Treatment Timing & Follow-up Acute SCI SCI 12 hours F/U 6m. Began 8/2013 N. America Multicenter 351 subjects. Began 6/2013 Canada 248 subjects
|
|
|
- Maximilian Logan
- 6 years ago
- Views:
Transcription
1 AOSpine N. Am Research Network, Reeve Foundation, Dept of Defense Rick Hansen Institute NCT Rick Hansen Institute U of Calgary Alberta Paraplegia Foundation NCT Ohio State Univ. NCT Riluzole 2 x 100 mg by mouth or feeding tube the first 24 hours followed by 2 x 50 mg for the following 13 days after injury vs. placebo in acute SCI Twice Daily IV Minocycline vs. Placebo for over seven days All patients receive decompressive spine surgery and Blood Pressure management per protocol 72 hour IV infusion of glyburide (RP-1127) started within 6 hours of SCI yr Age C4-C8 A, B, C 16yr Age C0-C8 A, B, C, D 18-70yr Age C4-C8 A, B, C SCI 12 hours SCI 12 hours SCI 6hrs Began 8/2013 N. America Multicenter 351 subjects Began 6/2013 Canada 248 subjects Began 8/2015 USA multisite 10 subjects Phase 2/3 Double-Blind Phase 3 Efficacy/Safety Change in ISNCSCI total motor score from baseline to 6months of F/U Efficacy/Safety ISNCSCI Motor Score recovery from baseline to examination between 3m and 1yr post injury; ISNCSCI Sensory Scores ; SCIM; QoL: SF-36 Preliminary Efficacy (n.s.) Multicenter Phase2/ 3 trial of riluzole vs. placebo for improving motor recovery in acute SCI 800 mg initial dose tapered 100mg each dose to 400mg then continued twice daily x 7days Single group early phase safety study of IV glyburide in acute SCI Vertex Pharmaceuticals NCT Kringle Pharma, Inc NCT Jan Schwab, Elise Kroner Fresnius Foundation, Charite University NCT Hotchkiss Brain Inst U of Calgary NCT VX-210 (3mg or 9mg dose) in fibrin sealant applied to the dura at the time of spinal decompression/stabilization surgery within 72hr of SCI IT injection of 0.6mg Hepatocyte Growth Factor (HGF) vs. placebo starting at 72hr post injury, then weekly x5 weeks Dolormin extra (ibuprofen) 2400mg daily (800mg 3 times per day) for 4 wks (6 subjects) or 12wks (6subjects) Medical management of blood pressure to target of mean arterial pressure 65mmHg vs. 85mmHg for 7days following SCI 14-75yr Age C4-6 Motor Level each side A, B 18-75yr Age C4-C8 A, B C4-T4 A, B 16yr Age C0-T12 A, B, C No Central Cord SCI 72hr SCI 72hr F/U 24wk 4d SCI 21d SCI 12hr Began 2/ subjects Began 6/2014 Japan 48 subjects Began 6/2014 Germany 12 subjects Began 3/2012 Calgary, Alberta 100 subjects Phase 2/3 Placebo Controlled Phase 1 Phase 3 ISNCSCI UEMS/Motor Level CUE-T GRASSP Pharmacokinetics Safety/Efficacy ASIA Motor Score Change 24wk Safety/Efficacy Severe Gastroduodenal Bleed Modified Ashworth Scale Neuropathic Pain Scale ISNCSCI ASIA motor score change ASIA sensory score change improvement SF-36 SCIM, FIM to determine whether VX-210 delivered during spinal surgery is effective in neurological recovery and functional capacity in persons with acute SCI of intrathecal HGF vs. placebo given within 72h then daily for 5 days Safety study of oral ibuprofen for 1 or 3 months in acute SCI also measuring ISNCSCI, spasticity and pain outcomes Non-inferiority study of hypotension avoidance vs. induced hypertension 1
2 Oregon Health and Science University Dept of Defense NCT Pharmacological management of blood pressure in persons with acute SCI; comparing BP kept in a higher range (85-90mmHg), vs. BP kept in a normal range (MAP 65-70mmHg) for 7 days 18yr Age C0-T8 A, B No Central Cord No Penetrating Injury Time post SCI ns Not yet recruit USA Multicenter 152 subjects Single Blind ASIA motor score change ASIA sensory score change Pain Scores QoL Satisfaction Score Cardiovascular Randomized Trial of Early Hemodynamic Management of Patients Following Acute Spinal Cord Injury, comparing 2 BP target ranges St. Joseph s Hosp NCT Moleac Pte Ltd. NCT Emory University Wings for Life NCT Emory University NICHD NCT Emory University US Dept of Defense NCT CSF Drainage (target IT pressure 10mmHg) and elevation of Mean Arterial Pressure (MAP) with norepinephrine (goal mmhg) vs. Elevation/maintenance of MAP alone with norepinephrine (goal 85-90mmHg) for 5d. NeuroAiD (a natural product combining several Chinese herbal ingredients) given in oral capsule form for 6 months; combined with standard rehabilitation therapies Effect of acute intermittent hypoxia (AIH, breathing air with low oxygen) vs. Room air (breathing air with normal oxygen) placebo on Leg Function following SCI Effect of acute intermittent hypoxia (AIH, breathing air with low oxygen) with caffeine or placebo vs. Room air (breathing air with normal oxygen) sham with caffeine or placebo on Leg Function following SCI (Caffeine Sub-study) 10 sessions of daily acute intermittent hypoxia (daih) vs. daily room air (dsham); ambulatory subjects in both groups will also receive 60 minutes of walking practice at a frequency of up to 5 days each week for 2 weeks 18-75yr Age C4-C8 A, B, C A, B 18-77yr Age C4-T12 C, D 18-77yr Age C2-T11 C, D C4-T11 Some motor function below neuro level B, C, D SCI 24h F/U 180d Acute/Subacute SCI 3d-4wk post SCI SCI>12m F/U 4m SCI>12m F/U 2weeks Subacute SCI SCI for 2-4m F/U 2weeks Began 10/2015 USA Arizona, Alabama 60 subjects Began 6/2015 Malaysia 30 subjects Began 10/2014 Atlanta 20 Subjects Began 10/2014 Atlanta 20 Subjects Began 10/2015 Atlanta, GA 125 subjects Phase 2B Phase 4 Case Series Double Blinded Placebo Controlled Crossover Double Blinded Placebo Controlled Crossover Change in IT Pressure ISNCSCI TMS UEMS, LEMS, sensory scores SCIM Pain ISNCSCI Motor/Sensory Scores SCIM SF-8 Change in over ground walking speed and endurance Change in over ground walking speed and endurance Muscle Strength Coordination Kinematics Force Production during walking TUG 6 minute walk test 10 meter walk test Pain, Spasticity Hypertension Autonomic Dysreflexia incidence to study the effect of CSF drainage and BP support in acute SCI Open label study of Chinese herbal supplement plus rehabilitation in acute/subacute SCI Repetitive Exposure of Intermittent Hypoxia to Enhance Walking Recovery in Persons With Chronic Spinal Cord Injury on the Effects of Caffeine and Low Oxygen Therapy on Leg Function in Human Spinal Cord Injury of daily acute intermittent hypoxia vs. sham (room air) in nonambulatory and ambulatory subacute incomplete SCI to determine effect on recovery of walking function 2
3 Emory University NICHD NCT Effect of acute intermittent hypoxia (AIH, breathing air with low oxygen) vs. Room air (breathing air with normal oxygen) sham on Leg Function following SCI 18-77yr Age C2-T11 C, D SCI>12m F/U 2weeks Began 10/2014 Atlanta 44 Subjects Double Blinded Placebo Controlled Crossover Change in over ground walking speed and endurance Muscle Strength Coordination Kinematics Force Production during walking BDNF, Apolipoprotein E Polymorphisms to gain understanding of underlying mechanisms of AIH effect on Leg Function after SCI University of Miami Miami Project NCT Asterias Biotherapeutics NCT Surgical implantation of autologous Schwann Cells harvested from the sural nerve of the participant transplanted into the epicenter of the participant s spinal cord injury Surgical spinal cord implantation of embryonic stem cell-derived Oligodendrocyte Progenitor Cells (AST-OPC1). Dose escalation with sequential cohorts receiving an 2, 10, or 20 million AST-OPC1 at a single time-point days after injury. SCI C5-T12 (Thoracic cohort followed by Cervical Cohort) A, B, C SCI 3cm length 18-69yr Age C5-C7 A, B SCI 12m Subacute SCI days post SCI Began 1/2015 Miami 10 Subjects Began 3/2015 USA multisite 35 subjects Phase 1 a Safety/Efficacy Change in ISNCSCI Exam from baseline to 12 months Imaging of the Spinal Cord Neuropathic Pain measure Others: SCIM, FIM, Neurophysiology, autonomic, etc. Safety Incidence of ISNCSCI UEMS Motor Level of the safety of autologous human Schwann cell (ahsc) transplantation in participants with chronic SCI receiving rehabilitation a Dose Escalation of AST-OPC1 in Subjects With Cervical A and B SCI Hospital Sao Rafael NCT Hospital Sao Rafael NCT Autologous Mesenchymal Cells (Bone Marrow) transplanted into the spinal cord via transcutaneous injection (location n.s.) Autologous bone marrow mesenchymal stem cell transplantation in patients with cervical chronic and complete spinal cord injury (location n.s.) SCI below T8 A C5-C7 A >6m 12m Began 1/2015 Brazil 5 subjects Began 10/2015 Brazil 10 subjects Phase 1 Sensory/motor examination LEMS/ change Urodynamics AE assessed by spinal cord Sensory Mapping Neuropathic Pain Small pilot trial to study percutaneous Spinal Cord injection of Mesenchymal SC Autologous Mesenchymal Stem Cells Transplantation in Subjects With Cervical Chronic Complete SCI Hospital Sao Rafael NCT Autologous mesenchymal cells transplantation. Two percutaneous injections (location n.s.) of mesenchymal stem cells, with a 3-month interval between the injections; vs. randomly assigned control group without any specific intervention T1-L2 A 12m Not yet recruit Brazil 40 subjects Phase 2 AE assessed by spinal cord Sensory Mapping Neuropathic Pain for the evaluation of autologous mesenchymal stem cell transplantation in thoracolumbar chronic complete SCI 3
4 Neurogen Brain and Spine Institute NCT Autologous Bone Marrow Mononuclear Cells administered by IT injection via lumbar puncture, followed by vigorous rehabilitation therapy 1-65yr Age SCI of any type Time post-sci Not Specified Began 8/2012 Mumbai, India 500 subjects Phase 2 nonrandomized Change in symptoms of SCI FIM Large Phase 2, Nonrandomized, open label, parallel group trial of BM stem cells Da Nang Hospital NCT University of Jordan NCT Autologous Bone Marrow Mononuclear Cells administered by IT injection via lumbar puncture Comparison of Autologous Mesenchymal Stem Cells derived from Bone Marrow vs. Adipose Tissue administered by three separate IT injections 20-60yr Age SCI Level N.S. A, B 18-79yr Age SCI Level N.S A, B, C Subacute-Chronic SCI 3wk to 1yr Subacute-Chronic SCI 2w Began 9/2016 Da Nang, Vietnam 30 subjects Began 11/2016 Aman, Jordan 14 subjects Randomized Safety/ ISNCSCI Motor/Sensory Safety/ ISNCSCI Open label, single group study of bone marrowderived mononuclear cells transplanted via lumbar puncture Comparison of autologous Bone Marrow-derived and Adipose Tissue-derived stem cells Ferrer Internacional NCT BioArctic Neuroscience AB NCT Sun Yat-Sen Univ. 3rd Affil. Hospital NCT Chinese Acad. of Sci University of CAPF Soochow University NCT Single intramedullary injection of FAB117-HC, a medicinal product containing human allogeneic adipose-derived adult mesenchymal stem cells in either 20 million or 40 million cell doses; Phase 2 includes untreated control group; treatment group receives highest tolerated dose from Phase 1 Surgical implantation of SC0806 (a biodegradable device with heparin-activated FGF1 and peripheral nerve implants); both surgical implant and control groups receive rehabilitation (walking training). Control subjects will be offered SC0806 treatment after completion of their rehabilitation IT administration of up to 1x 10 6 umbilical cord mesenchymal stem cells per kg, every month for 4 months Collagen scaffold transplanted into spinal cord after acute spinal cord injury T5-10 (Phase 1) T1-12 (Phase 2) A (Phase 1), A, B (Phase 2) T2-T11 A 18-60yr Age SCI Level n.s. A, B, C, D C5-T12 A Phase 1: hr Phase 2: 24-72hr 4m-48m post SCI F/U 18m Acute-Chronic 2w-1yr post-sci SCI 21d Not yet recruit Spain 46 subjects Began 6/2015 Sweden 27 subjects Began 1/2014 China 44 subjects Began 4/2015 Soochow, and Tianjin, China 10 subjects Randomized Phase 3 Phase 1 Safety/ ISNCSCI SSEP MEP Safety/ MEP improvement ISNCSCI ASIA score change EMG Electroneurophysiology SSEP, MEP of medicinal product containing allogeneic adipose-derived adult mesenchymal stem cells pulsed with H2O2, injected into SCI during clinical decompressive spine surgery Rehabilitation-controlled studying SC0806 (a biodegradable device with heparin-activated FGF1 and nerve implants) IT injection of umbilical cord blood mesenchymal stem cells Functional Neural Regeneration Collagen Scaffold Transplantation in Complete 4
5 Chinese Acad. of Sci University of CAPF NCT Surgical implantation of NeuroRegen scaffold with either 10 7 mesenchymal stem cells or 10 7 neural stem cells into the spinal cord in patients with chronic spinal cord injury. All patients have surgical removal of spinal cord scar tissue, and have post-operative comprehensive rehabilitation C5-T12 A Time after SCI n.s. Began 1/2016 Tianjin, China 30 Subjects SSEP/MEP FIM Bladder/Bowel Function Safety/Tolerability/AE to assess the efficacy & safety of mesenchymal stem cells or neural stem cells combined with NeuroRegen scaffold transplantation in patients with chronic SCI Chinese Acad. of Sci PLA Gen Hospital NCT NeuroRegen Scaffold with bone marrow mononuclear cell transplantation vs. intradural decompression and adhesiolysis in persons with chronic SCI 18-60yr Age Thoracic Level A Time after SCI n.s. Began 1/2016 Beijing, China 22 subjects SSEP/MEP FIM Bladder/Bowel Function Safety/Tolerability/AE comparing NeuroRegen scaffold with BM mononuclear cells vs. intradural decompression with lysis of adhesions InVivo Therapeutics NCT Surgical Implantation of PLGA Poly-L-Lysine Scaffold (Neuro-Spinal Scaffold) into the injured spinal cord in subjects with complete thoracic A spinal cord injury 16-70yr Age T2-T12 A Can receive implant within 96h of SCI contusion 4mm Able to receive implant 96h after SCI Began 4/ USA Centers 20 Subjects Phase 3 Motor Scores/Sensory Scores Bowel/Bladder Function Incidence of AE Humanitarian Device Exemption (HDE) Probable Benefit to demonstrate safety and probable benefit in support of future studies and an HDE application with subsequent FDA approval Washington U US Department of Defense NCT Brachialis branch to anterior interosseous nerve transfer Cervical SCI; No hand function; A, B, C or central cord syndrome; SCI>6mos >6m Began 10/2012 St. Louis, MO 20 subjects Phase not specified; Upper Extremity Strength (Manual Muscle Testing) DASH scale SF-36 Complication rates of peripheral nerve transfer for improving UE strength in patients with tetraplegia/no hand function U British Columbia NCT Supinator branch to posterior interosseous nerve transfer 18yr Age Cervical SCI 12m>SCI>6m ICSH m>SCI>6 Began 6/2012 Vancouver, BC 10 Subjects Phase 4 Upper Extremity Strength (Manual Muscle Testing) Active Range of Motion DASH scale of peripheral nerve transfer for improving UE strength in patients with tetraplegia 5
6 Kunming Tongren Hospital China SCI Network NCT Surgical decompression/untethering of the spinal cord, combined with daily intensive weight bearing rehabilitation compared to daily intensive weight bearing rehabilitation alone yr Age T1-T12 A SCI 12m Began 7/2015 Kunming, China 30 Subjects Single Blind Kunming Locomotor Scale WISCI SCIM Modified Ashworth, Pain Surgical Decompression/Untethering Combined With Weight Bearing Rehabilitation in Chronic Spinal Cord Injury Subjects Tokyo University NCT Early (<24h) vs. Delayed (>2wk) Decompression surgery for acute cervical SCI in patients with cervical canal stenosis without bony injury 20-79yr Age Cervical below C5 C Acute/Subacute Admitted within 48 hours of SCI Began 12/2011 Japan 100 subjects Safety/Efficacy ISNCSCI motor sensory examination; SCIM; walking ability Test of whether timing of spinal cord decompression is associated with neurological outcome in SCI without fracture/dislocation Nantes Univ Hosp NCT Randomized assignment to early (within 48hr) vs. delayed (at 15 days) spinal decompression surgery 18 yr Age C2-T1 A-D Contusive SCI on with narrow canal SCI eligible for surgery within 48hrs F/U 2yr Not yet enrolling France 72 subjects ISNCSCI TMS, UEMS, CUE WISCI II SF-36 AE/Complications to compare SCI outcomes of decompressive spine surgery within 48hr vs. surgery performed at 15 days This table is abstracted from the clinical trial registration website using the search term Spinal Cord Injury and is updated periodically. The most recent update occurred December 5, 2016 at which time the search found a total of 831 SCI trials. Of these, the status of 271 trials was known and listed as currently recruiting or not yet recruiting. The table includes 34 SCI trials from the search that: 1) are currently actively recruiting or soon-to-be recruiting subjects; 2) are interventional (testing an intervention/treatment) using drugs, cell therapies, surgery, or hypoxia; and 3) targeted neurological or related functional improvement of the spinal cord as outcome measures. Interventional clinical trials are routinely registered on based on legal requirements* and because scientific journals may require registration for publication of the trial results. Investigators may choose not to register some early phase trials and those testing behavioral interventions, even though they may be important and scientifically rigorous studies. *U.S. Public Law requires the registration and reporting of results of certain applicable clinical trials, i.e., controlled interventional clinical trials that are subject to FDA regulation and that involve a Drug or Biologic (other than Phase I investigations), or Device (other than small feasibility studies); Terms/Abbreviations 6
7 NCT number: all trials registered with are assigned a registration number that begins with NCT (e.g. NCT ). The number listed for the trials in the table can be used in the search field of to access the webpage describing the trial, the study centers, and contact information in more detail. : Clinical trials usually progress in phases from 1 to 4. (Note: trials that are not on the path to FDA/regulatory approval (e.g. trials of surgical techniques or rehabilitation therapies) may not have a phase designation.) 1. Phase 1 trials are usually first-in-human or first-in-disease/condition experiments that are intended to demonstrate feasibility (can it be done), safety (is it reasonably safe), and tolerability (are the side effects tolerable). Phase 1 trials usually do not include a comparison control group and as such, do not provide direct evidence of the interventions efficacy. Phase 1 trials usually enroll a small number of subjects and are commonly done at a single study center but may use a small number of collaborating centers. 2. Phase 2 trials follow successful completion of Phase 1. Phase 2 trials are used to develop information on intervention administration (how to give), dose (how much to give), timing (when and how long to give), effect of the intervention on the body (what does it do, beneficial or harmful). Phase 2 trials commonly utilize multiple study centers, many subjects, and include a randomized control group to provide direct information about efficacy and safety of the intervention. Phase 2 trials enable refinement of how to administer the intervention and how to measure its beneficial effects (what Outcome Measurement to use). 3. Phase 3 trials are conducted using the refined protocols developed from Phase 2 trials. Phase 3 trials are often termed pivotal studies because they are sufficiently well-designed and rigorously conducted that their results, if positive, can be used to make the case for regulatory approval (e.g. trials that lead to FDA approval for clinical use). Phase 3 trials almost always enroll large numbers of subjects (in the hundreds or more), use multiple study centers, and randomized control group design (with placebo control and double blinding if feasible). The FDA generally requires two successful confirmatory Phase 3 trials of an intervention for approval. 4. Phase 4 trials are conducted after regulatory (e.g. FDA) approval to gather additional safety and efficacy data. : a trial in which there is no attempt to conceal the identity of the intervention from the subjects; i.e. there is no blinding or masking of the intervention the subjects know that they are receiving either an active ingredient or a placebo. : Randomized Controlled Trial a clinical trial in which subjects are randomly (like flipping a coin) assigned to either receive the active treatment or an alternative (control). Welldesigned s minimize the influence of variables other than the intervention that might have an effect on the desired outcome. For this reason, they provide the best evidence of efficacy and safety. The most rigorous s utilize a placebo (inactive) control group and blinding (concealing active vs. control assignment) to minimize bias in the interpretation of study results. IV: intravenous administration of a drug by vein IT: intrathecal, within the subarachnoid space surrounding the spinal cord e.g. administration of a drug into the subarachnoid space which contains the cerebrospinal fluid (CSF) SQ: subcutaneous administration of a drug by injection beneath the skin F/U: follow-up 7
8 n.s.: not specified ISNCSCI: International Standards for Neurological Classification of Spinal Cord Injury sometimes referred to as the ASIA (American Spinal Injury Association) standards. This refers to the accepted international standards for performing motor/sensory physical examination of persons with spinal cord injury and the classification scheme for documenting the neurological level and the severity (completeness) of injury. TMS/UEMS/LEMS: Total Motor Score/Upper Extremity Motor Score/Lower Extremity Motor Score are components of the ISNCSCI that include the ASIA Motor Index Score (the TMS) and the sub-components of the UEMS and the LEMS which are commonly analyzed and reported separately. : the ASIA (American Spinal Injury Association) Impairment Scale is a component of the ISNCSCI that classifies the degree of motor/sensory sparing below the level of injury. The scale ranges from A (most severe, complete injury with no sparing of sensory/motor function in the sacral segments S4-S5 that innervate the anus/rectum) to E (normal). B describes sensory only sparing; C describes sensory and very weak motor sparing; D describes sensory and stronger but not normal motor sparing. Central Cord Syndrome/Cervical Central Cord Syndrome: motor incomplete cervical SCI in which the upper extremities are significantly more impaired than the lower extremities Frankel Scale: an older scale for classifying severity of injury that was modified in 1992 to create the. Kunming Locomotor Scale: a 10-grade Roman numeral locomotion scoring system describing ability to stand, ability to walk, and required support/devices. SCIM/SCIM II/: the Spinal Cord Independence Measure is a measure of a person s ability to perform certain activities independently; i.e. an outcome measure of a research subject s independence in the performance of a variety of specific activities. FIM: the Functional Independence Measure was developed to measure the burden of care in persons who were not independent in ADL, hygiene/self-care, and mobility. The FIM and its subscales have been used as an outcome measure of a research subject s independence in the performance of a variety of specific activities. EMG: the electromyogram refers to a physiological test of muscle and nerve function. GRASSP: Graded Redefined Assessment of Strength, Sensibility, and Prehension is a clinical measurement of upper limb function for use in person with tetraplegia (quadriplegia) ICSH: International Classification for Surgery of the Hand in Tetraplegia is a clinical measure of hand function used by surgeons performing reconstructive surgery to improve function in persons with tetraplegia 8
9 IANR-SCIFRS: the International Association of Neurorestoratology-Spinal Cord Injury Functional Rating Scale. Penn Spasm Frequency Scale: a measure of spasticity based on frequency of spasm occurrence VAS: Visual Analogue Scale a scale commonly used to assess the severity of pain DASH: Disability of Arm, Shoulder, Hand scale is a measure of the upper extremity function Ashworth/Modified Ashworth: a scale used to measure spasticity severity Barthel Index: a measure of performance in Activities of Daily Living (ADL) and Mobility SF-36: the Short Form-36 is a patient-reported survey of health status. The SF-36 is commonly used as a measure of Health-Related Quality of Life 9
Treatment Timing & Follow-up Acute SCI SCI 12 hours F/U 6m. Began 8/2013 N. America Multicenter 351 subjects. Began 6/2013 Canada 248 subjects
 AOSpine N. Am Research Network, Reeve Foundation, Dept of Defense Rick Hansen Institute NCT01597518 Rick Hansen Institute U of Calgary Alberta Paraplegia Foundation NCT01828203 Ohio State Univ. NCT02524379
AOSpine N. Am Research Network, Reeve Foundation, Dept of Defense Rick Hansen Institute NCT01597518 Rick Hansen Institute U of Calgary Alberta Paraplegia Foundation NCT01828203 Ohio State Univ. NCT02524379
Treatment Timing & Follow-up Acute SCI SCI 12 hours F/U 6m. Began 8/2013 N. America Multicenter 351 subjects. Began 6/2013 Canada 248 subjects
 AOSpine N. Am Research Network, Reeve Foundation, Dept of Defense Rick Hansen Institute NCT01597518 Rick Hansen Institute U of Calgary Alberta Paraplegia Foundation NCT01828203 Ohio State Univ. NCT02524379
AOSpine N. Am Research Network, Reeve Foundation, Dept of Defense Rick Hansen Institute NCT01597518 Rick Hansen Institute U of Calgary Alberta Paraplegia Foundation NCT01828203 Ohio State Univ. NCT02524379
Current clinical drug trials for improving functional outcomes in spinal cord injury (updated 4 March 2014)
 Current clinical drug trials for improving functional outcomes in spinal cord injury (updated 4 March 2014) Compiled by Ashley Cooper, Queensland Brain Institute 1. The Rick Hansen Institute Canada Inc
Current clinical drug trials for improving functional outcomes in spinal cord injury (updated 4 March 2014) Compiled by Ashley Cooper, Queensland Brain Institute 1. The Rick Hansen Institute Canada Inc
U.S. National Institutes of Health & Australian/New Zealand Clinical Trial Registry Database Records for Current Clinical Drug Trials
 SpinalCure Australia Tuesday 9 th January, 2018 U.S. National Institutes of Health & Australian/New Zealand Clinical Trial Registry Database Records for Current Clinical Drug Trials Improving Functional
SpinalCure Australia Tuesday 9 th January, 2018 U.S. National Institutes of Health & Australian/New Zealand Clinical Trial Registry Database Records for Current Clinical Drug Trials Improving Functional
Experimental treatments for spinal cord injury:
 Experimental treatments for spinal cord injury: WHAT YOU SHOULD KNOW VERSION 2 A GUIDE FOR PEOPLE LIVING WITH SPINAL CORD INJURY, THEIR FAMILY, FRIENDS AND HEALTH CARE PROFESSIONALS Authors John D Steeves
Experimental treatments for spinal cord injury: WHAT YOU SHOULD KNOW VERSION 2 A GUIDE FOR PEOPLE LIVING WITH SPINAL CORD INJURY, THEIR FAMILY, FRIENDS AND HEALTH CARE PROFESSIONALS Authors John D Steeves
Case Report Functional Recovery in Chronic Stage of Spinal Cord Injury by Neurorestorative Approach: A Case Report
 Case Reports in Surgery, Article ID 404207, 4 pages http://dx.doi.org/10.1155/2014/404207 Case Report Functional Recovery in Chronic Stage of Spinal Cord Injury by Neurorestorative Approach: A Case Report
Case Reports in Surgery, Article ID 404207, 4 pages http://dx.doi.org/10.1155/2014/404207 Case Report Functional Recovery in Chronic Stage of Spinal Cord Injury by Neurorestorative Approach: A Case Report
Time Frames Post-SCI Follow-up Subacute/Chronic SCI < 12 months F/U 8 weeks
 Kowloon Hospital, Hong Kong Polytechnic Univ. NCT01989806 Department of Veterans Affairs NCT01740128 University of Zurich NCT01147185 University of Ioannina NCT02031835 ed of Robotic-assisted body weight
Kowloon Hospital, Hong Kong Polytechnic Univ. NCT01989806 Department of Veterans Affairs NCT01740128 University of Zurich NCT01147185 University of Ioannina NCT02031835 ed of Robotic-assisted body weight
Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury
 Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury James Guest MD, PhD, FACS Clinical Professor of Neurological Surgery, Neurosurgery and the Miami Project to
Is Spinal Cord Repair a Reality? Schwann Cell Transplantation for Subacute Spinal Cord Injury James Guest MD, PhD, FACS Clinical Professor of Neurological Surgery, Neurosurgery and the Miami Project to
A Structural Service Plan: Towards Better and Safer Spine Surgeries. Department of Orthopaedics & Traumatology Tuen Mun Hospital
 A Structural Service Plan: Towards Better and Safer Spine Surgeries Department of Orthopaedics & Traumatology Tuen Mun Hospital Cheung KK Wong CY Chan Andrew Tse Alfred Chow YY Department of Orthopaedics
A Structural Service Plan: Towards Better and Safer Spine Surgeries Department of Orthopaedics & Traumatology Tuen Mun Hospital Cheung KK Wong CY Chan Andrew Tse Alfred Chow YY Department of Orthopaedics
regenerative medicine in the brain and the spinal cord spinal cord injuries
 regenerative medicine in the brain and the spinal cord spinal cord injuries primary and secondary events during SCI traumatic spinal cord injury (SCI) traumatic spinal cord injury (SCI) main goal is to
regenerative medicine in the brain and the spinal cord spinal cord injuries primary and secondary events during SCI traumatic spinal cord injury (SCI) traumatic spinal cord injury (SCI) main goal is to
Last Updated: July 20, 2016 Articles up-to-date as of: July 2015
 Reviewer ID: Christie Chan, John Zhu, Jeremy Mak, Kyle Diab Type of Outcome Measure: 10 Meter Walk Test (10MWT) Total articles: 21 Author ID Year Amatachaya Datta 2009 Ditunno et al. 2007 Duffell 2015
Reviewer ID: Christie Chan, John Zhu, Jeremy Mak, Kyle Diab Type of Outcome Measure: 10 Meter Walk Test (10MWT) Total articles: 21 Author ID Year Amatachaya Datta 2009 Ditunno et al. 2007 Duffell 2015
Interventional Pain Management
 Spinal Injections Can be beneficial for both chronic and acute pain depending on pathology Contraindications: Patient refusal Active infection Platelets less than 75 or inability to stop anticoagulation
Spinal Injections Can be beneficial for both chronic and acute pain depending on pathology Contraindications: Patient refusal Active infection Platelets less than 75 or inability to stop anticoagulation
Am I eligible for the TOPS study? Possibly, if you suffer from one or more of the following conditions:
 Am I eligible for the TOPS study? Possibly, if you suffer from one or more of the following conditions: Radiating leg pain Greater leg / buttock pain than back pain Severe pain sets in when walking as
Am I eligible for the TOPS study? Possibly, if you suffer from one or more of the following conditions: Radiating leg pain Greater leg / buttock pain than back pain Severe pain sets in when walking as
The physical, financial, and social sequelae of traumatic. Time is spine: a review of translational advances in spinal cord injury
 LITERATURE REVIEW J Neurosurg Spine 30:1 18, 2019 Time is spine: a review of translational advances in spinal cord injury JNSPG 75th Anniversary Invited Review Article *Jetan H. Badhiwala, MD, 1,2 Christopher
LITERATURE REVIEW J Neurosurg Spine 30:1 18, 2019 Time is spine: a review of translational advances in spinal cord injury JNSPG 75th Anniversary Invited Review Article *Jetan H. Badhiwala, MD, 1,2 Christopher
Objectives. SCI EDGE Outcome Recommenda3ons 1/23/12. Property of Kahn, Newman, Palma, Romney Tappan, Tefer3ller, Tseng, Weisbach 1
 Outcome Recommendations from the Neurology Section Spinal Cord Injury EDGE Taskforce Combined Sections Meeting of the American Physical Therapy Association, San Diego, CA January 21-24, 2013 SCI EDGE Taskforce
Outcome Recommendations from the Neurology Section Spinal Cord Injury EDGE Taskforce Combined Sections Meeting of the American Physical Therapy Association, San Diego, CA January 21-24, 2013 SCI EDGE Taskforce
Time Frames Post-SCI Follow-up Time post SCI N/S F/U 6wks. Chronic SCI SCI >12m F/U 16wks
 University of Ioannina NCT02031835 Hugo W. Moser Research Institute, Kennedy Krieger, Inc NCT02774603 North Norway Rehabilitation Center NCT00854555 Body Weight Supported Treadmill Training (BWSTT) as
University of Ioannina NCT02031835 Hugo W. Moser Research Institute, Kennedy Krieger, Inc NCT02774603 North Norway Rehabilitation Center NCT00854555 Body Weight Supported Treadmill Training (BWSTT) as
NVM5 NERVE MONITORING SYSTEM AN INTRODUCTION TO
 AN INTRODUCTION TO NVM5 NERVE MONITORING SYSTEM This booklet is designed to inform you about the use of NVM5 nerve monitoring in the course of your surgery. It is not meant to replace any personal conversations
AN INTRODUCTION TO NVM5 NERVE MONITORING SYSTEM This booklet is designed to inform you about the use of NVM5 nerve monitoring in the course of your surgery. It is not meant to replace any personal conversations
Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne
 Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne Michelle Krishnan MD, PhD. Translational Medicine Leader, Roche information is presented only for purposes of providing a
Roche/Genentech Anti-Myostatin Adnectin RG6206 Development Program in Duchenne Michelle Krishnan MD, PhD. Translational Medicine Leader, Roche information is presented only for purposes of providing a
Evidence-based on advanced therapy
 Evidence-based on advanced therapy A presentation to: ASCoN Workshop and Conference 7-10 th December 2017 Chiang Mai Thailand Assoc. Prof Dr Julia Patrick Engkasan Rehabilitation Physician University of
Evidence-based on advanced therapy A presentation to: ASCoN Workshop and Conference 7-10 th December 2017 Chiang Mai Thailand Assoc. Prof Dr Julia Patrick Engkasan Rehabilitation Physician University of
3/3/2016. International Standards for the Neurologic Classification of Spinal Cord Injury (ISNCSCI)
 International Standards for the Neurologic Classification of Spinal Cord Injury (ISNCSCI) American Spinal Injury Association International Spinal Cord Society Presented by Adam Stein, MD Chairman and Professor
International Standards for the Neurologic Classification of Spinal Cord Injury (ISNCSCI) American Spinal Injury Association International Spinal Cord Society Presented by Adam Stein, MD Chairman and Professor
Spinal Cord Stimulation for Multiple Sclerosis and Spinal Cord Injury
 Spinal Cord Stimulation for Multiple Sclerosis and Spinal Cord Injury R. Davis Neural Engineering Clinic, PO Box 480, Rockport, Maine, 04856, U.S.A. Introduction Since 1973, spinal cord stimulation (SCS)
Spinal Cord Stimulation for Multiple Sclerosis and Spinal Cord Injury R. Davis Neural Engineering Clinic, PO Box 480, Rockport, Maine, 04856, U.S.A. Introduction Since 1973, spinal cord stimulation (SCS)
Intra-operative neurologic injuries: Avoidance and prompt response
 Intra-operative neurologic injuries: Avoidance and prompt response James S. Harrop MD, FACS Professor Neurological and Orthopedic Surgery Director, Division of Spine and Peripheral Nerve Surgery Nsurg
Intra-operative neurologic injuries: Avoidance and prompt response James S. Harrop MD, FACS Professor Neurological and Orthopedic Surgery Director, Division of Spine and Peripheral Nerve Surgery Nsurg
New Approaches to Repair of Spinal Cord Injury
 New Approaches to Repair of Spinal Cord Injury 1 WHY IS THE SPINAL CORD SO VULNERABLE? Peripheral Nerve Spinal Cord Schwann Cell Myelin DRG Neuron Oligodendrocyte Myelin Collagen Axon 2 3 WHY IS THE SPINAL
New Approaches to Repair of Spinal Cord Injury 1 WHY IS THE SPINAL CORD SO VULNERABLE? Peripheral Nerve Spinal Cord Schwann Cell Myelin DRG Neuron Oligodendrocyte Myelin Collagen Axon 2 3 WHY IS THE SPINAL
Outcome Measures for Acute/Subacute Cervical Sensorimotor Complete (AIS-A) Spinal Cord Injury During a Phase 2 Clinical Trial
 Outcome Measures for Acute/Subacute Cervical Sensorimotor Complete (AIS-A) Spinal Cord Injury During a Phase 2 Clinical Trial John D. Steeves, 1 Daniel P. Lammertse, 2 John L.K. Kramer, 1 Naomi Kleitman,
Outcome Measures for Acute/Subacute Cervical Sensorimotor Complete (AIS-A) Spinal Cord Injury During a Phase 2 Clinical Trial John D. Steeves, 1 Daniel P. Lammertse, 2 John L.K. Kramer, 1 Naomi Kleitman,
Contemporary Management of Spinal Cord Injury
 Contemporary Management of Spinal Cord Injury Ali Salim, MD Professor of Surgery Chief, Division of Trauma, Burns, Surgical Critical Care, and Emergency General Surgery Disclosures I have nothing to disclose
Contemporary Management of Spinal Cord Injury Ali Salim, MD Professor of Surgery Chief, Division of Trauma, Burns, Surgical Critical Care, and Emergency General Surgery Disclosures I have nothing to disclose
CARE OF SPINAL CORD INJURY VICTIMS
 CARE OF SPINAL CORD INJURY VICTIMS Dr THIERRY ALBERT Centre de Rééducation et de Réadaptation pour Adulte de COUBERT Route de Liverdy, Coubert 77257 Brie comte robert, cedex talbert@ugecamif.fr Spinal
CARE OF SPINAL CORD INJURY VICTIMS Dr THIERRY ALBERT Centre de Rééducation et de Réadaptation pour Adulte de COUBERT Route de Liverdy, Coubert 77257 Brie comte robert, cedex talbert@ugecamif.fr Spinal
Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury. Original Policy Date
 MP 7.01.58 Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury Medical Policy Section Issue 12:2013 Original Policy Date 12:2013 Last Review
MP 7.01.58 Stimulation of the Sacral Anterior Root Combined with Posterior Sacral Rhizotomy in Patients with Spinal Cord Injury Medical Policy Section Issue 12:2013 Original Policy Date 12:2013 Last Review
Jessica Jameson MD Post Falls, ID
 Jessica Jameson MD Post Falls, ID Discuss the history of interventiona l pain Discuss previous tools to manage chronic pain Discuss current novel therapies to manage chronic pain and indications HISTORY
Jessica Jameson MD Post Falls, ID Discuss the history of interventiona l pain Discuss previous tools to manage chronic pain Discuss current novel therapies to manage chronic pain and indications HISTORY
Localizing and Characterizing Neural Plasticity in Spinal Cord Injury
 PVA Summer 2012 Expo The Effect of Rehabilitation on the Neurobiology of MS and SCI Las Vegas, NV, August 30, 2012 Localizing and Characterizing Neural Plasticity in Spinal Cord Injury Keith Tansey, MD,
PVA Summer 2012 Expo The Effect of Rehabilitation on the Neurobiology of MS and SCI Las Vegas, NV, August 30, 2012 Localizing and Characterizing Neural Plasticity in Spinal Cord Injury Keith Tansey, MD,
Lack of muscle control (Stroke, bladder control, neurological disorders) Mechanical movement therapist assisted
 By Lisa Rosenberg Electrical Current Stimulates muscles and nerves Produces movement Helps Individuals with Disabilities Lack of muscle control (Stroke, bladder control, neurological disorders) Passive
By Lisa Rosenberg Electrical Current Stimulates muscles and nerves Produces movement Helps Individuals with Disabilities Lack of muscle control (Stroke, bladder control, neurological disorders) Passive
ASIA impairment scale (AIS)
 Physiotherapy management of people with SCI: the essentials Lisa Harvey ASIA impairment scale (AIS) COMPLETE: No motor or sensory in S4-S5 segments Moorong Spinal Unit Royal Rehabilitation Centre Sydney
Physiotherapy management of people with SCI: the essentials Lisa Harvey ASIA impairment scale (AIS) COMPLETE: No motor or sensory in S4-S5 segments Moorong Spinal Unit Royal Rehabilitation Centre Sydney
Chapter 32. Objectives. Objectives 01/09/2013. Spinal Column and Spinal Cord Trauma
 Chapter 32 Spinal Column and Spinal Cord Trauma Prehospital Emergency Care, Ninth Edition Joseph J. Mistovich Keith J. Karren Copyright 2010 by Pearson Education, Inc. All rights reserved. Objectives 1.
Chapter 32 Spinal Column and Spinal Cord Trauma Prehospital Emergency Care, Ninth Edition Joseph J. Mistovich Keith J. Karren Copyright 2010 by Pearson Education, Inc. All rights reserved. Objectives 1.
Soleus 75 (6 ml) 0 (6 ml) 75 (6 ml. Tibialis posterior 75 (6 ml) 0 (6 ml) 75 (6 ml) Total 300 (24 ml) 0 (24 ml) 300 (24 ml) Dose: U (solution volume)
 Study No.: BTX108512 Title: A Multicenter Study to Evaluate the Efficacy and Safety in Patients with Post-Stroke lower Limb Spasticity Receiving a Double-Blind, -Controlled GSK1358820 Treatment Followed
Study No.: BTX108512 Title: A Multicenter Study to Evaluate the Efficacy and Safety in Patients with Post-Stroke lower Limb Spasticity Receiving a Double-Blind, -Controlled GSK1358820 Treatment Followed
Motor and Gait Improvement in Patients With Incomplete Spinal Cord Injury Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation
 Motor and Gait Improvement in Patients With Incomplete Spinal Cord Injury Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation J. Benito, MD, 1 H. Kumru, PhD, 1 N. Murillo, PhD, 1 U.
Motor and Gait Improvement in Patients With Incomplete Spinal Cord Injury Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation J. Benito, MD, 1 H. Kumru, PhD, 1 N. Murillo, PhD, 1 U.
Last Updated: July 26, 2012
 Reviewer ID: Nicole Elfring Type of Outcome Measure: Functional Independence Measure (FIM) Total articles: 27 Author ID Year Davidoff 1990 Segal et al. Marino et al. Yavuz et al. 1998 Study Design Setting
Reviewer ID: Nicole Elfring Type of Outcome Measure: Functional Independence Measure (FIM) Total articles: 27 Author ID Year Davidoff 1990 Segal et al. Marino et al. Yavuz et al. 1998 Study Design Setting
Odontoid Fractures and Other Cervical Trauma: Geriatric Considerations
 Odontoid Fractures and Other Cervical Trauma: Geriatric Considerations Vishal Khatri, MD Division of Spine Surgery Department of Orthopaedic Surgery Cooper Bone and Joint Institute Cooper University Health
Odontoid Fractures and Other Cervical Trauma: Geriatric Considerations Vishal Khatri, MD Division of Spine Surgery Department of Orthopaedic Surgery Cooper Bone and Joint Institute Cooper University Health
Fracture REduction Evaluation (FREE) Study
 Fracture REduction Evaluation (FREE) Study Efficacy and Safety of Balloon Kyphoplasty Compared with Non-surgical Care for Vertebral Compression Fracture (FREE): A Randomised Controlled Trial Wardlaw Lancet
Fracture REduction Evaluation (FREE) Study Efficacy and Safety of Balloon Kyphoplasty Compared with Non-surgical Care for Vertebral Compression Fracture (FREE): A Randomised Controlled Trial Wardlaw Lancet
Spinal Cord Injury. North American Spine Society Public Education Series
 Spinal Cord Injury North American Spine Society Public Education Series What Is a Spinal Cord Injury? A spinal cord injury is a condition that results from damage or trauma to the nerve tissue of the spine.
Spinal Cord Injury North American Spine Society Public Education Series What Is a Spinal Cord Injury? A spinal cord injury is a condition that results from damage or trauma to the nerve tissue of the spine.
Anatomy of the Spine. Figure 1. (left) The spine has three natural curves that form an S-shape; strong muscles keep our spine in alignment.
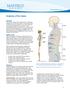 1 2 Anatomy of the Spine Overview The spine is made of 33 individual bony vertebrae stacked one on top of the other. This spinal column provides the main support for your body, allowing you to stand upright,
1 2 Anatomy of the Spine Overview The spine is made of 33 individual bony vertebrae stacked one on top of the other. This spinal column provides the main support for your body, allowing you to stand upright,
3/5/2014. Rehabilitation Technology versus Research Technology: Where/What is the Value?
 Technology Applied to SCI: The Value of Assistive Devices During SCI Recovery and While Living with SCI Mark S. Nash, Ph.D., FACSM Departments of Neurological Surgery and Rehabilitation Medicine Principal
Technology Applied to SCI: The Value of Assistive Devices During SCI Recovery and While Living with SCI Mark S. Nash, Ph.D., FACSM Departments of Neurological Surgery and Rehabilitation Medicine Principal
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Paraplegia: Exercise and Health Considerations. By: Juli and Trina
 Paraplegia: Exercise and Health Considerations By: Juli and Trina What is paraplegia? Paraplegia is impairment of motor and/or sensory function to the lower extremities, and sometimes the lower trunk Complete
Paraplegia: Exercise and Health Considerations By: Juli and Trina What is paraplegia? Paraplegia is impairment of motor and/or sensory function to the lower extremities, and sometimes the lower trunk Complete
Spinal injury. Structure of the spine
 Spinal injury Structure of the spine Some understanding of the structure of the spine (spinal column) and the spinal cord is important as it helps your Neurosurgeon explain about the part of the spine
Spinal injury Structure of the spine Some understanding of the structure of the spine (spinal column) and the spinal cord is important as it helps your Neurosurgeon explain about the part of the spine
In this presentation:
 Clinical Studies In this presentation: Total number of studies Publications Key clinical studies Israel: sub acute stroke Germany: sub acute stroke Italy: chronic stroke Japan: sub acute stroke New article
Clinical Studies In this presentation: Total number of studies Publications Key clinical studies Israel: sub acute stroke Germany: sub acute stroke Italy: chronic stroke Japan: sub acute stroke New article
New Zealand Spinal Cord Injury Registry. First Annual Report August 2016 to July 2017
 New Zealand Spinal Cord Injury Registry First Annual Report August 216 to July 217 2 The New Zealand Spinal Cord Injury Registry (NZSCIR) would like to acknowledge the spinal service clinicians and coordinators
New Zealand Spinal Cord Injury Registry First Annual Report August 216 to July 217 2 The New Zealand Spinal Cord Injury Registry (NZSCIR) would like to acknowledge the spinal service clinicians and coordinators
Spinal Cord Injury Transection Injury, Spinal Shock, and Hermiated Disc. Copyright 2014, 2011, 2006 by Saunders, an imprint of Elsevier, Inc.
 Spinal Cord Injury Transection Injury, Spinal Shock, and Hermiated Disc 1 Spinal Cord Injury Results from fracture and/or dislocation of vertebrae // Compresses, stretches, or tears spinal cord Cervical
Spinal Cord Injury Transection Injury, Spinal Shock, and Hermiated Disc 1 Spinal Cord Injury Results from fracture and/or dislocation of vertebrae // Compresses, stretches, or tears spinal cord Cervical
Innovations In Neuromodulation. Maged Guirguis, MD Director Of Research Pain Management
 Innovations In Neuromodulation Maged Guirguis, MD Director Of Research Pain Management 1 2 Pain Pathway 3 Gate Theory In the dorsal horn (where pain signals relay), there is a gate that opens and closes
Innovations In Neuromodulation Maged Guirguis, MD Director Of Research Pain Management 1 2 Pain Pathway 3 Gate Theory In the dorsal horn (where pain signals relay), there is a gate that opens and closes
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Spinal Cord Stimulation Hani N. Sabbah, Ph.D., FACC, FCCP, FAHA Professor of Medicine Wayne State University & Director of Cardiovascular Research
 Spinal Cord Stimulation Hani N. Sabbah, Ph.D., FACC, FCCP, FAHA Professor of Medicine Wayne State University & Director of Cardiovascular Research Henry Ford Health System Disclosure Information ESC 2011
Spinal Cord Stimulation Hani N. Sabbah, Ph.D., FACC, FCCP, FAHA Professor of Medicine Wayne State University & Director of Cardiovascular Research Henry Ford Health System Disclosure Information ESC 2011
The MOVE Trial Summary. Palovarotene FAQs. 1. What is the MOVE Trial?
 MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
MOVE TRIAL FAQS Contents The MOVE Trial Summary... 1 1. What is the MOVE Trial?... 1 Palovarotene FAQs... 1 2. What is palovarotene?... 1 3. What is an Orphan designation?... 2 4. What is a Fast Track
CRITICALLY APPRAISED PAPER (CAP)
 CRITICALLY APPRAISED PAPER (CAP) FOCUSED QUESTION To what extent do the effects of neuromuscular electrical stimulation (NMES) on motor recovery of the upper extremity after stroke persist after the intervention
CRITICALLY APPRAISED PAPER (CAP) FOCUSED QUESTION To what extent do the effects of neuromuscular electrical stimulation (NMES) on motor recovery of the upper extremity after stroke persist after the intervention
SCI EXAM & FUNCTIONAL PROGNOSIS
 SCI EXAM & FUNCTIONAL PROGNOSIS MARCH 20, 2015 JUAN L ASANZA, MD STAFF PHYSICIAN VA PUGET SOUND HEALTH CARE SYSTEM SPINAL CORD INJURY UNIVERSITY OF WASHINGTON PHYSICAL MEDICINE & REHABILITATION OBJECTIVES
SCI EXAM & FUNCTIONAL PROGNOSIS MARCH 20, 2015 JUAN L ASANZA, MD STAFF PHYSICIAN VA PUGET SOUND HEALTH CARE SYSTEM SPINAL CORD INJURY UNIVERSITY OF WASHINGTON PHYSICAL MEDICINE & REHABILITATION OBJECTIVES
Teresa Jacobson Kimberley, PhD, PT
 Teresa Jacobson Kimberley, PhD, PT Professor MGH Institute of Health Professions School of Rehabilitation and Health Sciences Department of Physical Therapy tkimberley@mgh.harvard.edu Disclosures Human
Teresa Jacobson Kimberley, PhD, PT Professor MGH Institute of Health Professions School of Rehabilitation and Health Sciences Department of Physical Therapy tkimberley@mgh.harvard.edu Disclosures Human
Curriculum Vitae Sushma Chandan, M.D. Revised 09/01/15
 Curriculum Vitae Sushma Chandan, M.D. Revised 09/01/15 Current Position Staff Physician PM&R Service Veterans Administration Medical Center, Atlanta, Georgia Assistant Professor Department of Rehabilitation
Curriculum Vitae Sushma Chandan, M.D. Revised 09/01/15 Current Position Staff Physician PM&R Service Veterans Administration Medical Center, Atlanta, Georgia Assistant Professor Department of Rehabilitation
NEWSLETTER. Technologies to Prioritise HORIZON SCANNING TECHNOLOGIES INCLUDED IN THIS NEWSLETTER
 HORIZON SCANNING Issue 3 / July 2016 NEWSLETTER Technologies to Prioritise The Institute for Safety, Compensation and Recovery Research (ISCRR) Horizon Scanning program is designed to identify new and
HORIZON SCANNING Issue 3 / July 2016 NEWSLETTER Technologies to Prioritise The Institute for Safety, Compensation and Recovery Research (ISCRR) Horizon Scanning program is designed to identify new and
2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves
 2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Joseph S. Cheng, M.D., M.S. Associate Professor of Neurological Surgery, Orthopedic Surgery, and Rehabilitation
2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Joseph S. Cheng, M.D., M.S. Associate Professor of Neurological Surgery, Orthopedic Surgery, and Rehabilitation
A Patient s Guide to Intraoperative Monitoring
 A Patient s Guide to Intraoperative Monitoring 228 West Main, Suite C Missoula, MT 59802 Phone: info@spineuniversity.com DISCLAIMER: The information in this booklet is compiled from a variety of sources.
A Patient s Guide to Intraoperative Monitoring 228 West Main, Suite C Missoula, MT 59802 Phone: info@spineuniversity.com DISCLAIMER: The information in this booklet is compiled from a variety of sources.
Disclosure. Esquenazi
 Learning Objectives At the conclusion of this activity, the participant will be able to: A. Understand the purpose and function of the ReWalk exoskeletal orthotic device. B. Understand the variation in
Learning Objectives At the conclusion of this activity, the participant will be able to: A. Understand the purpose and function of the ReWalk exoskeletal orthotic device. B. Understand the variation in
Dr Brigitta Brandner UCLH
 Dr Brigitta Brandner UCLH 2.5% paraplegia/paraparesis (EUROSTAR) Some studies up to 8% Immediate, recurrent and delayed 37% deficits are delayed: present 13 hours 91 days post op >50% will resolve with
Dr Brigitta Brandner UCLH 2.5% paraplegia/paraparesis (EUROSTAR) Some studies up to 8% Immediate, recurrent and delayed 37% deficits are delayed: present 13 hours 91 days post op >50% will resolve with
Neurologic improvement after thoracic, thoracolumbar, and lumbar spinal cord (conus medullaris) injuries
 Thomas Jefferson University Jefferson Digital Commons Department of Orthopaedic Surgery Faculty Papers Department of Orthopaedic Surgery 1-2011 Neurologic improvement after thoracic, thoracolumbar, and
Thomas Jefferson University Jefferson Digital Commons Department of Orthopaedic Surgery Faculty Papers Department of Orthopaedic Surgery 1-2011 Neurologic improvement after thoracic, thoracolumbar, and
Neuromodulation and Non- Pharmacological Approaches
 Multimodal approaches to pain management and potential synergies Neuromodulation and Non- Pharmacological Approaches Richard Wilson, MD Director, Division of Neurologic Rehabilitation MetroHealth Rehabilitation
Multimodal approaches to pain management and potential synergies Neuromodulation and Non- Pharmacological Approaches Richard Wilson, MD Director, Division of Neurologic Rehabilitation MetroHealth Rehabilitation
Asterias Biotherapeutics NYSE American: AST
 Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2014 Origination: 7/2013 Next Review: 1/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 9/2014 Origination: 7/2013 Next Review: 1/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
What is a spinal cord injury?
 Spinal Cord Injury What is a spinal cord injury? A spinal cord injury (SCI) is when the spinal cord is damaged Such damage causes 2 things: - loss or change of movement (paralysis) - loss or change of
Spinal Cord Injury What is a spinal cord injury? A spinal cord injury (SCI) is when the spinal cord is damaged Such damage causes 2 things: - loss or change of movement (paralysis) - loss or change of
Time Equals Neurons - Spinal Cord Injury Management in the first 4 Hours
 Time Equals Neurons - Spinal Cord Injury Management in the first 4 Hours William D. Whetstone M.D. Clinical Professor UCSF Department of Emergency Medicine SFGH ED Center for Neuro-Critical Emergencies
Time Equals Neurons - Spinal Cord Injury Management in the first 4 Hours William D. Whetstone M.D. Clinical Professor UCSF Department of Emergency Medicine SFGH ED Center for Neuro-Critical Emergencies
Introduction. Castellvi Spine Current FDA Trials: Disc Regeneration DISCLOSURE 5/27/2016
 Castellvi Spine Current FDA Trials: Disc Regeneration Dom Coric, M.D. Carolina Neurosurgery and Spine Associates Chief, Department of Neurosurgery, CMC Charlotte, NC 5/21/16 DISCLOSURE Spine Wave: Consultant/Stock/Royalties
Castellvi Spine Current FDA Trials: Disc Regeneration Dom Coric, M.D. Carolina Neurosurgery and Spine Associates Chief, Department of Neurosurgery, CMC Charlotte, NC 5/21/16 DISCLOSURE Spine Wave: Consultant/Stock/Royalties
Sir William Asher ANATOMY
 SPINAL CORD INJURY BASICS RELATED TO LIFE CARE PLANNING Lesson 1 Sir William Asher Picture the pathetic patient lying long abed, the urine leaking from his distended bladder, the lime draining from his
SPINAL CORD INJURY BASICS RELATED TO LIFE CARE PLANNING Lesson 1 Sir William Asher Picture the pathetic patient lying long abed, the urine leaking from his distended bladder, the lime draining from his
MRI of chronic spinal cord injury
 The British Journal of Radiology, 76 (2003), 347 352 DOI: 10.1259/bjr/11881183 E 2003 The British Institute of Radiology Pictorial review MRI of chronic spinal cord injury 1 K POTTER, FRCR and 1 A SAIFUDDIN,
The British Journal of Radiology, 76 (2003), 347 352 DOI: 10.1259/bjr/11881183 E 2003 The British Institute of Radiology Pictorial review MRI of chronic spinal cord injury 1 K POTTER, FRCR and 1 A SAIFUDDIN,
Chapter 12b. Overview
 Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
Chapter 12b Spinal Cord Overview Spinal cord gross anatomy Spinal meninges Sectional anatomy Sensory pathways Motor pathways Spinal cord pathologies 1 The Adult Spinal Cord About 18 inches (45 cm) long
AWARD NUMBER: W81XWH TITLE: Treatment of Pain and Autonomic Dysreflexia in Spinal Cord Injury with Deep Brain Stimulation
 AWARD NUMBER: W81XWH-12-1-0559 TITLE: Treatment of Pain and Autonomic Dysreflexia in Spinal Cord Injury with Deep Brain Stimulation PRINCIPAL INVESTIGATOR: Jonathan R. Jagid, M.D. CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-12-1-0559 TITLE: Treatment of Pain and Autonomic Dysreflexia in Spinal Cord Injury with Deep Brain Stimulation PRINCIPAL INVESTIGATOR: Jonathan R. Jagid, M.D. CONTRACTING ORGANIZATION:
Therapeutic Exercise And Manual Therapy For Persons With Lumbar Spinal Stenosis
 Therapeutic Exercise And Manual Therapy For Persons With Lumbar Spinal Stenosis The program consisted of manual therapy twice per week (eg, soft tissue and neural The components of the Boot Camp Program
Therapeutic Exercise And Manual Therapy For Persons With Lumbar Spinal Stenosis The program consisted of manual therapy twice per week (eg, soft tissue and neural The components of the Boot Camp Program
EDWARDS RESEARCH GRANTS AND SUPPORT
 EDWARDS RESEARCH GRANTS AND SUPPORT Edwards served as the principal investigator or co-investigator on a number of National and International grants and commissions since 2009 with accumulated earning
EDWARDS RESEARCH GRANTS AND SUPPORT Edwards served as the principal investigator or co-investigator on a number of National and International grants and commissions since 2009 with accumulated earning
Spinal Cord Injury. The nerves that go from the spinal cord to the arms, legs, chest, and abdomen are called peripheral nerves.
 Spinal Cord Injury Introduction Spinal cord injuries can be very devastating. More than 10,000 Americans experience spinal cord injuries each year, mainly due to auto or falling accidents. More than 200,000
Spinal Cord Injury Introduction Spinal cord injuries can be very devastating. More than 10,000 Americans experience spinal cord injuries each year, mainly due to auto or falling accidents. More than 200,000
Research Knowledge Forum SCI Q3 Report
 Research Knowledge Forum SCI 2015-Q3 Report Steering committee Michael Fehlings (Chairman) University of Toronto Toronto, Canada Start of Term: 01 Apr 2013 End of Term: 30 Jun 2016 Robert Grossman Bizhan
Research Knowledge Forum SCI 2015-Q3 Report Steering committee Michael Fehlings (Chairman) University of Toronto Toronto, Canada Start of Term: 01 Apr 2013 End of Term: 30 Jun 2016 Robert Grossman Bizhan
UPPER CERVICAL MYELOPATHY WITH METATROPIC DYSPLASIA
 UPPER CERVICAL MYELOPATHY WITH METATROPIC DYSPLASIA REPORT OF 4 SURGICAL CASES Yujiro Takeshita*, Kota Miyoshi**, Tomoaki Kitagawa** *Department of Orthopaedic and Spine Surgery, University of Tokyo, Japan
UPPER CERVICAL MYELOPATHY WITH METATROPIC DYSPLASIA REPORT OF 4 SURGICAL CASES Yujiro Takeshita*, Kota Miyoshi**, Tomoaki Kitagawa** *Department of Orthopaedic and Spine Surgery, University of Tokyo, Japan
2016 Top Papers in Critical Care
 2016 Top Papers in Critical Care Briana Witherspoon DNP, APRN, ACNP-BC Assistant Director of Advanced Practice, Neuroscience Assistant in Division of Critical Care, Department of Anesthesiology Neuroscience
2016 Top Papers in Critical Care Briana Witherspoon DNP, APRN, ACNP-BC Assistant Director of Advanced Practice, Neuroscience Assistant in Division of Critical Care, Department of Anesthesiology Neuroscience
ว ทยาการความก าวหน าในการร กษาผ ป วยบาดเจ บ กระด กส นหล งและไขส นหล ง. Piyawat Bintachitt, MD.
 ว ทยาการความก าวหน าในการร กษาผ ป วยบาดเจ บ กระด กส นหล งและไขส นหล ง Piyawat Bintachitt, MD. Thank you Outline Pathophysiology Neurological classification Imaging Airway management Cardiovascular management
ว ทยาการความก าวหน าในการร กษาผ ป วยบาดเจ บ กระด กส นหล งและไขส นหล ง Piyawat Bintachitt, MD. Thank you Outline Pathophysiology Neurological classification Imaging Airway management Cardiovascular management
A Patient s Guide to Cervical Foraminotomy
 A Patient s Guide to Cervical Foraminotomy 15195 Heathcote Blvd Suite 334 Haymarket, VA 20169 Phone: 703-369-9070 Fax: 703-369-9240 DISCLAIMER: The information in this booklet is compiled from a variety
A Patient s Guide to Cervical Foraminotomy 15195 Heathcote Blvd Suite 334 Haymarket, VA 20169 Phone: 703-369-9070 Fax: 703-369-9240 DISCLAIMER: The information in this booklet is compiled from a variety
Chapter 13. The Spinal Cord & Spinal Nerves. Spinal Cord. Spinal Cord Protection. Meninges. Together with brain forms the CNS Functions
 Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Spinal Cord Chapter 13 The Spinal Cord & Spinal Nerves Together with brain forms the CNS Functions spinal cord reflexes integration (summation of inhibitory and excitatory) nerve impulses highway for upward
Medical Policy Manual. Topic: Peripheral Subcutaneous Field Stimulation Date of Origin: April Section: Surgery Last Reviewed Date: April 2014
 Medical Policy Manual Topic: Peripheral Subcutaneous Field Stimulation Date of Origin: April 2013 Section: Surgery Last Reviewed Date: April 2014 Policy No: 188 Effective Date: July 1, 2014 IMPORTANT REMINDER
Medical Policy Manual Topic: Peripheral Subcutaneous Field Stimulation Date of Origin: April 2013 Section: Surgery Last Reviewed Date: April 2014 Policy No: 188 Effective Date: July 1, 2014 IMPORTANT REMINDER
Patient Information. Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System
 Patient Information Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Your doctor has recommended spinal fusion surgery using
Patient Information Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Spinal Fusion Using the ST360 or Silhouette Pedicle Screw System Your doctor has recommended spinal fusion surgery using
FOOT AND ANKLE ARTHROSCOPY
 FOOT AND ANKLE ARTHROSCOPY Information for Patients WHAT IS FOOT AND ANKLE ARTHROSCOPY? The foot and the ankle are crucial for human movement. The balanced action of many bones, joints, muscles and tendons
FOOT AND ANKLE ARTHROSCOPY Information for Patients WHAT IS FOOT AND ANKLE ARTHROSCOPY? The foot and the ankle are crucial for human movement. The balanced action of many bones, joints, muscles and tendons
22110 vertebral segment; cervical vertebral segment; thoracic vertebral segment; lumbar
 The following codes are authorized by Palladian Health for applicable product lines. Visit palladianhealth.com to request authorization and to access guidelines. Palladian Musculoskeletal Program Codes
The following codes are authorized by Palladian Health for applicable product lines. Visit palladianhealth.com to request authorization and to access guidelines. Palladian Musculoskeletal Program Codes
Management of Bone and Spinal Cord in Spinal Surgery.
 Management of Bone and Spinal Cord in Spinal Surgery. G. Saló, PhD, MD. Senior Consultant Spine Unit. Hospital del Mar. Barcelona. Ass. Prof. Universitat Autònoma de Barcelona. Introduction The management
Management of Bone and Spinal Cord in Spinal Surgery. G. Saló, PhD, MD. Senior Consultant Spine Unit. Hospital del Mar. Barcelona. Ass. Prof. Universitat Autònoma de Barcelona. Introduction The management
Patient Information MIS TLIF. Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine..............................................
Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine..............................................
3D titanium interbody fusion cages sharx. White Paper
 3D titanium interbody fusion cages sharx (SLM selective laser melted) Goal of the study: Does the sharx intervertebral cage due to innovative material, new design, and lordotic shape solve some problems
3D titanium interbody fusion cages sharx (SLM selective laser melted) Goal of the study: Does the sharx intervertebral cage due to innovative material, new design, and lordotic shape solve some problems
Facet Arthroplasty. Policy Number: Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019
 Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Update on early management of acute spinal cord injuries. Mr Jonathon L Richards Orthopaedic & Spinal surgeon
 Update on early management of acute spinal cord injuries Mr Jonathon L Richards Orthopaedic & Spinal surgeon Overview: Aetiology/Cost Pathophysiology Early surgical intervention Emerging therapies Steroids
Update on early management of acute spinal cord injuries Mr Jonathon L Richards Orthopaedic & Spinal surgeon Overview: Aetiology/Cost Pathophysiology Early surgical intervention Emerging therapies Steroids
Thoracic Spine Applied Anatomy. Jason Zafereo, PT, OCS, FAAOMPT
 Thoracic Spine Applied Anatomy Jason Zafereo, PT, OCS, FAAOMPT Clinical i l Orthopedic Rehabilitation ti Education Objectives Discuss concepts relevant to thoracic pain of red flag origin Discuss concepts
Thoracic Spine Applied Anatomy Jason Zafereo, PT, OCS, FAAOMPT Clinical i l Orthopedic Rehabilitation ti Education Objectives Discuss concepts relevant to thoracic pain of red flag origin Discuss concepts
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 3Q17 July August
 BRAND NAME Radicava GENERIC NAME edaravone MANUFACTURER MT Pharma America, Inc. DATE OF APPROVAL May 5, 2017 PRODUCT LAUNCH DATE May 5, 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
BRAND NAME Radicava GENERIC NAME edaravone MANUFACTURER MT Pharma America, Inc. DATE OF APPROVAL May 5, 2017 PRODUCT LAUNCH DATE May 5, 2017 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review
Ten Second Step Test" as a New Quantifiable Parameter of Cervical Myelopathy. Spine January 1, 2009; Volume 34; Issue 1; pp 82-86
 Ten Second Step Test" as a New Quantifiable Parameter of Cervical Myelopathy 1 Spine January 1, 2009; Volume 34; Issue 1; pp 82-86 Yukawa, Yasutsugu; Kato, Fumihiko; Ito, Keigo; Horie, Yumiko; Nakashima,
Ten Second Step Test" as a New Quantifiable Parameter of Cervical Myelopathy 1 Spine January 1, 2009; Volume 34; Issue 1; pp 82-86 Yukawa, Yasutsugu; Kato, Fumihiko; Ito, Keigo; Horie, Yumiko; Nakashima,
Randomised Controlled Trial for Low Back Pain
 INSPIRING THE WORLD TO MOVE WELL Randomised Controlled Trial for Low Back Pain A T 10 WEEKS, ViMove PATIENTS SHOWED SIGNIFICANT IMPROVEMENT IN ALL KEY MEASURES. IMPROVEMENTS WERE SUSTAINED OR IMPROVED
INSPIRING THE WORLD TO MOVE WELL Randomised Controlled Trial for Low Back Pain A T 10 WEEKS, ViMove PATIENTS SHOWED SIGNIFICANT IMPROVEMENT IN ALL KEY MEASURES. IMPROVEMENTS WERE SUSTAINED OR IMPROVED
Journal of Orthopaedic & Sports Physical Therapy. January 2012; Volume 42; Number 1; pp. 5-18
 1 Upper Cervical and Upper Thoracic Thrust Manipulation Versus Nonthrust Mobilization in Patients With Mechanical Neck Pain: A Multicenter Randomized Clinical Trial Journal of Orthopaedic & Sports Physical
1 Upper Cervical and Upper Thoracic Thrust Manipulation Versus Nonthrust Mobilization in Patients With Mechanical Neck Pain: A Multicenter Randomized Clinical Trial Journal of Orthopaedic & Sports Physical
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
Corporate Medical Policy
 Corporate Medical Policy Image-Guided Minimally Invasive Decompression (IG-MLD) for File Name: Origination: Last CAP Review: Next CAP Review: Last Review: image-guided_minimally_invasive_decompression_for_spinal_stenosis
Corporate Medical Policy Image-Guided Minimally Invasive Decompression (IG-MLD) for File Name: Origination: Last CAP Review: Next CAP Review: Last Review: image-guided_minimally_invasive_decompression_for_spinal_stenosis
REHABILITATION OF PATIENTS MANAGED IN ICU
 REHABILITATION OF PATIENTS MANAGED IN ICU RECOMMENDATIONS Safety to mobilize / exercise: on the website Recommendation 1 All critically ill patients nursed in ICU should be screened closely before active
REHABILITATION OF PATIENTS MANAGED IN ICU RECOMMENDATIONS Safety to mobilize / exercise: on the website Recommendation 1 All critically ill patients nursed in ICU should be screened closely before active
Neuromuscular Electrical Stimulator (NMES) Corporate Medical Policy
 Neuromuscular Electrical Stimulator (NMES) Corporate Medical Policy File name: Neuromuscular Electrical Stimulator (NMES) File Code: UM.NS.04 Origination: 05/01/2007 Last Review: 06/2018 Next Review: 06/2019
Neuromuscular Electrical Stimulator (NMES) Corporate Medical Policy File name: Neuromuscular Electrical Stimulator (NMES) File Code: UM.NS.04 Origination: 05/01/2007 Last Review: 06/2018 Next Review: 06/2019
Peripheral Subcutaneous Field Stimulation. Description
 Subject: Peripheral Subcutaneous Field Stimulation Page: 1 of 6 Last Review Status/Date: June 2016 Peripheral Subcutaneous Field Stimulation Description Peripheral subcutaneous field stimulation (PSFS,
Subject: Peripheral Subcutaneous Field Stimulation Page: 1 of 6 Last Review Status/Date: June 2016 Peripheral Subcutaneous Field Stimulation Description Peripheral subcutaneous field stimulation (PSFS,
Traumatic Spinal Cord Injury. 39 th CANP Annual Educational Conference March 18 th, :00pm-6:15pm Carl Wherry, ACNP-bc Amanda Severson, ACNP-bc
 Traumatic Spinal Cord Injury 39 th CANP Annual Educational Conference March 18 th, 2016 5:00pm-6:15pm Carl Wherry, ACNP-bc Amanda Severson, ACNP-bc Disclosures No conflicts of interest to disclose. Introduction
Traumatic Spinal Cord Injury 39 th CANP Annual Educational Conference March 18 th, 2016 5:00pm-6:15pm Carl Wherry, ACNP-bc Amanda Severson, ACNP-bc Disclosures No conflicts of interest to disclose. Introduction
Stand-Alone Technology. Reginald Davis, M.D., FAANS, FACS Director of Clinical Research
 Stand-Alone Technology Reginald Davis, M.D., FAANS, FACS Director of Clinical Research Disclosures Stand-Alone Devices Optio-C Stalif C Coalition Prevail ROI-C Technology Descriptions Optio-C A no profile,
Stand-Alone Technology Reginald Davis, M.D., FAANS, FACS Director of Clinical Research Disclosures Stand-Alone Devices Optio-C Stalif C Coalition Prevail ROI-C Technology Descriptions Optio-C A no profile,
