Chronic wound treatment with negative-pressure RECONSTRUCTIVE
|
|
|
- Herbert Harrison
- 5 years ago
- Views:
Transcription
1 RECONSTRUCTIVE Evaluation of Chronic Wound Treatment with the SNaP Wound Care System versus Modern Dressing Protocols Bruce Lerman, D.P.M. Leslie Oldenbrook, D.P.M. Shaundra L. Eichstadt, B.S. Justin Ryu, B.A. Kenton D. Fong, M.D. Peter J. Schubart, M.D., Ph.D. San Jose, Stanford, and Sunnyvale, Calif. Chronic wound treatment with negative-pressure wound therapy is an important clinical tool, with multiple published studies reporting improved granulation tissue formation and decreased time to wound healing. 1 3 Numerous negative-pressure wound therapy systems are available on the market today, including the KCI (Kinetic Concepts, Inc., San Antonio, Texas) wound vacuum-assisted closure device and the Smith & Nephew (London, United Kingdom) Renasys systems. However, these systems have a number of drawbacks, including their size and bulk, noise, From the O Connor Wound Care Clinic, O Connor Hospital; the Divisions of Plastic and Reconstructive Surgery and Vascular Surgery, Department of Surgery, Stanford University School of Medicine, Stanford University; and Spiracur, Inc. Received for publication February 8, 2010; accepted April 6, Copyright 2010 by the American Society of Plastic Surgeons DOI: /PRS.0b013e3181ea4559 Background: Traditional negative-pressure wound therapy systems use an electrically powered pump to generate negative pressure at the wound bed. The SNaP Wound Care System is a novel, ultraportable device that delivers negativepressure wound therapy without the use of an electrically powered pump. Methods: At an outpatient wound care clinic, 21 subjects with difficult-to-treat lower extremity ulcers received treatment with the SNaP System and were evaluated for wound healing for up to 4 months. Outcomes were then compared with 42 patient-matched controls treated at the same center with modern wound care protocols that included the use of Apligraf, Regranex, and skin grafting. Results: In the SNaP-treated group, 100 percent of subjects demonstrated improvement in wound size and 86 percent (18 of 21) exhibited a statistically significant healing trend (p 0.05). Using Kaplan-Meier estimates of wound healing, SNaP-treated subjects healed in an average of days from the start of SNaP treatment and the matched controls healed in an average of days from the start of conventional treatment. This significantly faster healing time represents a 50 percent absolute reduction in time to healing (p ) for subjects treated with the SNaP device. Conclusions: The findings reported here for the SNaP Wound Care System are similar to published reports for powered negative-pressure wound therapy devices for the treatment of highly challenging lower extremity wounds. This study suggests that the SNaP Wound Care System may be a useful addition to the techniques available to the wound care clinician. (Plast. Reconstr. Surg. 126: 1253, 2010.) need for an electrical power source, difficult procurement process, and cost. This study evaluates the SNaP (Smart Negative Pressure) Wound Care System (Spiracur, Inc., Sunnyvale, Calif.) (Fig. 1), a novel ultraportable negative-pressure wound therapy device that does not require an electrically powered pump. Instead, the SNaP System uses specialized springs to generate continuous negative pressure at the wound bed. Because of the special configuration of the springs, a near constant level of negative pressure is delivered over time to the wound, even in the face of exudates. The SNaP System consists of five basic elements: the cartridge, activation/reset key, hydro- Disclosure: This work was supported by a research grant from Spiracur Inc. Dr. Fong and Mr. Ryu are employees of Spiracur Inc. None of the other authors has any financial relationship with the company
2 Plastic and Reconstructive Surgery October 2010 PATIENTS AND METHODS Prospective observational and retrospective match-controlled clinical studies were performed to evaluate the safety and efficacy of the SNaP System for the treatment of difficult lower extremity wounds. The studies were performed at the O Connor Wound Care Center in San Jose, California, with the approval of the O Connor Hospital Institutional Review Board. Fig. 1. The SNaP Wound Care System. The 125-mmHg cartridge is shown. colloid dressing layer with integrated nozzle and tubing, holster with strap, and an antimicrobial gauze wound interface layer. The cartridge is currently capable of delivering three different preset pressure levels ( 75 mmhg, 100 mmhg, and 125 mmhg), and is small enough to be worn on a patient s leg, arm, or belt and completely hidden under normal clothing. The cartridge portion of the device weighs less than 3 ounces and has a canister capacity of approximately 60 ml for wound exudates. Because there is no electrical pump, operation of the SNaP System is silent. The SNaP System is also entirely disposable, eliminating the added administrative and support costs of the current rental-based system used by most electrically powered pumps. Previous studies have shown that negativepressure delivery by the SNaP System has similar characteristics to powered pumps in biomechanical testing and an animal wound-healing model. 4 In addition, an earlier clinical series at an academic outpatient clinic demonstrated that the SNaP System can be used safely and effectively for treating a subset of less refractory chronic wounds. 5 The goal of this study was to evaluate the SNaP System for the treatment of more difficult chronic wounds of the lower extremity. Specifically, we wanted to evaluate the impact of integrating the SNaP System as an adjunct to modern wound treatment protocols for diabetic and venous ulcers using both prospective observational analysis and retrospective match-controlled comparisons Prospective Observational Study Potential subjects were screened for eligibility on referral to or during routine treatment visits at the O Connor Wound Care Center. Written informed consent was obtained for those patients who met the study eligibility criteria, and negativepressure wound therapy was initiated using the SNaP System. Subjects were followed for up to 4 months or to wound closure, whichever came first. Wound measurements, wound photographs, adverse events, and treatment data were captured on a biweekly basis. Patients were included in the study if they were older than 18 years, presented with a chronic wound on the lower extremity less than 10 cm in greatest diameter and greater than 1.5 cm in narrowest diameter, and surrounded by 2 cm or more of intact epithelium around the wound edges. In addition, the wound must not have healed following greater than 14 days of use of traditional treatments. Patients were excluded from the study if they had active wound infection, no pedal pulse assessable by Doppler, a history of malignancy at the wound site, or thick eschar at the base of the wound after débridement, or if the wound location was not amenable to forming a reliable dressing seal. Also, patients were excluded if the ulcers were attributable to inflammatory conditions (i.e., pyoderma gangrenosum, rheumatoid arthritis, vasculitis, cryoglobulinemia, necrobiosis lipoidica diabeticorum, lupus or pancreatic panniculitis, cryofibrinogenemia, calcinosis cutis, scleroderma, or Raynaud syndrome); the patients had current warfarin anticoagulation; had wounds with exposed bone, blood vessels, or tendon; or were pregnant. In addition, patients unable to give informed consent or comply with study procedures (including lack of telephone access) were excluded from the study. All outpatients underwent standard wound care clinic intake evaluations, including history, physical examination, and wound assessment. Débridement of necrotic tissue was performed ac-
3 Volume 126, Number 4 SNaP Wound Care System cording to standard of care for the specific wound type. Duration of negative-pressure wound therapy was determined by the treating clinician, and negative-pressure wound therapy was discontinued if the clinician felt that adequate healing had occurred to no longer require its use. For the prospectively treated SNaP subjects, photographic wound surface area analysis was performed using imaging software (UTHSCSA Image Tool, Version 3.0; University of Texas Health Sciences Center, San Antonio, Texas). These tracing data were used to provide a precise assessment of wound surface area. A linear regression model based on the number of days in the study was performed to evaluate the trend toward healing of wounds treated with the SNaP System. Because of the variability between subjects and wound characteristics in the SNaP-treated group, the analysis was performed on a per-patient basis. Retrospective Comparison Study In the retrospective phase of the study, patients treated at the O Connor Wound Care Center over the past 4 years were screened by means of chart review to identify retrospective controls that matched the wound characteristics and comorbidities of the SNaP-treated subjects. The O Connor Wound Treatment Database allowed for potential matches to be generated based on wound type and wound size. Once potential matches were identified, charts were reviewed individually to identify a match based on patient age Table 1. Study Demographics ( 10 years), wound type (venous or diabetic), wound age ( 25 percent), initial wound size ( 25 percent surface area), and the presence or absence of diabetes and peripheral vascular disease. To be considered a match, the retrospective subject had to satisfy each of the matching criteria for a specific SNaP-treated subject. Two unique matches were identified for each SNaPtreated subject, and after confirming the match, all available wound treatment and demographic data were captured retrospectively for comparative wound-treatment analysis. To minimize bias in the retrospective group, matches were generated before reviewing wound treatment data and patient outcomes. The SNaP-treated subjects and the matched controls were grouped into a treatment and a control group, respectively, and a Kaplan-Meier survival analysis was performed to evaluate the differences in time required for healing. 6,7 As no photographic or tracing data were available for the historical controls, surface area measurements and closure data from the patient chart were used for both groups to allow for more similar comparisons. Wound characteristics and demographics were also compared to evaluate the degree of similarity between the groups. Data analyses were performed using SAS version 9.0 (SAS Institute, Inc., Cary, N.C.). RESULTS Subjects were enrolled prospectively into the first phase of the study starting in August of 2008, Match (n 42) SNaP (n 21) p Matching characteristics Age, yr Male 45.2% (19/42) 42.9% (9/21) Diabetes 57.1% (24/42) 57.1% (12/21) PVD 42.9% (18/42) 47.6% (10/21) Wound type diabetic 50.0% (21/42) 47.6% (10/21) Wound type venous 50.0% (21/42) 52.4% (11/21) Wound length, mm (42) (21) Wound width, mm (42) (21) Wound depth, mm (42) (21) Wound age, yr (40*) (20*) Comorbidities Immunosuppressive/steroid use 9.5% (4/42) 23.8% (5/21) History of osteomyelitis 4.8% (2/42) 23.8% (5/21) Previous amputation 14.3% (6/42) 9.5% (2/21) Smoker 20.0% (8/40) 42.9% (9/21) History of cancer 14.3% (6/42) 4.8% (1/21) Pulmonary disease 9.5% (4/42) 19.0% (4/21) Hypertension 88.1% (37/42) 76.2% (16/21) Hyperlipidemia 42.9% (18/42) 52.4% (11/21) PVD, peripheral vascular disease. *Patient 018 and patient 018 s matched controls were excluded as outliers (no close matches were found for a 19-year-old SNaP-treated wound). Fisher s exact test was used because of small samples sizes in either one or both groups. 1255
4 Plastic and Reconstructive Surgery October 2010 Table 2. Regression Analysis on SNaP-Treated Subjects Patient ID Slope p * * * * * * * * * * * * * * * * * * *p and the last subject completed treatment with the SNaP System in September of There were a total of 36 subjects enrolled prospectively in the first phase of the study, and 21 subjects completed treatment with the SNaP device. Of the 15 subjects that did not complete the study, two subjects were hospitalized for unrelated medical issues and subsequently removed from the study, six subjects were not compliant with the protocol requirements and follow-up, and seven subjects had complications that required premature termination of SNaP treatment. Of these seven subjects, one had an allergic skin reaction to the hydrocolloid dressing, one developed a wound infection requiring discontinuation of therapy, one was discontinued after bleeding from débridement prevented reapplication of the device, one was discontinued because of worsening lower extremity edema, and three patients developed maceration severe enough to require discontinuation of therapy. Data for these dropped patients were incomplete and thus not included in the final analysis. The demographic analysis for the SNaPtreated patients who completed the study revealed a group of highly refractory wounds (Table 1). The historical control subjects closely matched the treatment group for major wound-healing risk factors. Matching characteristics were expected to be close to identical, as they were the basis for the match; however, it is of note that the additional comorbidities captured were also similar between the groups. History of osteomyelitis was the only comorbidity that was found to be significantly different (p 0.05) between the groups. It should be noted that for wound age, one of the SNaP-treated patients had a wound present for 19 years, and this patient and his matches were excluded from the analysis because no matches could be identified with wound age of comparable duration. Prospective Results In the SNaP-treated group, 75 percent of subjects (21 of 28) participating in the study tolerated treatment without complications. The seven subjects that could not tolerate treatment were discontinued from the study because of complications and were considered treatment failures. No further data were captured for these patients. In Fig. 2. Normalized wound area tracing data over time for SNaP-treated patients. 1256
5 Volume 126, Number 4 SNaP Wound Care System the SNaP-treated subjects that tolerated treatment, 100 percent of subjects demonstrated improvement in wound size and 86 percent (18 of 21) exhibited a statistically significant healing trend (p 0.05). As shown in Table 2, regression analysis of wound healing over time for each of the 21 subjects resulted in consistently negative slopes of varying magnitude, demonstrating that although the speed of healing varied for each subject, all 21 subjects experienced a trend toward wound healing over the 4-month study period. Average treatment time using the SNaP System was weeks. Normalized average wound size is shown in Figure 2 with the SEM and SD. Case examples of some of the subjects treated are shown in Figures 3 through 7. Retrospective Comparison Kaplan-Meier estimates of wound healing at 1, 2, 3, and 4 months of treatment were 0, 20, 66.2, and 83.1 percent, respectively, for the SNaPtreated subjects. 6,7 Compared with the matched controls, there was a 47.4 percent absolute improvement in the percentage of wounds healed when subjects were treated with the SNaP device as compared with modern dressings over a 4-month period (Table 3). Because so few of the matched-controlled wounds healed in the 4-month time frame as compared with the SNaP-treated subjects, data beyond 4 months were gathered for the matched controls and were evaluated with a Kaplan-Meier survival analysis to provide a more meaningful comparison of the relative time it took for wounds to heal in each treatment group (Fig. 8). 6,7 For those subjects with wound healing, average time to healing was found to be significantly reduced (p ) in those subjects treated with the SNaP System as compared with matched subjects receiving modern dressings. In those reporting wound healing, the SNaPtreated subjects healed in an average of days from the start of SNaP treatment, and the matched controls healed in an average of days from the start of conventional treatment (p ). This significantly faster healing time represents a 50 percent absolute reduction in time to healing for those treated with the SNaP System. There was also less variation observed in the SNaPtreated subjects than in the matched controls, with an SD of 20 days as compared with the 63-day SD reported for the matched controls. It is of note that this reduced variability was identified in a group with half the sample size of the matched controls. When each individual SNaP-treated subject was analyzed as compared with their matched Fig. 3. Case 1. (Above)An88-year-oldwomanpresentedwith avenousulcer.comorbiditiesincludeddiabetesmellitus,peripheral arterial disease, rheumatoid arthritis, venous insufficiency, hypertension, asthma, and prednisone use. Wound history included diabetic/venous stasis ulcer present for over 1yearwithoutwoundclosuredespiteover1yearoftreatment in a wound care center with compression and multiple modern dressings/therapies, including topical Regranex. Negative-pressure wound therapy consisted of treatment with the SNaPWoundCareSystemfor11weeks.(Below)Woundclosure was achieved at 12 weeks following initiation of negativepressure wound therapy. controls, time to healing also appeared to be reduced in the SNaP-treated subjects as compared with the matched controls. The average difference in time to healing between each SNaP-treated subject and their matched control(s) was days (p ) in favor of the SNaP-treated subjects. Apligraf Application The difference in Apligraf application was also evaluated for each treatment group. Because the matched-controlled subjects were followed for a longer period than the SNaP-treated subjects, an 1257
6 Plastic and Reconstructive Surgery October 2010 Fig. 4. Case 2. (Left)A77-year-oldwomanpresentedwithamixeddiabetic/venous/arterialwound.Comorbiditiesincluded diabetes mellitus, severe peripheral arterial disease, smoking, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, bone cancer, and severe malnutrition (weight, 90 pounds). Wound history included a venous ulcer present for over 8 months, continuing to enlarge despite wound care with modern dressings and treatment with Apligraf (Organogenesis, Inc., Canton, Mass.). (Center)Negative-pressurewoundtherapyconsistedoftreatmentwiththeSNaPWoundCareSystem for 11 weeks until full granulation of the wound bed was achieved. Apligraf was then applied. (Right) Woundclosurewas achieved at 4 months following initiation of negative-pressure wound therapy. Fig. 5. Case 3. (Left) A 65-year-old man presented with a diabetic foot wound. Comorbidities included insulin-dependent diabetes mellitus, hypertension, and hyperlipidemia. Wound history included osteomyelitis and gangrene requiring second-toe amputation. Negative-pressure wound therapy consisted of treatment with the SNaP Wound Care System for 4 weeks. (Right) Wound closure was achieved at 5 weeks following initiation of negative-pressure wound therapy. absolute comparison of the number of Apligraf dressings applied was not appropriate. Rather, the rate of graft application was calculated for each subject based on the number of Apligraf dressings applied and the number of days in the study to provide an equal comparison of Apligraf dressing application in each group. In the SNaP-treated subjects, Apligraf dressing application occurred at less than half the rate ( grafts per day) than in the matched-controlled subjects ( grafts per day). This difference was statistically significant, with a value of p
7 Volume 126, Number 4 SNaP Wound Care System Fig. 6. Case 4. (Left) A68-year-oldmanpresentedwithadiabeticfootwound.Comorbiditiesincludeddiabetesmellitus, smoking, peripheral vascular disease, coronary artery disease, chronic obstructive pulmonary disease, hypertension, and hyperlipidemia. Wound history included trauma to dorsal foot from a door. (Center)Negative-pressurewoundtherapyconsisted of treatment with the SNaP Wound Care System for 3 weeks until full granulation of the wound bed was achieved. Apligraf was then applied. (Right) Woundclosurewasachievedatapproximately9weeksfollowinginitiationofnegativepressure wound therapy. Fig. 7. Case 5. (Left) A62-year-oldwomanpresentedwithadiabeticfootwound.Comorbiditiesincludedtype1diabetes mellitus, hypertension, and hyperlipidemia. Wound history included osteomyelitis and gangrene requiring second- and third-toe amputations. The wounds were slow to heal after 1 month of traditional dressings. (Center) Negative-pressure wound therapy consisted of treatment with the SNaP Wound Care System for 4 weeks until full granulation of the wound bed was achieved. Apligraf was then applied. (Right)Woundclosurewasachievedat10weeksafterinitiationofnegative-pressure wound therapy. Table 3. Kaplan-Meier Estimates of Wound Healing 1 Month (%) 2 Months (%) 3 Months (%) 4 Months (%) Match (n 42) SNaP (n 21) DISCUSSION Chronic wounds of the lower extremity, including diabetic foot ulcers and venous stasis ulcers, are major health care problems. Negative-pressure wound therapy has been shown to be effective in treating these types of wounds. 1,2 However, the most widely used negative-pressure wound therapy devices are bulky and noisy, require an electrical power 1259
8 Plastic and Reconstructive Surgery October 2010 Fig. 8. Kaplan-Meier survival analysis. 6,7 In those reporting wound healing, the SNaPtreated subjects healed in an average of days from the start of SNaP treatment, and the matched controls healed in an average of days from the start of conventional treatment (p ). This significantly faster healing time represents a 50 percent absolute reduction in time to healing for those treated with the SNaP System. source, are difficult to procure, and are costly to use. This study examines the clinical effectiveness of a new ultraportable negative-pressure wound therapy device with a prospective observational study and retrospective case-control comparison to modern wound care protocols. Patients treated in this study were a group with highly refractory lower extremity diabetic and venous ulcers. For these types of wounds, treatment with the SNaP System resulted in statistically significant reduced healing time and a higher rate of wound closure than treatment with standard modern dressing therapy. These results are similar to published reports treating similar wounds using electrically powered negative-pressure wound therapy devices. 1,2 In our clinic, we use multiple modalities to heal wounds, including the frequent use of Apligraf, a bilaminar tissue-engineered biological dressing that has established effectiveness in treating diabetic and venous ulcers. 8,9 Because the adherence and effectiveness of Apligraf and other tissue-engineered dressings depend on adequate wound bed preparation, negative-pressure wound therapy may play an important role in speeding the preparation of the wound bed before their use. Because Apligraf was used in both SNaP and control patient groups, the data from this study suggest that using Apligraf together with negativepressure wound therapy may result in better wound-healing outcomes than use of Apligraf alone. We successfully used the SNaP System to prepare wounds before initiating Apligraf treatment. Using this serial therapy approach, we found that the use of Apligraf and total healing times were decreased in those patients treated with the SNaP System. Although the early clinical outcomes with the SNaP Wound Care System may be similar to those of other powered systems, there are a number of less obvious potential advantages of the SNaP System over traditional negative-pressure wound therapy systems. In a comprehensive overview of negative-pressure therapy for the treatment of lower extremity ulcers, Capobianco and Zgonis 10 identify a number of key disadvantages of traditional negative-pressure wound therapy systems, including decreased patient autonomy, increased anxiety levels during treatment, high cost and unavailability of the therapy for patients in less medically sophisticated countries, and the potential for exsanguination. Because of its unique design, the SNaP System may prove to better address some of these other disadvantages of powered negativepressure systems. 1260
9 Volume 126, Number 4 SNaP Wound Care System The SNaP System is a gauze-based negative-pressure wound therapy system. Recent studies support that gauze-based systems have results comparable to foam dressings when used in negative-pressure wound therapy. 11 Using an in vivo porcine wound model, Borgquist et al. showed that the resultant microdeformation and macrodeformation of the wound bed after negative-pressure therapy are similar with foam and gauze. 12 Clinically, the data in this study support the findings of the retrospective evaluation performed by Campbell et al. that demonstrated similar clinical outcomes using a gauze-based negative-pressure wound therapy system to those reported with foam. 11 Although the data presented in this study are promising, several considerations should be taken into account when interpreting these results. First, retrospectively controlled studies are inherently limited by design. Second, patients treated in the SNaP arm of the study may have benefited from the effects of being in an experimental environment. Moreover, consistent with most longitudinal wound care studies, this study also had a relatively high dropout rate. Finally, study subjects had twice-weekly dressing changes while being treated with the SNaP System compared with the more variable follow-up in the control group. This may have also resulted in differences in débridement frequency. Although it is doubtful that the very large treatment effects seen in the data are solely attributable to the issues above, they must be considered in the interpretation of these results. CONCLUSIONS The SNaP Wound Care System can be an effective tool in treating highly refractory chronic diabetic and venous ulcers. The advantages in size and convenience for both patients and clinicians, as compared with powered devices, would make the SNaP Wound Care System for us a desirable choice for patients with appropriate wounds that would benefit from negative-pressure wound therapy. This study suggests that the SNaP Wound Care System may be a useful addition to the techniques available to the wound care clinician. An ongoing randomized controlled trial of the SNaP Wound Care System, which is currently in progress, may further elucidate the value of this novel system in the application of negative-pressure therapy for wound treatment. Peter J. Schubart, M.D., Ph.D. O Connor Wound Care Clinic 125 Ciro Avenue, Suite 201 San Jose, Calif schubart@ix.netcom.com REFERENCES 1. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet 2005;366: Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006;44: ; discussion Argenta LC, Morykwas MJ, Marks MW, DeFranzo AJ, Molnar JA, David LR. Vacuum-assisted closure: State of clinic art. Plast Reconstr Surg. 2006;117(Suppl):127S 142S. 4. Fong KD, Hu D, Eichstadt S, et al. The SNaP System: Biomechanical and animal model testing of a novel ultraportable NPWT system. Plast Reconstr Surg. 2010;125: Fong KD, Hu D, Eichstadt SL, et al. Initial clinical experience using a novel ultraportable negative pressure wound therapy device. Wounds (in press). 6. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley; Lee ET. Statistical Methods for Survival Data Analysis. 2nd ed. New York: Wiley; Edmonds M. Apligraf in the treatment of neuropathic diabetic foot ulcers. Int J Low Extrem Wounds 2009;8: Barber C, Watt A, Pham C, et al. Influence of bioengineered skin substitutes on diabetic foot ulcer and venous leg ulcer outcomes. J Wound Care 2008;17: Capobianco CM, Zgonis T. An overview of negative pressure wound therapy for the lower extremity. Clin Podiatr Med Surg. 2009;26: Campbell PE, Smith GS, Smith JM. Retrospective clinical evaluation of gauze-based negative pressure therapy. Int Wound J. 2008;5: Borgquist O, Ingemansson R, Malmsjö M. Negative pressure wound therapy using gauze and foam: An in-detail study of the effects on the wound bed including macro- and microdeformation, tissue ingrowth and wound bed histology. Paper presented at the 24th Annual Clinical Symposium on Advances in Skin & Wound Care; October 22 25, 2009; San Antonio, Texas. 1261
Negative pressure wound therapy (NPWT) is an important tool for
 ORIGINAL RESEARCH Initial Clinical Experience Using a Novel Ultraportable Negative Pressure Wound Therapy Device Kenton D. Fong, MD; 1,2 Dean Hu, MS; 2, Shaundra L. Eichstadt, BS; 1 Emily Gorell, MS; 4
ORIGINAL RESEARCH Initial Clinical Experience Using a Novel Ultraportable Negative Pressure Wound Therapy Device Kenton D. Fong, MD; 1,2 Dean Hu, MS; 2, Shaundra L. Eichstadt, BS; 1 Emily Gorell, MS; 4
The Use of the. in Clinical Practice
 The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
The Use of the SNAP Therapy System in Clinical Practice It s an ultraportable, mechanically-powered disposable NPWT. By Animesh Bhatia, DPM, CWS This article is written exclusively for PM and appears courtesy
METHODS. David G. Armstrong, DPM, MD, PhD 1 ; William A. Marston, MD 2 ; Alexander M. Reyzelman, DPM 3 ; Robert S. Kirsner, MD, PhD 4
 Wound Repair and Regeneration Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower
Wound Repair and Regeneration Comparison of negative pressure wound therapy with an ultraportable mechanically powered device vs. traditional electrically powered device for the treatment of chronic lower
QUICK GUIDE SNAP THERAPY SYSTEM
 QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
QUICK GUIDE SNAP THERAPY SYSTEM Clinical Pathway to SNAP System Full holistic assessment of patient and wound Is the wound type indicated for NPWT use without contraindications 1? SNAP System is indicated
SNAP THERAPY SYSTEM MONOGRAPH
 SNAP THERAPY SYSTEM MONOGRAPH Table of Contents Introduction...3 SNAP System Components Literature Review...4 Clinical Evidence Supporting SNAP System... 7 Technology for SNAP System.........................................11
SNAP THERAPY SYSTEM MONOGRAPH Table of Contents Introduction...3 SNAP System Components Literature Review...4 Clinical Evidence Supporting SNAP System... 7 Technology for SNAP System.........................................11
DO NOT DUPLICATE. Negative pressure wound therapy (NPWT) has revolutionized the
 Original research WOUNDS 2013;25(4):89 93 From the Aesthetic and Plastic Surgery Institute, University of California Irvine, Orange, CA and Long Beach Memorial Medical Center, Long Beach, CA Address correspondence
Original research WOUNDS 2013;25(4):89 93 From the Aesthetic and Plastic Surgery Institute, University of California Irvine, Orange, CA and Long Beach Memorial Medical Center, Long Beach, CA Address correspondence
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur
 VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
VACUUM ASSISTED CLOSURE (V.A.C.) THERAPY: Mr. Ismazizi Zaharudin Jabatan pembedahan Am Hospital Kuala Lumpur Learning Objectives Define Negative Pressure Wound Therapy (NPWT) Discuss guidelines for the
1/5. Introduction. Primary endpoint Time to reach readiness for closure by surgical intervention or left for closure by secondary intention
 1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
Negative Pressure Wound Therapy in the Outpatient Setting
 Negative Pressure Wound Therapy in the Outpatient Setting Policy Number: 1.01.16 Last Review: 9/2017 Origination: 7/2001 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Negative Pressure Wound Therapy in the Outpatient Setting Policy Number: 1.01.16 Last Review: 9/2017 Origination: 7/2001 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Negative Pressure Wound Therapy (NPWT)
 Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
Negative Pressure Wound Therapy (NPWT) Policy Number: Original Effective Date: MM.01.005 11/19/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 01/01/2015 Section: DME Place(s) of Service:
TECHNOLOGY ADVANCES. j 33. Theresa Hurd, 1, * Alan Rossington, 2 Paul Trueman, 2 and Jennifer Smith 2, *
 TECHNOLOGY ADVANCES A Retrospective Comparison of the Performance of Two Negative Pressure Wound Therapy Systems in the Management of Wounds of Mixed Etiology Theresa Hurd, 1, * Alan Rossington, 2 Paul
TECHNOLOGY ADVANCES A Retrospective Comparison of the Performance of Two Negative Pressure Wound Therapy Systems in the Management of Wounds of Mixed Etiology Theresa Hurd, 1, * Alan Rossington, 2 Paul
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial
 Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial David G. Armstrong, DPM, MD, PhD 1 ; William A. Marston,
Comparative effectiveness of mechanically and electrically powered negative pressure wound therapy devices: A multicenter randomized controlled trial David G. Armstrong, DPM, MD, PhD 1 ; William A. Marston,
Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series
 Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series Theresa Hurd, RN, MScN, PhD; Paul Trueman, BA, MA; and Alan
Use of a Portable, Single-use Negative Pressure Wound Therapy Device in Home Care Patients with Low to Moderately Exuding Wounds: A Case Series Theresa Hurd, RN, MScN, PhD; Paul Trueman, BA, MA; and Alan
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Vacuum Assisted Wound Closure (VAC) Page 1 of 32 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
Vacuum Assisted Wound Closure (VAC) Page 1 of 32 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
DISCOVERY EXPRESS. j 75. William A. Marston, 1, * David G. Armstrong, 2 Alexander M. Reyzelman, 3 and Robert S. Kirsner 4 INTRODUCTION
 DISCOVERY EXPRESS A Multicenter Randomized Controlled Trial Comparing Treatment of Venous Leg Ulcers Using Mechanically Versus Electrically Powered Negative Pressure Wound Therapy William A. Marston, 1,
DISCOVERY EXPRESS A Multicenter Randomized Controlled Trial Comparing Treatment of Venous Leg Ulcers Using Mechanically Versus Electrically Powered Negative Pressure Wound Therapy William A. Marston, 1,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Vacuum Assisted Wound Closure (VAC) Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
Vacuum Assisted Wound Closure (VAC) Page 1 of 25 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Vacuum Assisted Wound Closure (VAC) Professional Institutional Original
V.A.C. Therapy Safety Information
 Bringing Safety Home V.A.C. Therapy Safety Information Bleeding Precautions Dressing Change Frequency Foam Removal Important Information 2 Prior to use of V.A.C. Therapy System it is important for the
Bringing Safety Home V.A.C. Therapy Safety Information Bleeding Precautions Dressing Change Frequency Foam Removal Important Information 2 Prior to use of V.A.C. Therapy System it is important for the
Prediction of healing for post-operative diabetic foot wounds based on early wound area progression
 Diabetes Care Publish Ahead of Print, published online October 12, 2007 Prediction of healing for post-operative diabetic foot wounds based on early wound area progression Lawrence A Lavery, DPM, MPH 1
Diabetes Care Publish Ahead of Print, published online October 12, 2007 Prediction of healing for post-operative diabetic foot wounds based on early wound area progression Lawrence A Lavery, DPM, MPH 1
V.A.C. Therapy Patient Guide. Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you.
 V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
V.A.C. Therapy Patient Guide Are you suffering from a wound? Ask your doctor about V.A.C. Therapy and whether it may be right for you. kci1.com 800.275.4524 Table of Contents Wound Healing is a Process...2
Will it heal? How to assess the probability of wound healing
 Will it heal? How to assess the probability of wound healing Richard F. Neville, M.D. Professor of Surgery Chief, Division of Vascular Surgery George Washington University Limb center case 69 yr old male
Will it heal? How to assess the probability of wound healing Richard F. Neville, M.D. Professor of Surgery Chief, Division of Vascular Surgery George Washington University Limb center case 69 yr old male
Cahaba Medicare Policy Primer 1,2 for Apligraf
 Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Cahaba Medicare Policy Primer 1,2 for Apligraf MAC A: AL, GA & TN MAC B: AL, GA, & TN LCD# 31428 Indications Applied to partial- or full-thickness ulcers of the lower extremities (see individual product
Negative Pressure Wound Therapy in the Outpatient Setting. Original Policy Date 12:2013
 MP 1.01.11 Negative Pressure Wound Therapy in the Outpatient Setting Medical Policy Section Durable Medical Equipment Issue 12:2013` Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature
MP 1.01.11 Negative Pressure Wound Therapy in the Outpatient Setting Medical Policy Section Durable Medical Equipment Issue 12:2013` Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature
Role of Negative Pressure Therapy in Healing of Diabetic Foot Ulcers
 Journal of Surgery 2015; 3(2-1): 31-35 Published online May 8, 2015 (http://www.sciencepublishinggroup.com/j/js) doi: 10.11648/j.js.s.2015030201.17 ISSN: 2330-0914 (Print); ISSN: 2330-0930 (Online) Role
Journal of Surgery 2015; 3(2-1): 31-35 Published online May 8, 2015 (http://www.sciencepublishinggroup.com/j/js) doi: 10.11648/j.js.s.2015030201.17 ISSN: 2330-0914 (Print); ISSN: 2330-0930 (Online) Role
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema. Original Policy Date
 MP 2.02.12 End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
MP 2.02.12 End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
Palmetto Medicare Policy Primer
 Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Palmetto Medicare Policy Primer Medicare Jurisdiction (JM) NC, SC, WV & VA Application of Skin Substitutes LCD #L36466 Indications Presence of neuropathic diabetic foot ulcer(s) having failed to respond
Corporate Medical Policy
 Corporate Medical Policy Topical Negative Pressure Therapy for Wounds File Name: Origination: Last CAP Review: Next CAP Review: Last Review: topical_negative_pressure_therapy_for_wounds 12/1998 5/2018
Corporate Medical Policy Topical Negative Pressure Therapy for Wounds File Name: Origination: Last CAP Review: Next CAP Review: Last Review: topical_negative_pressure_therapy_for_wounds 12/1998 5/2018
Management of Complex Wounds with Vacuum Assisted Closure
 Management of Complex Wounds with Vacuum Assisted Closure Wendy McInnes Vascular / Wound Nurse Practitioner The Queen Elizabeth Hospital, Adelaide, South Australia Treasurer ANZSVN wendy.mcinnes@health.sa.gov.au
Management of Complex Wounds with Vacuum Assisted Closure Wendy McInnes Vascular / Wound Nurse Practitioner The Queen Elizabeth Hospital, Adelaide, South Australia Treasurer ANZSVN wendy.mcinnes@health.sa.gov.au
Use of Vacuum-assisted Wound Closure to Manage Limb Wounds in Patients Suffering from Acute Necrotizing Fasciitis
 Original Article Use of Vacuum-assisted Wound Closure to Manage Limb Wounds in Patients Suffering from Acute Necrotizing Fasciitis Wen-Shyan Huang, Shang-Chin Hsieh, Chun-Sheng Hsieh, Jen-Yu Schoung and
Original Article Use of Vacuum-assisted Wound Closure to Manage Limb Wounds in Patients Suffering from Acute Necrotizing Fasciitis Wen-Shyan Huang, Shang-Chin Hsieh, Chun-Sheng Hsieh, Jen-Yu Schoung and
See Policy CPT/HCPCS CODE section below for any prior authorization requirements
 Effective Date: 7/1/2018 Section: DME Policy No: 377 Medical Officer 7/1/18 Date Technology Assessment Committee Approved Date: 10/10; 10/13; 9/14: 9/15; 4/16 Medical Policy Committee Approved Date: 3/03;
Effective Date: 7/1/2018 Section: DME Policy No: 377 Medical Officer 7/1/18 Date Technology Assessment Committee Approved Date: 10/10; 10/13; 9/14: 9/15; 4/16 Medical Policy Committee Approved Date: 3/03;
Novitas Medicare Policy Primer
 Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Novitas Medicare Policy Primer Medicare Jurisdiction (JL and JH) AR, CO, LA, MS, NM, OK, TX, DC, DE, MD, NJ, & PA Counties of Arlington and Fairfax in Virginia and the city of Alexandria in Virginia Application
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude
 Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
NEGATIVE PRESURE WOUND THERAPY PROGRAM
 NEGATIVE PRESURE WOUND THERAPY PROGRAM WWW.USTOMWOUNDCARE.COM 866-802-0006/901-619-8897 SUPPORT@USTOM.COM TABLE OF CONTENTS 1. 2. 3. 4. To place an order: 1. Please visit our website 2. Please email to
NEGATIVE PRESURE WOUND THERAPY PROGRAM WWW.USTOMWOUNDCARE.COM 866-802-0006/901-619-8897 SUPPORT@USTOM.COM TABLE OF CONTENTS 1. 2. 3. 4. To place an order: 1. Please visit our website 2. Please email to
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers
 A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
The Risk. Background / Bias. Integrating Wound Care into a Limb Preservation Initiative 4/24/2009
 Stimulating Wound Granulation: Advances in NPWT and other Measures (Wound Bed Preparation) Charles Andersen MD, FACS, FAPWCA Clinical Prof of Surgery UW, USUHS Chief Vascular/Endovascular/ Limb Preservation
Stimulating Wound Granulation: Advances in NPWT and other Measures (Wound Bed Preparation) Charles Andersen MD, FACS, FAPWCA Clinical Prof of Surgery UW, USUHS Chief Vascular/Endovascular/ Limb Preservation
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU)
 Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Topical Oxygen Wound Therapy (MEDICAID)
 Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine
 Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine Dr. Julian Vitse, Montellier University Hospital, France Negative Pressure Wound
Novel Approaches for Accelerating Wound Healing Negative Pressure Wound Therapy in Accelerating Wound Healing Telemedicine Dr. Julian Vitse, Montellier University Hospital, France Negative Pressure Wound
Negative Pressure Wound Therapy
 Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
Origination: 6/29/04 Revised: 8/24/16 Annual Review: 11/10/16 Purpose: To provide Negative Pressure Wound Therapy (wound care treatment) guidelines for the Medical Department staff to reference when making
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP058 Section: Medical Benefit Policy Subject: Negative Pressure Wound Therapy I. Policy: Negative Pressure Wound Therapy II. Purpose/Objective: To provide a policy
POLICIES AND PROCEDURE MANUAL Policy: MP058 Section: Medical Benefit Policy Subject: Negative Pressure Wound Therapy I. Policy: Negative Pressure Wound Therapy II. Purpose/Objective: To provide a policy
Nanogen Aktiv. Naz Wahab MD, FAAFP, FAPWCA Nexderma
 Nanogen Aktiv Naz Wahab MD, FAAFP, FAPWCA Nexderma Patient BM 75 y.o female with a history of Type 2 Diabetes, HTN, Hypercholesterolemia, Renal insufficiency, Chronic back Pain, who had undergone a L3-L4
Nanogen Aktiv Naz Wahab MD, FAAFP, FAPWCA Nexderma Patient BM 75 y.o female with a history of Type 2 Diabetes, HTN, Hypercholesterolemia, Renal insufficiency, Chronic back Pain, who had undergone a L3-L4
Disclosures. Outpatient NPWT Options Free up Hospital Beds, but Do They Work? Objectives. Clinically Effective: Does it Work?
 4/16/16 Disclosures Consultant, Volcano Corporation Outpatient Options Free up Hospital Beds, but Do They Work? UCSF Vascular Symposium 16 Jonathan Labovitz, DPM Medical Director, Foot & Ankle Center Associate
4/16/16 Disclosures Consultant, Volcano Corporation Outpatient Options Free up Hospital Beds, but Do They Work? UCSF Vascular Symposium 16 Jonathan Labovitz, DPM Medical Director, Foot & Ankle Center Associate
Supporting healthcare professionals in taking control of the infection risk with ACTICOAT Flex TAKE CONTROL. of the infection risk in chronic wound
 Supporting healthcare professionals in taking control of the infection risk with ACTICOAT Flex TAKE CONTROL of the infection risk in chronic wound Introduction The impact of infection on patients is well
Supporting healthcare professionals in taking control of the infection risk with ACTICOAT Flex TAKE CONTROL of the infection risk in chronic wound Introduction The impact of infection on patients is well
PRODIGY Quick Reference Guide
 PRODIGY Quick Venous leg ulcer infected How do I assess a venous leg ulcer? Chronic venous insufficiency and venous hypertension result from damage to the valves in the veins of the leg and inadequate
PRODIGY Quick Venous leg ulcer infected How do I assess a venous leg ulcer? Chronic venous insufficiency and venous hypertension result from damage to the valves in the veins of the leg and inadequate
Durable Medical Equipment Providers
 August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
August 2009 Provider Bulletin Number 974 Durable Medical Equipment Providers Vacuum Assisted Wound Closure Therapy Negative pressure wound therapy (NPWT) must be requested and supplied by an enrolled durable
CGS Medicare Policy Primer
 CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
CGS Medicare Policy Primer Medicare Jurisdiction (J15) OH & KY Wound Application of Cellular and/or Tissue Based Products (CTPs) Lower Extremities #L36690 Indications Application of a CTP graft for lower
Policy Specific Section: Medical Necessity and Investigational / Experimental. June 13, 2001 January 1, 2015
 Medical Policy Negative Pressure Wound Therapy in the Outpatient Setting Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Durable Medical Equipment Original Policy Date:
Medical Policy Negative Pressure Wound Therapy in the Outpatient Setting Type: Medical Necessity and Investigational / Experimental Policy Specific Section: Durable Medical Equipment Original Policy Date:
WOUND CARE. By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare
 WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
Use of Non-Contact Low Frequency Ultrasound in Wound Care
 Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
ULCERS 1/12/ million diabetics in the US (2012) Reamputation Rate 26.7% at 1 year 48.3% at 3 years 60.7% at 5 years
 Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
NEGATIVE PRESSURE WOUND THERAPY
 NEGATIVE PRESSURE WOUND THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
NEGATIVE PRESSURE WOUND THERAPY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Coverage Summary. Wound Treatments
 Coverage Summary Wound Treatments Policy Number: W-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 02/18/2009 Approved by: UnitedHealthcare Medicare Benefit Interpretation
Coverage Summary Wound Treatments Policy Number: W-001 Products: UnitedHealthcare Medicare Advantage Plans Original Approval Date: 02/18/2009 Approved by: UnitedHealthcare Medicare Benefit Interpretation
Transmetatarsal amputation in an at-risk diabetic population: a retrospective study
 The Journal of Diabetic Foot Complications Transmetatarsal amputation in an at-risk diabetic population: a retrospective study Authors: Merribeth Bruntz, DPM, MS* 1,2, Heather Young, MD 3,4, Robert W.
The Journal of Diabetic Foot Complications Transmetatarsal amputation in an at-risk diabetic population: a retrospective study Authors: Merribeth Bruntz, DPM, MS* 1,2, Heather Young, MD 3,4, Robert W.
Role of free tissue transfer in management of chronic venous ulcer
 Original Article Role of free tissue transfer in management of chronic venous ulcer K. Murali Mohan Reddy, D. Mukunda Reddy Department of Plastic Surgery, Nizams Institute of Medical Sciences, India. Address
Original Article Role of free tissue transfer in management of chronic venous ulcer K. Murali Mohan Reddy, D. Mukunda Reddy Department of Plastic Surgery, Nizams Institute of Medical Sciences, India. Address
Successful Wound Management Strategies : An Introduction. Alex Khan, APRN ACNS-BC. Organization of Wound Care Nurses
 Successful Wound Management Strategies : An Introduction Alex Khan, APRN ACNS-BC Organization of Wound Care Nurses www.woundcarenurses.org Goals & Objectives The role and importance of wound care management
Successful Wound Management Strategies : An Introduction Alex Khan, APRN ACNS-BC Organization of Wound Care Nurses www.woundcarenurses.org Goals & Objectives The role and importance of wound care management
Journal of American Science 2014;10(12) Vacuum assisted closure [VAC] in management of diabetic foot
![Journal of American Science 2014;10(12) Vacuum assisted closure [VAC] in management of diabetic foot Journal of American Science 2014;10(12) Vacuum assisted closure [VAC] in management of diabetic foot](/thumbs/89/100997654.jpg) Vacuum assisted closure [VAC] in management of diabetic foot Hisham W. Anwar 1 and Ayman A. Al-Tramsy 2 1 Department of General Surgery, Faculty of Medicine, Al-Azhar University, Cairo, Egypt 2 Department
Vacuum assisted closure [VAC] in management of diabetic foot Hisham W. Anwar 1 and Ayman A. Al-Tramsy 2 1 Department of General Surgery, Faculty of Medicine, Al-Azhar University, Cairo, Egypt 2 Department
Premier Health Plan considers Negative Pressure Wound Therapy (NPWT) in the home setting medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
Premier Health Plan POLICY AND PROCEDURE MANUAL PA.009.PH Negative Pressure Wound Therapy This policy applies to the following lines of business: Premier Commercial Premier Employee Premier Health Plan
CASE 1: TYPE-II DIABETIC FOOT ULCER
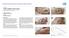 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
Genadyne A4 and foam to treat a postoperative debridment flank abscess
 Genadyne A4 and foam to treat a postoperative debridment flank abscess Michael S. DO, The Wound Healing Center Indianapolis, IN Cynthia Peebles RN D.O.N., Becky Beck RN Heartland at Prestwick NH Avon,
Genadyne A4 and foam to treat a postoperative debridment flank abscess Michael S. DO, The Wound Healing Center Indianapolis, IN Cynthia Peebles RN D.O.N., Becky Beck RN Heartland at Prestwick NH Avon,
Delayed Primary Closure of Diabetic Foot Wounds using the DermaClose RC Tissue Expander
 Delayed Primary Closure of Diabetic Foot Wounds using the DermaClose RC Tissue Expander TDavid L. Nielson, DPM 1, Stephanie C. Wu, DPM, MSc 2, David G. Armstrong, DPM,PhD 3 The Foot & Ankle Journal 1 (2):
Delayed Primary Closure of Diabetic Foot Wounds using the DermaClose RC Tissue Expander TDavid L. Nielson, DPM 1, Stephanie C. Wu, DPM, MSc 2, David G. Armstrong, DPM,PhD 3 The Foot & Ankle Journal 1 (2):
Vacuumed Assisted Closure
 Vacuumed Assisted Closure Louise Morris Lead Nurse in Tissue Viability Jackie Stephen-Haynes Consultant Nurse and senior Lecturer in Tissue Viability 2009 Aims and Objectives To develop an awareness of
Vacuumed Assisted Closure Louise Morris Lead Nurse in Tissue Viability Jackie Stephen-Haynes Consultant Nurse and senior Lecturer in Tissue Viability 2009 Aims and Objectives To develop an awareness of
Acute and Chronic WOUND ASSESSMENT. Wound Assessment OBJECTIVES ITEMS TO CONSIDER
 WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema
 End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
End-Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Partnering the burn community
 * At smith&nephew we seek imaginative solutions that improve wound outcomes for patients and at the same time conserve resources for healthcare systems. Partnering the burn community Dedicated to the management
* At smith&nephew we seek imaginative solutions that improve wound outcomes for patients and at the same time conserve resources for healthcare systems. Partnering the burn community Dedicated to the management
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
Negative Pressure Wound Therapy (NPWT) Using On-shelf Products for Treatment of Post-Traumatic Wounds: A Case Series
 Med. J. Cairo Univ., Vol. 80, No. 2, December: 87-93, 2012 www.medicaljournalofcairouniversity.com Negative Pressure Wound Therapy (NPWT) Using On-shelf Products for Treatment of Post-Traumatic Wounds:
Med. J. Cairo Univ., Vol. 80, No. 2, December: 87-93, 2012 www.medicaljournalofcairouniversity.com Negative Pressure Wound Therapy (NPWT) Using On-shelf Products for Treatment of Post-Traumatic Wounds:
Negative pressure wound therapy in surgical wounds: a prospective comparative study
 International Surgery Journal Chandrashekar S et al. Int Surg J. 2017 Oct;4(10):3272-3276 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174102
International Surgery Journal Chandrashekar S et al. Int Surg J. 2017 Oct;4(10):3272-3276 http://www.ijsurgery.com pissn 2349-3305 eissn 2349-2902 Original Research Article DOI: http://dx.doi.org/10.18203/2349-2902.isj20174102
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Efficacy of Velcro Band Devices in Venous and. Mixed Arterio-Venous Patients
 Efficacy of Velcro Band Devices in Venous and Mixed Arterio-Venous Patients T. Noppeney Center for Vascular Diseases: Outpatient Dept. Obere Turnstrasse, Dept. for Vascular Surgery Martha-Maria Hospital
Efficacy of Velcro Band Devices in Venous and Mixed Arterio-Venous Patients T. Noppeney Center for Vascular Diseases: Outpatient Dept. Obere Turnstrasse, Dept. for Vascular Surgery Martha-Maria Hospital
Negative pressure wound therapy: past, present and future
 International Wound Journal ISSN 1742-4801 REVIEW ARTICLE Negative pressure wound therapy: past, present and future Dennis P Orgill & Lauren R Bayer Division of Plastic Surgery, Brigham and Women s Hospital,
International Wound Journal ISSN 1742-4801 REVIEW ARTICLE Negative pressure wound therapy: past, present and future Dennis P Orgill & Lauren R Bayer Division of Plastic Surgery, Brigham and Women s Hospital,
Simple, gentle and affordable. *smith&nephew V1STA Negative Pressure Wound Therapy
 Simple, gentle and affordable *smith&nephew V1STA Negative Pressure Wound Therapy Smith & Nephew s extensive presence and portfolio means that for every wound, at every stage, there is an appropriate solution.
Simple, gentle and affordable *smith&nephew V1STA Negative Pressure Wound Therapy Smith & Nephew s extensive presence and portfolio means that for every wound, at every stage, there is an appropriate solution.
Negative Pressure Wound Therapy A Descriptive Study
 FEATURE Negative Pressure Wound Therapy A Descriptive Study Ann-Mari Wallin, RN; Lennart Boström, MD, PhD; Johanna Ulfvarson, RN, PhD; and Carin Ottosson, MD, PhD Abstract To address a persistent lack
FEATURE Negative Pressure Wound Therapy A Descriptive Study Ann-Mari Wallin, RN; Lennart Boström, MD, PhD; Johanna Ulfvarson, RN, PhD; and Carin Ottosson, MD, PhD Abstract To address a persistent lack
July 1, September 24, 2013
 Original Issue Date (Created): Most Recent Review Date (Revised): July 1, 2002 September 24, 2013 Effective Date: February 1, 2014 I. POLICY Powered negative pressure therapy systems should be used as
Original Issue Date (Created): Most Recent Review Date (Revised): July 1, 2002 September 24, 2013 Effective Date: February 1, 2014 I. POLICY Powered negative pressure therapy systems should be used as
Description. Section: Durable Medical Equipment Effective Date: July 15, 2016
 Last Review Status/Date: June 2016 Page: 1 of 18 Description Negative pressure wound therapy (NPWT) consists of the use of a negative pressure or suction device to aspirate and remove fluids, debris, and
Last Review Status/Date: June 2016 Page: 1 of 18 Description Negative pressure wound therapy (NPWT) consists of the use of a negative pressure or suction device to aspirate and remove fluids, debris, and
Venous Leg Ulcers. Care for Patients in All Settings
 Venous Leg Ulcers Care for Patients in All Settings Summary This quality standard focuses on care for people who have developed or are at risk of developing a venous leg ulcer. The scope of the standard
Venous Leg Ulcers Care for Patients in All Settings Summary This quality standard focuses on care for people who have developed or are at risk of developing a venous leg ulcer. The scope of the standard
Wound Care Evaluation by Kris Dalseg MS PT CWS CLT
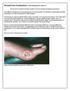 Wound Care Evaluation by Kris Dalseg MS PT CWS CLT This document is intended to describe a standard wound care evaluation for healthcare practitioners. In healthcare, all aspects of our treatment have
Wound Care Evaluation by Kris Dalseg MS PT CWS CLT This document is intended to describe a standard wound care evaluation for healthcare practitioners. In healthcare, all aspects of our treatment have
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
NPWT Case Series EXPERIENCES WITH INVIA MOTION. Precious life Progressive care. Invia Motion Negative Pressure Wound Therapy
 NPWT Case Series EXPERIENCES WITH INVIA MOTION Invia Motion Negative Pressure Wound Therapy Precious life Progressive care npwt_case_booklet_a4.indd 1 18.12.13 13:17 Chronic sacral pressure ulcer Case
NPWT Case Series EXPERIENCES WITH INVIA MOTION Invia Motion Negative Pressure Wound Therapy Precious life Progressive care npwt_case_booklet_a4.indd 1 18.12.13 13:17 Chronic sacral pressure ulcer Case
Advazorb. Hydrophilic foam dressing range
 Advazorb Hydrophilic foam dressing range Advazorb A comprehensive range of patient friendly, absorbent foam dressings Non-adhesive and atraumatic silicone adhesive options Designed to manage exudate whilst
Advazorb Hydrophilic foam dressing range Advazorb A comprehensive range of patient friendly, absorbent foam dressings Non-adhesive and atraumatic silicone adhesive options Designed to manage exudate whilst
Case 1. July 14, th week wound gel 3 cm x 2.5 cm = 7.5 cm². May 25, st wound gel on 290 days PI treatment 4 cm x 2.4 cm = 9.
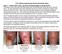 2.5% Sodium Hyaluronate Wound Gel Study Cases Case 1 Patient with Lower Leg Ulcer Not Responding to Compression This patient was a 50-year old male patient with nonhealing right lower leg since January
2.5% Sodium Hyaluronate Wound Gel Study Cases Case 1 Patient with Lower Leg Ulcer Not Responding to Compression This patient was a 50-year old male patient with nonhealing right lower leg since January
Vacuum-Assisted Closure of Perineal War Wound Related to Rectum
 Vacuum-Assisted Closure of Perineal War Wound Related to Rectum Nazım Gümüş, MD Plastic and Reconstructive Surgery Department, Adana Numune Research and Training Hospital, Adana, Turkey Correspondence:
Vacuum-Assisted Closure of Perineal War Wound Related to Rectum Nazım Gümüş, MD Plastic and Reconstructive Surgery Department, Adana Numune Research and Training Hospital, Adana, Turkey Correspondence:
Introduction. Follow standard infection control precautions
 Patient Handbook 1 TABLE OF CONTENTS 1. Introduction. 2 2. Indications for Use. 3 3. Contraindications... 3 4. Warnings 4 5. Precautions.. 6 6. Patient Information Guide. 6 7. Key Pad Features.. 7 8. Battery
Patient Handbook 1 TABLE OF CONTENTS 1. Introduction. 2 2. Indications for Use. 3 3. Contraindications... 3 4. Warnings 4 5. Precautions.. 6 6. Patient Information Guide. 6 7. Key Pad Features.. 7 8. Battery
Protocol. Negative Pressure Wound Therapy in the Outpatient Setting
 Protocol Negative Pressure Wound Therapy in the Outpatient Setting (10116) Medical Benefit Effective Date: 07/01/18 Next Review Date: 05/19 Preauthorization No Review Dates: 11/07, 09/08, 09/09, 09/10,
Protocol Negative Pressure Wound Therapy in the Outpatient Setting (10116) Medical Benefit Effective Date: 07/01/18 Next Review Date: 05/19 Preauthorization No Review Dates: 11/07, 09/08, 09/09, 09/10,
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
JMSCR Vol 06 Issue 03 Page March 2018
 www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
Velcro Compression Devices
 Velcro Compression Devices Joseph A. Caprini, MD, MS, FACS, RVT, FACCWS Louis W. Biegler Chair of Surgery NorthShore University HealthSystem, Evanston, IL Clinical Professor of Surgery University of Chicago
Velcro Compression Devices Joseph A. Caprini, MD, MS, FACS, RVT, FACCWS Louis W. Biegler Chair of Surgery NorthShore University HealthSystem, Evanston, IL Clinical Professor of Surgery University of Chicago
Management of Complex Avulsion Injuries of the Dorsum of the Foot and Ankle in Pediatric Patients by Using Local Delayed Flaps and Skin Grafts
 Management of Complex Avulsion Injuries of the Dorsum of the Foot and Ankle in Pediatric Patients by Using Local Delayed Flaps and Skin Grafts Ahmed Elshahat, MD Plastic Surgery Department, Ain Shams University,
Management of Complex Avulsion Injuries of the Dorsum of the Foot and Ankle in Pediatric Patients by Using Local Delayed Flaps and Skin Grafts Ahmed Elshahat, MD Plastic Surgery Department, Ain Shams University,
A Prospective Study of Negative Pressure Wound Therapy With Integrated Irrigation for the Treatment of Diabetic Foot Ulcers
 A Prospective Study of Negative Pressure Wound Therapy With Integrated Irrigation for the Treatment of Diabetic Foot Ulcers Charles M. Zelen, DPM, FACFAS, a,b Brian Stover, DPM, a David Nielson, DPM, a,b
A Prospective Study of Negative Pressure Wound Therapy With Integrated Irrigation for the Treatment of Diabetic Foot Ulcers Charles M. Zelen, DPM, FACFAS, a,b Brian Stover, DPM, a David Nielson, DPM, a,b
Negative Pressure Wound Therapy in the Treatment of Diabetic Foot Ulcers
 J Wound Ostomy Continence Nurs. 2014;41(3):233-237. Published by Lippincott Williams & Wilkins WOUND CARE Negative Pressure Wound Therapy in the Treatment of Diabetic Foot Ulcers A Systematic Review of
J Wound Ostomy Continence Nurs. 2014;41(3):233-237. Published by Lippincott Williams & Wilkins WOUND CARE Negative Pressure Wound Therapy in the Treatment of Diabetic Foot Ulcers A Systematic Review of
Negative-pressure wound therapy (NPWT) is
 WOU HEALING Negative-Pressure Wound Therapy: A Comprehensive Review of the Evidence Ersilia L. Anghel, BS, BA Paul J. Kim, DPM, MS, FACFAS Washington, D.C. Background: Negative-pressure wound therapy (NPWT)
WOU HEALING Negative-Pressure Wound Therapy: A Comprehensive Review of the Evidence Ersilia L. Anghel, BS, BA Paul J. Kim, DPM, MS, FACFAS Washington, D.C. Background: Negative-pressure wound therapy (NPWT)
Negative Pressure Wound Therapy
 Ontario Health Technology Assessment Series 2010; Vol. 10, No. 22 Negative Pressure Wound Therapy An Evidence Update Presented to the Ontario Health Technology Advisory Committee in September 2010 December
Ontario Health Technology Assessment Series 2010; Vol. 10, No. 22 Negative Pressure Wound Therapy An Evidence Update Presented to the Ontario Health Technology Advisory Committee in September 2010 December
Granulox Update on evidence and science
 Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
