Interobserver Agreement and Assay Reproducibility of Folate Receptor a Expression in Lung Adenocarcinoma
|
|
|
- Carol Clarke
- 6 years ago
- Views:
Transcription
1 Interobserver Agreement and Assay Reproducibility of Folate Receptor a Expression in Lung Adenocarcinoma A Prognostic Marker and Potential Therapeutic Target Ryan E. Bremer, PhD; Tatiana S. Scoggin, MS; Elizabeth B. Somers, BS; Daniel J. O Shannessy, PhD; David E. Tacha, PhD Context. Lung cancer is the leading cause of cancer deaths in the United States and globally. Folate-targeted drugs are among the promising new targeted therapies for lung cancer, provided predictive biomarkers can be identified for optimal patient selection. Objective. To evaluate the interobserver agreement and reproducibility of an immunohistochemistry assay for folate receptor a as a potential predictive marker for folate-targeted therapies. Design. Immunohistochemistry using anti folate receptor a antibody 26B3 was performed on formalin-fixed, paraffin-embedded tissues. The M-score, a semiquantitative measure of staining intensity and proportion of tumor cells staining, was determined for each specimen. Interobserver agreement was assessed using lung adenocarcinoma specimens stained at a single site and evaluated by 3 independent pathologists. Interinstrument reproducibility assessed 20 specimens stained by 3 different automated stainers. Interlaboratory agreement was determined on 5 specimens, repeatedly stained on each of 5 days, at 3 different study sites. Results. Folate receptor a expression was identified in 39 of 54 cases of lung adenocarcinoma (72%) and 4 of 37 cases of lung squamous cell carcinoma (11%). Agreement among 3 pathologists was found in 24 of 26 cases (92%). Interinstrument reproducibility was observed in 19 of 20 cases (95%). Agreement among 3 laboratories was found for 49 of 50 specimens (98%). Conclusions. Immunostaining of folate receptor a in lung adenocarcinomas is reproducible across staining platforms and among laboratories. Agreement among pathologists is achieved using a semiquantitative scoring method. An accurate and convenient method for determining folate receptor a expression offers a potentially invaluable tool for selecting patients for folate-targeted therapies. (Arch Pathol Lab Med. doi: /arpa OA) (Arch Pathol Lab Med. 2013;137: ; doi: /arpa OA) Lung cancer remains the leading cause of cancer deaths in Historically, the 5-year survival rates of lung cancer the world, with estimated deaths due to lung patients have been among the lowest, with reported survival cancer worldwide in In the United States, lung cancer rates of 6% to 18%. 3 However, as molecular targeted represents 28% of cancer-related deaths among men and 26% of cancer-related deaths of women, with an estimated total of therapies for lung cancer have reached the clinic, a new approach to treatment is emerging, offering the potential for new cases and deaths in 2011, exceeding the improvement in patient care and overall survival. 4 In an combined totals of colon, breast, and prostate cancers. 2 environment that already offers multiple options for targeted therapy, with more on the horizon, it will be critical to match patients to the most promising therapeutic option. In particular, diagnostic assessment of biomarkers Accepted for publication February 19, that are predictive of patient outcomes with a particular Published as an Early Online Release April 9, From the Department of Research & Development, Biocare targeted therapy will be of the utmost importance. Accurate Medical LLC, Concord, California (Drs Bremer and Tacha and Ms and specific measurement of predictive biomarkers, preferably using a familiar and robust methodology, will be a Scoggin); and the Department of Diagnostic Development, Morphotek Inc, Exton, Pennsylvania (Ms Somers and Dr O Shannessy). critical component of the treatment paradigm. Dr Tacha is a founder, chief scientific officer, and stockholder of Folate (folic acid) is an essential water-soluble vitamin, Biocare Medical, Concord, California. Dr Bremer and Ms Scoggin are employees of Biocare Medical. Ms Somers and Dr O Shannessy necessary for the biosynthesis of the purine nucleobases are employees of Morphotek, Exton, Pennsylvania. The authors have thymine and uracil and as a cofactor in certain metabolic no other relevant financial interest in the products or companies reactions. Because of its essential role in DNA synthesis and described in this article. repair mechanisms, folate may be required for the continued Portions of these data were presented as a poster at the annual growth and maintenance of malignant cells. As a result, the College of American Pathologists meeting; September 10, 2012; San Diego, California. folate pathway is an attractive objective for targeted Reprints: Ryan Bremer, PhD, Reagent Chemistry, Biocare Medical, therapies, including folate-linked chemotherapeutics and 4040 Pike Ln, Concord, CA ( rbremer@biocare.net). Arch Pathol Lab Med Vol 137, December 2013 humanized monoclonal antibodies. 5,6 Folate Receptor a in Lung Adenocarcinoma Bremer et al 1747
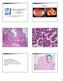
2 Folate receptor a (FRA) is a cell-surface glycoprotein responsible for the transport of folate across cell membranes. FRA has demonstrated restricted expression in polarized epithelial cells in a subset of normal tissues, as well as certain cancers of epithelial origin. 7 FRA expression has been identified in the majority of lung adenocarcinomas (LADCs) and serous (nonmucinous) ovarian and endometrial carcinomas, as well as a limited subset of breast cancers Analysis of messenger RNA levels in LADC has shown that a higher expression level of FRA is a positive prognostic factor for patient survival and disease-free survival, following surgical resection. 14 Conversely, increased FRA expression has been found to be a predictor of negative outcomes in ovarian, endometrial, and breast cancers. 15,16 Folate receptor a has been the target of therapeutic approaches that exploit expression in malignant lesions, including folate-conjugated chemotherapeutics and humanized anti-fra antibodies. 5,6 Currently, therapeutics of both types are in late-stage clinical development. In particular, an anti-fra monoclonal antibody, farletuzumab, is currently being evaluated as a therapeutic treatment in both non small cell LADC and ovarian cancer. 6 Immunohistochemistry of FRA In order to routinely evaluate FRA expression for clinical and research purposes, a new, highly specific mouse monoclonal anti-fra antibody, 26B3.F2, was developed and thoroughly characterized using immunohistochemistry (IHC) on formalin-fixed, paraffin-embedded tissues. 17 The 26B3 antibody identified expression of FRA in a variety of normal tissues, including pancreas, thyroid, hypophysis, breast, lung, salivary gland, kidney, and cervix. Notable malignant tissues that exhibited FRA expression included serous ovarian carcinoma, endometrial carcinoma, and the majority of cases of LADC. Importantly, IHC with 26B3 has demonstrated that FRA is a prognostic marker for patient outcomes in LADC, consistent with previous reports based on messenger RNA of FRA. 8,14,18 A semiquantitative determination of FRA expression based on staining intensity and the proportion of tumor stained (ie, M-score) was used to classify cases. 8 Receiver operating characteristic analysis identified an M-score of 10 (out of 50) as the optimal cutoff for discrimination of overall survival, with high FRA expression (M-score 10) associated with increased overall survival, and low FRA expression (M-score, 10) predictive of decreased overall survival. These results validate both the significance of FRA expression in LADC and the utility of the M-score, thus encouraging ongoing efforts to expand the use of FRA in characterizing cases of LADC. FRA as a Predictive Marker Optimal patient stratification, based on the determination of FRA expression, may be critical for the success of anti- FRA therapies currently in clinical development. Therefore, a sensitive, specific, and robust assay for determining FRA expression is required. Immunohistochemistry of formalinfixed, paraffin-embedded tissues would be the preferred method for FRA determination, because of its low cost, convenience, and routine use in clinical laboratories worldwide, provided that reproducibility across laboratories and instrument platforms can be established. Interlaboratory and interobserver agreement is imperative if FRA expression will be used as a predictive marker of efficacy for an anti-fra therapy or in assessing patient prognosis. Towards this goal, a protocol and reagents for IHC of formalin-fixed, paraffin-embedded tissues using anti-fra mouse monoclonal antibody 26B3 were developed and evaluated at 3 clinical sites and across different automated staining platforms. Initially, 30 slides stained at a single site were assessed by one pathologist from each of the 3 study sites. Each pathologist reported an M-score for each specimen, enabling direct evaluation of interobserver agreement on M-score when viewing the same stained slide. Subsequently, interlaboratory reproducibility of FRA staining was investigated by staining the same specimens at each of the 3 study sites, followed by determination of an M-score by the site pathologist. Finally, 20 cases were stained using 3 different automated staining instruments: Biocare s intellipath (Biocare Medical, Concord, California), Dako s Autostainer Plus (Dako, Carpinteria, California) and Dako s Autostainer Link, in addition to a manual staining method, and the M-scores compared for the same specimen stained by each method. MATERIALS AND METHODS Immunohistochemistry Formalin-fixed, paraffin-embedded whole tissues and tissue microarrays were analyzed by IHC. Tissue blocks were all obtained from archival sources, with no associated patient information, treatment, or follow-up data. Tissues were deparaffinized and rehydrated in the usual manner. Immunohistochemistry was performed with reagents provided by Biocare Medical (Folate Receptor Alpha IHC Assay Kit, BRI4006K), using the protocol provided in the kit instructions. Antigen retrieval was performed in a pressure cooker (958C/40 minutes; Decloaking Chamber; Biocare Medical), using a citrate-based retrieval solution (Diva Decloaker; Biocare Medical). Protein block (5 minutes) was followed by incubation with primary anti-fra 26B3 antibody (1 lg/ml), or negative control reagent (mouse immunoglobulin G1 j, 1lg/mL), for 30 minutes. Detection was performed using a secondary probe (10 minutes), followed by horseradish peroxidase conjugated polymer (10 minutes). Visualization with 3,3 0 -diaminobenzidine (5 minutes) identified both intracellular and membranous expression of FRA. Only membrane staining for FRA was considered to be positive FRA staining in the pathologist s interpretation of each specimen. Semiquantitative Scoring Membrane staining in the tumor of each stained specimen was evaluated by a site pathologist using the M-score, as previously reported. 8 Briefly, the M-score is a semiquantitative algorithm for scoring FRA expression based on the proportion of tumor cells staining at each intensity level (0, 1þ, 2þ, 3þ). The M-score is calculated as follows: M-score ¼ð3xþ2yþzÞ=6 where x is the percentage of tumor stained with intensity 3þ, y is the percentage of tumor stained with intensity 2þ, and z is the percentage of tumor stained with intensity 1þ. Observed M-scores may range from 0 (no tumor staining) to 50 (100% tumor staining at an intensity of 3þ). Sensitivity and Specificity of 26B3 in LADC and Squamous Cell Carcinoma Cases of LADC (n¼54) and lung squamous cell carcinoma (SqCC) (n ¼ 37) were stained with 26B3 using the standard protocol and reagents described above, using an intellipath stainer. Membrane staining of tumor cells was evaluated for each specimen 1748 Arch Pathol Lab Med Vol 137, December 2013 Folate Receptor a in Lung Adenocarcinoma Bremer et al
3 and determined to be positive for FRA if at least 10% of the tumor cells were stained at any intensity. Interobserver Agreement One specimen from each of 30 cases of LADC was stained with 26B3 and reviewed by 3 pathologists. Each pathologist determined an M-score for each specimen. Twenty-six of the cases were determined to be evaluable; 3 cases were found to lack sufficient tumor in the specimen, and 1 case contained a folded sample, which could not be reviewed. Agreement among pathologists was determined as described below. Interinstrument Reproducibility Twenty cases of LADC were stained using the standard protocol, by 4 different staining methods, including automated staining on Dako Autostainer, Dako PT Link 48 Autostainer, and Biocare intellipath, and a manual staining method. (Dako Target Retrieval Solution, ph 6, was used for retrieval on the Dako PT Link 18 Autostainer.) A single pathologist determined an M-score for each of the 80 slides. Interlaboratory and Interrun Reproducibility Five cases of LADC were stained on each of 5 days, at 3 independent study sites, in order to evaluate both interrun and interlaboratory reproducibility. All specimens were stained on both the Biocare intellipath and the Dako Autostainer on each of the 5 days. An independent pathologist at each study site determined an M-score for the 50 slides stained at that site (5 cases 3 5 days 3 2 instruments). Determination of Agreement For each study, agreement among observers, instruments, or repeated staining runs was determined by 2 different methods. First, using the previously established M-score 10 as the cutoff for establishing a favorable prognosis, each specimen was determined to be FRA positive if the M-score was 10 or greater, and FRA negative if the M-score was less than In this manner, the reliability of the IHC method at determining FRA expression for the purpose of predicting prognosis could be established. Additionally, in anticipation that future applications of IHC of FRA using 26B3 may stratify patients using different M-score criteria, agreement among the M-scores for each specimen was determined. A difference in M-scores of 15 was selected as an acceptable range for agreement among replicates of the same specimen in each study. M-scores that differed by more than 15 were recorded as not in agreement. In this manner, the reproducibility of M-score determination could be evaluated across the full range of possible M-scores, which may be useful for application in future studies. Overall percentage agreement was calculated in each study by dividing the number of specimens in agreement by the total number of specimens evaluated. The 95% confidence interval (CI) for the percentage agreement was calculated using the exact method of Clopper and Pearson. 19 RESULTS Sensitivity and Specificity in LADC and Lung SqCC Sensitivity and specificity of 26B3, using the established protocol and reagents, were determined using 54 cases of LADC and 37 cases of lung SqCC. The 26B3 was found to stain 39 of 54 cases of LADC (72%; 95% CI, 58% 84%) (Table 1). Significantly fewer cases of lung SqCC were positive for FRA expression with 26B3 (4 of 37; 11%; 95% CI, 3% 25%). The incidence of staining in LADC was consistent with previous reports. 8,9 However, 26B3 has been found to be more specific for LADC and to stain fewer cases of lung SqCC in this study (11%) and previously (14%) 8 than an alternative anti-fra antibody. 9 Given these differing results, studies are currently underway to further Table 1. Sensitivity of Anti-FRA Antibody 26B3 for Lung Adenocarcinoma (LADC) and Squamous Cell Carcinoma (SqCC), by Immunohistochemistry Anti-FRA 26B3 LADC Lung SqCC Positive cases/total cases 39/54 4/37 Sensitivity, % investigate staining in lung SqCC, in order to most accurately determine FRA expression levels. Immunohistochemistry with 26B3 results in membranous and intracellular staining, which varies in frequency and intensity from case to case, offering a convenient measure of FRA expression for each case (Figure 1, A through D). As expected, 26B3 also stains normal lung tissue (Figure 2). Interobserver Agreement Twenty-six cases of LADC were stained with 26B3 at a single study site and the slides were reviewed by 3 independent pathologists at 3 different sites. The M-scores determined by each pathologist for each case were evaluated for agreement. Using the FRA-positive/FRAnegative method of evaluating agreement, (ie, a specimen was determined FRA positive if the M-score is 10 or greater, and FRA negative if the M-score is less than 10; see Materials and Methods ), all 3 pathologists agreed on FRA expression in 23 of 26 (88%; 95% CI, 70% 97%) cases (Table 2). Two of the 3 instances of disagreement occurred in cases near the M-score ¼ 10 cutoff, with recorded M-scores of 9, 12, and 13 for one specimen and 5, 5, and 16 for the other specimen. In both cases, the interpretation among pathologists was consistent, with the expected differences in subjective interpretation accounting for the differing positive/negative results when FRA expression was near the cutoff value of M-score ¼ 10. The third case of disagreement arose from 1 pathologist s interpretation that 95% of the tumor in the sample exhibited 2þ staining, whereas the other 2 pathologists found 80% to 85% of the tumor sample had no staining for FRA. Direct comparison of explicit M-scores from each pathologist found agreement in 24 of 26 (92%; 95% CI, 75% 99%) cases, when an M-score range of 15 among pathologists was used as the acceptable limit for agreement (Table 2). One case of disagreement was the same case as that described above, with differing pathologist interpretation. The remaining case of disagreement exhibited more similar M-scores among pathologists (23, 28, 42); however, 1 pathologist similarly found 80% of the tumor to exhibit 3þ staining, whereas the other 2 pathologists allocated 40% to 50% of the tumor as 1þ or no staining. Interinstrument Reproducibility Folate receptor a staining of 20 cases of LADC was compared among specimens stained on 3 automated stainers (Dako Autostainer, Dako PT Link 48 Autostainer, and Biocare intellipath Autostainer), as well as a manual IHC staining method. Staining was performed at a single site and an M-score determined by a single pathologist. Seven of these cases were negative for FRA, whereas the remaining 13 cases exhibited a full range of FRA expression. Using the FRA-positive/FRA-negative method of evaluating agreement, 18 of 20 specimens (90%; 95% CI, 69% 98%) gave identical positive/negative results across all 4 staining methods (Table 2). One of the specimens lacking Arch Pathol Lab Med Vol 137, December 2013 Folate Receptor a in Lung Adenocarcinoma Bremer et al 1749
4 Figure 1. Anti folate receptor a (FRA) 26B3 staining of lung adenocarcinoma (LADC) with intensity 3þ (A), 2þ (B), 1þ (C), and LADC negative for FRA (D) (original magnifications 310 [A] and 320 [B through D]). complete agreement had M-scores near the M-score ¼ 10 cutoff (M-scores ¼ 8, 8, 8, 11). Although consistent staining was clearly observed across methods for this specimen, its FRA expression level near the M-score ¼ 10 cutoff places it in an intermediate range where a classification cannot be made with certainty. The other specimen lacking agreement exhibited a single staining failure on the Dako PT 48 Link Autostainer. In this case, M-scores by the other 3 staining methods were 35, 30, and 33, whereas the Dako PT 48 Link Autostainer specimen had an M-score of 2. Using the method of comparing all M-scores, 19 of 20 cases (95%; 95% CI ¼ 75% 99%) produced equivalent staining across all 4 staining methods when using a range of 15 as agreement in the M-scores for a given specimen among staining methods (Table 2). The only specimen lacking agreement was the same specimen as noted above, with an M-score ¼ 2 on the Dako PT 48 Link Autostainer. Figure 2. Anti folate receptor a 26B3 staining of normal lung (original Interrun and Interlaboratory Reproducibility Five cases of FRA-positive LADC were stained on each of 5 days, at 3 different study sites, and using 2 different instruments (Biocare intellipath and Dako Autostainer), in order to evaluate the reproducibility of FRA staining among runs on separate days, and, most importantly, among laboratories. Agreement among runs and among laboratories was performed using the method of direct comparisons of M-score, with a difference of 15 as the acceptable limit for agreement across M-scores for specimens stained on different days, or at different laboratories. In each case, staining the same specimen on repeated days produced highly consistent staining results. At each of magnification 310). the 3 study sites, there was 100% agreement (25 of 25; 1750 Arch Pathol Lab Med Vol 137, December 2013 Folate Receptor a in Lung Adenocarcinoma Bremer et al
5 Table 2. Interobserver Agreement and Interinstrument Reproducibility for Folate Receptor a (FRA) Staining With 26B3 Assay Kit Method for Evaluating Agreement FRA Positive, M-Score 10; Study FRA Negative, M-Score,10 Difference in M-Scores 15 Interobserver agreement, No./total (%) 23/26 (88) 24/26 (92) Interinstrument reproducibility, No./total (%) 18/20 (90) 19/20 (95) 95% CI ¼ 86% 100%) for all 5 cases among staining runs performed on the 5 different days at the site (Table 3). The same agreement was observed for specimens stained on both the Dako Autostainer and the Biocare intellipath. In order to evaluate interlaboratory reproducibility, the M- scores for each specimen were compared for each of 5 days. On all 5 days, 100% agreement was observed for all 5 specimens stained on the Biocare intellipath, among all 3 sites (25 of 25; 95% CI ¼ 86% 100%) (Table 3). Evaluation of specimens stained with the Dako Autostainer in each laboratory resulted in 100% agreement on 4 of 5 days (24 of 25; 95% CI ¼ 80% 99%). On a single day (day 2), one specimen from the Dako Autostainer differed in M-score among the 3 sites by 17 (M-score ¼ 30, 38, 47). Although this specimen was classified as not in agreement based on the study criteria, the observed M-score difference of 17 was only slightly outside the acceptable range of 15. In total, 50 comparisons were made among the 3 sites (5 specimens for 5 days, with 2 instruments). Among these specimens, 49 of 50 (98%; 95% CI ¼ 89% 99%) demonstrated agreement in M-score among laboratories. COMMENT Immunohistochemistry using anti-fra antibody 26B3 is a sensitive, specific, and reproducible method for the determination of FRA expression in LADC and lung SqCC. Use of the semiquantitative M-score as a measure of FRA levels demonstrated agreement in interpretation among pathologists and among specimens stained in different laboratories, as well as across various staining methods, during multiple days. Evaluation of FRA with 26B3 uses a convenient and routine IHC method that is familiar to laboratory personnel, and requires no further training or expertise than what is already available in anatomic pathology laboratories. Consistent staining of FRA with 26B3 among laboratories depends upon use of the same ancillary reagents (protein block, detection, chromogen) and a standard protocol; however, this is no more burdensome than what is currently performed for other predictive biomarkers. Interpretation of FRA expression depends upon the determination of percentage of the tumor staining at various intensities; although this practice is not routinely used for every specimen, pathologists are well prepared for such interpretation, without additional training. Importantly, the M-score in particular mitigates the minor differences in subjective interpretation that arise from assigning different values to each staining level among pathologists, resulting in a consistent scoring of FRA expression among observers. The reproducibility and agreement observed for FRA staining, with these protocols and reagents, supports its use as a method to routinely and conveniently evaluate FRA expression for the purposes of selecting patients for anti- FRA targeted treatments as well as for evaluating patient prognosis. Although ongoing clinical trials will evaluate the utility of FRA expression as a selection criteria for treatment, using FRA expression to assess the prognosis of LADC patients is a potentially valuable diagnostic option, currently available today, and supported by studies in the literature. 8,14,18 Anti-FRA 26B3 was found here, and previously, 8 to stain fewer cases of lung SqCC (11% and 14%, respectively) than previously reported for anti-fra antibody mab343 (51%). 9 Studies are currently underway to further investigate the staining of each of these antibodies in lung SqCC. Finally, FRA expression was found in 72% of LADC cases, using IHC with 26B3, consistent with previous reports. Importantly, this may identify a large subset of patients for whom an anti-fra targeted therapy may be valuable. The possibility of addressing the significant unmet medical need in lung cancer therapy encourages the continued development of anti-fra therapies and the diagnostic assays to identify the patients most likely to benefit from such treatments. The authors would like to thank Yao-Shi Fu, MD; Thomas Haas, DO; and Haodong Xu, MD, PhD, for determination of M-scores. References 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2): Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4): Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, CA Cancer J Clin. 2012;62(4): Cagle PT, Chirieac LR. Advances in treatment of lung cancer with targeted therapy. Arch Pathol Lab Med. 2012;136(5): Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13(3): Konner JA, Bell-McGuinn KM, Sabbatini P, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16(21): Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56(8): Table 3. Interrun Reproducibility and Interlaboratory Agreement for Folate Receptor a Staining With 26B3 Assay Kit Staining Instrument Study Biocare Medical intellipath a Dako Autostainer b Interrun reproducibility, No./total (%) 25/25 (100) 25/25 (100) Interlaboratory agreement, No./total (%) 25/25 (100) 24/25 (96) a Biocare Medical, Concord, California. b Dako, Carpinteria, California. Arch Pathol Lab Med Vol 137, December 2013 Folate Receptor a in Lung Adenocarcinoma Bremer et al 1751
6 8. O Shannessy DJ, Yu G, Smale R, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget. 2012;3(4): Cagle PT, Zhai QJ, Murphy L, Low PS. Folate receptor in adenocarcinoma and squamous cell carcinoma of the lung: potential target for folate-linked therapeutic agents [published online ahead of print September 14, 2012]. Arch Pathol Lab Med. 2013;137(2): Nunez MI, Behrens C, Woods D et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR mutation. J Thorac Oncol. 2012;7(5): Toffoli G, Cernigoi C, Russo A, Gallo A, Bagnoli M, Boiocchi M. Overexpression of folate binding protein in ovarian cancers. Int J Cancer. 1997; 74(2): O Shannessy DJ, Somers EB, Maltzman J, Smale R, Fu YS. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. SpringerPlus. 2012;1: O Shannessy DJ, Somers EB, Smale R, Fu YS. Expression of folate receptora (FRA) in gynecologic malignancies and its relationship to the tumor type. Int J Gynecol Pathol. In press. 14. Iwakiri S, Sonobe M, Nagai S, Hirata T, Wada H, Miyahara R. Expression status of folate receptor alpha is significantly correlated with prognosis in nonsmall-cell lung cancers. Ann Surg Oncol. 2008;15(3): Toffoli G, Russo A, Gallo A, et al. Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer. Int J Cancer. 1998;79(2): Hartmann LC, Keeney GL, Lingle WL, et al. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121(5): O Shannessy, DJ, Somers ES, Albone E, et al. Characterization of the human folate receptor alpha via novel antibody-based probes. Oncotarget. 2011; 2(12): Christoph DC, Reyna-Asuncion B, Hassan B, et al. Significance of folate receptor alpha and thymidylate synthase protein expression in patients with nonsmall-cell lung cancer treated with pemetrexed. J Thorac Oncol. 2013;8(1): Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26: Arch Pathol Lab Med Vol 137, December 2013 Folate Receptor a in Lung Adenocarcinoma Bremer et al
Original Article CREPT expression correlates with esophageal squamous cell carcinoma histological grade and clinical outcome
 Int J Clin Exp Pathol 2017;10(2):2030-2035 www.ijcep.com /ISSN:1936-2625/IJCEP0009456 Original Article CREPT expression correlates with esophageal squamous cell carcinoma histological grade and clinical
Int J Clin Exp Pathol 2017;10(2):2030-2035 www.ijcep.com /ISSN:1936-2625/IJCEP0009456 Original Article CREPT expression correlates with esophageal squamous cell carcinoma histological grade and clinical
Workflow. Connecting the Pieces For Total Patient Care
 Workflow Connecting the Pieces For Total Patient Care Biocare provides a full line of IHC and molecular pathology products for cancer and infectious disease diagnosis. From a full range of equipment: including
Workflow Connecting the Pieces For Total Patient Care Biocare provides a full line of IHC and molecular pathology products for cancer and infectious disease diagnosis. From a full range of equipment: including
High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer.
 Biomedical Research 2017; 28 (18): 7779-7783 ISSN 0970-938X www.biomedres.info High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer. Hu Song 1, Qi-yu Liu 2, Zhi-wei
Biomedical Research 2017; 28 (18): 7779-7783 ISSN 0970-938X www.biomedres.info High expression of fibroblast activation protein is an adverse prognosticator in gastric cancer. Hu Song 1, Qi-yu Liu 2, Zhi-wei
Immunotherapy in NSCLC Pathologist role
 Immunotherapy in NSCLC Pathologist role Pimpin Incharoen, M.D. Assistant Professor, Thoracic Pathology Department of Pathology, Ramathibodi Hospital Genetic alterations in NSCLC Khono et al, Trans Lung
Immunotherapy in NSCLC Pathologist role Pimpin Incharoen, M.D. Assistant Professor, Thoracic Pathology Department of Pathology, Ramathibodi Hospital Genetic alterations in NSCLC Khono et al, Trans Lung
Folate Receptor Alpha Expression in Lung Cancer: Diagnostic and Prognostic Significance
 / Oncotarget, April, Vol.3, No 4 Folate Receptor Alpha Expression in Lung Cancer: Diagnostic and Prognostic Significance Daniel J. O Shannessy 1, Gordon Yu 3, Robert Smale 5, Yao-Shi Fu 5, Sunil Singhal
/ Oncotarget, April, Vol.3, No 4 Folate Receptor Alpha Expression in Lung Cancer: Diagnostic and Prognostic Significance Daniel J. O Shannessy 1, Gordon Yu 3, Robert Smale 5, Yao-Shi Fu 5, Sunil Singhal
Expression of high affinity folate receptor in breast cancer brain metastasis
 /, Vol. 6, No. 30 Expression of high affinity folate receptor in breast cancer brain metastasis José Pablo Leone 1, Rohit Bhargava 2, Brian K. Theisen 2, Ronald L. Hamilton 2, Adrian V. Lee 3 and Adam
/, Vol. 6, No. 30 Expression of high affinity folate receptor in breast cancer brain metastasis José Pablo Leone 1, Rohit Bhargava 2, Brian K. Theisen 2, Ronald L. Hamilton 2, Adrian V. Lee 3 and Adam
Quantitative Image Analysis of HER2 Immunohistochemistry for Breast Cancer
 Quantitative Image Analysis of HER2 Immunohistochemistry for Breast Cancer Guideline from the College of American Pathologists Early Online Release Publication: Archives of Pathology & Laboratory Medicine
Quantitative Image Analysis of HER2 Immunohistochemistry for Breast Cancer Guideline from the College of American Pathologists Early Online Release Publication: Archives of Pathology & Laboratory Medicine
Coordinate Expression of Cytokeratins 7 and 20 in Prostate Adenocarcinoma and Bladder Urothelial Carcinoma
 Anatomic Pathology / CYTOKERATINS 7 AND 20 IN PROSTATE AND BLADDER CARCINOMAS Coordinate Expression of Cytokeratins 7 and 20 in Prostate Adenocarcinoma and Bladder Urothelial Carcinoma Nader H. Bassily,
Anatomic Pathology / CYTOKERATINS 7 AND 20 IN PROSTATE AND BLADDER CARCINOMAS Coordinate Expression of Cytokeratins 7 and 20 in Prostate Adenocarcinoma and Bladder Urothelial Carcinoma Nader H. Bassily,
Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression
 Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression Mona A. Abd-Elazeem, Marwa A. Abd- Elazeem Pathology department, Faculty of Medicine, Tanta
Claudin-4 Expression in Triple Negative Breast Cancer: Correlation with Androgen Receptors and Ki-67 Expression Mona A. Abd-Elazeem, Marwa A. Abd- Elazeem Pathology department, Faculty of Medicine, Tanta
Supplementary Online Content
 Supplementary Online Content Bell EH, Pugh SL, McElroy JP, et al. Molecular-based recursive partitioning analysis model for glioblastoma in the temozolomide era: a correlative analysis based on NRG Oncology
Supplementary Online Content Bell EH, Pugh SL, McElroy JP, et al. Molecular-based recursive partitioning analysis model for glioblastoma in the temozolomide era: a correlative analysis based on NRG Oncology
Epithelial cell-cell adhesion molecule (Ep-CAM)
 Assessment Run 3 011 Epithelial cell-cell adhesion molecule (Ep-CAM) Material The slide to be stained for Ep-CAM comprised: 1. Appendix,. Kidney, 3. Adrenal gland, 4. Lung carcinoid, 5 & 6. Renal clear
Assessment Run 3 011 Epithelial cell-cell adhesion molecule (Ep-CAM) Material The slide to be stained for Ep-CAM comprised: 1. Appendix,. Kidney, 3. Adrenal gland, 4. Lung carcinoid, 5 & 6. Renal clear
Assessment Run NKX3.1 (NKX3.1)
 Assessment Run 49 2017 NKX3.1 (NKX3.1) Material The slide to be stained for NKX3.1 comprised: 1. Testis 2. Appendix 3-4. Prostate adenocarcinoma 5. Prostate hyperplasia All tissues were fixed in 10% neutral
Assessment Run 49 2017 NKX3.1 (NKX3.1) Material The slide to be stained for NKX3.1 comprised: 1. Testis 2. Appendix 3-4. Prostate adenocarcinoma 5. Prostate hyperplasia All tissues were fixed in 10% neutral
Thyroid transcription factor-1 (TTF1) Assessment run
 Thyroid transcription factor- (TTF) Assessment run 39 203 The slide to be stained for TTF comprised:. Thyroid gland, 2. Liver, 3. Normal lung, 4. Lung adenocarcinoma 5. Colon adenocarcinoma, 6 & 7. Lung
Thyroid transcription factor- (TTF) Assessment run 39 203 The slide to be stained for TTF comprised:. Thyroid gland, 2. Liver, 3. Normal lung, 4. Lung adenocarcinoma 5. Colon adenocarcinoma, 6 & 7. Lung
1. Q: What has changed from the draft recommendations posted for public comment in November/December 2011?
 Frequently Asked Questions (FAQs) in regard to Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors 1. Q: What has changed from the draft recommendations
Frequently Asked Questions (FAQs) in regard to Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors 1. Q: What has changed from the draft recommendations
Estrogen receptor (ER)
 Material The slide to be stained for ER comprised: Assessment Run B26 2018 Estrogen receptor (ER) No. Tissue ER-positivity* ER-intensity* 1. Uterine cervix 80-90% Moderate to strong 2. Tonsil 1-5% Weak
Material The slide to be stained for ER comprised: Assessment Run B26 2018 Estrogen receptor (ER) No. Tissue ER-positivity* ER-intensity* 1. Uterine cervix 80-90% Moderate to strong 2. Tonsil 1-5% Weak
Improving quality of care for patients with ovarian and endometrial cancer Eggink, Florine
 University of Groningen Improving quality of care for patients with ovarian and endometrial cancer Eggink, Florine IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
University of Groningen Improving quality of care for patients with ovarian and endometrial cancer Eggink, Florine IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
microrna Presented for: Presented by: Date:
 microrna Presented for: Presented by: Date: 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions
microrna Presented for: Presented by: Date: 2 micrornas Non protein coding, endogenous RNAs of 21-22nt length Evolutionarily conserved Regulate gene expression by binding complementary regions at 3 regions
Assessment Run CK19
 Assessment Run 29 200 CK9 The slide to be stained for CK9 comprised:. Appendix, 2. Thyroid gland, 3. Pancreas, 4. Ductal breast carcinoma, 5. Esophagus, 6. Papillary thyroid carcinoma. All tissues were
Assessment Run 29 200 CK9 The slide to be stained for CK9 comprised:. Appendix, 2. Thyroid gland, 3. Pancreas, 4. Ductal breast carcinoma, 5. Esophagus, 6. Papillary thyroid carcinoma. All tissues were
Original Article Folate receptor α expression and significance in endometrioid endometrium carcinoma and endometrial hyperplasia
 Int J Clin Exp Pathol 2015;8(5):5633-5641 www.ijcep.com /ISSN:1936-2625/IJCEP0006892 Original Article Folate receptor α expression and significance in endometrioid endometrium carcinoma and endometrial
Int J Clin Exp Pathol 2015;8(5):5633-5641 www.ijcep.com /ISSN:1936-2625/IJCEP0006892 Original Article Folate receptor α expression and significance in endometrioid endometrium carcinoma and endometrial
A Newly Developed Uroplakin II Antibody With Increased Sensitivity in Urothelial Carcinoma of the Bladder
 A Newly Developed Uroplakin II Antibody With Increased Sensitivity in Urothelial Carcinoma of the Bladder Laura L. Hoang, PhD; David E. Tacha, PhD; Weimin Qi, MD, PhD; Charlie Yu, MD; Ryan E. Bremer, PhD;
A Newly Developed Uroplakin II Antibody With Increased Sensitivity in Urothelial Carcinoma of the Bladder Laura L. Hoang, PhD; David E. Tacha, PhD; Weimin Qi, MD, PhD; Charlie Yu, MD; Ryan E. Bremer, PhD;
Layered-IHC (L-IHC): A novel and robust approach to multiplexed immunohistochemistry So many markers and so little tissue
 Page 1 The need for multiplex detection of tissue biomarkers. There is a constant and growing demand for increased biomarker analysis in human tissue specimens. Analysis of tissue biomarkers is key to
Page 1 The need for multiplex detection of tissue biomarkers. There is a constant and growing demand for increased biomarker analysis in human tissue specimens. Analysis of tissue biomarkers is key to
Results you can trust
 PRODUCT I NF OR MAT ION pharmdx Results you can trust The first and only FDA-approved PD-L1 test to assess the magnitude of treatment effect on progression-free survival in melanoma patients from OPDIVO
PRODUCT I NF OR MAT ION pharmdx Results you can trust The first and only FDA-approved PD-L1 test to assess the magnitude of treatment effect on progression-free survival in melanoma patients from OPDIVO
Corporate Medical Policy
 Corporate Medical Policy Microarray-based Gene Expression Testing for Cancers of Unknown File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microarray-based_gene_expression_testing_for_cancers_of_unknown_primary
Corporate Medical Policy Microarray-based Gene Expression Testing for Cancers of Unknown File Name: Origination: Last CAP Review: Next CAP Review: Last Review: microarray-based_gene_expression_testing_for_cancers_of_unknown_primary
Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients
 /, Vol. 7, No. 14 Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients Leonora S.F. Boogerd 1,*, Martin C. Boonstra 1,*, Ann-Jean
/, Vol. 7, No. 14 Concordance of folate receptor-α expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients Leonora S.F. Boogerd 1,*, Martin C. Boonstra 1,*, Ann-Jean
Human epididymal protein 4 The role of HE4 in the management of patients presenting with pelvic mass Publication abstracts
 Human epididymal protein 4 The role of HE4 in the management of patients presenting with pelvic mass Publication abstracts Ovarian cancer is diagnosed annually in more than 200,000 women worldwide, with
Human epididymal protein 4 The role of HE4 in the management of patients presenting with pelvic mass Publication abstracts Ovarian cancer is diagnosed annually in more than 200,000 women worldwide, with
Dr. dr. Primariadewi R, SpPA(K)
 Curriculum Vitae Dr. dr. Primariadewi R, SpPA(K) Education : Medical Doctor from UKRIDA Doctoral Degree from Faculty of Medicine University of Indonesia Pathologist Specialist and Consultant from Faculty
Curriculum Vitae Dr. dr. Primariadewi R, SpPA(K) Education : Medical Doctor from UKRIDA Doctoral Degree from Faculty of Medicine University of Indonesia Pathologist Specialist and Consultant from Faculty
WHAT SHOULD WE DO WITH TUMOUR BUDDING IN EARLY COLORECTAL CANCER?
 CANCER STAGING TNM and prognosis in CRC WHAT SHOULD WE DO WITH TUMOUR BUDDING IN EARLY COLORECTAL CANCER? Alessandro Lugli, MD Institute of Pathology University of Bern Switzerland Maastricht, June 19
CANCER STAGING TNM and prognosis in CRC WHAT SHOULD WE DO WITH TUMOUR BUDDING IN EARLY COLORECTAL CANCER? Alessandro Lugli, MD Institute of Pathology University of Bern Switzerland Maastricht, June 19
Reflex Testing Guidelines for Immunotherapy in Non-Small Cell Lung Cancer
 Reflex Testing Guidelines for Immunotherapy in Non-Small Cell Lung Cancer Jimmy Ruiz, MD Assistant Professor Thoracic Oncology Program Wake Forest Comprehensive Cancer Center Disclosures I have no actual
Reflex Testing Guidelines for Immunotherapy in Non-Small Cell Lung Cancer Jimmy Ruiz, MD Assistant Professor Thoracic Oncology Program Wake Forest Comprehensive Cancer Center Disclosures I have no actual
FAQs for UK Pathology Departments
 FAQs for UK Pathology Departments This is an educational piece written for Healthcare Professionals FAQs for UK Pathology Departments If you would like to discuss any of the listed FAQs further, or have
FAQs for UK Pathology Departments This is an educational piece written for Healthcare Professionals FAQs for UK Pathology Departments If you would like to discuss any of the listed FAQs further, or have
Assessment Run B HER-2 IHC. HER-2/chr17 ratio**
 Assessment Run B2 20 HER-2 IHC Material The slide to be stained for HER-2 comprised the following 5 tissues: IHC HER-2 Score* (0, +, 2+,3+) FISH HER-2/chr7 ratio**. Breast ductal carcinoma 0..3 2. Breast
Assessment Run B2 20 HER-2 IHC Material The slide to be stained for HER-2 comprised the following 5 tissues: IHC HER-2 Score* (0, +, 2+,3+) FISH HER-2/chr7 ratio**. Breast ductal carcinoma 0..3 2. Breast
Assessment Run GATA3
 Assessment Run 44 2015 GATA3 Material The slide to be stained for GATA3 comprised: 1. Tonsil 2. Kidney, 3. Urothelial carcinoma, 4. Breast ductal carcinoma, 5. Colon adenocarcinoma All tissues were fixed
Assessment Run 44 2015 GATA3 Material The slide to be stained for GATA3 comprised: 1. Tonsil 2. Kidney, 3. Urothelial carcinoma, 4. Breast ductal carcinoma, 5. Colon adenocarcinoma All tissues were fixed
Cancers of unknown primary : Knowing the unknown. Prof. Ahmed Hossain Professor of Medicine SSMC
 Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
WT1, Estrogen Receptor, and Progesterone Receptor as Markers for Breast or Ovarian Primary Sites in Metastatic Adenocarcinoma to Body Fluids
 Anatomic Pathology / WT1, ESTROGEN RECEPTOR, AND PROGESTERONE RECEPTOR IN CYTOLOGY OF BODY FLUIDS WT1, Estrogen Receptor, and Progesterone Receptor as Markers for Breast or Ovarian Primary Sites in Metastatic
Anatomic Pathology / WT1, ESTROGEN RECEPTOR, AND PROGESTERONE RECEPTOR IN CYTOLOGY OF BODY FLUIDS WT1, Estrogen Receptor, and Progesterone Receptor as Markers for Breast or Ovarian Primary Sites in Metastatic
Assessment Run C1 2017
 Assessment Run C1 2017 PD-L1 The first assessment in this new NordiQC Companion module C1 focused on the accuracy of the PD-L1 IHC assays performed by the participating laboratories to identify patients
Assessment Run C1 2017 PD-L1 The first assessment in this new NordiQC Companion module C1 focused on the accuracy of the PD-L1 IHC assays performed by the participating laboratories to identify patients
Human Papillomavirus Testing in Head and Neck Carcinomas
 Human Papillomavirus Testing in Head and Neck Carcinomas Guideline from the College of American Pathologists Early Online Release Publication: Archives of Pathology & Laboratory Medicine 12/18/2017 Overview
Human Papillomavirus Testing in Head and Neck Carcinomas Guideline from the College of American Pathologists Early Online Release Publication: Archives of Pathology & Laboratory Medicine 12/18/2017 Overview
Supplementary Online Content
 Supplementary Online Content Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologistbased assessment of 4 immunohistochemistry assays for PD-L1 expression in non small cell lung
Supplementary Online Content Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologistbased assessment of 4 immunohistochemistry assays for PD-L1 expression in non small cell lung
VENTANA MMR IHC Panel Interpretation Guide for Staining of Colorectal Tissue
 VENTANA MMR IHC Panel Interpretation Guide for Staining of Colorectal Tissue VENTANA anti-mlh1 (M1) Mouse Monoclonal Primary Antibody VENTANA anti-pms2 (A16-4) Mouse Monoclonal Primary Antibody VENTANA
VENTANA MMR IHC Panel Interpretation Guide for Staining of Colorectal Tissue VENTANA anti-mlh1 (M1) Mouse Monoclonal Primary Antibody VENTANA anti-pms2 (A16-4) Mouse Monoclonal Primary Antibody VENTANA
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER. Guidelines for the assessment of mismatch. Colorectal Cancer
 COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer March 2017 1 Background Mismatch repair (MMR) deficiency is seen in approximately
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer March 2017 1 Background Mismatch repair (MMR) deficiency is seen in approximately
HER2 CISH pharmdx TM Kit Interpretation Guide Breast Cancer
 P A T H O L O G Y HER2 CISH pharmdx TM Kit Interpretation Guide Breast Cancer FROM CERTAINTY COMES TRUST For in vitro diagnostic use HER2 CISH pharmdx Kit HER2 CISH pharmdx Kit is intended for dual-color
P A T H O L O G Y HER2 CISH pharmdx TM Kit Interpretation Guide Breast Cancer FROM CERTAINTY COMES TRUST For in vitro diagnostic use HER2 CISH pharmdx Kit HER2 CISH pharmdx Kit is intended for dual-color
Clinical Utility of Diagnostic Tests
 Clinical Utility of Diagnostic Tests David A. Eberhard MD, PhD Director, Pre-Clinical Genomic Pathology, Lineberger Comprehensive Cancer Center Associate Professor, Depts. of Pathology and Pharmacology
Clinical Utility of Diagnostic Tests David A. Eberhard MD, PhD Director, Pre-Clinical Genomic Pathology, Lineberger Comprehensive Cancer Center Associate Professor, Depts. of Pathology and Pharmacology
RNA preparation from extracted paraffin cores:
 Supplementary methods, Nielsen et al., A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor positive breast cancer.
Supplementary methods, Nielsen et al., A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor positive breast cancer.
Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors
 Q: How is the strength of recommendation determined in the new molecular testing guideline? A: The strength of recommendation is determined by the strength of the available data (evidence). Strong Recommendation:
Q: How is the strength of recommendation determined in the new molecular testing guideline? A: The strength of recommendation is determined by the strength of the available data (evidence). Strong Recommendation:
Estrogen receptor (ER)
 Material The slide to be stained for ER comprised: Assessment B25 208 Estrogen receptor (ER) No. Tissue ER-positivity* ER-intensity*. Uterine cervix 80-90% Moderate to strong 2. Tonsil < 2-5% Weak to strong
Material The slide to be stained for ER comprised: Assessment B25 208 Estrogen receptor (ER) No. Tissue ER-positivity* ER-intensity*. Uterine cervix 80-90% Moderate to strong 2. Tonsil < 2-5% Weak to strong
HOW TO GET THE MOST INFORMATION FROM A TUMOR BIOPSY
 HOW TO GET THE MOST INFORMATION FROM A TUMOR BIOPSY 7 TH Annual New York Lung Cancer Symposium Saturday, November 10, 2012 William D. Travis, M.D. Attending Thoracic Pathologist Memorial Sloan Kettering
HOW TO GET THE MOST INFORMATION FROM A TUMOR BIOPSY 7 TH Annual New York Lung Cancer Symposium Saturday, November 10, 2012 William D. Travis, M.D. Attending Thoracic Pathologist Memorial Sloan Kettering
Carcinoembryonic antigen (CEA)
 Assessment Run 37 2013 Carcinoembryonic antigen (CEA) Material The slide to be stained for CEA comprised: 1. Appendix, 2. Liver, 3-4. Colon adenocarcinoma, 5. Urothelial carcinoma All tissues were fixed
Assessment Run 37 2013 Carcinoembryonic antigen (CEA) Material The slide to be stained for CEA comprised: 1. Appendix, 2. Liver, 3-4. Colon adenocarcinoma, 5. Urothelial carcinoma All tissues were fixed
Patterns of E.cadherin and Estrogen receptor Expression in Histological Sections of Sudanese Patients with Breast Carcinoma
 Patterns of E.cadherin and Estrogen receptor Expression in Histological Sections of Sudanese Patients with Breast Carcinoma Hadia. Mohammed. Abdalla. Abdalrhman *, Elsadig.A.Adam, Ayda.D.A.Allatif 3,'Namareg.E.Afadul
Patterns of E.cadherin and Estrogen receptor Expression in Histological Sections of Sudanese Patients with Breast Carcinoma Hadia. Mohammed. Abdalla. Abdalrhman *, Elsadig.A.Adam, Ayda.D.A.Allatif 3,'Namareg.E.Afadul
Histological Typing Of Cancer And Precancer Of The Oral Mucosa
 Histological Typing Of Cancer And Precancer Of The Oral Mucosa 1 / 7 2 / 7 3 / 7 Histological Typing Of Cancer And Within the last decade, histologic grading has become widely accepted as a powerful indicator
Histological Typing Of Cancer And Precancer Of The Oral Mucosa 1 / 7 2 / 7 3 / 7 Histological Typing Of Cancer And Within the last decade, histologic grading has become widely accepted as a powerful indicator
Cytokeratin 19 (CK19)
 Assessment Run 34 202 Cytokeratin 9 (CK9) Material The slide to be stained for CK9 comprised:. Thyroid gland, 2. Appendix, 3. Esophagus, 4. Papillary thyroid carcinoma, 5 & 6. Pancreatic neuroendocrine
Assessment Run 34 202 Cytokeratin 9 (CK9) Material The slide to be stained for CK9 comprised:. Thyroid gland, 2. Appendix, 3. Esophagus, 4. Papillary thyroid carcinoma, 5 & 6. Pancreatic neuroendocrine
Evolution of Pathology
 1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
Molecular Testing Updates. Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine
 Molecular Testing Updates Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine Keeping Up with Predictive Molecular Testing in Oncology: Technical
Molecular Testing Updates Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine Keeping Up with Predictive Molecular Testing in Oncology: Technical
ACCURACY OF IMMUNOHISTOCHEMISTRY IN EVALUATION
 POL J PATHOL 2011; 2: 95-100 ACCURACY OF IMMUNOHISTOCHEMISTRY IN EVALUATION OF MALIGNANT PLEURAL AND PERITONEAL EFFUSIONS FERESHTEH ENSANI, FARNAZ NEMATIZADEH, GITI IRVANLOU Department of Cytology, Cancer
POL J PATHOL 2011; 2: 95-100 ACCURACY OF IMMUNOHISTOCHEMISTRY IN EVALUATION OF MALIGNANT PLEURAL AND PERITONEAL EFFUSIONS FERESHTEH ENSANI, FARNAZ NEMATIZADEH, GITI IRVANLOU Department of Cytology, Cancer
April 5, :45 AM 1:45 PM MARRIOTT MARQUIS HOTEL & MARINA, MIRAMAR VIGNETTE 1 VIGNETTE 2 VIGNETTE 3* VIGNETTE 4* VIGNETTE 5*
 April 5, 2016 11:45 AM 1:45 PM MARRIOTT MARQUIS HOTEL & MARINA, MIRAMAR CHAIR: DANNY A. MILNER, JR., BRIGHAM & WOMEN S HOSPITAL, BOSTON, MA VIGNETTE 1 VIGNETTE 2 VIGNETTE 3* VIGNETTE 4* VIGNETTE 5* *VIGNETTES
April 5, 2016 11:45 AM 1:45 PM MARRIOTT MARQUIS HOTEL & MARINA, MIRAMAR CHAIR: DANNY A. MILNER, JR., BRIGHAM & WOMEN S HOSPITAL, BOSTON, MA VIGNETTE 1 VIGNETTE 2 VIGNETTE 3* VIGNETTE 4* VIGNETTE 5* *VIGNETTES
VENTANA PD-L1 (SP142) Assay Guiding immunotherapy in NSCLC
 VENTANA (SP142) Assay Guiding immunotherapy in NSCLC Hiker s path: VENTANA (SP142) Assay on non-small cell lung cancer tissue Location: Point Conception, CA VENTANA (SP142) Assay Assess NSCLC patient benefit
VENTANA (SP142) Assay Guiding immunotherapy in NSCLC Hiker s path: VENTANA (SP142) Assay on non-small cell lung cancer tissue Location: Point Conception, CA VENTANA (SP142) Assay Assess NSCLC patient benefit
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer
 Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
Correlation between expression and significance of δ-catenin, CD31, and VEGF of non-small cell lung cancer X.L. Liu 1, L.D. Liu 2, S.G. Zhang 1, S.D. Dai 3, W.Y. Li 1 and L. Zhang 1 1 Thoracic Surgery,
Case 1. Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno
 Case 1 Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno SAMO Interdisciplinary Workshop on Gynecological Tumors Lucern, October
Case 1 Pathology of gynecological cancer. What do we need to know (Case 1) Luca Mazzucchelli Istituto cantonale di patologia Locarno SAMO Interdisciplinary Workshop on Gynecological Tumors Lucern, October
SMH (Myosin, smooth muscle heavy chain)
 Material The slide to be stained for SMH comprised: Assessment Run 50 2017 SMH (Myosin, smooth muscle heavy chain) 1.Tonsil, 2. Esophagus, 3. Breast hyperplasia, 4. Breast ductal carcinoma in situ (DCIS),
Material The slide to be stained for SMH comprised: Assessment Run 50 2017 SMH (Myosin, smooth muscle heavy chain) 1.Tonsil, 2. Esophagus, 3. Breast hyperplasia, 4. Breast ductal carcinoma in situ (DCIS),
Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell lung Cancer (NSCLC) By CISH Technique
 Cancer and Clinical Oncology; Vol. 7, No. 1; 2018 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell
Cancer and Clinical Oncology; Vol. 7, No. 1; 2018 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell
Decipher Bladder Predicts Which Patients May Benefit from Neoadjuvant Chemotherapy Prior to Radical Cystectomy
 Decipher Bladder Predicts Which Patients May Benefit from Neoadjuvant Chemotherapy Prior to Cystectomy Contact the GenomeDx Customer Support Team 1.888.792.1601 (toll-free) customersupport@genomedx.com
Decipher Bladder Predicts Which Patients May Benefit from Neoadjuvant Chemotherapy Prior to Cystectomy Contact the GenomeDx Customer Support Team 1.888.792.1601 (toll-free) customersupport@genomedx.com
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER. Guidelines for the assessment of mismatch. Colorectal Cancer
 COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer January 2015 1 Background Mismatch repair (MMR) deficiency is seen in approximately
COLORECTAL PATHWAY GROUP, MANCHESTER CANCER Guidelines for the assessment of mismatch repair (MMR) status in Colorectal Cancer January 2015 1 Background Mismatch repair (MMR) deficiency is seen in approximately
Applications of IHC. Determination of the primary site in metastatic tumors of unknown origin
 Applications of IHC Determination of the primary site in metastatic tumors of unknown origin Classification of tumors that appear 'undifferentiated' by standard light microscopy Precise classification
Applications of IHC Determination of the primary site in metastatic tumors of unknown origin Classification of tumors that appear 'undifferentiated' by standard light microscopy Precise classification
Sry-related HMG-Box gene 10 (SOX10) protein is a
 A Newly Developed Mouse Monoclonal SOX10 Antibody Is a Highly Sensitive and Specific Marker for Malignant Melanoma, Including Spindle Cell and Desmoplastic Melanomas David Tacha, PhD; Weimin Qi, PhD, MD;
A Newly Developed Mouse Monoclonal SOX10 Antibody Is a Highly Sensitive and Specific Marker for Malignant Melanoma, Including Spindle Cell and Desmoplastic Melanomas David Tacha, PhD; Weimin Qi, PhD, MD;
AdnaNews. News from the meeting on Advances in Circulating Tumor Cells (ACTC), Reythymnon, Crete, Greece October 8-11, 2014.
 News from the meeting on Advances in Circulating Tumor Cells (ACTC), Reythymnon, Crete, Greece October 8-11, 2014. The ACTC meeting (www.actc2014.org) is together with the ISMRC meeting one of the most
News from the meeting on Advances in Circulating Tumor Cells (ACTC), Reythymnon, Crete, Greece October 8-11, 2014. The ACTC meeting (www.actc2014.org) is together with the ISMRC meeting one of the most
Thymidine Kinase 1: A Universal Marker for Cancer
 Cancer and Clinical Oncology; Vol. 2, No. 1; 2013 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Thymidine Kinase 1: A Universal Marker for Cancer Melissa M. Alegre
Cancer and Clinical Oncology; Vol. 2, No. 1; 2013 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Thymidine Kinase 1: A Universal Marker for Cancer Melissa M. Alegre
Product Introduction
 Product Introduction Product Codes: HCL026, HCL027 and HCL028 Contents Introduction to HER2 2 HER2 immunohistochemistry 3 Cell lines as controls 5 HER2 Analyte Control DR IHC 7 HER2 Analyte Control DR
Product Introduction Product Codes: HCL026, HCL027 and HCL028 Contents Introduction to HER2 2 HER2 immunohistochemistry 3 Cell lines as controls 5 HER2 Analyte Control DR IHC 7 HER2 Analyte Control DR
Senior of Histopathology Department at Khartoum, Radiation and Isotopes Center
 EUROPEAN ACADEMIC RESEARCH Vol. IV, Issue 2/ May 2016 ISSN 2286-4822 www.euacademic.org Impact Factor: 3.4546 (UIF) DRJI Value: 5.9 (B+) Immune Histochemical Evaluation of AMACR (P504S) in Prostatic Adenocarcinoma
EUROPEAN ACADEMIC RESEARCH Vol. IV, Issue 2/ May 2016 ISSN 2286-4822 www.euacademic.org Impact Factor: 3.4546 (UIF) DRJI Value: 5.9 (B+) Immune Histochemical Evaluation of AMACR (P504S) in Prostatic Adenocarcinoma
Higher expression of deoxyuridine triphosphatase (dutpase) may predict the metastasis potential of colorectal cancer
 1 Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan; 2 Department of Surgery, Kurume University School of Medicine, Kurume, Japan; 3 Biostatistics Center, Kurume University,
1 Department of Diagnostic Pathology, Kurume University Hospital, Kurume, Japan; 2 Department of Surgery, Kurume University School of Medicine, Kurume, Japan; 3 Biostatistics Center, Kurume University,
Measure Description. Denominator Statement
 CMS ID/CMS QCDR ID: CAP 18 Title: Mismatch Repair (MMR) or Microsatellite Instability (MSI) Biomarker Testing to Inform Clinical Management and Treatment Decisions in Patients with Primary or Metastatic
CMS ID/CMS QCDR ID: CAP 18 Title: Mismatch Repair (MMR) or Microsatellite Instability (MSI) Biomarker Testing to Inform Clinical Management and Treatment Decisions in Patients with Primary or Metastatic
Applying Risk Management Principles to QA in Surgical Pathology: From Principles to Practice
 Applying Risk Management Principles to QA in Surgical Pathology: From Principles to Practice Dr. Gregory Flynn CEO, Institute for Quality Management in Healthcare Managing Director, Quality Management
Applying Risk Management Principles to QA in Surgical Pathology: From Principles to Practice Dr. Gregory Flynn CEO, Institute for Quality Management in Healthcare Managing Director, Quality Management
Development of a Companion Diagnostic for Pembrolizumab in Non Small Cell Lung Cancer Using Immunohistochemistry for Programmed Death Ligand-1
 Development of a Companion Diagnostic for Pembrolizumab in Non Small Cell Lung Cancer Using Immunohistochemistry for Programmed Death Ligand-1 Marisa Dolled-Filhart, PhD; Charlotte Roach, BS; Grant Toland,
Development of a Companion Diagnostic for Pembrolizumab in Non Small Cell Lung Cancer Using Immunohistochemistry for Programmed Death Ligand-1 Marisa Dolled-Filhart, PhD; Charlotte Roach, BS; Grant Toland,
7/6/2015. Cancer Related Deaths: United States. Management of NSCLC TODAY. Emerging mutations as predictive biomarkers in lung cancer: Overview
 Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC-
 Supplemental material and methods Reagents. Hydralazine was purchased from Sigma-Aldrich. Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC- 133, human thyroid medullary
Supplemental material and methods Reagents. Hydralazine was purchased from Sigma-Aldrich. Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC- 133, human thyroid medullary
Assessment Run B HER2 IHC
 Assessment Run B24 2017 HER2 IHC Material The slide to be stained for HER2 comprised the following 5 materials: IHC: HER2 Score* (0, 1+, 2+, 3+) FISH: HER2 gene/chr 17 ratio** 1. Breast carcinoma, no.
Assessment Run B24 2017 HER2 IHC Material The slide to be stained for HER2 comprised the following 5 materials: IHC: HER2 Score* (0, 1+, 2+, 3+) FISH: HER2 gene/chr 17 ratio** 1. Breast carcinoma, no.
Vernieuwing en diagnostiek bij NSCLC: Immunotherapy: PD-L1 analyse: waar staan we
 9e avondsymposium: "Nieuwe ontwikkelingen in de behandeling van NSCLC" 9 november 2016, UMCG Vernieuwing en diagnostiek bij NSCLC: Immunotherapy: PD-L1 analyse: waar staan we Wim Timens Professor and Chair
9e avondsymposium: "Nieuwe ontwikkelingen in de behandeling van NSCLC" 9 november 2016, UMCG Vernieuwing en diagnostiek bij NSCLC: Immunotherapy: PD-L1 analyse: waar staan we Wim Timens Professor and Chair
THE IASLC/ERS/ATS ADENOCARCINOMA CLASSIFICATION RATIONALE AND STRENGTHS
 THE IASLC/ERS/ATS ADENOCARCINOMA CLASSIFICATION RATIONALE AND STRENGTHS PULMONARY PATHOLOGY SOCIETY USCAP, BALTIMORE, March 2, 2013 William D. Travis, M.D. Dept of Pathology, Memorial Sloan-Kettering Cancer
THE IASLC/ERS/ATS ADENOCARCINOMA CLASSIFICATION RATIONALE AND STRENGTHS PULMONARY PATHOLOGY SOCIETY USCAP, BALTIMORE, March 2, 2013 William D. Travis, M.D. Dept of Pathology, Memorial Sloan-Kettering Cancer
Immunohistochemistry on Fluid Specimens: Technical Considerations
 Immunohistochemistry on Fluid Specimens: Technical Considerations Blake Gilks Dept of Pathology University of British Columbia, Vancouver, BC, Canada Disclosures None Learning Objectives At the end of
Immunohistochemistry on Fluid Specimens: Technical Considerations Blake Gilks Dept of Pathology University of British Columbia, Vancouver, BC, Canada Disclosures None Learning Objectives At the end of
Buffered solution containing hydrogen peroxide, detergent and mol/l sodium azide.
 PD-L1 IHC 22C3 pharmdx SK006 50 tests for use with Autostainer Link 48 Intended Use For in vitro diagnostic use. PD-L1 IHC 22C3 pharmdx is a qualitative immunohistochemical assay using Monoclonal Mouse
PD-L1 IHC 22C3 pharmdx SK006 50 tests for use with Autostainer Link 48 Intended Use For in vitro diagnostic use. PD-L1 IHC 22C3 pharmdx is a qualitative immunohistochemical assay using Monoclonal Mouse
Brief Formalin Fixation and Rapid Tissue Processing Do Not Affect the Sensitivity of ER Immunohistochemistry of Breast Core Biopsies
 Brief Formalin Fixation and Rapid Tissue Processing Do Not Affect the Sensitivity of ER Immunohistochemistry of Breast Core Biopsies Victoria Sujoy, MD, Mehrdad Nadji, MD, and Azorides R. Morales, MD From
Brief Formalin Fixation and Rapid Tissue Processing Do Not Affect the Sensitivity of ER Immunohistochemistry of Breast Core Biopsies Victoria Sujoy, MD, Mehrdad Nadji, MD, and Azorides R. Morales, MD From
NEW IHC A n t i b o d i e s
 NEW IHC Antibodies TABLE OF CONTENTS NEW IHC ANTIBODIES from Cell Marque CITED1 (5H6).... 1 Claudin 7 (5D10F3).... 1 GATA1 (4F5).... 1 Transgelin (2A10C2).... 1 NEW IHC ANTIBODIES using RabMAb Technology
NEW IHC Antibodies TABLE OF CONTENTS NEW IHC ANTIBODIES from Cell Marque CITED1 (5H6).... 1 Claudin 7 (5D10F3).... 1 GATA1 (4F5).... 1 Transgelin (2A10C2).... 1 NEW IHC ANTIBODIES using RabMAb Technology
THE STUDY ON RELATIONSHIP BETWEEN CIGARETTE SMOKING AND THE p53 PROTEIN AND P21 PROTEIN EXPRESSION IN NON-SMALL LUNG CANCER
 ( Thmese Journal of ('ancer Research 8(3): 187-19L 1996. THE STUDY ON RELATIONSHIP BETWEEN CIGARETTE SMOKING AND THE p53 PROTEIN AND P21 PROTEIN EXPRESSION IN NON-SMALL LUNG CANCER ZhouBaosen )~j'#:~ lleanguang
( Thmese Journal of ('ancer Research 8(3): 187-19L 1996. THE STUDY ON RELATIONSHIP BETWEEN CIGARETTE SMOKING AND THE p53 PROTEIN AND P21 PROTEIN EXPRESSION IN NON-SMALL LUNG CANCER ZhouBaosen )~j'#:~ lleanguang
HercepTest for the Dako Autostainer Code K5207
 HercepTest for the Dako Autostainer Code K5207 9th edition For immunocytochemical staining. The kit is for 50 tests (100 slides). (126659-002) P04088US_02_K520721-5/2016.05 p. 1/54 Contents Page Intended
HercepTest for the Dako Autostainer Code K5207 9th edition For immunocytochemical staining. The kit is for 50 tests (100 slides). (126659-002) P04088US_02_K520721-5/2016.05 p. 1/54 Contents Page Intended
Quality in Control. ROS1 Analyte Control. Product Codes: HCL022, HCL023 and HCL024
 Quality in Control ROS1 Analyte Control Product Codes: HCL022, HCL023 and HCL024 Contents What is ROS1? 2 The Role of ROS1 in Cancer 3 ROS1 Assessment 3 ROS1 Analyte Control Product Details 4 ROS1 Analyte
Quality in Control ROS1 Analyte Control Product Codes: HCL022, HCL023 and HCL024 Contents What is ROS1? 2 The Role of ROS1 in Cancer 3 ROS1 Assessment 3 ROS1 Analyte Control Product Details 4 ROS1 Analyte
Quality Assurance and Quality Control in the Pathology Dept.
 Quality Assurance and Quality Control in the Pathology Dept. Judith Sandbank M.D. Pathology Assaf-Harofeh Medical Center ISRAEL jsandbank@asaf.health.gov.il 2 nd IBDC, 9 th February, 2012 Pathology as
Quality Assurance and Quality Control in the Pathology Dept. Judith Sandbank M.D. Pathology Assaf-Harofeh Medical Center ISRAEL jsandbank@asaf.health.gov.il 2 nd IBDC, 9 th February, 2012 Pathology as
Identifying ALK+ NSCLC patients for targeted treatment
 VENTANA (D5F3) CDx Assay Identifying + NSCLC patients for targeted treatment VENTANA (D5F3) CDx Assay Identify + NSCLC patients eligible for treatment with XORI, ZYKADIA or ALECENSA NSCLC tissue samples
VENTANA (D5F3) CDx Assay Identifying + NSCLC patients for targeted treatment VENTANA (D5F3) CDx Assay Identify + NSCLC patients eligible for treatment with XORI, ZYKADIA or ALECENSA NSCLC tissue samples
5/1/2009. Squamous Dysplasia/CIS AAH DIPNECH. Adenocarcinoma
 Pathological Assessment of Diagnostic Specimens Keith Kerr Department of Pathology Aberdeen University Medical School Aberdeen Royal Infirmary Foresterhill, Aberdeen, Scotland, UK Tumours of the Lung:
Pathological Assessment of Diagnostic Specimens Keith Kerr Department of Pathology Aberdeen University Medical School Aberdeen Royal Infirmary Foresterhill, Aberdeen, Scotland, UK Tumours of the Lung:
MEDICAL POLICY. Proprietary Information of Excellus Health Plan, Inc. A nonprofit independent licensee of the BlueCross BlueShield Association
 MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
Assessment Run
 Assessment Run 50 2017 S100 Material The slide to be stained for S100 comprised: 1. Appendix, 2. Tonsil, 3. Schwannoma, 4-5. Malignant melanoma, 6. Colon adenocarcinoma. All tissues were fixed in 10% neutral
Assessment Run 50 2017 S100 Material The slide to be stained for S100 comprised: 1. Appendix, 2. Tonsil, 3. Schwannoma, 4-5. Malignant melanoma, 6. Colon adenocarcinoma. All tissues were fixed in 10% neutral
Patient Selection: The Search for Immunotherapy Biomarkers
 Patient Selection: The Search for Immunotherapy Biomarkers Mark A. Socinski, MD Executive Medical Director Florida Hospital Cancer Institute Orlando, Florida Patient Selection Clinical smoking status Histologic
Patient Selection: The Search for Immunotherapy Biomarkers Mark A. Socinski, MD Executive Medical Director Florida Hospital Cancer Institute Orlando, Florida Patient Selection Clinical smoking status Histologic
Digital Pathology and CAP Guidelines
 Digital Pathology and CAP Guidelines Frequently asked questions The VENTANA family of digital pathology products empowers you with the convenience of a comprehensive image and workflow solution. When used
Digital Pathology and CAP Guidelines Frequently asked questions The VENTANA family of digital pathology products empowers you with the convenience of a comprehensive image and workflow solution. When used
The College of American Pathologists (CAP) offers these
 CAP Laboratory Improvement Programs Template for Reporting Results of Biomarker Testing of Specimens From Patients With Carcinoma of the Endometrium Teri A. Longacre, MD; Russell Broaddus, MD, PhD; Linus
CAP Laboratory Improvement Programs Template for Reporting Results of Biomarker Testing of Specimens From Patients With Carcinoma of the Endometrium Teri A. Longacre, MD; Russell Broaddus, MD, PhD; Linus
# Best Practices for IHC Detection and Interpretation of ER, PR, and HER2 Protein Overexpression in Breast Cancer
 #1034 - Best Practices for IHC Detection and Interpretation of ER, PR, and HER2 Protein Overexpression in Breast Cancer Richard W. Cartun, MS, PhD Andrew Ricci, Jr, MD Department of Pathology Hartford
#1034 - Best Practices for IHC Detection and Interpretation of ER, PR, and HER2 Protein Overexpression in Breast Cancer Richard W. Cartun, MS, PhD Andrew Ricci, Jr, MD Department of Pathology Hartford
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma
 Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma Y.-J. Hu 1, X.-Y. Luo 2, Y. Yang 3, C.-Y. Chen 1, Z.-Y. Zhang 4 and X. Guo 1 1 Department
The PAX8 gene is a member of the paired-box family of
 Assessment of the Utility of PAX8 Immunohistochemical Stain in Diagnosing Endocervical Glandular Lesions Li Liang, MD, PhD; Wenxin Zheng, MD; Jinsong Liu, MD, PhD; Sharon X. Liang, MD, PhD Context. PAX8,
Assessment of the Utility of PAX8 Immunohistochemical Stain in Diagnosing Endocervical Glandular Lesions Li Liang, MD, PhD; Wenxin Zheng, MD; Jinsong Liu, MD, PhD; Sharon X. Liang, MD, PhD Context. PAX8,
Breast cancer: IHC classification. Mogens Vyberg Professor of Clinical Pathology Director of NordiQC Aalborg University Hospital, Aalborg, Denmark
 Breast cancer: IHC classification Mogens Vyberg Professor of Clinical Pathology Director of NordiQC Aalborg University Hospital, Aalborg, Denmark http://upload.wikimedia.org/wikipedia/commons/1/1a/breast.svg
Breast cancer: IHC classification Mogens Vyberg Professor of Clinical Pathology Director of NordiQC Aalborg University Hospital, Aalborg, Denmark http://upload.wikimedia.org/wikipedia/commons/1/1a/breast.svg
Lung Anaplastic Lymphoma Kinase (lu-alk)
 Assessment Run 5 207 Lung Anaplastic Lymphoma Kinase (lu-alk) Material The slide to be stained for lu-alk comprised:. Appendix, 2. Tonsil, 3. Merkel cell carcinoma, 4. Anaplastic large cell lymphoma with
Assessment Run 5 207 Lung Anaplastic Lymphoma Kinase (lu-alk) Material The slide to be stained for lu-alk comprised:. Appendix, 2. Tonsil, 3. Merkel cell carcinoma, 4. Anaplastic large cell lymphoma with
Current Status of Biomarkers (including DNA Tumor Markers and Immunohistochemistry in the Laboratory Diagnosis of Tumors)
 Current Status of Biomarkers (including DNA Tumor Markers and Immunohistochemistry in the Laboratory Diagnosis of Tumors) Kael Mikesell, DO McKay-Dee Hospital May 14, 2015 Outline Update to DNA Testing
Current Status of Biomarkers (including DNA Tumor Markers and Immunohistochemistry in the Laboratory Diagnosis of Tumors) Kael Mikesell, DO McKay-Dee Hospital May 14, 2015 Outline Update to DNA Testing
Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano
 Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano Unit of Pathology Fondazione IRCCS Casa Sollievo della Sofferenza San Giovanni Rotondo, Foggia,Italy p.graziano@operapadrepio.it Disclosure
Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano Unit of Pathology Fondazione IRCCS Casa Sollievo della Sofferenza San Giovanni Rotondo, Foggia,Italy p.graziano@operapadrepio.it Disclosure
Study Finds Lung Cancer Subtype Test from UNC, Startup GeneCentric to be Accurate, Reproducible
 Study Finds Lung Cancer Subtype Test from UNC, Startup GeneCentric to be Accurate, Reproducible May 30, 2013 By Ben Butkus A team led by clinical researchers from the University of North Carolina, Chapel
Study Finds Lung Cancer Subtype Test from UNC, Startup GeneCentric to be Accurate, Reproducible May 30, 2013 By Ben Butkus A team led by clinical researchers from the University of North Carolina, Chapel
Reviewer's report. Version: 1 Date: 24 May Reviewer: Cathy Moelans. Reviewer's report:
 Reviewer's report Title: Validation of HER2 testing with core needle biopsy specimens from primary breast cancers in terms of interobserver reproducibility and concordance with surgically resected specimens
Reviewer's report Title: Validation of HER2 testing with core needle biopsy specimens from primary breast cancers in terms of interobserver reproducibility and concordance with surgically resected specimens
Ground Glass Opacities
 Ground Glass Opacities A pathologist s perspective Marie-Christine Aubry, M.D. Professor of Pathology Mayo Clinic Objectives Discuss the proposed new pathologic classification of adenocarcinoma with historical
Ground Glass Opacities A pathologist s perspective Marie-Christine Aubry, M.D. Professor of Pathology Mayo Clinic Objectives Discuss the proposed new pathologic classification of adenocarcinoma with historical
