MEDICAL POLICY SUBJECT: PHOTODYNAMIC THERAPY FOR EFFECTIVE DATE: 07/20/00 SUBFOVEAL CHOROIDAL NEOVASCULARIZATION
|
|
|
- Claribel Harvey
- 5 years ago
- Views:
Transcription
1 MEDICAL POLICY SUBJECT: PHOTODYNAMIC THERAPY FOR PAGE: 1 OF: 8 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an Essential Plan product, covers a specific service, medical policy criteria apply to the benefit. If a Medicare product covers a specific service, and there is no national or local Medicare coverage decision for the service, medical policy criteria apply to the benefit. POLICY STATEMENT: I. Based upon our criteria and assessment of peer-reviewed literature, photodynamic therapy with verteporfin has been medically proven effective and therefore medically appropriate for patients with a diagnosis of subfoveal choroidal neovascularization (CNV) associated with the following conditions: A. Age-related macular degeneration; B. Pathologic myopia; C. Chronic central serous chorioretinopathy; D. Choroidal hemangioma; or E. Ocular histoplasmosis syndrome. II. Based upon our criteria and assessment of peer-reviewed literature, photodynamic therapy with Verteporfin as yet, has not demonstrated a benefit to patient outcomes and is considered investigational as a treatment for patients with CNV for any other ophthalmologic condition not listed above. Refer to Corporate Medical Policy # regarding Photodynamic Therapy for Malignant Conditions. Refer to Corporate Medical Policy # Experimental and Investigational Services. Refer to FLRx drug policy regarding Neovascular (Wet) Age Related Macular Degeneration (Magugen :pegaptanib, Lucentis : ranibizumab, Avastin : benacizumab). POLICY GUIDELINES: I. Photodynamic therapy with verteporfin must be administered by an ophthalmologist who has completed a fellowship in vitreoretinal diseases and surgery. II. The specialist should evaluate the patient every 3 months. Repeat PDT therapy may be necessary to achieve optimal visual acuity if the abnormal blood vessels re-leak. On the average, 3-4 treatments are necessary during the first year of therapy with approximately 2 treatments during the second year. III. Verteporfin photodynamic therapy combined with ranibizumab/lucentis or bevacizumab/avastin will be considered in patients with predominantly classic lesions. Refer to FLRx drug policy regarding Neovascular (Wet) Age Related Macular Degeneration (Magugen :pegaptanib, Lucentis : ranibizumab, Avastin : benacizumab). IV. The Federal Employee Health Benefit Program (FEHBP/FEP) requires that procedures, devices or laboratory tests approved by the U.S. Food and Drug Administration (FDA) may not be considered investigational and thus these procedures, devices or laboratory tests may be assessed only on the basis of their medical necessity. A nonprofit independent licensee of the BlueCross BlueShield Association.
2 PAGE: 2 OF: 8 DESCRIPTION: Age-related macular degeneration (AMD) is a major cause of severe vision loss in people older than age 65 years. There are 2 forms of AMD: wet and dry. The dry form is the most common form and is characterized by yellow deposits in the retina, called drusen. The dry form can progress to the wet form, which is more aggressive and severe. Wet or exudative AMD is caused by the growth of abnormal leaky blood vessels (choroidal neovascularization or CNV) that eventually damage the macula. The macula is the area of the eye responsible for central vision, which is essential for most visual activities, including reading, driving, and recognizing faces. CNV associated with wet AMD may include classic or occult neovascular leakage patterns. Classic CNV is distinct or well demarcated during fluorescein angiography whereas occult CNV is obscured or poorly demarcated on fluorescein angiography. Choroidal neovascularization due to pathologic myopia is caused by abnormal blood vessels that grow under the center of the retina as a result of the abnormal elongation of the back of the eye associated with severe near-sightedness or myopia. Pathologic myopia generally occurs among people over 30 years of age and can result in a progressive loss of vision. Ocular histoplasmosis syndrome (OHS) is thought to be caused by Histoplasm capsulatum (a fungus found in the dust and soil of river valley regions) which is inhaled into the lungs and then spreads to the choroid layer of the eye forming scar tissue. In later years, OHS develops when the scar tissue forms fragile, abnormal blood vessels known as choroidal neovascularization. OHS is also known as Presumed OHS due to the fact that the fungus is rarely isolated or cultured from the eye. Central serous chorioretinopathy refers to an idiopathic disease in which there is a serous detachment of the macula due to leakage of fluid from the choriocapillaris through the retinal pigment epithelium. This condition is avascular; however, neovascularization can occur as a secondary complication. Although central serous chorioretinopathy often resolves spontaneously in 3 to 4 months, chronic or recurrent central serous chorioretinopathy can result in progressive decline of visual acuity. Central serous chorioretinopathy has been treated with medication and laser photocoagulation, but these treatments have limited efficacy. Choroidal hemangioma is an uncommon, benign vascular tumor, manifesting as an orange-red mass in the posterior pole of the eye. Visual loss may be progressive and irreversible because of chronic foveal detachment. Photodynamic therapy is a treatment modality designed to selectively occlude neovascular tissue. Therapy consists of intravenous injection of a photosensitizing agent followed by irradiation of the neovascular tissue with non-thermal light. It is thought that when the light activates the photosensitizer, it generates singlet oxygen, which leads to the selective destruction of new blood vessels. Visudyne (verteporfin) is the only FDA approved intravenous photosensitizing agent for the treatment of patients with choroidal neovascularization. Visudyne photodynamic therapy is a two-step process: it is injected intravenously and rapidly accumulates in the abnormal vessels in the eye. Activation of Visudyne by the non-thermal laser (usually within 5 minutes of the injection) results in a reduction in the growth and leakage of these abnormal blood vessels and a corresponding reduction or stabilization of vision loss, with minimal effects on the surrounding normal tissue. RATIONALE: Visudyne (verteporfin) is the only FDA approved intravenous photosensitizing agent for the treatment of patients with choroidal neovascularization. The FDA approved indications include classic CNV secondary to wet AMD, pathologic myopia and ocular histoplasmosis syndrome. Several clinical trials are currently underway investigating other photosensitizing agents in the treatment of subfoveal CNV. Publications of the TAP clinical trial have reported that photodynamic therapy can safely reduce the risk of vision loss in patients with age-related macular degeneration characterized by choroidal neovascularization up to 2 years
3 PAGE: 3 OF: 8 following the initial treatment. In an extension trial of the TAP trial, patients visual acuity was found to remain stable between the 24 th and 36 th month of follow-up, while the number of treatments required continued to decrease (1.4 treatments in the third year compared to 3.4 and 2.1 treatments received in the first and second years, respectively). The VIP trial primarily focused on the safety and efficacy of PDT in patients with predominantly occult lesions. While there was no significant difference in vision loss between the treatment and placebo group in the first 12 months, by 24 months a significantly lower percentage of those patients in the treatment group had lost vision. A second arm of the VIP trial investigated patients with CNV due to pathologic myopia. Beneficial outcomes regarding visual acuity were noted at 12 months (86% of verteporfin-treated patients lost less than 3 lines of vision, compared to 67% of patients receiving sham treatment). The FDA s 2001 decision to expand Visudyne therapy for ocular histoplasmosis was based on findings of a case study of 26 patients. Patients treated with verteporfin demonstrated a reduction in the number of episodes of severe visual acuity loss compared to historical control data. A recent analysis of the TAP and VIP studies found correlation between lesion size of minimally classic CNV and efficacy of verteporfin treatment. Patients with minimally classic CNV with lesions 4 disc sizes or smaller treated with verteporfin have a better visual acuity outcome after treatment than patients with larger areas of CNV. Preliminary results of the VIM study also support the use of verteporfin treatment in patients with minimally classic CNV with small disc areas. Quality evidence on use of PDT for central serous chorioretinopathy is limited. The available evidence indicates substantial numbers of adverse events with standard PDT. Reduced-dose PDT may result in improved anatomical outcomes for acute central serous chorioretinopathy, but clinically significant improvements in visual acuity have not been shown for this self-limiting disease. For chronic central serous chorioretinopathy, recent comparative studies of reduced fluence and reduced-dose PDT suggest a possible beneficial effect of this treatment. PDT has been reported to induce complete and irreversible occlusion of the microvasculature, although this may require more than one treatment. Several case series demonstrated encouraging visual and anatomical outcomes in 150 patients with circumscribed choroidal hemangioma who were treated with various PDT regimens. Based on numerous case reports and case series, PDT is being used in an attempt to decrease CNV of many different etiologies. For example, PDT has been reported to slow down, but not prevent or reverse, the progression of disease of CNV associated with polypoidal choroidal vasculopathy, angioid streaks, and inflammatory chorioretinal disease. There is insufficient evidence to support the use of PDT as monotherapy or in combination therapy for these other ophthalmologic disorders. As a result, PDT is considered investigational for ophthalmologic disorders other than AMD, chronic central serous chorioretinopathy, choroidal hemangioma, pathologic myopia, or presumed ocular histoplasmosis. CODES: Number Description Eligibility for reimbursement is based upon the benefits set forth in the member s subscriber contract. CODES MAY NOT BE COVERED UNDER ALL CIRCUMSTANCES. PLEASE READ THE POLICY AND GUIDELINES STATEMENTS CAREFULLY. Codes may not be all inclusive as the AMA and CMS code updates may occur more frequently than policy updates. CPT: Destruction of localized lesion of choroid (eg, choroidal neovascularization); photodynamic therapy (includes intravenous infusion) photodynamic therapy, second eye, at single session (List separately in addition to code for primary eye treatment)
4 PAGE: 4 OF: 8 Copyright 2018 American Medical Association, Chicago, IL HCPCS: J3396 Injection, verteporfin, 0.1 mg Occult histoplasmosis ICD10: H H Retinal neovascularization, eye (code range) REFERENCES: H35.32 Exudative age-related macular degeneration H44.20-H44.23 Degenerative myopia, eye (code range) Adjunctive Difclofenac with Verteporfin (ADD-V) Study Group, et al. Effect of adjunctive diclofenac with verteporfin therapy to treat choroidal neovascularization due to age-related macular degeneration: phase II study. Retina 2007 Jul- Aug;27(6): Ahmadieh H, et al. Single sessions photodynamic therapy combined with intravitreal bevacizumab and triamcinolone for neovascular age-related macular degeneration. BMC Ophthalmol 2007 Jun 7;7(10):1-6. *American Academy of Ophthalmology. Photodynamic therapy with verteporfin for age-related macular degeneration. Ophthalmol Dec 2000;107(12): American Academy of Ophthalmology. Preferred practice pattern. Age-related macular degeneration [ accessed 2/25/13. American Academy of Ophthalmology. Photodynamic therapy with verteporfin for age-related macular degeneration [ accessed 2/25/13. Antoszyk AN, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol 2008 May;145(5): Arias L, et al. Photodynamic therapy with intravitreal triamcinolone on predominantly classic choroidal neovascularization: one-year results of a randomized study. Ophthalmol 2006 Dec;113(12): Augustin AJ, et al. Verteporfin and intravitreal triamcinolone acetonide combination therapy for occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol 2006 Apr;141(4): *Axer-Siegel R, et al. Photodynamic therapy for occult choroidal neovascularization with pigment epithelium detachment in age-related macular degeneration. Arch Ophthalmol 2004 Apr;122(4): *Azab M, et al. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: meta-analysis of 2-year safety results in three randomized clinical trials: Treatment of age-related macular degeneration with photodynamic therapy and verteporfin in photodynamic therapy study. Report no. 4. Retina 2004 Feb;24(1):1-12. Azab M, et al. Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration: 2-year results of a randomized clinical trial. Arch Ophthal 2005 Apr;123(4): Bashshur ZF, et al. Intravitreal bevacizumab vs verteporfin photodynamic therapy for neovascular age-related macular degeneration. Arch Ophthalmol 2007 Oct;125(10): *Blinder KJ, et al. Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial VIP report no. 3. Ophthalmol 2003 Apr;110(4):
5 PAGE: 5 OF: 8 *Blinder KJ, et al. Effect of lesion size, visual acuity, and lesion composition on visual acuity with and without verteporfin therapy for choroidal neovascularization secondary to age-related macular degeneration: TAP and VIP report no.1. Am J Ophthalmol 2003 Sep;136(3): BlueCross BlueShield Association. Photodynamic therapy for subfoveal choroidal neovascularization. Medical Policy Reference Manual Policy # March 9. *BlueCross BlueShield Association Technology Evaluation Center (TEC). Photodynamic therapy for subfoveal neovascularization Dec;15(18). BlueCross BlueShield Association Technology Evaluation Center (TEC). Special report: Current and evolving strategies in the treatment of age-related macular degeneration Dec;20 (11). *Blumenkranz MS, et al. Verteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: three-year results of an open-label extension of 2 randomized clinical trials TAP Report no. 5. Arch Ophthalmol 2002 Oct;120(10): Boixadera A, et al. Prospective clinical trial evaluating the efficacy of photodynamic therapy for symptomatic circumscribed choroidal hemangioma. Ophthalmol 2009 Jan;116(1): *Bressler NM, et al. Verteporfin therapy in age-related macular degeneration (VAM): an open label multicenter photodynamic therapy study of 4,435 patients. Retina 2004 Aug;24(6): Bresslar SB, et al. Natural history of minimally classic subfoveal choroidal neovascular lesions in the treatment of agerelated macular degeneration with photodynamic therapy (TAP) investigation. Arch Ophthalmol 2004 Mar;122(3): *Bressler NM, et al. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol 2001 Feb;119(2): Bressler NM, et al. Verteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: four-year results of an open-label extension of 2 randomized clinical trials: TAP Report No. 7. Arch Ophthalmol 2005 Sep;123(9): Brown DM, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. NEJM 2006 Oct 5;355(14): Brown DM, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmol 2009 Jan;116(1): Browning AC, et al. Verteporfin photodynamic therapy of choroidal neovascularization in angioid streaks one-year results of a prospective case series. Ophthalmol 2005 Jul;112(7):1-5. Browning AC, et al. Verteporfin photodynamic therapy of choroidal neovascularization in angioid streaks: one year results of a prospective case series. Ophthalmol 2005 Jul;112(7): *Busquets MA, et al. Ocular photodynamic therapy with verteporfin for choroidal neovascularization secondary to ocular histoplasmosis syndrome. Retina 2003 Jun;23(3): Chan WM, et al. Combined photodynamic therapy and intravitreal triacinolone injection for the treatment of subfoveal choroidal neovascularization in age related macular degeneration: a comparative study. Br J Ophthalmol 2006 Mar;90(3): Chan WM, et al. Half-dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one-year results of a randomized controlled trial. Ophthalmol 2008 Oct;115(10):
6 PAGE: 6 OF: 8 Chaudhary V, et al. Triamcinolone acetonide as adjunctive treatment to verteporfin in neovascular age-related macular degeneration: a prospective randomized trial. Ophthalmol 2007 Dec;114(12): Dhalla MS, et al. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina 2006 Nov-Dec;26(9): *Do DV, et al. Large submacular hemorrhages after verteporfin therapy. Am J Ophthalmol 2004 Mar; 137(3): *Ergun E, et al. Photodynamic therapy with verteporfin in subfoveal choroidal neovascularization secondary to central serous chorioretinopathy. Arch Ophthalmol 2004 Jan;122(1): Glacet-Bernard A, et al. Photodynamic therapy vs limited macular translocation in the management of subfoveal choroidal neovascularization in pathologic myopia: a two-year study. Am J Ophthalmol 2007 Jan;143(1): Jurklies B, et al. Photodynamic therapy using verteporfin for choroidal neovascularization associated with angioid streaks-long-term effects. Ophthalmic Res 2006;38(4): Kaiser PK, et al. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: 5 year results of two randomized trials with an open-label extension: TAP report no. 8. Graefes Arch Clin Exp Ophthalmol 2006 Sep;244(9): *Karacorlu M, et al. Photodynamic therapy with verteporfin for choroidal neovascularization in patients with angioid streaks. Am J Ophthalmol 2002 Sep;134(3): Lam DS, et al. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization secondary to pathologic myopia- 1-year results of a prospective series. Eye 2005 Aug;19(8): Lazic R, et al. Verteporfin therapy and intravitreal bevacizumab combined and alone in choroidal neovascularization due to age-related macular degeneration. Ophthalmol 2007 Jun;114(6): Manku KK, et al. Factors influencing poor visual outcome in patients treated with photodynamic therapy for choroidal neovascularization secondary to age-related macular degeneration. Clin Experiment Ophthalmol 2007 May- Jun;35(4): Mennel S, et al. Ocular photodynamic therapy-standard applications and new applications (part1). Review of the literature and personal experience. Ophthalmologica 2007;221(4): Michels S, et al. Comparison of early retreatment with standard regimen in verteporfin therapy of neovascular agerelated macular degeneration. Ophthalmol 2005 Dec;112(12): Neovascular age-related macular degeneration, periocular corticosteroids, and photodynamic therapy (NAPP) trial research group, et al. Periocular triamcinolone and photodynamic therapy for subfoveal choroidal neovascularization in age-related macular degeneration. Ophthalmol 2007 Sep;114(9): Pece A, et al. Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: a long-term follow-up study. Am J Ophthalmol 2007 Mar;143(3): Potter MJ, et al. recurrence of choroidal neovascularization after photodynamic therapy in patients with age-related macular degeneration. Br J Ophthalmol 2007 Jun;91(6): *Rechtman E, et al. Intravitreal triamcinolone with photodynamic therapy for subfoveal choroidal neovascularization in age related macular degeneration. Br J Ophthalmol 2004 Mar;88(3): *Rogers AH, et al. Photodynamic therapy of idiopathic and inflammatory choroidal neovascularization in young adults. Ophthalmol 2003 Jul;110(7):
7 PAGE: 7 OF: 8 *Rosenfeld PJ, et al. Photodynamic therapy with verteporfin in ocular histoplasmosis: uncontrolled, open-label 2-year study. Ophthalmol 2004 Sep;111(9): Rosenfeld PJ, et al. Verteporfin therapy of subfoveal occult choroidal neovascularization in AMD using delayed light application: one-year results of the VALIO Study. Am J Ophthalmol 2007 Dec;144(6): Ruiz-Moreno JM, et al. Photodynamic therapy and high-dose intravitreal triacinolone to treat exudative age-related macular degeneration: 1-year outcomes. Retina 2006 Jul-Aug;26(6): *Saperstein DA, et al. Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin in the ocular histoplasmosis syndrome: one-year results of an uncontrolled, prospective case series. Ophthalmol 2002 Aug;109(8): Saperstein DA, et al. Verteporfin therapy for CNV secondary to OHS. Ophthalmol 2006 Dec;113(12): 2371.e1-3. *Schmidt-Erfurth U, et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by agerelated macular degeneration: results of re-treatments in a phase 1 and 2 study. Arch Ophthalmol 1999;117: Schmidt-Erfurth U, et al. Randomized multicenter trial of more intense and standard early verteporfin treatment of neovascular age-related macular degeneration. Ophthalmol 2008 Jan;115(1): Schmidt-Erfurth U, et al. Same-day administration of verteporfin and ranibizumab 0.5 mg in patients with choroidal neovascularization due to age-related macular degeneration. Br J Ophthalmol 2008 Dec;92912): Schnurrbusch UE, et al. Complications after photodynamic therapy. Arch Ophthalmol 2005 Oct;123(10): *Shaikh S, et al. Photodynamic therapy using verteporfin for choroidal neovascularization in angioid streaks. Am J Ophthalmol 2003 Jan;135(1):1-6. Sharp DM, et al. Photodynamic therapy with verteporfin for choroidal neovascularization due to age-related macular degeneration and other causes: a New Zealand outcomes study. Clin Experiment Ophthalmol 2007 Jan-Feb;32(1): Slakter JS, et al. Anecortave acetate (15 milligrams) versus photodynamic therapy for the treatment of subfoveal neovascularization in age-related macular degeneration. Ophthalmol 2006 Jan;113(1):3-13. *Spaide RF, et al. Combined photodynamic therapy with verteporfin and intravitreal tiamcinolone acetonide for choroidal neovascularization. Ophthalmol 2003 Aug;110(8): *Taban M, et al. Chronic central serous chorioretinopathy: photodynamic therapy. Am J Ophthalmol 2004 Jun;137(6): *Verteporfin in Photodynamic Therapy Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial - VIP report no.1. Ophthalmol 2001 May;108(5): *Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization- verteporfin in photodynamic therapy - report 2. Am J Ophthalmol 2001 May;131(5): Verteporfin Roundtable Participants. Guidelines for using verteporfin (Visudyne) in photodynamic therapy for choroidal neovascularization due to age-related macular degeneration and other causes: update. Retina 2005 Feb-Mar;25 (2): Virgili G, et al. Photodynamic therapy for nonsubfoveal choroidal neovascularization in 100 eyes with pathologic myopia. Am J Ophthalmol 2007 Jan;143(1):77-82.
8 PAGE: 8 OF: 8 Weigert G, et al. Intravitreal bevacizumab (Avastin) therapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age-related macular degeneration: 6-month results of a prospective, randomized, controlled clinical study. Br J Ophthalmol 2008 Mar;92(3): Wormald R, et al. Photodynamic therapy for neovascular macular degeneration. Cochrane database of systematic reviews Jul 18;(3):CD *key article KEY WORDS: Age-related macular degeneration, AMD, Visudyne, Verteporfin. CMS COVERAGE FOR MEDICARE PRODUCT MEMBERS There is currently a National Coverage Determination (NCD) for ocular photodynamic therapy and a Local Coverage Determination (LCD), Drugs and Biologics. Please refer to the following NCD and LCD websites for Medicare members: NCD: details.aspx?ncdid=349&ncdver=1&coverageselection=both&articletype=all&policytype=final&s=new+york+- +Upstate&CptHcpcsCode=36514&bc=gAAAABAAAAAA& LCD: al+government+services%2c+inc.+(13201%2c+a+and+b+and+hhh+mac%2c+j+- +K)&s=All&DocType=Active&bc=AggAAAQAAAAA&
Despite our growing knowledge of the
 OVERVIEW OF AVAILABLE TREATMENTS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Carl D. Regillo, MD ABSTRACT Although potential treatments for neovascular age-related macular degeneration represent
OVERVIEW OF AVAILABLE TREATMENTS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Carl D. Regillo, MD ABSTRACT Although potential treatments for neovascular age-related macular degeneration represent
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 9.03.20 Intraocular Radiation Therapy for Age-Related Macular Degeneration Photodynamic Therapy for Choroidal
FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 9.03.20 Intraocular Radiation Therapy for Age-Related Macular Degeneration Photodynamic Therapy for Choroidal
Clinical Trials Related to Age Related Macular Degeneration
 Clinical Trials Related to Age Related Macular Degeneration Kirti Singh MD, DNB, FRCS Kirti Singh MD, DNB, FRCS, Pooja Jain MBBS, Nitasha Ahir MBBS, Divya Jain MD, DNB Guru Nanak Eye Centre, Maulana Azad
Clinical Trials Related to Age Related Macular Degeneration Kirti Singh MD, DNB, FRCS Kirti Singh MD, DNB, FRCS, Pooja Jain MBBS, Nitasha Ahir MBBS, Divya Jain MD, DNB Guru Nanak Eye Centre, Maulana Azad
Photodynamic Therapy for Choroidal Neovascularization
 Photodynamic Therapy for Choroidal Neovascularization Policy Number: 9.03.08 Last Review: 10/2014 Origination: 10/2000 Next Review: 10/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Photodynamic Therapy for Choroidal Neovascularization Policy Number: 9.03.08 Last Review: 10/2014 Origination: 10/2000 Next Review: 10/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Description. Section: Other Effective Date: July 15, Subsection: Vision Original Policy Date: December 7, 2011 Subject: Page: 1 of 23
 Last Review Status/Date: June 2015 Page: 1 of 23 Description Photodynamic therapy (PDT) is a treatment modality designed to selectively occlude ocular choroidal neovascular tissue. The therapy is a 2-step
Last Review Status/Date: June 2015 Page: 1 of 23 Description Photodynamic therapy (PDT) is a treatment modality designed to selectively occlude ocular choroidal neovascular tissue. The therapy is a 2-step
Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283
 Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
The limited number of currently approved
 CLINICAL TRIALS OF VERTEPORFIN AND PEGAPTANIB: WHAT ARE THE RESULTS? * William F. Mieler, MD ABSTRACT Currently available treatment options for the management of choroidal neovascularization (CNV) in age-related
CLINICAL TRIALS OF VERTEPORFIN AND PEGAPTANIB: WHAT ARE THE RESULTS? * William F. Mieler, MD ABSTRACT Currently available treatment options for the management of choroidal neovascularization (CNV) in age-related
Clinical Policy Title: Ocular photodynamic therapy (OPDT) with Visudyne (verteporfin) for macular degeneration treatment
 Clinical Policy Title: Ocular photodynamic therapy (OPDT) with Visudyne (verteporfin) for macular degeneration treatment Clinical Policy Number: 10.02.04 Effective Date: January 1, 2016 Initial Review
Clinical Policy Title: Ocular photodynamic therapy (OPDT) with Visudyne (verteporfin) for macular degeneration treatment Clinical Policy Number: 10.02.04 Effective Date: January 1, 2016 Initial Review
MEDICAL POLICY SUBJECT: TRANSPUPILLARY THERMOTHERAPY. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: TRANSPUPILLARY EDITED DATE: 08/20/15, 08/18/16, 08/17/17 PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
MEDICAL POLICY SUBJECT: TRANSPUPILLARY EDITED DATE: 08/20/15, 08/18/16, 08/17/17 PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
CASE STUDIES CURRENT AND FUTURE TREATMENT OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Neil M.
 CURRENT AND FUTURE TREATMENT OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Neil M. Bressler, MD Dr Bressler: The overview articles in this monograph summarize safety, efficacy,
CURRENT AND FUTURE TREATMENT OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Neil M. Bressler, MD Dr Bressler: The overview articles in this monograph summarize safety, efficacy,
SUMMARY. Heather Casparis, MD,* and Neil M. Bressler, MD MARINA AND ANCHOR
 The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187
 Clinical Policy: (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03/16 Last Review Date: 03/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy Title: Ocular photodynamic therapy with Visudyne (verteporfin) for macular degeneration treatment
 Clinical Policy Title: Ocular photodynamic therapy with Visudyne (verteporfin) for macular degeneration treatment Clinical Policy Number: 10.02.04 Effective Date: January 1, 2016 Initial Review Date: August
Clinical Policy Title: Ocular photodynamic therapy with Visudyne (verteporfin) for macular degeneration treatment Clinical Policy Number: 10.02.04 Effective Date: January 1, 2016 Initial Review Date: August
Clinical Policy Title: Ocular photodynamic therapy (OPDT) with Visudyne (verteporfin) for macular degeneration treatment
 Clinical Policy Title: Ocular photodynamic therapy (OPDT) with Visudyne (verteporfin) for macular degeneration treatment Clinical Policy Number: 10.02.04 Effective Date: January 1, 2016 Initial Review
Clinical Policy Title: Ocular photodynamic therapy (OPDT) with Visudyne (verteporfin) for macular degeneration treatment Clinical Policy Number: 10.02.04 Effective Date: January 1, 2016 Initial Review
Retina Conference. Janelle Fassbender, MD, PhD University of Louisville Department of Ophthalmology and Visual Sciences 09/04/2014
 Retina Conference Janelle Fassbender, MD, PhD University of Louisville Department of Ophthalmology and Visual Sciences 09/04/2014 Subjective CC/HPI: 64 year old Caucasian female referred by outside ophthalmologist
Retina Conference Janelle Fassbender, MD, PhD University of Louisville Department of Ophthalmology and Visual Sciences 09/04/2014 Subjective CC/HPI: 64 year old Caucasian female referred by outside ophthalmologist
CLINICAL SCIENCES. Verteporfin Therapy for Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration
 CLINICAL SCIENCES Verteporfin Therapy for Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration Three-Year Results of an Open-Label Extension of 2 Randomized Clinical Trials TAP Report
CLINICAL SCIENCES Verteporfin Therapy for Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration Three-Year Results of an Open-Label Extension of 2 Randomized Clinical Trials TAP Report
FEEDER VESSEL TREATMENT
 THERAPEUTIC OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Donald J. D'Amico, MD Dr D Amico: The overview articles in this monograph highlight findings from clinical trials
THERAPEUTIC OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Donald J. D'Amico, MD Dr D Amico: The overview articles in this monograph highlight findings from clinical trials
Intraocular Radiation Therapy for Age-Related Macular Degeneration
 Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
Current indications for photodynamic therapy in medical retina practice
 réalités Current indications for photodynamic therapy in medical retina practice No. 254 September 2018 OPHTALMOLOGIQUES SUMMARY: In medical retina practice, the use of photodynamic therapy (PDT) revolutionised
réalités Current indications for photodynamic therapy in medical retina practice No. 254 September 2018 OPHTALMOLOGIQUES SUMMARY: In medical retina practice, the use of photodynamic therapy (PDT) revolutionised
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 6.01.10 Stereotactic Radiosurgery and Stereotactic Body Radiotherapy 8.01.10 Charged-Particle (Proton
FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 6.01.10 Stereotactic Radiosurgery and Stereotactic Body Radiotherapy 8.01.10 Charged-Particle (Proton
The Natural History of Occult Choroidal Neovascularisation Associated With Age-related Macular Degeneration. A Systematic Review
 Review Article 145 The Natural History of Occult Choroidal Neovascularisation Associated With Age-related Macular Degeneration. A Systematic Review Antonio Polito, 1 MD, Miriam Isola, 2 MHS, Paolo Lanzetta,
Review Article 145 The Natural History of Occult Choroidal Neovascularisation Associated With Age-related Macular Degeneration. A Systematic Review Antonio Polito, 1 MD, Miriam Isola, 2 MHS, Paolo Lanzetta,
Description. Section: Other Effective Date: July 15, Subsection: Vision Original Policy Date: September 13, 2011 Subject: Page: 1 of 18
 Last Review Status/Date: June 2014 Page: 1 of 18 Description Angiogenesis inhibitors (e.g., ranibizumab, bevacizumab, pegaptanib, aflibercept) are being evaluated for the treatment of disorders of choroidal
Last Review Status/Date: June 2014 Page: 1 of 18 Description Angiogenesis inhibitors (e.g., ranibizumab, bevacizumab, pegaptanib, aflibercept) are being evaluated for the treatment of disorders of choroidal
Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration.
 Thomas Jefferson University Jefferson Digital Commons Wills Eye Institute Papers Wills Eye Institute 4-1-2008 Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related
Thomas Jefferson University Jefferson Digital Commons Wills Eye Institute Papers Wills Eye Institute 4-1-2008 Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related
Coding Implications Revision Log. See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Stabilization of visual acuity with photodynamic therapy in eyes with chorioretinal anastomoses
 Graefe s Arch Clin Exp Ophthalmol (2004) 242:368 376 CLINICAL INVESTIGATION DOI 10.1007/s00417-003-0844-0 Rufino M. Silva José R. Faria de Abreu António Travassos José G. Cunha-Vaz Stabilization of visual
Graefe s Arch Clin Exp Ophthalmol (2004) 242:368 376 CLINICAL INVESTIGATION DOI 10.1007/s00417-003-0844-0 Rufino M. Silva José R. Faria de Abreu António Travassos José G. Cunha-Vaz Stabilization of visual
Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis)
 Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 2Q17 April May
 BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
Original Policy Date
 MP 9.03.08 Photocoagulation of Macular Drusen Medical Policy Section Miscellaneous Policies Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return
MP 9.03.08 Photocoagulation of Macular Drusen Medical Policy Section Miscellaneous Policies Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return
Intravenous diclofenac as prophylactic treatment for verteporfin-associated low back pain
 European Journal of Ophthalmology / Vol. 18 no. 5, 2008 / pp. 805-808 Intravenous diclofenac as prophylactic treatment for verteporfin-associated low back pain T. THEELEN, C.B. HOYNG Department of Ophthalmology,
European Journal of Ophthalmology / Vol. 18 no. 5, 2008 / pp. 805-808 Intravenous diclofenac as prophylactic treatment for verteporfin-associated low back pain T. THEELEN, C.B. HOYNG Department of Ophthalmology,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA68; The clinical and cost effectiveness of photodynamic therapy for age-related macular degeneration TA68 guidance
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA68; The clinical and cost effectiveness of photodynamic therapy for age-related macular degeneration TA68 guidance
Photodynamic Therapy for Treatment of Subfoveal Choroidal Neovascularization in Exudative Age-Related Macular Degeneration
 Clinical Medicine Journal Vol. 4, No. 4, 2018, pp. 60-66 http://www.aiscience.org/journal/cmj ISSN: 2381-7631 (Print); ISSN: 2381-764X (Online) Photodynamic Therapy for Treatment of Subfoveal Choroidal
Clinical Medicine Journal Vol. 4, No. 4, 2018, pp. 60-66 http://www.aiscience.org/journal/cmj ISSN: 2381-7631 (Print); ISSN: 2381-764X (Online) Photodynamic Therapy for Treatment of Subfoveal Choroidal
Although photocoagulation and photodynamic PROCEEDINGS PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION *
 PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
Photodynamic therapy (PDT) for Central Serous Retinopathy (CSR)/ Polypoidal Choroidal Vasculopathy (PCV)
 Photodynamic therapy (PDT) for Central Serous Retinopathy (CSR)/ Polypoidal Choroidal Vasculopathy (PCV) About photodynamic therapy for Retinal Conditions The macula is the central part of the retina at
Photodynamic therapy (PDT) for Central Serous Retinopathy (CSR)/ Polypoidal Choroidal Vasculopathy (PCV) About photodynamic therapy for Retinal Conditions The macula is the central part of the retina at
MONTHLY ADMINISTERED INTRAVITREOUS PHARmacotherapy
 Polypoidal Choroidal Vasculopathy Masquerading as Neovascular Age-Related Macular Degeneration Refractory to Ranibizumab ALEXANDROS N. STANGOS, JAGDEEP SINGH GANDHI, JAYASHREE NAIR-SAHNI, HEINRICH HEIMANN,
Polypoidal Choroidal Vasculopathy Masquerading as Neovascular Age-Related Macular Degeneration Refractory to Ranibizumab ALEXANDROS N. STANGOS, JAGDEEP SINGH GANDHI, JAYASHREE NAIR-SAHNI, HEINRICH HEIMANN,
VERTEPORFIN IN PHOTODYNAMIC THERAPY STUDY GROUP
 Verteporfin Therapy of Subfoveal Choroidal Neovascularization in Age-related Macular Degeneration: Two-year Results of a Randomized Clinical Trial Including Lesions With Occult With No Classic Choroidal
Verteporfin Therapy of Subfoveal Choroidal Neovascularization in Age-related Macular Degeneration: Two-year Results of a Randomized Clinical Trial Including Lesions With Occult With No Classic Choroidal
Department of Ophthalmology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
 Department of Ophthalmology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea Purpose: To investigate the factors that affect final vision following photodynamic therapy
Department of Ophthalmology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea Purpose: To investigate the factors that affect final vision following photodynamic therapy
Angiogenesis and Role of Anti-VEGF Therapy
 Review Article Angiogenesis and Role of Anti-VEGF Therapy P.S. Mahar, Azfar N. Hanfi, Aimal Khan Pak J Ophthalmol 2009, Vol. 25 No. 3.....................................................................................................
Review Article Angiogenesis and Role of Anti-VEGF Therapy P.S. Mahar, Azfar N. Hanfi, Aimal Khan Pak J Ophthalmol 2009, Vol. 25 No. 3.....................................................................................................
ANTIVASCULAR ENDOTHELIAL GROWTH FACTOR
 THE ROLE OF RANIBIZUMAB * Michael L. Klein, MD ABSTRACT The positive 1-year results of ranibizumab trials reported at the 23rd Annual Meeting of the American Society of Retina Specialists have generated
THE ROLE OF RANIBIZUMAB * Michael L. Klein, MD ABSTRACT The positive 1-year results of ranibizumab trials reported at the 23rd Annual Meeting of the American Society of Retina Specialists have generated
Intraocular Radiotherapy for Age- Related Macular Degeneration
 Intraocular Radiotherapy for Age- Related Macular Degeneration Policy Number: 9.03.20 Last Review: 9/2017 Origination: 9/2008 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Intraocular Radiotherapy for Age- Related Macular Degeneration Policy Number: 9.03.20 Last Review: 9/2017 Origination: 9/2008 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Evolving European guidance on the medical management of neovascular age related macular degeneration
 Evolving European guidance on the medical management of neovascular age related macular degeneration U Chakravarthy, G Soubrane, F Bandello, V Chong, C Creuzot-Garcher, S A Dimitrakos, II, J-F Korobelnik,
Evolving European guidance on the medical management of neovascular age related macular degeneration U Chakravarthy, G Soubrane, F Bandello, V Chong, C Creuzot-Garcher, S A Dimitrakos, II, J-F Korobelnik,
I n the Western world, age related macular degeneration
 207 SCIENTIFIC REPORT Clinicopathological findings of choroidal neovascularisation following verteporfin photodynamic therapy F Gelisken, B A Lafaut, W Inhoffen, M Voelker, S Grisanti, K U Bartz-Schmidt...
207 SCIENTIFIC REPORT Clinicopathological findings of choroidal neovascularisation following verteporfin photodynamic therapy F Gelisken, B A Lafaut, W Inhoffen, M Voelker, S Grisanti, K U Bartz-Schmidt...
Ophthalmologic Policy. Vascular Endothelial Growth Factor (VEGF) Inhibitors
 Ophthalmologic Policy UnitedHealthcare Commercial Drug Policy Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: 2016D0042H Effective Date: October 1, 2016 Table of Contents Page INSTRUCTIONS
Ophthalmologic Policy UnitedHealthcare Commercial Drug Policy Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: 2016D0042H Effective Date: October 1, 2016 Table of Contents Page INSTRUCTIONS
Clinical Study Fixed Monthly versus Less Frequent Ranibizumab Dosing and Predictors of Visual Response in Exudative Age-Related Macular Degeneration
 Ophthalmology Volume 12, Article ID 69641, 8 pages doi:1.1155/12/69641 Clinical Study Fixed Monthly versus Less Frequent Ranibizumab Dosing and Predictors of Visual Response in Exudative Age-Related Macular
Ophthalmology Volume 12, Article ID 69641, 8 pages doi:1.1155/12/69641 Clinical Study Fixed Monthly versus Less Frequent Ranibizumab Dosing and Predictors of Visual Response in Exudative Age-Related Macular
CMS Manual System Pub Medicare National Coverage Determinations
 CMS Manual System Pub. 100-03 Medicare National Coverage Determinations Department of Health & Human Services (DHHS) Centers for Medicare & Medicaid Services (CMS) Transmittal 9 Date: APRIL 1, 2004 CHANGE
CMS Manual System Pub. 100-03 Medicare National Coverage Determinations Department of Health & Human Services (DHHS) Centers for Medicare & Medicaid Services (CMS) Transmittal 9 Date: APRIL 1, 2004 CHANGE
Photodynamic Therapy (PDT) for agerelated
 Page 1 of 5 Photodynamic Therapy (PDT) for agerelated eye conditions Introduction This leaflet gives you information about Photodynamic Therapy (PDT) for the age--related eye conditions macular degeneration
Page 1 of 5 Photodynamic Therapy (PDT) for agerelated eye conditions Introduction This leaflet gives you information about Photodynamic Therapy (PDT) for the age--related eye conditions macular degeneration
CENTRAL SEROUS CHORIORETINOPATHY (CSC) IS
 Association Between the Efficacy of Half-Dose Photodynamic Therapy With Indocyanine Green Angiography and Optical Coherence Tomography Findings in the Treatment of Central Serous Chorioretinopathy MASSIMO
Association Between the Efficacy of Half-Dose Photodynamic Therapy With Indocyanine Green Angiography and Optical Coherence Tomography Findings in the Treatment of Central Serous Chorioretinopathy MASSIMO
Date approved: 04/18/18. Approved by: Pharmacy and Therapeutics Quality Management Subcommittee Effective Date: Department of Origin: Pharmacy
 Integrated Healthcare Services and Criteria Document: Reference #: PC/V001 Page: 1 of 9 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Integrated Healthcare Services and Criteria Document: Reference #: PC/V001 Page: 1 of 9 PRODUCT APPLICATION: PreferredOne Administrative Services, Inc. (PAS) ERISA PreferredOne Administrative Services,
Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin
 Graefe s Arch Clin Exp Ophthalmol (2005) 243: 973 979 CLINICAL INVESTIGATION DOI 10.1007/s00417-005-1139-4 Rufino M. Silva João Figueira M. Luz Cachulo Liliane Duarte José R. Faria de Abreu J. G. Cunha-Vaz
Graefe s Arch Clin Exp Ophthalmol (2005) 243: 973 979 CLINICAL INVESTIGATION DOI 10.1007/s00417-005-1139-4 Rufino M. Silva João Figueira M. Luz Cachulo Liliane Duarte José R. Faria de Abreu J. G. Cunha-Vaz
Digital Journal of Ophthalmology Digital Journal of Ophthalmology. Abstract
 Original Articles Combined intravitreal ranibizumab and verteporfin photodynamic therapy versus ranibizumab alone for the treatment of age-related macular degeneration Rosalia Giustolisi, MD, Nicoletta
Original Articles Combined intravitreal ranibizumab and verteporfin photodynamic therapy versus ranibizumab alone for the treatment of age-related macular degeneration Rosalia Giustolisi, MD, Nicoletta
A Treat and Extend Regimen Using Ranibizumab for Neovascular Age-Related Macular Degeneration
 A Treat and Extend Regimen Using Ranibizumab for Neovascular Age-Related Macular Degeneration Clinical and Economic Impact Omesh P. Gupta, MD, MBA, Gary Shienbaum, MD, Avni H. Patel, MD, Christopher Fecarotta,
A Treat and Extend Regimen Using Ranibizumab for Neovascular Age-Related Macular Degeneration Clinical and Economic Impact Omesh P. Gupta, MD, MBA, Gary Shienbaum, MD, Avni H. Patel, MD, Christopher Fecarotta,
Curriculum Vitae Ryan M. Rich, M.D.
 Retina Consultants of Southern Colorado, P.C. 3030 North Circle Drive, Suite 301 Colorado Springs, CO 80909 (719) 473-9595 DOB: March 20, 1972 Citizenship: USA CURRENT POSITION 2008-present Retina Consultants
Retina Consultants of Southern Colorado, P.C. 3030 North Circle Drive, Suite 301 Colorado Springs, CO 80909 (719) 473-9595 DOB: March 20, 1972 Citizenship: USA CURRENT POSITION 2008-present Retina Consultants
연령연관황반변성에서망막혈관종성증식과동반된망막색소상피박리의임상양상과일차적인광역학치료의결과
 연령연관황반변성에서망막혈관종성증식과동반된망막색소상피박리의임상양상과일차적인광역학치료의결과 40 Table. Clinical characteristics and results of patients undergoing photodynamic therapy for retinal angiomatous proliferation Patients No. Age/ sex Eye
연령연관황반변성에서망막혈관종성증식과동반된망막색소상피박리의임상양상과일차적인광역학치료의결과 40 Table. Clinical characteristics and results of patients undergoing photodynamic therapy for retinal angiomatous proliferation Patients No. Age/ sex Eye
Age-Related Macular Degeneration (AMD)
 Age-Related Macular Degeneration (AMD) What is the Macula? What is Dry AMD (Age-related Macular Degeneration)? Dry AMD is an aging process that causes accumulation of waste product under the macula leading
Age-Related Macular Degeneration (AMD) What is the Macula? What is Dry AMD (Age-related Macular Degeneration)? Dry AMD is an aging process that causes accumulation of waste product under the macula leading
Instudies of vascular endothelial growth factor
 MONITORING THERAPEUTIC EFFICACY IN THE ANTI-VEGF ERA* SriniVas R. Sadda, MD ABSTRACT One of the most important questions with vascular endothelial growth factor (VEGF) inhibitors for age-related macular
MONITORING THERAPEUTIC EFFICACY IN THE ANTI-VEGF ERA* SriniVas R. Sadda, MD ABSTRACT One of the most important questions with vascular endothelial growth factor (VEGF) inhibitors for age-related macular
Systemic bevacizumab (Avastin) therapy for exudative neovascular age-related macular degeneration. The BEAT-AMD-Study
 1 Department of Ophthalmology, The Ludwig Boltzmann Institute for Retinology and Biomicroscopic Lasersurgery, Rudolf Foundation Clinic, Vienna, Austria; 2 Department of Internal Medicine, Oncology, Rudolf
1 Department of Ophthalmology, The Ludwig Boltzmann Institute for Retinology and Biomicroscopic Lasersurgery, Rudolf Foundation Clinic, Vienna, Austria; 2 Department of Internal Medicine, Oncology, Rudolf
Anti VEGF Agents in Retinal Disorders Current Scenario
 Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Long-term Management of AMD. Motasem Al-latayfeh, MD Assistant Prof. Ophthalmology Hashemite University Jordan
 Long-term Management of AMD Motasem Al-latayfeh, MD Assistant Prof. Ophthalmology Hashemite University Jordan DEFINITION 1 Age-related macular degeneration (AMD) is a disorder of the macula characterized
Long-term Management of AMD Motasem Al-latayfeh, MD Assistant Prof. Ophthalmology Hashemite University Jordan DEFINITION 1 Age-related macular degeneration (AMD) is a disorder of the macula characterized
Clinically Significant Macular Edema (CSME)
 Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
Fluorescein Angiography
 Last revision: October 2011 by Luis Arias Fluorescein Angiography Authors: Luis Arias, MD Hospital Universitari de Bellvitge - University of Barcelona. Spain Jordi Monés, MD Institut de la Màcula i de
Last revision: October 2011 by Luis Arias Fluorescein Angiography Authors: Luis Arias, MD Hospital Universitari de Bellvitge - University of Barcelona. Spain Jordi Monés, MD Institut de la Màcula i de
NEOVASCULAR AGE-RELATED MACULAR DEGENERation
 Randomized, Double-Masked, -Controlled Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration: PIER Study Year 2 PREMA ABRAHAM, HUIBIN YUE, AND LAURA WILSON PURPOSE: To evaluate efficacy
Randomized, Double-Masked, -Controlled Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration: PIER Study Year 2 PREMA ABRAHAM, HUIBIN YUE, AND LAURA WILSON PURPOSE: To evaluate efficacy
OPHTHALMOLOGIC POLICY: VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) INHIBITORS
 UnitedHealthcare Commercial Medical Benefit Drug Policy OPHTHALMOLOGIC POLICY: VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) INHIBITORS Policy Number: PHA020 Effective Date: September 1, 2018 Table of Contents
UnitedHealthcare Commercial Medical Benefit Drug Policy OPHTHALMOLOGIC POLICY: VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) INHIBITORS Policy Number: PHA020 Effective Date: September 1, 2018 Table of Contents
Intraocular Radiation Therapy for Age-Related Macular Degeneration
 Intraocular Radiation Therapy for Age-Related Macular Degeneration Policy Number: 9.03.20 Last Review: 9/2014 Origination: 9/2008 Next Review: 9/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Intraocular Radiation Therapy for Age-Related Macular Degeneration Policy Number: 9.03.20 Last Review: 9/2014 Origination: 9/2008 Next Review: 9/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION. POLICY NUMBER: CATEGORY: Therapy/Rehabilitation
 MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
MEDICAL POLICY SUBJECT: COGNITIVE REHABILITATION PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
Original Article Intravitreal injections of ranibizumab in treatment of idiopathic choroidal neovascularization
 Int J Clin Exp Med 2016;9(2):3780-3784 www.ijcem.com /ISSN:1940-5901/IJCEM0017597 Original Article Intravitreal injections of ranibizumab in treatment of idiopathic choroidal neovascularization Donghua
Int J Clin Exp Med 2016;9(2):3780-3784 www.ijcem.com /ISSN:1940-5901/IJCEM0017597 Original Article Intravitreal injections of ranibizumab in treatment of idiopathic choroidal neovascularization Donghua
PHOTODYNAMIC THERAPY (PDT) WITH VERTEPORFIN IS
 Verteporfin and Intravitreal Triamcinolone Acetonide Combination Therapy for Occult Choroidal Neovascularization in Age-Related Macular Degeneration ALBERT J. AUGUSTIN, MD, AND URSULA SCHMIDT-ERFURTH,
Verteporfin and Intravitreal Triamcinolone Acetonide Combination Therapy for Occult Choroidal Neovascularization in Age-Related Macular Degeneration ALBERT J. AUGUSTIN, MD, AND URSULA SCHMIDT-ERFURTH,
Risk factors of a reduced response to ranibizumab treatment for neovascular age-related macular degeneration evaluation in a clinical setting
 Korb et al. BMC Ophthalmology 2013, 13:84 RESEARCH ARTICLE Open Access Risk factors of a reduced response to ranibizumab treatment for neovascular age-related macular degeneration evaluation in a clinical
Korb et al. BMC Ophthalmology 2013, 13:84 RESEARCH ARTICLE Open Access Risk factors of a reduced response to ranibizumab treatment for neovascular age-related macular degeneration evaluation in a clinical
Ophthalmology Macular Pathways
 Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
Ophthalmology Macular Pathways Age related Macular Degeneration Diabetic Macular Oedema Macular Oedema secondary to Central Retinal Macular Oedema secondary to Branch Retinal CNV associated with pathological
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Lucentis) Reference Number: CP.PHAR.186 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Lucentis) Reference Number: CP.PHAR.186 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy
 Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy Fawwaz Al Mamoori* Medical Retina Department, Eye Specialty Hospital,
Cronicon OPEN ACCESS EC OPHTHALMOLOGY Case Report IQ 532 Micropulse Green Laser treatment for Refractory Chronic Central Serous Retinopathy Fawwaz Al Mamoori* Medical Retina Department, Eye Specialty Hospital,
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- AIDED DETECTION (CAD) POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Bilateral Elevated Macular Lesions
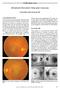 Challenging Case Bilateral Elevated Macular Lesions Section Editor: Alireza Ramezani, MD Case presentation A 65-year-old woman presented with decreased vision in both eyes of 2 months duration. She reported
Challenging Case Bilateral Elevated Macular Lesions Section Editor: Alireza Ramezani, MD Case presentation A 65-year-old woman presented with decreased vision in both eyes of 2 months duration. She reported
AGE-RELATED MACULAR DEGENERATION IS THE LEADing
 Photodynamic Therapy for Age-related Macular Degeneration in a Clinical Setting: Visual Results and Angiographic Patterns RUTH AXER-SIEGEL, MD, RITA EHRLICH, MD, YUVAL YASSUR, MD, IRIT ROSENBLATT, MD,
Photodynamic Therapy for Age-related Macular Degeneration in a Clinical Setting: Visual Results and Angiographic Patterns RUTH AXER-SIEGEL, MD, RITA EHRLICH, MD, YUVAL YASSUR, MD, IRIT ROSENBLATT, MD,
Name of Policy: Bevacizumab, Avastin
 Name of Policy: Bevacizumab, Avastin Policy #: 377 Latest Review Date: September 2013 Category: Pharmacology Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Bevacizumab, Avastin Policy #: 377 Latest Review Date: September 2013 Category: Pharmacology Policy Grade: B Background/Definitions: As a general rule, benefits are payable under Blue Cross
Subgroup Analysis of the MARINA Study of Ranibizumab in Neovascular Age-Related Macular Degeneration
 Subgroup Analysis of the MARINA Study of in Neovascular Age-Related Macular Degeneration David S. Boyer, MD, 1 Andrew N. Antoszyk, MD, 2 Carl C. Awh, MD, 3 Robert B. Bhisitkul, MD, PhD, 4 Howard Shapiro,
Subgroup Analysis of the MARINA Study of in Neovascular Age-Related Macular Degeneration David S. Boyer, MD, 1 Andrew N. Antoszyk, MD, 2 Carl C. Awh, MD, 3 Robert B. Bhisitkul, MD, PhD, 4 Howard Shapiro,
Drug Class Update: Vascular Endothelial Growth Factors
 Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35 Salem, Oregon 97301-1079 Phone 503-947-5220 Fax 503-947-1119
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
Contractor Information. LCD Information. Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426)
 Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
Local Coverage Determination (LCD): Ophthalmic Angiography (Fluorescein and Indocyanine Green) (L34426) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website.
CLINICAL SCIENCES. Photodynamic Therapy With Verteporfin. Observations on the Introduction of a New Treatment Into Clinical Practice
 CLINICAL SCIENCES Photodynamic Therapy With Verteporfin Observations on the Introduction of a New Treatment Into Clinical Practice Oliver D. Schein, MD, MPH; Neil M. Bressler, MD; Patrick Price, MD Objective:
CLINICAL SCIENCES Photodynamic Therapy With Verteporfin Observations on the Introduction of a New Treatment Into Clinical Practice Oliver D. Schein, MD, MPH; Neil M. Bressler, MD; Patrick Price, MD Objective:
Fundamentals of Retina Coding
 Fundamentals of Retina Coding Presented by: Joy Woodke, COE, OCS Sunday, April 2, 2017 ASRS Business of Retina Meeting Dallas, TX American Academy of Ophthalmic Executives Financial Disclosure Joy Woodke,
Fundamentals of Retina Coding Presented by: Joy Woodke, COE, OCS Sunday, April 2, 2017 ASRS Business of Retina Meeting Dallas, TX American Academy of Ophthalmic Executives Financial Disclosure Joy Woodke,
Management of Neovascular AMD
 Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Original Article Ranibizumab as needed therapy for wet age-related macular degeneration combined with serous pigment epithelial detachment
 Int J Clin Exp Med 2016;9(7):13650-13656 www.ijcem.com /ISSN:1940-5901/IJCEM0026582 Original Article Ranibizumab as needed therapy for wet age-related macular degeneration combined with serous pigment
Int J Clin Exp Med 2016;9(7):13650-13656 www.ijcem.com /ISSN:1940-5901/IJCEM0026582 Original Article Ranibizumab as needed therapy for wet age-related macular degeneration combined with serous pigment
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND (TRUS)
 MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION
 THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
THE ROLE OF anti-vegf IN DIABETIC RETINOPATHY AND AGE RELATED MACULAR DEGENERATION MOESTIDJAB DEPARTMENT OF OPHTHALMOLOGY SCHOOL OF MEDICINE AIRLANGGA UNIVERSITY DR SOETOMO HOSPITAL SURABAYA INTRODUCTION
Update on Central Serous Chorioretinopathy
 Update on Central Serous Chorioretinopathy Steve Bennett Retina Update 2017 No financial interest Off label use of Spironolactone, Eplerenone, Melatonin, Verteporfin, Rifampin 1 2 Classic Central Serous
Update on Central Serous Chorioretinopathy Steve Bennett Retina Update 2017 No financial interest Off label use of Spironolactone, Eplerenone, Melatonin, Verteporfin, Rifampin 1 2 Classic Central Serous
Optimizing the Anti-VEGF Treatment Strategy for Neovascular Age-Related Macular Degeneration: From Clinical Trials to Real-Life Requirements
 Perspective DOI: 10.1167/tvst.4.3.6 Optimizing the Anti-VEGF Treatment Strategy for Neovascular Age-Related Macular Degeneration: From Clinical Trials to Real-Life Requirements Irmela Mantel 1,2 1 Department
Perspective DOI: 10.1167/tvst.4.3.6 Optimizing the Anti-VEGF Treatment Strategy for Neovascular Age-Related Macular Degeneration: From Clinical Trials to Real-Life Requirements Irmela Mantel 1,2 1 Department
There are no published randomized, double-blind trials comparing aflibercept to other therapies in neovascular AMD.
 Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Acknowledgements. Outline. Who were von Hippel and Lindau? Eugen von Hippel German Ophthalmologist
 Ophthalmic Therapies & Standard of Care Acknowledgements Eric Jonasch, MD & Surena Matin, MD Collaborators Franco DeMonte, MD Marcy Johnson Ian McCutcheon, MD Chaan Ng, MD Nancy Perrier, MD Dawid Schellingerhout,
Ophthalmic Therapies & Standard of Care Acknowledgements Eric Jonasch, MD & Surena Matin, MD Collaborators Franco DeMonte, MD Marcy Johnson Ian McCutcheon, MD Chaan Ng, MD Nancy Perrier, MD Dawid Schellingerhout,
Photodynamic therapy. in the treatment of subfoveal choroidal neovascu larisation
 Photodynamic therapy SIMON HARDING in the treatment of subfoveal choroidal neovascu larisation Abstract Sub foveal choroidal neovascularisation (CNV) is a major cause of visual disability, with agerelated
Photodynamic therapy SIMON HARDING in the treatment of subfoveal choroidal neovascu larisation Abstract Sub foveal choroidal neovascularisation (CNV) is a major cause of visual disability, with agerelated
The Era of anti- - - VEGF Kirk L. Halvorson, OD
 The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
The Era of anti- - - VEGF Kirk L. Halvorson, OD Introduction: Anti- - - Vascular Endothelial Growth Factor (Anti- - - VEGF) medication is a relatively a new line of medications used in treating a variety
Since /01/2014 Private practice Ophthalmology, Retinal Medical Specialty and AMD, private office
 Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist SELARL CABINET DOCTEUR GONZALEZ 27, Boulevard des Minimes 31 200 Toulouse! 05 34 40 77 30 e-mail : cabinet.dr.gonzalez@wanadoo.fr Fax
Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist SELARL CABINET DOCTEUR GONZALEZ 27, Boulevard des Minimes 31 200 Toulouse! 05 34 40 77 30 e-mail : cabinet.dr.gonzalez@wanadoo.fr Fax
P. Stavrakas MD PhD. Consultant Ophthalmologist and vitreoretinal surgeon 2 nd Department of Ophthalmology University of Athens
 P. Stavrakas MD PhD Consultant Ophthalmologist and vitreoretinal surgeon 2 nd Department of Ophthalmology University of Athens Primary diagnose of exudative AMD Anti-VEGF monotherapy treatment Loss of
P. Stavrakas MD PhD Consultant Ophthalmologist and vitreoretinal surgeon 2 nd Department of Ophthalmology University of Athens Primary diagnose of exudative AMD Anti-VEGF monotherapy treatment Loss of
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health Technology Appraisal. Aflibercept for treating diabetic macular oedema.
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Aflibercept for treating diabetic macular oedema Final scope Final remit/appraisal objective To appraise the clinical and cost
2009 REIMBURSEMENT GUIDE, VISUCAM and VISUCAM NM/FA
 2009 REIMBURSEMENT GUIDE FF 450 PLUS PRO NM, VISUCAM and VISUCAM NM/FA Zeiss Fundus Cameras INTRODUCTION The following guide provides an overview of billing and reimbursement for procedures performed with
2009 REIMBURSEMENT GUIDE FF 450 PLUS PRO NM, VISUCAM and VISUCAM NM/FA Zeiss Fundus Cameras INTRODUCTION The following guide provides an overview of billing and reimbursement for procedures performed with
MEDICAL POLICY SUBJECT: CORNEAL ULTRASOUND PACHYMETRY. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: CORNEAL ULTRASOUND,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: CORNEAL ULTRASOUND,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Authors. Introduction. Introduction. Materials and Methods. Objective 10/27/2015
 Idiopathic Polypoidal Choroidal Vasculopathy (IPCV) in Thai Population Presenting with Choroidal Neovascularization (CNV) A multicenter study Authors Yonrawee Piyacomn 1, Chavakij Bhoomibunchoo 1, Yosanan
Idiopathic Polypoidal Choroidal Vasculopathy (IPCV) in Thai Population Presenting with Choroidal Neovascularization (CNV) A multicenter study Authors Yonrawee Piyacomn 1, Chavakij Bhoomibunchoo 1, Yosanan
MEDICAL POLICY SUBJECT: TUMOR CHEMORESISTANCE AND CHEMOSENSITIVITY ASSAYS. POLICY NUMBER: CATEGORY: Laboratory Test
 MEDICAL POLICY SUBJECT: TUMOR CHEMORESISTANCE AND PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: TUMOR CHEMORESISTANCE AND PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Clinical Policy: Implantable Miniature Telescope for Age Related Macular Degeneration Reference Number: CP.MP.517
 Clinical Policy: Implantable Miniature Telescope for Age Related Macular Reference Number: CP.MP.517 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Clinical Policy: Implantable Miniature Telescope for Age Related Macular Reference Number: CP.MP.517 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
