Credentialing of surgeons as interventionalists for carotid artery stenting: Experience from the leadin phase of CREST
|
|
|
- Aron Jennings
- 5 years ago
- Views:
Transcription
1 From the Society for Vascular Surgery Credentialing of surgeons as interventionalists for carotid artery stenting: Experience from the leadin phase of CREST Robert W. Hobson II, MD, a Virginia J. Howard, MSPH, b Gary S. Roubin, MD, PhD, c Robert D. Ferguson, MD, d Thomas G. Brott, MD, e George Howard, DrPh, b Alice J. Sheffet, PhD, f Jamie Roberts, LPN, e L. Nick Hopkins, MD, g and Wesley S. Moore, MD, h for the CREST Investigators,* Newark, NJ; Birmingham, Ala; New York, NY; Winston-Salem, NC; Jacksonville, Fla; Buffalo, NY; and Los Angeles, Calif Background: Credentialing of vascular surgeons to perform carotid artery stenting (CAS) continues to be a major issue confronting the specialty of Vascular Surgery. Cannulation of aortic arch branches, and placement of carotid antiembolic devices and stents constitute the major technical challenges to vascular surgeons becoming credentialed to perform CAS. The multicenter Carotid Revascularization Endarterectomy vs Stenting Trial (CREST), supported by the National Institute of Neurological Disorders and Stroke, National Institute of Health, reviews credentials of interventionalists, including surgeons, for the trial s lead-in phase of CAS to treat symptomatic (>50% stenosis) and asymptomatic (>70% stenosis). Methods: Vascular surgeons requesting participation in CREST must have achieved basic interventional credentialing criteria as recommended by the Society of Vascular Surgery. Each interventionalist is asked to submit notes and narrative summaries from a series of 10 to 30 CAS procedures for review by a multi-specialty review committee before being approved to participate in CREST. Thereafter, during the lead-in phase of CREST, each approved interventionalist is asked to perform CAS procedures using the study devices in as many as 20 patients. In this interim report from the CREST lead-phase, the association of specialty of operator (vascular surgeon, neurosurgeon, other specialist) and periprocedural stroke and death rate was examined in patients undergoing CAS. In addition, current enrollment volume in the lead-in phase by specialty of the principal investigator was examined. Results: Thirty-two of 134 (23.9%) CREST-credentialed interventionalists are vascular surgeons (n 22; 16.4%) or neurosurgeons (n 10; 7.5%). For events monitored through March 31, 2004, 789 patients had undergone CAS procedures performed by these 134 specialists. Thirty-day stroke and death rate was 4.6%, and myocardial infarction was observed in 1.1% of patients. Serious adverse events have not been clustered at individual institutions, and no significant differences have been observed between vascular surgeons or neurosurgeons and other credentialed specialists. Conclusions: Vascular surgeons with basic catheter and guide wire skills, particularly those who have incorporated diagnostic cerebral angiography into their practice, can be credentialed to perform CAS. Individuals or groups should devote a number of cases (n per surgeon) to CAS to accomplish this goal. Pending US Food and Drug Administration approval of devices and Center for Medicaid and Medicare Services reimbursement, institutional financial support for the performance of these procedures must be secured. The learning curve for CAS should not be considered so formidable as to discourage surgeons from adding these techniques of CAS to their procedural inventory. (J Vasc Surg 2004;40:952-7.) From the Department of Surgery, UMDNJ-NJ Medical School, a Newark, the Department of Epidemiology, University of Alabama at Birmingham, b the Department of Cardiology, Lenox Hill Hospital, c New York, Department of Radiology, Forsyth Medical Center, d Winston-Salem, Mayo Clinic, e Jacksonville, Department of Biostatistics, University of Alabama at Birmingham, b CREST Administrative Center, f Newark, Department of Surgery, State University of New York, g Buffalo, and Department of Surgery, University of California at Los Angeles. h Competition of interest: none. Presented at the Fifty-seventh Annual Meeting of the Society for Vascular Surgery, Anaheim, Calif, Jun 3-6, Additional material for this article may be found online at com/jvs. *Dr Hobson, Principal Investigator, CREST; Ms Howard, Project Director, CREST Statistical Analysis Center; Dr Roubin, Co-Principal Investigator, Intervention (Cardiology); Dr Ferguson, Co-Principal Investigator, Intervention (Interventional Radiology); Dr Brott, Co-Principal Investigator, CREST; Dr Howard, Co-Principal Investigator, Statistical Analysis Center; Dr Sheffet, Project Director, CREST Administrative Center; Ms Roberts, Recruitment Director, CREST; Dr Hopkins, Co-Principal Investigator (Neurosurgery); Dr Moore, Co-Principal Investigator (Vascular Surgery). 952 Carotid artery stenting (CAS) is a current option in the management of extracranial carotid occlusive disease. 1 Although patients at conventional risk continue to be referred for carotid endarterectomy (CEA), 2-6 a multidisciplinary panel that included vascular surgeons has identified current indications in patients considered at high risk for CEA in whom CAS is an appropriate management approach. 1 These indications include recurrent stenosis after previous CEA, severe medical comorbid conditions, radiation-induced carotid stenosis, and the occasional anatomically high (above C2) lesion. We agree with the American Heart Association consensus panel that in patients who are not in these high-risk groups, the few exceptions should undergo Reprint requests: Robert W. Hobson II, MD, CREST Administrative Center, UMDNJ-New Jersey Medical School, ADMC, Bldg 6, Rm 620, 30 Bergen St, Newark, NJ ( hobsonrw@umdnj.edu) /$30.00 Copyright 2004 by The Society for Vascular Surgery. doi: /j.jvs
2 JOURNAL OF VASCULAR SURGERY Volume 40, Number 5 Hobson et al 953 performance of CAS confined to well-controlled, randomized clinical trials. 7 While some vascular surgeons have begun to adopt the procedure in selected patients, concerns about attainment of skills and credentialing have limited opportunities for surgeons and other specialists. 8,9 The Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) is an ongoing, randomized, controlled, multicenter clinical trial in patients with symptomatic extracranial carotid disease, to assess the differential efficacy of CEA and CAS in preventing stroke, myocardial infarction, and death in the 30-day periprocedural period, and ipsilateral stroke during the follow-up period. 10 A lead-in phase is built into the CREST study design to provide clinical centers with start-up and credentialing periods, during which each eligible interventionalist will perform as many as 20 CAS procedures in patients. Patients with asymptomatic disease with stenosis of 70% or greater, and patients with symptomatic disease with stenosis 50% or greater are eligible for this lead-in phase. Results from each interventionalist are reviewed by the Interventional Management Committee (Table I), and committee approval is required before the interventionalist is certified to perform CAS in randomized patients. This report details the lead-in credentialing process for surgeons admitted to CREST as interventionalists. Enrollment into both the lead-in and randomization phases of CREST is ongoing, and this report is based on data from 51 sites that participated in the lead-in phase from November 3, 2000, through March 31, Data from the CREST lead-in phase were assessed, and results were compared among specialists. METHODS As of May 2004, 60 centers had been approved for inclusion in this multicenter, North American study. Enrollment is ongoing, and this report is based on data from 51 sites that have participated in the lead-in phase of CREST. The CAS procedure in CREST uses the ACCULINK carotid stent system (Advanced Cardiovascular Systems, Guidant Corp). In September 2001 the protocol was amended to include an embolic protection device, the ACCUNET embolic protection system (Guidant). The protocol and all amendments were approved by the institutional review boards of each participating institution and administrative unit, and informed consent was obtained from every potential subject before enrollment. Each interventionalist who applied for CREST certification was required to submit data on standardized forms about their last 10 to 30 CAS procedures performed with any device. These data were reviewed by the CREST Interventional Management Committee (IMC). As a guideline, 30-day stroke and death was to be below 6% to 8%, and technical features were to include use of wire systems rather than wires, 6F guide sheaths, and initial application of anti-embolic devices. After approval to join CREST and before enrolling patients in the lead-in phase, study interventionalists without previous experience with the ACCULINK or ACCUNET systems were required to Table I. Carotid Revascularization and Endarterectomy vs Stent Trial Interventional Management Committee Gary Roubin, MD, PhD, Committee Co-Chair Robert Ferguson, MD, Committee Co-Chair Stanley Barnwell, MD Thomas G. Brott, MD Elie Chakhtoura, MD Jonathan Goldstein, MD William Gray, MD Robert W. Hobson II, MD L. Nick Hopkins, MD Barry Katzen, MD Kenneth Rosenfield, MD participate in a specially designed Carotid Stent Operator Certification Program. This program consisted of intensive didactic and hands-on training that focused on the prerequisites for use of carotid stenting and embolic protection devices, and observation of live case demonstrations. The CAS procedures in the lead-in phase were then performed only by CREST-certified study interventionalists. Procedural details of CAS are summarized in Table II. Inclusion and exclusion criteria for CREST are summarized, respectively, in Tables III and IV, online only. All participants underwent cerebral angiography to ascertain carotid exclusionary disorders (Table IV, online only). Patients in the lead-in phase had symptomatic disease (symptoms of amaurosis fugax, transient ischemic attack, or stroke in the distribution of the study artery within the previous 180 days) or asymptomatic disease (absent cerebrovascular symptoms in the distribution of the carotid artery.) Patients with symptoms were to have discrete carotid artery stenoses of 50% or greater at angiography, and patients without symptoms were to have stenosis of 70% or greater at angiography. Patients were evaluated before the procedure, after CAS, and before discharge at 24 to 48 hours by the study neurologist, and the National Institutes of Health Stroke Scale was routinely administered. Follow-up evaluations were performed at 1 and 12 months, and annually thereafter, and included neurologic evaluation and the National Institutes of Health Stroke Scale. This report focuses on the 30-day periprocedural complications among specialists credentialed in CREST. The primary lead-in end points were stroke, death, or myocardial infarction during the periprocedural period (30 days after index procedure) or any stroke ipsilateral to the study artery during 1 year after the procedure. However, for safety monitoring several criteria for specific actions have been developed for rates of stroke and death during the 30-day periprocedural period. RESULTS Between January 2000 and March 31, 2004, CREST investigators enrolled 862 adult patients with CAS at 51 approved clinical sites, and complete data sets on stroke morbidity and mortality at 30 days post-procedure are based on an analysis of 789 cases.
3 954 Hobson et al JOURNAL OF VASCULAR SURGERY November 2004 Table II. Protocol for carotid artery stenting procedures Preprocedural (48 hours) aspirin (325 mg PO qd or bid) and clopidogrel (75 mg PO bid). Substitute ticlopidine (250 mg bid) if patient is unable to tolerate clopidogrel. Transfemoral approach. Heparinization to activated clotting time of 250 to 300 seconds (with introduction of anti-embolic device). 5F Vitek catheter for cannulation of aortic arch branches inch coated Terumo long exchange guide wire to external carotid artery. 6F guide sheath (100-cm long) to common carotid artery proximal to lesion; occasional use of inch Amplatz stiff guide wire is recommended to advance the Vitek catheter or 6F guide sheath into common carotid artery inch guide wire to cross common carotid-internal carotid stenosis, and placement of anti-embolic device (ACCUNET; Guidant); 3-mm or 4-mm low-profile balloon for pre-deployment dilatation as required. Deployment of nitinol self-expanding stent (ACCULINK; Guidant). Post-stenting dilatation with 5.0-mm to 5.5-mm balloon. Intermittent hand-injection angiography during procedure; use bony landmarks for balloon and stent placements. Use of femoral closure device, as recommend by individual interventionalists. Aspirin is continued indefinitely, and clopidogrel (or ticlopidine) is continued for a minimum of 2 to 4 weeks after carotid artery stenting. Fig 1. Distribution of interventionalists credentialed by the Carotid Revascularization Endarterectomy vs Stenting Trial (n 134). From a group of 134 CREST-credentialed interventionalists (Fig 1), vascular surgeons and neurosurgeons accounted for 32 of specialists (23.9%): 22 vascular surgeons (16.4%) and 10 neurosurgeons (7.5%). Other specialists were certified as CREST interventionalists as follows: cardiologists, 52 (38.8%); interventional neuroradiologists, 31 (23.1%), interventional radiologists, 15 (11.1%), and neurologists, 4 (3.0%). At the time of this report, only 106 of the credentialed interventionalists have been active in enrollment in the lead-in phase. Of the 22 vascular surgeons, only 14 have contributed thus far. The other 8 vascular surgical interventionalists are poised to increase case enrollment. Since accepted into the lead-in phase, vascular surgeons have performed 131 of 789 (17%) lead-in procedures (Fig 2). Thirty-day stroke and death rates for vascular surgeons and neurosurgeons as interventionalists was 5.3%, and 30- day stroke and death rates for all other specialists was 4.4%, which did not represent a significant difference (P.64). Acute myocardial infarction occurred in 1.1% of cases. Overall recruitment of lead-in patients has been significantly greater in institutions with vascular surgeons (n 15; 26.3%) or neurosurgeons (n 4, 7.0%) as principal investigators, compared with centers headed by other interventionalists. Centers with vascular or neurosurgical principal investigators have recruited 329 patients (36%) in the lead-in phase, and other specialists have recruited 583 patients (64%). Currently, surgical centers have been more productive than those headed by other specialists (Fig 3). DISCUSSION The interim record of the CREST surgical interventional group in the performance of CAS during the lead-in phase of CREST is essentially comparable to other reports from single institutional centers or registries, or randomized trial analyses. Thirty-day stroke and death rate for all interventionalists in CREST was 4.6% (vascular surgeons or neurosurgeons, 5.4%; other interventionalists, 4.4%; P 0.64), as compared with other published series. Roubin
4 JOURNAL OF VASCULAR SURGERY Volume 40, Number 5 Hobson et al 955 Fig 2. Percentage of lead-in procedures performed by vascular surgeons and neurosurgeons vs all other interventionalists (n 789). Fig 3. Productivity of centers with vascular surgeons as Principal Investigators vs other specialists as Principal Investigators (n 912). et al 11 eported an overall 30-day stroke and death rate of 7.1%, the Schneider investigators 12 reported 12.1%, the CAVATAS investigators 13 reported 10.0%, and the SAP- PHIRE trialists 14 reported 4.5%. In the CAS registry of CARESS, the 30-day stroke and death rate for CAS was 2%, and half of the procedures were performed by surgeons. 15 These data suggest that vascular surgeons and neurosurgeons who are willing to devote a portion of their endarterectomy procedures to CAS can gain skills if they have been credentialed in basic catheter and guide wire skills, particularly vascular surgeons skilled in peripheral angioplasty and stenting procedures. 16 Credentialing committees should take this volume of endovascular procedures into consideration in considering approval of vascular surgeons to perform CAS procedures. If the vascular surgeon or his or her group is willing to allocate a portion of their
5 956 Hobson et al JOURNAL OF VASCULAR SURGERY November 2004 CEA procedures to 1 member of the group, this process could require no more than 6 to 12 months, and might optimally include collaboration with a colleague from Cardiology or Interventional Radiology. Previous analysis of the learning curve for CREST suggested that an operator must perform a minimum of 15 procedures to achieve technical proficiency. 17 However, the number of interventionalists currently participating in CREST precludes a more reliable assessment. Only 15 operators performed 15 or more CAS procedures. Although no events were recorded in patients who underwent the 9th to 15th procedures in these operators series, the suggestion of a plateau at this level continues to have unreliability, on the basis of this lesser number of observations. Currently this is regarded clinically as the minimally acceptable number to initiate participation in CREST. Whether use of simulators 18 will contribute to attenuation of the learning curve was also discussed by the multidisciplinary panel. 8 Opinions varied, but all agreed that, with correlation of the value of simulation to clinical practice, this experience might be an excellent introduction to CAS in the hands of an otherwise experienced surgeon. Several features of the CREST credentialing process for surgeons as interventionalists should be emphasized. The vascular surgical group had satisfied basic minimal skills in catheter and guide wire technology. 16 Although some vascular surgeons have suggested that performance of aortic endografting should not be considered helpful before initiating a program in CAS, all of the panelists on a recently published roundtable discussion 8 agreed that experience in peripheral angioplasty and stenting, including performance of iliac, superficial femoral, and renal artery interventions, are excellent prerequisites for performance of CAS. The performance of 10 to 30 initial CAS procedures by each of the CREST surgeons suggests an innovative assessment of the importance of adding this catheter-based technology to their armamentarium. Approval of these interventionalists for the lead-in and randomization phases confirms the value of these initial procedures in the hands of skilled interventional surgeons. Although data on this group s performance with cerebral angiography was not recorded, it should be noted that this was not a prerequisite for credentialing in CREST. While it is the opinion of one of the authors (R.W.H.), which is shared by others, 8,9 that diagnostic cerebral angiography will assist an interventionalist who is preparing to perform CAS, the CREST IMC has not recommended diagnostic angiography as a prerequisite for interventionalists with documented basic skill levels. Productivity in enrollment by vascular surgeons and neurosurgeons in CREST was notable. Their networking with other specialties, including Neurology, probably accounts for these results. In addition, many supervise activities in noninvasive vascular laboratories, which are natural referral points for patients with symptomatic and asymptomatic extracranial carotid occlusive disease. These factors make surgeons ideal candidates to identify potential participants for randomized clinical trials of CAS versus CEA. Patients can be referred to endovascular surgical specialists with confidence that the procedure, CEA or CAS, will be tailored to their patients needs. Also, it would appear that the future success of CREST depends in no small measure on the coordinated efforts of these surgeons, who have made significant contributions to the recruitment of patients to this randomized clinical trial. The future format for education and credentialing of vascular surgeons and other specialists for CAS will be shaped by specialty societies 9 and manufacturers. 19 At the recent US Food and Drug Administration Device Panel meeting on CAS 19 one manufacturer (Johnson & Johnson, Cordis) outlined an ambitious plan that included introductory didactic courses, use of simulators, live case demonstrations, and ultimately performance of CAS procedures with observation by approved proctors. While this may become a great asset to vascular surgeons interested in performing CAS, implementation of this plan may not occur for 12 to 18 months. In the meantime, we recommend initiation of a plan as outlined by the CREST IMC and Executive Committees by individual surgeons and groups to achieve early skill levels, which may then be supplemented by training mandated by the Food and Drug Administration and the manufacturer. It is also the recommendation of the CREST investigators that the Society for Vascular Surgery sponsor and supervise an expert panel to define credentialing requirements for CAS by vascular surgeons. REFERENCES 1. Veith FJ, Amor M, Ohki T, Beebe HG, Bell PR, Bolia A, et al. Current status of carotid bifurcation angioplasty and stenting based on a consensus of opinion leaders. J Vasc Surg 2001;33(II suppl):s North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325: European Carotid Surgery Trialists Collaborative Group. MCR European Carotid Surgery Trial: interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet 1991;337: Mayberg MR, Wilson SE, Yatsu F, and VA Symptomatic Carotid Stenosis Group. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. JAMA 1991;266: Hobson RW, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, et al, and Veterans Affairs Cooperative Study Group. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. N Engl J Med 1993;328: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid stenosis. JAMA 1995; 273: Bettman MA, Katzen BT, Whisnant J, Cochairs; Brant-Zawadzki M, Broderick JP, et al. AHA Consensus Statement on Carotid Stenting and Angioplasty. Stroke 1998;29: Katzen BT, Ohki T, Gray WA, Smith JAM, Murphy KP. CAS accreditation roundtable. Endovasc Today 2004;3: Barr JD, Connors JJ III, Sacks D, Wojak JC, Becker GJ, Cardella JF, et al. Quality improvement guidelines for the performance of cervical carotid angioplasty and stent placement. Am J Neuroradiol 2003;24: Hobson RW II. Update on the Carotid Revascularization Endarterectomy vs Stent Trial (CREST) protocol. J Am Coll Surg 2002; 194(suppl):S9-14.
6 JOURNAL OF VASCULAR SURGERY Volume 40, Number 5 Hobson et al Roubin GS, New G, Iyer SS, Vitek JJ, Al-Mubarak N, Liu MW, et al. Immediate and late clinical outcomes of carotid artery stenting in patients with symptomatic and asymptomatic carotid artery stenosis. Circulation 2001;103: Alberts MJ, for Publications Committee of the WallStent. Results of a multicenter prospective randomized trial of carotid artery stenting versus carotid endarterectomy [abstract]. Stroke 2001;32: Brown MM, Rogers J, Bland JM, and CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Lancet 2001;357: Yadav JS. Stenting and angioplasty with protection in patients at high risk from endarterectomy: the SAPPHIRE study. Circulation 2002; 106:2986A. 15. CARESS Steering Committee. Carotid revascularization using endarterectomy or stenting systems (CARESS). J Endovasc Ther 2003;10: White RA, Hodgson KJ, Ahn SS, Hobson RW II, Veith FJ. Endovascular interventions training and credentialing for vascular surgeons. J Vasc Surg 1999;29: Al-Mubarak N, Roubin GS, Hobson RW II, Ferguson R, Brott T, Moore WS. Credentialing of stenting operators for the Carotid Revascularization Endarterectomy vs Stenting Trial (CREST) [abstract]. Stroke 2000;31: Hsu JH, Khanna A, Surowiec S, Illig K, Davies M, Shortell C, et al. Use of computer simulation for determining endovascular skill levels in a carotid stenting model. J Vasc Surg In press. 19. Public Advisory Committee of the FDA Circulatory System Device Panel meeting, Gaithersburg, Md, April 21, Submitted Jun 11, 2004; accepted Aug 19, Additional material for this article may be found online at
7 JOURNAL OF VASCULAR SURGERY 957.e1 Table III, online only. Inclusion criteria for lead-in patients Clinical inclusion criteria 1. Patient age Symptomatic, as evidenced by transient ischemic attack, amaurosis fugax, or minor or nondisabling stroke (in hemisphere supplied by target vessel), within 180 days of treatment date, or asymptomatic meeting angiographic criteria ( 70% stenosis). 3. No childbearing potential or negative results of pregnancy test within 1 week before study procedure. 4. Patient and physician agree that the patient will return for all required clinical contacts after study enrollment. 5. Patient informed of nature of study, and has provided written informed consent; approval of appropriate institutional review board or medical ethics committee of respective clinical site. Anatomic inclusion criteria (angiograms used to establish eligibility) 1. Discrete lesion located in internal carotid artery (with or without involvement of contiguous common carotid artery). 2. Carotid stenosis defined as 50% at angiography in symptomatic disease or 70% at angiography in asymptomatic disease. 3. Target internal carotid artery reference diameter must be visually estimated to be 4.0mm and 9.0 mm; may be reasonably estimated at angiography of contralateral artery. 4. Patients with bilateral carotid stenosis eligible. Nonrandomized stenosis may be managed in accordance with local Principal Investigator recommendation. (NOTE: Non-studied artery must be treated at least 30 days before randomization or 30 days after study procedure is completed.) 5. Expected ability to deliver stent to lesion (absence of excessive tortuosity).
8 957.e2 JOURNAL OF VASCULAR SURGERY Table IV, online only. Exclusion criteria for lead-in patients Clinical exclusion criteria 1. Evolving stroke. 2. History of intolerance or allergic reaction to any study medications, including aspirin, ticlopidine, and clopidogrel. (Patients must be able to tolerate a combination of aspirin and ticlopidine, or aspirin and clopidogrel.) 3. Active bleeding diathesis or coagulopathy, or patient refuses blood transfusions. 4. History of major ipsilateral stroke likely to confound study end points. 5. Severe dementia. 6. History of spontaneous intracranial hemorrhage within past 12 months. 7. Recent ( 7 days) stroke of sufficient magnitude (on computed tomography scan or magnetic resonance image) to place patient at risk for hemorrhagic conversion during procedure. 8. Hemorrhagic transformation of ischemic stroke within past 60 days. 9. Hemoglobin concentration 10 g/dl, platelet count 125,000/ L, uncorrected international normalized ratio 1.5, bleeding time 1 minute beyond upper limit of normal, or heparin-associated thrombocytopenia. 10. Any condition that precludes proper angiographic assessment or makes percutaneous arterial access unsafe (eg, morbid obesity, sustained systolic blood pressure 80 mm Hg). 11. Neurologic illness within past 2 years, characterized by fleeting or fixed neurologic deficit that cannot be differentiated from transient ischemic attack or stroke (eg, partial or secondarily generalized seizures, complicated or classic migraine, tumor or other space-occupying brain lesion, subdural hematoma, cerebral contusion or other posttraumatic lesion, intracranial infection, demyelinating disease, moderate to severe dementia, intracranial hemorrhage). 12. Patient actively participating in another drug or device trial (investigational new drug or investigational device exemption) who has not completed required protocol follow-up period. Patients may be enrolled only once in CREST, and may not participate in any other clinical trial during CREST follow-up period. 13. Inability to understand and cooperate with study procedures or provide informed consent. 14. Vertebrobasilar insufficiency symptoms only, without clearly identifiable symptoms referable to study carotid artery. 15. Knowledge of cardiac sources of emboli (eg, left ventricular aneurysm, atrial fibrillation, intracardiac filling defect, cardiomyopathy, aortic or mitral prosthetic heart valve, cardioversion of atrial fibrillation within 1 month before intervention, calcific aortic stenosis, endocarditis, mitral stenosis, atrial septal defect, atrial septal aneurysm, left atrial myxoma). 16. Myocardial infarction within previous 30 days. 17. Recent gastrointestinal bleed that would interfere with antiplatelet therapy. Anatomic exclusions (specific angiographic criteria for all lead-in patients) 1. Severe vascular tortuosity or anatomy that would preclude safe introduction of guiding catheter, guiding sheath, or stent placement. 2. Presence of a previously placed intravascular stent or graft in the ipsilateral distribution. 3. Presence of extensive or diffuse atherosclerotic disease involving aortic arch and proximal common carotid artery that would preclude safe introduction of guiding catheter or guiding sheath. 4. Intraluminal filling defect (defined as endoluminal lucency surrounded by contrast material, seen in multiple angiographic projections, in absence of angiographic evidence of calcification) not associated with ulcerated target lesion. 5. Ipsilateral intracranial or extracranial arterial stenosis greater in severity than lesion to be treated, cerebral aneurysm 5 mm, arteriovenous malformation of cerebral vasculature, or other abnormal angiographic findings that constitute contraindication to CAS. 6. Bilateral carotid stenosis if intervention is planned within 30-day CREST periprocedure period. 7. Occlusion (Thrombolysis in Myocardial Infarction Trial [TIMI 0]) string sign 1 cm of ipsilateral common or internal carotid artery. Well-delineated carotid artery dissection below carotid siphon. Ostial lesion of left or right common carotid artery.
CAROTID DEBATE High-Grade Asymptomatic Disease Should Be Repaired Selectively; Medical Management is NOT Enough
 Todd W GenslerMD April 28, 2018 CAROTID DEBATE High-Grade Asymptomatic Disease Should Be Repaired Selectively; Medical Management is NOT Enough DISCLOSURES I have no financial disclosures Presenter name
Todd W GenslerMD April 28, 2018 CAROTID DEBATE High-Grade Asymptomatic Disease Should Be Repaired Selectively; Medical Management is NOT Enough DISCLOSURES I have no financial disclosures Presenter name
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
CAROTID ARTERY ANGIOPLASTY Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
New Trials in Progress: ACT 1. Jon Matsumura, MD Cannes, France June 28, 2008
 New Trials in Progress: ACT 1 Jon Matsumura, MD Cannes, France June 28, 2008 Faculty Disclosure I disclose the following financial relationships: Consultant, CAS training director, and/or research grants
New Trials in Progress: ACT 1 Jon Matsumura, MD Cannes, France June 28, 2008 Faculty Disclosure I disclose the following financial relationships: Consultant, CAS training director, and/or research grants
Assessment of the procedural etiology of stroke resulting from carotid artery stenting
 Assessment of the procedural etiology of stroke resulting from carotid artery stenting 1. Study Purpose and Rationale: A. Background Stroke is the 3 rd leading cause of death in the United States and carries
Assessment of the procedural etiology of stroke resulting from carotid artery stenting 1. Study Purpose and Rationale: A. Background Stroke is the 3 rd leading cause of death in the United States and carries
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service
 M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
M AY. 6. 2011 10:37 A M F D A - C D R H - O D E - P M O N O. 4147 P. 1 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control
Carotid. The. Issue. Now approved by the FDA, carotid stenting moves into the spotlight in endovascular care.
 The Carotid Issue 33 Carotid Revascularization in 2004 By Thomas G. Brott, MD; Jamie Roberts, LPN, CRC; Robert W. Hobson II, MD; and Susan Hughes, BSN Now approved by the FDA, carotid stenting moves into
The Carotid Issue 33 Carotid Revascularization in 2004 By Thomas G. Brott, MD; Jamie Roberts, LPN, CRC; Robert W. Hobson II, MD; and Susan Hughes, BSN Now approved by the FDA, carotid stenting moves into
Corporate Medical Policy
 Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Corporate Medical Policy Endovascular Therapies for Extracranial Vertebral Artery Disease File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endovascular_therapies_for_extracranial_vertebral_artery_disease
Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary
 SOCIETY FOR VASCULAR SURGERY DOCUMENT Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary John J. Ricotta, MD, a Ali AbuRahma, MD, FACS, b
SOCIETY FOR VASCULAR SURGERY DOCUMENT Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: Executive summary John J. Ricotta, MD, a Ali AbuRahma, MD, FACS, b
AN EXPERIENCE WITH ANGIOPLASTY AND STENTING OF CAROTID ARTERY STENOSIS WITH EMBOLIC PROTECTION DEVICES
 Arch Iranian Med 2006; 9 (2): 138 143 Original Article AN EXPERIENCE WITH ANGIOPLASTY AND STENTING OF CAROTID ARTERY STENOSIS WITH EMBOLIC PROTECTION DEVICES Ali-Mohammad Haji-Zeinali MD *, Davood Kazemi-Saleh
Arch Iranian Med 2006; 9 (2): 138 143 Original Article AN EXPERIENCE WITH ANGIOPLASTY AND STENTING OF CAROTID ARTERY STENOSIS WITH EMBOLIC PROTECTION DEVICES Ali-Mohammad Haji-Zeinali MD *, Davood Kazemi-Saleh
AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS
 Pak Heart J ORIGINAL ARTICLE AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS 1 2 3 4 5 Abhishek Nemani, Arshad Ali, Arshad Rehan, Ali Aboufaris, Jabar Ali 1-4 Guthrie
Pak Heart J ORIGINAL ARTICLE AN ASSESSMENT OF INTER-RATER RELIABILITY IN THE TREATMENT OF CAROTID ARTERY STENOSIS 1 2 3 4 5 Abhishek Nemani, Arshad Ali, Arshad Rehan, Ali Aboufaris, Jabar Ali 1-4 Guthrie
Carotid Artery Stenting Versus
 Carotid Artery Stenting Versus Carotid Endarterectomy Seong-Wook Park, MD, PhD, FACC,, Seoul, Korea Stroke & Carotid artery stenosis Stroke & Carotid artery stenosis Cerebrovascular disease is one of the
Carotid Artery Stenting Versus Carotid Endarterectomy Seong-Wook Park, MD, PhD, FACC,, Seoul, Korea Stroke & Carotid artery stenosis Stroke & Carotid artery stenosis Cerebrovascular disease is one of the
Carotid Artery Stenting Today: A Few Updating Remarks
 Carotid Artery Stenting Today: A Few Updating Remarks Camilo R. Gomez, MD, MBA Director, Alabama Neurological Institute Birmingham, Alabama Disclaimer & Warning Company Pharmaceutical BMS-Sanofi-Aventis
Carotid Artery Stenting Today: A Few Updating Remarks Camilo R. Gomez, MD, MBA Director, Alabama Neurological Institute Birmingham, Alabama Disclaimer & Warning Company Pharmaceutical BMS-Sanofi-Aventis
TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS
 TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS Assistant Professor of Surgery Director of Carotid Interventions Division of Vascular & Endovascular Surgery Stony Brook University
TCAR: TransCarotid Artery Revascularization Angela A. Kokkosis, MD, RPVI, FACS Assistant Professor of Surgery Director of Carotid Interventions Division of Vascular & Endovascular Surgery Stony Brook University
ESC Congress 2011 SIMULTANEOUS HYBRID REVASCULARIZATION OF CAROTID AND CORONARY DISEASE INITIAL RESULTS OF A NEW THERAPEUTIC APPROACH
 ESC Congress 2011 SIMULTANEOUS HYBRID REVASCULARIZATION OF CAROTID AND CORONARY DISEASE IN PATIENTS WITH ACUTE CORONARY SYNDROME: INITIAL RESULTS OF A NEW THERAPEUTIC APPROACH AUTHORS: Marta Ponte 1, RICARDO
ESC Congress 2011 SIMULTANEOUS HYBRID REVASCULARIZATION OF CAROTID AND CORONARY DISEASE IN PATIENTS WITH ACUTE CORONARY SYNDROME: INITIAL RESULTS OF A NEW THERAPEUTIC APPROACH AUTHORS: Marta Ponte 1, RICARDO
Index. interventional.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A ACAS (Asymptomatic Carotid Atherosclerosis Study), 65 66 ACST (Asymptomatic Carotid Surgery Trial), 6 7, 65, 75 Age factors, in carotid
Index Note: Page numbers of article titles are in boldface type. A ACAS (Asymptomatic Carotid Atherosclerosis Study), 65 66 ACST (Asymptomatic Carotid Surgery Trial), 6 7, 65, 75 Age factors, in carotid
The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: Lessons learned and anticipated results
 Thomas L. Forbes, MD, Section Editor CLINICAL TRIALS UPDATE The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: Lessons learned and anticipated results Brajesh K. Lal,
Thomas L. Forbes, MD, Section Editor CLINICAL TRIALS UPDATE The Carotid Revascularization Endarterectomy vs. Stenting Trial completes randomization: Lessons learned and anticipated results Brajesh K. Lal,
Carotid Artery Stenting
 Carotid Artery Stenting Woong Chol Kang M.D. Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea Carotid Stenosis and Stroke ~25% of stroke is due to carotid disease, the reminder
Carotid Artery Stenting Woong Chol Kang M.D. Gil Medical Center, Gachon University of Medicine and Science, Incheon, Korea Carotid Stenosis and Stroke ~25% of stroke is due to carotid disease, the reminder
More than strokes occur
 Surgery vs Stent: Treatment for Carotid Artery Disease Imad A. Alhaddad, MD ABSTRACT PURPOSE: This article summarizes and compares the roles of surgery and stent in the management of carotid artery disease.
Surgery vs Stent: Treatment for Carotid Artery Disease Imad A. Alhaddad, MD ABSTRACT PURPOSE: This article summarizes and compares the roles of surgery and stent in the management of carotid artery disease.
Update on Carotid Stenting. John R. Laird Cardiovascular Research Institute Washington Hospital Center
 Update on Carotid Stenting John R. Laird Cardiovascular Research Institute Washington Hospital Center Carotid Stenting What a Crazy Idea! Pathogenesis of stroke Does it make sense to think that expansion
Update on Carotid Stenting John R. Laird Cardiovascular Research Institute Washington Hospital Center Carotid Stenting What a Crazy Idea! Pathogenesis of stroke Does it make sense to think that expansion
CLINICAL TIMELINE EVA-3S CREST ICSS SPACE SAPPHIRE
 Normal Risk Symptomatic Patients: Ongoing Debate CAS vs CEA John R. Laird, MD Professor of Medicine Medical Director of the Vascular Center University of California, Davis CLINICAL TIMELINE Randomized
Normal Risk Symptomatic Patients: Ongoing Debate CAS vs CEA John R. Laird, MD Professor of Medicine Medical Director of the Vascular Center University of California, Davis CLINICAL TIMELINE Randomized
Disclosures. State of the Art Management of Carotid Stenosis. NIH funding for clinical trials Consultant for Scientia Vascular and Medtronic
 State of the Art Management of Carotid Stenosis Mark R. Harrigan, MD UAB Stroke Center Professor of Neurosurgery, Neurology, and Radiology University of Alabama, Birmingham Disclosures NIH funding for
State of the Art Management of Carotid Stenosis Mark R. Harrigan, MD UAB Stroke Center Professor of Neurosurgery, Neurology, and Radiology University of Alabama, Birmingham Disclosures NIH funding for
CAROTID ARTERY ANGIOPLASTY
 CAROTID ARTERY ANGIOPLASTY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
CAROTID ARTERY ANGIOPLASTY Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and
Small in-stent Low Density on CT Angiography after Carotid Artery Stenting
 www.centauro.it Interventional Neuroradiology 14 (Suppl. 2): 41-46, 2008 Small in-stent Low Density on CT Angiography after Carotid Artery Stenting MIKA OKAHARA 1, HIRO KIYOSUE 2, JUNJI KASHIWAGI 1, SHINYA
www.centauro.it Interventional Neuroradiology 14 (Suppl. 2): 41-46, 2008 Small in-stent Low Density on CT Angiography after Carotid Artery Stenting MIKA OKAHARA 1, HIRO KIYOSUE 2, JUNJI KASHIWAGI 1, SHINYA
Carotid Artery Stenting (CAS) Pathophysiology. Technical Considerations. Plaque characteristics: relevant concepts. CAS and CEA
 Carotid Artery Stenting (CAS) Carotid Artery Stenting for Stroke Risk Reduction Matthew A. Corriere MD, MS, RPVI Assistant Professor of Surgery Department of Vascular and Endovascular Surgery Rationale:
Carotid Artery Stenting (CAS) Carotid Artery Stenting for Stroke Risk Reduction Matthew A. Corriere MD, MS, RPVI Assistant Professor of Surgery Department of Vascular and Endovascular Surgery Rationale:
Slide 1. Slide 2 Conflict of Interest Disclosure. Slide 3 Stroke Facts. The Treatment of Intracranial Stenosis. Disclosure
 Slide 1 The Treatment of Intracranial Stenosis Helmi Lutsep, MD Vice Chair and Dixon Term Professor, Department of Neurology, Oregon Health & Science University Chief of Neurology, VA Portland Health Care
Slide 1 The Treatment of Intracranial Stenosis Helmi Lutsep, MD Vice Chair and Dixon Term Professor, Department of Neurology, Oregon Health & Science University Chief of Neurology, VA Portland Health Care
UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE?
 UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE? Richard W. Petrella M.D. FACP,FACC,FASCI DEPARTMENT CHAIRMAN CVM&S UPMC HAMOT MEDICAL CENTER 1 LEARNING OBJECTIVES REVIEW THE RISK FACTORS FOR
UPMC HAMOT CAROTID ARTERY DISEASE WHERE DO WE GO FROM HERE? Richard W. Petrella M.D. FACP,FACC,FASCI DEPARTMENT CHAIRMAN CVM&S UPMC HAMOT MEDICAL CENTER 1 LEARNING OBJECTIVES REVIEW THE RISK FACTORS FOR
Carotid Endarterectomy versus Carotid Angioplasty Cui Bono *
 Eur J Vasc Endovasc Surg (2010) 39, S44eS48 Carotid Endarterectomy versus Carotid Angioplasty Cui Bono * W.S. Moore* Division of Vascular Surgery, David Geffen School of Medicine at UCLA, 200 UCLA Medical
Eur J Vasc Endovasc Surg (2010) 39, S44eS48 Carotid Endarterectomy versus Carotid Angioplasty Cui Bono * W.S. Moore* Division of Vascular Surgery, David Geffen School of Medicine at UCLA, 200 UCLA Medical
Guidelines for Ultrasound Surveillance
 Guidelines for Ultrasound Surveillance Carotid & Lower Extremity by Ian Hamilton, Jr, MD, MBA, RPVI, FACS Corporate Medical Director BlueCross BlueShield of Tennessee guidelines for ultrasound surveillance
Guidelines for Ultrasound Surveillance Carotid & Lower Extremity by Ian Hamilton, Jr, MD, MBA, RPVI, FACS Corporate Medical Director BlueCross BlueShield of Tennessee guidelines for ultrasound surveillance
Carotid artery stenting in the elderly: the time has come
 88 Journal of Geriatric Cardiology June 2007 Vol 4 No 2 Symposium: Review Article Carotid artery stenting in the elderly: the time has come Dipsu Patel, Neil E Strickman St. Luke s Episcopal Hospital/Texas
88 Journal of Geriatric Cardiology June 2007 Vol 4 No 2 Symposium: Review Article Carotid artery stenting in the elderly: the time has come Dipsu Patel, Neil E Strickman St. Luke s Episcopal Hospital/Texas
ORIGINAL CONTRIBUTION
 ORIGINAL CONTRIBUTION Safety of Latest-Generation Self-expanding Stents in Patients With NASCET-Ineligible Severe Symptomatic Extracranial Internal Carotid Artery Stenosis Italo Linfante, MD; Joshua A.
ORIGINAL CONTRIBUTION Safety of Latest-Generation Self-expanding Stents in Patients With NASCET-Ineligible Severe Symptomatic Extracranial Internal Carotid Artery Stenosis Italo Linfante, MD; Joshua A.
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine
 Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
Vivek R. Deshmukh, MD Director, Cerebrovascular and Endovascular Neurosurgery Chairman, Department of Neurosurgery Providence Brain and Spine Institute The Oregon Clinic Disclosure I declare that neither
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY BROCHURE
 STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY BROCHURE Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in
STONY BROOK UNIVERSITY HOSPITAL VASCULAR CENTER CREDENTIALING POLICY BROCHURE Per Medical Board decision March 18, 2008: These credentialing standards do NOT apply to peripheral angiography performed in
Carotid Artery Revascularization: Current Strategies. Shonda Banegas, D.O. Vascular Surgery Carondelet Heart and Vascular Institute September 6, 2014
 Carotid Artery Revascularization: Current Strategies Shonda Banegas, D.O. Vascular Surgery Carondelet Heart and Vascular Institute September 6, 2014 Disclosures None 1 Stroke in 2014 Stroke kills almost
Carotid Artery Revascularization: Current Strategies Shonda Banegas, D.O. Vascular Surgery Carondelet Heart and Vascular Institute September 6, 2014 Disclosures None 1 Stroke in 2014 Stroke kills almost
Carlo Setacci Chief Department of Surgery Vascular and Endovascular Unit University of Siena
 Which carotid procedures are required to grade the stroke risk? Carlo Setacci Chief Department of Surgery Vascular and Endovascular Unit University of Siena Faculty disclosure Carlo Setacci I have no financial
Which carotid procedures are required to grade the stroke risk? Carlo Setacci Chief Department of Surgery Vascular and Endovascular Unit University of Siena Faculty disclosure Carlo Setacci I have no financial
Extracranial Carotid Artery/Stenting
 Extracranial Carotid Artery/Stenting Policy Number: 7.01.68 Last Review: 6/2018 Origination: 4/2005 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Extracranial Carotid Artery/Stenting Policy Number: 7.01.68 Last Review: 6/2018 Origination: 4/2005 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Carotid Artery Stenting
 Carotid Artery Stenting JESSICA MITCHELL, ACNP CENTRAL ILLINOIS RADIOLOGICAL ASSOCIATES External Carotid Artery (ECA) can easily be identified from Internal Carotid Artery (ICA) by noticing the branches.
Carotid Artery Stenting JESSICA MITCHELL, ACNP CENTRAL ILLINOIS RADIOLOGICAL ASSOCIATES External Carotid Artery (ECA) can easily be identified from Internal Carotid Artery (ICA) by noticing the branches.
CAROTID STENTING A 2009 UPDATE. Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital
 CAROTID STENTING A 2009 UPDATE Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital TREATMENT FOR CAROTID STENOSIS Best medical management Antiplatelet therapy Antihypertensive
CAROTID STENTING A 2009 UPDATE Hoang Duong, MD Director of Interventional Neuroradiology Memorial Regional Hospital TREATMENT FOR CAROTID STENOSIS Best medical management Antiplatelet therapy Antihypertensive
Disclosures. CREST Trial: Summary. Lecture Outline 4/16/2015. Cervical Atherosclerotic Disease
 Disclosures Your Patient Has Carotid Bulb Stenosis and a Tandem Intracranial Stenosis: How Do SAMMPRIS and Other Evidence Inform Your Treatment? UCSF Vascular Symposium 2015 Steven W. Hetts, MD Associate
Disclosures Your Patient Has Carotid Bulb Stenosis and a Tandem Intracranial Stenosis: How Do SAMMPRIS and Other Evidence Inform Your Treatment? UCSF Vascular Symposium 2015 Steven W. Hetts, MD Associate
In-stent recurrent stenosis after carotid artery stenting: Life table analysis and clinical relevance
 From the Eastern Vascular Society In-stent recurrent stenosis after carotid artery stenting: Life table analysis and clinical relevance Brajesh K. Lal, MD, a Robert W. Hobson II, MD, a Jonathan Goldstein,
From the Eastern Vascular Society In-stent recurrent stenosis after carotid artery stenting: Life table analysis and clinical relevance Brajesh K. Lal, MD, a Robert W. Hobson II, MD, a Jonathan Goldstein,
Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective)
 Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective) T-Woei Tan, MD, FACS, RPVI Assistant Professor of Surgery Vascular and Endovascular Surgery Louisiana State University Health -
Carotid Endarterectomy vs. Carotid artery Stenting (Surgeon Perspective) T-Woei Tan, MD, FACS, RPVI Assistant Professor of Surgery Vascular and Endovascular Surgery Louisiana State University Health -
Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO
 Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO Goal of treatment of carotid disease Identify those at risk of developing symptoms Prevent patients at risk from developing symptoms Prevent
Carotid Artery Disease and What s Pertinent JOSEPH A PAULISIN DO Goal of treatment of carotid disease Identify those at risk of developing symptoms Prevent patients at risk from developing symptoms Prevent
Comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR study
 Comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR study (RADial access for CARotide artery stenting) Zoltán Ruzsa MD PhD et al. TCT 2013 Disclosure Statement of Financial
Comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR study (RADial access for CARotide artery stenting) Zoltán Ruzsa MD PhD et al. TCT 2013 Disclosure Statement of Financial
Carotid Artery Stenting
 Carotid Artery Stenting Natural history of the carotid stenosis Asymptomatic 80% carotid stenosis - 6% risk of stroke / year Symptomatic carotid stenosis have 10% risk of CVA at one year and 40% at 5 years
Carotid Artery Stenting Natural history of the carotid stenosis Asymptomatic 80% carotid stenosis - 6% risk of stroke / year Symptomatic carotid stenosis have 10% risk of CVA at one year and 40% at 5 years
2019 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule
 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
Contemporary Management of Carotid Disease What We Know So Far
 Contemporary Management of Carotid Disease What We Know So Far Ammar Safar, MD, FSCAI, FACC, FACP, RPVI Interventional Cardiology & Endovascular Medicine Disclosers NONE Epidemiology 80 % of stroke are
Contemporary Management of Carotid Disease What We Know So Far Ammar Safar, MD, FSCAI, FACC, FACP, RPVI Interventional Cardiology & Endovascular Medicine Disclosers NONE Epidemiology 80 % of stroke are
About 700,000 Americans each year suffer a new or recurrent stroke. On average, a stroke occurs every 45 seconds
 UCLA Stroke Center Stroke Facts About 700,000 Americans each year suffer a new or recurrent stroke On average, a stroke occurs every 45 seconds Stroke kills more than 150,000 people a year (1 of every
UCLA Stroke Center Stroke Facts About 700,000 Americans each year suffer a new or recurrent stroke On average, a stroke occurs every 45 seconds Stroke kills more than 150,000 people a year (1 of every
Carotid artery stents and embolic protection
 Regulation of Carotid Artery Stents and Embolic Protection Devices in the United States A history of, and perspectives on, FDA regulation of carotid stents and associated embolic protection devices over
Regulation of Carotid Artery Stents and Embolic Protection Devices in the United States A history of, and perspectives on, FDA regulation of carotid stents and associated embolic protection devices over
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Extracranial Carotid Artery Stenting Page 1 of 23 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Extracranial Carotid Artery Stenting Professional Institutional
Extracranial Carotid Artery Stenting Page 1 of 23 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Extracranial Carotid Artery Stenting Professional Institutional
Why I do not believe the
 Why I do not believe the EVA-3S Trial J Mocco,, MD Ken Snyder, MD,PhD LN Hopkins, MD University at Buffalo Neurosurgery LN Hopkins, MD Potential Conflicts Consultant & research support: Boston Scientific,
Why I do not believe the EVA-3S Trial J Mocco,, MD Ken Snyder, MD,PhD LN Hopkins, MD University at Buffalo Neurosurgery LN Hopkins, MD Potential Conflicts Consultant & research support: Boston Scientific,
Comparison of Five Major Recent Endovascular Treatment Trials
 Comparison of Five Major Recent Endovascular Treatment Trials Sample size 500 # sites 70 (100 planned) 316 (500 planned) 196 (833 estimated) 206 (690 planned) 16 10 22 39 4 Treatment contrasts Baseline
Comparison of Five Major Recent Endovascular Treatment Trials Sample size 500 # sites 70 (100 planned) 316 (500 planned) 196 (833 estimated) 206 (690 planned) 16 10 22 39 4 Treatment contrasts Baseline
CEREBRO VASCULAR ACCIDENTS
 CEREBRO VASCULAR S MICHAEL OPONG-KUSI, DO MBA MORTON CLINIC, TULSA, OK, USA 8/9/2012 1 Cerebrovascular Accident Third Leading cause of deaths (USA) 750,000 strokes in USA per year. 150,000 deaths in USA
CEREBRO VASCULAR S MICHAEL OPONG-KUSI, DO MBA MORTON CLINIC, TULSA, OK, USA 8/9/2012 1 Cerebrovascular Accident Third Leading cause of deaths (USA) 750,000 strokes in USA per year. 150,000 deaths in USA
2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY. MEASURE TYPE: Outcome
 Measure #344: Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Effective Clinical
Measure #344: Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major Complications (Discharged to Home by Post-Operative Day #2) National Quality Strategy Domain: Effective Clinical
Carotid Artery Stent: Is it ready for prime time?
 2010 CATH LAB SYMPOSIUM Carotid Artery Stent: Is it ready for prime time? Luis F. Tami, MD, FACC, FSCAI Interventional Cardiology and Vascular Medicine Memorial Regional Hospital August 2010 CAE and CAS
2010 CATH LAB SYMPOSIUM Carotid Artery Stent: Is it ready for prime time? Luis F. Tami, MD, FACC, FSCAI Interventional Cardiology and Vascular Medicine Memorial Regional Hospital August 2010 CAE and CAS
Original Article. Abstract. Introduction. Patients and Methods:
 Original Article Endovascular stenting for carotid artery stenosis: Results of local experience at Aga Khan University Hospital, Karachi Muhammad Azeem Uddin, Tanveer ul Haq, Ishtiaque Ahmed Chishty, Zafar
Original Article Endovascular stenting for carotid artery stenosis: Results of local experience at Aga Khan University Hospital, Karachi Muhammad Azeem Uddin, Tanveer ul Haq, Ishtiaque Ahmed Chishty, Zafar
Peter A. Soukas, M.D., FACC, FSVM, FSCAI, RPVI
 Peter A. Soukas, M.D., FACC, FSVM, FSCAI, RPVI Director, Peripheral Vascular Interventional Laboratory Director, Vascular & Endovascular Medicine Fellowship Program Assistant Professor of Medicine The
Peter A. Soukas, M.D., FACC, FSVM, FSCAI, RPVI Director, Peripheral Vascular Interventional Laboratory Director, Vascular & Endovascular Medicine Fellowship Program Assistant Professor of Medicine The
Extracranial Carotid Artery/Stenting
 Extracranial Carotid Artery/Stenting Policy Number: 7.01.68 Last Review: 6/2017 Origination: 4/2005 Next Review: 6/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Extracranial Carotid Artery/Stenting Policy Number: 7.01.68 Last Review: 6/2017 Origination: 4/2005 Next Review: 6/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
CY2015 Hospital Outpatient: Endovascular Procedure APCs and Complexity Adjustments
 CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
CY2015 Hospital Outpatient: Endovascular Procedure APCs Complexity Adjustments Comprehensive Ambulatory Payment Classifications (c-apcs) CMS finalized the implementation of 25 Comprehensive APC to further
Preoperative risk factors for carotid endarterectomy: Defining the patient at high risk
 Preoperative risk factors for carotid endarterectomy: Defining the patient at high risk Amy B. Reed, MD, a Peter Gaccione, MA, b Michael Belkin, MD, b Magruder C. Donaldson, MD, b John A. Mannick, MD,
Preoperative risk factors for carotid endarterectomy: Defining the patient at high risk Amy B. Reed, MD, a Peter Gaccione, MA, b Michael Belkin, MD, b Magruder C. Donaldson, MD, b John A. Mannick, MD,
Elective stenting of carotid artery stenosis in patients with severe coronary artery disease
 European Heart Journal (1998) 19, 1365 1370 Article No. hj981048 Elective stenting of carotid artery stenosis in patients with severe coronary artery disease J. Waigand, C. M. Gross, F. Uhlich, J. Krämer,
European Heart Journal (1998) 19, 1365 1370 Article No. hj981048 Elective stenting of carotid artery stenosis in patients with severe coronary artery disease J. Waigand, C. M. Gross, F. Uhlich, J. Krämer,
Carotid artery percutaneous treatment: back to the future Alberto Cremonesi MD, FESC
 Carotid artery percutaneous treatment: back to the future Alberto Cremonesi MD, FESC GVM Care & Research - Cardiovascular Department (Cotignola Italy) Hypothesis: Does CAS present similar outcomes than
Carotid artery percutaneous treatment: back to the future Alberto Cremonesi MD, FESC GVM Care & Research - Cardiovascular Department (Cotignola Italy) Hypothesis: Does CAS present similar outcomes than
Special Topic Section
 Special Topic Section Cerebrovasc Dis 2004;18:69 74 DOI: 10.1159/000078753 Received: March 8, 2004 Accepted: March 8, 2004 Published online: June 1, 2004 International Carotid Stenting Study: Protocol
Special Topic Section Cerebrovasc Dis 2004;18:69 74 DOI: 10.1159/000078753 Received: March 8, 2004 Accepted: March 8, 2004 Published online: June 1, 2004 International Carotid Stenting Study: Protocol
Management of Carotid Disease CHRISTOPHER LAU PGY-3 BROOKLYN VA
 Management of Carotid Disease CHRISTOPHER LAU PGY-3 BROOKLYN VA SUNY DOWNSTATE MEDICAL CENTER Case 61 year old male referred to Vascular Surgery for left internal carotid stenosis Presented with transient
Management of Carotid Disease CHRISTOPHER LAU PGY-3 BROOKLYN VA SUNY DOWNSTATE MEDICAL CENTER Case 61 year old male referred to Vascular Surgery for left internal carotid stenosis Presented with transient
Multicenter Evaluation of Carotid Artery Stenting With a Filter Protection System
 Journal of the American College of Cardiology Vol. 39, No. 5, 2002 2002 by the American College of Cardiology Foundation ISSN 0735-1097/02/$22.00 Published by Elsevier Science Inc. PII S0735-1097(02)01692-3
Journal of the American College of Cardiology Vol. 39, No. 5, 2002 2002 by the American College of Cardiology Foundation ISSN 0735-1097/02/$22.00 Published by Elsevier Science Inc. PII S0735-1097(02)01692-3
APPENDIX A NORTH AMERICAN SYMPTOMATIC CAROTID ENDARTERECTOMY TRIAL
 APPENDIX A Primary Findings From Selected Recent National Institute of Neurological Disorders and Stroke-Sponsored Clinical Trials That Have shaped Modern Stroke Prevention Philip B. Gorelick 178 NORTH
APPENDIX A Primary Findings From Selected Recent National Institute of Neurological Disorders and Stroke-Sponsored Clinical Trials That Have shaped Modern Stroke Prevention Philip B. Gorelick 178 NORTH
Carotid Artery Stenosis
 Evidence-Based Approach to Carotid Artery Stenosis Seong-Wook Park, MD Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea Carotid Artery Stenosis Carotid
Evidence-Based Approach to Carotid Artery Stenosis Seong-Wook Park, MD Division of Cardiology, Asan Medical Center University of Ulsan College of Medicine, Seoul, Korea Carotid Artery Stenosis Carotid
How to Build a Multi Disciplinary University Based Vascular Practice in the Same Division and Under the Same Leadership
 How to Build a Multi Disciplinary University Based Vascular Practice in the Same Division and Under the Same Leadership Ali F. AbuRahma, M.D. Professor of Surgery Chief, Vascular & Endovascular Surgery
How to Build a Multi Disciplinary University Based Vascular Practice in the Same Division and Under the Same Leadership Ali F. AbuRahma, M.D. Professor of Surgery Chief, Vascular & Endovascular Surgery
Pre-and Post Procedure Non-Invasive Evaluation of the Patient with Carotid Disease
 Pre-and Post Procedure Non-Invasive Evaluation of the Patient with Carotid Disease Michael R. Jaff, D.O., F.A.C.P., F.A.C.C. Assistant Professor of Medicine Harvard Medical School Director, Vascular Medicine
Pre-and Post Procedure Non-Invasive Evaluation of the Patient with Carotid Disease Michael R. Jaff, D.O., F.A.C.P., F.A.C.C. Assistant Professor of Medicine Harvard Medical School Director, Vascular Medicine
Review of clinical carotid stent procedural & long-term outcomes in. symptomatic asymptomatic. patients
 Review of clinical carotid stent procedural & long-term outcomes in symptomatic asymptomatic patients 1 Conflict of Interest Statement Within the past 12 months, I or my spouse have had a financial interest/arrangement
Review of clinical carotid stent procedural & long-term outcomes in symptomatic asymptomatic patients 1 Conflict of Interest Statement Within the past 12 months, I or my spouse have had a financial interest/arrangement
Treatment Considerations for Carotid Artery Stenosis. Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery
 Treatment Considerations for Carotid Artery Stenosis Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery 4.29.2016 There is no actual or potential conflict of interest in regards to this presentation
Treatment Considerations for Carotid Artery Stenosis Danielle Zielinski, RN, MSN, ACNP Rush University Neurosurgery 4.29.2016 There is no actual or potential conflict of interest in regards to this presentation
Two major randomised trials concerning the surgical treatment of carotid artery stenosis
 944 * See end of article for authors affiliations c CAROTID Correspondence to: Dr Carlo Di Mario, Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK; c.dimario@rbh.nthames.nhs.uk General cardiology
944 * See end of article for authors affiliations c CAROTID Correspondence to: Dr Carlo Di Mario, Royal Brompton Hospital, Sydney Street, London SW3 6NP, UK; c.dimario@rbh.nthames.nhs.uk General cardiology
Subclavian artery Stenting
 Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Subclavian artery Stenting Etiology Atherosclerosis Takayasu s arteritis Fibromuscular dysplasia Giant Cell Arteritis Radiation-induced Vascular Injury Thoracic Outlet Syndrome Neurofibromatosis Incidence
Subclavian and Vertebral Artery Angioplasty - Vertebro-basilar Insufficiency: Clinical Aspects and Diagnosis
 HOSPITAL CHRONICLES 2008, 3(3): 136 140 ORIGINAL ARTICLE Subclavian and Vertebral Artery Angioplasty - Vertebro-basilar Insufficiency: Clinical Aspects and Diagnosis Antonios Polydorou, MD Hemodynamic
HOSPITAL CHRONICLES 2008, 3(3): 136 140 ORIGINAL ARTICLE Subclavian and Vertebral Artery Angioplasty - Vertebro-basilar Insufficiency: Clinical Aspects and Diagnosis Antonios Polydorou, MD Hemodynamic
Carotid Revascularization
 Options for Carotid Disease Carotid Revascularization Wayne Causey, MD 2 nd Year Vascular Surgery Fellow Best medical therapy, Carotid Endarterectomy, and Carotid Stenting Who benefits from best medical
Options for Carotid Disease Carotid Revascularization Wayne Causey, MD 2 nd Year Vascular Surgery Fellow Best medical therapy, Carotid Endarterectomy, and Carotid Stenting Who benefits from best medical
Technology Assessment Report
 ICSI I NSTITUTE FOR CLINICAL S YSTEMS IMPROVEMENT Report The information contained in this ICSI Report is intended primarily for health professionals and the following expert audiences: physicians, nurses,
ICSI I NSTITUTE FOR CLINICAL S YSTEMS IMPROVEMENT Report The information contained in this ICSI Report is intended primarily for health professionals and the following expert audiences: physicians, nurses,
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
SCAI Fall Fellows Course Subclavian/Innominate Case Presentation
 SCAI Fall Fellows Course 2012 Subclavian/Innominate Case Presentation Daniel J. McCormick DO, FACC, FSCAI Director, Cardiovascular Interventional Therapy Pennsylvania Hospital University of Pennsylvania
SCAI Fall Fellows Course 2012 Subclavian/Innominate Case Presentation Daniel J. McCormick DO, FACC, FSCAI Director, Cardiovascular Interventional Therapy Pennsylvania Hospital University of Pennsylvania
a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers
 The study a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers Conflict of interest have the following potential conflicts of interest to report: Consulting
The study a physician-initiated study investigating the RoadSaver stent in carotid lesions Dr. Michel Bosiers Conflict of interest have the following potential conflicts of interest to report: Consulting
ROADSTER 2. SPONSOR: Silk Road Medical
 ROADSTER 2 CONDITION: Carotid artery disease PI: Jessica Titus, MD CONTACT INFO: Jo Anne Goldman JoAnne.Goldman@allina.com 612-863-3793 DESCRIPTION: The study is intended to evaluate real world usage of
ROADSTER 2 CONDITION: Carotid artery disease PI: Jessica Titus, MD CONTACT INFO: Jo Anne Goldman JoAnne.Goldman@allina.com 612-863-3793 DESCRIPTION: The study is intended to evaluate real world usage of
My Latest Take on RCT Data: When is CEA or CAS the Best Option? The Interventional Position
 LINC 2016 Leipzig, Jan 26-29, 2016 My Latest Take on RCT Data: When is CEA or CAS the Best Option? The Interventional Position Horst Sievert, Iris Grunwald CardioVasculäres Centrum Frankfurt - CVC, Frankfurt
LINC 2016 Leipzig, Jan 26-29, 2016 My Latest Take on RCT Data: When is CEA or CAS the Best Option? The Interventional Position Horst Sievert, Iris Grunwald CardioVasculäres Centrum Frankfurt - CVC, Frankfurt
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014
 Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
CAROTID ANGIOPLASTY AND STENTING UNDER PROTECTION IS BECOMING THE GOLD STANDARD TREATMENT IN HIGH AND LOW RISK PATIENTS
 CAROTID ANGIOPLASTY AND STENTING UNDER PROTECTION IS BECOMING THE GOLD STANDARD TREATMENT IN HIGH AND LOW RISK PATIENTS M. HENRY* MD, I. HENRY MD A. POLYDOROU MD, A.D. POLYDOROU MD M. HUGEL RN NANCY FRANCE
CAROTID ANGIOPLASTY AND STENTING UNDER PROTECTION IS BECOMING THE GOLD STANDARD TREATMENT IN HIGH AND LOW RISK PATIENTS M. HENRY* MD, I. HENRY MD A. POLYDOROU MD, A.D. POLYDOROU MD M. HUGEL RN NANCY FRANCE
Techniques for Carotid Interventon
 Techniques for Carotid Interventon Jay S. Yadav MD Cleveland, Ohio Disclosures Inventor of Angioguard; fixed and recurring payments from JNJ Advisor: JNJ, Guidant Understand the Patient What is the Cause
Techniques for Carotid Interventon Jay S. Yadav MD Cleveland, Ohio Disclosures Inventor of Angioguard; fixed and recurring payments from JNJ Advisor: JNJ, Guidant Understand the Patient What is the Cause
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Spontaneous Recanalization after Complete Occlusion of the Common Carotid Artery with Subsequent Embolic Ischemic Stroke
 Original Contribution Spontaneous Recanalization after Complete Occlusion of the Common Carotid Artery with Subsequent Embolic Ischemic Stroke Abstract Introduction: Acute carotid artery occlusion carries
Original Contribution Spontaneous Recanalization after Complete Occlusion of the Common Carotid Artery with Subsequent Embolic Ischemic Stroke Abstract Introduction: Acute carotid artery occlusion carries
BULgarian Carotid Artery Stenting versus Surgery Study (BULCASSS): Randomized single center trial
 BULgarian Carotid Artery Stenting versus Surgery Study (): Randomized single center trial Ivo Petrov, M. Konteva, H. Dimitrov, K. Kichukov Tokuda Hospital Sofia Cardiology Department Background Carotid
BULgarian Carotid Artery Stenting versus Surgery Study (): Randomized single center trial Ivo Petrov, M. Konteva, H. Dimitrov, K. Kichukov Tokuda Hospital Sofia Cardiology Department Background Carotid
Overview of Subclavian & Innominate Artery Interventions
 TCT 2016 Washington, DC, USA Tuesday November 1st, 2016 Peripheral vascular interventions Overview of Subclavian & Innominate Artery Interventions Dr Jacques Busquet Vascular & Endovascular Surgery Paris,
TCT 2016 Washington, DC, USA Tuesday November 1st, 2016 Peripheral vascular interventions Overview of Subclavian & Innominate Artery Interventions Dr Jacques Busquet Vascular & Endovascular Surgery Paris,
Endarterectomy for Mild Cervical Carotid Artery Stenosis in Patients With Ischemic Stroke Events Refractory to Medical Treatment
 Neurol Med Chir (Tokyo) 48, 211 215, 2008 Endarterectomy for Mild Cervical Carotid Artery Stenosis in Patients With Ischemic Stroke Events Refractory to Medical Treatment Two Case Reports Masakazu KOBAYASHI,
Neurol Med Chir (Tokyo) 48, 211 215, 2008 Endarterectomy for Mild Cervical Carotid Artery Stenosis in Patients With Ischemic Stroke Events Refractory to Medical Treatment Two Case Reports Masakazu KOBAYASHI,
Carotid Stenosis (carotid artery disease)
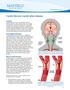 1 Carotid Stenosis (carotid artery disease) Overview Carotid stenosis is a narrowing of the carotid arteries, the two major arteries that carry oxygenrich blood from the heart to the brain. Also called
1 Carotid Stenosis (carotid artery disease) Overview Carotid stenosis is a narrowing of the carotid arteries, the two major arteries that carry oxygenrich blood from the heart to the brain. Also called
Surgical Treatment of Carotid Disease
 Department of Cardiothoracic & Vascular Surgery McGovern Medical School / The University of Texas Health Science Center at Houston Surgical Treatment of Carotid Disease The Old, the New, and the Future
Department of Cardiothoracic & Vascular Surgery McGovern Medical School / The University of Texas Health Science Center at Houston Surgical Treatment of Carotid Disease The Old, the New, and the Future
Algorithmic selection of emboli protection device during the procedure of carotid artery stunting
 Algorithmic selection of emboli protection device during the procedure of carotid artery stunting Yasuhiro Kawabata, Tetsuya Tsukahara, Shunichi Fukuda, Tomokazu Aoki, Satoru Kawarazaki Department of Neurosurgery,
Algorithmic selection of emboli protection device during the procedure of carotid artery stunting Yasuhiro Kawabata, Tetsuya Tsukahara, Shunichi Fukuda, Tomokazu Aoki, Satoru Kawarazaki Department of Neurosurgery,
REBEL. Platinum Chromium Coronary Stent System. Patient Information Guide
 REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
REBEL Patient Information Guide REBEL PATIENT INFORMATION GUIDE You have recently had a REBEL bare metal stent implanted in the coronary arteries of your heart. The following information is important for
Carotid Stenting and Surgery in 2016 in Russia
 Carotid Stenting and Surgery in 2016 in Russia Novosibirsk research institute of circulation pathology named by Meshalkin, Novosibirsk, Russia Starodubtsev V., Karpenko A., Ignatenko P. Annually in Russia
Carotid Stenting and Surgery in 2016 in Russia Novosibirsk research institute of circulation pathology named by Meshalkin, Novosibirsk, Russia Starodubtsev V., Karpenko A., Ignatenko P. Annually in Russia
Clinical Policy Bulletin: Angioplasty and Stenting of Extra-Cranial and Intra-Cranial Arteries
 Go Clinical Policy Bulletin: Angioplasty and Stenting of Extra-Cranial and Intra-Cranial Arteries Number: 0276 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Additional
Go Clinical Policy Bulletin: Angioplasty and Stenting of Extra-Cranial and Intra-Cranial Arteries Number: 0276 Policy *Please see amendment for Pennsylvania Medicaid at the end of this CPB. Additional
MEET 2007: Evaluation and treatment of the stroke and TIA patient for the non-neurointerventionist. neurointerventionist
 MEET 2007: Evaluation and treatment of the stroke and TIA patient for the non-neurointerventionist neurointerventionist Steve Ramee, MD Ochsner Medical Center New Orleans DISCLOSURE Nothing Nothing to
MEET 2007: Evaluation and treatment of the stroke and TIA patient for the non-neurointerventionist neurointerventionist Steve Ramee, MD Ochsner Medical Center New Orleans DISCLOSURE Nothing Nothing to
Why I m afraid of occlusive devices
 Why I m afraid of occlusive devices Cannes 28.06.2008 Carlo Cernetti Cardiology Department Mirano (Venice) MEET 2008 CANNES I HAVE NOT FINACIAL INTEREST/ARRANGEMENT OR AFFILIATION CONFLICT Obstructive
Why I m afraid of occlusive devices Cannes 28.06.2008 Carlo Cernetti Cardiology Department Mirano (Venice) MEET 2008 CANNES I HAVE NOT FINACIAL INTEREST/ARRANGEMENT OR AFFILIATION CONFLICT Obstructive
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS
 CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Wein T, Gladstone D (Writing Group Chairs) on Behalf of the PREVENTION of STROKE
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS Management of Extracranial Carotid Disease and Intracranial Atherosclerosis Wein T, Gladstone D (Writing Group Chairs) on Behalf of the PREVENTION of STROKE
Vascular Surgery Rotation Objectives for Junior Residents (PGY-1 and 2)
 Vascular Surgery Rotation Objectives for Junior Residents (PGY-1 and 2) Definition Vascular surgery is the specialty concerned with the diagnosis and management of congenital and acquired diseases of the
Vascular Surgery Rotation Objectives for Junior Residents (PGY-1 and 2) Definition Vascular surgery is the specialty concerned with the diagnosis and management of congenital and acquired diseases of the
WHI Form Report of Cardiovascular Outcome Ver (For items 1-11, each question specifies mark one or mark all that apply.
 WHI Form - Report of Cardiovascular Outcome Ver. 6. COMMENTS To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: OMB# 095-044 Exp: 4/06 -Affix label here- Clinical Center/ID:
WHI Form - Report of Cardiovascular Outcome Ver. 6. COMMENTS To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: OMB# 095-044 Exp: 4/06 -Affix label here- Clinical Center/ID:
Articles. Funding Medical Research Council, the Stroke Association, Sanofi-Synthélabo, European Union.
 Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial International
Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial International
