JAK2 inhibition has different therapeutic effects according to myeloproliferative neoplasm development in mice
|
|
|
- Dominick Bond
- 5 years ago
- Views:
Transcription
1 J. Cell. Mol. Med. Vol 19, No 11, 2015 pp JAK2 inhibition has different therapeutic effects according to myeloproliferative neoplasm development in mice Franck Debeurme a, Catherine Lacout a, Claudine Moratal b, Rebecca G. Bagley c, William Vainchenker a, Francisco Adrian c, Jean-Luc Villeval a, * a Inserm, U.1009, Institut Gustave Roussy (IGR), Universite Paris XI, Villejuif, France b ibv, CNRS UMR7277, INSERM U1091, Universite Nice-Sophia Antipolis, Nice, France c Sanofi Oncology, Cambridge, MA, USA Abstract Received: January 7, 2015; Accepted: April 3, 2015 JAK2 inhibition therapy is used to treat patients suffering from myeloproliferative neoplasms (MPN). Conflicting data on this therapy are reported possibly linked to the types of inhibitors or disease type. Therefore, we decided to compare in mice the effect of a JAK2 inhibitor, Fedratinib, in MPN models of increasing severity: polycythemia vera (PV), post-pv myelofibrosis (PPMF) and rapid post-essential thrombocythemia MF (PTMF). The models were generated through JAK2 activation by the JAK2 V617F mutation or MPL constant stimulation. JAK2 inhibition induced a correction of splenomegaly, leucocytosis and microcytosis in all three MPN models. However, the effects on fibrosis, osteosclerosis, granulocytosis, erythropoiesis or platelet counts varied according to the disease severity stage. Strikingly, complete blockade of fibrosis and osteosclerosis was observed in the PPMF model, linked to correction of MK hyper/dysplasia, but not in the PTMF model, suggesting that MF development may also become JAK2-independent. Interestingly, we originally found a decreased in the JAK2 V617F allele burden in progenitor cells from the spleen but not in other cell types. Overall, this study shows that JAK2 inhibition has different effects according to disease phenotypes and can (i) normalize platelet counts, (ii) prevent the development of marrow fibrosis/osteosclerosis at an early stage and (iii) reduce splenomegaly through blockage of stem cell mobilization in the spleen. Keywords: JAK2 inhibitor myeloproliferative neoplasms fibrosis preclinical murine models Introduction *Correspondence to: Jean-Luc VILLEVAL villeval@gustaveroussy.fr Classical BCR-ABL-negative myeloproliferative neoplasms (MPN) include polycythemia vera (PV), essential thrombocytemia (ET) and primary myelofibrosis (PMF). They are malignant hemopathies resulting from the transformation of a multipotent haematopoietic stem cell (HSC). The common mechanism of transformation is the constitutive activation of the cytokine receptor/jak2 pathway that leads to the myeloproliferation. The acquired point mutation JAK2 V617F is the most prevalent (95% of patients with PV and 60% of patients with ET or PMF) [1]. JAK2 V617F is a gain-of-function mutation leading to activation of cytokine receptor pathway, such as the erythropoietin or the thrombopoietin (MPL) receptors. Activating MPL W515X mutations are also observed in less than 10% PMF [2]. In a minority of MPN, lossof-function mutations are also observed for two negative regulators of the JAK/STAT pathways LNK and CBL [3, 4]. Very recently, wholeexome sequencing identified recurrent somatic mutations of calreticulin (CALR) [5, 6]. These mutations are found in ET and PMF patients devoid of JAK2 or MPL mutations but not in PV. Mutant CALR induces JAK2/STAT activation by a yet unknown mechanism and patients with CALR mutations respond to JAK2 inhibitors [7]. In addition to these driver or phenotypic mutations activating the JAK/ STAT pathway, a growing list of somatic mutations in genes that are commonly mutated in other haematological myeloid malignancies have been reported in patients with MPNs at frequencies from 1% to 25% for each of them. They affect genes involved in splicing, epigenetic regulation, or tumour suppressors that may have dual roles in both disease initiation and progression [8]. The discovery of JAK2 V617F has rapidly led to the development of JAK2 inhibitors with different kinase specificities and biological/clinical activities. Ruxolitinib has been approved for the treatment of myelofibrosis inducing major effects on splenomegaly, constitutional symptoms related to inflammation and quality of life but minor effects on survival and the JAK2 V617F clones. It has been recently tested in PV patients resistant to hydroxyurea offering a new option for the treatment of PV [9]. Fedratinib (also called SAR302503/TG101348) doi: /jcmm This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
2 J. Cell. Mol. Med. Vol 19, No 11, 2015 displays a higher degree of kinase selectivity for JAK2 versus the other JAK family members than ruxolitinib or other JAK2 inhibitors [10]. This small molecule has also shown efficacy in treating PMF patients with reduction in splenomegaly and normalization of blood counts [11]. It has been assessed in JAK2 V617F retrovirally transduced mice and KI mice [12, 13]. In these human PV-like mouse models, Fedratinib showed a reduction in white blood cells (WBC), spleen size, histological defects and erythroid dysplasia including tissue progenitor/precursors and haematocrit. An effect on allele burden was observed in the retroviral (RV) model, but no effect on disease-initiating cells in a KI model. Effect on platelets or fibrosis was not evaluated in these models that did not develop very abnormal levels of platelets or fibrosis [12 15]. In this study, we decided to test anti-jak2 therapeutic efficacy, using Fedratinib, in three different murine MPN models: PV, post-pv MF (PPMF) and post-et MF (PTMF). While some parameters, as splenomegaly, leucocytosis and erythroid hyperplasia varied in a similar way in all models, some responses involving platelets, granulocytes, fibrosis or osteosclerosis varied according to disease models and severity. JAK2 inhibition decreases the JAK2 V617F allele burden in progenitor cells from the spleen but not in mature cells or marrow progenitor cells. Overall, this study describes three preclinical models of MPN, recapitulates changes induced by a JAK2 inhibition and finally suggests that it could (i) prevent the development of fibrosis if applied in early-phases, (ii) normalize platelet count and (iii) reduce splenomegaly through reduction in stem cell mobilization from the marrow to the spleen. Materials and methods Generation of PV, PPMF and PTMF models The conditional flexed JAK2 KI mice have been previously described [16]. To express the mutation, KI mice were crossed with VavCre transgenic (TG) mice [16, 17]. These heterozygous recombined JAK2 V617F/WT mice with a wild type (WT) and a mutated Jak2 allele, termed as JAK2 V617F KI mice, were used to generate the PV or PPMF models (Fig. 1). The previously described TPO high mice [18] were used to generate the PTMF model (see Fig. 1 for details). Treatment and analysis of mice The Fedratinib powder was diluted in water containing 0.5% methylcellulose and 0.05% Tween 80. Solutions were administrated once a day (SID) at escalading doses by oral gavage. Maximum tolerated doses (MTD) decreased as disease severity increased and were evaluated, according to high mortality, at 150, 190 or 240 mg/kg (mpk) for the PTMF, PPMF or PV model respectively. Therefore, in the PV model, mice were treated for 15 weeks from 60 to 190 mpk (190 mpk for 8 weeks). In the PPMF model, mice were treated for around 10 weeks from 100 to 150 mpk (150 mpk for 4 weeks). In the PTMF model, mice were treated for 8 weeks from 60 to 120 mpk (120 mpk for 3 weeks) (Fig. 1). Haemoglobin (Hb), mean corpuscular or globular volume (MCV/MGV), haematocrit, red blood cell (RBC), platelet and WBC counts were determined using an automated counter (MS9; Schloessing Melet, Osny, France) on blood collected from the retro-orbital plexus in citrated tubes. BM cells were removed by flushing both femurs. Spleens were weighted and single cell suspensions were prepared. Histology and flow cytometry of blood, BM and spleen were used as described in supplemental data. Progenitor cell study Progenitor cell assays were carried out in 1 ml methylcellulose MethoCult 32/34 (Stemcell Technologies, Vancouver, Canada) without stimulus or maximally stimulated by interleukin 3 (IL3), IL6, TPO, SCF and EPO. Cultures in duplicate were scored after 2 days for colony-forming unit-erythroid (CFU-E) assays and 8 days for burst forming unit-erythroid (BFU-E) and CFU-granulocyte macrophage (CFU-GM) assays. Total progenitor cell number was calculated assuming that one femur represents 6% of the total marrow and from the total number of cells isolated from the spleen. Statistical analysis Results are presented as mean SEM and data were analysed with the two-tailed Student s t-test. Results Fedratinib treatment, pharmacokinetic (PK) and pharmacodynamic (PD) analysis To test the therapeutic utility and efficacy of Fedratinib in classical MPN, we treated mice developing diseases mimicking human PV, PPMF and PTMF (Fig. 1). Mice were treated as described in materials and methods for PD/PK analysis and determination of MTD. PK studies revealed in the PV model that a single dose of 120 or 240 mpk was able to maintain a plasma concentration above the IC 50 for 18 or >24 hrs respectively. IC 50 (190 nm) was determined, using the Phosphoflow assay, as the dose was able to decrease by half the level of P-Stat3 (or P-Stat5) induced by TPO in Baf3MPL cells. Using Western blot analysis as a PD assay, a dose of 190 mpk was able to completely suppress Stat3 and Stat5 phosphorylation observed in BM cells after a 10 min. Epo (10 U/ml) and IL3 (0.1 lg/ml) stimulation (data not shown). Effect on blood parameters In all MPN models, Fedratinib induced a reduction in WBC counts towards normal values after 20 days of treatment (Fig. 2). This was clearly observed for the high leucocytosis of PTMF mice, where a decrease from WBC/l to WBC/l was observed after 1 month of treatment. The drop was mainly in the PTMF model 2565
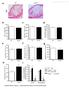
3 Fig. 1 Myeloproliferative neoplasms (MPN) animal models developed to test the therapeutic utility of Fedratinib. We developed three models of MPN corresponding to three degrees of disease severity. The polycythemia vera (PV) model is the milder one but it slowly evolves into post-pv myelofibrosis (PPMF), a more severe form of MPN with fibrosis, reduction in polycythemia and possible anaemia. The post-essential thrombocythemia MF (PTMF) form is the most severe form of MPN starting with initial thrombocytosis, leucocytosis and anaemia and progressively evolving into severe pancytopenia and premature death. The PV or PPMF murine models were derived from lethally irradiated recipient mice (9.5 Gy) transplanted with a mixture of BM cells (BMT) collected from JAK2 V617F KI (1/3) and WT (2/3) mice [16]. These mice develop a disease mimicking human PV evolving into severe PPMF around 7 months after transplantation [16] and were studied from 13 to 28 weeks after transplantation for the PV phenotype or from 22 to 32 weeks after transplantation for the PPMF phenotype. To monitor the response of neoplastic cells (also called JAK2 V617F allele burden) to the treatment, in the PPMF model, we transplanted a mixture of Ly5.1+2 WT cells and Ly5.2 JAK2 V617F KI cells into Ly5.1 WT recipient mice. JAK2 V617F allele burden was measured by monitoring the Ly5.2 allele by FACS analysis. Competitive WT cells and residual endogenous reconstitution from the WT recipient were measured using the Ly5.1+2 alleles or the Ly5.1 allele respectively. The PTMF model (called TPO high ) derives from the recipient mice transplanted with BM cells transduced with a retrovirus (RV) expressing the TPO gene. Severe PTMF quickly occurred around 3 months after transplantation [18]. Briefly, 4 days after 5-fluorouracil (5-FU) treatment (150 mg/kg), BM cells from two WT C57Bl/6 femurs were co-cultivated for 4 days with 10 5 MPZenTPO virus-producing GP/E-86 cells in 20 ml DMEM containing IL3, SCF and 20% FCS. Non-adherent cells were removed and injected into lethally irradiated congenic recipient mice. Mice were treated with Fedratinib as described in materials and methods by oral gavage Semel in Die (SID). because of a decrease in Gr1 + /CD11b + (from 26 3% to 6 1% after 20 days of treatment, P < 0.01). In contrast, in the PV and PPMF models, the percentage of Gr1 + /CD11b + or granulocytes increased, especially in the PV model (Fig. 2), and the modest drop in WBC was mainly because of a decrease in CD4 + cells (maximal drops from 35 1to172% at 63 days or from 19 1to133% at 31 days in the PV or PPMF models, respectively, P < 0.05). This difference maybe because of the type of circulating myeloid cells, immature (myelemia) in the TPO high model [18] and mature in the JAK2 V617F KI models. Platelet level responses to Fedratinib also varied according to the models (Fig. 2). In the PTMF model, thrombocytosis was initially observed (ET-like phenotype) but dropped concomitantly to the development of myelofibrosis (see vehicle). Fedratinib suppressed thrombocytosis after 3 weeks of treatment, but interestingly did not worsen thrombopenia observed with the vehicle-treated mice around 50 days after treatment (around platelets/ll). In the PV and PPMF models, a mild thrombopenia was observed and instead of worsening the phenotype, Fedratinib treatment normalized platelet counts. In the PPMF model, a durable increase was observed from 43 to 63 days 2566
4 J. Cell. Mol. Med. Vol 19, No 11, 2015 Fig. 2 Effect of Fedratinib on blood cell parameters in polycythemia vera (PV), post-pv myelofibrosis (PPMF) and rapid post-essential thrombocythemia MF (PTMF) mice. Blood parameters from Fedratinib (filled circles, n = 3 15)- and vehicle (open circles, n = 3 15)-treated mice were studied. WBC: white blood cells (10 3 cells/ll), granulocytes (%), platelets (10 3 / ll), RBC: red blood cells (10 6 cells/ll), MGV: mean globular volume (or mean corpuscular volume, MCV) (fl) and haematocrit (%) and haemoglobin (g/dl). The grey lines indicate the normal values measured in WT C57/Bl6 mice. Results are mean values SEM, *P (end-point) of treatment (P < 0.01). In the PV model, the same tendency towards a platelet level increase was observed after 93 days of treatment. Strikingly, the high level of RBC in the PV model dropped under Fedratinib treatment to progressively reach normal levels (Fig. 2). In the PPMF model, RBC numbers drop during the survey because of MF development and Fedratinib did not modify this drop. In the PTMF model, the very low RBC levels only slightly worsened during Fedratinib treatment. The MGV clearly increased in response to Fedratinib (Fig. 2) in all models. From the initial microcytosis because of iron deficiency in JAK2 V617F KI mice [19], animals even developed a macrocytosis for the PV model and reached normal MGV in both MF models. As a consequence, haematocrit and Hb levels (Fig. 2) were hardly modified by the treatment. Reticulocyte counts decreased in the PV (day 85) and PPMF (day 67) models from around 8 10% to around 3 4% (normal values around 3%) showing restraining of erythropoiesis in the JAK2 V617F models. In contrast, in the PTMF model, the values remained close to 10% for the Fedratinib- and vehicle-treated mice showing that Fedratinib did not worsen stressed erythropoiesis in high oxygen demand. Effect on BM and spleen BM cellularity was low (Fig. 3A) in all models, particularly in the PTMF model, and Fedratinib treatment did not increase it. Similarly, Fedratinib did not modify BM cell type composition. Compared to WT mice, the percentage of myeloid cells (Gr-1/Mac1 and Mac1) in the PV and 2567
5 PPMF models remained high while B cells were very low. In the PTMF model, the percentage of myeloid (especially Mac1 + ) cells remained high and those of erythroblasts remained low. Splenomegaly was observed in all models, particularly in the PPMF model (Fig. 3B). It was mainly because of erythroid hyperplasia in the PV/PPMF models and of myeloid hyperplasia in the PTMF model. Fedratinib sharply decreased spleen weight to almost normal values in all models. In the PV model, spleen cell composition almost returned to normal values with a large decrease in erythroblasts (Ter /CD71 + ) cells and an increase in the percentages of B and T cells. The same tendency was observed in the PPMF model but the level of erythroblasts still remained elevated. In very sharp contrast, the spleen cell composition in the PTMF model was unchanged by the treatment. Fedratinib had a profound effect on megakaryocyte (MK) hyperplasia except in the PTMF model. In the PV model, Fedratinib induced a potent decrease in MK density in the spleen but not in BM (Fig. S1A and B). Preliminary results also showed a reduction after treatment of the high ploidy observed in JAK2 V617F KI mice (mean ploidy from 20 2N before to 14 1N after treatment, WT mice 16 2N, n = 4). In the PPMF model, Fedratinib induced a decrease in MK density and size in spleen. In BM, only MK size was reduced (Fig. S1C). Total transforming growth factor (TGF)-b1, a MK-derived cytokine involved in fibrosis, tended to decrease in BM and spleen (Fig. S2). In the PTMF model, no effect in spleen and BM MK was observed, probably because of the extreme MK hyperplasia observed in the TPO high model (Fig. S1A and B). At the progenitor cell level, methylcellulose cultures revealed a decrease in the total high numbers of spleen CFU-GM, BFU-E and CFU-E in all models (P < 0.05) after Fedratinib treatment. In the BM, there was a decrease in the total CFU-E number only for the PV model and an increase in the total BFU-E number for the PPMF model, all other total number of BM progenitor cells showing no variation during treatment. In the PPMF model, flow cytometry analysis (Fig. 3D) showed that Fedratinib strikingly restored normal proportions of BM and spleen MEP, GMP, CMP and overall total progenitor cells (LK/ LSK cells) suggesting that defects in immature cells were corrected in contrast to, or before, progenies. Histology and Fibrosis of the BM and spleen In the PV and PPMF models, histology of the organs with the haematoxylin and eosin/safran (HES) staining reveals that Fedratinib strikingly allowed a return to a quasi-normal architecture of spleen and BM with lymphoid areas (white pulp) appearing in the spleen (Fig. 4A and B, Fig. S1A and B). In the PTMF model, no major effect was seen on BM or spleen architecture. In BM, osteoclerosis was observed in the PPMF and PTMF models during the treatment by vehicle. It was fully abolished with the Fedratinib treatment in the PPMF model but not in the PTMF model (Fig. 4A C). In the PV model, no fibrosis developed in the BM (Fig. 4C). In the PPMF model, no BM fibrosis occurred before treatment, but high-grade fibrosis was observed at end of treatment with vehicle (Fig. 4C). Strikingly no BM fibrosis and osteosclerosis were observed after 57 days of Fedratinib treatment (n = 6, Fig. 4C) showing that Fedratinib treatment prevented the development of BM fibrosis and osteosclerosis in this early or slowly developing MF model. However, no effect on BM fibrosis was observed in the PTMF model. Fedratinib treatment did not reduce spleen fibrosis whatever the model (Fig. 4D). At the opposite, an increase in spleen reticulin fibre density, compared to vehicle, was observed after Fedratinib treatment in the PV and PPMF models (Fig. 4D). In the PTMF model, no change in the massive network of spleen reticulin fibres was seen in Fedratinib-treated mice. Overall, Fedratinib contributed to the prevention of developing collagen producing cells or excess of fibre deposition in marrow. However, Fedratinib did not play this curative role in the rapid development of BM fibrosis of the PTMF model or in the treatment of spleen fibrosis in both models showing that JAK2-independent changes within the microenvironment play a crucial role in fibrosis or in the rapid TPO-induced development of fibrosis. JAK2 V617F allele burden Allele burden was assessed in the PPMF model using Ly5.1 WT recipient and Ly5.1+2 donor competitive mice. Myeloid cells (Mac-1/Gr- 1) from JAK2 V617F KI donor origin were identified by their Ly5.2 allotype (Fig. 1). Allele burden at start of treatment (22 weeks after BMT) in blood cells varied from 20% to 83% and increased with the evolution of the disease (Fig. 5A). The treatment had no effect on this evolution compared to vehicle. At the end of treatment, there was no significant difference in the percentage of myeloid cells (Gr1 + / CD11b + ) from KI origin assessed in the blood, but also in BM and spleen of Fedratinib- and vehicle-treated mice (Fig. 5B). However, focusing on progenitor cells by immuno-labelling, the treatment clearly induced a large decrease (>50%) in allele burden, i.e. the percentages of spleen Lin, LK and especially LSK from KI origin and only the percentages of BM CMP and GMP from KI origin (Fig. 5C and D). This result suggests that Fedratinib prevented the mobilization or development of KI stem cells and associated extramedullary haematopoiesis (EMH) in the spleen. This effect may have contributed to the decrease in splenomegaly. Discussion In this study, we investigated the effect of the selective JAK2 inhibitor Fedratinib [10], in mouse models of MPN driven by JAK2 V617F or TPO/MPL/JAK2 with increasing disease severity mimicking human PV, human PV with its evolution into PPMF and human ET with a rapid evolution into PTMF. According to the patient scoring system [20], leucocytosis, anaemia and blast counts [18] would classify our PPMF and PTMF models into low- and high-risk PMF groups respectively. In all models, JAK2 inhibition reduced the excess of blood leucocytes, platelets or RBC. Microcytosis was also reduced with decrease in erythroid hyperplasia, when observed. Splenomegaly was also considerably corrected. Interestingly, the effect of Fedratinib var- 2568
6 J. Cell. Mol. Med. Vol 19, No 11, 2015 Fig. 3 Effect of Fedratinib on BM and spleen cell composition of polycythemia vera (PV), post-pv myelofibrosis (PPMF) and rapid post-essential thrombocythemia MF (PTMF) mice. (A) BM cell numbers per femur (910 6 ) (top) and cell type percentages in vehicle (VEH, top, white column)-, Fedratinib (FED, middle, grey column)-treated mice (n = 6 7) and normal WT C57/Bl6 mice (WT, bottom, striped column) (n = 4). Cell types were determined by FACS analysis with antibodies directed against Gr-1, CD11b (Mac1), Terr119/CD71 (TER), CD3 (or CD4 and CD8) and B220. (B) Spleen weight and cell type percentages in vehicle (VEH, top)-, Fedratinib (FED, middle)- treated mice (n = 6 7) and normal WT C57/Bl6 mice (WT, bottom) (n = 4). Cell types were determined by FACS analysis as in A. Normal spleen cell composition was restored by Fedratinib in the PV model. (C) Progenitor cell per cent in BM and spleen from WT C57/Bl6 mice (WT, bottom) (n = 3) and from mice developing PPMF and treated by Fedratinib (FED) or vehicle (VH) (n = 4). Lin cell subpopulations (Gr1, CD11b, B220, CD3 and Ter119 negative) were identified according by FACS analysis: LSK (progenitor cells with multipotent activity, lin, c-kit +, Sca- 1 + ) and subpopulation of LK myeloid progenitor cells (Lin, c-kit + ) including MEP (megakaryocyte-erythroid progenitors, LK, CD34, FcRgII/III ), GMP (granulocytemacrophage progenitors, LK, CD34 +, FcRgII/III high) and CMP (common myeloid progenitors, LK, CD34 +, FcRgII/III low). Fedratinib restored normal proportions of MEP, GMP and CMP in BM and Spleen. All experiments were carried out at the end of treatment, after around 8, 10 or 15 weeks of treatment in the PTMF, PPMF or PV models respectively. Results represented the percent of cell types per singlet and are mean values SEM, *P A B C 2569
7 A B D C Fig. 4 Histology of WT mice and polycythemia vera (PV), post-pv myelofibrosis (PPMF) and rapid post-essential thrombocythemia MF (PTMF) mice treated by Fedratinib or vehicle. BM and spleen were stained with haematoxylin and eosin/safran (HES) (A and B) and silver (C and D). (A) BM HES staining 109 lens. BM osteosclerosis is shown in vehicle-treated PPMF and PTMF mice. Fedratinib-treated PPMF mice do not display osteosclerosis in contrast to Fedratinib-treated PTMF mice. High density of fibrotic tissues responsible for the low cellularity is shown in BM PTMF mice. (B) Spleen HES staining 109 (left) and 2.59 lens (right). Spleen from PV, PPMF and PTMF mice compared to WT mice are characterized by (i) clustered MK, (ii) fibrosis and (iii) hyperplasia of the myeloid red pulp and blending of the lymphoid white pulp areas (910 lens). Treatment with Fedratinib induced decreased in MK density and appearance of lymphoid areas in PPMF mice, slightly in PTMF mice and especially in PV mice (92.5 lens). (C) BM silver staining 109 (left) and 2.59 lens (right). No excess fibrosis is shown in WT and PV mice. High-grade BM fibrosis (large deposit of reticulin fibres) and osteosclerosis are shown in vehicle-treated PPMF and PTMF mice. Fedratinib-treated PPMF mice did not display fibrosis and osteosclerosis (n = 7) in contrast to vehicle-treated PPMF mice (n = 5) and Fedratinib/vehicle-treated PTMF mice, day 57 or 70. (D) Spleen silver staining 109 lens. High-grade fibrosis is shown in PV, PPMF and PTMF mice compared to WT mice. Fedratinib treatment did not reduce fibrosis but seemed to worsen it (especially around the blood vessels) in PV and PPMF mice. This effect may be because of shrinking of the spleen in treated animals. Images were obtained (109 or 2.59/0.3 Zeiss lens) using a Zeiss Axiophot microscope with a Zeiss AxioCam Mrc camera and the AxioVision Rel.4.3 acquisition software (Oberkochen, Germany). 2570
8 J. Cell. Mol. Med. Vol 19, No 11, 2015 A B C D Fig. 5 Effect of Fedratinib on JAK2 V617F allele burden in the PPMF model. Competitive grafts composed of 2/3 CD45.1 WT and 1/3 CD45.2 JAK2 V617F KI BM cells were transplanted into recipient mice. Mean percentage over 33% of CD45.2 cells at start of treatment (22 weeks after BMT) showed proliferative advantage of KI cells over WT cells. (A) Percentage of CD blood myeloid (CD11b + /Gr-1 + ) cells (from KI origin) determined by FACS in vehicle- (solid lines) or Fedratinib- (dashed lines) treated recipient mice. (B) Percentage of CD blood, BM and spleen myeloid cells (from KI origin) at end of treatment (n = 7) from animals treated by in vehicle (white column) or Fedratinib (grey column). (C) Percentage of CD Lin, LSK, LK, MEP, CMP and GMP cells in BM from animals treated by vehicle (white column) or Fedratinib (grey column), n = 4. (D) Percentage of CD Lin, LSK, LK, MEP, CMP and GMP cells in BM from animals treated by vehicle (white column) or Fedratinib (grey column), n = 4. Results show that Fedratinib only decreased JAK2 V617F allele burden in immature spleen cells. Results are mean values SEM, *P ied according to the models with increase in low levels of platelets observed in the PPMF model and decrease in high levels observed in the PTMF model. Similarly, the percentage of granulocytes decreased in the PTMF model but increased in the PV model. Interestingly, development of BM fibrosis and osteosclerosis was strikingly suppressed in the PPMF but not in the PTMF models. Paradoxically, spleen fibrosis was not affected in all models and even appeared worsened. Finally, no decrease in allele burden was observed at the exception of spleen early progenitors, suggesting that they contribute to the decrease in splenomegaly. Activation of JAK2, through the JAK2, MPL or CALR mutations, has been identified in the vast majority of classical BCR-ABL-negative MPN. In mice, these mutations generate the corresponding MPN phenotype observed in human [1, 2] (and personal communication), showing that JAK2 activation is the main pathological contributor towards MPN phenotype. Therefore, the development of JAK2 inhibitors has represented a real hope for the treatment of these diseases. Ruxolitinib, which inhibits JAK2 but also TYK2, JAK1 and JAK3 [10] at similar degrees demonstrated durable reduction in splenomegaly and constitutional symptoms with improved quality of life in comparison to placebo-controlled [21] or best available therapy [22] in phase 3 trials and an impact on survival for patients originally randomized to ruxolitinib compared with those originally randomized to placebo in both COMFORT trials [23]. Anaemia and thrombopenia were the main limitations and side effects. Ruxolitinib was approved by the FDA for the treatment of intermediate-2 and high-risk myelofibrosis. However, molecular remission including major drop in allele burden has not been demonstrated [21, 22, 24]. It appears as a good palliative drug for patients suffering from MF, but better drugs, including more selective JAK2 inhibitors, are expected to be more effective in treating MPN [25]. Fedratinib [11] displays a more JAK2-selective inhibitory activity than ruxolitinib [10]. A multi-centre phase I/II trial with high- or intermediate-risk primary or post-pv/et myelofibrosis demonstrated Fedratinib efficacy in reducing splenomegaly, normalizing WBC counts and significantly decreasing JAK2 V617F allele burden for some patients [11]. Main adverse events included diarrhoea (10%) and more often thrombocytopenia (24%) and anaemia (35%). Unfortunately, cases consistent with Wernicke s encephalopathy were reported in patients participating in Fedratinib clinical trials and the trials were put on clinical hold as directed by the FDA [26]. These events have been attributed to thiamine uptake inhibition [27]. 2571
9 Our MPN murine models were studied in transplanted animals to have homogeneous disease groups. Two models were developed from JAK2 V617F KI mice mimicking human PV and evolving into secondary MF (PPMF) [16]. The third model resulted from continuous MPL activation by TPO secretion from haematopoietic cells and models MPL W515L -positive human PMF or PTMF [18]. All models are characterized by splenomegaly with erythroid hyperplasia in the PV/PPMF models and myeloid hyperplasia and anaemia in the PTMF model. Fedratinib was very efficient in reducing erythroid hyperplasia in the PV/PPMF models as shown by the reduced number of MEP, spleen erythroblasts, circulating reticulocytes and RBC, confirming JAK2 inhibition as an option in PV treatment [9]. However, it was less efficient in reducing stressed erythropoiesis in the PTMF model and did not correct the anaemia, as we showed for the JAK1/2 inhibitor Momelotinib [28], possibly because of its JAK2 specificity [29]. Microcytosis, because of iron deficiency [19], was corrected in all models leading to minor effects on haematocrit and Hb levels. Fedratinib was also very efficient in reducing leucocytosis, but not myeloid hyperplasia in the marrow and spleen. MK hyperplasia was reduced in all models. Regarding platelets, it is not well understood why Fedratinib had the paradoxical effect in reducing thrombocytosis in the PTMF model, as expected from a JAK2 inhibitor, but reducing thrombopenia observed in the PPMF model. Increase number of platelets by a JAK2 inhibitor treatment has been reported in mice [30]. As we showed, the mechanism of action may be related to the dual opposite role of MPL inducing either proliferation or blockade of proliferation with low or high levels of stimulation respectively [31]. Thus, decrease in MPL/JAK2 stimulation may either increase or decrease platelet counts. Alternatively it may be because of restoration of MPL level and low JAK2 stimulation by a JAK2 inhibitor in JAK2 V617F cells [32]. No decrease in JAK2 V617F allele burden was observed in the PPMF model in blood, BM and spleen in agreement with reports in ruxolitinib-treated patients [21, 22] or mice [33]. However, when we focused on progenitor cells, we noticed a decrease in the percentage of LSK from neoplastic origin in the spleen but not in the BM. This result may suggest that JAK2 inhibition by Fedratinib specifically blocks the migration induced by JAK2 V617F of early BM cells into extramedullar sites resulting into EMH. Alternatively stem cells residing in the spleen may require a higher JAK2 activation than those residing in the BM possibly because of different stromal environments. These could be the elusive mechanisms resulting into the reduction in splenomegaly in human, a major effect of JAK2 inhibitors on one of the most debilitating symptoms of MF [34]. Our preliminary data show that spleen EMH in JAK2 V617F KI mice is partially dependent on the CXCR4/CXCL12 axis. The link between JAK2 V617F and CXCR4 is not well understood and there are controversial results concerning the level of CXCR4 on CD34 + cells and their response to CXCL12 [35]. Strikingly, PPMF mice treated with Fedratinib did not develop BM myelofibrosis or osteosclerosis in contrast to PTMF mice. Blockade of myelofibrosis was associated with a decrease in MK hyperplasia, large size and ploidy, suggesting correction of dysmegakaryopoiesis, and decrease in TGF-b1 concentrations in organs that may have contributed to the prevention of fibrosis [36]. In contrast, MK hyperplasia/dysplasia was maintained in the PTMF model during the treatment. Overall, this result shows that anti-jak2 therapy efficiently prevents excessive deposits of extracellular matrix (ECM) proteins and bone formation in low- but not high-risk PMF model and suggests that this effect is mediated through inhibition of MK hyperplasia and dysplasia. Another explanation is that JAK2 inhibition is not as efficient to inhibit TPO-stimulated MK than JAK2 V617F -stimulated MK and this maybe because of the rapid fibrosis development of this high-risk PMF/PTMF model or different signalling regulation [32, 37]. In contrast, worsening of spleen fibrosis that could have resulted from the shrinking of the organ was observed in all models. The meaning of this observation regarding human MPN remains elusive because, to our knowledge, spleen fibrosis is not reported in patients. The fact that spleen but not marrow fibrosis persisted strongly suggests that the microenvironment play a role in preventing or resorbing extra fibrous tissues during Fedratinib treatment. Reversion of fibrosis has been reported after BM transplantation and after interferon-a treatment for some patients [38]. It has not been reported in large clinical trials with ruxolitinib [22] even if post hoc analysis suggested that JAK1/2 inhibition retards the progression of BM fibrosis for some patients [39]. It would be interesting to know if this correction was associated to early disease stages. In JAK2 V617F overexpressing animal models, reduction in BM reticulin fibre density, without mention about spleen fibrosis or BM osteosclerosis, by several JAK2 inhibitors, including Fedratinib [12], Momelotinib [40], NS-018 [41], G6 [42] and R723 [43] was reported in mice displaying low-grade fibrosis or in early disease stages but not in models displaying high-grade fibrosis. These accumulating data that reinforce our results strongly suggest that correction of BM fibrosis and osteosclerosis development in early MPN phase is strictly mediated through inhibition of JAK2. In contrast, the fact that MK hyperplasia and fibrosis were only slightly modified by Fedratinib in our TPO HIGH models or by other JAK2 inhibitors in JAK2 V617F late-phase disease models [43] suggests that the development of advanced stage myelofibrosis and associated pathological megakaryopoiesis become progressively JAK2-independent in MPN and refractory to JAK2 inhibitors. Changes in the microenvironment are capable to induce a MK differentiation independent of the TPO/MPL/JAK2 axis as observed for c-mpl / mice when treated with 5-FU or by the association of CXCL12 and FGF4. Therefore, other agents acting on MK, the marrow microenvironment such as CXCR4 antagonists or anti-fibrotic drugs seems necessary to treat PMF at advanced stages when JAK2 inhibitors may not be sufficient. Overall, our study shows that JAK2 activation is responsible for deposition of ECM protein by accessory cells and that JAK2 inhibition may prevent this lethal evolution only if applied early in disease evolution. Overall, this study underlines that JAK2 inhibition, using a JAK2- selective inhibitor, may lead to different effects on fibrosis, osteosclerosis, granulocytosis, erythropoiesis or platelet counts according to MPN disease stage. It gives clues on the mechanisms on how JAK2 inhibition may modulate platelet counts, block marrow fibrosis and osteosclerosis and prevent mobilization of early neoplastic cells in extramedullary sites decreasing splenomegaly. Finally, it predicts that JAK2 inhibitors will be more efficacious with less major toxic effects in early/pre-fibrotic MF patients and low-risk rather than in high- or intermediate-risk PMF patients. 2572
10 J. Cell. Mol. Med. Vol 19, No 11, 2015 Acknowledgements The authors thank the staff of the animal facilities of the IGR directed by Patrick Gonin, Olivia Bawa for the histology, Pr Warren Alexander from the Walter Elisa Hall Institute, Melbourne, Australia for the VavCre mice. This work was supported by grants from Inserm, Gustave Roussy, Institut National du Cancer, Cancerop^ole Ile-de-France and by a funding from Ligue Nationale Contre le Cancer (labelled team 2009). Conflicts of interest The authors report no potential conflicts of interest. Author contribution JLV was the principal investigator and takes primary responsibility for the paper. FD, CL, CL and JLV performed the laboratory work and statistical analysis for this study; JLV, FA, WV, RB and S-MH co-ordinated the research; JLV, FD, FA and WV wrote the paper. Supporting information Additional Supporting Information may be found in the online version of this article: Data S1 Methods. Figure S1 MKC from WT, PV, PVMF and PMF mice treated by Fedratinib (FED) or vehicle (VEH) are identified in spleen or BM by immunochemistry using a peroxidase-conjugated anti-vwf polyclonal antibody. Figure S2 TGF-b1 levels in tissue fluids from PVMF mice treated by Fedratinib (FED) or vehicle (VEH). References 1. James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005; 434: Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006; 3: Oh ST, Simonds EF, Jones C, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010; 116: Grand FH, Hidalgo-Curtis CE, Ernst T, et al. Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood. 2009; 113: Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013; 369: Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013; 369: Passamonti F, Caramazza D, Maffioli M. JAK inhibitor in CALR-mutant myelofibrosis. N Engl J Med. 2014; 370: Tefferi A, Vainchenker W. Myeloproliferative neoplasms: molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011; 29: Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 Inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer. 2014; 120: Zhou T, Georgeon S, Moser R, et al. Specificity and mechanism-of-action of the JAK2 tyrosine kinase inhibitors ruxolitinib and SAR (TG101348). Leukemia. 2014; 28: Pardanani A, Gotlib JR, Jamieson C, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011; 29: Wernig G, Kharas MG, Okabe R, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia vera. Cancer Cell. 2008; 13: Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell. 2010; 17: Mullally A, Poveromo L, Schneider RK, et al. Distinct roles for long-term hematopoietic stem cells and erythroid precursor cells in a murine model of Jak2V617F-mediated polycythemia vera. Blood. 2012; 120: Wernig G, Mercher T, Okabe R, et al. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006; 107: Hasan S, Lacout C, Marty C, et al. JAK2V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNalpha. Blood. 2013; 122: Croker BA, Metcalf D, Robb L, et al. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004; 20: Villeval JL, Cohen-Solal K, Tulliez M, et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997; 90: Marty C, Lacout C, Martin A, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood. 2010; 116: Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009; 113: Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012; 366: Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012; 366: Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015; 100:
11 24. Tefferi A. Challenges facing JAK inhibitor therapy for myeloproliferative neoplasms. N Engl J Med. 2012; 366: Tefferi A, Litzow MR, Pardanani A. Longterm outcome of treatment with ruxolitinib in myelofibrosis. N Engl J Med. 2011; 365: Galli S, McLornan D, Harrison C. Safety evaluation of ruxolitinib for treating myelofibrosis. Expert Opin Drug Saf. 2014; 13: Zhang Q, Zhang Y, Diamond S, et al. The janus kinase 2 inhibitor fedratinib inhibits thiamine uptake: a putative mechanism for the onset of Wernicke s encephalopathy. Drug Metab Dispos. 2014; 42: Villeval J-L, Moratal C, Debeurme F, et al. Effect of the JAK1/2 inhibitor GS-0387 (momelotinib) on the anemia associated with a preclinical murine model of human primary myelofibrosis. Blood (ASH Annual Meeting Abstracts). 2013; 122(2851): Vainchenker W, Favale F. Myelofibrosis, JAK2 inhibitors and erythropoiesis. Leukemia. 2013; 27: Kraus M, Wang Y, Aleksandrowicz D, et al. Efficacious intermittent dosing of a novel JAK2 inhibitor in mouse models of polycythemia vera. PLoS ONE. 2012; 7: e Besancenot R, Chaligne R, Tonetti C, et al. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol. 2010; 8: e Pecquet C, Diaconu CC, Staerk J, et al. Thrombopoietin receptor down-modulation by JAK2 V617F: restoration of receptor levels by inhibitors of pathologic JAK2 signaling and of proteasomes. Blood. 2012; 119: Kubovcakova L, Lundberg P, Grisouard J, et al. Differential effects of hydroxyurea and INC424 on mutant allele burden and myeloproliferative phenotype in a JAK2-V617F polycythemia vera mouse model. Blood. 2013; 121: Randhawa J, Ostojic A, Vrhovac R, et al. Splenomegaly in myelofibrosis new options for therapy and the therapeutic potential of Janus kinase 2 inhibitors. J Hematol Oncol. 2012; 5: Guglielmelli P, Zini R, Bogani C, et al. Molecular profiling of CD34 + cells in idiopathic myelofibrosis identifies a set of disease-associated genes and reveals the clinical significance of Wilms tumor gene 1 (WT1). Stem Cells. 2007; 25: Chagraoui H, Komura E, Tulliez M, et al. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002; 100: Kota J, Caceres N, Constantinescu SN. Aberrant signal transduction pathways in myeloproliferative neoplasms. Leukemia. 2008; 22: Savona MR. Are we altering the natural history of primary myelofibrosis? Leuk Res. 2014; 38: Kvasnicka HM, Thiele J, Bueso-Ramos CE, et al. Effects of five-years of ruxolitinib therapy on bone marrow morphology in patients with myelofibrosis and comparison with best available therapy. ASH Annual Meeting Abstracts. 2013; 122: Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010; 115: Nakaya Y, Shide K, Naito H, et al. Effect of NS-018, a selective JAK2V617F inhibitor, in a murine model of myelofibrosis. Blood Cancer J. 2014; 4: e Kirabo A, Park SO, Wamsley HL, et al. The small molecule inhibitor G6 significantly reduces bone marrow fibrosis and the mutant burden in a mouse model of Jak2- mediated myelofibrosis. Am J Pathol. 2012; 181: Shide K, Kameda T, Markovtsov V, et al. R723, a selective JAK2 inhibitor, effectively treats JAK2V617F-induced murine myeloproliferative neoplasm. Blood. 2011; 117:
«Mice are not small people» A. Rangarajan & Weinberg R. Nature Reviews Cancer 3,
 «Mice are not small people» A. Rangarajan & Weinberg R. Nature Reviews Cancer 3, 952-959. 2003. Most common age-related neoplasm Humans in the West = Epithelial * Basal-cell carcinoma of skin * Breast
«Mice are not small people» A. Rangarajan & Weinberg R. Nature Reviews Cancer 3, 952-959. 2003. Most common age-related neoplasm Humans in the West = Epithelial * Basal-cell carcinoma of skin * Breast
JAK2 Inhibitors for Myeloproliferative Diseases
 JAK2 Inhibitors for Myeloproliferative Diseases Srdan (Serge) Verstovsek M.D., Ph.D. Associate Professor Department of Leukemia University of Texas MD Anderson Cancer Center Houston, Texas, USA Myeloproliferative
JAK2 Inhibitors for Myeloproliferative Diseases Srdan (Serge) Verstovsek M.D., Ph.D. Associate Professor Department of Leukemia University of Texas MD Anderson Cancer Center Houston, Texas, USA Myeloproliferative
Technical Bulletin No. 100
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 100 August 2, 2012 JAK2 AND MPL 515 MUTATIONAL ANALYSIS Contact: Dr. Jeffrey Wisotzkey, 717-851-1422 Technical Director, CPAL Jill A.
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 100 August 2, 2012 JAK2 AND MPL 515 MUTATIONAL ANALYSIS Contact: Dr. Jeffrey Wisotzkey, 717-851-1422 Technical Director, CPAL Jill A.
CLINICAL POLICY DEPARTMENT: Medical Management DOCUMENT NAME: JakafiTM REFERENCE NUMBER: NH.PHAR.98
 PAGE: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 6 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
WHO Update to Myeloproliferative Neoplasms
 WHO Update to Myeloproliferative Neoplasms Archana M Agarwal, MD, Associate Professor of Pathology University of Utah Department of Pathology/ARUP Laboratories Myeloproliferative Neoplasms The categories
WHO Update to Myeloproliferative Neoplasms Archana M Agarwal, MD, Associate Professor of Pathology University of Utah Department of Pathology/ARUP Laboratories Myeloproliferative Neoplasms The categories
London Cancer. Myelofibrosis guidelines. August Review August Version v1.0. Page 1 of 12
 London Cancer Myelofibrosis guidelines August 2013 Review August 2013 Version v1.0 Page 1 of 12 CONTENTS 1. DIAGNOSIS... 3 1a. BCSH (2012)... 3 1b. WHO (2009) diagnostic criteria for PMF:... 4 2. MOLECULAR
London Cancer Myelofibrosis guidelines August 2013 Review August 2013 Version v1.0 Page 1 of 12 CONTENTS 1. DIAGNOSIS... 3 1a. BCSH (2012)... 3 1b. WHO (2009) diagnostic criteria for PMF:... 4 2. MOLECULAR
Disclosure BCR/ABL1-Negative Classical Myeloproliferative Neoplasms
 Disclosure BCR/ABL1-Negative Classical Myeloproliferative Neoplasms Sonam Prakash declares affiliation with Incyte Corporation: Advisor for Hematopathology Publications Steering Committee Sonam Prakash,
Disclosure BCR/ABL1-Negative Classical Myeloproliferative Neoplasms Sonam Prakash declares affiliation with Incyte Corporation: Advisor for Hematopathology Publications Steering Committee Sonam Prakash,
Opportunities for Optimal Testing in the Myeloproliferative Neoplasms. Curtis A. Hanson, MD
 Opportunities for Optimal Testing in the Myeloproliferative Neoplasms Curtis A. Hanson, MD 2013 MFMER slide-1 DISCLOSURES: Relevant Financial Relationship(s) None Off Label Usage None 2013 MFMER slide-2
Opportunities for Optimal Testing in the Myeloproliferative Neoplasms Curtis A. Hanson, MD 2013 MFMER slide-1 DISCLOSURES: Relevant Financial Relationship(s) None Off Label Usage None 2013 MFMER slide-2
Heme 9 Myeloid neoplasms
 Heme 9 Myeloid neoplasms The minimum number of blasts to diagnose acute myeloid leukemia is 5% 10% 20% 50% 80% AML with the best prognosis is AML with recurrent cytogenetic abnormality AML with myelodysplasia
Heme 9 Myeloid neoplasms The minimum number of blasts to diagnose acute myeloid leukemia is 5% 10% 20% 50% 80% AML with the best prognosis is AML with recurrent cytogenetic abnormality AML with myelodysplasia
SIES_SOCIETÀ ITALIANA DI EMATOLOGIA SPERIMENTALE, October, 6-8, 2010 MEGAKARYOCYTOPOIESIS IN MYELOPROLIFERATIVE NEOPLASMS
 SIES_SOCIETÀ ITALIANA DI EMATOLOGIA SPERIMENTALE, October, 6-8, 2010 MEGAKARYOCYTOPOIESIS IN MYELOPROLIFERATIVE NEOPLASMS Vannucchi Alessandro M. Dipartimento di Area Critica Medico-Chirurgica, Sezione
SIES_SOCIETÀ ITALIANA DI EMATOLOGIA SPERIMENTALE, October, 6-8, 2010 MEGAKARYOCYTOPOIESIS IN MYELOPROLIFERATIVE NEOPLASMS Vannucchi Alessandro M. Dipartimento di Area Critica Medico-Chirurgica, Sezione
[COMPREHENSIVE GENETIC ASSAY PANEL ON
 2014 SN GENELAB AND RESEARCH CENTER DR. SALIL VANIAWALA, PH.D [COMPREHENSIVE GENETIC ASSAY PANEL ON MYELOPROLIFERATIVE NEOPLASMS] SN Genelab presents one of the most comprehensive genetic assay panel for
2014 SN GENELAB AND RESEARCH CENTER DR. SALIL VANIAWALA, PH.D [COMPREHENSIVE GENETIC ASSAY PANEL ON MYELOPROLIFERATIVE NEOPLASMS] SN Genelab presents one of the most comprehensive genetic assay panel for
Bone marrow histopathology in Ph - CMPDs. - the new WHO classification - Juergen Thiele Cologne, Germany
 Bone marrow histopathology in Ph - CMPDs - the new WHO classification - Juergen Thiele Cologne, Germany Current issues in MPNs concerning morphology 1.Prodromal stages of disease 2.Impact of histopathology
Bone marrow histopathology in Ph - CMPDs - the new WHO classification - Juergen Thiele Cologne, Germany Current issues in MPNs concerning morphology 1.Prodromal stages of disease 2.Impact of histopathology
Understanding how Jakafi (ruxolitinib) inhibits* overactive JAK pathway signaling
 Understanding how Jakafi (ruxolitinib) inhibits* overactive JAK pathway signaling * Jakafi, a kinase inhibitor, inhibits and (Janus-associated kinases 1 and 2), which mediate the signaling of cytokines
Understanding how Jakafi (ruxolitinib) inhibits* overactive JAK pathway signaling * Jakafi, a kinase inhibitor, inhibits and (Janus-associated kinases 1 and 2), which mediate the signaling of cytokines
Polycythemia Vera and other Myeloproliferative Neoplasms. A.Mousavi
 Polycythemia Vera and other Myeloproliferative Neoplasms A.Mousavi Chronic MPNs Multipotent hematopoietic progenitor cell is origin. Overproduction of one or more formed element of blood cells without
Polycythemia Vera and other Myeloproliferative Neoplasms A.Mousavi Chronic MPNs Multipotent hematopoietic progenitor cell is origin. Overproduction of one or more formed element of blood cells without
Myeloproliferative Disorders - D Savage - 9 Jan 2002
 Disease Usual phenotype acute leukemia precursor chronic leukemia low grade lymphoma myeloma differentiated Total WBC > 60 leukemoid reaction acute leukemia Blast Pro Myel Meta Band Seg Lymph 0 0 0 2
Disease Usual phenotype acute leukemia precursor chronic leukemia low grade lymphoma myeloma differentiated Total WBC > 60 leukemoid reaction acute leukemia Blast Pro Myel Meta Band Seg Lymph 0 0 0 2
How I Treat Myelofibrosis. Adam Mead, MD, PhD University of Oxford Oxford, United Kingdom
 How I Treat Myelofibrosis Adam Mead, MD, PhD University of Oxford Oxford, United Kingdom Primary Myelofibrosis Archiv Fur Pathol. 1879;78:475-96. 2 cases of leukemia with peculiar blood and marrow findings
How I Treat Myelofibrosis Adam Mead, MD, PhD University of Oxford Oxford, United Kingdom Primary Myelofibrosis Archiv Fur Pathol. 1879;78:475-96. 2 cases of leukemia with peculiar blood and marrow findings
CASE REPORT. Abstract. Introduction
 CASE REPORT The Amelioration of Myelofibrosis with Thrombocytopenia by a JAK1/2 Inhibitor, Ruxolitinib, in a Post-polycythemia Vera Myelofibrosis Patient with a JAK2 Exon 12 Mutation Kazuhiko Ikeda 1,2,
CASE REPORT The Amelioration of Myelofibrosis with Thrombocytopenia by a JAK1/2 Inhibitor, Ruxolitinib, in a Post-polycythemia Vera Myelofibrosis Patient with a JAK2 Exon 12 Mutation Kazuhiko Ikeda 1,2,
Transplants for MPD and MDS
 Transplants for MPD and MDS The question is really who to transplant, with what and when. Focus on myelofibrosis Jeff Szer Royal Melbourne Hospital Myelodysplasia Little needs to be said Despite new therapies
Transplants for MPD and MDS The question is really who to transplant, with what and when. Focus on myelofibrosis Jeff Szer Royal Melbourne Hospital Myelodysplasia Little needs to be said Despite new therapies
MALATTIE MIELOPROLIFERATIVE CRONICHE
 MALATTIE MIELOPROLIFERATIVE CRONICHE Dott. Roberto Latagliata Policlinico Umberto I Università Sapienza, Roma Highlights from EHA 2017: some points to address today WHO 2016 MPN classification: hot topics
MALATTIE MIELOPROLIFERATIVE CRONICHE Dott. Roberto Latagliata Policlinico Umberto I Università Sapienza, Roma Highlights from EHA 2017: some points to address today WHO 2016 MPN classification: hot topics
Polycythemia Vera and Essential Thombocythemia A Single Institution Experience
 INDIAN JOURNAL OF MEDICAL & PAEDIATRIC ONCOLOGY Vol. 29 No 4, 2008 7 Original Article-I Polycythemia Vera and Essential Thombocythemia A Single Institution Experience CECIL ROSS, NAVYA, VANAMALA AND KARUNA
INDIAN JOURNAL OF MEDICAL & PAEDIATRIC ONCOLOGY Vol. 29 No 4, 2008 7 Original Article-I Polycythemia Vera and Essential Thombocythemia A Single Institution Experience CECIL ROSS, NAVYA, VANAMALA AND KARUNA
Virtually every intracellular signal transduction
 MYELOPROLIFERATIVE DISORDERS Therapeutic potential of JAK2 inhibitors Srdan Verstovsek 1 1 The University of Texas M. D. Anderson Cancer Center, Houston, TX The discovery of an activating tyrosine kinase
MYELOPROLIFERATIVE DISORDERS Therapeutic potential of JAK2 inhibitors Srdan Verstovsek 1 1 The University of Texas M. D. Anderson Cancer Center, Houston, TX The discovery of an activating tyrosine kinase
Should Mutational Status in Primary Myelofibrosis (PMF) Guide Therapy..YES!!!
 Should Mutational Status in Primary Myelofibrosis (PMF) Guide Therapy..YES!!! Lindsay Anne Magura Rein, MD Division of Hematologic Malignancies and Cellular Therapy/BMT A Little Bit of History.. 1665 Advanced
Should Mutational Status in Primary Myelofibrosis (PMF) Guide Therapy..YES!!! Lindsay Anne Magura Rein, MD Division of Hematologic Malignancies and Cellular Therapy/BMT A Little Bit of History.. 1665 Advanced
Disclosures for Ayalew Tefferi
 Disclosures for Ayalew Tefferi Principal investigator role Employee Consultant Major Stockholder Speakers Bureau Scientific Advisory Board Janssen, Geron, Celgene, Sanofi-Aventis, Gilead Sciences, Incyte
Disclosures for Ayalew Tefferi Principal investigator role Employee Consultant Major Stockholder Speakers Bureau Scientific Advisory Board Janssen, Geron, Celgene, Sanofi-Aventis, Gilead Sciences, Incyte
Cooperation of germ line JAK2 mutations E846D and R1063H leads to erythroid hyperplasia with megakaryocytic atypia
 Cooperation of germ line JAK2 mutations E846D and R1063H leads to erythroid hyperplasia with megakaryocytic atypia Katarina Kapralova, Ph.D. Department of Biology Faculty of Medicine and Dentistry Palacký
Cooperation of germ line JAK2 mutations E846D and R1063H leads to erythroid hyperplasia with megakaryocytic atypia Katarina Kapralova, Ph.D. Department of Biology Faculty of Medicine and Dentistry Palacký
Molecular aberrations in MPN. and use in the clinic. Timothy Devos MD PhD
 Molecular aberrations in MPN and use in the clinic Timothy Devos MD PhD MB&C2017 24-3-2017 Introduction 1951: William Dameshek MPD MPN = clonal, hematopoietic stem cell disorders, proliferation in BM of
Molecular aberrations in MPN and use in the clinic Timothy Devos MD PhD MB&C2017 24-3-2017 Introduction 1951: William Dameshek MPD MPN = clonal, hematopoietic stem cell disorders, proliferation in BM of
What is next: Emerging JAK2 inhibitor combination studies. Alessandro M. Vannucchi. Section of Hematology, University of Florence, Italy
 ymposium JAK2 Inhibition in Myelofibrosis: What Can We Expect in the Clinic? 4 5 May 2012 Lisbon, Portugal What is next: Emerging JAK2 inhibitor combination studies Alessandro M. Vannucchi ection of Hematology,
ymposium JAK2 Inhibition in Myelofibrosis: What Can We Expect in the Clinic? 4 5 May 2012 Lisbon, Portugal What is next: Emerging JAK2 inhibitor combination studies Alessandro M. Vannucchi ection of Hematology,
Stifel Nicolaus 2013 Healthcare Conference. John Scarlett, M.D. Chief Executive Officer September 11, 2013
 Stifel Nicolaus 2013 Healthcare Conference John Scarlett, M.D. Chief Executive Officer September 11, 2013 1 forward-looking statements Except for the historical information contained herein, this presentation
Stifel Nicolaus 2013 Healthcare Conference John Scarlett, M.D. Chief Executive Officer September 11, 2013 1 forward-looking statements Except for the historical information contained herein, this presentation
MPNs: JAK2 inhibitors & beyond. Mohamed Abdelmooti (MD) NCI, Cairo University, Egypt
 MPNs: JAK2 inhibitors & beyond Mohamed Abdelmooti (MD) NCI, Cairo University, Egypt Myeloproliferative Neoplasms (MPNs) AGENDA: 1. Molecular biology 2. New WHO diagnostic criteria. 3. Risk stratification
MPNs: JAK2 inhibitors & beyond Mohamed Abdelmooti (MD) NCI, Cairo University, Egypt Myeloproliferative Neoplasms (MPNs) AGENDA: 1. Molecular biology 2. New WHO diagnostic criteria. 3. Risk stratification
MPN What's new in the morphological classification, grading of fibrosis and the impact of novel drugs
 MPN What's new in the morphological classification, grading of fibrosis and the impact of novel drugs Hans Michael Kvasnicka University of Frankfurt, Germany hans-michael.kvasnicka@kgu.de Disclosure of
MPN What's new in the morphological classification, grading of fibrosis and the impact of novel drugs Hans Michael Kvasnicka University of Frankfurt, Germany hans-michael.kvasnicka@kgu.de Disclosure of
Intro alla patologia. Giovanni Barosi. Fondazione IRCCS Policlinico San Matteo Pavia
 Settima Giornata Fiorentina dedicata ai pazienti con malattie mieloproliferative croniche Sabato 13 Maggio 2017 CRIMM Centro di Ricerca e Innovazione per le Malattie Mieloproliferative AOU Careggi Intro
Settima Giornata Fiorentina dedicata ai pazienti con malattie mieloproliferative croniche Sabato 13 Maggio 2017 CRIMM Centro di Ricerca e Innovazione per le Malattie Mieloproliferative AOU Careggi Intro
JAK inhibitors in Phmyeloproliferative
 Disclosures for A Pardanani Research Support/P.I. Employee Consultant Major Stockholder Speakers Bureau Scientific Advisory Board TargeGen, Cytopia/YM BioSciences, PharmaMar None None None None None Presentation
Disclosures for A Pardanani Research Support/P.I. Employee Consultant Major Stockholder Speakers Bureau Scientific Advisory Board TargeGen, Cytopia/YM BioSciences, PharmaMar None None None None None Presentation
Myeloid neoplasms. Early arrest in the blast cell or immature cell "we call it acute leukemia" Myoid neoplasm divided in to 3 major categories:
 Myeloid neoplasms Note: Early arrest in the blast cell or immature cell "we call it acute leukemia" Myoid neoplasm divided in to 3 major categories: 1. AML : Acute myeloid leukemia(stem cell with myeloid
Myeloid neoplasms Note: Early arrest in the blast cell or immature cell "we call it acute leukemia" Myoid neoplasm divided in to 3 major categories: 1. AML : Acute myeloid leukemia(stem cell with myeloid
MPL W515L K mutation
 MPL W515L K mutation BCR-ABL genotyping The exact chromosomal defect in Philadelphia chromosome is a translocation. Parts of two chromosomes, 9 and 22, switch places. The result is a fusion gene, created
MPL W515L K mutation BCR-ABL genotyping The exact chromosomal defect in Philadelphia chromosome is a translocation. Parts of two chromosomes, 9 and 22, switch places. The result is a fusion gene, created
Welcome to Master Class for Oncologists. Session 3: 9:15 AM - 10:00 AM
 Welcome to Master Class for Oncologists Session 3: 9:15 AM - 10:00 AM Miami, FL December 18, 2009 Myeloproliferative Neoplasms: Bringing Order to Complexity and Achieving Optimal Outcomes Speaker: Andrew
Welcome to Master Class for Oncologists Session 3: 9:15 AM - 10:00 AM Miami, FL December 18, 2009 Myeloproliferative Neoplasms: Bringing Order to Complexity and Achieving Optimal Outcomes Speaker: Andrew
Myelodysplastic syndrome (MDS) & Myeloproliferative neoplasms
 Myelodysplastic syndrome (MDS) & Myeloproliferative neoplasms Myelodysplastic syndrome (MDS) A multipotent stem cell that can differentiate into any of the myeloid lineage cells (RBCs, granulocytes, megakaryocytes)
Myelodysplastic syndrome (MDS) & Myeloproliferative neoplasms Myelodysplastic syndrome (MDS) A multipotent stem cell that can differentiate into any of the myeloid lineage cells (RBCs, granulocytes, megakaryocytes)
How I treat high risk myeloproliferative neoplasms. Francesco Passamonti Università dell Insubria Varese - Italy
 How I treat high risk myeloproliferative neoplasms Francesco Passamonti Università dell Insubria Varese - Italy How I treat high risk MF MF Treatment ELN 2018 Guidelines Anemia (Hb < 10 g/dl) Splenomegaly
How I treat high risk myeloproliferative neoplasms Francesco Passamonti Università dell Insubria Varese - Italy How I treat high risk MF MF Treatment ELN 2018 Guidelines Anemia (Hb < 10 g/dl) Splenomegaly
Classical Ph-1neg myeloproliferative neoplasms: Ruxolitinib in myelofibrosis. Francesco Passamonti Università degli Studi dell Insubria, Varese
 Classical Ph-1neg myeloproliferative neoplasms: Ruxolitinib in myelofibrosis Francesco Passamonti Università degli Studi dell Insubria, Varese DIPSS during f-up IPSS at diagnosis Diagnose MF and genotype
Classical Ph-1neg myeloproliferative neoplasms: Ruxolitinib in myelofibrosis Francesco Passamonti Università degli Studi dell Insubria, Varese DIPSS during f-up IPSS at diagnosis Diagnose MF and genotype
3. Pardanani A, et al. Leukemia 2009;23: Hedvat M, et al. Cancer Cell 2009;16:
 A Phase / Study of NS-8, an Oral JAK Inhibitor, in Patients with Primary Myelofibrosis (PMF), Post-Polycythemia Vera Myelofibrosis (ppv MF), or Post-Essential Thrombocythemia Myelofibrosis (pet MF) 8 Srdan
A Phase / Study of NS-8, an Oral JAK Inhibitor, in Patients with Primary Myelofibrosis (PMF), Post-Polycythemia Vera Myelofibrosis (ppv MF), or Post-Essential Thrombocythemia Myelofibrosis (pet MF) 8 Srdan
Polycthemia Vera (Rubra)
 Polycthemia Vera (Rubra) Polycthemia Vera (Rubra) Increased red cells Clonal Myeloid lineages also increased 2-13 cases per million Mean age: 60 years Sites of Involvement Bone marrow Peripheral blood
Polycthemia Vera (Rubra) Polycthemia Vera (Rubra) Increased red cells Clonal Myeloid lineages also increased 2-13 cases per million Mean age: 60 years Sites of Involvement Bone marrow Peripheral blood
Chronic Idiopathic Myelofibrosis (CIMF)
 Chronic Idiopathic Myelofibrosis (CIMF) CIMF Synonyms Agnogenic myeloid metaplasia Myelosclerosis with myeloid metaplasia Chronic granulocytic-megakaryocytic myelosis CIMF Megakaryocytic proliferation
Chronic Idiopathic Myelofibrosis (CIMF) CIMF Synonyms Agnogenic myeloid metaplasia Myelosclerosis with myeloid metaplasia Chronic granulocytic-megakaryocytic myelosis CIMF Megakaryocytic proliferation
Leukemia and subsequent solid tumors among patients with myeloproliferative neoplasms
 Leukemia and subsequent solid tumors among patients with myeloproliferative neoplasms Tiziano Barbui (tbarbui@asst-pg23.it Hematology and Research Foundation,Ospedale Papa Giovanni XXIII, Bergamo Italy
Leukemia and subsequent solid tumors among patients with myeloproliferative neoplasms Tiziano Barbui (tbarbui@asst-pg23.it Hematology and Research Foundation,Ospedale Papa Giovanni XXIII, Bergamo Italy
Disclosures. Myeloproliferative Neoplasms: A Case-Based Approach. Objectives. Myeloproliferative Neoplasms. Myeloproliferative Neoplasms
 Myeloproliferative Neoplasms: A Case-Based Approach Disclosures No conflicts of interests regarding the topic being presented Adam M. Miller, MD PGY-4 Resident Physician Department of Pathology and Laboratory
Myeloproliferative Neoplasms: A Case-Based Approach Disclosures No conflicts of interests regarding the topic being presented Adam M. Miller, MD PGY-4 Resident Physician Department of Pathology and Laboratory
JAK2 inhibitors do not affect stem cells present in the spleens of patients with myelofibrosis
 Regular Article MYELOID NEOPLASIA JAK2 inhibitors do not affect stem cells present in the spleens of patients with myelofibrosis Xiaoli Wang, 1 Fei Ye, 1 Joseph Tripodi, 1 Cing Siang Hu, 1 Jiajing Qiu,
Regular Article MYELOID NEOPLASIA JAK2 inhibitors do not affect stem cells present in the spleens of patients with myelofibrosis Xiaoli Wang, 1 Fei Ye, 1 Joseph Tripodi, 1 Cing Siang Hu, 1 Jiajing Qiu,
CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow
 White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
Effect of treatment with a JAK2 selective inhibitor, fedratinib, on bone marrow fibrosis in patients with myelofibrosis
 DOI 1.1186/s12967-15-6- RESEARCH Open Access Effect of treatment with a JAK2 selective inhibitor, fedratinib, on bone marrow fibrosis in patients with myelofibrosis Catriona Jamieson 1*, Robert Hasserjian
DOI 1.1186/s12967-15-6- RESEARCH Open Access Effect of treatment with a JAK2 selective inhibitor, fedratinib, on bone marrow fibrosis in patients with myelofibrosis Catriona Jamieson 1*, Robert Hasserjian
MANIFEST Phase 2 Enhancement / Expansion
 MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
MANIFEST Phase 2 Enhancement / Expansion Investor Conference Call Stellar Science, Breakthrough Medicine October 11, 2018 Forward-Looking Statements This presentation contains forward-looking statements
Supplement Material. Spleen weight (mg) LN cells (X106) Acat1-/- Acat1-/- Mouse weight (g)
 Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
Supplement Material A Spleen weight (mg) C Mouse weight (g) 1 5 1 2 9 6 3 2 5 2 1 5 Male LN cells (X16) 4 ** ** Female B 3 2 1 Supplemental Figure I. Spleen weight (A), Inguinal lymph node (LN) cell number
Decreased expression of PIAS1 and PIAS3 in essential thrombocythemia patients
 Decreased expression of PIAS1 and PIAS3 in essential thrombocythemia patients H.-H. Hsiao 1,2, Y.-C. Liu 1,2, M.-Y. Yang 3, Y.-F. Tsai 2, T.-C. Liu 1,2, C.-S. Chang 1,2 and S.-F. Lin 1,2 1 Faculty of Medicine,
Decreased expression of PIAS1 and PIAS3 in essential thrombocythemia patients H.-H. Hsiao 1,2, Y.-C. Liu 1,2, M.-Y. Yang 3, Y.-F. Tsai 2, T.-C. Liu 1,2, C.-S. Chang 1,2 and S.-F. Lin 1,2 1 Faculty of Medicine,
MYELOPROLIFARATIVE NEOPLASMS. Dr. Hasan Fahmawi, MRCP(UK), FRCP(Edin).
 MYELOPROLIFARATIVE NEOPLASMS Dr. Hasan Fahmawi, MRCP(UK), FRCP(Edin). These are a group of chronic conditions characterised by clonal proliferation of marrow precursor cells. PRV, essential thrombocyathaemia,
MYELOPROLIFARATIVE NEOPLASMS Dr. Hasan Fahmawi, MRCP(UK), FRCP(Edin). These are a group of chronic conditions characterised by clonal proliferation of marrow precursor cells. PRV, essential thrombocyathaemia,
Should Mutation Status in PMF Guide Therapy? No! Brady L. Stein, MD MHS Assistant Professor of Medicine Division of Hematology/Oncology
 Should Mutation Status in PMF Guide Therapy? No! Brady L. Stein, MD MHS Assistant Professor of Medicine Division of Hematology/Oncology Case presentation A 67 yo woman presents to transition care, as her
Should Mutation Status in PMF Guide Therapy? No! Brady L. Stein, MD MHS Assistant Professor of Medicine Division of Hematology/Oncology Case presentation A 67 yo woman presents to transition care, as her
Practical Considerations in the Treatment Myeloproliferative Neoplasms: Prognostication and Current Treatment Indy Hematology
 Practical Considerations in the Treatment Myeloproliferative Neoplasms: Prognostication and Current Treatment Indy Hematology Angela Fleischman MD PhD UC Irvine March 9, 2019 Disclosures: Angela Fleischman
Practical Considerations in the Treatment Myeloproliferative Neoplasms: Prognostication and Current Treatment Indy Hematology Angela Fleischman MD PhD UC Irvine March 9, 2019 Disclosures: Angela Fleischman
Treatment of polycythemia vera with recombinant interferon alpha (rifnα)
 Treatment of polycythemia vera with recombinant interferon alpha (rifnα) Richard T. Silver, MD Professor of Medicine Weill Cornell Medical College New York, New York Outline of Lecture What are interferons?
Treatment of polycythemia vera with recombinant interferon alpha (rifnα) Richard T. Silver, MD Professor of Medicine Weill Cornell Medical College New York, New York Outline of Lecture What are interferons?
Characterization of MPL-mutated myeloid neoplasms: a review of 224 MPL+ cases
 Article Characterization of MPL-mutated myeloid neoplasms: a review of 224 MPL+ cases Keming Lin 1,*, Gang Xu 1, Jie-Gen Jiang 1, Mayuko Imai 1, Zhao Wu 1, Paris Petersen 1, Kim Janatpour 1, and Bashar
Article Characterization of MPL-mutated myeloid neoplasms: a review of 224 MPL+ cases Keming Lin 1,*, Gang Xu 1, Jie-Gen Jiang 1, Mayuko Imai 1, Zhao Wu 1, Paris Petersen 1, Kim Janatpour 1, and Bashar
Sponsor / Company: Sanofi Drug substance(s): SAR302503
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None JAK2, MPL and CALR Testing for Myeloproliferative Neoplasms Description Somatic (acquired) genetic variants in JAK2, MPL,
FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None JAK2, MPL and CALR Testing for Myeloproliferative Neoplasms Description Somatic (acquired) genetic variants in JAK2, MPL,
New WHO Classification of Myeloproliferative Neoplasms
 New WHO Classification of Myeloproliferative Neoplasms Hans Michael Kvasnicka Senckenberg Institute of Pathology, University of Frankfurt, Germany hans-michael.kvasnicka@kgu.de Principles and rationale
New WHO Classification of Myeloproliferative Neoplasms Hans Michael Kvasnicka Senckenberg Institute of Pathology, University of Frankfurt, Germany hans-michael.kvasnicka@kgu.de Principles and rationale
Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms
 Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms Version: MPNBiomarkers 1.0.0.2 Protocol Posting Date: June 2017 This biomarker template is NOT required for accreditation
Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms Version: MPNBiomarkers 1.0.0.2 Protocol Posting Date: June 2017 This biomarker template is NOT required for accreditation
Myelodysplastic Syndromes Myeloproliferative Disorders
 Myelodysplastic Syndromes Myeloproliferative Disorders Myelodysplastic Syndromes characterized by maturation defects that are associated with ineffective hematopoiesis and a high risk of transformation
Myelodysplastic Syndromes Myeloproliferative Disorders Myelodysplastic Syndromes characterized by maturation defects that are associated with ineffective hematopoiesis and a high risk of transformation
BCR-ABL - LSK BCR-ABL + LKS - (%)
 Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Marker Clone BCR-ABL + LSK (%) BCR-ABL + LKS - (%) BCR-ABL - LSK (%) P value vs. BCR-ABL + LKS - P value vs. BCR-ABL - LSK CD2 RM2-5 12.9 ± 3.6 36.7 ± 6.5 19.3 ± 2.4 0.01 0.10 CD5 53-7.3 13.9 ± 3.2 20.8
Myelofibrosis a Reference Guide for Journalists
 Myelofibrosis a Reference Guide for Journalists What Is Myelofibrosis? Myelofibrosis is an uncommon blood cancer characterized by bone marrow scarring (fibrosis), enlarged spleen (splenomegaly), potential
Myelofibrosis a Reference Guide for Journalists What Is Myelofibrosis? Myelofibrosis is an uncommon blood cancer characterized by bone marrow scarring (fibrosis), enlarged spleen (splenomegaly), potential
Hematopoiesis. BHS Liège 27/1/2012. Dr Sonet Anne UCL Mont-Godinne
 Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
Hematopoiesis BHS Liège 27/1/2012 Dr Sonet Anne UCL Mont-Godinne Hematopoiesis: definition = all the phenomenons to produce blood cells Leukocytes = White Blood Cells Polynuclear = Granulocytes Platelet
Case Presentation. Attilio Orazi, MD
 Case Presentation Attilio Orazi, MD Weill Cornell Medical College/ NYP Hospital Department of Pathology and Laboratory Medicine New York, NY United States History 60 year old man presented with anemia
Case Presentation Attilio Orazi, MD Weill Cornell Medical College/ NYP Hospital Department of Pathology and Laboratory Medicine New York, NY United States History 60 year old man presented with anemia
Mouse models of myeloproliferative neoplasms: JAK of all grades
 Disease Models & Mechanisms 4, 311-317 (2011) doi:10.1242/dmm.006817 Mouse models of myeloproliferative neoplasms: JAK of all grades Juan Li 1, David G. Kent 1, Edwin Chen 1 and Anthony R. Green 1,2, In
Disease Models & Mechanisms 4, 311-317 (2011) doi:10.1242/dmm.006817 Mouse models of myeloproliferative neoplasms: JAK of all grades Juan Li 1, David G. Kent 1, Edwin Chen 1 and Anthony R. Green 1,2, In
New Advances in the Pathogenesis and Therapy of Essential Thrombocythemia
 MYELOPROLIFERATIVE DISORDERS New Advances in the Pathogenesis and Therapy of Essential Thrombocythemia Ross L. Levine 1,2 and Mark Heaney 2 1 Human Oncology and Pathogenesis Program; 2 Leukemia Service,
MYELOPROLIFERATIVE DISORDERS New Advances in the Pathogenesis and Therapy of Essential Thrombocythemia Ross L. Levine 1,2 and Mark Heaney 2 1 Human Oncology and Pathogenesis Program; 2 Leukemia Service,
Winship Cancer Institute of Emory University New Determinants and Approaches for MPN
 Winship Cancer Institute of Emory University New Determinants and Approaches for MPN Elliott F. Winton August 8, 2014 Sea Island, Georgia Outline: MPN Determinants, Approaches Diagnosis Prognosis Treatment
Winship Cancer Institute of Emory University New Determinants and Approaches for MPN Elliott F. Winton August 8, 2014 Sea Island, Georgia Outline: MPN Determinants, Approaches Diagnosis Prognosis Treatment
Nature Medicine doi: /nm.3150
 Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Nature Medicine doi:10.1038/nm.3150 Supplementary Table 1 Primer sequences used for q-pcr analysis Gene Forward primer Reverse primer Abca1 CAGCTTCCATCCTCCTTGTC CCACATCCACAACTGTCTGG Abcg1 GTACCATGACATCGCTGGTG
Chronic Myeloproliferative Disorders
 1 Chronic Myeloproliferative Disorders 15th 9 April2015 Polycythemia vera Essential thrombocythemia Idiopathic primary myelofibrosis 2 Learning objectives To appreciate types of polycythaemia (erythrocytosis)
1 Chronic Myeloproliferative Disorders 15th 9 April2015 Polycythemia vera Essential thrombocythemia Idiopathic primary myelofibrosis 2 Learning objectives To appreciate types of polycythaemia (erythrocytosis)
The spectrum of JAK2-positive myeloproliferative neoplasms
 THE SPECTRUM OF JAK2-POSITIVE MYELOPROLIFERATIVE NEOPLASMS:COMPLICATIONS AND THERAPEUTIC ADVANCES The spectrum of JAK2-positive myeloproliferative neoplasms Jean-Jacques Kiladjian 1 1 Saint-Louis Hospital
THE SPECTRUM OF JAK2-POSITIVE MYELOPROLIFERATIVE NEOPLASMS:COMPLICATIONS AND THERAPEUTIC ADVANCES The spectrum of JAK2-positive myeloproliferative neoplasms Jean-Jacques Kiladjian 1 1 Saint-Louis Hospital
Preliminary Results From a Phase I Dose Escalation Trial of Ruxolitinib and the PI3Kδ Inhibitor TGR-1202 in Myelofibrosis
 Preliminary Results From a Phase I Dose Escalation Trial of Ruxolitinib and the PI3Kδ Inhibitor TGR-1202 in Myelofibrosis Tamara K. Moyo, Andrew Sochacki, Gregory D. Ayers, Michael T. Byrne, Stephen A.
Preliminary Results From a Phase I Dose Escalation Trial of Ruxolitinib and the PI3Kδ Inhibitor TGR-1202 in Myelofibrosis Tamara K. Moyo, Andrew Sochacki, Gregory D. Ayers, Michael T. Byrne, Stephen A.
Biologia molecolare delle malattie mieloproliferative croniche Ph1-negative tipiche
 41 CONGRESSO NAZIONALE SIE Bologna 14-17 Ottobre 2007 Biologia molecolare delle malattie mieloproliferative croniche Ph1-negative tipiche Alessandro M. Vannucchi Dip.. di Ematologia, Università degli Studi
41 CONGRESSO NAZIONALE SIE Bologna 14-17 Ottobre 2007 Biologia molecolare delle malattie mieloproliferative croniche Ph1-negative tipiche Alessandro M. Vannucchi Dip.. di Ematologia, Università degli Studi
Managing ET in Tiziano Barbui MD
 Managing ET in 2019 Tiziano Barbui MD (tbarbui@asst-pg23.it) Hematology and Foundation for Clinical Research, Hospital Papa Giovanni XXIII Bergamo, Italy Managing ET in 2019 Establish diagnosis Risk Stratification
Managing ET in 2019 Tiziano Barbui MD (tbarbui@asst-pg23.it) Hematology and Foundation for Clinical Research, Hospital Papa Giovanni XXIII Bergamo, Italy Managing ET in 2019 Establish diagnosis Risk Stratification
Supplementary information. Supplementary figure 1. Flow chart of study design
 Supplementary information Supplementary figure 1. Flow chart of study design Supplementary Figure 2. Quantile-quantile plot of stage 1 results QQ plot of the observed -log10 P-values (y axis) versus the
Supplementary information Supplementary figure 1. Flow chart of study design Supplementary Figure 2. Quantile-quantile plot of stage 1 results QQ plot of the observed -log10 P-values (y axis) versus the
Related Policies None
 Medical Policy MP 2.04.60 BCBSA Ref. Policy: 2.04.60 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Medicine Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 2.04.60 BCBSA Ref. Policy: 2.04.60 Last Review: 07/25/2018 Effective Date: 07/25/2018 Section: Medicine Related Policies None DISCLAIMER Our medical policies are designed for informational
JAK2 V617F analysis. Indication: monitoring of therapy
 JAK2 V617F analysis BCR-ABL genotyping The exact chromosomal defect in Philadelphia chromosome is a translocation. Parts of two chromosomes, 9 and 22, switch places. The result is a fusion gene, created
JAK2 V617F analysis BCR-ABL genotyping The exact chromosomal defect in Philadelphia chromosome is a translocation. Parts of two chromosomes, 9 and 22, switch places. The result is a fusion gene, created
Supplementary Figure 1. Successful excision of genes from WBM lysates and
 Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
Supplementary Information: Supplementary Figure 1. Successful excision of genes from WBM lysates and survival of mice with different genotypes. (a) The proper excision of Pten, p110α, p110α and p110δ was
Evolving Management of Myelofibrosis
 Evolving Management of Myelofibrosis Srdan (Serge) Verstovsek M.D., Ph.D. Professor of Medicine Department of Leukemia University of Texas MD Anderson Cancer Center Houston, Texas, USA Why do we prognosticate?
Evolving Management of Myelofibrosis Srdan (Serge) Verstovsek M.D., Ph.D. Professor of Medicine Department of Leukemia University of Texas MD Anderson Cancer Center Houston, Texas, USA Why do we prognosticate?
Chi sono i candidati agli inibitori di JAK2
 Chi sono i candidati agli inibitori di JAK2 Francesco Passamon, Hematology, University Hospital Varese, Italy Ruxoli8nib (US approved in MF; EAP study and compassionate use in Italy) SAR302503 (phase 3
Chi sono i candidati agli inibitori di JAK2 Francesco Passamon, Hematology, University Hospital Varese, Italy Ruxoli8nib (US approved in MF; EAP study and compassionate use in Italy) SAR302503 (phase 3
Emerging diagnostic and risk stratification criteria
 PV STATE OF MIND Polycythemia vera: Emerging diagnostic and risk stratification criteria Rami S. Komrokji, MD Moffitt Cancer Center, Tampa, Florida Disclosure These slides were developed by Incyte Corporation
PV STATE OF MIND Polycythemia vera: Emerging diagnostic and risk stratification criteria Rami S. Komrokji, MD Moffitt Cancer Center, Tampa, Florida Disclosure These slides were developed by Incyte Corporation
Spleens of myelofibrosis patients contain malignant hematopoietic stem cells
 Research article Spleens of myelofibrosis patients contain malignant hematopoietic stem cells Xiaoli Wang, 1 Sonam Prakash, 2 Min Lu, 1 Joseph Tripodi, 1 Fei Ye, 1 Vesna Najfeld, 1 Yan Li, 1 Myron Schwartz,
Research article Spleens of myelofibrosis patients contain malignant hematopoietic stem cells Xiaoli Wang, 1 Sonam Prakash, 2 Min Lu, 1 Joseph Tripodi, 1 Fei Ye, 1 Vesna Najfeld, 1 Yan Li, 1 Myron Schwartz,
Novel drugs in MPNs: Histone-Deacetylase Inhibitors
 Novel drugs in MPNs: HistoneDeacetylase Inhibitors 1st Annual Florence MPN Meeting April 16, 2011 Guido Finazzi Chronic Myeloproliferative Neoplasm Unit Division of Hematology Ospedali Riuniti di Bergamo,
Novel drugs in MPNs: HistoneDeacetylase Inhibitors 1st Annual Florence MPN Meeting April 16, 2011 Guido Finazzi Chronic Myeloproliferative Neoplasm Unit Division of Hematology Ospedali Riuniti di Bergamo,
Ropeginterferon alpha-2b targets JAK2V617F-positive polycythemia vera cells in vitro and in vivo
 Verger et al. (2018) 8:94 DOI 10.1038/s41408-018-0133-0 ARTICLE Ropeginterferon alpha-2b targets JAK2V617F-positive polycythemia vera cells in vitro and in vivo Open Access Emmanuelle Verger 1, Juliette
Verger et al. (2018) 8:94 DOI 10.1038/s41408-018-0133-0 ARTICLE Ropeginterferon alpha-2b targets JAK2V617F-positive polycythemia vera cells in vitro and in vivo Open Access Emmanuelle Verger 1, Juliette
Prognostic models in PV and ET
 Prognostic models in PV and ET Francesco Passamonti Hematology, Varese, Italy Current risk stratification in PV and ET: statement from European LeukemiaNet consensus Age over 60 years Previuos thrombosis
Prognostic models in PV and ET Francesco Passamonti Hematology, Varese, Italy Current risk stratification in PV and ET: statement from European LeukemiaNet consensus Age over 60 years Previuos thrombosis
HENATOLYMPHOID SYSTEM THIRD YEAR MEDICAL STUDENTS- UNIVERSITY OF JORDAN AHMAD T. MANSOUR, MD. Part 4 MYELOID NEOPLASMS
 HENATOLYMPHOID SYSTEM THIRD YEAR MEDICAL STUDENTS- UNIVERSITY OF JORDAN AHMAD T. MANSOUR, MD Part 4 MYELOID NEOPLASMS Introduction: o Myeloid neoplasms are divided into three major categories: o Acute
HENATOLYMPHOID SYSTEM THIRD YEAR MEDICAL STUDENTS- UNIVERSITY OF JORDAN AHMAD T. MANSOUR, MD Part 4 MYELOID NEOPLASMS Introduction: o Myeloid neoplasms are divided into three major categories: o Acute
Case Workshop of Society for Hematopathology and European Association for Haematopathology
 Case 148 2007 Workshop of Society for Hematopathology and European Association for Haematopathology Robert P Hasserjian Department of Pathology Massachusetts General Hospital Boston, MA Clinical history
Case 148 2007 Workshop of Society for Hematopathology and European Association for Haematopathology Robert P Hasserjian Department of Pathology Massachusetts General Hospital Boston, MA Clinical history
Clinical trials with JAK inhibitors for essential thrombocythemia and polycythemia vera
 Clinical trials with JAK inhibitors for essential thrombocythemia and polycythemia vera Alessandro M. Vannucchi, MD Laboratorio Congiunto MMPC Department of Experimental and Clinical Medicine University
Clinical trials with JAK inhibitors for essential thrombocythemia and polycythemia vera Alessandro M. Vannucchi, MD Laboratorio Congiunto MMPC Department of Experimental and Clinical Medicine University
Greater Manchester and Cheshire Cancer Network
 Greater Manchester and Cheshire Cancer Network Guidelines for the diagnosis and treatment of primary myelofibrosis, post-essential thrombocythaemia myelofibrosis and post-polycythaemia myelofibrosis Tim
Greater Manchester and Cheshire Cancer Network Guidelines for the diagnosis and treatment of primary myelofibrosis, post-essential thrombocythaemia myelofibrosis and post-polycythaemia myelofibrosis Tim
Primary myelofibrosis (PMF) is a hematologic malignancy
 Brief Report Mature Survival Data for 176 Patients Younger Than With Primary Myelofibrosis Diagnosed Between 1976 and 5: Evidence for Survival Gains in Recent Rakhee Vaidya, MBBS; Sergio Siragusa, MD;
Brief Report Mature Survival Data for 176 Patients Younger Than With Primary Myelofibrosis Diagnosed Between 1976 and 5: Evidence for Survival Gains in Recent Rakhee Vaidya, MBBS; Sergio Siragusa, MD;
Histological evaluation of myeloproliferative neoplasms
 Journal of clinical and experimental hematopathology Vol. 58 No.2, 45-50, 2018 JC lin EH xp ematopathol Review Article Histological evaluation of myeloproliferative neoplasms Hideyo Fujiwara In 2017, the
Journal of clinical and experimental hematopathology Vol. 58 No.2, 45-50, 2018 JC lin EH xp ematopathol Review Article Histological evaluation of myeloproliferative neoplasms Hideyo Fujiwara In 2017, the
Guidelines for diagnosis and management of adult myeloproliferative neoplasms (PV, ET, PMF and HES)
 Guidelines for diagnosis and management of adult myeloproliferative neoplasms (PV, ET, PMF and HES) Author: Dr N Butt, Consultant Haematologist On behalf of the Haematology CNG Written: July 2010 Reviewed:
Guidelines for diagnosis and management of adult myeloproliferative neoplasms (PV, ET, PMF and HES) Author: Dr N Butt, Consultant Haematologist On behalf of the Haematology CNG Written: July 2010 Reviewed:
The ruxolitinib effect
 The ruxolitinib effect Greenfield, G., McPherson, S., Mills, K., & McMullin, M. F. (2018). The ruxolitinib effect: understanding how molecular pathogenesis and epigenetic dysregulation impact therapeutic
The ruxolitinib effect Greenfield, G., McPherson, S., Mills, K., & McMullin, M. F. (2018). The ruxolitinib effect: understanding how molecular pathogenesis and epigenetic dysregulation impact therapeutic
CALR-positive myeloproliferative disorder in a patient with Phpositive chronic myeloid leukemia in durable treatment-free remission: a case report
 Case Report Page 1 of 5 CALR-positive myeloproliferative disorder in a patient with Phpositive chronic myeloid leukemia in durable treatment-free remission: a case report Irene Dogliotti 1, Carmen Fava
Case Report Page 1 of 5 CALR-positive myeloproliferative disorder in a patient with Phpositive chronic myeloid leukemia in durable treatment-free remission: a case report Irene Dogliotti 1, Carmen Fava
MYELOPROLIFERATIVE NEOPLASMS
 9 : 2 MYELOPROLIFERATIVE NEOPLASMS Introduction William Dameshek in 1951 introduced the term Myeloproliferative disorders (MPD). This included polycythemia vera (PV), essential thrombocythemia (ET), primary
9 : 2 MYELOPROLIFERATIVE NEOPLASMS Introduction William Dameshek in 1951 introduced the term Myeloproliferative disorders (MPD). This included polycythemia vera (PV), essential thrombocythemia (ET), primary
WHO 2016/17 update on Myeloproliferative Neoplasms. Anna Ruskova Auckland City Hospital New Zealand
 WHO 2016/17 update on Myeloproliferative Neoplasms Anna Ruskova Auckland City Hospital New Zealand BLOOD, 19 MAY 2016 x VOLUME 127, NUMBER 20 2 2016/17 Classification of Myeloproliferative Neoplasms Chronic
WHO 2016/17 update on Myeloproliferative Neoplasms Anna Ruskova Auckland City Hospital New Zealand BLOOD, 19 MAY 2016 x VOLUME 127, NUMBER 20 2 2016/17 Classification of Myeloproliferative Neoplasms Chronic
The Internists Approach to Polycythemia and Implications of Uncontrolled Disease
 The Internists Approach to Polycythemia and Implications of Uncontrolled Disease Mary Jo K. Voelpel, DO, FACOI, MA, CS Associate Clinical Professor MSU-COM Disclosures NONE Overview 1. Objectives 2. Case
The Internists Approach to Polycythemia and Implications of Uncontrolled Disease Mary Jo K. Voelpel, DO, FACOI, MA, CS Associate Clinical Professor MSU-COM Disclosures NONE Overview 1. Objectives 2. Case
New Therapies for MPNs
 Pomalidomide and IMIDS in Myelofibrosis New Therapies for MPNs Fourth International Workshop on CML and MPN Natchez Louisiana Ruben A. Mesa, MD Professor of Medicine Mayo Clinic College of Medicine Director
Pomalidomide and IMIDS in Myelofibrosis New Therapies for MPNs Fourth International Workshop on CML and MPN Natchez Louisiana Ruben A. Mesa, MD Professor of Medicine Mayo Clinic College of Medicine Director
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
June Ruxolitinib for the second-line treatment of myelofibrosis (IPSS intermediate LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW
 LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Ruxolitinib for the second-line treatment of myelofibrosis (IPSS intermediate risk-1 or above) Ruxolitinib for the second-line treatment of myelofibrosis (IPSS
LONDON CANCER NEWS DRUGS GROUP RAPID REVIEW Ruxolitinib for the second-line treatment of myelofibrosis (IPSS intermediate risk-1 or above) Ruxolitinib for the second-line treatment of myelofibrosis (IPSS
Meeting Report. From December 8 to 11, 2012 at Atlanta, GA, U.S.A
 Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
Meeting Report Affiliation Department of Transfusion Medicine and Cell Therapy Name Hisayuki Yao Name of the meeting Period and venue Type of your presentation Title of your presentation The 54 th Annual
