Quelicin TM SUCCINYLCHOLINE CHLORIDE INJECTION, USP
|
|
|
- Jessie Osborne
- 5 years ago
- Views:
Transcription
1 Quelicin TM SUCCINYLCHOLINE CHLORIDE INJECTION, USP A short-acting depolarizing skeletal muscle relaxant. Fliptop Vial Rx only WARNING RISK OF CARDIAC ARREST FROM HYPERKALEMIC RHABDOMYOLYSIS There have been rare reports of acute rhabdomyolysis with hyperkalemia followed by ventricular dysrhythmias, cardiac arrest and death after the administration of succinylcholine to apparently healthy pediatric patients who were subsequently found to have undiagnosed skeletal muscle myopathy, most frequently Duchenne s muscular dystrophy. This syndrome often presents as peaked T-waves and sudden cardiac arrest within minutes after the administration of the drug in healthy appearing pediatric patients (usually, but not exclusively, males, and most frequently 8 years of age or younger). There have also been reports in adolescents. Therefore, when a healthy appearing infant or child develops cardiac arrest soon after administration of succinylcholine, not felt to be due to inadequate ventilation, oxygenation or anesthetic overdose, immediate treatment for hyperkalemia should be instituted. This should include administration of intravenous calcium, bicarbonate, and glucose with insulin, with hyperventilation. Due to the abrupt onset of this syndrome, routine resuscitative measures are likely to be unsuccessful. However, extraordinary and prolonged resuscitative efforts have resulted in successful resuscitation in some reported cases. In addition, in the presence of signs of malignant hyperthermia, appropriate treatment should be instituted concurrently. Since there may be no signs or symptoms to alert the practitioner to which patients are at risk, it is recommended that the use of succinylcholine in pediatric patients should be reserved for emergency intubation or instances where immediate securing of the airway is necessary, e.g., laryngospasm, difficult airway, full stomach, or for intramuscular use when a suitable vein is inaccessible (see PRECAUTIONS: Pediatric Use and DOSAGE AND ADMINISTRATION). This drug should be used only by individuals familiar with its actions, characteristics and hazards. DESCRIPTION Quelicin (Succinylcholine Chloride Injection, USP) is a sterile, nonpyrogenic solution to be used as a short-acting, depolarizing, skeletal muscle relaxant. See HOW SUPPLIED for summary of content and characteristics of the solutions. The solutions are for I.M. or I.V. use. Succinylcholine Chloride, USP is chemically designated C 14 H 30 Cl 2 N 2 O 4 and its molecular weight is It has the following structural formula: Succinylcholine is a diquaternary base consisting of the dichloride salt of the dicholine ester of succinic acid. It is a white, odorless, slightly bitter powder, very soluble in water. The drug is EN-2630 Page 1 of 9
2 incompatible with alkaline solutions but relatively stable in acid solutions. Solutions of the drug lose potency unless refrigerated. Solution intended for multiple-dose administration contains 0.18% methylparaben and 0.02% propylparaben as preservatives (List No. 6629). Solution intended for single-dose administration contains no preservatives. Unused solution should be discarded. Product not requiring dilution (multiple-dose fliptop vial) contains sodium chloride to render isotonic. May contain sodium hydroxide and/or hydrochloric acid for ph adjustment. ph is 3.6 (3.0 to 4.5). See table in HOW SUPPLIED for characteristics. Sodium Chloride, USP, chemically designated NaCl, is a white crystalline compound freely soluble in water. CLINICAL PHARMACOLOGY Succinylcholine is a depolarizing skeletal muscle relaxant. As does acetylcholine, it combines with the cholinergic receptors of the motor end plate to produce depolarization. This depolarization may be observed as fasciculations. Subsequent neuromuscular transmission is inhibited so long as adequate concentration of succinylcholine remains at the receptor site. Onset of flaccid paralysis is rapid (less than one minute after intravenous administration), and with single administration lasts approximately 4 to 6 minutes. Succinylcholine is rapidly hydrolyzed by plasma cholinesterase to succinylmonocholine (which possesses clinically insignificant depolarizing muscle relaxant properties) and then more slowly to succinic acid and choline (see PRECAUTIONS). About 10% of the drug is excreted unchanged in the urine. Succinylcholine levels were reported to be below the detection limit of 2 µg/ml after 2.5 minutes of an IV bolus dose of 1 or 2 mg/kg in fourteen (14) anesthetized patients. The paralysis following administration of succinylcholine is progressive, with differing sensitivities of different muscles. This initially involves consecutively the levator muscles of the face, muscles of the glottis and finally the intercostals and the diaphragm and all other skeletal muscles. Succinylcholine has no direct action on the uterus or other smooth muscle structures. Because it is highly ionized and has low fat solubility, it does not readily cross the placenta. Tachyphylaxis occurs with repeated administration (see PRECAUTIONS). Depending on the dose and duration of succinylcholine administration, the characteristic depolarizing neuromuscular block (Phase I block) may change to a block with characteristics superficially resembling a non-depolarizing block (Phase II block). This may be associated with prolonged respiratory muscle paralysis or weakness in patients who manifest the transition to Phase II block. When this diagnosis is confirmed by peripheral nerve stimulation, it may sometimes be reversed with anticholinesterase drugs such as neostigmine (see PRECAUTIONS). Anticholinesterase drugs may not always be effective. If given before succinylcholine is metabolized by cholinesterase, anticholinesterase drugs may prolong rather than shorten paralysis. Succinylcholine has no direct effect on the myocardium. Succinylcholine stimulates both autonomic ganglia and muscarinic receptors which may cause changes in cardiac rhythm, including cardiac arrest. Changes in rhythm, including cardiac arrest, may also result from vagal stimulation, which may occur during surgical procedures, or from hyperkalemia, particularly in pediatric patients (see PRECAUTIONS: Pediatric Use). These effects are enhanced by halogenated anesthetics. Succinylcholine causes an increase in intraocular pressure immediately after its injection and during the fasciculation phase, and slight increases which may persist after onset of complete paralysis (see WARNINGS). Succinylcholine may cause slight increases in intracranial pressure immediately after its injection and during the fasciculation phase (see PRECAUTIONS). EN-2630 Page 2 of 9
3 As with other neuromuscular blocking agents, the potential for releasing histamine is present following succinylcholine administration. Signs and symptoms of histamine mediated release such as flushing, hypotension and bronchoconstriction are, however, uncommon in normal clinical usage. Succinylcholine has no effect on consciousness, pain threshold or cerebration. It should be used only with adequate anesthesia (see WARNINGS). INDICATIONS AND USAGE Succinylcholine chloride is indicated as an adjunct to general anesthesia, to facilitate tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation. CONTRAINDICATIONS Succinylcholine is contraindicated in persons with personal or familial history of malignant hyperthermia, skeletal muscle myopathies and known hypersensitivity to the drug. It is also contraindicated in patients after the acute phase of injury following major burns, multiple trauma, extensive denervation of skeletal muscle, or upper motor neuron injury, because succinylcholine administered to such individuals may result in severe hyperkalemia which may result in cardiac arrest (see WARNINGS). The risk of hyperkalemia in these patients increases over time and usually peaks at 7 to 10 days after the injury. The risk is dependent on the extent and location of the injury. The precise time of onset and the duration of the risk period are not known. WARNINGS SUCCINYLCHOLINE SHOULD BE USED ONLY BY THOSE SKILLED IN THE MANAGEMENT OF ARTIFICIAL RESPIRATION AND ONLY WHEN FACILITIES ARE INSTANTLY AVAILABLE FOR TRACHEAL INTUBATION AND FOR PROVIDING ADEQUATE VENTILATION OF THE PATIENT, INCLUDING THE ADMINISTRATION OF OXYGEN UNDER POSITIVE PRESSURE AND THE ELIMINATION OF CARBON DIOXIDE. THE CLINICIAN MUST BE PREPARED TO ASSIST OR CONTROL RESPIRATION. TO AVOID DISTRESS TO THE PATIENT, SUCCINYLCHOLINE SHOULD NOT BE ADMINISTERED BEFORE UNCONSCIOUSNESS HAS BEEN INDUCED. IN EMERGENCY SITUATIONS, HOWEVER, IT MAY BE NECESSARY TO ADMINISTER SUCCINYLCHOLINE BEFORE UNCONSCIOUSNESS IS INDUCED. SUCCINYLCHOLINE IS METABOLIZED BY PLASMA CHOLINESTERASE AND SHOULD BE USED WITH CAUTION, IF AT ALL, IN PATIENTS KNOWN TO BE OR SUSPECTED OF BEING HOMOZYGOUS FOR THE ATYPICAL PLASMA CHOLINESTERASE GENE. Hyperkalemia: (SEE BOX WARNING) Succinylcholine should be administered with GREAT CAUTION to patients suffering from electrolyte abnormalities and those who may have massive digitalis toxicity, because in these circumstances succinylcholine may induce serious cardiac arrhythmias or cardiac arrest due to hyperkalemia. GREAT CAUTION should be observed if succinylcholine is administered to patients during the acute phase of injury following major burns, multiple trauma, extensive denervation of skeletal muscle, or upper motor neuron injury (see CONTRAINDICATIONS). The risk of hyperkalemia in these patients increases over time and usually peaks at 7 to 10 days after the injury. The risk is dependent on the extent and location of the injury. The precise time of onset and the duration of the risk period are undetermined. Patients with chronic abdominal infection, subarachnoid hemorrhage, or conditions causing degeneration of central and peripheral nervous systems should receive succinylcholine with GREAT CAUTION because of the potential for developing severe hyperkalemia. Anaphylaxis Severe anaphylactic reactions to neuromuscular blocking agents, including succinylcholine, have been reported. These reactions have, in some cases, been life-threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency EN-2630 Page 3 of 9
4 treatment, should be taken. Precautions should also be taken in those individuals who have had previous anaphylactic reactions to other neuromuscular blocking agents since cross-reactivity between neuromuscular blocking agents, both depolarizing and non-depolarizing, has been reported in this class of drugs. Malignant Hyperthermia: Succinylcholine administration has been associated with acute onset of malignant hyperthermia, a potentially fatal hypermetabolic state of skeletal muscle. The risk of developing malignant hyperthermia following succinylcholine administration increases with the concomitant administration of volatile anesthetics. Malignant hyperthermia frequently presents as intractable spasm of the jaw muscles (masseter spasm) which may progress to generalized rigidity, increased oxygen demand, tachycardia, tachypnea and profound hyperpyrexia. Successful outcome depends on recognition of early signs, such as jaw muscle spasm, acidosis, or generalized rigidity to initial administration of succinylcholine for tracheal intubation, or failure of tachycardia to respond to deepening anesthesia. Skin mottling, rising temperature and coagulopathies may occur later in the course of the hypermetabolic process. Recognition of the syndrome is a signal for discontinuance of anesthesia, attention to increased oxygen consumption, correction of acidosis, support of circulation, assurance of adequate urinary output and institution of measures to control rising temperature. Intravenous dantrolene sodium is recommended as an adjunct to supportive measures in the management of this problem. Consult literature references and the dantrolene prescribing information for additional information about the management of malignant hyperthermic crisis. Continuous monitoring of temperature and expired CO 2 is recommended as an aid to early recognition of malignant hyperthermia. Other: In both adults and pediatric patients the incidence of bradycardia, which may progress to asystole, is higher following a second dose of succinylcholine. The incidence and severity of bradycardia is higher in pediatric patients than adults. Whereas bradycardia is common in pediatric patients after an initial dose of 1.5 mg/kg, bradycardia is seen in adults only after repeated exposure. Pretreatment with anticholinergic agents (e.g., atropine) may reduce the occurrence of bradyarrhythmias. Succinylcholine causes an increase in intraocular pressure. It should not be used in instances in which an increase in intraocular pressure is undesirable (e.g., narrow angle glaucoma, penetrating eye injury) unless the potential benefit of its use outweighs the potential risk. Succinylcholine is acidic (ph = 3.5) and should not be mixed with alkaline solutions having a ph greater than 8.5 (e.g., barbiturate solutions). PRECAUTIONS: (SEE BOX WARNING) General: When succinylcholine is given over a prolonged period of time, the characteristic depolarization block of the myoneural junction (Phase I block) may change to a block with characteristics superficially resembling a non-depolarizing block (Phase II block). Prolonged respiratory muscle paralysis or weakness may be observed in patients manifesting this transition to Phase II block. The transition from Phase I to Phase II block has been reported in 7 of 7 patients studied under halothane anesthesia after an accumulated dose of 2 to 4 mg/kg succinylcholine (administered in repeated, divided doses). The onset of Phase II block coincided with the onset of tachyphylaxis and prolongation of spontaneous recovery. In another study, using balanced anesthesia (N 2 O/O 2 /narcotic-thiopental) and succinylcholine infusion, the transition was less abrupt, with great individual variability in the dose of succinylcholine required to produce Phase II block. Of 32 patients studied, 24 developed Phase II block. Tachyphylaxis was not associated with the transition to Phase II block, and 50% of the patients who developed Phase II block experienced prolonged recovery. When Phase II block is suspected in cases of prolonged neuromuscular blockade, positive diagnosis should be made by peripheral nerve stimulation, prior to administration of any anticholinesterase drug. Reversal of Phase II block is a medical decision which must be made upon the basis of the individual, clinical pharmacology and the experience and judgment of the physician. The presence of Phase II block is indicated by fade of responses to successive stimuli (preferably "train of four"). The use of an EN-2630 Page 4 of 9
5 anticholinesterase drug to reverse Phase II block should be accompanied by appropriate doses of an anticholinergic drug to prevent disturbances of cardiac rhythm. After adequate reversal of Phase II block with an anticholinesterase agent, the patient should be continually observed for at least 1 hour for signs of return of muscle relaxation. Reversal should not be attempted unless: (1) a peripheral nerve stimulator is used to determine the presence of Phase II block (since anticholinesterase agents will potentiate succinylcholine-induced Phase I block), and (2) spontaneous recovery of muscle twitch has been observed for at least 20 minutes and has reached a plateau with further recovery proceeding slowly; this delay is to ensure complete hydrolysis of succinylcholine by plasma cholinesterase prior to administration of the anticholinesterase agent. Should the type of block be misdiagnosed, depolarization of the type initially induced by succinylcholine (i.e., Phase I block) will be prolonged by an anticholinesterase agent. Succinylcholine should be employed with caution in patients with fractures or muscle spasm because the initial muscle fasciculations may cause additional trauma. Succinylcholine may cause a transient increase in intracranial pressure; however, adequate anesthetic induction prior to administration of succinylcholine will minimize this effect. Succinylcholine may increase intragastric pressure, which could result in regurgitation and possible aspiration of stomach contents. Neuromuscular blockade may be prolonged in patients with hypokalemia or hypocalcemia. Since allergic cross-reactivity has been reported in this class, request information from your patients about previous anaphylactic reactions to other neuromuscular blocking agents. In addition, inform your patients that severe anaphylactic reactions to neuromuscular blocking agents, including succinylcholine have been reported. Reduced Plasma Cholinesterase Activity: Succinylcholine should be used carefully in patients with reduced plasma cholinesterase (pseudocholinesterase) activity. The likelihood of prolonged neuromuscular block following administration of succinylcholine must be considered in such patients (see DOSAGE AND ADMINISTRATION). Plasma cholinesterase activity may be diminished in the presence of genetic abnormalities of plasma cholinesterase (e.g., patients heterozygous or homozygous for atypical plasma cholinesterase gene), pregnancy, severe liver or kidney disease, malignant tumors, infections, burns, anemia, decompensated heart disease, peptic ulcer, or myxedema. Plasma cholinesterase activity may also be diminished by chronic administration of oral contraceptives, glucocorticoids, or certain monoamine oxidase inhibitors and by irreversible inhibitors of plasma cholinesterase (e.g., organophosphate insecticides, echothiophate, and certain antineoplastic drugs). Patients homozygous for atypical plasma cholinesterase gene (1 in 2500 patients) are extremely sensitive to the neuromuscular blocking effect of succinylcholine. In these patients, a 5 to 10 mg test dose of succinylcholine may be administered to evaluate sensitivity to succinylcholine, or neuromuscular blockade may be produced by the cautious administration of a 1 mg/ml solution of succinylcholine by slow intravenous infusion. Apnea or prolonged muscle paralysis should be treated with controlled respiration. Drug Interactions: Drugs which may enhance the neuromuscular blocking action of succinylcholine include: promazine, oxytocin, aprotinin, certain non-penicillin antibiotics, quinidine, β-adrenergic blockers, procainamide, lidocaine, trimethaphan, lithium carbonate, magnesium salts, quinine, chloroquine, diethylether, isoflurane, desflurane, metoclopramide and terbutaline. The neuromuscular blocking effect of succinylcholine may be enhanced by drugs that reduce plasma cholinesterase activity (e.g., chronically administered oral contraceptives, glucocorticoids, or certain monoamine oxidase inhibitors) or by drugs that irreversibly inhibit plasma cholinesterase (see PRECAUTIONS). If other neuromuscular blocking agents are to be used during the same procedure, the possibility of a synergistic or antagonistic effect should be considered. EN-2630 Page 5 of 9
6 Carcinogenesis, Mutagenesis, Impairment of Fertility: There have been no long-term studies performed in animals to evaluate carcinogenic potential. Pregnancy: Teratogenic Effects: Pregnancy Category C. Animal reproduction studies have not been conducted with succinylcholine chloride. It is also not known whether succinylcholine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Succinylcholine should be given to a pregnant woman only if clearly needed. Nonteratogenic Effects: Plasma cholinesterase levels are decreased by approximately 24% during pregnancy and for several days postpartum. Therefore, a higher proportion of patients may be expected to show increased sensitivity (prolonged apnea) to succinylcholine when pregnant than when nonpregnant. Labor and Delivery: Succinylcholine is commonly used to provide muscle relaxation during delivery by caesarean section. While small amounts of succinylcholine are known to cross the placental barrier, under normal conditions the quantity of drug that enters fetal circulation after a single dose of 1 mg/kg to the mother should not endanger the fetus. However, since the amount of drug that crosses the placental barrier is dependent on the concentration gradient between the maternal and fetal circulations, residual neuromuscular blockade (apnea and flaccidity) may occur in the newborn after repeated high doses to, or in the presence of atypical plasma cholinesterase in, the mother. Nursing Mothers: It is not known whether succinylcholine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised following succinylcholine administration to a nursing woman. Pediatric Use: Safety and effectiveness of succinylcholine chloride have been established in pediatric patient age groups, neonate to adolescent. There are rare reports of ventricular dysrhythmias and cardiac arrest secondary to acute rhabdomyolysis with hyperkalemia in apparently healthy pediatric patients who receive succinylcholine (see BOX WARNING). Many of these pediatric patients were subsequently found to have a skeletal muscle myopathy such as Duchenne s muscular dystrophy whose clinical signs were not obvious. The syndrome often presents as sudden cardiac arrest within minutes after the administration of succinylcholine. These pediatric patients are usually, but not exclusively, males, and most frequently 8 years of age or younger. There have also been reports in adolescents. There may be no signs or symptoms to alert the practitioner to which patients are at risk. A careful history and physical may identify developmental delays suggestive of a myopathy. A preoperative creatine kinase could identify some but not all patients at risk. Due to the abrupt onset of this syndrome, routine resuscitative measures are likely to be unsuccessful. Careful monitoring of the electrocardiogram may alert the practitioner to peaked T-waves (an early sign). Administration of intravenous calcium, bicarbonate, and glucose with insulin, with hyperventilation have resulted in successful resuscitation in some of the reported cases. Extraordinary and prolonged resuscitative efforts have been effective in some cases. In addition, in the presence of signs of malignant hyperthermia, appropriate treatment should be initiated concurrently (see WARNINGS). Since it is difficult to identify which patients are at risk, it is recommended that the use of succinylcholine in pediatric patients should be reserved for emergency intubation or instances where immediate securing of the airway is necessary, e.g., laryngospasm, difficult airway, full stomach, or for intramuscular use when a suitable vein is inaccessible. As in adults, the incidence of bradycardia in pediatric patients is higher following the second dose of succinylcholine. The incidence and severity of bradycardia is higher in pediatric patients than adults. Pretreatment with anticholinergic agents, e.g., atropine, may reduce the occurrence of bradyarrhythmias. Geriatric Use: Clinical studies of Quelicin did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. EN-2630 Page 6 of 9
7 ADVERSE REACTIONS Adverse reactions to succinylcholine consist primarily of an extension of its pharmacological actions. Succinylcholine causes profound muscle relaxation resulting in respiratory depression to the point of apnea; this effect may be prolonged. Hypersensitivity reactions, including anaphylaxis, may occur in rare instances. The following additional adverse reactions have been reported: cardiac arrest, malignant hyperthermia, arrhythmias, bradycardia, tachycardia, hypertension, hypotension, hyperkalemia, prolonged respiratory depression or apnea, increased intraocular pressure, muscle fasciculation, jaw rigidity, postoperative muscle pain, rhabdomyolysis with possible myoglobinuric acute renal failure, excessive salivation, and rash. There have been post-marketing reports of severe allergic reactions (anaphylactic and anaphylactoid reactions) associated with use of neuromuscular blocking agents, including succinylcholine. These reactions, in some cases, have been life threatening and fatal. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency (See WARNINGS and PRECAUTIONS). OVERDOSAGE Overdosage with succinylcholine may result in neuromuscular block beyond the time needed for surgery and anesthesia. This may be manifested by skeletal muscle weakness, decreased respiratory reserve, low tidal volume, or apnea. The primary treatment is maintenance of a patent airway and respiratory support until recovery of normal respiration is assured. Depending on the dose and duration of succinylcholine administration, the characteristic depolarizing neuromuscular block (Phase I) may change to a block with characteristics superficially resembling a non-depolarizing block (Phase II) (see PRECAUTIONS). DOSAGE AND ADMINISTRATION The dosage of succinylcholine should be individualized and should always be determined by the clinician after careful assessment of the patient (see WARNINGS). Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Solutions which are not clear and colorless should not be used. Adults: For Short Surgical Procedures: The average dose required to produce neuromuscular blockade and to facilitate tracheal intubation is 0.6 mg/kg Quelicin (succinylcholine chloride) Injection given intravenously. The optimum dose will vary among individuals and may be from 0.3 to 1.1 mg/kg for adults. Following administration of doses in this range, neuromuscular blockade develops in about 1 minute; maximum blockade may persist for about 2 minutes, after which recovery takes place within 4 to 6 minutes. However, very large doses may result in more prolonged blockade. A 5 to 10 mg test dose may be used to determine the sensitivity of the patient and the individual recovery time (see PRECAUTIONS). For Long Surgical Procedures: The dose of succinylcholine administered by infusion depends upon the duration of the surgical procedure and the need for muscle relaxation. The average rate for an adult ranges between 2.5 and 4.3 mg per minute. Solutions containing from 1 to 2 mg per ml succinylcholine have commonly been used for continuous infusion. The more dilute solution (1 mg per ml) is probably preferable from the standpoint of ease of control of the rate of administration of the drug and, hence, of relaxation. This intravenous solution containing 1 mg per ml may be administered at a rate of 0.5 mg (0.5 ml) to 10 mg (10 ml) per minute to obtain the required amount of relaxation. The amount required per minute will depend upon the individual response as well as the degree of relaxation required. Avoid overburdening the circulation with a large volume of fluid. It is recommended that neuromuscular function be carefully monitored with a peripheral nerve stimulator when using succinylcholine by infusion in order to avoid overdose, detect EN-2630 Page 7 of 9
8 development of Phase II block, follow its rate of recovery, and assess the effects of reversing agents (see PRECAUTIONS). Intermittent intravenous injections of succinylcholine may also be used to provide muscle relaxation for long procedures. An intravenous injection of 0.3 to 1.1 mg/kg may be given initially, followed, at appropriate intervals, by further injections of 0.04 to 0.07 mg/kg to maintain the degree of relaxation required. Pediatrics: For emergency tracheal intubation or in instances where immediate securing of the airway is necessary, the intravenous dose of succinylcholine is 2 mg/kg for infants and small pediatric patients; for older pediatric patients and adolescents the dose is 1 mg/kg (see BOX WARNING and PRECAUTIONS: Pediatric Use). It is currently known that the effective dose of succinylcholine in pediatric patients may be higher than that predicted by body weight dosing alone. For example, the usual adult IV dose of 0.6 mg/kg is comparable to a dose of 2-3 mg/kg in neonates and infants to 6 months and 1-2 mg/kg in infants up to 2 years of age. This is thought to be due to the relatively large volume of distribution in the pediatric patient versus the adult patient. Rarely, I.V. bolus administration of succinylcholine in infants and pediatric patients may result in malignant ventricular arrythmias and cardiac arrest secondary to acute rhabdomyolysis with hyperkalemia. In such situations, an underlying myopathy should be suspected. Intravenous bolus administration of succinylcholine in infants or pediatric patients may result in profound bradycardia or, rarely, asystole. As in adults, the incidence of bradycardia in pediatric patients is higher following a second dose of succinylcholine. Whereas bradycardia is common in pediatric patients after an initial dose of 1.5 mg/kg, bradycardia is seen in adults only after repeated exposure. The occurrence of bradyarrhythmias may be reduced by pretreatment with atropine (see PRECAUTIONS: Pediatric Use). Intramuscular Use: If necessary, succinylcholine may be given intramuscularly to infants, older pediatric patients or adults when a suitable vein is inaccessible. A dose of up to 3 to 4 mg/kg may be given, but not more than 150 mg total dose should be administered by this route. The onset of effect of succinylcholine given intramuscularly is usually observed in about 2 to 3 minutes. Compatibility and Admixtures: Succinylcholine is acidic (ph 3.5) and should not be mixed with alkaline solutions having a ph greater than 8.5 (e.g., barbiturate solutions). Admixtures containing 1 to 2 mg/ml may be prepared by adding 1 g Quelicin to 1000 or 500 ml sterile solution, such as 5% Dextrose Injection, USP or 0.9% Sodium Chloride Injection, USP. Admixtures of Quelicin must be used within 24 hours after preparation. Aseptic techniques should be used to prepare the diluted product. Admixtures of Quelicin should be prepared for single patient use only. The unused portion of diluted Quelicin should be discarded. To prevent needle-stick injuries, needles should not be recapped, purposely bent, or broken by hand. HOW SUPPLIED Quelicin TM (Succinylcholine Chloride Injection, USP) is supplied as a clear, colorless solution in the following concentrations and packages: mg mosmol/ml NDC No. Container Size (ml) mg/ml (total) (calc.) Single-dose Fliptop Vial 10 in Multiple-dose Fliptop Vial Refrigeration of the undiluted agent will assure full potency until expiration date. All units carry a date of expiration. EN-2630 Page 8 of 9
9 Store in refrigerator 2 to 8 C (36 to 46 F). The multi-dose vials are stable for up to 14 days at room temperature without significant loss of potency. Revised: September, 2010 Printed in USA Hospira, Inc., Lake Forest, IL USA EN-2630 EN-2630 Page 9 of 9
PRESCRIBING INFORMATION QUELICIN. Neuromuscular Blocking Agent
 PRESCRIBING INFORMATION QUELICIN Succinylcholine Chloride Injection USP Neuromuscular Blocking Agent Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland, Québec H9J 2M5 DATE OF REVISION: June 23, 2017
PRESCRIBING INFORMATION QUELICIN Succinylcholine Chloride Injection USP Neuromuscular Blocking Agent Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland, Québec H9J 2M5 DATE OF REVISION: June 23, 2017
PROSTIGMIN (neostigmine bromide)
 PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
PROSTIGMIN (neostigmine bromide) TABLETS DESCRIPTION: Prostigmin (neostigmine bromide), an anticholinesterase agent, is available for oral administration in 15 mg tablets. Each tablet also contains gelatin,
A Nondepolarizing Neuromuscular Blocking (NMB) Agent
 DOSING GUIDE A Nondepolarizing Neuromuscular Blocking (NMB) Agent Easy to remember dosing for the 0.20 mg/kg adult intubating doses of NIMBEX 1 *: For every 10 kg, give 1 ml of NIMBEX (2 mg/ml concentration)
DOSING GUIDE A Nondepolarizing Neuromuscular Blocking (NMB) Agent Easy to remember dosing for the 0.20 mg/kg adult intubating doses of NIMBEX 1 *: For every 10 kg, give 1 ml of NIMBEX (2 mg/ml concentration)
DBL NALOXONE HYDROCHLORIDE INJECTION USP
 Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
Name of medicine Naloxone hydrochloride Data Sheet New Zealand DBL NALXNE HYDRCHLRIDE INJECTIN USP Presentation DBL Naloxone Hydrochloride Injection USP is a sterile, clear, colourless solution, free from
ADVERSE REACTIONS Most common adverse reactions during treatment: nausea, vomiting, and tachycardia. (6)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AKOVAZ safely and effectively. See full prescribing information for AKOVAZ. AKOVAZ (ephedrine sulfate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AKOVAZ safely and effectively. See full prescribing information for AKOVAZ. AKOVAZ (ephedrine sulfate
NEUROMUSCULAR BLOCKING AGENTS
 NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
NEUROMUSCULAR BLOCKING AGENTS Edward JN Ishac, Ph.D. Associate Professor, Pharmacology and Toxicology Smith 742, 828-2127, Email: eishac@vcu.edu Learning Objectives: 1. Understand the physiology of the
Core Safety Profile. Pharmaceutical form(s)/strength: Solution for Injection CZ/H/PSUR/0005/002 Date of FAR:
 Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
Core Safety Profile Active substance: Rocuronium bromide Pharmaceutical form(s)/strength: Solution for Injection P-RMS: CZ/H/PSUR/0005/002 Date of FAR: 31.05.2012 4.3 Contraindications Hypersensitivity
Rx only. wen-4121v03 Page 1 of 9
 Fentanyl Citrate Injection, USP 50 mcg (0.05 mg) fentanyl/ml FOR INTRAVENOUS OR INTRAMUSCULAR USE ONLY Ampul Fliptop Vial Protect From Light. Retain In Carton Until Time Of Use. Rx only DESCRIPTION Fentanyl
Fentanyl Citrate Injection, USP 50 mcg (0.05 mg) fentanyl/ml FOR INTRAVENOUS OR INTRAMUSCULAR USE ONLY Ampul Fliptop Vial Protect From Light. Retain In Carton Until Time Of Use. Rx only DESCRIPTION Fentanyl
NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container
 NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container R x only DESCRIPTION Normosol-R is a sterile, nonpyrogenic isotonic
NORMOSOL -R MULTIPLE ELECTROLYTES INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid Flexible Plastic Container R x only DESCRIPTION Normosol-R is a sterile, nonpyrogenic isotonic
(PP XI) Dr. Samir Matloob
 DRUGS ACTING ON THE CHOLINERGIC SYSTEM AND THE NEUROMUSCULAR BLOCKING DRUGS IV (NICOTINIC ANTAGONISTS) (PP XI) Dr. Samir Matloob Dept. of Pharmacology Baghdad College of Medicine Drugs acting on the cholinergic
DRUGS ACTING ON THE CHOLINERGIC SYSTEM AND THE NEUROMUSCULAR BLOCKING DRUGS IV (NICOTINIC ANTAGONISTS) (PP XI) Dr. Samir Matloob Dept. of Pharmacology Baghdad College of Medicine Drugs acting on the cholinergic
Suxamethonium Chloride Injection BP PRODUCT INFORMATION
 Suxamethonium Chloride Injection BP Product Information 1 (8) Suxamethonium Chloride Injection BP PRODUCT INFORMATION NAME OF DRUG The Australian Approved Name is suxamethonium chloride. The CAS number
Suxamethonium Chloride Injection BP Product Information 1 (8) Suxamethonium Chloride Injection BP PRODUCT INFORMATION NAME OF DRUG The Australian Approved Name is suxamethonium chloride. The CAS number
PACKAGE INSERT CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher mosmol/ml 680 mosmol/l Ca meq/ml ph
 CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher 0.68 mosmol/ml 680 mosmol/l Ca ++ 0.465 meq/ml ph 6.0 8.2 DESCRIPTION Calcium Gluconate Injection, USP is a sterile, nonpyrogenic supersaturated
CALCIUM GLUCONATE INJECTION, USP 10% Electrolyte Replenisher 0.68 mosmol/ml 680 mosmol/l Ca ++ 0.465 meq/ml ph 6.0 8.2 DESCRIPTION Calcium Gluconate Injection, USP is a sterile, nonpyrogenic supersaturated
Core Safety Profile. Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV FI/H/PSUR/0010/002 Date of FAR:
 Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
Core Safety Profile Active substance: Esketamine Pharmaceutical form(s)/strength: 5mg/ml and 25 mg/ml, Solution for injection, IM/IV P-RMS: FI/H/PSUR/0010/002 Date of FAR: 29.05.2012 4.3 Contraindications
Normosol -R and 5% Dextrose Injection MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid
 Normosol -R and 5% Dextrose Injection MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid R x only Flexible Plastic Container DESCRIPTION Normosol-R
Normosol -R and 5% Dextrose Injection MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP For Replacing Acute Losses of Extracellular Fluid R x only Flexible Plastic Container DESCRIPTION Normosol-R
Fentanyl Citrate Injection, USP CII
 Fentanyl Citrate Injection, USP CII R x only DESCRIPTION Fentanyl Citrate Injection is a sterile, non-pyrogenic solution for intravenous or intramuscular use as a potent narcotic analgesic. Each ml contains
Fentanyl Citrate Injection, USP CII R x only DESCRIPTION Fentanyl Citrate Injection is a sterile, non-pyrogenic solution for intravenous or intramuscular use as a potent narcotic analgesic. Each ml contains
FOR REPRESENTATIVE EDUCATION
 Neuromuscular Blockade in the ICU NIMBEX Indication 1 NIMBEX (cisatracurium besylate) is indicated as an adjunct to general anesthesia to facilitate tracheal intubation in adults and in pediatric patients
Neuromuscular Blockade in the ICU NIMBEX Indication 1 NIMBEX (cisatracurium besylate) is indicated as an adjunct to general anesthesia to facilitate tracheal intubation in adults and in pediatric patients
Sodium Bicarbonate Injection, USP
 Sodium Bicarbonate Injection, USP FOR THE CORRECTION OF METABOLIC ACIDOSIS AND OTHER CONDITIONS REQUIRING SYSTEMIC ALKALINIZATION. Plastic Ansyr II Syringe R x only DESCRIPTION Sodium Bicarbonate Injection,
Sodium Bicarbonate Injection, USP FOR THE CORRECTION OF METABOLIC ACIDOSIS AND OTHER CONDITIONS REQUIRING SYSTEMIC ALKALINIZATION. Plastic Ansyr II Syringe R x only DESCRIPTION Sodium Bicarbonate Injection,
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME SUXAMETHONIUM CHLORIDE INJECTION B.P. 50 mg/ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2 ml of solution contains 100 mg of suxamethonium chloride BP For
NEW ZEALAND DATA SHEET 1. PRODUCT NAME SUXAMETHONIUM CHLORIDE INJECTION B.P. 50 mg/ml 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 2 ml of solution contains 100 mg of suxamethonium chloride BP For
NERVOUS SYSTEM NERVOUS SYSTEM. Somatic nervous system. Brain Spinal Cord Autonomic nervous system. Sympathetic nervous system
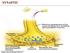 SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
SYNAPTIC NERVOUS SYSTEM NERVOUS SYSTEM CENTRAL NERVOUS SYSTEM PERIPHERAL NERVOUS SYSTEM Brain Spinal Cord Autonomic nervous system Somatic nervous system Sympathetic nervous system Parasympathetic nervous
Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise
 Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise 1. Read chapter 2. Review objectives (p.305) 3. Review key terms and definitions (p.305) Add: Cholinesterase inhibitor Vagal
Chapter 18 Neuromuscular Blocking Agents Study Guide and Application Exercise 1. Read chapter 2. Review objectives (p.305) 3. Review key terms and definitions (p.305) Add: Cholinesterase inhibitor Vagal
This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards.
 MIVACRON Injection (mivacurium chloride) This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. DESCRIPTION MIVACRON (mivacurium
MIVACRON Injection (mivacurium chloride) This drug should be administered only by adequately trained individuals familiar with its actions, characteristics, and hazards. DESCRIPTION MIVACRON (mivacurium
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1
 VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
Mepivacaine Hydrochloride Injection USP, 3%
 Mepivacaine Hydrochloride Injection USP, 3% R x only THESE SOLUTIONS ARE INTENDED FOR DENTAL USE ONLY. DESCRIPTION Mepivacaine hydrochloride, a tertiary amine used as a local anesthetic, is 1-methyl-2,6
Mepivacaine Hydrochloride Injection USP, 3% R x only THESE SOLUTIONS ARE INTENDED FOR DENTAL USE ONLY. DESCRIPTION Mepivacaine hydrochloride, a tertiary amine used as a local anesthetic, is 1-methyl-2,6
MUSCLE RELAXANTS. Mr. D.Raju, M.pharm, Lecturer
 MUSCLE RELAXANTS Mr. D.Raju, M.pharm, Lecturer Muscle Relaxants are classified as: I)Peripherally acting A.Neuromuscular blocking agents:- 1) Depolarizing muscle relaxants. 2) Non-depolarizing muscle relaxants
MUSCLE RELAXANTS Mr. D.Raju, M.pharm, Lecturer Muscle Relaxants are classified as: I)Peripherally acting A.Neuromuscular blocking agents:- 1) Depolarizing muscle relaxants. 2) Non-depolarizing muscle relaxants
Neuromuscular Junction
 Muscle Relaxants Neuromuscular Junction Cholinergic antagonists Neuromuscular-blocking agents (mostly nicotinic antagonists): interfere with transmission of efferent impulses to skeletal muscles. These
Muscle Relaxants Neuromuscular Junction Cholinergic antagonists Neuromuscular-blocking agents (mostly nicotinic antagonists): interfere with transmission of efferent impulses to skeletal muscles. These
Benztropine and trihexyphenidyl: Centrally acting antimuscarinic agents used for treatment of Parkinson disease & extrapyramidal symptoms.
 Scopolamine: Tertiary amine plant alkaloid. Produces peripheral effects similar to those of atropine. Unlike atropine, scopolamine has greater action on the CNS (observed at therapeutic doses). It has
Scopolamine: Tertiary amine plant alkaloid. Produces peripheral effects similar to those of atropine. Unlike atropine, scopolamine has greater action on the CNS (observed at therapeutic doses). It has
Demerol meperidine hydrochloride
 Demerol meperidine hydrochloride injection, USP Rx only DESCRIPTION Meperidine hydrochloride is ethyl 1-methyl-4-phenylisonipecotate hydrochloride, a white crystalline substance with a melting point of
Demerol meperidine hydrochloride injection, USP Rx only DESCRIPTION Meperidine hydrochloride is ethyl 1-methyl-4-phenylisonipecotate hydrochloride, a white crystalline substance with a melting point of
Pharmacology of the Neuromuscular Junction (NMJ)
 Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1
 9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
Ganglion blocking agents
 Ganglion blocking agents -out of date -Specifically act on the nicotinic receptors of both parasymphatetic and sympathetic ganglia - no selectivity toward PG or SG -These drugs are non-depolarizing, competitive
Ganglion blocking agents -out of date -Specifically act on the nicotinic receptors of both parasymphatetic and sympathetic ganglia - no selectivity toward PG or SG -These drugs are non-depolarizing, competitive
Pharmacology of the Neuromuscular Junction (NMJ)
 Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
Pharmacology of the Neuromuscular Junction (NMJ) Edward JN Ishac, Ph.D. Professor Smith Building, Room 742 eishac@vcu.edu 828 2127 Department of Pharmacology and Toxicology Medical College of Virginia
Succinycholine: Succinylcholine has no place in pediatric anesthesia. Wads Ames MBBS FRCA
 Succinycholine: Succinylcholine has no place in pediatric anesthesia Wads Ames MBBS FRCA Food And Drug Administration Created in 1906 Responsible for protecting and promoting public health through the
Succinycholine: Succinylcholine has no place in pediatric anesthesia Wads Ames MBBS FRCA Food And Drug Administration Created in 1906 Responsible for protecting and promoting public health through the
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
50% Concentrated Injection
 NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
NAME OF THE MEDICINE. The molecular weight of the compound is 246.5 and the CAS registry number is 10034-99-8. The molecular formula is MgSO4, 7H2O. DESCRIPTION MAGNESIUM SULFATE HEPTAHYDRATE 50% CONCENTRATED
P-RMS: NO/H/PSUR/0009/001
 Core Safety Profile Active substance: Propofol Pharmaceutical form(s)/strength: Emulsion for injection, infusion, 10mg/ml Emulsion for infusion, 20mg/ml P-RMS: NO/H/PSUR/0009/001 Date of FAR: 30.06.2011
Core Safety Profile Active substance: Propofol Pharmaceutical form(s)/strength: Emulsion for injection, infusion, 10mg/ml Emulsion for infusion, 20mg/ml P-RMS: NO/H/PSUR/0009/001 Date of FAR: 30.06.2011
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
PRESCRIBING INFORMATION. Dextrose Injection USP. (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher
 PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
PRESCRIBING INFORMATION Dextrose Injection USP (Concentrated Dextrose for Intravenous Administration) 50% (500 mg/ml) Fluid and Nutrient Replenisher Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland,
GALLIUM CITRATE Ga 67 INJECTION
 511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
MIVACRON GlaxoSmithKline
 MIVACRON GlaxoSmithKline Mivacurium QUALITATIVE AND QUANTITATIVE COMPOSITION Injection: Sterile solution containing 2 mg mivacurium per ml as mivacurium chloride, without an antimicrobial preservative,
MIVACRON GlaxoSmithKline Mivacurium QUALITATIVE AND QUANTITATIVE COMPOSITION Injection: Sterile solution containing 2 mg mivacurium per ml as mivacurium chloride, without an antimicrobial preservative,
3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container
 3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container Description 3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution
3% Sorbitol Urologic Irrigating Solution in UROMATIC Plastic Container Description 3% Sorbitol Urologic Irrigating Solution is a sterile, nonpyrogenic, nonhemolytic, electrically nonconductive solution
Cholinergic antagonists
 Cholinergic antagonists Cholinergic antagonists: They are also called anticholinergic drugs or cholinergic blockers, this group include: 1.Antimuscarinic agents ( atropine, ipratropium, scopolamine) 2.
Cholinergic antagonists Cholinergic antagonists: They are also called anticholinergic drugs or cholinergic blockers, this group include: 1.Antimuscarinic agents ( atropine, ipratropium, scopolamine) 2.
MAGNESIUM SULFATE IN WATER FOR INJECTION
 MAGNESIUM SULFATE IN WATER FOR INJECTION Flexible Plastic Container For Intravenous Use Only Rx only DESCRIPTION Magnesium Sulfate in Water for Injection is a sterile, nonpyrogenic solution of magnesium
MAGNESIUM SULFATE IN WATER FOR INJECTION Flexible Plastic Container For Intravenous Use Only Rx only DESCRIPTION Magnesium Sulfate in Water for Injection is a sterile, nonpyrogenic solution of magnesium
PRODUCT MONOGRAPH. 2% Lidocaine Hydrochloride Injection USP 20 mg/ml
 PRODUCT MONOGRAPH 2% Lidocaine Hydrochloride Injection USP 20 mg/ml 0.4 % Lidocaine Hydrochloride and 5% Dextrose Injection USP Lidocaine Hydrochloride and Dextrose Injection USP Lidocaine Hydrochloride
PRODUCT MONOGRAPH 2% Lidocaine Hydrochloride Injection USP 20 mg/ml 0.4 % Lidocaine Hydrochloride and 5% Dextrose Injection USP Lidocaine Hydrochloride and Dextrose Injection USP Lidocaine Hydrochloride
February 12, Re: 10% Calcium Chloride Injection, USP. Dear Healthcare Professional,
 Re: 10% Calcium Chloride Injection, USP Dear Healthcare Professional, In an effort to reduce medication errors and ensure patient safety, American Regent, Inc. is informing you of recent changes to the
Re: 10% Calcium Chloride Injection, USP Dear Healthcare Professional, In an effort to reduce medication errors and ensure patient safety, American Regent, Inc. is informing you of recent changes to the
Posology. Posology and Dosage Regimen: Factors Affecting Drug Dosage: 08/09/ ) Age: 1. Young s Rule, based on age:
 Posology and Dosage Regimen: Posology Posology: (Derived from the Greek posos- how much, and logos- science) is the branch of medicine/pharmacy dealing with doses. Posology is a branch of medical science
Posology and Dosage Regimen: Posology Posology: (Derived from the Greek posos- how much, and logos- science) is the branch of medicine/pharmacy dealing with doses. Posology is a branch of medical science
PRESCRIBING INFORMATION. 0.1 mg/ml. Sterile Solution. Anticholinergic
 1 PRESCRIBING INFORMATION Pr Atropine Sulfate Injection USP 0.1 mg/ml Sterile Solution Anticholinergic Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland, QC H9H 2M5 DATE OF REVISION: January 18, 2018
1 PRESCRIBING INFORMATION Pr Atropine Sulfate Injection USP 0.1 mg/ml Sterile Solution Anticholinergic Pfizer Canada Inc. 17300 Trans-Canada Highway Kirkland, QC H9H 2M5 DATE OF REVISION: January 18, 2018
PRODUCT INFORMATION. Colistin Link. Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial
 PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
PRODUCT INFORMATION Colistin Link Colistin 150 mg/2 ml (as colistimethate sodium) powder for injection vial For Intramuscular and Intravenous use. NAME OF THE MEDICINE Colistimethate sodium for injection,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ACURMIL 25 mg/2.5 ml solution for intravenous injection ACURMIL 50 mg/5 ml solution for intravenous injection 2. QUALITATIVE AND QUANTITATIVE
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT ACURMIL 25 mg/2.5 ml solution for intravenous injection ACURMIL 50 mg/5 ml solution for intravenous injection 2. QUALITATIVE AND QUANTITATIVE
Surgical Care at the District Hospital. EMERGENCY & ESSENTIAL SURGICAL CARE
 Surgical Care at the District Hospital 1 14 Practical Anesthesia Key Points 2 14.1 General Anesthesia Have a clear plan before starting anesthesia Never use an unfamiliar anesthetic technique in an emergency
Surgical Care at the District Hospital 1 14 Practical Anesthesia Key Points 2 14.1 General Anesthesia Have a clear plan before starting anesthesia Never use an unfamiliar anesthetic technique in an emergency
TRACRIUM GlaxoSmithKline
 TRACRIUM GlaxoSmithKline Atracurium QUALITATIVE AND QUANTITATIVE COMPOSITION Injection: A sterile solution containing 10 mg atracurium besylate per ml, without an antimicrobial preservative, supplied in
TRACRIUM GlaxoSmithKline Atracurium QUALITATIVE AND QUANTITATIVE COMPOSITION Injection: A sterile solution containing 10 mg atracurium besylate per ml, without an antimicrobial preservative, supplied in
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Citanest with Octapressin Dental 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains Prilocaine Hydrochloride 30 mg (54 mg/1.8
ROSOBAC-1GM / ROSOBAC-FORT
 ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ROSOBAC-1GM / ROSOBAC-FORT ROSOBAC - 1GM. COMPOSITION : Each vial contains Sterile Cefoperazone Sodium IP Eq. to Anhydrous Cefoperazone - Sterile Sulbactam Sodium USP Eq. to Anhydrous Sulbactam - ROSOBAC
ELSPAR (asparaginase) For injection, intravenous or intramuscular Initial U.S. Approval: 1978
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Elspar safely and effectively. See full prescribing information for Elspar. ELSPAR (asparaginase)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Elspar safely and effectively. See full prescribing information for Elspar. ELSPAR (asparaginase)
Sodium Chloride Injection, USP in VIAFLEX Plastic Container
 Sodium Chloride Injection, USP in VIAFLEX Plastic Container DESCRIPTION Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in single dose containers
Sodium Chloride Injection, USP in VIAFLEX Plastic Container DESCRIPTION Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in single dose containers
Neosynephrine. Name of the Medicine
 Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
LIDOCAINE HYDROCHLORIDE TOPICAL SOLUTION USP 4%
 LIDOCAINE HYDROCHLORIDE TOPICAL SOLUTION USP 4% PRODUCT OVERVIEW: Lidocaine Hydrochloride SOLUTION Rx only DESCRIPTION Lidocaine Hydrochloride Topical Solution USP 4% contains a local anesthetic agent
LIDOCAINE HYDROCHLORIDE TOPICAL SOLUTION USP 4% PRODUCT OVERVIEW: Lidocaine Hydrochloride SOLUTION Rx only DESCRIPTION Lidocaine Hydrochloride Topical Solution USP 4% contains a local anesthetic agent
EPINEPHRINE Injection, USP 1:1000 (1 mg/ml) Ampul Protect from light until ready to use.
 EPINEPHRINE Injection, USP 1:1000 (1 mg/ml) Ampul Protect from light until ready to use. DESCRIPTION Epinephrine Injection, USP 1:1000 is a sterile, nonpyrogenic solution. Each ml contains epinephrine
EPINEPHRINE Injection, USP 1:1000 (1 mg/ml) Ampul Protect from light until ready to use. DESCRIPTION Epinephrine Injection, USP 1:1000 is a sterile, nonpyrogenic solution. Each ml contains epinephrine
CLINICAL PHARMACOLOGY
 robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
robaxin / robaxin -7 50 (methocarbamol tablets, USP) Rx Only DESCRIPT ION robaxin /robaxin -750 (methocarbamol tablets, USP), a carbamate derivative of guaifenesin, is a central nervous system (CNS) depressant
ALFENTANIL INJECTION, USP Ampul
 ALFENTANIL INJECTION, USP Ampul R x only DESCRIPTION Alfentanil Injection, USP is an opioid analgesic chemically designated as N-[1-[2-(4-ethyl-4,5- dihydro-5-oxo-1h-tetrazol-1-yl)ethyl]-4-(methoxymethyl)-4-piperidinyl]-n-phenylpropanamide
ALFENTANIL INJECTION, USP Ampul R x only DESCRIPTION Alfentanil Injection, USP is an opioid analgesic chemically designated as N-[1-[2-(4-ethyl-4,5- dihydro-5-oxo-1h-tetrazol-1-yl)ethyl]-4-(methoxymethyl)-4-piperidinyl]-n-phenylpropanamide
New Zealand Data Sheet COLISTIN-LINK
 New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
New Zealand Data Sheet COLISTIN-LINK Colistimethate sodium equivalent to colistin 150mg powder for injection For intramuscular and intravenous use. Description Colistimethate sodium for injection, USP
CONTRAINDICATIONS None (4)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VAZCULEP safely and effectively. See full prescribing information for VAZCULEP. VAZCULEP (phenylephrine
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VAZCULEP safely and effectively. See full prescribing information for VAZCULEP. VAZCULEP (phenylephrine
200 mg/20 ml (10 mg/ml) in single-dose vials (3)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NIMBEX safely and effectively. See full prescribing information for NIMBEX. NIMBEX (cisatracurium
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use NIMBEX safely and effectively. See full prescribing information for NIMBEX. NIMBEX (cisatracurium
KHAPZORY (levoleucovorin) for injection, for intravenous use Initial U.S. Approval: 1952 (d,l-leucovorin)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KHAPZORY safely and effectively. See full prescribing information for KHAPZORY. KHAPZORY (levoleucovorin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KHAPZORY safely and effectively. See full prescribing information for KHAPZORY. KHAPZORY (levoleucovorin)
(angiotensin II) injection for intravenous infusion
 ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
SODIUM POLYSTYRENE SULFONATE Suspension, USP
 SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
DOBUTamine INJECTION, USP R x only
 DOBUTamine INJECTION, USP R x only DESCRIPTION Dobutamine Injection, USP is 1,2-benzenediol, 4-[2-[[3-(4-hydro-xyphenyl)-1- methylpropyl]amino]ethyl]-hydrochloride, (±). It is a synthetic catecholamine.
DOBUTamine INJECTION, USP R x only DESCRIPTION Dobutamine Injection, USP is 1,2-benzenediol, 4-[2-[[3-(4-hydro-xyphenyl)-1- methylpropyl]amino]ethyl]-hydrochloride, (±). It is a synthetic catecholamine.
NURSING DEPARTMENT CRITICAL CARE POLICY MANUAL CRITICAL CARE PROTOCOL USE OF PROPOFOL (DIPRIVAN) FOR VENTILATOR MANAGEMENT
 NURSING DEPARTMENT CRITICAL CARE POLICY MANUAL CRITICAL CARE PROTOCOL I. PURPOSE: To provide guidelines for the administration of Propofol, which is an anesthetic agent, indicated for the continuous intravenous
NURSING DEPARTMENT CRITICAL CARE POLICY MANUAL CRITICAL CARE PROTOCOL I. PURPOSE: To provide guidelines for the administration of Propofol, which is an anesthetic agent, indicated for the continuous intravenous
Neuromuscular Blockers
 Neuromuscular Blockers Joanne Leung joanneleung22@hotmail.com Oct 14, 2014 Objectives After this lecture, you should be able to: Describe the physiology of the neuromuscular junction Differentiate the
Neuromuscular Blockers Joanne Leung joanneleung22@hotmail.com Oct 14, 2014 Objectives After this lecture, you should be able to: Describe the physiology of the neuromuscular junction Differentiate the
RMP section VI.2 Elements for Public Summary
 RMP section VI.2 Elements for Public Summary Product: Atropin Stragen, 0,1 mg/ml solution for injection in pre-filled syringe Atropin Stragen, 0,2 mg/ml solution for injection in pre-filled syringe RMP:
RMP section VI.2 Elements for Public Summary Product: Atropin Stragen, 0,1 mg/ml solution for injection in pre-filled syringe Atropin Stragen, 0,2 mg/ml solution for injection in pre-filled syringe RMP:
ADULT DRUG REFERENCE Drug Indication Adult Dosage Precautions / Comments
 ADENOSINE Paroxysmal SVT 1 st Dose 6 mg rapid IV 2 nd & 3 rd Doses 12 mg rapid IV push Follow each dose with rapid bolus of 20 ml NS May cause transient heart block or asystole. Side effects include chest
ADENOSINE Paroxysmal SVT 1 st Dose 6 mg rapid IV 2 nd & 3 rd Doses 12 mg rapid IV push Follow each dose with rapid bolus of 20 ml NS May cause transient heart block or asystole. Side effects include chest
Chapter 18. Skeletal Muscle Relaxants (Neuromuscular Blocking Agents)
 Chapter 18 Skeletal Muscle Relaxants (Neuromuscular Blocking Agents) Uses of Neuromuscular Blocking Facilitate intubation Surgery Agents Enhance ventilator synchrony Reduce intracranial pressure (ICP)
Chapter 18 Skeletal Muscle Relaxants (Neuromuscular Blocking Agents) Uses of Neuromuscular Blocking Facilitate intubation Surgery Agents Enhance ventilator synchrony Reduce intracranial pressure (ICP)
Sodium Chloride Injection, USP in VIAFLO Plastic Container
 Sodium Chloride Injection, USP in VIAFLO Plastic Container DESCRIPTION Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in single dose containers
Sodium Chloride Injection, USP in VIAFLO Plastic Container DESCRIPTION Sodium Chloride Injection, USP is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in single dose containers
TERBUTALINE SULFATE INJECTION, USP
 451001C/Revised: March 2008 TERBUTALINE SULFATE INJECTION, USP Rx only A sterile aqueous solution for subcutaneous injection. DESCRIPTION: Terbutaline Sulfate Injection, USP, is a beta-adrenergic agonist
451001C/Revised: March 2008 TERBUTALINE SULFATE INJECTION, USP Rx only A sterile aqueous solution for subcutaneous injection. DESCRIPTION: Terbutaline Sulfate Injection, USP, is a beta-adrenergic agonist
Atropine Sulfate Injection, USP 0.1 mg/ml (Adult) 0.05 mg/ml (Pediatric) Ansyr Plastic Syringe
 Atropine Sulfate Injection, USP 0.1 mg/ml (Adult) 0.05 mg/ml (Pediatric) Ansyr Rx only DESCRIPTION Atropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution of atropine sulfate monohydrate
Atropine Sulfate Injection, USP 0.1 mg/ml (Adult) 0.05 mg/ml (Pediatric) Ansyr Rx only DESCRIPTION Atropine Sulfate Injection, USP is a sterile, nonpyrogenic isotonic solution of atropine sulfate monohydrate
NEW ZEALAND DATA SHEET. Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution containing 2.5 mg/ml Neostigmine methylsulphate.
 NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
NEW ZEALAND DATA SHEET NAME OF MEDICINE NEOSTIGMINE METHYLSULPHATE INJECTION B.P. Solution for injection 2.5 mg/ml. PRESENTATIONS Injection solution 2.5 mg/ml: a clear, colourless, particle-free solution
CLINICAL PHARMACOLOGY
 EPHEDRINE SULFATE- ephedrine sulfate injection, solution Sandoz Inc Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further
EPHEDRINE SULFATE- ephedrine sulfate injection, solution Sandoz Inc Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further
NEW ZEALAND DATA SHEET. Injection 2 mg/ml: a clear, colourless, particle-free solution containing 2 mg/ml pancuronium bromide.
 NEW ZEALAND DATA SHEET NAME OF MEDICINE Pancuronium Bromide B.P. Injection 2 mg/ml PRESENTATION Injection 2 mg/ml: a clear, colourless, particle-free solution containing 2 mg/ml pancuronium bromide. INDICATIONS
NEW ZEALAND DATA SHEET NAME OF MEDICINE Pancuronium Bromide B.P. Injection 2 mg/ml PRESENTATION Injection 2 mg/ml: a clear, colourless, particle-free solution containing 2 mg/ml pancuronium bromide. INDICATIONS
KEVEYIS (dichlorphenamide) tablets, for oral use Initial U.S. Approval: 1958
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KEVEYIS safely and effectively. See full prescribing information for KEVEYIS. KEVEYIS (dichlorphenamide)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KEVEYIS safely and effectively. See full prescribing information for KEVEYIS. KEVEYIS (dichlorphenamide)
PRESCRIBING INFORMATION. Sodium Bicarbonate Injection USP 42mg/ml (4.2%), 75mg/ml (7.5%), 84mg/ml (8.4%) Sterile solution ALKALIZER
 PRESCRIBING INFORMATION Sodium Bicarbonate Injection USP 42mg/ml (4.2%), 75mg/ml (7.5%), 84mg/ml (8.4%) Sterile solution Lifeshield ABBOJECT Single-dose Syringes and Flipp Vial ALKALIZER FOR CORRECTION
PRESCRIBING INFORMATION Sodium Bicarbonate Injection USP 42mg/ml (4.2%), 75mg/ml (7.5%), 84mg/ml (8.4%) Sterile solution Lifeshield ABBOJECT Single-dose Syringes and Flipp Vial ALKALIZER FOR CORRECTION
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion Cisatracurium Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion Cisatracurium Read all of this leaflet carefully before
Pain: 1-2µg/kg q30-60min prn. effects in 10 minutes. Contraindications: Morphine is preferred in. Duration of Action: minutes. renal failure.
 Procedural Sedation / Analgesia / Anaesthesia Chart - Page 1 Diazepam (Valium) Anxiolytic / Sedative Etomidate (Amidate) Hypnotic / Anesthetic Fentanyl Citrate (Sublimaze) Narcotic Analgesic Dose Pediatric:
Procedural Sedation / Analgesia / Anaesthesia Chart - Page 1 Diazepam (Valium) Anxiolytic / Sedative Etomidate (Amidate) Hypnotic / Anesthetic Fentanyl Citrate (Sublimaze) Narcotic Analgesic Dose Pediatric:
INTRAVENOUS SOLUTIONS WITH POTASSIUM CHLORIDE POTASSIUM CHLORIDE IN LACTATED RINGER S AND 5% DEXTROSE INJECTION, USP Flexible Plastic Container
 INTRAVENOUS SOLUTIONS WITH POTASSIUM CHLORIDE POTASSIUM CHLORIDE IN LACTATED RINGER S AND 5% DEXTROSE INJECTION, USP Flexible Plastic Container R x only DESCRIPTION Intravenous solutions with potassium
INTRAVENOUS SOLUTIONS WITH POTASSIUM CHLORIDE POTASSIUM CHLORIDE IN LACTATED RINGER S AND 5% DEXTROSE INJECTION, USP Flexible Plastic Container R x only DESCRIPTION Intravenous solutions with potassium
DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related to acetylcholine.
 BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related
BETHANECHOL CHLORIDE TABLETS, USP 5 mg, 10 mg, 25 mg and 50 mg Rx only DESCRIPTION Bethanechol chloride, USP, a cholinergic agent, is a synthetic ester which is structurally and pharmacologically related
Shaded areas=not MARKETED 24/2/09
 PACKAGE INSERT SCHEDULING STATUS Schedule 6 PROPRIETARY NAME AND DOSAGE FORM RAPIFEN 2 ml IV injection RAPIFEN 10 ml IV injection COMPOSITION Each ml contains alfentanil hydrochloride 0,544 mg (equivalent
PACKAGE INSERT SCHEDULING STATUS Schedule 6 PROPRIETARY NAME AND DOSAGE FORM RAPIFEN 2 ml IV injection RAPIFEN 10 ml IV injection COMPOSITION Each ml contains alfentanil hydrochloride 0,544 mg (equivalent
PRODUCT INFORMATION. TRACRIUM INJECTION atracurium besylate DESCRIPTION:
 PRODUCT INFORMATION TRACRIUM INJECTION (atracurium besylate) NAME OF THE DRUG: atracurium besylate DESCRIPTION: TRACRIUM (atracurium besylate) is an intermediate-duration, non-depolarising, skeletal muscle
PRODUCT INFORMATION TRACRIUM INJECTION (atracurium besylate) NAME OF THE DRUG: atracurium besylate DESCRIPTION: TRACRIUM (atracurium besylate) is an intermediate-duration, non-depolarising, skeletal muscle
 VITAMIN K1 - phytonadione injection, emulsion Cardinal Health ---------- Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K1 Ampul Rx only Protect from light. Keep ampuls in tray until
VITAMIN K1 - phytonadione injection, emulsion Cardinal Health ---------- Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K1 Ampul Rx only Protect from light. Keep ampuls in tray until
RYANODEX (dantrolene sodium) for injectable suspension, for intravenous use. Initial U.S. Approval: 1974
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RYANODEX safely and effectively. See full prescribing information for RYANODEX. RYANODEX (dantrolene
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use RYANODEX safely and effectively. See full prescribing information for RYANODEX. RYANODEX (dantrolene
What is MH? Malignant Hyperthermia (MH)! Malignant Hyperthermia (MH) Malignant Hyperthermia (MH) ! The underlying physical mechanismintracellular
 10/2/13 What is MH? Libby Morse BSN RN CPAN An inherited disorder of skeletal muscle triggered in susceptible humans in most instances by inhalation agents and/ or succinylcholine, resulting in hypermetabolism,
10/2/13 What is MH? Libby Morse BSN RN CPAN An inherited disorder of skeletal muscle triggered in susceptible humans in most instances by inhalation agents and/ or succinylcholine, resulting in hypermetabolism,
CSL Behring LLC Albuminar -25 US Package Insert Albumin (Human) USP, 25% Revised: 01/2008 Page 1
 Page 1 CSL Behring Albuminar -25 Albumin (Human) USP, 25% R x only DESCRIPTION Albuminar -25, Albumin (Human) 25%, is a sterile aqueous solution of albumin obtained from large pools of adult human venous
Page 1 CSL Behring Albuminar -25 Albumin (Human) USP, 25% R x only DESCRIPTION Albuminar -25, Albumin (Human) 25%, is a sterile aqueous solution of albumin obtained from large pools of adult human venous
Document 3 Summary of Data and Guidance for Medical Professionals
 Document 3 Summary of Data and Guidance for Medical Professionals Anvirzel Anvirzel is a novel antitumor compound extracted from the plant Nerium oleander, which belongs to the family Apocynaceae. Anvirzel
Document 3 Summary of Data and Guidance for Medical Professionals Anvirzel Anvirzel is a novel antitumor compound extracted from the plant Nerium oleander, which belongs to the family Apocynaceae. Anvirzel
45779C/Revised: April 2008 MANNITOL INJECTION, USP
 45779C/Revised: April 2008 MANNITOL INJECTION, USP 25% For Intravenous Use and Urologic Irrigation DESCRIPTION: Mannitol is a 6-carbon sugar alcohol and has the following structure: C 6 H 14 O 6 182.17
45779C/Revised: April 2008 MANNITOL INJECTION, USP 25% For Intravenous Use and Urologic Irrigation DESCRIPTION: Mannitol is a 6-carbon sugar alcohol and has the following structure: C 6 H 14 O 6 182.17
PRODUCT MONOGRAPH. Solution for Injection 20 mg/10 ml (2 mg/ml) Cisatracurium. Non-depolarizing Skeletal Neuromuscular Blocking Agent
 PRODUCT MONOGRAPH Pr CISATRACURIUM BESYLATE INJECTION Solution for Injection 20 mg/10 ml (2 mg/ml) Cisatracurium Non-depolarizing Skeletal Neuromuscular Blocking Agent This drug should be administered
PRODUCT MONOGRAPH Pr CISATRACURIUM BESYLATE INJECTION Solution for Injection 20 mg/10 ml (2 mg/ml) Cisatracurium Non-depolarizing Skeletal Neuromuscular Blocking Agent This drug should be administered
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
Final Core Safety Profile for propofol 10 mg/ml (1%) and 20 mg/ml (2%) emulsion for injection or infusion
 CSP Drug Substance Propofol Date September 04 2014 Final Core Safety Profile for propofol 10 mg/ml (1%) and 20 mg/ml (2%) emulsion for injection or infusion CORE SAFETY PROFILE 4.3 Contraindications Propofol
CSP Drug Substance Propofol Date September 04 2014 Final Core Safety Profile for propofol 10 mg/ml (1%) and 20 mg/ml (2%) emulsion for injection or infusion CORE SAFETY PROFILE 4.3 Contraindications Propofol
VENTOLIN RESPIRATOR SOLUTION
 VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
General anesthetics. Dr. Shamil AL-Noaimy Lecturer of Pharmacology Dept. of Pharmacology College of Medicine
 General anesthetics Dr. Shamil AL-Noaimy Lecturer of Pharmacology Dept. of Pharmacology College of Medicine Rationale General anesthesia is essential to surgical practice, because it renders patients analgesic,
General anesthetics Dr. Shamil AL-Noaimy Lecturer of Pharmacology Dept. of Pharmacology College of Medicine Rationale General anesthesia is essential to surgical practice, because it renders patients analgesic,
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml
 New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
New Zealand Datasheet Glycopyrronium Bromide and Neostigmine Metilsulfate 0.5mg/2.5mg per ml Solution for injection Glycopyrronium bromide 0.5 mg/ml & Neostigmine metilsulfate 2.5 mg/ml Presentation is
Potassium Chloride in 0.9% Sodium Chloride Injection, USP in Plastic Container
 PRESCRIBING INFORMATION Potassium Chloride in 0.9% Sodium Chloride Injection, USP in Plastic Container VIAFLEX Container 20mmol/L Potassium Chloride in Sodium in 0.9% Chloride Injection 40mmol/L Potassium
PRESCRIBING INFORMATION Potassium Chloride in 0.9% Sodium Chloride Injection, USP in Plastic Container VIAFLEX Container 20mmol/L Potassium Chloride in Sodium in 0.9% Chloride Injection 40mmol/L Potassium
