PATIENT GUIDE TO LUMIGAN 0.01%
|
|
|
- Anne Patrick
- 6 years ago
- Views:
Transcription
1 Treatment Tracker enclosed PATIENT GUIDE TO LUMIGAN 0.01% LUMIGAN (bimatoprost ophthalmic solution) 0.01% is used for the treatment of high eye pressure, also called intraocular pressure (IOP), in people with open-angle glaucoma or ocular hypertension. Important Safety Information LUMIGAN (bimatoprost ophthalmic solution) 0.01% has been reported to cause darkening (pigmentation) of eye color, eyelid skin, and eyelashes as well as increased growth of eyelashes. Please see additional Important Safety Information inside.
2 Introduction This guide will help you learn more about glaucoma and how LUMIGAN (bimatoprost ophthalmic solution) 0.01% may help to reduce high eye pressure, also known as intraocular pressure (IOP), for people who have open-angle glaucoma or ocular hypertension. It is designed to provide you with the education and support you need to better understand your condition and why it is important to use your LUMIGAN 0.01% ophthalmic solution each and every day. As a manufacturer of ophthalmic medications for more than 60 years, Allerganiscommittedtodevelopingeffectivetherapiesforglaucoma patients. Our mission is to help educate patients about the disease and the importance of treatment. 1. The Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4): Mayo Clinic Staff. Glaucoma: risk factors. Mayo Clinic website. Published July 17, Updated October 2, Accessed September 26, WebMD. Glaucoma and your eyes. WebMD website. Reviewed September 9, Accessed September 26, PubMed Health. Glaucoma. US National Library of Medicine website. Reviewed September 14, Accessed September 26, WebMD. Prostaglandin analogs for glaucoma. WebMD website. Updated May 5, Accessed September 26, Katz LJ, Cohen JS, Batoosingh AL, Felix C, Shu V, Schiffman RM. Twelve-month, randomized, controlled trial of bimatoprost 0.01%, %, and 0.03% in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2010;149(4): U.S. Food and Drug Administration. Drugs@FDA. Drug details: LUMIGAN 0.01%. FDA website: Accessed September 23, LUMIGAN Prescribing Information. 9. IMS Health, Inc. Vector One : National (VONA). September
3 Table of Contents About Glaucoma... 4 Treatment With LUMIGAN (bimatoprost ophthalmic solution) 0.01%... 8 How to Use LUMIGAN 0.01%...14 Going to Your Eye Doctor...16 Going to the Pharmacy...18 Caregiver Tips...20 Notes...22 Treatment Tracker...23 Visit for more information, savings offerings, and additional resources. Important Safety Information (continued) Pigmentation changes can increase as long as LUMIGAN 0.01% is used. After stopping LUMIGAN 0.01%, darkening of eye color is likely to be permanent, while darkening of the eyelid skin and eyelash changes may be reversible. The effects of increased darkening beyond 5 years are not known. Please see additional Important Safety Information on the following pages. 3
4 About Glaucoma What is glaucoma? Glaucoma is a group of eye diseases in which the optic nerves become damaged over time. The optic nerves are like 2 cables that send images from your eyes to your brain. When one or both of these nerves is damaged, vision may be impaired or lost. Are there different types of glaucoma? Yes. This booklet is for patients who have open-angle glaucoma or ocular hypertension, which may be treated with LUMIGAN (bimatoprost ophthalmic solution) 0.01%. Discuss with your doctor which type of glaucoma you have. Open-angle glaucoma is a type of glaucoma where the fluid (aqueous humor) in the eye cannot drain out properly, which may be due to dysfunction or obstruction of a special membrane (trabecular meshwork) Ocular hypertension is used to describe high eye pressure without optic nerve damage Important Safety Information (continued) When only one eye is treated, there is a possibility of eyelash changes in the eye treated with LUMIGAN 0.01%. 4
5 Who gets glaucoma? About 2.2 million Americans have open-angle glaucoma1 You may be at a higher risk of developing this type of glaucoma if you 2,3 : Are over 40 years of age Are of a certain ethnic background (ie, African American) Have diabetes Have a family history of or a relative with glaucoma Does glaucoma have any early warning signs? Unfortunately, open-angle glaucoma does not have any early warning signs. You cannot feel high eye pressure By the time you notice a change in your vision, the damage may have already occurred Regular appointments with your eye doctor are the only way to detect glaucoma early. Important Safety Information (continued) These changes may result in differences between the eyes in eyelash length, thickness, darkness, number of eyelashes, and/or direction of eyelash growth. These changes are usually reversible upon stopping LUMIGAN 0.01% therapy. Please see additional Important Safety Information on the following pages. 5
6 About Glaucoma Why is eye pressure important? A number of causes may contribute to glaucoma, but high pressure inside the eye, also known as high intraocular pressure (IOP), plays a very important role. Your eyes contain fluid (aqueous humor) that feeds them and keeps them healthy Normally, this fluid flows within the eye and drains freely In people who have glaucoma, the fluid does not drain properly The buildup of fluid causes pressure inside the eye to rise If IOP remains high too long, it can damage the optic nerve and lead to loss of vision Is there a cure for glaucoma? There is no cure for glaucoma; it is a chronic disease. However, treatment options are available for glaucoma patients. Treatment options include medicated eyedrops, implants, and surgery. Important Safety Information (continued) Avoid allowing the tip of the dispensing bottle to touch the eye, anything around the eye, fingers, or any other surface in order to avoid contamination by common bacteria known to cause eye infections. Serious damage to the eye and loss of vision may result from using contaminated solutions. Please see additional Important Safety Information on the following pages. 6
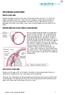
7 An eye without high pressure Retina Iris Lens Cornea Conjunctiva Optic nerve Sclera An eye with high eye pressure 4 Pressure inside eye Optic nerve 7
8 Treatment With LUMIGAN 0.01% What is LUMIGAN (bimatoprost ophthalmic solution) 0.01%? LUMIGAN 0.01% ophthalmic solution is an FDA-approved medicated eyedrop that belongs to a group of medications known as prostaglandin analogs, which are the most frequently used medicines for lowering high eye pressure. 5 Why did my doctor choose LUMIGAN 0.01%? Your doctor prescribed LUMIGAN 0.01%, which is used for the treatment of high eye pressure in patients with open-angle glaucoma or ocular hypertension. The effectiveness of LUMIGAN 0.01% has been proven in a large clinical study. 6 There is no FDA-approved generic version of LUMIGAN 0.01%, 7 and 0.01% is the only concentration of LUMIGAN available. 8
9 Make sure you receive LUMIGAN (bimatoprost ophthalmic solution) 0.01% strength at the pharmacy When you pick up your prescription at the pharmacy, be sure you receive the correct bottle LUMIGAN 0.01%. 7.5-mL, 5-mL, and 2.5-mL bottle sizes are available Ask your doctor to prescribe the largest covered bottle to help you save on co-pays Important Safety Information (continued) If you have eye surgery or develop any eye reactions (such as trauma or infection), immediately consult with your physician about continuing the use of LUMIGAN 0.01%. Please see additional Important Safety Information on the following pages. 9
10 Treatment With LUMIGAN 0.01% What should I expect with LUMIGAN (bimatoprost ophthalmic solution) 0.01%? A clinical study has shown that LUMIGAN 0.01% is effective for reducing high eye pressure in patients with open-angle glaucoma and ocular hypertension. The most commonly reported side effects were: Eye redness Eyelash growth Itchy eyes In clinical studies, about 0.5% to 3.0% of patients (about 1 to 3 out of 100) stopped therapy due to eye redness. After stopping LUMIGAN 0.01%, darkening of eye color is likely to be permanent, while darkening of the eyelid skin and eyelash changes may be reversible. The effects of increased darkening beyond 5 years are not known. These are not the only risks associated with LUMIGAN 0.01%. If you experience these or other side effects, contact your eye care professional. To avoid contamination of LUMIGAN 0.01% ophthalmic solution, do not allow anything to touch the tip of the bottle (including the surface of your eye, the area around the eye, your fingers, or anything else). Using contaminated solutions can cause serious damage to your eyes and result in vision loss. 10
11 Who should NOT use LUMIGAN (bimatoprost ophthalmic solution) 0.01%? LUMIGAN 0.01% is not recommended for children below 16 years of age because of safety concerns related to darkening of the iris and tissues around the eye. Immediately contact your eye care professional if you injure an eye, experience symptoms of an eye infection, or have an allergic reaction such as persistent itching or pain. Contact lenses can absorb the preservative in LUMIGAN 0.01% ophthalmic solution. Remove contact lenses before using LUMIGAN 0.01% and wait 15 minutes before reinserting the lenses. If you use more than one type of eyedrop, wait at least 5 minutes between using them. 11
12 Treatment With LUMIGAN 0.01% Remember to use LUMIGAN (bimatoprost ophthalmic solution) 0.01% once a day, every day Even if you feel fine, it s important to continue using your LUMIGAN 0.01% eyedrops every day as your doctor prescribed. Do not skip or stop treatment before speaking with your doctor. Make your medication part of your daily routine. Remember to: Associate using your eyedrops with other daily routines you ve established for yourself, such as brushing your teeth Set a daily clock or watch alarm that can remind you to use your eyedrops Ask a friend or family member to remind you when it s time to use your eyedrops 12
13 Where can I find more support? Friends and family Let them know you are using this medication Ask them for their support in helping you remember to use your LUMIGAN (bimatoprost ophthalmic solution) 0.01% eyedrops every day Your doctor, eye care technician/staff, and pharmacist Your eye care team can answer any questions you may have about your diagnosis and therapy with LUMIGAN 0.01% Visit Get more information about glaucoma and treatment with LUMIGAN 0.01% Print a $25 savings coupon Refill reminders Download the free MyDrops app for iphone from the App Store to help you keep up with treatment LUMIGAN 0.01% At Your Service patient support line Call LUMIGAN ( ) to have your questions answered by a member of our support team Important Safety Information (continued) If you wear contact lenses, remove them first, then wait 15 minutes after using LUMIGAN 0.01% ophthalmic solution before you put them back into your eyes. Please see additional Important Safety Information on the following pages. 13
14 How to Use LUMIGAN 0.01% Follow these 5 steps 1 Wash your hands. Tilt your head back and look at the ceiling.* 2 3 Using your index finger, gently pull down your lower eyelid to form a pocket. Gently squeeze 1 drop into the pocket. Do not let the bottle tip touch your eye, your fingers, or anything else. * If you wear contact lenses, remove them first, then wait 15 minutes after using LUMIGAN (bimatoprost ophthalmic solution) 0.01% ophthalmic solution before you put them back into your eyes. 8 14
15 4 5 Gently close your eyes and lightly press on the inside corners of your eyes. Then carefully blot away any excess liquid that may be on your skin. How to handle your LUMIGAN (bimatoprost ophthalmic solution) 0.01% bottle It is very important to handle your LUMIGAN 0.01% bottle carefully. Always wash your hands before putting the drops in your eyes. Don t allow anything to touch the tip not even your eyes or the areas around your eyes! Potentially harmful bacteria may enter the bottle and cause serious eye infections. If you think your LUMIGAN 0.01% ophthalmic solution bottle has become contaminated, contact your eye care professional. Important Safety Information (continued) The most common side effects are eye redness, growth of eyelashes, and itchy eyes. Please see additional Important Safety Information on the following pages. 15
16 Going to Your Eye Doctor Be an active member of your own glaucoma team! High eye pressure is a lifelong medical condition. Work with your eye doctor and his or her staff to get the treatment information you need. Tips for when you visit the eye doctor Don t be afraid to ask questions for support Bring a friend or family member Ask the office staff for referrals, written instructions, and printed materials Important Safety Information LUMIGAN (bimatoprost ophthalmic solution) 0.01% has been reported to cause darkening (pigmentation) of eye color, eyelid skin, and eyelashes as well as increased growth of eyelashes. Pigmentation changes can increase as long as LUMIGAN 0.01% is used. 16
17 Questions to ask your eye doctor How do you monitor whether damage to my optic nerve is getting worse over time? Should I watch for any particular symptoms and notify you if they occur? Should I make any lifestyle changes? What are the best long-term treatment options for my condition? What should I do if I miss a dose? What is my intraocular pressure, IOP? Is that good or bad? Could prescribing a larger bottle size for fewer refills help me to not miss drops and possibly reduce my average monthly co-pay? Important Safety Information (continued) After stopping LUMIGAN (bimatoprost ophthalmic solution) 0.01%, darkening of eye color is likely to be permanent, while darkening of the eyelid skin and eyelash changes may be reversible. The effects of increased darkening beyond 5 years are not known. Please see additional Important Safety Information on the following pages. 17
18 Going to the Pharmacy Always make sure you receive LUMIGAN (bimatoprost ophthalmic solution) 0.01% from the pharmacist LUMIGAN 0.01% is the medication prescribed by your doctor. There is no FDA-approved generic version of LUMIGAN 0.01%. 7 Get the answers you need to make an informed decision If the pharmacist talks to you about changing your prescription, there are some important questions you should ask. Important Safety Information (continued) When only one eye is treated, there is a possibility of eyelash changes in the eye treated with LUMIGAN 0.01%. These changes may result in differences between the eyes in eyelash length, thickness, darkness, number of eyelashes, and/or direction of eyelash growth. These changes are usually reversible upon stopping LUMIGAN 0.01% therapy. 18
19 Questions to ask the pharmacist Why are you suggesting an alternative medication? Do you know there s no FDA-approved generic version of LUMIGAN (bimatoprost ophthalmic solution) 0.01%? Have you contacted my doctor about this issue? If the pharmacist quotes a high cash payment, remember to ask: Have you run the prescription through my insurance? Are you quoting the full price or my out-of-pocket (cash) amount? Is there a larger bottle size available to save on my monthly co-pays? If this size isn t covered by my insurance, is another size covered? Important Safety Information (continued) Avoid allowing the tip of the dispensing bottle to touch the eye, anything around the eye, fingers, or any other surface in order to avoid contamination by common bacteria known to cause eye infections. Serious damage to the eye and loss of vision may result from using contaminated solutions. If you have eye surgery or develop any eye reactions (such as trauma or infection), immediately consult with your physician about continuing the use of LUMIGAN 0.01%. Please see additional Important Safety Information on the following pages. 19
20 Caregiver Tips If you care for someone who takes LUMIGAN (bimatoprost ophthalmic solution) 0.01% for open-angle glaucoma or ocular hypertension, these important tips can help him or her get the care intended by the doctor. Ensure the person you care for receives the correct medication at the pharmacy Always check the prescription at the pharmacy to ensure you get LUMIGAN 0.01% There is no FDA-approved generic substitute for LUMIGAN 0.01% 7 Important Safety Information (continued) If you wear contact lenses, remove them before using LUMIGAN 0.01%. Then wait 15 minutes after using LUMIGAN 0.01% before you put your contacts back into your eyes. 20
21 Help the person you care for remember to take his or her eyedrops every day Write him or her a note and leave it in a conspicuous place (such as on the bathroom mirror) If you provide meals, always provide the eyedrops after a certain meal, such as breakfast and dosing times Maintain a written schedule of medications, dosing amounts, Ensure he or she always has enough eyedrops on hand remind or help him or her to pick up a new prescription when necessary Refer to the list on page 12 for additional suggestions Ask at the doctor s office for any additional resources to help your loved one stay on LUMIGAN (bimatoprost ophthalmic solution) 0.01% therapy Important Safety Information (continued) The most common side effects are eye redness, growth of eyelashes, and itchy eyes. Please see additional Important Safety Information on the following pages. 21
22 Notes 22
23 Find your Treatment Tracker in this pocket 23
24 Your Treatment for High Eye Pressure Four important facts about LUMIGAN (bimatoprost ophthalmic solution) 0.01% 1. Proof: The effectiveness and tolerability of LUMIGAN 0.01% were proven in a large clinical trial 6 2. Experience: LUMIGAN 0.01% is the #1 dispensed branded glaucoma medication 9 3. Uniqueness: There is no FDA-approved generic version of the LUMIGAN 0.01% formulation 7 4. Savings: Larger bottles may help reduce your average monthly co-pay Available in 3 bottle sizes: 7.5 ml, 5 ml, and 2.5 ml Please see full Prescribing Information inside pocket on page 23. Visit for more information, savings offers, and additional resources Allergan, Inc., Irvine, CA marks owned by Allergan, Inc. iphone is a trademark and App Store is a service mark of Apple Inc., registered in the US and other countries. Vector One is a registered service mark owned by IMS Health, Inc. WebMD is a registered service mark of WebMD LLC. Re-order: APCXXXX
 TREATMENT TRACKER Indication LUMIGAN (bimatoprost ophthalmic solution) 0.01% is used for the reduction of high eye pressure, also called intraocular pressure (IOP), in people with open-angle glaucoma or
TREATMENT TRACKER Indication LUMIGAN (bimatoprost ophthalmic solution) 0.01% is used for the reduction of high eye pressure, also called intraocular pressure (IOP), in people with open-angle glaucoma or
YOUR VYZULTA TREATMENT GUIDE. Please see Important Safety Information on pages 1, 9, 10, 17 and 18. Please see accompanying Prescribing Information.
 YOUR VYZULTA TREATMENT GUIDE Please see Important Safety Information on pages 1, 9, 10, 17 and 18. Please see accompanying Prescribing Information. INDICATION VYZULTA TM (latanoprostene bunod ophthalmic
YOUR VYZULTA TREATMENT GUIDE Please see Important Safety Information on pages 1, 9, 10, 17 and 18. Please see accompanying Prescribing Information. INDICATION VYZULTA TM (latanoprostene bunod ophthalmic
PATIENT INFORMATION LEAFLET. PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM LUMIGAN 0,01 %, bimatoprost 0,1 mg/ml eye drops
 Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM LUMIGAN 0,01 %, bimatoprost 0,1 mg/ml eye drops Read all of this leaflet carefully
Page 1 of 6 SCHEDULING STATUS Schedule 4 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM LUMIGAN 0,01 %, bimatoprost 0,1 mg/ml eye drops Read all of this leaflet carefully
LUMIGAN EYE DROPS (bimatoprost 0.3 mg per ml)
 LUMIGAN EYE DROPS (bimatoprost 0.3 mg per ml) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about LUMIGAN eye drops. It does not contain all the available
LUMIGAN EYE DROPS (bimatoprost 0.3 mg per ml) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about LUMIGAN eye drops. It does not contain all the available
PATIENT INFORMATION LEAFLET
 Page 1 of 6 PATIENT INFORMATION LEAFLET SCHEDULING STATUS Schedule 4 Proprietary name, Strength and Pharmaceutical Form LUMIGAN 0,03 % Eye Drops (each ml contains bimatoprost 0,3 mg) Read all of this leaflet
Page 1 of 6 PATIENT INFORMATION LEAFLET SCHEDULING STATUS Schedule 4 Proprietary name, Strength and Pharmaceutical Form LUMIGAN 0,03 % Eye Drops (each ml contains bimatoprost 0,3 mg) Read all of this leaflet
Package Leaflet: Information for the user. LUMIGAN 0.3 mg/ml, eye drops, solution, in single-dose container Bimatoprost
 Package Leaflet: Information for the user LUMIGAN 0.3 mg/ml, eye drops, solution, in single-dose container Bimatoprost Read all of this leaflet carefully before you start using this medicine because it
Package Leaflet: Information for the user LUMIGAN 0.3 mg/ml, eye drops, solution, in single-dose container Bimatoprost Read all of this leaflet carefully before you start using this medicine because it
Glaucoma. What is glaucoma? Eye Words to Know. What causes glaucoma?
 2014 2015 Glaucoma What is glaucoma? Glaucoma is a disease that damages your eye s optic nerve. It usually happens when fluid builds up in the front part of your eye. That extra fluid increases the pressure
2014 2015 Glaucoma What is glaucoma? Glaucoma is a disease that damages your eye s optic nerve. It usually happens when fluid builds up in the front part of your eye. That extra fluid increases the pressure
WORLD GLAUCOMA WEEK What is Glaucoma? Can I develop glaucoma if I have increased eye pressure?
 WORLD GLAUCOMA WEEK What is Glaucoma? Glaucoma is a group of diseases that damage the optic nerve and can result in gradual vision loss and blindness. However, with early detection and treatment, you can
WORLD GLAUCOMA WEEK What is Glaucoma? Glaucoma is a group of diseases that damage the optic nerve and can result in gradual vision loss and blindness. However, with early detection and treatment, you can
Glaucoma What You Should Know
 Glaucoma What You Should Know U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Eye Institute The National Eye Institute (NEI) conducts and supports research that leads
Glaucoma What You Should Know U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES National Institutes of Health National Eye Institute The National Eye Institute (NEI) conducts and supports research that leads
N W KNOW. What You Need to Know. What You Know About Glaucoma Could Save Your Sight
 What You Need to Know is the second leading cause of blindness around the world, so it is important to understand its significance and treatment options. While glaucoma is not curable, there are several
What You Need to Know is the second leading cause of blindness around the world, so it is important to understand its significance and treatment options. While glaucoma is not curable, there are several
PRED FORTE 1% w/v Eye Drops, Suspension Prednisolone acetate
 Package leaflet: Information for the user PRED FORTE 1% w/v Eye Drops, Suspension Prednisolone acetate Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user PRED FORTE 1% w/v Eye Drops, Suspension Prednisolone acetate Read all of this leaflet carefully before you start using this medicine because it contains important
MAINTAINING COMPLIANCE IN GLAUCOMA PATIENTS. by : Abdalla El-Sawy, M.D. Professor of Ophthalmology, Benha Faculty of Medicine.
 MAINTAINING COMPLIANCE IN GLAUCOMA PATIENTS by : Abdalla El-Sawy, M.D. Professor of Ophthalmology, Benha Faculty of Medicine. The problem is especially critical for eye doctors who manage patients with
MAINTAINING COMPLIANCE IN GLAUCOMA PATIENTS by : Abdalla El-Sawy, M.D. Professor of Ophthalmology, Benha Faculty of Medicine. The problem is especially critical for eye doctors who manage patients with
BrinzoQuin Eye Drops 1.0%
 BrinzoQuin Eye Drops 1.0% Brinzolamide Consumer Medicine Information What Is In This Leaflet Read this leaflet carefully before you start to use BrinzoQuin Eye Drops. This leaflet has been written to answer
BrinzoQuin Eye Drops 1.0% Brinzolamide Consumer Medicine Information What Is In This Leaflet Read this leaflet carefully before you start to use BrinzoQuin Eye Drops. This leaflet has been written to answer
PACKAGE LEAFLET: INFORMATION FOR THE USER ACULAR 0.5% w/v EYE DROPS, SOLUTION (Ketorolac trometamol)
 PACKAGE LEAFLET: INFORMATION FOR THE USER ACULAR 0.5% w/v EYE DROPS, SOLUTION (Ketorolac trometamol) Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You may
PACKAGE LEAFLET: INFORMATION FOR THE USER ACULAR 0.5% w/v EYE DROPS, SOLUTION (Ketorolac trometamol) Read all of this leaflet carefully before you start using this medicine. - Keep this leaflet. You may
Package leaflet: Information for the user. PRED MILD STERILE OPHTHALMIC SUSPENSION 0.12% w/v Eye Drops, Suspension Prednisolone Acetate
 Package leaflet: Information for the user PRED MILD STERILE OPHTHALMIC SUSPENSION 0.12% w/v Eye Drops, Suspension Prednisolone Acetate Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user PRED MILD STERILE OPHTHALMIC SUSPENSION 0.12% w/v Eye Drops, Suspension Prednisolone Acetate Read all of this leaflet carefully before you start using this medicine
APO-Latanoprost Contains the active ingredient latanoprost
 APO-Latanoprost Contains the active ingredient latanoprost Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read this leaflet carefully before
APO-Latanoprost Contains the active ingredient latanoprost Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read this leaflet carefully before
PATIENT INFORMATION LEAFLET
 Page 1 of 6 PATIENT INFORMATION LEAFLET SCHEDULING STATUS Schedule 4 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM PRED FORTE Sterile Eye Suspension, prednisolone acetate 10 mg/ml sterile eye suspension
Page 1 of 6 PATIENT INFORMATION LEAFLET SCHEDULING STATUS Schedule 4 PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM PRED FORTE Sterile Eye Suspension, prednisolone acetate 10 mg/ml sterile eye suspension
ZADITEN Eye Drops 1.0%
 ZADITEN Eye Drops 1.0% Ketotifen Consumer Medicine Information What is in this leaflet Please read this leaflet carefully before you use Zaditen Eye Drops. This leaflet answers some common questions about
ZADITEN Eye Drops 1.0% Ketotifen Consumer Medicine Information What is in this leaflet Please read this leaflet carefully before you use Zaditen Eye Drops. This leaflet answers some common questions about
LET S TALK about Sticking with your treatment plan
 LET S TALK about Sticking with your treatment plan HOW ONGOING HIV CARE HELPS YOU LIVE A LONGER AND HEALTHIER LIFE Your treatment plan is vital to your overall health (and to reducing HIV transmission)
LET S TALK about Sticking with your treatment plan HOW ONGOING HIV CARE HELPS YOU LIVE A LONGER AND HEALTHIER LIFE Your treatment plan is vital to your overall health (and to reducing HIV transmission)
Pharmacy Advisor Program. Specialized Health Support
 Pharmacy Advisor Program Specialized Health Support Contents Your Health and Your CVS Caremark Pharmacy Advisor Pharmacist...3 Keys to Your Health....4 Getting the Most from Your Medication...6 Feeling
Pharmacy Advisor Program Specialized Health Support Contents Your Health and Your CVS Caremark Pharmacy Advisor Pharmacist...3 Keys to Your Health....4 Getting the Most from Your Medication...6 Feeling
Patient & Family Guide. Glaucoma Management.
 Patient & Family Guide Glaucoma Management 2018 www.nshealth.ca Glaucoma Management What is glaucoma? Your eyes are filled with a fluid made by the ciliary body (1). This fluid flows through the pupil
Patient & Family Guide Glaucoma Management 2018 www.nshealth.ca Glaucoma Management What is glaucoma? Your eyes are filled with a fluid made by the ciliary body (1). This fluid flows through the pupil
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION OPTIMYXIN Gramicidin and Polymyxin B Sulfate Oto-Ophthalmic Solution sterile Eye/Ear Drops Antibiotic Sandoz Canada Inc. Date of Revision: November 25, 2015 145 Jules-Léger Boucherville,
PRESCRIBING INFORMATION OPTIMYXIN Gramicidin and Polymyxin B Sulfate Oto-Ophthalmic Solution sterile Eye/Ear Drops Antibiotic Sandoz Canada Inc. Date of Revision: November 25, 2015 145 Jules-Léger Boucherville,
Get a grip on your glaucoma Booklet 1
 Information for Patients Glaucoma services Welcome! Get a grip on your glaucoma Booklet 1 Group-based Glaucoma information Course Session 1 What happens in session 1? Finding out what questions you have
Information for Patients Glaucoma services Welcome! Get a grip on your glaucoma Booklet 1 Group-based Glaucoma information Course Session 1 What happens in session 1? Finding out what questions you have
PATIENT INFORMATION LEAFLET
 Page 1 PATIENT INFORMATION LEAFLET Please read this leaflet carefully before using COMBIGAN. Keep this leaflet. You may need to read it again. If you have any further questions, please ask your doctor
Page 1 PATIENT INFORMATION LEAFLET Please read this leaflet carefully before using COMBIGAN. Keep this leaflet. You may need to read it again. If you have any further questions, please ask your doctor
Package leaflet: Information for the patient. IKERVIS 1 mg/ml, eye drops, emulsion ciclosporin
 Package leaflet: Information for the patient IKERVIS 1 mg/ml, eye drops, emulsion ciclosporin Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the patient IKERVIS 1 mg/ml, eye drops, emulsion ciclosporin Read all of this leaflet carefully before you start using this medicine because it contains important information
ACULAR EYE DROPS 5 mg/ml Eye Drops
 ACULAR EYE DROPS 5 mg/ml Eye Drops (ketorolac trometamol) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about ACULAR eye drops, including how to use the
ACULAR EYE DROPS 5 mg/ml Eye Drops (ketorolac trometamol) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about ACULAR eye drops, including how to use the
Ocular Hypertension. A Guide
 Ocular Hypertension A Guide Printed: July 2015 Review: July 2018 This free booklet is brought to you by the International Glaucoma Association (IGA), the charity for people with glaucoma. We haven t charged
Ocular Hypertension A Guide Printed: July 2015 Review: July 2018 This free booklet is brought to you by the International Glaucoma Association (IGA), the charity for people with glaucoma. We haven t charged
TOBREX TM EYE DROPS and EYE OINTMENT 0.3%
 TOBREX TM EYE DROPS and EYE OINTMENT 0.3% Tobramycin Consumer Medicine Information What is in this leaflet Read this leaflet carefully before you start to use Tobrex Eye Drops and Eye Ointment. This leaflet
TOBREX TM EYE DROPS and EYE OINTMENT 0.3% Tobramycin Consumer Medicine Information What is in this leaflet Read this leaflet carefully before you start to use Tobrex Eye Drops and Eye Ointment. This leaflet
Package leaflet: Information for the patient. Brimonidine Biogaran 2 mg/ml eye drops, solution. brimonidine tartrate
 Package leaflet: Information for the patient Brimonidine Biogaran 2 mg/ml eye drops, solution brimonidine tartrate Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient Brimonidine Biogaran 2 mg/ml eye drops, solution brimonidine tartrate Read all of this leaflet carefully before you start using this medicine because it contains
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. IOPIDINE 1% Apraclonidine Ophthalmic Solution, USP
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr IOPIDINE 1% Apraclonidine Ophthalmic Solution, USP Read this carefully before you start taking IOPIDINE 1% and each
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr IOPIDINE 1% Apraclonidine Ophthalmic Solution, USP Read this carefully before you start taking IOPIDINE 1% and each
glaucoma a closer look
 0AMERICAN ACADEMY OF OPHTHALMOLOGY The Eye M.D. Association 2012-2013 glaucoma a closer look WHAT IS GLAUCOMA? < Glaucoma is a disease of the optic nerve the part of the eye that carries the images we
0AMERICAN ACADEMY OF OPHTHALMOLOGY The Eye M.D. Association 2012-2013 glaucoma a closer look WHAT IS GLAUCOMA? < Glaucoma is a disease of the optic nerve the part of the eye that carries the images we
NEVANAC TM 0.1% Eye Drops
 NEVANAC TM 0.1% Eye Drops Nepafenac CONSUMER MEDICINE INFORMATION What is in this leaflet Please read this leaflet carefully before you use Nevanac Eye This leaflet answers some common questions about
NEVANAC TM 0.1% Eye Drops Nepafenac CONSUMER MEDICINE INFORMATION What is in this leaflet Please read this leaflet carefully before you use Nevanac Eye This leaflet answers some common questions about
Pilocarpine 2% w/v Eye Drops, solution. Pilocarpine 4% w/v Eye Drops, solution. Pilocarpine Hydrochloride
 PACKAGE LEAFLET: INFORMATION FOR THE USER Pilocarpine 2% w/v Eye Drops, solution Pilocarpine 4% w/v Eye Drops, solution Pilocarpine Hydrochloride (Referred to as Pilocarpine Eye Drops in this leaflet)
PACKAGE LEAFLET: INFORMATION FOR THE USER Pilocarpine 2% w/v Eye Drops, solution Pilocarpine 4% w/v Eye Drops, solution Pilocarpine Hydrochloride (Referred to as Pilocarpine Eye Drops in this leaflet)
Dorzolamide 20 mg/ml Eye Drops, Solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER Dorzolamide 20 mg/ml Eye Drops, Solution (Dorzolamide hydrochloride) Read all of this leaflet carefully before you start using this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE USER Dorzolamide 20 mg/ml Eye Drops, Solution (Dorzolamide hydrochloride) Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user. GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution Bimatoprost/timolol
 Package leaflet: Information for the user GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution Bimatoprost/timolol Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution Bimatoprost/timolol Read all of this leaflet carefully before you start using this medicine because it contains
Patient Information COSOPT PF (CO-sopt PEA EHF) (dorzolamide hydrochloride-timolol maleate ophthalmic solution) 2%/0.5%
 Patient Information COSOPT PF (CO-sopt PEA EHF) (dorzolamide hydrochloride-timolol maleate ophthalmic solution) 2%/0.5% Read this information before you start using COSOPT PF and each time you get a refill.
Patient Information COSOPT PF (CO-sopt PEA EHF) (dorzolamide hydrochloride-timolol maleate ophthalmic solution) 2%/0.5% Read this information before you start using COSOPT PF and each time you get a refill.
MAXIDEX TM Eye Ointment 0.1%
 MAXIDEX TM Eye Ointment 0.1% Dexamethasone CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Maxidex Eye Ointment. This leaflet answers some common
MAXIDEX TM Eye Ointment 0.1% Dexamethasone CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Maxidex Eye Ointment. This leaflet answers some common
Package leaflet: Information for the user Oftaquix 5 mg/ml eye drops, solution. Levofloxacin
 Package leaflet: Information for the user Oftaquix 5 mg/ml eye drops, solution Levofloxacin Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the user Oftaquix 5 mg/ml eye drops, solution Levofloxacin Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the user. Xalatan 50 micrograms/ml Eye drops, solution Latanoprost
 Package leaflet: Information for the user Xalatan 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user Xalatan 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains important
Treatments for Open-Angle Glaucoma. A Review of the Research for Adults
 Treatments for Open-Angle Glaucoma A Review of the Research for Adults Is This Information Right for Me? Yes, this information is for you if: Your eye doctor has said that you have open-angle glaucoma,
Treatments for Open-Angle Glaucoma A Review of the Research for Adults Is This Information Right for Me? Yes, this information is for you if: Your eye doctor has said that you have open-angle glaucoma,
What Maxitrol Eye Drops is used for
 MAXITROL Dexamethasone, Neomycin and Polymyxin B CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use This leaflet answers some common questions about
MAXITROL Dexamethasone, Neomycin and Polymyxin B CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use This leaflet answers some common questions about
DUOTRAV Eye Drops travoprost 0.004% and timolol 0.5%
 DUOTRAV Eye Drops travoprost 0.004% and timolol 0.5% Alcon (Australia) Pty Ltd Consumer Medicine Information What is in this Leaflet This leaflet answers some common questions about DUOTRAV Eye Drops.
DUOTRAV Eye Drops travoprost 0.004% and timolol 0.5% Alcon (Australia) Pty Ltd Consumer Medicine Information What is in this Leaflet This leaflet answers some common questions about DUOTRAV Eye Drops.
Type 2 Diabetes. Care for your body today for a healthier tomorrow
 Type 2 Diabetes Care for your body today for a healthier tomorrow Understanding diabetes You may already know that having diabetes means you have too much sugar in your blood. Why do you have high blood
Type 2 Diabetes Care for your body today for a healthier tomorrow Understanding diabetes You may already know that having diabetes means you have too much sugar in your blood. Why do you have high blood
Because the more you know, the better you ll feel.
 ABOUT ASTHMA Because the more you know, the better you ll feel. This booklet is designed to help you understand asthma and the things you can do every day to help control symptoms. As always, talk to your
ABOUT ASTHMA Because the more you know, the better you ll feel. This booklet is designed to help you understand asthma and the things you can do every day to help control symptoms. As always, talk to your
CIPROXIN HC Ear Drops
 CIPROXIN HC Ear Drops Ciprofloxacin and Hydrocortisone CONSUMER MEDICINE INFORMATION What is in this leaflet Please read this leaflet carefully before you use. This leaflet answers some common questions
CIPROXIN HC Ear Drops Ciprofloxacin and Hydrocortisone CONSUMER MEDICINE INFORMATION What is in this leaflet Please read this leaflet carefully before you use. This leaflet answers some common questions
GIVE THEM A DROP THAT LASTS Proven effective through 16 hours1-3
 FOR PATIENTS WITH ITCHING DUE TO ALLERGIC CONJUNCTIVITIS INDICATIONS AND USAGE LASTACAFT (alcaftadine ophthalmic solution) 0.25% is an H1 histamine receptor antagonist indicated for the prevention of itching
FOR PATIENTS WITH ITCHING DUE TO ALLERGIC CONJUNCTIVITIS INDICATIONS AND USAGE LASTACAFT (alcaftadine ophthalmic solution) 0.25% is an H1 histamine receptor antagonist indicated for the prevention of itching
MAXIDEX Eye Drops 0.1%
 MAXIDEX Eye Drops 0.1% Dexamethasone CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Maxidex Eye Drops. This leaflet answers some common questions
MAXIDEX Eye Drops 0.1% Dexamethasone CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Maxidex Eye Drops. This leaflet answers some common questions
health care quality Your Medicine: Play It Safe Medicine Record Form at the Learn more about how to take medicines safely. Use the
 Your Medicine: Play It Safe Learn more about how to take medicines safely. Use the Medicine Record Form at the back of this booklet to keep track of your medicines. Logo Here health care quality Have you
Your Medicine: Play It Safe Learn more about how to take medicines safely. Use the Medicine Record Form at the back of this booklet to keep track of your medicines. Logo Here health care quality Have you
MAXITROL Eye Ointment
 MAXITROL Dexamethasone, Neomycin and Polymyxin B CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Maxitrol. This leaflet answers some common questions
MAXITROL Dexamethasone, Neomycin and Polymyxin B CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Maxitrol. This leaflet answers some common questions
Zovirax Ophthalmic (eye) Ointment Acyclovir
 Zovirax Ophthalmic (eye) Ointment Acyclovir Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Zovirax ophthalmic ointment. It does not contain all the
Zovirax Ophthalmic (eye) Ointment Acyclovir Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Zovirax ophthalmic ointment. It does not contain all the
PATIENT INFORMATION LEAFLET. PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM RELESTAT, epinastine hydrochloride 0,5 mg/ml, eye drops
 Page 1 of 5 SCHEDULING STATUS Schedule 2 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM RELESTAT, epinastine hydrochloride 0,5 mg/ml, eye drops Read all of this leaflet
Page 1 of 5 SCHEDULING STATUS Schedule 2 PATIENT INFORMATION LEAFLET PROPRIETARY NAME, STRENGTH AND PHARMACEUTICAL FORM RELESTAT, epinastine hydrochloride 0,5 mg/ml, eye drops Read all of this leaflet
Glaucoma Short Paper 1: Medical Literature Review for a Case Study
 Glaucoma Short Paper 1: Medical Literature Review for a Case Study BBH 411W Devon Stobbe * The purpose of the writing is to fulfill course requirements for BBH 411W and to stand as a personal writing sample,
Glaucoma Short Paper 1: Medical Literature Review for a Case Study BBH 411W Devon Stobbe * The purpose of the writing is to fulfill course requirements for BBH 411W and to stand as a personal writing sample,
Package leaflet: Information for the patient. Verkazia 1 mg/ml eye drops emulsion ciclosporin
 Package leaflet: Information for the patient Verkazia 1 mg/ml eye drops emulsion ciclosporin Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: Information for the patient Verkazia 1 mg/ml eye drops emulsion ciclosporin Read all of this leaflet carefully before you start using this medicine because it contains important information
Written by Administrator Wednesday, 13 January :27 - Last Updated Thursday, 21 January :34
 angle closure glaucoma A type of glaucoma caused by a sudden and severe rise in eye pressure. Occurs when the pupil enlarges too much or too quickly, and the outer edge of the iris blocks the eye s drainage
angle closure glaucoma A type of glaucoma caused by a sudden and severe rise in eye pressure. Occurs when the pupil enlarges too much or too quickly, and the outer edge of the iris blocks the eye s drainage
ENIDIN EYE DROPS [brimonidine tartrate]
![ENIDIN EYE DROPS [brimonidine tartrate] ENIDIN EYE DROPS [brimonidine tartrate]](/thumbs/81/84520817.jpg) ENIDIN EYE DROPS [brimonidine tartrate] Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about ENIDIN eye drops, including how to use the eye It does not
ENIDIN EYE DROPS [brimonidine tartrate] Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about ENIDIN eye drops, including how to use the eye It does not
CONSUMER INFORMATION. Sandoz Travoprost / Timolol PQ ophthalmic solution travoprost and timolol ophthalmic solution with POLYQUAD * as preservative
 CONSUMER INFORMATION Pr Sandoz Travoprost / Timolol PQ ophthalmic solution travoprost and timolol ophthalmic solution with POLYQUAD * as preservative This leaflet is designed specifically for Consumers.
CONSUMER INFORMATION Pr Sandoz Travoprost / Timolol PQ ophthalmic solution travoprost and timolol ophthalmic solution with POLYQUAD * as preservative This leaflet is designed specifically for Consumers.
Do Not Reproduce. Things to Tell Your Health Care Provider
 Note: This CareKit does not replace expert medical care. 2 Things to Tell Your Health Care Provider Before medicine is prescribed, tell him or her: Medicines on your health plan s preferred drug list (formulary).
Note: This CareKit does not replace expert medical care. 2 Things to Tell Your Health Care Provider Before medicine is prescribed, tell him or her: Medicines on your health plan s preferred drug list (formulary).
Package leaflet: Information for the user. Relestat, 0.5 mg/ml, eye drops, solution Epinastine hydrochloride
 Package leaflet: Information for the user Relestat, 0.5 mg/ml, eye drops, solution Epinastine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user Relestat, 0.5 mg/ml, eye drops, solution Epinastine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important
Because the more you know, the better you ll feel.
 ABOUT ASTHMA Because the more you know, the better you ll feel. What You ll Find Attitudes and Beliefs Asthma What Is It? Where You ll Find It Page 4-5 This booklet is designed to help you understand asthma
ABOUT ASTHMA Because the more you know, the better you ll feel. What You ll Find Attitudes and Beliefs Asthma What Is It? Where You ll Find It Page 4-5 This booklet is designed to help you understand asthma
Making Your Treatment Work Long-Term
 Making Your Treatment Work Long-Term How to keep your treatment working... and why you don t want it to fail Regardless of the particular drugs you re taking, your drugs will only work when you take them.
Making Your Treatment Work Long-Term How to keep your treatment working... and why you don t want it to fail Regardless of the particular drugs you re taking, your drugs will only work when you take them.
Frequently Asked Questions about General Ophthalmology:
 1. Normal Eye Structure The eye is a slightly asymmetrical globe, about an inch in diameter. The parts of the eye include: Cornea (a clear dome over the iris), Iris (the pigmented part); Pupil (the black
1. Normal Eye Structure The eye is a slightly asymmetrical globe, about an inch in diameter. The parts of the eye include: Cornea (a clear dome over the iris), Iris (the pigmented part); Pupil (the black
MURINE HAYFEVER RELIEF 2% W/V EYE DROPS Sodium Cromoglicate
 MURINE HAYFEVER RELIEF 2% W/V EYE DROPS Sodium Cromoglicate Read this leaflet carefully before you start using this medicine. This medicine is available without a prescription, however you still need to
MURINE HAYFEVER RELIEF 2% W/V EYE DROPS Sodium Cromoglicate Read this leaflet carefully before you start using this medicine. This medicine is available without a prescription, however you still need to
SAFE MEDICATION USE FOR OLDER ADULTS
 SAFE MEDICATION USE FOR OLDER ADULTS INFORMATION FOR OLDER ADULTS, FAMILIES, AND CAREGIVERS READ THIS PAMPHLET TO LEARN: Why it is Important to Understand and Know How to Manage your Medications. The Different
SAFE MEDICATION USE FOR OLDER ADULTS INFORMATION FOR OLDER ADULTS, FAMILIES, AND CAREGIVERS READ THIS PAMPHLET TO LEARN: Why it is Important to Understand and Know How to Manage your Medications. The Different
VOLTAREN OPHTHA Eye Drops 0.1%
 VOLTAREN OPHTHA Eye Drops 0.1% Diclofenac sodium CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Voltaren Eye Drops. This leaflet answers some
VOLTAREN OPHTHA Eye Drops 0.1% Diclofenac sodium CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Voltaren Eye Drops. This leaflet answers some
What do I need to tell my doctor BEFORE I take this drug? If you have an allerg y to latanoprost or any other part of this drug.
 PATIENT & CAREGIVER EDUCATION Latanoprost Brand Names: US Xalatan; Xelpros Brand Names: Canada Monoprost; Xalatan What is this drug used for? It is used to treat glaucoma. It is used to lower high eye
PATIENT & CAREGIVER EDUCATION Latanoprost Brand Names: US Xalatan; Xelpros Brand Names: Canada Monoprost; Xalatan What is this drug used for? It is used to treat glaucoma. It is used to lower high eye
ABOUT TYPE 2 DIABETES
 ABOUT TYPE 2 DIABETES Because the more you know, the better you ll feel. What You ll Find Attitudes and Beliefs Type 2 Diabetes What Is It? Where You ll Find It Page 4-5 This booklet is designed to help
ABOUT TYPE 2 DIABETES Because the more you know, the better you ll feel. What You ll Find Attitudes and Beliefs Type 2 Diabetes What Is It? Where You ll Find It Page 4-5 This booklet is designed to help
APO-Olopatadine Eye Drops Contains the active ingredient olopatadine (as olopatadine hydrochloride)
 APO-Olopatadine Eye Drops Contains the active ingredient olopatadine (as olopatadine hydrochloride) Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
APO-Olopatadine Eye Drops Contains the active ingredient olopatadine (as olopatadine hydrochloride) Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet
HELPING LIFT YOU THROUGH YOUR JOURNEY WITH SYMPTOMATIC SARCOIDOSIS
 HELPING LIFT YOU THROUGH YOUR JOURNEY WITH SYMPTOMATIC SARCOIDOSIS Your Guide to Acthar SUPPORTING YOU FROM THE START OF YOUR TREATMENT H.P. Acthar Gel is a prescription medicine for people with symptoms
HELPING LIFT YOU THROUGH YOUR JOURNEY WITH SYMPTOMATIC SARCOIDOSIS Your Guide to Acthar SUPPORTING YOU FROM THE START OF YOUR TREATMENT H.P. Acthar Gel is a prescription medicine for people with symptoms
Enjoying life with the KAMRA
 Enjoying life with the KAMRA inlay. Your aftercare guide to clearer reading vision. AFTERCARE guide NATURAL vision Natural Vision For Everyday Living Congratulations on your decision to get the KAMRA inlay!
Enjoying life with the KAMRA inlay. Your aftercare guide to clearer reading vision. AFTERCARE guide NATURAL vision Natural Vision For Everyday Living Congratulations on your decision to get the KAMRA inlay!
Package leaflet: Information for the user. Carmellose sodium
 Package leaflet: Information for the user CELLUVISC 0.5% w/v, eye drops, solution, unit dose Carmellose sodium Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user CELLUVISC 0.5% w/v, eye drops, solution, unit dose Carmellose sodium Read all of this leaflet carefully before you start using this medicine because it contains
ALPHAGAN EYE DROPS BRIMONIDINE TARTRATE
 PACKAGE LEAFLET: INFORMATION FOR THE USER ALPHAGAN EYE DROPS BRIMONIDINE TARTRATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which
PACKAGE LEAFLET: INFORMATION FOR THE USER ALPHAGAN EYE DROPS BRIMONIDINE TARTRATE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which
DUOTRAV Eye Drops travoprost 0.004% and timolol 0.5% Consumer Medicine Information
 DUOTRAV travoprost 0.004% and timolol 0.5% Consumer Medicine Information What is in this Leaflet Read this leaflet carefully before you start to use DuoTrav Eye This leaflet answers some common questions
DUOTRAV travoprost 0.004% and timolol 0.5% Consumer Medicine Information What is in this Leaflet Read this leaflet carefully before you start to use DuoTrav Eye This leaflet answers some common questions
Save time at your check-in and register online before your appointment! It s as easy as 1-2-3
 Save time at your check-in and register online before your appointment! It s as easy as 1-2-3 1. Go online to www.blackhillseyes.com 2. Click this logo on our home page for the link to register: 3. Set-up
Save time at your check-in and register online before your appointment! It s as easy as 1-2-3 1. Go online to www.blackhillseyes.com 2. Click this logo on our home page for the link to register: 3. Set-up
FROM CATARACTS TO CLARITY
 Cathy Cataracts FROM CATARACTS TO CLARITY If you re 55 or older, you may have cataracts and not even know it. What You Need to Know Seeing Beyond the Symptoms Cataracts are one of the leading causes of
Cathy Cataracts FROM CATARACTS TO CLARITY If you re 55 or older, you may have cataracts and not even know it. What You Need to Know Seeing Beyond the Symptoms Cataracts are one of the leading causes of
Understanding. Glaucoma
 Understanding Glaucoma Contact us We re here to answer any questions you have about your eye condition or treatment. If you need further information about glaucoma or on coping with changes in your vision,
Understanding Glaucoma Contact us We re here to answer any questions you have about your eye condition or treatment. If you need further information about glaucoma or on coping with changes in your vision,
Package Leaflet - Information for the User. TOBRADEX 3 mg/ml/1 mg/ml Eye Drops, Suspension Tobramycin and Dexamethasone
 Package Leaflet - Information for the User TOBRADEX 3 mg/ml/1 mg/ml Eye Drops, Suspension Tobramycin and Dexamethasone Read all of this leaflet carefully before you start using this medicine because it
Package Leaflet - Information for the User TOBRADEX 3 mg/ml/1 mg/ml Eye Drops, Suspension Tobramycin and Dexamethasone Read all of this leaflet carefully before you start using this medicine because it
Vision Health: Conditions, Disorders & Treatments GLAUCOMA
 Vision Health: Conditions, Disorders & Treatments GLAUCOMA Glaucoma is a disease of the optic nerve, which transmits the images you see from the eye to the brain. The optic nerve is made up of many nerve
Vision Health: Conditions, Disorders & Treatments GLAUCOMA Glaucoma is a disease of the optic nerve, which transmits the images you see from the eye to the brain. The optic nerve is made up of many nerve
TRAVATAN Eye Drops 0.004% travoprost
 Eye Drops 0.004% travoprost Consumer Medicine Information What is in this leaflet Read this leaflet carefully before you start to use Travatan Eye Drops. This leaflet answers some common questions about
Eye Drops 0.004% travoprost Consumer Medicine Information What is in this leaflet Read this leaflet carefully before you start to use Travatan Eye Drops. This leaflet answers some common questions about
CONSUMER MEDICINE INFORMATION
 CONSUMER MEDICINE INFORMATION Arrow Dortim Dorzolamide 2% and Timolol 0.5% Eye Drops, Solution What is in this leaflet This leaflet answers some common questions about ARROW-DORTIM. It does not contain
CONSUMER MEDICINE INFORMATION Arrow Dortim Dorzolamide 2% and Timolol 0.5% Eye Drops, Solution What is in this leaflet This leaflet answers some common questions about ARROW-DORTIM. It does not contain
Revised: 07/2017. LUMIGAN (bimatoprost ophthalmic solution) 0.01% for topical ophthalmic use Initial U.S. Approval: 2001
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN 0.01% safely and effectively. See full prescribing information for LUMIGAN 0.01%. LUMIGAN
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use LUMIGAN 0.01% safely and effectively. See full prescribing information for LUMIGAN 0.01%. LUMIGAN
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. XIIDRA TM* Lifitegrast Ophthalmic Solution 5%
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION XIIDRA TM* Lifitegrast Ophthalmic Solution 5% Read this carefully before you start taking XIIDRA and each time you get
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION XIIDRA TM* Lifitegrast Ophthalmic Solution 5% Read this carefully before you start taking XIIDRA and each time you get
Pharmacy Advisor Program. Specialized Lung Health Support
 Pharmacy Advisor Program Specialized Lung Health Support Contents Lung Health and Your CVS Caremark Pharmacy Advisor Pharmacist...3 Knowing Your Asthma...4 Taking Care of Your Asthma...6 Knowing Your COPD...8
Pharmacy Advisor Program Specialized Lung Health Support Contents Lung Health and Your CVS Caremark Pharmacy Advisor Pharmacist...3 Knowing Your Asthma...4 Taking Care of Your Asthma...6 Knowing Your COPD...8
Selective Laser Trabeculoplasty (SLT)
 Selective Laser Trabeculoplasty (SLT) A treatment for Glaucoma Eye Department Patient information leaflet Introduction The aim of this information sheet is to help answer some of the questions you may
Selective Laser Trabeculoplasty (SLT) A treatment for Glaucoma Eye Department Patient information leaflet Introduction The aim of this information sheet is to help answer some of the questions you may
PATIENT INFORMATION LEAFLET
 ENFLUAT 0.4 % Sterile Eye Drops Applied dropwise to the eye. PATIENT INFORMATION LEAFLET Drug substance: Each 1ml contains 4 mg ketorolac tromethamine. Excipient(s): Benzalconium chloride, sodium chloride,
ENFLUAT 0.4 % Sterile Eye Drops Applied dropwise to the eye. PATIENT INFORMATION LEAFLET Drug substance: Each 1ml contains 4 mg ketorolac tromethamine. Excipient(s): Benzalconium chloride, sodium chloride,
Getting started on Otezla
 Getting started on Otezla Your guide to starting and staying on treatment Otezla (apremilast) is a prescription medicine approved for the treatment of patients with moderate to severe plaque psoriasis
Getting started on Otezla Your guide to starting and staying on treatment Otezla (apremilast) is a prescription medicine approved for the treatment of patients with moderate to severe plaque psoriasis
Advice following Cataract surgery Eye clinic
 Advice following Cataract surgery Eye clinic 01935 475 122 yeovilhospital.nhs.uk The plastic shield The plastic shield over your eye can be removed the following morning. However, you should wear the shield
Advice following Cataract surgery Eye clinic 01935 475 122 yeovilhospital.nhs.uk The plastic shield The plastic shield over your eye can be removed the following morning. However, you should wear the shield
TOBRADEX TM Eye Drops
 TOBRADEX TM Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Tobradex Eye This leaflet answers some common
TOBRADEX TM Eye Drops Tobramycin and Dexamethasone CONSUMER MEDICINE INFORMATION What is in this leaflet Read this leaflet carefully before you start to use Tobradex Eye This leaflet answers some common
ANSWERS TO YOUR MOST COMMON GLAUCOMA QUESTIONS
 For Your Eyes Only: ANSWERS TO YOUR MOST COMMON GLAUCOMA QUESTIONS www.kremereyecenter.com / 800-694-3937 1 Table of Contents Introduction... 3 Glaucoma Defined... 4 Symptoms of Glaucoma... 6 Treatment
For Your Eyes Only: ANSWERS TO YOUR MOST COMMON GLAUCOMA QUESTIONS www.kremereyecenter.com / 800-694-3937 1 Table of Contents Introduction... 3 Glaucoma Defined... 4 Symptoms of Glaucoma... 6 Treatment
Information Sheet 10. Medication Hints and Tips (Updated August 2014)
 An information sheet on Medication problems and how to prevent them. Memory Loss... People who suffer from dementia or Alzheimer s disease may simply forget to take their medications, causing them to skip
An information sheet on Medication problems and how to prevent them. Memory Loss... People who suffer from dementia or Alzheimer s disease may simply forget to take their medications, causing them to skip
Package leaflet: Information for the user. GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution, in single-dose container Bimatoprost/timolol
 Package leaflet: Information for the user GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution, in single-dose container Bimatoprost/timolol Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user GANFORT 0.3 mg/ml + 5 mg/ml eye drops, solution, in single-dose container Bimatoprost/timolol Read all of this leaflet carefully before you start using this medicine
Prescription Drug Options for Older Adults: Managing Your Medicines
 Prescription Drug Options for Older Adults: Managing Your Medicines As people age, the likelihood of taking medicines increases; studies show that the more medicines people take, the more likely they are
Prescription Drug Options for Older Adults: Managing Your Medicines As people age, the likelihood of taking medicines increases; studies show that the more medicines people take, the more likely they are
THE CHRONIC GLAUCOMAS
 THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
THE CHRONIC GLAUCOMAS WHAT IS GLAUCOMA People with glaucoma have lost some of their field of all round vision. It is often the edge or periphery that is lost. That is why the condition can be missed until
Glaucoma. How is Glaucoma Diagnosed? Glaucoma Testing
 Glaucoma How is Glaucoma Diagnosed? Glaucoma Testing There is no single test for glaucoma. The diagnosis is made by evaluating the patient from a number of perspectives, using specialized instruments.
Glaucoma How is Glaucoma Diagnosed? Glaucoma Testing There is no single test for glaucoma. The diagnosis is made by evaluating the patient from a number of perspectives, using specialized instruments.
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults
 East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
East and North Hertfordshire treatment pathway for primary open angle glaucoma and ocular hypertension in adults Introduction Glaucoma is a group of eye diseases causing optic nerve damage. In most cases
Understanding. Glaucoma. National Glaucoma Research
 Understanding National Research Understanding What is? is not just one disease, but a group of eye diseases that damage the optic nerve the bundle of nerve fibers that carries information from the eye
Understanding National Research Understanding What is? is not just one disease, but a group of eye diseases that damage the optic nerve the bundle of nerve fibers that carries information from the eye
Caregiver s guide to wet AMD
 Caregiver s guide to wet AMD Learn more about wet AMD (wet age-related macular degeneration) and how to support someone with wet AMD. Who is LUCENTIS for? LUCENTIS (ranibizumab injection) is a prescription
Caregiver s guide to wet AMD Learn more about wet AMD (wet age-related macular degeneration) and how to support someone with wet AMD. Who is LUCENTIS for? LUCENTIS (ranibizumab injection) is a prescription
Package leaflet: Information for the user. Latanoprost Pfizer 50 micrograms/ml Eye drops, solution Latanoprost
 Package leaflet: Information for the user Latanoprost Pfizer 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user Latanoprost Pfizer 50 micrograms/ml Eye drops, solution Latanoprost Read all of this leaflet carefully before you start using this medicine because it contains
Medication Use safety Training FOR SENIORS
 www.mustforseniors.org Medication Use safety Training FOR SENIORS Medication Use Safety Training for Seniors A National Education Campaign for Older Adults and Caregivers developed by the National Council
www.mustforseniors.org Medication Use safety Training FOR SENIORS Medication Use Safety Training for Seniors A National Education Campaign for Older Adults and Caregivers developed by the National Council
