Clinical Study Clinical Analysis of Algerian Patients with Pompe Disease
|
|
|
- Oswin Calvin Moody
- 5 years ago
- Views:
Transcription
1 Hindawi Publishing Corporation Journal of Neurodegenerative Diseases Volume 2017, Article ID , 7 pages Clinical Study Clinical Analysis of Algerian Patients with Pompe Disease Y. Sifi, 1,2 M. Medjroubi, 3 R. Froissart, 4 N. Taghane, 1,2 K. Sifi, 2,5 A. Benhabiles, 6 S. Lemai, 7 S. Semra, 8 H. Benmekhebi, 3 Z. Bouderda, 3 N. Abadi, 2,5 and A. Hamri 1,2 1 Service de Neurologie, FacultédeMédecine, Université Constantine 3, Constantine, Algeria 2 LaboratoiredeBiologieetdeGénétique Moléculaire, FacultédeMédecine, Université Constantine 3, Constantine, Algeria 3 Service de Pédiatrie, FacultédeMédecine, Université Constantine 3, Constantine, Algeria 4 Laboratoire des Maladies Héréditaires du Métabolisme, Biochimie et Biologie Moléculaire, Centre de Biologie et Pathologie Est, Hospices Civils de Lyon, Bron, France 5 LaboratoiredeBiochimie,FacultédeMédecine, Université Constantine 3, Constantine, Algeria 6 Service de Chirurgie Orthopédique, FacultédeMédecine, Université Constantine 3, Constantine, Algeria 7 Service Médecine Physique et Réadaptation Fonctionnelle, FacultédeMédecine, Université Constantine 3, Constantine, Algeria 8 Service de Réanimation Médicale, FacultédeMédecine, Université Constantine 3, Constantine, Algeria Correspondence should be addressed to Y. Sifi; sifimina@yahoo.fr Received 30 April 2016; Accepted 9 October 2016; Published 6 February 2017 Academic Editor: Brian Spencer Copyright 2017 Y. Sifi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Pompe s disease is a metabolic myopathy caused by a deficiency of acid alpha-glucosidase (GAA), also called acid maltase, an enzyme that degrades lysosomal glycogen. The clinical presentation of Pompe s disease is variable with respect to the age of onset and rate of disease progression. Patients with onset of symptoms in early infancy (infantile-onset Pompe disease (IOPD)) typically exhibit rapidly progressive hypertrophic cardiomyopathy and marked muscle weakness. Most of them die within the first year of life from cardiac and/or respiratory failure. In the majority of cases of Pompe s disease, onset of symptoms occurs after infancy, ranging widely from the first to sixth decade of life (late-onset Pompe s disease or LOPD). Progression of the disease is relentless and patients eventually progress to loss of ambulation and death due to respiratory failure. The objective of this study was to characterize the clinical presentation of 6 patients (3 with EOPD and the other 3 with LOPD) of 5 families from the East of Algeria. All our patients were diagnosed as having Pompe s disease based on biochemical confirmations of GAA deficiency by dried blood spots (DBS) and GAA gene mutations were analyzed in all patients who consented (n =4). Our results are similar to other ethnic groups. 1. Introduction Pompe s disease (PD), or glycogen storage disease type II (OMIM ) is an autosomal recessive disorder caused by a deficiency in the activity of the lysosomal enzyme acid alpha-glucosidase (GAA; EC ), an enzyme that degrades lysosomal glycogen. As a result, glycogen accumulates in lysosomes of many types of cells but accumulates predominantly in skeletal muscle fibers. The process is progressive and finally destroys the muscle architecture and function [1 4]. The incidence of PD varies in different ethnic groups andforthedifferentclinicalforms.therapidlyprogressive infantile-onset form has estimated frequencies of 1 : 138,000 in the Caucasian population [5], 1 : 50,000 in Taiwanese [6], and 1 : 31,000 in those of African ancestry [7]. The clinical presentation of PD variates according to the age of onset andrateofdiseaseprogression.theclassicinfantileform or infantile onset Pompe disease (IOPD) is characterized by progressive cardiac hypertrophy and rapid loss of muscle function. Symptoms are manifested shortly after birth and patients usually die within the first year of life [1, 7, 8]. In the majority of cases, onset of symptoms occurs after infancy, ranging widely from the first to sixth decade of life; this form is called late-onset Pompe s disease (LOPD). The progression of the disease in this form is relentless and patients eventually lose ambulation and then die due to respiratory failure [9, 10]. This phenotypic variation is associated with different levels of residual GAA activity; indeed GAA activity in infantile onset Pompe s disease (IOPD) is less than 1% of controls and is generally higher in (LOPD) [11 13]. The GAA gene
2 2 Journal of Neurodegenerative Diseases Demographic characteristics of the study population Table 1: Demographic characteristics of the study population. Case 1 Case 2 Case 3 Case 4 Case 5 Case 6 Age at presentation 31 years 27 years 35 years 5 months 11 months 5 months Symptomatic since 12years 21years 5years 4months 11months Birth Sex Male Female Male Male Female Male Consanguinity Present Present Present Present Absent Present Motor delay Absent Absent Absent Absent Absent Present Generalised hypotonia Absent Absent Absent Present Present Present Hepatomegaly Absent Absent Absent Present Absent Present Cardiomegaly Absent Absent Absent Present Absent Present (OMIM number ) has been localized to chromosome 17q25, 3 and a large number of mutations have been described to date, the majority being private. c T>G mutation affects about 40% of alleles in late-onset phenotypes [14, 15]. GAA mutation is not required for the diagnosis of (PD), but it is important for prenatal diagnosis (especially in IOPD families). Until recently there was no therapy for patients with PD other than supportive care. This has changed and the prognosis of PD has been modified considerably by enzyme replacement therapy (ERT) [16 18], but at least one-third of children do not respond to ERT and ultimately die or do not reach a normal motor milestone [19]. The diagnosis of PD is based on a low level of GAA confirmed through a quantitative enzyme (GAA) activity assay in tissue samples, including blood, skin fibroblasts, and muscle. Historically, skin fibroblasts and muscle biopsy have been the samples of choice for measuring GAA activity; both require invasive proceduresandtakeseveralweekstoobtainresults.new GAA enzyme activity assays using blood samples, including dried blood spot (DBS), are rapidly being adopted because of their accuracy, speed, cost, and convenience [8, 20, 21]. The aim of our study was to report the phenotype of six patients from 5 families with PD, all from the East of Algeria. 2. Patients and Methods Study Design The names of patients diagnosed with PD during the period from 2001 to 2013 were recruited from our centre of neurology and from the pediatric centre in Constantine. Informed consent has been signed by all patients or by their parents. Detailed pedigree charting was done and first degree relatives were examined. Patients were diagnosed as having PD based on biochemical confirmations of GAA deficiency by dried blood spots (DBS) (Centre de Référence des Maladies Héréditaires du Métabolisme, Hôpital Debrousse, Hospices Civils, Lyon, France). GAA gene mutations were analyzed in all patients who consented (n =4)(CentredeRéférence des Maladies Héréditaires du Métabolisme, Hôpital Debrousse, Hospices Civils, Lyon, France). Cross-reactive immunologic material or CRIM status was not determined. All patients provided a detailed medical history and underwent a clinical neurological examination. Motor performance was assessed using manual muscle testing (MMT). Serum creatine kinase (CK), serum glutamic oxaloacetic transaminase/aspartate transaminase (SGOT/AST), and serum glutamic pyruvic transaminase/alanine transaminase (SGPT/ALT) levels were determined during the initial presentation. One muscle biopsy was performed. Electrocardiography (ECG) and echocardiography as lung function tests were done on all patients. Our patients were treated with 20 mg/kg IV alglucosidase alfa (Myozyme, provided by Genzyme Corporation) biweekly according to previously published reports [22 25].OfthesepatientswhowerediagnosedtohavePD,three were of the IOPD variety and the three other of the LOPD category. The clinical profile of these cases is as shown in Table Results 3.1. Late-Onset Pompe s Disease Cases Patient Number 1. Patient number 1 is a 31-year-old man who developed since the age of 12 years a progressive pelvic muscle weakness responsible for difficulty in walking and climbing stairs; this was associated with a proximally predominant severe weakness of upper limb muscles at the age of 20 years. At that time a diagnosis of limb-girdle muscular dystrophywasthenmade.hewas23-year-oldwhenhewas seen again for exertional dyspnea and headaches when waking. Neurological examination revealed lumbar hyperlordosis with a waddling gait, a severe proximal weakness of lower limbs, upper limbs, and paraspinal muscles and amyotrophy in four limbs with moderate calves hypertrophy. Only the knee reflexes were absent. He was at grade 3 of Vignos andwaltonscales.ecgandechocardiographywerenormal; however lung function tests showed restrictive changes. SerumCKactivitywas4timesthenormalvalueandASTand ALT were 3 times the normal value. EMG showed myopathic changes, and a muscle biopsy revealed a dystrophic pattern. The activity of lysosomal acid maltase (acid alpha-glucosidase GAA) measured in leukocytes was reduced to about 6% of control values. Genetic studies revealed the presence of the mutationp.tyr543asporp.y543d(c.1627t>g) in the exon 11, that has never been described. Our patient was treated since 2007 with IV alglucosidase alfa (Myozyme), biweekly. But he had become wheelchair-dependent at the age of 27
3 Journal of Neurodegenerative Diseases Infantile Pompe s Disease Cases Figure 1: Family tree of patient number 1. years.thefamilyhistoryofthepatientnumber1revealedone affected parental cousin who died from respiratory problems at The family pedigree is shown in Figure Patient Number 2. Patientnumber2isthepaternal cousin of patient number 1; she was 27-year-old when she was first examined in our centre. The age of onset has been estimated at 21 years with progressive difficulty in walking and climbing stairs. Two years later respiratory symptoms were reported and dyspnea worsened when she was supine. She became wheelchair bound at 25 years. The neurologic examination indicated severe proximal predominant muscles weakness in four limbs causing the patient s dependence on awheelchair,aseverekyphoscoliosiswithtruncalmuscle weakness, contracture of knee and ankles articulations, and amyotrophy in four limbs. Deep tendon reflexes were absent. Shewasatgrade10ofVignosscaleand7ofWaltonscale. EMG was compatible with a myopathic disorder. Serum CK activity was 6 times the normal value and AST and ALT were 4timesthenormalvalue.ECGandechocardiographyshowed no cardiac abnormalities but lung function s tests showed restrictive changes. The activity of lysosomal acid maltase (acid alpha-glucosidase GAA) measured in leukocytes was very reduced. She did not benefit of genetic study. She was treated since 2007 with IV alglucosidase alfa (Myozyme), biweekly. She died 8 months later due to respiratory failure following respiratory tract infections Patient Number 3. Patient number 3 is a 35-years-old man; he was 23-year-old when he was first examined in our centre. The age of onset has been estimated at 5 years with a progressive pelvic muscle weakness responsible for difficulty in walking. Neurological examination found a severe weakness and proximal amyotrophy of lower limbs with a discreet weakness of upper limb muscles. He was at grade 3 ofvignosandwaltonscales.ckratewas5timesthenormal value and AST and ALT were normal. EMG was compatible with a myopathic disorder. ECG, echocardiography, and lung function tests were normal. The activity of lysosomal acid maltasemeasuredinleukocyteswassignificantlydecreased and the genetic studies revealed c t>g homozygous mutation in the intron 1. He was treated since 2013 with IV alglucosidasealfa(myozyme),biweekly.atthemomentheis stillambulatoryandabletoclimbstairs Patient Number 4. A 5-month-old boy was born at full term. His family history revealed that an older sister died of hypertrophic cardiomyopathy with congestive heart failureattheageoffourmonths.thechildwassenttothe pediatric centre because of a cardiac insuffisance; on examination, he had respiratory distress and tachycardia. There was marked hepatomegaly but no splenomegaly. A generalized hypotonia, decreased deep tendon reflexes, macroglossia, and swallowing disorders were found on neurological examination. Chest films showed cardiomegaly with a cardiothoracic ratio of about 0, 68%. The echocardiography demonstrated biventricular and interventricular hypertrophy but no left ventricular outflow tract obstruction. CK rate was 7 times thenormalvalueandastandaltwere2timesthenormal value. Quantitative blood alpha-glucosidase s level was null. Hedidnotbenefitofgeneticstudy.Thechildwastreatedsince 2012 with IV alglucosidase alfa (Myozyme), biweekly with medical treatment of the cardiomyopathy. After 06 months of treatment we noted stabilization of cardiomyopathy and swallowing disorders. A respiratory failure occurred on acute bronchiolitis four months later and he died at the age of 14 months Patient Number 5. A 11-month-old girl was hospitalized at the pediatric centre for progressively increasing respiratory distress associated with presumed viral infection. She was the second born child of healthy consanguineous parents. The pregnancy was uneventful. Physical examination during admission revealed a rapid breathing, a perioral cyanosis, and intercostal and subcostal retraction. Chest examination revealed no significant heart murmur but she had coarse breathing sounds. The heart had a normal rhythm. There was a peripheral and axial hypotonia. Deep tendon reflexes were conserved and cranial nerves were uninvolved. A roentgenogram of the chest revealed a diffuse infiltrative process involving the left lung field, but the ECG was normal. CK rate was 3 times the normal value and AST and ALT were 2 times the normal value, and the EMG was compatible with a myopathic disorder. PD was confirmed by deficient activity of acid-glucosidase (GAA) and the genetic studies revealed a homozygous intronic mutation (c G>A) located outside the consensus splice sites and that has never been described. Her response to antibiotic therapy was temporary and she died of pulmonary failure 15 days after her hospitalization Patient Number 6. A 5-month-old boy, the only son of consanguineous parents, was admitted at the pediatric centre because of heart failure due to a hypertrophic cardiomyopathy. After birth, feeding and swallowing difficulties were noticed by his parents. Physical examination upon admission revealed a mild cyanosis of the extremities and intercostal and subcostal retraction. We also noted generalized weakness associated with axial hypotonia and macroglossia. Deep tendon reflexes were abolished. Developmental milestones were delayed; the child could not support his head s weight. Abdominal ultrasound was in favor of
4 4 Journal of Neurodegenerative Diseases moderate hepatomegaly. Chest films showed cardiomegaly with a cardiothoracic ratio of about 0, 78%. CK, ALT, and AST rates were normal. Quantitative blood alpha-glucosidase s level was null. Genetic studies revealed missense mutation L328P that has never been described. He was treated with IV alglucosidase alfa (Myozyme), biweekly but he died at the age of 11 months following respiratory failure that occurred on acute bronchiolitis. 4. Discussion Pompe s disease is a progressive, debilitating, and often fatal neuromuscular disorder resulting from the deficiency of a lysosomal enzyme, acid alpha-glucosidase (GAA) [26]. The disease presents as a continuous clinical spectrum, although it can be broadly classified into IOPD and LOPD forms, according to the age of onset [27]. The IOPD form is the most aggressive and life-threatening form of the disease. Our results confirm and expand on earlier reports documenting the rapid progression and fatal course of this form. All our patients with EOPD presented a typical clinical course and the phenotype was homogenous; they usually present with symptoms within the first months of life and have a rapidly progressive disease course that was usually fatal by one year of age [28]. Generalized hypotonia was a universal feature and our 3 patients exhibited the pathognomonic frog like position. Heart involvement is frequent in IOPD and most infants developed massive and progressive cardiomegaly before age of 6 months [29 31]. Marked cardiomegaly and cardiomyopathy with progression to cardiac failure were observed in 2 of our 3 patients with EOPD. All our patients died during follow-up, and the median age at death was 12 months; cause of death was usually ascribed to cardiorespiratory complications, as reported elsewhere [27]. Other common features were observed, including alert look, hepatomegaly, and macroglossia which were seen in most of our infantile cases [32]. In comparison, patients with LOPD have a less rapid and more variable disease course, where symptoms may begin anywhere from infancy to adulthood. The age at the symptom onset, rate of progression, and sequence of respiratory and skeletal muscle involvement vary by each patient [33]. Our data correlates with the similar age of onset reported by the other studies [9, 34], but the age at diagnosis was considerably older than that in the previous studies. Lack of awareness and qualified diagnostic tests might be the reason for the late diagnosis [27]. The clinical presentation of our patients with LOPD was classical, characterized by progressive limb-girdle muscle weakness in all cases and diaphragm involvement in 2 patients, leading to respiratory insufficiency and early death in the patient number 2 in agreement with previous studies [19, 35]. Progressive dysfunction ofthediaphragmandancillarymusclesisthemajorcause of respiratory insufficiency in children and adults, leading to low lung capacity, blood gas abnormalities, and sleepdisordered breathing. In addition, children and adults may have recurrent respiratory infections due to impaired cough and ineffective airway clearance [34]. In some rare cases, respiratory muscle involvement with respiratory failure as the first and presenting symptom occurs [36]. Death in that case results from cardiorespiratory complications [19]. Progressive weakness of the proximal lower limb and paraspinal muscles contributes to difficulties when walking, running, climbing stairs, and doing other functional activities. Increasing weakness eventually leads to wheelchair dependency [34]. In our series two patients (patient numbers 1 and 2) lost ambulation attheageof27and25years,respectively.patientnumber3 has experienced mild muscle symptoms since childhood and he is still ambulatory. Many patients eventually require the use of a wheelchair [37]. The clinical symptoms of PD show a significant variability depending on the age at onset, extent of organ involvement, and rate of progression [1, 10, 32]. PD should be considered as part of the differential diagnosis for all children and adults presenting with limb-girdle muscle weakness and respiratory insufficiency without significant cardiomyopathy[8,21,38,39],butcardiomyopathieshasalso been reported in some cases; it includes rhythm disturbances, such as Wolff Parkinson White syndrome, electrocardiogram (ECG) abnormalities, and ascending aorta dilation [37, 40]. In contrast to patients with other neuromuscular disorders, LOPD patients who are still ambulatory may have respiratory insufficiency, which can go undiagnosed [41]. Patients should be specifically asked about nocturnal restlessness, frequent arousals, snoring, excessive daytime sleepiness, headache, and morning lethargy [42]; all these symptoms can orient to respiratory disorders as reported in patient number 1 who presented headaches when waking up. The diagnosisofpdissimpleifseveralmanifestationsarepresent butitmaybetrickyinthepresenceofisolatedsymptoms. This variability in disease severity is more probably explained by a different genetic background, because the median age at the time of data collection is approximately the same in different populations [19]. Additional level of residual GAA activity is considered to be associated with severity and rate of disease progression [11]. Typically, infants with littletonodetectableenzymeactivity(<1%) have a rapid and uniformly fatal disease course. Children and adults with low to moderate GAA activity (1 30%) generally have a less rapid and more variable disease course. Diagnosis of PD must be confirmed by biochemical testing. Diagnosis of PD is confirmed through quantitative enzyme GAA activity assayintissuesamples,includingblood,skinfibroblasts, and muscle [20]. New methods have been developed that assay GAA activity in DBS extracts [43]. These novel DBS methods not only are suitable for newborn screening of PD, butalsoofferarapid,noninvasivediagnostictest.muscle biopsy in PD shows the presence of vacuoles that stain positively for glycogen. Given the high anesthesia risk in these patients, muscle biopsies to make a diagnosis of infantile PD should be avoided [30]. In our study, CK levels were increased in 5/6 of our patients, according to the literary data [19], but their elevation is a nonspecific marker for PD [26]. AST and ALT rates were elevated in 4 patients; they reflect enzymes released from muscle [44]. Multiple types of mutations in the GAA gene (locus on 17q23) have been reported worldwide [45]. PD is caused by mutations in the humangenegaawhichislocatedonchromosome17and is transcribed into three RNA isoforms encoding the same protein. The gene contains 20 exons (transcript variant 1,
5 Journal of Neurodegenerative Diseases 5 NM ),andthefirstexonisnoncoded[46,47].The cdnaforgaaisgreaterthan3.6kbinlengthwidth2856 nucleotides of coding sequence and the resulting product is a protein of 952 amino acids [48]. More than 393 variations have been described in the GAA gene, 257 of which have been confirmed to be pathogenic ( [27]. Most mutations are extremely rare and limited to individual patients, but common mutations in some populations have been reported. In our study, we identified 3 novel allelic variants predicted to encode truncated forms of GAA:thehomozygousintronicmutation(c G>A), P. Tyr543Asp or p. y543d (c. 1627T>G) and the missense L328P mutation. The effect of the 3 novel mutations should be assessed by functional studies. In 2006, the Food and Drug Administration approved an enzyme replacement therapy (ERT), alglucosidase alfa (Myozyme), for the treatment of PD [22, 42]. The recommended dosage regime of Myozyme ranges from 10 mg/kg/week to mg/kg every two weeks, administered as an intravenous infusion. Initial reports of its usage suggest that the ERT has been well tolerated and an improvement in cardiac size and function, as measured by left ventricular mass index (LVMI) and fractional shortening, with a continued improvement in motor function has been recorded [49]. This product was commercialized in Algeria since 2007 and 5 out of 6 of our 6 patients received Myozyme. The treatment was well tolerated by all patients. In clinical studies, the majority of patients developed IgG antibodies to Myozyme, typically within 3 months of treatment. Thus, seroconversion is expected to occur in most patients treated with Myozyme. A tendency was observed for patients treated with a higher dose of Myozyme (40 mg/kg) to develop higher titers of antibody [50]. Our patients should be tested for IgE antibodies and antibody titers ought to be regularly monitored. 5. Conclusion Pompe s disease is now being increasingly diagnosed, due to definitive enzyme estimation facilities. The disease presents as a broad spectrum of clinical phenotypes, with varying rates of progression, symptom onset, degree of organ involvement, and severity. Diagnosis of PD can be accomplished by a simple blood test. With the recent availability of enzyme replacement therapy with Myozyme, the prognosis is likely to change for the better. Gene replacement therapy will eventuallybethecureforpompe sdiseaseandotherrare metabolic disorders. Competing Interests Alltheauthorshavenocompetinginterests. Acknowledgments The authors thank the Pompe families and clinicians who collaborated with them in this study. They are also grateful toprof.a.benhabiles,prof.s.lemai,anddr.s.semra. References [1]R.HirschhornandA.J.J.Reuser, Glycogenstoragedisease Type II: acid α-glucosidase (acid maltase) deficiency, in The Metabolic and Molecular Bases of Inherited Disease,C.R.Scriver, A. Beaudet, W. Sly, and D. Valle, Eds., McGraw-Hill Global Education Holdings, [2] A. G. Engel and R. Hirschhorn, Acid maltase deficiency, in Myology: Basic and Clinical, A. G. E. A. C. Franzini-Armstrong, Ed., vol. 1-2, p. 1937, McGraw-Hill, New York, NY, USA, 2nd edition, [3] B.L.Thurberg,C.L.Maloney,C.Vaccaroetal., Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease, Laboratory Investigation,vol. 86, no. 12, pp , [4] T. Fukuda, L. Ewan, M. Bauer et al., Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease, Annals of Neurology,vol.59,no.4,pp ,2006. [5] M.G.E.M.Ausems,J.Verbiest,M.M.P.Hermansetal., Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling, European Journal of Human Genetics,vol.7,no.6,pp ,1999. [6] C.-Y. Lin, B. Hwang, K.-J. Hsiao, and Y.-R. Jin, Pompe s disease in Chinese and prenatal diagnosis by determination of αglucosidase activity, Journal of Inherited Metabolic Disease,vol. 10,no.1,pp.11 17,1987. [7] P.S.Kishnani,W.-L.Hwu,H.Mandel,M.Nicolino,F.Yong,and D. Corzo, A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease, Journal of Pediatrics,vol.148,no.5,pp e2,2006. [8] H. M. P. Van den Hout, W. Hop, O. P. Van Diggelen et al., The natural course of infantile Pompe s disease: 20 original cases compared with 133 cases from the literature, Pediatrics,vol.112, no. 2, pp , [9]M.L.C.Hagemans,L.P.F.Winkel,P.A.VanDoornetal., Clinical manifestation and natural course of late-onset Pompe s disease in 54 Dutch patients, Brain, vol. 128, part 3, pp , [10] N. Pellegrini, P. Laforet, D. Orlikowski et al., Respiratory insufficiency and limb muscle weakness in adults with Pompe s disease, European Respiratory Journal,vol.26,no.6,pp , [11] A. T. van der Ploeg and A. J. Reuser, Pompe s disease, The Lancet,vol.372,no.9646,pp ,2008. [12] ACMG Work Group on Management of Pompe Disease, P. S. Kishnani, R. D. Steiner et al., Pompe disease diagnosis and management guideline, Genetics in Medicine, vol. 8, no. 5, pp , [13] A. J. Reuser, M. A. Kroos, M. M. Hermans et al., Glycogenosis type II (acid maltase deficiency), Muscle & Nerve. Supplement, vol. 3, pp. S61 S69, [14] A.L.E.Montalvo,B.Bembi,M.Donnarummaetal., Mutation profile of the GAA gene in 40 Italian patients with late onset glycogen storage disease type II, Human Mutation,vol.27,no. 10, pp , [15] P.R.Joshi,D.Gläser, S. Schmidt et al., Molecular diagnosis of German patients with late-onset glycogen storage disease type II, Journal of Inherited Metabolic Disease,vol.31,no.2,pp.S261 S265, [16] A. T. van der Ploeg, P. R. Clemens, D. Corzo et al., A randomized study of alglucosidase alfa in late-onset Pompe s
6 6 Journal of Neurodegenerative Diseases disease, The New England Journal of Medicine,vol.362,no.15, pp ,2010. [17] C. Regnery, C. Kornblum, F. Hanisch et al., 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy, Journal of Inherited Metabolic Disease,vol.35,no.5,pp ,2012. [18] C. Angelini, C. Semplicini, S. Ravaglia et al., Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years, Journal of Neurology,vol.259,no.5,pp ,2012. [19] P. Laforêt,K.Laloui,B.Grangeretal., TheFrenchPompe registry. Baseline characteristics of a cohort of 126 patients with adult Pompe disease, Revue Neurologique, vol. 169, no. 8-9, pp , [20] Pompe Disease Diagnostic Working Group, B. Winchester, D. Bali et al., Methods for a prompt and reliable laboratory diagnosis of Pompe disease: report from an international consensus meeting, Molecular Genetics and Metabolism,vol.93, no.3,pp , [21]L.E.Case,A.A.Beckemeyer,andP.S.Kishnani, Infantile Pompe disease on ERT-Update on clinical presentation, musculoskeletal management, and exercise considerations, American Journal of Medical Genetics, Part C: Seminars in Medical Genetics,vol.160,no.1,pp.69 79,2012. [22] A. Amalfitano, A. R. Bengur, R. P. Morse et al., Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial, Genetics in Medicine,vol.3,no.2,pp ,2001. [23] P. S. Kishnani, M. Nicolino, T. Voit et al., Chinese hamster ovary cell-derived recombinant human acid α-glucosidase in infantile-onset Pompe disease, Journal of Pediatrics,vol.149,no. 1, pp , [24]P.S.Kishnani,D.Corzo,M.Nicolinoetal., Recombinant human acid α-glucosidase: major clinical benefits in infantileonset Pompe disease, Neurology,vol.68,no.2,pp ,2007. [25] M. Nicolino, B. Byrne, J. E. Wraith et al., Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease, Genetics in Medicine, vol. 11, no. 3, pp , [26] M. G. E. M. Ausems, P. Lochman, O. P. Van Diggelen, H. K.PloosVanAmstel,A.J.J.Reuser,andJ.H.J.Wokke, A diagnostic protocol for adult-onset glycogen storage disease type II, Neurology,vol.52,no.4,pp ,1999. [27] L. Fu, W. Qiu, Y. Yu et al., Clinical and molecular genetic study of infantile-onset Pompe disease in Chinese patients: identification of 6 novel mutations, Gene, vol. 535, no. 1, pp , [28] L.P.F.Winkel,J.M.P.VanDenHout,J.H.J.Kamphovenet al., Enzyme replacement therapy in late-onset pompe s disease: athree-yearfollow-up, Annals of Neurology, vol.55,no.4,pp , [29] A. Jegadeeswari, V. Amuthan, R. A. Janarthanan, S. Murugan, and S. Balasubramanian, Two cases of Pompe s disease: case report and review of literature, Indian Heart Journal, vol. 64, no. 2, pp , [30] P. S. Kishnani, S. BurnsWechsler, and J. S. Li, Enzymedeficiency metabolic cardiomyopathies and the role of enzyme replacement therapy, Progress in Pediatric Cardiology, vol. 23, no. 1-2, pp , [31] O. I. I. Soliman, N. A. M. E. Van Der Beek, P. A. Van Doorn et al., Cardiac involvement in adults with Pompe disease, Journal of Internal Medicine,vol.264,no.4,pp ,2008. [32] U.Raju,S.C.Shaw,K.S.Rana,M.Sharma,andH.R.Ramamurthy, Pompe s disease in childhood: a metabolic myopathy, Medical Journal Armed Forces India, vol.66,no.1,pp.32 36, [33] L. E. Case and P. S. Kishnani, Physical therapy management of Pompe disease, Genetics in Medicine,vol.8,no.5,pp , [34] L.P.F.Winkel,M.L.C.Hagemans,P.A.VanDoornetal., The natural course of non-classic Pompe s disease; a review of 225 published cases, Journal of Neurology, vol.252,no.8,pp , [35] N. A. M. E. van der Beek, C. I. van Capelle, K. I. van der Veldenvan Etten et al., Rate of progression and predictive factors for pulmonary outcome in children and adults with Pompe disease, Molecular Genetics and Metabolism, vol. 104, no. 1-2, pp ,2011. [36] L.Burghaus,W.Liu,E.Neuen-Jacob,K.Gempel,andW.Haupt, Selective respiratory failure as a rare first symptom of Pompe s disease, Nervenarzt,vol.77,no.2,pp ,2006. [37] W. Müller-Felber,R.Horvath,K.Gempeletal., Lateonset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients, Neuromuscular Disorders, vol. 17, no. 9-10, pp , [38] E. J. Cupler, K. I. Berger, R. T. Leshner et al., Consensus treatment recommendations for late-onset pompe disease, Muscle & Nerve,vol.45,no.3,pp ,2012. [39] N. Preisler, Z. Lukacs, L. Vinge et al., Late-onset Pompe disease is prevalent in unclassified limb-girdle muscular dystrophies, Molecular Genetics and Metabolism,vol.110,no.3,pp , [40] S. Sacconi, J. D. Bocquet, S. Chanalet, V. Tanant, L. Salviati, and C. Desnuelle, Abnormalities of cerebral arteries are frequent in patients with late-onset Pompe disease, Journal of Neurology, vol. 257, no. 10, pp , [41] U. Mellies, F. Stehling, C. Dohna-Schwake, R. Ragette, H. Teschler, and T. Voit, Respiratory failure in Pompe disease: treatment with noninvasive ventilation, Neurology,vol.64,no. 8, pp , [42] U. K. Dhand and R. Dhand, Sleep disorders in neuromuscular diseases, Current Opinion in Pulmonary Medicine, vol. 12, no. 6, pp , [43] H. Zhang, H. Kallwass, S. P. Young et al., Comparison of maltose and acarbose as inhibitors of maltase-glucoamylase activity in assaying acid α-glucosidase activity in dried blood spots for the diagnosis of infantile Pompe disease, Genetics in Medicine,vol.8,no.5,pp ,2006. [44] M. T. Di Fiore, R. Manfredi, L. Marri, A. Zucchini, L. Azzaroli, and G. Manfredi, Elevation of transaminases as an early sign of late-onset glycogenosis type II, EuropeanJournalofPediatrics, vol.152,no.9,p.784,1993. [45] T.-M. Ko, W.-L. Hwu, Y.-W. Lin et al., Molecular genetic study of Pompe disease in Chinese patients in Taiwan, Human Mutation,vol.13,no.5,pp ,1999. [46] L. H. Hoefsloot, M. Hoogeveen-Westerveld, M. A. Kroos, J. van Beeumen, A. J. Reuser, and B. A. Oostra, Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex, EMBO Journal,vol. 7, no. 6, pp , [47] F. Martiniuk, M. Mehler, A. Pellicer et al., Isolation of a cdna for human acid α-glucosidase and detection of genetic heterogeneity for mrna in three α-glucosidase-deficient patients,
7 Journal of Neurodegenerative Diseases 7 Proceedings of the National Academy of Sciences of the United States of America,vol.83,no.24,pp ,1986. [48] F. Martiniuk, M. Mehler, S. Tzall, G. Meredith, and R. Hirschhorn, Sequence of the cdna and 5 -flanking region for human acid α-glucosidase, detection of an intron in the 5 untranslated leader sequence, definition of 18-bp polymorphisms, and differences with previous cdna and amino acid sequences, DNA and Cell Biology,vol.9,no.2,pp.85 94,1990. [49] L. Klinge, V. Straub, U. Neudorf, and T. Voit, Enzyme replacement therapy in classical infantile Pompe disease: results of a ten-month follow-up study, Neuropediatrics, vol. 36, no. 1, pp. 6 11, [50]M.RohrbachandJ.T.R.Clarke, Treatmentoflysosomal storage disorders: progress with enzyme replacement therapy, Drugs,vol.67,no.18,pp ,2007.
LSD WORKSHOP WHAT EVERY PEDIATRICIAN SHOULD KNOW. Amal Al Tenaiji Pediatric Metabolic Genetics SKMC Abu Dhabi
 LSD WORKSHOP WHAT EVERY PEDIATRICIAN SHOULD KNOW Amal Al Tenaiji Pediatric Metabolic Genetics SKMC Abu Dhabi CASE A 1 month old infant presents with difficulty feeding and respiratory distress. Mom and
LSD WORKSHOP WHAT EVERY PEDIATRICIAN SHOULD KNOW Amal Al Tenaiji Pediatric Metabolic Genetics SKMC Abu Dhabi CASE A 1 month old infant presents with difficulty feeding and respiratory distress. Mom and
Pompe. Lysosomal Disorders. Introduction
 Introduction disease is an inherited, genetic disorder which results in the lack of an enzyme 'acid alpha-glucosidase. disease is also known as acid maltase deficiency or glycogen storage disease type
Introduction disease is an inherited, genetic disorder which results in the lack of an enzyme 'acid alpha-glucosidase. disease is also known as acid maltase deficiency or glycogen storage disease type
Medication Policy Manual. Topic: Lumizyme, alglucosidase alfa Date of Origin: February 17, 2015
 Medication Policy Manual Policy No: dru392 Topic: Lumizyme, alglucosidase alfa Date of Origin: February 17, 2015 Committee Approval Date: March 13, 2015 Next Review Date: March 2016 Effective Date: July
Medication Policy Manual Policy No: dru392 Topic: Lumizyme, alglucosidase alfa Date of Origin: February 17, 2015 Committee Approval Date: March 13, 2015 Next Review Date: March 2016 Effective Date: July
Opinion 9 January 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 9 January 2013 The draft opinion adopted by the Transparency Committee on 7 November 2012 was the subject of a hearing
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 9 January 2013 The draft opinion adopted by the Transparency Committee on 7 November 2012 was the subject of a hearing
DEVELOPING A FOLLOW-UP FRAMEWORK FOR POMPE DISEASE
 DEVELOPING A FOLLOW-UP FRAMEWORK FOR POMPE DISEASE Presenter: Sarah Bradley, MS, CGC Genetic Counselor, NYS Newborn Screening Program Authors: S. Bradley, D. Kronn, B. Vogel, M. Caggana, J. Orsini, K.
DEVELOPING A FOLLOW-UP FRAMEWORK FOR POMPE DISEASE Presenter: Sarah Bradley, MS, CGC Genetic Counselor, NYS Newborn Screening Program Authors: S. Bradley, D. Kronn, B. Vogel, M. Caggana, J. Orsini, K.
Alglucosidase alfa: first available treatment for Pompe disease
 DRUG EVALUATION Alglucosidase alfa: first available treatment for Pompe disease Marc Nicolino Division of Pediatric Endocrinology & Metabolism, University Hospital Debrousse, 29 rue Soeur Bouvier, 69322
DRUG EVALUATION Alglucosidase alfa: first available treatment for Pompe disease Marc Nicolino Division of Pediatric Endocrinology & Metabolism, University Hospital Debrousse, 29 rue Soeur Bouvier, 69322
Infantile-onset glycogen storage disease type II
 Case Report 379 Infantile-Onset Glycogen Storage Disease Type II (Pompe Disease): Report of a Case with Genetic Diagnosis and Pathological Findings Yao-Tun Teng, MD; Wen-Jen Su, MD; Jia-Wei Hou 1, MD,
Case Report 379 Infantile-Onset Glycogen Storage Disease Type II (Pompe Disease): Report of a Case with Genetic Diagnosis and Pathological Findings Yao-Tun Teng, MD; Wen-Jen Su, MD; Jia-Wei Hou 1, MD,
Pompe Disease. Family Education Booklet. Division of Pediatric Genetics Metabolism and Genomic Medicine
 Pompe Disease Family Education Booklet Division of Pediatric Genetics Metabolism and Genomic Medicine Table of Contents What is Pompe disease?... 1 How common is Pompe disease?... 1 Basic genetic concepts...
Pompe Disease Family Education Booklet Division of Pediatric Genetics Metabolism and Genomic Medicine Table of Contents What is Pompe disease?... 1 How common is Pompe disease?... 1 Basic genetic concepts...
Background ORIGINAL ARTICLE
 J Inherit Metab Dis (2016) 39:383 390 DOI 10.1007/s10545-015-9912-y ORIGINAL ARTICLE Effects of a higher dose of alglucosidase alfa on ventilator-free survival and motor outcome in classic infantile Pompe
J Inherit Metab Dis (2016) 39:383 390 DOI 10.1007/s10545-015-9912-y ORIGINAL ARTICLE Effects of a higher dose of alglucosidase alfa on ventilator-free survival and motor outcome in classic infantile Pompe
The Changing Face of Infantile Pompe Disease: A Report of Five Patients from the UAE
 JIMD Reports DOI 10.1007/8904_2012_148 RESEARCH REPORT The Changing Face of Infantile Pompe Disease: A Report of Five Patients from the UAE Waseem Fathalla Elamin Ahmed Received: 02 July 2011 /Revised:
JIMD Reports DOI 10.1007/8904_2012_148 RESEARCH REPORT The Changing Face of Infantile Pompe Disease: A Report of Five Patients from the UAE Waseem Fathalla Elamin Ahmed Received: 02 July 2011 /Revised:
abstract SUPPLEMENT ARTICLE
 Management of Confirmed Newborn- Screened Patients With Pompe Disease Across the Disease Spectrum David F. Kronn, MD, a Debra Day-Salvatore, MD, b Wuh-Liang Hwu, MD, PhD, c Simon A. Jones, MBChB, BSc,
Management of Confirmed Newborn- Screened Patients With Pompe Disease Across the Disease Spectrum David F. Kronn, MD, a Debra Day-Salvatore, MD, b Wuh-Liang Hwu, MD, PhD, c Simon A. Jones, MBChB, BSc,
The quick motor function test: a new tool to rate clinical severity and motor function in Pompe patients
 DOI 10.1007/s10545-011-9388-3 ORIGINAL ARTICLE The quick motor function test: a new tool to rate clinical severity and motor function in Pompe patients Carine I. van Capelle & Nadine A. M. E. van der Beek
DOI 10.1007/s10545-011-9388-3 ORIGINAL ARTICLE The quick motor function test: a new tool to rate clinical severity and motor function in Pompe patients Carine I. van Capelle & Nadine A. M. E. van der Beek
Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report
 Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report Roshanak Jazayeri, MD, PhD Assistant Professor of Medical Genetics Faculty of Medicine, Alborz University of Medical Sciences
Genetic diagnosis of limb girdle muscular dystrophy type 2A, A Case Report Roshanak Jazayeri, MD, PhD Assistant Professor of Medical Genetics Faculty of Medicine, Alborz University of Medical Sciences
Corporate Medical Policy
 Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Corporate Medical Policy Genetic Testing for Duchenne and Becker Muscular Dystrophy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_duchenne_and_becker_muscular_dystrophy
Pompe Disease in Infants: Improving the Prognosis by Newborn Screening and Early Treatment. abstract
 Pompe Disease in Infants: Improving the Prognosis by Newborn Screening and Early Treatment WHAT S KNOWN ON THIS SUBJECT: Newborn screening program is now technically feasible in ultrarare diseases and
Pompe Disease in Infants: Improving the Prognosis by Newborn Screening and Early Treatment WHAT S KNOWN ON THIS SUBJECT: Newborn screening program is now technically feasible in ultrarare diseases and
YES NO UNKNOWN. Stage I: Rule-Out Dashboard Secondary Findings in Adults ACTIONABILITY PENETRANCE SIGNIFICANCE/BURDEN OF DISEASE NEXT STEPS
 Stage I: Rule-Out Dashboard GENE/GENE PANEL: GAA DISORDER: Glycogen storage disease II (GSD2) HGNC ID: 4065 OMIM ID: 232300 ACTIONABILITY PENETRANCE 1. Is there a qualifying resource, such as a practice
Stage I: Rule-Out Dashboard GENE/GENE PANEL: GAA DISORDER: Glycogen storage disease II (GSD2) HGNC ID: 4065 OMIM ID: 232300 ACTIONABILITY PENETRANCE 1. Is there a qualifying resource, such as a practice
Clinical Study Orthopedic Management of Patients with Pompe Disease: A Retrospective Case Series of 8 Patients
 e Scientific World Journal, Article ID 963861, 8 pages http://dx.doi.org/10.1155/2014/963861 Clinical Study Orthopedic Management of Patients with Pompe Disease: A Retrospective Case Series of 8 Patients
e Scientific World Journal, Article ID 963861, 8 pages http://dx.doi.org/10.1155/2014/963861 Clinical Study Orthopedic Management of Patients with Pompe Disease: A Retrospective Case Series of 8 Patients
Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease.
 Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease. Listen Observe Evaluate Test Refer Guide for primary care providers includes: Surveillance Aid: Assessing Weakness
Learn the steps to identify pediatric muscle weakness and signs of neuromuscular disease. Listen Observe Evaluate Test Refer Guide for primary care providers includes: Surveillance Aid: Assessing Weakness
Treatment Strategies for a Complex
 Treatment Strategies for a Complex Neuromuscular Disease: The Pompe Story Barry J. Byrne, MD, PhD Pediatrics and Powell Gene Therapy Center University of Florida, College of Medicine 2 4 Initial Presentation
Treatment Strategies for a Complex Neuromuscular Disease: The Pompe Story Barry J. Byrne, MD, PhD Pediatrics and Powell Gene Therapy Center University of Florida, College of Medicine 2 4 Initial Presentation
Muscular Dystrophies. Pinki Munot Consultant Paediatric Neurologist Great Ormond Street Hospital Practical Neurology Study days April 2018
 Muscular Dystrophies Pinki Munot Consultant Paediatric Neurologist Great Ormond Street Hospital Practical Neurology Study days April 2018 Definition and classification Clinical guide to recognize muscular
Muscular Dystrophies Pinki Munot Consultant Paediatric Neurologist Great Ormond Street Hospital Practical Neurology Study days April 2018 Definition and classification Clinical guide to recognize muscular
What s New in Newborn Screening?
 What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is administered by the Illinois Department of Public Health.
What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is administered by the Illinois Department of Public Health.
Understanding Late-Onset Pompe Disease
 Understanding Late-Onset Pompe Disease What Is a Lysosome? Millions of tiny units called cells make up the human body. Each cell has its own job to keep the body running. Within each cell, there are organelles,
Understanding Late-Onset Pompe Disease What Is a Lysosome? Millions of tiny units called cells make up the human body. Each cell has its own job to keep the body running. Within each cell, there are organelles,
Recombinant human acid -glucosidase
 Recombinant human acid -glucosidase Articles Major clinical benefits in infantile-onset Pompe disease P.S. Kishnani, MD*; D. Corzo, MD*; M. Nicolino, MD, PhD; B. Byrne, MD, PhD; H. Mandel, MD; W.L. Hwu,
Recombinant human acid -glucosidase Articles Major clinical benefits in infantile-onset Pompe disease P.S. Kishnani, MD*; D. Corzo, MD*; M. Nicolino, MD, PhD; B. Byrne, MD, PhD; H. Mandel, MD; W.L. Hwu,
LIMP BABIES - WHAT MAY THAT MEAN?
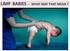 LIMP BABIES - WHAT MAY THAT MEAN? ARE WE LOOKING WHERE WE SHOULD? FLOPPY = LIMP = FLACCID = WEAK Poor head control when pulled C-posture sitting, or in ventral susp. Frog-leg lying Dropping arms Casey
LIMP BABIES - WHAT MAY THAT MEAN? ARE WE LOOKING WHERE WE SHOULD? FLOPPY = LIMP = FLACCID = WEAK Poor head control when pulled C-posture sitting, or in ventral susp. Frog-leg lying Dropping arms Casey
Spinal Muscular Atrophy: Case Study. Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting
 Spinal Muscular Atrophy: Case Study Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting approximately one in 6,000 babies. It is estimated that one in every 40 Americans carries
Spinal Muscular Atrophy: Case Study Spinal muscular atrophy (SMA) is a fairly common genetic disorder, affecting approximately one in 6,000 babies. It is estimated that one in every 40 Americans carries
Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating)
 Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating) Table 1: Appropriate indications (median score 7-9) Indication
Appropriate Use Criteria for Initial Transthoracic Echocardiography in Outpatient Pediatric Cardiology (scores listed by Appropriate Use rating) Table 1: Appropriate indications (median score 7-9) Indication
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2017 10/2018 10/2017 Description of Procedure or Service Spinal muscular atrophy
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2017 10/2018 10/2017 Description of Procedure or Service Spinal muscular atrophy
Nemaline (rod) myopathies
 Nemaline (rod) myopathies Nemaline, or rod, myopathies are a group of conditions which fall under the umbrella of congenital myopathies. They are characterised by rod-like structures in the muscle cells,
Nemaline (rod) myopathies Nemaline, or rod, myopathies are a group of conditions which fall under the umbrella of congenital myopathies. They are characterised by rod-like structures in the muscle cells,
Case Report GNE Myopathy in Turkish Sisters with a Novel Homozygous Mutation
 Case Reports in Neurological Medicine Volume 2016, Article ID 8647645, 4 pages http://dx.doi.org/10.1155/2016/8647645 Case Report GNE Myopathy in Turkish Sisters with a Novel Homozygous Mutation Gulden
Case Reports in Neurological Medicine Volume 2016, Article ID 8647645, 4 pages http://dx.doi.org/10.1155/2016/8647645 Case Report GNE Myopathy in Turkish Sisters with a Novel Homozygous Mutation Gulden
Myotubular (centronuclear) myopathy
 Myotubular (centronuclear) myopathy Myotubular, or centronuclear, myopathy falls under the umbrella of congenital myopathies. It is characterised by a specific pattern in the muscle tissue when viewed
Myotubular (centronuclear) myopathy Myotubular, or centronuclear, myopathy falls under the umbrella of congenital myopathies. It is characterised by a specific pattern in the muscle tissue when viewed
Muscular Dystrophy. Biol 405 Molecular Medicine
 Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Muscular Dystrophy Biol 405 Molecular Medicine Duchenne muscular dystrophy Duchenne muscular dystrophy is a neuromuscular disease that occurs in ~ 1/3,500 male births. The disease causes developmental
Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis
 DOI 10.1007/s00415-016-8219-8 REVIEW Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis Benedikt Schoser 1 Andrew
DOI 10.1007/s00415-016-8219-8 REVIEW Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis Benedikt Schoser 1 Andrew
Anaesthesia recommendations for patients suffering from Pompe disease
 orphananesthesia Anaesthesia recommendations for patients suffering from Pompe disease Disease name: Pompe Disease ICD 10: E74.0 Synonyms: Glycogen storage disease due to acid maltase deficiency, Glycogen
orphananesthesia Anaesthesia recommendations for patients suffering from Pompe disease Disease name: Pompe Disease ICD 10: E74.0 Synonyms: Glycogen storage disease due to acid maltase deficiency, Glycogen
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Amyotrophic Lateral Sclerosis 10 (ALS10) and Amyotrophic Lateral Sclerosis 6 (ALS6)
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Amyotrophic Lateral Sclerosis 10 (ALS10) and Amyotrophic Lateral Sclerosis 6 (ALS6)
Pompe disease (i.e., glycogen-storage disease type
 EXPANDING THE PHENOTYPE OF LATE-ONSET POMPE DISEASE: TONGUE WEAKNESS: A NEW CLINICAL OBSERVATION ALBERTO DUBROVSKY, MD, 1 JOSE CORDERI, PhT, 1 MIN LIN, PhD, 2 PRIYA S. KISHNANI, MBBS, MD, 3 and HARRISON
EXPANDING THE PHENOTYPE OF LATE-ONSET POMPE DISEASE: TONGUE WEAKNESS: A NEW CLINICAL OBSERVATION ALBERTO DUBROVSKY, MD, 1 JOSE CORDERI, PhT, 1 MIN LIN, PhD, 2 PRIYA S. KISHNANI, MBBS, MD, 3 and HARRISON
Clinical Summaries. CLN1 Disease, infantile onset and others
 Clinical Summaries CLN1 Disease, infantile onset and others The gene called CLN1 lies on chromosome 1. CLN1 disease is inherited as an autosomal recessive disorder, which means that both chromosomes carry
Clinical Summaries CLN1 Disease, infantile onset and others The gene called CLN1 lies on chromosome 1. CLN1 disease is inherited as an autosomal recessive disorder, which means that both chromosomes carry
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2018 10/2019 10/2018 Description of Procedure or Service Spinal muscular atrophy
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: nusinersen_spinraza 03/2017 10/2018 10/2019 10/2018 Description of Procedure or Service Spinal muscular atrophy
Clinical and molecular characterization of Korean children with infantile. and late-onset Pompe disease: 10 years of experience with enzyme
 Clinical and molecular characterization of Korean children with infantile and late-onset Pompe disease: 10 years of experience with enzyme replacement therapy at a single center Min-Sun Kim 1, Ari Song
Clinical and molecular characterization of Korean children with infantile and late-onset Pompe disease: 10 years of experience with enzyme replacement therapy at a single center Min-Sun Kim 1, Ari Song
Association of motor milestones and SMN2 copy and outcome in spinal muscular. atrophy types 0 4
 jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
jnnp-2016-314292 1 - SUPPLEMENTARY FILE - Methods and additional data on clinical characteristics and motor development Association of motor milestones and SMN2 copy and outcome in spinal muscular atrophy
Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1
 Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1 17 th April 2018 Adnan Manzur Consultant Paediatric Neurologist Dubowitz Neuromuscular Centre, GOSH & ICH,
Diagnosis, management and new treatments for Spinal Muscular Atrophy Special Focus: SMA Type 1 17 th April 2018 Adnan Manzur Consultant Paediatric Neurologist Dubowitz Neuromuscular Centre, GOSH & ICH,
What s New in Newborn Screening?
 What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is mandated and administered by the Illinois Department of
What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is mandated and administered by the Illinois Department of
Cardiac Considerations and Care in Children with Neuromuscular Disorders
 Cardiac Considerations and Care in Children with Neuromuscular Disorders - importance of early and ongoing treatment, management and available able medications. Dr Bo Remenyi Department of Cardiology The
Cardiac Considerations and Care in Children with Neuromuscular Disorders - importance of early and ongoing treatment, management and available able medications. Dr Bo Remenyi Department of Cardiology The
National Sleep Disorders Research Plan
 Research Plan Home Foreword Preface Introduction Executive Summary Contents Contact Us National Sleep Disorders Research Plan Return to Table of Contents SECTION 5 - SLEEP DISORDERS SLEEP-DISORDERED BREATHING
Research Plan Home Foreword Preface Introduction Executive Summary Contents Contact Us National Sleep Disorders Research Plan Return to Table of Contents SECTION 5 - SLEEP DISORDERS SLEEP-DISORDERED BREATHING
Profile, types, duration and severity of muscular dystrophy: a clinical study at a tertiary care hospital
 International Journal of Advances in Medicine Viswajyothi P et al. Int J Adv Med. 2018 Jun;5(3):700-704 http://www.ijmedicine.com pissn 2349-3925 eissn 2349-3933 Original Research Article DOI: http://dx.doi.org/10.18203/2349-3933.ijam20182126
International Journal of Advances in Medicine Viswajyothi P et al. Int J Adv Med. 2018 Jun;5(3):700-704 http://www.ijmedicine.com pissn 2349-3925 eissn 2349-3933 Original Research Article DOI: http://dx.doi.org/10.18203/2349-3933.ijam20182126
SUMMARY TABLE Referring to Part of the Dossier: Volume: Page: Reference:
 SYNOPSIS TITLE: An Open-Label, Multicenter, Multinational, Study of the Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of Recombinant Human Acid Alpha-Glucosidase Treatment in Patients > 6 and
SYNOPSIS TITLE: An Open-Label, Multicenter, Multinational, Study of the Safety, Efficacy, Pharmacokinetics, and Pharmacodynamics of Recombinant Human Acid Alpha-Glucosidase Treatment in Patients > 6 and
Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN
 Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN Birth History LL was born to a healthy first time mother with an uncomplicated pregnancy Delivered at 38 weeks via C-section due
Patient L.L. KRISTEN ARREDONDO, MD CHILD NEUROLOGY PGY5, UT SOUTHWESTERN Birth History LL was born to a healthy first time mother with an uncomplicated pregnancy Delivered at 38 weeks via C-section due
HYPERTROPHIC CARDIOMYOPATHY
 HYPERTROPHIC CARDIOMYOPATHY Most often diagnosed during infancy or adolescence, hypertrophic cardiomyopathy (HCM) is the second most common form of heart muscle disease, is usually genetically transmitted,
HYPERTROPHIC CARDIOMYOPATHY Most often diagnosed during infancy or adolescence, hypertrophic cardiomyopathy (HCM) is the second most common form of heart muscle disease, is usually genetically transmitted,
READ ORPHA.NET WEBSITE ABOUT BETA-SARCOGLYOCANOPATHY LIMB-GIRDLE MUSCULAR DYSTROPHIES
 READ ORPHA.NET WEBSITE ABOUT BETA-SARCOGLYOCANOPATHY LIMB-GIRDLE MUSCULAR DYSTROPHIES (LGMD) Limb-girdle muscular dystrophies (LGMD) are a heterogeneous group of genetically determined disorders with a
READ ORPHA.NET WEBSITE ABOUT BETA-SARCOGLYOCANOPATHY LIMB-GIRDLE MUSCULAR DYSTROPHIES (LGMD) Limb-girdle muscular dystrophies (LGMD) are a heterogeneous group of genetically determined disorders with a
abstract SUPPLEMENT ARTICLE
 The Initial Evaluation of Patients After Positive Newborn Screening: Recommended Algorithms Leading to a Confirmed Diagnosis of Pompe Disease Barbara K. Burton, MD, a David F. Kronn, MD, b Wuh-Liang Hwu,
The Initial Evaluation of Patients After Positive Newborn Screening: Recommended Algorithms Leading to a Confirmed Diagnosis of Pompe Disease Barbara K. Burton, MD, a David F. Kronn, MD, b Wuh-Liang Hwu,
Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses
 Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses Zoheb B. Kazi, 1 Sean N. Prater, 1 Joyce A. Kobori, 2 David Viskochil, 3 Carrie Bailey, 3 Renuka Gera, 4
Durable and sustained immune tolerance to ERT in Pompe disease with entrenched immune responses Zoheb B. Kazi, 1 Sean N. Prater, 1 Joyce A. Kobori, 2 David Viskochil, 3 Carrie Bailey, 3 Renuka Gera, 4
Neonatal Hypotonia Guideline Prepared by Dan Birnbaum MD August 27, 2012
 Neonatal Hypotonia Guideline Prepared by Dan Birnbaum MD August 27, 2012 Hypotonia: reduced tension or resistance to range of motion Localization can be central (brain), peripheral (spinal cord, nerve,
Neonatal Hypotonia Guideline Prepared by Dan Birnbaum MD August 27, 2012 Hypotonia: reduced tension or resistance to range of motion Localization can be central (brain), peripheral (spinal cord, nerve,
Neonatal Hypotonia. Encephalopathy acute No encephalopathy. Neurology Chapter of IAP
 The floppy infant assumes a frog legged position. On ventral suspension, the baby can not maintain limb posture against gravity and assumes the position of a rag doll. Encephalopathy acute No encephalopathy
The floppy infant assumes a frog legged position. On ventral suspension, the baby can not maintain limb posture against gravity and assumes the position of a rag doll. Encephalopathy acute No encephalopathy
Anatomy & Physiology
 1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
1 Anatomy & Physiology Heart is divided into four chambers, two atrias & two ventricles. Atrioventricular valves (tricuspid & mitral) separate the atria from ventricles. they open & close to control flow
Post-marketing experiences in Pompe disease. Chris van der Meijden, MD TREAT-NMD meeting 20 September 2016
 Post-marketing experiences in Pompe disease Chris van der Meijden, MD TREAT-NMD meeting 20 September 2016 Pompe disease Pompe disease - a clinical spectrum No α-glucosidase activity Hypertrophic cardiomyopathy
Post-marketing experiences in Pompe disease Chris van der Meijden, MD TREAT-NMD meeting 20 September 2016 Pompe disease Pompe disease - a clinical spectrum No α-glucosidase activity Hypertrophic cardiomyopathy
PAS-positive lymphocyte vacuoles can be used as diagnostic screening test for Pompe disease
 J Inherit Metab Dis (2010) 33:133 139 DOI 10.1007/s10545-009-9027-4 ORIGINAL ARTICLE PAS-positive lymphocyte vacuoles can be used as diagnostic screening test for Pompe disease Marloes L. C. Hagemans &
J Inherit Metab Dis (2010) 33:133 139 DOI 10.1007/s10545-009-9027-4 ORIGINAL ARTICLE PAS-positive lymphocyte vacuoles can be used as diagnostic screening test for Pompe disease Marloes L. C. Hagemans &
CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE. Dr. Bahar Naghavi
 2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
Mark Tarnopolsky, MD, PhD. Dept of Pediatrics (Neuromuscular and Neurometabolic Diseases), McMaster University, Hamilton, ON.
 Late Onset Pompe Disease - General Overview. Mark Tarnopolsky, MD, PhD. Dept of Pediatrics (Neuromuscular and Neurometabolic Diseases), McMaster University, Hamilton, ON. Learning Objectives. To review
Late Onset Pompe Disease - General Overview. Mark Tarnopolsky, MD, PhD. Dept of Pediatrics (Neuromuscular and Neurometabolic Diseases), McMaster University, Hamilton, ON. Learning Objectives. To review
POMPE DISEASE, THE MUST-NOT-MISS DIAGNOSIS: A REPORT OF 3 PATIENTS CASES OF THE MONTH
 CASES OF THE MONTH POMPE DISEASE, THE MUST-NOT-MISS DIAGNOSIS: A REPORT OF 3 PATIENTS ALBERTO DUBROVSKY, MD, 1 JOSE CORDERI, PT, 1 THEODORA KARASARIDES, BA, 2 and ANA LIA TARATUTO, MD, PhD 3 1 Department
CASES OF THE MONTH POMPE DISEASE, THE MUST-NOT-MISS DIAGNOSIS: A REPORT OF 3 PATIENTS ALBERTO DUBROVSKY, MD, 1 JOSE CORDERI, PT, 1 THEODORA KARASARIDES, BA, 2 and ANA LIA TARATUTO, MD, PhD 3 1 Department
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/29354 holds various files of this Leiden University dissertation. Author: Straathof, Chiara Title: dystrophinopathies : heterogeneous clinical aspects of
Cover Page The handle http://hdl.handle.net/1887/29354 holds various files of this Leiden University dissertation. Author: Straathof, Chiara Title: dystrophinopathies : heterogeneous clinical aspects of
Clinical Policy Bulletin: Nusinersen (Spinraza)
 Clinical Policy Bulletin: Nusinersen (Spinraza) Number: 0915 Policy *Pleasesee amendment forpennsylvaniamedicaidattheendofthiscpb. Note: REQUIRES PRECERTIFICATION.Footnotes for Precertification of nusinersen
Clinical Policy Bulletin: Nusinersen (Spinraza) Number: 0915 Policy *Pleasesee amendment forpennsylvaniamedicaidattheendofthiscpb. Note: REQUIRES PRECERTIFICATION.Footnotes for Precertification of nusinersen
Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit)
 Line of Business: All Lines of Business Effective Date: August 16, 2017 Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through
Line of Business: All Lines of Business Effective Date: August 16, 2017 Spinraza (Nusinersen) Drug Prior Authorization Protocol (Medical Benefit & Part B Benefit) This policy has been developed through
DNA Day Illinois 2013 Webinar: Newborn Screening and Family Health History. Tuesday, April 16, 2013
 DNA Day Illinois 2013 Webinar: Newborn Screening and Family Health History Tuesday, April 16, 2013 Objectives Recognize the importance & impact of newborn screening Describe the process of newborn screening
DNA Day Illinois 2013 Webinar: Newborn Screening and Family Health History Tuesday, April 16, 2013 Objectives Recognize the importance & impact of newborn screening Describe the process of newborn screening
Quality of life and participation in daily life of adults with Pompe disease receiving enzyme replacement therapy: 10 years of international follow-up
 J Inherit Metab Dis (2016) 39:253 260 DOI 10.1007/s10545-015-9889-6 ORIGINAL ARTICLE Quality of life and participation in daily life of adults with Pompe disease receiving enzyme replacement therapy: 10
J Inherit Metab Dis (2016) 39:253 260 DOI 10.1007/s10545-015-9889-6 ORIGINAL ARTICLE Quality of life and participation in daily life of adults with Pompe disease receiving enzyme replacement therapy: 10
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Leber congenital amaurosis OMIM number for disease 204000 Disease alternative
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Leber congenital amaurosis OMIM number for disease 204000 Disease alternative
Phenotypical variation within 22 families with Pompe disease
 Wens et al. Orphanet Journal of Rare Diseases 2013, 8:182 RESEARCH Phenotypical variation within 22 families with Pompe disease Stephan C A Wens 1,2, Carin M van Gelder 2,3, Michelle E Kruijshaar 2, Juna
Wens et al. Orphanet Journal of Rare Diseases 2013, 8:182 RESEARCH Phenotypical variation within 22 families with Pompe disease Stephan C A Wens 1,2, Carin M van Gelder 2,3, Michelle E Kruijshaar 2, Juna
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Loeys-Dietz Syndrome OMIM number for disease 609192; 608967; 610380; 610168 Disease
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name Loeys-Dietz Syndrome OMIM number for disease 609192; 608967; 610380; 610168 Disease
Echocardiographic Findings in Pompe s Disease with Left Ventricular Obstruction
 Clin. Cardiol. 8, 181-185 (1985) 0 Clinical Cardiology Publishing Co., Inc. Echocardiographic Findings in Pompe s Disease with Left Ventricular Obstruction Y. SHAPIR, M.D., N. ROGUIN, M.D Department of
Clin. Cardiol. 8, 181-185 (1985) 0 Clinical Cardiology Publishing Co., Inc. Echocardiographic Findings in Pompe s Disease with Left Ventricular Obstruction Y. SHAPIR, M.D., N. ROGUIN, M.D Department of
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Highly Specialised Technology Evaluation
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Highly Specialised Technology Evaluation Drisapersen for treating Duchenne muscular Draft scope (pre-referral) Draft remit/evaluation objective
Cigna Drug and Biologic Coverage Policy
 Cigna Drug and Biologic Coverage Policy Subject Nusinersen Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 5 References... 5 Effective Date... 10/15/2017 Next
Cigna Drug and Biologic Coverage Policy Subject Nusinersen Table of Contents Coverage Policy... 1 General Background... 2 Coding/Billing Information... 5 References... 5 Effective Date... 10/15/2017 Next
NIH Public Access Author Manuscript Neurotherapeutics. Author manuscript; available in PMC 2009 October 14.
 NIH Public Access Author Manuscript Published in final edited form as: Neurotherapeutics. 2008 October ; 5(4): 569 578. doi:10.1016/j.nurt.2008.08.009. Therapeutic approaches in Glycogen Storage Disease
NIH Public Access Author Manuscript Published in final edited form as: Neurotherapeutics. 2008 October ; 5(4): 569 578. doi:10.1016/j.nurt.2008.08.009. Therapeutic approaches in Glycogen Storage Disease
Case 1 A 65 year old college professor came to the neurology clinic referred by her family physician because of frequent falling. She had a history of
 Peripheral Nervous System Case 1 A 65 year old college professor came to the neurology clinic referred by her family physician because of frequent falling. She had a history of non-insulin dependent diabetes
Peripheral Nervous System Case 1 A 65 year old college professor came to the neurology clinic referred by her family physician because of frequent falling. She had a history of non-insulin dependent diabetes
Sialic Acid Storage Diseases
 Sialic Acid Storage Diseases Class: BIOL 10001 Instructor: Dr. Vivegananthan Submitted by: Lyndsay Grover Date Submitted: Thursday March 24 th, 2011 Introduction to Sialic Acid Storage Diseases Sialic
Sialic Acid Storage Diseases Class: BIOL 10001 Instructor: Dr. Vivegananthan Submitted by: Lyndsay Grover Date Submitted: Thursday March 24 th, 2011 Introduction to Sialic Acid Storage Diseases Sialic
A Randomized Study of Alglucosidase Alfa in Late-Onset Pompe s Disease
 The new england journal of medicine original article A Randomized Study of Alglucosidase Alfa in Late-Onset Pompe s Disease Ans T. van der Ploeg, M.D., Ph.D., Paula R. Clemens, M.D., Deyanira Corzo, M.D.,
The new england journal of medicine original article A Randomized Study of Alglucosidase Alfa in Late-Onset Pompe s Disease Ans T. van der Ploeg, M.D., Ph.D., Paula R. Clemens, M.D., Deyanira Corzo, M.D.,
1/28/2019. OSF HealthCare INI Care Center Team. Neuromuscular Disease: Muscular Dystrophy. OSF HealthCare INI Care Center Team: Who are we?
 Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
Neuromuscular Disease: Muscular Dystrophy Muscular Dystrophy Association (MDA) and OSF HealthCare Illinois Neurological Institute (INI) Care Center Team The Neuromuscular clinic is a designated MDA Care
The c.65-2a>g splice site mutation is associated with a mild phenotype in Danon disease due to the transcription of normal LAMP2 mrna
 Clin Genet 2016: 90: 366 371 Printed in Singapore. All rights reserved Short Report 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd CLINICAL GENETICS doi: 10.1111/cge.12724 The c.65-2a>g
Clin Genet 2016: 90: 366 371 Printed in Singapore. All rights reserved Short Report 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd CLINICAL GENETICS doi: 10.1111/cge.12724 The c.65-2a>g
Childhood Pompe disease: clinical spectrum and genotype in 31 patients
 van Capelle et al. Orphanet Journal of Rare Diseases (2016) 11:65 DOI 10.1186/s13023-016-0442-y RESEARCH Open Access Childhood Pompe disease: clinical spectrum and genotype in 31 patients C. I. van Capelle
van Capelle et al. Orphanet Journal of Rare Diseases (2016) 11:65 DOI 10.1186/s13023-016-0442-y RESEARCH Open Access Childhood Pompe disease: clinical spectrum and genotype in 31 patients C. I. van Capelle
Case Report Anomalous Left Main Coronary Artery: Case Series of Different Courses and Literature Review
 Case Reports in Vascular Medicine Volume 2013, Article ID 380952, 5 pages http://dx.doi.org/10.1155/2013/380952 Case Report Anomalous Left Main Coronary Artery: Case Series of Different Courses and Literature
Case Reports in Vascular Medicine Volume 2013, Article ID 380952, 5 pages http://dx.doi.org/10.1155/2013/380952 Case Report Anomalous Left Main Coronary Artery: Case Series of Different Courses and Literature
A Lawyer s Perspective on Genetic Screening Performed by Cryobanks
 A Lawyer s Perspective on Genetic Screening Performed by Cryobanks As a lawyer practicing in the area of sperm bank litigation, I have, unfortunately, represented too many couples that conceived a child
A Lawyer s Perspective on Genetic Screening Performed by Cryobanks As a lawyer practicing in the area of sperm bank litigation, I have, unfortunately, represented too many couples that conceived a child
SCOTTISH MUSCLE NETWORK DUCHENNE MUSCULAR DYSTROPHY TRANSITION SOME USEFUL THINGS TO KNOW ABOUT HEALTH AROUND ADOLESCENCE
 SCOTTISH MUSCLE NETWORK DUCHENNE MUSCULAR DYSTROPHY TRANSITION SOME USEFUL THINGS TO KNOW ABOUT HEALTH AROUND ADOLESCENCE 02 Changes in our lives can be stressful and leaving school and moving into the
SCOTTISH MUSCLE NETWORK DUCHENNE MUSCULAR DYSTROPHY TRANSITION SOME USEFUL THINGS TO KNOW ABOUT HEALTH AROUND ADOLESCENCE 02 Changes in our lives can be stressful and leaving school and moving into the
Agent(s) FDA Labeled Indications Dosage and Administration. Perinatal/Infantile-onset hypophosphatasia. Juvenile-onset hypophosphatasia
 Strensiq (asfotase alfa) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE Agent(s) FDA Labeled Indications Dosage and Administration Strensiq (asfotase alfa) injection Perinatal/Infantile-onset
Strensiq (asfotase alfa) Prior Authorization Program Summary FDA APPROVED INDICATIONS DOSAGE Agent(s) FDA Labeled Indications Dosage and Administration Strensiq (asfotase alfa) injection Perinatal/Infantile-onset
Fatty Acid Oxidation Disorders
 Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Genetic Fact Sheets for Parents Fatty Acid Oxidation Disorders Screening, Technology, and Research in Genetics is a multi-state project to improve information about the financial, ethical, legal, and social
Neuromuscular in the Pediatric Clinic: Recognition and Referral
 Neuromuscular in the Pediatric Clinic: Recognition and Referral Matthew Harmelink, MD Assistant Professor, Pediatric Neurology Medical College of Wisconsin Objectives: 1. Understand common presentations
Neuromuscular in the Pediatric Clinic: Recognition and Referral Matthew Harmelink, MD Assistant Professor, Pediatric Neurology Medical College of Wisconsin Objectives: 1. Understand common presentations
Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy
 DOI 10.1007/s10545-011-9404-7 ORIGINAL ARTICLE Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy C. M. van Gelder
DOI 10.1007/s10545-011-9404-7 ORIGINAL ARTICLE Facial-muscle weakness, speech disorders and dysphagia are common in patients with classic infantile Pompe disease treated with enzyme therapy C. M. van Gelder
Cardiac Disease in Organic Acidemias
 Cardiac Disease in Organic Acidemias Kathryn Chatfield, MD, PhD Assistant Professor of Pediatrics Division of Cardiology University of Colorado School of Medicine Children s Hospital Colorado Introduction
Cardiac Disease in Organic Acidemias Kathryn Chatfield, MD, PhD Assistant Professor of Pediatrics Division of Cardiology University of Colorado School of Medicine Children s Hospital Colorado Introduction
Cost-effectiveness of sebelipase alfa (Kanuma ) for the treatment of lysosomal acid lipase (LAL) deficiency infantile paediatric adult
 Cost-effectiveness of sebelipase alfa (Kanuma ) for the treatment of lysosomal acid lipase (LAL) deficiency The NCPE has issued a recommendation regarding the cost-effectiveness of sebelipase alfa (Kanuma
Cost-effectiveness of sebelipase alfa (Kanuma ) for the treatment of lysosomal acid lipase (LAL) deficiency The NCPE has issued a recommendation regarding the cost-effectiveness of sebelipase alfa (Kanuma
The role of the laboratory in diagnosing lysosomal disorders
 The role of the laboratory in diagnosing lysosomal disorders Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. Lysosomal disorders What
The role of the laboratory in diagnosing lysosomal disorders Dr Guy Besley, formerly Willink Biochemical Genetics Unit, Manchester Children s Hospital, Manchester M27 4HA, UK. Lysosomal disorders What
Primary Periodic Paralysis. A Complex Disorder. A Challenging Diagnosis.
 Primary Periodic Paralysis A Complex Disorder. A Challenging Diagnosis. A Complex Disorder A Group of Rare Channelopathies With Varying Subtypes and Triggers Primary periodic paralysis causes recurrent,
Primary Periodic Paralysis A Complex Disorder. A Challenging Diagnosis. A Complex Disorder A Group of Rare Channelopathies With Varying Subtypes and Triggers Primary periodic paralysis causes recurrent,
Hypotonia Care Pathway Update: Diagnosis Garey Noritz, MD...
 Hypotonia Care Pathway Update: Diagnosis Garey Noritz, MD Disclosure Information- AACPDM 72 nd Annual Meeting October 9-13, 2018 Speaker Name: Garey Noritz, MD Disclosure of Relevant Financial Relationships
Hypotonia Care Pathway Update: Diagnosis Garey Noritz, MD Disclosure Information- AACPDM 72 nd Annual Meeting October 9-13, 2018 Speaker Name: Garey Noritz, MD Disclosure of Relevant Financial Relationships
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier
 Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names Osteogenesis Imperfecta
Proposal form for the evaluation of a genetic test for NHS Service Gene Dossier Test Disease Population Triad Disease name and description (please provide any alternative names Osteogenesis Imperfecta
variant led to a premature stop codon p.k316* which resulted in nonsense-mediated mrna decay. Although the exact function of the C19L1 is still
 157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
Hypophosphatasia. Tom Blevins, MD Texas Diabetes and Endocrinology Austin, TX
 Hypophosphatasia Tom Blevins, MD Texas Diabetes and Endocrinology Austin, TX Hypophosphatasia Case Teeth 57 y/o male with a hyperlipidemia and hypogonadism. Noted to have an alkaline phosphatase of
Hypophosphatasia Tom Blevins, MD Texas Diabetes and Endocrinology Austin, TX Hypophosphatasia Case Teeth 57 y/o male with a hyperlipidemia and hypogonadism. Noted to have an alkaline phosphatase of
PAEDIATRIC EMQs. Andrew A Mallick Paediatrics.info.
 PAEDIATRIC EMQs Andrew A Mallick Paediatrics.info www.paediatrics.info Paediatric EMQs Paediatrics.info First published in the United Kingdom in 2012. While the advice and information in this book is believed
PAEDIATRIC EMQs Andrew A Mallick Paediatrics.info www.paediatrics.info Paediatric EMQs Paediatrics.info First published in the United Kingdom in 2012. While the advice and information in this book is believed
Spinal Muscular Atrophy in 2017
 Spinal Muscular Atrophy in 2017 Leigh Maria Ramos-Platt, MD Children s Hospital of Los Angeles University of Southern California Keck School of Medicine Disclosures MDA Care Center Grant In this presentation,
Spinal Muscular Atrophy in 2017 Leigh Maria Ramos-Platt, MD Children s Hospital of Los Angeles University of Southern California Keck School of Medicine Disclosures MDA Care Center Grant In this presentation,
Muscle Pathology Surgical Pathology Unknown Conference. November, 2008 Philip Boyer, M.D., Ph.D.
 Muscle Pathology Surgical Pathology Unknown Conference November, 2008 Philip Boyer, M.D., Ph.D. Etiologic Approach to Differential Diagnosis Symptoms / Signs / Imaging / Biopsy / CSF Analysis Normal Abnormal
Muscle Pathology Surgical Pathology Unknown Conference November, 2008 Philip Boyer, M.D., Ph.D. Etiologic Approach to Differential Diagnosis Symptoms / Signs / Imaging / Biopsy / CSF Analysis Normal Abnormal
Newborn Screening Study for AADC Deficiency Published. in Molecular Genetics and Metabolism
 Newborn Screening Study for AADC Deficiency Published in Molecular Genetics and Metabolism Study highlights frequency of AADC deficiency Cambridge, MA, June 2, 2016, 7:30 am EST - Agilis Biotherapeutics
Newborn Screening Study for AADC Deficiency Published in Molecular Genetics and Metabolism Study highlights frequency of AADC deficiency Cambridge, MA, June 2, 2016, 7:30 am EST - Agilis Biotherapeutics
Case Report Sinus Venosus Atrial Septal Defect as a Cause of Palpitations and Dyspnea in an Adult: A Diagnostic Imaging Challenge
 Case Reports in Medicine Volume 2015, Article ID 128462, 4 pages http://dx.doi.org/10.1155/2015/128462 Case Report Sinus Venosus Atrial Septal Defect as a Cause of Palpitations and Dyspnea in an Adult:
Case Reports in Medicine Volume 2015, Article ID 128462, 4 pages http://dx.doi.org/10.1155/2015/128462 Case Report Sinus Venosus Atrial Septal Defect as a Cause of Palpitations and Dyspnea in an Adult:
TEST INFORMATION Test: CarrierMap GEN (Genotyping) Panel: CarrierMap Expanded Diseases Tested: 311 Genes Tested: 299 Mutations Tested: 2647
 Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
Enzyme-Replacement Thera Infantile Pompe Disease:
 Enzyme-Replacement Thera Infantile Pompe Disease: in Classic Long-term outcomej dosingj and the role of antibodies Carin M. van Gelder Financial support for this thesis was obtained from ZonMw (the Netherlands
Enzyme-Replacement Thera Infantile Pompe Disease: in Classic Long-term outcomej dosingj and the role of antibodies Carin M. van Gelder Financial support for this thesis was obtained from ZonMw (the Netherlands
Cardiorespiratory changes in progressive
 British Heart Journal, 197I, 33, 533-537. Cardiorespiratory changes in progressive muscular dystrophy P. L. Wahi, K. C. Bhargava, and S. Mohindra From the Department of Medicine, Postgraduate Institute
British Heart Journal, 197I, 33, 533-537. Cardiorespiratory changes in progressive muscular dystrophy P. L. Wahi, K. C. Bhargava, and S. Mohindra From the Department of Medicine, Postgraduate Institute
