Page 1 of 5. Fast Facts. CTC v.4; AJCC 7 th ed. Herceptin provided
|
|
|
- Ethelbert Sharp
- 5 years ago
- Views:
Transcription
1 Page 1 f 5 NSABP B-47 - A Randmized Phase III Trial f Adjuvant Therapy Cmparing Chemtherapy Alne (Six Cycles f Dcetaxel Plus Cyclphsphamide r Fur Cycles f Dxrubicin Plus Cyclphsphamide Fllwed by Weekly Paclitaxel) t Chemtherapy Plus Trastuzumab in Wmen with Nde-Psitive r High-Risk Nde- Negative HER2-Lw Invasive Breast Cancer CTC v.4; AJCC 7 th ed. Herceptin prvided Fast Facts Althugh the guidelines belw are nt inclusin/exclusin criteria, investigatrs shuld cnsider each f these factrs when selecting patients fr the trial. Investigatrs shuld als cnsider all ther relevant factrs (medical and nn-medical), as well as the risks and benefits f the study therapy, when deciding if a patient is an apprpriate candidate fr this trial. Patients shuld have a life expectancy f at least 10 years, excluding their diagnsis f breast cancer. (Cmrbid cnditins shuld be taken int cnsideratin, but nt the diagnsis f breast cancer.) Wmen f reprductive ptential must agree t use an effective nn-hrmnal methd f cntraceptin (fr example cndms, sme intrauterine devices, diaphragms, tubal ligatin, vasectmized partner, r abstinence) during therapy and fr at least 6 mnths after the last dse f study therapy (chemtherapy r trastuzumab). Submissin f tumr samples frm the breast surgery is required fr all patients (see Sectin 7.1). Therefre, the lcal pathlgy department plicy regarding release f tumr samples must be cnsidered in the screening prcess. Patients whse tumr samples are lcated in a pathlgy department that by plicy will nt submit any samples fr research purpses shuld nt be apprached fr participatin in the B-47 trial. Patient eligibility 1. The patient must have signed and dated an IRB-apprved cnsent frm that cnfrms t federal and institutinal guidelines. 2. The patient must be female. 3. The patient must be 18 years ld. 4. The patient must have an ECOG perfrmance status f 0 r 1 (see Appendix A). 5. The tumr must be unilateral invasive adencarcinma f the breast n histlgic examinatin. 6. All f the fllwing staging criteria (using 7th editin f the AJCC Cancer Staging Manual) must be met: By pathlgic evaluatin, primary tumr must be pt1-3; By pathlgic evaluatin, ipsilateral ndes must be pn0, pn1 (pn1mi, pn1a, pn1b, pn1c), pn2a, pn2b, pn3a, r pn3b If pn0, ne f the fllwing criteria must be met: pt2 and ER negative and PgR negative; r pt2 and ER psitive (PgR status may be psitive r negative) and either grade 3 histlgy r Onctype DX Recurrence Scre f 25; r pt3 regardless f hrmne receptr status, histlgic grade, and Onctype DX 7. HER2 status f the primary tumr must be evaluated prir t randmizatin; all testing perfrmed must indicate that the tumr is HER2-lw as defined belw. IHC must be perfrmed and the IHC staining results must indicate a scre f 1+ (in situ hybridizatin [ISH] testing is nt required) r 2+ (ISH must als be perfrmed and must indicate that the tumr is HER2-lw as described belw). if ISH testing is perfrmed, test results must be as fllws and IHC must be 1+ r 2+: The rati f HER2 t CEP17 must be < 2.0 r, if a rati was nt perfrmed, the HER2 gene cpy number must be < 4 per nucleus. Nte: If the IHC staining intensity is reprted as a range, e.g., 0 t 1+ r 1+ t 2+, the higher intensity scre in the range shuld be used t determine eligibility.the patient must have undergne either a ttal mastectmy r breast-cnserving surgery (lumpectmy). (Patients wh have had a nipple-sparing mastectmy are eligible.) 8. Fr patients wh underg lumpectmy, the margins f the resected specimen must be histlgically free f invasive tumr and DCIS as determined by the lcal pathlgist. If pathlgic examinatin demnstrates tumr at the line f resectin, additinal perative prcedures may be perfrmed t btain clear margins. If tumr is still present at the resected margin after re-excisin(s), the patient must underg ttal mastectmy t be eligible. (Patients with margins psitive fr LCIS are eligible withut additinal resectin.) 9. Fr patients wh underg mastectmy, margins must be free f grss residual tumr. (Patients with micrscpic psitive margins are eligible as lng as pst-mastectmy RT f the chest wall will be administered.) 10. The patient must have cmpleted ne f the prcedures fr evaluatin f pathlgic ndal status listed belw. Sentinel lymphadenectmy alne: If pathlgic ndal staging based n sentinel lymphadenectmy is pn0 r pn1b; If pathlgic ndal staging based n sentinel lymphadenectmy is pn1mi r pn1a, the primary tumr must be T1 r T2 by pathlgic evaluatin and the ndal invlvement must be limited t 1 r 2 psitive ndes.
2 Page 2 f 5 Sentinel lymphadenectmy fllwed by remval f additinal nn-sentinel lymph ndes if the sentinel nde (SN) is psitive; r Axillary lymphadenectmy with r withut SN islatin prcedure. 11. The interval between the last surgery fr breast cancer (treatment r staging) and randmizatin must be n mre than 84 days. 12. The patient must have ER analysis perfrmed n the primary tumr prir t randmizatin. If ER analysis is negative, then PgR analysis must als be perfrmed. (Either the cre bipsy r surgical resectin specimen can be used fr ER/PgR testing.) Patients with a primary tumr that is hrmne receptr-psitive r receptr-negative are eligible. 13. The mst recent pstperative bld cunts, perfrmed within 6 weeks prir t randmizatin, must meet the fllwing criteria: ANC must be 1200/mm 3 ; Platelet cunt must be 100,000/mm 3 ; and Hemglbin must be 10 g/dl. 14. The fllwing criteria fr evidence f adequate hepatic functin must be met based n the results f the mst recent pstperative tests perfrmed within 6 weeks prir t randmizatin: ttal bilirubin must be ULN fr the lab unless the patient has a bilirubin elevatin > ULN t 1.5 x ULN due t Gilbert s disease r similar syndrme invlving slw cnjugatin f bilirubin; and alkaline phsphatase must be 2.5 x ULN fr the lab; and AST must be 1.5 x ULN fr the lab. Alkaline phsphatase and AST may nt bth be > the ULN. Fr example, if the alkaline phsphatase is > the ULN but 2.5 x ULN, the AST must be the ULN. If the AST is > the ULN but 1.5 x ULN, the alkaline phsphatase must be ULN. Nte: If ALT is perfrmed instead f AST (per institutin's standard practice), the ALT value must be 1.5 x ULN; if bth were perfrmed, the AST must be 1.5 x ULN. 15. Patients with AST r alkaline phsphatase > ULN are eligible fr inclusin in the study if liver imaging (CT, MRI, PET-CT, r PET scan) perfrmed within 90 days prir t randmizatin des nt demnstrate metastatic disease and the requirements in criterin are met. 16. Patients with alkaline phsphatase that is > ULN but 2.5 x ULN r unexplained bne pain are eligible fr inclusin in the study if a bne scan, PET-CT scan, r PET scan perfrmed within 90 days prir t randmizatin des nt demnstrate metastatic disease. 17. The mst recent pstperative serum creatinine perfrmed within 6 weeks prir t randmizatin must be ULN fr the lab. 18. LVEF assessment must be perfrmed within 90 days prir t randmizatin. LVEF assessment perfrmed by 2-D echcardigram is preferred; hwever, MUGA scan may be substituted based n institutinal preferences. Fr patients wh will receive the TC chemtherapy regimen, the LVEF must be 50% regardless f the cardiac imaging facility's lwer limit f nrmal. Fr patients wh will receive the AC WP chemtherapy regimen, the LVEF must be 55% regardless f the cardiac imaging facility's lwer limit f nrmal. Nte: Since the pre-entry LVEF serves as the baseline fr cmparing subsequent LVEF assessments, it is critical that this baseline study be an accurate assessment. If the baseline LVEF is > 70%, the investigatr is encuraged t have the accuracy f the initial LVEF result cnfirmed and repeat the test if the accuracy is uncertain. (See Sectins 5.2 and 5.3 fr LVEF instructins.) Patient ineligibility 1. Primary tumr with any f the fllwing HER2 testing results: IHC staining intensity: - 0 n all evaluatins f specimen - 3+ n evaluatins f any specimen ISH with a rati f HER2 f CEP n evaluatin f any specimen ISH result indicating HER2 gene cpy number 4 per nucleus n evaluatin f any specimen 2. T4 tumrs including inflammatry breast cancer. 3. Definitive clinical r radilgic evidence f metastatic disease. (Nte: Chest imaging [mandatry fr all patients] and ther imaging [if required] must have been perfrmed within 90 days prir t randmizatin.) 4. Synchrnus r previus cntralateral invasive breast cancer. (Patients with synchrnus and/r previus cntralateral DCIS r LCIS are eligible.) 5. Any previus histry f ipsilateral invasive breast cancer r ipsilateral DCIS. (Patients with synchrnus r previus ipsilateral LCIS are eligible.) 6. Histry f nn-breast malignancies (except fr in situ cancers treated nly by lcal excisin and basal cell and squamus cell carcinmas f the skin) within 5 years prir t randmizatin. 7. Previus therapy with anthracyclines, taxanes, r trastuzumab fr any malignancy. 8. Chemtherapy r HER2-targeted therapy administered fr the currently diagnsed breast cancer prir t randmizatin.
3 Page 3 f 5 9. Whle breast RT prir t randmizatin r partial breast RT that cannt be cmpleted n r befre the date f randmizatin (see Sectins 9.8 and ). 10. Cntinued endcrine therapy such as ralxifene r tamxifen (r ther SERM) r an armatase inhibitr. Patients are eligible if these medicatins are discntinued prir t randmizatin (see Sectin 9.9). 11. Any cntinued use f sex hrmnal therapy, e.g., birth cntrl pills, varian hrmne replacement therapy. Patients are eligible if these medicatins are discntinued prir t randmizatin (see Sectin 4.1). 12. Cardiac disease (histry f and/r active disease) that wuld preclude the use f the drugs included in the treatment regimens. This includes but is nt cnfined t: Active cardiac disease angina pectris that requires the current use f anti-anginal medicatin; ventricular arrhythmias except fr benign premature ventricular cntractins; supraventricular and ndal arrhythmias requiring a pacemaker r nt cntrlled with medicatin; cnductin abnrmality requiring a pacemaker; valvular disease with dcumented cmprmise in cardiac functin; and symptmatic pericarditis. Histry f cardiac disease mycardial infarctin dcumented by elevated cardiac enzymes r persistent reginal wall abnrmalities n assessment f LV functin; histry f dcumented CHF; and dcumented cardimypathy. 13. Hypertensin defined accrding t the fllwing ineligibility criteria: Fr patients wh will receive TC (regardless f the patient's age): Uncntrlled hypertensin defined as sustained systlic BP > 150 mmhg r diastlic BP > 90 mmhg. (Patients with initial BP elevatins are eligible if initiatin r adjustment f BP medicatin lwers pressure t meet entry criteria.) Fr patients < 50 years ld wh will receive AC WP: Uncntrlled hypertensin defined as sustained systlic BP > 150 mmhg r diastlic BP > 90 mmhg. Patients with initial BP elevatins are eligible if initiatin r adjustment f BP medicatin lwers pressure t meet entry criteria. Fr patients 50 years ld wh will receive AC WP: Uncntrlled hypertensin defined as sustained systlic BP > 150 mmhg r diastlic BP > 90 mmhg. Cntrlled hypertensin (systlic BP 150 mmhg and diastlic BP 90 mmhg), if anti-hypertensive medicatin(s) are needed. Nte: Patients wh are nt eligible based n the AC WP regimen BP criteria but wh meet the TC regimen BP criteria are eligible fr B-47, if the intended chemtherapy regimen is changed t TC. 14. Active hepatitis B r hepatitis C with abnrmal liver functin tests. 15. Intrinsic lung disease resulting in dyspnea. 16. Prly cntrlled diabetes mellitus. 17. Active infectin r chrnic infectin requiring chrnic suppressive antibitics. 18. Nervus system disrder (paresthesia, peripheral mtr neurpathy, r peripheral sensry neurpathy) grade 2, per the CTCAE v Cnditins that wuld prhibit administratin f crticsterids. 20. Chrnic daily treatment with crticsterids with a dse f 10 mg/day methylprednislne equivalent (excluding inhaled sterids). 21. Knwn hypersensitivity t any f the study drugs r excipients, e.g., plysrbate 80 and Cremphr EL. 22. Pregnancy r lactatin at the time f study entry. (Nte: Pregnancy testing must be perfrmed within 2 weeks prir t randmizatin accrding t institutinal standards fr wmen f childbearing ptential.) 23. Other nn-malignant systemic disease that wuld preclude the patient frm receiving study treatment r wuld prevent required fllw-up. 24. Psychiatric r addictive disrders r ther cnditins that, in the pinin f the investigatr, wuld preclude the patient frm meeting the study requirements. 25. Use f any investigatinal prduct within 30 days prir t randmizatin. Pre-study Parameters 1. Histry and physical including height, weight, perfrmance status, menstrual histry, menpausal status, BP, cncmitant meds assessment including BP meds 2. CBC with differential, AST, Alk Phs, ttal bilirubin, serum creatinine, pregnancy test fr wmen f child-bearing ptential 3. Ech (preferred) r MUGA, ECG 4. Chest imaging (CT r x-ray) (PET r PET/CT may be substituted); Liver imaging if AST > ULN; Bne nuclear imaging if Alk Phs > ULN
4 Page 4 f 5 5. Bilateral breast imaging (mammgram r MRI)
5 Page 5 f 5
NCI Version Date: (194) NSABP B-55/BIG 6-13
 Figure 1 Study Flw Chart ICF fr patients with unknwn BRCA status t underg central BRCA testing during, r prir t, neadjuvant/adjuvant chemtherapy Neadjuvant chemtherapy Minimum 6 cycles (cntaining anthracyclines,
Figure 1 Study Flw Chart ICF fr patients with unknwn BRCA status t underg central BRCA testing during, r prir t, neadjuvant/adjuvant chemtherapy Neadjuvant chemtherapy Minimum 6 cycles (cntaining anthracyclines,
ACRIN 6666 Screening Breast US Follow-up Assessment Form
 Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
WARNING: FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
 INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
BRCA1 and BRCA2 Mutations
 BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
PBTC-026: A Feasibility Study of SAHA Combined with Isotretinoin and Chemotherapy in Infants with Embryonal Tumors of the Central Nervous System
 PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
XX Abraxane 100 MG SUSR (CELGENE CORP)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING. Public Health Relevance. Highlights.
 HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Background 1. Definition Fibroadenoma: Group of hyperplastic breast lobules composed of stromal and epithelial elements
 Fibradenma Backgrund 1. Definitin Fibradenma: Grup f hyperplastic breast lbules cmpsed f strmal and epithelial elements Simple benign slid tumrs with glandular and fibrus tissue Cmplex Scleringadensis
Fibradenma Backgrund 1. Definitin Fibradenma: Grup f hyperplastic breast lbules cmpsed f strmal and epithelial elements Simple benign slid tumrs with glandular and fibrus tissue Cmplex Scleringadensis
XX Abraxane 100 MG SUSR (CELGENE CORP
 Medical Manual Apprved Revised: D Nt Implement until 6/30/2019 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP DESCRIPTION Paclitaxel is a natural prduct with antitumr
Medical Manual Apprved Revised: D Nt Implement until 6/30/2019 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP DESCRIPTION Paclitaxel is a natural prduct with antitumr
NPCR CLINICAL EDIT CHECKS
 NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
Rituxan (rituximab) Effective Date: 10/01/2015. Line(s) of Business: HMO; PPO; QUEST Integration Akamai Advantage
 Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
Rituxan (rituximab) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Effective Date: 10/01/2015 POLICY A. INDICATIONS The indicatins belw including FDA-apprved indicatins and cmpendial uses
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
Section 5. Study Procedures
 Sectin 5. Study Prcedures 5.1 Visit Lcatins... 1 5.2 Eligibility Determinatin... 1 5.3 Screening Visit... 2 5.3.1 Screening and Enrllment Timeframe... 2 5.3.2 Screening Visit Prcedures... 2 5.3.3 Screening
Sectin 5. Study Prcedures 5.1 Visit Lcatins... 1 5.2 Eligibility Determinatin... 1 5.3 Screening Visit... 2 5.3.1 Screening and Enrllment Timeframe... 2 5.3.2 Screening Visit Prcedures... 2 5.3.3 Screening
Continuous Quality Improvement: Treatment Record Reviews. Third Thursday Provider Call (August 20, 2015) Wendy Bowlin, QM Administrator
 Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
High Performance Network Quality Criteria for Designation
 Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
Selected quality measures include: Specialty Measure Descriptin Allergy / Immunlgy Asthma Drug Mgt Vaccine Pneumnia Vaccine High Perfrmance Netwrk Quality Criteria fr Designatin AvMed has selected certain
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
BP Thresholds for Medical Review
 BP Threshlds fr Medical Review Wmen presents t GP pstnatally with high bld pressure r referred t GP by midwife GP t review patient n the same day if BP>150/100. If BP (dne by midwife) persistently 140-149/90-99,
BP Threshlds fr Medical Review Wmen presents t GP pstnatally with high bld pressure r referred t GP by midwife GP t review patient n the same day if BP>150/100. If BP (dne by midwife) persistently 140-149/90-99,
Continuous Positive Airway Pressure (CPAP) and Respiratory Assist Devices (RADs) including Bi-Level PAP
 Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Request for Prior Authorization for Click here to enter text. Website Form Submit request via: Fax
 Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT
 SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG 6 II PRÉVENTION A Randmized Phase II Duble-Blind Placeb-Cntrlled Trials f Acetylsalicylic
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG 6 II PRÉVENTION A Randmized Phase II Duble-Blind Placeb-Cntrlled Trials f Acetylsalicylic
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION
 CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
Q&A. Collecting Cancer Data: Breast. Collecting Cancer Data: Breast 4/7/2011. NAACCR Webinar Series 1. Agenda
 Cllecting Cancer Data: Breast April 7, 2011 NAACCR 2010-2011 Webinar Series 1 Q&A Please submit all questins cncerning webinar cntent thrugh the Q&A panel 2 Agenda Overview Primary Site Reginal Lymph Ndes
Cllecting Cancer Data: Breast April 7, 2011 NAACCR 2010-2011 Webinar Series 1 Q&A Please submit all questins cncerning webinar cntent thrugh the Q&A panel 2 Agenda Overview Primary Site Reginal Lymph Ndes
ITP typically presents with the sudden appearance of a petechial rash, spontaneous bruising and/or bleeding in an otherwise well child.
 Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Measure Specific Guidelines for Comprehensive Diabetes Care (CDC)
 Measure Specific Guidelines fr Cmprehensive Diabetes Care (CDC) Descriptin: Members age 18-75 years f age with diabetes (Type 1 and Type 2)* that had all f the fllwing: *Members in hspice are excluded
Measure Specific Guidelines fr Cmprehensive Diabetes Care (CDC) Descriptin: Members age 18-75 years f age with diabetes (Type 1 and Type 2)* that had all f the fllwing: *Members in hspice are excluded
Coronary Artery Disease (CAD): Beta Blocker Therapy for CAD Patients with Prior Myocardial Infarction (MI) (NQF 0070)
 Crnary Artery Disease (CAD): Beta Blcker Therapy fr CAD Patients with Prir Mycardial Infarctin (MI) (NQF 0070) EMeasure Name Crnary Artery Disease EMeasure Id Pending (CAD): Beta Blcker Therapy fr CAD
Crnary Artery Disease (CAD): Beta Blcker Therapy fr CAD Patients with Prir Mycardial Infarctin (MI) (NQF 0070) EMeasure Name Crnary Artery Disease EMeasure Id Pending (CAD): Beta Blcker Therapy fr CAD
Protocol Abstract and Schema
 NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
Significance of Chronic Kidney Disease in 2015
 1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
Q 5: Is relaxation training better (more effective than/as safe as) than treatment as usual in adults with depressive episode/disorder?
 updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
Policy Guidelines: Genetic Testing for Carrier Screening and Reproductive Planning
 Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Plicy Guidelines: Genetic Testing fr Carrier Screening and Reprductive Planning Cntents Overview... 1 Cverage guidelines... 2 General cverage guidelines... 2 Rutine carrier screening... 2 Carrier screening
Four categories which guide further evaluation
 Unknwn Primary Updated May 2017 by Di Maria Jiang (PGY-5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Chistine Elser (Staff Medical Onclgist, University f Trnt) and Dr. Sct Dwden (Staff
Unknwn Primary Updated May 2017 by Di Maria Jiang (PGY-5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Chistine Elser (Staff Medical Onclgist, University f Trnt) and Dr. Sct Dwden (Staff
Ontario s Referral and Listing Criteria for Adult Lung Transplantation
 Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Ontari s Referral and Listing Criteria fr Adult Lung Transplantatin Versin 2.0 Trillium Gift f Life Netwrk Adult Lung Transplantatin Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The patient referral
Cambridge Breast Unit Protocols for anticoagulant management prior to breast or axillary biopsies or excisions.
 Prtcl CBU/POL/ JOINT POLICIES/008 Octber 2017 Prtcls fr anticagulant management prir t breast r axillary bipsies r excisins. 1. Scpe Fr use in the in bth screening and symptmatic breast services. 2. Purpse
Prtcl CBU/POL/ JOINT POLICIES/008 Octber 2017 Prtcls fr anticagulant management prir t breast r axillary bipsies r excisins. 1. Scpe Fr use in the in bth screening and symptmatic breast services. 2. Purpse
CHEMOPREVENTION in BREAST CANCER
 CHEMOPREVENTION in BREAST CANCER Ozlem Er, M.D. Assc Prf f Medical Onclgy ESMO Curse Essentials f Medical Onclgy Outline Risk Assessment Mlecular Targets ER psitive Breast Cancer Tamxifen Ralxifene Lasfxifene
CHEMOPREVENTION in BREAST CANCER Ozlem Er, M.D. Assc Prf f Medical Onclgy ESMO Curse Essentials f Medical Onclgy Outline Risk Assessment Mlecular Targets ER psitive Breast Cancer Tamxifen Ralxifene Lasfxifene
Referral Criteria: Inflammation of the Spine Feb
 Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
A Phase I Study of CEP-701 in Patients with Refractory Neuroblastoma NANT (01-03) A New Approaches to Neuroblastoma Therapy (NANT) treatment protocol.
 SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
TOP TIPS Lung Cancer Update Dr Andrew Wight Consultant respiratory Physician - WUTH
 Tpic Circulatin list In case f query please cntact Executive Summary TOP TIPS Lung Cancer Update Dr Andrew Wight Cnsultant respiratry Physician - WUTH All Wirral GP s JaneFletcher2@nhs.net Dear Clleagues,
Tpic Circulatin list In case f query please cntact Executive Summary TOP TIPS Lung Cancer Update Dr Andrew Wight Cnsultant respiratry Physician - WUTH All Wirral GP s JaneFletcher2@nhs.net Dear Clleagues,
23/11/2015. Introduction & Aims. Methods. Methods. Survey response. Patient Survey (baseline)
 Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
Nonclinical factors associated with premature termination of adjuvant chemotherapy for stage I-III breast cancer
 Nnclinical factrs assciated with premature terminatin f adjuvant chemtherapy fr stage I-III breast cancer Xia-Cheng Wu, Trevr Thmpsn, Meichin Hsieh, Meijia Zhu, Patricia Andrews, Michelle Lch, Timthy Styles,
Nnclinical factrs assciated with premature terminatin f adjuvant chemtherapy fr stage I-III breast cancer Xia-Cheng Wu, Trevr Thmpsn, Meichin Hsieh, Meijia Zhu, Patricia Andrews, Michelle Lch, Timthy Styles,
NCT ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: PRIST_L_ Study Code: PRISTINAMYCIN Date: Generic drug name:
 These results are supplied fr infrmatinal purpses nly. Prescribing decisins shuld be made based n the apprved package insert in the cuntry f prescriptin Spnsr/cmpany: sanfi-aventis ClinialTrials.gv Identifier:
These results are supplied fr infrmatinal purpses nly. Prescribing decisins shuld be made based n the apprved package insert in the cuntry f prescriptin Spnsr/cmpany: sanfi-aventis ClinialTrials.gv Identifier:
Study Design Open, three arm-stratified, non-randomized, prospective, multicentric study
 PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
BREAST CANCER Updated June 2016 by Dr. Nixon (PGY-5 Medical Oncology Resident, University of Calgary)
 BREAST CANCER Updated June 2016 by Dr. Nixn (PGY-5 Medical Onclgy Resident, University f Calgary) Reviewed by Dr. Jan-Willem Henning (Staff Medical Onclgist, Tm Baker Cancer Centre, University f Calgary),
BREAST CANCER Updated June 2016 by Dr. Nixn (PGY-5 Medical Onclgy Resident, University f Calgary) Reviewed by Dr. Jan-Willem Henning (Staff Medical Onclgist, Tm Baker Cancer Centre, University f Calgary),
Perjeta (pertuzumab) Document Number: IC I. Length of Authorization. Dosing Limits. Initial Approval Criteria
 Perjeta (pertuzumab) Last Review Date: 11/21/2017 Date f Origin: 11/01/2012 Dcument Number: IC-0096 Dates Reviewed: 12/2012, 3/2013, 6/2013, 9/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014, 3/2015,
Perjeta (pertuzumab) Last Review Date: 11/21/2017 Date f Origin: 11/01/2012 Dcument Number: IC-0096 Dates Reviewed: 12/2012, 3/2013, 6/2013, 9/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014, 3/2015,
2017 Optum, Inc. All rights reserved BH1124_112017
 1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
Guideline Number: NIA_CG_301 Last Revised Date: October 2014 Responsible Department: Implementation Date: October 2014 Clinical Operations
 Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
RANDOMIZED CONTROLLED TRIAL OF LUMBAR TRANSFORAMINAL EPIDURAL STEROID INJECTIONS
 RANDOMIZED CONTROLLED TRIAL OF LUMBAR TRANSFORAMINAL EPIDURAL STEROID INJECTIONS Study Design A blinded randmized cntrlled trial f lumbar transframinal epidural sterid injectins versus intramuscular saline
RANDOMIZED CONTROLLED TRIAL OF LUMBAR TRANSFORAMINAL EPIDURAL STEROID INJECTIONS Study Design A blinded randmized cntrlled trial f lumbar transframinal epidural sterid injectins versus intramuscular saline
Osteoporosis Fast Facts
 Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Triumeq (abacavir, dolutegravir and lamivudine) Product Backgrounder for US Media
 Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Clinical Study Synopsis
 Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH
 GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
HIP REPLACEMENT SURGERY (ARTHROPLASTY)
 Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
Prtcl: ORT015 Effective Date: June 1, 2017 HIP REPLACEMENT SURGERY (ARTHROPLASTY) Table f Cntents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE... 3 U.S.FOOD AND DRUG ADMINISTRATION
Indications and Limitations of Coverage and/or Medical back to top
 Fr services perfrmed n r after 09/15/2009 Original Determinatin Ending Date Revisin Effective Date Revisin Ending Date Indicatins and Limitatins f Cverage and/r Medical Necessity Indicatins Medicare cverage
Fr services perfrmed n r after 09/15/2009 Original Determinatin Ending Date Revisin Effective Date Revisin Ending Date Indicatins and Limitatins f Cverage and/r Medical Necessity Indicatins Medicare cverage
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
WHAT IS HEAD AND NECK CANCER FACT SHEET
 WHAT IS HEAD AND NECK CANCER FACT SHEET This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice
WHAT IS HEAD AND NECK CANCER FACT SHEET This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice
Folotyn (pralatrexate)
 Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
2018 Medical Association Poster Symposium Guidelines
 2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Reference: Patient A. Brenda WXXXXX Date of Birth: 4/15/57
 Reference: Patient A Brenda WXXXXX Date f Birth: 4/15/57 49 year ld white female patient presented n July 20, 2006 with chief cmplaint f stage 4 cancer, initially diagnsed in Octber, 2003 with Cervical
Reference: Patient A Brenda WXXXXX Date f Birth: 4/15/57 49 year ld white female patient presented n July 20, 2006 with chief cmplaint f stage 4 cancer, initially diagnsed in Octber, 2003 with Cervical
New Exception Status Benefits
 FEBRUARY 2019 Nva Sctia Frmulary Updates New Exceptin Status Benefits Prcysbi (cysteamine bitartrate) Nucala (meplizumab) Ocaliva (betichlic acid) Ravicti (glycerl phenylbutyrate) Taltz (ixekizumab) Criteria
FEBRUARY 2019 Nva Sctia Frmulary Updates New Exceptin Status Benefits Prcysbi (cysteamine bitartrate) Nucala (meplizumab) Ocaliva (betichlic acid) Ravicti (glycerl phenylbutyrate) Taltz (ixekizumab) Criteria
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018
 Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Wund Care Equipment and Supply Benefits t Change fr Texas Medicaid July 1, 2018 Infrmatin psted May 11, 2018 Nte: Texas Medicaid managed care rganizatins (MCOs) must prvide all medically necessary, Medicaid-cvered
Annual Principal Investigator Worksheet About Local Context
 Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
Sterilization. B.K. Merritt, MD. Second most common form of birth control after pills. Surgical (tubal ligation or removal)
 Sterilizatin B.K. Merritt, MD Overview Secnd mst cmmn frm f birth cntrl after pills Shuld never be cnsidered reversible Mirena IUD and Nexplann implant are bth reversible and are as effective r even mre
Sterilizatin B.K. Merritt, MD Overview Secnd mst cmmn frm f birth cntrl after pills Shuld never be cnsidered reversible Mirena IUD and Nexplann implant are bth reversible and are as effective r even mre
Field Epidemiology Training Program
 Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
2018 CMS Web Interface
 CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
Cancer of Unknown Primary (CUP) Pathways and Guidelines (v 2) London Cancer. April 2017
 Cancer f Unknwn Primary (CUP) Pathways and Guidelines (v 2) Lndn Cancer April 2017 The fllwing pathways and guidelines dcument has been cmpiled by the Lndn Cancer CUP technical subgrup and agreed by the
Cancer f Unknwn Primary (CUP) Pathways and Guidelines (v 2) Lndn Cancer April 2017 The fllwing pathways and guidelines dcument has been cmpiled by the Lndn Cancer CUP technical subgrup and agreed by the
Imaging tests allow the cancer care team to check for cancer and other problems inside the body.
 IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
IMAGING TESTS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between yu
Prostatitis - chronic - Management
 Prstatitis - chrnic - Management Scenari: Diagnsis f chrnic prstatitis Hw shuld I diagnse chrnic prstatitis? Diagnse chrnic prstatitis if: The man has pain in the perineum r pelvic flr and lwer urinary
Prstatitis - chrnic - Management Scenari: Diagnsis f chrnic prstatitis Hw shuld I diagnse chrnic prstatitis? Diagnse chrnic prstatitis if: The man has pain in the perineum r pelvic flr and lwer urinary
HEDIS. Healthcare Effectiveness Data & Information Set (HEDIS ) QUALITY MANAGEMENT PROGRAM SECTION 8
 HEDIS Healthcare Effectiveness Data & Infrmatin Set (HEDIS ) The HEDIS â audit cntains a cre set f perfrmance measures that prvide infrmatin abut custmer satisfactin, specific health care measures, and
HEDIS Healthcare Effectiveness Data & Infrmatin Set (HEDIS ) The HEDIS â audit cntains a cre set f perfrmance measures that prvide infrmatin abut custmer satisfactin, specific health care measures, and
TRANSPLANTATION AND CLINICAL IMMUNOLOGY. Proceedings of the Twenty-Second International Course, Lyon, May 1990
 -----.---.----~ Reprinted frm: TRANSPLANTATION AND CLINICAL IMMUNOLOGY VOLUME XXII Multiple Transplants Prceedings f the Twenty-Secnd Internatinal Curse, Lyn, 2-23 May 99 This publicatin was made pssible
-----.---.----~ Reprinted frm: TRANSPLANTATION AND CLINICAL IMMUNOLOGY VOLUME XXII Multiple Transplants Prceedings f the Twenty-Secnd Internatinal Curse, Lyn, 2-23 May 99 This publicatin was made pssible
Kadcyla (ado-trastuzumab emtansine) Document Number: IC-0092
 Kadcyla (ad-trastuzumab emtansine) Dcument Number: IC-0092 Last Review Date: 2/6/2018 Date f Origin: 05/16/2013 Dates Reviewed: 7/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014, 5/2015, 8/2015,
Kadcyla (ad-trastuzumab emtansine) Dcument Number: IC-0092 Last Review Date: 2/6/2018 Date f Origin: 05/16/2013 Dates Reviewed: 7/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014, 5/2015, 8/2015,
Male patients with pain, swelling or erythema please refer to the Acute Painful Scrotum pathway Female patients
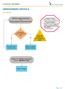 UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
UNDESCENDED TESTICLE ALGORITHM Unilateral undescended testicle OR Bilateral palpable undescended testicle Inclusin Criteria: Male patients w/ unilateral r bilateral undescended testicle(s) Exclusin Criteria:
Chronic Fatigue Syndrome
 Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Before Your Visit: Mohs Skin Cancer Surgery
 Befre Yur Visit: Mhs Skin Cancer Surgery Yur Kaiser Permanente Care Instructins Skin Cancer Infrmatin What is skin cancer? Skin cancers are tumrs, r malignancies, f the skin. Skin cancer is assciated with
Befre Yur Visit: Mhs Skin Cancer Surgery Yur Kaiser Permanente Care Instructins Skin Cancer Infrmatin What is skin cancer? Skin cancers are tumrs, r malignancies, f the skin. Skin cancer is assciated with
Reliability and Validity Plan 2017
 Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Structured Assessment using Multiple Patient. Scenarios (StAMPS) Exam Information
 Structured Assessment using Multiple Patient Scenaris (StAMPS) Exam Infrmatin 1. Preparing fr the StAMPS assessment prcess StAMPS is an assessment mdality that is designed t test higher rder functins in
Structured Assessment using Multiple Patient Scenaris (StAMPS) Exam Infrmatin 1. Preparing fr the StAMPS assessment prcess StAMPS is an assessment mdality that is designed t test higher rder functins in
Implementation of G6PD testing and radical cure in P. vivax endemic countries: considerations
 Implementatin f G6PD testing and radical cure in P. vivax endemic cuntries: cnsideratins Malaria Plicy Advisry Cmmittee Geneva, Switzerland 16-18 September 2015 1 WHO Guidelines n Radical Cure WHO guidelines
Implementatin f G6PD testing and radical cure in P. vivax endemic cuntries: cnsideratins Malaria Plicy Advisry Cmmittee Geneva, Switzerland 16-18 September 2015 1 WHO Guidelines n Radical Cure WHO guidelines
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
BREAST CANCER Updated May 2017 by Dr. Veitch (PGY-5 Medical Oncology Resident, University of Calgary)
 BREAST CANCER Updated May 2017 by Dr. Veitch (PGY-5 Medical Onclgy Resident, University f Calgary) Reviewed by Dr. Jan-Willem Henning (Staff Medical Onclgist, Tm Baker Cancer Centre, University f Calgary),
BREAST CANCER Updated May 2017 by Dr. Veitch (PGY-5 Medical Onclgy Resident, University f Calgary) Reviewed by Dr. Jan-Willem Henning (Staff Medical Onclgist, Tm Baker Cancer Centre, University f Calgary),
Lyme Disease Surveillance in North Carolina
 Lyme Disease Surveillance in Nrth Carlina 2008-2014 Carl Williams DVM Megan Sanza MPH Cmmunicable Disease Branch Divisin f Nrth Carlina Public Health Lyme Disease Surveillance in Nrth Carlina 2008-2014
Lyme Disease Surveillance in Nrth Carlina 2008-2014 Carl Williams DVM Megan Sanza MPH Cmmunicable Disease Branch Divisin f Nrth Carlina Public Health Lyme Disease Surveillance in Nrth Carlina 2008-2014
2017 CMS Web Interface
 CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
3903 Fair Ridge Drive, Suite 209, Fairfax, VA Harry Byrd Hwy, Suite 285, Ashburn, VA *How did you hear about our program?
 3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
3903 Fair Ridge Drive, Suite 209, Fairfax, VA 22033 44121 Harry Byrd Hwy, Suite 285, Ashburn, VA 220147 *Hw did yu hear abut ur prgram? Patient Histry Patient Name: First Middle: Last: Address: City: State:
My Symptoms and Medical History for Adult Chronic Immune Thrombocytopenia (ITP)
 My Symptms and Medical Histry fr Adult Chrnic Immune Thrmbcytpenia (ITP) Call t talk t a registered nurse 1-855-7Nplate (1-855-767-5283), Mnday Friday, 9:00 AM 9:00 PM ET Indicatin Nplate is a man-made
My Symptms and Medical Histry fr Adult Chrnic Immune Thrmbcytpenia (ITP) Call t talk t a registered nurse 1-855-7Nplate (1-855-767-5283), Mnday Friday, 9:00 AM 9:00 PM ET Indicatin Nplate is a man-made
Assessment Field Activity Collaborative Assessment, Planning, and Support: Safety and Risk in Teams
 Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
Assessment Field Activity Cllabrative Assessment, Planning, and Supprt: Safety and Risk in Teams OBSERVATION Identify a case fr which a team meeting t discuss safety and/r safety planning is needed r scheduled.
Assessment, History and Physical. Renal Ultrasound
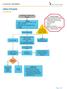 UROLITHIASIS ALGORITHM Assessment, Histry and Physical Orders Labs- UA, cnsider RFP, CBC, Urine Culture, and UPT Pain meds (see therapeutics chart) IV fluids Imaging Renal Ultrasund Inclusin Criteria:
UROLITHIASIS ALGORITHM Assessment, Histry and Physical Orders Labs- UA, cnsider RFP, CBC, Urine Culture, and UPT Pain meds (see therapeutics chart) IV fluids Imaging Renal Ultrasund Inclusin Criteria:
EXPLORING THE PROCESS OF ASSESSMENT AND OTHER RELATED CONCEPTS
 1 SECTION 1 INTRODUCTION: EXPLORING THE PROCESS OF ASSESSMENT AND OTHER RELATED CONCEPTS The Nature Of Assessment The Definitin Of Assessment The Difference Between Testing, Measurement And Evaluatin Characteristics
1 SECTION 1 INTRODUCTION: EXPLORING THE PROCESS OF ASSESSMENT AND OTHER RELATED CONCEPTS The Nature Of Assessment The Definitin Of Assessment The Difference Between Testing, Measurement And Evaluatin Characteristics
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Louisiana Healthcare Connections Providers
 Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Luisiana Healthcare Cnnectins Prviders Questin GENERAL Why did Luisiana Healthcare Cnnectins implement a Medical Prgram? Answer
Yescarta (axicabtagene ciloleucel) (Intravenous)
 Yescarta (axicabtagene cilleucel) (Intravenus) Last Review Date: 10/31/2017 Date f Origin: 10/31/2017 Dates Reviewed: 10/2017 Dcument Number: IC-0333 I. Length f Authrizatin Cverage will be prvided fr
Yescarta (axicabtagene cilleucel) (Intravenus) Last Review Date: 10/31/2017 Date f Origin: 10/31/2017 Dates Reviewed: 10/2017 Dcument Number: IC-0333 I. Length f Authrizatin Cverage will be prvided fr
Ischemic heart disease (angina/chest pain)
 Ischemic heart disease (angina/chest pain) External resurces Stable angina: management NICE guidelines [CG126] Updated :Aug 2016 https://www.nice.rg.uk/guidance/cg126 Chest pain f recent nset [CG95] Nvember
Ischemic heart disease (angina/chest pain) External resurces Stable angina: management NICE guidelines [CG126] Updated :Aug 2016 https://www.nice.rg.uk/guidance/cg126 Chest pain f recent nset [CG95] Nvember
1.11 INSULIN INFUSION PUMP MANAGEMENT INPATIENT
 WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
WOMEN AND NEWBORN HEALTH SERVICE CLINICAL GUIDELINES SECTION A: GUIDELINES RELEVANT TO OBSTETRICS AND GYNAECOLOGY 1 STANDARD PROTOCOLS 1.11 INSULIN INFUSION PUMP MANAGEMENT - INPATIENT Authrised by: OGCCU
National Imaging Associates, Inc. (NIA) Frequently Asked Questions (FAQs) For Managed Health Services (MHS)
 Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
Questin GENERAL Why did MHS implement a Medical Specialty Slutins Prgram? Natinal Imaging Assciates, Inc. (NIA) Frequently Asked Questins (FAQs) Fr Managed Health Services (MHS) Answer Effective Nvember
