Accepted Manuscript. S (12)00076-X Reference: MEDIMA 694
|
|
|
- Della Goodman
- 5 years ago
- Views:
Transcription
1 Accepted Manuscript An Integrated Framework for Finite-Element Modeling of Mitral Valve Biomechanics from Medical Images: Application to MitralClip Intervention Planning Tommaso Mansi, Ingmar Voigt, Bogdan Georgescu, Xudong Zheng, Etienne Assoumou Mengue, Michael Hackl, Razvan I. Ionasec, Thilo Noack, Joerg Seeburger, Dorin Comaniciu PII: S (12)00076-X DOI: Reference: MEDIMA 694 To appear in: Medical Image Analysis Received Date: 26 January 2012 Revised Date: 21 April 2012 Accepted Date: 18 May 2012 Please cite this article as: Mansi, T., Voigt, I., Georgescu, B., Zheng, X., Mengue, E.A., Hackl, M., Ionasec, R.I., Noack, T., Seeburger, J., Comaniciu, D., An Integrated Framework for Finite-Element Modeling of Mitral Valve Biomechanics from Medical Images: Application to MitralClip Intervention Planning, Medical Image Analysis (2012), doi: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
2 An Integrated Framework for Finite-Element Modeling of Mitral Valve Biomechanics from Medical Images: Application to MitralClip Intervention Planning Tommaso Mansi a,, Ingmar Voigt a,b, Bogdan Georgescu a, Xudong Zheng a, Etienne Assoumou Mengue a, Michael Hackl c, Razvan I. Ionasec a, Thilo Noack d, Joerg Seeburger d, Dorin Comaniciu a a Siemens Corporation, Corporate Research and Technology, Image Analytics and Informatics, Princeton, NJ, USA b Friedrich-Alexander-University, Pattern Recognition Lab, Erlangen, Germany c Friedrich-Alexander-University, Medizinische Fakultät, Erlangen, Germany d Heart Centre Leipzig, Department of Cardiac Surgery, Leipzig, Germany Abstract Treatment of mitral valve (MV) diseases requires comprehensive clinical evaluation and therapy personalization to optimize outcomes. Finite-element models (FEM) of MV physiology have been proposed to study the biomechanical impact of MV repair, but their translation into the clinics remains challenging. As a step towards this goal, we present an integrated framework for finite-element modeling of the MV closure based on patient-specific anatomies and boundary conditions. Starting from temporal medical images, we estimate a comprehensive model of the MV apparatus dynamics, including papillary tips, using a machine-learning approach. A detailed model of the open MV at end-diastole is then computed, which is finally closed according to a FEM of MV biomechanics. The motion of the mitral annulus and papillary tips are constrained from the image data for increased accuracy. A sensitivity analysis of our system shows that chordae rest length and boundary conditions have a significant influence upon the simulation results. We quantitatively test the generalization of our framework on 25 consecutive patients. Comparisons between the simulated closed valve and ground truth show encouraging results (average point-to-mesh distance: 1.49 ± 0.62 mm) tommaso.mansi@siemens.com Tel: Fax: College Road East, Princeton, NJ, USA ) Preprint submitted to Medical Image Analysis April 20, 2012
3 but also the need for personalization of tissue properties, as illustrated in three patients. Finally, the predictive power of our model is tested on one patient who underwent MitralClip by comparing the simulated intervention with the real outcome in terms of MV closure, yielding promising prediction. By providing an integrated way to perform MV simulation, our framework may constitute a surrogate tool for model validation and therapy planning. Keywords: Mitral Valve Apparatus, Patient-Specific Anatomy, Machine-Learning, Biomechanics, Finite-Element Model, Therapy Planning Introduction 1.1. Clinical Rationale: Preoperative Planning of Mitral Valve Repair The mitral valve (MV), located between the left atrium (LA) and the left ventricle (LV), controls the blood flow from the LA towards the LV. The MV is a complex cardiac structure comprising two leaflets, the mitral annulus and chordae tendineae. The leaflets are attached to the left heart through the fibrous mitral annulus. The other extremity, called free edge, is tethered to the papillary muscles through the chordae tendineae (Fig. 1). During diastole, the leaflets open as the blood enters the LV. When the myocardium starts to contract, the leaflets close to prevent the blood from going back to the atrium. The chordae tendineae tighten to ensure perfect closure. Left Atrium Left Atrium Aortic Valve Mitral Annulus Aortic Valve Posterior Leaflet Mitral Annulus Anterior Leaflet Posterior Leaflet Anterior Leaflet Left Ventricle Tendinae Chordae Left Ventricle Papillary Tips Figure 1: 3D+t transesophageal (TEE) ultrasound image of the mitral valve Mitral valve disease is one of the most common heart valve diseases (Iung and Vahanian, 2011), with a prevalence sharply increasing with age (from 0.5% to 9.3% between 18 and 75 year of age in the US (Roger et al., 2
4 )). Defects in leaflet morphology, chordae structure or ventricular asynchrony can result in incorrect MV closure. In this situation, the blood flows back towards the LA during systole, the so-called mitral regurgitation, thus decreasing cardiac efficiency. In severe cases, surgical intervention may be necessary to repair, or even replace the incompetent valve. The edge-to-edge technique, which consists in suturing the two mitral leaflets at the regurgitant hole, has demonstrated good clinical outcomes in patients with severe mitral insufficiency due to leaflet prolapse or calcified annulus (Maisano et al., 1998). Recently, a percutaneous device, called MitralClip, has been designed to attach the two leaflets through a catheter (Herrmann and Feldman, 2006). A careful preoperative planning is necessary to select patients and to determine the clipping sites. Strict guidelines have been defined (Feldman et al., 2009) but current selection criteria still lack predictive power with respect to complications and effectiveness of the therapy in specific patients. It is not uncommon to perform several attempts during intervention and, in some cases, decide to place two clips ( 30% of the patients) or even to abort the procedure due to complications ( 10% of the patients) (Feldman et al., 2009). Thus, there is a need for an efficient and predictive framework that can assist the clinicians in planning the MitralClip procedure and guide them during intervention Technical Background: Computational Models of the Mitral Valve Geometrical Models of MV Anatomy Comprehensive assessment of MV physiology requires detailed modeling of patient MV anatomy and dynamics from images. However, the complexity of MV anatomy and its fast dynamics make its accurate delineation from medical images difficult. Ultrasound images, and 3D+t transesophageal (TEE) in particular, are the images of choice to evaluate MV function in patients. They not only show the dynamics of the structure but also enable clinicians to compute diagnostic parameters through tedious and timeconsuming delineation, with little computational assistance (Chandra et al., 2011; Jassar et al., 2011). More automatic methods have been proposed to make MV assessment more efficient. Burlina et al. (2010) proposed an interactive algorithm based on thin-tissue detection and level-set deformable models to identify the MV and the LV endocardium in 3D TEE images. Detailed geometrical models were obtained, enabling the authors to perform patient-specific MV simulations. However, several user interactions were still necessary to guide the al- 3
5 gorithm and no automation was provided to estimate MV anatomy throughout the cardiac cycle consistently as the method deals with frames individually. Schneider et al. proposed a complex pipeline to automatically delineate the MV from 3D+t TEE images, obtaining results with promising accuracy. The method relied on mitral annulus detection and tracking (Schneider et al., 2011b), leaflet segmentation of the open valve (Schneider et al., 2011a) and leaflet tracking using a deformable model that handled contacts and chordae stresses (Schneider et al., 2011d). Temporal resampling of 3D+t TEE images acquired on multiple heartbeats was proposed to improve temporal consistency (Schneider et al., 2011c). However, it is not clear how the authors approach generalizes on large populations, with a wider spectrum of MV diseases, because of the numerous parameters to set. In (Ionasec et al., 2010), we provided a fast and accurate method based on machine learning to detect the MV on 3D+t TEE or CT images. The method has been recently extended to all heart valves (Grbić et al., 2010) and papillary tips (Voigt et al., 2011b). Biomechanical constraints have also been added to improve the robustness of MV tracking (Voigt et al., 2011a). The algorithm has been validated on hundreds of patients with various heart valve diseases, showing very good robustness and accuracy Biomechanical Models of MV Physiology Quantifying MV function might not be sufficient to plan the optimal treatment for a specific patient. Mechanical insights are necessary to predict how pathological MV dynamics will be modified after intervention. Furthermore, a comprehensive understanding of MV physiology is crucial in order to design long-term treatments that do not alter normal LV function. To address these questions, computational models of MV physiology have been proposed. Since the pioneering work of Kunzelman et al. (1993), several models have been proposed and new insights on MV function have been obtained. Two categories of computational MV models can be distinguished: structural models and fluid-structure interaction (FSI) models. Structural Models. Structural models aim to simulate the biomechanics of MV apparatus without directly considering the blood that flows across it. The standard approach is to use finite-element models (FEM) to solve the dynamics equation of MV leaflets, under chordae tension, surface pressure and boundary conditions (Kunzelman et al., 1993). Chordae were identified as non-linear tensile tissues very early (Kunzelman and Cochran, 1990). 4
6 Biaxial stress-stretch experiments revealed that leaflets are non-linear tissues, owing to crimped collagen fibers that unfold when the leaflet are under stress, and anisotropic, as these fibers are mostly oriented parallel to the mitral annulus (May-Newman and Yin, 1995; Grashow et al., 2006; Sacks et al., 2009). Thus, although some studies relied on anisotropic linear elasticity (Kunzelman et al., 1993; Schievano et al., 2009; Krishnamurthy et al., 2009), leaflets biomechanics are mostly modeled using hyperelasticity theory (May-Newman and Yin, 1998; Prot et al., 2007). These studies enabled to analyze leaflet stress distribution during valve closure (Prot et al., 2007; Votta et al., 2008; Stevanella et al., 2009) and to quantify the effects of material properties on valve closure (Prot et al., 2010). They also highlighted the key role of MV annulus motion and papillary tips position for optimal valve closure (Stevanella et al., 2009). Considered as passive tissues until recently, leaflets may actually be active as revealed by recent studies (Krishnamurthy et al., 2009; Stevanella et al., 2011a), responding to atrial contraction. Computational models simulating this property (Skallerud et al., 2011; Swanson et al., 2011) suggested that MV shape at systole may be driven by the systolic stiffening of the leaflets. Alternatives to FEM that require less computational power have been investigated for surgery training and therapy planning. In (Hammer et al., 2011), the authors simulated MV closure using an anisotropic mass-spring model whose stiffness is fitted to a typical leaflet stress-strain energy. The authors successfully tested their approach on ex-vivo pig data. Burlina et al. (2010) developed a stationary analysis framework for MV closure simulation based on shape-finding finite elements. Given a triangulated mesh, the algorithm finds the equilibrium position of its vertices by minimising a total energy that comprises external forces, contact forces, tethering forces and linear elastic energy. Sprouse et al. (2011) extended this approach to hyperelastic materials to estimate leaflet stresses. The approach was applied on two in-vivo cases, resulting in promising prediction errors of 3 mm although MV annulus was deformed based on global geometrical parameters. Fluid-Structure Interaction Models. While structural models focus on MV biomechanics, FSI models aim to study the interactions between the MV and the blood flow by coupling FEM MV models with computational fluid dynamics (CFD) of blood flow (Einstein et al., 2010). Common approaches rely on Lagrangian frameworks (Kunzelman et al., 2007; Lau et al., 2010) or immersed boundaries methods (Watton et al., 2008). While FSI models 5
7 are necessary for comprehensive analysis of MV physiology, they are significantly more complex to solve. Additionally, blood flow boundary conditions are required, which may be difficult to measure from standard clinical data. The standard approach is to immerse the MV into an idealized fluid domain (tubes (Watton et al., 2008), boxes (Einstein et al., 2010) or idealized ventricular geometries (Lau et al., 2010)), which may not reflect the actual condition of the patient. Recently, patient-specific LV geometry and dynamics from TEE ultrasound data have been used as boundary conditions to CFD solvers (Sprouse et al., 2009; Voigt et al., 2011b). However, fluid-to-solid interactions were not considered in these studies Computational Analysis of MV Repair As MV modeling reaches maturity, researchers are beginning to investigate the effects of therapies on MV function. Mitral annuloplasty was simulated on a patient-specific anatomy (Votta et al., 2007; Stevanella et al., 2011b), showing that FEM models can be used to determine ring size. Reported simulations were not compared with actual outcomes. Schievano et al. (2009) investigated two different techniques for percutaneous valve dilatation using a linear elastic model (Kunzelman et al., 1993) and idealized geometries. Structural changes due to edge-to-edge repair were also simulated (Votta et al., 2002; Avanzini, 2008). In (Avanzini et al., 2011), the authors concluded that MitralClip intervention yields leaflet stresses similar to those resulting after the surgical edge-to-edge procedure. Finally, FSI modeling of the edge-to-edge procedure on idealized valve geometry identified an increased fluid jet velocity due to the double orifice (Lau et al., 2011). However, all these studies could not be evaluated against clinical data on large populations due to their idealized framework or the tedious process to get patient-specific anatomical models Aim of the Study Translating computational models of MV physiology to clinical practice remains a tremendous challenge. One of the major difficulty is the lack of integrated and efficient framework for MV modeling based on patient data. Studies based on in-vivo patient images are being reported but timeconsuming manual delineations are still required, with no (Stevanella et al., 2011b; Conti et al., 2010) or partial automation (Votta et al., 2008; Burlina et al., 2010). Because of the user interactions, validation is still limited to 6
8 Patient Images MV Apparatus Estimation Volumetric Anatomical Model MV Closure Simulation Outcome Prediction Therapy Simulation Figure 2: The main steps of the proposed framework for MV closure simulation few subjects (usually 5). Therefore, current approaches can hardly scale up and may yield inconsistent results due to user variability. As a first step towards patient-specific MV FEM, we propose in this manuscript an integrated framework that combines efficient machine-learning methods with a biomechanical model of the valve apparatus to simulate MV function and therapies in patients. As illustrated in Fig. 2, and detailed in Sec. 2, our approach first estimates a comprehensive anatomical model of MV apparatus, including papillary tips, from the images. We then automatically build a detailed volumetric model comprising leaflet fibers, and simulate MV closure based on a biomechanical model. After parameter sensitivity analysis, we evaluated the generalization of our biomechanical model with respect to tissue properties, i.e. how accurately MV closure can be simulated by using patient geometry and boundary conditions but standard tissue parameters. MV closure was simulated on 25 consecutive patients and compared with ground truth, showing that good results can be achieved (mean point to mesh error: 1.49±0.62 mm) but also highlighting the importance of personalizing tissue biomechanical parameters. Finally, we applied our framework to MitralClip planning by simulating the intervention on the preoperative data of one retrospective patient. Comparison with the real outcome in terms of MV closure suggested a promising prediction power. This study extends our previous work (Mansi et al., 2011) as follows: i The anatomical model used to simulate MV closure is closer to experimental data reported in the literature, more especially leaflet thickness (Kunzelman et al., 2007; Conti et al., 2010). ii The biomechanical model is improved to consider tissue anisotropy. MV 7
9 fiber orientation is modeled as in (Prot et al., 2009). iii A sensitivity analysis of the main biomechanical parameters is carried out to identify the parameters to adjust first when calibrating the model. iv The generalization of the biomechanical model with respect to tissue properties is tested on 25 cases. v A first personalization of chordae biomechanics is reported on three cases. 2. Methods 2.1. Estimation of Mitral Valve Apparatus from 3D+t TEE Images In this study, MV anatomy is estimated from 3D+t TEE images. The model comprises (Fig. 3): the mitral annulus, the anterior and posterior leaflets (henceforth denoted AL and PL respectively), and the anterior and posterior papillary tips. To capture a broad spectrum of morphological variations, the model is parameterized by three coarse-to-fine components: i) Three transformations B for global location, orientation and scale over the cardiac cycle; ii) The trajectories of nine anatomical landmarks L(B) = (l 1...l 9 ) R 3 9 (two trigones, one posterior annulus mid-point, two commissures, two leaflet tips and two papillary tips); and iii) a triangulated surface mesh S LA (B,L) to represent the left atrial (LA) surface of both AL and PL. The position of the vertices of the LA surface is constrained by the anatomical landmarks, resulting in an anatomically consistent parameterization (Ω, u, v) that ensures intra- and inter-patient point correspondence (Ionasec et al., 2010). Ω is the vertex of the mitral annulus that is directly perpendicular to the anterior commissure, u (v resp.) is the curvilinear coordinate tangent (radial resp.) to the annulus. u res and v res are the resolutions along the u and v coordinates respectively. B, L(B) ands LA (L, B) are estimated from the images using a hierarchical discriminative learning algorithm (Fig. 4). The probability p(b,l,s I) knowing the image data I is incrementally modeled within the Marginal Space Learning (MSL) framework, based on the Probabilistic Boosting Tree (PBT). Intuitively, given a test image, MLS framework first finds position candidates around the MV based on Haar- and steerable features. The position candidates are then successively refined by rotation and scaling candidates. This defines a region of interest inside which the position of nine landmarks is estimated using the same strategy. Next, a point-distribution model of the MV surface is mapped according to the landmarks and deformed, within the learned space of shapes, according to boundary detectors 8
10 Figure 3: Anatomical model of the MV and sub-valvular apparatus that is estimated from clinical images. Leaflets are uniquely parametrized through the curvilinear coordinates (Ω, u, v) calculated from the detected landmarks estimated through PBT. Finally, the complete MV anatomy is tracked over the cardiac sequence using a manifold-based motion model. The reader is referred to (Zheng et al., 2008; Ionasec et al., 2010) for further details. 3D+t TEE Input Sequence Global Location and Rigid Motion Landmark Trajectories Full MV Apparatus Figure 4: The main steps of the automatic estimation pipeline. We first estimate the global position of the MV, then detect the MV landmarks inside this region of interest and finally map and deform triangulated surfaces to segment the MV leaflets Volumetric Anatomical Model of the MV Apparatus In a second stage, we extend the surface model by i) generating a thick, tetrahedral representation of MV leaflets, ii) adding MV leaflet fiber orientation to capture tissue anisotropy and iii) modeling MV chordae. 9
11 Figure 5: Left panel: Tetrahedral mesh of MV leaflets. Colors encode element tag for regional personalization. Right panel: Computational model of MV fiber orientation. Fibers are oriented mainly parallel to the annulus. On the anterior leaflet, fibers become radial close to the commissures. Colors encode fiber direction Tetrahedral Representation of MV Leaflets. MV biomechanics are computed using tetrahedral FEM (Sec. 2.3). The thick geometry of MV leaflets must therefore be modeled but due to the inconsistent quality of ultrasound images, accurate measurement of leaflet thickness from the images is still challenging. Instead, we compute the LV surface of the leaflets, S LV, by extruding the previously S LA towards the ventricle. The surfaces S LA and S LV are merged at the free edges and annulus to obtain the thick geometry. Leaflet thickness is directly controlled by the extrusion depth, here 1.32 mm and 1.26 mm for the AL and PL respectively, as in (Votta et al., 2008; Kunzelman et al., 2007). Tetrahedral elements are created between S LA and S LV by connecting the surface vertices. Thus, the number of tetrahedra is directly controlled by the parameters u res and v res, and point correspondence is ensured across time frames and patients. For regional personalization, each element is automatically tagged according to the leaflet it belongs to (Fig. 5, left panel). MV Fibers Modeling. Current in-vivo imaging technology cannot quantify the orientation of leaflet collagen fibers. Thus, their direction was modeled as in (Prot et al., 2009), following the experimental observations reported in (May-Newman and Yin, 1995). Fibers are mainly parallel to the annulus (circumferential direction). For the AL, the fibers close to the commissures gradually rotate to become perpendicular to the annulus (radial direction) (Fig. 5, right panel). MV Chordae Modeling. 28 marginal chordae are defined, evenly attached between papillary tips and leaflet free edges (Hammer et al., 2008; Votta et al., 10
12 Figure 6: Tendineae chordae and boundary conditions (Patient 25). Chordae are inserted in the leaflets at the same spatial location for all patients owing to the point correspondence. Mitral annulus and papillary tips motions are prescribed from image data ). Four basal chordae are attached to each leaflet, two for each papillary tip (Fig. 6). To avoid any bias in the evaluation, insertion points are identical across subjects owing to the point correspondence. More precisely, the insertion points of the marginal chordae are defined by (u, v) =(ku res /28,v res 1), where k [[0 : 27]]. Basal insertion points are defined by (u, v) = ((2i + 1)u res /8, (v res 1)/2) and (u, v) = ((2i+1)u res /8+1, (v res 1)/2), i [[0 : 3]] Biomechanical Model of Mitral Valve Apparatus Valve closure is simulated by solving the dynamics system: MÜ +C U +KU = F c + F p (1) 261 U is the displacement vector of the free vertices of the MV mesh, U the velocity vector and Ü the acceleration vector. M is the lumped mass matrix 262 (a uniform mass density ρ =1.04 g/ml is used, Table 1). K is the stiffness 263 matrix of the internal elastic forces. C is the damping matrix modeling the 264 viscosity of the system. It also increases system robustness under strong 265 collisions. In this work, C is a linear combination of stiffness and mass 266 matrices (Rayleigh damping), C = α M M+α K K. Non-reported experiments 267 identified α M =0.1s 1 and α K =0.1sas a good compromise between system 268 damping and robustness. F c and F p are the forces developed by the chordae 269 and heart pressure respectively. 11
13 Leaflet Passive Properties MV leaflets are modeled as linear, transverse isotropic elastic tissues (Hammer et al., 2008; Schievano et al., 2009; Krishnamurthy et al., 2009) as we are not directly interested in leaflet stresses during the entire cardiac cycle but we rather seek to predict how well they close during systole to assess possible residual regurgitant holes after intervention. Besides, recent works based on inverse-problem methods suggested that the leaflets may behave as linear materials in the range of physiological pressures (Krishnamurthy et al., 2009). In our experiments, different tissue properties are assigned to the AL and PL. The Young moduli corresponding to the tangent elastic modulus measured at large strains are used (values from (Schievano et al., 2009) and estimated experimentally in (Kunzelman et al., 1993)) (Table 1) Chordae Passive Properties Chordae are modeled by piecewise tensile springs between papillary tips, modeled as spatial points, and leaflet insertion points (Fig. 6). Between an insertion point v i,i {marginal, basal} and its corresponding papillary tip p i, we apply the force: f c (v i, p i,t)= k c,i (ɛ c,i,t) (L i (t) L i,0 ), (2) where L i is the current elongation v i (t) p i (t), ɛ c,i (t) =(L i (t) L i,0 )/L i,0 is the strain and L i,0 is the chordae rest length, defined as the distance between the papillary tips and the insertion points estimated at the end diastole time frame. At compression, when ɛ c,i (t) 0, k c,i =0g/mm 2 to model free compression. During tension, the non-linear response of the chordae (Fig. 7, left panel) is approximated using a piece-wise constant stiffness function k c,i (ɛ c,i,t). Spring tensile stiffnesses k c,i are calculated from the chordae Young moduli according to k c,i = A 0,i E c,i /L 0,i where A 0,i is the chordae cross-section at rest. The parameters used in this study were estimated experimentally in (Kunzelman and Cochran, 1990) and reported in Table External Loads, Boundary Conditions and Contacts We simulated valve dynamics between end of diastole, defined as the last time frame when the valve is seen fully open, and the first systolic frame, defined as the first frame when the valve is seen maximally closed (fully closed if not pathological). Since pressure values were unknown for the patients, we assumed a generic profile that increases from 0 mmhg to 120 mmhg (Fig. 7, 12
14 right panel), as in (Prot et al., 2009; Stevanella et al., 2011b; Hammer et al., 2011). The motions of the papillary tips and of the mitral annulus are prescribed from the automatic detection (Fig. 6). In practice the displacement and velocity of the prescribed vertices are projected at every time step of the simulation such that they correspond to the motion observed in the images. Finally, self-collisions are detected using a ray-casting approach (Hermann et al., 2008). At every time step, the distance between a leaflet vertex and the leaflets, along vertex normal, is calculated. If that distance is lower than an alarm distance d c =0.6mm, then a spring is added between the vertex and the collision point to ensure a minimal collision distance of 0.3 mm. Tangential interaction is modeled with a friction coefficient of Basal Marginal Stress (g/mm 2 ) Pressure (mmhg) Strain (%) Normalized Time Figure 7: Left panel: Stress-strain behavior of chordae. Linear approximation in dashed lines (data from (Kunzelman and Cochran, 1990)). Right panel: Pressure profile applied to the leaflets to simulate closure. The time is adjusted for each patient to match valve closure duration MitralClip Simulation Virtual mitral clipping is performed interactively, in real-time, on the open configuration of the preoperative anatomy. The pressures are disabled and mitral annulus and papillary tips are fixed to facilitate user interactions. The user first picks a vertex on each leaflet (Fig. 8A). A stiff spring (k clip = 1000 g/mm) is then created between the two vertices, which makes the leaflets progressively close at the clip location (Fig. 8B) according to their internal constitutive law and chordae tension. The stiffness of the spring is strong enough to hold the leaflets together and model the clip (Fig. 8C). 13
15 Table 1: Parameters of the biomechanical model Leaflets (from Schievano et al., 2009) Mass density ρ =1.04 g/ml Poisson ratio ν =0.488 AL Young s modulus E ALf =6.233 MPa, E ALf =2.350 MPa AL shear modulus G ff =1.369 MPa PL Young s modulus E PLf =2.087 MPa, E PLf =1.887 MPa PL shear modulus G ff =0.694 MPa Marginal Chordae (from Kunzelman and Cochran, 1990) Young s modulus (ɛ <2.5%) E c = 312 g/mm 2 Young s modulus (ɛ 2.5%) E c = 3406 g/mm 2 Cross-section A 0 =0.40 mm 2 Basal Chordae (from Kunzelman and Cochran, 1990) Young s modulus (ɛ <5%) E c =66g/mm 2 Young s modulus (ɛ 5%) E c = 2120 g/mm 2 Cross section A 0 =2.05 mm 2 General Time step dt =0.01 s Mesh resolution u res = v res =30 Number of marginal chordae 28 Number of basal chordae 4 k clip 1000 g/mm Once the clip is implanted, postoperative MV closure is simulated to assess the efficacy of the intervention in terms of MV regurgitation, just after clip release. To that end, we assume the ventricle motion to be similar to the preoperative motion, as only the morphology of the leaflets changed. Preoperative boundary conditions and ventricular pressures are applied on the clipped geometry to simulate closure Implementation The biomechanical model is implemented using SOFA 1,anopen-source soft-tissue intervention framework (Allard et al., 2007). Eq. (1) is solved using co-rotational linear tetrahedral finite elements to cope with large deformations and rotations (Nesme et al., 2005). The simulation time is scaled such that the simulated MV closure is ten times longer than what is observed
16 v AL v AL v PL v PL A B C v AL v PL Figure 8: Interactive simulation of MitralClip procedure. The user picks two vertices on each leaflet (v AL and v PL )(A). A spring is created between the picked vertices (blue lines) that will progressively (B) stitch the leaflets together (C) in the images (from 70 ms to 150 ms) to handle the strong and discontinuous contact forces, as in (Votta et al., 2008). An implicit Euler solver is used to update mesh positions (Baraff and Witkin, 1998). We finally stress that the pipeline is completely integrated, from model estimation to simulation, although the user can manually adjust the models if necessary. 3. Experiments and Results Three experiments were carried out. We first evaluated the accuracy of the anatomical model estimation. We then performed a sensitivity analysis of our biomechanical model, followed by a systematic evaluation on 25 consecutive subjects. We finally applied our framework to predict MitralClip intervention in one case for whom postoperative data was available Validation of the MV Anatomical Model The performance of the automatic estimation of the MV apparatus was evaluated on 200 3D+t TEE images from 120 patients with a wide range of diseases (MV prolapse, calcified mitral annulus, stenosis, ventricular dysfunction, etc.). Images were acquired with different angulation, field of view and resolution (spatial and temporal). Three-fold cross-validation against manual delineation yielded an average point-to-mesh error of 2.75 ± 0.86 mm. Qualitative evaluation showed that the MV was faithfully tracked throughout the cardiac sequence, even during valve closure and opening when the dynamics are fast (Fig. 9). Computation time was 4.8 s per 3D volume (Intel Core2Duo, 2.66GHz quad core, 2GB RAM). A thorough evaluation of model estimation is beyond the scope of this study, more comprehensive evaluations 15
17 Patient A Patient B Patient C Patient D Figure 9: Automatic detection of the MV apparatus on 3D+T TEE sequences of four patients with different diseases. The model accurately fits patients MV in the images. 358 can be found in (Ionasec et al., 2010; Grbić et al., 2010; Voigt et al., 2011b) Evaluation of MV Closure Simulation FEM Convergence Analysis We first determined the spatial resolution and the time step that are required for computationally efficient and accurate simulations. To this end, we quantified the numerical convergence of the dynamic system by computing the difference between the simulation results at a given spatial / temporal resolution and the result obtained at the finest discretization. Sub-millimetric errors were considered as satisfactory, compared to image resolution (between mm). The analyses were performed using the MV anatomy and boundary conditions of Patient 1. The parameters reported in Table 1 were used. The rest lengths of marginal and basal chordae estimated from the images were increased by 30% and 10% respectively to better capture the observed MV closure (see Sec and Fig. 17 for details and simulation accuracy with respect to the ground truth). Spatial Convergence Analysis. We first fixed the radial resolution v res =30 (Sec. 2.2) and simulated MV closure with the circumferential resolutions 16
18 u res = 30, v res =30 u res = 40, v res =30 u res = 50, v res =30 Point-to-mesh distance (in mm) Figure 10: Effect of the circumferential resolution u res on the simulation. Only millimetric differences were visible. Colors encode point-to-mesh distance computed with respect to the solution obtained at u res = 50, v res = u res =30, 40, 50, corresponding respectively to 9408, 12768, elements with 1.67 mm, 1.24 mm and 0.98 mm average edge-length. As one can see from Fig. 10, u res had sub-millimetric effects on the results, as confirmed by the point-to-mesh distance with respect to the result obtained with the finest mesh, i.e. u res =50(e ures=30 =0.48 ± 0.28 mm, e ures=40 =0.46 ± 0.25). We then fixed u res = 30 and used v res =10, 20, 30, corresponding respectively to 2688, 6048 and 9408 elements with 4.89 mm, 2.49 mm and 1.67 mm average edge-length. As it can be seen in Fig 11, the simulation at v res =10was significantly different from the others (point-to-mesh distance with the result obtained with v res = 30: e vres=10 =0.74 ± 0.85 mm). In particular, the PL position was different. Results at v res =20andv res = 30 were closer to each other (e vres=20 =0.31 ± 0.23 mm), suggesting numerical convergence at v res = 30. Those results also hold for non-reported simulations at higher v res and u res. Therefore, meshes generated with u res = v res = 30 (9408 elements, 1.67 mm average edge-length) were used for all subsequent simulations. Temporal Convergence Analysis. Although Euler implicit scheme theoretically allows large time steps dt, collision detection can be hampered if dt is too large, when displacement amplitude can be larger than collision alarm distance d c (Sec ). We investigated the effects of time discretization on 17
19 u res = 30, v res =10 u res = 30, v res =20 u res = 30, v res =30 Point-to-mesh distance (in mm) Figure 11: Effect of the radial resolution v res on the simulation. Atv res = 10, MV closure simulation yielded a totally different result (see posterior leaflet), while convergence can be seen for v res the simulations by computing MV closure with dt =10 1 s, 10 2 s, 10 3 s and 10 4 s. Fig. 12 reports the point-to-point distances between the closed MV computed with dt =10 1 s, 10 2 s and 10 3 s and the closed MV computed with dt =10 4 s. Numerical convergence was clearly reached at dt =10 3 s (mean error: 0.03 ± 0.04 mm, max.: 0.21 mm). dt =10 2 s yielded already voxel accuracy (mean error: 0.20 ± 0.20 mm, max.: 1.84 mm) but dt =0.1s was too large (mean error: 0.62 ± 0.61 mm, max.:3.50 mm). With that time step, inter-leaflet collisions were not detected due to the large displacements, and both leaflets interpenetrated (red zone in Fig. 12). dt =10 2 s seemed to be a good compromise between accuracy and computational efficiency Sensitivity Analysis of Biomechanical Model Parameters We analyzed the sensitivity of our framework with respect to boundary conditions and chordae configuration since these parameters were reported to influence significantly FEM (Stevanella et al., 2009) and mass-spring (Hammer et al., 2011) simulations. We used the same parameters as in the previous section, with u res = v res =30anddt =0.01 s. Sensitivity to Boundary Conditions. We evaluated the effects of boundary conditions on the computed MV closure by successively disabling mitral an- 18
20 dt =10 1 s dt =10 2 s dt =10 3 s Figure 12: Effect of the time step dt on the simulation. Colors encode point-to-point distance computed with the result obtained at dt =10 4 s. Convergence was achieved at dt =10 3 s but dt =10 2 s already yielded voxel accuracy nulus motion, papillary tips motion and both (Fig. 13). When the mitral annulus was fixed, the MV did not close anymore, with a significant regurgitant hole. When the papillary tips were fixed, the AL belly excessively billowed into the atrium. This experiment further confirm the importance of patient-specific boundary conditions, as previously suggested by (Stevanella et al., 2009) based on stress/strain analyses. Sensitivity to Marginal Chordae Properties. Chordae distribution can vary from patient to patient (Kunzelman and Cochran, 1990). We thus investigated the impact of the number of marginal chordae on the simulation by computing MV closure with 56, 28, 20, 16 and 12 marginal chordae evenly attached to the free edges. All other parameters were kept constant (Table 1). MV dynamics are quantified by computing the distance between the leaflet tips and the annulus plane, which was defined by the two trigones and the posterior annulus mid-point (Fig. 3). Not surprisingly, varying the number of marginal chordae modified leaflets dynamics (Fig. 14). For instance, AL free edge came closer to the mitral annulus as the number of chordae decreased, since the leaflet is less taut. This feature was also visible for the PL, although less evident due to smaller tip motion. As the maximum difference in tip displacement was 1.65 mm for the anterior tip and 0.64 mm for the posterior tip, we preferred to use 28 marginal chordae to be consistent 19
21 Ground truth at the same time point Simulation with all boundary conditions Point to mesh distance (in mm) More realistic coaptation and controlled billowing Simulation without prescribed annulus and papillary motion Simulation without prescribed annulus motion Simulation without prescribed papillary motion Incomplete closure Excessive billowing into atrium Figure 13: Effect of boundary conditions on the simulation. Simulations without any of the boundary conditions was significantly less accurate. Mitral annulus motion favored leaflet coaptation while papillary motion helped control anterior leaflet billowing. Distance measured with respect to ground truth with previous studies (Stevanella et al., 2011b). It should be noted though that local MV dynamics can be modified if chordae distribution is locally changed, to model chordae rupture for instance. We evaluated the sensitivity of the simulation with respect to chordae stiffness. MV closure was computed with stiffnesses varying from 30% to +30% of the standard values (Fig. 14, mid panel). We used 28 marginal chordae for that experiment. Interestingly, the variations were similar both in trends and amplitude to those obtained in the previous experiment (maximum difference in tip displacement 0.44 mm for the anterior tip, 0.46 mm for the posterior tip). This can be explained by the law of parallel springs, since chordae were always evenly distributed. In both experiments, all configurations yielded identical results at t< 40ms. During that time interval, all chordae were under compression (no tension), as papillaries and leaflet free edges were moving synchronously. Leaflets were thus free to move. Differences appeared only later, when chordae started to tense and control leaflets motion. Chordae rest length L i,0 (Sec ) was also reported to have a significant influence on MV dynamics (Hammer et al., 2011). In our framework, L i,0 is the distance between papillary tips and insertion points at end diastole. Computing MV closure with L i,0 ± 10%, 20% and 30% yielded significant 20
22 differences in simulation results (Fig. 14, right panels). By changing the rest length from 30% to +30%, differences in tip motion amplitude of mm for the anterior tip and 3.87 mm for the posterior tip were observed. When the rest length was too long, the leaflets were free to fold and the tips eventually attained the mitral annulus plane. When the rest length was too short, the leaflets did not close at all. It is therefore crucial to set up this parameter accordingly for each patient for accurate simulations. Based on these results, a three-step personalization strategy can be derived. First, insertion points distribution is assessed such that the main features of the closed valves are captured. This can be achieved by starting with a homogeneous distribution and then removing chordae apparently ruptured. The insertion points are then fixed and the rest length estimated individually such that the position of the free edges matches what is observed in the images. Finally chordae stiffnesses are adjusted to further refine the simulation results. The process is iterated until convergence Biomechanical Model Generalization with Respect to Tissue Properties Our integrated framework enabled us to evaluate the generalization of the biomechanical model in terms of tissue parameters on 25 consecutive patients. The objective was to assess how well MV closure can be captured using patient-specific anatomy and boundary conditions only, tissue properties being set according to Table 1. Patient Selection and Experimental Protocol. 25 patients were randomly selected from five hospitals, representing a large spectrum of heart diseases (MV diseases, aortic valve diseases, myocardium infarction, etc.). For these patients, standard 3D+t TEE images of the MV were acquired (image resolution: mm isotropic, number of frames: 7 28 (median: 14), covering the full cardiac cycle). MV closure was visible during two to four frames (average duration: s). The dynamic models of MV anatomy were automatically estimated on all time frames of the sequences. To minimize bias in the evaluation of the simulation, an expert (M.H.) carefully verified the estimated models. When errors were visible, the expert could interactively manipulate the landmarks and leaflet surfaces in user-friendly 2D views, the shape constraint and temporal model ensuring anatomical and temporal consistency automatically (Ionasec et al., 2010). In particular, papillary tips were carefully verified using all temporal information. The resulting models were then used as ground truth. 21
23 Anterior tip to mitral annulus plane distance (in mm) Number of Chordae Chordae Stiffness Chordae Rest Length Distance to Mitral Annulus (mm) Time (ms) Distance to Mitral Annulus (mm) % 80% 90% 100% 110% 120% 130% Time (ms) Distance to Mitral Annulus (mm) % 80% 90% 100% 110% 120% 130% Posterior tip to mitral annulus plane distance (in mm) Time (ms) Number of Chordae Chordae Stiffness Chordae Rest Length Distance to Mitral Annulus (mm) Time (ms) Distance to Mitral Annulus (mm) % 80% % 100% 9 110% 120% % Time (ms) Distance to Mitral Annulus (mm) % 80% % 100% 9 110% 120% % Time (ms) Figure 14: Effect of chordae parameters on the simulation. Chordae rest length had a significant influence on MV dynamics, compared to the number of marginal chordae and chordae stiffness. Chordae rest length can also alter MV dynamics by modifying inter-leaflet collisions (pink curve, right panels) The anatomical model at the time frame just before closure, which corresponds to the end-diastole time point, was used to simulate MV closure. Boundary conditions were estimated from the images. The pressure profile described in Sec was applied and simulation was scaled as described in Sec The simulated closed valve was compared to the ground truth estimated at the first time frame at which the MV was observed closed. MV Closure Simulation. Table 2 reports the average point-to-mesh distances from the simulations to the ground truth for every patient. Populationwise, the average error was 1.49 ± 0.62 mm, which was of the same order of magnitude of the automated detection and in the range of values reported in the literature (Burlina et al., 2010; Hammer et al., 2011; Sprouse et al., 2011). We qualitatively evaluated the results by ranking them as satisfactory if the 22
24 closure observed in the images was captured (Fig. 15), passable if closure was only partial (Fig. 16), or unsatisfactory if the valve was still open or not corresponding to the observation (Fig. 17). With those criteria, simulations were qualified as satisfactory in seven patients (28%, average point-to-mesh error: e mean =1.07 ± 0.36 mm, e max =1.66 mm), passable in nine patients (36%, e mean =1.38 ± 0.31 mm, e max =2.01 mm) and unsatisfactory in nine patients (36%, e mean =1.91 ± 0.77 mm, e max =3.13 mm). Table 2: Point-to-mesh distances between simulation and ground truth. Values reported as mean ± SD (95 th - percentile). The average error (1.49 ± 0.62 mm) was of the same order of magnitude as that of the automatic detection (2.75 ± 0.86 mm)., and denote satisfactory, passable and unsatisfactory simulations. Patient Point-to-Mesh Error (mm) Patient Point-to-Mesh Error (mm) ± 1.50 (4.92), ± 1.81 (5.78), ± 2.09 (6.23), ± 1.09 (3.61), ± 2.56 (8.19), ± 1.04 (3.54), ± 2.91 (8.77), ± 1.64 (4.73), ± 1.43 (4.29), ± 1.14 (3.83), ± 1.14 (3.78), ± 0.91 (2.95), ± 0.52 (1.58), ± 0.72 (2.32), ± 0.71 (2.49), ± 1.57 (4.79), ± 1.00 (3.20), ± 2.03 (6.31), ± 1.33 (4.05), ± 0.87 (2.80), ± 2.96 (9.14), ± 0.84 (2.61), ± 0.84 (2.61), ± 1.31 (4.38), ± 1.68 (5.23), The biomechanical model was able to capture MV closure in seven patients with an accuracy equivalent to the image resolution. The other cases confirmed that tissue properties required further personalization, in particular in a population of patients where leaflet properties and chordae tendineae configurations can vary significantly among individuals. Large errors were often due to non optimal chordae rest length, as observed in patient one and 17 for instance (Fig. 17, black arrows). In these two cases, the AL remained open, pulled down by tensed chordae. Although a complete inverse problem analysis of our population is out of the scope of this manuscript, we evaluated the ability of our model to capture valve closure for those two patients and for one case rated as satisfactory (Patient 10) by manually adjusting 23
25 chordae rest length based on the full dynamic data. Chordae were grouped per papillary and per type (marginal and basal), yielding four groups. For each group, chordae rest length was multiplied by a factor, which was determined by qualitatively minimizing the differences between the simulation results and the models computed from the images. The number of chordae and their stiffness were fixed to their default values. Fig 17 shows the results obtained before and after personalization for Patient one and 17. Fig 18, top row, shows the results for Patient 10. Point-to-mesh errors after personalization are reported in Table 3. As it can be seen from the figures, adjusting chordae rest length significantly improved the accuracy of MV closure prediction. Further improvement could be reached by adjusting all biomechanical parameters at once through inverse problem methods (Powell, 2008). Table 3: Point-to-mesh distance between simulation and ground truth after chordae rest length adjustment in three cases. Values reported as mean ± SD (95 th - percentile). Personalizing MV chordae rest length enabled us to better capture MV closure than with standard rest length (Table 2). Patient Point-to-Mesh Error (mm) ± 0.87 (2.96) ± 1.10 (3.40) ± 1.27 (4.25) Computation Time. Simulation was performed in 0.3 frames per second on a desktop machine (Intel Xeon, 2.40GHz octo-core, 4GB RAM), monocore execution, which amounts to 10 min. No particular optimizations were implemented. Simulation profiling showed that about 75% of computational costs were dedicated to collision handling. GPU-based collision methods (Courtecuisse et al., 2011) can therefore significantly speed-up the simulation. The entire process, from the 3D+t TEE images to anatomical model verification to the forward simulation (no personalization), took about 14 min. Chordae rest length personalization was achieved by running several forward simulations in parallel (six simulations at a time). The full process took less than one hour for the three cases reported in Table Simulation of MitralClip As it has been shown in the previous section, it is possible to find a set of parameters that would enable one to simulate the MV closure of a 24
26 Patient 07 Open MV Closed MV Simulation Patient 08 Patient 12 Point-to-mesh distance to ground truth (in mm) Figure 15: Simulated MV closure in three patients compared to observed MV shape. These cases were visually ranked as satisfactory. 25
27 Patient 16 Open MV Closed MV Simulation Patient 25 Point-to-mesh distance to ground truth (in mm) Figure 16: Simulated MV closure in two patients compared to observed MV shape. Partial MV closure was achieved in these cases, mainly because of an incomplete closure of posterior leaflet. 26
28 Open MV Patient 1 Simulation with Closed MV Standard Parameters Personalized Simulation Patient 17 Point-to-mesh distance to ground truth (in mm) Figure 17: Simulated MV closure in two patients compared to observed MV shape. Simulation with generic tissue parameters was not able to capture MV closure in these patients. Too short chordae prevented the anterior leaflet to close (black arrow). Adjusting chordae rest length significantly improved the results. 27
29 specific patient. Yet, the computational model may still not be able to predict changes in MV physiology after modifications of cardiovascular parameters or therapies. We tested the ability of our framework to predict the outcome of MV intervention based on preoperative data only. Intra-operative 3D+t TEE images of the MV before, during and after MitralClip release were available for Patient 10. We first improved the accuracy of the simulated MV closure before clip release by slightly personalizing chordae rest length (10% higher than the distance between papillary tips and leaflet free edges measured at end diastole) (Table 3). We then simulated the MitralClip intervention on the pre-clip anatomy as described in Sec 2.4. The virtual intervention was performed in real-time (2 frames per second). We finally applied the pressure profile on the ventricular surface and the preoperative boundary conditions to predict MV closure just after release of the device. Biomechanical parameters were kept unchanged. Fig 18 illustrates each step of the experiment. The model was able to simulate MV closure after the intervention with results similar to what was observed in the post-clip images, suggesting promising prediction power. One should remark that the difference in mitral annulus shape between the simulated clipped valve and the real outcome is due to the different boundary conditions. Here, preoperative boundary conditions were applied to reproduce a real-case scenario where therapy outcome is unknown Discussion and Conclusions 4.1. Discussion In this work, MV anatomy and boundary conditions were estimated from 3D+t TEE images. Our approach can be easily applied to other imaging modalities (Grbić et al., 2010). Although difficult to visualize, papillaries could be detected by our framework as the temporally-consistent detection algorithm was trained on annotations carefully verified by clinicians (Ionasec et al., 2010). Nonetheless, to avoid bias in the evaluation of the simulations, we utilized anatomical models that were verified and corrected by an expert (M.H.) when required. However, that manual interaction, which took about 1-2 minutes (Stiver et al., 2011), was not a limitation. Recent in-vivo studies highlighted the importance of AL shape in MV closure (Stevanella et al., 2011a). No general trend in AL shape could be observed in our population of patients, but the features reported in (Stevanella et al., 2011a) could be identified in some patients, in particular during systole 28
30 Open MV estimated from images prior to MitralClip Simulated MV closure with generic tissue properties Simulated MV Closure with adjusted chordae rest length Comparison with closed MV from pre MitralClip images MV closure urebetter captured after chordae rest length adjustment Comparison with real outcome from post MitralClip images Simulated post MitralClip MV closure with preop. p boundary conditions Simulation of MitralClip intervention on the preop. anatomy to replicate the actual intervention as seen in the postoperative tive images Virtual intervention on preoperative anatomy Real intervention on as seen on postoperative images Observed in post MitralClip MV closure Predict MV Vclosure Figure 18: The prediction power of the biomechanical model was evaluated by simulating MitralClip intervention on the preoperative anatomy of Patient 10 and comparing the result with the real outcome. The result was qualitatively consistent with the true postoperative outcome (Fig. 19) (these patterns were more difficult to distinguish during diastole). The AL was more concave towards the atrium near the annulus, and more convex close to the free edges in the septolateral direction. In the commissurecommissure direction, the surface was rather convex towards the atrium at the belly. Comprehensive analysis of that aspect and its consequence on leaflet strain and stress distribution will be the subject of a future study. Our sensitivity analysis showed that the motion of the mitral annulus and papillary tips significantly contributed to leaflet coaptation and systolic shape. The observed importance of boundary conditions agrees with other published studies on that aspect, as in (Stevanella et al., 2009). Estimating automatically these boundary conditions from the images is therefore a 29
31 A B B A Septolateral Direction A Commissure - Commissure Direction B B A Figure 19: Mitral valve compound curvature estimated from the images in Patient 1. See text for details key feature of our framework. We also found that chordae rest length had more impact on MV closure than chordae stiffness. Unfortunately, chordae length cannot be measured from the images directly as they are not visible. We thus approximated that parameter as the distance between papillary tips and leaflet free edges at end diastole. This strategy enabled us to simulate the observed MV coaptation in seven cases. For those patients, the initial, open MV configuration was already close to the closed configuration, and therefore the influence of chordae rest length was less significant. Simulated MV closure of valves widely open was more challenging. Co-rotational elements being able to simulate large deformations accurately, the issue was mainly related to chordae rest length, which were under-estimated by our imagebased algorithm, thus preventing the leaflet to close. By increasing the rest length, we managed to capture MV closure in three patients. Inverse problem approaches based on the full MV dynamics can therefore provide more accurate estimations of chordae rest length, although in-vivo validation would be challenging as chordae are not visible in images. In that work, the average point-to-mesh distance was used to evaluate simulation accuracy. That metric is commonly used in the literature, thus allowing performance comparison. Overall, our systematic analysis on 25 cases yielded an accuracy of the same order of magnitude as the one of the automatic detection. However, visual inspection was still necessary to assess if MV closure was correctly captured. More advanced metrics would thus be necessary when designing cost functions for inverse problem algorithms. A necessary way to evaluate computational models is to assess their ability to predict therapy outcome. This task is all the more challenging as parameters may not be unique. We thus simulated MitralClip intervention 30
32 in one patient and compared, with success, model prediction with the real outcome. The virtual intervention was intuitive to perform, our system being fast enough for smooth interactions. Since we were interested in simulating MV closure just after clip release, we considered preoperative data only to mimic a real scenario where postoperative data would not be available. Furthermore, our postoperative data were acquired when the patient was still in the operating room, with catheters and ultrasound probe still in-place, thus minimizing biases in the comparisons. We assumed non-significant tissue remodeling and kept tissue properties unchanged, although the device may have altered tissue integrity locally. Despite these assumptions, obtained results were promising, encouraging further work towards FEM-based therapy planning. While linear tetrahedra can suffer from locking, and thus under-estimate deformations, co-rotational tetrahedra have been designed to cope with large deformations and rotations with good accuracy, as quantified by benchmark tests (Nesme et al., 2005). Visually, the dynamics of our simulations did not show locking nor ghost torques, which appeared when using linear tetrahedra (non-reported experiments). Co-rotational tetrahedra could therefore constitute an alternative to more conventional hexahedra, as tetrahedra meshes are easier to build from complex patient-specific geometries. Quantitative comparisons between the two spatial discretizations are planned for future work Limitations In this study, MV leaflets were modeled as linear elastic material, whereas biaxial studies showed a strongly non-linear constitutive law (May-Newman and Yin, 1995). Co-rotational tetrahedra were used to cope with large deformations but tissue linearity may yield different tissue stress and strain distributions, which can lead to differences in predicted leaflet position during systole as tissue stretches under external forces. Our framework being modular, computationally efficient methods for hyperelastic materials could be integrated without significant modifications (Marchesseau et al., 2010). Yet, recent studies based on inverse problem suggested that leaflets may have linear behavior in-vivo (Krishnamurthy et al., 2008, 2009), but with Young s moduli higher than those estimated ex-vivo. Using linear elastic models for in-vivo simulations may thus be suitable. This study was performed with standard ex-vivo Young s moduli to be consistent with simulations reported in the literature. Preliminary experiments based on homogeneous in-vivo 31
33 Young s moduli, which were extrapolated to human physiology as detailed in (Krishnamurthy et al., 2008) and extrapolated to the PL, resulted in more accurate AL billowing, as already observed in (Skallerud et al., 2011). However, the overall MV had more difficulty to close, in particular the PL due to a stiffer commissure area. Spatially-varying Young s moduli may increase the accuracy of the simulation. Further investigations and experiments on patient-specific anatomies would be necessary to include these new findings into our model. We modeled leaflets as homogeneous tissues with standard thickness for all patients. It has been shown that leaflets are made up of three layers, with different material properties. Multi-layer models (Prot and Skallerud, 2009) can be beneficial for pathology understanding. However, they introduce additional parameters that may be difficult to estimate from clinical data. Additionally, pathologies can significantly disrupt local tissue properties and leaflet morphology. Modeling these variations will be extremely important to quantify MV biomechanics and predict therapies. Tissue calcification could be quantified from CT imaging for instance, or estimated indirectly from the MV dynamics using inverse problems. Leaflet thickness, which can have significant impact upon MV dynamics, could be estimated from the images. This is possible in CT but more challenging in ultrasound images, which are more prone to noise and artifacts. Tissue viscosity was neglected as we focused on MV closure during one heart cycle only, as in (Prot et al., 2007; Votta et al., 2008). Yet, published studies on livers reported larger deformations when viscosity is added to hyperelastic models (Marchesseau et al., 2010). Numerical and experimental studies are therefore needed to study the impact of viscosity on leaflet deformations. On the other hand, Rayleigh damping was used in our work to mimic the viscous medium in which the leaflet operates, although its parameters are not physiological. One could use leaflet velocity to estimate Rayleigh coefficients, although the same results could be achieved by modifying blood pressure and chordae stiffness. More detailed models that take into account blood flow dynamics and fluid-structure interaction could thus be more appropriate, offering more control on the modeled system as more physically grounded (Einstein et al., 2010). A generic pressure profile was used for all simulations. The choice of that profile was motivated by the fact that for all patients, the first frame in which the MV was maximally closed showed an already opened aortic valve. Since pressure traces were not available, we assumed the pressure 32
34 being already larger than the aortic pressure baseline ( 80 mmhg). Yet, the pressure should be personalized as it can vary from patient to patient. Future experiments will be carried out to adjust that parameter based on MV dynamics and flow information. Finally, only MV closure was considered in this study, to predict residual regurgitation after therapy. Full cycle simulation may provide insights on the entire MV dynamics and tissue stresses for long-term therapy prognosis. This aspect of MV function will be investigated in future work Perspectives Our framework opens new technical and clinical avenues. First, the difference between in-vivo and ex-vivo leaflet behavior is nowadays believed to be due to an active force that modifies tissue stiffness throughout the cardiac cycle (Krishnamurthy et al., 2009; Stevanella et al., 2011a; Swanson et al., 2011). That mechanism further controls the systolic shape of the AL, which confers a funnel shape on the left ventricle outflow tract and optimize flow motion towards the aorta. Integrating an active component in our biomechanical model will certainly provide further insights into MV physiology (Skallerud et al., 2011). However, additional parameters would be necessary to estimate, which may be challenging to estimate from standard clinical data. Furthermore, it is not clear whether explicitly modeling the active force would improve the predictive power of the model, compared to hyperelastic materials or linear elastic models with a time varying stiffness. Numerical studies with subject-specific geometries are necessary to assess that question. Model personalization is another challenge that needs to be addressed for patient-specific modeling. As demonstrated by the experiments herein, patient-specific geometries and boundary conditions are necessary but not sufficient to simulate MV closure. Tissue properties and chordae anatomy need to be estimated. As our framework is able to estimate dynamic MV models from images, a next step of our study will be to use inverse problem methods to estimate the tissue properties from MV dynamics. Estimating tissue parameters is challenging due to the large parameter space and parameter ambiguity. That is why it is important to use models with few parameters, which are physiologically meaningful and have direct control on the simulation results. Chordae rest length is an example of such a parameter, although its effect can be confused with those of the pressure (which could be solved if one has pressure traces for instance). Similar uniqueness issues arise for leaflet tissue properties, in particular if active stiffening is 33
35 considered. A solution would be to add more data to constrain the problem, such as dynamics images at different heart rates, flow measurements, etc. In this study we predicted leaflet closure after MitralClip based on preoperative data. Yet, the intervention can modify heart function as regurgitations are treated. Ventricular systolic pressure could have raised after mitral regurgitation repair as more blood was ejected into the aorta (no data was available), eventually resulting in changes of mitral annulus motion as the myocardium compensates for the increased pressure to preserve ejection fraction. A more detailed model coupling LV and MV (Wenk et al., 2010) may increase prediction accuracy, at the price of additional parameters to adjust. Similarly, it would be interesting to investigate the effects of the device. For instance, tissue properties may have been altered locally by the mechanical clipping. The device may also locally modify fluid patterns, and therefore alter pressure distribution. Numerical studies may provide insights on the effects of the device Conclusions Translating computational models of MV physiology to clinical practice is a tremendous challenge. Thanks to the high degree of automation, our system enables rapid and interactive simulations of MV function. The user can therefore test therapies in-silico and assess their outcome. That level of integration will enable one to evaluate the computational models of MV on larger populations, a necessary step before deploying these methods in clinical settings. Our system may thus constitute a first step towards clinical application of MV computational model. From a research point of view, the proposed framework paves the way to quantitative and systematic evaluation of computational models of the MV. There is growing evidence that patient-specific anatomical models are necessary to fully comprehend MV physiology, in particular in the light of recent in-vivo studies (Krishnamurthy et al., 2008; Stevanella et al., 2011a). By providing an integrated system for patient-specific modeling, our system may be used as input to more comprehensive inverse problem studies. In conclusion, we have presented an integrated framework for FEM modeling of MV closure in patients. The level of integration reached by our framework enabled us i) to perform a sensitivity analysis of MV FEM on patient-specific geometry; ii) to test the generalization of a biomechanical model in terms of tissue properties in 25 patients, which, to the best of our knowledge, constitutes the largest in-vivo FEM study reported so far; and iii) to simulate and predict MitralClip intervention outcome in terms of valve 34
36 closure in one patient. Our results suggest that FEM models may constitute surrogate tools for mitral valve repair planning. References Allard, J., Cotin, S., Faure, F., Bensoussan, P., Poyer, F., Duriez, C., Delingette, H., Grisoni, L., SOFA - an open source framework for medical simulation, in: Medicine Meets Virtual Reality (MMVR), pp Avanzini, A., A computational procedure for prediction of structural effects of edge-to-edge repair on mitral valve. Journal of biomechanical engineering 130, Avanzini, A., Donzella, G., Libretti, L., Functional and structural effects of percutaneous edge-to-edge double-orifice repair under cardiac cycle in comparison with suture repair. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine 225, Baraff, D., Witkin, A., Large steps in cloth simulation, in: Proceedings of the 25th annual conference on Computer graphics and interactive techniques, ACM. pp Burlina, P., Sprouse, C., DeMenthon, D., Jorstad, A., Juang, R., Contijoch, F., Abraham, T., Yuh, D., McVeigh, E., Patient-specific modeling and analysis of the mitral valve using 3d-tee. Information Processing in Computer-Assisted Interventions, Chandra, S., Salgo, I., Sugeng, L., Weinert, L., Settlemier, S., Mor-Avi, V., Lang, R., A three-dimensional insight into the complexity of flow convergence in mitral regurgitation: adjunctive benefit of anatomic regurgitant orifice area. American Journal of Physiology-Heart and Circulatory Physiology 301, H1015 H1024. Conti, C., Stevanella, M., Maffessanti, F., Trunfio, S., Votta, E., Roghi, A., Parodi, O., Caiani, E., Redaelli, A., Mitral valve modelling in ischemic patients: Finite element analysis from cardiac magnetic resonance imaging, in: Computers in Cardiology, IEEE. pp
37 Courtecuisse, H., Cotin, S., Allard, J., Soler, L., Gpu-based interactive simulation of liver resection, in: ACM SIGGRAPH 2011 Computer Animation Festival, ACM. pp Einstein, D., Del Pin, F., Jiao, X., Kuprat, A., Carson, J., Kunzelman, K., Cochran, R., Guccione, J., Ratcliffe, M., Fluid-structure interactions of the mitral valve and left heart: Comprehensive strategies, past, present and future. International Journal for Numerical Methods in Biomedical Engineering 26, Feldman, T., Kar, S., Rinaldi, M., Fail, P., Hermiller, J., Smalling, R., Whitlow, P., Gray, W., Low, R., Herrmann, H., et al., Percutaneous mitral repair with the mitraclip system: Safety and midterm durability in the initial everest (endovascular valve edge-to-edge repair study) cohort. Journal of the American College of Cardiology 54, Grashow, J., Yoganathan, A., Sacks, M., Biaixal stress stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Annals of biomedical engineering 34, Grbić, S., Ionasec, R., Vitanovski, D., Voigt, I., Wang, Y., Georgescu, B., Navab, N., Comaniciu, D., Complete valvular heart apparatus model from 4d cardiac ct, in: Medical Image Computing and Computer-Assisted Intervention MICCAI 2010, Springer. pp Hammer, P., del Nido, P., Howe, R., Anisotropic mass-spring method accurately simulates mitral valve closure from image-based models, in: Functional Imaging and Modeling of the Heart, Springer. pp Hammer, P., Vasilyev, N., Perrin, D., Del Nido, P., Howe, R., Fast image-based model of mitral valve closure for surgical planning, in: Computational Biomechanics for Medicine (MICCAI 08 Workshop), pp Hermann, E., Faure, F., Raffin, B., Ray-traced collision detection for deformable bodies, in: 3rd International Conference on Computer Graphics Theory and Applications, GRAPP Herrmann, H., Feldman, T., Percutaneous mitral valve edge-to-edge repair with the evalve mitraclip system: rationale and phase i results. EuroIntervention 1, A36 A39. 36
38 Ionasec, R., Voigt, I., Georgescu, B., Wang, Y., Houle, H., Vega-Higuera, F., Navab, N., Comaniciu, D., Patient-Specific Modeling and Quantification of the Aortic and Mitral Valves From 4-D Cardiac CT and TEE. Medical Imaging, IEEE Transactions on 29, Iung, B., Vahanian, A., Epidemiology of valvular heart disease in the adult. Nature Reviews Cardiology 8, Jassar, A., Brinster, C., Vergnat, M., Robb, J., Eperjesi, T., Pouch, A., Cheung, A., Weiss, S., Acker, M., Gorman III, J., et al., Quantitative mitral valve modeling using real-time three-dimensional echocardiography: Technique and repeatability. The Annals of thoracic surgery 91, Krishnamurthy, G., Ennis, D., Itoh, A., Bothe, W., Swanson, J., Karlsson, M., Kuhl, E., Miller, D., Ingels Jr, N., Material properties of the ovine mitral valve anterior leaflet in vivo from inverse finite element analysis. American Journal of Physiology-Heart and Circulatory Physiology 295, H1141 H1149. Krishnamurthy, G., Itoh, A., Bothe, W., Swanson, J., Kuhl, E., Karlsson, M., Craig Miller, D., Ingels, N., Stress strain behavior of mitral valve leaflets in the beating ovine heart. Journal of biomechanics 42, Kunzelman, K., Cochran, K., Mechanical properties of basal and marginal mitral valve chordae tendineae. ASAIO Journal 36, M405. Kunzelman, K., Cochran, R., Chuong, C., Ring, W., Verrier, E., Eberhart, R., Finite element analysis of the mitral valve. The Journal of heart valve disease 2, 326. Kunzelman, K., Einstein, D., Cochran, R., Fluid structure interaction models of the mitral valve: function in normal and pathological states. Philosophical Transactions of the Royal Society B: Biological Sciences 362, Lau, K., Diaz, V., Scambler, P., Burriesci, G., Mitral valve dynamics in structural and fluid-structure interaction models. Medical engineering & physics 32,
39 Lau, K., Díaz-Zuccarini, V., Scambler, P., Burriesci, G., Fluid structure interaction study of the edge-to-edge repair technique on the mitral valve. Journal of Biomechanics 44, Maisano, F., Torracca, L., Oppizzi, M., Stefano, P., d Addario, G., La Canna, G., Zogno, M., Alfieri, O., The edge-to-edge technique: a simplified method to correct mitral insufficiency. European Journal of Cardiothoracic Surgery 13, 240. Mansi, T., Voigt, I., Mengue, E., Ionasec, R., Georgescu, B., Noack, T., Seeburger, J., Comaniciu, D., Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure, in: Medical Image Computing and Computer-Assisted Intervention MICCAI 2011, Springer. pp Marchesseau, S., Heimann, T., Chatelin, S., Willinger, R., Delingette, H., Fast porous visco-hyperelastic soft tissue model for surgery simulation: application to liver surgery. Progress in Biophysics and Molecular Biology 103, May-Newman, K., Yin, F., Biaxial mechanical behavior of excised porcine mitral valve leaflets. American Journal of Physiology - Heart and Circulatory Physiology 269, H1319 H1327. May-Newman, K., Yin, F., A constitutive law for mitral valve tissue. Journal of biomechanical engineering 120, 38. Nesme, M., Payan, Y., Faure, F., Efficient, physically plausible finite elements. Eurographics (short papers), Powell, M., Developments of newuoa for minimization without derivatives. IMA journal of numerical analysis 28, Prot, V., Haaverstad, R., Skallerud, B., Finite element analysis of the mitral apparatus: annulus shape effect and chordal force distribution. Biomechanics and modeling in mechanobiology 8, Prot, V., Skallerud, B., Nonlinear solid finite element analysis of mitral valves with heterogeneous leaflet layers. Computational Mechanics 43,
40 Prot, V., Skallerud, B., Holzapfel, G., Transversely isotropic membrane shells with application to mitral valve mechanics. constitutive modelling and finite element implementation. International Journal for Numerical Methods in Engineering 71, Prot, V., Skallerud, B., Sommer, G., Holzapfel, G., On modelling and analysis of healthy and pathological human mitral valves: two case studies. Journal of the Mechanical Behavior of Biomedical Materials 3, Roger, V., Go, A., Lloyd-Jones, D., Adams, R., Berry, J., Brown, T., Carnethon, M., Dai, S., de Simone, G., Ford, E., et al., Heart disease and stroke statistics 2011 update : A report from the american heart association. Circulation 123, e18 e209. Sacks, M., David Merryman, W., Schmidt, D., On the biomechanics of heart valve function. Journal of biomechanics 42, Schievano, S., Kunzelman, K., Nicosia, M., Cochran, R., Einstein, D., Khambadkone, S., Bonhoeffer, P., Percutaneous mitral valve dilatation: Single balloon ver-sus double balloon. a finite element study. Journal of Heart Valve Disease 18, Schneider, R., Burke, W., Marx, G., del Nido, P., Howe, R., 2011a. Modeling mitral valve leaflets from three-dimensional ultrasound, in: Functional Imaging and Modeling of the Heart FIMH 2011, Springer. pp Schneider, R., Perrin, D., Vasilyev, N., Marx, G., del Nido, P., Howe, R., 2011b. Mitral annulus segmentation from four-dimensional ultrasound using a valve state predictor and constrained optical flow. Medical Image Analysis. Schneider, R., Perrin, D., Vasilyev, N., Marx, G., del Nido, P., Howe, R., 2011c. Real-time image-based rigid registration of three-dimensional ultrasound. Medical Image Analysis. Schneider, R., Tenenholtz, N., Perrin, D., Marx, G., del Nido, P., Howe, R., 2011d. Patient-specific mitral leaflet segmentation from 4D ultrasound, in: Medical Image Computing and Computer-Assisted Intervention MICCAI 2011, Springer. pp
41 Skallerud, B., Prot, V., Nordrum, I.S., Modeling active muscle contraction in mitral valve leaflets during systole: a first approach. Biomechanics and Modeling in Mechanobiology 10, Sprouse, C., DeMenthon, D., Gammie, J., Burlina, P., Patient-specific modeling of stress/strain for surgical planning and guidance, in: Engineering in Medicine and Biology Society, EMBC, 2011, IEEE. pp Sprouse, C., Yuh, D., Abraham, T., Burlina, P., Computational hemodynamic modeling based on transesophageal echocardiographic imaging, in: Engineering in Medicine and Biology Society, EMBC Annual International Conference of the IEEE, IEEE. pp Stevanella, M., Krishnamurthy, G., Votta, E., Swanson, J., Redaelli, A., Ingels, N., 2011a. Mitral leaflet modeling: Importance of in vivo shape and material properties. Journal of biomechanics 44, Stevanella, M., Maffessanti, F., Conti, C., Votta, E., Arnoldi, A., Lombardi, M., Parodi, O., Caiani, E., Redaelli, A., 2011b. Mitral valve patientspecific finite element modeling from cardiac mri: Application to an annuloplasty procedure. Cardiovascular Engineering and Technology, /s Stevanella, M., Votta, E., Redaelli, A., Mitral valve finite element modeling: Implications of tissues nonlinear response and annular motion. Journal of biomechanical engineering 131, Stiver, K., Calleja, A., Ionasec, R., Voigt, I., Thavendiranathan, P., Liu, S., Houle, H., De Michelis, N., Ryan, T., Vannan, M., Superior reproducibility of automated 3-d surgical anatomy of normal and abnormal mitral valve when compared to a manual approach, in: American Society of Echocardiography. Swanson, J., Krishnamurthy, G., Kvitting, J., Miller, D., Ingels Jr, N., Electromechanical coupling between the atria and mitral valve. American Journal of Physiology-Heart and Circulatory Physiology 300, H1267 H1273. Voigt, I., Mansi, T., Ionasec, R., Mengue, E., Houle, H., Georgescu, B., Hornegger, J., Comaniciu, D., 2011a. Robust physically-constrained modeling of the mitral valve and subvalvular apparatus, in: Medical Image 40
42 Computing and Computer-Assisted Intervention MICCAI 2011, Springer. pp Voigt, I., Mansi, T., Mihalef, V., Ionasec, R., Calleja, A., Mengue, E., Sharma, P., Houle, H., Georgescu, B., Hornegger, J., et al., 2011b. Patientspecific model of left heart anatomy, dynamics and hemodynamics from 4d tee: a first validation study, in: Functional Imaging and Modeling of the Heart FIMH 2011, Springer. pp Votta, E., Caiani, E., Veronesi, F., Soncini, M., Montevecchi, F., Redaelli, A., Mitral valve finite-element modelling from ultrasound data: a pilot study for a new approach to understand mitral function and clinical scenarios. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 366, Votta, E., Maisano, F., Bolling, S., Alfieri, O., Montevecchi, F., Redaelli, A., The geoform disease-specific annuloplasty system: a finite element study. The Annals of thoracic surgery 84, Votta, E., Maisano, F., Soncini, M., Redaelli, A., Montevecchi, F., Alfieri, O., et al., d computational analysis of the stress distribution on the leaflets after edge-to-edge repair of mitral regurgitation. The Journal of heart valve disease 11, 810. Watton, P., Luo, X., Yin, M., Bernacca, G., Wheatley, D., Effect of ventricle motion on the dynamic behaviour of chorded mitral valves. Journal of Fluids and Structures 24, Wenk, J., Zhang, Z., Cheng, G., Malhotra, D., Acevedo-Bolton, G., Burger, M., Suzuki, T., Saloner, D., Wallace, A., Guccione, J., et al., First finite element model of the left ventricle with mitral valve: insights into ischemic mitral regurgitation. The Annals of thoracic surgery 89, Zheng, Y., Barbu, A., Georgescu, B., Scheuering, M., Comaniciu, D., Four-chamber heart modeling and automatic segmentation for 3-d cardiac ct volumes using marginal space learning and steerable features. Medical Imaging, IEEE Transactions on 27,
43 Model Estimation From Images Anatomical Model Generation Simulation Mitral Valve Closure Virtual MitralClip Intervention in Preoperative Anatomy Intervention Outcome Prediction
Medical Image Analysis
 Medical Image Analysis 16 (2012) 1330 1346 Contents lists available at SciVerse ScienceDirect Medical Image Analysis journal homepage: www.elsevier.com/locate/media An integrated framework for finite-element
Medical Image Analysis 16 (2012) 1330 1346 Contents lists available at SciVerse ScienceDirect Medical Image Analysis journal homepage: www.elsevier.com/locate/media An integrated framework for finite-element
Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure
 Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure T. Mansi 1, I. Voigt 1,2, E. Assoumou Mengue 1, R. Ionasec 1, B. Georgescu 1, T. Noack 3, J. Seeburger 3, and D. Comaniciu 1 1
Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure T. Mansi 1, I. Voigt 1,2, E. Assoumou Mengue 1, R. Ionasec 1, B. Georgescu 1, T. Noack 3, J. Seeburger 3, and D. Comaniciu 1 1
Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure
 Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure T. Mansi 1,I.Voigt 1,2, E. Assoumou Mengue 1,R.Ionasec 1, B. Georgescu 1, T. Noack 3, J. Seeburger 3, and D. Comaniciu 1 1 Siemens
Towards Patient-Specific Finite-Element Simulation of MitralClip Procedure T. Mansi 1,I.Voigt 1,2, E. Assoumou Mengue 1,R.Ionasec 1, B. Georgescu 1, T. Noack 3, J. Seeburger 3, and D. Comaniciu 1 1 Siemens
Automated Volumetric Cardiac Ultrasound Analysis
 Whitepaper Automated Volumetric Cardiac Ultrasound Analysis ACUSON SC2000 Volume Imaging Ultrasound System Bogdan Georgescu, Ph.D. Siemens Corporate Research Princeton, New Jersey USA Answers for life.
Whitepaper Automated Volumetric Cardiac Ultrasound Analysis ACUSON SC2000 Volume Imaging Ultrasound System Bogdan Georgescu, Ph.D. Siemens Corporate Research Princeton, New Jersey USA Answers for life.
Multi-modal Validation Framework of Mitral Valve Geometry and Functional Computational Models
 Multi-modal Validation Framework of Mitral Valve Geometry and Functional Computational Models Sasa Grbic 1, Thomas F. Easley 2, Tommaso Mansi 1, Charles H. Bloodworth 2, Eric L. Pierce 2, Ingmar Voigt
Multi-modal Validation Framework of Mitral Valve Geometry and Functional Computational Models Sasa Grbic 1, Thomas F. Easley 2, Tommaso Mansi 1, Charles H. Bloodworth 2, Eric L. Pierce 2, Ingmar Voigt
Complete Valvular Heart Apparatus Model from 4D Cardiac CT
 Complete Valvular Heart Apparatus Model from 4D Cardiac CT Saša Grbić 1,2, Razvan Ionasec 1,2, Dime Vitanovski 2, Ingmar Voigt 2, Yang Wang 2, Bogdan Georgescu 2, Nassir Navab 1, and Dorin Comaniciu 2
Complete Valvular Heart Apparatus Model from 4D Cardiac CT Saša Grbić 1,2, Razvan Ionasec 1,2, Dime Vitanovski 2, Ingmar Voigt 2, Yang Wang 2, Bogdan Georgescu 2, Nassir Navab 1, and Dorin Comaniciu 2
Anisotropic Mass-Spring Method Accurately Simulates Mitral Valve Closure from Image-Based Models
 Anisotropic Mass-Spring Method Accurately Simulates Mitral Valve Closure from Image-Based Models Peter E. Hammer 1,2,3, Pedro J. del Nido 1, and Robert D. Howe 2 1 Department of Cardiac Surgery, Children
Anisotropic Mass-Spring Method Accurately Simulates Mitral Valve Closure from Image-Based Models Peter E. Hammer 1,2,3, Pedro J. del Nido 1, and Robert D. Howe 2 1 Department of Cardiac Surgery, Children
Finite Element Modeling of the Mitral Valve and Mitral Valve Repair
 Finite Element Modeling of the Mitral Valve and Mitral Valve Repair Iain Baxter A thesis submitted to the Faculty of Graduate and Postdoctoral Studies in partial fulfillment of the requirements for the
Finite Element Modeling of the Mitral Valve and Mitral Valve Repair Iain Baxter A thesis submitted to the Faculty of Graduate and Postdoctoral Studies in partial fulfillment of the requirements for the
Contents 1 Computational Haemodynamics An Introduction 2 The Human Cardiovascular System
 Contents 1 Computational Haemodynamics An Introduction... 1 1.1 What is Computational Haemodynamics (CHD)... 1 1.2 Advantages of CHD... 3 1.3 Applications in the Cardiovascular System... 4 1.3.1 CHD as
Contents 1 Computational Haemodynamics An Introduction... 1 1.1 What is Computational Haemodynamics (CHD)... 1 1.2 Advantages of CHD... 3 1.3 Applications in the Cardiovascular System... 4 1.3.1 CHD as
Patient-Specific Computational Simulation of the Mitral Valve Function Using Three-Dimensional Echocardiography
 Patient-Specific Computational Simulation of the Mitral Valve Function Using Three-Dimensional Echocardiography Y. Rim 1, S. T. Laing 1, P. Kee 1, K. B. Chandran 2, D. D. McPherson 1 and H. Kim 1 1 Department
Patient-Specific Computational Simulation of the Mitral Valve Function Using Three-Dimensional Echocardiography Y. Rim 1, S. T. Laing 1, P. Kee 1, K. B. Chandran 2, D. D. McPherson 1 and H. Kim 1 1 Department
Fast Image-Based Mitral Valve Simulation from Individualized Geometry
 Fast Image-Based Mitral Valve Simulation from Individualized Geometry Pierre-Frédéric Villard, Peter Hammer, Douglas Perrin, Pedro Del Nido, Robert Howe To cite this version: Pierre-Frédéric Villard, Peter
Fast Image-Based Mitral Valve Simulation from Individualized Geometry Pierre-Frédéric Villard, Peter Hammer, Douglas Perrin, Pedro Del Nido, Robert Howe To cite this version: Pierre-Frédéric Villard, Peter
8/31/2016. Mitraclip in Matthew Johnson, MD
 Mitraclip in 2016 Matthew Johnson, MD 1 Abnormal Valve Function Valve Stenosis Obstruction to valve flow during that phase of the cardiac cycle when the valve is normally open. Hemodynamic hallmark - pressure
Mitraclip in 2016 Matthew Johnson, MD 1 Abnormal Valve Function Valve Stenosis Obstruction to valve flow during that phase of the cardiac cycle when the valve is normally open. Hemodynamic hallmark - pressure
Two semilunar valves. Two atrioventricular valves. Valves of the heart. Left atrioventricular or bicuspid valve Mitral valve
 The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
The Heart 3 Valves of the heart Two atrioventricular valves Two semilunar valves Right atrioventricular or tricuspid valve Left atrioventricular or bicuspid valve Mitral valve Aortic valve Pulmonary valve
Automated Image Biometrics Speeds Ultrasound Workflow
 Whitepaper Automated Image Biometrics Speeds Ultrasound Workflow ACUSON SC2000 Volume Imaging Ultrasound System S. Kevin Zhou, Ph.D. Siemens Corporate Research Princeton, New Jersey USA Answers for life.
Whitepaper Automated Image Biometrics Speeds Ultrasound Workflow ACUSON SC2000 Volume Imaging Ultrasound System S. Kevin Zhou, Ph.D. Siemens Corporate Research Princeton, New Jersey USA Answers for life.
New concepts for mitral valve imaging
 Perspective New concepts for mitral valve imaging Thilo Noack 1 *, Philipp Kiefer 1 *, Razvan Ionasec 2, Ingmar Voigt 2, Tammaso Mansi 2, Marcel Vollroth 1, Michael Hoebartner 1, Martin Misfeld 1, Friedrich-Wilhelm
Perspective New concepts for mitral valve imaging Thilo Noack 1 *, Philipp Kiefer 1 *, Razvan Ionasec 2, Ingmar Voigt 2, Tammaso Mansi 2, Marcel Vollroth 1, Michael Hoebartner 1, Martin Misfeld 1, Friedrich-Wilhelm
The Edge-to-Edge Technique f For Barlow's Disease
 The Edge-to-Edge Technique f For Barlow's Disease Ottavio Alfieri, Michele De Bonis, Elisabetta Lapenna, Francesco Maisano, Lucia Torracca, Giovanni La Canna. Department of Cardiac Surgery, San Raffaele
The Edge-to-Edge Technique f For Barlow's Disease Ottavio Alfieri, Michele De Bonis, Elisabetta Lapenna, Francesco Maisano, Lucia Torracca, Giovanni La Canna. Department of Cardiac Surgery, San Raffaele
Outcomes of Mitral Valve Repair for Mitral Regurgitation Due to Degenerative Disease
 Outcomes of Mitral Valve Repair for Mitral Regurgitation Due to Degenerative Disease TIRONE E. DAVID, MD ; SEMIN THORAC CARDIOVASC SURG 19:116-120c 2007 ELSEVIER INC. PRESENTED BY INTERN 許士盟 Mitral valve
Outcomes of Mitral Valve Repair for Mitral Regurgitation Due to Degenerative Disease TIRONE E. DAVID, MD ; SEMIN THORAC CARDIOVASC SURG 19:116-120c 2007 ELSEVIER INC. PRESENTED BY INTERN 許士盟 Mitral valve
Basic principles of Rheumatic mitral valve Repair
 Basic principles of Rheumatic mitral valve Repair Prof. Gebrine El Khoury, MD DEPARTMENT OF CARDIOVASCULAR AND THORACIC SURGERY ST. LUC HOSPITAL - BRUSSELS, BELGIUM 1 Rheumatic MV disease MV repair confers
Basic principles of Rheumatic mitral valve Repair Prof. Gebrine El Khoury, MD DEPARTMENT OF CARDIOVASCULAR AND THORACIC SURGERY ST. LUC HOSPITAL - BRUSSELS, BELGIUM 1 Rheumatic MV disease MV repair confers
Finite Element Analysis for Edge-to-Edge Technique to Treat Post-Mitral Valve Repair Systolic Anterior Motion
 Acta of Bioengineering and Biomechanics Vol. 6, No. 4, 04 Original paper DOI: 0.577/ABB-0008-04-0 Finite Element Analysis for Edge-to-Edge Technique to Treat Post-Mitral Valve Repair Systolic Anterior
Acta of Bioengineering and Biomechanics Vol. 6, No. 4, 04 Original paper DOI: 0.577/ABB-0008-04-0 Finite Element Analysis for Edge-to-Edge Technique to Treat Post-Mitral Valve Repair Systolic Anterior
Effect of leaflet-to-chordae contact interaction on computational mitral valve evaluation
 Rim et al. BioMedical Engineering OnLine 2014, 13:31 RESEARCH Open Access Effect of leaflet-to-chordae contact interaction on computational mitral valve evaluation Yonghoon Rim, David D McPherson and Hyunggun
Rim et al. BioMedical Engineering OnLine 2014, 13:31 RESEARCH Open Access Effect of leaflet-to-chordae contact interaction on computational mitral valve evaluation Yonghoon Rim, David D McPherson and Hyunggun
Tissue Doppler Imaging in Congenital Heart Disease
 Tissue Doppler Imaging in Congenital Heart Disease L. Youngmin Eun, M.D. Department of Pediatrics, Division of Pediatric Cardiology, Kwandong University College of Medicine The potential advantage of ultrasound
Tissue Doppler Imaging in Congenital Heart Disease L. Youngmin Eun, M.D. Department of Pediatrics, Division of Pediatric Cardiology, Kwandong University College of Medicine The potential advantage of ultrasound
Design and Simulation of Blocked Blood Vessel for Early Detection of Heart Diseases
 Proceedings of the 215 2nd International Symposium on Physics and Technology of Sensors, 8-1th March, 215, Pune, India Design and Simulation of Blocked Blood Vessel for Early Detection of Heart Diseases
Proceedings of the 215 2nd International Symposium on Physics and Technology of Sensors, 8-1th March, 215, Pune, India Design and Simulation of Blocked Blood Vessel for Early Detection of Heart Diseases
Echocardiographic Evaluation of Primary Mitral Regurgitation
 Echocardiographic Evaluation of Primary Mitral Regurgitation Roberto M Lang, MD 0-10 o ME 4CH Med A2 P2 50-70 o Commissural P3 P1 A2 80-100 o ME 2CH P3 A2 A1 A1 125-135 o - ME Long axis P2 A2 P3 A3 P2
Echocardiographic Evaluation of Primary Mitral Regurgitation Roberto M Lang, MD 0-10 o ME 4CH Med A2 P2 50-70 o Commissural P3 P1 A2 80-100 o ME 2CH P3 A2 A1 A1 125-135 o - ME Long axis P2 A2 P3 A3 P2
Supervisors: Dr. ir. Matthieu De Beule, Prof. dr. ir. Benedict Verhegghe Counsellors: Tim Dezutter, dhr. Philippe Bertrand
 Simulation tools for mitral valve repair Viktoriya Antonova Supervisors: Dr. ir. Matthieu De Beule, Prof. dr. ir. Benedict Verhegghe Counsellors: Tim Dezutter, dhr. Philippe Bertrand Master's dissertation
Simulation tools for mitral valve repair Viktoriya Antonova Supervisors: Dr. ir. Matthieu De Beule, Prof. dr. ir. Benedict Verhegghe Counsellors: Tim Dezutter, dhr. Philippe Bertrand Master's dissertation
A novel mathematical technique to assess of the mitral valve dynamics based on echocardiography
 ORIGINAL ARTICLE A novel mathematical technique to assess of the mitral valve dynamics based on echocardiography Mersedeh Karvandi 1 MD, Saeed Ranjbar 2,* Ph.D, Seyed Ahmad Hassantash MD 2, Mahnoosh Foroughi
ORIGINAL ARTICLE A novel mathematical technique to assess of the mitral valve dynamics based on echocardiography Mersedeh Karvandi 1 MD, Saeed Ranjbar 2,* Ph.D, Seyed Ahmad Hassantash MD 2, Mahnoosh Foroughi
PREDICTION OF BLOOD FLOW VELOCITY AND LEAFLET DEFORMATION VIA 2D MITRAL VALVE MODEL
 Journal of Mechanical Engineering and Sciences (JMES) e-issn: 2231-8380; Volume 2, pp. 217-225, June 2012 FKM, Universiti Malaysia Pahang PREDICTION OF BLOOD FLOW VELOCITY AND LEAFLET DEFORMATION VIA 2D
Journal of Mechanical Engineering and Sciences (JMES) e-issn: 2231-8380; Volume 2, pp. 217-225, June 2012 FKM, Universiti Malaysia Pahang PREDICTION OF BLOOD FLOW VELOCITY AND LEAFLET DEFORMATION VIA 2D
Valve Analysis and Pathoanatomy: THE MITRAL VALVE
 : THE MITRAL VALVE Marc R. Moon, M.D. John M. Shoenberg Chair in CV Disease Chief, Cardiac Surgery Washington University School of Medicine, St. Louis, MO Secretary, American Association for Thoracic Surgery
: THE MITRAL VALVE Marc R. Moon, M.D. John M. Shoenberg Chair in CV Disease Chief, Cardiac Surgery Washington University School of Medicine, St. Louis, MO Secretary, American Association for Thoracic Surgery
Eva Maria Delmo Walter Takeshi Komoda Roland Hetzer
 Surgical repair of the congenitally malformed mitral valve leaflets in infants and children Eva Maria Delmo Walter Takeshi Komoda Roland Hetzer Deutsches Herzzentrum Berlin Germany Background and Objective
Surgical repair of the congenitally malformed mitral valve leaflets in infants and children Eva Maria Delmo Walter Takeshi Komoda Roland Hetzer Deutsches Herzzentrum Berlin Germany Background and Objective
Keywords: Angioplasty, Explicit finite elements method, Tube hidroforming, Stents.
 Blucher Mechanical Engineering Proceedings May 2014, vol. 1, num. 1 www.proceedings.blucher.com.br/evento/10wccm AN ANALYSIS OF THE CONTACT BETWEEN THE STENT AND THE ARTERY USING TUBE HIDROFORMING SIMULATION
Blucher Mechanical Engineering Proceedings May 2014, vol. 1, num. 1 www.proceedings.blucher.com.br/evento/10wccm AN ANALYSIS OF THE CONTACT BETWEEN THE STENT AND THE ARTERY USING TUBE HIDROFORMING SIMULATION
Accepted Manuscript. A Bad Trade: Mitral Regurgitation for Mitral Stenosis and Atrial Fibrillation
 Accepted Manuscript A Bad Trade: Mitral Regurgitation for Mitral Stenosis and Atrial Fibrillation Marc Gillinov, MD, Per Wierup, MD, PhD, Stephanie Mick, MD PII: S0022-5223(18)32281-5 DOI: 10.1016/j.jtcvs.2018.08.036
Accepted Manuscript A Bad Trade: Mitral Regurgitation for Mitral Stenosis and Atrial Fibrillation Marc Gillinov, MD, Per Wierup, MD, PhD, Stephanie Mick, MD PII: S0022-5223(18)32281-5 DOI: 10.1016/j.jtcvs.2018.08.036
Mechanics of the mitral valve
 Biomech Model Mechanobiol (2013) 12:1053 1071 DOI 10.1007/s10237-012-0462-z ORIGINAL PAPER Mechanics of the mitral valve A critical review, an in vivo parameter identification, and the effect of prestrain
Biomech Model Mechanobiol (2013) 12:1053 1071 DOI 10.1007/s10237-012-0462-z ORIGINAL PAPER Mechanics of the mitral valve A critical review, an in vivo parameter identification, and the effect of prestrain
Medical Engineering & Physics
 Medical Engineering & Physics 32 (2010) 1057 1064 Contents lists available at ScienceDirect Medical Engineering & Physics journal homepage: www.elsevier.com/locate/medengphy Mitral valve dynamics in structural
Medical Engineering & Physics 32 (2010) 1057 1064 Contents lists available at ScienceDirect Medical Engineering & Physics journal homepage: www.elsevier.com/locate/medengphy Mitral valve dynamics in structural
Part II: Fundamentals of 3D Echocardiography: Acquisition and Application
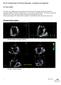 Part II: Fundamentals of 3D Echocardiography: Acquisition and Application Dr. Bruce Bollen 3D matrix array TEE probes provide options for both 2D and 3D imaging. Indeed, their utility in obtaining multiple
Part II: Fundamentals of 3D Echocardiography: Acquisition and Application Dr. Bruce Bollen 3D matrix array TEE probes provide options for both 2D and 3D imaging. Indeed, their utility in obtaining multiple
Verification and Validation Methodologies for Prosthetic Heart Valves: Review and Considerations. ASME V&V40 Subcommittee Heart Valve Subgroup
 Verification and Validation Methodologies for Prosthetic Heart Valves: Review and Considerations. ASME V&V40 Subcommittee Heart Valve Subgroup ASME V&V 40 Heart Valve Subgroup Members Andrew Rau (Exponent
Verification and Validation Methodologies for Prosthetic Heart Valves: Review and Considerations. ASME V&V40 Subcommittee Heart Valve Subgroup ASME V&V 40 Heart Valve Subgroup Members Andrew Rau (Exponent
Ο ΡΟΛΟΣ ΤΩΝ ΚΟΛΠΩΝ ΣΤΗ ΛΕΙΤΟΥΡΓΙΚΗ ΑΝΕΠΑΡΚΕΙΑ ΤΩΝ ΚΟΛΠΟΚΟΙΛΙΑΚΩΝ ΒΑΛΒΙΔΩΝ
 Ο ΡΟΛΟΣ ΤΩΝ ΚΟΛΠΩΝ ΣΤΗ ΛΕΙΤΟΥΡΓΙΚΗ ΑΝΕΠΑΡΚΕΙΑ ΤΩΝ ΚΟΛΠΟΚΟΙΛΙΑΚΩΝ ΒΑΛΒΙΔΩΝ Ανδρέας Κατσαρός Καρδιολόγος Επιµ. Α Καρδιοχειρ/κών Τµηµάτων Γ.Ν.Α. Ιπποκράτειο ΚΑΜΙΑ ΣΥΓΚΡΟΥΣΗ ΣΥΜΦΕΡΟΝΤΩΝ ΑΝΑΦΟΡΙΚΑ ΜΕ ΤΗΝ ΠΑΡΟΥΣΙΑΣΗ
Ο ΡΟΛΟΣ ΤΩΝ ΚΟΛΠΩΝ ΣΤΗ ΛΕΙΤΟΥΡΓΙΚΗ ΑΝΕΠΑΡΚΕΙΑ ΤΩΝ ΚΟΛΠΟΚΟΙΛΙΑΚΩΝ ΒΑΛΒΙΔΩΝ Ανδρέας Κατσαρός Καρδιολόγος Επιµ. Α Καρδιοχειρ/κών Τµηµάτων Γ.Ν.Α. Ιπποκράτειο ΚΑΜΙΑ ΣΥΓΚΡΟΥΣΗ ΣΥΜΦΕΡΟΝΤΩΝ ΑΝΑΦΟΡΙΚΑ ΜΕ ΤΗΝ ΠΑΡΟΥΣΙΑΣΗ
Complex Left Atrial (CLA) Best Practices for Navigant
 Complex Left Atrial (CLA) Best Practices for Navigant Ischemic Ventricular Tachycardia (VT) Best Practices for Navigant Ischemic VT Ablation Procedures Set Up In the absence of a septal defect or other
Complex Left Atrial (CLA) Best Practices for Navigant Ischemic Ventricular Tachycardia (VT) Best Practices for Navigant Ischemic VT Ablation Procedures Set Up In the absence of a septal defect or other
CLIP ΜΙΤΡΟΕΙ ΟΥΣ: ΠΟΥ ΒΡΙΣΚΟΜΑΣΤΕ;
 CLIP ΜΙΤΡΟΕΙ ΟΥΣ: ΠΟΥ ΒΡΙΣΚΟΜΑΣΤΕ; Επιµορφωτικά Σεµινάρια Ειδικευοµένων Καρδιολογίας 7 Απριλίου 2012 M Chrissoheris MD FACC THV Department HYGEIA Hospital Degenerative MR (DMR) Usually refers to an anatomic
CLIP ΜΙΤΡΟΕΙ ΟΥΣ: ΠΟΥ ΒΡΙΣΚΟΜΑΣΤΕ; Επιµορφωτικά Σεµινάρια Ειδικευοµένων Καρδιολογίας 7 Απριλίου 2012 M Chrissoheris MD FACC THV Department HYGEIA Hospital Degenerative MR (DMR) Usually refers to an anatomic
Despite advances in our understanding of the pathophysiology
 Suture Relocation of the Posterior Papillary Muscle in Ischemic Mitral Regurgitation Benjamin B. Peeler MD,* and Irving L. Kron MD,*, *Department of Cardiovascular Surgery, University of Virginia, Charlottesville,
Suture Relocation of the Posterior Papillary Muscle in Ischemic Mitral Regurgitation Benjamin B. Peeler MD,* and Irving L. Kron MD,*, *Department of Cardiovascular Surgery, University of Virginia, Charlottesville,
Construction of 4D Left Ventricle and Coronary Artery Models
 1 Construction of 4D Left Ventricle and Coronary Artery Models Dong Ping Zhang Supervisors: Prof Daniel Rueckert, Dr Eddie Edwards 2 1 Background Conventional heart surgery is one of the most invasive
1 Construction of 4D Left Ventricle and Coronary Artery Models Dong Ping Zhang Supervisors: Prof Daniel Rueckert, Dr Eddie Edwards 2 1 Background Conventional heart surgery is one of the most invasive
Simulating the Motion of Heart Valves Under Fluid Flows Induced by Cardiac Contraction
 Simulating the Motion of Heart Valves Under Fluid Flows Induced by Cardiac Contraction Eann A. Patterson Department of Mechanical Engineering, The University of Sheffield Mappin Street, Sheffield, S1 3JD
Simulating the Motion of Heart Valves Under Fluid Flows Induced by Cardiac Contraction Eann A. Patterson Department of Mechanical Engineering, The University of Sheffield Mappin Street, Sheffield, S1 3JD
Functional anatomy of the aortic root. ΔΡΟΣΟΣ ΓΕΩΡΓΙΟΣ Διεσθσνηής Καρδιοθωρακοτειροσργικής Κλινικής Γ.Ν. «Γ. Παπανικολάοσ» Θεζζαλονίκη
 Functional anatomy of the aortic root ΔΡΟΣΟΣ ΓΕΩΡΓΙΟΣ Διεσθσνηής Καρδιοθωρακοτειροσργικής Κλινικής Γ.Ν. «Γ. Παπανικολάοσ» Θεζζαλονίκη What is the aortic root? represents the outflow tract from the LV provides
Functional anatomy of the aortic root ΔΡΟΣΟΣ ΓΕΩΡΓΙΟΣ Διεσθσνηής Καρδιοθωρακοτειροσργικής Κλινικής Γ.Ν. «Γ. Παπανικολάοσ» Θεζζαλονίκη What is the aortic root? represents the outflow tract from the LV provides
Toward developing a finite element model of the mitral valve
 Toward developing a finite element model of the mitral valve I.A.E.W. van Loosdregt April 2011 July 2011 BMTE 11.22 Supervisors: R. Amini, Ph.D. (University of Pittsburgh) M.S. Sacks, Ph.D. (University
Toward developing a finite element model of the mitral valve I.A.E.W. van Loosdregt April 2011 July 2011 BMTE 11.22 Supervisors: R. Amini, Ph.D. (University of Pittsburgh) M.S. Sacks, Ph.D. (University
CFD Challenge: Simulation of Hemodynamics in a Patient-Specific Aortic Coarctation Model
 CFD Challenge: Simulation of Hemodynamics in a Patient-Specific Aortic Coarctation Model Background Coarctation of the aorta (CoA) accounts for 8%-11% of congenital heart defects, affecting tens of thousands
CFD Challenge: Simulation of Hemodynamics in a Patient-Specific Aortic Coarctation Model Background Coarctation of the aorta (CoA) accounts for 8%-11% of congenital heart defects, affecting tens of thousands
Effect of Congenital Anomalies of the Papillary Muscles on Mitral Valve Function
 J. Med. Biol. Eng. (2015) 35:104 112 DOI 10.1007/s40846-015-0011-1 ORIGINAL ARTICLE Effect of Congenital Anomalies of the Papillary Muscles on Mitral Valve Function Yonghoon Rim David D. McPherson Hyunggun
J. Med. Biol. Eng. (2015) 35:104 112 DOI 10.1007/s40846-015-0011-1 ORIGINAL ARTICLE Effect of Congenital Anomalies of the Papillary Muscles on Mitral Valve Function Yonghoon Rim David D. McPherson Hyunggun
3D Printing & Echocardiography
 ASE SOTA Feb 19, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Disclosures Personal
ASE SOTA Feb 19, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Disclosures Personal
MICCAI 2018 VISDMML Tutorial Image Analysis Meets Deep Learning and Visualization in Cardiology and Cardiac Surgery
 MICCAI 2018 VISDMML Tutorial Image Analysis Meets Deep Learning and Visualization in Cardiology and Cardiac Surgery Dr. Sandy Engelhardt Faculty of Computer Science, Mannheim University of Applied Sciences,
MICCAI 2018 VISDMML Tutorial Image Analysis Meets Deep Learning and Visualization in Cardiology and Cardiac Surgery Dr. Sandy Engelhardt Faculty of Computer Science, Mannheim University of Applied Sciences,
Introduction. Cardiac Imaging Modalities MRI. Overview. MRI (Continued) MRI (Continued) Arnaud Bistoquet 12/19/03
 Introduction Cardiac Imaging Modalities Arnaud Bistoquet 12/19/03 Coronary heart disease: the vessels that supply oxygen-carrying blood to the heart, become narrowed and unable to carry a normal amount
Introduction Cardiac Imaging Modalities Arnaud Bistoquet 12/19/03 Coronary heart disease: the vessels that supply oxygen-carrying blood to the heart, become narrowed and unable to carry a normal amount
Stretching Cardiac Myocytes: A Finite Element Model of Cardiac Tissue
 Megan McCain ES240 FEM Final Project December 19, 2006 Stretching Cardiac Myocytes: A Finite Element Model of Cardiac Tissue Cardiac myocytes are the cells that constitute the working muscle of the heart.
Megan McCain ES240 FEM Final Project December 19, 2006 Stretching Cardiac Myocytes: A Finite Element Model of Cardiac Tissue Cardiac myocytes are the cells that constitute the working muscle of the heart.
MITRAL VALVE FORCE BALANCE: A QUANTITATIVE ASSESSMENT OF ANNULAR AND SUBVALVULAR FORCES
 MITRAL VALVE FORCE BALANCE: A QUANTITATIVE ASSESSMENT OF ANNULAR AND SUBVALVULAR FORCES A Dissertation Presented to The Academic Faculty by Andrew W. Siefert In Partial Fulfillment of the Requirements
MITRAL VALVE FORCE BALANCE: A QUANTITATIVE ASSESSMENT OF ANNULAR AND SUBVALVULAR FORCES A Dissertation Presented to The Academic Faculty by Andrew W. Siefert In Partial Fulfillment of the Requirements
Commentary:Right Ventricular-Tricuspid Valve Interdependance And The Challenges For Structural Heart Valve Therapy
 Accepted Manuscript Commentary:Right Ventricular-Tricuspid Valve Interdependance And The Challenges For Structural Heart Valve Therapy Amedeo Anselmi, MD PhD PII: S0022-5223(19)30021-2 DOI: https://doi.org/10.1016/j.jtcvs.2018.12.094
Accepted Manuscript Commentary:Right Ventricular-Tricuspid Valve Interdependance And The Challenges For Structural Heart Valve Therapy Amedeo Anselmi, MD PhD PII: S0022-5223(19)30021-2 DOI: https://doi.org/10.1016/j.jtcvs.2018.12.094
Finite Element Analysis of Radius and Ulna. Eli Pavlatos April 24, 2013
 Finite Element Analysis of Radius and Ulna Eli Pavlatos April 24, 2013 Outline Review bone biomechanics bone behavior during fracture Stress analysis concepts properties of bone for models Fracture simulations
Finite Element Analysis of Radius and Ulna Eli Pavlatos April 24, 2013 Outline Review bone biomechanics bone behavior during fracture Stress analysis concepts properties of bone for models Fracture simulations
DEVELOPMENT OF A CRYO-ANCHORING AND RADIOFREQUENCY ABLATION CATHETER FOR PERCUTANEOUS TREATMENT OF MITRAL VALVE PROLAPSE. Steven Michael Boronyak
 DEVELOPMENT OF A CRYO-ANCHORING AND RADIOFREQUENCY ABLATION CATHETER FOR PERCUTANEOUS TREATMENT OF MITRAL VALVE PROLAPSE By Steven Michael Boronyak Dissertation Submitted to the Faculty of the Graduate
DEVELOPMENT OF A CRYO-ANCHORING AND RADIOFREQUENCY ABLATION CATHETER FOR PERCUTANEOUS TREATMENT OF MITRAL VALVE PROLAPSE By Steven Michael Boronyak Dissertation Submitted to the Faculty of the Graduate
3D Printing & Echocardiography
 Echo Hawaii Jan 18, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Rapid Prototyping
Echo Hawaii Jan 18, 2018 3D Printing & Echocardiography Stephen H. Little, MD John S. Dunn Chair in Cardiovascular Research and Education, Associate professor, Weill Cornell Medicine Rapid Prototyping
Development of a surgical simulation toolkit for mitral valve repair surgeries
 University of Iowa Iowa Research Online Theses and Dissertations Summer 2014 Development of a surgical simulation toolkit for mitral valve repair surgeries Piyusha Sanjay Gade University of Iowa Copyright
University of Iowa Iowa Research Online Theses and Dissertations Summer 2014 Development of a surgical simulation toolkit for mitral valve repair surgeries Piyusha Sanjay Gade University of Iowa Copyright
Computer based delineation and follow-up multisite abdominal tumors in longitudinal CT studies
 Research plan submitted for approval as a PhD thesis Submitted by: Refael Vivanti Supervisor: Professor Leo Joskowicz School of Engineering and Computer Science, The Hebrew University of Jerusalem Computer
Research plan submitted for approval as a PhD thesis Submitted by: Refael Vivanti Supervisor: Professor Leo Joskowicz School of Engineering and Computer Science, The Hebrew University of Jerusalem Computer
Percutaneous mitral annuloplasty. Francesco Maisano MD, FESC San Raffaele Hospital Milano, Italy
 Percutaneous mitral annuloplasty Francesco Maisano MD, FESC San Raffaele Hospital Milano, Italy Disclosure Consultant for Abbott, Medtronic, St Jude, Edwards, ValtechCardio Founder of 4Tech Surgical techniques
Percutaneous mitral annuloplasty Francesco Maisano MD, FESC San Raffaele Hospital Milano, Italy Disclosure Consultant for Abbott, Medtronic, St Jude, Edwards, ValtechCardio Founder of 4Tech Surgical techniques
Finite Element Analysis of Patient-Specific Mitral Valve with Mitral Regurgitation
 Finite Element Analysis of Patient-Specific Mitral Valve with Mitral Regurgitation Pham Thuy, Georgia Institute of Technology Fanwei Kong, Georgia Institute of Technology Caitlin Martin, Georgia Institute
Finite Element Analysis of Patient-Specific Mitral Valve with Mitral Regurgitation Pham Thuy, Georgia Institute of Technology Fanwei Kong, Georgia Institute of Technology Caitlin Martin, Georgia Institute
ICE: Echo Core Lab-CRF
 APPENDIX 1 ICE: Echo Core Lab-CRF Study #: - Pt Initials: 1. Date of study: / / D D M M M Y Y Y Y 2. Type of Study: TTE TEE 3. Quality of Study: Poor Moderate Excellent Ejection Fraction 4. Ejection Fraction
APPENDIX 1 ICE: Echo Core Lab-CRF Study #: - Pt Initials: 1. Date of study: / / D D M M M Y Y Y Y 2. Type of Study: TTE TEE 3. Quality of Study: Poor Moderate Excellent Ejection Fraction 4. Ejection Fraction
Original. Stresses and Strains Distributions in Three-Dimension Three-Layer Abdominal Aortic Wall Based on in vivo Ultrasound Imaging
 Original Stresses and Strains Distributions in Three-Dimension Three-Layer Abdominal Aortic Wall Based on in vivo Ultrasound Imaging P. Khamdaengyodtai 1, T. Khamdaeng 1, P. Sakulchangsatjatai 1, N. Kammuang-lue
Original Stresses and Strains Distributions in Three-Dimension Three-Layer Abdominal Aortic Wall Based on in vivo Ultrasound Imaging P. Khamdaengyodtai 1, T. Khamdaeng 1, P. Sakulchangsatjatai 1, N. Kammuang-lue
Percutaneous Mitral Valve Repair
 Indiana Chapter of ACC November 15 th,2008 Percutaneous Mitral Valve Repair James B Hermiller, MD, FACC The Care Group, LLC St Vincent Hospital Indianapolis, IN Mechanisms of Mitral Regurgitation Mitral
Indiana Chapter of ACC November 15 th,2008 Percutaneous Mitral Valve Repair James B Hermiller, MD, FACC The Care Group, LLC St Vincent Hospital Indianapolis, IN Mechanisms of Mitral Regurgitation Mitral
Posterior leaflet prolapse is the most common lesion seen
 Techniques for Repairing Posterior Leaflet Prolapse of the Mitral Valve Robin Varghese, MD, MS, and David H. Adams, MD Posterior leaflet prolapse is the most common lesion seen in degenerative mitral valve
Techniques for Repairing Posterior Leaflet Prolapse of the Mitral Valve Robin Varghese, MD, MS, and David H. Adams, MD Posterior leaflet prolapse is the most common lesion seen in degenerative mitral valve
Computer Aided Surgery (CAS) for Abdominal Aortic Aneurysm (AAA)
 Computer Aided Surgery (CAS) for Abdominal Aortic Aneurysm (AAA) Aurélien Dumenil Pierre Louat Thierry Marchal Michel Rochette 2009 ANSYS, Inc. All rights reserved. 1 ANSYS, Inc. Proprietary Outline Clinical
Computer Aided Surgery (CAS) for Abdominal Aortic Aneurysm (AAA) Aurélien Dumenil Pierre Louat Thierry Marchal Michel Rochette 2009 ANSYS, Inc. All rights reserved. 1 ANSYS, Inc. Proprietary Outline Clinical
MITRAL REGURGITATION ECHO PARAMETERS TOOL
 Comprehensive assessment of qualitative and quantitative parameters, along with the use of standardized nomenclature when reporting echocardiographic findings, helps to better define a patient s MR and
Comprehensive assessment of qualitative and quantitative parameters, along with the use of standardized nomenclature when reporting echocardiographic findings, helps to better define a patient s MR and
Certificate in Clinician Performed Ultrasound (CCPU) Syllabus. Rapid Cardiac Echo (RCE)
 Certificate in Clinician Performed Ultrasound (CCPU) Syllabus Rapid Cardiac Echo (RCE) Purpose: Rapid Cardiac Echocardiography (RCE) This unit is designed to cover the theoretical and practical curriculum
Certificate in Clinician Performed Ultrasound (CCPU) Syllabus Rapid Cardiac Echo (RCE) Purpose: Rapid Cardiac Echocardiography (RCE) This unit is designed to cover the theoretical and practical curriculum
PERCUTANEOUS MITRAL VALVE THERAPIES 13 TH ANNUAL CARDIAC, VASCULAR AND STROKE CARE CONFERENCE PIEDMONT ATHENS REGIONAL
 PERCUTANEOUS MITRAL VALVE THERAPIES 13 TH ANNUAL CARDIAC, VASCULAR AND STROKE CARE CONFERENCE PIEDMONT ATHENS REGIONAL DISCLOSURES I WILL BE DISCUSSING OFF-LABEL USAGE OF DEVICES RELATED TO TMVR OBJECTIVES
PERCUTANEOUS MITRAL VALVE THERAPIES 13 TH ANNUAL CARDIAC, VASCULAR AND STROKE CARE CONFERENCE PIEDMONT ATHENS REGIONAL DISCLOSURES I WILL BE DISCUSSING OFF-LABEL USAGE OF DEVICES RELATED TO TMVR OBJECTIVES
Imaging MV. Jeroen J. Bax Leiden University Medical Center The Netherlands Davos, feb 2015
 Imaging MV Jeroen J. Bax Leiden University Medical Center The Netherlands Davos, feb 2015 MV/MR: information needed on.. 1. MV anatomy 2. MR etiology - primary vs secondary 3. MR severity quantification
Imaging MV Jeroen J. Bax Leiden University Medical Center The Netherlands Davos, feb 2015 MV/MR: information needed on.. 1. MV anatomy 2. MR etiology - primary vs secondary 3. MR severity quantification
Atrioventricular valve repair: The limits of operability
 Atrioventricular valve repair: The limits of operability Francis Fynn-Thompson, MD Co-Director, Center for Airway Disorders Surgical Director, Pediatric Mechanical Support Program Surgical Director, Heart
Atrioventricular valve repair: The limits of operability Francis Fynn-Thompson, MD Co-Director, Center for Airway Disorders Surgical Director, Pediatric Mechanical Support Program Surgical Director, Heart
Transient stiffening of mitral valve leaflets in the beating heart
 Am J Physiol Heart Circ Physiol 298: H2221 H2225, 2010. First published April 16, 2010; doi:10.1152/ajpheart.00215.2010. Transient stiffening of mitral valve leaflets in the beating heart Gaurav Krishnamurthy,
Am J Physiol Heart Circ Physiol 298: H2221 H2225, 2010. First published April 16, 2010; doi:10.1152/ajpheart.00215.2010. Transient stiffening of mitral valve leaflets in the beating heart Gaurav Krishnamurthy,
Disclosure Statement of Financial Interest Saibal Kar, MD, FACC
 MitraClip Therapy Saibal Kar, MD, FACC, FAHA, FSCAI Director of Interventional Cardiac Research Program Director, Interventional Cardiology Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA
MitraClip Therapy Saibal Kar, MD, FACC, FAHA, FSCAI Director of Interventional Cardiac Research Program Director, Interventional Cardiology Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA
Quantifying Heart Valves: From Diagnostic to Personalized Valve Repair
 White Paper Quantifying Heart Valves: From Diagnostic to Personalized Valve Repair Tommaso Mansi, Ph.D. Helene Houle, BA, RDCS, FASE Ingmar Voigt Michael Szucs, BS Elizabeth Hunter, RDCS Saurabh Datta,
White Paper Quantifying Heart Valves: From Diagnostic to Personalized Valve Repair Tommaso Mansi, Ph.D. Helene Houle, BA, RDCS, FASE Ingmar Voigt Michael Szucs, BS Elizabeth Hunter, RDCS Saurabh Datta,
ΔΙΑΔΕΡΜΙΚΗ ΑΝΤΙΜΕΤΩΠΙΣΗ ΔΟΜΙΚΩΝ ΠΑΘΗΣΕΩΝ: Ο ΡΟΛΟΣ ΤΗΣ ΑΠΕΙΚΟΝΙΣΗΣ ΣΤΟ ΑΙΜΟΔΥΝΑΜΙΚΟ ΕΡΓΑΣΤΗΡΙΟ ΣΤΗΝ ΤΟΠΟΘΕΤΗΣΗ MITRACLIP
 ΔΙΑΔΕΡΜΙΚΗ ΑΝΤΙΜΕΤΩΠΙΣΗ ΔΟΜΙΚΩΝ ΠΑΘΗΣΕΩΝ: Ο ΡΟΛΟΣ ΤΗΣ ΑΠΕΙΚΟΝΙΣΗΣ ΣΤΟ ΑΙΜΟΔΥΝΑΜΙΚΟ ΕΡΓΑΣΤΗΡΙΟ ΣΤΗΝ ΤΟΠΟΘΕΤΗΣΗ MITRACLIP ΒΛΑΣΗΣ ΝΙΝΙΟΣ MD MRCP ΚΛΙΝΙΚΗ ΑΓΙΟΣ ΛΟΥΚΑΣ ΘΕΣΣΑΛΟΝΙΚΗ CONFLICT OF INTEREST PROCTOR
ΔΙΑΔΕΡΜΙΚΗ ΑΝΤΙΜΕΤΩΠΙΣΗ ΔΟΜΙΚΩΝ ΠΑΘΗΣΕΩΝ: Ο ΡΟΛΟΣ ΤΗΣ ΑΠΕΙΚΟΝΙΣΗΣ ΣΤΟ ΑΙΜΟΔΥΝΑΜΙΚΟ ΕΡΓΑΣΤΗΡΙΟ ΣΤΗΝ ΤΟΠΟΘΕΤΗΣΗ MITRACLIP ΒΛΑΣΗΣ ΝΙΝΙΟΣ MD MRCP ΚΛΙΝΙΚΗ ΑΓΙΟΣ ΛΟΥΚΑΣ ΘΕΣΣΑΛΟΝΙΚΗ CONFLICT OF INTEREST PROCTOR
MitraClip in the ICCU: Which Patient will Benefit?
 MitraClip in the ICCU: Which Patient will Benefit? DAVID MEERKIN STRUCTURAL A ND CONGENITAL HEART DISEASE UNIT SHAARE ZEDEK MEDICAL CENTER JERUSALEM Conflict of Interest No relevant disclosures Complex
MitraClip in the ICCU: Which Patient will Benefit? DAVID MEERKIN STRUCTURAL A ND CONGENITAL HEART DISEASE UNIT SHAARE ZEDEK MEDICAL CENTER JERUSALEM Conflict of Interest No relevant disclosures Complex
CFD as Non-Invasive Tool for Patient- Specific Treatment Support in Cardiology
 CFD as Non-Invasive Tool for Patient- Specific Treatment Support in Cardiology Katharina Vellguth U N I V E R S I T Ä T S M E D I Z I N B E R L I N Introduction [1] 2 Introduction 7.200 Heart valve surgeries
CFD as Non-Invasive Tool for Patient- Specific Treatment Support in Cardiology Katharina Vellguth U N I V E R S I T Ä T S M E D I Z I N B E R L I N Introduction [1] 2 Introduction 7.200 Heart valve surgeries
Mitral Valve Disease, When to Intervene
 Mitral Valve Disease, When to Intervene Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Current ACC/AHA guideline Stages
Mitral Valve Disease, When to Intervene Swedish Heart and Vascular Institute Ming Zhang MD PhD Interventional Cardiology Structure Heart Disease Conflict of Interest None Current ACC/AHA guideline Stages
In Silico Tumor Growth: Application to Glioblastomas
 In Silico Tumor Growth: Application to Glioblastomas Olivier Clatz 1, Pierre-Yves Bondiau 1, Hervé Delingette 1,Grégoire Malandain 1, Maxime Sermesant 1, Simon K. Warfield 2, and Nicholas Ayache 1 1 Epidaure
In Silico Tumor Growth: Application to Glioblastomas Olivier Clatz 1, Pierre-Yves Bondiau 1, Hervé Delingette 1,Grégoire Malandain 1, Maxime Sermesant 1, Simon K. Warfield 2, and Nicholas Ayache 1 1 Epidaure
Routine MitraClip. Image Guidance Step by Step
 Routine MitraClip Image Guidance Step by Step Douglas C. Shook, MD, FASE Director, Cardiothoracic Anesthesia Fellowship Director, Cardiac Interventional Anesthesia Department of Anesthesiology BRIGHAM
Routine MitraClip Image Guidance Step by Step Douglas C. Shook, MD, FASE Director, Cardiothoracic Anesthesia Fellowship Director, Cardiac Interventional Anesthesia Department of Anesthesiology BRIGHAM
King s Research Portal
 King s Research Portal DOI: 10.1016/j.amjcard.2016.03.028 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Eskandari, M.,
King s Research Portal DOI: 10.1016/j.amjcard.2016.03.028 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Eskandari, M.,
Surgical Repair of the Mitral Valve Presenter: Graham McCrystal Cardiothoracic Surgeon Christchurch Public Hospital
 Mitral Valve Surgical intervention Graham McCrystal Chairs: Rajesh Nair & Gerard Wilkins Surgical Repair of the Mitral Valve Presenter: Graham McCrystal Cardiothoracic Surgeon Christchurch Public Hospital
Mitral Valve Surgical intervention Graham McCrystal Chairs: Rajesh Nair & Gerard Wilkins Surgical Repair of the Mitral Valve Presenter: Graham McCrystal Cardiothoracic Surgeon Christchurch Public Hospital
Non-Newtonian pulsatile blood flow in a modeled artery with a stenosis and an aneurysm
 Non-Newtonian pulsatile blood flow in a modeled artery with a stenosis and an aneurysm I. Husain, C. Langdon and J. Schwark Department of Mathematics Luther College University of Regina Regina, Saskatchewan
Non-Newtonian pulsatile blood flow in a modeled artery with a stenosis and an aneurysm I. Husain, C. Langdon and J. Schwark Department of Mathematics Luther College University of Regina Regina, Saskatchewan
In-Silico approach on Offset placement of implant-supported bridges placed in bone of different density in Orthodontics.
 In-Silico approach on Offset placement of implant-supported bridges placed in bone of different density in Orthodontics. Chandrasenan.P 1, Vishnu.G 2, Akshay K Nair 3 1M Tech student, Department of Mechanical
In-Silico approach on Offset placement of implant-supported bridges placed in bone of different density in Orthodontics. Chandrasenan.P 1, Vishnu.G 2, Akshay K Nair 3 1M Tech student, Department of Mechanical
WHAT CAN WE LEARN FROM COMPUTER SIMULATION?
 WHAT CAN WE LEARN FROM COMPUTER SIMULATION? Ehud Raanani, MD Gil Marom, Phd Cardiothoracic Surgery, Sheba Medical Center Sackler School of Medicine, Biomechanical Engineering, Tel Aviv University Homburg,
WHAT CAN WE LEARN FROM COMPUTER SIMULATION? Ehud Raanani, MD Gil Marom, Phd Cardiothoracic Surgery, Sheba Medical Center Sackler School of Medicine, Biomechanical Engineering, Tel Aviv University Homburg,
ARTICLE IN PRESS. Journal of Biomechanics
 Journal of Biomechanics 42 (2009) 2697 2701 Contents lists available at ScienceDirect Journal of Biomechanics journal homepage: www.elsevier.com/locate/jbiomech www.jbiomech.com Regional stiffening of
Journal of Biomechanics 42 (2009) 2697 2701 Contents lists available at ScienceDirect Journal of Biomechanics journal homepage: www.elsevier.com/locate/jbiomech www.jbiomech.com Regional stiffening of
How does the heart pump? From sarcomere to ejection volume
 How does the heart pump? From sarcomere to ejection volume Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases University Leuven, Leuven, Belgium Course on deformation
How does the heart pump? From sarcomere to ejection volume Piet Claus Cardiovascular Imaging and Dynamics Department of Cardiovascular Diseases University Leuven, Leuven, Belgium Course on deformation
Myocardial Twist from X-ray Angiography
 Myocardial Twist from X-ray Angiography Can we Observe Left Ventricular Twist in Rotational Coronary Angiography? Tobias Geimer 1,2,, Mathias Unberath 1,2,, Johannes Höhn 1, Stephan Achenbach 3, Andreas
Myocardial Twist from X-ray Angiography Can we Observe Left Ventricular Twist in Rotational Coronary Angiography? Tobias Geimer 1,2,, Mathias Unberath 1,2,, Johannes Höhn 1, Stephan Achenbach 3, Andreas
Introduction. Aortic Valve. Outflow Tract and Aortic Valve Annulus
 Chapter 1: Surgical anatomy of the aortic and mitral valves Jordan RH Hoffman MD, David A. Fullerton MD, FACC University of Colorado School of Medicine, Department of Surgery, Division of Cardiothoracic
Chapter 1: Surgical anatomy of the aortic and mitral valves Jordan RH Hoffman MD, David A. Fullerton MD, FACC University of Colorado School of Medicine, Department of Surgery, Division of Cardiothoracic
Neuro-Inspired Statistical. Rensselaer Polytechnic Institute National Science Foundation
 Neuro-Inspired Statistical Pi Prior Model lfor Robust Visual Inference Qiang Ji Rensselaer Polytechnic Institute National Science Foundation 1 Status of Computer Vision CV has been an active area for over
Neuro-Inspired Statistical Pi Prior Model lfor Robust Visual Inference Qiang Ji Rensselaer Polytechnic Institute National Science Foundation 1 Status of Computer Vision CV has been an active area for over
Muscle-Tendon Mechanics Dr. Ted Milner (KIN 416)
 Muscle-Tendon Mechanics Dr. Ted Milner (KIN 416) Muscle Fiber Geometry Muscle fibers are linked together by collagenous connective tissue. Endomysium surrounds individual fibers, perimysium collects bundles
Muscle-Tendon Mechanics Dr. Ted Milner (KIN 416) Muscle Fiber Geometry Muscle fibers are linked together by collagenous connective tissue. Endomysium surrounds individual fibers, perimysium collects bundles
JOINT MEETING 2 Tricuspid club Chairpersons: G. Athanassopoulos, A. Avgeropoulou, M. Khoury, G. Stavridis
 JOINT MEETING 2 Tricuspid club Chairpersons: G. Athanassopoulos, A. Avgeropoulou, M. Khoury, G. Stavridis Similarities and differences in Tricuspid vs. Mitral Valve Anatomy and Imaging. Echo evaluation
JOINT MEETING 2 Tricuspid club Chairpersons: G. Athanassopoulos, A. Avgeropoulou, M. Khoury, G. Stavridis Similarities and differences in Tricuspid vs. Mitral Valve Anatomy and Imaging. Echo evaluation
4D Auto LAQ (Left Atrial Quantification)
 4D Auto LAQ (Left Atrial Quantification) Introduction There has been an increased interest in quantification of the left atrium (LA) for various types of diseases; e.g. heart failure, heart valve diseases,
4D Auto LAQ (Left Atrial Quantification) Introduction There has been an increased interest in quantification of the left atrium (LA) for various types of diseases; e.g. heart failure, heart valve diseases,
Fast Simulation of Mitral Annuloplasty for Surgical Planning
 Fast Simulation of Mitral Annuloplasty for Surgical Planning Neil A. Tenenholtz 1, Peter E. Hammer 1,2, Assunta Fabozzo 2,3, Eric N. Feins 2, Pedro J. del Nido 2, and Robert D. Howe 1 1 Harvard School
Fast Simulation of Mitral Annuloplasty for Surgical Planning Neil A. Tenenholtz 1, Peter E. Hammer 1,2, Assunta Fabozzo 2,3, Eric N. Feins 2, Pedro J. del Nido 2, and Robert D. Howe 1 1 Harvard School
ACUSON Sequoia C512 Echocardiography System The ultimate in imaging
 ACUSON Sequoia C512 Echocardiography System The ultimate in imaging Optimum imaging for every patient ACUSON Sequoia C512 Patient Specific Imaging Programmable Waveform Generator Coherent Pulse Formation
ACUSON Sequoia C512 Echocardiography System The ultimate in imaging Optimum imaging for every patient ACUSON Sequoia C512 Patient Specific Imaging Programmable Waveform Generator Coherent Pulse Formation
Historical perspective R1 黃維立
 Degenerative mitral valve disease refers to a spectrum of conditions in which morphologic changes in the connective tissue of the mitral valve cause structural lesions that prevent normal function of the
Degenerative mitral valve disease refers to a spectrum of conditions in which morphologic changes in the connective tissue of the mitral valve cause structural lesions that prevent normal function of the
Available online at ScienceDirect. Procedia Engineering 126 (2015 )
 Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 126 (2015 ) 441 445 7th International Conference on Fluid Mechanics, ICFM7 FSI modeling approach to develop right ventricle
Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 126 (2015 ) 441 445 7th International Conference on Fluid Mechanics, ICFM7 FSI modeling approach to develop right ventricle
A finite strain nonlinear human mitral valve model with fluid-structure interaction
 INTERNATIONAL JOURNAL FOR NUMERICAL METHODS IN BIOMEDICAL ENGINEERING Int. J. Numer. Meth. Biomed. Engng. 2014; 30:1597 1613 Published online in Wiley Online Library (wileyonlinelibrary.com)..2691 SELECTED
INTERNATIONAL JOURNAL FOR NUMERICAL METHODS IN BIOMEDICAL ENGINEERING Int. J. Numer. Meth. Biomed. Engng. 2014; 30:1597 1613 Published online in Wiley Online Library (wileyonlinelibrary.com)..2691 SELECTED
DISCLOSURE. Myocardial Mechanics. Relevant Financial Relationship(s) Off Label Usage
 7th Annual Team Echocardiography: The Heart of Cardiovascular Medicine Tissue Doppler, Strain, Speckle: What? How? Christopher J Kramer RDCS Aurora Medical Group Advanced Cardiovascular Services, Aurora
7th Annual Team Echocardiography: The Heart of Cardiovascular Medicine Tissue Doppler, Strain, Speckle: What? How? Christopher J Kramer RDCS Aurora Medical Group Advanced Cardiovascular Services, Aurora
LV FUNCTION ASSESSMENT: WHAT IS BEYOND EJECTION FRACTION
 LV FUNCTION ASSESSMENT: WHAT IS BEYOND EJECTION FRACTION Jamilah S AlRahimi Assistant Professor, KSU-HS Consultant Noninvasive Cardiology KFCC, MNGHA-WR Introduction LV function assessment in Heart Failure:
LV FUNCTION ASSESSMENT: WHAT IS BEYOND EJECTION FRACTION Jamilah S AlRahimi Assistant Professor, KSU-HS Consultant Noninvasive Cardiology KFCC, MNGHA-WR Introduction LV function assessment in Heart Failure:
Tissue Doppler and Strain Imaging
 Tissue Doppler and Strain Imaging Steven J. Lester MD, FRCP(C), FACC, FASE Relevant Financial Relationship(s) None Off Label Usage None 1 Objective way with which to quantify the minor amplitude and temporal
Tissue Doppler and Strain Imaging Steven J. Lester MD, FRCP(C), FACC, FASE Relevant Financial Relationship(s) None Off Label Usage None 1 Objective way with which to quantify the minor amplitude and temporal
On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve
 Biomech Model Mechanobiol DOI 10.1007/s10237-015-0674-0 ORIGINAL PAPER On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve Chung-Hao Lee 1 Jean-Pierre
Biomech Model Mechanobiol DOI 10.1007/s10237-015-0674-0 ORIGINAL PAPER On the effects of leaflet microstructure and constitutive model on the closing behavior of the mitral valve Chung-Hao Lee 1 Jean-Pierre
Repair or Replacement
 Surgical intervention post MitraClip Device: Repair or Replacement Saudi Heart Association, February 21-24 Rüdiger Lange, MD, PhD Nicolo Piazza, MD, FRCPC, FESC German Heart Center, Munich, Germany Division
Surgical intervention post MitraClip Device: Repair or Replacement Saudi Heart Association, February 21-24 Rüdiger Lange, MD, PhD Nicolo Piazza, MD, FRCPC, FESC German Heart Center, Munich, Germany Division
