PRODUCT INFORMATION. Codeine CAS: Molecular formula: C18H21NO3.H3PO4.1/2 H2O. MW:406.4
|
|
|
- Bernadette Lamb
- 5 years ago
- Views:
Transcription
1 PRODUCT INFORMATION i) Name of the medicine: NUROFEN PLUS ibuprofen 200mg and codeine phosphate hemihydrate 12.8mg Ibuprofen CAS: Molecular formula: C13H18O2. MW:206.3 Codeine CAS: Molecular formula: C18H21NO3.H3PO4.1/2 H2O. MW:406.4 Nurofen Plus tablets are gluten-free and lactose-free. ii) Description Ibuprofen: Chemical name: 2-(4-Isobutylphenyl) propionic acid. It is a white or almost white powder or crystals with a characteristic odour. Practically insoluble in water, soluble 1 in 1.5 of alcohol, 1 in 1 of chloroform, 1 in 2 of ether and 1 in 1.5 of acetone; soluble in aqueous solutions of alkali hydroxides and carbonates. Codeine phosphate hemihydrate: Chemical name: (5R,6S)-7, 8-didehydro-4,5-epoxy- 3-methoxy-N-methylmorphinan-6-ol dihydrogen orthophosphate hemihydrate. It is a small, colourless, odourless crystal or a white, odourless crystalline powder. Codeine phosphate is soluble in four parts water, slightly soluble in ethanol (96%), practically insoluble in chloroform and ether. A white capsule-shaped tablet, containing ibuprofen 200mg and codeine phosphate hemihydrate12.8mg. Also containing microcrystalline cellulose, sodium starch glycollate, hypromellose, pregelatinised maize starch, talc and Opaspray white colouring. iii) Pharmacology: Actions: Nurofen Plus PI 12/2017 1
2 It is thought that ibuprofen produces an antiinflammatory effect at least in part by inhibiting prostaglandin synthetase. Ibuprofen has shown antiinflammatory, analgesic and antipyretic activity in both animal and human studies. Codeine phosphate hemihydrate is a narcotic analgesic acting on central opiate receptors, although its pharmacological effects are thought to be largely due to its biotransformation to morphine. Pharmacokinetics: Ibuprofen Absorption. Ibuprofen is well absorbed after oral administration with peak serum levels occurring after 1 to 2 hours. Distribution. Apparent volume of distribution is 0.14L/kg. Ibuprofen and its metabolites readily cross the placental barrier in pregnant rabbits and rats. It is not known if the drug enters the cerebrospinal fluid. 99% of ibuprofen is protein bound. The high protein binding of the drug should be borne in mind when prescribing ibuprofen together with other protein bound drugs that bind to the same site on human serum albumin. Metabolism. 90% of ibuprofen is metabolised in the liver to produce two major metabolites, a hydroxylated and carboxylated compound. Excretion. Both the inactive metabolites and a small amount of unchanged ibuprofen are excreted rapidly and completely by the kidney with 95% of the administered dose eliminated in the urine within four hours of ingestion. The elimination half-life of ibuprofen is in the range 1.9 to 2.2 hours. Codeine Absorption: Codeine is well absorbed from the gastrointestinal tract and peak plasma concentrations are reached one hour after oral administration. Onset of action occurs in 15 to 30 minutes and analgesia is maintained for 4 to 6 hours. Distribution: Codeine is rapidly distributed to skeletal muscles, kidneys, liver, gastrointestinal tract, lungs, spleen and brain. It crosses the placenta and is distributed in low levels in breast milk. Metabolism: Codeine is metabolised mainly in the liver. The major metabolic pathway involves glucuronidation of codeine to codeine-6-glucuronide. Codeine can also undergo O- and N-demethylation catalysed by CYP2D6 and CYP3A4 respectively. About 10% of an administered dose of codeine is converted by O- demethylation to morphine, which subsequently undergoes glucuronidation to morphine-3 or morphine-6 glucuronide, or N-demethylation to normorphine. Approximately 8% of the general Australian population cannot convert codeine to the active metabolite morphine as they are deficient in the CYP2D6 enzyme. These patients are likely to obtain reduced pain relief from codeine. Codeine is also converted by N-demethylation to norcodeine, which subsequently undergoes glucuronidation to norcodeine glucuronide or O-demethylation to normorphine. Excretion: Codeine is excreted mainly by the kidneys. Of the excreted material in the urine 40-70% is free or conjugated codeine, 5-15% is free or conjugated morphine, and 10-20% is free or conjugated norcodeine. The plasma half-life of codeine is 2 to 4 hours. Only traces of codeine and its metabolites are found in the faeces Nurofen Plus PI 12/2017 2
3 iv) Clinical trials This section is not applicable v) Indications For the temporary relief of acute moderate pain and inflammation in patients over the age of 12 years ( see also Contraindications and Paediatric use). v) Contraindications Known hypersensitivity to ibuprofen, codeine or other opioid analgesics, or any of the excipients. Patients who have previously shown hypersensitivity reactions (e.g., asthma, rhinitis, angioedema, broncho-spasm or urticaria) in response to ibuprofen, acetylsalicylic acid (aspirin) or other non- steroidal antiinflammatory drugs (NSAIDs). Active, or a history of recurrent peptic ulcer/haemorrhage (two or more distinct episodes of proven ulceration or bleeding). As with other non-steroidal antiinflammatory agents, ibuprofen should not be used in active gastrointestinal bleeding or in the presence of peptic ulceration. Respiratory depression, chronic constipation and active alcoholism. Diarrhoea caused by pseudomembranous colitis or poisoning (until the causative organism or toxin has been eliminated from the gastrointestinal tract, since codeine may slow down the elimination, thereby prolonging the diarrhoea). Use with concomitant NSAIDs, including cyclo-oxygenase-2 specific inhibitors - increased risk of adverse reactions Heart or renal problems. (see Precautions). Severe hepatic impairment (see Precautions) During the last trimester of pregnancy (see Precautions). Concomitant treatment with Monoamine oxidase inhibitors (MAOIs) or within 14 days of stopping treatment. Use of codeine containing products is contraindicated in women during breast feeding see Precautions Use in Lactation).. In patients for whom it is known they are CYP2D6 ultra-rapid metabolisers (see Precautions CYP2D6 metabolism). In patients younger than 12 years (see Precautions Paediatric Use). Nurofen Plus PI 12/2017 3
4 In patients aged between years in whom respiratory function might be compromised, including post tonsillectomy and/or adenoidectomy for obstructive sleep apnoea, due to an increased risk of developing serious and life-threatening adverse reactions (see Precautions Paediatric Use). Treatment of perioperative pain in setting of coronary artery bypass surgery (CABG) vii) Precautions Effects on Fertility There is limited evidence that drugs which inhibit cyclo-oxygenase prostaglandin synthesis may cause impairment of female fertility by an effect on ovulation. This is reversible on withdrawal of treatment. Use in Pregnancy Category C: Inhibition of prostaglandin synthesis by ibuprofen may adversely affect pregnancy and/or the embryo/foetal development. During the first and second trimester of pregnancy, this product should not be given unless clearly necessary, and is contraindicated in the third trimester. During the third trimester, all prostaglandin synthesis inhibitors may expose the foetus to cardiopulmonary toxicity (with premature closure of the ductus arteriosus and pulmonary hypertension) and renal dysfunction, which may progress to renal failure with oligohydramnios. At the end of pregnancy, prostaglandin synthesis inhibitors may expose the mother and the neonate to possible prolongation of bleeding time and inhibition of uterine contractions, which may result in delayed or prolonged labour. Opioid analgesics cross the placenta. Regular use during pregnancy may cause physical dependence in the foetus, leading to withdrawal symptoms in the neonate. The use of codeine may prolong labour. Administration of codeine during labour may cause respiratory depression in the newborn infant. Nurofen Plus is contraindicated for use during the last trimester of pregnancy (see Contraindications). Use in Lactation The use of Nurofen Plus during breastfeeding is contraindicated (see also Precautions - CYP2D6 metabolism) due to risk of respiratory depression in the infant. Ibuprofen appears in breast milk in very low concentrations. Analgesic doses excreted in breast milk are generally low. However, infants of breastfeeding mothers taking codeine may have an increased risk of morphine overdose if the mother is an ultrarapid metabolizer of codeine Codeine is excreted into human breast milk. Codeine is partially metabolized by cytochrome P4502D6 (CYP2D6) into morphine, which is excreted into breast milk. If nursing mothers are CYP2D6 ultra-rapid metabolisers, higher levels of morphine may be present in their breast milk. This may result in symptoms of opioid toxicity in both mother and the breastfed infant. Life-threatening adverse events or neonatal death may occur even at therapeutic doses. Therefore, Nurofen Plus is contraindicated for use during breastfeeding. However, in circumstances where a breastfeeding mother requires codeine therapy, breastfeeding should be suspended and alternative arrangement should be made for feeding the infant for any period during codeine treatment. Breastfeeding mothers should be told how to recognize signs of high morphine levels in themselves and their babies. For example, in a mother, symptoms include extreme sleepiness and trouble caring for the baby. In the Nurofen Plus PI 12/2017 4
5 baby, symptoms include signs of increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness. Medical advice should be sought immediately. Use in the elderly Adverse effects may have more serious consequences in the elderly, and they may be more susceptible to the CNS depressant effects of opioids. Undesirable effects may be minimised by using the minimum effective dose for the shortest duration necessary to control symptoms. The elderly have an increased frequency of adverse reactions to NSAIDs especially gastrointestinal bleeding and perforation which may be fatal (see below). Gastrointestinal NSAIDS should be given with care to patients with a history of gastrointestinal disease (ulcerative colitis, Crohn's disease) as their condition may be exacerbated (See Adverse effects). Gastrointestinal bleeding, ulceration and perforation which can be fatal, has been reported with all NSAIDs at any time during treatment, with or without warning symptoms or a previous history of serious GI events. The risk of GI bleeding, ulceration or perforation is higher with increasing NSAID doses and patients with a history of ulcer, particularly if complicated with haemorrhage or perforation (See Contraindications) and in the elderly. These patients should commence treatment on the lowest dose available. Patients with a history of GI toxicity, particularly the elderly, patients with a history of gastrointestinal bleeding or perforation or peptic ulcer haemorrhage related to previous NSAID therapy should report any unusual abdominal symptoms (especially GI bleeding) particularly in the initial stages of treatment. Care is advised in the administration of NUROFEN PLUS to patients with obstructive bowel disorders, recent gastrointestinal surgery, gallstones, myasthenia gravis, a history of peptic ulcer or convulsions. Caution should be advised in patients receiving concomitant medications which could increase the risk of ulceration or bleeding, such as oral corticosteroids, anticoagulants such as warfarin, selective serotonin-reuptake inhibitors or anti-platelet agents such as aspirin (See Interactions with Other Medicines). When GI bleeding or ulceration occurs in patients receiving Nurofen Plus, the treatment should be withdrawn. Respiratory Bronchospasm may be precipitated in patients suffering from, or with a history of bronchial asthma or allergic disease. SLE and mixed connective tissue disease Use of ibuprofen in patients with systemic lupus erythematosus (SLE) and mixed connective tissue disease can increase the risk of aseptic meningitis. Nurofen Plus PI 12/2017 5
6 Hepatic As with other NSAIDs, elevations of one or more liver function tests may occur in up to 15% of patients. These abnormalities may progress, may remain essentially unchanged, or may resolve with continued therapy. Meaningful elevations (three times the upper limit of normal) of ALT or AST occurred in controlled clinical trials in less than 1% of patients. Nurofen Plus should be administered with caution in patients with hepatic impairment. Patients should be advised to remain alert for hepatotoxicity and be informed about the signs and/or symptoms of hepatotoxicity (e.g. nausea, fatigue, lethargy, pruritus, jaundice, abdominal tenderness in the right upper quadrant and flu-like symptoms) and the steps to take should these signs and/or symptoms occur. Renal Renal impairment as renal function may deteriorate, especially in dehydrated paediatric patients (see Contraindications and Undesirable Effects). Cardiovascular and cerebrovascular effects Observational studies have indicated that NSAIDs may be associated with an increased risk of serious cardiovascular events, including myocardial infarction and stroke, which may increase with dose or duration of use. Patients with cardiovascular disease, history of atherosclerotic cardiovascular disease or cardiovascular risk factors may also be at greater risk. Patients should be advised to remain alert for such cardiovascular events, even in the absence of previous cardiovascular symptoms. Patients should be informed about signs and/or symptoms of serious cardiovascular toxicity and the steps to take if they occur. Fluid retention, hypertension and oedema have been reported in association with NSAID therapy. Patients taking antihypertensives with NSAIDs may have an impaired antihypertensive response. Nurofen Plus should be administered with caution in patients with hypertension or fluid retention (see Contraindications heart failure). Dermatological Serious skin reactions, some of them fatal, including exfoliative dermatitis, Stevens- Johnson syndrome, and toxic epidermal necrolysis, have been reported very rarely in association with the use of NSAIDs (see Adverse Effects). Patients appear to be at highest risk of these reactions early in the course of therapy, the onset of the reaction occurring in the majority of cases within the first month of treatment. Nurofen Plus use should be discontinued at the first appearance of skin rash, mucosal lesions, or any other sign of hypersensitivity. CYP2D6 metabolism Nurofen Plus is contraindicated for use in patients who are CYP2D6 ultra-rapid metabolisers. Nurofen Plus PI 12/2017 6
7 Codeine is metabolised by the liver enzyme CYP2D6 into morphine, its active metabolite. If a patient has a deficiency or is completely lacking this enzyme an adequate analgesic effect will not be obtained. However if the patient is an extensive or ultra-rapid metabolizer there is an increased risk of developing side effects of opioid toxicity even at commonly prescribed doses. These patients convert codeine into morphine rapidly resulting in higher than expected serum morphine levels. General symptoms of opioid toxicity include confusion, somnolence, shallow breathing, small pupils, nausea, vomiting, constipation and lack of appetite. In severe cases this may include symptoms of circulatory and respiratory depression, which may be life-threatening and very rarely fatal Children are particularly susceptible due to their immature airway anatomy. Deaths have been reported in children with rapid metabolism who were given codeine for analgesia post adenotonsillectomy. Morphine can also be ingested by infants through breast milk, causing risk of respiratory depression to infants of rapid metaboliser mothers who take codeine. The prevalence of codeine ultra-rapid metabolism by CYP2D6 in children is not known, but is assumed to be similar to that reported in adults. The prevalence of ultrarapid metabolisers is estimated to be 1% in those of Chinese, Japanese and Hispanic descent, 3% in African Americans and 1%-10% in Caucasians. The highest prevalence (16%- 28%) occurs in North African, Ethiopian and Arab populations. Estimates of prevalence of ultra- rapid metabolisers in different populations are summarised below: Population Prevalence % African/Ethiopian 29% African American 3.4% to 6.5% Asian 1.2% to 2.0% Caucasian 3.6% to 6.5% Greek 6.0% Hungarian 1.9% Northern European 1.0 to 2.0% (See also the sections on Paediatric Use and Use in Lactation.) Paediatric Use Nurofen Plus is contraindicated for use in children: younger than 12 years. aged between years in whom respiratory function might be compromised, including post tonsillectomy and/or adenoidectomy for obstructive sleep apnoea. Respiratory depression and death have occurred in some children who received codeine following tonsillectomy and/or adenoidectomy and had evidence of being ultrarapid metabolisers of codeine due to a CYP2D6 polymorphism. Post-operative use in children: There have been reports in the published literature that codeine given post-operatively in children after tonsillectomy and/or adenoidectomy for obstructive sleep apnoea, led to rare, but life-threatening adverse Nurofen Plus PI 12/2017 7
8 events including death (see Contraindications). All children received doses of codeine that were within the appropriate dose range; however there was evidence that these children were either ultrarapid or extensive metabolisers in their ability to metabolise codeine to morphine. Children with compromised respiratory function: Codeine is contraindicated for use in children in whom respiratory function might be compromised including neuromuscular disorders, severe cardiac or respiratory conditions, upper respiratory or lung infections, multiple trauma or extensive surgical procedures. These factors may worsen symptoms of morphine toxicity. Other precautions As with other drugs of this class, ibuprofen may mask the usual signs of infection. Codeine may also obscure the diagnosis or the course of gastrointestinal diseases. NUROFEN PLUS should therefore be administered with caution in such situations. NUROFEN PLUS should be administered with caution in patients who have recently had gastrointestinal surgery, as codeine may reduce gastrointestinal motility. NUROFEN PLUS should be administered with caution in those with hypotension and /or hypothyroidism. The tablets should be used with caution in patients with raised intracranial pressure or head injury. Nurofen Plus PI 12/2017 8
9 Physical and/or psychological dependence may occur following prolonged administration of codeine. Tolerance may also develop following prolonged administration and irritability and restlessness may be experienced when the tablets are stopped NUROFEN PLUS should be administered with caution in patients with prostatic hypertrophy since codeine may cause urinary retention. Care is advised in the administration of NUROFEN PLUS to patients with adrenocortical insufficiency and also in patients with a history of drug abuse. The effect of ability to drive and use machinery Codeine may cause drowsiness. Opioid analgesics can impair mental function and cause blurred vision and dizziness. Rare side effects may include convulsions, hallucinations, blurred or double vision and orthostatic hypotension. Patients should be advised not to drive or operate machinery. viii) Interactions with Other Medicines Nurofen Plus should be avoided in combination with: Aspirin. Experimental data suggest that ibuprofen may inhibit the effect of low dose aspirin on platelet aggregation when they are dosed concomitantly. However, no clinically relevant effect is considered to be likely for occasional ibuprofen use.. Other NSAIDs: Including cyclooxygenase-2-selective inhibitors. Avoid the use of two or more NSAIDs as this may increase the risk of adverse effects. Nurofen Plus should be used with caution in combination with: Anticholinergics. Concurrent use of codeine and anticholinergic agents may increase the risk of severe constipation and/or urinary retention. Anticoagulants. Concurrent use of NSAIDs and warfarin has been associated with severe, sometimes fatal haemorrhage. The mechanism of this interaction is not known but may involve increased bleeding from NSAID-induced gastrointestinal ulceration or an additive effect of NSAID inhibition of platelet function with the anticoagulant effect of warfarin. NUROFEN PLUS should only be used in patients taking warfarin if absolutely necessary. Patients taking this combination must be closely monitored. Antidiarrhoeal and Anti-peristaltic agents: Concurrent use of codeine with antidiarrhoeal and antiperistaltic agents such as loperamide and kaolin may increase the risk of severe constipation. Nurofen Plus PI 12/2017 9
10 Antimuscarinics. Concomitant use of antimuscarinics or medications with muscarinic action, e.g. atropine and some antidepressants may result in increased risk of severe constipation which may lead to paralytic ileus and/or urinary retention. ACE inhibitors, diuretics and other antihypertensives. Ibuprofen, like other NSAIDs can reduce the antihypertensive effect of ACE inhibitors and beta-blockers with possible loss of blood pressure control and can attenuate the natriuretic effects of diuretics. Hypotensive effects of antihypertensive agents may be potentiated when used concurrently with codeine and lead to orthostatic hypotension. NSAIDs may diminish the effects of antihypertensives and diuretics. Diuretics can increase the risk of nephrotoxicity of NSAIDs The combined use of the three classes of drugs, diuretics, an ACE inhibiting drug (ACE inhibitor or angiotensin receptor antagonist) and an antiinflammatory drug (NSAID or cyclooxygenase-2 (COX-2) inhibitor) all at the same time increases the risk of renal impairment. The combination of drugs from these three classes should be used with caution particularly in elderly patients or those with pre-existing renal impairment. Antiperistaltic antidiarrhoeals (including kaolin, pectin, loperamide). Concurrent use of these agents with codeine may increase the risk of severe constipation Anti-platelet agents and selective serotonin reuptake inhibitors (SSRls). Increased risk of gastrointestinal bleeding (see Precautions) Cardiac glycosides. NSAIDs may exacerbate cardiac failure, reduce GFR and increase plasma glycoside levels. Care should therefore be taken in patients treated with cardiac glycosides. Central nervous system depressants. Codeine may potentiate the effects of CNS depressants. Ciclosporin. An increased risk of nephrotoxicity Cimetidine. Cimetidine inhibits the metabolism of opioid analgesics resulting in increased plasma concentrations. Corticosteroids. An increased risk of gastrointestinal ulceration or bleeding may occur with corticosteroids (see Precautions). Drugs that inhibit CYP2D6 such as quinidine, phenothiazines and antipsychotic agents. Can interfere with the metabolism of codeine to morphine, reducing the analgesic effect of codeine. Hydroxyzine. Concurrent use of hydroxyzine (anxiolytics) with codeine may result in increased analgesia as well as increased CNS depressant, sedative and hypotensive effects. Nurofen Plus PI 12/
11 Lithium. Ibuprofen has been shown to decrease the renal clearance and increase plasma concentrations of lithium. Lithium plasma concentrations should be monitored in patients on concurrent ibuprofen therapy. Metoclopramide, cisapride and domperidone: Codeine may antagonise the gastrointestinal effects of metoclopramide, cisapride and domperidone. Methotrexate. NSAIDs inhibit tubular secretion of methotrexate in animals. As a result, reduction in the clearance of methotrexate may occur. Use of high doses of methotrexate concomitantly with NSAIDs should be avoided. At low doses of methotrexate, caution should be used if ibuprofen is administered concomitantly. Mexiletine. Codeine may delay the absorption of mexiletine and thus reduce the antiarrhythmic effect of the latter. Mifepristone: NSAIDs should not be used for 8-12 days after mifepristone administration as NSAIDs can reduce the effect of mifepristone. Moclobemide: Risk of hypertensive crisis. Monoamine oxidase inhibitors (MAOIs). Concurrent administration or use within 14 days of ceasing monoamine oxidase inhibitors may enhance the potential respiratory depressant effects of codeine. CNS depression or excitation may occur if codeine is given to patients receiving monoamine oxidase inhibitors, or within two weeks of stopping treatment with them. Naloxone. Naloxone antagonises the analgesic, CNS and respiratory depressant effects of opioid analgesics. Naltrexone also blocks the therapeutic effect of opioids. NSAIDs and aspirin. Concurrent use of ibuprofen with aspirin or other NSAIDs can lead to increased gastrointestinal adverse effects. Neuromuscular blocking agents. The respiratory depressant effect caused by neuromuscular blocking agents may be additive to the central respiratory depressant effects of opioid analgesics. Opioid analgesics. Concurrent use of codeine and other opioid receptor agonists is usually inappropriate as additive CNS depression, respiratory depression and hypotensive effects may occur. Probenecid and phenytoin. Interactions may also occur with probenecid, antidiabetic medications and phenytoin. Quinolone antibiotics. Animal data indicate that NSAIDs can increase the risk of convulsions associated with Quinolone antibiotics. Patients taking NSAIDs and Quinolone may have an increased risk of developing convulsions. Quinidine. Quinidine can inhibit the analgesic effect of codeine Tacrolimus. Possible increased risk of nephrotoxicity when NSAIDs are given with Nurofen Plus PI 12/
12 Tacrolimus. Tranquillizers, sedatives and hypnotics. Codeine may potentiate the effects of these drugs. Zidovudine. Increased risk of haematological toxicity when NSAIDs are given with Zidovudine. There is evidence of an increased risk of haemarthroses and haematoma in HIV (+) haemophiliacs receiving concurrent treatment with Zidovudine and ibuprofen. Incompatibilities: Codeine has been reported to be incompatible with phenobarbitone sodium forming a codeine phenobarbitone complex, and with potassium-iodide, forming crystals of codeine periodide. Acetylation of codeine phosphate by acetylsalicylic acid (aspirin) has occurred in solid dosage forms containing the two drugs, even at low moisture levels. Interference with laboratory tests: Opioid analgesics interfere with a number of laboratory tests including plasma amylase, lipase, bilirubin, alkaline phosphatase, lactate dehydrogenase, alanine aminotransferase and aspartate aminotransferase. Opoids may also interfere with gastric emptying studies as they delay gastric emptying and with hepatobiliary imaging using technetium Tc 99m disofenin as opioid treatment may cause constriction of the sphincter of Oddi and increase biliary tract pressure. ix) Adverse effects: The most commonly observed adverse events are gastrointestinal in nature. Adverse events are mostly dose-dependent, in particular the risk of occurrence of gastrointestinal bleeding which is dependent on the dosage range and duration of treatment. Side effects from codeine are theoretical warnings based on drug class. No clinical data is available to determine frequency. Regular prolonged use of codeine is known to lead to addiction and symptoms of restlessness and irritability may result when treatment is then stopped. Prolonged use of a painkiller for headache can make them worse. The list of the following adverse events relates to those experienced with ibuprofen and codeine (maximum of 1200mg ibuprofen per day), for short-term use. In the treatment of chronic conditions, under long-term treatment, additional adverse events may occur. Adverse events which have been associated with ibuprofen and codeine are given below, tabulated by System Organ Class (SOC) and frequency. The frequencies of adverse effects are defined as follows: Very common: 1/10 Common: 1/100, <1/10 Nurofen Plus PI 12/
13 Uncommon: 1/1,000, <1/100 Rare: 1/10,000, <1/1,000 Very Rare: <1/10,000, including isolated reports. : cannot be estimated from the available data. Within each frequency grouping, adverse events are presented in order of decreased seriousness. System Organ Class Frequency Adverse Events Blood and Lymphatic System Disorders Very rare Haematopoietic disorders 1, alveolitis, pulmonary eosinophilia, pancreatitis, haemoglobin decreased, platelet aggregation 2 Immune System Disorders Uncommon Hypersensitivity with urticaria and pruritus 3 Metabolism and nutrition disorders Very rare Severe hypersensitivity reactions, including swelling face, swollen tongue and laryngeal oedema, dyspnoea, apnoea and tachycardia, (anaphylaxis, angioedema or severe shock) 3 Decreased appetite Psychiatric Disorders Depression, hallucination, confusional state, dependence, mood altered, restlessness, nightmares, irritability Nervous System Disorders Common Drowsiness Uncommon Very rare Headache, dizziness Aseptic meningitis 4, nervousness, tinnitus, depression, insomnia, disturbance in attention, affect lability, convulsions. Intracranial pressure increased, dyskinesia, miosis Eye Disorders Very rare Vision blurred, amblyopia, visual impairment 5 Ear and Labyrinth Disorders Rare Rare Dry eye Diplopia Hearing impaired Vertigo Cardiac Disorders Rare Palpitations 6 Cardiac failure, oedema, bradycardia, Vascular Disorders Rare Cerebrovascular accident 6, hypotension, cardiac failure congestive 7 Respiratory, Thoracic and Mediastinal Disorders Hypertension, orthostatic hypotension 6 Rare Rhinitis Respiratory tract reactivity comprising asthma, bronchospasm or dyspnoea 3 Nurofen Plus PI 12/
14 System Organ Class Frequency Adverse Events Respiratory depression, cough suppression Gastrointestinal Disorders Uncommon Abdominal pain, nausea, dyspepsia Rare Very rare Hepatobiliary Disorders Very rare Liver disorder 10 Skin and Subcutaneous Tissue Disorders Musculoskeletal and connective tissue Disorders Renal and Urinary Disorders General Disorders and Administration Site Conditions Diarrhoea, flatulence, constipation, vomiting, Dry mouth, gingival ulceration Peptic ulcer, gastrointestinal perforation or gastrointestinal haemorrhage, melaena, haematemesis 8. Mouth ulceration, gastritis. Exacerbation of ulcerative colitis and Crohn's disease 9 Biliary colic Uncommon Skin rash 3 Rare Very rare Skin exfoliation, alopecia, dermatitis exfoliative, photosensitivity reaction, rash maculopapular,hyperhidrosis Bullous reactions, including Stevens-Johnson Syndrome, erythema multiforme and toxic epidermal necrolysis 3 Flushing Muscle rigidity Very rare Acute renal failure 11 Renal colic,, dysuria 12 Hypothermia, fatigue, malaise Investigations Very rare Haemoglobin decreased Description of Selected Adverse Reactions 1 Examples include anaemia, neutropenia, aplastic anaemia, haemolytic anaemia, eosinophilia, reduction of haemoglobin and haematocrit leucopenia, thrombocytopenia, pancytopenia and agranulocytosis. First signs are fever, sore throat, superficial mouth ulcers, flu-like symptoms, severe exhaustion, unexplained bleeding and bruising 2 Reversible. 3 Hypersensitivity reactions have been reported following treatment with ibuprofen. These may consist of Nurofen Plus PI 12/
15 a) non-specific allergic reactions and anaphylaxis b) respiratory tract reactivity e.g. asthma, aggravated asthma, bronchospasm, dyspnea c) assorted skin disorders, including rashes of various types, pruritus, urticaria, purpura, angioedema and more rarely exfoliative and bullous dermatoses (including toxic epidermal necrolysis, Stevens-Johnson syndrome and erythema multiforme). Severe shock syndrome may be characterised by abdominal pain, fever, shivering, nausea and vomiting. Hepatotoxicity and aseptic meningitis which occur less frequently may also be hypersensitivity reactions. 4 The pathogenic mechanism of drug-induced aseptic meningitis is not fully understood. However, the available data on NSID related aseptic meningitis points to a hypersensitivity reaction (due to a temporal relationship with drug intake, and disappearance of symptoms after drug discontinuation). Of note, single cases of symptoms of aseptic meningitis (such as stiff, neck, headache, nausea, vomiting, fever or disorientation) have been observed during treatment with ibuprofen in patients with existing auto-immune disorders (such as systemic lupus erythematosus and mixed connective tissue disease). 5 Includes changes in visual colour perception 6 Clinical trial and epidemiological data suggest that use of ibuprofen (particularly at high doses 2400mg daily) and in long-term treatment may be associated with a small increased risk of arterial thrombotic events (e.g. myocardial infarction or stroke), (see section Precautions ). 7 In patients with compromised cardiac function. 8 Sometimes fatal, particularly in the elderly. 9 See section Precautions 10 Especially in long term treatment, including hepatotoxicity, hepatitis, jaundice, alterations of hepatic function tests, pancreatitis, duodenitis, oesophagitis, hepatorenal syndrome, hepatic necrosis, hepatic insufficiency 11 Especially in long-term use, associated with increased serum urea and oedema. Also includes papillary necrosis. Ibuprofen may cause cystitis and haematuria, interstitial nephritis, nephrotic syndrome, oliguria, tubular necrosis, glomerulonephritis, alteration in the renal function test, polyuria, anaphylaxis. 12 Increased frequency, decrease in amount. x) Dosage and administration: Dosage: Adults and children 12 years and over: Nurofen Plus PI 12/
16 Initial dose two tablets taken with fluid, then one or two tablets every 4 to 6 hours when necessary. Maximum 6 tablets in a 24-hour period. Nurofen Plus should not be used for more than three days at a time unless on medical advice, in which case the patient should be reviewed regularly with regards to efficacy, risk factors and ongoing need for treatment. Children: Do not give to children under 12 years. Nurofen Plus is contraindicated for use in children: younger than 12 years. aged between years in whom respiratory function might be compromised, including post tonsillectomy and/or adenoidectomy for obstructive sleep apnoea (See Paediatric Use and Contraindications). xi) Overdosage: Overuse of this product, defined as consumption of quantities in excess of the recommended dose, or consumption for a prolonged period, may lead to physical or psychological dependency. Symptoms of restlessness and irritability may result when treatment is stopped. Symptoms- The symptoms of overdose with ibuprofen include nausea, vomiting, abdominal pain, diarrhoea (rarely), headache, dizziness, drowsiness, nystagmus, vertigo, blurred vision, tinnitus and rarely, hypertension, metabolic acidosis, convulsions, excitation, disorientation, coma, renal failure, liver damage, hypotension, respiratory depression, cyanosis and loss of consciousness. Exacerbation of asthma is possible in asthmatics. Nausea and vomiting are prominent features of codeine overdose. Respiratory depression, excitability, convulsions, hypotension and loss of consciousness may occur with large codeine overdose. Central nervous system depression, including respiratory depression, may develop but is unlikely to be severe unless other sedative agents have been co-ingested, including alcohol, or the overdose is very large. The pupils may be pinpoint in size. Hypotension and tachycardia are possible. For information on the management of overdose, contact the Poisons Information Centre on xii) Presentation and storage conditions Dosage form White capsule-shaped tablets marked N+ on one side. Quantity, proportion or strength of each therapeutically active ingredient ibuprofen 200mg and codeine phosphate hemihydrate12.8mg Container type Aluminium blister packed in a carton Pack sizes Packs of *4, *6, *12, *24 and 30 tablets Nurofen Plus PI 12/
17 Storage conditions Store below 25 degrees Celsius. Nurofen Plus PI 12/
18 xiii) Name and address of sponsor: Reckitt Benckiser Sydney NSW Australia xiv) Poison Schedule of the medicine Nurofen Plus in packs *4, *6, *12, *24 and 30 tablets are S4 (Prescription Only Medicine) xv) Date of first inclusion in the Australian Register of Therapeutic Goods (ARTG) 17 February 2005 xvi) Date of most recent amendment December 2017 *Not available in Australia Nurofen Plus PI 12/
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION
 RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
RAFEN PLUS Ibuprofen 200mg/Codeine phosphate 12.8mg tablets PRODUCT INFORMATION Composition RAFEN PLUS tablet is a white to off-white capsule-shaped, biconvex tablet, containing ibuprofen 200mg and codeine
Panafen Plus Product Information
 Panafen Plus Product Information COMPOSITION Actives: Ibuprofen 200 mg, codeine phosphate 12.8 mg Inactives: cellulose-microcrystalline, vegetable oil-hydrogenated, sodium starch glycollate, silica-colloidal
Panafen Plus Product Information COMPOSITION Actives: Ibuprofen 200 mg, codeine phosphate 12.8 mg Inactives: cellulose-microcrystalline, vegetable oil-hydrogenated, sodium starch glycollate, silica-colloidal
Panafen Plus PRODUCT INFORMATION
 Panafen Plus PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Ibuprofen Codeine phosphate Ibuprofen Chemical structure: Chemical name: 2-(4-isobutylphenyl) propionic acid Molecular formula:
Panafen Plus PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Ibuprofen Codeine phosphate Ibuprofen Chemical structure: Chemical name: 2-(4-isobutylphenyl) propionic acid Molecular formula:
PRODUCT INFORMATION ACTACODE
 PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
PRODUCT INFORMATION ACTACODE NAME OF THE MEDICINE Actacode codeine linctus contains codeine phosphate 5 mg/ml. Codeine phosphate is (5R,6S)-7,8-didehydro-4,5-epoxy-3-methoxy-N-methylmorphinan-6-ol dihydrogen
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML)
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP DAY-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML AND CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML) 1. NAME OF THE MEDICINE Paracetamol and codeine phosphate hemihydrate.
Migraleve, Migraleve Pink and Migraleve Yellow Product Information
 Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
Migraleve, Migraleve Pink and Migraleve Yellow Product Information Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard Adverse events should also
PRODUCT INFORMATION. Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets
 PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
PRODUCT INFORMATION Sudafed* Sinus + Anti-inflammatory Pain Relief Caplets Product description Sudafed* Sinus + Anti-inflammatory Pain Relief caplets contain pseudoephedrine hydrochloride 30 mg and ibuprofen
Product Information - Australia APO-IBUPROFEN 400. NAME OF THE MEDICINE Ibuprofen The structural formula for ibuprofen is shown below:
 NAME OF THE MEDICINE Ibuprofen The structural formula for ibuprofen is shown below: APO-IBUPROFEN 400 Chemical formula: C13H18O2 Molecular weight: 206.3 CAS Number: 15687-27-1 DESCRIPTION Active ingredient:
NAME OF THE MEDICINE Ibuprofen The structural formula for ibuprofen is shown below: APO-IBUPROFEN 400 Chemical formula: C13H18O2 Molecular weight: 206.3 CAS Number: 15687-27-1 DESCRIPTION Active ingredient:
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
NEW ZEALAND DATA SHEET NUROFEN PLUS
 NEW ZEALAND DATA SHEET NUROFEN PLUS 1. TRADE NAME OF THE MEDICINAL PRODUCT NUROFEN PLUS Ibuprofen 200mg Codeine Phosphate Hemihydrate 12.8mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains
NEW ZEALAND DATA SHEET NUROFEN PLUS 1. TRADE NAME OF THE MEDICINAL PRODUCT NUROFEN PLUS Ibuprofen 200mg Codeine Phosphate Hemihydrate 12.8mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains
New Zealand Datasheet
 1. NAME OF MEDICINE PANAFEN PLUS New Zealand Datasheet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredients: Ibuprofen 200 mg, codeine phosphate 12.8 mg 3. PHARMACEUTICAL FORM Ibuprofen Chemical
1. NAME OF MEDICINE PANAFEN PLUS New Zealand Datasheet 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Active Ingredients: Ibuprofen 200 mg, codeine phosphate 12.8 mg 3. PHARMACEUTICAL FORM Ibuprofen Chemical
CSP Nabumetone ES/H/PSUR/0014/001. January 2010
 CSP Nabumetone ES/H/PSUR/0014/001 January 2010 CLINICAL PARTICULARS 4.1 Therapeutic indications Nabumetone is indicated in the symptomatic treatment of a variety of musculoskeletal disorders requiring
CSP Nabumetone ES/H/PSUR/0014/001 January 2010 CLINICAL PARTICULARS 4.1 Therapeutic indications Nabumetone is indicated in the symptomatic treatment of a variety of musculoskeletal disorders requiring
Read all of this leaflet carefully because it contains important information for you.
 Nurofen Plus Tablets Ibuprofen 200mg, Codeine Phosphate Hemihydrate 12.8mg ADVANCED DUAL ACTION FOR POWERFUL RELIEF Read all of this leaflet carefully because it contains important information for you.
Nurofen Plus Tablets Ibuprofen 200mg, Codeine Phosphate Hemihydrate 12.8mg ADVANCED DUAL ACTION FOR POWERFUL RELIEF Read all of this leaflet carefully because it contains important information for you.
PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
![PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate PRODUCT INFORMATION. (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate](/thumbs/81/82665370.jpg) NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
NORGESIC Orphenadrine citrate and paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: CAS number: 4682-36-4 Chemical structure: Orphenadrine citrate (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine
PRODUCT INFORMATION. Codral* Original Cold & Flu Tablets
 PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
PRODUCT INFORMATION Codral* Original Cold & Flu Tablets Product description Codral* Original Cold & Flu tablets contain paracetamol 500 mg, pseudoephedrine hydrochloride 30 mg and codeine phosphate 6 mg.
PRODUCT INFORMATION Panadeine EXTRA
 PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
PRODUCT INFORMATION Panadeine EXTRA COMPOSITION Each caplet brand of capsule-shaped tablet contains: Paracetamol 500 mg Codeine phosphate 15 mg and Maize Starch Purified Talc Pregelatinised Maize Starch
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Vitafen 100mg Film-coated Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Aceclofenac 100mg. For a full list of
1 NAME OF THE MEDICINAL PRODUCT Vitafen 100mg Film-coated Tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Aceclofenac 100mg. For a full list of
Elements for a Public Summary Overview of disease epidemiology
 VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
VI.2 VI.2.1 Elements for a Public Summary Overview of disease epidemiology Acute pain usually responds to medication and should settle in less than three months. Inadequate pain relief may lead to other
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 200 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 200 mg ibuprofen. Excipients:
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 200 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 200 mg ibuprofen. Excipients:
Patient Group Direction for the supply and/or administration of Ibuprofen 400mg tablets to patients attending NHS Borders services
 Patient Group Direction for the supply and/or administration of Ibuprofen 400mg tablets to patients attending NHS Borders services This document authorises the supply and/or administration of Ibuprofen
Patient Group Direction for the supply and/or administration of Ibuprofen 400mg tablets to patients attending NHS Borders services This document authorises the supply and/or administration of Ibuprofen
Your Pharmacy Paracetamol Plus, capsule shaped tablets (caplets)
 New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
New Zealand Data Sheet Product Description Your Pharmacy Plus, capsule shaped tablets (caplets) 500 mg per tablet & Codeine Phosphate 10 mg per tablet Blister packs of white, capsule-shaped tablets Composition
PROFESSIONAL INFORMATION
 SCHEDULING STATUS: S1 PROPRIETARY NAME AND DOSAGE FORM: Aleve Tablets COMPOSITION: Each tablet contains naproxen sodium 220 mg (equivalent to 200 mg naproxen) CATEGORY AND CLASS: A / 2.7 Antipyretic or
SCHEDULING STATUS: S1 PROPRIETARY NAME AND DOSAGE FORM: Aleve Tablets COMPOSITION: Each tablet contains naproxen sodium 220 mg (equivalent to 200 mg naproxen) CATEGORY AND CLASS: A / 2.7 Antipyretic or
Name Brufen Flu Tablets & Suspension Description For the relief of the symptoms of colds and flu. Active Ingredients:
 Name Brufen Flu Tablets & Suspension Description For the relief of the symptoms of colds and flu Active Ingredients: Brufen Flu each tablet (Film-coated) contains: Ibuprofen 200 mg Pseudoephedrine hydrochloride
Name Brufen Flu Tablets & Suspension Description For the relief of the symptoms of colds and flu Active Ingredients: Brufen Flu each tablet (Film-coated) contains: Ibuprofen 200 mg Pseudoephedrine hydrochloride
Paracetamol, codeine phosphate hemihydrate and promethazine hydrochloride.
 AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
AUSTRALIAN PRODUCT INFORMATION, PAINSTOP NIGHT-TIME PAIN RELIEVER (PARACETAMOL 120MG IN 5ML,CODEINE PHOSPHATE HEMIHYDRATE 5MG IN 5ML AND PROMETHAZINE HYDROCHLORIDE 6.5MG IN 5ML) 1. NAME OF THE MEDICINE
NEW ZEALAND DATA SHEET
 IBUPROFEN (Arrowcare) Ibuprofen Tablets 200mg Presentation NEW ZEALAND DATA SHEET White, capsule shaped, coated tablets with no markings. Uses Actions Ibuprofen is a non-steroidal anti-inflammatory agent.
IBUPROFEN (Arrowcare) Ibuprofen Tablets 200mg Presentation NEW ZEALAND DATA SHEET White, capsule shaped, coated tablets with no markings. Uses Actions Ibuprofen is a non-steroidal anti-inflammatory agent.
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 400 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 400 mg ibuprofen. For the
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Melfen 400 mg Film-coated Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 400 mg ibuprofen. For the
PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.
 Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
Product Information Page 1 PRODUCT INFORMATION APOHEALTH PARACETAMOL PLUS CODEINE & CALMATIVE paracetamol 500mg codeine phosphate 10mg and doxylamine succinate 5.1mg tablets NAME OF THE MEDICINE Paracetamol
NEW ZEALAND DATA SHEET ACUPAN TM. 3. PHARMACEUTICAL FORM White, round, biconvex, film-coated tablets (7 mm diameter) engraved APN on one face.
 1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
1. PRODUCT NAME ACUPAN 30 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains nefopam hydrochloride 30 mg. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Codeine Phosphate Tablets (PSM) 15 mg, 30 mg & 60 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Codeine Phosphate Hemihydrate BP 15 mg
1. PRODUCT NAME Codeine Phosphate Tablets (PSM) 15 mg, 30 mg & 60 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Codeine Phosphate Hemihydrate BP 15 mg
PRODUCT INFORMATION. Ammonium chloride is an expectorant that has an irritant effect on mucous membranes.
 PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
PRODUCT INFORMATION BENADRYL Original Oral Liquid (New Formula) Name of the Medicine Diphenhydramine hydrochloride Ammonium chloride The chemical name for diphenhydramine hydrochloride is 2-(diphenylmethoxy)-N,Ndimethylethanamine
Molecular formula: Molecular weight: C 8 H 9 NO 2 CAS Registry no.:
 Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
Parapane Paracetamol PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredient: Chemical name: Paracetamol N-(4-hydroxyphenyl) Structural formula: Molecular formula: 151.20 Molecular weight: C 8 H 9 NO
Ibuprofen 200 mg and Codeine Phosphate Hemihydrate 12.8 mg tablets
 CHEMISTS OWN IBUPROFEN PLUS CODEINE Ibuprofen 200 mg and Codeine Phosphate Hemihydrate 12.8 mg tablets PRODUCT INFORMATION NAME OF THE MEDICINE Chemists Own Ibuprofen Plus Codeine Ibuprofen 200 mg, Codeine
CHEMISTS OWN IBUPROFEN PLUS CODEINE Ibuprofen 200 mg and Codeine Phosphate Hemihydrate 12.8 mg tablets PRODUCT INFORMATION NAME OF THE MEDICINE Chemists Own Ibuprofen Plus Codeine Ibuprofen 200 mg, Codeine
CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/ January 2013
 CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/001 16 January 2013 1 4.2 Posology and method of administration (safety aspects only) Posology Elderly patients For oral preparations A dose adjustment
CORE SAFETY PROFILE OXYCODONE HYDROCHLORIDE NL/H/PSUR/0054/001 16 January 2013 1 4.2 Posology and method of administration (safety aspects only) Posology Elderly patients For oral preparations A dose adjustment
PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other.
 Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
Product description PANADOL COLD & FLU RELIEF ORIGINAL FORMULA is a white, capsule-shaped tablet with flat edges, one face marked with C&F, PANADOL on the other. Ingredients Active Ingredients: Paracetamol
NEOFEN 60 mg suppository
 PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER NEOFEN 60 mg suppository IBUPROFEN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 400 mg Tablets BP Ibuprofen 400 mg Caplets Ibuprofen 400 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 400 mg Tablets BP Ibuprofen 400 mg Caplets Ibuprofen 400 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet
Children Enteric coated tablet : 1-3 mg/kg per day in divided doses.
 Ultrafen Tablet/SR Tablet/Suppository/Gel Description Ultrafen is a preparation of Diclofenac is a non-steroidal antiinflammatory agent with marked analgesic, anti-inflammatory and antipyretic properties.
Ultrafen Tablet/SR Tablet/Suppository/Gel Description Ultrafen is a preparation of Diclofenac is a non-steroidal antiinflammatory agent with marked analgesic, anti-inflammatory and antipyretic properties.
Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml SI/H/PSUR/0002/002 Date of FAR:
 0BCore Safety Profile Active substance: Ceftibuten Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml P-RMS: SI/H/PSUR/0002/002 Date of FAR: 14.02.2013
0BCore Safety Profile Active substance: Ceftibuten Pharmaceutical form(s)/strength: Capsules, 200mg, 400mg, Oral suspensions, 90mg/5ml, 180mg/5ml, 36 mg/ml P-RMS: SI/H/PSUR/0002/002 Date of FAR: 14.02.2013
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Tablets Lloyds pharmacy Ibuprofen 200mg Caplets Ibuprofen 200 mg Caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Tablets Lloyds pharmacy Ibuprofen 200mg Caplets Ibuprofen 200 mg Caplets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Package Insert. Spasfree
 Package Insert Spasfree Product Summary 1. Name of the medicinal product Spasfree 2. Qualitative and quantitative composition Each film-coated tablet contains Aceclofenac 100 mg and drotaverine hydrochloride
Package Insert Spasfree Product Summary 1. Name of the medicinal product Spasfree 2. Qualitative and quantitative composition Each film-coated tablet contains Aceclofenac 100 mg and drotaverine hydrochloride
Each tablet contains 30mg Codeine Phosphate Ph Eur. Excipient with known effect: Also contains mg of lactose.
 1 NAME OF THE MEDICINAL PRODUCT Codeine Phosphate Tablets BP 30mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 30mg Codeine Phosphate Ph Eur. Excipient with known effect: Also contains
1 NAME OF THE MEDICINAL PRODUCT Codeine Phosphate Tablets BP 30mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 30mg Codeine Phosphate Ph Eur. Excipient with known effect: Also contains
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Codeine Phosphate 30 mg Tablets BP 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 30mg Codeine Phosphate. For the full
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Codeine Phosphate 30 mg Tablets BP 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 30mg Codeine Phosphate. For the full
Each tablet contains 15mg Codeine Phosphate Ph Eur
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Codeine Phosphate Tablets BP 15mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 15mg Codeine Phosphate Ph Eur For the
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Codeine Phosphate Tablets BP 15mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 15mg Codeine Phosphate Ph Eur For the
Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules.
 Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
Nausicalm Cyclizine lactate 50 mg/ml solution for injection Presentation Nausicalm solution for injection is a clear colourless solution, presented in 1 ml ampoules. Uses Actions Cyclizine is a piperazine
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Caplets Ibuprofen 200 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 200mg of Ibuprofen.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200 mg Caplets Ibuprofen 200 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 200mg of Ibuprofen.
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION
 Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
patient group direction
 NAPROXEN v01 1/10 NAPROXEN PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
NAPROXEN v01 1/10 NAPROXEN PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
NEW ZEALAND DATA SHEET. Each Paracetamol + Codeine contains 500 mg of paracetamol and 8 mg of codeine phosphate.
 NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
NEW ZEALAND DATA SHEET PARACETAMOL + CODEINE 1. Product Name Paracetamol + Codeine, 500 mg + 8 mg, tablets. 2. Qualitative and Quantitative Composition Each Paracetamol + Codeine contains 500 mg of paracetamol
Please note that the information highlighted in grey colour is the additional information specific to the concerned dosage form.
 Proposed Core Safety Profile for diclofenac systemic formulations, including solution for injection, sugar-coated tablets, prolonged release tablets, dispersible tablets, gastro-resistant tablets and suppositories
Proposed Core Safety Profile for diclofenac systemic formulations, including solution for injection, sugar-coated tablets, prolonged release tablets, dispersible tablets, gastro-resistant tablets and suppositories
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30
 PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
PRODUCT INFORMATION APO-Paracetamol/Codeine 500/30 NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate Chemical Structure Paracetamol MW 151.17 Codeine phosphate MW 406.37 CAS Numbers
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200mg Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Ibuprofen 200mg. Also contains lactose, sucrose
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen 200mg Tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Ibuprofen 200mg. Also contains lactose, sucrose
Advil Liqui - gels 200 Capsules
 1. NAME OF THE MEDICINAL PRODUCT Advil Liqui - gels 200 Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 200mg Ibuprofen For excipients, see 6.1 3. PHARMACEUTICAL FORM Capsules
1. NAME OF THE MEDICINAL PRODUCT Advil Liqui - gels 200 Capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 200mg Ibuprofen For excipients, see 6.1 3. PHARMACEUTICAL FORM Capsules
PRODUCT INFORMATION LOFENOXAL
 PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
PRODUCT INFORMATION LOFENOXAL Diphenoxylate hydrochloride 2.5 mg and Atropine sulfate 25 microgram Name of the medicines Diphenoxylate Hydrochloride: C30H32N2O2, HCl M. W. = 489.1 CAS registry no.: 3810-80-8
AVIOMARIN 50 mg tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER AVIOMARIN 50 mg tablets DIMENHYDRINATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER AVIOMARIN 50 mg tablets DIMENHYDRINATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
4.4 Special warnings and precautions for use
 SUMMARY OF PRODUCT CHARACTERISTICS 4.3 Contraindications Durogesic is contraindicated in patients with known hypersensitivity to fentanyl or to the excipients present in the patch. Acute or postoperative
SUMMARY OF PRODUCT CHARACTERISTICS 4.3 Contraindications Durogesic is contraindicated in patients with known hypersensitivity to fentanyl or to the excipients present in the patch. Acute or postoperative
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
1. Name of Medicinal Product Co-Codamol 8/500 mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Paracetamol 500.0 mg Codeine Phosphate 8.0 mg For excipients, see section 6.1 3 PHARMACEUTICAL FORM
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain
 TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
RANTUDIL F 60mg capsules
 PACKAGE LEAFLET: INFORMATION FOR THE USER RANTUDIL F 60mg capsules ACEMETACIN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER RANTUDIL F 60mg capsules ACEMETACIN This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE. Chemical structure: CAS 2 Registry Number: DESCRIPTION
 PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
PRODUCT INFORMATION PANADOL COLD & FLU MAX HOT LEMON POWDER NAME OF THE MEDICINE Active ingredient: Paracetamol Chemical structure: CAS 2 Registry Number: 103-90-2 DESCRIPTION Paracetamol is a white, crystalline
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Piroxicam 20mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 20mg of Piroxicam. For a full list of excipients,
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Piroxicam 20mg Capsules 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each capsule contains 20mg of Piroxicam. For a full list of excipients,
NEW ZEALAND DATA SHEET. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 200 mg of ibuprofen.
 1. PRODUCT NAME IBUPROFEN, coated tablets 200 mg, Teva NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 200 mg of ibuprofen. Excipient with known effect: Lactose
1. PRODUCT NAME IBUPROFEN, coated tablets 200 mg, Teva NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each coated tablet contains 200 mg of ibuprofen. Excipient with known effect: Lactose
Action Naproxen sodium, the active principle of Naproxan, has been developed as an analgesic because it is more rapidly absorbed than Naproxen.
 NAPROXAN Composition Each tablet contains Naproxen sodium 275 mg. Tablets Action Naproxen sodium, the active principle of Naproxan, has been developed as an analgesic because it is more rapidly absorbed
NAPROXAN Composition Each tablet contains Naproxen sodium 275 mg. Tablets Action Naproxen sodium, the active principle of Naproxan, has been developed as an analgesic because it is more rapidly absorbed
Action Rufenal contains a non - steroidal compound with pronounced antirheumatic, anti-inflammatory, analgesic and antipyretic properties.
 RUFENAL Composition Rufenal Injection Each ampoule of 3 ml contains Diclofenac sodium 75 mg. Ampoule, Tablets & Suppositories Rufenal 12.5 Suppositories Each suppository contains Diclofenac sodium 12.5
RUFENAL Composition Rufenal Injection Each ampoule of 3 ml contains Diclofenac sodium 75 mg. Ampoule, Tablets & Suppositories Rufenal 12.5 Suppositories Each suppository contains Diclofenac sodium 12.5
NEW ZEALAND DATASHEET
 NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
NEW ZEALAND DATASHEET COLDREX HOT REMEDY COLD & FLU HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS
 PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
PRODUCT INFORMATION MERSYNDOL FORTE TABLETS NAME OF THE MEDICINE Non-proprietary Name Paracetamol, codeine phosphate and doxylamine succinate DESCRIPTION Each tablet contains paracetamol 450 mg, codeine
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Brupro 200mg Film-coated tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 200 mg of ibuprofen. For the full list
1 NAME OF THE MEDICINAL PRODUCT Brupro 200mg Film-coated tablets Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 200 mg of ibuprofen. For the full list
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution DATA SHEET
 PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
PANADOL COLD & FLU MAX HOT LEMON Powder for Oral Solution Paracetamol (BP) 1000mg/sachet DATA SHEET Presentation Pale yellow, free flowing heterogeneous powder with and odour of lemon Indications Fast,
Core Safety Profile. Pharmaceutical form(s)/strength: Immediate release tablets 1 mg, 2 mg, 4 mg and 8 mg (IR) Date of FAR:
 Core Safety Profile Active substance: Doxazosin Pharmaceutical form(s)/strength: Immediate release tablets 1 mg, 2 mg, 4 mg and 8 mg (IR) P - RMS: DK/H/PSUR/0004/002 Date of FAR: 12.12.2011 4.3 Contraindications
Core Safety Profile Active substance: Doxazosin Pharmaceutical form(s)/strength: Immediate release tablets 1 mg, 2 mg, 4 mg and 8 mg (IR) P - RMS: DK/H/PSUR/0004/002 Date of FAR: 12.12.2011 4.3 Contraindications
PRODUCT INFORMATION PANADEINE FORTE
 PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
PRODUCT INFORMATION PANADEINE FORTE NAME OF THE MEDICINE Non-proprietary Name Paracetamol and codeine phosphate DESCRIPTION Each capsule-shaped tablet contains Paracetamol 500 mg, Codeine phosphate 30
0BCore Safety Profile. Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) NL/H/PSUR/0058/001 Date of FAR:
 0BCore Safety Profile Active substance: Omeprazole Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) P-RMS: NL/H/PSUR/0058/001 Date of FAR: 13.06.2013 4.2 Posology and method of administration
0BCore Safety Profile Active substance: Omeprazole Pharmaceutical form(s)/strength: Losec MUPS tablets 10, 20 mg (OTC) P-RMS: NL/H/PSUR/0058/001 Date of FAR: 13.06.2013 4.2 Posology and method of administration
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
PRODUCT INFORMATION. Sudafed* Sinus Day + Night Relief Tablets
 PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
PRODUCT INFORMATION Sudafed* Sinus Day + Night Relief Tablets Product description Sudafed* Sinus Day + Night Relief tablets contain two separate formulations: day tablets and night tablets. Each Sudafed*
SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ibuprofen 400mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Ibuprofen 400mg Also contains lactose, sucrose
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ibuprofen 400mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains Ibuprofen 400mg Also contains lactose, sucrose
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
APPROVED PACKAGE INSERT
 APPROVED PACKAGE INSERT SCHEDULING STATUS:S3 PROPRIETARY NAMES (AND DOSAGE FORMS): PONSTAN (CAPSULES) PONSTAN FORTE (TABLET) PONSTAN (SUSPENSION) PONSTAN (PAEDIATRIC SUPPOSITORIES) COMPOSITION: PONSTAN
APPROVED PACKAGE INSERT SCHEDULING STATUS:S3 PROPRIETARY NAMES (AND DOSAGE FORMS): PONSTAN (CAPSULES) PONSTAN FORTE (TABLET) PONSTAN (SUSPENSION) PONSTAN (PAEDIATRIC SUPPOSITORIES) COMPOSITION: PONSTAN
Each 5ml of Sinarest LP New Syrup contains: Phenylephrine
 Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
Composition: Each 5ml of Sinarest LP New Syrup contains: Paracetamol Phenylephrine Levocetrizine 250mg 5mg 1.25mg Pharmacokinetic properties: Paracetamol is readily absorbed from the gastrointestinal tract
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen Tablets BP 600 mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 600 mg of Ibuprofen. For the full list of
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Ibuprofen Tablets BP 600 mg 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 600 mg of Ibuprofen. For the full list of
Core Safety Profile. Pharmaceutical form(s)/strength: Capsules, 0.5 mg AT/H/PSUR/0028/001 Date of FAR:
 Core Safety Profile Active substance: Anagrelide hydrochloride Pharmaceutical form(s)/strength: Capsules, 0.5 mg P-RMS: AT/H/PSUR/0028/001 Date of FAR: 02.08.2010 4.3 Contraindications Hypersensitivity
Core Safety Profile Active substance: Anagrelide hydrochloride Pharmaceutical form(s)/strength: Capsules, 0.5 mg P-RMS: AT/H/PSUR/0028/001 Date of FAR: 02.08.2010 4.3 Contraindications Hypersensitivity
NOTOPAIN CAPLETS. Diclofenac Sodium + Paracetamol. Composition. Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg.
 NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
NOTOPAIN CAPLETS Diclofenac Sodium + Paracetamol Composition Each tablet contains: Diclofenac Sodium BP 50mg Paracetamol BP 500mg Pharmacology Phamacodynamics Diclofenac relieves pain and inflammation
Package leaflet: Information for the user CODEINE PHOSPHATE TABLETS BP 15mg and 30mg (Codeine Phosphate)
 Package leaflet: Information for the user CODEINE PHOSPHATE TABLETS BP 15mg and 30mg (Codeine Phosphate) Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the user CODEINE PHOSPHATE TABLETS BP 15mg and 30mg (Codeine Phosphate) Read all of this leaflet carefully before you start taking this medicine because it contains important
Iroko Pharmaceuticals Gains Additional Patents for ZORVOLEX and TIVORBEX TM
 Iroko Pharmaceuticals Gains Additional Patents for ZORVOLEX and TIVORBEX TM PHILADELPHIA, April 8, 2015 Iroko Pharmaceuticals, LLC, a global specialty pharmaceutical company dedicated to advancing the
Iroko Pharmaceuticals Gains Additional Patents for ZORVOLEX and TIVORBEX TM PHILADELPHIA, April 8, 2015 Iroko Pharmaceuticals, LLC, a global specialty pharmaceutical company dedicated to advancing the
Active ingredients: Paracetamol Codeine phosphate. Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
 Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
Codapane Paracetamol / Codeine phosphate PRODUCT INFORMATION NAME OF THE MEDICINE Active ingredients: Paracetamol Codeine phosphate Chemical names: N-(4-Hydroxyphenyl)acetamide 4,5a-epoxy-3-methoxy-17-methyl-7,8-
Immodium / loprarmide
 Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
Immodium / loprarmide IMODIUM (loperamide hydrochloride) is indicated for the control and symptomatic relief of acute nonspecific diarrhea and of chronic diarrhea associated with inflammatory bowel disease.
PRODUCT INFORMATION. Benadryl* for the Family Nightime Oral Liquid
 Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
Product description PRODUCT INFORMATION Benadryl* for the Family Nightime Oral Liquid Each 5 ml of Benadryl* Nightime oral liquid contains dextromethorphan hydrobromide 10 mg and diphenhydramine hydrochloride
RE: Shortage of DBL MORPHINE SULFATE 30mg/1mL injection BP ampoule and alternative supply arrangement under Section 19A of the Therapeutic Goods Act
 22 January 2019 RE: Shortage of DBL MORPHINE SULFATE 30mg/1mL injection BP ampoule and alternative supply arrangement under Section 19A of the Therapeutic Goods Act Dear Healthcare Professional, This notification
22 January 2019 RE: Shortage of DBL MORPHINE SULFATE 30mg/1mL injection BP ampoule and alternative supply arrangement under Section 19A of the Therapeutic Goods Act Dear Healthcare Professional, This notification
PANADOL EXTRA PRODUCT INFORMATION
 PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
PANADOL EXTRA PRODUCT INFORMATION DESCRIPTION Active Ingredients: Paracetamol 500 mg and Caffeine 65mg per tablet. Excipients: Maize starch Starch - Pregelatinised maize Povidone Potassium sorbate Purified
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
NEW ZEALAND DATA SHEET VERGO 16 1. Product Name Vergo 16, 16 mg, tablet. 2. Qualitative and Quantitative Composition Each tablet contains 16 mg of betahistine dihydrochloride. For the full list of excipients,
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
 AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
AUSTRALIAN PRODUCT INFORMATION APO-BETAHISTINE (BETAHISTINE DIHYDROCHLORIDE) 1 NAME OF THE MEDICINE Betahistine dihydrochloride. 2 AND 3 QUALITATIVE AND QUANTITATIVE COMPOSITION AND PHARMACEUTICAL FORM
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dihydrocodeine 30mg Tablets BP. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains dihydrocodeine tartrate 30 mg Also contains
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dihydrocodeine 30mg Tablets BP. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains dihydrocodeine tartrate 30 mg Also contains
European PSUR Work Sharing Project CORE SAFETY PROFILE. Lendormin, 0.25mg, tablets Brotizolam
 European PSUR Work Sharing Project CORE SAFETY PROFILE Lendormin, 0.25mg, tablets Brotizolam 4.2 Posology and method of administration Unless otherwise prescribed by the physician, the following dosages
European PSUR Work Sharing Project CORE SAFETY PROFILE Lendormin, 0.25mg, tablets Brotizolam 4.2 Posology and method of administration Unless otherwise prescribed by the physician, the following dosages
APO-PARACETAMOL/CODEINE 500/30
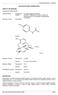 APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
APO-PARACETAMOL/CODEINE 500/30 NAME OF THE MEDICINE Paracetamol/Codeine 500/30. Chemical Name: Paracetamol: N-(4-Hydroxyphenyl) acetamide Codeine: (5R, 6S)-7, 8-didehydro-4,5-epoxy-3-methoxy-Nmethylmorphinan-6-ol
DATA SHEET. Paracetamol (BP) 500 mg and Phenylephrine Hydrochloride (BP) 5 mg
 COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
COLDREX PE PHENYLEPHRINE SINUS COLDREX PE PHENYLEPHRINE CONGESTION CLEAR PANADOL SINUS PAIN & CONGESTION RELIEF PANADOL COLD & FLU MAX + DECONGESTANT DATA SHEET Paracetamol (BP) 500 mg and Phenylephrine
DATA SHEET. PANADOL Mini Caps Capsule shaped tablet with a gelatin coating which is one half green and the other half white.
 PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
PANADOL TABLETS PANADOL MINI CAPS DATA SHEET Proprietary (Trade) Name: PANADOL Active ingredient: Paracetamol (BP) 500 mg/tablet PRESENTATIONS White, film-coated tablet with bevelled edge, shallow convex,
SANDOMIGRAN (pizotifen malate)
 SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
SANDOMIGRAN (pizotifen malate) S N CH 3 Pizotifen. COOH CH OH CH 2 COOH MALATE DESCRIPTION Pizotifen is a cycloheptathiophene derivative structurally related to cyproheptadine and the tricyclic antidepressants.
PRODUCT INFORMATION. Active ingredients Chemical structure CAS Registry Number. Paracetamol
 PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
PRODUCT INFORMATION PANADOL SINUS PAIN & CONGESTION RELIEF CAPLETS PANADOL COLD & FLU PLUS DECONGESTANT CAPLETS NAME OF THE MEDICINE Active ingredients Chemical structure CAS Registry Number Paracetamol
