FluGlucoScan Injection
|
|
|
- Basil Jefferson
- 5 years ago
- Views:
Transcription
1 PRODUCT MONOGRAPH FluGlucoScan Injection 18 F Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution / >0.5 GBq / vial Diagnostic Radiopharmaceutical Alberta Cancer Board Standard Life Center Jasper Avenue Edmonton, AB T5J 3N4 Control # Date of Approval : October 2, 2007
2 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION...3 SUMMARY PRODUCT INFORMATION...3 DESCRIPTION...3 INDICATIONS AND CLINICAL USE...5 CONTRAINDICATIONS...5 WARNINGS AND PRECAUTIONS...5 ADVERSE REACTIONS...7 DRUG INTERACTIONS...8 DOSAGE AND ADMINISTRATION...8 RADIATION DOSIMETRY...11 ACTION AND CLINICAL PHARMACOLOGY...12 OVERDOSAGE...14 STORAGE AND STABILITY...14 SPECIAL HANDLING INSTRUCTIONS...14 DOSAGE FORMS, COMPOSITION AND PACKAGING...14 PART II: SCIENTIFIC INFORMATION...15 PHARMACEUTICAL INFORMATION...15 CLINICAL TRIALS...17 DETAILED PHARMACOLOGY...23 TOXICOLOGY...24 REFERENCES...25 PART III: CONSUMER INFORMATION F-Fluorodeoxyglucose, 18 F-FDG Page 2 of 27
3 FluGlucoScan 1 Injection 18 F - Fluorodeoxyglucose PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Administration Intravenous DESCRIPTION Dosage Form / Strength Parenteral Solution / > 0.5 GBq/vial Clinically Relevant Nonmedicinal Ingredients Citrate buffer and / or Sodium Chloride, Water FluGlucoScan ( 18 F - Fluorodeoxyglucose; 18 F - FDG) Injection is an intravenous diagnostic radiopharmaceutical for Positron Emission Tomography (PET). The chemical name of the active ingredient in FluGlucoScan Injection is 2-deoxy-2-18 F-fluoro-Dglucose, which has a molecular formula of C 6 H FO 5 with a molecular weight of daltons and the following chemical structure: FluGlucoScan Injection is supplied as a multi-dose (20 ml), sterile, non-pyrogenic injection vial containing a calibrated amount of 18 F - FDG in approximately 16 ml of citrate buffer and / or saline without preservatives. The ph of the solution is between 4.5 and FluGlucoScan is a trademark owned by the Alberta Cancer Board. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 3 of 27
4 Physical Characteristics The radionuclide present in the drug substance is fluorine-18 ( 18 F), which decays by positron (ß + ) emission with a half life of minutes. The daughter product of this process is the stable radionuclide, oxygen-18 ( 18 O). The physical radiation emission data for fluorine-18 are summarised in Table 1. Table 1 Principle Emission Data for Fluorine-18 Radiation Emission Percentage per Disintegration Mean Energy (kev) Positron (ß + ) Gamma (γ)* * produced by positron annihilation External Radiation The specific gamma ray constant for Fluorine F18 is 0.3 (Gy/hr/kBq) at 1 cm. The half-value layer (HVL) for the 511 KeV photons is 4.1 mm lead (Pb). A range of values for the attenuation of radiation results from the interposition of various thickness of Pb. The range of attenuation coefficients for this radionuclide is listed in Table 2. To correct for the physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 3. Table 2 Radiation Attenuation of 511 kev Photons by Lead Shielding Shield Thickness (Pb) (mm) Coefficient of Attenuation Table 3 Physical Decay Chart for Fluorine-18 Minutes (Calibration time) Fraction Remaining F-Fluorodeoxyglucose, 18 F-FDG Page 4 of 27
5 INDICATIONS AND CLINICAL USE FluGlucoScan Injection is indicated in Positron Emission Tomography (PET) for diagnostic use in patients for: (1) the evaluation of pulmonary nodules to distinguish benign from malignant and the evaluation of non-small cell and small cell lung cancers for staging and restaging and; (2) the evaluation of colorectal cancer for recurrence, restaging, and distant metastases. For lung cancer evaluation, certain thoracic area non-cancerous lesions may show FluGlucoScan Injection uptake including acute and chronic infections (such as abscesses, tuberculosis and histoplasmosis), and inflammatory / granulomatous conditions (such as sarcoidosis, bronchiectasis, or post radiotherapy sites) that could mimic tumour accumulation. Absent or less intense relative uptake of FluGlucoScan Injection may be observed in specific lesions including bronchoalveolar, mucinous and lobular carcinoma as well as carcinoid and fibroadenoma sites. For colorectal cancer evaluation, certain abdominal / pelvic area non-cancerous lesions may show FluGlucoScan Injection uptake including sites of post radiation or post surgical inflammatory response, lesion site flare following chemotherapy, colonic adenomas and bladder diverticula that could mimic tumour accumulation. Absent or less intense relative uptake of FluGlucoScan Injection may be observed in specific lesions including mucinous carcinoma. Lesion size may also affect detectability based on relative 18 F-FDG accumulation and PET imaging system resolution, as it has been shown that 18 F-FDG PET imaging may have a lower sensitivity in evaluating lesion sizes of less than 1 cm. CONTRAINDICATIONS Patients who are hypersensitive to this drug or to any ingredient in the formulation or component of the container. For a complete listing of ingredients, see the Dosage Forms, Composition and Packaging section of the product monograph. WARNINGS AND PRECAUTIONS Precautions related to the handling of radioactive material must be observed in the handling and utilisation of this product including those concerning radioactive patients. Radiopharmaceuticals should only be used by those health professionals who are appropriately qualified in the use of radioactive prescribed substances in or on humans. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 5 of 27
6 Serious Warnings and Precautions Radiopharmaceuticals should be used only by those health professionals who are appropriately qualified in the use of radioactive prescribed substances in or on humans. FluGlucoScan Injection should not be administered to pregnant women unless it is considered that the benefits to be gained outweigh the potential hazards to the fetus. Where an assessment of the risk benefit ratio suggests the use of FluGlucoScan Injection in nursing women, formula feeding should be substituted for breast feeding for a period of 24 hours following the PET scan. General The product should be administered under the supervision of a health professional who is experienced in the use of radiopharmaceuticals. Appropriate management of therapy and complications is only possible when adequate diagnostic and treatment facilities are readily available. The radiopharmaceutical product may be received, used and administered only by authorised persons in designated clinical settings. Its receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licenses of local competent official organisations. As in the use of any other radioactive material, care should be taken to minimise radiation exposure to patients consistent with proper patient management, and to minimise radiation exposure to occupational workers and other persons FluGlucoScan Injection should be used within twelve hours from the time of calibration. Contamination The following measures should be taken for up to 12 hours after receiving the radiopharmaceutical product: Toilet should be used instead of urinal. Toilet should be flushed several times after use Special precautions such as bladder catheterisation should be taken following administration to incontinent patients to minimise the risk of radioactive contamination of clothing, bed linen and the patient's environment. Special Populations Diabetes Mellitus Patients with diabetes may need stabilisation of blood glucose on the day preceding and on the day of the FluGlucoScan Injection PET scan. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 6 of 27
7 Pregnant Women: Since adequate reproduction studies have not been performed in animals to determine whether this drug affects fertility in males or females, has teratogenic potential, or has other adverse reactions on the fetus, FluGlucoScan Injection should not be administered to pregnant women, unless it is considered that the benefits to be gained outweigh the potential hazards to the fetus. Ideally examinations using FluGlucoScan Injection, especially those elective in nature of women of childbearing capacity should be performed during the first ten days following the onset of menses. Nursing Women: Where an assessment of the risk benefit ratio suggests the use of this product in nursing women, formula feeding should be substituted for breast feeding for a period of 24 hours following the PET scan. Pediatrics (< 16 years of age): The safety and efficacy of FluGlucoScan Injection in pediatric patients have not been established. Geriatrics (> 75 years of age): There are no known limitations on the clinical use of FluGlucoScan Injection in geriatric patients. ADVERSE REACTIONS Adverse Drug Reaction Overview Reviews of the literature and the clinical trial data indicate that there are no known adverse drug reactions associated with the use of FluGlucoScan Injection. Clinical Trial Adverse Drug Reactions Because clinical trials are conducted under very specific conditions the adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice and should not be compared to the rates in the clinical trials of another drug. Adverse drug reaction information from clinical trials is useful for identifying drug-related adverse events and for approximating rates. A single, multi-phase (Phase I/II, Phase III and Extended Phase III) clinical trial was conducted in a variety of cancer patients and a total of 575 patients were evaluated for safety. No FluGlucoScan Injection related adverse reactions have been observed. FluGlucoScan Injection has also been used in approximately 1300 additional patients encompassing various cancer types. No FluGlucoScan Injection related adverse reactions have been observed. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 7 of 27
8 Less Common Clinical Trial Adverse Drug Reactions (<1%) There are no known clinical trial adverse drug reactions (< 1%) associated with FluGlucoScan Injection. Abnormal Hematologic and Clinical Chemistry Findings Evaluation of 21 patients in the Phase I/II stage of the clinical trial indicated that no clinically significant changes in laboratory measurements were associated with FluGlucoScan Injection. Post-Market Adverse Drug Reactions There are no known post-market adverse drug reactions associated with FluGlucoScan Injection. DRUG INTERACTIONS Drug-Drug Interactions Interactions with other drugs have not been established. Drug-Food Interactions Patients can not eat or drink (except water) for four hours prior to the administration of FluGlucoScan Injection in order to diminish insulin-induced glucose utilisation. Drug-Herb Interactions Interactions with herbal products have not been established. Drug-Laboratory Interactions Interactions with laboratory tests have not been established. DOSAGE AND ADMINISTRATION Dosing Considerations Although, the dose required for the imaging study is determined by the patient s weight and the acquisition parameters of each particular PET camera, a minimum dosage of 100 MBq is used as a guideline to ensure that the PET scan is of diagnostic quality. A maximum dosage of 740 MBq is defined as the upper dosing limit, as any amount of drug exceeding that dose would not improve the diagnostic quality of the PET scan while unnecessarily increasing the absorbed radiation dose to the patient. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 8 of 27
9 Dosage The recommended dose of FluGlucoScan Injection for an adult is dependent upon patient body weight and the requirements of the PET scanner used for a particular type of study, but falls within the range of MBq ( mci) by intravenous injection. For example, a typical PET scanner may require the administration of 5 MBq/kg of patient body weight for a whole body scan so that for a patient weighing 70 kg, the required dose would be 350 MBq (70 kg x 5 MBq/kg). The final patient dose should be calculated using proper decay factors from the time of calibration and measured by a suitable radioactivity calibration system before administration. Patients should receive a single dose per day of FluGlucoScan Injection, with sufficient time between doses to allow for substantial decay (both physical and biological) of previous administration(s). Administration Patients can not eat or drink (except water) four hours prior to the administration of FluGlucoScan Injection in order to stabilize blood glucose levels. Patients with diabetes should not eat or drink (except water) for four hours prior to the administration of FluGlucoScan Injection. Patients with diabetes should also avoid taking insulin two hours prior to receiving FluGlucoScan Injection. To ensure a stable glycemic state (blood glucose 10 mmol / L), the patient s blood glucose level should be checked prior to receiving FluGlucoScan Injection. Patients must be able to lie still for approximately one to two hours (sedation may be required) and, for certain scan types, may be required to raise their arms over their head. Proper hydration, a urinary catheter and / or a diuretic may be required to eliminate confusing urinary tract activity that may confound PET scan interpretation of the abdomen and /or pelvis. The patient should void prior to being positioned on the scanner table. Proper hydration and frequent urination is recommended following a PET examination to minimise radiation exposure to the bladder. Using appropriate shielding and aseptic technique, the appropriate amount of FluGlucoScan Injection should be drawn into an appropriately sized syringe and needle. The patient dose should be measured by a suitable radioactivity calibration system prior to administration. FluGlucoScan Injection, like other parenteral drug products, should be inspected visually for particulate matter and discolouration before administration whenever solution and container permit. Preparations containing particulate matter or discolouration should not be administered to the patient; rather they should be disposed of in a safe manner that is compliant with applicable regulations. Image Acquisition, Reconstruction and Interpretation The acquisition parameters for imaging with FluGlucoScan Injection will vary depending upon the type of PET scanner and images required. For limited field tomographic imaging using a dedicated PET camera, careful patient positioning will allow for the clear delineation of metabolic activity in lesions previously identified through physical or other imaging examinations. Emission imaging should begin approximately 30 to 60 minutes following administration of FluGlucoScan Injection. Emission image acquisition typically ranges from six to fifteen minutes, 18 F-Fluorodeoxyglucose, 18 F-FDG Page 9 of 27
10 collecting between five to fifteen million total counts depending upon the number of body positions required. Whole body imaging can be obtained with correction for photon attenuation, which requires acquisition of transmission images. Elimination of image artifacts requires the exact repositioning at each level of the patient during the acquisitions of both the transmissions and emission whole body images. For the determination of tumour metabolic rates, dynamic imaging using a dedicated PET system is recommended. Following the transmission image, a sequence of serial images is initiated at the time of use of FluGlucoScan Injection and continues for approximately 60 to 90 minutes. Standard transaxial images are reconstructed in the form of transaxial 128 x 128 pixel images or a pixel size of 4 to 5 mm. Image sets can be re-oriented into coronal and / or sagittal slices. The contiguous transaxial and / or coronal or sagittal slices can then be examined by visual inspection and interpreted relative to the normal physiological uptake of FluGlucoScan Injection in the brain, myocardium, liver, spleen, stomach, intestines, kidneys or urine. Increased or abnormal FluGlucoScan Injection uptake can signify neoplasms. Healing surgical wounds, infections, granulomatous tissue, or other inflammatory tissue responses may also show areas of increased FluGlucoScan Injection uptake. Practitioners should be appropriately trained in the interpretation of FluGlucoScan Injection PET images. Tumour metabolism can also be estimated using semi-quantitative or quantitative methods. The semi-quantitative estimate of tumour metabolism (i.e. standard uptake values [SUV]) is based on relative lesion radioactivity normalised to the injected dose and patient body weight. It requires a static emission image acquired following the plateau of FluGlucoScan Injection concentration levels (approximately 30 minutes), the total administered dose of FluGlucoScan Injection, and the patient s height and weight for measurement of lean body mass or of body surface area. Additional data which may be required include the measurement of arterial input function and the determination of the plasma FluGlucoScan Injection levels and glucose concentrations. A calibration factor will be required between scanner events in terms of (counts / pixel / sec) and in vitro measured activity concentrations in (counts / ml / sec). This can be accomplished by imaging a cylindrical phantom with a known concentration of positron emitter and by measuring the activity of an aliquot of the cylinder solution in a well counter. This measurement can be corrected for blood glucose concentration. Estimates of metabolic tumour rates, either using quantitative or semi-quantitative methods, are obtained by assigning regions of interest (ROI) to the tumour and the blood pool on the dynamically acquired images. The resulting time activity curves are then fitted with a tracer compartment model or submitted to graphical analysis in order to derive the phosphorylation of 18 F-2-deoxyglucose. Instructions for Preparation and Use The components of the vial are isotonic, sterile and non-pyrogenic. It is essential that the user follows the directions carefully and adheres to strict aseptic technique. Use aseptic technique and wear waterproof gloves throughout the entire preparation procedure. Make all transfers of radioactive solutions with an adequately shielded syringe and maintain adequate shielding around the vial during the useful life of the radioactive product. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 10 of 27
11 Directions for Quality Control The required quality control testing is performed on the product prior to release. RADIATION DOSIMETRY Estimated Absorbed Doses (mgy/mbq) from Intravenous Administration of 18 F-FDG 2 Absorbed dose per Unit Organ Activity Administered (mgy/mbq) Adult Adrenals Bladder Bone Surfaces Brain Breast Gall Bladder Stomach Small Intestine Colon Upper Large Intestine Lower Large Intestine Heart Kidney Liver Lungs Muscles Esophagus Ovaries Pancreas Red Marrow Skin Spleen Testes Thymus Thyroid Uterus Remaining Organs The International Commission on Radiological Protection (ICRP) 80. Radiation dose to patients from radiopharmaceuticals. Addendum 2 to ICRP publication 53. Ann ICRP 1998; 28:1,47, F-Fluorodeoxyglucose, 18 F-FDG Page 11 of 27
12 Effective Dose for 18 F-FDG 2 Parameter Effective Dose (msv/mbq) Age Group Adult ACTION AND CLINICAL PHARMACOLOGY Mechanism of Action 18 F-FDG is actively transported from blood to tissue in a manner similar to glucose, where it is phosphorylated by hexokinase to 18 F-FDG-6-phosphate. As 18 F-FDG-6-phosphate is not a substrate for subsequent glycolytic pathways, and has very low membrane permeability, the 18 F-FDG becomes trapped in tissue in proportion to the rate of glycolysis or glucose utilisation of that tissue. Imaging of the subject using a PET scanner takes advantage of the positron decay of 18 F to identify those tissues that have an abnormal accumulation of the radioisotope. Pharmacodynamics 18 F-FDG, as a glucose analogue, concentrates in cells that rely upon glucose as a primary energy source, or in cells whose dependence on glucose increases under pathophysiologic conditions. 18 F- FDG is transported through the cell membrane by facilitative glucose transporter proteins and is phosphorylated within the cell to 18 F-FDG-6-phosphate by the enzyme hexokinase. Once phosphorylated, it cannot exit the cell as it is not a suitable substrate for dephosphorylation by glucose-6-phosphate. Therefore, within a given tissue or pathophysiological process, the retention and clearance of 18 F-FDG reflect a balance involving glucose transporter proteins, hexokinase and glucose-6-phosphatase activities. When allowance is made for the kinetic differences between glucose and 18 F-FDG transport and phosphorylation, 18 F-FDG can be used to assess glucose metabolism. In comparison to background activity of the specific organ or tissue type, regions of decreased or absent uptake of 18 F-FDG reflect the decrease or absence of glucose metabolism. Regions of increased uptake (relative to background) of 18 F-FDG reflect greater than normal rates of glucose metabolism. In cancer, cells are generally characterised by enhanced glucose metabolism partially due to (1) an increase of the glucose transporters, (2) an increase rate of phosphorylation activity, (3) a reduction of phosphatase activity or, (4) a dynamic alteration in the balance among all of these processes. However, glucose metabolism of cancer as reflected by 18 F-FDG accumulation shows considerable variability. Depending upon the tumour type, stage and location, 18 F-FDG accumulation may be increased, normal or decreased. Also, inflammatory cells can have the same variability of uptake of 18 F-FDG. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 12 of 27
13 Pharmacokinetics Absorption: FluGlucoScan Injection is intended for intravenous administration only. Distribution: 18 F-FDG accumulates throughout the body in proportion to glucose metabolism. Due to their high glycolytic rates, the brain and heart generally exhibit the highest accumulations post-prandially, therefore a fasting state is desirable to minimise uptake in these organs. Other tissues that exhibit the potential for moderate glucose metabolic rates and therefore 18 F-FDG uptake are the liver, spleen, thyroid, gut and bone marrow. As active skeletal muscle will accumulate 18 F-FDG, a relaxed state, especially during the initial uptake phase, is important to minimise uptake in these organs. 18 F-FDG has been shown to accumulate in primary and metastatic tumours throughout the body, possibly related to the concentration of glucose transporters in the cell membrane, the tumour proliferation rate, the degree of tumour differentiation, and the number of viable cancer cells present in the tumour. Metabolism: 18 F-FDG is phosphorylated to 18 F-FDG-6-phosphate by hexokinase, with no significant further metabolism taking place within the duration of the PET scan. Excretion: 18 F-FDG is excreted unchanged in the urine (approximately 20 % of the administered activity is excreted within the first 2 hours), therefore the urinary tract can show intense accumulation of 18 F- FDG. Seventy-five (75) % of the administered activity of 18 F-FDG is retained with an effective half-life of 1.83 hours; 19% has an effective half-life of 0.26 hours and the remaining 6 % has an effective half-life of 1.53 hours. The time to peak concentration is approximately 30 minutes in highly metabolic tissues such as the brain. Since the time to peak concentration depends on the glucose metabolic rate and whole body clearance of 18 F-FDG, less metabolically active tissues such as many tumours may not reach peak concentrations until nearly 2 hours. The time also depends on the balance between the uptake of 18 F-FDG, the clearance of 18 F-FDG from the blood and radioactive decay. Special Populations and Conditions No data available. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 13 of 27
14 OVERDOSAGE Overdoses of FluGlucoScan Injection have not been reported. In case of overdose, the patient should be monitored for adverse drug reactions and managed as clinically indicated. STORAGE AND STABILITY The multi-dose vial containing FluGlucoScan Injection should be stored upright at room temperature in a shielded area until administration, within twelve hours from time of calibration. All regulations regarding the handling of radioactive material should be followed. SPECIAL HANDLING INSTRUCTIONS As in the use of any other radioactive material, care should be taken to minimise radiation exposure to patients consistent with proper patient management, and to minimise radiation exposure to occupational workers, consistent with ALARA. Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclide, and whose experience and training have been approved by the appropriate governmental agency authorised to license the use of radionuclides. DOSAGE FORMS, COMPOSITION AND PACKAGING FluGlucoScan Injection is supplied in a 20 ml, multi-dose, sterile, non-pyrogenic glass vial containing at least 0.5 GBq of no carrier added 18 F-fluorodeoxyglucose in approximately 16 ml of citrate buffer and / or saline. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 14 of 27
15 PHARMACEUTICAL INFORMATION PART II: SCIENTIFIC INFORMATION Drug Substance Proper name: Chemical name: 18 F-fluorodeoxyglucose, 18 F-FDG 2-deoxy- 18 F-fluoro-D-glucose Molecular formula and molecular mass: C 6 H F O 5, MW = Structural formula: Product Characteristics FluGlucoScan Injection is a clear, colourless solution supplied in a multi-dose (20 ml), sterile, nonpyrogenic glass vial containing at least 0.5 GBq of no carrier added 18 F-fluorodeoxyglucose in approximately 16 ml of citrate buffer and / or saline. Sodium citrate, saline and Sterile Water for Injection are used as formulation excipients. Product is prepared on a daily basis with expiry time of 12 hours from the calibration time noted on the label. The radionuclide present in the drug product is fluorine-18 ( 18 F), which decays by positron (β+) emission and has a half-life of minutes. The principal photons useful for diagnostic imaging are the 511 kev gamma photons, resulting from the interaction of the emitted positron with an electron (Table 4). Table 4 Principal Emission Data for Fluorine 18 Radiation / Emission Percent per Disintegration Mean Energy Positron (β+) kev Gamma (±)* kev *Produced by positron annihilation 18 F-Fluorodeoxyglucose, 18 F-FDG Page 15 of 27
16 The specific gamma ray constant for fluorine-18 is 0.3 Gy/hr/kBq (6.0 R/hr/mCi) at 1 cm. The lead shielding half value layer (HVL) for the 511 kev photons is 4.1 mm. A range of values for the attenuation of radiation results from the interposition of various thicknesses of lead shielding. The range of attenuation coefficients for this radionuclide is shown in Table 5. For example, the interposition of an 8.3 mm thickness of lead shielding, with a coefficient of attenuation of 0.25, will decrease the external radiation by 75%. Table 5 Radiation Attenuation of 511 kev Photons by Lead Shielding Lead Shield Thickness (mm) Coefficient of Attenuation For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 6. Table 6 Physical Decay Chart for Fluorine-18 *Calibration Time Minutes Fraction Remaining 0* The specific activity of no carrier added 18 F-FDG has been calculated to be approximately 63.3 GBq / nmol or 348 MBq / ng (1.7 Ci / nmol or 9.4 mci / ng) with a total calculated amount of 18 F- FDG present as approximately 1.58 nmol (or 287 ng) per vial at a concentration of approximately 17.9 ng / ml (or 98.8 pmol /ml). Citrate buffer (approximately 8.4 mg/ml) and / or sodium chloride ( mg/ml) are also present in the solution prepared using Sterile Water for Injection. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 16 of 27
17 CLINICAL TRIALS Study demographics and trial design Study Title: A Phase I/II, Phase III and Extended Phase III Study of 18 F-Fluorodeoxyglucose (FluGlucoScan Injection) in Patients with Cancer or Suspected Cancer (DX-FDG-001) The trial design was a combined continuous Phase I/II, Phase III, Extended Phase III, diagnostic imaging, controlled, open label, multi-centre, clinical trial in a broad cross-section of patients with oncologic disease. These included carcinoma of the brain, breast, colorectal, head & neck, lung, thyroid, esophageal, and lymphoma, melanoma, PRUNK and neuroendocrine tumours (Table 7). Approximately 1893 patients had been enrolled up to the cut-off date of September 29, 2005 and, of these, 575 were evaluated for safety and 331 were evaluated for efficacy. Demographic data for the patients evaluated for efficacy are presented in Table 8 Table 7 Demographic Data for Patients in Phase I/II, Phase III and Extended Phase III Studies included in NDS Parameter Phase I / II Phase III Extended Phase III Total patient numbers Gender Age (years) Indications and Distributions Female: 13 Male: 8 Mean: 63.5 Median: 65.2 Range: Brain: 1 Breast: 6 Lung: 7 Primary Unknown: 1 Thyroid: 1 Esophageal: 2 Lymphoma: * Female: 185 Male: 166 Mean: 57.9 Median: 58.9 Range: Brain: 7 Breast: 65 Colorectal: 34 Esophageal: 10 Head & Neck: 15 Lymphoma: 22 Lung: 140 Melanoma: 9 Neuroendocrine: 11 Primary Unknown: 12 Thyroid: 26 Female: 111 Male: 113 Mean: 63.8 Median: 64.6 Range: Colorectal: 147 Lung: 77 * sequential evaluable lung and colorectal cancer patients dose is calculated individually for each patient dependent upon patient weight, PET scanner type 18 F-Fluorodeoxyglucose, 18 F-FDG Page 17 of 27
18 Table 8 Demographic Data for Efficacy Patients from Phase III / Extended Phase III Study Disease Category Parameter Value Lung Cancer (SPN) Total Patient Numbers 125 Gender Total Patients Female: 64 Male: 61 Phase III Patients Female: 28 Male: 36 Extended Phase III Patient Female: 36 Male: 25 Age (years) Mean: 64.8 Median: 66.6 Range: ( ) Recurrent Colorectal Cancer Total Patient Numbers 148 Gender Total Patients Female: 65 Male: 83 Phase III Patients Female: 13 Male: 17 Extended Phase III Patient Female: 52 Male: 66 Age (years) Mean: 63.0 Median: 64.3 Range: ( ) Study results Safety results: Table 9 summarises the results of the safety analysis for FluGlucoScan Injection. Table 9 Summary of Safety Results from Clinical Trials Study # (Name) Primary Safety Endpoints Results Phase I/II Phase III/Extended Phase III Evaluation of safety by assessment of adverse events, vital signs, haematology and blood chemistry. Evaluation of safety by assessment of adverse events and vital signs. Twenty-one (21) patients were analysed for safety. No adverse reactions observed or reported and no significant changes observed in patient vital signs, haematology or blood chemistry due to administration of FluGlucoScan Injection. Five hundred and seventy-five (575) patients were analysed for safety. No adverse reactions were observed or reported and no significant changes observed in patient vital signs due to administration of FluGlucoScan Injection. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 18 of 27
19 A study of over 80, F-FDG injections (1) and the USP DI Product Monograph for Fludeoxyglucose F 18 Systemic (2) confirm that there are no known side/adverse effects associated with the use of 18 F-FDG establishing its safety profile. Efficacy Results: Final efficacy was determined from Phase III and Extended Phase III of the clinical trial as described intable 10. Table 10 Final Efficacy Analysis Summary Patient Source: Phase III Extended Phase III Patient Demographics (Tumour Type, Gender and Number) Sixty-four (64) patients (28 females and 36 males) with pulmonary nodules and thirty (30) patients (13 females and 17 males ) with recurrent colorectal cancer were included in the efficacy analysis. Sixty-one (61) patients (36 females and 25 males) with pulmonary nodules and one hundred eighteen (118) (52 females and 66 males) with recurrent colorectal cancer were included in the efficacy analysis. Primary Efficacy Endpoints Evaluation of efficacy by assessment of sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) of FluGlucoScan Injection for detection of solitary pulmonary nodules (SPN) and recurrent colorectal cancer compared to appropriate matched literature values. Diagnostic outcomes on a per patient basis were determined using FluGlucoScan Injection scan outcome values and all applicable clinical information. Sensitivity (ratio of true positive target lesions to total positive target lesions), specificity (ratio of true negative target lesions to total negative target lesions), accuracy (ratio of total correct studies to the total number of target lesions), PPV (ratio of true positive target lesions to total number of positive lesions) and NPV (ratio of true negative target lesions to total number of negative lesions) of FluGlucoScan Injection PET scans obtained were determined. Confidence intervals for sensitivity, specificity, accuracy, PPV and NPV were derived using the exact binomial calculations. Statistical comparison to the indication matched literature values (obtained from appropriate meta-analyses) was conducted using an exact binomial test. Table 11 and Table 12 shows the overall sensitivity, specificity, accuracy, PPV and NPV of FluGlucoScan Injection PET imaging for the final efficacy analysis population compared to corresponding literature values for patients with solitary pulmonary nodules (SPNs) or recurrent colorectal cancer, respectively. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 19 of 27
20 Table 11 Clinical Diagnostic Parameter Efficacy Results for Patients with SPNs Source Indication Diagnosis (Pulmonary Sensitivity 95.2 ( ) n = 1360 Specificity 75.2 ( ) n = 467 Accuracy 90.0 ( ) n = 1827 PPV 91.8 ( ) n = 1411 NPV 84.4 ( ) n = 416 Supporting Clinical Liter ature Diagnosis (Pulmonary 96.9 ( ) n = ( ) n = ( ) n = ( ) n = ( ) n = 50 Clinical Trial Data p-value * * Significantly different p < 0.05 n = number of patients evaluated; p-value = one-sided mid p - value Table 12 Clinical Diagnostic Parameter Efficacy Results for Patients with Recurrent Colorectal Cancer Source Supporting Clinical Litera ture Indication Recurrent Sensitivity 96.1 ( ) n = ( ) n = 102 Specificity 93.5 ( ) n = ( ) n = 46 Accuracy 94.7 ( ) n = ( ) n = 148 PPV 92.4 ( ) n = ( ) n = 103 NPV 96.7 ( ) n = ( ) n = 45 ACB Clinical Trial Data p-value n = number of patients evaluated; p-value = one-sided mid p - value 18 F-Fluorodeoxyglucose, 18 F-FDG Page 20 of 27
21 Lung Cancer Lung cancer is the leading cause of cancer related mortality in the western world, with approximately 170,000 new cases diagnosed each year in the U.S.A. In Canada, 29% of all cancer related deaths in males and 25% in females are attributable to cancer of the lung. Pulmonary nodules are typically encountered in patients with lung cancer, with many nodules incidentally discovered during work-up for unrelated signs or symptoms. Although suspicious nodules can frequently be addressed through percutaneous biopsy or thoracoscopic surgery; less definitive lesions often require serial imaging with conventional radiography (i.e. chest x-rays) or CT over a period of several months. Fortunately, the preferential uptake of FluGlucoScan Injection in metabolically active lung cancers can quickly differentiate malignant nodules from those of a benign nature thus minimising the amount of time required for diagnosis, reducing the number of invasive diagnostic procedures in the event of FluGlucoScan Injection negative lesions and permitting a timely implementation of patient therapy. When compared to the cumulative literature diagnostic parameter values (Table 11), FluGlucoScan Injection diagnostic parameter values for patients with pulmonary nodules demonstrate an absolute difference of not greater than 7% (except for the NPV - where the difference between FluGlucoScan Injection NPV and that of the cumulative literature is statistically significant and is greater by an absolute difference of 11.6%), indicating that FluGlucoScan Injection is indeed comparable to other marketed 18 F-FDG products for the diagnostic evaluation of pulmonary nodules. Staging and Restaging of Lung Cancer Once diagnosed with a primary lung cancer, patients undergo a series of staging procedures to determine the extent of their disease in accordance to the Tumour-Nodes-Metastases (TNM) classificatory scheme and to plan their optimal treatment strategy. Although CT is employed ubiquitously for the staging of primary lung cancer, it is limited by the inability to delineate tumour tissue from obstructive or inflammatory changes, differentiate benign accumulations of pleural fluid from those of a more malignant nature, and accurately characterize mediastinal and hilar lymph nodes. In contrast, the metabolic imaging capabilities of 18 F-FDG PET can accurately discriminate active tumour from atelectasis or pneumonia, verify the presence of malignant pleural effusions, and delineate the nodal spread of disease. Indeed the negative predictive values for the 18 F-FDG PET evaluation of mediastinal nodes are reported to be greater than 90.0%, suggesting that patients identified as 18 F -FDG PET-negative for metastases can proceed to surgery with confidence that their disease is localized (Table 13). For patients with recurrent disease, 18 F-FDG PET also proves useful in the identification of local intrathoracic recurrence and metastatic disease (Table 13). 18 F-FDG PET can accurately characterize new pulmonary nodules or masses in the ipsilateral or contralateral lung, identify distant metastatic disease sites and delineate post-therapeutic tissue changes from malignancies. In contrast, the proper evaluation of distant metastases with conventional imaging techniques requires numerous scans, including CT, MRI and skeletal scintigraphy. Serial CT studies are also required to document any post-therapeutic tissue changes, resulting in the delayed implementation of 18 F-Fluorodeoxyglucose, 18 F-FDG Page 21 of 27
22 appropriate patient therapy. Table 13 Summary of Supporting Clinical Literature Diagnostic Parameter Values for 18 F-FDG PET Staging and Restaging of Lung Cancer Source Supporting Clinical Literature Supporting Clinical Literature Indication Staging Restaging Sensitivity 84.6 ( ) n = ( ) n = 249 Specificity 87.9 ( ) n = ( ) n = 279 Accuracy 86.8 ( ) n = ( ) n = 528 PPV 78.4 ( ) n = ( ) n = 279 NPV 91.6 ( ) n = ( ) n = 249 The established comparability of FluGlucoScan Injection with literature 18 F-FDG in SPN evaluation (Table 11), the established literature benefit of 18 F-FDG in staging and restaging of lung cancer (Table 13) and the similar biochemical patterns in these tumour types supports the application of FluGlucoScan Injection in these indications. Thus the combination of clinical trial data and literature analysis establishes the use of FluGlucoScan Injection in all claimed indications for lung cancer. 18 F-Fluorodeoxyglucose, 18 F-FDG Page 22 of 27
23 Recurrent Colorectal Cancer It is estimated that 25-40% of patients treated surgically for a primary colorectal cancer will experience disease recurrence. In 20% of these cases, the recurrence is localized and therefore amenable to curative resection. Unfortunately the use of standard techniques, such as carcinoembryonic antigen (CEA) assays, CT and MRI, for the detection of disease recurrence is often hindered by poor accuracy. Scarring, fibrosis and other post-therapeutic tissue changes are often difficult to differentiate from malignancy using CT and MRI, requiring patients to undergo serial examinations over a period of several months before receiving the correct diagnosis. In contrast, 18 F-FDG PET can quickly and accurately discriminate malignancy from metabolically inactive tissue changes and identify hepatic, extrahepatic and distant metastases, facilitating patient diagnosis and treatment planning (Table 12). 18 F -FDG PET has a further advantage in that only a single study is required to make these determinations unlike other conventional imaging techniques. When compared to the cumulative literature diagnostic parameter values (Table 12), FluGlucoScan Injection diagnostic parameter values demonstrate a difference of not greater than 10%, indicating that FluGlucoScan Injection is indeed comparable to other marketed 18 F-FDG products for the diagnostic evaluation colorectal cancer recurrence and the restaging of disease. DETAILED PHARMACOLOGY The hydroxyl group on the second carbon of glucose can be substituted by a group such as hydrogen or fluorine without seriously compromising the kinetic and biochemical ability of the molecule to be actively transported through the cell membrane and to act as a substrate for the hexokinase enzyme. The 2-deoxy analogues of glucose are transported into the cell and metabolized quantitatively exactly like D-glucose up to the point in the glycolytic pathway where its anomalous structure prevents the final conversion of the 2-deoxyglucose-6-phosphate by phosphohexoseisomerase 1 (3). Gallagher et al. (4, 5) have studied the tissue distribution of 18 F-FDG in animals. In mice, 18 F-FDG distributes uniformly to the kidneys, heart, brain, lungs and liver initially and clears rapidly from all tissue except the heart where it remains constant for at least two hours and, to a lesser extent, in the brain where it decreases slowly from one to two hours. The rapid clearance of 18 F-FDG from the liver, lungs and kidneys, and its retention by the heart and brain is a result of metabolic trapping within these organs and is reflective of glucose utilisation. Urinary excretion of intact 18 F-FDG was 15 to 25% of injected dose at 90 minutes. Kearfott et al. (6) studied groups of mice (CD-1 strain) and rats (Sprague-Dawley strain) injected intravenous (IV) with tracer amounts of 18 F-FDG and sacrificed at 1, 5, 30, 60, and 120 minutes for tissue biodistribution analysis. They also studied two mongrel dogs with imaging at 40 to 80 minutes post IV injection of tracer doses for tissue biodistribution analysis with arterial blood sampling at 0 to 10, 15, 20, 30, 40, 45, 60, and 90 minutes. The dogs were sacrificed at 60 and 120 minutes. Tissue biodistribution (percent of injected dose per gram - %ID/gram) for blood, brain, liver, spleen, lung, heart, kidney, bone, muscle and bladder over the time periods indicated for mice and rats was reported. Tissue biodistribution (percent of injected dose per one percent of body weight - %ID/gram/1%body weight) for blood, brain (left and right hemisphere, cerebellum), liver, 18 F-Fluorodeoxyglucose, 18 F-FDG Page 23 of 27
24 spleen, lung, heart (left and right atria and ventricle), kidney, bone, muscle, and bladder wall for the dogs was determined. Tissue pharmacokinetic parameters in heart and brain were estimated. TOXICOLOGY Fluorodeoxyglucose (FDG) Bessell et al. (7) studied the toxicology of FDG injected intraperitoneally in mice and rats and reported the LD50 in mice as 600 mg/kg. Reivich et al. (8) studied the toxicology of FDG in mice and dogs. Mice were injected intraperitoneally with FDG (3 weekly doses of 14.3 mg/kg 3000 times the human dose). No effect was noted on animal weight, no gross or microscopic abnormalities were noted and no immediate or long term effects were noted. Dogs were injected intravenously with three doses of 0.72 mg/kg and showed no clinical signs or symptoms of adverse effects. No significant abnormalities were detected in the blood, urine or cerebrospinal fluid. No significant gross or microscopic abnormalities were noted in internal organs. Som et al.(9) studied the toxicology of FDG in mice and dogs. Mice were injected intraperitoneally with FDG (3 weekly doses of 14.3 mg/kg 3000 times the human dose). No effect was noted on animal weight and no gross or microscopic abnormalities were noted. Dogs were injected intravenously with three doses of 0.72 mg/kg and no significant abnormalities were detected in the blood, urine or cerebrospinal fluid. No significant gross or microscopic abnormalities were noted in internal organs. No abnormalities of body temperature, blood pressure, pulse or breathing were observed in the dogs. Acetonitrile, Ethanol, Acetone and Kryptofix 222 Potential impurities that have been observed in very small amounts in FluGlucoScan Injection are acetonitrile, acetone and Kryptofix 222 (Kryptofix) and therefore, the potential impact of their presence on product safety was assessed. The acetonitrile limit for FluGlucoScan Injection follows the ICH Q3C(R3) specification for this residual solvent and thus provides a large safety factor for exposure of the patient to this potentially toxic chemical. The ethanol limit for FluGlucoScan Injection follows the ICH Q3C(R3) specification for this residual solvent and thus provides a large safety factor for exposure of the patient to this low toxicity chemical. The acetone limit for FluGlucoScan Injection follows the ICH Q3C(R3) specification for this residual solvent and thus provides a large safety factor for exposure of the patient to this low toxicity chemical. The acute toxicity of Kryptofix has been evaluated in rats and mice (10). The LD 50 of an intravenous dose in mice was 35 mg/kg and of an intraperitoneal dose was 110 mg/kg. The LD 50 of an intravenous dose in rats was 32 mg/kg and of an intraperitoneal dose was 153 mg/kg. Doses of 18 F-Fluorodeoxyglucose, 18 F-FDG Page 24 of 27
25 up to mg/kg (route not specified) in rats demonstrated transient elevations in liver enzymes but no other histopathological changes were evident (11). The Kryptofix limit ( 50 µg/ml) for FluGlucoScan Injection follows the USP 30 specification and is more than two orders of magnitude below the lethal dose in rodents, delivered intravenously. Thus a more than reasonable safety margin is realised for the (worst case) Kryptofix content in FluGlucoScan Injection based on these assessments. REFERENCES 1. Silberstein EB. Positron-Emitting Radiopharmaceuticals: How Safe Are They? Cancer Biother Radiopharm 2001 Feb; 16(1): The United States Pharmacopeia Convention, Inc. USPDI Drug Advice for the Health Care Professional. Vol. 1. [monograph on the Internet]. Greenwood Village: Thomson Micromedex; [cited 2007 Feb 22]. Available from: 3. Pauwels EKJ, Sturm EJC, Bombardieri E, Cleton FJ, Stokkel MPM. Positron-emission tomography with [ 18 F]fluorodeoxyglucose: Part I. Biochemical uptake mechanism and its implication for clinical studies. J Cancer Res Clin Oncol 2000 Oct; 126(10): Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan C-N, Wolf AP. Metabolic Trapping as a Principle of Radiopharmaceutical Design: Some Factors Responsible for the Biodistribution of [ 18 F] 2-Deoxy-2-Fluoro-D-Glucose. J Nucl Med 1978 Oct; 19(10): Gallagher BM, Ansari A, Atkins H, Casella V, Christman DR, Fowler JS, et al. Radiopharmaceuticals XXVII. 18 F-Labeled 2-Deoxy-2-Fluoro-D-Glucose as a Radiopharmaceutical for Measuring Regional Myocardial Glucose Metabolism In Vivo: Tissue Distribution and Imaging Studies in Animals. J Nucl Med 1977 Oct; 18(10): Kearfott KJ, Elmaleh DR, Goodman M, Correira JA, Alpert NM, Ackerman RH, et al. Comparison of 2- and 3-18F-Fluoro-deoxy-D-glucose for Studies of Tissue Metabolism. Int J Nucl Med Biol 1984;11(1): Bessell EM, Courtenay VD, Foster AB, Jones M, Westwood JH. Some In Vivo and In Vitro Antitumour Effects of the Deoxyfluoro-D-Glucopyranoses. Eur J Cancer 1973 Jul; 9(7): Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, et al. The [ 18 F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Cir Res 1979 Jan; 44(1): Som P, Atkins HL, Bandoypadhyay D, Fowler JS, MacGregor RR, Matsui K, et al. A Fluorinated Glucose Analog, 2-fluoro-2-deoxy-D-glucose (F-18): Nontoxic Tracer for Rapid Tumor Detection. J Nucl Med 1980 Jul; 21(7): Baudot P, Jacque M, Robin M. Effect of a Diaza-Polyoxa-Macrobicylic Complexing Agent on the Urinary Elimination of Lead in Lead-Poisoned Rats. Toxicol Appl Pharmacol 1977 Jul; 41(1): Bauman M, Schäffer E, Greim H. Short-term Studies with the Cryptating Agent Hexaoxa-diazabicyclo-hexacosane in Rats. Arch Toxicol Suppl 1984; Suppl 7: F-Fluorodeoxyglucose, 18 F-FDG Page 25 of 27
26 PART III: CONSUMER INFORMATION FluGlucoScan Injection 18 F - Fluorodeoxyglucose, 18 F-FDG This leaflet is part III of a three-part Product Monograph published when FluGlucoScan Injection was approved for sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about FluGlucoScan Injection. Contact your doctor or pharmacist if you have any questions about the drug. ABOUT THIS MEDICATION What the medication is used for: FluGlucoScan Injection is a radioactive drug which is used in conjunction with a diagnostic Positron Emission Tomography (PET) scan to help your physician evaluate your cancer. What it does: FluGlucoScan Injection is a radioactive form of sugar with the radioisotope, Fluorine-18, attached to it. When it is injected into a vein, it is distributed throughout your body. Cancer cells require more sugar to function and, therefore, FluGlucoScan Injection will concentrate in them. A diagnostic scanning test, called a PET scan, uses the radioisotope in FluGlucoScan Injection to make whole-body images. These images can help your physician detect the presence and the location of cancer cells within your body. When it should not be used: The FluGlucoScan Injection should not be used if you: are pregnant or nursing are allergic to any components of FluGlucoScan Injection are diabetic with uncontrolled blood sugar levels What the medicinal ingredient is: F-18 Fluorodeoxyglucose (a radioactive form of sugar) What the important nonmedicinal ingredients are: Citrate buffer, Sodium Chloride (salt) and Water IWARNINGS AND PRECAUTIONSAND PRECAUTIONS Serious Warnings and Precautions BEFORE you receive FluGlucoScan Injection talk to your doctor or pharmacist if: you have any allergies to the FluGlucoScan Injection or its ingredients you have diabetes, as your blood sugar levels may have to be assessed prior having your PET scan with FluGlucoScan Injection you think you may be or are pregnant you are breast feeding your baby INTERACTIONS WITH THIS MEDICATION Interactions between FluGlucoScan Injection and other drugs, herbal remedies, and food or food products have not been established. PROPER USE OF THIS MEDICATION This product (FluGlucoScan Injection) will be administered under the supervision of a health professional who is experienced in the use of radiopharmaceuticals. Diabetic patients should ensure that their blood sugar levels are stable the day preceding and the day of the PET scan with FluGlucoScan Injection. You may be asked to eat nothing and drink only water for four hours before your scheduled PET scan with FluGlucoScan Injection. To decrease the radiation exposure to your bladder, you should drink plenty of water and urinate as often as possible when the PET scan is finished. SIDE EFFECTS AND WHAT TO DO ABOUT THEM No side effects have been associated with the use of FluGlucoScan Injection in clinical trials. SERIOUS SIDE EFFECTS, HOW OFTEN THEY HAPPEN AND WHAT TO DO ABOUT THEM. There are no known serious side effects associated with FluGlucoScan Injection. If you experience any unusual symptoms or allergic reactions (such as itching, rash, hives, elevated heart rate, nausea or vomiting), contact your physician or pharmacist. Because FluGlucoScan Injection is a radio-pharmaceutical, it should be used only by those health professionals who are appropriately qualified in the use of radioactive prescribed substances in or on humans. FluGlucoScan Injection should not be administered to pregnant women unless it is considered that the benefits to be gained outweigh the potential hazards to the fetus. FluGlucoScan Injection can be passed through breast milk to your nursing infant. To avoid unnecessary exposure of your infant to radiation, breast-feeding should be substituted with formula feeding for a period of 24 hours following the PET scan 18 F-Fluorodeoxyglucose, 18 F-FDG Page 26 of 27
CanTrace PRODUCT MONOGRAPH. Parenteral Solution, up to 1.4 GBq/mL (38.2 mci/ml) Diagnostic Radiopharmaceutical
 PRODUCT MONOGRAPH CanTrace 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, up to 1.4 GBq/mL (38.2 mci/ml) Diagnostic Radiopharmaceutical IPET Pharmaceuticals, Inc. Suite 880, 1090 West Georgia,
PRODUCT MONOGRAPH CanTrace 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, up to 1.4 GBq/mL (38.2 mci/ml) Diagnostic Radiopharmaceutical IPET Pharmaceuticals, Inc. Suite 880, 1090 West Georgia,
GALLIUM CITRATE Ga 67 INJECTION
 511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
INDICATIONS AND USAGE
 1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
Page 1 of CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
GLUCEPTATE. Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION
 27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
Sodium Iodide I 131 Solution. Click Here to Continue. Click Here to Return to Table of Contents
 Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Click Here to Continue. Click Here to Return to Table of Contents
 Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
PRODUCT MONOGRAPH. F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml. Diagnostic Radiopharmaceutical
 PRODUCT MONOGRAPH 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml Diagnostic Radiopharmaceutical Isologic Innovative Radiopharmaceuticals Ltd. Date of Preparation: 1855 32 nd Avenue,
PRODUCT MONOGRAPH 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml Diagnostic Radiopharmaceutical Isologic Innovative Radiopharmaceuticals Ltd. Date of Preparation: 1855 32 nd Avenue,
To report suspected adverse reactions to Axumin, call AXUMIN1 ( ) or contact FDA at FDA-1088 or
 An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
Sodium Fluoride F 18 Injection* PET/CT Imaging
 Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
Core SPC for Fludeoxyglucose ( 18 F) March 2005
 Core SPC for Fludeoxyglucose ( 18 F) March 2005 This FDG Core SPC has been prepared on the basis, and taking into account the available published scientific literature dated from more than 10 years. Then
Core SPC for Fludeoxyglucose ( 18 F) March 2005 This FDG Core SPC has been prepared on the basis, and taking into account the available published scientific literature dated from more than 10 years. Then
PHYSICAL CHARACTERISTICS
 BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
R: March, E D T P A. Kit for the Preparation of Technetium Tc 99m Pentetate Injection. DIAGNOSTIC - For Intravenous Use
 R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate)
 Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
FP/1-61//- p-o37 A mb. Precision Nuclear, LLC. July 7, 2011
 211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF)
 Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF) Overview Indication Sodium Fluoride F18 injection is a radioactive diagnostic agent for positron emission
Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF) Overview Indication Sodium Fluoride F18 injection is a radioactive diagnostic agent for positron emission
Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer
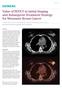 Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
Poltechnet GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER Poltechnet 8.0-175 GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution Read all of the leaflet carefully before you are given this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER Poltechnet 8.0-175 GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution Read all of the leaflet carefully before you are given this medicine because
Imaging Core Laboratory Standard Operating Procedure
 Imaging Core Laboratory Standard Operating Procedure Patient Preparation and FDG Administration Procedure for ACRIN FDG-PET Scans 1.0 PURPOSE To outline the procedure for patient preparation and for 18
Imaging Core Laboratory Standard Operating Procedure Patient Preparation and FDG Administration Procedure for ACRIN FDG-PET Scans 1.0 PURPOSE To outline the procedure for patient preparation and for 18
DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection. For Intravenous Use DIAGNOSTIC FOR SKELETAL IMAGING
 TECHNETIUM TC 99M MEDRONATE - medronic acid injection, powder, for solution Jubilant DraxImage Inc ---------- DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection For Intravenous
TECHNETIUM TC 99M MEDRONATE - medronic acid injection, powder, for solution Jubilant DraxImage Inc ---------- DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection For Intravenous
PRODUCT MONOGRAPH. FluorOHmet
 PRODUCT MONOGRAPH FluorOHmet ([ 18 F]Fluorodeoxyglucose, 18 F-FDG) Solution for Injection, 45-4240 mci (1.665-156.88 GBq) per multi-dose vial Diagnostic Radiopharmaceutical University of Ottawa Heart Institute
PRODUCT MONOGRAPH FluorOHmet ([ 18 F]Fluorodeoxyglucose, 18 F-FDG) Solution for Injection, 45-4240 mci (1.665-156.88 GBq) per multi-dose vial Diagnostic Radiopharmaceutical University of Ottawa Heart Institute
TABLE 1 Principal Radiation Emission Data Radiation Mean % per Disintegration Mean Energy (kev) Gamma
 KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
SUMMARY OF PRODUCT CHARACTERISTICS. for. BRIDATEC, kit for radiopharmaceutical preparation
 February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
PET/CT Frequently Asked Questions
 PET/CT Frequently Asked Questions General Q: Is FDG PET specific for cancer? A: No, it is a marker of metabolism. In general, any disease that causes increased metabolism can result in increased FDG uptake
PET/CT Frequently Asked Questions General Q: Is FDG PET specific for cancer? A: No, it is a marker of metabolism. In general, any disease that causes increased metabolism can result in increased FDG uptake
Positron Emission Tomography in Lung Cancer
 May 19, 2003 Positron Emission Tomography in Lung Cancer Andrew Wang, HMS III Patient DD 53 y/o gentleman presented with worsening dyspnea on exertion for the past two months 30 pack-year smoking Hx and
May 19, 2003 Positron Emission Tomography in Lung Cancer Andrew Wang, HMS III Patient DD 53 y/o gentleman presented with worsening dyspnea on exertion for the past two months 30 pack-year smoking Hx and
Core Summary of Product Characteristics for Fludeoxyglucose ( 18 F)
 Core Summary of Product Characteristics for Fludeoxyglucose ( 18 F) This fludeoxyglucose ( 18 F) Core SmPC has been prepared on the basis, and taking into account the available published scientific literature
Core Summary of Product Characteristics for Fludeoxyglucose ( 18 F) This fludeoxyglucose ( 18 F) Core SmPC has been prepared on the basis, and taking into account the available published scientific literature
1. OVERVIEW 2. RADIOPHARMACEUTICAL UTILIZED
 1. OVERVIEW F-18 Fluorodeoxyglucose Injection is indicated for positron emission tomography (PET) imaging in the following settings: a) Oncology: For assessment of abnormal glucose metabolism to assist
1. OVERVIEW F-18 Fluorodeoxyglucose Injection is indicated for positron emission tomography (PET) imaging in the following settings: a) Oncology: For assessment of abnormal glucose metabolism to assist
FDG-PET/CT for cancer management
 195 REVIEW FDG-PET/CT for cancer management Hideki Otsuka, Naomi Morita, Kyo Yamashita, and Hiromu Nishitani Department of Radiology, Institute of Health Biosciences, The University of Tokushima, Graduate
195 REVIEW FDG-PET/CT for cancer management Hideki Otsuka, Naomi Morita, Kyo Yamashita, and Hiromu Nishitani Department of Radiology, Institute of Health Biosciences, The University of Tokushima, Graduate
Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG
 Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG version 2.0, approved February 7, 1999 Authors: Heinrich R. Schelbert, MD, PhD (UCLA School of Medicine, Los Angeles, CA);
Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG version 2.0, approved February 7, 1999 Authors: Heinrich R. Schelbert, MD, PhD (UCLA School of Medicine, Los Angeles, CA);
Summary of Product Characteristics
 Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5)
 PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5) 1. (a) A radioisotope is an isotope that is unstable and will emit particles from the nucleus
PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5) 1. (a) A radioisotope is an isotope that is unstable and will emit particles from the nucleus
PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET
![PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET](/thumbs/92/110393678.jpg) PHYTACIS Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET CIS bio international, member of IBA Molecular group of companies T1800nH (T1800- T1817) 01/2017 IDENTIFICATION
PHYTACIS Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET CIS bio international, member of IBA Molecular group of companies T1800nH (T1800- T1817) 01/2017 IDENTIFICATION
The Use of PET Scanning in Urologic Oncology
 The Use of PET Scanning in Urologic Oncology Dr Nicholas C. Buchan Uro-oncology Fellow 1 2 Aims To understand the basic concepts underlying PET scanning. Understand the emerging role of PET Scanning for
The Use of PET Scanning in Urologic Oncology Dr Nicholas C. Buchan Uro-oncology Fellow 1 2 Aims To understand the basic concepts underlying PET scanning. Understand the emerging role of PET Scanning for
Molecular Imaging and Cancer
 Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
POSITRON EMISSION TOMOGRAPHY (PET)
 Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
General Nuclear Medicine
 General Nuclear Medicine What is General Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
General Nuclear Medicine What is General Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
Package leaflet: Information for the user. Elaprase 2 mg/ml concentrate for solution for infusion idursulfase
 Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Positron Emission Tomography Computed Tomography (PET/CT)
 Positron Emission Tomography Computed Tomography (PET/CT) What is Positron Emission Tomography Computed Tomography (PET/CT) Scanning? What are some common uses of the procedure? How should I prepare for
Positron Emission Tomography Computed Tomography (PET/CT) What is Positron Emission Tomography Computed Tomography (PET/CT) Scanning? What are some common uses of the procedure? How should I prepare for
VASCULOCIS 10 mg kit for radiopharmaceutical preparation
 VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
An Introduction to PET Imaging in Oncology
 January 2002 An Introduction to PET Imaging in Oncology Janet McLaren, Harvard Medical School Year III Basics of PET Principle of Physiologic Imaging: Allows in vivo visualization of structures by their
January 2002 An Introduction to PET Imaging in Oncology Janet McLaren, Harvard Medical School Year III Basics of PET Principle of Physiologic Imaging: Allows in vivo visualization of structures by their
Package leaflet: Information for the patient. DOPAVIEW 222 MBq/mL solution for injection. Fluorodopa (18F)
 PACKAGE LEAFLET 1 Package leaflet: Information for the patient 222 MBq/mL solution for injection Fluorodopa (18F) Read all of this leaflet carefully before you start using this medicine because it contains
PACKAGE LEAFLET 1 Package leaflet: Information for the patient 222 MBq/mL solution for injection Fluorodopa (18F) Read all of this leaflet carefully before you start using this medicine because it contains
VIII. 9. FDG-PET for Diagnosis of an Advanced Jejunal Adenocarcinoma with Distant Metastases, Compared with Gallium Scintigraphy
 CYRIC Annual Report 2003 VIII. 9. FDG-PET for Diagnosis of an Advanced Jejunal Adenocarcinoma with Distant Metastases, Compared with Gallium Scintigraphy Yamaura G., Yoshioka T., Yamaguchi K. *, Fukuda
CYRIC Annual Report 2003 VIII. 9. FDG-PET for Diagnosis of an Advanced Jejunal Adenocarcinoma with Distant Metastases, Compared with Gallium Scintigraphy Yamaura G., Yoshioka T., Yamaguchi K. *, Fukuda
Los Angeles Radiological Society 62 nd Annual Midwinter Radiology Conference January 31, 2010
 Los Angeles Radiological Society 62 nd Annual Midwinter Radiology Conference January 31, 2010 Self Assessment Module on Nuclear Medicine and PET/CT Case Review FDG PET/CT IN LYMPHOMA AND MELANOMA Submitted
Los Angeles Radiological Society 62 nd Annual Midwinter Radiology Conference January 31, 2010 Self Assessment Module on Nuclear Medicine and PET/CT Case Review FDG PET/CT IN LYMPHOMA AND MELANOMA Submitted
Table 1 Principal Radiation Emission Data * Disintegration. Gamma Ka 1 X-ray
 DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
ZANOSAR 1g. STREPTOZOCINE Lyophilised powder for solution for injection
 PACKAGE LEAFLET: INFORMATION FOR THE USER ZANOSAR 1g STREPTOZOCINE Lyophilised powder for solution for injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER ZANOSAR 1g STREPTOZOCINE Lyophilised powder for solution for injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
performed to help sway the clinician in what the appropriate diagnosis is, which can substantially alter the treatment of management.
 Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection
![TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection](/thumbs/74/70410941.jpg) TECHNESCAN SESTAMIBI Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection NAME OF THE MEDICINE Tetrakis (2-methoxyisobutylisonitrile) copper (I) tetrafluoroborate. The abbreviated notation
TECHNESCAN SESTAMIBI Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection NAME OF THE MEDICINE Tetrakis (2-methoxyisobutylisonitrile) copper (I) tetrafluoroborate. The abbreviated notation
PET IMAGING (POSITRON EMISSION TOMOGRAPY) FACT SHEET
 Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Dr Sneha Shah Tata Memorial Hospital, Mumbai.
 Dr Sneha Shah Tata Memorial Hospital, Mumbai. Topics covered Lymphomas including Burkitts Pediatric solid tumors (non CNS) Musculoskeletal Ewings & osteosarcoma. Neuroblastomas Nasopharyngeal carcinomas
Dr Sneha Shah Tata Memorial Hospital, Mumbai. Topics covered Lymphomas including Burkitts Pediatric solid tumors (non CNS) Musculoskeletal Ewings & osteosarcoma. Neuroblastomas Nasopharyngeal carcinomas
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
Breast Cancer PET/CT Imaging Protocol
 Breast Cancer PET/CT Imaging Protocol Scanning Protocol: Patients are scanned from the top of the neck through the pelvis. Arms-up position is used to avoid beam-hardening artifact in the chest and abdomen.
Breast Cancer PET/CT Imaging Protocol Scanning Protocol: Patients are scanned from the top of the neck through the pelvis. Arms-up position is used to avoid beam-hardening artifact in the chest and abdomen.
OSTEOCIS. PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate Read all of this leaflet carefully before you are given this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the user. Tobramycin 40 mg/ml Solution for Injection tobramycin
 Package leaflet: Information for the user Tobramycin 40 mg/ml Solution for Injection tobramycin Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the user Tobramycin 40 mg/ml Solution for Injection tobramycin Read all of this leaflet carefully before you start using this medicine because it contains important information
SUMMARY OF PRODUCT CHARACTERISTICS (DTPA)
 CMR Group of Companies info@isotope-cmr.com www.isotope-cmr.com SUMMARY OF PRODUCT CHARACTERISTICS (DTPA) 1. NAME OF THE MEDICINAL PRODUCT PENTATECH lyophilized powder for injection (DTPA) 2. QUALITATIVE
CMR Group of Companies info@isotope-cmr.com www.isotope-cmr.com SUMMARY OF PRODUCT CHARACTERISTICS (DTPA) 1. NAME OF THE MEDICINAL PRODUCT PENTATECH lyophilized powder for injection (DTPA) 2. QUALITATIVE
The Role of PET / CT in Lung Cancer Staging
 July 2004 The Role of PET / CT in Lung Cancer Staging Vlad Vinarsky, Harvard Medical School Year IV Patient AM HPI: 81 yo F p/w hemoptysis x 1 month LLL lesion on CXR, not responsive to Abx 35 pack-year
July 2004 The Role of PET / CT in Lung Cancer Staging Vlad Vinarsky, Harvard Medical School Year IV Patient AM HPI: 81 yo F p/w hemoptysis x 1 month LLL lesion on CXR, not responsive to Abx 35 pack-year
FDG PET/CT STAGING OF LUNG CANCER. Dr Shakher Ramdave
 FDG PET/CT STAGING OF LUNG CANCER Dr Shakher Ramdave FDG PET/CT STAGING OF LUNG CANCER FDG PET/CT is used in all patients with lung cancer who are considered for curative treatment to exclude occult disease.
FDG PET/CT STAGING OF LUNG CANCER Dr Shakher Ramdave FDG PET/CT STAGING OF LUNG CANCER FDG PET/CT is used in all patients with lung cancer who are considered for curative treatment to exclude occult disease.
Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates
![Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates](/thumbs/87/95592963.jpg) Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates Molecular Imaging Branch, NIMH Bldg. 1 Rm. B3-10 September 6 th, 2006 The
Whole-body biodistribution and radiation dosimetry estimates for the β-amyloid radioligand [ 11 C]MeS-IMPY in non-human primates Molecular Imaging Branch, NIMH Bldg. 1 Rm. B3-10 September 6 th, 2006 The
Amira K. Brown, Ph.D. Molecular Imaging Branch, NIMH Bldg. 1 Rm. B3-10
 Whole-body biodistribution and radiation dosimetry estimates for the metabotropic glutamate receptor subtype 5 (mglur5) radioligand [ 18 F]SP203 in nonhuman primates Amira K. Brown, Ph.D. Molecular Imaging
Whole-body biodistribution and radiation dosimetry estimates for the metabotropic glutamate receptor subtype 5 (mglur5) radioligand [ 18 F]SP203 in nonhuman primates Amira K. Brown, Ph.D. Molecular Imaging
Principles of nuclear metabolic imaging. Prof. Dr. Alex Maes AZ Groeninge Kortrijk and KULeuven Belgium
 Principles of nuclear metabolic imaging Prof. Dr. Alex Maes AZ Groeninge Kortrijk and KULeuven Belgium I. Molecular imaging probes A. Introduction - Chemical disturbances will precede anatomical abnormalities
Principles of nuclear metabolic imaging Prof. Dr. Alex Maes AZ Groeninge Kortrijk and KULeuven Belgium I. Molecular imaging probes A. Introduction - Chemical disturbances will precede anatomical abnormalities
Chapter 10. Summary, conclusions and future perspectives
 Chapter 10 Summary, conclusions and future perspectives 10.1 SUMMARY In this thesis, a new tumor imaging tracer in nuclear medicine is studied. This 123 tracer, L-3-[ I]Iodo-alpha-methyl-tyrosine (IMT),
Chapter 10 Summary, conclusions and future perspectives 10.1 SUMMARY In this thesis, a new tumor imaging tracer in nuclear medicine is studied. This 123 tracer, L-3-[ I]Iodo-alpha-methyl-tyrosine (IMT),
PET imaging of cancer metabolism is commonly performed with F18
 PCRI Insights, August 2012, Vol. 15: No. 3 Carbon-11-Acetate PET/CT Imaging in Prostate Cancer Fabio Almeida, M.D. Medical Director, Arizona Molecular Imaging Center - Phoenix PET imaging of cancer metabolism
PCRI Insights, August 2012, Vol. 15: No. 3 Carbon-11-Acetate PET/CT Imaging in Prostate Cancer Fabio Almeida, M.D. Medical Director, Arizona Molecular Imaging Center - Phoenix PET imaging of cancer metabolism
Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ])
![Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ]) Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ])](/thumbs/81/82771892.jpg) Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ]) Overview Indications The Prostate Cancer Study with an indium-111 labeled murine
Austin Radiological Association Nuclear Medicine Procedure PROSTATE CANCER STUDY (In-111-Capromab Pendetide [ProstaScint ]) Overview Indications The Prostate Cancer Study with an indium-111 labeled murine
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan SULFUR COLLOID Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan SULFUR COLLOID Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
SUMMARY OF PRODUCT CHARACTERISTICS. for. Stabilised Ceretec, kit for radiopharmaceutical preparation
 SUMMARY OF PRODUCT CHARACTERISTICS for Stabilised Ceretec, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT Stabilised Ceretec 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial
SUMMARY OF PRODUCT CHARACTERISTICS for Stabilised Ceretec, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT Stabilised Ceretec 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial
M A A. Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection. DIAGNOSTIC - For Intravenous Use
 R: March 1998 27248 0001E m TM M A A Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection DIAGNOSTIC - For Intravenous Use DESCRIPTION The kit consists of reaction vials which contain
R: March 1998 27248 0001E m TM M A A Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection DIAGNOSTIC - For Intravenous Use DESCRIPTION The kit consists of reaction vials which contain
Radiopharmacy. Prof. Dr. Çetin ÖNSEL. CTF Nükleer Tıp Anabilim Dalı
 Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
PRESCRIBING INFORMATION. BaciJect. Bacitracin for injection U.S.P. Powder for Solution. 50,000 Units/Vial. Antibiotic
 PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
Package leaflet: Information for the patient. Xofigo 1100 kbq/ml solution for injection radium Ra 223 dichloride
 Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end
Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end
Palliative treatment of bone metastases with samarium-153
 APPROVED BY: Z. Yang Page 1 of 5 Palliative treatment of bone metastases with samarium-153 Primary Indications: Rationale: To treat bone pain resulting from osteoblastic metastases as defined by bone scan.
APPROVED BY: Z. Yang Page 1 of 5 Palliative treatment of bone metastases with samarium-153 Primary Indications: Rationale: To treat bone pain resulting from osteoblastic metastases as defined by bone scan.
Contrast Materials Patient Safety: What are contrast materials and how do they work?
 Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
Molecular Imaging and Breast Cancer
 Molecular Imaging and Breast Cancer Breast cancer forms in tissues of the breast usually in the ducts, tubes that carry milk to the nipple, and lobules, the glands that make milk. It occurs in both men
Molecular Imaging and Breast Cancer Breast cancer forms in tissues of the breast usually in the ducts, tubes that carry milk to the nipple, and lobules, the glands that make milk. It occurs in both men
Package leaflet: Information for the user
 Package leaflet: Information for the user Emerade 150 micrograms solution for injection in pre-filled pen Emerade 300 micrograms solution for injection in pre-filled pen Emerade 500 micrograms solution
Package leaflet: Information for the user Emerade 150 micrograms solution for injection in pre-filled pen Emerade 300 micrograms solution for injection in pre-filled pen Emerade 500 micrograms solution
45 Hr PET Registry Review Course
 45 HR PET/CT REGISTRY REVIEW COURSE Course Control Document Timothy K. Marshel, MBA, R.T. (R), (N)(CT)(MR)(NCT)(PET)(CNMT) The PET/CT Training Institute, Inc. SNMMI-TS 028600-028632 45hr CEH s Voice Credits
45 HR PET/CT REGISTRY REVIEW COURSE Course Control Document Timothy K. Marshel, MBA, R.T. (R), (N)(CT)(MR)(NCT)(PET)(CNMT) The PET/CT Training Institute, Inc. SNMMI-TS 028600-028632 45hr CEH s Voice Credits
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
HEPATIC METASTASES. We can state 3 types of metastases depending on their treatment options:
 HEPATIC METASTASES 1. Definition Metastasis means the spread of cancer. Cancerous cells can separate from the primary tumor and enter the bloodstream or the lymphatic system (the one that produces, stores,
HEPATIC METASTASES 1. Definition Metastasis means the spread of cancer. Cancerous cells can separate from the primary tumor and enter the bloodstream or the lymphatic system (the one that produces, stores,
objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University
 objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University To determine the regions of physiologic activity To understand
objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University To determine the regions of physiologic activity To understand
FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 SOLUTION THERAPEUTIC safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 SOLUTION THERAPEUTIC safely and effectively. See full prescribing information
Package leaflet: Information for the patient. NEGABAN 1g, powder for solution for injection or infusion Temocillin
 Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient NEGABAN 1g, powder for solution for injection or infusion Temocillin Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
OSTEOCIS 3 mg kit for radiopharmaceutical preparation
 OSTEOCIS 3 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T2000nH 10/2016 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
OSTEOCIS 3 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T2000nH 10/2016 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Amyvid 800 MBq/mL solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution for injection contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Amyvid 800 MBq/mL solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution for injection contains
BRACCO DIAGNOSTICS. MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use
 BRACCO DIAGNOSTICS MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use DESCRIPTION Macrotec is a sterile, nonpyrogenic, lyophilized preparation of albumin
BRACCO DIAGNOSTICS MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use DESCRIPTION Macrotec is a sterile, nonpyrogenic, lyophilized preparation of albumin
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 PET Scanning: Oncologic Applications Page 1 of 88 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: Oncologic Applications
PET Scanning: Oncologic Applications Page 1 of 88 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: Oncologic Applications
Clinical indications for positron emission tomography
 Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
Package leaflet: Information for the patient. Trasylol 10,000 KIU/ml solution for injection or infusion. Aprotinin
 Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
An investigation of the effect of ionising radiation on nurses and their patients during dialysis
 International Scholars Journals African Journal of Nursing and Midwifery ISSN 2198-4638 Vol. 2 (7), pp. 548-552, September, 2015. Available online at www.internationalscholarsjournals.org International
International Scholars Journals African Journal of Nursing and Midwifery ISSN 2198-4638 Vol. 2 (7), pp. 548-552, September, 2015. Available online at www.internationalscholarsjournals.org International
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
SODIUM IODIDE I 131 CAPSULES THERAPEUTIC 01/ Page 1 of 11
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 CAPSULES THERAPEUTIC safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 CAPSULES THERAPEUTIC safely and effectively. See full prescribing information
Quantitation of Cerebral Glucose Utilization using the Arterial Input Function or the Standardized Uptake Value (SUV)
 Quantitation of Cerebral Glucose Utilization using the Arterial Input Function or the Standardized Uptake Value (SUV) Brian R. Moyer BRMoyer & Associates, LLC, Amherst, NH 03031 http://www.brmassocllc-org.com/
Quantitation of Cerebral Glucose Utilization using the Arterial Input Function or the Standardized Uptake Value (SUV) Brian R. Moyer BRMoyer & Associates, LLC, Amherst, NH 03031 http://www.brmassocllc-org.com/
Cardiac Nuclear Medicine
 Cardiac Nuclear Medicine What is Cardiac Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
Cardiac Nuclear Medicine What is Cardiac Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
PET/CT in oncology. Positron emission tomography
 Clinical Medicine 2012, Vol 12, No 4: 368 72 PET/CT in oncology Fahim-Ul-Hassan, SpR Nuclear Medicine, Guy s Hospital, London; Gary J Cook, professor of Clinical PET, KCL Division of Imaging Sciences &
Clinical Medicine 2012, Vol 12, No 4: 368 72 PET/CT in oncology Fahim-Ul-Hassan, SpR Nuclear Medicine, Guy s Hospital, London; Gary J Cook, professor of Clinical PET, KCL Division of Imaging Sciences &
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PACKAGE LEAFLET: INFORMATION FOR THE USER. Fibrovein 3%, 1%, 0.5% and 0.2% Solution for Injection Sodium tetradecyl sulphate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Fibrovein 3%, 1%, 0.5% and 0.2% Solution for Injection Sodium tetradecyl sulphate Read all of this leaflet carefully before you start using this medicine. Keep
PACKAGE LEAFLET: INFORMATION FOR THE USER Fibrovein 3%, 1%, 0.5% and 0.2% Solution for Injection Sodium tetradecyl sulphate Read all of this leaflet carefully before you start using this medicine. Keep
( 18 F) ( 40: 23 30, 2003) 3 1 ( ) 3) 18 F-FDG 1,2) 1,2) 5,6) 6,7) 14 C-FDG 18 F-FDG F-FDM-6-
 23 18 F-FDG 2- -2- -D- ( 18 F) * ** * * * 18 F-FDG 14 C 14 C-FDG B 75% FDG 18 F-FDG 18 F-FDG-6-18 F-FDG- 6-18 F-FDM-6- PET FDG 18 F-FDG FDG ( 40: 23 30, 2003) I. 2 18 [ 18 F]-2- -2- -D- ( 18 F-FDG ) (
23 18 F-FDG 2- -2- -D- ( 18 F) * ** * * * 18 F-FDG 14 C 14 C-FDG B 75% FDG 18 F-FDG 18 F-FDG-6-18 F-FDG- 6-18 F-FDM-6- PET FDG 18 F-FDG FDG ( 40: 23 30, 2003) I. 2 18 [ 18 F]-2- -2- -D- ( 18 F-FDG ) (
Package leaflet: Information for the user. Cyanokit 5 g powder for solution for infusion hydroxocobalamin
 Package leaflet: Information for the user Cyanokit 5 g powder for solution for infusion hydroxocobalamin Read all of this leaflet carefully before using this medicine because it contains important information
Package leaflet: Information for the user Cyanokit 5 g powder for solution for infusion hydroxocobalamin Read all of this leaflet carefully before using this medicine because it contains important information
