DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
|
|
|
- Cory Roberts
- 5 years ago
- Views:
Transcription
1 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32 C.F.R , the DoD P&T Committee is responsible for developing the Uniform Formulary (UF). Recommendations to the Director, TMA, on formulary status, pre-authorizations, and the effective date for a drug s change from formulary to non-formulary status receive comments from the Beneficiary Advisory Panel (BAP), which must be reviewed by the Director before making a final decision. II. SELF-MONITORING BLOOD GLUCOSE SYSTEM TEST STRIPS (SMBGS) P&T Comments A. SMBGS Test Strips Relative Clinical Effectiveness The P&T Committee evaluated the relative clinical effectiveness of the Self-Monitoring Blood Glucose Test Systems (SMBGS) test strips. The clinical evaluation included, but was not limited to, the requirements stated in the UF rule, 32 CFR (e)(1). The primary goal for the UF recommendation is to ensure uniform availability of quality SMBGS test strips across the MHS (MTF, TRRx, and TMOP points of service). SMBGS meters are not included as part of the TRICARE outpatient pharmacy benefit (they are included under the medical benefit) and are not the focus of the review; however provisions have been made to provide SMBGS meters at no cost to MHS beneficiaries. The FDA classifies SMBGS test strips and meters as medical devices, rather than drugs, thus the focus of the clinical effectiveness review centered on differences in the technical aspects/attributes among the products. The P&T Committee had previously determined that all SMBGS test strips considered for inclusion on the UF must meet minimum technical standards relating to accuracy, blood sample size, availability of testing sites other than the fingertips, result time, memory capacity, ease of use (e.g., calibration and coding, large visual display), manufacturer customer support services, downloading capabilities, availability of data management software, and size. The test strips included in the SMBGS class were those products approved by the FDA and available in the marketplace as of May Due to the complexity of evaluating the more than 40 commercially marketed SMBGS test strip brands, the number of test strips eligible of inclusion on the UF was determined by DoD P&T Committee minimum technical requirements, operational limitations of the existing TMOP and TRRx contract, and Federal Government contracting regulations. Relative Clinical Effectiveness Conclusion a) With regard to efficacy, all meters that are approved by the FDA for licensing in the USA must meet the FDA standard of accuracy, which is a total analytical error of <5%. The International Organization for Standardization (ISO) also has standards. All the SMBGS test strips meeting the minimum technical 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 1 of 12
2 requirements for inclusion on the UF met both FDA and ISO standards. There was insufficient published clinical trial data to determine if there were clinically relevant differences between the SMBGS test strips with regard to accuracy. The most common cause of inaccurate SMBGS test results is operator error. b) With regard to calibration and coding, the SMBGS test strips with the lowest risk of coding/calibration errors (as they do not require coding) are the Ascensia Contour and Freestyle Lite test strips. The Accu-chek Aviva, Precision Xtra, and TrueTrack test strips require insertion of a coding chip or strip. The One Touch Ultra test strip requires manual coding. c) With regard to blood sample size, the Freestyle Lite test strip requires 0.3 microliter (μl) blood; the Accu-check Aviva, Ascensia Contour, and Precision Xtra require 0.6 μl; and the One Touch Ultra and TrueTrack test strips require 1 μl blood. d) With regard to alternate site testing, the Accu-chek Aviva and Freestyle Lite strips are FDA-approved for testing at 5 alternate sites other than the fingertips, the Ascensia Contour strip is approved for 4 alternate sites, the Precision Xtra and One Touch Ultra strips are approved for 3 alternate sites, and the TrueTrack strip is approved for one alternate testing site other than the fingertips. e) With regard to test result time, the Accu-chek Aviva, Ascensia Contour, Freestyle Lite, Precision Xtra, and One Touch Ultra provide test results within 5 seconds, while the TrueTrack strips provide test results in 10 seconds. f) With regard to SMBGS test strip degradation due to heat and humidity, the Precision Xtra test strips are individually foil-wrapped; however patients with dexterity problems may have difficulty opening the foil wrappers. g) With regard to safety, the Accu-chek Aviva and Freestyle Lite SMBGS test strips employ technology using glucose dehydrogenase (GDH) pyrroloquinolinequinone, which may cause falsely elevated blood glucose readings in patients receiving concomitant therapy with icodextrin-containing substances (Extrarenal peritoneal dialysis solution and the IV immunoglobulin product Octagam). SMBGS strips using GDH nicotinamide adenine dinucleotide [Precision Xtra], GDH flavin adenine dinucleotide [Ascensia Contour] or glucose oxidase technology [One Touch Ultra and TrueTrack] do not interfere with Extrarenal or Octagam. h) With regard to special populations, those patients requiring intensive blood glucose monitoring (e.g., women with gestational diabetes, Type 1 diabetics, children and adults using insulin pumps) may prefer SMBGS test strips used in certain meters that can communicate wirelessly with insulin pumps. i) With regard to provider opinion, a survey of MTF providers reported that accuracy and small blood sample size were the two technical requirements considered most important when comparing SMBGS. j) With regard to therapeutic interchangeability, there is a high degree of therapeutic interchangeability between the SMBGS test strips meeting the DoD P&T Committee minimum technical requirements. 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 2 of 12
3 COMMITTEE ACTION: The P&T Committee voted to accept the clinical effectiveness B. SMBGS Test Strips Relative Cost Effectiveness In considering the relative cost-effectiveness of pharmaceutical agents in the SMBGS test strip class, the P&T Committee evaluated the costs of the agents in relation to the efficacy, safety, tolerability, and clinical outcomes of the other agents in the class. Information considered by the P&T Committee included but was not limited to sources of information listed in 32 CFR (e)(2). Relative Cost Effectiveness Conclusion: The relative clinical effectiveness evaluation concluded that for those SMBGS test strips meeting the minimum technical criteria, there were no clinically relevant differences between the agents. As a result, a cost minimization analysis (CMA) and budget impact analysis (BIA) were conducted. Based on the results of the cost analyses and other clinical and cost considerations, the P&T Committee concluded: a) Results from the CMAs for the condition sets for both the 3 or less and 4 or more included on the UF revealed that Ascensia Contour was the most cost effective SMBG system while One Touch Ultra was the least cost effective. The ranking of most to least cost effective SMBGS test strips based on prices submitted for each condition set was: Ascensia Contour >TrueTrack > Freestyle Lite > Precision Xtra > Accu-chek Aviva > OneTouch Ultra. b) The BIA evaluated the potential impact of scenarios with selected SMBGS products designated formulary or non-formulary on the UF. The BIA results showed that the scenario that designated the One Touch Ultra and True Track self SMBGS as non-formulary on the UF was more favorable to the MHS. COMMITTEE ACTION: The P&T Committee voted to accept the cost effectiveness C. SMBGS Test Strips Uniform Formulary Recommendation In view of the conclusions from the relative clinical effectiveness and relative cost effectiveness determinations of the SMBGS, and other relevant factors, the P&T Committee voted to recommend that: 1) Accu-chek Aviva, Precision Xtra, Freestyle Lite, and the Ascensia Contour SMBGS test strips be designated as formulary on the Uniform Formulary. 2) One Touch Ultra, TrueTrack, Accu-chek Comfort Curve, Accu-chek Compact Plus, Accu-chek Simplicity, Ascensia Autodisc, Ascensia Breeze 2, Ascensia Elite, Assure, Assure 3, Assure II, Assure Pro, Bd Test Strips, Chemstrip Bg, Control AST, Dextrostix Reagent, Easygluco, Easypro, Fast Take, Freestyle Test Strips (other than Freestyle Lite), Glucofilm, Glucolab, Glucometer Dex, Glucometer Elite, Glucose Test Strip, Glucostix, Optium, Precision Pcx, Precision Pcx Plus, Precision Q-I-D, Precision Sof-Tact, Prestige Smart System, Prodigy, Quicktek, Sidekick, Sof-Tact, Surestep, Surestep Pro, Test Strip, Relion Ultima, 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 3 of 12
4 Uni-Check, and all store/private label brands not specified as formulary in 1 above be designated as non-formulary on the UF. The SMBGS test strips are a medical device and subject to wholesale acquisition cost, rather than Federal Ceiling Price (FCP) pricing. D. SMBGS Test Strips Implementation Plan The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed, following a 120-day implementation period in the TRICARE Mail Order Pharmacy (TMOP) and TRICARE Retail Network Pharmacy Program (TRRx). The implementation period will begin immediately following the approval by the Director, TMA. III. SELF-MONITORING BLOOD GLUCOSE SYSTEM TEST STRIPS (SMBGS) BAP Comments A. SMBGS Test Strips Uniform Formulary Recommendation: In view of the conclusions from the relative clinical effectiveness and relative cost effectiveness determinations of the SMBGS test strips, and other relevant factors, the P&T Committee voted to recommend that: 1) Accu-chek Aviva, Precision Xtra, Freestyle Lite, and the Ascensia Contour SMBGS test strips be designated as formulary on the Uniform Formulary. 2) One Touch Ultra, TrueTrack, Accu-chek Comfort Curve, Accu-chek Compact Plus, Accu-chek Simplicity, Ascensia Autodisc, Ascensia Breeze 2, Ascensia Elite, Assure, Assure 3, Assure II, Assure Pro, Bd Test Strips, Chemstrip Bg, Control AST, Dextrostix Reagent, Easygluco, Easypro, Fast Take, Freestyle Test Strips (other than Freestyle Lite), Glucofilm, Glucolab, Glucometer Dex, Glucometer Elite, Glucose Test Strip, Glucostix, Optium, Precision Pcx, Precision Pcx Plus, Precision Q-I-D, Precision Sof-Tact, Prestige Smart System, Prodigy, Quicktek, Sidekick, Sof-Tact, Surestep, Surestep Pro, Test Strip, Relion Ultima, Uni-Check, and all store/private label brands not specified as formulary in 1 above be designated as non-formulary on the UF. 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 4 of 12
5 B. SMBGS Test Strips Implementation Plan: The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed, following a 120-day implementation period in the TRICARE Mail Order Pharmacy (TMOP) and TRICARE Retail Network Pharmacy Program (TRRx). The implementation period will begin immediately following the approval by the Director, TMA. IV. OVERACTIVE BLADDER DRUGS (OABs) P&T Comments A. Overactive Bladder Drugs Relative Clinical Effectiveness The DoD P&T Committee evaluated the clinical effectiveness of the Overactive Bladder Agents (OABs); this class was first reviewed for UF placement in February There are nine marketed anticholinergic drugs for overactive bladder (OAB) in the US, darifenacin (Enablex), oxybutynin immediate release (IR) (Ditropan, generics), oxybutynin extended release (ER) (Ditropan XL; generics), oxybutynin transdermal (Oxytrol patch) solifenacin (Vesicare), tolterodine IR (Detrol), tolterodine ER (Detrol LA), trospium IR (Sanctura) and trospium ER (Sanctura XR). The clinical evaluation included, but was not limited to, the requirements stated in the UF rule, 32 CFR (e)(1). All nine drugs are FDA approved for the treatment of OAB with symptoms of urge incontinence, urgency and urinary frequency. Oxybutynin ER is also approved for the treatment of patients aged 6-years and older with symptoms of detrusor overactivity associated with a neurological condition (e.g. spina bifida), but was not reviewed for this indication by the Committee. Only oxybutynin IR and ER are available in generic formulations. Military Health System expenditures for the OAB class exceeded $74 million from July 07 to June 08. Tolterodine ER (Detrol LA) is the highest utilized OAB agent at the MTFs, followed by oxybutynin ER (Ditropan XL, generics). Relative Clinical Effectiveness Conclusion: The P&T Committee concluded that: a) With regard to efficacy, evaluation of clinically relevant differences in efficacy of the OAB agents at relieving urinary symptoms is hampered by the high placebo response rate (30-50%), varying use of non-pharmacologic measures such as bladder training and behavioral modification, and differing outcome measures used in clinical trials. 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 5 of 12
6 b) With regard to efficacy at reducing the number of urge incontinent episodes, urgency episodes, and micturation frequency, the available evidence does not support clinically relevant differences between oxybutynin IR (Ditropan, generics), oxybutynin ER (Ditropan XL, generics), oxybutynin patch (Oxytrol), tolterodine IR (Detrol), tolterodine ER (Detrol LA), trospium IR (Sanctura), trospium ER (Sanctura XR), solifenacin (Vesicare), and darifenacin (Enablex). c) With regard to safety and tolerability, the following conclusions were made: There are no differences between the OAB drugs in terms of black box warnings (e.g., acute urinary or gastric retention, acute angle-closure glaucoma, and myasthenia gravis), listed in the product labeling. Oxybutynin IR had higher rates of withdrawals of therapy due to adverse events and occurrence of dry mouth then the other OAB agents, but no single agent has shown a clearly superior profile. The incidence of adverse events including dry mouth, and constipation, overall was lower with extended release preparations compared with immediate release formulations of the agents. The oxybutynin patch has been associated with pruritis and rash. The newer agents (trospium IR and ER, solifenacin, and darifenacin) do not appear to have a significantly lower incidence of dry mouth or constipation compared to extended-release forms of the older agents (oxybutynin ER, and tolterodine ER). All the OAB agents may cross the blood brain barrier and result in significant central nervous system effects, although this may be less likely with trospium IR and ER. Drug-drug interactions are less likely with trospium than the other agents. d) With regard to tolerability and persistence rates, persistence rates for OAB medications reported in the medical literature are in general low (<10%). A 2005 PEC analysis reported that only about 11% of MHS patients continued to obtain prescriptions for OAB medications on a regular basis after 1 year. When the analysis was updated for the August 2008 DoD P&T meeting, the reported 1-year persistence rate with OAB therapy was 14% overall. Generally higher persistence was seen for patients receiving newer agents and extended release versions of older agents, compared to those receiving immediate release versions of tolterodine and oxybutynin. About 28% of patients who were considered to be non-persistent continued to occasionally obtain prescription refills, consistent with use on an as needed rather than routine basis. e) With regard to special populations, only oxybutynin IR and oxybutynin ER are approved for use in children ages 6 years and older. For pregnancy, oxybutynin IR, oxybutynin ER, and the oxybutynin patch are labeled as category B drugs, while the other OAB drugs are labeled as category C drugs. COMMITTEE ACTION: The P&T Committee voted to accept the clinical effectiveness 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 6 of 12
7 B. Overactive Bladder Drugs Relative Cost Effectiveness In considering the relative cost-effectiveness of pharmaceutical agents in this class, the P&T Committee evaluated the costs of the agents in relation to the efficacy, safety, tolerability, and clinical outcomes of the other agents in the class. Information considered by the P&T Committee included but was not limited to sources of information listed in 32 CFR (e)(2). Relative Cost Effectiveness Conclusion: The relative clinical effectiveness evaluation concluded that the newer OAB drugs darifenacin and solifenacin and the extended release formulations had higher persistence rates in the MHS than oxybutynin IR and tolterodine IR. Therefore, the cost effectiveness of the OAB agents was evaluated by CMA, cost effectiveness analysis (CEA), and by budget impact analysis (BIA). Based on the results of the cost analyses and other clinical and cost considerations, the P&T Committee concluded the following: a) Results from the CMA for the immediate release OAB agents (oxybutynin IR [Ditropan, generics], tolterodine IR [Detrol], and trospium IR [Sanctura]) revealed that oxybutynin IR was the most cost effective immediate release OAB agent overall. b) Results from the CMA of extended release OAB agents (oxybutynin ER [Ditropan XL, generics], tolterodine ER [Detrol LA], trospium ER [Sanctura XR], oxybutynin transdermal [Oxytrol patch], darifenacin [Enablex], and solifenacin [Vesicare]) revealed that 1) trospium ER (Sanctura XR) was the most cost effective extended release OAB agent overall; and 2) when the price for generic formulations of oxybutynin ER (Ditropan XR) drops by 21.3% from the current price, oxybutynin ER will become the most cost-effective agent. c) The results from a CEA comparing immediate release vs. extended release agents revealed that patients are more persistent with therapy when taking extended release products than when taking immediate release products. This is done at a significantly higher incremental cost per day of persistence gained by taking extended release products. However, the incremental cost per day of persistence gained is ~ 18% lower than when compared to MHS costs in 2005 when the OAB drugs were previously reviewed for UF placement. d) The BIA evaluated the potential impact of scenarios with selected OAB agents designated formulary or non-formulary on the UF. Results from the BIA revealed that the scenario that designated tolterodine IR (Detrol) and trospium IR (Sanctura) as non-formulary under the UF was more favorable to the MHS. COMMITTEE ACTION: The P&T Committee voted to accept the cost effectiveness C. Overactive Bladder Drugs Uniform Formulary Recommendation In view of the conclusions from the relative clinical effectiveness and relative cost effectiveness determinations of the Overactive Bladder Drugs, and other relevant factors, 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 7 of 12
8 the P&T Committee, based upon its collective professional judgment, voted to recommend that: 1) Oxybutynin IR (Ditropan, generics), oxybutynin ER (Ditropan XL, generics), oxybutynin patch (Oxytrol), tolterodine ER (Detrol LA), solifenacin (Vesicare), trospium ER (Sanctura XR), and darifenacin (Enablex) be classified as formulary on the UF. 2) Tolterodine IR (Detrol) and trospium IR (Sanctura) be designated as non-formulary under the UF, based on cost effectiveness. All OAB drugs recommended for inclusion on the UF were covered by Uniform Formulary Voluntary Agreement for Retail Refunds (UF VARR) submissions at or below the Federal Ceiling Price (FCP). D. Overactive Bladder Drugs Implementation Plan - The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed following a 90-day implementation period in the TMOP and TRRx. The implementation period will begin immediately following the approval by the Director, TMA. V. OVERACTIVE BLADDER DRUGS BAP Comments A. Overactive Bladder Drugs Uniform Formulary Recommendation Taking into consideration of the conclusions from the relative clinical effectiveness conclusions and cost effectiveness determinations of the Overactive Bladder Drugs, and other relevant factors, the P&T Committee, based on its professional judgment, voted to recommend that oxybutynin IR (Ditropan, generics), oxybutynin ER (Ditropan XL, generics), oxybutynin patch (Oxytrol), tolterodine ER (Detrol LA), solifenacin (Vesicare), trospium ER (Sanctura XR), and darifenacin (Enablex) be classified as formulary on the UF, and that tolterodine IR (Detrol) and trospium IR (Sanctura) be designated as non-formulary under the UF, based on cost effectiveness. 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 8 of 12
9 B. Overactive Bladder Drugs Implementation Plan The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed following a 90-day implementation period in the TMOP and TRRx. The implementation period will begin immediately following the approval by the Director, TMA. VI. NEWLY APPROVED DRUGS Desvenlafaxine (Pristiq) P&T Comments A. Pristiq Relative Clinical Effectiveness - The desvenlafaxine clinical evaluation included, but was not limited to, the requirements stated in the UF rule, 32 CFR (e)(1). Desvenlafaxine (Pristiq) is a serotonin norepinephrine re-uptake inhibitor (SNRI) that is classified as part of the Antidepressant-1 (AD-1) drug class. The AD-1s were reviewed for Uniform Formulary (UF) placement in November Other SNRIs included on the UF are venlafaxine immediate release (Effexor, generics) and venlafaxine extended release (ER) (Effexor XR). Desvenlafaxine is FDA-approved for the treatment of major depressive disorder in adults. Desvenlafaxine is an extended release formulation of the major active metabolite of venlafaxine ER. Generic formulations of venlafaxine ER (Effexor XR) are expected in Relative Clinical Effectiveness Conclusion: The P&T Committee concluded that desvenlafaxine does not have a significant, clinically meaningful therapeutic advantage in terms of safety, effectiveness, or clinical outcomes over other AD-1 agents currently included on the UF. COMMITTEE ACTION: The P&T Committee voted to accept the clinical effectiveness B. Pristiq Relative Cost Effectiveness - The P&T Committee evaluated the relative cost effectiveness of desvenlafaxine (Pristiq) in relation to efficacy, safety, tolerability, and clinical outcomes of the other agents in the AD-1 class, particularly to the following medications: citalopram (Celexa, generics), sertraline (Zoloft, generics), venlafaxine (Effexor, generics), venlafaxine ER (Effexor XR), bupropion ER (Wellbutrin XL), and duloxetine (Cymbalta). Information considered by the P&T Committee included, but was not limited to sources of information listed in 32 CFR (e)(2). A cost minimization analysis (CMA) was employed to evaluate the cost effectiveness of desvenlafaxine relative to the UF AD-1s citalopram, sertraline, venlafaxine, and venlafaxine ER, and the Non-formulary (NF) AD-1s bupropion ER, and duloxetine. 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 9 of 12
10 VII. Results of the CMA showed that the projected weighted average daily cost of desvenlafaxine was significantly higher than its AD-1 class comparators. Relative Cost Effectiveness Conclusion: - The P&T Committee concluded that desvenlafaxine (Pristiq) is not cost effective relative to the other AD-1s included on the UF. COMMITTEE ACTION: The P&T Committee voted to accept the cost effectiveness C. Pristiq Uniform Formulary Recommendation - Taking into consideration the conclusions from the relative clinical effectiveness and relative cost effectiveness determinations, and other relevant factors, the P&T Committee, based upon its collective professional judgment, recommended that desvenlafaxine (Pristiq) be designated as nonformulary on the UF. This recommendation was based on the clinical effectiveness conclusion, and the determination that citalopram (Celexa, generics), sertraline (Zoloft, generics), venlafaxine (Effexor, generics), and venlafaxine ER (Effexor XR) remain the most cost effective AD-1 agents on the UF compared to desvenlafaxine. D. Pristiq Implementation Plan - The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed, following a 60-day implementation period in the TMOP and TRRx. The implementation period will begin immediately following approval by the Director, TMA. NEWLY APPROVED DRUGS Desvenlafaxine (Pristiq) BAP Comments A. Pristiq Uniform Formulary Recommendation The P&T Committee, based on its professional judgment, voted to recommend that desvenlafaxine (Pristiq) be classified as non-formulary on the Uniform Formulary. B. Pristiq Implementation Plan - The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed, following a 60-day implementation period in the TMOP and TRRx. The implementation period will begin immediately following approval by the Director, TMA. 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 10 of 12
11 VIII. NEWLY APPROVED DRUGS Nisoldipine geomatrix (Sular geomatrix) P&T Comments A. Sular geomatrix Relative Clinical Effectiveness - The nisoldipine geomatrix clinical evaluation included, but was not limited to, the requirements stated in the UF rule, 32 CFR (e)(1). Nisoldipine geomatrix (Sular geomatrix) is a dihydropyridine calcium channel blocker (DHP CCB) approved for treating hypertension. The CCBs were reviewed for UF placement at the August 2005 P&T Committee meeting. Other antihypertensive DHP CCBs included on the UF are amlodipine (Norvasc, generics), felodipine (Plendil, generics), nisoldipine coat core (Sular, generics), and nifedipine ER (Adalat CC, generics). Nisoldipine geomatrix employs a different extended-release mechanism than the original nisoldipine product, nisoldipine coat core; both products are dosed once daily. Generic formulations of the original coat core product recently became commercially available. The geomatrix delivery system allows for a 15% lower dosage than the coat core product. Relative Clinical Effectiveness Conclusion - The P&T Committee concluded that there is no evidence to suggest that there are clinically relevant differences in the efficacy, safety, and clinical outcomes of nisoldipine geomatrix (Sular geomatrix) compared to nisoldipine coat core, as both products contain the same active ingredient. Additionally, the Committee agreed that nisoldipine geomatrix does not have a significant, clinically meaningful therapeutic advantage in terms of safety, effectiveness, or clinical outcomes over other DHP CCB agents currently included on the UF. COMMITTEE ACTION: The P&T Committee voted to accept the clinical effectiveness B. Sular geomatrix Relative Cost Effectiveness The DoD P&T Committee evaluated the relative cost effectiveness of nisoldipine (Sular Geomatrix) in relation to efficacy, safety, tolerability, and clinical outcomes of other DHP CCBs, particularly to amlodipine (Norvasc, generics), felodipine (Plendil, generics) and nisoldipine (Sular coat core, generics). Information considered by the P&T Committee included, but was not limited to sources of information listed in 32 CFR (e)(2). A CMA was employed to determine the relative cost effectiveness of nisoldipine geomatrix relative to other UF DHP CCBs (nisoldipine coat core, felodipine, amlodipine). The results from the CMA revealed that the projected weighted average cost per day for therapy for nisoldipine geomatrix (Sular Geomatrix) is significantly higher than other UF CCBs amlodipine, felodipine, and nisoldipine (Sular coat core, generics). Relative Cost Effectiveness Conclusion: P&T Committee, based upon its collective professional judgment, voted that nisoldipine geomatrix (Sular Geomatrix) is not cost effective relative to other UF DHP CCB agents. COMMITTEE ACTION: The P&T Committee voted to accept the cost effectiveness 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 11 of 12
12 C. Sular geomatrix Uniform Formulary Recommendation - Taking into consideration the conclusions from the relative clinical effectiveness and relative cost effectiveness of nisoldipine geomatrix, and other relevant factors, the P&T Committee, based upon its collective professional judgment, voted to recommend that nisoldipine geomatrix (Sular geomatrix) be designated as non-formulary on the UF. This recommendation was based on the clinical effectiveness conclusion, and the determination that amlodipine (Norvasc, generics), felodipine (Plendil, generics) and generic nisoldipine coat core remain the most cost effective CCB agents on the UF compared to Sular Geomatrix. D. Sular geomatrix Implementation Plan - The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed, following a 60-day implementation period in the TMOP and TRRx. The implementation period will begin immediately following approval by the Director, TMA. IX. NEWLY APPROVED DRUGS Nisoldipine geomatrix (Sular geomatrix) BAP Comments A. Sular geomatrix Uniform Formulary Recommendation The P&T Committee, based on its professional judgment, voted to recommend that nisoldipine geomatrix (Sular geomatrix) be classified as non-formulary on the Uniform Formulary. B. Sular geomatrix Implementation Plan - The P&T Committee recommended an effective date of the first Wednesday one week after the minutes are signed, following a 60-day implementation period in the TMOP and TRRx. The implementation period will begin immediately following approval by the Director, TMA. 17 Sep 2008 Beneficiary Advisory Panel Background Information Page 12 of 12
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
Beneficiary Advisory Panel Handout Uniform Formulary Decisions 17 September 2008
 Beneficiary Advisory Panel Handout Uniform Formulary Decisions 17 September 2008 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical
Beneficiary Advisory Panel Handout Uniform Formulary Decisions 17 September 2008 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical
Uniform Formulary Beneficiary Advisory Panel Handout February 2006
 Uniform Formulary Beneficiary Advisory Panel Handout February 2006 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinicaleffectiveness
Uniform Formulary Beneficiary Advisory Panel Handout February 2006 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinicaleffectiveness
I. Uniform Formulary Review Process
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
II. UNIFORM FORMULARY CLASS REVIEWS Phosphodiesterase Type-5 (PDE-5) INHIBITORS FOR PULMONARY ARTERIAL HYPERTENSION (PAH) P&T Comments
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
Summary ofpanel Vote/Comments:
 14 August 2009 Executive Summary UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL COMMENTS 30 July 2009 The Unifonn Fonnulary (UF) Beneficiary Advisory Panel (BAP) commented on the recommendations from the
14 August 2009 Executive Summary UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL COMMENTS 30 July 2009 The Unifonn Fonnulary (UF) Beneficiary Advisory Panel (BAP) commented on the recommendations from the
I. UNIFORM FORMULARY REVIEW PROCESS
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code 1074g, as implemented
Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based
 Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based upon a medical history, and includes a focused physical exam (abdominal, neurological, pelvic in females and
Overactive bladder (OAB) affects approximately 15% of the adult population. Diagnosis is based upon a medical history, and includes a focused physical exam (abdominal, neurological, pelvic in females and
II. Angiotensin Receptor Blockers (ARBs) Drug Class Review
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE DOD BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32 C.F.R. 199.21,
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE DOD BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32 C.F.R. 199.21,
Uniform Formulary Beneficiary Advisory Panel (BAP) Meeting Summary January 9, 2013 Washington, D.C.
 Uniform Formulary Beneficiary Advisory Panel (BAP) Meeting Summary January 9, 2013 Washington, D.C. Panel Members Present: Deborah Fryar, National Military Family Association, Chairperson Kathryn Buchta,
Uniform Formulary Beneficiary Advisory Panel (BAP) Meeting Summary January 9, 2013 Washington, D.C. Panel Members Present: Deborah Fryar, National Military Family Association, Chairperson Kathryn Buchta,
Drugs for Overactive Bladder (OAB)
 ril 2014 Drugs for Overactive Bladder (OAB) FINAL CONSOLIDATED COMPREHENSIVE RESEARCH PLAN July 2015 FINAL COMPREHENSIVE RESEARCH PLAN 2 A. Introduction The objective of the drug class review on drugs
ril 2014 Drugs for Overactive Bladder (OAB) FINAL CONSOLIDATED COMPREHENSIVE RESEARCH PLAN July 2015 FINAL COMPREHENSIVE RESEARCH PLAN 2 A. Introduction The objective of the drug class review on drugs
Anticholinergic medication use for female overactive bladder in the ambulatory setting in the United States.
 Página 1 de 6 PubMed darifenacin vs solifenacin Display Settings:, Sorted by Recently Added Results: 5 1. Int Urogynecol J. 2013 Oct 25. [Epub ahead of print] Anticholinergic medication use for female
Página 1 de 6 PubMed darifenacin vs solifenacin Display Settings:, Sorted by Recently Added Results: 5 1. Int Urogynecol J. 2013 Oct 25. [Epub ahead of print] Anticholinergic medication use for female
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS. November 2009
 DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS I. CONVENING II. November 2009 The Department of Defense (DoD) Pharmacy and Therapeutics (P&T) Committee convened at 0800 hours
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS I. CONVENING II. November 2009 The Department of Defense (DoD) Pharmacy and Therapeutics (P&T) Committee convened at 0800 hours
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE DOD BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE DOD BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32 C.F.R. 199.21,
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE DOD BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32 C.F.R. 199.21,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Trintellix) Reference Number: CP.PMN.65 Effective Date: 05.01.15 Last Review Date: 08.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Trintellix) Reference Number: CP.PMN.65 Effective Date: 05.01.15 Last Review Date: 08.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy
Presentation Goals 4/14/2015. Pharmacology for Urinary Incontinence in Women. Medications Review anti muscarinic medications Focus on newer meds
 Presentation Goals Pharmacology for Urinary Incontinence in Women Medications Review anti muscarinic medications Focus on newer meds Introduce beta adrenergic medications Current Concepts in Drug Therapy
Presentation Goals Pharmacology for Urinary Incontinence in Women Medications Review anti muscarinic medications Focus on newer meds Introduce beta adrenergic medications Current Concepts in Drug Therapy
Overactive Bladder (OAB) Step Therapy Program Policy Number: Last Review: Origination: Next Review: Policy When Policy Topic is covered:
 Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Origination: 07/2013 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for brand name Overactive Bladder
Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Origination: 07/2013 Last Review: 11/2014 Next Review: 11/2015 Policy BCBSKC will provide coverage for brand name Overactive Bladder
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Type 2 Diabetes Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not routinely offered for adults with type 2 diabetes
Overactive Bladder (OAB) Step Therapy Program
 Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Last Review: 11/2018 Origination: 7/2013 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Overactive Bladder (OAB) Step Therapy Program Policy Number: 5.01.556 Last Review: 11/2018 Origination: 7/2013 Next Review: 11/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Clinical Policy: Vilazodone (Viibryd) Reference Number: CP.PMN.145 Effective Date: Last Review Date: Line of Business: HIM, Medicaid
 Clinical Policy: (Viibryd) Reference Number: CP.PMN.145 Effective Date: 08.01.12 Last Review Date: 08.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Viibryd) Reference Number: CP.PMN.145 Effective Date: 08.01.12 Last Review Date: 08.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy for
Country-Specific Glucose Monitor List
 Country-Specific Glucose Monitor List Country Name: United States Important Information This is a non-comprehensive list, current as of December 2017. Absence of a specific glucose monitor or test strips
Country-Specific Glucose Monitor List Country Name: United States Important Information This is a non-comprehensive list, current as of December 2017. Absence of a specific glucose monitor or test strips
The Management of Overactive Bladder Syndrome with Antimuscarinic Drugs
 The Management of Overactive Bladder Syndrome with Antimuscarinic Drugs Author Version Date Consultation Date of Ratification By JPG Shaista Hussain Joint Formulary Pharmacist V2 16.09.2014 Homerton University
The Management of Overactive Bladder Syndrome with Antimuscarinic Drugs Author Version Date Consultation Date of Ratification By JPG Shaista Hussain Joint Formulary Pharmacist V2 16.09.2014 Homerton University
SENSOR ELECTRODE/PRECISION (Also Medisense Companion)
 DIABETIC PSEUDO-DIN LIST - Revised Mar 2017 For Provinces not listed, please submit using the diabetic pseudo DIN listed in your provincial formulary or the AB/ON pseudo DIN (except for insulin). Pricing
DIABETIC PSEUDO-DIN LIST - Revised Mar 2017 For Provinces not listed, please submit using the diabetic pseudo DIN listed in your provincial formulary or the AB/ON pseudo DIN (except for insulin). Pricing
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose (SMBG) should be considered but not be routinely offered for adults with type 2 diabetes
WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT
 WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT PURPOSE This paper explores the science behind glucose test strips and proposes the format
WHITE PAPER LOOKING PAST THE WINDOW DRESSING: UNDERSTANDING BLOOD GLUCOSE TESTING STRIPS IN A FORMULARY ENVIRONMENT PURPOSE This paper explores the science behind glucose test strips and proposes the format
Beneficiary Advisory Panel Handout Uniform Formulary Decisions 08 Jan 2009
 Beneficiary Advisory Panel Handout Uniform Formulary Decisions 08 Jan 2009 PURE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical effectiveness
Beneficiary Advisory Panel Handout Uniform Formulary Decisions 08 Jan 2009 PURE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical effectiveness
Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline.
 Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. TARGET POPULATION Eligibility Decidable (Y or N) Inclusion Criterion non-neurogenic OAB Exclusion Criterion
Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. TARGET POPULATION Eligibility Decidable (Y or N) Inclusion Criterion non-neurogenic OAB Exclusion Criterion
Appendix 2 Drug Information
 Appendix 2 Drug Information Chemical Name: baclofen (bak-loe-fen) Brand Name: Lioresal (U.S. and Canada) Description: Baclofen acts on the central nervous system to relieve spasms, cramping, and tightness
Appendix 2 Drug Information Chemical Name: baclofen (bak-loe-fen) Brand Name: Lioresal (U.S. and Canada) Description: Baclofen acts on the central nervous system to relieve spasms, cramping, and tightness
II. UF CLASS REVIEWS NASAL ALLERGY DRUGS
 DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
Home Glucose Monitoring
 Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
Home Glucose Monitoring Blood Glucose Monitors Name (Manufacturer/Distributor) Size (inches) Weight (ounces) Active (Roche) 4.6 X 1.07 X 0.9 2.01 without Advantage (Roche) 3.3 X 2.8 X 0.8 1.8 without Test
Dr. Melissa Kagarise, PA C
 Dr. Melissa Kagarise, PA C This program has been supported by an educational grant from Pfizer Pharmaceuticals PharmCon is accredited by the Accreditation Council for Pharmacy Education as a provider of
Dr. Melissa Kagarise, PA C This program has been supported by an educational grant from Pfizer Pharmaceuticals PharmCon is accredited by the Accreditation Council for Pharmacy Education as a provider of
TREATMENT OF OVERACTIVE BLADDER IN ADULTS FUGA 2016 KGH
 TREATMENT OF OVERACTIVE BLADDER IN ADULTS FUGA 2016 KGH CONTENTS Overactive bladder (OAB) Treatment of OAB Beta-3 adrenoceptor agonist (Betmiga ) - Panacea? LASER treatment - a flash in the pan or the
TREATMENT OF OVERACTIVE BLADDER IN ADULTS FUGA 2016 KGH CONTENTS Overactive bladder (OAB) Treatment of OAB Beta-3 adrenoceptor agonist (Betmiga ) - Panacea? LASER treatment - a flash in the pan or the
It is the policy of health plans affiliated with Centene Corporation that Seroquel XR is medically necessary when the following criteria are met:
 Clinical Policy: (Seroquel XR) Reference Number: CP.PMN.64 Effective Date: 12.01.14 Last Review Date: 02.18 Line of Business: Commercial, Health Insurance Marketplace, Medicaid Revision Log See Important
Clinical Policy: (Seroquel XR) Reference Number: CP.PMN.64 Effective Date: 12.01.14 Last Review Date: 02.18 Line of Business: Commercial, Health Insurance Marketplace, Medicaid Revision Log See Important
DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS)
 Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Type 2 Diabetes DIABETES BLOOD GLUCOSE MONITORING AT HOME (ADULTS) Clinical Guideline Self-monitoring of blood glucose should be offered to all newly diagnosed type 2 diabetic patients as an integral part
Is There a Best Drug for Overactive Bladder in a Patient with Dementia?
 Is There a Best Drug for Overactive Bladder in a Patient with Dementia? Todd Semla, MS, Pharm.D., BCPS, FCCP, AGSF National PBM Clinical Pharmacy Program Manager Mental Health & Geriatrics U.S. Department
Is There a Best Drug for Overactive Bladder in a Patient with Dementia? Todd Semla, MS, Pharm.D., BCPS, FCCP, AGSF National PBM Clinical Pharmacy Program Manager Mental Health & Geriatrics U.S. Department
Neuromuscular Blocking Agents
 Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
Neuromuscular Blocking Agents DRUG POLICY This Prior Authorization request will be reviewed for medical necessity only. Benefits are subject to the terms and conditions of the patient s contract. Please
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
Evaluation of Accuracy and User Performance of the TRUE METRIX Self-Monitoring Blood Glucose System Summary Objectives: To demonstrate that the TRUE METRIX Self-Monitoring Blood Glucose System*, from Trividia
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
THE OVER-ACTIVE BLADDER (OAB)
 THE OVER-ACTIVE BLADDER (OAB) Passage of urine is caused by the bladder muscle contracting coordinated with the relaxation of the sphincter muscles, which is controlled by higher centers in the central
THE OVER-ACTIVE BLADDER (OAB) Passage of urine is caused by the bladder muscle contracting coordinated with the relaxation of the sphincter muscles, which is controlled by higher centers in the central
Urinary Incontinence for the Primary Care Provider
 Urinary Incontinence for the Primary Care Provider Diana J Scott FNP-BC https://youtu.be/gmzaue1ojn4 1 Assessment of Urinary Incontinence Urge Stress Mixed Other overflow, postural, continuous, insensible,
Urinary Incontinence for the Primary Care Provider Diana J Scott FNP-BC https://youtu.be/gmzaue1ojn4 1 Assessment of Urinary Incontinence Urge Stress Mixed Other overflow, postural, continuous, insensible,
Quick Guide to Common Antidepressants-Adults
 Quick Guide to Common Antidepressants-Adults Medication Therapeutic Range (mg/day) Initial Suggested Serotonin Reuptake Inhibitors (SSRIs) All available as generic FLUOXETINE (Prozac) CITALOPRAM (Celexa
Quick Guide to Common Antidepressants-Adults Medication Therapeutic Range (mg/day) Initial Suggested Serotonin Reuptake Inhibitors (SSRIs) All available as generic FLUOXETINE (Prozac) CITALOPRAM (Celexa
According to the INDICATIONS AND USAGE section of its FDA-approved product labeling (PI):
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Karen L. Walles, M.S. Assistant Director, Regulatory Affairs 3
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE Karen L. Walles, M.S. Assistant Director, Regulatory Affairs 3
GUIDELINE FOR SELF MONITORING OF BLOOD GLUCOSE IN ADULTS WITH DIABETES
 It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
It is recognised that self- monitoring of blood glucose (SMBG) may have an important role to play for some patients with. It can allow individuals to see what impact particular behaviours, such as dietary
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System
 Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Clinical Accuracy and User Performance of the TRUE METRIX AIR Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX AIR Blood Glucose Monitoring System, from Trividia
Report on New Patented Drugs - Vesicare
 Report on New Patented Drugs - Vesicare Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board's Excessive
Report on New Patented Drugs - Vesicare Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board's Excessive
Geriatric Urinary Incontinence
 Geriatric Urinary Incontinence Neil M. Resnick, MD Thomas Detre Professor of Medicine Chief, Division of Geriatric Medicine University of Pittsburgh/UPMC UI: The Problem Prevalence in elderly 33% Morbidity
Geriatric Urinary Incontinence Neil M. Resnick, MD Thomas Detre Professor of Medicine Chief, Division of Geriatric Medicine University of Pittsburgh/UPMC UI: The Problem Prevalence in elderly 33% Morbidity
Continence PGD transdermal oxybutynin Kentera patch 36mg
 Continence PGD transdermal oxybutynin Kentera patch 36mg Patient group direction for the supply of transdermal oxybutynin Kentera patch 36mg to patients suffering from urinary frequency, urgency or incontinence
Continence PGD transdermal oxybutynin Kentera patch 36mg Patient group direction for the supply of transdermal oxybutynin Kentera patch 36mg to patients suffering from urinary frequency, urgency or incontinence
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System
 Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
Evaluation of Accuracy and User Performance of the TRUE METRIX Blood Glucose Monitoring System Summary Objectives: To demonstrate that the TRUE METRIX Blood Glucose Monitoring System, from Trividia Health,
CADTH CANADIAN DRUG EXPERT COMMITTEE FINAL RECOMMENDATION
 CADTH CANADIAN DRUG EXPERT COMMITTEE FINAL RECOMMENDATION PROPIVERINE HYDROCHLORIDE (Mictoryl/Mictoryl Pediatric Duchesnay Inc.) Indication: Overactive bladder This document was originally issued on April
CADTH CANADIAN DRUG EXPERT COMMITTEE FINAL RECOMMENDATION PROPIVERINE HYDROCHLORIDE (Mictoryl/Mictoryl Pediatric Duchesnay Inc.) Indication: Overactive bladder This document was originally issued on April
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
Type 2 Diabetes Recommended SMBG
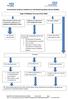 Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Herefordshire Diabetes Guideline for Self-Monitoring Blood Glucose (SMBG) Type 2 Diabetes Recommended SMBG Diet controlled/ metformin only/ combination of metformin and pioglitazone/dpp4/sglt2 or GLP1
Management of OAB. Lynsey McHugh. Consultant Urological Surgeon. Lancashire Teaching Hospitals
 Management of OAB Lynsey McHugh Consultant Urological Surgeon Lancashire Teaching Hospitals Summary Physiology Epidemiology Definitions NICE guidelines Evaluation Conservative management Medical management
Management of OAB Lynsey McHugh Consultant Urological Surgeon Lancashire Teaching Hospitals Summary Physiology Epidemiology Definitions NICE guidelines Evaluation Conservative management Medical management
Clinical Policy: Levomilnacipran (Fetzima) Reference Number: HIM.PA.125 Effective Date: Last Review Date: 11.18
 Clinical Policy: (Fetzima) Reference Number: HIM.PA.125 Effective Date: 12.01.17 Last Review Date: 11.18 Line of Business: HIM Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Fetzima) Reference Number: HIM.PA.125 Effective Date: 12.01.17 Last Review Date: 11.18 Line of Business: HIM Revision Log See Important Reminder at the end of this policy for important
CDEC FINAL RECOMMENDATION
 CDEC FINAL RECOMMENDATION MIRABEGRON (Myrbetriq Astellas Pharma Canada Inc.) Indication: Overactive Bladder Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that mirabegron be listed
CDEC FINAL RECOMMENDATION MIRABEGRON (Myrbetriq Astellas Pharma Canada Inc.) Indication: Overactive Bladder Recommendation: The Canadian Drug Expert Committee (CDEC) recommends that mirabegron be listed
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA
 BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Home monitoring of blood glucose
 Home Monitoring of Glucose and Blood Pressure JAMES R. TAYLOR, PharmD, CDE, and KENDALL M. CAMPBELL, MD, University of Florida, Gainesville, Florida Home monitoring of blood glucose and blood pressure
Home Monitoring of Glucose and Blood Pressure JAMES R. TAYLOR, PharmD, CDE, and KENDALL M. CAMPBELL, MD, University of Florida, Gainesville, Florida Home monitoring of blood glucose and blood pressure
ril 2014 FINAL CONSOLIDATED REPORT MARCH 2016 Treatment of Overactive Bladder
 ril 2014 FINAL CONSOLIDATED REPORT MARCH 2016 Treatment of Overactive Bladder FINAL REPORT 2 The (ODPRN) is funded to conduct drug class reviews as part of an initiative to modernize the public drug formulary
ril 2014 FINAL CONSOLIDATED REPORT MARCH 2016 Treatment of Overactive Bladder FINAL REPORT 2 The (ODPRN) is funded to conduct drug class reviews as part of an initiative to modernize the public drug formulary
Primary Care management of Overactive Bladder (OAB)
 DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Primary Care management of Overactive Bladder (OAB) Prescribing Tips All medicines for OAB have similar dose-related efficacy. More than one agent (up
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC) Primary Care management of Overactive Bladder (OAB) Prescribing Tips All medicines for OAB have similar dose-related efficacy. More than one agent (up
Drug Regimen Optimization
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Excluding Valsartan / Ramipril Prior authorization criteria logic: a description
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Excluding Valsartan / Ramipril Prior authorization criteria logic: a description
Common Antidepressant Medications for Adults
 (and Citalopram (Celexa) Escitalopram (Lexapro) Fluoxetine (Prozac) Fluoxetine Weekly (Prozac Weekly) 20 in AM w/ food (10 mg in elderly or those w/ panic disorder) 20 40 40 (If age >60yo, max 20) 10 10
(and Citalopram (Celexa) Escitalopram (Lexapro) Fluoxetine (Prozac) Fluoxetine Weekly (Prozac Weekly) 20 in AM w/ food (10 mg in elderly or those w/ panic disorder) 20 40 40 (If age >60yo, max 20) 10 10
Have You Ever Wondered
 Have You Ever Wondered A few facts about medication use and related falls The Number of Medications You Take & The Connection to Falls CONCERN: As you increase the number of medications that you take,
Have You Ever Wondered A few facts about medication use and related falls The Number of Medications You Take & The Connection to Falls CONCERN: As you increase the number of medications that you take,
System accuracy evaluation of FORA Test N Go Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013
 System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
System Accuracy Evaluation of Project number: Fora092614-01 System accuracy evaluation of Blood Glucose Monitoring System versus YSI 2300 STAT Plus glucose analyzer following ISO 15197:2013 Date: 13 th
STEP THERAPY IN MEDICARE PART D
 STEP THERAPY IN MEDICARE PART D Sarkis Kavarian, PharmD Candidate 15 Preceptor Dr. Craig Stern Pro Pharma Pharmaceutical Consultants, Inc. May 1 st, 2015 Objectives Why is this important? Medicare Part
STEP THERAPY IN MEDICARE PART D Sarkis Kavarian, PharmD Candidate 15 Preceptor Dr. Craig Stern Pro Pharma Pharmaceutical Consultants, Inc. May 1 st, 2015 Objectives Why is this important? Medicare Part
FDA Briefing Document for Nonprescription Drugs Advisory Committee
 FDA Briefing Document for Nonprescription Drugs Advisory Committee Oxybutynin Transdermal System NDA 202211 Proposed Indication: Treats overactive bladder in women Topic: Partial Rx-to-OTC switch for oxybutynin
FDA Briefing Document for Nonprescription Drugs Advisory Committee Oxybutynin Transdermal System NDA 202211 Proposed Indication: Treats overactive bladder in women Topic: Partial Rx-to-OTC switch for oxybutynin
Overactive Bladder Syndrome
 Overactive Bladder Syndrome behavioural modifications to pharmacological and surgical treatments Dr Jos Jayarajan Urologist Austin Health, Eastern Health Warringal Private, Northpark Private, Epworth Overactive
Overactive Bladder Syndrome behavioural modifications to pharmacological and surgical treatments Dr Jos Jayarajan Urologist Austin Health, Eastern Health Warringal Private, Northpark Private, Epworth Overactive
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other BUPROPION HCL WELLBUTRIN, 01653 WELLBUTRIN SR, WELLBUTRIN XL BUPROPION HBR APLENZIN 17050 16996 26198 CITALOPRAM CELEXA 10321 GPID 16344 HYDROBROMIDE DESVENLAFAXINE
Generic Brand HICL GCN Exception/Other BUPROPION HCL WELLBUTRIN, 01653 WELLBUTRIN SR, WELLBUTRIN XL BUPROPION HBR APLENZIN 17050 16996 26198 CITALOPRAM CELEXA 10321 GPID 16344 HYDROBROMIDE DESVENLAFAXINE
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary
 NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
NHS Greater Glasgow and Clyde Adult Blood Glucose and Combined Ketone Meter and Test Strip Formulary 2018-20 Primary Care and Acute Joint Formulary Blood Glucose Meter formulary developed by the Diabetes
Report on New Patented Drugs Enablex
 Report on New Patented Drugs Enablex Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the PMPRB s Excessive
Report on New Patented Drugs Enablex Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the PMPRB s Excessive
Ontario Drug Benefit Formulary/Comparative Drug Index
 Ministry of Health and Long-Term Care Ontario Drug Benefit Formulary/Comparative Drug Index Edition 42 Summary of Changes July 2016 Effective July 28, 2016 Drug Programs Policy and Strategy Branch Ontario
Ministry of Health and Long-Term Care Ontario Drug Benefit Formulary/Comparative Drug Index Edition 42 Summary of Changes July 2016 Effective July 28, 2016 Drug Programs Policy and Strategy Branch Ontario
Approval of Multiple Diabetes Management Products and Mesalazine proposals
 8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
8 June Approval of Multiple Diabetes Management Products and Mesalazine proposals PHARMAC is pleased to announce the approval of five agreements in relation to funding of blood glucose, blood glucose diagnostic
Uniform Formulary Beneficiary Advisory Panel Handout September 2005
 Uniform Formulary Beneficiary Advisory Panel Handout September 2005 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical-effectiveness
Uniform Formulary Beneficiary Advisory Panel Handout September 2005 PURPOSE: The purpose of this handout is to provide BAP Committee members with a reference document for the relative clinical-effectiveness
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Generic Brand HICL GCN Exception/Other BLOOD SUGAR DIAGNOSTIC DIABETIC TEST STRIPS 25200 NOTE: Requests for preferred blood glucose (diabetic) test strips manufactured by Abbott Diabetes Care (such as
Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing Pain
 Journal of Diabetes Science and Technology Volume 3, Issue 5, September 2009 Diabetes Technology Society ORIGINAL ARTICLES Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing
Journal of Diabetes Science and Technology Volume 3, Issue 5, September 2009 Diabetes Technology Society ORIGINAL ARTICLES Comparison of Lancing Devices for Self-Monitoring of Blood Glucose Regarding Lancing
Drug Class Review Agents for Overactive Bladder
 Drug Class Review Agents for Overactive Bladder Final Update 4 Report March 2009 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Drug Class Review Agents for Overactive Bladder Final Update 4 Report March 2009 The purpose of reports is to make available information regarding the comparative clinical effectiveness and harms of different
Innovations in Childhood Incontinence: Neurogenic and Functional Bladder Disorders
 TNe 10 Innovations in Childhood Incontinence: Neurogenic and Functional Bladder Disorders Stuart B. Bauer, MD President, ICCS Department of Urology Children s Hospital Boston Professor of Urology Harvard
TNe 10 Innovations in Childhood Incontinence: Neurogenic and Functional Bladder Disorders Stuart B. Bauer, MD President, ICCS Department of Urology Children s Hospital Boston Professor of Urology Harvard
Walsall CCG Supported Blood Glucose Meters April 2018
 Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
Meter Name Picture Type Strips Lancets Information Company PRIMARY CARE INITIATION TEE2+ Uncomplicated Type 2 diabetes patient on TEE2 test CareSens Lancets Easy to use. Large clear display Bluetooth functionality
URGE MOTOR INCONTINENCE
 URGE MOTOR INCONTINENCE URGE INCONTINENCE COMMONEST TYPE IN ELDERLY WOMEN Causes: 1 - Defects in CNS regulation Stroke Parkinson s disease Dementia (Alzheimer s and other types) Normopressure hydrocephalus
URGE MOTOR INCONTINENCE URGE INCONTINENCE COMMONEST TYPE IN ELDERLY WOMEN Causes: 1 - Defects in CNS regulation Stroke Parkinson s disease Dementia (Alzheimer s and other types) Normopressure hydrocephalus
M E M O R A N D U M. Please share this information as applicable within your organizations/practices.
 ESRD NETWORK 18 M E M O R A N D U M To: From: Subject: Facility Manager Lana Kacherova, QI Director Lisle Mukai, QI Coordinator FDA Recalls & Alerts Date: September 8, 2009 The Network is required to distribute
ESRD NETWORK 18 M E M O R A N D U M To: From: Subject: Facility Manager Lana Kacherova, QI Director Lisle Mukai, QI Coordinator FDA Recalls & Alerts Date: September 8, 2009 The Network is required to distribute
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
Multi User Model. Product Overview
 Multi User Model Product Overview Features and Benefits NO CODING EASY TO USE o o o Large LCD display. Large buttons with simple functions Easy Setup. Pre-Setup before shipping. Easy strip insertion. SMALL
Multi User Model Product Overview Features and Benefits NO CODING EASY TO USE o o o Large LCD display. Large buttons with simple functions Easy Setup. Pre-Setup before shipping. Easy strip insertion. SMALL
Blood Glucose Test Strip Guidelines
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Prescription Refill List Insulin and Related Supplies
 Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
Insulin and Related Supplies Name Date Local Pharamcy Name/Address : 30 day/ 5 refills 90 day/ 3 refills Mail Away Pharamacy Name/Address: 30 day/ 5 refills 90day / 3 refills *Check only those items that
TAKING LUVOX AND WELLBUTRIN TOGETHER
 TAKING LUVOX AND WELLBUTRIN TOGETHER Taking Luvox And Wellbutrin Together Wellbutrin make you sleepy Wellbutrin and low white blood cells Wellbutrin used with lexapro Wellbutrin xl full effect Wellbutrin
TAKING LUVOX AND WELLBUTRIN TOGETHER Taking Luvox And Wellbutrin Together Wellbutrin make you sleepy Wellbutrin and low white blood cells Wellbutrin used with lexapro Wellbutrin xl full effect Wellbutrin
Overactive Bladder. When to see a doctor. Normal bladder function
 Overactive Bladder Overactive bladder is a problem with bladder-storage function that causes a sudden urge to urinate. The urge may be difficult to stop, and overactive bladder may lead to the involuntary
Overactive Bladder Overactive bladder is a problem with bladder-storage function that causes a sudden urge to urinate. The urge may be difficult to stop, and overactive bladder may lead to the involuntary
Frequently Used PINs for Billing Purposes November 2018
 Diabetic Test Strips PIN Description 97799824 ACCU-CHEK ADVANTAGE STRIPS (100) 97799823 ACCU-CHEK ADVANTAGE STRIPS (50) 97799814 ACCU-CHEK AVIVA TEST STRIPS (100) 97799815 ACCU-CHEK AVIVA TEST STRIPS (50)
Diabetic Test Strips PIN Description 97799824 ACCU-CHEK ADVANTAGE STRIPS (100) 97799823 ACCU-CHEK ADVANTAGE STRIPS (50) 97799814 ACCU-CHEK AVIVA TEST STRIPS (100) 97799815 ACCU-CHEK AVIVA TEST STRIPS (50)
MTF Quarterly Webcast
 TMA Fort Sam Houston, TX MTF Quarterly Webcast LTC Stacia Spridgen Director, 1 Introduction Greetings from the PEC Purpose of the Quarterly MTF Webcast DCO Ground Rules Type questions into DCO system Put
TMA Fort Sam Houston, TX MTF Quarterly Webcast LTC Stacia Spridgen Director, 1 Introduction Greetings from the PEC Purpose of the Quarterly MTF Webcast DCO Ground Rules Type questions into DCO system Put
Treatment for Overactive Bladder
 ril 2014 Treatment for Overactive Bladder Stakeholder Review STAKEHOLDER REVIEW 2 GENERAL Comment: ODPRN should consider more prominently identifying one of the key limitations in their assessment and
ril 2014 Treatment for Overactive Bladder Stakeholder Review STAKEHOLDER REVIEW 2 GENERAL Comment: ODPRN should consider more prominently identifying one of the key limitations in their assessment and
Evaluation & Management of Overactive Bladder
 Evaluation & Management of Overactive Bladder Disclosures/Conflict of Interest/Bias I have no financial relationship with any of the companies that produce the products I m about to discuss I have no conflicts
Evaluation & Management of Overactive Bladder Disclosures/Conflict of Interest/Bias I have no financial relationship with any of the companies that produce the products I m about to discuss I have no conflicts
NICE Pathways bring together all NICE guidance, quality standards and other NICE information on a specific topic.
 bring together all NICE guidance, quality standards and other NICE information on a specific topic. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published.
bring together all NICE guidance, quality standards and other NICE information on a specific topic. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published.
Pharmacologic management of overactive bladder
 REVIEW Pharmacologic management of overactive bladder Sum Lam 1,2 lga Hilas 1,3 1 St. John s University, College of Pharmacy and Allied Health Professions, Department of Clinical Pharmacy Practice, Queens,
REVIEW Pharmacologic management of overactive bladder Sum Lam 1,2 lga Hilas 1,3 1 St. John s University, College of Pharmacy and Allied Health Professions, Department of Clinical Pharmacy Practice, Queens,
Prescription benefit updates Large group
 Prescription benefit updates Large group Moda Health s prescription program is a pharmacy benefit that offers members a choice of safe effective medication treatments. The program also helps you save money
Prescription benefit updates Large group Moda Health s prescription program is a pharmacy benefit that offers members a choice of safe effective medication treatments. The program also helps you save money
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INTERIM MEETING
 DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INTERIM MEETING Addendum December 17, 2013 I. UNIFORM FORMULARY (UF) DRUG CLASS REVIEWS A. Anti-Lipidemic-ls (LIP-ls) Background-New
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INTERIM MEETING Addendum December 17, 2013 I. UNIFORM FORMULARY (UF) DRUG CLASS REVIEWS A. Anti-Lipidemic-ls (LIP-ls) Background-New
STEP THERAPY CRITERIA
 STEP THERAPY This is a complete list of drugs that have written coverage determination policies. Drugs on this list do not indicate that this particular drug will be covered under your medical or prescription
STEP THERAPY This is a complete list of drugs that have written coverage determination policies. Drugs on this list do not indicate that this particular drug will be covered under your medical or prescription
Diagnosis & Management of Major Depression: A Review of What s Old and New. Cerrone Cohen, MD
 Diagnosis & Management of Major Depression: A Review of What s Old and New Cerrone Cohen, MD Why You re Treating So Much Mental Health 59% of Psychiatrists Are Over the Age of 55 AAMC 2014 Physician specialty
Diagnosis & Management of Major Depression: A Review of What s Old and New Cerrone Cohen, MD Why You re Treating So Much Mental Health 59% of Psychiatrists Are Over the Age of 55 AAMC 2014 Physician specialty
" EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING years of experience
 Commissie voor Klinische Biologie Commission de Biologie Clinique " EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING 8 3 years of experience CM Van Campenhout JC Libeer WIV/ISP/IPH Clinical
Commissie voor Klinische Biologie Commission de Biologie Clinique " EXTERNAL QUALITY EVALUATION PROGRAM FOR POCT GLUCOSE TESTING 8 3 years of experience CM Van Campenhout JC Libeer WIV/ISP/IPH Clinical
