IMMORTALIZATION OF MIP-GFP NEURONS FROM THE HYPOTHALAMUS AND NEURONAL CELL TYPES FROM HUMAN ISLET ISOLATES
|
|
|
- Preston Cooper
- 6 years ago
- Views:
Transcription
1 IMMORTALIZATION OF MIP-GFP NEURONS FROM THE HYPOTHALAMUS AND NEURONAL CELL TYPES FROM HUMAN ISLET ISOLATES BY ZI CHEN WANG A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Physiology University of Toronto Copyright - Zi Chen Wang 2013
2 IMMORTALIZATION OF MIP-GFP NEURONS FROM THE HYPOTHALAMUS AND NEURONAL CELL TYPES FROM HUMAN ISLET ISOLATES Zi Chen Wang Master of Science Department of Physiology University of Toronto 2013 Abstract- The mouse insulin I promoter construct was developed to eliminate the unintended hypothalamic activity seen in the rat insulin II promoter. Given the importance of the hypothalamus in the maintenance of energy homeostasis, the specificity of this construct must be validated. To investigate the possible promoter activity within the hypothalamus, primary hypothalamic cultures of fluorescent reporter mice were immortalized and sorted. The resulting mhypoa-mip/gfp cell lines confirmed the activity of the promoter within hypothalamic neurons and act as representative neuronal models. Initial characterization has determined the insulin responsive nature of these NPY neurons. Limited in vivo validations have confirmed the NPY nature of these neurons but were unable to pinpoint their localization within the hypothalamus. Subsequently, the technology utilized was adapted to attempt to generate immortalized human islet cells with limited success. The newly generated cell models can be utilized to refine the promoter construct and human islet immortalization process. ii
3 Acknowledgements I would like to express my deepest gratitude to Dr. Denise Belsham, without her aid, the timely completion of my degree would have been impossible. Denise, I hereby thank you for your early intervention and allowing me to transfer to your lab. You have provided a rich and welcoming environment as well as constant support and inspiration for the duration of my studies. Thanks to your efforts, I have been able to substantially improve my skills as a scientist and advance as a person. I am infinitely grateful for both the experience and the opportunity given to me. To Dr. Wheeler and Dr. Giacca, I would like to express gratitude for your invaluable guidance and refreshing ideas. Without your aid, my project would not be where it is today. To my fellow labmates and friends, thank you all for your ideas, support and patience. You guys have made these past 24 months fly by and I will always cherish the moments that I have spent with you. iii
4 Table of Contents Chapter 1- Introduction Overview Promoter and cell specificity Metabolic disorders and energy homeostasis Hypothalamus and feeding control Central effects of insulin on energy balance and obesity Studying dysregulation and tools available Primary cells and cultures Immortalized cell lines Toxin modified in vivo models/ targeted cell destruction Transgenic in vivo models using site specific recombination Revisiting cell specificity of Cre expression Accounting for RIP-Cre modifications in the hypothalamus The MIP-Cre transgenic mice Hypothesis and aims...24 Chapter 2- Materials and Methods Extraction of primary hypothalamic cells Immortalization of primary hypothalamic cells Fluorescent activated cell sorting Cell culture techniques Cell line experimental protocols Molecular screening Immunocytochemistry and cellular imaging Insulin sensitivity Effects of insulin treatment RNA isolation, cdna synthesis and quantitative real time RT-PCR RNA isolation cdna synthesis Real-Time RT-PCR...36 iv
5 2.6.4 Primers Immunofluorescence Immortalization of human islets Statistical analysis...39 Chapter 3- Results Generation of morphologically distinct GFP+ cell lines from primary hypothalamic cultures Gene expression profiles of mhypoa-mip/gfp cell lines Localization of GFP and NPY mhypoa-mip/gfp cells are insulin sensitive Differential regulation of NPY by insulin in the mhypoa-mip/gfp cell lines Primary hypothalamic cells also express NPY and GFP GFP signal was not detectable in the MIP-GFP brain via immunofluorescence Generation of immortal cells from human islets...48 Chapter 4- Discussion Summary and significance of findings Detectable activity of MIP within the hypothalamus MIP-GFP neurons Characterization of hypothalamic MIP neurons MIP neurons are involved in energy homeostasis In vivo validation of findings Immortalized human islet cells Limitations of the study Future studies Concluding remarks...72 References...74 v
6 Figure and Table Legend 1.1 Insulin promoters Rodent cell lines commonly used for β-cell research Cre-transgenic mice and mechanism of Cre-recombinase action Representative illustration of RIP-Cre expression within the mouse brain Antibodies used for ICC and IF Primers used for RT-PCR screening and quantitative RT-PCR analysis Proliferative factors and concentrations used for human islet immortalization Representative images of mhypoa-mip/gfp cell lines Gene expression profile of mhypoa-mip/gfp cell lines Representative blots of molecular screening GFP confirmation of cell lines via immunocytochemistry Intracellular NPY expression patterns of the cell lines Insulin sensitivity of the cell lines Differential regulation of orexigenic genes by insulin Detection of native GFP expression via IF Representative images of immortal human islet cells and gene expression...57 vi
7 List of abbreviations AgRP AKT ANOVA ARC AVP bp CART CEBPβ CNS CNTF CNTFR CRH DMEM DMH DRN EGF ERα ERβ FACs GABA agouti related peptide protein kinase B analysis of variance arcuate nucleus arginine vasopressin base pair cocaine and amphetamine related transcript CCAAT/enhancer binding protein β central nervous system ciliary neurotrophic factor ciliary neurotrophic factor receptor corticotropin-releasing hormone dulbecco's modified eagle medium dorsalmedial nucleus dorsal raphe nucleus epidermal growth factor estrogen receptor alpha estrogen receptor beta fluorescent activated cell sorting γ-aminobutyric acid vii
8 GFP (+) GHRH GHSR GLP1R GLP2R HBSS HGF hislet ICC ICV IHC IF IR IRS LH LINES MAPK MCxR mhypoa MIP NBA NPY NSE green fluorescent protein (positive) growth hormone releasing hormone ghrelin receptor glucagon-like peptide-1 receptor glucagon-like peptide-2 receptor hank's balanced salt solution hepatocyte growth factor human islet derived immunocytochemistry intracerebroventricular immunohistochemistry immunofluorescence insulin receptor insulin receptor substrate lateral hypothalamus long interspersed elements mitogen-activated protein kinases melanocortin x receptor mouse hypothalamic, adult derived mouse insulin I promoter neurobasal A neuropeptide Y neuron specific enolase viii
9 NTS ObRb PBS nucleus of the solitary tract leptin receptor, long form phosphate buffered saline PDX1 pancreatic and duodenal homeobox 1 PI3K PMSF POMC PVN qrt-pcr RIP RIP-Cre/ERT RIP-Cre Herr RIP-Cre Mgn ROS RT-PCR Phosphatidylinositide 3-kinases phenylmethanesulfonylfluoride proopiomelanocortin paraventricular nucleus quantitative real time- polymerase chain reaction rat insulin II promoter rat insulin II promoter-cre recombinase, inducible variant rat insulin II promoter-cre recombinase, Herrera laboratory variant rat insulin II promoter-cre recombinase, Magnuson laboratory variant reactive oxygen species real time- polymerase chain reaction STAT signal transducer and activator of transcription 3 SV40 T-ag T2DM VIP VMH x-msh β-gal SV40 Large T antigen type 2 diabetes mellitus vasoactive intestinal polypeptide ventromedial nucleus x-melanocyte stimulating hormone β-galactosidase ix
10 Chapter 1 Introduction 1
11 2 1 Introduction 1.1 Overview The advancement of technology has provided researchers with progressively more powerful tools for use in the sciences. Increased precision and capabilities in equipment, methods and models provides the basis for cutting edge research and development in the 21 st century. However, due to the complex nature of an organism, where multiple organ systems integrate and function in collaboration to regulate physiological functions, the study of disease and pathology becomes difficult. Moreover, all available technologies have yet to produce a cure for complex and prevalent diseases such as type 2 diabetes mellitus (T2DM) [264]. Thus, it became obvious that current scientific models and methodologies cannot provide conditions needed for the development of permanent solutions. Due to the complexity of diseases such as T2DM, both in vitro and in vivo models must be employed to study the molecular mechanisms and systemic effects of specific genes and proteins. Many in vivo models rely on cell-specific targeting to elucidate the functions and significance of genes and proteins within critical cell types such as the pancreatic β-cell. As the β-cell is a high-value target in diabetes research, β-cell-specific promoters were therefore used to drive overexpression or knockdown the genes and proteins of interest. In the field of diabetes, the rat insulin II promoter (RIP) [100, 193, 251] is among the most widely used to create transgenic animals. Thus, utilization of the RIP was thought to be able to achieve β-cell specific modifications and ultimately reveal the importance of specific genes and proteins to the overall pathology and development of diabetes. However, recent studies that utilized the RIP have reported promoter activity within the hypothalamus [43, 53, 129, 140, 161, 265] and suggested possible roles of these hypothalamic neurons in energy homeostasis. Given that the hallmarks of
12 3 T2DM are glucose intolerance and insulin resistance, where fundamental disturbances in energy homeostasis and metabolism are evident; promoter activity within hypothalamic neuronal populations involved in energy homeostasis makes the attribution of observed effects in energy balance to transgenic manipulations of the β-cell difficult. Especially given that attributes such as hyperphagia cannot be explained readily by modifications within the β-cell. Ultimately, these caveats lead to the development of models with utilization of the improved mouse insulin I promoter (MIP) to achieve greater β-cell specificity [101]. However, given the novelty of the improved promoter, significant validation efforts must be invested to determine the degree of improvement if any are present. Similarly, mechanistic studies rely on the availability of appropriate in vitro models to generate relevant and valid findings in the field of diabetes. Cell lines are often used due to their primary advantages of high availability and ease of use. While a number of rodent-derived β-cell models are readily available [184] for research use, each cell line exhibit distinct and significant differences when compared to isolated primary cells [224]. Additionally, significant differences have been noted to exist between rodent and human β-cells in terms of their resilience to damage [73]. Therefore, mechanisms elucidated by the rodent cell lines may not be applicable to humans because of their differences. Thus, the utilization of human cells would be able to provide a more relevant in vitro model for use in diabetes research. However, given that human donor islets are scarce and cannot divide in substantial quantity in vitro, the supply of human cells significantly restrict the quantity of research that can be conducted. This combined with a lack of available of human islet cell lines creates a significant bottleneck in the progression of mechanistic studies that can be performed in the field. However, given the perpetual advancements in available
13 4 technology, generation of human islet cells remain to be a possibility. Ultimately, the successful creation of the cell lines may open new avenues for human islet research. Due to the limitation of current models used in diabetes research, new models must be generated and thoroughly validated to ensure their improvements resolve the problems associated with previous iterations of cellular and systemic models. Therefore, the primary aim of this thesis is to determine whether the newly utilized MIP resolves the issue of hypothalamic activity as previously seen in the RIP by using novel methods. While the secondary aim of this thesis is to use the same technology to attempt to generate human islet cell lines in order to bypass the supply scarcity faced by research groups. 1.2 Promoters and cell specificity As previously eluded, cell-specific promoters can be used to drive overexpression or knockdown of genes and proteins in cells of interest. Given the heavy focus on β-cell within the diabetes field, the promoter of choice is often that of the insulin promoter. However, unlike humans which have a single copy of insulin located on chromosome 11 [102], rodents such as rats and mice have two equally expressed copies of the insulin genes with copies located on chromosome 1 for the rat [226] and chromosomes 7 and 19 for the mouse [59, 260]. While both genes produces functional insulin, the evolutionary origin of these two insulin genes gives rise to different promoter elements with differing regulatory properties [223]. Although two variants of the insulin genes and promoters are available, currently available transgenic models utilize the RIP (Figure 1.1). Previous studies have determined that the necessary regulatory elements of the rat insulin II gene to confer β-cell specific activity are located within approximately 500 base pairs upstream of the transcriptional start site [263],
14 5 where the proximal promoter construct appeared to be functionally equivalent to the native promoter [25, 54, 255]. This led to the development of β-cell-specific Cre-loxP systems under the rat insulin II promoter [194]. Subsequently, the RIP-Cre Herr [110], RIP-Cre Mgn [187] along with the Tamoxifen inducible RIP-Cre/ERT [66] were created via utilizing RIP constructs approximately 660 basepairs (bp) in length by the Herrera, Magnuson, and Melton laboratories respectively; thus these variants have been widely used in diabetes for β-cell-specific gene modification using the Cre-loxP system (refer to chapter 1.4.4). However, insulin gene expression itself is not limited within the pancreatic β-cells. Low levels of insulin synthesis and expression has been detected in multiple extrapancreatic tissues such as the liver [200], spleen [126], thymus [189, 250] and the brain [64]. Additionally, studies prior to the creation of the insulin promoter driven Cre models have demonstrated limited expression of insulin within regions of the brain [103, 133] such as the hypothalamus. Since insulin expression occurs outside of the pancreatic β-cells, its promoter must be active within these non-pancreatic tissues. Subsequently, RIP activity has been observed within regions of the brain such as the cortex and the hypothalamus (Figure 1.4) by multiple research groups [43, 53, 129, 140, 161, 265]. Given that the hypothalamus is also a critical brain region that regulates energy homeostasis, observed phenotypes of RIP-Cre mice such as increased appetite, lean and fat mass as well as insulin resistance becomes difficult to attribute solely to genetic manipulations within the pancreas due to the concurrent expression of Cre-recombinase in the pancreatic β-cells and the hypothalamus In addition to the concurrent promoter activity within the hypothalamus, different iterations of the RIP-Cre transgenic mice have been shown to differ in their RIP activity pattern within the hypothalamus [88, 228, 265]. These hypothalamic neurons with the concurrent RIP
15 6 activity have been demonstrated to contribute to the control of energy homeostasis, appetite control and body weight [55, 129, 202]. Additionally, no evidence exist to indicate that these RIP hypothalamic neurons are capable of de novo insulin synthesis, which also indicated that RIP activity is not restricted to insulin producing cells. Since distinct populations of appetite inducing and suppressing (orexigenic and anorexigenic, respectively) neuronal populations exist within the hypothalamus, the varying RIP activity between the different RIP-Cre mice leads to differential gene modification in distinct energy regulating neurons. By crossing RIP-Cre variants with β-galactosidase reporter mice (Figure 1.4) [265], RIP-Cre Herr mice have been found to have punctate reporter staining in hypothalamic regions surrounding the third ventricle, thalamus, as well as distinct staining in dorsal regions of the cortex; RIP-Cre Mgn exhibited significant staining in most of the hypothalamus as well as amygdala; RIP-Cre/ERT exhibited distinct staining within the hypothalamus surrounding the third ventricle, lateral thalamus and dorsal cortex. Ultimately, the resulting phenotype of these RIP transgenic mice may be dependent on the genetic modifications on these hypothalamic neuronal populations in addition to the conditional gene modification within the pancreatic β-cell, which inevitably depends on the variant of the RIP-Cre model used. However, since characterizations of these RIP neurons are scarce, the ability to attribute specific observed phenotypes to the RIP neurons becomes significantly diminished without further study of these neuronal populations. Ultimately, given the concurrent expression of Cre within both the brain and the pancreas, it is difficult to conclude whether evidence gathered using the current RIP-Cre transgenic model is due to either organ system. This is compounded by the varying but distinct pattern of RIP activity within the hypothalamus, which further confounds findings on the causes
16 7 of observed changes in energy homeostasis. Despite its ability to provide diabetes researchers with targeted genetic modification in vivo with good spatial and temporal control, improvements must be made to further increase the fidelity and specificity of genetic modifications obtained through the Cre-loxP system. 1.3 Metabolic disorders and energy homeostasis Given that diabetes results in reduced cellular uptake of glucose and excess in the bloodstream, its classification as a metabolic disorder ultimately indicate a significant imbalance in energy homeostasis. As energy balance is controlled by both neuronal and endocrine systems, its study must take into consideration multiple causes and effects presented in a highly complex and integrated system. In addition, given that type 2 diabetics often develop prior cases of metabolic syndrome [143], where insulin resistance and obesity is present in the individuals, additional obesity related factors such as hyperphagia and leptin resistance [6, 116] are indicative of malfunctions in multiple organ systems. Therefore, the ability to determine the overall significance of specific failures in the system can aid in the elucidation of pathological causes of the T2DM. However, as previously stated, concurrent modification of genes in both the hypothalamus and the pancreatic β-cell in transgenic RIP models is problematic because of the significant role that hypothalamic neurons play in energy regulation Hypothalamus and feeding control The hypothalamus plays a vital role in the homeostatic control of the mammalian organism. Situated between the thalamus and the pituitary along with its neural and hormonal connections to the hypophyseal portal system, the hypothalamus is responsible for regulating a
17 8 number of vital functions such as energy balance, thermoregulation, fluidic and electrolyte balance, blood pressure, reproduction [78]. Despite its size, the hypothalamus also receives multiple neuronal inputs from multiple brain regions such as the brainstem, amygdala, hippocampus and the medulla while output signals are sent to brain regions such as dorsal raphe nucleus (DRN) and nucleus of the solitary track (NTS) [78]. Moreover, the hypothalamus is a region with three distinct divisions, with medial and lateral regions within the anterior, posterior and tuberal hypothalamus, where each region is comprised of distinct neuronal populations with specific associated physiological roles. Early studies of the mid-20 th century have found that energy balance is significantly regulated by the hypothalamus, as large lesions within this structure gave rise to hyperphagia and significant weight gain in rodent models [113]. However, given that the hypothalamus is comprised of multiple regions itself, subsequent studies were aimed to pinpoint the regions responsible for this observed hyperphagia and obesity. Bilateral lesions of the ventromedial nucleus (VMH) resulted in similar obese phenotype observed previously [112, 114], and thereby indicated the role of the VMH in energy homeostasis. Similar studies with bilateral lesions to the lateral hypothalamus (LH) resulted in an opposite observed effect [7-9], where hypophagia and significant weight loss was observed. Moreover, it was observed that effects of LH lesions could override those of VMH lesions which resulted in the starvation of the animals. Additionally, targeted electrical stimulation of the dorsalmedial hypothalamus (DMH) produced hyperphagia similar to the earlier VMH lesion studies [23, 34], and lesions to the paraventricular nucleus (PVN) demonstrated hyperphagia and obesity [135] similar to those observed by its severance from afferent and efferent neuronal connections [97, 220].
18 9 In addition to these hypothalamic regions, the arcuate nucleus (ARC) contains one of the most critical systems in the regulation of feeding and energy balance [75, 205]. As energy homeostasis is maintained by orexigenic and anorexigenic neuronal subtypes involved in the respective stimulation and suppression of feeding. The ARC is the host of functionally opposing neuronal populations that have been extensively studied. Known as the agouti related peptide/ neuropeptide Y (AgRP/NPY) and proopiomelanocortin/ cocaine and amphetamine related transcript (POMC/CART) neurons, these neuronal populations are well known in their mechanisms in the regulation of feeding [248, 266]. Among the products of POMC/CART neurons, alpha and beta melanocyte-stimulating hormones (α- and β-msh) increases energy expenditure and suppresses food intake in both humans and rodents [24, 79, 134] by acting on type 3 and 4 melanocortin receptors (MC3R and MC4R) located in the ARC, PVN, DMH, LH, and VMH [1, 164]. Similarly, CART has been demonstrated to exert potent anorexigenic effects and was able to block the feeding response stimulated by orexigenic neuropeptides such as NPY [124, 128]. Conversely, AgRP is an inverse agonist of MC3R and MC4R, where its expression results in the blockade of anorexigenic effects of α-msh [177]. Likewise, NPY produced by the AgRP/NPY neurons is a powerful stimulator of food intake while simultaneously reduces energy expenditure [44, 231]. Moreover, a significant number of orexigenic and anorexigenic neuropeptides exist in the hypothalamus in addition to those expressed by AgRP/NPY and POMC/CART neurons. In addition to their roles in regulating functions such as water balance, hormone release, thermoregulation, blood pressure, stress response and circadian rhythm, neuropeptides such as oxytocin [13, 178, 235], neurotensin [49, 230], corticotropin-releasing hormone (CRH) [67, 127], urocortin [56, 249], vasoactive intestinal polypeptide (VIP) [93, 130], and arginine
19 10 vasopressin (AVP) [11, 235] have anorexigenic affects in the hypothalamus. Conversely, neuropeptides such as orexin [68, 208], galanin [40, 156], ghrelin [154, 179], as well as growth hormone releasing hormone (GHRH) [82, 175] have been shown to have orexigenic effects in the hypothalamus. Ultimately, it is obvious that a large number of neuronal populations in the hypothalamus are involved in energy homeostasis, and the unintentional modification of these neurons due to lack of promoter specificity may significantly confound the results obtained from intended β-cell-specific target genes Central effects of insulin on energy balance and obesity In addition to the key neuropeptides expressed within the hypothalamus, key metabolic hormones from the periphery such as insulin also have significant central actions which can regulate energy homeostasis [47, 48, 163]. Insulin secreted by the β-cell was determined to act within the hypothalamus to regulate energy balance and body weight. Insulin was found to act on the receptors located within the ARC, where phosphoinositide-3 kinase (PI3K) activation was found to mediate insulin induced anorexia [172]. Conversely, a reduction in insulin receptor expression within the hypothalamus was able to produce a hyperphagic, obese and insulin resistant phenotype [173]. Furthermore, insulin suppresses the expression of NPY [218], its release from the nerve terminals within the PVN [204] and pathways such as the GABAergic system [210], and ultimately reducing total energy intake and glucose influx. Additionally, the ability of peripheral insulin to act centrally to inhibit glucose production [174] combined with its ability to suppress NPY indicate that insulin is a key central regulator of energy balance. As insulin has critical actions in the hypothalamus to suppress the expression of orexigenic neuropeptides, resistance to its signaling negates its regulatory effect on energy
20 11 homeostasis [50, 160]. Moreover, high circulating levels of insulin combined with the presence of insulin resistance and obesity are seen with both metabolic syndrome and T2DM [158, 262]. Therefore, all indications point towards a highly complex interaction and integrative neuronal network linking the roles of the central nervous system (CNS) and peripheral organs and tissues in the overall energy homeostatic system, and thereby increasing the difficulty of research on topics such as diabetes and obesity. 1.4 Studying dysregulation and tools available Mammalian species are homeostatic in nature and therefore maintains energy sources such as glucose within a physiological range to ensure the survival of the animal. However, due to failures in homeostatic mechanisms in single or multiple organ systems, imbalances such as hyperglycemia or hypoglycemia occurs which drastically change the energy status of the animal, and energy dysregulation occurs as a result. However, the homeostatic failure is often comprised of multiple contributing factors which results in the pathological state. Therefore, the ability to examine specific genes and their expression within cells of interest can determine their importance to the development of pathology. Moreover, due to the significant integration between the neuronal and endocrine networks in the regulation of energy homeostasis, multiple models must be used to ensure that results obtained are due to manipulation of specific elements within the system. Currently, there are four major models that can be used to achieve the specificity necessary for the attribution of results to manipulations of specific genes and proteins. Primary cells and tissue, and cell lines present good in vitro models for the mechanistic research. While targeted in vivo cell destruction and targeted gene modification in mice provide unique working
21 12 in vivo models. Each method has its own advantages and caveats, therefore a combination of models can provide better insights into the significance of the gene in question and their mechanistic actions while ensuring that the results obtained were due to the intended manipulations as designed by the researcher Primary cells and cultures Given the unmodified nature of primary cells, their isolation permits in vitro experiments in a more controlled environment with limited fluctuations. The results obtained from these experiments are considered to be more biologically relevant, than those obtained from transformed cells which must be genetically manipulated for continual growth. Despite this advantage of being a better model for in vitro work, there are a significant number of challenges present to their use. First and foremost, the isolation of primary cells may be challenging with limited yield such that multiple organisms must be sacrificed to yield usable quantity. In the field of diabetes, rodent sourced pancreatic islets and cells are often isolated for experimentation. However, due to the non-dividing nature of pancreatic endocrine cells, isolated islet cells limit the number of experiments that can be done. Despite the development [131] and continual improvement of islet isolation process [137, 237], as well as improvement to the purification of β-cells [203, 225], their non-mitotic state combined with their limited lifespan dictates the necessity for a steady supply of islets for the continuation of experimentation. However, the application of various growth factors such as hepatocyte growth factor (HGF) [19, 108], epidermal growth factor (EGF) [117, 234], gastrin [198, 241], GLP-1/exendin-4 [139, 256], and γ-aminobutyric acid (GABA) [227] to β-cells have yielded statistically significant replication of primary cells, but not
22 13 to the extent in which enough cells can be generated for additional experimentation. For the field of neuroscience, similar challenges are posed to researchers. Unlike the pancreatic islet where distinct packets of endocrine cells exist, neural tissue is comprised of a mixture of heterogeneous astrocytes, oligodendrocytes, neurons and glia. Techniques exist to isolate specific subtypes of neural tissue [31, 119], however the isolation of individual populations of neurons requires the use of cell-specific markers and additional sorting. Thus, long term studies using either primary islet cells or neurons require significant commitments in both resources and time. Second, the maintenance of primary cells for the duration of the study poses significant challenges. In the case of pancreatic β-cells, the disruption of cellular matrix found within the islet following isolation causes a significant increase in β-cells apoptosis [257], thus the functional viability of the β-cells is typically less than a week following extraction. Conversely, cultured primary neurons have been demonstrated to be functionally viable for up to five months post isolation [192]. However, their functional viability requires significantly more maintenance and appropriate growth factors with periodic administration [95, 192]. Despite the advantage of being a good model for experimentation, the limited supply, difficulty and cost of working with primary cells limit their usefulness within research. Therefore, additional models must be used to confirm and complement findings obtained from primary cells Immortalized cell lines As the principle limitation of primary cells is in regards to their availability and long term viability, immortal cell lines provide independent and readily available model for physiology and pathophysiology studies. In addition to the perpetual proliferative state of cell lines, their immortality and ease of care have allowed researchers to obtain consistent and reproducible
23 14 results quickly. Due to their isolated nature and removal from the overall integrated system, cell lines are very useful for detailed in vitro studies on transcriptional events, protein function, cell signaling and therefore allow physiological, biochemical and pharmacological characterization of specific cell lineages. While cell lines provide a large quantity of cells for experimentation, the transformed nature of these cells result in several caveats that must be considered to provide physiologically and pathologically relevant findings. First, cell lines by definition are altered from their origin population, either by natural mutation or induced transformation. Induced transformation is achieved by either infection with the SV-40 T-antigen that drives cell proliferation [5] or exposure to high energy radiation to damage mitotic inhibitors, ultimately causing unregulated cellular division. While this transformation may increase the availability of specific cells to researchers, the transformed lines may not retain the necessary phenotype of the comparable primary cells. In diabetes, more than a dozen pancreatic β-cell lines exist from various rodent species as well as humans. The most widely used rodent-derived INS-1, MIN, RIN, HIT and TC cell lines (Table 1.2) all vary in glucose sensitivity, insulin content, and culture conditions [184, 224]. The T-antigen derived mouse insulinoma β-tc cells can produce the two rodent variants of proinsulin as well as the final cleaved insulin, but exhibit glucose-stimulated insulin secretion at sub-physiological glucose concentrations [72]. The similarly derived hamster insulinoma HIT cells exhibit glucose-stimulated insulin release curves comparable to the hamster islet, however the quantity of insulin released is times lower in comparison [209]. In contrast, the radiation derived rat RIN cells secrete both insulin and somatostatin but exhibit abnormal glucose transport and responses when compared to native β-cells [89]. Finally, the two most widely used rodent cell lines, the T-antigen derived mouse MIN6 cells and radiation derived rat
24 15 INS-1 cells both exhibit appropriate glucose-stimulated insulin secretion at physiological glucose concentrations. However, MIN6 and INS-1 must be cultured in the presence of nicotinamide [86, 157] and 2β-mercaptoethanol [14] respectively to maintain their secretory properties. In addition, MIN6 cells are known to spontaneously lose their ability to secrete insulin upon stimulation, and insulin secreted by INS-1 cells only amounts to approximately 20% to that of native β-cells. Ultimately, it must be accepted that the transformation process alters the phenotype of the origin cells and therefore studies conducted using cell lines may not be as physiologically and pathologically relevant. Therefore, cell lines are sometimes regarded as lesser models when compared to primary cells despite their advantages and their ability to generate a significant quantity of reproducible data in a very short period of time. Second, due to the number of available cell lines with varying individual characteristics, it is difficult to determine which cell line best represents in vivo conditions. The selection and usage of some cell lines has therefore been controversial, and the validity of the results obtained from these cell lines have been questioned [90, 122, 180]. Moreover, a select number of the most widely used cell lines for pharmacological testing were invalidated as these in vitro models were found to have no correlation with clinical samples [94]. This places additional questions on the validity of select cell lines to develop new pharmacological therapies. Therefore, because their alterations following the transformation process, careful selection from available cell lines must occur in order to produce valid and translatable results. Third, due to the differences observed between rodent and human islet cells, such as islet architecture and micro-environment [27, 32, 37], energy processing protein and enzyme levels [145], ion channel subtypes [28, 29], and sensitivity to oxidative stress [73], the cellular mechanisms eluded using rodent cell models may not be sufficiently similar to that of human
25 16 islet cells. Additionally, given that the islet is a mixture of different cell types composed of α-, β-, δ-, ε-, PP, and neuronal cells, which produce glucagon, insulin, somatostatin, ghrelin, pancreatic polypeptide and neuropeptides respectively [10, 74, 166, 167, 246], the study of multiple human cell types may provide additional insights into the intercellular interactions and functions. However, due to the limitations in previously available technology, the EndoC-βH1 cell line remains to be the only human islet cell line in existence [191]. Despite the existence of a single embryonically derived human β-cell line, no human islet derived cell lines exist for any other cell type found within the pancreatic islet. This combined with the limited supply of primary donor islets, the ability to perform sufficient studies on islet cells becomes severely diminished. Thus, it is imperative to provide readily available human islet cell models to researchers in order to gain additional insights into the development of islet pathology. Given the repertoire of growth factors that can induce proliferation of primary islet cells, the transformational properties of the SV-40 T-antigen, and the perpetual advancement of technology, new methods and techniques may be able to generate the critical human islet cell lines needed for diabetes research Toxin modified in vivo models/ targeted cell destruction Due to the complex interaction between the pancreatic endocrine system and the CNS, the best models must maintain this interaction as well as provide the necessary pathology for studying T2DM. In vivo models are therefore useful for research as they provide experimental conditions that are more reflective of living organisms. Specifically, targeted cell destruction provides researchers with pathophysiological models, in which therapies can be developed to mitigate or reverse the induced pathology via protective or regenerative means.
26 17 β-cell-specific toxins give researchers the ability to generate systemic models of type 1 and 2 diabetes with intact pancreatic-neuronal integration. Both alloxan and streptozotocin are known to induce pancreatic β-cell death upon administration, and their mechanisms of actions have been well established [236]. The rapid uptake of alloxan by the β-cell results in the inactivation of glucokinase [136], production of superoxide radicals [165], disturbances in intracellular calcium mobilization and concentrations [125, 258], and ultimately DNA fragmentation [207, 239] causing cellular destruction. However, due to the narrow range of doses that can be used with alloxan, where lower doses are ineffective and higher doses cause nonspecific cell destruction, it is used to generate type 1 diabetic models. In contrast, streptozotocin can be used to generate both type 1 and type 2 diabetic models due to its wider range of effective doses. Following glucose transporter 2 dependent uptake by the β-cell [216], streptozotocin impairs glucose oxidation [20], insulin biosynthesis [26] and exposes DNA to reactive oxygen species (ROS) [240] and alkylation [62, 76], ultimately causing the necrosis of the β-cell. Despite its cytotoxic effects, streptozotocin can be used differentially to produce varying pathologies. Multiple low doses of streptozotocin can be used to initiate immune mediated β-cell destruction and causes type 1 diabetes [201, 270]. Most notably, this effect can be amplified where prior administration of immunostimulants can increase the sensitivity of β-cell to the effects of streptozotocin [267]. In addition, administration of a single high dose of streptozotocin within 24 hours of birth can produce type 2 diabetic animals [185]. This state is characterized by a reduced level of insulin stores and impaired glucose tolerance [186], as well as a complete loss of glucose sensitivity of β-cells [96]. Given its versatility and availability, streptozotocin is often used to generate pathological model needed for research.
27 18 However, despite the ability to generate pathologically relevant models, significant differences exist between humans and rodents in both sensitivity as well as responses to both alloxan and streptozotocin. Alloxan administration to human islet grafts in vivo resulted in no observable decline in function or viability of the islets [247]. Similarly, streptozotocin administration to human islets as well as purified β-cells resulted in no decline of function or viability [73]. Therefore, human pathology appears to be more resilient against factors such as ROS and determination of relevant repair and/or defense mechanisms in rodents may only provide limited parallel to human pathology [197], and the obtained results ultimately be limited in its translational value for the development of effective therapies Transgenic in vivo models using site specific recombination As in vivo models are able to provide experimental conditions that maintain endogenous system interactions with intact feedback loops, their utilization can thus elucidate the role and importance of specific elements within the organism. While toxin induced in vivo models can provide pathological models and means to test protective and regenerative therapies, whole cell destruction limits the subset of elements that can be examined. Thus, to elucidate the role of specific genes and proteins, additional in vivo models have been developed to allow the insertion, deletion and additional modifications to DNA. The ability to drive overexpression or knock down of genes can ultimately determine their importance in the development of pathology. The development of site specific recombination provided the necessary tools to target small segments of DNA to elucidate their contribution in the control and expression of proteins, or the functional significance of the protein. In the in vivo model, this provides an opportunity to observe the consequences of gene modification while maintaining the overall integrity and
28 19 function of the host organism. Current methods require two key components to generate transgenic in vivo models with the designed modification. First, there must be an expressed enzyme to carry out the necessary modification to the DNA strands. Second, the DNA sequences of interest must be flanked by short sequences recognizable by the enzyme used. The phage derived Cre-recombinase enzyme [211], along with the bacterial derived Flp-recombinase enzyme [215] are among the most commonly used systems for transgenic studies. Of the recombination systems available, the Cre-loxP system is among the most used in transgenic animal research as it is both well studied and readily available. The principle working elements of this system are the P1 bacterial phage derived Cre-recombinase enzyme [212] and two loxp target sites. The Cre enzyme is a 38kDa protein with an optimal efficiency at 37ºC [36] with high binding affinity [196] for specific 34 bp loxp sites [99]. The two loxp sites flank the DNA sequence of interest, and are composed of two 13 base pair palindromic sites around an 8 base pair core sequence [232]. The Cre enzyme binds to these palindromic site and forms a tetramer to excise the enclosed DNA sequence [253] (Figure 1.3). Due to the necessity of both elements and the unlikely random occurrence of loxp sites, high specificity can thus be achieved. In addition to the high fidelity of the recombination event itself, the Cre-loxP system provides strict control in both spatial and temporal parameters. Since the recombination event requires both elements to function, restriction of either will result in null recombination. As genome modification such as insertion of loxp sites is unrestricted to specific cell types, the expression of Cre must be limited to obtain high spatial control. Therefore, the utilization of cellspecific promoters can thus spatially restrict Cre-expression to specific cell populations [12, 168]. As previously eluded, the most widely used promoters in the field of diabetes to obtain cells specificity are the RIP [100, 193, 251], along with the pancreatic and duodenal homeobox 1
29 20 (PDX1) promoter [3, 87]. Both promoters were thought to be highly specific to pancreatic β-cells and the mature β-cells of the endocrine pancreas respectively, therefore by placing the expression of Cre under these promoters, recombinase activity should be limited to β-cells. Furthermore, synthetic compounds could be used to gain temporal control of the activity of Cre [83]. Currently available methods utilize an additional mutated ligand binding domain of either estrogen or progesterone, where fusion with the Cre-recombinase allows the enzyme to translocate to the nucleus upon ligand binding. As the ligand binding domains have been mutated, only synthetic compounds such as tamoxifen [107] or RU-486 [123] can bind and initiate the translocation of the Cre to the nucleus. Therefore, such methods allow both high spatial and temporal control over Cre-mediated recombination in transgenic mice models. Ultimately, transgenic mice utilizing Cre mediated recombination provide good models to study energy dysregulation. Due to its ability to specifically target genes while maintaining overall integrity of the in vivo system, along with the ability to provide both temporal and spatial control over transgenic modification, results obtained using this model offers high integrity and validity. However, the specificity of this transgenic system is highly dependent on the promoter utilized to drive the system. Promoter activity outside of the target cells therefore result in failure of spatial control and may complicate interpretation of obtained results. 1.5 Revising cell specificity of Cre expression Despite the lack of de novo insulin synthesis in RIP neurons, the resulting expression of Cre in critical regions of the brain controlling energy homeostasis make results obtained from RIP-Cre models difficult to interpret. As both the pancreatic β-cell and the hypothalamus are both integral to the control of energy homeostasis, the significance of the genetic alterations in
30 21 the β-cell can only be concluded if the effect can be also determined within the concurrent hypothalamic populations Accounting for RIP-Cre modifications in the hypothalamus While it is possible to partially account for the role of the Cre-expressing hypothalamic neurons following recombination using RIP-Cre, the process requires the use of multiple models in conjunction with the study. In a recent study, in order to attribute the observed phenotype to hypothalamic RIP-Cre action [170], similar experiments utilizing the PDX1-Cre [229] as well as virus mediated hypothalamic specific modifications [233] were needed to arrive at the final conclusion. The study relied on the assumption that PDX1-Cre is β-cell specific and differential phenotypes observed between the RIP and PDX1 transgenic animals must be due to the neuronal populations of the RIP-Cre mice. Regardless, the inclusion of additional experiments in parallel models is limited in their ability to provide interpretation to findings especially when subtle variation between models such as the pattern of hypothalamic Cre activity is considered [265]. Alternatively, the role of the RIP-Cre positive hypothalamic neurons in the overall system can be partially elucidated by their identification. Recent developments have allowed specific ablation of RIP-Cre Herr hypothalamic neurons [202]. Specificity of ablation was achieved by utilizing the RIP-Cre itself to drive the expression of the diphtheria toxin receptor [35] followed by the ICV administration of the toxin [206]. This combined with trans-synaptic tracing experiments elucidated the orexigenic nature and role of these RIP neurons as well as the neuronal links that are established [30]. While these cells were not found to be of the NPY subtype, their projections to the PVN indicated their inhibitory actions on anorexigenic PVN neurons. Such information on the RIP-Cre neurons thus provides the partial ability to account for
31 22 the phenotypes associated with concurrent gene modification within the pancreatic β-cells as well as the RIP-Cre neurons. Despite the existence of these workaround methods, they can only provide limited insights into the observed results from concurrent β-cells and hypothalamic gene modifications. Thus, the best representative models of β-cell specific gene actions must utilize transgenic animals with true β-cell-specific actions The MIP-Cre transgenic mice On account of the extrapancreatic activity of the RIP-Cre, additional promoter constructs were developed in order to solve the specificity problem. As previously mentioned, rodent insulin is a result of two distinct genes located on separated chromosomes. While preceding studies have determined that insulin is indeed expressed in the brain in limited quantity, more recent findings indicate that only the insulin II variant is actively synthesized within neuronal tissue [146]. This finding matches previous in vivo models where systemic insulin I and II knock downs resulted in differential phenotypes [70]. More specifically, insulin I gene knockout resulted in little to no diabetes or immuno-infiltration of islets (insulitis) [16, 162, 169]. Conversely, insulin II gene knockout significantly accelerated the development of both insulitis and diabetes [242], increased autoimmune activity [39], as well as islet destruction [118]. Since diabetes only developed following insulin II knockout, where the loss of function of insulin I could be compensated by insulin II and not vice versa, it is evident that insulin I expression is more restricted to the pancreas than the currently used insulin II variant. Despite the existence of differential control of the two insulin genes within the β-cell [141] and slightly lower expression levels of insulin I [63, 259] mrna, its β-cell restricted nature makes it better suited as a β-cellspecific promoter that can used to drive Cre.
32 23 In addition, comparative analysis of insulin promoters [106] have shown additional regulatory elements within the promoter regions of insulin I when compared to insulin II [171]. Of these regulation elements, repressors such as the CCAAT/enhancer binding protein β (CEBPβ) [144, 222] as well as long interspersed elements (LINES) located between 2-4 kb upstream of the transcriptional start site [132] function to restrict insulin expression [71] (Figure 1.1). Thus, the additional regulatory elements in combination with the known restricted activity of the insulin I gene resulted in the development of transgenic mouse regulated under the mouse insulin I promoter (MIP), which is a fragment of the endogenous insulin I promoter approximately 8.5 kb in length. Recent developments in transgenic models have thus included this 8.5 kb fragment of the insulin promoter to drive Cre expression as a means to increase β-cell specificity. The resulting Tamoxifen inducible mouse strain, known as the MIP-Cre/ERT is therefore theoretically capable of achieving true β-cell-specific gene modification with no neuronal activity. Moreover, recent studies utilizing green fluorescent protein (GFP) and β-galactosidase (β-gal) reporters under the regulation of MIP have reported no observable activity within neuronal tissue [101, 265]. This finding combined with the high localization of the reporters with endogenous insulin [101, 181] would indicate a transgenic Cre model with high β-cell activity but no neuronal activity, which is required in the field of diabetes to examine the role of specific genes and their contribution to pathology in vivo with the necessary neuronal and pancreatic integration intact. However, with limited imaging resolution of these studies, small quantity of reporter-positive neurons may not have been detected, thus significant validation efforts must occur prior to the widespread availability of MIP models.
33 Hypothesis and aims Studies to date would indicate that the new MIP-Cre line of transgenic mice is highly specific to the pancreatic β-cell. While insulin I expression has not been reported in neural tissue, this transgenic construct only use an 8.5 kb fragment of the endogenous insulin I promoter. Additionally, specificity studies on this new transgenic model are limited and current studies utilized techniques with limited spatial resolution and may not be indicative of low level of MIP activity in the brain. Given the previous revelation that RIP activity is present in orexigenic neurons controlling energy homeostasis [202], it is necessary to determine the quantity of neuronal MIP activity prior to the commercial availability of the model. Ultimately, this will determine whether the use of the MIP can overcome the problem of concurrent expression within both the hypothalamus and the pancreatic β-cell, and if the MIP transgenic animals are able to provide the high fidelity β-cell-specific targeting necessary to determine the role of specific genes in the overall pathology of diabetes. Additionally, with the development of new methods and techniques, immortalization of adult human islet cell lines may now be possible by the combined utilization of growth factors to induce limited proliferation and T-antigen to drive cellular transformation in a manner similar to which immortalized hypothalamic neuronal models have been generated. Thus, the thesis aims are to determine whether the MIP construct is active within the hypothalamus of transgenic MIP-GFP reporter animals, characterize the identity of the cells if reporter activity is found, and to attempt to create human islet cell lines. Therefore, it is hypothesized that by immortalizing hypothalamic neuronal populations, MIP activity will be detected in the hypothalamus of the MIP-GFP transgenic mouse and similar techniques can be used to generate immortalized human islet models.
34 25 To test this hypothesis, four aims were established. First, primary hypothalamic cultures will be immortalized and GFP-positive cells isolated. Second, immortal GFP-positive cells will be characterized to determine their origin and function. Third, the immortal cells will be validated using primary GFP-positive cells directly obtained from the reporter mice. Fourth, attempts will be made to generate human islet cell lines from donor tissue.
35 26 Rat Insulin I C2 E2 A4 A3 CRE CAAT A2 C1 E1 A1 G1 GLE CEB Rat Insulin II (RIP) NCS A3 CRE CAAT A2 C1 E1 A1 G1 CEB Mouse Insulin I (MIP) LINEs Rat Insulin I Figure 1.1 Representative illustration of rodent insulin promoter variants. A1-4, C1-2, E1-2, G1, GAS-like element (GLE), Negative control sequence (NCS), camp response element (CRE), CAAT box, CAAT enhancer binding (CEB) and long interspersed elements (LINEs) are known regulatory elements of the insulin promoters upstream of the transcriptional start site (red arrow). Figure not drawn to scale and adapted from Melloul et al [155]. Blue, green and orange vertical lines represent approximately 600, 2000 and 4000 bp upstream of the transcriptional start site respectively.
36 27 β-cell line Origin Species Derived Methdology Cell Origin INS-1 Rat High energy radiation Insulinoma RIN Rat High energy radiation Insulinoma MIN Mouse SV40 T-antigen Insulinoma TC Mouse SV40 T-antigen Insulinoma HIT Hamster SV40 T-antigen Insulinoma Table 1.2 Most widely used rodent pancreatic β-cell lines within diabetes research; all cell lines were derived from insulinomas generated from either high energy radiation or expression of the SV40 T-antigen.
37 28 a. Parental 1 Parental 2 Promoter Cre-Recombinase loxp Target Gene loxp Progeny Promoter Cre-Recombinase loxp Target Gene loxp b. loxp site Cre-Recombinase Cre-Recombinase ATAACTTCGTATA ATGTATGC TATACGAAGTTAT Figure 1.3 Mechanisms of (a) generation of Cre-loxP transgenic mice and (b) Cre mediated recombination events. Transgenic animals are derived by crossing parental mice with Cre driven by cell specific promoters and loxed target gene. Upon expression of Cre-recombinase, each loxp site provides two binding sites for the enzyme around a core spacer sequence. Thus the pair of loxp sites provides four binding sites necessary for the formation of the Cre-recombinase tetramer needed to excise the target gene.
38 29 RIP-Cre Herr RIP-Cre Mgn RIP-Cre/ERT Figure 1.4 Representative illustration of RIP-Cre expression (red, X-gal staining) within coronal brain sections of the transgenic mice variants, figure adapted from Wicksteed et al [265].
39 Chapter 2 Material and Methods 30
40 31 2 Materials and Methods 2.1 Preparation of primary hypothalamic cells Primary hypothalamic tissue from approximately 8 week-old MIP-GFP CD1 mice were extracted surgically following the ethical and animal use guidelines set by the Animal Care Committee of the University of Toronto. The extracted tissue was dispersed using 0.25% trypsin solution without EDTA (Gibco, Burlington, Ontario, Canada) incubated at 37ºC (5% CO 2, humidified incubator) for 5 minutes. The trypsin was subsequently removed and deactivated using Neurobasal A medium (NBA, Gibco) containing 25 mm glucose, supplemented with 10% fetal bovine serum, 5% horse serum, 2% B27, 1% penicillin-streptomycin (FBS, HS, B27 and Pen-Strep, Gibco) and 2 mm L-glutamine (Sigmal-Aldrich, Oakville, Ontario, Canada). Cells were used for immortalization or sent for fluorescent activated cell sorting following the dissociation stage. 2.2 Immortalization of primary hypothalamic cells The primary cells extracted were plated on 60 mm tissue culture dishes and treated with 10 ng/ml of CNTF (R&D Systems, Minneapolis, Minnesota, USA) daily for approximately 9 days in Neurobasal A incubated at 37ºC (5% CO 2, humidified incubator) [21, 22]. Following the CNTF treatments, the cells were incubated with a custom retrovirus construct containing the SV40 Large T-antigen with the neomycin resistance gene twice over 48 hours in fresh Neurobasal A medium at 37ºC (5% CO 2, humidified incubator) [21, 33]. Following infection with the viral construct, surviving cells were given 48 hours to recover in fresh Neurobasal A medium. Subsequently, the cells were given three 100 ng/ml doses of geneticin (G418, Gibco) and incubated for 72 hours in the same incubation conditions mentioned above. Finally, cells that
41 32 survived the geneticin treatments were given fresh medium and were allowed to grow to 95% confluency and sent for fluorescent activated cell sorting (FACs). 2.3 Fluorescent activated cell sorting Primary and immortalized hypothalamic cells were suspended in Hank s Balanced Salt Solution (HBSS, without Ca 2+ and Mg 2+ ) supplemented with 1% bovine serum albumin and 25 mm HEPES adjusted to ph 7.0 (BSA and HEPES, Sigma-Aldrich) [213]. The cells were subjected to 100 micron low pressure sorts at 37ºC and 20 psi by the Faculty of Medicine Flow Cytometry Facility (Dionne White, University of Toronto, Toronto, Ontario, Canada) [111]. GFP emissions were collected using a 530/30 band pass filter with excitation at 488 nm. Cells positive for green fluorescent protein were collected in fresh Neurobasal A and used for either RNA extraction or cell culture. 2.4 Cell culture techniques Primary cells were grown and incubated in supplemented Neurobasal A medium as described in 2.1. FAC sorted cells were initially grown in the same Neurobasal A medium for 5 passages following the sort, but was subsequently grown using Dulbecco s Modified Eagle Medium (DMEM, Sigma-Aldrich) with 4.5 mm glucose, and supplemented with 5% FBS and 1% Pen-Strep. All cultures were kept in standard cell culture settings at 37ºC (5% CO 2, humidified incubator). All cells were grown in 100 mm tissue culture plates to 95% confluence prior being dissociated using 0.05% trypsin-edta (Sigma-Aldrich), resuspended and appropriately diluted to 60 mm plates with DMEM for further experimentation.
42 Cell line experimental protocols The immortalized MIP-GFP cell lines were used for four sets of experiments. First, mrna was isolated from the cells and subjected to gene expression screening. Second, the cells were subjected to cellular imaging via immunocytochemistry (ICC). Third, the cells were treated with insulin to determine insulin sensitivity. Last, the cells were treated with insulin to determine changes in mrna expression levels of relevant neuropeptides. For the first and second experiment sets, cells of no more than 5 passages after the FAC sorting process were used and grown with Neurobasal A. The third and last experiment sets utilized DMEM and all experiments were done with cell lines between passages In all experiments, cells were grown to a single 100 mm plate and were split to 60mm plates or coated glass slides a minimum of 48 hours prior to the start of the experiment. In addition, all experiments began with a minimum of 70% cellular confluency Molecular screening In the first experiment that determined the complement of neuropeptides and cellular markers, cells were split and cultured in Neurobasal A and given 24 hours to adhere to the culture plate and recover from the split. Following the recovery period, RNA was collected, purified and subsequently amplified using a One-Step RT-PCR kit (Invitrogen, Burlington, Ontario, Canada) with the following mixture: 200 ng of template RNA, 1 µl of gene specific primers (10 µm) along with 12.5 µl of 2X Reaction Mix, 1 µl of Superscript III RT/Platinum Taq enzyme mix, and adjusted to a total volume of 25 µl using RNAse free water [190]. The mixture was placed in the ABI 2720 thermo cycler (Applied Biosystems, Streetsville, Ontario, Canada) and subjected to the following profile: 30 minutes at 55ºC, 2 minutes at 94ºC,
43 34 40 cycles of 94ºC for 30 seconds, 60ºC for 30 seconds, and 68ºC for 1 minute, followed by 5 minutes at 68ºC and held at 4ºC. The final samples were mixed with loading dye (Thermo Scientific, Burlington, Ontario, Canada) and ran on a 2% agarose gel (Gibco) with 0.5 µg/ml ethidium bromide [58] Immunocytochemistry and cellular imaging The second experiment determined the cellular protein expression of neuropeptides found in the previous experiment. All cells were grown in Neurobasal A and plated to Poly-L-Lysine (Sigma-Aldrich) coated glass slides at least 48 hours prior to the start of the experiment [151]. Cells were fixed with 4% Paraformaldehyde (Electron Microscopy Sciences, Hatfield Pennsylvania, USA) for 15 minutes, permeated with 0.2% TX-100 (Sigma-Aldrich) for 15 minutes at room temperature and blocked with 5% BSA for 2 hours at room temperature. The cells were incubated with antigen specific primary antibodies (Table 2.1a) overnight at 4ºC and fluorescent secondary antibodies (Table 2.1b) at room temperature for 2 hours. The cells were subsequently mounted with anti-fade mounting medium (Life Technologies, Burlington, Ontario, Canada) and imaged using the Zeiss 510 LSM laser confocal fluorescent microscope (Carl Zeiss, Toronto, Ontario, Canada) Insulin sensitivity The third experiment determined the insulin sensitivity of the cell lines. All cells were grown in DMEM and split to 60 mm plates for treatment with insulin. 2 hours prior to the start of the experiments, the plates were rinsed with PBS and serum starved with FBS free DMEM for 2 hours. Insulin was added after 2 hours and protein was collected 15 minutes after treatment with
44 35 0, 1, 10, 100 nm insulin [150]. The plates were aspirated and 50 µl of premade lysis buffer (New England Biolabs, Whitby, Ontario, Canada) containing phosphatase and protease inhibitors cocktails as well as 1 mm PMSF (Sigma-Aldrich). The subsequent mixture was spun down at rpm at 4ºC for 15 minutes to enable collection of solubilized proteins. The protein was then quantified using a BCA protein assay kit (Thermo Scientific) and mixed with 4X SDS protein sample buffer (40% glycerol, 240 mm Tris/Hcl ph 6.8, 8% SDS, 0.04% bromophenol blue, 5% 2β-mercaptoethanol, Sigma-Aldrich). The protein with sample buffer was heated to 100ºC for 10 minutes and ran on a 10% Bis-Tris gel with a Tris-Glycine SDS running buffer. The proteins were then transferred to a 0.22um PVDF membrane, probed for phospho-akt activation (serine 473) and results normalized to total AKT using appropriate antibodies (9271S and 9272S, New England Biolabs) Effects of insulin treatment The experiment began 48 hours after the cells were split from 100 mm to 60 mm tissue culture plates. The cells were serum starved for 12 hours with FBS free DMEM and given vehicle/ phosphate buffered saline (PBS) or 10 nm insulin suspended in FBS free DMEM. RNA was isolated from the cells at 0, 2, 4, 6, 8, and 12 hours following initial treatment with vehicle or insulin and subjected to quantification via real time RT-PCR. 2.6 RNA isolation, cdna synthesis and quantitative real time RT-PCR RNA isolation RNA was isolated from GFP-positive primary cells using the Arcturus Picopure RNA isolation kit with sensitivity range from single cells up to 100 µg, and manufacturers protocol
45 36 were utilized (Applied Biosystems). In contrast, a modified guanidinium thiocynate method was used to isolate RNA from the cell lines [42]. For each 60 mm plate, a 1 ml lysate mixture consisting of 500 µl guanidium thiocynate, 500 µl water saturated phenol, 50 µl 2M sodium acetate and 3.6 µl 2β-mercaptoethanol (Sigma-Aldrich) was added following aspiration. The lysate and cell mixture was then transferred to a tube, and 100 µl of chloroform-isoamyl alcohol (49:1) (Sigma-Aldrich) was added to the mix. The sample was then subjected to a 15 second vortex followed by a 20 minute dissolving and re-suspension period on ice. This was followed by 30 minutes of centrifugation at rpm at 4ºC where the clear aqueous layer was transferred into a second tube with 375 µl of ethanol and 1M acetic acid mix (20:1). The solution was mixed and stored at -20ºC overnight (minimum of 16 hours) for RNA precipitation. The RNA was pelleted on the following day by spinning the mixture at rpm at 4ºC for a minimum of 40 minutes, the supernatant was discarded and the pellet was washed once using 75% ethanol. The pellet was centrifuged with the 75% ethanol at rpm at 4ºC for 15 minutes and remaining liquid was aspirated. Finally, the RNA was dried on ice and dissolved with a minimal amount of RNAse free water. All dissolved RNA samples were quantified via the NanoDrop 2000c (Thermo Scientific, Nepean, Ontario, Canada) cdna synthesis Since reverse transcriptase can amplify both mrna as well as genomic DNA, genomic DNA was degraded using the Turbo DNase kit (Ambion, Streetsville, Ontario, Canada) with the provided protocols. The DNase enzyme was deactivated with 17mM EDTA at 75ºC for 10 minutes and cdna was made from 2 µg of mrna using the High-Capacity cdna Reverse Transcription Kit (Applied Biosystems) with the provided protocol.
46 Real-Time RT-PCR An amplification mix of gene specific primers (10 µm, 0.4 µl), premade Platinum SYBR Green qpcr SuperMix-UDG w/rox (4 µl) (Invitrogen) and RNAse free water (1.6 µl) was used to amplify cdna (50 ng in 4 µl). Samples and amplification mix were loaded in triplicate into 384 well plates and placed in an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems). The reaction was subjected to the following thermo cycler profile: 50 ºC for 2 minutes, 95ºC for 2 minutes, 40 cycles of 95ºC for 15 seconds and 60ºC for 1 minute, followed by 95ºC for 15 seconds, 60ºC for 15 seconds and 95ºC for 15 seconds [153]. All outputs from the PCR system were analyzed using the provided ABI Sequence Detection System software (SDS version 2.4.1) Primers PrimerQuest from Integrated DNA Technologies (IDT, were used to design all primers used in this study. All designed primers were cross referenced with the National Centre for Biotechnology Information using the basic local alignment search tool (Primer BLAST, All new primers were tested and amplification products separated using a 2% agarose gel with the appropriate DNA ladder (Thermo Scientific). The primer products were subsequently isolated from the gel with the QIAquick Gel Extraction Kit (Qiagen, Mississagua, Ontario, Canada) and sent for sequencing at the Centre for Applied Genomics (Hospital for Sick Children, MaRS Centre, Toronto, Ontario, Canada). Results were cross referenced with BLAST to determine specificity.
47 Immunofluorescence (IF) MIP-GFP CD1 mice were anesthetized using isoflurane and subjected to cardiac perfusion using 20 ml of PBS followed by 20 ml of 4% PFA. The brains were then surgically extracted and subjected to additional fixation in 40 ml of 4% PFA for 24 hours. Subsequently, the brains were stepwise dehydrated using solutions of 15% and 30% sucrose in PBS for a minimum of 24 hours for each dehydration step. The dehydrated brains are then frozen in isopentane at -78ºC and embedded in Tissue-Tek O.C.T compound (Sakura Finetek, Torrance, California, USA), and placed in the cryostat for one hour to freeze to -20ºC slicing temperature. 25 micron slices were obtained sequentially and used for immunofluorescence [199]. Brain slices were washed using PBS with 0.06% Tween-20, and blocked using a PBS solution with 5% serum and 0.4% TX-100 for a minimum of 2 hours. Primary antibodies were incubated with a PBS solution with 2% serum and 0.4% TX-100 with antigen specific primary antibodies (Table 2.1a) at 4ºC for 24 hours, and fluorescent secondary antibodies (Table 2.1b) at room temperature for 2 hours. Brain slices were subsequently mounted with anti-fade mounting medium and imaged using the Zeiss 510 LSM laser confocal fluorescent microscope (Carl Zeiss) 2.8 Immortalization of human islets Human islets from donor tissue were obtained (Clinical Islet Laboratory, University of Alberta, Alberta, Canada) and were either dispersed with Accutase (Sigma-Aldrich) or left intact. The dispersed or whole islets were subsequently plated onto Poly-L-Lysine coated culture dishes and treated with growth factors (Table 2.3) along with 50 µg/ml gentamicin (Sigma-Aldrich) for approximately 9 days in RPMI-1640 supplemented with 10% FBS and 1% Pen-Strep (Gibco) at 37ºC (5% CO 2, humidified incubator). Post treatments with proliferative factors, the islet cells
48 39 were incubated with the SV40 T-antigen retrovirus twice over 48 hours in fresh RPMI-1640 at 37ºC (5% CO 2, humidified incubator), and given 48 hours to recover from the viral infection with fresh media. Subsequently, the cells were given two 50 ng/ml doses of geneticin (Gibco) and incubated for 48 hours. Finally, surviving cells were given fresh media and were allowed to grow. Cell viability following the immortalization process was assessed with Trypan Blue (Sigma-Aldrich) and an inverted light microscope. 2.9 Statistical analysis Relative mrna expression was calculated by normalizing the gene of interest to the internal control (Histone 3a). All values were subjected to the Grubb s outlier test and outliers are omitted in the final statistical calculation. The mean and standard error was then calculated for each time point of each treatment group. Two-way analysis of variance (ANOVA) was subsequently performed followed by the Holm-Sidak post hoc test using SigmaPlot 12 (Systat Software, San Jose, California, USA). Relative protein phosphorylation was calculated by normalizing the protein of interest to total protein expression. Mean and standard error was calculated for each treatment group following an outlier test. One-way ANOVA was performed followed by Dunnett s post hoc test using SigmaPlot 12. Data was considered to be statistically significant if p<0.05.
49 40 a. Antigen Host Concentration Supplier NPY (IF) Rabbit 1:250 Phoenix Pharmaceuticals (Burlingame, California, USA) GFP (IF) Chicken 1:2500 AbCam (Cambridge, Massachusetts, USA) NPY (ICC) Rabbit 1:250 Santa Cruz Biotechnology (Dallas, Texas, USA) GFP (ICC) Mouse 1:200 AbCam (Cambridge, Massachusetts, USA) b. Fluorophore Host Antigen Concentration LSM Filters Supplier Alexa Fluor 488 Donkey Mouse Ab 1:500 BP Life Technologies Alexa Fluor 488 Goat Rabbit Ab 1:500 BP Life Technologies Alexa Fluor 555 Goat Chicken Ab 1:500 BP Life Technologies DAPI Nuclear Stain N/A N/A 1:1000 LP 650 Life Technologies Table 2.1 Primary (a) and fluorescent antibodies/ stains (b) used for immunocytochemistry (ICC) and immunofluorescence (IF) with their corresponding antigen, host species and dilution ratios. Wavelength of band pass (BP) and long pass (LP) filters used on the laser scanning microscope (LSM, Zeiss-510) are given in nanometers.
50 41 Gene Forward Primer Reverse Primer Histone 3a GCAAGAGTGCGCCCTCTACTG GGCCTCACTTGCCTCGTGCAA T-Antigen AGAGGAATCTTTGCAGCTAA CTAAACACAGCATGACTCAA NSE CTGATGCTGGAGTTGGATG CTTCGCTGTTCTCCAGGATAT AgRP AGGGCATCAGAAGGCCTGACCAGG TTGAAGAAGCGGCAGTAGCACGT AVP GCCGTGGGCATCTGCTGCAGCGACG TCAGTAGACCCGGGGCTTGGCAGAA CART AGCTCCCGCCTGCGGCTGCT CAGTCACACAGCTTCCCGATCC CRH ATTCTGATCCGCATGGGTGAAGAATA TAATTAGGGGTATATAGGCTCTCTCC Galanin CATGCCATTGACAACCACAG GGATTGGCTTGAGGAGTTGG Ghrelin AGCATGCTCTGGATGGACATG AGGCCTGTCCGTGGTTACTTGT GHRH TTGTGATCCTCATCCTCACCAG ATCACTTTCCGGGCATACAG Insulin I AGAGACCATCAGCAAGCAGGTCAT TTCAGTGGCATTTACACGGTTGCC Insulin II TGTCAAGCAGCACCTTTGTGGTTC AGTGGTGGGTCTAGTTGCAGTAGT Neurotensin ATAGGAATGAACCTTCAGCTG GTAGGAGGCCCTCTTGAGTAT NPY TAGGTAACAAGCGAATGGGG ACATGGAAGGGTCTTCAAGC POMC TAGATGTGTGGAGCTGGTGC CAGTCAGGGGCTGTTCATCT Orexin CCTGAGCTCCAGGCACCATGAACT TGGTTACCGTTGGCCTGAAGGAGG Oxytocin ATCACCTACAGCGGATCTCAGACT AAGCGCGCTAAAGGTATTCCCAGA Urocortin GCGTCTTCAGCCCGTCCCCGGGGACAGAGT GCCGATCACTTGCCCACCGAATCGAATATG VIP TGATTCGTTTGCCAATGAGTGAC TGGATGACAGGATGCCGTTTGAAG CNTFR ATACTGCGAAGCTTAGAACTGGGC GCACAGTCACATTGAAGGTATTGG ERα GAATTCAATTCTGACAATCGACGCCAG GAATTCGTGCTTCAACATTCTCCCTCC ERβ GAATTCTAGCCACCCACTGCCAATCAT GAATTCCACACCTTTCTCTCCTGGATG IR GTGATACCAGAGCATAGGAG CTGTTCGGAACCTGATGAC GHSR ACCTGCTCTGCAAACTCTTCCAGT AACACCACCACAGCAAGCATCTTC GLP1R TTTGATGACTATGCCTGCTGG AGCCCATCCCACTGGTGTT GLP2R TGCTGGTTTCCATCAAGCAA ATCAGCTGCAAGGTGGACAA ObRb ATGACGCAGTGTACTGCTG GTGGCGAGTCAAGTGAACCT Gene Forward (SYBR) Reverse (SYBR) Histone 3a ATCTTCAAAAAGGCCAACCAGAT CGCTTCCAGAGTGCAGCTATT NPY CAGAAAACGCCCCCAGAA AAAAGTCGGGAGAACAAGTTTCATT AgRP CGGAGGTGCTAGATCCACAGA AGGACTCGTGCAGCCTTACAC VIP AGAAGCAAGCCTCAGTTCCT TGCTCCTTCAAACGGCATCC Table 2.2 Forward and reverse gene specific primers used for either molecular screening (One-Step RT-PCR) or mrna quantification (SYBR, quantitative Real-Time PCR).
51 42 Proliferative Factors Concentration Supplier HGF 25 ng/µl Sigma-Aldrich EGF 25 ng/µl Sigma-Aldrich Gastrin 25 ng/µl Sigma-Aldrich GABA 100 µm Sigma-Aldrich Exendin nm American Peptide Company (Sunnyvale, California, USA) Table 2.3 Hepatocyte growth factor (HGF), epidermal growth factor (EGF), gastrin, γ-aminobutyric acid (GABA) and Exendin-4 have been known to cause significant increases in the proliferative rate of pancreatic islet cells. These growth factors were applied in specified concentrations prior to the introduction of the SV40 T-antigen virus in the immortalization protocol.
52 Chapter 3 Results 43
53 44 3 Results 3.1 Generation of morphologically distinct GFP-positive cell lines from primary hypothalamic cultures Primary hypothalamic cultures were obtained from four MIP-GFP CD1 mice of approximately 8 weeks of age. Of the four cultures, three were successfully immortalized following viral infection and Geneticin selection. The three surviving cultures were FAC sorted and were all found to contain GFP-positive populations within the immortalized culture. These GFP-positive cells were named mhypoa-mip/gfp-1, mhypoa-mip/gfp-2, and mhypoa- MIP/GFP-3 and represent 693, 1869 and 469 of approximately 3 million cells in each respective sorting event (approximately 0.023%, 0.062% and 0.016% respectively). Following the recovery period post FAC sorting, the three lines were found to have distinct morphological characteristics. mhypoa-mip/gfp-1 was noted to have long singular projections (Figure 3.1) with a doubling time of approximately 48 hours. mhypoa-mip/gfp-2 was noted to be smaller in size, have multiple short projections (Figure 3.1) with a doubling time of approximately 36 hours. mhypoa-mip/gfp-3 was found to have a notably larger cell body, variable length of projections (Figure 3.1) and has a doubling time of approximately 72 hours. 3.2 Gene expression profiles of mhypoa-mip/gfp cell lines As the mhypoa-mip/gfp cell lines were newly generated in this study, no data were available on the origin, receptor complement or neuropeptide expression of these cells. All three lines were therefore subjected to screening via One-Step RT-PCR. Initial screening of the three lines found neuron specific markers, but none of the cells were found to express insulin mrna. In order to elucidate potential functionality and possible origin of these neurons, the cells were
54 45 subjected to a more detailed gene expression profile. Of the genes screened, the cells were found to express the feeding related neuropeptides NPY, AgRP and VIP, but did not express other hypothalamic neuropeptides such as POMC, CART, Galanin, Ghrelin, and Orexin (Table 3.2, Figure 3.3). This is paired with the expression of insulin receptor and long form of the leptin receptor (ObRb), which suggest that the MIP activity is present within key neurons that maintain energy homeostasis. 3.3 Localization of GFP and NPY Due to the lack of specific primers available for the detection of the GFP mrna transcript, combined with previous unsuccessful attempts of GFP detection via Western blotting, immunofluorescence was employed to both confirm the expression of GFP and to determine the cellular localization of the neuropeptides found in the screening,. All cell lines were found to express neuron specific enolase (NSE) (Table 3.2) confirming the neuronal phenotype of the cells. However, of the three cell lines, only mhypoa-mip/gfp-1 and 2 expressed sufficient GFP for immunocytochemical confirmation (Figure 3.4), while mhypoa-mip/gfp-3 did not express sufficient GFP fluorescence per given surface area of the cell to provide a distinctly positive image, and thus was excluded from additional experimentation. In addition, of the two cell lines confirmed to be GFP-positive by immunocytochemistry, all cells within the cell lines were found to be NPY positive and with punctate expression of the neuropeptide (Figure 3.5). 3.4 mhypoa-mip/gfp cells are insulin sensitive Given the presence of NPY and the insulin receptor, it was possible that insulin regulates NPY in these cells. Upon treatment with vehicle control, 0.1, 1, 10 or 100 nm of insulin, both
55 46 mhypoa-mip/gfp-1 and 2 cell lines were found to have a significantly elevated level of phosphor-akt at Serine 473 (Figure 3.6), as phosphorylation of this residue denotes the activation of the insulin signaling pathway and insulin sensitivity. In mhypoa-mip/gfp-1, treatment with 0.1 nm insulin did not induce a significant increase in AKT phosphorylation (0.11±0.04 treated vs. 0.11±0.05 vehicle, p> 0.05). However, with 1 nm insulin treatment, a significant increase in AKT phosphorylation was present (2.5 fold increase, 0.27±0.06 treated vs. 0.11±0.05 vehicle p<0.05). Similarly, 10 and 100 nm insulin treatment induced a significant increase in AKT phosphorylation of 6.8 and 11.7 fold respectively (0.75± nm treated vs. 0.11±0.05 vehicle and 1.29± nm treated vs 0.11±0.05 vehicle, p<0.001). In mhypoa-mip/gfp-2, treatment with 0.1 nm insulin also failed to induce a significant change in AKT phosphorylation (0.23±0.07 treated vs. 0.26±0.11 vehicle, p>0.05). Upon treatment with 1 nm insulin, a significant 1.8 fold increase was observed (0.49±0.11 treated vs. 0.26±0.11 vehicle, p<0.01). Similarly, treatment with 10 and 100 nm insulin also induced significant increases in AKT phosphorylation of 3.4 and 5.7 fold respectively (0.89± nm treated vs. 0.26±0.11 vehicle and 1.49± nm treated vs. 0.26±0.11, p<0.001). 3.5 Differential regulation of NPY by insulin in the mhypoa-mip/gfp cell lines To determine the effect of insulin on NPY and AgRP mrna levels in the mhypoa- MIP/GFP-1 and 2 lines, cells were treated with 10 nm insulin and RNA was collected at 0, 2, 4, 6, 8 and 12 hours. NPY and AgRP mrna levels were found to respond differentially upon insulin treatment.
56 47 In mhypoa-mip/gfp-1, treatment with 10 nm insulin had no effect on AgRP mrna levels at any of the time points (Figure 3.7a). However, NPY mrna was found to be up regulated by insulin and was significantly increased (1.25±0.07 treated vs. 0.86±0.05 vehicle, p<0.001) at 4 hours post treatment with 10 nm insulin (Figure 3.7a). This effect was not seen at any other time point within the 12 hour time course. In mhypoa-mip/gfp-2, 10 nm insulin treatment also had no effect on AgRP mrna levels at any time points (Figure 3.7b). But NPY mrna was found to be down regulated and was significantly decreased (0.8751±0.12 treated vs. 1.41±0.18 vehicle, p<0.05) at 8 hours post treatment with 10 nm insulin (Figure 3.7b). This repression in NPY mrna was not seen at any other time point within the 12 hour time course, although an overall trend of repression was noted at 2, 6 and 12 hours. 3.6 Primary hypothalamic cells also express NPY and GFP To determine whether GFP expression seen in the cell lines occurs endogenously within the hypothalamus of the mice, primary cells from five MIP-GFP CD1 mice were isolated, pooled and sent for FAC sorting directly after extraction. FAC sorting was able to isolate 1642 primary cells with GFP expression from approximately 1.7 million cells during the sort. In addition to obtaining primary cells with native GFP expression, RNA was isolated with the Arcturus Picopure kit to ensure mrna was isolated from the small number of cells and ran through quantitative RT-PCR, signal was detected for NPY (CT of 33 vs. 30 for Histone). Thus, this validates that MIP is indeed active within a subset of NPY positive cells of the hypothalamus.
57 GFP was not detectable in the MIP-GFP brain via immunofluorescence Since the hypothalamus of MIP-GFP CD1 mice contained GFP-positive cells, it was of interest to determine the localization of these cells and potential colocalization with neuropeptides such as NPY. 25 micron brain slices were obtained from four animals of approximately 12 weeks of age and subjected to immunofluorescence staining. 117 slices from the four mice and approximately 1.3 million hypothalamic cells were assessed. While NPY was detected and exhibited a distinct expression pattern as determined by confocal laser microscopy, no significant or distinct GFP signal was detected (Figure 3.8). This is in line with the previous finding that MIP activity is low within the hypothalamus of the MIP-GFP CD1 mice. Ultimately, the localization of GFP and NPY double positive neurons could not be determined. 3.8 Generation of immortal cells from human islets Human islets obtained from five donors were treated with proliferative factors (Table 2.3) and infected with the SV40 T-antigen virus. Of the immortalized islet cells, only those treated with EGF and gastrin displayed observable proliferation. Additionally, assessment of all surviving immortalized cultures approximately 4 weeks after viral infection with Trypan Blue resulted in negligible staining, denoting a lack of dead cells. The EGF and gastrin-treated proliferative cells were isolated and appeared to be of neuronal origin (Figure 3.9a). These islet cells were named hislet-1, subjected to screening for neuron specific enolase (NSE) and NPY, and were found to be positive for both (Figure 3.9b). However, with a doubling time in excess of two weeks, the islet cells were only subjected to a limited number of experiments and were frozen for future use.
58 49 mhypoa-mip/gfp-1 mhypoa-mip/gfp-2 mhypoa-mip/gfp-3 Figure 3.1 Morphologically distinct GFP+ immortal cell lines Representative images of the cell lines captured from live cells approximately 48 hours post cell passaging. Images were captured via a light microscope at 40X magnification.
59 50 a. Gene of Interest mhypoa-mip/gfp-1 mhypoa-mip/gfp-2 mhypoa-mip/gfp-3 Histone 3a T-Antigen NSE AgRP AVP CART CRH Galanin Ghrelin GHRH Insulin I Insulin II Neurotensin NPY POMC Orexin Oxytocin Urocortin VIP CNTFR ERα ERβ IR GHSR GLP1R GLP2R ObRb Table 3.2 Neuropeptides and receptors expressed by the immortal cell lines Table indicating the presence (+) or absence (-) of mrna transcripts for neuronal markers, SV-40 T-antigen, orexigenic and anorexigenic neuropeptides as well as receptors known to be involved in the regulation of feeding and energy homeostasis. RNA was isolated and amplified using gene specific primers listed in table 2.2 and one-step RT-PCR.
60 51 NPY Figure 3.3 Neuropeptides and receptors expressed by the immortal cell lines Representative blots of screening results from table 3.2. Hypothalamic RNA (Hypo) and non-template control (NTC) were utilized as positive and negative controls respectively for the screening. RNA was isolated and amplified using gene specific primers and semi quantitative RT-PCR. VIP AgRP IR ObRb CNTFR Insulin I Insulin II Histone
61 52 mhypoa-mip/gfp-1 mhypoa-mip/gfp-2 mhypoa-mip/gfp-3 mhypoa-gnrh/gfp (Control) Figure 3.4 GFP localization within the mhypoa-mip/gfp cells Representative ICC images of GFP expression within the mhypoa-mip/gfp cell lines compared to a known GFP positive control (mhypoa-gnrh/gfp cell line). Green and red staining represents cytoplasmic GFP expression and nucleus respectively. Images were obtained approximately 48 hours following passaging using the Zeiss 510 laser confocal microscope and antibodies listed in table 2.1
62 53 mhypoa-mip/gfp-1 mhypoa-mip/gfp-2 Figure 3.5 NPY localization within the mhypoa-mip/gfp cells Representative ICC images of NPY expression within the mhypoa-mip/gfp-1 and 2 cell lines. Red and blue staining represents cytoplasmic NPY expression and nucleus respectively. Images were obtained approximately 48 hours following passaging using the Zeiss 510 laser confocal microscope and antibodies listed in Table 2.1.
63 54 pakt AKT pakt AKT Figure 3.6 mhypoa-mip/gfp-1 and 2 are sensitive to 1-100nM of insulin AKT phosphorylation (Serine 473, with representative blots) following treatment with vehicle (black bar) or insulin (white bars) at doses of 0.1, 1, 10 and 100 nm. Cells were serum starved for 2 hours and protein was collected at 15 minutes following treatment and assessed by western blotting. Phosphorylation levels were normalized to total protein and represented as mean±se of 3 independent experiments. Statistical significance was considered at p<0.05 by one-way ANOVA with Dunnett s post hoc test.
64 55 a. MIP-GFP 1 10nM Insulin AgRP MIP-GFP 1 10nM Insulin NPY Relative AgRP mrna Vehicle 10nM Insulin Relative NPY mrna *** Vehicle 10nM Insulin 0.0 0h 2h 4h 6h Time Points 8h 12h n= h 2h 4h 6h Time Points 8h 12h ****= P<0.001 n=6 b. MIP-GFP 2 10nM Insulin AgRP MIP-GFP 2 10nM Insulin NPY Relative AgRP mrna Vehicle 10nM Insulin Relative NPY mrna * Vehicle 10nM Insulin 0.0 0h 2h 4h 6h Time Points 8h 12h n= h 2h 4h 6h Time Points 8h 12h * = P<0.05 n=6 Figure 3.7 Insulin regulation of orexigenic genes in the cell lines NPY and AgRP expression levels of (a) mhypoa-mip/gfp-1 and (b) mhypoa- MIP/GFP-2 cell lines over a 12 hour time course following treatment with vehicle (white bars) and 10 nm insulin (black bars). Cells were serum starved for 12 hours prior to the treatment of insulin or vehicle. RNA was collected at 0, 2, 4, 6, 8 and 12 hours following treatment and mrna was assessed by quantitative RT-PCR. All samples were run in triplicate and normalized to Histone 3a. All data is represented as mean±se of 6 independent experiments. Statistical significance was considered at p<0.05 by two-way ANOVA with Holm-Sidak post hoc test.
65 56 GFP, 5X Magnification GFP, 20X Magnification NPY, 5X Magnification NPY, 20X Magnification Figure 3.8 No hypothalamic GFP was detectable via immunofluorescence Representative immunofluorescence images probing for GFP (green, top images) and NPY (red, bottom images) localization in the hypothalamus of 12 week old MIP-GFP CD1 mice. While NPY exhibited punctate staining within the ARC and DMH, no GFP signal was detectable against nuclear staining (blue) at either 5X or 20 X magnifications. Images were obtained using the Zeiss 510 laser confocal microscope and antibodies listed in Table 2.1
66 57 a. a. T-antigen NSE NPY Figure 3.9 Immortal human islet cells and preliminary screening Representative images of the immortalized human islet cells hislet-1 at 40X magnification (a) captured after viral transformation and 48 hours following passaging respectively. The islet cell line were subjected to screening (b) for markers of interest and compared to the positive control of mhypoa-npy/gfp. RNA was isolated and amplified using gene specific primers and one-step RT-PCR.
CNS Control of Food Intake. Adena Zadourian & Andrea Shelton
 CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
CNS Control of Food Intake Adena Zadourian & Andrea Shelton Controlling Food Intake Energy Homeostasis (Change in body adiposity + compensatory changes in food intake) Background Information/Review Insulin
Insulin-Leptin Interactions
 Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Insulin-Leptin Interactions Ahmed S., Al-Azzam N., Cao B. Karshaleva B., Sriram S., Vu K. If you understand a system, you can predict it. Agenda - Energy homeostasis Overview of leptin and insulin Signaling
Ingestive Behaviors 21. Introduction. Page 1. control and story lines. (a review of general endocrinology) Integration (or the basic reflex arc model)
 Ingestive Behaviors 21 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Ingestive Behaviors 21 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction. Laura Gunter
 Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Neurophysiology of the Regulation of Food Intake and the Common Reward Pathways of Obesity and Addiction Laura Gunter The Brain as the Regulatory Center for Appetite The brain is the integration center
Internal Regulation II Energy
 Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Internal Regulation II Energy Reading: BCP Chapter 16 lookfordiagnosis.com Homeostasis Biologically, what is necessary for life is a coordinated set of chemical reactions. These reactions take place in
Motivation 1 of 6. during the prandial state when the blood is filled
 Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
Motivation 1 of 6 I. INTRODUCTION A. Motivation: a condition (usually internal) that initiates, activates, or maintains goal-directed behavior. B. Archery analogy 1. undrawn bow has no potential energy
LESSON 3.3 WORKBOOK. How do we decide when and how much to eat?
 Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Appetite The psychological desire to eat, driven by feelings of pleasure from the brain. Hunger The biological or physiological need to eat, caused by a release of hormones from the digestive tract. LESSON
Hypothalamus. Small, central, & essential.
 Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Hypothalamus Small, central, & essential. Summary: You can t live without a hypothalamus. Located at the junction between the brain stem and the forebrain Medial hypothalamus: interface between the brain
Chapter 20. Endocrine System Chemical signals coordinate body functions Chemical signals coordinate body functions. !
 26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
26.1 Chemical signals coordinate body functions Chapter 20 Endocrine System! Hormones Chemical signals Secreted by endocrine glands Usually carried in the blood Cause specific changes in target cells Secretory
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN. COGS 163 By: Pranav Singh Alexandra Villar
 THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
THE ROLE OF INSULIN RECEPTOR SIGNALING IN THE BRAIN COGS 163 By: Pranav Singh Alexandra Villar INTRODUCTION Insulin is a hormone produced in the pancreas by the islets of Langerhans that regulates the
Hormones. Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6
 Hormones Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6 Tel. 030-8385-6920 (Sekret.) 030-8385-6922 (direkt) e-mail: vhaucke@chemie.fu-berlin.de http://userpage.chemie.fu-berlin.de/biochemie/aghaucke/teaching.html
Hormones Prof. Dr. Volker Haucke Institut für Chemie-Biochemie Takustrasse 6 Tel. 030-8385-6920 (Sekret.) 030-8385-6922 (direkt) e-mail: vhaucke@chemie.fu-berlin.de http://userpage.chemie.fu-berlin.de/biochemie/aghaucke/teaching.html
Testosterone and other male hormones seem to be related to aggressive behavior in some species
 Testosterone and Male Aggression Testosterone and other male hormones seem to be related to aggressive behavior in some species In the fish species Oreochromis mossambicus, elevated levels have been found
Testosterone and Male Aggression Testosterone and other male hormones seem to be related to aggressive behavior in some species In the fish species Oreochromis mossambicus, elevated levels have been found
Ingestive Behaviors 33. Introduction. Page 1. control and story lines. (a review of general endocrinology) Integration (or the basic reflex arc model)
 Ingestive Behaviors 33 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Ingestive Behaviors 33 (a review of general endocrinology) A neuroendocrine system: components, a reflex arc, the endocrine system, the AN, endocrine / nervous systems as afferents and efferents, the theoretical
Chemical Regulation. Chapter 26. Testosterone and Male Aggression: Is There a Link? THE NATURE OF CHEMICAL REGULATION
 Chapter 6 Chemical Regulation PowerPoint Lectures for Biology: Concepts and Connections, Fifth Edition Campbell, Reece, Taylor, and Simon Testosterone and Male Aggression: Is There a Link? Among male animals,
Chapter 6 Chemical Regulation PowerPoint Lectures for Biology: Concepts and Connections, Fifth Edition Campbell, Reece, Taylor, and Simon Testosterone and Male Aggression: Is There a Link? Among male animals,
Chapter 1. General introduction. Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815
 Chapter 1 General introduction Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815 Chapter 1 B. Tiesjema, 2007 GENERAL INTRODUCTION NEURAL CIRCUITS INVOLVED IN ENERGY BALANCE
Chapter 1 General introduction Part of this chapter is adapted from Adan et.al., Br.J.Pharmacol. 2006;149:815 Chapter 1 B. Tiesjema, 2007 GENERAL INTRODUCTION NEURAL CIRCUITS INVOLVED IN ENERGY BALANCE
FLASH CARDS. Kalat s Book Chapter 10 Alphabetical
 FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
FLASH CARDS www.biologicalpsych.com Kalat s Book Chapter 10 Alphabetical AgRP AgRP Agouti-related peptide; synthesized in hypothalamus. Acts as an appetite stimulator. Also decreases metabolism. aldosterone
Growth Hormone, Somatostatin, and Prolactin 1 & 2 Mohammed Y. Kalimi, Ph.D.
 Growth Hormone, Somatostatin, and Prolactin 1 & 2 Mohammed Y. Kalimi, Ph.D. I. Growth Hormone (somatotropin): Growth hormone (GH) is a 191 amino acid single chain polypeptide (MW 22,000 daltons). Growth
Growth Hormone, Somatostatin, and Prolactin 1 & 2 Mohammed Y. Kalimi, Ph.D. I. Growth Hormone (somatotropin): Growth hormone (GH) is a 191 amino acid single chain polypeptide (MW 22,000 daltons). Growth
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control
 NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
NROSCI/BIOSC 1070 and MSNBIO 2070 September 11, 2017 Control Mechanisms 2: Endocrine Control Hormones are chemical messengers that are secreted into the blood by endocrine cells or specialized neurons.
Chapter 26. Hormones and the Endocrine System. Lecture by Edward J. Zalisko
 Chapter 26 Hormones and the Endocrine System PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture
Chapter 26 Hormones and the Endocrine System PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education, Inc. Lecture
Copyright 2017 The Guilford Press
 This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
This is a chapter excerpt from Guilford Publications. Eating Disorders and Obesity: A Comprehensive Handbook, Third Edition. Edited by Kelly D. Brownell and B. Timothy Walsh. Copyright 2017. Purchase this
BIOLOGY - CLUTCH CH.45 - ENDOCRINE SYSTEM.
 !! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
!! www.clutchprep.com Chemical signals allow cells to communicate with each other Pheromones chemical signals released to the environment to communicate with other organisms Autocrine signaling self-signaling,
BIOL212 Biochemistry of Disease. Metabolic Disorders - Obesity
 BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
BIOL212 Biochemistry of Disease Metabolic Disorders - Obesity Obesity Approx. 23% of adults are obese in the U.K. The number of obese children has tripled in 20 years. 10% of six year olds are obese, rising
Investigation of the role of nesfatin-1/nucb2 in the central nervous system. Ph.D. thesis Katalin Könczöl
 Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Investigation of the role of nesfatin-1/nucb2 in the central nervous system Ph.D. thesis Katalin Könczöl Semmelweis University János Szentágothai Doctoral School of Neurosciences Supervisor: Official reviewers:
Discussion & Conclusion
 Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
Discussion & Conclusion 7. Discussion DPP-4 inhibitors augment the effects of incretin hormones by prolonging their half-life and represent a new therapeutic approach for the treatment of type 2 diabetes
! acts via the autonomic nervous system. ! maintaining body weight within tight limits. ! ventromedial (VMN) ! arcuate (ARC) ! neuropeptide Y (NPY)
 Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Brain Regulates energy homeostatis Glucose Sensing in the Brain Seminar 2012 Gareth Price! acts in response to circulating signals of nutrient states! acts via the autonomic nervous system Ensures a balance
Leptin Intro/Signaling. ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph
 Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
Leptin Intro/Signaling ATeamP: Angelo, Anthony, Charlie, Gabby, Joseph Overview Intro to Leptin Definition & Sources Physiology Bound vs. Free Receptors Signaling JAK/STAT MAPK PI3K ACC Experimental findings
Chapter 24 Cholesterol, Energy Balance and Body Temperature. 10/28/13 MDufilho
 Chapter 24 Cholesterol, Energy Balance and Body Temperature 10/28/13 MDufilho 1 Metabolic Role of the Liver Hepatocytes ~500 metabolic functions Process nearly every class of nutrient Play major role in
Chapter 24 Cholesterol, Energy Balance and Body Temperature 10/28/13 MDufilho 1 Metabolic Role of the Liver Hepatocytes ~500 metabolic functions Process nearly every class of nutrient Play major role in
Chapter 16: Endocrine System 1
 Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Ch 16 Endocrine System Bi 233 Endocrine system Endocrine System: Overview Body s second great controlling system Influences metabolic activities of cells by means of hormones Slow signaling Endocrine glands
Leptin-Insulin Signaling in the Brain. BY TEAM CEPHALIC Aman Hamdard, Kevin Artiga, Megan Imreh, Ronald Baldonado, and Sharri Mo
 Leptin-Insulin Signaling in the Brain BY TEAM CEPHALIC Aman Hamdard, Kevin Artiga, Megan Imreh, Ronald Baldonado, and Sharri Mo Agenda Leptin in the Hypothalamus: Pathways and Roles Cross-talk between
Leptin-Insulin Signaling in the Brain BY TEAM CEPHALIC Aman Hamdard, Kevin Artiga, Megan Imreh, Ronald Baldonado, and Sharri Mo Agenda Leptin in the Hypothalamus: Pathways and Roles Cross-talk between
Ingestive Behavior: Feeding & Weight Regulation. Hypovolemic vs. Osmotic Thirst
 Ingestive Behavior: Feeding & Weight Regulation 1 Hypovolemic Thirst Receptors, CNS, Responses Salt Appetite Digestive components Glucose Homeostasis: Insulin & Glucagon Diabetes Mellitus 1 & 2 CNS Hypothalamic
Ingestive Behavior: Feeding & Weight Regulation 1 Hypovolemic Thirst Receptors, CNS, Responses Salt Appetite Digestive components Glucose Homeostasis: Insulin & Glucagon Diabetes Mellitus 1 & 2 CNS Hypothalamic
Endocrine System. Chapter 20. Endocrine Glands and Hormones. The Endocrine System. Endocrine glands
 Chapter 20 Endocrine System Endocrine Glands and Hormones The endocrine system consists of glands and tissues that secrete hormones Hormones are chemicals that affect other glands or tissues, many times
Chapter 20 Endocrine System Endocrine Glands and Hormones The endocrine system consists of glands and tissues that secrete hormones Hormones are chemicals that affect other glands or tissues, many times
Chapter 20 Endocrine System
 Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Chapter 20 Endocrine System The endocrine system consists of glands and tissues that secrete Hormones are chemicals that affect other glands or tissues, many times far away from the site of hormone production
Bi156 lecture 2, 1/6/12. Eating and weight regulation
 Bi156 lecture 2, 1/6/12 Eating and weight regulation Introduction: weight regulation in an affluent society In our society much effort and money is expended on regulation of weight. Failure to maintain
Bi156 lecture 2, 1/6/12 Eating and weight regulation Introduction: weight regulation in an affluent society In our society much effort and money is expended on regulation of weight. Failure to maintain
Endocrine System Notes
 Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Endocrine System Notes is the tendency to maintain a stable internal environment. - parts of the body that secrete hormones directly into the body. - parts of the body that make secretions which travel
Chapter 7 Conclusions
 VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
Human Anatomy, First Edition. Endocrine System. Chapter 20 Lecture Outline: Endocrine System. McKinley & O'Loughlin
 Human Anatomy, First Edition McKinley & O'Loughlin Chapter 20 Lecture Outline: Endocrine System 1 Endocrine System Endocrine system and the nervous system often work together to bring about homeostasis.
Human Anatomy, First Edition McKinley & O'Loughlin Chapter 20 Lecture Outline: Endocrine System 1 Endocrine System Endocrine system and the nervous system often work together to bring about homeostasis.
The Endocrine System. I. Overview of the Endocrine System. II. Three Families of Hormones. III. Hormone Receptors. IV. Classes of Hormone Receptor
 The Endocrine System I. Overview of the Endocrine System A. Regulates long term metabolic processes B. Releases hormones from endocrine cells 1. Hormones are chemicals 2. Alter metabolism of cells 3. Release
The Endocrine System I. Overview of the Endocrine System A. Regulates long term metabolic processes B. Releases hormones from endocrine cells 1. Hormones are chemicals 2. Alter metabolism of cells 3. Release
Autonomic regulation of islet hormone secretion
 Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Autonomic regulation of islet hormone secretion Implications for health and disease Billy & Bree Paper 1: Autonomic regulation of islet hormone secretion : Implications for health and disease By Team BBB
Motility Conference Ghrelin
 Motility Conference Ghrelin Emori Bizer, M.D. Division of Gastroenterology/Hepatology November 21, 2007 Ghrelin: Basics Hormone produced by the A-like A endocrine cells in the oxyntic mucosa (stomach body
Motility Conference Ghrelin Emori Bizer, M.D. Division of Gastroenterology/Hepatology November 21, 2007 Ghrelin: Basics Hormone produced by the A-like A endocrine cells in the oxyntic mucosa (stomach body
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 26 Hormones and the Endocrine System Multiple-Choice Questions
 Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 26 Hormones and the Endocrine System 26.1 Multiple-Choice Questions 1) Hormones are chemicals produced by the endocrine system that
Campbell's Biology: Concepts and Connections, 7e (Reece et al.) Chapter 26 Hormones and the Endocrine System 26.1 Multiple-Choice Questions 1) Hormones are chemicals produced by the endocrine system that
Peripubertal, leptin-deficient ob/ob female mice were used in an investigation of
 ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
ESSICK-BROOKSHIRE, ELIZABETH ANN, M.S. The Effects of Peripherally Administered 17-β Estradiol and BIBP3226, a NPY Y1 Receptor Antagonist, on Food Intake, Body Mass, Reproductive Development and Behavior
1 Neuroregulation of Appetite
 Chapter 1 / Neuroregulation of Appetite 3 1 Neuroregulation of Appetite Ofer Reizes, PhD, Stephen C. Benoit, PhD, and Deborah J. Clegg, PhD CONTENTS INTRODUCTION THE DUAL-CENTERS HYPOTHESIS CONTROL OF
Chapter 1 / Neuroregulation of Appetite 3 1 Neuroregulation of Appetite Ofer Reizes, PhD, Stephen C. Benoit, PhD, and Deborah J. Clegg, PhD CONTENTS INTRODUCTION THE DUAL-CENTERS HYPOTHESIS CONTROL OF
Hormones. BIT 230 Walsh Chapter 8
 Hormones BIT 230 Walsh Chapter 8 Hormones Regulatory molecules Affect all areas of metabolism Endocrine- hormones travel via the bloodstream to its target cell: true hormone Modern definition- any regulatory
Hormones BIT 230 Walsh Chapter 8 Hormones Regulatory molecules Affect all areas of metabolism Endocrine- hormones travel via the bloodstream to its target cell: true hormone Modern definition- any regulatory
Subject Index. hypothalamic-pituitary-adrenal axis 158. Atherosclerosis, ghrelin role AVP, see Arginine vasopressin.
 Subject Index Acromegaly, somatostatin analog therapy dopamine agonist combination therapy 132 efficacy 132, 133 overview 130, 131 receptor subtype response 131, 132 SOM30 studies 131, 132 ACTH, see Adrenocorticotropic
Subject Index Acromegaly, somatostatin analog therapy dopamine agonist combination therapy 132 efficacy 132, 133 overview 130, 131 receptor subtype response 131, 132 SOM30 studies 131, 132 ACTH, see Adrenocorticotropic
Endocrine Glands. Endocrine glands
 ENDOCRINOLOGY Endocrine Glands Endocrine glands Produce substances called hormones. Ductless glands, i.e., they release hormones directly into the bloodstream Hormones only act at their target tissue where
ENDOCRINOLOGY Endocrine Glands Endocrine glands Produce substances called hormones. Ductless glands, i.e., they release hormones directly into the bloodstream Hormones only act at their target tissue where
Prasad S. Dalvi. Copyright by Prasad S. Dalvi 2012
 Molecular Mechanisms Involved in Insulin- and Leptinmediated Regulation of Hypothalamic Proglucagon Gene Expression and Action of Glucagon-like Peptides on Hypothalamic Neuropeptides by Prasad S. Dalvi
Molecular Mechanisms Involved in Insulin- and Leptinmediated Regulation of Hypothalamic Proglucagon Gene Expression and Action of Glucagon-like Peptides on Hypothalamic Neuropeptides by Prasad S. Dalvi
Figure 1: The leptin/melanocortin pathway Neuronal populations propagate the signaling of various molecules (leptin, insulin, ghrelin) to control
 Leptin Deficiency Introduction The leptin/melanocortin pathway plays a key role in the hypothalamic control of food intake. It is activated following the systemic release of the adipokine leptin (LEP)
Leptin Deficiency Introduction The leptin/melanocortin pathway plays a key role in the hypothalamic control of food intake. It is activated following the systemic release of the adipokine leptin (LEP)
What systems are involved in homeostatic regulation (give an example)?
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS (Diabetes Mellitus Part 1): An Overview
An important function of the central nervous
 Perspectives in Diabetes Insulin Signaling in the Central Nervous System A Critical Role in Metabolic Homeostasis and Disease From C. elegans to Humans Daniel Porte, Jr., 1,2,3 Denis G. Baskin, 3 and Michael
Perspectives in Diabetes Insulin Signaling in the Central Nervous System A Critical Role in Metabolic Homeostasis and Disease From C. elegans to Humans Daniel Porte, Jr., 1,2,3 Denis G. Baskin, 3 and Michael
Chapter 12. Ingestive Behavior
 Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
Chapter 12 Ingestive Behavior Drinking a. fluid compartments b. osmometric thirst c. volumetric thirst Eating a. energy sources b. starting a meal c. stopping a meal d. eating disordersd Drinking a. fluid
UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY
 1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
1 UNIVERSITY OF PNG SCHOOL OF MEDICINE AND HEALTH SCIENCES DIVISION OF BASIC MEDICAL SCIENCES DISCIPLINE OF BIOCHEMISTRY AND MOLECULAR BIOLOGY GLUCOSE HOMEOSTASIS An Overview WHAT IS HOMEOSTASIS? Homeostasis
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 4,000 116,000 120M Open access books available International authors and editors Downloads Our
BIOL 2458 A&P II CHAPTER 18 SI Both the system and the endocrine system affect all body cells.
 BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
BIOL 2458 A&P II CHAPTER 18 SI 1 1. Both the system and the endocrine system affect all body cells. 2. Affect on target cells by the system is slow. Affect on target cells by the system is fast. INTERCELLULAR
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1
 GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
GENERAL CHARACTERISTICS OF THE ENDOCRINE SYSTEM FIGURE 17.1 1. The endocrine system consists of glands that secrete chemical signals, called hormones, into the blood. In addition, other organs and cells
6.6 HORMONES & REPRODUCTION
 6.6 HORMONES & REPRODUCTION Endocrine system Produces and releases hormones Hormones travel in the blood to target tissues Long distance communication between cells Endocrine Glands Blood stream Hormone
6.6 HORMONES & REPRODUCTION Endocrine system Produces and releases hormones Hormones travel in the blood to target tissues Long distance communication between cells Endocrine Glands Blood stream Hormone
Cell to Cell Communication
 Review #1 15 Review using OPAL figures Review using class web PDF Preview of test #1 Cell to Cell Communication 1 Communication Strategies endocrine neurocrine paracrine autocrine Endocrine System Overview
Review #1 15 Review using OPAL figures Review using class web PDF Preview of test #1 Cell to Cell Communication 1 Communication Strategies endocrine neurocrine paracrine autocrine Endocrine System Overview
Orexin and Sleep. Team: A Little Bit of Leptin
 Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Orexin and Sleep Team: A Little Bit of Leptin Intro to Orexin 1997 -Scripps Research Institute gene expression in the hypothalamus Found gene clone 35 - expression limited to the lateral hypothalamus NTs
Hormonal regulation of. Physiology Department Medical School, University of Sumatera Utara
 Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Hormonal regulation of nutrient metabolism Physiology Department Medical School, University of Sumatera Utara Homeostasis & Controls Successful compensation Homeostasis reestablished Failure to compensate
Chapter 26 Hormones and the
 Chapter 6 Hormones and the Endocrine System Introduction In lions, the hormone testosterone promotes the development and maintenance of male traits including growth and maintenance of the mane and increased
Chapter 6 Hormones and the Endocrine System Introduction In lions, the hormone testosterone promotes the development and maintenance of male traits including growth and maintenance of the mane and increased
Chapter 11 - Endocrine System
 Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
Chapter 11 - Endocrine System 11.1 Introduction A. The endocrine system is made up of the cells, tissues, and organs that secrete hormones into body fluids. B. The body has two kinds of glands, exocrine
Endocrine System. Chapter 9
 Endocrine System Chapter 9 Endocrine Organs Hormones Chemical messengers that are released from one tissue and transported through blood to a target tissue. Chemical classification: amino acids, steroids,
Endocrine System Chapter 9 Endocrine Organs Hormones Chemical messengers that are released from one tissue and transported through blood to a target tissue. Chemical classification: amino acids, steroids,
Hormonal Regulation of Food Intake
 Physiol Rev 85: 1131 1158, 2005; doi:10.1152/physrev.00015.2004. Hormonal Regulation of Food Intake SARAH STANLEY, KATIE WYNNE, BARBARA McGOWAN, AND STEPHEN BLOOM Endocrine Unit, Imperial College Faculty
Physiol Rev 85: 1131 1158, 2005; doi:10.1152/physrev.00015.2004. Hormonal Regulation of Food Intake SARAH STANLEY, KATIE WYNNE, BARBARA McGOWAN, AND STEPHEN BLOOM Endocrine Unit, Imperial College Faculty
Homeostasis Through Chemistry. The Endocrine System Topic 6.6
 Homeostasis Through Chemistry The Endocrine System Topic 6.6 Comparing NS & ES Animals have two systems of internal communication and regulation The nervous system Response time: Fast, quick Signals: electrical
Homeostasis Through Chemistry The Endocrine System Topic 6.6 Comparing NS & ES Animals have two systems of internal communication and regulation The nervous system Response time: Fast, quick Signals: electrical
9.3 Stress Response and Blood Sugar
 9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
9.3 Stress Response and Blood Sugar Regulate Stress Response Regulate Blood Sugar Stress Response Involves hormone pathways that regulate metabolism, heart, rate and breathing The Adrenal Glands a pair
Improving Diabetes Research: Moving Beyond Animal Models. Charu Chandrasekera, Ph.D. Anne Bunner, Ph.D.
 Improving Diabetes Research: Moving Beyond Animal Models Charu Chandrasekera, Ph.D. Anne Bunner, Ph.D. July 19, 2014 From Bench-to-Bedside Sulfonylurea Biguanide Dipeptidyl peptidase-4 inhibitor Glucagon-like
Improving Diabetes Research: Moving Beyond Animal Models Charu Chandrasekera, Ph.D. Anne Bunner, Ph.D. July 19, 2014 From Bench-to-Bedside Sulfonylurea Biguanide Dipeptidyl peptidase-4 inhibitor Glucagon-like
Endocrine System. Endocrine vs. Exocrine. Bio 250 Human Anatomy & Physiology
 Endocrine System Bio 250 Human Anatomy & Physiology Endocrine vs. Exocrine Endocrine glands secrete their products called hormones into body fluids (the internal environment) Exocrine glands secrete their
Endocrine System Bio 250 Human Anatomy & Physiology Endocrine vs. Exocrine Endocrine glands secrete their products called hormones into body fluids (the internal environment) Exocrine glands secrete their
Gut hormones KHATTAB
 Gut hormones PROF:ABD ALHAFIZ HASSAN KHATTAB Gut as an endocrine gland The talk will cover the following : Historical background. Why this subject is chosen. Gastro-intestinal hormones and their function.
Gut hormones PROF:ABD ALHAFIZ HASSAN KHATTAB Gut as an endocrine gland The talk will cover the following : Historical background. Why this subject is chosen. Gastro-intestinal hormones and their function.
18. PANCREATIC FUNCTION AND METABOLISM. Pancreatic secretions ISLETS OF LANGERHANS. Insulin
 18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
18. PANCREATIC FUNCTION AND METABOLISM ISLETS OF LANGERHANS Some pancreatic functions have already been discussed in the digestion section. In this one, the emphasis will be placed on the endocrine function
Islets of Langerhans consist mostly of insulin-producing β cells. They will appear to be densely labeled
 Are adult pancreatic beta cells formed by self-duplication or stem cell differentiation? Introduction Researchers have long been interested in how tissues produce and maintain the correct number of cells
Are adult pancreatic beta cells formed by self-duplication or stem cell differentiation? Introduction Researchers have long been interested in how tissues produce and maintain the correct number of cells
Chapter 45-Hormones and the Endocrine System. Simple Hormone Pathways
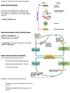 Chapter 45-Hormones and the Endocrine System Simple Hormone s Low ph in duodenum Hormones are released from an endocrine, travel through the bloodstream, and interact with the receptor or a target to cause
Chapter 45-Hormones and the Endocrine System Simple Hormone s Low ph in duodenum Hormones are released from an endocrine, travel through the bloodstream, and interact with the receptor or a target to cause
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION
 Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
Page 32 AP Biology: 2013 Exam Review CONCEPT 6 REGULATION 1. Feedback a. Negative feedback mechanisms maintain dynamic homeostasis for a particular condition (variable) by regulating physiological processes,
Endocrine secretion cells secrete substances into the extracellular fluid
 Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Animal Hormones Concept 30.1 Hormones Are Chemical Messengers Endocrine secretion cells secrete substances into the extracellular fluid Exocrine secretion cells secrete substances into a duct or a body
Endocrine System. Modified by M. Myers
 Endocrine System Modified by M. Myers 1 The Endocrine System 2 Endocrine Glands The endocrine system is made of glands & tissues that secrete hormones. Hormones are chemicals messengers influencing a.
Endocrine System Modified by M. Myers 1 The Endocrine System 2 Endocrine Glands The endocrine system is made of glands & tissues that secrete hormones. Hormones are chemicals messengers influencing a.
Insulin and the brain. Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD
 Insulin and the brain Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD 1921 Banting & Macleod Nobel Prize 1923 White, M. F. (2003) Science Berg, J. M., Tymoczko, J. L. and Stryer, L. (2007) Biochemistry
Insulin and the brain Mary ET Boyle, Ph. D. Department of Cognitive Science UCSD 1921 Banting & Macleod Nobel Prize 1923 White, M. F. (2003) Science Berg, J. M., Tymoczko, J. L. and Stryer, L. (2007) Biochemistry
Chapter 13 worksheet
 Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
Name: Chapter 13 worksheet The Endocrine System Please label the: hypothalamus pineal gland pituitary gland thyroid gland parathyroid gland thymus heart stomach liver adrenal glands kidneys pancreas small
NOTES 11.5: ENDOCRINE SYSTEM. Pages
 NOTES 11.5: ENDOCRINE SYSTEM Pages 1031-1042 ENDOCRINE SYSTEM Communication system that controls metabolism, growth, and development with hormones Maintains homeostasis Hormones: chemical messengers released
NOTES 11.5: ENDOCRINE SYSTEM Pages 1031-1042 ENDOCRINE SYSTEM Communication system that controls metabolism, growth, and development with hormones Maintains homeostasis Hormones: chemical messengers released
Chapter 6 Communication, Integration, and Homeostasis
 Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
Chapter 6 Communication, Integration, and Homeostasis About This Chapter Cell-to-cell communication Signal pathways Novel signal molecules Modulation of signal pathways Homeostatic reflex pathways Cell-to-Cell
Chapter 9 The Endocrine System and Hormone Activity
 Chapter 9 The Endocrine System and Hormone Activity Overview Coordinates and directs the activity of cells. Interacts with the nervous system Uses chemical messengers called hormones released by organs
Chapter 9 The Endocrine System and Hormone Activity Overview Coordinates and directs the activity of cells. Interacts with the nervous system Uses chemical messengers called hormones released by organs
Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells
 Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells Gérald J. Prud homme, MD, FRCPC Keenan Research Centre for Biomedical
Gamma-aminobutyric acid (GABA) treatment blocks inflammatory pathways and promotes survival and proliferation of pancreatic beta cells Gérald J. Prud homme, MD, FRCPC Keenan Research Centre for Biomedical
Endocrine System Hormones (Ch. 45)
 Endocrine System Hormones (Ch. 45) Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body daily homeostasis & regulation of large
Endocrine System Hormones (Ch. 45) Regulation Why are hormones needed? chemical messages from one body part to another communication needed to coordinate whole body daily homeostasis & regulation of large
BIOLOGY. CONCEPTS & CONNECTIONS Fourth Edition. Neil A. Campbell Jane B. Reece Lawrence G. Mitchell Martha R. Taylor. CHAPTER 26 Chemical Regulation
 BIOLOGY CONCEPTS & CONNECTIONS Fourth Edition Neil A. Campbell Jane B. Reece Lawrence G. Mitchell Martha R. Taylor CHAPTER 26 Chemical Regulation Modules 26.1 26.5 From PowerPoint Lectures for Biology:
BIOLOGY CONCEPTS & CONNECTIONS Fourth Edition Neil A. Campbell Jane B. Reece Lawrence G. Mitchell Martha R. Taylor CHAPTER 26 Chemical Regulation Modules 26.1 26.5 From PowerPoint Lectures for Biology:
Ch45: Endocrine System
 Ch45: Endocrine System Endocrine System Homeostasis is the tendency to maintain a stable internal environment. Function = coordinate and control the body with hormones to maintain homeostasis Works with
Ch45: Endocrine System Endocrine System Homeostasis is the tendency to maintain a stable internal environment. Function = coordinate and control the body with hormones to maintain homeostasis Works with
WEIGHT GAIN DURING MENOPAUSE EMERGING RESEARCH
 MENOPAUSE WHEN DOES IT OCCUR? The cessation of the menstrual cycle for one year. WEIGHT GAIN DURING MENOPAUSE EMERGING RESEARCH Jan Schroeder, Ph.D. Chair of The Department of Kinesiology California State
MENOPAUSE WHEN DOES IT OCCUR? The cessation of the menstrual cycle for one year. WEIGHT GAIN DURING MENOPAUSE EMERGING RESEARCH Jan Schroeder, Ph.D. Chair of The Department of Kinesiology California State
Understanding obesity by local overexpression of neuropeptides: a viral vector based approach
 Understanding obesity by local overexpression of neuropeptides: a viral vector based approach Cover photo: Chris Timmers Cover design: Marije Tiesjema Print: Gildeprint, Enschede ISBN: 978-90-39344675
Understanding obesity by local overexpression of neuropeptides: a viral vector based approach Cover photo: Chris Timmers Cover design: Marije Tiesjema Print: Gildeprint, Enschede ISBN: 978-90-39344675
Cell to Cell Communication
 Review #1 08 Review using OPAL figures Review using class web PDF Preview of test #1 Cell to Cell Communication 1 Communication Strategies endocrine neurocrine paracrine autocrine Endocrine System Overview
Review #1 08 Review using OPAL figures Review using class web PDF Preview of test #1 Cell to Cell Communication 1 Communication Strategies endocrine neurocrine paracrine autocrine Endocrine System Overview
Hormones and the Endocrine System Chapter 45. Intercellular communication. Paracrine and Autocrine Signaling. Signaling by local regulators 11/26/2017
 Hormones and the Endocrine System Chapter 45 Intercellular communication Endocrine signaling Local regulators Paracrine and autocrine signaling Neuron signaling Synaptic and neuroendocrine signaling Paracrine
Hormones and the Endocrine System Chapter 45 Intercellular communication Endocrine signaling Local regulators Paracrine and autocrine signaling Neuron signaling Synaptic and neuroendocrine signaling Paracrine
Gene therapy: State of the art. Ramon Gomis MD, PhD, MAE. Hospital Clínic. IDIBAPS. University of Barcelona.
 Gene therapy: State of the art Ramon Gomis MD, PhD, MAE. Hospital Clínic. IDIBAPS. University of Barcelona. Gene Therapy: First Steps Type 1 diabetes: An islet disease Alpha cell Beta cell Pancreatic
Gene therapy: State of the art Ramon Gomis MD, PhD, MAE. Hospital Clínic. IDIBAPS. University of Barcelona. Gene Therapy: First Steps Type 1 diabetes: An islet disease Alpha cell Beta cell Pancreatic
2) Storehouse for the hormones produced by the hypothalamus of the brain. 2)
 AP 2 Exam Chapter 16 Endocrie Due Wed. night 4/22 or Thurs. morning 4/23 Name: Matching; match the labeled organ with the most appropriate response or identification. Figure 16.1 Using Figure 16.1, match
AP 2 Exam Chapter 16 Endocrie Due Wed. night 4/22 or Thurs. morning 4/23 Name: Matching; match the labeled organ with the most appropriate response or identification. Figure 16.1 Using Figure 16.1, match
Monday, 7 th of July 2008 ( ) University of Buea MED30. (GENERAL ENDOCRINOLOGY) Exam ( )
 .. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
.. Monday, 7 th of July 2008 (8 30-11. 30 ) Faculty of Health Sciences University of Buea MED30 304 Programme in Medicine (GENERAL ENDOCRINOLOGY) Exam (2007-2008).. Multiple Choice Identify the letter
General Principles of Endocrine Physiology
 General Principles of Endocrine Physiology By Dr. Isabel S.S. Hwang Department of Physiology Faculty of Medicine University of Hong Kong The major human endocrine glands Endocrine glands and hormones
General Principles of Endocrine Physiology By Dr. Isabel S.S. Hwang Department of Physiology Faculty of Medicine University of Hong Kong The major human endocrine glands Endocrine glands and hormones
Homeostasis and Mechanisms of Weight Regulation
 Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Homeostasis and Mechanisms of Weight Regulation Purpose In this activity students will investigate how negative feedback mechanisms function to maintain homeostatic balance using a recently discovered
Control of Glucose Metabolism
 Glucose Metabolism Control of Glucose Metabolism The pancreas is both an exocrine and endocrine gland. It secretes digestive enzymes into the duodenum (exocrine) and 3 specific hormones into the bloodstream
Glucose Metabolism Control of Glucose Metabolism The pancreas is both an exocrine and endocrine gland. It secretes digestive enzymes into the duodenum (exocrine) and 3 specific hormones into the bloodstream
History of Investigation
 Acini - Pancreatic juice (1º) (2º) Secretions- neuronal and hormonal mechanisms 1) Secretin - bicarbonate rich 2) Cholecystokinin - enzyme rich Islets of Langerhans (contain 4 cell types) Alpha cells (α)-
Acini - Pancreatic juice (1º) (2º) Secretions- neuronal and hormonal mechanisms 1) Secretin - bicarbonate rich 2) Cholecystokinin - enzyme rich Islets of Langerhans (contain 4 cell types) Alpha cells (α)-
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
CHEMICAL COORDINATION & INTEGRATION
 CHEMICAL COORDINATION & INTEGRATION 1. The hormone responsible for Fight and Flight response is a) Adrenalin** b) Thyroxine c) ADH d) Oxytocin 2. The primary androgen produced by males is. a) Epinephrine
CHEMICAL COORDINATION & INTEGRATION 1. The hormone responsible for Fight and Flight response is a) Adrenalin** b) Thyroxine c) ADH d) Oxytocin 2. The primary androgen produced by males is. a) Epinephrine
Ch 11: Endocrine System
 Ch 11: Endocrine System SLOs Describe the chemical nature of hormones and define the terms proand prepro-hormone. Explain mechanism of action of steroid and thyroid hormones Create chart to distinguish
Ch 11: Endocrine System SLOs Describe the chemical nature of hormones and define the terms proand prepro-hormone. Explain mechanism of action of steroid and thyroid hormones Create chart to distinguish
The Endocrine System. The Endocrine System
 The Endocrine System Like nervous system, endocrine system provides communication and control. Messages are relayed from one cell to another via chemical messengers (hormones). Unlike nervous system which
The Endocrine System Like nervous system, endocrine system provides communication and control. Messages are relayed from one cell to another via chemical messengers (hormones). Unlike nervous system which
Human Biochemistry. Hormones
 Human Biochemistry Hormones THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM The ENDOCRINE SYSTEM = the organ system that regulates internal environment conditions by secreting hormones into
Human Biochemistry Hormones THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM THE ENDOCRINE SYSTEM The ENDOCRINE SYSTEM = the organ system that regulates internal environment conditions by secreting hormones into
Endocrine System. Chemical Control
 Endocrine System Chemical Control Endocrine System - the system that secretes hormones in the body - hormones can last for minutes or for hours - a major gland, once called the master gland, is the pituitary
Endocrine System Chemical Control Endocrine System - the system that secretes hormones in the body - hormones can last for minutes or for hours - a major gland, once called the master gland, is the pituitary
4/23/2018. Endocrine System: Overview. Endocrine System: Overview
 Endocrine System: Overview With nervous system, coordinates and integrates activity of body cells Influences metabolic activities via hormones transported in blood Response slower but longer lasting than
Endocrine System: Overview With nervous system, coordinates and integrates activity of body cells Influences metabolic activities via hormones transported in blood Response slower but longer lasting than
