Bradycardic and Proarrhythmic Properties of Sinus Node Inhibitors
|
|
|
- Ethan Francis
- 5 years ago
- Views:
Transcription
1 X/06/ $20.00 MOLECULAR PHARMACOLOGY Vol. 69, No. 4 Copyright 2006 The American Society for Pharmacology and Experimental Therapeutics 20701/ Mol Pharmacol 69: , 2006 Printed in U.S.A. Bradycardic and Proarrhythmic Properties of Sinus Node Inhibitors Juliane Stieber, Karen Wieland, Georg Stöckl, Andreas Ludwig, and Franz Hofmann Institut für Pharmakologie und Toxikologie der Technischen Universität München, München, Germany Received November 10, 2005; accepted December 30, 2005 The hyperpolarization-activated, cyclic nucleotide-gated cation current termed I h or I f is a prominent depolarizing current in a variety of neuronal and cardiac cells that show spontaneous electrical activity (Robinson and Siegelbaum, 2003; Baruscotti et al., 2005). In the heart, this current is especially found in the cardiac conduction system [e.g., in Purkinje fibers and sinoatrial node cells (pacemaker cells)], in which it is believed to mediate the -adrenergic stimulation of the heart rate via its direct modulation by camp. Four HCN channels underlie the native I f with an as-yet-unknown subtype assembly to produce the tissue- and cell-specific I f throughout the organism. In the sinoatrial node, HCN4 is the predominant subtype in all species, whereas HCN1, HCN2, and HCN3 are expressed at lower levels and with less specificity for the sinoatrial node compared with the myocardium (Marionneau et al., 2005). The physiological role of I f in the adult cardiac conduction This work was supported by grants from Deutsche Forschungsgemeinschaft and Fond der Chemie. Article, publication date, and citation information can be found at doi: /mol ABSTRACT Sinus node inhibitors reduce the heart rate presumably by blocking the pacemaker current I f in the cardiac conduction system. This pacemaker current is carried by four hyperpolarization-activated, cyclic nucleotide-gated cation (HCN) channels. We tested the potential subtype-specificity of the sinus node inhibitors cilobradine, ivabradine, and zatebradine using cloned HCN channels. All three substances blocked the slow inward current through human HCN1, HCN2, HCN3, and HCN4 channels. There was no subtype-specificity for the steady-state block, with mean IC 50 values of 0.99, 2.25, and 1.96 M for cilobradine, ivabradine, and zatebradine, respectively. Native I f, recorded from mouse sinoatrial node cells, was slightly more efficiently blocked by cilobradine (IC 50 value of 0.62 M) than were the HCN currents. The block of I f in sinoatrial node cells resulted in slower and dysrhythmic spontaneous action potentials. The in vivo action of these blockers was analyzed using telemetric ECG recordings in mice. Each compound reduced the heart rate dose-dependently from 600 to 200 bpm with ED 50 values of 1.2, 4.7, and 1.8 mg/kg for cilobradine, ivabradine, and zatebradine, respectively. -Adrenergic stimulation or forced physical activity only partly reversed this bradycardia. In addition to bradycardia, all three drugs induced increasing arrhythmia at concentrations greater than 5 mg/kg for cilobradine, greater than 10 mg/kg for zatebradine, or greater than 15 mg/kg for ivabradine. This dysrhythmic heart rate is characterized by periodic fluctuations of the duration between the T and P wave, resembling a form of sick sinus syndrome in humans. Hence, all available sinus node inhibitors possess an as-yetunrecognized proarrhythmic potential. system, especially the relevance of I f for heart rate regulation, has not been fully elucidated to date. HCN2 knockout mice show slight sinus arrhythmia at rest, but in these mice, I f in sinoatrial cells is only reduced, not eliminated (Ludwig et al., 2003). HCN4 knockout mice die during embryonal development (Stieber et al., 2003a). The heart of these embryos is beating but at a slow pace, which can be attributed to missing pacemaker potentials as a result of the almost complete lack of I f in the developing cardiac cells. However, this does not allow conclusions about the role of I f /HCN4 in the adult organism. There are no reports about a cardiac phenotype of HCN3 or HCN1 knockout mice. Sinus node inhibitors (SNIs) have been shown to block the native I f in cells of the cardiac conduction system and in neurons of various species (Bogaert et al., 1990; BoSmith et al., 1993; Bois et al., 1996), even before HCN channels have been identified as the underlying ion channels (Ludwig et al., 1998; Santoro et al., 1998) and thus as possible targets of sinus node inhibitors. The block of I f in sheep Purkinje fibers or rabbit sinoatrial node cells results in a slower or, in the case of Purkinje fibers, even abolished spontaneous slow ABBREVIATIONS: HCN, hyperpolarization-activated, cyclic nucleotide-gated cation channel; HEK, human embryonic kidney cells; AP, action potential; SAN, sinoatrial node; SNI, sinus node inhibitor; DDR, diastolic depolarization rate; MDP, maximal diastolic potential; CL, cycle length; CL-SD, standard deviation of the cycle length; AV, atrioventricular. 1328
2 Block of HCN Channels Results in Bradycardia and Arrhythmia 1329 depolarization. The slowing effect on sinoatrial node depolarization has been assumed consequently to be the reason for the bradycardic action of these substances (DiFrancesco and Camm, 2004). For several years, ivabradine, a member of the newer SNIs which also include cilobradine and zatebradine, has been tested in animals (Vilaine et al., 2003; Colin et al., 2004; Du et al., 2004; Monnet et al., 2004) and humans (Borer et al., 2003). It has been found that although this substance has a depressing effect on the heart rate, neuronal side effects are limited to occasional visual symptoms. This led to the clinical development of SNIs as specific bradycardic agents. In addition, SNIs could serve as a tool to study the effects of I f inhibition, specifically in the cardiac conduction system in vivo. In this study, we addressed two issues: first, we sought to determine the effects of an increasing inhibition of I f on the mammalian heart rate regulation; and second, we investigated whether sinus node inhibitors preferably target certain HCN subtypes. Materials and Methods Molecular Cloning of Human HCN Channels. Human HCN2 and HCN4 cdnas were originally cloned from the atrioventricular node region of a human heart (Ludwig et al., 1999), and human HCN1 and HCN3 cdnas were cloned from Whole Human Brain QUICK-Clone cdna (Clontech, Mountain View, CA) (Stieber et al., 2005). All cdnas were subcloned into the pcdna3 mammalian expression vector (Invitrogen, Karlsruhe, Germany). Functional Expression of HCN Channels and Electrophysiological Recordings. Expression of HCN channels in HEK293 cells and voltage-clamp experiments were basically done as described previously (Stieber et al., 2003b). In brief, HEK293 cells were transfected with expression vectors encoding one of the human HCN channels using FuGENE6 transfection reagent (Roche, Mannheim, Germany) according to the manufacturer s instructions (transfectant/dna ratio: 3:1, v/w) or by electroporation using a Bio-Rad system (Bio-Rad, München, Germany) according to the manufacturer s instructions. Cells were cultured on polylysated glass coverslips in a modified Dulbecco s modified Eagle s medium complete medium (Quantum 286; PAA Laboratories, Pasching, Austria) and kept at 37 C in 8.5% CO 2. Currents from transfected cells were recorded with the whole-cell patch-clamp recording technique at a temperature of 23 1 C. Patch pipettes were pulled from borosilicate glass and had a resistance of 2 to 5 M when filled with intracellular solution containing 10 mm NaCl, 30 mm KCl, 90 mm potassium aspartate, 1 mm MgSO 4, 5 mm EGTA, and 10 mm HEPES, with ph adjusted to 7.4 with KOH. During the recordings, cells were continuously superfused with extracellular (bath) solution containing 120 mm NaCl, 20 mm KCl, 1 mm MgCl 2, 1.8 mm CaCl 2, 10 mm HEPES, and 10 mm glucose, with ph adjusted to 7.4 with NaOH. All chemicals for these solutions were obtained from Sigma (Taufkirchen, Germany). Chemical structures of the sinus node inhibitors used are displayed in Fig. 1. Stock solutions (10 mm; in double-distilled H 2 O) of cilobradine (Boehringer, Biberach, Germany), ivabradine (Institut de Recherches Servier, Suresnes, France), zatebradine (Boehringer Ingelheim Pharma KG, Biberach, Germany), and verapamil (Tocris, Bristol, UK) were stored as aliquots at 20 C for a maximum of four weeks and were diluted into the extracellular solution to the appropriate concentration on the day of the experiment. Application of the drug to the cells was performed by complete exchange of the bath solution with bath solution containing the indicated drug concentration and continuous superfusing. The membrane potential was held at 40 mv. To elicit inward currents, hyperpolarizing steps to 100 mv were repeatedly applied. The protocol used to obtain use-dependent block was as follows (Fig. 2A): first, there was a drug wash-in period of 10 min; cells were clamped at 40 mv during this time. Then, there was repetition of the following activation/deactivation step: HCN1, 100 mv for 0.5 s/ 40 mv for 0.5 s; HCN2, 100 mv for2s/ 40 mv for 2 s; HCN3, 100 mv for 2 s/ 40 mv for 2 s; and HCN4, 100 mv for 10 s/ 40 mv for 10 s. The different durations were chosen to account for the different activation/deactivation kinetics of the HCN channels. At the end of the activation pulse, an open probability of 0.5 was reached for each HCN subtype. It is postulated that in this open configuration SNIs are able to bind to the blocking site inside the pore (DiFrancesco and Camm, 2004). Data were acquired using an Axopatch 200B amplifier and pclamp7 software or a MultiClamp 700A amplifier and pclamp9 software (all from Molecular Devices, Union City, CA). Analysis was done offline with Origin 6.0. (OriginLab Corp., Northampton, MA). HCN current amplitude was defined as the amplitude at the end of the activation pulse minus the amplitude of the initial lag. The first activation after the drug wash-in period was taken as baseline, and the amplitudes of the subsequent activations were used to calculate the percentage of block at each activation relative to the first activation. Maximal achievable block for each drug concentration was reached within 30 min. IC 50 values were obtained by fitting the logarithmically plotted data (percentage of block) with a sigmoidal fit/logistic model: y A 2 (A 1 A 2 )/(1 (x/x 0 ) p ), where x 0 corresponds to the IC 50 value. The Hill coefficient was defined as the slope of this logarithmic fit. Statistical significance was tested by comparing the data sets (percentage of block) for each drug concentration/ HCN subtype using the Student s t test. p values 0.05 were considered significant. Preparation of Sinoatrial Node Cells from Murine Hearts and Electrophysiological Recordings. Sinoatrial node (SAN) cells for electrophysiological recordings were basically prepared as described previously (Ludwig et al., 2003). In brief, 2- to 3-month-old C57/Bl6 mice were deeply anesthetized with 100 mg/kg ketamine (Ketavet; Pharmacia GmbH, Erlangen, Germany) and 10 mg/kg xylazine (Rompun; Bayer AG, Leverkusen, Germany), injected intraperitoneally. The beating hearts were quickly removed and placed in prewarmed Tyrode s solution containing 140 mm NaCl, 5.4 mm KCl, Fig. 1. Chemical structures and molecular weights (MW) of the SNIs cilobradine (DK-AH269), ivabradine (S ), and zatebradine (UL- FS49), and the related calcium-channel blocker verapamil. All substances in this study were used as hydrochloride salts.
3 1330 Stieber et al. Fig. 2. Use-dependent block of HCN currents and native I f. A, pulse protocol for the voltage-clamp measurements of HCN channels, expressed in HEK293 cells, and I f, recorded from isolated mouse sinoatrial node cells. Duration of activation/deactivation depends on HCN subtype as described under Materials and Methods. B, example of use-dependent block of cloned HCN channels, shown for HCN4 in the presence of three concentrations of cilobradine. Inward currents were elicited by applying the pulse protocol described in A. Different cells were used for each measurement. (Number)x in the graphics refers to the number of activations at which the current trace was elicited after application of the drug. No block was obtained with 0.01 M cilobradine after 50 activations, whereas with 5 M cilobradine, maximum block was achieved with less than 50 activations. In comparison, 50 M verapamil only induced a small, instant block of HCN4 channels. C, block of native I f by 5 M cilobradine. Maximum block for this concentration was achieved after 20 activations. D, comparison of use-dependent block of HCN1, HCN2, HCN3, and HCN4 channels and the native I f by 5 M cilobradine. All currents are blocked to the same extent, but only 20 to 40 activations are required for HCN3, HCN4, and I f to reach this block, whereas 300 to 500 activations are required for HCN1 and HCN2. Values are mean S.E.M., n 4 to 9, for each current. Squares, HCN1; circles, HCN2; triangles, HCN3; diamonds, HCN4; stars, I f. 1.8 mm CaCl 2, 1 mm MgCl 2, 5 mm HEPES, and 5.5 mm glucose, ph 7.4. The SAN region between the superior vena cava and the right atrium was excised, minced, and placed into modified Tyrode s solution containing 140 mm NaCl, 5.4 mm KCl, 0.2 mm CaCl 2, 0.5 mm MgCl 2, 1.2 mm KH 2 PO 4, 50 mm taurine, 5 mm HEPES, and 5.5 mm glucose, ph 6.9. Enzymatic digestion of the tissue was carried out for 30 min at 35 C with 1.75 mg/ml collagenase B, 0.4 mg/ml elastase (both from Roche, Mannheim, Germany), and 1 mg/ml bovine serum albumin added to the modified Tyrode s solution. After digestion, the modified Tyrode s solution was replaced by storage solution containing 25 mm KCl, 80 mm L-glutamic acid, 20 mm taurine, 10 mm KH 2 PO 4, 3 mm MgCl 2, 10 mm glucose, 10 mm HEPES, and 0.5 mm EGTA. The ph was adjusted to 7.4 with KOH. Cells were kept at least 3 h at 4 C in storage solution before they were slowly readapted to calcium-containing solutions. To compare cloned and native channel properties, I f from SAN cells was recorded in whole-cell mode under the same conditions as described above for HCN currents in HEK293 cells, especially at the same bath temperature of 23 1 C. The extracellular solution was the same as for recordings from HEK293 cells. The pulse length for repeated activation/deactivation of I f was 5 s/5 s. The intracellular solution for measurements on native cells contained 10 mm NaCl, 130 mm potassium aspartate, 0.04 mm CaCl 2, 2 mm Mg-ATP, 6.6 mm creatine phosphate, 0.1 mm sodium GTP, and 10 mm HEPES, ph 7.3. Spontaneous action potentials (APs) from SAN cells were recorded at 32 1 C using the perforated patch technique with 200 M amphotericin B added to the intracellular solution. Standard AP parameters were calculated as described previously (Mangoni and Nargeot, 2001). Telemetric ECG Recordings in Mice. All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals/U.S. National Institutes of Health. Male C57/ Bl6 mice were housed in single cages in a 12-h dark-light cycle environment with free access to food and water. Radiotelemetric ECG transmitters (DSI, St. Paul, MN) were implanted into the peritoneal cavity under general anesthesia with isoflurane/o 2. The ECG leads were sutured subcutaneously onto the upper right chest muscle and the upper left abdominal wall muscle. This resulted approximately in the lead II position of the ECG electrodes. The animals were allowed to recover for at least 2 weeks before the experiments. Stock solutions of cilobradine (4.5 mg/ml), ivabradine (4.5 mg/ml), zatebradine (4.5 mg/ml), and verapamil (2 mg/ml) were prepared in sterile 0.9% NaCl (Braun AG, Melsungen, Germany), kept at 20 C, and diluted with 0.9% NaCl to the desired concentrations in a volume of 100 l on the day of the experiment. Isoproterenol (Sigma, Taufkirchen, Germany) was dissolved in 0.9% NaCl less than 1 h before the injection. Data were acquired using the DSI acquisition system. ECG signals were sampled every minute for 20 s. After 1-h prerun (unless otherwise noted), the mice were injected intraperitoneally with the drug. The ECGs were recorded for 3 to 24 h thereafter. The animals were allowed to recover for at least 48 h between experiments because the effective half-life of the SNIs was up to 12 h compared with the postulated rather short plasma half-life of only 2 h (ivabradine/procoralan; Dr. R. Derwand, Servier Deutschland GmbH, personal communication). For forced physical activity, animals were trained to run on a custom-made, electrically driven treadmill at a speed of approximately 0.4 m/s and 20% ascending slope. As appropriate, before and after the injection of the drug, ECG parameters, heart rate, and heart rate variability were analyzed. The definitions of the ECG intervals are the following: R-R, duration from R to R; P-P, duration from P peak to P peak; PQ, end of P to beginning of Q; QT, beginning of Q to end of T, where end of T is the return of any T deviation to the isoelectric line; QTc, heart ratecorrected QT interval using the equation QT/( R-R/100) (Mitchell et al., 1998); and TP, end of T to beginning of the next ECG complex s P. The heart rate (in beats per minute) was calculated from the R-R duration (in milliseconds) as 60,000/R-R. The standard deviation of
4 Block of HCN Channels Results in Bradycardia and Arrhythmia 1331 the R-R duration (RR-SD) as a measure of heart rate variability was calculated as the standard deviation of the R-R durations during a 20-s period. ED 50 values were obtained by fitting the logarithmically plotted data (heart rate versus drug concentration) with a sigmoidal fit/logistic model: y A 2 (A 1 A 2 )/(1 (x/x 0 ) p ), where x 0 corresponds to ED 50 value. On the linear scale, R-R values were fitted with an exponential model: y A 1 e ( x/t1), RR-SD could be fitted with a sigmoidal (Boltzmann) model: y A 1 (A 2 A 1 )/ (1 10 (log(x0) x)p ). Results Efficient Block of All Four HCN Subtypes by SNIs. SNIs are known blockers of I f. However, because all four subtypes of HCN channels underlie I f in the heart, we wanted to know whether any of the sinus node inhibitors could be used to discriminate between HCN subtypes. The inward currents through all expressed HCN channels were blocked dose- and use-dependently by SNIs at low micromolar concentrations. For example, 0.01 M cilobradine did not inhibit current flow through HCN4 channels, whereas 1 and 5 M led to increasing inhibition (Fig. 2B). The development of the inhibition was rather slow. The channels had to be repeatedly activated before the maximal inhibition for the respective drug concentration could be observed. Verapamil, on the other hand, a known calcium-channel blocker from which the SNIs have been structurally derived, only induced a small, non use-dependent block of HCN channels at high concentrations (Fig. 2B, bottom). Similar to HCN currents, the native I f value of murine SAN cells was blocked efficiently by 5 M cilobradine (Fig. 2C). Again, the block developed rather slowly, because repeated activations were necessary for the block to occur. Comparison of the usedependent block for all HCN subtypes and I f (Fig. 2D) revealed a difference between the HCN subtypes with respect to the onset of the block. HCN1 and HCN2 channels needed 300 to 500 activations before the steady-state inhibition was reached, whereas HCN3, HCN4, and the I f were maximally inhibited after 20 to 40 activations. However, the steadystate inhibition reached for each concentration, an important measure for the effectiveness in vivo, did not seem to be influenced by this 10-fold difference in use-dependence (Fig. 3). The steady-state dose-response curves for HCN1- to -4 showed no subtype-specificity. However, there are differences in the efficiency of the individual SNIs (Table 1). Cilobradine was the most efficient substance with a mean IC 50 value of 0.99 M, followed by zatebradine (1.97 M) and ivabradine (2.2 M). The block of native I f by cilobradine was similarly efficient with an IC 50 value of 0.62 M under the same conditions, measured at room temperature (23 1 C). The slope of the dose-response curves was approximately 1 for each substance and channel (Table 1), suggesting one docking site per channel. Cilobradine Does Not Abolish Spontaneous Action Potentials in Isolated SAN Cells but Changes Their Shape, Frequency, and Variability. To test the effect of SNIs on action potentials, cilobradine as the most effective HCN channel blocker was chosen. Cilobradine (1 M) had a prominent effect on spontaneous action potentials recorded from isolated murine SAN cells (compare Fig. 4, A and B). The firing frequency was reduced to approximately half, apparently by prolongation of the duration between action potentials. Statistical analysis of the AP parameters indeed revealed a highly significant prolongation of the cycle lengths from to ms, n 6 (Fig. 4C), caused by a slowdown of the diastolic depolarization rate (DDR) from to 21 6 mv/s (Fig. 4E). The average maximal diastolic potential (MDP) at the beginning of the pacemaker potential was shifted toward more negative potentials, from to mv (Fig. 4D). This difference, however, was not significant, possibly because of a high variability of this parameter. The action potential duration (i.e., the duration from reaching the threshold potential at approximately 40 mv) to the return of the potential to the MDP was prolonged from to ms (Fig. 4F). This Fig. 3. Steady-state inhibition of HCN1, HCN2, HCN3, and HCN4 channels by cilobradine, ivabradine, and zatebradine and the inhibition of sinoatrial I f by cilobradine. Values are mean inhibition S.D. of currents at the indicated drug concentration determined after 500 activations for HCN1 and HCN2, after 50 activations for HCN3 and HCN4, and after 30 activations for I f. n numbers used to establish the curves are given in Table 1. Superimposed dose-response curves represent logistic fits of means. The meaning of symbols are the following, for all three: square/ short-dotted line, HCN1; circle/broken-dotted line, HCN2; triangle/dotted line, HCN3; diamond/broken line: HCN4; open stars/solid line, I f. TABLE 1 Effects of sinus node inhibitors on HCN channels and I f in sinoatrial node cells and in vivo IC 50 values and slopes of logistic fit (Hill coefficient) of the dose-response curves displayed in Fig. 3 for cilobradine, ivabradine, and zatebradine on expressed HCN channels. In addition, the IC 50 value for cilobradine on native I f recorded from isolated mouse sinoatrial node cells was determined. ED 50 values for the three drugs were determined by analyzing the bradycardic effect in mice (Fig. 6A). Numbers in parentheses give the number of mice/cells used to establish the dose-response curves. HCN Subtype IC 50 Hill Coefficient M Cilobradine, 1.2 mg/kg (10 animals) HCN1 (33) HCN2 (42) HCN3 (37) HCN4 (37) I f (28) Ivabradine, 4.7 mg/kg (6 animals) HCN1 (56) HCN2 (27) HCN3 (38) HCN4 (17) Zatebradine, 1.8 mg/kg (10 animals) HCN1 (27) HCN2 (26) HCN3 (24) HCN4 (22)
5 1332 Stieber et al. prolongation was caused by a slightly slower repolarization velocity of 0.47 versus 0.62 V/s in the absence of cilobradine (Fig. 4G). The slower repolarization velocity contributed only marginally to the slower AP rate. Because the impairment of repolarization is caused by a block of repolarizing potassium currents and might cause serious rhythm problems such as torsades-de-pointes arrhythmias, we tested the effect of a high dose of cilobradine (5 M) on the outward potassium currents of myocytes isolated from murine atria (data not shown). In agreement with findings by Goethals et al. (1993), cilobradine induced a small block of the fast-activating phase of repolarizing potassium currents (from 21 3 to pa/pf at 60 mv, n 12 cells). This reduction was significant at p 0.05 and could be responsible for the slightly delayed repolarization. The depolarization (upstroke) velocity, presumably carried by calcium and sodium currents, was not changed significantly by this SNI ( versus V/s; data not shown). Incubation with high concentrations of cilobradine up to 10 M did not slow the DDR further. However, the cycle lengths (CL) became increasingly variable. Figure 4H shows that 1 M cilobradine already increased significantly the S.D. of the CL. This increasing CL-SD corresponds to the increasing heart rate variability in mice as described below. SNIs Cause Bradycardia and Arrhythmia in Mice. Telemetric ECG recordings in mice allowed the analysis of the heart rate and ECG parameters in response to the application of SNIs in an unrestrained state for the animal. In- Fig. 4. Effect of 1 M cilobradine on spontaneous APs recorded from isolated murine sinoatrial node cells at 32 C. Example of APs recorded from the same cell before (A) and 60 s after (B) application of the SNI. Parameters shown in C to H are depicted in B. C to H, comparison of CL (C), MDP (D), DDR (E), action potential duration (APD, F), repolarization velocity (V Rep, G), and CL-SD (H) of APs recorded before ( bath ) and after ( cilobradine ) drug application. Values are mean S.D., n 6., p Of the AP parameters, all except for MDP differ significantly before and after the application of cilobradine. The increase in CL-SD (H) indicates increasing AP arrhythmia in the presence of the SNI.
6 Block of HCN Channels Results in Bradycardia and Arrhythmia 1333 traperitoneal injection of cilobradine dose-dependently reduced the heart rate and increased its variability (Fig. 5). Identical results were obtained with ivabradine and zatebradine but not with verapamil (Fig. 6A). The reduction of the heart rate started at doses of 0.5 mg/kg. The slowest heart rate that could be reached with the SNIs was approximately 200 bpm. No slower heart rates were reached, even at progressively higher doses. The ED 50 values for the decrease in heart rate were in a similar range, with cilobradine having the lowest ED 50 value with 1.2 mg/kg followed by zatebradine (1.8 mg/kg) and ivabradine (4.7 mg/kg) (Table 1). Again, the heart rate was reduced because the R-R interval durations increased in the presence of SNIs (Fig. 6B, top) and so did the variability of the R-R intervals, as demonstrated by the increasing standard deviation of the R-R intervals (Fig. 6B, bottom). Doses greater than 5 mg/kg of the SNIs, however, had an additional effect on the heart rate. They induced a periodic arrhythmia (Fig. 5, bottom). This type of arrhythmia started at approximately 7.5 mg/kg cilobradine, 10 mg/kg zatebradine, and 12.5 mg/kg ivabradine. The arrhythmia consisted of a periodic fluctuation of the R-R interval durations (Fig. 5, right bottom). This periodicity was characterized by cycles in which the P-P intervals got progressively shorter until one whole ECG complex was omitted, and the cycle started again with a longer P-P interval (Fig. 7, A and B). An AV conduction block did not seem to be the cause for the arrhythmia, because the PQ intervals showed no abnormal variations during one cycle of arrhythmia ( ms), and once a P wave occurred, all following peaks and waves of the ECG complex (Q, R, S, and T) were invariably present. The only interval of the ECG complex that was consistently and in parallel with the P-P interval (Fig. 7B) affected by increasing doses of cilobradine was the duration between the end of the T wave and the beginning of the following P wave. This TP interval is basically the diastolic interval and includes the (usually nonvisible) slow spontaneous depolarization of the sinus node that leads to the excitation of the atria, visible as P wave. After injection of saline, 2 mg/kg cilobradine, which induces bradycardia, and 10 mg/kg cilobradine, which induces bradycardia and arrhythmia, the TP duration changed significantly with every dose from a mean of 47 7 (control) to ms (2 mg/kg) to ms (10 mg/kg) (Fig. 7C). Ivabradine and zatebradine had qualitatively the same effects on the ECG parameters as cilobradine only that higher doses were needed in agreement with their higher ED 50 values. SNIs also prolonged the net QT duration, an effect caused by their impact on the heart rate. However, the heart rate-corrected QT durations are not prolonged even at high doses of SNIs (Fig. 7C). This result suggested that even though repolarization velocity and repolarizing potassium currents of single cardiac cells are slightly inhibited by a high dose of the drugs (demonstrated for cilobradine, Fig. 4), this Fig. 5. Examples of telemetric ECG recordings in freely moving mice after injection of an SNI. Left, recordings from a mouse 30 min after intraperitoneal injection with saline or 2, 5, or 10 mg/kg cilobradine. Right, the corresponding R-to-R interval durations obtained by peak-topeak analysis of the ECG complexes; 2 and 5 mg/kg cilobradine induce increasing bradycardia without abnormal arrhythmia, and 10 mg/kg cilobradine induces bradycardia and arrhythmia characterized by periodic fluctuations of the R-R durations. Fig. 6. Dose-dependent heart rate reduction and arrhythmia induction in mice by SNIs. A, heart rates (in beats per minute) 30 min after intraperitoneal injection of cilobradine, ivabradine, and zatebradine at the indicated doses. Symbols represent the mean S.D., and superimposed dose-response curves represent the logistic fits of the means. Mean heart rates after injection of verapamil are also given, but no fit was found because verapamil did not affect the heart rate at doses comparable with the SNIs; n 6 to 10 mice per dose and drug (also given in Table 1). The ED 50 value of ivabradine differs significantly at p from both cilobradine and zatebradine. B, R-R intervals (top) and the R-R standard deviation from beat to beat (bottom) as a means to describe arrhythmia after injection of indicated doses of the drugs. All drugs induce arrhythmia at doses greater than 5 mg/kg, with ivabradine requiring the highest and cilobradine the lowest dose for the arrhythmia to appear. Symbols represent the mean S.E.M., fits represent exponential fit at the top and sigmoidal fit at the bottom; circles/solid line, cilobradine; diamonds/ broken-dotted line, zatebradine; squares/dotted line, ivabradine. Note that for linear scales in B, n numbers are the same as in A.
7 1334 Stieber et al. Fig. 7. Effects of SNIs on the cardiac electrical activity in mice. A, enlarged ECG examples from Fig. 5, bottom, showing one cycle of the typical arrhythmia caused by a high dose of the SNI cilobradine. P, Q, and end of T (here: return of negative part of T deviation to isoelectric line), from which the parameters in B were calculated, are indicated in the bottom enlargement. B, P-P, TP, and PQ durations corresponding to the ECG stretch displayed in A, top. The duration of the P-P interval decreases with each heart beat until a pause occurs. This fluctuation in P-P durations is independent of the PQ durations but is caused by equally varying TP durations. C, statistical analysis of the TP-, PQ-, QT-, and QTc durations; n 6 mice, with analysis performed on a 20-s ECG stretch per animal, before ( unmodulated ) and after application of 2 or 10 mg/kg drug. The TP duration is highly significantly prolonged by SNIs, and fluctuations in the TP duration are responsible for the arrhythmia. SNIs also prolong the QT duration significantly, but the heart ratecorrected QTc duration is not significantly different., p 0.05;, p inhibition does not seem to change significantly the repolarization time in the whole animal at reasonable SNI doses. Impaired Up-Regulation of the Heart Rate after Inhibition of I f. Binding of camp to HCN channels has been implicated to participate in the sympathetic up-regulation of the beating frequency. To explore the role of I f in heart rate regulation at stress, we performed two sets of experiments in mice: pharmacological stimulation with isoproterenol, and forced physical activity. Injection of 0.5 mg/kg isoproterenol alone increased the heart rate from a mean of to bpm within 10 min (Fig. 8, A and C). After approximately 1 h, the heart rate returned to a resting value, which was not different from the heart rate after injection of NaCl as a control. Injection of 5 mg/kg cilobradine slowed the heart rate to a mean of bpm within 30 min, and this effect lasted for more than 3 h. It is noteworthy that the heart rate after injection of 5 mg/kg cilobradine and, 30 min later, 0.5 mg/kg isoproterenol (Fig. 8A, bottom) still increased, but not above a low resting value of bpm. Physical activity, like running on a treadmill, increased the heart rate to bpm, similar to an isoproterenol injection (Fig. 8, B and C). However, running after an injection of 5 mg/kg cilobradine increased the heart rate only to bpm, not further, again similar to the experiment with the pharmacological stimulation. These results indicated that an up-regulation of the heart rate under the condition of presumably blocked I f is still possible but is impaired. Because it was possible that the blocked I f could be simply unblocked by the binding of camp, we tested the effect of cilobradine on cloned HCN channels and native I f in the absence and presence of camp (Fig. 8D). Addition of the membrane-permeable camp analog on 8-bromoadenosine-cAMP, however, increased neither the steadystate HCN4 current in HEK293 cells nor the sinoatrial I f after the currents had been blocked by 1 M cilobradine. These findings suggest that a basic up-regulation of the heart rate is independent of I f, but faster heart rates require I f. Discussion The results of this study can be summarized as follows: 1) Cilobradine, ivabradine, and zatebradine block all four HCN channels with the same IC 50 value and provide no subtypespecific blocker. As reported previously for native I f (Bogaert et al., 1990; Bucchi et al., 2002), the block is use-dependent. The use-dependence is affected by the subtype of the channel; 2) cardiac rhythm in mice is decreased to a basal frequency and can not be further lowered even by supramaximal doses of SNIs; 3) high doses of SNIs induce periodic arrhythmia that has the characteristics of a second-degree sinoatrial block; and 4) the sympathetic stimulation of the heart rate remains possible but is impaired in the presence of an apparent complete block of I f by high doses of SNIs. These findings are in line with previous reports (Schram et al., 2002; Satoh, 2003; Mangoni et al., 2005) that regulation of the heart rate depends on several independent mechanisms. Our experiments showed that both the normal heart rate and up-regulation of the heart rate higher than resting values require an intact I f. A characteristic feature of I f and HCN channels is their direct modulation by camp (Robinson and Siegelbaum, 2003; Baruscotti et al., 2005). Binding of camp to HCN4 shifts the voltage dependence of channel opening to more positive values and increases thereby the
8 Block of HCN Channels Results in Bradycardia and Arrhythmia 1335 Fig. 8. Reduced sympathetic up-regulation of the heart rate in mice after application of an SNI. A, time course of heart rate after intraperitoneal injection of cilobradine and isoproterenol ( -adrenergic stimulation). A, top, heart rate after the injection of 100 l of 0.9% NaCl as control (NaCl, broken line), 5 mg/kg cilobradine (Cilo, solid line), or 0.5 mg/kg isoproterenol (Iso, broken-dotted line). Injection was done at time point 0 (vertical line). A, bottom, heart rate after cilobradine/isoproterenol injection; 5 mg/kg cilobradine was injected at t 0 (first vertical line), and 0.5 mg/kg isoproterenol was injected 30 min after cilobradine (second vertical line). The -adrenergic stimulation was only able to increase the heart rate from bradycardia (approximately 250 bpm) to a low-resting level of DDR in vivo. However, the persistence of a basic heart rate even when I f is maximally blocked suggests that I f is not responsible for the basic pacemaker action potential, supporting notions of other mechanisms for the generation of a slow spontaneous diastolic depolarization independent of I f, which include the activation of several depolarizing currents (I Ca,T, I Ca,L, I st, I Na/Ca, and calcium release from SR) and the inactivation of hyperpolarizing potassium currents. We cannot absolutely rule out that even in the presence of high doses of SNIs, some depolarizing current through HCN channels still flows, because there is always an equilibrium between blocked and unblocked channels. In contrast to this consideration, an up-regulation of the heart rate in the presence of SNIs was only possible from approximately 200 to 450 bpm, not further. This basic up-regulation upon sympathetic stimulation is most likely caused by a mechanism that does not involve I f but rather modulation of I st (Mitsuiye et al., 2000), I Ca currents, and/or Ca 2 release through ryanodine receptors (Satoh, 2003; Mangoni et al., 2005). The relation of blocked I f in the cardiac conduction system and bradycardia has been established and needs no further consideration (DiFrancesco and Camm, 2004; Baruscotti et al., 2005). However, the induction of very striking dysrhythmias by each tested SNI has not been described before. Although the sinus node inhibitors have been studied both in vivo (Borer et al., 2003; Vilaine et al., 2003; Colin et al., 2004; DiFrancesco and Camm, 2004; Du et al., 2004; Monnet et al., 2004) and in vitro (Goethals et al., 1993; Bogaert and Pittoors, 2003; DiFrancesco and Camm, 2004), reports of a changed variability of heart rates, AP cycle lengths, or diastolic depolarization rates are missing. This lack of information is probably due to the fact that in these previous studies, the drugs had been applied in a dosage and manner to study only the bradycardic effect of the compounds. In addition, many of the in vitro experiments were conducted on Purkinje fibers using electrically triggered action potentials (Bogaert and Pittoors, 2003), which, of course, disguises any spontaneous arrhythmia. We found similar arrhythmias both in the living mouse and, using the most efficient HCN blocker cilobradine, in isolated sinoatrial node cells, pointing to the conclusion that in the whole animal, the observed arrhythmia, like the bradycardia, originated in the leading pacemaker cell. Periodic arrhythmias are often associated with Wenckebach s periodicity. Normal Wenckebach s periodicity is typapproximately 450 bpm, compared with up to 750 bpm after the injection of isoproterenol without cilobradine; n 6 mice for each experiment, and each experiment was done twice. Values are mean S.D. B, heart rate before, during, and after a 10-min run on a treadmill. B, without (top) and with (bottom) injection of 5 mg/kg cilobradine before the run. Similar to the drug-induced -adrenergic stimulation, the SNI prevents up-regulation of the heart rate above a low-resting level. C, statistical analysis of the heart rate modulations shown in A and B. For this analysis, the heart rate reached 30 min after the injection at t 0 (A, top) or after the second injection (A, bottom) or the maximum reached during the run (B) was taken. Levels of significance are indicated. Broken-dotted lines/, p 0.05; Solid lines/, p 0.01; broken lines/, p D, HCN currents or I f, blocked by cilobradine, cannot be unblocked by camp. Partial block of HCN4 channels (left) or I f (right) by 1 M cilobradine was obtained by repeated activation steps to 100 mv as described in Fig. 2 and is given as residual current from unmodulated currents, which are set to 100%. In the presence of 1 M cilobradine, 100 M 8-bromoadenosine-cAMP was then added to the bath solution, whereas inward currents were continuously elicited by steps to 100 mv.
9 1336 Stieber et al. ical for a second-degree atrioventricular conduction block. In this condition, the AV conduction is impaired, leading to an elongation of the PQ interval from beat to beat until the interval becomes too long for conduction through the AV node to occur, resulting in one omitted QRS complex after a P wave. After that, a new Wenckebach s cycle with a normal PQ interval starts. In our study, the animals did not show any AV conduction delay. The PQ intervals did not show unusual variations from beat to beat, and isolated P waves without a following QRS complex did not occur. Rather, during one cycle of arrhythmia, the R-R intervals became progressively shorter until one whole ECG complex was left out. This is a sign for a second-degree sinoatrial block, sometimes called sinoatrial-wenckebach s periodicity. In accordance with this, the only ECG interval that was affected by SNIs was the TP duration, which represents the diastolic phase in which the sinoatrial excitation builds up, eventually leading to the excitation of the atria, visible as the P wave. With the data available to date, we propose two basic mechanisms that, probably in combination, could underlie this periodic disturbance of sinus node excitation after the application of SNIs. First, inhibition of I f has different effects on individual sinoatrial node cells because the sinus node is a heterogeneous structure. The cells in the center are postulated to function as leading pacemaker cells. Ion-channel density, including I f, increases from center to periphery (Kodama et al., 2004) and so does conduction velocity. The AP rate in the intact sinus node is highest in the center, complying with the function as leading pacemaking site, but, paradoxically, in isolated cells from the sinus node, the AP rate is higher in cells from the periphery. SNIs could have a stronger impact on the leading pacemaker cells with their smaller current density. Sooner or later, they fire too slowly, so cells from the periphery initiate the next beat. Without the depolarizations and regulations from the center, their firing rate gets faster and faster, until nonpacemaking atrial cells interfere or excitation-refractory is reached, causing the pause. Then, a slower pacemaker cell in the center can take over again. Second, the arrhythmia could arise in one leading pacemaker cell because SNIs have been shown to block I k and I Ca,L (but not I Ca,T ) at high concentrations in addition to I f in SAN cells (Goethals et al., 1993; DiFrancesco and Camm, 2004). The block of these currents was not very effective: 10 M zatebradine or ivabradine blocked I K by 18 and 16%, respectively, and I Ca,L by 7% and 18%, respectively. Still, this could lead to an imbalance of the ionic mechanisms usually working together in pacemaking: The block of depolarizing currents (I f and I Ca,L ) in sinoatrial node cells, or of the repolarizing I K in the myocardium, would result in a slower AP rate, but the block of the outward I K in sinoatrial node cells, on the other hand, would tend to accelerate the diastolic depolarization by keeping the membrane potential closer to the AP threshold potential. Depending on the degree of block of the individual currents, this results only in bradycardia or additional arrhythmia. Because a large I f is present in other parts of the cardiac conduction system as well, the question might arise of why the inhibition of this current does not influence conduction. I f probably has no influence on triggered action potentials. Although Purkinje fibers have a certain potential for spontaneous depolarizations, this activity is normally overridden by the oncoming depolarizations from faster firing cells. On the other hand, the inhibition of I f in the whole cardiac conduction system may be the reason for the lack of escape rhythms, even though no excitation of the sinus node was sometimes observed for several seconds (corresponding to up to 40 heart beats in the mouse) during the arrhythmias. We did find slight subtype-specific differences in the usedependent onset of the block, even when, in the case of HCN2 and HCN3, the same activation/deactivation times and thus exposure times to the drugs were used. This is in accordance with available data from other groups. The block of I f by cilobradine in sheep Purkinje fibers (Bogaert and Pittoors, 2003) or mouse sinoatrial node cells in our experiments or by ivabradine in rabbit sinoatrial node cells (Bucchi et al., 2002) was completed after 40 activations, whereas 150 to 200 activations were required to block the I h recorded from mouse dorsal root ganglion neurons (Raes et al., 1998). Assuming HCN4 to be the major HCN subtype of I f in the cardiac conduction system and HCN1 and HCN2 to be the major neuronal subtypes, the data match, even though the molecular basis for this remains to be documented. To study the effect of I f inhibition in vivo, the time to onset of action might be less important than the steady-state inhibition. There were no differences in the steady-state inhibition between the HCN subtypes, although the three used SNIs differed slightly in their IC 50 values. Cilobradine was the most effective HCN current blocker, followed by zatebradine and ivabradine. This order of efficiency is very interesting because it is exactly the order determined for both the induction of bradycardia and arrhythmia in vivo. This similarity suggests that both conditions are primarily caused by an increasing block of HCN channels despite the potential involvement of other ionic mechanism discussed above. In conclusion, SNIs cause bradycardia and arrhythmia primarily by inhibition of I f, which impairs the slow diastolic depolarization of sinoatrial pacemaker cells. The major physiological role of I f seems to be its contribution to the pacemaker potential at fast heart rates. Acknowledgments We thank Natalie Krahmer and Evi Kiermayer for some excellent electrophysiological recordings. Technical assistance from Anna Thomer is highly appreciated. References Baruscotti M, Bucchi A, and DiFrancesco F (2005) Physiology and pharmacology of the cardiac pacemaker ( funny ) current. Pharmacol Ther 107: Bogaert PP, Goethals M, and Simoens C (1990) Use- and frequency-dependent blockade by UL-FS 49 of the I f pacemaker current in sheep cardiac Purkinje fibres. Eur J Pharmacol 187: Bogaert PP and Pittoors F (2003) Use-dependent blockade of cardiac pacemaker current (I f ) by cilobradine and zatebradine. Eur J Pharmacol 478: Bois P, Bescond J, Renaudon B, and Lenfant J (1996) Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol 118: Borer JS, Fox K, Jaillon P, and Lerebours G (2003) Antiangial and antiischemic effects of ivabradine, an I f inhibitor, in stable angina. Circulation 107: BoSmith RE, Briggs I, and Sturgess NC (1993) Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (I f ) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol 110: Bucchi A, Baruscotti M, and DiFrancesco D (2002) Current-dependent block of rabbit sino-atrial node I f channels by Ivabradine. J Gen Physiol 120:1 13. Colin P, Ghaleh B, Monnet X, Hittinger L, and Berdeaux A (2004) Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther 308: DiFrancesco D and Camm JA (2004) Heart rate lowering by specific and selective I f current inhibition with ivabradine. Drugs 64: Du XJ, Feng X, Gao XM, Tan TP, Kiriazis H, and Dart AM (2004) I f channel inhibitor ivabradine lowers heart rate in mice with enhanced sympathoadrenergic activities. Br J Pharmacol 142: Goethals M, Raes A, and Bogaert PP (1993) Use-dependent block of the pacemaker
10 Block of HCN Channels Results in Bradycardia and Arrhythmia 1337 current I f in rabbit sinoatrial node cells by Zatebradine (UL-FS 49). Circulation 88: Kodama I, Honjo H, Dobrzynski H, and Boyett M (2004) Cellular mechanisms of sinoatrial activity, in Cardiac Electrophysiology. From Cell to Bedside (Zipes DP and Jalife J eds) pp , Saunders/Elsevier Inc., Philadelphia. Ludwig A, Budde T, Stieber J, Moosmang S, Wahl C, Holthoff K, Langebartels A, Wotjak C, Munsch T, Zong XG, et al. (2003) Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO (Eur Mol Biol Organ) J 22: Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, and Biel M (1999) Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO (Eur Mol Biol Organ) J 18: Ludwig A, Zong XG, Jeglitsch M, Hofmann F, and Biel M (1998) A family of hyperpolarization-activated mammalian cation channels. Nature (Lond) 393: Mangoni ME, Couette B, Marger L, Bourinet E, Striessnig J, and Nargeot J (2005) Voltage-dependent calcium channels and cardiac pacemaker activity: from ionic currents to genes. Prog Biophys Mol Biol 90: Mangoni ME and Nargeot N (2001) Properties of the hyperpolarization-activated current (I f ) in isolated mouse sino-atrial cells. Cardiovasc Res 52: Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J, Lei M, Escande D, and Demolombe S (2005) Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol (Lond) 562: Mitchell GF, Jeron A, and Koren G (1998) Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol 274:H747 H751. Mitsuiye T, Shinagawa Y, and Noma A (2000) Sustained inward current during pacemaker depolarization in mammalian sinoatrial node cells. Circ Res 87: Monnet X, Colin P, Ghaleh B, Hittinger L, Giudecelli J-F, and Berdeaux A (2004) Heart rate reduction during exercise-induced myocardial ischaemia and stunning. Eur Heart J 25: Raes A, Van de Vijver G, Goethals M, and Bogaert PP (1998) Use-dependent block of I h in mouse dorsal root ganglion neurons by sinus node inhibitors. Br J Pharmacol 125: Robinson RB and Siegelbaum SA (2003) Hyperpolarization-activated cation currents: From molecules to physiological function. Annu Rev Physiol 65: Santoro B, Liu D, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, and Tibbs GR (1998) Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93: Satoh H (2003) Sino-atrial nodal cells of mammalian hearts: Ionic currents and gene expression of pacemaker ionic channels. J Smooth Muscle Res 39: Schram G, Pourrier M, Melnyk P, and Nattel S (2002) Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res 90: Stieber J, Herrmann S, Feil S, Löster J, Feil R, Biel M, Hofmann F, and Ludwig A (2003a) The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart. Proc Natl Acad Sci USA 100: Stieber J, Stöckl G, Herrmann S, Hassfurth B, and Hofmann F (2005) Functional expression of the human HCN3 channel. J Biol Chem 280: Stieber J, Thomer A, Much B, Schneider A, Biel M, and Hofmann F (2003b) Molecular basis for the different activation kinetics of the pacemaker channels HCN2 and HCN4. J Biol Chem 278: Vilaine JP, Bidouard JP, Lesage L, Reure H, and Peglion JL (2003) Anti-ischemic effects of ivabradine, a selective heart rate-reducing agent, in exercise-induced myocardial ischemia in pigs. J Cardiovasc Pharmacol 42: Address correspondence to: Dr. Juliane Stieber, Institut für Pharmakologie und Toxikologie, TU München, Biedersteiner Str. 29, München, Germany. stieber@ipt.med.tu-muenchen.de
Cardiac Pacemaker (I f ) Current: Physiological and Pharmacological Properties
 HOSPITAL CHRONICLES 2006, SUPPLEMENT: 151 155 CARDIOLOGY UPDATE 2006 Cardiac Pacemaker (I f ) Current: Physiological and Pharmacological Properties Dario DiFrancesco, Ph.D. A B S T R A C T University of
HOSPITAL CHRONICLES 2006, SUPPLEMENT: 151 155 CARDIOLOGY UPDATE 2006 Cardiac Pacemaker (I f ) Current: Physiological and Pharmacological Properties Dario DiFrancesco, Ph.D. A B S T R A C T University of
Introduction. Circulation
 Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Introduction Circulation 1- Systemic (general) circulation 2- Pulmonary circulation carries oxygenated blood to all parts of the body carries deoxygenated blood to the lungs From Lt. ventricle aorta From
Heart rate reduction via selective funny channel blockers Annalisa Bucchi 1,2, Andrea Barbuti 1, Mirko Baruscotti 1 and Dario DiFrancesco 1
 Heart rate reduction via selective funny channel blockers Annalisa Bucchi 1,2, Andrea Barbuti 1, Mirko Baruscotti 1 and Dario DiFrancesco 1 The funny current, first described in cardiac pacemaker cells
Heart rate reduction via selective funny channel blockers Annalisa Bucchi 1,2, Andrea Barbuti 1, Mirko Baruscotti 1 and Dario DiFrancesco 1 The funny current, first described in cardiac pacemaker cells
Cardiac Properties MCQ
 Cardiac Properties MCQ Abdel Moniem Ibrahim Ahmed, MD Professor of Cardiovascular Physiology Cairo University 2007 1- Cardiac Valves: a- Prevent backflow of blood from the ventricles to the atria during
Cardiac Properties MCQ Abdel Moniem Ibrahim Ahmed, MD Professor of Cardiovascular Physiology Cairo University 2007 1- Cardiac Valves: a- Prevent backflow of blood from the ventricles to the atria during
Chapter 12: Cardiovascular Physiology System Overview
 Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
Chapter 12: Cardiovascular Physiology System Overview Components of the cardiovascular system: Heart Vascular system Blood Figure 12-1 Plasma includes water, ions, proteins, nutrients, hormones, wastes,
The Effects of Extracellular Calcium Removal on Sino-atrial Node Cells Treated with Potassium-depleted Solutions
 Short Communication Japanese Journal of Physiology, 36, 403-409, 1986 The Effects of Extracellular Calcium Removal on Sino-atrial Node Cells Treated with Potassium-depleted Solutions Shun-ichi MIYAMAE
Short Communication Japanese Journal of Physiology, 36, 403-409, 1986 The Effects of Extracellular Calcium Removal on Sino-atrial Node Cells Treated with Potassium-depleted Solutions Shun-ichi MIYAMAE
PART I. Disorders of the Heart Rhythm: Basic Principles
 PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
PART I Disorders of the Heart Rhythm: Basic Principles FET01.indd 1 1/11/06 9:53:05 AM FET01.indd 2 1/11/06 9:53:06 AM CHAPTER 1 The Cardiac Electrical System The heart spontaneously generates electrical
The "Pacemaker" Function of the Transient Outward Current in the Rabbit Myocardium
 Gen. Physiol. Biophys. (1988). 7. 235 242 235 The "Pacemaker" Function of the Transient Outward Current in the Rabbit Myocardium R. Z. GAINULLIN 1, N. I. KUKUSHKIN 1, R. E. KISELEVA 2 and E. A. SOSUNOV
Gen. Physiol. Biophys. (1988). 7. 235 242 235 The "Pacemaker" Function of the Transient Outward Current in the Rabbit Myocardium R. Z. GAINULLIN 1, N. I. KUKUSHKIN 1, R. E. KISELEVA 2 and E. A. SOSUNOV
Where are the normal pacemaker and the backup pacemakers of the heart located?
 CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
CASE 9 A 68-year-old woman presents to the emergency center with shortness of breath, light-headedness, and chest pain described as being like an elephant sitting on her chest. She is diagnosed with a
Cardiac arrhythmias. Janusz Witowski. Department of Pathophysiology Poznan University of Medical Sciences. J. Witowski
 Cardiac arrhythmias Janusz Witowski Department of Pathophysiology Poznan University of Medical Sciences A 68-year old man presents to the emergency department late one evening complaining of increasing
Cardiac arrhythmias Janusz Witowski Department of Pathophysiology Poznan University of Medical Sciences A 68-year old man presents to the emergency department late one evening complaining of increasing
Heart failure (HF) predisposes to life-threatening ventricular
 Ionic Remodeling of Sinoatrial Node Cells by Heart Failure Arie O. Verkerk, PhD; Ronald Wilders, PhD; Ruben Coronel, MD, PhD; Jan H. Ravesloot, PhD; E. Etienne Verheijck, PhD Background In animal models
Ionic Remodeling of Sinoatrial Node Cells by Heart Failure Arie O. Verkerk, PhD; Ronald Wilders, PhD; Ruben Coronel, MD, PhD; Jan H. Ravesloot, PhD; E. Etienne Verheijck, PhD Background In animal models
CARDIOVASCULAR SYSTEM
 CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
CARDIOVASCULAR SYSTEM Overview Heart and Vessels 2 Major Divisions Pulmonary Circuit Systemic Circuit Closed and Continuous Loop Location Aorta Superior vena cava Right lung Pulmonary trunk Base of heart
The action potential and the underlying ionic currents. Norbert Jost, PhD
 The action potential and the underlying ionic currents Norbert Jost, PhD The propagation of the stimulation in the heart Sinus node Left atria His Bundle Conduction velocity in m/s Time to arrive from
The action potential and the underlying ionic currents Norbert Jost, PhD The propagation of the stimulation in the heart Sinus node Left atria His Bundle Conduction velocity in m/s Time to arrive from
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1
 BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
BIPN100 F15 Human Physiology I (Kristan) Problem set #5 p. 1 1. Dantrolene has the same effect on smooth muscles as it has on skeletal muscle: it relaxes them by blocking the release of Ca ++ from the
The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart
 The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart Juliane Stieber*, Stefan Herrmann*, Susanne Feil*, Jana Löster, Robert
The hyperpolarization-activated channel HCN4 is required for the generation of pacemaker action potentials in the embryonic heart Juliane Stieber*, Stefan Herrmann*, Susanne Feil*, Jana Löster, Robert
Full file at
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What electrical event must occur for atrial kick to occur? 1) A) Atrial repolarization B) Ventricular
ELECTROCARDIOGRAPHY (ECG)
 ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
ELECTROCARDIOGRAPHY (ECG) The heart is a muscular organ, which pumps blood through the blood vessels of the circulatory system. Blood provides the body with oxygen and nutrients, as well as assists in
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Cardiac arrhythmias, also known as irregular heartbeat, occur
Differences in cardiac atrial and ventricular ion channels
 Differences in cardiac atrial and ventricular ion channels Norbert Jost, PhD Department of Pharmacology & Pharmacotherapy, University of Szeged Division for Cardiovascular Pharmacology, Hungarian Academy
Differences in cardiac atrial and ventricular ion channels Norbert Jost, PhD Department of Pharmacology & Pharmacotherapy, University of Szeged Division for Cardiovascular Pharmacology, Hungarian Academy
Is action potential threshold lowest in the axon?
 Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
Supplementary information to: Is action potential threshold lowest in the axon? Maarten H. P. Kole & Greg J. Stuart Supplementary Fig. 1 Analysis of action potential (AP) threshold criteria. (a) Example
THE CARDIOVASCULAR SYSTEM. Heart 2
 THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
THE CARDIOVASCULAR SYSTEM Heart 2 PROPERTIES OF CARDIAC MUSCLE Cardiac muscle Striated Short Wide Branched Interconnected Skeletal muscle Striated Long Narrow Cylindrical PROPERTIES OF CARDIAC MUSCLE Intercalated
Neuroscience 201A Problem Set #1, 27 September 2016
 Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
Neuroscience 201A Problem Set #1, 27 September 2016 1. The figure above was obtained from a paper on calcium channels expressed by dentate granule cells. The whole-cell Ca 2+ currents in (A) were measured
The effect of acidosis on the ECG of the rat heart
 The effect of acidosis on the ECG of the rat heart A. Aberra*, K. Komukai, F. C. Howarth and C. H. Orchard School of Biomedical Sciences, University of Leeds, Leeds LS2 9NL, UK, * Faculty of Medicine,
The effect of acidosis on the ECG of the rat heart A. Aberra*, K. Komukai, F. C. Howarth and C. H. Orchard School of Biomedical Sciences, University of Leeds, Leeds LS2 9NL, UK, * Faculty of Medicine,
CASE 10. What would the ST segment of this ECG look like? On which leads would you see this ST segment change? What does the T wave represent?
 CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
CASE 10 A 57-year-old man presents to the emergency center with complaints of chest pain with radiation to the left arm and jaw. He reports feeling anxious, diaphoretic, and short of breath. His past history
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels. Vahri Beaumont and Robert S. Zucker
 Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic I h channels Vahri Beaumont and Robert S. Zucker Background I h channels discovered in 1976 (Noma A. and Irisawa H.) Voltage-gated
Inhibitory effects of sevoflurane on pacemaking activity of sinoatrial node cells in guinea-pig heart
 2117..2135 British Journal of Pharmacology DOI:10.1111/j.1476-5381.2012.01914.x www.brjpharmacol.org RESEARCH PAPERbph_1914 Inhibitory effects of sevoflurane on pacemaking activity of sinoatrial node cells
2117..2135 British Journal of Pharmacology DOI:10.1111/j.1476-5381.2012.01914.x www.brjpharmacol.org RESEARCH PAPERbph_1914 Inhibitory effects of sevoflurane on pacemaking activity of sinoatrial node cells
Human TRPC6 Ion Channel Cell Line
 TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
UNDERSTANDING YOUR ECG: A REVIEW
 UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
UNDERSTANDING YOUR ECG: A REVIEW Health professionals use the electrocardiograph (ECG) rhythm strip to systematically analyse the cardiac rhythm. Before the systematic process of ECG analysis is described
Shock-induced termination of cardiac arrhythmias
 Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Abstract: Cardiac arrhythmias occur when blood flow to the
Shock-induced termination of cardiac arrhythmias Group members: Baltazar Chavez-Diaz, Chen Jiang, Sarah Schwenck, Weide Wang, and Jinglei Zhang Abstract: Cardiac arrhythmias occur when blood flow to the
The pacemaker function of the specialized myocytes of cardiac
 From Funny Current to HCN Channels: 20 Years of Excitation E. A. Accili, 3 C. Proenza, 3 M. Baruscotti, 1 and D. DiFrancesco 1,2 1 Dipartimento di Fisiologia e Biochimica Generali, Laboratorio di Fisiologia
From Funny Current to HCN Channels: 20 Years of Excitation E. A. Accili, 3 C. Proenza, 3 M. Baruscotti, 1 and D. DiFrancesco 1,2 1 Dipartimento di Fisiologia e Biochimica Generali, Laboratorio di Fisiologia
Fast Calcium Currents in Cut Skeletal Muscle Fibres of the Frogs Rana temporaria and Xenopus laevis
 Gen. Physiol. Biophys. (1988), 7, 651-656 65! Short communication Fast Calcium Currents in Cut Skeletal Muscle Fibres of the Frogs Rana temporaria and Xenopus laevis M. HENČĽK, D. ZACHAROVÁ and J. ZACHAR
Gen. Physiol. Biophys. (1988), 7, 651-656 65! Short communication Fast Calcium Currents in Cut Skeletal Muscle Fibres of the Frogs Rana temporaria and Xenopus laevis M. HENČĽK, D. ZACHAROVÁ and J. ZACHAR
QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY]
![QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY]](/thumbs/96/126998162.jpg) QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] Learning Objectives: Describe the ionic basis of action potentials in cardiac contractile and autorhythmic cells Explain the relationship
QUIZ/TEST REVIEW NOTES SECTION 1 CARDIAC MYOCYTE PHYSIOLOGY [CARDIOLOGY] Learning Objectives: Describe the ionic basis of action potentials in cardiac contractile and autorhythmic cells Explain the relationship
Pulmonary veins (PVs) have been demonstrated to be
 Effects of Rapid Atrial Pacing on the Arrhythmogenic Activity of Single Cardiomyocytes From Pulmonary Veins Implication in Initiation of Atrial Fibrillation Yi-Jen Chen, MD; Shih-Ann Chen, MD; Yao-Chang
Effects of Rapid Atrial Pacing on the Arrhythmogenic Activity of Single Cardiomyocytes From Pulmonary Veins Implication in Initiation of Atrial Fibrillation Yi-Jen Chen, MD; Shih-Ann Chen, MD; Yao-Chang
Chapter 3 subtitles Action potentials
 CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 3 subtitles Action potentials Introduction (3:15) This third chapter explains the calcium current triggered by the arrival of the action potential in
Ionic mechanisms underlying the negative chronotropic action of propofol on sinoatrial node automaticity in guinea pig heart
 British Journal of Pharmacology DOI:10.1111/bph.12936 www.brjpharmacol.org RESEARCH PAPER Ionic mechanisms underlying the negative chronotropic action of propofol on sinoatrial node automaticity in guinea
British Journal of Pharmacology DOI:10.1111/bph.12936 www.brjpharmacol.org RESEARCH PAPER Ionic mechanisms underlying the negative chronotropic action of propofol on sinoatrial node automaticity in guinea
The pacemaker current I f is present in both cardiac
 Dominant-Negative Suppression of HCN Channels Markedly Reduces the Native Pacemaker Current I f and Undermines Spontaneous Beating of Neonatal Cardiomyocytes Fikret Er, MD; Robert Larbig; Andreas Ludwig,
Dominant-Negative Suppression of HCN Channels Markedly Reduces the Native Pacemaker Current I f and Undermines Spontaneous Beating of Neonatal Cardiomyocytes Fikret Er, MD; Robert Larbig; Andreas Ludwig,
Cardiovascular system
 BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
BIO 301 Human Physiology Cardiovascular system The Cardiovascular System: consists of the heart plus all the blood vessels transports blood to all parts of the body in two 'circulations': pulmonary (lungs)
WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES
 WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES Michelle Henry Barton DVM, PhD, DACVIM University of Georgia, Athens, GA INTRODUCTION The purpose of this talk is to review
WHAT S THAT RHYTHM I AM HEARING? GUIDE TO AUSCULTATION OF ARRHYTHMIAS IN HORSES Michelle Henry Barton DVM, PhD, DACVIM University of Georgia, Athens, GA INTRODUCTION The purpose of this talk is to review
Step by step approach to EKG rhythm interpretation:
 Sinus Rhythms Normal sinus arrhythmia Small, slow variation of the R-R interval i.e. variation of the normal sinus heart rate with respiration, etc. Sinus Tachycardia Defined as sinus rhythm with a rate
Sinus Rhythms Normal sinus arrhythmia Small, slow variation of the R-R interval i.e. variation of the normal sinus heart rate with respiration, etc. Sinus Tachycardia Defined as sinus rhythm with a rate
Introduction to ECG Gary Martin, M.D.
 Brief review of basic concepts Introduction to ECG Gary Martin, M.D. The electrical activity of the heart is caused by a sequence of rapid ionic movements across cell membranes resulting first in depolarization
Brief review of basic concepts Introduction to ECG Gary Martin, M.D. The electrical activity of the heart is caused by a sequence of rapid ionic movements across cell membranes resulting first in depolarization
Cardiac muscle is different from other types of muscle in that cardiac muscle
 6 E X E R C I S E Cardiovascular Physiology O B J E C T I V E S 1. To define autorhythmicity, sinoatrial node, pacemaker cells, and vagus nerves 2. To understand the effects of the sympathetic and parasympathetic
6 E X E R C I S E Cardiovascular Physiology O B J E C T I V E S 1. To define autorhythmicity, sinoatrial node, pacemaker cells, and vagus nerves 2. To understand the effects of the sympathetic and parasympathetic
Antiarrhythmic Drugs
 Antiarrhythmic Drugs DR ATIF ALQUBBANY A S S I S T A N T P R O F E S S O R O F M E D I C I N E / C A R D I O L O G Y C O N S U L T A N T C A R D I O L O G Y & I N T E R V E N T I O N A L E P A C H D /
Antiarrhythmic Drugs DR ATIF ALQUBBANY A S S I S T A N T P R O F E S S O R O F M E D I C I N E / C A R D I O L O G Y C O N S U L T A N T C A R D I O L O G Y & I N T E R V E N T I O N A L E P A C H D /
Chapter 13 The Cardiovascular System: Cardiac Function
 Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
Chapter 13 The Cardiovascular System: Cardiac Function Overview of the Cardiovascular System The Path of Blood Flow through the Heart and Vasculature Anatomy of the Heart Electrical Activity of the Heart
ECG. Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13
 ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
ECG Prepared by: Dr.Fatima Daoud Reference: Guyton and Hall Textbook of Medical Physiology,12 th edition Chapters: 11,12,13 The Concept When the cardiac impulse passes through the heart, electrical current
Collin County Community College
 Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
Collin County Community College BIOL. 2402 Anatomy & Physiology WEEK 5 The Heart 1 The Heart Beat and the EKG 2 1 The Heart Beat and the EKG P-wave = Atrial depolarization QRS-wave = Ventricular depolarization
CARDIAC PHYSIOLOGY. Amelyn U. Ramos-Rafael,M.D. Functional Anatomy of the Heart
 CARDIAC PHYSIOLOGY Amelyn U. Ramos-Rafael,M.D. Functional Anatomy of the Heart 1 Functional Anatomy of The Heart The Atria relatively thin walled The Ventricles ventricular walls thicker than atrial walls
CARDIAC PHYSIOLOGY Amelyn U. Ramos-Rafael,M.D. Functional Anatomy of the Heart 1 Functional Anatomy of The Heart The Atria relatively thin walled The Ventricles ventricular walls thicker than atrial walls
Chapter 20 (2) The Heart
 Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Chapter 20 (2) The Heart ----------------------------------------------------------------------------------------------------------------------------------------- Describe the component and function of
Cardiac Telemetry Self Study: Part One Cardiovascular Review 2017 THINGS TO REMEMBER
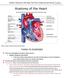 Please review the above anatomy of the heart. THINGS TO REMEMBER There are 3 electrolytes that affect cardiac function o Sodium, Potassium, and Calcium When any of these electrolytes are out of the normal
Please review the above anatomy of the heart. THINGS TO REMEMBER There are 3 electrolytes that affect cardiac function o Sodium, Potassium, and Calcium When any of these electrolytes are out of the normal
V. TACHYCARDIAS Rapid rhythm abnormalities
 V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
V. TACHYCARDIAS Rapid rhythm abnormalities Tachyarrhythmias currently account for up to 350,000 deaths annually in the US. In addition to these clearly dangerous rhythm disturbances, other forms of more
Bradydysrhythmias and Atrioventricular Conduction Blocks
 Emerg Med Clin N Am 24 (2006) 1 9 Bradydysrhythmias and Atrioventricular Conduction Blocks Jacob W. Ufberg, MD*, Jennifer S. Clark, MD Department of Emergency Medicine, Temple University School of Medicine,
Emerg Med Clin N Am 24 (2006) 1 9 Bradydysrhythmias and Atrioventricular Conduction Blocks Jacob W. Ufberg, MD*, Jennifer S. Clark, MD Department of Emergency Medicine, Temple University School of Medicine,
Electrophysiology and pacemaker function of the developing sinoatrial node
 Am J Physiol Heart Circ Physiol 293: H2613 H2623, 2007. First published September 7, 2007; doi:10.1152/ajpheart.00750.2007. Electrophysiology and pacemaker function of the developing sinoatrial node Mirko
Am J Physiol Heart Circ Physiol 293: H2613 H2623, 2007. First published September 7, 2007; doi:10.1152/ajpheart.00750.2007. Electrophysiology and pacemaker function of the developing sinoatrial node Mirko
MOLECULAR MEDICINE REPORTS 10: , 2014
 1576 Berberine attenuates spontaneous action potentials in sinoatrial node cells and the currents of human HCN4 channels expressed in Xenopus laevis oocytes HUI CHEN, YONGJUN CHEN, YANHONG TANG, JING YANG,
1576 Berberine attenuates spontaneous action potentials in sinoatrial node cells and the currents of human HCN4 channels expressed in Xenopus laevis oocytes HUI CHEN, YONGJUN CHEN, YANHONG TANG, JING YANG,
Collin County Community College. ! BIOL Anatomy & Physiology! WEEK 5. The Heart
 Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
Collin County Community College! BIOL. 2402 Anatomy & Physiology! WEEK 5 The Heart 1 (1578-1657) A groundbreaking work in the history of medicine, English physician William Harvey s Anatomical Essay on
Acta Physiologica Sinica
 , 1999 4, 51 (2), 187 192 187 Acta Physiologica Sinica 3 1998204222 1998206203 3 (No139500052) 3 3, 221002 3 3 3 3 3 (, 200031) ( Ito), 28 d (H28, 6 h/ d), Ito (16118 4161 6132 1135 pa/ pf, P < 0105),
, 1999 4, 51 (2), 187 192 187 Acta Physiologica Sinica 3 1998204222 1998206203 3 (No139500052) 3 3, 221002 3 3 3 3 3 (, 200031) ( Ito), 28 d (H28, 6 h/ d), Ito (16118 4161 6132 1135 pa/ pf, P < 0105),
Arrhythmias. 1. beat too slowly (sinus bradycardia). Like in heart block
 Arrhythmias It is a simple-dysfunction caused by abnormalities in impulse formation and conduction in the myocardium. The heart is designed in such a way that allows it to generate from the SA node electrical
Arrhythmias It is a simple-dysfunction caused by abnormalities in impulse formation and conduction in the myocardium. The heart is designed in such a way that allows it to generate from the SA node electrical
Assessment of pro-arrhythmic effects using Pluricyte Cardiomyocytes. on the ACEA xcelligence RTCA CardioECR
 Assessment of pro-arrhythmic effects using Pluricyte Cardiomyocytes on the ACEA xcelligence RTCA CardioECR Application Note Version 2.1 / March 2018 Contents 1. Introduction 1 2. Assessment of pro-arrhythmic
Assessment of pro-arrhythmic effects using Pluricyte Cardiomyocytes on the ACEA xcelligence RTCA CardioECR Application Note Version 2.1 / March 2018 Contents 1. Introduction 1 2. Assessment of pro-arrhythmic
Lab 2. The Intrinsic Cardiac Conduction System. 1/23/2016 MDufilho 1
 Lab 2 he Intrinsic Cardiac Conduction System 1/23/2016 MDufilho 1 Figure 18.13 Intrinsic cardiac conduction system and action potential succession during one heartbeat. Superior vena cava ight atrium 1
Lab 2 he Intrinsic Cardiac Conduction System 1/23/2016 MDufilho 1 Figure 18.13 Intrinsic cardiac conduction system and action potential succession during one heartbeat. Superior vena cava ight atrium 1
Supplementary Information
 Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
Hyperpolarization-activated cation channels inhibit EPSPs by interactions with M-type K + channels Meena S. George, L.F. Abbott, Steven A. Siegelbaum Supplementary Information Part 1: Supplementary Figures
New Agents for Heart Failure: Ivabradine Jeffrey S. Borer, MD
 New Agents for Heart Failure: Ivabradine Jeffrey S. Borer, MD Professor of Medicine, Cell Biology, Radiology and Surgery Director, The Howard Gilman Institute for Heart Valve Disease and the Schiavone
New Agents for Heart Failure: Ivabradine Jeffrey S. Borer, MD Professor of Medicine, Cell Biology, Radiology and Surgery Director, The Howard Gilman Institute for Heart Valve Disease and the Schiavone
EKG Abnormalities. Adapted from:
 EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
EKG Abnormalities Adapted from: http://www.bem.fi/book/19/19.htm Some key terms: Arrhythmia-an abnormal rhythm or sequence of events in the EKG Flutter-rapid depolarizations (and therefore contractions)
Management of the coronary patient in Roberto Ferrari
 Management of the coronary patient in 2011 Roberto Ferrari What is new in treatment of stable CAD? In the era of interventional cardiology, is chronic stable angina a rare disease? Stable angina pectoris
Management of the coronary patient in 2011 Roberto Ferrari What is new in treatment of stable CAD? In the era of interventional cardiology, is chronic stable angina a rare disease? Stable angina pectoris
ACTION POTENTIAL TRANSFER AT THE PURKINJE - VENTRICULAR JUNCTION: ROLE OF TRANSITIONAL CELLS
 1 of 4 ACTION POTENTIAL TRANSFER AT THE PURKINJE - VENTRICULAR JUNCTION: ROLE OF TRANSITIONAL CELLS Arie O. Verkerk 1, Marieke W. Veldkamp 2, Antoni C.G. van Ginneken 1,2, Ronald Wilders 1,3 1 Department
1 of 4 ACTION POTENTIAL TRANSFER AT THE PURKINJE - VENTRICULAR JUNCTION: ROLE OF TRANSITIONAL CELLS Arie O. Verkerk 1, Marieke W. Veldkamp 2, Antoni C.G. van Ginneken 1,2, Ronald Wilders 1,3 1 Department
Effects of Temperature, Stretch, and Various Drug Treatments on the
 Nicole Rodi Bio 235: Animal Physiology Heart Muscle Lab Report 10/24/2014 Effects of Temperature, Stretch, and Various Drug Treatments on the Cardiac Muscle Activity of Rana pipiens Abstract Mechanical
Nicole Rodi Bio 235: Animal Physiology Heart Muscle Lab Report 10/24/2014 Effects of Temperature, Stretch, and Various Drug Treatments on the Cardiac Muscle Activity of Rana pipiens Abstract Mechanical
11/10/2014. Muscular pump Two atria Two ventricles. In mediastinum of thoracic cavity 2/3 of heart's mass lies left of midline of sternum
 It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
It beats over 100,000 times a day to pump over 1,800 gallons of blood per day through over 60,000 miles of blood vessels. During the average lifetime, the heart pumps nearly 3 billion times, delivering
Funny channels in the control of cardiac rhythm and mode of action of selective blockers
 Pharmacological Research xxx (2006) xxx xxx Review Funny channels in the control of cardiac rhythm and mode of action of selective blockers Dario DiFrancesco University of Milano, Department of Biomolecular
Pharmacological Research xxx (2006) xxx xxx Review Funny channels in the control of cardiac rhythm and mode of action of selective blockers Dario DiFrancesco University of Milano, Department of Biomolecular
Gene annotation for heart rhythm. 1. Control of heart rate 2. Action Potential 3. Ion channels and transporters 4. Arrhythmia 5.
 Gene annotation for heart rhythm 1. Control of heart rate 2. Action Potential 3. Ion channels and transporters 4. Arrhythmia 5. EC coupling Control of heart rate Autonomic regulation of heart function
Gene annotation for heart rhythm 1. Control of heart rate 2. Action Potential 3. Ion channels and transporters 4. Arrhythmia 5. EC coupling Control of heart rate Autonomic regulation of heart function
A spontaneously active focus drives a model atrial sheet more easily than a model ventricular sheet
 Am J Physiol Heart Circ Physiol 279: H752 H763, 2000. A spontaneously active focus drives a model atrial sheet more easily than a model ventricular sheet RONALD W. JOYNER, 1 YANG-GAN WANG, 1 RONALD WILDERS,
Am J Physiol Heart Circ Physiol 279: H752 H763, 2000. A spontaneously active focus drives a model atrial sheet more easily than a model ventricular sheet RONALD W. JOYNER, 1 YANG-GAN WANG, 1 RONALD WILDERS,
Lecture outline. Electrical properties of the heart. Automaticity. Excitability. Refractoriness. The ABCs of ECGs Back to Basics Part I
 Lecture outline The ABCs of ECGs Back to Basics Part I Meg Sleeper VMD, DACVIM (cardiology) University of Florida Veterinary School Electrical properties of the heart Action potentials Normal intracardiac
Lecture outline The ABCs of ECGs Back to Basics Part I Meg Sleeper VMD, DACVIM (cardiology) University of Florida Veterinary School Electrical properties of the heart Action potentials Normal intracardiac
HCN channels in the heart: lessons from mouse mutants
 REVIEWbph_1798 501..509 British Journal of Pharmacology DOI:10.1111/j.1476-5381.2011.01798.x www.brjpharmacol.org HCN channels in the heart: lessons from mouse mutants S Herrmann, F Hofmann, J Stieber
REVIEWbph_1798 501..509 British Journal of Pharmacology DOI:10.1111/j.1476-5381.2011.01798.x www.brjpharmacol.org HCN channels in the heart: lessons from mouse mutants S Herrmann, F Hofmann, J Stieber
Differences in ionic currents between canine myocardial and Purkinje cells
 ORIGINAL RESEARCH Physiological Reports ISSN 2051-817X Differences in ionic currents between canine myocardial and Purkinje cells Mario Vassalle & Leonardo Bocchi Department of Physiology and Pharmacology,
ORIGINAL RESEARCH Physiological Reports ISSN 2051-817X Differences in ionic currents between canine myocardial and Purkinje cells Mario Vassalle & Leonardo Bocchi Department of Physiology and Pharmacology,
NEURONS Chapter Neurons: specialized cells of the nervous system 2. Nerves: bundles of neuron axons 3. Nervous systems
 NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
NEURONS Chapter 12 Figure 12.1 Neuronal and hormonal signaling both convey information over long distances 1. Nervous system A. nervous tissue B. conducts electrical impulses C. rapid communication 2.
Nerve. (2) Duration of the stimulus A certain period can give response. The Strength - Duration Curve
 Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
Nerve Neuron (nerve cell) is the structural unit of nervous system. Nerve is formed of large numbers of nerve fibers. Types of nerve fibers Myelinated nerve fibers Covered by myelin sheath interrupted
The Electrocardiogram
 The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
The Electrocardiogram Chapters 11 and 13 AUTUMN WEDAN AND NATASHA MCDOUGAL The Normal Electrocardiogram P-wave Generated when the atria depolarizes QRS-Complex Ventricles depolarizing before a contraction
Chapter 3 Neurotransmitter release
 NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
NEUROPHYSIOLOGIE CELLULAIRE CONSTANCE HAMMOND Chapter 3 Neurotransmitter release In chapter 3, we proose 3 videos: Observation Calcium Channel, Ca 2+ Unitary and Total Currents Ca 2+ and Neurotransmitter
Cellular Sinoatrial Node and Atrioventricular Node Activity in the Heart
 Cellular Sinoatrial Node and Atrioventricular Node Activity in the Heart HJ Jansen and TA Quinn, Dalhousie University, Halifax, NS, Canada RA Rose, Dalhousie University, Halifax, NS, Canada; University
Cellular Sinoatrial Node and Atrioventricular Node Activity in the Heart HJ Jansen and TA Quinn, Dalhousie University, Halifax, NS, Canada RA Rose, Dalhousie University, Halifax, NS, Canada; University
CRC 431 ECG Basics. Bill Pruitt, MBA, RRT, CPFT, AE-C
 CRC 431 ECG Basics Bill Pruitt, MBA, RRT, CPFT, AE-C Resources White s 5 th ed. Ch 6 Electrocardiography Einthoven s Triangle Chest leads and limb leads Egan s 10 th ed. Ch 17 Interpreting the Electrocardiogram
CRC 431 ECG Basics Bill Pruitt, MBA, RRT, CPFT, AE-C Resources White s 5 th ed. Ch 6 Electrocardiography Einthoven s Triangle Chest leads and limb leads Egan s 10 th ed. Ch 17 Interpreting the Electrocardiogram
Self-sustained contractile activity is a fundamental cardiac. Reviews. The Role of the Funny Current in Pacemaker Activity.
 Reviews This article is the introduction of a new thematic series on Mechanisms of Pacemaking in the Heart, which includes the following articles: Be Still, My Beating Heart Never! [2010;106:238 239] Development
Reviews This article is the introduction of a new thematic series on Mechanisms of Pacemaking in the Heart, which includes the following articles: Be Still, My Beating Heart Never! [2010;106:238 239] Development
a lecture series by SWESEMJR
 Electrolyte disturbances Hypokalaemia Decreased extracellular potassium increases excitability in the myocardial cells and consequently the effect of very severe hypokalaemia is ventricular arrhythmia.
Electrolyte disturbances Hypokalaemia Decreased extracellular potassium increases excitability in the myocardial cells and consequently the effect of very severe hypokalaemia is ventricular arrhythmia.
Chapter 14. Agents used in Cardiac Arrhythmias
 Chapter 14 Agents used in Cardiac Arrhythmias Cardiac arrhythmia Approximately 50% of post-myocardial infarction fatalities result from ventricular tachycarida (VT) or ventricular fibrillation (VF). These
Chapter 14 Agents used in Cardiac Arrhythmias Cardiac arrhythmia Approximately 50% of post-myocardial infarction fatalities result from ventricular tachycarida (VT) or ventricular fibrillation (VF). These
The duration of the canine ventricular action potential
 Contribution of I Kr to Rate-Dependent Action Potential Dynamics in Canine Endocardium Fei Hua, Robert F. Gilmour, Jr Abstract Previous modeling studies have suggested that the rapid component of the delayed
Contribution of I Kr to Rate-Dependent Action Potential Dynamics in Canine Endocardium Fei Hua, Robert F. Gilmour, Jr Abstract Previous modeling studies have suggested that the rapid component of the delayed
High Ca Content of Pacemaker Tissues in the Frog Heart
 Short Communication Japanese Journal of Physiology, 34, 1117-1121,1984 High Ca Content of Pacemaker Tissues in the Frog Heart Yasuichiro FUKUDA Department of Physiology II, School of Medicine, Chiba University,
Short Communication Japanese Journal of Physiology, 34, 1117-1121,1984 High Ca Content of Pacemaker Tissues in the Frog Heart Yasuichiro FUKUDA Department of Physiology II, School of Medicine, Chiba University,
Ncardia. Assessment of pro-arrhythmic effects in Pluricyte Cardiomyocytes. using the Axion BioSystems Maestro TM MEA system
 Ncardia Stem cell experts Assessment of pro-arrhythmic effects in Pluricyte Cardiomyocytes using the Axion BioSystems Maestro TM MEA system Application note Version 2.0 Contents 1. Introduction 1 2. Assessment
Ncardia Stem cell experts Assessment of pro-arrhythmic effects in Pluricyte Cardiomyocytes using the Axion BioSystems Maestro TM MEA system Application note Version 2.0 Contents 1. Introduction 1 2. Assessment
Cardiac physiology. b. myocardium -- cardiac muscle and fibrous skeleton of heart
 I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
I. Heart anatomy -- general gross. A. Size/orientation - base/apex B. Coverings D. Chambers 1. parietal pericardium 2. visceral pericardium 3. Layers of heart wall a. epicardium Cardiac physiology b. myocardium
BRIEF COMMUNICATION CALCIUM- AND VOLTAGE-ACTIVATED POTASSIUM CHANNELS IN HUMAN MACROPHAGES. frequency of channel opening increased with depolarization
 BRIEF COMMUNICATION CALCIUM- AND VOLTAGE-ACTIVATED POTASSIUM CHANNELS IN HUMAN MACROPHAGES ELAINE K. GALLIN Physiology Department, Armed Forces Radiobiology Research Institute, Bethesda, Maryland 20814
BRIEF COMMUNICATION CALCIUM- AND VOLTAGE-ACTIVATED POTASSIUM CHANNELS IN HUMAN MACROPHAGES ELAINE K. GALLIN Physiology Department, Armed Forces Radiobiology Research Institute, Bethesda, Maryland 20814
Sample Lab Report 1 from 1. Measuring and Manipulating Passive Membrane Properties
 Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
Sample Lab Report 1 from http://www.bio365l.net 1 Abstract Measuring and Manipulating Passive Membrane Properties Biological membranes exhibit the properties of capacitance and resistance, which allow
Systolic and Diastolic Currents of Injury
 Systolic and Diastolic Currents of Injury Figure 1 Action Potentials of Normal and Ischemic Tissue In Figure 1 above, the action potential of normal myocardium is represented by the solid lines and the
Systolic and Diastolic Currents of Injury Figure 1 Action Potentials of Normal and Ischemic Tissue In Figure 1 above, the action potential of normal myocardium is represented by the solid lines and the
Synaptic Integration
 Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Synaptic Integration 3 rd January, 2017 Touqeer Ahmed PhD Atta-ur-Rahman School of Applied Biosciences National University of Sciences and Technology Excitatory Synaptic Actions Excitatory Synaptic Action
Cardiovascular System Notes: Physiology of the Heart
 Cardiovascular System Notes: Physiology of the Heart Interesting Heart Fact Capillaries are so small it takes ten of them to equal the thickness of a human hair. Review What are the 3 parts of the cardiovascular
Cardiovascular System Notes: Physiology of the Heart Interesting Heart Fact Capillaries are so small it takes ten of them to equal the thickness of a human hair. Review What are the 3 parts of the cardiovascular
This presentation will deal with the basics of ECG description as well as the physiological basics of
 Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Snímka 1 Electrocardiography basics This presentation will deal with the basics of ECG description as well as the physiological basics of Snímka 2 Lecture overview 1. Cardiac conduction system functional
Department of medical physiology 7 th week and 8 th week
 Department of medical physiology 7 th week and 8 th week Semester: winter Study program: Dental medicine Lecture: RNDr. Soňa Grešová, PhD. Department of medical physiology Faculty of Medicine PJŠU Cardiovascular
Department of medical physiology 7 th week and 8 th week Semester: winter Study program: Dental medicine Lecture: RNDr. Soňa Grešová, PhD. Department of medical physiology Faculty of Medicine PJŠU Cardiovascular
Intro. Comp. NeuroSci. Ch. 9 October 4, The threshold and channel memory
 9.7.4 The threshold and channel memory The action potential has a threshold. In figure the area around threshold is expanded (rectangle). A current injection that does not reach the threshold does not
9.7.4 The threshold and channel memory The action potential has a threshold. In figure the area around threshold is expanded (rectangle). A current injection that does not reach the threshold does not
The Cardiovascular System
 Chapter 18 Part A The Cardiovascular System 1/19/16 1 Annie Leibovitz/Contact Press Images Similarities of Cardiac and Skeletal Muscle RMP Ion concentration Deploarization Action Potential Repolarization
Chapter 18 Part A The Cardiovascular System 1/19/16 1 Annie Leibovitz/Contact Press Images Similarities of Cardiac and Skeletal Muscle RMP Ion concentration Deploarization Action Potential Repolarization
Mathematical modeling of ischemia and infarction
 Mathematical modeling of ischemia and infarction Mostly based on Cimponeriu, Starmer and Bezerianos: A theoretical analysis of acute ischemia and infarction using ECG reconstruction on a 2-D model of myocardium
Mathematical modeling of ischemia and infarction Mostly based on Cimponeriu, Starmer and Bezerianos: A theoretical analysis of acute ischemia and infarction using ECG reconstruction on a 2-D model of myocardium
Electrocardiography I Laboratory
 Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
Introduction The body relies on the heart to circulate blood throughout the body. The heart is responsible for pumping oxygenated blood from the lungs out to the body through the arteries and also circulating
Investigation of human cardiovascular physiology is very interesting, but many
 6 E X E R C I S E Frog Cardiovascular Physiology O B J E C T I V E S 1. To list the properties of cardiac muscle as automaticity and rhythmicity, and to define each. 2. To explain the statement, Cardiac
6 E X E R C I S E Frog Cardiovascular Physiology O B J E C T I V E S 1. To list the properties of cardiac muscle as automaticity and rhythmicity, and to define each. 2. To explain the statement, Cardiac
Electrocardiography Abnormalities (Arrhythmias) 7. Faisal I. Mohammed, MD, PhD
 Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
Electrocardiography Abnormalities (Arrhythmias) 7 Faisal I. Mohammed, MD, PhD 1 Causes of Cardiac Arrythmias Abnormal rhythmicity of the pacemaker Shift of pacemaker from sinus node Blocks at different
Blocks classification by their constancy or steadiness
 Blocks classification by their constancy or steadiness By their constance the blocks can be: 1. Permanent 2. Temporary, transient or transitory 3. Intermittent (Okajima 1980): 3a) Dependent on heart rate:
Blocks classification by their constancy or steadiness By their constance the blocks can be: 1. Permanent 2. Temporary, transient or transitory 3. Intermittent (Okajima 1980): 3a) Dependent on heart rate:
Ask Mish. EKG INTERPRETATION part i
 EKG INTERPRETATION part i What is EKG? EKG or ECG= electrocardiogram(~graphy) means the recording of the heart electrical activity from Greek kardio= heart, graphein= to write cardiac cell physiology Cardiac
EKG INTERPRETATION part i What is EKG? EKG or ECG= electrocardiogram(~graphy) means the recording of the heart electrical activity from Greek kardio= heart, graphein= to write cardiac cell physiology Cardiac
ECG and Cardiac Electrophysiology
 ECG and Cardiac Electrophysiology Simon Some very basic electrophysiology Intracellular fluid: 10 mm Na, 140 mm K, etc. K Na-K ATPase Extracellular fluid: 140mM Na, 4mM K, etc. Na Ion gradient plus selective
ECG and Cardiac Electrophysiology Simon Some very basic electrophysiology Intracellular fluid: 10 mm Na, 140 mm K, etc. K Na-K ATPase Extracellular fluid: 140mM Na, 4mM K, etc. Na Ion gradient plus selective
Mr. Eknath Kole M.S. Pharm (NIPER Mohali)
 M.S. Pharm (NIPER Mohali) Drug Class Actions Therapeutic Uses Pharmacokinetics Adverse Effects Other Quinidine IA -Binds to open and inactivated Na+ -Decreases the slope of Phase 4 spontaneous depolarization
M.S. Pharm (NIPER Mohali) Drug Class Actions Therapeutic Uses Pharmacokinetics Adverse Effects Other Quinidine IA -Binds to open and inactivated Na+ -Decreases the slope of Phase 4 spontaneous depolarization
