-RA (1 + AT (1200 IU),
|
|
|
- Dinah Townsend
- 6 years ago
- Views:
Transcription
1 Treatment of Former Smokers With 9-cis-Retinoic Acid Reverses Loss of Retinoic Acid Receptor- Expression in the Bronchial Epithelium: Results From a Randomized Placebo-Controlled Trial Jonathan M. Kurie, Reuben Lotan, J. Jack Lee, Jin Soo Lee, Rodolfo C. Morice, Diane D. Liu, Xiao-Chun Xu, Fadlo R. Khuri, Jae Y. Ro, Walter N. Hittelman, Garrett L. Walsh, Jack A. Roth, John D. Minna, Waun Ki Hong Background: Loss of retinoic acid receptor beta (RAR- ) expression in the bronchial epithelium is considered a biomarker of preneoplasia. Retinoids can restore expression of this receptor and, presumably, halt the progression of carcinogenesis. This study was designed to investigate whether either of two retinoid-based regimens, 9-cis-retinoic acid (RA) or 13-cis-RA plus -tocopherol (AT), could reverse RAR- expression loss in former smokers after 3 months of treatment. Methods: Individuals (n = 226) who had smoked at least 20 pack-years and had ceased smoking for at least 12 months were randomly assigned to receive 3 months of daily oral 9-cis-RA (100 mg), 13-cis-RA (1 mg/kg) + AT (1200 IU), or placebo. Bronchoscopy and biopsy at six predetermined sites of the bronchial tree were performed before treatment and at 3 and 6 months thereafter. Specimens were evaluated for squamous metaplasia, dysplasia, and RAR- expression. McNemar s test was used to test changes in RAR- expression and squamous metaplasia within each treatment group, and a generalized estimating equations model was applied to model the treatment effect, adjusting for covariates. All statistical tests were two-sided. Results: A total of 177 assessable subjects completed at least 3 months of therapy and underwent at least the baseline and 3-month bronchoscopic evaluations with biopsies. RAR- was detected in 69.7% of all baseline biopsy samples, and metaplasia was evident in 6.9% of all baseline samples from 240 subjects. Restoration of RAR- expression (P =.03) and reduction of metaplasia (P =.01) were found in the 9-cis-RA group. After adjustment for years of smoking, packs/day smoked, and metaplasia, treatment with 9-cis-RA, but not with 13-cis-RA + AT, led to a statistically significant increase in RAR- expression compared with placebo (P =.03). Conclusion: 9-cis-RA treatment can restore RAR- expression in the bronchial epithelium of former smokers, raising the possibility that this retinoid has potential chemopreventive properties in former smokers. [J Natl Cancer Inst 2003;95:206 14] Lung cancer is the leading cause of cancer-related death in the United States (1), largely because most cases of lung cancer are diagnosed after the disease has metastasized and because current therapeutic approaches for advanced lung cancer have little effect on disease-related mortality. Surgical resection of the primary tumor results in long-term cure for only about 50% of patients with early-stage lung cancer; the remaining patients either experience disease recurrence or develop a second primary tumor in the aerodigestive tract (2). Clearly, given these findings, new treatment approaches are needed to reduce lung cancer-related mortality. In light of the ineffectiveness of current therapeutic strategies in treating clinically evident lung cancer, investigative research efforts have focused on lung cancer prevention. Because exposure to cigarette smoke confers the greatest risk for developing lung cancer, with approximately 90% of all lung cancers occurring among individuals who smoke, smoking cessation campaigns have been a major focus of preventive efforts. The relative risk of developing lung cancer declines in former smokers to approximately twice that of individuals who have never smoked by 20 years after smoking cessation; however, it remains elevated in these individuals indefinitely (3). There are 45 million former smokers in the United States, and 50% of newly diagnosed lung cancers occur in former smokers (3). These findings illustrate the importance of targeting former smokers for lung cancer prevention studies and the need to identify chemopreventive strategies that will further reduce lung cancer risk in former smokers. The agents most frequently studied in preclinical and clinical lung cancer chemoprevention studies thus far are the retinoids, which are natural and synthetic compounds related to vitamin A (retinol) and retinoic acid (RA) (4). Retinoids act by binding and transcriptionally activating nuclear retinoid receptors. Each of the two families of retinoid receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs), has three members (,, and ) (5). These receptors function as RXR:RAR heterodimers and RXR:RXR homodimers. Of the naturally occurring retinoids, all-trans-ra binds to RARs, and 9-cis-RA binds to both RARs and RXRs. 13-cis-RA binds to neither receptor type and is thought to bind after stereoisomerization to either all-trans-ra or 9-cis-RA, a process that occurs intracellularly (6). Because 9-cis-RA binds to both RARs and RXRs, it can activate RXR:RAR heterodimers, RXR:RXR homodimers, and Affiliations of authors: J. M. Kurie, R. Lotan, J. S. Lee, F. R. Khuri, W. K. Hong (Department of Thoracic/Head and Neck Medical Oncology), J. J. Lee, D. D. Liu (Department of Biostatistics), R. C. Morice (Department of Pulmonary Medicine), X.-C. Xu (Department of Cancer Prevention), J. Y. Ro (Department of Pathology), W. N. Hittelman (Department of Experimental Therapeutics), G. L. Walsh, J. A. Roth (Department of Thoracic and Cardiovascular Surgery), The University of Texas M. D. Anderson Cancer Center, Houston; J. D. Minna, Hamon Center for Therapeutic Oncology Research, University of Texas Southwestern Medical Center, Dallas. Correspondence to: Waun Ki Hong, M.D., Box 432, Department of Thoracic/ Head and Neck Medical Oncology, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX ( whong@mdanderson.org). See Notes following References. Journal of the National Cancer Institute, Vol. 95, No. 3, Oxford University Press 2003, all rights reserved. 206 ARTICLES Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003
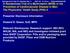
2 other nuclear receptor complexes in which the RXR is a ligandbinding partner, such as the vitamin D receptor and the peroxisome proliferator-activated receptor (5). Thus, because of its unique receptor-binding properties, 9-cis-RA has biologic effects that all-trans-ra and 13-cis-RA do not. Supporting this concept, single-agent 9-cis-RA was effective in the chemoprevention of mammary and prostate cancer in preclinical studies, and it enhanced the antitumor effects of cisplatin against human oral squamous carcinoma xenografts (7 9). Furthermore, Targretin (Ligand Pharmaceuticals, San Diego, CA) a synthetic retinoid that binds selectively to RXRs, also demonstrated efficacy in preclinical models of mammary cancer chemoprevention, suggesting that RXR is an important target for the chemoprevention of epithelial cancer (10,11). The retinoid that has demonstrated the greatest activity in the prevention of aerodigestive tract cancer is 13-cis-RA. In patients with a previous cancer of the head and neck region, high-dose 13-cis-RA treatment reduced the incidence of second primary tumors and decreased leukoplakia (i.e., premalignant oral lesions), which is evident on physical examination and microscopic evaluation of leukoplakia biopsies (12). This latter effect correlated with the ability of 13-cis-RA to increase the expression of RAR-, a retinoic acid-responsive gene whose expression is lost in premalignant epithelial lesions of several organs, including those of the aerodigestive tract (13,14). This chemopreventive activity of 13-cis-RA against head and neck cancer will require confirmation by large-scale chemoprevention trials, which are currently ongoing. Although effective in the prevention of head and neck cancer, retinoids have not yet been demonstrated to be active in the prevention of lung cancer. In fact, several large-scale trials, including the Beta-Carotene and Retinol Efficacy Trial (CARET), the Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study, and the National Cancer Institute-sponsored Intergroup Lung Study, have demonstrated that retinoids may actually enhance lung cancer incidence in individuals who smoke (15 17). In contrast, retinoid treatment reduced tumor recurrence and mortality in nonsmokers, and there was some evidence of benefit, albeit not statistically significant, in tumor recurrence and mortality in former smokers (17), suggesting that current and former smokers differ in their response to retinoids. Supporting these findings, 13-cis-RA treatment of current smokers had no effect on bronchial squamous metaplasia, a histologic abnormality associated with smoking, whereas 13-cis-RA treatment conferred some evidence of reduction in bronchial squamous metaplasia in former smokers (18). However, the number of former smokers enrolled in these studies was small, and additional investigations are needed to address whether former smokers may be a better target population than current smokers for retinoid-based chemoprevention studies. On the basis of these findings, we sought to investigate the activity of 9-cis-RA in the reversal of bronchial premalignancy in former smokers and to compare it with the activity of 13-cis- RA in combination with -tocopherol (AT), which has been shown to reduce the toxic effects of 13-cis-RA and to have intrinsic chemopreventive activity of its own (19,20). We focused on former smokers so that we could characterize the biology of the bronchial epithelium and its response to retinoids in the absence of continuous exposure to cigarette smoke carcinogens. Histologic evidence of bronchial premalignancy has been detected in only approximately 30% of former smokers (18). Therefore, we chose as the primary endpoint of this study the reversal of RAR- expression loss in the bronchial epithelium. We have found in preliminary studies that RAR- expression loss, a biomarker of bronchial premalignancy, is found in approximately 60% of former smokers. We hypothesized that in former smokers, loss of expression of RAR- is a marker of bronchial preneoplasia and that its restoration reflects suppression of bronchial preneoplasia. SUBJECTS AND METHODS Subjects A three-arm, randomized, double-blinded, placebo-controlled trial was conducted to compare the effects of 9-cis-RA (100 mg) with those of 13-cis-RA (1 mg/kg) plus AT (1200 IU) on loss of RAR- expression in bronchial epithelial biopsies from former smokers. For the purpose of this study, former smokers were defined as individuals who had a smoking history of at least 20 pack-years and who had stopped smoking at least 1 year before study entry. Subjects were identified through advertisements that were distributed to referring doctors and patients at The University of Texas M. D. Anderson Cancer Center, provided to local health fairs and community groups, and distributed through local and national media campaigns. To be eligible, subjects had to have adequate renal, hematologic, and hepatic function and must not have taken more than IU of vitamin A or other retinoids per day within 3 months of study entry. Subjects were allowed to have had a prior smoking-related cancer, but they had to have been tumor-free for 6 months before enrollment in the study. Subjects were required to abstain from consuming vitamin A dietary supplements during treatment on the study. This study was approved by the Institutional Review Board at The University of Texas M. D. Anderson Cancer Center and by the U.S. Department of Health and Human Services. Prior to being randomly assigned, all eligible subjects provided written informed consent. Trial Design This trial originally began as a two-arm trial to compare the activity of 13-cis-RA + AT with that of placebo in former smokers using metaplasia index as the primary endpoint. The eligibility criteria for the original trial were the same as those for the subsequent one, except for a requirement for detectable bronchial squamous metaplasia (i.e., a metaplasia index of >15%, dysplasia, or both) at the time of the baseline screening bronchoscopy. The treatment duration was 6 months in the original trial rather than the 3-month treatment duration for the present trial. Under the original trial design, 13 subjects were registered between November 21, 1995, and September 4, However, 10 subjects could not be randomly assigned after their baseline screening bronchoscopy because of an inadequate metaplasia index (i.e., 15%). After modification to the three-arm trial, a further 227 subjects were registered between February 1997 and May 2001, of whom 223 were eligible for random assignment. Of the four patients who were not randomly assigned, one had intercurrent illness, one had a complication from bronchoscopy and wished to drop out, one was ineligible because of high triglyceride levels, and one dropped out for personal reasons. Altogether, 240 subjects were registered in the trial, of whom 226 were randomly assigned to one of the treatment arms (Fig. 1). Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003 ARTICLES 207
3 Fig. 1. CONSORT flow diagram for subjects who were former smokers accrued into the trial. Subjects were randomly assigned to receive daily oral 9-cis-RA (retinoic acid), 13-cis-RA + -tocopherol (AT), or placebo. N C number of subjects completing 3 months or 6 months of treatment and who had a second and third bronchial biopsy. N R number of subjects with assessable retinoic acid receptor beta (RAR- ) expression. * One of these subjects was incorrectly crossed over to the 13-cis-RA + AT arm. Therefore, only 59 subjects were assessable for metaplasia index and 58 were assessable for RAR- expression. In the baseline screening bronchoscopy, biopsy samples were obtained from six predetermined sites in the bronchial tree the main carina, the bifurcation of the right upper lobe and mainstem bronchus, the bifurcation of the right middle lobe and right lower lobe, the bifurcation of the left upper lobe and the lingula, the medial bronchus of the right lower lobe, and the anterior bronchus of the left lower lobe. Subjects were seen monthly and evaluated for compliance with the trial protocol, drug-related toxic effects, and serum 13-cis-RA drug levels. Serum cotinine levels were drawn at baseline, 3 months, and 6 months to document compliance with smoking abstinence during the trial. Because toxicity data from prior phase I trials that included 9-cis-RA treatment of cancer patients did not extend beyond 3 months of treatment (21,22), the duration of retinoid treatment for this study was 3 months. At that time, a second bronchoscopic examination with biopsy was performed to evaluate the bronchial tree for evidence of change. Drug-related toxicity was graded according to the National Cancer Institute s Common Toxicity Criteria (CTC, version 2.0; forms/ctcv20_ pdf). Treatment Of the 226 eligible subjects, 75 were randomly assigned to the placebo group, 77 to the 13-cis-RA + AT group, and 74 to the 9-cis-RA group (Fig. 1). Treatment for all three groups was divided into twice-daily doses. Subjects took oral 9-cis-RA (or the placebo for it) in the evening and 13-cis-RA + AT (or the placebos for them) in the morning. 13-cis-RA was supplied by Hoffmann-LaRoche Pharmaceuticals (Nutley, NJ), AT by the Henkel Corporation (La Grange, IL), and 9-cis-RA by Ligand Pharmaceuticals (San Diego, CA). The trial also included a crossover design based on the results of the baseline and 3-month bronchoscopy examinations. If disease progression was evident (i.e., if development of bronchial dysplasia and/or the metaplasia index increased) the subject s trial arm code was unblinded and the subject s treatment group assignment was revealed. If the subject had been in the placebo group, he or she was crossed over to undergo 3 months of treatment with 13-cis-RA + AT. If the subject had been in the 13-cis-RA + AT group or the 9-cis-RA group, treatment was discontinued. Biopsy Specimens Specimens were fixed in buffered formalin, embedded in paraffin, and sliced into 4- m sections (23). Ten sections per biopsy site were stained with hematoxylin eosin and evaluated for the presence of dysplasia and squamous metaplasia by a pathologist (J. Y. Ro) blinded to the timing of specimen collection and the treatment group. Squamous metaplasia was quantified with a metaplasia index (23), in which the number of biopsy sections from each subject exhibiting squamous metaplasia was divided by the total number of sections examined for that time point, and the result was multiplied by 100. The 15% cutoff point for the metaplasia index was established based on the fact that 10 sections were analyzed from each biopsy, with six sites per subject. Typically, if metaplasia was seen in a biopsy, then it was seen in all 10 sections. Thus, this amounts to 10/60 (16.6%) sites. In this situation, one biopsy site with complete squamous metaplasia would place a subject over the 15% cutoff point. However, if one biopsy site has only partial squamous metaplasia, then the subject would be classified as being below the 15% cutoff point. In addition to its analysis as a continuous variable, squamous metaplasia was analyzed on a per-site basis as a binary variable. In Situ mrna Hybridization Studies RAR- mrna levels in tissue sections were analyzed by nonradioactive in situ hybridization (24). Briefly, digoxygeninlabeled antisense RAR- riboprobes were hybridized to 4- m sections of the formalin-fixed, paraffin-embedded biopsy specimens. Probe binding was visualized by incubating sections with peroxidase-conjugated antidigoxygenin antibodies followed by a peroxidase substrate. Specificity of binding was confirmed with digoxygenin-labeled sense probes. To control for RNA degradation, we used an antisense riboprobe for RXR-, a ubiquitous retinoid receptor. Expression of RAR- was analyzed in one section in each of the assessable (i.e., containing sufficient epithelium for interpretation) biopsy sites for each subject (i.e., up 208 ARTICLES Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003
4 to six sections per subject). RAR- mrna expression in the bronchial biopsy samples was quantified in terms of an RAR- index, in which the number of biopsy sections from each subject expressing RAR- mrna was divided by the total number of sections examined, and the result was multiplied by 100. In addition to its analysis as a continuous variable, RAR- was analyzed as a binary variable. Statistical Analysis Summary statistics, including frequency tabulation, means, standard deviations, median, and range, were given to characterize subject characteristics, expression of RAR-, and metaplasia index. The Wilcoxon rank sum test and Kruskal Wallis test were used to test the equal median of continuous variables among two and three treatment groups, respectively. The chisquare ( 2 ) test or Fisher s exact test was used to test the association between two categorical variables. McNemar s test and Wilcoxon signed-rank test (25) were used to test changes in RAR- expression and squamous metaplasia by subject over time within each treatment group. When the biopsy site was used as the unit of analysis (under the assumption that the site was nested within the subject), the generalized estimating equations model (26) was applied to model the treatment effect on RAR- expression and squamous metaplasia, adjusting for covariates, such as packs smoked per day and number of years of smoking. Changes in RAR- index within each group were considered a secondary outcome variable. Assuming a 25% difference in RAR- expression change from baseline to 3 months between the placebo and treatment groups, the study was designed to accrue 60 assessable subjects per group (i.e., n 180) to achieve 78% power to detect a treatment effect. All statistical tests were two-sided, with a 5% type I error rate. Statistical analysis was performed with standard statistical software, including SAS Release 8.1 (SAS Institute, Cary, NC) and S-Plus 2000 (Mathsoft, Inc., Seattle, WA). RESULTS Subject Characteristics Two hundred forty subjects were registered into the clinical trial, of whom 226 were eligible for being randomly assigned (Fig. 1). As required under the original eligibility criteria (before the protocol was revised), 10 of the original 13 subjects were not included because of an inadequate metaplasia index at baseline bronchoscopy. Three other subjects of the next 227 were excluded because of intervening health problems after bronchoscopic examination, and one was excluded because of personal reasons. The three treatment groups (placebo, 13-cis-RA + AT, and 9-cis-RA) were well balanced for age, sex, race, history of smoking-related cancer, numbers of pack-years and quit-years of smoking, and metaplasia index (Table 1). Twenty-two of the 226 subjects had had a prior smoking-related cancer, including 18 lung cancers, three head and neck cancers, and one bladder cancer. Of the 226 subjects, 46 withdrew from treatment before the 3-month time point 11 because of treatment-related toxic effects and 35 because of inconvenience (e.g., changes in job status or relocation out of the area). An additional three subjects were excluded because their second bronchoscopy was performed 6 months after rather than 3 months after treatment initiation. Thus, 177 subjects were assessable for response, on the basis of having completed at least 3 months of treatment and having undergone bronchoscopic examinations with biopsy at baseline and at the 3-month time point. Of these 177 subjects, 161 underwent a third bronchoscopic examination at the 6-month time point. Compliance With Protocol Protocol eligibility required that subjects had stopped smoking at least 12 months before being randomly assigned. Serum cotinine levels drawn at registration, at 3 months, and at 6 months indicated that more than 95% of the participants had serum levels in the range of nonsmokers (<1.0 ng/ml) or passive smokers (1 20 ng/ml) at all three measurement times. Based on serum cotinine data, seven of the 226 randomly assigned subjects either resumed smoking or smoked continuously during their participation in the study. Exclusion of these subjects from the data analysis did not change the results of the study (data not shown); therefore, we have included these seven subjects in this report. Among the 226 randomly assigned subjects, compliance with the treatment aspect of the protocol determined as having taken at least 80% of the prescribed doses according to a review of subjects monthly calendars and pill counts varied with treatment group. Patients treated with 9-cis-RA were less compliant than patients in the other treatment groups, particularly in the third month of treatment (Table 2). A chi-square test showed that compliance at the 3-month time point in the 9-cis-RA group (50%) was statistically significantly lower than in the placebo group (70%) (difference 20%, 95% confidence interval [CI] 5% to 38%; P.01) or the 13-cis-RA group (73%) (difference 23%, 95% CI 8% to 38%; P.004). The lower compliance among patients treated with 9-cis-RA may reflect the severe headaches reported by those receiving this drug. These headaches occurred early in the treatment regimen and typically did not improve with the use of nonsteroidal antiinflammatory agents. We also monitored compliance by measuring serum 13-cis- RA levels at the baseline, 3-month, and 6-month visits. At the time of the 3-month bronchoscopy, all but six subjects in the 13-cis-RA group had detectable 13-cis-RA levels in their serum (these six subjects were included in the analysis). None of the subjects in the 9-cis-RA or placebo groups had detectable 13- cis-ra levels. Compliance with treatment with 9-cis-RA was not monitored, because we did not have an assay to detect for it. Of the 177 subjects who were assessable for response, nine subjects in the 9-cis-RA group had evidence of disease progression (i.e., an increase in the metaplasia index, an increase in bronchial dysplasia, or both) at the 3-month time point. Nine subjects in the 13-cis-RA + AT group showed disease progression as manifested by an increase in the metaplasia index at the 3-month time point (one developed dysplasia), one of whom was mistakenly crossed over to the 13-cis-RA + AT group. Thirteen subjects in the placebo group had evidence of disease progression at the 3-month time point, 12 of whom crossed over to the 13-cis-RA + AT group, and one dropped out shortly after the 3-month time point. Toxic Effects Of the 226 randomly assigned subjects, 93 experienced grade 1 toxic effects, 89 experienced grade 2 toxic effects, 13 experienced grade 3 toxic effects, and none experienced grade 4 toxic effects or serious adverse events (grading was in accordance Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003 ARTICLES 209
5 Table 1. Characteristics of the 226 randomly assigned subjects according to treatment group* Characteristic Placebo (N 75) 13-cis-RA + AT (N 77) 9-cis-RA (N 74) P value Sex, n (%) Male 46 (61.3) 42 (54.6) 40 (54.1).60 Female 29 (38.7) 35 (45.5) 34 (45.9) Race, n (%) White 66 (88.0) 67 (87.0) 69 (93.2) African-American 6 (8.0) 6 (7.8) 4 (5.4).82 Hispanic 3 (4.0) 3 (3.9) 1 (1.4) Asian 0 (0.0) 1 (1.3) 0 (0.0) Smoking-related cancer, n (%) No 66 (88.0) 69 (89.6) 69 (93.2).54 Yes 9 (12.0) 8 (10.4) 5 (6.8) Age, y Mean ± SD 58.5 ± ± ± Median (range) 59.0 ( ) 56.5 ( ) 55.1 ( ) No. of years smoking Mean ± SD 30.1 ± ± ± Median (range) 30 (15 50) 25 (11 49) 25 (10 50) No. of packs smoked per day Mean ± SD 1.8 ± ± ± Median (range) 2 (1 4) 1.5 (0.8 4) 2 (0.8 4) No. of pack-years Mean ± SD 54.3 ± ± ± Median (range) 44 (20 152) 39 (20 136) 40 (20 136) No. of smoking quit-years Mean ± SD 10.1 ± ± ± Median (range) 9.6 ( ) 8.1 ( ) 8.4 ( ) Metaplasia index Mean ± SD 7.9 ± ± ± 12.3 Median (range) 0 ( ) 0 ( ) 0 ( ).51 Metaplasia index, n (%) 0 50 (66.7) 57 (74.0) 50 (67.6) (2.7) 1 (1.3) 6 (8.1) (30.7) 19 (24.7) 18 (24.3) *RA retinoic acid; AT -tocopherol; SD standard deviation. The Kruskal Wallis test was performed to test the equal median of continuous variables among the three treatment groups. The chi-square test (for sex and smoking-related cancer) or Fisher s exact test (for race) was performed to test the association between two categorical variables. All smoking-related cancers were lung cancers except for two head and neck cancers and one bladder cancer in the 13-cis-RA + AT group and one head and neck cancer in the 9-cis-RA group. Metaplasia index the number of biopsy sections from each subject exhibiting squamous metaplasia divided by the total number of sections examined for that time point multiplied by 100. Table 2. Compliance with treatment protocol* Table 3. Toxicity effects of treatment* Treatment group No. of subjects (N 226) Month of treatment Placebo 75 65% 61% 70% 13-cis-RA + AT 77 78% 68% 73% 9-cis-RA 74 61% 48% 50% *RA retinoic acid; AT -tocopherol. The percentage of subjects who complied with the treatment protocol during each month of treatment. Compliance was defined as a subject s having taken at least 80% of the prescribed doses according to a review of the subjects monthly calendars. Dropouts, missing compliance information, and drug withheld due to toxicity were considered noncompliance events. with the National Cancer Institute s Common Toxicity Criteria) (Table 3). Among the treatment groups, subjects in the 9-cis-RA group experienced the most grade 2 (46 subjects) and grade 3 (nine subjects) toxic effects. In general, the most common toxic effects were those typical of retinoid treatment, including skin rash, hypertriglyceridemia, headache, cheilitis, conjunctivitis, Treatment group No. of subjects (N 226) No. of subjects experiencing each grade of toxicity No. of subjects who dropped out due to toxicity Placebo cis-RA + AT cis-RA *Toxicity grade was in accordance with the National Cancer Institute s Common Toxicity Criteria. RA retinoic acid; AT -tocopherol. One subject had grade 2 arthralgia, one had grade 3 arthralgia, and one had grade 2 photosensitivity. One subject had grade 3 hypertriglyceridemia, one had grade 2 flu-like symptoms, four had grade 2 headaches, and two had grade 3 headaches. arthralgia, and myalgia. By protocol design, grade 2 or higher toxicity necessitated dose reductions of 50%. Treatment-related toxicity, therefore, necessitated dose reduction in 87 of 102 (85.3%) subjects (eight of nine subjects in the placebo group, ARTICLES Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003
6 of 38 subjects in the 13-cis-RA + AT group, and 49 of 55 subjects in the 9-cis-RA group). For the remaining 15 subjects with grade 2 or higher toxicities, dose was not reduced because of symptom resolution (four), attrition from the study (three), or detection of the toxicity at the time of treatment completion (eight). RAR- Expression in the Bronchial Epithelium The primary endpoint of this study was restoration of RAR- expression in the bronchial epithelium. RAR- mrna was detected at baseline in 69.7% (948 of 1357) of the biopsy samples from the 240 registered subjects. Of the 174 subjects who were assessable for response based on having biopsy samples assessable for RAR- expression at baseline and at the 3-month time point, 109 (62.6%) had loss of RAR- expression at baseline in at least one of the six biopsy sites sampled. We examined the effects of treatment on RAR- expression in two ways: as a continuous variable (i.e., RAR- index) and as a binary variable (i.e., loss of RAR- expression at any biopsy site). Although changes in RAR- index from baseline to the 3-month time point did not reach statistical significance within treatment groups, compared with the effect of placebo, the median change in receptor index was statistically significantly different from placebo for 9-cis-RA (P.045) but not for 13-cis-RA+AT(P.41) (Table 4). Table 4. Modulation of retinoic acid receptor beta (RAR- ) expression according to treatment group* Characteristic Placebo (N 59) 13-cis-RA+AT (N 64) 9-cis-RA (N 51) RAR- index Baseline ± ± ± (0 100) 66.7 (0 100) 80 (0 100) 3 months ± ± ± (0 100) 81.7 (0 100) 83.3 (0 100) Modulation 5.79 ± 5.89, 0.21 ± ± ( ) 0 ( ) 0 ( ) P value RAR- loss by subject +/ / / / P value** *RA retinoic acid; AT -tocopherol. RAR- index the percentage of six biopsy sites in each subject that was positive; shown as mean ± standard error and median (range). Modulation of RAR- index was calculated as (RAR- index at 3 months minus RAR- index at baseline); shown as mean ± standard error and median (range). Wilcoxon rank-sum test was performed to test equal modulation of RAR- index between placebo and 13-cis-RA + AT; P.41. Wilcoxon rank-rum test was performed to test equal modulation of RAR- index between placebo and 9-cis-RA; P.045. Wilcoxon signed-rank test was performed to test the statistical significance of the change in the median RAR- index modulation (3-month minus baseline) in each treatment group. Subjects were grouped according to RAR- expression at baseline and at 3 months (+/+, +/, /+, / ), + indicates that RAR- was detected in all six biopsy sites; indicates that RAR- was not detected in at least one biopsy site. Number of subjects with each combination of RAR- expression is given. **McNemar s test was performed to test the statistical significance of modulation of RAR- expression relative to that in other treatment groups over time. To determine the effect of treatment on loss of RAR- expression at any biopsy site, we grouped subjects in terms of whether their biopsy samples were positive (i.e., RAR- was detected in all six biopsy sites) or negative (i.e., RAR- was not detected in at least one biopsy site) for RAR- expression. We hypothesized that if a treatment was ineffective, the proportion of individuals who would convert from negative to positive over the 3-month treatment period would be equal to the proportion of individuals who would convert from positive to negative. This hypothesis held true for the placebo group, in which 14 subjects changed from negative to positive and 21 subjects changed from positive to negative (P.24), and the 13-cis-RA + AT group, in which 18 subjects changed from negative to positive and 13 subjects changed from positive to negative (P.37), but not for the 9-cis-RA group (P.03), in which 15 subjects changed from negative to positive and five changed from positive to negative (Table 4). In the analysis by site, the increase in RAR- expression in the 9-cis-RA group was statistically significantly different from that in the placebo group (odds ratio [OR] 1.72, 95% CI 1.05 to 2.78; P.03), after adjusting for smoking years, packs smoked per day, and the presence of squamous metaplasia (by the generalized estimating equations model, Table 5). Thus, 9-cis-RA was unique in its ability to increase RAR- expression in the bronchial epithelium. Squamous Metaplasia in the Bronchial Epithelium Overall, squamous metaplasia was detected at baseline in 6.9% of biopsy sites (96 of 1399) and 29.6% of subjects (71 of 240), which included biopsy specimens from all registered participants. Of the 1007 pairs (i.e., baseline and 3-month) of biopsy samples examined from the 177 subjects who were assessable for response, squamous metaplasia was detected at baseline in 9.2% of the placebo group (32 of 348 sites), 5.8% of the 13-cis-RA + AT group (21 of 359 sites), and 8.0% of the 9-cis- RA group (24 of 300 sites). Evidence of dysplasia was noted in only five biopsy samples from five subjects. At the 3-month time point, the mean percentage of biopsy sites with squamous metaplasia had decreased to 7.8% in the placebo group (27 of 348 sites), 3.6% in the 13-cis-RA + AT group (13 of 359 sites), and 4.7% in the 9-cis-RA group (14 of 300 sites). We also examined the effect of treatment on squamous meta- Table 5. Generalized estimating equations model for testing the effect of covariates on retinoic acid receptor beta (RAR- ) expression (N 174)* Covariates Estimate (SE) Odds ratio (95% CI) P value Packs smoked per day 0.14 (0.07) 0.87 (0.76 to 1.01).07 No. of years smoking 0.01 (0.006) 0.99 (0.98 to 1.00).06 Squamous metaplasia 0.90 (0.25) 2.46 (1.49 to 4.06) <.001 (1 versus 0) Treatment group 13-cis-RA + AT vs. placebo 0.31 (0.17) 0.73 (0.53 to 1.03).07 9-cis-RA vs. placebo 0.31 (0.18) 0.73 (0.51 to 1.05).08 Time (3 months vs. baseline) 0.15 (0.18) 0.86 (0.61 to 1.21).39 Group time interaction 13-cis-RA + AT 3 mo 0.14 (0.24) 1.15 (0.73 to 1.84).54 9-cis-RA 3 mo 0.54 (0.25) 1.72 (1.05 to 2.78).03 *RA retinoic acid; AT -tocopherol; SE standard error; CI confidence interval. 0 no squamous metaplasia; 1 squamous metaplasia. Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003 ARTICLES 211
7 plasia quantified as a continuous variable (metaplasia index) and as a binary variable (squamous metaplasia detected in at least one biopsy site). The mean metaplasia index declined from baseline to the 3-month time point in all three treatment groups (Table 6), but the declines were not statistically significant within treatment groups or when compared between treatment groups. The percentage of subjects with detectable squamous metaplasia declined in all three treatment groups, but this decline was statistically significant only in the 9-cis-RA group (P.01, Table 6). However, relative to the effect of placebo, the change in squamous metaplasia in the 9-cis-RA and 13-cis-RA + AT groups did not reach statistical significance (P.30 and P.42, respectively, by the generalized estimating equations model) (data not shown). Of the 12 subjects who crossed over to the 13-cis-RA group, 11 were assessable for the 6-month response and one subject dropped out before the 6-month evaluation. Three subjects had an increase in RAR- index and a decrease in metaplasia index, and eight subjects had no change or a decrease in RAR- index, of which six had a decrease and two had an increase in metaplasia index. The number of participants who crossed over is too small to permit making a conclusion about the effect of treatment on this subgroup. Table 6. Modulation of the level of detectable squamous metaplasia according to treatment group* Characteristic Placebo (N 61) 13-cis-RA+AT (N 64) 9-cis-RA (N 52) Metaplasia index Baseline 8.18 ± ± ± (0 40.0) 0 (0 40.0) 0 (0 50.0) 3 months 7.39 ± ± ± (0 63.3) 0 (0 33.3) 0 (0 40.0) Modulation 0.79 ± 2.26, 1.80 ± ± ( ) 0 ( ) 0 ( ) P value Squamous metaplasia by subject +/ / / / P value** *RA retinoic acid; AT -tocopherol. Metaplasia index is the percentage of six biopsy sites in each subject that was positive for squamous metaplasia; shown as mean ± standard error and median (range). Modulation of the metaplasia index was calculated as (metaplasia index at 3 months minus metaplasia index at baseline); shown as mean ± standard error and median (range). Wilcoxon rank-sum test was performed to test equal modulation of metaplasia index between placebo and 13-cis-RA + AT; P.74. Wilcoxon rank-sum test was performed to test equal modulation of metaplasia index between placebo and 9-cis-RA; P.68. Wilcoxon signed-rank test was performed to test the median metaplasia index modulation in each treatment group. Subjects were grouped according to the presence or absence of squamous metaplasia at the baseline and 3-month time points (+/+, +/, /+, / ). + indicated that squamous metaplasia was detected in at least one of six biopsy sites; indicated that squamous metaplasia was not detected in any of the six biopsy sites. Number of subjects with each combination of squamous metaplasia is given. **McNemar s test was performed to test the statistical significance of modulation of squamous metaplasia relative to that in the other treatment groups over time. DISCUSSION In this article, we present the first study, to our knowledge, in which people who have (according to self-report) quit smoking are the target population for the chemoprevention of bronchial premalignancy. From the perspective of directing future public health initiatives, the findings of our study are important, because we have shown both that clinical trials targeting former smokers are feasible and that 9-cis-RA can modulate a biomarker of bronchial preneoplasia RAR- expression in this population. The finding that 9-cis-RA can modulate a bronchial preneoplasia biomarker differs from those of prior retinoidbased studies in current smokers (15 18), which have demonstrated no benefit from chemopreventive intervention with respect to either lung cancer risk or reversal of biomarkers of bronchial premalignancy. Thus, the efficacy of retinoids in the chemoprevention of bronchial premalignancy may differ in current and former smokers. The primary endpoint of this study was restoration of RAR- expression in the bronchial epithelium. We observed that subjects in the 9-cis-RA group, but not those in the 13-cis-RA group, had statistically significantly increased RAR- expression compared with subjects in the placebo group. Several possible factors could have contributed to this unique activity of 9-cis-RA. First, unlike 13-cis-RA, which must be metabolized to all-trans-ra or 9-cis-RA before it can bind nuclear retinoid receptors (6), 9-cis-RA is a natural ligand for both RARs and RXRs. Thus, administration of 9-cis-RA may lead to higher levels of ligand available for receptor activation in the bronchial epithelium than can be achieved with administration of 13-cis- RA. Second, RAR- expression is activated transcriptionally by retinoids through a response element in its gene promoter that binds to RAR:RXR heterodimers. Given the ability of 9-cis-RA to bind to both types of retinoid receptor (i.e., RAR and RXR), it can, therefore, activate both components of the heterodimeric complex, which could activate RAR- gene transcription more potently than activation of either receptor alone (27). The inability of 13-cis-RA to increase RAR- expression in the bronchial epithelium in this study differs from the findings of a previous report (28). There are several possible explanations for this difference. First, the combination of 13-cis-RA with AT, as used in our study, could have influenced retinoid absorption, metabolism, or distribution. Second, the previous report (28) targeted current smokers. Evidence from our studies (29) demonstrates that the biology of the bronchial epithelium differs greatly between individuals who smoke and individuals who have stopped smoking. Cellular proliferation is higher among current smokers than it is among former smokers, and the characteristics of the proliferating bronchial epithelial cells differ with regard to their level of genomic instability (29). These two factors could influence retinoid-induced levels of RAR- in the bronchial epithelium. Clearly, additional work will be necessary to determine whether the changes in RAR- levels induced by 9-cis-RA correlate with their abilities to reduce lung cancer risk in former smokers. To begin to answer this question, we examined the effect of retinoid treatment on the level of bronchial squamous metaplasia in biopsy samples from the bronchial tree of former smokers. Comparing to placebo, neither 9-cis-RA nor 13-cis-RA + AT treatment had a statistically significant effect on squamous metaplasia in this study, but there was some evidence of benefit in reducing squamous metaplasia in the 9-cis-RA group. Under- 212 ARTICLES Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003
8 standing the activity of 9-cis-RA in the reversal of bronchial premalignancy may require the design of a study that tests its effects in individuals who have histologic evidence of bronchial premalignancy. Alternatively, biomarkers of bronchial premalignancy may be required that are more prevalent in former smokers than squamous metaplasia, which we observed in only 29.6% of subjects. Such studies will require correlation of RAR- expression with known biomarkers of bronchial epithelial premalignancy and novel biomarkers of premalignancy identified by proteomic and genomic analysis. The toxic effects of 9-cis-RA and 13-cis-RA + AT observed in this study were similar in nature, frequency, and severity to those observed in a previous report of 13-cis-RA therapy (18). In addition, headaches of similar nature and severity have also been observed in phase I studies of 9-cis-RA (21,22). The toxicities observed in this trial limit the usefulness of certain retinoids in chemoprevention strategies. For example, in patients with oral leukoplakia, 13-cis-RA treatment induces disease remission, but treatment cessation is associated with disease relapse (30). Thus, chronic administration of retinoids may be necessary to suppress the progression of premalignant lesions of the aerodigestive tract. If so, it will be necessary to develop agents that are both effective and well tolerated in prolonged use. Our finding that 9-cis-RA, a drug that activates both RARs and RXRs, was more effective at restoring RAR- expression than a 13-cis-RA-based regimen suggests that agents that activate both RXRs and RARs may be more effective in reversing bronchial premalignancy than RAR-selective agents. An RXRselective agonist, LGD1069, has demonstrated activity against non-small-cell lung cancer in early clinical trials (31). LGD1069 is relatively well tolerated, has been administered for prolonged periods in responding patients and, when administered at high doses, has RAR-agonistic activity (32). Based on our data, treatment with LGD1069 may augment expression of RAR- and thereby reverse bronchial premalignancy. Thus, we suggest that drugs that activate both RARs and RXRs should be tested in biomarker-based lung cancer chemoprevention trials. REFERENCES (1) Stewart AK, Bland KI, McGinnis LS Jr, Morrow M, Eyre HJ. Clinical highlights from the National Cancer Institute database. CA Cancer J Clin 2000;50: (2) Walsh GL, Pisters KM, Stevens C. Treatment of stage I lung cancer. Chest Surg Clin N Am 2001;11: (3) Burns DM. Primary prevention, smoking, and smoking cessation: implications for future trends in lung cancer prevention. Cancer 2000;89: (4) Kurie JM. The biologic basis for the use of retinoids in cancer prevention and treatment. Curr Opin Oncol 1999;11: (5) Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell 1995;83: (6) Marchetti MN, Samplo E, Bun H, Scoma H, Lacarelle B, Durand A. In vitro metabolism of three major isomers of retinoic acid in rats. Intersex and interstrain comparison. Drug Metab Dispos 1997;25: (7) Shalinsky DR, Bischoff ED, Gregory ML, Lamph WW, Heyman RA, Hayes JS, et al. Enhanced anitumor efficacy of cisplatin in combination with ALRT1057 (9-cis retinoic acid) in human oral squamous carcinoma xenografts in nude mice. Clin Cancer Res 1996;2: (8) McCormick DL, Rao KV, Steele VE, Lubet RA, Kelloff GJ, Bosland MC. Chemoprevention of rat prostate carcinogenesis by 9-cis retinoic acid. Cancer Res 1999;59: (9) Anzano MA, Byers SW, Smith JM, Peer CW, Mullen LT, Brown CC, et al. Prevention of breast cancer in the rat with 9-cis retinoic acid as a single agent and in combination with tamoxifen. Cancer Res 1994;54: (10) Bischoff ED, Gottardis MM, Moon TE, Heyman RA, Lamph WW. Beyond tamoxifen: the retinoid X receptor-selective ligand LGD1069 (TARGRE- TIN) causes complete regression of mammary carcinoma. Cancer Res 1998;58: (11) Gottardis MM, Bischoff ED, Shirley MA, Wagoner MA, Lamph WW, Heyman RA. Chemoprevention of mammary carcinoma by LGD1069 (Targretin): an RXR-selective ligand. Cancer Res 1996;56: (12) Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science 1997;278: (13) Lotan R, Xu XC, Lippman SM, Ro YJ, Lee JS, Lee JJ, et al. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its upregulation by isotretinoin. N Engl J Med 1995;332: (14) Xu XC, Sozzi G, Lee LS, Lee JJ, Pastorino U, Pilotti S, et al. Suppression of retinoic acid receptor beta in non-small-cell lung cancer in vivo: implications for lung cancer development. J Natl Cancer Inst 1997;89: (15) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 1994;330: (16) Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334: (17) Lippman SM, Lee JJ, Karp DD, Vokes EE, Benner SE, Goodman GE, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst 2001;93: (18) Lee JS, Lippman SM, Benner SE, Lee JJ, Ro JY, Lukeman JM, et al. Randomized placebo-controlled trial of isotretinoin in chemoprevention of bronchial squamous metaplasia. J Clin Oncol 1994;12: (19) Sawant SS, Kandarkar SV. Role of vitamins C and E as chemopreventive agents in the hamster cheek pouch treated with the oral carcinogen-dmba. Oral Dis 2000;6: (20) Dimery IW, Hong WK, Lee JJ, Guillory-Perez C, Pham F, Fritsche HA Jr, et al. Phase I trial of alpha-tocopherol effects on 13-cis retinoic acid toxicity. Ann Oncol 1997;8:85 9. (21) Kurie JM, Lee JS, Griffin T, Lippman SM, Drum P, Thomas MP, et al. Phase I trial of 9-cis retinoic acid in adults with solid tumors. Clin Cancer Res 1996;2: (22) Miller VA, Rigas JR, Benedetti FM, Verret AL, Tong WP, Kris MG, et al. Initial clinical trial of the retinoid receptor pan agonist 9-cis retinoic acid. Clin Cancer Res 1996;2: (23) Kurie JM, Lee JS, Khuri FR, Mao L, Morice RC, Lee JJ, et al. N-(4- hydroxyphenyl) retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium. Clin Cancer Res 2000;6: (24) Xu XC, Clifford JL, Hong WK, Lotan R. Detection of nuclear retinoic acid receptor mrna in histological tissue sections using nonradioactive in situ hybridization histochemistry. Diagn Mol Pathol 1994;3: (25) Agresti A. Categorical data analysis. New York (NY): John Wiley and Sons; p (26) Diggle PJ, Liang KY, Zeger SL, editors. Analysis of longitudinal data. New York (NY): Oxford University Press; p (27) Lotan R, Dawson MI, Zou CC, Jong L, Lotan D, Zou CP. Enhanced efficacy of combinations of retinoic acid- and retinoid X receptor-selective retinoids and alpha-interferon in inhibition of cervical carcinoma cell proliferation. Cancer Res 1995;55: (28) Xu XC, Lee JS, Lee JJ, Morice RC, Liu X, Lippman SM, et al. Nuclear retinoid acid receptor beta in bronchial epithelium of smokers before and during chemoprevention. J Natl Cancer Inst 1999;91: (29) Lee JJ, Liu D, Lee JS, Kurie JM, Khuri FR, Ibarguen H, et al. Long-term impact of smoking on lung epithelial proliferation in current and former smokers. J Natl Cancer Inst 2001;93: (30) Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis retinoic acid in the treatment of oral leukoplakia. N Engl J Med 1986;315: (31) Rizvi NA, Marshall JL, Dahut W, Ness E, Truglia JA, Loewen G, et al. A phase I study of LGD1069 in adults with advanced cancer. Clin Cancer Res 1999;5: (32) Dawson MI, Jong L, Hobbs PD, Cameron JF, Chao WR, Pfahl M, et al. 1. Journal of the National Cancer Institute, Vol. 95, No. 3, February 5, 2003 ARTICLES 213
Proliferative Changes in the Bronchial Epithelium of Former Smokers Treated With Retinoids
 ARTICLE Proliferative Changes in the Bronchial Epithelium of Former Smokers Treated With Retinoids Walter N. Hittelman, Diane D. Liu, Jonathan M. Kurie, Reuben Lotan, Jin Soo Lee, Fadlo Khuri, Heladio
ARTICLE Proliferative Changes in the Bronchial Epithelium of Former Smokers Treated With Retinoids Walter N. Hittelman, Diane D. Liu, Jonathan M. Kurie, Reuben Lotan, Jin Soo Lee, Fadlo Khuri, Heladio
Randomized Phase III Intergroup Trial of Isotretinoin to Prevent Second Primary Tumors in Stage I Non-Small-Cell Lung Cancer
 Randomized Phase III Intergroup Trial of Isotretinoin to Prevent Second Primary Tumors in Stage I Non-Small-Cell Lung Cancer Scott M. Lippman, J. Jack Lee, Daniel D. Karp, Everett E. Vokes, Steven E. Benner,
Randomized Phase III Intergroup Trial of Isotretinoin to Prevent Second Primary Tumors in Stage I Non-Small-Cell Lung Cancer Scott M. Lippman, J. Jack Lee, Daniel D. Karp, Everett E. Vokes, Steven E. Benner,
CHEMOPREVENTION OF HEAD AND NECK CANCER AND PROSTATE CANCER HUSEYIN ABALI, MD BASKENT UNIVERSITY MEDICAL ONCOLOGY ANKARA/TURKEY
 CHEMOPREVENTION OF HEAD AND NECK CANCER AND PROSTATE CANCER HUSEYIN ABALI, MD BASKENT UNIVERSITY MEDICAL ONCOLOGY ANKARA/TURKEY Definition Cancer chemoprevention is defined as the use of natural, synthetic,
CHEMOPREVENTION OF HEAD AND NECK CANCER AND PROSTATE CANCER HUSEYIN ABALI, MD BASKENT UNIVERSITY MEDICAL ONCOLOGY ANKARA/TURKEY Definition Cancer chemoprevention is defined as the use of natural, synthetic,
RETINOIDS, including vitamin A and its analogues,
 Vol. 332 No. 21 RETINOIC ACID RECEPTOR b IN PREMALIGNANT ORAL LESIONS 1405 SUPPRESSION OF RETINOIC ACID RECEPTOR IN PREMALIGNANT ORAL LESIONS AND ITS UP-REGULATION BY ISOTRETINOIN REUBEN LOTAN, PH.D.,
Vol. 332 No. 21 RETINOIC ACID RECEPTOR b IN PREMALIGNANT ORAL LESIONS 1405 SUPPRESSION OF RETINOIC ACID RECEPTOR IN PREMALIGNANT ORAL LESIONS AND ITS UP-REGULATION BY ISOTRETINOIN REUBEN LOTAN, PH.D.,
Predicting Cancer Development in Oral Leukoplakia: Ten Years of Translational Research 1
 1702 Vol. 6, 1702 1710, May 2000 Clinical Cancer Research Predicting Cancer Development in Oral Leukoplakia: Ten Years of Translational Research 1 J. Jack Lee, 2 Waun Ki Hong, Walter N. Hittelman, Li Mao,
1702 Vol. 6, 1702 1710, May 2000 Clinical Cancer Research Predicting Cancer Development in Oral Leukoplakia: Ten Years of Translational Research 1 J. Jack Lee, 2 Waun Ki Hong, Walter N. Hittelman, Li Mao,
Chemoprevention Strategies in Lung Carcinogenesis*
 Chemoprevention Strategies in Lung Carcinogenesis* Scott M. Lippman, M.D.; Steven E. Benner M.D.; and Waun Ki Hong, M.D. Chemoprevention entails using specific agents to suppress carcinogenesis and thereby
Chemoprevention Strategies in Lung Carcinogenesis* Scott M. Lippman, M.D.; Steven E. Benner M.D.; and Waun Ki Hong, M.D. Chemoprevention entails using specific agents to suppress carcinogenesis and thereby
Cancer Chemoprevention Part 1: Retinoids and Carotenoids and Other Classic Antioxidants
 Cancer Chemoprevention Part 1: Retinoids and Carotenoids and Other Classic Antioxidants Review Article [1] November 01, 1998 By Diljeet K. Singh, MD, MPH [2] and Scott M. Lippman, MD, FACP [3] Cancer chemoprevention
Cancer Chemoprevention Part 1: Retinoids and Carotenoids and Other Classic Antioxidants Review Article [1] November 01, 1998 By Diljeet K. Singh, MD, MPH [2] and Scott M. Lippman, MD, FACP [3] Cancer chemoprevention
Questions and Answers About Beta Carotene Chemoprevention Trials
 CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Questions and Answers
CANCER FACTS N a t i o n a l C a n c e r I n s t i t u t e N a t i o n a l I n s t i t u t e s o f H e a l t h D e p a r t m e n t o f H e a l t h a n d H u m a n S e r v i c e s Questions and Answers
OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER
 & OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER Interim Data Report of TRUST study on patients from Bosnia and Herzegovina
& OUR EXPERIENCES WITH ERLOTINIB IN SECOND AND THIRD LINE TREATMENT PATIENTS WITH ADVANCED STAGE IIIB/ IV NON-SMALL CELL LUNG CANCER Interim Data Report of TRUST study on patients from Bosnia and Herzegovina
J Clin Oncol 25: by American Society of Clinical Oncology INTRODUCTION
 VOLUME 25 NUMBER 21 JULY 20 2007 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Prediction of Prostate Cancer for Patients Receiving Finasteride: Results From the Prostate Cancer Prevention Trial
VOLUME 25 NUMBER 21 JULY 20 2007 JOURNAL OF CLINICAL ONCOLOGY O R I G I N A L R E P O R T Prediction of Prostate Cancer for Patients Receiving Finasteride: Results From the Prostate Cancer Prevention Trial
Antioxidants: Are We abusing It?
 10.5005/jp-journals-10011-1319 TG Shrihari et al REVIEW ARTICLE TG Shrihari, Vijeev Vasudevan, S Kailasam, Devaraju Devaiah, V Manjunath, GR Jagadish ABSTRACT Oral cancer holds the eighth position in the
10.5005/jp-journals-10011-1319 TG Shrihari et al REVIEW ARTICLE TG Shrihari, Vijeev Vasudevan, S Kailasam, Devaraju Devaiah, V Manjunath, GR Jagadish ABSTRACT Oral cancer holds the eighth position in the
Learning Objectives 9/9/2013. Hypothesis Testing. Conflicts of Interest. Descriptive statistics: Numerical methods Measures of Central Tendency
 Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Last-Chance Ambulatory Care Webinar Thursday, September 5, 2013 Learning Objectives For
Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Last-Chance Ambulatory Care Webinar Thursday, September 5, 2013 Learning Objectives For
9/4/2013. Decision Errors. Hypothesis Testing. Conflicts of Interest. Descriptive statistics: Numerical methods Measures of Central Tendency
 Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Pharmacotherapy Webinar Review Course Tuesday, September 3, 2013 Descriptive statistics:
Conflicts of Interest I have no conflict of interest to disclose Biostatistics Kevin M. Sowinski, Pharm.D., FCCP Pharmacotherapy Webinar Review Course Tuesday, September 3, 2013 Descriptive statistics:
Cancer Cell Research 14 (2017)
 Available at http:// www.cancercellresearch.org ISSN 2161-2609 Efficacy and safety of bevacizumab for patients with advanced non-small cell lung cancer Ping Xu, Hongmei Li*, Xiaoyan Zhang Department of
Available at http:// www.cancercellresearch.org ISSN 2161-2609 Efficacy and safety of bevacizumab for patients with advanced non-small cell lung cancer Ping Xu, Hongmei Li*, Xiaoyan Zhang Department of
Handbook 8. Targretin
 Handbook 8 Targretin 1. Chemical and Physical ArH), 8.03 (d, I= 8.1 Hz, 2H, ArH) Characteristics High-resolution mass spectrum: (FAB-MS): (M + H) calculated for C14H2902 349.2168, found 1.1 Nomenclature
Handbook 8 Targretin 1. Chemical and Physical ArH), 8.03 (d, I= 8.1 Hz, 2H, ArH) Characteristics High-resolution mass spectrum: (FAB-MS): (M + H) calculated for C14H2902 349.2168, found 1.1 Nomenclature
Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers
 日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer
 Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
PFIZER INC. Study Initiation Date and Completion Dates: 09 March 2000 to 09 August 2001.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Retrospective analysis to determine the use of tissue genomic analysis to predict the risk of recurrence in early stage invasive breast cancer.
 Retrospective analysis to determine the use of tissue genomic analysis to predict the risk of recurrence in early stage invasive breast cancer. Goal of the study: 1.To assess whether patients at Truman
Retrospective analysis to determine the use of tissue genomic analysis to predict the risk of recurrence in early stage invasive breast cancer. Goal of the study: 1.To assess whether patients at Truman
Novel RCC Targets from Immuno-Oncology and Antibody-Drug Conjugates
 Novel RCC Targets from Immuno-Oncology and Antibody-Drug Conjugates Christopher Turner, MD Vice President, Clinical Science 04 November 2016 Uveal Melanoma Celldex Pipeline CANDIDATE INDICATION Preclinical
Novel RCC Targets from Immuno-Oncology and Antibody-Drug Conjugates Christopher Turner, MD Vice President, Clinical Science 04 November 2016 Uveal Melanoma Celldex Pipeline CANDIDATE INDICATION Preclinical
Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008
 Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Special Report Temporal Trends in Demographics and Overall Survival of Non Small-Cell Lung Cancer Patients at Moffitt Cancer Center From 1986 to 2008 Matthew B. Schabath, PhD, Zachary J. Thompson, PhD,
Supplementary Online Content
 Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
Supplementary Online Content Regan EA, Lynch DA, Curran-Everett D, et al; Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. Published
Supplementary Information. Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent
 Supplementary Information Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent Authors: Susanne Kossatz a, Christian Brand a, Stanley Gutiontov b, Jonathan T.C. Liu c, Nancy
Supplementary Information Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent Authors: Susanne Kossatz a, Christian Brand a, Stanley Gutiontov b, Jonathan T.C. Liu c, Nancy
Product: Darbepoetin alfa Clinical Study Report: Date: 22 August 2007 Page 2 of 14145
 Date: 22 ugust 2007 Page 2 of 14145 2. SYNOPSIS Name of Sponsor: mgen Inc., Thousand Oaks, C, US Name of Finished Product: ranesp Name of ctive Ingredient: Darbepoetin alfa Title of Study: Randomized,
Date: 22 ugust 2007 Page 2 of 14145 2. SYNOPSIS Name of Sponsor: mgen Inc., Thousand Oaks, C, US Name of Finished Product: ranesp Name of ctive Ingredient: Darbepoetin alfa Title of Study: Randomized,
METRIC Study Key Eligibility Criteria
 The METRIC Study METRIC Study Key Eligibility Criteria The pivotal METRIC Study is evaluating glembatumumab vedotin in patients with gpnmb overexpressing metastatic triple-negative breast cancer (TNBC).
The METRIC Study METRIC Study Key Eligibility Criteria The pivotal METRIC Study is evaluating glembatumumab vedotin in patients with gpnmb overexpressing metastatic triple-negative breast cancer (TNBC).
Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell lung Cancer (NSCLC) By CISH Technique
 Cancer and Clinical Oncology; Vol. 7, No. 1; 2018 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell
Cancer and Clinical Oncology; Vol. 7, No. 1; 2018 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell
trial update clinical
 trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
trial update clinical by John W. Mucenski, BS, PharmD, Director of Pharmacy Operations, UPMC Cancer Centers The treatment outcome for patients with relapsed or refractory cervical carcinoma remains dismal.
Comparison of three mathematical prediction models in patients with a solitary pulmonary nodule
 Original Article Comparison of three mathematical prediction models in patients with a solitary pulmonary nodule Xuan Zhang*, Hong-Hong Yan, Jun-Tao Lin, Ze-Hua Wu, Jia Liu, Xu-Wei Cao, Xue-Ning Yang From
Original Article Comparison of three mathematical prediction models in patients with a solitary pulmonary nodule Xuan Zhang*, Hong-Hong Yan, Jun-Tao Lin, Ze-Hua Wu, Jia Liu, Xu-Wei Cao, Xue-Ning Yang From
Name of Active Ingredient: Fully human monoclonal antibody to receptor activator for nuclear factor-κb ligand
 Page 2 of 1765 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Denosumab (AMG 162) Name of Active Ingredient: Fully human monoclonal antibody to receptor activator for nuclear factor-κb
Page 2 of 1765 2. SYNOPSIS Name of Sponsor: Amgen Inc. Name of Finished Product: Denosumab (AMG 162) Name of Active Ingredient: Fully human monoclonal antibody to receptor activator for nuclear factor-κb
Chemoprevention of Lung Cancer
 CHEST Supplement DIAGNOSIS AND MANAGEMENT OF LUNG CANCER, 3RD ED: ACCP GUIDELINES Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice
CHEST Supplement DIAGNOSIS AND MANAGEMENT OF LUNG CANCER, 3RD ED: ACCP GUIDELINES Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice
The Association between Vitamin D and Lung Cancer Risk in Finnish Male Smokers. Julia Burkley Briarcliff High School
 The Association between Vitamin D and Lung Cancer Risk in Finnish Male Smokers. Julia Burkley Briarcliff High School 2 Burkley Table of Contents Introduction~ Pg. 2 Methods~ Pg. 4 Ethics Statement~ Pg.
The Association between Vitamin D and Lung Cancer Risk in Finnish Male Smokers. Julia Burkley Briarcliff High School 2 Burkley Table of Contents Introduction~ Pg. 2 Methods~ Pg. 4 Ethics Statement~ Pg.
Virtual Journal Club: Front-Line Therapy and Beyond Recent Perspectives on ALK-Positive Non-Small Cell Lung Cancer.
 Virtual Journal Club: Front-Line Therapy and Beyond Recent Perspectives on ALK-Positive Non-Small Cell Lung Cancer Reference Slides ALK Rearrangement in NSCLC ALK (anaplastic lymphoma kinase) is a receptor
Virtual Journal Club: Front-Line Therapy and Beyond Recent Perspectives on ALK-Positive Non-Small Cell Lung Cancer Reference Slides ALK Rearrangement in NSCLC ALK (anaplastic lymphoma kinase) is a receptor
Comparisons within randomised groups
 Comparisons within randomised groups Martin Bland Prof. of Health Statistics University of York Douglas G Altman Centre for Statistics in Medicine University of Oxford Presented to the Department of Health
Comparisons within randomised groups Martin Bland Prof. of Health Statistics University of York Douglas G Altman Centre for Statistics in Medicine University of Oxford Presented to the Department of Health
Head and Neck Chemoprevention: Recent Advances
 Head and Neck Chemoprevention: Recent Advances Scott M. Lippman, MD Significant clinical advances have been made in understanding the role of retinoids, particularly 13cRA, in the chemoprevention of head
Head and Neck Chemoprevention: Recent Advances Scott M. Lippman, MD Significant clinical advances have been made in understanding the role of retinoids, particularly 13cRA, in the chemoprevention of head
came from a carcinoma and in 12 from a sarcoma. Ninety lesions were intrapulmonary and the as the chest wall and pleura. Details of the primary
 Thorax 1982;37:366-370 Thoracic metastases MARY P SHEPHERD From the Thoracic Surgical Unit, Harefield Hospital, Harefield ABSTRACI One hundred and four patients are reviewed who were found to have thoracic
Thorax 1982;37:366-370 Thoracic metastases MARY P SHEPHERD From the Thoracic Surgical Unit, Harefield Hospital, Harefield ABSTRACI One hundred and four patients are reviewed who were found to have thoracic
A mouse model for oral squamous cell carcinoma
 4 A mouse model for oral squamous cell carcinoma Remilio A. L. Schoop Robert J. Baatenburg de Jong Abstract Despite recent advances, the prognosis of oral squamous cell carcinoma is still poor. Therapeutic
4 A mouse model for oral squamous cell carcinoma Remilio A. L. Schoop Robert J. Baatenburg de Jong Abstract Despite recent advances, the prognosis of oral squamous cell carcinoma is still poor. Therapeutic
Research. Breast cancer represents a major
 Research GENERAL GYNECOLOGY Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR) Carolyn D. Runowicz, MD; Joseph P. Costantino, DrPH; D.
Research GENERAL GYNECOLOGY Gynecologic conditions in participants in the NSABP breast cancer prevention study of tamoxifen and raloxifene (STAR) Carolyn D. Runowicz, MD; Joseph P. Costantino, DrPH; D.
Supplementary Methods: Omalizumab Trial This double-blind, randomized, placebo-controlled trial was conducted at the University of Utah Hospital and
 Supplementary Methods: Omalizumab Trial This double-blind, randomized, placebo-controlled trial was conducted at the University of Utah Hospital and Primary Children s Hospital, Salt Lake City, UT, both
Supplementary Methods: Omalizumab Trial This double-blind, randomized, placebo-controlled trial was conducted at the University of Utah Hospital and Primary Children s Hospital, Salt Lake City, UT, both
First-Line Ribociclib + Letrozole for Postmenopausal Women With HR+, HER2-, Advanced Breast Cancer: First Results From the Phase III MONALEESA-2 Study
 First-Line Ribociclib + Letrozole for Postmenopausal Women With HR+, HER2-, Advanced Breast Cancer: First Results From the Phase III MONALEESA-2 Study Abstract LBA1 Hortobagyi GN, Stemmer SM, Burris HA,
First-Line Ribociclib + Letrozole for Postmenopausal Women With HR+, HER2-, Advanced Breast Cancer: First Results From the Phase III MONALEESA-2 Study Abstract LBA1 Hortobagyi GN, Stemmer SM, Burris HA,
Visceral Pleural Invasion Is Not Predictive of Survival in Patients With Lung Cancer and Smaller Tumor Size
 GENERAL THORACIC Visceral Pleural Invasion Is Not Predictive of Survival in Patients With Lung Cancer and Smaller Tumor Size Elizabeth David, MD, Peter F. Thall, PhD, Neda Kalhor, MD, Wayne L. Hofstetter,
GENERAL THORACIC Visceral Pleural Invasion Is Not Predictive of Survival in Patients With Lung Cancer and Smaller Tumor Size Elizabeth David, MD, Peter F. Thall, PhD, Neda Kalhor, MD, Wayne L. Hofstetter,
Background and Analysis Objectives Methods and Approach
 Cigarettes, Tobacco Dependence, and Smoking Cessation: Project MOM Final Report Lorraine R. Reitzel, Ph.D., The University of Texas, MD Anderson Cancer Center Background and Analysis Objectives Background.
Cigarettes, Tobacco Dependence, and Smoking Cessation: Project MOM Final Report Lorraine R. Reitzel, Ph.D., The University of Texas, MD Anderson Cancer Center Background and Analysis Objectives Background.
Figure 1: PALLAS Study Schema. Endocrine adjuvant therapy may have started before randomization and be ongoing at that time.
 Figure 1: PALLAS Study Schema Endocrine adjuvant therapy may have started before randomization and be ongoing at that time. Approximately 4600 patients from approximately 500 global sites will be randomized
Figure 1: PALLAS Study Schema Endocrine adjuvant therapy may have started before randomization and be ongoing at that time. Approximately 4600 patients from approximately 500 global sites will be randomized
CheckMate 012: Safety and Efficacy of First Line Nivolumab and Ipilimumab in Advanced Non-Small Cell Lung Cancer
 CheckMate 12: Safety and Efficacy of First Line Nivolumab and Ipilimumab in Advanced Non-Small Cell Lung Cancer Abstract 31 Hellmann MD, Gettinger SN, Goldman J, Brahmer J, Borghaei H, Chow LQ, Ready NE,
CheckMate 12: Safety and Efficacy of First Line Nivolumab and Ipilimumab in Advanced Non-Small Cell Lung Cancer Abstract 31 Hellmann MD, Gettinger SN, Goldman J, Brahmer J, Borghaei H, Chow LQ, Ready NE,
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
Last Review Status/Date: December 2016 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
PFIZER INC. Study Initiation Date and Completion Dates: Information not available (Date of Statistical Report: 16 May 2004)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
A Randomized Trial of a Multivitamin (MVM) in the Prevention of Cardiovascular Disease in Men: The Physicians Health Study (PHS) II
 A Randomized Trial of a Multivitamin (MVM) in the Prevention of Cardiovascular Disease in Men: The Physicians Health Study (PHS) II Presenter Disclosure Information Howard D. Sesso, ScD, MPH Relevant Disclosures:
A Randomized Trial of a Multivitamin (MVM) in the Prevention of Cardiovascular Disease in Men: The Physicians Health Study (PHS) II Presenter Disclosure Information Howard D. Sesso, ScD, MPH Relevant Disclosures:
LUNG cancer is the leading cause of death from cancer
 115 THE NEW ENGLAND JOURNAL OF MEDICINE May 2, 1996 EFFECTS OF A COMBINATION OF BETA CAROTENE AND VITAMIN A ON LUNG CANCER AND CARDIOVASCULAR DISEASE GILBERT S. OMENN, M.D., PH.D., GARY E. GOODMAN, M.D.,
115 THE NEW ENGLAND JOURNAL OF MEDICINE May 2, 1996 EFFECTS OF A COMBINATION OF BETA CAROTENE AND VITAMIN A ON LUNG CANCER AND CARDIOVASCULAR DISEASE GILBERT S. OMENN, M.D., PH.D., GARY E. GOODMAN, M.D.,
José Baselga, MD, PhD
 i n t e r v i e w José Baselga, MD, PhD Dr Baselga is Physician-in-Chief at Memorial Sloan-Kettering Cancer Center in New York, New York. Tracks 1-15 Track 1 Track 2 Track 3 Track 4 Track 5 Track 6 Track
i n t e r v i e w José Baselga, MD, PhD Dr Baselga is Physician-in-Chief at Memorial Sloan-Kettering Cancer Center in New York, New York. Tracks 1-15 Track 1 Track 2 Track 3 Track 4 Track 5 Track 6 Track
Key words: autofluorescence bronchoscopy; early detection; occult lung cancer
 Cigarette Smoking, Preinvasive Bronchial Lesions, and Autofluorescence Bronchoscopy* Denis Moro-Sibilot, MD; Michel Jeanmart, MD; Sylvie Lantuejoul, MD; François Arbib, MD; Marie Hélène Laverrière, MD;
Cigarette Smoking, Preinvasive Bronchial Lesions, and Autofluorescence Bronchoscopy* Denis Moro-Sibilot, MD; Michel Jeanmart, MD; Sylvie Lantuejoul, MD; François Arbib, MD; Marie Hélène Laverrière, MD;
[(Lung Cancer)] [Author: David J. Stewart] Published On (February, 2011) By David J. Stewart READ ONLINE
![[(Lung Cancer)] [Author: David J. Stewart] Published On (February, 2011) By David J. Stewart READ ONLINE [(Lung Cancer)] [Author: David J. Stewart] Published On (February, 2011) By David J. Stewart READ ONLINE](/thumbs/82/85293069.jpg) [(Lung Cancer)] [Author: David J. Stewart] Published On (February, 2011) By David J. Stewart READ ONLINE Eric Seitelman, 2 Perri Donenfeld, 1 Kevin Kay, 1 Kazuaki Takabe, 2 Shahriyour Andaz, 1 and Stewart
[(Lung Cancer)] [Author: David J. Stewart] Published On (February, 2011) By David J. Stewart READ ONLINE Eric Seitelman, 2 Perri Donenfeld, 1 Kevin Kay, 1 Kazuaki Takabe, 2 Shahriyour Andaz, 1 and Stewart
Key words: chemoprevention; epidermal growth factor receptor; G1 cyclin; lung cancer; retinoids
 Lung Cancer Prevention* The Guidelines Konstantin H. Dragnev, MD; Diane Stover, MD, FCCP; and Ethan Dmitrovsky, MD Lung carcinogenesis is a chronic and multi-step process resulting in malignant lung tumors.
Lung Cancer Prevention* The Guidelines Konstantin H. Dragnev, MD; Diane Stover, MD, FCCP; and Ethan Dmitrovsky, MD Lung carcinogenesis is a chronic and multi-step process resulting in malignant lung tumors.
Statement on the safety of β-carotene use in heavy smokers 1
 EFSA Journal 2012;10(12):2953 ABSTRACT SCIENTIFIC OPINION Statement on the safety of β-carotene use in heavy smokers 1 EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) 2, European
EFSA Journal 2012;10(12):2953 ABSTRACT SCIENTIFIC OPINION Statement on the safety of β-carotene use in heavy smokers 1 EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) 2, European
SMOKING RELAPSE ONE YEAR AFTER DELIVERY AMONG WOMEN WHO QUIT SMOKING DURING PREGNANCY
 International Journal of Occupational Medicine and Environmental Health, 2005;8(2):59 65 SMOKING RELAPSE ONE YEAR AFTER DELIVERY AMONG WOMEN WHO QUIT SMOKING DURING PREGNANCY KINGA POLAŃSKA, WOJCIECH HANKE,
International Journal of Occupational Medicine and Environmental Health, 2005;8(2):59 65 SMOKING RELAPSE ONE YEAR AFTER DELIVERY AMONG WOMEN WHO QUIT SMOKING DURING PREGNANCY KINGA POLAŃSKA, WOJCIECH HANKE,
Sponsor. Novartis Pharmaceuticals Corporation Generic Drug Name. Agomelatine Therapeutic Area of Trial. Major depressive disorder Approved Indication
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major depressive disorder Approved Indication Investigational drug Study
2.0 Synopsis. ABT-333 M Clinical Study Report R&D/09/956
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
Pembrolizumab for Patients With PD-L1 Positive Advanced Carcinoid or Pancreatic Neuroendocrine Tumors: Results From the KEYNOTE-028 Study
 Pembrolizumab for Patients With PD-L1 Positive Advanced Carcinoid or Pancreatic Neuroendocrine Tumors: Results From the KEYNOTE-28 Study Abstract 427O Mehnert JM, Bergsland E, O Neil BH, Santoro A, Schellens
Pembrolizumab for Patients With PD-L1 Positive Advanced Carcinoid or Pancreatic Neuroendocrine Tumors: Results From the KEYNOTE-28 Study Abstract 427O Mehnert JM, Bergsland E, O Neil BH, Santoro A, Schellens
Principal Investigator and Study Center: F. Nobuoka, MD Ageo Medical Clinic, 3133 Haraichi, Ageo City, Saitama , Japan
 Page 1 APPENDIX 9: ZRHM-PK-05-JP CLINICAL STUDY SUMMARY The study was conducted in Japan from August to November 2013. Principles as defined in International Conference on Harmonization (ICH) Good Clinical
Page 1 APPENDIX 9: ZRHM-PK-05-JP CLINICAL STUDY SUMMARY The study was conducted in Japan from August to November 2013. Principles as defined in International Conference on Harmonization (ICH) Good Clinical
The Relation of Surgery for Prostatic Hypertrophy to Carcinoma of the Prostate
 American Journal of Epidemiology Vol. 138, No. 5 Copyright C 1993 by The Johns Hopkins University School of Hygiene and Public Health Printed in U.SA. All rights reserved The Relation of Surgery for Prostatic
American Journal of Epidemiology Vol. 138, No. 5 Copyright C 1993 by The Johns Hopkins University School of Hygiene and Public Health Printed in U.SA. All rights reserved The Relation of Surgery for Prostatic
Factorial Study Design 07/18/12
 Disclaimer: The following information is fictional and is only intended for the purposes of illustrating key concepts for results data entry in the Protocol Registration System (PRS). Example Factorial
Disclaimer: The following information is fictional and is only intended for the purposes of illustrating key concepts for results data entry in the Protocol Registration System (PRS). Example Factorial
Downloaded from:
 Evans, JR; Lawrenson, JG (2013) Dietary interventions for AMD: what do we know and what do we not know? The British journal of ophthalmology. ISSN 0007-1161 DOI: https://doi.org/10.1136/bjophthalmol- 2013-303134
Evans, JR; Lawrenson, JG (2013) Dietary interventions for AMD: what do we know and what do we not know? The British journal of ophthalmology. ISSN 0007-1161 DOI: https://doi.org/10.1136/bjophthalmol- 2013-303134
Joshua Klopper, Madeleine Kane, Antonio Jimeno and Bryan Haugen
 Joshua Klopper, Madeleine Kane, Antonio Jimeno and Bryan Haugen None Learning Objectives Understand the efficacy of long term bexarotene treatment for poorly differentiated thyroid cancer Understand the
Joshua Klopper, Madeleine Kane, Antonio Jimeno and Bryan Haugen None Learning Objectives Understand the efficacy of long term bexarotene treatment for poorly differentiated thyroid cancer Understand the
Sponsor / Company: Sanofi Drug substance(s): SAR (iniparib)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Beta-carotene in Multivitamins and the Possible Risk of Lung Cancer Among Smokers Versus Former Smokers
 150 Beta-carotene in Multivitamins and the Possible Risk of Lung Cancer Among Smokers Versus Former Smokers A Meta-analysis and Evaluation of National Brands Tawee Tanvetyanon, MD Gerold Bepler, MD, PhD
150 Beta-carotene in Multivitamins and the Possible Risk of Lung Cancer Among Smokers Versus Former Smokers A Meta-analysis and Evaluation of National Brands Tawee Tanvetyanon, MD Gerold Bepler, MD, PhD
Although the test that measures total prostate-specific antigen (PSA) has been
 ORIGINAL ARTICLE STEPHEN LIEBERMAN, MD Chief of Urology Kaiser Permanente Northwest Region Clackamas, OR Effective Clinical Practice. 1999;2:266 271 Can Percent Free Prostate-Specific Antigen Reduce the
ORIGINAL ARTICLE STEPHEN LIEBERMAN, MD Chief of Urology Kaiser Permanente Northwest Region Clackamas, OR Effective Clinical Practice. 1999;2:266 271 Can Percent Free Prostate-Specific Antigen Reduce the
Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific Journals
 ORIGINAL CONTRIBUTION Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific From the Division of Emergency Medicine, University of California, San Francisco, CA *
ORIGINAL CONTRIBUTION Citation Characteristics of Research Published in Emergency Medicine Versus Other Scientific From the Division of Emergency Medicine, University of California, San Francisco, CA *
3. Chantix [package insert]. New York, NY: Pfizer, Inc,; Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine
![3. Chantix [package insert]. New York, NY: Pfizer, Inc,; Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine 3. Chantix [package insert]. New York, NY: Pfizer, Inc,; Ramon JM, Morchon S, Baena A, Masuet-Aumatell C. Combining varenicline and nicotine](/thumbs/87/96607870.jpg) How can there be a warning regarding concomitant use of varenicline with nicotine replacement therapy yet patients can be on varenicline and smoke concurrently? April 20, 2017 The United States (US) Preventive
How can there be a warning regarding concomitant use of varenicline with nicotine replacement therapy yet patients can be on varenicline and smoke concurrently? April 20, 2017 The United States (US) Preventive
ClinicalTrials.gov Identifier: NCT Adi Diab 1, Nizar Tannir 1, Daniel Cho
 Pivot-02: Preliminary safety, efficacy and biomarker results from dose escalation of the Phase 1/2 study of CD-122-biased agonist NKTR-214 plus nivolumab in patients with locally advanced/metastatic melanoma,
Pivot-02: Preliminary safety, efficacy and biomarker results from dose escalation of the Phase 1/2 study of CD-122-biased agonist NKTR-214 plus nivolumab in patients with locally advanced/metastatic melanoma,
Early detection and treatment for Esophageal Cancer in Africa
 Early detection and treatment for Esophageal Cancer in Africa Dr Michael Mwachiro Tenwek Hospital NCI-IARC ESCC Tumor Workshop Where is Tenwek Hospital? Tenwek Nairobi Tenwek Hospital Identification of
Early detection and treatment for Esophageal Cancer in Africa Dr Michael Mwachiro Tenwek Hospital NCI-IARC ESCC Tumor Workshop Where is Tenwek Hospital? Tenwek Nairobi Tenwek Hospital Identification of
Immunotherapy for the Treatment of Head and Neck Cancers. Robert F. Taylor, MD Aurora Health Care
 Immunotherapy for the Treatment of Head and Neck Cancers Robert F. Taylor, MD Aurora Health Care Disclosures No relevant financial relationships to disclose I will be discussing non-fda approved indications
Immunotherapy for the Treatment of Head and Neck Cancers Robert F. Taylor, MD Aurora Health Care Disclosures No relevant financial relationships to disclose I will be discussing non-fda approved indications
Liver Transplantation for Alcoholic Liver Disease in the United States: 1988 to 1995
 Liver Transplantation for Alcoholic Liver Disease in the United States: 1988 to 1995 Steven H. Belle, Kimberly C. Beringer, and Katherine M. Detre T he Scientific Liver Transplant Registry (LTR) was established
Liver Transplantation for Alcoholic Liver Disease in the United States: 1988 to 1995 Steven H. Belle, Kimberly C. Beringer, and Katherine M. Detre T he Scientific Liver Transplant Registry (LTR) was established
Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective
 Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective Julie R. Brahmer, M.D. Associate Professor of Oncology The Sidney Kimmel Comprehensive
Management Guidelines and Targeted Therapies in Metastatic Non-Small Cell Lung Cancer: An Oncologist s Perspective Julie R. Brahmer, M.D. Associate Professor of Oncology The Sidney Kimmel Comprehensive
CHL 5225 H Advanced Statistical Methods for Clinical Trials. CHL 5225 H The Language of Clinical Trials
 CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
CHL 5225 H Advanced Statistical Methods for Clinical Trials Two sources for course material 1. Electronic blackboard required readings 2. www.andywillan.com/chl5225h code of conduct course outline schedule
This was a multinational, multicenter study conducted at 14 sites in both the United States (US) and Europe (EU).
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. NAME OF SPONSOR/COMPANY: Genzyme Corporation,
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. NAME OF SPONSOR/COMPANY: Genzyme Corporation,
Nasopharynx. 1. Introduction. 1.1 General Information and Aetiology
 Nasopharynx 1. Introduction 1.1 General Information and Aetiology The nasopharynx is the uppermost, nasal part of the pharynx. It extends from the base of the skull to the upper surface of the soft palate.
Nasopharynx 1. Introduction 1.1 General Information and Aetiology The nasopharynx is the uppermost, nasal part of the pharynx. It extends from the base of the skull to the upper surface of the soft palate.
Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells
 Supporting Information Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells Wei Zhao, Ji-Shi Wei, Peng Zhang, Jie Chen, Ji-Lie Kong,,, * Lian-Hua Sun, Huan-Ming
Supporting Information Self-assembled ZnO nanoparticle capsules for carrying and delivering isotretinoin to cancer cells Wei Zhao, Ji-Shi Wei, Peng Zhang, Jie Chen, Ji-Lie Kong,,, * Lian-Hua Sun, Huan-Ming
Labeled Uses: Treatment of Clostiridum Difficile associated diarrhea (CDAD)
 Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Brand Name: Dificid Generic Name: fidaxomicin Manufacturer 1,2,3,4,5 : Optimer Pharmaceuticals, Inc. Drug Class 1,2,3,4,5 : Macrolide Antibiotic Uses 1,2,3,4,5 : Labeled Uses: Treatment of Clostiridum
Imfinzi (durvalumab) (Intravenous)
 Imfinzi (durvalumab) (Intravenous) Last Review Date: 09/05/2018 Date of Origin: 05/30/2017 Dates Reviewed: 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 09/2018 Document Number: IC-0301 I. Length of Authorization
Imfinzi (durvalumab) (Intravenous) Last Review Date: 09/05/2018 Date of Origin: 05/30/2017 Dates Reviewed: 05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 09/2018 Document Number: IC-0301 I. Length of Authorization
Treatment A Placebo to match COREG CR 20 mg OD + Lisinopril 10 mg OD (Days 1-7) Placebo to match COREG CR 40 mg OD + Lisinopril 10 mg OD (Days 8-14)
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
PFIZER INC. GENERIC DRUG NAME and/or COMPOUND NUMBER: 5% Spironolactone Cream/ PF
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial
 Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial Gilbert S. Omenn, Gary E. Goodman, Mark D. Thornquist, John Balmes, Mark R. Cullen, Andrew
Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial Gilbert S. Omenn, Gary E. Goodman, Mark D. Thornquist, John Balmes, Mark R. Cullen, Andrew
Ripamonti C, et al. ASCO 2012 (Abstract 9005)
 ZOOM: A Prospective, Randomized Trial of Zoledronic Acid for Long-term Treatment in Patients With Bone-Metastatic Breast Cancer After 1 Year of Standard Zoledronic Acid Treatment D. Amadori, M. Aglietta,
ZOOM: A Prospective, Randomized Trial of Zoledronic Acid for Long-term Treatment in Patients With Bone-Metastatic Breast Cancer After 1 Year of Standard Zoledronic Acid Treatment D. Amadori, M. Aglietta,
Adjuvant Therapy in Locally Advanced Head and Neck Cancer. Ezra EW Cohen University of Chicago. Financial Support
 Adjuvant Therapy in Locally Advanced Head and Neck Cancer Ezra EW Cohen University of Chicago Financial Support This program is made possible by an educational grant from Eli Lilly Oncology, who had no
Adjuvant Therapy in Locally Advanced Head and Neck Cancer Ezra EW Cohen University of Chicago Financial Support This program is made possible by an educational grant from Eli Lilly Oncology, who had no
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
518 Vol. 12, , June 2003 Cancer Epidemiology, Biomarkers & Prevention
 518 Vol. 12, 518 526, June 2003 Cancer Epidemiology, Biomarkers & Prevention The Association between Lung and Prostate Cancer Risk, and Serum Micronutrients: Results and Lessons Learned from -Carotene
518 Vol. 12, 518 526, June 2003 Cancer Epidemiology, Biomarkers & Prevention The Association between Lung and Prostate Cancer Risk, and Serum Micronutrients: Results and Lessons Learned from -Carotene
Menthol Cigarettes, Smoking Cessation, Atherosclerosis and Pulmonary Function
 Center for Regulatory Effectiveness (CRE) assessment of the following research report: Menthol Cigarettes, Smoking Cessation, Atherosclerosis and Pulmonary Function By: Mark J. Pletcher, MD, MPH; Benjamin
Center for Regulatory Effectiveness (CRE) assessment of the following research report: Menthol Cigarettes, Smoking Cessation, Atherosclerosis and Pulmonary Function By: Mark J. Pletcher, MD, MPH; Benjamin
Handheld Radiofrequency Spectroscopy for Intraoperative Assessment of Surgical Margins During Breast-Conserving Surgery
 Last Review Status/Date: December 2014 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
Last Review Status/Date: December 2014 Page: 1 of 6 Intraoperative Assessment of Surgical Description Breast-conserving surgery as part of the treatment of localized breast cancer is optimally achieved
UPDATE FROM ASCO GU FEBRUARY 2018, SAN FRANCISCO, USA. Prof. David Pfister University Hospital of Cologne Germany RENAL CELL CARCINOMA
 UPDATE FROM ASCO GU FEBRUARY 2018, SAN FRANCISCO, USA Prof. David Pfister University Hospital of Cologne Germany RENAL CELL CARCINOMA DISCLAIMER Please note: The views expressed within this presentation
UPDATE FROM ASCO GU FEBRUARY 2018, SAN FRANCISCO, USA Prof. David Pfister University Hospital of Cologne Germany RENAL CELL CARCINOMA DISCLAIMER Please note: The views expressed within this presentation
PERIOPERATIVE TREATMENT OF NON SMALL CELL LUNG CANCER. Virginie Westeel Chest Disease Department University Hospital Besançon, France
 PERIOPERATIVE TREATMENT OF NON SMALL CELL LUNG CANCER Virginie Westeel Chest Disease Department University Hospital Besançon, France LEARNING OBJECTIVES 1. To understand the potential of perioperative
PERIOPERATIVE TREATMENT OF NON SMALL CELL LUNG CANCER Virginie Westeel Chest Disease Department University Hospital Besançon, France LEARNING OBJECTIVES 1. To understand the potential of perioperative
Rate of delivery of hyperbaric oxygen treatments does not affect response in soft tissue radionecrosis.
 Rate of delivery of hyperbaric oxygen treatments does not affect response in soft tissue radionecrosis. Submitted - 2/23/07; Accepted 4/23/07 N. B. HAMPSON 1, J. M. CORMAN 2 1 Center for Hyperbaric Medicine,
Rate of delivery of hyperbaric oxygen treatments does not affect response in soft tissue radionecrosis. Submitted - 2/23/07; Accepted 4/23/07 N. B. HAMPSON 1, J. M. CORMAN 2 1 Center for Hyperbaric Medicine,
UMEC/VI vs. UMEC in subjects who responded to UMEC UMEC/VI vs. VI in subjects who responded to VI
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Supplementary webappendix
 Supplementary webappendix This webappendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Kratz JR, He J, Van Den Eeden SK, et
Supplementary webappendix This webappendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Kratz JR, He J, Van Den Eeden SK, et
 PATCH Analysis Plan v1.2.doc Prophylactic Antibiotics for the Treatment of Cellulitis at Home: PATCH Analysis Plan for PATCH I and PATCH II Authors: Angela Crook, Andrew Nunn, James Mason and Kim Thomas,
PATCH Analysis Plan v1.2.doc Prophylactic Antibiotics for the Treatment of Cellulitis at Home: PATCH Analysis Plan for PATCH I and PATCH II Authors: Angela Crook, Andrew Nunn, James Mason and Kim Thomas,
SYNOPSIS (PROTOCOL WX17796)
 TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
TITLE OF THE STUDY A prospective, randomized, double-blind, placebo-controlled, parallel group, multicenter, 52-week trial to assess the efficacy and safety of adjunct MMF to achieve remission with reduced
Precision Medicine Lessons from meta-analyses of 70,253 patients
 Precision Medicine Lessons from meta-analyses of 70,253 patients Razelle Kurzrock, MD Senior Deputy Director, Clinical Science Director, Center for Personalized Cancer Therapy and Clinical Trials Office
Precision Medicine Lessons from meta-analyses of 70,253 patients Razelle Kurzrock, MD Senior Deputy Director, Clinical Science Director, Center for Personalized Cancer Therapy and Clinical Trials Office
Biology Based Cancer Interception
 Biology Based Cancer Interception Waun Ki Hong, M.D. American Cancer Society Professor Former Head Division of Cancer Medicine UT MD Anderson Cancer Center Disclosures SAB : Janssen,Medimmune,Molecular
Biology Based Cancer Interception Waun Ki Hong, M.D. American Cancer Society Professor Former Head Division of Cancer Medicine UT MD Anderson Cancer Center Disclosures SAB : Janssen,Medimmune,Molecular
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Erlotinib for the third or fourth-line treatment of NSCLC January 2012
 Disease background LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Erlotinib for the third or fourth-line treatment of NSCLC January 2012 Lung cancer is the second most common cancer in the UK (after breast),
Disease background LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Erlotinib for the third or fourth-line treatment of NSCLC January 2012 Lung cancer is the second most common cancer in the UK (after breast),
Preoperative Gleason score, percent of positive prostate biopsies and PSA in predicting biochemical recurrence after radical prostatectomy
 JBUON 2013; 18(4): 954-960 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Gleason score, percent of positive prostate and PSA in predicting biochemical
JBUON 2013; 18(4): 954-960 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Gleason score, percent of positive prostate and PSA in predicting biochemical
Epidemiology of Lung Cancer: Implications for screening and prevention
 Epidemiology of Lung Cancer: Implications for screening and prevention Hormuzd A. Katki, Ph.D. Senior Investigator Division of Cancer Epidemiology and Genetics (DCEG) I thank my DCEG colleagues Neil Caporaso
Epidemiology of Lung Cancer: Implications for screening and prevention Hormuzd A. Katki, Ph.D. Senior Investigator Division of Cancer Epidemiology and Genetics (DCEG) I thank my DCEG colleagues Neil Caporaso
