Healing of Chronic Venous Ulcers Is Not Enhanced by the Addition of Topical Repifermin (KGF-2) to Standardized Care
|
|
|
- Louisa Green
- 5 years ago
- Views:
Transcription
1 Healing of Chronic Venous Ulcers Is Not Enhanced by the Addition of Topical Repifermin (KGF-2) to Standardized Care Martin C. Robson, MD a,b Jason Hanfnt, DPM c Warren Garner, MD d Jeffrey Jenson, DPM e Thomas Serena, MD f Wyatt G. Payne, MD a,b Anthony Sussman, MD g Adrian Barbul, MD h Markete Limova, MD i Robert Snyder, DPM j Daniel J. Odenheimer, PhD k Diane M. Cooper, PhD k,l KGF-2 Venous Ulcer Study Group m a The Institute for Tissue Regeneration, Repair, and Rehabilitation, Department of Veterans Affairs Medical Center, Bay Pines, Florida b Division of Plastic Surgery, Department of Surgery, University of South Florida, Tampa, Florida c Foot and Ankle Institute of South Florida, Miami, Florida d LAC-USC Medical Center, Los Angeles, California e Diabetic Foot and Wound Center, Denver, Colorado f Penn North Center for Advanced Wound Care, Warren, Pennsylvania g Memorial Health University Medical Center, Savannah, Georgia h Siani Hospital of Baltimore, Baltimore, Maryland i Sierra Medical Research, Fresno, California j Taramac, Florida k Human Genome Sciences, Rockville, Maryland l Johnson and Johnson Wound Management, Somerville, New Jersey m The remaining investigators are listed in the acknowledgement at the end of the article. KEY WORDS: Growth factor, venous ulcer, KGF-2, compression therapy ABSTRACT Background: It is estimated that 2.5 million people in the United States have chronic venous ulcers. Standard care for these ulcers includes compression, 302 debridement of necrotic tissue, treatment of edema, and control of infection. There has been a search for additional therapeutic compounds and/or devices to enhance ulcer healing beyond that provided by standard care. A previous phase 2a clinical trial suggested repifermin (KGF-2) might enhance ulcer healing. Vol. 4, No. 2, 2004 The Journal of Applied Research
2 Materials and Methods: A randomized, double-blinded, parallel group, multicenter clinical trial was conducted to evaluate the safety and efficacy of topical repifermin treatment, for up to 26 weeks, in the healing of chronic venous ulcers in 352 patients. Results: The percent of ulcers achieving 100% closure by 20 weeks was not significantly different in ulcers treated with 60 µg/cm 2 or 120 µg/cm 2 of repifermin compared with placebo treatment. Similarly, there was no difference in total healing among the 3 treatment groups at 26 weeks or at 16 weeks. Time to closure was also statistically identical for the 3 treatment groups. Safety analyses showed that topical repifermin is well-tolerated. Conclusion: Healing of chronic venous ulcers is not enhanced by the addition of topical repifermin (KGF-2) to standardized care. INTRODUCTION It is estimated that two and a half million people in the United States have chronic venous ulcers. 1,2 These ulcers result in two million lost work days at a cost of two to four billion dollars for treatment. 1,2 Only 50 to 60% of venous ulcers will heal within 6 months of treatment. 3,4 Even if complete healing of the venous ulcer occurs, recurrence is a major clinical problem. It has been reported that up to 70% of venous ulcers can recur after complete healing. 5,6 The cornerstone of standard care of chronic venous ulcers involves application of compression therapy. 1,7 Other treatment includes debridement, treatment of edema, and control of infection. A more specific therapeutic approach would be to promote the proliferation and migration of epithelial cells to restore the epithelium. 7 Repifermin (a truncated form of Keratinocyte growth factor-2 [KGF-2]) selectively promotes proliferation and migration of keratinocytes in culture. 7 Repifermin has shown, in both in vitro and in vivo experiments, a highly selective action on epithelial cells. 7 In addition to promoting re-epithelialization by a direct effect on keratinocytes causing them to proliferate and migrate, repifermin may also stimulate granulation tissue formation by a direct effect on fibroblasts. 8 After extensive in vitro and in vivo preclinical studies suggested that repifermin would be effective in accelerating closure of wounds healing largely by the process of epithelialization, a phase 2a multi-center, randomized, double-blinded, parallel group, placebo-controlled, venous ulcer clinical trial was performed to assess the safety and efficacy of two different dose levels of repifermin (20 µg/cm 2 and 60 µg/cm 2 ) compared to placebo, following twice weekly topical applications for a period of 12 weeks. 7,9,10 Repifermin was shown to be safe and well-tolerated in the clinical trial. Venous ulcer healing was evaluated by a number of parameters including complete wound closure, partial wound closure, and percent area healed. There was no difference in total ulcer healing in 12 weeks in the two repifermin-treated groups compared to the placebo. However, trends in favor of repifermin were observed for many of the parameters, including the percentage of subjects achieving partial wound closure and total area of the ulcer healed. 7 The difference between repifermin and placebo treatment was statistically significant for the 75% healed endpoint when the results from the 2 repifermin treatment groups were combined. 7 The treatment effect of repifermin appeared more marked for a subgroup of patients with initial ulcer areas 15cm 2 and wound ages of 18 months. In this subgroup, The Journal of Applied Research Vol. 4, No. 2,
3 statistically more ulcers treated with repifermin reached 75% closure and 90% closure than did placebo-treated ulcers. The phase 2a clinical trial results suggested that there was a dose response effect with more patients with 90% or 100% closure being treated with the 60 µg/cm 2 dose of repifermin than with the 20 µg/cm 2 dose. Because ulcers in that trial tended to achieve 75% or 90% closure more frequently than 100% closure, it was concluded that 12 weeks may not have been a sufficient treatment period. Therefore, it was decided to perform a phase 2b clinical trial using a larger dose of repifermin and applying it for a longer period in an attempt to enhance healing of chronic venous ulcers. MATERIALS AND METHODS The study was designed to assess the efficacy and safety of 2 different doselevels of repifermin (60 µg/cm 2 and 120 µg/cm 2 ) compared with placebo following twice-weekly topical application for up to 26 weeks. Topical therapy was in addition to conventional compression therapy and a standardized protocol of good ulcer care. It was conducted using a randomized, double-blinded, parallel group, multi-center design. The treatment period was preceded by up to a 48 day screening phase for evaluation of subject eligibility and standardized wound care. Follow-up visits at 1 and 4 weeks after the last dose of study agent were used to evaluate wound healing and drug safety. In addition, a follow-up visit 13 weeks following the last dose of study agent was used to assess the durability of response for subjects who were healed during the trial or at the early follow-up visit. All participants provided informed consent prior to the start of the protocol. Each institution s Human Investigation Committee gave approval to the protocol before any patients were enrolled. Application of Growth Factor A truncated form of KGF-2 was used in this study. It was expressed in E. coli and was purified by a series of chromatography and filtration procedures. The repifermin was prepared as a sterile lyophilized product. Upon reconstitution with 5.0 ml of bacteriostatic water for injection, each vial contained 1.0 or 2.0 mg/ml of repifermin (depending on the applied dose), 2% glycine, 0.5% sucrose, 10 mm sodium citrate, 20mM sodium chloride, 1 mm ethylenediamine tetra acetic acid disodium salt, 0.12% methyl paraben, and 0.12% propyl paraben, ph 6.2. The placebo was also prepared as a sterile lyophilized product. Upon reconstitution with 5.0 ml of bacteriostatic water for injection, each vial contained identical additives as the repifermin vials and a ph of 6.2. Once the repifermin or placebo was reconstituted, it was used within 5 days. Subjects received 1 of 2 dose levels of repifermin (60 µg/cm 2 or 120 µg/cm 2 ) or placebo-control administered by topical spray twice weekly (separated by 2 to 3 days) for 26 weeks or until the ulcer healed, whichever was shorter. The ulcer edge was traced to determine its area on the first visit of each week. The area of the ulcer was used to calculate the amount of the study agent sprays for the subsequent week. The agent was sprayed 2 to 3 inches from the ulcer surface. Five minutes after application of the study agent, the wound was covered with a petrolatum-impregnated gauze (ADAP- TIC, Johnson and Johnson Wound Management, Somerville, NJ) and then the leg encased in a multi-layer sustained, graduated compression bandage system (DYNAFLEX, Johnson and Johnson Wound Management, Somerville, NJ) which was only removed by study personnel during a study visit. Protocol Design The primary objective of the study was 304 Vol. 4, No. 2, 2004 The Journal of Applied Research
4 Table 1. Inclusion Criteria To be eligible for entry into this study, a subject must have met the following inclusion criteria: Have evidence of venous insufficiency documented by venous duplex scanning or impedance plethysmography. Have a venous ulcer with a wound size of 3 to 25 cm 2. Have been in prescribed compression for at least 7 consecutive days immediately prior to randomization, but not more than 28 consecutive or non-consecutive days. Have a wound that has been unhealed continuously for 3 to 36 months prior to treatment. Be available for twice-weekly visits. Be willing and able to comply with the protocol. to evaluate the efficacy of topically administered repifermin in achieving complete wound closure within 20 weeks of initiating treatment. Secondary efficacy analyses included: the percentage of subjects achieving complete wound closure within 26 weeks of initiating treatment, the percentage of subjects achieving complete ulcer healing within 16 weeks, the time required to achieve complete ulcer healing, and the percent change in ulcer size from baseline to the final on-therapy wound measurement. In addition to the efficacy objectives, safety of topically administered repifermin was evaluated. Based on the assumption that approximately 50% of subjects in the placebo group would show complete healing during the 20 week treatment period, a sample size of at least 113 subjects per group would provide a greater than 80% power to detect an absolute 20% difference in complete ulcer closure rates between placebo and either of the repifermin treatment groups at the 2.5% level of significance using a 2 sided test. There was a screening period of up to 48 days prior to study enrollment. At the first visit of the screening period, the ulcer was debrided if necrotic tissue was present, to reveal a clean ulcer bed. The ulcer was photographed, traced for planimetry, and a quantitative tissue biopsy performed for bacterial analysis. 11 Following the biopsy, the ulcer was dressed with ADAPTIC gauze and placed in DYNAFLEX compression dressing. If the biopsy was not supportive of an active wound infection (background colony count of < 10 6 CFU/gram of tissue and no beta-hemolytic streptococci 12 ), compression was continued until the period of compression totaled at least 7 days at which time the application of the study treatment was initiated. If the biopsy was supportive of an active wound infection (bacterial count 10 6 CFU/gram of tissue or any betahemolytic streptococci), the infection was treated with systemic or topical antimicrobials for at least 10 days with the P.I. allowed to select a compression device of his/her choice which would facilitate self-application of a topical treatment by the subject. Up to 3 tissue biopsies were allowed to achieve an ulcer in bacterial balance. 12 Once the ulcer was in bacterial balance and protocol compression had been used for at least 7 days, the ulcer was re-photographed and traced for the randomization planimetry. Prior to randomization, eligibility criteria had to be met. Inclusion criteria for the study are enumerated in Table 1. Exclusion criteria are listed in Table 2. Measures of Treatment Efficacy and Statistics Efficacy evaluations were made using wound areas measured by computer planimetry from wound tracings. Analyses of all end points were performed on the intention-to-treat popula- The Journal of Applied Research Vol. 4, No. 2,
5 Table 2. Exclusion Criteria To be eligible for entry into the study, the subject must not: Have participated in a clinical trial of an investigational agent within the last 30 days. Have been treated with repifermin (KGF-2). Have the designated ulcer below the malleolus, on the foot, or above the base of the knee. Have had the study ulcer treated with Regranex (PDGF-BB) within the last 30 days. Have had the study ulcer treated at any time with a skin substitute or an autologous growth factor. Have had a surgical procedure to treat venous or arterial disease within the last 90 days. Have evidence of significant arterial insufficiency (an ankle brachial index of < 0.8). Subjects with an ABI > 1.2 must have a toe brachial index of > 0.6 or a supine transcutaneous oxygen measurement (TcPO 2 ) > 30 mmhg. Have clinical evidence of active infection at the ulcer site. Have a granulation tissue colony count 10 6 /gram of tissue or beta-hemolytic streptococci at any level. Have evidence of active vasculitis, cellulitis, or collagen vascular disease. Have a history of malignant neoplasm within the last 5 years, except for adequately treated cancers of the skin or uterine cervix. Have significant acute or chronic diseases (ie, cardiovascular, pulmonary, gastrointestinal, hepatic, renal, neurological, or infectious diseases), which are not adequately controlled by medical treatment as determined by the investigator s judgment. Have diabetes mellitus with a hemoglobin A1c 8%. Have an active skin disease, such as psoriasis, which could impair the ability to assess the wound. Have an allergy to the dressings used in the study. Require treatment to the study ulcer with any topical agent other than normal saline within 7 days of the first repifermin / placebo treatment. Require treatment of the study ulcer with topical lidocaine for anesthesia prior to study ulcer debridement after the first repifermin / placebo treatment. Require concomitant use of pentoxifylline or clopidrogrel bisulfate during the study. Have undergone enzymatic debridement at any time during the screening period. Have a known history of allergies to Escherichia coli-derived or paraben-containing products. Have any requirement for the use of systemic steroids or immunosuppressive or cytotoxic compounds during the period of the study. Expected to undergo hyperbaric oxygen therapy at any time during treatment or through the 4 week follow-up visit. Be a pregnant female or nursing mother. All females with an intact uterus, regardless of age must have a negative serum pregnancy test result at screening and use contraception during the study. During the first 20 weeks of the study be expected to miss 3 or more consecutive visits or be expected to miss 2 consecutive visits more than once. tion, defined as anyone randomized. The study was designed to test the null hypothesis that the percentage of subjects with complete ulcer closure at 20 weeks would be no different for the placebo group compared with the 60 µg/cm 2 repifermin-treated group or for the placebo group compared with the 120 µg/cm 2 repifermin-treated group. All tests were performed at the two-sided 2.5% level. All analyses were performed using the SAS software version 8.0 or later and R statistical software version 1.51 or later. All baseline covariants were assessed to determine differences between the treatment groups. For categorical variables like gender and race, the likelihood ratio chi-squared test was used. For continuous variables like age, 306 Vol. 4, No. 2, 2004 The Journal of Applied Research
6 Table 3. Study Completion Status Treatment Groups Placebo 60µg/cm 2 120µg/cm 2 P Value (n=117) (n=123) (n=112) Subjects completed 102 (87.2%) 105 (85.4%) 92 (82.1%) Subjects discontinued due to: Subject request 2 (1.7%) 6 (4.9%) 4 (3.6%) Adverse Event 6 (5.1%) 6 (4.9%) 4 (3.6%) Protocol Violation 1 (0.09%) 2 (1.6%) 6 (5.4%) Lost to Follow-up 2 (1.7%) 3 (2.4%) 2 (1.8%) Death 2 (1.7%) 2 (1.8%) Other 2 (1.7%) 1 (0.8%) 2 (1.8%) height, weight, duration, and size of ulcer, ANOVA methods were used to assess differences between treatments. The time to complete wound closure was compared across treatment groups using Kaplan-Meier methods. The percent change from baseline in the area healed was compared across the treatment groups using the ANOVA methods. Safety Assessment Safety evaluation criteria included the frequency, severity, and duration of adverse events (AE s), including changes from baseline of laboratory test parameters. The likelihood ratio chisquared test was utilized to test if the occurrence of AE s was similar across treatment groups. Changes from baseline to endpoint in laboratory parameters were assessed using ANOVA methods. RESULTS A total of 352 patients were enrolled into the trial by 55 different investigators. Nine investigators enrolled at least 11 patients each and accounted for 174 of the subjects. The treatment assortment for enrolled patients was 117 for placebo treatment, 123 for repifermin 60 µg/cm 2, and 112 for repifermin 120 µg/cm 2. The number of subjects that completed the study was 102 placebo, 105 repifermin 60 µg/cm 2, and 92 repifermin 120 µg/cm 2 (Table 3). As shown in Table 4, treatment groups were comparable for race, mean age, duration of the ulcer and size of the ulcer at time of randomization / enrollment into the study (P > 0.05). The percentage of males enrolled into the 120 µg/cm 2 repifermin treatment group was significantly less than the percentage of males randomized into the other 2 treatment groups (P = ). Efficacy Outcomes The percent of ulcers achieving complete closure by 20 weeks in the 60 µg/cm 2 repifermin treatment group (58.5%) or the 120 µg/cm 2 repifermin treatment group (51.8%) was not statistically different from the placebo-treated ulcers (61.5%) (Figure 1). The P values were and respectively. By 26 weeks of therapy more ulcers reached 100% healing in each treatment group but the differences between groups were not significant (P = for 60 µg/cm 2 repifermin vs placebo; P = for 120 µg/cm 2 repifermin vs placebo) (Figure 2). As expected, at 16 weeks, the closure rates were less, but still no statistical differences among treatment groups were demonstrated (Figure 3). Kaplan-Meier analysis of time to closure also showed no significant differences between placebo-treated ulcers and 60 µg/cm 2 repifermin (P = ) or 120 µg/cm 2 The Journal of Applied Research Vol. 4, No. 2,
7 Figure 1. The primary efficacy analysis of percent of subjects attaining 100% ulcer healing by 20 weeks of treatment demonstrated no significant differences among the 3 treatment groups. Figure 2. Extending treatment to 26 weeks increased the number of ulcers in each treatment group that attained complete ulcer closure, but showed no statistical differences among the 3 treatment groups. Figure 3. Earlier evaluation of complete ulcer healing at 16 weeks did not show acceleration of healing by any specific treatment group and there were no significant differences among the 3 treatment groups. repifermin (P = ) (Figure 4). The mean percent change in ulcer size from time of enrollment to week 20 was not statistically different for the 3 treatment groups (P > 0.05). Repifermin was well tolerated in this study. There were no significant differences in the number or severity of adverse events (AE s) reported among the 3 treatment groups (Table 5). Figure 4. Time to ulcer closure was statistically identical between the repifermin-treated ulcers and the placebo-treated ulcers. DISCUSSION Venous ulcers have a prevalence of approximately one percent in the United States, with approximately 50% of the patients having an ulcer history of 10 years. 13,14 Despite standard therapy with compression dressings, only 50 to 60% of venous ulcers heal completely within 6 months and the recurrence rate is as high as 70%. 3-6,15,16 Direct and indirect costs associated with the treatment 308 Vol. 4, No. 2, 2004 The Journal of Applied Research
8 Table 4. Demographics Placebo 60µg/cm 2 120µg/cm 2 P Value (n=117) (n=123) (n=112) Sex Male 81 (69.2%) 76 (61.8%) 59 (52.7%) Race White 78 (66.7%) 74 (60.2%) 69 (61.6%) Black 25 (21.4%) 30 (24.4%) 25 (22.3%) Hispanic 12 (1.7%) 1 (0.8%) 2 (1.8%) Other 2 (1.7%) 1 (0.8%) 2 (1.8%) Age (Years) Mean ± SD 61.0 ± ± ± 15.6 Duration of Ulcer (Months) Mean ± SD 11.8 ± ± ± 5.3 Median Range (3.0, 35.0) (3.0, 36.0) (3.0, 36.0 Ulcer size at randomization (cm 2 ) Mean + SD 9.9 ± ± ± 5.3 Median Range (3.0, 24.3) (3.0, 25.0) (2.4, 22.9) of these ulcers are considerable. 17 Patients affected with venous ulcers experience a decrease in their overall health-related quality of life, including severe pain, impaired mobility, limited work capacity, and negative emotions. 15,18 Treatment of venous stasis ulcers varies from multiple surgical procedures to the use of various topical agents combined with external pressure support to the legs. 19,20 Standard care for these ulcers includes compression therapy, debridement of necrotic tissue, treatment of edema, and control of infection. 1,2,12 In the United States, the standard compression dressing is either the Unna boot, a zinc-oxide impregnated inelastic dressing, the Duke boot with an added elastic bandage, or the multiple-ply compression dressing. 5,21 The method chosen for this study was the DYNAFLEX multi-layer, sustained and graduated compression device. This is considered a Class 3 (most supportive) dressing. 1 A recent meta-analysis of the compression therapy literature reveals that high compression such as used in this study was more effective than low compression, but failed to identify any particular procedure or product as more effective than the other. 1,16 High bacterial burdens have been shown to interfere with healing. 12 Each of the various processes of the wound healing scheme has been reported to be inhibited by high levels of bacteria. 22 Controlling bacterial levels is particularly important when proteins such as growth factors are used for wound treatment. Growth factors have been demonstrated to be degraded by the common bacteria that populate chronic wounds. 23 It has been recommended that the bacterial burden be controlled during conduct of clinical trials involving growth factors. 23,25 Based on the results of the Phase 2a clinical trial previously performed evaluating repifermin (KGF-2) at 20 µg/cm 2 and 60 µg/cm 2 for 12 weeks, it was hypothesized that larger doses given for a longer time period would enhance healing of venous ulcers beyond that The Journal of Applied Research Vol. 4, No. 2,
9 Table 5. Summary of Adverse Events Placebo 60 µg/cm µg/cm 2 P Value (n=117) (n=123) (n=112) At Least One AE 89 (76.1%) 83 (67.5%) 79 (70.5%) At Least One Related AE 9 (7.7%) 13 (10.6%) 10 (8.9%) At Least One Serious AE 19 (16.2%) 13 (10.6%) 10 (8.9%) At Least One Severe AE 12 (10.3%) 9 (7.3%) 12 (10.7%) TABLE 6. Subgroup Analysis Placebo 60 µg/cm2 120 µg/cm2 P Value vs P Value vs (n=117) (n=123) (n=112) 60 µg/cm2 120µg/cm2 15 cm2 64 / / / (66.7%) (64.7%) (57.3%) 3 18 months 63 / / / (69.2%) (59.6%) (54.3%) 15 cm2 and 55 / / / months (72.4%) (65.8%) (60.0%) provided by standardized care. 7 The data from this study show that was not the case. The 61.5% and 66.7% incidence of complete healing for the placebo-treated patients at 20 to 26 weeks respectively was comparable to the better outcomes for compression therapy in the literature. 1,3,4,16 These healing percentages were not enhanced by the addition of topical repifermin therapy (Figures 1 and 2). Similarly, time to closure was not speeded by the addition of repifermin (Figure 4), nor was the decrease in ulcer size. In the previously reported clinical trial using repifermin for the treatment of chronic venous ulcers, a subgroup of patients with ulcers 15 cm 2 in area and a wound age 18 months appeared to have a better response to repifermin. 7 Therefore, a subgroup analysis was performed on the data from the present study. Table 6 demonstrates that there were no significant differences demonstrated on the smaller ulcers. The placebo treatment actually did better than repifermin in the ulcers of shorter duration and this difference reached significance when the placebo-treated ulcers were compared with the 120 µg/cm 2 repifermin-treated ulcers (P = ). 310 When one combined ulcers of smaller size ( 15 cm 2 ) and shorter duration (3 to 18 months), no significant differences were seen among treatments (Table 6). The safety profile was expected. In the previous study of 94 patients treated with topical repifermin, it proved to be well-tolerated and non-immunogenic. 7 In conclusion, healing of chronic venous ulcers is not enhanced by the addition of topical repifermin (KGF-2) to standardized care. ACKNOWLEDGEMENT The remainder of investigators comprising the KGF-2 Venous Ulcer Study Group include: A. Abdullah, MD, D. Armstrong, DPM, J. Boykin, MD, L. Britt, DPM, G. Chin, MD, R. Clark, MD, W. Conlan, MD, A. D Audiffret, MD, B. Elkof, MD, V. Falanga, MD, D. Fiverson, MD, S. Galt, MD, V. Giacalone, DPM, K Given, MD, M. Glickman, MD, D. Goldman, MD, I Gordan, MD, J. Harmon, MD, J. Hoballah, MD, R. Kirsner, MD, B. Kraemer, MD, A Landsman, MD, J. Lantis, MD, T. Littlejohn, MD, W. Marston, MD, R. Matheson, MD, M. McAnaw, MD, P. Mlynarezyk, MD, G. Moheta, MD, H. Moosa, MD, M. Moursi, MD, S. Muluk, Vol. 4, No. 2, 2004 The Journal of Applied Research
10 MD, J. Page, DPM, M. Passman, MD, W. Pearce, MD, F. Pesa, MD, L. Phillips, MD, T. Phillips, MD, M. Rohrer, MD, J. Serfetti, MD, H. Sharata, MD, PhD., J. Starvosky, DPM, D. Vayser, DPM, R. Wasserman, MD. REFERENCES 1. Ennis JE, Meneses P. Standard, appropriate, and advanced care and medical-legal considerations: Part two venous ulcerations. Wounds. 2003;15: Kerstein MD, Gahtan V. Outcomes of venous ulcer care: Results of a longitudinal study. Ostomy/Wound Manage. 1998;44: Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with allogenic cultured human skin equivalent. Arch Dermatol. 1998;134: Lyon RT, Veith FJ, Bolton L, et al. Clinical benchmark for healing of chronic venous ulcers. Am J Surg. 1998;176: Falanga V. Care of venous ulcers. Ostomy/Wound Manage. 1999;45(suppl. 1A):33S-43S. 6. Hansson, C, Anderson E, Swanback G. A follow-up study of leg and foot ulcer patients. Acta Derm Venereol. (Stokh) 1987;67: Robson MC, Phillips TJ, Falanga V, et al. Randomized trial of topically applied repifermin (recombinant human keratinocyte growth factor 2) to accelerate healing in venous ulcers. Wound Rep Regen. 2001;9: Tagashira H, Harada H, Katsumata T, et al. Cloning of mouse FGF-1 and up-regulation of its gene expression during wound healing. Gene. 1997;197: Soler PM, Wright TE, Smith PD, et al. In vivo characterization of Keratinocyte growth factor-2 as a potential wound healing agent. Wound Rep Regen. 1999;7: Robson MC, Wright TE, Ko F, et al. Genomics-based KGF-2 (repifermin) and its receptors function effectively in infected wounds. J Appl Res. 2003;3: Heggers JP. Variations on a theme. In: Heggers JP, Robson MC, eds. Quantitative bacteriology: its role in the armamentarium of the surgeon. Boca Raton, Fl. CRC Press; 1991: Robson MC. Wound infection: A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am. 1997;77: Callam MJ, Harper DR, Dale JJ, Ruckley CV. Chronic ulcer of the leg. Clinical history. Brit Med J. 1987;294: Angle N, Bergan JJ. Chronic venous ulcer. Brit Med J. 1997;314: Kirsner R, Falanga V, Fiverson D, et al. Clinical experiences with a human skin equivalent for the treatment of venous leg ulcers: process and outcomes. Wounds. 1999;11: Cullum N, Nelson EA, Fletcher AW, Sheldon TA. Compression for venous leg ulcers (Cochrane Review). In: Update Software Ltd., eds. The Cochrane Library. Issue 4. Chichester, UK: John Wiley and Sons; Olin JW, Beusterein KM, Childs MB, et al. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med. 1999;4: Phillips T, Stanton B, Provan A, Lew R. A study of the impact of leg ulcers on quality of life: financial, social, and psychologic implications. J Am Acad Dermatol. 1991;25: DePalma RG, Kowallek DL. Venous ulceration: A cross-over study from nonoperative to operative treatment. J Vasc Surg. 1996;24: Bishop JB, Phillips LG, Mustoe TA, et al. A prospective, randomized, evaluator-blinded trial of two potential wound healing agents for the treatment of venous stasis ulcers. J Vasc Surg. 1992;16: Burton CS. Treatment of leg ulcers. Dermatol Clin. 1993;11: Robson MC, Stenberg BD, Heggers JP. Wound healing alterations caused by infection. Clin Plast Surg. 1990;17: Robson MC, Mustoe TA, Hunt TK. The future of recombinant growth factors in wound healing. Am J Surg. 1998;176(suppl):80S-82S. 24. Payne WG, Wright TE, Ko F, et al. Bacterial degradation of growth factors. J Appl Res. 2003;3: FDA Wound Healing Clinical Focus Group. Guidance for Industry: Chronic cutaneous ulcer and burn wounds developing products for treatment. Wound Rep Regen. 2001;9: The Journal of Applied Research Vol. 4, No. 2,
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU)
 Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Adjunctive Therapies: The Use of Skin Substitutes and Growth Factors in Venous Leg Ulceration (VLU) Sami Khan, MD FACS Associate Professor of Surgery Division of Plastic and Reconstructive Surgery SUNY-Stony
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
ULCERS 1/12/ million diabetics in the US (2012) Reamputation Rate 26.7% at 1 year 48.3% at 3 years 60.7% at 5 years
 Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
Jay Christensen D.P.M Advanced Foot and Ankle of Wisconsin 2-4% of the population at any given time will have ulcers 0.06-0.20% of the total population Average age of patients 70 years increased as more
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
Four Layer Versus Actico Cohesive Short Stretch Bandage Background
 Four Layer Versus Actico Cohesive Short Stretch Bandage Background Cochrane Systematic Review 6/2/01 "Five small studies found no difference in healing between multi-layer high compression (4-layer bandage
Four Layer Versus Actico Cohesive Short Stretch Bandage Background Cochrane Systematic Review 6/2/01 "Five small studies found no difference in healing between multi-layer high compression (4-layer bandage
A comprehensive study on effect of collagen dressing in diabetic foot ulcer
 Original Research Article A comprehensive study on effect of collagen dressing in diabetic foot ulcer Sivakumar 1, S. Shanmugam 2* 1 Associate Professor, 2 Senior Assistant Professor Department of General
Original Research Article A comprehensive study on effect of collagen dressing in diabetic foot ulcer Sivakumar 1, S. Shanmugam 2* 1 Associate Professor, 2 Senior Assistant Professor Department of General
A comprehensive study on effect of recombinant human epidermal growth factor gel in diabetic foot ulcer
 Original Research Article A comprehensive study on effect of recombinant human epidermal growth factor gel in diabetic foot ulcer S Vijayalakshmi * Associate Professor, Department of General Surgery, Govt.
Original Research Article A comprehensive study on effect of recombinant human epidermal growth factor gel in diabetic foot ulcer S Vijayalakshmi * Associate Professor, Department of General Surgery, Govt.
Mean percent reduction in ulcer area from baseline at six weeks 62 % SANTYL Ointment + supportive care* + sharp debridement 1 (P<0.
 Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
Evaluating two common adjuncts to sharp debridement in the treatment of diabetic foot ulcers Mean percent reduction in ulcer area from baseline at six weeks 62 % 40 % SANTYL Ointment + supportive care*
Lower Extremity Wound Evaluation and Treatment
 Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
Lower Extremity Wound Evaluation and Treatment Boni-Jo Silbernagel, DPM Describe effective lower extremity wound evaluation and treatment. Discuss changes in theories of treatment in wound care and implications
P. P.2-7 P R A C T I C A L
 P R A T I A L O U R S E The use of hyaluronic acid derivatives in the treatment of long-term non-healing wounds Systematic review and meta-analysis of RSs (randomized controlled studies) J. Voigt and V.
P R A T I A L O U R S E The use of hyaluronic acid derivatives in the treatment of long-term non-healing wounds Systematic review and meta-analysis of RSs (randomized controlled studies) J. Voigt and V.
Interesting Case Series. Skin Grafting in Pyoderma Gangrenosum
 Interesting Case Series Skin Grafting in Pyoderma Gangrenosum Marco Romanelli, MD, PhD, Agata Janowska, MD, Teresa Oranges, MD, and Valentina Dini, MD, PhD Department of Dermatology, University of Pisa,
Interesting Case Series Skin Grafting in Pyoderma Gangrenosum Marco Romanelli, MD, PhD, Agata Janowska, MD, Teresa Oranges, MD, and Valentina Dini, MD, PhD Department of Dermatology, University of Pisa,
Accelerate wound healing of acute and chronic wounds in patients with Diabetes: Experience from Mexico using supplementary haemoglobin spray.
 Accelerate wound healing of acute and chronic wounds in patients with Diabetes: Experience from Mexico using supplementary haemoglobin spray. Obdulia Lopez Lopez 1, Fredrik Elg 2 Peter Engels 3, 1 Universidad
Accelerate wound healing of acute and chronic wounds in patients with Diabetes: Experience from Mexico using supplementary haemoglobin spray. Obdulia Lopez Lopez 1, Fredrik Elg 2 Peter Engels 3, 1 Universidad
The Immediate and Delayed Post-Debridement Effects on Tissue Bacterial Wound Counts of Hypochlorous Acid Versus Saline Irrigation in Chronic Wounds
 The Immediate and Delayed Post-Debridement Effects on Tissue Bacterial Wound Counts of Hypochlorous Acid Versus Saline Irrigation in Chronic Wounds JohnM.Hiebert,MD,FACN,FACS,andMartinC.Robson,MD,FACS,Hon.FRCS,
The Immediate and Delayed Post-Debridement Effects on Tissue Bacterial Wound Counts of Hypochlorous Acid Versus Saline Irrigation in Chronic Wounds JohnM.Hiebert,MD,FACN,FACS,andMartinC.Robson,MD,FACS,Hon.FRCS,
Diabetic Foot Ulcer Treatment and Prevention
 Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Diabetic Foot Ulcer Treatment and Prevention Alexander Reyzelman DPM, FACFAS Associate Professor California School of Podiatric Medicine at Samuel Merritt University Diabetic Foot Ulcers One of the most
Nanogen Aktiv. Naz Wahab MD, FAAFP, FAPWCA Nexderma
 Nanogen Aktiv Naz Wahab MD, FAAFP, FAPWCA Nexderma Patient BM 75 y.o female with a history of Type 2 Diabetes, HTN, Hypercholesterolemia, Renal insufficiency, Chronic back Pain, who had undergone a L3-L4
Nanogen Aktiv Naz Wahab MD, FAAFP, FAPWCA Nexderma Patient BM 75 y.o female with a history of Type 2 Diabetes, HTN, Hypercholesterolemia, Renal insufficiency, Chronic back Pain, who had undergone a L3-L4
VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device
 VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device Calf Muscle Pump Dysfunction Therapy Increases blood flow, accelerates wound healing, and improves CVD and PAD symptoms Tomorrow s Technology
VeinOPlus Vascular Peripheral Vascular & Wound Therapy Device Calf Muscle Pump Dysfunction Therapy Increases blood flow, accelerates wound healing, and improves CVD and PAD symptoms Tomorrow s Technology
Use of Non-Contact Low Frequency Ultrasound in Wound Care
 Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Use of Non-Contact Low Frequency Ultrasound in Wound Care BLAIRE CHANDLER SEPTEMBER 29, 2015 VCU DPT CLASS OF 2016 Objectives Patient case overview Examine clinical evidence Review intervention of interest
Case study: A targeted approach to healing complex wounds using the geko device.
 Case study: A targeted approach to healing complex wounds using the geko device. Authors: Mr Sameh Dimitri Consultant Vascular and Endovascular Surgeon MSc FRCS (Eng Edin) Nikki Pavey Physiotherapist at
Case study: A targeted approach to healing complex wounds using the geko device. Authors: Mr Sameh Dimitri Consultant Vascular and Endovascular Surgeon MSc FRCS (Eng Edin) Nikki Pavey Physiotherapist at
2008 American Medical Association and National Committee for Quality Assurance. All Rights Reserved. CPT Copyright 2007 American Medical Association
 Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
Chronic Wound Care ASPS #1: Use of wound surface culture technique in patients with chronic skin ulcers (overuse measure) This measure may be used as an Accountability measure Clinical Performance Measure
ORIGINAL ARTICLE. Wound Healing Trajectories as Predictors of Effectiveness of Therapeutic Agents
 ORIGINAL ARTICLE Wound Healing Trajectories as Predictors of Effectiveness of Therapeutic Agents Martin C. Robson, MD; Donald P. Hill, PharmD; Matthew E. Woodske, BS; David L. Steed, MD Background: One
ORIGINAL ARTICLE Wound Healing Trajectories as Predictors of Effectiveness of Therapeutic Agents Martin C. Robson, MD; Donald P. Hill, PharmD; Matthew E. Woodske, BS; David L. Steed, MD Background: One
o Venous edema o Stasis ulcers o Varicose veins (not including spider veins) o Lipodermatosclerosis
 Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Wound Care Equipment and Supply Benefits to Change for Texas Medicaid July 1, 2018 Effective for dates of service on or after July 1, 2018, wound care equipment and supply benefits will change for Texas
Advancing the science of wound bed preparation
 Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
Advancing the science of wound bed preparation How Drawtex wound dressing works LevaFiber Technology provides three different types of action. Mechanisms of Action Capillary Action Hydroconductive Action
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
Dressings do not heal wounds properly selected dressings enhance the body s ability to heal the wound. Progression Towards Healing
 Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
Dressings in Wound Care: They Do Matter John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Dressings do not heal wounds properly selected
HOW TO APPLY EFFECTIVE MULTILAYER COMPRESSION BANDAGING
 HOW TO APPLY EFFECTIVE MULTILAYER COMPRESSION BANDAGING Alison Hopkins is Clinical Nurse Specialist, East London Wound Healing Centre, Tower Hamlets Primary Care Trust Compression therapy is essential
HOW TO APPLY EFFECTIVE MULTILAYER COMPRESSION BANDAGING Alison Hopkins is Clinical Nurse Specialist, East London Wound Healing Centre, Tower Hamlets Primary Care Trust Compression therapy is essential
Static Magnet device, 4UlcerCare, prevents Leg Ulcer Recurrence: Potential Cost Savings in leg ulcer management
 Static Magnet device, 4UlcerCare, prevents Leg Ulcer Recurrence: Potential Cost Savings in leg ulcer management Abstract Nyjon K. Eccles MRCP PhD The Chiron Clinic, 104 Harley St, London W1G 7JD info@chironclinic.com
Static Magnet device, 4UlcerCare, prevents Leg Ulcer Recurrence: Potential Cost Savings in leg ulcer management Abstract Nyjon K. Eccles MRCP PhD The Chiron Clinic, 104 Harley St, London W1G 7JD info@chironclinic.com
Defining Outcomes for Clinical Wound Research in Older Adults February 21, 2014
 Defining Outcomes for Clinical Wound Research in Older Adults February 21, 214 Harold Brem, MD Professor of Surgery Stony Brook University School of Medicine Chief, Division of Wound Healing and Regenerative
Defining Outcomes for Clinical Wound Research in Older Adults February 21, 214 Harold Brem, MD Professor of Surgery Stony Brook University School of Medicine Chief, Division of Wound Healing and Regenerative
Description. Section: Medicine Effective Date: April 15, 2015 Subsection: Medicine Original Policy Date: September 13, 2012 Subject:
 Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
1/5. Introduction. Primary endpoint Time to reach readiness for closure by surgical intervention or left for closure by secondary intention
 1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
1/5 Introduction Materials and methods Animal studies show that intermittent NPWT has potential to increase the rate of granulation tissue formation compared with adjustable intermittent (AI) NPWT 1 However,
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers
 A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
A Pilot Study of Oxygen Therapy for Acute Leg Ulcers Background: The concept of increasing the oxygen concentration in healing wounds developed originally with hyperbaric oxygen therapy and from the fact
4-layer compression bandaging system (includes microbe binding wound contact layer) Latex-free, 4-layer compression bandaging system
 JOBST Comprifore JOBST Comprifore at a glance: provides effective levels of sustained graduated compression provides built in safety and ease of application Insures compliance and maximum healing for cost
JOBST Comprifore JOBST Comprifore at a glance: provides effective levels of sustained graduated compression provides built in safety and ease of application Insures compliance and maximum healing for cost
Hemostasis Inflammatory Phase Proliferative/rebuilding Phase Maturation Phase
 The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
The presenters are staff members of the CHI Health St. Elizabeth Burn and Wound Center. Many of the products discussed are used in our current practice but we have no conflict of interest to disclose.
Efficacy of Velcro Band Devices in Venous and. Mixed Arterio-Venous Patients
 Efficacy of Velcro Band Devices in Venous and Mixed Arterio-Venous Patients T. Noppeney Center for Vascular Diseases: Outpatient Dept. Obere Turnstrasse, Dept. for Vascular Surgery Martha-Maria Hospital
Efficacy of Velcro Band Devices in Venous and Mixed Arterio-Venous Patients T. Noppeney Center for Vascular Diseases: Outpatient Dept. Obere Turnstrasse, Dept. for Vascular Surgery Martha-Maria Hospital
Discussion Topics. Calcium Alginates. DME For the Diabetic Foot 1/25/2017. Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA
 DME For the Diabetic Foot Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA Editorial Advisory Board, WOUNDS Board of Directors, American Society of Podiatric Surgeons Board of Directors, American Professional
DME For the Diabetic Foot Jeffrey D. Lehrman, DPM, FASPS, FACFAS, MAPWCA Editorial Advisory Board, WOUNDS Board of Directors, American Society of Podiatric Surgeons Board of Directors, American Professional
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology
 A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
A New Approach To Diabetic Foot Ulcers Using Keratin Gel Technology Farheen Walid, BA, Shrunjay R. Patel, BSc, Stephanie Wu, DPM, MS Center for Lower Extremity Ambulatory Research (CLEAR), Scholl College
Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, and Arterial Ulcers: A Systematic Review
 Department of Veterans Affairs Health Services Research & Development Service Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, and Arterial Ulcers: A Systematic Review November 2012 Prepared
Department of Veterans Affairs Health Services Research & Development Service Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, and Arterial Ulcers: A Systematic Review November 2012 Prepared
The influence of wound geometry on the measurement of wound healing rates in clinical trials
 The influence of wound geometry on the measurement of wound healing rates in clinical trials Daniel R. Gorin, MD, Paul R. Cordts, MD, Wayne W. LaMorte, MD, PhD, MPH, and James O. Menzoian, MD, Boston,
The influence of wound geometry on the measurement of wound healing rates in clinical trials Daniel R. Gorin, MD, Paul R. Cordts, MD, Wayne W. LaMorte, MD, PhD, MPH, and James O. Menzoian, MD, Boston,
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds
 Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Use of an Acellular Regenerative Tissue Matrix Over Chronic Wounds D. Heath Stacey, MD Northwest Arkansas Center for Plastic Surgery, Fayetteville, Ark Correspondence: dheathstacey@gmail.com Keywords:
Transmetatarsal amputation in an at-risk diabetic population: a retrospective study
 The Journal of Diabetic Foot Complications Transmetatarsal amputation in an at-risk diabetic population: a retrospective study Authors: Merribeth Bruntz, DPM, MS* 1,2, Heather Young, MD 3,4, Robert W.
The Journal of Diabetic Foot Complications Transmetatarsal amputation in an at-risk diabetic population: a retrospective study Authors: Merribeth Bruntz, DPM, MS* 1,2, Heather Young, MD 3,4, Robert W.
First Coast Service Options (FCSO) Medicare Policy Primer
 First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
First Coast Service Options (FCSO) Medicare Policy Primer Medicare Jurisdiction (JN) Florida, Puerto Rico and U.S. Virgin Islands Application of Skin Substitute Grafts for the treatment of DFU and VLU
Short-stretch or Four-layer Compression Bandages: An Overview of the Literature
 STUDENT FEATURE Short-stretch or Four-layer Compression Bandages: An Overview of the Literature Gisele Castonguay, BSN, RN Venous ulcers are a common, costly occurrence. Treatment typically includes the
STUDENT FEATURE Short-stretch or Four-layer Compression Bandages: An Overview of the Literature Gisele Castonguay, BSN, RN Venous ulcers are a common, costly occurrence. Treatment typically includes the
Prepares wounds to allow natural healing
 ADVANCED CARE OF ACUTE AND CHRONIC WOUNDS Prepares wounds to allow natural healing 0373 Medical Device Hyaluronic Acid and Collagenase COMBINED ACTION IN WOUND REPAIR Hyaluronic acid and collagenase promote
ADVANCED CARE OF ACUTE AND CHRONIC WOUNDS Prepares wounds to allow natural healing 0373 Medical Device Hyaluronic Acid and Collagenase COMBINED ACTION IN WOUND REPAIR Hyaluronic acid and collagenase promote
JMSCR Vol 06 Issue 03 Page March 2018
 www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
www.jmscr.igmpublication.org Impact Factor (SJIF): 6.379 Index Copernicus Value: 71.58 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v6i3.55 Thesis Paper A Prospective Comparative
Cytokine manipulation of the wound
 Clin Plastic Surg 30 (2003) 57 65 Cytokine manipulation of the wound Martin C. Robson, MD* Department of Surgery, University of South Florida, Tampa, FL 33620, USA * 3619 S.E. Cambridge Drive, Stuart,
Clin Plastic Surg 30 (2003) 57 65 Cytokine manipulation of the wound Martin C. Robson, MD* Department of Surgery, University of South Florida, Tampa, FL 33620, USA * 3619 S.E. Cambridge Drive, Stuart,
WOUND CARE. By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare
 WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
WOUND CARE By Laural Aiesi, RN, BSN Alina Kisiel RN, BSN Summit ElderCare PRESSURE ULCER DIABETIC FOOT ULCER VENOUS ULCER ARTERIAL WOUND NEW OR WORSENING INCONTINENCE CHANGE IN MENTAL STATUS DECLINE IN
elastic stockings or inelastic bandages for ulcer treatment
 ICC - Compression session May 14, 2015 elastic stockings or inelastic bandages for ulcer treatment Giovanni Mosti; Lucca; Italy DISCLOSURE: NO CONFLICT OF INTEREST leg ulcers 31.619 patients venous 47.6
ICC - Compression session May 14, 2015 elastic stockings or inelastic bandages for ulcer treatment Giovanni Mosti; Lucca; Italy DISCLOSURE: NO CONFLICT OF INTEREST leg ulcers 31.619 patients venous 47.6
Granulox Update on evidence and science
 Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
Understanding and Managing
 Understanding and Managing MM s in Wound Bed Brock Liden DM, ABM, FAWCA Learning Objectives Review the four sequential phases of normal wound healing and recognize the BENEFICIAL effects of CONTROLLED
Understanding and Managing MM s in Wound Bed Brock Liden DM, ABM, FAWCA Learning Objectives Review the four sequential phases of normal wound healing and recognize the BENEFICIAL effects of CONTROLLED
The Management of Lower Limb Oedema. Catherine Hammond CNS/CNE 2018
 The Management of Lower Limb Oedema Catherine Hammond CNS/CNE 2018 Causes of oedema Venous stasis Lymphoedema Heart Failure Dependency Liver and kidney failure Medications Cellulitis Low protein Under
The Management of Lower Limb Oedema Catherine Hammond CNS/CNE 2018 Causes of oedema Venous stasis Lymphoedema Heart Failure Dependency Liver and kidney failure Medications Cellulitis Low protein Under
Chronic wounds need an ideal microenvironment. NEXODYN TM can support the physiological healing process.
 Chronic wounds need an ideal microenvironment. can support the physiological healing process. Wound microenvironment of chronic wounds represents a major therapeutic challenge¹ The most relevant factors
Chronic wounds need an ideal microenvironment. can support the physiological healing process. Wound microenvironment of chronic wounds represents a major therapeutic challenge¹ The most relevant factors
Your guide to wound debridement and assessment. Michelle Greenwood. Lorraine Grothier. Lead Nurse, Tissue Viability, Walsall Healthcare NHS Trust
 Your guide to wound debridement and assessment Michelle Greenwood Lead Nurse, Tissue Viability, Walsall Healthcare NHS Trust Lorraine Grothier Clinical Nurse Specialist, Tissue Viability, Central Essex
Your guide to wound debridement and assessment Michelle Greenwood Lead Nurse, Tissue Viability, Walsall Healthcare NHS Trust Lorraine Grothier Clinical Nurse Specialist, Tissue Viability, Central Essex
Setting The setting was a hospital. The economic study was carried out in the USA.
 A cost analysis of a living skin equivalent in the treatment of diabetic foot ulcers Steinberg J, Beusterien K, Plante K, Nordin J, Chaikoff E, Arcona S, Kim J, Belletti D A Record Status This is a critical
A cost analysis of a living skin equivalent in the treatment of diabetic foot ulcers Steinberg J, Beusterien K, Plante K, Nordin J, Chaikoff E, Arcona S, Kim J, Belletti D A Record Status This is a critical
Solving the Compliance Riddle with Compression Garments Jeffrey D. Lehrman, DPM, FASPS, MAPWCA, CPC
 Solving the Compliance Riddle with Compression Garments Jeffrey D. Lehrman, DPM, FASPS, MAPWCA, CPC Advisor, APMA Coding Committee Advisor, APMA MACRA Task Force Expert Panelist, Codingline Fellow, American
Solving the Compliance Riddle with Compression Garments Jeffrey D. Lehrman, DPM, FASPS, MAPWCA, CPC Advisor, APMA Coding Committee Advisor, APMA MACRA Task Force Expert Panelist, Codingline Fellow, American
Source of effectiveness data The effectiveness evidence was derived from a review of published studies.
 Cost and cost effectiveness of venous and pressure ulcer protocols of care Kerstein M D, Gemmen E, van Rijswijk L, Lyder C H, Phillips T, Xakellis G, Golden K, Harrington C Record Status This is a critical
Cost and cost effectiveness of venous and pressure ulcer protocols of care Kerstein M D, Gemmen E, van Rijswijk L, Lyder C H, Phillips T, Xakellis G, Golden K, Harrington C Record Status This is a critical
Hyperbaric Oxygen Utilization in Wound Care
 Hyperbaric Oxygen Utilization in Wound Care Robert Barnes, MD, CWS Hyperbaric Center Sacred Heart Medical Center Riverbend Springfield, Oregon No relevant disclosures Diabetes and lower extremity wounds
Hyperbaric Oxygen Utilization in Wound Care Robert Barnes, MD, CWS Hyperbaric Center Sacred Heart Medical Center Riverbend Springfield, Oregon No relevant disclosures Diabetes and lower extremity wounds
CASE REPORT Use of a Hydroconductive Dressing to Treat a Traumatic Avulsive Injury of the Face
 CASE REPORT Use of a Hydroconductive Dressing to Treat a Traumatic Avulsive Injury of the Face Colin Jerome Perumal, BDS, a and Martin Robson, MD b a Department of Maxillofacial and Oral Surgery, Medical
CASE REPORT Use of a Hydroconductive Dressing to Treat a Traumatic Avulsive Injury of the Face Colin Jerome Perumal, BDS, a and Martin Robson, MD b a Department of Maxillofacial and Oral Surgery, Medical
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude
 Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
Evaluating the use of a topical haemoglobin spray as adjunctive therapy in non-healing chronic wounds a pilot study Liezl Naude Abstract Wound Management Specialist Eloquent Health & Wellness Centre, Pretoria,
Venous. Arterial. Neuropathic (e.g. diabetic foot ulcer) Describe Wound Types & Stages of. Pressure Ulcers. Identify Phases of Healing & Wound Care
 A dressing the situation at hand Describe Wound Types & Stages of Pressure Ulcers Identify Phases of Healing & Wound Care Goals Clarify Referral Protocol Lacerations- The goal is nearest to complete approximation
A dressing the situation at hand Describe Wound Types & Stages of Pressure Ulcers Identify Phases of Healing & Wound Care Goals Clarify Referral Protocol Lacerations- The goal is nearest to complete approximation
Postoperative Surgical Site Infection after Incisional Hernia Repair: Link to Previous Surgical Site Infection? Zulfiqar Ali, AG Rehan
 Original Article Postoperative Surgical Site Infection after Incisional Hernia Repair: Link to Previous Surgical Site Infection? Zulfiqar Ali, AG Rehan ABSTRACT Objective: Aim of the study was to determine
Original Article Postoperative Surgical Site Infection after Incisional Hernia Repair: Link to Previous Surgical Site Infection? Zulfiqar Ali, AG Rehan ABSTRACT Objective: Aim of the study was to determine
Disclosures. Critical Limb Ischemia. Vascular Testing in the CLI Patient. Vascular Testing in Critical Limb Ischemia UCSF Vascular Symposium
 Disclosures Vascular Testing in the CLI Patient None 2015 UCSF Vascular Symposium Warren Gasper, MD Assistant Professor of Surgery UCSF Division of Vascular Surgery Critical Limb Ischemia Chronic Limb
Disclosures Vascular Testing in the CLI Patient None 2015 UCSF Vascular Symposium Warren Gasper, MD Assistant Professor of Surgery UCSF Division of Vascular Surgery Critical Limb Ischemia Chronic Limb
DO NOT DUPLICATE. A Comparison of Collagenase to Hydrogel Dressings in Maintenance Debridement and Wound Closure.
 Original research WOUNDS 2012;24(11):317 322 From 1 Connecticut Clinical Nursing Associates, LLC, Plymouth, CT; 2 Federal Hill Plastic Surgery, Bristol, CT Address correspondence to: Catherine T. Milne,
Original research WOUNDS 2012;24(11):317 322 From 1 Connecticut Clinical Nursing Associates, LLC, Plymouth, CT; 2 Federal Hill Plastic Surgery, Bristol, CT Address correspondence to: Catherine T. Milne,
A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers
 International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg
International Wound Journal ISSN 1742-4801 ORIGINAL ARTICLE A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg
Multidisciplinary approach to BTK Y. Gouëffic, MD, PhD
 Multidisciplinary approach to BTK Y. Gouëffic, MD, PhD Department of vascular surgery, University Hospital of Nantes, France Response to the increased demand of hospital care Population is aging Diabetes
Multidisciplinary approach to BTK Y. Gouëffic, MD, PhD Department of vascular surgery, University Hospital of Nantes, France Response to the increased demand of hospital care Population is aging Diabetes
Wound Management. E. Foy White-Chu, MD, CWSP
 Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
Wound Management E. Foy White-Chu, MD, CWSP E. Foy White-Chu, MD, CWSP Assistant Professor, OHSU Wound Medical Director, VAPORHCS List the Four Principles of Wound Bed Preparation Determine safe debridement
Cost-effectiveness of becaplermin for nonhealing neuropathic diabetic foot ulcers Sibbald R G, Torrance G, Hux M, Attard C, Milkovich N
 Cost-effectiveness of becaplermin for nonhealing neuropathic diabetic foot ulcers Sibbald R G, Torrance G, Hux M, Attard C, Milkovich N Record Status This is a critical abstract of an economic evaluation
Cost-effectiveness of becaplermin for nonhealing neuropathic diabetic foot ulcers Sibbald R G, Torrance G, Hux M, Attard C, Milkovich N Record Status This is a critical abstract of an economic evaluation
Integra. PriMatrix Dermal Repair Scaffold PATIENT INFORMATION. Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1
 Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Integra PriMatrix Dermal Repair Scaffold PATIENT INFORMATION Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Your Path to Recovery Your health care provider has chosen to use
Data extraction. Specific interventions included in the review Dressings and topical agents in relation to wound healing.
 Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
Systematic reviews of wound care management: (2) dressings and topical agents used in the healing of chronic wounds Bradley M, Cullum N, Nelson E A, Petticrew M, Sheldon T, Torgerson D Authors' objectives
Emil Schmidt Wound Care specialist SDHB - Otago
 Prospective randomised trial of low frequency ultrasound debridement (LFUD) in management of lower limb wounds Krysa J, Schmidt E, Thomson I, van Rij A Emil Schmidt Wound Care specialist SDHB - Otago Background
Prospective randomised trial of low frequency ultrasound debridement (LFUD) in management of lower limb wounds Krysa J, Schmidt E, Thomson I, van Rij A Emil Schmidt Wound Care specialist SDHB - Otago Background
UC SF. Disclosures. Vascular Assessment of the Diabetic Foot. What are the best predictors of wound healing? None. Non-Invasive Vascular Studies
 Disclosures Vascular Assessment of the Diabetic Foot What are the best predictors of wound healing? None Shant Vartanian MD Assistant Professor of Vascular Surgery UCSF Vascular Symposium April 20, 2013
Disclosures Vascular Assessment of the Diabetic Foot What are the best predictors of wound healing? None Shant Vartanian MD Assistant Professor of Vascular Surgery UCSF Vascular Symposium April 20, 2013
A randomized trial of the Tubulcus multilayer bandaging system in the treatment of extensive venous ulcers
 From the American Venous Forum A randomized trial of the Tubulcus multilayer bandaging system in the treatment of extensive venous ulcers Dragan J. Milic, PhD, a Sasa S. Zivic, MD, a Dragan C. Bogdanovic,
From the American Venous Forum A randomized trial of the Tubulcus multilayer bandaging system in the treatment of extensive venous ulcers Dragan J. Milic, PhD, a Sasa S. Zivic, MD, a Dragan C. Bogdanovic,
Monitoring Prevent. Can Temperature. DFUs
 Can Temperature Monitoring Prevent DFUs Alexander Reyzelman DPM Associate Professor, California School of Podiatric Medicine at Samuel Merritt University Oakland, CA Co-Director, UCSF Center for Limb Preservation
Can Temperature Monitoring Prevent DFUs Alexander Reyzelman DPM Associate Professor, California School of Podiatric Medicine at Samuel Merritt University Oakland, CA Co-Director, UCSF Center for Limb Preservation
Regenerative Tissue Matrix in Treatment of Wounds
 Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Regenerative Tissue Matrix in Treatment of Wounds Learning Objectives Differentiate between reparative and regenerative healing Review surgical techniques for applying a regenerative tissue scaffold to
Smart Solutions for Serious Wounds. An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers.
 Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
Smart Solutions for Serious Wounds An advanced bilayer dermal regeneration matrix FDA approved for the treatment of diabetic foot ulcers. A New Approach to Diabetic Foot Ulcer Care Supported by Over Two
STPH Clinic for Wound Care and Hyperbaric Medicine
 STPH Clinic for Wound Care and Hyperbaric Medicine Ochsner/St. Tammany Partnership John Kessels, MD Medical Director St. Tammany Wound Center Chantal Lorio, DPM Associate Medical Director Ochsner Wound
STPH Clinic for Wound Care and Hyperbaric Medicine Ochsner/St. Tammany Partnership John Kessels, MD Medical Director St. Tammany Wound Center Chantal Lorio, DPM Associate Medical Director Ochsner Wound
STUDY. complication of diabetes, affecting 12% to 15% of patients. during their lifetime. 1,2 Diabetic foot ulcers (DFUs) impair quality
 STUDY Advanced Biological Therapies for Diabetic Foot Ulcers Robert S. Kirsner, MD, PhD; Robert Warriner, MD; Michelle Michela, MS; Laure Stasik, BA; Katherine Freeman, PhD Objective: To assess the clinical
STUDY Advanced Biological Therapies for Diabetic Foot Ulcers Robert S. Kirsner, MD, PhD; Robert Warriner, MD; Michelle Michela, MS; Laure Stasik, BA; Katherine Freeman, PhD Objective: To assess the clinical
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema. Original Policy Date
 MP 2.02.12 End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
MP 2.02.12 End Diastolic Pneumatic Compression Boot as a Treatment of Peripheral Vascular Disease or Lymphedema Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date
Ray Norris, Rachel Henchy
 Use of low frequency ultrasound therapy in the treatment of recalcitrant leg ulcers: case series This series of case reports looks at the efficacy of low frequency ultrasound using the MIST Therapy System
Use of low frequency ultrasound therapy in the treatment of recalcitrant leg ulcers: case series This series of case reports looks at the efficacy of low frequency ultrasound using the MIST Therapy System
Surgical Wounds & Incisions
 Surgical Wounds & Incisions A Comprehensive Review Assessment & Management Alex Khan APRN ACNS-BC MSN CWCN CFCN WCN-C Advanced Practice Nurse / Adult Clinical Nurse Specialist www.woundcarenurses.org 1
Surgical Wounds & Incisions A Comprehensive Review Assessment & Management Alex Khan APRN ACNS-BC MSN CWCN CFCN WCN-C Advanced Practice Nurse / Adult Clinical Nurse Specialist www.woundcarenurses.org 1
Consider the impact of Venous Disease Review elements in the workup and diagnosis of Venous Disease Review treatment considerations
 Gregory Bohn, MD FACS Clinical Symposium on Advances in Skin and Wound Care September 9-12, 2011 Gaylord National Washington, DC Consider the impact of Venous Disease Review elements in the workup and
Gregory Bohn, MD FACS Clinical Symposium on Advances in Skin and Wound Care September 9-12, 2011 Gaylord National Washington, DC Consider the impact of Venous Disease Review elements in the workup and
A Post-hoc Analysis of Reduction in Diabetic Foot Ulcer Size at 4 Weeks as a Predictor of Healing by 12 Weeks
 A Post-hoc Analysis of Reduction in Diabetic Foot Ulcer Size at 4 Weeks as a Predictor of Healing by 12 Weeks Robert J. Snyder, DPM, CWS; Matthew Cardinal, ME; Damien M. Dauphinée, DPM, FACFAS, CWS; and
A Post-hoc Analysis of Reduction in Diabetic Foot Ulcer Size at 4 Weeks as a Predictor of Healing by 12 Weeks Robert J. Snyder, DPM, CWS; Matthew Cardinal, ME; Damien M. Dauphinée, DPM, FACFAS, CWS; and
ALLIANCE OF WOUND CARE STAKEHOLDERS. Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015
 ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
ALLIANCE OF WOUND CARE STAKEHOLDERS Palmetto GBA Public Meeting Draft LCD on Application of Skin Substitutes (DL36466) October 13, 2015 1 ALLIANCE OF WOUND CARE Who is the Alliance? STAKEHOLDERS A non-profit
Arterial & Venous Ulcers. A Comprehensive Review Assessment & Management
 Arterial & Venous Ulcers A Comprehensive Review Assessment & Management 1 Objectives Understand Arterial & Venous disease Understand the etiology of lower extremities ulcers Understand assessment of lower
Arterial & Venous Ulcers A Comprehensive Review Assessment & Management 1 Objectives Understand Arterial & Venous disease Understand the etiology of lower extremities ulcers Understand assessment of lower
Case study: Young athlete suffering from PTS recovers from traumatic foot ulcer, following use of the geko TM device.
 Case study: Young athlete suffering from PTS recovers from traumatic foot ulcer, following use of the geko TM device.... Subject 34-year-old male, ex professional rugby player. Wound Type Lower left leg
Case study: Young athlete suffering from PTS recovers from traumatic foot ulcer, following use of the geko TM device.... Subject 34-year-old male, ex professional rugby player. Wound Type Lower left leg
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18
 Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
DO NOT DUPLICATE. Negative pressure wound therapy (NPWT) has revolutionized the
 Original research WOUNDS 2013;25(4):89 93 From the Aesthetic and Plastic Surgery Institute, University of California Irvine, Orange, CA and Long Beach Memorial Medical Center, Long Beach, CA Address correspondence
Original research WOUNDS 2013;25(4):89 93 From the Aesthetic and Plastic Surgery Institute, University of California Irvine, Orange, CA and Long Beach Memorial Medical Center, Long Beach, CA Address correspondence
Wound Healing Community Outreach Service
 Wound Healing Community Outreach Service Wound Management Education Plan January 2011 December 2011 Author: Michelle Gibb Nurse Practitioner Wound Management Wound Healing Community Outreach Service Institute
Wound Healing Community Outreach Service Wound Management Education Plan January 2011 December 2011 Author: Michelle Gibb Nurse Practitioner Wound Management Wound Healing Community Outreach Service Institute
Prediction of healing for post-operative diabetic foot wounds based on early wound area progression
 Diabetes Care Publish Ahead of Print, published online October 12, 2007 Prediction of healing for post-operative diabetic foot wounds based on early wound area progression Lawrence A Lavery, DPM, MPH 1
Diabetes Care Publish Ahead of Print, published online October 12, 2007 Prediction of healing for post-operative diabetic foot wounds based on early wound area progression Lawrence A Lavery, DPM, MPH 1
Setting The setting was primary care. The economic study was carried out in the UK and the USA.
 Validation of venous leg ulcer guidelines in the United States and United Kingdom McGuckin M, Waterman R, Brooks J, Cherry G, Porten L, Hurley S, Kerstein M D Record Status This is a critical abstract
Validation of venous leg ulcer guidelines in the United States and United Kingdom McGuckin M, Waterman R, Brooks J, Cherry G, Porten L, Hurley S, Kerstein M D Record Status This is a critical abstract
CLINICAL EVIDENCE Partial and Deep Partial Burns
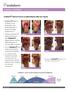 CLINICAL EVIDENCE Partial and Deep Partial Burns Endoform helps to improve re-epithelialization after burn injuries Endoform helps to facilitate tissue granulation and epithelialization in partial and
CLINICAL EVIDENCE Partial and Deep Partial Burns Endoform helps to improve re-epithelialization after burn injuries Endoform helps to facilitate tissue granulation and epithelialization in partial and
Making the Most of your Dressing Products Catherine Hammond CNS/CNE
 Making the Most of your Dressing Products 2013 Catherine Hammond CNS/CNE What do you need in your dressings cupboard? 2 Skin tear 3 4 Lack Confidence in Selecting Dressings? 5 Appropriate Use of Product
Making the Most of your Dressing Products 2013 Catherine Hammond CNS/CNE What do you need in your dressings cupboard? 2 Skin tear 3 4 Lack Confidence in Selecting Dressings? 5 Appropriate Use of Product
Level 2 Leg Ulcer Management Service. Service Level Agreement Background. Contents:
 Level 2 Leg Ulcer Management Service Service Level Agreement 2016-2019 Contents: 1. Background to Leg Ulcer Management Service 2. Service Details 3. Accreditation 4. Service Standards 5. Finance Details
Level 2 Leg Ulcer Management Service Service Level Agreement 2016-2019 Contents: 1. Background to Leg Ulcer Management Service 2. Service Details 3. Accreditation 4. Service Standards 5. Finance Details
Inflammation is Not the Enemy
 6/22/2017 Inflammation is Not the Enemy Sean Mulvaney, MD 1 6/22/2017 2 6/22/2017 Lascaux 7.4 Billion 3 This image cannot currently be displayed. 6/22/2017 Goals 4 ANTI INFLAMMATORY THERAPIES NSAIDS 5
6/22/2017 Inflammation is Not the Enemy Sean Mulvaney, MD 1 6/22/2017 2 6/22/2017 Lascaux 7.4 Billion 3 This image cannot currently be displayed. 6/22/2017 Goals 4 ANTI INFLAMMATORY THERAPIES NSAIDS 5
Summary ID#7029. Clinical Study Summary: Study F1D-MC-HGKQ
 CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
CT Registry ID# 7029 Page 1 Summary ID#7029 Clinical Study Summary: Study F1D-MC-HGKQ Clinical Study Report: Versus Divalproex and Placebo in the Treatment of Mild to Moderate Mania Associated with Bipolar
Hyperbarics in Diabetic Wound Care. Aurel Mihai, MD & Brian Kline, MD
 Hyperbarics in Diabetic Wound Care Aurel Mihai, MD & Brian Kline, MD Presentation Outline The Scope of the Problem Important Definitions Standard Wound Care Hyperbaric Oxygen as an Adjunct Diabetic Foot
Hyperbarics in Diabetic Wound Care Aurel Mihai, MD & Brian Kline, MD Presentation Outline The Scope of the Problem Important Definitions Standard Wound Care Hyperbaric Oxygen as an Adjunct Diabetic Foot
Patient Care Information
 Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Patient Care Information A Guide to Healing Diabetic Foot Ulcers Questions? Contact us: Clinician: Phone #: In case of emergency, dial 9-1-1 Dermal Regeneration Matrix Overview Diabetic foot ulcers are
Pressure Ulcers: 3 Keys to Pressure Ulcer Management. Evidence Based Prevention & Management. I have no financial conflicts of interest
 Pressure Ulcers: Evidence Based Prevention & Management Madhuri Reddy, MD MSc I have no financial conflicts of interest I have nothing to disclose financially 3 Keys to Pressure Ulcer Management 1 3 Keys
Pressure Ulcers: Evidence Based Prevention & Management Madhuri Reddy, MD MSc I have no financial conflicts of interest I have nothing to disclose financially 3 Keys to Pressure Ulcer Management 1 3 Keys
pressure of compression stockings matters (clinical importance of pressure)
 Classification of Compression Stockings ICC Meeting, Copenhagen, May 17, 2013. pressure of compression stockings matters (clinical importance of pressure) Giovanni Mosti; Lucca, Italy disclosure no conflict
Classification of Compression Stockings ICC Meeting, Copenhagen, May 17, 2013. pressure of compression stockings matters (clinical importance of pressure) Giovanni Mosti; Lucca, Italy disclosure no conflict
Case 1. July 14, th week wound gel 3 cm x 2.5 cm = 7.5 cm². May 25, st wound gel on 290 days PI treatment 4 cm x 2.4 cm = 9.
 2.5% Sodium Hyaluronate Wound Gel Study Cases Case 1 Patient with Lower Leg Ulcer Not Responding to Compression This patient was a 50-year old male patient with nonhealing right lower leg since January
2.5% Sodium Hyaluronate Wound Gel Study Cases Case 1 Patient with Lower Leg Ulcer Not Responding to Compression This patient was a 50-year old male patient with nonhealing right lower leg since January
Dr Paul Thibault. Phlebologist & Assistant Editor Phlebology (International Journal) Australasian College of Phlebology
 Dr Paul Thibault Phlebologist & Assistant Editor Phlebology (International Journal) Australasian College of Phlebology Prescribing Effective Compression and PTS Dr Paul Thibault Phlebologist, Newcastle,
Dr Paul Thibault Phlebologist & Assistant Editor Phlebology (International Journal) Australasian College of Phlebology Prescribing Effective Compression and PTS Dr Paul Thibault Phlebologist, Newcastle,
