METHODS. David G. Armstrong, DPM, MD, PhD 1 ; William A. Marston, MD 2 ; Alexander M. Reyzelman, DPM 3 ; Robert S. Kirsner, MD, PhD 4
|
|
|
- Emery Butler
- 5 years ago
- Views:
Transcription
1 Wound Repair and Regeneration Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower extremity ulcers: A multicenter randomized-controlled trial David G. Armstrong, DPM, MD, PhD 1 ; William A. Marston, MD 2 ; Alexander M. Reyzelman, DPM 3 ; Robert S. Kirsner, MD, PhD 4 1. Southern Arizona Limb Salvage Alliance (SALSA), University of Arizona College of Medicine, Tuscon, Arizona, 2. Division of Vascular Surgery, Department of Surgery, University of North Carolina School of Medicine, Chapel Hill, North Carolina, 3. Department of Medicine, California School of Podiatric Medicine at Samuel Merritt University, Oakland, California, and 4. Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, Miami, Florida Reprint requests: David G. Armstrong, Southern Arizona Limb Salvage Alliance (SALSA), University of Arizona College of Medicine. Tel: ; Fax: ; armstrong@usa.net Manuscript received: August 9, 2010 Accepted in final form: November 9, 2010 DOI: /j X x ABSTRACT The purpose of this study was to compare the ultraportable mechanically powered Smart Negative Pressure (SNaP s ) Wound Care System to the traditional electrically powered Vacuum-Assisted Closure (VAC s ) Therapy System in the treatment of chronic lower extremity wounds. This 12-center randomizedcontrolled trial of patients with noninfected, nonischemic, nonplantar lower extremity wounds had enrolled 65 patients, as of January 5, 2010, at the time of a planned interim analysis. Subjects were randomly assigned to treatment with either the SNaP s or VAC s Systems. The trial evaluated treatment for up to 16 weeks or till complete closure was achieved. Fifty-three patients (N527 SNaP s, N526 VAC s ) completed at least 4 weeks of therapy. Thirty-three patients (N518 SNaP s, N515 VAC s ) completed the study with either healing or 16 weeks of therapy. At the time of planned interim analysis, no significant differences (p50.99) in the proportion of subjects healed between the two devices evaluated were found. In addition, the percent wound size reduction between treatment groups was not significantly different at 4, 8, 12, and 16 weeks, with noninferiority analysis at 4 weeks of treatment reaching the < 0.05 significance level ( n p50.019). These interim data suggest no difference in wound closure between the SNaP s System and the VAC s System in the population studied. We look forward to the final analysis results. Chronic wounds of the lower extremity, including diabetic foot ulcers and venous stasis ulcers, are a major healthcare problem. 1 4 Negative pressure wound therapy (NPWT) has benefit in treating these types of wounds with improved granulation tissue formation and decreased time to healing compared with modern moist dressings. 5 8 Numerous NPWT systems are available, including the widely used VAC s Therapy System. However, utilization of these systems may be limited by their weight and bulk, need for an electrical power source, difficult procurement process, cost, and impact on patient quality of life. This study evaluates the Smart Negative Pressure (SNaP s ) Wound Care System, a novel light weight NPWT device that does not require an electrically powered pump. 9 Instead, the SNaP s System utilizes specialized springs to generate a preset continuous subatmospheric pressure level to the wound bed. Previous studies have shown that negative pressure delivered by the SNaP s System has similar characteristics to the VAC s Therapy System in biomechanical testing and in an animal wound-healing model. 10 Most recently, a study comparing SNaP s -treated patients to modern treatment protocols for highly refractory lower extremity ulcers demonstrated healing time improvements similar to studies using electrically powered NPWT devices. 11 This study was developed to directly compare the SNaP s Wound Care System to the VAC s Therapy System for the treatment of lower extremity chronic wounds in a prospective, multicenter, randomized-controlled study design. Importantly, this study was designed as a noninferiority trial with predetermined primary endpoints and a planned interim analysis. METHODS This study recruited subjects from 12 sites in the United States and was conducted in compliance with the 1975 Declaration of Helsinki and under presiding institutional review board approval. The study was registered at clinicaltrials.gov under number: NCT Wounds that healed > 30% during the first week after enrollment where excluded from the study, and the total period from enrollment to initiation of NPWT therapy was 2 weeks for both treatment conditions. Subjects were evaluated on a weekly basis to complete wound closure (defined as complete reepithelialization without drainage) or for up to Wound Rep Reg (2011) c 2011 by the Wound Healing Society 173
2 16 weeks of therapy. Dressing changes were performed following manufacturer recommended instructions and (2x/week for SNaP s, 3x/week for VAC s, or more). Wound debridement was performed as part of standard wound care. The exact techniques used by the investigators to debride wounds was left to the individual investigators as long as it was not an advanced modality (such as ultrasound). The only stipulations were that the same techniques for a given wound type should be used for both treatment conditions and the maximum frequency of allowable debridement for each wound was 1/week. The Profore s (Smith & Nephew, Durham, NC, USA) four layer compression bandage system was used for all venous ulcer patients. The standard off-loading orthotics used by the participating study centers were utilized for diabetic patients in both treatment conditions. Wound sizes were evaluated using wound tracings captured by the Visitrak wound measurement system (Smith & Nephew). Inclusion criteria included patients aged 18 years; lower extremity venous ulcer or diabetic ulcer with a surface area < 100 cm 2 but > 1cm 2,and < 10 cm in widest diameter. Wounds were to have been present for > 30 days despite appropriate wound care before admission. Admission criteria also required adequate blood perfusion defined as either transcutaneous oxygen measurements of the dorsum of the foot > 30 mmhg, skin perfusion pressure > 30 mmhg, or an ankle/brachial index between 0.7 and 1.2. The wound wasrequiredtobeinalocationamendabletocreationofan airtight seal using the provided dressings. Exclusion criteria included active infection (redness, swelling, pain, purulent exudate), untreated osteomyelitis, pregnancy, allergies to wound care products used in the study, etiologies of the wound that included malignancy, burn, collagen vascular disease, sickle cell, vasculopathy, or pyoderma gangrenosum. Further grounds for exclusion included a diagnosis of Active Charcot arthropathy, wound location on toes or plantar surface of foot, uncontrolled hyperglycemia (HbA1C > 12%), end-stage renal disease requiring dialysis, active chemotherapy treatment, previous treatment with a NPWT device, growth factors, hyperbaric oxygen, or bioengineered tissue product within 30 days of enrollment. Patients were not enrolled if they exhibited > 30% wound surface area reduction in size at 1 week after the screening visit. Randomization Randomization was accomplished using blocks of numbers from Pocock. 12 Stratification was performed by treatment center and wound type. Sealed opaque envelopes contained the assigned treatment and patient identification number. Envelopes were sequentially numbered before clinical trial site distribution. At patient randomization, treatment was assigned on the basis of the next sequentially labeled envelope. Patients were randomized to receive either the VAC device (K.C.I., San Antonio, TX) or the SnAP s device (Spiracur Inc., Palo Alto, CA). Statistical analysis To establish noninferiority to traditional NPWT, this study was designed assuming 80% wound closure with an 18.5% standard deviation (derived from previous study wounds treated with the SNaP s System 13 ) for both groups at 16 weeks using a margin of noninferiority of 12.5%. An interim analysis was planned using an a of 0.005, and the a to be used for the final analysis was adjusted to to account for this interim analysis. The statistical method applied was the two-sample Student s t test assuming the two treatment groups have approximately the same percent decrease in wound area. Percent decrease in wound area was evaluated at 4, 8, 12, and 16 weeks. If a wound was completely healed or a subject had discontinued before completion, the last observation was carried forward for the intent-to-treat analysis. To further evaluate the treatment effect, a Kaplan Meier survival analysis was performed comparing completed healing between treatment groups. Primary healing analysis was performed on the Visitrak data and the reported wound closure outcomes. Statistical analyses were performed using SAS version 9.1. Secondary outcome analysis Secondary endpoints such as time for dressing change and patient exit survey data were collected, and statistical analysis was performed to evaluate any differences in outcomes. Serious adverse events, device-related adverse events, and complications were also collected and reported. RESULTS Sixty-five patients were enrolled at the time of the planned interim analysis. Fifty-three patients (N527 SNaP s, N526 VAC s ) completed at least 4 weeks of therapy. Thirty-three patients (N518 SNaP s, N515 VAC s ) completed the study with either healing or 16 weeks of therapy. Table 1 shows patient demographics and comorbidities. In addition, of those patients with diabetes, 69.3% of patient randomized to the SNaP s -treatment group and 72.4% of those patients randomized to the VAC s -treatment group had loss of pressure sensation found on five-point Semmes-Weinstein 10 G monofilament testing. Percent decrease in wound area The percent decrease in wound area as measured from Visitrak tracings was compared for those subjects randomized to either treatment with the SNaP s System or the VAC s System, and results are shown in Table 2. At 4 weeks, the SNaP s -treated subjects demonstrated noninferiority n < 0.05 ( n p50.019) with SNaP s -treated subjects reporting 51.22% 41.47% wound closure and the VAC s -treated subjects reporting 37.27% 47.31% wound closure. As this was performed as part of the interim analysis, this did not meet the stop-criteria per the O Brien-Fleming stopping rule ( n p < 0.005). At 4, 8, 12, and 16 weeks, there were no statistical differences identified between treatment groups with a similar mean percent reduction in wound area and standard deviation. (Table 2). This indicated that both treatments had a similar effect on the wounds; however, two VAC s -treated patients who had > 100% increase in their wound size during treatment were excluded in this analysis as outliers because inclusion of their data would have heavily skewed the mean reductions in wound size in favor of the SNaP s System and increased the variance in the data set. To 174 Wound Rep Reg (2011) c 2011 by the Wound Healing Society
3 Table 1. Demographics and comorbidities account for early subject withdrawal and other confounding elements in the intent-to-treat analysis, the data were analyzed based on those subjects who completed the entire study protocol (either healed or had follow-up data through 16 weeks of treatment). Again, there were no statistically significant differences ( n p50.611) found between treatment groups with SNaP s -treated patients having a reduction of 85.03% SD and VAC s -treated patients having a reduction of 91.11% SD Table 2. Percent decrease in wound area Week VAC s Mean (%) SD SNaP s VAC s Mean (%) SD SNaP s Age Wound duration (months) Initial wound size (cm 2 ) Male (%) Race (% white) Current smoker (%) Diabetes mellitus (%) Venous stasis disease (%) Lower extremity edema (%) Peripheral arterial disease (%) Peripheral neuropathy (%) Respiratory disease (COPD/asthma) (%) Renal disease (%) Cardiac disease (%) Anticoagulation/coumadin (%) Immobility (%) Steroid use (%) Immunosuppressed (%) Poor nutrition (%) Other morbidity (%) VAC s, Vacuum-Assisted Closure; SNaP s, Smart Negative Pressure. f n f is for a test of noninferiority with a 12.5% noninferiority margin. is for a test of a difference between treatment groups. n Statistically significant ( n p < 0.05). VAC s, Vacuum-Assisted Closure; SNaP s, Smart Negative Pressure. Proportion of wounds healed and Kaplan Meier survival curve The proportion of wounds healed at 4, 8, 12, and 16 weeks is provided in Table 3 and the Kaplan Meier estimates are shown in Figure 1. There was no significant difference (p50.99) in the proportion of subjects healed over time, indicating that the effect of the SNaP s System was not significantly different than that of the VAC s System in promoting complete wound closure in the population studied. Device application time and exit survey data Time from initiation of dressing application as measured from the time an open wound bed was ready for a dressing application to the delivery of successful negative pressure to the wound bed without leaks was recorded during dressing changes. Mean application time for the VAC s Therapy System was minutes (SD minutes), while mean application time for the SNaP s System was 8.58 minutes (SD 5.23 minutes). The mean application time for the SNaP s System was significantly shorter ( n p5 < ) than that of the VAC s System at all timepoints (Figure 2). Response data concerning the user experience with their NPWT device were available on 25 (12 VAC s -treated and 13 SNaP s -treated) subjects upon exit from the study. These questions covered areas such as activities of daily living, mobility, sleep, noise disruption, social interactions, pain and, and perceived effectiveness and satisfaction with NPWT device treatment (Table 4). Importantly, for those patients randomized to VAC s Therapy treatment, 83.3% of the subjects surveyed were using the Acti-VAC s System, the lightest and most portable VAC s Therapy System available at the time of patient enrollment. The remaining 16.7% had treatment with the Freedom VAC s, the second most portable VAC s Therapy System. There were no differences in reported pain, perceived effectiveness, and patient satisfaction between the devices used to apply negative pressure. However, the SNaP s System interfered less with overall activity, sleep, and social interactions than the VAC s System. Adverse events and incidence of infection The proportion of patients experiencing one or more device-related adverse events was similar between the VAC s and SNaP s treatment groups (Table 5). Percent of patients with clinically determined infection was 3.0% vs. 6.3%, maceration was 12.1% vs. 9.4%, and allergic reaction to dressing material 3.0% vs. 3.1% for VAC s and SNaP s groups, respectively. Other device-related adverse events reported included increase in wound size > 100%, Table 3. Proportion of subjects healed Proportion healed Weeks of treatment VAC s (%) SNaP s (%) VAC s, Vacuum-Assisted Closure; SNaP s, Smart Negative Pressure. Wound Rep Reg (2011) c 2011 by the Wound Healing Society 175
4 Figure 1. Kaplan Meier Diagrams showing no significant difference (p50.99) in the proportion of subjects healed between the the SnaP s System and VAC s Therapy System treatment groups. VAC s, Vacuum-Assisted Closure; SNaP s, Smart Negative Pressure; NPWT, negative pressure wound therapy. redness due to possible venous thrombus and periwound skin erythema and swelling for the VAC s group (15.2%), and pain at the wound site for the SNaP s group (3.1%). DISCUSSION At least three randomized-controlled trials exist demonstrating the use of the VAC s Therapy System is superior to standard care for diabetic foot and venous leg ulcers. 5,6,8 Although these studies were not without limitations, NPWT is now routinely used to treat these types of wounds and evidence for its effectiveness continues to grow. Many NPWT devices are now commercially available, but the Agency for Healthcare Research and Quality Mean Application Time (min) Device Application Time VAC SNaP Week of Treatment Figure 2. Device application time. Mean device application time at initiation and all follow-up time points. SNaP s, Smart Negative Pressure; VAC, Vacuum-Assisted Closure. report in 2009 found that there were no published studies directly comparing one NPWT system to another, nor published head-to-head comparisons that were able to identify a significant distinction of one NPWT system or component over another. 14 To our knowledge this protocol represents the first prospective multicenter randomized trial comparing the efficacy of two negative pressure therapy systems in chronic wounds. In this planned interim analysis, the data available to date did not find significant differences in wound-healing outcomes between the SNaP s System and the VAC s Therapy System in the treatment of chronic lower extremity ulcers. Wound size healing data further showed noninferiority for those patients completing at least 4 weeks of therapy. When interpreting these data, several caveats need to be considered. First, it must be emphasized that this is an interim anaylsis. As such, the of the noninferiority analysis did not meet the stop-criteria per the O Brien- Fleming stopping rule of n p < Furthermore, two VAC s -treated patients who had wound size increases during the study of > 100% were excluded as outliers in the wound size analysis. Also, despite randomization of patients to treatment conditions, there were demographic differences found between the two treatment group with more SNaP s -treated patients having diabetes mellitus and peripheral vascular disease and VAC s -treated patients having on average older and larger wounds. Lastly, the frequency of dressing changes was different between the two treatment groups, as each system had dressing changes performed according to manufacturer recommended frequencies. It is important to note that while the data analysis from this initial group of patients does suggest equivalent healing outcomes, the findings may be different when the entire patient cohort is enroled. 176 Wound Rep Reg (2011) c 2011 by the Wound Healing Society
5 Table 4. Exit survey data Yes No exact test Activities of daily living 1. Did use of the NPWT device interfere with your ability to perform normal daily activity? VAC s %(N) 58.3 (7) 41.7 (5) n n SNaP s %(N) 7.7 (1) 92.3 (12) 1 20% 21 40% 41 60% 2. What percentage of your normal activities was negatively impacted by use of the NPWT device? VAC s %(N) 14.3 (1) 28.6 (2) 57.1 (4) SNaP s %(N) (1) 0.0 (0) 0.0 (0) Strongly disagree Disagree Neutral Agree Strongly agree 3. It was a burden to come to the wound care clinic for my dressing change VAC s %(N) 8.3 (1) 25.0 (3) 41.7 (5) 25.0 (3) 0.0 (0) SNaP s %(N) 30.8 (4) 23.1 (3) 23.1 (3) 7.7 (1) 15.4 (2) 4. The use of the NPWT device restricted many of my normal activities VAC s %(N) 0.0 (0) 16.7 (2) 25.0 (3) 50.0 (6) 8.3 (1) n n SNaP s %(N) 61.5 (8) 7.7 (1) 15.4 (2) 15.4 (2) 0.0 (0) 5. The NPWT device was cumbersome and interfered with my mobility VAC s %(N) 0.0 (0) 25.0 (3) 33.3 (4) 41.7 (5) 0.0 (0) n 4.28E 04 n SNaP s %(N) 61.5 (8) 30.8 (4) 7.7 (1) 0.0 (0) 0.0 (0) 6. I was able to work and do my normal daily activities while being treated with the NPWT device VAC s %(N) 0.0 (0) 33.3 (4) 33.3 (4) 33.3 (4) 0.0 (0) n n SNaP s %(N) 0.0 (0) 0.0 (0) 7.7 (1) 46.2 (6) 46.2 (6) 7. The NPWT device was easy to use VAC s %(N) 0.0 (0) 16.7 (2) 8.3 (1) 75.0 (9) 0.0 (0) n n SNaP s %(N) 0.0 (0) 0.0 (0) 0.0 (0) 61.5 (8) 38.5 (5) Less active Stayed the same More active 8. After treatment with the NPWT, how did your overall activity level change? VAC s %(N) 58.3 (7) 41.7 (5) 0.0 (0) n n SNaP s %(N) 7.7 (1) 84.6 (11) 7.7 (1) Strongly disagree Disagree Neutral Agree Strongly agree 9. The NPWT device was light-weight and portable VAC s %(N) 0.0 (0) 8.3 (1) 25.0 (3) 66.7 (8) 0.0 (0) n 5.19E 04 n SNaP s %(N) 0.0 (0) 0.0 (0) 0.0 (0) 30.8 (4) 69.2 (9) 10. I wish the NPWT device was more light-weight and portable VAC s %(N) 0.0 (0) 0.0 (0) 33.3 (4) 58.3 (7) 8.3 (1) n 7.22E 04 n SNaP s %(N) 30.8 (4) 46.2 (6) 15.4 (2) 7.7 (1) 0.0 (0) Wound Rep Reg (2011) c 2011 by the Wound Healing Society 177
6 Never Rarely Several times per day Most of the time All of the time exact test Noise from device 1. How often did the noise from the NPWT device bother you? VAC s %(N) 33.3 (4) 33.3 (4) 16.7 (2) 16.7 (2) 0.0 (0) n 4.58E 04 n SNaP s %(N) (13) 0.0 (0) 0.0 (0) 0.0 (0) 0.0 (0) Strongly disagree Disagree Neutral Agree Strongly agree 2. The noise from the NPWT device was bothersome VAC s %(N) 8.3 (1) 33.3 (4) 33.3 (4) 25.0 (3) 0.0 (0) n 3.27E 05 n SNaP s %(N) 92.3 (12) 7.7 (1) 0.0 (0) 0.0 (0) 0.0 (0) Never 1 2 nights per week 2 4 nights per week 4 6 nights per week Every night exact test Sleep disruption 1. How often did the use of the NPWT device disrupt your sleep? VAC s %(N) 41.7 (5) 33.3 (4) 25.0 (3) 0.0 (0) 0.0 (0) n n SNaP s %(N) 84.6 (11) 7.7 (1) 0.0 (0) 0.0 (0) 7.7 (1) 2. Did the noise from the NPWT device bother you when you were trying to go to sleep? VAC s %(N) 58.3 (7) 16.7 (2) 25.0 (3) 0.0 (0) 0.0 (0) n n SNaP s %(N) (13) 0.0 (0) 0.0 (0) 0.0 (0) 0.0 (0) Strongly disagree Disagree Neutral Agree Strongly agree 3. The use of the NPWT device was disruptive to my sleep VAC s %(N) 0.0 (0) 41.7 (5) 16.7 (2) 41.7 (5) 0.0 (0) n 4.72E 04 n SNaP s %(N) 61.5 (8) 30.8 (4) 0.0 (0) 0.0 (0) 7.7 (1) Never Rarely About half the time Most of the time Always exact test Social situations 1. In social situations, did you feel that other people noticed the NPWT device? VAC s %(N) 0.0 (0) 25.0 (3) 16.7 (2) 33.3 (4) 25.0 (3) n 9.33E 05 n SNaP s %(N) 69.2 (9) (4) 0.0 (0) 0.0 (0) 0.0 (0) 2. In social situations, were you ever bothered by other people noticing your NPWT device? VAC s %(N) 33.3 (4) 25.0 (3) 33.3 (4) 0.0 (0) 8.33 (1) n n SNaP s %(N) 84.6 (11) 15.4 (2) 0.0 (0) 0.0 (0) 0.0 (0) Strongly disagree Disagree Neutral Agree Strongly agree 3. The NPWT device was rarely noticed by other people in social situations VAC s %(N) 16.7 (2) 33.3 (4) 8.33 (1) 41.7 (5) 0.0 (0) n n SNaP s %(N) 0.0 (0) 0.0 (0) 23.1 (3) 30.8 (4) 46.2 (6) 178 Wound Rep Reg (2011) c 2011 by the Wound Healing Society
7 N Sum of scores Expected sum of scores SD of sum Wilcoxon test t test Pain and 1. What was your level of pain associated with dressing changes for the NPWT device? VAC s %(N) n SNaP s %(N) What was your level of pain associated with just wearing the NPWT device? VAC s %(N) n SNaP s %(N) What was your overall level of pain associated with treatment with the NPWT device? VAC s %(N) SNaP s %(N) Strongly disagree Disagree Neutral Agree Strongly agree exact test 4. The NPWT was comfortable to wear VAC s %(N) 8.3 (1) 25.0 (3) 33.3 (4) 33.3 (4) 0.0 (0) n n SNaP s %(N) 0.0 (0) 7.7 (1) 7.7 (1) 38.5 (5) 46.2 (6) No Minimal Low level of Moderate level of High level of 5. What was your overall from using the NPWT device? VAC s %(N) 16.7 (2) 33.3 (4) 16.7 (2) 25.0 (3) 8.3 (1) SNaP s %(N) 53.9 (7) 23.1 (3) 15.4 (2) 7.7 (1) 0.0 (0) Strongly disagree Disagree Neutral Agree Strongly agree exact test Perceived effectiveness/satisfaction 1. The use of the NPWT device helped my wound to heal faster VAC s %(N) 0.0 (0) 8.3 (1) 16.7 (2) 33.3 (4) 41.7 (5) SNaP s %(N) 7.7 (1) 0.0 (0) 30.8 (4) 15.4 (2) 46.2 (6) 2. I would use the NPWT device again on another wound in the future VAC s %(N) 0.0 (0) 0.0 (0) 33.3 (4) 33.3 (4) 33.3 (4) SNaP s %(N) 0.0 (0) 0.0 (0) 0.0 (0) 38.5 (5) 61.5 (8) N Sum of scores Expected sum of scores SD of sum Wilcoxon test t test 3. What was your overall level of satisfaction concerning the NPWT device used to treat your wound? VAC s %(N) SNaP s %(N) n p < NPWT, negative pressure wound therapy; VAC s, Vacuum-Assisted Closure; SNaP s, Smart Negative Pressure. Wound Rep Reg (2011) c 2011 by the Wound Healing Society 179
8 Table 5. Patients with device-related adverse events Although no differences in wound-healing efficacy were shown, the SNaP s System did show significant advantages with regards to time required for dressing application and patient survey data. The SNaP s System was applied on average in less than half the time it takes to apply the VAC s System. It is important to note that time was measured till successful activation of NPWT was achieved; thus, the data more accurately describe ease of use for the system (both successful dressing application and operation of the negative pressure source) and not just dressing application alone. Furthermore, survey data found less detrimental impact on daily activities, overall mobility level, social interactions, and sleep. These factors can significantly affect patients psychological well-being and may have effects on compliance and overall outcomes In addition to comparing two systems of NPWT, the results of this study may also add insight into the role of foam and gauze and their relative effectiveness with NPWT. Should full results confirm the results of the interim analysis based on the demonstration of noninferiority between the gauze-based SNaP s System and the foambased VAC s System, it would support, for at least a subset of chronic wounds, similar efficacy for both foam and gauze interfaces. Wounds enroled in this study were nonplantar extremity wounds, predominantly secondary to venous disease, and were therefore, generally more superficial. It is possible that the use of a foam interface may prove to have advantages for deeper wounds with exposed structures. However, a study comparing SNaP s -treated subjects to modern treatment protocols for highly refractory lower extremity ulcers demonstrated healing time improvements similar to studies using foam-based powered NPWT devices. 11 We look forward to completion of this study to further confirm or refute these interim findings. ACKNOWLEDGMENTS This study was sponsored by a grant from Spiracur Inc., manufacturer of the SnAP s device. Two authors (Drs. Armstrong and Marston) have received research funding from both Spiracur and K.C.I. REFERENCES VAC s (n533) SnaP s (N532) Infection 3.0% (1) 6.3% (2) Maceration 12.1% (4) 9.4% (3) Allergic reaction to dressing 3.0% (1) 3.1% (1) Other 15.2% (5) 3.1% (1) VAC s, Vacuum-Assisted Closure; SNaP s, Smart Negative Pressure. 1. Wu SC, Armstrong DG. Clinical outcome of diabetic foot ulcers treated with negative pressure wound therapy and the transition from acute care to home care. Int Wound J 2008; 5 (Suppl. 2): Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005; 293: Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol 2002; 46: Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009; 17: Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005; 366: Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006; 44: ; discussion Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum-assisted closure: state of clinic art. Plast Reconstr Surg 2006; 117 (Suppl.): 127S 142S. 8. Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008; 31: Lerman B, Oldenbrook L, Ryu J, Fong KD, Schubart PJ. The SNaP wound care system: a case series using a novel ultraportable negative pressure wound therapy device for the treatment of diabetic lower extremity wounds. J Diabetes Sci Technol 2010; 4: Fong KD, Hu D, Eichstadt S, Gupta DM, Pinto M, Gurtner GC, Longaker MT, Lorenz HP. The SNaP system: biomechanical and animal model testing of a novel ultraportable negative-pressure wound therapy system. Plast Reconstr Surg 2010; 125: Lerman B, Oldenbrook L, Eichstadt SL, Ryu J, Fong KD, Schubart PJ. Evaluation of chronic wound treatment with the SNaP wound care system versus modern dressing protocols. Plast Reconstr Surg 2010; 126: Pocock S. Clinical trial (a practical approach). Chichester: John Wiley and Sons, Fong KD, Hu D, Eichstadt BS, et al. Initial clinical experience using a novel ultraportable negative pressure wound therapy device. Wounds 2010; 22: ECRI, Negative pressure wound therapy devices technology assessment. Agency for Healthcare Research and Quality. Baltimore, MD: U.S. Department of Health and Human Services, Moffatt CJ, Franks PJ, Doherty DC, Smithdale R, Steptoe A. Psychological factors in leg ulceration: a case control study. Br J Dermatol 2009; 161: Apostoli A, Caula C. Pain and basic functional activities in a group of patients with cutaneous wounds under V.A.C. therapy in hospital setting. Prof Inferm 2008; 61: Leijnen M, Steenvoorde P, van Doorn L, Zeillemaker AM, da Costa SA, Oskam J. Does VAC increase the risk of venous thromboembolism? J Wound Care 2007; 16: Keskin M, Karabekmez FE, Yilmaz E, Tosun Z, Savaci N. Vacuum-assisted closure of wounds and anxiety. Scand J Plast Reconstr Surg Hand Surg 2008; 42: Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. J Clin Nurs 2009; 18: Wound Rep Reg (2011) c 2011 by the Wound Healing Society
Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial
 Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial David G. Armstrong, DPM, MD, PhD 1 ; William A. Marston,
Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial David G. Armstrong, DPM, MD, PhD 1 ; William A. Marston,
DISCOVERY EXPRESS. j 75. William A. Marston, 1, * David G. Armstrong, 2 Alexander M. Reyzelman, 3 and Robert S. Kirsner 4 INTRODUCTION
 DISCOVERY EXPRESS A Multicenter Randomized Controlled Trial Comparing Treatment of Venous Leg Ulcers Using Mechanically Versus Electrically Powered Negative Pressure Wound Therapy William A. Marston, 1,
DISCOVERY EXPRESS A Multicenter Randomized Controlled Trial Comparing Treatment of Venous Leg Ulcers Using Mechanically Versus Electrically Powered Negative Pressure Wound Therapy William A. Marston, 1,
Negative pressure wound therapy (NPWT) is an important tool for
 ORIGINAL RESEARCH Initial Clinical Experience Using a Novel Ultraportable Negative Pressure Wound Therapy Device Kenton D. Fong, MD; 1,2 Dean Hu, MS; 2, Shaundra L. Eichstadt, BS; 1 Emily Gorell, MS; 4
ORIGINAL RESEARCH Initial Clinical Experience Using a Novel Ultraportable Negative Pressure Wound Therapy Device Kenton D. Fong, MD; 1,2 Dean Hu, MS; 2, Shaundra L. Eichstadt, BS; 1 Emily Gorell, MS; 4
Chronic wound treatment with negative-pressure RECONSTRUCTIVE
 RECONSTRUCTIVE Evaluation of Chronic Wound Treatment with the SNaP Wound Care System versus Modern Dressing Protocols Bruce Lerman, D.P.M. Leslie Oldenbrook, D.P.M. Shaundra L. Eichstadt, B.S. Justin Ryu,
RECONSTRUCTIVE Evaluation of Chronic Wound Treatment with the SNaP Wound Care System versus Modern Dressing Protocols Bruce Lerman, D.P.M. Leslie Oldenbrook, D.P.M. Shaundra L. Eichstadt, B.S. Justin Ryu,
The Use of the. in Clinical Practice
 The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
1/5. Introduction. Primary endpoint Time to reach readiness for closure by surgical intervention or left for closure by secondary intention
 1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
QUICK GUIDE SNAP THERAPY SYSTEM
 QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
Prediction of healing for post-operative diabetic foot wounds based on early wound area progression
 Diabetes Care Publish Ahead of Print, published online October 12, 2007 Prediction of healing for post-operative diabetic foot wounds based on early wound area progression Lawrence A Lavery, DPM, MPH 1
Diabetes Care Publish Ahead of Print, published online October 12, 2007 Prediction of healing for post-operative diabetic foot wounds based on early wound area progression Lawrence A Lavery, DPM, MPH 1
TECHNOLOGY ADVANCES. j 33. Theresa Hurd, 1, * Alan Rossington, 2 Paul Trueman, 2 and Jennifer Smith 2, *
 TECHNOLOGY ADVANCES A Retrospective Comparison of the Performance of Two Negative Pressure Wound Therapy Systems in the Management of Wounds of Mixed Etiology Theresa Hurd, 1, * Alan Rossington, 2 Paul
TECHNOLOGY ADVANCES A Retrospective Comparison of the Performance of Two Negative Pressure Wound Therapy Systems in the Management of Wounds of Mixed Etiology Theresa Hurd, 1, * Alan Rossington, 2 Paul
Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series
 Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series Theresa Hurd, RN, MScN, PhD; Paul Trueman, BA, MA; and Alan
Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series Theresa Hurd, RN, MScN, PhD; Paul Trueman, BA, MA; and Alan
SNAP THERAPY SYSTEM MONOGRAPH
 SNAP THERAPY SYSTEM MONOGRAPH Table of Contents Introduction...3 SNAP System Components Literature Review...4 Clinical Evidence Supporting SNAP System... 7 Technology for SNAP System.........................................11
SNAP THERAPY SYSTEM MONOGRAPH Table of Contents Introduction...3 SNAP System Components Literature Review...4 Clinical Evidence Supporting SNAP System... 7 Technology for SNAP System.........................................11
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
Negative Pressure Wound Therapy in the Treatment of Diabetic Foot Ulcers
 J Wound Ostomy Continence Nurs. 2014;41(3):233-237. Published by Lippincott Williams & Wilkins WOUND CARE Negative Pressure Wound Therapy in the Treatment of Diabetic Foot Ulcers A Systematic Review of
J Wound Ostomy Continence Nurs. 2014;41(3):233-237. Published by Lippincott Williams & Wilkins WOUND CARE Negative Pressure Wound Therapy in the Treatment of Diabetic Foot Ulcers A Systematic Review of
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy
 Ontario Health Technology Assessment Series 2010; Vol. 10, No. 22 Negative Pressure Wound Therapy An Evidence Update Presented to the Ontario Health Technology Advisory Committee in September 2010 December
Ontario Health Technology Assessment Series 2010; Vol. 10, No. 22 Negative Pressure Wound Therapy An Evidence Update Presented to the Ontario Health Technology Advisory Committee in September 2010 December
Journal of American Science 2014;10(12) Vacuum assisted closure [VAC] in management of diabetic foot
![Journal of American Science 2014;10(12) Vacuum assisted closure [VAC] in management of diabetic foot Journal of American Science 2014;10(12) Vacuum assisted closure [VAC] in management of diabetic foot](/thumbs/89/100997654.jpg) Vacuum assisted closure [VAC] in management of diabetic foot Hisham W. Anwar 1 and Ayman A. Al-Tramsy 2 1 Department of General Surgery, Faculty of Medicine, Al-Azhar University, Cairo, Egypt 2 Department
Vacuum assisted closure [VAC] in management of diabetic foot Hisham W. Anwar 1 and Ayman A. Al-Tramsy 2 1 Department of General Surgery, Faculty of Medicine, Al-Azhar University, Cairo, Egypt 2 Department
DO NOT DUPLICATE. Negative pressure wound therapy (NPWT) has revolutionized the
 Original research WOUNDS 2013;25(4):89 93 From the Aesthetic and Plastic Surgery Institute, University of California Irvine, Orange, CA and Long Beach Memorial Medical Center, Long Beach, CA Address correspondence
Original research WOUNDS 2013;25(4):89 93 From the Aesthetic and Plastic Surgery Institute, University of California Irvine, Orange, CA and Long Beach Memorial Medical Center, Long Beach, CA Address correspondence
Delayed Primary Closure of Diabetic Foot Wounds using the DermaClose RC Tissue Expander
 Delayed Primary Closure of Diabetic Foot Wounds using the DermaClose RC Tissue Expander TDavid L. Nielson, DPM 1, Stephanie C. Wu, DPM, MSc 2, David G. Armstrong, DPM,PhD 3 The Foot & Ankle Journal 1 (2):
Delayed Primary Closure of Diabetic Foot Wounds using the DermaClose RC Tissue Expander TDavid L. Nielson, DPM 1, Stephanie C. Wu, DPM, MSc 2, David G. Armstrong, DPM,PhD 3 The Foot & Ankle Journal 1 (2):
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: July 3,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: July 3,
Role of Negative Pressure Therapy in Healing of Diabetic Foot Ulcers
 Journal of Surgery 2015; 3(2-1): 31-35 Published online May 8, 2015 (http://www.sciencepublishinggroup.com/j/js) doi: 10.11648/j.js.s.2015030201.17 ISSN: 2330-0914 (Print); ISSN: 2330-0930 (Online) Role
Journal of Surgery 2015; 3(2-1): 31-35 Published online May 8, 2015 (http://www.sciencepublishinggroup.com/j/js) doi: 10.11648/j.js.s.2015030201.17 ISSN: 2330-0914 (Print); ISSN: 2330-0930 (Online) Role
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Negative Pressure Wound Therapy in the Outpatient Setting
 Negative Pressure Wound Therapy in the Outpatient Setting Policy Number: 1.01.16 Last Review: 9/2017 Origination: 7/2001 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Negative Pressure Wound Therapy in the Outpatient Setting Policy Number: 1.01.16 Last Review: 9/2017 Origination: 7/2001 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Disclosures. Outpatient NPWT Options Free up Hospital Beds, but Do They Work? Objectives. Clinically Effective: Does it Work?
 4/16/16 Disclosures Consultant, Volcano Corporation Outpatient Options Free up Hospital Beds, but Do They Work? UCSF Vascular Symposium 16 Jonathan Labovitz, DPM Medical Director, Foot & Ankle Center Associate
4/16/16 Disclosures Consultant, Volcano Corporation Outpatient Options Free up Hospital Beds, but Do They Work? UCSF Vascular Symposium 16 Jonathan Labovitz, DPM Medical Director, Foot & Ankle Center Associate
Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine
 Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine Dr. Julian Vitse, Montellier University Hospital, France Negative Pressure Wound
Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine Dr. Julian Vitse, Montellier University Hospital, France Negative Pressure Wound
ACTIVE INCISION MANAGEMENT: A PLAN FOR PROTECTING YOUR SURGICAL RESULTS, YOUR PATIENTS AND YOUR HOSPITAL.
 ACTIVE INCISION MANAGEMENT: A PLAN FOR PROTECTING YOUR SURGICAL RESULTS, YOUR PATIENTS AND YOUR HOSPITAL. How active hands-on involvement in managing the incision healing process helps to protect your
ACTIVE INCISION MANAGEMENT: A PLAN FOR PROTECTING YOUR SURGICAL RESULTS, YOUR PATIENTS AND YOUR HOSPITAL. How active hands-on involvement in managing the incision healing process helps to protect your
Use of Non-Contact Low Frequency Ultrasound in Wound Care
 Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Haemoglobin. Granulox: TOPICAL HAEMOGLOBIN
 QUICK GUIDE Haemoglobin Granulox: TOPICAL HAEMOGLOBIN OXYGEN AND WOUND HEALING 1 Oxygen is needed at all phases of wound healing Insufficient wound tissue oxygenation is a major risk factor for delayed
QUICK GUIDE Haemoglobin Granulox: TOPICAL HAEMOGLOBIN OXYGEN AND WOUND HEALING 1 Oxygen is needed at all phases of wound healing Insufficient wound tissue oxygenation is a major risk factor for delayed
CASE 1: TYPE-II DIABETIC FOOT ULCER
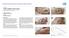 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology
 A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
V.A.C. Therapy Patient Guide. Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you.
 V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
Oxygen is required for normal cellular
 Article The role of topical oxygen therapy in the management of diabetic foot ulcer s in Scotland: round table recommendations Citation: Stang D, Young M, Wilson D et al (18) The role of topical oxygen
Article The role of topical oxygen therapy in the management of diabetic foot ulcer s in Scotland: round table recommendations Citation: Stang D, Young M, Wilson D et al (18) The role of topical oxygen
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 7/1/2018 Section: DME Policy No: 377 Medical Officer 7/1/18 Date Technology Assessment Committee Approved Date: 10/10; 10/13; 9/14: 9/15; 4/16 Medical Policy Committee Approved Date: 3/03;
Effective Date: 7/1/2018 Section: DME Policy No: 377 Medical Officer 7/1/18 Date Technology Assessment Committee Approved Date: 10/10; 10/13; 9/14: 9/15; 4/16 Medical Policy Committee Approved Date: 3/03;
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Efficacy of Vacuum-Assisted Closure Therapy versus Conventional Povidone Iodine Dressing in
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Efficacy of Vacuum-Assisted Closure Therapy versus Conventional Povidone Iodine Dressing in
JMSCR Vol 06 Issue 03 Page March 2018
 www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
Coverage Summary. Wound Treatments
 Coverage Summary Wound Treatments Policy Number: W-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 02/18/2009 Approved by: UnitedHealthcare Medicare Benefit Interpretation
Coverage Summary Wound Treatments Policy Number: W-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 02/18/2009 Approved by: UnitedHealthcare Medicare Benefit Interpretation
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP058 Section: Medical Benefit Policy Subject: Negative Pressure Wound Therapy I. Policy: Negative Pressure Wound Therapy II. Purpose/Objective: To provide a policy
POLICIES AND PROCEDURE MANUAL Policy: MP058 Section: Medical Benefit Policy Subject: Negative Pressure Wound Therapy I. Policy: Negative Pressure Wound Therapy II. Purpose/Objective: To provide a policy
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude
 Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
Mark Q. Niederauer, PhD 1, Joel E. Michalek, PhD 2, and David G. Armstrong, DPM, MD, PhD 3. Symposium/Special Issue
 695574DSTXXX10.1177/1932296817695574Journal of Diabetes Science and TechnologyNiederauer et al research-article2017 Symposium/Special Issue A Prospective, Randomized, Double-Blind Multicenter Study Comparing
695574DSTXXX10.1177/1932296817695574Journal of Diabetes Science and TechnologyNiederauer et al research-article2017 Symposium/Special Issue A Prospective, Randomized, Double-Blind Multicenter Study Comparing
Venous Leg Ulcers. Care for Patients in All Settings
 Venous Leg Ulcers Care for Patients in All Settings Summary This quality standard focuses on care for people who have developed or are at risk of developing a venous leg ulcer. The scope of the standard
Venous Leg Ulcers Care for Patients in All Settings Summary This quality standard focuses on care for people who have developed or are at risk of developing a venous leg ulcer. The scope of the standard
Use of Vacuum-assisted Wound Closure to Manage Limb Wounds in Patients Suffering from Acute Necrotizing Fasciitis
 Original Article Use of Vacuum-assisted Wound Closure to Manage Limb Wounds in Patients Suffering from Acute Necrotizing Fasciitis Wen-Shyan Huang, Shang-Chin Hsieh, Chun-Sheng Hsieh, Jen-Yu Schoung and
Original Article Use of Vacuum-assisted Wound Closure to Manage Limb Wounds in Patients Suffering from Acute Necrotizing Fasciitis Wen-Shyan Huang, Shang-Chin Hsieh, Chun-Sheng Hsieh, Jen-Yu Schoung and
NPWT Case Series EXPERIENCES WITH INVIA MOTION. Precious life Progressive care. Invia Motion Negative Pressure Wound Therapy
 NPWT Case Series EXPERIENCES WITH INVIA MOTION Invia Motion Negative Pressure Wound Therapy Precious life Progressive care npwt_case_booklet_a4.indd 1 18.12.13 13:17 Chronic sacral pressure ulcer Case
NPWT Case Series EXPERIENCES WITH INVIA MOTION Invia Motion Negative Pressure Wound Therapy Precious life Progressive care npwt_case_booklet_a4.indd 1 18.12.13 13:17 Chronic sacral pressure ulcer Case
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Determining Wound Diagnosis and Documentation Tips Job Aid
 Determining Wound Diagnosis and Job Aid 1 Coding Is this a traumatic injury from an accident? 800 Codes - Injury Section of the Coding Manual Code by specific site of injury. Only use for accidents or
Determining Wound Diagnosis and Job Aid 1 Coding Is this a traumatic injury from an accident? 800 Codes - Injury Section of the Coding Manual Code by specific site of injury. Only use for accidents or
NEGATIVE PRESURE WOUND THERAPY PROGRAM
 NEGATIVE PRESURE WOUND THERAPY PROGRAM WWW.USTOMWOUNDCARE.COM 866-802-0006/901-619-8897 SUPPORT@USTOM.COM TABLE OF CONTENTS 1. 2. 3. 4. To place an order: 1. Please visit our website 2. Please email to
NEGATIVE PRESURE WOUND THERAPY PROGRAM WWW.USTOMWOUNDCARE.COM 866-802-0006/901-619-8897 SUPPORT@USTOM.COM TABLE OF CONTENTS 1. 2. 3. 4. To place an order: 1. Please visit our website 2. Please email to
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Vacuum Assisted Wound Closure (VAC) Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
Vacuum Assisted Wound Closure (VAC) Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
Vacuumed Assisted Closure
 Vacuumed Assisted Closure Louise Morris Lead Nurse in Tissue Viability Jackie Stephen-Haynes Consultant Nurse and senior Lecturer in Tissue Viability 2009 Aims and Objectives To develop an awareness of
Vacuumed Assisted Closure Louise Morris Lead Nurse in Tissue Viability Jackie Stephen-Haynes Consultant Nurse and senior Lecturer in Tissue Viability 2009 Aims and Objectives To develop an awareness of
Accelerate wound healing of acute and chronic wounds in patients with Diabetes: Experience from Mexico using supplementary haemoglobin spray.
 Accelerate wound healing of acute and chronic wounds in patients with Diabetes: Experience from Mexico using supplementary haemoglobin spray. Obdulia Lopez Lopez 1, Fredrik Elg 2 Peter Engels 3, 1 Universidad
Accelerate wound healing of acute and chronic wounds in patients with Diabetes: Experience from Mexico using supplementary haemoglobin spray. Obdulia Lopez Lopez 1, Fredrik Elg 2 Peter Engels 3, 1 Universidad
ULCERS 1/12/ million diabetics in the US (2012) Reamputation Rate 26.7% at 1 year 48.3% at 3 years 60.7% at 5 years
 Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
V.A.C. VeraFlo Therapy can help.
 WOUND CARE IS COMPLEX & COSTLY Therapy can help. PROBLEM Complex wounds pose challenges to clinicians and patients in terms of achieving desired outcomes while managing healthcare costs. 1-5 OUTCOMES &
WOUND CARE IS COMPLEX & COSTLY Therapy can help. PROBLEM Complex wounds pose challenges to clinicians and patients in terms of achieving desired outcomes while managing healthcare costs. 1-5 OUTCOMES &
Management of Complex Wounds with Vacuum Assisted Closure
 Management of Complex Wounds with Vacuum Assisted Closure Wendy McInnes Vascular / Wound Nurse Practitioner The Queen Elizabeth Hospital, Adelaide, South Australia Treasurer ANZSVN wendy.mcinnes@health.sa.gov.au
Management of Complex Wounds with Vacuum Assisted Closure Wendy McInnes Vascular / Wound Nurse Practitioner The Queen Elizabeth Hospital, Adelaide, South Australia Treasurer ANZSVN wendy.mcinnes@health.sa.gov.au
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Vacuum Assisted Wound Closure (VAC) Page 1 of 32 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
Vacuum Assisted Wound Closure (VAC) Page 1 of 32 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
Monitoring Prevent. Can Temperature. DFUs
 Can Temperature Monitoring Prevent DFUs Alexander Reyzelman DPM Associate Professor, California School of Podiatric Medicine at Samuel Merritt University Oakland, CA Co-Director, UCSF Center for Limb Preservation
Can Temperature Monitoring Prevent DFUs Alexander Reyzelman DPM Associate Professor, California School of Podiatric Medicine at Samuel Merritt University Oakland, CA Co-Director, UCSF Center for Limb Preservation
A Prospective Study of Negative Pressure Wound Therapy With Integrated Irrigation for the Treatment of Diabetic Foot Ulcers
 A Prospective Study of Negative Pressure Wound Therapy With Integrated Irrigation for the Treatment of Diabetic Foot Ulcers Charles M. Zelen, DPM, FACFAS, a,b Brian Stover, DPM, a David Nielson, DPM, a,b
A Prospective Study of Negative Pressure Wound Therapy With Integrated Irrigation for the Treatment of Diabetic Foot Ulcers Charles M. Zelen, DPM, FACFAS, a,b Brian Stover, DPM, a David Nielson, DPM, a,b
Review of Negative Pressure Therapy as a Treatment for Diabetic Foot Ulcer
 Review of Negative Pressure Therapy as a Treatment for Diabetic Foot Ulcer Clare Henry Faculty of Education, Health and Community School of Nursing and Allied Health Liverpool John Moores University Abstract
Review of Negative Pressure Therapy as a Treatment for Diabetic Foot Ulcer Clare Henry Faculty of Education, Health and Community School of Nursing and Allied Health Liverpool John Moores University Abstract
Negative pressure wound therapy in surgical wounds: a prospective comparative study
 International Surgery Journal Chandrashekar S et al. Int Surg J. 2017 Oct;4(10):3272-3276 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174102
International Surgery Journal Chandrashekar S et al. Int Surg J. 2017 Oct;4(10):3272-3276 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174102
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Simple, gentle and affordable. *smith&nephew V1STA Negative Pressure Wound Therapy
 Simple, gentle and affordable *smith&nephew V1STA Negative Pressure Wound Therapy Smith & Nephew s extensive presence and portfolio means that for every wound, at every stage, there is an appropriate solution.
Simple, gentle and affordable *smith&nephew V1STA Negative Pressure Wound Therapy Smith & Nephew s extensive presence and portfolio means that for every wound, at every stage, there is an appropriate solution.
A comprehensive study on effect of collagen dressing in diabetic foot ulcer
 Original Research Article A comprehensive study on effect of collagen dressing in diabetic foot ulcer Sivakumar 1, S. Shanmugam 2* 1 Associate Professor, 2 Senior Assistant Professor Department of General
Original Research Article A comprehensive study on effect of collagen dressing in diabetic foot ulcer Sivakumar 1, S. Shanmugam 2* 1 Associate Professor, 2 Senior Assistant Professor Department of General
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur
 VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
STUDY. complication of diabetes, affecting 12% to 15% of patients. during their lifetime. 1,2 Diabetic foot ulcers (DFUs) impair quality
 STUDY Advanced Biological Therapies for Diabetic Foot Ulcers Robert S. Kirsner, MD, PhD; Robert Warriner, MD; Michelle Michela, MS; Laure Stasik, BA; Katherine Freeman, PhD Objective: To assess the clinical
STUDY Advanced Biological Therapies for Diabetic Foot Ulcers Robert S. Kirsner, MD, PhD; Robert Warriner, MD; Michelle Michela, MS; Laure Stasik, BA; Katherine Freeman, PhD Objective: To assess the clinical
Protocol. Negative Pressure Wound Therapy in the Outpatient Setting
 Protocol Negative Pressure Wound Therapy in the Outpatient Setting (10116) Medical Benefit Effective Date: 07/01/18 Next Review Date: 05/19 Preauthorization No Review Dates: 11/07, 09/08, 09/09, 09/10,
Protocol Negative Pressure Wound Therapy in the Outpatient Setting (10116) Medical Benefit Effective Date: 07/01/18 Next Review Date: 05/19 Preauthorization No Review Dates: 11/07, 09/08, 09/09, 09/10,
Re: Draft Technology Assessment Negative Pressure Wound Therapy Technologies (WNDT0913)
 701 Pennsylvania Avenue, Ste. 800 Washington, DC 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org July 16, 2014 Via Electronic Mail Only AHRQ Scientific Resource Center, Oregon EPC Mail code:
701 Pennsylvania Avenue, Ste. 800 Washington, DC 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org July 16, 2014 Via Electronic Mail Only AHRQ Scientific Resource Center, Oregon EPC Mail code:
Case study: A targeted approach to healing complex wounds using the geko device.
 Case study: A targeted approach to healing complex wounds using the geko device. Authors: Mr Sameh Dimitri Consultant Vascular and Endovascular Surgeon MSc FRCS (Eng Edin) Nikki Pavey Physiotherapist at
Case study: A targeted approach to healing complex wounds using the geko device. Authors: Mr Sameh Dimitri Consultant Vascular and Endovascular Surgeon MSc FRCS (Eng Edin) Nikki Pavey Physiotherapist at
Negative-pressure wound therapy (NPWT) is
 WOU HEALING Negative-Pressure Wound Therapy: A Comprehensive Review of the Evidence Ersilia L. Anghel, BS, BA Paul J. Kim, DPM, MS, FACFAS Washington, D.C. Background: Negative-pressure wound therapy (NPWT)
WOU HEALING Negative-Pressure Wound Therapy: A Comprehensive Review of the Evidence Ersilia L. Anghel, BS, BA Paul J. Kim, DPM, MS, FACFAS Washington, D.C. Background: Negative-pressure wound therapy (NPWT)
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers
 A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound
 ORIGINAL ARTICLES Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound A Pilot Study John G. Fleischli, DPM* Terese J. Laughlin, DPM* Jeffery W. Fleischli,
ORIGINAL ARTICLES Equine Pericardium Collagen Wound Dressing in the Treatment of the Neuropathic Diabetic Foot Wound A Pilot Study John G. Fleischli, DPM* Terese J. Laughlin, DPM* Jeffery W. Fleischli,
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
4-layer compression bandaging system (includes microbe binding wound contact layer) Latex-free, 4-layer compression bandaging system
 JOBST Comprifore JOBST Comprifore at a glance: provides effective levels of sustained graduated compression provides built in safety and ease of application Insures compliance and maximum healing for cost
JOBST Comprifore JOBST Comprifore at a glance: provides effective levels of sustained graduated compression provides built in safety and ease of application Insures compliance and maximum healing for cost
Advancing the science of wound bed preparation
 Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
Jonathan Brown Assignment 2 November 11, 2010
 1 Jonathan Brown Assignment 2 November 11, 2010 2 The Effectiveness of Removable Walking Casts and Total Contact Casts in Decreasing Healing Times of Diabetic Foot Ulcers Prepared by: jonathan.brown@gbcpando.com
1 Jonathan Brown Assignment 2 November 11, 2010 2 The Effectiveness of Removable Walking Casts and Total Contact Casts in Decreasing Healing Times of Diabetic Foot Ulcers Prepared by: jonathan.brown@gbcpando.com
VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device
 VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device Calf Muscle Pump Dysfunction Therapy Increases blood flow, accelerates wound healing, and improves CVD and PAD symptoms Tomorrow s Technology
VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device Calf Muscle Pump Dysfunction Therapy Increases blood flow, accelerates wound healing, and improves CVD and PAD symptoms Tomorrow s Technology
A randomized controlled trial comparing low cost vacuum assisted dressings and conventional dressing methods in the management of diabetic foot ulcers
 International Surgery Journal Dsouza C et al. Int Surg J. 2017 Dec;4(12):3858-3865 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20175142
International Surgery Journal Dsouza C et al. Int Surg J. 2017 Dec;4(12):3858-3865 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20175142
Four Layer Versus Actico Cohesive Short Stretch Bandage Background
 Four Layer Versus Actico Cohesive Short Stretch Bandage Background Cochrane Systematic Review 6/2/01 "Five small studies found no difference in healing between multi-layer high compression (4-layer bandage
Four Layer Versus Actico Cohesive Short Stretch Bandage Background Cochrane Systematic Review 6/2/01 "Five small studies found no difference in healing between multi-layer high compression (4-layer bandage
Negative Pressure Wound Therapy in the Outpatient Setting. Original Policy Date 12:2013
 MP 1.01.11 Negative Pressure Wound Therapy in the Outpatient Setting Medical Policy Section Durable Medical Equipment Issue 12:2013` Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature
MP 1.01.11 Negative Pressure Wound Therapy in the Outpatient Setting Medical Policy Section Durable Medical Equipment Issue 12:2013` Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature
V.A.C. Therapy Safety Information
 Bringing Safety Home V.A.C. Therapy Safety Information Bleeding Precautions Dressing Change Frequency Foam Removal Important Information 2 Prior to use of V.A.C. Therapy System it is important for the
Bringing Safety Home V.A.C. Therapy Safety Information Bleeding Precautions Dressing Change Frequency Foam Removal Important Information 2 Prior to use of V.A.C. Therapy System it is important for the
Premier Health Plan considers Negative Pressure Wound Therapy (NPWT) in the home setting medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Level 2 Leg Ulcer Management Service. Service Level Agreement Background. Contents:
 Level 2 Leg Ulcer Management Service Service Level Agreement 2016-2019 Contents: 1. Background to Leg Ulcer Management Service 2. Service Details 3. Accreditation 4. Service Standards 5. Finance Details
Level 2 Leg Ulcer Management Service Service Level Agreement 2016-2019 Contents: 1. Background to Leg Ulcer Management Service 2. Service Details 3. Accreditation 4. Service Standards 5. Finance Details
Supporting healthcare professionals in taking control of the infection risk with ACTICOAT Flex TAKE CONTROL. of the infection risk in chronic wound
 Supporting healthcare professionals in taking control of the infection risk with ACTICOAT Flex TAKE CONTROL of the infection risk in chronic wound Introduction The impact of infection on patients is well
Supporting healthcare professionals in taking control of the infection risk with ACTICOAT Flex TAKE CONTROL of the infection risk in chronic wound Introduction The impact of infection on patients is well
Clinical assessment of diabetic foot in 5 minutes
 Clinical assessment of diabetic foot in 5 minutes Assoc. Prof. N. Tentolouris, MD 1 st Department of Propaedeutic Internal Medicine Medical School Laiko General Hospital Leading Innovative Vascular Education
Clinical assessment of diabetic foot in 5 minutes Assoc. Prof. N. Tentolouris, MD 1 st Department of Propaedeutic Internal Medicine Medical School Laiko General Hospital Leading Innovative Vascular Education
Definitions and criteria
 Several disciplines are involved in the management of diabetic foot disease and having a common vocabulary is essential for clear communication. Thus, based on a review of the literature, the IWGDF has
Several disciplines are involved in the management of diabetic foot disease and having a common vocabulary is essential for clear communication. Thus, based on a review of the literature, the IWGDF has
JMSCR Vol 06 Issue 04 Page April 2018
 www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i4.15 Study of Vaccum Assisted Closure
www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i4.15 Study of Vaccum Assisted Closure
NEGATIVE PRESSURE WOUND THERAPY
 NEGATIVE PRESSURE WOUND THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
NEGATIVE PRESSURE WOUND THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Hyperbarics in Diabetic Wound Care. Aurel Mihai, MD & Brian Kline, MD
 Hyperbarics in Diabetic Wound Care Aurel Mihai, MD & Brian Kline, MD Presentation Outline The Scope of the Problem Important Definitions Standard Wound Care Hyperbaric Oxygen as an Adjunct Diabetic Foot
Hyperbarics in Diabetic Wound Care Aurel Mihai, MD & Brian Kline, MD Presentation Outline The Scope of the Problem Important Definitions Standard Wound Care Hyperbaric Oxygen as an Adjunct Diabetic Foot
CHAPTER 16 LOWER EXTREMITY. Amanda K Silva, MD and Warren Ellsworth, MD, FACS
 CHAPTER 16 LOWER EXTREMITY Amanda K Silva, MD and Warren Ellsworth, MD, FACS The plastic and reconstructive surgeon is often called upon to treat many wound problems of the lower extremity. These include
CHAPTER 16 LOWER EXTREMITY Amanda K Silva, MD and Warren Ellsworth, MD, FACS The plastic and reconstructive surgeon is often called upon to treat many wound problems of the lower extremity. These include
Policy Specific Section: Medical Necessity and Investigational / Experimental. June 13, 2001 January 1, 2015
 Medical Policy Negative Pressure Wound Therapy in the Outpatient Setting Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Durable Medical Equipment Original Policy Date:
Medical Policy Negative Pressure Wound Therapy in the Outpatient Setting Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Durable Medical Equipment Original Policy Date:
Diabetes Care Publish Ahead of Print, published online December 27, 2007
 Diabetes Care Publish Ahead of Print, published online December 27, 2007 Comparison of Negative Pressure Wound Therapy Utilizing Vacuum-Assisted Closure to Advanced Moist Wound Therapy in the Treatment
Diabetes Care Publish Ahead of Print, published online December 27, 2007 Comparison of Negative Pressure Wound Therapy Utilizing Vacuum-Assisted Closure to Advanced Moist Wound Therapy in the Treatment
Original Article Honey Dressing in Healing of Foot Ulcers Pak Armed Forces Med J 2018; 68 (1): Umar Bashir, Rasikh Maqsood, Hassan Shabbir,
 Open Access Original Article Honey Dressing in Healing of Foot Ulcers Pak Armed Forces Med J 2018; 68 (1): 34-38 COMPARING NEGATIVE PRESSURE WOUND TREATMENT WITH HONEY DRESSING IN HEALING OF FOOT ULCERS
Open Access Original Article Honey Dressing in Healing of Foot Ulcers Pak Armed Forces Med J 2018; 68 (1): 34-38 COMPARING NEGATIVE PRESSURE WOUND TREATMENT WITH HONEY DRESSING IN HEALING OF FOOT ULCERS
Cigna Medical Coverage Policy
 Cigna Medical Coverage Policy Subject Negative Pressure Wound Therapy/Vacuum-Assisted Closure (VAC) for Nonhealing Wounds Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information...
Cigna Medical Coverage Policy Subject Negative Pressure Wound Therapy/Vacuum-Assisted Closure (VAC) for Nonhealing Wounds Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information...
Ray Norris, Rachel Henchy
 Use of low frequency ultrasound therapy in the treatment of recalcitrant leg ulcers: case series This series of case reports looks at the efficacy of low frequency ultrasound using the MIST Therapy System
Use of low frequency ultrasound therapy in the treatment of recalcitrant leg ulcers: case series This series of case reports looks at the efficacy of low frequency ultrasound using the MIST Therapy System
DIABETES AND THE AT-RISK LOWER LIMB:
 DIABETES AND THE AT-RISK LOWER LIMB: Shawn M. Cazzell Disclosure of Commercial Support: Dr. Shawn Cazzell reports the following financial relationships: Speakers Bureau: Organogenesis Grants/Research Support:
DIABETES AND THE AT-RISK LOWER LIMB: Shawn M. Cazzell Disclosure of Commercial Support: Dr. Shawn Cazzell reports the following financial relationships: Speakers Bureau: Organogenesis Grants/Research Support:
Diabetic/Neuropathic Foot Ulcer Assessment Guide South West Regional Wound Care Program Last Updated April 7,
 Developed in collaboration with the Wound Care Champions, Wound Care Specialists, Enterostomal Nurses, and South West Regional Wound Care Program (SWRWCP) members from Long Term Care Homes, Hospitals,
Developed in collaboration with the Wound Care Champions, Wound Care Specialists, Enterostomal Nurses, and South West Regional Wound Care Program (SWRWCP) members from Long Term Care Homes, Hospitals,
Defining Outcomes for Clinical Wound Research in Older Adults February 21, 2014
 Defining Outcomes for Clinical Wound Research in Older Adults February 21, 214 Harold Brem, MD Professor of Surgery Stony Brook University School of Medicine Chief, Division of Wound Healing and Regenerative
Defining Outcomes for Clinical Wound Research in Older Adults February 21, 214 Harold Brem, MD Professor of Surgery Stony Brook University School of Medicine Chief, Division of Wound Healing and Regenerative
National Clinical Conference 2018 Baltimore, MD
 National Clinical Conference 2018 Baltimore, MD No relevant financial relationships to disclose Wound Care Referral The patient has been maximized from a vascular standpoint. She has no other options.
National Clinical Conference 2018 Baltimore, MD No relevant financial relationships to disclose Wound Care Referral The patient has been maximized from a vascular standpoint. She has no other options.
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology
 The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
The Power of a Hydroconductive Wound Dressing with LevaFiber Technology The first step in healing a chronic wound is to detoxify it by removing slough, necrotic tissue, exudate and bacteria, while keeping
