PRODUCT MONOGRAPH. F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml. Diagnostic Radiopharmaceutical
|
|
|
- Jodie Rodgers
- 6 years ago
- Views:
Transcription
1 PRODUCT MONOGRAPH 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, > 0.5 GBq /ml Diagnostic Radiopharmaceutical Isologic Innovative Radiopharmaceuticals Ltd. Date of Preparation: nd Avenue, Lachine, Quebec H8T 3J1 December 12th, 2016 Control #: Date of Approval: March 22, 2017
2 Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION... 3 SUMMARY PRODUCT INFORMATION... 3 DESCRIPTION... 3 Physical Characteristics... 3 External Radiation... 4 INDICATIONS AND CLINICAL USE... 4 CONTRAINDICATIONS... 4 WARNINGS AND PRECAUTIONS... 4 ADVERSE REACTIONS... 6 DRUG INTERACTIONS... 7 DOSAGE AND ADMINISTRATION... 7 Dosing considerations... 7 Dosage... 7 Adistration... 7 Image Acquisition and Interpretation... 7 Instructions for Preparation and Use... 8 Directions for Quality Control... 8 RADIATION DOSIMETRY... 8 OVERDOSAGE... 9 ACTION AND CLINICAL PHARMACOLOGY... 9 Mechanism of Action... 9 Pharmacodynamics Pharmacokinetics Special Populations and Conditions STORAGE AND STABILITY SPECIAL HANDLING INSTRUCTIONS DOSAGE FORMS, COMPOSITION AND PACKAGING PART II: SCIENTIFIC INFORMATION PHARMACEUTICAL INFORMATION Drug Substance Product Characteristics CLINICAL TRIALS DETAILED PHARMACOLOGY TOXICOLOGY REFERENCES PART III: CONSUMER INFORMATION Isologic Innovative Radiopharmaceuticals 2
3 PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY PRODUCT INFORMATION Route of Adistration Dosage Form / Strength Intravenous Parenteral solution / > 0.5 GBq /ml in up to 30 ml Clinically Relevant Non-medicinal Ingredients None Fludeoxyglucose F 18 Injection is a positron-emitting radiopharmaceutical used as an accessory to Positron Emission Tomography (PET) for the assessment of abnormal glucose metabolism. The active ingredient 2-deoxy-2-[ 18 F]fluoro-D-glucose, abbreviated [ 18 F]-FDG, differs from glucose only in having a radioactive fluorine ( 18 F) in the 2 position. The 18 F atom decays into 18 O, converting the molecule into D-[2-18 O]glucose, an isotopologue of glucose. CH 2 OH CH 2 OH CH 2 OH HO HO O OH OH HO HO 18 F O OH HO HO O 18 OH OH D-glucose 2-deoxy-2-[ 18 F]fluoro-D-glucose D-[2-18 O]glucose DESCRIPTION Physical Characteristics Fluorine F 18 decays by positron (β+) emission and has a half-life of utes. The fractions remaining at selected intervals after calibration are shown in Table 1. Table 1. Physical Decay Chart for Fluorine 18 F Time from Calibration Fraction remaining 91% 83% 75% 68% 62% 57% 47% 39% 32% 27% 22% The principal photons useful for diagnostic imaging are the 511 kev gamma photons, resulting from the interaction of the emitted positron with an electron (Table 2). Table 2. Principal Emission Data for Fluorine 18 F Radiation/Emission Photons per Disintegration Mean Energy Positron (β+) kev Gamma (±) kev Isologic Innovative Radiopharmaceuticals 3
4 External Radiation The specific gamma-ray constant for fluorine F 18 is 0.3 Gy/hr/kBq at 1cm. The narrow-beam attenuation half value layer is 4.1 mm for lead (and 3.4 cm for concrete). Broad-beam transmission factors at 511 kev for various thicknesses of lead are given in Table 3. Table 3. Broad-beam transmission factors at 511 kev in lead mm Pb Transmission INDICATIONS AND CLINICAL USE Fludeoxyglucose F 18 Injection is indicated as an accessory to positron emission tomography (PET) imaging, in patients with known or suspected abnormalities found by other testing modalities, for the assessment of abnormal glucose metabolism to assist in: The characterisation of solitary pulmonary nodules; The staging of lung cancer; The detection of recurrence in patients with previously diagnosed lung cancer; and The monitoring of the therapeutic response in patients with lung cancer. The uptake of FDG is not cancer-specific. False-positives may occur in non-malignant areas of high metabolic activity, such as infection, inflammation, granulomatous reactions, and tissue repair. False negative results may occur in malignant tumors with low glycolytic activity, in tumors with large mucinous components, in some bronchioloalveolar carcinomas (e.g., localized), in small-sized tumors (< 2 times system resolution), and in hyperglycemic states (See Dosage and Adistration, Image Interpretation) CONTRAINDICATIONS 18 F-FDG should not be adistered to patients who are hypersensitive to fludeoxyglucose F18. WARNINGS AND PRECAUTIONS Serious Warnings and Precautions Radiopharmaceuticals should be used only by those health professionals who are appropriately qualified in the use of radioactive prescribed substances in or on humans. 18 F-FDG should not be adistered to pregnant women unless it is considered that the benefits to be gained outweigh the potential hazards to the foetus. There is little secretion of 18 F-FDG in breast milk but there is a high uptake of 18 F-FDG in a lactating, suckled breast. Where an assessment of the risk benefit ratio suggests the use of 18 F-FDG in nursing woman, breastfeeding should be discontinued, and close contact between mother and infant avoided, for a period of 12 hours following an FDG PET scan. Isologic Innovative Radiopharmaceuticals 4
5 General Precautions related to the handling of radioactive material must be observed in the handling and utilisation of this product including those concerning radioactive patients. Only those health professionals who are appropriately qualified in the use of radioactive prescribed substances in or on humans shall use radiopharmaceuticals. The product should be adistered under the supervision of a health professional who is experienced in the use of radiopharmaceuticals. Appropriate management of therapy and complications is only possible when adequate diagnostic and treatment facilities are readily available. The radiopharmaceutical product may be received, used and adistered only by authorized persons in designated clinical settings. Its receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licenses of local competent official organizations. As in the use of any other radioactive material, care should be taken to imize radiation exposure to patients consistent with proper patient management, and to imize radiation exposure to occupational workers. Carcinogenesis and Mutagenesis Studies with Fludeoxyglucose F 18 Injection have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility (see also Pregnant women, below). Contaation The following measures should be taken for up to 12 hours after receiving the radiopharmaceutical product: Toilet should be used instead of urinal. Toilet should be flushed several times after use. Special precautions such as bladder catheterization should be taken following adistration to incontinent patients to imize the risk of radioactive contaation of clothing, bed linen and the patient's environment. Endocrine and Metabolism Use in patients with diabetes or hyperglycemia has not been well studied. It is recommended that patients be normoglycemic when undergoing PET imaging with Fludeoxyglucose F 18 Injection. Transport of Fludeoxyglucose F 18 into cells may be affected by fasting or by blood glucose changes associated with diabetes mellitus. Diabetic patients may need stabilization of blood glucose levels on the day before and on the day of adistration of Fludeoxyglucose F 18 Injection. Special Populations Pregnant Women: Ideally exaations using radiopharmaceuticals, especially those elective in nature of women of childbearing capability should be performed during the first ten days following the onset of menses. Since adequate reproduction studies have not been performed in animals to detere whether this drug affects fertility in males or females, has teratogenic potential, or has other adverse reactions on the fetus, this radiopharmaceutical preparation should not be adistered to pregnant women unless it is considered that the potential benefits outweigh the potential hazards to the fetus. Isologic Innovative Radiopharmaceuticals 5
6 The fetus would receive a radiation dose of 10 mgy * from a 455 MBq maternal dose (7.5 MBq/kg to a 60 kg women). This level of radiation can increase the risk of leukemia and other cancers by 40%. 1 The use of a lower dose of [ 18 F]-FDG, maternal hydration, and frequent voiding can reduce the radiation dose to the fetus. Nursing Women: There is little secretion of Fludeoxyglucose F 18 in breast milk but there is a high uptake of Fludeoxyglucose F 18 in a lactating, suckled breast 2. A higher radiation dose is received by the infant from close contact with the breast than from ingestion of radioactive milk. Breastfeeding should be discontinued, and close contact between mother and infant avoided, for a period of 12 hours following an FDG PET scan. Pediatrics: The safety and effectiveness of Fludeoxyglucose F 18 Injection in the approved indication has not been established in pediatric patients. Fludeoxyglucose F 18 injection has been safely and effectively used in pediatric patients for the identification of regions of abnormal glucose metabolism associated with foci of epileptic seizures. Geriatrics: Geriatric patients were included in the studies demonstrating the efficacy and safety of [ 18 F]- FDG in the approved indication. There are no known limitations on the clinical use of Fludeoxyglucose F 18 Injection in geriatric patients. ADVERSE REACTIONS Adverse Drug Reaction Overview A systematic review of the published literature, publicly available reference sources, and adverse drug reaction reporting systems indicated that adverse reactions have not been reported for Fludeoxyglucose F 18 Injection. In a large published study in 22 PET centres, no adverse reactions to positron-emitting radiopharmaceuticals, primarily 18 F-FDG, were reported retrospectively for 33,295 doses and prospectively for 47,876 doses. 3 Clinical Trial Adverse Drug Reactions Because clinical trials are conducted under very specific conditions the adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice and should not be compared to the rates in the clinical trials of another drug. Adverse drug reaction information from clinical trials is useful for identifying drug-related adverse events and for approximating rates. In a 7,710-patient prospective clinical trial, no adverse reactions attributable to FDG were reported. Less Common Clinical Trial Adverse Drug Reactions (<1%) In a 7,710-patient prospective clinical trial, there were 6 reports of skin rash (6/7710; 0.08%), without pruritus or other accompanying signs or symptoms, all reported 24 hours after the procedure. A relationship to FDG can neither be inferred nor denied. * Fetal dose is estimated at 2.2 x 10-2 mgy/mbq; see Radiation Dosimetry below Isologic Innovative Radiopharmaceuticals 6
7 Abnormal Hematologic and Clinical Chemistry Findings Not applicable. Post-Market Adverse Drug Reactions Not applicable. DRUG INTERACTIONS Interactions with drugs, food, herbs, and laboratory tests have not been established. DOSAGE AND ADMINISTRATION Dosing considerations Patients should fast for at least 4 hours prior to dosing. Blood glucose should be assessed prior to dosing. Hyperglycemic patients should have their blood glucose level normalized prior to dosing. Patients should be well hydrated and should be encouraged to drink sufficient amounts of water to permit frequent bladder emptying, especially immediately prior to and after the PET exaation. Patients should avoid all strenuous physical activity prior to the exaation and remain at rest between the injection and exaation. Dosage The optimal dose of [ 18 F]-FDG has not been systematically investigated and may vary according to the imaging equipment used, the delay from adistration to imaging, and to patient characteristics. The usual recommended adult dose is 7.5 MBq/kg (370 to 740 MBq). Adistration The dose of [ 18 F]-FDG should be measured by a suitable radioactivity calibration system prior to intravenous adistration. The intravenous injection should be on the contralateral side to the site of concern. Image Acquisition and Interpretation Image acquisition parameters and procedures will vary depending upon the clinical question and the type of equipment available. The optimal time from dosing to imaging has not been systematically investigated. Images are generally acquired 30 to 60 utes after the adistration of 18 F-FDG but later imaging times are also frequently used. Isologic Innovative Radiopharmaceuticals 7
8 Only experienced nuclear medicine physicians familiar with the normal and condition-specific physiological and anatomical variants of 18 F-FDG biodistribution should interpret 18 F-FDG-PET images. Physicians should be aware of patient preparation anomalies (e.g., marginally acceptable blood glucose level and heightened anxiety state), and relevant patient history (e.g. recent and current health problems, recent surgeries and radiation treatments, menstrual and lactation status). The uptake of [ 18 F]-FDG is not cancer-specific. False positive findings may occur in non-malignant areas of high metabolic activity, such as infection (mycobacterial, fungal, bacterial infection), inflammatory and especially granulomatous reactions (e.g., bronchiectasis, sarcoidosis, pleurodesis, radiotherapy sites, and active atheromas) and tissue repair (trauma, post- surgery). False-negatives have been reported in tumors with low glycolytic activity (e.g., adenomas; bronchioloalveolar, mucinous, and lobular carcinomas; carcinoid tumors, and fibroadenoma), and in small-sized tumors (< 1 cm). [ 18 F]-FDG competes with blood glucose for uptake; increased levels of blood glucose can interfere with the uptake of [ 18 F]-FDG by malignant cells and result in false negative findings. Instructions for Preparation and Use The components of the vial are sterile and non-pyrogenic. It is essential that the user follows the directions carefully and adheres to strict aseptic technique. Use aseptic technique and wear waterproof gloves throughout the entire preparation procedure. Make all transfers of radioactive solutions with an adequately shielded syringe and maintain adequate shielding around the vial during the useful life of the radioactive product. Directions for Quality Control The required quality control testing has been performed on the product prior to release. RADIATION DOSIMETRY Based on ICRP 80, the effective dose resulting from the adistration of 555 MBq of 18 F-FDG is 10.5 msv. The whole-body dose, based on MIRD dose estimates, is 6.7 mgy. For the critical organs bladder, heart, and brain, the (MIRD) estimated absorbed radiation doses from 555 MBq of 18 F-FDG are, respectively, 41 mgy, 38 mgy, and 26 mgy. Isologic Innovative Radiopharmaceuticals 8
9 The estimated absorbed radiation doses are shown in Table 4. Table 4. Estimated Absorbed Radiation Doses after IV 18 FDG Target organ mgy/mbq rad/mci Brain ± ± Heart wall ± ± Kidneys ± ± Liver ± ± Lungs ± ± Pancreas ± ± Red marrow ± ± Spleen ± ± Urinary bladder wall ± ± Ovaries ± ± Testes ± ± Whole body ± ± MIRD Dose Estimate Report No The fetal dose estimates are: 2.2 x 10-2 mgy/mbq in early pregnancy and at 3-months gestation, and 1.7 x 10-2 mgy/mbq at 6 and 9 months gestation. 5 OVERDOSAGE Cases of overdose are not known to have occurred with Fludeoxyglucose F 18. In case of overdose, the patient should be monitored and managed as clinically indicated. ACTION AND CLINICAL PHARMACOLOGY Mechanism of Action 18 F-FDG is transported in a manner similar to glucose from blood to tissue where it is phosphorylated by hexokinase to 18 F-FDG-6-phosphate. As 18 F-FDG-6-phosphate is not a substrate for subsequent glycolytic pathways, and has very low membrane permeability, 18 F-FDG becomes trapped in tissue in proportion to the rate of glycolysis or glucose utilisation of that tissue. Imaging of the subject using a positron emission tomography (PET) scanner takes advantage of the positron decay of 18 F to identify those tissues that have an abnormal accumulation of the radioisotope. In cancer, cells are generally characterised by enhanced glucose metabolism partially due to (1) an increase of the glucose transporters, (2) an increase rate of phosphorylation activity, (3) a reduction of phosphatase activity or, (4) a dynamic alteration in the balance among all of these processes. However, glucose metabolism of cancer as reflected by 18 F-FDG accumulation shows considerable variability. Depending upon the tumour type, stage and location, 18 F-FDG accumulation may be increased, normal or decreased. Also, inflammatory cells can have the same variability of uptake of 18 F-FDG. Isologic Innovative Radiopharmaceuticals 9
10 Pharmacodynamics At the concentrations used for diagnostic exaations, [ 18 F]-FDG does not have any pharmacodynamic activity. Pharmacokinetics Distribution: FDG is widely distributed in the body following intravenous adistration and equilibrates quickly between plasma and erythrocytes. 6 The brain, heart, and liver show the highest accumulation. The brain contains 3.9% of injected activity 33 utes after dosing 7 and 6.9% of cumulated activity. 8 Other cumulated activities are urinary bladder, 6.3%; liver, 4.4%; heart, 3.3%; kidney, 1.3%; lung, 0.9%; spleen 0.4%; and pancreas 0.3%. Based on these results, cumulated activities have been estimated for red marrow, 1.7%; testes, 0.4%; and ovaries, 0.01%. The majority of FDG distribution at 90 utes is in tissues other than the blood, brain, heart and liver. These other tissues (probably the skeletal muscle and gut) become increasingly important with time and account for approximately 75% of the cumulated activity. 6 Mean residence times have been calculated from human data for plasma, 0.17 ± 0.06; erythrocytes, 0.10 ± 0.03; heart, 0.13 ± 0.06; lungs, 0.08 ± 0.03; liver, 0.16 ± 0.06; whole brain, 0.24 ± 0.09; and bladder, 0.04 ± 0.017, with voids at 0.5, 1, 2, then every 2 hours. Whole body residence time is 2.41 ± Metabolism: 18 F-FDG is phosphorylated to 18 F-FDG-6-phosphate by hexokinase, with no further metabolism taking place. Excretion: 18 F-FDG is mostly excreted unchanged in the urine; approximately 20% of the adistered activity is recovered in the urine within the first 2 hours. 7,8 Special Populations and Conditions No data available. STORAGE AND STABILITY Fludeoxyglucose F 18 Injection should be stored upright in a lead shielded container at controlled room temperature. Fludeoxyglucose F 18 Injection should be used within 12 hours from the time of the end of synthesis. SPECIAL HANDLING INSTRUCTIONS As in the use of any other radioactive material, care should be taken to imize radiation exposure to Isologic Innovative Radiopharmaceuticals 10
11 patients consistent with proper patient management, and to imize radiation exposure to occupational workers. Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclide, and whose experience and training have been approved by the appropriate governmental agency authorised to license the use of radionuclides. DOSAGE FORMS, COMPOSITION AND PACKAGING Fludeoxyglucose F 18 Injection is supplied in a multi-dose, septum-caped, 30 ml, glass vial containing at least 0.5 GBq /ml (>18.5 mci)) of no carrier added 2-deoxy-2-[ 18 F]fluoro-D-glucose, at end of synthesis, in up to 30 ml. Isologic Innovative Radiopharmaceuticals 11
12 PART II: SCIENTIFIC INFORMATION PHARMACEUTICAL INFORMATION Drug Substance Proper name: Chemical name: Fludeoxyglucose 18 F 2-Deoxy-2-fluoro-D-glucose Molecular formula: C 6H FO 5 Molecular mass: daltons Structural formula: Physicochemical properties: clear, colorless solution. Product Characteristics Fludeoxyglucose F18 Injection is provided as a ready to use sterile, clear, colorless solution. Each ml contains at least 0.5 GBq (18.5 mci) of 2-deoxy-2-[ 18 F]fluoro-D glucose at the end of synthesis (EOS). The ph of the solution is between 4.5 to 7.5. The solution is packaged in a multiple-dose glass vial and does not contain any preservative. CLINICAL TRIALS Isologic conducted a single clinical trial in over 7,700 patients in a variety of oncology, neurology, and cardiovascular indications. The most common indications were the characterization of solitary pulmonary nodules (SPN), the staging and localization of lung cancer, and the diagnosis of relapse of lung cancer. A sub-group of 138 patients who underwent [ 18 ]-FDG-PET for investigation of solitary pulmonary nodules was selected for 'efficacy' analysis. The criteria for selection were that there be an acceptable "truth standard" to support the diagnosis, and that the patient's clinical follow-up be at the same institution. The mean (± sd) dose of 18 FDG was 524 ± 114 MBq, or 7.5 ± 0.6 MBq/kg. The mean time from dosing to imaging was 87.1 ± 20.8 utes. The pulmonary nodules assessed had a mean (±sd) size of 2.6 ± 1.7 Isologic Innovative Radiopharmaceuticals 12
13 cm. The smallest nodule was 0.8 cm and the largest was 8.3 cm. The overall prevalence of malignancy in this patient population was 71.0%. The "truth standard" was established by histopathology in 77% of patients; CT follow-up in 15%; X-ray follow-up in 4%, and clinical follow-up in 4%. X-ray, CT, and clinical follow-up of negative cases were generally for a period of 2 or more years. FDG-PET was 98% sensitive for the detection of malignancy in patients with solitary pulmonary nodules. There were only two false negatives in this sample of 138 patients. One lesion was a 1.1 cm metastasis of an adenocarcinoma and the other was a 2.0 cm bronchoalveolar cell carcinoma. Specificity was 77.5%. The nine false positives included one case each of bronchiolitis obliterans with organizing pneumonia (BOOP); lipid pneumonia; granulotamous inflammation; silico-anthracotic nodules; non-necrotizing granuloma and bronchiectasis; and pulmonary granulomatous reaction. In the two remaining cases, the pathology was either non-existent or not described. Table 4. Diagnostic Performance Mean CI 95% LL CI 95% UL Sensitivity 98.0% (96/98) 92.1% 99.6% Specificity 77.5% (31/40) 61.1% 88.6% PPV 91.4% 83.9% 95.8% NPV 93.9% 78.4% 98.9% Accuracy 92.0% 85.9% 95.8% These results are essentially identical to the results of a meta-analysis published by Gould, of 31 studies of FDG-PET in a total of 1474 focal pulmonary lesions 9. Gould concluded: "In current practice, FDG-PET operates at a point on the summary receiver operating characteristic curve that corresponds approximately to a sensitivity and specificity of 96.8% and 77.8%. An earlier tabulated summary of the FDG-PET literature was compiled by Gambhir. 10 For the diagnosis of lung disease, Ghambir's review included 23 publications in a total of 1255 patients. He reported an overall sensitivity of 96% and a specificity of 73%. DETAILED PHARMACOLOGY The hydroxyl group of the second carbon of glucose can be substituted by a group such as hydrogen or fluorine without seriously compromising the kinetic and biochemical ability of the molecule to be actively transported through the cell membrane and to act as a substrate for the hexokinase enzyme. The 2-deoxy analogues of glucose are transported into the cell and metabolized quantitatively exactly like D-glucose up the point in the glycolytic pathway where its anomalous structure prevents the final conversion of the 2- deoxyglucose-6-phosphate by phosphohexoseixomeriase. 11 In mice, 18 F-FDG distributes uniformly to the kidneys, heart, brain, lungs and liver initially and clears rapidly from all tissue except the heart where it remains constant for at least 2 hours and, to a lesser extent, in the brain where it decreases slowly from 1 to 2 hours. 12,13 The rapid clearance of 18 F-FDG from the liver, lungs and kidneys, and its retention by the heart and brain is a result of metabolic trapping within these organs and is reflective of glucose utilisation. Urinary excretion of intact 18 F-FDG was 15-25% of injected dose at 90 utes. Isologic Innovative Radiopharmaceuticals 13
14 In mice, FDG accumulates in organs and fluids as parent FDG (or FDG-6-phosphate) and FD-Mannose (or FD-Mannose-6-phosphate) and is excreted in the urine in both forms. 14 In rats, the percentages of 18 F-FDG and 18 F-FDG-6-phosphate 45 utes after injection were 68 and 33%, respectively in the liver; and 70 and 27%, respectively, in the kidney. 15 In mice bearing C3H mammary carcinoma the predoant metabolite observed in the tumour at 180 utes was 18 F-FDG-6-phosphate, with measurable quantities of other phosphorylated species. 16 TOXICOLOGY 'Cold' 2-deoxy-2-fluoro-D-glucose had an LD 50 of 600 mg/kg in both mice and rats when given by intraperitoneal injection in 5 or 6 consecutive daily doses. 17. Toxicity studies in mice given three doses of 14.3 mg/kg of FDG did not reveal any immediate or longterm effects as detered by routine observations, changes in body weight, and gross and histopathology of the internal organs. Toxicity studies in dogs injected with three doses of 0.72 mg/kg of FDG did not show any immediate or long-term effects. No significant abnormalities were detected in blood, urine, or CSF analyses, and no significant gross or microscopic abnormalities were detected in the heart, brain, spleen, liver, kidneys, lungs, ovaries, or intestines. 18. No long-term animal studies have been performed to evaluate carcinogenic or mutagenic potential or whether 18 F-FDG affects fertility in males or females. As with other radiopharmaceuticals that distribute intracellularly, there may be increased risk of chromosome damage from Auger electrons if nuclear uptake occurs. REFERENCES 1 International Commission on Radiological Protection. Pregnancy and Medical Radiation. ICRP Publication 84. Annals of the ICRP 2000;30(1):1 2 Hicks RJ, Binns D, Stabin MG. Pattern of uptake and excretion of (18)F-FDG in the lactating breast. J Nucl Med Aug;42(8): Silberstein EB. Prevalence of adverse reactions to positron emitting radiopharmaceuticals in nuclear medicine. Pharmacopeia Committee of the Society of Nuclear Medicine. J Nucl Med Dec;39(12): Hays MT, Watson EE, Thomas SR, Stabin M. MIRD dose estimate report no. 19: radiation absorbed dose estimates from (18)F-FDG. J Nucl Med 2002;43: Stabin MG. Proposed Addendum to Previously Published Fetal Dose Estimate Tables for 18 F-FDG. J Nucl Med 2004; 45: Hays MT, Segall GM. A mathematical model for the distribution of fluorodeoxyglucose in humans. J Nucl Med Aug;40(8): Isologic Innovative Radiopharmaceuticals 14
15 7 Jones SC, Alavi A, Christman D, Montanez I, Wolf AP, Reivich M. The radiation dosimetry of 2 [F-18]fluoro-2-deoxy-D-glucose in man. J Nucl Med Jul;23(7): Mejia AA, Nakamura T, Masatoshi I, Hatazawa J, Masaki M, Watanuki S. Estimation of absorbed doses in humans due to intravenous adistration of fluorine-18-fluorodeoxyglucose in PET studies. J Nucl Med Apr;32(4): Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA Feb 21;285(7): Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med May;42(5 Suppl):1S-93S 11 Pauwels EKJ, Sturm EJC, Bombardieri E, Cleton FJ, Stokkel MPM. Positron-emission tomography with [18F]fluorodeoxyglucose: Part I. Biochemical uptake mechanism and its implication for clinical studies J Cancer Res Clin Oncol 2000;126: Gallagher BM, Fowler JS, et al. Metabolic trapping as a principle of radiopharmaceuticals design: Some factors responsible for the biodistribution of [18F] 2-deoxy-2-fluoro-Dglucose. J Nucl Med 1978;19: Gallagher BM, Ansari A, Atkins H, et al., Radiopharmaceuticals XXVII. 18F-labelled 2- deoxy-2-fluoro-d-glucose as a radiopharmaceutical for measuring regional myocardial glucose metabolism in vivo: Tissue distribution and imaging studies in animals. J Nucl Med 1977;18: Kanazawa Y, Momozono Y, Ishikawa M, et al. Metabolic pathway of 2-deoxy-2-fluoro-Dglucose studied by F-19 NMR. Life Sciences 1986;39: Suolinna E-M, Haaparanta M, Paul R, et al. Metabolism of 2-[18F]fluoro-2-deoxyglucose in tumor-bearing rats: Chromatographic and enzymatic studies. Nucl Med Biol 1986;13: Kaarstad K, Bender D, Bentzen L, et al. Metabolic fate of 18F-FDG in mice bearing either SCCVII squamous cell carcinoma or C3H mammary carcinoma. J Nucl Med 2002; 43: Bessell EM, Courtney VD, Foster AB, et al. Some in vivo and in vitro antitumor effects of the deoxyfluoro-d-glucopyranoses. Eur J Cancer 1973;9: Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Fowler J, Hoffman E, Alavi A, Som P, Sokoloff L. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res Jan;44(1): Isologic Innovative Radiopharmaceuticals 15
16 PART III: CONSUMER INFORMATION Fludeoxyglucose 18 F Injection This leaflet is part III of a three-part "Product Monograph" published when Fludeoxyglucose 18 F Injection was approved for sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about Fludeoxyglucose 18 F Injection. Contact your doctor or pharmacist if you have any questions about the drug. ABOUT THIS MEDICATION What the medication is used for: Fludeoxyglucose 18 F Injection, or FDG, is used to study how different tissues in your body are using blood sugar (glucose). The approved use is to study abnormalities found in the lung by other tests (e.g., chest x-ray). What it does: Fludeoxyglucose 18 F Injection behaves just like blood sugar (glucose) but because it has a radioactive atom, its behavior can be followed with a special camera (PET). When it should not be used: FDG should not be used in patients allergic to FDG. What the medicinal ingredient is: Fludeoxyglucose 18 F What the important non-medicinal ingredients are: There are no important non-medicinal ingredients. WARNINGS AND PRECAUTIONS Serious Warnings and Precautions Fludeoxyglucose 18F Injection should be used only by those health professionals who are appropriately qualified in the use of radioactive prescribed substances in or on humans. 18 F-FDG should not be adistered to pregnant women unless it is considered that the benefits to be gained outweigh the potential hazards to the foetus. Breastfeeding should be discontinued, and close contact between mother and infant avoided, for a period of 12 hours following an FDG PET scan. BEFORE you receive Fludeoxyglucose 18 F Injection talk to your doctor or pharmacist if you think you might be pregnant or if you are diabetic. 18 F-FDG is not present in dangerous amounts in breast milk but high radioactivity is present in the breast tissue. Breastfeeding should be discontinued, and close contact between mother and infant avoided, for a period of 12 hours following an FDG PET scan. To help eliate Fludeoxyglucose 18 F Injection quickly after the procedure, you should drink a large glass of water and urinate frequently after you receive the injection. Males should use a toilet rather than a urinal. Toilets should be flushed several times. Hands should be thoroughly washed. If blood, urine or feces soil clothing, the clothing should be washed separately from other clothing. INTERACTIONS WITH THIS MEDICATION Drug-drug interactions with Fludeoxyglucose F 18 Injection have not been evaluated. PROPER USE OF THIS MEDICATION This product will be adistered under the supervision of a health professional who is experienced in the use of radiopharmaceuticals. Diabetic patients should ensure that their blood sugar levels are stable the day preceding and the day of the PET scan with 18F-FDG product. You may be asked to eat nothing and drink only water for four hours before your scheduled PET scan with I8F-FDG product. To decrease the radiation exposure to your bladder, you should drink plenty of water and urinate as often as possible when the PET scan is finished. SIDE EFFECTS AND WHAT TO DO ABOUT THEM Fludeoxyglucose F 18 Injection is called a 'tracer' meaning that it is given in very small doses and has no activity of its own. Other than possible adverse reactions related to receiving an injection, adverse reactions have not been reported for Fludeoxyglucose F 18 Injection. SERIOUS SIDE EFFECTS, HOW OFTEN THEY HAPPEN AND WHAT TO DO ABOUT THEM Reporting Side Effects You can report any suspected side effects associated with the use of health products to Health Canada by: Visiting the Web page on Adverse Reaction Reporting ( Isologic Innovative Radiopharmaceuticals 16
17 declaration/index-eng.php) for information on how to report online, by mail or by fax; or Calling toll-free at NOTE: Contact your health professional if you need information about how to manage your side effects. The Canada Vigilance Program does not provide medical advice MORE INFORMATION This document plus the full product monograph, prepared for health professionals can be found by contacting the sponsor, Isologic Innovative Radiopharmaceuticals Ltd at This leaflet was prepared by Isologic Innovative Radiopharmaceuticals Ltd. Last revised: December 12th, 2016 Isologic Innovative Radiopharmaceuticals 17
CanTrace PRODUCT MONOGRAPH. Parenteral Solution, up to 1.4 GBq/mL (38.2 mci/ml) Diagnostic Radiopharmaceutical
 PRODUCT MONOGRAPH CanTrace 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, up to 1.4 GBq/mL (38.2 mci/ml) Diagnostic Radiopharmaceutical IPET Pharmaceuticals, Inc. Suite 880, 1090 West Georgia,
PRODUCT MONOGRAPH CanTrace 18 F-Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution, up to 1.4 GBq/mL (38.2 mci/ml) Diagnostic Radiopharmaceutical IPET Pharmaceuticals, Inc. Suite 880, 1090 West Georgia,
GALLIUM CITRATE Ga 67 INJECTION
 511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
Sodium Iodide I 131 Solution. Click Here to Continue. Click Here to Return to Table of Contents
 Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
GLUCEPTATE. Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION
 27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
27194 0001E m TM GLUCEPTATE Technetium Tc 99m Gluceptate Kit DIAGNOSTIC DESCRIPTION The kit consists of reaction vials which contain the sterile, non-pyrogenic, nonradioactive ingredients necessary to
Click Here to Continue. Click Here to Return to Table of Contents
 Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
Hippuran I 131 Injection Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to Table
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
Page 1 of CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AXUMIN safely and effectively. See full prescribing information for AXUMIN. AXUMIN (fluciclovine
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
R: March, E D T P A. Kit for the Preparation of Technetium Tc 99m Pentetate Injection. DIAGNOSTIC - For Intravenous Use
 R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
R: March, 1998 27227 0002E m TM DESCRIPTION D T P A Kit for the Preparation of Technetium Tc 99m Pentetate Injection DIAGNOSTIC - For Intravenous Use Each kit consists of reaction vials which contain the
FluGlucoScan Injection
 PRODUCT MONOGRAPH FluGlucoScan Injection 18 F Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution / >0.5 GBq / vial Diagnostic Radiopharmaceutical Alberta Cancer Board Standard Life Center 1220 10405 Jasper
PRODUCT MONOGRAPH FluGlucoScan Injection 18 F Fluorodeoxyglucose ( 18 F-FDG) Parenteral Solution / >0.5 GBq / vial Diagnostic Radiopharmaceutical Alberta Cancer Board Standard Life Center 1220 10405 Jasper
INDICATIONS AND USAGE
 1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
1. INDICATIONS AND USAGE a) Axumin is indicated for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following
PHYSICAL CHARACTERISTICS
 BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
BRACCO DIAGNOSTICS L/4739/0 1 CHOLETEC Kit for the Preparation of Technetium Tc 99m Mebrofenin For Diagnostic Use DESCRIPTION Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture
FP/1-61//- p-o37 A mb. Precision Nuclear, LLC. July 7, 2011
 211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
211 JUL Ili A II 5b Precision Nuclear, LLC July 7, 211 Amendment to Citizen Petition docket no. FDA-211-P-337-1/CP filed on 5/6/211 I, Jon L. McReynolds, PharmD, representing Precision Nuclear, LLC submit
Sodium Fluoride F 18 Injection* PET/CT Imaging
 Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
Sodium Fluoride F 18 Injection* PET/CT Imaging for Prostate Cancer Sodium Fluoride F 18 Injection* ( 18 F NaF) PET/CT Imaging of Bone Metastases in Prostate Cancer About Prostate Cancer According to the
Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer
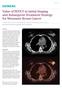 Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
Case Study Value of PET/CT in Initial Staging and Subsequent Treatment Strategy for Metastatic Breast Cancer By Dustin Osborne, PhD, and Yong Bradley, MD, University of Tennessee, Knoxville, TN, USA Data
Core SPC for Fludeoxyglucose ( 18 F) March 2005
 Core SPC for Fludeoxyglucose ( 18 F) March 2005 This FDG Core SPC has been prepared on the basis, and taking into account the available published scientific literature dated from more than 10 years. Then
Core SPC for Fludeoxyglucose ( 18 F) March 2005 This FDG Core SPC has been prepared on the basis, and taking into account the available published scientific literature dated from more than 10 years. Then
To report suspected adverse reactions to Axumin, call AXUMIN1 ( ) or contact FDA at FDA-1088 or
 An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
An Axumin scan can detect and localize recurrent prostate cancer Axumin is an FDA-approved diagnostic imaging agent, also known as a tracer, which may help your physician determine if and where your prostate
PRODUCT MONOGRAPH. FluorOHmet
 PRODUCT MONOGRAPH FluorOHmet ([ 18 F]Fluorodeoxyglucose, 18 F-FDG) Solution for Injection, 45-4240 mci (1.665-156.88 GBq) per multi-dose vial Diagnostic Radiopharmaceutical University of Ottawa Heart Institute
PRODUCT MONOGRAPH FluorOHmet ([ 18 F]Fluorodeoxyglucose, 18 F-FDG) Solution for Injection, 45-4240 mci (1.665-156.88 GBq) per multi-dose vial Diagnostic Radiopharmaceutical University of Ottawa Heart Institute
DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection. For Intravenous Use DIAGNOSTIC FOR SKELETAL IMAGING
 TECHNETIUM TC 99M MEDRONATE - medronic acid injection, powder, for solution Jubilant DraxImage Inc ---------- DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection For Intravenous
TECHNETIUM TC 99M MEDRONATE - medronic acid injection, powder, for solution Jubilant DraxImage Inc ---------- DRAXIMAGE MDP-25 Kit for the Preparation of Technetium Tc 99m Medronate Injection For Intravenous
TABLE 1 Principal Radiation Emission Data Radiation Mean % per Disintegration Mean Energy (kev) Gamma
 KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
KIT FOR THE PREPARATION OF TECHNETIUM TC 99M MEBROFENIN - mebrofenin injection, powder, lyophilized, for solution ---------- Kit for the Preparation of Technetium Tc 99m Mebrofenin DESCRIPTION Each multidose
The estimated absorbed doses from a bolus intravenous
 BASIC SCIENCE INVESTIGATIONS MIRD Dose Estimate Report No. 19: Radiation Absorbed Dose Estimates from F-FDG Marguerite T. Hays, MD 1,2 ; Evelyn E. Watson, BA 3 ; Stephen R. Thomas, PhD 4 ; and Michael
BASIC SCIENCE INVESTIGATIONS MIRD Dose Estimate Report No. 19: Radiation Absorbed Dose Estimates from F-FDG Marguerite T. Hays, MD 1,2 ; Evelyn E. Watson, BA 3 ; Stephen R. Thomas, PhD 4 ; and Michael
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate)
 Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
Austin Radiological Association Ga-68 NETSPOT (Ga-68 dotatate) Overview Ga-68 dotatate binds to somatostatin receptors, with highest affinity for subtype 2 receptors (sstr2). It binds to cells that express
Summary of Product Characteristics
 Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
Core Summary of Product Characteristics for Fludeoxyglucose ( 18 F)
 Core Summary of Product Characteristics for Fludeoxyglucose ( 18 F) This fludeoxyglucose ( 18 F) Core SmPC has been prepared on the basis, and taking into account the available published scientific literature
Core Summary of Product Characteristics for Fludeoxyglucose ( 18 F) This fludeoxyglucose ( 18 F) Core SmPC has been prepared on the basis, and taking into account the available published scientific literature
Positron Emission Tomography Computed Tomography (PET/CT)
 Positron Emission Tomography Computed Tomography (PET/CT) What is Positron Emission Tomography Computed Tomography (PET/CT) Scanning? What are some common uses of the procedure? How should I prepare for
Positron Emission Tomography Computed Tomography (PET/CT) What is Positron Emission Tomography Computed Tomography (PET/CT) Scanning? What are some common uses of the procedure? How should I prepare for
Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF)
 Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF) Overview Indication Sodium Fluoride F18 injection is a radioactive diagnostic agent for positron emission
Austin Radiological Association Nuclear Medicine Procedure PET SODIUM FLUORIDE BONE SCAN (F-18 NaF) Overview Indication Sodium Fluoride F18 injection is a radioactive diagnostic agent for positron emission
Imaging Core Laboratory Standard Operating Procedure
 Imaging Core Laboratory Standard Operating Procedure Patient Preparation and FDG Administration Procedure for ACRIN FDG-PET Scans 1.0 PURPOSE To outline the procedure for patient preparation and for 18
Imaging Core Laboratory Standard Operating Procedure Patient Preparation and FDG Administration Procedure for ACRIN FDG-PET Scans 1.0 PURPOSE To outline the procedure for patient preparation and for 18
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION. NeuraCeq. Florbetaben ( 18 F) 3 µg/ml Solution for Intravenous Administration
 PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION NeuraCeq Florbetaben ( 18 F) 3 µg/ml Solution for Intravenous Administration Diagnostic Radiopharmaceutical IsoLogic Innovative Radiopharmaceuticals
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION NeuraCeq Florbetaben ( 18 F) 3 µg/ml Solution for Intravenous Administration Diagnostic Radiopharmaceutical IsoLogic Innovative Radiopharmaceuticals
SUMMARY OF PRODUCT CHARACTERISTICS. for. BRIDATEC, kit for radiopharmaceutical preparation
 February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
February 9, 2010 SUMMARY OF PRODUCT CHARACTERISTICS for BRIDATEC, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT BRIDATEC 2. QUALITATIVE AND QUANTITATIVE COMPOSITION N-(3-bromo-2,4,6-trimethylphenylcarbamoyl
PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET
![PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET PHYTACIS. Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET](/thumbs/92/110393678.jpg) PHYTACIS Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET CIS bio international, member of IBA Molecular group of companies T1800nH (T1800- T1817) 01/2017 IDENTIFICATION
PHYTACIS Kit for the preparation of technetium [ 99m Tc] phytate injection USER PACKAGE LEAFLET CIS bio international, member of IBA Molecular group of companies T1800nH (T1800- T1817) 01/2017 IDENTIFICATION
Poltechnet GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER Poltechnet 8.0-175 GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution Read all of the leaflet carefully before you are given this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER Poltechnet 8.0-175 GBq, radionuclide generator Sodium pertechnetate ( 99m Tc) solution Read all of the leaflet carefully before you are given this medicine because
VIII. 9. FDG-PET for Diagnosis of an Advanced Jejunal Adenocarcinoma with Distant Metastases, Compared with Gallium Scintigraphy
 CYRIC Annual Report 2003 VIII. 9. FDG-PET for Diagnosis of an Advanced Jejunal Adenocarcinoma with Distant Metastases, Compared with Gallium Scintigraphy Yamaura G., Yoshioka T., Yamaguchi K. *, Fukuda
CYRIC Annual Report 2003 VIII. 9. FDG-PET for Diagnosis of an Advanced Jejunal Adenocarcinoma with Distant Metastases, Compared with Gallium Scintigraphy Yamaura G., Yoshioka T., Yamaguchi K. *, Fukuda
POSITRON EMISSION TOMOGRAPHY (PET)
 Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
OSTEOCIS. PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate Read all of this leaflet carefully before you are given this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT OSTEOCIS 3 mg kit for radiopharmaceutical preparation sodium oxidronate Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient. DOPAVIEW 222 MBq/mL solution for injection. Fluorodopa (18F)
 PACKAGE LEAFLET 1 Package leaflet: Information for the patient 222 MBq/mL solution for injection Fluorodopa (18F) Read all of this leaflet carefully before you start using this medicine because it contains
PACKAGE LEAFLET 1 Package leaflet: Information for the patient 222 MBq/mL solution for injection Fluorodopa (18F) Read all of this leaflet carefully before you start using this medicine because it contains
Table 1 Principal Radiation Emission Data * Disintegration. Gamma Ka 1 X-ray
 DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
DESCRIPTIONGeneralGlofil-125 (Sodium Iothalamate I-125 Injection) is a sterile, nonpyrogenic aqueous injection containing approximately 1 mg sodium iothalamate per ml, and 0.9 percent benzyl alcohol as
General Nuclear Medicine
 General Nuclear Medicine What is General Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
General Nuclear Medicine What is General Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
VASCULOCIS 10 mg kit for radiopharmaceutical preparation
 VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
VASCULOCIS 10 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T0200nH 12/2012 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
1. OVERVIEW 2. RADIOPHARMACEUTICAL UTILIZED
 1. OVERVIEW F-18 Fluorodeoxyglucose Injection is indicated for positron emission tomography (PET) imaging in the following settings: a) Oncology: For assessment of abnormal glucose metabolism to assist
1. OVERVIEW F-18 Fluorodeoxyglucose Injection is indicated for positron emission tomography (PET) imaging in the following settings: a) Oncology: For assessment of abnormal glucose metabolism to assist
Radiation Exposure to Staff Using PET/CT Facility
 Egyptian J. Nucl. Med., Vol. 8, No. 2, December 2013 1 Editorial Radiation Exposure to Staff Using PET/CT Facility Taalab, Kh; and Mohsen, Z Department of Nuclear Medicine, International Medical Center;
Egyptian J. Nucl. Med., Vol. 8, No. 2, December 2013 1 Editorial Radiation Exposure to Staff Using PET/CT Facility Taalab, Kh; and Mohsen, Z Department of Nuclear Medicine, International Medical Center;
Palliative treatment of bone metastases with samarium-153
 APPROVED BY: Z. Yang Page 1 of 5 Palliative treatment of bone metastases with samarium-153 Primary Indications: Rationale: To treat bone pain resulting from osteoblastic metastases as defined by bone scan.
APPROVED BY: Z. Yang Page 1 of 5 Palliative treatment of bone metastases with samarium-153 Primary Indications: Rationale: To treat bone pain resulting from osteoblastic metastases as defined by bone scan.
M A A. Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection. DIAGNOSTIC - For Intravenous Use
 R: March 1998 27248 0001E m TM M A A Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection DIAGNOSTIC - For Intravenous Use DESCRIPTION The kit consists of reaction vials which contain
R: March 1998 27248 0001E m TM M A A Kit for the Preparation of Technetium Tc 99m Albumin Aggregated Injection DIAGNOSTIC - For Intravenous Use DESCRIPTION The kit consists of reaction vials which contain
Cardiac Nuclear Medicine
 Cardiac Nuclear Medicine What is Cardiac Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
Cardiac Nuclear Medicine What is Cardiac Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5)
 PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5) 1. (a) A radioisotope is an isotope that is unstable and will emit particles from the nucleus
PHYSICS 2: HSC COURSE 2 nd edition (Andriessen et al) CHAPTER 20 Radioactivity as a diagnostic tool (pages 394-5) 1. (a) A radioisotope is an isotope that is unstable and will emit particles from the nucleus
Radiation Safety - Things You Need to Know
 Radiation Safety - Things You Need to Know Michael Casey Ph.D. Phlebotomy Autumn Seminar 13 th October 2012 Radiation is a form of energy transport What is Radiation? It is caused by electrical disturbances
Radiation Safety - Things You Need to Know Michael Casey Ph.D. Phlebotomy Autumn Seminar 13 th October 2012 Radiation is a form of energy transport What is Radiation? It is caused by electrical disturbances
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. (em-plis-city)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Empliciti TM (em-plis-city) (elotuzumab 300 & 400 mg powder for concentrate for solution for infusion) Read this
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Empliciti TM (em-plis-city) (elotuzumab 300 & 400 mg powder for concentrate for solution for infusion) Read this
SODIUM IODIDE I 131 CAPSULES THERAPEUTIC 01/ Page 1 of 11
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 CAPSULES THERAPEUTIC safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 CAPSULES THERAPEUTIC safely and effectively. See full prescribing information
FULL PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 SOLUTION THERAPEUTIC safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use SODIUM IODIDE I 131 SOLUTION THERAPEUTIC safely and effectively. See full prescribing information
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan SULFUR COLLOID Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan SULFUR COLLOID Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
Trifluridine Ophthalmic Solution, 1% Sterile
 Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
Trifluridine Ophthalmic Solution, 1% Sterile DESCRIPTION Trifluridine (also known as trifluorothymidine, F 3 TdR,F 3 T), is an antiviral drug for topical treatment of epithelial keratitis caused by herpes
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution)
 VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
VIROPTIC Ophthalmic Solution, 1% Sterile (trifluridine ophthalmic solution) PRODUCT OVERVIEW: VIROPTIC SOLUTION DESCRIPTION VIROPTIC is the brand name for trifluridine (also known as trifluorothymidine,
Positron Emission Tomography in Lung Cancer
 May 19, 2003 Positron Emission Tomography in Lung Cancer Andrew Wang, HMS III Patient DD 53 y/o gentleman presented with worsening dyspnea on exertion for the past two months 30 pack-year smoking Hx and
May 19, 2003 Positron Emission Tomography in Lung Cancer Andrew Wang, HMS III Patient DD 53 y/o gentleman presented with worsening dyspnea on exertion for the past two months 30 pack-year smoking Hx and
PRESCRIBING INFORMATION. BaciJect. Bacitracin for injection U.S.P. Powder for Solution. 50,000 Units/Vial. Antibiotic
 PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
PRESCRIBING INFORMATION BaciJect Bacitracin for injection U.S.P. Powder for Solution 50,000 Units/Vial Antibiotic SteriMax Inc. 2770 Portland Drive, Oakville, ON L6H 6R4 Date of Revision: July 30, 2018
( 18 F) ( 40: 23 30, 2003) 3 1 ( ) 3) 18 F-FDG 1,2) 1,2) 5,6) 6,7) 14 C-FDG 18 F-FDG F-FDM-6-
 23 18 F-FDG 2- -2- -D- ( 18 F) * ** * * * 18 F-FDG 14 C 14 C-FDG B 75% FDG 18 F-FDG 18 F-FDG-6-18 F-FDG- 6-18 F-FDM-6- PET FDG 18 F-FDG FDG ( 40: 23 30, 2003) I. 2 18 [ 18 F]-2- -2- -D- ( 18 F-FDG ) (
23 18 F-FDG 2- -2- -D- ( 18 F) * ** * * * 18 F-FDG 14 C 14 C-FDG B 75% FDG 18 F-FDG 18 F-FDG-6-18 F-FDG- 6-18 F-FDM-6- PET FDG 18 F-FDG FDG ( 40: 23 30, 2003) I. 2 18 [ 18 F]-2- -2- -D- ( 18 F-FDG ) (
FDG-PET/CT for cancer management
 195 REVIEW FDG-PET/CT for cancer management Hideki Otsuka, Naomi Morita, Kyo Yamashita, and Hiromu Nishitani Department of Radiology, Institute of Health Biosciences, The University of Tokushima, Graduate
195 REVIEW FDG-PET/CT for cancer management Hideki Otsuka, Naomi Morita, Kyo Yamashita, and Hiromu Nishitani Department of Radiology, Institute of Health Biosciences, The University of Tokushima, Graduate
Nuclear Medicine - Hepatobiliary
 Scan for mobile link. Nuclear Medicine - Hepatobiliary Hepatobiliary nuclear medicine imaging helps evaluate the parts of the biliary system, including the liver, gallbladder and bile ducts, using small
Scan for mobile link. Nuclear Medicine - Hepatobiliary Hepatobiliary nuclear medicine imaging helps evaluate the parts of the biliary system, including the liver, gallbladder and bile ducts, using small
Positron Emission Tomography - Computed Tomography (PET/CT)
 Scan for mobile link. Positron Emission Tomography - Computed Tomography (PET/CT) Positron emission tomography (PET) uses small amounts of radioactive materials called radiotracers, a special camera and
Scan for mobile link. Positron Emission Tomography - Computed Tomography (PET/CT) Positron emission tomography (PET) uses small amounts of radioactive materials called radiotracers, a special camera and
An Introduction to PET Imaging in Oncology
 January 2002 An Introduction to PET Imaging in Oncology Janet McLaren, Harvard Medical School Year III Basics of PET Principle of Physiologic Imaging: Allows in vivo visualization of structures by their
January 2002 An Introduction to PET Imaging in Oncology Janet McLaren, Harvard Medical School Year III Basics of PET Principle of Physiologic Imaging: Allows in vivo visualization of structures by their
See 17 for PATIENT COUNSELING INFORMATION Vial 1 (reaction vial with lyophilized powder) containing 40 mcg of dotatate (3)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NETSPOT safely and effectively. See full prescribing information for NETSPOT. NETSPOT (kit for the
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NETSPOT safely and effectively. See full prescribing information for NETSPOT. NETSPOT (kit for the
Radiopharmacy. Prof. Dr. Çetin ÖNSEL. CTF Nükleer Tıp Anabilim Dalı
 Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
Prof. Dr. Çetin ÖNSEL CTF Nükleer Tıp Anabilim Dalı What is Nuclear Medicine? Nuclear Medicine is the branch of medicine concerned with the use of radionuclides in the study and the diagnosis of diseases.
NEURACEQ (florbetaben F 18 injection), for intravenous use Initial U.S. Approval: 2014
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEURACEQ safely and effectively. See full prescribing information for NEURACEQ. NEURACEQ (florbetaben
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEURACEQ safely and effectively. See full prescribing information for NEURACEQ. NEURACEQ (florbetaben
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
RIMSO-50 PRODUCT MONOGRAPH. (Dimethyl Sulfoxide 500 mg/g) Intravesical Instillation for the Treatment of Interstitial Cystitis
 PRODUCT MONOGRAPH Pr RIMSO-50 (Dimethyl Sulfoxide 500 mg/g) Intravesical Instillation for the Treatment of Interstitial Cystitis Mylan Pharmaceuticals ULC 85 Advance Road Etobicoke, ON M8Z 2S6 Date of
PRODUCT MONOGRAPH Pr RIMSO-50 (Dimethyl Sulfoxide 500 mg/g) Intravesical Instillation for the Treatment of Interstitial Cystitis Mylan Pharmaceuticals ULC 85 Advance Road Etobicoke, ON M8Z 2S6 Date of
Ultra-TechneKow FM. Click Here to Continue. Click Here to Return to Table of Contents
 Ultra-TechneKow FM Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
Ultra-TechneKow FM Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
Principles of nuclear metabolic imaging. Prof. Dr. Alex Maes AZ Groeninge Kortrijk and KULeuven Belgium
 Principles of nuclear metabolic imaging Prof. Dr. Alex Maes AZ Groeninge Kortrijk and KULeuven Belgium I. Molecular imaging probes A. Introduction - Chemical disturbances will precede anatomical abnormalities
Principles of nuclear metabolic imaging Prof. Dr. Alex Maes AZ Groeninge Kortrijk and KULeuven Belgium I. Molecular imaging probes A. Introduction - Chemical disturbances will precede anatomical abnormalities
3 DOSAGE FORMS AND STRENGTHS
 PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PATADAY- olopatadine hydrochloride solution/ drops Alcon Laboratories, Inc. ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PATADAY safely
PET imaging of cancer metabolism is commonly performed with F18
 PCRI Insights, August 2012, Vol. 15: No. 3 Carbon-11-Acetate PET/CT Imaging in Prostate Cancer Fabio Almeida, M.D. Medical Director, Arizona Molecular Imaging Center - Phoenix PET imaging of cancer metabolism
PCRI Insights, August 2012, Vol. 15: No. 3 Carbon-11-Acetate PET/CT Imaging in Prostate Cancer Fabio Almeida, M.D. Medical Director, Arizona Molecular Imaging Center - Phoenix PET imaging of cancer metabolism
https://rm2.scinet.fda.gov/druglabel/rs/spl/by-id/207036/ html 1/12
 NEURACEQ - florbetaben f-18 injection, solution Piramal Imaging, SA ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEURACEQ safely and
NEURACEQ - florbetaben f-18 injection, solution Piramal Imaging, SA ---------- HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NEURACEQ safely and
Site Planning and Design of PET/CT Facilities. Melissa C. Martin, M.S., FACR, FAAPM AAPM Annual Meeting, Orlando, FL August 2, 2006
 Site Planning and Design of PET/CT Facilities Melissa C. Martin, M.S., FACR, FAAPM AAPM Annual Meeting, Orlando, FL August 2, 2006 Acknowledgements: AAPM Task Group #108 on PET and PET/CT Shielding Requirements
Site Planning and Design of PET/CT Facilities Melissa C. Martin, M.S., FACR, FAAPM AAPM Annual Meeting, Orlando, FL August 2, 2006 Acknowledgements: AAPM Task Group #108 on PET and PET/CT Shielding Requirements
The Use of PET Scanning in Urologic Oncology
 The Use of PET Scanning in Urologic Oncology Dr Nicholas C. Buchan Uro-oncology Fellow 1 2 Aims To understand the basic concepts underlying PET scanning. Understand the emerging role of PET Scanning for
The Use of PET Scanning in Urologic Oncology Dr Nicholas C. Buchan Uro-oncology Fellow 1 2 Aims To understand the basic concepts underlying PET scanning. Understand the emerging role of PET Scanning for
Description of the consecutive stages (which took place from December 2002 to July 2003)
 Radiation Protection Issues in a PET/CT Installation M. Coronado 1, R. Plaza 2, R. Couto 1, MD. Marin 1, C. Huerga 2, J. Coya 1, LM Martin Curto 1, M. Téllez de Cepeda 2 1 Servicio de Medicina Nuclear,
Radiation Protection Issues in a PET/CT Installation M. Coronado 1, R. Plaza 2, R. Couto 1, MD. Marin 1, C. Huerga 2, J. Coya 1, LM Martin Curto 1, M. Téllez de Cepeda 2 1 Servicio de Medicina Nuclear,
Research Article Kidney Modelling for FDG Excretion with PET
 Biomedical Imaging Volume 2007, Article ID 63234, 4 pages doi:10.1155/2007/63234 Research Article Kidney Modelling for FDG Excretion with PET Huiting Qiao, 1 Jing Bai, 1 Yingmao Chen, 2 and Jiahe Tian
Biomedical Imaging Volume 2007, Article ID 63234, 4 pages doi:10.1155/2007/63234 Research Article Kidney Modelling for FDG Excretion with PET Huiting Qiao, 1 Jing Bai, 1 Yingmao Chen, 2 and Jiahe Tian
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Medicinal product no longer authorised
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NeoSpect 47 micrograms, kit for radiopharmaceutical preparation. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT NeoSpect 47 micrograms, kit for radiopharmaceutical preparation. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains
Package Insert. Elkar
 Package Insert Elkar Product Summary 1. Name of the medicinal product Elkar 500 mg tablets 2. Qualitative and quantitative composition Each tablet contains levocarnitine 500 mg. 3. Pharmaceutical form
Package Insert Elkar Product Summary 1. Name of the medicinal product Elkar 500 mg tablets 2. Qualitative and quantitative composition Each tablet contains levocarnitine 500 mg. 3. Pharmaceutical form
SUMMARY OF PRODUCT CHARACTERISTICS. for. Stabilised Ceretec, kit for radiopharmaceutical preparation
 SUMMARY OF PRODUCT CHARACTERISTICS for Stabilised Ceretec, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT Stabilised Ceretec 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial
SUMMARY OF PRODUCT CHARACTERISTICS for Stabilised Ceretec, kit for radiopharmaceutical preparation 1. NAME OF THE MEDICINAL PRODUCT Stabilised Ceretec 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial
BRACCO DIAGNOSTICS. MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use
 BRACCO DIAGNOSTICS MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use DESCRIPTION Macrotec is a sterile, nonpyrogenic, lyophilized preparation of albumin
BRACCO DIAGNOSTICS MAGROTEC Kit for the Preparation of Technetium To 99m Albumin Aggregated Diagnostic For Intravenous Use DESCRIPTION Macrotec is a sterile, nonpyrogenic, lyophilized preparation of albumin
Your guide to NETSPOT
 Your guide to NETSPOT A tool to help your doctors diagnose and plan the treatment of neuroendocrine tumors (NETs) NETSPOT, after radiolabeling with Ga 68, is a radioactive diagnostic agent indicated for
Your guide to NETSPOT A tool to help your doctors diagnose and plan the treatment of neuroendocrine tumors (NETs) NETSPOT, after radiolabeling with Ga 68, is a radioactive diagnostic agent indicated for
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. Pentamidine Isetionate for Injection BP (pentamidine isetionate)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Pentamidine Isetionate for Injection BP (pentamidine isetionate) Read this carefully before you start taking Pentamidine
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr Pentamidine Isetionate for Injection BP (pentamidine isetionate) Read this carefully before you start taking Pentamidine
ZANOSAR 1g. STREPTOZOCINE Lyophilised powder for solution for injection
 PACKAGE LEAFLET: INFORMATION FOR THE USER ZANOSAR 1g STREPTOZOCINE Lyophilised powder for solution for injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER ZANOSAR 1g STREPTOZOCINE Lyophilised powder for solution for injection Read all of this leaflet carefully before you start using this medicine. Keep this leaflet.
Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG
 Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG version 2.0, approved February 7, 1999 Authors: Heinrich R. Schelbert, MD, PhD (UCLA School of Medicine, Los Angeles, CA);
Society of Nuclear Medicine Procedure Guideline for Tumor Imaging Using F-18 FDG version 2.0, approved February 7, 1999 Authors: Heinrich R. Schelbert, MD, PhD (UCLA School of Medicine, Los Angeles, CA);
OSTEOCIS 3 mg kit for radiopharmaceutical preparation
 OSTEOCIS 3 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T2000nH 10/2016 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
OSTEOCIS 3 mg kit for radiopharmaceutical preparation SUMMARY OF PRODUCT CHARACTERISTICS T2000nH 10/2016 CIS bio international, Member of IBA Molecular group of companies 1. NAME OF THE MEDICINAL PRODUCT
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked
TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection
![TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection TECHNESCAN SESTAMIBI NAME OF THE MEDICINE DESCRIPTION. Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection](/thumbs/74/70410941.jpg) TECHNESCAN SESTAMIBI Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection NAME OF THE MEDICINE Tetrakis (2-methoxyisobutylisonitrile) copper (I) tetrafluoroborate. The abbreviated notation
TECHNESCAN SESTAMIBI Kit for the preparation of Technetium [ 99m Tc] Sestamibi Injection NAME OF THE MEDICINE Tetrakis (2-methoxyisobutylisonitrile) copper (I) tetrafluoroborate. The abbreviated notation
STATE ENTERPRISE «RADIOPREPARAT» Generator of 99 Mo/ 99m Tc TECHNICAL DESCRIPTION AND INSTRUCTIONS ТSH
 INSTITUTE OF NUCLEAR PHYSICS, ACADEMY OF SCIENCE OF UZBEKISTAN STATE ENTERPRISE «RADIOPREPARAT» Generator of 99 Mo/ 99m Tc TECHNICAL DESCRIPTION AND INSTRUCTIONS ТSH 42-006-2008 Description and Composition
INSTITUTE OF NUCLEAR PHYSICS, ACADEMY OF SCIENCE OF UZBEKISTAN STATE ENTERPRISE «RADIOPREPARAT» Generator of 99 Mo/ 99m Tc TECHNICAL DESCRIPTION AND INSTRUCTIONS ТSH 42-006-2008 Description and Composition
ESKATA TM (hydrogen peroxide) topical solution Initial U.S. Approval: 2017
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ESKATA TM safely and effectively. See full prescribing information for ESKATA TM. ESKATA TM (hydrogen
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ESKATA TM safely and effectively. See full prescribing information for ESKATA TM. ESKATA TM (hydrogen
LUPIN LIMITED SAFETY DATA SHEET. Section 1: Identification. Abacavir Sulfate, Lamivudine and Zidovudine Tablets 300 mg, 150 mg and 300 mg
 LUPIN LIMITED SAFETY DATA SHEET Section 1: Identification Section 1, Identification Material Manufacturer Distributor Abacavir Sulfate, Lamivudine and Zidovudine Tablets 300 mg, 150 mg and 300 mg Lupin
LUPIN LIMITED SAFETY DATA SHEET Section 1: Identification Section 1, Identification Material Manufacturer Distributor Abacavir Sulfate, Lamivudine and Zidovudine Tablets 300 mg, 150 mg and 300 mg Lupin
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
PRESCRIBING INFORMATION Bacitracin USP (Bacitracin for Injection USP) For Topical or Intramuscular Use in Solution 50 000 IU Sterile Powder Pfizer Canada Inc Date of Preparation: 17,300 Trans-Canada Highway
Ultra-TechneKow DTE. Click Here to Continue. Click Here to Return to Table of Contents
 Ultra-TechneKow DTE Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
Ultra-TechneKow DTE Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table of
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Trandolapril Tablets 1 mg, 2 mg & 4 mg Tablets Lupin Limited Mumbai 400 098 INDIA Lupin Pharmaceuticals,
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Trandolapril Tablets 1 mg, 2 mg & 4 mg Tablets Lupin Limited Mumbai 400 098 INDIA Lupin Pharmaceuticals,
AN INTRODUCTION TO NUCLEAR MEDICINE
 AN INTRODUCTION TO NUCLEAR MEDICINE WITH RESPECT TO THYROID DISORDERS By: B.Shafiei MD Nuclear Physician Taleghani Medical Center Radioactive: An element with Unstable Nucleus (Excess Energy), can achieve
AN INTRODUCTION TO NUCLEAR MEDICINE WITH RESPECT TO THYROID DISORDERS By: B.Shafiei MD Nuclear Physician Taleghani Medical Center Radioactive: An element with Unstable Nucleus (Excess Energy), can achieve
Molecular Imaging and the Brain
 Molecular imaging technologies are playing an important role in neuroimaging, a branch of medical imaging, by providing a window into the living brain. Where CT and conventional MR imaging provide important
Molecular imaging technologies are playing an important role in neuroimaging, a branch of medical imaging, by providing a window into the living brain. Where CT and conventional MR imaging provide important
Unusual Radiation Safety Issues in Nuclear Medicine From Alphas to Fetuses
 Unusual Radiation Safety Issues in Nuclear Medicine From Alphas to Fetuses 59 th Annual Meeting Southwestern Chapter Society of Nuclear Medicine and Molecular Imaging Stephen Lokitz Center for Molecular
Unusual Radiation Safety Issues in Nuclear Medicine From Alphas to Fetuses 59 th Annual Meeting Southwestern Chapter Society of Nuclear Medicine and Molecular Imaging Stephen Lokitz Center for Molecular
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Amyvid 800 MBq/mL solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution for injection contains
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Amyvid 800 MBq/mL solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of solution for injection contains
The safety concerns defined with the reference product apply to Buprenorphine-Naloxone 2/0.5 mg, 8/2 mg, sublingual tablets.
 These products are indicated in the substitution treatment for opioid drug dependence, within a framework of medical, social and psychological treatment. The intention of the naloxone component is to deter
These products are indicated in the substitution treatment for opioid drug dependence, within a framework of medical, social and psychological treatment. The intention of the naloxone component is to deter
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
