Clinical Policy Title: Noninvasive testing for H. pylori
|
|
|
- Brice Dixon
- 5 years ago
- Views:
Transcription
1 Clinical Policy Title: Noninvasive testing for H. pylori Clinical Policy Number: Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17, 2016 Next Review Date: August 2017 Policy contains: Helicobacter pylori (H. pylori) infection. Urea breath testing (UBT). Stool antigen testing (SAT) or fecal antigen testing (FAT). Related policies: None. ABOUT THIS POLICY: AmeriHealth Caritas Iowa has developed clinical policies to assist with making coverage determinations. AmeriHealth Caritas Iowa s clinical policies are based on guidelines from established industry sources, such as the Centers for Medicare & Medicaid Services (CMS), state regulatory agencies, the American Medical Association (AMA), medical specialty professional societies, and peer-reviewed professional literature. These clinical policies along with other sources, such as plan benefits and state and federal laws and regulatory requirements, including any state- or plan-specific definition of medically necessary, and the specific facts of the particular situation are considered by AmeriHealth Caritas Iowa when making coverage determinations. In the event of conflict between this clinical policy and plan benefits and/or state or federal laws and/or regulatory requirements, the plan benefits and/or state and federal laws and/or regulatory requirements shall control. AmeriHealth Caritas Iowa s clinical policies are for informational purposes only and not intended as medical advice or to direct treatment. Physicians and other health care providers are solely responsible for the treatment decisions for their patients. AmeriHealth Caritas Iowa s clinical policies are reflective of evidence-based medicine at the time of review. As medical science evolves, AmeriHealth Caritas Iowa will update its clinical policies as necessary. AmeriHealth Caritas Iowa s clinical policies are not guarantees of payment. Coverage policy AmeriHealth Caritas Iowa considers the use of noninvasive diagnostic testing for H. pylori infection including serology, urea breath testing (UBT) or stool antigen testing (SAT) to be clinically proven and, therefore, medically necessary when any of the following criteria are met: Evaluation of new onset dyspepsia in the following groups: Those less than 55 years of age without alarm features, including bleeding, anemia, early satiety, unexplained weight loss, progressive dysphagia, odynophagia, persistent vomiting or family history of gastrointestinal cancers. Active peptic ulcer disease (PUD). Past history of peptic ulcers. Gastric mucosa-associate tissue lymphoma (MALT). Recurrent dyspeptic symptoms despite two weeks of antibiotic treatment. Recurrent dyspeptic symptoms suggestive of reinfection with H. pylori. Post-treatment eradication evaluation (no sooner than four weeks after completion of treatment and using either UBT or SAT) is only required in patients with: 1
2 PUD. Persistent dyspeptic symptoms. H. pylori-associated MALT lymphoma. Previously resected early gastric cancers. Limitations: All other uses of non-invasive diagnostic testing for H. pylori are not medically necessary, including any of the following: Screening individuals without documented upper gastrointestinal symptoms. Screening individuals with non-invasive techniques who have documented alarm features or are greater than 55 years of age, with new onset dyspepsia (upper gastrointestinal endoscopy is indicated). Screening individuals with non-invasive techniques who have had upper gastrointestinal endoscopy, with testing for H. pylori within preceding six weeks. Screening individuals without dyspeptic symptoms post-treatment (other than groups listed above). Screening using UBT or SAT for those without a two-week washout period of proton pump inhibitors (PPIs). Using UBT with carbon-14 in pregnant women or children. Using serologic testing as an eradication evaluation measure post-antibiotic treatment. Simultaneous testing using UBT and SAT concurrently. Alternative covered services: Clinical evaluation by physicians and appropriate standard diagnostic procedures. Background H. pylori is a gram-negative bacterium found in the luminal surface of the gastric epithelium and responsible for the inflammation of the underlying mucosa of the gastric epithelium. This bacterium is unique in its ability to survive in the acidic environment of the stomach because of its high urease enzyme that converts the urea of gastric juice to basic ammonia and carbon dioxide activity. H. pylori infection can be a cause of chronic gastritis, peptic ulcer disease and gastric malignancy. Estimates of the prevalence of H. pylori infection indicate that at least 50 percent of the world s population is affected (Tonkic 2012). Within the United States, prevalence estimates range from 30 percent to 40 percent of the general public. The likelihood of infection increases with age, lower socioeconomic status and male gender (Tonkic 2012). 2
3 Acquisition of the infection occurs mostly during childhood, due to poor living conditions. In the U.S., falling prevalence rates among children correlate with improved living conditions. The majority of individuals infected with H. pylori does not display clinically significant complications and will not require treatment (Tonkic 2012). Signs, symptoms, and conditions associated with H. pylori: Recognition, testing, and treatment for H. pylori can be difficult because of the variety of non-specific gastrointestinal signs and symptoms at presentation. H. pylori causes many symptoms of indigestion or dyspepsia defined as long-term pain in the upper abdomen, which can also be linked to non-ulcer dyspepsia or gastroesophageal reflux disease (GERD). The most frequent features reported in patients with H. pylori are heartburn or epigastric pain, burping, bloating or post-prandial fullness, nausea, vomiting, slow digestion or delayed gastric emptying and acid hyper secretion. H. pylori bacteria inflame the mucosa lining triggering an increase in gastrin, a hormone that stimulates stomach acid release. Elevated levels of gastrin cause excess acid secretion from all areas of the stomach (even those areas not infected). In turn, the high acidity, which further damages the mucosa lining of the stomach, causes ulceration and metaplasia and increases the risk for additional infection by the H. pylori bacteria. There is a strong association between the H. pylori infection, gastric cancer and gastric MALT lymphomas. Eradication of the infection provides a cure to 80 percent of duodenal ulcers unrelated to non-steroidal anti-inflammatory drugs (NSAIDs) and reduces progression of atrophic gastritis and localized gastric MALT lymphoma. Diagnosis of H. pylori: Proper eradication treatment can resolve symptoms of dyspepsia, if it is related to an underlying ulcer disease caused by H. pylori. However, screening is not recommended in all patients with symptoms of dyspepsia, as empiric treatment with acid suppression is effective among some populations. Further, many conditions, including GERD and non-ulcer dyspepsia, are not associated with H. pylori. There are numerous different diagnosis tests for H. pylori including endoscopy, serology, UBT and SAT. These tests are used for diagnosis and evaluation of eradication after treatment. The most invasive diagnostic tool is endoscopy, which entails a biopsy of cells from the pre-pyloric region or fundic region. Endoscopy is not required for diagnosis and is not often the test of choice to confirm diagnosis. Serologic tests identify the immunoglobulin G (IgG) antibodies response to H. pylori bacteria within an individual s blood. This type of testing has lower sensitivity at 85 percent and specificity at 79 percent. The results can be reported as equivocal. It is not useful in testing for eradication of the bacteria, as antibodies may persist for months beyond treatment despite eradication of the bacteria. A common noninvasive test is UBT; this test relies on the bacteria s ability to convert carbon dioxide to urea. UBT centers on labeling isotopes of carbon dioxide urea ingested by the patient. The test 3
4 compares a baseline sample with the post administration level of carbon dioxide using a mass spectrometer or infrared spectrophotometer. This test is commonly used to measure the success of treatment and is widely used, as the samples are easy to collect and can be sent to central laboratories. This reduces the need for expensive equipment at individual providers. Confirmation of diagnosis and eradication is also possible with SAT (or FAT). Identifying H. pylori-specific antigens using either monoclonal or polyclonal antibodies detects the presence of H. pylori. Compared to UBT, monoclonal testing is more sensitive and specific, less expensive, less affected by PPI use, and requires less equipment. Searches AmeriHealth Caritas Iowa searched PubMed and the databases of: UK National Health Services Centre for Reviews and Dissemination. Agency for Healthcare Research and Quality s National Guideline Clearinghouse and other evidence-based practice centers. The Centers for Medicare & Medicaid Services (CMS). We conducted searches on July 11, Search terms were: Helicobacter Infections/diagnosis (Mesh), diagnostic test, stool antigen and urea breath test. We included: Systematic reviews, which pool results from multiple studies to achieve larger sample sizes and greater precision of effect estimation than in smaller primary studies. Systematic reviews use predetermined transparent methods to minimize bias, effectively treating the review as a scientific endeavor, and are thus rated highest in evidence-grading hierarchies. Guidelines based on systematic reviews. Economic analyses, such as cost-effectiveness, and benefit or utility studies (but not simple cost studies), reporting both costs and outcomes sometimes referred to as efficiency studies which also rank near the top of evidence hierarchies. Findings Table 1 summarizes the findings from four systematic reviews of the clinical validity of multiple types of non-invasive H. pylori diagnostic tests. The second table summarizes the results of four systematic reviews of non-invasive testing, one randomized control trial (RCT) comparing diagnostic tests and four professional guidelines related to diagnosis and treatment of H. pylori. Serologic testing for H. pylori: 4
5 The results from available systematic reviews listed in the two tables indicate that serologic testing for antibodies linked to H. pylori infections demonstrate high sensitivity and specificity for initial diagnosis. These tests are especially useful in areas with high prevalence of the infection, but antibodies specifying this infection can differ locally, and the accuracy of this test can range (Childs 2000). Because of the cost effectiveness and ease of blood tests, this is a first line diagnostic measure in some places, but confirmation requires additional testing if the area has a low prevalence (Ling 2013, Chey 2007). Further serologic testing is unreliable for post-treatment eradication verification, as antibodies for H. pylori may remain in the blood for six to 12 months after eradication (Childs 2000). UBT testing: The evidence for UBT testing for screening for H. pylori in a test-and-treat strategy is based on three systematic reviews (Ling 2013, Ferwana 2015, Childs 2006) and professional guidelines. As shown in Table 1, UBT has high sensitivity and specificity, both > 90 percent and can also be used to evaluate if eradication treatments were successful. UBT is considered the gold standard for post-treatment testing (Childs 2000). This test can be prohibitively expensive and some facilities may not have the materials onsite to perform. UBT testing in women and children should only use carbon-13 isotopes, as they provide lower doses of radiation. Table 1. Pooled estimates of sensitivity and specificity of non-invasive tests for H. pylori diagnosis. Table 1. Pooled estimates of sensitivity and specificity of non-invasive tests for H. pylori diagnosis. Type of Test Serological UBT Monoclonal Stool Polyclonal stool Stool testing (with no differentiation Sensitivity Specificity Source % (95% CI) % (95% CI) Childs Ling ( ) 71.7 ( ) ICSI Childs Calvet (83 95) 89.5 (81 95) Ling ( ) 91.6 ( ) Ferwana (95 97) 93 (91 94) ICSI NICE Chey Gisbert (93 96) 97 (94 98) MAS ( ) depending on test Calvet (83 95) 93 (84 97) Chey Gisbert (80 85) 96 (94 97) ICSI
6 Table 1. Pooled estimates of sensitivity and specificity of non-invasive tests for H. pylori diagnosis. Type of Test to type) 95% CI, 95% confidence interval Source Sensitivity % (95% CI) Specificity % (95% CI) Policy updates: We identified two new professional guidelines from the American Gastroenterological Association (Yang 2015) and the American Society for Gastrointestinal Endoscopy (Shaukat 2015). Both addressed the role of endoscopy in managing dyspepsia. Their recommendations are consistent with the original policy. Therefore, no changes to the policy are warranted. Summary of clinical evidence: Citation Ferwana (2015) UBT. Shaukat (2015) for the ASGE The role of endoscopy in dyspepsia. Yang (2015) for the AGA Guidelines for Upper Gastrointestinal Biopsy to Evaluate Content, Methods, Recommendations Systematic review of 3,999 patients for the accuracy of UBT (Level B). Twenty-three studies pooled found UBT testing of Se = 96% and Sp = 93%. UBT showed significant discrimination ability between infected and non-infected states. May provide better comprehensive results than endoscopy because eliminate error with endoscopic biopsy when H. pylori has inconsistent distribution and does not rely on pathologist. Oral flora s urease activity may impact accuracy of results. Recommend initial endoscopy for new-onset dyspepsia in patients age 50 or those with alarm features (moderate quality evidence). Recommend dyspeptic patients age < 50 and without alarm features undergo either initial test and treat approach for H pylori or empiric therapy with a PPI, depending on the prevalence of H pylori infection in their population (moderate quality evidence). Recommend test and treat for H pylori prevalence > 20%. Recommend offering a trial of PPI acid suppression to dyspeptics age < 50, lack alarm features and H pylori negative (low quality evidence). Recommend performing endoscopy if H pylori negative and nonresponsive to empiric PPI (low quality evidence). Guidelines generally support test-and-treat approach to managing dyspepsia. Alternative non-endoscopic tests are available for the diagnosis of H. pylori, but a patient who is already undergoing an esophagogastroduodenoscopy would likely prefer not having to take additional time to undergo one of the alternative H. pylori tests. Cost of 6
7 Citation Dyspepsia in the Absence of Mucosal Lesions. National Institute for Health and Care Excellence (2014) Dyspepsia and GERD. Ling (2013) Content, Methods, Recommendations these alternative tests would offset the cost of diagnosing H. pylori infection endoscopically. Recommendations (Level C). Serological testing is not a viable option for post-treatment/eradication evaluation. Dyspepsia and acute gastrointestinal bleeding require same day referral to endoscopic procedure. Prior to UBT or SAT testing, patient must undergo two week washout out of PPIs, as they can affect results. Carbon-13 UBT in uninvestigated dyspepsia. Systematic review of 21 studies with 4,536 patients (Level A). Serology and UBT are comparably sensitive, but UBT is more sensitive. Clinically, serological is a first-line diagnostic test in the United States; UBT or SAT is also considered first-line diagnostic methods. Lack of standards in processing of UBT creates variability in results. Calvet (2009) SAT SAT is most accurate non-invasive test (Level B). One RCT (n = 199) found among patients with dyspepsia, SAT demonstrated the most accurate alternative to endoscopy compared to UBT. Chey (2007) for the American College of Gastroenterology (ACG) Guidelines for management of helicobacter infection. Level C evidence. Testing for proof of eradication post-treatment is required for those with PUD with persistent dyspeptic symptoms, H. pylori MALT lymphoma, and resected early gastric cancers. Test and treat in those with active PUD, past history of peptic ulcers, and gastric MALT, or those greater than 55 years of age without alarm symptoms. UBT is the most reliable method to test for post-treatment eradication. Post-treatment eradication testing should be at least four weeks after antibiotic regimen completion. Carbon-13 is preferred method of UBT in children and pregnant women, as lower doses of radiation. Cost of UBT testing is driven by expensive and rare equipment and costly labeled isotopes. SAT and UBT are interchangeable diagnostic methods. Use of serological testing for specific antibodies is acceptable in areas of high prevalence (including urban or immigrant areas) as the positive predictive value is good. In low prevalence areas (~20%), avoid relying on antibody or confirm with SAT or UBT before 7
8 Citation Content, Methods, Recommendations treatment. Gisbert (2006) FAT. Talley (2005) for the ACG Guidelines on management of dyspepsia. Institute for Clinical Systems Improvement (ICSI, 2004) Algorithm annotations for dyspepsia and GERD. Childs (2000) Systematic review of 2,499 patients (Level A). Pre-treatment pooled Se = 94%, Sp = 97%, monoclonal FAT had higher sensitivity (Se = 95%, Sp = 96%) versus polyclonal test (Se = 83%, Sp = 96%). Post-treatment evaluation shows distinct difference between monoclonal (Se=91%, Sp=95%) and polyclonal (Se = 76%, SP = 95%), as monoclonal is more sensitive. Monoclonal testing shows better distinction between infection and non-infection, with no equivocal response. Level C evidence. Those greater than 55 years of age, with alarm signs (bleeding, anemia, early satiety, unexplained weight loss, progressive dysphagia, odynophagia, persistent vomiting, and family history of gastrointestinal cancers) should be examined with endoscopy. When prevalence is greater than 10%, use test and treat strategy using non-invasive diagnostic methods; when prevalence is less than 10%, initiate empiric PPI treatment and revert to test and treat for H. pylori, if PPI therapy is unsuccessful. Serological tests require local validation; sensitivity and specificity can range depending on geography. Level C evidence. Symptoms persisting after four weeks of treatment require additional screening with endoscopy. Prior PUD is a predictor of recurrent ulcers; 30-95% of PUD are due to H. pylori, so testing is required for those with past medical history. Most cost effective first line test is serological. Noninvasive and post treatment testing. Systematic review. Serology is not accurate for all patient populations and requires local validation; can have six to 12-month lag time in detecting changes in infection status, although may be the cheapest option. UBT is non-invasive test of choice. Post-treatment testing is required in those with severe or complicated DU or GU, MALT lymphoma, and early gastric cancer. H. pylori treatment guided by testing is preferred choice, although cost savings are slow to see. Can lower cost of treatment further by using empiric treatment where there is high prevalence of infection and DU. 8
9 Glossary Dyspepsia General discomfort of the upper gastrointestinal tract, including upper abdominal pain, heartburn, acid reflux, nausea or vomiting. Endoscopy A non-surgical procedure to enable providers to examine interior structures, such as organs or joints using an endoscope, a flexible tube with a light and camera. Samples can be taken during this procedure. Gastric MALT lymphoma A cancer involving the mucosa-associated lymphoid tissue of the stomach that originates in B cells and is related to inflammation. Gastritis Inflammation of the lining of the stomach. May cause symptoms including upper abdominal pain, vomiting, nausea, bloating, loss of appetite or heart burn. Gastroesophageal reflux disease (GERD) A disease caused by chronic back-flow of acid from the stomach into the esophagus, causing heartburn and leading to irritation and possible damage to the lining of the esophagus. Histology The study of the anatomy of cells and tissues. In medicine, this microscopic study is performed by pathologists to correctly diagnose certain cancers and other diseases. Non-steroidal anti-inflammatory drugs (NSAIDs) A class of drugs with both analgesic and antiinflammatory effects. Serology Testing of blood serum for the presence of specific products that can serve as indicators of disease. References Professional society guidelines/other: Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007; 102(8): Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. National Institute for Health and Clinical Excellence (NICE) website. Published Sep Updated Nov Accessed July 11, Institute for Clinical Systems Improvement (ICSI) Health Care Guideline: Dyspepsia and GERD ICSI website. 9
10 I% pdf. Accessed July 11, Ling D. Carbon-13 urea breath test for Helicobacter pylori infection in patients with uninvestigated ulcerlike dyspepsia: an evidence-based analysis. Ont Health Technol Assess Ser. 2013; 13(19): Talley NJ, Vakil N, Practice Parameters Committee of the American College of Gastroenterology. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005; 100(10): Peer-reviewed references: Calvet X, Sánchez-delgado J, Montserrat A, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis. 2009; 48(10): Childs S, Roberts A, Meinecke-Schmidt V, dewit N, Rubin G. The management of Helicobacter pylori infection in primary care: a systematic review of the literature. Family Practice. 2000; 17(suppl 2): S6. Fashner J, Gitu AC. Diagnosis and Treatment of Peptic Ulcer Disease and H. pylori Infection. Am Fam Physician. 2015; 91(4): Ferwana M, Abdulmajeed I, Alhajiahmed A, et al. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015; 21(4): Gisbert JP, De la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol. 2006; 101(8): Mccoll KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010; 362(17): Tonkic A, Tonkic M, Lehours P, Mégraud F. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter. 2012; 17 Suppl 1: 1 8. Clinical trials: Searched clinicaltrials.gov on July 11, 2016 using terms: helicobacter pylori diagnosis Open Studies. 31 studies found, one relevant. Helicobacter Pylori Genome Project. ClinicalTrials.gov website. Published May 28, Updated May 28, Accessed July 11,
11 Endofaster Test for Helicobacter Pylori Detection in Patients on Proton Pump Inhibitor Therapy. ClinicalTrials.gov website. Published April 28, Updated April 28, Accessed July 11, The Effect of PPI Therapy on the Result of Helicobacter Pylori Diagnostic Tests. ClinicalTrials.gov website. Published January 16, Updated January 18, Accessed July 11, CMS National Coverage Determinations (NCDs): No NCDs identified as of the writing of this policy. Local Coverage Determinations (LCDs): No LCDs identified as of the writing of this policy. Commonly submitted codes Below are the most commonly submitted codes for the service(s)/item(s) subject to this policy. This is not an exhaustive list of codes. Providers are expected to consult the appropriate coding manuals and bill accordingly. CPT codes Description Comment Helicobacter pylori; breath test analysis for urease activity, non-radioactive isotope Helicobacter pylori, stool ICD-10 Code Description Comment A04.8 Helicobacter pylori (H. pylori) B96.81 H. pylori as the cause of diseases classified elsewhere C88.4 MALT lymphoma K27.9 Peptic ulcer, site unspecified R10.13 Dyspepsia HCPCS Level II N/A Description Comment 11
Clinical Policy Title: Noninvasive testing for H. pylori
 Clinical Policy Title: Noninvasive testing for H. pylori Clinical Policy Number: 08.01.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17, 2016 Next
Clinical Policy Title: Noninvasive testing for H. pylori Clinical Policy Number: 08.01.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17, 2016 Next
Clinical Policy Title: Noninvasive testing for H. pylori
 Clinical Policy Title: Noninvasive testing for H. pylori Clinical Policy Number: 08.01.05 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17, 2017 Next
Clinical Policy Title: Noninvasive testing for H. pylori Clinical Policy Number: 08.01.05 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17, 2017 Next
Clinical Policy Title: Breath Testing for H. Pylori
 Clinical Policy Title: Breath Testing for H. Pylori Clinical Policy Number: 08.01.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 19, 2015 Next Review
Clinical Policy Title: Breath Testing for H. Pylori Clinical Policy Number: 08.01.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 19, 2015 Next Review
Clinical Policy Title: Noninvasive testing for H. pylori
 Clinical Policy Title: Noninvasive testing for H. pylori Clinical Policy Number: 08.01.05 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: July 3, 2018 Next
Clinical Policy Title: Noninvasive testing for H. pylori Clinical Policy Number: 08.01.05 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: July 3, 2018 Next
Helicobacter Pylori Testing HELICOBACTER PYLORI TESTING HS-131. Policy Number: HS-131. Original Effective Date: 9/17/2009
 Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Easy Choice Health Plan, Inc. Harmony Health Plan of Illinois, Inc. Missouri Care, Inc. Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona, Inc. WellCare Health Insurance of Illinois,
Clinical Policy: Helicobacter Pylori Serology Testing Reference Number: CP.MP.153
 Clinical Policy: Reference Number: CP.MP.153 Effective Date: 12/17 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.153 Effective Date: 12/17 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: helicobacter_pylori_testing 01/01/2019 N/A 01/01/2020 01/01/2019 Policy Effective April 1, 2019 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: helicobacter_pylori_testing 01/01/2019 N/A 01/01/2020 01/01/2019 Policy Effective April 1, 2019 Description
MEDICAL POLICY EFFECTIVE DATE: 05/19/11 REVISED DATE: 05/24/12, 05/23/13 ARCHIVED DATE: 05/22/14 EDITED DATE: 05/28/15, 05/25/16, 05/18/17, 05/17/18
 MEDICAL POLICY SUBJECT: NON-INVASIVE HELICOBACTER PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: NON-INVASIVE HELICOBACTER PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Urea Breath Test for Diagnosis of Helicobactor pylori. Original Policy Date 12:2013
 MP 2.04.04 Urea Breath Test for Diagnosis of Helicobactor pylori Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date 12:2013 Return to Medical Policy Index
MP 2.04.04 Urea Breath Test for Diagnosis of Helicobactor pylori Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date 12:2013 Return to Medical Policy Index
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA o Patients of any age with ALARM signs should be referred through the 2-week referral system o Routine endoscopic investigation
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA o Patients of any age with ALARM signs should be referred through the 2-week referral system o Routine endoscopic investigation
Helicobacter 2008;13:1-6. Am J Gastroent 2007;102: Am J of Med 2004;117:31-35.
 An Update on Helicobacter pylori and Its Treatment Trenika Mitchell, PharmD, BCPS Clinical Assistant Professor University of Kentucky College of Pharmacy October 18, 2008 Objectives Review the epidemiology
An Update on Helicobacter pylori and Its Treatment Trenika Mitchell, PharmD, BCPS Clinical Assistant Professor University of Kentucky College of Pharmacy October 18, 2008 Objectives Review the epidemiology
Treatment of Helicobacter pylori Infection
 Treatment of Helicobacter pylori Infection Epidemiology of H. pylori infection (North America) Which are the high risk groups? Epidemiology of H. pylori infection (North America) Which are the high risk
Treatment of Helicobacter pylori Infection Epidemiology of H. pylori infection (North America) Which are the high risk groups? Epidemiology of H. pylori infection (North America) Which are the high risk
June By: Reza Gholami
 ACG/CAG guideline on Management of Dyspepsia June 2017 By: Reza Gholami DEFINITION OF DYSPEPSIA AND SCOPE OF THE GUIDELINE Dyspepsia was originally defined as any symptoms referable to the upper gastrointestinal
ACG/CAG guideline on Management of Dyspepsia June 2017 By: Reza Gholami DEFINITION OF DYSPEPSIA AND SCOPE OF THE GUIDELINE Dyspepsia was originally defined as any symptoms referable to the upper gastrointestinal
MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) MANAGEMENT OF DYSPEPSIA AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD) Routine endoscopic investigation of patients of any age, presenting with dyspepsia
Clinical Policy Title: Strep testing
 Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Management of dyspepsia and of Helicobacter pylori infection
 Management of dyspepsia and of Helicobacter pylori infection The University of Nottingham John Atherton Wolfson Digestive Diseases Centre University of Nottingham, UK Community management of dyspepsia
Management of dyspepsia and of Helicobacter pylori infection The University of Nottingham John Atherton Wolfson Digestive Diseases Centre University of Nottingham, UK Community management of dyspepsia
Non-Ulcer Dyspepsia: what is it? What can we do with these patients? Overview. Dyspepsia Definition. Functional Dyspepsia. Dyspepsia the Basics
 Non-Ulcer : what is it? What can we do with these patients? Temporal Changes and Geographic Variations in Developing Peptic Ulcer Disease Gastric Cancer 1900 Eamonn M M Quigley MD FACG Alimentary Pharmabiotic
Non-Ulcer : what is it? What can we do with these patients? Temporal Changes and Geographic Variations in Developing Peptic Ulcer Disease Gastric Cancer 1900 Eamonn M M Quigley MD FACG Alimentary Pharmabiotic
6/25/ % 20% 50% 19% Functional Dyspepsia Peptic Ulcer GERD Cancer Other
 Peptic Ulcer Disease and Dyspepsia John M. Inadomi, MD Professor of Medicine UCSF Chief, Clinical Gastroenterology San Francisco General Hospital Case History 49 y/o woman complains of several months of
Peptic Ulcer Disease and Dyspepsia John M. Inadomi, MD Professor of Medicine UCSF Chief, Clinical Gastroenterology San Francisco General Hospital Case History 49 y/o woman complains of several months of
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Management of Dyspepsia
 MPharm Programme Management of Dyspepsia Slide 1 of 28 Learning Objectives Understand the principles and wider implications underpinning evidence based therapeutics in the key clinical specialities Objectively
MPharm Programme Management of Dyspepsia Slide 1 of 28 Learning Objectives Understand the principles and wider implications underpinning evidence based therapeutics in the key clinical specialities Objectively
Clinical Policy Title: Cardiac rehabilitation
 Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
ACG Clinical Guideline: Treatment of Helicobacter pylori Infection
 ACG Clinical Guideline: Treatment of Helicobacter pylori Infection William D. Chey, MD, FACG 1, Grigorios I. Leontiadis, MD, PhD 2, Colin W. Howden, MD, FACG 3 and Steven F. Moss, MD, FACG 4 1 Division
ACG Clinical Guideline: Treatment of Helicobacter pylori Infection William D. Chey, MD, FACG 1, Grigorios I. Leontiadis, MD, PhD 2, Colin W. Howden, MD, FACG 3 and Steven F. Moss, MD, FACG 4 1 Division
American College of Gastroenterology Guideline on the Management of Helicobacter pylori Infection
 American Journal of Gastroenterology ISSN 0002-9270 C 2007 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2007.01393.x Published by Blackwell Publishing American College of Gastroenterology
American Journal of Gastroenterology ISSN 0002-9270 C 2007 by Am. Coll. of Gastroenterology doi: 10.1111/j.1572-0241.2007.01393.x Published by Blackwell Publishing American College of Gastroenterology
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab
 Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
Peptic Ulcer Disease Update
 Peptic Ulcer Disease Update Col Pat Storms RAM 2005 Disclosure Information 84th Annual AsMA Scientific Meeting Col Patrick Storms I have no financial relationships to disclose. I will discuss the following
Peptic Ulcer Disease Update Col Pat Storms RAM 2005 Disclosure Information 84th Annual AsMA Scientific Meeting Col Patrick Storms I have no financial relationships to disclose. I will discuss the following
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening
 Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Helicobacter and gastritis
 1 Helicobacter and gastritis Dr. Hala Al Daghistani Helicobacter pylori is a spiral-shaped gram-negative rod. H. pylori is associated with antral gastritis, duodenal (peptic) ulcer disease, gastric ulcers,
1 Helicobacter and gastritis Dr. Hala Al Daghistani Helicobacter pylori is a spiral-shaped gram-negative rod. H. pylori is associated with antral gastritis, duodenal (peptic) ulcer disease, gastric ulcers,
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Breast cancer index genetic testing
 Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Disclosures. Co-founder and Chief Science Officer, TechLab
 H. pylori testing Disclosures Co-founder and Chief Science Officer, TechLab Learning Objectives Evaluate the appropriate testing methodology by balancing performance, economics, and workflow. Discuss the
H. pylori testing Disclosures Co-founder and Chief Science Officer, TechLab Learning Objectives Evaluate the appropriate testing methodology by balancing performance, economics, and workflow. Discuss the
National Digestive Diseases Information Clearinghouse
 Gastritis National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is gastritis? Gastritis is a condition in which the stomach
Gastritis National Digestive Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is gastritis? Gastritis is a condition in which the stomach
Clinical Policy Title: Genicular nerve block
 Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Zoster (shingles) vaccine
 Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
The significance of Helicobacter pylori in the approach of dyspepsia in primary care Arents, Nicolaas Lodevikus Augustinus
 University of Groningen The significance of Helicobacter pylori in the approach of dyspepsia in primary care Arents, Nicolaas Lodevikus Augustinus IMPORTANT NOTE: You are advised to consult the publisher's
University of Groningen The significance of Helicobacter pylori in the approach of dyspepsia in primary care Arents, Nicolaas Lodevikus Augustinus IMPORTANT NOTE: You are advised to consult the publisher's
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Acid-Peptic Diseases of the Stomach and Duodenum Including Helicobacter pylori and NSAIDs Prof. Sheila Crowe
 Acid-Peptic Diseases of the Stomach and Duodenum Including Helicobacter pylori and NSAIDs 1 Division of Gastroenterology UC San Diego School of Medicine Clinical presentations of Helicobacter pylori infection
Acid-Peptic Diseases of the Stomach and Duodenum Including Helicobacter pylori and NSAIDs 1 Division of Gastroenterology UC San Diego School of Medicine Clinical presentations of Helicobacter pylori infection
PEPTIC ULCER DISEASE JOHN R SALTZMAN, MD. Director of Endoscopy Brigham and Women s Hospital Professor of Medicine Harvard Medical School
 PEPTIC ULCER DISEASE JOHN R SALTZMAN, MD Director of Endoscopy Brigham and Women s Hospital Professor of Medicine Harvard Medical School No disclosures Disclosures Overview Causes of peptic ulcer disease
PEPTIC ULCER DISEASE JOHN R SALTZMAN, MD Director of Endoscopy Brigham and Women s Hospital Professor of Medicine Harvard Medical School No disclosures Disclosures Overview Causes of peptic ulcer disease
Proton Pump Inhibitors Drug Class Prior Authorization Protocol
 Proton Pump Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: November 15, 2017 Effective Date: January 1, 2018 This policy has been developed through review
Proton Pump Inhibitors Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: November 15, 2017 Effective Date: January 1, 2018 This policy has been developed through review
Module 2 Heartburn Glossary
 Absorption Antacids Antibiotic Module 2 Heartburn Glossary Barrett s oesophagus Bloating Body mass index Burping Chief cells Colon Digestion Endoscopy Enteroendocrine cells Epiglottis Epithelium Absorption
Absorption Antacids Antibiotic Module 2 Heartburn Glossary Barrett s oesophagus Bloating Body mass index Burping Chief cells Colon Digestion Endoscopy Enteroendocrine cells Epiglottis Epithelium Absorption
EDUCATION PRACTICE. Persistent Helicobacter pylori Infection After a Course of Antimicrobial Therapy What s Next? Clinical Scenario.
 CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1086 1090 EDUCATION PRACTICE Persistent Helicobacter pylori Infection After a Course of Antimicrobial Therapy What s Next? RICHARD J. SAAD* and WILLIAM D.
CLINICAL GASTROENTEROLOGY AND HEPATOLOGY 2008;6:1086 1090 EDUCATION PRACTICE Persistent Helicobacter pylori Infection After a Course of Antimicrobial Therapy What s Next? RICHARD J. SAAD* and WILLIAM D.
What is the status of Sequential Therapy Versus Standard Triple- Drug Therapy in peptic ulcer disease in eradicating H pylori?
 What is the status of Sequential Therapy Versus Standard Triple- Drug Therapy in peptic ulcer disease in eradicating H pylori? Sequential Therapy Versus Standard Triple- Drug Therapy for Helicobacter pylori
What is the status of Sequential Therapy Versus Standard Triple- Drug Therapy in peptic ulcer disease in eradicating H pylori? Sequential Therapy Versus Standard Triple- Drug Therapy for Helicobacter pylori
594 Lewin, Weinstein, and Riddell s Gastrointestinal Pathology and Its Clinical Implications
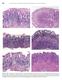 594 Lewin, Weinstein, and Riddell s Gastrointestinal Pathology and Its Clinical Implications Figure 13-20. Stages in the natural history of H. pylori. Biopsies from the antrum are on the left and the oxyntic
594 Lewin, Weinstein, and Riddell s Gastrointestinal Pathology and Its Clinical Implications Figure 13-20. Stages in the natural history of H. pylori. Biopsies from the antrum are on the left and the oxyntic
QUICK QUERIES. Topical Questions, Sound Answers
 QUICK QUERIES Topical Questions, Sound Answers Dyspepsia: An Evidence-Based Approach Alan B. R. Thomson, MD, PhD, FRCPC, FACP, FACG Presented at the University of Alberta s Medical Grand Rounds, University
QUICK QUERIES Topical Questions, Sound Answers Dyspepsia: An Evidence-Based Approach Alan B. R. Thomson, MD, PhD, FRCPC, FACP, FACG Presented at the University of Alberta s Medical Grand Rounds, University
Outline. Definition (s) Epidemiology Pathophysiology Management With an emphasis on recent developments
 Chronic Dyspepsia Eamonn M M Quigley MD FRCP FACP MACG FRCPI Lynda K and David M Underwood Center for Digestive Disorders Houston Methodist Hospital Houston, Texas Outline Definition (s) Epidemiology Pathophysiology
Chronic Dyspepsia Eamonn M M Quigley MD FRCP FACP MACG FRCPI Lynda K and David M Underwood Center for Digestive Disorders Houston Methodist Hospital Houston, Texas Outline Definition (s) Epidemiology Pathophysiology
Clinical Policy Title: Ketamine for treatment-resistant depression
 Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Helicobacter pylori: Diagnosis, treatment and risks of untreated infection
 Helicobacter pylori: Diagnosis, treatment and risks of untreated infection Klaus Mönkemüller Department of Gastroenterology, Hepatology und Infectius Diseases Otto-von-Guericke University, Magdeburg bb
Helicobacter pylori: Diagnosis, treatment and risks of untreated infection Klaus Mönkemüller Department of Gastroenterology, Hepatology und Infectius Diseases Otto-von-Guericke University, Magdeburg bb
Clinical Policy Title: Room humidifiers
 Clinical Policy Title: Room humidifiers Clinical Policy Number: 17.02.05 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review Date:
Clinical Policy Title: Room humidifiers Clinical Policy Number: 17.02.05 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review Date:
Guidelines for the Management of Dyspepsia and GORD. Gastroenterology/ Acute Adult Governance. Drugs and Therapeutics Committee
 Guidelines for the Management of Dyspepsia and GORD Document type: Version: 3.0 Author (name): Author (designation): Validated by Prescribing Dr. G. Lipscomb Date validated October 2015 Ratified by: Date
Guidelines for the Management of Dyspepsia and GORD Document type: Version: 3.0 Author (name): Author (designation): Validated by Prescribing Dr. G. Lipscomb Date validated October 2015 Ratified by: Date
Peptic ulcer disease Disorders of the esophagus
 Peptic ulcer disease Disorders of the esophagus Peptic ulcer disease Burning epigastric pain Exacerbated by fasting Improved with meals Ulcer: disruption of mucosal integrity >5 mm in size, with depth
Peptic ulcer disease Disorders of the esophagus Peptic ulcer disease Burning epigastric pain Exacerbated by fasting Improved with meals Ulcer: disruption of mucosal integrity >5 mm in size, with depth
TRANSPARENCY COMMITTEE OPINION. 13 December 2006
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 December 2006 HELIKIT 75 mg, powder for oral solution CIP : 343 132-1 Applicant : MAYOLY SPINDLER 13 Curea anhydrous
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 December 2006 HELIKIT 75 mg, powder for oral solution CIP : 343 132-1 Applicant : MAYOLY SPINDLER 13 Curea anhydrous
Peptic ulcer disease. Nomin-Erdene. D SOM-531
 Peptic ulcer disease Nomin-Erdene. D SOM-531 Learning objectives Stomach gross anatomy PUD Epidemiology Pathogenesis Clinical manifestation Diagnosing Treatment Complicated ulcer disease Surgical procedures
Peptic ulcer disease Nomin-Erdene. D SOM-531 Learning objectives Stomach gross anatomy PUD Epidemiology Pathogenesis Clinical manifestation Diagnosing Treatment Complicated ulcer disease Surgical procedures
CHAPTER 18. PEPTIC ULCER DISEASE, SELF-ASSESSMENT QUESTIONS. 1. Which of the following is not a common cause of peptic ulcer disease (PUD)?
 CHAPTER 18. PEPTIC ULCER DISEASE, SELF-ASSESSMENT QUESTIONS 1. Which of the following is not a common cause of peptic ulcer disease (PUD)? A. Chronic alcohol ingestion B. Nonsteroidal antiinflammatory
CHAPTER 18. PEPTIC ULCER DISEASE, SELF-ASSESSMENT QUESTIONS 1. Which of the following is not a common cause of peptic ulcer disease (PUD)? A. Chronic alcohol ingestion B. Nonsteroidal antiinflammatory
GI update. Common conditions and concerns my patients frequently asked about
 GI update Common conditions and concerns my patients frequently asked about Specific conditions I ll try to cover today 1. Colon polyps, colorectal cancer and colonoscopy 2. Crohn s disease 3. Peptic ulcer
GI update Common conditions and concerns my patients frequently asked about Specific conditions I ll try to cover today 1. Colon polyps, colorectal cancer and colonoscopy 2. Crohn s disease 3. Peptic ulcer
Management of dyspepsia in adults in primary care
 Dyspepsia Management of dyspepsia in adults in primary care June 2005. The recommendations on referral for endoscopy in this NICE guideline have been amended in line with the recommendation in the NICE
Dyspepsia Management of dyspepsia in adults in primary care June 2005. The recommendations on referral for endoscopy in this NICE guideline have been amended in line with the recommendation in the NICE
One-third of adults experience pain or discomfort in
 GASTROENTEROLOGY 2002;122:1270 1285 Dyspepsia Management in Primary Care: A Decision Analysis of Competing Strategies BRENNAN M. R. SPIEGEL,* NIMISH B. VAKIL, and JOSHUA J. OFMAN*, *Department of Medicine
GASTROENTEROLOGY 2002;122:1270 1285 Dyspepsia Management in Primary Care: A Decision Analysis of Competing Strategies BRENNAN M. R. SPIEGEL,* NIMISH B. VAKIL, and JOSHUA J. OFMAN*, *Department of Medicine
Functional Dyspepsia. Norbert Welkovics Heine van der Walt
 Norbert Welkovics Heine van der Walt Characteristics: Central abdomen Pain or discomfort Not associated with bowel movements No structural or biochemical abnormalty Definition Part of Gastroduodenal disorders
Norbert Welkovics Heine van der Walt Characteristics: Central abdomen Pain or discomfort Not associated with bowel movements No structural or biochemical abnormalty Definition Part of Gastroduodenal disorders
Updates in Evaluation and Management of Dyspepsia and H. Pylori Infection
 Updates in Evaluation and Management of Dyspepsia and H. Pylori Infection Isabel Lee, MD Associate Professor of Health Sciences UCSF Department of Family and Community Medicine Disclosures None 2 Session
Updates in Evaluation and Management of Dyspepsia and H. Pylori Infection Isabel Lee, MD Associate Professor of Health Sciences UCSF Department of Family and Community Medicine Disclosures None 2 Session
Clinical Policy: Fecal Calprotectin Assay Reference Number: CP.MP.135
 Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.135 Effective Date: 11/16 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Gilles Jequier. Commercial Director Organobalance, a Novozymes Company
 "Latest clinical Evidences showing that a proprietary Lactobacillus reuteri Strain can reduce the Symptoms associated with a Helicobacter pylori Infection" Gilles Jequier Commercial Director Organobalance,
"Latest clinical Evidences showing that a proprietary Lactobacillus reuteri Strain can reduce the Symptoms associated with a Helicobacter pylori Infection" Gilles Jequier Commercial Director Organobalance,
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering)
 Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
KK College of Nursing Peptic Ulcer Badil D ass Dass, Lecturer 25th July, 2011
 KK College of Nursing Peptic Ulcer Badil Dass, Lecturer 25 th July, 2011 Objectives: By the end of this lecture, the students t will be able to: Define peptic pp ulcer Describe the etiology and pathology
KK College of Nursing Peptic Ulcer Badil Dass, Lecturer 25 th July, 2011 Objectives: By the end of this lecture, the students t will be able to: Define peptic pp ulcer Describe the etiology and pathology
instrument. When 13C-UBT positive value is greater than or equal to / - 0.4, the the subject can be 1. Data and methods details are as follows:
 Application of 13 C-urea breath test in screening helicobacter pylori infection during health examination in Chengdu, Sichuan YANG Yan-hua. LIU Yu-ping. CHENG You-fu, SHUAI Ping. LU Qiao. ZHENG Xiao-xia,
Application of 13 C-urea breath test in screening helicobacter pylori infection during health examination in Chengdu, Sichuan YANG Yan-hua. LIU Yu-ping. CHENG You-fu, SHUAI Ping. LU Qiao. ZHENG Xiao-xia,
University Medical Center at Brackenridge. Gastroenterology Clinic Worksheet
 Gastroenterology Clinic Worksheet 1. GI Bleeding (occult or symptomatic) a. CBC b. Iron, Ferritin b. Medication history 2. Iron Deficiency Anemia and no evident source (if no iron deficiency consider hematological
Gastroenterology Clinic Worksheet 1. GI Bleeding (occult or symptomatic) a. CBC b. Iron, Ferritin b. Medication history 2. Iron Deficiency Anemia and no evident source (if no iron deficiency consider hematological
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening
 Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
The Nobel Prize in Physiology or Medicine for 2005
 The Nobel Prize in Physiology or Medicine for 2005 jointly to Barry J. Marshall and J. Robin Warren for their discovery of "the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer
The Nobel Prize in Physiology or Medicine for 2005 jointly to Barry J. Marshall and J. Robin Warren for their discovery of "the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer
Assessment of symptomatic response as predictor of Helicobacter pylori status following eradication therapy in patients with ulcer
 618 University Department of Medicine and Therapeutics, Western Infirmary, Glasgow G11 6NT, UK K E L McColl A El-Nujumi L S Murray E M El-Omar A Dickson A W Kelman T E Hilditch Correspondence to: Professor
618 University Department of Medicine and Therapeutics, Western Infirmary, Glasgow G11 6NT, UK K E L McColl A El-Nujumi L S Murray E M El-Omar A Dickson A W Kelman T E Hilditch Correspondence to: Professor
Helicobacter pylori 幽門螺旋桿菌 馬偕紀念醫院新竹分院一般內科, 肝膽腸胃科陳重助醫師
 Helicobacter pylori 幽門螺旋桿菌 馬偕紀念醫院新竹分院一般內科, 肝膽腸胃科陳重助醫師 Hp : Helicobacter pylori Part 1. Pathophysiology and immune response Pathogenesis of Hp infection Part 2. Clinical manifestation Part 3. Dx tests for
Helicobacter pylori 幽門螺旋桿菌 馬偕紀念醫院新竹分院一般內科, 肝膽腸胃科陳重助醫師 Hp : Helicobacter pylori Part 1. Pathophysiology and immune response Pathogenesis of Hp infection Part 2. Clinical manifestation Part 3. Dx tests for
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Ear tubes (tympanostomy)
 Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
Comparison of stool antigen and urea breath test (UBT) methods for detection of Helicobacter pylori infection and the risk factors
 International Journal of Current Research in Medical Sciences ISSN: 2454-5716 P-ISJN: A4372-3064, E -ISJN: A4372-3061 www.ijcrims.com Original Research Article Volume 3, Issue 12-2017 Comparison of stool
International Journal of Current Research in Medical Sciences ISSN: 2454-5716 P-ISJN: A4372-3064, E -ISJN: A4372-3061 www.ijcrims.com Original Research Article Volume 3, Issue 12-2017 Comparison of stool
Investigating dyspepsia Rocco Maurizio Zagari, Lorenzo Fuccio, Franco Bazzoli
 DATE: 11/5/2008-10:27:27 ID:(BMJ)zagr584193 /(Jouve)bmj-001985 DOI: 10.1136/bmj.a1400 Topic(s): Type: InSection:review-article For the full versions of these articles see bmj.com CLINICAL REVIEW Investigating
DATE: 11/5/2008-10:27:27 ID:(BMJ)zagr584193 /(Jouve)bmj-001985 DOI: 10.1136/bmj.a1400 Topic(s): Type: InSection:review-article For the full versions of these articles see bmj.com CLINICAL REVIEW Investigating
COMPUS OPTIMAL THERAPY REPORT. Supporting Informed Decisions. À l appui des décisions éclairées
 OPTIMAL THERAPY REPORT COMPUS Volume 1, Issue 5 March 2007 Current Practice Analysis Report for the Prescribing and Use of Proton Pump Inhibitors (PPIs) Supporting Informed Decisions À l appui des décisions
OPTIMAL THERAPY REPORT COMPUS Volume 1, Issue 5 March 2007 Current Practice Analysis Report for the Prescribing and Use of Proton Pump Inhibitors (PPIs) Supporting Informed Decisions À l appui des décisions
Clinical Policy Title: Pharmacogenomic tests for psychiatric medications
 Clinical Policy Title: Pharmacogenomic tests for psychiatric medications Clinical Policy Number: 02.02.01 Effective Date: October 1, 2015 Initial Review Date: April 15, 2015 Most Recent Review Date: May
Clinical Policy Title: Pharmacogenomic tests for psychiatric medications Clinical Policy Number: 02.02.01 Effective Date: October 1, 2015 Initial Review Date: April 15, 2015 Most Recent Review Date: May
Clinical Policy Title: Fecal biomarkers in inflammatory bowel disease
 Clinical Policy Title: Fecal biomarkers in inflammatory bowel disease Clinical Policy Number: 08.01.08 Effective Date: April 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: November
Clinical Policy Title: Fecal biomarkers in inflammatory bowel disease Clinical Policy Number: 08.01.08 Effective Date: April 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: November
Type of intervention Diagnosis. Economic study type Cost-effectiveness analysis.
 Noninvasive tests as a substitute for histology in the diagnosis of Helicobacter pylori infection Hahn M, Fennerty M B, Corless C L, Magaret N, Lieberman D A, Faigel D O Record Status This is a critical
Noninvasive tests as a substitute for histology in the diagnosis of Helicobacter pylori infection Hahn M, Fennerty M B, Corless C L, Magaret N, Lieberman D A, Faigel D O Record Status This is a critical
GASTROINTESTINAL AND ANTIEMETIC DRUGS. Submitted by: Shaema M. Ali
 GASTROINTESTINAL AND ANTIEMETIC DRUGS Submitted by: Shaema M. Ali GASTROINTESTINAL AND ANTIEMETIC DRUGS by: Shaema M. Ali There are four common medical conditions involving the GI system 1) peptic ulcers
GASTROINTESTINAL AND ANTIEMETIC DRUGS Submitted by: Shaema M. Ali GASTROINTESTINAL AND ANTIEMETIC DRUGS by: Shaema M. Ali There are four common medical conditions involving the GI system 1) peptic ulcers
Clinical Policy: Gastric Electrical Stimulation Reference Number: CP.MP.40
 Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.40 Effective Date: 09/09 Last Review Date: 10/16 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Epidemiology of Peptic Ulcer Disease
 Epidemiology of Peptic Ulcer Disease Introduction Peptic Ulcer Disease (PUD) is disruption of the mucosal integrity of the stomach and/or duodenum leading to a local defect or excavation due to active
Epidemiology of Peptic Ulcer Disease Introduction Peptic Ulcer Disease (PUD) is disruption of the mucosal integrity of the stomach and/or duodenum leading to a local defect or excavation due to active
Indigestion (dyspepsia)
 Commissioning pathways Indigestion (dyspepsia) Supplementary information to be read in conjunction with the pathway Reference Supplementary Information 1.1 Symptom Description Annual incidence 40%. Prevalence
Commissioning pathways Indigestion (dyspepsia) Supplementary information to be read in conjunction with the pathway Reference Supplementary Information 1.1 Symptom Description Annual incidence 40%. Prevalence
Clinical Policy Title: Computerized gait analysis
 Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Treating H. pylori in 2016
 Treating H. pylori in 2016 William D. Chey, MD, FACG Professor of Medicine University of Michigan The Case: A 38 yo Russian man presents with recurrent epigastric pain which occurs after meals and sometimes
Treating H. pylori in 2016 William D. Chey, MD, FACG Professor of Medicine University of Michigan The Case: A 38 yo Russian man presents with recurrent epigastric pain which occurs after meals and sometimes
Helicobacter Pylori Test-and-Treat Strategy for Management of Dyspepsia: A Comprehensive Review
 Citation: (2013) 4, e32; doi:10.1038/ctg.2013.3 & 2013 the American College of Gastroenterology All rights reserved 2155-384X/13 www.nature.com/ctg CLINICAL/NARRATIVE REVIEW Helicobacter Pylori Test-and-Treat
Citation: (2013) 4, e32; doi:10.1038/ctg.2013.3 & 2013 the American College of Gastroenterology All rights reserved 2155-384X/13 www.nature.com/ctg CLINICAL/NARRATIVE REVIEW Helicobacter Pylori Test-and-Treat
Fecoprevalence and determinants of Helicobacter pylor infection among asymptomatic children in Myanmar
 International Journal of Gastroenterology, Hepatology, Transplant & Nutrition Original Article Fecoprevalence and determinants of Helicobacter pylor infection among asymptomatic children in Myanmar Hnin
International Journal of Gastroenterology, Hepatology, Transplant & Nutrition Original Article Fecoprevalence and determinants of Helicobacter pylor infection among asymptomatic children in Myanmar Hnin
A Trip Through the GI Tract: Common GI Diseases and Complaints. Jennifer Curtis, MD
 A Trip Through the GI Tract: Common GI Diseases and Complaints Jennifer Curtis, MD Colon Cancer How does it develop? Most cancers arise from polyps Over time these can turn into cancer Combination of genetic
A Trip Through the GI Tract: Common GI Diseases and Complaints Jennifer Curtis, MD Colon Cancer How does it develop? Most cancers arise from polyps Over time these can turn into cancer Combination of genetic
Treatment and Screening of H. pylori Infection in Alaskan Populations
 Treatment and Screening of H. pylori Infection in Alaskan Populations Matthew F. Deraedt, Pharm.D. Lieutenant United States Public Health Service Alaska Native Medical Center Pharmacy Practice PGY-1 Resident
Treatment and Screening of H. pylori Infection in Alaskan Populations Matthew F. Deraedt, Pharm.D. Lieutenant United States Public Health Service Alaska Native Medical Center Pharmacy Practice PGY-1 Resident
1. Appropriateness of Gastroscopy: Dyspepsia 1
 Special Topic 579 1. Appropriateness of Gastroscopy: Dyspepsia 1 F. Froehlich *, M. Bochud **, J.-J. Gonvers*, R.W. Dubois***, J.-P. Vader **, V. Wietlisbach ***, B. Burnand ** * Policlinique Médicale
Special Topic 579 1. Appropriateness of Gastroscopy: Dyspepsia 1 F. Froehlich *, M. Bochud **, J.-J. Gonvers*, R.W. Dubois***, J.-P. Vader **, V. Wietlisbach ***, B. Burnand ** * Policlinique Médicale
Clinical Policy Title: Seasonal influenza testing
 Clinical Policy Title: Seasonal influenza testing Clinical Policy Number: 07.01.08 Effective Date: October 1, 2017 Initial Review Date: August 17, 2017 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Seasonal influenza testing Clinical Policy Number: 07.01.08 Effective Date: October 1, 2017 Initial Review Date: August 17, 2017 Most Recent Review Date: September 21, 2017 Next
Update on the pathological classification of gastritis. Hala El-Zimaity, M.D. M.S. Epidemiology McMaster University Hamilton, Ontario Canada
 Update on the pathological classification of gastritis Hala El-Zimaity, M.D. M.S. Epidemiology McMaster University Hamilton, Ontario Canada CLASSIFICATION GASTRITIS GASTROPATHY 1. Acute 2. Chronic 3. Uncommon
Update on the pathological classification of gastritis Hala El-Zimaity, M.D. M.S. Epidemiology McMaster University Hamilton, Ontario Canada CLASSIFICATION GASTRITIS GASTROPATHY 1. Acute 2. Chronic 3. Uncommon
Clinical Policy Title: Tactile breast imaging
 Clinical Policy Title: Tactile breast imaging Clinical Policy Number: 05.01.07 Effective Date: February 1, 2018 Initial Review Date: November 16, 2017 Most Recent Review Date: January 11, 2018 Next Review
Clinical Policy Title: Tactile breast imaging Clinical Policy Number: 05.01.07 Effective Date: February 1, 2018 Initial Review Date: November 16, 2017 Most Recent Review Date: January 11, 2018 Next Review
Setting The setting was secondary care. The economic study was carried out in Hong Kong, China.
 Cost-effectiveness of Helicobacter pylori "test and treat" for patients with typical reflux symptoms in a population with a high prevalence of H. pylori infection: a Markov model analysis You J H, Wong
Cost-effectiveness of Helicobacter pylori "test and treat" for patients with typical reflux symptoms in a population with a high prevalence of H. pylori infection: a Markov model analysis You J H, Wong
CHAPTER 11 Functional Gastrointestinal Disorders (FGID) Mr. Ashok Kumar Dept of Pharmacy Practice SRM College of Pharmacy SRM University
 CHAPTER 11 Functional Gastrointestinal Disorders (FGID) Mr. Ashok Kumar Dept of Pharmacy Practice SRM College of Pharmacy SRM University 1 Definition of FGID Chronic and recurrent symptoms of the gastrointestinal
CHAPTER 11 Functional Gastrointestinal Disorders (FGID) Mr. Ashok Kumar Dept of Pharmacy Practice SRM College of Pharmacy SRM University 1 Definition of FGID Chronic and recurrent symptoms of the gastrointestinal
Functional Dyspepsia
 Functional Dyspepsia American College of Gastroenterology Boston Massachusetts, June 2015 Brian E. Lacy, PhD, MD, FACG Professor of Medicine Geisel School of Medicine at Dartmouth Chief, Section of Gastroenterology
Functional Dyspepsia American College of Gastroenterology Boston Massachusetts, June 2015 Brian E. Lacy, PhD, MD, FACG Professor of Medicine Geisel School of Medicine at Dartmouth Chief, Section of Gastroenterology
Clinical Policy Title: Esophageal ph monitoring
 Clinical Policy Title: Esophageal ph monitoring Clinical Policy Number: CCP.1381 Effective Date: August 1, 2018 Initial Review Date: June 5, 2018 Most Recent Review Date: July 3, 2018 Next Review Date:
Clinical Policy Title: Esophageal ph monitoring Clinical Policy Number: CCP.1381 Effective Date: August 1, 2018 Initial Review Date: June 5, 2018 Most Recent Review Date: July 3, 2018 Next Review Date:
Research Article Reliability of Diagnostic Tests for Helicobacterpylori Infection
 Gastroenterology Research and Practice Volume 211, Article ID 946, 6 pages doi:1.1155/211/946 Research Article Reliability of Diagnostic Tests for Helicobacterpylori Infection S. Redéen, 1, 2 F. Petersson,
Gastroenterology Research and Practice Volume 211, Article ID 946, 6 pages doi:1.1155/211/946 Research Article Reliability of Diagnostic Tests for Helicobacterpylori Infection S. Redéen, 1, 2 F. Petersson,
