Evidence That Marek s Disease Virus Exists in a Latent State in a Sustainable Fibroblast Cell Line
|
|
|
- Erik Little
- 5 years ago
- Views:
Transcription
1 VIROLOGY 229, (1997) ARTICLE NO. VY Evidence That Marek s Disease Virus Exists in a Latent State in a Sustainable Fibroblast Cell Line AMIN A. ABUJOUB* and PAUL M. COUSSENS*,,1 Molecular Virology Laboratory, Departments of *Microbiology, and Animal Science, Michigan State University, East Lansing, Michigan Received May 22, 1996; returned to author for revision June 28, 1996; accepted January 7, 1997 Previously, we reported the development of two fibroblastic cell lines (MDV OU2.1 and OU2.2) infected with Marek s disease virus (MDV). The two cell lines, in nonconfluent continuous cultures, displayed characteristics consistent with MDV existing in a latent state. However, presence of distinct plaques in confluent cell monolayers and the ability to transfer cytolytic infection to susceptible birds and primary chick embryo fibroblasts, suggest that, if latent, the virus is easily reactivated from MDV OU2 cell lines. In this report, we present evidence which supports the hypothesis that MDV genomes in MDV OU2 cells are latent. PCR analyses and in vivo experiments demonstrate that CHCC-OU2 cells stabilize MDV so that serial in vitro passage does not attenuate the virus. Following two years of active culture, MDV genomes in MDV OU2 cells are still oncogenic, similar to that seen in MDV-lymphoblastoid cell lines. Expansion of the 132-bp repeat within MDV BamHI fragments H and D, typical of highly passaged serotype 1 MDV, has not been observed beyond two copies in MDV OU2 cells. Indirect immunofluorescence assays clearly demonstrate that MDV OU2 cells do not express glycoproteins B and I when subconfluent. However, upon reaching confluence these proteins are expressed in readily detectable amounts. Using RT-PCR we demonstrate that glycoproteins E and D are highly expressed in confluent MDV OU2 cells but absent from subconfluent cells, and MDV latency-associated transcripts (LATs), which are antisense to ICP4 transcripts and have been associated with latent MDV infection, are expressed in subconfluent MDV OU2 cells. Coincident with an increase in ICP4 expression, MDV LAT expression is down regulated when MDV OU2 cells become confluent Academic Press INTRODUCTION Marek s disease (MD) is a lymphoproliferative disease of chickens caused by Marek s disease virus (MDV). MDV is an avian herpesvirus, which is highly infectious and cell associated (Calnek and Witter, 1991). In infected birds, a lytic cycle of MDV replication takes place in differentiating epithelial cells and bursal lymphocytes. Infectious viruses are produced only in feather follicle epithelium and are shed with feather dander and dust (Witter et al., 1972; Calnek et al., 1970). Following an initial burst of lytic infection, T-lymphocytes become latently infected with MDV. Subsequently, chickens infected with virulent, oncogenic (sero- type 1) strains of MDV develop aggressive T-cell lympho- mas (Calnek and Witter, 1991). Evidence accumulated to date suggests that latent infection of T-lymphocytes is a prerequisite to malignant transformation and neoplastic dis- ease (Shek et al., 1983). Although MDV cannot produce fully infectious particles (enveloped virus) in tissue culture, cytopathic infections can be produced in monolayers of primary or secondary chicken and duck embryo fibroblasts (CEF and DEF, re- spectively) as well as chick kidney cells (CKC). CEF, CKC, and DEF support the lytic cycle of MDV in vitro (Schat et al., 1989). In addition, more than 90 lymphoblastoid cell 1 To whom reprint requests should be addressed. lines have been isolated and established from MD tumors (Schat et al., 1991; Akiyama and Kato, 1974; Powell et al., 1974; Calnek et al., 1978; Payne et al., 1981; Nazarian and Witter, 1975). MDV-transformed lymphoblastoid cells are immortalized cell lines which are latently infected with MDV and usually capable of transferring MDV to CEF or DEF in vitro and to susceptible chickens in vivo. In MDV lymphoblastoid cell lines a limited set of viral genes have been found to be transcribed (Maray et al., 1988; Sugaya et al., 1990). Transcriptional activity is lim- ited to approximately 20% of the MDV genome, primarily in repeat regions and adjacent sequences. Most transcripts found in MDV lymphoblastoid cells are derived from immediate early (IE) genes (Silver et al., 1979; Schat et al., 1989), suggesting that IE genes could have a signif- icant role in maintenance of latency. Latency-associated transcripts (LATs) which map antisense to IE genes have been described for many herpesviruses. Transcripts antisense to ICP4 have been reported for pseudorabies virus and have been postulated to be involved in latency (Priola et al., 1990). In the case of herpes simplex virus, LATs map antisense to the ICP0 gene (Feldman, 1991). Epstein Barr virus (EBV) latently infects human B- lymphocytes, causing both benign and malignant lymphoproliferations. Viral gene expression during latent infection is limited to 11 viral genes, 9 of which are translated. Six of these proteins are nuclear (Ep /97 $25.00 Copyright 1997 by Academic Press All rights of reproduction in any form reserved.
2 310 ABUJOUB AND COUSSENS exists in a continuous latent state in MDV OU2 cell lines and virus lytic cycle is activated upon confluency. Virus produced from MDV OU2 cell lines remained virulent and oncogenic after more than 30 in vitro passages. In addition, continuous cultivation in CHCC-OU2 cells appears to stabilize MDV genomes. For approximately 2 years of continuous culture, only a minor expansion of an unstable 132-bp DR sequence (het region) was de- tected in MDV OU2 cells. Our results suggest the MDV OU2 cell system may represent an ideal in vitro system to study factors involved in the switch between lytic and latent herpesvirus infections. In addition, stabilization of MDV genes will have important applications in production of recombinant and mutant MDV. stein Barr nuclear antigen (EBNA)-1, -2, -3A, -3B, -3C, -LP), and three latent membrane proteins (LMP)-1, -2A, -2B) (Lee, 1994). Reactivation of EBV replication is induced by ZEBRA (an immediate-early activator encoded by BZLF1 gene) (Countryman and Miller, 1985). BZLF1 is a transcription factor that can activate other viral genes and also affect cellular genes (Flemington and Speck, 1990). In MDV, LATs which map antisense to ICP4 have been detected (Li et al., 1994; Cantello et al., 1994; Mckie et al., 1995). These LAT transcripts appear to be expressed at higher levels in lymphoblastoid cell lines (MSB-1 and RPL1) and are down regulated in lytically infected cells (Li et al., 1994). Although it is possible that MDV LATs play a role in maintenance of latency, definitive evidence is still lacking. MDV can be rescued from some lymphoblastoid cell lines by cocultivation with primary or secondary CEF and DEF (Schat et al., 1989). In addition, some lymphoblastoid cell lines will induce MD upon injection into susceptible birds (Akiyama et al., 1973, Nazarian et al., 1977). Reactivation of cytolytic infection, however, varies with each cell line. Serial in vitro passage of virulent oncogenic MDV re- sults in loss of MDV tumorigenicity (Churchill and Chubb, 1969). Attenuation of MDV was strongly correlated with an expansion in two regions (BamHI-H and -D), present in MDV long terminal repeat (TR L ) and long inverted repeat (IR L ) regions, respectively (Silva and Witter, 1985; Fukuchi et al., 1985; Chen and Velicer, 1991). It was latter discovered that expansion was due to amplification of a 132-bp direct repeat (DR) sequence found within MDV BamHI-H and -D fragments (Maotani et al., 1986). Tumor induction studies in susceptible birds suggest that cloned virus populations which exhibit an amplification of the 132-bp repeat region have decreased tumorigenic capability. In contrast, viruses which do not contain am- plified BamHI-H or -D regions efficiently induce tumors in chickens (Fukuchi et al., 1985). Recently we reported development of fibroblastic cell lines (MDV OU2.1 and OU2.2) infected with serotype 1 MDV, strain Md11 (Abujoub and Coussens, 1995). MDV OU2 cell lines are similar to certain lymphoblastoid cell lines that MDV infection can be transferred to primary and secondary CEF monolayer culture and MD induced in susceptible birds (Abujoub and Coussens, 1995). However, MDV OU2 cell lines, unlike lymphoblastoid cell lines, are not transformed. Latency in many viruses results from lack of host factors critical for the expression of viral IE gene products (Garcia-Blanco and Cullen, 1991). Reactivation of these viruses from the latent state is not completely understood, but could be due to activation of specific cellular factors in response to an external stimuli. Stimulation will activate viral regulatory proteins and lead to a state of lytic infection. As a first step in examining the status of MDV in the MDV OU2 cell lines, we report here that MDV Cells and virus MATERIALS AND METHODS Preparation, propagation, and infection of CEF cells with MDV were performed as described previously (Glau- biger et al., 1983; Coussens and Velicer, 1988). The very virulent MDV strain Md11 was used at cell culture passage levels 15, 28, 35, 48, and 86 (Md11p15, Md11p28, Md11p35, Md11p48, and Md11p86, respectively). CHCC- OU2 (Ogura and Fujiwara, 1987), MDV OU2.2, and MDV OU2.1 cells were maintained as described previously (Abujoub and Coussens, 1995). MDV OU2 cells were used at cell culture passages 8, 12, 16, 23, and 31 (MDV OU2.2p8, MDV OU2.2p12, MDV OU2.2p16, MDV OU2.2p23, and MDV OU2.2p31) for PCR, and RT-PCR analyses. MDV OU2.1 and OU2.2 cells are passaged every days prior to formation of confluent mono- layers. Preparation of cellular DNA and polymerase chain reaction (PCR) Total cellular DNA was extracted from CEF, CEF/Md11, MSB-1, CHCC-OU2, and MDV OU2 cells by standard methods (Sambrook et al., 1989). Total cellular DNA was used as a template for PCR amplification of the 132-bp DR sequences. The upstream primer (5 -TGCGATGAA- AGTGCTATGGAGG-3 ) anneals 3-bp 5 to the 132-bp DR sequences, while the downstream primer (5 -GAGAAT- CCCTATGAGAAAGCGC-3 ) anneals 6 bp from the 3 end of the 132-bp DR sequences. PCR using these two primers amplifies a 317-bp fragment when two copies of the DR sequence are present (Silva, 1992). PCR conditions were slightly modified from those suggested by Silva (1992). Briefly, 200 ng of total cellular DNA was mixed with 20 mm of each dntp, 20 mm of each oligonucleotide primer pair, 10 ml 101PCR reaction buffer (Life Technologies, Gaithersburg, MD), 1.5 mm MgCl 2, and 1.0 U Taq polymerase (Life Technologies). PCR reactions were performed using a GeneAmp 9600 thermal cycler (Perkin Elmer Cetus, Norwalk, CT). Following an initial denatur-
3 MAREK S DISEASE IN A LATENT STATE IN FIBROBLASTIC CELLS 311 ing step at 95 for 5 min, DNA was amplified during for 15 min. A negative control without template and cycles of 95 for 30 sec, 67 for 30 sec, and 72 for 30 a positive control with Md11p35 DNA was included in sec. PCR reactions were completed by a final elongation each RT-PCR reaction. PCR conditions for glycoproteins step at 72 for 10 min. A negative control with CHCC-OU2 D and E were similar to those of MDV ICP4 and LATs, DNA was included in each PCR reaction. Amplification of except for using 10 mm of oligonucleotide primers D1 and an 850-bp fragment of the pp38 gene was performed as D2 (5 -TACGTGAATATGCCAACTGC-3 ) as an upstream previously described (Abujoub and Coussens, 1995). primer and oligonucleotide primers E1 and E2 (5 -GAA- PCR products were analyzed on 6% polyacrylamide gels, TCGCTAAGTCTGAATGG-3 ) as an upstream primer, restained with ethidium bromide, and photographed under spectively. Also, the annealing temperature has been ultraviolet light. Sizes of amplified fragments were determined changed to 56 to match the T m of these oligonucleotide by comparison to a 1-kb DNA ladder marker (Life primers. Technologies). Inoculation of chickens with cells and virus RNA isolation and reverse transcriptase PCR In vivo experiments were performed using specific (RT-PCR) pathogen-free chickens (SPF), obtained from SPAFAS (Chicago, IL). Chicks were divided into three groups of Total RNA was isolated from CHCC-OU2, MDV 5 chicks per group at 1 day of age, and groups inoculated OU2.2p31, Md11p35, and MSB-1 cells using the Trizole intraperitoneally with: (A) uninfected CHCCreagent (Life Technologies) according to the manufactur- OU2 cells, (B) 2000 plaque forming units (PFU) of MDV er s recommendation. Prior to use, RNA was treated with OU2.2p8 cells, or (C) 2000 PFU of MDV OU2.2p units RNase-free RQ1 DNAase (Promega, Madison, Birds were euthanized and necropsied upon severe WI) for 30 min at 37. Coupled cdna synthesis and PCR signs of morbidity. Blood was collected, and peripheral amplification of extracted RNAs was carried out in two blood leukocytes (PBLs) were isolated as described (Tarsteps. First, cdna synthesis was performed in 250 mm def and McQueen, 1993) and cocultivated with secondary Tris HCl (ph 8.3), 375 mm KCl, 15 mm MgCl 2, 100 mm CEF cells as an assay for production of viable virus. dithiothreitol, and 10 mm each datp, dctp, dgtp, and Various tissues, including heart, liver, kidney, and spleen dttp. First strand synthesis mixture also contained 0.5 were frozen for subsequent DNA isolation. Total tissue- U RNasin (Promega), 200 U Superscript II Reverse Transpecific (Kidney) DNA was isolated and used as template scriptase (Life Technologies), and 15 mg of total cellular for PCR amplification of the 132-bp DR sequence and for RNA. One micromolar of oligonucleotide corresponding an 850-bp region of MDV pp38 gene sequences. to nt (complementary) of M49 cdna clone of MDV (Li et al., 1994) (5 -CGTCGGACATGTTTCCAGATC- GCC-3 ) was used as a downstream primer in order to Indirect immunofluorescence antibody (IIFA) staining amplify MDV LATs transcripts, whereas 1 mm of oligonu- Uninfected CEF, CEF infected with Md11p15, CHCCcleotide corresponding to nt of M49 cdna clone OU2, and MDV OU2.2 cells, grown on glass cover slips of MDV (Li et al., 1994) (5 -CGTTGGACGGCTCGGCGG- in 35-mm tissue culture plates, were used for indirect ACTTGGG-3 ) was used as a downstream primer to immunofluorescence labeling according to standard proamplify ICP4 transcripts. Oligonucleotide primers D1 (5 - tocols ( Harlow and Lane, 1988; Hong et al., 1995). CEF AGTGAGTCCAGTGTAAACCATCC-3 ) and E1 (5 -CAG- and CHCC-OU2 cells were processed 3 days postplating AATGTCAATGTTGGATGCG-3 ) were used as down- on cover slips. CEF infected with Md11p15 were processed stream primers to amplify glycoproteins D and E transcripts, 1 day after displaying plaques characteristic of respectively. Reactions were incubated at 42 for MDV infection. MDV OU2.2 were processed either when 60 min, followed by enzyme inactivation for 15 min at they were 60 80% confluent (subconfluent) or 5 days 70. Second, the cdna synthesized in the first step was after forming a confluent monolayer (confluent). Cells used as a template for PCR reaction as follows: 10% of were washed with cold PBS, fixed and permeabilized the first strand reaction was amplified in 200 mm Tris with 2 ml of ice-cold acetone:methanol (1:1) for 3 min at HCl (ph 8.4), 500 mm KCl, 1.5 mm MgCl 2, 20 mm of room temperature with gentle agitation. Following three each dntp, and 10 mm each of oligonucleotide corre- washes with cold PBS, cells were preincubated with 3% sponding to nt of M49 cdna clone of MDV and BSA in PBS for 1 hr at room temperature. Anti-MDV/gB nt (complementary) (Li et al., 1994) for ampli- (Silva and Lee, 1984) monoclonal antibody ( generously fying MDV LATs and MDV ICP4. PCR reactions were provided by Dr. Lucy Lee, USDA-ADOL) was added to performed using a GeneAmp 2400 thermal cycler (Per- the cells at a 1:40 dilution in PBS and incubated for 1 hr kin Elmer Cetus). Following an initial denaturing step at room temperature. Anti-MDV/gI polyclonal antibody at 95 for 5 min, DNA was amplified during 30 cycles of (generously provided by Dr. Lee Velicer, Department of 95 for 45 sec, 62 for 45 sec, and 72 for 45 sec. PCR Microbiology, Michigan State University) was added to reactions were completed by a final elongation step at cells in a 1:20 dilution in PBS and incubated for 1 hr
4 312 ABUJOUB AND COUSSENS at room temperature. After extensive washing with PBS, respectively). Similarly, anti-mdv/gi (Brunovskis, and Velicer, sheep anti-mouse IgG (whole molecule) conjugated with 1995) polyclonal antibodies detected a protein consheep fluorescein-5 -isothiocyanate (FITC) (Sigma) was used as sistent with MDV gi in confluent MDV OU2.2p16, but not secondary antibody for cells incubated with anti-mdv/ in subconfluent cells (Figs. 3D and 3B, respectively). A gb as primary antibody. Goat anti-rabbit IgG (whole mole- similar protein was not detected in either subconfluent cule) conjugated with FITC (Sigma, St. Louis, MO) was or confluent CHCC-OU2 cells (A and C in Figs. 2 and 3). used as a secondary antibody for cells incubated with Uninfected CEF and Md11p15/CEF cells were used as anti-mdv/gi as a primary antibody. All secondary antibodies negative and positive controls, respectively (data not were diluted 1:20 in PBS. After extensive washing shown). Together, results of IIFA staining, Western blot with PBS, Slow-Fade (Molecular Probes, Eugene, OR) analysis (Abujoub and Coussens, 1995), and MDV OU2 was added prior to mounting to glass slides to minimize cell growth characteristics suggest that fully lytic growth quenching of fluorescence. Cells were photographed on of MDV and corresponding expression of late proteins an Olympus BH-2 fluorescence microscope. is initiated only after MDV OU2 cells form a confluent monolayer. RESULTS Detection of late structural glycoproteins expression MDV is stably maintained in subconfluent using RT-PCR MDV OU2 cells RT-PCR was used to compare MDV gene expression Recently we reported development of two fibroblastic between subconfluent and confluent MDV OU2 cells. Olicell lines (MDV OU2.2 and OU2.1) capable of supporting gonucleotide primers specific for glycoproteins E and D replication and growth of MDV (Abujoub and Coussens, amplified 530- and 560-bp fragments, respectively, from 1995). MDV OU2 cells cannot be visibly distinguished confluent MDV OU2 cells but not from subconfluent MDV from uninfected parental CHCC-OU2 cells when grown OU2 cells (Figs. 4A and 4B). A similar pattern of expresat a subconfluent level ( Figs. 1A and 1B). Subconfluent sion was detected in MD11p35 lytically infected CEF MDV OU2.2 cells do not display any signs of lytic MDV cells and no expression was detected in uninfected infection and exhibit a doubling time only slightly shorter CHCC-OU2 cells (Figs. 4A and 4B). No expression of gd than parental cells. Within 5 days of becoming confluent, was detected in MSB-1 cells but very low level of ge monolayers of MDV OU2 cells display plaques similar in expression was detected. This low level of expression appearance to those observed in MDV-infected primary may be due to the spontaneous viral reactivation within or secondary CEF cells (Figs. 1C and 1D). Thus MDV MSB-1 cells. OU2 cells have been viable in cell culture for almost 2 years and display plaques only when allowed to reach MDV LATs are expressed in subconfluent confluence. MDV OU2 cells Within MDV OU2 cells, MDV may exist in a latent state and is reactivated when cells become confluent. AlternamRNA s could be detected in MDV OU2 cell lines under We used RT-PCR to determine if antisense ICP4 tively, cytolytic infection of CHCC-OU2 cells by MDV may be offset by cellular growth in subconfluent cultures. In subconfluent and confluent conditions. Total cellular RNA this case, plaques become visible only when contact isolated from CHCC-OU2 cells, MDV-OU2 cells (subcon- inhibition decreases cellular growth. To distinguish becultures was used for RT-PCR to detect expression of fluent and confluent), MSB-1 cells, and Md11p35/CEF tween these possibilities, the nature of MDV infection in MDV OU2 cells was examined. MDV LATs. A 335-bp fragment corresponding to the 5 end of M49 cdna which maps antisense to the MDV Detection of viral proteins by IIFA staining ICP4 gene (Li et al., 1994) was detected in RNA isolated from subconfluent MDV OU2 cells, MSB-1 cells, and occasionally MDV OU2 cells express pp38 and pp14 in quantities in confluent MDV OU2 cells, but was not de- sufficient to be detected by Western blot analysis (Abu- tected in RNA isolated from lytically infected Md11p35/ joub and Coussens, 1995). However, expression of other CEF or uninfected CHCC-OU2 cells (Fig. 5A). A fragment MDV antigens, particularly those of structural glycopro- of approximately 230 bp amplified during RT-PCR of teins, was not detectable by Western blot analysis (Abujoub MSB-1 cells RNA may represent an alternatively spliced and Coussens, 1995). Therefore, we compared MDV variant of MDV LATs (Fig. 5A, lane 6). antigen expression by IIFA staining of subconfluent and To verify that products amplified in RT-PCR reactions confluent MDV OU2 cells. Anti-MDV/gB (Silva and Lee, were indeed antisense to the MDV ICP4 homolog gene, 1984) monoclonal antibody IAN 86 ( generously provided PCR products were transferred to a Zeta-probe memby Dr. Lucy Lee, USDA-ADOL) detected a protein consis- brane and probed with DNA corresponding to the MDV tent with MDV gb in confluent MDV OU2.2p16, but not ICP4 gene. Hybridization of radioactively labeled MDV in subconfluent MDV OU2.2p16 cells (Figs. 2D and 2B, ICP4 probes to RT-PCR products from reaction with MDV
5 MAREK S DISEASE IN A LATENT STATE IN FIBROBLASTIC CELLS 313 FIG. 1. Subconfluent MDV OU2.2p23 and MDV OU2.1p23 cells display a cobblestone appearance, similar to that seen with uninfected parental CHCC-OU2 cells (A and B, respectively). At 5 7 days after cells became confluent, numerous plaques consistent with MDV lytic infection are observed on cultures of MDV OU2.2p23 and MDV OU2.1p23 cells (C and D, respectively).
6 314 ABUJOUB AND COUSSENS FIG. 2. IIFA staining with monoclonal antibody IAN86 specific for MDV gb homologue. Subconfluent CHCC-OU2, subconfluent MDV OU2.2, confluent CHCC- OU2, and confluent MDV OU2.2 cells were grown on glass cover slips. Cells were fixed and stained as described under Materials and Methods. Sheep antimouse IgG conjugated with FITC (Sigma) was used as a secondary antibody. Cells were photographed on an Olympus BH-2 fluorescence microscope with a 401 objective and 3.31 photo eyepiece. (A) Subconfluent CHCC-OU2 cells. (B) Subconfluent MDV OU2.2 cells. (C) Confluent CHCC-OU2 cells. (D) Confluent MDV OU2.2 cells.
7 MAREK S DISEASE IN A LATENT STATE IN FIBROBLASTIC CELLS 315 FIG. 3. IIFA staining with polyclonal antisera against MDV gi homologue. Subconfluent CHCC-OU2, subconfluent MDV OU2.2, confluent CHCC-OU2, and confluent MDV OU2.2 cells were grown on glass cover slips. Cells were fixed and stained as described under Materials and Methods. Sheep anti-rabbit IgG conjugated with FITC (Sigma) was used as a secondary antibody. Cells were photographed on an Olympus BH-2 fluorescence microscope with a 40X objective and 3.3X photo eyepiece. (A) Subconfluent CHCC-OU2 cells. (B) Subconfluent MDV OU2.2 cells. (C) Confluent CHCC-OU2 cells. (D) Confluent MDV OU2.2 cells.
8 316 ABUJOUB AND COUSSENS OU2 and MSB-1 RNA (Fig. 5C) confirmed that these products were located within the ICP4 gene. No hybridization was detected in lanes containing RT-PCR reactions with CHCC-OU2 or Md11p35 RNA (Fig. 5C). As a control for DNA contamination, no hybridization was detected when RNA templates were used for PCR without prior addition of Superscript II Reverse Transcriptase (data not shown). There was no hybridization of ICP4-specific probe to the 230-bp band in LAT-specific or ICP4-specific RT-PCR reactions with MSB-1 RNA(Fig. 5A, lane 6), or to the 175- bp band in Md11p35/CEF and MSB-1 (Fig. 5B, lanes 5 FIG. 5. RT-PCR amplification of a 335-bp fragment of MDV LATs and ICP4 gene. (A) LAT-specific primers were used to amplify a 335-bp fragment using total RNA isolated from CHCC-OU2 (lane 2), Md11p35/CEF (lane 3), confluent MDV OU2.2p31 (lane 4), subconfluent MDV OU2.2p31 (lane 5), MSB-1 cells (lane 6). Lanes 7 and 8 are PCR negative and positive control, respectively. (B) Primers specific for the MDV ICP4 transcript were used for RT-PCR amplification of a 300-bp fragment using RNA isolated from CHCC-OU2 (lane 4), Md11p35/CEF (lane 5), confluent MDV OU2.2p31(lane 6), subconfluent MDV OU2.2p31 (lane 7), MSB-1 cells (lane 8). Lanes 2 and 3 represent PCR negative and positive controls, respectively. A 1-kb DNA ladder marker (Life Technologies) (lane 1) was used for size comparison. Numbers at the left indicate approximate sizes of amplified fragments. Smaller size fragments are due to nonspecific amplification and were not detected using Southern blot analysis (data not shown). (C) Southern blot hybridization of LATs RT-PCR. Using ICP4 gene as a probe, RT-PCR products from MSB-1 and Subconfluent MDV OU2 cell strongly hybridized to a product of the expected size. No hybridization was detected in lanes containing uninfected OU2, confluent MDV OU2 cells, and Md11p35/CEF. and 8), suggesting these bands represented nonspecific amplification products. Expression of MDV LATs in MDV OU2 cells combined with MDV protein expression patterns strongly suggest that MDV is in a latent state in these cells. Down regulation of MDV LATs in confluent MDV OU2 cells is associated with an increase in ICP4 gene expression as deduced from RT-PCR results (Fig. FIG. 4. RT-PCR amplification of a 530-bp fragment of ge and of a 5B, compare lanes 6 and 7). MDV ICP4 gene expression 560-bp fragment of gd transcripts. (A) Glycoprotein E-specific primers (E1 and E2) were used to amplify a 530-bp fragment using total RNA in subconfluent MDV OU2 cells was very low compared isolated from uninfected CHCC-OU2, Md11p35/CEF, confluent MDV to expression in Md11p35-infected CEF cells and conflu- OU2.2p33, MSB-1, and subconfluent MDV OU2.2p33 cells. (B) Glycopro- ent MDV OU2 cells (Fig. 5B, lanes 5 and 6). These results tein D-specific primers were used to amplify a 560-bp fragment using are consistent with previous evidence that ICP4 trantotal RNA isolated from uninfected CHCC-OU2, Md11p35/CEF, conflu- scripts are predominantly expressed in lytically infected ent MDV OU2.2p33, MSB-1, and subconfluent MDV OU2.2p33 cells. PCR-negative control was used in all reactions ( No Template lane). A cells and antisense transcripts are predominantly pro- 1-kb DNA ladder marker (Life Technologies) was used for size comparison. Numbers at the left indicate sizes of MW marker fragments. et al., duced in latently infected cells (Cantello et al., 1994; Li 1994).
9 MAREK S DISEASE IN A LATENT STATE IN FIBROBLASTIC CELLS 317 FIG. 6. PCR amplification of the 132-bp DR sequence. (A) DNA iso- lated from CHCC-OU2 (lane 2), Md11p15/CEF (lane 3), MDV OU2.2p12 (lane 4), MDV OU2.2p23 (lane 5), and Md11p86/CEF (lane 6) was used as template for PCR amplification. A 1-kb ladder marker (Life Technologies) (lane 1) was used for size comparison. DNA isolated from CHCC- OU2 cells (lane 2) served as negative control. (B) DNA isolated from Md11p15/CEF (lane 1), Md11p48/CEF (lane 2), and MDV OU2.2p28 (lane 3) was used as template for PCR amplification of DR sequences. Numbered arrows at the right indicate number of copies of the 132- bp DR sequence. Arrows at the left represent positions of selected bands from the 1-kb ladder marker (Life Technologies). Serial in vitro passage does not cause attenuation of MDV genomes in MDV OU2 cells sequence in MDV DNA was not significantly expanded following extended serial in vitro passage in MDV OU2 cells. In contrast, and as expected, the 132-bp DR region was expanded following serial in vitro passage in secondary CEF cells. Therefore, similar to lymphoblastoid cell lines, MDV OU2 cells appear to stabilize MDV DNA. Despite many years in culture, lymphoblastoid cell lines are still capable of inducing MD in susceptible birds. Upon PCR analysis, 3 5 copies of the 132-bp DR sequences (data not shown) were detected in MDV genomes from MSB-1 cells. To further verify stabilization of MDV het region DNA in MDV OU2 cells, an equal passage variant of Md11 was generated in CEF cells. Due to a switch in passage numbering upon transfer to the CHCC-OU2 culture system, Md11p48/CEF represents an equal number of passages as MDV OU2.2p28 cells. A comparison of equal passage MDV in MDV OU2 cells (MDV OU2.2p28) and CEF cells (Md11p48) (Fig. 6B, lanes 3 and 2, respectively) clearly demonstrated that the 132- bp DR sequence is stabilized within MDV OU2 cells (Fig. 6B, lane 3) relative to an equal passage counterpart cultivated on CEF cells (Fig. 6B, lane 2). More than seven copies of the 132-bp DR were detected in PCR amplifica- tion (Fig. 6A, lane 6, and Fig. 6B, lane 2) of DNA from CEF cells infected with Md11p86 and Md11p48, respectively. High passage MDV OU2 cells induce MD in susceptible chickens Early passages of MDV OU2.2 cells can induce MD in susceptible birds within 3 5 weeks following intraperitoneal injection (Abujoub and Coussens, 1995). Stabilization of het region DNA in MDV OU2 cells suggested that, as with MDV lymphoblastoid cells, MDV pathogenicity should also be preserved in long term cultures of MDV OU2 cells. To confirm that the MDV genomes were not attenuated after extended serial in vitro culture in MDV OU2 cells, SPF chickens were inoculated with either MDV OU2.2p8 (low passage) or p23 (high passage) cells A PCR assay developed by Silva (1992) was used to estimate the number of 132-bp DR sequences present in MDV genomes following serial in vitro passage of MDV OU2 cells and various control infections. Two hundred nanograms of total DNA from CHCC-OU2, Md11p15/CEF, Md11p28/CEF, Md11p48/CEF, Md11p86/CEF, MDV at 1 day of age. As a negative control, chickens were OU2.2p12, MDV OU2.2p23, and MDV OU2.2p28 cells was injected with parental CHCC-OU2 cells. Consistent with used as template for PCR amplification with a primer previous results, chickens injected with MDV OU2.2p8 set flanking the 132-bp DR sequence (het region). In all cells developed classical signs of MD by the end of the reactions, except the CHCC-OU2 negative control, it was fourth week postinoculation and either died or had to be possible to detect a 185-bp amplified fragment corresponding euthanized by the end of the fifth week due to widespread to one copy of the 132-bp DR (Fig. 6A, lane 3). paralysis. Necropsy revealed complete bursal atrophy A 317-bp fragment corresponding to two copies of the and splenomegaly in all birds inoculated with MDV 132-bp DR was predominant in reactions containing OU2.2p8. Chickens inoculated with MDV OU2.2 p23 also pathogenic Md11p15/CEF DNA as well as in DNA isolated displayed signs of MD within 3 5 weeks postinoculation. from MDV OU2.2p12 and p23 (Fig. 6A, lanes 3, 4, In addition to severe paralysis, birds inoculated with and 5). Although the 317-bp fragment was present in MDV OU2.2p23 cells displayed severe weight loss when DNA amplified from cells infected with Md11p86, the compared to CHCC-OU2- and MDV OU2.2p8-injected predominant PCR products were distributed over a range birds (data not shown). All birds in this group were eu- of bands representing between four and seven copies thanized by the end of the fifth week due to signs of of the 132-bp DR sequence (Fig. 6A, lane 6). severe illness. Upon necropsy, complete bursal atrophy Results of PCR analyses indicated that the 132-bp DR and early signs of tumor formation were observed in
10 318 ABUJOUB AND COUSSENS primed with a primer set specific for pp38 gene sequences amplified an 850-bp fragment in reactions containing DNA isolated from kidneys of birds inoculated with MDV OU2.2p8 (Fig. 7B, lanes 4 and 5) or MDV OU2.2p23 (Fig. 7B, lanes 6 and 7). No detectable MDV products were amplified in reactions containing DNA from kidneys of birds inoculated with CHCC-OU2 (Figs. 7A and 7B, lanes 2 and 3). The presence of MDV-specific PCR products indicated that both MDV OU2.2p8 and MDV OU2.2p23 cells were capable of transferring MDV to susceptible birds. As an additional test for disseminated viremia in infected birds, blood was collected at the time of euthanization, and peripheral blood leukocytes (PBLs) were isolated from whole blood, washed with PBS, and cocultivated with secondary CEF cells. CEF monolayers cocultivated with PBLs from MDV OU2.2p8 and p23-injected birds displayed plaques consistent with MDV infection 3 5 days postcultivation. In contrast, and as expected, no plaques were observed on CEF monolayers FIG. 7. PCR amplification of an 850-bp MDV pp38 gene. (A) DNA cocultivated with PBLs from chickens injected with isolated from kidneys of birds inoculated with CHCC-OU2 (lanes 2 and 3), MDV OU2.2p8 (lanes 4 and 5), and MDV OU2.2p23 (lanes 6 and 7) CHCC-OU2 cells. The disseminated viremia is an indicawas used as template for PCR amplification of the 132-bp DR region tion that MDV OU2 cells induced a systematic MDV infec- as described under Materials and Methods. DNA isolated from tion in the inoculated birds and the virus was not local- Md11p15 and p28/cef (lanes 8 and 9) served as positive controls for ized to the site of inoculation (intraperitoneal cavity). DNA isolation and PCR reaction. (B) DNA isolated from kidneys of birds inoculated with CHCC-OU2 (lanes 2 and 3), MDV OU2.2p8 (lanes 4 and 5), and MDV OU2.2p23 (lanes 6 and 7) was used as template for DISCUSSION PCR amplification of 850 bp as described under Materials and Methods. DNA isolated from Md11p15 and p28/cef (lanes 8 and 9) served The MDV strain Md11-infected fibroblastic cell lines as positive controls for DNA isolation and PCR reaction. A 1-kb ladder MDV OU2.1 and OU2.2 (Abujoub, and Coussens, 1995) marker (Life Technologies) (lane 1) was used for size comparisons. are permissive for MDV replication. In contrast with pri- Sizes of selected fragments are indicated at the left. mary CEF and DEF, these cell lines have an unlimited life span and have been in continuous culture for 2 years livers of dissected birds. In contrast, chickens inoculated under conditions similar to that used for primary fibro- with parental CHCC-OU2 cells showed no clinical signs blasts. Growth characteristics and appearance of MDV of MD. Control birds inoculated with CHCC-OU2 cells OU2 cells are indistinguishable from the parental CHCC- were euthanized at 16 weeks postinoculation. Necropsy OU2-immortalized fibroblastic cell line (Ogura and Fujiwara, revealed no signs of bursal atrophy, splenomegaly, or 1987). MDV OU2 cells display characteristics conrevealed tumor formation. sistent with MDV genomes existing in a latent state, similar To confirm that injected birds harbor MDV genomes, to that observed in MD-lymphoblastoid cell lines. total cellular DNA isolated from kidneys was used as However, the appearance of distinct plaques in confluent template for PCR amplification using a primer set specific cell monolayers is more consistent with a lytic infection for the 132-bp DR sequence (Silva, 1992) and for amplifi- (Abujoub, and Coussens, 1995). Thus, MDV OU2 cells cation of an 850-bp segment of the pp38 gene (Abujoub appear to switch from a latent to lytic infection, coinci- and Coussens, 1995). No PCR products were detected dent with cells reaching confluence. Both MDV OU2 cell when DNA isolated from two separate control bird kidneys lines are capable of transferring MDV infection to CEF was used as template (Fig. 7A, lanes 2 and 3). in culture and inducing clinical signs of MD in suscepti- In contrast, amplification products consistent with MDV ble birds. Virus yields from these cell lines is comparable sequences containing two to four copies of the 132-bp to or greater than yields produced by CEF cultures. Thus, DR sequence were amplified from MDV OU2.2p8-inoculated CHCC-OU2- and MDV-infected derivatives provide an exusing bird kidneys (Fig. 7A, lanes 4 and 5). PCR reactions cellent system for cultivation of MDV vaccine viruses, DNA isolated from MDV OU2.2p23-injected birds production of MDV mutants (with coordinated expression resulted in amplification of products consistent with two of essential genes), and a model for herpesvirus latency/ to five copies of the 132-bp DR sequence (Fig. 7A, lanes reactivation. 6 and 7). Md11p15/CEF DNA was used as a positive The overall aims of this study were to determine the control for PCR reactions (Fig. 7A, lane 8). PCR reactions status of MDV genomes in MDV OU2 cells and to exam-
11 MAREK S DISEASE IN A LATENT STATE IN FIBROBLASTIC CELLS 319 TABLE 1 that MDV LATs may serve to down regulate expression Summary of IIFA and RT-PCR Results of MDV ICP4. The factors responsible for the switch from a latent Expressed gene state in subconfluent cultures to a lytic infection in confluent monolayers is not understood. Generally, factors im- Cell type gb gi gd ge ICP4 LATs portant in determining latent or lytic infection cycles in MSB-1 ND ND 0 { / // many herpes viruses are cellular activators or repressors Md11/CEF // // // // // 0 which in turn activate or repress viral gene products Subconfluent MDV OU { // responsible for determining the pathway of viral infection Confluent MDV OU2 // // // // // { (Garcia-Blanco and Cullen, 1991). MDV OU2 cells are CHCC-OU similar to their parental CHCC-OU2 cells in being contact Note. ND, not determined; {, low level of expression; /, normal inhibited. It is possible that changes in cell growth assolevel of expression; //, high level of expression; 0, not detected. ciated with confluence and contact inhibition act to trig- ger reactivation of MDV leading to expression of the full complement of MDV genes and lytic replication. It is ine the effect of in vitro passage on stability of MDV tempting to speculate that such a trigger may be a regu- genomes and virus pathogenesis within MDV OU2 cells. lator (repressor) of MDV LAT expression. As in CEF cells, To achieve our first goal, we used IIFA staining and RT- lytic replication of MDV in confluent MDV OU2 cell cul- PCR to examine differential gene expression in subcon- tures appears to be of the productive restrictive class fluent versus confluent MDV OU2 cells. Our results, summarized with no infectious virions released into the culture fluid in Table 1, support the hypothesis that MDV (Abujoub and Coussens, unpublished observations) is in a latent state in MDV OU2 cells, similar to MDV Serial in vitro passage of virulent MDV strains on CEF lymphoblastoid cell lines. IIFA staining and RT-PCR data cells results in attenuation and loss of oncogenicity clearly demonstrate that expression of structural glycoproteins which is correlated with het region expansion. In this (glycoproteins B, I, D, and E) which are encoded report, we provide evidence that a highly variable MDV by late genes and are mainly expressed in lytically infected genome region is stabilized in MDV OU2 cells, and after cells, occurs only in confluent cell monolayers. over 2 years of continuous in vitro culture MDV from MDV This pattern of protein expression is consistent with MDV OU2 cells is still oncogenic. PCR analyses showed that genomes existing in a latent state in subconfluent MDV the number of 132-bp DR sequences did not change OU2 cells. MDV appears to be reactivated and a full cycle significantly in MDV genomes after more than 30 in vitro of lytic infection initiated after cells reach confluence. passages. Whereas, Md11 passed in CEF culture for Cantello et al. (1994) and Li et al. (1994) separately the same number of passages (Md11p48/CEF) exhibited reported the identification of transcripts (MDV LATs) that significant expansion of the 132-bp DR region. The number map antisense to the ICP4 homolog gene of MDV. MDV of 132-bp DR sequences in oncogenic MDV strains LATs are expressed at a significantly higher level in is typically between 2 and 5 copies, whereas the average lymphoblastoid cell lines (MSB-1 and RPL1) than in lytically number in strains attenuated by in vitro cultivation on infected cells. Based on these findings, it was spec- CEF cells is three to greater than seven copies. These ulated that MDV LATs may play a role in maintenance results suggest that, as with MDV lymphoblastoid cell of latency by negatively regulating MDV ICP4 expression lines MDV OU2 cell lines tend to stabilize the MDV genome. (Cantello et al., 1994; Li et al., 1994). Using RT-PCR, we However, CHCC-OU2 cells are nontumorigenic examined the level of expression of MDV LATs in MDV and thus may offer an attractive alternative for production OU2 cells from subconfluent cultures versus confluent of recombinant MDV which may be unstable in CEF culcultures. Expression of MDV LATs in subconfluent MDV tures. OU2 cells was comparable to that observed in MSB-1 Marek s disease can be induced by injection of MDVcells. MDV LAT expression is down regulated when cells infected cells into susceptible birds. Tumor incidence become confluent and was not detected or detected at can reach 100%, but is dependent on various natural and a very low levels when cells were allowed to display experimental conditions and factors, for example, virus plaques characteristic of lytic MDV infection. When the strain and dose, site of injection, age at primary expo- level of MDV ICP4 expression was examined, the level sure, and the genetic background of the birds (Calnek of ICP4 expression was inversely proportional to the level and Witter, 1991). Oncogenic serotype 1 MDV-infected of MDV LAT expression. The similarity between MDV CEF, DEF, and CKC cells as well as some producer MDlymphoblastoid LAT/ICP4 expression patterns in subconfluent MDV OU2 cell lines such as MSB-1, CU36, and cells to MSB-1 cells strongly supports our hypothesis CU41 are able to induce MD in susceptible birds. Similarly, that MDV exists in a latent state in MDV OU2 cells in MDV OU2 cells are capable of inducing MD in subconfluent cultures. In addition, our results confirm the susceptible birds. With serial in vitro passage, in CEF, hypothesis of Cantello et al. (1994) and Li et al. (1994) DEF, and CKC cells oncogenic MDV strains are rapidly
12 320 ABUJOUB AND COUSSENS attenuated. Nononcogenic MDV strains tend to lose povirus-homologous, (MDV) unique short region: alpha herpesvirus-homologous, fowlpox and MDV-specific genes. Virology 206, tency as vaccines with continuous in vitro cultivation. Calnek, B. W., and Witter, R. L. (1991). In Diseases of Poultry: Marek s Propagation in lymphoblastoid cells can stabilize viral Disease (B. W. Calnek, H. J. Barnes, C. W. Beard, W. M. Reid, and genomes and preserve the initial character of resident H. W. Yoder, Jr., Eds.), pp Iowa State Univ. Press, Ames, MDV strains. However, Lymphoblastoid cells are onco- IA. genic and have not been found tp harbor common vacinduced Calnek, B. W., Shek, W. R., and Schat, K. A. (1981). Spontaneous and herpesvirus genome expression in Marek s disease tumor cine strains of MDV. cell lines. Infect. Immunit. 34, Since, PCR analyses showed that the 132-bp DR re- Calnek, B. W., Murthy, K. K., and Schat, K. A. (1978). Establishment of gion of MDV was stabilized during prolonged cultivation Marek s disease lymphoblastoid cell lines from transplantable versus in MDV OU2 cells. It was important to determine if pro- primary lymphomas. Int. J. Cancer 21, longed cultivation of MDV in CHCC-OU2 cells could preepithelium: Calnek, B. W., Adldinger, H. K., and Kahn, D. E. (1970). Feather follicle A source of enveloped and infectious cell-free herpesvi- serve oncogenicity. Susceptible birds inoculated with rus from Marek s disease. Avian Dis. 14, MDV OU2.2p23 developed signs of MD, including severe Cantello, J. L., Anderson, A. S., and Morgan, R. W. (1994). Identification weight loss and paralysis similar to those inoculated with of latency-associated transcripts that map anitsense to the ICP4 MDV OU2.2p8. PCR amplification of DNA isolated from homolog gene of Marek s disease virus. J. Virol. 68, kidneys of infected birds demonstrated systemic presterminations Chen, X., and Velicer, L. F. (1991). Multiple bidirectional initiations and of transcription in Marek s disease virus long repeat ence of MDV and indicated that infectious virions conregions. J. Virol. 65, tained between two and five copies of the MDV 132-bp Churchill, A. E., and Chubb, R. C. (1969). The attenuation, with loss of DR sequences. Our PCR and in vivo data indicates that oncogenicity, of the herpes-type virus of Marek s disease (strain MDV genomes are stabilized within MDV OU2 cell lines, HPRS-16) on passage in cell culture. J. Gen. Virol. 4, similar to that seen in lymphoblastoid cell lines. However, Countryman, J., and Miller, G. (1985). Activation of expression of latent Epstein-Barr herpes virus after gene transfer with a small cloned unlike lymphoblastoid cell lines, the parental CHCC-OU2 subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA cells are nononcogenic (Abujoub and Coussens, 1995; 82, Ogura and Fujiwara, 1987). No evidence of tumor forma- Coussens, P. M., and Velicer, L. F. (1988). Structure and complete nucleotide tion or any illness in birds inoculated with CHCC-OU2 sequence of the Marek s disease herpesvirus gp parental cells up to 16 weeks postinoculation has been gene. J. Virol. 62, found. Cui, Z., Yan, D., and Lee, L. F. (1990). Marek s disease virus gene clones encoding virus-specific phosphorylated polypeptides and serological This report shows, for the first time, the presence of characterization of fusion proteins. Virus Genes. 4, latent MDV genomes in a sustainable fibroblast cell line. Feldman, L. T. (1991). The molecular biology of herpes simplex virus Establishment and characterization of these cell lines latency. In Herpesvirus Transcription and Its Regulation (E. K. will reduce many of the difficulties associated with MDV Wagner, Ed.), pp CRC Press Boca Raton, FL. Flemington, E., and Speck, S. H. (1990). Epstein-Barr virus BZLF1 trans experimentation and vaccine production. For example, activator induce the promoter of a cellular cognate gene. J. Virol. 64, studying differential gene expression between subcon fluent and confluent MDV OU2 cells will help in the Fukuchi, K., Tanaka, A., Schierman, L. W., Witter, R. L., and Nonoyama, search for key switches in MDV latency. A key difference M. (1985). The structure of Marek disease virus DNA: the presence noted in the present report between latent and lytic MDV of a unique expansion in non-pathogenic viral DNA. Proc. Natl. Acad. Sci. USA 82, OU2 infections is down regulation of MDV LAT expres- Garcia-Blanco, M. A., and Cullen, B. R. (1991). Molecular basis of lasion coincident with culture confluence. Regulation of tency in pathogenic human viruses. Science 254, these transcripts in subconfluent and confluent MDV Glaubiger, C., Nazerian, K., and Velicer, L. F. (1983). Marek s disease OU2 cells is currently under investigation. Stabilization herpesvirus. IV. Molecular characterization of Marek s disease herpesvirus of MDV genomes within MDV OU2 cell lines will make A antigen. J. Virol. 45, this system ideal for generation of mutants and recombi- Harlow, E., and Lane, D. (1988). Antibodies: A Laboratory Manual, pp Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. nant viruses. In addition, propagation of MDV vaccine Hong, Y., and Coussens, P. M. (1994). Identification of an immediatestrains on CHCC-OU2 cells, unlike on CEF, offers the early gene in the Marek s disease virus long internal repeat region possibility that continuous passage will not offset effi- which encodes a unique 14-Kilodalton polypeptide. J. Virol. 68, 3593 cacy of MDV vaccines Hong, Y., Frame, M., and Coussens, P. M. (1995). A 14-kDa immediateearly phosphoprotein is specifically expressed in cells infected with REFERENCES oncogenic Marek s disease virus strains and their attenuated derivatives. Virology 206, Abujoub, A., and Coussens, P. M. (1995). Development of a sustainable Kishi, M., Bradley, G., Jessip, J., Tanaka, A., and Nonoyama, M. (1991). chick cell line infected with Marek s disease virus. Virology 214, Inverted repeat of Marek s disease virus DNA possess a structure similar to that of the a sequence of herpes simplex virus DNA and Akiyama, Y., and Kato, S. (1974). Two cell lines from lymphomas of contain host cell telomere sequences. J. Virol. 65, Marek s disease. Biken J. 17, Lee, S. P. (1994). Control mechanisms of Epstein Barr virus persis- Akiyama, Y., Kato, S., and Iwa, N. (1973). Continuous cell culture from tence. Semin. Virol. 5, lymphoma of Marek s disease. Biken J. 16, Li, D. S., Pastorek, J., Zelnik, V., Smith, G., and Ross, L. J. N. (1994). Brunovskis, P., and Velicer, L. F. (1995). The Marek s disease virus Identification of novel transcripts complementary to the Marek s dis-
13 MAREK S DISEASE IN A LATENT STATE IN FIBROBLASTIC CELLS 321 ease virus homologue of the ICP4 gene of herpes simplex virus. J. A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Gen. Virol. 75, Spring Harbor, N.Y. Maotani, K., Kanamori, K., Ueda, S., and Hirai, K. (1986). Amplification Schat, K. A., Buckmaster, A., and Ross, L. N. J. (1989). Partial transcripof a tandem direct repeat within inverted repeats of Marek s disease tion map of Marek s disease herpesvirus in lytically infected cells virus DNA during serial in vitro passage. J. Virol. 58, and lymphoblastoid cell lines. Int. J. Cancer 44, Maray, T., Malkinson, M., and Becker, Y. (1988). RNA transcripts of Schat, K. A., Chen, C.-L. H., Calnek, B. W., and Char, D. (1991). Transfor- Marek s disease virus (MDV) serotype-1 in infected and transformed mation of T-lymphocytes subsets by Marek s disease herpesvirus. J. Virol. 65, cells. Virus Genes 2, Shek, W. R., Calnek, B. W., Schat, K. A., and Chen, C. H. (1983). Charac- Mckie, E. A., Ubukata, E., Hasegawa, S., Zhang, S., Nonoyama, M., and terization of Marek s disease virus-infected lymphocytes: discrimina- Tanaka, A. (1995). The transcripts from the sequences flanking the tion between cytolytically and latently infected cells. J. Natl. Cancer short component of Marek s disease virus during latent infection Inst. 70, from a unique family of 3 -coterminal RNAs. J. Virol. 69, Silva, R. F. (1992). Differentiation of pathogenic and non-pathogenic Nazerian, K., Stephens, E. A., Sharma, J. M., Lee, L. F., Galitis, M., and serotype 1 Marek s disease virus (MDV) by the polymerase chain Witter, R. L. (1977). A nonproducer T lymphoblastoid cell line from reaction amplification of a tandem direct repeats within the MDV Marek s disease transplantable tumor (JMV). Avian Dis. 21, genome. Avian Dis. 36, Nazerian, K., and Witter, R. L. (1975). Properties of a chicken lymph- Silva, R. F., and Lee, L. F. (1984). Monoclonal antibody-mediated immunoprecipitation oblastoid cell line from Marek s disease tumor. J. Natl. Cancer Inst. of proteins from cells infected with Marek s disease 54, virus or turkey herpesvirus. Virology 136, Ogura, H., and Fujiwara, T. (1987). Establishment and characterization Silva, R. F., and Witter, R. L. (1985). Genomic expansion of Marek s of a virus-free chick cell line. Acta Med. Okayama 41, disease virus DNA is associated with serial in vitro passage. J. Virol. Payne, L. N., Howes, K., Rennie, M., Bumstead, J. M., and Kidd, W. 54, (1981). Use of an agar culture technique for establishing lymphoid Silver, S., Tanaka, A., and Nonoyama, M. (1979). Transcription of the cell lines from Marek s disease lymphoma. Int. J. Cancer 28, 757 Marek s disease virus genome in a nonproductive chicken lymph- oblastoid cell line. Virology 93, Sugaya, K., Bradley, G., Nonoyama, M., and Tanaka, A. (1990). Latent Powell, P. C., Payne, L. N., Frazier, J. M., and Rennie, M. (1974). T lymphtranscripts of Marek s disease virus are clustered in the short and oblastoid cell lines from Marek s disease lymphomas. Nature 251, long repeat regions. J. Virol. 64, Tardef, G. N., and McQueen, J. M. (1993). South Eastern Organ Pro- Priola, S. A., Gustafson, D. P., Wagner, E. K., and Stevens, J. G. (1990). curement Foundation (Seopf) Manual, 3rd ed., Vol. 14, Section B, A major portion of the latent pseudorabies virus genome is tran- pp. 1 25, Richmond, VA. scribed in terminal ganglia of pigs. J. Virol. 64, Witter, R. L., Nazerian, K., and Solmon, J. J. (1972). Studies on the in vivo Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: replication of turkey herpesvirus. J. Natl. Cancer Inst. 49,
Development of a Sustainable Chick Cell Line Infected with Marek s Disease Virus
 VIROLOGY 214, 541 549 (1995) Development of a Sustainable Chick Cell Line Infected with Marek s Disease Virus AMIN ABUJOUB and PAUL M. COUSSENS 1 Molecular Virology Laboratory, Departments of Animal Science
VIROLOGY 214, 541 549 (1995) Development of a Sustainable Chick Cell Line Infected with Marek s Disease Virus AMIN ABUJOUB and PAUL M. COUSSENS 1 Molecular Virology Laboratory, Departments of Animal Science
Identification and Molecular Cloning of Marek s Disease Virus Type 3 Glycoprotein M and Glycoprotein K
 International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 1, Number 1 (2010), pp. 57-64 International Research Publication House http://www.irphouse.com Identification and Molecular
International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 1, Number 1 (2010), pp. 57-64 International Research Publication House http://www.irphouse.com Identification and Molecular
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection
 Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Determination of the temporal pattern and importance of BALF1 expression in Epstein-Barr viral infection Melissa Mihelidakis May 6, 2004 7.340 Research Proposal Introduction Apoptosis, or programmed cell
Corporation obtaining approval, the name of its representative, and the address of its main office
 Corporation obtaining approval, the name of its representative, and the address of its main office Name: The Chemo-Sero-Therapeutic Research Institute Applicant: Akinobu Funatsu, Director General Address:
Corporation obtaining approval, the name of its representative, and the address of its main office Name: The Chemo-Sero-Therapeutic Research Institute Applicant: Akinobu Funatsu, Director General Address:
Transcription of the Marek's Disease Virus Genome in Virus-
 JOURNAL OF VIROLOGY, Apr. 1979, p. 84-89 0022-538X/79/04-0084/06$02.00/0 Vol. 30, No. 1 Transcription of the Marek's Disease Virus Genome in Virus- Induced Tumors SANDRA SILVER, MARY SMITH, AND MEIHAN
JOURNAL OF VIROLOGY, Apr. 1979, p. 84-89 0022-538X/79/04-0084/06$02.00/0 Vol. 30, No. 1 Transcription of the Marek's Disease Virus Genome in Virus- Induced Tumors SANDRA SILVER, MARY SMITH, AND MEIHAN
Differential susceptibility to Marek s disease is associated with differences in number, but not phenotype or location, of pp38 M lymphocytes
 Journal of General Virology (1998), 79, 2795 2802. Printed in Great Britain...... Differential susceptibility to Marek s disease is associated with differences in number, but not phenotype or location,
Journal of General Virology (1998), 79, 2795 2802. Printed in Great Britain...... Differential susceptibility to Marek s disease is associated with differences in number, but not phenotype or location,
hemagglutinin and the neuraminidase genes (RNA/recombinant viruses/polyacrylamide gel electrophoresis/genetics)
 Proc. Natl. Acad. Sci. USA Vol. 73, No. 6, pp. 242-246, June 976 Microbiology Mapping of the influenza virus genome: Identification of the hemagglutinin and the neuraminidase genes (RNA/recombinant viruses/polyacrylamide
Proc. Natl. Acad. Sci. USA Vol. 73, No. 6, pp. 242-246, June 976 Microbiology Mapping of the influenza virus genome: Identification of the hemagglutinin and the neuraminidase genes (RNA/recombinant viruses/polyacrylamide
Identification of Virus-specific Polypeptides by Monoclonal Antibodies against Serotype 2 Marek's Disease Virus
 J. gen. Virol. (1989), 70, 2563-2571. Printed in Great Britain 2563 Key words: MDV2/MAbs/polypeptides Identification of Virus-specific Polypeptides by Monoclonal Antibodies against Serotype 2 Marek's Disease
J. gen. Virol. (1989), 70, 2563-2571. Printed in Great Britain 2563 Key words: MDV2/MAbs/polypeptides Identification of Virus-specific Polypeptides by Monoclonal Antibodies against Serotype 2 Marek's Disease
Herpesviruses. Virion. Genome. Genes and proteins. Viruses and hosts. Diseases. Distinctive characteristics
 Herpesviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped icosahedral capsid (T=16), diameter 125 nm Diameter of enveloped virion 200 nm Capsid
Herpesviruses Virion Genome Genes and proteins Viruses and hosts Diseases Distinctive characteristics Virion Enveloped icosahedral capsid (T=16), diameter 125 nm Diameter of enveloped virion 200 nm Capsid
Replication kinetics of Marek s disease vaccine virus in feathers and lymphoid tissues using PCR and virus isolation
 Journal of General Virology (2005), 86, 2989 2998 DOI 10.1099/vir.0.81299-0 Replication kinetics of Marek s disease vaccine virus in feathers and lymphoid tissues using PCR and virus isolation Susan J.
Journal of General Virology (2005), 86, 2989 2998 DOI 10.1099/vir.0.81299-0 Replication kinetics of Marek s disease vaccine virus in feathers and lymphoid tissues using PCR and virus isolation Susan J.
Recommended laboratory tests to identify influenza A/H5 virus in specimens from patients with an influenza-like illness
 World Health Organization Recommended laboratory tests to identify influenza A/H5 virus in specimens from patients with an influenza-like illness General information Highly pathogenic avian influenza (HPAI)
World Health Organization Recommended laboratory tests to identify influenza A/H5 virus in specimens from patients with an influenza-like illness General information Highly pathogenic avian influenza (HPAI)
Attenuation of Marek s Disease Virus by Deletion of Open Reading Frame RLORF4 but Not RLORF5a
 JOURNAL OF VIROLOGY, Sept. 2005, p. 11647 11659 Vol. 79, No. 18 0022-538X/05/$08.00 0 doi:10.1128/jvi.79.18.11647 11659.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Attenuation
JOURNAL OF VIROLOGY, Sept. 2005, p. 11647 11659 Vol. 79, No. 18 0022-538X/05/$08.00 0 doi:10.1128/jvi.79.18.11647 11659.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved. Attenuation
EBV infection B cells and lymphomagenesis. Sridhar Chaganti
 EBV infection B cells and lymphomagenesis Sridhar Chaganti How EBV infects B-cells How viral genes influence the infected B cell Differences and similarities between in vitro and in vivo infection How
EBV infection B cells and lymphomagenesis Sridhar Chaganti How EBV infects B-cells How viral genes influence the infected B cell Differences and similarities between in vitro and in vivo infection How
In Vitro and In Vivo Studies with Epstein-Barr
 A n n a l s o f C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, No. 6 Copyright 1973, Institute for Clinical Science In Vitro and In Vivo Studies with Epstein-Barr Virus (EBV)-------A Review
A n n a l s o f C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, No. 6 Copyright 1973, Institute for Clinical Science In Vitro and In Vivo Studies with Epstein-Barr Virus (EBV)-------A Review
Variation in the HindlII Restriction Fragments of DNA from the Chinese Tian Tan Strain of Vaccinia Virus
 J. gen. irol. (1985), 66, 1819-1823. Printed in Great Britain 1819 Key words: vaccinia virus~vaccine~restriction Jragrnent variation ariation in the Hindl Restriction Fragments of DNA from the Chinese
J. gen. irol. (1985), 66, 1819-1823. Printed in Great Britain 1819 Key words: vaccinia virus~vaccine~restriction Jragrnent variation ariation in the Hindl Restriction Fragments of DNA from the Chinese
Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection. Masoud Mardani M.D,FIDSA
 Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection Masoud Mardani M.D,FIDSA Shahidhid Bh BeheshtiMdi Medical lui Universityit Cytomegalovirus (CMV), Epstein Barr Virus(EBV), Herpes
Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection Masoud Mardani M.D,FIDSA Shahidhid Bh BeheshtiMdi Medical lui Universityit Cytomegalovirus (CMV), Epstein Barr Virus(EBV), Herpes
Yamaguchi University School of Medicine, Kogushi, Ube, Yamaguchi. growth in low serum, anchorage-independent growth in soft
 JOURNAL OF VIROLOGY, Sept. 1994, p. 6069-6073 Vol. 68, No. 9 0022-538X/94/$04.00+0 Copyright 1994, American Society for Microbiology Isolation of Epstein-Barr Virus (EBV)-Negative Cell Clones from the
JOURNAL OF VIROLOGY, Sept. 1994, p. 6069-6073 Vol. 68, No. 9 0022-538X/94/$04.00+0 Copyright 1994, American Society for Microbiology Isolation of Epstein-Barr Virus (EBV)-Negative Cell Clones from the
Lymphomagenesis in Marek's disease
 Avian Pathology ISSN: 0307-9457 (Print) 1465-3338 (Online) Journal homepage: http://www.tandfonline.com/loi/cavp20 Lymphomagenesis in Marek's disease Bruce W. Calnek To cite this article: Bruce W. Calnek
Avian Pathology ISSN: 0307-9457 (Print) 1465-3338 (Online) Journal homepage: http://www.tandfonline.com/loi/cavp20 Lymphomagenesis in Marek's disease Bruce W. Calnek To cite this article: Bruce W. Calnek
FEATHER TIP MONITORING OF MAREK S DISEASE VIRUS IN EXPERIMENTAL AND COMMERCIAL SETTINGS. Milos Markis
 FEATHER TIP MONITORING OF MAREK S DISEASE VIRUS IN EXPERIMENTAL AND COMMERCIAL SETTINGS by Milos Markis A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements
FEATHER TIP MONITORING OF MAREK S DISEASE VIRUS IN EXPERIMENTAL AND COMMERCIAL SETTINGS by Milos Markis A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements
CHARACTERISATION OF INFECTIOUS BURSAL DISEASE VIRUS AND DETERMINATION OF POSSIBLE VACCINE STRAIN(S) IN KENYA
 CHARACTERISATION OF INFECTIOUS BURSAL DISEASE VIRUS AND DETERMINATION OF POSSIBLE VACCINE STRAIN(S) IN KENYA Investigator: Dr. Mutinda, W.U (BVM, MSc.) Supervisors: Prof. P.N Nyaga, BVM, MSc, PhD Prof.
CHARACTERISATION OF INFECTIOUS BURSAL DISEASE VIRUS AND DETERMINATION OF POSSIBLE VACCINE STRAIN(S) IN KENYA Investigator: Dr. Mutinda, W.U (BVM, MSc.) Supervisors: Prof. P.N Nyaga, BVM, MSc, PhD Prof.
الحترمونا من خري الدعاء
 الحترمونا من خري الدعاء Instructions for candidates The examination consists of 30 multiple choice questions, each divided into 5 different parts. Each part contains a statement which could be true or
الحترمونا من خري الدعاء Instructions for candidates The examination consists of 30 multiple choice questions, each divided into 5 different parts. Each part contains a statement which could be true or
The humoral immune responses to IBV proteins.
 The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
The Infectious Cycle. Lecture 2 Biology W3310/4310 Virology Spring You know my methods, Watson --SIR ARTHUR CONAN DOYLE
 The Infectious Cycle Lecture 2 Biology W3310/4310 Virology Spring 2016 You know my methods, Watson --SIR ARTHUR CONAN DOYLE The Infectious Cycle Virologists divide the infectious cycle into steps to facilitate
The Infectious Cycle Lecture 2 Biology W3310/4310 Virology Spring 2016 You know my methods, Watson --SIR ARTHUR CONAN DOYLE The Infectious Cycle Virologists divide the infectious cycle into steps to facilitate
EBV Infection and Immunity. Andrew Hislop Institute for Cancer Studies University of Birmingham
 EBV Infection and Immunity Andrew Hislop Institute for Cancer Studies University of Birmingham EBV Introduction Large ds DNA virus Spread by saliva contact Lifelong infection Predominantly B-lymphotropic
EBV Infection and Immunity Andrew Hislop Institute for Cancer Studies University of Birmingham EBV Introduction Large ds DNA virus Spread by saliva contact Lifelong infection Predominantly B-lymphotropic
Coronaviruses cause acute, mild upper respiratory infection (common cold).
 Coronaviruses David A. J. Tyrrell Steven H. Myint GENERAL CONCEPTS Clinical Presentation Coronaviruses cause acute, mild upper respiratory infection (common cold). Structure Spherical or pleomorphic enveloped
Coronaviruses David A. J. Tyrrell Steven H. Myint GENERAL CONCEPTS Clinical Presentation Coronaviruses cause acute, mild upper respiratory infection (common cold). Structure Spherical or pleomorphic enveloped
Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR
 Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR Pages with reference to book, From 305 To 307 Irshad N. Soomro,Samina Noorali,Syed Abdul Aziz,Suhail Muzaffar,Shahid
Gastric Carcinoma with Lymphoid Stroma: Association with Epstein Virus Genome demonstrated by PCR Pages with reference to book, From 305 To 307 Irshad N. Soomro,Samina Noorali,Syed Abdul Aziz,Suhail Muzaffar,Shahid
VIRUSES AND CANCER Michael Lea
 VIRUSES AND CANCER 2010 Michael Lea VIRAL ONCOLOGY - LECTURE OUTLINE 1. Historical Review 2. Viruses Associated with Cancer 3. RNA Tumor Viruses 4. DNA Tumor Viruses HISTORICAL REVIEW Historical Review
VIRUSES AND CANCER 2010 Michael Lea VIRAL ONCOLOGY - LECTURE OUTLINE 1. Historical Review 2. Viruses Associated with Cancer 3. RNA Tumor Viruses 4. DNA Tumor Viruses HISTORICAL REVIEW Historical Review
Chapter 6- An Introduction to Viruses*
 Chapter 6- An Introduction to Viruses* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. 6.1 Overview of Viruses
Chapter 6- An Introduction to Viruses* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. 6.1 Overview of Viruses
Department of Animal and Poultry Sciences October 16, Avian Leukosis Virus Subgroup J. Héctor L. Santiago ABSTRACT
 Department of Animal and Poultry Sciences October 16, 2000 Avian Leukosis Virus Subgroup J Héctor L. Santiago ABSTRACT The avian leukosis viruses (ALV) are a class of retroviruses belonging to the avian
Department of Animal and Poultry Sciences October 16, 2000 Avian Leukosis Virus Subgroup J Héctor L. Santiago ABSTRACT The avian leukosis viruses (ALV) are a class of retroviruses belonging to the avian
Research Update: Avian Disease & Oncology Lab (ADOL) and SEPRL Endemic Poultry Virus Diseases (EPVD)
 Research Update: Avian Disease & Oncology Lab (ADOL) and SEPRL Endemic Poultry Virus Diseases (EPVD) John Dunn, Hans Cheng, Mohammad Heidari, Huanmin Zhang, Taejoong Kim, Stephen Spatz, Qingzhong Yu USDA-ARS-USPNRC
Research Update: Avian Disease & Oncology Lab (ADOL) and SEPRL Endemic Poultry Virus Diseases (EPVD) John Dunn, Hans Cheng, Mohammad Heidari, Huanmin Zhang, Taejoong Kim, Stephen Spatz, Qingzhong Yu USDA-ARS-USPNRC
Lecture 2: Virology. I. Background
 Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Lecture 2: Virology I. Background A. Properties 1. Simple biological systems a. Aggregates of nucleic acids and protein 2. Non-living a. Cannot reproduce or carry out metabolic activities outside of a
Activation of Gene Expression by Human Herpes Virus 6
 Activation of Gene Expression by Human Herpes Virus 6 M. E. M. Campbell and S. McCorkindale 1 Introduction Human herpes virus type 6 (HHV-6) was first detected by Salahuddin et al. [6] and has been isolated
Activation of Gene Expression by Human Herpes Virus 6 M. E. M. Campbell and S. McCorkindale 1 Introduction Human herpes virus type 6 (HHV-6) was first detected by Salahuddin et al. [6] and has been isolated
Polyomaviridae. Spring
 Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
Polyomaviridae Spring 2002 331 Antibody Prevalence for BK & JC Viruses Spring 2002 332 Polyoma Viruses General characteristics Papovaviridae: PA - papilloma; PO - polyoma; VA - vacuolating agent a. 45nm
The Threat of Marek s Disease Virus Is Expanding
 The Threat of Marek s Disease Virus Is Expanding The virus responsible for this lymphoproliferative disease is growing more virulent and evading control by available vaccines Kyle S. MacLea and Hans H.
The Threat of Marek s Disease Virus Is Expanding The virus responsible for this lymphoproliferative disease is growing more virulent and evading control by available vaccines Kyle S. MacLea and Hans H.
Pathogenicity and Antigenicity of Clones from Strains of Marek's Disease Virus and the Herpesvirus of Turkeys1
 INFEC11ON AND IMMUNITY, Feb. 1971, p. 295-33 Copyright 1971 American Society for Microbiology Vol. 3, No. 2 Printed in US.A. Pathogenicity and Antigenicity of Clones from Strains of Marek's Disease Virus
INFEC11ON AND IMMUNITY, Feb. 1971, p. 295-33 Copyright 1971 American Society for Microbiology Vol. 3, No. 2 Printed in US.A. Pathogenicity and Antigenicity of Clones from Strains of Marek's Disease Virus
CHAPTER 4 RESULTS. showed that all three replicates had similar growth trends (Figure 4.1) (p<0.05; p=0.0000)
 CHAPTER 4 RESULTS 4.1 Growth Characterization of C. vulgaris 4.1.1 Optical Density Growth study of Chlorella vulgaris based on optical density at 620 nm (OD 620 ) showed that all three replicates had similar
CHAPTER 4 RESULTS 4.1 Growth Characterization of C. vulgaris 4.1.1 Optical Density Growth study of Chlorella vulgaris based on optical density at 620 nm (OD 620 ) showed that all three replicates had similar
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
Product Manual. Omni-Array Sense Strand mrna Amplification Kit, 2 ng to 100 ng Version Catalog No.: Reactions
 Genetic Tools and Reagents Universal mrna amplification, sense strand amplification, antisense amplification, cdna synthesis, micro arrays, gene expression, human, mouse, rat, guinea pig, cloning Omni-Array
Genetic Tools and Reagents Universal mrna amplification, sense strand amplification, antisense amplification, cdna synthesis, micro arrays, gene expression, human, mouse, rat, guinea pig, cloning Omni-Array
Complete nucleotide sequence analysis of oncogenicity associated Gene pp38 of Serotype 1 Marek s disease virus isolates from India
 2018; SP1: 2197-2204 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2018; SP1: 2197-2204 P Suresh Assistant Professor, Department of Veterinary Microbiology, Veterinary College and Research Institute, Namakkal,
2018; SP1: 2197-2204 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2018; SP1: 2197-2204 P Suresh Assistant Professor, Department of Veterinary Microbiology, Veterinary College and Research Institute, Namakkal,
Virus and Prokaryotic Gene Regulation - 1
 Virus and Prokaryotic Gene Regulation - 1 We have discussed the molecular structure of DNA and its function in DNA duplication and in transcription and protein synthesis. We now turn to how cells regulate
Virus and Prokaryotic Gene Regulation - 1 We have discussed the molecular structure of DNA and its function in DNA duplication and in transcription and protein synthesis. We now turn to how cells regulate
Understanding Marek s Disease Immunity: A Continuing Challenge
 International Journal of Poultry Science 3 (1): 89-95, 2004 Asian Network for Scientific Information 2004 Understanding Marek s Disease Immunity: A Continuing Challenge K.A. Schat Unit of Avian Medicine,
International Journal of Poultry Science 3 (1): 89-95, 2004 Asian Network for Scientific Information 2004 Understanding Marek s Disease Immunity: A Continuing Challenge K.A. Schat Unit of Avian Medicine,
Chapter13 Characterizing and Classifying Viruses, Viroids, and Prions
 Chapter13 Characterizing and Classifying Viruses, Viroids, and Prions 11/20/2017 MDufilho 1 Characteristics of Viruses Viruses Minuscule, acellular, infectious agent having either DNA or RNA Cause infections
Chapter13 Characterizing and Classifying Viruses, Viroids, and Prions 11/20/2017 MDufilho 1 Characteristics of Viruses Viruses Minuscule, acellular, infectious agent having either DNA or RNA Cause infections
Isolation and identification of Mycoplasma gallisepticum in chickensbn from industrial farms in Kerman province
 Available online at http://www.ijabbr.com International journal of Advanced Biological and Biomedical Research Volume 2, Issue 1, 2014: 100-104 Isolation and identification of Mycoplasma gallisepticum
Available online at http://www.ijabbr.com International journal of Advanced Biological and Biomedical Research Volume 2, Issue 1, 2014: 100-104 Isolation and identification of Mycoplasma gallisepticum
Primary Isolation and Cultivation of Viruses
 Primary Isolation and Cultivation of Viruses Practical Medical Virology 450 MBIO 2017-18 01/10/2017 Amal Alghamdi Reham Alahmadi Dalia Alsrar 1 Diagnostic Virology Virus Isolation and Cultivation Viral
Primary Isolation and Cultivation of Viruses Practical Medical Virology 450 MBIO 2017-18 01/10/2017 Amal Alghamdi Reham Alahmadi Dalia Alsrar 1 Diagnostic Virology Virus Isolation and Cultivation Viral
Transcription and RNA processing
 Transcription and RNA processing Lecture 7 Biology W3310/4310 Virology Spring 2016 It is possible that Nature invented DNA for the purpose of achieving regulation at the transcriptional rather than at
Transcription and RNA processing Lecture 7 Biology W3310/4310 Virology Spring 2016 It is possible that Nature invented DNA for the purpose of achieving regulation at the transcriptional rather than at
THE CYTOPATHOGENIC ACTION OF BLUETONGUE VIRUS ON TISSUE CULTURES AND ITS APPLICATION TO THE DETECTION OF ANTIBODIES IN THE SERUM OF SHEEP.
 Onderstepoort Journal of Veterinary Research, Volume 27, Number 2, October, 1956. The Government Printer. THE CYTOPATHOGENIC ACTION OF BLUETONGUE VIRUS ON TISSUE CULTURES AND ITS APPLICATION TO THE DETECTION
Onderstepoort Journal of Veterinary Research, Volume 27, Number 2, October, 1956. The Government Printer. THE CYTOPATHOGENIC ACTION OF BLUETONGUE VIRUS ON TISSUE CULTURES AND ITS APPLICATION TO THE DETECTION
Investigations of Avian Leukosis Virus Subgroup J and Reticuloendotheliosis Virus Infections in Broiler Breeders in China
 Investigations of Avian Leukosis Virus Subgroup J and Reticuloendotheliosis Virus Infections in Broiler Breeders in China Cheng, Z.,* Zhang, H., Wang G., Liu, Q., Liu, J., Guo, H. and Zhou E. College of
Investigations of Avian Leukosis Virus Subgroup J and Reticuloendotheliosis Virus Infections in Broiler Breeders in China Cheng, Z.,* Zhang, H., Wang G., Liu, Q., Liu, J., Guo, H. and Zhou E. College of
BIT 120. Copy of Cancer/HIV Lecture
 BIT 120 Copy of Cancer/HIV Lecture Cancer DEFINITION Any abnormal growth of cells that has malignant potential i.e.. Leukemia Uncontrolled mitosis in WBC Genetic disease caused by an accumulation of mutations
BIT 120 Copy of Cancer/HIV Lecture Cancer DEFINITION Any abnormal growth of cells that has malignant potential i.e.. Leukemia Uncontrolled mitosis in WBC Genetic disease caused by an accumulation of mutations
Revaccination with Marek s Disease Vaccines Induces Productive Infection and Superior Immunity
 CLINICAL AND VACCINE IMMUNOLOGY, Feb. 2009, p. 184 193 Vol. 16, No. 2 1556-6811/09/$08.00 0 doi:10.1128/cvi.00201-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Revaccination
CLINICAL AND VACCINE IMMUNOLOGY, Feb. 2009, p. 184 193 Vol. 16, No. 2 1556-6811/09/$08.00 0 doi:10.1128/cvi.00201-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Revaccination
Animal hosts Natural host Laboratory animals Rabbits Mice Rats Hamsters Newborn or suckling rodents Animal models for viral pathogenesis 4 Growth of v
 Principles of Virology Department of Molecular Genetics & Microbiology Univ ersity of Florida, Gainesv ille, FL 1 Outline Virus cultivation Assay of viruses Virus genetics 2 Virus isolation Evidence of
Principles of Virology Department of Molecular Genetics & Microbiology Univ ersity of Florida, Gainesv ille, FL 1 Outline Virus cultivation Assay of viruses Virus genetics 2 Virus isolation Evidence of
In the Name of God. Talat Mokhtari-Azad Director of National Influenza Center
 In the Name of God Overview of influenza laboratory diagnostic technology: advantages and disadvantages of each test available Talat Mokhtari-Azad Director of National Influenza Center Tehran- Iran 1 1)
In the Name of God Overview of influenza laboratory diagnostic technology: advantages and disadvantages of each test available Talat Mokhtari-Azad Director of National Influenza Center Tehran- Iran 1 1)
Original Article Development and Sequence Analysis of a Cold-Adapted Strain of Influenza A/New Caledonia/20/1999(H1N1) Virus
 Iranian Journal of Virology 2011;5(4): 6-10 2011, Iranian Society for Virology Original Article Development and Sequence Analysis of a Cold-Adapted Strain of Influenza A/New Caledonia/20/1999(H1N1) Virus
Iranian Journal of Virology 2011;5(4): 6-10 2011, Iranian Society for Virology Original Article Development and Sequence Analysis of a Cold-Adapted Strain of Influenza A/New Caledonia/20/1999(H1N1) Virus
ISOLATION OF A SARCOMA VIRUS FROM A SPONTANEOUS CHICKEN TUMOR
 ISOLATION OF A SARCOMA VIRUS FROM A SPONTANEOUS CHICKEN TUMOR Shigeyoshi ITOHARA, Kouichi HIRATA, Makoto INOUE, Masanori Veterinary Pathology, Faculty of Agriculture, Yamaguchi University* HATSUOKA, and
ISOLATION OF A SARCOMA VIRUS FROM A SPONTANEOUS CHICKEN TUMOR Shigeyoshi ITOHARA, Kouichi HIRATA, Makoto INOUE, Masanori Veterinary Pathology, Faculty of Agriculture, Yamaguchi University* HATSUOKA, and
Characterization of Non-oncogenic Marek's Disease Virus-infected and Turkey Herpesvirus-infected Lymphocytes
 J. gen. Virol. (1982), 63, 333-341. Printed in Great Britain 333 Key words: turkey herpesvirus/mdv/lyraphocytes Characterization of Non-oncogenic Marek's Disease Virus-infected and Turkey Herpesvirus-infected
J. gen. Virol. (1982), 63, 333-341. Printed in Great Britain 333 Key words: turkey herpesvirus/mdv/lyraphocytes Characterization of Non-oncogenic Marek's Disease Virus-infected and Turkey Herpesvirus-infected
Viruses Tomasz Kordula, Ph.D.
 Viruses Tomasz Kordula, Ph.D. Resources: Alberts et al., Molecular Biology of the Cell, pp. 295, 1330, 1431 1433; Lehninger CD Movie A0002201. Learning Objectives: 1. Understand parasitic life cycle of
Viruses Tomasz Kordula, Ph.D. Resources: Alberts et al., Molecular Biology of the Cell, pp. 295, 1330, 1431 1433; Lehninger CD Movie A0002201. Learning Objectives: 1. Understand parasitic life cycle of
IDENTIFICATION OF MAREK S DISEASE VIRUS (MDV) AND HERPESVIRUS OF TURKEY (HVT) USING DUPLEX POLYMERASE CHAIN REACTION (PCR) APPROACH
 IDENTIFICATION OF MAREK S DISEASE VIRUS (MDV) AND HERPESVIRUS OF TURKEY (HVT) USING DUPLEX POLYMERASE CHAIN REACTION (PCR) APPROACH RISZA HARTAWAN Indonesian Research Center for Veterinary Science Jl.
IDENTIFICATION OF MAREK S DISEASE VIRUS (MDV) AND HERPESVIRUS OF TURKEY (HVT) USING DUPLEX POLYMERASE CHAIN REACTION (PCR) APPROACH RISZA HARTAWAN Indonesian Research Center for Veterinary Science Jl.
Identification of Microbes Lecture: 12
 Diagnostic Microbiology Identification of Microbes Lecture: 12 Electron Microscopy 106 virus particles per ml required for visualization, 50,000-60,000 magnification normally used. Viruses may be detected
Diagnostic Microbiology Identification of Microbes Lecture: 12 Electron Microscopy 106 virus particles per ml required for visualization, 50,000-60,000 magnification normally used. Viruses may be detected
Ali Alabbadi. Bann. Bann. Dr. Belal
 31 Ali Alabbadi Bann Bann Dr. Belal Topics to be discussed in this sheet: Particles-to-PFU Single-step and multi-step growth cycles Multiplicity of infection (MOI) Physical measurements of virus particles
31 Ali Alabbadi Bann Bann Dr. Belal Topics to be discussed in this sheet: Particles-to-PFU Single-step and multi-step growth cycles Multiplicity of infection (MOI) Physical measurements of virus particles
Epidemiology of a Herpesvirus of Turkeys: Possible Sources and Spread of Infection in Turkey Flocks
 INFECTION AND IMMUNITY, OCt. 1971, p. 356-361 Copyright @ 1971 American Society for Microbiology Vol. 4, No. 4 Printed in U.S.A. Epidemiology of a Herpesvirus of Turkeys: Possible Sources and Spread of
INFECTION AND IMMUNITY, OCt. 1971, p. 356-361 Copyright @ 1971 American Society for Microbiology Vol. 4, No. 4 Printed in U.S.A. Epidemiology of a Herpesvirus of Turkeys: Possible Sources and Spread of
Chapter 4 Cellular Oncogenes ~ 4.6 -
 Chapter 4 Cellular Oncogenes - 4.2 ~ 4.6 - Many retroviruses carrying oncogenes have been found in chickens and mice However, attempts undertaken during the 1970s to isolate viruses from most types of
Chapter 4 Cellular Oncogenes - 4.2 ~ 4.6 - Many retroviruses carrying oncogenes have been found in chickens and mice However, attempts undertaken during the 1970s to isolate viruses from most types of
CDC website:
 Hepatitis B virus CDC website: http://www.cdc.gov/ncidod/diseases/hepatitis/slideset/hep_b/slide_1.htm Relevance Key Features es of Hepatitis t B Virus 250 million people infected worldwide. In areas of
Hepatitis B virus CDC website: http://www.cdc.gov/ncidod/diseases/hepatitis/slideset/hep_b/slide_1.htm Relevance Key Features es of Hepatitis t B Virus 250 million people infected worldwide. In areas of
Transcription and RNA processing
 Transcription and RNA processing Lecture 7 Biology 3310/4310 Virology Spring 2018 It is possible that Nature invented DNA for the purpose of achieving regulation at the transcriptional rather than at the
Transcription and RNA processing Lecture 7 Biology 3310/4310 Virology Spring 2018 It is possible that Nature invented DNA for the purpose of achieving regulation at the transcriptional rather than at the
making LT protection safer and easier
 making LT protection safer and easier 1 vaccine up to 3 immunities 4 Vectormune FP-LT and. Vectormune FP-LT is a genetically engineered live fowl pox virus vaccine carrying 2 immunorelevant genes from
making LT protection safer and easier 1 vaccine up to 3 immunities 4 Vectormune FP-LT and. Vectormune FP-LT is a genetically engineered live fowl pox virus vaccine carrying 2 immunorelevant genes from
L. J. N. Ross,~ M. M. Binns,:~ P. Tyers, J. Pastorek, V. Zelnik and S. Scott~
 Journal of General Virology (1993), 74, 371-377. Printed in Great Britain 371 Construction and properties of a turkey herpesvirus recombinant expressing the Marek's disease virus homologue of glycoprotein
Journal of General Virology (1993), 74, 371-377. Printed in Great Britain 371 Construction and properties of a turkey herpesvirus recombinant expressing the Marek's disease virus homologue of glycoprotein
Skin involvement in lymphomas caused by Marek s disease virus infection in Silkie chickens
 522462VDIXXX10.1177/1040638714522462Skin involvement in lymphomas in SilkiesLiu et al. research-article2014 Case Report Skin involvement in lymphomas caused by Marek s disease virus infection in Silkie
522462VDIXXX10.1177/1040638714522462Skin involvement in lymphomas in SilkiesLiu et al. research-article2014 Case Report Skin involvement in lymphomas caused by Marek s disease virus infection in Silkie
Viral Genetics. BIT 220 Chapter 16
 Viral Genetics BIT 220 Chapter 16 Details of the Virus Classified According to a. DNA or RNA b. Enveloped or Non-Enveloped c. Single-stranded or double-stranded Viruses contain only a few genes Reverse
Viral Genetics BIT 220 Chapter 16 Details of the Virus Classified According to a. DNA or RNA b. Enveloped or Non-Enveloped c. Single-stranded or double-stranded Viruses contain only a few genes Reverse
Temperature-Sensitive Mutants Isolated from Hamster and
 JOURNAL OF VIROLOGY, Nov. 1975, p. 1332-1336 Copyright i 1975 American Society for Microbiology Vol. 16, No. 5 Printed in U.S.A. Temperature-Sensitive Mutants Isolated from Hamster and Canine Cell Lines
JOURNAL OF VIROLOGY, Nov. 1975, p. 1332-1336 Copyright i 1975 American Society for Microbiology Vol. 16, No. 5 Printed in U.S.A. Temperature-Sensitive Mutants Isolated from Hamster and Canine Cell Lines
Diagnostic Considerations for Marek s Disease. Frederic J. Hoerr, DVM, PhD AMEVEA - Argentina Colon, Entre Rios May 15, 2013
 Diagnostic Considerations for Marek s Disease Frederic J. Hoerr, DVM, PhD AMEVEA - Argentina Colon, Entre Rios May 15, 2013 Control of Marek s Disease Accurate diagnosis Apply appropriate procedures to
Diagnostic Considerations for Marek s Disease Frederic J. Hoerr, DVM, PhD AMEVEA - Argentina Colon, Entre Rios May 15, 2013 Control of Marek s Disease Accurate diagnosis Apply appropriate procedures to
Differential diagnosis of infectious laryngotracheitis from other avian respiratory diseases by a simplified PCR procedure
 ELSEVIER Journal of Virological Methods 50 (1994) 313-322 Journal of Virological Methods Differential diagnosis of infectious laryngotracheitis from other avian respiratory diseases by a simplified PCR
ELSEVIER Journal of Virological Methods 50 (1994) 313-322 Journal of Virological Methods Differential diagnosis of infectious laryngotracheitis from other avian respiratory diseases by a simplified PCR
X/01/$ DOI: /JVI Copyright 2001, American Society for Microbiology. All Rights Reserved.
 JOURNAL OF VIROLOGY, Apr. 2001, p. 3753 3765 Vol. 75, No. 8 0022-538X/01/$04.00 0 DOI: 10.1128/JVI.75.8.3753 3765.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Route of Simian
JOURNAL OF VIROLOGY, Apr. 2001, p. 3753 3765 Vol. 75, No. 8 0022-538X/01/$04.00 0 DOI: 10.1128/JVI.75.8.3753 3765.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Route of Simian
Fayth K. Yoshimura, Ph.D. September 7, of 7 HIV - BASIC PROPERTIES
 1 of 7 I. Viral Origin. A. Retrovirus - animal lentiviruses. HIV - BASIC PROPERTIES 1. HIV is a member of the Retrovirus family and more specifically it is a member of the Lentivirus genus of this family.
1 of 7 I. Viral Origin. A. Retrovirus - animal lentiviruses. HIV - BASIC PROPERTIES 1. HIV is a member of the Retrovirus family and more specifically it is a member of the Lentivirus genus of this family.
AviagenBrief. Marek s Disease Control in Broiler Breeders
 AviagenBrief January 2018 Marek s Disease Control in Broiler Breeders Author: A. Gregorio Rosales DVM, MS, PhD, DACPV - Poultry Health Consultant Introduction Marek s Disease Virus (MDV), a highly infectious
AviagenBrief January 2018 Marek s Disease Control in Broiler Breeders Author: A. Gregorio Rosales DVM, MS, PhD, DACPV - Poultry Health Consultant Introduction Marek s Disease Virus (MDV), a highly infectious
Influenza or flu is a
 Clinical and Research Area Infectious Diseases Influenza Virus Types A and B Influenza or flu is a respiratory illness that is caused by influenza viruses. Influenza viruses type A and type B cause seasonal
Clinical and Research Area Infectious Diseases Influenza Virus Types A and B Influenza or flu is a respiratory illness that is caused by influenza viruses. Influenza viruses type A and type B cause seasonal
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Viral vector vaccines expressing nucleoprotein and phosphoprotein genes of avian bornaviruses ameliorate homologous challenge infections in cockatiels and common canaries Marita
SUPPLEMENTARY INFORMATION Viral vector vaccines expressing nucleoprotein and phosphoprotein genes of avian bornaviruses ameliorate homologous challenge infections in cockatiels and common canaries Marita
AVIAN VIRAL TUMORS I. MAREK'S DISEASE
 DEFINITION The several viral neoplastic diseases of chickens and turkeys, although previously considered a "complex", are actually distinct disease entities. In some cases a single tumor virus strain can
DEFINITION The several viral neoplastic diseases of chickens and turkeys, although previously considered a "complex", are actually distinct disease entities. In some cases a single tumor virus strain can
Chapter 13 Viruses, Viroids, and Prions. Biology 1009 Microbiology Johnson-Summer 2003
 Chapter 13 Viruses, Viroids, and Prions Biology 1009 Microbiology Johnson-Summer 2003 Viruses Virology-study of viruses Characteristics: acellular obligate intracellular parasites no ribosomes or means
Chapter 13 Viruses, Viroids, and Prions Biology 1009 Microbiology Johnson-Summer 2003 Viruses Virology-study of viruses Characteristics: acellular obligate intracellular parasites no ribosomes or means
DETECTION AND SEROTYPING OF MAREKS DISEASE VIRUS IN DISEASED CHICKENS IN ABEOKTA
 ISSN: Print - 2277-0593 Online - 2315-7461 FUNAAB 2017 Journal of Natural Science, Engineering and Technology DETECTION AND SEROTYPING OF MAREKS DISEASE VIRUS IN DISEASED CHICKENS IN ABEOKTA 1O.O. ONI,
ISSN: Print - 2277-0593 Online - 2315-7461 FUNAAB 2017 Journal of Natural Science, Engineering and Technology DETECTION AND SEROTYPING OF MAREKS DISEASE VIRUS IN DISEASED CHICKENS IN ABEOKTA 1O.O. ONI,
Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell
 Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell Wenxin Li Department of Biological Sciences Fordham University Abstract MEFV is a human gene that codes for an
Differentiation-induced Changes of Mediterranean Fever Gene (MEFV) Expression in HL-60 Cell Wenxin Li Department of Biological Sciences Fordham University Abstract MEFV is a human gene that codes for an
Lab 3: Pathogenesis of Virus Infections & Pattern 450 MIC PRACTICAL PART SECTION (30397) MIC AMAL ALGHAMDI 1
 Lab 3: Pathogenesis of Virus Infections & Pattern 450 MIC PRACTICAL PART SECTION (30397) 2018 450 MIC AMAL ALGHAMDI 1 Learning Outcomes The pathogenesis of viral infection The viral disease pattern Specific
Lab 3: Pathogenesis of Virus Infections & Pattern 450 MIC PRACTICAL PART SECTION (30397) 2018 450 MIC AMAL ALGHAMDI 1 Learning Outcomes The pathogenesis of viral infection The viral disease pattern Specific
Chapter 5. Virus isolation and identification of measles and rubella in cell culture
 Chapter 5. Virus isolation and identification of measles and rubella in cell culture In this chapter: 5.1. Recommended cell line for measles and rubella virus isolation 5.2. Propagation of Vero/hSLAM cells
Chapter 5. Virus isolation and identification of measles and rubella in cell culture In this chapter: 5.1. Recommended cell line for measles and rubella virus isolation 5.2. Propagation of Vero/hSLAM cells
19/06/2013. Viruses are not organisms (do not belong to any kingdom). Viruses are not made of cells, have no cytoplasm, and no membranes.
 VIRUSES Many diseases of plants and animals are caused by bacteria or viruses that invade the body. Bacteria and viruses are NOT similar kinds of micro-organisms. Bacteria are classified as living organisms,
VIRUSES Many diseases of plants and animals are caused by bacteria or viruses that invade the body. Bacteria and viruses are NOT similar kinds of micro-organisms. Bacteria are classified as living organisms,
Identification of the Site of Epstein-Barr Virus Persistence In Vivo as a Resting B Cell
 JOURNAL OF VIROLOGY, July 1997, p. 4882 4891 Vol. 71, No. 7 0022-538X/97/$04.00 0 Copyright 1997, American Society for Microbiology Identification of the Site of Epstein-Barr Virus Persistence In Vivo
JOURNAL OF VIROLOGY, July 1997, p. 4882 4891 Vol. 71, No. 7 0022-538X/97/$04.00 0 Copyright 1997, American Society for Microbiology Identification of the Site of Epstein-Barr Virus Persistence In Vivo
Structural vs. nonstructural proteins
 Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
Why would you want to study proteins associated with viruses or virus infection? Receptors Mechanism of uncoating How is gene expression carried out, exclusively by viral enzymes? Gene expression phases?
PERSISTENT INFECTIONS WITH HUMAN PARAINFLUENZAVIRUS TYPE 3 IN TWO CELL LINES
 71 PERSISTENT INFECTIONS WITH HUMAN PARAINFLUENZAVIRUS TYPE 3 IN TWO CELL LINES Harold G. Jensen, Alan J. Parkinson, and L. Vernon Scott* Department of Microbiology & Immunology, University of Oklahoma
71 PERSISTENT INFECTIONS WITH HUMAN PARAINFLUENZAVIRUS TYPE 3 IN TWO CELL LINES Harold G. Jensen, Alan J. Parkinson, and L. Vernon Scott* Department of Microbiology & Immunology, University of Oklahoma
Viral reproductive cycle
 Lecture 29: Viruses Lecture outline 11/11/05 Types of viruses Bacteriophage Lytic and lysogenic life cycles viruses viruses Influenza Prions Mad cow disease 0.5 µm Figure 18.4 Viral structure of capsid
Lecture 29: Viruses Lecture outline 11/11/05 Types of viruses Bacteriophage Lytic and lysogenic life cycles viruses viruses Influenza Prions Mad cow disease 0.5 µm Figure 18.4 Viral structure of capsid
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
numbe r Done by Corrected by Doctor
 numbe r 5 Done by Mustafa Khader Corrected by Mahdi Sharawi Doctor Ashraf Khasawneh Viral Replication Mechanisms: (Protein Synthesis) 1. Monocistronic Method: All human cells practice the monocistronic
numbe r 5 Done by Mustafa Khader Corrected by Mahdi Sharawi Doctor Ashraf Khasawneh Viral Replication Mechanisms: (Protein Synthesis) 1. Monocistronic Method: All human cells practice the monocistronic
Epstein-Barr Virus Polypeptides: Identification of Early Proteins and Their Synthesis and Glycosylation
 JOURNAL OF VIROLOGY, Aug. 1981, p. 651-655 0022-538X/81/080651-05$02.00/0 Vol. 39, No. 2 Epstein-Barr Virus Polypeptides: Identification of Early Proteins and Their Synthesis and Glycosylation ROBERT J.
JOURNAL OF VIROLOGY, Aug. 1981, p. 651-655 0022-538X/81/080651-05$02.00/0 Vol. 39, No. 2 Epstein-Barr Virus Polypeptides: Identification of Early Proteins and Their Synthesis and Glycosylation ROBERT J.
SHORT COMMUNICATION. Human Papillomavirus Type 11 E1 Ú E4 and L1 Proteins Colocalize in the Mouse Xenograft System at Multiple Time Points
 VIROLOGY 214, 259 263 (1995) SHORT COMMUNICATION Human Papillomavirus Type 11 E1 Ú E4 and L1 Proteins Colocalize in the Mouse Xenograft System at Multiple Time Points DARRON R. BROWN,*,,1 JANINE T. BRYAN,
VIROLOGY 214, 259 263 (1995) SHORT COMMUNICATION Human Papillomavirus Type 11 E1 Ú E4 and L1 Proteins Colocalize in the Mouse Xenograft System at Multiple Time Points DARRON R. BROWN,*,,1 JANINE T. BRYAN,
125. Identification o f Proteins Specific to Friend Strain o f Spleen Focus forming Virus (SFFV)
 No. 101 Proc. Japan Acad., 54, Ser. B (1978) 651 125. Identification o f Proteins Specific to Friend Strain o f Spleen Focus forming Virus (SFFV) By Yoji IKAWA,*} Mitsuaki YOSHIDA,*) and Hiroshi YosHIKURA**>
No. 101 Proc. Japan Acad., 54, Ser. B (1978) 651 125. Identification o f Proteins Specific to Friend Strain o f Spleen Focus forming Virus (SFFV) By Yoji IKAWA,*} Mitsuaki YOSHIDA,*) and Hiroshi YosHIKURA**>
Diagnostic Guide for Marek s Disease and other Tumors
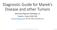 Diagnostic Guide for Marek s Disease and other Tumors Veterinary Diagnostic Pathology, LLC Frederic J. Hoerr, DVM, PhD fred.hoerr@gmail.com; 334-750-7566; www.vetdx.com CF.416.1.TumorHistopathologyDiagnosticGuide.FJH.08.26.2013
Diagnostic Guide for Marek s Disease and other Tumors Veterinary Diagnostic Pathology, LLC Frederic J. Hoerr, DVM, PhD fred.hoerr@gmail.com; 334-750-7566; www.vetdx.com CF.416.1.TumorHistopathologyDiagnosticGuide.FJH.08.26.2013
Section 6. Junaid Malek, M.D.
 Section 6 Junaid Malek, M.D. The Golgi and gp160 gp160 transported from ER to the Golgi in coated vesicles These coated vesicles fuse to the cis portion of the Golgi and deposit their cargo in the cisternae
Section 6 Junaid Malek, M.D. The Golgi and gp160 gp160 transported from ER to the Golgi in coated vesicles These coated vesicles fuse to the cis portion of the Golgi and deposit their cargo in the cisternae
Dr. Ahmed K. Ali. Outcomes of the virus infection for the host
 Lec. 9 Dr. Ahmed K. Ali Outcomes of the virus infection for the host In the previous few chapters we have looked at aspects of the virus replication cycle that culminate in the exit of infective progeny
Lec. 9 Dr. Ahmed K. Ali Outcomes of the virus infection for the host In the previous few chapters we have looked at aspects of the virus replication cycle that culminate in the exit of infective progeny
EBV and Infectious Mononucleosis. Infectious Disease Definitions. Infectious Diseases
 Infectious Disease Definitions Infection when a microorganism invades a host and multiplies enough to disrupt normal function by causing signs and symptoms Pathogencity ability of an organism to cause
Infectious Disease Definitions Infection when a microorganism invades a host and multiplies enough to disrupt normal function by causing signs and symptoms Pathogencity ability of an organism to cause
Epstein-Barr Virus BART MicroRNAs Are Produced from a Large Intron prior to Splicing
 JOURNAL OF VIROLOGY, Sept. 2008, p. 9094 9106 Vol. 82, No. 18 0022-538X/08/$08.00 0 doi:10.1128/jvi.00785-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Epstein-Barr Virus BART
JOURNAL OF VIROLOGY, Sept. 2008, p. 9094 9106 Vol. 82, No. 18 0022-538X/08/$08.00 0 doi:10.1128/jvi.00785-08 Copyright 2008, American Society for Microbiology. All Rights Reserved. Epstein-Barr Virus BART
Laboratory diagnosis of congenital infections
 Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
AP Biology. Viral diseases Polio. Chapter 18. Smallpox. Influenza: 1918 epidemic. Emerging viruses. A sense of size
 Hepatitis Viral diseases Polio Chapter 18. Measles Viral Genetics Influenza: 1918 epidemic 30-40 million deaths world-wide Chicken pox Smallpox Eradicated in 1976 vaccinations ceased in 1980 at risk population?
Hepatitis Viral diseases Polio Chapter 18. Measles Viral Genetics Influenza: 1918 epidemic 30-40 million deaths world-wide Chicken pox Smallpox Eradicated in 1976 vaccinations ceased in 1980 at risk population?
Persistent Infection of MDCK Cells by Influenza C Virus: Initiation and Characterization
 J. gen. Virol. (199), 70, 341-345. Printed in Great Britain 341 Key words: influenza C virus/interferon/persistent infection Persistent Infection of MDCK Cells by Influenza C Virus: Initiation and Characterization
J. gen. Virol. (199), 70, 341-345. Printed in Great Britain 341 Key words: influenza C virus/interferon/persistent infection Persistent Infection of MDCK Cells by Influenza C Virus: Initiation and Characterization
Diagnostic Methods of HBV and HDV infections
 Diagnostic Methods of HBV and HDV infections Zohreh Sharifi,ph.D Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine Hepatitis B-laboratory diagnosis Detection
Diagnostic Methods of HBV and HDV infections Zohreh Sharifi,ph.D Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine Hepatitis B-laboratory diagnosis Detection
Epstein-Barr Virus: Stimulation By 5 '-Iododeoxy uridine or 5 '-Brom odeoxy uridine in Human Lymphoblastoid Cells F ro m a Rhabdom yosarcom a*
 A n n a ls o f C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, No. 6 Copyright 1973, Institute for Clinical Science Epstein-Barr Virus: Stimulation By 5 '-Iododeoxy uridine or 5 '-Brom odeoxy
A n n a ls o f C l i n i c a l L a b o r a t o r y S c i e n c e, Vol. 3, No. 6 Copyright 1973, Institute for Clinical Science Epstein-Barr Virus: Stimulation By 5 '-Iododeoxy uridine or 5 '-Brom odeoxy
