Treatment of Pressure Ulcers
|
|
|
- Maurice Hamilton
- 6 years ago
- Views:
Transcription
1 CLINICAL REVIEW CLINICIAN S CORNER Treatment of Pressure Ulcers A Systematic Review Madhuri Reddy, MD, MSc Sudeep S. Gill, MD, MSc Sunila R. Kalkar, MBBS, MD Wei Wu, MSc Peter J. Anderson, BA Paula A. Rochon, MD, MPH PRESSURE ULCERS ARE REGIONS OF localized damage to the skin and underlying tissues that usually develop over bony prominences such as the sacrum or heels. - These lesions are an important source of suffering for patients and their caregivers. Pressure ulcer prevalence varies widely depending on patient factors (eg, age, physical impairments) and treatment setting. -7 Treatment strategies for pressure ulcers can be both costly and complex. Hundreds of different mattresses and local wound care products are currently promoted, and few have been evaluated in randomized controlled trials (RCTs). It remains unclear which of the many available treatments promote the most effective healing of pressure ulcers. 8- While several effective strategies to prevent pressure ulcers exist, 6 many patients continue to develop them. This is especially true in high-risk settings such as acute care hospitals, in which patients have reduced mobility., Thus, clinicians require an understanding of effective treatment options. We examined the evidence supporting interventions for the treatment of pressure ulcers. CME available online at and questions on p 68. Context Many treatments for pressure ulcers are promoted, but their relative efficacy is unclear. Objective To systematically review published randomized controlled trials (RCTs) evaluating therapies for pressure ulcers. Data s and Study Selection The databases of MEDLINE, EMBASE, and CINAHL were searched (from inception through August, 8) to identify relevant RCTs published in the English language. Data Extraction Methodological characteristics and outcomes were extracted by investigators. Data Synthesis A total of RCTs met inclusion criteria. Of these, 8 did not provide sufficient information about authors potential financial conflicts of interest. Methodological quality was variable. Most trials were conducted in acute care (8 [7%]), mixed care (5 [%]), or long-term care ( [%]) settings. Among RCTs evaluating support surfaces, no clear evidence favored one support surface over another. No trials compared a specialized support surface with a standard mattress and repositioning. Among 7 RCTs evaluating nutritional supplements, higher-quality trial found that protein supplementation of long-term care residents improved wound healing compared with placebo (improvement in Pressure Ulcer Scale for Healing mean [SD] score of.55 [.66] vs. [.], respectively; P.5). Other nutritional supplement RCTs showed mixed results. Among 5 RCTs evaluating absorbent wound dressings, found calcium alginate dressings improved healing compared with dextranomer paste (mean wound surface area reduction per week,.9 cm vs.7 cm, respectively; P.). No other dressing was superior to alternatives. Among 9 RCTs evaluating biological agents, several trials reported benefits with different topical growth factors. However, the incremental benefit of these biological agents over less expensive standard wound care remains uncertain. No clear benefit was identified in RCTs evaluating adjunctive therapies including electric current, ultrasound, light therapy, and vacuum therapy. Conclusions Little evidence supports the use of a specific support surface or dressing over other alternatives. Similarly, there is little evidence to support routine nutritional supplementation or adjunctive therapies compared with standard care. JAMA. 8;(): METHODS The databases of MEDLINE, EMBASE, and CINAHL were searched from inception through August, 8, to identify Author Affiliations: Department of Medicine, Hebrew Rehabilitation Center, Division of Gerontology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts (Dr Reddy); Division of Geriatric Medicine, Departments of Medicine and Community Health and Epidemiology, Queen s University, Kingston, Ontario, Canada (Dr Gill); Kunin-Lunenfeld Applied Research Unit-Baycrest, Toronto, Ontario, Canada (Drs Kalkar and Rochon and Messrs Wu and Anderson); Department of Medicine, University of relevantrcts.thefollowingsearchterms wereused: pressureulcer, pressuresore, decubitus, bedsore, chronic wound, treatment, therapy, management, randomized, and Toronto, Toronto, Ontario, Canada (Dr Rochon); and Institute for Clinical Evaluative Sciences, Toronto, Ontario, Canada (Dr Rochon). Corresponding Author: Madhuri Reddy, MD, MSc, Hebrew Rehabilitation Center, Center St, Boston, MA (Madhuri.Reddy@hrca.harvard.edu). Clinical Review Section Editor: Mary McGrae McDermott, MD, Contributing Editor. We encourage authors to submit papers for consideration as a Clinical Review. Please contact Mary McGrae McDermott, MD, at mdm68@northwestern.edu. 8 American Medical Association. All rights reserved. (Reprinted) JAMA, December, 8 Vol, No. 67 Downloaded From: on 6//8
2 clinical trials. A hand search also was performed to identify any other articles. Inclusion criteria were RCTs published in the English language that reported objective, clinicallyrelevantoutcomemeasures suchashealingratesorwoundsize. When thesearchwasnotlimitedtostudiespublished in the English language, non English-language trials were found: one initalian(9participants) andanother in Japanese (9 participants). 5 Because few non English-language trials were foundandthetotalnumberofparticipants in these trials was small, this study was limited to RCTs published in the English language. Studies that evaluated chronic wounds other than pressure ulcers or assessed only adverse events or secondary outcomes(eg, pain) wereexcluded. There wastoomuchclinicalheterogeneityinthe individual RCTs to permit meaningful pooling of the data in a meta-analysis. s of funding were extracted from the trials using a method described by Als-Nielsen et al. 6 We also determined if the RCTs reported any potential author conflicts of interest. Information was extracted regarding participant age, population studied, and treatment setting. Trials used different terms to describe treatment settings. These terms were grouped as follows: acute care, long-term care, palliative care, rehabilitation, ambulatory care, and home care. Pressure ulcers at the beginning of each trial are described by stage unless the trial used different terminology (such as superficial or deep). The included RCTs were categorized into groups depending on whether they investigated the management of underlying contributing factors, the effects of local wound care, or adjunctive therapies. This approach was selected because wound specialists approach pressure ulcer management sequentially; first, reduce or eliminate underlying contributing factors (support surfaces and nutritional supplementation), then provide local wound care (wound dressings and biological agents), and finally consider adjunctive therapies (eg, vacuum therapy). 7 A criterion standard for quantifying outcomes in ulcer healing has not been established. 8,9 Surrogate end points (eg, amount of granulation tissue, degree of debridement, and bacterial burden) do not directly measure healing and may not correlate with healing., Measurement of wound surface area, including wound depth and undermining (ie, tunneling under the skin), is a reliable and valid method of assessing wound healing. 9 Therefore, only studies that calculated wound size with wound volume and/or surface area, used evaluation tools that incorporated these measurements, or used complete wound healing as end points were included. Individual trials used various terms to describe outcomes. Some trials used scales such as Pressure Ulcer Scale for Healing or Pressure Sore Status Tool. For simplicity, remaining terms were classified into categories: complete wound healing (ie, proportion of individuals whose wounds healed), time to healing (ie, time to complete wound healing), and wound surface area (ie, changes over time). Methodological quality of the RCTs was determined using 6 elements from thechecklisttoevaluateareportofanonpharmacological trial (CLEAR NPT) ( /Emi57/docs/usersguidelines.pdf) that are relevant to therapies for pressure ulcers: () adequate allocation sequence generation (ie, use of an appropriate method to generate the randomization sequence); () concealed treatment allocation; () adequate participant blinding; () adequate outcome assessor blinding; (5) comparable rates of other treatments and care in each randomized group (eg, frequency of dressing changes); and (6) intention-to-treat analysis. If these elements were not explicitly reported, they were considered not performed. Three authors (M.R., S.R.K., W.W.) independently rated each RCT and reached consensus. Trials meeting or more of the CLEAR NPT criteria were considered good quality. Trials meeting or less of the CLEAR NPT criteria were considered suboptimal. We also assessed which articles reported a sample size justification to determine whether RCTs were adequately powered to detect either clinically important differences or equivalence of compared treatments. 5 Specialized support surfaces such as mattresses and cushions redistribute a patient s weight over skin and subcutaneous tissues as it presses against a bed or chair surface. 6 A reduction of pressure between the body and the support surface is considered helpful in healing pressure ulcers. The distinction between types of support surfaces is important because costs vary widely. Support surfaces were categorized using the National Pressure Ulcer Advisory Panel classification system 6 : nonpowered (support surfaces such as foam that do not need electricity, previously known as static) and powered (support surfaces such as rotating beds that require electricity, previously known as dynamic). An overlay is a support surface designed to be placed on top of another support surface. Powered support surfaces are generally more expensive than nonpowered surfaces. 6 Standard hospital mattresses (ie, not a specialized support surface) usually incur a -time cost of less than $, but specialized support surfaces (frequently rented) can range from less than $5 per month for nonpowered mattress overlays to more than $5 per month for some powered support surfaces. 7 Randomized controlled trials that described nutritional supplementation by any method (eg, enterally or parenterally) were included. Local wound care dressings were categorized by function rather than form (eg, films or gels). 8 Because many dressings perform more than function, they were categorized based on their primary purpose: exudate absorbing (eg, foams), debriding (eg, collagenase), hydrating (eg, hydrocolloids), antimicrobial (eg, silver, povidone-iodine), and other (eg, did not fit any of these categories, fit in category, or function was unclear). Adjunctive therapies were defined as modalities that neither directly address the underlying contributing factors nor primarily address local wound care (eg, vacuum therapy). 68 JAMA, December, 8 Vol, No. (Reprinted) 8 American Medical Association. All rights reserved. Downloaded From: on 6//8
3 RESULTS The search identified 87 abstracts, from which relevant RCTs were selected. The flow diagram shows an overview of the study selection process (FIGURE). The RCTs included 5889 participants. Only 5 trials involved more than participants 9- and provided a sample size justification. 9-,5,9,,,-57 Thirty-eight of the trials took place in acute care (7%), 5 in mixed settings (%), in long-term care (%), 6 in rehabilitation (6%), in ambulatory care (%), in home care (%), in palliative care (%), and did not mention their treatment setting (%). Twenty-two trials (.%) included only participants older than 6 years or described participants as elderly and trials (.7%) included only participants with spinal cord injuries. Forty-five trials reported funding by the for-profit manufacturers of the products under evaluation (.7%), 5 reported funding from nonprofit peer-reviewed granting agencies only (.6%), reported funding from for-profit and nonprofit organizations (.6%), and 9 did not indicate sources of funding (8.%). Eighty-three trials (8.6%) did not provide sufficient information about authors potential financial conflicts of interest. Three authors (M.R., S.K., W.W.) independently rated each RCT on CLEAR NPT items. Initial agreement was 8% (9% for adequate description of generation of allocation sequences, 8% for treatment allocation concealment, 8% for adequate participant blinding, 87% for adequate blinding of outcome assessors, 68% for co-interventions same in each group, and 9% for intentionto-treat analysis). Differences were resolved by consensus. Sixteen of the trials (5.5%) met or more of the CLEAR NPT criteria. Nineteen RCTs (57 participants) evaluated interventions for underlying contributing factors. Twelve RCTs( participants) evaluated support surfaces (TABLE). 9,,9-,-6,58-6 Noneevaluated the effects of repositioning alone. Six RCTs directly compared powered (eg, alternating pressure) with nonpowered (eg, foam) support surfaces.,,,5,58,59 Russell et al and Day and Leonard found no differences in pressure ulcer healing between powered and nonpowered support surfaces. The remaining studies, which met fewer quality criteria than Russell et al, suggested that powered support surfaces were superior to nonpowered support surfaces,,5,58,59 although RCT did not report statistical significance. 58 Inconsistent findings in these 6 studies result in persistent uncertainty regarding the benefit of powered support surfaces. Five RCTs compared different types of powered mattresses. Evans et al 6 and Nixon et al found no differences in ulcer healing between the powered support surfaces they compared. Allman et al foundthatulcersurfaceareadecreased with an air-fluidized mattress but increased on an alternating pressure mattress (median changes,. vs.5 cm [95% confidence interval for the difference, 9. to.6 cm ]; P=.). Seven RCTs (58 participants) evaluated nutritional supplements (TABLE ) All were oral supplements, but contents varied in each trial. Lee et al 6 evaluated ulcer healing over 8 weeks in long-term care residents randomized to either a collagen protein supplement or placebo combined with standard care. Healing was measured with the Pressure Ulcer Scale for Healing (=healed, 7=worst possible score). 8 Individuals randomized to the supplement had better healing than those randomized to placebo (mean [SD] improvement in Pressure Ulcer Scale for Healing score,.55 [.66] vs. [.], respectively; P.5). ter Riet et al 6 compared high-dose (5 mg twice daily) with low-dose ( mg twice daily) vitamin C given for either weeks or until pressure ulcer healing (whichever came first) and found no differences in wound closure rates or mean change in ulcer surface area per week. In contrast, Taylor et al 66 found that 5 mg of vitamin C twice daily was better than placebo (mean reduction in pressure ulcer area, Figure. Flow Diagram of Included and Excluded Studies 88 Abstracts identified 87 MEDLINE, EMBASE, and CINAHL Hand search 8 Potentially relevant RCTs identified and screened 6 Non-RCTs excluded 8 Excluded Focused on prevention not treatment 5 Inadequate information about outcome measures or used proxy outcome measures Not pressure ulcers (other medical conditions, wounds other than pressure ulcers, or examined treatment of surrounding skin only) 9 Duplicate studies Abstract only Did not use human participants RCTs included (5889 participants) 9 Underlying contributing factors 6 Local wound care Adjunctive therapies RCT indicates randomized controlled trial. 8% vs.7%; P.5). Several factors may explain the disparate findings. The study by ter Riet et al, 6 but not the study by Taylor et al, 66 provided an adequate description of generation of allocation sequences and used an intention-to-treat analysis. In addition, the study by ter Riet et al 6 included more patients (88 vs in Taylor et al 66 ), was multicentered, and had longer follow-up ( vs weeks, respectively). Thus, the value of vitamin C supplementation in pressure ulcer treatment remains uncertain. Two other trials that found beneficial effects for nutritional supplements had suboptimal quality. 6,6 One of these did not report statistical significance. 6 Desneves et al 6 compared diets in a study of6patients: standardhospitaldiet, standard hospital diet plus high protein, and standard hospital diet plus high protein witharginine, zinc, andantioxidants. The first groups did not achieve significant improvements in pressure ulcer healing as measured by the Pressure Ulcer Scale for Healing, but the third group did. 8 American Medical Association. All rights reserved. (Reprinted) JAMA, December, 8 Vol, No. 69 Downloaded From: on 6//8
4 Eleven of 9 studies (57.9%) evaluating underlying contributing factors adequately described the generation of random allocation sequences. Of the 9 studies, 8 indicated that participants were randomized using concealed allocation (.%). Because blinding may be difficult when studying support surfaces, ratings for the CLEAR NPT item regarding participant blinding were not Table. Randomized Controlled Trials Evaluating Support Surfaces as an Underlying Contributing Factor to Pressure Ulcer Severity a Groen et al, Rosenthal et al, Russell et al, Branom, 58 Mulder et al, Day and Leonard, 99 Ferrell et al, 5 99 Nixon et al, 6 Study; Age b ; ; 6 y 7; ; LAL mattress, 69. y; SFMO, 68.6 y; APM, 7. y 99; 58; APM, 8. y; FMO, 79.8 y ; 8; 6- y 9; 9; NA 8; 8; 8 y 8; 75 y ; 55 y h Evans et al, 6 ; 65 y Land et al, 6 Russell et al, 9 Allman et al, 987 7; y 8; ; age described as elderly 7; 65; 8 y Duration of Treatment c - wk - wk mean: wk long-term -8 wk wk - wk -8 wk 6 wk long-term 67 wk long-term wk 7 wk - Pressure Ulcer Severity at Baseline; Intervention d Nonpowered vs Nonpowered specialized foam mattress vs water mattress Primary Outcome Measures of Treatment Effect e Complete wound healing: 5% for specialized foam mattress vs 8.% for water mattress; this difference is not significant Powered vs Nonpowered Mean (SD) Pressure Sore Status Score LAL mattress vs improvement: 8. (.5) for LAL mattress vs SFMO vs APM g. (.5) for APM (P.); mean (SD) time to complete healing:.8 (.) mo (95% CI,.-.65) for LAL mattress vs.55 (.) mo (95% CI,.-.98) for SFMO vs. (.) mo (95% CI,.9-.58) for APM I, APM vs FMO LAL mattress vs air and foam mattress g LAL mattress g vs SFMO LAL mattress vs foam overlay LAL mattress g vs specialized foam mattress Wound surface area overall ulcer progress: 7.% for APM vs 7.7% for FMO (P =.67) Wound surface area mean (SD) rate of wound closure per week: 5.% (.7%) for LAL mattress vs 9.% (.8%) for air and foam mattress Wound surface area reduction in wound size: 77% more for LAL mattress vs SFMO (P.) of Trial f Wound surface area (P.5) Wound surface area median reduction: 9. mm /d for LAL mattress vs.5 mm /d for specialized foam mattress (P.) Powered vs Powered II; Complete wound healing:.% for APM vs.7% APM vs alternating for alternating pressure overlay (P =.75) pressure overlay APM No. vs APM No. APM vs APM or overlay types of APM and cushion combination I, air-fluidized mattress g vs APM covered with foam Wound surface area median absolute reduction per day:. cm for APM No. vs.8 cm for APM No. (P =.57) Wound surface area: no significant difference in healing sores Complete wound healing: improvement in heel ulcers only (P =.) Wound surface area median changes in surface area:. cm for air-fluidized mattress vs.5 cm for APM covered with foam (P =.) Abbreviations: APM, alternating pressure mattress; CI, confidence interval; FMO, fluid mattress overlay; LAL, low air loss; NA, data not available; SFMO, specialized foam mattress overlay. a Support surface groups: nonpowered, does not require electricity (eg, foam mattress); powered, requires electricity (eg, rotating bed). b Eligible age to participate in study is provided unless participant age is only available. c Duration of treatment is expressed to nearest week. d Different systems were used in the studies to stage pressure ulcer severity, but most systems rely on -stage categorization with higher numbers representing more severe ulcers. e Complete wound healing defined as the proportion of ulcers in the study group that healed during the intervention period; time to healing defined as the time to complete wound healing; wound surface area changes defined as surface area measurements before and after treatment. f Maximum score of 5 and determined by the criteria on the checklist to evaluate a report of a nonpharmacological trial. See Methods section for description of criteria. g Indicates effective intervention for treatment of pressure ulcers. h A total of 97 patients were enrolled in this trial; were studied for treatment of pressure ulcers and the rest were studied for prevention purposes JAMA, December, 8 Vol, No. (Reprinted) 8 American Medical Association. All rights reserved. Downloaded From: on 6//8
5 included in Table. It is feasible, however, to blind participants in nutritional supplement trials but this was done in only of 7 trials. In all 9 RCTs evaluating underlying contributing factors, it was feasible to perform blinded outcome assessments, and this was described in trials (57.9%). Co-interventions were described as consistent in all treatment groups among of the 9 studies (7.7%). Intention-to-treat analyses were described in only of these 9 studies (5.8%). One support surface study 6 met all 5 CLEAR NPT criteria, nutrition study 6 met 5 of 6 criteria, and two,6 of the 9 RCTs met criteria. Table. Randomized Controlled Trials Evaluating Nutritional Supplementation as an Underlying Contributing Factor to Pressure Ulcer Severity Lee et al, 6 6 Desneves et al, 6 5 Benati et al, 6 ter Riet et al, Myers et al, Taylor et al, Norris and Reymolds, Study; Age a 89; 7; NA 6; ; 7-9 y 6; 7-9 y 88; 77; NA 95; 8; - y ; 5-88 y ; ; -88 y Duration of Treatment b 8wk wk wk long-term wk wk wk wk Pressure Ulcer Severity at Baseline; Intervention c collagen protein f vs placebo standard hospital diet vs standard hospital diet plus high protein vs standard hospital diet plus high protein plus arginine, zinc, and vitamin C f standard hospital diet vs standard hospital diet plus high protein vs standard hospital diet plus high protein plus arginine, zinc, and antioxidants f vitamin C ( mg twice daily) plus placebo ultrasound vs vitamin C ( mg twice daily) plus ultrasound vs vitamin C (5 mg twice daily) plus placebo ultrasound vs vitamin C (5 mg twice daily) plus ultrasound I, standard care g plus standard diet vs consistent wound care vs controlled nutritional support vs consistent wound care plus controlled nutritional support vitamin C (5 mg twice daily) f vs placebo twice daily zinc sulfate vs placebo Primary Outcome Measures of Treatment Effect d Mean (SD) changes in PUSH at 8 wk:.55 (.66) for collagen protein vs. (.) for placebo (P.5) Mean (SD) PUSH score reduction from baseline to week : 8.7 (.) to 7. (.5) for standard hospital diet vs 8. (.5) to 6. (.) for standard hospital diet plus high protein vs 9. (.) to.6 (.6) for standard hospital diet plus high protein plus arginine, zinc, and vitamin C (P.5) of Trial e Pressure Sore Status Tool score: NA Wound surface area mean absolute healing rates:. cm /wk for intervention group vs.7 cm /wk for control group Adjusted mean change in ulcer size on wound surface area:.7 for standard care plus standard diet vs.76 for consistent wound care vs.6 for controlled nutritional support vs. for consistent wound care plus controlled nutritional support; there were no group differences in healing Mean reduction in wound surface area after mo: 8% for vitamin C (5 mg twice daily) vs.7% for placebo twice daily (P.5) Wound surface area mean net change of ulcer volume:. ml for zinc sulfate vs 6. ml for placebo (P.8) Abbreviations: NA, data not available; PUSH, Pressure Ulcer Score for Healing. a Eligible age to participate in study is provided unless participant age is only available. b Duration of treatment is expressed to nearest week. c Different systems were used in the studies to stage pressure ulcer severity, but most systems rely on -stage categorization with higher numbers representing more severe ulcers. d PUSH score range:, healed and 7, worst possible score. Wound surface area defined as changes of surface area measurements before and after treatment. e Maxiumum score of 6 and determined by the criteria on the checklist to evaluate a report of a nonpharmacological trial. See Methods section for description of criteria. f Indicates effective intervention for treatment of pressure ulcers. g Standard care refers to various topical treatments in accordance with the participating institution and/or guidelines. 5 8 American Medical Association. All rights reserved. (Reprinted) JAMA, December, 8 Vol, No. 65 Downloaded From: on 6//8
6 Table. Randomized Controlled Trials Evaluating Absorbent Wound Dressings for Local Wound Care Alvarez et al, 68 Püllen et al, Burgos et al, 8 Müller et al, 8 Burgos et al, 69 Mulder et al, 7 99 Parish and Collins, Amione et al, 7 5 Sayag et al, Sipponen et al, 57 8 Price et al, 7 Engdahl, 7 98 Brown-Etris et al, 75 8 Motta et al, Seeley et al, Day et al, Study; Age a 8; 6; 8 y 5; 78; 5 y ; 6; 55 y ; ; y ; 7; 55 y 67; 6; 8 y 7; 8-7 y ; 8; 8 y 9; 6; 6 y 7; ; y 58; 5; radiant heat, 75.7 y; alginate, 69.8 y ; 69-9 y 7; 7; 8 y ; -76 y ; 9; 8 y ; 96; 8 y Duration of Treatment b wk Acute care and rehabilitation; wk 8wk 6-6 wk wk ambulatory 8wk -6 wk ambulatory 6wk Ambulatory 8wk wk home -6 wk - wk Long-term, home, and ambulatory 8wk Home 8wk Ambulatory -8 wk wk (mean) Pressure Ulcer Severity at Baseline; Intervention c Primary Outcome Measure of Treatment Effect d Debriding vs Debriding Wound surface area: no significantly different rate of collagenase vs reduction in wound area papain-ureachlorophyllin copper collagenase vs fibrinolysin or deoxyribonuclease III; collagenase daily vs collagenase every d Debriding vs Hydrating IV; collagenase f vs hydrocolloid III; collagenase vs hydrocolloid Wound surface area reduction of 6.7% for collagenase vs 57.% for fibrinolysin or deoxyribonuclease (P=.) Mean (SD) reduction in wound surface area from 7.7 (8.6) cm to.6 (7.) cm for collagenase daily vs from. (.) cm to 5. (9.9) cm for collagenase every d (P=.6) Complete wound healing: 9.7% for collagenase vs 6.6% for hydrocolloid (P.5) Wound surface area reduction of 8.% for collagenase vs 7.7% for hydrocolloid (P=.75) Debriding vs Hydrating vs Other Mean reduction in wound surface area per week of 8.% for hydrogel vs hydrocolloid hydrogel vs.% for hydrocolloid vs 5.% for moist saline vs moist saline gauze gauze (P=.89) Debriding vs Absorbent vs Other Wound surface area reduction of 5.5% for collagenase vs collagenase vs 85.7% for dextranomer vs % for sugar and egg white dextranomer f vs (collagenase vs dextranomer, P.; dextranomer vs sugar and egg white sugar and egg white, P.) Absorbent vs Absorbent Wound surface area: no significant differences in foam vs foam with percentage decrease in ulcer area wound-contact layer calcium alginate f vs dextranomer Mean reduction in wound surface area per week of.9 cm for calcium alginate vs.7 cm for dextranomer (P.) of Trial e Absorbent vs Other Complete wound healing of 9% for resin salve vs % for resin salve f vs sodium sodium carboxymethylcellulose hydrocolloid carboxymethylcellulose polymer (P=.) hydrocolloid polymer Wound surface area mean reduction (P=.8) radiant heat dressing g vs alginate dextranomer powder vs moist saline gauze Hydrating vs Hydrating transparent absorbent acrylic dressing vs hydrocolloid hydrogel dressing vs hydrocolloid hydrocellular dressing vs hydrocolloid hydrocolloid (triangleshaped) f vs hydrocolloid (oval-shaped) Wound surface area reduction of.5% for dextranomer powder vs 8.% for moist saline gauze; this difference is not statistically significant Complete wound healing of 6.% for transparent absorbent acrylic dressing vs 59.5% for hydrocolloid (P=.96) Complete wound healing of % for hydrogel dressing vs % for hydrocolloid; the overall healing rates of wounds were not statistically significant between the groups Wound surface area mean reduction of 5% for hydrocellular dressing vs 5% for hydrocolloid (P=.) Wound surface area reduction in ulcer width of % for triangle-shaped vs 7% for oval-shaped hydrocolloid (P =.) 5 (continued) 65 JAMA, December, 8 Vol, No. (Reprinted) 8 American Medical Association. All rights reserved. Downloaded From: on 6//8
7 Table. Randomized Controlled Trials Evaluating Absorbent Wound Dressings for Local Wound Care (continued) Hondé et al, 7 99 Darkovich et al, Belmin et al, 5 Sopata et al, 79 Colin et al, 996 Yastrub, 8 Kim et al, Hollisaz et al, 5 Graumlich et al, 5 Seaman et al, 8 Matzen et al, Chang et al, Thomas et al, Colwell et al, Kraft et al, Xakellis and Chrischilles, Study; Age a 68; 9; 65 y 9; -98 y ; 77; 65 y ; 9; -88 y 5; 96; 5-98 y 5; ; 65 y ; hydrocolloid, 5.5 y; moist gauze, 6.9 y 8; 8; 6.6 y 65; 5; 8 y 5; ; change, 78 y; hydrocolloid, 66 y ; ; -97 y ; 8 y ; ; 8 y 9; 7; 8- y 8; 7; 8-78 y 9; ; hydrocolloid, 77. y; moist gauze, 8.5 y Duration of Treatment b -8 wk longterm 8 wk 8wk Palliative 8 wk Acute care h ; - wk wk Rehabilitation; wk (mean) Long-term and home care h ; 8wk 8 wk Long-term and home wk Ambulatory wk -8 wk Long-term and home wk -8 wk long-term wk wk Pressure Ulcer Severity at Baseline; Intervention c Primary Outcome Measure of Treatment Effect d Hydrating vs Hydrating Complete wound healing of 8.% for hydrocolloid vs hydrocolloid vs 5.8% for copolymer membrane (P =.) copolymer membrane f IorII; hydrogel dressing f vs hydrocolloid Complete wound healing of % for hydrogel dressing vs % for hydrocolloid Hydrating vs Absorbent Mean (SD) reduction in wound surface area of.6 (.9) hydrocolloid for 8 wk vs cm and. (7.) cm for hydrocolloid vs 5. (5.7) calcium alginate for cm and 7.6 (7.) cm for sequential group, at and wk and then 8 wk, respectively (P.) hydrocolloid for wk f polyurethane foam vs hydrogel wafer I, hydrogel f vs dextranomer Mean (SD) healing rate for wound surface area ulcers of. (.) cm /d (stage II) and. (.7) cm /d (stage III) for polyurethane foam vs.67 (.7) cm /d (stage II) and. (.) cm /d (stage III) for hydrogel wafer (P.5) Wound surface area median reduction in wound area of 5% for hydrogel vs 7% for dextranomer (P =.) Hydrating vs Antimicrobial II; Mean PUSH of. for polymeric membrane dressing polymeric membrane vs.6 for antibiotic ointment (P.) dressing f vs antibiotic ointment IorII; hydrocolloid vs moist povidone-iodine gauze Hydrating vs Other IorII; hydrocolloid f vs phenytoin cream vs moist saline gauze collagen vs hydrocolloid Complete wound healing of 8.8% for hydrocolloid vs 77.8% for moist povidone-iodine gauze; the healing rates of the groups were not statistically significant Complete wound healing of 7.% for hydrocolloid vs % for phenytoin cream vs 6.7% for moist saline gauze (P.5) Complete wound healing of 5% for collagen vs 5% for hydrocolloid (P=.89) Complete wound healing of 5% for change indicator change indicator f vs vs 6% for hydrocolloid alginate (P=.) hydrocolloid alginate hydrocolloid gel vs moist saline gauze f hydrocolloid vs moist saline gauze hydrogel vs moist saline gauze hydrocolloid f vs moist saline gauze polyurethane foam f vs moist saline gauze hydrocolloid vs moist saline gauze Mean (SD) reduction in wound surface area of 6% (%) for hydrocolloid gel vs 6% (6%) for moist saline gauze (P.) Wound surface area mean reduction of % for hydrocolloid vs 9% for moist saline gauze (P=.) Complete wound healing of 6% for hydrogel vs 6% for moist saline gauze (P=.9) Wound surface area complete wound healing of % for hydrocolloid vs % for moist saline gauze Complete wound healing of % for polyurethane foam vs % for moist saline gauze Complete wound healing of 89% for hydrocolloid vs 86% for moist saline gauze; median time to healing: 9 d for hydrocolloid vs d for moist saline gauze (P=.) of Trial e (continued) 8 American Medical Association. All rights reserved. (Reprinted) JAMA, December, 8 Vol, No. 65 Downloaded From: on 6//8
8 Table. Randomized Controlled Trials Evaluating Absorbent Wound Dressings for Local Wound Care (continued) Brod et al, Oleske et al, Sebern, Rhodes et al, 9 Yapucu Günes and Eser, 9 7 Kaya et al, 9 5 Gerding and Browning, 9 99 Moberg et al, Shamimi et al, 96 8 Subbanna et al, 56 7 Thomas et al, 97 5 Meaume et al, 98 Kloth et al, 99 Small et al, 5 Kuflik et al, Whitney et al, Study; Age a ; 8; age described as elderly 6; 5; 5-9 y 8; transparent dressing, 76. y; moist gauze, 7. y 7; 9; 6 y 6; 6; 8 y 7; 7; 6-56 y 7; NA 5; ; 5-97 y 8; 8; 8 y 8; 6; phenytoin,. y; saline,.6 y ; ; radiant heat, 7. y; hydrocolloid and/or alginate, 77. y 8; 65 y 5; ; radiant heat, 78. y; standard care, 77.9 y 58; ; 8 y 9; 5; age described as elderly ; 9; 8 y Duration of Treatment b 6 wk (median) wk Home 8wk - wk 5 wk Acute care h ; - wk wk -8 wk 8wk Rehabilitation h ; wk Rehabilitation, long-term, and ambulatory wk 8 wk long-term wk Home 6 wk Long-term care and rehabilitation; 6wk Acute, long-term, and home 8 wk Pressure Ulcer Severity at Baseline; Intervention c Hydrating vs Other hydrocolloid vs polyhema IorII; occlusive polyurethane dressing vs moist saline gauze transparent moisture-permeable dressing f vs moist saline gauze Antimicrobial vs Hydrating vs Other II; phenytoin suspension f vs hydrocolloid vs triple antibiotic ointment Primary Outcome Measure of Treatment Effect d Complete wound healing of 6% for hydrocolloid vs 5% for polyhema (P=.5) Mean wound surface area of. cm for occlusive polyurethane dressing vs 7.7 cm for moist saline gauze (P.5) Median reduction in wound surface area of % for transparent moisture-permeable dressing vs 5% for moist saline gauze (P.) Mean (SD) time to healing of 5. (.) d for phenytoin suspension vs 5.8 (9.6) d for hydrocolloid vs 5.8 (8.5) d for triple antibiotic ointment (P=.5) Antimicrobial vs Other Mean changes in PUSH from.5 to.6 for ethoxydiaminoacridine ethoxydiaminoacridine and nitrofurazone vs from and nitrofurazone vs 5. to 6.55 for honey dressing (P.) honey dressing f I, II, or III; povidone-iodine gauze vs hydrogel f IorII; oxyquinoline f vs lanolin or petrolateum Deep or superficial; cadexomer iodine f vs standard care i Other vs Other semelil gel f vs standard care i II; phenytoin solution vs normal saline radiant heat dressing f vs hydrocolloid and/or alginate II; soft silicone vs hydropolymer radiant heat dressing f,g vs standard care i Wound surface area epithelialization of 5% for povidone-iodine gauze vs 8% for hydrogel (P=.) Complete stage II wound healing of.5% for oxyquinoline vs.8% for lanolin or petrolateum (P.5) Mean decrease of wound surface ulcer area of.9% for cadexomer iodine vs 9.6% for standard care (P.) Mean (SD) reduction in wound surface area of 8. (85.) cm for semelil gel (78.%) vs.8 (6.) cm for standard care (6.%) (P.) Mean (SD) reduction in PUSH of 9.5 (7.7) for phenytoin solution vs.9 (.9) for normal saline (P=.6) Complete wound healing of 57% for radiant heat dressing vs % for hydrocolloid and/or alginate (P=.6) Complete wound healing of % for soft silicone (8/8) vs 5% for hydropolymer (/) Wound surface area reduction of.5 cm /wk for radiant heat dressing vs. cm /wk for standard care (P.) of Trial e hydrogel or foam or transparent film vs standard care i Complete wound healing (P=.5) IorII; Complete wound healing of 9% for active ointment active ointment (with live (9/) vs % for placebo (/) yeast cell derivative) f vs placebo radiant heat dressing f,g vs standard care i Wound surface area mean reduction of. cm /d for radiant heat dressing vs. cm /d for standard care (P=.) (continued) 65 JAMA, December, 8 Vol, No. (Reprinted) 8 American Medical Association. All rights reserved. Downloaded From: on 6//8
9 Table. Randomized Controlled Trials Evaluating Absorbent Wound Dressings for Local Wound Care (continued) Study; Age a Duration of Treatment b Pressure Ulcer Severity at Baseline; Intervention c Other vs Other LeVasseur and IorII; Helme, active cream (extract of 99 barley) vs placebo Guthrie, 988 Agren and Stromberg, Knudsen et al, 98 Gerber and Van Ort, 979 Van Ort and Gerber, ; ; active cream, 8.5 y; placebo, 8.5 y 8; 5; 77.9 y 8; 8; 6-9 y 6; 8; -57 y ; 9; y ; ; 9-9 y long-term 6wk 6 wk ambulatory 8 wk Acute care h ; wk wk wk Deep or superficial; zinc salt spray and aluminum hydroxide or vitamin A ointment f vs zinc salt spray vs aluminum hydroxide or vitamin A ointment vs placebo spray and ointment streptokinasestreptodornase vs zinc oxide dialysate f vs placebo topical insulin vs standard care i topical insulin f vs standard care i Primary Outcome Measure of Treatment Effect d Mean (SD) time to healing of 8. (.) d for active cream vs 9. (.6) d for placebo (P=.8) Wound surface area reduction of 9.% for zinc salt spray and aluminum hydroxide or vitamin A ointment vs.% for zinc salt spray vs 8.7% for aluminum hydroxide or vitamin A ointment vs.% for placebo Wound surface area median reduction of 8.7% for streptokinase-streptodornase vs.% for zinc oxide (P.5) of Trial e Wound surface area reduction on th and th days, respectively, of 9% and 8% for dialysate vs 8% and 59% for placebo (P.5) Wound surface area (P=.) Complete wound healing (P=.5) Abbreviations: NA, data not available; PUSH, Pressure Ulcer Score for Healing. a Eligible age to participate in study is provided unless participant age is only available. b Duration of treatment is expressed to nearest week. c Different systems were used in the studies to stage pressure ulcer severity, but most systems rely on -stage categorization with higher numbers representing more severe ulcers. d PUSH score range:, healed and 7, worst possible score. Wound surface area defined as changes of surface area measurements before and after treatment; complete wound healing defined as the proportion of ulcers in the study group that healed during the intervention period; and time to healing defined as the time to complete wound healing. e Maximum score of 6 and determined by the criteria on the checklist to evaluate a report of a nonpharmacological trial. See Methods section for description of criteria. f Indicates effective intervention for treatment of pressure ulcers. g Radiant heat dressing has been removed from the market due to safety concerns. h Indicates treatment for spinal cord injuries. i Standard care refers to various topical treatments in accordance with the participating institution and/or guidelines. Sixty-three RCTs ( participants) evaluated interventions targeting local wound care. Fifty-four RCTs (857 participants) evaluated wound dressings (TABLE).,,-8,8-5,55-57,68-5 Fiveofthe 7 highest-quality RCTs of wound dressings found no difference in wound healing with the products they compared: collagenase vs fibrinolysin or deoxyribonuclease, collagenase vs hydrocolloid, radiant heat dressing vs hydrocolloid and/or alginate and phenytoin solution vsnormalsaline.,5,56,69,97 Sayagetal 9 performedamulticenteredtrialof9patients aged 6 years or older with pressure ulcers in acute care. They found that mean wound surface area reduction per week was.9 cm (SD,.5) in wounds treatedwithcalciumalginateand.7cm (SD,.) in wounds treated with dextranomer paste (P.). Gerding and Browning 9 foundoxyquinolineimproved wound healing compared with lanolin or petrolatum. However, lanolin may cause allergic contact dermatitis and has fallen out of favor in chronic wound treatment. 6,7 No debriding agent was consistently superior to other dressings for wound healing.,8,68,69,7 Nine RCTs (7 participants) evaluated biological agents (TABLE ).,8-5 Three trials examined the effects of platelet-derived growth factors. The trial that met the most CLEAR NPT criteria was performed by Rees et al, which compared doses of recombinant human platelet derived growth factor with placebo. The incidence of complete healing was greater in all recombinant human platelet derived growth factor groups (P. in all groups) compared with placebo. In another trial, nerve growth factor improved healing when compared with placebo at 6-week follow-up (mean [SD] reduction in pressure ulcer area, 78 [9] vs 85 [8] mm ; P=.). Of the 6 studies examining local wound care, adequately described the generation of random allocation sequences (.9%) and reported that participants were randomized using concealed allocation (.6%). Only 5 of the 6 studies (.8%) described adequate participant blinding. Adequate blinding of outcome assessors was described in studies (6.5%). Cointerventions were equally applied in 8 studies (.%), and intention-totreat analyses were performed in only studies (5.9%). None of the 6 studies examining local wound care fulfilled all 6 CLEAR NPT criteria. 8 American Medical Association. All rights reserved. (Reprinted) JAMA, December, 8 Vol, No. 655 Downloaded From: on 6//8
10 Table. Randomized Controlled Trials Evaluating Biological Agents for Local Wound Care Nisi et al, 8 5 Payne et al, 9 Rees et al, 999 Mustoe et al, 99 Robson et al, 99 Landi et al, Hirshberg et al, Robson et al, Robson et al, 5 99 Study; Age a 8; 5-85 y ; ; 8 y ; ; 8 y 5; ; age described as elderly ; ; -56 y 8; 6; 75-9 y ; 8; 8 y 6; 8-7 y 5; 9; 8-65 y Duration of Treatment b -8 wk - wk 6 wk long-term wk wk 6 wk Ambulatory 6 wk 5wk wk Pressure Ulcer Severity at Baseline; Intervention c Wound-Environment Modulators protease-modulating matrix vs petrolatum-soaked gauze Skin Substitutes III; fibroblast-derived dermal replacement plus standard care f vs standard care f Platelet-Derived Growth Factors recombinant platelet-derived growth factor BB ( µg/g once daily) alternated with placebo every h g vs recombinant platelet-derived growth factor BB ( µg/g once daily) alternated with placebo every h g vs recombinant platelet-derived growth factor BB ( µg/g every h g )vs placebo every h recombinant platelet-derived growth factor BB ( vs µg/ml) g vs placebo IorII; recombinant platelet-derived growth factor BB ( µg/ml) vs recombinant platelet-derived growth factor BB ( µg/ml) vs recombinant plateletderived growth factor BB ( µg/ml) g vs placebo Other Growth Factors II, III, IV, or V; nerve growth factor g vs placebo transforming growth factor beta ( µg/cm ) vs transforming growth factor beta (.5 µg/cm ) vs placebo granulocyte-macrophage/colonystimulating factor for d and then basic fibroblast growth factor vs granulocyte-macrophage/ colony-stimulating factor vs basic fibroblast growth factor g vs placebo basic fibroblast growth factor g vs placebo Primary Outcome Measure of Treatment Effect d Complete healing of 9% for protease-modulating matrix vs 7% for petrolatum-soaked gauze (P =.59) Complete wound healing of % for fibroblast-derived dermal replacement plus standard care vs % for standard care (P.5) Complete wound healing of % for µg/g of recombinant platelet-derived growth factor BB vs 9% for µg/g of recombinant platelet-derived growth factor BB vs % for placebo (P =.5 and P =.8, respectively) Wound surface area for ulcers in the recombinant platelet-derived growth factor BB groups were significantly smaller in volume vs placebo group (P =.9) After 8 d, mean volume of ulcer on wound surface area (vs day ): 6.% for recombinant platelet-derived growth factor BB ( µg/ml) vs.8% for placebo Mean (SD) wound surface area reduction at 6 wk: 78 (9) mm for nerve growth factor vs 85 (8) mm for placebo (P =.) Mean relative wound surface area:. cm for transforming growth factor beta ( µg/ cm ) vs. cm for transforming growth factor beta (.5 µg/cm )vs.7 cm for placebo (P.5) Wound surface area: basic fibroblast growth factor had significantly more patients than placebo with 85% closure (P =.) and 9% closure (P =.) Wound surface area: 6% of patients achieved a 7% volume reduction for basic fibroblast growth factor vs 9% for placebo (P =.5) of Trial e Abbreviation: NA, data not available. a Eligible age to participate in study is provided unless participant age is only available. b Duration of treatment is expressed to nearest week. c Different systems were used in the studies to stage pressure ulcer severity, but most systems rely on -stage categorization with higher numbers representing more severe ulcers. d Complete wound healing defined as the proportion of ulcers in the study group that healed during the intervention period; and wound surface area defined as changes of surface area measurements before and after treatment. e Maximum score of 6 and determined by the criteria on the checklist to evaluate a report of a nonpharmacological trial. See Methods section for description of criteria. f Standard care refers to various topical treatments in accordance with the participating institution and/or guidelines. g Indicates effective intervention for treatment of pressure ulcers. 656 JAMA, December, 8 Vol, No. (Reprinted) 8 American Medical Association. All rights reserved. Downloaded From: on 6//8
11 Table 5. Randomized Controlled Trials Evaluating Adjunctive Therapies for Local Wound Care Study; Age a Length of Follow-up b Pressure Ulcer Severity at Baseline; Intervention c Vacuum Therapy Wanner et al, 6 ; Rehabilitation f ; mean: wk vacuum therapy vs moist gauze -77 y Ford et al, 7 Adunsky and Ohry, 5 5 Adegoke and Badmos, 8 Wood et al, 9 99 Griffin et al, 99 Asbjornsen et al, 99 Kloth and Feedar, 988 ter Riet et al, 995 McDiarmid et al, 985 Salzberg et al, Comorosan et al, 6 99 Taly et al, 7 Lucas et al, 7 Dehlin et al, Iordanou et al, 8 8; ; 8-8 y 6; 8; 8 y 7; 6; -6 y 7; -95 y ; 7; -7 y ; 6; 7-9 y 6; -89 y 88; median age: 8 y ; 8; 8 y ; 9; -69 y ; ; 6-8 y 5; 5; 8-65 y 86; 79; 9- y ; 6; 65 y 55; ; 7-85 y ambulatory 6wk Long-term care and rehabilitation; 8- wk Acute care f ; wk Acute care and rehabilitation; 8 wk Rehabilitation f ; - wk -6 wk -6 wk wk 9 wk Acute care f ; wk wk Rehabilitation f ; 5 wk 6 wk ambulatory wk wk vacuum therapy vs cadexomer iodine or papain-ureachlorophyllin copper Electric Current III; direct current vs placebo direct current Primary Outcome Measures of Treatment Effect d Wound surface area: no difference in time to reach 5% of initial wound volume between groups Wound surface area mean reduction in ulcer volume of 5.8% for vacuum therapy vs.% for cadexomer iodine or papain-urea-chlorophyllin copper (P =.6) Complete wound healing of 5.7% for direct current vs 5.7% for placebo direct current (P =.8) IV; Wound surface area reduction of.% for interrupted direct current g vs interrupted direct current vs.6% for placebo interrupted direct current placebo interrupted direct current pulsed low-intensity direct current g vs placebo pulsed low-intensity direct current high-voltage pulsed direct current vs placebo high-voltage pulsed direct current transcutaneous electrical nerve stimulation vs placebo transcutaneous electrical nerve stimulation IV; high-voltage pulsed current g vs placebo high-voltage pulsed current Ultrasound II; ultrasound vs placebo ultrasound ultrasound vs placebo ultrasound Electromagnetic Therapy electromagnetic therapy g vs placebo electromagnetic therapy Wound surface area reduction of 8% within 8 wk (7.9% for pulsed low-intensity direct current vs.9% for placebo pulsed low-intensity direct current); P. Wound surface area reduction at day of 8 for high-voltage pulsed direct current vs 9 for placebo high-voltage pulsed direct current (P =.5) Wound surface area reduction in size of for transcutaneous electrical nerve stimulation vs 9 for placebo transcutaneous electrical nerve stimulation Wound surface area reduction per week of 5% for high-voltage pulsed current vs.6% for placebo high-voltage pulsed current Wound surface area reduction of % for ultrasound vs % for placebo ultrasound (P =.6) Median healing time of d for ultrasound vs 6 d for placebo ultrasound (P =.8) Complete wound healing of 8.% for electromagnetic therapy vs % for placebo electromagnetic therapy at wk (P =.) Complete wound healing of % for standard standard care h vs standard care plus care vs 85% for standard care plus electromagnetic therapy g vs electromagnetic therapy vs % for standard care plus placebo standard care plus placebo after wk Laser laser and moist saline gauze vs moist saline gauze III; low-level laser vs standard care h Light monochromatic phototherapy vs placebo I, II, or III; polarized light g vs standard care h Mean (SD) complete wound healing of.5 (.6) wk for laser and moist saline gauze vs.78 (.) wk for moist saline gauze (P =.); PSST score (P =.57) Wound surface area absolute wound size reduction (P =.) Complete wound healing reduction in ulcer area (P =.8); time to healing (P =.9) Mean reduction in wound surface area from.8 to.6 cm for polarized light vs from. to. cm for standard care of Trial e 5 5 (continued) 8 American Medical Association. All rights reserved. (Reprinted) JAMA, December, 8 Vol, No. 657 Downloaded From: on 6//8
12 Table 5. Randomized Controlled Trials Evaluating Adjunctive Therapies for Local Wound Care (continued) Schubert, 9 Wills et al, 98 Burke et al, 998 Shamimi et al, 8 Nussbaum et al, 99 Study; Age a 7; 59; 65 y 8; 6; 6- y ; ; NA 8; 8; 6 y ; 6; 5-6 y Length of Follow-up b wk wk wk wk Rehabilitation f ; - wk Pressure Ulcer Severity at Baseline; Intervention c Light monochromatic light plus cadexomer iodine or hydrocolloid g vs cadexomer iodine or hydrocolloid Superficial; UV light g vs placebo UV light Hydrotherapy moist saline gauze plus whirlpool g vs moist saline gauze Other intravenous semelil g vs intravenous normal saline laser and standard care h vs ultrasound and UV-C plus standard care g vs standard care Primary Outcome Measures of Treatment Effect d Wound surface area reduction per week of 9.8% for monochromatic light plus cadexomer iodine or hydrocolloid vs.% for cadexomer iodine or hydrocolloid; there was a 9% higher healing rate for monochromatic light (P =.5) Mean time to complete healing of 6. wk for superficial UV light vs 8. for placebo UV light (P.) Wound surface area: of wounds improved with moist saline gauze plus whirlpool vs 5 of 8 wounds improved with moist saline gauze only (P.5) Mean (SD) reduction in wound surface area of. (57.) cm (8.%) for intravenous semelil vs.8 (6.) cm (6.%) for intravenous normal saline (P.) Mean weekly reduction in wound surface area of.7% for laser and standard care vs 5.5% for ultrasound and UV-C plus standard care vs.% for standard care (P =.) of Trial e Abbreviations: NA, data not available; PSST, Pressure Sore Status Test. a Eligible age to participate in study is provided unless participant age is only available. b Duration of treatment is expressed to nearest week. c Different systems were used in the studies to stage pressure ulcer severity, but most systems rely on -stage categorization with higher numbers representing more severe ulcers. d The PSST score range is between and 65 with lower total score indicating better wound appearance. Complete wound healing defined as the proportion of ulcers in the study group that healed during the intervention period; time to healing defined as the time to complete wound healing; and wound surface area defined as changes of surface area measurements before and after treatment. e Maximum score of 6 and determined by the criteria on the checklist to evaluate a report of a nonpharmacological trial. See Methods section for description of criteria. f Indicates treatment for spinal cord injuries. g Indicates effective intervention for treatment of pressure ulcers. h Standard care refers to various topical treatments in accordance with the participating institution and/or guidelines. One study 9 of dressings met 5 of the 6 criteria, 6 studies,5,56,69,9,97 of dressings met of the 6 criteria, and studies, of biological agents met of the 6 criteria. Fourteen of the 6 RCTs (.%) did not meet any of the CLEAR NPT criteria.* Twenty-one RCTs (987 participants) evaluated adjunctive therapies (TABLE 5).,7,5,6- Among the goodquality RCTs examining adjunctive therapies, there were no benefits to the interventions, which included electric current (vs placebo electric current), 5 laser 7 (vs moist saline gauze), and ultrasound (vs placebo ultrasound). Two RCTs examined electromagnetic therapy and found improvements in wound healing compared with placebo or standard care, 5,6 butof *References, 8, 55, 7, 8, 8, 86-88, 9, 9, 95, 8. these RCTs did not report statistical significance. 6 Four trials examined light therapy,,8- with the highestquality trial demonstrating no improvement in healing with light therapy compared with placebo therapy. Two RCTs studied vacuum therapy and found no improvement in wound healing compared with cadexomer iodine, papain-ureachlorophyllin copper, or moist gauze. 6,7 Of the studies evaluating adjunctive therapies, 5 adequately described the generation of random allocation sequences (.8%) and provided information indicating that participants were randomized with concealed allocation (9.5%). Thirteen of the studies (6.9%) blinded participants adequately. Adequate blinding of outcome assessors was described in of the studies (66.7%). Co-interventions were balanced between groups in 6 of the studies (76.%). Intention-to-treat analyses were performed in only studies (9.5%). None of the studies examining local wound care fulfilled all 6 criteria from the CLEAR NPT checklist. Two studies 5,7 of adjunctive therapies met 5 of the 6 criteria and study met of 6 CLEAR NPT criteria. COMMENT Fundamental to chronic wound care are managing the underlying contributing factors, local wound care, and adjunctive therapies. Guidelines for the practical management of pressure ulcers are available from the Wound Healing Society ( /86575/issue). Management of underlying contributing factors is likely 658 JAMA, December, 8 Vol, No. (Reprinted) 8 American Medical Association. All rights reserved. Downloaded From: on 6//8
Pressure Ulcer Treatment
 Pressure Ulcer Treatment an educational webinar December 8, 2015 Webinar Producer Sue Brooks Online Production Assistant Web Page Manager Expert Synchronous Webinar Producer Webinar Guidelines 1 hour presentation
Pressure Ulcer Treatment an educational webinar December 8, 2015 Webinar Producer Sue Brooks Online Production Assistant Web Page Manager Expert Synchronous Webinar Producer Webinar Guidelines 1 hour presentation
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Centre for Clinical Practice
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Centre for Clinical Practice Review consultation document Review of Clinical Guideline (CG29) Pressure ulcers: the management of pressure ulcers in
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Centre for Clinical Practice Review consultation document Review of Clinical Guideline (CG29) Pressure ulcers: the management of pressure ulcers in
Seeking effective interventions to treat complex wounds: an overview of systematic reviews
 Tricco et al. BMC Medicine (2015) 13:89 DOI 10.1186/s12916-015-0288-5 RESEARCH ARTICLE Open Access Seeking effective interventions to treat complex wounds: an overview of systematic reviews Andrea C Tricco
Tricco et al. BMC Medicine (2015) 13:89 DOI 10.1186/s12916-015-0288-5 RESEARCH ARTICLE Open Access Seeking effective interventions to treat complex wounds: an overview of systematic reviews Andrea C Tricco
Palliative Care. EPUAP/NPUAP Publish New Pressure Ulcer Guidelines for. Treatment. Improving Quality of Care Based on CMS Guidelines 39
 Treatment EPUAP/NPUAP Publish New Pressure Ulcer Guidelines for Palliative Care Dealing with the end of a loved one s life is difficult enough, but when wound and skin care issues are involved, the decisions
Treatment EPUAP/NPUAP Publish New Pressure Ulcer Guidelines for Palliative Care Dealing with the end of a loved one s life is difficult enough, but when wound and skin care issues are involved, the decisions
INTRODUCTION TO WOUND DRESSINGS
 WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
WOUND CARE INTRODUCTION TO WOUND DRESSINGS JEC 2017 Wound Care Successfully completed specialized skills training in Wound Management. WOUND CONDITIONS & SYMBOLS BY COLOURS Yellow Black Necrotic tissue
Authors' objectives To assess the value of treatments for foot ulcers in patients with Type 2 diabetes mellitus.
 A systematic review of foot ulcer in patients with Type 2 diabetes mellitus - II: treatment Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A Authors' objectives To assess the value of treatments
A systematic review of foot ulcer in patients with Type 2 diabetes mellitus - II: treatment Mason J, O'Keeffe C, Hutchinson A, McIntosh A, Young R, Booth A Authors' objectives To assess the value of treatments
Data extraction. Specific interventions included in the review Dressings and topical agents in relation to wound healing.
 Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
SDMA Categorisation of Wound Care and Associated Products
 Version 7 - February 2015 TAPES AND TRADITIONAL DRESSINGS Traditional Wound Dressings Wound Dressings Packs Swabs Swabs Swab Products Adhesive Tapes Taping Sheets Absorbent Wadding Absorbent Dressings
Version 7 - February 2015 TAPES AND TRADITIONAL DRESSINGS Traditional Wound Dressings Wound Dressings Packs Swabs Swabs Swab Products Adhesive Tapes Taping Sheets Absorbent Wadding Absorbent Dressings
Appropriate Dressing Selection For Treating Wounds
 Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
Appropriate Dressing Selection For Treating Wounds Criteria to Consider for an IDEAL DRESSING Exudate Management Be able to provide for moist wound healing by absorbing exudate or adding moisture Secure
NPUAP Mission. Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries. npuap.org
 Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries Margaret Goldberg, MSN, RN, CWOCN June 29, 2016 NPUAP Mission The National Pressure Ulcer Advisory Panel (NPUAP) serves
Clinical Practice Guidelines: Wound Dressings for the Management of Pressure Injuries Margaret Goldberg, MSN, RN, CWOCN June 29, 2016 NPUAP Mission The National Pressure Ulcer Advisory Panel (NPUAP) serves
Acute and Chronic WOUND ASSESSMENT. Wound Assessment OBJECTIVES ITEMS TO CONSIDER
 WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
WOUND ASSESSMENT Acute and Chronic OBJECTIVES Discuss classification systems and testing methods for pressure ulcers, venous, arterial and diabetic wounds List at least five items to be assessed and documented
Categorisation of Wound Care and Associated Products
 Categorisation of Wound Care and Associated Products Version 9 March 2018 Surgical Dressing Manufacturers Association 2018 TAPES AND TRADITIONAL DRESSINGS Wound Dressings Swabs Taping Traditional Wound
Categorisation of Wound Care and Associated Products Version 9 March 2018 Surgical Dressing Manufacturers Association 2018 TAPES AND TRADITIONAL DRESSINGS Wound Dressings Swabs Taping Traditional Wound
Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types. Summary
 Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
Dressing selection Agenda (45 minutes) Some questions for you. Which wound dressing? Dressing categories/types Summary Which wound dressing poster Ref: Which wound dressing? Practice Nursing, September
CASE 1: TYPE-II DIABETIC FOOT ULCER
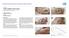 CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
CASE 1: TYPE-II DIABETIC FOOT ULCER DIABETIC FOOT ULCER 48 YEAR-OLD MALE Mr. C., was a 48-year old man with a history of Type-II diabetes over the past 6 years. The current foot ulcer with corresponding
Effective Health Care Program
 Comparative Effectiveness Review Number 90 Effective Health Care Program Pressure Ulcer Treatment Strategies: Comparative Effectiveness Executive Summary Background Uninterrupted pressure exerted on the
Comparative Effectiveness Review Number 90 Effective Health Care Program Pressure Ulcer Treatment Strategies: Comparative Effectiveness Executive Summary Background Uninterrupted pressure exerted on the
Uncovering the Pressure Ulcer Coverup Rhonda Kistler RN MS CWON Wound Care Concepts Gentell
 Uncovering the Pressure Ulcer Coverup Rhonda Kistler RN MS CWON Wound Care Concepts Gentell Objectives Identify the stages of pressure ulcer according to the depth of tissue destruction. Discuss the differences
Uncovering the Pressure Ulcer Coverup Rhonda Kistler RN MS CWON Wound Care Concepts Gentell Objectives Identify the stages of pressure ulcer according to the depth of tissue destruction. Discuss the differences
TITLE: Australian Sheepskins for the Management of Pressure Ulcers: A Review of the Clinical-Effectiveness, Cost-Effectiveness, and Guidelines
 TITLE: Australian Sheepskins for the Management of Pressure Ulcers: A Review of the Clinical-Effectiveness, Cost-Effectiveness, and Guidelines DATE: 21 July 2009 CONTEXT AND POLICY ISSUES: Pressure ulcers,
TITLE: Australian Sheepskins for the Management of Pressure Ulcers: A Review of the Clinical-Effectiveness, Cost-Effectiveness, and Guidelines DATE: 21 July 2009 CONTEXT AND POLICY ISSUES: Pressure ulcers,
Beyond the Basics ImprovingYour Wound Care Knowledge. Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN
 Beyond the Basics ImprovingYour Wound Care Knowledge Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN Projects and Posters These resources were developed by creative VA nurses who had no special
Beyond the Basics ImprovingYour Wound Care Knowledge Berna Goldentyer RN, BSN, CWOCN Kathy Hugen RN, BSN, CWOCN Projects and Posters These resources were developed by creative VA nurses who had no special
International Pressure Ulcer Guidelines Update Aamir Siddiqui, MD, FACS Division of Plastic Surgery Henry Ford Hospital Detroit MI
 International Pressure Ulcer Guidelines Update 2015 Aamir Siddiqui, MD, FACS Division of Plastic Surgery Henry Ford Hospital Detroit MI Disclosure Aamir Siddiqui has listed no financial interest/arrangement
International Pressure Ulcer Guidelines Update 2015 Aamir Siddiqui, MD, FACS Division of Plastic Surgery Henry Ford Hospital Detroit MI Disclosure Aamir Siddiqui has listed no financial interest/arrangement
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Pressure Ulcers: 3 Keys to Pressure Ulcer Management. Evidence Based Prevention & Management. I have no financial conflicts of interest
 Pressure Ulcers: Evidence Based Prevention & Management Madhuri Reddy, MD MSc I have no financial conflicts of interest I have nothing to disclose financially 3 Keys to Pressure Ulcer Management 1 3 Keys
Pressure Ulcers: Evidence Based Prevention & Management Madhuri Reddy, MD MSc I have no financial conflicts of interest I have nothing to disclose financially 3 Keys to Pressure Ulcer Management 1 3 Keys
Galen ( A.D) Advanced Wound Dressing
 Galen (120-201A.D) Advanced Wound Dressing Wounds heal optimally in a moist environment นพ.เก งกาจ ว น ยโกศล Wound assessment Ideal wound dressing Type of wound Clinical appearance Wound location Measurement
Galen (120-201A.D) Advanced Wound Dressing Wounds heal optimally in a moist environment นพ.เก งกาจ ว น ยโกศล Wound assessment Ideal wound dressing Type of wound Clinical appearance Wound location Measurement
Silver Dressings. Sajida Khatri PrescQIPP Primary Care Lead.
 Silver Dressings Sajida Khatri PrescQIPP Primary Care Lead www.prescqipp.info Available at: www.prescqipp.info/silverdressings 2 Introduction PrescQIPP Silver dressings bulletin published in March 2014
Silver Dressings Sajida Khatri PrescQIPP Primary Care Lead www.prescqipp.info Available at: www.prescqipp.info/silverdressings 2 Introduction PrescQIPP Silver dressings bulletin published in March 2014
Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community-based patients with chronic leg ulceration
 Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community-based patients with chronic leg ulceration Harding K G, Price P, Robinson B, Thomas S, Hofman D Record Status
Cost and dressing evaluation of hydrofiber and alginate dressings in the management of community-based patients with chronic leg ulceration Harding K G, Price P, Robinson B, Thomas S, Hofman D Record Status
7/2/2015 A.Shahrokhi 1
 7/2/2015 A.Shahrokhi 1 PRESSURE ULCER MANAGEMENT A.Shahrokhi,MSc Qazvin University of Medical Sciences 7/2/2015 A.Shahrokhi 3 Noticeable Facts Significant Prevalence 10% to 18% in acute care Cause of Death
7/2/2015 A.Shahrokhi 1 PRESSURE ULCER MANAGEMENT A.Shahrokhi,MSc Qazvin University of Medical Sciences 7/2/2015 A.Shahrokhi 3 Noticeable Facts Significant Prevalence 10% to 18% in acute care Cause of Death
Anseong Factory : 70-17, Wonam-ro, Wongok-myeon, Anseong-si, Gyeonggi-do , REPUBLIC OF KOREA
 Care for tomorrow The Solution for Management HQ & Factory : 7, Hyeongjero4Beon-gil, Namsa-myeon, Cheoin-gu, Yong-in-si, Gyeonggi-do 449-884, REPUBLIC OF KOREA TEL: +8-3-33-33 / FAX: +8-3-33-34 Anseong
Care for tomorrow The Solution for Management HQ & Factory : 7, Hyeongjero4Beon-gil, Namsa-myeon, Cheoin-gu, Yong-in-si, Gyeonggi-do 449-884, REPUBLIC OF KOREA TEL: +8-3-33-33 / FAX: +8-3-33-34 Anseong
Wound Dressing. Choosing the Right Dressing
 Wound Dressing Choosing the Right Dressing Benefits of using the correct Drsg Helps create the optimal wound environment Increases healing rates Reduces pain Decreases infection rates Cost effective Care
Wound Dressing Choosing the Right Dressing Benefits of using the correct Drsg Helps create the optimal wound environment Increases healing rates Reduces pain Decreases infection rates Cost effective Care
Consider the possibility of pressure ulcer development
 Douglas Fronzaglia II, DO, MS LECOM Institute for Successful Aging LECOM Institute for Advanced Wound Care and Hyperbaric Medicine Consider the possibility of pressure ulcer development 1 Identify ulcer
Douglas Fronzaglia II, DO, MS LECOM Institute for Successful Aging LECOM Institute for Advanced Wound Care and Hyperbaric Medicine Consider the possibility of pressure ulcer development 1 Identify ulcer
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Management in the Elderly
 Wound Management in the Elderly Stephanie Yates, MSN, ANP, ANP-BC, CWOCN Nurse Practitioner/CNS Duke University Medical Center Durham, NC stephanie.yates@duke.edu Skin Condition Key quality indicator To
Wound Management in the Elderly Stephanie Yates, MSN, ANP, ANP-BC, CWOCN Nurse Practitioner/CNS Duke University Medical Center Durham, NC stephanie.yates@duke.edu Skin Condition Key quality indicator To
SUPPORT SURFACES AND POSITIONING
 SUPPORT SURFACES AND POSITIONING American Medical Technologies Irvine, CA 1 Disclaimer The information presented herein is provided for educational and informational purposes only and to promote the safeand-effective
SUPPORT SURFACES AND POSITIONING American Medical Technologies Irvine, CA 1 Disclaimer The information presented herein is provided for educational and informational purposes only and to promote the safeand-effective
System-wide assessment of wound care interventions: A scoping review
 System-wide assessment of wound care interventions: A scoping review Prepared by: Andrea C. Tricco, Jesmin Antony, Elise Cogo, Paul A. Kahn, Alana Harrington, Afshin Vafaei, Geetha Sanmugalingham, Charlotte
System-wide assessment of wound care interventions: A scoping review Prepared by: Andrea C. Tricco, Jesmin Antony, Elise Cogo, Paul A. Kahn, Alana Harrington, Afshin Vafaei, Geetha Sanmugalingham, Charlotte
Additional file 6: CEA Sensitivity Analyses, Uncertainty of Results and Incremental Variabilities CEA (Original Yr of Values)
 Additional file 6: CEA Sensitivity Analyses, Uncertainty of Results and Incremental Variabilities CEA (Original Yr of Values) Intervention vs. Comparator Sensitivity Analyses a Uncertainty of Result b
Additional file 6: CEA Sensitivity Analyses, Uncertainty of Results and Incremental Variabilities CEA (Original Yr of Values) Intervention vs. Comparator Sensitivity Analyses a Uncertainty of Result b
Making the Most of your Dressing Products Catherine Hammond CNS/CNE
 Making the Most of your Dressing Products 2013 Catherine Hammond CNS/CNE What do you need in your dressings cupboard? 2 Skin tear 3 4 Lack Confidence in Selecting Dressings? 5 Appropriate Use of Product
Making the Most of your Dressing Products 2013 Catherine Hammond CNS/CNE What do you need in your dressings cupboard? 2 Skin tear 3 4 Lack Confidence in Selecting Dressings? 5 Appropriate Use of Product
IAD and its Severity Instrument
 IAD and its Severity Instrument Designed and validated by WOC nurses and their faculty 2 WOC nurses established initial face validity Content and criterion validity via 9 WOC nurses in North Central Region
IAD and its Severity Instrument Designed and validated by WOC nurses and their faculty 2 WOC nurses established initial face validity Content and criterion validity via 9 WOC nurses in North Central Region
DRESSING SELECTION SIMPLIFIED
 10 DRESSING SELECTION SIMPLIFIED It must be recognised that no one dressing provides the optimum environment for the healing of all wounds (Mahoney, 2015) DRESSING SELECTION SIMPLIFIED Selecting the correct
10 DRESSING SELECTION SIMPLIFIED It must be recognised that no one dressing provides the optimum environment for the healing of all wounds (Mahoney, 2015) DRESSING SELECTION SIMPLIFIED Selecting the correct
ד"ר בוריס פונצ' קי PRESSURE ULCERS
 ד"ר בוריס פונצ' קי 25.12.2013 PRESSURE ULCERS International EPUAP-NPUAP Pressure Ulcer Definition: (European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel, 2010).. is localized
ד"ר בוריס פונצ' קי 25.12.2013 PRESSURE ULCERS International EPUAP-NPUAP Pressure Ulcer Definition: (European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel, 2010).. is localized
Source of effectiveness data The effectiveness evidence was derived from a review of published studies.
 Cost and cost effectiveness of venous and pressure ulcer protocols of care Kerstein M D, Gemmen E, van Rijswijk L, Lyder C H, Phillips T, Xakellis G, Golden K, Harrington C Record Status This is a critical
Cost and cost effectiveness of venous and pressure ulcer protocols of care Kerstein M D, Gemmen E, van Rijswijk L, Lyder C H, Phillips T, Xakellis G, Golden K, Harrington C Record Status This is a critical
In today s healthcare arena, the prevention and treatment
 Constant Force Technology versus Low-Air-Loss Therapy in the Treatment of Pressure Ulcers Raquel Branom, RN, BSN, CWOCN, and Laurie M. Rappl, PT, CWS ABSTRACT Least costly but most effective has never
Constant Force Technology versus Low-Air-Loss Therapy in the Treatment of Pressure Ulcers Raquel Branom, RN, BSN, CWOCN, and Laurie M. Rappl, PT, CWS ABSTRACT Least costly but most effective has never
Dextranomer- Clinical Evaluation
 1.1 Clinical - Clinical Evaluation The efficacy and safety of dextranomer, in the form of both beads and paste, has been demonstrated in scientific literature 11. A summary table supporting the equivalence
1.1 Clinical - Clinical Evaluation The efficacy and safety of dextranomer, in the form of both beads and paste, has been demonstrated in scientific literature 11. A summary table supporting the equivalence
A GUIDE TO THE TREATMENT OF PRESSURE ULCERS FROM GRADE 1 GRADE 4
 A GUIDE TO THE TREATMENT OF PRESSURE ULCERS FROM GRADE 1 GRADE 4 Gill Wicks, Nurse Consultant, Tissue Viability for Wiltshire Primary Care Trust and Lecturer at University of West England Pressure ulcers
A GUIDE TO THE TREATMENT OF PRESSURE ULCERS FROM GRADE 1 GRADE 4 Gill Wicks, Nurse Consultant, Tissue Viability for Wiltshire Primary Care Trust and Lecturer at University of West England Pressure ulcers
LABETTE COMMUNITY COLLEGE BRIEF SYLLABUS. Please check with the LCC bookstore for the required texts for this class.
 LABETTE COMMUNITY COLLEGE BRIEF SYLLABUS SPECIAL NOTE: This brief syllabus is not intended to be a legal contract. A full syllabus will be distributed to students at the first class session. TEXT AND SUPPLEMENTARY
LABETTE COMMUNITY COLLEGE BRIEF SYLLABUS SPECIAL NOTE: This brief syllabus is not intended to be a legal contract. A full syllabus will be distributed to students at the first class session. TEXT AND SUPPLEMENTARY
Pressure Ulcer Prevention Guidelines
 EUROPEAN PRESSURE ULCER ADVISORY PANEL Pressure Ulcer Prevention Guidelines INTRODUCTION Pressure damage is common in many healthcare settings across Europe, affecting all age groups, and is costly both
EUROPEAN PRESSURE ULCER ADVISORY PANEL Pressure Ulcer Prevention Guidelines INTRODUCTION Pressure damage is common in many healthcare settings across Europe, affecting all age groups, and is costly both
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Assessment and Management of Pressure Injuries for the Interprofessional Team Third Edition RNAO Best Practice Guideline Launch
 Assessment and Management of Pressure Injuries for the Interprofessional Team Third Edition RNAO Best Practice Guideline Launch October 4, 2016 12:00 pm to 1:00pm Who will be speaking today? Irmajean Bajnok,
Assessment and Management of Pressure Injuries for the Interprofessional Team Third Edition RNAO Best Practice Guideline Launch October 4, 2016 12:00 pm to 1:00pm Who will be speaking today? Irmajean Bajnok,
Clinical Policy Title: Air fluidized beds
 Clinical Policy Title: Air fluidized beds Clinical Policy Number: 16.02.10 Effective Date: May 1, 2018 Initial Review Date: March 6, 2018 Most Recent Review Date: April 10, 2018 Next Review Date: April
Clinical Policy Title: Air fluidized beds Clinical Policy Number: 16.02.10 Effective Date: May 1, 2018 Initial Review Date: March 6, 2018 Most Recent Review Date: April 10, 2018 Next Review Date: April
Wound Care for Hospice Patients
 Wound Care for Hospice Patients Kristen Lyn Brodrick, RN, BSN, CHPN,CWCN No financial disclosures. Unique Population Patients needing hospice/palliative care are often at risk for developing multiple skin
Wound Care for Hospice Patients Kristen Lyn Brodrick, RN, BSN, CHPN,CWCN No financial disclosures. Unique Population Patients needing hospice/palliative care are often at risk for developing multiple skin
Wound Management. E. Foy White-Chu, MD, CWSP
 Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
Pressure ulcers: guideline development and economic modelling Legood R, McInnes E
 Pressure ulcers: guideline development and economic modelling Legood R, McInnes E Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each
Pressure ulcers: guideline development and economic modelling Legood R, McInnes E Record Status This is a critical abstract of an economic evaluation that meets the criteria for inclusion on NHS EED. Each
WOUND CARE. By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare
 WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
How Wounds Heal: A Guide for the Wound-care Novice
 C L I N I C A L P R A C T I C E How Wounds Heal: A Guide for the Wound-care Novice BY Christine Pearson Christine Pearson, RN, IIWCC, is a wound clinician for Vancouver Coastal Health and has worked in
C L I N I C A L P R A C T I C E How Wounds Heal: A Guide for the Wound-care Novice BY Christine Pearson Christine Pearson, RN, IIWCC, is a wound clinician for Vancouver Coastal Health and has worked in
Traditional Silicone Technology
 Innovative Non-Silicone Low Trauma Adhesives versus Traditional Silicone Technology A Review and Comparison Alan Neil Medical Industry Consultant Advanced Wound Care Lohmann Corporation 2016 Chronic wound
Innovative Non-Silicone Low Trauma Adhesives versus Traditional Silicone Technology A Review and Comparison Alan Neil Medical Industry Consultant Advanced Wound Care Lohmann Corporation 2016 Chronic wound
Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, and Arterial Ulcers: A Systematic Review
 Department of Veterans Affairs Health Services Research & Development Service Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, and Arterial Ulcers: A Systematic Review November 2012 Prepared
Department of Veterans Affairs Health Services Research & Development Service Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, and Arterial Ulcers: A Systematic Review November 2012 Prepared
Post-surgical wound management of pilonidal cysts by using a haemoglobin spray
 Post-surgical wound management of pilonidal cysts by using a haemoglobin spray Nesat Mustafi 1 & Peter Engels 2 1 Wound center, Nordwest Hospital, Frankfurt, Germany, 2 Bergisch Gladbach, Germany Objective:
Post-surgical wound management of pilonidal cysts by using a haemoglobin spray Nesat Mustafi 1 & Peter Engels 2 1 Wound center, Nordwest Hospital, Frankfurt, Germany, 2 Bergisch Gladbach, Germany Objective:
DMEPOS: hospital beds, bed accessories, and pressurereducing
 ACTION: Final DATE: 07/02/2018 10:03 AM 5160-10-18 DMEPOS: hospital beds, bed accessories, and pressurereducing support surfaces. (A) Definitions and explanations. (1) "Group 1," "group 2," and "group
ACTION: Final DATE: 07/02/2018 10:03 AM 5160-10-18 DMEPOS: hospital beds, bed accessories, and pressurereducing support surfaces. (A) Definitions and explanations. (1) "Group 1," "group 2," and "group
JMSCR Vol 06 Issue 03 Page March 2018
 www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
Downloaded from:
 Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Arnup, SJ; Forbes, AB; Kahan, BC; Morgan, KE; McKenzie, JE (2016) The quality of reporting in cluster randomised crossover trials: proposal for reporting items and an assessment of reporting quality. Trials,
Negative Pressure Wound Therapy
 Ontario Health Technology Assessment Series 2010; Vol. 10, No. 22 Negative Pressure Wound Therapy An Evidence Update Presented to the Ontario Health Technology Advisory Committee in September 2010 December
Ontario Health Technology Assessment Series 2010; Vol. 10, No. 22 Negative Pressure Wound Therapy An Evidence Update Presented to the Ontario Health Technology Advisory Committee in September 2010 December
CURRENT CONCEPTS IN PRESSURE INJURY PREVENTION AND CARE
 CURRENT CONCEPTS IN PRESSURE INJURY PREVENTION AND CARE JOIE WHITNEY, PHD, RN, CWCN, FAAN PROFESSOR BIOBEHAVIORAL NURSING AND HEALTH SYSTEMS UNIVERSITY OF WASHINGTON HARBORVIEW ENDOWED PROFESSOR IN CRITICAL
CURRENT CONCEPTS IN PRESSURE INJURY PREVENTION AND CARE JOIE WHITNEY, PHD, RN, CWCN, FAAN PROFESSOR BIOBEHAVIORAL NURSING AND HEALTH SYSTEMS UNIVERSITY OF WASHINGTON HARBORVIEW ENDOWED PROFESSOR IN CRITICAL
Current Concepts in Burn Rehabilitation
 Current Concepts in Burn Rehabilitation 7 th Congress of the Baltic Association of Rehabilitation Tallinn, Estonia September 2010 R. Scott Ward, PT, PhD Professor and Chair Department of Physical Therapy
Current Concepts in Burn Rehabilitation 7 th Congress of the Baltic Association of Rehabilitation Tallinn, Estonia September 2010 R. Scott Ward, PT, PhD Professor and Chair Department of Physical Therapy
Understanding our report and advice: Antimicrobial wound dressings (AWDs) for chronic wounds
 Understanding our report and advice: Antimicrobial wound dressings (AWDs) for chronic wounds Health technology assessment report 13 December 2015 Healthcare Improvement Scotland 2015 Published December
Understanding our report and advice: Antimicrobial wound dressings (AWDs) for chronic wounds Health technology assessment report 13 December 2015 Healthcare Improvement Scotland 2015 Published December
DRESSING SELECTION. Rebecca Aburn MN NP Candidate
 DRESSING SELECTION Rebecca Aburn MN NP Candidate Should be individually tailored in conjunction with the patient to meet their individual needs. WOUND MANAGEMENT: Comprehensive health assessment Wound
DRESSING SELECTION Rebecca Aburn MN NP Candidate Should be individually tailored in conjunction with the patient to meet their individual needs. WOUND MANAGEMENT: Comprehensive health assessment Wound
Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis Kantor J, Margolis D J
 Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis Kantor J, Margolis D J Record Status This is a critical abstract of an economic evaluation that meets the criteria
Treatment options for diabetic neuropathic foot ulcers: a cost-effectiveness analysis Kantor J, Margolis D J Record Status This is a critical abstract of an economic evaluation that meets the criteria
PRESSURE ULCERS REPRESENT A
 REVIEW CLINICIAN S CORNER Preventing Pressure Ulcers: A Systematic Review Madhuri Reddy, MD, MSc Sudeep S. Gill, MD, MSc Paula A. Rochon, MD, MPH See also Patient Page. CME available online at www.jama.com
REVIEW CLINICIAN S CORNER Preventing Pressure Ulcers: A Systematic Review Madhuri Reddy, MD, MSc Sudeep S. Gill, MD, MSc Paula A. Rochon, MD, MPH See also Patient Page. CME available online at www.jama.com
Bear of a Problem: Who should be sleeping in which bed?
 Bear of a Problem: Who should be sleeping in which bed? Dianne Mackey MSN, RN, CWOCN Wound/Skin Coordinator Kaiser Permanente San Diego California Evan Call MS, CSN (NRM) Adjunct Faculty Weber State University
Bear of a Problem: Who should be sleeping in which bed? Dianne Mackey MSN, RN, CWOCN Wound/Skin Coordinator Kaiser Permanente San Diego California Evan Call MS, CSN (NRM) Adjunct Faculty Weber State University
I ve a drawer full of dressings i don t know how to use!
 I ve a drawer full of dressings i don t know how to use! Introduction: Originating from battlefield medicine much of what we use today is an evolution of material science combined with our understanding
I ve a drawer full of dressings i don t know how to use! Introduction: Originating from battlefield medicine much of what we use today is an evolution of material science combined with our understanding
A comprehensive study on effect of collagen dressing in diabetic foot ulcer
 Original Research Article A comprehensive study on effect of collagen dressing in diabetic foot ulcer Sivakumar 1, S. Shanmugam 2* 1 Associate Professor, 2 Senior Assistant Professor Department of General
Original Research Article A comprehensive study on effect of collagen dressing in diabetic foot ulcer Sivakumar 1, S. Shanmugam 2* 1 Associate Professor, 2 Senior Assistant Professor Department of General
South West Regional Wound Care Toolkit F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE)
 F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE) F.5 SURGICAL WOUND (CLOSED AND OPEN) 5.1 Background to Etiology Closed surgical wounds are well-approximated with a palpable healing ridge
F. PRINCIPLES OF TREATMENT BASED ON ETIOLOGY (TREAT THE CAUSE) F.5 SURGICAL WOUND (CLOSED AND OPEN) 5.1 Background to Etiology Closed surgical wounds are well-approximated with a palpable healing ridge
Disclosures for Tarik Alam. Wound Bed Preparation. Wound Prognosis. Session Objectives. Debridement 4/26/2015
 Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
Disclosures for Tarik Alam Challenges in Managing Bioburden and Devitalized Tissue Tarik Alam RN, BScN, ET, MClSc(WH) Enterostomal Therapy Nurse tarikalam@hotmail.com Clinical Affairs Manager for Hollister
Tissue Viability Service Wound Management Primary Care Formulary 2017
 Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
Wound Care Program for Nursing Assistants-
 Wound Care Program for Nursing Assistants- Wound Cleansing,Types & Presentation Elizabeth DeFeo, RN, WCC, OMS, CWOCN Wound, Ostomy, & Continence Specialist ldefeo@cornerstonevna.org Outline/Agenda At completion
Wound Care Program for Nursing Assistants- Wound Cleansing,Types & Presentation Elizabeth DeFeo, RN, WCC, OMS, CWOCN Wound, Ostomy, & Continence Specialist ldefeo@cornerstonevna.org Outline/Agenda At completion
WOUND DRESSING IN DIABETIC FOOT
 Chapter XII WOUND DRESSING IN DIABETIC FOOT OVERVIEW OF DRESSINGS AND WOUNDS FUNCTIONS OF DRESSING TYPES OF DRESSING SELECTION OF DRESSING MATERIAL TOPICAL AGENTS AND ANTISEPTIC CLEANSERS NEWER OPTIONS
Chapter XII WOUND DRESSING IN DIABETIC FOOT OVERVIEW OF DRESSINGS AND WOUNDS FUNCTIONS OF DRESSING TYPES OF DRESSING SELECTION OF DRESSING MATERIAL TOPICAL AGENTS AND ANTISEPTIC CLEANSERS NEWER OPTIONS
We look forward to serving you.
 ADVANCED CARE GEMCORE360 offers healthcare professionals a simple, clear and cost-effective wound care range while ensuring excellent clinical outcomes for their patients. 1 At GEMCO Medical, we strive
ADVANCED CARE GEMCORE360 offers healthcare professionals a simple, clear and cost-effective wound care range while ensuring excellent clinical outcomes for their patients. 1 At GEMCO Medical, we strive
DEBRIDEMENT. Professor Donald G. MacLellan Executive Director Health Education & Management Innovations
 DEBRIDEMENT Professor Donald G. MacLellan Executive Director Health Education & Management Innovations DEBRIDEMENT Principles - CSD Methods of Debridement Biopsy options PRINCIPLES OF WOUND MANAGEMENT
DEBRIDEMENT Professor Donald G. MacLellan Executive Director Health Education & Management Innovations DEBRIDEMENT Principles - CSD Methods of Debridement Biopsy options PRINCIPLES OF WOUND MANAGEMENT
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU)
 Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Tissue Viability Service Wound Management Primary Care Formulary 2017
 Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
Tissue Viability Service Wound Management Primary Care Formulary 2017 WMPF/TVS: March 2017 Review date: March 2019 Product Group Current Product Sizes Price per Item Hydrogel 1st Activheal Hydrogel 2nd
This is a repository copy of Dressings and topical agents for treating pressure ulcers (Protocol).
 This is a repository copy of Dressings and topical agents for treating pressure ulcers (Protocol). White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/118145/ Version: Accepted
This is a repository copy of Dressings and topical agents for treating pressure ulcers (Protocol). White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/118145/ Version: Accepted
Workshop on Debridement
 MEDICINSK TEKNIK I SÅRDIAGNOSTIK OCH SÅRBEHANDLING Monday, 20 th of april 2015 Stockholm Sweden Workshop on Debridement Sources for best practice Reference documents available in several languages : WHY
MEDICINSK TEKNIK I SÅRDIAGNOSTIK OCH SÅRBEHANDLING Monday, 20 th of april 2015 Stockholm Sweden Workshop on Debridement Sources for best practice Reference documents available in several languages : WHY
Link between effectiveness and cost data The costing was undertaken prospectively on the same patient sample that provided the effectiveness data.
 Randomized clinical study of hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns Caruso D M, Foster K N, Blome-Eberwein S A, Twomey J A, Herndon D N, Luterman
Randomized clinical study of hydrofiber dressing with silver or silver sulfadiazine in the management of partial-thickness burns Caruso D M, Foster K N, Blome-Eberwein S A, Twomey J A, Herndon D N, Luterman
Wound Assessment & Treatment
 Wound Assessment & Treatment Cathy Lyle Advanced Practice Nurse Providence Care, SMOL site LTC Physicians CME June 2011 Outline l Is it healing? l Will it heal? l What colour is it? l How wet is it? l
Wound Assessment & Treatment Cathy Lyle Advanced Practice Nurse Providence Care, SMOL site LTC Physicians CME June 2011 Outline l Is it healing? l Will it heal? l What colour is it? l How wet is it? l
WOUND DRESSING Daily Dressing Packets
 AMERIGEL WOUND DRESSING Daily Dressing Packets P R O D U C T I N F O R M AT I O N MSDS APPLICATION PROTOCOLS AmeriGel WOUND DRESSING Daily Dressing Packets A HYDROGEL WITH A UNIQUE AUTOLYTIC DEBRIDER Diabetic
AMERIGEL WOUND DRESSING Daily Dressing Packets P R O D U C T I N F O R M AT I O N MSDS APPLICATION PROTOCOLS AmeriGel WOUND DRESSING Daily Dressing Packets A HYDROGEL WITH A UNIQUE AUTOLYTIC DEBRIDER Diabetic
TIME CONCEPT AND LOCAL WOUND MANAGEMENT
 TIME CONCEPT AND LOCAL WOUND MANAGEMENT B. BRAUN WOUND CARE INTRODUCTION: TIME is a global care framework used to implement appropriate care plans and promote wound healing Tissue Management Inflammation
TIME CONCEPT AND LOCAL WOUND MANAGEMENT B. BRAUN WOUND CARE INTRODUCTION: TIME is a global care framework used to implement appropriate care plans and promote wound healing Tissue Management Inflammation
Mean percent reduction in ulcer area from baseline at six weeks 62 % SANTYL Ointment + supportive care* + sharp debridement 1 (P<0.
 Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
Wound and Ostomy Care: Basics and Troubleshooting
 Wound and Ostomy Care: Basics and Troubleshooting Catherine Clarey-Sanford, PhD, RN, CWOCN Conflict of Interest No conflict of interest exists No commercial interest No financial benefits Specific wound
Wound and Ostomy Care: Basics and Troubleshooting Catherine Clarey-Sanford, PhD, RN, CWOCN Conflict of Interest No conflict of interest exists No commercial interest No financial benefits Specific wound
NPWT Case Series EXPERIENCES WITH INVIA MOTION. Precious life Progressive care. Invia Motion Negative Pressure Wound Therapy
 NPWT Case Series EXPERIENCES WITH INVIA MOTION Invia Motion Negative Pressure Wound Therapy Precious life Progressive care npwt_case_booklet_a4.indd 1 18.12.13 13:17 Chronic sacral pressure ulcer Case
NPWT Case Series EXPERIENCES WITH INVIA MOTION Invia Motion Negative Pressure Wound Therapy Precious life Progressive care npwt_case_booklet_a4.indd 1 18.12.13 13:17 Chronic sacral pressure ulcer Case
Is noncontact normothermic wound therapy cost effective for the treatment of stages 3 and 4 pressure ulcers Macario A, Dexter F
 Is noncontact normothermic wound therapy cost effective for the treatment of stages 3 and 4 pressure ulcers Macario A, Dexter F Record Status This is a critical abstract of an economic evaluation that
Is noncontact normothermic wound therapy cost effective for the treatment of stages 3 and 4 pressure ulcers Macario A, Dexter F Record Status This is a critical abstract of an economic evaluation that
A film-dressing therapy [Open Wet-dressing Therapy (OWT)] for pressure ulcers. -a revolution of end of life care-
![A film-dressing therapy [Open Wet-dressing Therapy (OWT)] for pressure ulcers. -a revolution of end of life care- A film-dressing therapy [Open Wet-dressing Therapy (OWT)] for pressure ulcers. -a revolution of end of life care-](/thumbs/84/90807340.jpg) http://www.pressure-ulcer.net/ A film-dressing therapy [Open Wet-dressing Therapy (OWT)] for pressure ulcers. -a revolution of end of life care- Takeo SAIO (Fuji Toranomon Health Promotion Center) Shunichi
http://www.pressure-ulcer.net/ A film-dressing therapy [Open Wet-dressing Therapy (OWT)] for pressure ulcers. -a revolution of end of life care- Takeo SAIO (Fuji Toranomon Health Promotion Center) Shunichi
Study selection Study designs of evaluations included in the review Randomised controlled trials (RCTs) were eligible for inclusion in the review.
 Preventive intervention possibilities in radiotherapy- and chemotherapy-induced oral mucositis: results of meta-analyses Stokman M A, Spijkervet F K, Boezen H M, Schouten J P, Roodenburg J L, de Vries
Preventive intervention possibilities in radiotherapy- and chemotherapy-induced oral mucositis: results of meta-analyses Stokman M A, Spijkervet F K, Boezen H M, Schouten J P, Roodenburg J L, de Vries
Basic Dressing Categories
 Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
Category of Dressing Examples Advantages/Indications Disadvantages/Contraindications Hydrofiber Aquacel AG - ConvaTec Aquacel - Convatec Excellent for absorbing excess exudate These dressings form a gel
Case. Wounds. Fundamentals of Ulcer Care. Dr. Mark Meissner Wound Case Study. Compression and Ulcer Healing Cullum NA, Cochrane Reviews 2001
 Case Wounds Mark H. Meissner, MD University of Washington School of Medicine 65 yr old male physician Recurrent R medial malleolar ulcer over 5 years New R lateral malleolar ulcer Intermittently wearing
Case Wounds Mark H. Meissner, MD University of Washington School of Medicine 65 yr old male physician Recurrent R medial malleolar ulcer over 5 years New R lateral malleolar ulcer Intermittently wearing
Guidelines for the Treatment of Pressure Ulcers (Adapted from EPUAP & NPUAP 2009)
 Guidelines for the Treatment of Pressure Ulcers (Adapted from EPUAP & NPUAP 2009) This guidance should be read in conjunction with your local dressing formulary and anti-biotic prescribing guidelines.
Guidelines for the Treatment of Pressure Ulcers (Adapted from EPUAP & NPUAP 2009) This guidance should be read in conjunction with your local dressing formulary and anti-biotic prescribing guidelines.
2. Advanced wound therapies... 4 (i) Maggots... 4 (ii) Negative Pressure Wound Therapy (NPWT)... 4
 Contents: Wound management Medicines Formulary 1. Interactive dressings... 2 (i) Hydrocolloid dressings... 2 (ii) Hydrogel dressings... 2 (iii) Alginate dressings... 2 (iv) Fibrous absorbent dressings...
Contents: Wound management Medicines Formulary 1. Interactive dressings... 2 (i) Hydrocolloid dressings... 2 (ii) Hydrogel dressings... 2 (iii) Alginate dressings... 2 (iv) Fibrous absorbent dressings...
Foam dressings have frequently
 The practical use of foam dressings Efficient and cost-effective management of excessive exudate continues to challenge clinicians. Foam dressings are commonly used in the management of moderate to heavily
The practical use of foam dressings Efficient and cost-effective management of excessive exudate continues to challenge clinicians. Foam dressings are commonly used in the management of moderate to heavily
Research 101: Developing Critical Evaluation Skills
 R E S E A R C H Research 101: Developing Critical Evaluation Skills BY M. Gail Woodbury M. Gail Woodbury, BScPT, MSc, PhD, is an Investigator, Program in Rehabilitation and Geriatric Care, Lawson Health
R E S E A R C H Research 101: Developing Critical Evaluation Skills BY M. Gail Woodbury M. Gail Woodbury, BScPT, MSc, PhD, is an Investigator, Program in Rehabilitation and Geriatric Care, Lawson Health
Incontinence Associated Dermatitis. Moisture Associated Dermatitis 8/31/2017. Goals of Presentation. Differentiating and Controlling
 Incontinence Associated Dermatitis Moisture Associated Dermatitis Differentiating and Controlling Goals of Presentation This presentation will attempt to: Identify causes and risk factors for IAD and MASD
Incontinence Associated Dermatitis Moisture Associated Dermatitis Differentiating and Controlling Goals of Presentation This presentation will attempt to: Identify causes and risk factors for IAD and MASD
Open Wound( 개방창상 ) 피부나점막의손상이있는경우 ex)abrasion, Burn,Laceration 등 Closed Wound( 폐쇄창상 ) 피부나점막의손상이없는내부조직의손상 ex)closed Fracture, Ligament tear 등
 신체조직의연속성이파괴된상태 Open Wound( 개방창상 ) 피부나점막의손상이있는경우 ex)abrasion, Burn,Laceration 등 Closed Wound( 폐쇄창상 ) 피부나점막의손상이없는내부조직의손상 ex)closed Fracture, Ligament tear 등 Partial Thickness Skin Injury - dermis 의일부만손상을입은경우
신체조직의연속성이파괴된상태 Open Wound( 개방창상 ) 피부나점막의손상이있는경우 ex)abrasion, Burn,Laceration 등 Closed Wound( 폐쇄창상 ) 피부나점막의손상이없는내부조직의손상 ex)closed Fracture, Ligament tear 등 Partial Thickness Skin Injury - dermis 의일부만손상을입은경우
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist.
 Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
Systematic reviews and meta-analyses of observational studies (MOOSE): Checklist. MOOSE Checklist Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease:
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology
 A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
Advazorb. Hydrophilic foam dressing range
 Advazorb Hydrophilic foam dressing range Advazorb A comprehensive range of patient friendly, absorbent foam dressings Non-adhesive and atraumatic silicone adhesive options Designed to manage exudate whilst
Advazorb Hydrophilic foam dressing range Advazorb A comprehensive range of patient friendly, absorbent foam dressings Non-adhesive and atraumatic silicone adhesive options Designed to manage exudate whilst
Description. Section: Medicine Effective Date: April 15, 2015 Subsection: Medicine Original Policy Date: September 13, 2012 Subject:
 Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
