Facilitation and Attenuation of a Visceral Nociceptive Reflex From the Rostroventral Medulla in the Rat
|
|
|
- Henry Raymond O’Neal’
- 5 years ago
- Views:
Transcription
1 GASTROENTEROLOGY 2002;122: Facilitation and Attenuation of a Visceral Nociceptive Reflex From the Rostroventral Medulla in the Rat MIN ZHUO and G. F. GEBHART Department of Pharmacology, College of Medicine, The University of Iowa, Iowa City, Iowa Background & Aims: Noxious inputs from somatic tissue are subject to biphasic descending modulation from the rostroventral medulla (RVM). In the present study, we investigated descending facilitatory and inhibitory influences from the RVM on a visceral nociceptive reflex. Methods: The visceromotor response (VMR), a contraction of peritoneal musculature during noxious colorectal distention (80 mm Hg, 20 seconds), was quantified as the integrated electromyogram. Results: At 22 sites in the RVM, electrical stimulation produced biphasic effects, facilitating the VMR at low (5, 10, and 25 A) and inhibiting it at greater (>50 A) intensities of stimulation. Electrical stimulation at all intensities tested (5 200 A) in other sites in the RVM only inhibited (30 sites) or only facilitated (12 sites) the VMR to colorectal distention. Activation of glutamatergic receptors in the RVM replicated the effects of electrical stimulation. Reversible blockage (intraspinal lidocaine injection) or irreversible transection of spinal funiculi revealed that descending facilitatory influences from the RVM were conveyed in the ventrolateral/ventral funiculus, whereas descending inhibitory influences were contained in the dorsolateral funiculi. Conclusions: Spinal visceral nociceptive reflexes are subject to facilitatory modulation from the RVM, providing the basis for a mechanism by which visceral sensations can be enhanced from supraspinal sites. Spinal visceral nociceptive transmission, like somatic nociceptive transmission, is subjective to descending inhibitory influences from supraspinal structures (e.g., periaqueductal gray [PAG], nucleus raphe magnus [NRM], and nuclei reticularis gigantocellularis [NGC]). For example, responses to intraperitoneal injection of hypertonic saline in rats are attenuated by electrical stimulation in the PAG. 1 Chemical activation of cell bodies in the rostroventral medulla (RVM) similarly has been reported to attenuate responses to visceral stimulation. 2,3 In support, spinal dorsal horn neuron responses (including spinoreticular and spinothalamic tract neurons) to visceral afferent small myelinated and unmyelinated fiber stimulation or noxious colorectal distention are inhibited by electrical stimulation and/or glutamate microinjection into the PAG, NRM, or NGC. 4 8 Descending modulation of spinal visceral nociceptive transmission is tonically active and includes both inhibitory and facilitatory influences on spinal neurons. Reversible cold block of the cervical spinal cord revealed that spinal viscerosomatic neurons are under tonic descending inhibitory influences from supraspinal structures. 5,8,9 12 Removal of the tonic inhibition produced increases in spontaneous activity and/or responses of spinal neurons to visceral or visceral nerve stimuli. In addition, tonic descending facilitatory influences have been documented. Tattersall et al. 12 reported that 44% of viscerosomatic neurons recorded from thoracic segments of the cat spinal cord gave attenuated or no responses to splanchnic nerve stimulation during cervical cold block. Ness and Gebhart 11 noted that 7 of 20 neurons recorded from T13-L2 segments of the rat spinal cord gave reduced responses to noxious colorectal distention during reversible cervical spinal cold block. Euchner-Wamser et al. 13 and Hummel et al. 14 reported that T2-4 spinal neurons in the rat exhibited reduced responses to esophageal distention during reversible cold block and lower airway irritant stimulation, respectively, after spinal cord transection. Despite evidence that the viscera are subject to tonic descending facilitatory influences, inhibitory modulation of spinal visceral nociception has been the focus of most investigations. There has been no systemic characterization of descending facilitatory modulation from the RVM on spinal nociceptive reflexes or nociceptive neurons despite documentation of similar modulation of somatic reflexes and neurons. For example, it has been shown that electrical stimulation and/or glutamate mi- Abbreviations used in this paper: DLF, dorsolateral funiculus; EMG, electromyographic; NGC, nuclei reticularis gigantocellularis; NRM, nucleus raphe magnus; PAG, periaqueductal gray; RVM, rostroventral medulla; SRF, stimulus-response function; VF, ventral funiculus; VLF, ventrolateral funiculus; VMR, visceromotor response by the American Gastroenterological Association /02/$35.00 doi: /gast
2 1008 ZHUO AND GEBHART GASTROENTEROLOGY Vol. 122, No. 4 croinjection in the NGC and NGC facilitates the nociceptive tail-flick reflex as well as spinal nociceptive transmission We undertook the present study to systematically characterize descending modulatory (facilitatory and/or inhibitory) influences from the RVM on the visceromotor response to noxious colorectal distention (80 mm Hg) and on intensity coding of colorectal input. The visceromotor response, so named by Mackenzie, 22 is a contraction of the peritoneal musculature in response to distention of the colon. It is, as defined by Sherrington, 23 a pseudaffective reflex organized in the brainstem. Materials and Methods Animals Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing g were used. Rats were initially anesthetized with pentobarbital sodium (45 mg/kg Nembutal; Abbott Laboratories, Abbott Park, IL) administrated intraperitoneally. A femoral arterial catheter was placed for monitoring arterial blood pressure and heart rate, and a femoral venous catheter was placed for subsequent pentobarbital infusion. All wound margins were coated with a local anesthetic ointment. Rats were subsequently maintained at a light level of anesthesia (corneal and flexion reflexes present) by continuous intravenous infusion of pentobarbital (5 10 mg kg 1 h 1 ). 15,24 Body temperature was maintained at C by a water circulating heating pad. Colorectal Distention The noxious visceral stimulus used was distention with air of the descending colon and rectum by a 6 7-cm long, 2 3-cm diameter flaccid, flexible latex balloon inserted via the anus. The outside diameter of the balloon when inflated was greater than the intraluminal diameter of the colon of the rat. The end of the balloon was placed 1 cm from the anus and kept in place by taping the balloon catheter to the base of the tail. The balloon catheter was connected to a distention control device. Colorectal distention was initiated by opening a solenoid gate to a constant pressure air reservoir, and constant pressure stimuli were given every 3 minutes (10, 20, 40, 60, 80, or 100 mm Hg, 20-second duration). Visceromotor Response Recording electrodes constructed from fine barbed endodontic branches (Kerr, Romulus, MI) connected to hook-up wire were placed in the external oblique musculature just above the inguinal ligament to record electromyographic (EMG) activity. EMG activity was amplified and filtered, counted, and integrated using a window discriminator. A voltage threshold was set for each recording session such that no potentials exceeded it under basal conditions. The voltage threshold was not changed during an experiment. The visceromotor response (VMR) to colorectal distention ( mm Hg, 20 seconds) was taken as the increase in EMG activity above the preset voltage threshold. 25 In most experiments, the effect of electrical or glutamate stimulation in the RVM was tested on the VMR to a noxious intensity of colorectal distention (80 mm Hg). 25 In experiments in which the effect of electrical stimulation on intensity coding was examined, intensities of distention were mm Hg. Because the total number of counts varies between rats, summary data are reported as a percentage of the control response to colorectal distention, determined for each rat as the mean of 3 consecutive trials (80 or 100 mm Hg) given 3 minutes apart (100%). 25 Brain Stimulation and Glutamate Microinjection Focal electrical brain stimulation, A, consisted of continuous 100-Hz constant current cathodal pulses of 100- s duration. Brain stimulation was started 5 seconds before and continued during the 20 seconds of colorectal distention. This stimulation paradigm is the same as used in previous studies, which was determined experimentally to require the lowest intensity of stimulation in the NRM, NGC, and NGC to inhibit the tail-flick reflex. 15,24 Monopolar stimulating electrodes (34 gauge, 0.15-mm diameter), guided stereotaxically in the vertical plane (incisor bar at 3.3 mm), 26 were inserted into the brain through a 26-gauge (0.45-mm outer diameter) guide cannula. The electrodes were cut to extend 2 mm beyond the tip of the guide cannula. The stimulating electrode was lowered to a site in the RVM and the effect of stimulation on the VMR to colorectal distention was tested at 2 3 sites in an experiment, 0.5 mm apart, in the same electrode track. Testing the effect of electrical stimulation always preceded testing for effects of glutamate, which typically was tested 5 10 minutes after electrical stimulation. The effects of electrical stimulation did not outlast the duration of stimulation by more than seconds. Monosodium-L-glutamate (5 or 50 nmol, ph 6.7) was microinjected into the medulla in a volume of 0.5 L via an injection cannula (33 gauge, 0.20-mm outer diameter) inserted through the same 26-gauge guide cannula and extending 2 mm beyond its tip. Injection of glutamate was done by an electrically driven syringe pump at a speed of 0.5 L/1.5 min. The progress of the microinjection was continuously monitored by following the movement of an air bubble in a length of calibrated tubing between the injection syringe and the injection cannula. Ventrolateral Funiculus Lidocaine Injection The cervical spinal cord was exposed in some experiments to investigate spinal pathways of descending modulation. Two 26-gauge cannulae, 2.0-mm apart, were advanced into the cervical spinal cord (C1-C3) in the coronal plane to penetrate the pia matter. Microinjection of lidocaine (4%, 0.5 L) was made into the ipsilateral and/or contralateral ventrolateral funiculus (VLF) through a 33-gauge injection cannula inserted through the 26-gauge guide cannula. The injection cannula extended 2 mm beyond the end of the guide cannula.
3 April 2002 FACILITATION OF VISCERAL PAIN 1009 This procedure produced a reversible functional block in the ventral spinal cord. 18,27,28 Spinal Dorsolateral Funiculus Transection To transect the dorsolateral funiculus (DLF), a small pledget of Gelfoam (Pharmacia and UpJohn Co., Kalamazoo, MI) soaked in dilute lidocaine was applied briefly to the cervical spinal cord before transection. The ipsilateral and/or contralateral DLF was then cut using a pair of fine scissors. A reversible drop in arterial blood pressure was usually produced by DLF transection (see Results); all measurements were made only when arterial blood pressure recovered to near the pretransection baseline (30 60 minutes later). Histology At the end of the experiments, rats were killed with an intravenous overdose of pentobarbital sodium. Anodal electrolytic lesions were made in the brainstem and spinal cord to mark the sites of brain stimulation and spinal lidocaine injection, respectively. The brain and appropriate regions of the spinal cord were removed and fixed in 10% formalin, frozen, and cut in 40- m coronal sections, mounted on glass slides, and stained with H&E for histologic verification of the sites of brainstem stimulation and spinal lidocaine injection. Sites of brainstem stimulation and lidocaine injection are illustrated on standard brainstem sections adapted from the atlas of Paxinos and Watson. 26 The extent of transection of the DLF was reconstructed. Data and Statistics Data are presented as the mean value 1 SEM. Statistical comparisons were made using either one-way or two-way analysis of variance (Newman-Keuls test for post hoc comparisons). Student t test was applied for comparisons between groups. In all cases, P 0.05 was considered significant. Results General Peritoneal musculature contraction to noxious colorectal distention (80 mm Hg, 20 seconds), measured as the integrated EMG, was used to quantify the VMR (Figure 1). Electrical stimulation in the RVM produced both facilitatory and inhibitory effects on responses to noxious distention (80 mm Hg). At 33 of 75 sites in the RVM, stimulation produced intensity-dependent inhibition of responses to noxious distention (Figure 2). At 22 of the remaining 42 sites, electrical stimulation produced biphasic effects, facilitating the VMR at lesser intensities (5 50 A) and inhibiting responses at greater intensities ( 50 A). At 12 other sites, electrical stimulation only facilitated responses to noxious colorectal distention at all intensities tested (5 200 A). Electrical stimulation at 8 sites did not affect the VMR to noxious distention (unfilled squares in Figure 2). Figure 1. Example of peritoneal musculature contraction to noxious colorectal distention (80 mm Hg; 20 seconds) quantified as the electromyogram (EMG). The EMG and corresponding peristimulus time histogram (1-second bin width) are illustrated. The horizontal dotted line represents the discriminator threshold. Biphasic Modulation Twenty-two sites in which stimulation produced biphasic effects were located in the NGC (n 11), NGC (n 7), and raphe nuclei (obscurus, n 1; pallidus, n 1; and magnus, n 2). An example of stimulation-produced biphasic effects is given in Figure 3A; the data are summarized in Figure 4. In the example, the VMR to a noxious intensity of distention (80 mm Hg) during stimulation at 10 A in the NGC is 141.4% of the control response, whereas stimulation at 200 A in the same site attenuated the VMR to 63.8% of control. Overall, stimulation in these 22 sites facilitated the VMR to noxious distention at low intensities (5, 10, and 25 A) and inhibited responses at greater intensities ( A). Most modulatory sites of stimulation were found in the NGC or NGC, and we compared the effects of stimulation in these 2 sites. At 11 sites in the NGC, electrical stimulation at 25 A increased the VMR to a mean % of control; at 7 sites in the NGC, stimulation at the same intensity increased responses to a mean % of control. Similarly, inhibitory modulation produced by electrical stimulation at greater intensities also did not reveal any significant differences between sites in the NGC or NGC. Stimulation at 100 A in the NGC or NGC
4 1010 ZHUO AND GEBHART GASTROENTEROLOGY Vol. 122, No. 4 Figure 2. Summary of sites in the rostroventral medulla where electrical stimulation produced only inhibition (E), only facilitation (Œ), or biphasic modulation ( ) of the visceromotor response to noxious colorectal distention (80 mm Hg, 20 seconds). Sites where electrical stimulation did not produce effects are also shown ( ). Stimulation sites were reconstructed from histologic sections of the rat brainstem and are summarized on coronal brain sections 26 referenced to distance in millimeters posterior to Bregma (Bregma being most rostral). NPGCl, nucleus reticularis paragigantocellularis lateralis; Pyr, pyramidal tract; Sp5, spinal trigeminal tract; and VII, facial nucleus. inhibited responses to noxious distention to a comparable extent (mean % and mean % of control, respectively). Inhibitory Modulation Thirty of the 32 sites at which only inhibition was produced at all intensities of stimulation tested were located in the RVM, including the NGC (n 15), NGC (n 9), and raphe nuclei (obscuris, n 1; magnus, n 5). Other sites were found in the predorsal bundle (n 1) and the medial lemniscus (n 1). An example of stimulation-produced inhibition is given in Figure 3B; data from the 30 experiments are summarized in Figure 4. In this example, the VMR to 80 mm Hg Figure 3. Examples of (A) biphasic and (B) inhibitory modulation of the visceromotor response produced by electrical stimulation in the RVM. (A and B) Peristimulus time histograms (1-second bin width) of the visceromotor responses to noxious colorectal distention (80 mm Hg) before and during stimulation (intensities given). The period of distention (20 seconds) is indicated by the horizontal bar, and the period of RVM stimulation (25 seconds) is indicated by the arrows. (C) Graphic representation of the data in A and B; the point above 0 represents the response to distention (total counts in 20 seconds) in the absence of stimulation. (D) Brainstem stimulation sites, illustrated on a representative coronal brain section. 26 Abbreviations are the same as in Figure 2.
5 April 2002 FACILITATION OF VISCERAL PAIN 1011 Figure 4. Summary of descending modulation of the visceromotor response from the RVM. (A C) Visceromotor responses to noxious colorectal distention (80 mm Hg) are presented as a percentage of the control response (total counts in 20 seconds) against the intensity of stimulation in the RVM for (A) inhibitory, (B) facilitatory, and (C) biphasic modulation. (D) Summary of data in A, B, and C. distention during stimulation at 25 and 50 A inthe NGC was reduced to 42.7% and 1.8% of the control response to distention. As summarized in Figure 4D, electrical stimulation in these 30 sites produced intensity-dependent inhibition of the VMR to noxious colorectal distention. Because most sites of stimulation were in the NGC or NGC, we compared the effects of stimulation in these 2 sites. At sites in the NGC (n 15) and NGC (n 9), electrical stimulation at 50 A inhibited the VMR to a mean % and a mean % of control, respectively. Facilitatory Modulation The 12 sites in the RVM where only facilitatory effects were produced by stimulation were located in the NGC (n 10) and NGC (n 2). Electrical stimulation at 25 A facilitated VRMs to noxious colorectal distention to a mean % of control. At the greatest intensity of stimulation tested (200 A), the VMR to distention was facilitated to a mean % of the control response to distention. The magnitude of facilitation was not dependent on the intensity of electrical stimulation (F[5,31] 1.94). Data from these 12 sites are summarized in Figure 4. Electrical stimulation at 8 sites in the brainstem (Figure 2) did not affect responses to 80 mm Hg colorectal distention at the intensities tested (5 200 A). Seven of these 8 sites were located in the NGC, near or adjacent to sites of stimulation that did modulate the VMR. Resting EMG Activity At 21 sites in the RVM where electrical stimulation produced biphasic modulation of VMRs to noxious distention, resting EMG activity (mean 5 2 counts/second) was not significantly affected by stimulation at the intensities tested (5 200 A; F [6,96]
6 1012 ZHUO AND GEBHART GASTROENTEROLOGY Vol. 122, No. 4 Figure 5. Effects of stimulation in the RVM on SRFs to graded colorectal distention. (A and C) Control SRFs of individual animals in the absence of stimulation in the brainstem. Data are plotted as the response to distention (total counts in 20 seconds) against distention pressure. (B and D) Summary of effects of (B) facilitatory and (D) inhibitory stimulation in the RVM on the mean SRFs of data illustrated in A and C, respectively. Data are presented as mean response in the absence (E, control) and presence ( ) of stimulation. (E) Sites of facilitatory ( and N) and inhibitory (E and N) stimulation. 1.18). Similarly, at the 30 sites in the RVM where electrical stimulation only inhibited VMRs, electrical stimulation did not affect resting EMG activity (mean 5 1 counts/second; F[5,116] 1.33). At the 12 sites where electrical stimulation only facilitated VMRs to noxious distention, resting EMG activity (mean 2 1 counts/second) was not significantly affected at intensities of stimulation between 5 and 50 A (F[4,37] 2.01). However, at greater intensities (100 and 200 A), electrical stimulation significantly increased resting EMG activity to 23 9 counts/second and counts/second, respectively (F[2,20] 5.68; P 0.01). Intensity Coding Visceromotor responses to graded colorectal distention were positively accelerating functions ( mm Hg; 20 seconds). Stimulus-response functions (SRFs) and their modulation by a facilitatory intensity of stimulation in the RVM are presented in Figure 5A and B, respectively. Electrical stimulation at a mean intensity of A (n 5) increased VMRs to 100 mm Hg colorectal distention to a mean % of control, and produced, between mm Hg distention, a parallel shift of the SRF to the left without changing its slope (31 6 counts/mm Hg vs total counts/mm Hg). SRFs and their modulation by an inhibitory intensity of stimulation in the RVM are presented in Figure 5C and D, respectively. Electrical stimulation at a mean intensity of A significantly attenuated VMRs to distention, shifting the SRF rightward without changing the slope of the SRF (mean 36 5 counts vs counts/mm Hg). Latencies to Effect The apparent latency for stimulation-produced facilitation and inhibition was determined by using a cumulative sum technique 29 and bin-by-bin analysis of response. 30 Brainstem stimulation was given during a relatively stable VMR to 80 mm Hg colorectal distention. The first 500-ms period of recording was used to generate a reference baseline, and the cumulative sum of the VMR between 500 and 1500 ms after the onset of stimulation in the RVM was plotted. The latency to effect was defined as the time from the onset of stimulation to the time when the cumulative sum of the histogram began to depart steadily from the reference baseline. In a total of 10 experiments, the apparent latency to inhibition by electrical stimulation at a mean intensity of A was ms (range, ms). In 5 other experiments, the apparent mean latency to facilitation by electrical stimulation at a mean intensity of A was determined to be ms (range, ms), which is significantly longer than the latency to inhibition of the visceromotor reflex (t 2.65; P 0.05).
7 April 2002 FACILITATION OF VISCERAL PAIN 1013 Figure 6. Example of the inhibitory effect of glutamate microinjection (50 nmol) into the RVM on the visceromotor response to noxious colorectal distention (80 mm Hg). (A) Peristimulus time histograms (1-second bin width) illustrating responses to distention before (top) and 1, 4, 7, and 10 minutes after glutamate microinjection. (B) Graphic representation of data in A. The point above C represents the baseline response to distention (total counts in 20 seconds) and the points above stim indicate inhibitory effects produced by electrical stimulation (intensities given) at the same site in the brainstem. Glutamate (50 nmol) was subsequently given into the same site as stimulation and inhibited the response to distention. (C) Site of brainstem stimulation/glutamate microinjection. Abbreviations are the same as in Figure 2. Glutamate-Produced Effects Because electrical stimulation nonselectively activates cell bodies and fibers of passage in the RVM, glutamate was microinjected into the RVM to more selectively activate cells. L-glutamate microinjection into the RVM produced both facilitation and inhibition of VMRs to noxious colorectal distention reproducing the effects of electrical stimulation given at the same site. Figure 6 shows reversible glutamate-produced inhibition equivalent to that produced by 200 A stimulation at the same site in the NGC. Resting EMG activity in this example was not significantly affected by glutamate microinjection (Figure 6A), suggesting a selective effect of glutamate on the VMR to noxious colorectal distention. Summary data are shown in Figure 7, where the locations of injection in Figure 7C also reflect dose-dependent effects. The response to 80 mm Hg distention 1 minute after microinjection of 50 nmol L-glutamate was significantly inhibited to a mean % of control (Figure 7A and B). Glutamate microinjection at a low dose (5 nmol) in the RVM significantly facilitated the VMR to a mean % of control (t 3.35; P 0.05). Glutamate-produced effects were rapid onset, short in duration, and not associated with effects on resting EMG activity (F[4,25] 1.49). Control responses to 80 mm Hg colorectal distention in these experiments did not differ between those sites from which facilitatory or inhibitory effects were produced by glutamate (mean total counts/20 seconds vs. mean total counts/20 seconds, respectively; t 1.07). The effect of glutamate is likely caused by reversible activation of its receptors. In 9 experiments, a second Figure 7. Summary of glutamate-produced effects. (A) Mean peristimulus time histograms (PSTHs; 1-second bin width) representing the mean visceromotor response before glutamate administration (unfilled PSTHs) and at 1 minute after glutamate administration (filled PSTHs). The period of distention (20 seconds) is indicated below by the horizontal bar. (B) Graphic representation of the data in A and time course of effect. The data are presented as a percentage of the control response (total counts in 20 seconds). (C) Brainstem sites for glutamate microinjection at a low dose (5 nmol; ) and a greater dose (50 nmol; E). At 3 sites (indicated by N), both doses of glutamate (5 and 50 nmol) were tested.
8 1014 ZHUO AND GEBHART GASTROENTEROLOGY Vol. 122, No. 4 Figure 8. Summary of reproducibility of glutamate-produced facilitation and inhibition. (A) Mean peristimulus time histograms (PSTHs; 1-second bin width) representing the mean visceromotor responses before glutamate administration (unfilled PSTHs) and at 1 minute after glutamate administration (filled PSTHs). The period of distention (20 seconds) is indicated below by the horizontal bar. On the left, mean responses before and after the first glutamate administration at doses of 5 or 50 nmol are illustrated on top and bottom, respectively. Responses before and after the second glutamate administration at the same site are illustrated on the right top and bottom, respectively. (B) Graphic illustrations of the data in A expressed as a percentage of control responses to distention. (C) Summary of sites where glutamate at a low dose (5 nmol; E) or greater dose (50 nmol; ) was administered. At 2 sites (N), both low and high doses of glutamate were tested. Abbreviations are the same as in Figure 2. microinjection of glutamate at the same site and dose (5 or 50 nmol) was administered after the VMR to distention had recovered to control after the first glutamate microinjection. As shown in Figure 8, 5 nmol of glutamate facilitated VMRs to 80 mm Hg, 20 seconds of distention, from a mean baseline total counts/20 seconds (n 4) to % of control at a mean 3 1 minute (range, 1 4 minutes) after glutamate administration. Fifteen minutes later, when the VMR to noxious distention returned to the preinjection baseline ( total counts/20 seconds), a second glutamate microinjection at the same dose (5 nmol) was given into the same site. A similar magnitude of facilitation (t 0.17) was produced (to % of control) within 4 minutes (mean 3 1 minute). In 5 other experiments, 50 nmol of glutamate inhibited the VMR to 80 mm Hg distention from a baseline total counts/20 seconds to % of control at a mean 3 1 minute (range, 1 4 minutes) after administration of glutamate. The response to 80 mm Hg distention returned to baseline 15 minutes later ( total counts/20 seconds). A second microinjection of glutamate at the same dose into the same 5 sites produced a similar magnitude of inhibition (to % of control; t 0.4). Spinal Pathways Mediating Descending Modulation To investigate descending pathways mediating stimulation-produced effects, lidocaine (4%, 0.5 L) was microinjected into the VLF/ventral funiculus (VF) at the cervical level of the spinal cord. The effect of lidocaine injection was evaluated between 5 and 30 minutes after injection. 18,27,31,32 In 5 rats, resting EMG activity was not significantly affected by either unilateral (n 5) or subsequent bilateral (n 4) lidocaine microinjection. The post-lidocaine responses were taken as control to assess the effects of RVM stimulation. Lidocaine microinjection into the VLF/VF on the same side as RVM stimulation ( A, n 5) completely abolished the facilitatory effect of stimulation on the VMR to distention (from % of control before to % of control after ipsilateral
9 April 2002 FACILITATION OF VISCERAL PAIN 1015 Figure 9. Summary of effects of unilateral (ipsilateral or contralateral) lidocaine microinjection into the ventral part of the spinal cord. (A and B) Effects on stimulation-produced facilitation and inhibition of the visceromotor response, respectively. The mean response to noxious colorectal distention (80 mm Hg) is presented as a percentage of control (total counts in 20 seconds). Stimulation-produced effects before lidocaine microinjection are indicated by unfilled bars. (C) Histologic reconstruction of sites in the spinal cord for lidocaine microinjection. (D) Brainstem stimulation sites for inhibitory (E) or facilitatory ( ) modulation. At 5 sites (indicated by N), both facilitatory and inhibitory modulation produced by stimulation at lesser and greater intensities, respectively, was tested. Abbreviations are the same as in Figure 2. *Significantly less than corresponding control (P 0.05). lidocaine) (Figure 9A). Lidocaine microinjection into the VLF/VF contralateral to the site of stimulation in the RVM ( A, n 3) abolished the facilitatory effect of stimulation on the VMR (from % of control to % of control) (Figure 9A) produced by stimulation. In 5 experiments, the inhibitory effect on the VMR produced by electrical stimulation in the same sites at greater intensities (mean A) was not significantly affected ( % of control before vs % of control after) by lidocaine microinjection into the VLF/VF (n 3 for ipsilateral; n 2 for contralateral) (Figure 9B). To investigate involvement of the DLF in descending modulation from the RVM, stimulation-produced facilitation and/or inhibition of VMRs to 80 mm Hg colorectal distention was studied before and after bilateral transections of the DLFs. Electrical stimulation produced inhibition (at a mean A) from 5 sites in the RVM was significantly attenuated by bilateral transections of the DLFs (from % before to % of control after; Figure 10). In 2 experiments, bilateral DLF transection not only completely abolished the inhibitory effect of RVM stimulation, but electrical stimulation at the same intensities (25 or 50 A), which produced inhibition (to 58.7% and 11.7% of control, respectively) before bilateral DLF transections, now facilitated the VMR to noxious colorectal distention after transections of the DLFs (to 110.6% and 125.2% of control, respectively). In 3 experiments, stimulation-produced facilitation of VMRs to 80 mm Hg distention was not affected by ipsilateral (n 2) or contralateral (n 1) DLF transections ( % before vs % of control after) (Figure 10). Discussion The present study is the first of which we are aware to characterize descending facilitatory and inhibitory influences from the RVM on a visceral nociceptive reflex. An important finding is that spinal visceral input is subject to facilitatory modulation, providing the basis of a mechanism that could enhance visceral perceptions in the absence of noxious visceral stimulation. We found that electrical stimulation in the RVM produced inten-
10 1016 ZHUO AND GEBHART GASTROENTEROLOGY Vol. 122, No. 4 modulation of spinal visceral transmission. These descending influences were found to have different latencies to effect and different functional anatomies. Descending inhibitory effects from the RVM are mediated primarily by pathways traveling in the dorsolateral spinal cord; descending facilitatory effects are mediated by pathways traveling in the ventral/ventrolateral spinal cord. Finally, descending modulation, whether inhibitory or facilitatory, was linked to the stimulus; resting EMG activity was unaffected by either electrical stimulation or glutamate microinjection in the RVM. These findings are important in the context of functional bowel disorders characterized by sensations of bloating, discomfort, and pain in the absence of organ pathology. The function or balance between normal inhibitory and facilitatory modulatory influences may be altered in functional bowel disorder patients, giving rise to unpleasant sensations from otherwise normal physiologic stimuli (e.g., organ filling or storage). Figure 10. Summary of effects of bilateral transection of the DLFs. (A) Effects on stimulation-produced facilitatory and inhibitory effects. Responses to noxious colorectal distention (80 mm Hg) are presented as percentage of control (total counts in 20 seconds)., Stimulation-produced effects before bilateral DLF transections. (B) Brainstem stimulation sites for inhibitory (E) or facilitatory ( ) modulation. At 1 site (N), both facilitatory and inhibitory modulation produced by stimulation at lesser and greater intensities, respectively, were tested. (C) Histologic reconstruction of DLF transections. Abbreviations are the same as in Figure 2. *Significantly different from corresponding control (P 0.05). sity-dependent biphasic modulation (i.e., facilitation at lesser intensities and inhibition at greater intensities of stimulation), intensity-dependent inhibition, or facilitation of the VMR to colorectal distention. Importantly, activation of glutamatergic receptors in the RVM also facilitated or inhibited the VMR to colorectal distention, indicating that cell bodies in the RVM contribute to Descending Inhibition Descending inhibitory modulation from the PAG or NRM on spinal visceral nociceptive transmission has been reported, 4,6 8 and roles for the NGC and NGC in descending inhibitory modulation of spinal somatic nociceptive transmission have been documented Consistent with these previous studies of somatic nociception, the present study documented that electrical stimulation in the RVM, principally the NGC and NGC, produced intensity-dependent inhibition of responses to noxious colorectal distention. The inhibitory effects were replicated by glutamate microinjection (50 nmol) into the same sites at which electrical stimulation was effective, providing evidence for the contribution of glutamate receptors in the RVM in the effects produced. This is in good accord with the inhibitory effects produced by glutamate microinjection into the RVM on the nociceptive tail flick reflex, 15,18,21,33 35 spinal dorsal horn neuron responses to noxious somatic stimuli, 18,21 and on spinal visceral nociceptive transmission. 8 Descending Facilitation Descending facilitatory influences from the RVM on spinal somatic nociceptive transmission also have been systematically characterized. As in earlier studies of facilitatory modulation of the nociceptive tail flick reflex 15 and somatic nociceptive transmission, 21 we documented here that visceral nociceptive responses are subject to facilitatory influences descending from the RVM. Facilitation of the VMR to noxious colorectal distention was shown to be produced by low intensities of electrical stimulation at some and all intensities of stimulation at
11 April 2002 FACILITATION OF VISCERAL PAIN 1017 other sites in the RVM. As with descending inhibition, glutamate microinjection into sites at which electrical stimulation facilitated responses to distention also significantly increased responses to distention. The effects of glutamate were concentration-dependent (facilitation of responses at 5 nmol and inhibition of responses at 50 nmol), time-limited, and reproducible. In related studies, 2 we determined that the exaggerated VMR to colorectal distention of the inflamed rat colon involves a glutamate receptor in the RVM. As recently reviewed, 36 we believe that the RVM is important to both the development and maintenance of hyperalgesia, including visceral hyperalgesia. That glutamate microinjected into the RVM facilitates the visceromotor response to noxious colorectal distention supports that hypothesis. Latency to Descending Modulatory Effects In previous work, we documented that facilitation of either somatic nociceptive reflexes 15,37 or spinal nociceptive transmission 18,21,31 occurs at a significantly longer latency than does inhibition of the same reflexes/ neurons. For example, vagal afferent stimulation 31 or stimulation in the RVM 18,21 that decreased the latency of the nociceptive tail flick reflex or increased responses of spinal neurons to somatic stimuli were produced at latencies of greater than 200 ms after the onset of vagal afferent stimulation or RVM stimulation. Consistent with this earlier work, the apparent latency for stimulation-produced facilitation of the VMR in the present study was 261 ms (compared with a latency to inhibition of 153 ms). This may be explained on the basis of the different descending spinal pathways used by inhibitory (dorsal) and facilitatory (ventral) influences engaged in the RVM. Given the relatively short distance between the RVM and lumbar spinal cord in the rat, this seems an unlikely explanation. We noted in earlier work that descending facilitation, but not inhibition, produced by vagal afferent stimulation was abolished in decerebrated rats, 31 implicating sites rostral to the midbrain in descending facilitation of spinal nociceptive transmission. Intensity Coding Descending inhibitory and facilitatory influences from the RVM on the intensity coding of spinal somatic nociceptive transmission are parametrically different. Descending inhibitory influences produced by stimulation in the RVM significantly reduce the slope of the SRF (without changing the threshold for responses), whereas descending facilitatory influences produce a parallel, leftward shift of the SRF. 15,18,21 These results suggest that different mechanisms are responsible for descending facilitatory and inhibitory effects. That descending facilitatory and inhibitory influences are contained in different spinal pathways (see below) and are mediated by different spinal neurotransmitter receptors 17 reinforces the inference that that mechanisms of inhibitory and facilitatory effects are different. The present study also suggests that modulation of somatic and visceral nociception differ. In the present study of modulation of visceral nociception, stimulation in the RVM at facilitatory intensities produced a parallel, leftward shift of the SRF 18,21 as has been previously documented for facilitatory modulation of somatic nociception from the RVM (see our previous reports 15,18,21 and Gebhart 38 for review). In contrast to earlier studies of modulation of somatic nociception, in which stimulation in the RVM at inhibitory intensities reduced the slope of the SRF without significantly increasing response threshold, stimulation in the RVM at inhibitory intensities in the present study shifted the SRF of the VMR rightward without affecting the slope of the encoding function. The extrapolated VMR threshold increased from about 10 to more than 30 mm Hg. This difference between inhibitory modulation of somatic and visceral nociception is supported by other reports. In a study of spinal cord neuron responses to colorectal distention, Ness and Gebhart 8 found a similar rightward shift in the SRFs of the spinal neurons studied. Recently, we 39 further documented that stimulation in the RVM at inhibitory intensities similarly shifted to the right in a parallel fashion spinal neuron responses to colorectal distention. This difference from our previous studies of somatic nociceptive modulation in the rat 15,18,21 may represent differences between somatic and visceral nociceptive processing in pre- vs. postsynaptic effects or indicate a specific arrangement of bulbospinal neuronal terminals on spinal neurons. 40 Resolution of this potentially important issue requires additional studies. Spinal Pathways for Facilitation and Inhibition In the present study, stimulation-produced facilitation of the VMR from the RVM was selectively abolished by lidocaine microinjection into the ventral part of the spinal cord. Descending inhibition of the VMR was unaffected during local anesthetic blockage of the VLF/ VF. In contrast, descending inhibitory effects from the RVM were selectively affected by transection of the DLFs. A similar separation of inhibitory and facilitatory influences descending from the brainstem has been noted previously 18,21 and further supports the existence of independent, active systems of spinal inhibition and facilitation of somatic and visceral nociceptive transmission.
12 1018 ZHUO AND GEBHART GASTROENTEROLOGY Vol. 122, No. 4 In conjunction with other evidence (see Urban and Gebhart 36 for recent overview), the present findings further support a contribution to pain facilitation from the brainstem. As suggested previously (e.g., Fields 41 and Gebhart 42 ), such descending facilitation may contribute to increased pain in the presence of modest input or even pain in the absence of activation of nociceptors, including visceral nociceptors. Accordingly, the altered sensations that characterize functional bowel disorders could be sustained by central, supraspinal mechanisms that enhance and lead to misinterpretation of normal, nonpainful visceral input. References 1. Giesler GJ, Liebskind JC. Inhibition of visceral pain by electrical stimulation in the periaqueductal gray matter. Pain 1976;2: Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain 1998;78: Urban MO, Coutinho SV, Gebhart GF. Biphasic modulation of visceral nociception by neurotensin in rat rostral ventromedial medulla. J Pharmacol Exp Ther 1999;290: Ammons WS, Blair RW, Foreman RD. Raphe magnus inhibition of primate T1-T2 spinothalamic cells with cardiopulmonary visceral input. Pain 1984;20: Cervero F, Lumb BM, Tattersall JEH. Supraspinal loops that mediate visceral inputs to thoracic spinal cord neurons in the cat: involvement of descending pathways from raphe and reticular formation. Neurosci Lett 1985;56: Chandler MJ, Garrison DW, Brennan TJ, Foreman RD. Effects of chemical and electrical stimulation of the midbrain on feline T2-T6 afferent input. Brain Res 1989;496: Chapman CD, Ammons WS, Foreman RD. Raphe magnus inhibition of feline T1-T4 spinoreticular tract cell responses to visceral and somatic inputs. J Neurophysiol 1985;52: Ness TJ, Gebhart GF. Quantitative comparison of inhibition of visceral and cutaneous spinal nociceptive transmission from the midbrain and medulla in the rat. J Neurophysiol 1987;58: Akeyson EW, Kneupfer MM, Schramm LP. Splanchnic input to thoracic spinal neurons and its supraspinal modulation in the rat. Brain Res 1990;536: Cervero F. Supraspinal connections of neurons in the thoracic spinal cord of the cat: ascending projections and effects of descending impulses. Brain Res 1983;275: Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T 13 -L 2 spinal cord of the rat. J Neurophysiol 1988;60: Tattersall JEH, Cervero F, Lumb BM. Effects of reversible spinalization on the visceral input to viscerosomatic neurons in the lower thoracic spinal cord of the cat. J Neurophysiol 1986;56: Euchner-Wamser I, Sengupta JN, Gebhart GF, Meller ST. Characterization of responses of T 2 -T 4 spinal cord neurons to esophageal distension in the rat. J Neurophysiol 1993;69: Hummel T, Sengupta JN, Meller ST, Gebhart GF. Responses of T 2-4 spinal cord neurons to irritation of the lower airways in the rat. Am J Physiol 1997;273:R1147 R Zhuo M, Gebhart GF. Characterization of descending inhibition and facilitation from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Pain 1990;42: Zhuo M, Gebhart GF. Spinal cholinergic and monoaminergic receptors mediate descending inhibition from the nuclei reticularis gigantocellularis pars alpha in the rat. Brain Res 1990;535: Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res 1991;550: Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol 1997;78: McCreery DB, Bloedel JR, Hames EG. Effects of stimulating in raphe nuclei and in reticular formation on response of spinothalamic neurons to mechanical stimuli. J Neurophysiol 1979;42: Haber LH, Martin RF, Chung JM, Willis WD. Inhibition and excitation of primate spinothalamic tract neurons by stimulation in region of nucleus reticularis gigantocellularis. J Neurophysiol 1980;43: Zhuo M, Gebhart GF. Characterization of descending facilitation and inhibition of spinal nociceptive transmission from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. J Neurophysiol 1992;67: Mackenzie J. Symptoms and their management. London: Shaw, Sherrington CS. The integrative action of the nervous system. New Haven: Yale University Press, Sandkühler J, Gebhart GF. Characterization of inhibition of a spinal nociceptive reflex by stimulation medially and laterally in the midbrain and medulla in the pentobarbital-anesthetized rat. Brain Res 1984;305: Coutinho SV, Meller ST, Gebhart GF. Intracolonic zymosan produces visceral hyperalgesia in the rat that is mediated by spinal NMDA and non-nmda receptors. Brain Res 1996;736: Paxinos G, Watson C. The rat brain in stereotaxic coordinates (3rd ed.). New York: Academic Press, Jones SL, Gebhart GF. Spinal pathways mediating tonic, coeruleospinal, and raphe-spinal descending inhibition in the rat. J Neurophysiol 1987;58: Ren K, Randich A, Gebhart GF. Electrical stimulation of cervical vagal afferents. I. Central relays for modulation of spinal nociceptive transmission. J Neurophysiol 1990;64: Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 1978;45: Gerhart KD, Yezierski RP, Fang ZR, Willis WD. Inhibition of primate spinothalamic tract neurons by stimulation in ventral posterior lateral (VPLc) thalamic nucleus: possible mechanisms. J Neurophysiol 1983;49: Ren K, Randich A, Gebhart GF. Vagal afferent modulation of spinal nociceptive transmission in the rat. J Neurophysiol 1989; 62: Sandkühler J, Fu Q-G, Zimmermann M. Spinal pathways mediating tonic or stimulation-produced descending inhibition from the periaqueductal gray or nucleus raphe magnus are separate in the cat. J Neurophysiol 1987;58: Aimone LD, Gebhart GF. Stimulation-produced spinal inhibition from the midbrain in the rat is mediated by an excitatory amino acid transmitter in the medial medulla. J Neurosci 1986;6: Jensen TS, Yaksh TL. Spinal monoamine and opiate systems partly mediate the antinociceptive effects produced by glutamate at brainstem sites. Brain Res 1984;321: Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 1991;14: Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A 1999;96:
13 April 2002 FACILITATION OF VISCERAL PAIN Ren K, Randich A, Gebhart GF. Vagal afferent modulation of a nociceptive reflex in rats: involvement of spinal opioid and monoamine receptors. Brain Res 1988;446: Gebhart GF. Modulatory effects of descending systems on spinal dorsal horn neurons. In: Yaksh TL, ed. Spinal afferent processing. Plenum, 1986: Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol 2002;88:in press. 40. Carstens E, Klumpp D, Zimmermann M. Differential inhibitory effects of medial and lateral midbrain stimulation of spinal neuronal discharges to noxious skin heating in the cat. J Neurophysiol 1980;43: Fields HL. Is there a facilitating component to central pain modulation? Am Pain Soc J 1992;1: Gebhart GF. Can endogenous systems produce pain? Am Pain Soc J 1992;1: Received September 13, Accepted December 5, Address requests for reprints to: G. F. Gebhart, Ph.D., Department of Pharmacology, College of Medicine, Bowen Science Building, The University of Iowa, Iowa City, Iowa gf-gebhart@uiowa.edu; fax: (319) Supported by NIH grant DA Dr. Zhuo s current address is: Departments of Anesthesiology, Anatomy and Neurobiology and Psychiatry, Washington University Pain Center, Washington University, School of Medicine, St. Louis, Missouri The authors thank Susan Birely for secretarial assistance and Michael Burcham for preparation of the graphics.
The Role of Dorsal Columns Pathway in Visceral Pain
 Physiol. Res. 53 (Suppl. 1): S125-S130, 2004 The Role of Dorsal Columns Pathway in Visceral Pain J. PALEČEK Department of Functional Morphology, Institute of Physiology, Academy of Sciences of the Czech
Physiol. Res. 53 (Suppl. 1): S125-S130, 2004 The Role of Dorsal Columns Pathway in Visceral Pain J. PALEČEK Department of Functional Morphology, Institute of Physiology, Academy of Sciences of the Czech
Receptors and Neurotransmitters: It Sounds Greek to Me. Agenda. What We Know About Pain 9/7/2012
 Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
Receptors and Neurotransmitters: It Sounds Greek to Me Cathy Carlson, PhD, RN Northern Illinois University Agenda We will be going through this lecture on basic pain physiology using analogies, mnemonics,
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System
 The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
The Pain System The Neural Basis of Nociceptive Transmission in the Mammalian Nervous System Pain and Headache Vol. 8 Series Editor Philip L. Gildenberg, Houston, Tex. KARGER S.Karger Basel Miinchen: Paris
Medical Neuroscience Tutorial
 Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Pain Pathways Medical Neuroscience Tutorial Pain Pathways MAP TO NEUROSCIENCE CORE CONCEPTS 1 NCC1. The brain is the body's most complex organ. NCC3. Genetically determined circuits are the foundation
Plasticity in Excitatory Amino Acid Receptor-Mediated Descending Pain Modulation after Inflammation
 0022-3565/02/3002-513 520$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 300, No. 2 Copyright 2002 by The American Society for Pharmacology and Experimental Therapeutics 4316/960579
0022-3565/02/3002-513 520$3.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 300, No. 2 Copyright 2002 by The American Society for Pharmacology and Experimental Therapeutics 4316/960579
Pain classifications slow and fast
 Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
Pain classifications slow and fast Fast Pain Slow Pain Sharp, pricking (Aδ) fiber Short latency Well localized Short duration Dull, burning (C) fiber Slower onset Diffuse Long duration Less emotional Emotional,
San Francisco Chronicle, June 2001
 PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
PAIN San Francisco Chronicle, June 2001 CONGENITAL INSENSITIVITY TO PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional)
Department of Neurology/Division of Anatomical Sciences
 Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Spinal Cord I Lecture Outline and Objectives CNS/Head and Neck Sequence TOPIC: FACULTY: THE SPINAL CORD AND SPINAL NERVES, Part I Department of Neurology/Division of Anatomical Sciences LECTURE: Monday,
Descending modulation in persistent pain: an update
 Pain 100 (2002) 1 6 Topical review Descending modulation in persistent pain: an update Ke Ren, Ronald Dubner* www.elsevier.com/locate/pain Department of Oral and Craniofacial Biological Sciences, Program
Pain 100 (2002) 1 6 Topical review Descending modulation in persistent pain: an update Ke Ren, Ronald Dubner* www.elsevier.com/locate/pain Department of Oral and Craniofacial Biological Sciences, Program
PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus. MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive
 PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
PAIN PAIN IS A SUBJECTIVE EXPERIENCE: It is not a stimulus MAJOR FEATURES OF THE PAIN EXPERIENCE: Sensory discriminative Affective (emotional) Cognitive MEASUREMENT OF PAIN: A BIG PROBLEM Worst pain ever
Supraspinal contributions to hyperalgesia
 Proc. Natl. Acad. Sci. USA Vol. 96, pp. 7687 7692, July 1999 Colloquium Paper This paper was presented at the National Academy of Sciences colloquium The Neurobiology of Pain, held December 11 13, 1998,
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 7687 7692, July 1999 Colloquium Paper This paper was presented at the National Academy of Sciences colloquium The Neurobiology of Pain, held December 11 13, 1998,
Somatic Sensory System I. Background
 Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Somatic Sensory System I. Background A. Differences between somatic senses and other senses 1. Receptors are distributed throughout the body as opposed to being concentrated at small, specialized locations
Chapter 16. Sense of Pain
 Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
Chapter 16 Sense of Pain Pain Discomfort caused by tissue injury or noxious stimulation, and typically leading to evasive action important /// helps to protect us lost of pain in diabetes mellitus = diabetic
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
SOMATOSENSORY SYSTEMS: Pain and Temperature Kimberle Jacobs, Ph.D. Sensory systems are afferent, meaning that they are carrying information from the periphery TOWARD the central nervous system. The somatosensory
211MDS Pain theories
 211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
211MDS Pain theories Definition In 1986, the International Association for the Study of Pain (IASP) defined pain as a sensory and emotional experience associated with real or potential injuries, or described
Pharmacology of Pain Transmission and Modulation
 Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
Pharmacology of Pain Transmission and Modulation 2 Jürg Schliessbach and Konrad Maurer Nociceptive Nerve Fibers Pain is transmitted to the central nervous system via thinly myelinated Aδ and unmyelinated
Andreea Ilinca Cotoi. A thesis submitted to the Centre for Neuroscience Studies. In conformity with the requirements for
 BEHAVIORAL AND NEURAL CORRELATES OF CONDITIONED PAIN MODULATION IN THE HUMAN BRAINSTEM AND CERVICAL SPINAL CORD USING FUNCTIONAL MAGNETIC RESONANCE IMAGING by Andreea Ilinca Cotoi A thesis submitted to
BEHAVIORAL AND NEURAL CORRELATES OF CONDITIONED PAIN MODULATION IN THE HUMAN BRAINSTEM AND CERVICAL SPINAL CORD USING FUNCTIONAL MAGNETIC RESONANCE IMAGING by Andreea Ilinca Cotoi A thesis submitted to
CHAPTER 10 THE SOMATOSENSORY SYSTEM
 CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
CHAPTER 10 THE SOMATOSENSORY SYSTEM 10.1. SOMATOSENSORY MODALITIES "Somatosensory" is really a catch-all term to designate senses other than vision, hearing, balance, taste and smell. Receptors that could
Characterization of Biphasic Modulation of Spinal Nociceptive Transmission by Neurotensin in the Rat Rostral Ventromedial Medulla
 Characterization of Biphasic Modulation of Spinal Nociceptive Transmission by Neurotensin in the Rat Rostral Ventromedial Medulla Department of Pharmacology, College of Medicine, University of Iowa, Iowa
Characterization of Biphasic Modulation of Spinal Nociceptive Transmission by Neurotensin in the Rat Rostral Ventromedial Medulla Department of Pharmacology, College of Medicine, University of Iowa, Iowa
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D.
 Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
Somatosensory Physiology (Pain And Temperature) Richard M. Costanzo, Ph.D. OBJECTIVES After studying the material of this lecture the student should be familiar with: 1. The relationship between nociception
The anatomy and physiology of pain
 The anatomy and physiology of pain Charlotte E Steeds Abstract Pain is an unpleasant experience that results from both physical and psychological responses to injury. A complex set of pathways transmits
The anatomy and physiology of pain Charlotte E Steeds Abstract Pain is an unpleasant experience that results from both physical and psychological responses to injury. A complex set of pathways transmits
Descending facilitation: From basic science to the treatment of chronic pain
 Review Article Descending facilitation: From basic science to the treatment of chronic pain Molecular Pain Volume 13: 1 12! The Author(s) 2017 Reprints and permissions: sagepub.com/journalspermissions.nav
Review Article Descending facilitation: From basic science to the treatment of chronic pain Molecular Pain Volume 13: 1 12! The Author(s) 2017 Reprints and permissions: sagepub.com/journalspermissions.nav
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007)
 Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Somatic Sensation (MCB160 Lecture by Mu-ming Poo, Friday March 9, 2007) Introduction Adrian s work on sensory coding Spinal cord and dorsal root ganglia Four somatic sense modalities Touch Mechanoreceptors
Sensory coding and somatosensory system
 Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Sensory coding and somatosensory system Sensation and perception Perception is the internal construction of sensation. Perception depends on the individual experience. Three common steps in all senses
Pain. Pain. Pain: One definition. Pain: One definition. Pain: One definition. Pain: One definition. Psyc 2906: Sensation--Introduction 9/27/2006
 Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Pain Pain Pain: One Definition Classic Paths A new Theory Pain and Drugs According to the international Association for the Study (Merskey & Bogduk, 1994), Pain is an unpleasant sensory and emotional experience
Fig Cervical spinal nerves. Cervical enlargement C7. Dural sheath. Subarachnoid space. Thoracic. Spinal cord Vertebra (cut) spinal nerves
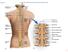 Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Fig. 13.1 C1 Cervical enlargement C7 Cervical spinal nerves Dural sheath Subarachnoid space Thoracic spinal nerves Spinal cord Vertebra (cut) Lumbar enlargement Medullary cone T12 Spinal nerve Spinal nerve
Pain and Temperature Objectives
 Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Pain and Temperature Objectives 1. Describe the types of sensory receptors that transmit pain and temperature. 2. Understand how axon diameter relates to transmission of pain and temp information. 3. Describe
Biomechanics of Pain: Dynamics of the Neuromatrix
 Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
Biomechanics of Pain: Dynamics of the Neuromatrix Partap S. Khalsa, D.C., Ph.D. Department of Biomedical Engineering The Neuromatrix From: Melzack R (1999) Pain Suppl 6:S121-6. NIOSH STAR Symposium May
Posterior White Column-Medial Lemniscal Pathway
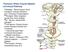 Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
Posterior White Column-Medial Lemniscal Pathway Modality: Discriminative Touch Sensation (include Vibration) and Conscious Proprioception Receptor: Most receptors except free nerve endings Ist Neuron:
1 The Physiology of Pain
 1 The Physiology of Pain Rohit Juneja and Siân Jaggar Introduction Definitions Key Messages Pain is still underdiagnosed and undertreated. Pain is a subjective experience and may even be present in the
1 The Physiology of Pain Rohit Juneja and Siân Jaggar Introduction Definitions Key Messages Pain is still underdiagnosed and undertreated. Pain is a subjective experience and may even be present in the
Internal Organisation of the Brainstem
 Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Internal Organisation of the Brainstem Major tracts and nuclei of the brainstem (Notes) The brainstem is the major pathway for tracts and houses major nuclei, that contain sensory, motor and autonomics
Somatosensory System. Steven McLoon Department of Neuroscience University of Minnesota
 Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Somatosensory System Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Dr. Riedl s review session this week: Tuesday (Oct 10) 4-5pm in MCB 3-146B 2 Sensory Systems Sensory
Physiological Society Symposium Nociceptors as Homeostatic Afferents: Central Processing
 Physiological Society Symposium Nociceptors as Homeostatic Afferents: Central Processing Distinct central representations of inescapable and escapable pain: observations and speculation Kevin A. Keay *
Physiological Society Symposium Nociceptors as Homeostatic Afferents: Central Processing Distinct central representations of inescapable and escapable pain: observations and speculation Kevin A. Keay *
Spinal Cord Organization. January 12, 2011
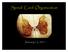 Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
Spinal Cord Organization January 12, 2011 Spinal Cord 31 segments terminates at L1-L2 special components - conus medullaris - cauda equina no input from the face Spinal Cord, Roots & Nerves Dorsal root
 Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Spinal Cord Tracts DESCENDING SPINAL TRACTS: Are concerned with somatic motor function, modification of ms. tone, visceral innervation, segmental reflexes. Main tracts arise form cerebral cortex and others
Separate populations of neurons in the rostral ventromedial medulla project to the spinal cord and to the dorsolateral pons in the rat
 Pacific University CommonKnowledge Faculty Scholarship (PHRM) School of Pharmacy 2004 Separate populations of neurons in the rostral ventromedial medulla project to the spinal cord and to the dorsolateral
Pacific University CommonKnowledge Faculty Scholarship (PHRM) School of Pharmacy 2004 Separate populations of neurons in the rostral ventromedial medulla project to the spinal cord and to the dorsolateral
SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE
 Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Dental Neuroanatomy Thursday, February 3, 2011 Suzanne S. Stensaas, PhD SOMATIC SENSATION PART I: ALS ANTEROLATERAL SYSTEM (or SPINOTHALAMIC SYSTEM) FOR PAIN AND TEMPERATURE Reading: Waxman 26 th ed, :
Table of Contents: Chapter 1 The organization of the spinal cord Charles Watson and Gulgun Kayalioglu
 Table of Contents: Chapter 1 The organization of the spinal cord Charles Watson and Gulgun Kayalioglu The gross anatomy of the spinal cord Spinal cord segments Spinal nerves Spinal cord gray and white
Table of Contents: Chapter 1 The organization of the spinal cord Charles Watson and Gulgun Kayalioglu The gross anatomy of the spinal cord Spinal cord segments Spinal nerves Spinal cord gray and white
SOMATOSENSORY SYSTEMS AND PAIN
 SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
SOMATOSENSORY SYSTEMS AND PAIN A 21 year old man presented with a stab wound of the right side of the neck (Panel A). Neurological examination revealed right hemiplegia and complete right-sided loss of
The Somatosensory System
 The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
The Somatosensory System Reading: BCP Chapter 12 cerebrovortex.com Divisions of the Somatosensory System Somatosensory System Exteroceptive External stimuli Proprioceptive Body position Interoceptive Body
BIOH111. o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system
 BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Integumentary system o Skeletal system o Muscle system o Nervous system o Endocrine system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
Nervous System C H A P T E R 2
 Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Nervous System C H A P T E R 2 Input Output Neuron 3 Nerve cell Allows information to travel throughout the body to various destinations Receptive Segment Cell Body Dendrites: receive message Myelin sheath
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal:
 Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotorarea: uses external
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014
 UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
UNIVERSITY OF JORDAN FACULTY OF MEDICINE DEPARTMENT OF PHYSIOLOGY & BIOCHEMISTRY NEUROPHYSIOLOGY (MEDICAL), SPRING 2014 Textbook of Medical Physiology by: Guyton & Hall, 12 th edition 2011 Eman Al-Khateeb,
Theme 2: Cellular mechanisms in the Cochlear Nucleus
 Theme 2: Cellular mechanisms in the Cochlear Nucleus The Cochlear Nucleus (CN) presents a unique opportunity for quantitatively studying input-output transformations by neurons because it gives rise to
Theme 2: Cellular mechanisms in the Cochlear Nucleus The Cochlear Nucleus (CN) presents a unique opportunity for quantitatively studying input-output transformations by neurons because it gives rise to
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline
 Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Chapter 11 Introduction to the Nervous System and Nervous Tissue Chapter Outline Module 11.1 Overview of the Nervous System (Figures 11.1-11.3) A. The nervous system controls our perception and experience
Cortical Control of Movement
 Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
Strick Lecture 2 March 24, 2006 Page 1 Cortical Control of Movement Four parts of this lecture: I) Anatomical Framework, II) Physiological Framework, III) Primary Motor Cortex Function and IV) Premotor
SENSORY (ASCENDING) SPINAL TRACTS
 SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
SENSORY (ASCENDING) SPINAL TRACTS Dr. Jamila El-Medany Dr. Essam Eldin Salama OBJECTIVES By the end of the lecture, the student will be able to: Define the meaning of a tract. Distinguish between the different
Lecturer. Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014
 Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
Lecturer Prof. Dr. Ali K. Al-Shalchy MBChB/ FIBMS/ MRCS/ FRCS 2014 Dorsal root: The dorsal root carries both myelinated and unmyelinated afferent fibers to the spinal cord. Posterior gray column: Long
themes G. F. GEBHART Department of Pharmacology, The University of Iowa, College of Medicine, Iowa City, Iowa 52242
 Am J Physiol Gastrointest Liver Physiol 278: G834 G838, 2000. themes Pathobiology of Visceral Pain: Molecular Mechanisms and Therapeutic Implications IV. Visceral afferent contributions to the pathobiology
Am J Physiol Gastrointest Liver Physiol 278: G834 G838, 2000. themes Pathobiology of Visceral Pain: Molecular Mechanisms and Therapeutic Implications IV. Visceral afferent contributions to the pathobiology
Pain Pathways. Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH
 Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Pain Pathways Dr Sameer Gupta Consultant in Anaesthesia and Pain Management, NGH Objective To give you a simplistic and basic concepts of pain pathways to help understand the complex issue of pain Pain
Lesson 33. Objectives: References: Chapter 16: Reading for Next Lesson: Chapter 16:
 Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lesson 33 Lesson Outline: Nervous System Structure and Function Neuronal Tissue Supporting Cells Neurons Nerves Functional Classification of Neuronal Tissue Organization of the Nervous System Peripheral
Lecture VIII. The Spinal Cord, Reflexes and Brain Pathways!
 Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Reflexes and Brain Bio 3411! Monday!! 1! Readings! NEUROSCIENCE 5 th ed: Review Chapter 1 pp. 11-21;!!Read Chapter 9 pp. 189-194, 198! THE BRAIN ATLAS 3 rd ed:! Read pp. 4-17 on class web site! Look at
Human Anatomy. Autonomic Nervous System
 Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit. Supplementary Information. Adam W. Hantman and Thomas M.
 Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
Clarke's Column Neurons as the Focus of a Corticospinal Corollary Circuit Supplementary Information Adam W. Hantman and Thomas M. Jessell Supplementary Results Characterizing the origin of primary
SOMATOSENSORY SYSTEMS
 SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
SOMATOSENSORY SYSTEMS Schematic diagram illustrating the neural pathways that convey somatosensory information to the cortex and, subsequently, to the motor system. Double arrows show reciprocal connections.
I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts.
 Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Descending Tracts I: To describe the pyramidal and extrapyramidal tracts. II: To discuss the functions of the descending tracts. III: To define the upper and the lower motor neurons. 1. The corticonuclear
Isoflurane can indirectly depress lumbar dorsal horn activity in the goat via action within the brain
 British Journal of Anaesthesia 82 (2): 244 9 (1999) Isoflurane can indirectly depress lumbar dorsal horn activity in the goat via action within the brain S. Jinks 1, J. F. Antognini 2 *, E. Carstens 1,
British Journal of Anaesthesia 82 (2): 244 9 (1999) Isoflurane can indirectly depress lumbar dorsal horn activity in the goat via action within the brain S. Jinks 1, J. F. Antognini 2 *, E. Carstens 1,
Chapter 16. APR Enhanced Lecture Slides
 Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Spinal Cord Protection. Chapter 13 The Spinal Cord & Spinal Nerves. External Anatomy of Spinal Cord. Structures Covering the Spinal Cord
 Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Spinal Cord Protection Chapter 13 The Spinal Cord & Spinal Nerves We are only going to cover Pages 420-434 and 447 Together with brain forms the CNS Functions spinal cord reflexes integration (summation
Chapter 17 Nervous System
 Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Chapter 17 Nervous System 1 The Nervous System Two Anatomical Divisions Central Nervous System (CNS) Brain and Spinal Cord Peripheral Nervous System (PNS) Two Types of Cells Neurons Transmit nerve impulses
Human Anatomy. Spinal Cord and Spinal Nerves
 Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
Human Anatomy Spinal Cord and Spinal Nerves 1 The Spinal Cord Link between the brain and the body. Exhibits some functional independence from the brain. The spinal cord and spinal nerves serve two functions:
PAIN MODULATION. numerical value. adjectives. DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH
 PAIN MODULATION numerical value adjectives DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH OBJECTIVES At the end of this lecture you should be able to describe:
PAIN MODULATION numerical value adjectives DR SYED SHAHID HABIB Professor & Consultant Dept. of Physiology College of Medicine & KKUH OBJECTIVES At the end of this lecture you should be able to describe:
Anatomical Substrates of Somatic Sensation
 Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Anatomical Substrates of Somatic Sensation John H. Martin, Ph.D. Center for Neurobiology & Behavior Columbia University CPS The 2 principal somatic sensory systems: 1) Dorsal column-medial lemniscal system
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
*Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord.
 *somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
*somatic sensations : PAIN *Anteriolateral spinothalamic tract (STT) : a sensory pathway that is positioned anteriorly and laterally in the spinal cord. *This pathway carries a variety of sensory modalities:
Thalamus and Sensory Functions of Cerebral Cortex
 Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Thalamus and Sensory Functions of Cerebral Cortex I: To describe the functional divisions of thalamus. II: To state the functions of thalamus and the thalamic syndrome. III: To define the somatic sensory
Does intravenous administration of GABA A receptor antagonists induce both descending antinociception and touch-evoked allodynia?
 Pain 76 (1998) 327 336 Does intravenous administration of GABA A receptor antagonists induce both descending antinociception and touch-evoked allodynia? Natsu Koyama*, Fumihiko Hanai, Toshikatsu Yokota
Pain 76 (1998) 327 336 Does intravenous administration of GABA A receptor antagonists induce both descending antinociception and touch-evoked allodynia? Natsu Koyama*, Fumihiko Hanai, Toshikatsu Yokota
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Spinal Cord Injury Pain. Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018
 Spinal Cord Injury Pain Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018 Objectives At the conclusion of this session, participants should be able to: 1. Understand the difference between nociceptive
Spinal Cord Injury Pain Michael Massey, DO CentraCare Health St Cloud, MN 11/07/2018 Objectives At the conclusion of this session, participants should be able to: 1. Understand the difference between nociceptive
Auditory and Vestibular Systems
 Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Auditory and Vestibular Systems Objective To learn the functional organization of the auditory and vestibular systems To understand how one can use changes in auditory function following injury to localize
Neural Basis of Motor Control
 Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Neural Basis of Motor Control Central Nervous System Skeletal muscles are controlled by the CNS which consists of the brain and spinal cord. Determines which muscles will contract When How fast To what
Spinal Interneurons. Control of Movement
 Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
Control of Movement Spinal Interneurons Proprioceptive afferents have a variety of termination patterns in the spinal cord. This can be seen by filling physiologically-identified fibers with HRP, so their
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy
 By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
By Dr. Saeed Vohra & Dr. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific
Neural Integration I: Sensory Pathways and the Somatic Nervous System
 15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
15 Neural Integration I: Sensory Pathways and the Somatic Nervous System PowerPoint Lecture Presentations prepared by Jason LaPres Lone Star College North Harris An Introduction to Sensory Pathways and
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D.
 SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
SOMATOSENSORY SYSTEMS: Conscious and Non-Conscious Proprioception Kimberle Jacobs, Ph.D. Divisions of Somatosensory Systems The pathways that convey sensory modalities from the body to consciousness are
Arterial Blood Supply
 Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
Arterial Blood Supply Brain is supplied by pairs of internal carotid artery and vertebral artery. The four arteries lie within the subarachnoid space Their branches anastomose on the inferior surface of
ANAT2010. Concepts of Neuroanatomy (II) S2 2018
 ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
ANAT2010 Concepts of Neuroanatomy (II) S2 2018 Table of Contents Lecture 13: Pain and perception... 3 Lecture 14: Sensory systems and visual pathways... 11 Lecture 15: Techniques in Neuroanatomy I in vivo
Descending Facilitation From the Rostral Ventromedial Medulla Maintains Visceral Pain in Rats With Experimental Pancreatitis
 GASTROENTEROLOGY 2006;130:2155 2164 Descending Facilitation From the Rostral Ventromedial Medulla Maintains Visceral Pain in Rats With Experimental Pancreatitis LOUIS P. VERA PORTOCARRERO, JENNIFER X.
GASTROENTEROLOGY 2006;130:2155 2164 Descending Facilitation From the Rostral Ventromedial Medulla Maintains Visceral Pain in Rats With Experimental Pancreatitis LOUIS P. VERA PORTOCARRERO, JENNIFER X.
Spinal cord. We have extension of the pia mater below L1-L2 called filum terminale
 Spinal cord Part of the CNS extend from foramen magnum to the level of L1-L2 (it is shorter than the vertebral column) it is covered by spinal meninges. It is cylindrical in shape. It s lower end become
Spinal cord Part of the CNS extend from foramen magnum to the level of L1-L2 (it is shorter than the vertebral column) it is covered by spinal meninges. It is cylindrical in shape. It s lower end become
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves
 Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
Human Anatomy - Problem Drill 11: The Spinal Cord and Spinal Nerves Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper as needed,
AKA a painful lecture by Colleen Blanchfield, MD Full Circle Neuropsychiatric Wellness Center
 A lecture on PAIN AKA a painful lecture by Colleen Blanchfield, MD Full Circle Neuropsychiatric Wellness Center 1 Overview of the lecture Anatomy of the Pain Tract How a painful stimulus travels from the
A lecture on PAIN AKA a painful lecture by Colleen Blanchfield, MD Full Circle Neuropsychiatric Wellness Center 1 Overview of the lecture Anatomy of the Pain Tract How a painful stimulus travels from the
Chapter 13. The Spinal Cord & Spinal Nerves. Spinal Cord. Spinal Cord Protection. Meninges. Together with brain forms the CNS Functions
 Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. Pain is always subjective
 Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
Pain & Acupuncture What is Pain? An unpleasant sensory and emotional experience associated with actual or potential tissue damage. NOCICEPTION( the neural processes of encoding and processing noxious stimuli.)
Module H NERVOUS SYSTEM
 Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Module H NERVOUS SYSTEM Topic from General functions of the nervous system Organization of the nervous system from both anatomical & functional perspectives Gross & microscopic anatomy of nervous tissue
Gross Morphology of Spinal Cord
 Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
Gross Morphology of Spinal Cord Lecture Objectives Describe the gross anatomical features of the spinal cord. Describe the level of the different spinal segments compared to the level of their respective
Motor Control, Pain, Somatic Dysfunction, Core Stability. Richard G. Schuster, DO Shawn Kerger, DO, FAOASM 19 September 2016 OMED 2016 Anaheim, CA
 Motor Control, Pain, Somatic Dysfunction, Core Stability Richard G. Schuster, DO Shawn Kerger, DO, FAOASM 19 September 2016 OMED 2016 Anaheim, CA How do we move? Decision to move (Executive or Cognitive
Motor Control, Pain, Somatic Dysfunction, Core Stability Richard G. Schuster, DO Shawn Kerger, DO, FAOASM 19 September 2016 OMED 2016 Anaheim, CA How do we move? Decision to move (Executive or Cognitive
Thermoreceptors (hot & cold fibers) Temperature gated Na+ channels. Adaptation in thermoreceptors
 Thermoreceptors (hot & cold fibers) Temperature gated Na+ channels Adaptation in thermoreceptors Response of a cold receptor Drugs can mimic sensory stimuli Response of taste nerve to cold water or menthol
Thermoreceptors (hot & cold fibers) Temperature gated Na+ channels Adaptation in thermoreceptors Response of a cold receptor Drugs can mimic sensory stimuli Response of taste nerve to cold water or menthol
Spinal Cord- Medulla Spinalis. Cuneyt Mirzanli Istanbul Gelisim University
 Spinal Cord- Medulla Spinalis Cuneyt Mirzanli Istanbul Gelisim University Spinal Column Supports the skull, pectoral girdle, upper limbs and thoracic cage by way of the pelvic girdle. Transmits body weight
Spinal Cord- Medulla Spinalis Cuneyt Mirzanli Istanbul Gelisim University Spinal Column Supports the skull, pectoral girdle, upper limbs and thoracic cage by way of the pelvic girdle. Transmits body weight
Joint Session with ACOFP, AOASM and AAO: Motor Control, Pain, Somatic Dysfunction, Core Stability. Shawn R. Kerger, DO, FAOSM Richard G.
 Joint Session with ACOFP, AOASM and AAO: Motor Control, Pain, Somatic Dysfunction, Core Stability Shawn R. Kerger, DO, FAOSM Richard G. Schuster, DO Motor Control, Pain, Somatic Dysfunction, Core Stability
Joint Session with ACOFP, AOASM and AAO: Motor Control, Pain, Somatic Dysfunction, Core Stability Shawn R. Kerger, DO, FAOSM Richard G. Schuster, DO Motor Control, Pain, Somatic Dysfunction, Core Stability
By the end of this lecture the students will be able to:
 UNIT VII: PAIN Objectives: By the end of this lecture the students will be able to: Review the concept of somatosensory pathway. Describe the function of Nociceptors in response to pain information. Describe
UNIT VII: PAIN Objectives: By the end of this lecture the students will be able to: Review the concept of somatosensory pathway. Describe the function of Nociceptors in response to pain information. Describe
J. Physiol. (I957) I35, (Received 20 July 1956) The interpretation ofthe experimental results ofthe preceding paper (Matthews
 263 J. Physiol. (I957) I35, 263-269 THE RELATIVE SENSITIVITY OF MUSCLE NERVE FIBRES TO PROCAINE BY PETER B. C. MATTHEWS AND GEOFFREY RUSHWORTH From the Laboratory of Physiology, University of Oxford (Received
263 J. Physiol. (I957) I35, 263-269 THE RELATIVE SENSITIVITY OF MUSCLE NERVE FIBRES TO PROCAINE BY PETER B. C. MATTHEWS AND GEOFFREY RUSHWORTH From the Laboratory of Physiology, University of Oxford (Received
Primary Functions. Monitor changes. Integrate input. Initiate a response. External / internal. Process, interpret, make decisions, store information
 NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
NERVOUS SYSTEM Monitor changes External / internal Integrate input Primary Functions Process, interpret, make decisions, store information Initiate a response E.g., movement, hormone release, stimulate/inhibit
Neural Control of Lower Urinary Tract Function. William C. de Groat University of Pittsburgh Medical School
 Neural Control of Lower Urinary Tract Function William C. de Groat University of Pittsburgh Medical School Disclosures Current funding: NIH Grants, DK093424, DK-091253, DK-094905, DK-090006. Other financial
Neural Control of Lower Urinary Tract Function William C. de Groat University of Pittsburgh Medical School Disclosures Current funding: NIH Grants, DK093424, DK-091253, DK-094905, DK-090006. Other financial
Virtually everyone has experienced pain in one
 Transfer of Advances in Sciences into Dental Education Recent Insights into Brainstem Mechanisms Underlying Craniofacial Pain Barry J. Sessle, B.D.S., M.D.S., B.Sc., Ph.D., F.R.S.C., D.Sc. (honorary) Abstract:
Transfer of Advances in Sciences into Dental Education Recent Insights into Brainstem Mechanisms Underlying Craniofacial Pain Barry J. Sessle, B.D.S., M.D.S., B.Sc., Ph.D., F.R.S.C., D.Sc. (honorary) Abstract:
Copyright McGraw-Hill Education. Permission required for reproduction or display. C1. Cervical spinal ner ves. Thor acic. T12 Spinal nerve rootlets
 Fig. 13.1 C1 Cervical enlar gem ent C7 Cervical spinal ner ves Dural sheath Subarachnoi d space Thor acic spinal ner ves Vertebra (cut) Lum bar enlar gem ent Medullar y T12 rootlets cone Posterior median
Fig. 13.1 C1 Cervical enlar gem ent C7 Cervical spinal ner ves Dural sheath Subarachnoi d space Thor acic spinal ner ves Vertebra (cut) Lum bar enlar gem ent Medullar y T12 rootlets cone Posterior median
Biology 218 Human Anatomy
 Chapter 21 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of Sensations (p. 652) 1. Sensation is the conscious or subconscious awareness of external or internal stimuli. 2. For a sensation
Chapter 21 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Overview of Sensations (p. 652) 1. Sensation is the conscious or subconscious awareness of external or internal stimuli. 2. For a sensation
discharge rate as intravesical pressure was raised. Some cells received inputs from only (Received 28 January 1981)
 J. Physiol. (1982), 322, pp. 21-34 21 With 5 text-figures Printed in Great Britain TWO GROUP OF SPINAL INTERNEURONES THAT RESPOND TO STIMULATION OF THE ABDOMINAL VISCERA OF THE CAT BY S. B. McMAHON AND
J. Physiol. (1982), 322, pp. 21-34 21 With 5 text-figures Printed in Great Britain TWO GROUP OF SPINAL INTERNEURONES THAT RESPOND TO STIMULATION OF THE ABDOMINAL VISCERA OF THE CAT BY S. B. McMAHON AND
Reticular Formation George R. Leichnetz, Ph.D.
 Reticular Formation George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the anatomical and functional organization of the brainstem reticular formation into three general regions: median (raphe), medial
Reticular Formation George R. Leichnetz, Ph.D. OBJECTIVES 1. To understand the anatomical and functional organization of the brainstem reticular formation into three general regions: median (raphe), medial
Neurobiological Basis of Acupuncture. Dr Marc Petitpierre Association Genevoise des Médecins Acupuncteurs
 Neurobiological Basis of Acupuncture Dr Marc Petitpierre Association Genevoise des Médecins Acupuncteurs Training Course in Sexual and Reproductive Health Research Geneva, February 2009 A misunderstanding
Neurobiological Basis of Acupuncture Dr Marc Petitpierre Association Genevoise des Médecins Acupuncteurs Training Course in Sexual and Reproductive Health Research Geneva, February 2009 A misunderstanding
