Outpatient Cardiac Rehabilitation
|
|
|
- Wilfrid Lambert
- 6 years ago
- Views:
Transcription
1 Last Review Date: May 12, 2017 Number: MG.MM.ME.26bC3v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth the clinical evidence that the patient meets the criteria for the treatment or surgical procedure. Without this documentation and information, EmblemHealth will not be able to properly review the request for prior authorization. The clinical review criteria expressed below reflects how EmblemHealth determines whether certain services or supplies are medically necessary. EmblemHealth established the clinical review criteria based upon a review of currently available clinical information (including clinical outcome studies in the peer-reviewed published medical literature, regulatory status of the technology, evidence-based guidelines of public health and health research agencies, evidence-based guidelines and positions of leading national health professional organizations, views of physicians practicing in relevant clinical areas, and other relevant factors). EmblemHealth expressly reserves the right to revise these conclusions as clinical information changes, and welcomes further relevant information. Each benefit program defines which services are covered. The conclusion that a particular service or supply is medically necessary does not constitute a representation or warranty that this service or supply is covered and/or paid for by EmblemHealth, as some programs exclude coverage for services or supplies that EmblemHealth considers medically necessary. If there is a discrepancy between this guideline and a member's benefits program, the benefits program will govern. In addition, coverage may be mandated by applicable legal requirements of a state, the Federal Government or the Centers for Medicare & Medicaid Services (CMS) for Medicare and Medicaid members. All coding and web site links are accurate at time of publication. EmblemHealth Services Company LLC, ( EmblemHealth ) has adopted the herein policy in providing management, administrative and other services to HIP Health Plan of New York, HIP Insurance Company of New York, Group Health Incorporated and GHI HMO Select, related to health benefit plans offered by these entities. All of the aforementioned entities are affiliated companies under common control of EmblemHealth Inc. Definition Cardiac rehabilitation Phase I Phase II Phase III A comprehensive program of medical evaluation, prescribed exercise, cardiac risk factor modification, education and counseling designed to restore certain patients with coronary heart disease to active and productive lives. Cardiac rehabilitation, as described in the medical literature, is divided into 3 phases and consists of a series of supervised exercise sessions with continuous electrocardiograph monitoring. Clinically optimal results are obtained if these sessions are conducted 3-times per week over a 12-to-18- week period. The immediate in-hospital post-cardiac event phase. The immediate post-hospitalization outpatient recuperation phase. The long-term maintenance phase. Guideline This guideline encompasses outpatient, post-hospital cardiac rehabilitation or Phase II. Members are eligible for coverage of outpatient cardiac rehabilitation when any of the following criteria are applicable: 1 Status post coronary artery bypass graft (CABG) surgery. 2 Status post heart or heart-lung transplant. 3 Status post heart valve repair or replacement. 4 Status post percutaneous transluminal angioplasty (PTA) or stent placement. 5 Members begin the program within 12 months of an acute myocardial infarction (MI). 6 Members have stable angina. 7 Members have stable, chronic heart failure (defined as left ventricular ejection fraction of 35% and New York Heart Association [NYHA] class II to IV symptoms despite being on optimal heart failure therapy for at least six weeks. Stable is defined as not having undergone recent [ 6 weeks] or planned [ 6 months] major cardiovascular hospitalizations or procedures).
2 Page 2 of 5 Limitations 1 Facilities Cardiac rehabilitation programs may be provided either by the outpatient department of a hospital or a physician-directed clinic. 1 Coverage for either program is subject to the following conditions: a. The facility meets the definition of a hospital outpatient department or a physician-directed clinic (e.g., a physician is on the premises and available to perform medical duties at all times the facility is open, and each patient is under the care of a hospital or clinic physician). b. The facility has available for immediate use all the necessary cardiopulmonary emergency diagnostic and therapeutic life-saving equipment accepted by the medical community as medically necessary (e.g., oxygen, cardiopulmonary resuscitation equipment or defibrillator). c. The program is conducted in an area set aside for the exclusive use of the program while it is in session. d. The program is staffed by personnel necessary to conduct the program safely and effectively who are trained in both basic and advanced life-support techniques and exercise therapy for coronary disease. Services of nonphysician personnel must be furnished under the direct supervision of a physician. Direct supervision means that a physician must be in the exercise program area and immediately available and accessible for all emergencies. It does not require that a physician be physically present in the exercise room itself. e. The nonphysician personnel are employees of the physician, hospital or clinic conducting the program and their services are incident to a physician's professional services. 2 Diagnoses a. For MI, the program entry date must be within 12 months of the infarction date. b. For CABG, program initiation should be early enough to have a restorative effect on the recuperative process; therefore, the entry date should be within 6 months of the CABG procedure. c. For angina, all patients must have a pre-entry stress test that is positive for exercise-induced ischemia within 6 months of starting cardiac rehabilitation. d. A positive stress test in this context implies a junctional depression of 2 mm or more with associated slowly rising ST segment, or 1 mm horizontal or downsloping ST segment depressions. e. Over the years, nuclear perfusion studies have supplanted standard electrocardiogram (ECG or EKG) treadmill tests as a means of evaluating ischemic heart disease, especially for patients who have abnormal rest ECGs; therefore, the positive stress test also includes perfusion studies that demonstrate ischemia. A stress ECG, however, is regarded as a comparable test to nuclear perfusion studies for ischemia evaluation. Patients with an abnormal stress ECG demonstrating ischemia may also be included in this group. f. For post-heart valve repair or replacement patients, the program should be early enough to provide restorative benefit; therefore, the date of entry must be within 6 months of surgery. g. For post-pta or stent replacement patients, the program should be early enough to provide a restorative benefit; therefore, the date of entry must be within 6 months of surgery. 1 Link to CMS-approved Intensive Cardiac Rehabilitation Programs: Information/MedicareApprovedFacilitie/ICR.html.
3 Page 3 of 5 h. For post-heart or heart-lung transplant patients, as special and complex posttransplant management problems may occur, program entry is extended to within 1 year. 3 Frequency and Duration a. Services provided in connection with a cardiac rehabilitation exercise program may be considered reasonable and necessary for up to 36 sessions. Patients generally receive 2 to 3 sessions per week for 12 to 18 weeks. b. Services at a frequency of less than 2 to 3 sessions per week will be considered not medically necessary. c. For the purposes of this guideline, Phase II is divided into Phase IIA and Phase IIB. Phase IIA is the initial outpatient cardiac rehabilitation, not to exceed 3 sessions per week for 12 to 18 weeks. d. Phase IIB consists of an additional series of 36 sessions in 12 to 18 weeks and will only be allowed if determined to be medically necessary. The total number of allowable sessions is 72. Phase IIB benefits must meet additional medical necessity criteria; specifically, there must be clear demonstration that the patient is benefiting from cardiac rehabilitation and that the exit criterion has not been met. 4 Exit criterion a. This guideline establishes an exit criterion to be met when the patient has achieved a stable exercise tolerance level of 7 metabolic equivalents (METS). In the American Heart Association s functional classification, Class I, or normal function status, begins at 7 METS; therefore, completion of 6 minutes of exercise during a treadmill or stress imaging test (utilizing the Bruce protocol without significant ischemia or dysrhythmia) is a reasonable exit criterion. b. For patients post valvuloplasty or valve replacement, coverage will be provided for Phase IIA only, as there is no data demonstrating that extension beyond 36 sessions is reasonable and necessary. c. The posttransplant patient poses a special challenge for the cardiac rehabilitation team. Issues such as deconditioning and cachectic deterioration may complicate the definition of a reasonable exit criterion; therefore, the Plan will use a peak oxygen consumption (VO2) of greater than 90% of the predicted as the exit criterion for phase IIA. Patients whose VO2 is less than 90% of the predicted may qualify for the additional phase IIB benefits. 5 Other services Exclusions a. Evaluation and management services, ECGs and other diagnostic services may be covered on the day of cardiac rehabilitation if these services are separate and distinct from the cardiac rehabilitation program and are medically necessary. b. Forms of counseling (i.e., dietary counseling and stress management) are not separately reimbursed. The Plan does not regard outpatient cardiac rehabilitation as medically necessary when any of the following are applicable: 1 Lack of continuous ECG monitoring. 2 Presence of congestive heart failure in the absence of other conditions. 3 Presence of unstable angina. 4 Continuation of > 72 sessions for > 36 weeks.
4 Page 4 of 5 Applicable Procedure Codes Physician services for outpatient cardiac rehabilitation; with continuous ECG monitoring (per session) G0422 G0423 S9472 Intensive cardiac rehabilitation; with or without continuous ECG monitoring with exercise, per session Intensive cardiac rehabilitation; with or without continuous ECG monitoring; without exercise, per session Cardiac rehabilitation program, non-physician provider, per diem Applicable Diagnosis Codes I20.8 Other forms of angina pectoris I20.9 Angina pectoris, unspecified I21.01 ST elevation (STEMI) myocardial infarction involving left main coronary artery I21.02 ST elevation (STEMI) myocardial infarction involving left anterior descending coronary artery I21.09 ST elevation (STEMI) myocardial infarction involving other coronary artery of anterior wall I21.11 ST elevation (STEMI) myocardial infarction involving right coronary artery I21.19 ST elevation (STEMI) myocardial infarction involving other coronary artery of inferior wall I21.21 ST elevation (STEMI) myocardial infarction involving left circumflex coronary artery I21.29 ST elevation (STEMI) myocardial infarction involving other sites I21.3 ST elevation (STEMI) myocardial infarction of unspecified site I21.4 Non-ST elevation (NSTEMI) myocardial infarction I21.A1 Myocardial infarction type 2 (Eff. 10/01/2017) I21.A9 Other myocardial infarction type (Eff. 10/01/2017) I22.0 Subsequent ST elevation (STEMI) myocardial infarction of anterior wall I22.1 Subsequent ST elevation (STEMI) myocardial infarction of inferior wall I22.2 Subsequent non-st elevation (NSTEMI) myocardial infarction I22.8 Subsequent ST elevation (STEMI) myocardial infarction of other sites I22.9 Subsequent ST elevation (STEMI) myocardial infarction of unspecified site I24.8 Other forms of acute ischemic heart disease I24.9 Acute ischemic heart disease, unspecified I25.10 Atherosclerotic heart disease of native coronary artery without angina pectoris I Atherosclerotic heart disease of native coronary artery with angina pectoris with documented spasm I Atherosclerotic heart disease of native coronary artery with other forms of angina pectoris I Atherosclerotic heart disease of native coronary artery with unspecified angina pectoris I25.2 Old myocardial infarction I25.5 Ischemic cardiomyopathy I25.6 Silent myocardial ischemia I Atherosclerosis of coronary artery bypass graft(s), unspecified, with angina pectoris with documented spasm I Atherosclerosis of coronary artery bypass graft(s), unspecified, with other forms of angina pectoris I Atherosclerosis of coronary artery bypass graft(s), unspecified, with unspecified angina pectoris I Atherosclerosis of autologous vein coronary artery bypass graft(s) with angina pectoris with documented spasm I Atherosclerosis of autologous vein coronary artery bypass graft(s) with other forms of angina pectoris I Atherosclerosis of autologous vein coronary artery bypass graft(s) with unspecified angina pectoris I Atherosclerosis of autologous artery coronary artery bypass graft(s) with angina pectoris with documented spasm
5 Page 5 of 5 I Atherosclerosis of autologous artery coronary artery bypass graft(s) with other forms of angina pectoris I Atherosclerosis of autologous artery coronary artery bypass graft(s) with unspecified angina pectoris I Atherosclerosis of nonautologous biological coronary artery bypass graft(s) with angina pectoris with documented spasm I Atherosclerosis of nonautologous biological coronary artery bypass graft(s) with other forms of angina pectoris I Atherosclerosis of nonautologous biological coronary artery bypass graft(s) with unspecified angina pectoris I Atherosclerosis of native coronary artery of transplanted heart with angina pectoris with documented spasm I Atherosclerosis of native coronary artery of transplanted heart with other forms of angina pectoris I Atherosclerosis of native coronary artery of transplanted heart with unspecified angina pectoris I Atherosclerosis of bypass graft of coronary artery of transplanted heart with angina pectoris with documented spasm I Atherosclerosis of bypass graft of coronary artery of transplanted heart with other forms of angina pectoris I Atherosclerosis of bypass graft of coronary artery of transplanted heart with unspecified angina pectoris I Atherosclerosis of other coronary artery bypass graft(s) with angina pectoris with documented spasm I Atherosclerosis of other coronary artery bypass graft(s) with other forms of angina pectoris I Atherosclerosis of other coronary artery bypass graft(s) with unspecified angina pectoris I25.89 Other forms of chronic ischemic heart disease I25.9 Chronic ischemic heart disease, unspecified Z94.1 Heart transplant status Z94.2 Lung transplant status Z94.3 Heart and lungs transplant status Z95.1 Presence of aortocoronary bypass graft Z95.2 Presence of prosthetic heart valve Z95.3 Presence of xenogenic heart valve Z95.4 Presence of other heart-valve replacement Z95.5 Presence of coronary angioplasty implant and graft Z Presence of heart assist device Z Presence of fully implantable artificial heart Z98.61 Coronary angioplasty status Z Other specified postprocedural states References Centers for Medicare & Medicaid Services. National Coverage Determination (NCD) for Cardiac Rehabilitation Programs (20.10) Accessed May 12, 2017 Centers for Medicare & Medicaid Services. Decision Memo for Cardiac Rehabilitation (CR) Programs - Chronic Heart Failure (CAG N) Accessed May 12, Decision Memo for Intensive Cardiac Rehabilitation (ICR) Program - Benson-Henry Institute Cardiac Wellness Program (CAG N) Accessed May 12, Specialty-matched clinical peer review.
Automatic External Defibrillators
 Last Review Date: April 21, 2017 Number: MG.MM.DM.10dC3v4 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: April 21, 2017 Number: MG.MM.DM.10dC3v4 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Cardiac Rehabilitation
 Easy Choice Health Plan Harmony Health Plan of Illinois Missouri Care Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona OneCare (Care1st Health Plan Arizona, Inc.) Staywell of Florida
Easy Choice Health Plan Harmony Health Plan of Illinois Missouri Care Ohana Health Plan, a plan offered by WellCare Health Insurance of Arizona OneCare (Care1st Health Plan Arizona, Inc.) Staywell of Florida
Title: Automatic External Defibrillators Division: Medical Management Department: Utilization Management
 Retired Date: Page 1 of 7 1. POLICY DESCRIPTION: Automatic External Defibrillators 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
Retired Date: Page 1 of 7 1. POLICY DESCRIPTION: Automatic External Defibrillators 2. RESPONSIBLE PARTIES: Medical Management Administration, Utilization Management, Integrated Care Management, Pharmacy,
Clinical Policy Title: Cardiac rehabilitation
 Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
11/19/2013. Cardiac Rehabilitation Coverage and Documentation Requirements. Phases of Cardiac Rehabilitation. Phase II
 Cardiac Rehabilitation Coverage and Documentation Requirements Phases of Cardiac Rehabilitation Phase I: Acute in-hospital phase of CR Phase II: is the initial outpatient phase of the program Phase III:
Cardiac Rehabilitation Coverage and Documentation Requirements Phases of Cardiac Rehabilitation Phase I: Acute in-hospital phase of CR Phase II: is the initial outpatient phase of the program Phase III:
Contractor Information. LCD Information. Local Coverage Determination (LCD): Cardiac Rehabilitation (L34412) Document Information
 Local Coverage Determination (LCD): Cardiac Rehabilitation (L34412) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): Cardiac Rehabilitation (L34412) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Clinical Policy: Cardiac Biomarker Testing for Acute Myocardial Infarction Reference Number: CP.MP.156
 Clinical Policy: Reference Number: CP.MP.156 Effective Date: 12/17 Last Review Date: 12/17 See Important Reminder at the end of this policy for important regulatory and legal information. Description The
Clinical Policy: Reference Number: CP.MP.156 Effective Date: 12/17 Last Review Date: 12/17 See Important Reminder at the end of this policy for important regulatory and legal information. Description The
Contractor Information
 FUTURE Local Coverage Determination (LCD): Cardiac Rehabilitation (L34412) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please Note: Future Effective
FUTURE Local Coverage Determination (LCD): Cardiac Rehabilitation (L34412) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Please Note: Future Effective
CMS Limitations Guide - Cardiovascular Services
 CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Populations Interventions Comparators Outcomes Individuals: With diagnosed heart disease. rehabilitation
 Protocol Cardiac Rehabilitation in the Outpatient Setting (80308) Medical Benefit Effective Date: 01/01/17 Next Review Date: 05/18 Preauthorization No Review Dates: 07/07, 07/08, 05/09, 05/10, 05/11, 05/12,
Protocol Cardiac Rehabilitation in the Outpatient Setting (80308) Medical Benefit Effective Date: 01/01/17 Next Review Date: 05/18 Preauthorization No Review Dates: 07/07, 07/08, 05/09, 05/10, 05/11, 05/12,
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Cardiac Rehabilitation in the Outpatient Setting Page 1 of 16 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cardiac Rehabilitation in the Outpatient Setting Professional
Cardiac Rehabilitation in the Outpatient Setting Page 1 of 16 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cardiac Rehabilitation in the Outpatient Setting Professional
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: August 20, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): January 28, 2014 Effective Date: August 20, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Topical Oxygen Wound Therapy (MEDICAID)
 Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Topical Oxygen Wound Therapy (MEDICAID) Last Review Date: September 8, 2017 Number: MG.MM.DM.15C8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or
Home Pulse Oximetry for Infants and Children
 Last Review Date: April 21, 2017 Number: MG.MM.DM.12aC2v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: April 21, 2017 Number: MG.MM.DM.12aC2v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
CMS Limitations Guide - Cardiovascular Services
 CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Cardiovascular Services
 CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Cardiovascular Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
Common Codes for ICD-10
 Common Codes for ICD-10 Specialty: Cardiology *Always utilize more specific codes first. ABNORMALITIES OF HEART RHYTHM ICD-9-CM Codes: 427.81, 427.89, 785.0, 785.1, 785.3 R00.0 Tachycardia, unspecified
Common Codes for ICD-10 Specialty: Cardiology *Always utilize more specific codes first. ABNORMALITIES OF HEART RHYTHM ICD-9-CM Codes: 427.81, 427.89, 785.0, 785.1, 785.3 R00.0 Tachycardia, unspecified
Cardiology/Cardiothoracic
 Cardiology/Cardiothoracic ICD-9-CM to ICD-10-CM Code Mapper 800-334-5724 www.contexomedia.com 2013 ICD-9-CM 272.0 Pure hypercholesterolemia 272.2 Mixed hyperlipidemia 272.4 Other and hyperlipidemia 278.00
Cardiology/Cardiothoracic ICD-9-CM to ICD-10-CM Code Mapper 800-334-5724 www.contexomedia.com 2013 ICD-9-CM 272.0 Pure hypercholesterolemia 272.2 Mixed hyperlipidemia 272.4 Other and hyperlipidemia 278.00
Lipoprotein Subclassification Testing for Screening, Evaluation and Monitoring of Cardiovascular Disease
 for Screening, Evaluation and Monitoring of Cardiovascular Disease Last Review Date: June 9, 2017 Number: MG.MM.LA.40Cv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The
for Screening, Evaluation and Monitoring of Cardiovascular Disease Last Review Date: June 9, 2017 Number: MG.MM.LA.40Cv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The
Lnformation Coverage Guidance
 Lnformation Coverage Guidance Coverage Indications, Limitations, and/or Medical Necessity Abstract: B-type natriuretic peptide (BNP) is a cardiac neurohormone produced mainly in the left ventricle. It
Lnformation Coverage Guidance Coverage Indications, Limitations, and/or Medical Necessity Abstract: B-type natriuretic peptide (BNP) is a cardiac neurohormone produced mainly in the left ventricle. It
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Cardiac Rehabilitation in the Outpatient Setting Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cardiac Rehabilitation in the Outpatient Setting Professional
Cardiac Rehabilitation in the Outpatient Setting Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Cardiac Rehabilitation in the Outpatient Setting Professional
Yescarta (axicabtagene ciloleucel)
 Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Intrathecal Baclofen for CNS Spasticity
 Intrathecal Baclofen for CNS Spasticity Last Review Date: October 13, 2017 Number: MG.MM.ME.31bC7 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary
Intrathecal Baclofen for CNS Spasticity Last Review Date: October 13, 2017 Number: MG.MM.ME.31bC7 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary
MedStar Health considers External Counterpulsation Therapy (ECP) medically necessary for the following indications:
 MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.107.MH External Counterpulsation Therapy This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar
MedStar Health, Inc. POLICY AND PROCEDURE MANUAL MP.107.MH External Counterpulsation Therapy This policy applies to the following lines of business: MedStar Employee (Select) MedStar MA DSNP CSNP MedStar
Process Measure: Screening for Adult Obstructive Sleep Apnea
 Process Measure: Screening for Adult Obstructive Sleep Apnea Measure Description Description Type of Measure All patients aged 18 years and older at high risk for obstructive sleep apnea (OSA) with documentation
Process Measure: Screening for Adult Obstructive Sleep Apnea Measure Description Description Type of Measure All patients aged 18 years and older at high risk for obstructive sleep apnea (OSA) with documentation
Cardiology Documentation in an ICD-10 World
 Cardiology Documentation in an ICD-10 World Providence Little Company of Mary Medical Center - Torrance July 10, 2015 Andrew H. Dombro, MD Internist/Hospitalist Regional Medical Director JA Thomas, a Nuance
Cardiology Documentation in an ICD-10 World Providence Little Company of Mary Medical Center - Torrance July 10, 2015 Andrew H. Dombro, MD Internist/Hospitalist Regional Medical Director JA Thomas, a Nuance
Chelation Therapy Last Review Date: February 10, 2017 Number: MG.MM.ME.48C3 Medical Guideline Disclaimer Definition Guideline Limitations/Exclusions
 Last Review Date: February 10, 2017 Number: MG.MM.ME.48C3 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: February 10, 2017 Number: MG.MM.ME.48C3 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
MEDICAL POLICY SUBJECT: CARDIAC REHABILITATION
 MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 6 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Baxdela (delafloxacin) for Injection
 Last Review Date: July 25th, 2018 Number: MG.MM.PH.53 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: July 25th, 2018 Number: MG.MM.PH.53 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
TYPE II MI. KC ACDIS LOCAL CHAPTER March 8, 2016
 TYPE II MI KC ACDIS LOCAL CHAPTER March 8, 2016 TYPE 2 MI DEFINITION: Acute coronary syndrome (ACS) encompasses a continuum of myocardial ischemia and infarction, which can make the diagnostic and coding
TYPE II MI KC ACDIS LOCAL CHAPTER March 8, 2016 TYPE 2 MI DEFINITION: Acute coronary syndrome (ACS) encompasses a continuum of myocardial ischemia and infarction, which can make the diagnostic and coding
MEDICAL POLICY SUBJECT: CARDIAC REHABILITATION. POLICY NUMBER: CATEGORY: Therapy/ Rehabilitation
 MEDICAL POLICY SUBJECT: CARDIAC REHABILITATION, 08/25/17 PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: CARDIAC REHABILITATION, 08/25/17 PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
WV BUREAU FOR MEDICAL SERVICES PRESENTATION FOR WV ASSOCIATION OF OPTOMETRIC PHYSICIANS
 WV BUREAU FOR MEDICAL SERVICES PRESENTATION FOR WV ASSOCIATION OF OPTOMETRIC PHYSICIANS Tammy Pritt-Jones, Director, Office of Transportation DeeAnn Price, Program Manager, Office of Professional Services
WV BUREAU FOR MEDICAL SERVICES PRESENTATION FOR WV ASSOCIATION OF OPTOMETRIC PHYSICIANS Tammy Pritt-Jones, Director, Office of Transportation DeeAnn Price, Program Manager, Office of Professional Services
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL REVASCULARIZATION
 MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
MEDICAL POLICY SUBJECT: TRANSMYOCARDIAL 7/21/05, 05/18/06, 03/15/07, 02/21/08,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply.
Blepharoplasty. Definitions
 Last Review Date: June 9, 2017 Number: MG.MM.SU.10eC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: June 9, 2017 Number: MG.MM.SU.10eC5 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Cardiovascular Nuclear Medicine (L35083) MP-055-MC-PA Medical Management Provider Notice Date: 05/01/2018 Issue Date: 06/01/2018
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Cardiovascular Nuclear Medicine (L35083) MP-055-MC-PA Medical Management Provider Notice Date: 05/01/2018 Issue Date: 06/01/2018
Premier Health Plan considers Intravascular Ultrasound (IVUS) for Coronary Vessels medically necessary for the following indications:
 Premier Health Plan POLICY AND PROCEDURE MANUAL MP.091.PH - Intravascular Ultrasound for Coronary Vessels This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
Premier Health Plan POLICY AND PROCEDURE MANUAL MP.091.PH - Intravascular Ultrasound for Coronary Vessels This policy applies to the following lines of business: Premier Commercial Premier Employee Premier
MEDICAL POLICY SUBJECT: CARDIAC REHABILITATION. POLICY NUMBER: CATEGORY: Therapy/ Rehabilitation
 MEDICAL POLICY SUBJECT: CARDIAC REHABILITATION, 08/25/17, 06/28/18 PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
MEDICAL POLICY SUBJECT: CARDIAC REHABILITATION, 08/25/17, 06/28/18 PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial
Therapeutic Shoes for Diabetics
 Last Review Date: August 11, 2017 Number: MG.MM.DM.03bC8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: August 11, 2017 Number: MG.MM.DM.03bC8v2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders
 C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
C1: Medical Standards for Safety Critical Workers with Cardiovascular Disorders GENERAL ISSUES REGARDING MEDICAL FITNESS-FOR-DUTY 1. These medical standards apply to Union Pacific Railroad (UPRR) employees
Quality Payment Program: Cardiology Specialty Measure Set
 Measure Title * Reportable via PINNACLE α Reportable via Diabetes Collaborative CQMC v1.0 Measure High Priority Measure Cross Cutting Measure Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor
Measure Title * Reportable via PINNACLE α Reportable via Diabetes Collaborative CQMC v1.0 Measure High Priority Measure Cross Cutting Measure Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor
CMS Limitations Guide - Radiology Services
 CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
CMS Limitations Guide - Radiology Services Starting October 1, 2015, CMS will update their existing medical necessity limitations on tests and procedures to correspond to ICD-10 codes. This limitations
2015 Facility and Physician Billing Guide Heart Valve Technologies
 2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
2015 Facility and Physician Billing Guide Heart Valve Technologies PHYSICIAN BILLING CODES Clinicians use Current Procedural Terminology (CPT 1 ) codes to bill for procedures and services. Each CPT code
Anatomy of the Heart and the. ICD-10 Codes
 Anatomy of the Heart and the Diseases ICD-10 Codes Sharon J. Oliver CPC, CPMA, CPC-I All Rights Reserved 1 Anatomy of the Heart Pulmonary Tricuspid (AV) Valve Mitral Aortic Semilunar Valve Chordae Tendineae
Anatomy of the Heart and the Diseases ICD-10 Codes Sharon J. Oliver CPC, CPMA, CPC-I All Rights Reserved 1 Anatomy of the Heart Pulmonary Tricuspid (AV) Valve Mitral Aortic Semilunar Valve Chordae Tendineae
Re: National Coverage Analysis (NCA) for Implantable Cardioverter Defibrillators (CAG R4)
 December 20, 2017 Ms. Tamara Syrek-Jensen Director, Coverage & Analysis Group Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: National Coverage Analysis (NCA) for
December 20, 2017 Ms. Tamara Syrek-Jensen Director, Coverage & Analysis Group Centers for Medicare & Medicaid Services 7500 Security Boulevard Baltimore, MD 21244 Re: National Coverage Analysis (NCA) for
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT JANUARY 24, 2012
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201203 JANUARY 24, 2012 The IHCP to reimburse implantable cardioverter defibrillators separately from outpatient implantation Effective March 1, 2012, the
Contractor Information. LCD Information. Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Document Information
 Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Local Coverage Determination (LCD): HOMOCYSTeine Level, Serum (L34419) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information Contractor
Specific Basic Standards for Osteopathic Fellowship Training in Cardiology
 Specific Basic Standards for Osteopathic Fellowship Training in Cardiology American Osteopathic Association and American College of Osteopathic Internists BOT 07/2006 Rev. BOT 03/2009 Rev. BOT 07/2011
Specific Basic Standards for Osteopathic Fellowship Training in Cardiology American Osteopathic Association and American College of Osteopathic Internists BOT 07/2006 Rev. BOT 03/2009 Rev. BOT 07/2011
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Cardiovascular Nuclear Medicine (L33960) MP-055-MC-KY Medical Management Provider Notice Date: 05/01/2018 Issue Date: 06/01/2018
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Cardiovascular Nuclear Medicine (L33960) MP-055-MC-KY Medical Management Provider Notice Date: 05/01/2018 Issue Date: 06/01/2018
2019 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule
 ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
ABBOTT REIMBURSEMENT GUIDE CMS Physician Fee Schedule This document and the information contained herein is for general information purposes only and is not intended and does not constitute legal, reimbursement,
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090)
 Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Local Coverage Determination (LCD) for Cardiac Catheterization (L29090) Contractor Information Contractor Name First Coast Service Options, Inc. Contractor Number 09102 Contractor Type MAC - Part B LCD
Quality Payment Program: Cardiology Specialty Measure Set
 Quality Payment Program: Cardiology Specialty Set Title Number CMS Reporting Method(s) Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor or Angiotensin Receptor Blocker (ARB) Therapy for
Quality Payment Program: Cardiology Specialty Set Title Number CMS Reporting Method(s) Heart Failure (HF): Angiotensin- Converting Enzyme (ACE) Inhibitor or Angiotensin Receptor Blocker (ARB) Therapy for
Acute Myocardial Infarction. Willis E. Godin D.O., FACC
 Acute Myocardial Infarction Willis E. Godin D.O., FACC Acute Myocardial Infarction Definition: Decreased delivery of oxygen and nutrients to the myocardium Myocardial tissue necrosis causing irreparable
Acute Myocardial Infarction Willis E. Godin D.O., FACC Acute Myocardial Infarction Definition: Decreased delivery of oxygen and nutrients to the myocardium Myocardial tissue necrosis causing irreparable
2017 Cardiology Survival Guide
 2017 Cardiology Survival Guide Chapter 4: Cardiac Catheterization/Percutaneous Coronary Intervention A cardiac catheterization involves a physician inserting a thin plastic tube (catheter) into an artery
2017 Cardiology Survival Guide Chapter 4: Cardiac Catheterization/Percutaneous Coronary Intervention A cardiac catheterization involves a physician inserting a thin plastic tube (catheter) into an artery
Cardiac Rehabilitation & Exercise Training in Congenital Heart Disease. Jidong Sung Division of Cardiology Sungkyunkwan University School of Medicine
 Cardiac Rehabilitation & Exercise Training in Congenital Heart Disease Jidong Sung Division of Cardiology Sungkyunkwan University School of Medicine Cardiac rehabilitation Agency of Health Care Policy
Cardiac Rehabilitation & Exercise Training in Congenital Heart Disease Jidong Sung Division of Cardiology Sungkyunkwan University School of Medicine Cardiac rehabilitation Agency of Health Care Policy
Acute Myocardial Infarction
 Acute Myocardial Infarction Hafeza Shaikh, DO, FACC, RPVI Lourdes Cardiology Services Asst.Program Director, Cardiology Fellowship Associate Professor, ROWAN-SOM Acute Myocardial Infarction Definition:
Acute Myocardial Infarction Hafeza Shaikh, DO, FACC, RPVI Lourdes Cardiology Services Asst.Program Director, Cardiology Fellowship Associate Professor, ROWAN-SOM Acute Myocardial Infarction Definition:
Cardiac Rehabilitation in the Outpatient Setting. Description
 Subject: Cardiac Rehabilitation in the Outpatient Setting Page: 1 of 10 Last Review Status/Date: September 2014 Cardiac Rehabilitation in the Outpatient Setting Description Cardiac rehabilitation refers
Subject: Cardiac Rehabilitation in the Outpatient Setting Page: 1 of 10 Last Review Status/Date: September 2014 Cardiac Rehabilitation in the Outpatient Setting Description Cardiac rehabilitation refers
Cardiac Rehabilitation for Heart Failure Patients. Jia Shen MD, MPH Assistant Professor of Medicine UC San Diego Health System
 Cardiac Rehabilitation for Heart Failure Patients Jia Shen MD, MPH Assistant Professor of Medicine UC San Diego Health System Disclosures There are no conflict of interests related to this presentation.
Cardiac Rehabilitation for Heart Failure Patients Jia Shen MD, MPH Assistant Professor of Medicine UC San Diego Health System Disclosures There are no conflict of interests related to this presentation.
Coronary intravascular ultrasound (IVUS)
 2017 Coding and Medicare payment guide Coronary intravascular ultrasound (IVUS) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered from third-party sources
2017 Coding and Medicare payment guide Coronary intravascular ultrasound (IVUS) All coding, coverage, billing and payment information provided herein by Philips Volcano is gathered from third-party sources
Supplementary Material to Mayer et al. A comparative cohort study on personalised
 Suppl. Table : Baseline characteristics of the patients. Characteristic Modified cohort Non-modified cohort P value (n=00) Age years 68. ±. 69.5 ±. 0. Female sex no. (%) 60 (0.0) 88 (.7) 0.0 Body Mass
Suppl. Table : Baseline characteristics of the patients. Characteristic Modified cohort Non-modified cohort P value (n=00) Age years 68. ±. 69.5 ±. 0. Female sex no. (%) 60 (0.0) 88 (.7) 0.0 Body Mass
DIGOXIN THERAPEUTIC DRUG ASSAY
 A18.84 Tuberculosis of heart E00.0 Congenital iodine-deficiency syndrome, neurological type E00.1 Congenital iodine-deficiency syndrome, myxedematous type E00.2 Congenital iodine-deficiency syndrome, mixed
A18.84 Tuberculosis of heart E00.0 Congenital iodine-deficiency syndrome, neurological type E00.1 Congenital iodine-deficiency syndrome, myxedematous type E00.2 Congenital iodine-deficiency syndrome, mixed
Program Metrics. New Unique ID. Old Unique ID. Metric Set Metric Name Description. Old Metric Name
 Program Metrics The list below includes the metrics that will be calculated by the PINNACLE Registry for the outpatient office setting. These include metrics for, Atrial Fibrillation, Hypertension and.
Program Metrics The list below includes the metrics that will be calculated by the PINNACLE Registry for the outpatient office setting. These include metrics for, Atrial Fibrillation, Hypertension and.
Value of Cardiac Rehabilitation for Improving Patient Outcomes
 Value of Cardiac Rehabilitation for Improving Patient Outcomes Pam R. Taub MD, FACC Director of Step Family Cardiac Wellness and Rehabilitation Center Associate Professor of Medicine UC San Diego Health
Value of Cardiac Rehabilitation for Improving Patient Outcomes Pam R. Taub MD, FACC Director of Step Family Cardiac Wellness and Rehabilitation Center Associate Professor of Medicine UC San Diego Health
Introducing the COAPT Trial
 physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
physician INFORMATION Eligible patients Symptomatic functional mitral regurgitation 3+ Not suitable candidates for open mitral valve surgery NYHA functional class II, III, or ambulatory IV Introducing
CARDIOLOGY IMAGING PROGRAM
 CARDIOLOGY IMAGING PROGRAM TABLE OF CONTENTS. OVERVIEW............................................................................................. 618..... MEMBERS........... EXEMPT.......... FROM.......
CARDIOLOGY IMAGING PROGRAM TABLE OF CONTENTS. OVERVIEW............................................................................................. 618..... MEMBERS........... EXEMPT.......... FROM.......
Ischaemic heart disease. IInd Chair and Clinic of Cardiology
 Ischaemic heart disease IInd Chair and Clinic of Cardiology Definition Syndrome due to chronic insufficient oxygen supply to myocardial cells Nomenclature: ischaemic heart disease (IHD), coronary artery
Ischaemic heart disease IInd Chair and Clinic of Cardiology Definition Syndrome due to chronic insufficient oxygen supply to myocardial cells Nomenclature: ischaemic heart disease (IHD), coronary artery
Clinical Policy: Microvolt T-Wave Alternans Testing Reference Number: CP.MP.212
 Clinical Policy: Reference Number: CP.MP.212 Effective Date: 03/05 Last Review Date: 09/17 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
Clinical Policy: Reference Number: CP.MP.212 Effective Date: 03/05 Last Review Date: 09/17 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications
12 Lead EKG Chapter 4 Worksheet
 Match the following using the word bank. 1. A form of arteriosclerosis in which the thickening and hardening of the vessels walls are caused by an accumulation of fatty deposits in the innermost lining
Match the following using the word bank. 1. A form of arteriosclerosis in which the thickening and hardening of the vessels walls are caused by an accumulation of fatty deposits in the innermost lining
National Medical Policy
 National Medical Policy Subject: Policy Number: Outpatient Cardiac Rehabilitation NMP530 Effective Date*: April 2014 Updated: January 2017 This National Medical Policy is subject to the terms in the IMPORTANT
National Medical Policy Subject: Policy Number: Outpatient Cardiac Rehabilitation NMP530 Effective Date*: April 2014 Updated: January 2017 This National Medical Policy is subject to the terms in the IMPORTANT
Clinical Policy: Total Artificial Heart Reference Number: CP.MP.127
 Clinical Policy: Reference Number: CP.MP.127 Effective Date: 12/16 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.127 Effective Date: 12/16 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Sample page. Medical. Cross Coder. Essential links from CPT codes to ICD-10-CM and HCPCS
 Cross Coder 2018 Medical Essential links from CPT codes to ICD-10-CM and HCPCS POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com. Contents Introduction...i
Cross Coder 2018 Medical Essential links from CPT codes to ICD-10-CM and HCPCS POWER UP YOUR CODING with Optum360, your trusted coding partner for 32 years. Visit optum360coding.com. Contents Introduction...i
Cardiovascular Disease
 Cardiovascular Disease Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars on the 3 rd Wednesday of each month to address topics related to risk adjustment
Cardiovascular Disease Session Guidelines This is a 15 minute webinar session for CNC physicians and staff CNC holds webinars on the 3 rd Wednesday of each month to address topics related to risk adjustment
Cardiovascular nuclear imaging employs non-invasive techniques to assess alterations in coronary artery flow, and ventricular function.
 National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA Original Date: October 2015 Page 1 of 9 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes:
National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA Original Date: October 2015 Page 1 of 9 FOR CMS (MEDICARE) MEMBERS ONLY CPT4 Codes:
Consensus Core Set: Cardiovascular Measures Version 1.0
 Consensus Core Set: Cardiovascular s NQF 0330 Hospital 30-day, all-cause, riskstandardized readmission rate (RSRR) following heart failure hospitalization 0229 Hospital 30-day, all-cause, riskstandardized
Consensus Core Set: Cardiovascular s NQF 0330 Hospital 30-day, all-cause, riskstandardized readmission rate (RSRR) following heart failure hospitalization 0229 Hospital 30-day, all-cause, riskstandardized
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014
 Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
Indications of Coronary Angiography Dr. Shaheer K. George, M.D Faculty of Medicine, Mansoura University 2014 Indications for cardiac catheterization Before a decision to perform an invasive procedure such
4. Which survey program does your facility use to get your program designated by the state?
 STEMI SURVEY Please complete one survey for each TCD designation you have in your facility. There would be a maximum of three surveys completed if your facility was designated as a trauma, stroke and STEMI
STEMI SURVEY Please complete one survey for each TCD designation you have in your facility. There would be a maximum of three surveys completed if your facility was designated as a trauma, stroke and STEMI
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Cardiology Documentation ICD-10 Analysis
 Cardiology Documentation ICD-10 Analysis Presenters Ghazala Q. Sharieff MD, MBA Corporate Director, Physician Outreach and Medical Management, Scripps Health Allison Hager-Faster ICD-10 Project Manager;
Cardiology Documentation ICD-10 Analysis Presenters Ghazala Q. Sharieff MD, MBA Corporate Director, Physician Outreach and Medical Management, Scripps Health Allison Hager-Faster ICD-10 Project Manager;
12 Lead Electrocardiogram (ECG) PFN: SOMACL17. Terminal Learning Objective. References
 12 Lead Electrocardiogram (ECG) PFN: SOMACL17 Slide 1 Terminal Learning Objective Action: Communicate knowledge of 12 Lead Electrocardiogram (ECG) Condition: Given a lecture in a classroom environment
12 Lead Electrocardiogram (ECG) PFN: SOMACL17 Slide 1 Terminal Learning Objective Action: Communicate knowledge of 12 Lead Electrocardiogram (ECG) Condition: Given a lecture in a classroom environment
Performance and Quality Measures 1. NQF Measure Number. Coronary Artery Disease Measure Set
 Unless indicated, the PINNACLE Registry measures are endorsed by the American College of Cardiology Foundation and the American Heart Association and may be used for purposes of health care insurance payer
Unless indicated, the PINNACLE Registry measures are endorsed by the American College of Cardiology Foundation and the American Heart Association and may be used for purposes of health care insurance payer
Imaging ischemic heart disease: role of SPECT and PET. Focus on Patients with Known CAD
 Imaging ischemic heart disease: role of SPECT and PET. Focus on Patients with Known CAD Hein J. Verberne Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands International Conference
Imaging ischemic heart disease: role of SPECT and PET. Focus on Patients with Known CAD Hein J. Verberne Academic Medical Center, University of Amsterdam, Amsterdam, Netherlands International Conference
Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality)
 2017 Coding and Medicare payment guide Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality) All coding, coverage, billing and payment information provided herein by Philips Volcano
2017 Coding and Medicare payment guide Fractional Flow Reserve (FFR) and instant wave-free Ratio (The ifr modality) All coding, coverage, billing and payment information provided herein by Philips Volcano
1. Skin care: Cleansing, lubrication, debriding and administration of antimicrobial therapy.
 Lymphedema Treatment Last Review Date: October 13, 2017 Number: MG.MM.ME.05aC9 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Lymphedema Treatment Last Review Date: October 13, 2017 Number: MG.MM.ME.05aC9 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Mortality. p =
 Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Resolution by the Federal Joint Committee on an amendment to the Pharmaceutical Directive (AM-RL): Appendix XII Resolutions on the benefit assessment of pharmaceuticals with new active ingredients, in
Coronary Interventions Indications, Treatment Options and Outcomes
 Coronary Interventions Indications, Treatment Options and Outcomes A talk should be like a woman s skirt long enough to cover the subject, but short enough to keep it interesting. Coronary anatomy Physiology
Coronary Interventions Indications, Treatment Options and Outcomes A talk should be like a woman s skirt long enough to cover the subject, but short enough to keep it interesting. Coronary anatomy Physiology
Rebuilding and Reinvigorating Cardiac Rehabilitation in 2018
 Rebuilding and Reinvigorating Cardiac Rehabilitation in 2018 Pam R. Taub MD, FACC Director of Step Family Cardiac Wellness and Rehabilitation Center Associate Professor of Medicine UC San Diego Health
Rebuilding and Reinvigorating Cardiac Rehabilitation in 2018 Pam R. Taub MD, FACC Director of Step Family Cardiac Wellness and Rehabilitation Center Associate Professor of Medicine UC San Diego Health
The importance of follow-up after a cardiac event: CARDIAC REHABILITATION. Dr. Guy Letcher
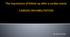 The importance of follow-up after a cardiac event: CARDIAC REHABILITATION Dr. Guy Letcher The National Medicare Experience Mortality After Angioplasty 225,915 patients Mortality After Bypass Surgery 357,885
The importance of follow-up after a cardiac event: CARDIAC REHABILITATION Dr. Guy Letcher The National Medicare Experience Mortality After Angioplasty 225,915 patients Mortality After Bypass Surgery 357,885
Bone Mineral Density Studies in Adult Populations
 Bone Mineral Density Studies in Adult Populations Last Review Date: July 14, 2017 Number: MG.MM.RA10aC6 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician
Bone Mineral Density Studies in Adult Populations Last Review Date: July 14, 2017 Number: MG.MM.RA10aC6 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician
Cardiovascular nuclear imaging employs non-invasive techniques to assess alterations in coronary artery flow, and ventricular function.
 National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
National Imaging Associates, Inc. Clinical guidelines CARDIOVASCULAR NUCLEAR MEDICINE -MYOCARDIAL PERFUSION IMAGING -MUGA CPT4 Codes: Refer to pages 6-9 LCD ID Number: L33960 J 15 = KY, OH Responsible
Drs. Rottman, Salloum, Campbell, Muldowney, Hong, Bagai, Kronenberg
 Rotation: or: Faculty: Coronary Care Unit (CVICU) Dr. Jeff Rottman Drs. Rottman, Salloum, Campbell, Muldowney, Hong, Bagai, Kronenberg Duty Hours: Mon Fri, 7 AM to 7 PM, weekend call shared with consult
Rotation: or: Faculty: Coronary Care Unit (CVICU) Dr. Jeff Rottman Drs. Rottman, Salloum, Campbell, Muldowney, Hong, Bagai, Kronenberg Duty Hours: Mon Fri, 7 AM to 7 PM, weekend call shared with consult
VENTRICULAR ASSIST DEVICES AND TOTAL ARTIFICIAL HEARTS
 Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-86 Effective Date: 03/26/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Surgery Policy Number: IV-86 Effective Date: 03/26/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP230 Section: Medical Benefit Policy Subject: Outpatient Pulmonary Rehabilitation I. Policy: Outpatient Pulmonary Rehabilitation II. Purpose/Objective: To provide
POLICIES AND PROCEDURE MANUAL Policy: MP230 Section: Medical Benefit Policy Subject: Outpatient Pulmonary Rehabilitation I. Policy: Outpatient Pulmonary Rehabilitation II. Purpose/Objective: To provide
Fellows on this rotation are expected to attend nuclear conferences and multimodality imaging conference.
 Rotation: Imaging 1 Imaging 1 provides COCATS Level 1 experience for nuclear cardiology (including SPECT and PET) and cardiac CT. Fellows will administer, process, and read cardiac nuclear studies with
Rotation: Imaging 1 Imaging 1 provides COCATS Level 1 experience for nuclear cardiology (including SPECT and PET) and cardiac CT. Fellows will administer, process, and read cardiac nuclear studies with
INTRO LEFT VENTRICULAR ASSIST DEVICE (LVAD) ACUTE MCS MECHANICAL CIRCULATORY SUPPORT (MCS)
 ABBOTT CODING GUIDE MECHANICAL CIRCULATORY SUPPORT (MCS) LEFT VENTRICULAR ASSIST DEVICE (LVAD), (HEARTMATE II OR HEARTMATE 3 LVADS) ACUTE MCS (CENTRIMAG OR PEDIMAG PUMPS) Effective January 1, 2019 MECHANICAL
ABBOTT CODING GUIDE MECHANICAL CIRCULATORY SUPPORT (MCS) LEFT VENTRICULAR ASSIST DEVICE (LVAD), (HEARTMATE II OR HEARTMATE 3 LVADS) ACUTE MCS (CENTRIMAG OR PEDIMAG PUMPS) Effective January 1, 2019 MECHANICAL
Chapter 4: Cardiovascular Disease in Patients With CKD
 Chapter 4: Cardiovascular Disease in Patients With CKD Introduction Cardiovascular disease is an important comorbidity for patients with chronic kidney disease (CKD). CKD patients are at high-risk for
Chapter 4: Cardiovascular Disease in Patients With CKD Introduction Cardiovascular disease is an important comorbidity for patients with chronic kidney disease (CKD). CKD patients are at high-risk for
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33200-37186, 37195-37785, 92950-93272, 93303-93581,
Supplementary Online Content
 Supplementary Online Content Valle JA, Tamez H, Abbott JD, et al. Contemporary use and trends in unprotected left main coronary artery percutaneous coronary intervention in the United States: an analysis
Supplementary Online Content Valle JA, Tamez H, Abbott JD, et al. Contemporary use and trends in unprotected left main coronary artery percutaneous coronary intervention in the United States: an analysis
Clinical Policy: Pediatric Heart Transplant
 Clinical Policy: Reference Number: CP.MP.138 Review Date: 01/19 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Clinical Policy: Reference Number: CP.MP.138 Review Date: 01/19 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Practice-Level Executive Summary Report
 PINNACLE Registry Metrics 0003, Test Practice_NextGen [Rolling: 1st April 2015 to 31st March 2016 ] Generated on 5/11/2016 11:37:35 AM American College of Cardiology Foundation National Cardiovascular
PINNACLE Registry Metrics 0003, Test Practice_NextGen [Rolling: 1st April 2015 to 31st March 2016 ] Generated on 5/11/2016 11:37:35 AM American College of Cardiology Foundation National Cardiovascular
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Cardiac Event Detection (L33952) MP-054-MC-KY Medical Management Provider Notice Date: 05/01/2018 Issue Date: 06/01/2018 Effective
Policy Name: Policy Number: Responsible Department(s): CLINICAL MEDICAL POLICY Cardiac Event Detection (L33952) MP-054-MC-KY Medical Management Provider Notice Date: 05/01/2018 Issue Date: 06/01/2018 Effective
Chapter 4 Section 9.1
 Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
Surgery Chapter 4 Section 9.1 Issue Date: August 26, 1985 Authority: 32 CFR 199.4(c)(2) and (c)(3) 1.0 CPT 1 PROCEDURE CODES 33010-33130, 33140, 33141, 33361-33369, 33200-37186, 37195-37785, 92950-93272,
