Panafen Plus Product Information
|
|
|
- Buddy Owen
- 5 years ago
- Views:
Transcription
1 Panafen Plus Product Information COMPOSITION Actives: Ibuprofen 200 mg, codeine phosphate 12.8 mg Inactives: cellulose-microcrystalline, vegetable oil-hydrogenated, sodium starch glycollate, silica-colloidal anhydrous, lactose, cellulose-powdered, hypromellose, macrogol 400. DESCRIPTION Ibuprofen Chemical name: 2-(4-isobutylphenyl) propionic acid Molecular formula: C 13 H 18 O 2 MW: CAS: Ibuprofen is a white or almost white powder or crystals with a characteristic odour. Practically insoluble in water, soluble 1 in 1.5 of alcohol, 1 in 1 of chloroform, 1 in 2 of ether and 1 in 1.5 of acetone; soluble in aqueous solutions of alkali hydroxides and carbonates. Codeine phosphate. Chemical name: (5R,6S)-7, 8-didehydro-4,5- epoxy-3-methoxy-nmethylmorphinan-6-ol dihydrogen orthophosphate hemihydrate Molecular formula: C 18 H 21 NO 3 H 3 PO 4. ½H 2 O MW: CAS: Codeine phosphate is a small, colourless, odourless crystal or a white, odourless crystalline powder. It is soluble in four parts water, slightly soluble in ethanol (96%), practically insoluble in chloroform and ether.
2 PHARMACOLOGY It is thought that ibuprofen produces an anti-inflammatory effect at least in part by inhibiting prostaglandin synthetase. Ibuprofen has shown antiinflammatory, analgesic and antipyretic activity in both animal and human studies. Codeine phosphate is a narcotic analgesic acting on central opiate receptors, although its pharmacological effects are thought to be largely due to its biotransformation to morphine. Pharmacokinetics Absorption: Ibuprofen is well absorbed after oral administration with peak serum levels occurring after one to two hours. Codeine is well absorbed from the gastrointestinal tract and peak plasma concentrations are reached one hour after oral administration. Onset of action occurs in 15 to 30 minutes and analgesia is maintained for four to six hours. Distribution: The apparent volume of distribution for ibuprofen is 0.14 L/kg. Ibuprofen and its metabolites readily cross the placental barrier in pregnant rabbits and rats. It is not known if the drug enters the cerebrospinal fluid. 99% of ibuprofen is protein bound. The high protein binding of the drug should be borne in mind when prescribing ibuprofen together with other protein bound drugs that bind to the same site on human serum albumin. Codeine is rapidly distributed to skeletal muscles, kidneys, liver, gastrointestinal tract, lungs, spleen and brain. It crosses the placenta and is distributed in low levels in breast milk. Metabolism: 90% of ibuprofen is metabolised in the liver to produce two major metabolites, a hydroxylated and carboxylated compound. Codeine is metabolised mainly in the liver. The major metabolic pathway involves glucuronidation of codeine to codeine-6-glucuronide. Codeine can also undergo O- and N-demethylation catalysed by CYP2D6 and CYP3A4 respectively. About 10% of an administered dose of codeine is converted by O-demethylation to morphine, which subsequently undergoes glucuronidation to morphine-3 or morphine-6 glucuronide, or N-demethylation to normorphine. Approximately 5 to 10% of the Caucasian population cannot convert codeine to morphine as they are deficient in the CYP2D6 enzyme. These patients are likely to obtain reduced pain relief from codeine. Codeine is also converted by N-demethylation to norcodeine, which subsequently undergoes glucuronidation to norcodeine glucuronide or O-demethylation to normorphine. Excretion: Both the inactive metabolites of ibuprofen and a small amount of unchanged ibuprofen are excreted rapidly and completely by the kidney with 95% of the administered dose eliminated in the urine within four hours of ingestion. The elimination half-life of ibuprofen is in the range 1.9 to 2.2 hours.
3 Codeine is excreted mainly by the kidneys. Of the excreted material in the urine, 40 to 70% is free or conjugated codeine, 5 to 15% is free or conjugated morphine and 10 to 20% is free or conjugated norcodeine. The plasma halflife of codeine is two to four hours. Only traces of codeine and its metabolites are found in the faeces. INDICATIONS The temporary relief of strong pain and discomfort associated with migraine headache, tension headache, period pain, toothache, cold & flu symptoms, back or muscular pain, arthritis and neuralgia. Reduces fever. CONTRAINDICATIONS Known hypersensitivity to ibuprofen, codeine or other opioid analgesics or any of the excipients. Hypersensitivity (e.g. asthma, rhinitis or urticaria) to aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). Panafen Plus should not be used in patients with gastrointestinal bleeding or with an active or previous peptic ulcer. Panafen Plus should not be used in severe hepatic, renal or heart failure. Respiratory depression, chronic constipation and active alcoholism. Diarrhoea caused by pseudomembranous colitis or poisoning (until the causative organism or toxin has been eliminated from the gastrointestinal tract, since codeine may slow down the elimination and thereby prolonging the diarrhoea). Panafen Plus should not be taken in those using concomitant NSAIDs including cyclo-oxygenase-2 (Cox-2) specific inhibitors (see Drug Interactions). PRECAUTIONS Undesirable effects may be minimised by using the minimum effective dose for the shortest possible duration. Panafen Plus should be administered with caution to patients with asthma, and especially those patients who have not taken an NSAID before. Panafen Plus should be administered with caution to patients with a history of hypertension or cardiac impairment as fluid retention, hypertension and
4 oedema have been reported with NSAID therapy. Gastrointestinal (GI) bleeding, ulceration or perforation, which can be fatal, has been reported with all NSAIDs at any time during treatment, with or without warning symptoms or a previous history of serious GI events. When GI bleeding or ulceration occurs in patients receiving ibuprofen, the treatment should be withdrawn and patients should consult a doctor (see Adverse Reactions) Panafen Plus should be used with caution in patients with chronic inflammatory intestinal disease (ulcerative colitis. Crohn s Disease), obstructive bowel disorders or acute abdominal conditions, as these conditions may be exacerbated. Use with caution in patients with a history of cholecystectomy as Panafen Plus may cause acute pancreatitis in some patients. Use with caution in patients with Systemic Lupus Erythematosus (SLE) and mixed connective tissue disease due to increase risk of aseptic meningitis. Panafen Plus should be administered with caution to patients with renal impairment. In patients with renal impairment, renal function should be monitored since it may deteriorate following the use of any NSAID. Panafen Plus should be administered with caution to patients with hepatic impairment. As with other drugs of this class, ibuprofen may mask the usual signs of infection. Codeine may also obscure the diagnosis or the course of gastrointestinal diseases. Panafen Plus should therefore be administered with caution in such situations. Panafen Plus should be administered with caution to patients who have recently had gastrointestinal surgery, as codeine may reduce gastrointestinal motility. Prolonged or excessive consumption can result in dependence (see Adverse Reactions). Panafen Plus should be administered with caution to those with hypotension and/or hypothyroidism. The caplets should be used with caution in patients with CNS depression, raised intracranial pressure or head injury, since codeine may increase the risk of respiratory depression and further elevate intracranial pressure. Panafen Plus should be administered with caution to patients with prostatic hypertrophy since codeine may cause urinary retention.
5 Use in the elderly NSAIDs should be used with particular caution in elderly patients who are more prone to adverse events. The elderly may also be more susceptible to the CNS depressant effects of opioids. Effect on female fertility There is some evidence that drugs which inhibit cyclooxygenase/prostaglandin synthesis may cause impairment of female fertility by an effect on ovulation. This is reversible upon withdrawal of treatment. Use in pregnancy (Category C) NSAIDs inhibit prostaglandin synthesis and, when given during the latter part of pregnancy, may cause closure of the foetal ductus arteriosus, foetal renal impairment, inhibition of platelet aggregation and delay labour and birth. Continuous treatment with NSAIDs during the last trimester of pregnancy should only be given on sound indications. During the last few days before expected birth, agents with an inhibitory effect on prostaglandin synthesis should be avoided. Based on animal studies and limited clinical experience, there is no evidence to suggest foetal abnormalities associated with the use of codeine. However, Panafen Plus caplets should be avoided during pregnancy. Prolonged highdose use of codeine prior to delivery may produce codeine withdrawal symptoms in the neonate. Use in lactation In limited studies, ibuprofen appears in breast milk in very limited concentrations and is unlikely to affect the breastfed infant adversely. Codeine is excreted in breast milk. The use of Panafen Plus caplets should be avoided if possible during lactation and should not be used while breastfeeding unless prescribed by a doctor. In nursing mothers, who are ultra-rapid metabolisers of codeine, higher than expected serum and breast milk morphine levels can occur. Morphine toxicity in babies can cause excessive somnolence, hypotonia and difficulty breastfeeding or breathing. In sever cases of respiratory depression death can occur. The lowest effective dose should be used, for the shortest period of time. Nursing mothers should be informed about carefully monitoring the infant during treatment for any sign and symptom of morphine toxicity such as increase drowsiness or sedation, difficulty breastfeeding, breathing difficulties and decrease tone and seeking immediate medical care if such symptoms or signs are noticed. Effect on ability to drive or operate machinery Codeine may cause dizziness or drowsiness. Those affected should not drive or operate machinery.
6 Interactions Ibuprofen-containing products should not be used in combination with other NSAIDs including aspirin and cyclo-oxygenase-2 (Cox-2) specific inhibitors as these may increase the risk of adverse effects (see Contraindications) Ibuprofen may inhibit the antiplatelet effect of low dose aspirin. Patients on low dose aspirin should be instructed to consult their doctor or pharmacist before taking ibuprofen. Ibuprofen-containing products should be used with caution in combination with the following drugs as interactions have been reported: ACE inhibitors, beta-blockers and diuretics: Ibuprofen, like other NSAIDs, can reduce the antihypertensive effect of ACE inhibitors and beta-blockers with possible loss of blood pressure control and can attenuate the natriuretic effects of diuretics. Anticoagulants: Concurrent use of NSAIDs and warfarin has been associated with severe, sometimes fatal, haemorrhage. The mechanism of this interaction is not known but may involve increased bleeding from NSAIDinduced gastrointestinal ulceration or an additive effect of NSAID inhibition of platelet function with the anticoagulant effect of warfarin. Panafen Plus should only be used in patients taking warfarin if absolutely necessary. Patients taking this combination must be closely monitored. Cardiac glycosides: NSAIDs may increase plasma glycoside levels. Care should therefore be taken in patients treated with cardiac glycosides. Corticosteroids: An increased risk of gastrointestinal bleeding may occur with corticosteroids. Lithium: Ibuprofen has been shown to decrease the renal clearance and increase plasma concentrations of lithium. Lithium plasma concentrations should be monitored in patients on concurrent ibuprofen therapy. Methotrexate: NSAIDs inhibit tubular secretion of methotrexate in animals. As a result, reduction in the clearance of methotrexate may occur. Use of high doses of methotrexate concomitantly with NSAIDs should be avoided. At low doses of methotrexate, caution should be used if ibuprofen is administered concomitantly. Monoamine oxidase inhibitors (MAOIs): Concurrent administration or use within 14 days of ceasing monoamine oxidase inhibitors may enhance the potential respiratory depressant effects of codeine. Cyclosporin: Increased risk of nephrotoxicity. Hypoglycemic agents (oral): Inhibition of metabolism of sulfonylurea drugs, prolonged half-life and increased risk of hypoglycaemia.
7 Quinolones: NSAIDs can increase the risk of convulsions associated with quinolone antibiotics. Zidovudine: Increased risk of haemarthroses and haematoma in HIV (+) haemophiliacs. Selective serotonin reuptake inhibitors (SSRIs): Increased risk of gastrointestinal bleeding. Codeine-containing products should be used with caution in combination with the following drugs as interactions have been reported. Anticholinergics: Concurrent use of codeine and anticholinergic agents may increase the risk of severe constipation and/or urinary retention. Antihypertensives: Hypotensive effects of antihypertensive agents may be potentiated when used concurrently with codeine and lead to orthostatic hypotension. Antiperistaltic antidiarrhoeals (including kaolin, pectin, loperamide): Concurrent use of these agents with codeine may increase the risk of severe constipation. Central nervous system depressants: Codeine may potentiate the effects of CNS depressants including alcohol, anaesthetics, hypnotics, sedatives, tricyclic antidepressants and phenothiazines. Metoclopramide & domperidone: Codeine may antagonise the effects of these antiemetics gastrointestinal motility. Monoamine oxidase inhibitors (MAOIs): Concurrent administration or use within 14 days of ceasing monoamine oxidase inhibitors may enhance the potential respiratory depressant effects of codeine. Opioid analgesics: Concurrent use of codeine and other opioid receptor agonists is usually inappropriate as additive CNS depression, respiratory depression and hypotensive effects may occur. Probenecid and phenytoin: Interactions may also occur with probenecid, antidiabetic medications and phenytoin. Quinidine: Interferes with the metabolism of codeine to morphine, lowering the analgesic effect of codeine. It is possible that interactions could occur between drugs that can inhibit CYP2D6 (such as quinidine, phenothiazines and antipsychotic agents) and codeine.
8 ADVERSE REACTIONS Adverse reactions reported from extensive post-marketing experience are listed below by System Organ Class and frequency. The following convention has been utilised for the classification of undesirable effects: very common ( 1/10), common ( 1/100 and <1/10), uncommon ( 1/1000 and <1/100), rare ( 1/10,000 and <1/1000), very rare (<1/10,000), not known (cannot be estimated from the available data). Ibuprofen The following list of adverse effects related to those experienced with ibuprofen at OTC doses, for short term use. The treatment of chronic conditions, under long-term treatment, additional adverse effects may occur. Gastrointestinal disorders: Uncommon: abdominal pain, nausea, dyspepsia. Rare: diarrhoea, flatulence, constipation and vomiting. Very rare: peptic ulcer, perforation or gastrointestinal haemorrhage, sometimes fatal, particularly in the elderly. Exacerbation of ulcerative colitis and Crohn s disease. Nervous system disorders: Common: headache, dizziness, hearing disturbance (tinnitus). Skin and subcutaneous tissue disorders: Uncommon: skin rashes and itching. Very rare: severe forms of skin reactions, exfoliative and bullous dermatitoses such as erythema multiforme and epidermal necrolysis can occur. Renal and urinary disorders: Very rare: acute renal failure, papillary necrosis, especially in long-term use, associated with increased serum urea and oedema. Hepatobiliary disorders: Very rare: liver disorders. Blood and lymphatic system disorders: Very rare: haematopoietic disorders (anaemia, leucopenia, thrombocytopenia, pancytopenia, agranulocytosis). First signs are fever, sore throat, superficial mouth ulcers, flu-like symptoms, sever exhaustion, unexplained bleeding and bruising. Immune system disorders: Uncommon: hypersensitivity reactions including urticaria and pruritus. Very rare: severe hypersensitivity reactions. Symptoms could be: facial, tongue and laryngeal swelling, dyspnoea, tachycardia, hypotension (anaphylaxis, angioedema or severe shock), exacerbation of asthma and bronchospasm. Others with unknown frequency: Oedema, hypertension, and cardiac failure, have been reported in association with NSAID treatment. In patients with existing auto-immune disorders (such as systemic lupus erythematosus, mixed connective tissue disease) during treatment with ibuprofen, single cases of symptoms of aseptic meningitis, such as stiff neck, headache, nausea, vomiting, fever or disorientation have been observed.
9 Codeine Psychiatric disorders: Not known: drug dependency can occur after prolonged use of codeine at higher doses. Gastrointestinal disorders: Not known: constipation, nausea, vomiting, dyspepsia, dry mouth, acute pancreatitis in patients with a history of cholecystectomy. Nervous system disorder: Not known: dizziness, worsening of headache with prolonged use and drowsiness. DOSAGE AND ADMINISTRATION Adults and children 12 years and over Initial dose two caplets taken with fluid, then one or two caplets every four hours when necessary. Maximum six caplets in a 24 hour period. Children Not recommended for children under 12 years. OVERDOSAGE Ibuprofen In children ingestion of more than 400mg/kg may cause symptoms. In adults the dose response effect is less clear cut. The half-life in overdose is hours. Most patients who have ingested clinically important amounts of NSAIDs will develop no more that nausea, vomiting, epigastric pain, or more rarely diarrhoea. Tinnitus, headache and gastrointestinal bleeding are also possible. In more severe poisoning, toxicity is seen in the central nervous system, manifesting as drowsiness, occasionally excitation and disorientation or coma. Occasionally patients develop convulsions. In serious poisoning metabolic acidosis may occur and the prothrombin time/inr may be prolonged, probably due to interference with the actions of circulating clotting factors. Acute renal failure and liver damage may occur. Exacerbation of asthma is possible in asthmatics. Treatment should be symptomatic and supportive including the maintenance of a clear airway and monitoring of cardiac and vital signs until stable. Activated charcoal may reduce absorption of the drug if given within one or two hours after ingestion. In patients who are not fully conscious or have impaired gag reflex, consideration should be given to administering activated charcoal via a nasogastric tube, once the airway is protected. If frequent or prolonged, convulsions should be treated with intravenous diazepam or lorazepam. Give bronchodilators for asthma.
10 Codeine An overdose of codeine is characterised, in the first phase, by nausea and vomiting. An acute depression of the respiratory centre can cause cyanosis, slower breathing, drowsiness, ataxia and, more rarely, pulmonary oedema. Respiratory pauses, miosis, convulsion, collapse and urine retention as well as signs of histamine release have been observed as well. Treatment should include general symptomatic and supportive measures including a clear airway and monitoring of vital signs until stable. Activated charcoal may reduce absorption of the drug if given within one or two hours after ingestion. In patients who are not fully conscious or have impaired gag reflex, consideration should be given to administering activated charcoal via a nasogastric tube, once the airway is protected. Give naloxone if coma or respiratory depression is present. Observe for at least four hours after ingestion. The Poisons Information Centre can also be contacted (telephone ) for current information on the treatment of oral overdoses. PRESENTATION Caplets (white, capsule shaped tablets, marked with a + sign surrounded by an oval): 15 and 30. POISONS SCHEDULE S3: Pharmacist Only Medicine 15 and 30: SPONSOR GlaxoSmithKline Australia Pty Ltd (t/a GlaxoSmithKline Consumer Healthcare) 82 Hughes Avenue ERMINGTON NSW 2115 Date of TGA approval: November, 2009 Panafen is a registered trade mark of the GlaxoSmithKline group of companies.
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION
 RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
Panafen Plus PRODUCT INFORMATION
 Panafen Plus PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Ibuprofen Codeine phosphate Ibuprofen Chemical structure: Chemical name: 2-(4-isobutylphenyl) propionic acid Molecular formula:
Panafen Plus PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Ibuprofen Codeine phosphate Ibuprofen Chemical structure: Chemical name: 2-(4-isobutylphenyl) propionic acid Molecular formula:
New Zealand Datasheet
 1. NAME OF MEDICINE PANAFEN PLUS New Zealand Datasheet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredients: Ibuprofen 200 mg, codeine phosphate 12.8 mg 3. PHARMACEUTICAL FORM Ibuprofen Chemical
1. NAME OF MEDICINE PANAFEN PLUS New Zealand Datasheet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredients: Ibuprofen 200 mg, codeine phosphate 12.8 mg 3. PHARMACEUTICAL FORM Ibuprofen Chemical
PRODUCT INFORMATION Panadeine EXTRA
 PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION. Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets
 PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION
 Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
Molecular formula: Molecular weight: C 8 H 9 NO 2 CAS Registry no.:
 Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
Migraleve, Migraleve Pink and Migraleve Yellow Product Information
 Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
PRODUCT INFORMATION. Codral* Original Cold & Flu Tablets
 PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML)
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.
 Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE. Chemical structure: CAS 2 Registry Number: DESCRIPTION
 PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
PRODUCT INFORMATION ACTACODE
 PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
Panafen Plus Ibuprofen 200 mg and Codeine Phosphate 12.8 mg
 Ibuprofen 200 mg and Codeine Phosphate 12.8 mg Consumer Medicine Information Please read this information before you start taking this medicine. What is in this leaflet This leaflet answers some common
Ibuprofen 200 mg and Codeine Phosphate 12.8 mg Consumer Medicine Information Please read this information before you start taking this medicine. What is in this leaflet This leaflet answers some common
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS
 AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
AUSTRALIAN PRODUCT INFORMATION PANADOL OPTIZORB TABLETS (PARACETAMOL) TABLETS PANADOL OPTIZORB CAPLETS (PARACETAMOL) TABLETS 1 NAME OF THE MEDICINE Paracetamol 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Active ingredients: Paracetamol Codeine phosphate. Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
 Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
NEW ZEALAND DATASHEET
 NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
PRODUCT INFORMATION. Codeine CAS: Molecular formula: C18H21NO3.H3PO4.1/2 H2O. MW:406.4
 PRODUCT INFORMATION i) Name of the medicine: NUROFEN PLUS ibuprofen 200mg and codeine phosphate hemihydrate 12.8mg Ibuprofen CAS: 15687-27-1 Molecular formula: C13H18O2. MW:206.3 Codeine CAS: 41444-62-6
PRODUCT INFORMATION i) Name of the medicine: NUROFEN PLUS ibuprofen 200mg and codeine phosphate hemihydrate 12.8mg Ibuprofen CAS: 15687-27-1 Molecular formula: C13H18O2. MW:206.3 Codeine CAS: 41444-62-6
Elements for a Public Summary Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
NEOFEN 60 mg suppository
 PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Advil Liqui - gels 200 Capsules
 1. NAME OF THE MEDICINAL PRODUCT Advil Liqui - gels 200 Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 200mg Ibuprofen For excipients, see 6.1 3. PHARMACEUTICAL FORM Capsules
1. NAME OF THE MEDICINAL PRODUCT Advil Liqui - gels 200 Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 200mg Ibuprofen For excipients, see 6.1 3. PHARMACEUTICAL FORM Capsules
Read all of this leaflet carefully because it contains important information for you.
 Nurofen Plus Tablets Ibuprofen 200mg, Codeine Phosphate Hemihydrate 12.8mg ADVANCED DUAL ACTION FOR POWERFUL RELIEF Read all of this leaflet carefully because it contains important information for you.
Nurofen Plus Tablets Ibuprofen 200mg, Codeine Phosphate Hemihydrate 12.8mg ADVANCED DUAL ACTION FOR POWERFUL RELIEF Read all of this leaflet carefully because it contains important information for you.
PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other.
 Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution DATA SHEET
 PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
Children Enteric coated tablet : 1-3 mg/kg per day in divided doses.
 Ultrafen Tablet/SR Tablet/Suppository/Gel Description Ultrafen is a preparation of Diclofenac is a non-steroidal antiinflammatory agent with marked analgesic, anti-inflammatory and antipyretic properties.
Ultrafen Tablet/SR Tablet/Suppository/Gel Description Ultrafen is a preparation of Diclofenac is a non-steroidal antiinflammatory agent with marked analgesic, anti-inflammatory and antipyretic properties.
APO-PARACETAMOL/CODEINE 500/30
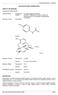 APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
PANADOL EXTRA PRODUCT INFORMATION
 PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PROFESSIONAL INFORMATION
 SCHEDULING STATUS: S1 PROPRIETARY NAME AND DOSAGE FORM: Aleve Tablets COMPOSITION: Each tablet contains naproxen sodium 220 mg (equivalent to 200 mg naproxen) CATEGORY AND CLASS: A / 2.7 Antipyretic or
SCHEDULING STATUS: S1 PROPRIETARY NAME AND DOSAGE FORM: Aleve Tablets COMPOSITION: Each tablet contains naproxen sodium 220 mg (equivalent to 200 mg naproxen) CATEGORY AND CLASS: A / 2.7 Antipyretic or
PRODUCT INFORMATION. Ammonium chloride is an expectorant that has an irritant effect on mucous membranes.
 PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
NEW ZEALAND DATA SHEET
 IBUPROFEN (Arrowcare) Ibuprofen Tablets 200mg Presentation NEW ZEALAND DATA SHEET White, capsule shaped, coated tablets with no markings. Uses Actions Ibuprofen is a non-steroidal anti-inflammatory agent.
IBUPROFEN (Arrowcare) Ibuprofen Tablets 200mg Presentation NEW ZEALAND DATA SHEET White, capsule shaped, coated tablets with no markings. Uses Actions Ibuprofen is a non-steroidal anti-inflammatory agent.
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Vitafen 100mg Film-coated Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Aceclofenac 100mg. For a full list of
1 NAME OF THE MEDICINAL PRODUCT Vitafen 100mg Film-coated Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Aceclofenac 100mg. For a full list of
CSP Nabumetone ES/H/PSUR/0014/001. January 2010
 CSP Nabumetone ES/H/PSUR/0014/001 January 2010 CLINICAL PARTICULARS 4.1 Therapeutic indications Nabumetone is indicated in the symptomatic treatment of a variety of musculoskeletal disorders requiring
CSP Nabumetone ES/H/PSUR/0014/001 January 2010 CLINICAL PARTICULARS 4.1 Therapeutic indications Nabumetone is indicated in the symptomatic treatment of a variety of musculoskeletal disorders requiring
PRODUCT INFORMATION PANADEINE FORTE
 PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
NEW ZEALAND DATA SHEET ACUPAN TM. 3. PHARMACEUTICAL FORM White, round, biconvex, film-coated tablets (7 mm diameter) engraved APN on one face.
 1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 400 mg Tablets BP Ibuprofen 400 mg Caplets Ibuprofen 400 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 400 mg Tablets BP Ibuprofen 400 mg Caplets Ibuprofen 400 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Caplets Ibuprofen 200 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 200mg of Ibuprofen.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Caplets Ibuprofen 200 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 200mg of Ibuprofen.
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
Product Information - Australia APO-IBUPROFEN 400. NAME OF THE MEDICINE Ibuprofen The structural formula for ibuprofen is shown below:
 NAME OF THE MEDICINE Ibuprofen The structural formula for ibuprofen is shown below: APO-IBUPROFEN 400 Chemical formula: C13H18O2 Molecular weight: 206.3 CAS Number: 15687-27-1 DESCRIPTION Active ingredient:
NAME OF THE MEDICINE Ibuprofen The structural formula for ibuprofen is shown below: APO-IBUPROFEN 400 Chemical formula: C13H18O2 Molecular weight: 206.3 CAS Number: 15687-27-1 DESCRIPTION Active ingredient:
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Tablets Lloyds pharmacy Ibuprofen 200mg Caplets Ibuprofen 200 mg Caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Tablets Lloyds pharmacy Ibuprofen 200mg Caplets Ibuprofen 200 mg Caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
MESULID 100 REVISED PRODUCT INFORMATION
 MESULID 100 REVISED PRODUCT INFORMATION COMPOSITION Each caplet of MESULID 100 contains: Nimesulide 100 mg. Inactive Ingredients Lactose, microcrystalline cellulose, sodium starch glycolate, hydrogenated
MESULID 100 REVISED PRODUCT INFORMATION COMPOSITION Each caplet of MESULID 100 contains: Nimesulide 100 mg. Inactive Ingredients Lactose, microcrystalline cellulose, sodium starch glycolate, hydrogenated
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200mg Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Ibuprofen 200mg. Also contains lactose, sucrose
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200mg Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Ibuprofen 200mg. Also contains lactose, sucrose
Ibuprofen 200 mg and Codeine Phosphate Hemihydrate 12.8 mg tablets
 CHEMISTS OWN IBUPROFEN PLUS CODEINE Ibuprofen 200 mg and Codeine Phosphate Hemihydrate 12.8 mg tablets PRODUCT INFORMATION NAME OF THE MEDICINE Chemists Own Ibuprofen Plus Codeine Ibuprofen 200 mg, Codeine
CHEMISTS OWN IBUPROFEN PLUS CODEINE Ibuprofen 200 mg and Codeine Phosphate Hemihydrate 12.8 mg tablets PRODUCT INFORMATION NAME OF THE MEDICINE Chemists Own Ibuprofen Plus Codeine Ibuprofen 200 mg, Codeine
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 200 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 200 mg ibuprofen. Excipients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 200 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 200 mg ibuprofen. Excipients:
DATA SHEET. PANADOL Mini Caps Capsule shaped tablet with a gelatin coating which is one half green and the other half white.
 PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
Metabolism Paracetamol is metabolised in the liver and excreted in the urine mainly as glucuronide and sulphate conjugates.
 FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
NOTOPAIN CAPLETS. Diclofenac Sodium + Paracetamol. Composition. Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg.
 NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
Paracetamol, codeine phosphate hemihydrate and promethazine hydrochloride.
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
patient group direction
 NAPROXEN v01 1/10 NAPROXEN PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
NAPROXEN v01 1/10 NAPROXEN PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS
 PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
PANADOL OSTEO PRODUCT INFORMATION
 PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
PANADOL OSTEO PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION White to off-white film coated capsule shaped tablets with
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
NEW ZEALAND DATA SHEET NUROFEN PLUS
 NEW ZEALAND DATA SHEET NUROFEN PLUS 1. TRADE NAME OF THE MEDICINAL PRODUCT NUROFEN PLUS Ibuprofen 200mg Codeine Phosphate Hemihydrate 12.8mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains
NEW ZEALAND DATA SHEET NUROFEN PLUS 1. TRADE NAME OF THE MEDICINAL PRODUCT NUROFEN PLUS Ibuprofen 200mg Codeine Phosphate Hemihydrate 12.8mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 400 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 400 mg ibuprofen. For the
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 400 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 400 mg ibuprofen. For the
NEW ZEALAND DATA SHEET. Each Paracetamol + Codeine contains 500 mg of paracetamol and 8 mg of codeine phosphate.
 NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
Name Brufen Flu Tablets & Suspension Description For the relief of the symptoms of colds and flu. Active Ingredients:
 Name Brufen Flu Tablets & Suspension Description For the relief of the symptoms of colds and flu Active Ingredients: Brufen Flu each tablet (Film-coated) contains: Ibuprofen 200 mg Pseudoephedrine hydrochloride
Name Brufen Flu Tablets & Suspension Description For the relief of the symptoms of colds and flu Active Ingredients: Brufen Flu each tablet (Film-coated) contains: Ibuprofen 200 mg Pseudoephedrine hydrochloride
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES
 PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
PRODUCT INFORMATION PANADOL TABLETS PANADOL MINI CAPS PANADOL SUPPOSITORIES NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol 103-90-2 DESCRIPTION Paracetamol is
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
1. PRODUCT NAME PANADOL TABLETS, Paracetamol 500 mg, film coated tablet PANADOL MINI CAPS, Paracetamol 500 mg, coated tablet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active ingredient: Paracetamol (BP)
NEW ZEALAND DATA SHEET. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 200 mg of ibuprofen.
 1. PRODUCT NAME IBUPROFEN, coated tablets 200 mg, Teva NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 200 mg of ibuprofen. Excipient with known effect: Lactose
1. PRODUCT NAME IBUPROFEN, coated tablets 200 mg, Teva NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 200 mg of ibuprofen. Excipient with known effect: Lactose
PANADOL SINUS DAY & NIGHT TABLETS PRODUCT INFORMATION
 Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
Product description PANADOL SINUS DAY tablet is a white, film-coated capsule-shaped tablet with flat edges, front and back faces marked with DAY. PANADOL SINUS NIGHT tablet is a white, film-coated round
PRODUCT INFORMATION PRODEINE 15 H 3 CO. Paracetamol MW Codeine phosphate MW
 PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
PRODUCT INFORMATION PRODEINE 15 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate. Chemical Structure HO O H N CH 3 NH CH 3 H H 3 CO Paracetamol MW 151.17 Codeine phosphate MW
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY
 TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
TRAPADOL INJECTION FOR I.V./I.M. USE ONLY Composition : Each 2ml. contains : Tramadol Hydrochloride I.P. Water for injection I.P. 100mg. q.s. CLINICAL PHARMACOLOGY : Pharmacodynamics Tramadol is a centrally
Action Naproxen sodium, the active principle of Naproxan, has been developed as an analgesic because it is more rapidly absorbed than Naproxen.
 NAPROXAN Composition Each tablet contains Naproxen sodium 275 mg. Tablets Action Naproxen sodium, the active principle of Naproxan, has been developed as an analgesic because it is more rapidly absorbed
NAPROXAN Composition Each tablet contains Naproxen sodium 275 mg. Tablets Action Naproxen sodium, the active principle of Naproxan, has been developed as an analgesic because it is more rapidly absorbed
Your Pharmacy Paracetamol Plus, capsule shaped tablets (caplets)
 New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
DATA SHEET. Paracetamol (BP) 500 mg and Phenylephrine Hydrochloride (BP) 5 mg
 COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
Action Rufenal contains a non - steroidal compound with pronounced antirheumatic, anti-inflammatory, analgesic and antipyretic properties.
 RUFENAL Composition Rufenal Injection Each ampoule of 3 ml contains Diclofenac sodium 75 mg. Ampoule, Tablets & Suppositories Rufenal 12.5 Suppositories Each suppository contains Diclofenac sodium 12.5
RUFENAL Composition Rufenal Injection Each ampoule of 3 ml contains Diclofenac sodium 75 mg. Ampoule, Tablets & Suppositories Rufenal 12.5 Suppositories Each suppository contains Diclofenac sodium 12.5
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flarin 200 mg Soft Capsules Ibuprofen
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flarin 200 mg Soft Capsules Ibuprofen Read all of this leaflet carefully before you start taking this product. This medicine is available without prescription.
PACKAGE LEAFLET: INFORMATION FOR THE USER Flarin 200 mg Soft Capsules Ibuprofen Read all of this leaflet carefully before you start taking this product. This medicine is available without prescription.
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Diclofenac Orifarm, 11.6 mg/g gel. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram Diclofenac Orifarm gel contains 11.6 mg (1.16%)
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Diclofenac Orifarm, 11.6 mg/g gel. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 gram Diclofenac Orifarm gel contains 11.6 mg (1.16%)
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flurbiprofen Sandoz sinaasappel suikervrij 8,75 mg, zuigtabletten 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each lozenge contains 8.75
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flurbiprofen Sandoz sinaasappel suikervrij 8,75 mg, zuigtabletten 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each lozenge contains 8.75
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30
 PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules.
 Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
2. QUALITATIVE AND QUANTITATIVE COMPOSITION. Each capsule contains PARACETAMOL 500mg For a full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PARACETAMOL 500mg CAPSULES Boots Paracetamol 500mg Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains PARACETAMOL
Each 5ml of Sinarest LP New Syrup contains: Phenylephrine
 Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN BURANA 40 MG/ML ORAL SUSPENSION DATE: , VERSION 1
 PUBLIC SUMMARY OF RISK MANAGEMENT PLAN BURANA 40 MG/ML ORAL SUSPENSION DATE: 15-5-2015, VERSION 1 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Musculoskeletal pain: Musculoskeletal
PUBLIC SUMMARY OF RISK MANAGEMENT PLAN BURANA 40 MG/ML ORAL SUSPENSION DATE: 15-5-2015, VERSION 1 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Musculoskeletal pain: Musculoskeletal
NAME OF THE MEDICINAL PRODUCT IMODIUM CAPS. QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule.
 NAME OF THE MEDICINAL PRODUCT IMODIUM CAPS QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule. PHARMACEUTICAL FORM AND DESCRIPTION Capsules, hard White powder filled
NAME OF THE MEDICINAL PRODUCT IMODIUM CAPS QUALITATIVE AND QUANTITATIVE COMPOSITION 2 mg loperamide hydrochloride (HCl) per capsule. PHARMACEUTICAL FORM AND DESCRIPTION Capsules, hard White powder filled
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
RANTUDIL F 60mg capsules
 PACKAGE LEAFLET: INFORMATION FOR THE USER RANTUDIL F 60mg capsules ACEMETACIN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER RANTUDIL F 60mg capsules ACEMETACIN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PRODUCT INFORMATION. Benadryl* for the Family Nightime Oral Liquid
 Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
Etopan XL 600 mg tablets
 Package leaflet: Information for the user Etopan XL 600 mg tablets etodolac Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep
Package leaflet: Information for the user Etopan XL 600 mg tablets etodolac Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. Keep
SUMMARY OF PRODUCT CHARACTERISTICS. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Lacrofarm, powder for oral solution, sachet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each sachet contains the following active ingredients:
PRODUCT INFORMATION. Sudafed* Sinus + Allergy & Pain Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus + Allergy & Pain Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
PRODUCT INFORMATION Sudafed* Sinus + Allergy & Pain Relief Tablets Name of the Medicine Paracetamol CAS 2 Registry Number: 103-90-2 Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 Triprolidine
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications
 Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
Annex I: Proposed Core Safety Profile (CSP) 4.3 Contraindications Hypersensitivity to cefuroxime or to any of the excipients listed in section 6.1. Patients with known hypersensitivity to cephalosporin
XTRAM. Composition Xtram 50 mg capsule Each capsule contains Tramadol HCl 50 mg
 XTRAM Composition Xtram 50 mg capsule Each capsule contains Tramadol HCl 50 mg Capsules Action Tramadol is a centrally acting synthetic analgesic of the aminocyclohexanol group with opioid-like effects.
XTRAM Composition Xtram 50 mg capsule Each capsule contains Tramadol HCl 50 mg Capsules Action Tramadol is a centrally acting synthetic analgesic of the aminocyclohexanol group with opioid-like effects.
PACKAGE LEAFLET: INFORMATION FOR THE USER. EASOFEN MAX STRENGTH 400mg FILM-COATED TABLETS. Ibuprofen
 PACKAGE LEAFLET: INFORMATION FOR THE USER EASOFEN MAX STRENGTH 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking Easofen Max Strength Tablets because it contains
PACKAGE LEAFLET: INFORMATION FOR THE USER EASOFEN MAX STRENGTH 400mg FILM-COATED TABLETS Ibuprofen Read all of this leaflet carefully before you start taking Easofen Max Strength Tablets because it contains
Package Insert. Spasfree
 Package Insert Spasfree Product Summary 1. Name of the medicinal product Spasfree 2. Qualitative and quantitative composition Each film-coated tablet contains Aceclofenac 100 mg and drotaverine hydrochloride
Package Insert Spasfree Product Summary 1. Name of the medicinal product Spasfree 2. Qualitative and quantitative composition Each film-coated tablet contains Aceclofenac 100 mg and drotaverine hydrochloride
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
0BCore Safety Profile. Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) NL/H/PSUR/0058/001 Date of FAR:
 0BCore Safety Profile Active substance: Omeprazole Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) P-RMS: NL/H/PSUR/0058/001 Date of FAR: 13.06.2013 4.2 Posology and method of administration
0BCore Safety Profile Active substance: Omeprazole Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) P-RMS: NL/H/PSUR/0058/001 Date of FAR: 13.06.2013 4.2 Posology and method of administration
