Langworthy, Kolb & Lewis, 1940, and Kuru, 1965). The function of the sympathetic innervation, on the other hand, is still uncertain.
|
|
|
- Collin Underwood
- 5 years ago
- Views:
Transcription
1 J. Physiol. (1972), 220, pp With 7 text-ftgurew Printed in Great Britain SYMPATHETIC INHIBITION OF THE URINARY BLADDER AND OF PELVIC GANGLIONIC TRANSMISSION IN THE CAT BY WILLIAM C. DE GROAT AND WILLIAM R. SAUM From the Department of Pharmacology, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, U.S.A. (Received 16 June 1971) SUMMARY 1. Electrophysiological techniques were utilized to study the mechanisms underlying adrenergic inhibition in the urinary bladder of the cat. 2. It has been shown that catecholamines administered by close intraarterial injection or released endogenously by electrical stimulation of the hypogastric nerves elicit two distinct inhibitory responses in the bladder: (1) a direct depression of the vesical smooth muscle and (2) a depression of transmission in vesical parasympathetic ganglia. 3. Pharmacological studies revealed that the inhibitory mechanisms were mediated via different adrenergic receptors: fl-receptors on the smooth muscle and a-receptors in the parasympathetic ganglia. 4. We have been unable, however, to demonstrate that either of these mechanisms is activated by naturally occurring sympathetic firing. INTRODUCTION The urinary bladder receives an innervation from both the parasympathetic and sympathetic divisions of the autonomic nervous system (Langley & Anderson, 1895, 1896). The parasympathetic pathway represents the principal excitatory input to the bladder; and its integrity is essential for the normal performance of micturition (see reviews by Langworthy, Kolb & Lewis, 1940, and Kuru, 1965). The function of the sympathetic innervation, on the other hand, is still uncertain. It is well known that electrical stimulation of the sympathetic fibres (hypogastrics) inhibits bladder contractions; however, evidence for a similar action by naturally occurring sympathetic activity has been obtained in only a few investigations. Gjone (1965) and Edvardsen (1968a) noted that in acute experiments on cats transaction of the vesical sympathetic nerves resulted in an enhancement of spontaneous and reflexly evoked contractions of the bladder. They proposed therefore that the sympathetic fibres exerted
2 298 WILLIAM C. DE GROAT AND WILLIAM B. SAUM a tonic inhibitory influence on the bladder. Their findings and conclusions, however, are in contrast to a considerable body of earlier data which indicated that the sympathetic pathways were relatively unimportant in the regulation of bladder function (see Langworthy et al. 1940, for references). The mechanisms underlying sympathetic inhibitory action in the bladder are also poorly understood. According to traditional concepts, sympathetic influences are mediated by a direct action of the adrenergic transmitter on the vesical smooth muscle cells. Recently, however, it was suggested (Hamberger & Norberg, 1965a) that sympathetic inhibition might also result from a depression of ganglionic transmission in the parasympathetic excitatory pathway to the bladder. This proposal was based on the finding that adrenergic terminals in the bladder were located primarily in the intramural nerve plexus, often in apparent synaptic contact with the parasympathetic (cholinergic) ganglion cells, and only sparsely in the layers of the detrusor muscle. Since exogenous catecholamines depress ganglionic transmission (see reviews by Volle, 1966; de Groat, 1967; Libet, 1970), the possibility exists that endogenously released catecholamines might have a similar action. The present study was undertaken to examine by more direct means the site of sympathetic-induced inhibition in the urinary bladder of the cat. We utilized electrophysiological techniques (de Groat & Ryall, 1969; de Groat, 1970) to monitor transmission in pelvic ganglia, while simultaneously recording intravesical pressure. In the majority of animals we were able to demonstrate a depression of pelvic ganglionic transmission during stimulation of the hypogastric nerves. However, we conclude, on the basis of pharmacological data, that under the conditions of our experiments, this action must have contributed only slightly to sympathetic depression of bladder activity. Preliminary accounts of some of these observations have been published (de Groat & Saum, 1971a, b). METHODS Experiments were performed on cats of both sexes anaesthetized either with chloralose (50-70 mg/kg, i.v.) or with a mixture of sodium diallylbarbiturate (70 mg/kg), urethane (280 mg/kg) and monoethylurea (280 mg/kg) administered intraperitoneally. Chloralose anaesthesia was induced with halothane. Following intubabation of the trachea, the urinary bladder and its neural innervation were exposed through a mid line abdominal incision. Hypogastric nerves on both sides and one or two preganglionic nerves to the inferior mesenteric ganglia were dissected free from surrounding connective tissue. Branches of the pelvic nerve (preganglionic) were freed at a point 2-3 cm from the neck of the bladder. In some experiments the pelvic nerves were crushed or sectioned central to the point of isolation and in other experiments the nerves were left intact. Ganglia were identified on the surface of the
3 SYMPATHETIC INHIBITION OF URINARY BLADDER 299 bladder and post-ganglionic fibres were freed of connective tissue and sectioned cm distal to the ganglia. The urinary bladder was cannulated by one of two different methods. In some cats (primarily females) a polyethylene tube (i.d. 2 mm) was introduced into the urethra either through the external orifice or through an incision in the urethra and passed into the bladder. The cannula was secured in place by a ligature around the urethra. In other cats an incision was made in the funds of the bladder and a thin-walled rubber condom mounted on the end of a flexible tube (i.d. 6'4 mm) was inserted into the lumen (de Groat & Ryall, 1968). The cannulae were filled with physiological saline solution and connected to a pressure transducer to record the pressure within the bladder. The cannulae could also be connected via a three-way stopcock to a reservoir of large surface area, the height of which could be adjusted to maintain a constant pressure in the bladder over a range of 0-60 cm H20. The ureters were ligated; and in many experiments the spinal cord was transacted in the lower thoracic or lower lumbar region. The inferior mesenteric artery was cannulated for close intra-arterial administration of drugs to the bladder and the external iliac arteries were ligated to increase the amount of drug reaching the bladder. Skin flaps were tied to a metal frame supporting the animal and the area was covered with warmed paraffin oil. Isolated nerves were mounted on bipolar platinum or silver electrodes for stimulation and recording. Stimulation was produced by rectangular pulses of msec duration at varying frequencies and intensities. Action potentials were displayed on an oscilloscope and photographed on 35 mm film. In some experiments potentials were also averaged on a Computer of Average Transients (CAT), the output of which was then plotted on an X-Y paper recorder. Most animals were paralysed with gallamine triethiodide and artificially respired. End-tidal C02, which was monitored continuously with a Beckman Medical Gas Analyzer, was maintained at approximately 4% by varying the rate and depth of respiration. Systemic blood pressure was measured from the carotid artery or femoral artery with a strain gauge pressure transducer. The animals' temperature was maintained at 36-38o C with the aid of a heating pad. End-tidal C02, blood pressure, and bladder pressure were displayed on a rectilinear multichannel paper recorder. The following drugs were used: (-)-adrenaline hydrochloride, (± )-isoprenaline hydrochloride, (-)-noradrenaline bitartrate (NADR), dopamine hydrochloride, y-aminobutyric acid (GABA), propranolol hydrochloride, 1-(p-nitrophenyl)-2- isopropyl aminoethanol hydrochloride (INPEA), dihydroergotamine methanesulphonate (DHE), phentolamine methanesulphonate, phenoxybenzamine hydrochloride, picrotoxin, tetraethylammonium bromide, guanethidine monosulphate, bretylium tosylate, atropine sulphate. Doses are expressed as the salt when compounds were administered in this form and refer to l.a. administration unless otherwise indicated. RESULTS Adrenergic inhibition in vesical ganglia As illustrated in Fig. 1, electrical stimulation of the pelvic nerve elicited a bimodal response on post-ganglionic fibres arising from vesical parasympathetic ganglia. The response consisted of a short latency, transient potential (marked by an arrow in record A) and a longer latency, more prolonged discharge. The late discharge in contrast to the early response was (1) facilitated during repetitive stimulation (2-20 c/s), (2) depressed at high frequencies of stimulation (40-60 c/s) and (3) completely blocked by
4 300 WILLIAM C. DE GROAT AND WILLIAM R. SAUM the administration of ganglionic blocking agents, e.g. tetraethylammonium or y-aminobutyric acid (GABA; de Groat, 1970). We assume, therefore, that the late discharge was activity in post-ganglionic fibres, while the early response represented firing in vesical afferents or preganglionic through-fibres. The amplitude of the post-ganglionic discharge was used as a monitor of ganglionic transmission. A B C D a 50 msec 9-0 e 0 to _ UBO E W WZ Frequency of stimulation (c/s) Fig. 1. Depression of transmission in pelvic ganglia by stimulation of the ipsilateral hypogastric nerve (HGN) at various frequencies. A is the control discharge recorded on a vesical post-ganglionic nerve filament in response to stimulation of the pelvic nerve (0.5 c/s) at submaximal intensity (2 V). Arrow denotes through-fibre response. In B and C the post-ganglionic discharge was depressed by stimulation of the HGN (10 V) at 5 c/s and 20 c/s, respectively. D is a plot of the depression of the amplitude of the post-ganglionic discharge (ordinate) against frequency of HGN-stimulation (abscissa). Vertical calibration in A-C is 200,uV, negativity upwards. Stimulation of sympathetic nerves. In a majority of experiments (twentyfive of thirty-seven) repetitive stimulation of the hypogastric nerve (HGN) ipsilateral to the recording site or stimulation of preganglionic nerves to the ipsilateral inferior mesenteric ganglion (IMG) depressed the evoked post-ganglionic firing on vesical nerve filaments, but did not block the through-fibre responses (Fig. 1). Stimulation of the contralateral HGN
5 SYMPATHETIC INHIBITION OF URINARY BLADDER 301 produced inhibition in three of eight experiments. However, the inhibition was abolished by crushing or sectioning the HGN central to the site of stimulation; a finding which suggests that the inhibition was evoked by an axon or Sokownin reflex through the opposite IMG and HGN (see Langworthy et al. 1940), rather than by fibres crossing at the level of the bladder. Stimulation of the HGN at intensities greater than V (0.05 msec) also elicited firing on vesical nerve filaments at latencies of msec. Since the firing was resistant to ganglionic blocking agents, A B C D A ] E f0 msec 50 msec 0 Zi 0 -c W._ To C >!R._ 0 4 to Intensity of stimulation (V) Fig. 2. Depression of transmission in pelvic ganglia by stimulation of the ipsilateral HGN at various intensities. A, B, C and D are the post-ganglionic C-fibre responses recorded on the HGN to antidromic stimulation of the HGN at 5, 9, 15 and 30 V, respectively. The threshold for the C-fibre response was 10 V. E is a plot of the depression of the amplitude of the pelvic post-ganglionic discharge (ordinate) against intensity of HGN stimulation (abscissa) at 20 c/s. Vertical calibration is 250 #V, negativity upwards. it is assumed that it occurred as a result of stimulation of afferent fibres or sympathetic post-ganglionic axons. The calculated conduction velocity in this pathway was m/sec. 30
6 302 WILLIAM C. DE GROAT AND WILLIAM R. SAUM The inhibitory responses to HGN-stimulation were apparent at intensities of stimulation below those which produced a detectable discharge on vesical nerves. Thresholds ranged from 2 to 5 V (0.05 msec duration), while maximal inhibition was generally produced by stimuli between 8 and 12 V (Fig. 2). Similar threshold and maximal values were obtained when stimulating preganglionic nerves to the IMG. Based on direct electrical recordings from the HGN it would appear that this range of voltages activates preganglionic B-fibres but excites relatively few post-ganglionic C-fibres (Fig. 2). It seems, therefore, that the sympathetic inhibitory pathway is comprised in part of preganglionic axons which pass directly through the IMG to make synapses with neurones in the pelvic plexus. Control HGN-STIM PREG-STIM 1MG-TRANS I 4'Ss X S 100 msec Fig. 3. Depression of transmission in pelvic ganglia during stimulation of either the ipsilateral hypogastric nerve (HGN-STINI, 10 V, 20 c/s) or preganglionic nerves (PREG-STIM, 10 V, 20 c/s) to the ipsilateral inferior mesenteric ganglion (IMG). Records I and II were taken, respectively, before and after the topical application of nicotine hydrochloride (0.1 % solution) to the IMG. This concentration of nicotine blocked synaptic transmission in the IMG (record II, IMG-TRANS). Pelvic ganglionic transmission was recorded as in Fig. 1. Note that the initial through-fibre response was not depressed during the inhibition. IMG-TRANS: the postganglionic response recorded on the ipsilateral HGN to stimulation (10 V, 0 5 c/s) of two preganglionic nerves to the ipsilateral IMG. Vertical calibration is 100,V, negativity upwards. The existence of a non-synaptic pathway through the IMG is also indicated by results shown in Fig. 3. In this experiment, stimulation of either the HGN or a preganglionic nerve to the IMG depressed pelvic ganglionic transmission (records I). After the topical application of nicotine to the IMG, in a concentration which completely blocked IMG-transmission (records II), stimulation at both sites produced the same degree of inhibition. Clearly, in this animal, the preganglionic axons made the majority of their synaptic connexions peripheral to the IMG. Similar results were
7 SYMPATHETIC INHIBITION OF URINARY BLADDER 303 obtained in another experiment; however, in two additional experiments the local application of nicotine reduced the inhibition to preganglionic stimulation without altering the response to HGN stimulation. These findings suggest that neurones in the IMG are also involved in the inhibitory pathway. The ganglionic inhibition elicited by activation of the sympathetic A Control I -l B 1-3 sec 4-1 sec 5-8 sec I C D I t_ A]- E I-,1 0- L cl. C._ 0a- bo ba Do LI bo LI 0.C so (0 u Interval (sec) Fig. 4. Time course of the inhibitory effect in pelvic ganglia to activation of the HGN with trains of stimuli (100 msec train duration, 20 V, 200 c/s intra-train frequency). A is a control response evoked by stimulation of the pelvic nerve (0-16 c/s) at submaximal stimulation (2 V). Top trace in this and remaining records is taken at slow sweep to show the time relation between the HGN-stimulus train (at the beginning of the sweep) and the pelvic-evoked response (at the arrow). Bottom trace is the pelvic-evoked response on an expanded sweep. Interval (in sec) between the start of the HGN-train and the pelvic response is above each record. E is a plot of the depression of the amplitude of the pelvic discharge (ordinate) against the interval between the start of the train and the occurrence of the pelvic discharge. For the upper traces the horizontal calibration represents 2 sec in A and B, 5 sec in C and 10 sec in D. For the lower traces the calibration represents 100 msec in A-C and 200 msec in D. Vertical calibration represents 240,uV for top tracings and 200 #V for bottom tracings.
8 304 WILLIAM C. DE GROAT AND WILLIAM R. SAUM fibres was only observed at frequencies of stimulation above 2 c/s and commonly reached a maximum at frequencies of c/s (Fig. 1). The inhibition was detectable within 1-5 see after the onset of stimulation, persisted for the period of stimulation (15-40 sec) and for 5-40 see after the termination of the stimulus. A facilitation of transmission lasting 1-2 min occurred during the recovery period in approximately 50% of the experiments. In two experiments, hypogastric nerve stimulation (HGS) elicited only a facilitation of transmission without a preceding inhibition. Ganglionic inhibition was also produced in a few experiments by stimulating the HGN with short trains of pulses ( msec train duration, c/s intra-train frequency). The inhibition occurred at a considerable latency ( msec after the beginning of the train) and persisted for 1-5 see following the stimulus (Fig. 4). The degree of HGN-induced inhibition was inversely related to the level of excitation in the pelvic ganglionic pathway. For example, inhibition was marked when the pelvic nerves were stimulated at submaximal intensities and at low frequencies (0*5-1 c/s), whereas little if any inhibition occurred when the pelvic excitatory stimulus was applied at supramaximal intensities and at higher frequencies (5-10 c/s). In addition not all ganglia exhibited inhibition. The degree of inhibition not only varied in different animals, but also varied between different post-ganglionic fibres in the same animal. Occasionally it was observed that inhibition was prominent on one post-ganglionic fibre and not detectable on another. There was no obvious relationship between the presence of inhibition and the anatomical location of the ganglia or the post-ganglionic fibres. Sympathetic inhibition of pelvic ganglionic transmission was not altered by the administration of fl-adrenergic blocking agents (fl-aba, propranolol, mg; INPEA, 0*1-2 mg) (Fig. 5A), atropine (10-100,ug) or picrotoxin ( ,ug), but, as illustrated in Fig. 5B, was completely antagonized by ac-adrenergic blocking agents (az-aba, dihydroergotamine, ,ug; phentolamine, 04-1 mg). The antagonism persisted for the duration of the experiment (4-6 hr). C-ABAs also reduced or abolished the post-inhibitory facilitation in two of seven experiments. Propranolol reduced the facilitation in two of three experiments. Response to injected catecholamines. The I.A. administration of adrenaline (ADR, 01-10,ug), noradrenaline (NADR, 01-10/%g) and dopamine (DA, 1-20 jug) mimicked the depressant actions of HGS (Fig. 5). Threshold doses to produce a detectable inhibition (10-15% reduction in the amplitude of the discharge) varied in different experiments; however, the order of potency was invariably ADR > NADR > DA. NADR was generally 5-10 times more potent than DA. The depressant effects of the catechol-
9 SYMPATHETIC INHIBITION OF URINARY BLADDER 305 mines appeared 2-10 sec after injection and persisted for 30 sec to 4 min depending upon the dose and the state of the animal's circulation. Isoprenaline (1-10 jug), an amine with potent beta adrenergic actions, exhibited no ganglionic depressant actions, but occasionally produced a slight enhancement of ganglionic transmission (Fig. 5A). A Control HGN-STIM NADR ISO A B Control HGN-STIM NADR Dopamine Fig msec Effects of electrical stimulation of the hypogastric nerve (HGN- STIM 20 V, 20 c/s) and injected catecholamines on transmission in pelvic ganglia. Recordings were made on a post-ganglionic nerve filament on the surface of the urinary bladder. Discharges were elicited by electrical stimulation of the ipsilateral pelvic nerve (1.5 V, 0 5 c/s). A: records I and II are, respectively, before and after the administration of propranolol (0.5 mg). Isoprenaline (ISO) and noradrenaline (NADR) were administered in doses of 1 and 5 ug, respectively. B: records I and II were obtained, respectively, before and after the administration of dihydroergotamine (150 #ug). Noradrenaline (NADR) and dopamine (DA) were administered in doses of 5 /zg. Vertical calibration is the same as in Fig. 1 and applies to records A and B. Like the depression produced by HGS, the ganglionic depressant actions of the catecholamines were antagonized by a-abas, dihydroergotamine (50-200,ug) and phentolamine ( ,ug), but were unaffected by,-abas or picrotoxin (Fig. 5). On the other hand, the effects of another ganglionic depressant, GABA (1-100 jug; de Groat, 1970), were reduced by picrotoxin ( ug) but were not altered by either o- or /3-ABAs. The
10 306 WILLIAM C. DE GROAT AND WILLIAM R. SAUM depressant actions ofadr and NADR unlike those of DA and GABA were often followed by a facilitation of transmission. The latter action was not consistently altered by adrenergic blocking agents. In some experiments, it was reduced or completely abolished by dihydroergotamine, whereas in others it was antagonized by propranolol. In other experiments it was not affected by either type of blocking agent. Adrenergic modulation of vesical activity Stimulation of the sympathetic nerves. In most experiments (eighteen of twenty) repetitive stimulation of the HGNs or preganglionic nerves to the IMG elicited an initial transient rise in intravesical pressure (i.e. contraction of the bladder) followed by a fall in pressure to below control levels (see Langworthy et al. 1940; or Edvardsen, 1968c, for references to earlier studies). The contractile response was of short duration (20-30 see) even during continued stimulation, whereas the secondary inhibitory effect persisted during prolonged stimulation (2-3 min) and was detectable for a period of see following the termination of the stimulus. The threshold stimulus to produce a detectable vesical excitatory or inhibitory response varied in different animals from 2 to 5 V (0.05 msec duration), at frequencies of 2-5 c/s. Maximal responses were generally produced by stimulation at V, c/s. In comparison to the vesical excitatory response elicited by pelvic nerve stimulation (peak intravesical pressures reaching cm H20), the excitatory response to HGS was relatively small, commonly attaining peak intravesical pressures of cm H20. It was present following bilateral transaction of the pelvic nerve or after complete transaction of the spinal cord at T1O to T12, a procedure which blocks parasympathetic excitatory reflexes to the bladder (de Groat & Ryall, 1969). Thus, the response to HGS was not dependent upon a central parasympathetic outflow to the vesical smooth muscle. Interestingly, however, stimulation of the HGN in intact animals often induced reflex parasympathetic firing, presumably as a result of activation of vesical afferents during the initial contractile response. The reflex parasympathetic firing complicated the analysis of adrenergic responses; and, thus, most experiments were conducted in animals with pelvic nerves or spinal cord transacted. The vesical contractions to HGS were reduced in amplitude immediately following a spontaneously occurring bladder contraction (in intact animals) or following a contraction elicited by electrical stimulation of the pelvic nerves. This inhibitory interaction would suggest either that the pelvic and hypogastric fibres activate the same population of smooth muscle cells (refractoriness, thereby accounting for the depression) or
11 SYMPATHETIC INHIBITION OF URINARY BLADDER 307 that an inhibitory mechanism is activated following a bladder contraction. This particular phenomenon was not studied in further detail. The vesical contractions elicited by HGS were increased in duration following the administration of propranolol (0 1-1 mg) and INPEA, (1-2 mg) (Edvardsen, 1968b). The contractions were blocked by guanethidine (0.7-2 mg), an agent which blocks the release of the adrenergic transmitter (Boura & Green, 1965), but they were not altered by the A I HGN-STIM NADR ISO I HGN-STIM NADR ISO I 30 - min U, X ~~~ i min Fig. 6. Effects of electrical stimulation of the hypogastric nerve (HGN- STIM, 20 V, 20 c/s) and injected catecholamines on bladder contractions evoked by intermittent stimulation of the pelvic nerve (15 V, 10 c/s every 30 see for a period of 5 sec). A: records I and II were obtained, respectively, before and after the administration of propranolol (0.5 mg). HGN-STIM was applied during the period indicated by the bar under each record. Note the small bladder contraction which appears at the beginning of each period of HGN-STIM. Noradrenaline (NADR) and isoprenaline (ISO) were injected at the arrows in doses of 5,ug. B: records I and II were obtained, respectively, before and after the administration of dihydroergotamine (100,ug). Doses of NADR and ISO are the same as in A. Horizontal calibration is 1 min per division for both A and B. administration of dihydroergotamine ( mg) or phenoxybenzamine (0.5-2 mg). The latter finding confirms an earlier report by Sigg & Sigg (1964), but conflicts with the results of Edvardsen (1968b) which indicated that an a-aba blocked the vesical contraction to HGS. The discrepancy
12 308 WILLIAM C. DE GROAT AND WILLIAM R. SAUM between our results and those of Edvardsen (1968b) is difficult to explain since we have never observed an antagonism even with very large doses of the blocking agents, i.e times the dose required to block adrenergic inhibition in pelvic ganglia. If the bladder contractions to HGS are indeed mediated via oc-adrenergic receptors, then these receptors must have different pharmacological characteristics than those receptors which are involved in ganglionic inhibition. The vesical inhibitory effects to HGS were evident as a decrease in the frequency and amplitude of spontaneously occurring bladder contractions or as a decrease in the amplitude of contractions evoked by intermittent electrical stimulation of a pelvic nerve (see Fig. 6). The intrinsic activity of the vesical smooth muscle which was present following bilateral transection of the pelvic nerves or transaction of the spinal cord at the lower thoracic levels was also inhibited by HGS. Depending on the frequency and intensity of stimulation, the inhibition ranged up to 100% depression and persisted for as long as 7 min following the cessation of stimulation. The inhibitory effects of HGS were antagonized by the administration of propranolol (0-5-2 mg) (Fig. 6A), INPEA (0-5-2 mg) and guanethidine (1-2 mg). The antagonism by propranolol was rapid in onset and persisted for 2-3 hr. On the other hand, a-abas (dihydroergotamine, phentolamine, phenoxybenzamine) did not modify the inhibition (Fig. 6B). Response to injected catecholamines. The I.A. administration of NADR ( /,g), ADR ( Mug) and isoprenaline ( /tg) to the urinary bladder depressed spontaneously occurring and pelvic nerve-evoked contractions ofthe bladder, decreased the resting bladder 'tone' and depressed the low amplitude contractions of the decentralized bladder. The depression occurred within 20 sec of the injection and persisted for 1-5 min depending upon the dose administered. Dopamine (0.5-50,sg) did not alter the activity of the urinary bladder. The depressant effects were reduced or completely blocked by the fl-abas but were unaffected by the various ac-abas mentioned in preceding sections of this paper (Fig. 6). The monoamines never produced a detectable contraction of the bladder under any of the conditions encountered in these experiments. GABA ug) produced a transient (20-40 sec) depression (30-50% reduction in peak amplitude) of spontaneous and evoked bladder contractions without producing a reduction in bladder 'tone'. Picrotoxin ( #g) blocked the depressant actions of GABA in three of six experiments. In some experiments, GABA also evoked a transient contraction of the bladder; this response was not studied in detail.
13 SYMPATHETIC INHIBITION OF URINARY BLADDER 309 Physiological role of sympathetic inhibitory mechanisms in the regulation of bladder activity If sympathetic fibres produce a tonic inhibition of the vesical smooth muscle or the vesical parasympathetic ganglion cells, then it might be expected that a surgical orpharmacological interruption ofthe sympathetic pathways would enhance the activity of these cells. Experiments were conducted to examine this possibility. It was found that neither spontaneous or pelvic nerve-evoked contractions of the bladder nor transmission in pelvic ganglia were consistently altered by transactions of the HGN or by the administration of o- or,-adrenergic blocking agents. It was observed as reported previously by Sigg & Sigg (1964), that dihydroergotamine increased base line pressure ('tone') in the bladder; however, this effect occurred in decentralized, as well as normally innervated preparations and would therefore appear to be related to a direct excitatory effect on the vesical smooth muscle rather than to a block of adrenergic inhibition. Other C-ABAs did not produce this effect. The administration of propranolol (0.5-2 mg) enhanced bladder activity and 'tone' in only a small percentage of experiments in which it was tested. Large doses of propranolol (2-3 mg) exerted direct depressant actions on pelvic ganglionic transmission. This action has been observed in sympathetic ganglia following the administration of other,-abas (de Groat & Volle, 1966a, b) and probably represents a local anaesthetic or membrane stabilizing action, which is unrelated to adrenergic blocking properties of the drugs. DISCUSSION It has been shown in the present investigation that catecholamines administered by close I.A. injection or released endogenously during stimulation of the hypogastric nerves (HGN) elicit two distinct inhibitory responses in the urinary bladder of the cat (see summary diagram in Fig. 7). One type of inhibition, which was apparent as a depression of spontaneous or evoked bladder contractions, was antagonized by /J-ABAs. Since this inhibition could be demonstrated in normally innervated as well as decentralized unstimulated bladder preparations, it must have been mediated by a direct action on the non-neural elements in the bladder, i.e. the vesical smooth muscle cells. This will be termed 'post-junctional inhibition'. The second type of inhibition occurred in the parasympathetic ganglia on the surface of the urinary bladder. This inhibition was unaffected by fl-abas, but was completely antagonized by ac-abas. Thus, adrenergic modulation of bladder activity seems to involve depressant actions at different sites in the vesical neuromuscular apparatus and also
14 310 WILLIAM C. DE GROAT AND WILLIAM R. SAUM actions on different adrenergic receptors: f-receptors in vesical smooth muscle and c-receptors in parasympathetic ganglia. Adrenergic inhibition in ganglia. The inhibition observed in parasympathetic ganglia is of particular interest, since it represents the first demonstration of ganglionic blockade by an adrenergic synaptic mechanism. The existence of such an inhibitory mechanism has been a subject of speculation for many years (Marrazzi, 1939; Biilbring, 1944) and is now supported by a considerable body of experimental evidence. Catecholamines are present in sympathetic and parasympathetic ganglia in nerve Adrenergic inhibition of the bladder f-adrenergic Noradrenaline I blocking agents Isoprenaline I Pre Adrenergic inhibitory neurone % ~~~~~Post oamn Noradrenaline /Dopamine. _a-adrenergic Bladder \ / ~~~~~~Pelvic ganglion Fig. 7. Diagrammatic representation of receptors mediating adrenergic inhibition in the urinary bladder of the cat. The dashed line from the adrenergic inhibitory neurone indicates that the post-ganglionic axons to the bladder and the pelvic ganglia do not necessarily originate from the same adrenergic inhibitory neurone. See Discussion for further details. terminals which form basket-like synaptic structures around the ganglion cells (Hamberger & Norberg, 1965a, b; Jacobowitz, 1970); and catecholamine-like substances are released into the venous effluent of isolated perfused ganglia during preganglionic stimulation (Biilbring, 1944). Exogenous catecholamines are potent ganglionic depressants, which hyperpolarize sympathetic ganglion cells (Lundberg, 1952; de Groat &
15 SYMPATHETIC INHIBITION OF URINARY BLADDER 311 Volle, 1966a, b; Libet & Kobayashi, 1969); and there is evidence from the studies of Eccles & Libet (1961) and more recently by Libet and coworkers (see review by Libet, 1970, for references) that endogenously released catecholamines have a similar hyperpolarizing action. It is tempting to speculate, therefore, that inhibition observed in vesical ganglia is also mediated by a hyperpolarization of the ganglion cells. However, a presynaptic inhibitory mechanism cannot be eliminated (Christ & Nishi, 1971). An anatomical substrate for adrenergic inhibition in vesical ganglia has been described by Hamberger & Norberg (1965a, b), El-Badawi (1967) and El-Badawi & Schenk (1971). Using histochemical techniques these investigators showed that adrenergic terminals occur in close apposition to the vesical cholinergic ganglion cells. Since the terminals did not degenerate following section of the HGNs or the lumbar sympathetic chain, it was concluded that they were derived from adrenergic neurones located in the pelvic plexus. We now suggest that these neurones receive synaptic connexions from preganglionic fibres in the HGN. This proposal is based on two observations. First, that the threshold stimulus (2-5 V) for producing inhibition in pelvic ganglia was below the threshold for activating unmyelinated post-ganglionic fibres and was similar on both the HGNs and on preganglionic fibres to the IMG. Secondly, that in one half of the experiments, complete block of transmission in the IMG by the topical application of nicotine did not reduce adrenergic inhibition elicited by stimulation of preganglionic nerves. This proposed pathway is also supported by the histological findings of Kuntz & Moseley (1936) and Moseley (1936) that a large percentage of pelvic ganglia receive a preganglionic input via the HGNs. By analogy with synaptic transmission on to other peripheral adrenergic neurones, it is reasonable to assume that the inhibitory neurones in the pelvic plexus were activated by a cholinergic mechanism. Furthermore, since atropine did not block ganglionic inhibition to HGS it would appear that these neurones are excited via nicotinic receptors. This is in contrast to the Eccles & Libet model for sympathetic ganglia (Libet, 1970) in which adrenergic inhibitory neurones are supposedly excited via muscarinic receptors. Relative importance of ganglionic and 'post-junctional inhibition' in the bladder. Of the two adrenergic inhibitory mechanisms 'post-junctional inhibition' (fl-type) obviously had the greatest influence on bladder activity under the conditions existing in the present experiments. Depression by HGS of pelvic nerve-evoked bladder contractions was invariably completely blocked by /5-ABAs and unaffected by o-abas. The failure of ganglionic inhibition (z-type) to alter bladder activity is probably related
16 312 WILLIAM C. DE GROAT AND WILLIAM R. SAUM both to the nature of the inhibitory mechanism and to the parameters of stimulation which we employed. Ganglionic inhibition was a relatively weak mechanism, which could be demonstrated in only 70% of the preparations and only when the excitatory input to the parasympathetic ganglia was at low level, i.e. submaximal stimulation at low frequencies (0.5-1 c/s). This mode of stimulation was unsuitable for evoking reproducible activity of the entire bladder; and in most experiments it was necessary to stimulate the pelvic nerve at higher intensities and frequencies (5-15 c/s). Under these conditions, ganglionic inhibition evoked by HGS, or by injected catecholamines, was undetectable; and depression of bladder activity was mediated exclusively by a direct inhibition of the vesical smooth muscle. It should be noted, however, that these findings do not necessarily indicate that ganglionic inhibition is unimportant in the regulation of bladder function. The synchronous type of activity which was elicited in these experiments on both inhibitory and excitatory pathways is far removed from the asynchronous discharge which occurs physiologically. It is therefore difficult to estimate the relative importance of the two types of inhibition under more physiological conditions. Physiological role of ganglionic and 'poet-junctional inhibition' in the regulation of bladder activity. Adrenergic inhibition in vesical parasympathetic ganglia and smooth muscle occurred at frequencies of HGS within the physiological range of sympathetic firing; it is therefore conceivable that these inhibitory responses might also be elicited by physiological sympathetic activity. Indeed, Edvardsen (1968a) and Gjone (1965) noted that bladder contractions were enhanced following section of the HGNs and on this basis suggested that the sympathetic produced a tonic inhibitory action on the bladder. Since Edvardsen (1968a) obtained this result only when bladder pressure was high he proposed that afferent activity from the bladder maintained a reflex discharge in vesical sympathetic inhibitory fibres. Recently, a vesico-sympathetic reflex has been demonstrated electrophysiologically (Lalley, de Groat & McLain, 1971); however, in the present experiments we have been unable to show that this pathway produces inhibition in the bladder. For example, neither ganglionic transmission nor evoked or spontaneous bladder activity was consistently changed by transaction of the HGNs. Following the administration of dihydroergotamine, we have observed an increase in bladder contractions and tone; however, this was noted in normally innervated as well as decentralized unstimulated bladders and was not duplicated by other x-abas. Therefore, the action seems attributable to a direct effect of the agent on vesical smooth muscle rather than an antagonism of a ganglionic inhibitory mechanism (see also Sigg & Sigg, 1964). The administration of propranolol enhanced bladder activity and tone in only a small
17 SYMPATHETIC INHIBITION OF URINARY BLADDER 313 percentage of the experiments. This action was observed more consistently by Edvardsen (1968c) and was ascribed to an antagonism of adrenergic inhibition in the vesical smooth muscle. In summary, the present experiments demonstrate the potentiality for two types of adrenergic inhibition in the urinary bladder of the cat; however, they provided little evidence that these mechanisms are functionally important in the anaesthetized animal. The discrepancies in this regard between our results and those of Edvardsen (1968a, c) and Gjone (1965) are difficult to explain. The authors would like to thank Miss E. La Rocco for her expert technical assistance. This investigation was supported in part by grant NB from the National Institute of Neurological Diseases and Stroke. We would also like to thank the Ciba, Sandoz and Selvi Pharmaceutical Companies for their kind donation of drugs. REFERENCES BOurRA, A. L. A. & GREEN, A. F. (1965). Adrenergic neurone blocking agents. A. Rev. Pharmac. 5, BULBRING, E. (1944). The action of adrenaline on transmission in the superior cervical ganglion. J. Physiol. 103, CHRIST, D. D. & NismI, S. (1971). Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit. Br. J. Pharmac. 41, DE GROAT, W. C. (1967). Actions of the catecholamines in sympathetic ganglia. Circulation Res , supply. 3, DE GROAT, W. C. (1970). The actions of y-aminobutyric acid and related amino acids on mammalian autonomic ganglia. J. Pharmac. exp. Ther. 172, DE GROAT, W. C. & RYALL, R. W. (1968). Recurrent inhibition in sacral parasympathetic pathways to the bladder. J. Physiol. 196, DE GROAT, W. C. & RYALL, R. W. (1969). Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J. Phy8iol. 200, DE GROAT, W. C. & SAum, W. R. (1971a). Sympathetic-inhibition of the urinary bladder and of pelvic parasympathetic ganglionic transmission in the cat. Fedn Proc. 30, 656. DE GROAT, W. C. & SAuIm, W. R. (1971b). Adrenergic inhibition in mammalian parasympathetic ganglia. Nature, New Biol. 231, DE GROAT, W. C. & VOLIE, R. L. (1966a). The actions of the catecholamines on transmission in the superior cervical ganglion of the cat. J. Pharmac. exp. Ther. 154, DE GROAT, W. C. & VOLLiE, R. L. (1966b). Interactions between the catecholamines and ganglionic stimulating agents in sympathetic ganglia. J. Pharmac. exp. Ther. 154, Eccrms, R. M. & LIBET, B. (1961). Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J. Physiol. 157, EDVARDSEN, P. (1968 a). Nervous control of urinary bladder in cats. I. The collecting phase. Acta physiol. 8cand. 72, EDVARDSEN, P. (1968b). Nervous control of urinary bladder in cats. IV. Effects of autonomic blocking agents on responses to peripheral nerve stimulation. Acta physiol. 8cand. 72,
18 314 WILLIAM C. DE GROAT AND WILLIAM R. SAUM EDVARDSEN, P. (1968c). Nervous control of urinary bladder in cats. III. Effects of autonomic blocking agents in the intact animal. Acta phy8iol. 8cand. 72, EL-BADAwI, A. (1967). Histochemical studies of the organization of the peripheral autonomic nervous system. Fedn Proc. 26, 794. EL-BADAWI, A. & ScHENK, E. A. (1971). A new theory of the innervation of bladder musculature. Part 3. Post-ganglionic synapses in uretero-vesico-urethral autonomic pathways. J. Urol. 105, GJONE, R. (1965). Peripheral autonomic influence on the motility of the urinary bladder in the cat. I. Rhythmic contractions. Acta phy8iol. 8cand. 65, HAmBERGER, B. & NORBERG, K.-A. (1965a). Adrenergic synaptic terminalsandnerve cells in bladder ganglia of the cat. Int. J. Neuropharmac. 4, HmBERGER, B. & NORBERG, K.-A. (1965b). Studies on some systems of adrenergic synaptic terminals in the abdominal ganglia of the cat. Acta phy8iol. 8cand. 65, JACOBOWITZ, D. (1970). Catecholamine fluorescence studies of adrenergic neurons and chromaffin cells in sympathetic ganglia. Fedn Proc. 29, KUNTZ, A. & MosErEY, R. L. (1936). An experimental analysis of the pelvic auto. nomic ganglia in the cat. J. comp. Neurol. 64, Kuxu, M. (1965). Nervous control of micturition. Physiol. Rev. 45, LALLEY, P. M., DE GROAT, W. C. & McLAIN, P. L. (1971). Reflex firing on the hypogastric nerve (HGN) in response to stimulation of vesical afferent fibers. Fedn Proc. 30, 324. LANGLEY, J. N. & ANDERSON, H. K. (1895). The innervation of the pelvic and adjoining viscera. Part II. The bladder. J. Physiol. 19, LANGLEY, J. N. & ANDERSON, H. K. (1896). The innervation of the pelvic and adjoining viscera. Part VII. Anatomical observations. J. Physiol. 20, LANGWORTHY, 0. R., KOLB, L. C. & LEWIs, L. G. (1940). Physiology of Micturition. Baltimore: Williams and Wilkins Company. LrBET, B. (1970). Generation of slow inhibitory and excitatory postsynaptic potentials. Fedn Proc. 29, LIBET, B. & KOBAYASHI, H. (1969). Generation of adrenergic and cholinergic potentials in sympathetic ganglion cells. Science, N.Y. 164, LUNDBERG, A. (1952). Adrenaline and transmission in the sympathetic ganglion of the cat. Acta physiol. 8cand. 26, MARRAZZI, A. S. (1939). Electrical studies on the pharmacology of autonomic synapses. II. The action of a sympathomimetic drug (epinephrine) on sympathetic ganglia. J. Pharmac. exp. Ther. 65, MOsELEY, R. L. (1936). Preganglionic connections of the intramural ganglia of the urinary bladder. Proc. Soc. exp. Biol. Med. 34, SIGG, E. B. & SIGG, T. D. (1964). Sympathetic stimulation and blockade of the urinary bladder in cat. Int. J. Neuropharmac. 3, VOLLE, R. L. (1966). Modification by drugs of synaptic mechanisms in autonomic ganglia. Pharmac. Rev. 18,
sympathetic innervation to the colon but was blocked by interruption of the sacral
 J. Physiol. (1978), 276, pp. 481-500 481 With 10 text-figures Printed in Great Britain THE SACRAL PARASYMPATHETIC REFLEX PATHWAY REGULATING COLONIC MOTILITY AND DEFAECATION IN THE CAT BY W. C. DE GROAT
J. Physiol. (1978), 276, pp. 481-500 481 With 10 text-figures Printed in Great Britain THE SACRAL PARASYMPATHETIC REFLEX PATHWAY REGULATING COLONIC MOTILITY AND DEFAECATION IN THE CAT BY W. C. DE GROAT
Action of drugs on denervated myoepithelial cells of salivary glands
 Br. J. Pharmac. (1973), 48, 73-79. Action of drugs on denervated myoepithelial cells of salivary glands N. EMMELIN AND A. THULIN Institute of Physiology, University of Lund, Sweden Summary 1. The pressure
Br. J. Pharmac. (1973), 48, 73-79. Action of drugs on denervated myoepithelial cells of salivary glands N. EMMELIN AND A. THULIN Institute of Physiology, University of Lund, Sweden Summary 1. The pressure
ASYNCHRONOUS POSTGANGLIONIC FIRING FROM THE CAT SUPERIOR CERVICAL SYMPATHETIC GANGLION TREATED WITH NEOSTIGMINE
 Brit. J. Pharmacol. (1963), 20, 214-220. ASYNCHRONOUS POSTGANGLIONIC FIRING FROM THE CAT SUPERIOR CERVICAL SYMPATHETIC GANGLION TREATED WITH NEOSTIGMINE BY C. TAKESHIGE AND R. L. VOLLE From the Department
Brit. J. Pharmacol. (1963), 20, 214-220. ASYNCHRONOUS POSTGANGLIONIC FIRING FROM THE CAT SUPERIOR CERVICAL SYMPATHETIC GANGLION TREATED WITH NEOSTIGMINE BY C. TAKESHIGE AND R. L. VOLLE From the Department
THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION
 Brit. J. Pharmacol. (1963), 20, 74-82. THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION BY J. H. BURN AND D. F. WEETMAN From the Biological Research Laboratories,
Brit. J. Pharmacol. (1963), 20, 74-82. THE EFFECT OF ESERINE ON THE RESPONSE OF THE VAS DEFERENS TO HYPOGASTRIC NERVE STIMULATION BY J. H. BURN AND D. F. WEETMAN From the Biological Research Laboratories,
THE ACTION OF NICOTINE ON THE CILIARY GANGLION
 Brit. J. Pharmnacol. (1952), 7, 665. THE ACTION OF NICOTINE ON THE CILIARY GANGLION BY BRENDA M. SCHOFIELD From the Department of Pharmacology, University of Oxford (Received June 7, 1952) The existing
Brit. J. Pharmnacol. (1952), 7, 665. THE ACTION OF NICOTINE ON THE CILIARY GANGLION BY BRENDA M. SCHOFIELD From the Department of Pharmacology, University of Oxford (Received June 7, 1952) The existing
Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit
 Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
Br. J. Pharmac. (1971), 41, 331-338. Effects of adrenaline on nerve terminals in the superior cervical ganglion of the rabbit D. D. CHRIST AND S. NISHI Neurophysiology Laboratory, Department of Pharmacology,
THE ACTION OF GUANETHIDINE WITH PARTICULAR REFERENCE TO THE SYMPATHETIC NERVOUS SYSTEM
 Brit. J. Pharinacol. (1963), 20, 171-177. THE ACTION OF GUANETHIDINE WITH PARTICULAR REFERENCE TO THE SYMPATHETIC NERVOUS SYSTEM BY G. F. ABERCROMBIE AND B. N. DAVIES From the Department of Physiology,
Brit. J. Pharinacol. (1963), 20, 171-177. THE ACTION OF GUANETHIDINE WITH PARTICULAR REFERENCE TO THE SYMPATHETIC NERVOUS SYSTEM BY G. F. ABERCROMBIE AND B. N. DAVIES From the Department of Physiology,
LEAKAGE OF TRANSMITTERS IN SALIVARY GLANDS
 Brit. J. Pharmacol. (1964), 22, 119-125. LEAKAGE OF TRANSMITTERS IN SALIVARY GLANDS BY N. ASSARSON AND N. EMMELIN From the Institute of Physiology, University of Lund, Sweden (Received October 8, 1963)
Brit. J. Pharmacol. (1964), 22, 119-125. LEAKAGE OF TRANSMITTERS IN SALIVARY GLANDS BY N. ASSARSON AND N. EMMELIN From the Institute of Physiology, University of Lund, Sweden (Received October 8, 1963)
1,1-Dimethyl-4-phenylpiperazinium iodide (DMPP) is known to have a depolarizing
 Brit. J. Pharmacol. (1965) 24, 375-386. AN ANALYSIS OF THE BLOCKING ACTION OF DIMETHYLPHENYLPIPERAZINIUM IODIDE ON THE INHIBITION OF ISOLATED SMALL INTESTINE PRODUCED BY STIMULATION OF THE SYMPATHETIC
Brit. J. Pharmacol. (1965) 24, 375-386. AN ANALYSIS OF THE BLOCKING ACTION OF DIMETHYLPHENYLPIPERAZINIUM IODIDE ON THE INHIBITION OF ISOLATED SMALL INTESTINE PRODUCED BY STIMULATION OF THE SYMPATHETIC
(Received 10 April 1956)
 446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
446 J. Physiol. (I956) I33, 446-455 A COMPARISON OF FLEXOR AND EXTENSOR REFLEXES OF MUSCULAR ORIGIN BY M. G. F. FUORTES AND D. H. HUBEL From the Department ofneurophysiology, Walter Reed Army Institute
Human Anatomy. Autonomic Nervous System
 Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
Human Anatomy Autonomic Nervous System 1 Autonomic Nervous System ANS complex system of nerves controls involuntary actions. Works with the somatic nervous system (SNS) regulates body organs maintains
THE EFFECT OF CYCLOPROPANE, HALOTHANE AND ETHER ON SYMPATHETIC GANGLIONIC TRANSMISSION
 Brit. J. Anaesth. (1966), 38, 3 THE EFFECT OF CYCLOPROPANE, HALOTHANE AND ETHER ON SYMPATHETIC GANGLIONIC TRANSMISSION BY T. J. BlSCOE* AND R. A. MlLLARf Agricultural Research Council Institute of Animal
Brit. J. Anaesth. (1966), 38, 3 THE EFFECT OF CYCLOPROPANE, HALOTHANE AND ETHER ON SYMPATHETIC GANGLIONIC TRANSMISSION BY T. J. BlSCOE* AND R. A. MlLLARf Agricultural Research Council Institute of Animal
Principles of Anatomy and Physiology
 Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
Principles of Anatomy and Physiology 14 th Edition CHAPTER 15 The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems The somatic nervous system includes both sensory and motor
Physiologic Anatomy and Nervous Connections of the Bladder
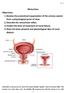 Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
Micturition Objectives: 1. Review the anatomical organization of the urinary system from a physiological point of view. 2. Describe the micturition reflex. 3. Predict the lines of treatment of renal failure.
number Done by Corrected by Doctor
 number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
number 13 Done by Tamara Wahbeh Corrected by Doctor Omar Shaheen In this sheet the following concepts will be covered: 1. Divisions of the nervous system 2. Anatomy of the ANS. 3. ANS innervations. 4.
Department of Physiology, Okayama University Medical School
 The Japanese Journal of Physiology 15, pp.243-252, 1965 Department of Physiology, Okayama University Medical School BAYLISS and STARLING 1) and others 6, 7, 9, 12, 14, 15) have reported that the stimulation
The Japanese Journal of Physiology 15, pp.243-252, 1965 Department of Physiology, Okayama University Medical School BAYLISS and STARLING 1) and others 6, 7, 9, 12, 14, 15) have reported that the stimulation
Autonomic Division of NS
 Autonomic Division of NS Compare and contrast the structures of the sympathetic and the parasympathetic divisions, including functions and neurotransmitters. Show the levels of integration in the ANS,
Autonomic Division of NS Compare and contrast the structures of the sympathetic and the parasympathetic divisions, including functions and neurotransmitters. Show the levels of integration in the ANS,
Autonomic Nervous System
 Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Autonomic Nervous System Autonomic nervous system organization Sympathetic Nervous System division of the autonomic nervous system that arouses the body, mobilizing its energy in stressful situations
Chapter 15: The Autonomic Nervous System. Copyright 2009, John Wiley & Sons, Inc.
 Chapter 15: The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems Comparison of Somatic and Autonomic Nervous Systems Anatomy of Autonomic Motor Pathways Preganglionic neuron
Chapter 15: The Autonomic Nervous System Comparison of Somatic and Autonomic Nervous Systems Comparison of Somatic and Autonomic Nervous Systems Anatomy of Autonomic Motor Pathways Preganglionic neuron
Biology 218 Human Anatomy
 Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
Chapter 20 Adapted form Tortora 10 th ed. LECTURE OUTLINE A. Introduction (p. 632) 1. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. 2.
suggesting that the release of noradrenaline from sympathetic fibres was dependent on the concentration of Ca2+ outside the fibre.
 214 J. Phy8iol. (1965), 181, pp. 214-223 With 4 text-figurem Printed in Great Britain THE RELEASE OF NORADRENALINE FROM SYMPATHETIC FIBRES IN RELATION TO CALCIUM CONCENTRATION BY J. H. BURN AND W. R. GIBBONS
214 J. Phy8iol. (1965), 181, pp. 214-223 With 4 text-figurem Printed in Great Britain THE RELEASE OF NORADRENALINE FROM SYMPATHETIC FIBRES IN RELATION TO CALCIUM CONCENTRATION BY J. H. BURN AND W. R. GIBBONS
discharge rate as intravesical pressure was raised. Some cells received inputs from only (Received 28 January 1981)
 J. Physiol. (1982), 322, pp. 21-34 21 With 5 text-figures Printed in Great Britain TWO GROUP OF SPINAL INTERNEURONES THAT RESPOND TO STIMULATION OF THE ABDOMINAL VISCERA OF THE CAT BY S. B. McMAHON AND
J. Physiol. (1982), 322, pp. 21-34 21 With 5 text-figures Printed in Great Britain TWO GROUP OF SPINAL INTERNEURONES THAT RESPOND TO STIMULATION OF THE ABDOMINAL VISCERA OF THE CAT BY S. B. McMAHON AND
ELECTRICAL RESPONSES OF THE CAT NICTITATING MEMBRANE
 Brit. J. Pharmacol. (1964), 22, 558-576. THE EFFECTS OF PHYSOSTIGMINE ON THE MECHANICAL AND ELECTRICAL RESPONSES OF THE CAT NICTITATING MEMBRANE BY W. C. BOWMAN, B. A. CALLINGHAM AND A. W. CUTHBERT* From
Brit. J. Pharmacol. (1964), 22, 558-576. THE EFFECTS OF PHYSOSTIGMINE ON THE MECHANICAL AND ELECTRICAL RESPONSES OF THE CAT NICTITATING MEMBRANE BY W. C. BOWMAN, B. A. CALLINGHAM AND A. W. CUTHBERT* From
tetrodotoxin. jejunum which inhibited the myogenic spontaneous activity blocked did not reduce the output of acetylcholine.
 J. Phy8iol. (1967), 189, pp. 317-327 317 With 4 text-figure8 Printed in Great Britain INHIBITION OF GASTROINTESTINAL MOVEMENT BY SYMPATHETIC NERVE STIMULATION: THE SITE OF ACTION BY M. D. GERSHON From
J. Phy8iol. (1967), 189, pp. 317-327 317 With 4 text-figure8 Printed in Great Britain INHIBITION OF GASTROINTESTINAL MOVEMENT BY SYMPATHETIC NERVE STIMULATION: THE SITE OF ACTION BY M. D. GERSHON From
SUPERSENSITIVITY OF THE SUBMAXILLARY GLAND FOLLOWING EXCLUSION OF THE POSTGANGLIONIC PARASYMPATHETIC NEURONE
 Brit. J. Pharmacol. (1960), 15, 356. SUPERSENSITIVITY OF THE SUBMAXILLARY GLAND FOLLOWING EXCLUSION OF THE POSTGANGLIONIC PARASYMPATHETIC NEURONE BY N. EMMELIN From the Institute of Physiology, University
Brit. J. Pharmacol. (1960), 15, 356. SUPERSENSITIVITY OF THE SUBMAXILLARY GLAND FOLLOWING EXCLUSION OF THE POSTGANGLIONIC PARASYMPATHETIC NEURONE BY N. EMMELIN From the Institute of Physiology, University
(Received 7 December 1982)
 J. Physiol. (1983), 344, pp. 293-304 293 With 6 text-figures Printed in Great Britain PATTERNS OF INNERVATION OF NEURONES IN THE INFERIOR MESENTERIC GANGLION OF THE CAT BY Y. JULR*, J. KRIERt AND J. H.
J. Physiol. (1983), 344, pp. 293-304 293 With 6 text-figures Printed in Great Britain PATTERNS OF INNERVATION OF NEURONES IN THE INFERIOR MESENTERIC GANGLION OF THE CAT BY Y. JULR*, J. KRIERt AND J. H.
Dopamine as a Synaptic Transmitter and Modulator in Sympathetic Ganglia: A Different Mode of Synaptic Action*
 Proceedings of the National Academy of Sciences Vol. 67, No. 2, pp. 667-673, October 1970 Dopamine as a Synaptic Transmitter and Modulator in Sympathetic Ganglia: A Different Mode of Synaptic Action* Benjamin
Proceedings of the National Academy of Sciences Vol. 67, No. 2, pp. 667-673, October 1970 Dopamine as a Synaptic Transmitter and Modulator in Sympathetic Ganglia: A Different Mode of Synaptic Action* Benjamin
Chapter 16. APR Enhanced Lecture Slides
 Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 16 APR Enhanced Lecture Slides See separate PowerPoint slides for all figures and tables pre-inserted into PowerPoint without notes and animations. Copyright The McGraw-Hill Companies, Inc. Permission
INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE
 Br. J. Pharmac. Chemother. (1966), 28, 207-211. INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE BY J. AMARO, P. S. GUTH AND L. WANDERLINDER From the Department of Pharmacology,
Br. J. Pharmac. Chemother. (1966), 28, 207-211. INHIBITION OF AUDITORY NERVE ACTION POTENTIALS BY ACETYLCHOLINE AND PHYSOSTIGMINE BY J. AMARO, P. S. GUTH AND L. WANDERLINDER From the Department of Pharmacology,
PHARMACOLOGICAL STUDY OF THE ANOCOCCYGEUS MUSCLE OF
 Br. J. Pharmac. (198). 71, 35-4 PHARMACOLOGICAL STUDY OF TH ANOCOCCYGUS MUSCL OF TH DOG A.R. DHPOUR, M.A. KHOYI, H. KOUTCHKI & M.R. ZARRINDAST Department of Pharmacology, Faculty of Medicine, University
Br. J. Pharmac. (198). 71, 35-4 PHARMACOLOGICAL STUDY OF TH ANOCOCCYGUS MUSCL OF TH DOG A.R. DHPOUR, M.A. KHOYI, H. KOUTCHKI & M.R. ZARRINDAST Department of Pharmacology, Faculty of Medicine, University
DEPOLARIZATION OF NORMAL AND PREGANGLIONICALLY DENERVATED SUPERIOR CERVICAL GANGLIA BY STIMULANT DRUGS
 Brit. J. Pharmacol. (1966), 26, 511-520. DEPOLARIZATION OF NORMAL AND PREGANGLIONICALLY DENERVATED SUPERIOR CERVICAL GANGLIA BY STIMULANT DRUGS BY D. A. BROWN From the Department of Pharmacology, Medical
Brit. J. Pharmacol. (1966), 26, 511-520. DEPOLARIZATION OF NORMAL AND PREGANGLIONICALLY DENERVATED SUPERIOR CERVICAL GANGLIA BY STIMULANT DRUGS BY D. A. BROWN From the Department of Pharmacology, Medical
Subsequently, Cunningham, Guttmann, Whitteridge & Wyndham (1953) remarked
 300 J. Physiol. (I957) I38, 300-306 EFFECT OF BLADDER DISTENSION ON ARTERIAL BLOOD PRESSURE AND RENAL CIRCULATION IN ACUTE SPINAL CATS BY S. R. MUKHERJEE* From the Department of Physiology, University
300 J. Physiol. (I957) I38, 300-306 EFFECT OF BLADDER DISTENSION ON ARTERIAL BLOOD PRESSURE AND RENAL CIRCULATION IN ACUTE SPINAL CATS BY S. R. MUKHERJEE* From the Department of Physiology, University
Cocaine, anticholinesterases and hexamethonium do not appear to
 J. Physiol. (1963), 167, pp. 505-514 505 With 8 text-figures Printed in Great Britain PHARMAOLOGIAL EXPERIMENTS ON THE RELEASE OF THE SYMPATHETI TRANSMITTER BY A. G. H. BLAKELEY,* G. L. BROWN AND. B. FERRY
J. Physiol. (1963), 167, pp. 505-514 505 With 8 text-figures Printed in Great Britain PHARMAOLOGIAL EXPERIMENTS ON THE RELEASE OF THE SYMPATHETI TRANSMITTER BY A. G. H. BLAKELEY,* G. L. BROWN AND. B. FERRY
susceptibility of either the axons in the dorsal and ventral roots, or the intramedullary
 213 J. Physiol. (31958) I40, 2I3-2I9 THE SITE OF ACTION OF PROCAINE ON THE ISOLATED SPINAL CORD OF THE FROG BY M. HARMEL AND J. L. MALCOLM From the Department of Physiology, State University of New York,
213 J. Physiol. (31958) I40, 2I3-2I9 THE SITE OF ACTION OF PROCAINE ON THE ISOLATED SPINAL CORD OF THE FROG BY M. HARMEL AND J. L. MALCOLM From the Department of Physiology, State University of New York,
CHAPTER 15 LECTURE OUTLINE
 CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
CHAPTER 15 LECTURE OUTLINE I. INTRODUCTION A. The autonomic nervous system (ANS) regulates the activity of smooth muscle, cardiac muscle, and certain glands. B. Operation of the ANS to maintain homeostasis,
Human Anatomy & Physiology
 PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 14 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
PowerPoint Lecture Slides prepared by Barbara Heard, Atlantic Cape Community College Ninth Edition Human Anatomy & Physiology C H A P T E R 14 Annie Leibovitz/Contact Press Images 2013 Pearson Education,
Autonomic Nervous System. Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry
 Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
Autonomic Nervous System Lanny Shulman, O.D., Ph.D. University of Houston College of Optometry Peripheral Nervous System A. Sensory Somatic Nervous System B. Autonomic Nervous System 1. Sympathetic Nervous
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD
 AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
AUTONOMIC NERVOUS SYSTEM PART I: SPINAL CORD How is the organization of the autonomic nervous system different from that of the somatic nervous system? Peripheral Nervous System Divisions Somatic Nervous
Introduction to Autonomic
 Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
Part 2 Autonomic Pharmacology 3 Introduction to Autonomic Pharmacology FUNCTIONS OF THE AUTONOMIC NERVOUS SYSTEM The autonomic nervous system (Figure 3 1) is composed of the sympathetic and parasympathetic
Actions of prostaglandin F20 on the splenic vascular and capsular smooth muscle in the dog
 Br. J. Pharmac. (1971), 41, 1-7 Actions of prostaglandin F20 on the splenic vascular and capsular smooth muscle in the dog B. N. DAVIES ADi P. G. WITHRINGTON Department of Physiology, Medical College of
Br. J. Pharmac. (1971), 41, 1-7 Actions of prostaglandin F20 on the splenic vascular and capsular smooth muscle in the dog B. N. DAVIES ADi P. G. WITHRINGTON Department of Physiology, Medical College of
Human Anatomy and Physiology - Problem Drill 15: The Autonomic Nervous System
 Human Anatomy and Physiology - Problem Drill 15: The Autonomic Nervous System Question No. 1 of 10 Which of the following statements is correct about the component of the autonomic nervous system identified
Human Anatomy and Physiology - Problem Drill 15: The Autonomic Nervous System Question No. 1 of 10 Which of the following statements is correct about the component of the autonomic nervous system identified
The Nervous System: Autonomic Nervous System
 17 The Nervous System: Autonomic Nervous System PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction The autonomic nervous system functions
17 The Nervous System: Autonomic Nervous System PowerPoint Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska Introduction The autonomic nervous system functions
J. Physiol. (I956) I33,
 232 J. Physiol. (I956) I33, 232-242 A STUDY OF THE EFFECT OF THE PATTERN OF ELECTRICAL STIMULATION OF THE AORTIC NERVE ON THE REFLEX DEPRESSOR RESPONSES By W. W. DOUGLAS, J. M. RITCHIE AND W. SCHAUMANN*
232 J. Physiol. (I956) I33, 232-242 A STUDY OF THE EFFECT OF THE PATTERN OF ELECTRICAL STIMULATION OF THE AORTIC NERVE ON THE REFLEX DEPRESSOR RESPONSES By W. W. DOUGLAS, J. M. RITCHIE AND W. SCHAUMANN*
MODIFICATIONS BY PROPRANOLOL OF THE RESPONSE OF ISOLATED RABBIT ATRIA TO ENDOGENOUS AND EXOGENOUS NORADRENALINE
 Br. J. Pharmac. Chemother. (1968), 32, 539-545. MODIFICATIONS BY PROPRANOLOL OF THE RESPONSE OF ISOLATED RABBIT ATRIA TO ENDOGENOUS AND EXOGENOUS NORADRENALINE BY K. SHIMAMOTO AND N. TODA From the Department
Br. J. Pharmac. Chemother. (1968), 32, 539-545. MODIFICATIONS BY PROPRANOLOL OF THE RESPONSE OF ISOLATED RABBIT ATRIA TO ENDOGENOUS AND EXOGENOUS NORADRENALINE BY K. SHIMAMOTO AND N. TODA From the Department
Technologies and architectures" Stimulator, electrodes, system flexibility, reliability, security, etc."
 March 2011 Introduction" Basic principle (Depolarization, hyper polarization, etc.." Stimulation types (Magnetic and electrical)" Main stimulation parameters (Current, voltage, etc )" Characteristics (Muscular
March 2011 Introduction" Basic principle (Depolarization, hyper polarization, etc.." Stimulation types (Magnetic and electrical)" Main stimulation parameters (Current, voltage, etc )" Characteristics (Muscular
Lujain Hamdan. Ayman Musleh & Yahya Salem. Mohammed khatatbeh
 12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
12 Lujain Hamdan Ayman Musleh & Yahya Salem Mohammed khatatbeh the last lecture, we have studied the differences between the two divisions of the ANS: sympathetic and parasympathetic pathways which work
ANATOMY & PHYSIOLOGY - CLUTCH CH THE AUTONOMIC NERVOUS SYSTEM.
 !! www.clutchprep.com ANATOMY & PHYSIOLOGY - CLUTCH CONCEPT: THE AUTONOMIC NERVOUS SYSTEM: DIVISIONS AND STRUCTURE The Autonomic Nervous System and its Divisions: Autonomic Nervous System (ANS) controls
!! www.clutchprep.com ANATOMY & PHYSIOLOGY - CLUTCH CONCEPT: THE AUTONOMIC NERVOUS SYSTEM: DIVISIONS AND STRUCTURE The Autonomic Nervous System and its Divisions: Autonomic Nervous System (ANS) controls
Integrated Cardiopulmonary Pharmacology Third Edition
 Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
Integrated Cardiopulmonary Pharmacology Third Edition Chapter 3 Pharmacology of the Autonomic Nervous System Multimedia Directory Slide 19 Slide 37 Slide 38 Slide 39 Slide 40 Slide 41 Slide 42 Slide 43
Chp. 16: AUTONOMIC N.S. (In Review: Peripheral N. S.)
 Chp. 16: AUTONOMIC N.S. (In Review: Peripheral N. S.) Peripheral nerves contain both motor and sensory neurons Among the motor neurons, some of these are somatic and innervate skeletal muscles while some
Chp. 16: AUTONOMIC N.S. (In Review: Peripheral N. S.) Peripheral nerves contain both motor and sensory neurons Among the motor neurons, some of these are somatic and innervate skeletal muscles while some
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons. Chad Smurthwaite & Jordan Shellmire
 Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
Chapter 45: Synapses Transmission of Nerve Impulses Between Neurons Chad Smurthwaite & Jordan Shellmire The Chemical Synapse The most common type of synapse used for signal transmission in the central
ACTIONS OF BRETYLIUM AND GUANETHIDINE ON THE UPTAKE AND RELEASE OF [3H]-NORADRENALINE
![ACTIONS OF BRETYLIUM AND GUANETHIDINE ON THE UPTAKE AND RELEASE OF [3H]-NORADRENALINE ACTIONS OF BRETYLIUM AND GUANETHIDINE ON THE UPTAKE AND RELEASE OF [3H]-NORADRENALINE](/thumbs/74/71109260.jpg) Brit. J. Pharmacol. (1962), 18, 161-166. ACTIONS OF BRETYLIUM AND GUANETHIDINE ON THE UPTAKE AND RELEASE OF [3H]-NORADRENALINE BY G. HERTTING,* J. AXELROD AND R. W. PATRICK From the Laboratory of Clinical
Brit. J. Pharmacol. (1962), 18, 161-166. ACTIONS OF BRETYLIUM AND GUANETHIDINE ON THE UPTAKE AND RELEASE OF [3H]-NORADRENALINE BY G. HERTTING,* J. AXELROD AND R. W. PATRICK From the Laboratory of Clinical
Chapter 14 The Autonomic Nervous System Chapter Outline
 Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
Chapter 14 The Autonomic Nervous System Chapter Outline Module 14.1 Overview of the Autonomic Nervous System (Figures 14.1 14.3) A. The autonomic nervous system (ANS) is the involuntary arm of the peripheral
The Nervous System. Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output
 The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
The Nervous System Nervous System Functions 1. gather sensory input 2. integration- process and interpret sensory input 3. cause motor output The Nervous System 2 Parts of the Nervous System 1. central
A CENTRAL NORADRENERGIC MECHANISM RESPONSIBLE FOR MODULATION OF THE ARTERIAL BARORECEPTOR REFLEX IN CATS
 www.kopfinstruments.com A CENTRAL NORADRENERGIC MECHANISM RESPONSIBLE FOR MODULATION OF THE ARTERIAL BARORECEPTOR REFLEX IN CATS V. S. EREMEEV, Ph.D. R. S. KHRUSTALEVA, Ph.D. V. A. TSYRLIN, Ph.D. Yu. I.
www.kopfinstruments.com A CENTRAL NORADRENERGIC MECHANISM RESPONSIBLE FOR MODULATION OF THE ARTERIAL BARORECEPTOR REFLEX IN CATS V. S. EREMEEV, Ph.D. R. S. KHRUSTALEVA, Ph.D. V. A. TSYRLIN, Ph.D. Yu. I.
I. Neural Control of Involuntary Effectors. Chapter 9. Autonomic Motor Nerves. Autonomic Neurons. Autonomic Ganglia. Autonomic Neurons 9/19/11
 Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
Chapter 9 I. Neural Control of Involuntary Effectors The Autonomic Nervous System Lecture PowerPoint Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Autonomic
bradykinin. sympathetic, splanchnic and hypogastric nerves after intra-arterial doses of
 Quarterly Journal of Experimental Physiology (1977) 62, 11-17 EFFECTS OF BRADYKININ MEDIATED BY AUTONOMIC EFFERENT NERVES. By K. FLOYD, VERITY E. HICK, JuTHIKA KOLEY and J. F. B. MORRISON. From the Department
Quarterly Journal of Experimental Physiology (1977) 62, 11-17 EFFECTS OF BRADYKININ MEDIATED BY AUTONOMIC EFFERENT NERVES. By K. FLOYD, VERITY E. HICK, JuTHIKA KOLEY and J. F. B. MORRISON. From the Department
Divisions of ANS. Divisions of ANS 2 Divisions dualing innervate most organs. Autonomic Nervous System (Chapter 9)
 Autonomic Nervous System (Chapter 9) Autonomic Nervous System (ANS) general properties anatomy Autonomic Effects on Target Organs Subs of Nervous System Central nervous system Brain Spinal cord Peripheral
Autonomic Nervous System (Chapter 9) Autonomic Nervous System (ANS) general properties anatomy Autonomic Effects on Target Organs Subs of Nervous System Central nervous system Brain Spinal cord Peripheral
6I2.368:6I developmental and physiological connection between the large bowel and. (From the Institute of Physiology, the University, Glasgow.
 422 6I2.368:6I2.898 THE NERVOUS CONTROL OF THE CAUDAL REGION OF THE LARGE BOWEL IN THE CAT. BY R. C. GARRY. (From the Institute of Physiology, the University, Glasgow.) To find the influence of a nervous
422 6I2.368:6I2.898 THE NERVOUS CONTROL OF THE CAUDAL REGION OF THE LARGE BOWEL IN THE CAT. BY R. C. GARRY. (From the Institute of Physiology, the University, Glasgow.) To find the influence of a nervous
Drugs Affecting The Autonomic Nervous System(ANS)
 Drugs Affecting The Autonomic Nervous System(ANS) ANS Pharmacology Lecture 1 Dr. Hiwa K. Saaed College of Pharmacy, University of Sulaimani 2018-2019 AUTOMATIC NERVOUS SYSTEM (ANS) The ANS is the major
Drugs Affecting The Autonomic Nervous System(ANS) ANS Pharmacology Lecture 1 Dr. Hiwa K. Saaed College of Pharmacy, University of Sulaimani 2018-2019 AUTOMATIC NERVOUS SYSTEM (ANS) The ANS is the major
The Autonomic Nervous
 Autonomic Nervous System The Autonomic Nervous Assess Prof. Fawzia Al-Rouq System Department of Physiology College of Medicine King Saud University LECTUR (1) Functional Anatomy & Physiology of Autonomic
Autonomic Nervous System The Autonomic Nervous Assess Prof. Fawzia Al-Rouq System Department of Physiology College of Medicine King Saud University LECTUR (1) Functional Anatomy & Physiology of Autonomic
skeletal muscle, it was concluded that the vasodilatation is brought about by
 289 J. Physiol. (I954) I23, 289-3 THE EFFECTS OF NICOTINE ON THE BLOOD VESSELS OF SKELETAL MUSCLE IN THE CAT. AN INVESTIGATION OF VASOMOTOR AXON REFLEXES BY S. M. HILTON From the Physiological Laboratory,
289 J. Physiol. (I954) I23, 289-3 THE EFFECTS OF NICOTINE ON THE BLOOD VESSELS OF SKELETAL MUSCLE IN THE CAT. AN INVESTIGATION OF VASOMOTOR AXON REFLEXES BY S. M. HILTON From the Physiological Laboratory,
Franklin, 1933; Waterman, 1933]; indeed, the only negative findings, [Waterman, 1933]. Inasmuch, then, as Donegan was misled with
![Franklin, 1933; Waterman, 1933]; indeed, the only negative findings, [Waterman, 1933]. Inasmuch, then, as Donegan was misled with Franklin, 1933; Waterman, 1933]; indeed, the only negative findings, [Waterman, 1933]. Inasmuch, then, as Donegan was misled with](/thumbs/91/106294335.jpg) 381 6I2.I34:6I2.893 THE CONSTRICTOR RESPONSE OF THE INFERIOR VENA CAVA TO STIMULATION OF THE SPLANCHNIC NERVE BY K. J. FRANKLIN AND A. D. McLACHLIN (From the University Department of Pharmacology, Oxford)
381 6I2.I34:6I2.893 THE CONSTRICTOR RESPONSE OF THE INFERIOR VENA CAVA TO STIMULATION OF THE SPLANCHNIC NERVE BY K. J. FRANKLIN AND A. D. McLACHLIN (From the University Department of Pharmacology, Oxford)
Autonomic Nervous System DR JAMILA EL MEDANY
 Autonomic Nervous System DR JAMILA EL MEDANY OBJECTIVES At the end of the lecture, students should be able to: Define the autonomic nervous system. Describe the structure of autonomic nervous system Trace
Autonomic Nervous System DR JAMILA EL MEDANY OBJECTIVES At the end of the lecture, students should be able to: Define the autonomic nervous system. Describe the structure of autonomic nervous system Trace
auriculo-temporal nerve. The secretory response to various cholinesterase
 Quarterly Journal of Experimental Phy8iology (1974) 59, 11-17 THE SECRETORY INNERVATION OF THE PAROTID GLAND OF THE CAT: AN UNEXPECTED COMPONENT. By J. EKSTROM and N. EMMELIN. From the Institute of Physiology,
Quarterly Journal of Experimental Phy8iology (1974) 59, 11-17 THE SECRETORY INNERVATION OF THE PAROTID GLAND OF THE CAT: AN UNEXPECTED COMPONENT. By J. EKSTROM and N. EMMELIN. From the Institute of Physiology,
Nervous Systems: Diversity & Functional Organization
 Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
Nervous Systems: Diversity & Functional Organization Diversity of Neural Signaling The diversity of neuron structure and function allows neurons to play many roles. 3 basic function of all neurons: Receive
A Cardiocardiac Sympathovagal Reflex in the Cat
 A Cardiocardiac Sympathovagal Reflex in the Cat By Peter J. Schwartz, Massimo Pagani, Federico Lombardi, Alberto Malliani, and Arthur M. Brown ABSTRACT The reflex changes in single cardiac vagal efferent
A Cardiocardiac Sympathovagal Reflex in the Cat By Peter J. Schwartz, Massimo Pagani, Federico Lombardi, Alberto Malliani, and Arthur M. Brown ABSTRACT The reflex changes in single cardiac vagal efferent
Composed by Natalia Leonidovna Svintsitskaya, Associate professor of the Chair of Human Anatomy, Candidate of Medicine
 Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
Theoretical background to the study of the autonomic nervous system. Sympathetic and parasympathetic divisions of the autonomic nervous system. Features of the structure, function Composed by Natalia Leonidovna
I. Autonomic Nervous System (ANS) A. Dual Innervation B. Autonomic Motor Pathway 1. Preganglionic Neuron a. Preganglionic Fibers (Axons) (1)
 I. Autonomic Nervous System (ANS) A. Dual Innervation B. Autonomic Motor Pathway 1. Preganglionic Neuron a. Preganglionic Fibers (Axons) (1) Acetylcholine - ACh 2. Ganglion (Ganglia) 3. Ganglionic Neuron
I. Autonomic Nervous System (ANS) A. Dual Innervation B. Autonomic Motor Pathway 1. Preganglionic Neuron a. Preganglionic Fibers (Axons) (1) Acetylcholine - ACh 2. Ganglion (Ganglia) 3. Ganglionic Neuron
The Autonomic Nervous System
 The Autonomic Nervous System Responsible for control of visceral effectors and visceral reflexes: smooth muscle, glands, the heart. e.g. blood pressure, cardiac output, plasma glucose The autonomic system
The Autonomic Nervous System Responsible for control of visceral effectors and visceral reflexes: smooth muscle, glands, the heart. e.g. blood pressure, cardiac output, plasma glucose The autonomic system
POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS
 J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
J. exp. Biol. (1980), 85, 343-347 343 With a figures Printed in Great Britain POSTSYNAPTIC INHIBITION OF CRAYFISH TONIC FLEXOR MOTOR NEURONES BY ESCAPE COMMANDS BY J. Y. KUWADA, G. HAGIWARA AND J. J. WINE
THE NATURE OF THE ATRIAL RECEPTORS RESPONSIBLE FOR A REFLEX INCREASE IN ACTIVITY IN EFFERENT CARDIAC SYMPATHETIC NERVES
 Quaterly Journal of Experimental Physiology (1982), 67, 143-149 Printed in Great Britain THE NATURE OF THE ATRIAL RECEPTORS RESPONSIBLE FOR A REFLEX INCREASE IN ACTIVITY IN EFFERENT CARDIAC SYMPATHETIC
Quaterly Journal of Experimental Physiology (1982), 67, 143-149 Printed in Great Britain THE NATURE OF THE ATRIAL RECEPTORS RESPONSIBLE FOR A REFLEX INCREASE IN ACTIVITY IN EFFERENT CARDIAC SYMPATHETIC
Ch 9. The Autonomic Nervous System
 Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
Ch 9 The Autonomic Nervous System SLOs Review the organization of the ANS Describe how neural regulation of smooth and cardiac muscles differs from that of skeletal muscles Describe the structure and innervation
Autonomic Nervous System Dr. Ali Ebneshahidi
 Autonomic Nervous System Dr. Ali Ebneshahidi Nervous System Divisions of the nervous system The human nervous system consists of the central nervous System (CNS) and the Peripheral Nervous System (PNS).
Autonomic Nervous System Dr. Ali Ebneshahidi Nervous System Divisions of the nervous system The human nervous system consists of the central nervous System (CNS) and the Peripheral Nervous System (PNS).
(Received 8 December 1966)
 J. Physiol. (1967), 189, pp. 545-550 545 With 2 text-figure8 Printed in Great Britain FUSIMOTOR STIMULATION AND THE DYNAMIC SENSITIVITY OF THE SECONDARY ENDING OF THE MUSCLE SPINDLE BY M. C. BROWN, I.
J. Physiol. (1967), 189, pp. 545-550 545 With 2 text-figure8 Printed in Great Britain FUSIMOTOR STIMULATION AND THE DYNAMIC SENSITIVITY OF THE SECONDARY ENDING OF THE MUSCLE SPINDLE BY M. C. BROWN, I.
(Received 16 September 1954)
 603 J. Physiol. (I955) 127, 603-66 THE TEMPORAL COURSE OF THE EFFECTS OF POST- GANGLIONIC AXOTOMY ON THE INFERIOR MESENTERIC GANGLION OF THE CAT BY G. H. ACHESON AND J. REMOLINA From the Department of
603 J. Physiol. (I955) 127, 603-66 THE TEMPORAL COURSE OF THE EFFECTS OF POST- GANGLIONIC AXOTOMY ON THE INFERIOR MESENTERIC GANGLION OF THE CAT BY G. H. ACHESON AND J. REMOLINA From the Department of
Drugs Affecting the Autonomic Nervous System-1. Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia
 Drugs Affecting the Autonomic Nervous System-1 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia The autonomic nervous system, along with the endocrine system,
Drugs Affecting the Autonomic Nervous System-1 Assistant Prof. Dr. Najlaa Saadi PhD Pharmacology Faculty of Pharmacy University of Philadelphia The autonomic nervous system, along with the endocrine system,
AUTONOMIC NERVOUS SYSTEM (ANS):
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical 1 st year students, 2017/2018. ++++++++++++++++++++++++++++++++++++++++++++++++++ Textbook of Medical Physiology,
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical 1 st year students, 2017/2018. ++++++++++++++++++++++++++++++++++++++++++++++++++ Textbook of Medical Physiology,
The Autonomic Nervous System Outline of class lecture for Physiology
 The Autonomic Nervous System Outline of class lecture for Physiology 1 After studying the endocrine system you should be able to: 1. Describe the organization of the nervous system. 2. Compare and contrast
The Autonomic Nervous System Outline of class lecture for Physiology 1 After studying the endocrine system you should be able to: 1. Describe the organization of the nervous system. 2. Compare and contrast
2.4 Autonomic Nervous System
 2.4 Autonomic Nervous System The ANS regulates visceral activities normally outside the realm of consciousness and voluntary control: Circulation. Digestion. Sweating. Pupillary size. The ANS consists
2.4 Autonomic Nervous System The ANS regulates visceral activities normally outside the realm of consciousness and voluntary control: Circulation. Digestion. Sweating. Pupillary size. The ANS consists
The Nervous System: Autonomic Nervous System Pearson Education, Inc.
 17 The Nervous System: Autonomic Nervous System Introduction The autonomic nervous system: Functions outside of our conscious awareness Makes routine adjustments in our body s systems The autonomic nervous
17 The Nervous System: Autonomic Nervous System Introduction The autonomic nervous system: Functions outside of our conscious awareness Makes routine adjustments in our body s systems The autonomic nervous
Adrenergic fibres in the human intestine
 Gut, 1968, 9, 678-682 Adrenergic fibres in the human intestine L. CAPURSO,1 C. A. FRIEDMANN, AND A. G. PARKS From the Research Department, St Mark's Hospital, London, and the London Hospital, Whitechapel,
Gut, 1968, 9, 678-682 Adrenergic fibres in the human intestine L. CAPURSO,1 C. A. FRIEDMANN, AND A. G. PARKS From the Research Department, St Mark's Hospital, London, and the London Hospital, Whitechapel,
Neuropsychiatry Block
 Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
Neuropsychiatry Block Physiology of the Autonomic Nervous System By Laiche Djouhri, PhD Dept. of Physiology Email: ldjouhri@ksu.edu.sa Ext:71044 References The Autonomic Nervous System and the Adrenal
Synaptic Transmission
 Synaptic Transmission Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Synaptic transmission involves the release
Synaptic Transmission Graphics are used with permission of: Pearson Education Inc., publishing as Benjamin Cummings (http://www.aw-bc.com) Page 1. Introduction Synaptic transmission involves the release
EFFECT OF ANTIMUSCARINIC AGENTS ON THE CONTRACTILE
 Br. J. Pharmac. (1981), 73,829-835 EFFECT OF ANTIMUSCARINIC AGENTS ON THE CONTRACTILE RESPONSES TO CHOLINOMIMETICS IN THE RAT ANOCOCCYGEUS MUSCLE SHEILA A. DOGGRELL Department of Pharmacology & Clinical
Br. J. Pharmac. (1981), 73,829-835 EFFECT OF ANTIMUSCARINIC AGENTS ON THE CONTRACTILE RESPONSES TO CHOLINOMIMETICS IN THE RAT ANOCOCCYGEUS MUSCLE SHEILA A. DOGGRELL Department of Pharmacology & Clinical
General organization of central and peripheral components of the nervous system
 General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
General organization of central and peripheral components of the nervous system Today we are focusing on the ANS Part of ANS?? Life depends on the innervation of the viscera... all the rest is biological
REVERSAL OF NICOTINE ACTION ON THE INTESTINE BY ATROPINE
 Brit. J. Pharmacol. (1951), 6, 31 1. REVERSAL OF NICOTINE ACTION ON THE INTESTINE BY ATROPINE BY N. AMBACHE AND J. EDWARDS From the Medical Research Council, Ophthalmological Research Unit, Institute Ophthalmology,
Brit. J. Pharmacol. (1951), 6, 31 1. REVERSAL OF NICOTINE ACTION ON THE INTESTINE BY ATROPINE BY N. AMBACHE AND J. EDWARDS From the Medical Research Council, Ophthalmological Research Unit, Institute Ophthalmology,
J. Physiol. (I957) I35, (Received 20 July 1956) The interpretation ofthe experimental results ofthe preceding paper (Matthews
 263 J. Physiol. (I957) I35, 263-269 THE RELATIVE SENSITIVITY OF MUSCLE NERVE FIBRES TO PROCAINE BY PETER B. C. MATTHEWS AND GEOFFREY RUSHWORTH From the Laboratory of Physiology, University of Oxford (Received
263 J. Physiol. (I957) I35, 263-269 THE RELATIVE SENSITIVITY OF MUSCLE NERVE FIBRES TO PROCAINE BY PETER B. C. MATTHEWS AND GEOFFREY RUSHWORTH From the Laboratory of Physiology, University of Oxford (Received
T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland
 AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
AUTONOMOUS NEURAL REGULATION T. Laitinen Departments of Physiology and Clinical Physiology, University of Kuopio and Kuopio University Hospital, Kuopio, Finland Keywords: Autonomic nervous system, sympathetic
Ganglionic Blockers. Ganglion- blocking agents competitively block the action of
 Ganglionic Blockers Ganglion- blocking agents competitively block the action of acetylcholine and similar agonists at nicotinic (Nn) receptors of both parasympathetic and sympathetic autonomic ganglia.
Ganglionic Blockers Ganglion- blocking agents competitively block the action of acetylcholine and similar agonists at nicotinic (Nn) receptors of both parasympathetic and sympathetic autonomic ganglia.
Relation across the Receptor.afferent in a Tonic Electroreceptor of Marine Catfish
 No. 6] Proc. Japan Acad., 51 (1975) 485 102. Input. output Synapses Relation across the Receptor.afferent in a Tonic Electroreceptor of Marine Catfish By Shun-ichi UMEKITA, Yoshiko SUGAWARA, and Shosaku
No. 6] Proc. Japan Acad., 51 (1975) 485 102. Input. output Synapses Relation across the Receptor.afferent in a Tonic Electroreceptor of Marine Catfish By Shun-ichi UMEKITA, Yoshiko SUGAWARA, and Shosaku
Sympathetic Nervous System
 Sympathetic Nervous System Lecture Objectives Review the subdivisions of the nervous system. Review the general arrangement and compare the sympathetic and parasympathetic parts. Describe the following
Sympathetic Nervous System Lecture Objectives Review the subdivisions of the nervous system. Review the general arrangement and compare the sympathetic and parasympathetic parts. Describe the following
Chronotropic and Inotropic Effects of 3 Kinds of Alpha-Adrenergic Blockers on the Isolated Dog Atria
 Chronotropic and Inotropic Effects of 3 Kinds of Alpha-Adrenergic Blockers on the Isolated Dog Atria Shigetoshi CHIBA, M.D., Yasuyuki FURUKAWA, M.D., and Hidehiko WATANABE, M.D. SUMMARY Using the isolated
Chronotropic and Inotropic Effects of 3 Kinds of Alpha-Adrenergic Blockers on the Isolated Dog Atria Shigetoshi CHIBA, M.D., Yasuyuki FURUKAWA, M.D., and Hidehiko WATANABE, M.D. SUMMARY Using the isolated
Portions from Chapter 6 CHAPTER 7. The Nervous System: Neurons and Synapses. Chapter 7 Outline. and Supporting Cells
 CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
CHAPTER 7 The Nervous System: Neurons and Synapses Chapter 7 Outline Neurons and Supporting Cells Activity in Axons The Synapse Acetylcholine as a Neurotransmitter Monoamines as Neurotransmitters Other
BIOH111. o Cell Module o Tissue Module o Skeletal system o Muscle system o Nervous system o Endocrine system o Integumentary system
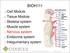 BIOH111 o Cell Module o Tissue Module o Skeletal system o Muscle system o Nervous system o Endocrine system o Integumentary system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
BIOH111 o Cell Module o Tissue Module o Skeletal system o Muscle system o Nervous system o Endocrine system o Integumentary system Endeavour College of Natural Health endeavour.edu.au 1 Textbook and required/recommended
EE 791 Lecture 2 Jan 19, 2015
 EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
EE 791 Lecture 2 Jan 19, 2015 Action Potential Conduction And Neural Organization EE 791-Lecture 2 1 Core-conductor model: In the core-conductor model we approximate an axon or a segment of a dendrite
STUDIES ON SYMPATHETIC MECHANISMS IN ISOLATED INTESTINAL AND VAS DEFERENS PREPARATIONS
 Brit. J. Pharmacol. (1962), 19, 85-98. STUDIES ON SYMPATHETIC MECHANISMS IN ISOLATED INTESTINAL AND VAS DEFERENS PREPARATIONS BY G. A. BENTLEY* From the Nicholas Institute for Medical and Veterinary Research,
Brit. J. Pharmacol. (1962), 19, 85-98. STUDIES ON SYMPATHETIC MECHANISMS IN ISOLATED INTESTINAL AND VAS DEFERENS PREPARATIONS BY G. A. BENTLEY* From the Nicholas Institute for Medical and Veterinary Research,
AND PARTIALLY INNERVATED BLADDER
 Br. J. Pharmac. (1981), 73, 837-842 ATROPINE AND MICTURITION RESPONSES BY RATS WITH INTACT AND PARTIALLY INNERVATED BLADDER F.G. CARPENTER Department of Pharmacology, U.S.A. University of Alabama in Birmingham
Br. J. Pharmac. (1981), 73, 837-842 ATROPINE AND MICTURITION RESPONSES BY RATS WITH INTACT AND PARTIALLY INNERVATED BLADDER F.G. CARPENTER Department of Pharmacology, U.S.A. University of Alabama in Birmingham
McSwiney and Wadge [1930] described the effects on the stomach of
![McSwiney and Wadge [1930] described the effects on the stomach of McSwiney and Wadge [1930] described the effects on the stomach of](/thumbs/83/87976616.jpg) 6I2.328:6I2.898 THE SYMPATHETIC INNERVATION OF THE STOMACH. II. The effect of stimulation of the peri-arterial nerves on the stomach and small intestine. BY B. A. McSWINEY AND J. M. ROBSON. (Department
6I2.328:6I2.898 THE SYMPATHETIC INNERVATION OF THE STOMACH. II. The effect of stimulation of the peri-arterial nerves on the stomach and small intestine. BY B. A. McSWINEY AND J. M. ROBSON. (Department
Chapter 15 Lecture Outline
 Chapter 15 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright McGraw-Hill Education. Permission required for reproduction or
Chapter 15 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright McGraw-Hill Education. Permission required for reproduction or
EFFECT OF DENERVATION AND OF COCAINE ON THE ACTION OF SYMPATHOMIMETIC AMINES
 Brit. J. Pharmacol. (1960), 15, 328. EFFECT OF DENERVATION AND OF COCAINE ON THE ACTION OF SYMPATHOMIMETIC AMINES BY B. C. R. STROMBLAD From the Institute of Physiology, Lund, Sweden (RECEIVED FEBRUARY
Brit. J. Pharmacol. (1960), 15, 328. EFFECT OF DENERVATION AND OF COCAINE ON THE ACTION OF SYMPATHOMIMETIC AMINES BY B. C. R. STROMBLAD From the Institute of Physiology, Lund, Sweden (RECEIVED FEBRUARY
