Integra Total Foot System 2
|
|
|
- Dominick Grant
- 5 years ago
- Views:
Transcription
1 Instructions for Use CAUTION: U.S. federal law restricts this device to sale by or on the order of a physician. Integra Total Foot System 2 DESCRIPTION The Integra Total Foot System (TFS2) is a system containing bone plates and screws designed for use in the skeletally mature foot. The plates and screws are intended to be used for stabilization and fixation of fresh fractures, intra-articular and extra-articular fractures, joint depression and multi-fragmentary fractures. The subject devices can also be used in revision procedures, joint fusion, and in reconstructing the small bones of the feet. Available plates, screws and instrumentation are packaged as a single system and organized around the types of plates described below: Fore Foot Plates 1. Open Wedge Plates The plates are offered with stems ranging from 2-5mm in 0.5mm increments, a 6mm stem, or without a stem. 2. Universal Forefoot Plates The plates are available in 7 sizes: 14mm, 16mm, 18mm, 20mm, 22mm, 24mm, and 30mm. 3. MPJ Fusion Plates The MPJ Fusion Plate is offered in medium and long lengths and left and right configurations. 4. Tarsalis Plates The 2-Hole Straight Tarsalis Plate is offered in short and long lengths. The 3-Hole and 10-Hole Straight Plates are included in the system as standard lengths. The 4- Hole Straight Tarsalis Plate is offered in both standard and short lengths. The Crescentic Tarsalis Plate is offered in left and right configurations. The 4-Hole Diamond Plate is available in left and right configurations. The T-Shaped Tarsalis Plates are offered in 4-hole, 5-hole, and, 6-hole configurations. The 5-hole designs are provided in straight, left, and right configurations. The 6-hole is only provided in straight configuration. OPTIONAL: Upon request, the 6-hole, 7-hole and 8-hole T-Shaped Tarsalis Plates are available. The 6-hole T-Shaped Tarsalis Plate is available in left and right. The 7-hole and 8- hole T-Shaped Tarsalis Plates are only available in left and right configurations. Rear Foot Plates 1. Lapidus Plates The plate is designed with a 0-6mm step in 1 mm increments (7 sizes) to displace the bones. 2. Interpositioning Plates The plate is offered with or without a stem to space apart two bones or a joint, with stems being offered in widths 2-12mm in 2mm increments. 3. Universal Rearfoot Plates The plates are available in 7 sizes: 14mm, 16mm, 18mm, 20mm, 22mm, 24mm, and 30mm.
2 4. Rearfoot Reconstruction Plates The plates are available in 7-hole, 9-hole and 14-hole versions. 5. Flat line Arthrodesis Plates The plates are available in 12mm, 14mm and 16mm sizes. 6. Dwyer Displacement Plates This plate is offered with three different step sizes; 6mm, 8mm, and 10mm 7. Calcaneus Plates The calcaneus plates are available in small, medium, extra small, mini and mini-long sizes. 8. Fibular Plates The Fibular plates are available in 3-hole, 4-hole, 5-hole, 6-hole, 7-hole, 8- hole, 10-hole, and 12-hole versions. The Fibular Tubular plates are available 4-hole, 6-hole, 8-hole, 10-hole, and 12-hole versions. MATERIAL The implants included in the Integra TFS2 are manufactured from Ti-6Al-4V Titanium Alloy (ASTM F136). The instrumentation is manufactured from biocompatible materials. INDICATIONS FOR USE The Integra TFS2 is intended for skeletally mature patients for the following: Stabilization and fixation of fresh fractures. Intra and extra articular fractures, joint depression, and multi-fragmentary fractures. Revision procedures, joint fusion and reconstruction of small bones of the feet. CONTRAINDICATIONS Plates and screws are contraindicated in: active infection, conditions which tend to retard healing such as blood supply limitations, previous infections, insufficient quantity or quality of bone to permit stabilization of the fracture complex, conditions that restrict the patient s ability or willingness to follow postoperative instructions during the healing process and foreign body sensitivity. Cases with malignant primary or metastatic tumors which preclude adequate bone support or screw fixations, unless supplemental fixation or stabilization methods are utilized. Foreign body sensitivity where material sensitivity is suspected, appropriate tests should be made and sensitivity ruled out prior to implementations. These implants are intended as a guide to normal healing, and are NOT intended to replace normal body structure or bear the weight of the body in the presence of incomplete bone healing. Delayed unions or non-unions in the presence of load bearing or weight bearing might eventually cause the implant to break due to metal fatigue. All
3 metal surgical implants are subjected to repeated stress in use, which can result in metal fatigue. WARNINGS AND PRECAUTIONS No metallic surgical implant should be reused. Any metal implant, once used, should be discarded. Even though it appears undamaged, it may already have small defects and internal stress patterns which may lead to fatigue failure. Correct handling of the implant is extremely important. Avoid contouring metallic implants whenever possible. If necessary, or allowed by design, the device should not be bent sharply, reverse bent, notched or scratched. All of these operations can produce defects in the surface finish and internal stress concentrations, which may become the focal point for eventual failure of the appliance. If metal plates or other metallic devices are to be used together with the TFS2, all such devices should be manufactured from a metal that has a similar composition to avert possibility of galvanic corrosion or other metallic reactions. Correct selection of the implant is extremely important. The potential for success in fracture fixation is increased by the selection of the proper size, shape and design of the implants. The patient s anatomy and indication will determine the size of the TFS2 plate to be used. The size and shape of the human bones presents limiting restrictions on the size and strength of implants. Postoperative care is extremely important. The patient must be warned that noncompliance with postoperative instructions could lead to breakage of the implant requiring revision surgery to remove the device. The use of TFS2 provides the surgeon a means of bone fixation and helps generally in the management of fractures and reconstructive surgeries. The implants are intended as a guide to normal healing and are NOT intended to replace normal body structure or bear the weight of the body in the presence of incomplete bone healing. Delayed unions or nonunions in the presence of load bearing or weight bearing might eventually cause the implant to break due to metal fatigue. All metal surgical implants are subject to repeated stress in use which can result in metal fatigue. Failure to immobilize a delayed union or nonunion of bone will result in excessive and repeated stresses which are transmitted by the body to any temporary internal fixation device prior to the healing of the fracture. Due to normal metal fatigue, these stresses can cause eventual bending or breakage of the device. Therefore, it is important that immobilization of the fracture site is maintained until firm bony union (confirmed by clinical and roentgenographic examination) is established.
4 No partial weight bearing or non-weight bearing device can be expected to withstand the unsupported stresses of full weight bearing. Until firm bone union is achieved, the patient should employ adequate external support and restrict physical activities which would place stress upon the implant or allow movement at the fracture site and delay healing. Detailed written instructions on the use and limitations of the device should be given to the patient. If partial weight bearing is recommended or required prior to firm bony union, the patient must be warned that bending or breakage of the device are complications which may occur as a result of the weight bearing or muscle activity. An active patient or a debilitated or demented patient who cannot properly utilize weight support devices may be particularly at risk during postoperative rehabilitation. While the surgeon must make the final decision on implant removal, whenever possible and practical for the individual patient, fixation devices should be removed once their service as an aid to healing is accomplished, particularly in younger more active patients. The MR environment presents risks to patients with metal implants. Review of the available literature documents that metal implants may heat resulting in tissue damage and may migrate out of position. They may also cause artifact affecting image quality. Physicians should take these risks into consideration when recommending MRI imaging for patients with metal implants. Note: Integra TFS2 has not been evaluated for safety and compatibility in the MR environment. The Integra TFS2 has not been tested for heating or migration in the MR environment. REPROCESSING INSTRUCTIONS The Integra TFS2 Instrument Tray and its contents are provided non-sterile, and must be cleaned and sterilized prior to use. All reusable instruments must be cleaned, inspected, and sterilized between uses. Always immediately clean and decontaminate all devices that have been soiled. Reusable instruments can be used indefinitely if not damaged, worn, or deteriorated, and should be inspected before each use for these conditions. DO NOT use broken, damaged, malfunctioning or deteriorated instruments. Examples of unacceptable wear or deterioration include any cracking, bending, corrosion, missing components, or visible wear which could impact function or performance (e.g. dull cutting edges). After cleaning the Ratchet Handle, a non-silicone lubricant should be used per the manufacturer s instructions for best results and to ensure proper working condition. Verify that all instruments required for use are present in the case. For manual cleaning, devices should be grouped according to similar metals before subsequent processing in order to prevent galvanic corrosion. In addition, it is not recommended to use chloride containing cleaning solutions since its use has been linked to corrosion of metallic instruments, especially stainless steel. Please also note the following: Disinfect and clean devices immediately after use in order to avoid device encrustations.
5 Solutions used for cleaning must always be prepared in accordance with the manufacturer s instructions. Never use metal brushes or metal sponges for manual cleaning. Use a suitably sized brush for cleaning lumens, cannulations, blind holes, and cavities, making sure that every part of the inner surface can be properly accessed. Clean jointed instruments in closed as well as open positions. Disassemble instruments as far as possible before cleaning. Be sure to arrange the items so that the water can easily flow out of cannulations, blind holes, and cavities. For instruments with long or narrow lumens, standard processing should be used only if the hot disinfectant can flow easily through the lumens and safe rinsing is guaranteed. The cases/trays used for cleaning must always be loaded correctly to ensure proper cleaning. After cleaning, check instruments for cleanliness (visible dirt). This especially applies to cannulated instruments or those with blind holes and crevices. To ensure proper instrument functioning, verify that all movable parts have been thoroughly cleaned. Pay special attention to slots, ratchets, joints and box locks, narrow lumens, blind holes, and other areas that are hard to access. Demineralized or distilled water should be used for the final rinse. MANUAL CLEANING INSTRUCTIONS The following steps should be completed in sequence: a) Prepare a neutral ph enzymatic detergent as per the manufacturer s recommendation (e.g. Enzol prepared at 1 oz. per gallon of lukewarm deionized water). b) Disassemble instruments to lowest level. c) Rinse instruments under lukewarm running water to remove all gross soil. Use a soft bristled brush to aid in the brushing. Agitate the instruments under the running water. Agitation includes actuating all movable parts such as opening and closing hinges and moving the instruments around under the running water. Use a clean soft bristled brush and/or pipe cleaner to brush and aid in the rinse for the exterior and interior of instruments. Use a syringe to flush any lumens. d) Fully immerse each device in the prepared detergent and allow it to soak for a minimum of two minutes. e) After soaking the devices, scrub them using a soft bristle brush and circular strokes to remove any visible soil. Pay particular attention to all the areas where the soil could be imbedded (i.e. grooves, crevices, lumens, blind holes). Use a syringe to flush lumens and a pipe cleaner to clean lumens and holes. Perform cleaning under the surface of the prepared detergent solution to limit aerosolization of the cleaning fluid and soil, as well as for worker and environmental safety. f) Rinse devices in lukewarm water for a minimum of one and a half (1.5) minutes to remove any detergent residuals. In accordance with Step C, agitate the instruments under the running water, being sure to actuate all movable parts, and using a soft bristled brush for internal and exterior device surfaces.
6 g) Prepare a neutral ph enzymatic detergent (eg. Enzol ) in a sonicator, as per the manufacturer s recommendation using lukewarm water. Fully immerse the devices in the detergent and sonicate for 10 minutes. h) After sonication, rinse the devices with running lukewarm water (use the highest grade of water available, distilled or deionized water is recommended) for three (3) minutes. Agitate the instruments under the running water, being sure to actuate all movable parts, and using a clean soft bristled brush for internal and exterior device surfaces, and flush all lumens with a syringe. i) Dry the devices using a clean lint free cloth and visually examine to determine if all adherent visible soil has been removed. j) Repeat the above cleaning procedure, if visible debris is detected. AUTOMATED IFU CLEANING PROCEDURE The following steps should be completed in sequence: a) Prepare an enzymatic detergent using lukewarm deionized water as per the manufacturer s recommendation. b) Fully immerse the devices and allow to soak for a minimum of two (2) minutes. c) Following the soak time, flush any lumens of the device using a syringe. d) Rinse the devices under lukewarm running deionized water for a minimum of one (1) minute, while agitating the devices. Agitation includes actuating all movable parts, such as opening and closing hinges and moving the devices around under the running water. e) Use a clean soft bristled brush and/or pipe cleaner to brush and aid in the rinse for the exterior and interior of device components. Use a syringe to flush any lumens. f) Place the devices back into the designated locations of the case/tray, and load the case/tray set into an automated washer (Steris 444 or equivalent). g) The washer cycle parameters are as follows: Phase Recirculation Time (Min.) Water Temperature Detergent Pre-Wash 1 02:00 Cold Water NA Enzyme Enzymatic 01:00 Hot Water Wash Cleaner Wash 1 02:00 60 C Neutral Detergent Rinse 1 10:00 Hot Water** NA **Note: The highest grade of water available should be used during the final rinse cycle. h) After washing, dry the devices using a clean lint free cloth and visually examine to determine if all adherent visible soil has been removed. i) Repeat the cleaning procedure if visible debris is detected.
7 INSPECTION AFTER CLEANING Following cleaning, the instruments must be macroscopically clean, i.e. free from visible dirt or deposits. All movable parts, working tips and blades (scissors) should be inspected with particular care. STERILITY The Integra TFS2 instrumentation are supplied non-sterile and must be sterilized before use. Do not reuse implants. They are single use only. Visually inspect the instruments under room lighting conditions to ensure instruments are suitable for use and free of debris. Sterilize with steam sterilization. The following cycles are recommended for wrapped goods: Cycle: Domestic EMEA Pre-vacuum Gravity Pre-vacuum Pre-vacuum Temperature: (274 F) 132 C (274 F) 132 C 134 C 134 C Exposure Time: 4 mins 15 mins 3 mins 18 mins Drying Time: 60 minutes 60 minutes 65 minutes 65 minutes ADVERSE EVENTS SURGICAL PROCEDURE Loosening, bending, cracking or fracture of the screw or loss of fixation in bone attributable to nonunion. Loss of anatomic position with nonunion or malunion with rotation or angulation. Infection, both deep and superficial. Allergies and other reaction to the device material. A Surgical Technique brochure is available which outlines the basic procedure for device implantation and the use of specialized surgical instrumentation. It is the responsibility of the surgeon to be familiar with the procedure before use of these products. Each surgeon must evaluate the appropriateness of the surgical technique used based on personal medical training and experience. Copies of the surgical technique may be obtained online at by contacting customer service at , or by contacting your local sales representative. TRAINING It is the responsibility of the surgeon to obtain the necessary training prior to implanting this implant. The surgeon should have specific training, experience, and thorough familiarity with the use of cannulated, non-cannulated, headless, and snap-off screws. The surgeon must exercise reasonable judgment when deciding which screw type to use for specific indications.
8 MANUFACTURED BY: Ascension Orthopedics Inc Cameron Road Austin, Texas USA Voice: Fax: AUTHORIZED REPRESENTATIVE: Integra LifeSciences Services France Immeuble Séquoia 2 97 Allée Alexandre Borodine Parc Technologique de la Porte des Alpes Saint Priest, France Voice +33 (0) Fax +33 (0)
9 Symbol Description Do not re-use Compliance with EU legislation Consult Instructions for use Manufacturer For prescription use only Implant plate and screw material is Ti-6AI-4V Titanium Alloy (ASTM F136) Reference Number Batch Number Authorized EC Representative in the European Community Quantity Non Sterile Integra and the Integra logo are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United States and/or other countries. Enzol is a trademark of Johnson & Johnson Integra LifeSciences Corporation. All rights reserved. Printed in the USA. LC Rev A
Lapidus Arthrodesis System Instructions for Use
 Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Lapidus Arthrodesis System Instructions for Use Description The AlignMATE Lapidus Arthrodesis System consists of bone plates and bone screws (locking, non-locking and interfragmentary), which are intended
Pinit Plate Small Bone Fusion System Bone Plate & Screw System
 Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Pinit Plate Small Bone Fusion System Bone Plate & Screw System Description The Pinit Plate Small Bone Fusion System consists of 2-hole bone plates made available in three length options and two thickness
Technique Guide Small Bone Fusion System
 Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
Technique Guide Small Bone Fusion System The Pinit Plate Small Bone Fusion System is a super low profile, modular bone plate and screw system designed to stabilize a bunionectomy with a medial to lateral
LBL-014. Rev
 Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
Package Insert Z- CLAMP ISP System Device Description: The Z-CLAMP ISP System is supplemental fixation device consisting of a variety of shapes and sizes of one-level lumbar and sacral plates and screws.
OBSOLETE. Visit for the latest version. Ankle Plating System 3 PKGI-79-B EFFECTIVE
 Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
Ankle Plating System 3 MediMark Europe Sarl. 11 rue Emile ZOLA. BP 2332 38033 GRENOBLE CEDEX 2 FRANCE +33.4.76.86.43.22 Acumed LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124-9432 +1.503.627.9957 acumed.net
M6-C Artificial Cervical Disc Surgical Instruments
 CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
CARE AND HANDLING INSTRUCTIONS Orthofix Inc. 3451 Plano Parkway Lewisville, Texas 75056-9453 U.S.A. 1-214-937-3199 1-888-298-5700 www.orthofix.com OSI-CustomerService@Orthofix.com Spinal Kinetics LLC,
2. Active systemic infection or infection localized to the site of the proposed implantation is contraindications to implantation.
 OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
OIC Pedicle Screw System! The Orthopaedic Implant Company 316 California Ave #701 Reno, NV 89509 USA System Contents: Non-Sterile Implants Single Use Only Non-Sterile Instruments - Reusable 2 Caution:
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert
 X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
X-spine Aranax Anterior Cervical Plating System Instructions for Use / Package Insert GENERAL INFORMATION The Aranax Cervical Plating System consists of screws and plates offered in various sizes so that
Reprocessing Guide. Autoclavable Arthroscope and Hardware Sterilization Tray
 Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Reprocessing Guide Autoclavable Arthroscope and Hardware Sterilization Tray 0233032116 Contents Introduction...2 Intended Use of Sterilization Trays...4 Warnings...5 Cautions...5 Instructions...6 Sterilization
Technique Guide Lapidus Arthrodesis System
 Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
Technique Guide Lapidus Arthrodesis System HVA Angle IMA Angle The AlignMate Lapidus Arthrodesis System features low-profile, anatomically pre-contoured Bone Plates with either a combination of locking
For use by an Accredited Orthopaedic Surgeon only
 Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
Page 1 of 5 For use by an Accredited Orthopaedic Surgeon only 1. Purpose: External fixators are intended to aid in surgical stabilization following operative procedures to treat fractures, enable correction
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System
 X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
X-spine Systems, Inc. Silex Sacroiliac Joint Fusion System GENERAL INFORMATION The Silex Sacroiliac Joint Fusion System consists of different diameter bone screws in various lengths and thread configurations
Surgical Technique Guide
 Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Surgical Technique Guide Flow-FX, LLC - 815.531.4424-9110 Darvin Drive, Mokena, IL 60448 - www.flow-fx.net Contents Implant Overview 1 Implant Features and Benefits 2 Instrument Overview 3 Surgical Technique
Velocity Orthopedics Instrument Cleaning and Sterilization
 Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
Velocity Orthopedics Instrument Cleaning and Sterilization It is important to read the Instructions For Use in its entirety prior to using the product. Caution: Federal law (USA) restricts this device
EVOLVE TRIAD SYSTEM
 EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EN EVOLVE TRIAD SYSTEM 146884-2 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information option.
EVOLVE TRIAD BONE SCREWS
 EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
EN EVOLVE TRIAD BONE SCREWS 146886-1 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing Information
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
0086 Instructions for Use RCS Anterior Buttress Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM
 For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE MATRIXRIB FIXATION SYSTEM 10/16 GP2685-E-CAN DESCRIPTION The MatrixRIB Fixation System consists of locking plates, locking screws,
X-spine Systems, Inc. Axle Interspinous Fusion System
 X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Axle Interspinous Fusion System GENERAL INFORMATION The Axle Interspinous Fusion System of X-spine Systems, Inc., is an internal fixation device for spinal surgery. Various sizes
X-spine Systems, Inc. Spider Cervical Plating System
 X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Spider Cervical Plating System GENERAL DESCRIPTION The Spider Cervical Plating System consists of screws and plates offered in various sizes so that adaptations can be made to take
X-spine Systems, Inc. Certex Spinal Fixation System
 X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
X-spine Systems, Inc. Certex Spinal Fixation System GENERAL INFORMATION The Certex Spinal Fixation System consists of screws, hooks, rods, plates and connectors. Various sizes of these implants are available
SI-BONE ifuse Implant System Instruments Hospital Cleaning and Sterilization Instructions 0344
 HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
HOW SUPPLIED The SI-BONE instruments are supplied non-sterile and must be cleaned and sterilized prior to use. The ifuse Implant is provided separately and supplied sterile. Please refer to the ifuse System
The following languages are included in this packet:
 DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
DARCO SIMONS-PLANTAR LAPIDUS PLATE 150857-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System
 X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Irix-A Lumbar Integrated Fusion System GENERAL INFORMATION The Irix-A Lumbar Integrated Fusion System is a stand-alone intervertebral fusion device to restore biomechanical height
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System
 X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
X-spine Systems, Inc. Butrex Lumbar Buttress Plating System GENERAL DESCRIPTION The Butrex Lumbar Buttress Plating System is intended for anterior screw fixation to the L1 to S1 spine. The Butrex System
DARCO MIS FOREFOOT SCREW
 DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
DARCO MIS FOREFOOT SCREW 140418-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) - Chinese (sch)
It is intended that the implants be removed after successful fusion.
 INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
INSTRUCTIONS FOR USE Solanas Posterior Stabilization System GENERAL INFORMATION: The Solanas Posterior Stabilization System facilitates the surgical correction of spinal deformities by providing temporary
X-spine Systems, Inc. Fixcet Spinal Facet Screw System
 X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
X-spine Systems, Inc. Fixcet Spinal Facet Screw System GENERAL INFORMATION The Fixcet Spinal Facet Screw System is designed to provide bilateral, transfacet fixation of the spinal facet joint in the lumbar
REDUCT Headless Compression Screw System
 REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
REDUCT Headless Compression Screw System INSTRUCTIONS FOR USE : For use by physicians only. Federal Law restricts this device to sale by or on the order of a physician. Failure to follow instructions may
Page 1 of 7 Doc. No.: IU/15 Rev. No.: 05 Rev. Date:03MAY2018. Instructions for use for ATLAS Tibial Fracture(TF) Nail
 Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
Page 1 of 7 Important Inmation on ATLAS Tibial Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: The ATLAS TFN (Tibial Fracture Nail) is designed to handle tibial fracture
INTELLIGENT SPINAL SYSTEM
 INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
INTELLIGENT SPINAL SYSTEM I. Introduction II. Product Specification III. Surgical Technique IV. Ordering Information V. IFU for Lospa IS SPINAL SYSTEM The LOSPA IS spinal system consists of
Metallic Internal Fixation Devices The following languages are included in this packet:
 Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Metallic Internal Fixation Devices 150848-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Integra. surgical technique. Advansys Midfoot Plating System. eng. D.L.P. Dorsal Lisfranc Plate. M.L.P. Medial Lisfranc Plate
 eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
eng Integra Advansys Midfoot Plating System surgical technique D.L.P. Dorsal Lisfranc Plate M.L.P. Medial Lisfranc Plate Table of Contents Advansys Medial Lisfranc Plate (DLP)... 4 Indications... 4 Contraindications...
SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM The following languages are included in this packet:
 EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
EN SIDEKICK EZ FRAME EXTERNAL FIXATION SYSTEM 149369-0 English (en) The following languages are included in this packet: For additional languages, visit our website www.wmt.com. Then click on the Prescribing
INSTRUCTIONS FOR USE AND CARE
 DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
DIAMONDS INSTRUCTIONS FOR USE AND CARE DIAMONDS, MULTI-USE, FRICTION GRIP Quala Diamonds are a rotary cutting device made of stainless steel and coated with diamond particles on the working end. It is
EXTERNAL FIXATION SYSTEMS The following languages are included in this packet:
 EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
EXTERNAL FIXATION SYSTEMS 150860-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch)
Important Information on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only
 0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
0434 Page 1 of 6 Instructions use Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking
X-spine Systems, Inc. Calix PC Spinal Implant System
 X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
X-spine Systems, Inc. Calix PC Spinal Implant System Y IMPORTANT NOTE: The user acknowledges that he/she has read and agreed to the conditions in this insert, which are to be considered as contractual.
Technique Guide KISSloc Suture System
 Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
Technique Guide KISSloc Suture System The KISSloc Suture System consists of a strong self-cinching suture assembly, a unique load-dispersing Arrow Plate and procedure specific instrumentation. Simple,
RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON
 ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
ACUTE Innovations LLC RibLoc U Plus CHEST WALL PLATING SYSTEM FOR THE PERSONAL ATTENTION OF THE OPERATING SURGEON INSTRUCTIONS FOR USE DESCRIPTION The ACUTE Innovations RibLoc U Plus Chest Wall Plating
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
Instructions for Use AccuFit Lateral Plate System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE
 ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
ORTHOLOC 3Di PLANTAR LAPIDUS PLATE 152130-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese
Instructions for use for Bone Plates, Bone Screws, Intramedullary Nails, Pins and Wires
 For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
For use by an Accredited Orthopaedic Surgeon only IMPORTANT MEDICAL INFORMATION Page 1 of 6 1. Purpose: are intended to aid in surgical stabilization following operative procedures to treat fractures,
Revision A Date:
 Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
Biomet Trauma 56 East Bell Drive P.O. Box 587 Warsaw, Indiana 46581 USA 01-50-4053 Revision A Date: 2016-06 Biomet Unite3D Bridge with OsseoTi Technology ATTENTION OPERATING SURGEON DESCRIPTION Biomet
DISTAL RADIUS. Instructions for Use
 DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
DISTAL RADIUS Instructions for Use CAUTION: FEDERAL LAW RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN. Table of Contents 1. INTENDED USE... 3 2. DEVICE DESCRIPTION... 3 3. METHOD OF
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician
 Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
Instructions for Use Reform POCT System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY There is no express or
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE
 English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
English G EMINU S Select Distal Radius S ystem INSTRUCTIONS FOR USE : For use by physicians only. Caution: Federal Law restricts this device to sale by or on the order of a physician. Failure to follow
Flexible Fragment Fixation. Surgical Technique
 Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Flexible Fragment Fixation Surgical Technique 2 F 3 Flexible Fragment Fixation The F 3 Fragment Plating System offers low profile, yet strong fixation in a locked plating construct that can be contoured
Aesculap Implant Systems
 Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
Aesculap Implant Systems Aesculap Implant Systems Spine USA Instructions for use/technical description Page 1 of 9 A 2 1 4 B 3 Page 2 of 9 USA Aesculap Implant Systems Instrument Legend A Rigid Inserter
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System
 X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
X-spine Systems, Inc. Xpress Minimally Invasive Pedicle Screw System GENERAL INFORMATION The Xpress Minimally Invasive Pedicle Screw System consists of rods, pedicle screws, screw caps and hand instruments.
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
 0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
0086 Instructions for Use ShurFit Anterior Cervical Interbody Fusion System Caution: Federal (USA) law restricts this device to sale by or on the order of a physician DISCLAIMER OF WARRANTY AND LIMITATION
BIOFOAM BONE WEDGE
 BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
BIOFOAM BONE WEDGE 150837-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM
 METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
METATARSAL DECOMPRESSION IMPLANT (MDI) SYSTEM 152381-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE
 OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
OPTIMUS CUSTOM SPINE OPTIMUS: STAND-ALONE ANTERIOR LUMBAR DEVICE CAUTION: Federal law (U.S.A.) restricts this device to sale by or on the order of a licensed physician. CONSISTS OF STERILE AND NON-STERILE
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. ACDF Anterior Cervical Discectomy and Fusion
 SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
SURGICAL TECHNIQUE GUIDE TM BRECKENRIDGE Intervertebral Body/VBR Fusion System ACDF Anterior Cervical Discectomy and Fusion The following general Surgical Technique Guide is for illustrative purposes only.
CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM
 EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
EN CLAW II POLYAXIAL COMPRESSION PLATING SYSTEM 150871-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português
MATERIAL SAFETY DATA SHEET (MSDS) Information about stainless steel for Surgical/Dental/Orthodontic Instruments.
 MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
MATERIAL SAFETY DATA SHEET (MSDS) THE INFORMATION BELOW IS BELIEVED TO BE ACCURATE AND REPRESENTS THE BEST INFORMATION CURRENTYLY AVAILABLE TO US. HOWEVER, WE MAKE NO WARRANTY OF MERCHANTABILITY OR ANY
Zavation Z-Link Cervical
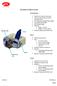 Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Zavation Z-Link Cervical Interbody Plate: Interbody Plate Screw Quarter turn locks for each screw Locks use same driver as used for inserting screws 30 caudal and cephalad biased angles 15 midline biased
Integra. Uni CP Compression Plating System PRODUCTS FOR SALE IN EUROPE, MIDDLE EAST AND AFRICA ONLY
 TM Integra Uni CP Compression Plating System S U R G I C A L T E C H N I Q U E Uni CP Compression Plating System Table of contents Indications...03 Contraindications...03...03 Surgical Technique... 04
TM Integra Uni CP Compression Plating System S U R G I C A L T E C H N I Q U E Uni CP Compression Plating System Table of contents Indications...03 Contraindications...03...03 Surgical Technique... 04
large qwix Positioning and fixation screws Surgical Technique lower large qwix English PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY
 large qwix Positioning and fixation screws Dia. 7.5 mm Dia. 5.5 mm large qwix English Surgical Technique orthopedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY LARGE qwix
large qwix Positioning and fixation screws Dia. 7.5 mm Dia. 5.5 mm large qwix English Surgical Technique orthopedics lower extremity PRODUCTS FOR SALE IN EUROPE, MIDDLE-EAST and AFRICA ONLY LARGE qwix
The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental fixation is used.
 Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Instructions for Aesculap Implant Systems A-Space SIBD Spinal System Indications The A-Space SIBD Spinal System is a stand-alone device intended to be used with the four supplied bone screws if no supplemental
Integra. Solustaple Standard staple SURGICAL TECHNIQUE. Uni-Clip Compression staple. Products for sale in Europe, Middle-East and Africa only.
 eng Integra Solustaple Standard staple SURGICAL TECHNIQUE Uni-Clip Compression staple Table of Contents Solustaple Standard staple...04 Implants Details...04 Indications...04 Instruments Details...04 Surgical
eng Integra Solustaple Standard staple SURGICAL TECHNIQUE Uni-Clip Compression staple Table of Contents Solustaple Standard staple...04 Implants Details...04 Indications...04 Instruments Details...04 Surgical
Precautionary Statement ( )
 Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
Precautionary Statement (21282008) Biomet Sports Medicine, Inc. 21282008 4861 E. Airport Dr. Rev. A Ontario, CA 91761 Date: 06/07 Sleeve and Button Soft Tissue Devices Utilizing ZipLoop Technology ATTENTION
OPERATIVE TECHNIQUE. CONSTRUX Mini PTC. Mini PTC Spacer System
 OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
OPERATIVE TECHNIQUE CONSTRUX Mini PTC Mini PTC Spacer System TABLE OF CONTENTS Introduction 1 Operative Technique 2 Part Numbers 6 Indications For Use 7 INTRODUCTION 1 INTRODUCTION The CONSTRUX Mini PTC
Integra. DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE
 Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
Integra DigiFuse Cannulated Intramedullary Fusion System SURGICAL TECHNIQUE Table of Contents Design Rationale... 2 System Features... 2 Indications... 2 Contraindications... 2 Surgical Technique...3 Step
The Omega LIF System devices are made from Titanium alloy Ti6Al4V ELI, ASTM F136.
 Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
Omega Lumbar Interbody Fusion Device Package Insert CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only. DESCRIPTION:
MICA SCREWS For additional information and translations please contact the manufacturer or local distributor.
 MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
MICA SCREWS 152580-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe (tk)
D. The following factors are of extreme importance to the eventual success of the procedure.
 Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
Reprocessed by Stryker Sustainability Solutions 1810 W. Drake Drive Tempe, AZ 85283 sustainability.stryker.com phone: 888.888.3433 English REPROCESSED EXTERNAL FIXATION DEVICES ATTENTION OPERATING SURGEON
eng Integra surgical technique Large Qwix Products for sale in Europe, Middle-East and Africa only.
 eng Integra Large Qwix surgical technique Positionning Positioning and Fixation fixation Screw screw Table of Contents Concept...04 Indications...04 Contraindications...04 Implant details...05 Description...06
eng Integra Large Qwix surgical technique Positionning Positioning and Fixation fixation Screw screw Table of Contents Concept...04 Indications...04 Contraindications...04 Implant details...05 Description...06
ATEC Porous Ti System
 ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
ATEC Porous Ti System GENERAL INFORMATION: The ATEC Porous Ti System is an intervertebral body fusion device with implants of various lengths, widths, heights, and degrees of lordosis to accommodate individual
Amendia Interbody Fusion Devices PACKAGE INSERT
 LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
LB-182 Rev 0 Amendia Interbody Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use
Zeus Intervertebral Body Fusion Devices PACKAGE INSERT
 LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
LB-119 Rev 4 Zeus Intervertebral Body Fusion Devices PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single
TM TM Surgical Technique
 TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
TM TM Surgical Technique TABLE OF CONTENTS Reli SP Spinous Plating System Overview Device Description Implant Features Indications Instruments Access Instruments Preparation Instruments Insertion Instruments
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM
 ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
ORTHOLOC 3Di ANKLE FUSION PLATING SYSTEM 150884-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt)
STABILIZATION AND FRACTURE FIXATION
 STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
STABILIZATION AND FRACTURE FIXATION 150838-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) Türkçe
BONE GRAFT WASHER _Rev A IMPORTANT INFORMATION ON THE BONE GRAFT WASHER
 0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
0381080_Rev A BONE GRAFT WASHER IMPORTANT INFORMATION ON THE BONE GRAFT WASHER 03/2008 Medtronic Sofamor Danek USA, Inc. 1800 Pyramid Place Memphis, Tennessee 38132 Telephone: 800-933-2625 (in U.S.A.)
Manufacturer s information
 WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
WARNINGS: Observe the standard German accident prevention regulations (UVV) We are not aware of any warnings if the instructions for the devices and disinfection and cleaning agents to be used are followed.
IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY)
 IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY) IMPORTANT NOTE Intramedullary nails provide an alternative to open reduction
IMPORTANT MEDICAL INFORMATION Advanced Orthopaedic Solutions INTRAMEDULLARY NAILS Warnings and Precautions (SINGLE USE ONLY) IMPORTANT NOTE Intramedullary nails provide an alternative to open reduction
TMJ Fossa-Eminence Prosthesis System. Instructions for Use. For partial reconstruction (hemiarthroplasty) of the temporomandibular joint
 TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
TMJ Fossa-Eminence Prosthesis System Instructions for Use For partial reconstruction (hemiarthroplasty) of the temporomandibular joint Stock TMJ Fossa-Eminence Prosthesis System RIGHT SIDE (REF: FER-01
Spartan S 3 Facet Screw System PACKAGE INSERT
 LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
LB-004 Rev 6 Spartan S 3 Facet Screw System PACKAGE INSERT CAUTION: Federal law (USA) restricts these devices to sale by or on the order of a physician. Implants and disposable instruments single use only.
Zimmer Anterior Buttress Plate System. Surgical Technique
 Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Zimmer Anterior Buttress Plate System Surgical Technique 2 Zimmer Anterior Buttress Plate System Surgical Technique Zimmer Anterior Buttress Plate System Surgical Technique Description, Indications & Contraindications...
Indications: ATLAS HF Nail is indicated for fractures of the femur including: Contraindications:
 Page 1 of 6 Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking nail with
Page 1 of 6 Important Inmation on ATLAS Hip Fracture Nail For use by an Accredited Orthopaedic Surgeon only Device Description: Atlas Hip Fracture (HF) Nail is an intramedullary interlocking nail with
OPERATIVE TECHNIQUE. anterior cervical plating system
 OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
OPERATIVE TECHNIQUE 3º anterior cervical plating system Introduction 1 Pre-Operative Technique 2 Oerative Technique 3 Instructions for Use 12 Part Numbers 16 The surgical technique shown is for illustrative
Endoscopic Technology. Operating Manual. Cannula system
 Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
Endoscopic Technology Operating Manual AlphaPort Cannula system This manual contains proprietary information that is protected by copyright. All rights are reserved. This manual or excerpts thereof may
M Manufacturer by: Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants
 Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
Guide for Cleaning, Sterilization and Storage of Instruments for Caldera Medical Desara Product Family of Implants M Manufacturer by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S.
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL
 ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
ASFORA ANTERIOR CERVICAL PLATE SYSTEM (AACP) SURGICAL PROCEDURE MANUAL Contents: Introduction Indications Contraindications Warnings Precautions Implant Overview Instruments Overview Surgical Technique
CANNULATED SCREW SYSTEM The following languages are included in this packet:
 CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
CANNULATED SCREW SYSTEM 137183-1 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) -Chinese (sch) Türkçe
Asnis. Micro Cannulated screw system. Xpress operative technique
 Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Asnis Micro Cannulated screw system Xpress operative technique Asnis Micro Cannulated screw system Table of contents Indications, precautions & contraindications 3 Operative technique 4 This publication
Salto Talaris Total Ankle Prosthesis
 Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Instructions for Use Salto Talaris Total Ankle Prosthesis Federal (USA) law restricts this device to sale by or on the order of a physician. Important The manufacturer recommends that all personnel responsible
Duraloc CONSTRAINED LINER
 SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
SURGICAL TECHNIQUE Duraloc CONSTRAINED LINER A COMPREHENSIVE ACETABULAR REVISION SYSTEM DURALOC CONSTRAINED LINER Introduction Dislocation is the most common postoperative complication in total hip reconstruction.
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE. This Package Insert is for product distributed in the US only.
 TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
TC-PLUS Primary Knee IMPORTANT MEDICAL INFORMATION SPECIAL NOTE This Package Insert is for product distributed in the US only. The component material is provided on the outside carton label. Components
PRO-DENSE Bone Graft Substitute The following languages are included in this packet:
 EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
EN PRO-DENSE Bone Graft Substitute 133486-11 The following languages are included in this packet: English (en) For additional languages, visit our website www.wmt.com Then click on the Prescribing Information
Talar Dome System Surgical Technique
 Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
Talar Dome System Surgical Technique CAP TALAR DOME RESURFACING HEMIARTHROPLASTY IMPLANT Surgical Technique Guide Description The HemiCAP Contoured Articular Prosthetic incorporates an articular resurfacing
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE. Intervertebral Body/VBR Fusion System. Oblique Lumbar Interbody Fusion
 SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
SURGICAL TECHNIQUE GUIDE BRECKENRIDGE TM Intervertebral Body/VBR Fusion System Oblique Lumbar Interbody Fusion The following general Surgical Technique Guide is for illustrative purposes only. As with
PRO-DENSE BONE GRAFT SUBSTITUTE
 PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
PRO-DENSE BONE GRAFT SUBSTITUTE 152917-0 The following languages are included in this packet: For additional languages, visit our website www.wright.com. Then click on the option. For additional information
Scope This policy applies to all personnel and departments that clean, prepare and/or sterilize items intended for patient care use.
 Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Dental Sterilization Procedures Policy Number VIM4(4)-10 Purpose The purpose of this policy is to ensure patient and employee safety when using instruments with potential for exposure to bloodborne pathogens
Headless Compession Screw 2.5 / 3.0
 SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
SURGICAL TECHNIQUE Headless Compession Screw 2.5 / 3.0 Titanium or Stainless Steel Cannulated Headless Design Multiple Thread Options Torx Driver Sterile and Non-Sterile Options Simple Instrumentation
IPP-ON. Interphalangeal implant. Surgical Technique. l o w e r e x tre mit y. Or t h o pa e d i c s. IPP-ON English
 IPP-ON Interphalangeal implant IPP-ON English Surgical Technique Or t h o pa e d i c s l o w e r e x tre mit y IPP-ON Rationale Indications The proximal interphalangeal fusion is a challenging surgery.
IPP-ON Interphalangeal implant IPP-ON English Surgical Technique Or t h o pa e d i c s l o w e r e x tre mit y IPP-ON Rationale Indications The proximal interphalangeal fusion is a challenging surgery.
METAL HEMI SYSTEM
 METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
METAL HEMI SYSTEM 152206-0 The following languages are included in this packet: English (en) Deutsch (de) Nederlands (nl) Français (fr) Español (es) Italiano (it) Português (pt) 中文 - Chinese (sch) Türkçe
Customized Cranial and Craniofacial Implants. Instructions For Use. 7000revD_Instructions_For_Use. Page 1 of 8
 Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
Customized Cranial and Craniofacial Implants Instructions For Use Page 1 of 8 Table of Contents INDICATIONS... 3 DESCRIPTION OF DEVICE... 3 CONDITIONS OF USE... 3 CONTRAINDICATIONS... 3 KELYNIAM GLOBAL
