Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
|
|
|
- Laurel Hawkins
- 5 years ago
- Views:
Transcription
1 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider Submitting laboratory: Birmingham RGC 1. Disorder/condition approved name (please provide UK spelling if different from US) and symbol as published on the OMIM database (alternative names will be listed on the UKGTN website). If NGS panel test, please provide a test name & the number of unique conditions across the whole of the panel test. If this submission is for a panel test please complete the Excel spread sheet, Appendix 1, available for download from the UKGTN website, and list all of the conditions grouped by sub panels if applicable. The full Cholestasis panel contains 24 genes for hepatic conditions presenting in the neonatal period in which cholestasis is the presenting feature. Liver conditions in neonates are rare but significant, with all conditions being potentially life limiting. Diagnosis can be difficult at such extremes of age when invasive procedures such as liver, skin and muscle biopsy are practically limited but also may not yield diagnostic certainty. A genetic diagnosis will aid timely appropriate therapy and avoid the need for such invasive procedures. Testing is divided into panel A (more common conditions and those with low GGT cholestasis) and panel B (which are the extremely rare conditions including those with neonatal sclerosing cholangitis). If genetic testing in panel A is negative then panel B will be analysed. See Appendix 1 for details 2. OMIM number for disorder/condition If a panel test see 1 above. If a number of subpanels exist with different clinical entry points e.g. cancer panel test but different subpanels for different types of cancer (breast cancer, colon, phaeochromocytoma), then please list the sub panels here providing name of each sub panel. See Appendix 1 for details. 3a. Disorder/condition to help commissioners to understand the impact of this condition please provide, in laymen s terms (e.g tubes in the kidney (renal tubule) or low sugar in the blood (hypoglycaemia), a brief (2-5 sentences/no more than 50 words) description of how the disorder(s) affect individuals and prognosis. Jaundice (yellowness in the eyes and skin) is the presenting clinical symptom of serious neonatal liver disease. Jaundice is due to the build of bilirubin due to poor bile flow. Poor bile flow through the liver results in scarring of the liver leading to liver failure. Some conditions have effective specific treatment, whilst others are life limiting requiring liver transplant and yet others are multisystem diseases in which liver transplant would be futile. An accurate diagnosis is therefore essential. 3b. Disorder/condition if required please expand on the description of the disorder provided in answer to Q3a. The majority of cholestatic conditions are due to single gene defects in genes that encode for enzymes that facilitate the movement of bile across the liver, the production of the correct type of bile or due to the build up of storage substrate within the liver so reducing the ability to excrete bile. All the conditions are rare and in the neonatal period indistinguishable by their biochemical profile and clinical features. Panel A This panel contains the conditions which are more frequent and includes the conditions:- Progressive familial intrahepatic cholestasis 1, 2, 3 and 4 and nuclear receptor FXR: these conditions present with cholestasis in the neonatal period but can also present as intermittent
2 jaundice in young adults (when it is known as benign recurrent intrahepatic cholestasis), during pregnancy (intrahepatic cholestasis of pregnancy which can be harmful for the fetus) or with gallstones in adults. When presenting in childhood the symptoms include severe fat soluble vitamin deficiency (which leads to bleeding, fractured bones, poor eye development and poor neurological development), poor nutrition which often requires supportive treatment with specialised formulas, severe pruritis (itching) which can be mutilating and the progression of liver disease to cirrhosis requiring liver transplant. In PFIC1 other organs are involved resulting in intractable diarrhoea, hearing loss and pancreatitis. In this group of conditions there is a clear management pathway, including the need for liver transplant in childhood. Arthrogryposis, Renal Dysfunction and Cholestasis Syndrome (ARC): this condition is multisystem and fatal in the first year of life. Severe cholestasis, renal failure and contractures of the arms and legs are usually present at birth. Liver biopsy in this cohort is contraindicated due to high risk of life threatening bleeding due to a platelet aggregation abnormality that cannot be detected on usual routine testing. Alpha 1 antitrypsin deficiency: this condition has a clear management pathway that includes intensive support for the liver and transplantation if necessary. Lifelong there is a risk of developing early onset emphysema. Alagille syndrome: This condition often presents with severe cholestasis in the newborn period requiring intensive management of nutrition with specialist milk formulas, management of mutilating pruritis and fat deposition within the skin (xanthomas) and severe fat soluble vitamin deficiency. This condition is a multisystem disease for which liver transplantation is an option if required. Intensive support for the liver long term is essential. The identification of the condition enables appreciation and specific management of the other organs that may be involved such as cardiac disease, kidney disease and the development of vascular aneurysms. The liver involvement occasionally may be minimal and the disorder may be first appreciated in adulthood. Niemann Pick type C: this is an extremely difficult condition to identify in the neonatal period. This is a storage disease in which the clinical presentation can be wide ranging. In the neonatal period it presents with chronic liver disease or acute liver failure. In older children or adults it presents with neurological disease including dementia and in all cases is life limiting. Liver transplantation is not usually indicated due to the multisystem life limiting features. Liver and bone marrow biopsies in the neonatal period do not often show the typical storage cells needed to make the diagnosis and hence genetic testing is more appropriate. Citrin deficiency: can present with cholestasis in newborns or with less common adult-onset disease. Further symptoms in newborns can include low blood sugar, abnormal ammonia production and fat accumulation within the liver. Poor growth is a common clinical feature and specialised milk formulas are required. In adult onset disease there is fat and iron deposition within the liver resulting in hepatitis. Panel B This panel contains the conditions that are rare but important causes of cholestasis. Bile salt synthesis disorders: there are numerous genes involved in the formation of bile and defects in any can cause clinical features. The presentation is wide ranging from severe neonatal liver failure to mild fat soluble vitamin deficiency. There are specific treatments available which can ameliorate the disease. Zellwegers syndrome: This is due to defects in the peroxisomes leading to multisystem, including neurological, disease. It is often fatal in childhood and liver transplant is contraindicated. Hereditary fructose intolerance: This is a difficult to diagnose condition that presents with hypoglycaemia, jaundice, hepatomegaly, bleeding, and renal failure. Dietary exclusion of fructose improves the clinical features. Neonatal sclerosing cholangitis: these are rare conditions resulting in rapidly progressive biliary cirrhosis and decompensated liver disease. The conditions are difficult to differentiate from other causes of cholestasis in the neonatal period. Intensive support and liver transplant are the management options.
3 4. Disorder/condition mode of inheritance If this submission is for a panel test, please complete the mode of inheritance for each condition in the Excel spread sheet appendix 1 and if there is only one mode of inheritance across all conditions, please state it here or if it varies please provide proportion split here. See Appendix 1 5. Gene approved name(s) and symbol as published on HGNC database (alternative names will be listed on the UKGTN website) If this submission is for a panel test please complete the Excel spread sheet, Appendix 1, available for download from the UKGTN website, and list all of the genes grouped by sub panels if applicable. See Appendix 1 6a. OMIM number(s) for gene(s) If a panel test see 5. above See Appendix 1 6b. HGNC number(s) for gene(s) If a panel test see 5. above See Appendix 1 7a. Gene description(s) If this submission is for a panel test, please provide total number of genes and if there are subpanels, please also list the number genes per sub panel. The panel consists of 24 genes that have been shown to be associated with one or more of the phenotypic subgroups of cholestasis (see Appendix 1): Cholestasis subpanel with all common genes (panel A) 13 genes Cholestasis subpanel with rare genes (panel B) 11 genes There are two panels both containing genes with disorders of the same phenotypes; panel A has the more common genes. If there are no mutations in panel A then panel B can be tested (following request by the referring clinician). 7b. Number of amplicons to provide this test (molecular) or type of test (cytogenetic) (n/a for panel tests) 7c. GenU band (based on 2016 version) that this test is assigned to for index case testing. For NGS panel tests if there are sub panels, please provide GenU per subpanel. Cholestasis common genes panel A GenU H Cholestasis rare genes panel B GenU G 8. Mutational spectrum for which you test including details of known common mutations (n/a for panel tests) 9a. Technical method(s) please describe the test. The enrichment method used is the Illumina TruSight One sequencing panel (TSO). TSO is used to capture and sequence the exonic regions of 4,813 genes on a HiSeq2500 sequencer, using Illumina sequencing technology. 24 samples run simultaneously, with the aim of achieving at least 20x vertical coverage. The target region is coding exons and exon/intron boundaries to include splice sites (+/-5). A total of 4,813 genes are sequenced. Only the genes associated with the phenotypes described in Appendix 1 are analysed.
4 For the analysis and filtering of the results, an in house pipeline has been developed, that uses the Ruffus framework for running and tracking the processing of multiple files simultaneously (details at section 11). Variant assessment and classification has been according to ACGS Best Practise Guidelines, and will shortly be by the ACMG Best Practise Guidelines which were recently adopted by the professional body ACGS (November 2015). All variants reported are confirmed by Sanger sequencing. 9b. For panel tests, please specify the strategy for dealing with gaps in coverage. Our in house pipeline produces a file for each patient that highlights any exons that may have not been covered to the desirable standard (20x read depth). The desired quality threshold is 97% of bases within the regions of interest are covered at least 20x. Any gaps that may be considered important will be covered by sanger sequencing, i.e. for any of the core genes: ABCB4, ABCB11, ATP8B1, NPC1, NPC2, SLC25A13, VIPAS39 and VPS33B, or in cases that there is insufficient coverage in a recessive gene which fits with the patient s phenotype and one pathogenic variant has been identified. Users will be informed if there are exons not sufficiently covered within the report, if relevant to the clinical condition. Further testing of gaps in coverage will be performed in liaison with users and on consideration of clinical presentation. This will be done by Sanger sequencing. 9c. Does the test include MLPA? (For panel tests, please provide this information in appendix 1) See Appendix 1 9d. If NGS is used, does the lab adhere to the Association of Clinical Genetic Science Best Practice Guidelines for NGS? Yes. 10. Is the assay to be provided by the lab or is it to be outsourced to another provider? If to be outsourced, please provide the name of the laboratory and a copy of their ISO certificate or their CPA number. Provided by laboratory. 11. Validation process Please explain how this test has been validated for use in your laboratory, including calculations of the sensitivity and specificity for the types of mutations reported to cause the clinical phenotype. Note that the preferred threshold for validation and verification is 95% sensitivity (with 95% Confidence Intervals). Your internal validation documentation can be submitted as an appendix (and will be included in the published Gene Dossier available on the website). The validation information should include data on establishing minimum read depth and horizontal coverage for the regions of interest, reproducibility of the pipeline, accuracy of variant calling, filtering of common variants and artefacts. If this submission is for a panel test, please provide a summary of evidence of instrument and pipeline validation and complete the tables below. If the performance of the sub panels is expected to vary significantly to the data provided, please provide further details. Instrument used: Illumina HiSeq2500 This is in use in the laboratory for other applications; it is used according to the manufacturer s instructions. There are quality checks during the run and these are performed according to manufacturer s instructions and recorded according to the laboratory practice Inter-run comparison has been carried out by using the Genome in a Bottle version 1 (GIAB) in three independent runs and one patient s sample in three independent runs; intra-run comparison was performed by the inclusion of one sample in duplicate; all results were concordant. Maintenance of the analyser and training of staff using this piece of equipment follows laboratory practice and is recorded according to UKAS standards. Analysis pipeline:
5 An in house pipeline has been developed, that uses the Ruffus framework for running and tracking the processing of multiple files simultaneously. Embedded within this framework are several programs that manipulate the data: (i) Trimmomatic for quality trimming of reads (ii) BWA mem for alignment of reads to hg19 (iii) Samblaster for duplicate marking (iv) Abra for realigning (v) Platypus for variant calling (vi) Annovar for variant annotation (vii) PLINK for IBS calculations (viii) Picard to calculate hybridisation and mapping metrics (ix) Custom Python code and bedtools to calculate coverage (x) Python pandas to produce Excel files. (xi) Samtools, vcftools, bedtools, used throughout to modify and manipulate intermediate files The pipeline was assessed by comparing the previously detected and known variants by different methods with the ones produced by this assay; pipeline v0.2.2 produced satisfactory results, identifying all known single nucleotide variants (SNVs) or insertion-deletions (in-dels) included in the coding regions of the genes of interest. No CNV controls have been tested; this assay will not test for CNVs currently, and this is clearly reported in the main body of the report. A generic validation of the Illumina TSO enrichment chemistry and the pipeline was initially performed - 4 independent runs with 89 patients. These included patients with various conditions, as well as some patients with small insertion-deletion mutations in genes associated with other conditions. All of these patients had previously been tested using Fluidigm and Roche454, TSCA and MiSeq or Sanger sequencing protocols, and had known SNVs or in-dels. Minimum read depth was established as 20x, and horizontal coverage was established by averaging read depths of regions of interest over more than 20 patients. The percentage mean coverage for each of the cholestasis-related genes is listed in the table in Appendix 1. Sensitivity 99.65% (CI 98.07% to 99.99%) Read depth minimum cut off: 20x Previously tested Number of patient samples 92 patients; 89 unique (1 tested in triplicate, 1 tested in duplicate) NGS test concordant results 1293 variants (not unique) NGS False negative 2 variants (not unique) Unique variants (total) a SNV Indel (1bp to 24bp) a CNV Details of false negative. (a) This variant is an intronic indel polymorphism in a repetitive region. The alignment tools left align (5 ) deletions/duplications whereas HGVS 3 aligns. When 5 aligned this variant falls outside our ROI so is not called by the pipeline. This is a limitation of the analysis. This variant was missed in two samples. No clinical significance for this particular variant, but this is a limitation of the technology. The Genome in a Bottle (GIAB) was run in three independent runs; all results for the 238 genes currently in service in the laboratory were concordant and are shown in the table below: 238 genes (these are genes currently in service in the laboratory) at 20x coverage, 95.7% is covered, with a stdev of 1.45% Specificity 99.65% (CI 98.07% to Run 001 Run 002 Run variants
6 Variant confirmed by other method NGS False positive Number of patient samples with a variant 92 patients; 89 unique (1 tested 1 detected by NGS in triplicate, 1 tested in duplicate) Unique variants (total) a SNV Indel (1bp to 24 bp) 51 1 CNV (a) This variant had low coverage and was seen in a low percentage of reads. Sanger confirmation is always performed prior to reporting a variant. Therefore this variant would not have been reported. The Genome in a Bottle (GIAB) reference data was filtered for variants for regions within the TSO assay, coding sequences +/-5, and was compared with our TSO GIAB run data for the same regions, and with a variant quality of >20. This was compared using Hap.py a comparison software written by Illumina: Input Name Reference SNV Precision SNV Recall Indel Precision Indel Recall TSO-GIAB-1 NIST Genome in a 99.91% 92.84% 89.15% 77.18% Bottle high confidence calls Het=4,274 Het=176 v2.18 Hom=2,765 Hom=98 TSO-GIAB-2 NIST Genome in a Bottle high confidence calls v % Het=4,269 Hom=2, % 89.82% Het=175 Hom= % TSO-GIAB-3 NIST Genome in a Bottle high confidence calls v % Het=4,252 Hom=2, % 89.23% Het=192 Hom= % This data presents the intersect and not total number of variants. The sensitivity/recall figure should be considered in context, as we would expect to see variable coverage across the whole clinical exome, and therefore that makes the comparison to a WGS gold standard difficult. This data shown above is in line with published data, if not slightly better; please see references below. This gene dossier is, however, for a small subset of genes from the clinical exome, and therefore the data above relating to detection of variants within those specific genes is more relevant. Since this original validation described above, the TSO chemistry and the pipeline have been used to screen >1000 patients for other disorders. References Linderman et al. BMC Medical Genomics 2014, 7:20 Analytical validation of whole exome and whole genome sequencing for clinical applications Cornish A & Chittibabu Guda. BioMed Research International Volume 2015 (2015), Article ID A Comparison of Variant Calling Pipelines Using Genome in a Bottle as a Reference 12a. Are you providing this test already? Yes, we have previously provided Sanger sequencing for the core genes listed and have recently moved on to NGS which includes the additional genes listed in Appendix 1. We have had Gene Dossiers for PFIC1, 2, 3, NPC1,2, citrullinemia type 2 and arthrogryposis, renal dysfunction and cholestasis approved since b. If yes, how many reports have you produced? Sanger Based Tests NGS Based Tests
7 12c. Number of reports with a pathogenic (or likely pathogenic) mutation identified? Sanger Based Tests NGS Based Tests d. Please provide the time period in which these reports have been produced and whether in a research or a full clinical diagnostic setting. All reports have been issued under a diagnostic setting, and we have been providing a diagnostic service for the core cholestasis genes since a. Is there specialised local clinical/research expertise for this disorder? Yes 13b. If yes, please provide details Dr Jane Hartley, Consultant in Paediatric Hepatology at Birmingham Children s Hospital. Dr Gideon Hirschfield, Senior Lecturer and Consultant Hepatologist at University Hospital Birmingham. 14. If using this form as an Additional Provider application, please explain why you wish to provide this test as it is already available from another provider.
8 EPIDEMIOLOGY 15. Estimated prevalence and/or incidence of conditions in the general UK population For panel tests, please provide estimates for the conditions grouped by phenotypes being tested. Prevalence is total number of persons with the condition(s) in a defined population at a specific time (i.e. new and existing cases). e.g. CF prevalence approx. 12 per 100,000 with UK population of approx. 63 million the prevalence of affected individuals in the UK is 7560 Incidence is total number of newly identified cases in a year in a defined population. e.g. CF incidence 1/2650 live births in a UK population with 724,000 live births in a year = 273 new cases a year Please identify the information on which this is based. It is currently not possible to estimate accurately the prevalence of individual cholestatic conditions in the UK population. Liver disease in childhood is rare and each individual condition is rare. Different ethnic populations within the UK will also have different incidences of the conditions. There is also marked phenotypic variability that compounds the difficulty in estimating the prevalence. There is some limited information regarding the incidence of some types of cholestasis and this is listed below. The true incidence of PFIC is not known, but it is considered a rare disease with an estimated incidence of 1/50,000 to 1/100,000 births [1]. Originally described in the Japanese population the estimated frequency of citrullineamia was 1 in 17,000 based on a carrier frequency of 1 in 65. In the UK, the overall carrier frequency is unknown but expected to be significantly lower. However, relatively high proportion of patients originate from the UK Pakistani community, hence in this ethnic group the carrier frequency might be higher than in the rest of the UK population [2]. The prevalence of NPC has been estimated at 1:150,000 in Western Europe; the prevalence in early life is probably underestimated, owing to its nonspecific presentations [3]. The incidence of ALGS is 1:30,000-1:50,000 live births, but due to the variable phenotype, it probably remains underdiagnosed [4] [1] Davit-Spraul et al. Orphanet Journal of Rare Diseases 2009, 4:1 [2] Hutchin et al. J Inherit Metab Dis. 2009;32 Suppl 1:S151 5 [3] Vanier et al. Orphanet J Rare Dis. 2010;5:16. [4] Kamath et al J Med Genet Estimated gene frequency (Carrier frequency or allele frequency) Please identify the information on which this is based. n/a for panel tests. 17. Estimated penetrance of the condition. Please identify the information on which this is based n/a for panel tests 18. Estimated prevalence of conditions in the population of people that will be tested. n/a for panel tests.
9 INTENDED USE 19. Please tick either yes or no for each clinical purpose listed. Panel Tests: a panel test would not be used for pre symptomatic testing, carrier testing and pre natal testing as the familial mutation would already be known in this case and the full panel would not be required. Diagnosis Yes No Treatment Yes No Prognosis & management Yes No Presymptomatic testing (n/a for Panel Tests) Yes No Carrier testing for family members (n/a for Panel Tests) Yes No Prenatal testing (n/a for Panel Tests) Yes No TEST CHARACTERISTICS 20. Analytical sensitivity and specificity The analytical sensitivity of a test is the proportion of positive results correctly identified by the test (true positive/true positive + false negative). The analytical specificity of a test is the proportion of negative results correctly identified by the test (true negative/true negative + false positive). This should be based on your own laboratory data for (a) the specific test being applied for or (b) the analytical sensitivity and specificity of the method/technique to be used in the case of a test yet to be set up. Please specify any types of mutations reported to cause the clinical phenotype that cannot be detected by the test. Note that the preferred threshold is 95% sensitivity (with 95% Confidence Intervals). Details in section 11. Analytical sensitivity 99.65% (CI 98.07% to 99.99%) Analytical specificity 99.65% (CI 98.07% to 99.99%) Copy number variation cannot be identified using this test 21. Clinical sensitivity and specificity of test in target population The clinical sensitivity of a test is the probability of a positive test result when condition is known to be present; the clinical specificity is the probability of a negative test result when disorder is known to be absent. The denominator in this case is the number with the disorder (for sensitivity) or the number without condition (for specificity). Please provide the best estimate. UKGTN will request actual data after one year service. For a panel test, the expected percentage diagnostic yield for the test in the target population can be presented as an alternative to clinical sensitivity and specificity? It is not currently possible to have an accurate estimation of the clinical sensitivity; if the condition is associated with one of the genes included in the panel, then the sensitivity would be >98% as CNV is expected to be very rare besides the genes that we will cover with MLPA. Based on our previous experience (Question12b and 12c), there is a positive finding in the range of 19% to 24% of the cases, however due to the recessive mode of inheritance this is not directly translated to diagnostic yield. An estimated diagnostic yield is around 10%. 22. Clinical validity (positive and negative predictive value in the target population) The clinical validity of a genetic test is a measure of how well the test predicts the presence or absence of the phenotype, clinical condition or predisposition. It is measured by its positive predictive value (the probability of getting the condition given a positive test) and negative predictive value (the probability of not getting the condition given a negative test). Not currently requested for panel tests 23. Testing pathway for tests where more than one gene is to be tested sequentially Please include your testing strategy if more than one gene will be tested and data on the expected proportions of positive results for each part of the process. Please illustrate this with a flow diagram. This will be added to the published Testing Criteria. n/a for panel tests
10 CLINICAL UTILITY 24. How will the test change the management of the patient and/or alter clinical outcome? Please summarise in 2-3 sentences no more than 50 words. An accurate diagnosis can be challenging in neonates with cholestasis and no distinguishing biochemical or clinical features. This test will provide the opportunity for an earlier diagnosis and avoid the need for invasive procedures, such as liver biopsy that is of high risk and does not provide diagnostic certainty. Earlier diagnosis enables earlier appropriate management, listing for transplant or appropriate counselling if the condition is fatal. 25. Please provide full description on likely impact on management of patient and describe associated benefits for family members. If there are any cost savings AFTER the diagnosis, please detail them here. Currently a neonate with cholestasis is provided with supportive management whilst attempts at a diagnosis are made. Making the specific diagnosis can be challenging. The infants are often poorly grown therefore making invasive procedures technically difficult. Invasive procedures may also be contraindicated when the liver disease is advanced and therefore risk of bleeding is too high. In some conditions the diagnostic yield at this young age, from liver or bone marrow, is also poor. Neonates would need to have a general anaesthetic to undergo the investigations which may then require intensive care. When a specific diagnosis is made it will enable appropriate management of the child. Some conditions have specific therapies (PFIC s, Alagille syndrome, bile salt synthesis disorders, citrullinaemia, and hereditary fructose intolerance) that need to be started in a timely manner to ameliorate the disease. Other conditions will require supportive therapy until the need for liver transplant becomes necessary. Others have severe multisystem involvement in which cases liver transplant would be futile hence extensive counselling of the family would be necessary for end of life care. An accurate diagnosis will enable specific genetic counselling for the families for the future. 26a. If this test was not available, what would be the consequences for patients and family members? Please describe in not more than 50 of words. It would take longer to make the diagnosis or a diagnosis might not ever be reached including where the condition would be fatal. A patient would need invasive investigations that may not even be diagnostic. These would cause extended anxiety for parents. There is a risk of not starting treatment in a timely manner or an inappropriate treatment may be undertaken, e.g liver transplant, this would have a direct impact on life expectancy and morbidity. There will also be a missed opportunity to provide counselling for family members. 26b. The consequences for patients and family members if this test was not available if required please expand on the response provided in question 26a. In both the adult and especially in the paediatric setting we have difficulty in obtaining samples from ill children. Our essential alternative tests such as liver biopsy may not be practically feasible due to contraindications such as coagulopathy and ascites. In ARC syndrome there is a platelet aggregation disorder and hence a biopsy would cause potentially fatal bleeding. A blood sample for DNA is always feasible and hence the genetic panels to provide a definitive diagnosis are essential. Some of the conditions are rapidly fatal hence only a blood test for DNA would be available for testing. The impact on the family to have diagnostic certainty is extremely positive. Specific counselling can be provided and support groups can be accessed. Prenatal testing may also become available for the family. For the clinician, a definite diagnosis will facilitate the counselling of the families and the appropriateness of liver transplant. This is extremely important in conditions such as Niemann Pick C and ARC syndrome in which the condition is not confined to the liver but is multisystem and the child is not expected to live outside of infancy. Therefore a liver transplant, a complex and difficult procedure, with a nationally scarce organ, would not be in the best interest of the child. Niemann Pick C is difficult to diagnose in infancy due to its heterogeneous clinical picture (although always cholestatic) and the other investigations such as liver biopsy and bone marrow biopsy may not demonstrate storage cells at such a young age.
11 27. Is there an alternative means of diagnosis or prediction that does not involve molecular diagnosis? If so (and in particular if there is a biochemical test), please state the added advantage of the molecular test. The diagnosis of PFIC can be made by immunohistological staining of a liver biopsy. The staining however requires interpretation and is not readily available. Liver biopsies only sample very small areas of the liver and in some conditions the findings may be patchy therefore biopsies, as well as clinically high risk for the patient, may not provide the diagnostic certainty that a molecular test can. Bile salt synthesis defects can be detected by urinary mass spectroscopy. This is a reliable test but can be influenced by common medications that the child may be on. It can also be extremely difficult to obtain an uncontaminated sample of urine from a neonate. Alpha 1 antitrypsin deficiency can be diagnosed by looking at the level in the blood, although it is also an acute phase response protein and therefore levels have to be interpreted with caution in acute illness. The protein phenotype can also provide a diagnosis. Niemann Pick C can be diagnosed by identifying the storage cells on liver and bone marrow biopsy. The cells however are difficult to see before the age of 3 months when many children would have presented resulting in false negative results. Filipin staining of skin fibroblasts if positive can aim making the diagnosis but again there are false negatives results. Plasma oxysterols can be diagnostic but are not useful in the neonatal period. There are clinical features associated with Alagille syndrome and ARC syndrome which would lead to a clinical diagnosis however a molecular diagnosis to aid genetic counselling is necessary as there are no other diagnostic tests. 28. Please list any genes where the main phenotype associated with that gene is unrelated to the phenotype being tested by the panel. For example, lung cancer susceptibility when testing for congenital cataract because ERCC6 gene (primarily associated with lung cancer) is included in a panel test for congenital cataract. To the best of our knowledge the genes included in this panel are not associated with any phenotype that may not be part of the referral reason. 29. If testing highlights a condition that is very different from that being tested for, please outline your strategy for dealing with this situation. 30. If a panel test, is this replacing an existing panel/multi gene test and/or other tests currently carried out by your lab e.g. Noonan Spectrum Disorders 12 Gene Panel replaced multigene Sanger test for KRAS, RAF1, PTPN11 and SOS1? If so, please provide details below. Yes, this test has replaced sanger sequencing for PFIC1,2,3, NPC1,2, citrullinemia type 2 and arthrogryposis, renal dysfunction and cholestasis. 31. Please describe any specific ethical, legal or social issues with this particular test. There are no specific ethical, legal or social issues with this particular test.
12 32. REAL LIFE CASE STUDY Please provide a case study that illustrates the benefits of this test A 6 week old baby girl born to non consanguineous Caucasian parents. She was the second child to this family all of who were healthy. At 6 weeks she had her primary vaccinations. Within an hour her leg had swelled and appeared markedly bruised. She was taken to hospital where blood tests showed her to be jaundiced, with biochemical hepatitis and her clotting was so deranged that it was unrecordable. She was referred to a tertiary hepatology centre. Her liver was supported with medium chain triglyceride fats milk and fat soluble vitamins, whilst a diagnosis was being considered. She was unable to undergo an invasive liver biopsy due to her abnormal clotting. She therefore had a sample analysed for the PFIC genes as she had a low GGT cholestasis. Compound heterozygous mutations were identified in ABCB11 so confirming the diagnosis to be PFIC2. By having this diagnosis:- 1. the child avoided an invasive and clinically high risk procedure 2. a firm diagnosis was made 3. genetic counselling regarding future pregnancies could be discussed 4. a typical clinical course could be discussed with the family including the need for liver transplant in childhood. This enabled them to have peer support through suitable charities and gain psychology help. 5. The diagnosis enabled heightened monitoring for the development of hepatocellular carcinoma 6. Specific medication to prevent severe pruritis and xanthoma formation (cholestyramine) was commenced
13 UKGTN Testing Criteria Test name: Cholestasis Disorders 24 Gene Exome Panel Approved name and symbol of disorder/condition(s): For panel tests: See website listing Approved name and symbol of gene(s): For panel tests: See website listing OMIM number(s): OMIM number(s): Referrals will only be accepted from one of the following: Referrer Consultant Adult Hepatologist Consultant Paediatric Hepatologist Consultant in Paediatric Metabolic Medicine Consultant Clinical Geneticist Tick if this refers to you. Minimum criteria required for testing to be appropriate as stated in the Gene Dossier: Criteria Neonatal conjugated hyperbilirubinaemia where multifactorial and infective causes have been excluded AND one of the following: - Low GGT cholestasis developing at any age - Intrahepatic cholestasis of pregnancy - Family history of gallstones and drug induced liver disease Tick if this patient meets criteria Additional Information: At risk family members where familial mutation is known do not require a full panel test but should be offered analysis of the known mutation. If the sample does not fulfil the clinical criteria or you are not one of the specified types of referrer and you still feel that testing should be performed please contact the laboratory to discuss testing of the sample.
14 IS IT A REASONABLE COST TO THE PUBLIC? 36. Based on experience what will be the national (UK wide) expected activity for requesting this test, per annum, for: Index cases : 120. Family members where mutation is known : 30 If a NGS panel test, it is recognised that the full panel will not be used to test family members where the familial mutation is known. Please provide expected number of tests to inform completion of Q If your laboratory does not have capacity to provide the full national need please suggest how the national requirement may be met. For example, are you aware of any other labs (UKGTN members or otherwise) offering this test to NHS patients on a local area basis only? This question has been included in order to gauge if there could be any issues in equity of access for NHS patients. If you are unable to answer this question please write unknown. The laboratory can meet the anticipated activity. 38. In order to establish the potential costs/savings that could be realised in the diagnostic care pathway, please list the tests/procedures that are no longer required to make a diagnosis for index cases where index cases would have the molecular genetic test proposed in this gene dossier at an earlier stage in the pathway. It is the tests/procedures that would be stopped for patients that are eligible for the gene test. This information will be used to calculate the overall investment / savings required in Q39 Example: The introduction of a 95 gene panel for syndromic and non syndromic hearing loss would allow those patients who are recognised early enough in their pathway to diagnosis to be offered the genetic test instead of having sequential gene tests for individual genes already available and repeated ECGs, ERGs & renal ultrasounds as part of the diagnostic pathway although these may still be required as part of management after diagnosis. Imaging procedures Laboratory pathology tests (other than molecular/cyto genetic test proposed in this Gene Dossier) Physiological tests (e.g. ECG) Other investigations/procedures (e.g. biopsy) Associated inpatient stays in the diagnostic pathway Total cost of tests/procedures to be stopped (please write n/a if the genetic test does not replace any other tests procedures in the diagnostic care pathway) If any of the tests/procedures listed above would be carried out on individuals after having the genetic test because the genetic test did not pick up a pathogenic mutation (i.e. negatives), please indicate the costs for these tests to continue to diagnosis. Type of test Cost ( ) n/a n/a For example a panel test replaces single gene tests that have been included above, but after the panel test an individual that tests negative would not need to have these single gene tests, because the genes were on the NGS panel. The test may replace other diagnostic tests such as liver biopsies but only in some patients and not all because these invasive tests are contra indicated. In the cases where a genetic diagnosis is achieved, it will influence the management of the patient and their family. For example, the identification of pathogenic mutations facilitates optimal medical
15 management to support the liver and listing for liver transplant at the best possible time. The consequence in the delay in diagnosis include prolonged liver disease, failure to thrive, recurrent hypoglycaemia, family stress. Diagnostic uncertainty can lead to reduced input of intensive nutritional support, repeated hospitalisation for invasive procedures such as liver biopsy, inability to be able to prognosticate and therefore provide advice as to timing of biliary diversion or transplantation. It will also enable the appropriate use of donated organs so as to avoid transplantation in children in whom this is a futile procedure whilst enabling the organ to be used to prolong life in another child. 39. Please complete the Excel spread sheet available to download from the UKGTN website to calculate the estimated investment or savings, based on the expected annual activity of index & family cases (Q36 above) and using the information provided in Q38. Cost neutral 40. Please indicate the healthcare outcomes that apply to this test after diagnosis. It is recognised that all tests recommended by the UKGTN for NHS service improve clinical management and, if a familial mutation is found, allows for prenatal testing and therefore these are not included in the list below. This information provides a useful guide to commissioners on the utility of the test. If there are sub panels please indicate if the same healthcare outcomes apply to all the sub panels or, if required, copy and paste the table below and complete for each sub panel. Healthcare outcomes 1. Alerts significant clinical co-morbidities Yes 2. Reduces mortality/saves lives Yes 3. Avoids irreversible harm Yes 4. Avoids diagnostic procedures/tests (some of which may be Yes invasive) and/or multiple hospital appointments 5. Avoids incorrect management (e.g. medication or treatment) Yes that could be harmful 6. Confirms targeted therapy/management Yes 7. Earlier diagnosis allowing commencement of treatment earlier Yes with associated improved prognosis 8. Enables access to educational and social support Yes 9. At risk family members that test negative for a familial mutation can be discharged from follow up n/a 10. At risk family members that test positive for a familial mutation Yes have appropriate follow up Does this apply to this test?
Submitting Laboratory: London NE RGC GOSH
 Submitting laboratory: London NE RGC GOSH 1. Disorder/condition approved name (please provide UK spelling if different from US) and symbol as published on the OMIM database (alternative names will be listed
Submitting laboratory: London NE RGC GOSH 1. Disorder/condition approved name (please provide UK spelling if different from US) and symbol as published on the OMIM database (alternative names will be listed
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease Disease alternative names please provide any alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease Disease alternative names please provide any alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Exeter RGC Approved: Sept 2013 1. Disorder/condition
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Exeter RGC Approved: Sept 2013 1. Disorder/condition
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Leber congenital amaurosis OMIM number for disease 204000 Disease alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Leber congenital amaurosis OMIM number for disease 204000 Disease alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: Cambridge RGC Approved: September 2012
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: Cambridge RGC Approved: September 2012
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Choroideremia OMIM number for disease 303100 Disease alternative names please
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Choroideremia OMIM number for disease 303100 Disease alternative names please
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name HEMOCHROMATOSIS, TYPE 4; HFE4 OMIM number for disease #606069 Disease alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name HEMOCHROMATOSIS, TYPE 4; HFE4 OMIM number for disease #606069 Disease alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Epileptic encephalopathy, early infantile 4. OMIM number for disease 612164 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Epileptic encephalopathy, early infantile 4. OMIM number for disease 612164 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Parkinson disease 8, automsomal dominant OMIM number for disease 607060 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Parkinson disease 8, automsomal dominant OMIM number for disease 607060 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Amyotrophic Lateral Sclerosis 10 (ALS10) and Amyotrophic Lateral Sclerosis 6 (ALS6)
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Amyotrophic Lateral Sclerosis 10 (ALS10) and Amyotrophic Lateral Sclerosis 6 (ALS6)
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: Birmingham RGC Approved: September 2012
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: Birmingham RGC Approved: September 2012
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease 231300 Disease alternative names Please provide any alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease 231300 Disease alternative names Please provide any alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease 147920 Disease alternative names Please provide any alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease 147920 Disease alternative names Please provide any alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider Submitting laboratory: Sheffield RGC 1. Disorder/condition approved name (please provide UK spelling
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider Submitting laboratory: Sheffield RGC 1. Disorder/condition approved name (please provide UK spelling
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names Osteogenesis Imperfecta
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names Osteogenesis Imperfecta
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: London North East RGC GOSH Approved: September
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: London North East RGC GOSH Approved: September
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Genetic Causes of Hypothyroidism 1. Loss of function mutations in TSHR cause thyroid
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Genetic Causes of Hypothyroidism 1. Loss of function mutations in TSHR cause thyroid
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Cardiff SAS Porphyria Approved: Sept
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Cardiff SAS Porphyria Approved: Sept
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Loeys-Dietz Syndrome OMIM number for disease 609192; 608967; 610380; 610168 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Loeys-Dietz Syndrome OMIM number for disease 609192; 608967; 610380; 610168 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider Submitting laboratory: Leeds RGC 1. Disorder/condition approved name (please provide UK spelling if different
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider Submitting laboratory: Leeds RGC 1. Disorder/condition approved name (please provide UK spelling if different
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Submitting laboratory: Bristol RGC 1. Disorder/condition approved name and symbol as published on the OMIM database (alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Submitting laboratory: Bristol RGC 1. Disorder/condition approved name and symbol as published on the OMIM database (alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: London North East RGC GOSH Approved: September
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: London North East RGC GOSH Approved: September
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS (PFIC)
 The Childhood Liver Disease Research Network strives to provide information and support to individuals and families affected by liver disease through its many research programs. PROGRESSIVE FAMILIAL INTRAHEPATIC
The Childhood Liver Disease Research Network strives to provide information and support to individuals and families affected by liver disease through its many research programs. PROGRESSIVE FAMILIAL INTRAHEPATIC
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
NGS Types of gene dossier applications UKGTN can evaluate
 NGS Types of gene dossier applications UKGTN can evaluate Jo Whittaker on behalf of the Genetic Test Evaluation Working Group & UKGTN Project team NGS used in a number of ways Replacement technology for
NGS Types of gene dossier applications UKGTN can evaluate Jo Whittaker on behalf of the Genetic Test Evaluation Working Group & UKGTN Project team NGS used in a number of ways Replacement technology for
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: Manchester RGC Approved: September 2013
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISEASE/CONDITION POPULATION TRIAD Submitting laboratory: Manchester RGC Approved: September 2013
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider Submitting laboratory: London NE RGC GOSH 1. Disorder/condition approved name (please provide UK spelling
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider Submitting laboratory: London NE RGC GOSH 1. Disorder/condition approved name (please provide UK spelling
Application to be an additional provider for existing test on the NHS Directory of Molecular Genetic Testing Additional Provider form
 Application to be an additional provider for existing test on the NHS Directory of Molecular Genetic Testing Additional Provider form Disease: Gene: Cystic Fibrosis (CF) (carrier testing in reproductive
Application to be an additional provider for existing test on the NHS Directory of Molecular Genetic Testing Additional Provider form Disease: Gene: Cystic Fibrosis (CF) (carrier testing in reproductive
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) Glucocorticoid-remediable
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) Glucocorticoid-remediable
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Polycystic Kidney Disease, Autosomal Dominant OMIM number for disease 173900 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Polycystic Kidney Disease, Autosomal Dominant OMIM number for disease 173900 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names you wish listed) (A)-Testing
Corporate Medical Policy
 Corporate Medical Policy Invasive Prenatal (Fetal) Diagnostic Testing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: invasive_prenatal_(fetal)_diagnostic_testing 12/2014 3/2018
Corporate Medical Policy Invasive Prenatal (Fetal) Diagnostic Testing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: invasive_prenatal_(fetal)_diagnostic_testing 12/2014 3/2018
BILIARY ATRESIA. What is biliary atresia?
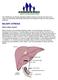 The Childhood Liver Disease Research Network strives to provide information and support to individuals and families affected by liver disease through its many research programs. BILIARY ATRESIA What is
The Childhood Liver Disease Research Network strives to provide information and support to individuals and families affected by liver disease through its many research programs. BILIARY ATRESIA What is
Genetics/Genomics: role of genes in diagnosis and/risk and in personalised medicine
 Genetics/Genomics: role of genes in diagnosis and/risk and in personalised medicine Lynn Greenhalgh, Macmillan Cancer and General Clinical Geneticist Cancer Genetics Service Cancer is common 1 in
Genetics/Genomics: role of genes in diagnosis and/risk and in personalised medicine Lynn Greenhalgh, Macmillan Cancer and General Clinical Geneticist Cancer Genetics Service Cancer is common 1 in
Benefits and pitfalls of new genetic tests
 Benefits and pitfalls of new genetic tests Amanda Krause Division of Human Genetics, NHLS and University of the Witwatersrand Definition of Genetic Testing the analysis of human DNA, RNA, chromosomes,
Benefits and pitfalls of new genetic tests Amanda Krause Division of Human Genetics, NHLS and University of the Witwatersrand Definition of Genetic Testing the analysis of human DNA, RNA, chromosomes,
Golden Helix s End-to-End Solution for Clinical Labs
 Golden Helix s End-to-End Solution for Clinical Labs Steven Hystad - Field Application Scientist Nathan Fortier Senior Software Engineer 20 most promising Biotech Technology Providers Top 10 Analytics
Golden Helix s End-to-End Solution for Clinical Labs Steven Hystad - Field Application Scientist Nathan Fortier Senior Software Engineer 20 most promising Biotech Technology Providers Top 10 Analytics
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Bristol RGC Approved: Sept 2013 1. Disorder/condition
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier/Additional Provider TEST DISORDER/CONDITION POPULATION TRIAD Submitting laboratory: Bristol RGC Approved: Sept 2013 1. Disorder/condition
No mutations were identified.
 Hereditary High Cholesterol Test ORDERING PHYSICIAN PRIMARY CONTACT SPECIMEN Report date: Aug 1, 2017 Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA Kelly Peters Sample Medical Group 123
Hereditary High Cholesterol Test ORDERING PHYSICIAN PRIMARY CONTACT SPECIMEN Report date: Aug 1, 2017 Dr. Jenny Jones Sample Medical Group 123 Main St. Sample, CA Kelly Peters Sample Medical Group 123
MEDICAL GENOMICS LABORATORY. Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG)
 Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits
 AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
AVENIO family of NGS oncology assays ctdna and Tumor Tissue Analysis Kits Accelerating clinical research Next-generation sequencing (NGS) has the ability to interrogate many different genes and detect
CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE. Dr. Bahar Naghavi
 2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
Metabolic Liver Disease
 Metabolic Liver Disease Peter Eichenseer, MD No relationships to disclose. Outline Overview Alpha-1 antitrypsin deficiency Wilson s disease Hereditary hemochromatosis Pathophysiology Clinical features
Metabolic Liver Disease Peter Eichenseer, MD No relationships to disclose. Outline Overview Alpha-1 antitrypsin deficiency Wilson s disease Hereditary hemochromatosis Pathophysiology Clinical features
Assessing Laboratory Performance for Next Generation Sequencing Based Detection of Germline Variants through Proficiency Testing
 Assessing Laboratory Performance for Next Generation Sequencing Based Detection of Germline Variants through Proficiency Testing Karl V. Voelkerding, MD Professor of Pathology University of Utah Medical
Assessing Laboratory Performance for Next Generation Sequencing Based Detection of Germline Variants through Proficiency Testing Karl V. Voelkerding, MD Professor of Pathology University of Utah Medical
CYSTIC FIBROSIS. The condition:
 CYSTIC FIBROSIS Both antenatal and neonatal screening for CF have been considered. Antenatal screening aims to identify fetuses affected by CF so that parents can be offered an informed choice as to whether
CYSTIC FIBROSIS Both antenatal and neonatal screening for CF have been considered. Antenatal screening aims to identify fetuses affected by CF so that parents can be offered an informed choice as to whether
Alpha-1 Antitrypsin Deficiency: Liver Disease
 Alpha-1 Antitrypsin Deficiency: Liver Disease Who is at risk to develop Alpha-1 liver disease? Alpha-1 liver disease may affect children and adults who have abnormal Alpha-1 antitrypsin genes. Keys to
Alpha-1 Antitrypsin Deficiency: Liver Disease Who is at risk to develop Alpha-1 liver disease? Alpha-1 liver disease may affect children and adults who have abnormal Alpha-1 antitrypsin genes. Keys to
Performance Characteristics BRCA MASTR Plus Dx
 Performance Characteristics BRCA MASTR Plus Dx with drmid Dx for Illumina NGS systems Manufacturer Multiplicom N.V. Galileïlaan 18 2845 Niel Belgium Table of Contents 1. Workflow... 4 2. Performance Characteristics
Performance Characteristics BRCA MASTR Plus Dx with drmid Dx for Illumina NGS systems Manufacturer Multiplicom N.V. Galileïlaan 18 2845 Niel Belgium Table of Contents 1. Workflow... 4 2. Performance Characteristics
Dhanpat Jain Yale University School of Medicine, New Haven, CT
 Dhanpat Jain Yale University School of Medicine, New Haven, CT Case history 15 years old female presented with fatigue. Found to have features suggestive of cirrhosis with esophageal varices, splenomegaly
Dhanpat Jain Yale University School of Medicine, New Haven, CT Case history 15 years old female presented with fatigue. Found to have features suggestive of cirrhosis with esophageal varices, splenomegaly
Amino Acid Disorders. Disorder name: Tyrosinemia, type 1. What is tyrosinemia 1? Genetic Fact Sheets for Parents
 Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease 312060 Disease alternative names please provide any alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name OMIM number for disease 312060 Disease alternative names please provide any alternative
Form 3. Template for a full review process for a condition being considered for addition to the newborn/child screening panel
 Form 3. Template for a full review process for a condition being considered for addition to the newborn/child screening panel **Note: please specify the basis for each answer and rely on published evidence
Form 3. Template for a full review process for a condition being considered for addition to the newborn/child screening panel **Note: please specify the basis for each answer and rely on published evidence
Practical challenges that copy number variation and whole genome sequencing create for genetic diagnostic labs
 Practical challenges that copy number variation and whole genome sequencing create for genetic diagnostic labs Joris Vermeesch, Center for Human Genetics K.U.Leuven, Belgium ESHG June 11, 2010 When and
Practical challenges that copy number variation and whole genome sequencing create for genetic diagnostic labs Joris Vermeesch, Center for Human Genetics K.U.Leuven, Belgium ESHG June 11, 2010 When and
AVENIO ctdna Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB
 Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
Analysis Kits The complete NGS liquid biopsy solution EMPOWER YOUR LAB Analysis Kits Next-generation performance in liquid biopsies 2 Accelerating clinical research From liquid biopsy to next-generation
MEDICAL GENOMICS LABORATORY. Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG)
 Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with receipt) Saliva (OGR-575 DNA Genotek;
Peripheral Nerve Sheath Tumor Panel by Next-Gen Sequencing (PNT-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with receipt) Saliva (OGR-575 DNA Genotek;
Below, we included the point-to-point response to the comments of both reviewers.
 To the Editor and Reviewers: We would like to thank the editor and reviewers for careful reading, and constructive suggestions for our manuscript. According to comments from both reviewers, we have comprehensively
To the Editor and Reviewers: We would like to thank the editor and reviewers for careful reading, and constructive suggestions for our manuscript. According to comments from both reviewers, we have comprehensively
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Submitting laboratory: Bristol RGC 1. Disorder/condition approved name and symbol as published on the OMIM database (alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Submitting laboratory: Bristol RGC 1. Disorder/condition approved name and symbol as published on the OMIM database (alternative
MEDICAL GENOMICS LABORATORY. Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG)
 Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with
Non-NF1 RASopathy panel by Next-Gen Sequencing and Deletion/Duplication Analysis of SPRED1 (NNP-NG) Ordering Information Acceptable specimen types: Blood (3-6ml EDTA; no time limitations associated with
EVALUATION & LISTING. Your Child s Liver Transplant Evaluation. What is the Liver?
 EVALUATION & LISTING Your Child s Liver Transplant Evaluation The University of Michigan is a national leader in liver transplantation, as well as the surgical and medical management of patients with liver
EVALUATION & LISTING Your Child s Liver Transplant Evaluation The University of Michigan is a national leader in liver transplantation, as well as the surgical and medical management of patients with liver
What Is Cirrhosis? CIRRHOSIS. Cirrhosis occurs when the liver is. by chronic conditions and diseases. permanently scarred or injured
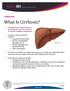 What Is Cirrhosis? Cirrhosis occurs when the liver is permanently scarred or injured by chronic conditions and diseases. Common causes of cirrhosis include: Long-term alcohol abuse. Chronic viral hepatitis
What Is Cirrhosis? Cirrhosis occurs when the liver is permanently scarred or injured by chronic conditions and diseases. Common causes of cirrhosis include: Long-term alcohol abuse. Chronic viral hepatitis
Breast and ovarian cancer in Serbia: the importance of mutation detection in hereditary predisposition genes using NGS
 Breast and ovarian cancer in Serbia: the importance of mutation detection in hereditary predisposition genes using NGS dr sc. Ana Krivokuća Laboratory for molecular genetics Institute for Oncology and
Breast and ovarian cancer in Serbia: the importance of mutation detection in hereditary predisposition genes using NGS dr sc. Ana Krivokuća Laboratory for molecular genetics Institute for Oncology and
YES NO UNKNOWN PENETRANCE ACTIONABILITY SIGNIFICANCE/BURDEN OF DISEASE NEXT STEPS. YES (Proceed to Stage II) YES ( 1 of above)
 Stage I: Rule-Out Dashboard GENE/GENE PANEL: ATP7B DISORDER: Wilson Disease HGNC ID: 870 OMIM ID: 277900 ACTIONABILITY 1. Is there a qualifying resource, such as a practice guideline or systematic review,
Stage I: Rule-Out Dashboard GENE/GENE PANEL: ATP7B DISORDER: Wilson Disease HGNC ID: 870 OMIM ID: 277900 ACTIONABILITY 1. Is there a qualifying resource, such as a practice guideline or systematic review,
DNA-seq Bioinformatics Analysis: Copy Number Variation
 DNA-seq Bioinformatics Analysis: Copy Number Variation Elodie Girard elodie.girard@curie.fr U900 institut Curie, INSERM, Mines ParisTech, PSL Research University Paris, France NGS Applications 5C HiC DNA-seq
DNA-seq Bioinformatics Analysis: Copy Number Variation Elodie Girard elodie.girard@curie.fr U900 institut Curie, INSERM, Mines ParisTech, PSL Research University Paris, France NGS Applications 5C HiC DNA-seq
Alpha-1 Liver Disease
 Alpha-1 Liver Disease Jeffrey Teckman, M.D. Professor of Pediatrics and Biochemistry Associate Chair of Pediatrics for Research Director, Pediatric Gastroenterology and Hepatology St. Louis University
Alpha-1 Liver Disease Jeffrey Teckman, M.D. Professor of Pediatrics and Biochemistry Associate Chair of Pediatrics for Research Director, Pediatric Gastroenterology and Hepatology St. Louis University
Dr Rick Tearle Senior Applications Specialist, EMEA Complete Genomics Complete Genomics, Inc.
 Dr Rick Tearle Senior Applications Specialist, EMEA Complete Genomics Topics Overview of Data Processing Pipeline Overview of Data Files 2 DNA Nano-Ball (DNB) Read Structure Genome : acgtacatgcattcacacatgcttagctatctctcgccag
Dr Rick Tearle Senior Applications Specialist, EMEA Complete Genomics Topics Overview of Data Processing Pipeline Overview of Data Files 2 DNA Nano-Ball (DNB) Read Structure Genome : acgtacatgcattcacacatgcttagctatctctcgccag
Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research
 Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research Application Note Authors John McGuigan, Megan Manion,
Mutation Detection and CNV Analysis for Illumina Sequencing data from HaloPlex Target Enrichment Panels using NextGENe Software for Clinical Research Application Note Authors John McGuigan, Megan Manion,
Hepatocytes produce. Proteins Clotting factors Hormones. Bile Flow
 R.J.Bailey MD Hepatocytes produce Proteins Clotting factors Hormones Bile Flow Trouble.. for the liver! Trouble for the Liver Liver Gall Bladder Common Alcohol Hep C Fatty Liver Cancer Drugs Viruses Uncommon
R.J.Bailey MD Hepatocytes produce Proteins Clotting factors Hormones Bile Flow Trouble.. for the liver! Trouble for the Liver Liver Gall Bladder Common Alcohol Hep C Fatty Liver Cancer Drugs Viruses Uncommon
Identifying Mutations Responsible for Rare Disorders Using New Technologies
 Identifying Mutations Responsible for Rare Disorders Using New Technologies Jacek Majewski, Department of Human Genetics, McGill University, Montreal, QC Canada Mendelian Diseases Clear mode of inheritance
Identifying Mutations Responsible for Rare Disorders Using New Technologies Jacek Majewski, Department of Human Genetics, McGill University, Montreal, QC Canada Mendelian Diseases Clear mode of inheritance
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
Introduction of an NGS gene panel into the Haemato-Oncology MPN service
 Introduction of an NGS gene panel into the Haemato-Oncology MPN service Dr. Anna Skowronska, Dr Jane Bryon, Dr Samuel Clokie, Dr Yvonne Wallis and Professor Mike Griffiths West Midlands Regional Genetics
Introduction of an NGS gene panel into the Haemato-Oncology MPN service Dr. Anna Skowronska, Dr Jane Bryon, Dr Samuel Clokie, Dr Yvonne Wallis and Professor Mike Griffiths West Midlands Regional Genetics
Genetics update and implications for (General) Practice
 Genetics update and implications for (General) Practice May 12 th 2018 Women s Health Symposium Clearwater Estate Dr Kate Gibson MB BCh, MRCP, FRACP Topics NZ Clinical Genetics delivery New Technologies
Genetics update and implications for (General) Practice May 12 th 2018 Women s Health Symposium Clearwater Estate Dr Kate Gibson MB BCh, MRCP, FRACP Topics NZ Clinical Genetics delivery New Technologies
Investigating rare diseases with Agilent NGS solutions
 Investigating rare diseases with Agilent NGS solutions Chitra Kotwaliwale, Ph.D. 1 Rare diseases affect 350 million people worldwide 7,000 rare diseases 80% are genetic 60 million affected in the US, Europe
Investigating rare diseases with Agilent NGS solutions Chitra Kotwaliwale, Ph.D. 1 Rare diseases affect 350 million people worldwide 7,000 rare diseases 80% are genetic 60 million affected in the US, Europe
Cryptogenic Cirrhosis: An Approach To The Diagnosis In The Era Of Molecular and Genomic Medicine
 Cryptogenic Cirrhosis: An Approach To The Diagnosis In The Era Of Molecular and Genomic Medicine Introduction and historical perspective: Cryptogenic cirrhosis(cc) is defined as cases of cirrhosis where
Cryptogenic Cirrhosis: An Approach To The Diagnosis In The Era Of Molecular and Genomic Medicine Introduction and historical perspective: Cryptogenic cirrhosis(cc) is defined as cases of cirrhosis where
How many disease-causing variants in a normal person? Matthew Hurles
 How many disease-causing variants in a normal person? Matthew Hurles Summary What is in a genome? What is normal? Depends on age What is a disease-causing variant? Different classes of variation Final
How many disease-causing variants in a normal person? Matthew Hurles Summary What is in a genome? What is normal? Depends on age What is a disease-causing variant? Different classes of variation Final
September 23, The Role of In Vitro Diagnostic Tests in Pediatric Master Protocol Development
 The Role of In Vitro Diagnostic Tests in Pediatric Master Protocol Development September 23, 2016 Anand Pathak, MD, PhD, MPH Medical Officer Molecular Genetics Branch Division of Molecular Genetics and
The Role of In Vitro Diagnostic Tests in Pediatric Master Protocol Development September 23, 2016 Anand Pathak, MD, PhD, MPH Medical Officer Molecular Genetics Branch Division of Molecular Genetics and
Fatty Acid Oxidation Disorders
 Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
The Deciphering Development Disorders (DDD) project: What a genomic approach can achieve
 The Deciphering Development Disorders (DDD) project: What a genomic approach can achieve RCP ADVANCED MEDICINE, LONDON FEB 5 TH 2018 HELEN FIRTH DM FRCP DCH, SANGER INSTITUTE 3,000,000,000 bases in each
The Deciphering Development Disorders (DDD) project: What a genomic approach can achieve RCP ADVANCED MEDICINE, LONDON FEB 5 TH 2018 HELEN FIRTH DM FRCP DCH, SANGER INSTITUTE 3,000,000,000 bases in each
Approach to a case of Neonatal Cholestasis
 Approach to a case of Neonatal Cholestasis Ira Shah (Co-Incharge, Consultant Pediatrician, Pediatric Liver Clinic) Gunjan Narkhede (Resident in Pediatric Liver Clinic) Pediatric Hepatobiliary Clinic, B.J.Wadia
Approach to a case of Neonatal Cholestasis Ira Shah (Co-Incharge, Consultant Pediatrician, Pediatric Liver Clinic) Gunjan Narkhede (Resident in Pediatric Liver Clinic) Pediatric Hepatobiliary Clinic, B.J.Wadia
Community and Mental Health Services. Palliative Care. Criteria and
 Community and Mental Health Services Specialist Palliative Care Service Referral Criteria and Guidance November 2018 Specialist Palliative Care Service Referrals These guidelines cover referrals for patients
Community and Mental Health Services Specialist Palliative Care Service Referral Criteria and Guidance November 2018 Specialist Palliative Care Service Referrals These guidelines cover referrals for patients
Biliary Atresia. Who is at risk for biliary atresia?
 Biliary Atresia Biliary atresia is a life-threatening condition in infants in which the bile ducts inside or outside the liver do not have normal openings. Bile ducts in the liver, also called hepatic
Biliary Atresia Biliary atresia is a life-threatening condition in infants in which the bile ducts inside or outside the liver do not have normal openings. Bile ducts in the liver, also called hepatic
Talking Genomes with Your Patients. Meagan Cochran, MS, CGC Certified Genetic Counselor HudsonAlpha Institute for Biotechnology
 Talking Genomes with Your Patients Meagan Cochran, MS, CGC Certified Genetic Counselor HudsonAlpha Institute for Biotechnology Objectives Review the importance of physician familiarity with genomic testing
Talking Genomes with Your Patients Meagan Cochran, MS, CGC Certified Genetic Counselor HudsonAlpha Institute for Biotechnology Objectives Review the importance of physician familiarity with genomic testing
NHS public health functions agreement Service specification No.1 Neonatal hepatitis B immunisation programme
 NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
NHS public health functions agreement 2017-18 Service specification No.1 Neonatal hepatitis B immunisation programme NHS public health functions agreement 2017-18 Service specification No.1Neonatal hepatitis
Chapter 26. Newborn Screening
 Chapter 26 Newborn Screening Severe Combined Immune Deficiency (SCID) leads to life-threatening infections unless the immune system can be restored through a bone marrow transplant, enzyme replacement
Chapter 26 Newborn Screening Severe Combined Immune Deficiency (SCID) leads to life-threatening infections unless the immune system can be restored through a bone marrow transplant, enzyme replacement
Disorder name: Argininemia / Arginase deficiency Acronym: ARG 1 deficiency
 Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Genetic Fact Sheets for Parents Amino Acid Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues
Molecular Pathology Evaluation Panel and Molecular Pathology Consortium Advice Note
 Molecular Pathology Evaluation Panel and Molecular Pathology Consortium Advice Note MPEP/MPC Advice Note 2016-02 June 2016 Test evaluated: Tumour Protein p53 (TP53) Molecular Pathology Evaluation Panel
Molecular Pathology Evaluation Panel and Molecular Pathology Consortium Advice Note MPEP/MPC Advice Note 2016-02 June 2016 Test evaluated: Tumour Protein p53 (TP53) Molecular Pathology Evaluation Panel
PROGRESS: Beginning to Understand the Genetic Predisposition to PSC
 PROGRESS: Beginning to Understand the Genetic Predisposition to PSC Konstantinos N. Lazaridis, MD Associate Professor of Medicine Division of Gastroenterology and Hepatology Associate Director Center for
PROGRESS: Beginning to Understand the Genetic Predisposition to PSC Konstantinos N. Lazaridis, MD Associate Professor of Medicine Division of Gastroenterology and Hepatology Associate Director Center for
GATA6 Syndrome. rarechromo.org
 GATA6 Syndrome rarechromo.org What is GATA6 syndrome? GATA6 syndrome occurs when one of a person s two copies of the GATA6 gene doesn t work as it should. Our DNA is formed by 3 billion letters that contain
GATA6 Syndrome rarechromo.org What is GATA6 syndrome? GATA6 syndrome occurs when one of a person s two copies of the GATA6 gene doesn t work as it should. Our DNA is formed by 3 billion letters that contain
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Highly Specialised Technology Evaluation
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
General Guidelines for Health professionals
 General Guidelines for Health professionals Page 1 Haemochromatosis Introduction Hereditary haemochromatosis (HH) now easily screened for as most symptomatic individuals are homozygous for the C282Y mutation
General Guidelines for Health professionals Page 1 Haemochromatosis Introduction Hereditary haemochromatosis (HH) now easily screened for as most symptomatic individuals are homozygous for the C282Y mutation
CentoXome FUTURE'S KNOWLEDGE APPLIED TODAY
 CentoXome FUTURE'S KNOWLEDGE APPLIED TODAY More genetic information requires cutting-edge interpretation techniques Whole Exome Sequencing For some patients, the combination of symptoms does not allow
CentoXome FUTURE'S KNOWLEDGE APPLIED TODAY More genetic information requires cutting-edge interpretation techniques Whole Exome Sequencing For some patients, the combination of symptoms does not allow
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary tract infection: diagnosis, treatment and long-term management of urinary tract infection in children 1.1 Short title
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary tract infection: diagnosis, treatment and long-term management of urinary tract infection in children 1.1 Short title
SETPEG GENETIC TESTING GUIDELINES Version 1.0, 5 th October 2017
 SETPEG GENETIC TESTING GUIDELINES Version 1.0, 5 th October 2017 1. The Epilepsy Genetic Diagnostic & Counselling Service at King s Health Partners Professor Deb Pal PhD MRCP (Consultant) deb.pal@nhs.net
SETPEG GENETIC TESTING GUIDELINES Version 1.0, 5 th October 2017 1. The Epilepsy Genetic Diagnostic & Counselling Service at King s Health Partners Professor Deb Pal PhD MRCP (Consultant) deb.pal@nhs.net
Fatty Acid Oxidation Disorders
 Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Specialist Palliative Care Service Referral Criteria and Guidance
 Specialist Palliative Care Service Referral Criteria and Guidance Specialist Palliative Care Service Referrals These guidelines cover referrals for patients with progressive terminal illness, whether
Specialist Palliative Care Service Referral Criteria and Guidance Specialist Palliative Care Service Referrals These guidelines cover referrals for patients with progressive terminal illness, whether
Framework on the feedback of health-related findings in research March 2014
 Framework on the feedback of health-related findings in research March 2014 Summary Scope and background In the course of a study involving human participants, researchers may make a finding that has potential
Framework on the feedback of health-related findings in research March 2014 Summary Scope and background In the course of a study involving human participants, researchers may make a finding that has potential
Analysis with SureCall 2.1
 Analysis with SureCall 2.1 Danielle Fletcher Field Application Scientist July 2014 1 Stages of NGS Analysis Primary analysis, base calling Control Software FASTQ file reads + quality 2 Stages of NGS Analysis
Analysis with SureCall 2.1 Danielle Fletcher Field Application Scientist July 2014 1 Stages of NGS Analysis Primary analysis, base calling Control Software FASTQ file reads + quality 2 Stages of NGS Analysis
NGS panels in clinical diagnostics: Utrecht experience. Van Gijn ME PhD Genome Diagnostics UMCUtrecht
 NGS panels in clinical diagnostics: Utrecht experience Van Gijn ME PhD Genome Diagnostics UMCUtrecht 93 Gene panels UMC Utrecht Cardiovascular disease (CAR) (5 panels) Epilepsy (EPI) (11 panels) Hereditary
NGS panels in clinical diagnostics: Utrecht experience Van Gijn ME PhD Genome Diagnostics UMCUtrecht 93 Gene panels UMC Utrecht Cardiovascular disease (CAR) (5 panels) Epilepsy (EPI) (11 panels) Hereditary
Disorder name: Severe Combined Immunodeficiency Acronym: SCID
 Genetic Fact Sheets for Parents Other Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues surrounding
Genetic Fact Sheets for Parents Other Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social issues surrounding
GENETIC TESTING AND COUNSELING FOR HERITABLE DISORDERS
 Status Active Medical and Behavioral Health Policy Section: Laboratory Policy Number: VI-09 Effective Date: 03/17/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
Status Active Medical and Behavioral Health Policy Section: Laboratory Policy Number: VI-09 Effective Date: 03/17/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members
