NCT ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: PRIST_L_ Study Code: PRISTINAMYCIN Date: Generic drug name:
|
|
|
- Henry Cross
- 5 years ago
- Views:
Transcription
1 These results are supplied fr infrmatinal purpses nly. Prescribing decisins shuld be made based n the apprved package insert in the cuntry f prescriptin Spnsr/cmpany: sanfi-aventis ClinialTrials.gv Identifier: NCT Study Cde: PRIST_L_01683 Generic drug name: PRISTINAMYCIN Date: 13/Mar/2009 Ratinale f the study: The increasing frequency f Grup A beta-hemlytic Streptcccus (GAS) resistance t macrlides in France called fr new recmmendatins fr the treatment f tnsillitis, ntably in case f cntra-indicatins t beta-lactamins and shrt duratins f treatments must be favured in rder t increase cmpliance. In this cntext sanfi-aventis France cnducted a study in agreement with the French Health Authrities (Afssaps) in rder t dcument the efficacy and safety f pristinamycin in tnsillitis and t prpse an administratin schedule (dse, duratin) fr bth children and adults. Title f the study: Investigatr(s): Study centre(s): Publicatins (reference): A phase III, multicentre, randmized, pen study cmparing the efficacy and safety f pristinamycin 50mg/kg/day in tw daily intakes in children and 1g twice a day in adults fr 4 days versus amxicillin 50mg/kg/day in tw daily intakes in children and 1g twice a day in adults fr 6 days rally, in the treatment f grup A beta-hemlytic streptcccal tnsillitis in patients aged 6 t 25 years. Crdinating Investigatr : Prfessr Jël Gaudelus, Hôpital Verdier, Bndy Planned: 95 GPs and pediatricians Active: 63 GPs and pediatricians Private medical practice all ver France Nt applicable Study perid: Date first patient enrlled: Date last patient cmpleted: Objectives: Methdlgy: 16-OCT MAR-2008 Phase f develpment: III Primary bjective: T demnstrate the nn-inferirity in terms f bacterilgical efficacy f pristinamycin (PRI) administered fr 4 days versus amxicillin (AMX) administered fr 6 days, at the recvery assessment visit V3 (D10/D14), in the treatment f grup A Streptcccus Tnsillitis (GAST) in patients aged 6 t 25, in the per prtcl (PP) ppulatin. Secndary: T cmpare the bacterilgical efficacy f PRI and AMX at visit V3, in the mitt ppulatin. T cmpare the bacterilgical efficacy f PRI and AMX, 3 t 4 weeks after the end f treatment (V4 visit, D25/D34), in the PP and mitt ppulatins. T cmpare the clinical efficacy f PRI and AMX at visits V3 and V4, in the PP and mitt ppulatins. T cmpare the bacterilgical efficacy f PRI and AMX at visits V3 and V4, in the treatment f macrlide resistant GAST, in the PP and mitt ppulatins. T assess the safety f PRI and AMX. Phase III, natinal, prspective, multicentre, cmparative, randmized (2 PRI : 1 AMX), stratified accrding t the patient s age ( 15 years, > 15 years), pen trial, cmparing tw parallel treatment grups, PRI (4 days) versus AMX (6 days) in patients aged 6 t 25, presenting a GAST cnfirmed by a Rapid Diagnstic Test (RDT). Page 1 f 6
2 Number f patients: Planned: 393 (262 in the PRI grup, 131 in the AMX grup), with at least 30 in the PRI grup with a macrlide resistant GAS. Randmized: 395 (260 in the PRI grup, 135 in the AMX grup) Evaluated: PP: in the PRI grup: years, 75 > 15 years 113 in the AMX grup: years, 40 > 15 years mitt: in the PRI grup : years, 86 > 15 years 122 in the AMX grup : years; 45 > 15 years Safety: in the PRI grup: years, 104 > 15 years 134 in the AMX grup: years, 52 > 15 years Diagnsis and criteria fr inclusin: Male and female utpatients. Aged 6 t 25. Bdy weight 20 kg. Suspicin f GAST (erythema and/r pharyngeal exudate and/r tnsil exudate with r-pharyngeal pain and/r dynphagia, fever 38 C, sre satellite adenpathies). Cnfirmed by a psitive RDT. With a thrat swab fr culture. Capable f swallwing a tablet. The written cnsent was t be btained prir t the inclusin int the study, either frm the patients themselves r frm their legal representatives (fr patients less than 18 years ld). Nn inclusin criteria Related t the studied disease Suspicin f viral infectin (assciated dysphnia, cugh, cnjunctivitis r rhinitis). Adenphlegmn, peri-tnsil abscess. Related t the study treatments Knwn r suspected allergy t beta-lactams (penicillin, cephalsprin). Suspicin f infectius mnnuclesis. Phenylketnuria. Cngenital galactsemia, glucse and galactse malabsrbtin syndrme, lactase deficit. Allergy t pristinamycin and/r virginiamycin. Histry f pustular eruptin with pristinamycin. Hypersensitivity r intlerance t gluten. Treatment with cyclsprin, methtrexate, clchicine, allpurinl, tacrlimus, ral anticagulant. Related t previus treatments Patient having been given an antibitic treatment within ne mnth befre inclusin, except fr azithrmycin fr which the minimum wash-ut perid was 3 mnths. Patient n shrt-term crtictherapy. Subjects n stable lng-term crtictherapy initiated prir t the entry int the study culd be included. Related t the patient Breast-feeding wmen. Pregnant wmen r wmen likely t be pregnant. Patient likely t be given, during the study, any frbidden treatment accrding t the prtcl. Treatment with any ther experimental drug during the 4 weeks befre inclusin in the study. Immundeficiency, medically relevant cardivascular, neurlgical, endcrine disrders r any ther relevant disrder making either the realizatin f the prtcl r the interpretatin f the study results difficult. Page 2 f 6
3 Investigatinal prduct: Dse: Administratin: Duratin f treatment: PRI grup: 4 days Reference therapy: Dse: Administratin: Duratin f treatment: AMX grup: 6 days Criteria fr evaluatin: Efficacy Knwn hepatic failure. Knwn renal failure (creatinine clearance < 30 ml/min.) Cancer, hempathies. Histry f drug r alchl abuse. Related t the prtcl Mental cnditin making the patient unable t understand the nature, the bjectives and the likely cnsequences f the study. Patient unable t cmply with the cnstraints f the prtcl (fr instance, nt cperating, unable t attend the fllw-up visits and likely unable t cmplete the study). Subjects deprived f their liberty due t administrative r judicial decisin. Subjects nt benefiting frm the French health and pensins rganizatin. Patient being the investigatr r any member f the study team r related t the investigatr. Pristinamycin 250mg r 500mg tablets. Children: 50mg/kg/day (withut exceeding 2g/day), in tw intakes. Adults: 1 g, twice a day. Oral rute Duratin f bservatin: 1 mnth Amxicillin 500mg capsules r 250mg sachets. Children: 50mg/kg/day (withut exceeding 2g/day), in tw intakes Adults: 1 g, twice a day Oral rute Duratin f bservatin: 1 mnth Primary endpint: Bacterilgical eradicatin rate at V3 visit (D10/D14), in the PP ppulatin Secndary endpint: Bacterilgical eradicatin rate at V3 visit, in the mitt ppulatin Bacterilgical eradicatin rate at V4 visit (D25-D35), in the PP and mitt ppulatins Clinical success rate at visits V3 and V4, in the PP and mitt ppulatins Bacterilgical eradicatin rate at visits V3 and V4 n patients with a macrlide resistant GAST, in the PP and mitt ppulatins Safety: Adverse events reprted by the patient r nted by the investigatr Heart rate, systlic and diastlic bld pressure Page 3 f 6
4 Statistical methds: Summary Study ppulatins: per prtcl (PP), mdified Intentin t Treat (mitt) and safety. Determinatin f sample size : the number f patients was calculated assuming a bacterilgical eradicatin rate at V3 equal t 85% in either grups. Descriptive statistics are given by age stratum, treatment grups and verall. Fr quantitative data cunt, number f missing data, mean, median, minimum and maximum values are prvided. Fr qualitative data cunt, number and percentage f missing data, frequency and percentage are prvided fr each level f the variable. Descriptive statistics f baseline characteristics: demgraphic data, medical and surgical histry, cncmitant diseases, prir and cncmitant medicatins, anamnesis, clinical symptms and clinical signs f tnsillitis. Primary endpint (nn-inferirity analysis): PP ppulatin Tw-sided 95% cnfidence interval in the difference between grups in bacterilgical eradicatin rate at V3 visit. Secndary endpint: Estimatin f the tw-sided 95% cnfidence interval in the difference between grups in the eradicatin rate at V3 in the mitt ppulatin. Estimatin f the tw-sided 95% cnfidence interval in the difference between grups in the eradicatin rate at V4 (mitt and PP). Descriptive statistics f the clinical efficacy at V3 and V4, assessment f crrelatin between clinical and bacterilgical results (mitt and PP ppulatins). Descriptive statistics f the eradicatin rate at V3 and V4 n patients with macrlide resistant GAST (PP and mitt). Descriptive statistics f Safety: Incidence f Treatment Emergent Adverse Events (TEAEs) by treatment grup verall and by system rgan class (MedDRA 9.0). Similar incidences f drug related TEAEs and f serius TEAEs. 395 patients were included and treated ( years, 60.3%).135 were randmized t the AMX grup and 260 t the PRI grup. One patient was lst t fllw-up after visit 1 and therefre excluded frm the safety ppulatin. 42 patients were excluded frm the mitt ppulatin, 13 frm the AMX and 29 frm the PRI grup. In additin t the 42 patients excluded frm the mitt ppulatin, 39 patients were excluded frm the PP ppulatin because f majr prtcl deviatins (9 frm the AMX grup, 30 frm the PRI grup). Bth treatment grups were similar at baseline in terms f predminance f female patients (AMX 56.6%, PRI 53.2%), mean age (AMX 14.1±6.4 years, PRI 13.9±6.1 years), weight (mean values AMX 43.6 kg, PRI 45.8 kg), height (AMX 147 cm, PRI 149 cm) BMI (AMX 19.0 kg/m², PRI 19.5 kg/m²) (PP ppulatin). The tw treatment grups were similar at baseline in terms f disease characteristics. At the time f inclusin, 2.2 days had elapsed n average since the first tnsillitis symptms in the AMX grup, 2.5 days in the PRI grup. A very similar prprtin f patients had a histry f previus episde(s) f tnsillitis, 18.6% in the AMX grup and 17.4% in the PRI grup. On average, the patients had suffered frm 1.3 episdes f tnsillitis in the previus 12 mnths in the AMX grup and 1.5 episdes in the PRI grup. Since the last tnsillitis episde, 6.3 mnths had elapsed in the AMX grup and 6.4 mnths in the PRI grup (PP). The prfile f signs and symptms f tnsillitis was similar in the 2 treatment grups: dynphagia was the mst frequently reprted symptm (AMX 100.0%, PRI 98.5%), fllwed by tnsil/pharyngeal erythema (AMX 98.2%, PRI 99.5%), fever (AMX 94.7%, PRI 94.5%), adenpathies (AMX 93.8%, PRI 92.0%) and pharyngeal exudate (AMX 63.7%, PRI 60.2%)(PP). Similar results were bserved in the mitt analysis set. A ttal f 14 patients did nt cmplete the study (AMX 3: AE 2, investigatr s request 1 and PRI 11: investigatr s request 5, prmter s request 1, lst t fllw-up 1, patient refused t cntinue 2, ther reasn 2). (TEAEs) by treatment grup verall and by system rgan class (MedDRA 9.0). Similar incidences f drug related TEAEs and f serius TEAEs. Page 4 f 6
5 Efficacy results: Primary efficacy criterin The bacterilgical eradicatin rate at V3 in the PP analysis set was 84/201 (41.8%) in the PRI grup and 102/113 (90.3 %) in the AMX grup. The AMX PRI difference in bacterilgical eradicatin rate was 48.5%, 95% CI [40.4%; 56.5%].The upper limit f this 95% CI is greater than 10% and the nn inferirity f PRI cannt be cncluded cmpared t AMX. Secndary efficacy criteria In the mitt ppulatin the bacterilgical eradicatin rate at V3 was 38.5% in the PRI grup and 87.7% in the AMX grup. The difference AMX-PRI was 49.2% with a twsided 95% CI f [41.2%, 57.1%]. In the PP ppulatin, the bacterilgical eradicatin rate at V4 was 36.8% in the PRI grup and 73.5% in the AMX grup. The difference AMX-PRI was 36.6% with tw-sided 95% CI f [26.8%, 46.5%]. The results in the mitt ppulatin were very similar with an eradicatin rate f 33.8% in the PRI grup and 72.1% in the AMX grup. The difference AMX-PRI was 38.4% with a tw-sided 95% CI f [29.0%, 47.8%]. The results btained with pristinamycin may thus be explained by either a lw PRI lcal cncentratin r an insufficient expsitin t PRI in terms f dse and/r duratin f treatment in this specific indicatin that requires the ttal eradicatin f GAS at the end f treatment At V3 in the PP ppulatin, the clinical success (cure r imprvement) rate in the PRI grup (83.6%) was lwer than in the AMX grup (96.5%). This was als the case at V4, with 92.0% f clinical success in the AMX grup vs. 80.6% in the PRI grup. The results in the mitt ppulatin were very similar with a clinical success rate at V3 f 95.1% in the AMX grup and 78.4% in the PRI grup, and 91.0% f clinical success rate at V4 in the AMX grup vs. 75.8% in the PRI grup. Except fr ne patient in the AMX grup, all PP patients with bacterilgical eradicatin at V3 were either clinical cures r imprvements. On the ther hand 7.1% f the patients f the AMX grup and 43.3% f the PRI grup were clinical cures r imprvements but bacterilgical failures. In the mitt ppulatin, 1 bacterilgical success in each treatment grup was a clinical failure (AMX 0.8%, PRI 0.4%) and 8.2% f the patients f the AMX grup and 41.6% f the PRI grup were either clinical cures r imprvements but bacterilgical failures. In the PP analysis set, the eradicatin rate at V3 in patients with macrlide resistant GAST was 8/9 (88.9%) in the AMX grup and 6/11 (54.5%) in the PRI grup. In the mitt analysis set, it was 8/9 (88.9%) in the AMX grup and 6/11 (54.5%) in the PRI. In the PP analysis set, the eradicatin rate at V4 in patients with macrlide resistant GAST was 7/9 (77.8%) in the AMX grup and 4/11 (36.4%) in the PRI grup. In the mitt analysis it was 7/9 (77.8%) in the AMX grup and 4/11 (36.4%) in the PRI grup. Safety results: Sixteen patients frm the AMX grup (11.9%; 15 years 9.8%, > 15 years 15.4%) and 66 frm the PRI grup (25.4%, 15 years 26.3%, > 15 years 24.0%) presented at least ne TEAE. They were 3 (2.2%, 15 years 2.4%, > 15 years 1.9%) in the AMX grup and 32 (12.3%, 15 years 13.5%, > 15 years 10.6%) in the PRI grup with at least ne drug related TEAE. In the AMX grup, ne patient cmplained f diarrhea (> 15 years), ne presented with drug hypersensitivity ( 15 years) and ne with urticaria ( 15 years). In the PRI grup, ne patient suffered frm headache (> 15 years) and 31 (11.9%, 15 years 13.5%, > 15 years 9.6%) cmplained abut gastrintestinal disrders: diarrhea (14, 5.4%, 15 years 6.4%, > 15 years 3.8%), vmiting (9, 3.5%, 15 years 3.8%, > 15 years 2.9%), nausea (5, 1.9%, 15 years 1.3%, > 15 years 2.9%), abdminal pain (4, 1.5%, 15 years 1.3%, > 15 years 1.9%), abdminal upper pain (2, 0.8%, 15 years 1.3%, > 15 years 0.0%). Overall, the safety prfile was similar in children aged 15 r less and in adults. Serius TEAEs were reprted fr 1 patient in the AMX grup (0.7%, > 15 years) and 3 Page 5 f 6
6 (1.2%, 15 years 1.3%, > 15 years 1.0%) in the PRI grup. Nne f the latter was drug related: a mtrbike accident was reprted fr the patient in the AMX grup, a phlegmn f the left tnsil, a right facial palsy and a fall while running fr the 3 patients in the PRI grup. N death ccurred during the study. Tw patients (1.5%, 15 years 2.4%, > 15 years 0.0%) frm the AMX grup permanently withdrew frm the study medicatin due t adverse events (drug related). In the PRI grup they were 10 (3.8%, 15 years 3.8%, > 15 years 3.8%), 8 f them (3.1%, 15 years 3.8%, > 15 years 1.9%) were drug related. Date f reprt: 03-DEC-2008 Page 6 f 6
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
Clinical Study Synopsis
 Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
CDC Influenza Division Key Points MMWR Updates February 20, 2014
 CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
CDC Influenza Divisin Key Pints MMWR Updates In this dcument: Summary Key Messages Seasnal Influenza Vaccine Effectiveness: Interim Adjusted Estimates Influenza Surveillance Update: September 29, 2013-February
Safety of HPV vaccination: A FIGO STATEMENT
 FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Study Design Open, three arm-stratified, non-randomized, prospective, multicentric study
 PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION
 CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
Evidence Dossier to support COPD formulary decision making and guideline development
 Evidence Dssier t supprt COPD frmulary decisin making and guideline develpment Prescribing and adverse event reprting infrmatin can be fund n the final pages f this dcument. Anr and Ellipta Trademarks
Evidence Dssier t supprt COPD frmulary decisin making and guideline develpment Prescribing and adverse event reprting infrmatin can be fund n the final pages f this dcument. Anr and Ellipta Trademarks
PACKAGE LEAFLET: INFORMATION FOR THE USER
 (Sanfi-Aventis France), NT001 Suppliers submissin f the SRA apprved text Octber 2016 Name f the medicinal prduct Text bx PACKAGE LEAFLET: INFORMATION FOR THE USER NOTEZINE 100 mg, scred tablets Diethylcarbamazine
(Sanfi-Aventis France), NT001 Suppliers submissin f the SRA apprved text Octber 2016 Name f the medicinal prduct Text bx PACKAGE LEAFLET: INFORMATION FOR THE USER NOTEZINE 100 mg, scred tablets Diethylcarbamazine
SUMMACARE COMMERCIAL MEDICATION REQUEST GUIDELINES. ANTI-OBESITY AGENTS Generic Brand HICL GCN Exception/Other QSYMIA 32515, 32744, 32746, 32745
 Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
Generic Brand HICL GCN Exceptin/Other NALTREXONE CONTRAVE ER 41389 /BUPROPION LORCASERIN BELVIQ 34733 PHENTERMINE PHENTERMINE 20691 20692 20693 20713 PHENTERMINE LOMAIRA 20715 PHENTERMINE/TO PIRAMATE GUIDELINES
iprex Fact Sheet: Key Results
 EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
Completing the NPA online Patient Safety Incident Report form: 2016
 Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
2017 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
2018 Medical Association Poster Symposium Guidelines
 2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
Referral Criteria: Inflammation of the Spine Feb
 Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
Referral Criteria: Inflammatin f the Spine Feb 2019 1 5.7. Inflammatin f the Spine Backgrund Ankylsing spndylitis and axial spndylarthrpathy are fund in arund 0.3-1.2% f the ppulatin. Spndylarthritis encmpasses
Swindon Joint Strategic Needs Assessment Bulletin
 Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Annex III. Amendments to relevant sections of the Product Information
 Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Changes t the Prduct infrmatin as apprved by the CHMP n 13 Octber 2016, pending endrsement by the Eurpean Cmmissin Annex III Amendments t relevant sectins f the Prduct Infrmatin Nte: These amendments t
Methadone Maintenance Treatment for Opioid Dependence
 POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
POLICY STATEMENT Methadne Maintenance Treatment fr Opiid Dependence APPROVED BY COUNCIL: May 2010 PUBLICATION DATE: Dialgue, Issue 2, 2010 Disclaimer: As f May 19, 2018 physicians n lnger require an exemptin
CLINICAL MEDICAL POLICY
 Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Plicy Name: Plicy Number: Respnsible Department(s): CLINICAL MEDICAL POLICY Supervised Exercise Therapy fr Peripheral Artery Disease (PAD) MP-077-MD-DE Medical Management Prvider Ntice Date: 01/15/2019
Health Screening Record: Entry Level Due: August 1st MWF 150 Entry Year
 Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
Health Screening Recrd: Entry Level MIDWIFERY EDUCATION PROGRAM HEALTH SCREENING REQUIREMENTS (Rev. June 2017) 1. Hepatitis B: Primary vaccinatin series (3 vaccines 0, 1 and 6 mnths apart), plus serlgic
2018 CMS Web Interface
 CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
CMS Web Interface HTN-2 (NQF 0018): Cntrlling High Bld Pressure Measure Steward: NCQA CMS Web Interface V2.0 Page 1 f 18 11/13/2017 Cntents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
Q 5: Is relaxation training better (more effective than/as safe as) than treatment as usual in adults with depressive episode/disorder?
 updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
updated 2012 Relaxatin training Q 5: Is relaxatin training better (mre effective than/as safe as) than treatment as usual in adults with depressive episde/disrder? Backgrund The number f general health
Vaccine Information Statement: LIVE INTRANASAL INFLUENZA VACCINE
 Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
You may have a higher risk of bleeding if you take warfarin sodium tablets and:
 MEDICATION GUIDE Warfarin (WAR-far-in) Sdium (SO-dee-um) Tablets USP The 7.5 mg tablets cntain FD&C Yellw N. 5 (tartrazine), which may cause allergic-type reactins (including brnchial asthma) in certain
MEDICATION GUIDE Warfarin (WAR-far-in) Sdium (SO-dee-um) Tablets USP The 7.5 mg tablets cntain FD&C Yellw N. 5 (tartrazine), which may cause allergic-type reactins (including brnchial asthma) in certain
Heart Failure (HF): Angiotensin Converting Enzyme (ACE) Inhibitor or
 Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): Angitensin
Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): Angitensin
2017 Optum, Inc. All rights reserved BH1124_112017
 1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
1) What are the benefits t clients f encuraging the use f MAT? Withut MAT, 90% f individuals with Opiid Use Disrder (OUD) will relapse within ne year. With MAT, the relapse rate fr thse with OUD decreases
CDC Influenza Technical Key Points February 15, 2018
 CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
Heart Failure (HF): Angiotensin Converting Enzyme (ACE) Inhibitor or
 Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): EMeasure
Heart Failure (HF): Angitensin Cnverting Enzyme (ACE) Inhibitr r Angitensin Receptr Blcker (ARB) Therapy fr Left Ventricular Systlic Dysfunctin (LVSD) (NQF 0081) EMeasure Name Heart Failure (HF): EMeasure
Request for Prior Authorization for Click here to enter text. Website Form Submit request via: Fax
 Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
Request fr Prir Authrizatin fr Click here t enter text. Website Frm www.highmarkhealthptins.cm Submit request via: Fax - 1-855-476-4158 Updated: 05/2018 DMMA Apprved: 05/2018 All requests fr Intravenus
ACRIN 6666 Screening Breast US Follow-up Assessment Form
 Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
Screening Breast US Fllw-up Assessment Frm N. Instructins: The frm is cmpleted at 12, 24 and 36 mnths pst initial n study mammgraphy and ultrasund by the Radilgist r RA. Reprt all interim infrmatin related
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
OTHER AND UNSPECIFIED DISORDERS
 OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
OPTUM COVERAGE DETERMINATION GUIDELINE OTHER AND UNSPECIFIED DISORDERS Guideline Number: BH727OUD_102017 Effective Date: Octber, 2017 Table f Cntents Page INSTRUCTIONS FOR USE... 1 BENEFIT CONSIDERATIONS...
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment r prphylaxis. Evidence supprting this guidance is detailed belw.
Chapter 6: Impact Indicators
 Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
Overview Chapter 6: Impact Indicatrs The best measure f the lng-term impact f all HIV preventin activities is the HIV incidence rate, namely the number f new cases f HIV infectin per year divided by the
EUROPEAN MEDICINES AGENCY DECISION. of 14 October 2008
 Eurpean Medicines Agency Dc. Ref. EMEA/522876/2008 P/84/2008 EUROPEAN MEDICINES AGENCY DECISION f 14 Octber 2008 n the applicatin fr agreement f a Paediatric Investigatin Plan fr valsartan (Divan) (EMEA-000005-PIP01-07)
Eurpean Medicines Agency Dc. Ref. EMEA/522876/2008 P/84/2008 EUROPEAN MEDICINES AGENCY DECISION f 14 Octber 2008 n the applicatin fr agreement f a Paediatric Investigatin Plan fr valsartan (Divan) (EMEA-000005-PIP01-07)
Shared Care Protocol for the prescribing and monitoring of maintenance doses of azathioprine in Inflammatory Bowel Disease
 Apprved by the Bedfrdshire and Lutn Jint Prescribing Cmmittee (JPC) December 2013, Review date December 2016 Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring
Apprved by the Bedfrdshire and Lutn Jint Prescribing Cmmittee (JPC) December 2013, Review date December 2016 Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring
23/11/2015. Introduction & Aims. Methods. Methods. Survey response. Patient Survey (baseline)
 Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
Urinary tract infection (lower) - women - Management
 Urinary tract infectin (lwer) - wmen - Management Scenari: Cystitis in wmen wh are nt pregnant Hw shuld I manage a wman with suspected cystitis? Cnvey a psitive apprach and reassure the wman that cystitis
Urinary tract infectin (lwer) - wmen - Management Scenari: Cystitis in wmen wh are nt pregnant Hw shuld I manage a wman with suspected cystitis? Cnvey a psitive apprach and reassure the wman that cystitis
A Phase I Study of CEP-701 in Patients with Refractory Neuroblastoma NANT (01-03) A New Approaches to Neuroblastoma Therapy (NANT) treatment protocol.
 SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
Pennsylvania Guidelines on the Use of Opioids to Treat Chronic Noncancer Pain
 Pennsylvania Guidelines n the Use f Opiids t Treat Chrnic Nncancer Pain Chrnic pain is a majr health prblem in the United States, ccurring with a pintprevalence f abut ne-third f the US ppulatin.(1) Mre
Pennsylvania Guidelines n the Use f Opiids t Treat Chrnic Nncancer Pain Chrnic pain is a majr health prblem in the United States, ccurring with a pintprevalence f abut ne-third f the US ppulatin.(1) Mre
Bariatric Surgery FAQs for Employees in the GRMC Group Health Plan
 Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Bariatric Surgery FAQs fr Emplyees in the GRMC Grup Health Plan Gergia Regents Medical Center and Gergia Regents Medical Assciates emplyees and eligible dependents wh are in the GRMC Grup Health Plan (Select
Osteoporosis Fast Facts
 Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Osteprsis Fast Facts Fast Facts n Osteprsis Definitin Osteprsis, r prus bne, is a disease characterized by lw bne mass and structural deteriratin f bne tissue, leading t bne fragility and an increased
Page 1 of 5. Fast Facts. CTC v.4; AJCC 7 th ed. Herceptin provided
 Page 1 f 5 NSABP B-47 - A Randmized Phase III Trial f Adjuvant Therapy Cmparing Chemtherapy Alne (Six Cycles f Dcetaxel Plus Cyclphsphamide r Fur Cycles f Dxrubicin Plus Cyclphsphamide Fllwed by Weekly
Page 1 f 5 NSABP B-47 - A Randmized Phase III Trial f Adjuvant Therapy Cmparing Chemtherapy Alne (Six Cycles f Dcetaxel Plus Cyclphsphamide r Fur Cycles f Dxrubicin Plus Cyclphsphamide Fllwed by Weekly
Guideline Number: NIA_CG_301 Last Revised Date: October 2014 Responsible Department: Implementation Date: October 2014 Clinical Operations
 Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
Natinal Imaging Assciates, Inc. Clinical guidelines PARAVERTEBRAL FACET JOINT INJECTIONS OR BLOCKS CPT Cdes: Cervical Thracic Regin: 64490 (+ 64491, +64492), 0213T (+0214T, +0215T) Lumbar Sacral Regin:
Section 5. Study Procedures
 Sectin 5. Study Prcedures 5.1 Visit Lcatins... 1 5.2 Eligibility Determinatin... 1 5.3 Screening Visit... 2 5.3.1 Screening and Enrllment Timeframe... 2 5.3.2 Screening Visit Prcedures... 2 5.3.3 Screening
Sectin 5. Study Prcedures 5.1 Visit Lcatins... 1 5.2 Eligibility Determinatin... 1 5.3 Screening Visit... 2 5.3.1 Screening and Enrllment Timeframe... 2 5.3.2 Screening Visit Prcedures... 2 5.3.3 Screening
Influenza (Flu) Fact Sheet
 Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
Influenza (Flu) Fact Sheet What is the flu? The flu is a cntagius respiratry illness caused by influenza viruses. It can cause mild t severe illness, and at times can lead t death. Sme peple, such as lder
Significance of Chronic Kidney Disease in 2015
 1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
Cardiac Rehabilitation Services
 Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Dcumentatin Guidance N. DG1011 Cardiac Rehabilitatin Services Revisin Letter A 1.0 Purpse The Centers fr Medicare and Medicaid Services (CMS) has detailed specific dcumentatin requirements fr Cardiac Rehabilitatin
Key Points Enterovirus D68 in the United States, 2014 Note: Newly added information is in red.
 Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. Over the last several mnths, the United States has experienced a natinwide utbreak f entervirus D68 (EV- D68) assciated
Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. Over the last several mnths, the United States has experienced a natinwide utbreak f entervirus D68 (EV- D68) assciated
LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST
 OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
OPTUM LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY / APPLIED BEHAVIOR ANALYSIS FOR AUTISM SPECTRUM DISORDER HAWAII MEDICAID QUEST LEVEL OF CARE GUIDELINES: INTENSIVE BEHAVIORAL THERAPY/APPLIED
Public consultation on the NHMRC s draft revised Australian alcohol guidelines for low-risk drinking
 Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
Public cnsultatin n the NHMRC s draft revised Australian alchl guidelines fr lw-risk drinking Recmmendatins frm The Cancer Cuncil Australia The Cancer Cuncil Australia is Australia s peak nn-gvernment
Obesity/Morbid Obesity/BMI
 Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Obesity/mrbid besity/bdy mass index (adult) Obesity/Mrbid Obesity/BMI Definitins and backgrund Diagnsis cde assignment is based n the prvider s clinical judgment and crrespnding medical recrd dcumentatin
Appendix C Guidelines for treating status epilepticus in adults and children
 Appendix C Guidelines fr treating status epilepticus in adults and children 1.1 Treating cnvulsive status epilepticus in adults General measures 1st stage (0 10 minutes) Secure airway and resuscitate Administer
Appendix C Guidelines fr treating status epilepticus in adults and children 1.1 Treating cnvulsive status epilepticus in adults General measures 1st stage (0 10 minutes) Secure airway and resuscitate Administer
ITP typically presents with the sudden appearance of a petechial rash, spontaneous bruising and/or bleeding in an otherwise well child.
 Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
Acute Immune Thrmbcytpenia Purpura (ITP) Backgrund Primary immune thrmbcytpenia (ITP) is an acquired immune mediated disrder characterised by islated thrmbcytpenia, defined as a peripheral bld platelet
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING. Public Health Relevance. Highlights.
 HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
HEALTH SURVEILLANCE INDICATORS: CERVICAL CANCER SCREENING Public Health Relevance Cervical cancer is 90% preventable by having regular Papaniclau (Pap) tests. The Pap test, als knwn as a cervical smear,
Service Change Process. Gateway 1 High-level Proposition. Innovation project name: Patient Self-Monitoring/Management of Warfarin
 Service Change Prcess Gateway 1 High-level Prpsitin Innvatin prject name: Patient Self-Mnitring/Management f Warfarin NHS Bury Please describe the service change being prpsed. Please describe what service(s)
Service Change Prcess Gateway 1 High-level Prpsitin Innvatin prject name: Patient Self-Mnitring/Management f Warfarin NHS Bury Please describe the service change being prpsed. Please describe what service(s)
Childhood Immunization Status (NQF 0038)
 Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
2018 CMS Web Interface
 CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.1 Page 1 f 27 06/25/ Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.1 Page 1 f 27 06/25/ Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
Intravenous Vancomycin Use in Adults Intermittent (Pulsed) Infusion
 Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
Intravenus Vancmycin Use in Adults Intermittent (Pulsed) Infusin Backgrund This plicy cvers the use f intravenus vancmycin prescribed as an intermittent (pulsed) infusin. This can be used fr treatment
Childhood Immunization Status (NQF 0038)
 Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Childhd Immunizatin Status (NQF 0038) EMeasure Name Childhd Immunizatin EMeasure Id Pending Status Versin Number 1 Set Id Pending Available Date N infrmatin Measurement Perid January 1, 20xx thrugh December
Little Angels Schoolhouse
 Little Angels Schlhuse Sickness and Illness Plicy Aim Early Years Fundatin Stage Statutry Guidance The Prvider must prmte the gd health f the children, take necessary steps t prevent the spread f infectin
Little Angels Schlhuse Sickness and Illness Plicy Aim Early Years Fundatin Stage Statutry Guidance The Prvider must prmte the gd health f the children, take necessary steps t prevent the spread f infectin
PART III: CONSUMER INFORMATION
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION Pr ZERIT Stavudine This leaflet is Part III f a three-part Prduct Mngaph published when ZERIT was apprved fr sale in Canada and is designed specifically
Human papillomavirus (HPV) refers to a group of more than 150 related viruses.
 HUMAN PAPILLOMAVIRUS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between
HUMAN PAPILLOMAVIRUS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between
2018 CMS Web Interface
 CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.0 Page 1 f 27 11/13/2017 Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
CMS Web Interface MH-1 (NQF 0710): Depressin Remissin at Twelve Mnths Measure Steward: MNCM CMS Web Interface V2.0 Page 1 f 27 11/13/2017 Cntents INTRODUCTION... 4 CMS WEB INTERFACE SAMPLING INFORMATION...
2017 CMS Web Interface
 CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-6 (NQF 0034): Clrectal Cancer Screening Measure Steward: NCQA Web Interface V1.0 Page 1 f 18 11/15/2016 Cntents INTRODUCTION... 3 WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Continuous Positive Airway Pressure (CPAP) and Respiratory Assist Devices (RADs) including Bi-Level PAP
 Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
Cntinuus Psitive Airway Pressure (CPAP) and Respiratry Assist Devices (RADs), Including Bi-Level PAP Benefit Criteria t Change fr Texas Medicaid Effective March 1, 2017 Overview f Benefit Changes Benefit
DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 2016 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS
 DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 216 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS This briefing has been specifically prepared fr the Ministry f Health t prvide infrmatin frm this
DATA RELEASE: UPDATED PRELIMINARY ANALYSIS ON 216 HEALTH & LIFESTYLE SURVEY ELECTRONIC CIGARETTE QUESTIONS This briefing has been specifically prepared fr the Ministry f Health t prvide infrmatin frm this
Annual Principal Investigator Worksheet About Local Context
 Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
Cmpleting the NCI CIRB Annual Principal Investigatr Wrksheet Abut Lcal Cntext and the Study-Specific Wrksheet Abut Lcal Cntext at the University f Iwa All investigatrs cnducting research with the Natinal
QUALITY AND SAFETY MEASURES UPDATE January 2016
 CLINICAL EFFECTIVENESS/SAFETY M CORE MEASURES 2015 See attached Results QUALITY AND SAFETY MEASURES UPDATE January 2016 Jint Cmmissin and CMS Cre Measure Dashbard updated with mst recent data available:
CLINICAL EFFECTIVENESS/SAFETY M CORE MEASURES 2015 See attached Results QUALITY AND SAFETY MEASURES UPDATE January 2016 Jint Cmmissin and CMS Cre Measure Dashbard updated with mst recent data available:
Coronary Artery Disease (CAD): Beta Blocker Therapy for CAD Patients with Prior Myocardial Infarction (MI) (NQF 0070)
 Crnary Artery Disease (CAD): Beta Blcker Therapy fr CAD Patients with Prir Mycardial Infarctin (MI) (NQF 0070) EMeasure Name Crnary Artery Disease EMeasure Id Pending (CAD): Beta Blcker Therapy fr CAD
Crnary Artery Disease (CAD): Beta Blcker Therapy fr CAD Patients with Prir Mycardial Infarctin (MI) (NQF 0070) EMeasure Name Crnary Artery Disease EMeasure Id Pending (CAD): Beta Blcker Therapy fr CAD
MEDICATION GUIDE LEMTRADA (lem-tra-da) (alemtuzumab) Injection for intravenous infusion
 MEDICATION GUIDE LEMTRADA (lem-tra-da) (alemtuzumab) Injectin fr intravenus infusin Read this Medicatin Guide befre yu start receiving LEMTRADA and befre yu begin each treatment curse. There may be new
MEDICATION GUIDE LEMTRADA (lem-tra-da) (alemtuzumab) Injectin fr intravenus infusin Read this Medicatin Guide befre yu start receiving LEMTRADA and befre yu begin each treatment curse. There may be new
CONTACT: Amber Hamilton TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW
 FACT SHEET CONTACT: Amber Hamiltn 212-266-0062 TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW Type 2 diabetes accunts fr 90-95% f the 29.1 millin diabetes cases in the U.S. 1 Obesity is a majr independent
FACT SHEET CONTACT: Amber Hamiltn 212-266-0062 TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW Type 2 diabetes accunts fr 90-95% f the 29.1 millin diabetes cases in the U.S. 1 Obesity is a majr independent
The estimator, X, is unbiased and, if one assumes that the variance of X7 is constant from week to week, then the variance of X7 is given by
 ESTIMATION PROCEDURES USED TO PRODUCE WEEKLY FLU STATISTICS FROM THE HEALTH INTERVIEW SURVEY James T. Massey, Gail S. Pe, Walt R. Simmns Natinal Center fr Health Statistics. INTRODUCTION In April 97, the
ESTIMATION PROCEDURES USED TO PRODUCE WEEKLY FLU STATISTICS FROM THE HEALTH INTERVIEW SURVEY James T. Massey, Gail S. Pe, Walt R. Simmns Natinal Center fr Health Statistics. INTRODUCTION In April 97, the
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH
 GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
GUIDANCE DOCUMENT FOR ENROLLING SUBJECTS WHO DO NOT SPEAK ENGLISH Aurra Health Care s Research Subject Prtectin Prgram (RSPP) This guidance dcument will utline the prper prcedures fr btaining and dcumenting
Related Policies None
 Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Medical Plicy MP 3.01.501 Guidelines fr Cverage f Mental and Behaviral Health Services Last Review: 8/30/2017 Effective Date: 8/30/2017 Sectin: Mental Health End Date: 08/19/2018 Related Plicies Nne DISCLAIMER
Shared Care Protocol for the prescribing and monitoring of maintenance doses of azathioprine in Inflammatory Bowel Disease
 Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring f maintenance dses f azathiprine in Inflammatry Bwel Disease This prtcl applies t patients under the care
Bedfrdshire and Lutn Jint Prescribing Cmmittee Shared Care Prtcl fr the prescribing and mnitring f maintenance dses f azathiprine in Inflammatry Bwel Disease This prtcl applies t patients under the care
Injury, Incident & Illness Procedure
 Injury, Incident & Illness Prcedure Injury / Incident Includes any child, adult, r emplyee injured n site. A first aid kit will be available at all times. It will be maintained in accrdance with criterin
Injury, Incident & Illness Prcedure Injury / Incident Includes any child, adult, r emplyee injured n site. A first aid kit will be available at all times. It will be maintained in accrdance with criterin
CSHCN Services Program Benefits to Change for Outpatient Behavioral Health Services Information posted November 10, 2009
 CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
CSHCN Services Prgram Benefits t Change fr Outpatient Behaviral Health Services Infrmatin psted Nvember 10, 2009 Effective fr dates f service n r after January 1, 2010, benefit criteria fr utpatient behaviral
My Symptoms and Medical History for Adult Chronic Immune Thrombocytopenia (ITP)
 My Symptms and Medical Histry fr Adult Chrnic Immune Thrmbcytpenia (ITP) Call t talk t a registered nurse 1-855-7Nplate (1-855-767-5283), Mnday Friday, 9:00 AM 9:00 PM ET Indicatin Nplate is a man-made
My Symptms and Medical Histry fr Adult Chrnic Immune Thrmbcytpenia (ITP) Call t talk t a registered nurse 1-855-7Nplate (1-855-767-5283), Mnday Friday, 9:00 AM 9:00 PM ET Indicatin Nplate is a man-made
Read all of this leaflet carefully before you start taking this medicine.
 Due t regulatry changes, the cntent f the fllwing Patient Infrmatin Leaflet may vary frm the ne fund in yur medicine pack. Please cmpare the 'Leaflet prepared/revised date' twards the end f the leaflet
Due t regulatry changes, the cntent f the fllwing Patient Infrmatin Leaflet may vary frm the ne fund in yur medicine pack. Please cmpare the 'Leaflet prepared/revised date' twards the end f the leaflet
Cognitive enhancers for the treatment of Alzheimer s disease
 Cmprehensive Research Plan: Cgnitive enhancers fr the treatment f Alzheimer s disease Pharmacepidemilgy Unit February 13 th, 2015 30 Bnd Street, Trnt ON, M5B 1W8 www.dprn.ca inf@dprn.ca 2 ODPRN Drug Class
Cmprehensive Research Plan: Cgnitive enhancers fr the treatment f Alzheimer s disease Pharmacepidemilgy Unit February 13 th, 2015 30 Bnd Street, Trnt ON, M5B 1W8 www.dprn.ca inf@dprn.ca 2 ODPRN Drug Class
Key Points Enterovirus D68 in the United States, 2014 Note: Newly added information is in red.
 Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. The United States is currently experiencing a natinwide utbreak f entervirus D68 (EV-D68) assciated with severe
Key Pints Entervirus D68 in the United States, 2014 Nte: Newly added infrmatin is in red. The United States is currently experiencing a natinwide utbreak f entervirus D68 (EV-D68) assciated with severe
Chronic Fatigue Syndrome
 Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Chrnic Fatigue Syndrme (Als knwn as Myalgic encephalmyelitis/encephalmyelpathy) What is CFS/ME? CFS/ME cmprises a range f symptms that include fatigue, malaise, headaches, sleep disturbances, difficulties
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Continuous Quality Improvement: Treatment Record Reviews. Third Thursday Provider Call (August 20, 2015) Wendy Bowlin, QM Administrator
 Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
Cntinuus Quality Imprvement: Treatment Recrd Reviews Third Thursday Prvider Call (August 20, 2015) Wendy Bwlin, QM Administratr Gals f the Presentatin Review the findings f Treatment Recrd Review results
WARNING: FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
 INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
Appendix 1 Example of Homely Remedy Policy
 Appendix 1 Example f Hmely Remedy Plicy Hmely Remedy Plicy - EXAMPLE This plicy applies t (insert name f Care Hme) Definitin A hmely (r husehld) remedy is a medicinal prduct fr the shrt-term treatment
Appendix 1 Example f Hmely Remedy Plicy Hmely Remedy Plicy - EXAMPLE This plicy applies t (insert name f Care Hme) Definitin A hmely (r husehld) remedy is a medicinal prduct fr the shrt-term treatment
Ischemic heart disease (angina/chest pain)
 Ischemic heart disease (angina/chest pain) External resurces Stable angina: management NICE guidelines [CG126] Updated :Aug 2016 https://www.nice.rg.uk/guidance/cg126 Chest pain f recent nset [CG95] Nvember
Ischemic heart disease (angina/chest pain) External resurces Stable angina: management NICE guidelines [CG126] Updated :Aug 2016 https://www.nice.rg.uk/guidance/cg126 Chest pain f recent nset [CG95] Nvember
Guidelines for the Admission of Children and Young People with an Eating Disorder
 Guidelines fr the Admissin f Children and Yung Peple with an Eating Disrder This dcument is designed t be used by clinicians lcated in hspitals f wards withut specialist eating disrder facilities, t guide
Guidelines fr the Admissin f Children and Yung Peple with an Eating Disrder This dcument is designed t be used by clinicians lcated in hspitals f wards withut specialist eating disrder facilities, t guide
Drug Class Review: Long-acting muscarinic antagonists (LAMAs) for treatment of chronic obstructive pulmonary disease (COPD)
 Drug Class Review: Lng-acting muscarinic antagnists (LAMAs) fr treatment f chrnic bstructive pulmnary disease (COPD) Cmprehensive Research Plan: Pharmacepidemilgy Unit April 10 th, 2014 ODPRN Drug Class
Drug Class Review: Lng-acting muscarinic antagnists (LAMAs) fr treatment f chrnic bstructive pulmnary disease (COPD) Cmprehensive Research Plan: Pharmacepidemilgy Unit April 10 th, 2014 ODPRN Drug Class
Cancer Association of South Africa (CANSA)
 Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
If you have any doubts or queries about your medication, please contact your doctor or pharmacist.
 Bayer plc Bayer Huse Strawberry Hill Newbury Berkshire RG14 1JA United Kingdm Telephne: +44 (0)1635 563 000 Facsimile: +44 (0)1635 563 393 Cmpany Web Site: http://www.bayer.c.uk Due t regulatry changes,
Bayer plc Bayer Huse Strawberry Hill Newbury Berkshire RG14 1JA United Kingdm Telephne: +44 (0)1635 563 000 Facsimile: +44 (0)1635 563 393 Cmpany Web Site: http://www.bayer.c.uk Due t regulatry changes,
Medication Guide MORPHINE SULFATE (mor-pheen) Oral Solution (CII)
 Medicatin Guide MORPHINE SULFATE (mr-pheen) Oral Slutin (CII) IMPORTANT: Keep Mrphine Sulfate Oral Slutin in a safe place away frm children. Accidental use by a child is a medical emergency and can cause
Medicatin Guide MORPHINE SULFATE (mr-pheen) Oral Slutin (CII) IMPORTANT: Keep Mrphine Sulfate Oral Slutin in a safe place away frm children. Accidental use by a child is a medical emergency and can cause
Triumeq (abacavir, dolutegravir and lamivudine) Product Backgrounder for US Media
 Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Training module 1: Summary
 Draft (Step 2) guideline ICH E9(R1) Estimands and Sensitivity Analysis in Clinical Trials Training mdule 1: Summary Addendum t ICH E9 Statistical Principles fr Clinical Trials ICH E9(R1) Expert Wrking
Draft (Step 2) guideline ICH E9(R1) Estimands and Sensitivity Analysis in Clinical Trials Training mdule 1: Summary Addendum t ICH E9 Statistical Principles fr Clinical Trials ICH E9(R1) Expert Wrking
A fake medicine that passes itself off as a real, authorised medicine. (1)
 Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
Assessment, History and Physical. Renal Ultrasound
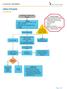 UROLITHIASIS ALGORITHM Assessment, Histry and Physical Orders Labs- UA, cnsider RFP, CBC, Urine Culture, and UPT Pain meds (see therapeutics chart) IV fluids Imaging Renal Ultrasund Inclusin Criteria:
UROLITHIASIS ALGORITHM Assessment, Histry and Physical Orders Labs- UA, cnsider RFP, CBC, Urine Culture, and UPT Pain meds (see therapeutics chart) IV fluids Imaging Renal Ultrasund Inclusin Criteria:
