Integra. Uni CP Compression Plating System PRODUCTS FOR SALE IN EUROPE, MIDDLE EAST AND AFRICA ONLY
|
|
|
- Egbert Wood
- 6 years ago
- Views:
Transcription
1 TM Integra Uni CP Compression Plating System S U R G I C A L T E C H N I Q U E
2 Uni CP Compression Plating System Table of contents Indications...03 Contraindications Surgical Technique Step n 1: Surgical approach Step n 2: Positioning trial plate Step n 3: Plate contouring Step n 4: Assembling the drill guides Step n 5: Drilling and hole preparation Step n 6: Screw insertion Step n 7: Compression X-Rays Instructions for use...07 References PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST AND AFRICA ONLY
3 Indications The UNI CP compression plates are indicated for fixation of bone fractures or for bone reconstruction: Arthrodesis in foot and ankle surgery. Fractures management in the foot and ankle. Mono or bi cortical osteotomies in the foot and ankle. Contraindications The implant should not be used in a patient who has currently, or who has a history of: Local or systemic acute or chronic inflammation, Active infection or inflammation, Suspected or documented metal allergy or intolerance. 3 plate configurations:» 2 hole design (17, 20, 25, and 30 mm interaxis)» 4 hole design (20, 25, and 30 mm interaxis)» 4 hole T shape design (20 mm interaxis) Range of screw lengths» 12 mm to 40 mm in 2 mm increments Surfix Locking Technology Material:» Plates: Stainless steel 316L (ISO / ASTM F138&139)» Screws: Titanium alloy Ti 6Al 4V (ISO / ASTM F136) 3
4 Trial plate t et u Implant sizer 2 and 4 holes Surgical technique This technique has been developed with the help of Stephen Conti, MD. NEWDEAL as the manufacturer of this device, does not practice medicine and does not recommend this or any other surgical technique for use on a specific patient. The surgeon who performs any implant procedure is responsible for determining and using the appropriate techniques for implanting the device in each patient. 1. Surgical approach The articular surfaces should be prepared using standard technique to resect the necessary amount of cartilage and, if necessary, to remove bone graft material. Obtain adequate reduction and provisional fixation using guide wires or reduction forceps. 2. Positioning trial plate Use the trial implant ( , ) to determine the ideal plate configuration and position. Depending on the indication, the surgical exposure may not accommodate the trial plate. Place the graduated end of the trial over the larger bone (fragment), under the soft tissue to minimize irritation. HINT 1mm guide wires can be placed through the trial to maintain plate alignment if desired. 4
5 K wire diam. 1.0 mm, L. 100 mm Bending Forceps diam. 3.5 mm hole, L. 171 mm Drilling guide diam. 2.7 mm Screwdriver/Hex. diam. 2.0 mm, L. 180 mm Holder 3. Plate contouring The chosen plate can be contoured, prior to application, to better fit the patient s anatomy. A set of two plate benders ( ) are included to aid in this process. It is important to position the benders in the locking holes to protect the locking mechanical properties of the plate design. If this is not the case, the intermediate locking threads may be damaged or deformed, thus preventing optimal functioning of the lock-screw mechanism. WARNING The plate will weaken with excessive bending. The Uni CP plate may be bent only once and should not be bent excessively. Attach the drill guides to the appropriately contoured plate. 4. Assembling the drill guides Assemble the drilling guides to the bended plate. 5. Drilling and hole preparation Position the plate in the desired location. An implant holder ( ) can be secured to any one of the drill guides to aid in this process. 1 mm guide wires can then be inserted into the wire holes in the plate for temporary fixation. HINT If 1mm guide wires were introduced through the trial instrument, the plate can be introduced over them. 5
6 Drill AO diam. 2.7 mm, L. 190 mm Depth gauge diam. 3.5 mm screws Screwdriver/Hex. diam. 2.0 mm, L. 180 mm Screwdriver/ AO hex. diam. 2.0 mm, L. 76 mm Compression forceps 6. Screw insertion For each hole, the following steps must be followed as shown in figures A to F: Fig. 6 b Fig. 6 a Prepare holes with the 2.7 mm drill ( ) through the drilling guide. The screw length can be read from the calibrated scale on the drill. The depth is read from the top side of the drilling guide. Fig. 6 d Insert the screw into the prepared hole until the plate is at the desired position relative to the bone. The screw should be fully seated in the plate. Clean the threaded hole before and after introducing the screw. Maintain co axiality between the screw and the threaded hole. Fig. 6 c Fig. 6 e Assemble the lock screw to the appropriate screwdriver. The lock screw should be inserted after each screw, and before preparation and insertion of the subsequent screw. This prevents potential damage to thread. WARNING Steps a to f should be completed for each screw before starting preparation of the subsequent screw(s). If not, the axes of the screw and the prepared hole may be misaligned. 7. Compression After all screws are locked in place, compress the Uni CP plate using the compression forcep (spreading) instrument ( ) Upon opening the diamond designed bridge, the compressive forces will pull the ends of the plate toward one another 8. X-Rays 6 Chamfer the drill hole with the screwdriver. Ensure that the threaded hole is not damaged when performing the chamfering. Alternately, measure the necessary screw length using the length gauge ( ), after having removed the drill guide. Surgical Technique Uni CP Compression Plating System PRODUCTS FOR SALE IN EUROPE, MIDDLE EAST AND AFRICA ONLY Fig. 6 f Locking: Fully seat the lock screw with the screwdriver. The lock screw should be flush with the top of the plate when it is fully inserted Drilling guide
7 INSTRUCTIONS FOR USE In accordance with the directive 93/42/EEC relative to medical devices, this product must be handled and/or implanted by WELL- TRAINED and QUALIFIED PERSONS, AWARE OF THESE INSTRUCTIONS FOR USE. The osteosynthesis systems manufactured by Newdeal, are designed for the fi xation of fractures, fusions and osteotomies, more especially for foot, ankle and hand surgery. Material: Plates: 316L (ISO / ASTM F138&139) Screws: Ti-6Al-4V (ISO / ASTM F136) Indications For fi xation of bone fractures or for bone reconstruction. Examples include: Arthrodesis in hand or foot surgery Fractures management in the foot or hand Mono or Bi-cortical osteotomies in the foot or hand Distal or proximal metatarsal or metacarpal osteotomies Fixation of osteotomies for Hallux Valgus treatment (such as Scarf, Chevron, etc.) The size of the chosen staple should be adapted to the specifi c indication. The UNI-CP Plates have to be fi xed with the Surfi x Locking System screws and washers diam. 3.5 mm. Contraindications The implant should not be used in a patient who has currently, or who has a history of: Local or systemic acute or chronic infl ammation; Active infection or infl ammation; Suspected or documented metal allergy or intolerance. Warnings Serious post-operative complications may occur from use of the implant in a patient who: Lacks good general physical condition; Has severe osteoporosis; Demonstrates physiologic or anatomic anomalies; Has immunological responses, sensitization, or hypersensitivity to foreign materials; Systemic or metabolic disorders. Precautions for use Physician must determine if implant is appropriate for patients who have any of the following conditions: Drug and/or alcohol and/or smoke addiction and/or abuse, Infectious disease, Malignancy, Local bone tumors, Compromised wound healing, Obesity, Demonstrated psychological instability, displayed a lack of understanding, inappropriate motivation, or attitude, Unwillingness to accept the possibility of multiple surgeries for revision or replacement, Lacks an understanding that a metallic implant is not as strong as normal healthy bone and will bend, loosen, or fracture if excessive demand is placed on it, Lacks an understanding that their preoperative capacity may not be fully recovered even after successful implantation, Knowledge of surgical techniques, proper reduction, selection and placement of implants, and post-operative patient management are considerations essential to a successful outcome. Criteria for patient selection are the responsibility of the surgeon. Information contained within this document should be taken into consideration during the selection process. Recognition of the appropriate indications and contraindications and the selection of the proper surgical procedures and techniques determined to be best for the patient are the responsibility of the surgeon. Each surgeon must evaluate the appropriateness of the procedure and instruments used during the procedure based on his or her own training and experience. The surgeon should discuss with the patient prior to surgery possible risks, precautions, warnings, consequences, complications, and adverse reactions associated with the surgical procedure and implantation of the device. Each patient must be evaluated by the surgeon to determine the specifi c risk/benefi t relationship in light of the patient s condition and the surgeon s practice, training, experience, and knowledge of the related medical literature. Complications with the use of osteosynthesis systems have been reported in the medical literature. Any patient undergoing a surgical procedure is subject to intra-operative and postoperative complications. Each patient s tolerance to surgery, medication, and implantation of a foreign object may be different. Possible risks, adverse reactions, and complications associated with surgery and the use of osteosynthesis systems should be discussed with and understood by the patient prior to surgery. The implant is made from metallic alloys; therefore, it is subject to possible reactions and complications, including those listed herein. The patient should not be led to unrealistic expectations as to the performance or results that the surgery and implant can provide. The patient should be informed that the life expectancy of the device is unpredictable once implanted, and that successful results cannot be guaranteed. IT IS THE RESPONSIBILITY OF THE SURGEON TO PROVIDE THE PATIENT WITH INFORMATION PRIOR TO SURGERY. Complications may include but are not limited to: Pain, discomfort, or abnormal sensations due to presence of the implant, Bending, loosening, and/or breakage, which could make removal impracticable or diffi cult, Risk of additional injury from post-operative trauma, Migration of the implant position or implant material resulting in injury, Bone loss due to stress shielding. Side effects may include but are not limited to: Infections, Hematoma, Allergy, Thrombosis, Bone non-union or delayed union. Adverse effects may necessitate re-operation, revision or removal surgery, arthrodesis of the involved joint, and /or amputation of the limb. Implant removal should be followed by adequate postoperative management to avoid fracture or re-fracture. Interference risks during medical imaging: MRI/SCANNER: ask the patient to systematically mention that he/ she was implanted with a metallic device. Packaging - sterility This product is sold either sterile or non sterile. The sterilization method is specifi ed on the packaging. Components sterilized by radiation are exposed to a minimum of 25 kgy of gamma irradiation. If the product is not labeled «STERILE», it must be sterilized prior to use, in compliance with current regulations. If the product has been removed from packaging but not used, it may be re-sterilized. Check packaging and labeling integrity before use. The sterility is guaranteed as long as the packaging has not been damaged or opened and before the expiration date. Do not use any implant for which the packaging has been opened or damaged outside the operating theatre. Inner packaging should be handled under sterile conditions (persons/instruments). Use of the products The surgeon must use the instrumentation recommended in accordance with the operative technique available from the manufacturer. The medical device must be used in compliance with the use of the profession and the standard of art. Do not attempt a surgical procedure with faulty, damaged or suspect instruments or implants. Inspect all components preoperatively to assure utility. Alternate fi xation methods should be available intraoperatively. Opening of the instruments set must be done according to aseptic condition. When handling the implants, avoid any contact with other material or tools which may damage the implant surface. Under no circumstances should the implant be modifi ed. The multi-component devices (such as plates-screws systems) should only associate the appropriated Newdeal products and should never be used in conjunction with components of any other manufacturers, as these products may not be compatible. The company accepts no responsibility for such use. Specifi c cautions for plates The plates should never been excessively bent, nor reverse bent. Re-use of the implants Orthopedic implants already implanted must never be re-used. The company accepts no responsibility for such re-use. Re-sterilization of non-implanted implants and sterilization of non-sterile products Unless supplied sterile and clearly labeled as such, all implants and instruments must be steam autoclaved prior to use in surgery. Resterilization is only allowed for non-implanted products. Remove delivery packaging in compliance with current regulations to sterilize non-sterile products. Ostesynthesis implants manufactured by Newdeal are recommended to be sterilized by the steam autoclaving procedure regularly used in the hospital. The implants can be sterilized several times in the same conditions. The following two methods have been validated by the manufacturer: Newdeal Stainless Steel sterilization trays CYCLE GRAVITY DISPLACEMENT 5 PULSES [MAXIMUM 900 MBAR; MINIMUM 200 MBAR] PRE-VACUUM 3 PULSES [MAXIMUM 26.0 PSIG (2.8 BAR); MINIMUM 10 INHG (339 MBAR)] MINIMUM TEMPERATURE 134 C (273 F) 132 C (270 F) EXPOSURE TIME 18 MINUTES 4 MINUTES PURGE MINUTE VACUUM DRYING 20 MINUTES 20 MINUTE These sterilization parameters assume that all instruments have been properly decontaminated prior to sterilization. The parameters are validated to sterilize specifi c confi gurations as noted in the tray markings. If other products are added to the tray or to the sterilizer, the recommended parameters may not be valid and new cycle parameters may need to be validated by the user. The autoclave must be properly installed, maintained and calibrated. Other sterilization method and cycles may also be used. However, individuals or hospitals not using the recommended method are advised to validate the alternative method using appropriate laboratory techniques. EtO sterilization or cold sterilization techniques are not recommended. Information related to postoperative care The patient should be advised that a second more minor procedure for the removal of the implants is usually necessary. While the surgeon must make the fi nal decision regarding implant removal, wherever possible and practical for the individual patient, fi xation devices should be removed once their service as an aid to healing is accomplished. In the absence of a bursa or pain, removal of the implant in elderly or debilitated patients is not suggested. Postoperative instructions to patients and appropriate nursing care are critical. Early weight bearing substantially increases implant loading and increases the risk of loosening, bending, or breaking the device. Patients who are obese or non-compliant, as well as patients who could be predisposed to delayed or non-union must have auxiliary support. Even after full healing, the patient should be cautioned that refracture is more likely with the implant in place and soon after its removal, rather than later, when the voids in the bone left by implant removal have been fi lled in completely. Patients should be cautioned against unassisted activity that requires walking or lifting. Postoperative care and physical therapy should be structured to prevent loading of the operative extremity until stability is evident. The patient should be encouraged to report to his/her surgeon any unusual changes of the operated extremity. If evidence suggests loosening of the implant (particular pain and progressive changes in the radiographs) an intensifi ed schedule of check-ups is advised and new warning and instructions to the patient may be appropriate regarding further activity restrictions. The patient should be encouraged to receive prompt medical attention for any infection that could occur, whether at the operated-member level or elsewhere in the body. Storage Store in dry place. Product information disclosure Liability Newdeal, an Integra LifeSciences Company, has exercised reasonable care in the selection of materials and the manufacture of these products and warrants that the products are free from manufacturing defects. Newdeal excludes all other warranties, whether expressed or implied, including but not limited to, any implied warranties of merchantability or fi tness for a particular purpose.newdeal shall not be liable for any incidental or consequential loss, damage, or expense, directly or indirectly arising from use of this product. Newdeal neither assumes nor authorizes any person to assume for it any other or additional liability or responsibility in connection with these products. Newdeal intends that this device should be used only by physicians having received proper training in orthopedic surgery technique for use of the device. WARNING This device is not approved for screw attachment or fi xation to the posterior elements (pedicles) of the cervical, thoracic or lumbar spine. INFORMATION Should any information regarding the products or their uses be required, please contact your representative or distributor or directly contact the manufacturer. 7
8 Uni CP Compression Plating System Surfix Titanium screw diam. 3.5 mm + lock screw Reference s Length 12 mm s Length 14 mm s Length 16 mm s Length 18 mm s Length 20 mm s Length 22 mm s Length 24 mm s Length 26 mm s Length 28 mm s Length 30 mm s Length 32 mm s Length 34 mm s Length 36 mm s Length 38 mm s Length 40 mm s Lock screw diam 3 5 mm s s s 2 holes 17 mm 2 holes 25 mm 2 holes 30 mm s s s s 4 holes 20 mm 4 holes 25 mm 4 holes 30 mm 4 holes 20mm T shape Compression stainless steel plate Reference Container Instruments Reference Reference Screwdriver / Hex diam 2 0 mm, L 180 mm Container Screwdriver / AO, hex diam 2 0 mm, L 76 mm Base Drilling guide, diam 2 7 mm Lid Drill, AO diam 2 7 mm, L 190 mm Module K wire, diam 1 0 mm, L 100 mm Bending forceps, diam 3 5 mm hole, L 171 mm Depth gauge, diam 3 5 mm screws Compression forceps Implant sizer for T and U plates * Holder Trial plate for 2 and 4 holes plates 9 Integra LifeSciences Services (France) SAS Sales & Marketing EMEA Immeuble Séquoia 2 97 allée Alexandre Borodine Parc technologique de la Porte des Alpes Saint Priest FRANCE +33 (0) fax +33 (0) emea info@integralife com integralife.com Distributed by Customer Service International: +33 (0) (0) (Fax) csemea@integralife com United Kingdom : csuk ortho@integralife com France: +33 (0) (0) (Fax) cs ortho@integralife com Benelux: +32 (0) (0) (Fax) custsvcbenelux@integralife com Switzerland: +41 (0) (0) (Fax) custsvcsuisse@integralife com Newdeal SAS Immeuble Séquoia 2 97 allée Alexandre Borodine Parc technologique de la Porte des Alpes Saint Priest FRANCE +33 (0) fax +33 (0) newdeal@newdeal info www newdeal info 2010 Integra LifeSciences Corporation All rights reserved ILS PRODUCTS FOR SALE IN EUROPE, MIDDLE EAST and AFRICA ONLY Availability of these products might vary from a given country or region to another, as a result of specific local regulatory approval or clearance requirements for sale in such country or region Always refer to the appropriate instructions for use for complete clinical instructions Non contractual document The manufacturer reserves the right, without prior notice, to modify the products in order to improve their quality WARNING: Applicable laws restrict these products to sale by or on the order of a physician Surfix, UNI CP, Integra and the Integra logo are trademarks or registered trademarks of Integra LifeSciences Corporation or its subsidiaries
ADVANSYS Midfoot plating system
 T.T.C. Tibio-Talo-Calcaneus plate TTC ENGLISH ADVANSYS Midfoot plating system Surgical technique Or t h o pa e d i c s LOWER EXTREMITY T.T.C. (DORSAL LISFRANC PLATE) Introduction Indications The Newdeal
T.T.C. Tibio-Talo-Calcaneus plate TTC ENGLISH ADVANSYS Midfoot plating system Surgical technique Or t h o pa e d i c s LOWER EXTREMITY T.T.C. (DORSAL LISFRANC PLATE) Introduction Indications The Newdeal
Integra. surgical technique. Advansys Midfoot Plating System. eng. D.L.P. Dorsal Lisfranc Plate. M.L.P. Medial Lisfranc Plate
 eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
large qwix Positioning and fixation screws Surgical Technique lower large qwix English PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY
 large qwix Positioning and fixation screws Dia. 7.5 mm Dia. 5.5 mm large qwix English Surgical Technique orthopedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY LARGE qwix
large qwix Positioning and fixation screws Dia. 7.5 mm Dia. 5.5 mm large qwix English Surgical Technique orthopedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY LARGE qwix
IPP-ON. Interphalangeal implant. Surgical Technique. l o w e r e x tre mit y. Or t h o pa e d i c s. IPP-ON English
 IPP-ON Interphalangeal implant IPP-ON English Surgical Technique Or t h o pa e d i c s l o w e r e x tre mit y IPP-ON Rationale Indications The proximal interphalangeal fusion is a challenging surgery.
IPP-ON Interphalangeal implant IPP-ON English Surgical Technique Or t h o pa e d i c s l o w e r e x tre mit y IPP-ON Rationale Indications The proximal interphalangeal fusion is a challenging surgery.
Integra. Solustaple Standard staple SURGICAL TECHNIQUE. Uni-Clip Compression staple. Products for sale in Europe, Middle-East and Africa only.
 eng Integra Solustaple Standard staple SURGICAL TECHNIQUE Uni-Clip Compression staple Table of Contents Solustaple Standard staple...04 Implants Details...04 Indications...04 Instruments Details...04 Surgical
eng Integra Solustaple Standard staple SURGICAL TECHNIQUE Uni-Clip Compression staple Table of Contents Solustaple Standard staple...04 Implants Details...04 Indications...04 Instruments Details...04 Surgical
eng Integra surgical technique Large Qwix Products for sale in Europe, Middle-East and Africa only.
 eng Integra Large Qwix surgical technique Positionning Positioning and Fixation fixation Screw screw Table of Contents Concept...04 Indications...04 Contraindications...04 Implant details...05 Description...06
eng Integra Large Qwix surgical technique Positionning Positioning and Fixation fixation Screw screw Table of Contents Concept...04 Indications...04 Contraindications...04 Implant details...05 Description...06
Integra. ADVANSYS Plating System SURGICAL TECHNIQUE
 Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
Integra ADVANSYS Plating System SURGICAL TECHNIQUE Table of Contents Dorsal Lisfranc Plate Indications...2 Contraindications...2 Description...2 Surgical Technique...3 Surgical Site Preparation...3 Step
SURGICAL TECHNIQUE. Compression screw FOREFOOT SOLUTIONS
 SURGICAL TECHNIQUE Compression screw FOREFOOT SOLUTIONS SCARF OSTEOTOMY The Scarf osteotomy consists of a horizontal cut and two transversal cuts of the first metatarsal, allowing for a broad range of
SURGICAL TECHNIQUE Compression screw FOREFOOT SOLUTIONS SCARF OSTEOTOMY The Scarf osteotomy consists of a horizontal cut and two transversal cuts of the first metatarsal, allowing for a broad range of
HALLU-FIX ENGLISH. HALLU -FIX M.T.P. Arthrodesis System. Surgical Technique
 HALLU-FIX ENGLISH HALLU -FIX M.T.P. Arthrodesis System Surgical Technique Or t h o pa e d i c s LOWER EXTREMITY HALLU-FIX Table of content Introduction...03 Indications...03 Contre-indications...03 Surgical
HALLU-FIX ENGLISH HALLU -FIX M.T.P. Arthrodesis System Surgical Technique Or t h o pa e d i c s LOWER EXTREMITY HALLU-FIX Table of content Introduction...03 Indications...03 Contre-indications...03 Surgical
TIBIAXYS. A u n i q u e p l a t i n g s y s t e m for distal tibial osteotomy. Surgical technique
 TIBIAXYS A u n i q u e p l a t i n g s y s t e m for distal tibial osteotomy TIBIAXYS ENGLISH Surgical technique osteotomy / fractures Or t h o pae d i c s LOWER EXTREMITY PRODUCTS FOR SALE IN EUROPE,
TIBIAXYS A u n i q u e p l a t i n g s y s t e m for distal tibial osteotomy TIBIAXYS ENGLISH Surgical technique osteotomy / fractures Or t h o pae d i c s LOWER EXTREMITY PRODUCTS FOR SALE IN EUROPE,
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Headless Compession Screw 4.5 / 6.5 SURGICAL TECHNIQUE. Titanium or Stainless Steel. Cannulated Headless Design. Multiple Thread Options.
 Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
Headless Compession Screw SURGICAL TECHNIQUE 4.5 / 6.5 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
ACP. Anterior Cervical Plate System SURGICAL TECHNIQUE
 ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
ACP Anterior Cervical Plate System SURGICAL TECHNIQUE ACP TABLE OF CONTENTS INTRODUCTION 4 INDICATIONS AND CONTRAINDICATIONS 5 WARNINGS AND PRECAUTIONS 6 IMPLANT DESCRIPTION 7 INSTRUMENTS 10 SURGICAL
eng Integra surgical technique TIBIAXYS Osteotomy - Arthrodesis Products for sale in Europe, Middle-East and Africa only.
 eng Integra TIBIAXYS Osteotomy - Arthrodesis surgical technique Distal Tibia Osteotomy Plating System OSTEOTOMY Ankle Arthrodesis Double Plating System ARTHRODESIS Table of Contents Description... 4 Main
eng Integra TIBIAXYS Osteotomy - Arthrodesis surgical technique Distal Tibia Osteotomy Plating System OSTEOTOMY Ankle Arthrodesis Double Plating System ARTHRODESIS Table of Contents Description... 4 Main
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
AxSOS. Locking Plate System. Operative Technique. Small Fragment Basic Fragment
 AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment Stryker Plating Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5 Compression Plates 5 Reconstruction and 1/3
AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment Stryker Plating Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5 Compression Plates 5 Reconstruction and 1/3
AxSOS Locking Plate System
 AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment 1 2 Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5mm Compression Plates 5 Reconstruction and 1/3 Tubular Locking
AxSOS Locking Plate System Operative Technique Small Fragment Basic Fragment 1 2 Contents Page 1. Introduction 4 2. Features & Benefits 5 4 and 5mm Compression Plates 5 Reconstruction and 1/3 Tubular Locking
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
MetaFix Ludloff Plate
 Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
Merete MetaFix Ludloff Plate Low Profile Locking Bone Plate System Surgical Technique and Ordering Information - Content - Content 1. Description.................................................. 3 2.
O.S.2-C & O.S.2-V. Static Staple - Stainless steel
 O.S.2-C & O.S.2-V Compression Staple PEEK Static Staple - Stainless steel 2 O.S.2 - TABLE OF CONTENTS O.S.2-C 3 Product description 4 Indications 8 Surgical technique 9 O.S.2-V 13 Product description 14
O.S.2-C & O.S.2-V Compression Staple PEEK Static Staple - Stainless steel 2 O.S.2 - TABLE OF CONTENTS O.S.2-C 3 Product description 4 Indications 8 Surgical technique 9 O.S.2-V 13 Product description 14
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Table of Contents.
 surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
surgical technique The Ambassador TM Anterior Cervical Plate System is a versatile system of implants and instruments with a variety of sizes to provide optimal anatomic compatibility. The integrated cam
ACP1 CERVICAL PLATE SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE II.
 I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
I. ACP1 CERVICAL PLATE II. SPINAL SYSTEM SURGICAL TECHNIQUE GUIDE I. Introduction The Gold Standard Orthopaedics, LLC ACP1 Spinal System was designed with surgeons to incorporate strength, functionality,
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
EasyStep. Operative technique
 Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
Operative technique EasyStep - Step staple This publication sets forth detailed recommended procedures for using Stryker Osteosynthesis devices and instruments. It offers guidance that you should heed,
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Zimmer Small Fragment Universal Locking System. Surgical Technique
 Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
Zimmer Small Fragment Universal Locking System Surgical Technique Zimmer Small Fragment Universal Locking System 1 Zimmer Small Fragment Universal Locking System Surgical Technique Table of Contents Introduction
3.5 mm LCP Extra-articular Distal Humerus Plate
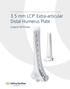 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Extra-articular Distal Humerus Plate Surgical Technique Table of Contents Introduction 3.5 mm LCP Extra-articular Distal Humerus
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation.
 LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Technique Guide LCP Small Fragment System Table of Contents Introduction
A U X I L I A R Y C O N N E C T O R S Surgical Technique
 A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
A U X I L I A R Y C O N N E C T O R S Surgical Technique AUXILIARY CONNECTORS ISSYS LP Auxiliary Connectors The ISSYS LP auxiliary connectors were designed to provide medial-lateral variability for the
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
3.5 mm LCP Olecranon Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Olecranon Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
SMV Scientific Bone Plate and Screw System Surgical Technique
 SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
SMV Scientific Bone Plate and Screw System Surgical Technique Description: The SMV Scientific Bone Plate and Screw System consists of non-locking plates and bone screw fasteners in a variety of lengths,
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
DARCO. Bow 2 Plate SURGIC AL TECHNIQUE
 DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
DARCO Bow 2 Plate SURGIC AL TECHNIQUE Contents 2 Preface 3 Chapter 1 4 Chapter 2 5 6 7 8 9 Appendix 10 10 11 Intended Use Indications/Contraindications Design Rationale Preoperative Planning Surgical Technique
Low Bend Distal Tibia Plates
 Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Part of the DePuy Synthes Locking Compression Plate (LCP ) System 3.5 mm LCP Low Bend Medial Distal Tibia Plates Surgical Technique Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia
Technique Guide. 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
Technique Guide 3.5 mm LCP Low Bend Medial Distal Tibia Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Low Bend Medial Distal Tibia Plates
TIBIAXYS ANKLE FUSION
 TIBIAXYS ANKLE FUSION SURGICAL TECHNIQUE TIBIAXYS Ankle Fusion Plate features Anatomically contoured plates The plates are designed to approximate the patient s bony and soft tissue anatomy The plate designs
TIBIAXYS ANKLE FUSION SURGICAL TECHNIQUE TIBIAXYS Ankle Fusion Plate features Anatomically contoured plates The plates are designed to approximate the patient s bony and soft tissue anatomy The plate designs
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
Hinged Laminoplasty System surgical technique
 BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
BlackbirdTM Hls Hinged Laminoplasty System surgical technique Blackbird TM Hls The ChoiceSpine Blackbird Hinged Laminoplasty System (HLS) design eliminates fitting plates through trial and error bending.
OBSOLETED. LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation.
 LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Surgical Technique LCP Small Fragment System This publication
LCP Medial Distal Tibia Plate, without Tab. The Low Profile Anatomic Fixation System with Angular Stability and Optimal Screw Orientation. Surgical Technique LCP Small Fragment System This publication
SURGICAL TECHNIQUE GUIDE TRESTLE. Anterior Cervical Plating System
 SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
SURGICAL TECHNIQUE GUIDE TRESTLE Anterior Cervical Plating System 2 SURGICAL TECHNIQUE GUIDE SURGICAL TECHNIQUE GUIDE System Features Large window enables visualization of graft site and end plates Screw
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique
 PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
PediLoc 3.5mm and 4.5mm Contour Femur Plate Surgical Technique Surgical Technique Contour Femur Plate The technique description herein is made available to the healthcare professional to illustrate the
Technique Guide. 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
Technique Guide 3.5 mm LCP Olecranon Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 3.5 mm LCP Olecranon Plates 2 AO Principles 3 Indications 3 Clinical
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
3.5 mm Locking Attachment Plate
 For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
For Treatment of Periprosthetic Fractures 3.5 mm Locking Attachment Plate Surgical Technique Table of Contents Introduction 3.5 mm Locking Attachment Plate 2 Indications 4 Surgical Technique Preparation
O.S.2-C. Compression staple PEEK
 3 O.S.2-C Compression staple PEEK 4 O.S.2 -C - PRODUCT DESCRIPTION (1/4) The O.S.2 staples are indicated for fixation of arthrodesis, osteotomies and fractures in hand or foot surgery. The size of the
3 O.S.2-C Compression staple PEEK 4 O.S.2 -C - PRODUCT DESCRIPTION (1/4) The O.S.2 staples are indicated for fixation of arthrodesis, osteotomies and fractures in hand or foot surgery. The size of the
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY)
 IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY) IMPORTANT NOTE Intramedullary nails provide an alternative to open reduction
IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY) IMPORTANT NOTE Intramedullary nails provide an alternative to open reduction
MetaCun DuoMetaCun. Midfoot Fusion Locking Plate System. Surgical Technique and Ordering Information
 MetaCun DuoMetaCun Midfoot Fusion Locking Plate System Surgical Technique and Ordering Information 2 Table of contents Table of contents Description... 4 Indications for use...5 Contraindications...5 Surgical
MetaCun DuoMetaCun Midfoot Fusion Locking Plate System Surgical Technique and Ordering Information 2 Table of contents Table of contents Description... 4 Indications for use...5 Contraindications...5 Surgical
Gallery Laminoplasty Spine System
 Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Surgical Technique Gallery Laminoplasty Spine System A smart, simple to use system with intuitive design features. Smart Plate Design Three hole, cobra head design Plates with hook or standard plates available
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine.
 Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
Thoracolumbar Spine Locking Plate (TSLP) System. A low-profile plating system for anterior stabilization of the thoracic and lumbar spine. Technique Guide Instruments and implants approved by the AO Foundation
3.0 mm Cannulated Screw System. Surgical Technique
 3.0 mm Cannulated Screw System Surgical Technique 3 3.0 mm Cannulated Screw System Surgical Technique 3.0 mm Cannulated Screw System The 3.0 mm Cannulated Screw System is part of a series of cannulated
3.0 mm Cannulated Screw System Surgical Technique 3 3.0 mm Cannulated Screw System Surgical Technique 3.0 mm Cannulated Screw System The 3.0 mm Cannulated Screw System is part of a series of cannulated
Technique Guide. 2.7 mm/3.5 mm LCP Distal Fibula Plates. Part of the Synthes locking compression plate (LCP) system.
 Technique Guide 2.7 mm/3.5 mm LCP Distal Fibula Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 2.7 mm/3.5 mm LCP Distal Fibula Plates 2 AO Principles
Technique Guide 2.7 mm/3.5 mm LCP Distal Fibula Plates. Part of the Synthes locking compression plate (LCP) system. Table of Contents Introduction 2.7 mm/3.5 mm LCP Distal Fibula Plates 2 AO Principles
CHARLOTTE. 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE
 CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce
CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGIC A L T ECHNIQUE CHARLOTTE 3.0 and 4.3 Multi-Use Compression Screws SURGICAL TECHNIQUE Surgical Technique as described by: Robert Anderson, MD Bruce
Peanut Growth Control Plating System
 Surgical Technique Peanut Growth Control Plating System An Innovative Approach To Hemi-Epiphysiodesis Provides controlled growth of healthy epiphysis, restoring proper anatomic alignment Advanced instrumentation
Surgical Technique Peanut Growth Control Plating System An Innovative Approach To Hemi-Epiphysiodesis Provides controlled growth of healthy epiphysis, restoring proper anatomic alignment Advanced instrumentation
Surgical Technique. Calcaneal Locking Plate
 Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Calcaneal Locking Plate PERI-LOC Locked Plating System Calcaneal Locking Plate Surgical TechniqueCatalog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
KATALYST. Bipolar Radial Head System. Surgical Technique. orthopedics. KATALYST English. PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY
 KATALYST Bipolar Radial Head System KATALYST English Surgical Technique orthopedics UPPER extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY KATALYST Introduction Description The Katalyst
KATALYST Bipolar Radial Head System KATALYST English Surgical Technique orthopedics UPPER extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY KATALYST Introduction Description The Katalyst
Surgical Technique. Clavicle Locking Plate
 Surgical Technique Clavicle Locking Plate PERI-LOC Locked Plating System Clavicle Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient Positioning...4
Surgical Technique Clavicle Locking Plate PERI-LOC Locked Plating System Clavicle Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient Positioning...4
Surgical Technique. Distal Humerus Locking Plate
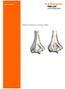 Surgical Technique Distal Humerus Locking Plate PERI-LOC Locked Plating System Distal Humerus Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Distal Humerus Locking Plate PERI-LOC Locked Plating System Distal Humerus Locking Plate Surgical Technique Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Complex process High costs
 MTP With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking
MTP With a non sterile standard kit Calling on medical staff Constraints Complex traceability Contracted out sterilization Suppliers deadline Complex process High costs 1 Delivery 2 Storage 3 Unpacking
A New Benchmark in Tibial Fixation
 PediLoc T I B I A S U R G I C A L T E C H N I Q U E A New Benchmark in Tibial Fixation Multiple plate designs for more options in osteotomies and fracture fixation Engineered from exclusive data resulting
PediLoc T I B I A S U R G I C A L T E C H N I Q U E A New Benchmark in Tibial Fixation Multiple plate designs for more options in osteotomies and fracture fixation Engineered from exclusive data resulting
Surgical Technique. Anterolateral and Medial Distal Tibia Locking Plates
 Surgical Technique Anterolateral and Medial Distal Tibia Locking Plates PERI-LOC Periarticular Locked Plating System Anterolateral and Medial Distal Tibia Locking Plates Surgical Technique Contents Product
Surgical Technique Anterolateral and Medial Distal Tibia Locking Plates PERI-LOC Periarticular Locked Plating System Anterolateral and Medial Distal Tibia Locking Plates Surgical Technique Contents Product
NCB Distal Femur System. Surgical Technique
 NCB Distal Femur System Surgical Technique NCB Distal Femur System Surgical Technique 3 Surgical Technique NCB Distal Femur System Table of Contents Introduction 4 Indications 8 Preoperative Planning
NCB Distal Femur System Surgical Technique NCB Distal Femur System Surgical Technique 3 Surgical Technique NCB Distal Femur System Table of Contents Introduction 4 Indications 8 Preoperative Planning
Telya. Varisation staple. Surgical Technique. Irish Distributer: O'Mara Medical Tel
 Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
Surgical Technique Indications The varisation is used to stabilize an osteotomy of the great toe proximal phalanx (Akin osteotomy), in order to correct : Hallux valgus interphalangeus, Phalangeal pronation,
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
PediLoc Tibia Surgical Technique
 PediLoc Tibia PediLoc Tibia Surgical Technique The technique description herein is made available to the healthcare professional to illustrate the author s suggested treatment for an uncomplicated procedure.
PediLoc Tibia PediLoc Tibia Surgical Technique The technique description herein is made available to the healthcare professional to illustrate the author s suggested treatment for an uncomplicated procedure.
Integra. DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE
 Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
Instructions for Use Reprocessed Arthroscopic Shavers
 Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
Reprocessed by Instructions for Use Reprocessed Arthroscopic Shavers Reprocessed Device for Single Use Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician. STERILE
PROXIMAL TIBIAL PLATE
 SURGICAL NÁSTROJE TECHNIQUE PRO ARTROSKOPII PROXIMAL INSTRUMENTS TIBIAL FOR PLATE ARTHROSCOPY Proximal Tibial Plate Description of medical device The Proximal Tibial Plate is used in epyphyseal and metaphyseal
SURGICAL NÁSTROJE TECHNIQUE PRO ARTROSKOPII PROXIMAL INSTRUMENTS TIBIAL FOR PLATE ARTHROSCOPY Proximal Tibial Plate Description of medical device The Proximal Tibial Plate is used in epyphyseal and metaphyseal
Cervical Screw System
 Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
Cervical Screw System LnK Posterior Cervical Screw The LnK Cervical Fixation System provides a top-loading, simple and secure system for rigid fixation. The LnK Cervical Fixation system includes instrumentation
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM The following languages are included in this packet:
 ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
ENDO-FUSE INTRA-OSSEOUS FUSION SYSTEM 137900-5 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese
Distal Radius Plate Instrument and Implant Set. Discontinued December 2017 DSUS/TRM/0916/1063(1)
 Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
Distal Radius Plate Instrument and Implant Set Surgical Technique Discontinued December 2017 DSUS/TRM/0916/1063(1) The Distal Radius Plates Indications For fixation of fractures and osteotomies, including
Surgical Technique. Olecranon Locking Plate
 Surgical Technique Olecranon Locking Plate PERI-LOC Locked Plating System Olecranon Locking Plate Surgical Techniquealog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
Surgical Technique Olecranon Locking Plate PERI-LOC Locked Plating System Olecranon Locking Plate Surgical Techniquealog Infor Table of Contents Introduction...2 Indications...3 Plate Features...3 Patient
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Conventus CAGE PH Surgical Techniques
 Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
Conventus CAGE PH Surgical Techniques Conventus Orthopaedics The Conventus CAGE PH (PH Cage) is a permanent implant comprised of an expandable scaffold, made from nitinol and titanium, which is deployed
LCP Medial Proximal Tibial Plate 3.5. Part of the Synthes small fragment Locking Compression Plate (LCP) system.
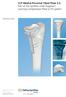 LCP Medial Proximal Tibial Plate 3.5. Part of the Synthes small fragment Locking Compression Plate (LCP) system. Technique Guide This publication is not intended for distribution in the USA. Instruments
LCP Medial Proximal Tibial Plate 3.5. Part of the Synthes small fragment Locking Compression Plate (LCP) system. Technique Guide This publication is not intended for distribution in the USA. Instruments
Integra Total Foot System 2
 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system
The Flower Proximal Humerus Plate
 The Flower Proximal Humerus Plate PROCEDURE GUIDE www.flowerortho.com The Flower Upper Extremity Application PROXIMAL HUMERUS PLATE SMALL BONE PLATES FOUR CORNER FUSION PLATE ANATOMIC DISTAL RADIUS PLATE
The Flower Proximal Humerus Plate PROCEDURE GUIDE www.flowerortho.com The Flower Upper Extremity Application PROXIMAL HUMERUS PLATE SMALL BONE PLATES FOUR CORNER FUSION PLATE ANATOMIC DISTAL RADIUS PLATE
PediLoc 3.5mm and 4.5mm Bowed Femur Plate Surgical Technique
 PediLoc 3.5mm and 4.5mm Bowed Femur Plate Surgical Technique 2957 Bow Broch_REV_B.indd 1 2/10/11 12:47 PM Surgical Technique Bowed Femur Plate The technique description herein is made available to the
PediLoc 3.5mm and 4.5mm Bowed Femur Plate Surgical Technique 2957 Bow Broch_REV_B.indd 1 2/10/11 12:47 PM Surgical Technique Bowed Femur Plate The technique description herein is made available to the
HawkeyeTM Peek. surgical technique
 HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
HawkeyeTM Peek surgical technique Introduction The ChoiceSpine HAWKEYE Vertebral Body Replacement (VBR) System is intended for use in the thoracolumbar spine (T1 - L5) to replace a collapsed, damaged,
Surgical Technique. Apache Anterior Lumbar Interbody Fusion
 Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Surgical Technique Apache Anterior Lumbar Interbody Fusion 2 Table of Contents Page Preoperative Planning 4 Patient Positioning 4 Disc and Endplate Preparation 4 Distraction/Size Selection 5 Implantation
Integra SURGICAL TECHNIQUE. MemoFix Super Elastic Nitinol Staple System. Super Elastic Nitinol Staple System
 Integra MemoFix Super Elastic Nitinol Staple System SURGICAL TECHNIQUE Super Elastic Nitinol Staple System Table of Contents Design Rationale...2 System Features...2 Indications...2 Contraindications...2
Integra MemoFix Super Elastic Nitinol Staple System SURGICAL TECHNIQUE Super Elastic Nitinol Staple System Table of Contents Design Rationale...2 System Features...2 Indications...2 Contraindications...2
The Flower Forefoot PROCEDURE GUIDE.
 The Flower Forefoot PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE MTP
The Flower Forefoot PROCEDURE GUIDE www.flowerortho.com The Flower Foot & Ankle Application NC FUSION PLATE 2-HOLE COMPRESSION PLATE TMT FUSION PLATE LAPIDUS FUSION PLATE COMPRESSION T-PLATE, OBLIQUE MTP
Royal Oak Cervical Plate System
 Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
Royal Oak Cervical Plate System Manufactured by Nexxt Spine, Inc. Royal Oak Cervical Plate System INTRODUCTION FEATURES AND BENEFITS Table of Contents SURGICAL TECHNIQUE Step 1. Patient Positioning Step
The Calcaneal Plate. The Synthes non-locking solution for the Calcaneus.
 The Calcaneal Plate. The Synthes non-locking solution for the Calcaneus. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation.
The Calcaneal Plate. The Synthes non-locking solution for the Calcaneus. Surgical Technique This publication is not intended for distribution in the USA. Instruments and implants approved by the AO Foundation.
Ratcheting Compression Plating System. Surgical Technique
 Ratcheting Compression Plating System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches
Ratcheting Compression Plating System Surgical Technique Acumed is a global leader of innovative orthopaedic and medical solutions. We are dedicated to developing products, service methods, and approaches
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use
 Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Aesculap CeSpace TM PEEK and CeSpace TM XP Spinal Implant System Instructions for Use Page 1 of 5 Indications for use: When used as a Vertebral Body Replacement Device: The CeSpace PEEK and CeSpace XP
Memory Staple. Surgical Technique
 Memory Staple Surgical Technique Contents Product The Memory Staple is an ideal form of fixation for arthrodesis, osteotomy and fracture fixation. Made from Nitinol memory alloy, the BioPro Memory Staple
Memory Staple Surgical Technique Contents Product The Memory Staple is an ideal form of fixation for arthrodesis, osteotomy and fracture fixation. Made from Nitinol memory alloy, the BioPro Memory Staple
