COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
|
|
|
- Melina Wheeler
- 5 years ago
- Views:
Transcription
1 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 26 June 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON THE CLINICAL DEVELOPMENT OF FIBRINOLYTIC MEDICINAL PRODUCTS IN THE TREATMENT OF PATIENTS WITH ST SEGMENT ELEVATION ACUTE MYOCARDIAL INFARCTION (STEMI) DISCUSSION IN THE EFFICACY WORKING PARTY May 2001 October 2002 TRANSMISSION TO CPMP November 2002 RELEASE FOR CONSULTATION November 2002 DEADLINE FOR COMMENTS February 2003 DISCUSSION IN THE EFFICACY WORKING PARTY April 2003 TRANSMISSION TO CPMP June 2003 ADOPTION BY CPMP June 2003 DATE FOR COMING INTO OPERATION December Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) Fax (44-20) mail@emea.eu.int Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged
2 POINTS TO CONSIDER ON THE CLINICAL DEVELOPMENT OF FIBRINOLYTIC MEDICINAL PRODUCTS IN THE TREATMENT OF PATIENTS WITH ST SEGMENT ELEVATION ACUTE MYOCARDIAL INFARCTION (STEMI) PREAMBLE This document intends to address the EU regulatory position on the main topics of the clinical development of new fibrinolytics in the treatment of patients with ST segment elevation acute myocardial infarction (STEMI). The scope of the present document is restricted to fibrinolytic therapy and will not consider other drugs intended to be used in the acute or chronic treatment of patients with STEMI. However, it must be understood that, under the term fibrinolytic therapy, this document refers to the whole fibrinolytic, anticoagulant and antiplatelet strategy under which each specific fibrinolytic agent has demonstrated an optimised benefit/risk ratio. On the other hand, the recanalisation strategy in patients with acute coronary syndrome (both STEMI and NSTEMI) is considered a rapidly evolving field where new combination strategies (revascularisation procedures, fibrinolytics, GPIIb/IIIa antagonists, LMWH, etc) are currently being assessed. In this regard, there is still little regulatory experience for giving specific recommendations on all possible strategies and study designs. Consequently, requesting scientific advice on case-by-case basis is recommended. This Note for Guidance document should be read in conjunction with the Directives 75/18/EEC, as amended, as well as in conjunction with other pertinent regulatory European and ICH documents, with special emphasis on: Note for Guidance on General Considerations for Clinical Trials (CPMP/ICH/291/95) Note for Guidance on Good Clinical Practice (CPMP/ICH/135/95) Note for Guidance on Dose Response Information to support Drug Registration (CPMP/ICH/378/95) Note for Guidance on Statistical Principles for Clinical Trials (CPMP/ICH/363/96) Note for Guidance on Choice of Control Group for Clinical Trials (CPMP/ICH/364/96) Points to Consider on Switching between Superiority and Non-inferiority (CPMP/EWP/482/99) Note for Guidance on the Investigation of Drug Interactions (CPMP/EWP/560/95) Points to Consider on the Clinical Investigation of New Medicinal Products for the Treatment of Unstable Angina Pectoris or Non-Q-Wave Myocardial Infarction (CPMP/EWP/570/98). There is no current regulatory guidance on this field. 1. BACKGROUND Myocardial infarction (MI) is (rapid development of) myocardial necrosis due to a critical imbalance between the oxygen supply and demand of the myocardium. The most common cause of MI is acute coronary occlusion, at the site of previous narrowing of the epicardial blood vessels due to atherosclerotic plaques, plaque rupture with subsequent exposure of the basement membrane and platelet aggregation, fibrin accumulation, thrombus formation, haemorrhage into the plaque. Varying degrees of vasospasm may also occur. This can result 1/7
3 in partial or complete occlusion of the vessel and subsequent myocardial ischaemia and necrosis. MI is one of the leading causes of death in most developed countries. Its incidence varies greatly within the EU, ranging in males from 200 cases per inhabitants per year in the Mediterranean area up to more than 800 cases per inhabitants per year in northern countries as Finland or the United Kingdom. The event rate in females is considerably lower ranging from 20 to 250 cases per women depending on the geographic region. This male predilection tends to dilute over 70 years. The overall mortality from MI at 30 days remains high (around 25%) and has not substantially changed over the last 15 years, mainly at the expense of pre-hospital deaths. Ninety percent of deaths occur within the first 24 hours of symptoms onset and most of them within the first 60 minutes. Nevertheless, recanalisation therapies (pharmacological or interventional) and supportive pharmacological approaches (antiplatelets, heparins, ACE inhibitors, beta-blockers) have yielded a substantial decrease of in-hospital mortality of patients with MI. In this sense, fibrinolytic treatment leads to a relative reduction of in-hospital mortality by around 20% as compared to placebo for those patients who are candidates for reperfusion therapy (50% of the patients who arrive alive to the hospital). All fibrinolytic agents act directly, or indirectly, as plasminogen activators. Two main groups can be considered: fibrin-specific agents (e.g. Alteplase -tpa-, Reteplase -rpa-, Tenecteplase -TNK-tPA-) and non-fibrin specific agents (streptokinase -SK-, urokinase -UK-). In the last decades the benefit of the use of fibrinolytics in patients suffering from STEMI has been consistently established, indicating that early therapy with drugs targeting coronary recanalisation decreases infarct size and improves patient s survival. A meta-analysis of initial clinical trials comparing streptokinase, alteplase and anistreplase with placebo definitely showed that fibrinolytic therapy is efficacious within 12 hours of symptom onset. Since then, extensive clinical experience has been gained with these and other fibrinolytics drugs like reteplase, tenecteplase, etc. 2. SELECTION OF PATIENTS 2.1. Inclusion criteria Patients should normally present with symptoms suggesting myocardial necrosis (chest pain lasting at least minutes) and ECG changes suggesting coronary arterial occlusion (ST segment elevation of 0.1 mv in two or more limb leads or 0.2 mv in two or more contiguous precordial leads). Although not included in some clinical trials, patients with symptoms and left bundle branch block -LBBB- not known to be previously present are also candidates for recanalisation therapy. Biochemical markers for myocardial necrosis (i.e. CK, CK-MB and troponins) are not required to start fibrinolytic treatment and, consequently, as inclusion criterion in clinical trials. However, they should be recorded as an indicator of myocardial damage. A clear relationship exists between the time window (from symptom onset to fibrinolysis) and the therapeutic benefit. The greatest benefit occurs within the first 3 h. Treatment benefit beyond 12 hours is considered marginal. The GUSTO I study showed that front-loaded regimen of alteplase has survival benefit (1%) over SK in STEMI with symptom onset 6 hours, although it is associated with a slight increase of intracranial haemorrhage ( % absolute higher risk than SK). Most clinical trials performed in recent years only included patients within a 6-hour symptom window. Nevertheless, there is no regulatory reason for not considering the inclusion of patients beyond this time-period (i.e. >6-12 hours from symptom 2/7
4 onset), thus reflecting the recommendations issued in currently available STEMI management guidelines. In any case, the consequences of the decision finally adopted on this issue regarding the study design, the selection of the comparator and the planned claim should be considered thoroughly. (See section 4). There is no reason for excluding patients according to their demographic characteristics or infarction location. Treatment benefit is observed and clinically relevant regardless of gender, age, body weight and regional practices, although the proportionate reduction in mortality may differ Exclusion criteria The benefit of fibrinolytic therapy in patients with NSTEMI or with isolated ST-segment depression has not been established, and there is some data suggesting potential harm from fibrinolytic therapy in these patients. Therefore, unless the purpose of the trial is targeting this specific population for new efficacy and safety data, these patients should be excluded. Patients with absolute contraindications to fibrinolytic therapy should also be excluded from clinical trials. 3. EFFICACY CRITERIA 3.1. Efficacy criteria in initial therapeutic studies Angiographic endpoints (TIMI flow grades) Dose finding studies are usually based on angiographic measurements, using the TIMI (Thrombolysis in Myocardial Infarction) perfusion grades as the major evaluation criteria. In principle, the rate of TIMI 3 flow (complete recanalisation) of the infarct related artery at 90 minutes is normally considered the most relevant angiographic endpoint, as it has been shown to correlate with an improved outcome in terms of mortality and left ventricular function. However, an earlier evaluation of the patency pattern (i.e. 30 and 60 minutes) may provide important information on the speed of recanalisation. Whatever is the timepoint selected as primary outcome, it must be properly justified and pre-specified in the clinical trial protocol. Angiograms should preferably undergo central blinded reading, particularly when open designs become unavoidable. It must be emphasised that some drugs providing higher complete recanalisation rates than alteplase have failed to demonstrate any additional survival benefit. Therefore, for the time being, angiographic evaluations are not considered as surrogate for survival when assessing fibrinolytic drugs. Electrocardiographic endpoints (ST segment elevation resolution) There is limited regulatory experience on the use of the resolution of ST elevation as a marker of recanalisation in phase II clinical trials with fibrinolytics. According to published data, it might be a good indicator of cardiac reperfusion subsequent to fibrinolytic therapy. Moreover, this endpoint can be measured also in centres without angiographic facilities. Therefore, the use of ST resolution might be acceptable as an indicator of myocardial reperfusion as a part of the dose finding package for new fibrinolytic agent. However, its use as the sole basis for the selection of the dose to be assayed in phase III clinical trials is discouraged until more information on the reliability of this variable as a predictor of recanalisation becomes available. 3/7
5 3.2. Efficacy criteria in main therapeutic studies Primary efficacy endpoint All cause mortality is the most relevant endpoint in clinical trials. Short-term (30/35-day) all cause mortality is the primary efficacy endpoint in studies assessing the efficacy of fibrinolytic drugs in patients with STEMI. Medium-term mortality (6-month/1-year) should also be recorded in order to assess the maintenance of the short-term effect Secondary efficacy endpoints In-hospital mortality, myocardial reinfarction, heart failure, left ventricular function, ventricular tachyarrhithmias, and the data related to the need for rescue (emergent or planned) recanalisation procedures (PTCA, stent and or CABG) should also be collected. As regional differences in revascularisation policies are likely to occur in multicentre multinational clinical trials, it is advisable to state in the study protocol the a priori criteria for revascularisation. A blind adjudicating committee is strongly recommended thus assuring homogeneous criteria for endpoint assessment. 4. SAFETY CRITERIA 4.1. Stroke Intracranial haemorrhage is the main safety issue related to fibrinolytic treatment. Strokes should be carefully recorded and a CT-scan or other suitable neuroimaging technique performed in order to assess their aetiology (ischaemic or haemorrhagic). The safety database should be large enough to determine any relevant increased risk of haemorrhagic stroke associated with the use of the new agent as compared to the reference drug. In addition, an adequate benefit/risk assessment according to age and gender, paying special attention to the elderly should be performed. Pivotal trials should allow to characterise adequately overall, haemorrhagic and disabling stroke rates in order to rule out any clinically relevant excess risk of intracranial haemorrhage with the new drug. A margin for such an excess risk that is considered as clinically relevant should be clearly stated in advance in the clinical trial protocol. 4.2 Other safety criteria The rates of bleeding events, either serious or no serious, the site of bleeding and the need of blood transfusions and the number of transfused units should also be recorded. Bleeding events should be categorised according to an acknowledged classification system (e.g. TIMI classification). It is strongly advisable to use the same classification throughout the whole clinical development program. Other cardiovascular adverse events like clinically relevant arrhythmia (idio-ventricular rhythm and ventricular fibrillation may occur at the time of reperfusion), cardiogenic shock, severe heart failure (Killip III), sustained hypotension, acute mitral regurgitation, acute ventricular septal defect, pericarditis, pulmonary embolism, and tamponade, among others, should be carefully recorded. Efforts should be made to identify clinical circumstances with an excess risk for bleeding or presenting certain cardiovascular adverse event (e.g. gender, age, bodyweight, invasive procedures, Killip class, time after the onset of symptoms, etc). Anaphylaxis and hypersensitivity reactions should carefully be recorded and analysed. Furthermore, the potential antibody response against the drug should be characterised, including determination of neutralising activity. 4/7
6 5. CRITICAL ISSUES WHEN DESIGNING CLINICAL TRIALS 5.1. Initial therapeutic studies The objective of this development phase is to find the optimal dosage regimen of the new agent and comparing its angiographic performance with a reference treatment. The dose finding strategy is normally expected to include at least 2 clinical trials (as described below). However, if properly justified by the applicant, alternative approaches might be considered acceptable. Thus initially, and starting from the lowest effective dose, increasing doses should be evaluated until a plateau effect on patency is reached or unacceptable safety problems occur. This process might be well performed in a sequential, open clinical trial. Appropriate rescue strategies should be planned for those patients treated with the lowest dose-levels of the new fibrinolytic regimen. Based on the results from this initial study, a comparative study is requested. Dose-ranging study should be conducted in parallel groups comparing at least three doses of the tested drug to an active control group. This study would preferably be double blind, although it is recognised that masking completely different treatment schedules might be difficult. In any case, a blind central reading of angiographic measures is considered unavoidable. Whenever other endpoints different from angiographic readings (i.e. ST resolution) the blinding of the study is considered of paramount importance. The identified doses with a predicted positive benefit/risk relationship should be preferably compared with the most active treatment actually available. At present, front-loaded alteplase, which has demonstrated higher coronary artery patency rates at 90 minutes compared with SK, is consider to be the reference comparator. This type of study is usually of medium size (several hundred of patients) to gather safety data related to morbidity and mortality Main therapeutic studies Selection of the comparator and study hypothesis The use of fibrinolytics in STEMI results in reduced mortality, therefore, placebo-controlled trials are considered ethically unacceptable, except in subpopulations where the clinical benefit of these compounds is not well established. Thus, main therapeutic studies should be parallel, double blind and active controlled. Most of the clinical and research experience in this field has been gained with either SK or alteplase. Initial clinical trials comparing streptokinase with placebo definitely showed that fibrinolytic therapy is efficacious in STEMI within the first 12 hours after symptom onset. Subsequently, a front-loaded regimen of alteplase showed a significant survival benefit in patients with STEMI symptom onset 6 hours over SK. Ideally, a new fibrinolytic agent would be expected to show superiority over the control arm. In this case, both SK and alteplase (or even any of the available fibrinolytic drugs) are considered as acceptable comparators. Under this hypothesis, the inclusion of patients with 0-12 hours of symptoms is acceptable and strongly recommended. Taking into account the above-mentioned consideration, it is expected that standard noninferiority trials with new fibrinolytic agents should be performed using front loaded alteplase as the active comparator. In order to preserve the fairness of the comparison and assure assay sensitivity, the target population should be the one for which the therapeutic benefit with the control drug is expected to be optimal (i.e. onset of symptoms 6 hours). For other target 5/7
7 populations (i.e. for patients with symptoms onset 0-12 hours) superiority trials are highly desirable. Considering that the body of evidence on the benefit/risk ratio for fibrinolytic therapy in STEMI beyond 6 hours is considerably weaker than between 0-6 hours, a case-bycase approach is recommended and nearly unavoidable Considerations when establishing a clinically relevant difference and a noninferiority margin In principle any superiority in terms of mortality over of an active comparator could be regarded as clinically relevant, provided that the safety profile is also acceptable. When defining an acceptable non-inferiority margin, the variability in the observed mortality proportions must be taken into consideration and, therefore a conservative approach to defining the non-inferiority margin is advisable. Both relative and absolute margins should be specified in the protocol, and the most conservative of these for the observed event rates adopted for the final analysis. In the recent past differences of 14% relative or 1% absolute (whichever proves smallest) have been accepted. These margins were based on all cause mortality rates at day 30 close to 6.5%-7%. In this situation, both the relative and the absolute criterion produce fairly identical requirements for estimating the sample size needed for a new study. Should in the future, medical progress further reduce mortality the aforementioned recommendations need to be revised Concomitant treatments With clot-specific fibrinolytic agents such as tpa, rpa and TNK-PA, immediate administration of heparin after administration of the fibrinolytic agent clearly diminishes reoclusion after successful recanalisation. With the non-specific plasminogen activators, however, such as SK, it is unclear if heparin is beneficial. Patients allocated to the control arm should be administered background antithrombotic therapy according to the standards recommended for the selected comparator. The administration of antithrombotic drugs should not interfere with the blindness of the study and double dummy techniques should be applied when necessary. All patients should receive appropriate background therapy, including antiplatelets and, when not contraindicated, betablockers and ACE inhibitors. Statins, when indicated, should also be considered. Treatment strategy in acute coronary syndrome is a fast evolving field. A number of major therapeutic options will need to be factored when designing clinical trials in this therapeutic area: Policy about PCI (primary rescue, early/late systematic, late conservative) and, in conjunction with PCI, the need for additional antithrombotic treatments (i.e. thienopyridines, GP IIb IIIa receptor antagonists, LMWH). Policy about other anti-thrombotic agents Pre-hospital and or hospital setting Necessity and relevance of predefined subgroup analysis Analysis of both mortality and stroke rates in specific predefined subgroups according (among others) to age, time to onset of treatment, infarct location, body weight, gender, and region (if applicable) will be required. It is anticipated that the effect of a new fibrinolytic will be shown to be consistent across all strata, and the purpose of these analyses is to confirm that fact. Claims of efficacy on specific subgroup of patients are not acceptable for drugs unable to 6/7
8 demonstrate to be effective and safe in the entire population studied. On the other hand, even if efficacy is satisfactorily shown for the whole population of the study, specific subgroups of patients could be excluded from the indication if the benefit-risk ratio is observed to be unfavourable for them or if not adequately represented. 7/7
Management of Acute Myocardial Infarction
 Management of Acute Myocardial Infarction Prof. Hossam Kandil Professor of Cardiology Cairo University ST Elevation Acute Myocardial Infarction Aims Of Management Emergency care (Pre-hospital) Early care
Management of Acute Myocardial Infarction Prof. Hossam Kandil Professor of Cardiology Cairo University ST Elevation Acute Myocardial Infarction Aims Of Management Emergency care (Pre-hospital) Early care
When the learner has completed this module, she/he will be able to:
 Thrombolytics and Myocardial Infarction WWW.RN.ORG Reviewed September 2017, Expires September 2019 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2017
Thrombolytics and Myocardial Infarction WWW.RN.ORG Reviewed September 2017, Expires September 2019 Provider Information and Specifics available on our Website Unauthorized Distribution Prohibited 2017
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 16 December 1999 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON CLINICAL
Acute coronary syndromes
 Acute coronary syndromes 1 Acute coronary syndromes Acute coronary syndromes results primarily from diminished myocardial blood flow secondary to an occlusive or partially occlusive coronary artery thrombus.
Acute coronary syndromes 1 Acute coronary syndromes Acute coronary syndromes results primarily from diminished myocardial blood flow secondary to an occlusive or partially occlusive coronary artery thrombus.
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 2004 CPMP/EWP/3020/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 2004 CPMP/EWP/3020/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
Acute myocardial infarction. Cardiovascular disorders. main/0202_new 02/03/06. Search date August 2004 Nicholas Danchin and Eric Durand
 main/0202_new 02/03/06 Acute myocardial infarction Search date August 2004 Nicholas Danchin and Eric Durand QUESTIONS Which treatments improve outcomes in acute myocardial infarction?...4 Which treatments
main/0202_new 02/03/06 Acute myocardial infarction Search date August 2004 Nicholas Danchin and Eric Durand QUESTIONS Which treatments improve outcomes in acute myocardial infarction?...4 Which treatments
Current Advances and Best Practices in Acute STEMI Management A pharmacoinvasive approach
 Current Advances and Best Practices in Acute STEMI Management A pharmacoinvasive approach Frans Van de Werf, MD, PhD University Hospitals, Leuven, Belgium Frans Van de Werf: Disclosures Research grants
Current Advances and Best Practices in Acute STEMI Management A pharmacoinvasive approach Frans Van de Werf, MD, PhD University Hospitals, Leuven, Belgium Frans Van de Werf: Disclosures Research grants
Reflection paper on assessment of cardiovascular safety profile of medicinal products
 25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP)
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use plondon, 20 February 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) APPENDIX TO THE NOTE FOR
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use plondon, 20 February 2003 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) APPENDIX TO THE NOTE FOR
The restoration of coronary flow after an
 Pharmacological Reperfusion in Acute Myicardial Infarction after ASSENT 3 and GUSTO V [81] DANIEL FERREIRA, MD, FESC Serviço de Cardiologia, Hospital Fernando Fonseca, Amadora, Portugal Rev Port Cardiol
Pharmacological Reperfusion in Acute Myicardial Infarction after ASSENT 3 and GUSTO V [81] DANIEL FERREIRA, MD, FESC Serviço de Cardiologia, Hospital Fernando Fonseca, Amadora, Portugal Rev Port Cardiol
Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular and metabolic diseases Draft
 1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
Facilitated Percutaneous Coronary Intervention in Acute Myocardial Infarction. Is it beneficial to patients?
 Facilitated Percutaneous Coronary Intervention in Acute Myocardial Infarction Is it beneficial to patients? Seung-Jea Tahk, MD. PhD. Suwon, Korea Facilitated PCI.. background Degree of coronary flow at
Facilitated Percutaneous Coronary Intervention in Acute Myocardial Infarction Is it beneficial to patients? Seung-Jea Tahk, MD. PhD. Suwon, Korea Facilitated PCI.. background Degree of coronary flow at
Cardiovascular Disorders Lecture 3 Coronar Artery Diseases
 Cardiovascular Disorders Lecture 3 Coronar Artery Diseases By Prof. El Sayed Abdel Fattah Eid Lecturer of Internal Medicine Delta University Coronary Heart Diseases It is the leading cause of death in
Cardiovascular Disorders Lecture 3 Coronar Artery Diseases By Prof. El Sayed Abdel Fattah Eid Lecturer of Internal Medicine Delta University Coronary Heart Diseases It is the leading cause of death in
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS
 CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 04 CPMP/EWP/2986/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
European Medicines Agency Evaluation of Medicines for Human Use London, 29 July 04 CPMP/EWP/2986/03 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE FOR GUIDANCE ON CLINICAL INVESTIGATION OF
2018 Acute Coronary Syndrome. Robert Bender, DO, FACOI, FACC Central Maine Heart and Vascular Institute
 2018 Acute Coronary Syndrome Robert Bender, DO, FACOI, FACC Central Maine Heart and Vascular Institute Definitions: Acute Myocardial Ischemia Unstable Angina Non-ST-Elevation MI (NSTEMI) }2/3 ST-Elevation
2018 Acute Coronary Syndrome Robert Bender, DO, FACOI, FACC Central Maine Heart and Vascular Institute Definitions: Acute Myocardial Ischemia Unstable Angina Non-ST-Elevation MI (NSTEMI) }2/3 ST-Elevation
APPENDIX F: CASE REPORT FORM
 APPENDIX F: CASE REPORT FORM Instruction: Complete this form to notify all ACS admissions at your centre to National Cardiovascular Disease Registry. Where check boxes are provided, check ( ) one or more
APPENDIX F: CASE REPORT FORM Instruction: Complete this form to notify all ACS admissions at your centre to National Cardiovascular Disease Registry. Where check boxes are provided, check ( ) one or more
STREAM - ONE YEAR MORTALITY STRATEGIC REPERFUSION EARLY AFTER MYOCARDIAL INFARCTION. STREAM 1Y AHA 2013 P Sinnaeve
 STREAM - ONE YEAR MORTALITY STRATEGIC REPERFUSION EARLY AFTER MYOCARDIAL INFARCTION PCI Hospital Ambulance/ER STREAM design STEMI
STREAM - ONE YEAR MORTALITY STRATEGIC REPERFUSION EARLY AFTER MYOCARDIAL INFARCTION PCI Hospital Ambulance/ER STREAM design STEMI
Acute Coronary Syndrome
 Acute Coronary Syndrome Vik Gongidi, DO FACOI, FACC Indian River Medical Center Vero Beach, FL Slides adapted from Robert Bender, DO, FACOI, FACC Definition: Acute Myocardial Ischemia Unstable Angina Non-ST-Elevation
Acute Coronary Syndrome Vik Gongidi, DO FACOI, FACC Indian River Medical Center Vero Beach, FL Slides adapted from Robert Bender, DO, FACOI, FACC Definition: Acute Myocardial Ischemia Unstable Angina Non-ST-Elevation
Critical Review Form Therapy Objectives: Methods:
 Critical Review Form Therapy Clinical Trial Comparing Primary Coronary Angioplasty with Tissue-Plasminogen Activator for Acute Myocardial Infarction (GUSTO-IIb), NEJM 1997; 336: 1621-1628 Objectives: To
Critical Review Form Therapy Clinical Trial Comparing Primary Coronary Angioplasty with Tissue-Plasminogen Activator for Acute Myocardial Infarction (GUSTO-IIb), NEJM 1997; 336: 1621-1628 Objectives: To
Subsequent management and therapies
 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation Subsequent management and therapies Marco Valgimigli, MD, PhD University of Ferrara ITALY
ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation Subsequent management and therapies Marco Valgimigli, MD, PhD University of Ferrara ITALY
Myocardial Infarction In Dr.Yahya Kiwan
 Myocardial Infarction In 2007 Dr.Yahya Kiwan New Definition Of Acute Myocardial Infarction The term of myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting
Myocardial Infarction In 2007 Dr.Yahya Kiwan New Definition Of Acute Myocardial Infarction The term of myocardial infarction should be used when there is evidence of myocardial necrosis in a clinical setting
FastTest. You ve read the book now test yourself
 FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
FastTest You ve read the book...... now test yourself To ensure you have learned the key points that will improve your patient care, read the authors questions below. The answers will refer you back to
Acute Coronary Syndromes
 Overview Acute Coronary Syndromes Rabeea Aboufakher, MD, FACC, FSCAI Section Chief of Cardiology Altru Health System Grand Forks, ND Epidemiology Pathophysiology Clinical features and diagnosis STEMI management
Overview Acute Coronary Syndromes Rabeea Aboufakher, MD, FACC, FSCAI Section Chief of Cardiology Altru Health System Grand Forks, ND Epidemiology Pathophysiology Clinical features and diagnosis STEMI management
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO CONSIDER ON MISSING DATA
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 15 November 2001 CPMP/EWP/1776/99 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 15 November 2001 CPMP/EWP/1776/99 COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) POINTS TO
Update on Antithrombotic Therapy in Acute Coronary Syndrome
 Update on Antithrombotic Therapy in Acute Coronary Syndrome Laura Tsang November 13, 2006 Objectives: By the end of this session, you should understand: The role of antithrombotics in ACS Their mechanisms
Update on Antithrombotic Therapy in Acute Coronary Syndrome Laura Tsang November 13, 2006 Objectives: By the end of this session, you should understand: The role of antithrombotics in ACS Their mechanisms
Acute Coronary Syndrome. Sonny Achtchi, DO
 Acute Coronary Syndrome Sonny Achtchi, DO Objectives Understand evidence based and practice based treatments for stabilization and initial management of ACS Become familiar with ACS risk stratification
Acute Coronary Syndrome Sonny Achtchi, DO Objectives Understand evidence based and practice based treatments for stabilization and initial management of ACS Become familiar with ACS risk stratification
UPDATE ON THE MANAGEMENTACUTE CORONARY SYNDROME. DR JULES KABAHIZI, Psc (Rwa) Lt Col CHIEF CONSULTANT RMH/KFH 28 JUNE18
 UPDATE ON THE MANAGEMENTACUTE CORONARY SYNDROME DR JULES KABAHIZI, Psc (Rwa) Lt Col CHIEF CONSULTANT RMH/KFH 28 JUNE18 INTRODUCTION The clinical entities that comprise acute coronary syndromes (ACS)-ST-segment
UPDATE ON THE MANAGEMENTACUTE CORONARY SYNDROME DR JULES KABAHIZI, Psc (Rwa) Lt Col CHIEF CONSULTANT RMH/KFH 28 JUNE18 INTRODUCTION The clinical entities that comprise acute coronary syndromes (ACS)-ST-segment
2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction
 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction Developed in Collaboration with American College of Emergency Physicians and Society for Cardiovascular Angiography and
2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction Developed in Collaboration with American College of Emergency Physicians and Society for Cardiovascular Angiography and
2010 ACLS Guidelines. Primary goals of therapy for patients
 2010 ACLS Guidelines Part 10: Acute Coronary Syndrome Present : 內科 R1 鍾伯欣 Supervisor: F1 吳亮廷 991110 Primary goals of therapy for patients of ACS Reduce the amount of myocardial necrosis that occurs in
2010 ACLS Guidelines Part 10: Acute Coronary Syndrome Present : 內科 R1 鍾伯欣 Supervisor: F1 吳亮廷 991110 Primary goals of therapy for patients of ACS Reduce the amount of myocardial necrosis that occurs in
Quinn Capers, IV, MD
 Heart Attacks Mended Hearts Presentation, January, 2017 Quinn Capers, IV, MD Associate Professor of Medicine (Cardiovascular Medicine) Director, Transradial Coronary Interventions Division of Cardiovascular
Heart Attacks Mended Hearts Presentation, January, 2017 Quinn Capers, IV, MD Associate Professor of Medicine (Cardiovascular Medicine) Director, Transradial Coronary Interventions Division of Cardiovascular
DISCUSSION QUESTION - 1
 CASE PRESENTATION 87 year old male No past history of diabetes, HTN, dyslipidemia or smoking Very active Medications: omeprazole for heart burn Admitted because of increasing retrosternal chest pressure
CASE PRESENTATION 87 year old male No past history of diabetes, HTN, dyslipidemia or smoking Very active Medications: omeprazole for heart burn Admitted because of increasing retrosternal chest pressure
Learning Objectives. Epidemiology of Acute Coronary Syndrome
 Cardiovascular Update: Antiplatelet therapy in acute coronary syndromes PHILLIP WEEKS, PHARM.D., BCPS-AQ CARDIOLOGY Learning Objectives Interpret guidelines as they relate to constructing an antiplatelet
Cardiovascular Update: Antiplatelet therapy in acute coronary syndromes PHILLIP WEEKS, PHARM.D., BCPS-AQ CARDIOLOGY Learning Objectives Interpret guidelines as they relate to constructing an antiplatelet
Cangrelor: Is it the new CHAMPION for PCI? Robert Barcelona, PharmD, BCPS Clinical Pharmacy Specialist, Cardiac Intensive Care Unit November 13, 2015
 Cangrelor: Is it the new CHAMPION for PCI? Robert Barcelona, PharmD, BCPS Clinical Pharmacy Specialist, Cardiac Intensive Care Unit November 13, 2015 Objectives Review the pharmacology and pharmacokinetic
Cangrelor: Is it the new CHAMPION for PCI? Robert Barcelona, PharmD, BCPS Clinical Pharmacy Specialist, Cardiac Intensive Care Unit November 13, 2015 Objectives Review the pharmacology and pharmacokinetic
Diagnosis and Management of Acute Myocardial Infarction
 Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
Practitioner Education Course
 2015 Practitioner Education Course ST Elevation Myocardial Infarction 2 Pathology Concept of vulnerable plaque Mild Atheroma Diagnosis IVUS OCT 3 Diagnosis This is based on : Clinical History ECG Changes.
2015 Practitioner Education Course ST Elevation Myocardial Infarction 2 Pathology Concept of vulnerable plaque Mild Atheroma Diagnosis IVUS OCT 3 Diagnosis This is based on : Clinical History ECG Changes.
Coronary Interventions Indications, Treatment Options and Outcomes
 Coronary Interventions Indications, Treatment Options and Outcomes A talk should be like a woman s skirt long enough to cover the subject, but short enough to keep it interesting. Coronary anatomy Physiology
Coronary Interventions Indications, Treatment Options and Outcomes A talk should be like a woman s skirt long enough to cover the subject, but short enough to keep it interesting. Coronary anatomy Physiology
Scottish Medicines Consortium
 Scottish Medicines Consortium bivalirudin 250mg for injection or infusion (Angiox ) (156/05) Nycomed UK Ltd No. 4 February, 2005 The Scottish Medicines Consortium has completed its assessment of the above
Scottish Medicines Consortium bivalirudin 250mg for injection or infusion (Angiox ) (156/05) Nycomed UK Ltd No. 4 February, 2005 The Scottish Medicines Consortium has completed its assessment of the above
Acute Myocardial Infarction. Willis E. Godin D.O., FACC
 Acute Myocardial Infarction Willis E. Godin D.O., FACC Acute Myocardial Infarction Definition: Decreased delivery of oxygen and nutrients to the myocardium Myocardial tissue necrosis causing irreparable
Acute Myocardial Infarction Willis E. Godin D.O., FACC Acute Myocardial Infarction Definition: Decreased delivery of oxygen and nutrients to the myocardium Myocardial tissue necrosis causing irreparable
Supplementary material 1. Definitions of study endpoints (extracted from the Endpoint Validation Committee Charter) 1.
 Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
Rationale, design, and baseline characteristics of the SIGNIFY trial: a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease without clinical
The Strategic Reperfusion Early After STEMI study Implications for clinical practice
 The Strategic Reperfusion Early After STEMI study Implications for clinical practice Robert C. Welsh, MD, FRCPC Associate Professor of Medicine Director, Adult Cardiac Catheterization and Interventional
The Strategic Reperfusion Early After STEMI study Implications for clinical practice Robert C. Welsh, MD, FRCPC Associate Professor of Medicine Director, Adult Cardiac Catheterization and Interventional
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT
 European Medicines Agency Evaluation of Medicines for Human Use London, 17 February 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON NON-CLINICAL AND CLINICAL DEVELOPMENT OF
European Medicines Agency Evaluation of Medicines for Human Use London, 17 February 2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) DRAFT GUIDELINE ON NON-CLINICAL AND CLINICAL DEVELOPMENT OF
Continuing Medical Education Post-Test
 Continuing Medical Education Post-Test Based on the information presented in this monograph, please choose one correct response for each of the following questions or statements. Record your answers on
Continuing Medical Education Post-Test Based on the information presented in this monograph, please choose one correct response for each of the following questions or statements. Record your answers on
Unstable angina and NSTEMI
 Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
Thrombolysis in Acute Myocardial Infarction
 CHAPTER 70 Thrombolysis in Acute Myocardial Infarction J. S. Hiremath Introduction Reperfusion of the occluded coronary artery at the earliest is the most important aim of management of STEMI. Once a flow
CHAPTER 70 Thrombolysis in Acute Myocardial Infarction J. S. Hiremath Introduction Reperfusion of the occluded coronary artery at the earliest is the most important aim of management of STEMI. Once a flow
OUTCOME OF THROMBOLYTIC AND NON- THROMBOLYTIC THERAPY IN ACUTE MYOCARDIAL INFARCTION
 OUTCOME OF THROMBOLYTIC AND NON- THROMBOLYTIC THERAPY IN ACUTE MYOCARDIAL INFARCTION FEROZ MEMON*, LIAQUAT CHEEMA**, NAND LAL RATHI***, RAJ KUMAR***, NAZIR AHMED MEMON**** OBJECTIVE: To compare morbidity,
OUTCOME OF THROMBOLYTIC AND NON- THROMBOLYTIC THERAPY IN ACUTE MYOCARDIAL INFARCTION FEROZ MEMON*, LIAQUAT CHEEMA**, NAND LAL RATHI***, RAJ KUMAR***, NAZIR AHMED MEMON**** OBJECTIVE: To compare morbidity,
STEMI 2014 YAHYA KIWAN. Consultant Cardiologist Head Of Cardiology Belhoul Specialty Hospital
 STEMI 2014 YAHYA KIWAN Consultant Cardiologist Head Of Cardiology Belhoul Specialty Hospital Aspiration Thrombectomy Manual aspiration thrombectomy is reasonable for patients undergoing primary PCI. I
STEMI 2014 YAHYA KIWAN Consultant Cardiologist Head Of Cardiology Belhoul Specialty Hospital Aspiration Thrombectomy Manual aspiration thrombectomy is reasonable for patients undergoing primary PCI. I
PRIMARY CORONARY ANGIOPLASTY VERSUS INTRAVENOUS THROMBOLYSIS FOR ACUTE MYOCARDIAL INFARCTION - A COMPARATIVE STUDY AT QUEEN ALIA HEART INSTITUTE
 PRIMARY CORONARY ANGIOPLASTY VERSUS INTRAVENOUS THROMBOLYSIS FOR ACUTE MYOCARDIAL INFARCTION - A COMPARATIVE STUDY AT QUEEN ALIA HEART INSTITUTE Walid Sawalha MD, MBBS (Lond), MRCP(UK)* ABSTRACT Objectives:
PRIMARY CORONARY ANGIOPLASTY VERSUS INTRAVENOUS THROMBOLYSIS FOR ACUTE MYOCARDIAL INFARCTION - A COMPARATIVE STUDY AT QUEEN ALIA HEART INSTITUTE Walid Sawalha MD, MBBS (Lond), MRCP(UK)* ABSTRACT Objectives:
Angina Luis Tulloch, MD 03/27/2012
 Angina Luis Tulloch, MD 03/27/2012 Acute coronary syndromes ACS STE > 1 mm, new LBBB* Increased cardiac enzymes STEMI Yes Yes NSTEMI No Yes UA No No *Recognize Wellen s sign/syndrome, posterior wall MI,
Angina Luis Tulloch, MD 03/27/2012 Acute coronary syndromes ACS STE > 1 mm, new LBBB* Increased cardiac enzymes STEMI Yes Yes NSTEMI No Yes UA No No *Recognize Wellen s sign/syndrome, posterior wall MI,
Chest Pain. Dr Robert Huggett Consultant Cardiologist
 Chest Pain Dr Robert Huggett Consultant Cardiologist Outline Diagnosis of cardiac chest pain 2016 NICE update on stable chest pain Assessment of unstable chest pain/acs and MI definition Scope of the
Chest Pain Dr Robert Huggett Consultant Cardiologist Outline Diagnosis of cardiac chest pain 2016 NICE update on stable chest pain Assessment of unstable chest pain/acs and MI definition Scope of the
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
Belinda Green, Cardiologist, SDHB, 2016
 Acute Coronary syndromes All STEMI ALL Non STEMI Unstable angina Belinda Green, Cardiologist, SDHB, 2016 Thrombus in proximal LAD Underlying pathophysiology Be very afraid for your patient Wellens
Acute Coronary syndromes All STEMI ALL Non STEMI Unstable angina Belinda Green, Cardiologist, SDHB, 2016 Thrombus in proximal LAD Underlying pathophysiology Be very afraid for your patient Wellens
Ticagrelor compared with clopidogrel in patients with acute coronary syndromes the PLATO trial
 compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
bivalirudin 250mg powder for concentrate for solution for injection or infusion (Angiox) SMC No. (638/10) The Medicines Company
 bivalirudin 250mg powder for concentrate for solution for injection or infusion (Angiox) SMC No. (638/10) The Medicines Company 06 August 2010 The Scottish Medicines Consortium (SMC) has completed its
bivalirudin 250mg powder for concentrate for solution for injection or infusion (Angiox) SMC No. (638/10) The Medicines Company 06 August 2010 The Scottish Medicines Consortium (SMC) has completed its
Controversies in Cardiac Pharmacology
 Controversies in Cardiac Pharmacology Thomas D. Conley, MD FACC FSCAI Disclosures I have no relevant relationships with commercial interests to disclose. 1 Doc, do I really need to take all these medicines?
Controversies in Cardiac Pharmacology Thomas D. Conley, MD FACC FSCAI Disclosures I have no relevant relationships with commercial interests to disclose. 1 Doc, do I really need to take all these medicines?
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON EVALUATION OF ANTICANCER MEDICINAL PRODUCTS IN MAN
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 24 July 2003 EMEA/ COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON EVALUATION
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 24 July 2003 EMEA/ COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON EVALUATION
Scottish Medicines Consortium
 Scottish Medicines Consortium bivalirudin, 250mg powder for concentrate for solution for injection or infusion (Angiox ) No. (516/08) The Medicines Company UK Ltd 07 November 2008 The Scottish Medicines
Scottish Medicines Consortium bivalirudin, 250mg powder for concentrate for solution for injection or infusion (Angiox ) No. (516/08) The Medicines Company UK Ltd 07 November 2008 The Scottish Medicines
Acute Coronary Syndrome
 Acute Coronary Syndrome Clinical Manifestation of CAD Silent Ischemia/asymptomatic Stable Angina Acute Coronary Syndrome (Non- STEMI/UA and STEMI) Arrhythmias Heart Failure Sudden Death Pain patterns with
Acute Coronary Syndrome Clinical Manifestation of CAD Silent Ischemia/asymptomatic Stable Angina Acute Coronary Syndrome (Non- STEMI/UA and STEMI) Arrhythmias Heart Failure Sudden Death Pain patterns with
Ischemic heart disease
 Ischemic heart disease Introduction In > 90% of cases: the cause is: reduced coronary blood flow secondary to: obstructive atherosclerotic vascular disease so most of the time it is called: coronary artery
Ischemic heart disease Introduction In > 90% of cases: the cause is: reduced coronary blood flow secondary to: obstructive atherosclerotic vascular disease so most of the time it is called: coronary artery
TYPE II MI. KC ACDIS LOCAL CHAPTER March 8, 2016
 TYPE II MI KC ACDIS LOCAL CHAPTER March 8, 2016 TYPE 2 MI DEFINITION: Acute coronary syndrome (ACS) encompasses a continuum of myocardial ischemia and infarction, which can make the diagnostic and coding
TYPE II MI KC ACDIS LOCAL CHAPTER March 8, 2016 TYPE 2 MI DEFINITION: Acute coronary syndrome (ACS) encompasses a continuum of myocardial ischemia and infarction, which can make the diagnostic and coding
WHI Form Report of Cardiovascular Outcome Ver (For items 1-11, each question specifies mark one or mark all that apply.
 WHI Form - Report of Cardiovascular Outcome Ver. 6. COMMENTS To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: OMB# 095-044 Exp: 4/06 -Affix label here- Clinical Center/ID:
WHI Form - Report of Cardiovascular Outcome Ver. 6. COMMENTS To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: OMB# 095-044 Exp: 4/06 -Affix label here- Clinical Center/ID:
Clopidogrel When For What For How Long. T Benjanuwattra Chiang Mai Heart Cent
 Clopidogrel When For What For How Long T Benjanuwattra Chiang Mai Heart Cent Evidence Based Medicine I don t want to put you to sleep But want you to be fully alert Atherothrombosis: A Generalized and
Clopidogrel When For What For How Long T Benjanuwattra Chiang Mai Heart Cent Evidence Based Medicine I don t want to put you to sleep But want you to be fully alert Atherothrombosis: A Generalized and
Medical Management of Acute Coronary Syndrome: The roles of a noncardiologist. Norbert Lingling D. Uy, MD Professor of Medicine UERMMMCI
 Medical Management of Acute Coronary Syndrome: The roles of a noncardiologist physician Norbert Lingling D. Uy, MD Professor of Medicine UERMMMCI Outcome objectives of the discussion: At the end of the
Medical Management of Acute Coronary Syndrome: The roles of a noncardiologist physician Norbert Lingling D. Uy, MD Professor of Medicine UERMMMCI Outcome objectives of the discussion: At the end of the
Management of acute myocardial infarction in patients presenting with ST-segment elevation
 European Heart Journal (2003) 24, 28 66 Task Force Report Management of acute myocardial infarction in patients presenting with ST-segment elevation The Task Force on the Management of Acute Myocardial
European Heart Journal (2003) 24, 28 66 Task Force Report Management of acute myocardial infarction in patients presenting with ST-segment elevation The Task Force on the Management of Acute Myocardial
Thrombolysis in Cardiology to whom? Professor Steen D. Kristensen, MD, DMSc, FESC Department of Cardiology
 Thrombolysis in Cardiology to whom? Professor Steen D. Kristensen, MD, DMSc, FESC Department of Cardiology UNIVERSITY OF AARHUS 1 COI Speakers fee: Aspen, AZ, Bayer, BMS/Pfizer Departmental research grant:
Thrombolysis in Cardiology to whom? Professor Steen D. Kristensen, MD, DMSc, FESC Department of Cardiology UNIVERSITY OF AARHUS 1 COI Speakers fee: Aspen, AZ, Bayer, BMS/Pfizer Departmental research grant:
Improving the Outcomes of
 Improving the Outcomes of STEMI Shelley Valaire, ACP; and Robert Welsh, MD, FRCPC Presented at the University of Alberta s 6th Annual Cardiology Update for General Practitioners and Internists, Edmonton,
Improving the Outcomes of STEMI Shelley Valaire, ACP; and Robert Welsh, MD, FRCPC Presented at the University of Alberta s 6th Annual Cardiology Update for General Practitioners and Internists, Edmonton,
Continuing Medical Education Post-Test
 Continuing Medical Education Post-Test Based on the information presented in this monograph, please choose one correct response for each of the following questions or statements. Record your answers on
Continuing Medical Education Post-Test Based on the information presented in this monograph, please choose one correct response for each of the following questions or statements. Record your answers on
(For items 1-12, each question specifies mark one or mark all that apply.)
 Form 121 - Report of Cardiovascular Outcome Ver. 9.2 COMMENTS -Affix label here- Member ID: - - To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: - Central Case No.:
Form 121 - Report of Cardiovascular Outcome Ver. 9.2 COMMENTS -Affix label here- Member ID: - - To be completed by Physician Adjudicator Date Completed: - - (M/D/Y) Adjudicator Code: - Central Case No.:
ST-elevation myocardial infarctions (STEMIs)
 Guidelines for Treating STEMI: Case-Based Questions As many as 25% of eligible patients presenting with STEMI do not receive any form of reperfusion therapy. The ACC/AHA guidelines highlight steps to improve
Guidelines for Treating STEMI: Case-Based Questions As many as 25% of eligible patients presenting with STEMI do not receive any form of reperfusion therapy. The ACC/AHA guidelines highlight steps to improve
Objectives. Acute Coronary Syndromes; The Nuts and Bolts. Overview. Quick quiz.. How dose the plaque start?
 Objectives Acute Coronary Syndromes; The Nuts and Bolts Michael P. Gulseth, Pharm. D., BCPS Pharmacotherapy II Spring 2006 Compare and contrast pathophysiology of unstable angina (UA), non-st segment elevation
Objectives Acute Coronary Syndromes; The Nuts and Bolts Michael P. Gulseth, Pharm. D., BCPS Pharmacotherapy II Spring 2006 Compare and contrast pathophysiology of unstable angina (UA), non-st segment elevation
TRANSPARENCY COMMITTEE OPINION. 2 April 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 2 April 2008 LOVENOX 6,000 IU anti-xa/0.6 ml, injectable solution (S.C.) in prefilled syringe Box of 2 (CIP: 364 690-3)
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 2 April 2008 LOVENOX 6,000 IU anti-xa/0.6 ml, injectable solution (S.C.) in prefilled syringe Box of 2 (CIP: 364 690-3)
Effect of upstream clopidogrel treatment in patients with ST-segment elevation myocardial infarction undergoing primary PCI
 Effect of upstream clopidogrel treatment in patients with ST-segment elevation myocardial infarction undergoing primary PCI Dr Sasha Koul, MD Dept of Cardiology, Lund University Hospital, Lund, Sweden
Effect of upstream clopidogrel treatment in patients with ST-segment elevation myocardial infarction undergoing primary PCI Dr Sasha Koul, MD Dept of Cardiology, Lund University Hospital, Lund, Sweden
A Randomized Trial Evaluating Clinically Significant Bleeding with Low-Dose Rivaroxaban vs Aspirin, in Addition to P2Y12 inhibition, in ACS
 A Randomized Trial Evaluating Clinically Significant Bleeding with Low-Dose Rivaroxaban vs Aspirin, in Addition to P2Y12 inhibition, in ACS Magnus Ohman MB, on behalf of the GEMINI-ACS-1 Investigators
A Randomized Trial Evaluating Clinically Significant Bleeding with Low-Dose Rivaroxaban vs Aspirin, in Addition to P2Y12 inhibition, in ACS Magnus Ohman MB, on behalf of the GEMINI-ACS-1 Investigators
Acute Myocardial Infarction
 Acute Myocardial Infarction Hafeza Shaikh, DO, FACC, RPVI Lourdes Cardiology Services Asst.Program Director, Cardiology Fellowship Associate Professor, ROWAN-SOM Acute Myocardial Infarction Definition:
Acute Myocardial Infarction Hafeza Shaikh, DO, FACC, RPVI Lourdes Cardiology Services Asst.Program Director, Cardiology Fellowship Associate Professor, ROWAN-SOM Acute Myocardial Infarction Definition:
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
The First 12 Hours. ST-Segment Elevation AMI: Introduction. Definitions
 ST-Segment Elevation AMI: The First 12 Hours Acute myocardial infarction (AMI) accounts for half of the deaths due to ischemic heart disease and is associated with significant use of resources. Because
ST-Segment Elevation AMI: The First 12 Hours Acute myocardial infarction (AMI) accounts for half of the deaths due to ischemic heart disease and is associated with significant use of resources. Because
ST Elevation Myocardial Infarction (STEMI) Reperfusion Order Set
 Form Title Form Number CH-0454 2018, Alberta Health Services, CKCM This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The license does not
Form Title Form Number CH-0454 2018, Alberta Health Services, CKCM This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. The license does not
ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
Adults With Diagnosed Diabetes
 Adults With Diagnosed Diabetes 1990 No data available Less than 4% 4%-6% Above 6% Mokdad AH, et al. Diabetes Care. 2000;23(9):1278-1283. Adults With Diagnosed Diabetes 2000 4%-6% Above 6% Mokdad AH, et
Adults With Diagnosed Diabetes 1990 No data available Less than 4% 4%-6% Above 6% Mokdad AH, et al. Diabetes Care. 2000;23(9):1278-1283. Adults With Diagnosed Diabetes 2000 4%-6% Above 6% Mokdad AH, et
Myocardial infarction
 CHAPTER-I CARDIOVASCULAR SYSTEM Myocardial infarction SUB: PHARMACOTHERAPEUTICS-I CODE:T0820006 Dr. Venugopal Pharm.D Assistant Professor Department of Pharm.D Kriahna Teja Pharmacy College,Tirupati. Definition
CHAPTER-I CARDIOVASCULAR SYSTEM Myocardial infarction SUB: PHARMACOTHERAPEUTICS-I CODE:T0820006 Dr. Venugopal Pharm.D Assistant Professor Department of Pharm.D Kriahna Teja Pharmacy College,Tirupati. Definition
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 15 November 2007 Doc. Ref. EMEA/CHMP/EWP/517497/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED
European Medicines Agency London, 15 November 2007 Doc. Ref. EMEA/CHMP/EWP/517497/2007 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON CLINICAL EVALUATION OF MEDICINAL PRODUCTS USED
Draft Agreed by Cardiovascular Working Party 25 Jan Adoption by CHMP for release for consultation 17 Feb 2011
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 London, 25 January 2011 EMA/CHMP/68875/2011 Committee for Medicinal Products for Human Use (CHMP) Concept paper on the need for a guideline on clinical investigation
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 London, 25 January 2011 EMA/CHMP/68875/2011 Committee for Medicinal Products for Human Use (CHMP) Concept paper on the need for a guideline on clinical investigation
Medicine Dr. Omed Lecture 2 Stable and Unstable Angina
 Medicine Dr. Omed Lecture 2 Stable and Unstable Angina Risk stratification in stable angina. High Risk; *post infarct angina, *poor effort tolerance, *ischemia at low workload, *left main or three vessel
Medicine Dr. Omed Lecture 2 Stable and Unstable Angina Risk stratification in stable angina. High Risk; *post infarct angina, *poor effort tolerance, *ischemia at low workload, *left main or three vessel
Role of Clopidogrel in Acute Coronary Syndromes. Hossam Kandil,, MD. Professor of Cardiology Cairo University
 Role of Clopidogrel in Acute Coronary Syndromes Hossam Kandil,, MD Professor of Cardiology Cairo University ACS Treatment Strategies Reperfusion/Revascularization Therapy Thrombolysis PCI (with/ without
Role of Clopidogrel in Acute Coronary Syndromes Hossam Kandil,, MD Professor of Cardiology Cairo University ACS Treatment Strategies Reperfusion/Revascularization Therapy Thrombolysis PCI (with/ without
The Window for Fibrinolysis. Frans Van de Werf, MD, PhD Leuven, Belgium
 The Window for Fibrinolysis Frans Van de Werf, MD, PhD Leuven, Belgium ESC STEMI Guidelines : December 2008 Reperfusion Therapy: Fibrinolytic Therapy Recommendations Class LOE In the absence of contraindications
The Window for Fibrinolysis Frans Van de Werf, MD, PhD Leuven, Belgium ESC STEMI Guidelines : December 2008 Reperfusion Therapy: Fibrinolytic Therapy Recommendations Class LOE In the absence of contraindications
Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United
 Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United States, totaling about 750,000 individuals annually
Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United States, totaling about 750,000 individuals annually
12 Lead EKG Chapter 4 Worksheet
 Match the following using the word bank. 1. A form of arteriosclerosis in which the thickening and hardening of the vessels walls are caused by an accumulation of fatty deposits in the innermost lining
Match the following using the word bank. 1. A form of arteriosclerosis in which the thickening and hardening of the vessels walls are caused by an accumulation of fatty deposits in the innermost lining
Pathophysiology of ACS
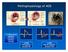 Pathophysiology of ACS ~ 2.0 MM patients admitted to CCU or telemetry annually 0.6 MM ST-segment elevation MI 1.4 MM Non-ST-segment elevation ACS NSTEMI vs STEMI VANQWISH Boden et al N Engl J Med 1998;338:1785-1792
Pathophysiology of ACS ~ 2.0 MM patients admitted to CCU or telemetry annually 0.6 MM ST-segment elevation MI 1.4 MM Non-ST-segment elevation ACS NSTEMI vs STEMI VANQWISH Boden et al N Engl J Med 1998;338:1785-1792
Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of pulmonary arterial hypertension
 5 December 2011 EMA/CHMP/213972/2010 Committee for Medicinal Products for Human use (CHMP) Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of
5 December 2011 EMA/CHMP/213972/2010 Committee for Medicinal Products for Human use (CHMP) Paediatric addendum to CHMP guideline on the clinical investigations of medicinal products for the treatment of
NEBRASKA STEMI CONFERENCE 2015 Dr. Doug Kosmicki. 2013, American Heart Association
 NEBRASKA STEMI CONFERENCE 2015 Dr. Doug Kosmicki 2013, American Heart Association 1 Dr. Doug Kosmicki Reperfusion Strategies Disclosure Information Report any disclosure information of conflicts of interest.
NEBRASKA STEMI CONFERENCE 2015 Dr. Doug Kosmicki 2013, American Heart Association 1 Dr. Doug Kosmicki Reperfusion Strategies Disclosure Information Report any disclosure information of conflicts of interest.
PRASUGREL HYDROCHLORIDE (Effient Eli Lilly Canada Inc.) Indication: Acute Coronary Syndrome
 CEDAC FINAL RECOMMENDATION PRASUGREL HYDROCHLORIDE (Effient Eli Lilly Canada Inc.) Indication: Acute Coronary Syndrome Recommendation: The Canadian Expert Drug Advisory Committee (CEDAC) recommends that
CEDAC FINAL RECOMMENDATION PRASUGREL HYDROCHLORIDE (Effient Eli Lilly Canada Inc.) Indication: Acute Coronary Syndrome Recommendation: The Canadian Expert Drug Advisory Committee (CEDAC) recommends that
What is a myocardial infarction and how do we treat it? Paul Das Consultant Cardiologist North Wales Cardiac Centre Glan Clwyd Hospital
 What is a myocardial infarction and how do we treat it? Paul Das Consultant Cardiologist North Wales Cardiac Centre Glan Clwyd Hospital What is a myocardial infarction? THEY AINT WHAT THEY USED TO BE Case
What is a myocardial infarction and how do we treat it? Paul Das Consultant Cardiologist North Wales Cardiac Centre Glan Clwyd Hospital What is a myocardial infarction? THEY AINT WHAT THEY USED TO BE Case
Acute Coronary syndrome
 Acute Coronary syndrome 7th Annual Pharmacotherapy Conference ACS Pathophysiology rupture or erosion of a vulnerable, lipidladen, atherosclerotic coronary plaque, resulting in exposure of circulating blood
Acute Coronary syndrome 7th Annual Pharmacotherapy Conference ACS Pathophysiology rupture or erosion of a vulnerable, lipidladen, atherosclerotic coronary plaque, resulting in exposure of circulating blood
ICH Topic S1B Carcinogenicity: Testing for Carcinogenicity of Pharmaceuticals. Step 5
 European Medicines Agency March 1998 CPMP/ICH/299/95 ICH Topic S1B Carcinogenicity: Testing for Carcinogenicity of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON CARCINOGENICITY: TESTING FOR CARCINOGENICITY
European Medicines Agency March 1998 CPMP/ICH/299/95 ICH Topic S1B Carcinogenicity: Testing for Carcinogenicity of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON CARCINOGENICITY: TESTING FOR CARCINOGENICITY
Technology appraisal guidance Published: 23 July 2014 nice.org.uk/guidance/ta317
 Prasugrel with percutaneous coronary intervention ention for treating acute coronary syndromes Technology appraisal guidance Published: 23 July 2014 nice.org.uk/guidance/ta317 NICE 2018. All rights reserved.
Prasugrel with percutaneous coronary intervention ention for treating acute coronary syndromes Technology appraisal guidance Published: 23 July 2014 nice.org.uk/guidance/ta317 NICE 2018. All rights reserved.
Ticagrelor compared with clopidogrel in patients with acute coronary syndromes the PLATelet Inhibition and patient Outcomes trial
 compared with clopidogrel in patients with acute coronary syndromes the PLATelet Inhibition and patient Outcomes trial Outcomes in patients with and planned PCI Ph.Gabriel Steg*, Stefan James, Robert A
compared with clopidogrel in patients with acute coronary syndromes the PLATelet Inhibition and patient Outcomes trial Outcomes in patients with and planned PCI Ph.Gabriel Steg*, Stefan James, Robert A
9/24/2013. Thrombolytics in 2013: Never Say Never. September 19 th, 2013 Scott M Lilly, MD PhD. Clinical Case
 September 19 th, 2013 Scott M Lilly, MD PhD Thrombolytics in 2013: Never Say Never Clinical Case 2 1 Evolution of STEMI Therapy The importance of absolute rest in bed for several days is clear James B
September 19 th, 2013 Scott M Lilly, MD PhD Thrombolytics in 2013: Never Say Never Clinical Case 2 1 Evolution of STEMI Therapy The importance of absolute rest in bed for several days is clear James B
CHAPTER-I MYOCARDIAL INFARCTION
 CHAPTER-I MYOCARDIAL INFARCTION Definition A myocardial infarction, more commonly known as MI or acute myocardial infarction (AMI) or heart attack is a condition where there is interruption of blood supply
CHAPTER-I MYOCARDIAL INFARCTION Definition A myocardial infarction, more commonly known as MI or acute myocardial infarction (AMI) or heart attack is a condition where there is interruption of blood supply
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE CHMP GUIDELINE ON CLINICAL EVALUATION OF NEW VACCINES ANNEX: SPC REQUIREMENTS
 European Medicines Agency London, 18 October 2006 EMEA/CHMP/VWP/382702/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE CHMP GUIDELINE ON CLINICAL EVALUATION OF NEW VACCINES ANNEX: SPC REQUIREMENTS
European Medicines Agency London, 18 October 2006 EMEA/CHMP/VWP/382702/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE CHMP GUIDELINE ON CLINICAL EVALUATION OF NEW VACCINES ANNEX: SPC REQUIREMENTS
