SYSTEM ELIGIBILITY BATTERY LONGEVITY
|
|
|
- Linda Blake
- 5 years ago
- Views:
Transcription
1 SYSTEM ELIGIBILITY BATTERY LONGEVITY Neurostimulation systems for deep brain stimulation Reference manual! USA Rx only 0123
2
3 Explanation of symbols on product and package labeling for non-usa audiences. For USA audiences only 0123 Conformité Européenne (European Conformity). This symbol means that the device fully complies with AIMD Directive 90/385EEC. SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 1
4 Medtronic, Activa PC, Activa RC, Kinetra, and Soletra are registered trademarks of Medtronic, Inc. 2 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
5 Table of contents Introduction 4 System Eligibility 4 Test stimulation devices 4 Test stimulation using the Model External Neurostimulator 5 Test stimulation using the Model 3625 Test Stimulator 8 Screening for patient eligibility 8 Battery longevity and maintenance 11 Estimating how frequently the battery requires charging 11 Tips for maximizing battery longevity and recharge intervals 11 Activa PC Neurostimulator battery longevity 11 Estimating battery longevity for the Activa PC Neurostimulator 12 Activa RC Neurostimulator battery longevity 20 Refer to the indications sheet for indications and related information. Refer to the appropriate information for prescribers booklet for contraindications, warnings, precautions, adverse events summary, individualization of treatment, patient selection, use in specific populations, resterilization, and component disposal. Refer to the device implant manual for device description, package contents, device specifications, and instructions for use.! USA Refer to the clinical summary booklet packaged with the neurostimulator for information on the clinical study results of the neurostimulation system and individualization of treatment. SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 3
6 Introduction This manual is divided into two parts: System eligibility: For movement disorders therapies, this part describes how to use test stimulation to aid in neurostimulator selection. Battery longevity and maintenance: This part describes how to maximize longevity and recharge intervals and how to estimate longevity based on programmed settings. System Eligibility For movement disorders therapies, you can use test stimulation results to guide you in selecting an appropriate implantable neurostimulator for your patient. When you find settings that, during test stimulation, provide the maximum symptom suppression available to your patient, identify the implantable neurostimulator that can most closely approximate those parameters. Remember to take into account the fact that not all neurostimulators have identical capabilities. For example, some neurostimulators will accommodate two leads, while others will not. Prior to test stimulation and implant, the following should also be considered: Patient capability (eg, can the patient effectively use the recharger and/or the patient programmer associated with the neurostimulator considered for use) Cosmetics (eg, is the neurostimulator too large to be comfortable for the patient) Different implantable neurostimulators have different limitations for various combinations of pulse width, rate, and amplitude. You might find that a specific combination of these parameters is accepted during test stimulation, but cannot be duplicated by all available implantable neurostimulators. Additionally, replacement of one type of neurostimulator with another can result in required parameter changes. For example, replacement of a Soletra neurostimulator with a Kinetra neurostimulator typically requires an increase of 25% in programmed voltage to maintain equivalent symptom suppression. Finally, remember that not all implantable neurostimulators offer identical expanded features. Test stimulation devices The Model External Neurostimulator and Model 3625 Test Stimulator can be used to assess the suitability of an Activa PC Model 37601, Activa RC Model 37612, Kinetra Model 7428, or Soletra Model 7426 Neurostimulator. Refer to Table 1 to identify: 4 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
7 which implantable neurostimulators to consider based on the number of programs that successful test stimulation results require. when to use the test stimulation maximum amplitude tables. Table 1. Test stimulation devices and implantable neurostimulators Test stimulation device Test stimulation compared to implantable neurostimulators Model External Neurostimulator For constant voltage mode, not all parameter settings available on the external neurostimulator during test stimulation can be duplicated with an Activa PC implantable neurostimulator. Refer to Table 2 for Activa PC constant voltage eligibility data. The Activa RC implantable neurostimulator is capable of duplicating all programming parameters available on the external neurostimulator. For constant current mode, parameter settings available on the external neurostimulator during test stimulation are identical to those available on all implantable neurostimulators. Model 3625 Test Stimulator Not all parameter settings available on the test stimulator during test stimulation can be duplicated with all available implantable neurostimulators. Refer to the programming parameter information for Kinetra and Soletra neurostimulators in the appropriate neurostimulator implant manual and in the Model 3625 Test Stimulator manual. Programming instructions for the Model External Neurostimulator are located in the Model 8870 programming guide for Activa RC and Activa PC neurostimulation systems. User instructions for the Model 3625 Test Stimulator are located in the Model DBS Lead Manual. Test stimulation using the Model External Neurostimulator Neurostimulation systems may have limited stimulation adjustments available with certain combinations of high amplitude, pulse width, and rate settings. A message will appear on the clinician programmer if you try to program these types of parameter combinations with the Model External Neurostimulator. You should lower the amplitude and: decrease the rate, or increase the pulse width(s), or change the selected electrodes, or reposition the lead(s). SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 5
8 Refer to Table 2 or Table 3 for a presentation of specific parameter combinations that will be accepted when you program the Model Activa PC Neurostimulator or the Model Activa RC Neurostimulator. Note: Because testing with the Model External Neurostimulator and the Model 3625 Test Stimulator is performed one hemisphere at a time, you might see acceptable test stimulation with single-hemisphere testing but still encounter limited available programming parameters during bilateral use. Table 2. Model Activa PC Nonrechargeable Neurostimulator Maximum programmable amplitude at high rate/pulse width combinations using constant voltage No. of programs intended for use Rate (Hz) Pulse width (µsec) a V 10.5V 10.5V 10.5V 10.5V 10.5V V 10.5V 10.5V 10.5V 10.5V 10.0V V 10.5V 10.5V 10.5V 10.5V 9.3V V 10.5V 10.5V 10.5V 10.15V 8.75V V 10.5V 10.5V 10.5V 9.5V 8.1V V 10.5V 10.5V 10.4V 9.15V 7.75V V 10.5V 10.5V 10.05V 8.85V 7.4V V 10.5V 10.5V 9.75V 8.55V 7.1V V 10.5V 10.3V 9.35V 8.1V 6.7V 2 b V 10.5V 10.5V 10.5V 10.5V 10.5V V 10.5V 10.5V 10.5V 10.5V 10.0V V 10.5V 10.5V 10.5V 10.5V 9.3V V 10.5V 10.5V 10.5V 9.5V 8.1V V 10.5V 10.5V 10.05V 8.85V 7.4V V 10.5V 10.45V 9.45V 8.25V 6.8V V 10.5V 10.3V 9.35V 8.1V 6.7V V 10.5V 9.85V 8.9V 7.65V 6.25V V 10.5V 9.35V 8.4V 7.15V 5.75V V 10V 8.85V 7.9V 6.65V 5.25V V 9.55V 8.4V 7.45V 6.2V 4.8V V 9.1V 7.95V 7V 5.75V 4.35V V 8.5V 7.35V 6.4V 5.15V 3.75V 3 or 4 c V 10.5V 10.5V 10.5V 10.5V 10.5V 3 or V 10.5V 10.5V 10.5V 10.5V 9.75V 3 or V 10.5V 10.5V 10.5V 9.5V 8.1V 3 or V 10.5V 10.45V 9.45V 8.25V 6.8V 3 or V 10.5V 9.35V 8.4V 7.15V 5.75V 3 or V 9.55V 8.4V 7.45V 6.2V 4.8V 6 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
9 Table 2. Model Activa PC Nonrechargeable Neurostimulator Maximum programmable amplitude at high rate/pulse width combinations using constant voltage (continued) No. of programs intended for use Rate (Hz) Pulse width (µsec) or V 8.7V 7.55V 6.6V 5.35V 3.95V 3 or V 8.5V 7.35V 6.4V 5.15V 3.75V a In this instance, the lead configuration is 1X4 and only one program is defined. b In this instance, the lead configuration is 2X4, and one program is defined per lead/hemisphere. c In this instance, the lead configuration is 2X4, and a second program is in use on one or both leads or in one or both hemispheres. Table 3. Model Activa RC Rechargeable Neurostimulator Maximum programmable amplitude at high rate/pulse width combinations No. of programs intended for use Rate (Hz) Pulse width (µsec) a V 10.5 V 10.5 V 10.5 V 10.5 V 10.5 V V 10.5 V 10.5 V 10.5 V 10.5 V V V 10.5 V 10.5 V 10.5 V 10.5 V V V 10.5 V 10.5 V 10.5 V 10.5 V 10.1 V V 10.5 V 10.5 V 10.5 V 10.4 V 9.9 V 2 b V 10.5 V 10.5 V 10.5 V V 10.5 V V 10.5 V 10.5 V 10.5 V 10.5 V V V 10.5 V 10.5 V 10.5 V 10.5 V 9.95 V V 10.5 V 10.5 V 10.5 V 10.5 V 9.9 V V 10.5 V 10.5 V 10.5 V 10.5 V 9.7 V V 10.5 V 10.5 V 10.5 V 10.4 V 9.45 V V 10.5 V 10.5 V 10.5 V V 9.20 V V 10.5 V 10.5 V 10.5 V 9.9 V 8.95 V V 10.5 V 10.5 V 10.5 V 9.65 V 8.7 V V 10.5 V 10.5 V V 9.25 V 8.3 V 3 or 4 c V 10.5 V 10.5 V 10.5 V 10.5 V 10.5 V 3 or V 10.5 V 10.5 V 10.5 V 10.5 V 9.95 V 3 or V 10.5 V 10.5 V 10.5 V 10.5 V 9.45 V 3 or V 10.5 V 10.5 V 10.5 V 9.90 V 8.95 V 3 or V 10.5 V 10.5 V V 9.35 V 8.45 V SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 7
10 Table 3. Model Activa RC Rechargeable Neurostimulator Maximum programmable amplitude at high rate/pulse width combinations (continued) No. of programs intended for use Rate (Hz) Pulse width (µsec) or V 10.5 V 10.5 V 9.95 V 9.25 V 8.3 V a In this instance, the lead configuration is 1X4 and only one program is defined. b In this instance, the lead configuration is 2X4, and one program is defined per lead/hemisphere. c In this instance, the lead configuration is 2X4, and a second program is in use on one or both leads or in one or both hemispheres. Remember that the amplitude available with one program on the external neurostimulator might be greater than the amplitude available on the implantable neurostimulator when all programs are taken into consideration. If satisfactory symptom suppression cannot be achieved within the parameters provided in the test stimulation maximum amplitude tables, that neurostimulator is not an appropriate device for the patient. Test stimulation using the Model 3625 Test Stimulator When using a Model 3625 Test Stimulator: If the test stimulation amplitude exceeds the maximum amplitude for a specific rate and pulse width, reduce the rate until you are at or below a rate/pulse width/amplitude displayed in the table. If the test stimulation pulse width is greater than 450 microseconds, reduce the pulse width to 450 microseconds and gradually increase the amplitude to confirm satisfactory symptom suppression. A five to ten percent increase in amplitude should be expected. If the test stimulation rate is greater than 250 Hz, reduce the rate to 250 Hz and gradually increase the amplitude to confirm satisfactory symptom suppression. Screening for patient eligibility For Kinetra and Soletra Neurostimulators, when performing test stimulation with either the Model External Neurostimulator or the Model 3625 Test Stimulator, verify that the screening parameters do not exceed the maximum amplitude and pulse width combinations shown in Table 4 and Table 5. If test stimulation is unsuccessful, the system should not be implanted. Table 4. Maximum amplitude (volts) available for specific rate/pulse width combinations on the Model 7428 Kinetra Neurostimulator a Pulse width (µseconds) Rate (Hz) ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
11 Table 4. Maximum amplitude (volts) available for specific rate/pulse width combinations on the Model 7428 Kinetra Neurostimulator a (continued) Pulse width (µseconds) a This table is based on data collected with a 1000-ohm resistive load. If the screening rate is between table settings, use the higher rate. SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 9
12 Table 5. Maximum amplitude (volts) available for specific rate/pulse width combinations on the Model 7426 Soletra Neurostimulators a Pulse width (µseconds) Rate (Hz) a This table is based on data collected with a 1000-ohm resistive load. If the screening rate is between table settings, use the higher rate. When assessing the suitability of a neurostimulation system, ensure parameter settings used to achieve symptom suppression are not within the charge density warning area shown in Figure 1. Note: The amplitude and pulse width limits in Figure 1 were computed for resistances ranging from 500 to 2000 ohms, lead surface areas of 0.06 cm 2, and a charge density threshold of 30 microcoulombs/cm 2 /phase. Amplitude (v) Pulse width (µsec) Figure 1. Charge density with Parkinson's disease clinical mean resistance. 10 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
13 Battery longevity and maintenance The Activa RC neurostimulator rechargeable battery requires that a minimum charge be maintained in order for the battery to reach maximum longevity. Estimating how frequently the battery requires charging The amount of time before the battery requires charging is affected by patient use, the number of active programs, and the stimulation settings in each program. The majority of patients can expect to recharge their neurostimulators every ten days. Higher stimulation settings will require more frequent recharging sessions. Patients should define a recharge schedule that meets their individual needs while maintaining a charge level that is capable of sustaining programmed stimulation settings. Battery longevity and brain target selection # Caution: Stimulation settings for systems implanted in the internal Globus Pallidus (GPi) may be higher than stimulation settings for systems implanted in the Subthalmic Nucleus (STN). Consequently, when implanted in the GPi, rechargeable systems may need to be charged more frequently and nonrechargeable systems may have reduced battery longevity compared to systems implanted in the STN. Tips for maximizing battery longevity and recharge intervals To maximize the battery longevity of a non-rechargeable implantable neurostimulator or the recharge interval of a rechargeable implantable neurostimulator, follow these tips: Use fewer programs, if applicable. If medically appropriate and effective in managing symptom control, program cycling to provide the shortest amount of time for effective stimulation. Use the minimum number of electrodes necessary for effective symptom suppression. Note: Adhere to the guidelines in the Model 7426 Soletra Neurostimulator literature for programming cycling. Use the lowest effective settings for amplitude, rate, and pulse width. For the same parameters, bipolar stimulation provides longer battery life than unipolar stimulation. Activa PC Neurostimulator battery longevity The battery of an Activa PC Neurostimulator can last for months or years, depending on the following factors: SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 11
14 Programmed parameters (ie, amplitude, rate, pulse width, number of active electrodes used, number of programs per lead) System impedance Hours per day of stimulation The degree of patient control over programmable stimulation parameters Estimating battery longevity for the Activa PC Neurostimulator The following formula estimates the approximate period of time that a new Activa PC Neurostimulator battery will last. The estimation is based on the settings for one group. Expected values 1. The estimation formula is based on expected programmed values, the modes of operation, the measured impedance, and how often the therapy is used: Program 1 Program 2 Program 3 Program 4 Amplitude: V/mA Amplitude: V/mA Amplitude: V/mA Amplitude: V/mA Rate a : Hz Rate a : Hz Rate a : Hz Rate a : Hz Pulse width: μsec Pulse width: μsec Pulse width: μsec Pulse width: μsec Impedance: ohms Impedance: ohms Impedance: ohms Impedance: ohms Modes of operation and use Stimulation/day: Hours/day Cycle ON/OFF times: ON (if not applicable, cycling ratio equals 1) OFF a Rates for Programs 1, 2, 3, and 4 are the same. Program 1 (P1) 2. Using the amplitude, rate, and pulse width, locate the energy use (EU) for Program 1 from Table 6 (if constant voltage is applied) or from Table 8 (if constant current is applied): 3. Locate the Impedance Correction Factor (ICF) for Program 1 based on the measured impedance from Table 7 (constant voltage) or Table 9 (constant current):. 4. Compute the Program 1 Factor (P1F): P1EU P1ICF = P1F 12 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
15 Program 2 (P2) 5. For a second program, repeat steps 1 3 to obtain the Program 2 Factor (P2F): P2EU P2ICF = P2F Program 3 (P3) 6. For a third program, repeat steps 1 3 to obtain the Program 3 Factor (P3F): P3EU P3ICF = P3F Program 4 (P4) 7. For a fourth program, repeat steps 1 3 to obtain the Program 4 Factor (P4F): P4EU P4ICF = P4F Computing the multi-program factor (MPF) 8. Compute the MPF (use 0 for unused programs): P1F + P2F + P3F + P4F = MPF 9. Determine the Usage Correction Factor (UCF) using the hours of stimulation per day and the cycle ON/OFF times: Usage Correction Factor = Usage Ratio Cycling Ratio (if applicable) Usage Ratio = hours of stimulation per day 24 hours Cycling Ratio = cycle ON time (cycle ON time + cycle OFF time) 10. Compute the adjusted Energy Use value: MPF UCF + [24.4 x (1 - UCF )] = Adjusted Energy Use 11. Use the adjusted Energy Use value and determine the battery longevity from Figure 2. Following are examples for estimating battery longevity for the Activa PC Neurostimulator. Calculating battery longevity for the Activa PC Neurostimulator in constant voltage mode The patient's neurostimulator is programmed with amplitude, rate, and pulse width settings that equate to an energy use figure of 89 for Program 1 and 67 for Program 2 (refer to Table 6 to obtain the energy use figure). The patient uses the neurostimulator 16 hours per day in continuous mode. The neurostimulator impedance is 1000 ohms for Program 1 and 750 ohms for Program 2. The resulting impedance correction factors (ICRs) from Table 7 are: Program 1: Impedance = 1000 ohms; ICR = 1.00 Program 2: Impedance = 750 ohms; ICR = 1.20 SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 13
16 Program 1 (P1) P1EU P1ICF P1F 89 X 1.00 = Program 2 (P2) P2EU P2ICF P2F 67 X 1.20 = Computing the multiprogram factor (use 0 for unused programs) P1F P2F P3F P4F MPF = Usage correction factor = Usage ratio X Cycling ratio Usage ratio = 16 hours of stimulation / 24 hours = 0.66 Cycling ratio = Continuous = 1.0 Usage correction factor = 0.66 X 1.0 = 0.66 Adjusted energy use value for four programs: MPF UCF UCF AEU X [24.4 X (1-0.66)] = Use the adjusted energy use to find the battery longevity from the graph in Figure 2. Calculating battery longevity for the Activa PC Neurostimulator in constant current mode The patient's neurostimulator is programmed with amplitude, rate, and pulse width settings that equate to an energy use figure of 65 for Program 1 and 77 for Programs 2, 3, and 4 (refer to Table 7 to obtain the energy use figure). The patient uses the neurostimulator 16 hours per day in cycling mode (10 seconds on/10 seconds off). The neurostimulator impedance is 1000 ohms for program 1, 1400 ohms for program 2, 1200 ohms for program 3, and 1100 ohms for program 4. The resulting impedance correction factors (ICRs) from Table 9 are: Program 1: Impedance = 1000 ohms; ICR = 1.00 Program 2: Impedance = 1400 ohms; ICR = 1.32 Program 3: Impedance = 1200 ohms; ICR = 1.16 Program 4: Impedance = 1100 ohms; ICR = ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
17 Program 1 (P1) P1EU P1ICF P1F 65 X 1.00 = Program 2 (P2) P2EU P2ICF P2F 77 X 1.32 = Program 3 (P3) P3EU P3ICF P3F 77 X 1.16 = Program 4 (P4) P4EU P4ICF P4F 77 X 1.08 = Computing the multiprogram factor P1F P2F P3F P4F MPF = Usage correction factor = Usage ratio X Cycling ratio Usage ratio = 16 hours of stimulation / 24 hours = 0.66 Cycling ratio = 10 seconds on / (10 seconds on + 10 seconds off) = 0.5 Usage correction factor = 0.66 X 0.5 = 0.33 Adjusted energy use value for four programs: MPF UCF UCF AEU X [24.4 X (1-0.33)] = Use the adjusted energy use to find the battery longevity from the graph in Figure 2. SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 15
18 Longevity Estimate (years) Adjusted Energy Use Figure 2. Activa PC neurostimulator longevity estimates (years) for energy use. 16 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
19 Table 6. Activa PC Neurostimulator energy use a for constant voltage Pulse width Amplitude (V) Rate NA NA NA NA NA NA SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 17
20 Table 6. Activa PC Neurostimulator energy use a for constant voltage (continued) Pulse width Amplitude (V) Rate NA NA NA NA a Use values that are closest to the expected values (round to the next highest value). Table 7. Activa PC Neurostimulator final impedance correction factor (ICF) for constant voltage a Impedance ICF b c 0.50 a Use value closest to the measured impedance value (round to the next lowest impedance value). b With a programmed amplitude of 1V, calculated longevity may overestimate actual longevity by 1 to 2 years. c Calculated longevity may overestimate actual longevity by 1 to 2 years. 18 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
21 Table 8. Activa PC Neurostimulator energy use a for constant current Pulse width Amplitude (ma) Rate NA NA NA NA NA NA NA NA NA NA a Use values that are closest to the expected values (round to the next highest value). SYSTEM ELIGIBILITY, BATTERY LONGEVITY ENGLISH 19
22 Table 9. Activa PC Neurostimulator final impedance correction factor (ICF) for constant current a Impedance ICF 500 b b a Use value closest to the measured impedance value (round to the next highest impedance value). b With a programmed amplitude of 1 ma, calculated longevity may overestimate actual longevity by 1 to 2 years. Activa RC Neurostimulator battery longevity The Activa RC neurostimulator battery will provide 9 years of operation. Over time, the neurostimulator battery will need more frequent recharges. Like all rechargeable batteries, use over time and repeated recharge cycles reduce the maximum charge capacity of the neurostimulator battery. When the neurostimulator battery reaches 8 years of operation, the elective replacement indicator (ERI) message is displayed in the Observations box on the clinician programmer screen and on the patient programmer screen. A replacement neurostimulator is needed within 12 months. At 9 years, the end of service (EOS) message is displayed in the Observations box on the clinician programmer screen, the patient programmer screen, and the recharger screen. At this time, the neurostimulator no longer provides stimulation and a replacement neurostimulator is needed. 20 ENGLISH SYSTEM ELIGIBILITY, BATTERY LONGEVITY
23 Contacts: Asia: Medtronic International Ltd. Tel Fax Medtronic Asia Ltd. Tel. (02) Fax (02) Australia: Medtronic Australasia Pty. Ltd. Tel Fax Toll free Austria: Medtronic Österreich GmbH Tel Fax Belgium: Medtronic Belgium S.A. Tel Fax Canada: Medtronic of Canada Ltd. Tel. (1905) Fax (1905) Czech Republic: Medtronic Czechia s.r.o. Tel Fax Denmark: Medtronic Danmark A/S Tel Fax Finland: Medtronic Finland OY/LTD Tel. (09) Fax (09) France: Medtronic France S.A.S. Tel Fax Germany: Medtronic GmbH Tel. (0211) Fax (0211) Greece: Medtronic Hellas S.A. Tel Fax Hungary: Medtronic Hungária Kft. Tel Fax Ireland: Medtronic Ireland Ltd. Tel. (01) Fax (01) Italy: Medtronic Italia SpA Tel Fax Tel Fax Japan: Medtronic Japan Tel Fax Latin America: Medtronic, Inc. Tel. (1305) Fax (1786) Norway: Medtronic Norge AS Tel Fax Poland: Medtronic Poland Sp. z.o.o. Tel. (022) Fax (022) Portugal: Medtronic Portugal, Lda. Tel Fax Russia: Medtronic Russia Tel. (8495) Fax (8495) Spain: Medtronic Ibérica, S.A. Tel Fax Sweden: Medtronic AB Tel Fax Switzerland: Medtronic (Schweiz) AG Tel Fax The Netherlands: Medtronic B.V. Tel. (045) Fax (045) U.K.: Medtronic U.K. Ltd. Tel Fax USA: Medtronic, Inc. Tel. (1-763) Toll-free: (1-800) Fax (1-763)
24 Manufacturer Medtronic, Inc. 710 Medtronic Parkway Minneapolis, MN USA Internet: Tel Fax Medtronic E.C. Authorized Representative/Distributed by Medtronic B.V. Earl Bakkenstraat PJ Heerlen The Netherlands Tel Fax Europe/Africa/Middle East Headquarters Medtronic International Trading Sàrl Route du Molliau 31 Case Postale CH-1131 Tolochenaz Switzerland Internet: Tel Fax Asia-Pacific Medtronic International Ltd. Suite /F, Manulife Plaza The Lee Gardens, 33 Hysan Avenue Causeway Bay Hong Kong Tel Fax Contacts for specific countries are listed inside this cover. *M929534A001* Medtronic, Inc All Rights Reserved M929534A001
RestoreSensor Implant manual. Multi-program rechargeable neurostimulator. ! USA Rx only
 RestoreSensor 37714 Multi-program rechargeable neurostimulator Implant manual! USA Rx only 2010 Explanation of symbols on product or package labeling Refer to the appropriate product for symbols that
RestoreSensor 37714 Multi-program rechargeable neurostimulator Implant manual! USA Rx only 2010 Explanation of symbols on product or package labeling Refer to the appropriate product for symbols that
MRI guidelines for Medtronic neurostimulation systems for chronic pain
 MRI guidelines for Medtronic neurostimulation systems for chronic pain See "START HERE" section before conducting MRI. Instructions for use! USA Rx only Explanation of symbols on product or package labeling
MRI guidelines for Medtronic neurostimulation systems for chronic pain See "START HERE" section before conducting MRI. Instructions for use! USA Rx only Explanation of symbols on product or package labeling
Activa RC and Activa PC
 Activa RC and Activa PC neurostimulators for deep brain stimulation From the company with the most experience in DBS The Activa Family Innovative programming platform More choices for patients With the
Activa RC and Activa PC neurostimulators for deep brain stimulation From the company with the most experience in DBS The Activa Family Innovative programming platform More choices for patients With the
Tell the doctor who prescribed your MRI scan that you have an implanted Medtronic neurostimulation system.
 Before Your I For patients with Medtronic neurostimulation systems for chronic pain The following materials are intended to be used by implant physicians with patients who are to receive Medtronic neurostimulation
Before Your I For patients with Medtronic neurostimulation systems for chronic pain The following materials are intended to be used by implant physicians with patients who are to receive Medtronic neurostimulation
Neurostimulation Systems
 Neurostimulation Systems Expanding the Array of Pain Control Solutions PROVEN EFFECTIVE FOR SIMPLE PAIN ITREL 3 SYSTEM For simple neuropathic pain Totally implantable for enhanced quality of life Easy
Neurostimulation Systems Expanding the Array of Pain Control Solutions PROVEN EFFECTIVE FOR SIMPLE PAIN ITREL 3 SYSTEM For simple neuropathic pain Totally implantable for enhanced quality of life Easy
InterStim Therapy InterStim II Model 3058 Neurostimulator InterStim Model 3023 Neurostimulator
 InterStim Therapy InterStim II Model 3058 Neurostimulator InterStim Model 3023 Neurostimulator 3058 3023 Implant manual Rx only Explanation of symbols on product or package labeling Open here Do not reuse
InterStim Therapy InterStim II Model 3058 Neurostimulator InterStim Model 3023 Neurostimulator 3058 3023 Implant manual Rx only Explanation of symbols on product or package labeling Open here Do not reuse
ITREL 3 Neurostimulator
 ITREL 3 Neurostimulator 7425 Implant manual c Rx only 1995 Explanation of symbols on product or package labeling Refer to the appropriate product for symbols that apply. L k r w h m Open here Do not reuse
ITREL 3 Neurostimulator 7425 Implant manual c Rx only 1995 Explanation of symbols on product or package labeling Refer to the appropriate product for symbols that apply. L k r w h m Open here Do not reuse
Micra MC1VR01. MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR)
 Micra MC1VR01 MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR) MRI Technical Manual The following list includes trademarks or registered
Micra MC1VR01 MRI procedural information for Micra Model MC1VR01 MR Conditional single chamber transcatheter pacing device (VVIR) MRI Technical Manual The following list includes trademarks or registered
PATIENT PROGRAMMER 37642
 PATIENT PROGRAMMER 37642 Medtronic DBS Therapy user manual Activa PC Model 37601 Activa RC Model 37612 Activa SC Model 37602 Activa SC Model 37603! USA Rx only 2008 Medtronic, Activa, and SoftStart/Stop
PATIENT PROGRAMMER 37642 Medtronic DBS Therapy user manual Activa PC Model 37601 Activa RC Model 37612 Activa SC Model 37602 Activa SC Model 37603! USA Rx only 2008 Medtronic, Activa, and SoftStart/Stop
Activa RC Neurostimulator MEDTRONIC DBS CHARGING EXPERIENCE
 Activa RC Neurostimulator MEDTRONIC DBS CHARGING EXPERIENCE MEDTRONIC DBS CHARGING EXPERIENCE CONTENTS The 37651 charging system Quick guide Charging system components Checking the battery status Charger
Activa RC Neurostimulator MEDTRONIC DBS CHARGING EXPERIENCE MEDTRONIC DBS CHARGING EXPERIENCE CONTENTS The 37651 charging system Quick guide Charging system components Checking the battery status Charger
Medtronic DBS Therapy Implanted neurostimulators
 Medtronic, Activa, Soletra, and Kinetra are registered trademarks of Medtronic, Inc. DBS is a trademark of Medtronic, Inc. Medtronic DBS Therapy Implanted neurostimulators Information for prescribers c
Medtronic, Activa, Soletra, and Kinetra are registered trademarks of Medtronic, Inc. DBS is a trademark of Medtronic, Inc. Medtronic DBS Therapy Implanted neurostimulators Information for prescribers c
INL No. A0075 Project Medtronic Patient Information Leaflet Description 16 page booklet
 INL No. A0075 Project Medtronic Patient Information Leaflet Description 16 page booklet www.inl-agency.com PATIENT INFORMATION BOOKLET Endovascular stent grafts: A treatment for Abdominal Aortic Aneurysms
INL No. A0075 Project Medtronic Patient Information Leaflet Description 16 page booklet www.inl-agency.com PATIENT INFORMATION BOOKLET Endovascular stent grafts: A treatment for Abdominal Aortic Aneurysms
Advisa MRI and Ensura MRI SureScan pacing systems
 Advisa MRI and Ensura MRI SureScan pacing systems MR Conditional dual chamber and single chamber SureScan pacing systems with SureScan leads MRI Technical Manual The following list includes trademarks
Advisa MRI and Ensura MRI SureScan pacing systems MR Conditional dual chamber and single chamber SureScan pacing systems with SureScan leads MRI Technical Manual The following list includes trademarks
Canada Medtronic of Canada Ltd Kitimat Road Mississauga, Ontario L5N 1W3 Canada Tel Fax
 What PATIENTS About TUNA Say Therapy TUNA THERAPY I would consider it to be a complete success. I have essentially no residual problems of any kind. I m back to the way I was before I ever had any symptoms
What PATIENTS About TUNA Say Therapy TUNA THERAPY I would consider it to be a complete success. I have essentially no residual problems of any kind. I m back to the way I was before I ever had any symptoms
POSTOPERATIVE PATIENT MANAGEMENT
 POSTOPERATIVE PATIENT MANAGEMENT INTRODUCTION Deep brain stimulation of the internal globus pallidus (GPi) or the subthalamic nucleus (STN) effectively reduces parkinsonian motor symptoms and alleviates
POSTOPERATIVE PATIENT MANAGEMENT INTRODUCTION Deep brain stimulation of the internal globus pallidus (GPi) or the subthalamic nucleus (STN) effectively reduces parkinsonian motor symptoms and alleviates
P6KE Series DO-15. Mechanical Data
 DO-15 Features: Plastic package. Exceeds environmental standards of MIL-STD-19500. 600W surge capability at 10 x 1000µs waveform, duty cycle: 0.01%. Excellent clamping capability. Low zener impedance.
DO-15 Features: Plastic package. Exceeds environmental standards of MIL-STD-19500. 600W surge capability at 10 x 1000µs waveform, duty cycle: 0.01%. Excellent clamping capability. Low zener impedance.
Medtronic, Inc.
 Recharging System Quick Guide for Chronic pain www.medtronic.com Medtronic, Inc. 710 Medtronic Parkway Minneapolis, MN 55432-5604 USA Tel: 763-505-5000 Toll-free: 1-800-328-0810 UC201002746a EN 2013 Medtronic,
Recharging System Quick Guide for Chronic pain www.medtronic.com Medtronic, Inc. 710 Medtronic Parkway Minneapolis, MN 55432-5604 USA Tel: 763-505-5000 Toll-free: 1-800-328-0810 UC201002746a EN 2013 Medtronic,
INL No. A0083 Project Medtronic Thoracic patients guide Description Version 18
 INL No. A0083 Project Medtronic Thoracic patients guide Description Version 18 www.inl-agency.com PATIENT INFORMATION BOOKLET Endovascular Stent Grafts: A treatment for Thoracic Aortic disease Table of
INL No. A0083 Project Medtronic Thoracic patients guide Description Version 18 www.inl-agency.com PATIENT INFORMATION BOOKLET Endovascular Stent Grafts: A treatment for Thoracic Aortic disease Table of
Resolute Integrity. Independent Conformability Assessment
 Resolute Integrity Independent Conformability Assessment Stent Conformability and its Clinical Importance Immediate post-deployment stent geometry determines neointimal thickness independently of arterial
Resolute Integrity Independent Conformability Assessment Stent Conformability and its Clinical Importance Immediate post-deployment stent geometry determines neointimal thickness independently of arterial
SS3 Series. Controlled Avalanche Power Diodes. Features: Mechanical Data: SMC/DO-214AB. Page 1 28/03/06 V1.0
 SMC/DO-214AB Features: For surface mounted application. Metal to silicon rectifier, majority carrier conduction. Low forward voltage drop. Easy pick and place. High surge current capability. Epitaxial
SMC/DO-214AB Features: For surface mounted application. Metal to silicon rectifier, majority carrier conduction. Low forward voltage drop. Easy pick and place. High surge current capability. Epitaxial
BEFORE AND AFTER YOUR MRI SCAN. For patients with St. Jude Medical neurostimulation systems for chronic pain
 BEFORE AND AFTER YOUR MRI SCAN For patients with St. Jude Medical neurostimulation systems for chronic pain Preparing for your MRI Depending on what type of neurostimulation system you have, you may be
BEFORE AND AFTER YOUR MRI SCAN For patients with St. Jude Medical neurostimulation systems for chronic pain Preparing for your MRI Depending on what type of neurostimulation system you have, you may be
IMPLANTABLE MAGNETIC TRANSCUTANEOUS BONE CONDUCTION HEARING SYSTEM REIMBURSEMENT CODING GUIDE
 IMPLANTABLE MAGNETIC TRANSCUTANEOUS BONE CONDUCTION HEARING SYSTEM REIMBURSEMENT CODING GUIDE This document provides general reimbursement information to assist in obtaining coverage and reimbursement
IMPLANTABLE MAGNETIC TRANSCUTANEOUS BONE CONDUCTION HEARING SYSTEM REIMBURSEMENT CODING GUIDE This document provides general reimbursement information to assist in obtaining coverage and reimbursement
HRM Series PCB Power Relays
 Features: SPCO contacts. Sealed to allow washing after flow soldering. Specifications: Coil Data: Nominal Voltage Nominal Power consumption : 3V dc to 24V dc. : 540mW to 720mW. Contact Data: Contact Arrangement
Features: SPCO contacts. Sealed to allow washing after flow soldering. Specifications: Coil Data: Nominal Voltage Nominal Power consumption : 3V dc to 24V dc. : 540mW to 720mW. Contact Data: Contact Arrangement
PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD
 PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD WHAT IS DEEP BRAIN STIMULATION? WHY SHOULD YOU CONSIDER DBS SURGERY FOR YOUR PATIENTS? HOW DOES DBS WORK? DBS electrical stimulation overrides abnormal
PACEMAKERS ARE NOT JUST FOR THE HEART! Ab Siadati MD WHAT IS DEEP BRAIN STIMULATION? WHY SHOULD YOU CONSIDER DBS SURGERY FOR YOUR PATIENTS? HOW DOES DBS WORK? DBS electrical stimulation overrides abnormal
508 the number of suicide deaths in deaths per 100,000 people was the suicide rate in Suicide deaths in 2013 by gender
 An overview of suicide statistics This document summarises information about suicide deaths in New Zealand covering up to 13. It does not attempt to explain causes of suicidal behaviour or causes of changes
An overview of suicide statistics This document summarises information about suicide deaths in New Zealand covering up to 13. It does not attempt to explain causes of suicidal behaviour or causes of changes
HER103, HER107 Power Diodes - Fast Recovery
 High Efficiency DO-41 Features: Fast reverse recovery time, t rr. Low forward voltage drop, V F. Low cost axial packages. High current capability. High reliability. High surge current capability. Mechanical
High Efficiency DO-41 Features: Fast reverse recovery time, t rr. Low forward voltage drop, V F. Low cost axial packages. High current capability. High reliability. High surge current capability. Mechanical
Clinical Data Summary: Avoid FFS Study
 Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
Atrial Pacing Lead with 1.1 mm Tip-to-Ring Spacing Clinical Data Summary: Avoid FFS Study A Multi-center, Randomized, Prospective Clinical Study Designed to Evaluate the 1699T Lead Clinical Data Summary:
Good Laboratory Practice. EU-Serbia screening meeting Brussels, 19 June 2014
 Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
VARIAN ONCOLOGY. RapidArc. One revolution is all it takes.
 VARIAN ONCOLOGY RapidArc. One revolution is all it takes. Previous approaches to arc IMRT therapy have been restrictive. Some are limited by machine design, and deliver treatments in the axial plane only,
VARIAN ONCOLOGY RapidArc. One revolution is all it takes. Previous approaches to arc IMRT therapy have been restrictive. Some are limited by machine design, and deliver treatments in the axial plane only,
NobelProcera Implant Bar Overdenture complete range of fixed and removable solutions.
 NobelProcera Implant Bar Overdenture EXPERIENCE THE NEW WORLD OF CAD/CAM DENTISTRY 2 NobelProcera Implant Bar Overdenture NobelProcera Implant Bar Overdenture complete range of fixed and removable solutions.
NobelProcera Implant Bar Overdenture EXPERIENCE THE NEW WORLD OF CAD/CAM DENTISTRY 2 NobelProcera Implant Bar Overdenture NobelProcera Implant Bar Overdenture complete range of fixed and removable solutions.
DRAINAGE. Merit.com. Corporate Headquarters, South Jordan, Utah USA. Austria Norway
 AGE Corporate Headquarters, South Jordan, Utah USA Merit Medical Systems, Inc. 1600 West Merit Parkway South Jordan, Utah 84095 1.801.253.1600 1.800.35.MERIT Merit Medical Europe, Middle East, & Africa
AGE Corporate Headquarters, South Jordan, Utah USA Merit Medical Systems, Inc. 1600 West Merit Parkway South Jordan, Utah 84095 1.801.253.1600 1.800.35.MERIT Merit Medical Europe, Middle East, & Africa
Surgical Treatment of Movement Disorders. Surgical Treatment of Movement Disorders. New Techniques: Procedure is safer and better
 Surgical Treatment of Movement Stephen Grill, MD, PHD Johns Hopkins University and Parkinson s and Movement Center of Maryland Surgical Treatment of Movement Historical Aspects Preoperative Issues Surgical
Surgical Treatment of Movement Stephen Grill, MD, PHD Johns Hopkins University and Parkinson s and Movement Center of Maryland Surgical Treatment of Movement Historical Aspects Preoperative Issues Surgical
Global Trade in Lightweight Coated Writing Paper TradeData International Pty Ltd (www.tradedata.net) Page 1 5/18/2015
 Page 1 5/18/2015 An Analysis of Global Trade in Lightweight paper, coated with inorganic substances, used for writing etc., of which more than 10% by weight of total fibre content consists of fibres obtained
Page 1 5/18/2015 An Analysis of Global Trade in Lightweight paper, coated with inorganic substances, used for writing etc., of which more than 10% by weight of total fibre content consists of fibres obtained
Member State Marketing autorisation Holder Invented Name Strength Pharmaceutical form Route of administration
 ANNEX I LIST OF THE NAMES, PHARMACEUTICAL FORM, STRENGTH OF THE MEDICINAL PRODUCTS, ROUTE OF ADMINISTRATION, MARKETING AUTHORISATION HOLDERS IN THE MEMBER STATES 1 Member State Marketing autorisation Holder
ANNEX I LIST OF THE NAMES, PHARMACEUTICAL FORM, STRENGTH OF THE MEDICINAL PRODUCTS, ROUTE OF ADMINISTRATION, MARKETING AUTHORISATION HOLDERS IN THE MEMBER STATES 1 Member State Marketing autorisation Holder
Before Your MRI FOR PATIENTS WITH MEDTRONIC NEUROSTIMULATION SYSTEMS FOR CHRONIC PAIN
 Before Your I FOR PATIENTS WITH MEDTRONIC NEUROSTIMULATION SYSTEMS FOR CHRONIC PAIN Before Your I Depending on the type of neurostimulation system you have, you may be eligible for either a full-body or
Before Your I FOR PATIENTS WITH MEDTRONIC NEUROSTIMULATION SYSTEMS FOR CHRONIC PAIN Before Your I Depending on the type of neurostimulation system you have, you may be eligible for either a full-body or
List of nationally authorised medicinal products
 11 February 2016 EMA/255959/2016 Procedure Management and Committees Support Active substance: calcitonin salmon, synthetic analogue of eel calcitonin Procedure No.: PSUSA/00000494/201506 30 Churchill
11 February 2016 EMA/255959/2016 Procedure Management and Committees Support Active substance: calcitonin salmon, synthetic analogue of eel calcitonin Procedure No.: PSUSA/00000494/201506 30 Churchill
NobelDesign 1.3 Installation guide
 NobelDesign 1.3 Installation guide 2 NobelDesign 1.3 Installation guide // English Disclaimer of liability: This product is part of an overall concept and may only be used in conjunction with the associated
NobelDesign 1.3 Installation guide 2 NobelDesign 1.3 Installation guide // English Disclaimer of liability: This product is part of an overall concept and may only be used in conjunction with the associated
Bio-Rad Laboratories HEMOGLOBIN Testing Bio-Rad A1c. VARIANT II TURBO Link. Fully-Automated HbA 1c Testing
 Bio-Rad Laboratories HEMOGLOBIN Testing Bio-Rad A1c VARIANT II TURBO Link Fully-Automated HbA 1c Testing Bio-Rad Laboratories HEMOGLOBIN Testing VARIANT II TURBO Link Efficient streamlined process Powerful
Bio-Rad Laboratories HEMOGLOBIN Testing Bio-Rad A1c VARIANT II TURBO Link Fully-Automated HbA 1c Testing Bio-Rad Laboratories HEMOGLOBIN Testing VARIANT II TURBO Link Efficient streamlined process Powerful
Health Care Providers:
 Health Care Providers: 1. What is SERVE-HF? SERVE-HF is a multinational, multi-center, randomized controlled trial designed to assess whether treatment of predominantly Central Sleep Apnea with Adaptive
Health Care Providers: 1. What is SERVE-HF? SERVE-HF is a multinational, multi-center, randomized controlled trial designed to assess whether treatment of predominantly Central Sleep Apnea with Adaptive
Louisville '19 Attachment #69
 Telephone Meeting Approved and why I propose Using zoom to fulfill both Phone and Virtual video meeting Formats. The first established phone meeting Sanctioned by Gamblers Anonymous (listed on Trustee
Telephone Meeting Approved and why I propose Using zoom to fulfill both Phone and Virtual video meeting Formats. The first established phone meeting Sanctioned by Gamblers Anonymous (listed on Trustee
Lead and Extension Insertion Tool. Model Clinician's Manual
 Lead and Extension Insertion Tool Model 1803 Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE
Lead and Extension Insertion Tool Model 1803 Clinician's Manual Unless otherwise noted, indicates that the name is a trademark of, or licensed to, St. Jude Medical or one of its subsidiaries. ST. JUDE
EUROPEAN AEROSOL PRODUCTION
 Introduction EUROPEAN AEROSOL PRODUCTION 2017 EU European production Market share Production by segment Worldwide production / FEA, 2018. 1 INTRODUCTION More than 5.7 billion over 16 billion units globally
Introduction EUROPEAN AEROSOL PRODUCTION 2017 EU European production Market share Production by segment Worldwide production / FEA, 2018. 1 INTRODUCTION More than 5.7 billion over 16 billion units globally
St. Jude Medical 8-Channel Adapter. Clinician's Manual
 St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
St. Jude Medical 8-Channel Adapter Clinician's Manual CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. For U.S. California Only: Proposition 65, a State of California
Summary of Results for Laypersons
 What was the Study Called? Summary of Results for Laypersons A Randomized, Double-blind, Parallel-group, Placebo- and Active-controlled, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability
What was the Study Called? Summary of Results for Laypersons A Randomized, Double-blind, Parallel-group, Placebo- and Active-controlled, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability
The first and only fully-automated, multiplexed solution for Measles, Mumps, Rubella and Varicella-zoster virus antibody testing
 Bio-Rad Laboratories BioPlex 2200 System BioPlex 2200 MMRV IgG Kit The first and only fully-automated, multiplexed solution for Measles, Mumps, Rubella and Varicella-zoster virus antibody testing Bio-Rad
Bio-Rad Laboratories BioPlex 2200 System BioPlex 2200 MMRV IgG Kit The first and only fully-automated, multiplexed solution for Measles, Mumps, Rubella and Varicella-zoster virus antibody testing Bio-Rad
FOURIER STUDY GREYLOCK PRESS: CTS PRODUCT SAMPLE - FOURIER YES. Did the study achieve its main objective?
 FOURIER STUDY Did the study achieve its main objective? 2 15% 1 5% 9.8% YES FOURIER compared Repatha with placebo in patients who were taking a statin and had hardening or narrowing of the arteries and
FOURIER STUDY Did the study achieve its main objective? 2 15% 1 5% 9.8% YES FOURIER compared Repatha with placebo in patients who were taking a statin and had hardening or narrowing of the arteries and
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS. Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring
 MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
MEDTRONIC CARELINK NETWORK FOR PACEMAKERS Comparison between the Medtronic CareLink Network for Pacemakers and Transtelephonic Monitoring Transtelephonic Monitoring Transmission What can you determine
Arrow-Clark VectorFlow Hemodialysis Catheter Because in Chronic Hemodialysis Catheters, the Tip Matters
 Arrow-Clark VectorFlow Hemodialysis Catheter Because in Chronic Hemodialysis Catheters, the Tip Matters Because in chronic hemodialysis catheters, the tip matters Designed for Performance Tip design optimizes
Arrow-Clark VectorFlow Hemodialysis Catheter Because in Chronic Hemodialysis Catheters, the Tip Matters Because in chronic hemodialysis catheters, the tip matters Designed for Performance Tip design optimizes
Cardiac Assessment Controls
 Bio-Rad Laboratories CARDIAC ASSESSMENT CONTROLS Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
Bio-Rad Laboratories CARDIAC ASSESSMENT CONTROLS Cardiac Assessment Controls Value Assigned for Clinical Laboratory and Point of Care Test Systems Solving the Challenges of Troponin I Testing for the Central
Immunohematology. IH-QC Modular System. Select. Combine. Control.
 Immunohematology IH-QC Modular System Select. Combine. Control. IH-QC Modular System Select. Combine. Control. Transfusion guidelines recommend regular checking of test materials, test methods, local working
Immunohematology IH-QC Modular System Select. Combine. Control. IH-QC Modular System Select. Combine. Control. Transfusion guidelines recommend regular checking of test materials, test methods, local working
Smokefree Policies in Europe: Are we there yet?
 Smokefree Policies in Europe: Are we there yet? 14 April 2015, 9:00 10:30am Rue de l Industrie 24, 1040 Brussels Permanent Partners: Temporary Partners: The research for the SFP Smokefree Map was partially
Smokefree Policies in Europe: Are we there yet? 14 April 2015, 9:00 10:30am Rue de l Industrie 24, 1040 Brussels Permanent Partners: Temporary Partners: The research for the SFP Smokefree Map was partially
PATIENT PROGRAMMER 97740
 PATIENT PROGRAMMER 97740 Pain therapy user manual for neurostimulation system models 37022, 37701, 37702, 37703, 37704, 37711, 37712, 37713, 37714, 97702, 97712, 97713, 97714! USA Rx only 2013 Medtronic,
PATIENT PROGRAMMER 97740 Pain therapy user manual for neurostimulation system models 37022, 37701, 37702, 37703, 37704, 37711, 37712, 37713, 37714, 97702, 97712, 97713, 97714! USA Rx only 2013 Medtronic,
Engagement in language assessment / Regions of Europe
 Summary table: Engagement in language / Regions of This table lists the statistically significant differences in the engagement in activities by the respondents from different s of : If the word or appears
Summary table: Engagement in language / Regions of This table lists the statistically significant differences in the engagement in activities by the respondents from different s of : If the word or appears
Pharmaceutical, Medical and Health-related Government and Regulatory bodies around the world.
 1 International International Conference on Harmonization (ICH) World Health Organization (WHO) 2 Argentina National Administration of Drugs, Food and medical Technology. Australia s Department of health
1 International International Conference on Harmonization (ICH) World Health Organization (WHO) 2 Argentina National Administration of Drugs, Food and medical Technology. Australia s Department of health
Access to treatment and disease burden
 Access to treatment and disease burden Robert Flisiak Department of Infectious Diseases and Hepatology Medical University in Białystok, Poland Moulin de Vernègues, 27-29 August 2015 Disclosures Advisor
Access to treatment and disease burden Robert Flisiak Department of Infectious Diseases and Hepatology Medical University in Białystok, Poland Moulin de Vernègues, 27-29 August 2015 Disclosures Advisor
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500)
 1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500) ONLY NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro
1.5 Tesla and 3 Tesla Magnetic Resonance Imaging (MRI) Guidelines for the Senza System (IPG1000 and IPG1500) ONLY NEVRO CORP. All questions or concerns about Nevro products should be forwarded to: Nevro
This document is a preview generated by EVS
 EESTI STANDARD EVS-EN 1640:2004 Stomatoloogia. Meditsiinivahendid stomatoloogias. Aparatuur Dentistry - Medical devices for dentistry - Equipment EESTI STANDARDIKESKUS EESTI STANDARDI EESSÕNA NATIONAL
EESTI STANDARD EVS-EN 1640:2004 Stomatoloogia. Meditsiinivahendid stomatoloogias. Aparatuur Dentistry - Medical devices for dentistry - Equipment EESTI STANDARDIKESKUS EESTI STANDARDI EESSÕNA NATIONAL
BioPlex 2200 Infectious Disease Panels
 BioPlex 2200 System BioPlex 2200 Infectious Disease Panels An Expanding Multiplexed Assay Menu Lyme HIV Ag-Ab MMV IgM Syphilis Total & RPR MMRV EBV HSV-1 & HSV-2 EBV IgM ToRC IgM ToRC Leading the way with
BioPlex 2200 System BioPlex 2200 Infectious Disease Panels An Expanding Multiplexed Assay Menu Lyme HIV Ag-Ab MMV IgM Syphilis Total & RPR MMRV EBV HSV-1 & HSV-2 EBV IgM ToRC IgM ToRC Leading the way with
Terms and Conditions. VISA Global Customer Assistance Services
 Terms and Conditions VISA Global Customer Assistance Services Visa Global Customer Assistance Services (VGCAS) 1 The Visa Global Customer Assistance Services are co-ordinated by the Global Assistance Centre
Terms and Conditions VISA Global Customer Assistance Services Visa Global Customer Assistance Services (VGCAS) 1 The Visa Global Customer Assistance Services are co-ordinated by the Global Assistance Centre
PARKINSON S SYMPTOM TRACKER
 PARKINSON S SYMPTOM You can help your doctor make good treatment decisions by tracking your symptoms. A well-kept Symptom Tracker provides a clear picture of when you are taking your medications, when
PARKINSON S SYMPTOM You can help your doctor make good treatment decisions by tracking your symptoms. A well-kept Symptom Tracker provides a clear picture of when you are taking your medications, when
WCPT COUNTRY PROFILE December 2017 HUNGARY
 WCPT COUNTRY PROFILE December 2017 HUNGARY HUNGARY NUMBERS WCPT Members Practising physical therapists 727 Total number of physical therapist members in your member organisation 4,000 Total number of practising
WCPT COUNTRY PROFILE December 2017 HUNGARY HUNGARY NUMBERS WCPT Members Practising physical therapists 727 Total number of physical therapist members in your member organisation 4,000 Total number of practising
WCPT COUNTRY PROFILE December 2017 SWEDEN
 WCPT COUNTRY PROFILE December 2017 SWEDEN SWEDEN NUMBERS WCPT Members Practising physical therapists 11,043 Total number of physical therapist members in your member organisation 17,906 Total number of
WCPT COUNTRY PROFILE December 2017 SWEDEN SWEDEN NUMBERS WCPT Members Practising physical therapists 11,043 Total number of physical therapist members in your member organisation 17,906 Total number of
Understand the New 2019 Neurostimulator Analysis-Programming CPT Coding Structure and Associated Relative Value Units
 Understand the New 2019 Neurostimulator Analysis-Programming CPT Coding Structure and Associated Relative Units The American Academy of Neurology (AAN) presents the following case studies to help you understand
Understand the New 2019 Neurostimulator Analysis-Programming CPT Coding Structure and Associated Relative Units The American Academy of Neurology (AAN) presents the following case studies to help you understand
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
D7.1 Report summarising results of survey of EU countries to identify volumes and trends in relation to the import and export of stem cells
 Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
Disclaimer: The content of this Deliverable represents the views of the author only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission and/or the
PATIENT CONTROLLER ST. JUDE MEDICAL UNDERSTANDING IMPORTANT FEATURES ON YOUR
 UNDERSTANDING IMPORTANT FEATURES ON YOUR ST. JUDE MEDICAL PATIENT CONTROLLER THINGS TO KNOW BEFORE AND AFTER AN MRI OR SURGICAL PROCEDURE WHEN YOU HAVE A ST. JUDE MEDICAL PROCLAIM NEUROSTIMULATION SYSTEM
UNDERSTANDING IMPORTANT FEATURES ON YOUR ST. JUDE MEDICAL PATIENT CONTROLLER THINGS TO KNOW BEFORE AND AFTER AN MRI OR SURGICAL PROCEDURE WHEN YOU HAVE A ST. JUDE MEDICAL PROCLAIM NEUROSTIMULATION SYSTEM
To provide information on the new standard, a number of EOG members formed a committee to compile information on the following basis:
 EN 13537 Sleeping Bag Standard Information for Retailers 30.12.2004 Disclaimer This information sheet has been provided by the EOG to help companies assess the implications of the new EN 13537 norm on
EN 13537 Sleeping Bag Standard Information for Retailers 30.12.2004 Disclaimer This information sheet has been provided by the EOG to help companies assess the implications of the new EN 13537 norm on
GLP in the European Union Ecolabel detergents, GLP and accreditation
 GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
MENTAL HEALTH CARE. OECD HCQI Expert meeting 17 th of May, Rie Fujisawa
 MENTAL HEALTH CARE OECD HCQI Expert meeting 17 th of May, 2013 Rie Fujisawa Mental health indicators Any hospital readmissions for patients with schizophrenia Same hospital readmissions for patients with
MENTAL HEALTH CARE OECD HCQI Expert meeting 17 th of May, 2013 Rie Fujisawa Mental health indicators Any hospital readmissions for patients with schizophrenia Same hospital readmissions for patients with
List of nationally authorised medicinal products
 5 November 2015 EMA/230130/2016 Procedure Management and Committees Support Active substance: cabergoline Procedure no.: PSUSA/00000477/201503 30 Churchill Place Canary Wharf London E14 5EU United Kingdom
5 November 2015 EMA/230130/2016 Procedure Management and Committees Support Active substance: cabergoline Procedure no.: PSUSA/00000477/201503 30 Churchill Place Canary Wharf London E14 5EU United Kingdom
WCPT COUNTRY PROFILE December 2017 SERBIA
 WCPT COUNTRY PROFILE December 2017 SERBIA SERBIA NUMBERS WCPT Members Practising physical therapists 622 Total number of physical therapist members in your member organisation 3,323 Total number of practising
WCPT COUNTRY PROFILE December 2017 SERBIA SERBIA NUMBERS WCPT Members Practising physical therapists 622 Total number of physical therapist members in your member organisation 3,323 Total number of practising
ACCESS + NAVIGATION + STABILIZATION
 ACCESS + NAVIGATION + STABILIZATION Merit Medical s intuitive platform is designed to treat vertebral compressions fractures, and can provide rapid and lasting pain relief 1 with the most advanced targeted
ACCESS + NAVIGATION + STABILIZATION Merit Medical s intuitive platform is designed to treat vertebral compressions fractures, and can provide rapid and lasting pain relief 1 with the most advanced targeted
Model 5392 EPG Temporary Pacer
 Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
Model 5392 EPG Temporary Pacer Compatible Components Reference Card 5392 Surgical Cables 5487 Disposable, short 5487L Disposable, long 5832S Reusable, small clip 5833S 5833SL Disposable, small clip, short
Pocket Adaptor Kit FOR SPINAL CORD STIMULATION. Tip Cards
 Pocket Adaptor Kit FOR SPINAL CORD STIMULATION Tip Cards model 74001 (1 4) model 74002 (2 4) The Pocket Adaptor Tip Cards are to be used for educational purposes only. Please refer to the Pocket Adaptor
Pocket Adaptor Kit FOR SPINAL CORD STIMULATION Tip Cards model 74001 (1 4) model 74002 (2 4) The Pocket Adaptor Tip Cards are to be used for educational purposes only. Please refer to the Pocket Adaptor
DRAINAGE. MeritEMEA.com
 DRAINAGE MeritEMEA.com Thoracentesis Biliary Drainage Nephrostomy Paracentesis Abscess Drainage COMPLETE DRAINAGE SOLUTIONS Merit Medical offers an integrated suite of products designed to support your
DRAINAGE MeritEMEA.com Thoracentesis Biliary Drainage Nephrostomy Paracentesis Abscess Drainage COMPLETE DRAINAGE SOLUTIONS Merit Medical offers an integrated suite of products designed to support your
Understanding the CareLink TM Therapy Management Dashboard Report
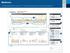 3 2 1 5 6 7 4 1 This is a great place to start recognizing trends. In this example you may see plenty of events that result in a low glucose trend. A low glucose is identified as a value less than 70mg/dL.
3 2 1 5 6 7 4 1 This is a great place to start recognizing trends. In this example you may see plenty of events that result in a low glucose trend. A low glucose is identified as a value less than 70mg/dL.
Radiotherapy and Radiosurgery
 RapidArc Radiotherapy and Radiosurgery RapidArc Faster. Sharper. Smarter. * It s true for the latest generation of RapidArc technology, and it can be true for your clinic. The oncology landscape has shifted
RapidArc Radiotherapy and Radiosurgery RapidArc Faster. Sharper. Smarter. * It s true for the latest generation of RapidArc technology, and it can be true for your clinic. The oncology landscape has shifted
The Facts about Biphasic Defibrillation
 The Facts about Biphasic Defibrillation Introduction In reference to the SMART Biphasic waveform, Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care, published by the American
The Facts about Biphasic Defibrillation Introduction In reference to the SMART Biphasic waveform, Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care, published by the American
TAKE CONTROL OF YOUR FUTURE
 NEUROSTIMULATION SYSTEMS FOR PAIN THERAPY Brief Summary: Product manuals must be reviewed prior to use for detailed disclosure. Indications Implantable neurostimulation systems - A Medtronic implantable
NEUROSTIMULATION SYSTEMS FOR PAIN THERAPY Brief Summary: Product manuals must be reviewed prior to use for detailed disclosure. Indications Implantable neurostimulation systems - A Medtronic implantable
ACCESS + NAVIGATION + STABILIZATION
 ACCESS + NAVIGATION + STABILIZATION Merit Medical s intuitive platform is designed to treat vertebral compressions fractures, and can provide rapid and lasting pain relief 1 with the most advanced targeted
ACCESS + NAVIGATION + STABILIZATION Merit Medical s intuitive platform is designed to treat vertebral compressions fractures, and can provide rapid and lasting pain relief 1 with the most advanced targeted
MRI GUIDELINES FOR INSPIRE THERAPY
 MRI GUIDELINES FOR INSPIRE THERAPY Clinician s Manual The following is a trademark of Inspire Medical Systems, Inc.: Inspire This product and/or the use of this product in a method may be covered by one
MRI GUIDELINES FOR INSPIRE THERAPY Clinician s Manual The following is a trademark of Inspire Medical Systems, Inc.: Inspire This product and/or the use of this product in a method may be covered by one
 http://www.sis.se http://www.sis.se http://www.sis.se http://www.sis.se http://www.sis.se Provläsningsexemplar / Preview SVENSK STANDARD SS-EN 980 Fastställd 2003-05-23 Utgåva 2 Grafiska symboler för märkning
http://www.sis.se http://www.sis.se http://www.sis.se http://www.sis.se http://www.sis.se Provläsningsexemplar / Preview SVENSK STANDARD SS-EN 980 Fastställd 2003-05-23 Utgåva 2 Grafiska symboler för märkning
Bio-Rad Laboratories HEMOGLOBIN Testing Bio-Rad A1c. VARIANT II TURBO Link System. Fully-Automated HbA 1c Testing
 Bio-Rad Laboratories HEMOGLOBIN Testing Bio-Rad A1c VARIANT II TURBO Link System Fully-Automated HbA 1c Testing Bio-Rad Laboratories HEMOGLOBIN Testing VARIANT II TURBO Link System Efficient streamlined
Bio-Rad Laboratories HEMOGLOBIN Testing Bio-Rad A1c VARIANT II TURBO Link System Fully-Automated HbA 1c Testing Bio-Rad Laboratories HEMOGLOBIN Testing VARIANT II TURBO Link System Efficient streamlined
Overview of drug-induced deaths in Europe - What does the data tell us?
 Overview of drug-induced deaths in Europe - What does the data tell us? João Matias, Isabelle Giraudon, Julián Vicente EMCDDA expert group on the key-indicator Drug-related deaths and mortality among drug
Overview of drug-induced deaths in Europe - What does the data tell us? João Matias, Isabelle Giraudon, Julián Vicente EMCDDA expert group on the key-indicator Drug-related deaths and mortality among drug
Tandvård Medicintekniska produkter för tandvård Dentala implantat. Dentistry Medical devices for dentistry Dental implants
 SVENSK STANDARD SS-EN 1642:2005 Fastställd 2005-04-15 Utgåva 2 Tandvård Medicintekniska produkter för tandvård Dentala implantat Dentistry Medical devices for dentistry Dental implants ICS 11.060.15 Språk:
SVENSK STANDARD SS-EN 1642:2005 Fastställd 2005-04-15 Utgåva 2 Tandvård Medicintekniska produkter för tandvård Dentala implantat Dentistry Medical devices for dentistry Dental implants ICS 11.060.15 Språk:
A Closer Look. LATITUDE NXT Alerts SUMMARY. Alerts. Red Alerts
 A Closer Look SUMMARY Boston Scientific s LATITUDE NXT Patient Management System enables a clinician to periodically monitor patient and device information remotely via a Communicator placed in the patient
A Closer Look SUMMARY Boston Scientific s LATITUDE NXT Patient Management System enables a clinician to periodically monitor patient and device information remotely via a Communicator placed in the patient
Differences make a Difference
 Differences make a Difference The European Mens Health Forum: Overview European Mens Health: An oxymoron? Mens use of services: Self-care & navigation Good practice: Wot does work? A Storm is coming: Man
Differences make a Difference The European Mens Health Forum: Overview European Mens Health: An oxymoron? Mens use of services: Self-care & navigation Good practice: Wot does work? A Storm is coming: Man
Amoxicillin 40 mg Clavulanic acid 10 mg. Clavulanic acid 10 mg. Clavulanic acid 10 mg. Clavulanic acid 10 mg
 Annex I List of the names, pharmaceutical form, strength of the veterinary medicinal product, animal species, route of applicant/marketing authorisation holder in the member states 1/10 Member State EU/EEA
Annex I List of the names, pharmaceutical form, strength of the veterinary medicinal product, animal species, route of applicant/marketing authorisation holder in the member states 1/10 Member State EU/EEA
2015 ST. JUDE MEDICAL THERAPY CODING GUIDE Cardiac Pacemakers
 2015 ST. JUDE MEDICAL THERAPY CODING GUIDE Cardiac Pacemakers This guide provides physician and hospital coding and reimbursement information for cardiac pacemaker procedures. In addition, St. Jude Medical
2015 ST. JUDE MEDICAL THERAPY CODING GUIDE Cardiac Pacemakers This guide provides physician and hospital coding and reimbursement information for cardiac pacemaker procedures. In addition, St. Jude Medical
NobelActive. The NobelActive technical story
 The NobelActive technical story The NobelActive technical story NobelActive origins The life cycle of NobelActive began following years of research and development on a self-drilling and bone-condensing
The NobelActive technical story The NobelActive technical story NobelActive origins The life cycle of NobelActive began following years of research and development on a self-drilling and bone-condensing
The success of any Inter-
 Sacral Nerve Neuromodulation (InterStim ) Part II: Review of Programming Helen Rittenmeyer The success of any Inter- Stim Sacral Nerve Neuromodulation program depends on the positioning of the chronic
Sacral Nerve Neuromodulation (InterStim ) Part II: Review of Programming Helen Rittenmeyer The success of any Inter- Stim Sacral Nerve Neuromodulation program depends on the positioning of the chronic
CHANGE OF DETAILS FORM CROSS-BORDER DISTANCE SALES OF TOBACCO PRODUCTS, ELECTRONIC CIGARETTES AND/OR REFILL CONTAINERS
 For completion by Office Premises Ref Processed Verified CHANGE OF DETAILS FORM CROSS-BORDER DISTANCE SALES OF TOBACCO PRODUCTS, ELECTRONIC CIGARETTES AND/OR REFILL CONTAINERS EHO This form relates to
For completion by Office Premises Ref Processed Verified CHANGE OF DETAILS FORM CROSS-BORDER DISTANCE SALES OF TOBACCO PRODUCTS, ELECTRONIC CIGARETTES AND/OR REFILL CONTAINERS EHO This form relates to
Biology Report. Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis?
 Biology Report Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis? Introduction Tuberculosis is a serious disease caused by the bacterium Mycobacterium
Biology Report Is there a relationship between Countries' Human Development Index (HDI) level and the incidence of tuberculosis? Introduction Tuberculosis is a serious disease caused by the bacterium Mycobacterium
WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017
 WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017 Mary L. Yost 404-520-6652 , LLC 23 Ridge Rd. Beaufort, SC 20097 Copyright Pending 2017 All rights reserved,
WESTERN EUROPE PREVALENCE AND INCIDENCE OF PERIPHERAL ARTERY DISEASE AND CRITICAL LIMB ISCHEMIA 2017 Mary L. Yost 404-520-6652 , LLC 23 Ridge Rd. Beaufort, SC 20097 Copyright Pending 2017 All rights reserved,
