Original article. R. Biffi, 1 F. de Braud, 2 F. Orsi, 3 S. Pozzi, 1 S. Mauri, 1 A. Goldhirsch, 2 F. Nole 2 & B. Andreoni 1
|
|
|
- Vincent Sharp
- 6 years ago
- Views:
Transcription
1 Annals of Oncology 9: , Kluwer Academic Publishers. Printed in the Netherlands. Original article Totally implantable central venous access ports for long-term chemotherapy A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days R. Biffi, 1 F. de Braud, 2 F. Orsi, 3 S. Pozzi, 1 S. Mauri, 1 A. Goldhirsch, 2 F. Nole 2 & B. Andreoni 1 Divisions of 'General Surgery, 2 Medical Oncology. 3 Radiology, European Institute of Oncology, Milano, Italy Summary Background: A few data are available from analyses of the complications and costs of central venous access ports for chemotherapy. This prospective study deals with the complications and global costs of central venous ports connected to a Groshong catheter for deliverance of long-term chemotherapy. Patients and methods: Patients with a variety of solid neoplastic diseases requiring chemotherapy who were undergoing placement of implantable ports over a 30-month period (1 October 1994 to 31 March 1997) have been prospectively studied. Follow-up continued until the device was removed or the study was closed (30 September 1997); patients with uneventful implant experience and subsequent follow-ups of less than 180 days were not considered for this study. A single port, constructed of titanium and silicone rubber (Dome Port, Bard Inc., Salt Lake City, USA), was used, connected to an 8 F silastic Groshong catheter tubing (Bard Inc., Salt Lake City, USA). Two-hundred ninety-six devices were placed in the operating room under fluoroscopic control even in the patients treated and monitored in a day-hospital setting; 37 of them were in an angiographic suite. A central venous access form was filled in by the operator after the procedure and all ports were followed prospectively for devicerelated and overall complications. The average purchase cost of the device was obtained from the hospital charges, based on the costs applied during the 30- month period of the study. Insertion and maintenance costs were estimated by obtaining the charges for an average T1AP implant and its subsequent use; the costs of complication management were assessed analytically. The total cost of each device was defined as the purchase cost plus the insertion cost plus the maintenance cost plus the cost of treatment of the complications, if any. The cost of removing the TIAP was also included in the economic analysis when required by the treatment of the complication. Results: Three hundred thirty-three devices, for a total of 79,178 days in situ, were placed in 328 patients. Five patients received second devices after removal of the first. In all cases the follow-up was appropriate (median 237 days, range ). Early complications included 10 pneumothoraxes (3.4%; six tube-thoracostomies were applied, 1.8%) and six revisions for port and/or catheter malfunction (overall early complications = 16, 4.48%). Late complications comprised five instances of catheter rupture and embolization (1.5%, episodes/1000 days of use), five of venous thrombosis (1.5%, episodes/ 1000 days of use), one of pocket infection (0.3%, episodes/ 1000 days of use), and eight of port-related bacteremia (2.4%, episodes/1000 days of use). The infections were caused by coagulase-negative Staphylococcus aureus (five cases), Bacillus subtilis (one case), Streptococcus lactaceae (one case) and an unknown agent (one case); port removal was necessary in six of eight cases. The total cost per patient treated for a six-month period, consisting of the costs of purchase and implantation, treatment of early and late complications, and of maintenance of the device, is US$1,970. Conclusions: This study represents the largest published series of patients with totally implantable access ports connected to a Groshong catheter. We have shown that US$2,000 are sufficient to cover six months of chemotherapy in one patient using the most expensive commercially available implantable port. According to the present study, totally implantable access ports connected to a Groshong catheter are associated with high purchase and insertion costs, a low complication rate and low maintenance costs. These data support their increasing use in current oncologic medical practice. Key words: central venous catheters, chemotherapy, Groshong catheter, ports Introduction Many cancer patients require long-term central venous access for the safe delivery of chemotherapeutic agents, transfusion of blood and blood products, and the performiance of laboratory tests. The choice of the device is usually made by the treating physician on the basis of retrospective reviews of the morbidity and longevity of various devices, hospital or surgical availability, as well as personal experience. Together with percutaneous tunneled catheters, such as those of Hickman and Broviac, there has been increased demand for totally implantable access ports (TIAP) because they need no external dressing, allow patient activity and require only monthly
2 768 flushes of heparinized saline to keep the catheter patent. An obvious disadvantage of these devices is the fact that they are much more expensive than tunneled catheters; moreover, the crude cost of the commercially available devices does not take into account the additional costs of catheter maintenance and the treatment of possible complications. There are only a few studies and only little data concerning the total cost of these systems, especially when prospectively assessed [1]; in addition, most studies do not exhibit sufficient follow-up, whereas it is well known that a number of late port-related complications arise some months after implantation. Only a little economic information about these devices and their related morbidity could lead to an underestimation of their global cost. The purpose of this study is to provide clinicians with data on the total cost of TIAP for long-term chemotherapy, derived from a prospective study of 333 devices with a minimum of 180 days of follow-up. Patients and methods Three hundred thirty-three ports placed in patients at the European Institute of Oncology in Milan during the 30-month period from 1 October 1994 to 30 March 1997 for chemotherapy of solid tumours were followed prospectively for device-related and overall complications. The main tumour types were breast (approximately 50%), colorectal, gastric, pancreatic, head and neck, and sarcoma. The chemotherapy regimens used were: VFUP (vinorelbine-fluorouracil-cisplatin), VEFU (vinorelbine-epirubicin-fluorouracil), ECF (epirubicincisplatin-fluorouracil), 5-FU continuous infusion + lederfolin, 5-FU continuous infusion + lederfolin + oxaliplatin, and ifosfamide continuous-infusion. Follow-up continued until the device was removed, the patient died or the study was closed (30 September 1997); patients with uneventful implant and subsequent follow-up of less than 180 days were not considered for this study. Two-hundred ninety-six devices were implanted in the operating room under fluoroscopic control, even if the patient was treated and monitored in a day-hospital setting, and 37 in an angiographic suite. Preoperative evaluation included a history and physical examination that focused on possible anatomic pitfalls (clavicle fracture, cervical or mediastinal adenopathy, chest wall tumours, presence of rotation flaps as part of head and neck reconstructive surgery), body habitus and vascular access history (side used and pneumothorax history, previous line infection). The only laboratory studies requested as absolutely necessary were a complete blood count, including platelet count and differential, and coagulation tests. All of the patients had chest radiographs for preoperative identification of mass lesions or anatomic anomalies. The patients received a local anesthesia, without routine additional intravenous sedation; a single dose (2 g) of Cefazoline sodium was given intravenous 15 minutes before implant. No breaks in operative technique or instrument sterility were documented; a confirmatory chest X-ray was always obtained after the placement and all patients were checked by a physician before discharge. To minimize possible variables related to the devices adopted, a single type of port was used, constructed of titanium and silicone rubber (Dome Port, Bard Inc., Salt Lake City, USA) with 8 F silastic Groshong catheter tubing (Bard Inc., Salt Lake City, USA). Developed in 1978, the Groshong catheter is a silicone rubber device with a three-position pressure-sensitive valve near the distal tip. The valve opens only with positive or negative pressure, effectively preventing spontaneous reflux of blood or inadvertent air embolism. This unique feature renders this catheter the most expensive of the commercially available ones. A central venous access form was filled in by the operator after the procedure. Data from the follow-up of these patients were entered into the form and collected in a software registry. The time interval between implant of catheter and first chemotherapy delivered through the device was usually 24 hours; if an early complication was found (e.g., pneumothorax), the start of chemotherapy was delayed until resolution of the problem (generally hours after implantation). Complications were divided into two main categories: (1) early (intraoperative and postimplantation period to first use), and (2) late complications (occurring after the first chemotherapy course given through the device). Blood screening for bacteremia has not been performed at regular intervals, since blood sampling for microbiology was obtained when clinically suggested (unexplained fever and/or signs of sepsis). Criteria for the diagnosis of device-related bacteremia were defined as: a) greater than a 10-fold increase in colony-forming units (CFU) of bacteria per ml of blood obtained through the device in comparison to peripheral blood cultures; b) greater than 1,000 CFUs of bacteria obtained through the device, in the absence of peripheral blood cultures; or c) positive catheter tip culture upon removal in the appropriate clinical setting. Device-related bacteremia or fungemia was considered cured when culture results were negative at the termination of antibiotic therapy and no evidence of clinical infection had been seen by two weeks later. Port pocket infection was defined as induration, erythema, and tenderness around the port with culture-positive material aspirated from the port pocket. Cutaneous site infection was defined as induration, erythema, or tenderness and exudate at the port surface needle access site. Thrombosis was identified with ultrasound and/or venography when clinically suggested by progressive arm or facial swelling. The average purchase cost of the devices was obtained from the hospital charges, based on the prices applied by the manufacturer during the 30-month period of the study. Insertion and maintenance costs were estimated by obtaining the charges for an average TIAP implant and its subsequent use. Although not strictly recommended by the manufacturer, implanted ports have been filled with sterile heparinized saline after each use to prevent clot formation and catheter blockage. The following schedule was adopted: after each infusion of medication or blood withdrawal: 20 ml sterile normal saline, then 5 ml heparinized saline (50 IU/ml); for ports not in use: 5 ml of heparinized saline (50 IU/ml), once every 28 days. This maintenance programme has been carried out on an outpatient basis by a experienced nursing staff. The costs of complication management were assessed analytically, providing the overall amount of related costs when more than one case of a complication was observed. The total cost for each device was defined as the purchase cost plus the insertion cost plus the maintenance cost plus the cost of complication treatments, if any. The costs of removing the TIAP and new implanting were also included in the economic analysis when required by the treatment of the complication. Other charges incurred by the patients in this study were not included in the cost analysis, because it was not possible to accurately attribute them to the TIAP or to other ongoing problems (e.g., febrile neutropenia). Results Three hundred thirty-three devices, comprising a total of 79,178 days in situ, were placed in 328 patients (five patients underwent a second placement after removal of the first device). All patients received at least one cycle of chemotherapy through the TIAP. Adequate follow-up was obtained in all cases (median 237 days, range ). Table 1 summarizes pertinent population data and route for central venous access; we observed no TIAP-
3 769 Table 1. Population characteristics. Table 2. Cost of implanting a TIAP in the present series* Number of ports 333 Parameter Cost USS Number of patients Age (in years) Median Range Male: female ratio Days in situ Median Overall Percutaneous catheterization Surgical venous cut-down (cephalic vein) : , SlafT Device Supplies 1 Fluoroscopy Anesthesia/monitoring 1 OR/recovery room 1 Laboratory tests Chest X-ray One day of day-hospital stay Total I related deaths in this series. Six of 10 patients in whom a pneumothorax was seen as a complication of the TIAP placement underwent a tube-thoracostomy, with no additional morbidity (our policy was to insert a chest-tube when the pneumothorax was large (more than 30%) and /or symptoms were relevant). Four patients of this series had an accidental arterial puncture during the implant procedure, which, however, caused no significant complications or additional cost. In four cases we had to revise the implant, without removal, because of malfunction of the catheter due to a narrowing of the lumen (two cases), dislocation into the controlateral brachio-cephalic vein (one case) and ipsilateral giugular vein (one case). An early catheter obstruction was detected in two cases. Catheter rupture and embolization occurred in five cases (1.5%, 0.063/1000 days of port use); two patients had palpitations and chest discomfort 66 ± 18 days after implant, during a pause between subsequent chemotherapy cycles, leading to chest X-ray and diagnosis. Three patients were completely asymptomatic and incidental chest radiographic examination performed for re-staging detected the catheter embolization. Retrieval of embolizing catheters was accomplished without additional morbidity by venous transfemoral approach using a modified Dormia device to seize the catheter piece and pull it out. The incidence of catheter-associated venous thrombosis was 1.5% in this series (five patients, 0.063/1000 days of port use); all of the patients exhibited ipsilateral arm swelling, without neurologic difficulties in the extremity. The interval between implantation and the onset of clinically evident venous thrombosis was less than 30 days in all patients. Duplex ultrasonography yielded a positive result, confirmed by a standard contrast venogram of the ipsilateral extremity. As the catheters were still needed, patients were treated with intravenous continuous infusion of heparin for three days or low molecular weight heparin subcutaneous for five days, then oral anticoagulation was begun and full anticoagulation was continued for three months. The first patient died 11 months after port placement for progression of her neoplastic disease, whereas the rest are still alive with a progressive disease nine to 20 months after implant. Port pocket infection, usually caused by gram-posi- * Estimate is based on a 40-minute OR procedure. tive cocci, suggests direct inoculation or migration of organisms along the accessing needle as the primary mechanism; such infection occurred in one case (0.3%, 0.012/1000 days of port use) and was successfully treated by administration of appropriate antibiotics, first with empiric coverage and then targeted for the cultured organism (coagulase-negative Staphylococcus aures). Eight patients suffered port-related bacteremia (2.4%, 0.101/1000 days of port use), leading to port removal in six cases (1.8%); causative agents were Bacillus subtilis (one case, port removed), coagulase-negative Staphylococcus aures (five cases, four ports removed, one retained after elimination of the clinical sepsis syndrome by antibiotic therapy), Streptococcus lactaceae (one case, retained after elimination of the clinical sepsis syndrome by antibiotic therapy) and an unknown agent (port removed for clinical sepsis in another hospital). The median number of days (SD) before start of the infection was 165 [20]. Table 2 shows the costs as determined by this study of implanting a TIAP; our initial experience in the use of TIAP connected to Groshong catheter in cancer patients undergoing chemotherapy has been presented elsewhere in a paper dealing with a prospectively-studied consecutive series of 178 unselected oncology patients treated in a single Institution [2]. The costs of diagnosis and management of early complications are listed in Table 3; as expected, the costs have proven to be much higher when a tube thoracostomy was required for the proper treatment of a pneumothorax (six patients out of 10; US$1827 vs. 535). Early revision of the devices, mostly caused by kinking/ obstruction or dislocation of the catheters, accounted for a total of six patients, and required a new operatingroom procedure. The total cost for its management was USS3825. The global cost for diagnosing and treating early complications was USS 16,927. Catheter rupture and embolization were observed in five of the patients in this series; the costs for their management (interventional radiology), totaling USS5913, are listed in Table 4a. Table 4b lists the analytically-assessed costs for treatment of venous thrombosis, which took place in five patients of the series and
4 770 Table 3. Early complications. Costs of diagnosis and management 3a Pneumothorax. Management: observation (four patients; average values) Chest X-ray: 1 (24 hours) + 1 (48 hours) + 1 (one week) $ 135 One day of in-hospital stay $400 Subtotal SS3S Total (x4) $2140 3b Pneumothorax. Management: chest tube (six patients; average values) Chest-X ray 1 + I (24 hours) + 1 (one after drain removal) + 1 (one week) Thoracic trocar drain Bottle Connecting tube Four days of in-hospital stay Subtotal Total (x6) $180 $9.8 $20.9 $16.3 $1600 $1827 $10,962 3c Early malfunction or obstruction of the device (six patients; average values) Staff $76.5 Revision of the device (operating theatre, supplies etc.) $398 One day of day-hospital stay $ 163 Subtotal $637.5 Total (x6) $3825 Global costs of early complications (3a + 3b + 3c) $16,927 amounted to US$9199. Pocket infection was observed in one case in which treatment was on an outpatient basis, which limited the cost to US$85 (Table 4c). The costs for treatment of skin erosion without infection, which was also detected in one case and required a new implant procedure in the operating theatre were considerably higher (US$872, Table 4d). Tables 4e and f shows the costs of treatment of portrelated bacteremia; it cost more than US$2000 to treat one patient suffering from this complication, either when the port was removed after a proper course of antibiotics or when antibiotics alone were administered. Finally, Table 5 summarizes the total cost of US$1970 per patient, treated for a six-month period, including the cost of purchase and implantation, the cost of treatment of early and late complications and device maintenance cost. Discussion The use of central venous access devices has become an essential component of the treatment of many medical disorders. It is estimated that several million devices are inserted each year, facilitating many emerging therapies, including long-term chemotherapy. TIAP are the last type of long-term venous access developed, after the proposal of tunneled, cuffed silastic catheters by Broviac Table 4. Late complications. Costs of diagnosis and management. 4a Catheter rupture and migration (six patients; average values) Chest X-ray for diagnosis $45 Removal of the catheter through the femoral vein: - diazepam 5 mg $2.6 - heparin 2500 IU $2.1 - angiographic catheter ' $ snare loop catheter $ contrast medium $ introducer $ angiographic wire $ stop cock $6.5 - angiographic suite (30') $ staff and supplies $138 One day of day-hospital stay $ 163 Cost for removal of the port $196 Subtotal $1182 Total (x5) $5913 4b Venous thrombosis (five patients; average values) Real-time US scan (at diagnosis and four weeks later) $457 Low molecular weight heparin s.c. for five days $81 Oral anticoagulant therapy for three months $23.5 Laboratory monitoring of coagulation tests $78.4 Three days of in-hospital stay $ 1200 Subtotal $1840 Total (x5) $ c Pocket Infection (one patient) Amoxicillin + clavulanic acid (2 g/day for 10 days) $ 15 Laboratoty tests (isolation, susceptibility, others) $70 Total $85 4d Skin erosion without infection (one patient) Four outpatient-clinic visits and supplies $235 One day of day-hospital stay $ 163 Reimplant of the device $474.5 Total $ e Port-related bacteremia. Management: removal after a proper course of antibiotic therapy (six patients; average values) Blood culture and susceptibility test $40 Removal of the device $196 Antibiotic treatment (mean cost) $305 Four days of in-hospital stay $1600 Subtotal $2141 Total (x6) $12,846 4f Port-related bacteremia. Management: antibiotic therapy alone (two patients; average values) Blood culture and susceptibility test $40 Antibiotic treatment (mean cost) $305 Two days of in-hospital stay $800 Subtotal $1145 Total (x2) $2290 Global costs of late complications (4a + 4b + 4c + 4d+4e + 4f) $31,206
5 771 Table 5. Global cost of purchase, implant and maintenance of T1AP in this series Cost of single port purchase and implanting (Table 2) Number of implanted ports Global cost of devices' purchase and implanting $ $529,803 % Cost of treatment of early complications (Table 3a-c) $16, Cost of treatment of late complications (Table 4a-f) $31, Global cost of complications'treatment $48, Global cost of port implanting and complication's management (3 + 6) Number of patients treated $577, Global cost for each patient treated $ Device maintenance cost (six months, per patient) $ Global cost for each patient treated (six months ) $ and Hickman; the rationale for choice of the port derives from the obvious observation that a route of bacterial invasion in patients with Broviac/Hickman catheters is the open wound maintained by the catheter's presence. When compared to those of tunneled catheters, port infections tend to be uncommon, although results are sometimes conflicting [3] and most series reflect differences in the type of adopted device and the patients being treated rather than any inherent superiority of one device over another. Another important point is the cost of purchase and use of these devices; a case-control study comparing durability and cost between TIAP and percutaneous silicone catheters showed a significantly greater total cost of TIAP (US$2233 vs. 1453) [4]; however, as the TIAP were found to function for a significantly longer time, they proved less expensive on a per diem basis, with a break point that occurs at approximately six months, the minimum follow-up of the present study. The major cost of TIAP compared to tunnneled-external devices should be balanced against the theoretical advantage of the former in terms of reduced complication rate and greater durability. An analytical assessment of the costs of purchase, implant and maintenance of TIAP has been carried out in the present study, with reference to the present situation of actual costs in the European Institute of Oncology in Milano. As a consequence, these costs might not completely reflect national, provincial or other price agreements or reimbursements, and may not be comparable with those applied in other countries or institutions. Moreover, the costs deriving from the proper treatment of every complication related to implant and use of such devices have been included. Importantly, the purchase cost of the device we have adopted in this series has proven to be the highest among the commercially available ones because of the distinguishing characteristics of the catheter. The early complications of subclavian venipunclure include pneumothorax, hemothorax, air embolism and arterial perforation causing a clinically relevant bleeding. Only pneumothorax has been observed in the present series (10 cases, 3.3%; with six of 10 = 1.8% of tube-thoracostomies), while we had no cases of significant hemorrhage related to TIAP placement. According to the literature, pneumothorax occurs in 1% to 4% of subclavian line insertions; careful attention to anatomic landmarks and operator experience during the placement of central venous catheters are most important for avoidance of this complication during subclavian vein catheterization. From published reports and in our experience as many as 50% of asymptomatic, small pneumothoraxes (less than 30% of the pleural space) can be treated conservatively, without tube thoracostomy, with a significant reduction of the costs (USS535 vs. $1827, Table 3a and b). Surgical cutdown of the cephalic vein in the deltoid-pectoralis space was adopted as an effective alternative option to the percutaneous approach to subclavian vein in 36 cases (10.8%), particularly in patients with difficult anatomical landmarks (for instance, obesity or previous surgery in the region). This approach averts the risk of pneumothorax, but significantly increases the median time required to complete the procedure as well as the stress for the conscious patient. Since the percutaneous placement can be performed much more quickly and with safety, comparable to that of an open venous cutdown, our policy was to confine the open approach to difficult cases, after uneventful attempts at percutaneous incannulation. Occasionally, in patients with refractory thrombocytopenia who do not respond well to platelet transfusion but need long-term venous access, venous cutdown on the cephalic as well as external jugular vein may be the safest approach. Although 'blind' insertion of the catheter could avert the additional cost of ultrasound or fluoroscopy, the routine use of fluoroscopy in this series allowed us to limit to six cases (1.8%) the time-consuming, patientstressing and very expensive replacements after implant, due to an incorrect catheter position (most often ipsilateral internal jugular or controlateral brachiocephalic vein) or to the kinking of a tube. Infection is the most common complication of venous access catheters and the leading reason for their removal. Catheter-related infections are reported in 11% to 45% of patients with Hickman catheters [5, 6], 0% to 22% of patients with TIAP [7] and 7% to 32% of patients with Groshong catheters [5, 7, 8-12]. Device-related morbidity from the literature is difficult to compare because of varying definitions and dissimilar patient populations. While several retrospective studies have noted higher infection rates for external devices compared to TIAP in select patient populations [13], a prospective randomized study was unable to demonstrate a
6 772 statistically significant difference in infections [6]. They appeared much higher for both Hickman catheters and ports than in many other reports, probably due in part to a study population that included many patients with hematologic malignancies. The time period of follow-up in the study may also introduce bias, because other studies have shown a progressive difference in infection rates between the two types of venous access devices after six months, which should therefore be considered the gold-standard period of follow-up for evaluating the long-term effectiveness of these devices. However, in a randomized study of infectious morbidity in patients with solid tumors, TIAP were shown to be associated with fewer infections than were catheters [14], Data from our work, derived from a prospective not randomized study, support the conclusions of most retrospective papers: the infectious morbidity related to TIAP is reasonably low, even in patients undergoing cytostatic treatments for solid tumors. The mechanisms of devicerelated infection may explain why TIAP are less likely to be associated with infection than are tunneled catheters. Migration of skin flora through the cutaneous insertion site with catheter colonization is supported by the finding that gram-positive organisms, especially coagulasenegative staphylococci, are responsible for a significant percentage of the cases of device-related bacteremia in patients with catheters. Compared with catheters, TIAP are irrigated less frequently, require no home care, and are less prone to environmental or cutaneous contamination when not accessed. All these factors may contribute to the reduced incidence of infections associated with TIAP. The incidence of catheter-related symptomatic venous thrombosis has proven very low in this study (1.5%, Table 4b); no useful data are available from retrospective analyses of clinical and autopsy reports in the medical literature, where the incidence varied from 0% to 50% [15, 16]. In a controlled randomized trial, prospective venography.was performed as part of a study of prophylactic low-dose warfarin treatment, with a symptomatic thrombosis rate in untreated patients of 12.5% and an overall rate (symptomatic + silent) of 38% (15 of 40) [17]. Another prospective study in cancer patients with Port-a-Cath subclavian venous catheters reported a 62% rate of upper extremity deep vein thrombosis in the control group, and 6% in patients taking 2500 IU subcutaneous of Fragmin once daily for 90 days (relative risk 6.75, P = 0.002, Fisher exact test [18]). Clinical data derived from our study are not fully comparable, due to differences in adopted devices; however, they do not support the routine use of lowdose anticoagulants in patients bearing a TIAP, the cost of which would be significantly higher than that of the proper treatment of 1.5% venous thromboses complicating the post-implant course (extended up to six months); this cost is estimated at US$40 per patient throughout six months (US$13,120 to prophylactically treat 328 patients for a minimum of six months), without taking into account the possible costs related to diagnosis and treatment of the side effects of anticoagulants. Venous access devices placed through a subclavian vein catheterisation may be compressed by neighboring bony structures, leading to biomaterial fatigue, catheter fracture at the compression point and embolization of the fragment into the central venous system. This complication occurred in five of the patients in our series; it has also been called 'catheter pinch-off and the radiologic finding of acute compression of the catheter is quite typical, indicating a catheter at high risk that should be removed as soon as possible [19]. Clinically it may be associated with intermittent catheter dysfunction, and can be improved by changes in the patient's shoulder position; this situation is not specific and can also be commonly seen when a fibrin sheath has formed around the catheter tip [20]. The most important factor in avoiding this complication is technique, such that the subclavian vein is always accessed lateral to the bend in the clavicle, far away from the ligament joining the clavicle and the first rib. However, catheter fracture and embolization is also possible when the pinch-off sign is not seen, and precisely that was observed in at least four patients of our series. The complication may be completely asymptomatic, and there are no reported fatalities from it in the medical literature. Table 4a shows the relatively low cost of treating this complication: of course, the cost of a new implant has to be added, should this choice be adopted. In summary, we have shown that US$2000 is sufficient to give a patient six months of chemotherapy using the most expensive commercially available TIAP, except for the crude costs of the drugs. Single institution data might differ from those gathered at other centres and might not apply to most practitioners; however, the results of this study are particularly relevant because they have been obtained through an accurate cost analysis of the largest published clinical series of TIAP connected to a Groshong catheter. According to the present study, TIAP connected to a Groshong catheter are associated with high purchase and insertion charges, a low rate of complications and low maintenance costs. Although optimal management of complications, optimal device and optimal timing of implant have not been addressed by the present paper (these need to be defined by multicentric randomised trials), the results of this study support the increasing use of TIAP in current oncology medical practice. References 1. Broadwater JR, Henderson MJ, Smith GJ et al. Outpatient percutaneous central venous access in cancer patients. Am J Surg 1990; 160: Biffi R, Corrado F, De Braud F ct al. Long-term, totally implantable central venous access ports connected to Groshong catheter for chemotherapy of solid tumours: Experience on 178 cases using a single type of device. Eur J Cancer 1997; 33: Brothers TE,Von Moll LK., Niederhuber JE et al. Experience with
7 773 subcutaneous infusion ports in the three hundred patients. Surg Gynecol Obstet 1988; 166: McCready D, Broadwater R, Ross M et al. A case-control comparison of durability and cost between implanted reservoir and percutaneous catheters in cancer patients. J Surg Res 1991; 51: Pasquale MD, Campbell JM, Magnant CM. Groshong versus Hickman catheters. Surg Gynecol Obsiel 1992; 174: Mueller BU, Skelton J, Callender DPE et al. A prospective randomized trial comparing the infectious and noninfectious complications of an externalized catheter versus a subcutaneously implanted device in cancer patients. J Clin Oncol 1992; 10: Gleeson NC, Fiorica JV, Mark JE et al. Externalized Groshong catheters and Hickman ports for central venous access in gynecologic oncology patients. Gynecol Oncol 1993; 51: Malviya VK., Gunter D, Gove N, Malone JM Jr. Vascular access in gynecologic cancer using the Groshong right atrial catheter. Gynecol Oncol 1989; 33: Delmore JE, Horbelt DV, Jack BL, Roberts RK. Experience with the Groshong long-term central venous catheter. Gynecol Oncol 1989; 34: Hull JE, Hunter CS, Luiken GA. The Groshong catheter: Initial experience and early results of imaging-guided placement. Radiology 1992; 185: Burnett AF, Lossef SV, Barth KH et al. Insertion of Groshong central venous catheters utilizing fluoroscopic techniques. Gynecol Oncol 1994; 52: Holloway RW, Orr JW. An evaluation of Groshong central venous catheters on a gynecologic oncology service. Gynecol Oncol 1995; 56: Greene FL, Moore W, Strikland G, McFarland J. Comparison of a totally implantable access device for chemotherapy (Port-A- Cath) and long-term percutaneous catheterization (Broviac). South MedJ 1988; 81: Groeger JS, Lucas AB, Thaler HT et al. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Ann Intern Med 1993; 119: Home III MK, May DJ, Alexander HR et al. Venographic surveillance of tunneled venous access devices in adult oncology patients. Ann Surg Oncol 1995; 2: Haire WD, Lieberman RP, Edney J et al. Hickman catheterinduced thoracic vein thrombosis: Frequency and long-term sequelae in patients receiving high-dose chemotherapy and marrow transplantation. Cancer 1990; 66: Bern MM, Lokich JJ.Wallach SR et al.very low doses of warfarin can prevent thrombosis in central venous catheters. Ann Intern Med 1990; 112: Monreal M, Alastrue A, Rull M et al. Upper extremity deep vein thrombosis in cancer patients with venous access devices - prophylaxis with a low molecular weight heparin (Fragmin). Thromb Haemost 1996; 75: Hinke DH, Zandt-Stastny DA, Goodman LR et al. Pinch-off syndrome: A complication of implantable subclavian venous access devices. Radiology 1990; 177: Starkhammar H, Benglsson M, Morales O. Fibrin sleeve formation afer long term brachial catheterisation with an implantable port device. A prospective venographic study. Eur J Surg 1992; 158: Received 26 February 1998; accepted 26 May Correspondence to: Roberto Biffi, MD Division of General Surgery European Institute of Oncology Via Ripamonti Milano Italy rbifli@ieo.cilea.it
8
Mary Lou Garey MSN EMT-P MedFlight of Ohio
 Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Mary Lou Garey MSN EMT-P MedFlight of Ohio Function Prolonged and frequent access to venous circulation Allows for patient to carry on normal life; decrease number of needle sticks Medications, parenteral
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Unit 11. Objectives. Indications for IV Therapy. Intravenous Access Devices & Common IV Fluids. 3 categories. Maintenance Replacement Restoration
 Unit 11 Fluids, Electrolytes and Acid Base Imbalances Intravenous Access Devices & Common IV Fluids Objectives Review the purpose and types of intravenous (IV) therapy. Recall the nursing care related
Unit 11 Fluids, Electrolytes and Acid Base Imbalances Intravenous Access Devices & Common IV Fluids Objectives Review the purpose and types of intravenous (IV) therapy. Recall the nursing care related
Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case Report
 Kosin Medical Journal 2016;31:167-172. https://doi.org/10.7180/kmj.2016.31.2.167 KMJ Case Report Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case
Kosin Medical Journal 2016;31:167-172. https://doi.org/10.7180/kmj.2016.31.2.167 KMJ Case Report Catheter Fracture of a Totally Implantable Venous Device Due to Pinch Off Syndrome in Breast Cancer: A Case
Spontaneous Migration of a Central Line Catheter into the Heart. Saeed Al Hindi, MD, CABS, FRCSI* Khulood Al-Saad, MD**
 Bahrain Medical Bulletin, Vol. 29, No. 3, September 2007 Spontaneous Migration of a Central Line Catheter into the Heart Saeed Al Hindi, MD, CABS, FRCSI* Khulood Al-Saad, MD** Background: Spontaneous migration
Bahrain Medical Bulletin, Vol. 29, No. 3, September 2007 Spontaneous Migration of a Central Line Catheter into the Heart Saeed Al Hindi, MD, CABS, FRCSI* Khulood Al-Saad, MD** Background: Spontaneous migration
Overview of CVADs. Type of device commonly used. Dwell time Flushing requirement Associated complications. lumens
 Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
Source: Clinical Skills Management of Vascular Access Devices Pre-course handbook. Adapted with permission from NHS Lothian Employee and Education Development Team. Overview of CVADs Type of device Veins
JKSS. Pinch-off syndrome INTRODUCTION CASE REPORTS
 CASE REPORT pissn 2233-7903 eissn 2093-0488 Journal of the Korean Surgical Society Pinch-off syndrome Jin-Beom Cho, Il-Young Park, Ki-Young Sung, Jong-Min Baek, Jun-Hyun Lee, Do-Sang Lee Department of
CASE REPORT pissn 2233-7903 eissn 2093-0488 Journal of the Korean Surgical Society Pinch-off syndrome Jin-Beom Cho, Il-Young Park, Ki-Young Sung, Jong-Min Baek, Jun-Hyun Lee, Do-Sang Lee Department of
Totally implantable venous access system (TIVAS) Complicated by Tracheo-Venous Fistula
 Totally implantable venous access system (TIVAS) Complicated by Tracheo-Venous Fistula Samer Khaled, M.D., Vladimir Gotlieb, M.D., and Arunbai Patel, M.D. Citation: Khaled S, Gotlieb V, Patel A. Totally
Totally implantable venous access system (TIVAS) Complicated by Tracheo-Venous Fistula Samer Khaled, M.D., Vladimir Gotlieb, M.D., and Arunbai Patel, M.D. Citation: Khaled S, Gotlieb V, Patel A. Totally
Advancing Lives and the Delivery of Health Care. The High-Flow Port Designed for Apheresis
 Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Advancing Lives and the Delivery of Health Care The High-Flow Port Designed for Apheresis Optimized for Long Device Life Bench Tested up to 1,000 Accesses 1 Bard is proud to introduce the first and only
Central Line Care and Management
 Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Line Care and Management What is a Central Line/ CVAD? (central venous access device) A vascular infusion device that terminates at or close to the heart or in one of the great vessels (aorta,
Central Venous Access Devices. Stephanie Cunningham Amy Waters
 Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
Central Venous Access Devices Stephanie Cunningham Amy Waters 5 Must Know Facts About CVAD s 1) What are CVAD s? 2) What are CVAD s used for? 3) How are these devices put in? 4) What are the complications
THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS)
 Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Children s Hospital for Wales Department of Paediatric Respiratory Medicine and Cystic Fibrosis THE CARE AND MAINTENANCE OF TOTALLY IMPLANTED VENOUS ACCESS DEVICES (PORTS) This protocol applies to the
Transcatheter Aortic Valve Implantation Procedure (TAVI)
 Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
Page 1 of 5 Procedure (TAVI) Introduction Aortic stenosis (AS) is a common heart valve problem associated with heart failure and death. Surgical valve repair or replacement is recommended if AS patients
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Vaxcel Implantable Ports Valved and Non-Valved. A Patient s Guide
 Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Vaxcel Implantable Ports Valved and Non-Valved A Patient s Guide Vaxcel Implantable Port This pamphlet provides some answers to questions you may have about your implantable port and how to care for it
Insertion of a totally implantable vascular access device (TIVAD)
 Insertion of a totally implantable vascular access device (TIVAD) What is a TIVAD? A TIVAD is a long hollow tube that is inserted into one of the large veins in your body. One end of the tube sits in a
Insertion of a totally implantable vascular access device (TIVAD) What is a TIVAD? A TIVAD is a long hollow tube that is inserted into one of the large veins in your body. One end of the tube sits in a
AV ACESS COMPLICATIONS. Ass. Prof. Dr. Habas
 AV ACESS COMPLICATIONS Ass. Prof. Dr. Habas COMPLICATION AVF IS CONSIDERED A MINOR PROCEDURE INCIDENCE OF COMPLICATION- 20-27% MANY A COMPLICATION LEADS TO FAILURE OF FISTULA LOSS OF SITE AND VEIN FOR
AV ACESS COMPLICATIONS Ass. Prof. Dr. Habas COMPLICATION AVF IS CONSIDERED A MINOR PROCEDURE INCIDENCE OF COMPLICATION- 20-27% MANY A COMPLICATION LEADS TO FAILURE OF FISTULA LOSS OF SITE AND VEIN FOR
Totally implantable venous devices (TIVDs) significantly
 Case Report 425 Catheter Fracture and Cardiac Migration - An Unusual Fracture Site of Totally Implantable Venous Devices: Report of Two Cases Chia-Lo Chang, MD; Hong-Hwa Chen, MD; Shung-Eing Lin, MD Totally
Case Report 425 Catheter Fracture and Cardiac Migration - An Unusual Fracture Site of Totally Implantable Venous Devices: Report of Two Cases Chia-Lo Chang, MD; Hong-Hwa Chen, MD; Shung-Eing Lin, MD Totally
Permanent central venous catheters: complications and strategies using different accesses.
 Permanent central venous catheters: complications and strategies using different accesses. Poster No.: C-1038 Congress: ECR 2015 Type: Educational Exhibit Authors: M. D. Ferrer-Puchol, R. Ramiro, E. Garcia-Oliver,
Permanent central venous catheters: complications and strategies using different accesses. Poster No.: C-1038 Congress: ECR 2015 Type: Educational Exhibit Authors: M. D. Ferrer-Puchol, R. Ramiro, E. Garcia-Oliver,
Totally indwelling venous access devices
 Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
Totally indwelling venous access devices Leeds MDT. April, 2008. Totally indwelling venous access devices [online]. Department of Respiratory Medicine, St James's University Hospital, UK. Available from
Procedures/Risks:central venous catheter
 Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
Appendix E: Overview of Vascular
 Appendix E: Overview of Vascular 56 Peripheral Short Catheter, less than 3 inches (7.5 cm) in length; over-the-needle catheter is most common. Inserted by percutaneous venipuncture, generally into a hand
Appendix E: Overview of Vascular 56 Peripheral Short Catheter, less than 3 inches (7.5 cm) in length; over-the-needle catheter is most common. Inserted by percutaneous venipuncture, generally into a hand
A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports
 Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
Disclosures A Primer on Central Venous Access: Peripherally-Inserted Central Catheters, Tunneled Catheters, and Subcutaneous Ports No conflicts of interest relevant to this presentation Jason W. Pinchot,
You have a what, inside you?
 Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Costal Emergency Medicine Conference You have a what, inside you? Less than mainstream medical devices encountered in the ED. Eric Ossmann, MD, FACEP Associate Professor Duke University Medical Center
Port Design. Page 1. Port Placement, Removal, and Management. Selecting a Vascular Access Device. Thomas M. Vesely, MD
 Non-Dialysis Procedures Port Placement, Removal, and Management Thomas M. Vesely, MD Saint Louis, Missouri Selecting a Vascular Access Device Duration of use Number of lumens Frequency used Blood flow
Non-Dialysis Procedures Port Placement, Removal, and Management Thomas M. Vesely, MD Saint Louis, Missouri Selecting a Vascular Access Device Duration of use Number of lumens Frequency used Blood flow
Pediatric ER Half-day Rounds October 12, 2011 Dr. Karen Bailey
 Pediatric ER Half-day Rounds October 12, 2011 Dr. Karen Bailey Objectives to identify various enteral and vascular access lines what do they look like? indications & contraindications proper placement
Pediatric ER Half-day Rounds October 12, 2011 Dr. Karen Bailey Objectives to identify various enteral and vascular access lines what do they look like? indications & contraindications proper placement
Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore
 CENTRAL VENOUS CATHETERIZATION Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore OBJECTIVES Introduction Indications and Contraindications Complications Technique Basic principles Specifics by Site
CENTRAL VENOUS CATHETERIZATION Dr. prakruthi Dept. of anaesthesiology, Rrmch, bangalore OBJECTIVES Introduction Indications and Contraindications Complications Technique Basic principles Specifics by Site
Portacath Insertion. Patient Information Leaflet
 Portacath Insertion Patient Information Leaflet What is a Portacath? A Portacath (also referred to as Port, Chemoport or Power Port) is an implanted central venous access device (CVAD) recommended for
Portacath Insertion Patient Information Leaflet What is a Portacath? A Portacath (also referred to as Port, Chemoport or Power Port) is an implanted central venous access device (CVAD) recommended for
Prevent Bloodstream Infections by Using Appropriate Devices
 37 Prevent Bloodstream Infections by Using Appropriate Devices Situation Catheter-associated infections include exit, tunnel, pocket and bloodstream infections. In the United States, when these types of
37 Prevent Bloodstream Infections by Using Appropriate Devices Situation Catheter-associated infections include exit, tunnel, pocket and bloodstream infections. In the United States, when these types of
Intravenous Catheter Complications
 Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
Vascular Access Device-Related Infection Inadequate skin antisepsis prior to VAD insertion Acute onset of fever, chills, and hypotension. No other apparent source of Notify Prescriber immediately Obtain
IV Fluids. Nursing B23. Objectives. Serum Osmolality
 IV Fluids Nursing B23 Objectives Discuss the purpose of IV Discuss nursing interventions in IV therapy Identify complications of IV therapy Differentiate between peripheral line, central line, and PICC
IV Fluids Nursing B23 Objectives Discuss the purpose of IV Discuss nursing interventions in IV therapy Identify complications of IV therapy Differentiate between peripheral line, central line, and PICC
IV Drug Delivery Systems used in Cancer Care
 IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
IV Drug Delivery Systems used in Cancer Care Cheri Constantino-Shor, RN, MSN, CRNI Seattle Cancer Care Alliance Nursing Staff Development Coordinator Presentation Objective Describe drug delivery devices
The High-Flow Port Designed & Indicated for Apheresis
 The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
The High-Flow Port Designed & Indicated for Apheresis Advancing Lives and the Delivery of Health Care Bard is proud to introduce the first and only high-flow, power-injectable port designed and indicated
IV Fluids Nursing B23 Objectives Serum Osmolality 275 to 295 Isotonic
 1 IV Fluids Nursing B23 2 Objectives 3 Serum Osmolality Serum osmolality solute concentration of a solution Higher osmolality means greater pulling power for water Normal serum osmolality is 275 to 295
1 IV Fluids Nursing B23 2 Objectives 3 Serum Osmolality Serum osmolality solute concentration of a solution Higher osmolality means greater pulling power for water Normal serum osmolality is 275 to 295
External Ref: Andres, D.A., et al. Catheter Pinch-Off Syndrome: Recognition and Management.
 Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Complications With Intravenous
Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services Department: Fairview Home Infusion Manual: Policy and Procedure Manual Category: Home Infusion Subject: Complications With Intravenous
Children's (Pediatric) PICC Line Placement
 Scan for mobile link. Children's (Pediatric) PICC Line Placement A peripherally inserted central catheter (PICC line) is most often used to deliver medication over a long period. The doctor or nurse inserts
Scan for mobile link. Children's (Pediatric) PICC Line Placement A peripherally inserted central catheter (PICC line) is most often used to deliver medication over a long period. The doctor or nurse inserts
Instructions For Use. Implantable Ports. with Open-Ended and Groshong Catheters
 Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Instructions For Use Implantable Ports with Open-Ended and Groshong Catheters 3 Implantable Ports with Open-Ended and Groshong Catheters 1 Description The BardPort, SlimPort, and X-Port implantable ports
Central venous catheters
 Follow the link from the online version of this article to obtain certified continuing medical education credits Central venous catheters Reston N Smith, 1 Jerry P Nolan 2 1 North Bristol NHS Trust, Bristol,
Follow the link from the online version of this article to obtain certified continuing medical education credits Central venous catheters Reston N Smith, 1 Jerry P Nolan 2 1 North Bristol NHS Trust, Bristol,
Case Report The Sheared Central Venous Catheter?
 Case Reports in Anesthesiology Volume 2011, Article ID 379827, 4 pages doi:10.1155/2011/379827 Case Report The Sheared Central Venous Catheter? Harihar V. Hegde, 1 Vijay G. Yaliwal, 1 Shyamsundar K. Joshi,
Case Reports in Anesthesiology Volume 2011, Article ID 379827, 4 pages doi:10.1155/2011/379827 Case Report The Sheared Central Venous Catheter? Harihar V. Hegde, 1 Vijay G. Yaliwal, 1 Shyamsundar K. Joshi,
The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections M A R C H 15, 2017
 The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections D A R C Y DOELLMAN M S N, RN, CRNI, VA - BC M A R C H 15, 2017 LOUISVILLE, KENTUCKY Cincinnati Children s Hospital 642
The Impact of Catheter Occlusion in Central Line Associated Bloodstream Infections D A R C Y DOELLMAN M S N, RN, CRNI, VA - BC M A R C H 15, 2017 LOUISVILLE, KENTUCKY Cincinnati Children s Hospital 642
Catheter-directed Thrombolysis
 Scan for mobile link. Catheter-directed Thrombolysis Catheter-directed thrombolysis treats vascular blockages and improves blood flow by dissolving abnormal blood clots. A blood clot, or thrombus, can
Scan for mobile link. Catheter-directed Thrombolysis Catheter-directed thrombolysis treats vascular blockages and improves blood flow by dissolving abnormal blood clots. A blood clot, or thrombus, can
Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
Victoria Chapman BS, RN, HP (ASCP)
 Victoria Chapman BS, RN, HP (ASCP) Considerations: Age Sex Body Composition Hydration Status Chemotherapy Use Access History Considerations: Immunosuppression Use Chemotherapy Frequency of plasma exchanges
Victoria Chapman BS, RN, HP (ASCP) Considerations: Age Sex Body Composition Hydration Status Chemotherapy Use Access History Considerations: Immunosuppression Use Chemotherapy Frequency of plasma exchanges
Identification and Management of Central Vascular Access Device Complications
 Identification and Management of Central Vascular Access Device Complications David Hahn, MD Interventional Radiology NorthShore University HealthSystem Evanston, Illinois Disclosures Consultant, AngioDynamics,
Identification and Management of Central Vascular Access Device Complications David Hahn, MD Interventional Radiology NorthShore University HealthSystem Evanston, Illinois Disclosures Consultant, AngioDynamics,
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5530 Entity: Fairview Pharmacy Services
UTERINE FIBROID EMBOLIZATION
 INTERVENTIONAL RADIOLOGY PROTOCOLS UTERINE FIBROID EMBOLIZATION Interventional Radiology Tower Health Medical Group offers the option to treat uterine fibroids with fibroid embolization (UFE), an alternative
INTERVENTIONAL RADIOLOGY PROTOCOLS UTERINE FIBROID EMBOLIZATION Interventional Radiology Tower Health Medical Group offers the option to treat uterine fibroids with fibroid embolization (UFE), an alternative
2013 Coding Changes. Diagnostic Radiology. Nuclear Medicine
 2013 Coding Changes The principal coding changes affecting Radiologists in 2013 occur in the Interventional Radiology Section of the AMA/CPT Manual. As in the past, we continue to see the Relative Update
2013 Coding Changes The principal coding changes affecting Radiologists in 2013 occur in the Interventional Radiology Section of the AMA/CPT Manual. As in the past, we continue to see the Relative Update
Per-Q-Cath* PICC Catheters with Excalibur Introducer* System
 Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
Bard Access Systems Per-Q-Cath* PICC and Catheters with Excalibur Introducer* System Instructions For Use Table of Contents Table of Contents Page Contents 1 Product Description, Indications & Contraindications
ESPEN Congress Brussels How to take care of central venous access devices (CVAD)? Eva Johansson
 ESPEN Congress Brussels 2005 How to take care of central venous access devices (CVAD)? Eva Johansson How to take care of central venous access devices (CVAD)? Eva Johansson, RN, PhD Division of Hematology
ESPEN Congress Brussels 2005 How to take care of central venous access devices (CVAD)? Eva Johansson How to take care of central venous access devices (CVAD)? Eva Johansson, RN, PhD Division of Hematology
IV therapy. By: Susan Mberenga, RN, MSN. Copyright 2016, 2013, 2010, 2006, 2002 by Saunders, an imprint of Elsevier Inc.
 IV therapy By: Susan Mberenga, RN, MSN 1 IV Therapy Types of solutions Isotonic Hypotonic Hypertonic Caution: Too rapid or excessive infusion of any IV fluid has the potential to cause serious problems
IV therapy By: Susan Mberenga, RN, MSN 1 IV Therapy Types of solutions Isotonic Hypotonic Hypertonic Caution: Too rapid or excessive infusion of any IV fluid has the potential to cause serious problems
Document No. BMB/IFU/40 Rev No. & Date 00 & 15/11/2017 Issue No & Date 01 & 15/11/2017
 Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
Central Venous Catheter Device Description Multi-lumen catheters incorporate separate, non-communicating vascular access lumens within a single catheter body. Minipunctur Access Sets And Trays: Used for
ESPEN Congress Florence 2008
 ESPEN Congress Florence 2008 Complications of Central Venous Catheters EPIDEMIOLOGY & DIAGNOSIS & PREVENTION Federico Bozzetti (Italy) CVC COMPLICATIONS EPIDEMIOLOGY & DIAGNOSIS & PREVENTION Federico Bozzetti
ESPEN Congress Florence 2008 Complications of Central Venous Catheters EPIDEMIOLOGY & DIAGNOSIS & PREVENTION Federico Bozzetti (Italy) CVC COMPLICATIONS EPIDEMIOLOGY & DIAGNOSIS & PREVENTION Federico Bozzetti
Prevention of thrombosis
 Prevention of thrombosis Massimo Lamperti MD, MBA Chief of General Anaesthesia Department Anaesthesiology Institute Cleveland Clinic Abu Dhabi Clinical Professor of Anaesthesiology Cleveland Clinic Lerner
Prevention of thrombosis Massimo Lamperti MD, MBA Chief of General Anaesthesia Department Anaesthesiology Institute Cleveland Clinic Abu Dhabi Clinical Professor of Anaesthesiology Cleveland Clinic Lerner
Surgical approach for DVT. Division of Vascular Surgery Department of Surgery Seoul National University College of Medicine
 Surgical approach for DVT Seung-Kee Min Division of Vascular Surgery Department of Surgery Seoul National University College of Medicine Treatment Options for Venous Thrombosis Unfractionated heparin &
Surgical approach for DVT Seung-Kee Min Division of Vascular Surgery Department of Surgery Seoul National University College of Medicine Treatment Options for Venous Thrombosis Unfractionated heparin &
RESTORING PATENCY TO CENTRAL VENOUS ACCESS DEVICES
 RESTORING PATENCY TO CENTRAL VENOUS ACCESS DEVICES Indications Venous access is poor Intravenous therapy involves venous sclerosants Ambulatory chemotherapy given as an outpatient Repeated sampling, or
RESTORING PATENCY TO CENTRAL VENOUS ACCESS DEVICES Indications Venous access is poor Intravenous therapy involves venous sclerosants Ambulatory chemotherapy given as an outpatient Repeated sampling, or
Over the Wire Technique vs. Modified Seldinger Technique in Insertion of PICC
 Over the Wire Technique vs. Modified Seldinger Technique in Insertion of PICC Deniz Kasikci Department of Radiology, Jena University Hospital Friedrich-Schiller-University, Jena, Germany Disclosure Speaker
Over the Wire Technique vs. Modified Seldinger Technique in Insertion of PICC Deniz Kasikci Department of Radiology, Jena University Hospital Friedrich-Schiller-University, Jena, Germany Disclosure Speaker
Central Venous Line Insertion
 Central Venous Line Insertion Understand the indications and risks of CVC insertion Understand and troubleshoot the seldinger technique Understand available sites and select the appropriate site for clinical
Central Venous Line Insertion Understand the indications and risks of CVC insertion Understand and troubleshoot the seldinger technique Understand available sites and select the appropriate site for clinical
ORIGINAL INVESTIGATION. Single-Lumen Subcutaneous Ports Inserted by Interventional Radiologists in Patients Undergoing Chemotherapy
 ORIGINAL INVESTIGATION Single-Lumen Subcutaneous Ports Inserted by Interventional Radiologists in Patients Undergoing Chemotherapy Incidence of Infection and Outcome of Attempted Catheter Salvage Delia
ORIGINAL INVESTIGATION Single-Lumen Subcutaneous Ports Inserted by Interventional Radiologists in Patients Undergoing Chemotherapy Incidence of Infection and Outcome of Attempted Catheter Salvage Delia
Venous Access and Ports. Helen Starosta
 Venous Access and Ports Helen Starosta Venous access and ports Peripheral IV access Arterio-Venous Fistula Central venous access Peripherally Inserted Central Catheter (PICC) Non Tunnelled Central Venous
Venous Access and Ports Helen Starosta Venous access and ports Peripheral IV access Arterio-Venous Fistula Central venous access Peripherally Inserted Central Catheter (PICC) Non Tunnelled Central Venous
2018 HEMODIALYSIS CATHETERS CODING AND REIMBURSEMENT GUIDE
 2018 HEMODIALYSIS CATHETERS CODING AND REIMBURSEMENT GUIDE Contents Overview of Central Venous Access s for Hemodialysis 2 Procedures Using Hemodialysis s 2 Physician Reimbursement for Hemodialysis s 3
2018 HEMODIALYSIS CATHETERS CODING AND REIMBURSEMENT GUIDE Contents Overview of Central Venous Access s for Hemodialysis 2 Procedures Using Hemodialysis s 2 Physician Reimbursement for Hemodialysis s 3
Patient Information. Peripheral Arterial Disease and the Lutonix 035 Balloon. Advancing Lives and the Delivery of Health Care
 Patient Information Peripheral Arterial Disease and the Lutonix 035 Balloon Advancing Lives and the Delivery of Health Care Contents Peripheral Arterial Disease (PAD) Peripheral Arterial Disease (PAD)
Patient Information Peripheral Arterial Disease and the Lutonix 035 Balloon Advancing Lives and the Delivery of Health Care Contents Peripheral Arterial Disease (PAD) Peripheral Arterial Disease (PAD)
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
The Roles and Responsibilities of Nurse Before and After Laparoscopic Urologic Surgery
 + The Roles and Responsibilities of Nurse Before and After Laparoscopic Urologic Surgery Elif GEZGINCI Gulhane Military Medical Academy School of Nursing Ankara 1 + 2 PREOPERATİVE + Preoperative (Patient
+ The Roles and Responsibilities of Nurse Before and After Laparoscopic Urologic Surgery Elif GEZGINCI Gulhane Military Medical Academy School of Nursing Ankara 1 + 2 PREOPERATİVE + Preoperative (Patient
Long-Term Central Venous Access
 June 01, 2016 By Stephen P. Povoski, MD [1] The use of multidrug chemotherapy and bone marrow transplantation in cancer treatment has made the utilization of reliable, long-term venous access (LTVA) an
June 01, 2016 By Stephen P. Povoski, MD [1] The use of multidrug chemotherapy and bone marrow transplantation in cancer treatment has made the utilization of reliable, long-term venous access (LTVA) an
Central venous access devices for children with lysosomal storage disorders
 Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Central venous access devices for children with lysosomal storage disorders This information explains about central
Peripherally Inserted Central Catheter (PICC) Booklet
 Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
Aintree University Hospital FT PICC Booklet: a real world example This local booklet is an example used in the NICE medical technology guidance adoption support resource for SecurAcath for securing percutaneous
Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE
 DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
DESCRIPTION: Dignity Dual Power Injectable Implantable Infusion Port INSTRUCTIONS FOR USE The Dignity Dual power injectable implantable infusion port is an implantable access device designed to provide
The HeRO Graft. Shawn M. Gage, PA Division of Vascular Surgery Duke University Medical Center
 The HeRO Graft Shawn M. Gage, PA Division of Vascular Surgery Duke University Medical Center Faculty Disclosure I disclose the following financial relationships: CryoLife/Hemosphere, Inc. & W.L. Gore and
The HeRO Graft Shawn M. Gage, PA Division of Vascular Surgery Duke University Medical Center Faculty Disclosure I disclose the following financial relationships: CryoLife/Hemosphere, Inc. & W.L. Gore and
Research Article Can we predict the Position of Central Venous Catheter Tip Following Cannulation of Internal Jugular Vein?
 Cronicon OPEN ACCESS ANAESTHESIA Research Article Can we predict the Position of Central Venous Catheter Tip Following Cannulation of Internal Jugular Vein? Pradeep Marur Venkategowda 1, Surath Manimala
Cronicon OPEN ACCESS ANAESTHESIA Research Article Can we predict the Position of Central Venous Catheter Tip Following Cannulation of Internal Jugular Vein? Pradeep Marur Venkategowda 1, Surath Manimala
Petite Wound Drainage System. 13mm CWV Drainage System. CWSump. CWV with Trocar
 CWVDrain Designed to assure optimum cosmetic results Tapered, hubless design provides atraumatic insertion and removal Internal ribs prevent collapsing or kinking Large 150cc capacity reservoir decreases
CWVDrain Designed to assure optimum cosmetic results Tapered, hubless design provides atraumatic insertion and removal Internal ribs prevent collapsing or kinking Large 150cc capacity reservoir decreases
NOTE: Deep Vein Thrombosis (DVT) Risk Factors
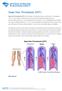 Deep Vein Thrombosis (DVT) Deep Vein Thrombosis (DVT) is the formation of a blood clot, known as a thrombus, in the deep leg vein. It is a very serious condition that can cause permanent damage to the
Deep Vein Thrombosis (DVT) Deep Vein Thrombosis (DVT) is the formation of a blood clot, known as a thrombus, in the deep leg vein. It is a very serious condition that can cause permanent damage to the
PROGNOSIS AND SURVIVAL
 CANCER ASSOCIATED THROMBOSIS PROGNOSIS AND SURVIVAL Since French internist Armand Trousseau reported the occurrence of mysterious thrombotic disorders in cancer patients in the mid-19th century, the link
CANCER ASSOCIATED THROMBOSIS PROGNOSIS AND SURVIVAL Since French internist Armand Trousseau reported the occurrence of mysterious thrombotic disorders in cancer patients in the mid-19th century, the link
PARENTERAL NUTRITION: VASCUAR ACCESS DEVICE SELECTION
 PARENTERAL NUTRITION: VASCUAR ACCESS DEVICE SELECTION Winifred Magambo-Gasana Vascular Access Nurse Practitioner Oxford University Hospitals NHS Foundation Trust Aim An overview of the range of Vascular
PARENTERAL NUTRITION: VASCUAR ACCESS DEVICE SELECTION Winifred Magambo-Gasana Vascular Access Nurse Practitioner Oxford University Hospitals NHS Foundation Trust Aim An overview of the range of Vascular
ESPEN Congress Florence 2008
 ESPEN Congress Florence 2008 PN Guidelines presentation PN Guidelines in home parenteal nutrition M. Staun (Denmark) ESPEN-guidelines for home parenteral nutrition (HPN) Michael Staun, Andre Van Gossum,
ESPEN Congress Florence 2008 PN Guidelines presentation PN Guidelines in home parenteal nutrition M. Staun (Denmark) ESPEN-guidelines for home parenteral nutrition (HPN) Michael Staun, Andre Van Gossum,
Superior vena cava stent
 Radiology department Superior vena cava stent Introduction This leaflet tells you about the procedure known as superior vena cava (SVC) stent insertion. It explains what is involved and what the benefits
Radiology department Superior vena cava stent Introduction This leaflet tells you about the procedure known as superior vena cava (SVC) stent insertion. It explains what is involved and what the benefits
Totally implantable venous access devices in children with cystic fibrosis: incidence and type of complications
 Thorax 1998;53:285 289 285 Thoracic Medicine J Deerojanawong SMSawyer C F Robertson Radiology A M Fink Surgery K B Stokes Royal Children s Hospital, Parkville, Melbourne, Victoria 3052, Australia Correspondence
Thorax 1998;53:285 289 285 Thoracic Medicine J Deerojanawong SMSawyer C F Robertson Radiology A M Fink Surgery K B Stokes Royal Children s Hospital, Parkville, Melbourne, Victoria 3052, Australia Correspondence
INDIANA HEALTH COVERAGE PROGRAMS
 INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
INDIANA HEALTH COVERAGE PROGRAMS PROVIDER CODE TABLES Note: Due to possible changes in Indiana Health Coverage Programs (IHCP) policy or national coding updates, inclusion of a code on the code tables
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS
 PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS As a single-source provider, Bard Access Systems sets the standard for implanted ports and vascular access devices. We make it possible for
PORTS SETTING THE STANDARD WITH A COMPREHENSIVE FAMILY OF PORTS As a single-source provider, Bard Access Systems sets the standard for implanted ports and vascular access devices. We make it possible for
Patient guide: pfm Nit-Occlud PDA coil occlusion system. Catheter occlusion of. Patent Ductus Arteriosus. with the
 Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
Patient guide: Catheter occlusion of Patent Ductus Arteriosus with the pfm Nit-Occlud PDA coil occlusion system pfm Produkte für die Medizin - AG Wankelstr. 60 D - 50996 Cologne Phone: +49 (0) 2236 96
CVAD Flushing Literature
 CVAD Flushing Literature Aquino, V. M., E. S. Sandler, et al. (2002). "A prospective double-blind randomized trial of urokinase flushes to prevent bacteremia resulting from luminal colonization of subcutaneous
CVAD Flushing Literature Aquino, V. M., E. S. Sandler, et al. (2002). "A prospective double-blind randomized trial of urokinase flushes to prevent bacteremia resulting from luminal colonization of subcutaneous
NYU School of Medicine Department of Radiology Rotation-Specific House Staff Evaluation
 Vascular & Interventional Radiology Rotation 1 Core competency in vascular and interventional radiology during the first resident rotation consists of clinical objectives, technical objectives and image
Vascular & Interventional Radiology Rotation 1 Core competency in vascular and interventional radiology during the first resident rotation consists of clinical objectives, technical objectives and image
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide)
 Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Central Venous Catheter Care and Maintenance (includes catheter troubleshooting guide) A Guide for Patients in the Home Phone Number: Nurse/Contact: Central Venous Catheters This manual is a guide for
Thrombin injection vs Conventional Surgical Repair in Treatment of Iatrogenic Post-cath Femoral Artery Pseudoaneurysm (IFAP)
 Kasr El Aini Journal of Surgery VOL., 11, NO 3 September 2010 31 Thrombin injection vs Conventional Surgical Repair in Treatment of Iatrogenic Post-cath Femoral Artery Pseudoaneurysm (IFAP) Farghaly A,
Kasr El Aini Journal of Surgery VOL., 11, NO 3 September 2010 31 Thrombin injection vs Conventional Surgical Repair in Treatment of Iatrogenic Post-cath Femoral Artery Pseudoaneurysm (IFAP) Farghaly A,
Transjugular Liver Biopsy UHB is a no smoking Trust
 Transjugular Liver Biopsy UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm Introduction, benefits and alternatives
Transjugular Liver Biopsy UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm Introduction, benefits and alternatives
Carry this card with you at all times. Show this card to any medical professional treating you. Patient Implant Card. Option ELITE Vena Cava Filter.
 Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
Patient Guide A Safe Option for a Healthier You! P/N: P-2017-0175-00 Rev B 1. Static magnetic field of 3 Tesla or less. 2. Spatial gradient magnetic field of 720 Gauss/cm or less. 3. Maximum whole body
2. Indications Infusion of hyperosmolar medication, e.g. TPN. Administration of vasoactive/irritant drugs.
 Policy and Procedure for Insertion and care of Peripherally Inserted Central Catheters by Neonatal Staff (see Ch 8 TPN) 1. Introduction The peripherally inserted central catheter (PICC) is an intravenous
Policy and Procedure for Insertion and care of Peripherally Inserted Central Catheters by Neonatal Staff (see Ch 8 TPN) 1. Introduction The peripherally inserted central catheter (PICC) is an intravenous
Vascular access device selection & placement. Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University
 Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
Vascular access device selection & placement Alisa Seangleulur, MD Anesthesiology Department, Faculty of Medicine, Thammasat University How to make the right choice of vascular access device.. Peripheral
3. Urinary Catheters. Indications. Methods of Bladder Catheterization. Hashim Hashim
 3. Urinary Catheters Hashim Hashim Indications Urinary catheters are used to drain urine from the bladder. The main indications are: A. Diagnostic Measure post-void residual in the absence of ultrasound
3. Urinary Catheters Hashim Hashim Indications Urinary catheters are used to drain urine from the bladder. The main indications are: A. Diagnostic Measure post-void residual in the absence of ultrasound
Kristin Wise, MD, FHM Division of General Internal Medicine and Geriatrics Hospital Medicine 2013
 Kristin Wise, MD, FHM Division of General Internal Medicine and Geriatrics Hospital Medicine 2013 Objectives for CVC Placement Understand the indications and contraindications Determine appropriate CVC
Kristin Wise, MD, FHM Division of General Internal Medicine and Geriatrics Hospital Medicine 2013 Objectives for CVC Placement Understand the indications and contraindications Determine appropriate CVC
Determination of complication rate of PICC lines in Oncological Patients
 Original Article Determination of complication rate of PICC lines in Oncological Patients Ghulam Haider, Shiyam Kumar, Basit Salam, Nehal Masood, Asim Jamal, Yasmeen Abdul Rasheed Section Hematology, Oncology
Original Article Determination of complication rate of PICC lines in Oncological Patients Ghulam Haider, Shiyam Kumar, Basit Salam, Nehal Masood, Asim Jamal, Yasmeen Abdul Rasheed Section Hematology, Oncology
Health Bites Breast Cancer. Breast Cancer. Normal breast
 Health Bites Breast Cancer Breast Cancer Normal breast The normal breast tissue varies in size and shape. The breasts rest in front of the rib cage. The breasts are made up of fatty tissue, milk ducts
Health Bites Breast Cancer Breast Cancer Normal breast The normal breast tissue varies in size and shape. The breasts rest in front of the rib cage. The breasts are made up of fatty tissue, milk ducts
Choosing the right access for long term parenteral nutrition: PICC lines or tunnelled catheters G. Goossens (BE)
 ESPEN Congress Lisbon 2015 HOW TO MAKE HOME PARENTERAL NUTRITION SAFER Choosing the right access for long term parenteral nutrition: PICC lines or tunnelled catheters G. Goossens (BE) Choosing the right
ESPEN Congress Lisbon 2015 HOW TO MAKE HOME PARENTERAL NUTRITION SAFER Choosing the right access for long term parenteral nutrition: PICC lines or tunnelled catheters G. Goossens (BE) Choosing the right
Sid Bhende MD Sentara Vascular Specialists April 28 th Dialysis Access Review: Understanding the Access Options our Patients Face
 Sid Bhende MD Sentara Vascular Specialists April 28 th 2018 Dialysis Access Review: Understanding the Access Options our Patients Face Disclosures Dialysis Background Why is it important? Outline National
Sid Bhende MD Sentara Vascular Specialists April 28 th 2018 Dialysis Access Review: Understanding the Access Options our Patients Face Disclosures Dialysis Background Why is it important? Outline National
Ultrasound Guided Vascular Access. 7/25/2016
 Ultrasound Guided Vascular Access 7/25/2016 www.ezono.com 1 Objectives Indications for insertion of central and peripheral lines Complications associated with procedures Role of ultrasound in vascular
Ultrasound Guided Vascular Access 7/25/2016 www.ezono.com 1 Objectives Indications for insertion of central and peripheral lines Complications associated with procedures Role of ultrasound in vascular
1 Description. 2 Indications. 3 Warnings ASPIRATION CATHETER
 Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Page 1 of 5 ASPIRATION CATHETER Carefully read all instructions prior to use, observe all warnings and precautions noted throughout these instructions. Failure to do so may result in complications. STERILE.
Ultrasound-guided infraclavicular axillary vein cannulation for central venous access {
 British Journal of Anaesthesia 93 (2): 188 92 DOI: 10.1093/bja/aeh187 Advance Access publication June 25, 2004 Ultrasound-guided infraclavicular axillary vein cannulation for central venous access { A.
British Journal of Anaesthesia 93 (2): 188 92 DOI: 10.1093/bja/aeh187 Advance Access publication June 25, 2004 Ultrasound-guided infraclavicular axillary vein cannulation for central venous access { A.
Advocate Christ Medical Center CVC Placement Certification Course
 Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
Advocate Christ Medical Center CVC Placement Certification Course July 12th, 2012 Hannah Watts, MD Medical Simulation Director Modified August 10, 2017 Taajwar Khan, MD Chief Resident of Internal Medicine
Is it Necessary to Verify Blood Return in Monthly Port Flushes?
 Is it Necessary to Verify Blood Return in Monthly Port Flushes? Gloria B. Ascoli, RN, CRNI, Amy C. Brown, BSN, RN, Jessica L. Cooper, BSN, RN, Allison N. Crawford, BSN, RN, CRNI Background Research Aims
Is it Necessary to Verify Blood Return in Monthly Port Flushes? Gloria B. Ascoli, RN, CRNI, Amy C. Brown, BSN, RN, Jessica L. Cooper, BSN, RN, Allison N. Crawford, BSN, RN, CRNI Background Research Aims
