Protocol for the Examination of Specimens From Patients With Sarcoma of the Uterus
|
|
|
- Charla Anderson
- 6 years ago
- Views:
Transcription
1 Protocol for the Examination of Specimens From Patients With Sarcoma of the Uterus Protocol applies to sarcomas of the uterus. Based on AJCC/UICC TNM, 7 th edition and FIGO 2009 Annual Report Protocol web posting date: December 2013 Procedures Hysterectomy Myomectomy Authors Christopher N. Otis, MD Department of Pathology, Baystate Medical Center (Tufts University School of Medicine), Springfield, Massachusetts Areli Cuevas Ocampo, MD Department of Pathology, Baystate Medical Center (Tufts University School of Medicine), Springfield, Massachusetts Marisa R. Nucci, MD Department of Pathology, Brigham and Women s Hospital, Boston, Massachusetts W. Glenn McCluggage, MD Department of Pathology, Belfast Health and Social Care Trust, Belfast, United Kingdom For the Members of the Cancer Committee, College of American Pathologists
2 2013 College of American Pathologists (CAP). All rights reserved. The College does not permit reproduction of any substantial portion of these protocols without its written authorization. The College hereby authorizes use of these protocols by physicians and other health care providers in reporting on surgical specimens, in teaching, and in carrying out medical research for nonprofit purposes. This authorization does not extend to reproduction or other use of any substantial portion of these protocols for commercial purposes without the written consent of the College. The CAP also authorizes physicians and other health care practitioners to make modified versions of the Protocols solely for their individual use in reporting on surgical specimens for individual patients, teaching, and carrying out medical research for non-profit purposes. The CAP further authorizes the following uses by physicians and other health care practitioners, in reporting on surgical specimens for individual patients, in teaching, and in carrying out medical research for non-profit purposes: (1) Dictation from the original or modified protocols for the purposes of creating a text-based patient record on paper, or in a word processing document; (2) Copying from the original or modified protocols into a text-based patient record on paper, or in a word processing document; (3) The use of a computerized system for items (1) and (2), provided that the Protocol data is stored intact as a single text-based document, and is not stored as multiple discrete data fields. Other than uses (1), (2), and (3) above, the CAP does not authorize any use of the Protocols in electronic medical records systems, pathology informatics systems, cancer registry computer systems, computerized databases, mappings between coding works, or any computerized system without a written license from the CAP. Any public dissemination of the original or modified Protocols is prohibited without a written license from the CAP. The College of American Pathologists offers these protocols to assist pathologists in providing clinically useful and relevant information when reporting results of surgical specimen examinations of surgical specimens. The College regards the reporting elements in the Surgical Pathology Cancer Case Summary portion of the protocols as essential elements of the pathology report. However, the manner in which these elements are reported is at the discretion of each specific pathologist, taking into account clinician preferences, institutional policies, and individual practice. The College developed these protocols as an educational tool to assist pathologists in the useful reporting of relevant information. It did not issue the protocols for use in litigation, reimbursement, or other contexts. Nevertheless, the College recognizes that the protocols might be used by hospitals, attorneys, payers, and others. Indeed, effective January 1, 2004, the Commission on Cancer of the American College of Surgeons mandated the use of the data elements of the protocols as part of its Cancer Program Standards for Approved Cancer Programs. Therefore, it becomes even more important for pathologists to familiarize themselves with these documents. At the same time, the College cautions that use of the protocols other than for their intended educational purpose may involve additional considerations that are beyond the scope of this document. The inclusion of a product name or service in a CAP publication should not be construed as an endorsement of such product or service, nor is failure to include the name of a product or service to be construed as disapproval. 2
3 CAP Uterine Sarcoma Version Code The definition of the version code can be found at Version: Uterine Sarcoma Summary of Changes This is a new protocol. 3
4 CAP Approved Surgical Pathology Cancer Case Summary Protocol web posting date: December 2013 UTERUS: Hysterectomy and Myomectomy, With or Without Other Organs or Tissues Select a single response unless otherwise indicated. Specimen (select all that apply) Uterine corpus Cervix Right ovary Left ovary Right fallopian tube Left fallopian tube Left parametrium Right parametrium Peritoneum Vaginal cuff Omentum Other (specify): Not specified Procedure (select all that apply) Supracervical hysterectomy Simple hysterectomy Radical hysterectomy Myomectomy Right oophorectomy Left oophorectomy Right salpingectomy Left salpingectomy Right salpingo-oophorectomy Left salpingo-oophorectomy Omentectomy Peritoneal biopsies Other (specify): Not specified Lymph Node Sampling (select all that apply) Performed Pelvic lymph nodes Paraaortic lymph nodes Other (specify): Not performed Not known + Data elements preceded by this symbol are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 4
5 CAP Approved Specimen Integrity Hysterectomy specimen (intact) Hysterectomy specimen without cervix Morcellated hysterectomy specimen Myomectomy (intact) Morcellated myomectomy specimen Other (specify): Tumor Site Fundus Lower uterine segment/isthmus Cervix Other (specify): Tumor Size Greatest dimension: cm + Additional dimensions: x cm Cannot be determined Histologic Type (select all that apply) (Notes B, C, D) Leiomyosarcoma Low-grade endometrial stromal sarcoma # Low-grade endometrial stromal sarcoma with: Smooth muscle differentiation Sex cord elements Glandular elements Other (specify): High-grade endometrial stromal sarcoma Undifferentiated uterine/endometrial sarcoma Adenosarcoma Adenosarcoma with: Rhabdomyoblastic differentiation Cartilagenous differentiation Osseous differentiation Other heterologous element (specify): Adenosarcoma with sarcomatous overgrowth Other (specify): # Low-grade endometrial sarcoma is distinguished from benign endometrial stromal nodule by infiltration into the surrounding myometrium and/or lymphovascular invasion. Minor marginal irregularity in the form of tongues <3 mm (up to 3) is allowable for an endometrial stromal nodule. This protocol does not apply to endometrial stromal nodule. Histologic Grade Leiomyosarcoma (Note D) Not applicable Endometrial Stromal Sarcoma (Note C) Low grade High grade Cannot be assessed + Data elements preceded by this symbol are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 5
6 CAP Approved Adenosarcoma (Note B) (select all that apply) Low grade High grade With sarcomatous overgrowth Cannot be assessed Myometrial Invasion (only for adenosarcoma) Cannot be determined (explain): Tumor is limited to the endometrium or cervical surface without myometrial invasion Tumor invades less than or equal to 50% ( 50%) total myometrial thickness Tumor invades greater than 50% (>50%) total myometrial thickness Involvement of Cervix Cannot be determined Tumor involves the glandular surface of the cervix only Tumor invades the cervical stromal connective tissue Extent of Involvement of Other Organs (select all that apply) Not applicable Right ovary Involved Left ovary Involved Right fallopian tube Involved Left fallopian tube Involved Vaginal cuff Involved Right parametrium Involved Left parametrium Involved Omentum Involved Other (specify): + Data elements preceded by this symbol are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 6
7 CAP Approved Margins Cannot be assessed Uninvolved by sarcoma + Distance of sarcoma from closest margin: mm + Specify margin: Involved by sarcoma Specify margin(s): Lymph-Vascular Invasion Not identified Present Indeterminate + Peritoneal Ascitic Fluid + Negative for malignancy + Atypical and/or suspicious (explain): + Malignant (positive for malignancy) + Unsatisfactory/nondiagnostic (explain): + Peritoneal Washing + Negative for malignancy + Atypical and/or suspicious (explain): + Malignant (positive for malignancy) + Unsatisfactory/nondiagnostic (explain): Lymph Nodes No nodes submitted or found Right pelvic lymph nodes: Number examined: Number cannot be determined (explain): Number involved: Number cannot be determined (explain): Left pelvic lymph nodes: Number examined: Number cannot be determined (explain): Number involved: Number cannot be determined (explain): Paraaortic lymph nodes: Number examined: Number cannot be determined (explain): Number involved: Number cannot be determined (explain): Lymph nodes (other, specify): Number examined: Number cannot be determined (explain): Number involved: Number cannot be determined (explain): + Data elements preceded by this symbol are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 7
8 CAP Approved Pathologic Staging (ptnm [FIGO]) TNM Descriptors (required only if applicable) (select all that apply) m (multiple primary tumors) r (recurrent) y (posttreatment) Leiomyosarcoma, Endometrial Stromal Sarcoma, and Undifferentiated Endometrial Sarcoma/Uterine Sarcoma Primary Tumor (pt) ptx [--]: Primary tumor cannot be assessed pt0 [--]: No evidence of primary tumor pt1 [I]: Tumor is limited to the uterus pt1a [IA]: Tumor is 5 cm or less ( 5 cm) in greatest dimension pt1b [IB]: Tumor is greater than 5 cm (>5 cm) in greatest dimension pt2 [II]: Tumor extends beyond the uterus, but is within the pelvis (tumor extends to extrauterine pelvic tissue) pt2a [IIA]: Tumor involves the adnexa pt2b [IIB]: Tumor involves other pelvic tissue pt3 [III]: Tumor invades abdominal tissues (not just protruding into the abdomen) pt3a [IIIA]: Tumor invades abdominal tissues at one site pt3b [IIIB]: Tumor invades abdominal tissues at more than one site pt4 [IVA]: Tumor invades bladder mucosa and/or rectum Regional Lymph Nodes (pn) pnx: Cannot be assessed pn0: No regional lymph node metastasis pn1 [IIIC]: Regional lymph node metastasis to pelvic lymph nodes Distant Metastasis (pm) Not applicable pm1 [IVB]: Distant metastasis (excluding adnexa, pelvic and abdominal tissues) Specify site(s), if known: Adenosarcoma Primary Tumor (pt) ptx [--]: Primary tumor cannot be assessed pt0 [--]: No evidence of primary tumor pt1 [I]: Tumor is limited to the uterus pt1a [IA]: Tumor is limited to the endometrium/endocervix without myometrial invasion pt1b [IB]: Tumor invades less than or equal to 50% ( 50%) total myometrial thickness pt1c [IC]: Tumor invades greater than 50% (>50%) total myometrial thickness pt2 [II]: Tumor extends beyond the uterus, but is within the pelvis (tumor extends to extrauterine pelvic tissue) pt2a [IIA]: Tumor involves the adnexa pt2b [IIB]: Tumor involves other pelvic tissue pt3 [III]: Tumor invades abdominal tissues (not just protruding into the abdomen) pt3a [IIIA]: Tumor invades abdominal tissues at one site pt3b [IIIB]: Tumor invades abdominal tissues at more than one site pt4 [IVA]: Tumor invades bladder mucosa and/or rectum + Data elements preceded by this symbol are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 8
9 CAP Approved Regional Lymph Nodes (pn) pnx: Cannot be assessed pn0: No regional lymph node metastasis pn1 [IIIC]: Regional lymph node metastasis to pelvic lymph nodes Distant Metastasis (pm) Not applicable pm1 [IVB]: Distant metastasis (excluding adnexa, pelvic and abdominal tissues) Specify site(s), if known: + Ancillary Studies + Specify: + Not performed + Comment(s) + Data elements preceded by this symbol are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 9
10 Background Documentation Explanatory Notes A. Carcinosarcoma Carcinosarcoma (malignant mixed mullerian tumor) is excluded from the uterine sarcoma diagnostic category as it is considered in tumors of the endometrial epithelium. B. Adenosarcoma According to World Health Organization (WHO) criteria, mitotic activity in the mesenchymal component in excess of 2 or more per 10 high-power fields (HPFs) is required for a diagnosis of adenosarcoma, but others use a cut-off of 4 per 10 HPFs. 1-5 However, given the multiple and well-known problems associated with counting mitotic figures and the fact that the number of mitoses may be variable from area to area, in practice, if the characteristic leaf-like architecture of adenosarcoma is present with periglandular cuffing resulting in a cambium layer, a diagnosis of adenosarcoma should be strongly considered with mitotic counts <2 per 10 HPFs or even in the absence of mitotic figures. In adenosarcomas without sarcomatous overgrowth, it is recommended to record on the pathology report whether the stromal component is morphologically low grade or high grade. Even though there are no studies showing that this is of prognostic significance, anecdotal evidence suggests that even a small focus of high-grade sarcoma may result in an adverse behavior. It is suggested that the parameter of nuclear atypia be used to distinguish between low grade and high grade. In low-grade neoplasms, the atypia should be akin to that seen in low-grade endometrial stromal sarcoma. Sarcomatous overgrowth in adenosarcoma is defined as the presence of pure sarcoma, usually high grade and without an epithelial component, occupying at least 25% of the tumor. 6 Adenosarcomas rarely exhibit lymphovascular invasion unless associated with deep myometrial invasion or sarcomatous overgrowth. The depth of myometrial invasion is important in the substaging of stage I adenosarcomas (tumor confined to the uterus). 7 Stage IA tumors are limited to the endometrium or endocervix with no myometrial involvement, stage IB equates to less than or half of myometrial invasion and stage 1C equates to more than one half myometrial invasion. This staging system is similar to the 1988 FIGO staging system for carcinomas of the uterine corpus. Since low-grade endometrial stromal sarcoma and leiomyosarcoma are predominantly myometrial-based lesions, myometrial invasion per se is not used in the staging of these neoplasms In most adenosarcomas with a low-grade stromal component without sarcomatous overgrowth, the stromal element expresses estrogen receptor (ER), progesterone receptor (PgR), CD10, and WT1, is negative ( wild-type ) with p53 and exhibits a low MIB1 proliferation index. 2,4 Thus, the immunophenotype resembles that of low-grade endometrial stromal sarcoma. Smooth muscle actin and desmin may also be positive. In areas of high-grade sarcoma and of sarcomatous overgrowth, the mesenchymal component exhibits a higher MIB1 proliferation index and may be p53 positive/aberrant. There is usually loss of expression of the cell differentiation markers ER, PgR and CD10, the immunophenotype being similar to that of an undifferentiated sarcoma. Rhabdomyosarcomatous elements in adenosarcomas express desmin and sometimes the skeletal muscle markers myogenin and myod1. Sex cord-like elements may express inhibin and calretinin. C. Endometrial Stromal Sarcoma Even though in the past endometrial stromal sarcomas (ESS) were classified as low grade (LG) and high grade (HG) based on mitotic activity, the largest and most comprehensive review of these tumors by Chang and colleagues in1990 showed that mitotic activity was not predictive of outcome in stage I tumors 8. Thus, the diagnosis of HG-ESS was discouraged in those tumors that resemble proliferativephase endometrial stroma but in which the mitotic index exceeded 10 per 10 HPF. Currently many expert gynecologic pathologists, without any proven basis outside of personal experience, make the 10
11 Background Documentation diagnosis of HG-ESS when there is a transition from high-grade undifferentiated sarcoma to areas that can be recognized as conventional LG-ESS. 9 However, recently, a subset of cases previously diagnosed as HG-ESSs has been histologically and genetically defined by Lee, Nucci and colleagues. 10,11 In these tumors, the high-grade areas are characterized by cells with a round cell-epithelioid appearance and high-grade cytologic features which often are associated with areas that have the appearance of the fibroblastic variant of low-grade conventional ESS. 10 These tumors have been shown to have a novel genetic fusion between YWHAE and FAM22A/B and harbor t(10;17)(q22;p13). The high-grade areas of the tumor express cyclin D1 but lose CD10, ER, and PgR expression (in contrast to the conventional lowgrade areas) consistent with a high-grade sarcoma. 10 It is important to recognize these tumors as they have an intermediate prognosis between LGESS and undifferentiated uterine sarcoma (UUS) and appear not to respond to the usual treatment for low-grade ESS. Low-grade ESS, high-grade ESS and UUS all exist and should be separately diagnosed, although UUS should be a diagnosis of exclusion (leiomyosarcomas and other high-grade sarcomas, for example rhabdomyosarcoma, should be excluded). Molecular testing is diagnostically unnecessary in conventional ESS and in USS, but is useful in confirming the diagnosis of HG-ESS in tumors with a round cell-epithelioid appearance that can be associated with areas that have the appearance of the fibroblastic variant of conventional LG-ESS. D. Leiomyosarcoma By definition, uterine leiomyosarcoma (LMS) is a highly malignant neoplasm with survival rates depending upon the extent of spread. For tumors confined to the uterine corpus, size plays a significant role in prognosis. Despite differences in survival rates, it is clear that stage is a significant factor related to outcome. Histologic grade, however, has not been consistently identified as a significant prognostic parameter. 12 The utility of grading uterine LMS is controversial and no universally accepted grading system exist. 5 In 2011, Veras et al 13 tried to characterize "low-grade uterine leiomyosarcomas" as a clinicopathological entity but came to the conclusion that this can be diagnosed only retrospectively at present. 13 Furthermore, when the Stanford criteria are strictly applied, all tumors classified as leiomyosarcomas, should be regarded intrinsically as high grade. 13,14 Conventional uterine LMS is a cellular tumor composed of fascicles of spindle-shaped cells exhibiting smooth muscle differentiation with moderate to severe pleomorphism. Usually coagulative tumor cell necrosis (CTCN) is present and mitoses exceed 10-15/10 HPF. 14 Two LMS subtypes included in the WHO classification deserve special attention as their pathologic features differ from those of ordinary spindle cell LMS. Epithelioid leiomyosarcoma (E-LMS) is composed predominantly of round or polygonal cells with eosinophilic to clear cytoplasm exhibiting nested, plexiform or corded growth patterns. Nuclear atypia may be only mild and necrosis may be absent. Mitotic rate is generally 3/10 HPF and most tumors infiltrate adjacent myometrium. Myxoid leiomyosarcoma (M-LMS) may be grossly gelatinous, microscopically hypocellular with a predominant myxoid stroma and often has a low mitotic rate. In the absence of severe cytologic atypia and high mitotic activity, both epithelioid and myxoid LMS are diagnosed as sarcomas based on their infiltrative borders. 12 Ancillary Studies in the Differential Diagnosis Immunoreactivity for smooth muscle actin, muscle specific actin, calponin, desmin, h-caldesmon and heavy chain smooth muscle myosin are commonly seen in uterine LMS. Desmin expression may be focal Similarly, E-LMS and M-LMS may demonstrate lesser degrees of immunoreactivity for these markers. Cell cycle related markers Ki-67, p53, and p16 are usually overexpressed in LMS compared to leiomyoma. 18 Cytokeratins and EMA may be focally positive in LMS, especially in the epithelioid variant. E. Undifferentiated Uterine/Endometrial Sarcoma Undifferentiated uterine/endometrial sarcoma (UUS) is a high-grade sarcoma that lacks specific differentiation. Histopathologically these tumors show marked cellular pleomorphism and abundant 11
12 Background Documentation mitotic activity with atypical forms. They lack the typical growth pattern and vascularity of low-grade ESS and displace the myometrium in contrast to the infiltrative pattern of low-grade ESS. They often resemble the sarcomatous component of a carcinosarcoma. These sarcomas are most often aneuploid with an S-phase fraction greater than 10%, and are negative for ER and PgR, 10 Nucci et al proposed that high-grade ESS with the novel fusion gene YWHAE-FAM22 should be distinguished from undifferentiated uterine/endometrial sarcoma. F. Other Other differential diagnostic considerations included in spindle/sarcomatous lesions primary to the uterus include perivascular epithelioid cell tumor (PEComa). PEComa belongs to a group of tumors characterized by both melanocytic and smooth muscle differentiation, and should be recognized separately from smooth muscle tumors The TNM staging system for uterine sarcoma endorsed by the American Joint Committee on Cancer (AJCC) and the parallel system formulated by the International Federation of Gynecology and Obstetrics (FIGO) are recommended, as shown below. According to AJCC/International Union Against Cancer (UICC) convention, the designation T refers to a primary tumor that has not been previously treated. The symbol p refers to the pathologic classification of the TNM, as opposed to the clinical classification, and is based on gross and microscopic examination. pt entails a resection of the primary tumor or biopsy adequate to evaluate the highest pt category, pn entails removal of nodes adequate to validate lymph node metastasis, and pm implies microscopic examination of distant lesions. Clinical classification (ctnm) is usually carried out by the referring physician before treatment during initial evaluation of the patient or when pathologic classification is not possible. Pathologic staging is usually performed after surgical resection of the primary tumor. Pathologic staging depends on pathologic documentation of the anatomic extent of disease, whether or not the primary tumor has been completely removed. If a biopsied tumor is not resected for any reason (eg, when technically infeasible) and if the highest T and N categories or the M1 category of the tumor can be confirmed microscopically, the criteria for pathologic classification and staging have been satisfied without total removal of the primary cancer. It is important to note that in uterine sarcoma, as in cancer of other organs, the validity of T stage depends upon the adequacy and completeness of the surgical staging. TNM Classification and FIGO Staging System for Leiomyosarcoma, Endometrial Stromal Sarcoma and Undifferentiated Uterine Sarcoma TNM FIGO Category Stage Definition Primary Tumor ptx [--]: Primary tumor cannot be assessed pt0 [--]: No evidence of primary tumor pt1 [I]: Tumor is limited to the uterus pt1a [IA]: Tumor is 5 cm or less ( 5 cm) in greatest dimension pt1b [IB]: Tumor is greater than 5 cm (>5 cm) in greatest dimension pt2 [II]: Tumor extends beyond the uterus, but is within the pelvis (tumor extends to extrauterine pelvic tissue) pt2a [IIA]: Tumor involves the adnexa pt2b [IIB]: Tumor involves other pelvic tissue 12
13 Background Documentation pt3 [III]: Tumor invades abdominal tissues (not just protruding into the abdomen) pt3a [IIIA]: Tumor invades abdominal tissues at one site pt3b [IIIB]: Tumor invades abdominal tissues at more than one site pt4 [IVA]: Tumor invades bladder mucosa and/or rectum Regional Lymph Nodes (pn) # pnx: Cannot be assessed pn0: No regional lymph node metastasis pn1 [IIIC]: Regional lymph node metastasis to pelvic lymph nodes # Regional lymph nodes include the pelvic, obturator, internal iliac (hypogastric), external iliac, common iliac, paraaortic, presacral, and parametrial lymph nodes. Distant Metastasis (pm) pm0 No distant metastasis (no pathologic M0; use clinical M to complete stage group) pm1 [IVB]: Distant metastasis (excluding adnexa, pelvic and abdominal tissues) Adenosarcoma TNM FIGO Category Stage Definition Primary Tumor ptx [--]: Primary tumor cannot be assessed pt0 [--]: No evidence of primary tumor pt1 [I]: Tumor is limited to the uterus pt1a [IA]: Tumor is limited to the endometrium/endocervix without myometrial invasion pt1b [IB]: Tumor invades less than or equal to 50% ( 50%) total myometrial thickness pt1c [IC] Tumor invades greater than 50% (>50%) total myometrial thickness pt2 [II]: Tumor extends beyond the uterus, but is within the pelvis (tumor extends to extrauterine pelvic tissue) pt2a [IIA]: Tumor involves the adnexa pt2b [IIB]: Tumor involves other pelvic tissue pt3 [III]: Tumor invades abdominal tissues (not just protruding into the abdomen) pt3a [IIIA]: Tumor invades abdominal tissues at one site pt3b [IIIB]: Tumor invades abdominal tissues at more than one site pt4 [IVA]: Tumor invades bladder mucosa and/or rectum Regional Lymph Nodes (pn) # pnx: Cannot be assessed pn0: No regional lymph node metastasis pn1 [IIIC]: Regional lymph node metastasis to pelvic lymph nodes # Regional lymph nodes include the pelvic, obturator, internal iliac (hypogastric), external iliac, common iliac, paraaortic, presacral, and parametrial lymph nodes. Distant Metastasis (pm) pm0 No distant metastasis (no pathologic M0; use clinical M to complete stage group) pm1 [IVB]: Distant metastasis (excluding adnexa, pelvic and abdominal tissues) TNM Stage Groupings Stage 0 Tis N0 M0 13
14 Background Documentation Stage IA # T1a N0 M0 Stage IB # T1b N0 M0 Stage IC ## T1c N0 M0 Stage II T2 N0 M0 Stage IIIA T3a N0 M0 Stage IIIB T3b N0 M0 Stage IIIC T1-T3 N1 M0 Stage IVA T4 Any N M0 Stage IVB Any T Any N M1 # Stage IA and IB for adenosarcoma differ from those applied to leiomyosarcoma and endometrial stromal sarcoma ## Stage IC does not apply for leiomyosarcoma and endometrial stromal sarcoma. TNM Descriptors For identification of special cases of TNM or ptnm classifications, the m suffix and y, r, and a prefixes are used. Although they do not affect the stage grouping, they indicate cases needing separate analysis. The m suffix indicates the presence of multiple primary tumors in a single site and is recorded in parentheses: pt(m)nm. The y prefix indicates those cases in which classification is performed during or after initial multimodality therapy (ie, neoadjuvant chemotherapy, radiation therapy, or both chemotherapy and radiation therapy). The ctnm or ptnm category is identified by a y prefix. The yctnm or yptnm categorizes the extent of tumor actually present at the time of that examination. The y categorization is not an estimate of tumor before multimodality therapy (ie, before initiation of neoadjuvant therapy). The r prefix indicates a recurrent tumor when staged after a documented disease-free interval and is identified by the r prefix: rtnm. The a prefix designates the stage determined at autopsy: atnm. Additional Descriptors Residual Tumor (R) Tumor remaining in a patient after therapy with curative intent (eg, surgical resection for cure) is categorized by a system known as R classification, shown below. RX R0 R1 R2 Presence of residual tumor cannot be assessed No residual tumor Microscopic residual tumor Macroscopic residual tumor For the surgeon, the R classification may be useful to indicate the known or assumed status of the completeness of a surgical excision. For the pathologist, the R classification is relevant to the status of the margins of a surgical resection specimen. That is, tumor involving the resection margin on pathologic examination may be assumed to correspond to residual tumor in the patient and may be classified as macroscopic or microscopic according to the findings at the specimen margin(s). 14
15 Background Documentation References 1. Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990;21: Gallardo A, Prat J. Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am J Surg Pathol. 2009;33: McCluggage WG. Mullerian adenosarcoma of the female genital tract. Adv Anat Pathol. 2010;17: Soslow RA, Ali A, Oliva E. Mullerian adenosarcomas: an immunophenotypic analysis of 35 cases. Am J Surg Pathol. 2008;32: Tavassoli FA, Devilee P. World Health Organization Classification of Tumors, Pathology and Genetics. Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press, Clement PB. Mullerian adenosarcomas of the uterus with sarcomatous overgrowth. A clinicopathological analysis of 10 cases. Am J Surg Pathol. 1989;13: Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104: Chang KL, Crabtree GS, Lim-Tan SK, Kempson RL, Hendrickson MR. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol. 1990;14: Ohta Y, Suzuki T, Omatsu M, et al. Transition from low-grade endometrial stromal sarcoma to highgrade endometrial stromal sarcoma. Int J Gynecol Pathol. 2010;29: Lee CH, Marino-Enriquez A, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36: Lee CH, Ou WB, Marino-Enriquez A, et al fusion oncogenes in high-grade endometrial stromal sarcoma. Proc Natl Acad Sci U S A. 2012;109: Nucci MR. Tumors of the female genital tract, part d myometrium. In: Fletcher C, ed. Diagnostic Histopathology of Tumors. Philidelphia, PA: Chruchil Livingston Elsevier; 2007: Veras E, Zivanovic O, Jacks L, Chiappetta D, Hensley M, Soslow R. "Low-grade leiomyosarcoma" and late-recurring smooth muscle tumors of the uterus: a heterogenous collection of frequently misdiagnosed tumors associated with an overall favorable prognosis relative to conventional uterine leiomyosarcomas. Am J Surg Pathol. 2011;35: Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms: a clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18: D'Angelo E, Spagnoli LG, Prat J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Hum Pathol. 2009;40: Nucci MR, Harburger D, Koontz J, Dal Cin P, Sklar J. Molecular analysis of the JAZF1-JJAZ1 gene fusion by RT-PCR and fluorescence in situ hybridization in endometrial stromal neoplasms. Am J Surg Pathol. 2007;31: Oliva E, Young RH, Amin MB, Clement PB. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol. 2002;26: Chen L, Yang B. Immunohistochemical analysis of p16, p53, and Ki-67 expression in uterine smooth muscle tumors. Int J Gynecol Pathol. 2008;27: Argani P, Aulmann S, Illei PB, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34: Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29: Hornick JL, Fletcher CD. PEComa: what do we know so far? Histopathology. 2006;48:
16 Background Documentation Bibliography Chiang S, Ali R, Melnyk N, et al. Frequency of known gene rearrangements in endometrial stromal tumors. Am J Surg Pathol. 2011;35: Chiang S, Oliva E. Cytogenetic and molecular aberrations in endometrial stromal tumors. Hum Pathol. 2011;42: Hrzenjak A, Moinfar F, Tavassoli FA, et al. JAZF1/JJAZ1 gene fusion in endometrial stromal sarcomas: molecular analysis by reverse transcriptase-polymerase chain reaction optimized for paraffinembedded tissue. J Mol Diagn. 2005;7: Huang HY, Ladanyi M, Soslow RA. Molecular detection of JAZF1-JJAZ1 gene fusion in endometrial stromal neoplasms with classic and variant histology: evidence for genetic heterogeneity. Am J Surg Pathol. 2004;28: Koontz JI, Soreng AL, Nucci M, et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci U S A. 2001;98: Kurihara S, Oda Y, Ohishi Y, et al. Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J Surg Pathol. 2008;32: Micci F, Panagopoulos I, Bjerkehagen B, Heim S. Consistent rearrangement of chromosomal band 6p21 with generation of fusion genes JAZF1/PHF1 and EPC1/PHF1 in endometrial stromal sarcoma. Cancer Res. 2006;66: Micci F, Walter CU, Teixeira MR, et al. Cytogenetic and molecular genetic analyses of endometrial stromal sarcoma: nonrandom involvement of chromosome arms 6p and 7p and confirmation of JAZF1/JJAZ1 gene fusion in t(7;17). Cancer Genet Cytogenet. 2003;144: Nucci MR, Harburger D, Koontz J, Dal Cin P, Sklar J. Molecular analysis of the JAZF1-JJAZ1 gene fusion by RT-PCR and fluorescence in situ hybridization in endometrial stromal neoplasms. Am J Surg Pathol. 2007;31: Nucci MR, O'Connell JT, Huettner PC, Cviko A, Sun D, Quade BJ. h-caldesmon expression effectively distinguishes endometrial stromal tumors from uterine smooth muscle tumors. Am J Surg Pathol. 2001;25: Oliva E, de Leval L, Soslow RA, Herens C. High frequency of JAZF1-JJAZ1 gene fusion in endometrial stromal tumors with smooth muscle differentiation by interphase FISH detection. Am J Surg Pathol. 2007;31: Oliva E, Young RH, Amin MB, Clement PB. An immunohistochemical analysis of endometrial stromal and smooth muscle tumors of the uterus: a study of 54 cases emphasizing the importance of using a panel because of overlap in immunoreactivity for individual antibodies. Am J Surg Pathol. 2002;26: Yilmaz A, Rush DS, Soslow RA. Endometrial stromal sarcomas with unusual histologic features: a report of 24 primary and metastatic tumors emphasizing fibroblastic and smooth muscle differentiation. Am J Surg Pathol. 2002;26:
Protocol for the Examination of Specimens From Patients With Primary Sarcoma of the Uterus
 Protocol for the Examination of Specimens From Patients With Primary Sarcoma of the Uterus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Protocol for the Examination of Specimens From Patients With Primary Sarcoma of the Uterus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
UTERINE SARCOMA EXAMPLE OF A UTERINE SARCOMA USING PROPOSED TEMPLATE
 UTERINE SARCOMA EXAMPLE OF A UTERINE SARCOMA USING PROPOSED TEMPLATE Case: Adenosarcoma with heterologous elements and stromal overgrowth o TAH, BSO, omentectomy, staging biopsies of cul-de-sac, bladder
UTERINE SARCOMA EXAMPLE OF A UTERINE SARCOMA USING PROPOSED TEMPLATE Case: Adenosarcoma with heterologous elements and stromal overgrowth o TAH, BSO, omentectomy, staging biopsies of cul-de-sac, bladder
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
Uterine Cervix. Protocol applies to all invasive carcinomas of the cervix.
 Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Staging and Treatment Update for Gynecologic Malignancies
 Staging and Treatment Update for Gynecologic Malignancies Bunja Rungruang, MD Medical College of Georgia No disclosures 4 th most common new cases of cancer in women 5 th and 6 th leading cancer deaths
Staging and Treatment Update for Gynecologic Malignancies Bunja Rungruang, MD Medical College of Georgia No disclosures 4 th most common new cases of cancer in women 5 th and 6 th leading cancer deaths
Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart
 Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart Protocol applies to primary malignant cardiac tumors. Hematolymphoid neoplasms are not included. No AJCC/UICC
Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart Protocol applies to primary malignant cardiac tumors. Hematolymphoid neoplasms are not included. No AJCC/UICC
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
64 YO lady THBSO for prolapse At gross : A 3 cm endometrial polyp in the fundus
 Case 6 64 YO lady THBSO for prolapse At gross : A 3 cm endometrial polyp in the fundus Numerous irregular, large glands with leaf-like pattern Large glands with broad-based papillary infolding into the
Case 6 64 YO lady THBSO for prolapse At gross : A 3 cm endometrial polyp in the fundus Numerous irregular, large glands with leaf-like pattern Large glands with broad-based papillary infolding into the
What really matters When and Why. Pathology of Uterine Mesenchymal Lesions. Nafisa Wilkinson London
 What really matters When and Why Pathology of Uterine Mesenchymal Lesions Nafisa Wilkinson London Patient centred approach immunohistochemistry Histological diagnosis Next generation sequencing Genetic
What really matters When and Why Pathology of Uterine Mesenchymal Lesions Nafisa Wilkinson London Patient centred approach immunohistochemistry Histological diagnosis Next generation sequencing Genetic
Department of Pathology, Royal Group of Hospitals Trust, Belfast, Northern Ireland.
 UTERINE ADENOSARCOMA W Glenn McCluggage Department of Pathology, Royal Group of Hospitals Trust, Belfast, Northern Ireland. Definition of Adenosarcoma: A mixed tumor composed of benign neoplastic glandular
UTERINE ADENOSARCOMA W Glenn McCluggage Department of Pathology, Royal Group of Hospitals Trust, Belfast, Northern Ireland. Definition of Adenosarcoma: A mixed tumor composed of benign neoplastic glandular
UTERINE SARCOMAS CURRENT THERAPEUTIC OPTIONS
 Review Journal of Translational Medicine and Research, volume 19, no. 1-2, 2014 UTERINE SARCOMAS CURRENT THERAPEUTIC OPTIONS N. Bacalbaæa 1, A. Traistaru 2, I. Bãlescu 3 1 Carol Davila University of Medicine
Review Journal of Translational Medicine and Research, volume 19, no. 1-2, 2014 UTERINE SARCOMAS CURRENT THERAPEUTIC OPTIONS N. Bacalbaæa 1, A. Traistaru 2, I. Bãlescu 3 1 Carol Davila University of Medicine
STAGE CATEGORY DEFINITIONS
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
CyclinD1 Positive High-Grade Endometrial Stromal Sarcoma: A Fascinating Entity!
 Case Report DOI: 10.21276/APALM.1530 CyclinD1 Positive High-Grade Endometrial Stromal Sarcoma: A Fascinating Entity! Divya Shelly*, Imtiaz Ahmed, Sampath K. Srinivasagowda and Reena Bharadwaj Department
Case Report DOI: 10.21276/APALM.1530 CyclinD1 Positive High-Grade Endometrial Stromal Sarcoma: A Fascinating Entity! Divya Shelly*, Imtiaz Ahmed, Sampath K. Srinivasagowda and Reena Bharadwaj Department
Definition of Synoptic Reporting
 Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Gynecologic Evening Specialty Conference. Karuna Garg, MD University of California San Francisco
 Gynecologic Evening Specialty Conference Karuna Garg, MD University of California San Francisco Disclosure of Relevant Financial Relationships The USCAP requires that anyone in a position to influence
Gynecologic Evening Specialty Conference Karuna Garg, MD University of California San Francisco Disclosure of Relevant Financial Relationships The USCAP requires that anyone in a position to influence
New Cancer Cases By Site Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3%
 Uterine Malignancy New Cancer Cases By Site 2010 Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3% Cancer Deaths By Site 2010 Lung 26% Breast 15% Colo-Rectal 9% Pancreas 7%
Uterine Malignancy New Cancer Cases By Site 2010 Breast 28% Lung 14% Colo-Rectal 10% Uterus 6% Thyroid 5% Lymphoma 4% Ovary 3% Cancer Deaths By Site 2010 Lung 26% Breast 15% Colo-Rectal 9% Pancreas 7%
Endometrium. Protocol applies to all carcinomas of the endometrium. Procedures Cytology (No Accompanying Checklist) Biopsy Curettage Hysterectomy
 Endometrium Protocol applies to all carcinomas of the endometrium. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Endometrium Protocol applies to all carcinomas of the endometrium. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
This protocol is intended to assist pathologists in providing
 Protocol for the Examination of Specimens From Patients With Carcinomas of the Endometrium A Basis for Checklists Steven G. Silverberg, MD, for the Members of the Cancer Committee, College of American
Protocol for the Examination of Specimens From Patients With Carcinomas of the Endometrium A Basis for Checklists Steven G. Silverberg, MD, for the Members of the Cancer Committee, College of American
ESS: Pathologic Insights
 GEIS XVI INTERNATIONAL SYMPOSIUM Seville 4th October 2018 ESS: Pathologic Insights Sílvia Bagué The Royal Marsden Hospital London (United Kingdom) I have no conflicts of interest Endometrial stromal sarcoma
GEIS XVI INTERNATIONAL SYMPOSIUM Seville 4th October 2018 ESS: Pathologic Insights Sílvia Bagué The Royal Marsden Hospital London (United Kingdom) I have no conflicts of interest Endometrial stromal sarcoma
3/25/2019. Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates
 J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates Signs
J. Anthony Rakowski D.O., F.A.C.O.O.G. MSU SCS Board Review Coarse Rare uterine cancers ~3% Leiomyosarcoma Carcinosarcoma (MMMT) Endometrial Stromal Sarcomas Aggressive tumors High Mortality Rates Signs
Urinary Bladder, Ureter, and Renal Pelvis
 Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Protocol applies to melanoma of cutaneous surfaces only.
 Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Descriptor Definition Author s notes TNM descriptors Required only if applicable; select all that apply multiple foci of invasive carcinoma
 S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
CME/SAM. Diagnosis of Endometrial Stromal Tumors. A Clinicopathologic Study of 25 Biopsy Specimens With Identification of Problematic Areas
 AJCP / Original Article Diagnosis of Endometrial Stromal Tumors A Clinicopathologic Study of 25 Biopsy Specimens With Identification of Problematic Areas Sten Stemme, MD, PhD, 1 Mehran Ghaderi, PhD, 1
AJCP / Original Article Diagnosis of Endometrial Stromal Tumors A Clinicopathologic Study of 25 Biopsy Specimens With Identification of Problematic Areas Sten Stemme, MD, PhD, 1 Mehran Ghaderi, PhD, 1
Uterine Mesenchymal Tumors from a Gynaecological Point of View: A Mini-Review
 EC Gynaecology Special Issue - 2017 Uterine Mesenchymal Tumors from a Gynaecological Point of View: A Mini-Review Mini Review Dr. Huseyin Aydogmus, Dr. Servet Gencdal, Dr. Nihan Gencdal and Dr. Serpil
EC Gynaecology Special Issue - 2017 Uterine Mesenchymal Tumors from a Gynaecological Point of View: A Mini-Review Mini Review Dr. Huseyin Aydogmus, Dr. Servet Gencdal, Dr. Nihan Gencdal and Dr. Serpil
Small Intestine. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition
 Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Standards and datasets for reporting cancers Dataset for histopathological reporting of uterine sarcomas. September 2018
 Standards and datasets for reporting cancers Dataset for histopathological reporting of uterine sarcomas September 2018 Authors: Professor W Glenn McCluggage, Royal Group of Hospitals, Belfast Professor
Standards and datasets for reporting cancers Dataset for histopathological reporting of uterine sarcomas September 2018 Authors: Professor W Glenn McCluggage, Royal Group of Hospitals, Belfast Professor
Protocol for the Examination of Specimens From Patients With Carcinoma of the Endometrium
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Endometrium Based on AJCC/UICC TNM, 7 th edition and FIGO 2008 Annual Report Protocol web posting date: October 2013 Procedure
Protocol for the Examination of Specimens From Patients With Carcinoma of the Endometrium Based on AJCC/UICC TNM, 7 th edition and FIGO 2008 Annual Report Protocol web posting date: October 2013 Procedure
05/07/2018. Types of challenges. Challenging cases in uterine pathology. Case 1 ` 65 year old female Post menopausal bleeding Uterine Polyp
 Types of challenges Challenging cases in uterine pathology Nafisa Wilkinson Gynaecological Pathologist UCLH London Lack of complete history often, NO clinical history at all! Cases from other centres often
Types of challenges Challenging cases in uterine pathology Nafisa Wilkinson Gynaecological Pathologist UCLH London Lack of complete history often, NO clinical history at all! Cases from other centres often
Colon and Rectum. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition
 Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
STUMPed for a Diagnosis Contemporary Management of Uterine Sarcomas
 UCSF Helen Diller Family Comprehensive Cancer Center Disclosures I have no financial disclosures STUMPed for a Diagnosis Contemporary Management of Uterine Sarcomas Lee-may Chen, MD Department of Obstetrics,
UCSF Helen Diller Family Comprehensive Cancer Center Disclosures I have no financial disclosures STUMPed for a Diagnosis Contemporary Management of Uterine Sarcomas Lee-may Chen, MD Department of Obstetrics,
Diagnostic problems in uterine smooth muscle tumors
 Diagnostic problems in uterine smooth muscle tumors Marina Kos Ljudevit Jurak Clinical Department of Pathology, Clinical Hospital Center Sestre milosrdnice, Zagreb Institute of Pathology, University of
Diagnostic problems in uterine smooth muscle tumors Marina Kos Ljudevit Jurak Clinical Department of Pathology, Clinical Hospital Center Sestre milosrdnice, Zagreb Institute of Pathology, University of
MPH Quiz. 1. How many primaries are present based on this pathology report? 2. What rule is this based on?
 MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
MPH Quiz Case 1 Surgical Pathology from hysterectomy performed July 11, 2007 Final Diagnosis: Uterus, resection: Endometrioid adenocarcinoma, Grade 1 involving most of endometrium, myometrial invasion
Procedures Needle Biopsy Transurethral Prostatic Resection Suprapubic or Retropubic Enucleation (Subtotal Prostatectomy) Radical Prostatectomy
 Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
Original Article Endometrial stromal sarcoma: a clinicopathological analysis of 14 cases
 Int J Clin Exp Pathol 2018;11(5):2799-2804 www.ijcep.com /ISSN:1936-2625/IJCEP0073760 Original Article Endometrial stromal sarcoma: a clinicopathological analysis of 14 cases Fuqiang Wang *, Ruixue Lei
Int J Clin Exp Pathol 2018;11(5):2799-2804 www.ijcep.com /ISSN:1936-2625/IJCEP0073760 Original Article Endometrial stromal sarcoma: a clinicopathological analysis of 14 cases Fuqiang Wang *, Ruixue Lei
Clinicopathologic correlation of endometrial stromal sarcomas: a retrospective study of 42 cases
 Original Article Clinicopathologic correlation of endometrial stromal sarcomas: a retrospective study of 42 cases Fengjie Wang 1,2, Yongfen Yi 1, Yi Luo 3, Jing Chen 1 1 Department of Pathology, Molecular
Original Article Clinicopathologic correlation of endometrial stromal sarcomas: a retrospective study of 42 cases Fengjie Wang 1,2, Yongfen Yi 1, Yi Luo 3, Jing Chen 1 1 Department of Pathology, Molecular
Cervical Cancer: 2018 FIGO Staging
 Cervical Cancer: 2018 FIGO Staging Jonathan S. Berek, MD, MMS Laurie Kraus Lacob Professor Stanford University School of Medicine Director, Stanford Women s Cancer Center Senior Scientific Advisor, Stanford
Cervical Cancer: 2018 FIGO Staging Jonathan S. Berek, MD, MMS Laurie Kraus Lacob Professor Stanford University School of Medicine Director, Stanford Women s Cancer Center Senior Scientific Advisor, Stanford
Thyroid Gland. Protocol applies to all malignant tumors of the thyroid gland, except lymphomas.
 Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Uveal Melanoma. Protocol applies to malignant melanoma of the uvea.
 Uveal Melanoma Protocol applies to malignant melanoma of the uvea. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Uveal Melanoma Protocol applies to malignant melanoma of the uvea. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Fallopian Tube. Protocol applies to all invasive carcinomas of the fallopian tube.
 Fallopian Tube Protocol applies to all invasive carcinomas of the fallopian tube. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology
Fallopian Tube Protocol applies to all invasive carcinomas of the fallopian tube. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology
Mody. AIS vs. Invasive Adenocarcinoma of the Cervix
 Common Problems in Gynecologic Pathology Michael T. Deavers, M.D. Houston Methodist Hospital, Houston, Texas Common Problems in Gynecologic Pathology Adenocarcinoma in-situ (AIS) of the Cervix vs. Invasive
Common Problems in Gynecologic Pathology Michael T. Deavers, M.D. Houston Methodist Hospital, Houston, Texas Common Problems in Gynecologic Pathology Adenocarcinoma in-situ (AIS) of the Cervix vs. Invasive
57th Annual HSCP Spring Symposium 4/16/2016
 An Unusual Malignant Spindle Cell Lesion to Involve the Breast Erinn Downs-Kelly, D.O. Associate Professor of Pathology University of Utah & ARUP Laboratories No disclosures Case 39 y/o female with no
An Unusual Malignant Spindle Cell Lesion to Involve the Breast Erinn Downs-Kelly, D.O. Associate Professor of Pathology University of Utah & ARUP Laboratories No disclosures Case 39 y/o female with no
What is endometrial cancer?
 Uterine cancer What is endometrial cancer? Endometrial cancer is the growth of abnormal cells in the lining of the uterus. The lining is called the endometrium. Endometrial cancer usually occurs in women
Uterine cancer What is endometrial cancer? Endometrial cancer is the growth of abnormal cells in the lining of the uterus. The lining is called the endometrium. Endometrial cancer usually occurs in women
Endometrial Stromal Sarcoma Arising from Endometrial Polyp: A Case Report
 Kobe J. Med. Sci., Vol. 64, No. 2, pp. E36-E42, 2018 Endometrial Stromal Sarcoma Arising from Endometrial Polyp: A Case Report SAKI SATO 1, YOJIRO OJIMA 1, MASATOSHI KANDA 1, TOMOHIKO KIZAKI 2 and NORIYUKI
Kobe J. Med. Sci., Vol. 64, No. 2, pp. E36-E42, 2018 Endometrial Stromal Sarcoma Arising from Endometrial Polyp: A Case Report SAKI SATO 1, YOJIRO OJIMA 1, MASATOSHI KANDA 1, TOMOHIKO KIZAKI 2 and NORIYUKI
Uterine sarcomas. Nomonde Mbatani 1,2 Alexander B. Olawaiye 3 Jaime Prat 4, * Abstract 1 INTRODUCTION FIGO CANCER REPORT 2018
 DOI: 10.1002/ijgo.12613 FIGO CANCER REPORT 2018 Uterine sarcomas Nomonde Mbatani 1,2 Alexander B. Olawaiye 3 Jaime Prat 4, * 1 Department of Obstetrics and Gynecology, Groote Schuur Hospital/ University
DOI: 10.1002/ijgo.12613 FIGO CANCER REPORT 2018 Uterine sarcomas Nomonde Mbatani 1,2 Alexander B. Olawaiye 3 Jaime Prat 4, * 1 Department of Obstetrics and Gynecology, Groote Schuur Hospital/ University
Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES CASE 1: FEMALE REPRODUCTIVE
 Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES PHYSICAL EXAMINATION CASE 1: FEMALE REPRODUCTIVE 3/5 Patient presents through the emergency room with
Please complete prior to the webinar. HOSPITAL REGISTRY WEBINAR FEMALE REPRODUCTIVE SYSTEM EXERCISES PHYSICAL EXAMINATION CASE 1: FEMALE REPRODUCTIVE 3/5 Patient presents through the emergency room with
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas.
 Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum
 Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th
Protocol for the Examination of Specimens From Patients With Primary Tumors of the Ovary, Fallopian Tube, or Peritoneum Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th
Endometrial Stromal Tumors
 Endometrial Stromal Tumors WHO Categories: Endometrial Stromal Nodule (ESN) Endometrial Stromal Sarcoma, low grade (LGESS) Endometrial Stromal Sarcoma, high grade (HGESS) Undifferentiated Uterine Sarcoma
Endometrial Stromal Tumors WHO Categories: Endometrial Stromal Nodule (ESN) Endometrial Stromal Sarcoma, low grade (LGESS) Endometrial Stromal Sarcoma, high grade (HGESS) Undifferentiated Uterine Sarcoma
International Journal of Case Reports in Medicine
 International Journal of Case Reports in Medicine Vol. 2013 (2013), Article ID 665097, 28 minipages. DOI:10.5171/2013.665097 www.ibimapublishing.com Copyright 2013 Hemalatha A. L., Varna I, Deepthi B.
International Journal of Case Reports in Medicine Vol. 2013 (2013), Article ID 665097, 28 minipages. DOI:10.5171/2013.665097 www.ibimapublishing.com Copyright 2013 Hemalatha A. L., Varna I, Deepthi B.
L ARYNX S TAGING F ORM
 CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
Protocol for the Examination of Specimens From Patients With Carcinoma and Carcinosarcoma of the Endometrium
 Protocol for the Examination of Specimens From Patients With Carcinoma and Carcinosarcoma of the Endometrium Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
Protocol for the Examination of Specimens From Patients With Carcinoma and Carcinosarcoma of the Endometrium Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
Carcinoma of the Urinary Bladder Histopathology
 Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Disclosure. Case. Mixed Tumors of the Uterine Corpus and Cervix. I have nothing to disclose
 Mixed Tumors of the Uterine Corpus and Cervix Marisa R. Nucci, M.D. Division of Women s and Perinatal Pathology Department of Pathology Brigham and Women s Hospital Boston, MA UCSF Current Issues in Anatomic
Mixed Tumors of the Uterine Corpus and Cervix Marisa R. Nucci, M.D. Division of Women s and Perinatal Pathology Department of Pathology Brigham and Women s Hospital Boston, MA UCSF Current Issues in Anatomic
Protocol applies to specimens from patients with Wilms tumor (nephroblastoma) or other renal tumors of childhood.
 Wilms Tumor Protocol applies to specimens from patients with Wilms tumor (nephroblastoma) or other renal tumors of childhood. Procedures Cytology (No Accompanying Checklist) Incisional Biopsy (Needle or
Wilms Tumor Protocol applies to specimens from patients with Wilms tumor (nephroblastoma) or other renal tumors of childhood. Procedures Cytology (No Accompanying Checklist) Incisional Biopsy (Needle or
Type I. Type II. Excess estrogen Lynch Endometrioid adenocarcinoma PTEN. High grade More aggressive Serous, Clear Cell p53
 Type I Excess estrogen Lynch Endometrioid adenocarcinoma PTEN Type II High grade More aggressive Serous, Clear Cell p53 Stage I IA IB Stage II Stage III IIIA IIIB IIIC IIIC1 IIIC2 Stage IV IVA IVB nodes
Type I Excess estrogen Lynch Endometrioid adenocarcinoma PTEN Type II High grade More aggressive Serous, Clear Cell p53 Stage I IA IB Stage II Stage III IIIA IIIB IIIC IIIC1 IIIC2 Stage IV IVA IVB nodes
Retinoblastoma. Protocol applies to retinoblastoma only.
 Retinoblastoma Protocol applies to retinoblastoma only. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Retinoblastoma Protocol applies to retinoblastoma only. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
M ALIGNANT MELANOMA OF THE UVEA STAGING FORM
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
Uterus Malignancies /5/15
 Collecting Cancer Data: Uterus 2014-2015 NAACCR Webinar Series February 5, 2015 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
Collecting Cancer Data: Uterus 2014-2015 NAACCR Webinar Series February 5, 2015 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
The International Federation of Gynecology and Obstetrics (FIGO) updated the staging
 Continuing Education Column Revised FIGO Staging System Hee Sug Ryu, MD Department of Obstetrics and Gynecology, Ajou University School of Medicine E - mail : hsryu@ajou.ac.kr J Korean Med Assoc 2010;
Continuing Education Column Revised FIGO Staging System Hee Sug Ryu, MD Department of Obstetrics and Gynecology, Ajou University School of Medicine E - mail : hsryu@ajou.ac.kr J Korean Med Assoc 2010;
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy
 Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Diagnostically Challenging Cases in Gynecologic Pathology
 Diagnostically Challenging Cases in Gynecologic Pathology Eric C. Huang, M.D., Ph.D. Department of Pathology and Laboratory Medicine University of California, Davis Medical Center Case 1 Presentation 38
Diagnostically Challenging Cases in Gynecologic Pathology Eric C. Huang, M.D., Ph.D. Department of Pathology and Laboratory Medicine University of California, Davis Medical Center Case 1 Presentation 38
Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do?
 Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Uterine Cervix. Protocol applies to all invasive carcinomas of the cervix.
 Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Low-Grade Periductal Stromal of Breast: a case report
 Low-Grade Periductal Stromal of Breast: a case report Rosanna Nenna 1 Cosimo Damiano Inchingolo 1 Domenico Palmieri 2 Annalisa De Lucia 1 Giusy Elicio 1 Pina Miscioscia 1 ( 1 ) U.O.C. di Anatomia Patologica,
Low-Grade Periductal Stromal of Breast: a case report Rosanna Nenna 1 Cosimo Damiano Inchingolo 1 Domenico Palmieri 2 Annalisa De Lucia 1 Giusy Elicio 1 Pina Miscioscia 1 ( 1 ) U.O.C. di Anatomia Patologica,
Uterine Cervix. Protocol applies to all invasive carcinomas of the cervix.
 Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2004 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2004 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
S1.04 Principal clinician. G1.01 Comments. G2.01 *Specimen dimensions (prostate) S2.02 *Seminal vesicles
 Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST)
 Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: December 2014 Procedures Biopsy Resection
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: December 2014 Procedures Biopsy Resection
Testis. Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies.
 Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Endometrial Stromal Sarcoma
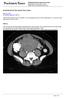 May 26, 2011 By Sushila Ladumor, MD [1] Endometrial stromal sarcoma (ESS) is a rare malignant tumor of the endometrium, occurring in the age group of 40-50 years. History The 50-year-old, female patient
May 26, 2011 By Sushila Ladumor, MD [1] Endometrial stromal sarcoma (ESS) is a rare malignant tumor of the endometrium, occurring in the age group of 40-50 years. History The 50-year-old, female patient
International Society of Gynecological Pathologists Symposium 2007
 International Society of Gynecological Pathologists Symposium 2007 Anais Malpica, M.D. Department of Pathology The University of Texas M.D. Anderson Cancer Center Grading of Ovarian Cancer Histologic grade
International Society of Gynecological Pathologists Symposium 2007 Anais Malpica, M.D. Department of Pathology The University of Texas M.D. Anderson Cancer Center Grading of Ovarian Cancer Histologic grade
Uterine Malignancies. Collecting Cancer Data: Uterine Malignancies 10/7/2010. NAACCR Webinar Series 1. Questions. Fabulous Prizes!!!
 Uterine October 7, 2010 NAACCR 2010-2011 Webinar Series Session 1 1 Questions Please use the Q&A panel to submit your questions Send questions to All Panelist 2 Fabulous Prizes!!! 3 NAACCR 2010-2011 Webinar
Uterine October 7, 2010 NAACCR 2010-2011 Webinar Series Session 1 1 Questions Please use the Q&A panel to submit your questions Send questions to All Panelist 2 Fabulous Prizes!!! 3 NAACCR 2010-2011 Webinar
The Good Uterine Sarcomas: What Do You Need to Know
 The Good Uterine Sarcomas: What Do You Need to Know Anais Malpica, M.D. Departments of Pathology and Gynecologic Oncology The University of Texas M.D. Anderson Cancer Center Matthew Powell, M.D. Division
The Good Uterine Sarcomas: What Do You Need to Know Anais Malpica, M.D. Departments of Pathology and Gynecologic Oncology The University of Texas M.D. Anderson Cancer Center Matthew Powell, M.D. Division
Staging. Carcinoma confined to the corpus. Carcinoma confined to the endometrium. Less than ½ myometrial invasion. Greater than ½ myometrial invasion
 5 th of June 2009 Background Most common gynaecological carcinoma in developed countries Most cases are post-menopausal Increasing incidence in certain age groups Increasing death rates in the USA 5-year
5 th of June 2009 Background Most common gynaecological carcinoma in developed countries Most cases are post-menopausal Increasing incidence in certain age groups Increasing death rates in the USA 5-year
Endometrial sarcomas: an immunohistochemical and JAZF1 re-arrangement study in low-grade and undifferentiated tumors
 & 2013 USCAP, Inc. All rights reserved 0893-3952/13 $32.00 95 Endometrial sarcomas: an immunohistochemical and JAZF1 re-arrangement study in low-grade and undifferentiated tumors Kiran Jakate 1, Farshad
& 2013 USCAP, Inc. All rights reserved 0893-3952/13 $32.00 95 Endometrial sarcomas: an immunohistochemical and JAZF1 re-arrangement study in low-grade and undifferentiated tumors Kiran Jakate 1, Farshad
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Gynecological sarcoma
 Gynecological sarcoma Therapy of the gynecological sarcoma Treatment gynecologist s update view Frédéric Amant MD PhD Gynecologic Oncology, KU Leuven, Belgium Center Gynecologic Oncology Amsterdam (CGOA),
Gynecological sarcoma Therapy of the gynecological sarcoma Treatment gynecologist s update view Frédéric Amant MD PhD Gynecologic Oncology, KU Leuven, Belgium Center Gynecologic Oncology Amsterdam (CGOA),
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Interactive Staging Bee
 Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain
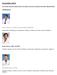 December 2016 59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain Contributed by: Divya Sharma, MD. Fellow, Gastrointestinal Pathology, Department of Pathology
December 2016 59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain Contributed by: Divya Sharma, MD. Fellow, Gastrointestinal Pathology, Department of Pathology
Page # 1. Endometrium. Cellular Components. Anatomical Regions. Management of SIL Thomas C. Wright, Jr. Most common diseases:
 Endometrium Pathology of the Endometrium Thomas C. Wright Columbia University, New York, NY Most common diseases: Abnormal uterine bleeding Inflammatory conditions Benign neoplasms Endometrial cancer Anatomical
Endometrium Pathology of the Endometrium Thomas C. Wright Columbia University, New York, NY Most common diseases: Abnormal uterine bleeding Inflammatory conditions Benign neoplasms Endometrial cancer Anatomical
Protocol for the Examination of Specimens from Patients with Carcinoma of the Fallopian Tube
 Protocol for the Examination of Specimens from Patients with Carcinoma of the Fallopian Tube Protocol applies to all carcinomas presumed to be arising from the mucosa of the fallopian tube. Based on AJCC/UICC
Protocol for the Examination of Specimens from Patients with Carcinoma of the Fallopian Tube Protocol applies to all carcinomas presumed to be arising from the mucosa of the fallopian tube. Based on AJCC/UICC
Current staging of endometrial carcinoma with MR imaging
 Current staging of endometrial carcinoma with MR imaging Poster No.: C-1436 Congress: ECR 2015 Type: Educational Exhibit Authors: M. Magalhaes, H. Donato, C. B. Marques, P. Gomes, F. Caseiro Alves; Coimbra/PT
Current staging of endometrial carcinoma with MR imaging Poster No.: C-1436 Congress: ECR 2015 Type: Educational Exhibit Authors: M. Magalhaes, H. Donato, C. B. Marques, P. Gomes, F. Caseiro Alves; Coimbra/PT
Diplomate of the American Board of Pathology in Anatomic and Clinical Pathology
 A 33-year-old male with a left lower leg mass. Contributed by Shaoxiong Chen, MD, PhD Assistant Professor Indiana University School of Medicine/ IU Health Partners Department of Pathology and Laboratory
A 33-year-old male with a left lower leg mass. Contributed by Shaoxiong Chen, MD, PhD Assistant Professor Indiana University School of Medicine/ IU Health Partners Department of Pathology and Laboratory
Staging for Residents, Nurses, and Multidisciplinary Health Care Team
 Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Case Report Serous Ovarian Carcinoma Recurring as Malignant Mixed Mullerian Tumor
 Case Reports in Obstetrics and Gynecology Volume 2015, Article ID 612824, 5 pages http://dx.doi.org/10.1155/2015/612824 Case Report Serous Ovarian Carcinoma Recurring as Malignant Mixed Mullerian Tumor
Case Reports in Obstetrics and Gynecology Volume 2015, Article ID 612824, 5 pages http://dx.doi.org/10.1155/2015/612824 Case Report Serous Ovarian Carcinoma Recurring as Malignant Mixed Mullerian Tumor
Chapter 2 Staging of Breast Cancer
 Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Uterine Tumors Resembling Ovarian Sex Cord Tumor in Postmenopausal Woman
 DOI 10.1007/s13224-014-0545-0 CASE REPORT in Postmenopausal Woman Byun Jung Mi Kim Ki Tae Yoon Hye Kyoung Jeong Dae Hoon Kim Young Nam Lee Kyung Bok Sung Moon Su Received: 14 January 2014 / Accepted: 22
DOI 10.1007/s13224-014-0545-0 CASE REPORT in Postmenopausal Woman Byun Jung Mi Kim Ki Tae Yoon Hye Kyoung Jeong Dae Hoon Kim Young Nam Lee Kyung Bok Sung Moon Su Received: 14 January 2014 / Accepted: 22
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Ovarian mucinous borderline tumor accompanied by LGESS with myxoid change: a case report and literature review
 https://doi.org/10.1186/s40001-017-0295-4 European Journal of Medical Research CASE REPORT Open Access Ovarian mucinous borderline tumor accompanied by LGESS with myxoid change: a case report and literature
https://doi.org/10.1186/s40001-017-0295-4 European Journal of Medical Research CASE REPORT Open Access Ovarian mucinous borderline tumor accompanied by LGESS with myxoid change: a case report and literature
NAACCR Webinar Series /7/17
 COLLECTING CANCER DATA: UTERUS 2017 2018 NAACCR WEBINAR SERIES Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this webinar
COLLECTING CANCER DATA: UTERUS 2017 2018 NAACCR WEBINAR SERIES Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching this webinar
Protocol for the Examination of Specimens From Patients With Thymoma and Thymic Carcinoma
 Protocol for the Examination of Specimens From Patients With Thymoma and Thymic Carcinoma Protocol applies to thymic epithelial tumors located in any area of the mediastinum. No AJCC/UICC TNM Staging System
Protocol for the Examination of Specimens From Patients With Thymoma and Thymic Carcinoma Protocol applies to thymic epithelial tumors located in any area of the mediastinum. No AJCC/UICC TNM Staging System
North of Scotland Cancer Network Clinical Management Guideline for Carcinoma of the Uterine Cervix
 THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
THIS DOCUMENT North of Scotland Cancer Network Carcinoma of the Uterine Cervix UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Prepared by A Kennedy/AG Macdonald/Others Approved by NOT APPROVED Issue date April
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina
 Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
CPC on Cervical Pathology
 CPC on Cervical Pathology Dr. W.K. Ng Senior Medical Officer Department of Clinical Pathology Pamela Youde Nethersole Eastern Hospital Cervical Smear: High Grade SIL (CIN III) Cervical Smear: High Grade
CPC on Cervical Pathology Dr. W.K. Ng Senior Medical Officer Department of Clinical Pathology Pamela Youde Nethersole Eastern Hospital Cervical Smear: High Grade SIL (CIN III) Cervical Smear: High Grade
A 25 year old female with a palpable mass in the right lower quadrant of her abdomen
 May 2016 A 25 year old female with a palpable mass in the right lower quadrant of her abdomen Contributed by: Paul Ndekwe, MD, Resident Physician, Indiana University School of Department of Pathology and
May 2016 A 25 year old female with a palpable mass in the right lower quadrant of her abdomen Contributed by: Paul Ndekwe, MD, Resident Physician, Indiana University School of Department of Pathology and
The new FIGO classification in endometrial carcinoma
 The new FIGO classification in endometrial carcinoma Poster No.: C-1073 Congress: ECR 2012 Type: Educational Exhibit Authors: A. IGLESIAS CASTAÑON, M. Arias Gonzales, J. Mañas Uxó, 1 2 1 2 2 2 B. NIETO
The new FIGO classification in endometrial carcinoma Poster No.: C-1073 Congress: ECR 2012 Type: Educational Exhibit Authors: A. IGLESIAS CASTAÑON, M. Arias Gonzales, J. Mañas Uxó, 1 2 1 2 2 2 B. NIETO
47. Melanoma of the Skin
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Protocol for the Examination of Specimens From Patients With Gestational Trophoblastic Malignancy
 Protocol for the Examination of Specimens From Patients With Gestational Trophoblastic Malignancy Protocol applies to all gestational trophoblastic malignancies. Based on AJCC/UICC TNM, 7th edition, and
Protocol for the Examination of Specimens From Patients With Gestational Trophoblastic Malignancy Protocol applies to all gestational trophoblastic malignancies. Based on AJCC/UICC TNM, 7th edition, and
