The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm: extended observations 2 years after trial closure
|
|
|
- Rodney Craig
- 5 years ago
- Views:
Transcription
1 European Heart Journal (2008) 29, doi: /eurheartj/ehm583 CLINICAL RESEARCH Prevention and epidemiology The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm: extended observations 2 years after trial closure Peter S. Sever*, Neil R. Poulter 1, Bjorn Dahlof 2, Hans Wedel 3, Gareth Beevers 4, Mark Caulfield 5, Rory Collins 6, Sverre E. Kjeldsen 7,8, Arni Kristinsson 9, Gordon McInnes 10, Jesper Mehlsen 11, Markku S. Nieminen 12, Eoin T. O Brien 13, and Jan Östergren 14 on behalf of the ASCOT Investigators 1 International Centre for Circulatory Health, Imperial College London, 59 North Wharf Road, London W2 1PG, UK; 2 Sahlgrenska University Hospital/Östra, Göteborg, Sweden; 3 Nordic School of Public Health, Göteborg, Sweden; 4 City Hospital, Birmingham, UK; 5 St Bartholomew s and the London, Queen Mary s School of Medicine, London, UK; 6 Oxford University, Oxford, UK; 7 Ullevål Sykehus, Oslo, Norway; 8 University of Michigan, Ann Arbor, MI, USA; 9 University Hospital, Reykjavik, Iceland; 10 University of Glasgow, Glasgow, UK; 11 H S Frederiksberg Hospital, Frederiksberg, Denmark; 12 University Central Hospital, Helsinki, Finland; 13 Beaumont Hospital and Royal College of Surgeons, Dublin, Ireland; 14 Karolinska Hospital, Stockholm, Sweden Received 8 June 2007; revised 31 October 2007; accepted 22 November 2007; Online-publish-ahead-of-print 5 January 2008 See page 425 for the editorial comment on this article (doi: /eurheartj/ehm628) Aims To determine the cardiovascular benefits in those originally assigned atorvastatin in the Anglo-Scandinavian Cardiac Outcomes Trial 2.2 years after closure of the lipid-lowering arm of the trial (ASCOT-LLA).... Methods The Blood Pressure Lowering Arm of the ASCOT trial (ASCOT-BPLA) compared two different antihypertensive and results treatment strategies on cardiovascular outcomes. ASCOT-LLA was a double-blind placebo-controlled trial of atorvastatin in those enrolled into ASCOT-BPLA with total cholesterol concentrations at baseline of 6.5 mmol/l. A total of hypertensive patients were enrolled in ASCOT-BPLA and were further assigned either atorvastatin, 10 mg, or placebo. ASCOT-LLA was stopped prematurely after a median 3.3 years follow-up because of substantial cardiovascular benefits in those assigned atorvastatin. Trial physicians were invited to offer atorvastatin to all ASCOT-LLA patients until the end of ASCOT-BPLA. The primary outcome of ASCOT-LLA was combined fatal coronary heart disease (CHD) or non-fatal myocardial infarction. Secondaryoutcomesincludedallcoronaryevents, allcardiovasculareventsandprocedures, fatalandnon-fatalstroke, cardiovascular mortality, all cause mortality, development of chronic stable angina, heart failure, and peripheral arterial disease. By the end of ASCOT-LLA, there was a 36% relative risk reduction in primary events (n ¼ 254) in favour of atorvastatin [hazard ratio (HR) 0.64, 95% CI: , P ¼ ]. At the end of ASCOT-BPLA, 2.2 years later, despite extensive crossovers from and to statin usage, the relative risk reduction in primary events (n ¼ 412) among those originally assigned atorvastatin remained at 36% (HR 0.64, 95% CI: , P ¼ ). For almost all other endpoints, risk reductions also remained essentially unchanged and in the case of all cause mortality, the risk reduction of 15% now achieved borderline statistical significance (P ¼ 0.02).... Conclusion Carry-over benefits from those originally assigned atorvastatin but no longer taking the drug may account for unchanged relative risk reductions in most cardiovascular endpoints observed 2 years after ASCOT-LLA closed Keywords Coronary and stroke events Atorvastatin ASCOT-LLA Extension * Corresponding author. Tel: þ , Fax: þ p.sever@imperial.ac.uk Published on behalf of the European Society of Cardiology. All rights reserved. & The Author For permissions please journals.permissions@oxfordjournals.org. The online version of this article has been published under an open access model. Users are entitled to use, reproduce, disseminate, or display the open access version of this article for non-commercial purposes provided that the original authorship is properly and fully attributed; the Journal, Learned Society and Oxford University Press are attributed as the original place of publication with correct citation details given; if an article is subsequently reproduced or disseminated not in its entirety but only in part or as a derivative work this must be clearly indicated. For commercial re-use, please contact journals.permissions@oxfordjournals.org.
2 500 P.S. Sever et al Introduction In 2003, we reported the outcome of the lipid-lowering arm of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT-LLA), 1 a placebo-controlled randomized trial of the effects of atorvastatin 10 mg daily in the primary prevention of coronary heart disease (CHD) in hypertensive subjects who had a total cholesterol level 6.5 mmol/l. The trial was stopped prematurely after a median 3.3 years follow-up due to substantial benefits of atorvastatin on the primary endpoint of non-fatal myocardial infarction and fatal CHD, together with significant reductions in several other cardiovascular endpoints. ASCOT-LLA was part of a factorially designed trial in which hypertensive patients with no prior history of CHD were initially randomized to one of two antihypertensive treatment strategies (a beta-blocker adding a thiazide diuretic as required or a dihydropyridine calcium channel blocker (CCB), adding an angiotensin converting enzyme inhibitor as required 2,3 (ASCOT-BPLA). After the termination of LLA, subjects continued in BPLA for a further 2.2 years when the trial was stopped on the recommendation of the Data Safety Monitoring Board (DSMB), owing to substantial mortality benefits in favour of the CCB-based treatment strategy. The present report evaluates the cardiovascular outcomes of those subjects originally assigned either atorvastatin or placebo in the LLA and followed-up to the end of BPLA (5.5 years). Methods The detailed ASCOT protocol, including study design, organization, clinical measurements, power calculations, recruitment rates, and baseline characteristics has been published, 2 and further detailed information is available on the ASCOT website ( In summary, the trial was an independent, investigator-led, multicentre study with a prospective, randomized parallel group design incorporating by way of a 2 2 factorial approach, a comparison of two antihypertensive treatment regimens and in a large subgroup, atorvastatin with placebo. Patients eligible for inclusion into the LLA had to be eligible for the blood pressure-lowering arm (BPLA) and have total cholesterol concentrations of 6.5 mmol/l or less, and not currently taking a statin or a fibrate. This population consisted of men and women aged between 40 and 79 years at randomization, with either untreated hypertension defined as systolic blood pressure of 160 mmhg or more, diastolic blood pressure 100 mmhg or more, or both, or treated hypertension with systolic blood pressure 140 mmhg or more, diastolic blood pressure 90 mmhg or more, or both. Trial participants were also required to have at least three of the following risk factors for cardiovascular disease: male sex, aged 55 years or older, smoking, left ventricular hypertrophy, other specified abnormalities on electrocardiogram, type II diabetes, peripheral arterial disease, previous stroke or transient ischaemic attack, microalbuminuria or proteinuria, ratio of plasma total cholesterol to HDL-cholesterol concentration of 6 or higher or family history of premature CHD. Exclusion criteria included (among others): previous myocardial infarction; currently treated angina; a cerebrovascular event within the previous 3 months; fasting triglycerides 4.5 mmol/ L; heart failure; uncontrolled arrhythmias; or any clinically important haematological or biochemical abnormality on routine screening. The study conformed to good clinical practice guidelines 4 and was carried out in accordance with the Declaration of Helsinki. 5 The protocol and all subsequent amendments to the protocol were reviewed and ratified by central and regional ethics review boards in the UK and by national ethics and statutory bodies in Ireland and the Nordic countries (Sweden, Denmark, Iceland, Norway, and Finland). Patients were recruited between February 1998 and May Eligibility criteria were established and written informed consent was obtained about 4 weeks before randomization. Blood pressure was measured using standard procedures; non-fasting blood samples were collected and 12-lead electrocardiograms were assessed centrally. After the 4-week run-in period, eligible recruits were randomized, and they underwent a physical examination; blood pressure, heart rate, and 12-lead electrocardiogram were again recorded. Fasting blood samples were obtained for total cholesterol, HDL cholesterol, triglyceride, and glucose levels. Management of the BPLA is detailed elsewhere. 2 Patients were randomly assigned one of two antihypertensive regimens. Of the patients randomized to one of the two antihypertensive regimens, with a non-fasting cholesterol concentration 6.5 mmol/l at the initial screening visit, who were untreated with a statin or fibrate were further randomly assigned, by computer (using minimization procedures), to receive atorvastatin 10 mg daily or matching placebo. Baseline characteristics of participants in these two randomized groups were well matched and have previously been reported. 1 3 At each follow-up visit, antihypertensive drug therapy was titrated to achieve target blood pressures (,140/90 mmhg for non-diabetic patients and,130/80 mmhg for diabetic patients); information was recorded about adverse events and any new cardiovascular events or procedures, including the cause for any hospital admission. Information on potential endpoints was reviewed by a blinded endpoint committee. Early closure of the lipid-lowering arm The DSMB decided a priori to use the symmetric Haybittle-Peto statistical boundary (critical value Z ¼ 3) as a guideline for deciding to recommend early termination of the trial, which has the added advantage that no material adjustment to the final P-values is required. 6 On 2 September 2002, the DSMB recommended that the LLA of the trial be stopped on the grounds that atorvastatin had resulted in a highly significant reduction in the primary endpoint compared with placebo and a significant reduction in the incidence of stroke. 1 This recommendation was ratified by the Steering Committee, whereupon all patients in LLA were recalled between October and December 2002, for a final end-of-study visit. Trial physicians were invited to offer atorvastatin 10 mg daily to all patients in LLA until the end of BPLA, which was stopped between December 2004 and June 2005 on the recommendation of the DSMB. Neither trial physicians nor patients were aware of whether they had formerly been assigned atorvastatin or placebo. Statistical methods We estimated that a total of at least patients followed-up for an average of 5 years were required in ASCOT-BPLA. Of these, we estimated about 9,000 patients would be assigned atorvastatin 10 mg or placebo. In LLA, we calculated power to be 90% (b ¼ 0.10) to detect a relative effect of 30% on the primary endpoint of atorvastatin 10 mg compared with placebo. This calculation assumed a significance level of 1% (a ¼ 0.01) and a yearly endpoint rate in the placebo group of 13 per 1000 for 5 years of treatment. For the extension of 2.2 years after ASCOT-LLA close-out and with 150 more primary endpoints,
3 The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm 501 statistical power calculation showed that the study had 83% power with a significance level of 5% for a reduction of 36% in hazard. We compared the time to first primary endpoint event in the atorvastatin and placebo groups on an intention-to-treat basis. All analyses excluded endpoints deemed invalid by the Endpoint Committee, with statistical censoring enforced at the end of the study or at death. Analysis of percentage time on statin was restricted to all those subjects who had a date when the patient was last seen or known alive in the study (disposition date), which fell 6 months after the lipid closeout visit or later. Time on statin was calculated as the number of days on statin between 6 months after the lipid close-out and the disposition date or 6 months after 1 October 2002 and the disposition date for those with no lipid close-out visit. The date used to indicate a silent myocardial infarction was taken as the mean time between the dates of two electrocardiograms, the first of which showed no myocardial infarction and the second of which did. The intent to treat rule was strictly applied. Patients were analysed according to their randomly allocated treatment group, regardless of later treatment. Thus, a non-compliant patient belonged to the initial randomized group. A patient who had switched drug was analysed as if he/she belonged to the original randomized group. A patient who was lost to follow-up is censored at the time of last visit. For the main analyses, we used log-rank procedures and Cox s proportional hazards model to calculate confidence intervals. 7 The proportionality of hazard ratio (HR) by time was investigated by using an interaction term of time and the indicator of treatment. No such interactions in time or between periods were found for primary and secondary endpoints. For tertiary endpoints, however, the HR seemed to change in the extended period. Thus, for tertiary endpoints, the HRs should be interpreted as mean estimates over the periods. Cumulative incidence curves were generated by the Kaplan Meier method for all major endpoints in the active and placebo groups. Results The baseline characteristics of trial participants randomized to either atorvastatin 10 mg or placebo were well matched (Table 1). Participants were mainly white (95%) and male (81%), with a mean age of 63 years. Baseline blood pressure and lipid subfraction values were identical in the two groups. LLA was stopped prematurely after patient-years of follow-up (median 3.3 years) when complete information was obtained on (98.8%) of the patients originally randomized. Of the remainder, vital status was obtained on all but 17 patients. Following closure of LLA and by the end of BPLA, complete information was available on patients originally randomized (Figure 1). However, between the closure of LLA and the subsequent closure of BPLA there was substantial drop-in and drop-out of statin therapy among those originally randomized to placebo and atorvastatin, respectively. Consequently, at the closure of BPLA, of those originally assigned atorvastatin, 63% were still taking it, and of those originally assigned placebo, 56% were taking atorvastatin (Table 2). A small percentage of additional patients were receiving other statins (4 and 7%, respectively Table 2). In a further analysis estimating extensive statin use as taking statins.90% of follow-up time, and minimal statin use as,10% of follow-up time, of those originally assigned atorvastatin 50% continued to use statins extensively and 29% minimally. Of Table 1 Baseline characteristics Atorvastatin, Placebo, n n Demographics and clinical characteristics Woman 979 (18.9%) 963 (18.7%) Age (years) (36.4%) 1853 (36.1%) (63.6%) 3284 (63.9%) Mean (SD) 63.1 (8.5) 63.2 (8.6) White 4889 (94.6%) 4863 (94.7%) Current smoker 1718 (33.2%) 1656 (32.2%) Alcohol consumption (unit/week) 8.0 (11.3) 8.2 (12.0) Systolic blood pressure (mmhg) (17.7) (18.0) Diastolic blood pressure (mmhg) 95.0 (10.3) 95.0 (10.3) Heart rate (b.p.m.) 71.3 (12.8) 71.8 (12.6) BMI (kg/m 2 ) 28.6 (4.7) 28.7 (4.6) Total cholesterol (mmol/l) 5.5 (0.8) 5.5 (0.8) LDL cholesterol (mmol/l) 3.4 (0.7) 3.4 (0.7) HDL cholesterol (mmol/l) 1.3 (0.4) 1.3 (0.4) Triglycerides (mmol/l) 1.7 (0.9) 1.6 (0.9) Glucose (mmol/l) 6.2 (2.1) 6.2 (2.1) Creatinine (mmol/l) 99.0 (16.9) 99.0 (16.4)... Medical history Previous stroke/transient 485 (9.4%) 516 (10.0%) ischaemic attack (TIA) Diabetes a 1370 (26.5%) 1370 (26.7%) leftventricular hypertrophy (LVH) 1194 (23.1%) 1192 (23.2%) (according to ECG or ECHO) a ECG abnormalities other than 1206 (23.3%) 1177 (22.9%) LVH a Peripheral vascular disease 261 (5.1%) 253 (4.9%) Other relevant cardiovascular 188 (3.6%) 207 (4.0%) disease Mean (SD) number of risk factors 3.7 (0.9) 3.7 (0.9)... Drug therapy Previous antihypertensive treatments None 1021 (19.8%) 996 (19.4%) (44.8%) 2279 (44.4%) (35.5%) 1862 (36.2%) Lipid-lowering therapy 41 (0.8%) 52 (1.0%) Aspirin use 929 (18.0%) 902 (17.6%) a Includes information from investigator, ECG and glucose values. those originally assigned placebo, the figures were 49 and 32%, respectively. Compared with placebo at the end of LLA (3.3 years), total cholesterol and LDL-cholesterol concentrations among those allocated were around 1.0 mmol/l lower than those allocated placebo (Table 3, Figure 2A). However, by the end of BPLA, total and LDL-cholesterol concentrations had lowered in those formerly assigned placebo and had slightly increased in those formerly assigned atorvastatin, such that these values were almost identical in the two groups (Table 3, Figures 2A and B).
4 502 P.S. Sever et al Figure 1 Trial profile Table 2 Total number of subjects and total per cent of subjects on atorvastatin and other statins respectively at lipid close-out visit and final visit. All LLA subjects by atorvastatin and placebo subgroups Atorvastatin, n Placebo, n Atorvastatin, n (%) Any statins, n (%) Atorvastatin, n (%) Any statins, n (%)... Lipid close-out visit 4113 (82.6%) 4167 (83.7%) 415 (8.4) 635 (12.9%) Final visit 3122 (62.7%) 3322 (66.7%) 2752 (56.0%) 3089 (62.8%) Concentrations of HDL cholesterol were similar in both groups throughout the trial. Compared with placebo, atorvastatin reduced triglycerides by about 0.2 mmol/l at the closure of LLA (Table 3, Figure 2C), but by the end of BPLA, concentrations were almost identical (Table 3, Figure 2D) in the two groups. Blood pressures were similar throughout the trial in those assigned atorvastatin and placebo and by the end of the trial had fallen to 137/78 in both treatment groups. At 3.3 years (the end of ASCOT- LLA), the primary endpoint of non-fatal myocardial infarction and fatal CHD was significantly lower by 35% (HR 0.65 [95% CI: ], P ¼ , in the atorvastatin group compared with the placebo group. (Table 3, Figure 3) Note that late reported endpoints which occurred during LLA are now included. There is a highly significant further divergence during the 2.2 years extension period for the primary outcome despite lipid levels achieving similar valves by the end of 5.5 years of follow-up. After 5.5 years, and with a further 150 primary endpoints, the relative risk reduction of 36% was unchanged (HR % CI: , P, ) (Table 4, Figure 3). There were also significant reductions in a number of secondary endpoints at the end of LLA which were sustained until the end of BPLA, including total cardiovascular events and procedures (23% vs. 19%); total coronary events (29% vs. 27%), fatal and non-fatal stroke (21% vs. 23%), and all cause mortality (13% vs. 15%), respectively (Table 4 and Figures 3 and 4). Owing to the increase in the number of deaths by the end of the extended period of observation, the risk reduction in all cause mortality associated with assignment to atorvastatin achieved borderline significance (P ¼ 0.02). For the primary endpoint, cumulative event rates continued to fall following the closure of LLA in both those originally assigned atorvastatin and placebo. Similarly, event rates during the follow-up from 3.3 to 5.5 years also fell in both groups of patients (Table 4). The number of serious adverse events and rates of liver enzyme abnormalities between those assigned atorvastatin or placebo did not differ at any stage of the trial.
5 The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm 503 Table 3 Plasma concentrations of lipid fractions by visit and treatment Total cholesterol LDL cholesterol HDL cholesterol Triglycerides Atorvastatin Placebo Atorvastatin Placebo Atorvastatin Placebo Atorvastatin Placebo Mean (SD) n Mean (SD) n Mean (SD) n Mean (SD) n Mean (SD) n Mean (SD) n mean (SD) n Mean (SD) n... Baseline 5.48 (0.78) (0.78) (0.72) (0.72) (0.37) (0.36) (0.92) (0.87) year 4.16 (0.83) (0.85) (0.69) (0.76) (0.37) (0.35) (0.85) (0.98) year 4.14 (0.81) (0.89) (0.68) (0.78) (0.37) (0.36) (0.78) (0.88) year 4.18 (0.85) (0.90) (0.71) (0.80) (0.37) (0.36) (0.75) (0.94) 3812 Lipid close-out 4.21 (0.84) (0.91) (0.72) (0.81) (0.37) (0.37) (0.72) (0.87) year 4.48 (1.04) (1.04) (0.94) (0.94) (0.38) (0.37) (0.81) (0.85) year 4.39 (1.00) (0.98) (0.90) (0.88) (0.38) (0.37) (0.80) (0.77) 3962 Final visit 4.31 (0.96) (0.95) (0.85) (0.84) (0.39) (0.40) (1.01) (0.82) 3956 First five rows based are on LLA-locked data, last three rows are based on BPLA-locked data. Discussion At the time of the early closure of LLA, it was considered appropriate in the light of the substantial benefits reported with atorvastatin, to recommend to trial physicians that active therapy be offered to all those included in ASCOT-LLA until the end of BPLA. In the event, it was surprising, given the evidence base, that there were physicians and/or patients who chose either not to continue or not to start atorvastatin at the end of LLA. Thus, by the end of BPLA 60% of the patients originally assigned either placebo or atorvastatin were taking atorvastatin or other statin. This is reflected by the equalization of total and LDL-cholesterol concentrations by the end of the trial. Assuming drop-in to statin therapy occurred at a constant rate throughout the extended follow-up of just over 2 years, it can be predicted that this should have accounted for a relative risk reduction in primary event rates of around 19% in this group from the end of LLA to the end of BPLA. This compared with the observed reduction in this group from the end of the LLA to the end of the BPLA of 15%. Estimates of this type are, however, problematic and potentially confounded by accumulating benefits from reductions in blood pressure. Further analyses taking account of extensive or minimal use of any statins during follow-up, including atorvastatin, do not alter these conclusions. If there was no carry-over benefit from atorvastatin in those stopping active treatment at the end of LLA, one might have expected a modest rise in event rate in this group during the final phase of the trial. In fact, event rates continued to decline suggesting substantial and important carry-over benefits on cessation of active treatment. If ASCOT-LLA had proceeded for 5.5 years with continuing assignment to either atorvastatin or placebo, the risk reductions in cardiovascular events associated with atorvastatin would have been expected to continue at the same rates reported at the end of LLA. The cardiovascular event rates reported between the closures of LLA and BPLA were almost identical to those at the termination of LLA, despite substantial changes to active treatment rates after the closure of LLA among those originally allocated to placebo and atorvastatin. Presumably, carry-over benefits from statin among those who dropped out of therapy and continuing benefits of those remaining on statins resulted in similar relative risk benefits at the end of BPLA compared with those at the conclusion of LLA. Also, attrition of susceptibles in the placebo phase of the trial could contribute to reduced benefits on cardiovascular event rates among new-statin users. One effect of the early termination of LLA was that the study had reduced power to evaluate the effect of atorvastatin on all cause mortality. However, the extended follow-up presented here involves a significantly increased number of deaths and thereby power is restored such that the risk reduction associated with atorvastatin now achieves borderline statistical significance for a secondary endpoint. Post-trial follow-up observations have been reported from the double blind Scandinavian Simvastatin Survival Study (4S). 8 During an extended 2 year follow-up after the termination of 4S, about 80% of patients originally assigned placebo were given simvastatin and there were fewer coronary and cardiovascular
6 504 P.S. Sever et al Figure 2 Lipid profiles over time throughout double blind and follow-up period for total cholesterol (A), LDL cholesterol (B), HDL cholesterol (C), and triglycerides (D) deaths in those originally assigned simvastatin, but differences in event rates between those originally assigned statin and placebo were less than those reported at the end of the double blind trial. However, because of relatively small numbers of additional deaths compared with those reported in the double blind trial, overall HRs for coronary and cardiovascular deaths changed little by the end of the extended follow-up (0.57 vs and 0.64 vs. 0.67, respectively). In a subsequent analysis of coronary and cardiovascular deaths during a 5 year extension, 9 the number of deaths between those originally assigned simvastatin and placebo was very similar, probably due to widespread statin use in both groups. The benefits conferred by statin in those originally assigned placebo presumably contributed to the reduction in relative risk between statin and placebo for coronary and cardiovascular deaths between the end of the double blind trial and the end of the 10 year follow-up (0.57 vs and 0.64 vs. 0.83, respectively). In the Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) study, 10 patients with previous coronary disease were randomly assigned pravastatin or placebo and were followed-up for 6 years. Most (97%) had 2 years extended follow-up during which 86% of those assigned placebo took openlabelled pravastatin. During this period, patients originally assigned pravastatin, although achieving cholesterol levels almost identical with those originally assigned placebo continued to demonstrate lower events rates for CHD deaths or non-fatal myocardial infarction (25%), stroke (24%), cardiovascular mortality (24%), and all cause mortality (24%). These relative risk reductions during the extended follow-up period are similar to those observed during the double-blind randomized placebo controlled main trial. A recent report of the 10-year post-trial follow-up of survivors of the West of Scotland Coronary Prevention Study has demonstrated that 5 years in trial treatment with pravastatin was associated with a significant reduction in coronary events for a subsequent 10 years in men with hypercholesterolaemia who had no history of myocardial infarction. 11 Five years after the completion of the trial 37% of those formerly assigned placebo or pravastatin were being treated with a statin, but there was no information on the comparability of lipid levels at the end of the 10-year follow-up. Nevertheless, it is reasonable to assume that they would be similar in the two groups. In a further study, The Assessment of LEscol in Renal Transplantation, the ALERT trial, fluvastatin was compared with placebo in renal transplant recipients. The primary composite endpoint of cardiac death, non-fatal myocardial infarction and coronary
7 Table 4 Cardiovascular events during LLA trial (locked data), extension and followed to final visit, atorvastatin vs. placebo LLA a LLA extension Followed to final visit a Atorvastatin Placebo Unadjusted Atorvastatin Placebo Unadjusted Atorvastatin Placebo Unadjusted Hazard ratio Hazard ratio Hazard ratio (95% CI) (95% CI) (95% CI) n (%) Rate b n (%) Rate b P-value n (%) Rate b n (%) Rate b P-value n (%) Rate b n (%) Rate b P-value... Primary endpoint Non-fatal MI (incl 104 (2.0%) (3.1%) ( ) 59 (1.2%) (1.9%) ( ) 163 (3.2%) (4.8%) ( ) silent) þ fatal CHD P ¼ P ¼ P ¼, Secondary endpoints Total CV events and procedures Total coronary endpoint Non-fatal MI (excl silent) þ fatal CHD 410 (7.9%) (10.2%) ( ) P ¼ (3.8%) (5.3%) ( ) P ¼ (1.7%) (2.7%) ( ) P ¼ All cause mortality 186 (3.6%) (4.1%) ( ) P ¼ Cardiovascular mortality Fatal and non-fatal stroke 75 (1.5%) (1.7%) ( ) P ¼ (2.1%) (2.7%) ( ) P ¼ (5.1%) (5.8%) ( ) P ¼ (2.5%) (3.3%) ( ) P ¼ (1.1%) (1.8%) ( ) P ¼ (4.0%) (4.8%) ( ) P ¼ (1.3%) (1.5%) ( ) P ¼ (1.1%) (1.5%) ( ) P ¼ (12.5%) (15.3%) ( ) P ¼, (6.1%) (8.3%) ( ) P ¼, (2.8%) (4.4%) ( ) P ¼, (7.5%) (8.7%) ( ) P ¼ (2.7%) (3.2%) ( ) P ¼ (3.2%) (4.1%) ( ) P ¼ Fatal and non-fatal 43 (0.8%) (0.8%) ( ) 33 (0.7%) (0.7%) ( ) 76 (1.5%) (1.5%) ( ) heart failure P ¼ P ¼ P ¼ Tertiary endpoints Silent MI 15 (0.3%) (0.3%) ( ) P ¼ Unstable angina 22 (0.4%) (0.5%) ( ) P ¼ Chronic stable angina Peripheral arterial disease Life threatening arrhythmias Develop. diabetes mellitus Develop. renal impairment 42 (0.8%) (1.4%) ( ) P ¼ (0.9%) (1.0%) ( ) P ¼ (0.2%) (0.1%) ( ) P ¼ (7.3%) (6.7%) ( ) P ¼ (2.8%) (2.6%) ( ) P ¼ a Late reported endpoints occurred during LLA are included as well. b Per 1000 patient years. 4 (0.1%) (0.1%) ( ) P ¼ (0.4%) (0.2%) ( ) P ¼ (0.6%) (0.8%) ( ) P ¼ (0.6%) (0.7%) ( ) P ¼ (0.1%) (0.1%) ( ) P ¼ (2.5%) (3.2%) ( ) P ¼ (2.0%) (1.9%) ( ) P ¼ (0.4%) (0.5%) ( ) P ¼ (0.8%) (0.7%) ( ) P ¼ (1.4%) (2.1%) ( ) P ¼ (1.5%) (1.6%) ( ) p ¼ (0.3%) (0.2%) ( ) P ¼ (9.6%) (9.5%) ( ) P ¼ (4.6%) (4.4%) ( ) P ¼ The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm 505
8 506 P.S. Sever et al Figure 3 Cumulative incidence of primary endpoint of non-fatal myocardial infarction and fatal coronary heart disease (A), fatal and non-fatal stroke (B), total cardiovascular events and procedures (C), and all cause mortality (D). Late reported endpoints occurring during lipid-lowering arm are now included intervention procedures was non-significantly reduced by 17% at the end of the formal follow-up period of 5.4 years. 12 However, despite widespread use of statins in those formerly assigned placebo during an extended 2 year period of observation and equalization of cholesterol levels, a significant risk reduction of 21% in the primary endpoint in those originally assigned fluvastatin was observed. 13 These observations on extended periods of observation after several trials provide further convincing evidence that withdrawal from active statin treatment is followed by sustained treatment benefits in those with or without prior coronary heart disease events. These observations indicate that the costeffectiveness of statins will be greater than that previously estimated. 14 Our findings during short-term extended follow-up of ASCOT-LLA, albeit with substantial numbers of fatal and non-fatal events reported, are similar to those of the 2-year extended follow-up of the 4S trial 8 and the LIPID trial. 10 Although a degree of protective carry-over effect afforded by statins, presumably resulting from plaque stabilization, could reasonably be expected over the short-term, it might be predicted that given sufficient extended follow-up, assuming treatment rates equalize in those originally assigned placebo and statin, that event rates would converge, as witnessed in the longer term follow-up of 4S. Acknowledgements P.S.S., B.D., N.R.P., H.W., constituting the executive committee and members of the steering committee designed the study, wrote the protocol, supervised the undertaking of the study, coordinated data collection, wrote the analysis plan, supervised the analyses, interpreted the results, and wrote the report. G.B., M.C., R.C., S.E.K., A.K., G.M., J.M., M.N., E.T.O., and J.Ö., as members of the steering committee, approved the protocol and analysis plan, supervised the undertaking of the study, and had input to the report. Conflict of interest. P.S.S., B.D., N.R.P., H.W., G.B., M.C., R.C., S.E.K., A.K., G.M., J.M., M.N., E.T.O., and J.Ö. have served as consultants or received travel expenses, or payment for speaking at meetings, or funding for research from one or more pharmaceutical companies that market blood pressure lowering or lipid-lowering drugs, including Pfizer for ASCOT.
9 The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm 507 Figure 4 Effects of atorvastatin and placebo on primary, secondary and tertiary endpoints at the end of lipid-lowering arm (3.3 years), during the lipid-lowering arm-extension and at the end of the trial. Area of squares is proportional to amount of statistical information. Point estimates of hazard ratios are given with 95% CI. MI, myocardial infarction; CHD, coronary heart disease Funding The principal funding source (Pfizer) had two non-voting members on the steering Committee. Data analyses and preparation of the report were done independently of the funding source. Funding to pay the Open Access publication charges for this article was provided by Pfizer New York. References 1. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O Brien E, Östergren J, for the ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361: Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, McInnes GT, Mehlsen J, Nieminen M, O Brien E, Östergren J, for the ASCOT investigators. Rationale, design, methods and baseline demography of participants of the Anglo-Scandinavian Cardiac Outcomes Trial. ASCOT investigators. J Hypertens 2001;6: Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O Brien E, Östergren J, for the ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366: ICH Harmonisation Tripartite Guidelines for Good Clinical Practice (GCP).. Surrey, Brookwood Medical Publications; ISBN World Medical Association Declaration of Helsinki. 1996; Republic of South Africa. 6. Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomised clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer 1976;34: Cox DR. Regression models and life-tables. J R Stat Soc B 1972;34: Pedersen TR, Wilhelmsen L, Faergeman O, Strandberg TE, Thorgeirsson G, Troedsson L, Kristianson J, Berg K, Cook TJ, Haghfelt T, Kjekshus J, Miettinen T, Olsson AG, Pyörälä K, Wedel H, on behalf of the Scandinavian Simvastatin Survival Study Group. Follow-up study of patients randomised in the Scandinavian Simvastatin Survival Study (4S) of cholesterol lowering. Am J Cardiol 2000;86: Strandberg TE, Pyörälä K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, Pedersen TR, Kjekshus J, for the 4S Group. Mortality and incidence of cancer during 10-year follow-up of the
10 508 P.S. Sever et al Scandinavian Simvastatin Survival Study (4S). Lancet 2004;364: The LIPID Study Group. Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet 2002; 359: Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM. Long-term follow up of the West of Scotland Coronary Prevention Study. N Engl J Med 2007;357: Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, Grunhagen-Riska C, Madsen S, Neumayer H, Cole E, Maes B, Ambuhl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebocontrolled trial. Lancet 2003;361: Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Gronhagen-Riska C, Neumayer H, Maes B, Ambuhl P, Hartmann A, Staffler B, Jardine AG. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant 2005;5: Lindgren P, Buxton M, Kahan T, Poulter NR, Dahlöf B, Sever PS, Wedel H, Jönsson B, for the ASCOT investigators. Costeffectiveness of atorvastatin for the prevention of coronary and stroke events: an economic analysis of the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm (ASCOT-LLA). Eur J Cardiovasc Prev Rehabil 2005;12:29 36.
Potential synergy between lipid-lowering and blood-pressure-lowering in the Anglo-Scandinavian Cardiac Outcomes Trial
 European Heart Journal (2006) 27, 2982 2988 doi:10.1093/eurheartj/ehl403 Clinical research Coronary heart disease Potential synergy between lipid-lowering and blood-pressure-lowering in the Anglo-Scandinavian
European Heart Journal (2006) 27, 2982 2988 doi:10.1093/eurheartj/ehl403 Clinical research Coronary heart disease Potential synergy between lipid-lowering and blood-pressure-lowering in the Anglo-Scandinavian
Reduction in Cardiovascular Events With Atorvastatin in 2,532 Patients With Type 2 Diabetes
 Pathophysiology/Complications O R I G I N A L A R T I C L E Reduction in Cardiovascular Events With Atorvastatin in 2,532 Patients With Type 2 Diabetes Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering
Pathophysiology/Complications O R I G I N A L A R T I C L E Reduction in Cardiovascular Events With Atorvastatin in 2,532 Patients With Type 2 Diabetes Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering
Articles. Vol 366 September 10,
 Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) Neil R Poulter,
Role of blood pressure and other variables in the differential cardiovascular event rates noted in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) Neil R Poulter,
CLINICAL DECISION USING AN ARTICLE ABOUT TREATMENT JOSEFINA S. ISIDRO LAPENA MD, MFM, FPAFP PROFESSOR, UPCM
 CLINICAL DECISION USING AN ARTICLE ABOUT TREATMENT JOSEFINA S. ISIDRO LAPENA MD, MFM, FPAFP PROFESSOR, UPCM Principles of Decision Making Knowing the patient s true state is often unnecessary Does my patient
CLINICAL DECISION USING AN ARTICLE ABOUT TREATMENT JOSEFINA S. ISIDRO LAPENA MD, MFM, FPAFP PROFESSOR, UPCM Principles of Decision Making Knowing the patient s true state is often unnecessary Does my patient
Cholesterol lowering intervention for cardiovascular prevention in high risk patients with or without LDL cholesterol elevation
 TrialResults-center.org www.trialresultscenter.org Cholesterol lowering intervention for cardiovascular prevention in high risk patients with or without LDL cholesterol elevation A systematic review and
TrialResults-center.org www.trialresultscenter.org Cholesterol lowering intervention for cardiovascular prevention in high risk patients with or without LDL cholesterol elevation A systematic review and
The role of statins in patients with arterial hypertension
 Invited review The role of statins in patients with arterial hypertension Trygve B. Tjugen 1, Sigrun Halvorsen 1, Reidar Bjørnerheim 1, Sverre E. Kjeldsen 1, 2 1University of Oslo, Department of Cardiology,
Invited review The role of statins in patients with arterial hypertension Trygve B. Tjugen 1, Sigrun Halvorsen 1, Reidar Bjørnerheim 1, Sverre E. Kjeldsen 1, 2 1University of Oslo, Department of Cardiology,
Antihypertensive Trial Design ALLHAT
 1 U.S. Department of Health and Human Services Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic National Institutes
1 U.S. Department of Health and Human Services Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic National Institutes
Is there a mechanism of interaction between hypertension and dyslipidaemia?
 Is there a mechanism of interaction between hypertension and dyslipidaemia? Neil R Poulter International Centre for Circulatory Health NHLI, Imperial College London Daegu, Korea April 2005 Observational
Is there a mechanism of interaction between hypertension and dyslipidaemia? Neil R Poulter International Centre for Circulatory Health NHLI, Imperial College London Daegu, Korea April 2005 Observational
Talking about blood pressure
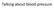 Talking about blood pressure Mrs Khan 56 BP 158/99 BMI 32 Total cholesterol 5.4 (HDL 0.8) HbA1c 43 She has been promising to do more exercise and eat more healthily for the last 2 years but her weight
Talking about blood pressure Mrs Khan 56 BP 158/99 BMI 32 Total cholesterol 5.4 (HDL 0.8) HbA1c 43 She has been promising to do more exercise and eat more healthily for the last 2 years but her weight
Traitements associés chez l hypertendu: Statines, Aspirine
 Traitements associés chez l hypertendu: Statines, Aspirine Pr Jean-Jacques Mourad CHU Avicenne, Université Paris 13, Bobigny DU HTA, Mars 2012 jean-jacques.mourad@avc.aphp.fr Global Mortality 2000: Impact
Traitements associés chez l hypertendu: Statines, Aspirine Pr Jean-Jacques Mourad CHU Avicenne, Université Paris 13, Bobigny DU HTA, Mars 2012 jean-jacques.mourad@avc.aphp.fr Global Mortality 2000: Impact
Data Alert. Vascular Biology Working Group. Blunting the atherosclerotic process in patients with coronary artery disease.
 1994--4 Vascular Biology Working Group www.vbwg.org c/o Medical Education Consultants, LLC 25 Sylvan Road South, Westport, CT 688 Chairman: Carl J. Pepine, MD Eminent Scholar American Heart Association
1994--4 Vascular Biology Working Group www.vbwg.org c/o Medical Education Consultants, LLC 25 Sylvan Road South, Westport, CT 688 Chairman: Carl J. Pepine, MD Eminent Scholar American Heart Association
How would you manage Ms. Gold
 How would you manage Ms. Gold 32 yo Asian woman with dyslipidemia Current medications: Simvastatin 20mg QD Most recent lipid profile: TC = 246, TG = 100, LDL = 176, HDL = 50 What about Mr. Williams? 56
How would you manage Ms. Gold 32 yo Asian woman with dyslipidemia Current medications: Simvastatin 20mg QD Most recent lipid profile: TC = 246, TG = 100, LDL = 176, HDL = 50 What about Mr. Williams? 56
APPENDIX B: LIST OF THE SELECTED SECONDARY STUDIES
 APPENDIX B: LIST OF THE SELECTED SECONDARY STUDIES Main systematic reviews secondary studies on the general effectiveness of statins in secondary cardiovascular prevention (search date: 2003-2006) NICE.
APPENDIX B: LIST OF THE SELECTED SECONDARY STUDIES Main systematic reviews secondary studies on the general effectiveness of statins in secondary cardiovascular prevention (search date: 2003-2006) NICE.
LIST OF ABBREVIATIONS
 Diabetes & Endocrinology 2005 Royal College of Physicians of Edinburgh Diabetes and lipids 1 G Marshall, 2 M Fisher 1 Research Fellow, Department of Cardiology, Glasgow Royal Infirmary, Glasgow, Scotland,
Diabetes & Endocrinology 2005 Royal College of Physicians of Edinburgh Diabetes and lipids 1 G Marshall, 2 M Fisher 1 Research Fellow, Department of Cardiology, Glasgow Royal Infirmary, Glasgow, Scotland,
Should beta blockers remain first-line drugs for hypertension?
 1 de 6 03/11/2008 13:23 Should beta blockers remain first-line drugs for hypertension? Maros Elsik, Cardiologist, Department of Epidemiology and Preventive Medicine, Monash University and The Alfred Hospital,
1 de 6 03/11/2008 13:23 Should beta blockers remain first-line drugs for hypertension? Maros Elsik, Cardiologist, Department of Epidemiology and Preventive Medicine, Monash University and The Alfred Hospital,
In the Literature 1001 BP of 1.1 mm Hg). The trial was stopped early based on prespecified stopping rules because of a significant difference in cardi
 Is Choice of Antihypertensive Agent Important in Improving Cardiovascular Outcomes in High-Risk Hypertensive Patients? Commentary on Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators.
Is Choice of Antihypertensive Agent Important in Improving Cardiovascular Outcomes in High-Risk Hypertensive Patients? Commentary on Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators.
Hypertension Update 2009
 Hypertension Update 2009 New Drugs, New Goals, New Approaches, New Lessons from Clinical Trials Timothy C Fagan, MD, FACP Professor Emeritus University of Arizona New Drugs Direct Renin Inhibitors Endothelin
Hypertension Update 2009 New Drugs, New Goals, New Approaches, New Lessons from Clinical Trials Timothy C Fagan, MD, FACP Professor Emeritus University of Arizona New Drugs Direct Renin Inhibitors Endothelin
Supplementary Online Content
 Supplementary Online Content Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med. 2012;172(12):IRA120005.
Supplementary Online Content Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med. 2012;172(12):IRA120005.
Original Scientific Paper. Copyright European Society of Cardiology. Unauthorized reproduction of this article is prohibited.
 Original Scientific Paper Cost-effectiveness of atorvastatin for the prevention of coronary and stroke events: an economic analysis of the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm (ASCOT-LLA)
Original Scientific Paper Cost-effectiveness of atorvastatin for the prevention of coronary and stroke events: an economic analysis of the Anglo-Scandinavian Cardiac Outcomes Trial lipid-lowering arm (ASCOT-LLA)
Evaluation of C-reactive protein prior to and on-treatment as a predictor of benefit
 Evaluation of C-reactive protein prior to and on-treatment as a predictor of benefit from atorvastatin. A cohort analysis from the Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering Arm Peter S Sever,
Evaluation of C-reactive protein prior to and on-treatment as a predictor of benefit from atorvastatin. A cohort analysis from the Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering Arm Peter S Sever,
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Cholesterol Treatment Trialists Collaboration.
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Cholesterol Treatment Trialists Collaboration.
York, New York, USA. Received 22 March 2010 Revised 5 October 2010 Accepted 17 November 2010
 592 Original article Impact of atorvastatin among older and younger patients in the Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering Arm David J. Collier a, Neil R. Poulter b, Björn Dahlöf c, Peter
592 Original article Impact of atorvastatin among older and younger patients in the Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering Arm David J. Collier a, Neil R. Poulter b, Björn Dahlöf c, Peter
DECLARATION OF CONFLICT OF INTEREST
 DECLARATION OF CONFLICT OF INTEREST Is there a mortality risk associated with aspirin use in heart failure? Results from a large community based cohort Margaret Bermingham, Mary-Kate Shanahan, Saki Miwa,
DECLARATION OF CONFLICT OF INTEREST Is there a mortality risk associated with aspirin use in heart failure? Results from a large community based cohort Margaret Bermingham, Mary-Kate Shanahan, Saki Miwa,
Disclosures. Diabetes and Cardiovascular Risk Management. Learning Objectives. Atherosclerotic Cardiovascular Disease
 Disclosures Diabetes and Cardiovascular Risk Management Tony Hampton, MD, MBA Medical Director Advocate Aurora Operating System Advocate Aurora Healthcare Downers Grove, IL No conflicts or disclosures
Disclosures Diabetes and Cardiovascular Risk Management Tony Hampton, MD, MBA Medical Director Advocate Aurora Operating System Advocate Aurora Healthcare Downers Grove, IL No conflicts or disclosures
Rikshospitalet, University of Oslo
 Rikshospitalet, University of Oslo Preventing heart failure by preventing coronary artery disease progression European Society of Cardiology Dyslipidemia 29.08.2010 Objectives The trends in cardiovascular
Rikshospitalet, University of Oslo Preventing heart failure by preventing coronary artery disease progression European Society of Cardiology Dyslipidemia 29.08.2010 Objectives The trends in cardiovascular
Outcomes and Perspectives of Single-Pill Combination Therapy for the modern management of hypertension
 Outcomes and Perspectives of Single-Pill Combination Therapy for the modern management of hypertension Prof. Massimo Volpe, MD, FAHA, FESC, Chair of Cardiology, Department of Clinical and Molecular Medicine
Outcomes and Perspectives of Single-Pill Combination Therapy for the modern management of hypertension Prof. Massimo Volpe, MD, FAHA, FESC, Chair of Cardiology, Department of Clinical and Molecular Medicine
Metformin should be considered in all patients with type 2 diabetes unless contra-indicated
 November 2001 N P S National Prescribing Service Limited PPR fifteen Prescribing Practice Review PPR Managing type 2 diabetes For General Practice Key messages Metformin should be considered in all patients
November 2001 N P S National Prescribing Service Limited PPR fifteen Prescribing Practice Review PPR Managing type 2 diabetes For General Practice Key messages Metformin should be considered in all patients
Statins in the Treatment of Type 2 Diabetes Mellitus: A Systematic Review.
 ISPUB.COM The Internet Journal of Cardiovascular Research Volume 7 Number 1 Statins in the Treatment of Type 2 Diabetes Mellitus: A Systematic Review. C ANYANWU, C NOSIRI Citation C ANYANWU, C NOSIRI.
ISPUB.COM The Internet Journal of Cardiovascular Research Volume 7 Number 1 Statins in the Treatment of Type 2 Diabetes Mellitus: A Systematic Review. C ANYANWU, C NOSIRI Citation C ANYANWU, C NOSIRI.
Andrew Cohen, MD and Neil S. Skolnik, MD INTRODUCTION
 2 Hyperlipidemia Andrew Cohen, MD and Neil S. Skolnik, MD CONTENTS INTRODUCTION RISK CATEGORIES AND TARGET LDL-CHOLESTEROL TREATMENT OF LDL-CHOLESTEROL SPECIAL CONSIDERATIONS OLDER AND YOUNGER ADULTS ADDITIONAL
2 Hyperlipidemia Andrew Cohen, MD and Neil S. Skolnik, MD CONTENTS INTRODUCTION RISK CATEGORIES AND TARGET LDL-CHOLESTEROL TREATMENT OF LDL-CHOLESTEROL SPECIAL CONSIDERATIONS OLDER AND YOUNGER ADULTS ADDITIONAL
The CARI Guidelines Caring for Australians with Renal Impairment. Cardiovascular Risk Factors
 Cardiovascular Risk Factors ROB WALKER (Dunedin, New Zealand) Lipid-lowering therapy in patients with chronic kidney disease Date written: January 2005 Final submission: August 2005 Author: Rob Walker
Cardiovascular Risk Factors ROB WALKER (Dunedin, New Zealand) Lipid-lowering therapy in patients with chronic kidney disease Date written: January 2005 Final submission: August 2005 Author: Rob Walker
egfr > 50 (n = 13,916)
 Saxagliptin and Cardiovascular Risk in Patients with Type 2 Diabetes Mellitus and Moderate or Severe Renal Impairment: Observations from the SAVOR-TIMI 53 Trial Supplementary Table 1. Characteristics according
Saxagliptin and Cardiovascular Risk in Patients with Type 2 Diabetes Mellitus and Moderate or Severe Renal Impairment: Observations from the SAVOR-TIMI 53 Trial Supplementary Table 1. Characteristics according
Beneficial effect of early initiation of lipid-lowering therapy following renal transplantation
 Nephrol Dial Transplant (25) 2: 974 98 doi:1.193/ndt/gfh735 Advance Access publication 15 March 25 Original Article Beneficial effect of early initiation of lipid-lowering therapy following renal transplantation
Nephrol Dial Transplant (25) 2: 974 98 doi:1.193/ndt/gfh735 Advance Access publication 15 March 25 Original Article Beneficial effect of early initiation of lipid-lowering therapy following renal transplantation
Statins in the elderly : Is there a rationale?
 Statins in the elderly : Is there a rationale? Pr B Boland After a communication by Dr. Manfred Gogol EAMA, Sion, June, 2006 1 RCTs with Statins Meta-Analysis, 1999 182 abstracts or research papers 29
Statins in the elderly : Is there a rationale? Pr B Boland After a communication by Dr. Manfred Gogol EAMA, Sion, June, 2006 1 RCTs with Statins Meta-Analysis, 1999 182 abstracts or research papers 29
Lifetime clinical and economic benefits of statin-based LDL lowering in the 20-year Followup of the West of Scotland Coronary Prevention Study
 Lifetime clinical and economic benefits of statin-based LDL lowering in the 20-year Followup of the West of Scotland Coronary Prevention Study Harvey White Green Lane Cardiovascular Service and Cardiovascular
Lifetime clinical and economic benefits of statin-based LDL lowering in the 20-year Followup of the West of Scotland Coronary Prevention Study Harvey White Green Lane Cardiovascular Service and Cardiovascular
Should we prescribe aspirin and statins to all subjects over 65? (Or even all over 55?) Terje R.Pedersen Oslo University Hospital Oslo, Norway
 Should we prescribe aspirin and statins to all subjects over 65? (Or even all over 55?) Terje R.Pedersen Oslo University Hospital Oslo, Norway The Polypill A strategy to reduce cardiovascular disease by
Should we prescribe aspirin and statins to all subjects over 65? (Or even all over 55?) Terje R.Pedersen Oslo University Hospital Oslo, Norway The Polypill A strategy to reduce cardiovascular disease by
New Lipid Guidelines. PREVENTION OF CARDIOVASCULAR DISEASE IN WOMEN: Implications of the New Guidelines for Hypertension and Lipids.
 PREVENTION OF CARDIOVASCULAR DISEASE IN WOMEN: Implications of the New Guidelines for Hypertension and Lipids Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Disclosure No relevant
PREVENTION OF CARDIOVASCULAR DISEASE IN WOMEN: Implications of the New Guidelines for Hypertension and Lipids Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine Disclosure No relevant
The future of coronary heart disease prevention
 The future of coronary heart disease prevention David S Wald MA MD MRCP, Consultant Cardiologist and Senior Lecturer, Wolfson Institute of Preventive Medicine, and London Chest Hospital Barts and The London,
The future of coronary heart disease prevention David S Wald MA MD MRCP, Consultant Cardiologist and Senior Lecturer, Wolfson Institute of Preventive Medicine, and London Chest Hospital Barts and The London,
Articles. Funding UK Medical Research Council, British Heart Foundation, Merck & Co, Roche Vitamins.
 Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20 536 high-risk individuals: a randomised controlled trial Heart Protection Study Collaborative
Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20 536 high-risk individuals: a randomised controlled trial Heart Protection Study Collaborative
Slide notes: References:
 1 2 3 Cut-off values for the definition of hypertension are systolic blood pressure (SBP) 135 and/or diastolic blood pressure (DBP) 85 mmhg for home blood pressure monitoring (HBPM) and daytime ambulatory
1 2 3 Cut-off values for the definition of hypertension are systolic blood pressure (SBP) 135 and/or diastolic blood pressure (DBP) 85 mmhg for home blood pressure monitoring (HBPM) and daytime ambulatory
The Indian Polycap Study 1 & 2 (TIPS 1 & 2) and The International Polycap Study 3 & 4 (TIPS 3 & 4)
 The Indian Polycap Study 1 & 2 (TIPS 1 & 2) and The International Polycap Study 3 & 4 (TIPS 3 & 4) Denis Xavier MD, MSc Professor and Head, Pharmacology, St. John's Medical College Coordinator, Division
The Indian Polycap Study 1 & 2 (TIPS 1 & 2) and The International Polycap Study 3 & 4 (TIPS 3 & 4) Denis Xavier MD, MSc Professor and Head, Pharmacology, St. John's Medical College Coordinator, Division
Weigh the benefit of statin treatment: LDL & Beyond
 Weigh the benefit of statin treatment: LDL & Beyond Duk-Woo Park, MD, PhD Heart Institute, University of Ulsan College of Medicine, Asan Medical, Seoul, Korea FOURIER Further cardiovascular OUtcomes Research
Weigh the benefit of statin treatment: LDL & Beyond Duk-Woo Park, MD, PhD Heart Institute, University of Ulsan College of Medicine, Asan Medical, Seoul, Korea FOURIER Further cardiovascular OUtcomes Research
Prevention of Atrial Fibrillation and Heart Failure in the Hypertensive Patient
 Prevention of Atrial Fibrillation and Heart Failure in the Hypertensive Patient The Issue of Primary Prevention of A.Fib. (and Heart Failure) and not the Prevention of Recurrent A.Fib. after Electroconversion
Prevention of Atrial Fibrillation and Heart Failure in the Hypertensive Patient The Issue of Primary Prevention of A.Fib. (and Heart Failure) and not the Prevention of Recurrent A.Fib. after Electroconversion
CLINICAL OUTCOME Vs SURROGATE MARKER
 CLINICAL OUTCOME Vs SURROGATE MARKER Statin Real Experience Dr. Mostafa Sherif Senior Medical Manager Pfizer Egypt & Sudan Objective Difference between Clinical outcome and surrogate marker Proper Clinical
CLINICAL OUTCOME Vs SURROGATE MARKER Statin Real Experience Dr. Mostafa Sherif Senior Medical Manager Pfizer Egypt & Sudan Objective Difference between Clinical outcome and surrogate marker Proper Clinical
The TNT Trial Is It Time to Shift Our Goals in Clinical
 The TNT Trial Is It Time to Shift Our Goals in Clinical Angioplasty Summit Luncheon Symposium Korea Assoc Prof David Colquhoun 29 April 2005 University of Queensland, Wesley Hospital, Brisbane, Australia
The TNT Trial Is It Time to Shift Our Goals in Clinical Angioplasty Summit Luncheon Symposium Korea Assoc Prof David Colquhoun 29 April 2005 University of Queensland, Wesley Hospital, Brisbane, Australia
h i g h b l o o d p r e s s u r e
 h i g h b l o o d p r e s s u r e where are we at? The recent literature has raised doubts about the role of ßblockers for lowering blood pressure and the New Zealand Guidelines Group is updating the Assessment
h i g h b l o o d p r e s s u r e where are we at? The recent literature has raised doubts about the role of ßblockers for lowering blood pressure and the New Zealand Guidelines Group is updating the Assessment
New evidences in heart failure: the GISSI-HF trial. Aldo P Maggioni, MD ANMCO Research Center Firenze, Italy
 New evidences in heart failure: the GISSI-HF trial Aldo P Maggioni, MD ANMCO Research Center Firenze, Italy % Improving survival in chronic HF and LV systolic dysfunction: 1 year all-cause mortality 20
New evidences in heart failure: the GISSI-HF trial Aldo P Maggioni, MD ANMCO Research Center Firenze, Italy % Improving survival in chronic HF and LV systolic dysfunction: 1 year all-cause mortality 20
Changing lipid-lowering guidelines: whom to treat and how low to go
 European Heart Journal Supplements (2005) 7 (Supplement A), A12 A19 doi:10.1093/eurheartj/sui003 Changing lipid-lowering guidelines: whom to treat and how low to go C.M. Ballantyne Section of Atherosclerosis,
European Heart Journal Supplements (2005) 7 (Supplement A), A12 A19 doi:10.1093/eurheartj/sui003 Changing lipid-lowering guidelines: whom to treat and how low to go C.M. Ballantyne Section of Atherosclerosis,
Presented by Terje R. Pedersen Oslo Disclosure: Research grants and/or speaker- / consulting fees from Merck, MSP, Astra-Zeneca, Pfizer
 Presented by Terje R. Pedersen Oslo Disclosure: Research grants and/or speaker- / consulting fees from Merck, MSP, Astra-Zeneca, Pfizer Patients Randomized by Country 187 UK n=187 Norway n=425 Finland
Presented by Terje R. Pedersen Oslo Disclosure: Research grants and/or speaker- / consulting fees from Merck, MSP, Astra-Zeneca, Pfizer Patients Randomized by Country 187 UK n=187 Norway n=425 Finland
The underestimated risk of
 Earn 3 CPD Points online The underestimated risk of hypertension Dr David Webb Johannesburg Introduction The high and increasing worldwide burden of hypertension is a major global health challenge. Hypertension
Earn 3 CPD Points online The underestimated risk of hypertension Dr David Webb Johannesburg Introduction The high and increasing worldwide burden of hypertension is a major global health challenge. Hypertension
Lessons from Recent Atherosclerosis Trials
 Lessons from Recent Atherosclerosis Trials Han, Ki Hoon MD PhD Asan Medical Center Seoul, Korea Change of concept Primary vs. secondary prevention Low risk vs. High risk High Risk CHD and equivalents CHD
Lessons from Recent Atherosclerosis Trials Han, Ki Hoon MD PhD Asan Medical Center Seoul, Korea Change of concept Primary vs. secondary prevention Low risk vs. High risk High Risk CHD and equivalents CHD
Effects of a perindopril-based blood pressure lowering regimen on cardiac outcomes among patients with cerebrovascular disease
 European Heart Journal (2003) 24, 475 484 Effects of a perindopril-based blood pressure lowering regimen on cardiac outcomes among patients with cerebrovascular disease PROGRESS Collaborative Group 1*
European Heart Journal (2003) 24, 475 484 Effects of a perindopril-based blood pressure lowering regimen on cardiac outcomes among patients with cerebrovascular disease PROGRESS Collaborative Group 1*
The JUPITER trial: What does it tell us? Alice Y.Y. Cheng, MD, FRCPC January 24, 2009
 The JUPITER trial: What does it tell us? Alice Y.Y. Cheng, MD, FRCPC January 24, 2009 Learning Objectives 1. Understand the role of statin therapy in the primary and secondary prevention of stroke 2. Explain
The JUPITER trial: What does it tell us? Alice Y.Y. Cheng, MD, FRCPC January 24, 2009 Learning Objectives 1. Understand the role of statin therapy in the primary and secondary prevention of stroke 2. Explain
Data Analysis Plan for assessing clinical efficacy and safety of ER niacin/laropiprant in the HPS2-THRIVE trial
 Data Analysis Plan for assessing clinical efficacy and safety of ER niacin/laropiprant in the HPS2-THRIVE trial 1 Background This Data Analysis Plan describes the strategy, rationale and statistical methods
Data Analysis Plan for assessing clinical efficacy and safety of ER niacin/laropiprant in the HPS2-THRIVE trial 1 Background This Data Analysis Plan describes the strategy, rationale and statistical methods
The target blood pressure in patients with diabetes is <130 mm Hg
 Controversies in hypertension, About Diabetes diabetes and and metabolic Cardiovascular syndrome Risk ESC annual congress August 29, 2011 The target blood pressure in patients with diabetes is
Controversies in hypertension, About Diabetes diabetes and and metabolic Cardiovascular syndrome Risk ESC annual congress August 29, 2011 The target blood pressure in patients with diabetes is
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd.
 rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
rosuvastatin, 5mg, 10mg, 20mg, film-coated tablets (Crestor ) SMC No. (725/11) AstraZeneca UK Ltd. 09 September 2011 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Treating Hypertension in Individuals with Diabetes
 Treating Hypertension in Individuals with Diabetes Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any
Treating Hypertension in Individuals with Diabetes Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced, copied, stored, or transmitted in any form or by any
ESC Geoffrey Rose Lecture on Population Sciences Cholesterol and risk: past, present and future
 ESC Geoffrey Rose Lecture on Population Sciences Cholesterol and risk: past, present and future Rory Collins BHF Professor of Medicine & Epidemiology Clinical Trial Service Unit & Epidemiological Studies
ESC Geoffrey Rose Lecture on Population Sciences Cholesterol and risk: past, present and future Rory Collins BHF Professor of Medicine & Epidemiology Clinical Trial Service Unit & Epidemiological Studies
Guidelines on cardiovascular risk assessment and management
 European Heart Journal Supplements (2005) 7 (Supplement L), L5 L10 doi:10.1093/eurheartj/sui079 Guidelines on cardiovascular risk assessment and management David A. Wood 1,2 * 1 Cardiovascular Medicine
European Heart Journal Supplements (2005) 7 (Supplement L), L5 L10 doi:10.1093/eurheartj/sui079 Guidelines on cardiovascular risk assessment and management David A. Wood 1,2 * 1 Cardiovascular Medicine
Clinical Updates in the Treatment of Hypertension JNC 7 vs. JNC 8. Lauren Thomas, PharmD PGY1 Pharmacy Practice Resident South Pointe Hospital
 Clinical Updates in the Treatment of Hypertension JNC 7 vs. JNC 8 Lauren Thomas, PharmD PGY1 Pharmacy Practice Resident South Pointe Hospital Objectives Review the Eighth Joint National Committee (JNC
Clinical Updates in the Treatment of Hypertension JNC 7 vs. JNC 8 Lauren Thomas, PharmD PGY1 Pharmacy Practice Resident South Pointe Hospital Objectives Review the Eighth Joint National Committee (JNC
The treatment of coronary heart disease (CHD) The Health Economics of the Treatment of Hyperlipidemia and Hypertension J. McMurray
 AJH 1999;12:99S 104S The Health Economics of the Treatment of Hyperlipidemia and Hypertension J. McMurray In the current economic climate it is important to demonstrate that healthcare resources are being
AJH 1999;12:99S 104S The Health Economics of the Treatment of Hyperlipidemia and Hypertension J. McMurray In the current economic climate it is important to demonstrate that healthcare resources are being
Management of Lipid Disorders and Hypertension: Implications of the New Guidelines
 Management of Lipid Disorders and Hypertension Management of Lipid Disorders and Hypertension: Implications of the New Guidelines Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine
Management of Lipid Disorders and Hypertension Management of Lipid Disorders and Hypertension: Implications of the New Guidelines Robert B. Baron MD MS Professor and Associate Dean UCSF School of Medicine
ARTICLE. Abstract Aims/hypothesis We estimated the cost-effectiveness of atorvastatin treatment in the primary prevention of cardiovascular
 Diabetologia (2007) 50:733 740 DOI 10.1007/s00125-006-0561-4 ARTICLE Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative
Diabetologia (2007) 50:733 740 DOI 10.1007/s00125-006-0561-4 ARTICLE Cost-effectiveness of primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes: results from the Collaborative
Cedars Sinai Diabetes. Michael A. Weber
 Cedars Sinai Diabetes Michael A. Weber Speaker Disclosures I disclose that I am a Consultant for: Ablative Solutions, Boston Scientific, Boehringer Ingelheim, Eli Lilly, Forest, Medtronics, Novartis, ReCor
Cedars Sinai Diabetes Michael A. Weber Speaker Disclosures I disclose that I am a Consultant for: Ablative Solutions, Boston Scientific, Boehringer Ingelheim, Eli Lilly, Forest, Medtronics, Novartis, ReCor
A bs tr ac t. n engl j med 357;15 october 11,
 The new england journal of medicine established in 1812 october 11, 2007 vol. 357 no. 15 Long-Term Follow-up of the West of Scotland Coronary Prevention Study Ian Ford, Ph.D., Heather Murray, M.Sc., Chris
The new england journal of medicine established in 1812 october 11, 2007 vol. 357 no. 15 Long-Term Follow-up of the West of Scotland Coronary Prevention Study Ian Ford, Ph.D., Heather Murray, M.Sc., Chris
GALECTIN-3 PREDICTS LONG TERM CARDIOVASCULAR DEATH IN HIGH-RISK CORONARY ARTERY DISEASE PATIENTS
 GALECTIN-3 PREDICTS LONG TERM CARDIOVASCULAR DEATH IN HIGH-RISK CORONARY ARTERY DISEASE PATIENTS Table of Contents List of authors pag 2 Supplemental figure I pag 3 Supplemental figure II pag 4 Supplemental
GALECTIN-3 PREDICTS LONG TERM CARDIOVASCULAR DEATH IN HIGH-RISK CORONARY ARTERY DISEASE PATIENTS Table of Contents List of authors pag 2 Supplemental figure I pag 3 Supplemental figure II pag 4 Supplemental
Polypharmacy - arrhythmic risks in patients with heart failure
 Influencing sudden cardiac death by pharmacotherapy Polypharmacy - arrhythmic risks in patients with heart failure Professor Dan Atar Head, Dept. of Cardiology Oslo University Hospital Ullevål Norway 27.8.2012
Influencing sudden cardiac death by pharmacotherapy Polypharmacy - arrhythmic risks in patients with heart failure Professor Dan Atar Head, Dept. of Cardiology Oslo University Hospital Ullevål Norway 27.8.2012
Does High-Intensity Pitavastatin Therapy Further Improve Clinical Outcomes?
 Late Breaking Clinical Trial Session at AHA 2017 Does High-Intensity Pitavastatin Therapy Further Improve Clinical Outcomes? The REAL-CAD Study in 13,054 Patients With Stable Coronary Artery Disease Takeshi
Late Breaking Clinical Trial Session at AHA 2017 Does High-Intensity Pitavastatin Therapy Further Improve Clinical Outcomes? The REAL-CAD Study in 13,054 Patients With Stable Coronary Artery Disease Takeshi
Mineralocorticoids. Effect of Spironolactone on Blood Pressure in Subjects With Resistant Hypertension
 Mineralocorticoids Effect of Spironolactone on Blood Pressure in Subjects With Resistant Hypertension Neil Chapman, Joanna Dobson, Sarah Wilson, Björn Dahlöf, Peter S. Sever, Hans Wedel, Neil R. Poulter,
Mineralocorticoids Effect of Spironolactone on Blood Pressure in Subjects With Resistant Hypertension Neil Chapman, Joanna Dobson, Sarah Wilson, Björn Dahlöf, Peter S. Sever, Hans Wedel, Neil R. Poulter,
Health technology The use of the antihypertensive drug losartan for the prevention of stroke.
 Cost effectiveness of losartan in patients with hypertension and LVH: an economic evaluation for Sweden of the LIFE trial Jonsson B, Carides G W, Burke T A, Dasbach E J, Lindholm L H, Dahlof B Record Status
Cost effectiveness of losartan in patients with hypertension and LVH: an economic evaluation for Sweden of the LIFE trial Jonsson B, Carides G W, Burke T A, Dasbach E J, Lindholm L H, Dahlof B Record Status
LDL cholesterol and cardiovascular outcomes?
 LDL cholesterol and cardiovascular outcomes? Prof Kausik Ray, BSc (hons), MBChB, FRCP, MD, MPhil (Cantab), FACC, FESC Professor of Cardiovascular Disease Prevention St Georges University of London Honorary
LDL cholesterol and cardiovascular outcomes? Prof Kausik Ray, BSc (hons), MBChB, FRCP, MD, MPhil (Cantab), FACC, FESC Professor of Cardiovascular Disease Prevention St Georges University of London Honorary
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Treatment to reduce cardiovascular risk: multifactorial management
 Treatment to reduce cardiovascular risk: multifactorial management Matteo Anselmino, MD PhD Assistant Professor San Giovanni Battista Hospital Division of Cardiology, Department of Internal Medicine University
Treatment to reduce cardiovascular risk: multifactorial management Matteo Anselmino, MD PhD Assistant Professor San Giovanni Battista Hospital Division of Cardiology, Department of Internal Medicine University
How Low Do We Go? Update on Hypertension
 How Low Do We Go? Update on Beth L. Abramson, MD, FRCPC, FACC As presented at the University of Toronto s Saturday at the University Session (September 2003) Arecent World Health Organization report states
How Low Do We Go? Update on Beth L. Abramson, MD, FRCPC, FACC As presented at the University of Toronto s Saturday at the University Session (September 2003) Arecent World Health Organization report states
Efficacy of beta-blockers in heart failure patients with atrial fibrillation: An individual patient data meta-analysis
 Efficacy of beta-blockers in heart failure patients with atrial fibrillation: An individual patient data meta-analysis Dipak Kotecha, MD PhD on behalf of the Selection of slides presented at the European
Efficacy of beta-blockers in heart failure patients with atrial fibrillation: An individual patient data meta-analysis Dipak Kotecha, MD PhD on behalf of the Selection of slides presented at the European
Preventing the cardiovascular complications of hypertension
 European Heart Journal Supplements (2004) 6 (Supplement H), H37 H42 Preventing the cardiovascular complications of hypertension Peter Trenkwalder* Department of Internal Medicine, Starnberg Hospital, Ludwig
European Heart Journal Supplements (2004) 6 (Supplement H), H37 H42 Preventing the cardiovascular complications of hypertension Peter Trenkwalder* Department of Internal Medicine, Starnberg Hospital, Ludwig
ALLHAT. Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic
 1 U.S. Department of Health and Human Services National Institutes of Health Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker
1 U.S. Department of Health and Human Services National Institutes of Health Major Outcomes in High Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker
The Clinical Unmet need in the patient with Diabetes and ACS
 The Clinical Unmet need in the patient with Diabetes and ACS Professor Kausik Ray (UK) BSc(hons), MBChB, MD, MPhil, FRCP (lon), FRCP (ed), FACC, FESC, FAHA Diabetes is a global public health challenge
The Clinical Unmet need in the patient with Diabetes and ACS Professor Kausik Ray (UK) BSc(hons), MBChB, MD, MPhil, FRCP (lon), FRCP (ed), FACC, FESC, FAHA Diabetes is a global public health challenge
ICSS Safety Results NOT for PUBLICATION. June 2009 ICSS ICSS ICSS ICSS. International Carotid Stenting Study: Main Inclusion Criteria
 Safety Results NOT for The following slides were presented to the Investigators Meeting on 22/05/09 and most of them were also presented at the European Stroke Conference on 27/05/09 They are NOT for in
Safety Results NOT for The following slides were presented to the Investigators Meeting on 22/05/09 and most of them were also presented at the European Stroke Conference on 27/05/09 They are NOT for in
CVD risk assessment using risk scores in primary and secondary prevention
 CVD risk assessment using risk scores in primary and secondary prevention Raul D. Santos MD, PhD Heart Institute-InCor University of Sao Paulo Brazil Disclosure Honoraria for consulting and speaker activities
CVD risk assessment using risk scores in primary and secondary prevention Raul D. Santos MD, PhD Heart Institute-InCor University of Sao Paulo Brazil Disclosure Honoraria for consulting and speaker activities
Optimizing risk assessment of total cardiovascular risk What are the tools? Lars Rydén Professor Karolinska Institutet Stockholm, Sweden
 Optimizing risk assessment of total cardiovascular risk What are the tools? Lars Rydén Professor Karolinska Institutet Stockholm, Sweden Cardiovascular Disease Prevention (CVD) Three Strategies for CVD
Optimizing risk assessment of total cardiovascular risk What are the tools? Lars Rydén Professor Karolinska Institutet Stockholm, Sweden Cardiovascular Disease Prevention (CVD) Three Strategies for CVD
Metoprolol Succinate SelokenZOC
 Metoprolol Succinate SelokenZOC Blood Pressure Control and Far Beyond Mohamed Abdel Ghany World Health Organization - Noncommunicable Diseases (NCD) Country Profiles, 2014. 1 Death Rates From Ischemic
Metoprolol Succinate SelokenZOC Blood Pressure Control and Far Beyond Mohamed Abdel Ghany World Health Organization - Noncommunicable Diseases (NCD) Country Profiles, 2014. 1 Death Rates From Ischemic
How to Reduce CVD Complications in Diabetes?
 How to Reduce CVD Complications in Diabetes? Chaicharn Deerochanawong M.D. Diabetes and Endocrinology Unit Department of Medicine Rajavithi Hospital, Ministry of Public Health Framingham Heart Study 30-Year
How to Reduce CVD Complications in Diabetes? Chaicharn Deerochanawong M.D. Diabetes and Endocrinology Unit Department of Medicine Rajavithi Hospital, Ministry of Public Health Framingham Heart Study 30-Year
The -adrenergic blocking drug doxazosin lowers blood. Hypertension
 Hypertension Effect of Doxazosin Gastrointestinal Therapeutic System as Third-Line Antihypertensive Therapy on Blood Pressure and Lipids in the Anglo-Scandinavian Cardiac Outcomes Trial Neil Chapman, FRCP;
Hypertension Effect of Doxazosin Gastrointestinal Therapeutic System as Third-Line Antihypertensive Therapy on Blood Pressure and Lipids in the Anglo-Scandinavian Cardiac Outcomes Trial Neil Chapman, FRCP;
MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebocontrolled
 Articles MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebocontrolled trial Heart Protection Study Collaborative Group* Summary Background
Articles MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebocontrolled trial Heart Protection Study Collaborative Group* Summary Background
VALUE OF ACEI IN THE MANAGEMENT OF HYPERTENSION
 VALUE OF ACEI IN THE MANAGEMENT OF HYPERTENSION Dr Catherine BESEME Paris 6 th December 2005 6 th International Congress of Bangladesh Society of Medicine Hypertension is a risk factor at the source, with
VALUE OF ACEI IN THE MANAGEMENT OF HYPERTENSION Dr Catherine BESEME Paris 6 th December 2005 6 th International Congress of Bangladesh Society of Medicine Hypertension is a risk factor at the source, with
Appendix F: Clinical evidence tables
 378 Appendix F: F.1 Blood pressure variability STUDY 1 P. M. Rothwell, S. C. Howard, E. Dolan, E. O'Brien, J. E. Dobson, B. Dahlof, N. R. Poulter, and P. S. Sever. Effects of beta blockers and calciumchannel
378 Appendix F: F.1 Blood pressure variability STUDY 1 P. M. Rothwell, S. C. Howard, E. Dolan, E. O'Brien, J. E. Dobson, B. Dahlof, N. R. Poulter, and P. S. Sever. Effects of beta blockers and calciumchannel
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Patient characteristics Intervention Comparison Length of followup
 ISCHAEMIA TESTING CHAPTER TESTING FOR MYCOCARDIAL ISCHAEMIA VERSUS NOT TESTING FOR MYOCARDIAL ISCHAEMIA Ref ID: 4154 Reference Wienbergen H, Kai GA, Schiele R et al. Actual clinical practice exercise ing
ISCHAEMIA TESTING CHAPTER TESTING FOR MYCOCARDIAL ISCHAEMIA VERSUS NOT TESTING FOR MYOCARDIAL ISCHAEMIA Ref ID: 4154 Reference Wienbergen H, Kai GA, Schiele R et al. Actual clinical practice exercise ing
Drug Class Review on HMG-CoA Reductase Inhibitors (Statins)
 Drug Class Review on HMG-CoA Reductase Inhibitors () Final Report June 2004 Mark Helfand, MD, MPH Susan Carson, MPH Cathy Kelley, PharmD Oregon Evidence-based Practice Center Oregon Health & Science University
Drug Class Review on HMG-CoA Reductase Inhibitors () Final Report June 2004 Mark Helfand, MD, MPH Susan Carson, MPH Cathy Kelley, PharmD Oregon Evidence-based Practice Center Oregon Health & Science University
REVEAL: Randomized placebo-controlled trial of anacetrapib in 30,449 patients with atherosclerotic vascular disease
 REVEAL: Randomized placebo-controlled trial of anacetrapib in 30,449 patients with atherosclerotic vascular disease Martin Landray and Louise Bowman on behalf of the HPS 3 / TIMI 55 - REVEAL Collaborative
REVEAL: Randomized placebo-controlled trial of anacetrapib in 30,449 patients with atherosclerotic vascular disease Martin Landray and Louise Bowman on behalf of the HPS 3 / TIMI 55 - REVEAL Collaborative
2003 World Health Organization (WHO) / International Society of Hypertension (ISH) Statement on Management of Hypertension.
 2003 World Health Organization (WHO) / International Society of Hypertension (ISH) Statement on Management of Hypertension Writing Group: Background Hypertension worldwide causes 7.1 million premature
2003 World Health Organization (WHO) / International Society of Hypertension (ISH) Statement on Management of Hypertension Writing Group: Background Hypertension worldwide causes 7.1 million premature
Felix Vallotton Ball (1899) LDL-C management in Asian diabetes: moderate vs. high intensity statin --- a lesson from EMPATHY study
 Felix Vallotton Ball (1899) LDL-C management in Asian diabetes: moderate vs. high intensity statin --- a lesson from EMPATHY study Conflict of interest disclosure None Committee of Scientific Affairs Committee
Felix Vallotton Ball (1899) LDL-C management in Asian diabetes: moderate vs. high intensity statin --- a lesson from EMPATHY study Conflict of interest disclosure None Committee of Scientific Affairs Committee
NICE QIPP about Lipitor. Robert Trotter. Clinical Effectiveness Consultant
 NICE QIPP about Lipitor Robert Trotter Clinical Effectiveness Consultant LIP2894c Date of preparation: April 2009 Prescribing information for atorvastatin is available on the last slide Roadmap Background
NICE QIPP about Lipitor Robert Trotter Clinical Effectiveness Consultant LIP2894c Date of preparation: April 2009 Prescribing information for atorvastatin is available on the last slide Roadmap Background
EDMS #4298 Version 1.0
 Randomized EValuation of the Effects of Anacetrapib through Lipid-modification (HPS3/TIMI55 REVEAL) Data Analysis Plan Version 1.0 1 Table of Contents 1 VERSION HISTORY... 2 2 INTRODUCTION... 3 2.1 BACKGROUND...
Randomized EValuation of the Effects of Anacetrapib through Lipid-modification (HPS3/TIMI55 REVEAL) Data Analysis Plan Version 1.0 1 Table of Contents 1 VERSION HISTORY... 2 2 INTRODUCTION... 3 2.1 BACKGROUND...
Supplementary Online Content
 Supplementary Online Content Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and
Supplementary Online Content Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE General practice Indicators for the NICE menu
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE General practice Indicators for the NICE menu Indicator area: Pulse rhythm assessment for AF Indicator: NM146 Date: June 2017 Introduction There is evidence
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE General practice Indicators for the NICE menu Indicator area: Pulse rhythm assessment for AF Indicator: NM146 Date: June 2017 Introduction There is evidence
Cost effectiveness of statin therapy for the primary prevention of coronary heart disease in Ireland Nash A, Barry M, Walshe V
 Cost effectiveness of statin therapy for the primary prevention of coronary heart disease in Ireland Nash A, Barry M, Walshe V Record Status This is a critical abstract of an economic evaluation that meets
Cost effectiveness of statin therapy for the primary prevention of coronary heart disease in Ireland Nash A, Barry M, Walshe V Record Status This is a critical abstract of an economic evaluation that meets
Articles. Funding Pfizer, Servier Research Group, and Leo Laboratories.
 Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled
Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled
John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam
 Latest Insights from the JUPITER Study John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam Inflammation, hscrp, and Vascular Prevention
Latest Insights from the JUPITER Study John J.P. Kastelein MD PhD Professor of Medicine Dept. of Vascular Medicine Academic Medial Center / University of Amsterdam Inflammation, hscrp, and Vascular Prevention
By Prof. Khaled El-Rabat
 What is The Optimum? By Prof. Khaled El-Rabat Professor of Cardiology - Benha Faculty of Medicine HT. Introduction Despite major worldwide efforts over recent decades directed at diagnosing and treating
What is The Optimum? By Prof. Khaled El-Rabat Professor of Cardiology - Benha Faculty of Medicine HT. Introduction Despite major worldwide efforts over recent decades directed at diagnosing and treating
