A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function
|
|
|
- Shavonne Marilynn Short
- 5 years ago
- Views:
Transcription
1 A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function 21 Nature America, Inc. All rights reserved. Josef S Ozer 1,,7, Frank Dieterle 2,7, Sean Troth 3, Elias Perentes 2, André Cordier 2, Pablo Verdes 2, Frank Staedtler 2, Andreas Mahl 2, Olivier Grenet 2, Daniel R Roth 2, Daniel Wahl 2, François Legay 2, Daniel Holder, Zoltan Erdos 1, Katerina Vlasakova 1, Hong Jin 1, Yan Yu 1, Nagaraja Muniappa 3, Tom Forest 1, Holly K Clouse, Spencer Reynolds 1, Wendy J Bailey 1, Douglas T Thudium 1, Michael J Topper 1, Thomas R Skopek 1, Joseph F Sina 1, Warren E Glaab 1, Jacky Vonderscher 2,, Gérard Maurer 2, Salah-Dine Chibout 2, Frank D Sistare 1 & David L Gerhold 1 The Predictive Safety Testing Consortium s first regulatory submission to qualify kidney safety biomarkers revealed two deficiencies. To address the need for biomarkers that monitor recovery from agent-induced renal damage, we scored changes in the levels of urinary biomarkers in rats during recovery from renal injury induced by exposure to carbapenem A or gentamicin. All biomarkers responded to histologic tubular toxicities to varied degrees and with different kinetics. After a recovery period, all biomarkers returned to levels approaching those observed in uninjured animals. We next addressed the need for a serum biomarker that reflects general kidney function regardless of the exact site of renal injury. Our assay for serum cystatin C is more sensitive and specific than serum creatinine (SCr) or blood urea nitrogen (BUN) in monitoring generalized renal function after exposure of rats to eight nephrotoxicants and two hepatotoxicants. This sensitive serum biomarker will enable testing of renal function in animal studies that do not involve urine collection. Acute kidney injury caused by a variety of chemicals, including contrast agents, amine antibiotics and chemotherapeutics, poses an important problem in clinical settings. It is also of particular relevance during drug development and the optimization of candidates in preclinical and clinical trials. Acute kidney injury is typically diagnosed by monitoring SCr and BUN, the levels of which elevate only after nearly half of functional human kidney capacity has been compromised 1. More sensitive renal functional biomarkers would enable more reliable diagnosis of druginduced acute kidney injury and intervention by providing earlier and more reliable signs of injury 2. Renal diagnostic biomarkers would also enable safer and easier-to-monitor therapeutic treatments with narrower margins between safety and efficacy than for markers currently used for clinical and preclinical applications. Additionally, these biomarkers might be used to diagnose particular forms of kidney injury 3,. The first biomarker qualification submission (Voluntary exploratory Data Submission, VXDS) brought forward by the Predictive Safety Testing Consortium (PSTC) set out to address the need for improved markers of nephrotoxicity with the initial goal of qualifying markers for preclinical applications during drug development and the eventual goal of translating these markers for use in clinical settings 8. The initial submission process revealed two limitations in the studies that we address here. The first gap concerns the need for recovery studies to demonstrate that reversibility of histopathologic renal lesions could be similarly monitored by biomarker changes. To address this need, we conducted two treatment-recovery studies in rats. Both involved measuring levels of a panel of seven renal tubular safety biomarkers, many of which were submitted in this VXDS application. The second gap was to identify a more sensitive serum biomarker of renal function, which allows general monitoring of impaired renal function. As renal injury most often is manifested by damage to the proximal tubule, injury to other parts of the organ is difficult to track in the absence of an improved biomarker that detects impaired functional capacity. As preclinical pharmaceutical studies routinely include blood collection, a sensitive serum biomarker would enable retrospective testing in animal studies that do not involve urine collection. Furthermore, interpretation of a serum biomarker data is less complex in controlled animal studies than in clinical patients with prevalent comorbidities. Most drug-induced acute renal toxicity primarily affects the sensitive proximal tubule epithelium. Acute necrosis of moderate numbers of proximal tubule cells is a reversible process, where regeneration of a contiguous proximal tubule layer restores the integrity of the tubule 9. Such regeneration comprises reversible kidney injury and is accompanied 1 Department of Investigative Laboratory Sciences, Safety Assessment, Merck Research Laboratories, West Point, Pennsylvania, USA. 2 Translational Sciences, Institutes for BioMedical Research,, Basel, Switzerland. 3 Department of Pathology, Safety Assessment, Merck Research Laboratories, West Point, Pennsylvania, USA. Department of Biometrics, Merck Research Laboratories, West Point, Pennsylvania, USA. Department of Exploratory Toxicology, Safety Assessment, Merck Research Laboratories, West Point, Pennsylvania, USA. Present addresses: Pharmacokinetics, Dynamics, and Metabolism, PGRD, Pfizer, Andover Laboratories, Andover, Massachusetts, USA (J.S.O.) and Molecular Medicine Labs, Group Research, Hoffmann-La Roche, Basel, Switzerland (J.V.). 7 These authors contributed equally to this work. Correspondence should be addressed to D.L.G. (david_gerhold@merck.com). Received 9 October 29; accepted 22 March 21; published online 1 May 21; doi:1.138/nbt VOLUME 28 NUMBER MAY 21 nature biotechnology
2 21 Nature America, Inc. All rights reserved. by limited inflammatory response. However, it is not known whether biomarkers respond acutely and return to baseline during recovery, or whether certain biomarkers remain elevated beyond baseline levels during regenerative processes. Urinary biomarkers for which assays are available in the rat include markers of functional deficits and proximal tubule dysfunction (such as albumin), biomarkers lost from dead or injured cells (such as glutathione-s-transferase α (GSTα)), and actively secreted proteins that are either induced or repressed as a result of injury. The last class include kidney injury molecule 1 (Kim-1), osteopontin (OPN), neutrophil gelatinase associated lipocalin/lipocalin 2 (NGAL/LCN2), clusterin (CLU) and trefoil factor 3 (TFF3). Albumin is a well-established biomarker of glomerular and proximal tubule cell dysfunction. GSTα is a detoxification enzyme, associated with the apical membrane of proximal tubule cells that is lost into the urine acutely upon injury 1. Kim-1 is an extracellular protein anchored in the membrane of proximal tubule cells that is cleaved by a metalloprotease and excreted into urine. Evidence obtained using rats and humans indicates that Kim-1 responds both sensitively and dynamically to proximal tubule injury from a variety of sources 11,12. OPN is secreted by a variety of cells and organs upon injury as part of an inflammatory response. LCN2 is secreted by a variety of epithelial cells and binds siderophores capable of chelating iron. Mice lacking LCN2 are susceptible to Escherichia coli infections but are not susceptible to renal damage resulting from reperfusionischemia 13. CLU is a glycoprotein secreted by a variety of cell types and organs, notably dedifferentiated tubular cells in the kidney. Secreted CLU is thought to play a cellular pro-survival function 1 1. TFF3 is a small secreted mucin and hormone that shows reduced urine excretion in response to acute kidney injury and promotes survival and differentiation of epithelial cells in several tissues,17. We show that levels of Kim-1, CLU, OPN, LCN2, albumin, GSTα and TFF3 change dynamically after treatment-related renal injury and return to baseline levels upon recovery. Whereas current urinary biomarkers for nephrotoxicity respond primarily to damage of either the proximal tubule or glomerulus, a functional serum biomarker would enable tracking of renal injury from those and other locations of injury such as the distal tubule. An improved functional renal marker will add value for monitoring injury, relative to markers that leak from injured cells or markers that reflect a response to injury, even if other renal injury markers, such as Kim-1, LCN2 and albumin, are more sensitive for their specialized applications. Serum cystatin C (S-cystatin C) is a renal function marker that is rapidly gaining increased use in clinical applications, but has not been tested and qualified in preclinical studies. Cystatin C is a nonglycosylated low-molecular protein with a molecular weight of 13 kda. It is continuously produced by all nucleated cells and functions as a housekeeping factor 18. S-cystatin C is directly and freely filtered from blood into the glomerulus, and is therefore an ideal estimator of the glomerular filtration rate due to (i) greatly reduced impact of age, sex, muscle mass, dehydration state and circadian rhythm on S-cystatin C levels in contrast to SCr; (ii) an unhindered straightforward filtration of cystatin C by glomeruli; and (iii) an absence of tubular secretion or extra-renal clearance in contrast to SCr 19. It has been shown in clinical studies that S-cystatin C either outperforms or performs similarly to SCr for the estimation of the glomerular filtration rate in broad contexts of kidney injury (e.g., acute kidney injury and chronic kidney disease and glomerular function impairment) The US Food and Drug Administration (FDA) approval of an assay to measure S-cystatin C shows the assay s increasing importance and value in clinical practice 2. As an extension of drug-induced renal injury as reported with urinary biomarkers,2 27, this study also involved a systematic preclinical qualification assessment of the merits of using S-cystatin C as a marker for kidney function. RESULTS Reversible tubular injury with carbapenem A treatment We treated rats for 3 d with carbapenem A, a potent renal tubular toxicant in rats and a discontinued candidate antibiotic 28, and then followed this with a 1-d recovery period to measure biomarker responses during treatment and recovery from drug-induced kidney damage. Modest treatment-related increases in kidney weights were observed in rats dosed with carbapenem A (1 mg/kg/d). Renal cortical pallor was observed with tubular degeneration, necrosis and regeneration observed at multiple time points. Histomorphologic renal changes consisted of tubular epithelial degeneration and necrosis of the deep cortex, beginning on day 1 with cumulative injury and peaking in severity on day (Supplementary Fig. 1). Renal tubular epithelial degeneration was most severe on days 1 and 2, progressing predominantly to necrosis by day (Supplementary Fig. 1). Regeneration of tubular epithelium was first observed on day with the peak response on day 8 (Supplementary Fig. 1). Regeneration, very slight interstitial fibrosis and minimal inflammation were present in males between days 3 and 18 in response to tubular damage (Supplementary Fig. 1). Tubular dilatation was sporadically identified. Very slight to moderate tubular proteinaceous cast accumulation was observed on days 1, 2 and in males and females within the cortex and/or medulla, which was attributable to tubule damage. Necrosis and degeneration, a designated tubular histomorphologic change, peaked on days 2 and, and was more severe in males than females (Supplementary Fig. 2). A necrosis and degeneration tubular histomorphologic scatter plot indicates the severity grade at necropsy for individual animals (Figs. 1 and 2, color scale). Urinary biomarkers monitor carbapenem A induced nephrotoxicity and recovery For the carbapenem A reversibility studies, serum samples were collected on days 2,, 8 and 18, whereas urine samples were collected at days 2,, 8 and 1. Traditional serum clinical chemistry markers BUN and SCr were plotted for individual animals by study day and were correlated to overall tubular histomorphologic change on a severity scale of 1 to (Fig. 1 and Supplementary Figs. 1 and 2). Previous reports 7 indicate that a >1.2-fold increase in SCr relative to the mean from concurrent controls is considered positive for injury (9% specificity, receiver operating characteristic (ROC) curve exclusion model). All carbapenem A control animal SCr values are below the threshold cut-off (Fig. 1, dotted red line). At day 2, all animals with histomorphologic change (grades 2 and 3) show SCr values elevated between 1.7- and fivefold relative to controls (Fig. 1). At day, five of seven animals with histomorphologic change (grades 3 and ) are elevated between 1.7- and sevenfold. At day 8, three of seven rats with histomorphologic change (grade 1) are between 1.3- and twofold elevated, whereas no animals showed SCr elevations at day 18 (Fig. 1). BUN values of carbapenem A treated rats showed high similarity to the SCr data, except that the positive value cutoff was at 1.7-fold elevation (9% specificity, exclusion model) (Supplementary Fig. 2). Urinary biomarker values were determined by enzyme-linked immunosorbent assay (ELISA) or MesoScale Discovery methods, plotted individually by study day and correlated to histomorphologic change on a severity scale of 1 to. Previous experience 7 indicates that changes in Kim-1 abundance >1.9-fold relative to concurrent controls is positive for injury at 9% specificity (exclusion model). Most carbapenem A individual control-animal Kim-1 values were below nature biotechnology VOLUME 28 NUMBER MAY 21 87
3 21 Nature America, Inc. All rights reserved. Figure 1 For carbapenem A treated rats, correlation of urinary ELISA and MesoScale Discovery biomarker levels with histomorphologic change. Male or female Sprague Dawley rats were administered carbapenem A at 1 mg/kg/d (groups of five rats/dose/time point for up to 3 d). The animals were euthanized on days 2,, 8 or 18 for toxicity evaluation and urinary ELISA and immunoturbometric biomarker levels (TFF3 and albumin, respectively) and MesoScale Discovery GSTα levels were measured (ng/ml) and normalized to urinary creatinine. Treated male (T:M, square), treated female (T:F, circle), vehicle male (V:M, star), and vehicle female (V:F, triangle) are indicated. TFF3 (top left), albumin (top right), and GSTα (bottom left) abundances are shown as fold-change relative to the average of concurrent controls. SCr change (bottom right) is indicated as fold-change relative to the average of concurrent controls. Red dotted line indicates threshold from ROC analysis. The severity grades of histopathologic change after carbapenem A treatment are indicated on a scale of (no observed pathology) to with the the group mean 1.9-fold change threshold, except for two animals at day 1 (Fig. 2). All animals with histomorphologic changes at day 2 (grades 2 and 3), showed Kim-1 elevations above threshold. At day (grades 3 and ), levels of Kim-1 were elevated more than eightfold relative to concurrent vehicle controls samples (Fig. 2). At day 8, all animals with histomorphologic change (grades 1 and 2) and four of five animals with histomorphologic changes (grade 1) at day 1 showed Kim-1 elevations above threshold (Fig. 2). Changes in urinary CLU >1.8-fold relative to concurrent controls are considered to indicate injury (9% specificity, exclusion model). With the exception of one animal at day 1, all carbapenem A individual control D2 D Carbapenem A D8 V:F V:M TFF3 D1 T:F T:M GSTα D2 D D8 D D D2 Albumin animal CLU values were at or below the 1.8-fold mean change threshold, except one animal at day 1 (Fig. 2). At days 2 and, CLU levels were elevated above the threshold in all animals with histomorphologic change (Fig. 2). At day 8, CLU levels in five of eight animals with histomorphologic change were between seven and nearly -fold elevated, with one additional animal just above the threshold. In contrast, at day 1, only three of eight animals are above the threshold for CLU (Fig. 2). Fold-changes in urinary OPN relative to concurrent controls that would be considered positive for injury have not yet been determined. Nonetheless, a twofold elevation was used based upon the data observed on day 1, where control animals were placed below the D Histo N&D D8 D1 SCr D D8 D18 indicated grades displayed as the following color: grade (white), grade 1 (yellow), grade 2 (orange), grade 3 (red), grade (blue), grade (black). The histomorphologic change is shown at each necropsy day and vehicle-treated animals (control) are shown in white. Renal tubular necrosis and degeneration is shown in all biomarker panels except SCr, which is correlated to the renal composite, an overall score of tubular damage 29. TFF3 control levels are high and are reduced with toxicity. TFF3 levels are displayed as fold-change in the negative direction (minus ). Figure 2 Correlation of urinary MesoScale Discovery biomarker levels with histomorphologic change for carbapenem A-treated rats. Male or female Sprague Dawley rats were administered carbapenem A at 1 mg/kg/d (groups of five rats/dose/ time point for up to 3 d) and the animals were euthanized on days 2,, 8 or 18 for toxicity evaluation and measurement and normalization of urinary biomarker levels (Kim-1, LCN2, OPN and CLU) (ng/ml) relative to urinary creatinine. Treated male (T:M, square), treated female (T:F, circle), vehicle male (V:M, star) and vehicle female (V:F, triangle) are indicated. Abundances of Kim-1 (top left), LCN2 (top right), OPN (bottom left) and CLU (bottom right) are shown as foldchange relative to the average of concurrent controls. Red dotted line indicates threshold from ROC analysis 7 (data not shown). The severity grades of histopathologic change after carbapenem A treatment are indicated on a scale of (no observed pathology) to with the indicated grades displayed as the following D2 D Carbapenem A D8 V:F V:M Kim-1 D1 T:F T:M OPN D2 D D8 D D D Histo N&D D8 LCN2 D1 CLU D2 D D8 D1 color: grade (white), grade 1 (yellow), grade 2 (orange), grade 3 (red), grade (blue), grade (black). The histomorphologic change is shown at each necropsy day and vehicle-treated animals (control) are shown in white. Renal tubular necrosis and degeneration are shown in all panels. 88 VOLUME 28 NUMBER MAY 21 nature biotechnology
4 Figure 3 Correlation of urinary ELISA- and MesoScale Discovery-derived biomarker levels with histomorphologic change in gentamicin-treated rats. Male Sprague Dawley rats were administered gentamicin at 12 mg/kg/d to groups of five rats/dose/time point for 9 d and the animals were euthanized either on day 1 (upper panel) or 39 (lower panel) for toxicity evaluation. Urinary biomarker levels of albumin (ALB), CLU, GSTα, Kim-1, LCN2, OPN were measured (ng/ml) and serum chemistry parameters BUN and SCr were determined. Treated male (T:M, square), vehicle male (V:M, circle) and treated average (T:A, black triangle) are indicated. Urinary biomarker and serum chemistry values are shown as fold-change relative to the average of concurrent controls. The severity grades of histopathologic change after gentamicin treatment are indicated on a scale of (no observed pathology) to with the indicated grades displayed as the following color: grade (white), grade 1 (yellow), grade 2 (orange), grade 3 (red), grade (blue), grade (black). Renal tubular necrosis and degeneration at day 1 (top panel) and regeneration at day 39 (bottom panel) is shown. Fold-change is relative to day 1 control group average Histo T:M V:M T:A 2 21 Nature America, Inc. All rights reserved. threshold (~92% specificity; Fig. 2). Thus, all carbapenem A control animal OPN values are below this arbitrary threshold. At day 2, three of eight animals with histomorphologic change are above this threshold, whereas at day, seven of eight animals with histomorphologic change are above this threshold (Fig. 2). At day 8, values of half of the animals with histomorphologic change are above this threshold, whereas at day 1, values from two animals are above this threshold (Fig. 2). LCN2 fold changes >2. relative to concurrent controls are considered positive for injury at 9% specificity (F.D., unpublished exclusion model data). All carbapenem A individual control animal LCN2 values are at or below the 2.-fold change threshold (Fig. 2). At day 2, seven of eight animals with histomorphologic change are between 18- and -fold (27 ng/ml upper limit of quantification, ULOQ) elevated for LCN2 (Fig. 2). At day, seven of eight animals with histomorphologic change are between - and 1-fold elevated for LCN2 whereas at day 8, half the animals with histomorphologic change are above the threshold and at day 1 no animals are above the threshold (Fig. 2). Urinary TFF3 reductions <% relative to concurrent controls are considered positive for injury (9% specificity, exclusion model). Nearly all the carbapenem A control animal TFF3 values are above the.-fold change threshold (Fig. 1). At day 2, all animals with histomorphologic change show between.2 and.3-fold change (that is, and 3- fold, respectively) for TFF3 (Fig. 1). At day, all animals with histomorphologic change show between.2 and.3-fold change for TFF3 (Fig. 1). At day 8, six of eight animals with histomorphologic change are beyond the.-fold threshold and at day 1, three animals are beyond the.-fold cutoff for TFF3 as well as one control animal (Fig. 1). Albumin values elevated above 1.9-fold are considered positive (9% specificity, exclusion model). All animals with histomorphologic change at days 2 (>1-fold) and showed albumin changes that were above the 1.9-fold cutoff, whereas several day-8 animals were moderately above the threshold, and day-1 animals also showed subtle elevations (Fig. 1). GSTα similarly has a determined threshold value of 1.8-fold, with 2 exploratory renal toxicity studies (J.S.O. and D.L.G., unpublished observations). All treated day-2 animals with histomorphologic change appear to have elevations of GSTα above threshold, whereas half of the treated animals showed modest elevations at day and no changes of this biomarker were seen at later study times (Fig. 1). Reversible tubular injury with gentamicin treatment We observed increases in treatment-related kidney weight increases (group average, 33%) on day 1 in high-dose (12 mg/kg/d for 9 d) gentamicin-treated rats. These correlated with bilateral renal enlargement and pallor. There were no significant gross or organ weight BUN SCr ALB CLU GSTα Kim-1 LCN2 OPN findings in the mg/kg/d dose group or at any other time point in the study, including after the 29-d recovery period. Moderate to severe renal tubular degeneration, necrosis and regeneration were observed by histomorphology on day 1 at the high dose (12 mg/kg/d 9 d) (Supplementary Fig. 3). After the 29-d recovery period, tubular changes at the high dose (12 mg/kg/d 9 d) were limited to very slight regeneration, indicating nearly complete recovery (Supplementary Fig. 3). One rat, which received a low-dose gentamicin (/mg/kg/d) treatment for 9 d had very slight tubular regeneration on day 1. Treatment-related focal areas of very slight to slight interstitial inflammation were noted at day 1. Urinary biomarkers monitor gentamicin-induced nephrotoxicity and recovery In the gentamicin time course study, renal tubular necrosis and degeneration were observed at day 1 in the 12 mg/kg/d dose group, whereas BUN and SCr elevations were more modest compared to those seen in the carbapenem A study. The urinary biomarker panel (albumin, CLU, GSTα, Kim-1 and LCN2) showed large elevations more than tenfold with Kim-1 increases nearly -fold (Fig. 3). TFF3 fold-change reductions were very small in this dose group (not shown), whereas OPN showed modest elevations (about fivefold) (Fig. 3). Considering that similar grade histomorphologic change at day 1 was observed for necrosis and degeneration and regeneration, biomarker responses can not be readily assigned to specific designations of histomorphologic change (Fig. 3 and Supplementary Fig. ). Twenty-nine days of recovery after gentamicin treatment showed both that serum chemistry markers and the urinary biomarker panel values returned to baseline with no observable necrosis and degeneration (Fig. 3 and data not shown). Thus, none of the biomarker levels appeared to correlate with observed grade 1 regeneration seen at study day 39 (Fig. 3). Cystatin C as a serum marker of kidney dysfunction We induced a variety of renal lesions in rats by treating them with one of eight nephrotoxicants cisplatin, gentamicin, tacrolimus (Protopic, Prograf), vancomycin, furosemide (Lasix), lithium (Eskalith), doxorubicin nature biotechnology VOLUME 28 NUMBER MAY 21 89
5 Table 1 Overview of the design of the studies Test compound Dose levels, route, regimen Necropsy/histopath. (d) Urine collection times (d) Blood/plasma (d) Animals strain n per group n total Study data set Nephrotoxicants 21 Nature America, Inc. All rights reserved. Carbapenem A Gentamicin sulfate Gentamicin sulfate Vancomycin hydrochloride Doxorubicin chlorhydrate Furosemide Lithium carbonate Cisplatin Puromycin dihydrochloride Tacrolimus/ FK ANIT Methapyrilene hydrochloride, 1 mg/kg i.v. 1 daily (3 d) ml/kg, 12 mg/kg 1 daily (9 d) ml/kg, 3, 7, 1 mg/kg 1 daily ml/kg, 7, 1, 21 mg/kg 1 daily injection ml/kg, 2.,., 7. mg/kg i.v. Once at day 1 ml/kg,, 9, 18 mg/kg oral gavage 2 daily ml/kg, 1, 2, 3 mequ/kg oral gavage 1 daily ml/kg,., 1, 3 mg/kg Once at day 1 ml/kg, 1, 2, mg/kg 1 daily 1 ml/kg, 9, 12, 1, 1 daily ml/kg,, 1, 3 mg/kg Oral gavage daily ml/kg, 1, 3, mg/kg Oral gavage 1 daily ml/kg 2, (3 d dosed), 8 (3 d dosed), 18 (3 d dosed) 1, 1 (9 d dosed), 39 (9 d dosed) , 3, 7, , 7, 1, , 7, 1, Hepatotoxicants 7 1, 3, 7, ,, 8, 18 Sprague Dawley 2 (V), (T) (male and female) 8 Merck 1, 39 Sprague Dawley , 7, 1, 22 Han Wistar 9 3, 7, 1, 21 Han Wistar Merck Merck i.v., intravenous;, intraperitoneal; mequ/kg, milli equivalent/kg. (Doxil, Adriamycin) or puromycin that reflect different modes of toxicity (Table 1). In contrast, two hepatotoxicants (alpha-naphtylisothiocyanate (ANIT) and methapyrilene) not induce kidney injury (Supplementary Table 1). For 92 animals in the ten studies, we measured BUN and Scr levels by a clinical chemistry analyzer and scored S-cystatin C abundance as part of a multiplexed protein assay. Kidney injury was assessed by histopathology, applying a systematic grading system (grade ) and a controlled lexicon to describe the types of lesions and the exact localization 29. Exemplary photos of the variety of the lesions observed are provided elsewhere in this issue. In the ideal case, a renal functional marker should capture functional changes resulting from all types of kidney injury. Therefore, all observed major drug-induced lesions were integrated into one composite histopathology bin using the highest grade of all lesions reported for that animal. In particular, we integrated lesions all along the nephron categorized as tubular injury (degeneration, necrosis, apoptosis, cell sloughing), tubular regeneration (basophilia, mitosis), intratubular casts (granular, leukocytic, hyaline, mineral), tubular dilatation, glomerular alterations (mesangial proliferation, glomerular vacuolization, glomerular fibrosis) and interstitial fibrosis (cortex and medulla). We next did ROC inclusion and exclusion analyses similar to those outlined elsewhere in this issue 29. The results of the ROC exclusion analysis (nephrotoxicant-dosed animals with a renal injury reported versus animals not dosed with a nephrotoxicant and without a reported renal injury) are shown in Figure and in Supplementary Tables 1 and 2. These data highlight the superiority of S-cystatin C compared to use of SCr and BUN for diagnosing renal injury. For all observed histopathology grades, S-cystatin C has the highest AUC (exclusion,.79; inclusion,.7) compared to the current peripheral standards SCr (exclusion,.8; inclusion,.) and BUN (exclusion,.7; inclusion,.2). It is also demonstrated by statistical methods that S-cystatin C clearly outperformed both clinical chemistry parameters in the exclusion analysis (differences of AUCs with P <.1). The fact that the differences of AUCs between S-cystatin C and SCr and BUN were smaller for the inclusion analysis (P =. for SCr and P =.2 for BUN) can be attributed to the fact that S-cystatin C was initially increased in 9 VOLUME 28 NUMBER MAY 21 nature biotechnology
6 21 Nature America, Inc. All rights reserved. Figure ROC curves for the inclusion and exclusion analysis with eight different nephrotoxicant studies and two different hepatotoxicant studies from. (a d) The sensitivity and specificity of BUN, SCr, and S-cystatin C with respect to a composite histopathology score includes data involving all histopathology grades (a), histopathology grade to 3 (b), histopathology grade to 2 (c) and histopathology grade and 1 (d). (e,f) Area under the curve (e) and sensitivity (f) (at 9% specificity) of BUN, SCr, and S-cystatin C compared to the gold standard, histopathology. Animal numbers, n. Negative: n = 322. Positive: all, n = 23; to 3, n = 21; to 2, n = 2; to 1, n = 127. a number of nephrotoxicant-dosed animals, when histopathology was not observed at early necropsies, which was scored as false positives in the inclusion analysis. Figure also shows the diagnostic performance in terms of AUCs and sensitivity for 9% specificity when only low-grade histopathology was compared between experimental and control animals (grade 1 to 3 versus grade, grade 1 to 2 versus grade, and in particular grade 1 versus grade ). In all cases, S-cystatin C shows an unquestionably improved performance relative to BUN and SCr for all studies. When restricting pathology to low-severity grades, S-cystatin C also shows an improved diagnostic performance relative to both SCr and BUN (Supplementary Table 1). In Figures and, the levels of S-cystatin C, BUN and SCr are shown as fold-changes for all animals whereby each data point is coded by the highest grade of renal lesion reported and the horizontal ascr levels (fold-change) BUN levels (fold-change) S-cystatin C levels (fold-change) b c Sensitivity Sensitivity Rand. SCr.8 BUN.97 Cst3.788 Rand. SCr.7 BUN.9 Cst specificity 1 specificity Cisplatin Gentamicin Vancomycin Tacrolimus Puromycin 3 a b e Rand. SCr. BUN. Cst specificity Sensitivity Sensitivity specificity c d f Rand. SCr.72 BUN. Cst All to 3 to 2 and 1 Histopathology grade subsets Cst3 SCr BUN Cst3 SCr BUN All to 3 to 2 and 1 Histopathology grade subsets line represents the thresholds for 9% specificity (exclusion: 1.21-fold for S-cystatin C, 1.21-fold for BUN and 1.12-fold for SCr). Looking at the color-coded dots with respect to the thresholds in the plots allows detection of true and false positives and true and false negatives on a study-by-study basis (the main histopathology findings behind the plotted grades are briefly listed after the compound name). In the cisplatin study (tubular injury and regeneration in the tubular segments S1-S3, tubular dilatation in cortex and medulla, intratubular hyaline casts in thick ascending tubules), S-cystatin C performed best in identifying animals with lesions of grades 3 and and a number of animals with grades 1 and 2 lesions. BUN changes detected injury in only a few high-grade animals, whereas SCr detected injury in a few low-grade and some high-grade lesions. Also, for gentamicin treatment (tubular injury and regeneration in S1-S2), S-cystatin C detected all grade 3 and lesions and a number of grade 1 and 2 lesions, whereas BUN did not detect drug-induced lesions. In contrast, in the vancomycin study (tubular injury and regeneration in S3 and thick ascending tubules, tubular dilatation in cortex and medulla, intratubular hyaline casts), both SCr and BUN outperformed S-cystatin C, which shows some false-negative grade 3 animals at the last termination time point. However, some low-dosed animals without histopathology findings have increased S-cystatin C values, which might reflect a signal earlier than histopathology observations. In the tacrolimus study (tubular regeneration in thick ascending tubules and distal tubules, intratubular mineralization in S3 and thick ascending tubules, juxtaglomerular apparatus hypertrophy) and in the puromycin study (glomerular alterations/damage, tubular injury and Figure Levels of S-cystatin C, BUN and SCr observed in individual animals. (a c) Correlation of S-cystatin C (a), BUN (b) and SCr (c) levels with severity grades of histopathology for 7 animals in five studies (cisplatin, gentamicin, vancomycin, tacrolimus and puromycin) involving Han Wistar rats. All values are represented as fold-changes versus the average values of study-matched and time-matched control animals on a logarithmic scale. The animals are ordered by study, within each study by dose group (with increasing doses) and within each dose group by termination time point (with increasing time). The symbols and the colors represent the histopathology readout (no histopathology finding observed (red), grade 1 (green), grade 2 (blue), grade 3 (orange) and grade (black) on a grade severity scale). The magenta lines represent the thresholds determined for 9% specificity in the ROC analysis for all histopathology grades (1.29 for S-cystatin C, 1.28 for BUN and for SCr) nature biotechnology VOLUME 28 NUMBER MAY 21 91
7 21 Nature America, Inc. All rights reserved. Figure Levels of S-cystatin C, BUN and SCr observed in individual animals. (a c) Correlation of S-cystatin C (a), BUN (b) and SCr (c) levels with severity grades of histopathology for 7 animals in five studies (doxorubicin, lithium, furosemide, methapyrilene and ANIT) involving Han Wistar rats. All values are represented as fold-changes versus the average values of study-matched and time-matched control animals on a logarithmic scale. The animals are ordered by study, within each study by dosegroup (with increasing doses) and within each dose-group by termination time point (with increasing time). The symbols and the colors represent the histopathology readout [no histopathology finding observed (red), grade 1 (green), grade 2 (blue), grade 3 (orange) on a grade severity scale]. The magenta lines represent the thresholds determined for 9% specificity in the ROC analysis for all histopathology grades (1.29 for S-cystatin C, 1.28 for BUN and for SCr). regeneration in S1-S3, intratubular hyaline casts all along the nephron, tubular dilatation in cortex and medulla), S-cystatin C detects most animals with positive histopathology and is increased in certain dosed animals without observed histopathology changes, perhaps revealing early symptoms of injury or prodromal processes. For the doxorubicin study (tubular injury and regeneration in S1-S3 and thick ascending tubules, intratubular casts from S1 to thick ascending tubules, tubular dilation in cortex, medulla and papilla), S-cystatin C elevations were seen in most high-grade animals. However, SCr did not distinguish kidney toxicity at all, showing significantly decreased values even below the control values, which might be ascribed to the high unspecific general cytotoxicity of doxorubicin. Yet, in the lithium study (tubular injury and regeneration in collecting duct, tubular dilatation in cortex and medulla), SCr identified slightly more animals with positive histopathology than S-cystatin C. In the furosemide study (tubular injury and regeneration in S3, regeneration in thick ascending tubules, intratubular casts and/or mineralization in S3), BUN outperformed SCr and S-cystatin C, which missed some animals, mainly with grade 1 lesions. In this study, all three markers provide sensitive (low-dose group) and earlier (mid-dose group early time points) assessment of kidney injury compared to histopathology in this study. For the hepatotoxicant methapyrilene (only spontaneous regeneration changes observed), none of the three markers showed false-positive measurements or increased levels for the animals with spontaneous regeneration lesions. Similarly for ANIT, S-cystatin C and BUN revealed instances of spontaneous regeneration, whereas SCr shows systematically a number of false positives in the highdose group. The increased SCr levels might be explained by a druginduced muscle breakdown supported by reduced body weights of the animals. In summary, when compared with the current standards BUN and SCr, S-cystatin C elevation detected more animals with renal injury in five of eight nephrotoxicant studies compared to BUN and SCr, which each showed better performance than S-cystatin C in just two studies. Except for some animals in the vancomycin study, S-cystatin C levels showed a notable correlation with the severity grade of histopathologic lesions. In several studies, S-cystatin C was more sensitive and showed changes even earlier than observed with histopathology, visible as groups of nephrotoxicant animals with systematically increased S-cystatin C relative to control animals. Finally, no significant specificity issues were identified for S-cystatin C with these studies in contrast to SCr, which shows systematic false positives for the animals dosed with the hepatotoxicant ANIT. The visual inspection on an animal-by-animal basis reconfirms the statistical ROC analysis, which shows that S-cystatin C outperformed SCr and BUN. S-cystatin C levels (fold-change) b levels (fold-change) BUN levels (fold-change) ascr c Doxorubicin Lithium Furosemide Methapyrilene ANIT DISCUSSION Urinary biomarkers hold considerable promise for monitoring potential adverse effects on kidney integrity and function in both clinical and nonclinical settings in the absence of biopsy. Ultimate clinical biomarker utility would monitor both the progression and recovery from onset of renal injury. This necessitates preclinical demonstration of injury reversibility both for the biomarker signal(s) and histopathologic observation. Our studies to evaluate the utility of a panel of biomarkers to monitor the reversibility of kidney damage after cessation of drug treatment address a key limitation identified by regulatory authorities during evaluation of the first VXDS of safety biomarkers for kidney toxicity. This is the first report to use a broad panel of urinary biomarker values to demonstrate that renal injury can be monitored at both the point where toxicity begins and when it reverses after the withdrawal of treatment. S-cystatin C shows improved sensitivity and specificity over the historical standard markers SCr and BUN, allowing renal injuries other than proximal tubular and glomerular damage to be monitored for drug development and for pediatric and geriatric clinical populations, where the standards are less optimal for monitoring. Carbapenem A treatment (3 d)-related tubular injury was tracked well by serum chemistry marker elevations up to day collection and returned toward baseline at study days 8 and 18 (Fig. 1 and Supplementary Fig. 2). If a similar clinical trial were designed with serum sampling at 1 or 2 weeks, then little information identifying renal injury would have been revealed. By contrast, in the carbapenem A study at day 8, urinary Kim-1, OPN, CLU and TFF3 values showed large fold-change alterations for most histopathologic-positive animals and at day 1, Kim-1, CLU, OPN and TFF3 values still revealed elevations above threshold (Figs. 1 and 2). The panel of urinary markers adds information to the clinical chemistry markers in the carbapenem A time course where all urinary markers show a trend 92 VOLUME 28 NUMBER MAY 21 nature biotechnology
8 21 Nature America, Inc. All rights reserved. toward baseline at study day 1. Urinary albumin and GSTα showed similar time-course elevations to clinical chemistry markers and a dynamic range in the 1-fold range that far exceeded that of SCr and BUN (Fig. 1 and Supplementary Fig. 2). The urinary markers albumin and GSTα appear to be useful for the monitoring of rapid onset renal injury that occurs soon after drug administration. BUN and SCr elevations were less elevated in the gentamicin time course compared to the carbapenem A study or the majority of studies from the VXDS submission and might be considered borderline positive 7. With the exception of TFF3, the urinary biomarker panel (albumin, CLU, GSTα, Kim-1, LCN2 and to a lesser degree OPN) showed large elevations at day 1 with gentamicin treatment (Fig. 3). An analysis on day 29 after cessation of gentamicin treatment revealed that the serum chemistry markers and the urinary biomarker panel values approached baseline with no observable necrosis and degeneration injury and limited grade 1 regeneration and fibrosis. Thus, the urinary biomarker panel (albumin, CLU, GSTα, Kim-1 and LCN2) in the gentamicin time course added value to subtle SCr and BUN changes to monitor renal injury, repair and function. In conclusion, the use of the urinary renal toxicity biomarker panel enables injury monitoring for a broad context of study designs and potential sampling time points. No single marker is likely to be applied universally across many possible renal injury contexts. For example, GSTα appears to be an excellent early toxicity biomarker for epithelial necrosis. In contrast, Kim-1 and clusterin levels persist during regeneration and appear to reflect the triggering and continuation of the repair process. Elevations in levels of albumin correlate strictly with early loss of function seen after tubular epithelial necrosis and degeneration. Measurement of all the renal injury markers measured in parallel enables the investigator to capture critical information with regard to renal toxicity, repair and function, from study start to finish. There are often limitations regarding frequency of sample collection due to study design and dosing requirements. Thus, use of a biomarker panel insures that data generated are not dependent upon preconceived viewpoints regarding the expected performance of any single marker for a given study. Measuring the panel of injury markers maximizes the level of interpretation from a study design compared to a single marker being deployed in a monitoring study. Multiple markers, however, require more expertise to interpret many biomarker signals compared to just one or two. Clinical decisions are often made with a few critical markers rather than a large panel. Integration of these advantages and concerns will be resolved with experience, in addition to appropriate models and algorithms. The second gap identified during the qualification and submission of renal safety biomarkers was a concern regarding whether particular renal injuries respond sensitively toward only specific types of lesions. For example, Kim-1 is specifically expressed in proximal tubules only in the case of proximal tubular injury but may have limited sensitivity to lesions in other compartments of the kidney. A panel of biomarkers that respond collectively and complement one another with respect to potential kidney injuries in various nephron segments would be a tremendous advantage to localize renal lesions. An alternative view is that a comprehensive panel of specific markers would be needed to cover every compartment and every possible lesion and injury in the kidney to monitor overall renal safety. This report presents an alternative to such localized markers. Novel renal function markers, such as S-cystatin C, monitor the general function of the kidney. The current standards BUN and SCr are both kidney function markers, but both markers are faced with several limitations, including extra-glomerular (SCr can be cleared trans-proximal tubules) clearance, variability in production, limited sensitivity, and poor specificity 1. The panel of biomarkers evaluated here and assessed in accompanying manuscripts extend the diagnostic capabilities to a variety of acute toxicant injuries at increased sensitivity and reliability. S-cystatin C has gained increasing use for clinical purposes, including treatment of chronic kidney diseases, such as diabetic nephropathy, kidney transplantation, treatment of elderly and pediatric populations (muscle mass changes), detection of acute renal failure and prediction of cardiovascular-associated risks. Several reviews and meta-analyses have demonstrated that S-cystatin C is more sensitive, specific and reliable than SCr looking at dosing regimens and clinical outcome Yet, no preclinical qualification has been performed because of the lack of available assays for the rat. In addition, it has been elucidated here, that a qualified biomarker in preclinical studies can be crucial for the translation of potentially preclinical nephrotoxic drugs safely into human 7. We have described a substantial analysis to close gaps in the preclinical qualification of this promising renal function biomarker using the statistical analyses and assessments established in the first VXDS of renal injury markers. We have demonstrated that the marker outperformed the current standards SCr and BUN both in terms of sensitivity and robustness. This reconfirms in the rat what has been described in numerous publications about the clinical use in humans of S-cystatin C In addition, the preclinical qualification is anchored in a histopathologic readout of the target organ, which is not available in most clinical situations. It was demonstrated that S-cystatin C as a renal function marker can detect various types of drug-induced lesions in various compartments of the kidney independent from the mode of nephrotoxicity also in the case of minimal injury (grade 1). From a preclinical perspective, another advantage of S-cystatin C is its measurement in blood. Urine collection in preclinical studies can be tedious and is not as routinely collected in drug development compared to serum clinical chemistry analyses. The results of the biological qualification of S-cystatin C as a preclinical functional biomarker presented in this manuscript may have several effects on drug development. First of all, a sensitive and reproducible assay is available for measuring S-cystatin C in rats. Second, the sensitivity and specificity of the biomarker was demonstrated and rules, such as thresholds with associated specificity and sensitivity, were established for its use in routine preclinical drug development. Finally, this work establishes the necessary bridge to its clinical use. We will submit these data to regulatory bodies in an attempt to close both gaps of the first VXDS intended to qualify biomarkers to monitor nephrotoxicity as part of the rolling qualification process. The recovery data will allow an extension of the context of the preclinically qualified biomarkers to monitor reversibility of lesions. S-cystatin C can monitor renal function in rat good laboratory practice studies and be used as a translational biomarker for early clinical trials in a narrow context. Taken together, the results presented should help to promote the use of these additional renal safety tests in both drug development and routine clinical practice. Methods Methods and any associated references are available in the online version of the paper at Note: Supplementary information is available on the Nature Biotechnology website. Acknowledgments S. Leuillet and B. Palate (Centre International de Toxicologie (CIT)) kindly performed studies and the histopathology assessment and J. Mapes (RBM) developed the S-cystatin C assay. We thank G. Miller and nature biotechnology VOLUME 28 NUMBER MAY 21 93
9 21 Nature America, Inc. All rights reserved. P. Srinivasa for helpful comments on the manuscript. Z.E., K.V. and W.E.G. kindly shared unpublished observations for GST alpha. AUTHOR CONTRIBUTIONS J.S.O., F.D., W.J.B., M.J.T., T.R.S., J.F.S., W.E.G., E.P., A.C., F.S., A.M., O.G., D.R.R., F.L., S.-D.C., G.M., J.V., D.L.G., F.D.S. and D.W. designed research; Z.E., T.F., N.M., E.P., D.R.R., S.T., H.K.C., S.R., D.T.T., K.V. and H.J. performed research; Z.E. and K.V. contributed new reagents/analytic tools; J.S.O., D.H., N.M., W.E.G., F.D., Y.Y., G.M., P.V., A.C., D.L.G. and F.D.S. analyzed data; and J.S.O., S.T., Z.E., K.V., F.D., D.L.G. and F.D.S. wrote the paper. COMPETING FINANCIAL INTERESTS The authors declare competing financial interests: details accompany the full-text HTML version of the paper at Published online at Reprints and permissions information is available online at reprintsandpermissions/. 1. Bonventre, J.V. et al. Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 28, 3 (21). 2. Ferguson, M.A., Vaidya, V.S. & Bonventre, J.V. Biomarkers of nephrotoxic acute kidney injury. Toxicology 2, (28). 3. Vaidya, V.S., Ramirez, V., Ichimura, T., Bobadilla, N.A. & Bonventre, J.V. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal Physiol. 29, F17 F29 (2).. Han, W.K. et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 73, (28).. Yu, Y., Jin, H., Holder, D., Ozer, J.S. & Villarreal, S. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat. Biotechnol. 28, 7 77 (21).. Dieterle, F. et al. Urinary clusterin, cystatin C, β2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat. Biotechnol. 28, 3 9 (21). 7. Vaidya, V.S. et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 28, 78 8 (21). 8. Mattes, W.B. & Walker, E.G. Translational toxicology and the work of the predictive safety testing consortium. Clin. Pharmacol. Ther. 8, (29). 9. Razzaque, M.S. & Taguchi, T. Cellular and molecular events leading to renal tubulointerstitial fibrosis. Med. Electron Microsc. 3, 8 8 (22). 1. Westhuyzen, J. et al. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol. Dial. Transplant. 18, 3 1 (23). 11. Bailly, V. et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 277, (22). 12. Ichimura, T., Hung, C.C., Yang, S.A., Stevens, J.L. & Bonventre, J.V. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Renal Physiol. 28, F2 F3 (2). 13. Berger, T. et al. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 13, (2). 1. Silkensen, J.R., Agarwal, A., Nath, K.A., Manivel, J.C. & Rosenberg, M.E. Temporal induction of clusterin in cisplatin nephrotoxicity. J. Am. Soc. Nephrol. 8, 32 3 (1997). 1. Orlandi, A. et al. Modulation of clusterin isoforms is associated with all-trans retinoic acid-induced proliferative arrest and apoptosis of intimal smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2, (2). 1. Vaidya, V.S. & Bonventre, J.V. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin. Drug Metab. Toxicol. 2, (2). 17. Hoffmann, W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell. Mol. Life Sci. 2, (2). 18. Mussap, M. & Plebani, M. Biochemistry and clinical role of human cystatin C. Crit. Rev. Clin. Lab. Sci. 1, 7 (2). 19. Takuwa, S., Ito, Y., Ushijima, K. & Uchida, K. Serum cystatin-c values in children by age and their fluctuation during dehydration. Pediatr. Int., (22). 2. Madero, M., Sarnak, M.J. & Stevens, L.A. Serum cystatin C as a marker of glomerular filtration rate. Curr. Opin. Nephrol. Hypertens. 1, 1 1 (2). 21. Dharnidharka, V.R., Kwond, C. & Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am. J. Kidney Dis., (22). 22. Shlipak, M.G., Praught, M.L. & Sarnak, M.J. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr. Opin. Nephrol. Hypertens. 1, (2). 23. Herget-Rosenthal, S. et al. Early detection of acute renal failure by serum cystatin C. Kidney Int., (2). 2. Anonymous US Food and Drug Administration Agency 1(k) Substantial equivalence determination decision summary device only. (FDA, Rockville, Maryland, USA, 27) < 2. Sing, T., Sander, O., Beerenwinkel, N. & Lengauer, T. ROCR: visualizing classifier performance in R. Bioinformatics 21, (2). 2. Hanley, J.A. & McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 13, 29 3 (1982). 27. DeLong, E.R., DeLong, D.M. & Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics, (1988). 28. Rosen, H. et al. Reduced immunotoxicity and preservation of antibacterial activity in a releasable side-chain carbapenem antibiotic. Science 283, 73 7 (1999). 29. Sistare, F.D. et al. Towards consensus practices to qualify safety biomarkers for use in early drug development. Nat. Biotechnol. 28, (21). 9 VOLUME 28 NUMBER MAY 21 nature biotechnology
COMMITTEE FOR HUMAN MEDICINAL PRODUCTS FINAL REPORT ON THE PILOT JOINT EMEA/FDA VXDS EXPERIENCE ON QUALIFICATION OF NEPHROTOXICITY BIOMARKERS.
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 3 July 28 Doc. Ref. EMEA/25885/28 Rev. 1 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS FINAL REPORT ON THE PILOT JOINT
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 3 July 28 Doc. Ref. EMEA/25885/28 Rev. 1 COMMITTEE FOR HUMAN MEDICINAL PRODUCTS FINAL REPORT ON THE PILOT JOINT
Pharmacogenomics/biomarker consultation
 PMDA's Summary of their Assessment Review of Seven Renal Safety Biomarkers Submitted in August 2010 by the Critical Path Institute's Predictive Safety Testing Consortium (PSTC). Unofficial English translation
PMDA's Summary of their Assessment Review of Seven Renal Safety Biomarkers Submitted in August 2010 by the Critical Path Institute's Predictive Safety Testing Consortium (PSTC). Unofficial English translation
SAFE-T consortium. DIKI BM Summary Data Package. Novel clinical biomarkers of Drug-Induced Kidney Injury
 1 SAFE-T consortium Summary Data Package Novel clinical biomarkers of Drug-Induced Kidney Injury The Drug induced kidney injury work package of Innovative Medicines Initiative SAFE-T Consortium Document
1 SAFE-T consortium Summary Data Package Novel clinical biomarkers of Drug-Induced Kidney Injury The Drug induced kidney injury work package of Innovative Medicines Initiative SAFE-T Consortium Document
Collaborative Approaches for Developing Kidney Safety Biomarkers
 Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Collaborative Approaches for Developing Kidney Safety Biomarkers John Michael Sauer, PhD Executive Director of the Predictive Safety Testing Consortium (PSTC) C-Path: A Public-Private Partnership Act as
Outline. Overview of Current Biomarkers in Kidney Disease
 Overview of Current Biomarkers in Kidney Disease Joseph V. Bonventre MD PhD Chief, Renal Division Chief, Biomedical Engineering Division Brigham and Women s Hospital Harvard Medical School Disclosure:
Overview of Current Biomarkers in Kidney Disease Joseph V. Bonventre MD PhD Chief, Renal Division Chief, Biomedical Engineering Division Brigham and Women s Hospital Harvard Medical School Disclosure:
Research Article. KIM-1 as a biomarker to predict and diagnose Acute Kidney Injury (AKI)
 Available online wwwjocprcom Journal of Chemical and Pharmaceutical Research, 216, 8(4):56-61 Research Article ISSN : 975-7384 CODEN(USA) : JCPRC5 KIM-1 as a biomarker to predict and diagnose Acute Kidney
Available online wwwjocprcom Journal of Chemical and Pharmaceutical Research, 216, 8(4):56-61 Research Article ISSN : 975-7384 CODEN(USA) : JCPRC5 KIM-1 as a biomarker to predict and diagnose Acute Kidney
Record of the Consultation on Pharmacogenomics/Biomarkers
 This English version of the record of the consultation has been published by PMDA. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. (Attachment
This English version of the record of the consultation has been published by PMDA. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. (Attachment
Conflict of Interest Statement
 Specific Aspects and Approaches for Regulatory Evaluation of Pharmaceuticals in Two-Year Rodent Carcinogenicity Studies James A. Popp Stratoxon LLC Morgantown, PA Tel: 610.286.7592 popp@stratoxon.com Conflict
Specific Aspects and Approaches for Regulatory Evaluation of Pharmaceuticals in Two-Year Rodent Carcinogenicity Studies James A. Popp Stratoxon LLC Morgantown, PA Tel: 610.286.7592 popp@stratoxon.com Conflict
Urinary biomarkers in acute kidney injury. Max Bell MD, PhD Karolinska University Hospital Solna/Karolinska Institutet
 Urinary biomarkers in acute kidney injury Max Bell MD, PhD Karolinska University Hospital Solna/Karolinska Institutet Development of AKI-biomarkers Early markers of AKI, do we need them? GFR drop Normal
Urinary biomarkers in acute kidney injury Max Bell MD, PhD Karolinska University Hospital Solna/Karolinska Institutet Development of AKI-biomarkers Early markers of AKI, do we need them? GFR drop Normal
Biomarkers of renal diseases. By Dr. Gouse Mohiddin Shaik
 By Dr. Gouse Mohiddin Shaik Introduction Renal system performs several functions Excretory Waste products like urea, creatinine, drug, toxins clearance Regulatory Water, electrolyte and acid base balance
By Dr. Gouse Mohiddin Shaik Introduction Renal system performs several functions Excretory Waste products like urea, creatinine, drug, toxins clearance Regulatory Water, electrolyte and acid base balance
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach
 Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Kidneys and Homeostasis
 16 The Urinary System The Urinary System OUTLINE: Eliminating Waste Components of the Urinary System Kidneys and Homeostasis Urination Urinary Tract Infections Eliminating Waste Excretion Elimination of
16 The Urinary System The Urinary System OUTLINE: Eliminating Waste Components of the Urinary System Kidneys and Homeostasis Urination Urinary Tract Infections Eliminating Waste Excretion Elimination of
Heart Failure and Cardio-Renal Syndrome 1: Pathophysiology. Biomarkers of Renal Injury and Dysfunction
 CRRT 2011 San Diego, CA 22-25 February 2011 Heart Failure and Cardio-Renal Syndrome 1: Pathophysiology Biomarkers of Renal Injury and Dysfunction Dinna Cruz, M.D., M.P.H. Department of Nephrology San Bortolo
CRRT 2011 San Diego, CA 22-25 February 2011 Heart Failure and Cardio-Renal Syndrome 1: Pathophysiology Biomarkers of Renal Injury and Dysfunction Dinna Cruz, M.D., M.P.H. Department of Nephrology San Bortolo
The Urinary System. BIOLOGY OF HUMANS Concepts, Applications, and Issues. Judith Goodenough Betty McGuire
 BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 16 The Urinary System Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
BIOLOGY OF HUMANS Concepts, Applications, and Issues Fifth Edition Judith Goodenough Betty McGuire 16 The Urinary System Lecture Presentation Anne Gasc Hawaii Pacific University and University of Hawaii
Discovery & Validation of Kidney Injury Biomarkers
 Dublin Academic Medical Centre Discovery & Validation of Kidney Injury Biomarkers Patrick Murray, MD, FASN, FRCPI, FJFICMI Professor, University College Dublin, Mater Misericordiae University Hospital,
Dublin Academic Medical Centre Discovery & Validation of Kidney Injury Biomarkers Patrick Murray, MD, FASN, FRCPI, FJFICMI Professor, University College Dublin, Mater Misericordiae University Hospital,
Introduction to Clinical Diagnosis Nephrology
 Introduction to Clinical Diagnosis Nephrology I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Professor of Medicine and Physiology and Functional Genomics University of Florida College
Introduction to Clinical Diagnosis Nephrology I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Professor of Medicine and Physiology and Functional Genomics University of Florida College
Surgical Pathology Report
 Louisiana State University Health Sciences Center Department of Pathology Shreveport, Louisiana Accession #: Collected: Received: Reported: 6/1/2012 09:18 6/2/2012 09:02 6/2/2012 Patient Name: Med. Rec.
Louisiana State University Health Sciences Center Department of Pathology Shreveport, Louisiana Accession #: Collected: Received: Reported: 6/1/2012 09:18 6/2/2012 09:02 6/2/2012 Patient Name: Med. Rec.
Nephron Structure inside Kidney:
 In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
In-Depth on Kidney Nephron Structure inside Kidney: - Each nephron has two capillary regions in close proximity to the nephron tubule, the first capillary bed for fluid exchange is called the glomerulus,
RENAL HISTOPATHOLOGY
 RENAL HISTOPATHOLOGY Peter McCue, M.D. Department of Pathology, Anatomy & Cell Biology Sidney Kimmel Medical College There are no conflicts of interest. 1 Goals and Objectives! Goals Provide introduction
RENAL HISTOPATHOLOGY Peter McCue, M.D. Department of Pathology, Anatomy & Cell Biology Sidney Kimmel Medical College There are no conflicts of interest. 1 Goals and Objectives! Goals Provide introduction
BCH 450 Biochemistry of Specialized Tissues
 BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
BCH 450 Biochemistry of Specialized Tissues VII. Renal Structure, Function & Regulation Kidney Function 1. Regulate Extracellular fluid (ECF) (plasma and interstitial fluid) through formation of urine.
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D.
 RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
RENAL SYSTEM 2 TRANSPORT PROPERTIES OF NEPHRON SEGMENTS Emma Jakoi, Ph.D. Learning Objectives 1. Identify the region of the renal tubule in which reabsorption and secretion occur. 2. Describe the cellular
A08 Using Kidney Biomarkers for AKI 2: Differential Diagnosis, Interventions and Prognosis
 A08 Using Kidney Biomarkers for AKI 2: Differential Diagnosis, Interventions and Prognosis Kent Doi, MD, PhD Emergency and Critical Care Medicine, The Univ of Tokyo, Japan Using kidney biomarkers: Key
A08 Using Kidney Biomarkers for AKI 2: Differential Diagnosis, Interventions and Prognosis Kent Doi, MD, PhD Emergency and Critical Care Medicine, The Univ of Tokyo, Japan Using kidney biomarkers: Key
Novel Biomarkers in Critically Ill Patients and the Emergency Room
 Novel Biomarkers in Critically Ill Patients and the Emergency Room Jay L. Koyner MD Section of Nephrology University of Chicago Research Funding: NIDDK, Abbvie, Astute, Argutus Outline Background / Pitfalls
Novel Biomarkers in Critically Ill Patients and the Emergency Room Jay L. Koyner MD Section of Nephrology University of Chicago Research Funding: NIDDK, Abbvie, Astute, Argutus Outline Background / Pitfalls
NGAL, a new markers for acute kidney injury
 NGAL, a new markers for acute kidney injury Prof. J. Delanghe, MD, PhD Dept. Clinical Chemistry Ghent University Lecture Feb 8, 2011 Serum creatinine is an inadequate marker for AKI. > 50% of renal
NGAL, a new markers for acute kidney injury Prof. J. Delanghe, MD, PhD Dept. Clinical Chemistry Ghent University Lecture Feb 8, 2011 Serum creatinine is an inadequate marker for AKI. > 50% of renal
Evaluation of KIM-1 and NGAL as Early Indicators for Assessment of Gentamycin- Induced Nephrotoxicity In Vivo and In Vitro
 2016 The Author(s). 2016 Published The Author(s) by S. Karger AG, Basel www.karger.com/kbr Published by S. Karger AG, Basel 911 www.karger.com/kbr Luo Accepted: et al.: Evaluation October 10, of 2016 Tissue
2016 The Author(s). 2016 Published The Author(s) by S. Karger AG, Basel www.karger.com/kbr Published by S. Karger AG, Basel 911 www.karger.com/kbr Luo Accepted: et al.: Evaluation October 10, of 2016 Tissue
renoprotection therapy goals 208, 209
 Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Subject Index Aldosterone, plasminogen activator inhibitor-1 induction 163, 164, 168 Aminopeptidases angiotensin II processing 64 66, 214 diabetic expression 214, 215 Angiotensin I intrarenal compartmentalization
Histopathology: Hypertension and diabetes in the kidney These presentations are to help you identify basic histopathological features.
 Histopathology: Hypertension and diabetes in the kidney These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need
Histopathology: Hypertension and diabetes in the kidney These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you need
1. Urinary System, General
 S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
S T U D Y G U I D E 16 1. Urinary System, General a. Label the figure by placing the numbers of the structures in the spaces by the correct labels. 7 Aorta 6 Kidney 8 Ureter 2 Inferior vena cava 4 Renal
Renal Disease and PK/PD. Anjay Rastogi MD PhD Division of Nephrology
 Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Renal Disease and PK/PD Anjay Rastogi MD PhD Division of Nephrology Drugs and Kidneys Kidney is one of the major organ of drug elimination from the human body Renal disease and dialysis alters the pharmacokinetics
Non-protein nitrogenous substances (NPN)
 Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Non-protein nitrogenous substances (NPN) A simple, inexpensive screening test a routine urinalysis is often the first test conducted if kidney problems are suspected. A small, randomly collected urine
Interesting case seminar: Native kidneys Case Report:
 Interesting case seminar: Native kidneys Case Report: Proximal tubulopathy and light chain deposition disease presented as severe pulmonary hypertension with right-sided cardiac dysfunction and nephrotic
Interesting case seminar: Native kidneys Case Report: Proximal tubulopathy and light chain deposition disease presented as severe pulmonary hypertension with right-sided cardiac dysfunction and nephrotic
Urinary System Laboratory
 Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Urinary System Laboratory 1 Adrenal gland Organs of The Urinary System Renal artery and vein Kidney Ureter Urinary bladder Figure 26.1 2 Urethra Functions of the urinary system organs: Urethra expels urine
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University
 Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
Renal System Dr. Naim Kittana Department of Biomedical Sciences Faculty of Medicine & Health Sciences An-Najah National University Declaration The content and the figures of this seminar were directly
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015
 RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
RNPDC CCNP Anatomy and Physiology: Renal System Pre-Quiz 2015 1. In which abdominal cavity do the kidneys lie? a) Peritoneum. b) Anteperitoneal. c) Retroperitoneal. d) Parietal peritoneal 2. What is the
A Comparison Of Diagnostic Accuracy Of Cystatin C With Creatinine In The Sample Of Patient Of T2 DM With Diabetic Nephropathy
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 7 Ver. V (July. 2017), PP 53-57 www.iosrjournals.org A Comparison Of Diagnostic Accuracy Of
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 7 Ver. V (July. 2017), PP 53-57 www.iosrjournals.org A Comparison Of Diagnostic Accuracy Of
ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
HYDRATION & EXERCISE : IMPLICATIONS FOR KIDNEY HEALTH
 64 TH AMERICAN COLLEGE OF SPORTS MEDICINE ANNUAL MEETING HYDRATION & EXERCISE : IMPLICATIONS FOR KIDNEY HEALTH May 31 st 5.30pm-7.00pm Room 103 Colorado Convention Center Denver AGENDA Session chaired
64 TH AMERICAN COLLEGE OF SPORTS MEDICINE ANNUAL MEETING HYDRATION & EXERCISE : IMPLICATIONS FOR KIDNEY HEALTH May 31 st 5.30pm-7.00pm Room 103 Colorado Convention Center Denver AGENDA Session chaired
Discovery and development of regenerative medicine products comprised of autologous cells and biomaterials. ISCT September 28, 2010 San Francisco, CA
 Discovery and development of regenerative medicine products comprised of autologous cells and biomaterials ISCT September 28, 2010 San Francisco, CA 1 Tengion s products catalyze regeneration INPUTS OUTPUTS
Discovery and development of regenerative medicine products comprised of autologous cells and biomaterials ISCT September 28, 2010 San Francisco, CA 1 Tengion s products catalyze regeneration INPUTS OUTPUTS
The principal functions of the kidneys
 Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
Renal physiology The principal functions of the kidneys Formation and excretion of urine Excretion of waste products, drugs, and toxins Regulation of body water and mineral content of the body Maintenance
RENAL FUNCTION BIOMARKERS
 HERNÁN TRIMARCHI HOSPITAL BRITÁNICO DE BUENOS AIRES ARGENTINA 2015 1 DISCLOSURES Served as a consultant and/or has received lecture honoraria from: ALEXION BRISTOL MYERS SQUIBB GENZYME NOVARTIS PFIZER
HERNÁN TRIMARCHI HOSPITAL BRITÁNICO DE BUENOS AIRES ARGENTINA 2015 1 DISCLOSURES Served as a consultant and/or has received lecture honoraria from: ALEXION BRISTOL MYERS SQUIBB GENZYME NOVARTIS PFIZER
Histopathology: Glomerulonephritis and other renal pathology
 Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
Histopathology: Glomerulonephritis and other renal pathology These presentations are to help you identify basic histopathological features. They do not contain the additional factual information that you
URINARY SYSTEM. Urinary System
 URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
URINARY SYSTEM Urinary System Kidney Functions Excretion Regulation of blood volume and pressure Regulation of electrolyte and ph levels Kidney Structure Gross Anatomy Fibrous Capsule Renal Cortex Renal
Outline Urinary System. Urinary System and Excretion. Urine. Urinary System. I. Function II. Organs of the urinary system
 Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Outline Urinary System Urinary System and Excretion Bio105 Chapter 16 Renal will be on the Final only. I. Function II. Organs of the urinary system A. Kidneys 1. Function 2. Structure III. Disorders of
Kidney injury molecule-1 expression is closely associated with renal allograft damage
 Kidney injury molecule-1 expression is closely associated with renal allograft damage Lianlian Song 1,2, Lijuan Xue 1, Jinyu Yu 1, Jun Zhao 1, Wenlan Zhang 1, Yaowen Fu 1 * 1 Urology and Nephrology Center,
Kidney injury molecule-1 expression is closely associated with renal allograft damage Lianlian Song 1,2, Lijuan Xue 1, Jinyu Yu 1, Jun Zhao 1, Wenlan Zhang 1, Yaowen Fu 1 * 1 Urology and Nephrology Center,
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology
 Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Human Physiology - Problem Drill 17: The Kidneys and Nephronal Physiology Question No. 1 of 10 Instructions: (1) Read the problem statement and answer choices carefully, (2) Work the problems on paper
Renal-Related Questions
 Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
Renal-Related Questions 1) List the major segments of the nephron and for each segment describe in a single sentence what happens to sodium there. (10 points). 2) a) Describe the handling by the nephron
EXCRETION QUESTIONS. Use the following information to answer the next two questions.
 EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
EXCRETION QUESTIONS Use the following information to answer the next two questions. 1. Filtration occurs at the area labeled A. V B. X C. Y D. Z 2. The antidiuretic hormone (vasopressin) acts on the area
The Excretory System
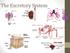 The Excretory System The excretory system The excretory system includes the skin, lungs and kidneys which all release metabolic wastes from the body. The kidneys, skin and the lungs are the principle organs
The Excretory System The excretory system The excretory system includes the skin, lungs and kidneys which all release metabolic wastes from the body. The kidneys, skin and the lungs are the principle organs
Case - Acute Renal Failure
 Case - Acute Renal Failure 73 yo diabetic F w hx of mild HBP but normal renal function develops infection of R foot. Over 1 week fever, chills, inflammation swelling of her R foot and leg. She takes Motrin
Case - Acute Renal Failure 73 yo diabetic F w hx of mild HBP but normal renal function develops infection of R foot. Over 1 week fever, chills, inflammation swelling of her R foot and leg. She takes Motrin
CHAPTER 25 URINARY. Urinary system. Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1. functions
 CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
CHAPTER 25 URINARY Kidneys 2 Ureters 2 Urinary Bladder 1 Urethra 1 fluid waste elimination secretion of wastes control blood volume and BP control blood ph electrolyte levels RBC levels hormone production
The CARI Guidelines Caring for Australasians with Renal Impairment. Specific management of IgA nephropathy: role of steroid therapy GUIDELINES
 Specific management of IgA nephropathy: role of steroid therapy Date written: July 2005 Final submission: September 2005 Author: Merlin Thomas GUIDELINES Steroid therapy may protect against progressive
Specific management of IgA nephropathy: role of steroid therapy Date written: July 2005 Final submission: September 2005 Author: Merlin Thomas GUIDELINES Steroid therapy may protect against progressive
014 Chapter 14 Created: 9:25:14 PM CST
 014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
014 Chapter 14 Created: 9:25:14 PM CST Student: 1. Functions of the kidneys include A. the regulation of body salt and water balance. B. hydrogen ion homeostasis. C. the regulation of blood glucose concentration.
Renal Transporters- pathophysiology of drug - induced renal disorders. Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November
 Renal Transporters- pathophysiology of drug - induced renal disorders Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November Renal Failure Up to 25% of acute renal failure is drug induced
Renal Transporters- pathophysiology of drug - induced renal disorders Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November Renal Failure Up to 25% of acute renal failure is drug induced
describe the location of the kidneys relative to the vertebral column:
 Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
Basic A & P II Dr. L. Bacha Chapter Outline (Martini & Nath 2010) list the three major functions of the urinary system: by examining Fig. 24-1, list the organs of the urinary system: describe the location
BIOACTIVE RENAL CELLS AUGMENT KIDNEY FUNCTION IN A RODENT MODEL OF CHRONIC KIDNEY DISEASE
 BIOACTIVE RENAL CELLS AUGMENT KIDNEY FUNCTION IN A RODENT MODEL OF CHRONIC KIDNEY DISEASE Rusty Kelley, PhD Regenerative Medicine & Biology Tengion Labs May 24, 2010 ISCT Annual Meeting, Philadelphia PA
BIOACTIVE RENAL CELLS AUGMENT KIDNEY FUNCTION IN A RODENT MODEL OF CHRONIC KIDNEY DISEASE Rusty Kelley, PhD Regenerative Medicine & Biology Tengion Labs May 24, 2010 ISCT Annual Meeting, Philadelphia PA
Professor and Director. Children s Hospital of Richmond
 Evaluation of AKI in term and premature infants Timothy E. Bunchman Professor and Director Pediatric Nephrology & Transplantation Children s Hospital of Richmond Virginia Commonwealth Univ. School of Medicine
Evaluation of AKI in term and premature infants Timothy E. Bunchman Professor and Director Pediatric Nephrology & Transplantation Children s Hospital of Richmond Virginia Commonwealth Univ. School of Medicine
Acute Kidney Injury for the General Surgeon
 Acute Kidney Injury for the General Surgeon UCSF Postgraduate Course in General Surgery Maui, HI March 20, 2011 Epidemiology & Definition Pathophysiology Clinical Studies Management Summary Hobart W. Harris,
Acute Kidney Injury for the General Surgeon UCSF Postgraduate Course in General Surgery Maui, HI March 20, 2011 Epidemiology & Definition Pathophysiology Clinical Studies Management Summary Hobart W. Harris,
Kidney Physiology. Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed
 Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Kidney Physiology Mechanisms of Urine Formation TUBULAR SECRETION Eunise A. Foster Shalonda Reed The purpose of tubular secrection To dispose of certain substances that are bound to plasma proteins. To
Copyright 2009 Pearson Education, Inc. Copyright 2009 Pearson Education, Inc. Figure 19-1c. Efferent arteriole. Juxtaglomerular apparatus
 /6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
/6/0 About this Chapter Functions of the Kidneys Anatomy of the urinary system Overview of kidney function Secretion Micturition Regulation of extracellular fluid volume and blood pressure Regulation of
The Urinary S. (Chp. 10) & Excretion. What are the functions of the urinary system? Maintenance of water-salt and acidbase
 10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
10.1 Urinary system The Urinary S. (Chp. 10) & Excretion 10.1 Urinary system What are the functions of the urinary system? 1. Excretion of metabolic wastes (urea, uric acid & creatinine) 1. Maintenance
Interest of NGAL as early marker of Acute Kidney Injury CLINIQUES UNIVERSITAIRES SAINT-LUC
 Interest of NGAL as early marker of Acute Kidney Injury P Wallemacq, Clinical Chemistry Department, M Mourad, Surgery and Abdominal Transplantation Cliniques universitaires St Luc, Université Catholique
Interest of NGAL as early marker of Acute Kidney Injury P Wallemacq, Clinical Chemistry Department, M Mourad, Surgery and Abdominal Transplantation Cliniques universitaires St Luc, Université Catholique
A. Correct! Flushing acids from the system will assist in re-establishing the acid-base equilibrium in the blood.
 OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
OAT Biology - Problem Drill 16: The Urinary System Question No. 1 of 10 1. Which of the following would solve a drop in blood ph? Question #01 (A) Decreased retention of acids. (B) Increased excretion
Nephrology - the study of the kidney. Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system
 Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Urinary System Nephrology - the study of the kidney Urology - branch of medicine dealing with the male and female urinary systems and the male reproductive system Functions of the Urinary System 1. Regulation
Chapter 11 Lecture Outline
 Chapter 11 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright 2016 McGraw-Hill Education. Permission required for reproduction
Chapter 11 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright 2016 McGraw-Hill Education. Permission required for reproduction
Kidney Functions Removal of toxins, metabolic wastes, and excess ions from the blood Regulation of blood volume, chemical composition, and ph
 The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
The Urinary System Urinary System Organs Kidneys are major excretory organs Urinary bladder is the temporary storage reservoir for urine Ureters transport urine from the kidneys to the bladder Urethra
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1
 BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
BIPN100 F15 Human Physiology (Kristan) Problem Set #8 Solutions p. 1 1. a. Proximal tubule. b. Proximal tubule. c. Glomerular endothelial fenestrae, filtration slits between podocytes of Bowman's capsule.
Na + Transport 1 and 2 Linda Costanzo, Ph.D.
 Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Na + Transport 1 and 2 Linda Costanzo, Ph.D. OBJECTIVES: After studying this lecture, the student should understand: 1. The terminology applied to single nephron function, including the meaning of TF/P
Kidney Structure. Renal Lobe = renal pyramid & overlying cortex. Renal Lobule = medullary ray & surrounding cortical labryinth.
 Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
Kidney Structure Capsule Hilum ureter renal pelvis major and minor calyxes renal and vein segmental arteries interlobar arteries arcuate arteries interlobular arteries Medulla renal pyramids cortical/renal
AKI: definitions, detection & pitfalls. Jon Murray
 AKI: definitions, detection & pitfalls Jon Murray Previous conventional definition Acute renal failure (ARF) An abrupt and sustained decline in renal excretory function due to a reduction in glomerular
AKI: definitions, detection & pitfalls Jon Murray Previous conventional definition Acute renal failure (ARF) An abrupt and sustained decline in renal excretory function due to a reduction in glomerular
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *)
 DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1
 BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
BIPN100 F15 Human Physiology (Kristan) Lecture 18: Endocrine control of renal function. p. 1 Terms you should understand by the end of this section: diuresis, antidiuresis, osmoreceptors, atrial stretch
Biomarkers for Kidney Disease
 Biomarkers for Kidney Disease Lessons From Modeling Lupus Nephritis Brad H. Rovin MD, FASN Professor of Medicine and Pathology Director, Division of Nephrology 1 DISCLOSURE STATEMENTS Dr. Rovin has the
Biomarkers for Kidney Disease Lessons From Modeling Lupus Nephritis Brad H. Rovin MD, FASN Professor of Medicine and Pathology Director, Division of Nephrology 1 DISCLOSURE STATEMENTS Dr. Rovin has the
CONTROLLING THE INTERNAL ENVIRONMENT
 AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
AP BIOLOGY ANIMAL FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROLLING THE INTERNAL ENVIRONMENT KIDNEY AND NEPHRON NEPHRON FUNCTIONS Animal Form & Function Activity #5 page 1 NEPHRON STRUCTURE NEPHRON
Cystatin C: A New Approach to Improve Medication Dosing
 Cystatin C: A New Approach to Improve Medication Dosing Erin Frazee Barreto, PharmD, MSc, FCCM Assistant Professor of Pharmacy and Medicine Kern Scholar, Center for the Science of Health Care Delivery
Cystatin C: A New Approach to Improve Medication Dosing Erin Frazee Barreto, PharmD, MSc, FCCM Assistant Professor of Pharmacy and Medicine Kern Scholar, Center for the Science of Health Care Delivery
Urinary System Organization. Urinary System Organization. The Kidneys. The Components of the Urinary System
 Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
Urinary System Organization The Golden Rule: The Job of The Urinary System is to Maintain the Composition and Volume of ECF remember this & all else will fall in place! Functions of the Urinary System
A. Incorrect! The urinary system is involved in the regulation of blood ph. B. Correct! The urinary system is involved in the synthesis of vitamin D.
 Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Human Anatomy - Problem Drill 22: The Urinary System Question No. 1 of 10 1. Which of the following statements about the functions of the urinary system is not correct? Question #01 (A) The urinary system
Cystatin C (serum, plasma, urine)
 Cystatin C (serum, plasma, urine) 1 Name and description of analyte 1.1 Name of analyte Cystatin C (serum, plasma and urine) 1.2 Alternative names Cystatin 3, post-gamma-globulin, neuroendocrine basic
Cystatin C (serum, plasma, urine) 1 Name and description of analyte 1.1 Name of analyte Cystatin C (serum, plasma and urine) 1.2 Alternative names Cystatin 3, post-gamma-globulin, neuroendocrine basic
Human Urogenital System 26-1
 Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
Human Urogenital System 26-1 Urogenital System Functions Filtering of blood, Removal of wastes and metabolites Regulation of blood volume and composition concentration of blood solutes ph of extracellular
NIH Public Access Author Manuscript Transplant Proc. Author manuscript; available in PMC 2010 July 14.
 NIH Public Access Author Manuscript Published in final edited form as: Transplant Proc. 1990 February ; 22(1): 17 20. The Effects of FK 506 on Renal Function After Liver Transplantation J. McCauley, J.
NIH Public Access Author Manuscript Published in final edited form as: Transplant Proc. 1990 February ; 22(1): 17 20. The Effects of FK 506 on Renal Function After Liver Transplantation J. McCauley, J.
2) This is a Point and Click question. You must click on the required structure.
 Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
Class: A&P2-1 Description: Test: Excretory Test Points: 144 Test Number: 28379 Printed: 31-March-10 12:03 1) This is a Point and Click question. You must click on the required structure. Click on the Bowman's
THE URINARY SYSTEM. The cases we will cover are:
 THE URINARY SYSTEM The focus of this week s lab will be pathology of the urinary system. Diseases of the kidney can be broken down into diseases that affect the glomeruli, tubules, interstitium, and blood
THE URINARY SYSTEM The focus of this week s lab will be pathology of the urinary system. Diseases of the kidney can be broken down into diseases that affect the glomeruli, tubules, interstitium, and blood
RENAL BIOMARKERS: TECHNOLOGIES AND GLOBAL MARKETS
 RENAL BIOMARKERS: TECHNOLOGIES AND GLOBAL MARKETS BIO138A January 2015 Cheng-Yuk Lee Project Analyst ISBN: 1-62296-003-3 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 024 USA 866-285-7215 (toll-free
RENAL BIOMARKERS: TECHNOLOGIES AND GLOBAL MARKETS BIO138A January 2015 Cheng-Yuk Lee Project Analyst ISBN: 1-62296-003-3 BCC Research 49 Walnut Park, Building 2 Wellesley, MA 024 USA 866-285-7215 (toll-free
Excretion and Water Balance
 Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
Excretion and Water Balance In the body, water is found in three areas, or compartments: Plasma, the liquid portion of the blood without the blood cells, makes up about 7 percent of body fluid. The intercellular
organs of the urinary system
 organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
organs of the urinary system Kidneys (2) bean-shaped, fist-sized organ where urine is formed. Lie on either sides of the vertebral column, in a depression beneath peritoneum and protected by lower ribs
Urinary System BIO 250. Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat. Routes of Waste Elimination
 Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Urinary System BIO 250 Waste Products of Metabolism Urea Carbon dioxide Inorganic salts Water Heat Routes of Waste Elimination Skin: Variable amounts of heat, salts, and water; small amounts of urea and
Urinary System kidneys, ureters, bladder & urethra
 Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Urinary System kidneys, ureters, bladder & urethra Kidney Function Filters blood removes waste products conserves salts, glucose, proteins, nutrients and water Produces urine Endocrine functions regulates
Lab Activity 31. Anatomy of the Urinary System. Portland Community College BI 233
 Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
Lab Activity 31 Anatomy of the Urinary System Portland Community College BI 233 Urinary System Organs Kidneys Urinary bladder: provides a temporary storage reservoir for urine Paired ureters: transport
THE URINARY SYSTEM. The cases we will cover are:
 THE URINARY SYSTEM The focus of this week s lab will be pathology of the urinary system. Diseases of the kidney can be broken down into diseases that affect the glomeruli, tubules, interstitium, and blood
THE URINARY SYSTEM The focus of this week s lab will be pathology of the urinary system. Diseases of the kidney can be broken down into diseases that affect the glomeruli, tubules, interstitium, and blood
Urinary System. Dr. Ahmed Maher Dr. Ahmed Manhal
 Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Urinary System Dr. Ahmed Maher Dr. Ahmed Manhal Presentation Map Kidney (cortex & medulla). Nephron. Duct system. Juxtaglomerular apparatus. Ureter, bladder & urethra. Definition & General Structure The
Excretory Lecture Test Questions Set 1
 Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Excretory Lecture Test Questions Set 1 1. The separation and ejection of metabolic wastes, usually in aqueous solution, is: a. reabsorption b. secretion c. filtration d. excretion e. endocrinology 2. Besides
Jo Abraham MD Division of Nephrology University of Utah
 Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance
 Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
Human Anatomy and Physiology - Problem Drill 23: The Urinary System, Fluid, Electrolyte and Acid-Base Balance Question No. 1 of 10 Which of the following statements about the functions of the urinary system
PRINCIPLE OF URINALYSIS
 PRINCIPLE OF URINALYSIS Vanngarm Gonggetyai Objective Can explain : the abnormalities detected in urine Can perform : routine urinalysis Can interprete : the results of urinalysis Examination of urine
PRINCIPLE OF URINALYSIS Vanngarm Gonggetyai Objective Can explain : the abnormalities detected in urine Can perform : routine urinalysis Can interprete : the results of urinalysis Examination of urine
Use the following diagram to answer the next question. 1. In the diagram above, pressure filtration occurs in a. W b. X c. Y d. Z
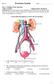 Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
Part A: Multiple Choice Questions Value: 32 Marks Suggested time: 40 minutes Instructions: For each question select the best answer and record your choice on the Scantron card provided. Using an HB pencil,
An In Vitro Alternative For Predicting Systemic Toxicity. By: James McKim, Ph.D., DABT Chief Science Officer
 An In Vitro Alternative For Predicting Systemic Toxicity By: James McKim, Ph.D., DABT Chief Science Officer What is Systemic Toxicity and What do We Want From Alternative Methods? Toxicity that occurs
An In Vitro Alternative For Predicting Systemic Toxicity By: James McKim, Ph.D., DABT Chief Science Officer What is Systemic Toxicity and What do We Want From Alternative Methods? Toxicity that occurs
Ch17-18 Urinary System
 Ch17-18 Urinary System Main Function: Filter the blood Other Functions: maintain purity and consistency of internal fluids eliminates nitrogenous wastes, toxins, and drugs from the body regulates blood
Ch17-18 Urinary System Main Function: Filter the blood Other Functions: maintain purity and consistency of internal fluids eliminates nitrogenous wastes, toxins, and drugs from the body regulates blood
Urinary Physiology. Chapter 17 Outline. Kidney Function. Chapter 17
 Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Urinary Physiology Chapter 17 Chapter 17 Outline Structure and Function of the Kidney Glomerular Filtration Reabsorption of Salt and Water Renal Plasma Clearance Renal Control of Electrolyte and Acid-Base
Renal Function and Associated Laboratory Tests
 Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
19. RENAL PHYSIOLOGY ROLE OF THE URINARY SYSTEM THE URINARY SYSTEM. Components and function. V BS 122 Physiology II 151 Class of 2011
 19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
19. RENAL PHYSIOLOGY THE URINARY SYSTEM Components and function The urinary system is composed of two kidneys, the functionally filtering apparatus, which connect through two tubular structures called
