PDAC. Mouse models of PDAC Proof that mutations in KRAS are a critical event in PDAC development came from mouse models in which expression of oncogenic Kras (Kras G12D ) at endogenous levels was targeted to pancreatic epithelium using the pancreatic-specific promoters Pdx-1 or p48/ptf1a [9]. Pdx- 1;Cre;LSL-Kras G12D and p48 +/Cre ;LSL-Kras G12D mice developed the full spectrum of PanIN lesions with complete penetrance. These mouse models demonstrated that physiological levels of Kras G12D are sufficient to drive PanIN development and are excellent model systems for investigating the molecular events that occur in the human disease. Although termed ductal adenocarcinoma, the cell of origin for PDAC remains elusive. The Pdx1-Cre;LSL- Kras G12D model is a valuable tool to study PanIN progression, but it renders the identification of the cell of origin for PDAC challenging because all pancreatic epithelial cells undergo Kras activation, including acinar, ductal, and centroacinar cells, as well as the cells comprising islets. Both PanIN lesions and PDAC express markers of ductal differentiation and morphologically resemble ductal cells, initially leading investigators to believe PDAC initiates in ductal cells. However, targeted expression of oncogenic Kras under control of the ductal-specific cytokeratin 19 promoter failed to produce PanIN lesions in a mouse model [10]. More recent studies confirm that ductal cells expressing Kras G12D are largely refractory to PanIN development [11]. By contrast, mouse models have demonstrated that targeting oncogenic Kras expression to acinar and centroacinar cells of the exocrine compartment in the pancreas, as well as endocrine lineages, results in the development of 320 Trends in Molecular Medicine May 2013, Vol. 19, No. 5
2 PanIN lesions. The first study to demonstrate that the exocrine lineage gives rise to PanIN lesions utilized Nestin-Cre transgenic mice [12]. Nestin is expressed later than Pdx-1 during pancreatic development, and marks exocrine progenitor cells. Targeting oncogenic Kras to a Nestinpositive cell lineage resulted in the development of PanIN lesions at the same frequency as in Pdx1-Cre;LSL- Kras G12D mice. Additional mouse models have identified the cell types within the exocrine compartment that are susceptible to oncogenic Kras-induced PanIN formation. Embryonic expression of an oncogenic allele of Kras (Kras G12V ) in acinar and centroacinar cells leads to the development of PanIN lesions and PDAC [13], although mature acinar and centroacinar cells in adult animals were refractory to Kras-driven PanIN development unless the mice were subjected to chronic chemically induced pancreatitis. Studies employing a second-generation mouse model found that targeting the expression of a different Kras mutation (Kras G12D ) to adult acinar cells did result in the spontaneous induction of PanIN lesions [14]. Overall, both studies conclude that acinar cells expressing oncogenic Kras give rise to PanINs, suggesting that acinar cells are a potential cell of origin for PDAC development. The development of lesions from adult acinar cells expressing Kras G12D highlights the plastic nature of mature pancreatic acinar cells and suggests that acinar-toductal metaplasia (ADM), the replacement of acinar tissue with ductal lesions, may be a precursor to the development of PanIN lesions. This process has been proposed to occur through a variety of mechanisms, including selective proliferation of ductal cells, differentiation of a stem cell population, or transdifferentiation of acinar cells to ductal cells [15]. In vitro, acinar cells can transdifferentiate to a ductal phenotype [16], and recent studies have shown that oncogenic Kras expression is sufficient to induce transdifferentiation in vitro even in the absence of exogenous growth factors [17,18]. Acinar-to-ductal transdifferentiation has also been documented to occur in vivo using lineage tracing models [19] when metaplasia is induced by chronic pancreatitis. However, acinar-to-ductal transdifferentiation only accounts for a minority of metaplastic lesions, approximately 12% [19], and therefore it is possible that either expansion of ductal cells or transdifferentiation of centroacinar cell populations accounts for increased metaplastic ductal lesions. To this point, a mouse model relying on pancreatic-specific Pten deletion provides evidence that the expansion of centroacinar cells accounts for ductal metaplasia [20]. Observations of human tumor samples suggest that acinar-to-ductal metaplasia does occur [21], but it remains unknown if these lesions progress to PanIN development. Recently, atypical flat lesions (AFLs) arising in areas of ADM in Ptf1a-Cre;Kras G12D mice have been proposed as direct PDAC precursors and as an alternative to the PanIN PDAC progression model [22]. More work is needed to identify ALFs in human tumors and determine their relationship to PanINs. In addition to acinar and centroacinar cells, endocrine cells are also susceptible to oncogenic Kras-induced transformation in the context of pancreatic injury [23]. These mouse models bring to light two important aspects of PDAC development. First, oncogenic Kras appears to be crucial for the deregulation of pancreatic differentiation. Multiple pancreatic cell lineages, including both exocrine and endocrine cells, can give rise to PanIN lesions when expressing oncogenic Kras. Second, pancreatitis provides a permissive environment for the development of PDAC and may be required in the absence of additional genetic lesions, such as mutations in tumor suppressor genes. This conclusion is especially relevant to human disease because chronic pancreatitis is a strong risk factor for the development of PDAC [24]. Many questions remain concerning how changes induced by pancreatitis affect the different cell lineages and the cellular environment to promote PanIN development, but recent work examining the effects of pancreatitis on acinar cells has revealed potential mechanisms. In the context of caerulein-induced pancreatitis, acinar cells decrease the expression of acinar markers and assume a genetic program resembling embryonic pancreatic precursors [25]. In wild type mice, the acinar compartment is rapidly repopulated following the cessation of caerulein pancreatitis. However, acinar cells expressing oncogenic Kras fail to regenerate and remain in a persistent state of dedifferentiation, ultimately resulting in PanIN development. The mechanism responsible for blocked regeneration appears to be inhibition of b-catenin signaling [26]. Further work is needed to establish how oncogenic Kras and pancreatitis synergize to promote PanIN development in the endocrine compartment. Notch signaling in the pancreas The Notch signaling pathway is a highly evolutionarily conserved pathway that mediates cell-to-cell communication (Figure 1). Among the diverse functions regulated by Notch signaling, some of the most well-documented functions include the maintenance of stem cell populations, determination of cell fate decisions, and the regulation of proliferation and apoptosis. Transcriptional targets of Notch signaling appear to be context-dependent. Two of the best-characterized targets are basic helix loop helix transcriptional repressors, the hairy enhancer of split (HES) and hairyrelated transcription factor (HEY) families. Notch signaling regulates multiple cell fate decisions during pancreatic development, but the main function appears to be maintaining a pool of undifferentiated, progenitor-like cells. Deletion of the genes encoding the Notch pathway ligand Dll1 or the DNA-binding protein RBJ-Jk leads to an accelerated differentiation of pancreatic progenitor cells into endocrine cells [27]. Hes1 knockout mice exhibit a similar phenotype, revealing that the effects of Notch signaling are potentially mediated through this target [28]. As expected, activation of the Notch pathway during development has the converse effect: both exocrine and endocrine differentiation programs are arrested and cells remain in a progenitor-like state [29]. At later stages of pancreatic development, Notch signaling appears to regulate exocrine cell differentiation in a complex manner. Deletion of Rbpj in pancreatic progenitors at embryonic day 10.5 causes a lack of acinar tissue and the appearance of large duct-like structures [30]. 321
3 Delta/Jagged Notch ADAM10 or TACE GSIs γ-secretase NICD Target genes repressed Maml Target genes ac ve CSL Corepressors TRENDS in Molecular Medicine Figure 1. Gamma-secretase activity is required during activation of the Notch signaling pathway. In mammals, the main components of the Notch pathway include the four transmembrane Notch receptors (Notch1 4) and their cognate ligands, Delta-like (DLL) 1, -3, and -4, and Jagged1 and -2. Notch receptors expressed on the cell surface bind to the Delta and Jagged family of ligands on an adjacent cell, after which Notch undergoes a series of proteolytic cleavages. The first cleavage is mediated by either TACE (tumor necrosis factor-a-converting enzyme) or ADAM10 (A Disintegrin and metalloproteinase domain-containing protein 10), followed by a second cleavage mediated by the gamma-secretase complex. This final cleavage releases the Notch intracellular domain (NICD) from the cell membrane, allowing this domain to translocate to the nucleus. Once in the nucleus, NICD binds to the transcription factor CSL (CBF-1, Suppressor of Hairless, Lag-2), displacing corepressors and recruiting transcriptional activators, including the coactivator Mastermind-like1 (Maml). Gamma-secretase inhibitors (GSIs) block the cleavage of Notch receptors by the gamma-secretase complex, inhibiting the release of NICD from the cell membrane. Similar results are seen in Jag1-deficient mice, which display malformed pancreatic ducts and acinar cell death [31]. Surprisingly, pancreatic-specific deletion of the Notch1 and Notch2 alleles does not lead to gross abnormalities in development, suggesting a Notch-independent function of Rbpj [30,32,33]. Alternatively, activation of Notch3 or Notch4 may compensate for loss of Notch1 and Notch2. Although the previous studies indicate that Notch signaling promotes acinar differentiation, other studies lead to opposing conclusions. Esni et al. have demonstrated that ectopic Notch activation in explant cultures inhibits acinar differentiation [34]. Furthermore, experiments in zebrafish demonstrate that the loss of Notch signaling accelerates acinar differentiation [34]. The discrepancies in these studies may result from lossof-function versus gain-of-function approaches as well as differences in the model systems. More recently, Notch signaling was shown to initiate ductal cell differentiation over endocrine specification depending on pathway activation thresholds [35]. Clearly, more work is needed to thoroughly understand how Notch signaling regulates exocrine cell differentiation. Unlike pancreatic development, very little is known about the role of Notch signaling in the adult organ (Figure 2). Most studies suggest that the expression and activation of Notch receptors are downregulated in the adult organ under normal physiological conditions [8,25]. An exception to this may be terminal duct and centroacinar cells, which express Hes1 [20,36]. Recent work indicates that Notch signaling in Hes1 + centroacinar cells functions to suppress acinar cell differentiation. Upon deletion of Rbpj in Hes1 + cells, centroacinar cells rapidly transform to acinar cells [36]. However, other studies reveal a distinct function for Notch signaling in the adult pancreas following injury. In response to chemically induced pancreatitis, the majority of exocrine tissue is lost and the surviving acinar cells induce genes associated with a progenitor-like phenotype, among them Notch1, Notch2, Jagged2, and Hes1 [25]. Deletion of Notch1 in the pancreatic epithelium impairs acinar regeneration following acute pancreatitis, indicating that Notch signaling plays a role in pancreatic homeostasis [32]. Finally, emerging evidence indicates that different Notch receptors may have nonoverlapping functions and are expressed in unique cellular compartments of the pancreas [37]. Using transgenic Notch1-GFP and a Notch2 lacz knockin reporter mice, it has been shown that Notch1 expression is observed primarily in acinar cells and Notch2 expression is localized to ductal cells. Interestingly, these results contrast with a previous study showing that Notch signaling is restricted to centroacinar cells [38]. The discrepancies described above highlight the need to directly determine where individual Notch receptors are expressed in the adult pancreas. Future studies await the development of antibodies sensitive enough to detect the endogenous proteins in tissue sections. Notch and cancer The Notch signaling pathway exerts both oncogenic and tumor suppressive functions, depending on the cellular context. Notch receptors have been identified as oncogenes 322
4 Acinar Cells Transgenic mouse models indicate acinar cells express Notch1. Notch1 is required for acinar regenera on following pancrea s. Centroacinar Cells (CACs) A transgenic reporter mouse demonstrates Notch ac vity in CACs. The Notch target gene, Hes1, is expressed in CACs. Notch signaling may func on to maintain CAC iden ty and inhibit acinar cell differen a on. Ductal cells A knockin mouse model indicates ductal cells express Notch2. TRENDS in Molecular Medicine Figure 2. Notch receptors are expressed in the mature exocrine pancreas. The mature pancreas is composed of exocrine and endocrine compartments, with the exocrine compartment consisting of acinar, ductal, and centroacinar cells. Notch receptors are known to play critical roles during pancreatic development; however, their role and expression patterns in the mature pancreas remain to be fully defined. Genetically engineered mouse models as well as immunohistochemical analysis indicate that Notch receptors are expressed and activated in mature exocrine cells. in multiple tumors, including leukemia, breast, colorectal, cervical, lung, and oral squamous cell carcinoma (reviewed in [39]). Although few activating mutations in Notch receptors have been identified in solid human tumors [40], high levels of NOTCH1 and JAG1 expression correlate with poor patient prognosis in lung and breast cancer [41,42]. In human samples, Notch pathway components are highly expressed in pancreatic adenocarcinoma [8], renal cell carcinoma [43], and prostate cancer [44] compared with control tissue. Furthermore, expression of NUMB, a negative regulator of Notch activity, is frequently lost in breast and non-small cell lung cancer [45]. These data imply that Notch pathway activation is induced in a variety of solid human malignancies by mechanisms other than activating mutations. The finding that Notch signaling is activated in multiple human tumors has led to interest in therapeutically targeting these pathways. The most widely used method to globally inhibit Notch signaling is the use of gamma-secretase inhibitors (GSIs), which block the cleavage of Notch at the cell membrane, inhibiting release of the transcriptionally active Notch intracellular domain (NICD) subunit. Recently, this approach has proven effective in inhibiting lung tumor progression in a mouse model [46]. Although GSIs are currently being tested in clinical trials for multiple types of cancer, previous clinical studies of treatments for Alzheimer s disease reveal that the compounds have significant toxicity, including gastrointestinal bleeding. Given that Notch signaling is required to maintain undifferentiated cells in intestinal crypts [47], it has been suggested that on-target effects, that is, the global Notch inhibition by GSIs, cause this toxicity. A second caveat to the use of GSIs is that the gamma-secretase complex is responsible for catalyzing the proteolysis of over 100 additional substrates in addition to Notch receptors [48], making it difficult to attribute antitumor effects exclusively to a blockade in Notch signaling. Finally, Notch receptors also have known tumor suppressor functions, discussed below, that might be impaired by GSI treatment. In response to the above issues, monoclonal antibodies capable of selectively inhibiting specific Notch receptors have been developed. Antibodies that specifically antagonize Notch1 or Notch2 inhibit tumor growth in vivo by decreasing cell proliferation and increasing apoptosis [49]. Furthermore, treatment with either the anti-notch1 or anti-notch2 antibody alone does not result in severe intestinal toxicity. Based on their efficacy and limited toxicity, Notch antibodies are currently being developed as treatments for multiple cancers, including T cell acute lymphoblastic leukemia (T-ALL) and solid tumors [50]. Although Notch was originally identified as an oncogene, recent studies have also demonstrated tumor suppressive effects for Notch receptors, illustrating the highly context-dependent nature of the pathway. The first conclusive evidence showing that Notch1 acts as a tumor suppressor came from studies in skin, where the loss of both Notch1 alleles leads to the development of spontaneous basal cell carcinomas in mice [51]. Although initial studies indicated that Notch1 functions as a tumor suppressor in a cell-autonomous manner, more recent work has highlighted a non-cell-autonomous mechanism [52]. Notch1 may function as a tumor suppressor in human skin cancers as well. Multiple components of the Notch signaling pathway, including NOTCH1, NOTCH2, and JAGGED1, show reduced expression in human basal cell carcinoma samples [53]. Further evidence supporting a role for NOTCH in human skin cancers came from the results of clinical trials studying semagacestat, a GSI, as a treatment for Alzheimer s disease. The trial revealed that patients taking the GSI had an increased risk of developing skin cancer [54]. In addition to non-melanoma skin cancers, Notch has been implicated as a tumor suppressor in prostate cancer, hepatocellular carcinoma, and small cell lung cancer [55], and loss-of-function mutations have been identified in human chronic myelomonocytic leukemia (CMML) and 323
5 Table 1. Mouse models reveal Notch receptors are implicated in PanIN and PDAC development Receptor Mouse model Role in PanIN/PDAC development Refs Notch1 Pdx1Cre ERT2 ;Kras G12D ;Rosa26NIC Notch1-ICD expression accelerates PanIN development [57] Pdx1-Cre;Kras G12D ;Notch1 lox/lox Notch1 deletion accelerates PanIN development [30] Ptf1a-Cre;Kras G12D ;Notch1 lox/lox Notch1 deletion decreases median survival [33] Notch2 Ptf1a-Cre;Kras G12D ;Notch2 lox/lox Notch2 deletion inhibits PanIN development and increases median survival [33] squamous cell carcinoma samples [56 58]. Somatic inactivating mutations in NICASTRIN and APH1, components of the gamma-secretase complex, as well as mutations in NOTCH2 and MAML1 were identified in a panel of human CMML samples [56]. Furthermore, mouse models of CMML show that loss of Notch signaling alters hematopoietic stem cell differentiation, resulting in the accumulation of monocyte progenitors and a CMML-like disease. A similar study analyzing human head and neck squamous cell carcinoma (HNSCC) tumors identified inactivating mutations in NOTCH1 in 15% of patients, with the majority of the mutations occurring in the same region of the protein, N-terminal to the transmembrane domain. Importantly, 9 of the 21 samples with NOTCH1 mutations in this study possess inactivating mutations in both alleles, supporting the notion that NOTCH1 acts as a classical tumor suppressor [57]. Finally, loss-of-function mutations in NOTCH1 and NOTCH2 are also seen in human cutaneous squamous cell carcinoma and lung squamous cell carcinoma [58]. Interestingly, the majority of these mutations are heterozygous, implying that NOTCH may act as a haploinsufficient tumor suppressor. Notch signaling and pancreatic cancer The role of Notch signaling in pancreatic cancer remains unresolved evidence supporting both oncogenic and tumor suppressive functions exists (Figure 3). In support of an oncogenic role for Notch signaling in PDAC, multiple pathway components are upregulated in pancreatic cancer samples compared with normal pancreatic epithelium, including Notch receptors, ligands, and targets [8]. Furthermore, analysis of the Pdx1-Cre;LSL-Kras G12D mouse model revealed increased expression of the Notch target Hes1 in PanIN lesions compared with normal ducts [9]. Although these results do not establish a causative role for Notch signaling, they do indicate that the pathway is inappropriately activated during PDAC development. However, a more recent global genomic analysis of the core signaling pathways activated in a panel of human pancreatic cancer samples revealed no evidence for overexpression of Notch receptors or classical target genes [59]. Studies relying on mouse models have also revealed both oncogenic and tumor suppressive functions for the Notch receptors (Table 1). Recently, work from our group demonstrated that Notch1 suppresses PanIN formation in a mouse model of PDAC (Pdx1-Cre;LSL-Kras G12D ;Notch1 lox/lox ) [33], a result supported by findings showing that deletion of both Notch1 alleles in ptf1a +/Cre ;LSL-Kras G12D mice caused a slight decrease in median survival [37]. By contrast, other groups have identified an oncogenic role for Notch1: coactivation of Kras G12D and Notch1-ICD in mature acinar cells led to significantly higher numbers of PanIN lesions compared with activation of Kras G12D alone [60]. However, expression of Notch1-ICD, in the absence of oncogenic Kras, fails to alter acinar cell differentiation or induce PanIN lesions, suggesting that activation of Notch1 alone is not sufficient to drive tumorigenesis. Several differences exist between these models that may account for the conflicting conclusions, including the timing of Cremediated recombination and overexpression of the activated form of Notch1 to supraphysiological levels. This possibility was recently illustrated in mammary epithelial cultures, where varying levels of NICD expression caused distinct responses: high NICD levels inhibited proliferation, whereas low Notch activity caused a hyperproliferative response [61], illustrating the crucial nature of Notch levels in regulating downstream effector pathways. The role of Notch signaling in PanIN development has also been investigated using GSIs [38]. Pdx1-Cre;LSL- Kras G12D ;p53 lox/+ mice treated with a GSI are refractory to PDAC development, supporting an oncogenic role for Notch signaling. Although this study proposes that Notch signaling is required for PanIN progression, more work is needed to identify which Notch receptor is responsible for inhibiting tumor progression and if other GSI targets are involved. Dissecting which Notch receptors are involved in PDAC is especially relevant given recent work revealing that individual receptors have opposing roles in pancreatic cancer development. Whereas deletion of both Notch1 alleles in mice expressing oncogenic Kras accelerates PanIN progression and causes a slight decrease in median survival, deleting Notch2 prolongs survival and delays PDAC development, shifting the spectrum of lesions towards development of mucinous cystic-like neoplasms [33,37]. The opposing outcomes observed upon Notch1 or Notch2 ablation may be explained by unique downstream targets or differential expression patterns of the receptors. Clearly, the above results indicate that different Notch receptors possess discrete functions in PDAC development, warranting caution in using a global Notch inhibition approach for treatment purposes. An additional explanation for the differences between the studies is that Notch signaling possesses opposing functions during PanIN initiation and progression. In the Pdx1-Cre;LSL- Kras G12D ;Notch1 lox/lox model, Notch is inactivated concurrently with activation of K-ras G12D at the point of PanIN initiation, whereas in the Pdx1-Cre;LSL-Kras G12D ;p53 lox/ + mice the GSI treatment is initiated after PanIN lesions have been established. Therefore, the possibility cannot be excluded that Notch signaling may function to inhibit PanIN development early but act to promote PanIN progression at later stages once lesions are established. A more recent study examining the role of Notch signaling in radiologically evident pancreatic tumors demonstrated 324
6 Role in PDAC Oncogene Tumor Suppressor No Effect Approach Notch1 gain-offunc on in the context of Kras G12D, embryonic and mature acinar cells Embryonic dele on of Notch2 in the context of Kras G12D GSI treatment at early PanIN stage Embryonic dele on of Notch1 in the context of Kras G12D GSI treatment at advanced PDAC stage Results Advanced PanIn lesions and acinarto-ductal reprogramming Inhibi on of PanIN progression Inhibi on of PanIN progression Advanced PanIN lesions No effect as a monothearpy TRENDS in Molecular Medicine Figure 3. Mouse models reveal dual roles for Notch receptors. Mouse models reveal that Notch receptors either promote or inhibit pancreatic intraepithelial neoplasia (PanIN) development depending on the context. Models differ on the Notch receptor being targeted, timing of genetic events, and the cell types targeted. Additional studies employing gamma-secretase inhibitors (GSIs) reveal that Notch receptors function to inhibit PanIN advancement early during disease progression and appear to play a minimal role in advanced pancreatic ductal adenocarcinoma (PDAC) stages. that GSI treatment failed to extend lifespan [62], implying that Notch signaling may not be involved in the maintenance of advanced pancreatic tumors. Hence, more studies are needed to define the effects of GSI treatment on PanIN initiation and progression before these drugs should be considered for clinical use. A final area of debate is the role of Notch signaling in ADM. Notch signaling has been implicated in ADM given that ectopic expression of NICD promotes transdifferentiation in explant culture models [8,63]. Notch is activated by epidermal growth factor receptor (EGFR) activation, and is required for growth factor induced ADM [8]. However, this does not appear to be the case in vivo because expression of Notch1-ICD alone has no effect on acinar cell differentiation. Further, the Notch pathway is consistently upregulated and activated in the absence of EGFR [60,64]. This result is supported by work showing that deletion of Notch1 has no effect on ADM in vitro [18]. One difference between these studies is the status of Kras: the Miyamoto model induces ADM using ectopic EGFR activation in the context of wild type Kras, while subsequent studies utilize Kras G12D expression. These results suggest that activation of Kras and downstream signaling pathways is capable of overriding a requirement for Notch signaling. Furthermore, in Pdx1-Cre;LSL-Kras G12D ;p53 lox/+ mice, treatment with a GSI does not suppress the abundance of metaplastic ducts, despite decreasing the prevalence of PanINs. In addition, in a slightly different in vitro model of ADM using isolated rat acinar cells, inhibition of Notch signaling by a GSI increased the proliferation of metaplastic exocrine cells in a Hes1-independent manner [65]. Finally, recent studies have demonstrated that the ductal fate determinant Sox9 is a key regulator of oncogenic Kras-induced acinar reprogramming [11]. As Notch signaling controls Sox9 expression, this may represent a critical effector of Notch-mediated ADM. Overall, additional studies are needed to clarify the role of various Notch receptors in the development of PDAC. As is the case in the pancreas, there is evidence from other organs indicating that Notch receptors have distinct functions that are cell type- and context-dependent. In the skin, chimeric deletion of Notch1 leads to the spontaneous development of epidermal tumors, whereas deletion of either Notch2 or Notch3 has no phenotypic effect [52]. Recent studies analyzing Notch transcriptional complexes have revealed differential responses for Notch1 and Notch2 depending on promoter architecture, further supporting distinct roles for each receptor [66]. Finally, there is a need to carefully evaluate the assays currently used to assess Notch activity and develop readouts specific to the different receptors. For example, Hes1 is commonly used as a surrogate for activation of the signaling pathway, but Hes1 expression does not necessarily correlate with Notch activation and does not distinguish between Notch receptors. Indeed, the inhibition of Notch signaling in the pancreas does not lead to decreased Hes1 expression [33,37,65], and Hes1 expression fails to recapitulate the effects of activated Notch expression [8,38]. 325
7 Finally, Hes1 can be activated in a Notch-independent manner [67]. Hence, it will be beneficial to identify all downstream effectors of Notch signaling specific to the pancreas in order to accurately assess pathway activation. Several lines of evidence indicate a functional interaction between Notch and Ras in development and cancer. Notch and Ras have been shown to cooperate or antagonize one another depending on cellular context [68]. Notch signaling is required for Ras-induced transformation of fibroblasts and for tumor formation in a mouse mammary tumor model [6,7]. By contrast, Notch1 deletion is required for primary keratinocytes expressing oncogenic Hras to form tumors when injected into nude mice [51]. Given that Kras and Notch signaling are deregulated in PDAC, it is imperative to determine if functional interactions exist between these two critical signaling pathways. Concluding remarks There is now considerable interest in the use of GSIs to treat pancreatic cancer and other malignancies. However, conflicting results on the function of Notch signaling in PDAC warrant further investigation into the long-term effects of this class of compounds (Box 1). Preclinical animal models testing GSI treatment for pancreatic cancer Box 1. Outstanding questions What is the cell of origin for PanIN lesions and ultimately PDAC? During the development and progression of PanIN the pancreas undergoes a shift from a predominantly acinar epithelium to one composed mainly of ductal structures, but despite the term ductal adenocarcinoma, the cell of origin for PDAC remains elusive. Mouse models have demonstrated that targeting oncogenic Kras to both acinar and centroacinar cells, as well as endocrine lineages, can lead to development of PanIN lesions, but more work is needed to identify the cell of origin in the human disease. Are individual Notch receptors expressed in specific compartments in the adult pancreas? The location of Notch receptor expression in the adult quiescent pancreas remains undefined. Most studies are in agreement that Notch receptor expression in the adult pancreas is reduced to nearly undetectable levels, but recent studies propose that individual Notch receptors are expressed in specific compartments of the adult pancreas. Using transgenic reporter mice, Notch1 appears to be localized mainly to acinar tissue, whereas Notch2 is present in ductal and centroacinar cells [37]. However, these results are in conflict with a previous study demonstrating that Notch signaling is restricted to centroacinar cells [38]. These discrepancies highlight the need to further elucidate where individual Notch receptors are expressed in the adult pancreas. What are the downstream mediators of Notch signaling in the pancreas? Notch signaling exerts specific functions depending on the tissue type and developmental stage. The classical mediators of Notch signaling, the Hes and Hey family of transcription factors, are commonly used as surrogates for pathway activation, yet multiple other targets exist. To accurately assess Notch pathway activation during PanIN development and progression, it will be crucial to identify all downstream effectors, as well as receptorspecific targets. What effect, if any, will GSI treatment have on PDAC patients? Recent studies show that individual Notch receptors have opposing roles in the context of pancreatic cancer and normal development. Therefore, global inhibition of the signaling pathway by GSIs may have either beneficial or detrimental effects on PDAC progression. Clearly, more studies are needed to clarify the roles of individual Notch receptors in PDAC progression prior to employing GSIs as a therapeutic approach. have demonstrated mixed results, showing that early treatment with GSIs inhibits PanIN development [38], whereas treatment of advanced tumors fails to prolong survival [62]. Given that most patients are initially diagnosed at an advanced stage of the disease, it is possible that treatment with GSIs alone will fail to significantly improve patient outcomes. A more significant concern is that the use of GSIs may increase the risk of developing PanIN lesions and, ultimately, PDAC. This concern is strengthened by recent Phase III clinical trials testing GSI treatment for Alzheimer s disease, the results of which showed that patients receiving the drug had a higher incidence of developing skin carcinomas, presumably due to inhibition of Notch signaling [54]. Thus, it is conceivable that GSI treatment may increase the risk for developing PanIN lesions. One population specifically at risk would be patients suffering from pancreatitis, a common risk factor for the development of PDAC. Therefore, it is imperative to fully understand the function of Notch signaling pathways during PanIN development to better inform treatment decisions with GSIs. References 1 Ottenhof, N.A. et al. (2011) Molecular characteristics of pancreatic ductal adenocarcinoma. Pathol. Res. Int. 2011, Maitra, A. and Hruban, R.H. (2008) Pancreatic cancer. Annu. Rev. Pathol. 3, Bardeesy, N. and DePinho, R.A. (2002) Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2, Almoguera, C. et al. (1988) Most human carcinomas of the exocrine pancreas contain mutant c-k-ras genes. Cell 53, Vakiani, E. and Solit, D.B. (2011) KRAS and BRAF: drug targets and predictive biomarkers. J. Pathol. 223, Weijzen, S. et al. (2002) Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat. Med. 8, Kiaris, H. et al. (2004) Modulation of Notch signaling elicits signature tumors and inhibits hras1-induced oncogenesis in the mouse mammary epithelium. Am. J. Pathol. 165, Miyamoto, Y. et al. (2003) Notch mediates TGFa-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 3, Hingorani, S.R. et al. (2003) Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, Brembeck, F.H. et al. (2003) The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 63, Kopp, J.L. et al. (2012) Identification of Sox9-dependent acinar-toductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell 22, Carriere, C. et al. (2007) The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc. Natl. Acad. Sci. U.S.A. 104, Guerra, C. et al. (2007) Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, Habbe, N. et al. (2008) Spontaneous induction of murine pancreatic intraepithelial neoplasia (mpanin) by acinar cell targeting of oncogenic Kras in adult mice. Proc. Natl. Acad. Sci. U.S.A. 105, Tosh, D. and Slack, J.M. (2002) How cells change their phenotype. Nat. Rev. Mol. Cell Biol. 3, Means, A.L. et al. (2005) Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132, Scotti, M.L. et al. (2012) Protein kinase C iota regulates pancreatic acinar-to-ductal metaplasia. PLoS ONE 7, e
8 18 Avila, J.L. et al. (2012) Notch1 is not required for acinar-to-ductal metaplasia in a model of Kras-induced pancreatic ductal adenocarcinoma. PLoS ONE 7, e Strobel, O. et al. (2007) In vivo lineage tracing defines the role of acinarto-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 133, Stanger, B.Z. et al. (2005) Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 8, Parsa, I. et al. (1985) Ductal metaplasia of human exocrine pancreas and its association with carcinoma. Cancer Res. 45, Aichler, M. et al. (2012) Origin of pancreatic ductal adenocarcinoma from atypical flat lesions: a comparative study in transgenic mice and human tissues. J. Pathol. 226, Gidekel Friedlander, S.Y. et al. (2009) Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 16, Morris, J.P.T. et al. (2010) KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat. Rev. Cancer 10, Jensen, J.N. et al. (2005) Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology 128, Morris, J.P.T. et al. (2010) Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest. 120, Apelqvist, A. et al. (1999) Notch signalling controls pancreatic cell differentiation. Nature 400, Jensen, J. et al. (2000) Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, Murtaugh, L.C. et al. (2003) Notch signaling controls multiple steps of pancreatic differentiation. Proc. Natl. Acad. Sci. U.S.A. 100, Nakhai, H. et al. (2008) Conditional ablation of Notch signaling in pancreatic development. Development 135, Golson, M.L. et al. (2009) Ductal malformation and pancreatitis in mice caused by conditional Jag1 deletion. Gastroenterology 136, Siveke, J.T. et al. (2008) Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology 134, Hanlon, L. et al. (2010) Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res. 70, Esni, F. et al. (2004) Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development 131, Shih, H.P. et al. (2012) A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development 139, Kopinke, D. et al. (2012) Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev. Biol. 362, Mazur, P.K. et al. (2010) Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc. Natl. Acad. Sci. U.S.A. 107, Plentz, R. et al. (2009) Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 136, Ranganathan, P. et al. (2011) Notch signalling in solid tumours: a little bit of everything but not all the time. Nat. Rev. Cancer 11, Lobry, C. et al. (2011) Oncogenic and tumor suppressor functions of Notch in cancer: it s NOTCH what you think. J. Exp. Med. 208, Donnem, T. et al. (2010) Prognostic impact of Notch ligands and receptors in nonsmall cell lung cancer: coexpression of Notch-1 and vascular endothelial growth factor-a predicts poor survival. Cancer 116, Reedijk, M. et al. (2005) High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 65, Sjolund, J. et al. (2008) Suppression of renal cell carcinoma growth by inhibition of Notch signaling in vitro and in vivo. J. Clin. Invest. 118, Santagata, S. et al. (2004) JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 64, Pece, S. et al. (2011) NUMB-ing down cancer by more than just a NOTCH. Biochim. Biophys. Acta 1815, Maraver, A. et al. (2012) Therapeutic effect of gamma-secretase inhibition in KrasG12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell 22, van Es, J.H. et al. (2005) Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, Groth, C. et al. (2010) Pharmacological analysis of Drosophila melanogaster gamma-secretase with respect to differential proteolysis of Notch and APP. Mol. Pharmacol. 77, Wu, Y. et al. (2010) Therapeutic antibody targeting of individual Notch receptors. Nature 464, Groth, C. and Fortini, M.E. (2012) Therapeutic approaches to modulating Notch signaling: current challenges and future prospects. Semin. Cell Dev. Biol. 23, Nicolas, M. et al. (2003) Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 33, Demehri, S. et al. (2009) Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell 16, Thelu, J. et al. (2002) Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 2, 7 54 Extance, A. (2010) Alzheimer s failure raises questions about diseasemodifying strategies. Nat. Rev. Drug Discov. 9, Koch, U. and Radtke, F. (2007) Notch and cancer: a double-edged sword. Cell. Mol. Life Sci. 64, Klinakis, A. et al. (2011) A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature 473, Agrawal, N. et al. (2011) Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, Wang, N.J. et al. (2011) Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl. Acad. Sci. U.S.A. 108, Jones, S. et al. (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, De La, O.J. et al. (2008) Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. U.S.A. 105, Mazzone, M. et al. (2010) Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 107, Cook, N. et al. (2012) Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma. J. Exp. Med. 209, Sawey, E.T. et al. (2007) Matrix metalloproteinase 7 controls pancreatic acinar cell transdifferentiation by activating the Notch signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 104, Ardito, C.M. et al. (2012) EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell 22, Rooman, I. et al. (2006) Expression of the Notch signaling pathway and effect on exocrine cell proliferation in adult rat pancreas. Am. J. Pathol. 169, Yuan, Z. et al. (2012) Characterization of CSL (CBF-1, Su(H), Lag-1) mutants reveals differences in signaling mediated by Notch1 and Notch2. J. Biol. Chem. 287, Hashimoto, T. et al. (2006) VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development 133, Sundaram, M.V. (2005) The love hate relationship between Ras and Notch. Genes Dev. 19,
Quantification of early stage lesions for loss of p53 should be shown in the main figures.
 Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Reviewer #1 (Remarks to the Author): Expert in prostate cancer The manuscript "Clonal dynamics following p53 loss of heterozygosity in Kras-driven cancers" uses a number of novel genetically engineered
Pancreatic intraepithelial
 Pancreatic intraepithelial neoplasia (PanIN) Markéta Hermanová St. Anne s University Hospital Brno Faculty of Medicine, Masaryk University Precursor lesions of invasive pancreatic cancer Pancreatic intraepithelial
Pancreatic intraepithelial neoplasia (PanIN) Markéta Hermanová St. Anne s University Hospital Brno Faculty of Medicine, Masaryk University Precursor lesions of invasive pancreatic cancer Pancreatic intraepithelial
Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma
 Article Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma Janel L. Kopp, 1,6 Guido von Figura, 2,6 Erin Mayes,
Article Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma Janel L. Kopp, 1,6 Guido von Figura, 2,6 Erin Mayes,
Journal Club. 03/04/2012 Lama Nazzal
 Journal Club 03/04/2012 Lama Nazzal NOTCH and the kidneys Is an evolutionarily conserved cell cell communication mechanism. Is a regulator of cell specification, differentiation, and tissue patterning.
Journal Club 03/04/2012 Lama Nazzal NOTCH and the kidneys Is an evolutionarily conserved cell cell communication mechanism. Is a regulator of cell specification, differentiation, and tissue patterning.
Enterprise Interest Nothing to declare
 Enterprise Interest Nothing to declare Update of mixed tumours of the GI tract, the pancreas and the liver Introduction to the concept of mixed tumours and clinical implication Jean-Yves SCOAZEC Surgical
Enterprise Interest Nothing to declare Update of mixed tumours of the GI tract, the pancreas and the liver Introduction to the concept of mixed tumours and clinical implication Jean-Yves SCOAZEC Surgical
Generating Mouse Models of Pancreatic Cancer
 Generating Mouse Models of Pancreatic Cancer Aom Isbell http://www2.massgeneral.org/cancerresourceroom/types/gi/index.asp Spring/Summer 1, 2012 Alexandros Tzatsos, MD PhD Bardeesy Lab: Goals and Objectives
Generating Mouse Models of Pancreatic Cancer Aom Isbell http://www2.massgeneral.org/cancerresourceroom/types/gi/index.asp Spring/Summer 1, 2012 Alexandros Tzatsos, MD PhD Bardeesy Lab: Goals and Objectives
Multistep nature of cancer development. Cancer genes
 Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
Multistep nature of cancer development Phenotypic progression loss of control over cell growth/death (neoplasm) invasiveness (carcinoma) distal spread (metastatic tumor) Genetic progression multiple genetic
Genetically engineered mouse models of pancreatic adenocarcinoma
 MOLECULAR ONCOLOGY 7 (2013) 232e247 available at www.sciencedirect.com www.elsevier.com/locate/molonc Review Genetically engineered mouse models of pancreatic adenocarcinoma Carmen Guerra*, Mariano Barbacid*
MOLECULAR ONCOLOGY 7 (2013) 232e247 available at www.sciencedirect.com www.elsevier.com/locate/molonc Review Genetically engineered mouse models of pancreatic adenocarcinoma Carmen Guerra*, Mariano Barbacid*
Cutting to the chase: How pathogenic mutations cause Alzheimer s
 Cutting to the chase: How pathogenic mutations cause Alzheimer s The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published
Cutting to the chase: How pathogenic mutations cause Alzheimer s The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published
From crypt stem cell to colorectal cancer
 19 3 2007 6 Chinese Bulletin of Life Sciences Vol. 19, No. 3 Jun., 2007 1004-0374(2007)03-0321-05 ( 510405) Wnt Notch BMP R735.35; R730.21 A From crypt stem cell to colorectal cancer WEN Bin*, CHEN Weiwen
19 3 2007 6 Chinese Bulletin of Life Sciences Vol. 19, No. 3 Jun., 2007 1004-0374(2007)03-0321-05 ( 510405) Wnt Notch BMP R735.35; R730.21 A From crypt stem cell to colorectal cancer WEN Bin*, CHEN Weiwen
 Rath, N., and Olson, M. (2016) Regulation of pancreatic cancer aggressiveness by stromal stiffening. Nature Medicine, 22(5), pp. 462-463. There may be differences between this version and the published
Rath, N., and Olson, M. (2016) Regulation of pancreatic cancer aggressiveness by stromal stiffening. Nature Medicine, 22(5), pp. 462-463. There may be differences between this version and the published
MBios 401/501: Lecture 12.1 Signaling IV. Slide 1
 MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
MBios 401/501: Lecture 12.1 Signaling IV Slide 1 Pathways that require regulated proteolysis 1. Notch and Delta 2. Wnt/ b-catenin 3. Hedgehog 4. NFk-B Our last topic on cell signaling are pathways that
KRAS: ONE ACTOR, MANY POTENTIAL ROLES IN DIAGNOSIS
 UNIVERSITÀ DEGLI STUDI DI PALERMO Scuola di Specializzazione in Biochimica Clinica Direttore Prof. Marcello Ciaccio KRAS: ONE ACTOR, MANY POTENTIAL ROLES IN DIAGNOSIS Loredana Bruno KRAS gene Proto-oncogene
UNIVERSITÀ DEGLI STUDI DI PALERMO Scuola di Specializzazione in Biochimica Clinica Direttore Prof. Marcello Ciaccio KRAS: ONE ACTOR, MANY POTENTIAL ROLES IN DIAGNOSIS Loredana Bruno KRAS gene Proto-oncogene
Neoplasia 18 lecture 6. Dr Heyam Awad MD, FRCPath
 Neoplasia 18 lecture 6 Dr Heyam Awad MD, FRCPath ILOS 1. understand the role of TGF beta, contact inhibition and APC in tumorigenesis. 2. implement the above knowledge in understanding histopathology reports.
Neoplasia 18 lecture 6 Dr Heyam Awad MD, FRCPath ILOS 1. understand the role of TGF beta, contact inhibition and APC in tumorigenesis. 2. implement the above knowledge in understanding histopathology reports.
Pancreatic Adenocarcinoma: What`s hot
 Pancreatic Adenocarcinoma: What`s hot Eva Karamitopoulou-Diamantis Institute of Pathology University of Bern 11.09.2018, 30th ECP, Bilbao Pancreatic Cancer and the Microbiome The Pancreatic Cancer Microbiome
Pancreatic Adenocarcinoma: What`s hot Eva Karamitopoulou-Diamantis Institute of Pathology University of Bern 11.09.2018, 30th ECP, Bilbao Pancreatic Cancer and the Microbiome The Pancreatic Cancer Microbiome
ANAT3231: lectures overview
 ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
CHAPTER 6 SUMMARIZING DISCUSSION
 CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
CHAPTER 6 SUMMARIZING DISCUSSION More than 20 years ago the founding member of the Wnt gene family, Wnt-1/Int1, was discovered as a proto-oncogene activated in mammary gland tumors by the mouse mammary
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
ANAT3231: lectures overview
 ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
ANAT3231: lectures overview Stem Cell Biology Stem Cell Technology Resources: http://php.med.unsw.edu.au/cell biology/ Essential Cell Biology 3 rd edition Alberts Dr Annemiek Beverdam School of Medical
Select problems in cystic pancreatic lesions
 Disclosure Select problems in cystic pancreatic lesions Five Prime Therapeutics shareholder Adicet Bio shareholder Bristol-Meyer Squibb advisory board grace.kim@ucsf.edu Pancreatic cystic lesions Intraductal
Disclosure Select problems in cystic pancreatic lesions Five Prime Therapeutics shareholder Adicet Bio shareholder Bristol-Meyer Squibb advisory board grace.kim@ucsf.edu Pancreatic cystic lesions Intraductal
Cancer Biology Dynamical Cell Systems
 The Institute of Cancer Research PHD STUDENTSHIP PROJECT PROPOSAL PROJECT DETAILS Project Title: SUPERVISORY TEAM Primary Supervisor: The forces behind pancreatic cancer; and changing them as a therapeutic
The Institute of Cancer Research PHD STUDENTSHIP PROJECT PROPOSAL PROJECT DETAILS Project Title: SUPERVISORY TEAM Primary Supervisor: The forces behind pancreatic cancer; and changing them as a therapeutic
Bihong Zhao, M.D, Ph.D Department of Pathology
 Bihong Zhao, M.D, Ph.D Department of Pathology 04-28-2009 Is tumor self or non-self? How are tumor antigens generated? What are they? How does immune system respond? Introduction Tumor Antigens/Categories
Bihong Zhao, M.D, Ph.D Department of Pathology 04-28-2009 Is tumor self or non-self? How are tumor antigens generated? What are they? How does immune system respond? Introduction Tumor Antigens/Categories
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION. Androgen deprivation therapy is the most used treatment of de novo or recurrent
 CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
CHAPTER VII CONCLUDING REMARKS AND FUTURE DIRECTION Stathmin in Prostate Cancer Development and Progression Androgen deprivation therapy is the most used treatment of de novo or recurrent metastatic PCa.
Tumor suppressor genes D R. S H O S S E I N I - A S L
 Tumor suppressor genes 1 D R. S H O S S E I N I - A S L What is a Tumor Suppressor Gene? 2 A tumor suppressor gene is a type of cancer gene that is created by loss-of function mutations. In contrast to
Tumor suppressor genes 1 D R. S H O S S E I N I - A S L What is a Tumor Suppressor Gene? 2 A tumor suppressor gene is a type of cancer gene that is created by loss-of function mutations. In contrast to
Mouse Models of K-RAS- and B-RAFinduced
 Mouse Models of K-RAS- and B-RAFinduced Cancer Martin O. Bergö, Professor Sahlgrenska Cancer Center University of Gothenburg www.gu.se Why models? Why mice? Critical to understanding pathogenesis and identifying
Mouse Models of K-RAS- and B-RAFinduced Cancer Martin O. Bergö, Professor Sahlgrenska Cancer Center University of Gothenburg www.gu.se Why models? Why mice? Critical to understanding pathogenesis and identifying
Introduction. Cancer Biology. Tumor-suppressor genes. Proto-oncogenes. DNA stability genes. Mechanisms of carcinogenesis.
 Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Cancer Biology Chapter 18 Eric J. Hall., Amato Giaccia, Radiobiology for the Radiologist Introduction Tissue homeostasis depends on the regulated cell division and self-elimination (programmed cell death)
Neoplasia part I. Dr. Mohsen Dashti. Clinical Medicine & Pathology nd Lecture
 Neoplasia part I By Dr. Mohsen Dashti Clinical Medicine & Pathology 316 2 nd Lecture Lecture outline Review of structure & function. Basic definitions. Classification of neoplasms. Morphologic features.
Neoplasia part I By Dr. Mohsen Dashti Clinical Medicine & Pathology 316 2 nd Lecture Lecture outline Review of structure & function. Basic definitions. Classification of neoplasms. Morphologic features.
Cancer. The fundamental defect is. unregulated cell division. Properties of Cancerous Cells. Causes of Cancer. Altered growth and proliferation
 Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Cancer The fundamental defect is unregulated cell division. Properties of Cancerous Cells Altered growth and proliferation Loss of growth factor dependence Loss of contact inhibition Immortalization Alterated
Notch signaling. Ramray Bhat 6/09/2017
 Notch signaling Ramray Bhat 6/09/2017 Lecture 1 introduction, signaling fundamentals, receptor ligand structure, cleavage Lecture 2 Introduction to non canonical signaling, Notch signaling in development:
Notch signaling Ramray Bhat 6/09/2017 Lecture 1 introduction, signaling fundamentals, receptor ligand structure, cleavage Lecture 2 Introduction to non canonical signaling, Notch signaling in development:
Development of Carcinoma Pathways
 The Construction of Genetic Pathway to Colorectal Cancer Moriah Wright, MD Clinical Fellow in Colorectal Surgery Creighton University School of Medicine Management of Colon and Diseases February 23, 2019
The Construction of Genetic Pathway to Colorectal Cancer Moriah Wright, MD Clinical Fellow in Colorectal Surgery Creighton University School of Medicine Management of Colon and Diseases February 23, 2019
Computational Systems Biology: Biology X
 Bud Mishra Room 1002, 715 Broadway, Courant Institute, NYU, New York, USA L#5:(October-18-2010) Cancer and Signals Outline 1 2 Outline 1 2 Cancer is a disease of malfunctioning cells. Cell Lineage: Adult
Bud Mishra Room 1002, 715 Broadway, Courant Institute, NYU, New York, USA L#5:(October-18-2010) Cancer and Signals Outline 1 2 Outline 1 2 Cancer is a disease of malfunctioning cells. Cell Lineage: Adult
Concomitant Pancreatic Activation of Kras G12D and Tgfa Results in Cystic Papillary Neoplasms Reminiscent of Human IPMN
 Article Concomitant Pancreatic Activation of Kras G12D and Tgfa Results in Cystic Papillary Neoplasms Reminiscent of Human IPMN Jens T. Siveke, 1 Henrik Einwächter, 1 Bence Sipos, 2 Clara Lubeseder-Martellato,
Article Concomitant Pancreatic Activation of Kras G12D and Tgfa Results in Cystic Papillary Neoplasms Reminiscent of Human IPMN Jens T. Siveke, 1 Henrik Einwächter, 1 Bence Sipos, 2 Clara Lubeseder-Martellato,
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
B Cells Promote Pancreatic Tumorigenesis
 B Cells Promote Pancreatic Tumorigenesis The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Roghanian,
B Cells Promote Pancreatic Tumorigenesis The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher Roghanian,
Summary and Concluding Remarks
 Summary and Concluding Remarks Chapter 6 The intestinal epithelium provides an excellent model system for investigating molecular mechanisms regulating cell lineage establishment, stem cell proliferation,
Summary and Concluding Remarks Chapter 6 The intestinal epithelium provides an excellent model system for investigating molecular mechanisms regulating cell lineage establishment, stem cell proliferation,
Virchow s Hypothesis lymphorecticular infiltration of cancer reflected the origin of cancer at sites of inflammation
 Virchow s Hypothesis 1863 lymphorecticular infiltration of cancer reflected the origin of cancer at sites of inflammation Barrett s esophagus/ Esophageal adenocarcinoma PSC / Cholangiocarcinoma Viral hepatitis
Virchow s Hypothesis 1863 lymphorecticular infiltration of cancer reflected the origin of cancer at sites of inflammation Barrett s esophagus/ Esophageal adenocarcinoma PSC / Cholangiocarcinoma Viral hepatitis
Stem Cells. Induced Stem Cells
 Induced Stem Cells Stem Cells Mouse and human somatic cells can either be reprogrammed to a pluripotent state or converted to another lineage with a combination of transcription factors suggesting that
Induced Stem Cells Stem Cells Mouse and human somatic cells can either be reprogrammed to a pluripotent state or converted to another lineage with a combination of transcription factors suggesting that
Endocrine Pancreas Development and Regeneration: Noncanonical Ideas From Neural Stem Cell Biology
 314 Diabetes Volume 65, February 2016 Jimmy Masjkur, 1 Steven W. Poser, 1 Polyxeni Nikolakopoulou, 1 George Chrousos, 2 Ronald D. McKay, 3 Stefan R. Bornstein, 1 Peter M. Jones, 4 and Andreas Androutsellis-Theotokis
314 Diabetes Volume 65, February 2016 Jimmy Masjkur, 1 Steven W. Poser, 1 Polyxeni Nikolakopoulou, 1 George Chrousos, 2 Ronald D. McKay, 3 Stefan R. Bornstein, 1 Peter M. Jones, 4 and Andreas Androutsellis-Theotokis
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Mice( Dissertation_ 全文 ) https://doi.org/ /doctor.k19
 Impact of Sox9 Dosage and Hes1-medi TitleControlling the Plasticity of Adult Mice( Dissertation_ 全文 ) Author(s) Hosokawa, Shinichi Citation Kyoto University ( 京都大学 ) Issue Date 2015-07-23 URL https://doi.org/10.14989/doctor.k19
Impact of Sox9 Dosage and Hes1-medi TitleControlling the Plasticity of Adult Mice( Dissertation_ 全文 ) Author(s) Hosokawa, Shinichi Citation Kyoto University ( 京都大学 ) Issue Date 2015-07-23 URL https://doi.org/10.14989/doctor.k19
The secret origins and surprising fates of pancreas tumors
 Carcinogenesis vol.35 no.7 pp.1436 1440, 2014 doi:10.1093/carcin/bgu056 Advance Access publication February 28, 2014 Review The secret origins and surprising fates of pancreas tumors Jennifer M.Bailey,
Carcinogenesis vol.35 no.7 pp.1436 1440, 2014 doi:10.1093/carcin/bgu056 Advance Access publication February 28, 2014 Review The secret origins and surprising fates of pancreas tumors Jennifer M.Bailey,
Pancreatic Cytopathology: The Solid Neoplasms
 Pancreatic Cytopathology: The Solid Neoplasms Syed Z. Ali, M.D. Professor of Pathology and Radiology Director of Cytopathology The Johns Hopkins Hospital Baltimore, Maryland Pancreatic Cytopathology: Past,
Pancreatic Cytopathology: The Solid Neoplasms Syed Z. Ali, M.D. Professor of Pathology and Radiology Director of Cytopathology The Johns Hopkins Hospital Baltimore, Maryland Pancreatic Cytopathology: Past,
Description of Procedure or Service. Policy. Benefits Application
 Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
oncogenes-and- tumour-suppressor-genes)
 Special topics in tumor biochemistry oncogenes-and- tumour-suppressor-genes) Speaker: Prof. Jiunn-Jye Chuu E-Mail: jjchuu@mail.stust.edu.tw Genetic Basis of Cancer Cancer-causing mutations Disease of aging
Special topics in tumor biochemistry oncogenes-and- tumour-suppressor-genes) Speaker: Prof. Jiunn-Jye Chuu E-Mail: jjchuu@mail.stust.edu.tw Genetic Basis of Cancer Cancer-causing mutations Disease of aging
FINALIZED SEER SINQ QUESTIONS
 0076 Source 1: WHO Class CNS Tumors pgs: 33 MP/H Rules/Histology--Brain and CNS: What is the histology code for a tumor originating in the cerebellum and extending into the fourth ventricle described as
0076 Source 1: WHO Class CNS Tumors pgs: 33 MP/H Rules/Histology--Brain and CNS: What is the histology code for a tumor originating in the cerebellum and extending into the fourth ventricle described as
number Done by Corrected by Doctor Maha Shomaf
 number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
number 19 Done by Waseem Abo-Obeida Corrected by Abdullah Zreiqat Doctor Maha Shomaf Carcinogenesis: the molecular basis of cancer. Non-lethal genetic damage lies at the heart of carcinogenesis and leads
Characteristics of Cancer Stem Cells (CSCs)
 GENReports: Market & Tech Analysis Characteristics of Cancer Stem Cells (CSCs) > Enal Razvi, Ph.D. Biotechnology Analyst, Managing Director Select Biosciences, Inc. enal@selectbio.us! Topic,IntroducEon,and,Scope!
GENReports: Market & Tech Analysis Characteristics of Cancer Stem Cells (CSCs) > Enal Razvi, Ph.D. Biotechnology Analyst, Managing Director Select Biosciences, Inc. enal@selectbio.us! Topic,IntroducEon,and,Scope!
Isoprenylcysteine carboxylmethyltransferase deficiency exacerbates KRAS-driven pancreatic neoplasia via Notch suppression
 Research article Isoprenylcysteine carboxylmethyltransferase deficiency exacerbates KRAS-driven pancreatic neoplasia via Notch suppression Helen Court, 1,2,3,4 Marc Amoyel, 3 Michael Hackman, 5 Kyoung
Research article Isoprenylcysteine carboxylmethyltransferase deficiency exacerbates KRAS-driven pancreatic neoplasia via Notch suppression Helen Court, 1,2,3,4 Marc Amoyel, 3 Michael Hackman, 5 Kyoung
Early Embryonic Development
 Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
Early Embryonic Development Maternal effect gene products set the stage by controlling the expression of the first embryonic genes. 1. Transcription factors 2. Receptors 3. Regulatory proteins Maternal
11/18/2015. Gloria Su, Ph.D. Path G4500. Cancer Statistics 2015 (Siegel et al, CA Cancer J CLIN 2015) The Pancreatic Cancer Burden
 Mouse Modeling for Human Pancreatic Cancer Gloria Su, Ph.D. gs2157@columbia.edu, ICRC 10-04 Associate Professor Departments of Pathology and Otolaryngology/Head and Neck Surgery Columbia University College
Mouse Modeling for Human Pancreatic Cancer Gloria Su, Ph.D. gs2157@columbia.edu, ICRC 10-04 Associate Professor Departments of Pathology and Otolaryngology/Head and Neck Surgery Columbia University College
mirna Dr. S Hosseini-Asl
 mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
mirna Dr. S Hosseini-Asl 1 2 MicroRNAs (mirnas) are small noncoding RNAs which enhance the cleavage or translational repression of specific mrna with recognition site(s) in the 3 - untranslated region
Appendix 4: WHO Classification of Tumours of the pancreas 17
 S3.01 The WHO histological tumour type must be recorded. CS3.01a The histological type of the tumour should be recorded based on the current WHO classification 17 (refer to Appendices 4-7). Appendix 4:
S3.01 The WHO histological tumour type must be recorded. CS3.01a The histological type of the tumour should be recorded based on the current WHO classification 17 (refer to Appendices 4-7). Appendix 4:
AP VP DLP H&E. p-akt DLP
 A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
A B AP VP DLP H&E AP AP VP DLP p-akt wild-type prostate PTEN-null prostate Supplementary Fig. 1. Targeted deletion of PTEN in prostate epithelium resulted in HG-PIN in all three lobes. (A) The anatomy
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San
 Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
609G: Concepts of Cancer Genetics and Treatments (3 credits)
 Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
Master of Chemical and Life Sciences Program College of Computer, Mathematical, and Natural Sciences 609G: Concepts of Cancer Genetics and Treatments (3 credits) Text books: Principles of Cancer Genetics,
Signaling Vascular Morphogenesis and Maintenance
 Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Signaling Vascular Morphogenesis and Maintenance Douglas Hanahan Science 277: 48-50, in Perspectives (1997) Blood vessels are constructed by two processes: vasculogenesis, whereby a primitive vascular
Human Lung Cancer Pathology and Cellular Biology Mouse Lung Tumor Workshop
 Human Lung Cancer Pathology and Cellular Biology Mouse Lung Tumor Workshop Jan 7 th and 8 th, 2014 Brigitte Gomperts, MD University of California, Los Angeles Lung Structure and Function Airway Epithelial
Human Lung Cancer Pathology and Cellular Biology Mouse Lung Tumor Workshop Jan 7 th and 8 th, 2014 Brigitte Gomperts, MD University of California, Los Angeles Lung Structure and Function Airway Epithelial
MicroRNA expression profiling and functional analysis in prostate cancer. Marco Folini s.c. Ricerca Traslazionale DOSL
 MicroRNA expression profiling and functional analysis in prostate cancer Marco Folini s.c. Ricerca Traslazionale DOSL What are micrornas? For almost three decades, the alteration of protein-coding genes
MicroRNA expression profiling and functional analysis in prostate cancer Marco Folini s.c. Ricerca Traslazionale DOSL What are micrornas? For almost three decades, the alteration of protein-coding genes
Gross appearance of nodular hyperplasia in material obtained from suprapubic prostatectomy. Note the multinodular appearance and the admixture of
 Tiền liệt tuyến Tiền liệt tuyến Gross appearance of nodular hyperplasia in material obtained from suprapubic prostatectomy. Note the multinodular appearance and the admixture of solid and microcystic areas.
Tiền liệt tuyến Tiền liệt tuyến Gross appearance of nodular hyperplasia in material obtained from suprapubic prostatectomy. Note the multinodular appearance and the admixture of solid and microcystic areas.
Can we classify cancer using cell signaling?
 Can we classify cancer using cell signaling? Central hypotheses (big ideas) Alterations to signaling genes would cause leukemic cells to react in an inappropriate or sensitized manner to environmental
Can we classify cancer using cell signaling? Central hypotheses (big ideas) Alterations to signaling genes would cause leukemic cells to react in an inappropriate or sensitized manner to environmental
Molecular classification of breast cancer implications for pathologists. Sarah E Pinder
 Molecular classification of breast cancer implications for pathologists Sarah E Pinder Courtesy of CW Elston Histological types Breast Cancer Special Types 17 morphological special types 25-30% of all
Molecular classification of breast cancer implications for pathologists Sarah E Pinder Courtesy of CW Elston Histological types Breast Cancer Special Types 17 morphological special types 25-30% of all
Animal Tissue Culture SQG 3242 Biology of Cultured Cells. Dr. Siti Pauliena Mohd Bohari
 Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Animal Tissue Culture SQG 3242 Biology of Cultured Cells Dr. Siti Pauliena Mohd Bohari The Culture Environment Changes of Cell s microenvironment needed that favor the spreading, migration, and proliferation
Triple Negative Breast Cancer
 Triple Negative Breast Cancer Prof. Dr. Pornchai O-charoenrat Division of Head-Neck & Breast Surgery Department of Surgery Faculty of Medicine Siriraj Hospital Breast Cancer Classification Traditional
Triple Negative Breast Cancer Prof. Dr. Pornchai O-charoenrat Division of Head-Neck & Breast Surgery Department of Surgery Faculty of Medicine Siriraj Hospital Breast Cancer Classification Traditional
Cancer Genetics. What is Cancer? Cancer Classification. Medical Genetics. Uncontrolled growth of cells. Not all tumors are cancerous
 Session8 Medical Genetics Cancer Genetics J avad Jamshidi F a s a U n i v e r s i t y o f M e d i c a l S c i e n c e s, N o v e m b e r 2 0 1 7 What is Cancer? Uncontrolled growth of cells Not all tumors
Session8 Medical Genetics Cancer Genetics J avad Jamshidi F a s a U n i v e r s i t y o f M e d i c a l S c i e n c e s, N o v e m b e r 2 0 1 7 What is Cancer? Uncontrolled growth of cells Not all tumors
Cell Polarity and Cancer
 Cell Polarity and Cancer Pr Jean-Paul Borg Email: jean-paul.borg@inserm.fr Features of malignant cells Steps in Malignant Progression Cell polarity, cell adhesion, morphogenesis and tumorigenesis pathways
Cell Polarity and Cancer Pr Jean-Paul Borg Email: jean-paul.borg@inserm.fr Features of malignant cells Steps in Malignant Progression Cell polarity, cell adhesion, morphogenesis and tumorigenesis pathways
Determination Differentiation. determinated precursor specialized cell
 Biology of Cancer -Developmental Biology: Determination and Differentiation -Cell Cycle Regulation -Tumor genes: Proto-Oncogenes, Tumor supressor genes -Tumor-Progression -Example for Tumor-Progression:
Biology of Cancer -Developmental Biology: Determination and Differentiation -Cell Cycle Regulation -Tumor genes: Proto-Oncogenes, Tumor supressor genes -Tumor-Progression -Example for Tumor-Progression:
Contents. Preface XV Acknowledgments XXI List of Abbreviations XXIII About the Companion Website XXIX
 Contents Preface XV Acknowledgments XXI List of Abbreviations XXIII About the Companion Website XXIX 1 General Aspects of Signal Transduction and Cancer Therapy 1 1.1 General Principles of Signal Transduction
Contents Preface XV Acknowledgments XXI List of Abbreviations XXIII About the Companion Website XXIX 1 General Aspects of Signal Transduction and Cancer Therapy 1 1.1 General Principles of Signal Transduction
Pancreatic ductal adenocarcinoma (PDAC) is often characterized
 The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia Catherine Carrière*, Elliott S. Seeley*, Tobias Goetze*, Daniel S. Longnecker, and Murray Korc* *Departments
The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia Catherine Carrière*, Elliott S. Seeley*, Tobias Goetze*, Daniel S. Longnecker, and Murray Korc* *Departments
NEOPLASIA. 3. Which of the following tumour is benign a. Chondrosarcoma b. Osteochondroma c. Chondroblastoma d. Ewing s tumour e.
 NEOPLASIA 1. malignant neoplasms a. are independent of hormonal influence b. are always composed of homogenous cell lines c. arise from differentiated cells by a process of anaplasia d. display abnormal
NEOPLASIA 1. malignant neoplasms a. are independent of hormonal influence b. are always composed of homogenous cell lines c. arise from differentiated cells by a process of anaplasia d. display abnormal
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Targeting Oncogenic Drivers
 Targeting Oncogenic Drivers Yujie Zhao Alex A. Adjei Department of Medicine, Roswell Park Cancer Institute, Buffalo, N.Y., USA Abstract Cancer is a genetic disease caused by a series of somatic and/or
Targeting Oncogenic Drivers Yujie Zhao Alex A. Adjei Department of Medicine, Roswell Park Cancer Institute, Buffalo, N.Y., USA Abstract Cancer is a genetic disease caused by a series of somatic and/or
Recent advances in breast cancers
 Recent advances in breast cancers Breast cancer is a hetrogenous disease due to distinct genetic alterations. Similar morphological subtypes show variation in clinical behaviour especially in response
Recent advances in breast cancers Breast cancer is a hetrogenous disease due to distinct genetic alterations. Similar morphological subtypes show variation in clinical behaviour especially in response
Tumor responses (patients responding/ patients treated)
 Table 1. ACT clinical trial tumor responses and toxicities. a Target antigen Cancer(s) Receptor type Tumor responses (patients responding/ patients treated) Immune-mediated toxicities (patients experiencing
Table 1. ACT clinical trial tumor responses and toxicities. a Target antigen Cancer(s) Receptor type Tumor responses (patients responding/ patients treated) Immune-mediated toxicities (patients experiencing
Dr Rodney Itaki Lecturer Anatomical Pathology Discipline. University of Papua New Guinea School of Medicine & Health Sciences Division of Pathology
 Neoplasia Dr Rodney Itaki Lecturer Anatomical Pathology Discipline University of Papua New Guinea School of Medicine & Health Sciences Division of Pathology General Considerations Overview: Neoplasia uncontrolled,
Neoplasia Dr Rodney Itaki Lecturer Anatomical Pathology Discipline University of Papua New Guinea School of Medicine & Health Sciences Division of Pathology General Considerations Overview: Neoplasia uncontrolled,
James C. Fleet, PhD Professor Dept of Nutrition Science Purdue University
 James C. Fleet, PhD Professor Dept of Nutrition Science Purdue University Overview What are we trying to model? Vitamin D biology Cancer Is there in vivo proof of principle for vitamin D/cancer relationship?
James C. Fleet, PhD Professor Dept of Nutrition Science Purdue University Overview What are we trying to model? Vitamin D biology Cancer Is there in vivo proof of principle for vitamin D/cancer relationship?
s u p p l e m e n ta ry i n f o r m at i o n
 Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
Figure S1 Characterization of tet-off inducible cell lines expressing GFPprogerin and GFP-wt lamin A. a, Western blot analysis of GFP-progerin- or GFP-wt lamin A- expressing cells before induction (0d)
ONCOGENESIS A multistep process comprised of
 by Dr JO Serrentino The seminar/webinar is for the purposes of continuing education and available to registrants only. No part of this material may be duplicated in any format without the expressed written
by Dr JO Serrentino The seminar/webinar is for the purposes of continuing education and available to registrants only. No part of this material may be duplicated in any format without the expressed written
Adenocarcinoma of the pancreas
 Adenocarcinoma of the pancreas SEMINARS IN DIAGNOSTIC PATHOLOGY 31 (2014) 443 451 Ralph H.Hruban, MD, David S. Klimstra, MD Paola Parente Anatomia Patologica Casa Sollievo della Sofferenza San Giovanni
Adenocarcinoma of the pancreas SEMINARS IN DIAGNOSTIC PATHOLOGY 31 (2014) 443 451 Ralph H.Hruban, MD, David S. Klimstra, MD Paola Parente Anatomia Patologica Casa Sollievo della Sofferenza San Giovanni
Cancer and Oncogenes Bioscience in the 21 st Century. Linda Lowe-Krentz
 Cancer and Oncogenes Bioscience in the 21 st Century Linda Lowe-Krentz December 1, 2010 Just a Few Numbers Becoming Cancer Genetic Defects Drugs Our friends and family 25 More mutations as 20 you get older
Cancer and Oncogenes Bioscience in the 21 st Century Linda Lowe-Krentz December 1, 2010 Just a Few Numbers Becoming Cancer Genetic Defects Drugs Our friends and family 25 More mutations as 20 you get older
Transformation of Normal HMECs (Human Mammary Epithelial Cells) into Metastatic Breast Cancer Cells: Introduction - The Broad Picture:
 Transformation of Normal HMECs (Human Mammary Epithelial Cells) into Metastatic Breast Cancer Cells: Introduction - The Broad Picture: Spandana Baruah December, 2016 Cancer is defined as: «A disease caused
Transformation of Normal HMECs (Human Mammary Epithelial Cells) into Metastatic Breast Cancer Cells: Introduction - The Broad Picture: Spandana Baruah December, 2016 Cancer is defined as: «A disease caused
Hyperplastische Polyps Innocent bystanders?
 Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
Biochemistry of Carcinogenesis. Lecture # 35 Alexander N. Koval
 Biochemistry of Carcinogenesis Lecture # 35 Alexander N. Koval What is Cancer? The term "cancer" refers to a group of diseases in which cells grow and spread unrestrained throughout the body. It is difficult
Biochemistry of Carcinogenesis Lecture # 35 Alexander N. Koval What is Cancer? The term "cancer" refers to a group of diseases in which cells grow and spread unrestrained throughout the body. It is difficult
Columbia College of P&S Sarah Huang Hans Snoeck biorxiv ; doi: https://doi.org/ /261461
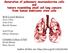 Generation of pulmonary neuroendocrine cells and tumors resembling small cell lung cancers from human embryonic stem cells Weill Cornell Medicine Joyce Chen Arun Unni Harold Varmus Asaf Poran Olivier Elemento
Generation of pulmonary neuroendocrine cells and tumors resembling small cell lung cancers from human embryonic stem cells Weill Cornell Medicine Joyce Chen Arun Unni Harold Varmus Asaf Poran Olivier Elemento
Early cell death (FGF) B No RunX transcription factor produced Yes No differentiation
 Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
Solution Key - Practice Questions Question 1 a) A recent publication has shown that the fat stem cells (FSC) can act as bone stem cells to repair cavities in the skull, when transplanted into immuno-compromised
IDENTIFICATION OF BIOMARKERS FOR EARLY DIAGNOSIS OF BREAST CANCER"
 IDENTIFICATION OF BIOMARKERS FOR EARLY DIAGNOSIS OF BREAST CANCER" Edmond Marzbani, MD December 2, 2008 Early diagnosis of cancer Many solid tumors are potentially curable if diagnosed at an early stage
IDENTIFICATION OF BIOMARKERS FOR EARLY DIAGNOSIS OF BREAST CANCER" Edmond Marzbani, MD December 2, 2008 Early diagnosis of cancer Many solid tumors are potentially curable if diagnosed at an early stage
VIII Curso Internacional del PIRRECV. Some molecular mechanisms of cancer
 VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
VIII Curso Internacional del PIRRECV Some molecular mechanisms of cancer Laboratorio de Comunicaciones Celulares, Centro FONDAP Estudios Moleculares de la Celula (CEMC), ICBM, Facultad de Medicina, Universidad
Neoplasia 2018 Lecture 2. Dr Heyam Awad MD, FRCPath
 Neoplasia 2018 Lecture 2 Dr Heyam Awad MD, FRCPath ILOS 1. List the differences between benign and malignant tumors. 2. Recognize the histological features of malignancy. 3. Define dysplasia and understand
Neoplasia 2018 Lecture 2 Dr Heyam Awad MD, FRCPath ILOS 1. List the differences between benign and malignant tumors. 2. Recognize the histological features of malignancy. 3. Define dysplasia and understand
Update in Salivary Gland Pathology. Benjamin L. Witt University of Utah/ARUP Laboratories February 9, 2016
 Update in Salivary Gland Pathology Benjamin L. Witt University of Utah/ARUP Laboratories February 9, 2016 Objectives Review the different appearances of a selection of salivary gland tumor types Establish
Update in Salivary Gland Pathology Benjamin L. Witt University of Utah/ARUP Laboratories February 9, 2016 Objectives Review the different appearances of a selection of salivary gland tumor types Establish
Crosstalk between Adiponectin and IGF-IR in breast cancer. Prof. Young Jin Suh Department of Surgery The Catholic University of Korea
 Crosstalk between Adiponectin and IGF-IR in breast cancer Prof. Young Jin Suh Department of Surgery The Catholic University of Korea Obesity Chronic, multifactorial disorder Hypertrophy and hyperplasia
Crosstalk between Adiponectin and IGF-IR in breast cancer Prof. Young Jin Suh Department of Surgery The Catholic University of Korea Obesity Chronic, multifactorial disorder Hypertrophy and hyperplasia
Molecular and genetic analysis of stromal fibroblasts in prostate cancer
 Final report ESMO Translational Research Fellowship 2010-2011 Molecular and genetic analysis of stromal fibroblasts in prostate cancer Michalis Karamouzis Host Institute Department of Biological Chemistry,
Final report ESMO Translational Research Fellowship 2010-2011 Molecular and genetic analysis of stromal fibroblasts in prostate cancer Michalis Karamouzis Host Institute Department of Biological Chemistry,
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 19: ,
 INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 19: 197-201, 2007 197 WNT antagonist, DKK2, is a Notch signaling target in intestinal stem cells: Augmentation of a negative regulation system for canonical
INTERNATIONAL JOURNAL OF MOLECULAR MEDICINE 19: 197-201, 2007 197 WNT antagonist, DKK2, is a Notch signaling target in intestinal stem cells: Augmentation of a negative regulation system for canonical
BASIC LIVER, PANCREAS, AND BILIARY TRACT
 GASTROENTEROLOGY 2009;136:1741 1749 BASIC LIVER, BILIARY TRACT Inhibition of -Secretase Activity Inhibits Tumor Progression in a Mouse Model of Pancreatic Ductal Adenocarcinoma RUBEN PLENTZ,* JI SUN PARK,*
GASTROENTEROLOGY 2009;136:1741 1749 BASIC LIVER, BILIARY TRACT Inhibition of -Secretase Activity Inhibits Tumor Progression in a Mouse Model of Pancreatic Ductal Adenocarcinoma RUBEN PLENTZ,* JI SUN PARK,*
Src-INACTIVE / Src-INACTIVE
 Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Biology 169 -- Exam 1 February 2003 Answer each question, noting carefully the instructions for each. Repeat- Read the instructions for each question before answering!!! Be as specific as possible in each
Cancer and Oncogenes Bioscience in the 21 st Century. Linda Lowe-Krentz October 11, 2013
 Cancer and Oncogenes Bioscience in the 21 st Century Linda Lowe-Krentz October 11, 2013 Just a Few Numbers Becoming Cancer Genetic Defects Drugs Our friends and family 200 180 160 140 120 100 80 60 40
Cancer and Oncogenes Bioscience in the 21 st Century Linda Lowe-Krentz October 11, 2013 Just a Few Numbers Becoming Cancer Genetic Defects Drugs Our friends and family 200 180 160 140 120 100 80 60 40
A class of genes that normally suppress cell proliferation. p53 and Rb..ect. suppressor gene products can release cells. hyperproliferation.
 Tumor Suppressor Genes A class of genes that normally suppress cell proliferation. p53 and Rb..ect Mutations that inactivate the tumor suppressor gene products can release cells from growth suppression
Tumor Suppressor Genes A class of genes that normally suppress cell proliferation. p53 and Rb..ect Mutations that inactivate the tumor suppressor gene products can release cells from growth suppression
Cancer. Questions about cancer. What is cancer? What causes unregulated cell growth? What regulates cell growth? What causes DNA damage?
 Questions about cancer What is cancer? Cancer Gil McVean, Department of Statistics, Oxford What causes unregulated cell growth? What regulates cell growth? What causes DNA damage? What are the steps in
Questions about cancer What is cancer? Cancer Gil McVean, Department of Statistics, Oxford What causes unregulated cell growth? What regulates cell growth? What causes DNA damage? What are the steps in
p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs
 p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs 2013, Katerina I. Leonova et al. Kolmogorov Mikhail Noncoding DNA Mammalian
p53 cooperates with DNA methylation and a suicidal interferon response to maintain epigenetic silencing of repeats and noncoding RNAs 2013, Katerina I. Leonova et al. Kolmogorov Mikhail Noncoding DNA Mammalian
Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma
 Research article Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma Hideaki Ijichi, 1 Anna Chytil, 2,3 Agnieszka E. Gorska,
Research article Inhibiting Cxcr2 disrupts tumor-stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma Hideaki Ijichi, 1 Anna Chytil, 2,3 Agnieszka E. Gorska,
Molecular Markers in Acute Leukemia. Dr Muhd Zanapiah Zakaria Hospital Ampang
 Molecular Markers in Acute Leukemia Dr Muhd Zanapiah Zakaria Hospital Ampang Molecular Markers Useful at diagnosis Classify groups and prognosis Development of more specific therapies Application of risk-adjusted
Molecular Markers in Acute Leukemia Dr Muhd Zanapiah Zakaria Hospital Ampang Molecular Markers Useful at diagnosis Classify groups and prognosis Development of more specific therapies Application of risk-adjusted
