Detection of Colorectal Serrated Polyps by Stool DNA Testing: Comparison with Fecal Immunochemical Testing for Occult Blood (FIT)
|
|
|
- Randell Alexander
- 6 years ago
- Views:
Transcription
1 Detection of Colorectal Serrated Polyps by Stool DNA Testing: Comparison with Fecal Immunochemical Testing for Occult Blood (FIT) Russell I. Heigh 1 *, Tracy C. Yab 2, William R. Taylor 2, Fareeda T. N. Hussain 3, Thomas C. Smyrk 4, Douglas W. Mahoney 5, Michael J. Domanico 6, Barry M. Berger 6, Graham P. Lidgard 6, David A. Ahlquist 2 1 Division of Gastroenterology at Mayo Clinic, Scottsdale, Arizona, United States of America, 2 Division of Gastroenterology and Hepatology at Mayo Clinic, Rochester, Minnesota, United States of America, 3 Mayo Medical School, Rochester, Minnesota, United States of America, 4 Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester Minnesota, United States of America, 5 Department of Biomedical Statistics and Informatics, Mayo Clinic, Rochester Minnesota, United States of America, 6 Exact Sciences Corporation, Madison, Wisconsin, United States of America Abstract Objectives: Precursors to 1/3 of colorectal cancer (CRC), serrated polyps have been under-detected by screening due to their inconspicuous, non-hemorrhagic, and proximal nature. A new multi-target stool DNA test (multi-target sdna) shows high sensitivity for both CRC and advanced adenomas. Screen detection of serrated polyps by this approach requires further validation. We sought to assess and compare noninvasive detection of sessile serrated polyps (SSP) $1 cm by sdna and an occult blood fecal immunochemical test (FIT). Methods: In a blinded prospective study, a single stool sample used for both tests was collected from 456 asymptomatic adults prior to screening or surveillance colonoscopy (criterion standard). All 29 patients with SSP$1 cm were included as cases and all 232 with no neoplastic findings as controls. Buffered stool samples were processed and frozen on receipt; Exact Sciences performed sdna in batches using optimized analytical methods. The sdna multi-marker panel targets methylated BMP3 (mbmp3) and NDRG4, mutant KRAS, b-actin, and hemoglobin. FIT (Polymedco OC-FIT Check) was performed in separate lab #2 days post defecation and evaluated at cutoffs of 50 (FIT-50) and 100 ng/ml (FIT-100). Results: Median ages: cases 61 (range 57 77), controls 62 (52 70), p = NS. Women comprised 59% and 51%, p = NS, respectively. SSP median size was 1.2 cm (1 3 cm), 93% were proximal, and 64% had synchronous diminutive polyps. Among multi-target sdna markers, mbmp3 proved highly discriminant for detection of SSP$1 cm (AUC = 0.87, p, ); other DNA markers provided no incremental sensitivity. Hemoglobin alone showed no discrimination (AUC = 0.50, p = NS). At matched specificities, detection of SSP$1 cm by stool mbmp3 was significantly greater than by FIT-50 (66% vs 10%, p = ) or FIT-100 (63% vs 0%, p,0.0001). Conclusions: In a screening and surveillance setting, SSP$1 cm can be detected noninvasively by stool assay of exfoliated DNA markers, especially mbmp3. FIT appears to have no value in SSP detection. Citation: Heigh RI, Yab TC, Taylor WR, Hussain FTN, Smyrk TC, et al. (2014) Detection of Colorectal Serrated Polyps by Stool DNA Testing: Comparison with Fecal Immunochemical Testing for Occult Blood (FIT). PLoS ONE 9(1): e doi: /journal.pone Editor: Antonio Moschetta, University of Bari & Consorzio Mario Negri Sud, Italy Received September 24, 2013; Accepted November 29, 2013; Published January 20, 2014 Copyright: ß 2014 Heigh et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: This study was funded by grants from the Charles Oswald Foundation, Helen Vandendriesche, and Exact Sciences Corporation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Competing Interests: Potential Competing Interests: Mayo Clinic has licensed related intellectual property to and is a minor equity investor in Exact Sciences. Dr. Ahlquist, Ms. Yab, Ms. Hussain, and Mr. Taylor are inventors on licensed technology. Dr. Ahlquist is a scientific advisor to Exact Sciences. Drs. Berger, Lidgard and Domanico are employees of Exact Sciences. This study was funded by grants from the Charles Oswald Foundation, Helen Vandendriesche, and Exact Sciences Corporation. This does not alter the authors adherence to all the PLOS ONE policies on sharing data and materials. * heigh.russell@mayo.edu Introduction Prevention of colorectal cancer (CRC) by screening rests on effective detection of the critical precursor lesions. Based on the assumption that CRC evolves primarily via the established adenoma-to-carcinoma pathway [1], screening has historically focused on detecting adenomas as the exclusive precursor lesion. However, it has become increasingly apparent in recent years that many CRCs arise from serrated polyps, which differ biologically and clinically from conventional adenomas [2,3]. Serrated polyps, often classified in the past as innocuous hyperplastic polyps and disregarded, are now thought to represent the essential progenitor of up to 1/3 of all CRC [4 6]. Sessile serrated adenomas or polyps (SSPs) represent the subset of serrated polyps at greatest risk of CRC progression and may grow more rapidly than conventional adenomas [4 6]. SSPs are typically right-sided, often flat and endoscopically inconspicuous, and relatively more common in women and the elderly [6 9]. Unlike conventional adenomas, SSPs are commonly associated with BRAF mutations [3,10]; while both SSPs and conventional PLOS ONE 1 January 2014 Volume 9 Issue 1 e85659
2 adenomas frequently harbor aberrantly methylated genes [6,11,12]. SSPs that progress often acquire dysplasia, a transition accompanied by microsatellite instability [4,8,13,14]; and remnants of SSP tissue can be demonstrated at margins of right-sided CRC [13,15]. Data on SSP detection by conventional screening tools are limited, but indirect evidence suggests that their effectiveness is suboptimal. Despite trends of increasing polypectomy rates and reductions in left-sided CRC, population-based studies show that intensified screening has had proportionately less effect on the incidence of right-sided CRC [16]. Sigmoidoscopy is inherently limited to visualization of the left colorectum, and its programmatic application has little impact on proximal CRC [17,18]. Fecal blood testing, whether by guaiac [19,20] or immunochemical [21,22] methods, exhibits low detection rates for adenomas, especially proximal ones; large screening studies reveal that guaiac-type fecal blood testing has had negligible or minimal effect on CRC incidence [20,21]; and it is not known if they can detect SSPs which are largely non-hemorrhagic. Even colonoscopy appears to detect right-sided precursor lesions less well than leftsided ones, despite its capacity to directly inspect the entire colorectum [23 25]. The relatively lower effectiveness of current screening approaches to affect proximal colon cancer may relate to the nature of right-sided precursor lesions and to our difficulties in detecting them with current tools. Stool DNA testing represents a biologically rational candidate approach to the screen detection of SSPs that merits further evaluation. With the improved analytical sensitivity of next generation assays, stool DNA testing has been shown to detect advanced adenomas at high rates [26 29], and neoplasm site does not appear to affect test sensitivity [20,27]. Preliminary reports on selected patients suggest that stool DNA testing detects large SSPs at sensitivities comparable to those for adenomas. In one small study using archived stool specimens [30], both mutant BRAF and selected methylated gene markers were elevated in stools from patients harboring large SSPs. In the other preliminary report [31], an optimized pre-commercial multi-marker stool DNA test (sdna), which targets aberrantly methylated BMP3 and NDRG4 genes, detected 60% of SSP$1 cm at 90% specificity. In the present prospective study, we sought to assess the noninvasive detection of SSP by stool DNA testing in asymptomatic persons undergoing screening or surveillance colonoscopic examination of the colon. Our specific aims were to 1) evaluate the performance of a pre-commercial multi-target stool DNA test (multi-target sdna) for detection of SSP$1 cm, 2) determine which multi-target sdna markers contribute most to SSP detection based on stool and tissue analyses, and 3) compare SSP detection rates by assay of exfoliated markers using stool DNA testing with those by assay of occult bleeding using a quantitative fecal immunochemical test (FIT). We hypothesized that asymptomatic SSP lesions $1 cm would exfoliate markers at rates sufficient to be detected by sdna testing but would rarely bleed and not be meaningfully detected by FIT. Methods This blinded cross-sectional study was approved by the Mayo Clinic Institutional Review Board on September 7, 2010 and conducted at Mayo Clinic facilities in Scottsdale AZ and Rochester MN. All stool and tissue assays were performed by technicians unaware of clinical source of samples. The Mayo Clinic Institutional Review Board deemed this study of deidentified biospecimens to be of minimal risk. Using a form approved by the Mayo Clinic Institutional Review Board, written informed consent was obtained prior to specimen collection. Participants in Stool Study Participants comprised consenting asymptomatic adults scheduled during for a screening or polyp surveillance colonoscopy, which served as the criterion standard. Patients were excluded if they had (1) a prior colorectal resection, (2) inflammatory bowel disease, Lynch syndrome, familial adenomatous polyposis, or other high risk conditions for CRC, (3) colonoscopy that was incomplete or associated with a poor preparation, or (4) a prior screening examination was done within 5 years. All patients found to have a SSP$1 cm without a synchronous advanced adenoma or CRC were designated as cases. The presence of synchronous small or diminutive polyps did not exclude cases; rather, stratified stool data analyses were performed on patient subsets with and without synchronous small or diminutive polyps. Data were not included on isolated lesions smaller than 1 cm in this proof-of-concept study. All patients found to be free of colorectal polyps or tumors were designated as controls. Stool Collection and Storage A single stool from each participant was prospectively collected prior to cathartic cleansing for colonoscopy using a bucket container mounted to the toilet seat. Patients sampled stools for FIT analyses using probe devices; and then added a preservative solution to the whole stool and promptly mailed the sealed container along with FIT tubes to the processing laboratories, as described [28]. Upon receipt, stools were homogenized, aliquoted, and frozen at 280uC for subsequent batch performance of the multi-marker sdna assay. The commercial FIT assay was performed upon receipt (see below). Stools received.3 days after defecation were disqualified and not tested. Stool Assay Methods Stool DNA tests. Stool processing, assay methods, and primer sequences have been described in detail [28,29,31,32]. This pre-commercial multi-target sdna assay was performed at Exact Sciences (Madison WI) and included the following recent innovations: automation, direct gene capture from fecal supernatant, an optimized rapid bisulfite treatment process, a panel of broadly informative DNA markers (mbmp3, mndrg4, mutant KRAS, and b-actin) assayed in multi-plex by the analyticallysensitive quantitative allele-specific real-time target and signal amplification (QuARTS) method, a proprietary quantitative fecal hemoglobin assay, and use of a logistic regression model for analysis. Results for the sdna test were designated as positive or negative by the manufacturer based on their pre-established logistic algorithm. Individual tumor markers from the multi-target sdna panel were normalized to stool b-actin (a marker of total human DNA) and evaluated separately as well; specificity cutoffs were selected to match those of FIT in the comparison studies. Fecal Immunochemical Test for occult blood (FIT). A quantitative commercially available FIT (OC-FIT CHEK, Polymedco, NY) was performed at Mayo Clinic using an automated reading device. FIT results were evaluated at the manufacturer s recommended specificity cutoff of 100 ng hemoglobin/ml buffer (FIT-100) and also at a cutoff of 50 ng/ml (FIT-50). Tissue analyses. To determine whether SSP lesions represent the likely origin of methylated markers in stool, a blinded independent tissue study was performed on SSP lesions $1cm colonoscopically-removed from 20 unique case patients [median PLOS ONE 2 January 2014 Volume 9 Issue 1 e85659
3 age 61 (range 30 83), 68% women] and on normal colon mucosa biopsies from 20 unique control patients without visible colorectal lesions [median age 54 (range 30 81), 70% women]. Following micro-dissection of paraffin or frozen tissue slides, DNA was extracted in usual fashion. Bisulfite treatment and marker assay methods were as described above for stool. Quantitative levels were normalized to b-actin. Statistical Analysis Associations between test positivity and clinical characteristics were assessed using the Chi-square test. Comparison of sensitivities between tests was done at matched specificities using McNemar s test for paired proportion. The non-parametric Wilcoxon Rank Sums Test was used to test the association between continuous marker values and clinical characteristics. The discriminant accuracy of each marker was estimated as the area under the ROC curve (AUC) [33]. Linear combination of markers in the multi-target sdna test for the prediction of disease status was assessed using logistic regression. To avoid over fitting of the data, the final set of predictors used within the logistic regression model was determined from fitting an Elastic Net regression model with all the markers; and the most discriminant marker or set of markers was selected to have the lowest cross-validated prediction error [34]. Results Patient and Lesion Characteristics for Stool Study From 456 asymptomatic patients undergoing screening or surveillance colonoscopy, we identified 29 individuals with SSP$1 cm who served as cases and 232 free of polyps who served as controls. Age and sex distributions were similar between groups (Table 1). In cases, median SSP size was 1.2 cm (range 1 3), 28/29 (93%) were located proximal to the splenic flexure, and only one SSP contained dysplasia (Table 1). Synchronous small or diminutive polyps were present in 64% of cases. Table 1. Patient and Lesion Characteristics for Stool Study. SSP Cases (29) a Normal Controls (232) b Patient Demographics Age in years, median (range) 62 (57 77) 61 (52 70) Sex, % women SSP Features Size in cm, median (range) 1.4 ( ) Right sided, % c 93 Dysplasia present, % 3 Synchronous small polyps, % d 64 Abbreviation:, not applicable. a Cases comprised patients with at least one SSP (sessile serrated polyp) $1 cm found on screening or surveillance colonoscopy and without synchronous advanced adenomas or CRC. b Control patients had no pathology (no CRC, colorectal polyps, hemorrhagic lesions, or inflammation) on screening or surveillance colonoscopy. c Right-sided location was defined as proximal to the splenic flexure. d Patients found to harbor synchronous polyps (adenomatous or serrated),1 cm in size were included as cases. doi: /journal.pone t001 SSP Detection by the Multi-target Stool DNA Test Test accuracy. Using the manufacturer s current cutoff, the sensitivity of the sdna test for detection of SSP$1 cm was 55% (95% CI: 36 74) and the specificity was 91% (95% CI: 87 94). Effect of covariates. The SSP detection rate did not differ significantly between case subsets with and without synchronous small or diminutive polyps; sensitivities were 60% and 50%, respectively (p = 0.4). Within the narrow size range of SSP cases identified, the detection rate was 55% for the 11 patients with lesions cm and 53% for those 17 with lesions cm, p = 0.9. Neither age nor sex influenced SSP detection rates (data not shown). Contribution of component markers to SSP detection. Among the multi-target sdna markers, mbmp3 proved most discriminant for SSP detection, with an AUC of 0.87 (95% CI: ), p, The other informative DNA markers, mndrg4 (AUC 0.79; 95% CI; ; p,0.0001) and mutant KRAS (AUC 0.64; 95% CI: ; p = ), did not provide statistically significant incremental sensitivity in this study above that provided by mbmp3. Of note, fecal hemoglobin alone showed no discrimination (AUC = 0.50; 95% CI: ; p = ). Tissue Confirmation of SSP Discrimination by Methylation Markers At the tissue level, each methylated marker alone discriminated SSP from normal colon mucosa almost completely (Figure 1). The median mbmp3 level in SSP lesions (24 relative units (range 0 78) was.200 times greater than in normal colon mucosa (0.11 (range ), p, The median mndrg4 level in SSP lesions (15 (range )) was.70 times higher than in normal colon mucosa (0.21 (0 0.8)), p, Comparison of Stool DNA Testing and FIT for SSP Detection Stool assay of mbmp3 (an exfoliated DNA marker) was selected for comparison against FIT, which measures fecal hemoglobin (an occult bleeding marker), to best evaluate the diagnostic yield of these markers that enter stool via biologically distinct mechanisms. At matched specificities, stool assay of mbmp3 was substantially and significantly more sensitive for detection of SSP$1 cm than FIT (Figure 2). At 95% specificity cutoffs, stool assay of mbmp3 detected 63% of SSP lesions compared to 0% by FIT-100, p,0.0001; at 91% specificities, stool assay of mbmp3 detected 66% compared to 10% by FIT-50, p = Discussion Based on this prospective case-control study on asymptomatic patients undergoing screening or surveillance colonoscopy, we demonstrate that stool DNA testing represents a feasible approach to the noninvasive detection of SSP$1 cm. Stool assay of mbmp3, a component of the multi-target stool DNA test, primarily accounted for the high SSP detection rates. Given our prior demonstrations that advanced adenomas can be detected at high rates by a next generation multi-target stool DNA test [26 29,31,32], these new findings suggest broadened value of this tool for CRC prevention through the detection of critical precursor lesions from both major molecular pathways of carcinogenesis. In contrast to stool DNA testing, FIT essentially failed to detect SSPs. This finding is perhaps not surprising given the biology of these precursor lesions. SSPs are typically sessile or flat, nonulcerated, and without hemorrhagic features [5,6,9]. In contrast, while SSPs may not bleed, our findings demonstrate that they do PLOS ONE 3 January 2014 Volume 9 Issue 1 e85659
4 Figure 1. Tissue levels of aberrantly methylated genes. Tissue levels of methylated BMP3 (mbmp3) and NDRG4 (mndrg4) genes are compared in normal colorectal mucosa, n = 20 unique control patients, and sessile serrated polyps (SSP), n = 20 unique case patients. Marker levels are normalized to b-actin (a marker of total human DNA) and expressed in relative units. Levels were substantially and significantly higher in SSP than normal colon for both mbmp3 (p,0.0001) and mndrg4 (p,0.0001). doi: /journal.pone g001 exfoliate at rates sufficient enough to allow their noninvasive detection by assay of altered DNA in stool. The specific point sensitivities by sdna testing for detection of SSP$1 cm that we observed could translate to substantial practical value. Repeat screening with a test having a point sensitivity of 55 67% yields a potential program sensitivity exceeding 90% after the third screening round [35]. These programatic detection rates may compare favorably with conventional colonoscopy done every 10 years, particularly given the reported wide range in operator variation with colonoscopy [36]. Accurate detection of gastrointestinal neoplasms by stool assay of exfoliated markers requires high analytical sensitivity and discriminant markers. The QuARTS assay method used in this study achieves more than 100-fold higher sensitivity than earlier generation stool DNA assays [26-29,31,32] and, as we observed in this study, provides the critical analytical power to detect lowabundance DNA markers exfoliated into stool from SSPs. SSP detection by the multi-target stool DNA test was primarily accounted for by mbmp3. Consistent with the stool observations, we found in our tissue study that mbmp3 levels highly Figure 2. Detection of SSP by stool assay of mbmp3 and by fecal immunochemical testing (FIT). FIT sensitivity was evaluated at the conventional cutoff of 100 ng hemoglobin/ml buffer (FIT-100) and at 50 ng/ml (FIT-50). Specificity cutoffs for stool DNA marker mbmp3 were selected to match those for both FIT-100 and FIT-50 so that sensitivities could be most meaningfully compared. doi: /journal.pone g002 PLOS ONE 4 January 2014 Volume 9 Issue 1 e85659
5 discriminated SSP from normal colon mucosa. Perhaps because of the low background levels of mbmp3 in normal control stools, assay of this marker alone achieved slightly higher SSP detection rates than did assay of the full marker panel in this study. Strengths of this study included prospective stool collections from well-characterized patients undergoing colonoscopy for average risk screening or polyp surveillance, a blinded design, state-of-the-art assay technology, and use of discriminant methylation markers. Study limitations comprised the relatively narrow and small SSP size range, as the median size was only 1.2 cm, and a paucity of lesions containing dysplasia which likely represent those at greatest risk of progression. Despite these SSP characteristics, the majority were detected by the multi-target sdna test. Furthermore, the observed differences in SSP detection rates between the stool DNA testing and FIT were substantial and highly significant. Sample size in this study did not permit robust covariate analyses. We demonstrate proof-of-concept for SSP detection by stool DNA testing. Clinical applications of these findings can be considered and further explored. Incorporating markers specific for SSP into stool DNA tests designed for general CRC screening has potential to expand the effectiveness of this approach for CRC prevention, as both major types of precursor lesions could be targeted. Furthermore, given that colonoscopy has had a proportionately lower impact on incidence and mortality from References 1. Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61: Jass JR (2001) Serrated route to colorectal cancer: back street or super highway? J Pathol 193: Jass JR (2005) Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol 2: Snover DC (2011) Update on the serrated pathway to colorectal carcinoma. Hum Pathol 42: Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP (2005) Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol 124: Noffsinger AE (2009) Serrated polyps and colorectal cancer: new pathway to malignancy Annu Rev Pathol 4: Lazarus R, Junttila OE, Karttunen TJ, Makinen MJ (2005) The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol 123: Lash RH, Genta RM, Schuler CM (2010) Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 63: Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, et al. (2012) Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 107: Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, et al. (2006) High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 131: Park SJ, Rashid A, Lee JH, Kim SG, Hamilton SR, et al. (2003) Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol 162: Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC 3rd, et al. (2007) Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol 5: Sheridan TB, Fenton H, Lewin MR, Burkart AL, Iacobuzio-Donahue CA, et al. (2006) Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions caught in the act. Am J Clin Pathol 126: Oka S, Tanaka S, Hiyama T, Ito M, Kitadai Y, et al. (2004) Clinicopathologic and endoscopic features of colorectal serrated adenoma: differences between polypoid and superficial types. Gastrointest Endosc 59: Makinen MJ, George SM, Jernvall P,Makela J, Vihko P, et al. (2001) Colorectal carcinoma associated with serrated adenoma: prevalence, histological features, and prognosis. J Pathol 193: Gupta AK, Melton LJ 3rd, Petersen GM, Timmons LJ, Vege SS, et al. (2005) Changing trends in the incidence, stage, survival, and screen-detection of colorectal cancer: a population-based study. Clin Gastroenterol Hepatol 3: Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, et al. (2010) Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 375: right-sided than left-sided CRC [23 25] and that the majority of interval CRC cases are right-sided [37], a rationale could be made for the complementary use of stool DNA testing between screening colonoscopies, which are recommended at a frequency of every 10 years. While an economic analysis of an enhanced colorectal neoplasia detection strategy is beyond the scope of this study, once accurate assumptions of costs and test performance characteristics are available, modeling of new approaches will be instructive. The multi-target sdna test is currently being evaluated in a prospective study involving.10,000 patients from the screen setting using colonoscopy as the criterion standard (the DEEP-C Study), and data from this large study should provide important and robust validation of this noninvasive screening approach. Author Contributions Conceived and designed the experiments: DAA. Performed the experiments: RIH DAA. Analyzed the data: RIH DAA DWM. Contributed reagents/materials/analysis tools: TCY WRT FTNH MJD GPL. Wrote the paper: RIH DAA FTNH. Guarantors: RIH DAA. Patient recruitment oversight: RIH. Data review: RIH TCY. Performance of tissue and stool assays: TCY. Primer designs and assistance with assays: WRT. Laboratory analyses: FTNH. Pathology review and oversight: TCS. Statistical analyses: DWM. Assay performance: MJD. Design and performance of assays: GPL. Study design and oversight: DAA. Procurement of funding: DAA. Manuscript review: TCY WRT TCS DWM MJD BB GPL. 18. Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, et al. (2012) Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 366: Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME, et al. (2004) Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 351: Ahlquist DA, Sargent DJ, Loprinzi CL,Levin TR, Rex DK, et al. (2008) Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med 149: Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, et al. (2005) A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology 129: Park DI, Ryu S, Kim YH, Lee SH, Lee CK, et al. (2010) Comparison of guaiacbased and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol 105: Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, et al. (2010) Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst 102: Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M (2011) Protection from colorectal cancer after colonoscopy: a population-based, casecontrol study. Ann Intern Med 54: Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, et al. (2009) Association of colonoscopy and death from colorectal cancer. Ann Intern Med 150: Zou H, Taylor WR, Harrington JJ, Hussain FT, Cao X, et al. (2009) High detection rates of colorectal neoplasia by stool DNA testing with a novel digital melt curve assay. Gastroenterology 136: Ahlquist D ZH, Domanico M, Mahoney D, Lidgard G (2010) Next generation stool DNA testing for detection of colorectal neoplasia: Early marker evaluation. Presented at AACR Meeting, Philadelphia, PA. 28. Ahlquist DA, Zou H, Domanico M, Mahoney DW, Yab TC, et al. (2012) Nextgeneration stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology 142: Ahlquist DA, Taylor WR, Mahoney DW, Zou H, Domanico M, et al. (2012) The stool DNA test is more accurate than the plasma septin 9 test in detecting colorectal neoplasia. Clin Gastroenterol Hepatol 10: Hussain FT, Yab TC, Harrington JJ, et al. (2010) Noninvasive Detection of Serrated Colorectal Polyps by Stool Assay of Methylated Vimentin and Mutant BRAF Genes. In: DDW. 31. Lidgard GP DM, Bruinsma JJ, Light J, Gagrat ZD, Oldham-Haltom RL, et al. (2012) An Optimized Molecular Stool Test for Colorectal Cancer Screening: Evaluation of an Automated Analytic Platform and Logistic Algorithm. In: AACR Frontiers in Cancer Prevention; 2012; San Francisco, CA. 32. Lidgard GP DM, Bruinsma JJ, Gagrat ZD, Oldham-Haltom RL, Fourrier KD, et al. (2012) An optimized multi-marker stool test for colorectal cancer screening: initial clinical appraisal. Gastroenterology 142: S1. PLOS ONE 5 January 2014 Volume 9 Issue 1 e85659
6 33. DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44: Zou H, Hastie T (2005) Regularization and variable selection via the elastic net. J R Statist Soc B 67: Berger BM, Ahlquist DA (2012) Stool DNA screening for colorectal neoplasia: biological and technical basis for high detection rates. Pathology 44: Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK, et al. (2011) Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol 9: Singh H TD, Xue L, Targownik LE, Bernstein CN (2006) Risk of Developing Colorectal Cancer Following a Negative Colonoscopy Examination: evidence for a 10-year interval between colonoscopies. JAMA 295: PLOS ONE 6 January 2014 Volume 9 Issue 1 e85659
Stool DNA Screening for Colorectal Cancer. David Ahlquist, MD Carrol M Gatton Professor of Digestive Science Mayo Clinic, Rochester MN
 Stool DNA Screening for Colorectal Cancer David Ahlquist, MD Carrol M Gatton Professor of Digestive Science Mayo Clinic, Rochester MN Disclosures Relationship with Exact Sciences Mayo Clinic Equity investor
Stool DNA Screening for Colorectal Cancer David Ahlquist, MD Carrol M Gatton Professor of Digestive Science Mayo Clinic, Rochester MN Disclosures Relationship with Exact Sciences Mayo Clinic Equity investor
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening. Summary
 Page: 1 of 11 Last Review Status/Date: March 2015 Technique for Colorectal Cancer Screening Summary Detection of genetic abnormalities associated with colorectal cancer in stool samples has been proposed
Page: 1 of 11 Last Review Status/Date: March 2015 Technique for Colorectal Cancer Screening Summary Detection of genetic abnormalities associated with colorectal cancer in stool samples has been proposed
A TEST FOR COLORECTAL CANCER THAT IS 92% SENSITIVE AND NON-INVASIVE. Stool DNA test
 A TEST FOR COLORECTAL CANCER THAT IS 92% SENSITIVE AND NON-INVASIVE Stool DNA test THE NEW NON-INVASIVE SCREENING TEST FOR COLORECTAL CANCER Sensitive Clinically proven 1 Easy to use FDA approved COLOGUARD
A TEST FOR COLORECTAL CANCER THAT IS 92% SENSITIVE AND NON-INVASIVE Stool DNA test THE NEW NON-INVASIVE SCREENING TEST FOR COLORECTAL CANCER Sensitive Clinically proven 1 Easy to use FDA approved COLOGUARD
IEHP UM Subcommittee Approved Authorization Guidelines Colorectal Cancer Screening with Cologuard TM for Medicare Beneficiaries
 for Medicare Beneficiaries Policy: Based on our review of the available evidence, the IEHP UM Subcommittee adopts the use of Cologuard TM - a multi-target stool DNA test as a colorectal cancer screening
for Medicare Beneficiaries Policy: Based on our review of the available evidence, the IEHP UM Subcommittee adopts the use of Cologuard TM - a multi-target stool DNA test as a colorectal cancer screening
Policy Specific Section: March 1, 2005 January 30, 2015
 Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Medical Policy Fecal DNA Analysis for Colorectal Cancer Screening Type: Investigational / Experimental Policy Specific Section: Laboratory/Pathology Original Policy Date: Effective Date: March 1, 2005
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Analysis of Human DNA in Stool Samples as a Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Analysis of Human DNA in Stool Samples as a Technique for
Analysis of Human DNA in Stool Samples as a Page 1 of 11 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Analysis of Human DNA in Stool Samples as a Technique for
Colorectal Cancer Screening and Surveillance
 1 Colorectal Cancer Screening and Surveillance Jeffrey Lee MD, MAS Assistant Clinical Professor of Medicine University of California, San Francisco jeff.lee@ucsf.edu Objectives Review the various colorectal
1 Colorectal Cancer Screening and Surveillance Jeffrey Lee MD, MAS Assistant Clinical Professor of Medicine University of California, San Francisco jeff.lee@ucsf.edu Objectives Review the various colorectal
Noninvasive Molecular Detection of Colorectal Neoplasia
 Disclosures Noninvasive Molecular Detection of Colorectal Next Generation Approaches David Ahlquist August 7, 29 Relationship with Exact Sciences Mayo Clinic is equity investor Potential for future royalties
Disclosures Noninvasive Molecular Detection of Colorectal Next Generation Approaches David Ahlquist August 7, 29 Relationship with Exact Sciences Mayo Clinic is equity investor Potential for future royalties
ABSTRACT. Background An accurate, noninvasive test could improve the effectiveness of colorectal-cancer screening.
 The new england journal of medicine established in 1812 april 3, 2014 vol. 370 no. 14 Multitarget Stool DNA Testing for Colorectal-Cancer Screening Thomas F. Imperiale, M.D., David F. Ransohoff, M.D.,
The new england journal of medicine established in 1812 april 3, 2014 vol. 370 no. 14 Multitarget Stool DNA Testing for Colorectal-Cancer Screening Thomas F. Imperiale, M.D., David F. Ransohoff, M.D.,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Analysis of Human DNA in Stool Samples as a Page 1 of 12 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Analysis of Human DNA in Stool Samples as a Technique for
Analysis of Human DNA in Stool Samples as a Page 1 of 12 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Analysis of Human DNA in Stool Samples as a Technique for
Synchronous and Subsequent Lesions of Serrated Adenomas and Tubular Adenomas of the Colorectum
 Tsumura T, et al 1 Synchronous and Subsequent Lesions of Serrated Adenomas and Tubular Adenomas of the Colorectum T. Tsumura a T. Hiyama d S. Tanaka b M. Yoshihara d K. Arihiro c K. Chayama a Departments
Tsumura T, et al 1 Synchronous and Subsequent Lesions of Serrated Adenomas and Tubular Adenomas of the Colorectum T. Tsumura a T. Hiyama d S. Tanaka b M. Yoshihara d K. Arihiro c K. Chayama a Departments
Early detection and screening for colorectal neoplasia
 Early detection and screening for colorectal neoplasia Robert S. Bresalier Department of Gastroenterology, Hepatology and Nutrition. The University of Texas. MD Anderson Cancer Center. Houston, Texas U.S.A.
Early detection and screening for colorectal neoplasia Robert S. Bresalier Department of Gastroenterology, Hepatology and Nutrition. The University of Texas. MD Anderson Cancer Center. Houston, Texas U.S.A.
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines
 Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Alberta Colorectal Cancer Screening Program (ACRCSP) Post Polypectomy Surveillance Guidelines June 2013 ACRCSP Post Polypectomy Surveillance Guidelines - 2 TABLE OF CONTENTS Background... 3 Terms, Definitions
Pharmacogenomic and Metabolite Markers for Patients Treated with Thiopurines
 Pharmacogenomic and Metabolite Markers for Patients Treated with Thiopurines Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
Pharmacogenomic and Metabolite Markers for Patients Treated with Thiopurines Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana,
References. Valorization
 Valorization 179 Valorization 180 Valorization Colorectal cancer (CRC) is a major burden on the health care system with over 1,4millionnewlydiagnosedpatientsandalmost700,000deathsannually. 1 Becauseof
Valorization 179 Valorization 180 Valorization Colorectal cancer (CRC) is a major burden on the health care system with over 1,4millionnewlydiagnosedpatientsandalmost700,000deathsannually. 1 Becauseof
Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it
 Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it Douglas K Rex 1 MD, MACG 1. Indiana University School of Medicine Division of Gastroenterology/Hepatology
Retroflexion and prevention of right-sided colon cancer following colonoscopy: How I approach it Douglas K Rex 1 MD, MACG 1. Indiana University School of Medicine Division of Gastroenterology/Hepatology
Citation for published version (APA): Wijkerslooth de Weerdesteyn, T. R. (2013). Population screening for colorectal cancer by colonoscopy
 UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
UvA-DARE (Digital Academic Repository) Population screening for colorectal cancer by colonoscopy de Wijkerslooth, T.R. Link to publication Citation for published version (APA): Wijkerslooth de Weerdesteyn,
Cologuard Screening for Colorectal Cancer
 Pending Policies - Medicine Cologuard Screening for Colorectal Cancer Print Number: MED208.056 Effective Date: 08-15-2016 Coverage: I.Cologuard stool DNA testing may be considered medically necessary for
Pending Policies - Medicine Cologuard Screening for Colorectal Cancer Print Number: MED208.056 Effective Date: 08-15-2016 Coverage: I.Cologuard stool DNA testing may be considered medically necessary for
Accepted Article. Association between the location of colon polyps at baseline and surveillance colonoscopy - A retrospective study
 Accepted Article Association between the location of colon polyps at baseline and surveillance colonoscopy - A retrospective study Ana Oliveira, Paulo Freire, Paulo Souto, Manuela Ferreira, Sofia Mendes,
Accepted Article Association between the location of colon polyps at baseline and surveillance colonoscopy - A retrospective study Ana Oliveira, Paulo Freire, Paulo Souto, Manuela Ferreira, Sofia Mendes,
Clinical Policy: DNA Analysis of Stool to Screen for Colorectal Cancer
 Clinical Policy: to Screen for Colorectal Cancer Reference Number: CP.MP.125 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding
Clinical Policy: to Screen for Colorectal Cancer Reference Number: CP.MP.125 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding
Summary. Cezary ŁozińskiABDF, Witold KyclerABCDEF. Rep Pract Oncol Radiother, 2007; 12(4):
 Rep Pract Oncol Radiother, 2007; 12(4): 201-206 Original Paper Received: 2006.12.19 Accepted: 2007.04.02 Published: 2007.08.31 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
Rep Pract Oncol Radiother, 2007; 12(4): 201-206 Original Paper Received: 2006.12.19 Accepted: 2007.04.02 Published: 2007.08.31 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
Update on Exact Sciences Molecular CRC Screening Test. November 16 th, 2011
 Update on Exact Sciences Molecular CRC Screening Test November 16 th, 2011 0 Safe Harbor Statement Certain matters contained in this presentation, other than historical information, consist of forward-looking
Update on Exact Sciences Molecular CRC Screening Test November 16 th, 2011 0 Safe Harbor Statement Certain matters contained in this presentation, other than historical information, consist of forward-looking
General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer
 General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer Complexities of Pathological Assessment: Serrated Polyps/Adenomas Carolyn Compton, MD, PhD Professor of Life Sciences,
General Session 7: Controversies in Screening and Surveillance in Colorectal Cancer Complexities of Pathological Assessment: Serrated Polyps/Adenomas Carolyn Compton, MD, PhD Professor of Life Sciences,
Protocol. This trial protocol has been provided by the authors to give readers additional information about their work.
 Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA
Protocol This trial protocol has been provided by the authors to give readers additional information about their work. Protocol for: Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget stool DNA
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS What is CRC? CRC (CRC) is cancer of the large intestine (colon), the lower part of the digestive system. Rectal cancer is cancer of the last several inches of the colon. Together,
FREQUENTLY ASKED QUESTIONS What is CRC? CRC (CRC) is cancer of the large intestine (colon), the lower part of the digestive system. Rectal cancer is cancer of the last several inches of the colon. Together,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer
 Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer David A. Lieberman, 1 Douglas K. Rex, 2 Sidney J. Winawer,
The Importance of Complete Colonoscopy and Exploration of the Cecal Region
 The Importance of Complete Colonoscopy and Exploration of the Cecal Region Kuangi Fu, Takahiro Fujii, Takahisa Matsuda, and Yutaka Saito 2 2.1 The Importance of a Complete Colonoscopy Ever since case-control
The Importance of Complete Colonoscopy and Exploration of the Cecal Region Kuangi Fu, Takahiro Fujii, Takahisa Matsuda, and Yutaka Saito 2 2.1 The Importance of a Complete Colonoscopy Ever since case-control
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening
 Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Sessile serrated polyps: Cancer risk and appropriate surveillance
 REVIEW CME CREDIT EDUCATIONAL OBJECTIVE: Readers will understand the role of the recently recognized serrated neoplasia pathway in the development of colorectal cancer ROHIT MAKKAR, MD St. Michael s Hospital,
REVIEW CME CREDIT EDUCATIONAL OBJECTIVE: Readers will understand the role of the recently recognized serrated neoplasia pathway in the development of colorectal cancer ROHIT MAKKAR, MD St. Michael s Hospital,
Colorectal Cancer Screening
 Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
Recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer Colorectal Cancer Screening Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Levin TR, Lieberman D, Robertson
There is No One Best CRC Screening Test: The Proof and the Benefits of Getting FIT
 There is No One Best CRC Screening Test: The Proof and the Benefits of Getting FIT James E. Allison, MD, FACP, AGAF Clinical Professor of Medicine Emeritus University of California San Francisco Emeritus
There is No One Best CRC Screening Test: The Proof and the Benefits of Getting FIT James E. Allison, MD, FACP, AGAF Clinical Professor of Medicine Emeritus University of California San Francisco Emeritus
Measuring the quality of colonoscopy: Where are we now and where are we going?
 Measuring the quality of colonoscopy: Where are we now and where are we going? Timothy D. Imler, MD Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, Indiana
Measuring the quality of colonoscopy: Where are we now and where are we going? Timothy D. Imler, MD Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, Indiana
ADVANCES IN FAECAL DNA TESTING FOR COLORECTAL CANCER SCREENING: A LITERATURE REVIEW FOR PRIMARY CARE PROVIDERS
 ADVANCES IN FAECAL DNA TESTING FOR COLORECTAL CANCER SCREENING: A LITERATURE REVIEW FOR PRIMARY CARE PROVIDERS *Louise Babikow, Adelle Grant McAuley, Jenny Calhoun University of Pennsylvania, Philadelphia,
ADVANCES IN FAECAL DNA TESTING FOR COLORECTAL CANCER SCREENING: A LITERATURE REVIEW FOR PRIMARY CARE PROVIDERS *Louise Babikow, Adelle Grant McAuley, Jenny Calhoun University of Pennsylvania, Philadelphia,
Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:1341 1346 Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia JUN KATO,* TAMIYA MORIKAWA,* MOTOAKI KURIYAMA,*
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2009;7:1341 1346 Combination of Sigmoidoscopy and a Fecal Immunochemical Test to Detect Proximal Colon Neoplasia JUN KATO,* TAMIYA MORIKAWA,* MOTOAKI KURIYAMA,*
C olorectal adenomas are reputed to be precancerous
 568 COLORECTAL CANCER Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese Y Yamaji, T Mitsushima, H Ikuma, H Watabe, M Okamoto, T
568 COLORECTAL CANCER Incidence and recurrence rates of colorectal adenomas estimated by annually repeated colonoscopies on asymptomatic Japanese Y Yamaji, T Mitsushima, H Ikuma, H Watabe, M Okamoto, T
Colorectal Cancer Screening: A Clinical Update
 11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
11:05 11:45am Colorectal Cancer Screening: A Clinical Update SPEAKER Kevin A. Ghassemi, MD Presenter Disclosure Information The following relationships exist related to this presentation: Kevin A. Ghassemi,
Optimal Colonoscopy Surveillance Interval after Polypectomy
 REVIEW Clin Endosc 2016;49:359-363 http://dx.doi.org/10.5946/ce.2016.080 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Optimal Colonoscopy Surveillance Interval after Polypectomy Tae Oh Kim Department
REVIEW Clin Endosc 2016;49:359-363 http://dx.doi.org/10.5946/ce.2016.080 Print ISSN 2234-2400 On-line ISSN 2234-2443 Open Access Optimal Colonoscopy Surveillance Interval after Polypectomy Tae Oh Kim Department
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
CRC Risk Factors. U.S. Adherence Rates Cancer Screening. Genetic Model of Colorectal Cancer. Epidemiology and Clinical Consequences of CRC
 10:45 11:45 am Guide to Colorectal Cancer Screening SPEAKER Howard Manten M.D. Presenter Disclosure Information The following relationships exist related to this presentation: Howard Manten MD: No financial
10:45 11:45 am Guide to Colorectal Cancer Screening SPEAKER Howard Manten M.D. Presenter Disclosure Information The following relationships exist related to this presentation: Howard Manten MD: No financial
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean?
 The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
Carol A. Burke, MD, FACG
 Updated Guidelines for CRC C Screening and Surveillance Carol A. Burke MD, FACG, FASGE, FACP Cleveland Clinic, Cleveland, OH Gastroenterology t 2012;143:844 143 Gut 2010;59:666 1 Caveat for all Recommendations
Updated Guidelines for CRC C Screening and Surveillance Carol A. Burke MD, FACG, FASGE, FACP Cleveland Clinic, Cleveland, OH Gastroenterology t 2012;143:844 143 Gut 2010;59:666 1 Caveat for all Recommendations
removal of adenomatous polyps detects important effectively as follow-up colonoscopy after both constitute a low-risk Patients with 1 or 2
 Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Supplementary Table 1. Study Characteristics Author, yr Design Winawer et al., 6 1993 National Polyp Study Jorgensen et al., 9 1995 Funen Adenoma Follow-up Study USA Multi-center, RCT for timing of surveillance
Colorectal Cancer Screening: Colonoscopy, Potential and Pitfalls. Disclosures: None. CRC: still a major public health problem
 Colorectal Cancer Screening: Colonoscopy, Potential and Pitfalls Disclosures: None Jonathan P. Terdiman, M.D. Professor of Clinical Medicine University of California, San Francisco CRC: still a major public
Colorectal Cancer Screening: Colonoscopy, Potential and Pitfalls Disclosures: None Jonathan P. Terdiman, M.D. Professor of Clinical Medicine University of California, San Francisco CRC: still a major public
The Natural History of Right-Sided Lesions
 The Natural History of Right-Sided Lesions Jasper L.A. Vleugels Dept of Gastroenterology and Hepatology, Academic Medical Center, Amsterdam, the Netherlands. None Disclosures Agenda Is there evidence that
The Natural History of Right-Sided Lesions Jasper L.A. Vleugels Dept of Gastroenterology and Hepatology, Academic Medical Center, Amsterdam, the Netherlands. None Disclosures Agenda Is there evidence that
Research Article Adenoma and Polyp Detection Rates in Colonoscopy according to Indication
 Hindawi Gastroenterology Research and Practice Volume 2017, Article ID 7207595, 6 pages https://doi.org/10.1155/2017/7207595 Research Article Adenoma and Polyp Detection Rates in Colonoscopy according
Hindawi Gastroenterology Research and Practice Volume 2017, Article ID 7207595, 6 pages https://doi.org/10.1155/2017/7207595 Research Article Adenoma and Polyp Detection Rates in Colonoscopy according
Page 1. Is the Risk This High? Dysplasia in the IBD Patient. Dysplasia in the Non IBD Patient. Increased Risk of CRC in Ulcerative Colitis
 Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
Screening for Colorectal Neoplasia in Inflammatory Bowel Disease Francis A. Farraye MD, MSc Clinical Director, Section of Gastroenterology Co-Director, Center for Digestive Disorders Boston Medical Center
Serrated Polyps, Part 2: Their Mechanisms and Management Ryan C. Romano, DO
 Polyps, Part 2: Their Mechanisms and Management Ryan C. Romano, DO In the prelude to this article ( Polyps Part I: Their Confusing History) we discussed the evolution of colorectal serrated polyp classification,
Polyps, Part 2: Their Mechanisms and Management Ryan C. Romano, DO In the prelude to this article ( Polyps Part I: Their Confusing History) we discussed the evolution of colorectal serrated polyp classification,
Objectives. Definitions. Colorectal Cancer Screening 5/8/2018. Payam Afshar, MS, MD Kaiser Permanente, San Diego. Colorectal cancer background
 Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Colorectal Cancer Screening Payam Afshar, MS, MD Kaiser Permanente, San Diego Objectives Colorectal cancer background Colorectal cancer screening populations Colorectal cancer screening modalities Colonoscopy
Five-Year Risk of Colorectal Neoplasia after Negative Screening Colonoscopy
 The new england journal of medicine original article Five-Year Risk of Colorectal Neoplasia after Negative Screening Colonoscopy Thomas F. Imperiale, M.D., Elizabeth A. Glowinski, R.N., Ching Lin-Cooper,
The new england journal of medicine original article Five-Year Risk of Colorectal Neoplasia after Negative Screening Colonoscopy Thomas F. Imperiale, M.D., Elizabeth A. Glowinski, R.N., Ching Lin-Cooper,
Colon Cancer Screening. Layth Al-Jashaami, MD GI Fellow, PGY 4
 Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colon Cancer Screening Layth Al-Jashaami, MD GI Fellow, PGY 4 -Colorectal cancer (CRC) is a common and lethal cancer. -It has the highest incidence among GI cancers in the US, estimated to be newly diagnosed
Colon Cancer Screening & Surveillance. Amit Patel, MD PGY-4 GI Fellow
 Colon Cancer Screening & Surveillance Amit Patel, MD PGY-4 GI Fellow Epidemiology CRC incidence and mortality rates vary markedly around the world. Globally, CRC is the third most commonly diagnosed cancer
Colon Cancer Screening & Surveillance Amit Patel, MD PGY-4 GI Fellow Epidemiology CRC incidence and mortality rates vary markedly around the world. Globally, CRC is the third most commonly diagnosed cancer
Colon Polyps: Detection, Inspection and Characteristics
 Colon Polyps: Detection, Inspection and Characteristics Stephen Kim, M.D. Assistant Professor of Medicine Interventional Endoscopy Services UCLA Division of Digestive Diseases September 29, 2018 1 Disclosures
Colon Polyps: Detection, Inspection and Characteristics Stephen Kim, M.D. Assistant Professor of Medicine Interventional Endoscopy Services UCLA Division of Digestive Diseases September 29, 2018 1 Disclosures
Hyperplastische Polyps Innocent bystanders?
 Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
Hyperplastische Polyps Innocent bystanders?? K. Geboes P th l i h O tl dk d Pathologische Ontleedkunde, KULeuven Content Historical Classification Relation Hyperplastic polyps carcinoma The concept cept
Accepted Manuscript. En bloc resection for mm polyps to reduce post-colonoscopy cancer and surveillance. C. Hassan, M. Rutter, A.
 Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
Accepted Manuscript En bloc resection for 10-20 mm polyps to reduce post-colonoscopy cancer and surveillance C. Hassan, M. Rutter, A. Repici PII: S1542-3565(19)30412-4 DOI: https://doi.org/10.1016/j.cgh.2019.04.022
Colonoscopy MM /01/2010. PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient
 Colonoscopy Policy Number: Original Effective Date: MM.12.003 12/01/2010 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient
Colonoscopy Policy Number: Original Effective Date: MM.12.003 12/01/2010 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient
Sessile Serrated Polyps
 Årsmøtet i Den norske Patologforening 2014 Sessile Serrated Polyps Tor J. Eide Oslo Universitetssykehus The term serrated include a group of lesions with a sawtoothlike appearance of the crypts and the
Årsmøtet i Den norske Patologforening 2014 Sessile Serrated Polyps Tor J. Eide Oslo Universitetssykehus The term serrated include a group of lesions with a sawtoothlike appearance of the crypts and the
Neoplastic Colon Polyps. Joyce Au SUNY Downstate Grand Rounds, October 18, 2012
 Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Serrated polyps of the colorectum: is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice?
 Virchows Arch (2007) 450:613 618 DOI 10.1007/s00428-007-0413-8 ORIGINAL ARTICLE Serrated polyps of the colorectum: is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice?
Virchows Arch (2007) 450:613 618 DOI 10.1007/s00428-007-0413-8 ORIGINAL ARTICLE Serrated polyps of the colorectum: is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice?
Title Description Type / Priority
 Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Merit-based Incentive Payment system (MIPS) 2019 Qualified Clinical Data Registry (QCDR) Measure Specifications Summary Listing of QCDR measures supported by the NHCR Measure # NHCR4 NHCR5 GIQIC12 GIQIC15
Douglas K. Rex, MD Indiana University Hospital Indianapolis, IN
 Serrated Adenomas: What do they mean and what to do about them? Douglas K. Rex, MD Indiana University Hospital Indianapolis, IN Colorectal Cancer Molecular Basis Pathway Frequency Genes MSI Precursor Speed
Serrated Adenomas: What do they mean and what to do about them? Douglas K. Rex, MD Indiana University Hospital Indianapolis, IN Colorectal Cancer Molecular Basis Pathway Frequency Genes MSI Precursor Speed
Serrated Polyps and a Classification of Colorectal Cancer
 Serrated Polyps and a Classification of Colorectal Cancer Ian Chandler June 2011 Structure Serrated polyps and cancer Molecular biology The Jass classification The familiar but oversimplified Vogelsteingram
Serrated Polyps and a Classification of Colorectal Cancer Ian Chandler June 2011 Structure Serrated polyps and cancer Molecular biology The Jass classification The familiar but oversimplified Vogelsteingram
The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy
 ORIGINAL RESEARCH GASTROENTEROLOGY // INTERNAL MEDICINE The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy Răzvan Opaschi 1, Simona Băţagă 1, Ioan Macarie 2, Imola Török
ORIGINAL RESEARCH GASTROENTEROLOGY // INTERNAL MEDICINE The Detection of Proximal Colon Polyps and Its Importance in Screening Colonoscopy Răzvan Opaschi 1, Simona Băţagă 1, Ioan Macarie 2, Imola Török
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
CASE DISCUSSION: The Patient with Dysplasia: Surgery or Active Surveillance? Noa Krugliak Cleveland, MD David T. Rubin, MD
 CASE DISCUSSION: The Patient with Dysplasia: Surgery or Active Surveillance? Noa Krugliak Cleveland, MD David T. Rubin, MD Disclosure Statement NKC: No relevant conflicts to disclose. DTR: No relevant
CASE DISCUSSION: The Patient with Dysplasia: Surgery or Active Surveillance? Noa Krugliak Cleveland, MD David T. Rubin, MD Disclosure Statement NKC: No relevant conflicts to disclose. DTR: No relevant
Frequency of coexistent carcinoma in sessile serrated adenoma/polyps and traditional serrated adenomas removed by endoscopic resection
 THIEME E451 Frequency of coexistent carcinoma in sessile serrated adenoma/polyps and traditional serrated adenomas removed by endoscopic resection Authors Hirotsugu Saiki 1, Tsutomu Nishida 1, Masashi
THIEME E451 Frequency of coexistent carcinoma in sessile serrated adenoma/polyps and traditional serrated adenomas removed by endoscopic resection Authors Hirotsugu Saiki 1, Tsutomu Nishida 1, Masashi
In its October 5, 2015, draft recommendation (draft
 USPSTF Colorectal Cancer Screening Guidelines: An Extended Look at Multi-Year Interval Testing Barry M. Berger, MD, FCAP; Marcus A. Parton, SB; and Bernard Levin, MD, FACP Managed Care & Healthcare Communications,
USPSTF Colorectal Cancer Screening Guidelines: An Extended Look at Multi-Year Interval Testing Barry M. Berger, MD, FCAP; Marcus A. Parton, SB; and Bernard Levin, MD, FACP Managed Care & Healthcare Communications,
Colorectal Cancer Screening: Cost-Effectiveness and Adverse events October, 2005
 Colorectal Cancer Screening: Cost-Effectiveness and Adverse events October, 2005 David Lieberman MD Chief, Division of Gastroenterology Oregon Health and Science University Portland VAMC Portland, Oregon
Colorectal Cancer Screening: Cost-Effectiveness and Adverse events October, 2005 David Lieberman MD Chief, Division of Gastroenterology Oregon Health and Science University Portland VAMC Portland, Oregon
Sarvenaz Moosavi, 1 Robert Enns, 1 Laura Gentile, 2 Lovedeep Gondara, 2 Colleen McGahan, 2 and Jennifer Telford Introduction
 Canadian Gastroenterology and Hepatology Volume 2016, Article ID 5914048, 5 pages http://dx.doi.org/10.1155/2016/5914048 Research Article Comparison of One versus Two Fecal Immunochemical Tests in the
Canadian Gastroenterology and Hepatology Volume 2016, Article ID 5914048, 5 pages http://dx.doi.org/10.1155/2016/5914048 Research Article Comparison of One versus Two Fecal Immunochemical Tests in the
2. Describe pros/cons of screening interventions (including colonoscopy, CT colography, fecal tests)
 Learning Objectives 1. Review principles of colon adenoma/cancer biology that permit successful prevention regimes 2. Describe pros/cons of screening interventions (including colonoscopy, CT colography,
Learning Objectives 1. Review principles of colon adenoma/cancer biology that permit successful prevention regimes 2. Describe pros/cons of screening interventions (including colonoscopy, CT colography,
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening
 Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication
 UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
UvA-DARE (Digital Academic Repository) Serrated polyps of the colon and rectum Hazewinkel, Y. Link to publication Citation for published version (APA): Hazewinkel, Y. (2014). Serrated polyps of the colon
Evolving Issues in Colonoscopy. May 19, This 3rd part of the lectures today will be presented by: Stanley H. Weiss, MD, FACP, FACE
 Evolving Issues in Colonoscopy May 19, 2011 This 3rd part of the lectures today will be presented by: Stanley H. Weiss, MD, FACP, FACE Professor, Preventive Medicine & Community Health, UMDNJ NJMS Professor,
Evolving Issues in Colonoscopy May 19, 2011 This 3rd part of the lectures today will be presented by: Stanley H. Weiss, MD, FACP, FACE Professor, Preventive Medicine & Community Health, UMDNJ NJMS Professor,
Inflammatory bowel disease (IBD) patients have a higher risk of
 Serrated Colorectal Lesions in Patients With Inflammatory Bowel Disease Alyssa M. Parian, MD, and Mark G. Lazarev, MD Dr Parian and Dr Lazarev are assistant professors of medicine at Johns Hopkins University
Serrated Colorectal Lesions in Patients With Inflammatory Bowel Disease Alyssa M. Parian, MD, and Mark G. Lazarev, MD Dr Parian and Dr Lazarev are assistant professors of medicine at Johns Hopkins University
Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER
 Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer
 Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer Douglas K. Rex, MD, MACG 1, C. Richard Boland, MD 2, Jason A. Dominitz,
EARLY DETECTION OF COLORECTAL CANCER. Epidemiology of CRC
 Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Razvan I. Arsenescu, MD Assistant Professor of Medicine Division of Digestive Diseases EARLY DETECTION OF COLORECTAL CANCER Epidemiology of CRC Colorectal cancer (CRC) is a common and lethal disease Environmental
Benchmarking For Colonoscopy. Technology and Technique to Improve Adenoma Detection
 Benchmarking For Colonoscopy Technology and Technique to Improve Adenoma Detection Objectives 1. Review the latest data on performance characteristics and efficacy for colon cancer prevention 2. Highlight
Benchmarking For Colonoscopy Technology and Technique to Improve Adenoma Detection Objectives 1. Review the latest data on performance characteristics and efficacy for colon cancer prevention 2. Highlight
Joint Session with ACOFP and Cancer Treatment Centers of America (CTCA): Cancer Screening: Consensus & Controversies. Ashish Sangal, M.D.
 Joint Session with ACOFP and Cancer Treatment Centers of America (CTCA): Cancer Screening: Consensus & Controversies Ashish Sangal, M.D. Cancer Screening: Consensus & Controversies Ashish Sangal, MD Director,
Joint Session with ACOFP and Cancer Treatment Centers of America (CTCA): Cancer Screening: Consensus & Controversies Ashish Sangal, M.D. Cancer Screening: Consensus & Controversies Ashish Sangal, MD Director,
GIQIC18 Appropriate follow-up interval of not less than 5 years for colonoscopies with findings of 1-2 tubular adenomas < 10 mm
 GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
GI Quality Improvement Consortium, Ltd. (GIQuIC) 1 Following is an overview of the clinical quality measures in GIQuIC that can be reported to CMS for the Quality performance category of the Merit-Based
Endoscopic Corner CASE 1. Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R
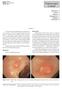 170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
170 Endoscopic Corner Kimtrakool S Aniwan S Linlawan S Muangpaisarn P Sallapant S Rerknimitr R CASE 1 A 54-year-old woman underwent a colorectal cancer screening. Her fecal immunochemical test was positive.
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE
 SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
SCREENING FOR BOWEL CANCER USING FLEXIBLE SIGMOIDOSCOPY REVIEW APPRAISAL CRITERIA FOR THE UK NATIONAL SCREENING COMMITTEE The Condition 1. The condition should be an important health problem Colorectal
Large Colorectal Adenomas An Approach to Pathologic Evaluation
 Anatomic Pathology / LARGE COLORECTAL ADENOMAS AND PATHOLOGIC EVALUATION Large Colorectal Adenomas An Approach to Pathologic Evaluation Elizabeth D. Euscher, MD, 1 Theodore H. Niemann, MD, 1 Joel G. Lucas,
Anatomic Pathology / LARGE COLORECTAL ADENOMAS AND PATHOLOGIC EVALUATION Large Colorectal Adenomas An Approach to Pathologic Evaluation Elizabeth D. Euscher, MD, 1 Theodore H. Niemann, MD, 1 Joel G. Lucas,
Rx Only. Detecting Cancer In Blood.
 Epi procolon is an FDA-approved blood test for colorectal cancer screening for patients who are unwilling or unable to be screened by recommended methods. Rx Only Intended Use, Contraindications, Warnings,
Epi procolon is an FDA-approved blood test for colorectal cancer screening for patients who are unwilling or unable to be screened by recommended methods. Rx Only Intended Use, Contraindications, Warnings,
Sequential screening in the early diagnosis of colorectal cancer in the community
 Journal of Public Health: From Theory to Practice https://doi.org/10.1007/s10389-019-01024-0 ORIGINAL ARTICLE Sequential screening in the early diagnosis of colorectal cancer in the community Ming-sheng
Journal of Public Health: From Theory to Practice https://doi.org/10.1007/s10389-019-01024-0 ORIGINAL ARTICLE Sequential screening in the early diagnosis of colorectal cancer in the community Ming-sheng
ACG Clinical Guideline: Colorectal Cancer Screening
 ACG Clinical Guideline: Colorectal Cancer Screening Douglas K. Rex, MD, FACG 1, David A. Johnson, MD, FACG 2, Joseph C. Anderson, MD 3, Phillip S. Schoenfeld, MD, MSEd, MSc (Epi), FACG 4, Carol A. Burke,
ACG Clinical Guideline: Colorectal Cancer Screening Douglas K. Rex, MD, FACG 1, David A. Johnson, MD, FACG 2, Joseph C. Anderson, MD 3, Phillip S. Schoenfeld, MD, MSEd, MSc (Epi), FACG 4, Carol A. Burke,
Determining the adenoma detection rate and adenomas per colonoscopy by photography alone: proof-of-concept study
 Original article 245 Determining the adenoma detection rate and adenomas per colonoscopy by photography alone: proof-of-concept study Authors Institution Douglas K. Rex, Kyle Hardacker, Margaret MacPhail,
Original article 245 Determining the adenoma detection rate and adenomas per colonoscopy by photography alone: proof-of-concept study Authors Institution Douglas K. Rex, Kyle Hardacker, Margaret MacPhail,
Quality in Endoscopy: Can We Do Better?
 Quality in Endoscopy: Can We Do Better? Erik Rahimi, MD Assistant Professor Division of Gastroenterology, Hepatology, and Nutrition UT Health Science Center at Houston McGovern Medical School Ertan Digestive
Quality in Endoscopy: Can We Do Better? Erik Rahimi, MD Assistant Professor Division of Gastroenterology, Hepatology, and Nutrition UT Health Science Center at Houston McGovern Medical School Ertan Digestive
Colorectal Cancer Screening and Surveillance
 Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0148 Colorectal Cancer Screening and Surveillance Table of Contents Related Coverage Resources
Medical Coverage Policy Effective Date...10/15/2017 Next Review Date...10/15/2018 Coverage Policy Number... 0148 Colorectal Cancer Screening and Surveillance Table of Contents Related Coverage Resources
Screening for Colorectal Cancer
 clinical practice Screening for Colorectal Cancer David A. Lieberman, M.D. This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies
clinical practice Screening for Colorectal Cancer David A. Lieberman, M.D. This Journal feature begins with a case vignette highlighting a common clinical problem. Evidence supporting various strategies
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Colorectal cancer screening
 26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
26 Colorectal cancer screening BETHAN GRAF AND JOHN MARTIN Colorectal cancer is theoretically a preventable disease and is ideally suited to a population screening programme, as there is a long premalignant
Sessile Serrated Polyps: An Important Route to Colorectal Cancer
 Focused Review 1585 Sessile Serrated Polyps: An Important Route to Colorectal Cancer Matthew F. Kalady, MD Abstract The serrated neoplastic pathway accounts for approximately 30% of colorectal cancers.
Focused Review 1585 Sessile Serrated Polyps: An Important Route to Colorectal Cancer Matthew F. Kalady, MD Abstract The serrated neoplastic pathway accounts for approximately 30% of colorectal cancers.
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING
 CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
Background: The value of narrow band imaging (NBI) for detecting serrated lesions is
 Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial Douglas K. Rex, Ryan Clodfelter, Farrah Rahmani, Hala Fatima, Toyia N. James-Stevenson,
Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial Douglas K. Rex, Ryan Clodfelter, Farrah Rahmani, Hala Fatima, Toyia N. James-Stevenson,
Surveying the Colon; Polyps and Advances in Polypectomy
 Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Surveying the Colon; Polyps and Advances in Polypectomy Educational Objectives Identify classifications of polyps Describe several types of polyps Verbalize rationale for polypectomy Identify risk factors
Molecular features of colorectal polyps presenting Kudo s type II mucosal crypt pattern: are they based on the same mechanism of tumorigenesis?
 E171 Molecular features of colorectal polyps presenting Kudo s type II mucosal crypt pattern: are they based on the same mechanism of tumorigenesis? Authors Kensuke Shinmura 1, Kazuo Konishi 1, Toshiko
E171 Molecular features of colorectal polyps presenting Kudo s type II mucosal crypt pattern: are they based on the same mechanism of tumorigenesis? Authors Kensuke Shinmura 1, Kazuo Konishi 1, Toshiko
FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test
 FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test LIMBACH-LABORATORY H E I D E L B E R G H J Roth H Schmidt-Gayk Estimated incidence of cancer in Europe and European Union, 2006 Limbach
FECAL OCCULT BLOOD TEST (FOBT) Common Guaiac versus Immunochemical Test LIMBACH-LABORATORY H E I D E L B E R G H J Roth H Schmidt-Gayk Estimated incidence of cancer in Europe and European Union, 2006 Limbach
Structured Follow-Up after Colorectal Cancer Resection: Overrated. R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007
 Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Structured Follow-Up after Colorectal Cancer Resection: Overrated R. Taylor Ripley University of Colorado Grand Rounds April 23, 2007 Guidelines for Colonoscopy Production: Surveillance US Multi-Society
Serrated Colorectal Polyps New Challenges to Old Dogma. Kenneth Batts, M.D. Abbott Northwestern Hospital Minneapolis, MN
 Serrated Colorectal Polyps New Challenges to Old Dogma Kenneth Batts, M.D. Abbott Northwestern Hospital Minneapolis, MN A Sneak Preview.... This was in the good old days: Adenomas HPPs Mixed Polyps A Sneak
Serrated Colorectal Polyps New Challenges to Old Dogma Kenneth Batts, M.D. Abbott Northwestern Hospital Minneapolis, MN A Sneak Preview.... This was in the good old days: Adenomas HPPs Mixed Polyps A Sneak
