Finite Element Analysis of Patient-Specific Mitral Valve with Mitral Regurgitation
|
|
|
- Owen Paul
- 5 years ago
- Views:
Transcription
1 Finite Element Analysis of Patient-Specific Mitral Valve with Mitral Regurgitation Pham Thuy, Georgia Institute of Technology Fanwei Kong, Georgia Institute of Technology Caitlin Martin, Georgia Institute of Technology Qian Wang, Georgia Institute of Technology Charles Primiano, Hartford Hospital Raymond McKay, Hartford Hospital John Elefteriades, Yale New Haven Medical Center Wei Sun, Emory University Journal Title: Cardiovascular Engineering and Technology Volume: Volume 8, Number 1 Publisher: Springer Verlag (Germany) , Pages 3-16 Type of Work: Article Post-print: After Peer Review Publisher DOI: /s Permanent URL: Final published version: Copyright information: 2017, Biomedical Engineering Society. Accessed September 30, :59 AM EDT
2 Finite Element Analysis of Patient-Specific Mitral Valve with Mitral Regurgitation Thuy Pham 1, Fanwei Kong 1, Caitlin Martin 1, Qian Wang 1, Charles Primiano 2, Raymond McKay 2, John Elefteradies 3, and Wei Sun 1 1 Tissue Mechanics Laboratory, The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA 2 Cardiology Department of Hartford Hospital, Hartford, CT 3 Aortic Institute of Yale-New Haven Hospital, New Haven, CT Abstract HHS Public Access Author manuscript Published in final edited form as: Cardiovasc Eng Technol March ; 8(1): doi: /s Functional mitral regurgitation (FMR) is a significant complication of left ventricular (LV) dysfunction and strongly associated with a poor prognosis. In this study, we developed a patientspecific finite element (FE) model of the mitral apparatus in a FMR patient which included: both leaflets with thickness, annulus, chordae tendineae, and chordae insertions on the leaflets and origins on the papillary muscles. The FE model incorporated human age- and gender- matched anisotropic hyperelastic material properties, and MV closure at systole was simulated. The model was validated by comparing the FE results from valve closure simulation with the in vivo geometry of the MV at systole. It was found that the FE model could not replicate the in vivo MV geometry without the application of tethering pre-tension force in the chordae at diastole. Upon applying the pre-tension force and performing model optimization by adjusting the chordal length, position, and leaflet length, a good agreement between the FE model and the in vivo model was established. Not only were the chordal forces high at both diastole and systole, but the tethering force on the anterior papillary muscle was higher than that of the posterior papillary muscle, which resulted in an asymmetrical gap with a larger orifice area at the anterolateral commissure resulting in MR. The analyses further show that high peak stress and strain were found at the chordal insertions where large chordal tethering forces were found. This study shows that the pre-tension tethering force plays an important role in accurately simulating the MV dynamics in this FMR patient, particularly in quantifying the degree of leaflet coaptation and stress distribution. Due to the complexity of the disease, the patient-specific computational modeling procedure of FMR patients presented should be further evaluated using a large patient cohort. However, this study provides useful insights into the MV biomechanics of a FMR patient, and could serve as a tool to assist in pre-operative planning for MV repair or replacement surgical or interventional procedures. Correspondent author's contact information: Wei Sun, Ph.D., The Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Technology Enterprise Park, Room 206, 387 Technology Circle, Atlanta, GA , Phone: (404) , Fax: (404) , wei.sun@bme.gatech.edu. Conflicts of Interest: None. Ethical Approval: No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
3 Pham et al. Page 2 Keywords Multi-slice Computed Tomography; Mitral valve; Mitral Regurgitation; Chordae Tendineae; Finite Element Simulation; Patient-Specific 1. Introduction Functional mitral regurgitation (FMR) is a significant complication of left ventricular (LV) dysfunction and strongly associated with a poor prognosis in patients with heart failure. Ischemic mitral regurgitation (IMR) is a form of FMR that results from ventricular abnormality while the mitral leaflets and subvavular apparatus are normal. In IMR, pathological LV remodeling contributes to the apical, posterior, and lateral displacement of papillary muscles, with reduced LV contractility and abnormal annular contraction. The displacement of papillary muscles results in traction on the mitral leaflets and restriction on leaflet motion. Moreover, inadequate closure of the mitral valve (MV) contributes to increased tethering forces and reduced leaflet closing force 1,2. Severe FMR is associated with high risk of heart failure 3 and mortality 4. Currently FMR is typically treated through annuloplasty with an undersized ring, but mitral insufficiency can recur in a significant number of cases 5,6. Other treatments include surgical leaflet resection, chordal shortening or replacement, and edge-to-edge repair The highly complex structure and the interrelated function among the valve substructures, as well as the continuous remodeling mechanism of MR, make MV treatment challenging. Particularly for MV repair, the success of a technique may be associated with increased leaflet stress, mitral annulus force, and chordal forces Therefore, it is important to understand the in vivo mechanics and dynamics of the MV for MR patients, in order to assess the effectiveness of surgical repair strategies and determine the optimal strategy available for specific patients. Computational simulations using numerical analysis methods have been useful in assessing the MV mechanics under normal and diseased conditions as well as evaluating surgical MV repair techniques. A number of studies compared different geometries of annuloplasty rings on MR models. Recent advancements in clinical imaging modalities have enabled the development of patient-specific models. MV repair by annuloplasty and MitraClip intervention was studied using patient-specific MV models derived from 3D transesophageal echocardiography (TEE) data However, due to the limited resolution of TEE data, the locations of chordal insertion points and single-point chordal origins on the papillary muscles in these models were assumed. Our recent study has shown that papillary muscle and mitral annulus motions from diastole to systole are essential to accurately simulate leaflet closing behaviors in healthy patient-specific MV models 22. Realistic chordae and leaflet reconstruction has been achieved based on Micro-CT, but Micro-CT cannot be applied in clinical settings 23. Hence, in this study, we reconstructed a patient-specific MV computational model from CT image data for a MR patient which includes the motion of the chordal origins and annulus from diastole to systole, as well as accurate leaflet geometry and thickness.
4 Pham et al. Page 3 Despite the presence of traction on the MV leaflets caused by displacement of the papillary muscles in FMR, the initial geometries, constructed from clinical images, were assumed stress-free in previous studies In this study, we introduced the tethering pre-tension force, the tension existing in the chordae tendineae prior to systole, which plays an important role in accurately simulating the MV dynamics in this FMR patient. Our model incorporated age- and gender- matched anisotropic hyperelastic material models to accurately describe the MV tissue behavior, and the model was validated by comparing the FE results with the in vivo CT images at systole. MV measurements and forces generated during diastole and systole were obtained and compared to the literature. 2. Patient and Methods 2. 1 Patient Information Full-phase cardiac multi-slice Computed Tomography (MSCT) scans of a 71 year old male patient were collected from Hartford Hospital (Hartford, CT). The use of de-identified patient imaging data for this study was approved by the Institutional Review Board at Hartford Hospital (Hartford, CT). The patient was referred for transcatheter aortic valve replacement (TAVR) due to severe aortic stenosis and was also diagnosed with severe MR from Echocardiogram images. Fig. 1A shows the MR jet during systole. The MV leaflets appeared to be mildly thickened. Particularly, the posterior mitral leaflet appeared abnormal it was tented apically and stationary in systole (Fig. 1B) and diastole (Fig. 1C). From the M-mode (motion mode) ultrasound images the restricted posterior leaflet motion and reduced leaflet copatation was visualized. The LV thickness was normal, but the chamber was dilated with severe regional global hypokinesis, such that the inferior wall was akinetic. The left ventricular ejection fraction (LVEF) was estimated to be 25%, and the LV systolic pressure was 114 mmhg at the time of imaging. The MR radius measured by proximal isovelocity surface area (PISA) was 0.6 cm. The MR Alias velocity was 38.5 cm/s. The left ventricular outflow tract (LVOT) cross-sectional area was 3.8 cm 2 and the stroke volume from the apical 2-chamber view was 33 ml Model reconstruction CT images Full-volume CT image data with ECG gating were acquired with a GE LightSpeed 64-channel volume CT scanner, with a collimation of mm. The rotation time of 375 ms resulted in a temporal resolution of less than 200 ms depending on the heart rate and pitch. A total of 2,430 images were collected and reconstructed into 10 phases throughout a cardiac cycle. The images from both middle diastole and middle systole phases were used in the analysis, and will be referred to as simply diastole and systole in the subsequent text. Image segmentation was performed using Avizo (Version 8.0, Burlington, MA) software, where the anterior mitral leaflet (AML), posterior mitral leaflet (PML), mitral annulus (MA), chordae tendineae (chords), papillary muscles (PPMs), chordae insertion points and chordae origins were visualized, identified, and manually segmented 22. The surface geometries in both diastolic and systolic phases were then imported into HyperMesh (Altair Engineering, Inc., MI) software for 3D mesh generation.
5 Pham et al. Page 4 Mitral annulus and leaflets The aorta-mitral fibrous region of the anterior annulus was identified as the junction between the aortic leaflet and the AML, and between the left and right trigones. The posterior annulus was identified at the hinge point of the PML, separating the left atrium from the LV. The anterolateral and posteromedial commissural leaflets were partially visible in CT images; thus, spline surfaces were used to generate the complete geometries of these leaflets. Two layers of solid elements (eight-node hexahedral C3D8I and six-node tetrahedral C3D6 elements) were used to model the leaflets. The morphology of the leaflet was clearly visible, thus, the entire leaflet thickness was traced and measured. For the AML, the average thickness values for the leaflet belly and edge were 1.26 mm and 2.09 mm respectively. For the PML, the average thickness values of the leaflet belly and edge were 1.31 mm and 1.57 mm respectively. The leaflet belly and edge regions were identified as previously described 24. Chordae Tendineae A total of 24 chords were visible on CT images. Chords were classified into five groups 22,25 : anterior strut (AS), anterior basal (AB), anterior marginal (AM), posterior basal (PB), and posterior marginal (PM). Chords originated from the anterolateral and posterolateral papillary muscles and inserted into the ventricular surfaces of the leaflets. Contraction of the LV also triggers the contraction of the papillary muscles. Thus, the locations of the chordal insertions and origins change from one phase to another. The chordal insertions and origins were therefore identified in both diastolic and systolic phases and their dynamic motion was accounted for in the simulation. The basal chordal insertion points on the PML were not visible in MSCT images due to the severe tethering, therefore the initial locations were approximated based on published data 25,26. The total of 18 chordal origins could be detected. The chordal distribution is shown in Table 1. The chords were modeled using 3D stress/displacement truss elements (two-node linear T3D2 elements), with cross-sectional area values of 0.71 mm 2, 2.05 mm 2, and 0.38 mm 2 assigned to the basal, strut, and marginal chordae, respectively 27. Branching of the chordae at the insertion points on the leaflets was modeled by creating fork-shaped truss elements, to avoid stress concentration on the leaflets. Each chord was modeled with up to 10 elements with average element length of 1.5 mm Constitutive Modeling of Mitral Tissues The material properties of the MV apparatus were obtained from an age- and gendermatched cadaver heart, i.e., 69 year old patient, from our previously published studies of human MV leaflets 24 and chordae tendineae 27 material characterization. Figure 2 shows the stress-strain curves from an equibiaxial protocol of the AML and PML leaflets in both directions and of four chords (AB was assumed to be equivalent to PB). An anisotropic hyperelastic material model was adopted to characterize the mechanical behaviors of the mitral leaflet tissues. The model is based on the fiber-reinforced hyperelastic material model proposed by Holzapfel et al 28,29. Briefly, the mitral tissues were assumed to be composed of a matrix material with two families of embedded fibers, each with a preferred direction. The fiber directions can be mathematically described using two unit vectors. The strain invariant I 1 is used to describe the matrix material; the strain invariant I 4i is used to describe the
6 Pham et al. Page 5 properties of the fiber families and equals to the squares of the stretches in the fiber directions. The strain energy function W can be expressed as where, C 10, C 01, k 1, k 2 and D are material constants. C 10 and C 01 are used to describe the matrix material. D is the material constant that introduces the near incompressibility, while k 1 is a positive material constant with the dimensions of stress and k 2 is a dimensionless parameter. In addition, a dispersion parameter κ was used to describe the distribution of fiber orientation. When κ=0, the fibers are perfectly aligned (no dispersion). When κ=1/3, the fibers are randomly distributed and the material becomes isotropic. The anisotropic hyperelastic material model was implemented into Abaqus 6.13(SIMULIA, Providence, RI) with a user sub-routine VUMAT 30. Details on the model implementation were previously published by our group 22,30,31. Local coordinate systems were defined for each leaflet to include fiber orientations for each region. The isotropic hyperelastic Ogden material model was used to characterize the mechanical properties of chords based on the experimental data 27. The material constants were determined using SYSTAT (Systat Software Inc., Chicago, IL) with the Marquardt- Levenberg algorithm to fit the uniaxial test results of chordae tendineae to the Ogden model. Table 2 lists the patient information and the fitted material parameters for the mitral tissues. A material sensitivity analysis was performed to investigate the effect of different material properties on MV response. The material properties of the MV leaflets and chordae were obtained from our previous publication 24,27. These material properties were either more compliant or stiffer than the one with age- and gender-matched, to observe whether the extremities would affect the results Boundary and loading conditions The motion and dynamics of the MV during closing was simulated in two steps. In the first step, the dynamic motions of the mitral annulus from diastole to systole were simulated by imposing kinematic displacement of the nodes along the mitral annulus. The chordal origin nodes were also displaced between the two phases. In the second step, time-varying physiological transvalvular pressure loading, with a maximum systolic pressure of 114 mmhg, was applied on the ventricular surfaces of the AML and PML. All simulations were performed in ABAQUS Explicit (Version 6.13) Model Optimization and Validation To validate the FE model accuracy, the distance error between the deformed FE model and the true image model obtained from systolic MSCT images was computed in MATLAB. (1)
7 Pham et al. Page 6 The distance errors were obtained by first projecting the deformed (systolic) FE model on to the true image model, then the distance error could be computed as the point-to-mesh distance between the nodes of the deformed FE mesh and their corresponding projected nodes on the true image model. A point-to-mesh distance greater than 2 mm was considered unacceptable and required further model adjustment. The method of calculating the distance error based on the point-to-mesh difference between the FE deformed and true image model has been recently published by our group in a previous publication 32. Due to the limitation of the CT image resolution, it was not possible to detect all of the chords or the accurate chordal length. Therefore, the number of chords and their lengths were estimated in the initial model and required optimization. The model was adjusted until the deformed FE model could closely match the true image model. Figure 4 illustrates the FE model optimization workflow, showing the FE simulation steps and criteria for model adjustment. If the surface of the valve leaflets were protruding towards the left atrium in FE deformed model at systole, with distance errors larger than 2 mm, the chords in the vicinity were shortened. If a chord was excessively tensioned and stretched the leaflet, it was then elongated. The chordal lengths were adjusted while keeping the insertion and origin points unchanged. After 20+ iterations, the length of the chords had been minimized, as shown in Fig. 5A, yet several regions on the deformed FE model still did not match the true image model, particularly the belly region of the AML, the entire PML, and the two commissural leaflets, see Fig. 5C & 5D. The point-to-mesh distance map in Fig. 5B shows the regions with straightened chords that exhibited errors greater than 2 mm. These large errors indicated that adjusting the chordal length alone was insufficient to accurately simulate the systolic MV deformation. Therefore, an existing chordal tethering force at diastole due to dilatation of the LV in this FMR patient, which could further restrict the leaflet motion, was assumed. This pre-existing force was generated by shortening the length of the tethered chords by 1.99 mm on average in a total of 8 chords. The chordal origins were translated towards the annulus plane along the vector going from the chordal origin point to the insertion point by the magnitude of the chordal shortening. Hence, an additional step of pretension was added to the simulation process, where the translated origins were displaced from their adjusted locations to their original locations identified from MSCT to generate the pre-tension in the chords, see Fig. 4. Approximately 50 iterations were performed to optimize the chordal lengths and degree of pre-tension. The application of pre-tension pulled and elongated the PML towards the papillary muscles. Therefore, the PML in deformed FE model was longer than in the true image model. In order to compensate for the post-pretension leaflet elongation, the degree of radial elongation throughout the PML was measured, and the un-deformed PML geometry was then shrunk radially by the same elongation ratio. After this adjustment, the FE deformed PML geometry could match that obtained from in vivo MSCT images at diastole. The PML thickness at the P2 section before and after the leaflet shrinking was 1.32 and 1.17, respectively, which is within the CT image resolution of mm. Therefore, we ignored the leaflet thickness change.
8 Pham et al. Page Data analysis 3. Results MV measurements in diastole and systole were made from the FE and true image. The anteroposterior (AP) distance was measured as the distance between the midpoint of the intertrigonal region and the midpoint of the posterior annulus 22. The intercommissural (IC) distance was measured as the distance between the two commissure leaflets. The distances between the PM tips were measured in both the apical-base and lateral-medial directions. The total mitral annular perimeter and area and the lengths of the anterior and posterior annuli were measured. The coaptation depth and length were calculated as the perpendicular distance from the annular plane down to the coaptation point, and from the coaptation point to the leaflet edges, respectively 33. The annular height was measured as the maximal vertical distance between the highest (anterior or posterior) and lowest (anterolateral or posteromedial) points 32. The systolic MV orifice areas for both the FE deformed and true image model were measured and compared. The tenting area and volume during mid systole were also measured during systole 34,35. The reaction force at the chordal origins, as well as the stress and strain on the leaflets were output from the FE simulation Patient valve geometry measurements Table 3 displays the MV measurements obtained from the MSCT images at diastole and systole. The measurements were compared to those in literature for normal and FMR patients. At diastole, the anterior and posterior mitral leaflets areas in the FE model were mm 2 and mm 2 respectively. The maximum radial lengths of the AML and PML were 30.0 mm and 15.4 mm, respectively. The typical saddle-shaped annulus was not present in this model. In contrast, the annulus appeared almost flat, with little change in the annular height from diastole to systole. Figure 3 displays the division of the mitral valve leaflets into three regions, where 1 is the anterolateral, 2 is the middle, and 3 is the posteromedial section. The table in Fig. 3 includes the tenting angle of the leaflets during systole. Large PML tenting angles of 53 and 73 degrees at P1 and P2 respectively, prevented coaptation with the AML at the leaflet free-edge. Distortion of the papillary muscle geometries was evident (Table 3): the distance between the APM and the left trigone of mm was greater than the distance between the PPM and the right trigone of mm. These values, especially the APM to left trigone distance, deviated from the normal ranges measured experimentally, where the APM to left trigone distance is 23.5 ± 3.7 mm and the PPM to right trigone distance is 23.5 ± 4.0 mm 36. The distance between the APM and PPM was laterally and 0.14 apically at diastole, and mm laterally and 0.42 mm apically in systole FE Simulation Results Figure 6 shows the final optimized geometries of the MV after performing chordal optimization, applying chordal pre-tension, applying annulus and papillary boundary conditions, and applying systolic pressure. The atrial, sagittal and isometric views of the valve in Figs. 6A to C demonstrated a good agreement between the FE deformed MV and the true image model at systole. During peak systole, a large regurgitant orifice was visible at the P1 and partial P2 regions. Figure 6D displays an overlay of the 2D projections of the
9 Pham et al. Page 8 regurgitant orifice in the FE deformed and true image models at systole. The 2D areas from the FE model and true image model were and mm 2, respectively. At diastole, the total chordal tethering forces were and 2.70 N for APM and PPM, respectively. At the peak systolic pressure, the total forces on the APM and PPM were 9.41 N and 4.50 N, respectively. The average systolic chordal tension forces were compared to in vitro force data obtained from Jimenez et al. 37 in Table 4. The mean point-to-mesh distance between the FE deformed model and the true image model was 0.96 ± 0.82 mm, indicating a good agreement between the two models. The model can capture most of the AML leaflet deformation, from the fibrosa toward the belly region. Larger errors between 2 and 4 mm were found along the free edge of the commissure leaflets. Most regions of the PML were well captured including the tethered regions of P1 and partial P2, with less than 1 mm errors. The largest error was located at the free edge region of the posteromedial commissure leaflet (P3). Figure 7 shows the stress and strain distributions of the MV components at peak systolic pressure. After removing the 1% peak stress 38, the maximum principal stress (MPS) of the AML was 1.62 MPa at the left and right trigone regions on the ventricular surface of AML. On the AML leaflet, high stresses were also distributed along the fibrosa region towards the strut chord insertions. The MPS of the PML was 1.51 MPa, and similar to the AML, was located at the P2 annular region. The maximum principal strain (MPe) for the AML was 0.40 at the trigones, and a high strain value of 0.28 was observed at the tethered chord insertion regions. The MPe for the PML was 0.36 at the tethered chord insertion regions, near the free-edge and basal locations. Overall, at the regions with significant tethering, stress and strain on PML (P1 and P2 regions) exceeded that of AML, for instance, peak values were ranging from 0.15 to 0.32 strain and 0.22 to 1.51 MPa stress compared to 0.03 to 0.15 strain and to 0.53 MPa on AML. High stress and large stretch on PML correspond to the phenomenon of posterior leaflet motion restriction observed clinically. The stress-strain curves in Fig. 8a & b show that for AML the Material 3 (blue) was much softer than the original Material 1 (red) in both circumferential and radial directions, and the Material 2 (green) was stiffer than the original Material 1 in the radial direction but comparable in the circumferential direction. Similarly, the PML tissue properties of Material 3 were softer in both directions, and the Material 2 was stiffer in the radial direction. The simulation results in Fig. 8c show that without applying pretension tethering forces, there was more coaptation at the regurgitant zone the leaflets were almost closed in the original and Material 3 models. With pretension, as shown in Fig. 8d, the Material 2 exhibited a larger gap, whereas smaller gap was observed in the softer Material 3 model. Furthermore, as shown in Fig. 8e & f, the effects of varying material properties were negligible for PML where there was no change in distance error among the three models. For AML, however, the softer Material 3 exhibited a largest mean distance error compared to others. The difference in distance error between with pretension (yellow) and without pretension (red) was more pronounced in PML compared to AML. The systolic chordal forces of the models with stiffer and softer materials were comparable to that of the original model. The chordal
10 Pham et al. Page 9 4. Discussion systolic forces on APM were 9.41 N, N, and 8.07 N for the original, stiffer and softer materials, respectively. The chordal systolic forces on PPM were 4.50 N, 3.94 N and 4.77 N on PPM, for the original, stiffer and softer materials, respectively. However, the diastolic chordal forces showed an exceedingly large variation depending on material properties. Therefore, applying pretension on chords and leaflets were effective to mimic valve closure for a range of human material properties, whereas highly matched material properties are required to obtain reliable pretension chordal forces at diastole. In this study, we developed a patient-specific MV model of a patient with severe MR. Echo images showed the patient exhibited a large MR yet, restricted posterior leaflet motion and dilated LV. Both restricted PML and LV enlargement resulted in large PML angles at P1 and P2 locations. According to a study by Magne et al. 39, a patient with this large PML angles might have significant risk factors of recurrent MR and poor long-term outcomes after MV repair. The FE model developed in this study was able to accurately captured PML tethering by accounting for the pre-tension tethering forces in the chordae tendineae and the mitral leaflets. We observed high chordal forces on the tethering papillary muscles at both diastole and systole, as well as increased stress and strain on the tethering PML. Early experiments have suggested that tension on chordae tendineae and mitral leaflets existed not only at systole but also diastole 40,41. For MR patient, LV dilation and apical displacement of papillary muscles could cause more traction on leaflets and thus prevent proper coaptation. Systolic chordal forces as well as leaflet stress and strain values have been studied both experimentally and computationally by simulating an MR state of mitral valve 17,30,37,42. However, few studies have looked into the tension that exists in diastole in IMR patient due to papillary muscle traction, and MV models at diastole state have mostly been assumed stress free 17,19,30,43. For this patient, a lack of annulus and ventricular contraction, large tenting angle and area were observed from MSCT images, as well as restricted PML motion from diastole to systole. From our simulation, we found that ignoring the tension in chords and leaflets during diastole would overestimate the leaflet coaptation for this MR patient, and 84% larger in average point-to-mesh distance error compared to the model with pretension. The results indicated that the existing pre-tension force in chords and leaflets at diastole was essential in accurately replicating the in vivo MV geometries at systole. Altered balance between chordal tethering force and leaflet coaptation force upon transvalvular pressure during systole contributes to improper leaflet coaptation 44. We found a higher chordal tethering force on APM (16.30 N at diastole and 9.41 N at systole) than on PPM (2.70 N at diastole and 4.50 N at systole). The imbalanced force distribution between two PPMs contributed to the larger regurgitant gap on the anterolateral side in systole. Moreover, the tethering force, required in diastole in order to match with the true systole geometry obtained from in vivo MSCT scans, caused a posteriorly shifted coaptation line as well as large tenting angle on PML. We also observed a decreased in the total chordal force on APM from diastole to systole. That could be due to the reduced relative distance between the APM tip and annulus in systole, which was observed from the MSCT images for this
11 Pham et al. Page 10 patient. Despite of the lack of saddle shape, the annulus for this patient moved towards the apex in systole due to active contraction 37,40,41. The reduction of relative distance released the tension on chords and leaflets, counteracting the coaptation force due to pressure in systole. A material sensitivity analysis was performed using a stiffer set and a softer set of material properties. For each material set, the point-to-mesh distance errors from simulations with pretension and without pretension were compared. Increased distance errors were observed on models without pretention for all materials, together with incorrectly increased coaptation and leaflet protrusion toward the left atrium, see Figure 8. The systolic chordal forces were comparable with the original model (11.72 N and 8.07 N on APM; 3.94 N and 4.77 N on PPM, for stiffer and softer materials, respectively). However, the diastolic chordal force showed large variation depending on material properties. Therefore, applying pretension on chords and leaflets were effective to mimic valve closure for a range of human material properties, whereas highly matched material properties are required to obtain reliable pretension chordal forces at diastole. Although the leaflet and chordae tendineae materials were age- and gender- matched, they were not determined from the same MR patient. Therefore, we investigated the effects of different sets of material parameters on the patient MV function and on the pretension forces. We showed that different material properties showed different MV response. However, variation in material parameters were negligible in the affected tethering region, in which the PML of this model. Furthermore, distance errors were higher on models without pretention, incorrectly increased coaptation and leaflet protrusion towards the left atrium. A large difference in distance error between with pretension and without pretension on PML compared to AML also indicated more error could be obtained at the tethering region of the valve leaflets when pretension is not considered. Nevertheless, material properties determined inversely from CT images would be ideal to obtain accurate biomechanics data. We have demonstrated that pre-tension force existed within this MR patient who had LV dilation and leaflet tethering. However, we only analyzed one patient in this study. Functional MR is a complicated disease with a large spectrum of etiologies ranges from upper region of annular dilatation, leaflet alternation due to tethering, to lower left ventricular distortion. Thus, patient-specific computational modeling of MR patient, particularly functional MR group, need to be further studied and the modeling procedures should be further evaluated using a large patient cohort. Although chords were traced from MSCT images, due to limited resolution of the current cardiac images, image digitalization, segmentation and model optimization methods were utilized to adjust chordal length. The number of chords, chordal insertion and origins, and branching patterns were determined based on CT images which were insufficiently clear to capture detailed structure and prone to human digitization errors. Active stiffening of AML was not accounted for in our model, while modeling active muscle contraction may prevent billowing of AML and provide better match between the deformed geometry and the ground truth 45.
12 Pham et al. Page Conclusion Acknowledgments References This study shows that the incorporation of pre-tension tethering forces improved modeling accuracy and allowed evaluation of MV dynamic function. A computational method was carried out to optimize the chordal length in combination of adjusting the leaflet height to model the effect of pre-tension. The results obtained show that the simulated model can closely replicate the in vivo geometry of the MV at systole from MSCT images. We found that not only the chordal forces were high at both diastole and systole, the total chordal tethering force on the anterior papillary muscle was higher than that of the posterior papillary muscle, which resulted in an asymmetrical gap with larger orifice area at the anterolateral commissure that caused MR. The analyses show that high peak stress and strain were observed at the chordal insertions where large chordal forces were found. This study provides a quantitative evaluation of MV function in a FMR patient that could be useful in pre-procedural planning for an effective MV repair or replacement surgical or interventional treatment. Research for this project was funded in part by NIH HL and HL Fanwei Kong was also supported in part by the Petit Scholar program at Georgia Institute of Technology. 1. Iung B. Management of ischaemic mitral regurgitation. Heart. 2003; 89: [PubMed: ] 2. Piérard LA, Carabello BA. Ischaemic mitral regurgitation: pathophysiology, outcomes and the conundrum of treatment Trichon BH, Felker GM, Shaw LK, Cabell CH, O'Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. American Journal of Cardiology. 2003; 91: [PubMed: ] 4. Taramasso M, Maisano F. Valvular disease: Functional mitral regurgitation: should all valves be replaced? Nat Rev Cardiol. 2016; 13: [PubMed: ] 5. Tahta SA, Oury JH, Maxwell JM, Hiro SP, Duran CM. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis. 2002; 11: discussion [PubMed: ] 6. McGee EC Jr, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. The Journal of thoracic and cardiovascular surgery. 2004; 128: [PubMed: ] 7. Glower DD. Surgical Approaches to Mitral Regurgitation. Journal of the American College of Cardiology. 2012; 60: [PubMed: ] 8. Acker MA, Parides MK, Perrault LP, et al. Mitral-Valve Repair versus Replacement for Severe Ischemic Mitral Regurgitation. New England Journal of Medicine. 2014; 370: [PubMed: ] 9. Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. New England Journal of Medicine. 2016; 374: [PubMed: ] 10. Nicolini F, Agostinelli A, Vezzani A, et al. Surgical treatment for functional ischemic mitral regurgitation: current options and future trends. Acta Biomed. 2015; 86: [PubMed: ] 11. de Oliveira J, Antunes MJ. Mitral valve repair: better than replacement. Heart. 2006; 92: [PubMed: ]
13 Pham et al. Page Bothe W, Kvitting JP, Swanson JC, et al. How do annuloplasty rings affect mitral leaflet dynamic motion? Eur J Cardiothorac Surg. 2010; 38: [PubMed: ] 13. Jensen MO, Jensen H, Smerup M, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation. 2008; 118:S [PubMed: ] 14. Laing G, Dupont PE. Beating-heart Mitral Valve Chordal Replacement. Conference Proceedings. 2011; 2011: [PubMed: ] 15. Maisano F, Redaelli A, Soncini M, et al. An Annular Prosthesis for the Treatment of Functional Mitral Regurgitation: Finite Element Model Analysis of a Dog Bone Shaped Ring Prosthesis. The Annals of Thoracic Surgery. 2005; 79: [PubMed: ] 16. Kunzelman KS, Reimink MS, Cochran RP. Flexible versus rigid ring annuloplasty for mitral valve annular dilatation: a finite element model. J Heart Valve Dis. 1998; 7: [PubMed: ] 17. Votta E, Maisano F, Bolling SF, et al. The Geoform Disease-Specific Annuloplasty System: A Finite Element Study. The Annals of Thoracic Surgery. 2007; 84: [PubMed: ] 18. Wong VM, Wenk JF, Zhang Z, et al. The effect of mitral annuloplasty shape in ischemic mitral regurgitation: a finite element simulation. Ann Thorac Surg. 2012; 93: [PubMed: ] 19. Choi A, Rim Y, Mun JS, Kim H. A novel finite element-based patient-specific mitral valve repair: virtual ring annuloplasty. Biomed Mater Eng. 2014; 24: [PubMed: ] 20. Stevanella M, Maffessanti F, Conti C, et al. Mitral Valve Patient-Specific Finite Element Modeling from Cardiac MRI: Application to an Annuloplasty Procedure. Cardiovascular Engineering and Technology. 2011; 2: Mansi T, Voigt I, Georgescu B, et al. An integrated framework for finite-element modeling of mitral valve biomechanics from medical images: application to MitralClip intervention planning. Med Image Anal. 2012; 16: [PubMed: ] 22. Wang Q, Sun W. Finite element modeling of mitral valve dynamic deformation using patientspecific multi-slices computed tomography scans. Ann Biomed Eng. 2013; 41: [PubMed: ] 23. Lee, CH., Oomen, PJA., Rabbah, JP., et al. Functional Imaging and Modeling of the Heart: 7th International Conference, FIMH 2013, London, UK, June 20-22, 2013 Proceedings. Sébastien, OurselinDaniel, Rueckert, Nicolas, Smith, editors. Springer Berlin; Heidelberg: p Pham T, Sun W. Material Properties of Aged Human Mitral Valve Leaflets. J Biomed Mater Res A. 2014; 102: [PubMed: ] 25. Lam JH, Ranganathan N, Wigle ED, Silver MD. Morphology of the human mitral valve. I. Chordae tendineae: a new classification. Circulation. 1970; 41: [PubMed: ] 26. Wang Q, Sun W. Finite Element Modeling of Mitral Valve Dynamic Deformation Using Patient- Specific Multi-Slices Computed Tomography Scans. Ann Biomed Eng. 2013; 41: [PubMed: ] 27. Zuo K, Pham T, Li K, et al. Characterization of biomechanical properties of aged human and ovine mitral valve chordae tendineae. J Mech Behav Biomed Mater. 2016; 62: [PubMed: ] 28. Holzapfel GA, Gasser TC, Ogden RW. A New Constitutive Framework for Arterial Wall Mechanics and a Comparative Study of Material Models. Journal of elasticity and the physical science of solids. 2000; 61: Gasser TC, Ogden RW, Holzapfel GA. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. Journal of The Royal Society Interface. 2006; 3: Wang Q, Primiano C, Sun W. Can isolated annular dilatation cause significant ischemic mitral regurgitation? Another look at the causative mechanisms. Journal of Biomechanics. 2014; 47: [PubMed: ] 31. Sun W, Sacks MS. Finite element implementation of a generalized Fung-elastic constitutive model for planar soft tissues. Biomech Model Mechanobiol. 2005; 4: [PubMed: ] 32. Liang L, Kong F, Martin C, et al. Machine learning based 3-D geometry reconstruction and modeling of aortic valve deformation using 3-D computed tomography images. International Journal for Numerical Methods in Biomedical Engineering. 2016:n/a n/a.
14 Pham et al. Page Gogoladze G, Dellis SL, Donnino R, et al. Analysis of the mitral coaptation zone in normal and functional regurgitant valves. Ann Thorac Surg. 2010; 89: [PubMed: ] 34. Ciarka A, Braun J, Delgado V, et al. Predictors of Mitral Regurgitation Recurrence in Patients With Heart Failure Undergoing Mitral Valve Annuloplasty. The American journal of cardiology. 2010; 106: [PubMed: ] 35. Dudzinski DM, Hung J. Echocardiographic assessment of ischemic mitral regurgitation. Cardiovascular Ultrasound. 2014; 12:46. [PubMed: ] 36. Sakai T, Okita Y, Ueda Y, et al. Distance between mitral anulus and papillary muscles: anatomic study in normal human hearts. J Thorac Cardiovasc Surg. 1999; 118: [PubMed: ] 37. Jimenez JH, Soerensen DD, He Z, Ritchie J, Yoganathan AP. Mitral valve function and chordal force distribution using a flexible annulus model: an in vitro study. Ann Biomed Eng. 2005; 33: [PubMed: ] 38. Pham, TM., DeHerrera, M., Sun, W. Mechanics of Biological Systems and Materials, Volume 2: Proceedings of the 2011 Annual Conference on Experimental and Applied Mechanics. Tom, Proulx, editor. Springer; New York: p Magne J, Pibarot P, Dagenais F, et al. Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation. 2007; 115: [PubMed: ] 40. Rushmer RF, Finlayson BL, Nash AA. Movements of the mitral valve. Circ Res. 1956; 4: [PubMed: ] 41. Yellin EL, Peskin C, Yoran C, et al. Mechanisms of mitral valve motion during diastole. American Journal of Physiology - Heart and Circulatory Physiology. 1981; 241:H Jimenez JH, Soerensen DD, He Z, He S, Yoganathan AP. Effects of a saddle shaped annulus on mitral valve function and chordal force distribution: an in vitro study. Ann Biomed Eng. 2003; 31: [PubMed: ] 43. Mansi T, Voigt I, Assoumou Mengue E, et al. Towards patient-specific finite-element simulation of MitralClip procedure. Med Image Comput Comput Assist Interv. 2011; 14: [PubMed: ] 44. Nielsen SL, Nygaard H, Fontaine AA, et al. Chordal force distribution determines systolic mitral leaflet configuration and severity of functional mitral regurgitation. Journal of the American College of Cardiology. 1999; 33: [PubMed: ] 45. Skallerud B, Prot V, Nordrum IS. Modeling active muscle contraction in mitral valve leaflets during systole: a first approach. Biomechanics and Modeling in Mechanobiology. 2011; 10: [PubMed: ] 46. Kunzelman KS, Cochran RP, Verrier ED, Eberhart RC. Anatomic basis for mitral valve modelling. J Heart Valve Dis. 1994; 3: [PubMed: ] 47. Jimenez JH, Soerensen DD, He Z, Ritchie J, Yoganathan AP. Mitral Valve Function and Chordal Force Distribution Using a Flexible Annulus Model: An In Vitro Study. Annals of Biomedical Engineering. 2005; 33: [PubMed: ]
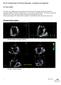
15 Pham et al. Page 14 Figure 1. Patient Echo images of the mitral valve at a) systole and b) diastole, showing the posterior leaflet (PML) is tenting, c) the Doppler Color Echo image displays a large MR jet during systole, d) 3D reconstructed Finite Element (FE) model of leaflets (red) and chordae tendineae (blue) from true CT images (yellow), and e) CT image of the valve during diastole, showing a tethered chord which can be captured in the FE model. AML Anterior Mitral Leaflet; PML Posterior Mitral Leaflet; PPM Posterior Papillary Muscle; APM Anterior Papillary Muscle; MD Middle Diastole; MSCT Multislice CT scan; MR Mitral Regurgitation.
16 Pham et al. Page 15 Figure 2. a) Stress-strain curves of anterior mitral (AML) and posterior mitral leaflet (PML) leaflets under equibiaxial stretch in both circumferential (CIRC) and radial (RAD) directions. The lines show the model fitting results comparing against experimental data; and b) Uniaxial stress-strain curves of anterior marginal (AM), anterior strut (AS), anterior basal (AB), posterior basal (PB) and posterior marginal (PM) chords, and the last data points (arrows) represent the ultimate failure strength.
17 Pham et al. Page 16 Figure 3. Leaflet tenting angles, areas and volumes at three regions, (1) anterolateral, (2) middle, and (3) posteromedial, during systole. Yellow lines indicates commissure clefts and red line is the middle point of the posterior annulus.
18 Pham et al. Page 17 Figure 4. Flow chart of the Finite Element model optimization.
19 Pham et al. Page 18 Figure 5. a) FE model after chordal length optimization at diastole; b) The distance error map between the deformed FE model and the true image model at middle systole; c) top and d) crosssectional views of deformed FE model overlapping with true image model from CT images at systole after applied the left ventricle pressure and mitral annulus and papillary muscle dynamic motions; the regions of unmatched geometries are labeled with asterisk, in the belly of anterior mitral leaflet (AML) and posterior mitral leaflet (PML).
20 Pham et al. Page 19 Figure 6. a) Atrial, b) sagittal and c) isometric views of the deformed FE model (orange for anterior mitral leaflet, light pink for posterior mitral leaflet, and dark red for anterior fibrosa) overlapped with the true image model from MSCT images (green) at middle systole, d) the projected orifice areas of the deformed FE model (orange) and middle-systolic geometry from MSCT images (green); e) atrial and f) ventricular surface views of the calculated distance error maps at middle systole.
21 Pham et al. Page 20 Figure 7. a) Stress and b) strain distributions of the optimized model of the mitral valve leaflets showing the atrial and ventricular surfaces and tethering locations on the posterior mitral leaflet. Black arrows show the stress at trigone regions. Red arrows show the stress at tethering regions.
22 Pham et al. Page 21 Figure 8. Stress-strain data of a) AML and b) PML leaflets from three patients under equibiaxial stretch in both circumferential (CIRC) and radial (RAD) directions; the deformed FE model c) with pretension and d) without pretension using the three different material parameter sets; d) the comparison of point-to-mesh distance error between the models with different material properties; e) the average distance errors of the deformed FE models with or without applying pre-tension
23 Pham et al. Page 22 Table 1 Number of chordal origins on each papillary muscle. Current FE Model Kunzelmen et al. 46 From anterolateral papillary muscle To anterior mitral leaflet ± 1.4 To posterior mitral leaflet ± 1.7 From posterolateral papillary muscle To anterior mitral leaflet ± 1.5 To posterior mitral leaflet ± 1.4
24 Pham et al. Page 23 Table 2 Material parameters for mitral leaflets and chordae tendineae. Mitral Leaflets C 01 K 1 k 2 C 10 Kappa Theta( O ) D R 2 AML e PML e Chordae Tendineae U1(Mpa) A1 U2(Mpa) A2 U3(Mpa) A3 R 2 AM AS PB PM AML Anterior Mitral Leaflet; PML Posterior Mitral Leaflet; AM Anterior marginal; AS Anterior strut; AB Anterior basal; PB Posterior basal; PM Posterior Marginal.
25 Pham et al. Page 24 Table 3 Mitral valve measurements and comparison to 3D Echocardiography by Grewal et al [1] and by Gogoladze et al. [2]. Raw MSCT images FE model (NP) FE model (P) Literature Unit (mm) Diastole Systole Systole Systole NORMAL Diastole [1] NORMAL Systole [1] NORMAL systole [2] FMR systole [2] Annular Perimeter ± ± ID ± ± AD ± ± ± ± 4.20 Intertrigonnal dist AH ± ± L C ± ± 2.2 D C ± ± 3.3 APM PPM distance Lateral Apical APM to left trigone PPM to right trigone ID Intercommissural Diameter, AD Anteroposterior Diameter; AH Annular Height; LC Coaptation Length; DC Coaptation Depth; NP No pretension, P Pretention.
Patient-Specific Computational Simulation of the Mitral Valve Function Using Three-Dimensional Echocardiography
 Patient-Specific Computational Simulation of the Mitral Valve Function Using Three-Dimensional Echocardiography Y. Rim 1, S. T. Laing 1, P. Kee 1, K. B. Chandran 2, D. D. McPherson 1 and H. Kim 1 1 Department
Patient-Specific Computational Simulation of the Mitral Valve Function Using Three-Dimensional Echocardiography Y. Rim 1, S. T. Laing 1, P. Kee 1, K. B. Chandran 2, D. D. McPherson 1 and H. Kim 1 1 Department
Effect of Congenital Anomalies of the Papillary Muscles on Mitral Valve Function
 J. Med. Biol. Eng. (2015) 35:104 112 DOI 10.1007/s40846-015-0011-1 ORIGINAL ARTICLE Effect of Congenital Anomalies of the Papillary Muscles on Mitral Valve Function Yonghoon Rim David D. McPherson Hyunggun
J. Med. Biol. Eng. (2015) 35:104 112 DOI 10.1007/s40846-015-0011-1 ORIGINAL ARTICLE Effect of Congenital Anomalies of the Papillary Muscles on Mitral Valve Function Yonghoon Rim David D. McPherson Hyunggun
Valve Analysis and Pathoanatomy: THE MITRAL VALVE
 : THE MITRAL VALVE Marc R. Moon, M.D. John M. Shoenberg Chair in CV Disease Chief, Cardiac Surgery Washington University School of Medicine, St. Louis, MO Secretary, American Association for Thoracic Surgery
: THE MITRAL VALVE Marc R. Moon, M.D. John M. Shoenberg Chair in CV Disease Chief, Cardiac Surgery Washington University School of Medicine, St. Louis, MO Secretary, American Association for Thoracic Surgery
Effect of leaflet-to-chordae contact interaction on computational mitral valve evaluation
 Rim et al. BioMedical Engineering OnLine 2014, 13:31 RESEARCH Open Access Effect of leaflet-to-chordae contact interaction on computational mitral valve evaluation Yonghoon Rim, David D McPherson and Hyunggun
Rim et al. BioMedical Engineering OnLine 2014, 13:31 RESEARCH Open Access Effect of leaflet-to-chordae contact interaction on computational mitral valve evaluation Yonghoon Rim, David D McPherson and Hyunggun
A novel mathematical technique to assess of the mitral valve dynamics based on echocardiography
 ORIGINAL ARTICLE A novel mathematical technique to assess of the mitral valve dynamics based on echocardiography Mersedeh Karvandi 1 MD, Saeed Ranjbar 2,* Ph.D, Seyed Ahmad Hassantash MD 2, Mahnoosh Foroughi
ORIGINAL ARTICLE A novel mathematical technique to assess of the mitral valve dynamics based on echocardiography Mersedeh Karvandi 1 MD, Saeed Ranjbar 2,* Ph.D, Seyed Ahmad Hassantash MD 2, Mahnoosh Foroughi
How to assess ischaemic MR?
 ESC 2012 How to assess ischaemic MR? Luc A. Pierard, MD, PhD, FESC, FACC Professor of Medicine Head, Department of Cardiology University Hospital Sart Tilman, Liège ESC 2012 No conflict of interest Luc
ESC 2012 How to assess ischaemic MR? Luc A. Pierard, MD, PhD, FESC, FACC Professor of Medicine Head, Department of Cardiology University Hospital Sart Tilman, Liège ESC 2012 No conflict of interest Luc
Outline. EuroScore II. Society of Thoracic Surgeons Score. EuroScore II
 SURGICAL RISK IN VALVULAR HEART DISEASE: WHAT 2D AND 3D ECHO CAN TELL YOU AND WHAT THEY CAN'T Ernesto E Salcedo, MD Professor of Medicine University of Colorado School of Medicine Director of Echocardiography
SURGICAL RISK IN VALVULAR HEART DISEASE: WHAT 2D AND 3D ECHO CAN TELL YOU AND WHAT THEY CAN'T Ernesto E Salcedo, MD Professor of Medicine University of Colorado School of Medicine Director of Echocardiography
8/31/2016. Mitraclip in Matthew Johnson, MD
 Mitraclip in 2016 Matthew Johnson, MD 1 Abnormal Valve Function Valve Stenosis Obstruction to valve flow during that phase of the cardiac cycle when the valve is normally open. Hemodynamic hallmark - pressure
Mitraclip in 2016 Matthew Johnson, MD 1 Abnormal Valve Function Valve Stenosis Obstruction to valve flow during that phase of the cardiac cycle when the valve is normally open. Hemodynamic hallmark - pressure
JOINT MEETING 2 Tricuspid club Chairpersons: G. Athanassopoulos, A. Avgeropoulou, M. Khoury, G. Stavridis
 JOINT MEETING 2 Tricuspid club Chairpersons: G. Athanassopoulos, A. Avgeropoulou, M. Khoury, G. Stavridis Similarities and differences in Tricuspid vs. Mitral Valve Anatomy and Imaging. Echo evaluation
JOINT MEETING 2 Tricuspid club Chairpersons: G. Athanassopoulos, A. Avgeropoulou, M. Khoury, G. Stavridis Similarities and differences in Tricuspid vs. Mitral Valve Anatomy and Imaging. Echo evaluation
Finite Element Analysis for Edge-to-Edge Technique to Treat Post-Mitral Valve Repair Systolic Anterior Motion
 Acta of Bioengineering and Biomechanics Vol. 6, No. 4, 04 Original paper DOI: 0.577/ABB-0008-04-0 Finite Element Analysis for Edge-to-Edge Technique to Treat Post-Mitral Valve Repair Systolic Anterior
Acta of Bioengineering and Biomechanics Vol. 6, No. 4, 04 Original paper DOI: 0.577/ABB-0008-04-0 Finite Element Analysis for Edge-to-Edge Technique to Treat Post-Mitral Valve Repair Systolic Anterior
Outcomes of Mitral Valve Repair for Mitral Regurgitation Due to Degenerative Disease
 Outcomes of Mitral Valve Repair for Mitral Regurgitation Due to Degenerative Disease TIRONE E. DAVID, MD ; SEMIN THORAC CARDIOVASC SURG 19:116-120c 2007 ELSEVIER INC. PRESENTED BY INTERN 許士盟 Mitral valve
Outcomes of Mitral Valve Repair for Mitral Regurgitation Due to Degenerative Disease TIRONE E. DAVID, MD ; SEMIN THORAC CARDIOVASC SURG 19:116-120c 2007 ELSEVIER INC. PRESENTED BY INTERN 許士盟 Mitral valve
Index. B B-type natriuretic peptide (BNP), 76
 Index A ACCESS-EU registry, 158 159 Acute kidney injury (AKI), 76, 88 Annular enlargement, RV, 177 178 Annuloplasty chordal cutting, 113 complete ring, 99 etiology-specific ring, 100 evolution, 98 flexible
Index A ACCESS-EU registry, 158 159 Acute kidney injury (AKI), 76, 88 Annular enlargement, RV, 177 178 Annuloplasty chordal cutting, 113 complete ring, 99 etiology-specific ring, 100 evolution, 98 flexible
Χειρουργική Αντιμετώπιση της Ανεπάρκειας της Μιτροειδούς Βαλβίδας
 Χειρουργική Αντιμετώπιση της Ανεπάρκειας της Μιτροειδούς Βαλβίδας Dr Χρήστος ΑΛΕΞΙΟΥ MD, PhD, FRCS(Glasgow), FRCS(CTh), CCST(UK) Consultant Cardiothoracic Surgeon Normal Mitral Valve Function Mitral Regurgitation
Χειρουργική Αντιμετώπιση της Ανεπάρκειας της Μιτροειδούς Βαλβίδας Dr Χρήστος ΑΛΕΞΙΟΥ MD, PhD, FRCS(Glasgow), FRCS(CTh), CCST(UK) Consultant Cardiothoracic Surgeon Normal Mitral Valve Function Mitral Regurgitation
Mitral Valve Repair for Functional Mitral Regurgitation- Description of A New Technique and Classification System
 Case Report Mitral Valve Repair for Functional Mitral Regurgitation- Description of A New Technique and Classification System Antonio Chiricolo 1*, Leonard Y Lee 2 1 Department of Anesthesiology, Rutgers
Case Report Mitral Valve Repair for Functional Mitral Regurgitation- Description of A New Technique and Classification System Antonio Chiricolo 1*, Leonard Y Lee 2 1 Department of Anesthesiology, Rutgers
Cardiac ultrasound protocols
 Cardiac ultrasound protocols IDEXX Telemedicine Consultants Two-dimensional and M-mode imaging planes Right parasternal long axis four chamber Obtained from the right side Displays the relative proportions
Cardiac ultrasound protocols IDEXX Telemedicine Consultants Two-dimensional and M-mode imaging planes Right parasternal long axis four chamber Obtained from the right side Displays the relative proportions
Techniques for ischemic mitral valve disease: An Update. Stanford CV Surgery
 Techniques for ischemic mitral valve disease: An Update Conflict of Interest Disclosure Grant/ Research Support: NHLBI RO1 HL67025 Consulting Fees/Honoraria: Stanford PI PARTNER Trial, Edwards Lifesciences
Techniques for ischemic mitral valve disease: An Update Conflict of Interest Disclosure Grant/ Research Support: NHLBI RO1 HL67025 Consulting Fees/Honoraria: Stanford PI PARTNER Trial, Edwards Lifesciences
Echocardiographic Evaluation of Primary Mitral Regurgitation
 Echocardiographic Evaluation of Primary Mitral Regurgitation Roberto M Lang, MD 0-10 o ME 4CH Med A2 P2 50-70 o Commissural P3 P1 A2 80-100 o ME 2CH P3 A2 A1 A1 125-135 o - ME Long axis P2 A2 P3 A3 P2
Echocardiographic Evaluation of Primary Mitral Regurgitation Roberto M Lang, MD 0-10 o ME 4CH Med A2 P2 50-70 o Commissural P3 P1 A2 80-100 o ME 2CH P3 A2 A1 A1 125-135 o - ME Long axis P2 A2 P3 A3 P2
Imaging MV. Jeroen J. Bax Leiden University Medical Center The Netherlands Davos, feb 2015
 Imaging MV Jeroen J. Bax Leiden University Medical Center The Netherlands Davos, feb 2015 MV/MR: information needed on.. 1. MV anatomy 2. MR etiology - primary vs secondary 3. MR severity quantification
Imaging MV Jeroen J. Bax Leiden University Medical Center The Netherlands Davos, feb 2015 MV/MR: information needed on.. 1. MV anatomy 2. MR etiology - primary vs secondary 3. MR severity quantification
3D Printing & Echocardiography
 ASE SOTA Feb 19, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Disclosures Personal
ASE SOTA Feb 19, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Disclosures Personal
Late secondary TR after left sided heart disease correction: is it predictibale and preventable
 Late secondary TR after left sided heart disease correction: is it predictibale and preventable Gilles D. Dreyfus Professor of Cardiothoracic surgery Nath J, et al. JACC 2004 PREDICT Incidence of secondary
Late secondary TR after left sided heart disease correction: is it predictibale and preventable Gilles D. Dreyfus Professor of Cardiothoracic surgery Nath J, et al. JACC 2004 PREDICT Incidence of secondary
The Edge-to-Edge Technique f For Barlow's Disease
 The Edge-to-Edge Technique f For Barlow's Disease Ottavio Alfieri, Michele De Bonis, Elisabetta Lapenna, Francesco Maisano, Lucia Torracca, Giovanni La Canna. Department of Cardiac Surgery, San Raffaele
The Edge-to-Edge Technique f For Barlow's Disease Ottavio Alfieri, Michele De Bonis, Elisabetta Lapenna, Francesco Maisano, Lucia Torracca, Giovanni La Canna. Department of Cardiac Surgery, San Raffaele
Ο ΡΟΛΟΣ ΤΩΝ ΚΟΛΠΩΝ ΣΤΗ ΛΕΙΤΟΥΡΓΙΚΗ ΑΝΕΠΑΡΚΕΙΑ ΤΩΝ ΚΟΛΠΟΚΟΙΛΙΑΚΩΝ ΒΑΛΒΙΔΩΝ
 Ο ΡΟΛΟΣ ΤΩΝ ΚΟΛΠΩΝ ΣΤΗ ΛΕΙΤΟΥΡΓΙΚΗ ΑΝΕΠΑΡΚΕΙΑ ΤΩΝ ΚΟΛΠΟΚΟΙΛΙΑΚΩΝ ΒΑΛΒΙΔΩΝ Ανδρέας Κατσαρός Καρδιολόγος Επιµ. Α Καρδιοχειρ/κών Τµηµάτων Γ.Ν.Α. Ιπποκράτειο ΚΑΜΙΑ ΣΥΓΚΡΟΥΣΗ ΣΥΜΦΕΡΟΝΤΩΝ ΑΝΑΦΟΡΙΚΑ ΜΕ ΤΗΝ ΠΑΡΟΥΣΙΑΣΗ
Ο ΡΟΛΟΣ ΤΩΝ ΚΟΛΠΩΝ ΣΤΗ ΛΕΙΤΟΥΡΓΙΚΗ ΑΝΕΠΑΡΚΕΙΑ ΤΩΝ ΚΟΛΠΟΚΟΙΛΙΑΚΩΝ ΒΑΛΒΙΔΩΝ Ανδρέας Κατσαρός Καρδιολόγος Επιµ. Α Καρδιοχειρ/κών Τµηµάτων Γ.Ν.Α. Ιπποκράτειο ΚΑΜΙΑ ΣΥΓΚΡΟΥΣΗ ΣΥΜΦΕΡΟΝΤΩΝ ΑΝΑΦΟΡΙΚΑ ΜΕ ΤΗΝ ΠΑΡΟΥΣΙΑΣΗ
3D Printing & Echocardiography
 Echo Hawaii Jan 18, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Rapid Prototyping
Echo Hawaii Jan 18, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Rapid Prototyping
MITRAL VALVE FORCE BALANCE: A QUANTITATIVE ASSESSMENT OF ANNULAR AND SUBVALVULAR FORCES
 MITRAL VALVE FORCE BALANCE: A QUANTITATIVE ASSESSMENT OF ANNULAR AND SUBVALVULAR FORCES A Dissertation Presented to The Academic Faculty by Andrew W. Siefert In Partial Fulfillment of the Requirements
MITRAL VALVE FORCE BALANCE: A QUANTITATIVE ASSESSMENT OF ANNULAR AND SUBVALVULAR FORCES A Dissertation Presented to The Academic Faculty by Andrew W. Siefert In Partial Fulfillment of the Requirements
Despite advances in our understanding of the pathophysiology
 Suture Relocation of the Posterior Papillary Muscle in Ischemic Mitral Regurgitation Benjamin B. Peeler MD,* and Irving L. Kron MD,*, *Department of Cardiovascular Surgery, University of Virginia, Charlottesville,
Suture Relocation of the Posterior Papillary Muscle in Ischemic Mitral Regurgitation Benjamin B. Peeler MD,* and Irving L. Kron MD,*, *Department of Cardiovascular Surgery, University of Virginia, Charlottesville,
Part II: Fundamentals of 3D Echocardiography: Acquisition and Application
 Part II: Fundamentals of 3D Echocardiography: Acquisition and Application Dr. Bruce Bollen 3D matrix array TEE probes provide options for both 2D and 3D imaging. Indeed, their utility in obtaining multiple
Part II: Fundamentals of 3D Echocardiography: Acquisition and Application Dr. Bruce Bollen 3D matrix array TEE probes provide options for both 2D and 3D imaging. Indeed, their utility in obtaining multiple
Finite Element Modeling of the Mitral Valve and Mitral Valve Repair
 Finite Element Modeling of the Mitral Valve and Mitral Valve Repair Iain Baxter A thesis submitted to the Faculty of Graduate and Postdoctoral Studies in partial fulfillment of the requirements for the
Finite Element Modeling of the Mitral Valve and Mitral Valve Repair Iain Baxter A thesis submitted to the Faculty of Graduate and Postdoctoral Studies in partial fulfillment of the requirements for the
The Effect of Mitral Annuloplasty Shape in Ischemic Mitral Regurgitation: A Finite Element Simulation
 The Effect of Mitral Annuloplasty Shape in Ischemic Mitral Regurgitation: A Finite Element Simulation Vincent M. Wong, BS, Jonathan F. Wenk, PhD, Zhihong Zhang, MS, Guangming Cheng, MD, PhD, Gabriel Acevedo-Bolton,
The Effect of Mitral Annuloplasty Shape in Ischemic Mitral Regurgitation: A Finite Element Simulation Vincent M. Wong, BS, Jonathan F. Wenk, PhD, Zhihong Zhang, MS, Guangming Cheng, MD, PhD, Gabriel Acevedo-Bolton,
Hemodynamic Assessment. Assessment of Systolic Function Doppler Hemodynamics
 Hemodynamic Assessment Matt M. Umland, RDCS, FASE Aurora Medical Group Milwaukee, WI Assessment of Systolic Function Doppler Hemodynamics Stroke Volume Cardiac Output Cardiac Index Tei Index/Index of myocardial
Hemodynamic Assessment Matt M. Umland, RDCS, FASE Aurora Medical Group Milwaukee, WI Assessment of Systolic Function Doppler Hemodynamics Stroke Volume Cardiac Output Cardiac Index Tei Index/Index of myocardial
MitraClip in the ICCU: Which Patient will Benefit?
 MitraClip in the ICCU: Which Patient will Benefit? DAVID MEERKIN STRUCTURAL A ND CONGENITAL HEART DISEASE UNIT SHAARE ZEDEK MEDICAL CENTER JERUSALEM Conflict of Interest No relevant disclosures Complex
MitraClip in the ICCU: Which Patient will Benefit? DAVID MEERKIN STRUCTURAL A ND CONGENITAL HEART DISEASE UNIT SHAARE ZEDEK MEDICAL CENTER JERUSALEM Conflict of Interest No relevant disclosures Complex
ΔΙΑΔΕΡΜΙΚΗ ΑΝΤΙΜΕΤΩΠΙΣΗ ΔΟΜΙΚΩΝ ΠΑΘΗΣΕΩΝ: Ο ΡΟΛΟΣ ΤΗΣ ΑΠΕΙΚΟΝΙΣΗΣ ΣΤΟ ΑΙΜΟΔΥΝΑΜΙΚΟ ΕΡΓΑΣΤΗΡΙΟ ΣΤΗΝ ΤΟΠΟΘΕΤΗΣΗ MITRACLIP
 ΔΙΑΔΕΡΜΙΚΗ ΑΝΤΙΜΕΤΩΠΙΣΗ ΔΟΜΙΚΩΝ ΠΑΘΗΣΕΩΝ: Ο ΡΟΛΟΣ ΤΗΣ ΑΠΕΙΚΟΝΙΣΗΣ ΣΤΟ ΑΙΜΟΔΥΝΑΜΙΚΟ ΕΡΓΑΣΤΗΡΙΟ ΣΤΗΝ ΤΟΠΟΘΕΤΗΣΗ MITRACLIP ΒΛΑΣΗΣ ΝΙΝΙΟΣ MD MRCP ΚΛΙΝΙΚΗ ΑΓΙΟΣ ΛΟΥΚΑΣ ΘΕΣΣΑΛΟΝΙΚΗ CONFLICT OF INTEREST PROCTOR
ΔΙΑΔΕΡΜΙΚΗ ΑΝΤΙΜΕΤΩΠΙΣΗ ΔΟΜΙΚΩΝ ΠΑΘΗΣΕΩΝ: Ο ΡΟΛΟΣ ΤΗΣ ΑΠΕΙΚΟΝΙΣΗΣ ΣΤΟ ΑΙΜΟΔΥΝΑΜΙΚΟ ΕΡΓΑΣΤΗΡΙΟ ΣΤΗΝ ΤΟΠΟΘΕΤΗΣΗ MITRACLIP ΒΛΑΣΗΣ ΝΙΝΙΟΣ MD MRCP ΚΛΙΝΙΚΗ ΑΓΙΟΣ ΛΟΥΚΑΣ ΘΕΣΣΑΛΟΝΙΚΗ CONFLICT OF INTEREST PROCTOR
The Key Questions in Mitral Valve Interventions. Where Are We in 2018?
 The Key Questions in Mitral Valve Interventions Where Are We in 2018? Gilles D. DREYFUS, MD, FRCS, FESC Professor of Cardiothoracic Surgery 30 GIORNATE CARDIOLOGICHE TORINESI - OCT 2018 Are guidelines
The Key Questions in Mitral Valve Interventions Where Are We in 2018? Gilles D. DREYFUS, MD, FRCS, FESC Professor of Cardiothoracic Surgery 30 GIORNATE CARDIOLOGICHE TORINESI - OCT 2018 Are guidelines
Functional Ischaemic Mitral Regurgitation: CABG + MV Replacement. Prakash P Punjabi. FRCS(Eng),FESC,MS,MCh,FCCP, Diplomate NBE
 Functional Ischaemic Mitral Regurgitation: CABG + MV Replacement Prakash P Punjabi FRCS(Eng),FESC,MS,MCh,FCCP, Diplomate NBE Consultant Cardiothoracic Surgeon Imperial College Healthcare NHS Trust Hammersmith
Functional Ischaemic Mitral Regurgitation: CABG + MV Replacement Prakash P Punjabi FRCS(Eng),FESC,MS,MCh,FCCP, Diplomate NBE Consultant Cardiothoracic Surgeon Imperial College Healthcare NHS Trust Hammersmith
Medical Engineering & Physics
 Medical Engineering & Physics 32 (2010) 1057 1064 Contents lists available at ScienceDirect Medical Engineering & Physics journal homepage: www.elsevier.com/locate/medengphy Mitral valve dynamics in structural
Medical Engineering & Physics 32 (2010) 1057 1064 Contents lists available at ScienceDirect Medical Engineering & Physics journal homepage: www.elsevier.com/locate/medengphy Mitral valve dynamics in structural
Multi-modal Validation Framework of Mitral Valve Geometry and Functional Computational Models
 Multi-modal Validation Framework of Mitral Valve Geometry and Functional Computational Models Sasa Grbic 1, Thomas F. Easley 2, Tommaso Mansi 1, Charles H. Bloodworth 2, Eric L. Pierce 2, Ingmar Voigt
Multi-modal Validation Framework of Mitral Valve Geometry and Functional Computational Models Sasa Grbic 1, Thomas F. Easley 2, Tommaso Mansi 1, Charles H. Bloodworth 2, Eric L. Pierce 2, Ingmar Voigt
Introduction. Aortic Valve. Outflow Tract and Aortic Valve Annulus
 Chapter 1: Surgical anatomy of the aortic and mitral valves Jordan RH Hoffman MD, David A. Fullerton MD, FACC University of Colorado School of Medicine, Department of Surgery, Division of Cardiothoracic
Chapter 1: Surgical anatomy of the aortic and mitral valves Jordan RH Hoffman MD, David A. Fullerton MD, FACC University of Colorado School of Medicine, Department of Surgery, Division of Cardiothoracic
MITRAL REGURGITATION ECHO PARAMETERS TOOL
 Comprehensive assessment of qualitative and quantitative parameters, along with the use of standardized nomenclature when reporting echocardiographic findings, helps to better define a patient s MR and
Comprehensive assessment of qualitative and quantitative parameters, along with the use of standardized nomenclature when reporting echocardiographic findings, helps to better define a patient s MR and
Anisotropic Mass-Spring Method Accurately Simulates Mitral Valve Closure from Image-Based Models
 Anisotropic Mass-Spring Method Accurately Simulates Mitral Valve Closure from Image-Based Models Peter E. Hammer 1,2,3, Pedro J. del Nido 1, and Robert D. Howe 2 1 Department of Cardiac Surgery, Children
Anisotropic Mass-Spring Method Accurately Simulates Mitral Valve Closure from Image-Based Models Peter E. Hammer 1,2,3, Pedro J. del Nido 1, and Robert D. Howe 2 1 Department of Cardiac Surgery, Children
Ischemic Mitral Regurgitation
 Ischemic Mitral Regurgitation Jean-Louis J. Vanoverschelde, MD, PhD Université catholique de Louvain Brussels, Belgium Definition Ischemic mitral regurgitation is mitral regurgitation due to complications
Ischemic Mitral Regurgitation Jean-Louis J. Vanoverschelde, MD, PhD Université catholique de Louvain Brussels, Belgium Definition Ischemic mitral regurgitation is mitral regurgitation due to complications
MEMO 3D. The true reflection of the mitral annulus. Natural physiological 3D motion
 MEMO 3D TM The true reflection of the mitral annulus Natural physiological 3D motion Imagine... if there was one annuloplasty ring that provided an optimal solution across the entire spectrum of mitral
MEMO 3D TM The true reflection of the mitral annulus Natural physiological 3D motion Imagine... if there was one annuloplasty ring that provided an optimal solution across the entire spectrum of mitral
ECHOCARDIOGRAPHY DATA REPORT FORM
 Patient ID Patient Study ID AVM - - Date of form completion / / 20 Initials of person completing the form mm dd yyyy Study period Preoperative Postoperative Operative 6-month f/u 1-year f/u 2-year f/u
Patient ID Patient Study ID AVM - - Date of form completion / / 20 Initials of person completing the form mm dd yyyy Study period Preoperative Postoperative Operative 6-month f/u 1-year f/u 2-year f/u
Percutaneous Mitral Valve Intervention: QuantumCor Device
 Percutaneous Mitral Valve Intervention: QuantumCor Device RICHARD R. HEUSER, MD, FACC, FACP, FESC Director Of Cardiology, St. Luke s Medical Center, Phoenix, Arizona Medical Director, Phoenix Heart Center,
Percutaneous Mitral Valve Intervention: QuantumCor Device RICHARD R. HEUSER, MD, FACC, FACP, FESC Director Of Cardiology, St. Luke s Medical Center, Phoenix, Arizona Medical Director, Phoenix Heart Center,
How does the heart pump? From sarcomere to ejection volume
 How does the heart pump? From sarcomere to ejection volume Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases University Leuven, Leuven, Belgium Course on deformation
How does the heart pump? From sarcomere to ejection volume Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases University Leuven, Leuven, Belgium Course on deformation
Quantitation of right ventricular dimensions and function
 SCCS Basics of cardiac assessment Quantitation of right ventricular dimensions and function Tomasz Kukulski, MD PhD Dept of Cardiology, Congenital Heart Disease and Electrotherapy Silesian Medical University
SCCS Basics of cardiac assessment Quantitation of right ventricular dimensions and function Tomasz Kukulski, MD PhD Dept of Cardiology, Congenital Heart Disease and Electrotherapy Silesian Medical University
Functional Mitral Regurgitation
 Club 35 - The best in heart valve disease - Functional Mitral Regurgitation Steven Droogmans, MD, PhD UZ Brussel, Jette, Belgium 08-12-2011 Euroecho & other Imaging Modalities 2011 No conflicts of interest
Club 35 - The best in heart valve disease - Functional Mitral Regurgitation Steven Droogmans, MD, PhD UZ Brussel, Jette, Belgium 08-12-2011 Euroecho & other Imaging Modalities 2011 No conflicts of interest
Accepted Manuscript. S (12)00076-X Reference: MEDIMA 694
 Accepted Manuscript An Integrated Framework for Finite-Element Modeling of Mitral Valve Biomechanics from Medical Images: Application to MitralClip Intervention Planning Tommaso Mansi, Ingmar Voigt, Bogdan
Accepted Manuscript An Integrated Framework for Finite-Element Modeling of Mitral Valve Biomechanics from Medical Images: Application to MitralClip Intervention Planning Tommaso Mansi, Ingmar Voigt, Bogdan
Posterior leaflet prolapse is the most common lesion seen
 Techniques for Repairing Posterior Leaflet Prolapse of the Mitral Valve Robin Varghese, MD, MS, and David H. Adams, MD Posterior leaflet prolapse is the most common lesion seen in degenerative mitral valve
Techniques for Repairing Posterior Leaflet Prolapse of the Mitral Valve Robin Varghese, MD, MS, and David H. Adams, MD Posterior leaflet prolapse is the most common lesion seen in degenerative mitral valve
Degenerative Mitral Regurgitation: Etiology and Natural History of Disease and Triggers for Intervention
 Degenerative Mitral Regurgitation: Etiology and Natural History of Disease and Triggers for Intervention John N. Hamaty D.O. FACC, FACOI November 17 th 2017 I have no financial disclosures Primary Mitral
Degenerative Mitral Regurgitation: Etiology and Natural History of Disease and Triggers for Intervention John N. Hamaty D.O. FACC, FACOI November 17 th 2017 I have no financial disclosures Primary Mitral
Three-Dimensional P3 Tethering Angle at the Heart of Future Surgical Decision Making in Ischemic Mitral Regurgitation
 Accepted Manuscript Three-Dimensional P3 Tethering Angle at the Heart of Future Surgical Decision Making in Ischemic Mitral Regurgitation Wobbe Bouma, MD PhD, Robert C. Gorman, MD PII: S0022-5223(18)32805-8
Accepted Manuscript Three-Dimensional P3 Tethering Angle at the Heart of Future Surgical Decision Making in Ischemic Mitral Regurgitation Wobbe Bouma, MD PhD, Robert C. Gorman, MD PII: S0022-5223(18)32805-8
Ischemic Mitral Regurgitation: A Quantitative Three-Dimensional Echocardiographic Analysis
 Ischemic Mitral Regurgitation: A Quantitative Three-Dimensional Echocardiographic Analysis Mathieu Vergnat, MD, Arminder S. Jassar, MBBS, Benjamin M. Jackson, MD, Liam P. Ryan, MD, Thomas J. Eperjesi,
Ischemic Mitral Regurgitation: A Quantitative Three-Dimensional Echocardiographic Analysis Mathieu Vergnat, MD, Arminder S. Jassar, MBBS, Benjamin M. Jackson, MD, Liam P. Ryan, MD, Thomas J. Eperjesi,
Overview of Surgical Approach to Mitral Valve Disease : Why Repair? Steven F. Bolling, MD Cardiac Surgery University of Michigan
 Overview of Surgical Approach to Mitral Valve Disease : Why Repair? Steven F. Bolling, MD Cardiac Surgery University of Michigan Degenerative MR is not Functional MR 2o - Functional MR : Ventricular Problem!!
Overview of Surgical Approach to Mitral Valve Disease : Why Repair? Steven F. Bolling, MD Cardiac Surgery University of Michigan Degenerative MR is not Functional MR 2o - Functional MR : Ventricular Problem!!
Direct Annuloplasty with QuantumCor: Device Evolution, Techniques and First-in-Man Results
 Direct Annuloplasty with QuantumCor: Device Evolution, Techniques and First-in-Man Results RICHARD R. HEUSER, MD, FACC, FACP, FESC, FSCAI Chief of Cardiology, St. Luke s Medical Center, Phoenix, Arizona
Direct Annuloplasty with QuantumCor: Device Evolution, Techniques and First-in-Man Results RICHARD R. HEUSER, MD, FACC, FACP, FESC, FSCAI Chief of Cardiology, St. Luke s Medical Center, Phoenix, Arizona
Percutaneous Mitral Valve Repair: What Can We Treat and What Should We Treat
 Percutaneous Mitral Valve Repair: What Can We Treat and What Should We Treat Innovative Procedures, Devices & State of the Art Care for Arrhythmias, Heart Failure & Structural Heart Disease October 8-10,
Percutaneous Mitral Valve Repair: What Can We Treat and What Should We Treat Innovative Procedures, Devices & State of the Art Care for Arrhythmias, Heart Failure & Structural Heart Disease October 8-10,
PART II ECHOCARDIOGRAPHY LABORATORY OPERATIONS ADULT TRANSTHORACIC ECHOCARDIOGRAPHY TESTING
 PART II ECHOCARDIOGRAPHY LABORATORY OPERATIONS ADULT TRANSTHORACIC ECHOCARDIOGRAPHY TESTING STANDARD - Primary Instrumentation 1.1 Cardiac Ultrasound Systems SECTION 1 Instrumentation Ultrasound instruments
PART II ECHOCARDIOGRAPHY LABORATORY OPERATIONS ADULT TRANSTHORACIC ECHOCARDIOGRAPHY TESTING STANDARD - Primary Instrumentation 1.1 Cardiac Ultrasound Systems SECTION 1 Instrumentation Ultrasound instruments
Two semilunar valves. Two atrioventricular valves. Valves of the heart. Left atrioventricular or bicuspid valve Mitral valve
 The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
ISCHEMIC/FUNCTIONAL MR
 ISCHEMIC/FUNCTIONAL MR Mitral valve annuloplasty and papillary muscle relocation oriented by 3-dimensional transesophageal echocardiography for severe functional mitral regurgitation Khalil Fattouch, MD,
ISCHEMIC/FUNCTIONAL MR Mitral valve annuloplasty and papillary muscle relocation oriented by 3-dimensional transesophageal echocardiography for severe functional mitral regurgitation Khalil Fattouch, MD,
New Cardiovascular Devices and Interventions: Non-Contrast MRI for TAVR Abhishek Chaturvedi Assistant Professor. Cardiothoracic Radiology
 New Cardiovascular Devices and Interventions: Non-Contrast MRI for TAVR Abhishek Chaturvedi Assistant Professor Cardiothoracic Radiology Disclosure I have no disclosure pertinent to this presentation.
New Cardiovascular Devices and Interventions: Non-Contrast MRI for TAVR Abhishek Chaturvedi Assistant Professor Cardiothoracic Radiology Disclosure I have no disclosure pertinent to this presentation.
Appendix II: ECHOCARDIOGRAPHY ANALYSIS
 Appendix II: ECHOCARDIOGRAPHY ANALYSIS Two-Dimensional (2D) imaging was performed using the Vivid 7 Advantage cardiovascular ultrasound system (GE Medical Systems, Milwaukee) with a frame rate of 400 frames
Appendix II: ECHOCARDIOGRAPHY ANALYSIS Two-Dimensional (2D) imaging was performed using the Vivid 7 Advantage cardiovascular ultrasound system (GE Medical Systems, Milwaukee) with a frame rate of 400 frames
What echo measurements are key prior to MitraClip?
 APHP CHU Bichat - Claude Bernard What echo measurements are key prior to MitraClip? Eric Brochet,MD Cardiology Department Hopital Bichat Paris France No disclosure Conflict of interest Case 69 y.o man
APHP CHU Bichat - Claude Bernard What echo measurements are key prior to MitraClip? Eric Brochet,MD Cardiology Department Hopital Bichat Paris France No disclosure Conflict of interest Case 69 y.o man
CLIP ΜΙΤΡΟΕΙ ΟΥΣ: ΠΟΥ ΒΡΙΣΚΟΜΑΣΤΕ;
 CLIP ΜΙΤΡΟΕΙ ΟΥΣ: ΠΟΥ ΒΡΙΣΚΟΜΑΣΤΕ; Επιµορφωτικά Σεµινάρια Ειδικευοµένων Καρδιολογίας 7 Απριλίου 2012 M Chrissoheris MD FACC THV Department HYGEIA Hospital Degenerative MR (DMR) Usually refers to an anatomic
CLIP ΜΙΤΡΟΕΙ ΟΥΣ: ΠΟΥ ΒΡΙΣΚΟΜΑΣΤΕ; Επιµορφωτικά Σεµινάρια Ειδικευοµένων Καρδιολογίας 7 Απριλίου 2012 M Chrissoheris MD FACC THV Department HYGEIA Hospital Degenerative MR (DMR) Usually refers to an anatomic
LV FUNCTION ASSESSMENT: WHAT IS BEYOND EJECTION FRACTION
 LV FUNCTION ASSESSMENT: WHAT IS BEYOND EJECTION FRACTION Jamilah S AlRahimi Assistant Professor, KSU-HS Consultant Noninvasive Cardiology KFCC, MNGHA-WR Introduction LV function assessment in Heart Failure:
LV FUNCTION ASSESSMENT: WHAT IS BEYOND EJECTION FRACTION Jamilah S AlRahimi Assistant Professor, KSU-HS Consultant Noninvasive Cardiology KFCC, MNGHA-WR Introduction LV function assessment in Heart Failure:
Original. Stresses and Strains Distributions in Three-Dimension Three-Layer Abdominal Aortic Wall Based on in vivo Ultrasound Imaging
 Original Stresses and Strains Distributions in Three-Dimension Three-Layer Abdominal Aortic Wall Based on in vivo Ultrasound Imaging P. Khamdaengyodtai 1, T. Khamdaeng 1, P. Sakulchangsatjatai 1, N. Kammuang-lue
Original Stresses and Strains Distributions in Three-Dimension Three-Layer Abdominal Aortic Wall Based on in vivo Ultrasound Imaging P. Khamdaengyodtai 1, T. Khamdaeng 1, P. Sakulchangsatjatai 1, N. Kammuang-lue
Advanced Multi-Layer Speckle Strain Permits Transmural Myocardial Function Analysis in Health and Disease:
 Advanced Multi-Layer Speckle Strain Permits Transmural Myocardial Function Analysis in Health and Disease: Clinical Case Examples Jeffrey C. Hill, BS, RDCS Echocardiography Laboratory, University of Massachusetts
Advanced Multi-Layer Speckle Strain Permits Transmural Myocardial Function Analysis in Health and Disease: Clinical Case Examples Jeffrey C. Hill, BS, RDCS Echocardiography Laboratory, University of Massachusetts
Tissue Doppler Imaging in Congenital Heart Disease
 Tissue Doppler Imaging in Congenital Heart Disease L. Youngmin Eun, M.D. Department of Pediatrics, Division of Pediatric Cardiology, Kwandong University College of Medicine The potential advantage of ultrasound
Tissue Doppler Imaging in Congenital Heart Disease L. Youngmin Eun, M.D. Department of Pediatrics, Division of Pediatric Cardiology, Kwandong University College of Medicine The potential advantage of ultrasound
On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve
 Biomech Model Mechanobiol DOI 10.1007/s10237-015-0674-0 ORIGINAL PAPER On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve Chung-Hao Lee 1 Jean-Pierre
Biomech Model Mechanobiol DOI 10.1007/s10237-015-0674-0 ORIGINAL PAPER On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve Chung-Hao Lee 1 Jean-Pierre
Percutaneous Mitral Valve Repair
 Indiana Chapter of ACC November 15 th,2008 Percutaneous Mitral Valve Repair James B Hermiller, MD, FACC The Care Group, LLC St Vincent Hospital Indianapolis, IN Mechanisms of Mitral Regurgitation Mitral
Indiana Chapter of ACC November 15 th,2008 Percutaneous Mitral Valve Repair James B Hermiller, MD, FACC The Care Group, LLC St Vincent Hospital Indianapolis, IN Mechanisms of Mitral Regurgitation Mitral
Σεμινάρια Ομάδων Εργασίας 2017 Ανεπάρκεια μιτροειδούς μυξωματώδους αιτιολογίας
 Σεμινάρια Ομάδων Εργασίας 2017 Ανεπάρκεια μιτροειδούς μυξωματώδους αιτιολογίας Μυτάς Δημήτρης MD, PhD Επιμ Α ΕΣΥ Σισμανόγλειο Γενικό Νοσοκομείο Αττικής Δηλώνω υπεύθυνα ότι η παρούσα ομιλία δεν επιχορηγείται
Σεμινάρια Ομάδων Εργασίας 2017 Ανεπάρκεια μιτροειδούς μυξωματώδους αιτιολογίας Μυτάς Δημήτρης MD, PhD Επιμ Α ΕΣΥ Σισμανόγλειο Γενικό Νοσοκομείο Αττικής Δηλώνω υπεύθυνα ότι η παρούσα ομιλία δεν επιχορηγείται
Normal TTE/TEE Examinations
 Normal TTE/TEE Examinations Geoffrey A. Rose, MD FACC FASE Sanger Heart & Vascular Institute Before you begin imaging... Obtain the patient s Height Weight BP PLAX View PLAX View Is apex @ 9-10 o clock?
Normal TTE/TEE Examinations Geoffrey A. Rose, MD FACC FASE Sanger Heart & Vascular Institute Before you begin imaging... Obtain the patient s Height Weight BP PLAX View PLAX View Is apex @ 9-10 o clock?
Disclosure Statement of Financial Interest Saibal Kar, MD, FACC
 MitraClip Therapy Saibal Kar, MD, FACC, FAHA, FSCAI Director of Interventional Cardiac Research Program Director, Interventional Cardiology Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA
MitraClip Therapy Saibal Kar, MD, FACC, FAHA, FSCAI Director of Interventional Cardiac Research Program Director, Interventional Cardiology Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA
MITRAL STENOSIS. Joanne Cusack
 MITRAL STENOSIS Joanne Cusack BSE Breakdown Recognition of rheumatic mitral stenosis Qualitative description of valve and sub-valve calcification and fibrosis Measurement of orifice area by planimetry
MITRAL STENOSIS Joanne Cusack BSE Breakdown Recognition of rheumatic mitral stenosis Qualitative description of valve and sub-valve calcification and fibrosis Measurement of orifice area by planimetry
Development of a surgical simulation toolkit for mitral valve repair surgeries
 University of Iowa Iowa Research Online Theses and Dissertations Summer 2014 Development of a surgical simulation toolkit for mitral valve repair surgeries Piyusha Sanjay Gade University of Iowa Copyright
University of Iowa Iowa Research Online Theses and Dissertations Summer 2014 Development of a surgical simulation toolkit for mitral valve repair surgeries Piyusha Sanjay Gade University of Iowa Copyright
Revealing new insights. irotate electronic rotation and xplane adjustable biplane imaging. Ultrasound cardiology. irotate and xplane
 Ultrasound cardiology irotate and xplane Revealing new insights irotate electronic rotation and xplane adjustable biplane imaging Annemien van den Bosch and Jackie McGhie Department of Cardiology, Erasmus
Ultrasound cardiology irotate and xplane Revealing new insights irotate electronic rotation and xplane adjustable biplane imaging Annemien van den Bosch and Jackie McGhie Department of Cardiology, Erasmus
Echocardiographic Evaluation of the Cardiomyopathies. Stephanie Coulter, MD, FACC, FASE April, 2016
 Echocardiographic Evaluation of the Cardiomyopathies Stephanie Coulter, MD, FACC, FASE April, 2016 Cardiomyopathies (CMP) primary disease intrinsic to cardiac muscle Dilated CMP Hypertrophic CMP Infiltrative
Echocardiographic Evaluation of the Cardiomyopathies Stephanie Coulter, MD, FACC, FASE April, 2016 Cardiomyopathies (CMP) primary disease intrinsic to cardiac muscle Dilated CMP Hypertrophic CMP Infiltrative
When should we intervene surgically in pediatric patient with MR?
 When should we intervene surgically in pediatric patient with MR? DR.SAUD A. BAHAIDARAH CONSULTANT, PEDIATRIC CARDIOLOGY ASSISTANT PROFESSOR OF PEDIATRICS HEAD OF CARDIOLOGY AND CARDIAC SURGERY UNIT KAUH
When should we intervene surgically in pediatric patient with MR? DR.SAUD A. BAHAIDARAH CONSULTANT, PEDIATRIC CARDIOLOGY ASSISTANT PROFESSOR OF PEDIATRICS HEAD OF CARDIOLOGY AND CARDIAC SURGERY UNIT KAUH
Effect of loading and geometry on functional parameters
 Effect of loading and geometry on functional parameters Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases Leuven University, Leuven, Belgium 5 th European Echocardiography
Effect of loading and geometry on functional parameters Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases Leuven University, Leuven, Belgium 5 th European Echocardiography
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Morphometric Analysis of Annulo - Papillary Distances in Left Ventricle of Human Hearts S.Kavitha
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Morphometric Analysis of Annulo - Papillary Distances in Left Ventricle of Human Hearts S.Kavitha
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/20135 holds various files of this Leiden University dissertation. Author: Braun, Jerry Title: Surgical treatment of functional mitral regurgitation Issue
Cover Page The handle http://hdl.handle.net/1887/20135 holds various files of this Leiden University dissertation. Author: Braun, Jerry Title: Surgical treatment of functional mitral regurgitation Issue
좌심실수축기능평가 Cardiac Function
 Basic Echo Review Course 좌심실수축기능평가 Cardiac Function Seonghoon Choi Cardiology Hallym university LV systolic function Systolic function 좌심실수축기능 - 심근의수축으로심실에서혈액을대동맥으로박출하는기능 실제임상에서 LV function 의의미 1Diagnosis
Basic Echo Review Course 좌심실수축기능평가 Cardiac Function Seonghoon Choi Cardiology Hallym university LV systolic function Systolic function 좌심실수축기능 - 심근의수축으로심실에서혈액을대동맥으로박출하는기능 실제임상에서 LV function 의의미 1Diagnosis
Certificate in Clinician Performed Ultrasound (CCPU) Syllabus. Rapid Cardiac Echo (RCE)
 Certificate in Clinician Performed Ultrasound (CCPU) Syllabus Rapid Cardiac Echo (RCE) Purpose: Rapid Cardiac Echocardiography (RCE) This unit is designed to cover the theoretical and practical curriculum
Certificate in Clinician Performed Ultrasound (CCPU) Syllabus Rapid Cardiac Echo (RCE) Purpose: Rapid Cardiac Echocardiography (RCE) This unit is designed to cover the theoretical and practical curriculum
Historical perspective R1 黃維立
 Degenerative mitral valve disease refers to a spectrum of conditions in which morphologic changes in the connective tissue of the mitral valve cause structural lesions that prevent normal function of the
Degenerative mitral valve disease refers to a spectrum of conditions in which morphologic changes in the connective tissue of the mitral valve cause structural lesions that prevent normal function of the
Imaging to select patients for Transcatheter TV
 Imaging to select patients for Transcatheter TV Jeroen J Bax Dept of Cardiology Leiden Univ Medical Center The Netherlands San Diego, february 2018 Research grants: Medtronic, Biotronik, Boston Scientific,
Imaging to select patients for Transcatheter TV Jeroen J Bax Dept of Cardiology Leiden Univ Medical Center The Netherlands San Diego, february 2018 Research grants: Medtronic, Biotronik, Boston Scientific,
WHAT CAN WE LEARN FROM COMPUTER SIMULATION?
 WHAT CAN WE LEARN FROM COMPUTER SIMULATION? Ehud Raanani, MD Gil Marom, Phd Cardiothoracic Surgery, Sheba Medical Center Sackler School of Medicine, Biomechanical Engineering, Tel Aviv University Homburg,
WHAT CAN WE LEARN FROM COMPUTER SIMULATION? Ehud Raanani, MD Gil Marom, Phd Cardiothoracic Surgery, Sheba Medical Center Sackler School of Medicine, Biomechanical Engineering, Tel Aviv University Homburg,
2/2/2011. Strain and Strain Rate Imaging How, Why and When? Movement vs Deformation. Doppler Myocardial Velocities. Movement. Deformation.
 Strain and Strain Rate Imaging How, Why and When? João L. Cavalcante, MD Advanced Cardiac Imaging Fellow Cleveland Clinic Foundation Disclosures: No conflicts of interest Movement vs Deformation Movement
Strain and Strain Rate Imaging How, Why and When? João L. Cavalcante, MD Advanced Cardiac Imaging Fellow Cleveland Clinic Foundation Disclosures: No conflicts of interest Movement vs Deformation Movement
Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure
 Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure T. Mansi 1, I. Voigt 1,2, E. Assoumou Mengue 1, R. Ionasec 1, B. Georgescu 1, T. Noack 3, J. Seeburger 3, and D. Comaniciu 1 1
Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure T. Mansi 1, I. Voigt 1,2, E. Assoumou Mengue 1, R. Ionasec 1, B. Georgescu 1, T. Noack 3, J. Seeburger 3, and D. Comaniciu 1 1
Andrew W. Siefert, Ph.D. Professional Profile
 6205 Shiloh Crossing, Suite C Alpharetta, GA 30005 Phone (770) 886-3207 asiefert@inscitech.com Andrew W. Siefert, Ph.D. Professional Profile Dr. Siefert s area of expertise is in medical device design
6205 Shiloh Crossing, Suite C Alpharetta, GA 30005 Phone (770) 886-3207 asiefert@inscitech.com Andrew W. Siefert, Ph.D. Professional Profile Dr. Siefert s area of expertise is in medical device design
Functional anatomy of the aortic root. ΔΡΟΣΟΣ ΓΕΩΡΓΙΟΣ Διεσθσνηής Καρδιοθωρακοτειροσργικής Κλινικής Γ.Ν. «Γ. Παπανικολάοσ» Θεζζαλονίκη
 Functional anatomy of the aortic root ΔΡΟΣΟΣ ΓΕΩΡΓΙΟΣ Διεσθσνηής Καρδιοθωρακοτειροσργικής Κλινικής Γ.Ν. «Γ. Παπανικολάοσ» Θεζζαλονίκη What is the aortic root? represents the outflow tract from the LV provides
Functional anatomy of the aortic root ΔΡΟΣΟΣ ΓΕΩΡΓΙΟΣ Διεσθσνηής Καρδιοθωρακοτειροσργικής Κλινικής Γ.Ν. «Γ. Παπανικολάοσ» Θεζζαλονίκη What is the aortic root? represents the outflow tract from the LV provides
Functional mitral regurgitation (MR), which occurs as a
 Geometric Differences of the Mitral Apparatus Between Ischemic and Dilated Cardiomyopathy With Significant Mitral Regurgitation Real-Time Three-Dimensional Echocardiography Study Jun Kwan, MD; Takahiro
Geometric Differences of the Mitral Apparatus Between Ischemic and Dilated Cardiomyopathy With Significant Mitral Regurgitation Real-Time Three-Dimensional Echocardiography Study Jun Kwan, MD; Takahiro
Regurgitant Lesions. Bicol Hospital, Legazpi City, Philippines July Gregg S. Pressman MD, FACC, FASE Einstein Medical Center Philadelphia, USA
 Regurgitant Lesions Bicol Hospital, Legazpi City, Philippines July 2016 Gregg S. Pressman MD, FACC, FASE Einstein Medical Center Philadelphia, USA Aortic Insufficiency Valve anatomy and function LVOT and
Regurgitant Lesions Bicol Hospital, Legazpi City, Philippines July 2016 Gregg S. Pressman MD, FACC, FASE Einstein Medical Center Philadelphia, USA Aortic Insufficiency Valve anatomy and function LVOT and
Chapter 24: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines Ashwin Nathan, MD, Saif Anwaruddin, MD, FACC Penn Medicine
 Chapter 24: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines Ashwin Nathan, MD, Saif Anwaruddin, MD, FACC Penn Medicine Mitral regurgitation, regurgitant flow between the
Chapter 24: Diagnostic workup and evaluation: eligibility, risk assessment, FDA guidelines Ashwin Nathan, MD, Saif Anwaruddin, MD, FACC Penn Medicine Mitral regurgitation, regurgitant flow between the
Quantitation of Mitral Valve Tenting in Ischemic Mitral Regurgitation by Transthoracic Real-Time Three-Dimensional Echocardiography
 Journal of the American College of Cardiology Vol. 45, No. 5, 2005 2005 by the American College of Cardiology Foundation ISSN 0735-1097/05/$30.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2004.11.048
Journal of the American College of Cardiology Vol. 45, No. 5, 2005 2005 by the American College of Cardiology Foundation ISSN 0735-1097/05/$30.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2004.11.048
AATS Annual Meeting Seattle, WA Irving Kron, M.D. Professor and Chairman Department of Surgery University of Virginia Hospital Charlottesville,
 AATS Annual Meeting Seattle, WA Irving Kron, M.D. Professor and Chairman Department of Surgery University of Virginia Hospital Charlottesville, Virginia AATS Ischemic MR Guideline Writing Group Roster
AATS Annual Meeting Seattle, WA Irving Kron, M.D. Professor and Chairman Department of Surgery University of Virginia Hospital Charlottesville, Virginia AATS Ischemic MR Guideline Writing Group Roster
Transient stiffening of mitral valve leaflets in the beating heart
 Am J Physiol Heart Circ Physiol 298: H2221 H2225, 2010. First published April 16, 2010; doi:10.1152/ajpheart.00215.2010. Transient stiffening of mitral valve leaflets in the beating heart Gaurav Krishnamurthy,
Am J Physiol Heart Circ Physiol 298: H2221 H2225, 2010. First published April 16, 2010; doi:10.1152/ajpheart.00215.2010. Transient stiffening of mitral valve leaflets in the beating heart Gaurav Krishnamurthy,
Back to Basics: Common Errors In Quantitation In Everyday Practice
 Back to Basics: Common Errors In Quantitation In Everyday Practice Deborah Agler, ACS, RDCS, FASE October 9, 2017 ASE: Echo Florida Rebecca T. Hahn, MD Director of Interventional Echocardiography Professor
Back to Basics: Common Errors In Quantitation In Everyday Practice Deborah Agler, ACS, RDCS, FASE October 9, 2017 ASE: Echo Florida Rebecca T. Hahn, MD Director of Interventional Echocardiography Professor
PROSTHETIC VALVE BOARD REVIEW
 PROSTHETIC VALVE BOARD REVIEW The correct answer D This two chamber view shows a porcine mitral prosthesis with the typical appearance of the struts although the leaflets are not well seen. The valve
PROSTHETIC VALVE BOARD REVIEW The correct answer D This two chamber view shows a porcine mitral prosthesis with the typical appearance of the struts although the leaflets are not well seen. The valve
CAS SECTION 3 PASSPORT
 CAS SECTION 3 PASSPORT Section 3 activities are awarded to CPD activities that provide assessment of knowledge or performance. Each one hour provides 3 credits under the Approved self-assessment and simulation
CAS SECTION 3 PASSPORT Section 3 activities are awarded to CPD activities that provide assessment of knowledge or performance. Each one hour provides 3 credits under the Approved self-assessment and simulation
Valvular Heart Disease in Clinical Practice
 Valvular Heart Disease in Clinical Practice Michael Y. Henein Editor Valvular Heart Disease in Clinical Practice 123 Editor Michael Y. Henein Consultant Cardiologist Umea Heart Centre Umea University
Valvular Heart Disease in Clinical Practice Michael Y. Henein Editor Valvular Heart Disease in Clinical Practice 123 Editor Michael Y. Henein Consultant Cardiologist Umea Heart Centre Umea University
Tissue Doppler and Strain Imaging
 Tissue Doppler and Strain Imaging Steven J. Lester MD, FRCP(C), FACC, FASE Relevant Financial Relationship(s) None Off Label Usage None 1 Objective way with which to quantify the minor amplitude and temporal
Tissue Doppler and Strain Imaging Steven J. Lester MD, FRCP(C), FACC, FASE Relevant Financial Relationship(s) None Off Label Usage None 1 Objective way with which to quantify the minor amplitude and temporal
Really Less-Invasive Trans-apical Beating Heart Mitral Valve Repair: Which Patients?
 Really Less-Invasive Trans-apical Beating Heart Mitral Valve Repair: Which Patients? David H. Adams, MD Cardiac Surgeon-in-Chief Mount Sinai Health System Marie Josée and Henry R. Kravis Professor and
Really Less-Invasive Trans-apical Beating Heart Mitral Valve Repair: Which Patients? David H. Adams, MD Cardiac Surgeon-in-Chief Mount Sinai Health System Marie Josée and Henry R. Kravis Professor and
When Does 3D Echo Make A Difference?
 When Does 3D Echo Make A Difference? Wendy Tsang, MD, SM Assistant Professor, University of Toronto Toronto General Hospital, University Health Network 1 Practical Applications of 3D Echocardiography Recommended
When Does 3D Echo Make A Difference? Wendy Tsang, MD, SM Assistant Professor, University of Toronto Toronto General Hospital, University Health Network 1 Practical Applications of 3D Echocardiography Recommended
